STUDY. Dosing With 5% Imiquimod Cream 3 Times per Week for the Treatment of Actinic Keratosis
|
|
|
- Suzan Parker
- 5 years ago
- Views:
Transcription
1 STUDY Dosing With 5% Imiquimod Cream 3 Times per Week for the Treatment of Actinic Keratosis Results of Two Phase 3, Randomized, Double-blind, Parallel-Group, Vehicle-Controlled Trials Neil Korman, MD, PhD; Ron Moy, MD; Mark Ling, MD, PhD; Robert Matheson, MD; Stacy Smith, MD; Scott McKane, MS; James H. Lee, MD, PhD Objective: To evaluate the efficacy and safety of 5% imiquimod cream compared with vehicle in the treatment of actinic keratosis (AK). Design: Two phase 3 randomized, double-blind, parallelgroup, vehicle-controlled studies. Setting: Twenty-six ambulatory care offices, including dermatologists in private practice or research centers. Patients: Four hundred ninety-two patients, 18 years and older, with 4 to 8 AK lesions in a 25-cm 2 treatment area on the face or the balding scalp were randomized; an additional 162 patients underwent screening but were ineligible. Interventions: Patients applied 5% imiquimod (Aldara) or vehicle cream to the treatment area once daily, 3 times per week, for 16 weeks, followed by an 8-week posttreatment period. Main Outcome Measurements: Complete clearance rate (proportion of patients at the 8-week posttreatment visit with no clinically visible AK lesions in the treatment area), partial clearance rate (proportion of patients at the 8-week posttreatment visit with a 75% reduction in the number of baseline AK lesions in the treatment area), and frequency and severity of adverse events and local skin reactions were measured. Results: Complete and partial clearance rates for imiquimod-treated patients (48.3% and 64.0%, respectively) were clinically and statistically significantly higher than for vehicle-treated patients (7.2% and 13.6%, respectively). The median percentage reduction of baseline lesions was 86.6% for the imiquimod-treated group and 14.3% for the vehicletreated group. Conclusion: The 5% imiquimod cream dosed 3 times weekly for 16 weeks is safe and effective for the treatment of AK. Arch Dermatol. 2005;141: Author Affiliations are listed at the end of this article. Financial Disclosure: Drs Moy, Ling, Matheson, and Smith received support from 3M Pharmaceuticals for performing clinical trials. Drs Smith and Ling have received funding (eg, research support and honoraria) from companies with competing products. Drs Korman, Moy, and Matheson received clinical trial support and honoraria from 3M Pharmaceuticals. This research was conducted with financial support from 3M Pharmaceuticals, St Paul, Minn. ACTINIC KERATOSES (AKS) are epidermal lesions consisting of dysplastic keratinocytes that generally occur in fair-skinned individuals with long-term exposure to UV radiation. 1,2 Although the exact pathogenesis of AK is unknown, long-term UV radiation is known to produce local and systemic immunosuppression, mutations in the p53 tumor suppressor gene, and DNA See also pages 447 and 507 pyrimidine covalent dimers, each of which are believed to contribute to the development of AK. 3,4 The most common current therapies for AK include cryosurgery, curettage with or without electrosurgery, and topical fluorouracil. Despite the widespread use of these therapies, most have limitations such as inconvenience, pain, and scarring. Furthermore, none of the current therapies target a critical component underlying the disease pathogenesis: the suppression of the immune response. CME course available at The only treatment for AK that works by enhancing the immune response against dysplastic cells is 5% imiquimod cream (Aldara; 3M Pharmaceuticals, St Paul, Minn). Imiquimod has been shown to induce the production of interferon, tumor necrosis factor, and interleukin (IL) 12 (IL- 12), with a resulting cytokine cascade that may induce and/or support a cytotoxic T- lymphocyte immune response. 5 Ongoing research has recently shown that imi- 467
2 quimod may also act through Toll-like receptor 7 to stimulate rapid synthesis and release of cytokines from monocytes, macrophages, and dendritic cells. Toll-like receptors are receptors that recognize special patterns on immune cell surfaces and are considered essential components of the human innate immune response. 6 Since imiquimod works through an immunological mechanism, regimens with infrequent dosing for an extended duration have been evaluated to minimize potentially intolerable adverse effects. To date, 5 large randomized, double-blind, parallel-group, vehiclecontrolled studies have been conducted to evaluate the efficacy and safety of topically applied 5% imiquimod cream vs vehicle cream for the treatment of AK lesions on the face and balding scalp. Two of these studies evaluated once-daily dosing 2 times per week for 16 weeks; the other 3 studies evaluated once-daily dosing 3 times per week for 16 weeks. The complete and partial clearance rates for both studies that evaluated dosing 2 times per week and for one of those that evaluated dosing 3 times per week have been reported. 7,8 The efficacy end point in that previously reported study of dosing 3 times per week included histological confirmation and clinical lesion assessments. The results of the remaining 2 studies that evaluated the regimen of dosing 3 times per week are presented herein. METHODS STUDY POPULATION The study population consisted of otherwise healthy men and women 18 years or older with a clinical diagnosis of 4 to 8 AK lesions within a contiguous 25-cm 2 treatment area on the face or the balding scalp as previously described. 7 STUDY DESIGN Two independent, randomized, double-blind, parallel-group, vehicle-controlled studies collectively enrolled patients from 26 US study centers (13 centers per study). The primary and secondary objectives of these studies were to evaluate the efficacy and safety of 5% imiquimod cream, respectively, compared with vehicle cream in the treatment of AK lesions on the face or the balding scalp when the cream was applied once daily 3 times per week for 16 weeks. The studies consisted of prestudy ( 2 weeks), treatment (16 weeks), and posttreatment (8 weeks) periods. Patients underwent assessment during treatment weeks 1, 2, 4, 6, 8, 10, 12, 14, and 16 and posttreatment weeks 4 and 8. At the treatment initiation visit, enrolled patients were assigned the next sequential patient study number and the corresponding study cream, which was randomized and labeled, on the basis of a computer-generated randomization schedule (stratified by study center). Patients were randomized to imiquimod or to vehicle cream in a 1:1 ratio. At the treatment initiation visit, the treatment area and the location of the 4 to 8 baseline AK lesions were recorded and mapped. Patients were instructed to apply the study cream (imiquimod or vehicle) to the entire treatment area at the same time of day (just before normal sleeping hours), and the cream was to remain in place for approximately 8 hours. Rest periods from study cream were allowed at the discretion of the investigator, but did not alter the length of the 16-week treatment period. Patients who discontinued treatment were asked to return for an assessment of their AK lesions at the 8-week posttreatment visit. All study procedures and informed consent documents received approval from the respective institutional review boards, and all enrolled patients signed informed consent forms. EFFICACY MEASUREMENTS Actinic keratosis lesions were counted at the 4-, 8-, and 16-week treatment visits and the 8-week posttreatment visit. No differentiation was made between the baseline and new AK lesions. The primary variable was the complete clearance rate, defined as the proportion of patients at the 8-week posttreatment visit with no clinically visible AK lesions in the treatment area. The secondary efficacy variable was the partial clearance rate, defined as the proportion of patients at the 8-week posttreatment visit with at least a 75% reduction in the number of baseline AK lesions in the treatment area. An additional measurement of clearance was the median percentage of reduction of baseline AK lesions at the 8-week posttreatment visit. SAFETY MEASUREMENTS Safety was monitored by reviewing concomitant medication use and assessing the incidence and severity of adverse events and local skin reactions. For the purposes of these studies, local skin reactions were differentiated and prospectively collected independently from adverse events (spontaneously reported). Local skin reactions in the treatment and surrounding areas were clinically categorized as erythema, edema, erosion/ulceration, scabbing/ crusting, weeping/exudation, vesicles, and flaking/scaling/ dryness. The intensity of an investigator-assessed local skin reaction was rated, with 0 indicating none; 1, mild; 2, moderate; and 3, severe. Reactions within the treatment area that were not assessed as local skin reactions were reported as adverse events. At the treatment initiation and 8-week posttreatment visits, investigators performed skin quality assessments of the treatment area. Characteristics assessed included skin surface (roughness/ dryness/scaliness), hyperpigmentation, hypopigmentation, mottled or irregular pigmentation (hyperpigmentation and hypopigmentation), degree of scarring, and degree of atrophy. After visual, clinical, and tactile examinations of the treatment area, the investigator coded the intensity of each characteristic (with 0 indicating none; 1, mild; 2, moderate; and 3, severe). STATISTICAL ANALYSIS The primary data set analyzed was the intent-to-treat data set, which consisted of combined data from the 2 studies and included all randomized patients. For each study, 94 patients per treatment group were required to have at least 90% power to detect a difference in complete clearance rates of 35% for the imiquimod group vs 14% for the vehicle group, with a type I error rate of.05. Assuming a 20% dropout rate, 120 patients per treatment group were required, giving a total of 240 patients per study. There were no interim analyses or stopping rules. The Cochran-Mantel-Haenszel test was used to compare the treatment groups (imiquimod vs vehicle) with respect to complete clearance rate; the test was performed at the.05 level of significance. The difference in complete clearance rates (imiquimod minus vehicle) with an associated 95% confidence interval (CI) was calculated. The same analyses were performed on partial clearance as described for complete clearance. The Cochran-Armitage test for trend in proportions (2 sided) examined relationships between complete clearance and the most intense local skin reaction experienced by patients. 468
3 492 Patients Randomized 242 Received 5% Imiquimod Cream 250 Received Vehicle Cream 210 Completed Treatment Period 32 Withdrew in Treatment Period 237 Completed Treatment Period 13 Withdrew in Treatment Period 209 Completed 8-wk Posttreatment 18 Completed 8-wk Posttreatment 234 Completed 8-wk Posttreatment 1 Completed 8-wk Posttreatment 1 Did Not Complete 8-wk Posttreatment 14 Did Not Complete 8-wk Posttreatment 3 Did Not Complete 8-wk Posttreatment 12 Did Not Complete 8-wk Posttreatment Figure 1. Patient accountability for the combined application studies of dosing 3 times per week. Adverse events were compared between treatment groups using the Fisher exact test. Wilcoxon rank sum tests were used to compare treatment groups with respect to local skin reaction assessments. For skin quality assessments, Wilcoxon signed rank tests were used to assess the significance of withintreatment changes from baseline, and Wilcoxon rank sum tests were used to assess the significance of between-treatment changes from baseline. RESULTS The combined studies enrolled 492 patients; 242 patients were randomized to 5% imiquimod cream and 250 patients were randomized to vehicle cream (Figure 1). Enrollment for both studies began in August 2001, and all study procedures were completed by August Table 1. Summary of Patient Demographics* Treatment Group 5% Imiquimod Cream Vehicle Cream Variable (n = 242) (n = 250) Sex Female 32 (13.2) 29 (11.6) Male 210 (86.6) 221 (88.4) Age, y Mean ± SD 66.7 ± ± 9.9 Range White race 242 (100) 250 (100) Skin type (Fitzpatrick) I-II 157 (64.9) 149 (59.6) III-VI 85 (35.1) 101 (40.4) P Value DEMOGRAPHICS The median age of the patients was 67 years. All were white; 61 (12.4%) were female; and 431 (87.6%) were male. The sex distribution observed in this study was consistent with previous epidemiological data. 9 There were no distribution differences for sex, age, race, or skin type between treatment groups (Table 1). EFFICACY Complete clearance occurred in 117 (48.3%) of 242 patients in the imiquimod group and 18 (7.2%) of 250 patients in the vehicle group (P.001). The difference in complete clearance rates was 41.1% (95% CI, 34.1%- 48.2%). Partial clearance occurred in 155 (64.0%) of imiquimod-treated patients and 34 (13.6%) of vehicletreated patients (P.001). Differences for the complete and partial clearance rates in the imiquimod groups were observed between the 2 studies; complete clearance rates were 56.4% vs 40.8% and partial clearance rates were 71.8% vs 56.8%. No differences in baseline characteristics or demographics of the patients were noted between the 2 studies (data not shown). *Unless otherwise indicated, data are expressed as number (percentage) of patients. P values for sex and skin type are calculated by means of the Fisher exact test; for age, by means of analysis of variance. For the imiquimod group, there was a statistically significant observed association between complete and partial clearance rates and the intensity of local skin reactions. Specifically, clearance rates tended to increase as the intensity of erythema increased (Figure 2). The number of patients who had an increase in AK lesion count at any point in the treatment period was higher in the imiquimod group (103/242 [42.6%]) compared with the vehicle group (55/250 [22.0%]). The complete clearance rate was slightly higher for imiquimodtreated patients who had an increase in AK lesion count over baseline (56/103 [54.4%]) compared with imiquimod-treated patients who did not have an increase in lesion count (61/139 [43.9%]). At the 8-week posttreatment visit, the median percentage of reduction in AK lesion count was 86.6% for the imiquimod group and 14.3% for the vehicle group. This means that half of the patients in the imiquimod group had at least an 86.6% reduction in the number of baseline AK lesions. 469
4 Complete Clearance Rate, % % None (n = 4) 25% Mild (n = 36) 46% Moderate (n = 121) Erythema Intensity 65% Severe (n = 80) Figure 2. Complete clearance by most intense erythema in the 5% imiquimod cream treated subjects. Cochran-Armitage test for trend P.001 (2 sided). There was a trend for complete clearance to increase as the intensity of erythema increased. Table 2. Application Site Reactions Possibly or Probably Related to Study Drug Reported by at Least 1% of Patients Application Site Reaction Treatment Group, No. (%)* 5% Imiquimod Cream (n = 242) Vehicle Cream (n = 250) P Value Itching at target site 70 (28.9) 10 (4.0).001 Burning at target site 18 (7.4) 2 (0.8).001 Itching at remote site 17 (7.0) 3 (1.2).001 Pain at target site 9 (3.7) Tenderness at target site 5 (2.1) 2 (0.8).28 Infection at target site 4 (1.7) 0.06 Burning at remote site 4 (1.7) 0.06 Stinging at target site 3 (1.2) 1 (0.4).37 Swollen at remote site 3 (1.2) 0.12 Tenderness at remote site 3 (1.2) 0.12 *Both study treatments were applied 3 times per week. Target site refers to the treatment area; remote site refers to the area surrounding the treatment area and beyond. Calculated by means of the Fisher exact test. SAFETY Adverse Events Adverse events occurred in both the imiquimod (176/242 [72.7%]) and vehicle (149/250 [59.6%]) treatment groups. At least 1 adverse event considered by the investigator as possibly or probably related to the study drug was reported by 104 (43.0%) of imiquimod-treated patients and 22 (8.8%) of vehicle-treated patients (P.001). The most frequently reported adverse event was application site reaction, with itching at target site reported most often. Application site reactions were reported by 94 (38.8%) of imiquimod-treated patients and 18 (7.2%) of vehicle-treated patients (P.001). The most frequently reported application site reactions that were considered possibly or probably related to study cream are listed by type in Table 2. Other adverse events showing statistically significant differences between the imiquimod and vehicle groups were fever (7 patients [2.9%] vs 1 patient [0.4%]; P=.04) and postoperative pain (9 patients [3.7%] vs 2 patients [0.8%]; P=.03). The adverse events of postoperative pain reported by patients included references to dental procedures (5 patients), unspecified operation (2 subjects), and squamous cell carcinoma removal from the ear of a subject randomized to the treatment of the scalp, hammer toe surgery, arthroscopic knee surgery, and kidney surgery (1 patient each). None of the adverse events of postoperative pain were considered by the investigator to be related to study drug. Forty-two serious adverse events were reported by 20 patients (12 imiquimod- and 8 vehicle-treated patients); none of the serious adverse events were considered by the investigator to be related to study drug. No deaths occurred in these studies. Local Skin Reactions Local skin reactions were common and occurred in both treatment groups. Both imiquimod- and vehicle-treated patients experienced severe local skin reactions; the rate of severe local skin reactions was higher in the imiquimod group. Table 3 includes the most intense Table 3. Three Most Frequent Investigator-Assessed Local Skin Reactions Skin Reactions, No. (%)* Erythema Flaking/Scaling/Dryness Scabbing/Crusting Summary by Intensity Imiquimod Vehicle Imiquimod Vehicle Imiquimod Vehicle Most intense during the study None 4/241 (1.7) 29/250 (11.6) 13/241 (5.4) 41/250 (16.4) 32/241 (13.3) 140/250 (56.0) Mild 36/241 (14.9) 158/250 (63.2) 88/241 (36.5) 152/250 (60.8) 60/241 (24.9) 95/250 (38.0) Moderate 121/241 (50.2) 63/250 (25.2) 119/241 (49.4) 56/250 (22.4) 83/241 (34.4) 15/250 (6.0) Severe 80/241 (33.2) 0/250 21/241 (8.7) 1/250 (0.4) 66/241 (27.4) 0/250 Any during study (mild, moderate, or severe) 237/241 (98.3) 221/250 (88.4) 228/241 (94.6) 209/250 (83.6) 209/241 (86.7) 110/250 (44.0) Baseline score (none) 73/242 (30.2) 76/249 (30.5) 71/242 (29.3) 78/249 (31.3) 210/242 (86.8) 217/248 (87.5) 8-wk Posttreatment visit score (none) 130/226 (57.5) 101/235 (43.0) 165/226 (73.0) 94/235 (40.0) 224/226 (99.1) 208/235 (88.5) *Imiquimod administered as 5% imiquimod cream. One imiquimod-treated patient did not undergo an assessment of local skin reactions after treatment initiation, so the denominator for the imiquimod group is 241 rather than 242. Baseline data were missing (not collected) for 1 vehicle-treated patient for flaking/scaling/dryness, and for 2 vehicle-treated patients for scabbing/crusting. 470
5 investigator-assessed local skin reactions in the treatment area during the course of the study (excluding the treatment initiation visit), the percentage of patients with any reaction at any time after treatment initiation, the number of patients with no reaction at treatment initiation, and the number of patients with no reaction at the 8-week posttreatment visit. Overall, local skin reactions were well tolerated and generally resolved or diminished in intensity after cessation of treatment (Figure 3). A Discontinuations From Study Patients could have discontinued (withdrawn) from the treatment period, the posttreatment period, or both. Patients who withdrew from the treatment period did not restart treatment, but were asked to return for posttreatment procedures. During the treatment period, 32 (13.2%) of 242 imiquimod-treated patients and 13 (5.2%) of 250 vehicle-treated patients discontinued from the study. Fifteen imiquimod-treated patients and 15 vehicle-treated patients discontinued during the posttreatment period. Table 4 summarizes the patients who discontinued during the treatment and posttreatment periods, stratified by reason for the discontinuation. B Rest Periods Rest periods were taken by 99 (40.9%) of 242 imiquimodtreated patients and 2 (0.8%) of 250 vehicle-treated patients. Of the patients taking rest periods, 86 (86.9%) of the 99 imiquimod-treated patients and 2 (100%) of the 2 vehicle-treated patients resumed treatment after their last rest period. C Skin Quality Assessment In general, the 8-week posttreatment assessments for each skin quality category indicated more patients with less intense scores than those with more intense scores after treatment with imiquimod. Scores for scarring and skin surface categories showed statistically significant within-treatment shifts from baseline to 8 weeks after treatment for the imiquimod group. Statistical significance was also found when scores for skin surface were compared between imiquimod and vehicle groups. Each significant shift was due to imiquimodtreated patients having less intense scores at 8 weeks after treatment than at baseline. Of the 226 imiquimodtreated patients with both initiation and 8-week posttreatment skin surface assessments, 9 (4.0%) had an increase and 115 (50.9%) had a decrease in intensity at the 8-week posttreatment assessment compared with baseline. Because of inconsistencies in the investigators interpretation of hyperpigmentation, hypopigmentation, and mottled pigmentation, these data are not presented. COMMENT The results from these large clinical studies and previously published studies confirm that 5% imiquimod cream is a safe and effective treatment option for AK. Unlike Figure 3. Resolution of actinic keratosis (AK) lesions after treatment with 5% imiquimod cream. A, Baseline count of 5 AK lesions in treatment area. B, At treatment week 8, 6 AK lesions, mild edema, erosion/ulceration, flaking/scaling/dryness, scabbing/crusting, and moderate erythema are seen. C, At 8 weeks after treatment, complete clearance (0 lesions) and no local skin reactions are seen. other treatments for AK, imiquimod is an immunebased therapy. Imiquimod has been shown to stimulate the immune system by activating antigen-presenting cells such as monocytes/macrophages and dendritic cells to produce interferon and other cytokines and chemokines. Imiquimod appears to mediate these cellular effects through a Toll-like receptor, leading to the activation of transcription factor nuclear factor B, which then culminates in the transcription of a number of cytokines including tumor necrosis factor, IL-1, IL-6, IL-8, and IL These cytokines stimulate several other aspects of the innate immune response and are important for directing the adaptive immune response. The enhancement of the immune response is an ideal therapeu- 471
6 Table 4. Patients Discontinued From the Treatment and Posttreatment Periods for Any Reason* Treatment Group, No. (%) Treatment Period Posttreatment Period Primary Reason for Discontinuation Imiquimod (n = 242) Vehicle (n = 250) Imiquimod (n = 242) Vehicle (n = 250) Adverse event 10 (4.1) 6 (2.4) 2 (0.8) 4 (1.6) Lost to follow-up 2 (0.8) 3 (1.2) 2 (0.8) 4 (1.6) Decreased Personal 8 (3.3) 4 (1.6) 8 (3.3) 6 (2.4) Noncompliance 1 (0.4) Other (0.4) 1 (0.4) Local skin reaction 10 (4.1) 0 1 (0.4) 0 Entry criteria violated 1 (0.4) 0 1 (0.4) 0 Total 32 (13.2) 13 (5.2) 15 (6.2) 15 (6.0) *Patients could have been discontinued from the treatment period, the posttreatment period, or both. Imiquimod administered as 5% imiquimod cream. Of the 6 patients lost to follow-up, 1 moved out of state, 4 did not respond after repeated telephone calls and letters, and 1 was coded as unknown reason. Other was specified for 1 imiquimod-treated patient for use of exclusionary drugs and treatment with liquid nitrogen and for 1 vehicle-treated patient for treatment of actinic keratosis during the posttreatment period. tic approach for AK. The pathogenesis of AK involves suppression of the immune response against the abnormal cells that can be caused by long-term UV light exposure. Imiquimod treatment can reverse this immunosuppression, which potentially could reduce the rate of recurrences and the possibility of malignant transformation. The complete and partial clearance rates for the imiquimod-treated patients were 48.3% and 64.0%, respectively. The clinical response with imiquimod dosing 3 times per week was higher than the response seen with dosing 2 times per weekg 7 ; however, the magnitude of the increase was not as large as expected for a dosing regimen that was 50% higher. The modest difference in the clinical response between the 2 dosing regimens may be partly related to the low complete clearance seen in one of the studies evaluating dosing 3 times per week (40.8% vs 56.4%). No methodological explanation for this difference was apparent. The difference was most likely the result of random variation seen with clinical studies. However, the complete clearance rate from the third study that also evaluated dosing 3 times per week was 57.1%. 8 These data suggest that the 56% clearance rate is more likely the true efficacy for this dosing regimen. If the actual efficacy rate for dosing 3 times per week is closer to the 56%, the incremental benefit of higher doses of imiquimod may be clinically justified in some patients. The end point of complete clearance was a rigorous study end point that underestimated the clinical effectiveness of this treatment. For example, patients who experienced resolution of 7 of 8 lesions were still considered treatment failures because they did not achieve complete clearance. A more clinically useful measurement of the benefit is the median percentage of reduction in the number of lesions counted at baseline for each patient. The median percentage of reduction in baseline lesions was greater than 86% in the studies of dosing 3 times per week. This compares favorably with reported lesion clearance rates of pharmacological and nonpharmacological treatments for AK. 13 More frequent dosing (3 vs 2 times per week) was associated with more local skin and application site reactions, more rest periods, and more subjects who discontinued treatment owing to local skin reactions (10/242 [4%] for imiquimod 3 times per week). 7 However, the reactions were generally well tolerated and either resolved or diminished in intensity after cessation of treatment. This pharmacodynamic response is consistent with the immune mechanism of imiquimod. Futhermore, complete and partial clearance rates tended to increase as the intensity level of erythema increased. Therefore, based on the appearance of local skin reactions and their association with clearance, local skin reactions can be considered an extension of the pharmacological effects of imiquimod cream. This pharmacological response contributed to one of the limitations of this study. Although the study design was double blind, the pharmacodynamic effect of imiquimod treatment may have biased the investigators assessments; however, when there is an evident pharmacodynamic response, bias is inherent in the study. An additional benefit from imiquimod treatment appears to be improved skin quality compared with vehicle treatment. Although this phenomenon has been observed with nonspecific treatments that induce inflammation, the cytokines observed after imiquimod treatment have been reported to mediate wound healing and tissue remodeling Therefore, an improved cosmetic outcome may be another potential benefit of imiquimod treatment. Because imiquimod works by enhancing the innate and adaptive immune responses, individual patient responses will vary depending on many intrinsic and extrinsic factors, such as the number and responsiveness of Langerhans cells and extent of solar damage. Although it is not known which factors will predict a patient s response to imiquimod, it is reassuring to clinicians that many different dosing regimens appear to be safe and effective. CONCLUSIONS Dosing with 5% imiquimod cream 3 times per week for 16 weeks was a safe and effective regimen for the treat- 472
7 ment of AK. The overall efficacy was higher for dosing 3 times per week than for 2 times per week; however, the rate of local skin reactions was also higher. These results, in addition to results from previously published studies, suggest that many different dosing regimens are effective and that dosing could be tailored to minimize drug exposure and adverse effects. Accepted for Publication: April 27, Author Affiliations: Department of Dermatology, University Hospitals of Cleveland, Case Western Reserve University, Cleveland, Ohio (Dr Korman); Skin Cancer and Dermatologic Surgery Medical Group and Westwood Ambulatory Center, Los Angeles, Calif (Dr Moy); MedaPhase, Inc, Newnan, Ga (Dr Ling); Oregon Medical Research Center, Portland (Dr Matheson); Therapeutics Inc, La Jolla, Calif (Dr Smith); and 3M Pharmaceuticals, St Paul, Minn (Mr McKane and Dr Lee). Correspondence: Neil Korman, MD, PhD, Department of Dermatology, University Hospitals of Cleveland, Euclid Ave, Cleveland, OH (njk2@po.cwru.edu). Group Information: The Imiquimod AK Investigators include the following: Neil Korman, MD, PhD (coordinating investigator); Ron Moy, MD (coordinating investigator); Debra Breneman, MD; Armand Cognetta, MD; Ponciano Cruz, MD; Frank Dunlap, MD; Ruth Gilboa, MD; Marc Green, MD; Karen Harkaway, MD; Christopher Huerter, MD; Michael Jarratt, MD; Steven Kempers, MD; Debra Liu, MD; Mark Ling, MD, PhD; Keith Loven, MD; Robert Matheson, MD; Jennie Muglia, MD; Keyvan Nouri, MD; Charles Phillips, MD; Tania Phillips, MD; Elyse Rafal, MD; Paul Ratner, MD; Toivo Rist, MD; Michael Scannon, MD; Stacy Smith, MD; and James Swinehart, MD. Acknowledgment: We thank Nanda Gosala, MD, for medical monitoring; Mary Owens, MD, for editorial contributions; Terry L. Fox, MS, for statistical analyses support; and Amy Brown for manuscript preparation. REFERENCES 1. Frost CA, Green AC. Epidemiology of solar keratoses. Br J Dermatol. 1994;131: Heaphy MR Jr, Ackerman AB. The nature of solar keratosis: a critical review in historical perspective. J Am Acad Dermatol. 2000;43: Edwards L, Levine N, Weidner M, Piepkorn M, Smiles K. Effect of intralesional alpha 2-interferon on actinic keratoses. Arch Dermatol. 1986;122: Yantsos VA, Conrad N, Zabawski E, Cockerell CJ. Incipient intraepidermal cutaneous squamous cell carcinoma: a proposal for reclassifying and grading solar (actinic) keratoses. Semin Cutan Med Surg. 1999;18: Slade HB, Owens ML, Tomai MA, Miller RL. Imiquimod 5% cream (Aldara ). Expert Opin Investig Drugs. 1998;7: Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002; 3: Lebwohl M, Dinehart S, Whiting D, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol. 2004;50: Szeimies RM, Gerritsen MJP, Gupta G, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from a phase III, randomized, doubleblind, vehicle-controlled, clinical trial with histology. J Am Acad Dermatol. In press. 9. Fleischer AB Jr, Feldman SR, White RE, Leshin B, Byington R. Procedures for skin diseases performed by physicians in 1993 and 1994: analysis of data from the National Ambulatory Medical Care Survey. J Am Acad Dermatol. 1997; 37: Imbertson LM, Beaurline JM, Couture AM, et al. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S J Invest Dermatol. 1998;110: Fujisawa H, Shivji GM, Kondo S, et al. Effect of a novel topical immunomodulator, S-28463, on keratinocyte cytokine gene expression and production. J Interferon Cytokine Res. 1996;16: Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195: Szeimies RM, Karrer S, Radakovic-Fijan S, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol. 2002;47: Duncan M, Berman B. -Interferon is the lymphokine and -interferon the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J Exp Med. 1985;162: Granstein RD, Flotte TJ, Amento EP. Interferons and collagen production. JInvest Dermatol. 1990;95:75S-80S. 16. Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:
Scottish Medicines Consortium
 Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Scottish Medicines Consortium imiquimod 5% cream (Aldara) No. (385/07) Meda Pharmaceuticals Ltd 04 April 2008 The Scottish Medicines Consortium has completed its assessment of the above product and advises
Diagnosis and Management of Actinic Keratosis (AKs)
 Diagnosis and Management of Actinic Keratosis (AKs) Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
Diagnosis and Management of Actinic Keratosis (AKs) Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada
Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM
 Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Dilemmas and Challenges in Skin Cancer Therapies and Management Field vs Lesional Therapies for AKs 3/2/2019, 9:00-12 AM Roger I. Ceilley, M.D. Clinical Professor of Dermatology The University of Iowa
Clinical Trial Report Synopsis. Efficacy and Safety of LEO in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up
 Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Clinical Trial Report Synopsis Efficacy and Safety of LEO 43204 in Field Treatment of Actinic Keratosis on Face or Chest including 12-month follow-up Design of trial: A phase 3, multi-centre, randomised,
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary
 Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
Topical Diclofenac Gel, Fluorouracil Cream, Imiquimod Cream, and Ingenol Gel Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1-8 Topical Diclofenac Gel Indication
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 26 November 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: 349 204-4) Applicant: MEDA PHARMA imiquimod ATC
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 26 November 2008 ALDARA 5%, cream Box of 12 sachets of 250 mg (CIP: 349 204-4) Applicant: MEDA PHARMA imiquimod ATC
Developing the next generation of dermatology products to treat serious skin diseases
 Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer www.peplin.com Forward Looking Statements This presentation contains
Developing the next generation of dermatology products to treat serious skin diseases Tom Wiggans Chairman and Chief Executive Officer www.peplin.com Forward Looking Statements This presentation contains
Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses
 Curr Derm Rep (2013) 2:113 117 DOI 10.1007/s13671-013-0046-x MEDICAL SURGERY (CJ MILLER, SECTION EDITOR) Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses Stephanie Jacks & Kasie
Curr Derm Rep (2013) 2:113 117 DOI 10.1007/s13671-013-0046-x MEDICAL SURGERY (CJ MILLER, SECTION EDITOR) Ingenol Mebutate: An Emerging Therapy in the Treatment of Actinic Keratoses Stephanie Jacks & Kasie
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A.
 fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
fluorouracil 0.5% / salicylic acid 10% cutaneous solution (Actikerall ) SMC No. (728/11) Almirall S.A. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Picato 150 micrograms/gram gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of gel contains 150 mcg of ingenol mebutate.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Picato 150 micrograms/gram gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of gel contains 150 mcg of ingenol mebutate.
TOPICAL TREATMENT OF ACTINIC KERATOSIS
 TOPICAL TREATMENT OF ACTINIC KERATOSIS Gary Goldenberg, MD Goldenberg Dermatology, PC Assistant Clinical Professor of Dermatology The Icahn School of Medicine at Mount Sinai Hospital Conflicts of Interest
TOPICAL TREATMENT OF ACTINIC KERATOSIS Gary Goldenberg, MD Goldenberg Dermatology, PC Assistant Clinical Professor of Dermatology The Icahn School of Medicine at Mount Sinai Hospital Conflicts of Interest
Clinical Trial Report Synopsis
 Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Clinical Trial Report Synopsis Efficacy and Safety of Ingenol Mebutate Gel in Field Treatment of Actinic Keratosis on Full Face, Balding Scalp or Approximately 250 cm 2 on the Chest Design of trial: Part
Ingenol Mebutate: A Succinct Review of a Succinct Therapy
 Dermatol Ther (Heidelb) (2014) 4:157 164 DOI 10.1007/s13555-014-0061-2 REVIEW Ingenol Mebutate: A Succinct Review of a Succinct Therapy David Rhys Alchin To view enhanced content go to www.dermtherapy-open.com
Dermatol Ther (Heidelb) (2014) 4:157 164 DOI 10.1007/s13555-014-0061-2 REVIEW Ingenol Mebutate: A Succinct Review of a Succinct Therapy David Rhys Alchin To view enhanced content go to www.dermtherapy-open.com
A Comparison of the Efficacy of Ablative Fractional Laser-assisted Photodynamic Therapy according to the Density of Ablative Laser Channel
 A Comparison of the Efficacy of Ablative Fractional Laser-assisted Photodynamic Therapy according to the Density of Ablative Laser Channel in the Treatment of Actinic Keratosis Yeo-Rye Cho, Jeong-Hwan
A Comparison of the Efficacy of Ablative Fractional Laser-assisted Photodynamic Therapy according to the Density of Ablative Laser Channel in the Treatment of Actinic Keratosis Yeo-Rye Cho, Jeong-Hwan
IMO 3100, an antagonist of Toll like receptor (TLR) 7 and TLR9, demonstrates clinical activity in psoriasis patients
 IMO 3100, an antagonist of Toll like receptor (TLR) 7 and TLR9, demonstrates clinical activity in psoriasis patients with 4 weeks of treatment in a Phase 2a trial A. B. Kimball 1, J. Krueger 2, T. Sullivan
IMO 3100, an antagonist of Toll like receptor (TLR) 7 and TLR9, demonstrates clinical activity in psoriasis patients with 4 weeks of treatment in a Phase 2a trial A. B. Kimball 1, J. Krueger 2, T. Sullivan
A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD
 A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD 8 Practical Dermatology February 007 Multiple treatment approaches exist for actinic
A by-the-numbers assessment of therapies considers efficacy, convenience, cosmesis, and cost. By Amy Forman Taub, MD 8 Practical Dermatology February 007 Multiple treatment approaches exist for actinic
BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS
 BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS 25 Skin lesions Miri Seiberg, PhD 26 Skin lesions A part of the skin that has an abnormal growth or appearance compared
BL-5010P A NOVEL PRE-FILLED APPLICATOR FOR THE NON-SURGICAL REMOVAL OF SKIN LESIONS 25 Skin lesions Miri Seiberg, PhD 26 Skin lesions A part of the skin that has an abnormal growth or appearance compared
Osteopathic Dermatology
 Osteopathic Dermatology Teodor Huzij, DO, FACN Reagan Anderson, DO, FAOCD, FASMS, Certificate of Added Qualification for Mohs Surgery, MPH, MCS, Program Director for RVU/CDI Dermatology Residency Program
Osteopathic Dermatology Teodor Huzij, DO, FACN Reagan Anderson, DO, FAOCD, FASMS, Certificate of Added Qualification for Mohs Surgery, MPH, MCS, Program Director for RVU/CDI Dermatology Residency Program
Molluscum contagiosum (MC) is a common
 THERAPEUTICS FOR THE CLINICIAN Effectiveness of Imiquimod Cream 5% for Treating Childhood Molluscum Contagiosum in a Double-blind, Randomized Pilot Trial Amy U. Theos, MD; Rebecca Cummins, MD; Nanette
THERAPEUTICS FOR THE CLINICIAN Effectiveness of Imiquimod Cream 5% for Treating Childhood Molluscum Contagiosum in a Double-blind, Randomized Pilot Trial Amy U. Theos, MD; Rebecca Cummins, MD; Nanette
Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: A double-blind, randomized, vehicle-controlled study*
 Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: A double-blind, randomized, vehicle-controlled study* John K. Geisse, MD, a Phoebe Rich, MD, b Amit Pandya, MD, c Kenneth Gross,
Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: A double-blind, randomized, vehicle-controlled study* John K. Geisse, MD, a Phoebe Rich, MD, b Amit Pandya, MD, c Kenneth Gross,
AWMSG SECRETARIAT ASSESSMENT REPORT. Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel. Reference number: 1392 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel Reference number: 1392 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
AWMSG SECRETARIAT ASSESSMENT REPORT Ingenol mebutate (Picato ) 150 micrograms/g gel and 500 micrograms/g gel Reference number: 1392 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics
Opinion 26 June 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 26 June 2013 PICATO 150 µg/g, gel 3 tubes of 0.47 g (CIP: 34009 268 528-4 9) PICATO 500 µg/g, gel 2 tubes of 0.47
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 26 June 2013 PICATO 150 µg/g, gel 3 tubes of 0.47 g (CIP: 34009 268 528-4 9) PICATO 500 µg/g, gel 2 tubes of 0.47
Richard Turner Consultant Dermatologist
 Old Problems & New Treatments Photo Album by Administrator Richard Turner Consultant Dermatologist Plan for tonight? Refresher on SCC and solar keratosis How to distinguish the two Classic therapy than
Old Problems & New Treatments Photo Album by Administrator Richard Turner Consultant Dermatologist Plan for tonight? Refresher on SCC and solar keratosis How to distinguish the two Classic therapy than
Skin cancer is the most common of all cancer PROCEEDINGS CLINICAL UPDATE ON ACTINIC KERATOSIS* Murad Alam, MD ABSTRACT
 CLINICAL UPDATE ON ACTINIC KERATOSIS* Murad Alam, MD ABSTRACT Cutaneous squamous cell carcinoma (SCC) accounts for approximately 250 000 cancer cases each year in the United States, but is associated with
CLINICAL UPDATE ON ACTINIC KERATOSIS* Murad Alam, MD ABSTRACT Cutaneous squamous cell carcinoma (SCC) accounts for approximately 250 000 cancer cases each year in the United States, but is associated with
A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ. Övermark, Meri.
 https://helda.helsinki.fi A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ Övermark, Meri 2016 Övermark, M, Koskenmies, S & Pitkanen, S 2016, ' A Retrospective Study of Treatment of
https://helda.helsinki.fi A Retrospective Study of Treatment of Squamous Cell Carcinoma In situ Övermark, Meri 2016 Övermark, M, Koskenmies, S & Pitkanen, S 2016, ' A Retrospective Study of Treatment of
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee
 I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
I have a skin lump doc! What s next? 12 th August 2017 Dr. Sue-Ann Ho Ju Ee Some thoughts Is this skin cancer? How common is this? How likely is this in this patient? What happens next if it s something
Clinical characteristics
 Skin Cancer Fernando Vega, MD Seattle Healing Arts Clinical characteristics Precancerous lesions Common skin cancers ACTINIC KERATOSIS Precancerous skin lesions Actinic keratoses Dysplastic melanocytic
Skin Cancer Fernando Vega, MD Seattle Healing Arts Clinical characteristics Precancerous lesions Common skin cancers ACTINIC KERATOSIS Precancerous skin lesions Actinic keratoses Dysplastic melanocytic
STUDY. Topical 5-Aminolevulinic Acid Combined With Intense Pulsed Light in the Treatment of Photoaging
 STUDY Topical 5-Aminolevulinic Acid Combined With Intense Pulsed Light in the Treatment of Photoaging Jeffrey S. Dover, MD, FRCPC; Ashish C. Bhatia, MD; Brigitte Stewart; Kenneth A. Arndt, MD Background:
STUDY Topical 5-Aminolevulinic Acid Combined With Intense Pulsed Light in the Treatment of Photoaging Jeffrey S. Dover, MD, FRCPC; Ashish C. Bhatia, MD; Brigitte Stewart; Kenneth A. Arndt, MD Background:
CLINICAL SCIENCE. doi: /S
 CLINICS 2009;64(10):961-6 CLINICAL SCIENCE TREATMENT OF CUTANEOUS TUMORS WITH TOPICAL 5% IMIQUIMOD CREAM Sabrina Sisto Alessi, Jose Antonio Sanches, Walmar Roncalli de Oliveira, Maria Cristina Messina,
CLINICS 2009;64(10):961-6 CLINICAL SCIENCE TREATMENT OF CUTANEOUS TUMORS WITH TOPICAL 5% IMIQUIMOD CREAM Sabrina Sisto Alessi, Jose Antonio Sanches, Walmar Roncalli de Oliveira, Maria Cristina Messina,
Interesting Case Series. Aggressive Tumor of the Midface
 Interesting Case Series Aggressive Tumor of the Midface Adrian Frunza, MD, Dragos Slavescu, MD, and Ioan Lascar, MD, PhD Bucharest Emergency Clinical Hospital, Bucharest University School of Medicine,
Interesting Case Series Aggressive Tumor of the Midface Adrian Frunza, MD, Dragos Slavescu, MD, and Ioan Lascar, MD, PhD Bucharest Emergency Clinical Hospital, Bucharest University School of Medicine,
SYNOPSIS. Issue Date: 25 Oct 2011
 SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
SYNOPSIS Issue Date: 25 Oct 2011 Name of Sponsor/Company Name of Finished Product Name of Active Ingredient(s) Janssen Research & Development STELARA Ustekinumab Protocol No.: Title of Study: Study Name:
Cutaneous Malignancies: A Primer COPYRIGHT. Marissa Heller, M.D.
 Cutaneous Malignancies: A Primer Marissa Heller, M.D. Associate Director of Dermatologic Surgery Department of Dermatology Beth Israel Deaconess Medical Center December 10, 2016 Skin Cancer Non-melanoma
Cutaneous Malignancies: A Primer Marissa Heller, M.D. Associate Director of Dermatologic Surgery Department of Dermatology Beth Israel Deaconess Medical Center December 10, 2016 Skin Cancer Non-melanoma
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Assistant Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Clinical Study Report Synopsis
 This document has been dovmloaded from www.leo-pharma.c.om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
This document has been dovmloaded from www.leo-pharma.c.om subject to the terms of use state on the website. It contains data and results regarding approved and non-approved uses, formulations or treatment
Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects *
 Investigation 529 s Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects * Maria Isabel Ramos Saraiva 1,3, Larissa Karine Leite Portocarrero 1, Marcella Amaral Horta
Investigation 529 s Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects * Maria Isabel Ramos Saraiva 1,3, Larissa Karine Leite Portocarrero 1, Marcella Amaral Horta
1) Photodynamic therapy with topical 5 aminolevulinic acid is considered medically necessary and is covered for the treatment of:
 Medical Policy Title: Photodynamic Therapy ARBenefits Approval: 10/26/2011 for Dermatologic Conditions Effective Date: 01/01/2012 Document: ARB0282:02 Revision Date: 03/20/2013 Code(s): 96567 Photodynamic
Medical Policy Title: Photodynamic Therapy ARBenefits Approval: 10/26/2011 for Dermatologic Conditions Effective Date: 01/01/2012 Document: ARB0282:02 Revision Date: 03/20/2013 Code(s): 96567 Photodynamic
PATHOLOGICAL TYPES OF SCARS AND THEIR IMPACT ON TREATMENT. Dr. Tarek Ahmed Said Professor of Plastic Surgery Cairo University 2015
 PATHOLOGICAL TYPES OF SCARS AND THEIR IMPACT ON TREATMENT Dr. Tarek Ahmed Said Professor of Plastic Surgery Cairo University 2015 Pathological Types of Scars Atrophic Scars Normotrophic Scars Hypertrophic
PATHOLOGICAL TYPES OF SCARS AND THEIR IMPACT ON TREATMENT Dr. Tarek Ahmed Said Professor of Plastic Surgery Cairo University 2015 Pathological Types of Scars Atrophic Scars Normotrophic Scars Hypertrophic
The no t-so-simple cutaneous wart
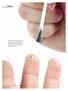 Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
Salicylic acid is a commonly used over-the-counter treatment for nongenital warts. Clearance rates of up to 75% have been reported with salicylic acid. istockphoto 10 Clinical practice guide Dermatology
This PDF is available for free download from a site hosted by Medknow Publications
 Net Study Comparison of clinical efficacy of topical tazarotene.1% cream with topical clobetasol propionate.5% cream in chronic plaque psoriasis: A double-blind, randomized, right-left comparison study
Net Study Comparison of clinical efficacy of topical tazarotene.1% cream with topical clobetasol propionate.5% cream in chronic plaque psoriasis: A double-blind, randomized, right-left comparison study
Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine
![Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Product Information Australia APO-IMIQUIMOD CREAM. NAME OF THE MEDICINE Imiquimod. 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine](/thumbs/83/88391058.jpg) APO-IMIQUIMOD CREAM NAME OF THE MEDICINE Imiquimod. Chemical Name: 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Structural Formula: Molecular Formula: C 14 H 16 N 4 Molecular Weight: 240.3 CAS
APO-IMIQUIMOD CREAM NAME OF THE MEDICINE Imiquimod. Chemical Name: 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine Structural Formula: Molecular Formula: C 14 H 16 N 4 Molecular Weight: 240.3 CAS
Living Beyond Cancer Skin Cancer Detection and Prevention
 Living Beyond Cancer Skin Cancer Detection and Prevention Cutaneous Skin Cancers Identification Diagnosis Treatment options Prevention What is the most common cancer in people? What is the most common
Living Beyond Cancer Skin Cancer Detection and Prevention Cutaneous Skin Cancers Identification Diagnosis Treatment options Prevention What is the most common cancer in people? What is the most common
Treatment of Mild to Moderate Psoriasis with Reliéva, a Mahonia aquifolium Extract A Double-Blind, Placebo-Controlled Study
 American Journal of Therapeutics 13, 121 126 (2006) Treatment of Mild to Moderate Psoriasis with Reliéva, a Mahonia aquifolium Extract A Double-Blind, Placebo-Controlled Study Steve Bernstein, Howard Donsky,*
American Journal of Therapeutics 13, 121 126 (2006) Treatment of Mild to Moderate Psoriasis with Reliéva, a Mahonia aquifolium Extract A Double-Blind, Placebo-Controlled Study Steve Bernstein, Howard Donsky,*
Have a Voice in Your Choice!
 Have a Voice in Your Choice! BLU-U Blue Light Photodynamic Therapy The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is
Have a Voice in Your Choice! BLU-U Blue Light Photodynamic Therapy The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is
STUDY. Laser-Mediated Photodynamic Therapy of Actinic Keratoses
 STUDY Laser-Mediated Photodynamic Therapy of Actinic Keratoses Macrene R. Alexiades-Armenakas, MD, PhD; Roy G. Geronemus, MD Objective: To assess the safety and efficacy of the longpulsed pulsed dye laser
STUDY Laser-Mediated Photodynamic Therapy of Actinic Keratoses Macrene R. Alexiades-Armenakas, MD, PhD; Roy G. Geronemus, MD Objective: To assess the safety and efficacy of the longpulsed pulsed dye laser
STUDY. Differences in Biopsy Techniques of Actinic Keratoses by Plastic Surgeons and Dermatologists. the most common reasons
 STUDY Differences in Biopsy Techniques of Actinic Keratoses by Plastic Surgeons and Dermatologists A Histologically Controlled Pilot Study Klaus Sellheyer, MD; Wilma F. Bergfeld, MD Objective: To compare
STUDY Differences in Biopsy Techniques of Actinic Keratoses by Plastic Surgeons and Dermatologists A Histologically Controlled Pilot Study Klaus Sellheyer, MD; Wilma F. Bergfeld, MD Objective: To compare
Medical Policy. MP Dermatologic Applications of Photodynamic Therapy
 Medical Policy MP 2.01.44 BCBSA Ref. Policy: 2.01.44 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.47 Light Therapy for Psoriasis 8.01.06 Oncologic Applications
Medical Policy MP 2.01.44 BCBSA Ref. Policy: 2.01.44 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.47 Light Therapy for Psoriasis 8.01.06 Oncologic Applications
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
New Medicines Committee Briefing May 2015
 New Medicines Committee Briefing May 2015 Picato (ingenol mebutate) 150 or 500 mcg/g gel for treatment of nonhyperkeratotic, non-hypertrophic actinic keratosis (AK) in adults. Picato is to be reviewed
New Medicines Committee Briefing May 2015 Picato (ingenol mebutate) 150 or 500 mcg/g gel for treatment of nonhyperkeratotic, non-hypertrophic actinic keratosis (AK) in adults. Picato is to be reviewed
Know who is at risk: LOOK! for ABCDs, rapidly changing lesions, do a biopsy when indicated
 Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
Lindy P. Fox, MD Associate Professor Director, Hospital Consultation Service Department of Dermatology University of California, San Francisco Applies to adults without history of malignancy or premalignant
30 Actinic Keratosis (Solar Keratosis)
 30 Actinic Keratosis (Solar Keratosis) CLINICAL APPLICATION QUESTIONS A 65-year-old white man is seen at your office for multiple scaling lesions over his face, ears, neck, and the V of the chest. These
30 Actinic Keratosis (Solar Keratosis) CLINICAL APPLICATION QUESTIONS A 65-year-old white man is seen at your office for multiple scaling lesions over his face, ears, neck, and the V of the chest. These
Imiquimod to Treat Different Cancers of the Epidermis
 COMMUNICATIONS AND BRIEF REPORTS Imiquimod to Treat Different Cancers of the Epidermis JAN EKLIND, MD, n ULRIKE TARTLER, MD, w JAN MASCHKE, MD, w PETER LIDBRINK, MD, n ULRICH R. HENGGE, MD w n Department
COMMUNICATIONS AND BRIEF REPORTS Imiquimod to Treat Different Cancers of the Epidermis JAN EKLIND, MD, n ULRIKE TARTLER, MD, w JAN MASCHKE, MD, w PETER LIDBRINK, MD, n ULRICH R. HENGGE, MD w n Department
Low-fluence Q-switched neodymium-doped yttrium aluminum garnet (1064nm) laser for the treatment of facial melasma in local population
 Original Article Low-fluence Q-switched neodymium-doped yttrium aluminum garnet (1064nm) laser for the treatment of facial melasma in local population Tahir Kamal, Usma Iftikhar* Department of Dermatology,
Original Article Low-fluence Q-switched neodymium-doped yttrium aluminum garnet (1064nm) laser for the treatment of facial melasma in local population Tahir Kamal, Usma Iftikhar* Department of Dermatology,
The future of Skin Cancer Treatment
 The future of Skin Cancer Treatment What is ZaicaDerm? Fully formulated cream that delivers Tea Tree Oil in a transdermal format Using proprietary technology, ZaicaDerm, delivers 10% Tea Tree Oil below
The future of Skin Cancer Treatment What is ZaicaDerm? Fully formulated cream that delivers Tea Tree Oil in a transdermal format Using proprietary technology, ZaicaDerm, delivers 10% Tea Tree Oil below
Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest risk.
 Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
Basics of Skin Cancer Detection and Treatment of Non- Melanoma Skin Cancers Large majority caused by sun exposure Often sun exposure before age 20 Persons who burn easily and tan poorly are at greatest
Opinion 6 March 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 6 March 2013 EFFALA 8 mg, medicated plaster B/4 sachets (CIP code: 34009 397 996 4 3) B/8 sachets (CIP code: 34009
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 6 March 2013 EFFALA 8 mg, medicated plaster B/4 sachets (CIP code: 34009 397 996 4 3) B/8 sachets (CIP code: 34009
Actinic Keratoses and Bowen s disease
 Actinic Keratoses and Bowen s disease Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Actinic Keratoses and Bowen s disease Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Clinical Study Report Synopsis
 Clinical Study Report Synopsis An Explorative Clinical Trial to Evaluate an Intra Patient Comparison Design of Topical Agents in Adults with Mild to Moderate Atopic Dermatitis Design of trial: Single-centre,
Clinical Study Report Synopsis An Explorative Clinical Trial to Evaluate an Intra Patient Comparison Design of Topical Agents in Adults with Mild to Moderate Atopic Dermatitis Design of trial: Single-centre,
OBSERVATION. Decreased Skin Cancer After Cessation of Therapy With Transplant-Associated Immunosuppressants
 OBSERVATION Decreased Skin Cancer After Cessation of Therapy With Transplant-Associated Immunosuppressants Clark C. Otley, MD; Brett M. Coldiron, MD; Thomas Stasko, MD; Glenn D. Goldman, MD Background:
OBSERVATION Decreased Skin Cancer After Cessation of Therapy With Transplant-Associated Immunosuppressants Clark C. Otley, MD; Brett M. Coldiron, MD; Thomas Stasko, MD; Glenn D. Goldman, MD Background:
Product Information ALDARA TM
 Product Information ALDARA TM NAME OF THE MEDICINE is 1-(2-methylpropyl)-1H-imidazo [4,5-c]quinolin-4-amine. It is an odourless, white to off-white crystalline solid. has a molecular formula of C 14 H
Product Information ALDARA TM NAME OF THE MEDICINE is 1-(2-methylpropyl)-1H-imidazo [4,5-c]quinolin-4-amine. It is an odourless, white to off-white crystalline solid. has a molecular formula of C 14 H
Cancer Medicine. Using topical imiquimod for the management of positive in situ margins after melanoma resection. Introduction.
 Cancer Medicine ORIGINAL RESEARCH Open Access Using topical imiquimod for the management of positive in situ margins after melanoma resection Amrita S. Pandit 1, Erik J. Geiger 2, Stephan Ariyan 2, Deepak
Cancer Medicine ORIGINAL RESEARCH Open Access Using topical imiquimod for the management of positive in situ margins after melanoma resection Amrita S. Pandit 1, Erik J. Geiger 2, Stephan Ariyan 2, Deepak
Skin lesions The Good and the Bad. Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry
 Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
Skin lesions The Good and the Bad Dr Virginia Hubbard Ipswich Hospital NHS Trust Barts and the London School of Medicine and Dentistry Case 1 32 year old woman Australian Lesion on back New hair growing
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 PAGE 1 of 5 PURPOSE To assure that DOP inmates with skin lesions are receiving appropriate Primary Care for their lesions POLICY All DOP Primary Care Providers are expected to follow this guideline and/or
PAGE 1 of 5 PURPOSE To assure that DOP inmates with skin lesions are receiving appropriate Primary Care for their lesions POLICY All DOP Primary Care Providers are expected to follow this guideline and/or
Dermatology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI Memorial
 Dermatology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI Memorial Cutaneous Oncology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI
Dermatology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI Memorial Cutaneous Oncology for the PCP Deanna G. Brown, MD, FAAD Susong Dermatology Consulting Staff at CHI
Pre-Cancerous Skin Lesions & Skin Cancer
 Pre-Cancerous Skin Lesions & Skin Cancer Ian D.R. Landells, MD, FRCPC Clinical Associate Professor Memorial University of Newfoundland Medical Director Dermatology Nexus Clinical Research St. John s, NL
Pre-Cancerous Skin Lesions & Skin Cancer Ian D.R. Landells, MD, FRCPC Clinical Associate Professor Memorial University of Newfoundland Medical Director Dermatology Nexus Clinical Research St. John s, NL
Clearance in vulvar lichen sclerosus: a realistic treatment endpoint or a chimera?
 DOI: 10.1111/jdv.14516 JEADV SHORT REPORT Clearance in vulvar lichen sclerosus: a realistic treatment endpoint or a chimera? A. Borghi,* A. Virgili, S. Minghetti, G. Toni, M. Corazza Dipartimento di Scienze
DOI: 10.1111/jdv.14516 JEADV SHORT REPORT Clearance in vulvar lichen sclerosus: a realistic treatment endpoint or a chimera? A. Borghi,* A. Virgili, S. Minghetti, G. Toni, M. Corazza Dipartimento di Scienze
Cutaneous Cryotherapy in Maxillofacial Surgery
 CURRENT THERAPY Cutaneous Cryotherapy in Maxillofacial Surgery Phillip J. Ameerally, BDS, MBBS (HONS), FDSRCS, FRCS (Maxillofacial),* and Graham B. Colver, DM, FRCP, FRCPE J Oral Maxillofac Surg 65:1785-1792,
CURRENT THERAPY Cutaneous Cryotherapy in Maxillofacial Surgery Phillip J. Ameerally, BDS, MBBS (HONS), FDSRCS, FRCS (Maxillofacial),* and Graham B. Colver, DM, FRCP, FRCPE J Oral Maxillofac Surg 65:1785-1792,
Historically, cryosurgery has been the treatment of choice
 New Therapeutic Options for Actinic Keratosis and Basal Cell Carcinoma James E. Sligh, Jr, MD, PhD n Abstract Actinic keratosis (AK) is a common premalignant skin lesion that is frequently treated by cryosurgery.
New Therapeutic Options for Actinic Keratosis and Basal Cell Carcinoma James E. Sligh, Jr, MD, PhD n Abstract Actinic keratosis (AK) is a common premalignant skin lesion that is frequently treated by cryosurgery.
THE EFFECTS OF REPEATED SUB-ERYTHEMAL EXPOSURES OF UVR ON HUMAN IMMUNITY
 THE EFFECTS OF REPEATED SUB-ERYTHEMAL EXPOSURES OF UVR ON HUMAN IMMUNITY Joanna Narbutt Department of Dermatology Medical University of Lodz, Lodz, Poland Photoimmunosuppression ULTRAVIOLET RADIATION DNA
THE EFFECTS OF REPEATED SUB-ERYTHEMAL EXPOSURES OF UVR ON HUMAN IMMUNITY Joanna Narbutt Department of Dermatology Medical University of Lodz, Lodz, Poland Photoimmunosuppression ULTRAVIOLET RADIATION DNA
It has been estimated that 90% of individuals
 Famciclovir for Cutaneous Herpesvirus Infections: An Update and Review of New Single-Day Dosing Indications Manju Chacko, MD; Jeffrey M. Weinberg, MD Infections with herpes simplex virus (HSV) types 1
Famciclovir for Cutaneous Herpesvirus Infections: An Update and Review of New Single-Day Dosing Indications Manju Chacko, MD; Jeffrey M. Weinberg, MD Infections with herpes simplex virus (HSV) types 1
PRODUCT INFORMATION METVIX
 PRODUCT INFORMATION METVIX NAME OF THE MEDICINE Methyl aminolevulinate (as hydrochloride). Structural formula: O OCH 3 NH 3 + Cl - O CAS number: 79416-27-6 DESCRIPTION Metvix cream contains 160 mg/g of
PRODUCT INFORMATION METVIX NAME OF THE MEDICINE Methyl aminolevulinate (as hydrochloride). Structural formula: O OCH 3 NH 3 + Cl - O CAS number: 79416-27-6 DESCRIPTION Metvix cream contains 160 mg/g of
Scottish Medicines Consortium
 Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
TCA 35% for Actinic Keratoses. Emily C Keller MD, FAAD Annual AAD Meeting 2019
 TCA 35% for Actinic Keratoses Emily C Keller MD, FAAD Annual AAD Meeting 2019 DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Emily C Keller, MD F116 Superficial, Medium, and Deep Chemical Peeling and Relevance
TCA 35% for Actinic Keratoses Emily C Keller MD, FAAD Annual AAD Meeting 2019 DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY Emily C Keller, MD F116 Superficial, Medium, and Deep Chemical Peeling and Relevance
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions
 Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use.
![NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. NDA /S-001. ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use.](/thumbs/89/100134179.jpg) ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. DESCRIPTION Aldara is the brand name for imiquimod which is an immune response modifier. Each gram of the 5%
ALDARA [al dar a] (imiquimod) Cream, 5% For Dermatologic Use Only - Not for Ophthalmic Use. DESCRIPTION Aldara is the brand name for imiquimod which is an immune response modifier. Each gram of the 5%
Fractional CO 2 Laser Skin Resurfacing for the Treatment of Sun-Damaged Skin and Actinic Keratoses COS DERM
 Case Report Fractional CO 2 Laser Skin Resurfacing for the Treatment of Sun-Damaged Skin and Actinic Keratoses LindaSusan Marcus, MD; Neal Carlin, BS; Robert Carlin, BS, MA It is important to realize that
Case Report Fractional CO 2 Laser Skin Resurfacing for the Treatment of Sun-Damaged Skin and Actinic Keratoses LindaSusan Marcus, MD; Neal Carlin, BS; Robert Carlin, BS, MA It is important to realize that
ACNE IS A COMMON DISORder,
 ORIGINAL ARTICLE Evaluation of Acne Scar Treatment With a 1450-nm Midinfrared Laser and 30% Trichloroacetic Acid Peels Paul J. Carniol, MD; Jyothi Vynatheya; Eric Carniol Objective: To evaluate the efficacy
ORIGINAL ARTICLE Evaluation of Acne Scar Treatment With a 1450-nm Midinfrared Laser and 30% Trichloroacetic Acid Peels Paul J. Carniol, MD; Jyothi Vynatheya; Eric Carniol Objective: To evaluate the efficacy
SPECIAL TOPIC. Institut National de la Sante et de la Recherche Medicale (INSERM U895), Nice, France c
 November 2011 1260 Volume 10 Issue 1i Copyright 2011 ORIGINAL ARTICLES Journal of Drugs in Dermatology SPECIAL TOPIC A Pilot Study Using Reflectance Confocal Microscopy (RCM) in the Assessment of a Novel
November 2011 1260 Volume 10 Issue 1i Copyright 2011 ORIGINAL ARTICLES Journal of Drugs in Dermatology SPECIAL TOPIC A Pilot Study Using Reflectance Confocal Microscopy (RCM) in the Assessment of a Novel
Keratolytics and Other Topical Dermatological Agents
 DRUG POLICY Keratolytics and Other Topical Dermatological Agents BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered.
DRUG POLICY Keratolytics and Other Topical Dermatological Agents BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered.
Cryotherapy of 2 weeks versus 3 weeks interval for Common warts
 Cryotherapy of 2 weeks versus 3 weeks interval for Common warts Received: 7/7/2011 Accepted: 5/8/2012 Intiha Mohamed Almosuly * shadan Hassan Mohammed * Abstract Background and objective: Cryotherapy with
Cryotherapy of 2 weeks versus 3 weeks interval for Common warts Received: 7/7/2011 Accepted: 5/8/2012 Intiha Mohamed Almosuly * shadan Hassan Mohammed * Abstract Background and objective: Cryotherapy with
Multi-Application Platform. Summary of Peer-reviewed Articles for Various Clinical Indications April 2016
 Multi-Application Platform Summary of Peer-reviewed Articles for Various Clinical Indications April 2016 Various Clinical Indications Atrophic acne scars and acne Photo-aged skin, pigmentation & hyperpigmentation
Multi-Application Platform Summary of Peer-reviewed Articles for Various Clinical Indications April 2016 Various Clinical Indications Atrophic acne scars and acne Photo-aged skin, pigmentation & hyperpigmentation
TOP-LINE DATA FROM THE RANDOMIZED PHASE 2 IMPULSE STUDY IN SMALL-CELL LUNG CANCER (SCLC): IMMUNOTHERAPEUTIC MAINTENANCE TREATMENT WITH LEFITOLIMOD
 Abstract #1527O TOP-LINE DATA FROM THE RANDOMIZED PHASE 2 IMPULSE STUDY IN SMALL-CELL LUNG CANCER (SCLC): IMMUNOTHERAPEUTIC MAINTENANCE TREATMENT WITH LEFITOLIMOD M. Thomas, S. Ponce-Aix, A. Navarro Mendivil,
Abstract #1527O TOP-LINE DATA FROM THE RANDOMIZED PHASE 2 IMPULSE STUDY IN SMALL-CELL LUNG CANCER (SCLC): IMMUNOTHERAPEUTIC MAINTENANCE TREATMENT WITH LEFITOLIMOD M. Thomas, S. Ponce-Aix, A. Navarro Mendivil,
This was a multinational, multicenter study conducted at 14 sites in both the United States (US) and Europe (EU).
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. NAME OF SPONSOR/COMPANY: Genzyme Corporation,
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. NAME OF SPONSOR/COMPANY: Genzyme Corporation,
To order reprints or e-prints of JDD articles please contact JDD
 June 2017 534 VOLUME 16 ISSUE 6 Copyright 2017 ORIGINAL ARTICLE Journal of Drugs in Dermatology The Efficacy and Safety of Azelaic Acid 15% Foam in the Treatment of Truncal Acne Vulgaris Lauren K. Hoffman
June 2017 534 VOLUME 16 ISSUE 6 Copyright 2017 ORIGINAL ARTICLE Journal of Drugs in Dermatology The Efficacy and Safety of Azelaic Acid 15% Foam in the Treatment of Truncal Acne Vulgaris Lauren K. Hoffman
CLINICAL REPORT MATERIALS AND METHODS. Acta Derm Venereol 2005; 85:
 Acta Derm Venereol 2005; 85: 424 428 CLINICAL REPORT A Randomized Multicenter Study to Compare Two Treatment Regimens of Topical Methyl Aminolevulinate (Metvix H )-PDT in Actinic Keratosis of the Face
Acta Derm Venereol 2005; 85: 424 428 CLINICAL REPORT A Randomized Multicenter Study to Compare Two Treatment Regimens of Topical Methyl Aminolevulinate (Metvix H )-PDT in Actinic Keratosis of the Face
Final Clinical Study Report. to the Dossier SYNOPSIS. Final Clinical Study Report for Study AI463110
 BMS-475 AI463 Name of Sponsor/Company: Bristol-Myers Squibb Individual Study Table Referring to the Dossier For National Authority Use Only) Name of Finished Product: Baraclude Name of Active Ingredient:
BMS-475 AI463 Name of Sponsor/Company: Bristol-Myers Squibb Individual Study Table Referring to the Dossier For National Authority Use Only) Name of Finished Product: Baraclude Name of Active Ingredient:
Original Policy Date
 MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
Actinic keratosis (AK): Dr Sarma s simple guide
 Actinic keratosis (AK): Dr Sarma s simple guide Actinic keratosis is a very common lesion that you will see in your day-to-day practice. First, let me explain the name Actinic keratosis. It means keratosis
Actinic keratosis (AK): Dr Sarma s simple guide Actinic keratosis is a very common lesion that you will see in your day-to-day practice. First, let me explain the name Actinic keratosis. It means keratosis
Vitiligo is a skin depigmentation disorder
 THERPEUTICS FOR THE CLINICIN Tacrolimus Ointment 0.1% Produces Repigmentation in Patients With Vitiligo: Results of a Prospective Patient Series Emil. Tanghetti, MD The cause of the selective melanocyte
THERPEUTICS FOR THE CLINICIN Tacrolimus Ointment 0.1% Produces Repigmentation in Patients With Vitiligo: Results of a Prospective Patient Series Emil. Tanghetti, MD The cause of the selective melanocyte
Aldara. Aldara (imiquimod) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.19 Subject: Aldara Page: 1 of 5 Last Review Date: March 17, 2017 Aldara Description Aldara (imiquimod)
ABC/3TC/ZDV ABC PBO/3TC/ZDV
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel: Report of Two Cases
 Dermatol Ther (Heidelb) (2016) 6:81 87 DOI 10.1007/s13555-016-0094-9 CASE REPORT Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel:
Dermatol Ther (Heidelb) (2016) 6:81 87 DOI 10.1007/s13555-016-0094-9 CASE REPORT Dermoscopy and Reflectance Confocal Microscopy for Monitoring the Treatment of Actinic Keratosis with Ingenol Mebutate Gel:
DNA vaccine, peripheral T-cell tolerance modulation 185
 Subject Index Airway hyperresponsiveness (AHR) animal models 41 43 asthma inhibition 45 overview 41 mast cell modulation of T-cells 62 64 respiratory tolerance 40, 41 Tregs inhibition role 44 respiratory
Subject Index Airway hyperresponsiveness (AHR) animal models 41 43 asthma inhibition 45 overview 41 mast cell modulation of T-cells 62 64 respiratory tolerance 40, 41 Tregs inhibition role 44 respiratory
A Network Meta-Analysis of the Relative Efficacy of Treatments for Actinic Keratosis of the Face or Scalp in Europe
 A Network Meta-Analysis of the Relative Efficacy of Treatments for Actinic Keratosis of the Face or Scalp in Europe Stefan Vegter 1,2 *, Keith Tolley 3 1 University of Groningen, Department of Pharmacy,
A Network Meta-Analysis of the Relative Efficacy of Treatments for Actinic Keratosis of the Face or Scalp in Europe Stefan Vegter 1,2 *, Keith Tolley 3 1 University of Groningen, Department of Pharmacy,
Extreme dermatoheliosis: How to approach the severely sun damaged patient
 Extreme dermatoheliosis: How to approach the severely sun damaged patient Anokhi Jambusaria MD, MSCE Staff Dermatologist Baylor Scott and White Health Round Rock, TX I have no relevant conflicts of interest
Extreme dermatoheliosis: How to approach the severely sun damaged patient Anokhi Jambusaria MD, MSCE Staff Dermatologist Baylor Scott and White Health Round Rock, TX I have no relevant conflicts of interest
Topical tretinoin: its use in daily practice to reverse photoageing
 British Journal of Dermatology (1990) 122, Supplement 35, 87-91. Topical tretinoin: its use in daily practice to reverse photoageing M.T.GOLDFARB, C.N.ELLIS AND J.J.VOORHEES Dermatopharmacology Unit, Department
British Journal of Dermatology (1990) 122, Supplement 35, 87-91. Topical tretinoin: its use in daily practice to reverse photoageing M.T.GOLDFARB, C.N.ELLIS AND J.J.VOORHEES Dermatopharmacology Unit, Department
SYNOPSIS. ER OROS Paliperidone: Clinical Study Report R SCH-301
 SYNOPSIS Protocol No.: R076477-SCH-301 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study With an Open-Label Extension Evaluating Extended Release OROS Paliperidone in
SYNOPSIS Protocol No.: R076477-SCH-301 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study With an Open-Label Extension Evaluating Extended Release OROS Paliperidone in
Periocular skin cancer
 Periocular skin cancer Information for patients Skin cancer involving the skin of the eyelid or around the eye is called a periocular skin cancer. Eyelid skin cancers occur most often on the lower eyelid,
Periocular skin cancer Information for patients Skin cancer involving the skin of the eyelid or around the eye is called a periocular skin cancer. Eyelid skin cancers occur most often on the lower eyelid,
STUDY. Facial Resurfacing for Nonmelanoma Skin Cancer Prophylaxis
 STUDY Facial Resurfacing for Nonmelanoma Skin Cancer Prophylaxis Basil M. Hantash, MD, PhD; Daniel B. Stewart, MD, PhD; Zachary A. Cooper, MD; Wingfield E. Rehmus, MD, MPH; R. James Koch, MD; Susan M.
STUDY Facial Resurfacing for Nonmelanoma Skin Cancer Prophylaxis Basil M. Hantash, MD, PhD; Daniel B. Stewart, MD, PhD; Zachary A. Cooper, MD; Wingfield E. Rehmus, MD, MPH; R. James Koch, MD; Susan M.
An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma
 An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma Pearl E. Grimes, MD Melasma is a common disorder of hyperpigmentation typically characterized by relatively symmetric
An Efficacy Study of 3 Commercially Available Hydroquinone 4% Treatments for Melasma Pearl E. Grimes, MD Melasma is a common disorder of hyperpigmentation typically characterized by relatively symmetric
