SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
|
|
|
- Eugenia Stephens
- 5 years ago
- Views:
Transcription
1 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction.
2 SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice for excellent onlay or suture performance Superior handling No adhesions * Non-animal derived, no risk of transmissible diseases Non-pyrogenic MR safe SYNTHECEL Dura Repair from clinical study. Courtesy of Barrow Neurosurgical Associates, Ltd. Conformable SYNTHECEL Dura Repair thickness is similar to human dura 1,2 and conforms to the brain. Suturable SYNTHECEL Dura Repair is excellent in sutureability. 3 *observed in clinical study. 1. Ontano SE, et al. Mechanical behavior of human dura mater. Transcripts from the BED Bioeng Con- ASME 29: , Sacks MS, et al. Local mechanical anisotropy in human cranial dura mater allografts. J Biomech Eng 120: , Rosen, Charles L., et al. Results of the Prospective, Randomized, Multicenter Clinical Trial Evaluating a Biosynthesized Cellulose Graft for Repair of Dural Defects. Neurosurgery Nov;69(5): ; discussion Control group: Duraform Dural Graft implant (15 units), Duragen II Dural Regeneration Matrix (8 units), Duragen Dural Graft Matrix (10 units), Durepair Regeneration Matrix (2 units), Duragen Plus (1 unit), and Preclude Dura Substitute (1 unit).
3 SYNTHECEL Dura Repair performance was demonstrated in a prospective, randomized, controlled study *. Superior Seal Quality Superior Strength No adhesions reported Superior Seal Quality SYNTHECEL Dura Repair demonstrated statistically significant seal quality. Superior Strength SYNTHECEL Dura Repair demonstrated statistically significant strength. *Rosen, Charles L., et al. Results of the Prospective, Randomized, Multicenter Clinical Trial Evaluating a Biosynthesized Cellulose Graft for Repair of Dural Defects. Neurosurgery Nov;69(5): ; discussion Control group: Duraform Dural Graft implant (15 units), Duragen II Dural Regeneration Matrix (8 units), Duragen Dural Graft Matrix (10 units), Durepair Regeneration Matrix (2 units), Duragen Plus (1 unit), and Preclude Dura Substitute (1 unit).
4 Naturally Formed, Biosynthesized Cellulose. SYNTHECEL Dura Repair is composed of biosynthesized cellulose and water. Layers, biosynthesized cellulose Interconnected, cellulose fibers Naturally formed by Gluconacetobacter xylinus
5 INSTRUCTIONS FOR USE Package: Provided sterile and must not be reused or re-sterilized. Do not use past expiration date, if package has been compromised or if the implant is dry. Storage: Store at room temperature, must not be frozen (5 C/41 F) or exposed to temperatures above 40 C/104 F. If there is a in the temperature indicator window, product is ok to use. If there is an X in the temperature indicator window, do not use the product. Application: Step 1: Open carton and outer pouch. Pass sterile, inner pouch onto the sterile field. Rinse surgical gloves to remove glove powder prior to handling the implant. Implant is packaged wet and ready to use. Protective plastic sheeting prevents implant from drying out. No rinsing or soaking for re-hydration is required. Step 2: When ready to use, open inner pouch and remove implant using aseptic technique. Immediately prior to use, remove and discard the protective plastic sheeting from both sides of the implant using aseptic technique. Do not allow implant to dry out prior to implantation. Step 3: Apply implant with or without sutures. Trim and size the implant to completely cover the defect with an overlap of sufficient size to allow sutures (if desired) to be placed along the margin of the implant 3 to 4 mm from the edge. Tensionless atraumatic sutures may be used if desired. Use non-absorbable, smallest appropriate diameter suture with a tapered (non- cutting) needle of appropriate size to anchor the material. Final suture selection should be determined by surgeon preference and the nature of the dural repair. A watertight closure should be achieved to minimize the possibility of CSF leakage. Ensure the edges of the implant are not folded. Allow sufficient size so that suturing does not place implant or surrounding dura under tension. Implant can be repositioned if required prior to suturing. Use caution when repositioning the implant to avoid tears. Step 4: Close remaining cranial layers to preference and clinical need. Discard all unused pieces of SYNTHECEL Dura Repair. Please refer to package insert for complete indications, contraindications, warnings and precautions.
6 SYNTHECEL Dura Repair A Size for all your Clinical Needs. Product Code Size Units Per A SC S 1 in x 1 in (2.5 cm x 2.5 cm) 1 B SC S 1 in x 3 in (2.5 cm x 7.5 cm) 1 C SC S 2 in x 2 in (5.0 cm x 5.0 cm) 1 D SC S 3 in x 3 in (7.5 cm x 7.5 cm) 1 E SC S 4 in x 5 in (10.0 cm x 12.0 cm) 1 Images shown are to scale in inches. Products are sold sterile. B 1 (2.5 cm) 3 (7.5 cm) E D C A 1 (2.5 cm) 2 (5.0 cm) 1 (2.5 cm) 3 (7.5 cm) 4 (10.0 cm) 2 (5.0 cm) 3 (7.5 cm) 5 (12.0 cm) Indications : SYNTHECEL Dura Repair is intended for use as a dura replacement for the repair of dura mater in adults. Contraindications : SYNTHECEL Dura Repair must not be implanted in patients who have known allergy or sensitivity to the implant materials (cellulose). WARNINGS : The safety and effectiveness of SYNTHECEL Dura Repair has not been studied in: Patients that are less than 18 years of age. Patients who also required use of dural adhesive or sealant. Patients who had systemic infection (e.g. urinary tract infection, active pneumonia) or evidence of any surgical site infection, fever > 38.3 C (101 F), positive blood culture and/or a positive chest x-ray for acute infectious process. Patients who were acute cranial trauma surgical cases. Patients who had chemotherapy and/or radiation treatment within 12 weeks prior to surgery, or had chemotherapy and/or radiation treatment planned 10 weeks post surgery. Patients who had compromised immune system or autoimmune disease. PRECAUTIONS : SYNTHECEL Dura Repair is provided sterile and should not be resterilized. Do not use if packaging or seal has been damaged or opened. SYNTHECEL Dura Repair should be stored at room temperature; must not be frozen (5 C/41 F) or exposed to temperatures above 40 C/104 F. SYNTHECEL Dura Repair is intended for single patient use only. Discard opened or unused product. Immediately prior to use: remove and discard the protective plastic sheeting from both sides of the device using aseptic technique. Do not allow implant to dry out prior to implantation. Tension should be avoided if suturing the SYNTHECEL Dura Repair. Ensure the edges of the implant are not folded. SYNTHECEL Dura Repair should be used with caution in restricted areas of the skull, (e.g., posterior fossa) due to the potential for local mass effect. Manufactured in the United States by: Synthes, Inc 1302 Wrights Lane East West Chester, PA Telephone: (610) To order: (800) Limited Warranty and Disclaimer: DePuy Synthes CMF products are sold with a limited warranty to the original purchaser against defects in workmanship and materials. Any other express or implied warranties, including warranties of merchantability or fitness, are hereby disclaimed. WARNING: In the USA, this product has labelling limitations. See package insert for complete information. Rx Only Not available for sale in Canada. DePuy Synthes CMF, a division of DOI All rights reserved. DSUS/CMF/0314/0011(1)
SYNPOR POROUS POLYETHYLENE IMPLANTS. For craniofacial and orbital augmentation and reconstruction
 SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
CRANIAL RECONSTRUCTION SOLUTIONS
 CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
CRANIAL RECONSTRUCTION SOLUTIONS CRANIAL RECONSTRUCTION SOLUTIONS Your partner of CHOiCE at depuy Synthes CMF, we are dedicated to providing solutions for your individual patient needs. We do this through
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
Lyoplant Onlay. Adding another layer of confidence to every procedure. Aesculap Neurosurgery
 Adding another layer of confidence to every procedure Aesculap Neurosurgery Adding another layer of confidence to every procedure For more than 140 years, Aesculap Neurosurgery has developed products that
Adding another layer of confidence to every procedure Aesculap Neurosurgery Adding another layer of confidence to every procedure For more than 140 years, Aesculap Neurosurgery has developed products that
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
matrixwave tm mmf expand your possibilities A novel system that expands and compresses to achieve maxillomandibular fixation
 matrixwave tm mmf expand your possibilities A novel system that expands and compresses to achieve maxillomandibular fixation Designed for adaptability, versatility, and patient comfort MatrixWAVE TM MMF
matrixwave tm mmf expand your possibilities A novel system that expands and compresses to achieve maxillomandibular fixation Designed for adaptability, versatility, and patient comfort MatrixWAVE TM MMF
Neurosurgery is complex. DuraGen is the simple choice.
 Neurosurgery is complex. is the simple choice. Family of Grafts Dural Regeneration Matrix Keep Closure Simple. A Breakthrough in Dural Repair A Pioneer in Dural Regeneration Matrices For almost forty years,
Neurosurgery is complex. is the simple choice. Family of Grafts Dural Regeneration Matrix Keep Closure Simple. A Breakthrough in Dural Repair A Pioneer in Dural Regeneration Matrices For almost forty years,
Technique Guide. SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction.
 Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
Technique Guide SynPOR Porous Polyethylene Implants. For craniofacial and orbital augmentation and reconstruction. Table of Contents Introduction SynPOR Porous Polyethylene Implants 2 Indications and Contraindications
CONFORM FlEX. Demineralized cancellous bone
 CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
CONFORM FlEX Demineralized cancellous bone TABlE OF CONTENTS introduction 2 FEATURES AND BENEFiTS 4 PROCESSiNg AND PACKAgiNg information 5 MUSCUlOSKElETAl TRANSPlANT FOUNDATiON (MTF) 6 ORDERiNg information
Mini External Fixator
 Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
Lag Screw Device Intended for symphyseal fracture fixation of the mandible
 Lag Screw Device Intended for symphyseal fracture fixation of the mandible SUrgicaL TecHNiqUe Lag Screw Device Intended for symphyseal fracture fixation of the mandible Simplifies the lag screw fixation
Lag Screw Device Intended for symphyseal fracture fixation of the mandible SUrgicaL TecHNiqUe Lag Screw Device Intended for symphyseal fracture fixation of the mandible Simplifies the lag screw fixation
CONFORM SHEET. Demineralized cancellous bone
 CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
CONFORM SHEET Demineralized cancellous bone TABLE OF CONTENTS INTRODUCTION 2 FEATURES AND BENEFITS 4 HYDRATION INFORMATION 5 PROCESSING AND PACKAGING INFORMATION 6 MUSCULOSKELETAL TRANSPLANT FOUNDATION
DuraMatrix Collagen Dura Substitute Membrane. Manufactured by: Collagen Matrix, Inc., Franklin Lakes, New Jersey
 DuraMatrix Collagen Dura Substitute Membrane Manufactured by: Collagen Matrix, Inc., Franklin Lakes, New Jersey What is DuraMatrix? Onlay or Sutured Implantation Balanced Implant Resorption vs. Host Tissue
DuraMatrix Collagen Dura Substitute Membrane Manufactured by: Collagen Matrix, Inc., Franklin Lakes, New Jersey What is DuraMatrix? Onlay or Sutured Implantation Balanced Implant Resorption vs. Host Tissue
orthodontic Bone anchor (oba) SYStem
 orthodontic Bone anchor (oba) SYStem Skeletal implants for the orthodontic movement of teeth SurgIcal technique Table of Contents Introduction Orthodontic Bone Anchor (OBA) System 2 Indications and Contraindications
orthodontic Bone anchor (oba) SYStem Skeletal implants for the orthodontic movement of teeth SurgIcal technique Table of Contents Introduction Orthodontic Bone Anchor (OBA) System 2 Indications and Contraindications
Titanium Wire With Barb and Needle
 For Canthal Tendon Procedures Titanium Wire With Barb and Needle Surgical Technique Table of Contents Introduction Titanium Wire With Barb and Needle 2 Indications 2 Surgical Technique Preoperative Planning
For Canthal Tendon Procedures Titanium Wire With Barb and Needle Surgical Technique Table of Contents Introduction Titanium Wire With Barb and Needle 2 Indications 2 Surgical Technique Preoperative Planning
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
3.5 mm Clavicle Hook Plates
 A Single Solution for Lateral Clavicle Fractures and Acromioclavicular Joint Dislocations 3.5 mm Clavicle Hook Plates Surgical Technique Discontinued December 2017 DSUS/TRM/1016/1126(1) Table of Contents
A Single Solution for Lateral Clavicle Fractures and Acromioclavicular Joint Dislocations 3.5 mm Clavicle Hook Plates Surgical Technique Discontinued December 2017 DSUS/TRM/1016/1126(1) Table of Contents
Technique Guide. SynPOR HD Ocular Spheres. For augmentation or reconstruction.
 Technique Guide SynPOR HD Ocular Spheres. For augmentation or reconstruction. Table of Contents Introduction SynPOR HD Ocular Spheres 2 Indications and Contraindications 3 Surgical Technique Sizing and
Technique Guide SynPOR HD Ocular Spheres. For augmentation or reconstruction. Table of Contents Introduction SynPOR HD Ocular Spheres 2 Indications and Contraindications 3 Surgical Technique Sizing and
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
DuraMatrix portfolio. Restore with confidence
 DuraMatrix portfolio Restore with confidence History Since 2003, Stryker has partnered with Collagen Matrix, Inc. to provide our customers with quality products that are safe and effective in the restoration
DuraMatrix portfolio Restore with confidence History Since 2003, Stryker has partnered with Collagen Matrix, Inc. to provide our customers with quality products that are safe and effective in the restoration
P e r i - G u a r d I N S T R U C C I O N E S D E U S O M O D E D E M P L O I... 6 I S T R U Z I O N I P E R L U S O
 MS-12788D Peri-Guard CE IFU:12788b.qxd 12/12/2011 2:49 PM Page 1 P e r i - G u a r d I N S T R U C T I O N S F O R U S E......... 2 M O D E D E M P L O I.............. 6 G E B R A U C H S A N L E I T U
MS-12788D Peri-Guard CE IFU:12788b.qxd 12/12/2011 2:49 PM Page 1 P e r i - G u a r d I N S T R U C T I O N S F O R U S E......... 2 M O D E D E M P L O I.............. 6 G E B R A U C H S A N L E I T U
Integra. TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE
 Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
SHOULDER EXTRACTION INSTRUMENTS
 SHOULDER EXTRACTION INSTRUMENTS PRODUCT OVERVIEW Introducing the orthopaedic industry s first dedicated Shoulder Extraction Instrument set. DePuy Synthes Joint Reconstruction has created this set of instruments
SHOULDER EXTRACTION INSTRUMENTS PRODUCT OVERVIEW Introducing the orthopaedic industry s first dedicated Shoulder Extraction Instrument set. DePuy Synthes Joint Reconstruction has created this set of instruments
DISTRACTION PRODUCT OVERVIEW. For a wide variety of facial applications
 DISTRACTION PRODUCT OVERVIEW For a wide variety of facial applications DISTRACTION PRODUCT OVERVIEW. STRONG, MODULAR, VERSATILE CRANIOFACIAL DISTRACTION External Midface Distractor Distraction of the maxilla,
DISTRACTION PRODUCT OVERVIEW For a wide variety of facial applications DISTRACTION PRODUCT OVERVIEW. STRONG, MODULAR, VERSATILE CRANIOFACIAL DISTRACTION External Midface Distractor Distraction of the maxilla,
SPINE BIOMATERIALS Crop Bleed
 A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
A comprehensive guide to biomaterials offered by Posterolateral DBX PUTTY DBX STRIP DBX MIX DBX INJECT chronos Strip chronos Granules chronos Preforms PROCURE CHOICE Marrow Aspiration Kit Bone Marrow Aspiration
Technique Guide. Cranial Clamps with Application Instrument. Quick and stable fixation of cranial bone flaps following a craniotomy.
 Technique Guide Cranial Clamps with Application Instrument. Quick and stable fixation of cranial bone flaps following a craniotomy. Table of Contents Introduction Cranial Clamps with Application Instrument
Technique Guide Cranial Clamps with Application Instrument. Quick and stable fixation of cranial bone flaps following a craniotomy. Table of Contents Introduction Cranial Clamps with Application Instrument
SELF-CENTERING Bipolar/Modular Cathcart Unipolar
 SELF-CENTERING Bipolar/Modular Cathcart Unipolar Modular Endo Heads Surgical Technique Responding To The Surgeon The SELF-CENTERING Bipolar and Modular Cathcart Unipolar Endo Heads add versatility to the
SELF-CENTERING Bipolar/Modular Cathcart Unipolar Modular Endo Heads Surgical Technique Responding To The Surgeon The SELF-CENTERING Bipolar and Modular Cathcart Unipolar Endo Heads add versatility to the
RAPIDSORB RAPID RESORBABLE FIXATION SYSTEM. A new resorbable approach to Alveolar bone augmentation
 RAPIDSORB RAPID RESORBABLE FIXATION SYSTEM A new resorbable approach to Alveolar bone augmentation RAPIDSORB RAPID RESORBABLE FIXATION SYSTEM Bone Graft Containment The RapidSorb Rapid Resorbable Fixation
RAPIDSORB RAPID RESORBABLE FIXATION SYSTEM A new resorbable approach to Alveolar bone augmentation RAPIDSORB RAPID RESORBABLE FIXATION SYSTEM Bone Graft Containment The RapidSorb Rapid Resorbable Fixation
HeliMEND Advanced. Absorbable Collagen Membrane. Instructions for Use
 HeliMEND Advanced Absorbable Collagen Membrane Instructions for Use 2 Indications HeliMEND Advanced absorbable collagen membrane is an absorbable, implantable material that is indicated for guided tissue
HeliMEND Advanced Absorbable Collagen Membrane Instructions for Use 2 Indications HeliMEND Advanced absorbable collagen membrane is an absorbable, implantable material that is indicated for guided tissue
Instructions for Use. Wright Medical Technology 1023 Cherry Road Memphis, TN USA
 Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
Instructions for Use Wright Medical Technology 1023 Cherry Road Memphis, TN 38117 USA 1-800-238-7117 Processed from Donated Human Tissue for Wright Medical Technology by LifeCell Corporation One Millennium
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Distal Radius Sterile Kit. Optimization And Efficiency You Can Rely On
 Distal Radius Sterile Kit Optimization And Efficiency You Can Rely On Introducing The Distal Radius Sterile Kit Improved OR Workflow The Distal Radius Sterile Kit provides high-quality single-use implants
Distal Radius Sterile Kit Optimization And Efficiency You Can Rely On Introducing The Distal Radius Sterile Kit Improved OR Workflow The Distal Radius Sterile Kit provides high-quality single-use implants
got you covered. Codman s The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions.
 Codman s got you covered. The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions. AN INTEGRA LIFESCIENCES COMPANY The Leader in Dural
Codman s got you covered. The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions. AN INTEGRA LIFESCIENCES COMPANY The Leader in Dural
V a s c u - G u a r d
 MS 12786C IFU, Vascu Guard CE:12786b 12/14/2011 12:01 PM Page 1 V a s c u - G u a r d I N S T R U C T I O N S F O R U S E........... 2 M O D E D E M P L O I................. 6 G E B R A U C H S A N L E
MS 12786C IFU, Vascu Guard CE:12786b 12/14/2011 12:01 PM Page 1 V a s c u - G u a r d I N S T R U C T I O N S F O R U S E........... 2 M O D E D E M P L O I................. 6 G E B R A U C H S A N L E
NovoSorb BTM. A unique synthetic biodegradable wound scaffold. Regenerating tissue. Changing lives.
 NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
NovoSorb BTM A unique synthetic biodegradable wound scaffold Regenerating tissue. Changing lives. Overview NovoSorb BTM is a unique synthetic biodegradable wound scaffold that delivers good cosmetic and
MatrixNEURO Cranial Plating System
 The Next Generation Cranial Plating System MatrixNEURO Cranial Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION MatrixNEURO Cranial Plating System 2 MatrixNEURO Reconstruction Mesh 6 MatrixNEURO
The Next Generation Cranial Plating System MatrixNEURO Cranial Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION MatrixNEURO Cranial Plating System 2 MatrixNEURO Reconstruction Mesh 6 MatrixNEURO
Trochanter Stabilization Plate for DHS Implants
 Extends DHS Plate Construct to Help Stabilize Greater Trochanter Trochanter Stabilization Plate for DHS Implants Surgical Technique Table of Contents Introduction Trochanter Stabilization Plate for DHS
Extends DHS Plate Construct to Help Stabilize Greater Trochanter Trochanter Stabilization Plate for DHS Implants Surgical Technique Table of Contents Introduction Trochanter Stabilization Plate for DHS
Restoration not just repair
 DURAGEN ENGLISH Restoration not just repair r e g e n e r at i v e t e c h n o l o g y PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY. More than a graft DuraGen is a matrix for repair of the
DURAGEN ENGLISH Restoration not just repair r e g e n e r at i v e t e c h n o l o g y PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY. More than a graft DuraGen is a matrix for repair of the
eptfe Vascular Graft Instructions for Use
 TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
SYNPOR HD FACIAL SHAPE SYSTEM SURGICAL TECHNIQUE. For the augmentation or reconstruction of the craniomaxillofacial skeleton
 SYNPOR HD FACIAL SHAPE SYSTEM For the augmentation or reconstruction of the craniomaxillofacial skeleton SURGICAL TECHNIQUE Table of Contents Introduction SynPOR HD Facial Shape System 2 Indications and
SYNPOR HD FACIAL SHAPE SYSTEM For the augmentation or reconstruction of the craniomaxillofacial skeleton SURGICAL TECHNIQUE Table of Contents Introduction SynPOR HD Facial Shape System 2 Indications and
For Distal Femur Fractures. 95º Condylar Plate. Quick Reference Chart
 For Distal Femur Fractures 95º Condylar Plate Quick Reference Chart 95 Condylar Plate. Quick reference chart for distal femur fractures. Insert guide wires Fix condylar fragments with 6.5 mm cancellous
For Distal Femur Fractures 95º Condylar Plate Quick Reference Chart 95 Condylar Plate. Quick reference chart for distal femur fractures. Insert guide wires Fix condylar fragments with 6.5 mm cancellous
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER. Magnifuse. Bone Graft
 POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER Magnifuse Bone Graft PREPARATION INSTRUCTIONS Soft tissue should be completely removed from the posterior elements of the spinal segments
POSTERIOR LUMBAR FUSION SURGICAL TECHNIQUE USING A BONE VOID FILLER Magnifuse Bone Graft PREPARATION INSTRUCTIONS Soft tissue should be completely removed from the posterior elements of the spinal segments
The Synthetic Self-Adhesive Barrier to Cerebrospinal Fluid Leaks
 The Synthetic SelfAdhesive Barrier to Cerebrospinal Fluid Leaks TissuePatchl has a fused multilaminate structure containing patented reactive polymers for chemical bonding to tissue surface, and a nonadhesive
The Synthetic SelfAdhesive Barrier to Cerebrospinal Fluid Leaks TissuePatchl has a fused multilaminate structure containing patented reactive polymers for chemical bonding to tissue surface, and a nonadhesive
SFX Cross Connector System. Surgical Technique Guide
 SFX Cross Connector System Surgical Technique Guide Introduction The EXPEDIUM SFX Cross Connector System redefines ease-of-use, implant versatility, and construct security with an advanced top-loading
SFX Cross Connector System Surgical Technique Guide Introduction The EXPEDIUM SFX Cross Connector System redefines ease-of-use, implant versatility, and construct security with an advanced top-loading
BACTISEAL Endoscopic Ventricular Catheter (REF & )
 BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
Drillable Fast Set Putty
 Norian Drillable Fast Set Putty Fiber reinforced calcium phosphate bone void filler Surgical Technique Indications and Contraindications* Indications Norian Drillable Fast Set Putty is intended for bony
Norian Drillable Fast Set Putty Fiber reinforced calcium phosphate bone void filler Surgical Technique Indications and Contraindications* Indications Norian Drillable Fast Set Putty is intended for bony
Dentoalveolar Bone Fixation System. Bone fixation sets for the office.
 Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Compact Modular Customized Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Who we are Synthes is a global medical
Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Compact Modular Customized Dentoalveolar Bone Fixation System. Bone fixation sets for the office. Who we are Synthes is a global medical
RECLAIM REVISION HIP SYSTEM
 RECLAIM REVISION HIP SYSTEM Where Strength and Modularity Connect DESIGN RATIONALE System Overview RECLAIM Modular Revision Hip System WHERE STRENGTH & MODULARITY CONNECT 2 Offset Options Proximal Body
RECLAIM REVISION HIP SYSTEM Where Strength and Modularity Connect DESIGN RATIONALE System Overview RECLAIM Modular Revision Hip System WHERE STRENGTH & MODULARITY CONNECT 2 Offset Options Proximal Body
RAPIDSORB RAPID RESORBABLE FIxATION SySTEm
 RAPIDSORB RAPID RESORBABLE FIxATION SySTEm For craniofacial reconstructionruction PRODUCT OVERVIEW RapidSorb Rapid Resorbable Fixation System Features Rapid resorbable implants maintain appropriate fixation
RAPIDSORB RAPID RESORBABLE FIxATION SySTEm For craniofacial reconstructionruction PRODUCT OVERVIEW RapidSorb Rapid Resorbable Fixation System Features Rapid resorbable implants maintain appropriate fixation
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Doc no: IFU/CPL Issue date: Rev no: 00 Rev date: 1
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE
 Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE Features Easily shaped and molded for implantation into defects positioned at difficult angles Hardens in a warm, wet environment, reducing the need to
Calcium Phosphate Bone Void Filler TECHNIQUE GUIDE Features Easily shaped and molded for implantation into defects positioned at difficult angles Hardens in a warm, wet environment, reducing the need to
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system.
 T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
T-PAL Spacer System. Transforaminal posterior atraumatic lumbar spacer system. One instrument, one technique Accommodates both open and MIS approaches A PEEK implant that works with patient anatomy Streamlined
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
PRODUCT CATALOG SPEED SPEEDSHIFT SPEEDARC SPEEDTITAN SPEEDTRIAD HAMMERLOCK 2 BME ELITE
 PRODUCT CATALOG SPEED SPEEDSHIFT SPEEDARC SPEEDTITAN SPEEDTRIAD HAMMERLOCK 2 BME ELITE Limited Warranty and Disclaimer: DePuy Synthes products are sold with a limited warranty to the original purchaser
PRODUCT CATALOG SPEED SPEEDSHIFT SPEEDARC SPEEDTITAN SPEEDTRIAD HAMMERLOCK 2 BME ELITE Limited Warranty and Disclaimer: DePuy Synthes products are sold with a limited warranty to the original purchaser
Case Study. TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis.
 Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
Case Study TRAM Flap Reconstruction with an Associated Complication. Repair using DermaMatrix Acellular Dermis. TRAM Flap Reconstruction with an Associated Complication Challenge Insulin-dependent diabetes
3.5 mm LCP Extra-articular Distal Humerus Plate
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Control. Control Through VISCOSITY Control Through DELIVERY Control Through SIMPLICITY
 Control. Now that s CONFIDENCE. Control Through VISCOSITY Control Through DELIVERY Control Through SIMPLICITY Control throughviscosity Controlled Fill Cement Interdigitation Low viscosity cement Trabecular
Control. Now that s CONFIDENCE. Control Through VISCOSITY Control Through DELIVERY Control Through SIMPLICITY Control throughviscosity Controlled Fill Cement Interdigitation Low viscosity cement Trabecular
Trephine System. Principle-based anterior lumbar interbody fusion.
 Trephine System. Principle-based anterior lumbar interbody fusion. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Trephine System 2 AO Principles
Trephine System. Principle-based anterior lumbar interbody fusion. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Trephine System 2 AO Principles
DermaSpan Acellular Dermal Matrix. Reinforcement of Ruptured Posterior Tibial Tendon Repair. Surgical Protocol by Charles Zelen, DPM, FACFAS
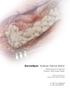 DermaSpan Acellular Dermal Matrix Reinforcement of Ruptured Posterior Tibial Tendon Repair Surgical Protocol by Charles Zelen, DPM, FACFAS One Surgeon. One Patient. Over 1 million times per year, Biomet
DermaSpan Acellular Dermal Matrix Reinforcement of Ruptured Posterior Tibial Tendon Repair Surgical Protocol by Charles Zelen, DPM, FACFAS One Surgeon. One Patient. Over 1 million times per year, Biomet
Large Distractor Femur
 Fracture Reduction and Provisional Stabilization Large Distractor Femur Surgical Technique Table of Contents Introduction Standard Femoral Distraction 2 Large Distractor System 4 Surgical Technique Prepare
Fracture Reduction and Provisional Stabilization Large Distractor Femur Surgical Technique Table of Contents Introduction Standard Femoral Distraction 2 Large Distractor System 4 Surgical Technique Prepare
Bone Preservation Stem
 TRI-LOCK Bone Preservation Stem Featuring GRIPTION Coating Surgical Technique Implant Geometry Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
TRI-LOCK Bone Preservation Stem Featuring GRIPTION Coating Surgical Technique Implant Geometry Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
Doc no: IFU/CC Issue date: Rev no: 04 Rev date:
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Chromic Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Chromic Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
HP KNEE EXTRACTION Instrumentation. Product Overview
 HP KNEE EXTRACTION Instrumentation Product Overview HP Extraction Knee Instruments DePuy Synthes Joint Reconstruction is working with Symmetry Medical to distribute Advanced Knee Extraction instruments.
HP KNEE EXTRACTION Instrumentation Product Overview HP Extraction Knee Instruments DePuy Synthes Joint Reconstruction is working with Symmetry Medical to distribute Advanced Knee Extraction instruments.
Titanium Wire with Barb and Needle. Surgical Technique Guide for Canthal Tendon Procedures.
 Titanium Wire with Barb and Needle. Surgical Technique Guide for Canthal Tendon Procedures. Technique Guide This publication is not intended for distribution in the USA. Instruments and implants approved
Titanium Wire with Barb and Needle. Surgical Technique Guide for Canthal Tendon Procedures. Technique Guide This publication is not intended for distribution in the USA. Instruments and implants approved
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Versatile grafting Solutions
 Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
Versatile grafting Solutions A Canadian company serving Canadian dentists since 1997 Here s why your colleagues are calling us for their bone regeneration needs Founded in 1997 Citagenix has been providing
ALLOPURE. Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE
 ALLOPURE Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE Contents Chapter 1 4 Chapter 2 5 5 5 Chapter 3 6 6 7 8 8 Appendix A 9 Introduction Intended Use Indications
ALLOPURE Bicortical Allograft Wedges Specific for Evans and Cotton Osteotomies SURGICAL TECHNIQUE Contents Chapter 1 4 Chapter 2 5 5 5 Chapter 3 6 6 7 8 8 Appendix A 9 Introduction Intended Use Indications
2.0 mm Mandible Locking Plate System
 Advanced Plating System for Trauma, Microvascular Reconstruction, and Orthognathic Surgery 2.0 mm Mandible Locking Plate System Surgical Technique TABLE OF CONTENTS INTRODUCTION 2.0 mm Mandible Locking
Advanced Plating System for Trauma, Microvascular Reconstruction, and Orthognathic Surgery 2.0 mm Mandible Locking Plate System Surgical Technique TABLE OF CONTENTS INTRODUCTION 2.0 mm Mandible Locking
3.5 mm LCP Olecranon Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15. Instructions for Use
 Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15 Instructions for Use For U.S. California Only: Proposition 65, a State of California
Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15 Instructions for Use For U.S. California Only: Proposition 65, a State of California
Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas. Reprocessed Device for Single Use.
 Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Tissue Sparing. DEsign Rationale
 Tissue Sparing Solutions DEsign Rationale TISSUE SPARING SOLUTIONS Instruments designed to accommodate your surgical approach preference and implant choice Enabling efficient, repeatable, tissue-sparing
Tissue Sparing Solutions DEsign Rationale TISSUE SPARING SOLUTIONS Instruments designed to accommodate your surgical approach preference and implant choice Enabling efficient, repeatable, tissue-sparing
Variable Angle LCP Locking Technology
 BUILDING ON SUCCESS Variable Angle LCP Locking Technology The Evolution of Plating Technology Round Screw Hole Dynamic Compression Plate Limited-Contact Dynamic Compression Less Invasive Stabilization
BUILDING ON SUCCESS Variable Angle LCP Locking Technology The Evolution of Plating Technology Round Screw Hole Dynamic Compression Plate Limited-Contact Dynamic Compression Less Invasive Stabilization
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
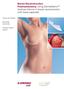 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
VERSALOK SURGICAL TECHNIQUE FOR ROTATOR CUFF REPAIR SURGICAL TECHNIQUE VERSATILITY STRENGTH SPEED
 VERSALOK SURGICAL TECHNIQUE FOR ROTATOR CUFF REPAIR VERSATILITY Allows a variety of suture configurations including suture spanning to meet the demands of various types of tear configurations STRENGTH
VERSALOK SURGICAL TECHNIQUE FOR ROTATOR CUFF REPAIR VERSATILITY Allows a variety of suture configurations including suture spanning to meet the demands of various types of tear configurations STRENGTH
The Ceterix NovoStitch Disposable Suture Passer. Do Not Reuse
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
T-PLIF Spacer. An allograft spacer for transforaminal posterior lumbar interbody fusion.
 Processed by MTF, designed and available through.. An allograft spacer for transforaminal posterior lumbar interbody fusion. The has been engineered to meet the specific demands of transforaminal posterior
Processed by MTF, designed and available through.. An allograft spacer for transforaminal posterior lumbar interbody fusion. The has been engineered to meet the specific demands of transforaminal posterior
VIPER PRIME System Cadaver Time Study
 VIPER PRIME System Cadaver Time Study White Paper August 24, 2017 1. INTRODUCTION Minimally invasive surgical techniques to perform spinal stabilization have gained popularity in recent years due to the
VIPER PRIME System Cadaver Time Study White Paper August 24, 2017 1. INTRODUCTION Minimally invasive surgical techniques to perform spinal stabilization have gained popularity in recent years due to the
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
READ BEFORE USING. Contents:
 READ BEFORE USING The Liveyon ReGen product is derived from voluntarily donated human umbilical cord. ReGen can only be sold to, and used by licensed physicians. Read this entire package insert carefully
READ BEFORE USING The Liveyon ReGen product is derived from voluntarily donated human umbilical cord. ReGen can only be sold to, and used by licensed physicians. Read this entire package insert carefully
ÆLITE Composites. Bisco. Instructions for Use. Light- Cured. U.S. Patent: 6,709,271
 Bisco ÆLITE Composites 0459 Light- Cured Instructions for Use U.S. Patent: 6,709,271 IN-131R6 Rev. 4/16 BISCO, Inc. 1100 W. Irving Park Road Schaumburg, IL 60193 U.S.A. 847-534-6000 1-800-247-3368 Caution:
Bisco ÆLITE Composites 0459 Light- Cured Instructions for Use U.S. Patent: 6,709,271 IN-131R6 Rev. 4/16 BISCO, Inc. 1100 W. Irving Park Road Schaumburg, IL 60193 U.S.A. 847-534-6000 1-800-247-3368 Caution:
The Fusion of. Strength, Design. Performance. and
 The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
DMF Page 63. Description
 DMF Page 60 DMF Page 61 DMF Page 62 4 GB Description The SPONGOSTAN Absorbable Haemostatic Gelatin Powder is a sterile, water-insoluble, porcine gelatin absorbable powder intended for haemostatic use by
DMF Page 60 DMF Page 61 DMF Page 62 4 GB Description The SPONGOSTAN Absorbable Haemostatic Gelatin Powder is a sterile, water-insoluble, porcine gelatin absorbable powder intended for haemostatic use by
COR. Precision Targeting Cartilage Repair System. Arthroscopic Technique for Repair of Osteochondral Defects
 COR Precision Targeting Cartilage Repair System Arthroscopic Technique for Repair of Osteochondral Defects Planning the Procedure An 18-gauge spinal needle is initially used to plan a perpendicular approach
COR Precision Targeting Cartilage Repair System Arthroscopic Technique for Repair of Osteochondral Defects Planning the Procedure An 18-gauge spinal needle is initially used to plan a perpendicular approach
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
