Confocal scanning laser microscopy (CSLM) allows
|
|
|
- Sara Simpson
- 5 years ago
- Views:
Transcription
1 View of Normal Human Skin In Vivo as Observed Using Fluorescent Fiber-Optic Confocal Microscopic Imaging ORIGINAL ARTICLE Lucinda D. Swindle, wz Steven G. Thomas,z Michael Freeman, y and Peter M. Delaneyz The Skin Center, Suite 5, Queensland, Australia; ythe Gold Coast Hospital, Queensland, Australia; zoptiscan,victoria, Australia; wdermatology Service, Memorial Sloan-Kettering Cancer Center, New York, New York, USA Fluorescence confocal scanning laser microscopy, using a miniaturized handheld scanner, was performed to visualize the microscopic architecture of normal human epidermis in vivo. Fluorescein sodium (B20 ll of 0.2% wt/vol) was administered via intradermal injection to normal skin on the volar forearm of 22 patients. The skin was imaged continuously from 1 to 15 min after injection. Fluorescein was excited at 488 nm and the uorescent emission was detected at 4505 nm. In each subject, a series of images was collected at increasing depth, from super cial stratum corneum to papillary dermis. Features observed in confocal images were compared to those seen in hematoxylin- and eosinstained sections of skin. The confocal images demonstrated the architecture of super cial skin in the horizontal plane. There was a transition in keratinocyte size, shape, and morphology with progressive imaging into the deeper epidermal layers. Super cial dermis and microscopic capillaries with blood ow were easily observed. The morphologic patterns associated with the major cell types of the epidermis were consistent with those known from conventional histology. We report the ability of in vivo uorescence point scanning laser confocal microscopy to produce real-time, high-resolution images of the microscopic architecture of normal human epidermis using a noninvasive imaging technology. J Invest Dermatol 121:706 ^712, 2003 Manuscript received October 11, 2002; revised March 10, 2003; accepted for publication April 28, 2003 Reprint requests to: Peter Delaney, Optiscan, PO Box 1066, Mt Waverley MDC, Victoria, Australia, peterd@optiscan.com This work was performed at The Skin Center under ethics approval from the Gold Coast Hospital. Nevertheless, the manuscript and images were prepared when the rst author s current a liation was Memorial Sloan Kettering Cancer Center. Abbreviation: CSLM, confocal scanning laser microscopy. Confocal scanning laser microscopy (CSLM) allows visualization of subsurface detail in living human skin by means of its ability to perform optical sectioning (New et al, 1991). The technology optically rejects out of focus light and only detects light from the focal plane. The focal plane is positioned below the tissue surface to enable subsurface imaging. The confocal technique enables preservation of the tissue s physical structure whilst being studied in its physiologic state in vivo and can also observe dynamic processes both in real time (Papworth et al, 1998) and by sequential imaging of the same site (Gonzalez et al, 1999a; Aghassi et al, 2000b). In contrast, light microscopy of biopsy specimens requires an extensive preparative protocol ( xation, sectioning, mounting, and staining), giving rise to the potential for artifacts (Bloom and Fawcett, 1975) and disruption of the cell s native structure and environment. Furthermore, histologic sections from a biopsy only demonstrate tissue morphology at one point in time. Fiber-optic elements may be used in various ways to miniaturize or bring exibility to the implementation of confocal microscopes (Gmitro and Aziz, 1993; Delaney and Harris, 1995; Juskaitis et al, 1997; Lianget al, 2002). This study utilized an implementation that uses one single-moded optical ber acting as a spatial lter (pinhole) in both the illumination and the detection light paths. This results in optical sectioning performance comparable to bulk optical implementations of point scanning confocal microscopes (Delaney and Harris, 1995; Delaney et al, 1994a). This ber-optic approach has enabled miniaturization of the scanner and objective optics of the confocal microscope into a handheld device that provides exibility and mobility, thus being convenient for in vivo and clinical use. Imaging of human epidermis and super cial dermis in vivo has been demonstrated using re ectance confocal microscopy (Rajadhyaksha et al, 1999; Corcu et al, 2001). Such use of re ectance confocal imaging has exploited inherent di erences in the re ectivity (or refractive index) of microstructures within and between cells to produce contrast, at speci c illumination wavelengths. For example, in these studies, strong image contrast is associated with distribution of naturally occurring components of tissue such as melanin or keratin, which have been shown to strongly backscatter at near-infrared wavelengths (Rajadhyaksha et al, 1995). Several studies have established the relationship between re ectance confocal images and known pathologies (Gonzalez et al, 1999a, b, c; Aghassi et al, 2000a; Busam et al, 2001a,b; Langley et al, 2001; Rajadhyaksha et al, 2001; Charles et al, 2002; Gonzalez and Tannous, 2002; Sauermann et al, 2002). There are limited reports of in vivo uorescence confocal microscopy utilizing exogenous uorophores in human skin to date (Swindle et al, 2002; Thorn Leeson et al, 2002). CSLM in uorescence mode relies on the di erential distribution of endogenous or exogenous uorescent molecules ( uorophores) to produce contrast (Delaney et al, 1993). A laser light source, at an appropriate wavelength, is used to excite the uorophore, and the emitted uorescence signal is detected simultaneously X/03/$ Copyright r 2003 by The Society for Investigative Dermatology, Inc. 706
2 VOL. 121, NO. 4 OCTOBER 2003 FLUORESCENCE CONFOCAL MICROSCOPY OF HUMAN SKIN 707 Fluorescence confocal microscopy has been used to visualize selectively stained structures in the skin ex vivo and in vitro.veiro and Cummins (1994) highlighted the potential for imaging targeted skin structures at the subcellular level, such as nuclei or lipids, with the use of an exogenous contrast agent. The uorescent agents used, however, were not suitable candidates for in vivo studies. In vivo uorescence confocal microscopy studies using animal models have demonstrated a range of potential clinical applications for in vivo uorescence CSLM. These have included characterization of epidermal layers and subcellular structure of skin (Bussau et al, 1998), mucous crypt architecture and subcellular detail of colon (Delaney et al, 1994b), blood ow in super cial vasculature of the skin (Delaney et al, 1993; Papworth et al, 1998), changes in microvasculature in skin pathologies (Vo et al, 2000; Anikijenko et al, 2001), and imaging of human melanoma using speci c labeled antibodies (Anikijenko et al, 2001).To date,however, these potential applications have not been tested in humans. This study examines the potential of uorescence CSLM, using a miniaturized handheld scanner in combination with an exogenous uorescent contrast agent, to provide high-resolution images of human skin in vivo. MATERIALS AND METHODS Subjects Twenty-two patients (17 men and 5 women) ranging from 26 to 83 years of age comprised the study sample. Ethics approval was granted from the Gold Coast Hospital Ethics Committee and informed written consent was obtained from each patient. All patients were Caucasian, with skin types ranging from I to IV. Patients recruited were those attending the Dermatology Clinic for routine skin examination or excision of sun-related atypical lesions at other skin sites. Clinically normal, nonsun-damaged skin on the inner forearm was imaged with the confocal microscope. In vivo uorescence confocal microscopy The imaging site on the volar surface of the forearm was prepared with a standard alcoholic preparation (AlcoWIPE, Erie Scienti c, Portsmouth, NH) before the introduction of contrast agent. Approximately 20 ml of a 0.2% solution of uorescein sodium was injected intradermally using a 30-gauge hypodermic needle. One drop of the same 0.2% uorescein sodium solution was also applied topically to the same site to ensure adequate labeling of the stratum corneum. A drop of immersion oil (Olympus) was applied to the imaging site to reduce surface scattering e ects. Imaging was performed continuously from 1 to 15 min after introduction of the uorescent contrast agent. This time frame was chosen after observations in a pilot study showed variation in the distribution of contrast agent within the viable cell layers after 20 min. In the pilot study it was observed that the uorescein was initially con ned to the extracellular spaces (bright cell borders and darker cytoplasm) and by approximately 20 min had redistributed considerably in some areas (bright cytoplasm and darker intercellular spaces). Beyond 30 min, image quality had deteriorated signi cantly with loss of consistent contrast between cellular structures. Therefore, the optimal time for this study to demonstrate consistent images was considered to be from injection time to 15 min later. The imaging system comprised a modi ed confocal microscope (F900e personal confocal microscope system, Optiscan Pty Ltd, Australia) tted with a novel miniaturized, vibrating ber, handheld scanner (Delaney and Harris, 1995). The scanner was placed against the skin with gentle pressure to stabilize the tissue relative to the scanner optics. The cohesive movement of the scanner head with the subject minimized movement artifact in the image. The tissue was illuminated with blue laser light (488 nm, o0.5 mw at the skin) with detection above 505 nm to capture the green uorescent signal. The scanner included a customized objective lens system with an e ective numerical aperture of approximately 0.5 and a scanned eld of view of mm. Images of pixels were captured from this eld of view and displayed at two frames per second. In pilot work, these parameters and the above-described protocol for uorescein administration were found to result in strong uorescent signal. At the instrument settings used to image the bright uorescein signal, auto uorescence was undetectable. Representative images at each layer of the epidermis were captured by varying the imaging depth, which was controlled by a lever on the scanner. Single scans of the tissue were captured, each representing one focal plane within the tissue. There was no processing of the images with software before analysis and reproduction for this paper. Histology Histologic correlation was not sought from each individual patient because this was clinically normal skin being imaged and therefore biopsy was not indicated. One representative sample of normal skin was obtained from the normal margins of an excision of a pigmented lesion on the ventral forearm. This normal skin was xed, sectioned vertically, and stained with hematoxylin and eosin as per conventional histologic technique. This allowed comparison of gross morphology in the confocal images to the more common histologic view of skin. Because the confocal images are an en face view, we also cut and stained sections of this normal skin in the horizontal plane to view with the conventional light microscope. This was helpful in the interpretation of the view o ered by the confocal images. RESULTS In vivo uorescence confocal microscopy demonstrates the microscopic architecture of normal human skin The image sets taken for all 22 patients showed a clearly recognizable epidermal architecture, with images taken from the stratum corneum to the basal layer (Fig 1). The papillary dermis was viewed in all patients. Owing to the fact that the skin surface and the underlying layers of the epidermis undulate, whereas the focal plane of the confocal microscope is at, most images feature focal regions of cells from di erent layers (Figs 2, 3). The named layers of the epidermis were identi ed in the confocal images by the morphologic characteristics of cells in each layer (which are well known from histology), together with their location relative to nearby distinctive structures (such as dermal papillae) and visual similarity to corresponding histology sections. Image quality improved over time, peaking between 3 and 5 min after introduction of the contrast agent. Skin surface structures are clearly labeled with topical uorescein The results (Fig 1a-j) show confocal images at the same site at progressive depths from stratum corneum to papillary dermis. The super cial corneocytes (Figs 1a, 4) uoresced avidly immediately after application of the topical uorescein. There was no temporal change in uorescein distribution in this layer of nonviable cells. The images of super cial planes showed irregular islands of large (15^30 mm), polygonal, attened, anucleated corneocytes that overlap at their edges. There was variation from a relatively neat, at array of cells in the deeper stratum compactum (Fig 4) to disorganized, folded, sloughing debris in the super cial stratum dysjunctum (Fig 1a). The residual nuclei or remnants of apoptotic nuclei were noted in the deeper layers of stratum corneum, as the junction of stratum corneum and granulosum was approached (Fig 4). At the skin surface, a brightly labeled hair (Fig 4) could often be seen emerging from a hair follicle. At all depths, the hair follicles were seen as large black empty ring structures (Fig 1a,c,e,g,i), where contrast agent had not penetrated. The black follicles could be traced down through the depth of the epidermis and were useful landmarks for orientation. Wrinkles and creases in the topography of the skin were seen as bright uorescent crevices near the surface as a result of pooling of topically applied uorescein (Fig 5). Fluorescence confocal microscopy of the viable epidermis results in consistent, reproducible images As depth was increased into the viable epidermal layers, large keratinocytes (20^30 mm) were arranged in a at, cohesive array (Figs 1c, 5). Initially, the uorescein was con ned predominantly to the extracellular spaces (Fig 1c). At the end of the imaging time (15 min), when uorescein had begun to appear to redistribute to an intracellular location, these cells demonstrated a distinct cytoplasm containing ne, bright staining granules and a dark, large nucleus (Fig 5). The granular pattern in the cytoplasm is
3 708 SWINDLE ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY consistent with the increased number of cytoplasmic organelles (keratohyaline granules and membrane coating granules) observed in the stratum granulosum in conventional histology (Fig 1d). It should be noted that in all subjects a very thin, acellular, darker layer was brie y visible as imaging depth was increased from the stratum corneum to granulosum (Fig 3a). Therefore, a region was observed which excluded uorescein, both from above (topical) and from beneath (injected), consistent with the barrier function expected for the upper epidermis. Keratinocytes in the underlying layers were markedly smaller (10^25 mm) and arranged in a distinctive honeycomb-like pattern (Fig 1e). Contiguous, morphologically similar cells characterized these layers, their size and location consistent with the stratum spinosum. In real-time imaging, as layers within stratum spinosum were imaged from super cial to deep, a decrease in Figure 2. This vertical histology section illustrates the perpendicular view (en face) acquired by confocal imaging. Each planar confocal image (thick black line) may capture multiple di erent cell layers of the undulating epidermis. S, stratum spinosum; B, basal layer; D, dermis; G, stratum granulosum; C, stratum corneum. 20 objective lens. cell size was appreciated (Fig 1g). Of particular note in the deeper regions of viable epidermis was the frequent presence of as yet unidenti ed, strongly uorescent, variable-sized spots (see Fig 1g and Discussion). As imaging depth progressed toward the basal layer, the bright, homogeneous, acellular round hilltops of papillary dermis were observed interposed among the keratinocytes (Figs 1g,i, 3a, 6a, 7a), as also demonstrated in comparative horizontal histologic sections (Figs 1h,j, 3b, 6b, 7b). Small (7^12 mm), densely packed basal keratinocytes were circumferentially arranged around the papillae (Figs 1i, 6). As the dermal papillae were sectioned deeper with confocal slices, the images showed these round papillae increasing in size. Many of the individual basal cells surrounding the papillae were noted to have distinctly darker cytoplasm compared to cells in other layers (see Fig 6a and Discussion). The approximate region of the dermoepidermal junction was predictable, with the presence of two factors, namely when the keratinocytes had reached minimal size and the bright Figure 1. The microarchitecture of horizontal (en face) confocal sections (a,c,e,g,i) visually corresponds with horizontal hematoxylinand eosin-stained histologic sections of epidermis and papillary dermis (b,d,f,h,j). The confocal images are captured at the same skin site, but at di erent depths below the skin surface. The distribution of the uorescein is predominantly extracellular. A hair follicle (F) can be seen at all depths as a black ring structure. Artifact on the skin surface (oil bubbles and debris), and shadowing in the deeper layers from surface artifact (A) is distinguishable from normal skin structure. The changing morphology from stratum corneum to papillary dermis includes (a,b) large (15^30 mm) polygonal anucleated corneocytes. The bright areas are clumped and sloughing corneocytes. (c,d) Large (20^30 mm) rounded keratinocytes. The granular cytoplasm of cells at this layer is seen in the hematoxylin and eosin section (d). Correlating granular cytoplasm is seen in confocal images when the uorescein has begun to redistribute to an intracellular location (see also Fig 5). (e^h) Honeycomb-like array of smaller (10^25 mm) keratinocytes in the spinous layer. A decrease in cell size was appreciated between upper (e,f) and lower (g,h) spinous layers. The dermal papillae hilltops (D) are seen in the lower spinous level (g,h). Strongly uorescent, variable-sized spots in the confocal image (bottom left of g) have not been correlated with histology. (i,j) Small (7^12 mm) basal cells are arranged circumferentially around circular dermal papillae projections. The cytoplasm of basal cells is often darker than keratinocytes in upper layers, especially in subjects with darker skin (see also Fig 6). Bar, 25 mm.
4 VOL. 121, NO. 4 OCTOBER 2003 FLUORESCENCE CONFOCAL MICROSCOPY OF HUMAN SKIN 709 Figure 4. The surface topography showed clumps of sloughing corneocytes ( ), uorescein-labeled hairs emerging from follicles (H), and surface artifact (black arrows, oil bubbles next to hair; and white spots, debris). The slightly deeper cells in stratum compactum (upper region) have small central darker circles that are nuclear remnants (white arrows). Bar, 25 mm. Figure 3. This in vivo confocal image (a) and horizontal histology section (b) show regions of cells from di erent layers. C, stratum corneum; G, stratum granulosum; S, stratum spinosum; B, basal layer; D, dermis; F, hair follicle. A bright hair at the surface has caused a shadow in the layers beneath (a, double-headed white arrow). When imaging from super cial to deep, a thin, acellular layer was passed through at the level of deep stratum corneum ( ) where neither topical uorescein from above nor intradermal uorescein from below had labeled and therefore appearing darker. Bar, 25 mm. homogeneous hilltops of the papillary dermis were in view. Capillary loops seen clearly in the dermal papillae (Fig 7) displayed the dynamic process of blood ow when imaging in vivo. Tumbling blood cells were visible as black disks contrasting with bright plasma in the capillary lumen (Fig 7a). The plasma appeared to be a predominant form of clearance for the contrast agent, as the capillary structures became more uorescent over time, whereas conversely, the contrast faded in the epidermal layers. In skin of (certain) subjects with relatively thin epidermis, imaging into super cial reticular dermis was possible. As maximal depth of uorescence was approached, by shifting the depth of the focal plane further into the tissue, the deepest of the basal keratinocytes were seen in the troughs of rete ridges and the individual dermal papillae coalesced into continuous bright sheets of reticular dermis. Artifact in the confocal images is distinguishable from normal skin structure Sources of artifact in the confocal images included spherical aberration caused by oil bubbles at the surface (Figs 1, 4) and bright streaks or spots caused by debris Figure 5. This confocal image shows keratinocytes at the junction of the deep stratum corneum and stratum granulosum. Some cells exhibit a granular cytoplasm (white arrows) and central dark nuclei (black arrows) when the uorescein has begun to redistribute from an extracellular to an intracellular location. Wrinkles and creases (W) appear as bright lines where topical uorescein has pooled. Bar, 25 mm. sticking to the glass coverslip (Fig 4). These were quite distinct and recognizable structures and therefore not confused with normal skin structure. Bright structures at the surface with large amounts of uorescent emission, such as hairs, clumps of sloughing corneocytes, and wrinkles, tended to cause shadowing in the layers directly beneath. (Figs 1c,e,g, 3) DISCUSSION In vivo confocal microscopy is being widely established as a useful investigative tool in the study of skin morphology and skin diseases. To date, the majority of reports of in vivo confocal microscopy of human skin have used re ectance mode. We report the feasibility of producing real-time, high-resolution images of intact living human skin, using a novel handheld uorescence laser scanning confocal microscope in combination with an exogenous
5 710 SWINDLE ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY Figure 6. In the confocal image (a), dermal papillae (D) project into the overlying epidermis and appear as bright, regular, round, or elliptical structures. The lower left region shows the tips of dermal papillae (white arrows). A single layer of darker basal cells (black arrows) is arranged circumferentially around each papillae, as also seen in the comparative horizontal histology section (b). Bar, 25 mm. uorophore acting as a contrast agent. The results have demonstrated the epidermal cell layers with subcellular detail and we have observed dynamic processes occurring within the super cial layers of the dermis. The imaging patterns observed using a uorescent contrast agent were consistent and reproducible among the study sample. The morphologic patterns associated with major cell types of the epidermis were recognizable and consistent with histologic skin sections viewed by conventional microscopy. Certain other features were not identi ed with con dence and will require future detailed correlative studies with histology. Of particular note in the deeper regions of viable epidermis was the frequent presence of strongly uorescent, variable-sized spots (3^5 mm) (Fig 1g). These were initially suspected to be nuclei; however, their variation in size and inconsistency in distribution did not support this. It is postulated that these spots are extracellular uorescein that has pooled owing to di culty tra cking through desmosomal adhesions between cells. Alternatively, they may be the result of phagocytosis of the contrast agent into intracellular compartments by keratinocytes. Many of the cells at the basal layer (which were seen surrounding the dermal papillae hilltops) had signi cantly darker cytoplasm compared to keratinocytes in the other layers (Fig 6a). The appearance and location of these cells is consistent with that of melanocytes or heavily melanized basal keratinocytes sitting at the expected location immediately above the dermoepidermal junction. This observation of darker cytoplasm at the basal level Figure 7. A capillary lumen (white arrow) is clearly visible in the dermal papillae (D) in the confocal image (a). Dark blood cells (black arrows) contrast with bright plasma in the lumen; so dynamic blood ow can be visualized in real-time imaging. The horizontal histology section gives a corresponding static view of capillaries (b). Bar, 25 mm. was enhanced in individuals with darker pigmentation (skin types III and IV) compared to lightly pigmented skin (types I and II). While the keratinocytes comprising the majority of the epidermal cell matrix could be identi ed with con dence, conclusive identi cation of particular cell types (e.g., melanocytes and Langerhans cells) and associated subcellular structures will need to be validated with further detailed morphologic examination and the use of immunologically targeted cell markers. With in vivo confocal microscopy, subsurface imaging of tissue layers is achieved by noninvasive optical sectioning. The view of the tissue is parallel to the surface of the skin, which is orthogonal to the typical vertical orientation used in conventional histology (Fig 2). This en face view of skin requires new techniques of analysis, interpretation, and description that should ultimately complement and expand on the information gained by conventional techniques and other skin imaging modalities. In vivo re ectance confocal imaging already has substantial momentum in the pursuit of the new paradigm of noninvasive skin imaging. This technique has been used to investigate in ammatory, keratinocytic, and pigmented skin lesions. Re ectance microscopy, as described in the majority of literature reports to date, exploits the distribution of naturally occurring refractive index variations and chemicals within skin to provide a source of contrast. For example melanin provides contrast when illuminated with light in the infrared spectrum (Rajadhyaksha et al, 1995). In the absence of exogenous re ective contrast agents, there is a limited potential to extract additional information from the tissue. Recent studies have been directed toward nding suitable contrast agents for re ectance imaging that may overcome this limitation (Rajadhyaksha et al, 2001; Zuluaga et al, 2002). The technique described in this study involves the use of an exogenous uorescent contrast agent, and like the multitude of stains
6 VOL. 121, NO. 4 OCTOBER 2003 FLUORESCENCE CONFOCAL MICROSCOPY OF HUMAN SKIN 711 used in conventional histology, di erent uorescent contrast agents and protocols would have the ability to highlight di erent structures. This could greatly expand the range of applications and usefulness of in vivo uorescence CSLM. The pharmacokinetic processes that occur in living tissue such as metabolism by enzymes and clearance via vascular and lymphatic channels, or the permeability characteristics of individual cells will a ect the distribution of contrast agent, and would therefore need to be taken into account in the interpretation of imaging patterns. These same processes also o er the potential to observe numerous dynamic functional aspects of living skin in vivo, through the selection of appropriate contrast agents and protocols. The use of intradermally administered uorescein in this study represents just one of myriad possible protocols and staining patterns. For example, an area of much potential for uorescence confocal microscopy lies in the ability to target speci c subcellular molecules (including proteins) and therefore to monitor speci c pathologic and immune processes over time. Preliminary studies using targeted uorescent probes in animal models of melanoma have demonstrated that pathologic protein overexpression can be microscopically observed in vivo (Anikijenko et al, 2001). The use of an exogenous contrast agent, however, also presents complexities in the interpretation of image contrast and may also impact the state of the tissue itself (Cullander, 1999; Rajadhyaksha and Gonzalez, 2003). Local physiologic factors such as clearance and redistribution, ph, and chemical quenching will all impact the pattern of uorescence observed (Rajadhyaksha and Gonzalez, 2003; Sjoback et al, 1995; Song et al, 1995). Interestingly, such factors also make this imaging method a form of functional imaging, in which such interactions may be useful in the study of physiologic factors that in uence them. Phototoxic e ects causing potential negative alterations in the in vivo cellular environment are also a factor for consideration in uorescence confocal microscopy (Saetzler et al, 1997; Zhang et al, 1997). In this study, no observations of photo bleaching were made, and no adverse events were reported in relation to the administration of sodium uorescein or confocal microscopy. The particular conditions employed in our study relating to use of a clinically well-established uorophore, low energy of illumination (by comparison to conventional usage of uorescein), limited lens numerical aperture and relatively fast, and transient scanning on any particular microscopic eld of view minimized the impact of such factors on our results. Imaging depth is one of the key technical limitations that will de ne the scope of clinical applications for which the technology will be useful. The limit in depth is associated with the wavelength of laser light used. The 488-nm argon ion laser used in this study only allowed penetration and detection at the level of super cial papillary dermis and occasionally super cial reticular dermis. Optical coherence tomography, high-frequency ultrasound, and magnetic resonance imaging have superior depth penetration compared to in vivo confocal microscopy, but do not o er the resolution that enables the cellular detail of confocal microscopy (Tadrous, 2000). If confocal microscopy is to be useful in the study of pathologic processes that occur in the reticular dermis, these limitations will have to be overcome (for example, through the use of near-infrared laser sources in combination with exogenous uorophores excited by such wavelengths). The single-frame images captured and shown in our results are only representative of some of the information available via uorescent confocal imaging. Imaging in real-time with operator interaction is preferable, providing a better appreciation of the relationships between structures observed in a continuum of images and spanning the undulating microarchitecture of the skin. It also allows real-time viewing of dynamic processes such as erythrocyte movement through capillaries. Development of tools for enhanced image review (e.g., capture and review of video streams) may facilitate further applications of this technology, which are enabled by this real-time aspect. Fiber-optic uorescence CSLM is currently an investigational imaging tool. CSLM is a widely accepted technique in the research eld with major advancements in technology in recent years. Further investigations are needed, however, to ascertain a future role in the eld of clinical dermatology. These initial results are compelling in their demonstration of real-time, microscopic views of living human skin in vivo, using a exible handheld scanner. It will be a valuable tool in the study of diseases of the epidermis and dermoepidermal junction, whereas its capacity to target speci c uorescent molecules lends itself to application within the domains of skin-related pharmacology, immunology, and molecular biology. The challenge in developing this emerging imaging technique lies in the establishment of standard lexicons for image collection and interpretation, so that the new information may be integrated with that from existing accepted techniques, such as dermoscopy and histology. With this in mind, the expanding eld of noninvasive skin imaging will continue to gain momentum. Preliminary in vivo and ex vivo studies using uorescent contrast agents to investigate cell morphology within a variety of benign and malignant skin lesions suggest that there are observable and noteworthy di erences in labeling patterns between normal skin and pathologic skin (Swindle et al, 2002). These early observations require further investigation and validation. We thank Milind Rajadhyaksha for his assistance with the preparation of images for the manuscript.we thank Christina Lohmann and Klaus Busam from the pathology department at Memorial Sloan-Kettering Cancer Center for their assistance with the preparation and interpretation of histology sections. REFERENCES Aghassi D, Anderson RR, Gonzalez S: Confocal laser microscopic imaging of actinic keratoses in vivo: A preliminary report. JAmAcadDermatol43:42^48, 2000a Aghassi D, Anderson RR, Gonzalez S: Time-sequence histologic imaging of lasertreated cherry angiomas with in vivo confocal microscopy. J Am Acad Dermatol 43:37^41, 2000b Anikijenko P, Vo LT, Murr ER, et al: In vivo detection of small subsurface melanomas in athymic mice using noninvasive ber optic confocal imaging. JInvest Dermatol 117:1442^1448, 2001 Bloom W, Fawcett DW: ATextbook of Histology, 10th ed. Philadelphia: Saunders, 1975: 10 Busam KJ, Charles C, Lee G, Halpern AC: Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol 14:862^868, 2001a Busam KJ, Hester K, Charles C, Sachs DL, Antonescu CR, Gonzalez S, Halpern AC: Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol 137: 923^929, 2001b Bussau LJ, Vo LT, Delaney PM, Papworth GD, Barkla DH, King RG: Fibre optic confocal imaging (FOCI) of keratinocytes, blood vessels and nerves in hairless mouse skin in vivo. JAnat192:187^194, 1998 Charles CA, Marghoob AA, Busam KJ, Clark-Loeser L, Halpern AC: Melanoma or pigmented basal cell carcinoma. A clinical-pathologic correlation with dermoscopy, in vivo confocal scanning laser microscopy, and routine histology. Skin ResTechnol 8:282^287, 2002 Corcu P, Chaussepied C, Madry G, Hadjur C: Skin optics revisited by in vivo confocal microscopy, melanin and sun exposure. JCosmetSci52:91^102, 2001 Cullander C: Fluorescent probes for confocal microscopy. Methods Mol Biol 122:59^73, 1999 Delaney PM, Harris MR: Fibreoptics in confocal microscopy. In: Pawley EJ, (eds). Handbook of Biological Confocal Microscopy. New York: Plenum, 1995 Delaney PM, Harris MR, King RG: A bre optic laser scanning confocal microscope suitable for uorescence imaging. Appl Opt 33:573^577, 1994a Delaney PM, Harris MR, King RG: Novel microscopy using bre optic confocal imaging and its suitability for subsurface blood vessel imaging in vivo. Clin Exp Pharmacol Physiol 20:197^198, 1993 Delaney PM, King RG, Lambert JR, Harris MR: Fibre optic confocal imaging (FOCI) for subsurface microscopy of the colon in vivo. J Anat 184:157^160, 1994b Gmitro A, Aziz D: Confocal microscopy through a ber-optic imaging bundle. Opt Lett 18:565^567, 1993
7 712 SWINDLE ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY Gonzalez S, Gonzalez E,White WM, Rajadhyaksha M, Anderson RR: Allergic contact dermatitis: Correlation of in vivo confocal imaging to routine histology. J Am Acad Dermatol 40:708^713, 1999a Gonzalez S, Rajadhyaksha M, Gonzalez-Serva A, White WM, Anderson RR: Confocal re ectance imaging of folliculitis in vivo: Correlation with routine histology. J Cutan Pathol 26:201^205, 1999b Gonzalez S, Rajadhyaksha M, Rubinstein G, Anderson RR: Characterization of psoriasis in vivo by re ectance confocal microscopy. J Med 30:337^356, 1999c Gonzalez S, Tannous Z: Real-time, in vivo confocal re ectance microscopy of basal cell carcinoma. JAmAcadDermatol47:869^874, 2002 Juskaitis T, Wilson T, Watson TF: Real-time white light re ection confocal microscopy using a bre-optic bundle. Scanning 19:15^19, 1997 Langley RG, Rajadhyaksha M, Dwyer PJ, Sober AJ, Flotte TJ, Anderson RR: Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo. JAm Acad Dermatol 45:365^376, 2001 Liang C, Sung KB, Richards-Kortum RR, Descour MR: Design of a high-numerical-aperture miniature microscope objective for an endoscopic ber confocal re ectance microscope. Appl Opt 41:4603^4610, 2002 New K, Petroll W, Boyde A, et al: In vivo imaging of human teeth and skin using real-time confocal microscopy. Scanning 13:369^372, 1991 Papworth GD, Delaney PM, Bussau LJ,Vo LT, King RG: In vivo bre optic confocal imaging of microvasculature and nerves in the rat vas deferens and colon. JAnat192:489^495, 1998 Rajadhyaksha M, Gonzalez S: Real-time in vivo confocal uorescence microscopy. In: Myeck MA, Pogue B, (eds). Handbook of Biomedical Fluorescence. New York: Dekker, 2003: p. 143^179 Rajadhyaksha M, Gonzalez S, Zavislan JM, Anderson RR, Webb RH: In vivo confocal scanning laser microscopy of human skin. II. Advances in instrumentation and comparison with histology. JInvestDermatol113:293^303, 1999 Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR: In vivo confocal scanning laser microscopy of human skin: Melanin provides strong contrast. JInvestDermatol104:946^952, 1995 Rajadhyaksha M, Menaker G, Flotte T, Dwyer PJ, Gonzalez S: Confocal examination of non melanoma cancers in thick skin excisions to potentially guide Mohs micrographic surgery without frozen histopathology. JInvestDermatol 117:1137^1143, 2001 Saetzler RK, Jallo J, Lehr HA, Philips CM, Vasthare U, Arfors KE, Tuma RF: Intravital uorescence microscopy; Impact of light-induced phototoxicity on adhesion of uorescently labeled leukocytes. J Histochem Cytochem 45:505^513, 1997 Sauermann K, Gambichler T, Wilmert M, Rotterdam S, Stucker M, Altmeyer P, Ho mann K: Investigation of basal cell carcinoma by confocal laser scanning microscopy in vivo. Skin ResTechnol 8:141^147, 2002 Sjoback R, Nygren J, Kubista M: Absorbtion and uorescence properties of uorescein. Spectrochim Acta A 51:7, 1995 Song L, Hennink EJ, Young IT, Tanke HJ: Photobleaching kinetics of uorescein in quantitative uorescence microscopy. Biophys J 68:2588^2600, 1995 Swindle LD, Delaney PM, Freeman M, et al: View of human skin in vivo as observed using uorescence confocal microscopic imaging [abstract]. Ann DermatolVenereol 129:1S602, 2002 Tadrous PJ: Methods for imaging the structure and function of living tissues and cells. 3. Confocal microscopy and micro-radiology. J Pathol 191:345^354, 2000 Thorn Leeson D, Lynn Meyers C, Subramanyan K: In vivo confocal uorescence imaging of skin surface cellular morphology [abstract]. OSA Biomedical Topical Meetings Technical Digest, Optical Society of America: Washington DC: 2002: PD8-1 Veiro JA, Cummins PG: Imaging of skin epidermis from various origins using confocal laser scanning microscopy. Dermatology 189:16^22, 1994 Vo LT, Papworth GD, Delaney PM, Barkla DH, King RG: In vivo mapping of the vascular changes in skin burns of anaesthetised mice by bre optic confocal imaging (FOCI). J Dermatol Sci 23:46^52, 2000 Zhang JL, Yokoyama S, Ohhashi T: Inhibitory e ects of uorescein isothiocyanate photoactivation on lymphatic pump activity. Microvasc Res 54:99^107, 1997 Zuluaga AF, Drezek R, Collier T, Lotan R, Follen M, Richards-Kortum R: Contrast agents for confocal microscopy: How simple chemicals a ect confocal images of normal and cancer cells in suspension. JBiomedOpt7:398^403, 2002
Morphologic Features of Melanocytes, Pigmented Keratinocytes, and Melanophages by In Vivo Confocal Scanning Laser Microscopy
 Morphologic Features of Melanocytes, Pigmented Keratinocytes, and Melanophages by In Vivo Confocal Scanning Laser Microscopy Klaus J. Busam, M.D., Carlos Charles, M.D., Grace Lee, M.D., Allan C Halpern,
Morphologic Features of Melanocytes, Pigmented Keratinocytes, and Melanophages by In Vivo Confocal Scanning Laser Microscopy Klaus J. Busam, M.D., Carlos Charles, M.D., Grace Lee, M.D., Allan C Halpern,
Diagnosis of Lentigo Maligna Melanoma. Steven Q. Wang, M.D. Memorial Sloan-Kettering Cancer Center Basking Ridge, NJ
 Diagnosis of Lentigo Maligna Melanoma Steven Q. Wang, M.D. Memorial Sloan-Kettering Cancer Center Basking Ridge, NJ Conflict of Interest: None Topics Epidemiology and Natural History Clinical and Histologic
Diagnosis of Lentigo Maligna Melanoma Steven Q. Wang, M.D. Memorial Sloan-Kettering Cancer Center Basking Ridge, NJ Conflict of Interest: None Topics Epidemiology and Natural History Clinical and Histologic
Reflectance-Mode Confocal Microscopy for the In Vivo Characterization of Pagetoid Melanocytosis in Melanomas and Nevi
 See related Commentary on page vii Reflectance-Mode Confocal Microscopy for the In Vivo Characterization of Pagetoid Melanocytosis in Melanomas and Nevi Giovanni Pellacani, Anna Maria Cesinaro,w and Stefania
See related Commentary on page vii Reflectance-Mode Confocal Microscopy for the In Vivo Characterization of Pagetoid Melanocytosis in Melanomas and Nevi Giovanni Pellacani, Anna Maria Cesinaro,w and Stefania
Histopathology: skin pathology
 Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Diagnostic Applicability of In Vivo Confocal Laser Scanning Microscopy in Melanocytic Skin Tumors
 See related Commentaries on pages v, vi and viii Diagnostic Applicability of In Vivo Confocal Laser Scanning Microscopy in Melanocytic Skin Tumors Armin Gerger, Silvia Koller, Thomas Kern, Cesare Massone,
See related Commentaries on pages v, vi and viii Diagnostic Applicability of In Vivo Confocal Laser Scanning Microscopy in Melanocytic Skin Tumors Armin Gerger, Silvia Koller, Thomas Kern, Cesare Massone,
Actinic keratosis (AK) is the most common precancerous
 Confocal laser microscopic imaging of actinic keratoses in vivo: A preliminary report David Aghassi, MD, R. Rox Anderson, MD, and Salvador González, MD Boston, Massachusetts Background: Real-time near-infrared
Confocal laser microscopic imaging of actinic keratoses in vivo: A preliminary report David Aghassi, MD, R. Rox Anderson, MD, and Salvador González, MD Boston, Massachusetts Background: Real-time near-infrared
Sensitivity and Specificity of Confocal Laser-Scanning Microscopy for In Vivo Diagnosis of Malignant Skin Tumors
 193 Sensitivity and Specificity of Confocal Laser-Scanning Microscopy for In Vivo Diagnosis of Malignant Skin Tumors Armin Gerger, MD 1 Silvia Koller, MD 2 Wolfgang Weger, MD 2 Erika Richtig, MD 2 Helmut
193 Sensitivity and Specificity of Confocal Laser-Scanning Microscopy for In Vivo Diagnosis of Malignant Skin Tumors Armin Gerger, MD 1 Silvia Koller, MD 2 Wolfgang Weger, MD 2 Erika Richtig, MD 2 Helmut
VivoSight Imaging for Dermatologists
 VivoSight Imaging for Dermatologists VivoSight: The new real time imaging tool VivoSight Image of Skin Stratum corneum 160 µm Stratum granulosum Stratum spinosum Epidermis-dermis junction Papillary dermis
VivoSight Imaging for Dermatologists VivoSight: The new real time imaging tool VivoSight Image of Skin Stratum corneum 160 µm Stratum granulosum Stratum spinosum Epidermis-dermis junction Papillary dermis
Skin (Integumentary System) Wheater, Chap. 9
 Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
Skin (Integumentary System) Wheater, Chap. 9 Skin (Integument) Consists of skin and associated derivatives Largest organ of body (21 ft 2 ; 9 lbs.; has 11 miles of blood vessels) Functions: Protection
The application of confocal microscopy for optical sectioning
 Skin Imaging With Reflectance Confocal Microscopy Kishwer S. Nehal, MD,*, Dan Gareau, PhD,* and Milind Rajadhyaksha, PhD* Confocal microscopy is a new imaging modality for noninvasive real-time tissue
Skin Imaging With Reflectance Confocal Microscopy Kishwer S. Nehal, MD,*, Dan Gareau, PhD,* and Milind Rajadhyaksha, PhD* Confocal microscopy is a new imaging modality for noninvasive real-time tissue
XF Microlens Optic and XD Microlens Compression Optic for Non-Ablative Fractional Skin Treatment with the Palomar Icon System
 Optic and XD Microlens Compression Optic for Non-Ablative Fractional Skin Treatment with the Palomar Icon System Sean Doherty, M.D., 1 Brooke Seckel, M.D., 1 James Childs Ph.D., 2 David Tabatadze Ph.D.,
Optic and XD Microlens Compression Optic for Non-Ablative Fractional Skin Treatment with the Palomar Icon System Sean Doherty, M.D., 1 Brooke Seckel, M.D., 1 James Childs Ph.D., 2 David Tabatadze Ph.D.,
OBSERVATION. Detection of Clinically Amelanotic Malignant Melanoma and Assessment of Its Margins by In Vivo Confocal Scanning Laser Microscopy
 OBSERVATION Detection of Clinically Amelanotic Malignant Melanoma and Assessment of Its Margins by In Vivo Confocal Scanning Laser Microscopy Klaus J. Busam, MD; Katherine Hester, MD; Carlos Charles, MD;
OBSERVATION Detection of Clinically Amelanotic Malignant Melanoma and Assessment of Its Margins by In Vivo Confocal Scanning Laser Microscopy Klaus J. Busam, MD; Katherine Hester, MD; Carlos Charles, MD;
B. Incorrect! The ectoderm does not produce the dermis. C. Incorrect! The dermis is derived from the mesoderm.
 Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
Human Anatomy - Problem Drill 04: The Integumentary System Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. From the inner cell
Histopathology of Melanoma
 THE YALE JOURNAL OF BIOLOGY AND MEDICINE 48, 409-416 (1975) Histopathology of Melanoma G. J. WALKER SMITH Department ofpathology, Yale University School ofmedicine, 333 Cedar Street, New Haven, Connecticut
THE YALE JOURNAL OF BIOLOGY AND MEDICINE 48, 409-416 (1975) Histopathology of Melanoma G. J. WALKER SMITH Department ofpathology, Yale University School ofmedicine, 333 Cedar Street, New Haven, Connecticut
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs
 Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Unit 4 - The Skin and Body Membranes 1
 Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
2/5/2019. Organ System: Skin or Integumentary System. Hypodermis (or superficial fascia) Integumentary System - Learn and Understand
 Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
Pathology of the skin. 2nd Department of Pathology, Semmelweis University
 Pathology of the skin 2nd Department of Pathology, Semmelweis University Histology of the skin Epidermis: Stratum corneum Stratum granulosum Stratum spinosum Stratum basale Dermis: papillary and reticular
Pathology of the skin 2nd Department of Pathology, Semmelweis University Histology of the skin Epidermis: Stratum corneum Stratum granulosum Stratum spinosum Stratum basale Dermis: papillary and reticular
Disclosure. Objectives. PAFP CME Conference Lou Mancano MD, FAAFP Reading Health System November 18, 2016
 PAFP CME Conference Lou Mancano MD, FAAFP Reading Health System November 18, 2016 1 Disclosure The speaker has no conflict of interest, financial agreement, or working affiliation with any group or organization.
PAFP CME Conference Lou Mancano MD, FAAFP Reading Health System November 18, 2016 1 Disclosure The speaker has no conflict of interest, financial agreement, or working affiliation with any group or organization.
In vivo confocal scanning laser microscopy of pigmented Spitz nevi: Comparison of in vivo confocal images with dermoscopy and routine histopathology
 In vivo confocal scanning laser microscopy of pigmented Spitz nevi: Comparison of in vivo confocal images with dermoscopy and routine histopathology Giovanni Pellacani, MD, a Anna Maria Cesinaro, MD, b
In vivo confocal scanning laser microscopy of pigmented Spitz nevi: Comparison of in vivo confocal images with dermoscopy and routine histopathology Giovanni Pellacani, MD, a Anna Maria Cesinaro, MD, b
Introduction. Skin and Body Membranes. Cutaneous Membranes Skin 9/14/2017. Classification of Body Membranes. Classification of Body Membranes
 Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
4 Skin and Body Membranes Study Guide
 Name: SKIN AND BODY MEMBRANES: 4 Skin and Body Membranes Study Guide Period: Body membranes, which cover body surfaces, line its cavities, and form protective sheets around organs, fall into two major
Name: SKIN AND BODY MEMBRANES: 4 Skin and Body Membranes Study Guide Period: Body membranes, which cover body surfaces, line its cavities, and form protective sheets around organs, fall into two major
Ch. 4: Skin and Body Membranes
 Ch. 4: Skin and Body Membranes I. Body Membranes A. Function of body membranes 1. Cover body surfaces 2. Line body cavities 3. Form protective sheets around organs II. Classification of Body Membranes
Ch. 4: Skin and Body Membranes I. Body Membranes A. Function of body membranes 1. Cover body surfaces 2. Line body cavities 3. Form protective sheets around organs II. Classification of Body Membranes
HIGH-RESOLUTION OPTICAL COHERENCE TOMOGRAPHY FOR THE DIAGNOSIS OF ACTINIC KERATOSIS
 Romanian Reports in Physics XX, XYZ (2018) HIGH-RESOLUTION OPTICAL COHERENCE TOMOGRAPHY FOR THE DIAGNOSIS OF ACTINIC KERATOSIS A.G. PEHOIU 1, I. POPESCU 2, C. GIURCANEANU 1,2, A.M. FORSEA 1,2 1 Carol Davila
Romanian Reports in Physics XX, XYZ (2018) HIGH-RESOLUTION OPTICAL COHERENCE TOMOGRAPHY FOR THE DIAGNOSIS OF ACTINIC KERATOSIS A.G. PEHOIU 1, I. POPESCU 2, C. GIURCANEANU 1,2, A.M. FORSEA 1,2 1 Carol Davila
Confocalist. Why this is important? No Relevant Conflict of Interest Dermpath Lab
 Confocal Application in Practice Everyday (CAPE) AAD F109: Imaging in San Diego 2/18/2018 Jane M. Grant-Kels, MD Founding Chair Emeritus Department of Dermatology Professor of Dermatology, Pathology, &
Confocal Application in Practice Everyday (CAPE) AAD F109: Imaging in San Diego 2/18/2018 Jane M. Grant-Kels, MD Founding Chair Emeritus Department of Dermatology Professor of Dermatology, Pathology, &
Assisting diagnosis of melanoma through the noninvasive biopsy of skin lesions
 Assisting diagnosis of melanoma through the noninvasive biopsy of skin lesions Symon D Oyly Cotton Ela Claridge School of Computer Science, The University of Birmingham Birmingham B15 2TT, UK Per Hall
Assisting diagnosis of melanoma through the noninvasive biopsy of skin lesions Symon D Oyly Cotton Ela Claridge School of Computer Science, The University of Birmingham Birmingham B15 2TT, UK Per Hall
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS
 Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
Lab 7: Integumentary System Hamilton ANSWERS TO PRE- LAB ASSIGNMENTS Pre-Lab Activity 1: 1. a. epidermis b. dermis c. hypodermis d. adipose tissue e. hair f. sebaceous gland g. sweat gland 2. a Pre-Lab
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
INTEGUMENTARY 1-Epidermis, 2-Dermis, Structure of thick and thin skin I- Epidermis . Stratum basale
 INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
LARYNGEAL DYSPLASIA. Tomas Fernandez M; 3 rd year ENT resident, Son Espases University Hospital
 LARYNGEAL DYSPLASIA Tomas Fernandez M; 3 rd year ENT resident, Son Espases University Hospital INTRODUCTION Laryngeal cancer constitutes 1-2% of all malignancies diagnosed worldwide Survival is related
LARYNGEAL DYSPLASIA Tomas Fernandez M; 3 rd year ENT resident, Son Espases University Hospital INTRODUCTION Laryngeal cancer constitutes 1-2% of all malignancies diagnosed worldwide Survival is related
Integumentary System
 Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Appendix : Dermoscopy
 Go Back to the Top To Order, Visit the Purchasing Page for Details APP Appendix : Dermoscopy Dermoscopy, also known as dermatoscopy, epiluminoscopy and epiluminescent microscopy, is an effective non-invasive
Go Back to the Top To Order, Visit the Purchasing Page for Details APP Appendix : Dermoscopy Dermoscopy, also known as dermatoscopy, epiluminoscopy and epiluminescent microscopy, is an effective non-invasive
Chapter 5: Integumentary System
 Chapter 5: Integumentary System I. Overview of the Integumentary System A. List the five major functions of the integumentary system: 1. 2. 3. 4. 5. Il. Skin A. Epidermis 1. The epidermis consists of 2.
Chapter 5: Integumentary System I. Overview of the Integumentary System A. List the five major functions of the integumentary system: 1. 2. 3. 4. 5. Il. Skin A. Epidermis 1. The epidermis consists of 2.
(From The Rockefeller Institute) Materials and Methods. Observations with the Electron Microscope
 ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
Skin and Body Membranes
 4 Skin and Body Membranes PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Skin and Body Membranes
4 Skin and Body Membranes PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Skin and Body Membranes
Basics in Dermoscopy
 Basics in Dermoscopy Manal Bosseila Professor of Dermatology, Cairo University Member of European Academy Dermatology & Venereology EADV Member of International Dermoscopy Society IDS Member of Aesthetic
Basics in Dermoscopy Manal Bosseila Professor of Dermatology, Cairo University Member of European Academy Dermatology & Venereology EADV Member of International Dermoscopy Society IDS Member of Aesthetic
Clinics in Dermatology
 Clinics in Dermatology Copy of e-mail Notification bw Proofs of CID ===== Dear Author: The proof of your article to be published by Elsevier Science in Clinics in Dermatology can be viewed from: http://rapidproof.cadmus.com/rapidproof/retrieval/index.jsp
Clinics in Dermatology Copy of e-mail Notification bw Proofs of CID ===== Dear Author: The proof of your article to be published by Elsevier Science in Clinics in Dermatology can be viewed from: http://rapidproof.cadmus.com/rapidproof/retrieval/index.jsp
Integumentary System. Integumentary System
 1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
Chapter 4 Opener Pearson Education, Inc.
 Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
22/04/2015. Dermoscopy of Melanoma. Ilsphi Browne. Overview
 Dermoscopy of Melanoma Ilsphi Browne Overview The device Dermoscopic criteria (terminology) Colour Patterns Global features Local features Approach to diagnosing pigmented lesions Other uses in general
Dermoscopy of Melanoma Ilsphi Browne Overview The device Dermoscopic criteria (terminology) Colour Patterns Global features Local features Approach to diagnosing pigmented lesions Other uses in general
ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE
 ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE THAT WE USE WHEN A SAMPLE IS SO BIG THAT YOU CAN T
ALL PHOTOS ARE IDENTIFIED IN THE LOWER RIGHT CORNER WITH THE MAGNIFICATION POWER THAT THE PHOTO WAS TAKEN WITH. SCAN - THIS IS A VERY LOW POWER IMAGE THAT WE USE WHEN A SAMPLE IS SO BIG THAT YOU CAN T
Dermoscopy: Recognizing Top Five Common In- Office Diagnoses
 Dermoscopy: Recognizing Top Five Common In- Office Diagnoses Vu A. Ngo, DO Department of Family Medicine and Dermatology Choctaw Nation Health Services Authority Learning Objectives Introduction to dermoscopy
Dermoscopy: Recognizing Top Five Common In- Office Diagnoses Vu A. Ngo, DO Department of Family Medicine and Dermatology Choctaw Nation Health Services Authority Learning Objectives Introduction to dermoscopy
Ch 4. Skin and Body Membranes
 Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Ch 5: Integumentary System
 Ch 5: Integumentary System You gotta have skin; All you really need is skin. Skin's the thing, that if you've got it outside, It helps keep your insides in. Alan Sherman (1924-1973) Developed by John Gallagher,
Ch 5: Integumentary System You gotta have skin; All you really need is skin. Skin's the thing, that if you've got it outside, It helps keep your insides in. Alan Sherman (1924-1973) Developed by John Gallagher,
DEBRIDEMENT: ANATOMY and PHYSIOLOGY. Professor Donald G. MacLellan Executive Director Health Education & Management Innovations
 DEBRIDEMENT: ANATOMY and PHYSIOLOGY Professor Donald G. MacLellan Executive Director Health Education & Management Innovations ANATOMY and PHYSIOLOGY Epidermal Layers ECM Structure Dermis Structure Skin
DEBRIDEMENT: ANATOMY and PHYSIOLOGY Professor Donald G. MacLellan Executive Director Health Education & Management Innovations ANATOMY and PHYSIOLOGY Epidermal Layers ECM Structure Dermis Structure Skin
Integumentary System (Script) Slide 1: Integumentary System. Slide 2: An overview of the integumentary system
 Integumentary System (Script) Slide 1: Integumentary System Slide 2: An overview of the integumentary system Skin is the body s largest and heaviest organ making up 15% of body weight. Most skin is 1 to
Integumentary System (Script) Slide 1: Integumentary System Slide 2: An overview of the integumentary system Skin is the body s largest and heaviest organ making up 15% of body weight. Most skin is 1 to
Dermoscopy. Enhanced Diagnostic Ability: Pigmented Lesions. Ted Rosen, MD Baylor College of Medicine Houston, Texas
 Dermoscopy Enhanced Diagnostic Ability: Pigmented Lesions Ted Rosen, MD Baylor College of Medicine Houston, Texas Faculty Disclosure Statement No conflicts relevant to this workshop! Sir William Osler
Dermoscopy Enhanced Diagnostic Ability: Pigmented Lesions Ted Rosen, MD Baylor College of Medicine Houston, Texas Faculty Disclosure Statement No conflicts relevant to this workshop! Sir William Osler
SKIN HISTOLOGY the microscopic anatomy of the Integument. Mikrogeo. com
 SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
SKIN HISTOLOGY the microscopic anatomy of the Integument Mikrogeo. com Hair follicles, sweat glands, sebaceous glands (even teeth) are products of the epidermis,embryologically speaking ectododerm, that
Melanoma-Back to Basics I Thought I Knew Ya! Paul K. Shitabata, M.D. Dermatopathologist APMG
 Melanoma-Back to Basics I Thought I Knew Ya! Paul K. Shitabata, M.D. Dermatopathologist APMG At tumor board, a surgeon insists that all level II melanomas are invasive since they have broken through the
Melanoma-Back to Basics I Thought I Knew Ya! Paul K. Shitabata, M.D. Dermatopathologist APMG At tumor board, a surgeon insists that all level II melanomas are invasive since they have broken through the
Skin. Kristine Krafts, M.D.
 Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Skin Kristine Krafts, M.D. Skin Lecture Objectives Describe the functions of skin. Describe the structure, location and function of the cell types found in epidermis: keratinocytes, melanocytes, Langerhans
Epidermis. Integumentary system
 Epidermis the doctor mentioned at the begging of the lecture that the slides is from different sources and has information and details that is enough for us so we don t have to go back and read from the
Epidermis the doctor mentioned at the begging of the lecture that the slides is from different sources and has information and details that is enough for us so we don t have to go back and read from the
Integumentary System and Body Membranes
 Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
Integumentary System and Body Membranes The Skin and its appendages hair, nails, and skin glands Anatomy/Physiology NHS http://www.lab.anhb.uwa.edu.au/mb140/corepages/integumentary/integum.htm I. System
Chapter 5: The Integumentary System - Introduction and Epidermis
 Chapter 5: The Integumentary System - Introduction and Epidermis The Integument Means Covering Composed: Skin Hair Nails Sweat glands Oil glands The Integument Thickness 1.5 4 mm (or more) Weight 9 11
Chapter 5: The Integumentary System - Introduction and Epidermis The Integument Means Covering Composed: Skin Hair Nails Sweat glands Oil glands The Integument Thickness 1.5 4 mm (or more) Weight 9 11
The Integumentary System: An Overview
 The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
The Integumentary System: An Overview Functions: Protective covering Helps regulate body temperature Retards water loss from deeper tissues Houses sensory receptors Synthesizes biochemicals Excretes small
Actinic keratosis (AK): Dr Sarma s simple guide
 Actinic keratosis (AK): Dr Sarma s simple guide Actinic keratosis is a very common lesion that you will see in your day-to-day practice. First, let me explain the name Actinic keratosis. It means keratosis
Actinic keratosis (AK): Dr Sarma s simple guide Actinic keratosis is a very common lesion that you will see in your day-to-day practice. First, let me explain the name Actinic keratosis. It means keratosis
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 5 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
Integumentary System. Packet #12
 Integumentary System Packet #12 Introduction Skin/Integument Skin, considered an organ, is the major component of the integumentary system. The integumentary system is also composed of other accessory
Integumentary System Packet #12 Introduction Skin/Integument Skin, considered an organ, is the major component of the integumentary system. The integumentary system is also composed of other accessory
Chapter 5 The Integumentary System. Copyright 2009, John Wiley & Sons, Inc. 1
 Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more
 Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more common on the trunk; but extremities, head and neck are
Benign and malignant epithelial lesions: Seborrheic keratosis: A common benign pigmented epidermal tumor occur in middle-aged or older persons more common on the trunk; but extremities, head and neck are
Chapter 6 Skin and the Integumentary System. Skin Cells. Layers of Skin. Epidermis Dermis Subcutaneous layer beneath dermis not part of skin
 Chapter 6 Skin and the Integumentary System Composed of several tissues Maintains homeostasis Protective covering Retards water loss Regulates body temperature Houses sensory receptors Contains immune
Chapter 6 Skin and the Integumentary System Composed of several tissues Maintains homeostasis Protective covering Retards water loss Regulates body temperature Houses sensory receptors Contains immune
6/17/2018. Breaking Bad (Part 1) Dermoscopy of Brown(ish) Things. Bad?
 Breaking Bad (Part 1) Dermoscopy of Brown(ish) Things Jennie T. Clarke, MD ssociate Professor of Dermatology University of Utah School of Medicine Bad? 1 Brown(ish) Things Bad Melanoma Pigmented basal
Breaking Bad (Part 1) Dermoscopy of Brown(ish) Things Jennie T. Clarke, MD ssociate Professor of Dermatology University of Utah School of Medicine Bad? 1 Brown(ish) Things Bad Melanoma Pigmented basal
Chapter 5. Integumentary System 5-1
 Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
Chapter 5 Integumentary System 5-1 Structures that are part of the integument Skin Hair Nails Glands Overview of Functions Protection Sensation Temperature regulation Vitamin D production Excretion Immunity
The Integumentary System
 The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system (See if you can name some appendages) A fatty layer (hypodermis) lies deep
The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system (See if you can name some appendages) A fatty layer (hypodermis) lies deep
Overview of the Integumentary System. Lab #7. Layers of the epidermis are known as strata. Organization of the Epidermis: Layers of the Epidermis
 Overview of the Integumentary System Lab #7 Integumentary System Organization of the Epidermis: Layers of the epidermis are known as strata Figure 5 2 Layers of the Epidermis Top: Free surface of skin
Overview of the Integumentary System Lab #7 Integumentary System Organization of the Epidermis: Layers of the epidermis are known as strata Figure 5 2 Layers of the Epidermis Top: Free surface of skin
Effect of Propecia on the Hair Follicle in Male Androgenetic Alopecia: A Confocal Laser Scanning Microscopy and Video Imaging Study
 Effect of Propecia on the Hair Follicle in Male Androgenetic Alopecia: A Confocal Laser Scanning Microscopy and Video Imaging Study Investigators: Department of Dermatology, University of Minnesota, Minneapolis,
Effect of Propecia on the Hair Follicle in Male Androgenetic Alopecia: A Confocal Laser Scanning Microscopy and Video Imaging Study Investigators: Department of Dermatology, University of Minnesota, Minneapolis,
Dermoscopy. Sir William Osler. Dermoscopy. Dermoscopy. Melanoma USA Primary Care Update Faculty Disclosure Statement
 Diagnostic Ability: Pigmented Lesions Ted Rosen, MD Baylor College of Medicine Houston, Texas Enhanced 2010 Primary Care Update Faculty Disclosure Statement Ted Rosen, MD Speakers Bureau: Abbott, Amgen,
Diagnostic Ability: Pigmented Lesions Ted Rosen, MD Baylor College of Medicine Houston, Texas Enhanced 2010 Primary Care Update Faculty Disclosure Statement Ted Rosen, MD Speakers Bureau: Abbott, Amgen,
CHAPTER 5 INTEGUMENTARY
 CHAPTER 5 INTEGUMENTARY skin under the skin other stuff cutaneous layer hypodermis (subcutaneous) accessory structures Cutaneous layer = skin epithelial layers = connective tissue layer = dermis Subcutaneous
CHAPTER 5 INTEGUMENTARY skin under the skin other stuff cutaneous layer hypodermis (subcutaneous) accessory structures Cutaneous layer = skin epithelial layers = connective tissue layer = dermis Subcutaneous
The Integumentary System
 The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis
The Integumentary System The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis
ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION*
 ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION* The description of the lesion in the title of this rcport is intentionally non-committal. Diagnosed clinically as a lentigo, it was removed as
ELECTRON MICROSCOPY OF A SMALL PIGMENTED CUTANEOUS LESION* The description of the lesion in the title of this rcport is intentionally non-committal. Diagnosed clinically as a lentigo, it was removed as
Ex. 7: Integumentary
 Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Collin County Community College BIOL. 2401 Ex. 7: Integumentary. Skin or Integument Consists of three major regions Epidermis outermost superficial region Dermis middle region Hypodermis (superficial fascia)
Skin and Body Membranes
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 4 Skin and Body Membranes Slides 4.1 4.32 Lecture Slides in PowerPoint by Jerry L. Cook Skin and Body Membranes Function
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 4 Skin and Body Membranes Slides 4.1 4.32 Lecture Slides in PowerPoint by Jerry L. Cook Skin and Body Membranes Function
insight Exceptional SKINTELL in-depth, non-invasive dermatological solution HealthCare
 Optical Coherence Tomography insight Exceptional SKINTELL in-depth, non-invasive dermatological solution HealthCare From outside, exceptional insight inside SKINTELL from Agfa HealthCare enables you to
Optical Coherence Tomography insight Exceptional SKINTELL in-depth, non-invasive dermatological solution HealthCare From outside, exceptional insight inside SKINTELL from Agfa HealthCare enables you to
Skin. Lecture #14. Ref:
 Skin Lecture #14 Ref: http://www.ccunix.ccu.edu.tw/~chenmsl/tea/skin_910721.htm Structure of Skin 1. Epidermis 2. Dermis 3. Subcutis 4. Hair follicle 5. Sebaceous gland 6. Sweat gland Skin Largest human
Skin Lecture #14 Ref: http://www.ccunix.ccu.edu.tw/~chenmsl/tea/skin_910721.htm Structure of Skin 1. Epidermis 2. Dermis 3. Subcutis 4. Hair follicle 5. Sebaceous gland 6. Sweat gland Skin Largest human
Integument. Squamous Cell Carcinoma. Melanoma. Largest organ 30% of all clinical diagnoses 1/3 of all tumors
 Squamous Cell Carcinoma Integument Largest organ 30% of all clinical diagnoses 1/3 of all tumors Melanoma Epidermis Stratified, squamous keratinized epithelium Derived from ectoderm Appendages hair follicles
Squamous Cell Carcinoma Integument Largest organ 30% of all clinical diagnoses 1/3 of all tumors Melanoma Epidermis Stratified, squamous keratinized epithelium Derived from ectoderm Appendages hair follicles
Cornell Notes Name: Date: Topic: CH 4
 *We are revisiting Ch 3B on body tissues (Connective) prior to our study of Ch 4 Integumentary. Start on p.90 I. Connective Tissue A. Functions of Connective 1. Protection 2. Support 3. Binding Together
*We are revisiting Ch 3B on body tissues (Connective) prior to our study of Ch 4 Integumentary. Start on p.90 I. Connective Tissue A. Functions of Connective 1. Protection 2. Support 3. Binding Together
STUDY. Microscopic In Vivo Description of Cellular Architecture of Dermoscopic Pigment Network in Nevi and Melanomas
 STUDY Microscopic In Vivo Description of Cellular Architecture of Dermoscopic Pigment Network in Nevi and Melanomas Giovanni Pellacani, MD; Anna Maria Cesinaro, MD; Caterina Longo, MD; Costantino Grana,
STUDY Microscopic In Vivo Description of Cellular Architecture of Dermoscopic Pigment Network in Nevi and Melanomas Giovanni Pellacani, MD; Anna Maria Cesinaro, MD; Caterina Longo, MD; Costantino Grana,
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Skin and Body Membranes 4 Body Membranes Function of body membranes Cover body surfaces Line body cavities
In-vivo histopathological study of YouLaser MT interaction with the skin. A laser device emitting combined 1540 and nm wavelengths.
 Histology report No. 02/04-12, date April 2nd, 2012 Page 1 In-vivo histopathological study of YouLaser MT interaction with the skin. A laser device emitting combined 1540 and 10600 nm wavelengths. Paolo
Histology report No. 02/04-12, date April 2nd, 2012 Page 1 In-vivo histopathological study of YouLaser MT interaction with the skin. A laser device emitting combined 1540 and 10600 nm wavelengths. Paolo
Laser Effects on Skin Melanin
 Modern Applied Science January, 2009 Laser Effects on Skin Melanin Khalid M. Omar Universiti Sains Malaysia, School of Physics 11800 Penang, Malaysia E-mail: khalhadithi@yahoo.com Khaled A. Al-Khaza leh,
Modern Applied Science January, 2009 Laser Effects on Skin Melanin Khalid M. Omar Universiti Sains Malaysia, School of Physics 11800 Penang, Malaysia E-mail: khalhadithi@yahoo.com Khaled A. Al-Khaza leh,
Figure 6.1 Transparency Master 37
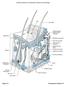 Pore Hair shaft Epidermis Dermal papillae (papillary layer of dermis) Meissner s corpuscle Dermis Free nerve ending Reticular layer of dermis Sebaceous (oil) gland Arrector pili muscle Sensory nerve fiber
Pore Hair shaft Epidermis Dermal papillae (papillary layer of dermis) Meissner s corpuscle Dermis Free nerve ending Reticular layer of dermis Sebaceous (oil) gland Arrector pili muscle Sensory nerve fiber
STUDY. Morphologic Features of Melanophages Under In Vivo Reflectance Confocal Microscopy
 STUDY Morphologic Features of Melanophages Under In Vivo Reflectance Confocal Microscopy Pascale Guitera, MD; Ling-Xi L. Li, MD, PhD; Richard A. Scolyer, MD; Scott W. Menzies, MS, PhD Objectives: To determine
STUDY Morphologic Features of Melanophages Under In Vivo Reflectance Confocal Microscopy Pascale Guitera, MD; Ling-Xi L. Li, MD, PhD; Richard A. Scolyer, MD; Scott W. Menzies, MS, PhD Objectives: To determine
Experiment Note the locations of the epidermis, dermis, dermal papillae, and the sweat glands. Note that fat cells that comprise the
 Experiment 1 Examining Skin, Bones and Muscle Histology Experiment Inventory Skin Digital Slide Images Cortical (Compact) Bone Digital Slide Image Trabecular (Spongy) Bone Digital Slide Image Cardiac Muscle
Experiment 1 Examining Skin, Bones and Muscle Histology Experiment Inventory Skin Digital Slide Images Cortical (Compact) Bone Digital Slide Image Trabecular (Spongy) Bone Digital Slide Image Cardiac Muscle
SPECIAL TOPIC. Institut National de la Sante et de la Recherche Medicale (INSERM U895), Nice, France c
 November 2011 1260 Volume 10 Issue 1i Copyright 2011 ORIGINAL ARTICLES Journal of Drugs in Dermatology SPECIAL TOPIC A Pilot Study Using Reflectance Confocal Microscopy (RCM) in the Assessment of a Novel
November 2011 1260 Volume 10 Issue 1i Copyright 2011 ORIGINAL ARTICLES Journal of Drugs in Dermatology SPECIAL TOPIC A Pilot Study Using Reflectance Confocal Microscopy (RCM) in the Assessment of a Novel
Fluorescence spectroscopy and microscopy of cutaneous tumours correlation between micro- and macro- spectral measurements
 Fluorescence spectroscopy and microscopy of cutaneous tumours correlation between micro- and macro- spectral measurements E. Borisova 1, L. Avramov 1, M. Lomova 2, O. Semyachkina-Glushkovskaya 2, D. Gorin
Fluorescence spectroscopy and microscopy of cutaneous tumours correlation between micro- and macro- spectral measurements E. Borisova 1, L. Avramov 1, M. Lomova 2, O. Semyachkina-Glushkovskaya 2, D. Gorin
The Integementary System. The Skin & Its Parts
 The Integementary System The Skin & Its Parts General Structure 2. Accessory structures: hair, nails, exocrine glands 1. Cutaneous membrane: various layers Major Functions 1. Protection 2. Temperature
The Integementary System The Skin & Its Parts General Structure 2. Accessory structures: hair, nails, exocrine glands 1. Cutaneous membrane: various layers Major Functions 1. Protection 2. Temperature
Figure 4.1. Using Figure 4.1, identify the following: 1) The region that contains adipose tissue is indicated by letter. Diff: 2 Page Ref: 115
 Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 4 Skin and Body Membranes Short Answer Figure 4.1 Using Figure 4.1, identify the following: 1) The region that contains adipose tissue is indicated
Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 4 Skin and Body Membranes Short Answer Figure 4.1 Using Figure 4.1, identify the following: 1) The region that contains adipose tissue is indicated
THE INTEGUMENTARY SYSTEM. Body Membranes & Skin
 THE INTEGUMENTARY SYSTEM Body Membranes & Skin TYPES OF MEMBRANES Epithelial Membranes includes layer of epithelial cells and connective tissue Serous Cutaneous Mucous Connective Tissue Membranes solely
THE INTEGUMENTARY SYSTEM Body Membranes & Skin TYPES OF MEMBRANES Epithelial Membranes includes layer of epithelial cells and connective tissue Serous Cutaneous Mucous Connective Tissue Membranes solely
DIFFERENCES IN DERMOSCOPIC IMAGES FROM NON-POLARIZED DERMOSCOPE AND POLARIZED DERMOSCOPE INFLUENCE THE DIAGNOSTIC ACCURACY AND CONFIDENCE LEVEL.
 DIFFERENCES IN DERMOSCOPIC IMAGES FROM NON-POLARIZED DERMOSCOPE AND POLARIZED DERMOSCOPE INFLUENCE THE DIAGNOSTIC ACCURACY AND CONFIDENCE LEVEL. 1. Steven Q. Wang MD 1 (wangs@mskcc.org) 2. Stephen W. Dusza
DIFFERENCES IN DERMOSCOPIC IMAGES FROM NON-POLARIZED DERMOSCOPE AND POLARIZED DERMOSCOPE INFLUENCE THE DIAGNOSTIC ACCURACY AND CONFIDENCE LEVEL. 1. Steven Q. Wang MD 1 (wangs@mskcc.org) 2. Stephen W. Dusza
STUDY. Confocal Examination of Untreated Fresh Specimens From Basal Cell Carcinoma
 STUDY Confocal Examination of Untreated Fresh Specimens From Basal Cell Carcinoma Implications for Microscopically Guided Surgery Armin Gerger, MD; Michael Horn, MD; Silvia Koller, MD; Wolfgang Weger,
STUDY Confocal Examination of Untreated Fresh Specimens From Basal Cell Carcinoma Implications for Microscopically Guided Surgery Armin Gerger, MD; Michael Horn, MD; Silvia Koller, MD; Wolfgang Weger,
This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur.
 Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
The Integumentary System
 The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
The Integumentary System Integument is skin Skin and its appendages make up the integumentary system A fatty layer (hypodermis) lies deep to it Two distinct regions Epidermis Dermis PHL 212 1 Function
SKIN. 3. How is the skin structured around the finger joints to allow for flexible movement of the fingers?
 SKIN Objectives for Exam #1: 1. List various skin structures and describe their functions. 2. Describe skin responses to increases and decreases in body temperature. 3. Provide examples of various skin
SKIN Objectives for Exam #1: 1. List various skin structures and describe their functions. 2. Describe skin responses to increases and decreases in body temperature. 3. Provide examples of various skin
Chapter 5 The Integumentary System. Copyright 2009, John Wiley & Sons, Inc. 1
 Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Malignant tumors of melanocytes : Part 3. Deba P Sarma, MD., Omaha
 Malignant tumors of melanocytes : Part 3 Deba P Sarma, MD., Omaha Let s go over one case of melanoma using the following worksheet. Of the various essential information that needs to be included in the
Malignant tumors of melanocytes : Part 3 Deba P Sarma, MD., Omaha Let s go over one case of melanoma using the following worksheet. Of the various essential information that needs to be included in the
Handheld optical coherence tomography device for photodynamic therapy
 Article Optics February 2012 Vol.57 No.5: 450 454 doi: 10.1007/s11434-011-4864-6 Handheld optical coherence tomography device for photodynamic therapy WANG TianShi 1, WANG ChengMing 1, HUANG NaiYan 2,
Article Optics February 2012 Vol.57 No.5: 450 454 doi: 10.1007/s11434-011-4864-6 Handheld optical coherence tomography device for photodynamic therapy WANG TianShi 1, WANG ChengMing 1, HUANG NaiYan 2,
Reflectance Confocal Microscopy Real-Time In Vivo Imaging of Basal and Squamous Cell Carcinomas
 Journal of Analytical Oncology, 2012, 1, 155-163 155 Reflectance Confocal Microscopy Real-Time In Vivo Imaging of Basal and Squamous Cell Carcinomas Anna Haydee Chacon 1,*, Uzma Farooq 1, Katlein Franca
Journal of Analytical Oncology, 2012, 1, 155-163 155 Reflectance Confocal Microscopy Real-Time In Vivo Imaging of Basal and Squamous Cell Carcinomas Anna Haydee Chacon 1,*, Uzma Farooq 1, Katlein Franca
Layers of the Skin *
 OpenStax-CNX module: m63171 1 Layers of the Skin * Ildar Yakhin Based on Layers of the Skin by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License
OpenStax-CNX module: m63171 1 Layers of the Skin * Ildar Yakhin Based on Layers of the Skin by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License
