Most Recent Revision and Approval Date: April 2016 Copyright MedStar Health, 2012
|
|
|
- Ursula Brown
- 6 years ago
- Views:
Transcription
1 Management of Hypercholesterolemia Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions regarding the care of their patients. They are not a substitute for individual judgment brought to each clinical situation by the patient s primary care provider in collaboration with the patient. As with all clinical reference resources, they reflect the best understanding of the science of medicine at the time of publication, but should be used with the clear understanding that continued research may result in new knowledge and recommendations. The following guideline is based on the report by American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ATP IV), published in November Statins have been shown to prevent fatal and non-fatal ASCVD events (except in those w/ NYHA class II-IV heart failure or chronic dialysis patients). They reduce secondary-prevention all-cause mortality; there is mounting evidence that they improve primary-prevention all-cause mortality risk in high-risk patients. The ATP IV proposed treatment method abandons a treat to target paradigm and embraces a method of using the maximum tolerated statin intensity in the groups known to benefit. Prior proposed approaches to statin treatment lack supporting RCT data. Global Risk Assessment for Primary Prevention using the new Pooled Cohort Equations to estimate 10-year ASCVD (first occurrence nonfatal and fatal MI and fatal stroke) risk in non-hispanic white and black patients without clinical ASCVD identifies those most likely to benefit from statins for primary prevention. The few existing studies of non-statin cholesterol lowering drugs do not demonstrate significant risk reduction above statin treatment alone and their use should be confined to select patients based on clinical judgment. The ATP IV guidelines continue to recommend a heart healthy diet, regular exercise, avoidance of tobacco products, and maintenance of a healthy weight and stress that all of these interventions were included as background therapy of RCTs of pharmacological cholesterol therapy. Statin Benefit Groups There are four groups of age 21 yo men and non-pregnant/non-nursing women for whom statin atherosclerotic cardiovascular disease benefit clearly exceeds adverse event risk (w/o NYHA II-IV HF and/or on HD). ATP IV guidelines recommend which intensity statin should be initiated in these cases, with some caveats: 1) Individuals with clinical ASCVD (ACS, h/o MI, stable or unstable angina, coronary or arterial revascularization, CVA, TIA or PAD presumed atherosclerotic) High-Intensity statin preferred 2) Individuals with LDL-C >= 190 mg/dl High-Intensity statin preferred 3) Individuals years of age with diabetes and LDL-C mg/dl: If 10-yr ASCVD risk >= 7.5% High-Intensity Statin preferred, otherwise use Moderate-Intensity 4) Individuals years of age without diabetes or clinical ASCVD and with LDL mg/dl and an estimated 10-year ASCVD risk of 7.5% or higher Moderate to High- Intensity Statin When risk is 5-7.5%, there can still be net benefit with moderate-intensity statin therapy but should be weighed against possible adverse events and treatment should be individualized 1) Rate of excess diabetes onset varies by statin intensity; for moderate-intensity is it ~0.1 excess cases per 100 treated patients per yr; for high intensity it is ~0.3
2 2) Myopathy (~ 0.01 excesses case/100 treated/yr) and hemorrhagic stroke (~ 0.01 excesses cases/100 treated/ yr) While ATP IV endorses the new clinical calculator, concerns have been raised as the Pooled Cohort Equations still lack validation in certain ethnic groups. Further studies may help clarify the calculator s broad utility. All calculators have benefits and drawbacks; clinician judgment may be used in choice of calculator, but the clinician should be well acquainted with the calculator they are using. For practical purposes, the provider may decide to quantify risk using another calculator, for example: Framingham Coronary Heart Disease 10-year Risk which is validated in whites, African Americans and Hispanic women, but may be less accurate in certain patients and does not estimate stroke risk Framingham General Cardiovascular Disease 10-year Risk which is based on data from primarily white patients Nontraditional risk factors may be taken into account when considering statin initiation for selected individuals when use of the Pooled Cohort Equations alone does not seem to be useful. These include: LDL-C >=160 mg/dl or other evidence of genetic hyperlipidemia, family h/o premature ASCVD (first degree male relative with onset < 55 yo or female < 65 yo), hs-crp 2 mg/dl, CAC score > = 300 Agatson units or 75 th percentile for age, sex, and ethnicity, ABI < 0.9, or elevated lifetime risk of ASCVD. Clinician decisions should also take into account patient preferences and individualized risks and benefits. The age at which screening should begin should be based on an individual s other cardiac risk factors and desire to be screened. Screening may begin in non-pregnant adults at any age but no later than age 40 (the age at which statin therapy for primary prevention is recommended). 10-year risk should be re-evaluated every 4-6 yrs between years old. The development of diabetes or clinical ASCVD should prompt evaluation as well. Statin Choice Choose medication and dose to achieve the desired LDL-C reduction intensity. High-Intensity Statin Therapy Moderate-Intensity Statin Low-Intensity Statin Therapy Therapy Lowers LDL-C >=50% Lowers LDL-C 30-50% Lowers LDL-C < 30% Atorvastatin mg ($165- $173) Rosuvastatin mg ($298) * Never tested in RCT Atorvastatin mg ($116- $165) Rosuvastatin 5-10 mg ($298)Simvastatin mg ($84-$147) -FDA does not recommend use of simvastatin 80 mg due to increased risk of myopathy Pravastatin mg ($203) Lovastatin 40 mg ($128) Fluvastatin XL 80 mg* Simvastatin 10 mg*($84) Pravastatin mg ($96-$138) Lovastatin 20 mg ($71) Fluvastatin mg * ($150) Pitavastatin 1 mg* ($252) Modification of Statin Choice 1) Since the following patient characteristics predispose to adverse statin effects, a moderate-intensity
3 statin should be used: A) Multiple or serious co-morbidities, including impaired renal/ hepatic function B) H/o previous statin intolerance or muscle disorder C) Unexplained elevation of ALT > 3 x upper limit of normal D) Patient characteristics or concomitant use of medicines affecting statin metabolism E) Age >= 75 yo 1) Fewer people > 75 were included in the reviewed RCTs but evidence supports continuing tolerated statins. The small amount of available data does not clearly support starting high-intensity statins for secondary prevention; a larger amount of data does support the use of moderate-intensity statins. 2) Few data in this group indicate a primary prevention benefit, so one must consider risk and benefits; Pooled Cohort Equations can be used in ages ) A lower intensity than recommended statin may be considered for other compelling indications including a history of hemorrhagic stroke or Asian ancestry 3) ATP IV makes no recommendations regarding initiation or discontinuation of statins in NYHA II-IV ischemic systolic HF or in patients on maintenance hemodialysis as they have been identified to be less likely to benefit from statin therapy. Initial Evaluation for those not currently on a statin 1. Clinical ASCVD: fasting (preferred) lipid panel, ALT, CK (if indicated) a. CK should not routinely be measured during statin therapy b. Baseline measurement of CK may be reasonable if there is concern for risk based on personal or family history of statin intolerance or muscle disease, clinical presentation, or concomitant drug treatment that may increase myopathy risk c. During statin treatment, it is reasonable to measure CK in individuals with muscle symptoms (pain, tenderness, stiffness, cramping, weakness, generalized fatigue) d. Routine monitoring of transaminases during statin therapy is no longer recommended. It is reasonable, however, to re-measure ALT in the setting of unusual fatigue, weakness, appetite loss, abdominal pain, dark urine, jaundice/icterus. For elevations in ALT > 3 times upper limit of normal, further investigation and either reducing statin dose, change to a different statin or stopping the medication are warranted. 2. No Clinical ASCVD: as above and screen for diabetes with HgbA1c or fasting glucose if diabetes status unknown 3. Evaluate for secondary causes as appropriate, particularly if Triglycerides are 500 mg/dl or LDL-C 190 mg/dl. Common Secondary Causes of Hyperlipidemia Seen in Clinical Practice Secondary Cause Elevated LDL-C Elevated Triglyceride Diet Drugs Saturated or trans-fat, weight gain, anorexia Diuretics, cyclosporine, glucocorticoids, amiodarone Weight gain, very low-fat diets, high intake of refined carbohydrates, excessive alcohol intake Oral estrogens, glucocorticoids, bile acid sequestrants, protease inhibitors, retinoic acid, anabolic steroids, sirolimus, raloxifeine, tamoxifen, most beta blockers (carvedilol most favorable) effect on lipid profile),
4 Diseases Biliary obstructions, nephrotic syndrome Nephrotic syndrome, chronic renal failure, lidodystrophies Disorders, altered metabolism Hypothyroidism, obesity, pregnancy Diabetes (poorly controlled), hypothyroidism, obesity, pregnancy Monitoring Therapy 1) Lipid panel 4-12 weeks after starting statin to determine adherence and then every 3-12 months as clinically indicated a. High-intensity statin therapy generally results in >=50% decrease from untreated baseline (if baseline is unknown, LDL-C < 100 has generally been observed) b. Moderate-intensity statin therapy generally results in 30-50% reduction c. Percent reduction may be used to indicate adherence (but can also indicate biologic variability); attention should be paid to adherence and lifestyle therapy, evaluation and treatment for secondary causes; clinical judgment should be used to decide if any therapy should be increased 2) A decrease in statin dose may be considered when 2 consecutive LDL-C values are < 40 mg/dl 3) It may be harmful to initiate or increase a simvastatin dose to 80 mg/dl due to the risk of rhabdomyolysis; lovastatin should be avoided in the setting of several medicines and dose limitations exist for other medicines; make sure to check labeling 4) Current diabetes screening guidelines should be maintained for those on statins 5) A review of manufacturer s prescribing information may be useful prior to initiation of any cholesterol lowering drug 6) To evaluate and treat muscle symptoms: a. Obtain a history of baseline symptoms prior to starting therapy b. For unexplained severe symptoms, discontinue statin and evaluate CK, Cr, UA c. For mild-moderate symptoms i. Discontinue statin until symptoms can be evaluated ii. Evaluate for conditions that might increase risk (hypothyroidism, reduced renal or hepatic function, rheumatologic disorder like polymyalgia rheumatica, steroid myopathy, vitamin D deficiency, primary muscle disease) 1. If symptoms resolve and there is no contraindication, give the same statin at an original or lower dose and observe for symptoms a. If causal relationship exists, discontinue original statin and when symptoms resolve, use a low dose of different statin. Pravastatin and fluvastatin are the statins with the least intrinsic muscle toxicity. b. Once that dose is tolerated, it can be gradually increased 2. If symptoms do not resolve after 2 mo without statin, or CK does not return to normal, consider other causes 3. If the statin is determined to not be the cause, or if the predisposing condition has been treated, the original statin at the original dose can be resumed 7) For presentation with a confusional state or memory impairment, it may be reasonable to evaluate for nonstatin causes (e.g. exposure to other drugs, systemic, or neuropsychiatric causes) in addition to possible statin adverse effects 8) Statins used in combination with other cholesterol-lowering drug therapies may require more intensive monitoring 9) Even lower-intensity statin therapy can reduce ASCVD events, so maximum intensity that does not cause adverse events should be used
5 10) Adverse events involving statins should be reported to the FDA MedWatch program Non-statin Therapy Non-statin therapy can be considered in high-risk patients (including those with clinical ASCVD less than 75 yo, those with LDL-C > 190 or diabetes) who have a less-than-anticipated response to statins, or are unable to tolerate the recommended statin intensity; clinicians should preferentially prescribe drugs w/ RCT proof of ASCVD risk reduction that exceeds risk of adverse effects 1) Niacin a. Obtain transaminases, fasting glucose or A1c and uric acid before initiation, during uptitration to maintenance dose, then every 6 months b. Niacin should not be used if LFTs are > 2-3 x ULN; there are persistent severe cutaneous symptoms, persistent hyperglycemia, acute gout, unexplained abdominal pain, or GI symptoms, or if new onset atrial fibrillation or weight loss occurs c. If an adverse effect occurs, risk: benefit ratio must be considered before restarting d. To reduce cutaneous symptoms: i. Start low dose and titrate over weeks as tolerated ii. Take w/ food or premedicate w/ 325 mg ASA 30 min prior to dose iii. If using extended-release preparation: increase from 500 mg to 2000 mg/day over 4-8 weeks, <= weekly iv. If using immediate-release preparation: increase from 100 mg TID and up-titrate to 3g/daily, in 2-3 divided doses 2) Bile Acid Sequestrants a. Do not use if baseline fasting trig > 300 mg/dl or type III hyperlipoproteinemia; fasting lipid should be obtained at baseline, at 3 mo and then Q6-12 mo b. May be used with caution if baseline trig is with fasting lipids at 6 weeks; discontinue if triglycerides exceed 400 mg/dl 3) Cholesterol-Absorption Inhibitor (Ezetimibe) a. Reasonable to obtain transaminases at baseline; when co-administered with statin, monitor LFTs as clinically indicated and stop if ALT > 3x ULN 4) Fibrates a. Gemfibrozil should not be initiated in patients on statin therapy due to increased risk of muscle symptoms and rhabdomyolysis b. Fenofibrate concurrent use with statin therapy is no longer recommended. FDA has deemed that benefits of combined therapy do not outweigh risks. 5) Omega-3 Fatty Acids a. If EPA and/or DHA are used for trig > 500 mg/dl, evaluate in setting of GI disturbance, skin changes, bleeding 6) New Non-statin therapy options: PCSK9 Inhibitors a. Evolocumab (Repatha, Repatha SureClick) and Alirocumab (Praluent) b. Reduce LDL_C by as much as 60% in patients on statins c. Indications i. Homozygous familial hypercholesterolemia: Adjunct to diet and other LDL-lowering therapies (eg, statins, ezetimibe, LDL apheresis) for the treatment of patients with
6 homozygous familial hypercholesterolemia (HoFH) who require additional lowering of LDL-C. (evolocumab only) ii. Hyperlipidemia, primary: Adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (CVD), who require additional lowering of low density lipoprotein cholesterol (LDL-C). (evolocumab and alirocumab) d. Administered subcutaneously monthly evolocumab (Repatha) or every 2 weeks alirocumab e. (Praluent). Evolocumab does have an alternate q 2 week regimen for primary hyperlipidemia) f. Repatha (140 mg) $651 per dose; Praluent (75 mg 150 mg) $672 per dose; Repatha utilizing monthly dosing regimen $1853 Lipid Lifestyle Management Guideline A separate Lifestyle Management Guideline was also issued, with the following recommendations for adults who would benefit from LDL-C lowering: consume a dietary pattern high in vegetables, fruits, and whole grains with low-fat dairy products, poultry, fish, legumes, nontropical vegetable oils and nuts; limit sweets, sugar-sweetened beverages, and red meat. The DASH dietary plan, USDA Food Pattern (which offers lacto-ovo vegetarian and vegan options), and AHA Diet are plans that can achieve these recommendations. This pattern should be individualized, keeping patient preferences and other dietary needs in mind. The pattern should aim to achieve 5-6% of calories from saturated fat and reduce the percent of calories from saturated and trans-fat. Aerobic exercise of moderate or vigorous intensity should be performed 3-4 sessions per week, an average of 40 minutes per session. The separate Guideline on Lifestyle Management published in 2013 has specific recommendations for patients who would benefit from LDL-C lowering and blood pressure lowering and can be referred to for more details. Patient education: Eating_UCM_001188_SubHomePage.jsp References Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Lloyd-Jone DM, Blum CB, McBride P, Eckel RH, Schwartz JS, Goldberg AC, Shero ST, Gordon D, Smith Jr SC, Levy D, Watson K, Wilson PWF, 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Risk in Adults, Journal of the American College of Cardiology (2013), doi: /j.jacc Eckel RH, Jakicic JM, Ard JD, Hubbard VS, de Jesus JM, Lee IM, Lichtenstein AH, Loria CM, Millen
7 BE, Houston Miller N, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TW, Yanovski SZ AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 00: FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs, PL Detail-Document, Common Cardiovascular Risk Calculators. Pharmacist s Letter/Prescriber s Letter. January PL Detail-Document, Common Cardiovascular Risk Calculators. Pharmacist s Letter/Prescriber s Letter. January 2014 Alirocumab (Praluent) to Lower LDL-Cholesterol. The Medical Letter August 17, 2015 Evolucumab (Repatha) A Second PCSK9 Inhibitor to Lower LDL-Cholesterol. The Medical Letter. October 12, Medications for Cholesterol Reduction HMG- CoA Reductase Inhibitors (Statins) Average LDL-C reduction: Low Intensity <30%, 30% to <50%, High Intensity 50% Drug Dose Comments /Safety Atorvastatin mg daily (see guideline pages 3-5 for additional (Lipitor and others) ($116-$173) High Intensity mg daily Only one RCT recommendations) with 40 mg dose; down-titration if unable to tolerate 80 mg dose Baseline measurement of CK is reasonable for individuals believed to be at increased risk for adverse muscle events During statin therapy, it is reasonable to
8 Fluvastatin (Lescol) ($150) Fluvastatin XL (Lescol XL)($150) Lovastatin (Mevacor) ($71) Lovastatin extended release (Altoprev) ($656) Pitavastatin (Livalo) ($252) Pravastatin(Pravacho l ($96-$138) Low Intensity mg daily 40 mg twice daily 80 mg daily measure CK in individuals with muscle symptoms, including pain, tenderness, stiffness, cramping, weakness, or generalized fatigue. Low Intensity Low Intensity Low Intensity 20 mg daily 40 mg daily (60 mg dose is not included in ACC/AHA guidelines) 1 mg daily 2-4 mg daily mg daily mg daily Baseline measurement of hepatic transaminase levels (ALT) should be performed before initiating statin therapy. During statin therapy, it is reasonable to measure hepatic function if symptoms suggesting hepatotoxicity arise Individuals receiving statin therapy should be evaluated for new-onset diabetes mellitus Rosuvastatin (Crestor)($298) Simvastatin (Zocor) ($84) High Intensity Low Intensity 5-10 mg daily mg daily 10 mg daily mg daily 80 mg dose is not recommended due to increased risk of myopathy, including rhabdomyolysis If unexplained severe muscle symptoms or fatigue develop during statin therapy, promptly discontinue the statin and address the possibility of rhabdomyolysis by evaluating CK, creatinine, and a urinalysis for myoglobinuria. Cost per month for normal dose range Significant Statin Drug Interactions
9 Atorvastatin Use with caution in patients in patients taking strong CYP34A inhibitors. Consider alternate agents. Avoid with atorvastatin: Cyclosporine Gemfibrozil Tipranavir plus ritonavir Telaprevir Itraconazole Use with caution and use with the lowest atorvastatin dose necessary: Lopinavir + ritonavir Amiodarone Fluvastatin Lovastatin Do not exceed 20 mg daily atorvastatin with the following agents: Darunavir + ritonavir Fosamprenavir Fosamprenavir + ritonavir Saquinavir + ritonavir Administer 1 hr before or at least 4 hours after cholestyramine or colestipol Use statins with caution with niacin 1000 mg/day Experts suggest avoiding grapefruit with atorvastatin Do not exceed fluvastatin 20 mg twice daily (fluvastatin may be least likely to interact): Cyclosporine Administer 1 hr before or at least 4 hours after cholestyramine or colestipol Avoid with fluvastatin: Gemfibrozil, Fenofibrate Use statins with caution with niacin 1000 mg/day Use with caution in patients in patients taking strong CYP34A inhibitors. Consider alternate agents. Contraindicated with lovastatin: Itraconazole Ketoconazole Posaconazole Erythromycin Clarithromycin Telithromycin HIV protease inhibitors Boceprevir Telaprevir Nefazodone Avoid with lovastatin: Cyclosporine Gemfibrozil Do not exceed 20 mg lovastatin daily with: Danazol
10
11 Pitavastatin Pravastatin Rosuvastatin Simvastatin Diltiazem Verapamil Clarithromycin Administer 1 hr before or at least 4 hours after cholestyramine or colestipol. Use statins with caution with niacin 1000 mg/day. Limit extended release niacin to 2000 mg and lovastatin dose to 40mg daily when used in combination. Experts suggest avoiding grapefruit with lovastatin. Contraindicated with pitavastin: Cyclosporine Limit dose to 1 mg daily with: Erythromycin Limit dose to 2 mg daily with: Rifampin Administer 1 hr before or at least 4 hours after cholestyramine or colestipol Avoid use with pravastatin: Gemfibrozil Do not exceed pravastatin 20 mg daily: Cyclopsorine Do not exceed pravastatin 40 mg daily: Clarithromycin Azithromycin Use statins with caution with niacin 1000 mg/day Do not exceed rosuvastatin 5 mg: Cyclosporine Do not exceed rosuvastatin 10 mg daily: Atazanavir ± ritonavir Lopinavir + ritonavir Avoid use with rosuvastatin: Gemfibrozil Administer 1 hr before or at least 4 hours after cholestyramine or colestipol Use statins with caution with niacin 1000 mg/day Contraindicated with simvastatin: HIV protease inhibitors Boceprevir Telaprevir Itraconcazole Ketoconazole Posaconazole Danazol Clarithromycin Erythromycin Do not exceed simvastatin 20 mg: Amiodarone Amlopidine Administer 1 hr before or at least 4 hours after cholestyramine or colestipol Use statins with caution with niacin 1000 mg/day. Limit extended release niacin to 2000 mg and simvastatin dose to 40mg daily when used in combination Experts suggest avoiding grapefruit with simvastatin. 9 of 11
12 Nonstatins Clinicians treating high-risk patients who have a less-than-anticipated response to statins, who are unable to tolerate a less-than-recommended intensity of a statin, or who are completely statin intolerant may consider the addition of a nonstatin cholesterol-lowering therapy. Drug Dose Other Selective Cholesterol Absorption When ezetimibe is Inhibitor coadministered with a statin, Ezetimibe (Zetia) ($312) 10 mg every day monitor transaminase levels as clinically indicated, and discontinue ezetimibe if persistent ALT elevations >3 times ULN occur Bile Acid Sequestrants Cholestyramine granules Questran granules $200 Questran Cans $137 Cholestyramine Light packets Questran Light $187 Initial: 4g every day Usual: 4 g 2-6 times a day Prevalite packets $214 Initial: 5 g every day-twice a day Usual: g divided Colestipol (Colestid) Colestipol granules $117 Colestipol tablets $148 Colesevelam (Welchol) $622 Initial: 2 g one-two times daily Usual: 2-16 g/day 3.75 g (oral suspension or 6 tabs) once daily or 1.875g (3 tabs) twice daily BAS should not be used in individuals with baseline fasting triglyceride levels 300 mg/dl or type III hyperlipoproteinemia Use BAS with caution if baseline triglyceride levels are 250 to 299 mg/dl, and evaluate a fasting lipid panel in 4 to 6 weeks after initiation. Discontinue the BAS if triglycerides exceed 400 mg/dl. The bile acid sequestrant should be taken 1 hour after or 4 hours before other medications due to binding interactions Fibrates Gemfibrozil Lopid (Parke Davis) $ mg twice a day Gemfibrozil should not be initiated in patients on statin therapy because of an increased risk for muscle symptoms and rhabdomyolysis Niacin (Most niacin products are available over the counter) Initial: 100 mg three times a day. Niacin should not be used if: Hepatic transaminase elevations are higher than 2 to 3 times ULN. Persistent severe cutaneous symptoms, persistent hyperglycemia, acute gout or unexplained abdominal pain or gastrointestinal symptoms occur. 11 of 11
13 Immediate-release Niacor($4 generic) Extended-release Niaspan($21 generic) Usual: 2-6 g in 3 divided doses Initial: 500 mg every evening Usual: 1-2 g every evening New-onset atrial fibrillation or weight loss occurs Combination Products Ezetimibe/Simvastatin (Vytorin) $310 Initial: 10/20 mg daily Usual: 10/20 mg 10/40 mg daily (See individual agents) Ezetimibe/Atorvastatin (Liptruzet) $198 Initial: 10/10mg or 10/20 mg daily Maximum dose: 10/80 mg daily Omega 3 Fatty Acids Omega-3-acid ethyl esters (Lovaza )$348 PCSK9 Inhibitors Evolocumab (Repatha, Repatha SureClick) $1302-$1853 4g/day as single dose or 2 divided doses with or without food Hyperlipidemia, primary: SubQ: 140 mg every 2 weeks or 420 mg once monthly Homozygous familial hypercholesterolemia: SubQ: 420 mg once monthly If used for the management of severe hypertriglyceridemia, defined as triglycerides 500 mg/dl, it is reasonable to evaluate the patient for gastrointestinal disturbances, skin changes, and bleeding. Most common side effect: >10%: Respiratory: Nasopharyngitis (6% to 11%) Influenza 8-9% Hypersensitivity reactions have been reported. Alirocumab (Praluent) $1344 Hyperlipidemia SubQ: 75 mg once every 2 weeks, may increase to 150 mg every 2 weeks if inadequate response is not achieved in 4-8 weeks. Most common side effect: injection site reaction (7%), Influenza (6%), Diarrhea 5%. Liver enzyme disorder 3% Hypersensitivity reactions have been reported. Cost = monthly cost AWP) 4/2014, 4/2016 Most Recent Revision and Approval Date: 4/2016 April 2018 Ambulatory Best Practice Condition: Hypercholesterolemia 12 of 11
2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2
 2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2 GOALS ACC/AHA as publisher of guidelines Determining which patients are appropriate for statin therapy The treatment
2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2 GOALS ACC/AHA as publisher of guidelines Determining which patients are appropriate for statin therapy The treatment
Major recommendations for statin therapy for ASCVD prevention
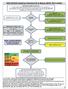 2013 A/AHA Guidelines holesterol Rx to Reduce ASVD Risk in Adults Major recommendations for statin therapy for ASVD prevention *% in LDL can be used as indication of response & adherence to Rx but is not
2013 A/AHA Guidelines holesterol Rx to Reduce ASVD Risk in Adults Major recommendations for statin therapy for ASVD prevention *% in LDL can be used as indication of response & adherence to Rx but is not
Cardiovascular Risk and Dyslipidemia Management Clinician Guide SEPTEMBER 2017
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Cardiovascular Risk and Dyslipidemia Management Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide is based on the 2017 KP National
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Cardiovascular Risk and Dyslipidemia Management Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide is based on the 2017 KP National
Pharmacy Drug Class Review
 Pharmacy Drug Class Review January 22, 2014 Authored By: Christina Manciocchi, Pharm.D. BCACP Disclaimer: Specific agents may have variations Edited By: Richard J. Kraft, Pharm.D.BCPS NEW CHOLESTEROL GUIDELINES
Pharmacy Drug Class Review January 22, 2014 Authored By: Christina Manciocchi, Pharm.D. BCACP Disclaimer: Specific agents may have variations Edited By: Richard J. Kraft, Pharm.D.BCPS NEW CHOLESTEROL GUIDELINES
Lipid Therapy: Statins and Beyond. Ivan Anderson, MD RIHVH Cardiology
 Lipid Therapy: Statins and Beyond Ivan Anderson, MD RIHVH Cardiology Outline The cholesterol hypothesis and lipid metabolism The Guidelines 4 Groups that Benefit from Lipid therapy Initiation and monitoring
Lipid Therapy: Statins and Beyond Ivan Anderson, MD RIHVH Cardiology Outline The cholesterol hypothesis and lipid metabolism The Guidelines 4 Groups that Benefit from Lipid therapy Initiation and monitoring
4/24/15. AHA/ACC 2013 Guideline Key Points
 Review of the ACC/AHA 2013 Guidelines Anita Ralstin, MS, CNS, CNP Next Step Health Consultant, LLC 1! Discuss the rationale for the change in lipid guidelines and how that affects the decision to implement
Review of the ACC/AHA 2013 Guidelines Anita Ralstin, MS, CNS, CNP Next Step Health Consultant, LLC 1! Discuss the rationale for the change in lipid guidelines and how that affects the decision to implement
High ( 50%) Restrictions mg 20-40mg PA; TS ⱡ 15 ⱡ
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/9/2017 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/16, 5/15, 2/14, 5/12, LOB AFFECTED: Medi-Cal
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/9/2017 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/16, 5/15, 2/14, 5/12, LOB AFFECTED: Medi-Cal
It is the policy of health plans affiliated with PA Health & Wellness that Vytorin is medically necessary when the following criteria are met:
 Clinical Policy: Ezetimibe and Simvastatin (Vytorin) Reference Number: PA.CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 07.18 Revision Log Description Ezetimibe/simvastatin (Vytorin ) contains ezetimibe,
Clinical Policy: Ezetimibe and Simvastatin (Vytorin) Reference Number: PA.CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 07.18 Revision Log Description Ezetimibe/simvastatin (Vytorin ) contains ezetimibe,
Conflicts of interest. What's the Skinny on the Lipid Guidelines? Key Differences. Are you applying the new ACC/AHA Lipid guidelines in your practice?
 Conflicts of interest What's the Skinny on the Lipid Guidelines? The presenter has no relevant conflicts of interest to disclose. Kathleen Vest, PharmD, CDE, BCACP At the end of this presentation, pharmacist
Conflicts of interest What's the Skinny on the Lipid Guidelines? The presenter has no relevant conflicts of interest to disclose. Kathleen Vest, PharmD, CDE, BCACP At the end of this presentation, pharmacist
Acute Coronary Syndromes (ACS)
 Sally A. Arif, Pharm.D., BCPS (AQ Cardiology) Assistant Professor of Pharmacy Practice Midwestern University, Chicago College of Pharmacy Cardiology Clinical Specialist, Rush University Medical Center
Sally A. Arif, Pharm.D., BCPS (AQ Cardiology) Assistant Professor of Pharmacy Practice Midwestern University, Chicago College of Pharmacy Cardiology Clinical Specialist, Rush University Medical Center
Conflict of Interest Disclosure. Learning Objectives. Learning Objectives. Guidelines. Update on Lifestyle Guidelines
 Conflict of Interest Disclosure Updates for the Ambulatory Care Pharmacist: Dyslipidemia and CV Risk Assessment No conflicts of interest to disclose 2014 Updates to the Updates in Ambulatory Care Pharmacy
Conflict of Interest Disclosure Updates for the Ambulatory Care Pharmacist: Dyslipidemia and CV Risk Assessment No conflicts of interest to disclose 2014 Updates to the Updates in Ambulatory Care Pharmacy
Non-Statin Lipid-Lowering Agents M Holler - Last updated: 10/2016
 Drug/Class Cholestyramine (Questran) Bile acid sequestrant Generic? Lipid Effects Y/N (monotherapy) Y LDL : 9% (4 g to 8 ; 21% (16 g to 20 ; 23% to 28% (>20 HDL : 4% to 8% (16 to 24 TG : 11% to 28% (4
Drug/Class Cholestyramine (Questran) Bile acid sequestrant Generic? Lipid Effects Y/N (monotherapy) Y LDL : 9% (4 g to 8 ; 21% (16 g to 20 ; 23% to 28% (>20 HDL : 4% to 8% (16 to 24 TG : 11% to 28% (4
Today s Recommendations
 Today s Recommendations 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk (45 pages) 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults (14 pages)
Today s Recommendations 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk (45 pages) 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults (14 pages)
Disclosures. Overview 9/30/ ACC/AHA Guidelines on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults
 2013 ACC/AHA Guidelines on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults 2014 AAHP Fall Seminar Sherry Myatt, PharmD, BCPS Assistant Director of Pharmacy for
2013 ACC/AHA Guidelines on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults 2014 AAHP Fall Seminar Sherry Myatt, PharmD, BCPS Assistant Director of Pharmacy for
2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults
 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation,
2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation,
Lipids & Hypertension Update
 Lipids & Hypertension Update No financial disclosures Michael W. Cullen, MD, FACC Senior Associate Consultant, Assistant Professor of Medicine Mayo Clinic Department of Cardiovascular Diseases 34 th Annual
Lipids & Hypertension Update No financial disclosures Michael W. Cullen, MD, FACC Senior Associate Consultant, Assistant Professor of Medicine Mayo Clinic Department of Cardiovascular Diseases 34 th Annual
Pharmacy Management Drug Policy
 SUBJECT: ; Praluent (alirocumab), Repatha (evolocumab) POLICY NUMBER: Pharmacy-61 EFFECTIVE DATE: 8/15 LAST REVIEW DATE: 9/22/2017 If the member s subscriber contract excludes coverage for a specific service
SUBJECT: ; Praluent (alirocumab), Repatha (evolocumab) POLICY NUMBER: Pharmacy-61 EFFECTIVE DATE: 8/15 LAST REVIEW DATE: 9/22/2017 If the member s subscriber contract excludes coverage for a specific service
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.16.08 Subject: Repatha Page: 1 of 8 Last Review Date: September 18, 2015 Repatha Description Repatha
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.16.08 Subject: Repatha Page: 1 of 8 Last Review Date: September 18, 2015 Repatha Description Repatha
2013 Cholesterol Guidelines. Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc.
 2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Adult Certified Nurse Practitioner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
Learning Objectives. Patient Case
 Joseph Saseen, Pharm.D., FASHP, FCCP, BCPS Professor and Vice Chair, Department of Clinical Pharmacy University of Colorado Anschutz Medical Campus Learning Objectives Identify the 4 patient populations
Joseph Saseen, Pharm.D., FASHP, FCCP, BCPS Professor and Vice Chair, Department of Clinical Pharmacy University of Colorado Anschutz Medical Campus Learning Objectives Identify the 4 patient populations
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 8 Last Review Date: December 2, 2016 Repatha Description Repatha (evolocumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 8 Last Review Date: December 2, 2016 Repatha Description Repatha (evolocumab)
2013 Cholesterol Guidelines. Anna Broz MSN, RN, CNP, AACC Cer=fied Adult Nurse Prac==oner North Ohio Heart, Inc.
 2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Cer=fied Adult Nurse Prac==oner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
2013 Cholesterol Guidelines Anna Broz MSN, RN, CNP, AACC Cer=fied Adult Nurse Prac==oner North Ohio Heart, Inc. Disclosures Speaker Gilead Sciences NHLBI Charge to the Expert Panel Evaluate higher quality
New Cholesterol Guidelines What the LDL are we supposed to do now?!
 New Cholesterol Guidelines What the LDL are we supposed to do now?! Michael D. Shapiro Assistant Professor of Medicine and Radiology Knight Cardiovascular Institute Oregon Health & Science University 2013
New Cholesterol Guidelines What the LDL are we supposed to do now?! Michael D. Shapiro Assistant Professor of Medicine and Radiology Knight Cardiovascular Institute Oregon Health & Science University 2013
Clinical Policy: Lomitapide (Juxtapid) Reference Number: ERX.SPA.170 Effective Date:
 Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
PREV E E V N E TI T O I N
 PREVENTION OF ATHEROSCLEROTIC CARDIOVASCULAR DISEASE SHANE JOHNSON,, PHARM. D. OBJECTIVES 1. Define atherosclerotic cardiovascular disease(s) and describe the disease progression. 2. Identify three independent
PREVENTION OF ATHEROSCLEROTIC CARDIOVASCULAR DISEASE SHANE JOHNSON,, PHARM. D. OBJECTIVES 1. Define atherosclerotic cardiovascular disease(s) and describe the disease progression. 2. Identify three independent
Cholesterol. Medicines To Help You
 Medicines To Help You Cholesterol Use this guide to help you talk to your doctor, pharmacist, or nurse about your cholesterol medicines. The guide lists all of the FDA-approved products now available to
Medicines To Help You Cholesterol Use this guide to help you talk to your doctor, pharmacist, or nurse about your cholesterol medicines. The guide lists all of the FDA-approved products now available to
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Vytorin) Reference Number: CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Vytorin) Reference Number: CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: September 15, 2017 Repatha Description Repatha
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: September 15, 2017 Repatha Description Repatha
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: November 30, 2018 Repatha Description Repatha (evolocumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: November 30, 2018 Repatha Description Repatha (evolocumab)
Vincent J. Caracciolo, MD FACC FOMA May 2014
 Vincent J. Caracciolo, MD FACC FOMA May 2014 Goals of the Guidelines National Heart, lung and Blood Institute ( NHLBI) collaborated with ACC/AHA to develop guidelines a.) assess CV risk, b.) lifestyle
Vincent J. Caracciolo, MD FACC FOMA May 2014 Goals of the Guidelines National Heart, lung and Blood Institute ( NHLBI) collaborated with ACC/AHA to develop guidelines a.) assess CV risk, b.) lifestyle
STATIN UTILIZATION MANAGEMENT CRITERIA
 STATIN UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: HMG Co-A Reductase Inhibitors & Combinations Agents which require prior review: Advicor (niacin extended-release/lovastatin) Crestor (rosuvastatin)(5mg,10mg,
STATIN UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: HMG Co-A Reductase Inhibitors & Combinations Agents which require prior review: Advicor (niacin extended-release/lovastatin) Crestor (rosuvastatin)(5mg,10mg,
If yes, continue to #2. If no, do not approve. DENIAL TEXT: See the initial denial text at the end of the guideline.
 Generic Brand HICL GCN Exception/Other LOMITAPIDE JUXTAPID 39883 This drug requires a written request for prior authorization. All requests for Juxtapid (lomitapide) require review by a pharmacist prior
Generic Brand HICL GCN Exception/Other LOMITAPIDE JUXTAPID 39883 This drug requires a written request for prior authorization. All requests for Juxtapid (lomitapide) require review by a pharmacist prior
Update on the Treatment of Hyperlipidemia
 Update on the Treatment of Hyperlipidemia Wafaa Abou-Zeineddine, PharmD Medstar Washington Hospital Center Washington Metropolitan Society of Health Systems Pharmacists May 30, 2015 Please MUTE phones
Update on the Treatment of Hyperlipidemia Wafaa Abou-Zeineddine, PharmD Medstar Washington Hospital Center Washington Metropolitan Society of Health Systems Pharmacists May 30, 2015 Please MUTE phones
Drug Class Monograph
 Drug Class Monograph Class: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor Drugs: Praluent (alirocumab), Repatha (evolocumab) Line of Business: Medi-Cal Effective Date: February 17, 2016
Drug Class Monograph Class: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor Drugs: Praluent (alirocumab), Repatha (evolocumab) Line of Business: Medi-Cal Effective Date: February 17, 2016
Clinical Policy: Evolocumab (Repatha) Reference Number: CP.CPA.269 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: (Repatha) Reference Number: CP.CPA.269 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Repatha) Reference Number: CP.CPA.269 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
High ( 50%) Restrictions mg 20-40mg Simvastatin (Zocor) 10mg 20-40mg $1.66 Pravastatin (Pravachol) mg $6.
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/8/2018 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/17, 5/16, 5/15, 2/14, LOB AFFECTED: Medi-Cal
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/8/2018 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/17, 5/16, 5/15, 2/14, LOB AFFECTED: Medi-Cal
PCSK9 Agents Drug Class Prior Authorization Protocol
 PCSK9 Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical
PCSK9 Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of medical
Clinical Policy: Evolocumab (Repatha) Reference Number: ERX.SPMN.184 Effective Date: 01/2017
 Clinical Policy: (Repatha) Reference Number: ERX.SPMN.184 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Clinical Policy: (Repatha) Reference Number: ERX.SPMN.184 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Lipid Guidelines Who, What, and How Low. Anita Ralstin, MS, CNP Next Step Health Consultant, LLC New Mexico Heart Institute
 Lipid Guidelines Who, What, and How Low Anita Ralstin, MS, CNP Next Step Health Consultant, LLC New Mexico Heart Institute Disclosures! None Objectives! List factors used in screening for dyslipidemia
Lipid Guidelines Who, What, and How Low Anita Ralstin, MS, CNP Next Step Health Consultant, LLC New Mexico Heart Institute Disclosures! None Objectives! List factors used in screening for dyslipidemia
Objectives. Having completed the learning activities, the participant will be able to: Dyslipidemia: The latest treatment recommendations
 Objectives Dyslipidemia: The latest treatment recommendations Margaret A. Fitzgerald, DNP, FNP-BC, NP-C, FAANP, CSP, FAAN, DCC President, Fitzgerald Health Education Associates, Inc. North Andover, MA
Objectives Dyslipidemia: The latest treatment recommendations Margaret A. Fitzgerald, DNP, FNP-BC, NP-C, FAANP, CSP, FAAN, DCC President, Fitzgerald Health Education Associates, Inc. North Andover, MA
Dyslipidemia in the light of Current Guidelines - Do we change our Practice?
 Dyslipidemia in the light of Current Guidelines - Do we change our Practice? Dato Dr. David Chew Soon Ping Senior Consultant Cardiologist Institut Jantung Negara Atherosclerotic Cardiovascular Disease
Dyslipidemia in the light of Current Guidelines - Do we change our Practice? Dato Dr. David Chew Soon Ping Senior Consultant Cardiologist Institut Jantung Negara Atherosclerotic Cardiovascular Disease
B. Patient has not reached the percentage reduction goal with statin therapy
 Managing Cardiovascular Risk: The Importance of Lowering LDL Cholesterol and Reaching Treatment Goals for LDL Cholesterol The Role of the Pharmacist Learning Objectives 1. Review the role of lipid levels
Managing Cardiovascular Risk: The Importance of Lowering LDL Cholesterol and Reaching Treatment Goals for LDL Cholesterol The Role of the Pharmacist Learning Objectives 1. Review the role of lipid levels
FORTH VALLEY. LIPID LOWERING GUIDELINE v5 2016
 FORTH VALLEY LIPID LOWERING GUIDELINE v5 2016 This guideline applies to people over 16 years of age. This guideline is not intended to serve as a standard of medical care or be applicable in every situation.
FORTH VALLEY LIPID LOWERING GUIDELINE v5 2016 This guideline applies to people over 16 years of age. This guideline is not intended to serve as a standard of medical care or be applicable in every situation.
Clinical Policy: Mipomersen (Kynamro) Reference Number: ERX.SPA.171 Effective Date:
 Clinical Policy: (Kynamro) Reference Number: ERX.SPA.171 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Kynamro) Reference Number: ERX.SPA.171 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Praluent. Praluent (alirocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.06 Subject: Praluent Page: 1 of 10 Last Review Date: September 20, 2018 Praluent Description Praluent
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.06 Subject: Praluent Page: 1 of 10 Last Review Date: September 20, 2018 Praluent Description Praluent
CPE Session 7. Update on Clinical Practice Guidelines and Best Evidence in the Management of Hyperlipidemia and Cardiovascular Risk Reduction
 CPE Session 7 Update on Clinical Practice Guidelines and Best Evidence in the Management of Hyperlipidemia and Cardiovascular Risk Reduction Saturday, April 25, 2015 ACPE UAN 0128-0000-15-027-L01-P 1.0
CPE Session 7 Update on Clinical Practice Guidelines and Best Evidence in the Management of Hyperlipidemia and Cardiovascular Risk Reduction Saturday, April 25, 2015 ACPE UAN 0128-0000-15-027-L01-P 1.0
Management of Post-transplant hyperlipidemia
 Management of Post-transplant hyperlipidemia B. Gisella Carranza Leon, MD Assistant Professor of Medicine Lipid Clinic - Vanderbilt Heart and Vascular Institute Division of Diabetes, Endocrinology and
Management of Post-transplant hyperlipidemia B. Gisella Carranza Leon, MD Assistant Professor of Medicine Lipid Clinic - Vanderbilt Heart and Vascular Institute Division of Diabetes, Endocrinology and
Clinical Policy: Alirocumab (Praluent) Reference Number: CP.CPA.268 Effective Date: Last Review Date: 11.17
 Clinical Policy: (Praluent) Reference Number: CP.CPA.268 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Praluent) Reference Number: CP.CPA.268 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
No relevant financial relationships
 MANAGEMENT OF LIPID DISORDERS Balancing Benefits and harms Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial relationships baron@medicine.ucsf.edu
MANAGEMENT OF LIPID DISORDERS Balancing Benefits and harms Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial relationships baron@medicine.ucsf.edu
PIEDMONT ACCESS TO HEALTH SERVICES, INC. Guidelines for Screening and Management of Dyslipidemia
 PIEDMONT ACCESS TO HEALTH SERVICES, INC. Policy Number: 01-09-021 SUBJECT: Guidelines for Screening and Management of Dyslipidemia EFFECTIVE DATE: 04/2008 REVIEWED/REVISED: 04/12/10, 03/17/2011, 4/10/2012,
PIEDMONT ACCESS TO HEALTH SERVICES, INC. Policy Number: 01-09-021 SUBJECT: Guidelines for Screening and Management of Dyslipidemia EFFECTIVE DATE: 04/2008 REVIEWED/REVISED: 04/12/10, 03/17/2011, 4/10/2012,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Statin Therapy Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Statin Therapy Prime Therapeutics will review Prior Authorization requests. Prior Authorization
Statin Therapy Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Statin Therapy Prime Therapeutics will review Prior Authorization requests. Prior Authorization
Antihyperlipidemic Drugs
 Antihyperlipidemic Drugs Hyperlipidemias. Hyperlipoproteinemias. Hyperlipemia. Hypercholestrolemia. Direct relationship with acute pancreatitis and atherosclerosis Structure Lipoprotein Particles Types
Antihyperlipidemic Drugs Hyperlipidemias. Hyperlipoproteinemias. Hyperlipemia. Hypercholestrolemia. Direct relationship with acute pancreatitis and atherosclerosis Structure Lipoprotein Particles Types
How to Handle Statin Intolerance in the High Risk Patient
 How to Handle Statin Intolerance in the High Risk Patient Thomas D. Conley, MD FACC FSCAI Disclosures: None 1 Definition of High Risk Primary Prevention ASCVD Risk Calculator Adults >21 yrs, LDL 190 mg/dl
How to Handle Statin Intolerance in the High Risk Patient Thomas D. Conley, MD FACC FSCAI Disclosures: None 1 Definition of High Risk Primary Prevention ASCVD Risk Calculator Adults >21 yrs, LDL 190 mg/dl
Royal Wolverhampton Hospital Adult Lipid Lowering Therapy Guidelines Lipid Lowering Therapy for the Prevention of Cardiovascular Disease
 Royal Wolverhampton Hospital Adult Lipid Lowering Therapy Guidelines 1 This guideline is intended to assist rational and cost-effective prescribing of lipid regulating medications across both primary and
Royal Wolverhampton Hospital Adult Lipid Lowering Therapy Guidelines 1 This guideline is intended to assist rational and cost-effective prescribing of lipid regulating medications across both primary and
Disclosure. No relevant financial relationships. Placebo-Controlled Statin Trials
 MANAGEMENT OF HYPERLIPIDEMIA AND CARDIOVASCULAR RISK IN WOMEN: Balancing Benefits and Harms Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial
MANAGEMENT OF HYPERLIPIDEMIA AND CARDIOVASCULAR RISK IN WOMEN: Balancing Benefits and Harms Disclosure Robert B. Baron, MD MS Professor and Associate Dean UCSF School of Medicine No relevant financial
Confusion about guidelines: How should we treat lipids?
 Confusion about guidelines: How should we treat lipids? Anne Carol Goldberg, MD, FACP, FAHA, FNLA Professor of Medicine Washington University School of Medicine American College of Physicians Missouri
Confusion about guidelines: How should we treat lipids? Anne Carol Goldberg, MD, FACP, FAHA, FNLA Professor of Medicine Washington University School of Medicine American College of Physicians Missouri
Cholesterol Management Roy Gandolfi, MD
 Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
New Guidelines in Dyslipidemia Management
 The Third IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2017 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
The Third IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2017 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
What do the guidelines say about combination therapy?
 What do the guidelines say about combination therapy? Christie M. Ballantyne, MD Center for Cardiovascular Disease Prevention Methodist DeBakey Heart & Vascular Center Baylor College of Medicine Houston,
What do the guidelines say about combination therapy? Christie M. Ballantyne, MD Center for Cardiovascular Disease Prevention Methodist DeBakey Heart & Vascular Center Baylor College of Medicine Houston,
MOLINA HEALTHCARE OF CALIFORNIA
 MOLINA HEALTHCARE OF CALIFORNIA HIGH BLOOD CHOLESTEROL IN ADULTS GUIDELINE Molina Healthcare of California has adopted the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel
MOLINA HEALTHCARE OF CALIFORNIA HIGH BLOOD CHOLESTEROL IN ADULTS GUIDELINE Molina Healthcare of California has adopted the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel
2013 ACC AHA LIPID GUIDELINE JAY S. FONTE, MD
 2013 ACC AHA LIPID GUIDELINE JAY S. FONTE, MD How do you interpret my blood test results? What are our targets for these tests? Before the ACC/AHA Lipid Guidelines A1c:
2013 ACC AHA LIPID GUIDELINE JAY S. FONTE, MD How do you interpret my blood test results? What are our targets for these tests? Before the ACC/AHA Lipid Guidelines A1c:
Introduction. Objective. Critical Questions Addressed
 Introduction Objective To provide a strong evidence-based foundation for the treatment of cholesterol for the primary and secondary prevention of ASCVD in women and men Critical Questions Addressed CQ1:
Introduction Objective To provide a strong evidence-based foundation for the treatment of cholesterol for the primary and secondary prevention of ASCVD in women and men Critical Questions Addressed CQ1:
New Guidelines in Dyslipidemia Management
 The Fourth IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2018 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
The Fourth IAS-OSLA Course on Lipid Metabolism and Cardiovascular Risk Muscat, Oman, February 2018 New Guidelines in Dyslipidemia Management Dr. Khalid Al-Waili, MD, FRCPC, DABCL Senior Consultant Medical
Clinical Policy: Mipomersen (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017
 Clinical Policy: (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Clinical Policy: (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Review current guideline recommendations for lipid-lowering therapy
 Breakout Session #3 New Paradigms in the Management of Dyslipidemia Review current guideline recommendations for lipid-lowering therapy Dr Meral KAYIKCIOGLU Ege University Medical School, Cardiology Dept,
Breakout Session #3 New Paradigms in the Management of Dyslipidemia Review current guideline recommendations for lipid-lowering therapy Dr Meral KAYIKCIOGLU Ege University Medical School, Cardiology Dept,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Repatha) Reference Number: HIM.PA.SP46 Effective Date: 01.01.18 Last Review Date: Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this
Clinical Policy: (Repatha) Reference Number: HIM.PA.SP46 Effective Date: 01.01.18 Last Review Date: Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this
Lipids What s new? Meera Jain, MD Providence Portland Medical Center
 Lipids 2016- What s new? Meera Jain, MD Providence Portland Medical Center 1 Can I trust the ASCVD risk calculator? Do harms outweigh benefits in primary prevention? Is there anything besides a statin?
Lipids 2016- What s new? Meera Jain, MD Providence Portland Medical Center 1 Can I trust the ASCVD risk calculator? Do harms outweigh benefits in primary prevention? Is there anything besides a statin?
Clinical Policy: Evolocumab (Repatha) Reference Number: ERX.SPA.169 Effective Date:
 Clinical Policy: (Repatha) Reference Number: ERX.SPA.169 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Repatha) Reference Number: ERX.SPA.169 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
2.3 CONTACT HOURS. Managing. By Kristine Anne Scordo, PhD, RN, ACNP-BC, FAANP
 2.3 CONTACT HOURS 2.3 CONTACT HOURS Managing hyperlipidemia The updated cholesterol treatment guidelines Abstract: The ACC/AHA 2013 cholesterol treatment guidelines focus on lowering the risk of heart
2.3 CONTACT HOURS 2.3 CONTACT HOURS Managing hyperlipidemia The updated cholesterol treatment guidelines Abstract: The ACC/AHA 2013 cholesterol treatment guidelines focus on lowering the risk of heart
Drug Prior Authorization Guideline PCSK9 Inhibitors -
 Drug Prior Authorization Guideline PCSK9 Inhibitors - REPATHA (evolocumab) PRALUENT (alirocumab) PA9911 Covered Service: Yes when meets criteria below Prior Authorization Required: Yes-as shown below Additional
Drug Prior Authorization Guideline PCSK9 Inhibitors - REPATHA (evolocumab) PRALUENT (alirocumab) PA9911 Covered Service: Yes when meets criteria below Prior Authorization Required: Yes-as shown below Additional
No relevant financial relationships
 MANAGEMENT OF LIPID DISORDERS: WHERE DO WE STAND WITH THE NEW PRACTICE GUIDELINES? Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Disclosure No relevant financial relationships
MANAGEMENT OF LIPID DISORDERS: WHERE DO WE STAND WITH THE NEW PRACTICE GUIDELINES? Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Disclosure No relevant financial relationships
CARDIOVASCULAR DISEASE WHAT IS IT? WHAT IS THE ROLE OF CHOLESTEROL? HOW CAN WE REDUCE RISK?
 1 CARDIOVASCULAR DISEASE WHAT IS IT? WHAT IS THE ROLE OF CHOLESTEROL? HOW CAN WE REDUCE RISK? Perry J Weinstock, MD, F.A.C.C. Head, Division of Cardiovascular Disease Director of Clinical Cardiology Cooper
1 CARDIOVASCULAR DISEASE WHAT IS IT? WHAT IS THE ROLE OF CHOLESTEROL? HOW CAN WE REDUCE RISK? Perry J Weinstock, MD, F.A.C.C. Head, Division of Cardiovascular Disease Director of Clinical Cardiology Cooper
Joshua Shepherd PA-C, MMS, MT (ASCP)
 Joshua Shepherd PA-C, MMS, MT (ASCP) None What is Cholesterol? Why cholesterol is it important? Review the National Cholesterol Education Programs guidelines (NCEP-ATPIII) Discuss New guidelines from the
Joshua Shepherd PA-C, MMS, MT (ASCP) None What is Cholesterol? Why cholesterol is it important? Review the National Cholesterol Education Programs guidelines (NCEP-ATPIII) Discuss New guidelines from the
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2063-8 Program Prior Authorization/Medical Necessity Medication Repatha (evolocumab) P&T Approval Date 5/2015, 9/2015, 11/2015,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2063-8 Program Prior Authorization/Medical Necessity Medication Repatha (evolocumab) P&T Approval Date 5/2015, 9/2015, 11/2015,
Prevention Updates and Paradigm Shifts
 Prevention Updates and Paradigm Shifts Andrew Freeman, MD, FACC Director of Clinical Cardiology and Operations National Jewish Health Assistant Professor of Medicine National Jewish Health and University
Prevention Updates and Paradigm Shifts Andrew Freeman, MD, FACC Director of Clinical Cardiology and Operations National Jewish Health Assistant Professor of Medicine National Jewish Health and University
Lecture 36 Dyslipidemia Therapeutics Barry LIPIDS:
 LIPIDS: PATHOPHYSIOLOGY: TC or LDL-C = CVD HDL-C = CVD Association between TG and CVD not established o HyperTG associated with pancreatitis o Reducing TG w/ drug therapy doesn t CVD TYPES OF DYSLIPIDEMIA:
LIPIDS: PATHOPHYSIOLOGY: TC or LDL-C = CVD HDL-C = CVD Association between TG and CVD not established o HyperTG associated with pancreatitis o Reducing TG w/ drug therapy doesn t CVD TYPES OF DYSLIPIDEMIA:
Approach to Dyslipidemia among diabetic patients
 Approach to Dyslipidemia among diabetic patients Farzad Hadaegh, MD, Professor of Internal Medicine & Endocrinology Prevention of Metabolic Disorders Research Center, Research Institute for Endocrine Sciences
Approach to Dyslipidemia among diabetic patients Farzad Hadaegh, MD, Professor of Internal Medicine & Endocrinology Prevention of Metabolic Disorders Research Center, Research Institute for Endocrine Sciences
Lipid Management: The Next Level How Will the New ACC/AHA Guidelines Change My Practice
 Lipid Management: The Next Level How Will the New ACC/AHA Guidelines Change My Practice Vera Bittner, MD, MSPH Professor of Medicine Section Head, Preventive Cardiology Medical Director, Cardiac Rehabilitation
Lipid Management: The Next Level How Will the New ACC/AHA Guidelines Change My Practice Vera Bittner, MD, MSPH Professor of Medicine Section Head, Preventive Cardiology Medical Director, Cardiac Rehabilitation
Supplement Table 2. Categorization of Statin Intensity Based on Potential Low-Density Lipoprotein Cholesterol Reduction
 Supplement: Tables Supplement Table 1. Study Eligibility Criteria Supplement Table 2. Categorization of Statin Intensity Based on Potential Low-Density Lipoprotein Cholesterol Reduction Supplement Table
Supplement: Tables Supplement Table 1. Study Eligibility Criteria Supplement Table 2. Categorization of Statin Intensity Based on Potential Low-Density Lipoprotein Cholesterol Reduction Supplement Table
Strategies for Managing Dyslipidemia Patients with Residual CVD Risk
 Strategies for Managing Dyslipidemia Patients with Residual CVD Risk Acknowledgements We acknowledge the work of Lynne T. Braun, PhD, CNP, FAHA, FPCNA, FAAN in the development of this presentation. Disclosures:
Strategies for Managing Dyslipidemia Patients with Residual CVD Risk Acknowledgements We acknowledge the work of Lynne T. Braun, PhD, CNP, FAHA, FPCNA, FAAN in the development of this presentation. Disclosures:
Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date:
 Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See
Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See
Dyslipidemia: What s New In The Guidelines?
 1 Dyslipidemia: What s New In The Guidelines? Rhonda Cooper-DeHoff, Pharm D, MS Associate Professor Colleges of Pharmacy and Medicine University of Florida Disclosures 2 No actual or perceived conflicts
1 Dyslipidemia: What s New In The Guidelines? Rhonda Cooper-DeHoff, Pharm D, MS Associate Professor Colleges of Pharmacy and Medicine University of Florida Disclosures 2 No actual or perceived conflicts
Lipid Management C. Samuel Ledford, MD Interventional Cardiology Chattanooga Heart Institute
 Lipid Management 2018 C. Samuel Ledford, MD Interventional Cardiology Chattanooga Heart Institute Disclosures No Financial Disclosures Disclosures I am an Interventional Cardiologist I put STENTS in for
Lipid Management 2018 C. Samuel Ledford, MD Interventional Cardiology Chattanooga Heart Institute Disclosures No Financial Disclosures Disclosures I am an Interventional Cardiologist I put STENTS in for
ATP IV: Predicting Guideline Updates
 Disclosures ATP IV: Predicting Guideline Updates Daniel M. Riche, Pharm.D., BCPS, CDE Speaker s Bureau Merck Janssen Boehringer-Ingelheim Learning Objectives Describe at least two evidence-based recommendations
Disclosures ATP IV: Predicting Guideline Updates Daniel M. Riche, Pharm.D., BCPS, CDE Speaker s Bureau Merck Janssen Boehringer-Ingelheim Learning Objectives Describe at least two evidence-based recommendations
Dyslipedemia New Guidelines
 Dyslipedemia New Guidelines New ACC/AHA Prevention Guidelines on Blood Cholesterol November 12, 2013 Mohammed M Abd El Ghany Professor of Cardiology Cairo Universlty 1 1 0 Cholesterol Management Pharmacotherapy
Dyslipedemia New Guidelines New ACC/AHA Prevention Guidelines on Blood Cholesterol November 12, 2013 Mohammed M Abd El Ghany Professor of Cardiology Cairo Universlty 1 1 0 Cholesterol Management Pharmacotherapy
VI.2 Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology This medicine is used to lower levels of total cholesterol, LDL cholesterol ( bad cholesterol), and fatty substances called triglycerides
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology This medicine is used to lower levels of total cholesterol, LDL cholesterol ( bad cholesterol), and fatty substances called triglycerides
Lipid Management 2013 Statin Benefit Groups
 Clinical Integration Steering Committee Clinical Integration Chronic Disease Management Work Group Lipid Management 2013 Statin Benefit Groups Approved by Board Chair Signature Name (Please Print) Date
Clinical Integration Steering Committee Clinical Integration Chronic Disease Management Work Group Lipid Management 2013 Statin Benefit Groups Approved by Board Chair Signature Name (Please Print) Date
Highlights of the new blood pressure and cholesterol guidelines: A whole new philosophy. Jeremy L. Johnson, PharmD, BCACP, CDE, BC-ADM
 Highlights of the new blood pressure and cholesterol guidelines: A whole new philosophy Jeremy L. Johnson, PharmD, BCACP, CDE, BC-ADM OSHP 2014 Annual Meeting Oklahoma City, OK April 4, 2014 1 Objectives
Highlights of the new blood pressure and cholesterol guidelines: A whole new philosophy Jeremy L. Johnson, PharmD, BCACP, CDE, BC-ADM OSHP 2014 Annual Meeting Oklahoma City, OK April 4, 2014 1 Objectives
Long-Term Complications of Diabetes Mellitus Macrovascular Complication
 Long-Term Complications of Diabetes Mellitus Macrovascular Complication Sung Hee Choi MD, PhD Professor, Seoul National University College of Medicine, SNUBH, Bundang Hospital Diabetes = CVD equivalent
Long-Term Complications of Diabetes Mellitus Macrovascular Complication Sung Hee Choi MD, PhD Professor, Seoul National University College of Medicine, SNUBH, Bundang Hospital Diabetes = CVD equivalent
Clinical Policy: Lomitapide (Juxtapid) Reference Number: ERX.SPA.170 Effective Date:
 Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Common Repatha Documentation Requirements for Patients With Primary Hyperlipidemia and Established CVD 1,2
 Established CVD Common Repatha Documentation Requirements for Patients With Primary Hyperlipidemia and Established CVD 1,2 Primary and Secondary Diagnosis Codes Primary Diagnosis: Primary hyperlipidemia
Established CVD Common Repatha Documentation Requirements for Patients With Primary Hyperlipidemia and Established CVD 1,2 Primary and Secondary Diagnosis Codes Primary Diagnosis: Primary hyperlipidemia
Lipid Panel Management Refresher Course for the Family Physician
 Lipid Panel Management Refresher Course for the Family Physician Objectives Understand the evidence that was evaluated to develop the 2013 ACC/AHA guidelines Discuss the utility and accuracy of the new
Lipid Panel Management Refresher Course for the Family Physician Objectives Understand the evidence that was evaluated to develop the 2013 ACC/AHA guidelines Discuss the utility and accuracy of the new
Hyperlipidemia: Past and Present. Rebecca Khaimova, PharmD The Brooklyn Hospital Center
 Hyperlipidemia: Past and Present Rebecca Khaimova, PharmD The Brooklyn Hospital Center Rkhaimova@tbh.org Conflicts of Interest None to disclose Learning Objectives for Pharmacist Describe the pathophysiology
Hyperlipidemia: Past and Present Rebecca Khaimova, PharmD The Brooklyn Hospital Center Rkhaimova@tbh.org Conflicts of Interest None to disclose Learning Objectives for Pharmacist Describe the pathophysiology
EVOLOCUMAB Generic Brand HICL GCN Exception/Other EVOLOCUMAB REPATHA 42378
 Generic Brand HICL GCN Exception/Other EVOLOCUMAB REPATHA 42378 This drug requires a written request for prior authorization. All requests for Repatha (evolocumab) require review by a pharmacist prior
Generic Brand HICL GCN Exception/Other EVOLOCUMAB REPATHA 42378 This drug requires a written request for prior authorization. All requests for Repatha (evolocumab) require review by a pharmacist prior
How would you manage Ms. Gold
 How would you manage Ms. Gold 32 yo Asian woman with dyslipidemia Current medications: Simvastatin 20mg QD Most recent lipid profile: TC = 246, TG = 100, LDL = 176, HDL = 50 What about Mr. Williams? 56
How would you manage Ms. Gold 32 yo Asian woman with dyslipidemia Current medications: Simvastatin 20mg QD Most recent lipid profile: TC = 246, TG = 100, LDL = 176, HDL = 50 What about Mr. Williams? 56
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2062-8 Program Prior Authorization/Medical Necessity Medication Praluent (alirocumab) P&T Approval Date 5/2015, 8/2015, 9/2015,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2062-8 Program Prior Authorization/Medical Necessity Medication Praluent (alirocumab) P&T Approval Date 5/2015, 8/2015, 9/2015,
REPATHA (PCSK9 INHIBITORS)
 REPATHA (PCSK9 INHIBITS) Indications: PCSK9 Inhibitors are indicated for treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease as
REPATHA (PCSK9 INHIBITS) Indications: PCSK9 Inhibitors are indicated for treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease as
Antihyperlipidemic drugs
 Antihyperlipidemic drugs The clinically important lipoproteins are LDL low density lipoprotein, VLDL very low density lipoprotein, HDL high density lipoprotein. Hyperlipidemia may caused 1. by individual
Antihyperlipidemic drugs The clinically important lipoproteins are LDL low density lipoprotein, VLDL very low density lipoprotein, HDL high density lipoprotein. Hyperlipidemia may caused 1. by individual
Elements for a public summary
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Cardiovascular disease (CVD) is responsible for one-third of global deaths and is a leading and increasing contributor to the
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Cardiovascular disease (CVD) is responsible for one-third of global deaths and is a leading and increasing contributor to the
