The MiniMed Continuous Glucose Monitoring System. JOHN J. MASTROTOTARO, Ph.D.
|
|
|
- Ann Madlyn Richard
- 6 years ago
- Views:
Transcription
1 DIABETES TECHNOLOGY & THERAPEUTICS Volume 2, Supplement 1, 2000 Mary Ann Liebert, Inc. The MiniMed Continuous Glucose Monitoring System JOHN J. MASTROTOTARO, Ph.D. DIABETES is a major source of morbidity, mortality, and economic expense. All patients with type 1 diabetes and an estimated 3 4 million patients in the United States with type 2 diabetes must take insulin to control their glucose levels. The majority of these patients selfmonitor their blood glucose one or more times each day, using fingerstick blood sampling and analysis with a portable glucose meter device. Approximately 200,000 type 1 diabetes patients in the United States practice intensive insulin therapy, requiring four or more blood glucose measurements each day coupled with either multiple daily injections of insulin or an insulin pump. The Diabetes Control and Complications Trial (DCCT) clearly demonstrated the importance of frequent self-monitoring of blood glucose (SMBG) in attaining tight glycemic con- trol. 1 Patients undergoing intensive therapy had a 39 76% reduced occurrence of long-term complications as compared to patients treated with conventional therapy. The primary drawback of intensive therapy was a threefold increase in the occurrence of severe hypoglycemia, despite performing four or more SMBG tests per day. Consequently, methods to improve the ability to achieve intensive control without hypoglycemia are being explored. There is a tremendous need to develop and commercialize a truly simple, accurate method of measuring glucose that can provide a basis for more accurate and directed disease selfmanagement. Generally, it is not practical to perform SMBG frequently enough throughout the day to accurately identify every blood glucose excursion. MiniMed has taken a first step in advancing the practice of glucose self-monitoring by developing a short-term, continuous glucose sensor. The sensor is inserted subcutaneously and is capable of reliable operation for up to 3 days, followed by replacement with a new sensor at a different location, if necessary. The assay method is based on electrochemical detection of glucose through its reaction with glucose oxidase. Data are collected once every 5 min by a pager-sized monitor device and can be periodically downloaded into a computer for analysis and interpretation. In future product iterations, the sensor will function as a hypoglycemia and hyperglycemia alarm, by notifying the user when blood glucose levels reach preselected thresholds (such as 60 mg/dl and 200 mg/dl), and will provide real-time glucose readouts. The alarm feature will be especially important in patients with hypoglycemic unawareness, which is believed to occur in anywhere from 25% to 50% of patients with type 1 diabetes, 2 especially those who have neuropathy or those without complications who are following intensive glycemic control regimens. 3 Fanelli et al. 4 have shown that meticulous control to prevent low glucose excursions can result in a significant recovery of hypoglycemic awareness. DESCRIPTION OF THE DEVICE The MiniMed Continuous Glucose Monitoring System (CGMS; MiniMed Inc., Northridge, CA; is a Holter-style (hardwired) sensor system comprised of four components: (1) a pager-sized glucose monitor, MiniMed Inc., Northridge, California. S-13
2 S-14 MASTROTOTARO (2) a sterile, disposable subcutaneous glucose sensing device with an external electrical connector, (3) a connecting cable, and (4) a communication device (Com-Station) enabling data stored in the monitor to be downloaded to a personal computer. The CGMS (Fig. 1) uses a subcutaneous sensor to continuously monitor interstitial glucose levels in the range of mg/dl. The function of the glucose monitor is to acquire, display, and store signals from the subcutaneous glucose sensor. The glucose sensor signal is acquired every 10 sec, with an average of the signals saved in memory every 5 min. The CGMS monitor, which is powered by two standard AAA alkaline batteries, can acquire and store up to 2 weeks of information. The Com-Station is designed to retrieve glucose data from the glucose monitor and download it via infrared pulses to a personal computer (PC) through a serial port (Fig. 2). Once glucose data from the monitor have been downloaded, the CGMS Com-Station software can be used to analyze and display the data. The data can be viewed as graphical data and summary statistics, or as numerical data. The CGMS is used primarily by the healthcare professional (HCP) to gather continuous glucose data on patients already diagnosed with diabetes mellitus. The data collected by the system, when downloaded to a PC, can be useful to the HCP in managing a patient s diabetes, by identifying patterns of high or low glucose concentrations. This information may be used to adjust insulin administration patterns, change the patient s diet, or improve patient education efforts. This information is particularly beneficial for patients whose diabetes is difficult to manage due to rapidly fluctuating glucose levels. The device is also quickly gaining acceptance among clinical researchers as a primary endpoint reflecting glycemic control. FIG. 1. Continuous glucose monitoring system. SET-UP AND USE OF THE CGMS The first CGMS product does not provide glucose values to the patient in real time. Instead, the CGMS is used by the HCP as a tool to provide retrospective information on daily glucose excursions during the period of use. The HCP inserts the sensor and programs the monitor at the office and then sends the patient home wearing the device. Patients continue to self-monitor their blood glucose as instructed by their HCP and enter these blood glucose values into the CGMS. Event markers for meals, FIG. 2. Monitor placement in the Com-Station.
3 MINIMED CONTINUOUS GLUCOSE MONITORING SYSTEM S-15 insulin administration, and exercise can also be entered. After wearing a sensor for up to 3 days, patients return to the medical office where the data from the CGMS is downloaded to a PC for review. Data are presented in the form of daily glucose trend plots and a summary table of average glucose levels, glucose ranges, and standard deviations. The HCP then can make adjustments to the therapeutic regimens based on continuous, accurate measurements. SENSOR CALIBRATION The CGMS sensor, when inserted subcutaneously, measures an electrical current in nanoamperes (na) that is related to the interstitial glucose concentration. Although the exact relationship between interstitial and capillary blood glucose remains to be uncovered, clinical studies have shown that subcutaneous sensor measurements generally follow fingerstick meter measurements and venous blood laboratory values. 5 9 These studies have shown that the concentrations of glucose in the interstitial fluid are typically lower than those in blood, and changes in interstitial glucose may be detected either earlier or later than those occurring in the blood. Nevertheless, numerous studies in humans have demonstrated the value of measuring glucose in the interstitial fluid Rebrin et al. 14 found that subcutaneous sensor measurements of interstitial glucose consistently predicted plasma glucose independent of increases in endogenous or exogenous insulin and without correction for delays in interstitial glucose equilibration. The CGMS instructions recommend that at least four SMBG meter readings be entered into the monitor each day that the CGMS is used. These blood glucose readings are used to calibrate the sensor s na readings at the time the data are downloaded to a PC and the graphing utility is run. The retrospective calibration approach uses a modified form of linear regression to determine calibration factors (slope and offset). A separate calibration is performed for each calendar day of CGMS data. The calibration is based upon a linear regression of all of the meter entries with corresponding valid sensor readings (na). FORMAT OF DATA DISPLAY FOR THE HEALTHCARE PROFESSIONAL Once the CGMS data are downloaded to a PC using the Com-Station, the HCP has the ability to review summary statistics, daily trend plots, and a Modal day plot of the sensor and meter data. Evaluation of optimal accuracy criteria Based on clinical research of the CGMS, two criteria for optimal accuracy have been established: (1) a correlation between the sensor and meter readings of at least 0.79, and (2) a mean absolute difference of no more than 28%. For each calendar day, the correlation coefficient between the meter readings and the calculated sensor glucose values is automatically calculated. The mean absolute difference is calculated by taking, for each pair of data, the absolute difference between the meter glucose reading and the sensor glucose measurement, dividing by the meter value, and then averaging across pairs within a day. The optimal accuracy criteria are then applied independently to each day of sensor data. If the sensor fails to meet these criteria, a warning message appears on the sensor profile and as a note to the summary statistics for that day. This message also appears if there are fewer than three meter-sensor data pairs for that day, because the correlation coefficient in such a case cannot be meaningfully calculated. Any statistic that fails to meet the criteria is identified in three ways: (1) the statistic is shaded in the summary table for easy identification, (2) an x will appear next to the date in the summary table and in the worksheet tab of the daily graph, and (3) the optimal accuracy message will appear as a footnote to the summary table and on the daily graph. Creation of data summary Summary statistics are calculated for each calendar day and for all dates combined. The sum-
4 S-16 MASTROTOTARO 1 n/a FIG. 3. mary table includes three sections: Optimal Accuracy Criteria, Sensor, and Meter. The Optimal Accuracy Criteria includes the number of paired meter readings, correlation coefficient Example of data summary table. (r), and mean absolute difference (%). The sensor and meter sections each include the number of readings, average value, standard deviation, and range of glucose values. These FIG. 4. Example of daily graph.
5 MINIMED CONTINUOUS GLUCOSE MONITORING SYSTEM S-17 summary statistics are placed in a Data Summary worksheet within the patient file (Fig. 3). Creation of daily graphs A scatter plot is created for each available calendar day in the data set (Fig. 4). Each graph plots real time on the horizontal axis (with a fixed 24-h range from midnight to midnight) and blood glucose from both the CGMS and the meter on the vertical axis, with a fixed range of mg/dl. User-entered events (meals, insulin, exercise, and other) are also plotted and are positioned on the graph outside the range of blood glucose values, so that the data markers for these events do not interfere with the profiles. Creation of modal day graph FIG. 5. A composite graph is generated that superimposes all daily sensor profiles from a data download on a single graph. Each day of sensor data appears in a different color, and the graph legend lists the dates being plotted. This graph, known as a Modal Day plot, may be used to identify consistent patterns of glucose excursion across days of sensor use (Fig. 5). Example of modal day graph. CONCLUSION The MiniMed CGMS is the first ambulatory continuous glucose monitoring system with FDA approval. For the first time, healthcare professionals can evaluate glucose trends and the effects of various intensive treatment modalities during normal activities of daily living. Its effect on the treatment of diabetes could have a similar impact as the advent of self-monitoring of blood glucose. REFERENCES 1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of longterm complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329: Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. Diabetes Care 1995; 18: Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS. Defective glucose counter-regulation after strict glycemic control of insulin dependent diabetes mellitus. N Engl J Med 1987;316: Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Di Vincenzo A, Modarelli F, Ciofetta M, Lepore M, An-
6 S-18 nibale B, Torlone E, Perriello G, De Feo P, Santeusanio F, Brunetti P, Bolli GB. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia 1994;37: Pfeiffer EF, Meyerhoff C, Bischof F, Keck FS, Kerner W. One line continuous monitoring of subcutaneous tissue glucose is feasible by combining portable glucosensor with microdialysis. Horm Metab Res 1993; 25: Pickup JC, Shaw GS, Claremont DJ. In vivo molecular sensing in diabetes mellitus: an implantable glucose sensor with direct electron transfer. Diabetologia 1989;32: Sternberg FC, Meyerhoff C, Mennel FJ, Hayer H, Bischof F, Pfeiffer EF. Does fall in tissue glucose precede fall in blood glucose? Diabetologia 1996;39: Thomas K, Kiwit M, Kerner W. Glucose concentration in human subcutaneous adipose tissue: comparison between forearm and abdomen. Exp Clin Endocrinol Diabetes 1998;106: Thomé-Duret V, Resch G, Gangnerau MN, Lemonnier F, Klein JC, Zhang Y, Wilson GS. Use of a subcutaneous glucose sensor to detect decreases in glucose concentration prior to observation in blood. Anal Chem 1996;68: MASTROTOTARO 10. Bantle J, Thomas W. Glucose monitoring using interstitial fluid [Abstract]. Diabetes 1997;46: Fisher U. Continuous in vivo monitoring in diabetes: the subcutaneous glucose concentration. Acta Anaesthesiol Scand 1995;39(suppl 104): Ginsberg BH. The FDA Panel advises approval of the first continuous glucose sensor. Diabetes Technol Ther 1999;2: Johnson KW, Mastrototaro JJ, Howey DC, Brunelle RL, Burden-Brady PL, Bryan NA, Andrew CC, Rowe HM, Allen DJ, Noffke BW, McMaham WC, Morff RJ, Lipson D, Nevin RS. In vivo evaluation of an electroenzymatic glucose sensor implanted in subcutaneous tissue. Biosens Bioelectron 1992;7: Rebrin K, Steil GM, Van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol 1992;277:E561 E571. Address reprint requests to: John Mastrototaro, Ph.D. MiniMed Inc Devonshire Street Northridge, CA johnm@minimed.com
Use of Continuous Glucose Monitoring in the Detection and Prevention of Hypoglycemia
 Journal of Diabetes Science and Technology Volume 1, Issue 1, January 2007 Diabetes Technology Society SYMPOSIUM Use of Continuous Glucose Monitoring in the Detection and Prevention of Hypoglycemia Howard
Journal of Diabetes Science and Technology Volume 1, Issue 1, January 2007 Diabetes Technology Society SYMPOSIUM Use of Continuous Glucose Monitoring in the Detection and Prevention of Hypoglycemia Howard
Reproducibility of Glucose Measurements Using the Glucose Sensor
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Reproducibility of Glucose Measurements Using the Glucose Sensor MURIEL METZGER, MD 1 GIL LEIBOWITZ, MD 1 JULIO WAINSTEIN,
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Reproducibility of Glucose Measurements Using the Glucose Sensor MURIEL METZGER, MD 1 GIL LEIBOWITZ, MD 1 JULIO WAINSTEIN,
Subcutaneous glucose monitoring with GlucoDay : comparison of the results to those obtained with the Endocrine Artificial Pancreas
 HORMONES 2010, 9(2):145-150 Research paper Subcutaneous glucose monitoring with GlucoDay : comparison of the results to those obtained with the Endocrine Artificial Pancreas George Dimitriadis 1, Eleni
HORMONES 2010, 9(2):145-150 Research paper Subcutaneous glucose monitoring with GlucoDay : comparison of the results to those obtained with the Endocrine Artificial Pancreas George Dimitriadis 1, Eleni
A Prospective Evaluation of Insulin Dosing Recommendations in Patients with Type 1 Diabetes at Near Normal Glucose Control: Bolus Dosing
 Journal of Diabetes Science and Technology Volume 1, Issue 1, January 2007 Diabetes Technology Society ORIGINAL ARTICLES A Prospective Evaluation of Insulin Dosing Recommendations in Patients with Type
Journal of Diabetes Science and Technology Volume 1, Issue 1, January 2007 Diabetes Technology Society ORIGINAL ARTICLES A Prospective Evaluation of Insulin Dosing Recommendations in Patients with Type
Agreement between Glucose Trends Derived from Three Simultaneously Worn Continuous Glucose Sensors
 Journal of Diabetes Science and Technology Volume 2, Issue 5, September 2008 Diabetes Technology Society ORIGINAL ARTICLES Agreement between Glucose Trends Derived from Three Simultaneously Worn Continuous
Journal of Diabetes Science and Technology Volume 2, Issue 5, September 2008 Diabetes Technology Society ORIGINAL ARTICLES Agreement between Glucose Trends Derived from Three Simultaneously Worn Continuous
Abstract CLINICAL APPLICATIONS. Objectives: Methods: Results: Conclusion: Journal of Diabetes Science and Technology
 Journal of Diabetes Science and Technology Volume 1, Issue 3, May 2007 Diabetes Technology Society CLINICAL APPLICATIONS Combined Insulin Pump Therapy with Real-Time Continuous Glucose Monitoring Significantly
Journal of Diabetes Science and Technology Volume 1, Issue 3, May 2007 Diabetes Technology Society CLINICAL APPLICATIONS Combined Insulin Pump Therapy with Real-Time Continuous Glucose Monitoring Significantly
Continuous glucose monitoring has been an elusive
 Timing of Changes in Interstitial and Venous Blood Glucose Measured With a Continuous Subcutaneous Glucose Sensor Michael S. Boyne, David M. Silver, Joy Kaplan, and Christopher D. Saudek The objective
Timing of Changes in Interstitial and Venous Blood Glucose Measured With a Continuous Subcutaneous Glucose Sensor Michael S. Boyne, David M. Silver, Joy Kaplan, and Christopher D. Saudek The objective
Prediction of severe hypoglycemia. Additional information for this article can be found in an online appendix at
 Diabetes Care In Press, published online March 15, 2007 Prediction of severe hypoglycemia Received for publication 3 July 2006 and accepted in revised form 7 March 2007. Additional information for this
Diabetes Care In Press, published online March 15, 2007 Prediction of severe hypoglycemia Received for publication 3 July 2006 and accepted in revised form 7 March 2007. Additional information for this
Diabetes Care 26: , 2003
 Emerging Treatments and Technologies O R I G I N A L A R T I C L E The Continuous Glucose Monitoring System Is Useful for Detecting Unrecognized Hypoglycemias in Patients With Type 1 and Type 2 Diabetes
Emerging Treatments and Technologies O R I G I N A L A R T I C L E The Continuous Glucose Monitoring System Is Useful for Detecting Unrecognized Hypoglycemias in Patients With Type 1 and Type 2 Diabetes
Clinical Value and Evidence of Continuous Glucose Monitoring
 Clinical Value and Evidence of Continuous Glucose Monitoring 9402313-012 Objective To review the clinical value and the recent clinical evidence for Professional and Personal CGM Key Points CGM reveals
Clinical Value and Evidence of Continuous Glucose Monitoring 9402313-012 Objective To review the clinical value and the recent clinical evidence for Professional and Personal CGM Key Points CGM reveals
Anneloes Kerssen, a Harold W. de Valk, b Gerard H.A. Visser a. D RCOG 2004 BJOG: an International Journal of Obstetrics and Gynaecology
 BJOG: an International Journal of Obstetrics and Gynaecology September 2004, Vol. 111, pp. 919 924 DOI: 10.1111/j.1471-0528.2004.00203.x Day-to-day glucose variability during pregnancy in women with Type
BJOG: an International Journal of Obstetrics and Gynaecology September 2004, Vol. 111, pp. 919 924 DOI: 10.1111/j.1471-0528.2004.00203.x Day-to-day glucose variability during pregnancy in women with Type
Long-term effects of continuous glucose monitoring on HbA 1c levels: An audit
 Long-term effects of continuous glucose monitoring on Julie Brake Continuous glucose monitoring (CGM) has become a common and useful tool in diabetes care. To understand whether a 72-hour glucose profile
Long-term effects of continuous glucose monitoring on Julie Brake Continuous glucose monitoring (CGM) has become a common and useful tool in diabetes care. To understand whether a 72-hour glucose profile
Limitations of Conventional Methods of Self-Monitoring of Blood Glucose
 Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Limitations of Conventional Methods of Self-Monitoring of Blood Glucose Lessons learned from 3 days of continuous glucose sensing in pediatric
Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Limitations of Conventional Methods of Self-Monitoring of Blood Glucose Lessons learned from 3 days of continuous glucose sensing in pediatric
Figure 2.1: Glucose meter
 CHAPTER TWO: MONITORING TECHNOLOGIES 2.1 Introduction Glucose monitoring is a method of self-testing glucose (blood sugar) levels for the management of diabetes. Traditionally, it involves pricking the
CHAPTER TWO: MONITORING TECHNOLOGIES 2.1 Introduction Glucose monitoring is a method of self-testing glucose (blood sugar) levels for the management of diabetes. Traditionally, it involves pricking the
Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond)
 Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond) 9501366-011 20110401 Objectives Understand the need for insulin pumps and CGMS in managing
Diabetes II Insulin pumps; Continuous glucose monitoring system (CGMS) Ernest Asamoah, MD FACE FACP FRCP (Lond) 9501366-011 20110401 Objectives Understand the need for insulin pumps and CGMS in managing
UvA-DARE (Digital Academic Repository) Hypoglycaemia in diabetes Schopman, J.E. Link to publication
 UvA-DARE (Digital Academic Repository) Hypoglycaemia in diabetes Schopman, J.E. Link to publication Citation for published version (APA): Schopman, J. E. (013). Hypoglycaemia in diabetes General rights
UvA-DARE (Digital Academic Repository) Hypoglycaemia in diabetes Schopman, J.E. Link to publication Citation for published version (APA): Schopman, J. E. (013). Hypoglycaemia in diabetes General rights
Continuous Glucose Monitoring (CGM)
 Continuous Glucose Monitoring (CGM) Date of Origin: 02/2001 Last Review Date: 07/26/2017 Effective Date: 07/26/2017 Dates Reviewed: 04/2004, 04/2005, 03/2006, 11/2006, 12/2007, 03/2008, 09/2008, 04/2009,
Continuous Glucose Monitoring (CGM) Date of Origin: 02/2001 Last Review Date: 07/26/2017 Effective Date: 07/26/2017 Dates Reviewed: 04/2004, 04/2005, 03/2006, 11/2006, 12/2007, 03/2008, 09/2008, 04/2009,
Corporate Medical Policy
 Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Continuous Glucose Monitoring Devices Pharmacy Policy
 Line of Business: All Line of Business Effective date: August 16, 2017 Revision date: August 16, 2017 Continuous Glucose Monitoring Devices Pharmacy Policy This policy has been developed through review
Line of Business: All Line of Business Effective date: August 16, 2017 Revision date: August 16, 2017 Continuous Glucose Monitoring Devices Pharmacy Policy This policy has been developed through review
Continuous Glucose Monitoring and the Reality of Metabolic Control in Preschool Children With Type 1 Diabetes
 Emerging Treatments and Technologies O R I G I N A L A R T I C L E Continuous Glucose Monitoring and the Reality of Metabolic Control in Preschool Children With Type 1 Diabetes GEORGE S. JEHA, MD 1,2 LEFKOTHEA
Emerging Treatments and Technologies O R I G I N A L A R T I C L E Continuous Glucose Monitoring and the Reality of Metabolic Control in Preschool Children With Type 1 Diabetes GEORGE S. JEHA, MD 1,2 LEFKOTHEA
Reduced Awareness of Hypoglycemia in Adults With IPPM
 O R I G I N A L A R T I C L E Reduced Awareness of Hypoglycemia in Adults With IPPM A prospective study of hypoglycemic frequency and associated symptoms WILLIAM L. CLARKE, MD DANIEL J. Cox, PHD LINDA
O R I G I N A L A R T I C L E Reduced Awareness of Hypoglycemia in Adults With IPPM A prospective study of hypoglycemic frequency and associated symptoms WILLIAM L. CLARKE, MD DANIEL J. Cox, PHD LINDA
Hypoglycemia Detection and Prediction Using Continuous Glucose Monitoring A Study on Hypoglycemic Clamp Data
 Journal of Diabetes Science and Technology Volume 1, Issue 5, September 2007 Diabetes Technology Society SYMPOSIUM Hypoglycemia Detection and Prediction Using Continuous Glucose Monitoring A Study on Hypoglycemic
Journal of Diabetes Science and Technology Volume 1, Issue 5, September 2007 Diabetes Technology Society SYMPOSIUM Hypoglycemia Detection and Prediction Using Continuous Glucose Monitoring A Study on Hypoglycemic
Today s Goals 10/6/2017. New Frontiers in Diabetes Technology. Disclosures
 New Frontiers in Diabetes Technology Marie E. McDonnell, MD Director, Brigham and Women's Diabetes Program Division of Endocrinology, Diabetes and Hypertension Brigham and Women s Hospital Today s Goals
New Frontiers in Diabetes Technology Marie E. McDonnell, MD Director, Brigham and Women's Diabetes Program Division of Endocrinology, Diabetes and Hypertension Brigham and Women s Hospital Today s Goals
CareLink. software REPORT REFERENCE GUIDE. Management Software for Diabetes
 CareLink Management Software for Diabetes software REPORT REFERENCE GUIDE How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used
CareLink Management Software for Diabetes software REPORT REFERENCE GUIDE How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used
Early Patient and Clinician Experiences with Continuous Glucose Monitoring
 Feature Article / Early Experiences with CGM Early Patient and Clinician Experiences with Continuous Glucose Monitoring David K. Bloomgarden, MD, FACE; Janine Freeman, RD, LD, CDE; and Elizabeth DeRobertis,
Feature Article / Early Experiences with CGM Early Patient and Clinician Experiences with Continuous Glucose Monitoring David K. Bloomgarden, MD, FACE; Janine Freeman, RD, LD, CDE; and Elizabeth DeRobertis,
Pumps & Sensors made easy. OPADA ALZOHAILI MD FACE Endocrinology Assistant Professor Wayne State University
 Pumps & Sensors made easy OPADA ALZOHAILI MD FACE Endocrinology Assistant Professor Wayne State University DeFronzo RA. Diabetes. 2009;58:773-795. Ominous Octet Relationship of b-cell Dysfunction and Development
Pumps & Sensors made easy OPADA ALZOHAILI MD FACE Endocrinology Assistant Professor Wayne State University DeFronzo RA. Diabetes. 2009;58:773-795. Ominous Octet Relationship of b-cell Dysfunction and Development
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Roughly 20% - 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the US FDA estimated
1979 Advances in Diabetes Care Technologies 2015 Introduction Roughly 20% - 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the US FDA estimated
Report Reference Guide
 Report Reference Guide How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used to generate the sample reports was from sample patient
Report Reference Guide How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used to generate the sample reports was from sample patient
Welcome to CareLink Pro
 Reference Guide Welcome to CareLink Pro This guide was developed to serve as a reference for obtaining patient data and reviewing CareLink Pro reports. Getting Started with CareLink Pro Adding New Patients
Reference Guide Welcome to CareLink Pro This guide was developed to serve as a reference for obtaining patient data and reviewing CareLink Pro reports. Getting Started with CareLink Pro Adding New Patients
The Realities of Technology in Type 1 Diabetes
 The Realities of Technology in Type 1 Diabetes May 6, 2017 Rosanna Fiallo-scharer, MD Margaret Frederick, RN Disclosures I have no conflicts of interest to disclose I will discuss some unapproved treatments
The Realities of Technology in Type 1 Diabetes May 6, 2017 Rosanna Fiallo-scharer, MD Margaret Frederick, RN Disclosures I have no conflicts of interest to disclose I will discuss some unapproved treatments
NEW TECHNOLOGIES FOR MANAGING DIABETES ANGELA THOMPSON DNP, FNP-C, BC-ADM, CDE, FAANP
 NEW TECHNOLOGIES FOR MANAGING DIABETES ANGELA THOMPSON DNP, FNP-C, BC-ADM, CDE, FAANP No commercial support or sponsorship was received for this project I have nothing to disclose OBJECTIVES Identify at
NEW TECHNOLOGIES FOR MANAGING DIABETES ANGELA THOMPSON DNP, FNP-C, BC-ADM, CDE, FAANP No commercial support or sponsorship was received for this project I have nothing to disclose OBJECTIVES Identify at
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE
 A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
FreeStyle Lite A Blood Glucose Meter That Requires No Coding
 Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
Understanding the Assessment and Progress Report
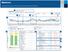 Understanding the ssessment and Progress Report ssessment and Progress 8//2-8/7/2 (7 Days) 3 7/2/2-7/27/2 (7 Days) 4 25-75% -% verage 2 4: M- 5:4 M Hyperglycemic patterns() :45 PM- :3 PM 2 3 :2 M- :5 M
Understanding the ssessment and Progress Report ssessment and Progress 8//2-8/7/2 (7 Days) 3 7/2/2-7/27/2 (7 Days) 4 25-75% -% verage 2 4: M- 5:4 M Hyperglycemic patterns() :45 PM- :3 PM 2 3 :2 M- :5 M
Comparison of the Numerical and Clinical Accuracy of Four Continuous Glucose Monitors
 Emerging Treatments and Technologies O R I G I N A L A R T I C L E Comparison of the Numerical and Clinical Accuracy of Four Continuous Glucose Monitors BORIS KOVATCHEV, PHD 1 STACEY ANDERSON, MD 1 2 LUTZ
Emerging Treatments and Technologies O R I G I N A L A R T I C L E Comparison of the Numerical and Clinical Accuracy of Four Continuous Glucose Monitors BORIS KOVATCHEV, PHD 1 STACEY ANDERSON, MD 1 2 LUTZ
Real-Time Continuous Glucose Monitoring: From Application to Evaluation
 Real-Time Continuous Glucose Monitoring: From Application to Evaluation Gary Scheiner MS, CDE Owner/Director, Integrated Diabetes Services 333 E. Lancaster Ave., Suite 24 Wynnewood, PA 1996 (877) 735-3648
Real-Time Continuous Glucose Monitoring: From Application to Evaluation Gary Scheiner MS, CDE Owner/Director, Integrated Diabetes Services 333 E. Lancaster Ave., Suite 24 Wynnewood, PA 1996 (877) 735-3648
Report Reference Guide. THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1
 Report Reference Guide THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1 How to use this guide Each type of CareLink report and its components are described in the following sections.
Report Reference Guide THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1 How to use this guide Each type of CareLink report and its components are described in the following sections.
Advances in Diabetes Care Technologies
 Advances in Diabetes Care Technologies 1979 2015 Introduction Roughly 20% to 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the U.S. FDA
Advances in Diabetes Care Technologies 1979 2015 Introduction Roughly 20% to 30% of patients with T1DM and fewer than 1% of insulin-treated patients with T2DM use an insulin pump In 2007, the U.S. FDA
Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid. Original Policy Date
 MP 1.01.15 Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid Medical Policy Section Durable Medical Equipment Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed
MP 1.01.15 Continuous or Intermittent Monitoring of Glucose in Interstitial Fluid Medical Policy Section Durable Medical Equipment Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed
The Current Environment of CGM Technologies. Barry H. Ginsberg, M.D., Ph.D.
 Journal of Diabetes Science and Technology Volume 1, Issue 1, January 2007 Diabetes Technology Society SYMPOSIUM The Current Environment of CGM Technologies Introduction Barry H., M.D., Ph.D. This presentation
Journal of Diabetes Science and Technology Volume 1, Issue 1, January 2007 Diabetes Technology Society SYMPOSIUM The Current Environment of CGM Technologies Introduction Barry H., M.D., Ph.D. This presentation
State of California Health and Human Services Agency Department of Health Care Services
 State of California Health and Human Services Agency Department of Health Care Services JENNIFER KENT Director EDMOND G. BROWN JR Governor DATE: N.L.: 03-0317 Index: Benefits TO: ALL COUNTY CALIFORNIA
State of California Health and Human Services Agency Department of Health Care Services JENNIFER KENT Director EDMOND G. BROWN JR Governor DATE: N.L.: 03-0317 Index: Benefits TO: ALL COUNTY CALIFORNIA
Subjects and Methods 2017, 64 (8),
 2017, 64 (8), 827-832 Careful readings for a flash glucose monitoring system in nondiabetic Japanese subjects: individual differences and discrepancy in glucose concentrarion after glucose loading Keiko
2017, 64 (8), 827-832 Careful readings for a flash glucose monitoring system in nondiabetic Japanese subjects: individual differences and discrepancy in glucose concentrarion after glucose loading Keiko
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017 Section: DME Place(s)
Continuous Glucose Monitors for Diabetes Management
 Continuous Glucose Monitors for Diabetes Management Ryan Huang, DO PGY II, Sonia Garcia-Jayne, DO PGY II Mandeep Gill, DO PGY I, Justin Leeka, DO, PGY I, Catherine Nguyen OMS IV Family Medicine Residency,
Continuous Glucose Monitors for Diabetes Management Ryan Huang, DO PGY II, Sonia Garcia-Jayne, DO PGY II Mandeep Gill, DO PGY I, Justin Leeka, DO, PGY I, Catherine Nguyen OMS IV Family Medicine Residency,
OneTouch Reveal web app Report Reference Guide
 OneTouch Reveal web app Report Reference Guide Your step-by-step guide to setting up and using the OneTouch Reveal web app OneTouch Verio meter OneTouch Verio Flex meter OneTouch Verio IQ meter OneTouch
OneTouch Reveal web app Report Reference Guide Your step-by-step guide to setting up and using the OneTouch Reveal web app OneTouch Verio meter OneTouch Verio Flex meter OneTouch Verio IQ meter OneTouch
MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps
 POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 02/13/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization
POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 02/13/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization
ORIGINAL ARTICLE. Use of Freestyle Libre Pro TM Flash Glucose Monitoring System in Different Clinical Situations at a Diabetes Centre
 18 Journal of The Association of Physicians of India Vol. 65 August 217 ORIGINAL ARTICLE Use of Freestyle Libre Pro TM Flash itoring System in Different Clinical Situations at a Diabetes Centre Bangalore
18 Journal of The Association of Physicians of India Vol. 65 August 217 ORIGINAL ARTICLE Use of Freestyle Libre Pro TM Flash itoring System in Different Clinical Situations at a Diabetes Centre Bangalore
Ultra Blue. Test Strips UNISTRIP. Charlotte, NC V2-10/25/2018 TECHNOLOGIES,LLC
 VS UNISTRIP TECHNOLOGIES,LLC Charlotte, NC 28269 1-866-889-6229 V2-10/25/2018 UniStrip Generic Blood Glucose VS OneTouch Ultra Blue 1. Purpose This study is intended to evaluate the difference in values
VS UNISTRIP TECHNOLOGIES,LLC Charlotte, NC 28269 1-866-889-6229 V2-10/25/2018 UniStrip Generic Blood Glucose VS OneTouch Ultra Blue 1. Purpose This study is intended to evaluate the difference in values
Norbert Hermanns, PhD 1,2, Beatrix Schumann, MD 2, Bernhard Kulzer, PhD 1,2, and Thomas Haak, MD 1,2. Original Article
 524105DSTXXX10.1177/1932296814524105Journal of Diabetes Science and TechnologyHermanns et al research-article2014 Original Article The Impact of Continuous Glucose Monitoring on Low Interstitial Glucose
524105DSTXXX10.1177/1932296814524105Journal of Diabetes Science and TechnologyHermanns et al research-article2014 Original Article The Impact of Continuous Glucose Monitoring on Low Interstitial Glucose
POPULATION TRACKER - DREAMED USER GUIDE
 POPULATION TRACKER - DREAMED USER GUIDE November 2018 IFU-0011 05 TABLE OF CONTENTS TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Glooko Intended Use... 1 DreaMed Intended Use...
POPULATION TRACKER - DREAMED USER GUIDE November 2018 IFU-0011 05 TABLE OF CONTENTS TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Glooko Intended Use... 1 DreaMed Intended Use...
QUESTION 4. WHAT CLINICAL DATA ARE CURRENTLY AVAILABLE TO SUPPORT EXPANDED CGM COVERAGE BY PAYERS AS PERTAINS TO QUESTIONS 1 AND 3?
 500 1 QUESTION 4. WHAT CLINICAL DATA ARE CURRENTLY AVAILABLE TO SUPPORT EXPANDED CGM COVERAGE BY PAYERS AS PERTAINS TO QUESTIONS 1 AND 3? WHAT ADDITIONAL DATA ARE NEEDED? AACE/ACE CGM Consensus Conference:
500 1 QUESTION 4. WHAT CLINICAL DATA ARE CURRENTLY AVAILABLE TO SUPPORT EXPANDED CGM COVERAGE BY PAYERS AS PERTAINS TO QUESTIONS 1 AND 3? WHAT ADDITIONAL DATA ARE NEEDED? AACE/ACE CGM Consensus Conference:
Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements
 Diabetes Care In Press, published online March 2, 2007 Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements SHORT RUNNING
Diabetes Care In Press, published online March 2, 2007 Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements SHORT RUNNING
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2016 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2016 Section: DME Place(s)
REPORT INTERPRETATION
 REPORT INTERPRETATION: Interpreting ipro Professional Continuous Glucose Monitoring (CGM) Reports and Making Therapy Adjustments TARGET AUDIENCE The audience for this section is physicians, mid-level practitioners,
REPORT INTERPRETATION: Interpreting ipro Professional Continuous Glucose Monitoring (CGM) Reports and Making Therapy Adjustments TARGET AUDIENCE The audience for this section is physicians, mid-level practitioners,
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
THE MINIMED 670G SYSTEM SCHOOL NURSE GUIDE
 THE MINIMED 670G SYSTEM SCHOOL NURSE GUIDE Indicated for type 1 patients 14 and over. Prescription required. WARNING: Medtronic performed an evaluation of the MiniMed 670G system and determined that it
THE MINIMED 670G SYSTEM SCHOOL NURSE GUIDE Indicated for type 1 patients 14 and over. Prescription required. WARNING: Medtronic performed an evaluation of the MiniMed 670G system and determined that it
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness
 Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Outline of Material Introduction
Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Outline of Material Introduction
Glycemic Variability:
 Glycemic Variability: Do the Differences Make a Difference? Kim L Kelly, PharmD, BCPS, FCCP Define Variability VERY LOW GLYCEMIC VARIABILITY LOW GLYCEMIC VARIABILITY HIGH GLYCEMIC VARIABILITY So what s
Glycemic Variability: Do the Differences Make a Difference? Kim L Kelly, PharmD, BCPS, FCCP Define Variability VERY LOW GLYCEMIC VARIABILITY LOW GLYCEMIC VARIABILITY HIGH GLYCEMIC VARIABILITY So what s
Medtronic MiniMed Insulin Infusion Pumps
 Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
The recovery of cognitive function following hypoglycemia
 RIEF REPORT Delayed Recovery of Cognitive Function Following Hypoglycemia in Adults With Type 1 Diabetes Effect of Impaired Awareness of Hypoglycemia Nicola N. Zammitt, 1 Roderick E. Warren, 1 Ian J. Deary,
RIEF REPORT Delayed Recovery of Cognitive Function Following Hypoglycemia in Adults With Type 1 Diabetes Effect of Impaired Awareness of Hypoglycemia Nicola N. Zammitt, 1 Roderick E. Warren, 1 Ian J. Deary,
Accuracy Requirements for a Hypoglycemia Detector: An Analytical Model to Evaluate the Effects of Bias, Precision, and Rate of Glucose Change
 Journal of Diabetes Science and Technology Volume 1, Issue 5, September 2007 Diabetes Technology Society SYMPOSIUM Accuracy Requirements for a Hypoglycemia Detector: An Analytical Model to Evaluate the
Journal of Diabetes Science and Technology Volume 1, Issue 5, September 2007 Diabetes Technology Society SYMPOSIUM Accuracy Requirements for a Hypoglycemia Detector: An Analytical Model to Evaluate the
Diabetes Care 25: , 2002
 Pathophysiology/Complications O R I G I N A L A R T I C L E The Glucose Area Under the Profiles Obtained With Continuous Glucose Monitoring System Relationships With HbA lc in Pediatric Type 1 Diabetic
Pathophysiology/Complications O R I G I N A L A R T I C L E The Glucose Area Under the Profiles Obtained With Continuous Glucose Monitoring System Relationships With HbA lc in Pediatric Type 1 Diabetic
OneTouch Reveal Web Application. User Manual for Healthcare Professionals Instructions for Use
 OneTouch Reveal Web Application User Manual for Healthcare Professionals Instructions for Use Contents 2 Contents Chapter 1: Introduction...4 Product Overview...4 Intended Use...4 System Requirements...
OneTouch Reveal Web Application User Manual for Healthcare Professionals Instructions for Use Contents 2 Contents Chapter 1: Introduction...4 Product Overview...4 Intended Use...4 System Requirements...
First steps for success.
 First steps for success. Getting to know continuous glucose monitoring (CGM). The Animas Vibe System is approved for persons age 2 and older. Important Safety Information The Animas Vibe Insulin Pump and
First steps for success. Getting to know continuous glucose monitoring (CGM). The Animas Vibe System is approved for persons age 2 and older. Important Safety Information The Animas Vibe Insulin Pump and
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide FreeStyle LibreLink app A FreeStyle Libre product IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide FreeStyle LibreLink app A FreeStyle Libre product IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the
Reconstruction of Glucose in Plasma from Interstitial Fluid Continuous Glucose Monitoring Data: Role of Sensor Calibration
 Journal of Diabetes Science and Technology Volume 1, Issue 5, September 2007 Diabetes Technology Society SYMPOSIUM Reconstruction of Glucose in Plasma from Interstitial Fluid Continuous Glucose Monitoring
Journal of Diabetes Science and Technology Volume 1, Issue 5, September 2007 Diabetes Technology Society SYMPOSIUM Reconstruction of Glucose in Plasma from Interstitial Fluid Continuous Glucose Monitoring
Continuous glucose monitoring (CGM) systems enable
 DIA-2014-0375-ver9-Wang_1P.3d 05/05/15 2:45pm Page 1 DIABETES TECHNOLOGY & THERAPEUTICS Volume 17, Number 8, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/dia.2014.0375 DIA-2014-0375-ver9-Wang_1P Type: original-article
DIA-2014-0375-ver9-Wang_1P.3d 05/05/15 2:45pm Page 1 DIABETES TECHNOLOGY & THERAPEUTICS Volume 17, Number 8, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/dia.2014.0375 DIA-2014-0375-ver9-Wang_1P Type: original-article
The role of a three-day trial of continuous glucose monitoring on HbA1C in noncompliant type 1 diabetic adolescents and teenagers
 The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects The role of a three-day trial of continuous glucose monitoring on HbA1C in noncompliant type 1 diabetic
The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects The role of a three-day trial of continuous glucose monitoring on HbA1C in noncompliant type 1 diabetic
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 4/1/2018 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 4/1/2018 Section: DME Place(s)
1. Continuous Glucose Monitoring
 1. Continuous Glucose Monitoring 1. Physiology of interstitial fluid glucose 2. Comparison of CGM and self-monitored blood glucose (SMBG) data 3. Insulin dosing indication in BGM vs. CGM & the FDA 4. Protection
1. Continuous Glucose Monitoring 1. Physiology of interstitial fluid glucose 2. Comparison of CGM and self-monitored blood glucose (SMBG) data 3. Insulin dosing indication in BGM vs. CGM & the FDA 4. Protection
10:20 AM March 5, B 100% 235 u. 124 mg/dl 3 HRS INSULIN ON BOARD:
 DEXCOM G5 MOBILE CGM COMPATIBLE B 100% 235 u INSULIN ON BOARD: OPTIONS 10:20 AM March 5, 2017 400 350 300 250 200 150 100 50 1.1 u 1:09 hrs BOLUS 124 mg/dl 3 HRS The pump that gets updated, not outdated.
DEXCOM G5 MOBILE CGM COMPATIBLE B 100% 235 u INSULIN ON BOARD: OPTIONS 10:20 AM March 5, 2017 400 350 300 250 200 150 100 50 1.1 u 1:09 hrs BOLUS 124 mg/dl 3 HRS The pump that gets updated, not outdated.
DISCLAIMER: ECHO Nevada emphasizes patient privacy and asks participants to not share ANY Protected Health Information during ECHO clinics.
 DISCLAIMER: Video will be taken at this clinic and potentially used in Project ECHO promotional materials. By attending this clinic, you consent to have your photo taken and allow Project ECHO to use this
DISCLAIMER: Video will be taken at this clinic and potentially used in Project ECHO promotional materials. By attending this clinic, you consent to have your photo taken and allow Project ECHO to use this
Continuous Glucose Monitoring: The Future of Diabetes Management
 In Brief Continuous glucose monitoring (CGM) technology has the potential to revolutionize diabetes care in the near future because of the real-time feedback it provides about therapeutic interventions
In Brief Continuous glucose monitoring (CGM) technology has the potential to revolutionize diabetes care in the near future because of the real-time feedback it provides about therapeutic interventions
Artificial Pancreas Device Systems. Populations Interventions Comparators Outcomes Individuals: With type 1 diabetes
 Protocol Artificial Pancreas Device Systems Medical Benefit Effective Date: 07/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18, 05/18, 01/19 Preauthorization
Protocol Artificial Pancreas Device Systems Medical Benefit Effective Date: 07/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18, 05/18, 01/19 Preauthorization
Artificial Pancreas Device Systems. Populations Interventions Comparators Outcomes. pump. pump
 Protocol Artificial Pancreas Device Systems (10130) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18 Preauthorization is required.
Protocol Artificial Pancreas Device Systems (10130) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18 Preauthorization is required.
Updates in Diabetes Technology
 Updates in Diabetes Technology Jessica Kirk, MSN, RN, CPN, CDE Nurse Manager, Endo ECHO No disclosures Disclosures 1 Objectives Distinguish patients appropriate for continuous glucose monitoring and insulin
Updates in Diabetes Technology Jessica Kirk, MSN, RN, CPN, CDE Nurse Manager, Endo ECHO No disclosures Disclosures 1 Objectives Distinguish patients appropriate for continuous glucose monitoring and insulin
Continuous Glucose Monitoring (CGM)
 Continuous Glucose Monitoring (CGM) Date of Origin: 02/2001 Last Review Date: 08/22/2018 Effective Date: 08/22/2018 Dates Reviewed: 04/2004, 04/2005, 03/2006, 11/2006, 12/2007, 03/2008, 09/2008, 04/2009,
Continuous Glucose Monitoring (CGM) Date of Origin: 02/2001 Last Review Date: 08/22/2018 Effective Date: 08/22/2018 Dates Reviewed: 04/2004, 04/2005, 03/2006, 11/2006, 12/2007, 03/2008, 09/2008, 04/2009,
MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps
 POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 05/24/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization
POLICY: PG0177 ORIGINAL EFFECTIVE: 12/01/08 LAST REVIEW: 05/24/18 MEDICAL POLICY Continuous Glucose Monitoring Systems and Insulin Pumps GUIDELINES This policy does not certify benefits or authorization
Assessing the value of the Ambulatory Glucose Profile in clinical practice
 Assessing the value of the Ambulatory Glucose Profile in clinical practice STEPHAN MATTHAEI Abstract Glycated haemoglobin (HbA 1c) is a measure of mean blood glucose levels over time. It is not a good
Assessing the value of the Ambulatory Glucose Profile in clinical practice STEPHAN MATTHAEI Abstract Glycated haemoglobin (HbA 1c) is a measure of mean blood glucose levels over time. It is not a good
Using the Bolus Wizard Calculator
 9501179-011 Using the Bolus Wizard Calculator Objective Describe the features and benefits of the Bolus Wizard Calculator Key Points The Bolus Wizard: Estimates high blood glucose corrections using the
9501179-011 Using the Bolus Wizard Calculator Objective Describe the features and benefits of the Bolus Wizard Calculator Key Points The Bolus Wizard: Estimates high blood glucose corrections using the
Accuracy of the 5-Day FreeStyle Navigator Continuous Glucose Monitoring System. Comparison with frequent laboratory reference measurements
 Emerging Treatments and Technologies O R I G I N A L A R T I C L E Accuracy of the 5-Day FreeStyle Navigator Continuous Glucose Monitoring System Comparison with frequent laboratory reference measurements
Emerging Treatments and Technologies O R I G I N A L A R T I C L E Accuracy of the 5-Day FreeStyle Navigator Continuous Glucose Monitoring System Comparison with frequent laboratory reference measurements
Receiver and App Setup
 Continuous Glucose Monitoring System Receiver and App Setup For training videos visit dexcom.com/medicare Overview Your Dexcom G5 Continuous Glucose Monitoring (CGM) System is made up of: Transmitter (Sensor
Continuous Glucose Monitoring System Receiver and App Setup For training videos visit dexcom.com/medicare Overview Your Dexcom G5 Continuous Glucose Monitoring (CGM) System is made up of: Transmitter (Sensor
INSPIRED BY GIVE HIM MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs.
 INSPIRED BY GIVE HIM MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs. * NOW APPROVED FOR AGES 7 AND UP. Waterproof to a depth
INSPIRED BY GIVE HIM MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs. * NOW APPROVED FOR AGES 7 AND UP. Waterproof to a depth
Continuous Glucose Monitoring System. Receiver Setup
 Continuous Glucose Monitoring System Receiver Setup Overview Your Dexcom G5 Continuous Glucose Monitoring (CGM) System is made up of: Receiver What do I do with it? Review your readings. Set and receive
Continuous Glucose Monitoring System Receiver Setup Overview Your Dexcom G5 Continuous Glucose Monitoring (CGM) System is made up of: Receiver What do I do with it? Review your readings. Set and receive
Continuous Glucose Monitoring: Changing Diabetes Behavior in Real Time and Retrospectively
 Journal of Diabetes Science and Technology Volume 2, Issue 3, May 2008 Diabetes Technology Society CONTROVERSIES in Continuous Glucose Monitoring Continuous Glucose Monitoring: Changing Diabetes Behavior
Journal of Diabetes Science and Technology Volume 2, Issue 3, May 2008 Diabetes Technology Society CONTROVERSIES in Continuous Glucose Monitoring Continuous Glucose Monitoring: Changing Diabetes Behavior
Continuous Glucose Monitoring
 Continuous Glucose Monitoring What is Continuous Glucose Monitoring? Blood glucose meters measure glucose in your blood and glucose sensors measure glucose levels in the fluid around the cells They are
Continuous Glucose Monitoring What is Continuous Glucose Monitoring? Blood glucose meters measure glucose in your blood and glucose sensors measure glucose levels in the fluid around the cells They are
Continuous or Intermittent Glucose Monitoring (CGMS) in Interstitial Fluid Corporate Medical Policy
 Continuous or Intermittent Glucose Monitoring (CGMS) in Interstitial Fluid Corporate Medical Policy File name: Continuous or Intermittent Glucose Monitoring (CGMS) in Interstitial Fluid File code: UM.DME.07
Continuous or Intermittent Glucose Monitoring (CGMS) in Interstitial Fluid Corporate Medical Policy File name: Continuous or Intermittent Glucose Monitoring (CGMS) in Interstitial Fluid File code: UM.DME.07
Blood Glucose Awareness Training (BGAT-2)
 Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Blood Glucose Awareness Training (BGAT-2) Long-term benefits DANIEL J. COX, PHD 1 LINDA GONDER-FREDERICK, PHD 1 WILLIAM POLOKY, PHD 2 3 DAVID
Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Blood Glucose Awareness Training (BGAT-2) Long-term benefits DANIEL J. COX, PHD 1 LINDA GONDER-FREDERICK, PHD 1 WILLIAM POLOKY, PHD 2 3 DAVID
INSPIRED BY GIVE HER MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs.
 INSPIRED BY GIVE HER MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs. * NOW APPROVED FOR AGES 7 AND UP. MORE TIME HERE TO USING
INSPIRED BY GIVE HER MORE. The MiniMed 670G system is the world s first insulin delivery system that automatically adapts to your child s needs. * NOW APPROVED FOR AGES 7 AND UP. MORE TIME HERE TO USING
Dexcom Current and Future Technology Innovations. Jorge Valdes Chief Technical Officer
 Dexcom Current and Future Technology Innovations 2015 Jorge Valdes Chief Technical Officer G5 App Sensor (Same as G4) Indicated for adults and children down to Age 2 years Tiny insertion needle (26Ga)
Dexcom Current and Future Technology Innovations 2015 Jorge Valdes Chief Technical Officer G5 App Sensor (Same as G4) Indicated for adults and children down to Age 2 years Tiny insertion needle (26Ga)
Coverage Guidelines. Continuous Glucose Monitors (CGMs)
 Coverage Guidelines Continuous Glucose Monitors (CGMs) Disclaimer: Please note that Baptist Health Plan Coverage Guidelines may be updated throughout the year. A printed version may not be most up to date
Coverage Guidelines Continuous Glucose Monitors (CGMs) Disclaimer: Please note that Baptist Health Plan Coverage Guidelines may be updated throughout the year. A printed version may not be most up to date
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
James J. Chamberlain, MD 1, Dana Dopita, RN, MSN, CDE 1, Emily Gilgen, RD, CD, CDE 1, and Annie Neuman, MPA, PA-C 1.
 604633DSTXXX10.1177/1932296815604633Journal of Diabetes Science and TechnologyChamberlain et al research-article2015 Original Article Impact of Frequent and Persistent Use of Continuous Glucose Monitoring
604633DSTXXX10.1177/1932296815604633Journal of Diabetes Science and TechnologyChamberlain et al research-article2015 Original Article Impact of Frequent and Persistent Use of Continuous Glucose Monitoring
FAQs for HCP segment New Instructions for Dexcom G5 Mobile Continuous Glucose Monitoring (CGM) System Non-Adjunctive Indication
 FAQs for HCP segment New Instructions for Dexcom G5 Mobile Continuous Glucose Monitoring (CGM) System Non-Adjunctive Indication Q1. The Dexcom G5 Mobile System is the first CGM System to receive FDA approval
FAQs for HCP segment New Instructions for Dexcom G5 Mobile Continuous Glucose Monitoring (CGM) System Non-Adjunctive Indication Q1. The Dexcom G5 Mobile System is the first CGM System to receive FDA approval
Preventing Hypoglycemia Using Predictive Alarm Algorithms and Insulin Pump Suspension
 DIABETES TECHNOLOGY & THERAPEUTICS Volume 11, Number 2, 29 Mary Ann Liebert, Inc. DOI: 1.189/dia.28.32 Preventing Hypoglycemia Using Predictive Alarm Algorithms and Insulin Pump Suspension Bruce Buckingham,
DIABETES TECHNOLOGY & THERAPEUTICS Volume 11, Number 2, 29 Mary Ann Liebert, Inc. DOI: 1.189/dia.28.32 Preventing Hypoglycemia Using Predictive Alarm Algorithms and Insulin Pump Suspension Bruce Buckingham,
Clinical Evaluation for. Embrace No Code Blood Glucose Monitoring System
 Embrace No Code Report Clinical Evaluation for Embrace No Code Blood Glucose Monitoring System Applicant: Apex Biotechnology Corp. Test Date: 2007/8/28~2007/9/21 Clinical Site: SCHMIDT Group Practice Clinic
Embrace No Code Report Clinical Evaluation for Embrace No Code Blood Glucose Monitoring System Applicant: Apex Biotechnology Corp. Test Date: 2007/8/28~2007/9/21 Clinical Site: SCHMIDT Group Practice Clinic
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System
 Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
