Financial Disclosures
|
|
|
- Myles Booth
- 5 years ago
- Views:
Transcription
1 Financial Disclosures DB is a scientific advisor for Regeneron/ Bayer and Genentech/ Roche & a DB member of the Regeneron Combination Products Steering Committee RCH receives grant support from Regeneron/ Bayer and Genentech/ Roche
2 Combination Therapy with Intravitreal Nesvacumab and Aflibercept for Diabetic Macula Edema David M. Brown, MD On behalf of the RUBY Investigators Retina Consultants of Houston Houston Methodist Hospital Houston, Texas USA
3 Take Home Messages Angiopoetin/ Tie 2 Cascade activated and important in diabetic retinopathy BCVA Equivalent Combo Better Anatomy Combo Improves DRS Co-formulated product disadvantaged in FDA registration
4 Ang-1 & Ang-2 Discovery 1 Davis et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell Dec 27;87(7): Maisonpierre et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science Jul 4;277(5322):55-60.
5 ANGIOPOIETIN/TIE2 SIGNALING PATHWAY ANGPT2 ANGPT1 ANGPT2 Tie2 is an endothelial cell-specific tyrosine kinase receptor to which two ligands bind Integrin α5β1 TIE1 TIE2 VEPTP Ang1 PIP 2 PIP 3 Expressed in normal adult tissues to help maintain vascular integrity GRB2 PI3K Ang2 Ras DOKR ABIN2 SHP2 AKT Only expressed under pathological FAK ERK1 and ERK2 RAF1 MEK Nck PAK IKK NF-κB ICAM1 VCAM1 E-selectin enos Survivin Caspase conditions. Modifies Tie-2 signaling in complex, context-dependent way. Endothelial cell proliferation and migration Inflammation Endothelial cell survival interaction Thurston and Daly. Cold Spring Harb Perspect Med 2012;2:a Jones et al. Nature Reviews Molecular Cell Biology 2, (April 2001) Eklund, Lauri et al. "Angiopoietin signaling in the vasculature." Experimental cell research (2013):
6 ANGIOPOIETIN/TIE2 SIGNALING PATHWAY ANGPT2 TIE2 Tie2 is an endothelial cell-specific tyrosine kinase receptor to which two ligands bind Ang1 GRB2 PI3K Ang2 Expressed in normal adult tissues to help maintain vascular integrity Ras DOKR ABIN2 SHP2 Only expressed under pathological ERK1 and ERK2 RAF1 MEK Nck PAK IKK NF-κB ICAM1 VCAM1 E-selectin enos conditions. Modifies Tie-2 signaling in complex, context-dependent way. Endothelial cell proliferation and migration Inflammation Endothelial cell survival interaction Thurston and Daly. Cold Spring Harb Perspect Med 2012;2:a Jones et al. Nature Reviews Molecular Cell Biology 2, (April 2001) Eklund, Lauri et al. "Angiopoietin signaling in the vasculature." Experimental cell research (2013):
7 Ang-2 Levels Elevated in Human Vitreous RVO > DR > AMD Vitreous levels in newly diagnosed patients Data courtesy of Roche US Medical Affairs
8 EFFECT OF IVT ADMINISTRATION OF NESVACUMAB, ALONE OR IN COMBINATION WITH AFLIBERCEPT IN A RETINAL VASCULAR DEVELOPMENT MODEL Nesvacumab Area of the superficial retinal plexus Nesvacumab Total vessel length superficial retinal plexus P4 (post natal day 4); P6 (post natal day 6)
9 NESVACUMAB INCREASED DURATION OF ANTI-LEAK ACTION OF AFLIBERCEPT IN PRECLINICAL MODEL OF CHRONIC VASCULAR LEAK Single IVT injection of Aflibercept or Nesvacumab or both co-formulated Treatment Week
10 NESVACUMAB/AFLIBERCEPT (CO-FORMULATED ANTI-ANG2 + ANTI-VEGF) Nesvacumab/aflibercept is a co-formulated drug product consisting of the fully human mab, REGN910, and the fusion protein, aflibercept
11 Study Design Baseline - Week 12 Multiple-dose, double masked, randomized, controlled study in patients with DME Randomized 1:2:3 Key Eligibility Criteria Clinically significant DME with central involvement BCVA ETDRS letter score equivalent to 20/40 to 20/320 Intravitreal anti-vegf 3 months from screening Panretinal laser photocoagulation or macular laser photocoagulation 3 months from screening Intraocular or periocular corticosteroids in the study eye 4 months from screening LD Combo Nesvacumab (3 mg) + aflibercept (2 mg) q4 weeks (n=50) HD Combo Nesvacumab (6 mg) + aflibercept (2 mg) q4 weeks (n=100) Intravitreal aflibercept injection (2mg) q4 weeks (n=152) Week 12 LD: Low Dose; HD: High Dose Week 36 (End of Study)
12 Study Design Week 12 Week 36 LD Combo Nesvacumab (3 mg) + aflibercept (2 mg) q4 weeks (n=50) HD Combo Nesvacumab (6 mg) + aflibercept (2 mg) q4 weeks (n=100) Intravitreal aflibercept injection (2mg) q4 weeks (n=152) Re-randomization at Week 12 LD q8 HD q8 HD q12 q8 q12 HD q8 Week 36 (End of Study) Stratification for re-randomization based on VA outcomes at week 12 LD: Low Dose; HD: High Dose
13 Patients Disposition and Demographics LD HD Total (n=50) (n=100) (n=152) (N=302) Patients completing Week 12, n(%) 46 (92.0%) 97 (97.0%) 148 (97.4%) 291 (96.4%) Mean Age, years (SD) 62.1 (8.90) 62.4 (10.37) 59.5(10.24) 60.9(10.15) Female, n (%) 21 (42.0%) 49 (49.0%) 68 (44.7%) 138 (45.7%) Race, n (%) White 37 (74.0%) 87 (87.0%) 121 (79.6%) 245 (81.1%) Black or African American 11 (22.0%) 8 (8.0%) 19 (12.5%) 38 (12.6%) Asian 1 (2.0%) 2 (2.0%) 4 (2.6%) 7 (2.3%) American Indian or Alaska Native 1 (2.0%) 0 3 (2.0%) 4 (1.3%) Native Hawaiian or Other Pacific Islander 0 1 (1.0%) 0 1 (0.3%) Other (2.0%) 3 (1.0%) Not Reported 0 2 (2.0%) 2 (1.3%) 4 (1.3%) SAF
14 Baseline Disease Characteristics LD HD Total (n=47) (n=99) (n=150) (N=296) Mean Baseline Hemoglobin A1C (SD) 8.5 (1.86) 7.8 (1.61) 8.1 (1.86) 8.0 (1.79) Mean Diabetes Duration, years (SD) 17.6 (10.93) 17.5 (11.22) 15.8 (10.69) 16.7 (10.90) Diabetes Type, n (%) Type 1 2 (4.3%) 5 (5.1%) 11 (7.3%) 18 (6.1%) Type 2 45 (95.7%) 94 (94.9%) 139 (92.7%) 278 (93.9%) Prior Treatment for DME/DR*, Study Eye, n % 27 (57.4%) 40 (40.4%) 58 (38.7%) 125 (42.2%) Prior Focal or Grid Laser 19 (40.4%) 27 (27.3%) 36 (24.0%) 82 (27.7%) Prior Intravitreal Anti-VEGF 12 (25.5%) 28 (28.3%) 27 (18%) 67 (22.6%) Prior Intravitreal Steroids 4 (8.5%) 7 (7.1%) 7 (4.7%) 18 (6.1%) FAS *Patients could have had more than one treatment
15 Baseline Disease Characteristics LD HD Total (n=47) (n=99) (n=150) (N=296) Mean ETDRS BCVA, letters (SD) 57.7 (11.13) 60.6 (11.11) 58.7 (10.78) 59.2 (10.96) Mean CRT, um (SD) (152.78) (151.77) (151.27) (151.80) Diabetic Retinopathy Severity Score, n (%) 10, (3.0%) 1 (0.7%) 4 (1.4%) (21.3%) 14 (14.1%) 17 (11.3%) 41 (13.9%) (21.3%) 15 (15.2%) 37 (24.7%) 62 (20.9%) 47 9 (19.1%) 34 (34.3%) 46 (30.7%) 89 (30.1%) (27.7%) 19 (19.2%) 35 (23.3%) 67 (22.6%) 61 1 (2.1%) 2 (2.0%) 4 (2.7%) 7 (2.4%) 65 1 (2.1%) 7 (7.1%) 3 (2.0%) 11 (3.7%) 71 1 (2.1%) 4 (4.0%) 5 (3.3%) 10 (3.4%) 75 1 (2.1%) 0 1 (0.7%) 2 (0.7%) FAS, 3 patients (1 in each group) were ungradable for DRSS and are not included.
16 Mean Change in Best-Corrected Visual Acuity Baseline - Week LD (n=47) HD (n=99) (n=150) ETDRS Letters 10 5 p = (95% CI: -4.84, 0.67) p = (95% CI: -2.10, 2.18) Weeks FAS, LOCF
17 Mean Central Retinal Thickness Baseline - Week 12 Absolute Change LD (n=47) HD (n=99) (n=150) 0-50 Weeks LD (n=47) HD (n=99) (n=150) 450 μm 400 μm FAS, LOCF Weeks * p= ( , 5.61) * p= ( , -4.74) *
18 Proportion of Patients With Complete Resolution of Fluid at the Foveal Center at Week % Proportion of Patients 80% 60% 40% 20% 59.6% * 66.3% 53.7% 0% LD n=47 FAS, Patients with no intraretinal or subretinal fluid at the foveal center on SD-OCT LOCF HD n=99 n=150 * p=
19 Proportion of Patients With Normalization of Macular Thickness (CRT 300 μm) at Week % 80% Proportion of Patients 60% 40% 20% 40.4% * 57.6% 35.3% 0% LD n=47 HD n=99 n=150 * p= FAS, LOCF
20 Proportion of Patients With 2 Step Improvement in DRSS at Week % Total DRSS 47 Moderately Severe Non-Proliferative Diabetic Retinopathy or Worse DRSS 53 Severe Non-Proliferative Diabetic Retinopathy or Worse Proportion of Patients 80% 60% 40% 20% 13.3% = 6.1% 21.3% 15.2% 24.0% 30.2% = 8.2% 22.0% 25.0% 48.4% = 14.4% 34.0% 0% LD n=47 HD n=99 n=150 LD n=25 HD n=63 n=89 LD n=17 HD n=32 n=48 FAS, LOCF
21 Week 36
22 Study Design Week 12 Week 36 LD Combo Nesvacumab (3 mg) + aflibercept (2 mg) q4 weeks (n=50) HD Combo Nesvacumab (6 mg) + aflibercept (2 mg) q4 weeks (n=100) Intravitreal aflibercept injection (2mg) q4 weeks (n=152) Re-randomization at Week 12 LD q8 HD q8 HD q12 q8 q12 HD q8 Week 36 (End of Study) Stratification for re-randomization based on VA outcomes at week 12 LD: Low Dose; HD: High Dose
23 Patient Disposition LD q8 HD q8 HD q12 q8 q12 HD q8 Number of patients in the Secondary Randomization Set, n (%) (n=45) (n=44) (n=52) (n=46) (n=48) (n=49) Number of patients completing week 36, n (%) 44 (97.8%) 42 (95.5%) 50 (96.2%) 46 (100%) 43 (89.6%) 45 (91.8%) FAS -Secondary randomization set
24 Dose Exposure Through Week 36 LD q8 HD q8 HD q12 q8 q12 HD q8 (n=45) (n=44) (n=52) (n=46) (n=48) (n=49) Number of Planned Injections, n Mean Number of Injections, n (SD) 7.2* (0.92) 5.9 (0.35) 5.1* (0.58) 5.9 (0.45) 4.8 (0.63) 5.8 (0.44) *~10% and 50% of patients received per protocol dosing in the LD q8 and HD q12 groups, respectively. SAF-Secondary randomization set
25 Mean Change in Best-Corrected Visual Acuity Baseline Week LD q8 (n=45) HD q8 (n=44) HD q12 (n=52) q8 (n=46) q12 (n=48) to HD q8 (n=49) 11.9 ETDRS Letters Weeks FAS -Secondary randomization set LOCF
26 Mean Change in Central Retinal Thickness Baseline Week Weeks LD q8 (n=45) HD q8 (n=44) HD q12 (n=52) q8 (n=46) q12 (n=48) to HD q8 (n=49) μm FAS -Secondary randomization set LOCF *p<0.05 vs q * *
27 Proportion of Patients with Complete Resolution of Fluid at the Foveal Center at Week 32* 100% Proportion of Patients 80% 60% 40% 20% 81.6% 81.0% 61.0% 61.8% 56.1% 0% HD q8 n=38 HD q12 n=21 q8 n=41 q12 n=34 HD q8 n=41 *8 or 12 weeks from the last study treatment FAS -Secondary randomization set, OC Per Protocol Set
28 Proportion of Patients With Complete Resolution of Fluid at the Foveal Center at Week 36 Combined q8+q12 Groups 100% * 90.4% Proportion of Patients 80% 60% 40% 20% 74.0% 0% n=75/83 HD n=57/77 * p= FAS -Secondary randomization set, Patients with no intraretinal or subretinal fluid at the foveal center on SD=-OCT. OC
29 Patients Maintaining* Complete Resolution of Fluid at the Foveal Center through Week 36 Combined q8+q12 Groups Proportion of Patients 100% 80% 60% 40% 20% * 79.5% 57.1% 0% N=66/83 HD n=44/77 * Defined as reaching No fluid at the foveal center and maintaining that status for all subsequent study visits. FAS -Secondary randomization Set, OC * p=
30 Proportion of Patients with Normalization of Macular Thickness (CRT 300 μm) at Week 36 Combined q8+q12 Groups 100% Proportion of Patients 80% 60% 40% 20% * 74.7% 56.6% 0% n=62/83 HD n=43/76 FAS- Secondary randomization set OC * p=
31 Patients Maintaining* Normalization of Macular Thickness (CRT 300 µm) through Week 36 Combined q8+q12 Groups 100% Proportion of Patients 80% 60% 40% 20% * 66.3% 42.1% 0% n=55/83 HD n=32/76 * Defined as reaching CRT<=300 and maintaining <=300 for all subsequent study visits. FAS -Secondary randomization Set, OC * p=
32 OCT Dry = Real World Durability
33 Proportion of Patients With 2 Step Improvement in DRSS at Week 36 Combined q8+q12 Groups 100% Total DRSS 47 Moderately Severe Non-Proliferative Diabetic Retinopathy or Worse DRSS 53 Severe Non-Proliferative Diabetic Retinopathy or Worse Proportion of Patients 80% 60% 40% 20% 34.0% = 8.2% 25.8% 47.6% = 11.9% 35.7% 64.5% = 24.5% 40.0% 0% FAS- Secondary randomization set LOCF HD n=96 n=94 HD n=63 n=56 HD n=31 n=25
34 Proportion of Patients With 3 Step Improvement in DRSS at Week 36 Combined q8+q12 Groups 100% Total DRSS 47 Moderately Severe Non-Proliferative Diabetic Retinopathy or Worse DRSS 53 Severe Non-Proliferative Diabetic Retinopathy or Worse Proportion of Patients 80% 60% 40% 20% 0% FAS- Secondary randomization set LOCF 11.7% 7.5% 17.5% 10.7% 29.0% HD n=96 = 4.2% n=94 HD n=63 = 6.8% n=56 HD n=31 = 13.0% 16.0% n=25
35 Safety
36 Most Frequent Ocular Adverse Events Through Week 36 LD q8 HD q8 HD q12 q8 q12 HD q8 No. of patients with at least 1 AE, n (%) (n=46) (n=44) (n=53) (n=47) (n=49) (n=49) 14 (30.4%) 12 (27.3%) 19 (35.8%) 10 (21.3%) 11 (22.4%) 17 (34.7%) Vitreous detachment 0 4 (9.1%) 3 (5.7%) 1 (2.1%) 0 4 (8.2%) Conjunctival hemorrhage 4 (8.7%) 1 (2.3%) 1 (1.9%) 6 (12.8%) 2 (4.1%) 3 (6.1%) Cataract 1 (2.2%) (4.3%) 2 (4.1%) 2 (4.1%) Eye pain 2 (4.3%) 1 (2.3%) 2 (3.8%) 0 2 (4.1%) 2 (4.1%) Punctate keratitis 0 1 (2.3%) (4.1%) Visual acuity reduced 1 (2.2%) 1 (2.3%) 1 (1.9%) 0 1 (2.0%) 2 (4.1%) Vitreous hemorrhage 0 1 (2.3%) 1 (1.9%) (4.1%) Vitreous floaters 1 (2.2%) 0 2 (3.8%) 1 (2.1%) 2 (4.1%) 1 (2.0%) Dry eye 0 1 (2.3%) 4 (7.5%) Retinal exudates 1 (2.2%) 0 3 (5.7%) 0 2 (4.1%) 0 SAF-Secondary randomization set; >4% in any treatment group.
37 Anti-Platelet Trialists Collaboration-Defined Arterial Thromboembolic Events Through Week 36 LD q8 HD q8 HD q12 q8 q12 HD q8 No. of patients w/ at least 1 AE, n (%) (n=46) (n=44) (n=53) (n=47) (n=49) (n=49) 3 (6.5%) 0 2 (3.8%) 2 (4.3%) 1 (2.0%) 0 Non-fatal MI 1 (2.2%) 0 1 (1.9%) 1 (2.1%) 1 (2.0%) 0 Non-fatal Stroke 1 (2.2%) 0 1 (1.9%) Vascular Death 2 (4.3%) (2.1%) 0 0 SAF-Secondary randomization set
38 Conclusions Ocular and systemic safety consistent with monotherapy BCVA Equivalent Combo Better Anatomy Combo Improves DRS Co-formulated product disadvantaged in FDA registration
Combination Therapy with Intravitreal Nesvacumab+Aflibercept in Diabetic Macular Edema: The Phase 2 RUBY Trial
 Combination Therapy with Intravitreal Nesvacumab+Aflibercept in Diabetic Macular Edema: The Phase 2 RUBY Trial Jeffrey S. Heier, MD On behalf of the RUBY Investigators Ophthalmic Consultants of Boston
Combination Therapy with Intravitreal Nesvacumab+Aflibercept in Diabetic Macular Edema: The Phase 2 RUBY Trial Jeffrey S. Heier, MD On behalf of the RUBY Investigators Ophthalmic Consultants of Boston
Front Line Diabetic Retinopathy What Not to Miss and Why
 Front Line Diabetic Retinopathy What Not to Miss and Why David M Brown MD FACS Clinical Professor of Ophthalmology Blanton Eye Institute, Houston Methodist Hospital Baylor College of Medicine Retina Consultants
Front Line Diabetic Retinopathy What Not to Miss and Why David M Brown MD FACS Clinical Professor of Ophthalmology Blanton Eye Institute, Houston Methodist Hospital Baylor College of Medicine Retina Consultants
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 2Q17 April May
 BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Outcomes in Diabetic Macular Edema (DME) in Patients Who Used Systemic Dipeptidyl Peptidase-4 (DPP-4) Inhibitors in the VISTA and VIVID trials
 Outcomes in Diabetic Macular Edema (DME) in Patients Who Used Systemic Dipeptidyl Peptidase-4 (DPP-4) Inhibitors in the VISTA and VIVID trials Ehsan Rahimy, MD 1 ; Keith Baker, MD 2 ; Desmond Thompson,
Outcomes in Diabetic Macular Edema (DME) in Patients Who Used Systemic Dipeptidyl Peptidase-4 (DPP-4) Inhibitors in the VISTA and VIVID trials Ehsan Rahimy, MD 1 ; Keith Baker, MD 2 ; Desmond Thompson,
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
2/1/2017. Agenda. David F. Williams, MD, MBA. History of Current Treatment. Current Treatment/Wet AMD Anti-VEGF Drugs. History of Current Treatment
 UPDATE ON CLINICAL TRIALS FOR AMD David F. Williams, MD, MBA Agenda Brief History of Current Rx Recently Completed/New CTs: Wet AMD Recently Completed/New CTs: Dry AMD Current Treatment/Wet AMD Anti-VEGF
UPDATE ON CLINICAL TRIALS FOR AMD David F. Williams, MD, MBA Agenda Brief History of Current Rx Recently Completed/New CTs: Wet AMD Recently Completed/New CTs: Dry AMD Current Treatment/Wet AMD Anti-VEGF
New Developments in the treatment of Diabetic Retinopathy
 New Developments in the treatment of Diabetic Retinopathy B. Jeroen Klevering University Medical Centre Nijmegen - The Netherlands Topics Management of diabetic retinopathy Interventions a. primary (prevention)
New Developments in the treatment of Diabetic Retinopathy B. Jeroen Klevering University Medical Centre Nijmegen - The Netherlands Topics Management of diabetic retinopathy Interventions a. primary (prevention)
Drug Class Update: Vascular Endothelial Growth Factors
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Ophthalmic VEGF Inhibitors. Eylea (aflibercept), Macugen (pegaptanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Diabetic Macular Edema Treatment in the 21st Century
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Treatment of visual impairment due to macular edema secondary to branch retinal vein occlusion (BRVO):
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Treatment of visual impairment due to macular edema secondary to branch retinal
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Treatment of visual impairment due to macular edema secondary to branch retinal
Charles C. Wykoff MD PhD Rahul N. Khurana MD
 HDWallpapers Suprachoroidal Triamcinolone Acetonide with & without Intravitreal Aflibercept for DME: Results of the 6 Month Prospective Phase 1/2 Hulk trial Blanton Eye Institute Charles C. Wykoff MD PhD
HDWallpapers Suprachoroidal Triamcinolone Acetonide with & without Intravitreal Aflibercept for DME: Results of the 6 Month Prospective Phase 1/2 Hulk trial Blanton Eye Institute Charles C. Wykoff MD PhD
Diagnosis and treatment of diabetic retinopathy. Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City
 Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Health Technology Appraisal. Aflibercept for treating diabetic macular oedema.
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
Diabetic Retinopatathy
 Diabetic Retinopatathy Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl Zeiss Meditec Arctic DX Macula Risk Advanced
Diabetic Retinopatathy Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl Zeiss Meditec Arctic DX Macula Risk Advanced
Diabetic Retinopathy: Recent Advances in Treatment and Treatment Approaches
 Diabetic Retinopathy: Recent Advances in Treatment and Treatment Approaches Dr. David Wong Associate Professor Retina Specialist, Department of Ophthalmology & Vision Sciences, University of Toronto, Canada
Diabetic Retinopathy: Recent Advances in Treatment and Treatment Approaches Dr. David Wong Associate Professor Retina Specialist, Department of Ophthalmology & Vision Sciences, University of Toronto, Canada
There are no published randomized, double-blind trials comparing aflibercept to other therapies in neovascular AMD.
 Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial
 ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema With Vision Impairment
 9:30 AM Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema With Vision Impairment Lee Jampol, MD OBJECTIVE To assess subsequent visual and anatomic outcomes of eyes with
9:30 AM Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema With Vision Impairment Lee Jampol, MD OBJECTIVE To assess subsequent visual and anatomic outcomes of eyes with
Diabetic eye disease. Diabetic retinopathy. Sam S. Dahr, M.D. Retina Center of Oklahoma.
 Diabetic eye disease Sam S. Dahr, M.D. Retina Center of Oklahoma www.rcoklahoma.com Downloaded from: The Retina (on 28 May 2007 12:48 AM) 2007 Elsevier Diabetic retinopathy Downloaded from: The Retina
Diabetic eye disease Sam S. Dahr, M.D. Retina Center of Oklahoma www.rcoklahoma.com Downloaded from: The Retina (on 28 May 2007 12:48 AM) 2007 Elsevier Diabetic retinopathy Downloaded from: The Retina
EyeGene Inc. Contact Information Korea Health Industry Development Institute
 EG-Mirotin: First-in-Class recombinant polypeptide novel subcutaneous drug containing RGD-motif targeted to suppress edema and vascular leakage for Nonproliferative Diabetic Retinopathy (NPDR) EyeGene
EG-Mirotin: First-in-Class recombinant polypeptide novel subcutaneous drug containing RGD-motif targeted to suppress edema and vascular leakage for Nonproliferative Diabetic Retinopathy (NPDR) EyeGene
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England. Retinal Vein Occlusion (RVO) with Macular oedema (MO)
 Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema
 Investor Update Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First Major Treatment Advance in More Than 25 Years for Sight-Threatening Condition
Investor Update Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First Major Treatment Advance in More Than 25 Years for Sight-Threatening Condition
Class Update: Vascular Endothelial Growth Factors
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
The Human Eye. Cornea Iris. Pupil. Lens. Retina
 The Retina Thin layer of light-sensitive tissue at the back of the eye (the film of the camera). Light rays are focused on the retina then transmitted to the brain. The macula is the very small area in
The Retina Thin layer of light-sensitive tissue at the back of the eye (the film of the camera). Light rays are focused on the retina then transmitted to the brain. The macula is the very small area in
Clinically Significant Macular Edema (CSME)
 Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab,
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab,
EFFICACY OF INTRAVITREAL TRIAMCINOLONE ACETONIDE FOR THE TREATMENT OF DIABETIC MACULAR EDEMA
 Basrah Journal Of Surgery EFFICACY OF INTRAVITREAL TRIAMCINOLONE ACETONIDE FOR THE TREATMENT OF DIABETIC MACULAR EDEMA Salah Zuhair Abed Al-Asadi MB,ChB, FICMS, Lecturer, Department of Surgery, College
Basrah Journal Of Surgery EFFICACY OF INTRAVITREAL TRIAMCINOLONE ACETONIDE FOR THE TREATMENT OF DIABETIC MACULAR EDEMA Salah Zuhair Abed Al-Asadi MB,ChB, FICMS, Lecturer, Department of Surgery, College
Supplementary Online Content
 Supplementary Online Content Koh A, Lai TYY, Takahashi K, et al; the EVEREST II Study Group. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal
Supplementary Online Content Koh A, Lai TYY, Takahashi K, et al; the EVEREST II Study Group. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal
Clinical Trials in Diabetic Retinopathy. Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D.
 1 Clinical Trials in Diabetic Retinopathy 2018 Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D. Bascom Palmer Eye Institute 900 N.W. 17th Street Miami, FL 33136 Phone: (305) 326-6118 Fax: (305) 326-6417
1 Clinical Trials in Diabetic Retinopathy 2018 Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D. Bascom Palmer Eye Institute 900 N.W. 17th Street Miami, FL 33136 Phone: (305) 326-6118 Fax: (305) 326-6417
Eyes on Diabetics: How to Avoid Blindness in Diabetic Patient
 Eyes on Diabetics: How to Avoid Blindness in Diabetic Patient Rova Virgana FK Unpad Pusat Mata Nasional RS Mata Cicendo Bandung Eye Center (Hospital and Clinic) PIT IDI Jabar 2018 Keys Facts from WHO
Eyes on Diabetics: How to Avoid Blindness in Diabetic Patient Rova Virgana FK Unpad Pusat Mata Nasional RS Mata Cicendo Bandung Eye Center (Hospital and Clinic) PIT IDI Jabar 2018 Keys Facts from WHO
Comparison of BRVO and CRVO management
 Comparison of BRVO and CRVO management Francesco Bandello, MD, FEBO Department of Ophthalmology University Vita-Salute Scientific Institute San Raffaele Milan, Italy 1 Financial Disclosure Advisory Board
Comparison of BRVO and CRVO management Francesco Bandello, MD, FEBO Department of Ophthalmology University Vita-Salute Scientific Institute San Raffaele Milan, Italy 1 Financial Disclosure Advisory Board
Phase 1 Study Results
 The Novel Trap Inhibitor of Vascular Endothelial Growth Factors C/D, OPT-302, and Ranibizumab, an anti-vegf-a Agent, for Wet Age-Related Macular Degeneration: Phase 1 Study Results John A. Wells, III MD
The Novel Trap Inhibitor of Vascular Endothelial Growth Factors C/D, OPT-302, and Ranibizumab, an anti-vegf-a Agent, for Wet Age-Related Macular Degeneration: Phase 1 Study Results John A. Wells, III MD
VEGF Trap-Eye: New Data Confirm Successes in the Treatment of Age-related Macular Degeneration
 Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Final Phase 2 Results Presented at Retina Society Meeting VEGF Trap-Eye: New Data Confirm Successes in the Treatment
Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Final Phase 2 Results Presented at Retina Society Meeting VEGF Trap-Eye: New Data Confirm Successes in the Treatment
Management of Neovascular AMD
 Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION
 THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION MOESTIDJAB DEPARTMENT OF OPHTHALMOLOGY SCHOOL OF MEDICINE AIRLANGGA UNIVERSITY DR SOETOMO HOSPITAL SURABAYA INTRODUCTION
THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION MOESTIDJAB DEPARTMENT OF OPHTHALMOLOGY SCHOOL OF MEDICINE AIRLANGGA UNIVERSITY DR SOETOMO HOSPITAL SURABAYA INTRODUCTION
Vascular Disease Ocular Manifestations of Systemic Hypertension
 Vascular Disease Ocular Manifestations of Systemic Hypertension Maynard L. Pohl, OD, FAAO Pacific Cataract & Laser Institute 10500 NE 8 th Street, Suite 1650 Bellevue, WA 98004 USA 425-462-7664 Cerebrovascular
Vascular Disease Ocular Manifestations of Systemic Hypertension Maynard L. Pohl, OD, FAAO Pacific Cataract & Laser Institute 10500 NE 8 th Street, Suite 1650 Bellevue, WA 98004 USA 425-462-7664 Cerebrovascular
Is OCT-A Needed As An Investigative Tool During The Management Of Diabetic Macular Edema
 Is OCT-A Needed As An Investigative Tool During The Management Of Diabetic Macular Edema Ayman M Khattab MD, FRCS Professor of Ophthalmology Cairo University Diabetic Macular Edema (DME) Diabetic macular
Is OCT-A Needed As An Investigative Tool During The Management Of Diabetic Macular Edema Ayman M Khattab MD, FRCS Professor of Ophthalmology Cairo University Diabetic Macular Edema (DME) Diabetic macular
Intravitreal Ranibizumab or Triamcinolone Acetonide in Combination with Laser Photocoagulation for Diabetic Macular Edema
 Diabetic Retinopathy Clinical Research Network Intravitreal Ranibizumab or Triamcinolone Acetonide in Combination with Laser Photocoagulation for Diabetic Macular Edema Version 6.0 May 21, 2010 Note: See
Diabetic Retinopathy Clinical Research Network Intravitreal Ranibizumab or Triamcinolone Acetonide in Combination with Laser Photocoagulation for Diabetic Macular Edema Version 6.0 May 21, 2010 Note: See
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO
 EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
The Era of anti- - - VEGF Kirk L. Halvorson, OD
 The Era of anti- - - VEGF Kirk L. Halvorson, OD Introduction: Anti- - - Vascular Endothelial Growth Factor (Anti- - - VEGF) medication is a relatively a new line of medications used in treating a variety
The Era of anti- - - VEGF Kirk L. Halvorson, OD Introduction: Anti- - - Vascular Endothelial Growth Factor (Anti- - - VEGF) medication is a relatively a new line of medications used in treating a variety
Marcus Gonzales, OD, FAAO Cedar Springs Eye Clinic
 Marcus Gonzales, OD, FAAO Cedar Springs Eye Clinic 25.6 million adults 11.3% of the adult population 10.9 million adults 65 years and older 26.9% of this age population 79 million people are Pre-diabetic!!
Marcus Gonzales, OD, FAAO Cedar Springs Eye Clinic 25.6 million adults 11.3% of the adult population 10.9 million adults 65 years and older 26.9% of this age population 79 million people are Pre-diabetic!!
Corporate Presentation August 2018
 Corporate Presentation August 218 Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words
Corporate Presentation August 218 Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words
Anti-VEGF drugs & CATT Results. in AMD. Apollo Hospitals, Hyderabad. Mallika Goyal, MD
 Anti-VEGF drugs & CATT Results in AMD Mallika Goyal, MD Apollo Hospitals, Hyderabad Vascular Endothelial Growth Factor Napoleone Ferrara at Genentech 1989 identified & cloned the gene Protein that causes
Anti-VEGF drugs & CATT Results in AMD Mallika Goyal, MD Apollo Hospitals, Hyderabad Vascular Endothelial Growth Factor Napoleone Ferrara at Genentech 1989 identified & cloned the gene Protein that causes
Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration
 Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration International
Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Regeneron start additional Phase 3 Study for VEGF Trap-Eye in Wet Age-related Macular Degeneration International
*Pleasesee amendment forpennsylvaniamedicaid at the endofthis CPB.
 1 of 134 Number: 0701 Policy *Pleasesee amendment forpennsylvaniamedicaid at the endofthis CPB. Aetna considers pegaptanib sodium injection (Macugen) medically necessary for the treatment of individuals
1 of 134 Number: 0701 Policy *Pleasesee amendment forpennsylvaniamedicaid at the endofthis CPB. Aetna considers pegaptanib sodium injection (Macugen) medically necessary for the treatment of individuals
Anti Vascular Endothelial Growth Factor Pharmacotherapy for Diabetic Macular Edema
 Ophthalmic Technology Assessment Anti Vascular Endothelial Growth Factor Pharmacotherapy for Diabetic Macular Edema A Report by the American Academy of Ophthalmology Allen C. Ho, MD, Ingrid U. Scott, MD,
Ophthalmic Technology Assessment Anti Vascular Endothelial Growth Factor Pharmacotherapy for Diabetic Macular Edema A Report by the American Academy of Ophthalmology Allen C. Ho, MD, Ingrid U. Scott, MD,
Treatment for Central-Involved Diabetic Macular Edema in Eyes with Very Good Visual Acuity
 Diabetic Retinopathy Clinical Research Network Treatment for Central-Involved Diabetic Macular Edema in Eyes with Very Good Visual Acuity Version 3.0 April 18, 2014 Treatment of CIDME in Eyes with Good
Diabetic Retinopathy Clinical Research Network Treatment for Central-Involved Diabetic Macular Edema in Eyes with Very Good Visual Acuity Version 3.0 April 18, 2014 Treatment of CIDME in Eyes with Good
CURRENT TRENDS IN DIABETIC MACULAR EDEMA TREATMENT. Muge R. Kesen, MD
 CURRENT TRENDS IN DIABETIC MACULAR EDEMA TREATMENT Muge R. Kesen, MD No relevant financial interest or relationships DISCLOSURE OBJECTIVES Current trends (evidence based) Review of clinical trials Diabetic
CURRENT TRENDS IN DIABETIC MACULAR EDEMA TREATMENT Muge R. Kesen, MD No relevant financial interest or relationships DISCLOSURE OBJECTIVES Current trends (evidence based) Review of clinical trials Diabetic
Diabetic Retinopathy: Managing the Extremes. J. Michael Jumper, MD West Coast Retina
 Diabetic Retinopathy: Managing the Extremes J. Michael Jumper, MD West Coast Retina Case 1: EC 65 y.o. HM No vision complaints Meds: Glyburide Metformin Pioglitazone Va: 20/20 OU 20/20 Case 2: HS 68 y.o.
Diabetic Retinopathy: Managing the Extremes J. Michael Jumper, MD West Coast Retina Case 1: EC 65 y.o. HM No vision complaints Meds: Glyburide Metformin Pioglitazone Va: 20/20 OU 20/20 Case 2: HS 68 y.o.
 measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
Diabetic maculopathy 11/ An update on. Miss Vasuki Sivagnanavel
 Miss Vasuki Sivagnanavel Consultant Ophthalmologist An update on Diabetic maculopathy Despite advances in the management of diabetes, diabetic retinopathy is already the commonest cause of blindness among
Miss Vasuki Sivagnanavel Consultant Ophthalmologist An update on Diabetic maculopathy Despite advances in the management of diabetes, diabetic retinopathy is already the commonest cause of blindness among
6.4 Postmarketing Experience (AMD) 8 USE IN SPECIFIC POPULATIONS (RVO)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUCENTIS safely and effectively. See full prescribing information for LUCENTIS. LUCENTIS (ranibizumab
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUCENTIS safely and effectively. See full prescribing information for LUCENTIS. LUCENTIS (ranibizumab
News Release - 1/5 - Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd.
 News Release Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd. Intravitreal VEGF Inhibitor EYLEA (aflibercept) Solution for Intravitreal Injection Released on the Market for the Treatment of Wet Age-Related
News Release Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd. Intravitreal VEGF Inhibitor EYLEA (aflibercept) Solution for Intravitreal Injection Released on the Market for the Treatment of Wet Age-Related
Diabetic Macular Oedema To treat or not to treat?
 Diabetic Macular Oedema To treat or not to treat? Ms Ranjit Sandhu MBBS MRCOphth MD FRCOphth Consultant Ophthalmic Surgeon Cataract Surgery, Medical Retina & Uveitis The Luton and Dunstable University
Diabetic Macular Oedema To treat or not to treat? Ms Ranjit Sandhu MBBS MRCOphth MD FRCOphth Consultant Ophthalmic Surgeon Cataract Surgery, Medical Retina & Uveitis The Luton and Dunstable University
Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant
 Ophthalmol Ther (2015) 4:51 58 DOI 10.1007/s40123-015-0028-0 CASE REPORT Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant Thomas Bertelmann
Ophthalmol Ther (2015) 4:51 58 DOI 10.1007/s40123-015-0028-0 CASE REPORT Long-Term Follow-Up of Patient with Diabetic Macular Edema Receiving Fluocinolone Acetonide Intravitreal Implant Thomas Bertelmann
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Eylea 40 mg/ml solution for injection in pre-filled syringe. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Eylea 40 mg/ml solution for injection in pre-filled syringe. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: Not Applicable
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Optical Coherence Tomography: Pearls for the Anterior Segment Surgeon Basic Science Michael Stewart, M.D.
 Optical Coherence Tomography: Pearls for the Anterior Segment Surgeon Basic Science Michael Stewart, M.D. Disclosure OCT Optical Coherence Tomography No relevant financial relationships I will refer to
Optical Coherence Tomography: Pearls for the Anterior Segment Surgeon Basic Science Michael Stewart, M.D. Disclosure OCT Optical Coherence Tomography No relevant financial relationships I will refer to
Diabetic Retinopathy Update 2017
 Diabetic Retinopathy Update 2017 Basis for DR management standards for past 40 years Diabetic Retinopathy Study (DRS) 1971-1989 and Early Treatment of Diabetic Retinopathy Study (ETDRS) 1979-1990 Sundeep
Diabetic Retinopathy Update 2017 Basis for DR management standards for past 40 years Diabetic Retinopathy Study (DRS) 1971-1989 and Early Treatment of Diabetic Retinopathy Study (ETDRS) 1979-1990 Sundeep
Diabetic Retinopathy A Presentation for the Public
 Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
The Diabetic Retinopathy Clinical Research Network. Management of DME in Eyes with PDR
 The Diabetic Retinopathy Clinical Research Network Management of DME in Eyes with PDR 1 What Has Been Learned? Diabetic Retinopathy Treatment Protocol F: Results suggest that clinically meaningful differences
The Diabetic Retinopathy Clinical Research Network Management of DME in Eyes with PDR 1 What Has Been Learned? Diabetic Retinopathy Treatment Protocol F: Results suggest that clinically meaningful differences
Anti VEGF Agents in Retinal Disorders Current Scenario
 Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Dexamethasone posterior segment drug delivery system (Ozurdex) for diabetic macular oedema
 Dexamethasone posterior segment drug delivery system (Ozurdex) for diabetic macular oedema This technology summary is based on information available at the time of research and a limited literature search.
Dexamethasone posterior segment drug delivery system (Ozurdex) for diabetic macular oedema This technology summary is based on information available at the time of research and a limited literature search.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Eylea 40 mg/ml solution for injection in pre-filled syringe. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Eylea 40 mg/ml solution for injection in pre-filled syringe. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for
FLUOCINOLONE ACETONIDE: STEROID LONG ACTING
 FLUOCINOLONE ACETONIDE: STEROID LONG ACTING Giuseppe Querques, MD PhD Department of Ophthalmology, IRCCS Ospedale San Raffaele, University Vita Salute San Raffaele, Milan, Italy Financial Disclosure ADVISORY
FLUOCINOLONE ACETONIDE: STEROID LONG ACTING Giuseppe Querques, MD PhD Department of Ophthalmology, IRCCS Ospedale San Raffaele, University Vita Salute San Raffaele, Milan, Italy Financial Disclosure ADVISORY
Diabetic Retinopathy
 Diabetic Retinopathy Diabetes can be classified into type 1 diabetes mellitus and type 2 diabetes mellitus, formerly known as insulin-dependent diabetes mellitus, and non-insulin diabetes mellitus, respectively.
Diabetic Retinopathy Diabetes can be classified into type 1 diabetes mellitus and type 2 diabetes mellitus, formerly known as insulin-dependent diabetes mellitus, and non-insulin diabetes mellitus, respectively.
Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis)
 Aggiornamento biotecnologico: MoAb anti VEGF Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis) Giulia Germena AMD DRY (non-neovascular) Atrophic cell death in the macula No leakage WET
Aggiornamento biotecnologico: MoAb anti VEGF Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis) Giulia Germena AMD DRY (non-neovascular) Atrophic cell death in the macula No leakage WET
Efficacy of intravitreal bevacizumab (Avastin TM ) for shortterm treatment of diabetic macular edema
 111 ORIGINAL Efficacy of intravitreal bevacizumab (Avastin TM ) for shortterm treatment of diabetic macular edema Toshihiko Nagasawa, Takeshi Naito, Shingo Matsushita, Hiroyuki Sato, Takashi Katome, and
111 ORIGINAL Efficacy of intravitreal bevacizumab (Avastin TM ) for shortterm treatment of diabetic macular edema Toshihiko Nagasawa, Takeshi Naito, Shingo Matsushita, Hiroyuki Sato, Takashi Katome, and
Facts About Diabetic Eye Disease
 Facts About Diabetic Eye Disease Points to Remember 1. Diabetic eye disease comprises a group of eye conditions that affect people with diabetes. These conditions include diabetic retinopathy, diabetic
Facts About Diabetic Eye Disease Points to Remember 1. Diabetic eye disease comprises a group of eye conditions that affect people with diabetes. These conditions include diabetic retinopathy, diabetic
Drugs vs. DME. Protocol T, the latest in a string of landmark studies by the Diabetic Retinopathy
 3 Anti-VEGF Drugs vs. DME The latest study from DRCR.net delivers important clinical information, but questions remain. BY MIRIAM KARMEL, CONTRIBUTING WRITER alfred t. kamajian Protocol T, the latest in
3 Anti-VEGF Drugs vs. DME The latest study from DRCR.net delivers important clinical information, but questions remain. BY MIRIAM KARMEL, CONTRIBUTING WRITER alfred t. kamajian Protocol T, the latest in
PART 1: GENERAL RETINAL ANATOMY
 PART 1: GENERAL RETINAL ANATOMY General Anatomy At Ora Serrata At Optic Nerve Head Fundoscopic View Of Normal Retina What Is So Special About Diabetic Retinopathy? The WHO definition of blindness is
PART 1: GENERAL RETINAL ANATOMY General Anatomy At Ora Serrata At Optic Nerve Head Fundoscopic View Of Normal Retina What Is So Special About Diabetic Retinopathy? The WHO definition of blindness is
Bayer to showcase latest Ophthalmology research at EURETINA 2017
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 17 th European Society of Retina Specialists (EURETINA) Congress Bayer to showcase
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 17 th European Society of Retina Specialists (EURETINA) Congress Bayer to showcase
Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study
 Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study Peter A. Campochiaro, MD, 1 Charles C. Wykoff, MD, 2 David M. Brown, MD, 2 David S. Boyer, MD, 3 Mark Barakat,
Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study Peter A. Campochiaro, MD, 1 Charles C. Wykoff, MD, 2 David M. Brown, MD, 2 David S. Boyer, MD, 3 Mark Barakat,
SUMMARY. Heather Casparis, MD,* and Neil M. Bressler, MD MARINA AND ANCHOR
 The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
Treatment of Diabetic Macular Edema without Intravitreal injections
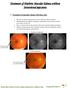 Treatment of Diabetic Macular Edema without Intravitreal injections 1. Treatment of macular edema with laser only This 60 year old female presented on 02-01-2007 for retinal evaluation. She had proliferative
Treatment of Diabetic Macular Edema without Intravitreal injections 1. Treatment of macular edema with laser only This 60 year old female presented on 02-01-2007 for retinal evaluation. She had proliferative
New Insights on Treating Diabetic Retinopathy & Diabetic Macular Edema from the 2018 AAO Meeting
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
NEOVASCULAR AGE-RELATED MACULAR DEGENERation
 Randomized, Double-Masked, -Controlled Trial of Ranibizumab for Neovascular Age-Related Macular Degeneration: PIER Study Year 2 PREMA ABRAHAM, HUIBIN YUE, AND LAURA WILSON PURPOSE: To evaluate efficacy
Randomized, Double-Masked, -Controlled Trial of Ranibizumab for Neovascular Age-Related Macular Degeneration: PIER Study Year 2 PREMA ABRAHAM, HUIBIN YUE, AND LAURA WILSON PURPOSE: To evaluate efficacy
VASCULAR ENDOTHELIAL GROWTH FACTOR INHIBITORS
 VASCULAR ENDOTHELIAL GROWTH FACTOR INHIBITORS FOR OPHTHALMIC USE Policy Number: 2016D0001B Effective Date: January 1, 2016 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION 2 ZALTRAP
VASCULAR ENDOTHELIAL GROWTH FACTOR INHIBITORS FOR OPHTHALMIC USE Policy Number: 2016D0001B Effective Date: January 1, 2016 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION 2 ZALTRAP
Opinion 4 December 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 4 December 2013 LUCENTIS 10 mg/ml, solution for injection Vial of 0.23 ml (CIP: 34009 378 101 5 9) Applicant: Novartis
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 4 December 2013 LUCENTIS 10 mg/ml, solution for injection Vial of 0.23 ml (CIP: 34009 378 101 5 9) Applicant: Novartis
Diabetic Retinopathy. Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012
 Diabetic Retinopathy Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012 Outline Statistics Anatomy Categories Assessment Management Risk factors What do you need to do? Objectives Summarize the
Diabetic Retinopathy Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012 Outline Statistics Anatomy Categories Assessment Management Risk factors What do you need to do? Objectives Summarize the
Macular edema (ME) is the most common
 MANAGEMENT OF RETINAL VEIN OCCLUSIONS * Peter A. Campochiaro, MD ABSTRACT Macular edema (ME) is the most common cause of reduced vision in patients with retinal vein occlusions (RVOs). The primary cause
MANAGEMENT OF RETINAL VEIN OCCLUSIONS * Peter A. Campochiaro, MD ABSTRACT Macular edema (ME) is the most common cause of reduced vision in patients with retinal vein occlusions (RVOs). The primary cause
VMA at the macula resulting in VMT
 Ocriplasmina for pharmacologic treatment in VMT Teresio Avitabile 1 Introduction PVD is a normal, physiologic process that occurs with aging; however, in some cases, PVD is incomplete Incomplete PVD localized
Ocriplasmina for pharmacologic treatment in VMT Teresio Avitabile 1 Introduction PVD is a normal, physiologic process that occurs with aging; however, in some cases, PVD is incomplete Incomplete PVD localized
FULL PRESCRIBING INFORMATION 1 INDICATIONS AND USAGE LUCENTIS is indicated for the treatment of patients with:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUCENTIS safely and effectively. See full prescribing information for LUCENTIS. Diabetic Macular
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUCENTIS safely and effectively. See full prescribing information for LUCENTIS. Diabetic Macular
Evaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular age-related macular degeneration.
 University of Nebraska Medical Center DigitalCommons@UNMC Journal Articles: Ophthalmology Ophthalmology 12-2012 Evaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular
University of Nebraska Medical Center DigitalCommons@UNMC Journal Articles: Ophthalmology Ophthalmology 12-2012 Evaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular
ZEISS AngioPlex OCT Angiography. Clinical Case Reports
 Clinical Case Reports Proliferative Diabetic Retinopathy (PDR) Case Report 969 PROLIFERATIVE DIABETIC RETINOPATHY 1 1-year-old diabetic female presents for follow-up of proliferative diabetic retinopathy
Clinical Case Reports Proliferative Diabetic Retinopathy (PDR) Case Report 969 PROLIFERATIVE DIABETIC RETINOPATHY 1 1-year-old diabetic female presents for follow-up of proliferative diabetic retinopathy
Applications of Sustained Release Delivery Systems in Ocular Disease
 Applications of Sustained Release Delivery Systems in Ocular Disease Formulation and Delivery Systems For Peptide and Protein 2012 December 5, 2012 Christopher A. Rhodes, Ph.D. Principal, Christopher A
Applications of Sustained Release Delivery Systems in Ocular Disease Formulation and Delivery Systems For Peptide and Protein 2012 December 5, 2012 Christopher A. Rhodes, Ph.D. Principal, Christopher A
1/25/2018. Case Management Strategies in Diabetic Retinopathy. Case Study #1: Severe DME. DDOS: 3/31/2016 Va 20/400. Disclosures
 Case Management Strategies in Diabetic Retinopathy Disclosures No financial conflict of interest Will discuss off label use of intraocular Bevacizumab (Avastin) for Diabetic Retinopathy Sundeep Dev, MD
Case Management Strategies in Diabetic Retinopathy Disclosures No financial conflict of interest Will discuss off label use of intraocular Bevacizumab (Avastin) for Diabetic Retinopathy Sundeep Dev, MD
ANTI-VEGF THERAPY FOR DIABETIC MACULAR EDEMA
 January/February 2015 Supplement to ANTI-VEGF THERAPY FOR DIABETIC MACULAR EDEMA A roundtable discussion with Franck Fajnkuchen, MD, and Paolo Lanzetta, MD Case report by Paolo Lanzetta, MD The answers
January/February 2015 Supplement to ANTI-VEGF THERAPY FOR DIABETIC MACULAR EDEMA A roundtable discussion with Franck Fajnkuchen, MD, and Paolo Lanzetta, MD Case report by Paolo Lanzetta, MD The answers
Subclinical Diabetic Macular Edema Study
 Diabetic Retinopathy Clinical Research Network Subclinical Diabetic Macular Edema Study Version 2.0 March 21, 2006 Subclinical DME Protocol v2 0 3-21-06.doc Table of Contents Chapter 1. Background Information
Diabetic Retinopathy Clinical Research Network Subclinical Diabetic Macular Edema Study Version 2.0 March 21, 2006 Subclinical DME Protocol v2 0 3-21-06.doc Table of Contents Chapter 1. Background Information
Clinical Outcomes After Intravitreal Bevacizumab Injection for Diabetic Macular Edema
 Original Article Clinical Outcomes After Intravitreal Bevacizumab Injection for Diabetic Macular Edema Karen Joyce G. Castro, MD, Marie Joan V. Loy, MD International Eye Institute St. Luke s Medical Center
Original Article Clinical Outcomes After Intravitreal Bevacizumab Injection for Diabetic Macular Edema Karen Joyce G. Castro, MD, Marie Joan V. Loy, MD International Eye Institute St. Luke s Medical Center
Vascular Endothelial Growth Factor (VEGF) Inhibitors Ocular Use Drug Class Monograph (Medical Benefit)
 Vascular Endothelial Growth Factor (VEGF) Inhibitors Ocular Use Drug Class Monograph (Medical Benefit) Line of Business: Medi-Cal Effective Date: May 17, 2017 Revision Date: May 17, 2017 This policy has
Vascular Endothelial Growth Factor (VEGF) Inhibitors Ocular Use Drug Class Monograph (Medical Benefit) Line of Business: Medi-Cal Effective Date: May 17, 2017 Revision Date: May 17, 2017 This policy has
Diabetic Retinopathy Clinical Research Network
 Diabetic Retinopathy Clinical Research Network Intravitreous Anti-VEGF Treatment for Prevention of Vision Threatening Diabetic Retinopathy in Eyes at High Risk Version 4.0 September 19, 2017 Anti-VEGF
Diabetic Retinopathy Clinical Research Network Intravitreous Anti-VEGF Treatment for Prevention of Vision Threatening Diabetic Retinopathy in Eyes at High Risk Version 4.0 September 19, 2017 Anti-VEGF
Intravitreal Corticosteroid Implants
 Intravitreal Corticosteroid Implants Policy Number: 9.03.23 Last Review: 4/2018 Origination: 07/2015 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Intravitreal Corticosteroid Implants Policy Number: 9.03.23 Last Review: 4/2018 Origination: 07/2015 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Please see accompanying full Prescribing Information. November 2011
 Fact Sheet For (aflibercept) Injection WHAT IS?, an inhibitor of vascular endothelial growth factor (VEGF), was discovered and developed by Regeneron Pharmaceuticals, Inc. The U.S. Food and Drug Administration
Fact Sheet For (aflibercept) Injection WHAT IS?, an inhibitor of vascular endothelial growth factor (VEGF), was discovered and developed by Regeneron Pharmaceuticals, Inc. The U.S. Food and Drug Administration
ILUVIEN IN DIABETIC MACULAR ODEMA
 1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
Age-related Macular Degeneration (AMD) Update. Michael W. Stewart, MD Professor and Chairman Department of Ophthalmology Mayo Clinic Florida
 Age-related Macular Degeneration (AMD) Update Michael W. Stewart, MD Professor and Chairman Department of Ophthalmology Mayo Clinic Florida Disclosure Alkahest Consultant Allergan Research Support Bayer
Age-related Macular Degeneration (AMD) Update Michael W. Stewart, MD Professor and Chairman Department of Ophthalmology Mayo Clinic Florida Disclosure Alkahest Consultant Allergan Research Support Bayer
