Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES
|
|
|
- Juliana Chase
- 6 years ago
- Views:
Transcription
1 Citagenix Catalogue 2017 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES
2 100% Allograft Hydratable Inductive Matrix NEW Create your customized osteoinductive graft by combining PentOS OI Max, a 100% allograft Inductive Collagen Matrix (ICM) with your choice of blood, BMA, PRP, I-PRF or simply saline to form a moldable graft which is cell friendly, independent laboratory in vitro studies show to be able to maintain between 96% and 116% cell viability after 72 hours. PENTOS OI Max is an osteoinductive matrix - not diluted by any carriers. FEATURES AND BENEFITS Mixed graft can be extruded through a syringe Demonstrated Osteoinductivity - up to 40x native BMP vs the BMP-2 control - each lot histologically demonstrates all 5 elements of bone formation Demonstrated Osteoconductivity ph balanced non cytotoxic Shown to form bone post-sterilization in-vivo Resists copious irrigation 5 year shelf life at ambient storage PentOS OI Max can resist copious irrigation Available jar sizes: 1cc and 2.5cc Create your own osteoinductive graft Mixed with saline or blood concentrate Can be extruded through a syringe CitagenixUSA.com PentOS-OI.com
3 CONTENTS 2017 MEMBRANES BONE GRAFTS PENTOS OI Proven to form bone Page 6 C-GRAFT PUTTY & C-BLAST PUTTY Demineralized Bone Matrix Page 7 RAPTOS Allograft bone particulates and bone blocks Page 8 ECLIPSE Synthetic resorbable bone substitute Page 9 10 NEOMEM Resorbable Bovine Collagen Membrane Page 11 NEOMEM FLEXPLUS Resorbable Porcine Collagen Membrane Page 12 NEODERM Natural elasticity of human acellular dermis Page 13 4 new product WOUND DRESSINGS 14 NEOPLUG Cylindrical shape adapts easily to surgical sites Page 14 NEOCOTE Thicker sponge shape protects soft tissue Page 14 NEOTAPE Thinner tape like design for more intricate uses Page info@citagenix.com CitagenixUSA.com 3
4 Visit our website at CitagenixUSA.com ICM Versatile Grafting Solutions DBM DBM Regenerative properties Description PentOS OI Max 100% Allograft Inductive Collagen Matrix (ICM) Create your customized osteoinductive graft by combining PentOS OI Max with your choice of blood, blood cell concentrate or simply saline to form a moldable graft which can be extruded though a syringe. PentOS OI Max is an osteoinductive matrix - not diluted by any carriers. PentOS OI Putty PentOS OI Putty is made from 100% human bone without any additional diluent or carriers to diminish its osteoinductive properties. EVERY lot used to manufacture PentOS OI products is verified to be osteoinductive and histologically demonstrates all five elements of bone formation. C-Graft Putty Demineralized Bone Matrix in CMC - a natural, plant derived, carrier for superior handling. C-Blast Putty A composite graft product that combines osteoinductive DBM and osteoconductive cancellous particles in CMC. Characteristics Osteoinductive - up to 40x native BMP vs the BMP-2 control - each lot histologically demonstrates all 5 elements of bone formation Osteoconductive Cell friendly over 96% cell viability ph balanced non cytotoxic Shown to form bone post-sterilization in-vivo Resists copious irrigation 100% allograft - no additional carrier 100% bone Verified to be osteoinductive Osteoconductive Excellent handling characteristics Resists irrigation Sterilized using low-dose gamma irradiation Ready to use Stored at room temperature No inert carrier up to 19x native BMP vs the BMP-2 control C-Graft Putty: DBM with natural CMC carrier Excellent handling Great pricing Ready to use Open bore syringe C-Blast Putty: Demineralized Bone Matrix (DBM) with Cancellous Bone and CMC carrier Osteoinductive and osteoconductive Applications An osteoinductive base for composite graft preparation Bone defects and sites requiring a high level of osteoinduction Periodontal defects Implant site development Coronal defects around immediate implants Extraction site repair Implant dehiscence defects Sinus lift procedures Moderate localized ridge defects Periodontal defects Implant site development Coronal defects around immediate implants Extraction site repair Implant dehiscence defects Sinus lift procedures Moderate localized ridge defects Mixing options May be hydrated with saline, blood, BMA, PRP or other biological preparations as the clinician deems appropriate. May be used alone or mixed with Raptos allograft bone and/or Eclipse synthetic bone. May be used alone or mixed with Raptos allograft bone and/or Eclipse synthetic bone. Availability Available jar sizes: 1cc and 2.5cc Putty in a syringe. 0.5 cc, 1 cc, 2.5 cc and 5 cc Putty in a syringe. 0.3 cc, 0.5 cc, 1 cc and 3 cc DBM Demineralized Bone Matrix ICM Inductive Collagen Matrix
5 Versatile Grafting Solutions ALLOGRAFT ALLOGRAFT SYNTHETIC Regenerative properties Raptos Allograft Cancellous, cortical, cortico-cancellous and demineralized irradiated bone particulates that offer an osteoconductive scaffold for bone regeneration. Premium Blend This cortical mineralized/demineralized (70/30) blend combines two of the most popular allograft particles used in dentistry, all in a single syringe! PentOS OI Flex Demineralized, osteoinductive 100% cortical bone veneer graft. When hydrated, the veneer graft becomes pliable and easily adapts to graft sites that would be difficult to treat with particulate or putty grafts. Eclipse Synthetic resorbable bone substitute that uses the mineral building blocks naturally found in human bone. Granules: 80%ß-tcp - 20%HA Putty: HA + ß-tcp + Hydrogel Description Osteoconductive scaffold Remodels completely to host bone Mineralized graft material Osteoinductive demineralized Cortical veneer 1 mm thick Can be fixated with screws, tacks and sutures Freeze-dried Sterilized using low-dose gamma irradiation Has a shelf-life of 5 years from the date of sterilization Macro and Micro pores allow complete remodeling Completely remodels to host bone while preserving volume (12-18 months*) * Resorption and bone regeneration times are estimates as each patient and case is unique. Characteristics Mineralized component in a composite graft As a graft extender Osseous defects Onlay grafting for the repair of shallow bone defects or bone loss To augment thin or missing bone over root form implants Orbital floor repair Cases where it is difficult to place particulate grafts Ridge preservation Extraction site repair Sinus lift procedures Ridge augmentation Osseous defects Periodontal defects Applications May be used alone or mixed with DBM Putty for osteoinductive potential. May be used alone or in combination with other graft materials. May be used alone or mixed with DBM Putty for osteoinductive potential. Mixing options Available in syringes in a variety of sizes. Available in these sizes: 10x10 mm, 15x15 mm and 17x10 mm Granules available in vials or syringes. Putty available in syringes. Availability CMC Carboxymethylcellulose carrier 5
6 100% Bone. Proven to form Bone. Every Lot. PENTOS OI ALLOGRAFTS MUST DEMONSTRATE ALL FIVE ELEMENTS OF BONE FORMATION BEFORE THEY ARE RELEASED FOR USE. Post sterilization in-vivo testing on each lot must demonstrate the presence of new bone, bone marrow, osteocytes, cartilage and chondrocytes within 28 days in an athymic rat model otherwise we can t call it PentOS OI. In-vitro tests of PentOS OI have demonstrated native levels of BMP-2 up to 19 times the native levels in the control. 100% Allograft Hydratable Inductive Matrix Create your customized osteoinductive graft by combining PentOS OI Max, a 100% allograft Inductive Collagen Matrix (ICM) with your choice of blood, BMA, PRP, I-PRF or simply saline to form a moldable graft which - is cell friendly - Independent laboratory in vitro studies show to be able to maintain between 96% and 116% cell viability after 72 hours. PENTOS OI Max is an osteoinductive matrix - not diluted by any carriers. Available jar sizes: 1cc and 2.5cc PentOS OI Max can resist copious irrigation 100% Allograft DBM Putty PentOS OI Putty is made from 100% human bone without any additional diluent or carriers to diminish its osteoinductive properties. EVERY lot used to manufacture PentOS OI products is verified to be osteoinductive and histologically demonstrate all five elements of bone formation. Proven to form bone. Every time. It s that simple! 100% Allograft Flexible Veneer Graft PentOS OI Flex is a 1 mm thick osteoinductive, demineralized cortical bone graft. When hydrated, this veneer graft becomes pliable and easily adapts to graft sites that would be difficult to treat with particulate or putty grafts. These characteristics make PentOS OI Flex ideally suited for onlay grafting for the repair of shallow bone defects or bone loss. Available sizes: 10x10mm, 15x15mm and 17x10mm 100% Allograft Compressible Bone Matrix PentOS OI Sponge is made of cancellous and demineralized bone, it is available as strips or blocks. Strips: 20x10x10mm and 20x15x7mm Blocks: 10mm³ and 12mm³ Visit our microsite at PentOS-OI.com 6
7 DBM with or without cancellous chips. Demineralized Bone Matrix (DBM) Putty + CMC DBM CMC Demineralized Bone Matrix Putty + CMC + Cancellous bone chips DBM Cancellous CMC CMC CARRIER Natural Plant Derived Carrier Carboxymethylcellulose (CMC) A polysaccharide hydrogel derived from linters of the cotton seed Commonly used in food, cosmetics and pharmaceutical preparations Excellent biocompatibility does not interfere with cell mediated bone formation Odourless, tasteless and nontoxic High viscosity physiologically inert carrier which maintains its interface at the graft site Malleable - can be molded and placed into any shaped defect Excellent dimensional stability does not significantly shrink nor expand Will not migrate from the graft site Resorbs slowly in harmony with new bone formation allowing for cellular and vascular infiltration Haemostatic effect when grafted into a bleeding site FDA classifies CMC as GRAS (generally regarded as safe) Features Quality products Excellent handling Great pricing Ready to use Room temperature storage Open bore syringes E-beam sterilization Double sealed blister packaging Common applications Socket preservation Extraction site repair Implant dehiscence defects Sinus lift procedures Moderate localized ridge defects Available syringe sizes: 0.3 cc 0.5 cc 1 cc 3 cc AVAILABLE IN 0.3 cc SYRINGES 7 Visit our microsite at Citagenix-DBM.com
8 All your bone allografts... under one wing! RAPTOS IRRADIATED BONE PARTICULATES, A NATURAL SCAFFOLD FOR BONE REGENERATION. RAPTOS allograft mineralized bone grafts offer an osteoconductive platform for bone regeneration and Raptos demineralized particles offer an osteoinductive boost. Used either alone or as part of a composite bone graft, allograft bone particulates supply a natural framework facilitating the attachment of osteogenic precursor cells. Premium Blend - This Cortical Mineralized/Demineralized blend combines two of the most popular allograft particles used in dentistry, all in a single syringe! SAFETY INFORMATION Each donor is thoroughly evaluated using medical/ social history questions, medical records, blood tests, culture results, physical examination and autopsy reports (when performed). This process is used to ensure the donor is suitable for donation by allowing to recognize and exclude potential diseases or medical conditions that are unacceptable. Specific lab tests are performed for Syphilis, Hepatitis B and C, HIV and other viruses. All of the donor chart information is evaluated by individuals trained in tissue banking, prior to the processing of the tissue. Tissue grafts are rinsed and soaked in various solutions to minimize transmission of bacteria and viruses. Processing and packaging of the tissue are performed using aseptic technique and occur in a clean room. As an added margin of safety, a low dose gamma irradiation after final packaging of the musculoskeletal grafts is utilized. AATB accredited source and mandatory CTO registered with Health Canada. Raptos prefilled syringes are available in cancellous, cortical, cortico-cancellous,demineralized and Premium Blend particulates ( µm): 0.25 cc, 0.5 cc & 1.0 cc Visit our microsite at Raptos.com 8
9 The science of balanced bone regeneration. SYNTHETIC RESORBABLE BONE SUBSTITUTE GRANULES & PUTTY For patients who express concerns with allografts and xenografts Completely remodels to host bone 25 years of clinical experience ECLIPSE GRANULES & PUTTY 100% SYNTHETIC - COMPLETELY TRANSFORMS TO NATURAL BONE Using the mineral building blocks naturally found in human bone, Eclipse goes a step beyond the realm of everyday hydroxyapatite (HA) and tricalcium phosphate (TCP) with its unique combination of micro and macroporosity technology. 70% overall porosity 2/3 macroporosity 1/3 microporosity Granules: Eclipse Granules, a 100% synthetic (20% HA, 80% TCP), costeffective bone graft matrix used as an alternative to biologic osteoinductive products. Putty: Eclipse Putty is an innovative and moldable bone graft, composed of Hydroxyapatite (HA), Beta Tricalcium Phosphate (ß-TCP) and a hydrogel. Microporosity (pores <10μm) allows the diffusion of biological fluids, by the capillary action, into the three-dimensional matrix. Inside the fine pores of the matrix, biological fluids dissolve ß-TCP producing calcium phosphate ions that are released back into the biological fluids. This saturation of ions leads to precipitation of apatite crystal identical to that of the natural bone minerals. Shortly afterwards, this process creates a surface that promotes bone cell adhesion. MICRO MACRO Granules ( mm) available in syringes of 0.5cc & 1.0cc or in vials of 0.25cc, 0.5cc & 1.0cc Granules ( mm) available in vials of 2.0cc Putty available in syringes of 0.5cc & 1.0cc Macropores (300 to 600 μm) promote a deep invasion of osteogenic cells by osteoconduction. Through the same resorption and formation cycle as the natural bone remodeling process, new bone increases progressively at the expense of Eclipse. 9 Visit our website at CitagenixUSA.com
10 Visit our website at CitagenixUSA.com A wide variety of membrane options BOVINE PORCINE ALLOGRAFT Description Neomem Resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. Neomem FlexPlus Single layer collagen membrane derived from porcine peritoneum. Neoderm Acellular human dermis available in thicknesses of μm (thin) and μm (thick). Characteristics Cell occlusive Allows nutrient transfer Optimized flexibility and rigidity Either side can be placed towards the soft tissue or bone Easily trimmed and placed, wet or dry Gamma irradiated to sterilize Fixated with sutures or tacks Nonpyrogenic Suture pull out strength between 290g and 350g High suture pull out strength Excellent draping Lower inflammation 3-4 month resorption Easy handling Human derived dermal grafts are decellularized to remove cellular components and preserve the biological properties that promote revascularization and repair. Neoderm acelluar dermis, has the natural elasticity of human skin. Neoderm is minimally processed to remove epidermal and dermal cells while preserving the extracellular matrix of the dermis. The grafts are freeze-dried and sterilized. GTR GBR GTR GBR Replacement of damaged or inadequate tissue for the repair, reinforcement, or supplemental support of soft tissue defects. Applications Resorption 26 to 38 weeks 3 to 4 months 3 to 4 months (thin) 4 to 6 months (thick) Availability 15x20, 20x30 & 30x40 mm 15x20, 20x30 & 30x40 mm 10x10, 10x20, 10x40 & 20x40 mm in either thin ( μm) or thick ( μm) GP (01/2017) GTR Guided Tissue Regeneration GBR Guided Bone Regeneration 10
11 The standard in membrane technology. NEOMEM MEMBRANE IS A TYPE 1 BOVINE COLLAGEN MATRIX INDICATED FOR USE IN GUIDED TISSUE PROCEDURES TO ENHANCE WOUND HEALING. Advantages Higher mechanical strength for membrane stabilization in situ using either sutures or resorbable tacks. Longer in vivo stability and resorption time, weeks, for sustained function. Optimized flexibility and rigidity for better space maintenance which allows for desired tissue in-growth. Either side of the Neomem can be placed towards the soft tissue or bone. Easily trimmed and placed, wet or dry. NEOMEM RESORPTION RATE Available in convenient sizes 15x20 mm 20x30 mm 30x40 mm NeoMem is sterilized by irradiation and packaged in double pouches. EXCEPTIONAL CLINICAL PERFORMANCE Suture pull out strength between 290g and 350g Cell occlusive - retards epithelium down growth Macromolecular pore size allows nutrient transfer WHY MAKE NEOMEM YOUR MEMBRANE OF CHOICE? Highly purified type I collagen fibres derived from bovine Achilles tendon Nonpyrogenic Excellent safety profile Easy in situ placement 11 Visit our microsite at Neomem-membrane.com
12 Strong performance with a supple touch. NEOMEM FLEXPLUS - Native porcine peritoneum Neomem FlexPlus is a single layer collagen membrane derived from porcine peritoneum. Suture pullout testing has shown this membrane to be biomechanically strong yet conformability evaluations demonstrate the ability to adapt to the contour of the surgical site. Neomem FlexPlus hydrates quickly and handling characteristics make surgical placement easy. It can be repositioned easily and placed wet or dry and with either side up. Additionally, in-vitro testing of Neomem FlexPlus shows low tissue inflammation and foreign body giant cell response. INFLAMMATION SCORE Bio-Gide Neomem FlexPlus 4 weeks 8 weeks 12 weeks 16 weeks GIANT CELLS SCORE Bio-Gide Neomem FlexPlus 4 weeks 8 weeks 12 weeks 16 weeks *Published in Science, Technology, Innovation, Feb. 1-5, 2015 All trademarks are the property of their respective owners. Compared to Bio-Gide : Stronger Neomem FlexPlus has 3 times the suture pull out strength compare to Bio-Gide and resorption time of 3-4 months. Neomem FlexPlus can be repositioned once placed. Supple Lack of memory means that Neomem FlexPlus drapes and conforms to the defect site and since both sides are the same, it is easy to place. Better Initial Resorption Rate Compared to Bio-Gide, Neomem FlexPlus initial in-vivo resorption profile suggests that Neomem FlexPlus is more stable. Less Inflammation In-vivo testing also demonstrates that Neomem FlexPlus produced a lower level of early inflammation than Bio-Gide and Neomem FlexPlus shows a lower level of giant cell response. Advantages: High suture pull out strength Excellent draping Lower inflammation 3-4 month resorption Easy handling Available in convenient sizes: 15x20, 20x30 & 30x40 mm Visit our microsite at Neomem-membrane.com 12
13 Natural elasticity of human acellular dermis. NEODERM IS HUMAN ACELLULAR DERMIS, WHICH HAS NATURAL ELASTICITY. These human derived dermal grafts are decellularized to remove cellular components and preserve the biological properties that promote revascularization and repair. Neoderm acellular dermis, has the natural elasticity of human skin. Neoderm is minimally processed to remove epidermal and dermal cells while preserving the extracellular matrix of the dermis. The grafts are freeze-dried and sterilized providing 5 years of shelf life at room temperature. Neoderm can be used to replace damaged or inadequate tissue for the repair, reinforcement, or supplemental support of soft tissue defects. Neoderm is available in a variety of sizes and thickness, and can be shaped with scissors or a scalpel. Neoderm is ideal for the replacement of damaged or inadequate tissue for the repair, reinforcement, or supplemental support of soft tissue defects. Resorption 3 to 4 months (thin) 4 to 6 months (thick) Available in convenient sizes in either thin ( µm) or thick ( µm) 10x10 mm 10x20 mm 10x40 mm 20x40 mm 13 Visit our website at CitagenixUSA.com
14 Collagen Dental Wound Dressings Our Resorbable Wound Dressings are absorbent and porous collagen matrices engineered from purified collagen that is derived from bovine dermis tissue. The thickness and pore structure of these devices allow them to absorb fluid and blood at the defect site. 10 mm x 20 mm Cylindrical shape adapts easily to surgical sites 20 x 40 x 3 mm Thicker sponge shape protects soft tissue 25 x 75 x 1 mm Thinner tape like design for more intricate uses Product Features: Applied directly to the wound and protect the wound and delicate new tissue Aids in wound healing Available in plug, cote and tape form Supplied sterile, non-pyrogenic and for single use only Essentially resorbs within 30 days of placement Indications: Denture sores Oral ulcers (non-infected or viral) Periodontal surgical wounds Suture sites Burns Extraction sites Surgical wounds Traumatic wounds new product Visit our website at CitagenixUSA.com 14
15 Visit our microsites to find out everything you need to know. The complete information on our products. Optimized for perfect viewing on your mobile device or on your computer. Documents, case studies, videos & more at your fingertips.
16 Our management and sales directors are experts in their field with over 20 years of experience in dental devices and grafting materials. Our Customer Service Team is made up of members with extensive experience in regenerative products to help our customers get answers and results. One of the reasons our customers rely on us is because most of our orders are shipped the same day when placed before 3 PM EST info@citagenix.com CitagenixUSA.com GP (04/2017)
Bone & Membrane. Catalogue Biologic Regenerative Products ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES
 Bone & Membrane Biologic Regenerative Products Catalogue 2016 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES PTFE Surgical Sutures Bone & Membrane Biologic Regenerative Products
Bone & Membrane Biologic Regenerative Products Catalogue 2016 ALLOGRAFTS BONE SUBSTITUTES BONE GRANULES & BLOCKS RESORBABLE MEMBRANES PTFE Surgical Sutures Bone & Membrane Biologic Regenerative Products
Versatile grafting Solutions
 Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
A wide variety of membrane options
 A wide variety of membrane options BOVINE Bovine collagen membrane Neomem is a resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. The macromolecular
A wide variety of membrane options BOVINE Bovine collagen membrane Neomem is a resorbable collagen membrane derived from highly purified type I collagen fibres from bovine Achilles tendon. The macromolecular
Innovative Range of Regenerative Solutions
 TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
TM Innovative Range of Regenerative Solutions MIS Implant Technologies Ltd. All rights reserved. Optimal volumes and quality of hard and soft tissue are required to satisfy the goals of oral rehabilitation
FDBA(Freeze Dried Bone Allograft) SureOss Cortical Bone - Powder / Chip. OsteOss Cortical with Cancellous Bone - Powder / Chip
 PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
PERIODONTAL Bone & Skin Allograft Products FDBA(Freeze Dried Bone Allograft) SureOss - Powder / Chip OsteOss Cortical with - Powder / Chip DFDBA(Demineralized Freeze Dried Bone Allograft) SureOss -D Demineralized
Biomaterials Line. MIS Corporation. All Rights Reserved.
 7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
7. Biomaterials Line MIS Corporation. All Rights Reserved. 6. MIS s Quality System complies with international quality standards: ISO 13485: 2003 - Quality Management System for Medical Devices, ISO 9001:
More than bone regeneration. A total solution.
 More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
More than bone regeneration. A total solution. More than a dental implant company. A total solution. When it comes to treatment options, your patients want positive results both functionally and esthetically.
DRIVING THE FUTURE. About HOSPITAL INNOVATIONS. Strong commitment to healthcare innovation
 idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
BEGO BIOMATERIALS When the result counts
 BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
BEGO BIOMATERIALS When the result counts Partners in Progress INTRO Challenge what exists get the right answers We practise systematic thinking with a passion and we are never satisfied with the status
A WIDE RANGE OF REGENERATIVE SOLUTIONS
 A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
A WIDE RANGE OF REGENERATIVE SOLUTIONS INDICATIONS: 1/ SOCKET AND RIDGE PRESERVATION 2/ FILLING OF EXTRACTION SOCKETS Biomaterials offers portfolio of regenerative materials for implantology, aimed at
HOSPITAL INNOVATIONS
 idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
idente Catalogue About HOSPITAL INNOVATIONS Since 2008, Hospital Innovations has been supplying a growing range of specialist products for use in orthopaedic and corrective surgery; with an emphasis on
SPINE BIOMATERIALS Crop Bleed
 A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
BIOMATERIAL COLLECTION
 BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Allograft Bone Grafting DirectGen 1-2 Bone Grafting DirectGen Putty 3 Bone Grafting DirectGen FLEX
BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Allograft Bone Grafting DirectGen 1-2 Bone Grafting DirectGen Putty 3 Bone Grafting DirectGen FLEX
BioVin Collagen Membrane
 BioVin Collagen Membrane Resorbable cross-linked collagen membrane BioVin Bovine Bone Bovine Bone Substitute OToss Synthetic Bone Synthetic Resorbable Biphasic Calcium Phosphate OToss Synthetic Bone Inject
BioVin Collagen Membrane Resorbable cross-linked collagen membrane BioVin Bovine Bone Bovine Bone Substitute OToss Synthetic Bone Synthetic Resorbable Biphasic Calcium Phosphate OToss Synthetic Bone Inject
THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options
 THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options 2 MEDLINE BUILD ON QUALITY. Successful bone fusion and osteogenesis depend on a number of factors
THE BUILDING BLOCKS OF BONE FUSION. Medline Demineralized Bone Allografts Safe and Effective Grafting Options 2 MEDLINE BUILD ON QUALITY. Successful bone fusion and osteogenesis depend on a number of factors
PERIODONTAL Bone & Skin Allograft Products
 PERIODONTAL Bone & Skin Allograft Products SureOss Family SureOss Cortical Bone - / SureOss Plus Cortical Bone with CA - / SureOss Collagen Cortical Bone with Collagen - Sponge Block SureOss Paste Cortical
PERIODONTAL Bone & Skin Allograft Products SureOss Family SureOss Cortical Bone - / SureOss Plus Cortical Bone with CA - / SureOss Collagen Cortical Bone with Collagen - Sponge Block SureOss Paste Cortical
DEMINERALIZED BONE MATRIX
 DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
DEMINERALIZED BONE MATRIX DEMINERALIZED BONE MATRIX GRAFT OVERVIEW BioAdapt is a pre-formed demineralized bone matrix (DBM) comprised of 00 percent donated human musculoskeletal tissue. BioAdapt DBM provides
Guided Tissue and Bone Regeneration
 Guided Tissue and Bone Regeneration One toolbox for your needs! Tefguide 9000 742 401 12x24 mm pc 1 Osgide 9000 701 520 15 x 20 mm pc 1 Osbone 9000 800 255 250-1000 μm 0.25 cc 5 Product Specifications
Guided Tissue and Bone Regeneration One toolbox for your needs! Tefguide 9000 742 401 12x24 mm pc 1 Osgide 9000 701 520 15 x 20 mm pc 1 Osbone 9000 800 255 250-1000 μm 0.25 cc 5 Product Specifications
BIOMATERIAL COLLECTION
 BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Xenograft Bone Grafting DirectOss 1 Allograft Bone Grafting DirectGen 2-3 Bone Grafting DirectGen Putty
BIOMATERIAL COLLECTION www.implantdirect.com Innovation. Quality. Service. Value. INDEX GRAFTING PAGE Xenograft Bone Grafting DirectOss 1 Allograft Bone Grafting DirectGen 2-3 Bone Grafting DirectGen Putty
Inion BioRestore. Bone Graft Substitute. Product Overview
 Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Product Information. MIS Corporation. All Rights Reserved.
 Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
REGENERATION BY DESIGN
 REGENERATION BY DESIGN ALLOGRAFT, XENOGRAFT, PUTT Y & BIOACTIVE GLASS FOR BONE REGENERATION INSTRUMENTS FOR COLLECTING BONE BARRIER MEMBRANES FOR GUIDED REGENERATION GUIDED IMPLANT TREATMENT Wide Selection
REGENERATION BY DESIGN ALLOGRAFT, XENOGRAFT, PUTT Y & BIOACTIVE GLASS FOR BONE REGENERATION INSTRUMENTS FOR COLLECTING BONE BARRIER MEMBRANES FOR GUIDED REGENERATION GUIDED IMPLANT TREATMENT Wide Selection
Product Catalog. Dental Bone & Tissue Regeneration
 Product Catalog Dental Bone & Tissue Regeneration Bone graft regeneration Guided bone substitutes regeneration Osteogenesis Controlled resorption rate Angiogenesis Long term volume stability Remodeling
Product Catalog Dental Bone & Tissue Regeneration Bone graft regeneration Guided bone substitutes regeneration Osteogenesis Controlled resorption rate Angiogenesis Long term volume stability Remodeling
Symbios Xenograft Granules Porcine Bone Graft Material
 Symbios Xenograft Granules Porcine Bone Graft Material 32671122-USX-1607_Symbios Highlight Brochure.indd 1 2016-09-27 12:12 Symbios Xenograft Granules Porcine bone graft material Symbios Xenograft Granules
Symbios Xenograft Granules Porcine Bone Graft Material 32671122-USX-1607_Symbios Highlight Brochure.indd 1 2016-09-27 12:12 Symbios Xenograft Granules Porcine bone graft material Symbios Xenograft Granules
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute.
 chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
chronos Bone Void Filler. Beta-Tricalcium Phosphate ( β-tcp) bone graft substitute. Osteoconductive Resorbable Synthetic chronos Bone Void Filler chronos granules and preforms are synthetic, porous, osteoconductive,
Alpha-Bio's GRAFT complete portfolio bone substitutes resorbable membranes best combination of quality & value with efficient, easy-to-use products
 Alpha-Bio Tec, a world leader in advanced implantology solutions, is proud to present its growing biomaterials product line: Alpha-Bio's GRAFT, which has taken a leading position in the global dental biomaterials
Alpha-Bio Tec, a world leader in advanced implantology solutions, is proud to present its growing biomaterials product line: Alpha-Bio's GRAFT, which has taken a leading position in the global dental biomaterials
Bone Grafting for Socket Preservation
 Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Bone Grafting for Socket Preservation Dr. Karl R. Koerner Normal extraction facial bone loss. Excessive force. Commonly the thickness of facial bone. Hussain, A. et al. Ridge preservation comparing a nonresorbable
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management Human Histology OSTEON II 26.5 months Regeneration Products information Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON
Regeneration Bone Grafting & Soft Tissue Management Human Histology OSTEON II 26.5 months Regeneration Products information Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON
Cerasorb M DENTAL. O:\Zulassung\Cerasorb Dental Kanada 2013\Texte\Cerasorb M Dental final IFU docx
 Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
Cerasorb M DENTAL Resorbable, pure-phase beta-tricalcium phosphate matrix with interconnecting porosity for bone regeneration for use in dental and maxillofacial surgery DESCRIPTION: Cerasorb M DENTAL
botiss biomaterials bone & tissue regeneration collacone max Innovative composite matrix socket preservation form-fitting resorbable composite
 bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 Socket preservation safeguarding your sockets collacone.. max flexbone*
bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 Socket preservation safeguarding your sockets collacone.. max flexbone*
(866) Oragen Particulate Bone. and Tape. Calcium Sulfate Hemihydrate. Membranes. NuOss
 (866)229.6025 Oragen Particulate Bone Membranes DBM Putty Calcium Sulfate Hemihydrate Collagen Foam, Plugs and Tape NuOss Bone Particulate Calcium Sulfate DBM Put t y Membranes Collagen Sutures 1 O r d
(866)229.6025 Oragen Particulate Bone Membranes DBM Putty Calcium Sulfate Hemihydrate Collagen Foam, Plugs and Tape NuOss Bone Particulate Calcium Sulfate DBM Put t y Membranes Collagen Sutures 1 O r d
botiss dental bone & tissue regeneration biomaterials collacone max Innovative composite matrix socket preservation form-fitting resorbable composite
 dental bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 botiss regeneration system maxresorb flexbone* collacone.. max
dental bone & tissue regeneration botiss biomaterials socket preservation Innovative composite matrix form-fitting resorbable composite 1 botiss regeneration system maxresorb flexbone* collacone.. max
Procedure Manual and Catalog
 Procedure Manual and Catalog TM Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption. Although
Procedure Manual and Catalog TM Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption. Although
Step-by-Step Step-by-Step. Internal Hex. Implant System MAKE IT SIMLE
 4. 7. Step-by-Step Cemented Step-by-Step Bridge BONDBONE Using Abutments Internal Hex. Implant System MAKE IT SIMLE MIS Corporation. All Rights Reserved. Published by MIS, which reserves the right to ameliorate
4. 7. Step-by-Step Cemented Step-by-Step Bridge BONDBONE Using Abutments Internal Hex. Implant System MAKE IT SIMLE MIS Corporation. All Rights Reserved. Published by MIS, which reserves the right to ameliorate
REGENERATIONTIME. A Case Report by. Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor
 A Case Report by Dr. Daniele Cardaropoli Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor The Situation An adult female patient presented with an endodontic/prosthetic failure
A Case Report by Dr. Daniele Cardaropoli Ridge Augmentation and Delayed Implant Placement on an Upper Lateral Incisor The Situation An adult female patient presented with an endodontic/prosthetic failure
OssiMend Bone Graft Matrix
 OssiMend All-Natural Mineral-Collagen Bone Grafting Matrix Anorganic bone mineral and type I collagen Highly purified, biocompatible matrix Osteoconductive Osteoinductive and Osteogenic in conjunction
OssiMend All-Natural Mineral-Collagen Bone Grafting Matrix Anorganic bone mineral and type I collagen Highly purified, biocompatible matrix Osteoconductive Osteoinductive and Osteogenic in conjunction
Developments in bone grafting in veterinary orthopaedics part one
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Developments in bone grafting in veterinary orthopaedics part one Author : John Innes, Peter Myint Categories : Vets Date
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Developments in bone grafting in veterinary orthopaedics part one Author : John Innes, Peter Myint Categories : Vets Date
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Cytoflex Barrier Membrane Clinical Evaluation
 Cytoflex Barrier Membrane Clinical Evaluation Historical Background Guided tissue regeneration is a well established concept in the repair of oral bone defects. The exclusion of soft tissue epithelial
Cytoflex Barrier Membrane Clinical Evaluation Historical Background Guided tissue regeneration is a well established concept in the repair of oral bone defects. The exclusion of soft tissue epithelial
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management OSTEON TM II Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II 08 OSTEON TM 12 ORTHOPEDIC OSTEON TM 14
Regeneration Bone Grafting & Soft Tissue Management OSTEON TM II Table of Contents Bone Graft Material OSTEON TM II Collagen 04 OSTEON TM Collagen 06 OSTEON TM II 08 OSTEON TM 12 ORTHOPEDIC OSTEON TM 14
BONE GRAFTING. Product Catalog
 BONE GRAFTING Product Catalog PRODUCT LINE Titanium Mesh Surgitime Titanium Titanium Foil Surgitime Titanium Seal Polytetraflouroethylene Membrane Surgitime PTFE Bone Screws Fixation Set We innovate to
BONE GRAFTING Product Catalog PRODUCT LINE Titanium Mesh Surgitime Titanium Titanium Foil Surgitime Titanium Seal Polytetraflouroethylene Membrane Surgitime PTFE Bone Screws Fixation Set We innovate to
The Essential Choice. With the Benefits of Biologic Predictability
 The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26
 P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
P105 Predictable Bone Grafting for Site Preparation for Implants and Prosthetics Workshop JAMES GRISDALE, DDS THURSDAY, FEBRUARY 26 Please complete the speaker evaluation form in the Midwinter Meeting
Regeneration Bone Grafting & Soft Tissue Management
 Regeneration Bone Grafting & Soft Tissue Management 1 Regeneration Bone Grafting & Soft Tissue Management Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON Collagen 5 6
Regeneration Bone Grafting & Soft Tissue Management 1 Regeneration Bone Grafting & Soft Tissue Management Table of contents Bone Graft Material OSTEON II OSTEON OSTEON II Collagen OSTEON Collagen 5 6
SYNPOR POROUS POLYETHYLENE IMPLANTS. For craniofacial and orbital augmentation and reconstruction
 SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
Grafting Material. Grafting Material MEMBRANE US$ OUSIA Bio Adapt B Memb 15x20 1pk. OUSIA Bio Adapt B Memb 20x30 1pk
 www.usdentaldepot.com - sales@usdentaldepot.com - 1 1-866-625-5558 - 1-954-874-6325 MEMBRANE OUSIA - 123152 Bio Adapt B Memb 15x20 1pk OUSIA - Bio Adapt barrier membrane- 15x20-1 per box - # OUSB1 OUSIA
www.usdentaldepot.com - sales@usdentaldepot.com - 1 1-866-625-5558 - 1-954-874-6325 MEMBRANE OUSIA - 123152 Bio Adapt B Memb 15x20 1pk OUSIA - Bio Adapt barrier membrane- 15x20-1 per box - # OUSB1 OUSIA
The Essential Choice. With the Benefits of Biologic Predictability
 The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
The Essential Choice With the Benefits of Biologic Predictability Documented, Reliable, Experienced is the essential choice for your daily regenerative needs. Throughout our long history and dedication
OSSIX PLUS The Resorbable Collagen Membrane Instructions for Use for OSSIX PLUS
 OSSIX PLUS The Resorbable Collagen Membrane Instructions for Use for OSSIX PLUS DESCRIPTION OSSIX PLUS is a biodegradable and biocompatible collagen membrane intended for use during the process of guided
OSSIX PLUS The Resorbable Collagen Membrane Instructions for Use for OSSIX PLUS DESCRIPTION OSSIX PLUS is a biodegradable and biocompatible collagen membrane intended for use during the process of guided
Surgical Solutions USA Regenerative Product Catalog USA
 RPC Surgical Solutions Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030 $230
RPC Surgical Solutions Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030 $230
Bone augmentation with biomaterials
 Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
Patient information dental bone & tissue regeneration botiss biomaterials Bone augmentation with biomaterials established safe natural X100 Implantation stability is crucial for success Atrophy of the
REASONS TO USE R.T.R.
 3 REASONS TO USE R.T.R. AFTER EACH EXTRACTION Fully resorbable ß-TCP material RTR 3raisons 120x280.indd 1 16/06/15 10:52 1AVOID SPONTANEOUS RIDGE RESORPTION After tooth extraction, spontaneous healing
3 REASONS TO USE R.T.R. AFTER EACH EXTRACTION Fully resorbable ß-TCP material RTR 3raisons 120x280.indd 1 16/06/15 10:52 1AVOID SPONTANEOUS RIDGE RESORPTION After tooth extraction, spontaneous healing
CONFORM FlEX. Demineralized cancellous bone
 CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
The Original remains unique.
 The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
The Original remains unique. Geistlich leading regeneration 2A, 2B Geistlich is the world leader in regenerative dentistry. We transform natural biomaterials into safe and reliable treatment methods that
THE WOUND SOLUTION. (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix)
 (Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
(Freeze dried Acellular Dermal Matrix) (Cryopreserved Acellular Dermal Matrix) What is / CGCryoDerm?? /CGCryoDerm are processed by the CGBio co. and is available through Daewoong Bio. /CGCryoDerm are an
international biologics product catalog
 international biologics product catalog 99.2% average implant success rate 1 BioHorizons is committed to developing evidence-based and scientifically-proven products. This commitment started with the launch
international biologics product catalog 99.2% average implant success rate 1 BioHorizons is committed to developing evidence-based and scientifically-proven products. This commitment started with the launch
botiss dental bone & tissue regeneration biomaterials mucoderm 3D-Regenerative Tissue Graft strictly biologic
 dental bone & tissue regeneration botiss biomaterials 3DRegenerative Tissue Graft strictly biologic mucoderm Soft Tissue Graft Indications mucoderm is a collagen tissue matrix derived of animal dermis
dental bone & tissue regeneration botiss biomaterials 3DRegenerative Tissue Graft strictly biologic mucoderm Soft Tissue Graft Indications mucoderm is a collagen tissue matrix derived of animal dermis
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
BIOCOLLAGEN TECHNICAL SHEET
 Pag. 1/5 TECHNICAL SHEET Pag. 2/5 Disposable sterile device Description Membrane/felt/gel/collagen paste. Product constituents / MeRG/ Type I equine collagen (from Achilles tendon). GEL: Type I equine
Pag. 1/5 TECHNICAL SHEET Pag. 2/5 Disposable sterile device Description Membrane/felt/gel/collagen paste. Product constituents / MeRG/ Type I equine collagen (from Achilles tendon). GEL: Type I equine
THE NEXT FRONTIER OF BONE REGENERATION. where Technology meets Nature
 THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
THE NEXT FRONTIER OF BONE REGENERATION where Technology meets Nature SmartBone is a new hybrid bioactive bone substitute specifically developed for bone regeneration in reconstructive surgery. SmartBone
ADVANCED BONE GRAFT SYSTEM OVERVIEW
 ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
( ) 2009;28(2):89-94
 ( ) 2009;28(2):89-94 Osseointegration is important in the functional aspect, however, esthetics is also important, especially in the maxillary anterior region. An adequate surgical technique is necessary
( ) 2009;28(2):89-94 Osseointegration is important in the functional aspect, however, esthetics is also important, especially in the maxillary anterior region. An adequate surgical technique is necessary
Comparative Animal Study of OssiMend and Healos 13,14
 Comparative Animal Study of OssiMend and Healos 13,14 Objective This study was conducted to evaluate the use of OssiMend as compared with Healos in combination with autologous bone marrow as a bone grafting
Comparative Animal Study of OssiMend and Healos 13,14 Objective This study was conducted to evaluate the use of OssiMend as compared with Healos in combination with autologous bone marrow as a bone grafting
Nova Scotia Provincial Blood Coordinating Program Provincial Dental Board of Nova Scotia. Toolkit for Allograft Usage In Dental Practice
 Nova Scotia Provincial Blood Coordinating Program Provincial Dental Board of Nova Scotia Toolkit for Allograft Usage In Dental Practice September 29, 2017 1 A. Preamble This document has been developed
Nova Scotia Provincial Blood Coordinating Program Provincial Dental Board of Nova Scotia Toolkit for Allograft Usage In Dental Practice September 29, 2017 1 A. Preamble This document has been developed
THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS. Collagenated heterologous cortico-cancellous bone mix + TSV Gel GTO I N S P I R E D B Y N A T U R E
 GTO THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS Collagenated heterologous cortico-cancellous bone mix + TSV Gel R E G E N E R A T I O N S C I E N C E I N S P I R E D B Y N A T U R E A unique biotechnology
GTO THE NEW STANDARD OF EXCELLENCE IN BIOMATERIALS Collagenated heterologous cortico-cancellous bone mix + TSV Gel R E G E N E R A T I O N S C I E N C E I N S P I R E D B Y N A T U R E A unique biotechnology
DiaDent Group International DIA.DENT DiaRoot BioAggregate. Root Canal Repair Material
 DiaDent Group International 1.877.DIA.DENT www.diadent.com DiaRoot BioAggregate Root Canal Repair Material PRECISION. PURITY. RESULTS ABOUT DIAROOT... DiaRoot BioAggregate Root Canal Repair Material is
DiaDent Group International 1.877.DIA.DENT www.diadent.com DiaRoot BioAggregate Root Canal Repair Material PRECISION. PURITY. RESULTS ABOUT DIAROOT... DiaRoot BioAggregate Root Canal Repair Material is
Symbios. Product Catalog
 Symbios Product Catalog Solutions for all your regenerative needs The comprehensive portfolio for hard and soft tissue regeneration provides ease of use and safety for dental clinicians and their patients.
Symbios Product Catalog Solutions for all your regenerative needs The comprehensive portfolio for hard and soft tissue regeneration provides ease of use and safety for dental clinicians and their patients.
BONE void FILLER. Beta-tricalcium phosphate ( b-tcp) bone graft substitute. OvERvIEW BROCHURE
 chronos BONE void FILLER Beta-tricalcium phosphate ( b-tcp) bone graft substitute OvERvIEW BROCHURE chronos STRIP chronos Strip is a synthetic bone void filler manufactured from chronos Beta-Tricalcium
chronos BONE void FILLER Beta-tricalcium phosphate ( b-tcp) bone graft substitute OvERvIEW BROCHURE chronos STRIP chronos Strip is a synthetic bone void filler manufactured from chronos Beta-Tricalcium
Bringing you Geistlich biocompatibility with improved application and handling benefits. Your combination for success
 Bringing you Geistlich biocompatibility with improved application and handling benefits Your combination for success Geistlich Combi-Kit Collagen: Combining ease and predictablility Geistlich Combi-Kit
Bringing you Geistlich biocompatibility with improved application and handling benefits Your combination for success Geistlich Combi-Kit Collagen: Combining ease and predictablility Geistlich Combi-Kit
The advantages of atelo-collagenated, lyophilized (non-heated), bovine bone graft composite. Dr. med. Sami Watad
 The advantages of atelo-collagenated, lyophilized (non-heated), bovine bone graft composite Dr. med. Sami Watad it s a term that describes the physicochemical deletion of the antigenic terminal peptide
The advantages of atelo-collagenated, lyophilized (non-heated), bovine bone graft composite Dr. med. Sami Watad it s a term that describes the physicochemical deletion of the antigenic terminal peptide
Pre op Failed endodontic treatment with sinus involvement.
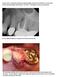 Case #1 of 10 consecutive extraction sockets grafted with Socket Graft Putty, covered with Socket Seal and sealed with Periacryl. I D # HEU This patient is a 66 year old female. Pre op Failed endodontic
Case #1 of 10 consecutive extraction sockets grafted with Socket Graft Putty, covered with Socket Seal and sealed with Periacryl. I D # HEU This patient is a 66 year old female. Pre op Failed endodontic
RPC. Surgical Solutions USA Regenerative Product Catalog
 RPC Surgical Solutions USA Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030
RPC Surgical Solutions USA Regenerative Product Catalog RESORBABLE COLLAGEN MEMBRANES (BOVINE) CYTOPLAST RTM Collagen Resorption time of 26-38 weeks RTM1520 $185 15mm x 20mm Membranes per box: 2 RTM2030
Limited bone availability makes implant placement challenging
 Bone Grafting: Essential Indications and Techniques in Implant Dentistry Limited bone availability makes implant placement challenging and sometimes unpredictable. Candidates for implant therapy must have
Bone Grafting: Essential Indications and Techniques in Implant Dentistry Limited bone availability makes implant placement challenging and sometimes unpredictable. Candidates for implant therapy must have
BONE AUGMENTATION AND GRAFTING
 1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
1 A Computer-Guided Bone Block Harvesting Procedure: A Proof-of-Principle Case Report and Technical Notes Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review
CONFORM SHEET. Demineralized cancellous bone
 CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
B U I L D S T R O N G B O N E F A S T
 B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
B U I L D S T R O N G B O N E F A S T Putty MIS Particulate Morsels A BIOACTIVE SYNTHETIC BONE FOR FASTER HEALING NovaBone is a 100% bioactive synthetic material composed from elements that occur naturally
We Want to Keep You Smiling. Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide
 We Want to Keep You Smiling Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong Bone for a Healthier Smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
We Want to Keep You Smiling Bone Regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong Bone for a Healthier Smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
Bone augmentation with maxgraft
 Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Patient information bone & tissue regeneration botiss biomaterials Bone augmentation with maxgraft established safe X100 natural Implantation stability is crucial for success Atrophy of the jaw bone loss
Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier. MEDLINEUNITE Bioactive Bone Graft
 Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier Principles of Bone Healing Reparative Phase Healing Cascade 1. Cellular infiltration and migration to site (fibroblasts, macrophages,
Bioactive Glass Biphasic β-tcp & HA Granules Alkylene Oxide Polymer Carrier Principles of Bone Healing Reparative Phase Healing Cascade 1. Cellular infiltration and migration to site (fibroblasts, macrophages,
Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS
 Page 1 of 5 Issue Date: March 2003, Posted On: 8/1/2005 Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS The extraction of teeth creates a
Page 1 of 5 Issue Date: March 2003, Posted On: 8/1/2005 Multi-Modality Anterior Extraction Site Grafting Increased Predictability for Aesthetics Michael Tischler, DDS The extraction of teeth creates a
Management of a complex case
 2 Soft- and hard-tissue reconstruction of a severely deficient site prior to implant placement: a case report Management of a complex case Younes Khosroshahy, DDS, MFDS RCS (Eng), Dip Imp Dent RCSEd, Blue
2 Soft- and hard-tissue reconstruction of a severely deficient site prior to implant placement: a case report Management of a complex case Younes Khosroshahy, DDS, MFDS RCS (Eng), Dip Imp Dent RCSEd, Blue
Inion GTR Biodegradable Membrane System
 Inion GTR Biodegradable Membrane System The concept of GTR/GBR Intended use The Inion GTR Biodegradable Membrane System is intended to be used as a barrier membrane in dental guided tissue regeneration
Inion GTR Biodegradable Membrane System The concept of GTR/GBR Intended use The Inion GTR Biodegradable Membrane System is intended to be used as a barrier membrane in dental guided tissue regeneration
BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE The following languages are included in this packet:
 BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE 150815-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
BONE VOID FILLERS CONTAINING DONATED HUMAN TISSUE 150815-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
ORTHOPEDIC Bone & Skin Tissue Products
 ORTHOPEDIC Bone & Skin Tissue Products DBM Products SurFuse TM Demineralized Bone Matrix - Gel / Putty ExFuse TM Demineralized Bone Matrix with Cancellous Bone - Gel / Putty BellaFuse TM Inserter Demineralized
ORTHOPEDIC Bone & Skin Tissue Products DBM Products SurFuse TM Demineralized Bone Matrix - Gel / Putty ExFuse TM Demineralized Bone Matrix with Cancellous Bone - Gel / Putty BellaFuse TM Inserter Demineralized
Instructions for Use. Wright Medical Technology 1023 Cherry Road Memphis, TN USA
 Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Redefining Regeneration
 Redefining Regeneration Taking Volume to the MAX One Product, One Treatment, Real VOLUME Buccal Bone Loss Socket Preservation Lateral / Vertical Augmentation Grafting Material Scaffold Barrier (4-6 months)
Redefining Regeneration Taking Volume to the MAX One Product, One Treatment, Real VOLUME Buccal Bone Loss Socket Preservation Lateral / Vertical Augmentation Grafting Material Scaffold Barrier (4-6 months)
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Maryland AGD AE and Socket Grafting 2015
 The Goodacre Study 5 year retrospective study looked at Crown & Bridge Caries Single crowns 1% FPD abutments 17% Periodontal Involvement Single crowns
The Goodacre Study 5 year retrospective study looked at Crown & Bridge Caries Single crowns 1% FPD abutments 17% Periodontal Involvement Single crowns
THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH
 A L L O S O U R C E THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. AlloSource, Centennial, CO B A S I C S C I E N
A L L O S O U R C E THE BIOMECHANICS OF ALLOMEND ACELLULAR DERMAL MATRIX: SUTURE RETENTION STRENGTH Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. AlloSource, Centennial, CO B A S I C S C I E N
AttraX. Portfolio. Surface Drives Performance
 Portfolio Surface Drives Performance Advanced Biomaterial Microarchitecture Drives Bone Formation Traditional calcium phosphate materials generally do not give rise to bone formation when implanted in
Portfolio Surface Drives Performance Advanced Biomaterial Microarchitecture Drives Bone Formation Traditional calcium phosphate materials generally do not give rise to bone formation when implanted in
We want to keep you smiling. Bone regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide
 We want to keep you smiling Bone regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong bone for a healthier smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
We want to keep you smiling Bone regeneration with Geistlich Bio-Oss and Geistlich Bio-Gide Strong bone for a healthier smile Strong and healthy teeth provide a feeling of well-being, self-confidence and
Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE
 Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE Features Easily shaped and molded for implantation into defects positioned at difficult angles Hardens in a warm, wet environment, reducing the need to
Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE Features Easily shaped and molded for implantation into defects positioned at difficult angles Hardens in a warm, wet environment, reducing the need to
Product Information. When one option is not enough.
 Product Information Biomaterials@Straumann When one option is not enough. Content BONE SUBSTITUTES Straumann AlloGraft granules Processed human allograft 6 MEMBRANES Straumann Membrane Plus Collagen membrane
Product Information Biomaterials@Straumann When one option is not enough. Content BONE SUBSTITUTES Straumann AlloGraft granules Processed human allograft 6 MEMBRANES Straumann Membrane Plus Collagen membrane
Comprehensive Bone Regeneration Solutions
 Comprehensive Bone Regeneration Solutions COMPREHENSIVE BONE REGENERATION SOLUTIONS Achieving functional and esthetic results when placing a dental implant requires an adequate amount of quality bone.
Comprehensive Bone Regeneration Solutions COMPREHENSIVE BONE REGENERATION SOLUTIONS Achieving functional and esthetic results when placing a dental implant requires an adequate amount of quality bone.
A new approach with an in-situ self-hardening grafting material
 74 Bone grafting with simultaneous early implant placement A new approach with an in-situ self-hardening grafting material MINAS LEVENTIS 1,2, PHD; PETER FAIRBAIRN 1,3, BDS; ORESTIS VASILIADIS 2,4, DDS
74 Bone grafting with simultaneous early implant placement A new approach with an in-situ self-hardening grafting material MINAS LEVENTIS 1,2, PHD; PETER FAIRBAIRN 1,3, BDS; ORESTIS VASILIADIS 2,4, DDS
Puros Cancellous Particulate Allograft & Puros Block Allograft
 Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
Puros Cancellous Particulate Allograft & Puros Block Allograft Puros Cancellous Particulate Allograft The Natural Choice For Healthy 1 Bone Growth. 1. Proven, Predictable Regeneration Acts as an osteoconductive
Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR
 Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR Patented Jet-Spraying technology: Full barrier effect during 4 weeks, and complete resorption in 6 months PATENTED
Resorbable bilayer synthetic membrane Biomimetic tissue-engineered matrix for GBR and GTR Patented Jet-Spraying technology: Full barrier effect during 4 weeks, and complete resorption in 6 months PATENTED
B&B DENTAL. implant company MATERIAL FOR BONE REGENERATION
 B&B DENTAL implant company MATERIAL FOR BONE REGENERATION NOVOCOR PLUS The Novocor Plus medical device comprises grains of natural coral with a low surface/volume, ranging from 200 to 500 mm. The natural
B&B DENTAL implant company MATERIAL FOR BONE REGENERATION NOVOCOR PLUS The Novocor Plus medical device comprises grains of natural coral with a low surface/volume, ranging from 200 to 500 mm. The natural
Case reports by Dr. Roland Török IMPLANT INSTITUTE TÖRÖK. botiss. dental bone & tissue regeneration. biomaterials.
 Case reports by Dr. Roland Török dental bone & tissue regeneration Dr. Med. STOM. roland török IMPLANT INSTITUTE TÖRÖK botiss biomaterials strictly biologic botiss BTR system: BONE bovine block & granules:
Case reports by Dr. Roland Török dental bone & tissue regeneration Dr. Med. STOM. roland török IMPLANT INSTITUTE TÖRÖK botiss biomaterials strictly biologic botiss BTR system: BONE bovine block & granules:
THE NEXT GENERATION OF REGENERATION
 THE NEXT GENERATION OF REGENERATION Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption.
THE NEXT GENERATION OF REGENERATION Why SynthoGraft? SynthoGraft offers a unique structure which provides stability, while its micro-porosity allows for rapid vascularization and subsequent resorption.
INNOVATE INVOLVE INVENT. Innovasis Bone Products
 INNOVATE INVOLVE INVENT Innovasis Bone Products Company Overview Innovasis is a rapidly growing company engaged in the research, development, manufacturing, and marketing of spinal implant devices and
INNOVATE INVOLVE INVENT Innovasis Bone Products Company Overview Innovasis is a rapidly growing company engaged in the research, development, manufacturing, and marketing of spinal implant devices and
