Front Line Diabetic Retinopathy What Not to Miss and Why
|
|
|
- Lenard McCoy
- 5 years ago
- Views:
Transcription
1 Front Line Diabetic Retinopathy What Not to Miss and Why David M Brown MD FACS Clinical Professor of Ophthalmology Blanton Eye Institute, Houston Methodist Hospital Baylor College of Medicine Retina Consultants of Houston Houston, TX USA 1 Relevant Disclosures Consultant/ Grant Support: Regeneron, Bayer, Senju, Allergan, Optos, Zeiss, Heidelberg, OHR, Biotime, Gemini, Genentech/Roche, Novartis, Apellis, Regenxbio, Chengdu Kanghong Biotechnology Co-patent holder on OPTOS de-warping algorithms Genentech/ Roche & OPTOS provided support for Trex DME & DAVE DMB had full control of the presentation 2 Case Study 1 57 Year Old Female Blurry Vision OS Diabetic for 15 years- Hemoglobin A1c = 9.2 Refraction- Plano OD-2/ OS- 2/
2 ETDRS Standardized Eye Chart Outcomes Measure for Clinical Trials Early Treatment Diabetic Retinopathy Study Vision Gain 7 8 ETDRS: Vision Loss Macular Laser for DME Standard of care No impact on underlying disease progression Reduces risk of vision loss, but few patients experience visual improvement Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. - Arch Ophthalmol - 1-DEC-1985; 3(12): Focal Laser Grid Laser Courtesy of Dr Donald D Amico Anti-VEGF Key Studies: Ranibizumab RISE/RIDE: 2 parallel phase III, multicenter, double-masked, sham-injection controlled, randomized studies Assessed safety and efficacy of intravitreal ranibizumab for the treatment of DME In the third year, patients who had received sham therapy were eligible to switch to treatment with ranibizumab
3 Sustained Improvement With Ongoing Anti-VEGF Therapy Mean change in BCVA from baseline (pooled) Effects of Treatment Delay Mean change in BCVA from baseline (pooled) Severe VA loss (15 letters) significantly reduced Rapid improvements in vision & anatomy maintained for 3 years Brown D et al, RISE and RIDE Research Group. Ophthalmology. 213;12: With crossover to 1 year of.5 mg ranibizumab therapy at third year, original sham treatment group s visual gains were lower than those seen in first year of ranibizumab-treated groups (2.8 vs.6 and 11.1 letters) Delayed treatment reduced magnitude of VA benefits of anti-vegf therapy Brown D et al. RISE and RIDE Research Group. Ophthalmology. 213;12: year-old Male With DME Treated With Monthly Ranibizumab Baseline Snellen = 2/ Month 36 Snellen = 2/5 August, 212 First & only FDA approved medication for DME Changed the standard of care for the first time in > 25 years Open Label Extension RISE/RIDE OLE Maintenance of Vision Boyer D, AAO Poster Boyer D, AAO Poster
4 8 Frequent Visits Multiple Injections 2/8 2/32 Long-Term Management Following RIDE & RISE Treatment Frequency Beyond 3 Years Patients (N = 5) %: injections Change VA Months Mean.5 injections Annualized 3.8 injections Anti-VEGF Injections Number of Ranibizumab Injections During OLE (mean follow-up: 1.1 months) OLE Ranibizumab Exposure Anti-VEGF Key Studies: Aflibercept VIVID-DME and VISTA-DME: assessed safety and efficacy of aflibercept vs laser alone in the treatment of DME Treatment groups: intravitreal aflibercept monthly, every 2 months (after 5 initial monthly injections), or laser photocoagulation 21 Boyer D, AAO Poster Korobelnik JF, Brown DM, et al. Ophthalmology. 21 Jul 8. Study Sites Japan Mean Change in BCVA Primary Analysis (LOCF) Europe Austria, Czech Republic, Denmark, France, Germany, Hungary, Italy, Poland, Spain USA ETDRS letters VIVID VISTA Control 2q 2q8 *P<.1 vs control *P<.1 vs control 11.7*.3* 1.6.5*.* VIVID 73 Centers Patients Australia VISTA 5 Centers 61 Patients Week Compared with baseline Primary analysis (LOCF): excludes patients who received rescue treatment; P values are not confirmatory VIVID FAS: control: n=132; IAI 2q: n=136; IAI 2q8: n=135; VISTA FAS: control: n=15; IAI 2q: n=15; IAI 2q8: n=
5 Proportion of Patients Gaining 15 Letters at Week 18 Primary Analysis (LOCF) Proportion of Patients Losing 15 Letters VIVID VISTA Proportion of patients % 8% 6% % 2% % 18.9% VIVID *P<.1 vs control 1.2%* 2.2%* 2.9%* 13.6% VISTA *P<.1 vs control Control 35.8%* 2q 2q8 Proportion of Patients 5% % 3% 2% %.6% 9.1%.7%.%.6%.7% Laser 2q 2q8 Compared with baseline Primary analysis (LOCF): excludes patients who received rescue treatment; P values are not confirmatory VIVID FAS: control: n=132; IAI 2q: n=136; IAI 2q8: n=135; VISTA FAS: control: n=15; IAI 2q: n=15; IAI 2q8: n=151 % 25 VIVID FAS- Laser: n=132; 2q: n=136; 2q8: n=135 VISTA FAS- Laser: n=15; 2q: n=15; 2q8: n=151 Compared to baseline; LOCF 26 Long-Term Management Following VISTA Treatment Frequency Beyond 3 Years July 29, 21 Diabetic Macula Edema Number of Patients % 23% 27% 75% None 1 to 5 6 to 11 to to 2 >2 Number of PRN Injections 12% Year 1 Weighted Mean =.5 Year 2 Weighted Mean = 3. Cumulative Weighted Mean = 7.7 8% 5% 27 Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Study of Aflibercept, Bevacizumab, or Ranibizumab for DME Supported through a cooperative agreement from the Baseline Randomization Randomly Assigned Eyes (one per participant): N = 66 Aflibercept (2. mg) N = 22 Bevacizumab (1.25 mg) N = 218 Ranibizumab (.3 mg) N = 218 National Eye Institute; National Institute of Diabetes and Digestive and Kidney Diseases; National Institutes of Health, Department of Health and Human Services EY1231, EY1229, EY18817 One Year N = 28 (93%) N = 26 (9%) N = 26 (9%) One Year Excluding Deaths 9% 97% 96%
6 *P values adjusted for baseline visual acuity and multiple evaluations. 1. Diabetic Retinopathy Clinical Research Network. N Engl J Med. 215;372(13): Wells JA et al. Ophthalmology. 216 Feb 27. [Epub ahead of print] 52 Week Treatment Group Comparison*: Aflibercept vs Bevacizumab P=.69 Aflibercept vs Ranibizumab P=.69 Ranibizumab vs Bevacizumab P=.69 Week Treatment Group Comparison*: Aflibercept vs Bevacizumab P=.51 Aflibercept vs Ranibizumab P=.51 Ranibizumab vs Bevacizumab P=.31 *P values adjusted for baseline visual acuity and multiple evaluations. 1. Diabetic Retinopathy Clinical Research Network. N Engl J Med. 215;372(13): Wells JA et al. Ophthalmology. 216 Feb 27. [Epub ahead of print] 52 Week Treatment Group Comparison*: Aflibercept vs Bevacizumab P<.1 Aflibercept vs Ranibizumab P=.3 Ranibizumab vs Bevacizumab P=.21 Week Treatment Group Comparison*: Aflibercept vs Bevacizumab P=.2 Aflibercept vs Ranibizumab P=.18 Ranibizumab vs Bevacizumab P=.18 Visual acuity gains varied based on anti-vegf at the primary endpoint of 1 year and were maintained through 2 years 1,2 Overall Mean Change in Visual Acuity Letter Score from Baseline to 2 Years Visual acuity gains varied by baseline visual acuity Mean Change in Visual Acuity Letter Score from Baseline to 2 Years by Baseline Visual Acuity Mean Change From Baseline in Visual Acuity Letter Score Aflibercept Bevacizumab Ranibizumab Primary Endpoint: 52 Week Treatment Week Treatment Group Comparison*: Group Comparision*: Aflibercept vs Bevacizumab P=.2 Aflibercept vs Bevacizumab P<.1 Aflibercept vs Ranibizumab P=.7 Aflibercept vs Ranibizumab P=.3 Ranibizumab vs Bevacizumab P=.11 2 Ranibizumab vs Bevacizumab P= Week Mean Change in Visual Acuity Letter Score /32 2/ (51% of participants) Week /5 or Worse (9% of participants) Week Aflibercept Bevacizumab Case Study 2 35 Year Old Male- Failed DPS test Just need glasses Diabetic for 5 years- Hemoglobin A1c = Unknown Doesn t want dilation-- Need to work tonight Refraction ish 2/25 OU
7 Diabetic Retinopathy 25% of patients with diabetes have some retinopathy 5,, in US Leading cause of visual loss & new-onset blindness 2 6 Pathophysiology of Diabetic Retinopathy Capillary pericyte loss Endothelial cell loss Nonfunctional acellular capillaries Capillary basement membrane thickening Microaneurysm formation Neovascularization Normal vessels Diabetic vessels 37 Frank RN. Etiologic mechanisms in diabetic retinopathy. In: Ryan SJ, ed. Retina, Schachat AP and Murphy RP, eds vol. 2 Medical Retina, St. Louis, 199, Mosby Photos copyright acknowledgement to publication. 38 Retinopathy Progression and HbA1c Diabetic Retinopathy: More Prevalent With Longer Disease Duration and Higher A1C Levels N = 333 Patients With Diabetic Retinopathy (%) < y -8 y >8 y Duration of Diabetes <6.3 >7.5 A1C 39 DCCT Research Group. N Engl J Med. 1993;329: Chart-based analysis of study patients with known type 2 diabetes. A1C = glycosylated hemoglobin. Tapp RJ, et al. Diabetes Care. 23;26: Prevalence of Proliferative Retinopathy Type I DM 15 years duration: 3% Treatments > 9% Effective at Preventing Blindness Earlier Tx = Better Outcomes Type II DM Receiving insulin: 15 years duration: 15% 2% Not receiving insulin: 15 years duration: 5% % Redrawn from Klein R, Klein BEK, et al. Arch Ophthalmol 2:52 526, 198 in Frank RN. Etiologic mechanisms in diabetic retinopathy. In Ryan SJ, ed: Retina, Schachat AP and Murphy RP, eds. vol. 2 Medical Retina, St. Louis, 199, Mosby, p
8 3 5 6 Protocol S Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: Randomized Clinical Trial DRCR.net Study Design Randomized, multi-center clinical trial (55 Sites) Study eye(s) meeting all of the following criteria (a participant can have 2 study eyes): PDR No history of PRP Best corrected visual acuity letter score 2 (~Snellen equivalent 2/32 or better) Eyes with or without central-involved DME were eligible Primary Objective: Compare the efficacy and safety of PRP with that of intravitreous ranibizumab (.5-mg in.5 ml) for proliferative diabetic retinopathy (PDR) Diabetic Retinopathy Clinical Research Network (DRCR.net): 8
9 Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreal Ranibizumab for Proliferative Diabetic Retinopathy PRP Baseline then additional PRP if size or amount of NV increased Ranibizumab Q through W2 with deferral option at W16 & W2 if NV resolved After W2 = Q if NV improved or worsened, deferral if NV resolved or stable after 2 consecutive injections Mean Number of Injections 5-Year Completers Only Ranibizumab Group (N = 117) PRP Group (N = 123) Year Year Year Year Year Cumulative Through 5 Years Gross JG, et al. JAMA Ophthalmol. 218;136(): Visual Acuity Visual Acuity at 5-Years Ranibizumab (N = 117) PRP (N = 123) Mean letter score 8 81 ~Snellen Equivalent, Mean 2/25 2/25 Median letter score (25 th, 75 th percentile) ~Snellen Equivalent, Median 8 (89, 78) 2/2 (2/16, 2/32) 8 (89, 77) 2/2 (2/16, 2/32) Mean Change in VA Letter Score Mean Changes in VA From Baseline Over Time - Overall Cohort 15 5 Adjusted Mean Difference at 5 Years: +.6 letters 95% Confidence Interval: (-2.3, +3.5), P =.68 Ranibizumab PRP N = 191 Visit Week N = 117 N = 23 N = 123 Outlying values were truncated to 3 SD from the mean Mean Change in Cumulative Visual Field Total Point Score ( ) - Overall Cohort Mean Change in Cumulative Visual Field Total Point Score ( ) - Overall Cohort Mean Change in Cumulative Visual Field Total Point Score (db) Ranibizumab PRP 52 N = 81 N = 1 Visit Week N = 86 N = 38 Outlying values were truncated to 3 SD from the mean Mean Change in Cumulative Visual Field Total Point Score (db) Year Adjusted Mean Difference: 28 db 95% Confidence Interval (-9, 8), P =. Ranibizumab PRP N = 81 N = 1 Visit Week N = 86 N = 38 Outlying values were truncated to 3 SD from the mean
10 DR Adverse Events: Over 5 Years Ranibizumab (N = 117) PRP (N = 123) Any Retinal detachment, % 6% 15% Retinal Detachment involving Center of the Macula, % 1% % Neovascular Glaucoma, % 3% % Neovascularization of the Iris, % 3% 1% Vitreous Hemorrhage, % 8% 6% Vitrectomy, % 11% 19% Adjusted Difference (95% CI) -9% (-1%, -%) -3% (-7%, %) -2% (-6%, 2%) 1% (-1%, 3%) 2% (-6%, 11%) -7% (-1%, -1%) CLARITY: 22 UK Centers Phase 2b Trial PRP versus aflibercept for PDR Included treatment naïve PDR (53%) and patients with PDR previously treated with PRP (7%) Different from Protocol S which only included treatment naïve Excluded patients with DME Different from Protocol S which included both PDR with and without DME CLARITY: Study Population CLARITY (N=232) Adults with type 1 or 2 diabetes mellitus; active PDR (defined as treatment naive OR persistent retinal neovascularization after initial PRP requiring additional PRP); no baseline macular edema; and BCVA letter score 5 (~Snellen equivalent 6/2 or better) Randomized 1:1 Aflibercept arm PRP arm (n=116) (n=116) Treatment-naive Active PDR Treatment-naive Active PDR PDR post initial PRP PDR post initial PRP n=6 (52%) n=56 (8%) n=63 (5%) n=53 (6%) Week 52: Primary endpoint Primary outcome: Change in BCVA from baseline to Week 52 in the study eye of the aflibercept group relative to the PRP group CLARITY VA Results At 52-weeks (primary outcome): BCVA difference between groups = letters (P<.1) Laser group lost 2.9 letters Aflibercept group gained 1.3 letters At 12-weeks (secondary outcome): BCVA difference between groups = 2.3 letters Laser group lost.9 letters Aflibercept group gained 1.5 letters Other Findings in CLARITY: Presence of Macular Edema at Week 52 CST and cube volume increased significantly in the PRP group compared to aflibercept At 52-weeks: 89% of patients in the aflibercept group did not have CME 71% of patients in the PRP group did not have CME Proportion of patients (%) (Pearson s chi-squared test comparing multiple categories between arms) 89% P=.7 9% n=93 n=9 Aflibercept No macular edema Central macular edema 3% n=3 71% 21% n=7 n=22 n=8 PRP (n=) (n=5 1 ) 8% Non-central macular edema Case Study 3 2 Year Old School Teacher- Annual Exam Diabetic for 12 years- Hemoglobin A1c = 8.1 Refraction /2 OU 6
11 61 62 Classification of Diabetic Retinopathy
12 Microaneurysms, Dot/ Blot Hemorrhages IRMA, Venous Beading, Soft Exudates (CWS) Hard Exudates NVE NVD Vitreous Heme Fibrosis Standard Fields Modified for DRS Modified for ETDRS Modified for DRS 199s- Modified for ETDRS 69 The ETDRS DR Severity Scale is a well established and validated scale for evaluation of DR severity DR severity is graded based on 7-field fundus photography, a standardized method for detection of DR First applications of standardized retinal imaging to clinical trials 3-degree overlapping stereo photographs: 9 3% ETDRS Diabetic Retinopathy Severity Scale Illustrated Using Images from RIDE/RISE DRSS = Highly Predictive of PDR Development, 12 DR absent 1, 15, 2 DR questionable 35 Mild NPDR 3 Moderate NPDR 7 Moderately Severe NPDR 53 Severe NPDR 6, 61 Mild PDR 65 Moderate PDR 71 High-risk PDR 75 High-risk PDR 81 Advanced PDR 85 Advanced PDR Eyes Progressing to PDR 8% 66% 71% 8% 52% 5 Years 26% 3 Years 1 Year Level 35 Level 3 Level 7 Level 53 Mild NPDR Moderate NPDR Moderately Severe NPDR Severe NPDR Baseline Severity of DR ETDRS Report 12. Ophthalmology 1991 N at 1 year = 1958; 3 years = 176; 5 years =
13 Incidence of DME Increases With DR Severity Progressive Drop in Health-Related QOL When DRSS >3 9 Patients With DME, % % Mild NPDR (n = 228) 39% Moderate NPDR (n = 11) 77% Severe NPDR (n = 7) NEI-VFQ-25 Scores Driving Difficulty Score NEI-VFQ-25 Composity Score / 2/<2 2/2 31/<31 31/31 37/<37 37/37 3/<3 3/3 7/<7 7/7 DR Severity (ETDRS Scale) 53/<53 53/53 6/<6+ 6+/6+ Worse QOL Ranibizumab: Slowing Progression of PDR in RIDE/RIDE Time to Development of PDR Event Crossover: sham to ranibizumab Pharmaceutical Dosing Can Slow Progression to PDR Cumulative probabilities calculated using the Kaplan-Meier method. Progression was defined by (1) progression from NPDR (DR severity level < 6) at baseline to PDR (DR severity level 6) at a later time point, (2) need for PRP laser, (3) vitreous hemorrhage (AE or slit lamp grade at baseline to > at a later time point, () cases identified by ophthalmoscopy, (5) vitrectomy, (6) iris neovascularization AE, or (7) retinal neovascularization AE. Case Study: 3-Step DR Improvement from RIDE/RISE in a Ranibizumab-Treated Patient With PDR at Baseline Pharmaceutical Dosing Can Improve DR Severity Screening High risk PDR (71A) Month 36 Mild NPDR (35E) 6 Level Improvement 13
14 DR Severity Improvements During RIDE/RISE DRSS Improvements 3 Years DR Severity Improvements During VISTA/VIVID 2-Step DRSS Improvements 3 Years Patients, % Sham/Crossover (n = 257).3 mg RBZ (n = 25).5 mg RBZ (n = 252) 1/3 rd Month Month Month Month 6 Month 12 Month 2 Month Month 36 2-Step Improvement 3-Step Improvement IAI 2q8 IAI 2q Control VIVID *P<.1 versus control 17.% 7.8%.3% * IAI 2q % *.% 2.%.% 6.% 8.%.% * Proportion of Patients IAI 2q8 Control VISTA *P<.5 versus control **P<.1 versus control % 3.% **.% 2.%.% 6.% 8.%.% Proportion of Patients In RIDE/RISE the Benefit of Ranibizumab on DR was Greatest in Patients With Baseline Moderately Severe or Severe NPDR (Level 7/53) 8 Sham Ranibizumab.3 mg Ranibizumab.5 mg 2-Step DR Severity Improvement at Month 2 78% 81% ETDRS Diabetic Retinopathy Severity Scale Illustrated Using Images from RIDE/RISE, 12 DR absent 1, 15, 2 DR questionable 35 Mild NPDR 3 Moderate NPDR 7 Moderately Severe NPDR 53 Severe NPDR Patients, % 6 2 1% 36% 31% 16% % 12% 7% 35/ /53 Without Prior PRP 6, 61 Mild PDR 65 Moderate PDR 71 High-risk PDR 75 High-risk PDR 81 Advanced PDR 85 Advanced PDR Baseline DR Severity Level n = DR, diabetic retinopathy; PRP, pan-retinal photocoagulation. >75% of Ranibizumab-Treated Patients With Moderately Severe or Severe NPDR (Level 7/53) had a 2-Step DR Improvement by Month 12 Patients, % Sham/ Crossover (n = 86) Ranibizumab.3 mg (n = 88) Ranibizumab.5 mg (n = 7) Month 2-Step Improvement a 3-Step Improvement b Management of NPDR without DME PANORAMA a All sham vs ranibizumab comparisons for 2-step improvement, P <.1. b All sham vs ranibizumab comparisons from month 12 on for 3-step improvement, P.2. 1
15 PANORAMA Study Design Inclusion & Exclusion Criteria Phase 3, Double-masked, Randomized, Study of the Efficacy & Safety of Intravitreal Aflibercept in patients with moderately severe to severe NPDR (DRSS Level 7 and 53) N=2 Sham N=133 + after 3 initial monthly doses and 1 q8 interval *after 5 initial monthly doses, flexible treatment schedule after week 52 **Patients were stratified by baseline DRSS level Group 1 IAI 2 mg Q16 weeks + N=135 Week 2 and Week 52 Primary Endpoint Proportion of patients improving 2 steps on DRSS Groups 1 & 2 combined Group 2 IAI 2 mg Q8 weeks* N=13 Follow up through Week Wykoff, CC. Key points from the Phase 3 PANORAMA Study. (July 218) American Society of Retina Specialists, Annual Meeting, Vancouver, BC, CA. Inclusion Moderately severe to severe NPDR (DRSS levels 7 or 53), confirmed by the central reading center, in whom PRP could be safely deferred for 6 months BCVA ETDRS letter score of 69 letters ( Snellen equivalent of 2/) Exclusion Presence of DME threatening the center of the macula Evidence of retinal neovascularization Any prior treatment with: Focal or grid laser photocoagulation or PRP Systemic or intravitreal anti-vegf agents Intraocular steroids Current ASNV, vitreous hemorrhage, or traction retinal detachment HbA1c >12% or HbA1c 12% with uncontrolled diabetes mellitus Uncontrolled blood pressure History of cerebrovascular accident or myocardial infarction within 6 months of study start Dosing Schedule Week: BL SHAM* O O O O O O O O O O Group 1* X X X O X O X O X O Group 2* X X X X X X X X X + X=active injection, O=sham injection 1 dose difference between Group 1 & 2 through week 2 +Group 2 (Q8) group continues PRN through Week based on DRSS level *Patients progressing to PDR/ASNV or CI-DME were eligible for rescue treatment (IAI or laser) at the discretion of the investigator. Data for patients receiving rescue treatment was censored from the time of rescue. Baseline Demographics Sham Group 1 Group 2 All IAI Total N (FAS, SAF) Age (years (SD)) 55.8 (.31) 55. (11.13) 55.8 (.19) 55.6 (.66) 55.7 (.53) Women # (%) 6 (8.1%) 6 (.%) 53 (39.6%) 113 (2.%) 177 (.%) Race # (%) White 7 (8.5%) 99 (73.3%) (77.6%) 23 (75.5%) 3 (77.1%) Black or African American 13 (9.8%) 16 (11.9%) 12 (9.%) 28 (.%) 1 (.2%) Asian (3.%) 12 (8.9%) 7 (5.2%) 19 (7.1%) 23 (5.7%) Other 9 (6.8%) 8 (5.9%) 11 (8.2%) 19 (7.1%) 28 (7.%) Hemoglobin A1C (%) 8.5 (1.5) 8.6 (1.69) 8. (1.6) 8.5 (1.66) 8.5 (1.62) Duration of Diabetes (years (SD)) 15.5 (9.3) 13.7 (8.61) 1. (9.69) 13.8 (9.15) 1. (9.2) Diabetes Type (92.5%) 121 (89.6%) 12 (92.5%) 25 (91.1%) 368 (91.5%) Group 1: 3 monthly doses followed by 1 Q8 interval then Q16, Group 2: 5 monthly doses then Q8 Baseline Disease Characteristics & Disposition N (FAS/SAF) ETDRS BCVA (letters) Mean (SD) Snellen Equivalent CRT(microns) Mean (SD) Sham Group 1 Group 2 All IAI Total 82.7 (6.3) 2/ (38.1) 82.2 (6.63) 2/ (3.3) 82.3 (5.15) 2/ (31.59) 82.3 (5.93) 2/ (32.9) 82. (5.96) 2/ (3.82) Diabetic Retinopathy Severity Score (DRSS) Level 7 99 (7.%) 2 (75.6%) 1 (75.%) 23 (75.5%) 32 (75.1%) Level 53 3 (25.6%) 33 (2.%) 33 (2.6%) 66 (2.5%) (2.9%) Number of Injections Treatment Experience # Active Injections (out of for Group 1 and 5 for Group 2) Number of Patients who Completed at Week (89.5%) 129 (95.6%) 132 (98.5%) 261 (97.%) 38 (9.5%) Group 1: 3 monthly doses followed by 1 Q8 interval then Q16, Group 2: 5 monthly doses then Q8 Group 1: 3 monthly doses followed by 1 Q8 interval then Q16, Group 2: 5 monthly doses then Q8 Sham n=133, Group 1 n=135, Group 2 n=13, All IAI n=269 15
16 Proportion of Patients with 2-Step Improvement from Baseline in DRSS Mean Change in Best Corrected Visual Acuity Proportion of Patients ** ** * ETDRS letters /133 83/135 7/13 157/269 Group 1: 3 monthly doses followed by 1 Q8 interval then Q16, Group 2: 5 monthly doses then Q8 Sham n=133, Group 1 n=135, Group 2 n=13, All IAI n=269 Week Group 1: 3 monthly doses followed by 1 Q8 interval then Q16, Group 2: 5 monthly doses then Q8 Sham n=133, Group 1 n=135, Group 2 n=13, All IAI n=269 Nominal p =.57 All IAI vs. sham Nominal p =.19 Group 1 vs. sham Nominal p =.139 Group 2 vs. sham Mean Change in Central Retinal Thickness Safety Events Through Week 2 μm Sham All IAI N (FAS/SAF) Ocular TEAEs ( 3%) Conjunctival hemorrhage 5 (3.8%) 32 (11.9%) Vitreous floaters 1 (.8%) 1 (5.2%) Diabetic retinal edema 2 (15.%) 11 (.1%) Eye pain 2 (1.5%) 11 (.1%) Diabetic retinopathy (3.%) 1 (.%) Week Nominal p <.1 All vs. sham Group 1: 3 monthly doses followed by 1 Q8 interval then Q16, Group 2: 5 monthly doses then Q8 Sham n=133, Group 1 n=135, Group 2 n=13, All IAI n=269 Non Ocular Events Patients with 1 APTC, n (%) 2 (1.5%) 1 (.%) Deaths 3 (2.3%) One Serious ocular AE of iris neovascularization occurred in 1 patient One ocular AE of vitreal cells occurred in 1 patient, which was considered mild Proportion of Patients Developing a Vision Threatening Complication (VTC) or Center Involved (CI)-DME through Week 2 Sham Group 1 Group 2 All IAI N (SAF) % Patients Developing PDR/ASNV or CI-DME % Developing PDR/ASNV 3/133 (25.6%) 18/133 (13.5% % Developing CI-DME + 18/125 (1.%) 5/135 (3.7%) 2/135 (1.5%) 3/128 (2.3%) 7/13 (5.2%) 1/13 (.7%) 6/128 (.7%) 12/269 (.5%) 3/269 (1.1%) 9/256 (3.5%) PANORAMA Week 52 Outcomes Proportion of Patients PDR/ASNV or PDR/ASNV CI-DME + CI-DME + Group 1: 3 monthly doses followed by 1 Q8 interval then Q16, Group 2: 5 monthly doses then Q8. Exploratory analysis. VTC = PDR/ASNV PDR/ASNV: Proliferative Diabetic Retinopathy/Anterior Segment Neovascularization; CI-DME: Central involved DME + CI-DME evaluable set excluded patients who, at baseline, both had CRT>3μm and qualitative evidence of CI-DME as assessed by the reading center. 16
17 Proportion of Patients with 2-Step Improvement from Baseline in DRSS Proportion of Patients with 2-Step Improvement from Baseline in DRSS Proportion of Patients % 9% 8% 8% 7% 65% 6% 5% p<.1 vs. sham % 3% 2% 15% % % Sham Group 1 Q16 Group 2 Q8 Aflibercept Improves Diabetic Retinopathy and Reduces Vision Threatening Complications in Phase 3 Trial; October 25, Proportion of Patients Developing a Vision Threatening Complication (VTC) or Center Involved (CI)-DME 5% % 1% Front Line Diabetic Retinopathy What Not to Miss and Why Proportion of Patients % 35% 3% 25% 2% 15% % 5% % 11% p<.1 vs. sham DME PDR NPDR % Sham Group 1 Q16 Group 2 Q8 Aflibercept Improves Diabetic Retinopathy and Reduces Vision Threatening Complications in Phase 3 Trial; October 25,
Diabetic Retinopathy: Recent Advances in Treatment and Treatment Approaches
 Diabetic Retinopathy: Recent Advances in Treatment and Treatment Approaches Dr. David Wong Associate Professor Retina Specialist, Department of Ophthalmology & Vision Sciences, University of Toronto, Canada
Diabetic Retinopathy: Recent Advances in Treatment and Treatment Approaches Dr. David Wong Associate Professor Retina Specialist, Department of Ophthalmology & Vision Sciences, University of Toronto, Canada
Diagnosis and treatment of diabetic retinopathy. Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City
 Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
Diabetic Retinopathy: Managing the Extremes. J. Michael Jumper, MD West Coast Retina
 Diabetic Retinopathy: Managing the Extremes J. Michael Jumper, MD West Coast Retina Case 1: EC 65 y.o. HM No vision complaints Meds: Glyburide Metformin Pioglitazone Va: 20/20 OU 20/20 Case 2: HS 68 y.o.
Diabetic Retinopathy: Managing the Extremes J. Michael Jumper, MD West Coast Retina Case 1: EC 65 y.o. HM No vision complaints Meds: Glyburide Metformin Pioglitazone Va: 20/20 OU 20/20 Case 2: HS 68 y.o.
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 2Q17 April May
 BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
1/25/2018. Case Management Strategies in Diabetic Retinopathy. Case Study #1: Severe DME. DDOS: 3/31/2016 Va 20/400. Disclosures
 Case Management Strategies in Diabetic Retinopathy Disclosures No financial conflict of interest Will discuss off label use of intraocular Bevacizumab (Avastin) for Diabetic Retinopathy Sundeep Dev, MD
Case Management Strategies in Diabetic Retinopathy Disclosures No financial conflict of interest Will discuss off label use of intraocular Bevacizumab (Avastin) for Diabetic Retinopathy Sundeep Dev, MD
Financial Disclosures
 Financial Disclosures DB is a scientific advisor for Regeneron/ Bayer and Genentech/ Roche & a DB member of the Regeneron Combination Products Steering Committee RCH receives grant support from Regeneron/
Financial Disclosures DB is a scientific advisor for Regeneron/ Bayer and Genentech/ Roche & a DB member of the Regeneron Combination Products Steering Committee RCH receives grant support from Regeneron/
Outcomes of Intravitreal Anti-VEGF Therapy for Diabetic Macular Edema in Routine Clinical Practice
 Outcomes of Intravitreal Anti-VEGF Therapy for Diabetic Macular Edema in Routine Clinical Practice John D. Pitcher, MD 1,2 ; Andrew A. Moshfeghi, MD 3 ; Genevieve Lucas, B. Comm. 4 ; Nick Boucher, BS 4
Outcomes of Intravitreal Anti-VEGF Therapy for Diabetic Macular Edema in Routine Clinical Practice John D. Pitcher, MD 1,2 ; Andrew A. Moshfeghi, MD 3 ; Genevieve Lucas, B. Comm. 4 ; Nick Boucher, BS 4
Diabetic Retinopatathy
 Diabetic Retinopatathy Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl Zeiss Meditec Arctic DX Macula Risk Advanced
Diabetic Retinopatathy Jay M. Haynie, OD, FAAO Financial Disclosure I have received honoraria or am on the advisory board for the following companies: Carl Zeiss Meditec Arctic DX Macula Risk Advanced
Treatment for Central-Involved Diabetic Macular Edema in Eyes with Very Good Visual Acuity
 Diabetic Retinopathy Clinical Research Network Treatment for Central-Involved Diabetic Macular Edema in Eyes with Very Good Visual Acuity Version 3.0 April 18, 2014 Treatment of CIDME in Eyes with Good
Diabetic Retinopathy Clinical Research Network Treatment for Central-Involved Diabetic Macular Edema in Eyes with Very Good Visual Acuity Version 3.0 April 18, 2014 Treatment of CIDME in Eyes with Good
Diabetic Retinopathy Clinical Research Network
 Diabetic Retinopathy Clinical Research Network Intravitreous Anti-VEGF Treatment for Prevention of Vision Threatening Diabetic Retinopathy in Eyes at High Risk Version 4.0 September 19, 2017 Anti-VEGF
Diabetic Retinopathy Clinical Research Network Intravitreous Anti-VEGF Treatment for Prevention of Vision Threatening Diabetic Retinopathy in Eyes at High Risk Version 4.0 September 19, 2017 Anti-VEGF
Diabetic Retinopathy Update 2017
 Diabetic Retinopathy Update 2017 Basis for DR management standards for past 40 years Diabetic Retinopathy Study (DRS) 1971-1989 and Early Treatment of Diabetic Retinopathy Study (ETDRS) 1979-1990 Sundeep
Diabetic Retinopathy Update 2017 Basis for DR management standards for past 40 years Diabetic Retinopathy Study (DRS) 1971-1989 and Early Treatment of Diabetic Retinopathy Study (ETDRS) 1979-1990 Sundeep
Diabetic Macular Edema Treatment in the 21st Century
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Eyes on Diabetics: How to Avoid Blindness in Diabetic Patient
 Eyes on Diabetics: How to Avoid Blindness in Diabetic Patient Rova Virgana FK Unpad Pusat Mata Nasional RS Mata Cicendo Bandung Eye Center (Hospital and Clinic) PIT IDI Jabar 2018 Keys Facts from WHO
Eyes on Diabetics: How to Avoid Blindness in Diabetic Patient Rova Virgana FK Unpad Pusat Mata Nasional RS Mata Cicendo Bandung Eye Center (Hospital and Clinic) PIT IDI Jabar 2018 Keys Facts from WHO
Diabetic Retinopathy
 Diabetic Retinopathy Diabetes can be classified into type 1 diabetes mellitus and type 2 diabetes mellitus, formerly known as insulin-dependent diabetes mellitus, and non-insulin diabetes mellitus, respectively.
Diabetic Retinopathy Diabetes can be classified into type 1 diabetes mellitus and type 2 diabetes mellitus, formerly known as insulin-dependent diabetes mellitus, and non-insulin diabetes mellitus, respectively.
Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema With Vision Impairment
 9:30 AM Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema With Vision Impairment Lee Jampol, MD OBJECTIVE To assess subsequent visual and anatomic outcomes of eyes with
9:30 AM Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema With Vision Impairment Lee Jampol, MD OBJECTIVE To assess subsequent visual and anatomic outcomes of eyes with
Combination Therapy with Intravitreal Nesvacumab+Aflibercept in Diabetic Macular Edema: The Phase 2 RUBY Trial
 Combination Therapy with Intravitreal Nesvacumab+Aflibercept in Diabetic Macular Edema: The Phase 2 RUBY Trial Jeffrey S. Heier, MD On behalf of the RUBY Investigators Ophthalmic Consultants of Boston
Combination Therapy with Intravitreal Nesvacumab+Aflibercept in Diabetic Macular Edema: The Phase 2 RUBY Trial Jeffrey S. Heier, MD On behalf of the RUBY Investigators Ophthalmic Consultants of Boston
Clinical Trials in Diabetic Retinopathy. Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D.
 1 Clinical Trials in Diabetic Retinopathy 2018 Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D. Bascom Palmer Eye Institute 900 N.W. 17th Street Miami, FL 33136 Phone: (305) 326-6118 Fax: (305) 326-6417
1 Clinical Trials in Diabetic Retinopathy 2018 Harry W. Flynn Jr., M.D. Nidhi Relhan Batra, M.D. Bascom Palmer Eye Institute 900 N.W. 17th Street Miami, FL 33136 Phone: (305) 326-6118 Fax: (305) 326-6417
8/8/17. Objectives. Proliferative Diabetic Retinopathy (PDR) Examining the Diabetic Patient: What Matters Most
 Objectives Examining the Diabetic Patient: What Matters Most Jordan Keith, OD, FAAO Minneapolis, MN Simplify strategy for evaluating the eyes of diabetics Identify the threats to vision and treatments
Objectives Examining the Diabetic Patient: What Matters Most Jordan Keith, OD, FAAO Minneapolis, MN Simplify strategy for evaluating the eyes of diabetics Identify the threats to vision and treatments
Clinically Significant Macular Edema (CSME)
 Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Charles C. Wykoff MD PhD Rahul N. Khurana MD
 HDWallpapers Suprachoroidal Triamcinolone Acetonide with & without Intravitreal Aflibercept for DME: Results of the 6 Month Prospective Phase 1/2 Hulk trial Blanton Eye Institute Charles C. Wykoff MD PhD
HDWallpapers Suprachoroidal Triamcinolone Acetonide with & without Intravitreal Aflibercept for DME: Results of the 6 Month Prospective Phase 1/2 Hulk trial Blanton Eye Institute Charles C. Wykoff MD PhD
Epidemiology and Pathophysiology of Diabetic Retinopathy
 Epidemiology and Pathophysiology of Diabetic Retinopathy Vincent Reppucci, MD Director, Retina Service Mt. Sinai St. Luke s-roosevelt Hospital Attending Physician, Retina Service New York Eye and Ear Infirmary
Epidemiology and Pathophysiology of Diabetic Retinopathy Vincent Reppucci, MD Director, Retina Service Mt. Sinai St. Luke s-roosevelt Hospital Attending Physician, Retina Service New York Eye and Ear Infirmary
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME
 Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Clinical results of OPT-302 (VEGF-C/D Trap ) Combination Treatment in namd and DME Ophthalmology Innovation Summit @ AAO, October 25 2018 Megan Baldwin PhD, CEO & Managing Director Disclaimer Investment
Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington
 Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington I Jay M. Haynie, OD, FAAO have received honoraria from the following companies: Reichert Technologies Notal Vision Carl Zeiss Meditec
Jay M. Haynie, O.D.; F.A.A.O. Olympia Tacoma Renton Kennewick Washington I Jay M. Haynie, OD, FAAO have received honoraria from the following companies: Reichert Technologies Notal Vision Carl Zeiss Meditec
Marcus Gonzales, OD, FAAO Cedar Springs Eye Clinic
 Marcus Gonzales, OD, FAAO Cedar Springs Eye Clinic 25.6 million adults 11.3% of the adult population 10.9 million adults 65 years and older 26.9% of this age population 79 million people are Pre-diabetic!!
Marcus Gonzales, OD, FAAO Cedar Springs Eye Clinic 25.6 million adults 11.3% of the adult population 10.9 million adults 65 years and older 26.9% of this age population 79 million people are Pre-diabetic!!
Outcomes of Anti-VEGF Therapy for Neovascular Age-Related Macular Degeneration in Routine Clinical Practice
 Outcomes of Anti-VEGF Therapy for Neovascular Age-Related Macular Degeneration in Routine Clinical Practice Andrew A. Moshfeghi, MD, MBA 1 ; John D. Pitcher, MD 2,3 ; Genevieve Lucas, B. Comm. 4 ; Nick
Outcomes of Anti-VEGF Therapy for Neovascular Age-Related Macular Degeneration in Routine Clinical Practice Andrew A. Moshfeghi, MD, MBA 1 ; John D. Pitcher, MD 2,3 ; Genevieve Lucas, B. Comm. 4 ; Nick
Diabetic Retinopathy Clinical Research Network
 Diabetic Retinopathy Clinical Research Network Prompt Panretinal Photocoagulation Versus Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy (Protocol
Diabetic Retinopathy Clinical Research Network Prompt Panretinal Photocoagulation Versus Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy (Protocol
Diabetic Retinopathy. Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012
 Diabetic Retinopathy Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012 Outline Statistics Anatomy Categories Assessment Management Risk factors What do you need to do? Objectives Summarize the
Diabetic Retinopathy Barry Emara MD FRCS(C) Giovanni Caboto Club October 3, 2012 Outline Statistics Anatomy Categories Assessment Management Risk factors What do you need to do? Objectives Summarize the
Overview of the Pathogenesis of Diabetic Retinopathy
 Activity presentations are considered intellectual property. These slides may not be published or posted online without permission from Vindico Medical Education (cme@vindicocme.com). Please be respectful
Activity presentations are considered intellectual property. These slides may not be published or posted online without permission from Vindico Medical Education (cme@vindicocme.com). Please be respectful
The Diabetic Retinopathy Clinical Research Network. Management of DME in Eyes with PDR
 The Diabetic Retinopathy Clinical Research Network Management of DME in Eyes with PDR 1 What Has Been Learned? Diabetic Retinopathy Treatment Protocol F: Results suggest that clinically meaningful differences
The Diabetic Retinopathy Clinical Research Network Management of DME in Eyes with PDR 1 What Has Been Learned? Diabetic Retinopathy Treatment Protocol F: Results suggest that clinically meaningful differences
 measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
The Human Eye. Cornea Iris. Pupil. Lens. Retina
 The Retina Thin layer of light-sensitive tissue at the back of the eye (the film of the camera). Light rays are focused on the retina then transmitted to the brain. The macula is the very small area in
The Retina Thin layer of light-sensitive tissue at the back of the eye (the film of the camera). Light rays are focused on the retina then transmitted to the brain. The macula is the very small area in
DIABETIC RETINOPATHY
 DIABETIC RETINOPATHY C. L. B. Canny, MD FRCSC Diabetic retinopathy is the most serious eye manifestation of diabetes and is responsible for most of the blindness caused by diabetes. Diabetic retinopathy
DIABETIC RETINOPATHY C. L. B. Canny, MD FRCSC Diabetic retinopathy is the most serious eye manifestation of diabetes and is responsible for most of the blindness caused by diabetes. Diabetic retinopathy
New Developments in the treatment of Diabetic Retinopathy
 New Developments in the treatment of Diabetic Retinopathy B. Jeroen Klevering University Medical Centre Nijmegen - The Netherlands Topics Management of diabetic retinopathy Interventions a. primary (prevention)
New Developments in the treatment of Diabetic Retinopathy B. Jeroen Klevering University Medical Centre Nijmegen - The Netherlands Topics Management of diabetic retinopathy Interventions a. primary (prevention)
Past ocular history. DME Case 1. Patient presents blurred VA. Hemoglobin A1c 11.5% -- patient states sugars have not been in good control
 Past ocular history Patient presents blurred VA DME Case 1 Hemoglobin A1c 11.5% -- patient states sugars have not been in good control PDR with macular edema OU Rishi Singh MD Cleveland Clinic OD OS 1
Past ocular history Patient presents blurred VA DME Case 1 Hemoglobin A1c 11.5% -- patient states sugars have not been in good control PDR with macular edema OU Rishi Singh MD Cleveland Clinic OD OS 1
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
EyePACS Grading System (Part 3): Detecting Proliferative (Neovascular) Diabetic Retinopathy. George Bresnick MD MPA Jorge Cuadros OD PhD
 EyePACS Grading System (Part 3): Detecting Proliferative (Neovascular) Diabetic Retinopathy George Bresnick MD MPA Jorge Cuadros OD PhD Anatomy of the eye: 3 Normal Retina Retinal Arcades Macula Optic
EyePACS Grading System (Part 3): Detecting Proliferative (Neovascular) Diabetic Retinopathy George Bresnick MD MPA Jorge Cuadros OD PhD Anatomy of the eye: 3 Normal Retina Retinal Arcades Macula Optic
Outcomes in Diabetic Macular Edema (DME) in Patients Who Used Systemic Dipeptidyl Peptidase-4 (DPP-4) Inhibitors in the VISTA and VIVID trials
 Outcomes in Diabetic Macular Edema (DME) in Patients Who Used Systemic Dipeptidyl Peptidase-4 (DPP-4) Inhibitors in the VISTA and VIVID trials Ehsan Rahimy, MD 1 ; Keith Baker, MD 2 ; Desmond Thompson,
Outcomes in Diabetic Macular Edema (DME) in Patients Who Used Systemic Dipeptidyl Peptidase-4 (DPP-4) Inhibitors in the VISTA and VIVID trials Ehsan Rahimy, MD 1 ; Keith Baker, MD 2 ; Desmond Thompson,
There are no published randomized, double-blind trials comparing aflibercept to other therapies in neovascular AMD.
 Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Diabetic Retinopathy Clinical Research Network
 Diabetic Retinopathy Clinical Research Network Prompt Panretinal Photocoagulation Versus Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy Version
Diabetic Retinopathy Clinical Research Network Prompt Panretinal Photocoagulation Versus Intravitreal Ranibizumab with Deferred Panretinal Photocoagulation for Proliferative Diabetic Retinopathy Version
Drug Class Update: Vascular Endothelial Growth Factors
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Dr Dianne Sharp Ophthalmologist Retina Specialists, Parnell Greenlane Clinical Centre
 Dr Dianne Sharp Ophthalmologist Retina Specialists, Parnell Greenlane Clinical Centre 11:00-11:55 WS #115: The Revolution in Macular Degeneration Management 12:05-13:00 WS #127: The Revolution in Macular
Dr Dianne Sharp Ophthalmologist Retina Specialists, Parnell Greenlane Clinical Centre 11:00-11:55 WS #115: The Revolution in Macular Degeneration Management 12:05-13:00 WS #127: The Revolution in Macular
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
New Insights on Treating Diabetic Retinopathy & Diabetic Macular Edema from the 2018 AAO Meeting
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab,
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab,
PART 1: GENERAL RETINAL ANATOMY
 PART 1: GENERAL RETINAL ANATOMY General Anatomy At Ora Serrata At Optic Nerve Head Fundoscopic View Of Normal Retina What Is So Special About Diabetic Retinopathy? The WHO definition of blindness is
PART 1: GENERAL RETINAL ANATOMY General Anatomy At Ora Serrata At Optic Nerve Head Fundoscopic View Of Normal Retina What Is So Special About Diabetic Retinopathy? The WHO definition of blindness is
Paradigm Shift in the treatment of Diabetic Retinopathy. Haytham I. S. Salti, MD Associate Professor
 Paradigm Shift in the treatment of Diabetic Retinopathy Haytham I. S. Salti, MD Associate Professor Disclosure No financial interests related to the subject matter of this talk This presentation includes
Paradigm Shift in the treatment of Diabetic Retinopathy Haytham I. S. Salti, MD Associate Professor Disclosure No financial interests related to the subject matter of this talk This presentation includes
DIABETIC RETINOPATHY (DR) PREFERRED PRACTICE PATTERNS (PPP) Philippines 2016
 DIABETIC RETINOPATHY (DR) PREFERRED PRACTICE PATTERNS (PPP) Philippines 2016 The Diabetic Retinopathy (DR) Preferred Practice Patterns (PPP) Philippines: 2016 was prepared by the VitreoRetina Society of
DIABETIC RETINOPATHY (DR) PREFERRED PRACTICE PATTERNS (PPP) Philippines 2016 The Diabetic Retinopathy (DR) Preferred Practice Patterns (PPP) Philippines: 2016 was prepared by the VitreoRetina Society of
Comparison of BRVO and CRVO management
 Comparison of BRVO and CRVO management Francesco Bandello, MD, FEBO Department of Ophthalmology University Vita-Salute Scientific Institute San Raffaele Milan, Italy 1 Financial Disclosure Advisory Board
Comparison of BRVO and CRVO management Francesco Bandello, MD, FEBO Department of Ophthalmology University Vita-Salute Scientific Institute San Raffaele Milan, Italy 1 Financial Disclosure Advisory Board
Subclinical Diabetic Macular Edema Study
 Diabetic Retinopathy Clinical Research Network Subclinical Diabetic Macular Edema Study Version 2.0 March 21, 2006 Subclinical DME Protocol v2 0 3-21-06.doc Table of Contents Chapter 1. Background Information
Diabetic Retinopathy Clinical Research Network Subclinical Diabetic Macular Edema Study Version 2.0 March 21, 2006 Subclinical DME Protocol v2 0 3-21-06.doc Table of Contents Chapter 1. Background Information
Diabetic Retinopathy
 Diabetic Retinopathy Diabetes mellitus is one of the leading causes of irreversible blindness worldwide. In the United States, it is the most common cause of blindness in people younger than 65 years.
Diabetic Retinopathy Diabetes mellitus is one of the leading causes of irreversible blindness worldwide. In the United States, it is the most common cause of blindness in people younger than 65 years.
Annual Optometry Academe Sunday, August 9 th University of MO St. Louis. Diabetes and Retinopathy 3 Hours CEE COPE# SD
 Annual Optometry Academe Sunday, August 9 th University of MO St. Louis Diabetes and Retinopathy 3 Hours CEE COPE# 4648 SD Michael W. Stewart, MD Mayo Clinic Jacksonville, FL Disclosure Diabetes Mellitus
Annual Optometry Academe Sunday, August 9 th University of MO St. Louis Diabetes and Retinopathy 3 Hours CEE COPE# 4648 SD Michael W. Stewart, MD Mayo Clinic Jacksonville, FL Disclosure Diabetes Mellitus
FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema
 Investor Update Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First Major Treatment Advance in More Than 25 Years for Sight-Threatening Condition
Investor Update Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First Major Treatment Advance in More Than 25 Years for Sight-Threatening Condition
RANZCO Screening and Referral Pathway for Diabetic Retinopathy #
 RANZCO Screening and Referral Pathway for Diabetic Retinopathy # Patient Presents a. Screen for Diabetic Retinopathy every 2 years b. Begin screening at diagnosis of Diabetes * Clinical Modifi ers Yearly
RANZCO Screening and Referral Pathway for Diabetic Retinopathy # Patient Presents a. Screen for Diabetic Retinopathy every 2 years b. Begin screening at diagnosis of Diabetes * Clinical Modifi ers Yearly
Scott M. Pfahler D.O. Dayton Vitreo-Retinal Associates AOCOO-HNS Palm Springs, CA 2012
 Scott M. Pfahler D.O. Dayton Vitreo-Retinal Associates AOCOO-HNS Palm Springs, CA 2012 Proliferative Diabetic Retinopathy Laser Treatments Medical Treatment Surgical Treatment Diabetic Macular Edema Laser
Scott M. Pfahler D.O. Dayton Vitreo-Retinal Associates AOCOO-HNS Palm Springs, CA 2012 Proliferative Diabetic Retinopathy Laser Treatments Medical Treatment Surgical Treatment Diabetic Macular Edema Laser
Diabetic eye disease. Diabetic retinopathy. Sam S. Dahr, M.D. Retina Center of Oklahoma.
 Diabetic eye disease Sam S. Dahr, M.D. Retina Center of Oklahoma www.rcoklahoma.com Downloaded from: The Retina (on 28 May 2007 12:48 AM) 2007 Elsevier Diabetic retinopathy Downloaded from: The Retina
Diabetic eye disease Sam S. Dahr, M.D. Retina Center of Oklahoma www.rcoklahoma.com Downloaded from: The Retina (on 28 May 2007 12:48 AM) 2007 Elsevier Diabetic retinopathy Downloaded from: The Retina
Mean Change in Visual Acuity Over 2 Years Baseline Visual Acuity 20/50 or Worse
 DRCRnet Randomized Trial of Intravitreous Aflibercept versus Intravitreous Bevacizumab + Deferred Aflibercept for Treatment of Central-Involved DME: Protocol AC Chirag Jhaveri, MD 1 Background Aflibercept
DRCRnet Randomized Trial of Intravitreous Aflibercept versus Intravitreous Bevacizumab + Deferred Aflibercept for Treatment of Central-Involved DME: Protocol AC Chirag Jhaveri, MD 1 Background Aflibercept
FA Conference. Lara Rosenwasser Newman, M.D. 10/2/14 University of Louisville Department of Ophthalmology and Visual Sciences
 FA Conference Lara Rosenwasser Newman, M.D. 10/2/14 University of Louisville Department of Ophthalmology and Visual Sciences Patient Presentation CC: (sent by optometrist) Blurry/foggy vision HPI: 62 yo
FA Conference Lara Rosenwasser Newman, M.D. 10/2/14 University of Louisville Department of Ophthalmology and Visual Sciences Patient Presentation CC: (sent by optometrist) Blurry/foggy vision HPI: 62 yo
Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial
 ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
ASX and Media Release 3 January 2018 Opthea Initiates OPT-302 Diabetic Macular Edema Clinical Trial Melbourne, Australia; January 3 2018 Opthea Limited (ASX:OPT), a late stage biopharmaceutical company
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
Diabetic and the Eye: An Introduction
 Diabetic and the Eye: An Introduction Lawrence Iu FRCSEd (Ophth), FCOphthHK, FHKAM (Ophthalmology) Department of Ophthalmology, Grantham Hospital & Queen Mary Hospital Background Diabetes mellitus (DM)
Diabetic and the Eye: An Introduction Lawrence Iu FRCSEd (Ophth), FCOphthHK, FHKAM (Ophthalmology) Department of Ophthalmology, Grantham Hospital & Queen Mary Hospital Background Diabetes mellitus (DM)
FRANZCO, MD, MBBS. Royal Darwin Hospital
 Diabetes and Eye By Dr. Nishantha Wijesinghe FRANZCO, MD, MBBS Consultant Ophthalmologist Royal Darwin Hospital 98% of Diabetics do not need to suffer from severe visual loss Yet Diabetic eye disease is
Diabetes and Eye By Dr. Nishantha Wijesinghe FRANZCO, MD, MBBS Consultant Ophthalmologist Royal Darwin Hospital 98% of Diabetics do not need to suffer from severe visual loss Yet Diabetic eye disease is
Facts About Diabetic Eye Disease
 Facts About Diabetic Eye Disease Points to Remember 1. Diabetic eye disease comprises a group of eye conditions that affect people with diabetes. These conditions include diabetic retinopathy, diabetic
Facts About Diabetic Eye Disease Points to Remember 1. Diabetic eye disease comprises a group of eye conditions that affect people with diabetes. These conditions include diabetic retinopathy, diabetic
Diabetic Retinopathy WHAT IS DIABETIC RETINOPATHY? WHAT CAUSES DIABETIC RETINOPATHY? WHAT ARE THE STAGES OF DIABETIC RETINOPATHY?
 Diabetic Retinopathy WHAT IS DIABETIC RETINOPATHY? Diabetic retinopathy affects 8 million Americans with diabetes. A leading cause of blindness in American adults, it is caused by damage to the small blood
Diabetic Retinopathy WHAT IS DIABETIC RETINOPATHY? Diabetic retinopathy affects 8 million Americans with diabetes. A leading cause of blindness in American adults, it is caused by damage to the small blood
Diabetic Retinopathy Clinical Research Network
 Diabetic Retinopathy Clinical Research Network Intravitreal Ranibizumab or Triamcinolone Acetonide as Adjunctive Treatment to Panretinal Photocoagulation for Proliferative Diabetic Retinopathy Version
Diabetic Retinopathy Clinical Research Network Intravitreal Ranibizumab or Triamcinolone Acetonide as Adjunctive Treatment to Panretinal Photocoagulation for Proliferative Diabetic Retinopathy Version
Use of Scanning Laser Ophthalmoscope Microperimetry in Clinically Significant Macular Edema in Type 2 Diabetes Mellitus
 Use of Scanning Laser Ophthalmoscope Microperimetry in Clinically Significant Macular Edema in Type 2 Diabetes Mellitus Fumihiko Mori, Satoshi Ishiko, Norihiko Kitaya, Taiichi Hikichi, Eiichi Sato, Akira
Use of Scanning Laser Ophthalmoscope Microperimetry in Clinically Significant Macular Edema in Type 2 Diabetes Mellitus Fumihiko Mori, Satoshi Ishiko, Norihiko Kitaya, Taiichi Hikichi, Eiichi Sato, Akira
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO
 EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
EU Regulatory workshop Ophthalmology clinical development and scientific advice. Industry view on DME and macular edema secondary to RVO Yehia Hashad, M.D. Vice President and Global Therapeutic Area Head
Serious Eye diseases, New treatments. Mr. M. Usman Saeed MBBS, FRCS, FRCOphth Consultant Ophthalmologist
 Serious Eye diseases, New treatments Mr. M. Usman Saeed MBBS, FRCS, FRCOphth Consultant Ophthalmologist 5 major causes of loss of vision Cataracts Glaucoma Macular degeneration Retinal Vein occlusions
Serious Eye diseases, New treatments Mr. M. Usman Saeed MBBS, FRCS, FRCOphth Consultant Ophthalmologist 5 major causes of loss of vision Cataracts Glaucoma Macular degeneration Retinal Vein occlusions
Brampton Hurontario Street Brampton, ON L6Y 0P6
 Diabetic Retinopathy What is Diabetic Retinopathy Diabetic retinopathy is one of the leading causes of blindness world-wide. Diabetes damages blood vessels in many organs of the body including the eyes.
Diabetic Retinopathy What is Diabetic Retinopathy Diabetic retinopathy is one of the leading causes of blindness world-wide. Diabetes damages blood vessels in many organs of the body including the eyes.
SUMMARY. Heather Casparis, MD,* and Neil M. Bressler, MD MARINA AND ANCHOR
 The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
Preventing Avoidable Vision loss from Diabetic Retinopathy in Indian Country
 Diabetes in Indian Country- 2017 Preventing Avoidable Vision loss from Diabetic Retinopathy in Indian Country Albuquerque, NM 20 September2017 Mark B. Horton, OD, MD Director, IHS/JVN Teleophthalmology
Diabetes in Indian Country- 2017 Preventing Avoidable Vision loss from Diabetic Retinopathy in Indian Country Albuquerque, NM 20 September2017 Mark B. Horton, OD, MD Director, IHS/JVN Teleophthalmology
Diabetic Retinopathy A Presentation for the Public
 Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
Supplement to March Ranibizumab for Visual Impairment in DME: An Overview of The Evidence SPONSORED BY NOVARTIS PHARMA AG
 Supplement to March 2018 Ranibizumab for Visual Impairment in DME: An Overview of The Evidence SPONSORED BY NOVARTIS PHARMA AG Ranibizumab for Visual Impairment in DME: An Overview of The Evidence BY PROF.
Supplement to March 2018 Ranibizumab for Visual Impairment in DME: An Overview of The Evidence SPONSORED BY NOVARTIS PHARMA AG Ranibizumab for Visual Impairment in DME: An Overview of The Evidence BY PROF.
Drugs vs. DME. Protocol T, the latest in a string of landmark studies by the Diabetic Retinopathy
 3 Anti-VEGF Drugs vs. DME The latest study from DRCR.net delivers important clinical information, but questions remain. BY MIRIAM KARMEL, CONTRIBUTING WRITER alfred t. kamajian Protocol T, the latest in
3 Anti-VEGF Drugs vs. DME The latest study from DRCR.net delivers important clinical information, but questions remain. BY MIRIAM KARMEL, CONTRIBUTING WRITER alfred t. kamajian Protocol T, the latest in
Diabetic retinopathy (DR) is the leading PROCEEDINGS EVIDENCE-BASED DATA IN THE TREATMENT OF DIABETIC RETINOPATHY*
 EVIDENCE-BASED DATA IN THE TREATMENT OF DIABETIC RETINOPATHY* Lloyd Paul Aiello, MD, PhD, and Jennifer K. Sun, MD, MPH ABSTRACT There is a wide array of research into the pathophysiology and treatment
EVIDENCE-BASED DATA IN THE TREATMENT OF DIABETIC RETINOPATHY* Lloyd Paul Aiello, MD, PhD, and Jennifer K. Sun, MD, MPH ABSTRACT There is a wide array of research into the pathophysiology and treatment
Intravitreal Ranibizumab or Triamcinolone Acetonide in Combination with Laser Photocoagulation for Diabetic Macular Edema
 Diabetic Retinopathy Clinical Research Network Intravitreal Ranibizumab or Triamcinolone Acetonide in Combination with Laser Photocoagulation for Diabetic Macular Edema Version 6.0 May 21, 2010 Note: See
Diabetic Retinopathy Clinical Research Network Intravitreal Ranibizumab or Triamcinolone Acetonide in Combination with Laser Photocoagulation for Diabetic Macular Edema Version 6.0 May 21, 2010 Note: See
Diabetic Retinopathy Screening in Hong Kong. Dr. Rita Gangwani M.S, FRCS (Ophth), FCOphth(HK), FHKAM Eye Institute, The University of Hong Kong
 Diabetic Retinopathy Screening in Hong Kong Dr. Rita Gangwani M.S, FRCS (Ophth), FCOphth(HK), FHKAM Eye Institute, The University of Hong Kong Co-Investigators Prof. David Wong Prof. Sarah McGhee Dr. Wico
Diabetic Retinopathy Screening in Hong Kong Dr. Rita Gangwani M.S, FRCS (Ophth), FCOphth(HK), FHKAM Eye Institute, The University of Hong Kong Co-Investigators Prof. David Wong Prof. Sarah McGhee Dr. Wico
FROM OUTDATED TO UPDATED Eminence-Based Medicine
 FROM OUTDATED TO UPDATED Eminence-Based Medicine Evidence-Based Medicine A REVIEW OF KEY CLINICAL TRIALS Anthony DeWilde, OD FAAO 1 EMINENCE BASED MEDICINE 2 EVIDENCE BASED MEDICINE 3 4 CLINICAL TRIALS
FROM OUTDATED TO UPDATED Eminence-Based Medicine Evidence-Based Medicine A REVIEW OF KEY CLINICAL TRIALS Anthony DeWilde, OD FAAO 1 EMINENCE BASED MEDICINE 2 EVIDENCE BASED MEDICINE 3 4 CLINICAL TRIALS
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England. Retinal Vein Occlusion (RVO) with Macular oedema (MO)
 Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
Retinal Vein Occlusion (RVO) Treatment pathway- Northeast England (Royal Victoria Infirmary, Sunderland Eye Infirmary, James Cook University Hospital, Darlington Memorial Hospital, University Hospital
Faculty. Learning Objectives
 Faculty H. Eric Cannon, PharmD, FAMCP Chief of Pharmacy SelectHealth Salt Lake City, Utah Rishi P. Singh, MD Staff Physician Cleveland Clinic Cole Eye Institute Associate Professor of Ophthalmology Case
Faculty H. Eric Cannon, PharmD, FAMCP Chief of Pharmacy SelectHealth Salt Lake City, Utah Rishi P. Singh, MD Staff Physician Cleveland Clinic Cole Eye Institute Associate Professor of Ophthalmology Case
Class Update: Vascular Endothelial Growth Factors
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Treatment of Diabetic Macular Edema without Intravitreal injections
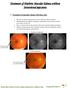 Treatment of Diabetic Macular Edema without Intravitreal injections 1. Treatment of macular edema with laser only This 60 year old female presented on 02-01-2007 for retinal evaluation. She had proliferative
Treatment of Diabetic Macular Edema without Intravitreal injections 1. Treatment of macular edema with laser only This 60 year old female presented on 02-01-2007 for retinal evaluation. She had proliferative
Targeting Inflammation in Diabetic Macular Edema: From Basic Science to Clinical Trials to Clinical Practice
 Targeting Inflammation in Diabetic Macular Edema: From Basic Science to Clinical Trials to Clinical Practice Baruch D Kuppermann, MD, PhD Professor of Ophthalmology and Biomedical Engineering Director,
Targeting Inflammation in Diabetic Macular Edema: From Basic Science to Clinical Trials to Clinical Practice Baruch D Kuppermann, MD, PhD Professor of Ophthalmology and Biomedical Engineering Director,
ILUVIEN IN DIABETIC MACULAR ODEMA
 1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
*Pleasesee amendment forpennsylvaniamedicaid at the endofthis CPB.
 1 of 134 Number: 0701 Policy *Pleasesee amendment forpennsylvaniamedicaid at the endofthis CPB. Aetna considers pegaptanib sodium injection (Macugen) medically necessary for the treatment of individuals
1 of 134 Number: 0701 Policy *Pleasesee amendment forpennsylvaniamedicaid at the endofthis CPB. Aetna considers pegaptanib sodium injection (Macugen) medically necessary for the treatment of individuals
Study of 189 Cases of Diabetic Retinopathy at CMC Larkana
 Original Article Study of 189 Cases of Diabetic Retinopathy at CMC Larkana Shahid Jamal Siddiqui, Sayed Imtiaz Ali Shah, Abdul Qadir Shaikh, Mohammed Yousuf Depar, Safder Ali Abbassi Pak J Ophthalmol 2007,
Original Article Study of 189 Cases of Diabetic Retinopathy at CMC Larkana Shahid Jamal Siddiqui, Sayed Imtiaz Ali Shah, Abdul Qadir Shaikh, Mohammed Yousuf Depar, Safder Ali Abbassi Pak J Ophthalmol 2007,
The Natural History of Diabetic Retinopathy and How Primary Care Makes A Difference
 The Natural History of Diabetic Retinopathy and How Primary Care Makes A Difference We will discuss - How exactly does blood sugar control affect retinopathy? - What are other factors that we measure in
The Natural History of Diabetic Retinopathy and How Primary Care Makes A Difference We will discuss - How exactly does blood sugar control affect retinopathy? - What are other factors that we measure in
Medical Retina 2011 Nicholas Lee
 Medical Retina 2011 Nicholas Lee 1 Diabetic Retinopathy Epidemiology 1000 registered blind each year 2% diabetics registered as blind (8% of all Blind Registrations) 42% with Mild Background DR will progress
Medical Retina 2011 Nicholas Lee 1 Diabetic Retinopathy Epidemiology 1000 registered blind each year 2% diabetics registered as blind (8% of all Blind Registrations) 42% with Mild Background DR will progress
Diabetes & Your Eyes
 Diabetes & Your Eyes Diabetes is a disease that occurs when the pancreas does not secrete enough insulin or the body is unable to process it properly. Insulin is the hormone that regulates the level of
Diabetes & Your Eyes Diabetes is a disease that occurs when the pancreas does not secrete enough insulin or the body is unable to process it properly. Insulin is the hormone that regulates the level of
Diabetic Retinopathy Clinical Research Network
 Diabetic Retinopathy Clinical Research Network Peripheral Diabetic Retinopathy (DR) Lesions on Ultrawide-field Fundus Images and Risk of DR Worsening Over Time Version 4.0 July 21, 2017 peripheral dr lesions
Diabetic Retinopathy Clinical Research Network Peripheral Diabetic Retinopathy (DR) Lesions on Ultrawide-field Fundus Images and Risk of DR Worsening Over Time Version 4.0 July 21, 2017 peripheral dr lesions
Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema
 Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema V Swetha E Jeganathan 1,2 * and Karen Madill 3 1 Department of Ophthalmology,
Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report Inherent Challenges in Managing Long Standing Refractory Diabetic Macular Edema V Swetha E Jeganathan 1,2 * and Karen Madill 3 1 Department of Ophthalmology,
Macular edema (ME) is the most common
 MANAGEMENT OF RETINAL VEIN OCCLUSIONS * Peter A. Campochiaro, MD ABSTRACT Macular edema (ME) is the most common cause of reduced vision in patients with retinal vein occlusions (RVOs). The primary cause
MANAGEMENT OF RETINAL VEIN OCCLUSIONS * Peter A. Campochiaro, MD ABSTRACT Macular edema (ME) is the most common cause of reduced vision in patients with retinal vein occlusions (RVOs). The primary cause
INTRODUCTION AND SYMPTOMS
 CHAPTER 1 INTRODUCTION AND SYMPTOMS Introduction of Diabetic Retinopathy Diabetic retinopathy (DR) is a potentially blinding complication of diabetes. It is defined as presence of one or more definite
CHAPTER 1 INTRODUCTION AND SYMPTOMS Introduction of Diabetic Retinopathy Diabetic retinopathy (DR) is a potentially blinding complication of diabetes. It is defined as presence of one or more definite
Anti Vascular Endothelial Growth Factor Pharmacotherapy for Diabetic Macular Edema
 Ophthalmic Technology Assessment Anti Vascular Endothelial Growth Factor Pharmacotherapy for Diabetic Macular Edema A Report by the American Academy of Ophthalmology Allen C. Ho, MD, Ingrid U. Scott, MD,
Ophthalmic Technology Assessment Anti Vascular Endothelial Growth Factor Pharmacotherapy for Diabetic Macular Edema A Report by the American Academy of Ophthalmology Allen C. Ho, MD, Ingrid U. Scott, MD,
aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer
 aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
aflibercept 40mg/mL solution for injection (Eylea ) SMC No. (1074/15) Bayer 07 August 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014
 From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014 1. This course is designed to review the important ophthalmic literature that was released between October 2013
From Outdated to Updated: A Review of Important Clinical Trials in Ocular Disease from 2014 1. This course is designed to review the important ophthalmic literature that was released between October 2013
Understanding Diabetic Retinopathy
 Understanding Diabetic Retinopathy What Is Diabetic Retinopathy? Diabetes damages blood vessels in the rear of the eye. This condition is called diabetic retinopathy. It can lead to vision loss or blindness.
Understanding Diabetic Retinopathy What Is Diabetic Retinopathy? Diabetes damages blood vessels in the rear of the eye. This condition is called diabetic retinopathy. It can lead to vision loss or blindness.
CURRENT TRENDS IN DIABETIC MACULAR EDEMA TREATMENT. Muge R. Kesen, MD
 CURRENT TRENDS IN DIABETIC MACULAR EDEMA TREATMENT Muge R. Kesen, MD No relevant financial interest or relationships DISCLOSURE OBJECTIVES Current trends (evidence based) Review of clinical trials Diabetic
CURRENT TRENDS IN DIABETIC MACULAR EDEMA TREATMENT Muge R. Kesen, MD No relevant financial interest or relationships DISCLOSURE OBJECTIVES Current trends (evidence based) Review of clinical trials Diabetic
