Differential Effects of PPAR-! Activation vs. Chemical or Genetic Reduction of DPP-4 Activity on Murine Bone Quality. Kimberly Anne Kyle
|
|
|
- Hollie Price
- 5 years ago
- Views:
Transcription
1 Differential Effects of PPAR-! Activation vs. Chemical or Genetic Reduction of DPP-4 Activity on Murine Bone Quality by Kimberly Anne Kyle A thesis submitted in conformity with the requirements for the degree of Master of Science Graduate Department of Laboratory Medicine and Pathobiology University of Toronto " Copyright by Kimberly Anne Kyle (2010) i
2 Differential Effects of PPAR-! Activation vs. Chemical or Genetic Reduction of DPP-4 Activity on Murine Bone Quality Master of Science Kimberly Kyle Laboratory Medicine and Pathobiology University of Toronto 2010 Abstract This study characterized the effects of two anti-diabetic drugs, a thiazolidinedione (TZD) and a Dipeptidyl Peptidase-4 (DPP-4) inhibitor on bone quality in a glucose intolerant mouse model. Bone quality in a DPP-4 -/- mouse model was also examined. Bone quality was evaluated through densitometry, mechanical testing and techniques to assess remodeling, structural and mineral properties. TZD treatment negatively affected trabecular mechanical properties in male, female and ovariectomized female (OVX) mice. Male mice exhibited the greatest effect due to TZD treatment with reduced vertebral vbmd, trabecular structure and bone formation. DPP-4 inhibitor treatment improved vertebral vbmd and trabecular architecture in female mice but improvements were lost in females following OVX. Male, female and OVX mice experienced increased trabecular mineralization due to DPP-4 inhibitor treatment. Genetic inactivation of DPP-4 did not produce a major bone phenotype in male and female mice but lead to reduced femoral geometry and mechanics in OVX mice. ii
3 ACKNOWLEDGEMENTS There are several people I would like to acknowledge for the important roles they played in the accomplishment of this work. To Dr. Marc Grynpas, who is an exceptional mentor and offers each of his students a generosity and support that pushes them to excel. I would like to thank Dr. Daniel Drucker, who generously offered his time and expertise to provide important feedback as well as answer any questions I may have. I would also like to thank Dr. Rita Kandel and Dr. Harry Elsholtz whom offered expert advice and suggestions as well as serving as crucial members of my committee. A special thanks to Richard Cheung, who played an essential role in my initial arrival in Grynpasland as a coop student and has an to prove it. Grynpasland would definitely not be the same without you. To Dr. Mircea Dumitriu for his Who s Who expertise in image analysis as well as the universe but more importantly, for being a friend and mentor. Thank you to Doug Holmyard for his invaluable assistance with BSE imaging and Dr. Laurie Baggio for her expertise as well as managing the generation of all mice used in this study. Dr. Thomas Willet deserves thanks for his managerial role in the initial Merck study as well as the coop students that aided in the project. Finally, thank you to my Grynpas labmates, both past and present, for providing a wonderfully supportive and exciting work environment. Of the past students, Dr. Lisa Wise-Milestone who returned on more than one occasion to train me and Dr. Sidney Omelon for our many talks on science and beyond. Of the present, Laura Sardone and Adeline Ng, if laughter is the best medicine, we may have overdosed. I would like to give special thanks to the wonderful friends that have helped me through my master s journey. Each of you helped me ride the ups and downs of life. I would like to especially acknowledge Rachel Bonner, Trista Hallman, Michael Jewer, Justin Parreno, Keira Pereira and Chrystia Wynnyckyj. I lost a stride that each of you helped me get back. You provided me with a foundation I needed and am very much grateful for. I love each of you like family. Rachel Bonner, you were a wonderful roommate through my masters and have heard so much about my research you may understand my project as much as I do. Michael Jewer, you have provided an invaluable friendship, you are always there to hear any worry I have and provide laughter at any minute of every day. Chrystia Wynnyckyj, you have given me exceptional support both as a colleague and as a loyal friend. I am lucky to have each of you in my life. Finally, I would like to thank my mother and father. To consider myself blessed to be your daughter is an understatement. Having you both as my parents is like winning the lottery. You have both put your everything into raising me. You offer me more support than I could ever ask for and only wish that I be the best that I can be. Just know that I strive to succeed because of how amazing you both are. I love you more than anything. iii
4 TABLE OF CONTENTS CHAPTER 1: INTRODUCTION AND BACKGROUND Motivation and Rationale Bone Biology Bone Structure Bone Composition Bone Remodeling Bone Diseases Osteoporosis Bone Quality Bone Mineral Density (BMD) Bone Mechanical Properties Structural Properties Material Properties Bone Remodeling Type 2 Diabetes Mellitus (T2DM) T2DM and Bone T2DM and Bone Quality Obesity Insulin Hyperglycemia Thiazolidinediones (TZD) TZDs and Bone Mechanism of Action Dipeptidyl Peptidase-4 (DPP-4) Inhibition DPP-4 Knockout Mice DPP-4 Inhibition and Bone Mouse Model. 33 CHAPTER 2: HYPOTHESIS AND OBJECTIVES Introduction Anti-Diabetic Drug Study Genetic Inactivation Study..38 CHAPTER 3: MATERIALS AND METHODS Introduction Mice and Experimental Design Anti-Diabetic Drug Study Genetic Inactivation Study Limitations of Mice Sacrifice and Dissection Experimental Assessment of Bone Quality Bone Mineral Density Dual Energy X-Ray Absorptiometry Volumetric BMD and Bone Structural Properties Micro-Computed Tomography Strut Analysis Bone Mechanical Properties 51 iv
5 3.5.1 Three-Point Bending Vertebral Compression Femoral Neck Fracture Testing Evaluation of Bone Remodeling Dynamic Histomorphometry Osteoclast Staining Osteoclast Nuclei Counting Evaluation of Bone Mineral Properties Quantitative Backscattered Electron Imaging (BSE) Statistical Analysis Anti-Diabetic Drug Study Genetic Inactivation Study. 68 CHAPTER 4: RESULTS FROM ANTI-DIABETIC DRUG STUDY Introduction Objective 1: Effects from TZD Treatment, Pioglitazone, on Bone Quality Weight Observed Phenotype Bone Colour Bone Densitometry Areal Bone Mineral Density Volumetric Bone Mineral Density Bone Mechanical Properties Femoral Geometry Three-Point Bending Vertebral Geometry Vertebral Compression Femoral Neck Fracture Structural Properties Observed Phenotype Bone Marrow Bone Formation Bone Resorption Bone Mineral Properties Total Trabecular Cortical Objective 2: Effects from DPP-4 Inhibitor Treatment, Sitagliptin, on Bone Quality Weight Bone Densitometry Areal Bone Mineral Density Volumetric Bone Mineral Density Bone Mechanical Properties Femoral Geometry Three-Point Bending Vertebral Geometry Vertebral Compression Femoral Neck Fracture Structural Properties v
6 4.2.5 Bone Formation Bone Resorption Bone Mineral Properties Total Trabecular Cortical Summary of Anti-Diabetic Drug Study Objective 1: Effects from TZD Treatment, Pioglitazone, on Bone Quality Objective 2: Effects from DPP-4 Inhibitor Treatment, Sitagliptin, on Bone Quality 113 CHAPTER 5: RESULTS FROM GENETIC INACTIVATION STUDY Introduction Objective 3: Effects of Genetic Inactivation of DPP-4 on Bone Quality Weight Bone Densitometry Areal Bone Mineral Density Volumetric Bone Mineral Density Bone Mechanical Properties Femoral Geometry Three-Point Bending Vertebral Geometry Vertebral Compression Femoral Neck Fracture Structural Properites Bone Formation Bone Resorption Bone Mineral Properties Total Trabecular Cortical Summary of Genetic Inactivation Study 135 CHAPTER 6: DISCUSSION Introduction Anti-Diabetic Drug Study Objective 1: Effects of TZD Treatment, Pioglitazone, on Bone Quality Objective 2: Effects of DPP-4 Inhibitor Treatment, Sitagliptin, on Bone Quality Anti-Diabetic Drug Study: Concluding Summary Genetic Inactivation Study Objective 3: Effects of Genetic Inactivation of DPP-4 on Bone Quality DPP-4 Inhibition and Bone Methodological Limitations Mice vi
7 6.4.2 Dual Energy X-Ray Absorptiometry Three-Point Bending Vertebral Compression Femoral Neck Fracture Histomorphometry and Strut Analysis Micro-Computed Tomography..167 CHAPTER 7: CONCLUSIONS Introduction Conclusions from the Anti-Diabetic Drug Study Pioglitazone Treatment on Bone Quality Sitagliptin Treatment on Bone Quality Conclusions from the Genetic Inactivation Study..171 CHAPTER 8: FUTURE WORK Introduction Future Area of Investigation: Effects of Pioglitazone and Sitagliptin on Bone Future Area of Investigation: Effects of Genetic Inactivation of DPP-4 on Bone REFERENCES 176 APPENDIX Figure A1. a) Pioglitazone-treated females Fat cells pushing against osteoclasts b) Vehicle control females Normal osteoclasts and bone marrow vii
8 LIST OF TABLES Table 1.1 Table 3.1 Table 3.2 Table 3.3 Table 3.4 Table 4.1 Table 4.2 Table 4.3 Table 4.4 Table 4.5 Table 4.6 Table 4.7 Table 4.8 Table 4.9 Table 4.10 Table 4.11 Table 4.12 Table 4.13 Table 4.14 Table 4.15 Table 4.16 Table 4.17 Table 4.18 Table 4.19 Table 4.20 Table 4.21 Table 4.22 in vitro and in vivo peptides cleaved by DPP-4 26 Sample sizes for anti-diabetic drug study...42 Sample sizes for genetic-inactivation study 44 Bone formation parameters...61 Osteoclast staining parameters..63 Weight for Pioglitazone-treated and control mice.70 DEXA results for Pioglitazone-treated and control mice..72 Femoral and vertebral vbmd for Pioglitazone-treated and control mice...72 Geometrical properties of right femora for Pioglitazonetreated and control mice Three-point bending results for Pioglitazone-treated and control mice..74 Geometrical properties of L6 vertebrae for Pioglitazonetreated and control mice Vertebral compression results for Pioglitazone-treated and control mice Femoral neck fracture results for Pioglitazone-treated and control mice D trabecular bone structural properties for Pioglitazonetreated and control mice D trabecular bone structural properties for Pioglitazonetreated and control mice Bone formation parameters for Pioglitazone-treated and control mice Osteoclast staining results for Pioglitazone-treated and control mice Results from BSE imaging of total bone for Pioglitazonetreated and control mice Results from BSE imaging of trabecular bone for Pioglitazonetreated and control mice Results from BSE imaging of cortical bone for Pioglitazonetreated and control mice Summary of results from Pioglitazone treatment in mice..91 Weight for Sitagliptin-treated and control mice.92 DEXA results for Sitagliptin-treated and control mice..93 Femoral and vertebral vbmd for Sitagliptin-treated and control mice Geometrical properties of right femora for Sitagliptintreated and control mice Three-point bending results for Sitagliptin-treated and control mice. 95 Geometry of the L6 vertebrae for Sitagliptin-treated and control mice viii
9 Table 4.23 Table 4.24 Table 4.25 Table 4.26 Table 4.27 Table 4.28 Table 4.29 Table 4.30 Table 4.31 Table 4.32 Table 5.1 Table 5.2 Table 5.3 Table 5.4 Table 5.5 Table 5.6 Table 5.7 Table 5.8 Table 5.9 Table 5.10 Table 5.11 Table 5.12 Table 5.13 Table 5.14 Table 5.15 Table 5.16 Vertebral compression results for Sitagliptin-treated and control mice Femoral neck fracture results for Sitagliptin-treated and control mice D trabecular bone structural properties for Sitagliptintreated and control mice D trabecular bone structural properties for Sitagliptintreated and control mice. 100 Bone formation parameters for Sitagliptin-treated and control mice. 101 Osteoclast staining results for Sitagliptin-treated and control mice. 102 Results from BSE imaging of total bone for Sitagliptintreated and control mice. 104 Results from BSE imaging of trabecular bone for Sitagliptintreated and control mice. 107 Results from BSE imaging of cortical bone for Sitagliptintreated and control mice. 109 Summary of results from Sitagliptin-treatment in mice Weight for DPP-4 KO and WT mice..115 DEXA results for DPP-4 KO and WT mice Femoral and vertebral vbmd for DPP-4 KO and WT mice Geometrical properties for right femora for DPP-4 KO and WT mice Three-point bending results for DPP-4 KO and WT mice..119 Geometrical properties of L6 vertebrae for DPP-4 KO and WT mice Vertebral compression results for DPP-4 KO and WT mice Femoral neck fracture results for DPP-4 KO and WT mice D Trabecular bone structural properties for DPP-4 KO and WT mice D Trabecular bone structural properties for DPP-4 KO and WT mice Bone formation parameters for DPP-4 KO and WT mice..124 Osteoclast staining results for DPP-4 KO and WT mice. 125 Results from BSE imaging of total bone for DPP-4 KO and WT mice Results from BSE imaging of trabecular bone for DPP-4 KO and WT mice Results from BSE imaging of cortical bone for DPP-4 KO and WT mice Summary of results for DPP-4 KO and WT mice ix
10 LIST OF FIGURES Figure 1.1 Figure 1.2 Figure 1.3 Figure 1.4 Figure 1.5 Figure 1.6 Figure 1.7 Figure 1.8 Figure 1.9 Figure 1.10 Figure 1.11 Figure 1.12 Figure 3.1 Figure 3.2 Figure 3.3 Figure 3.4 Figure 3.5 Figure 3.6 Figure 3.7 Figure 3.8 Figure 3.9 Figure 3.10 Figure 3.11 Figure 3.12 Figure 3.13 Figure 3.14 Figure 3.15 Figure 3.16 Physiology of a) long bone and b) cuboid bone.4 Haversian system in cortical bone...5 Hierarchical structure of collagen molecules and fibers...6 Bone remodeling cycle...8 Normal trabecular bone architecture versus osteoporotic trabecular bone architecture.. 11 Relationship between bone fragility and various properties of bone. 12 Representation of combined features in the development of T2DM..17 Multitude of diabetes mechanisms thought to reduce bone quality...21 The effects of PPAR-! agonist in muscle, liver and adipose tissue 22 Proposed TZD mechanisms in a) bone formation and b) bone resorption Amino acid sequence of GIP and GLP-1 with point of cleavage by DPP Posttranslational processing of proglucagon.30 Anti-diabetic drug study timeline..43 Genetic inactivation study timeline Summary of experimental techniques used to assess bone quality.. 46 PIXImus scan of femora and vertebrae 48 a) Cross-section of midpoint with labeled medio-lateral and anterior-posterior axis b) Micro-CT scan of femur 49 a) Cross-sectional view of scanned vertebrae b) Micro-CT scan of vertebrae. 50 Thresholded image with schematic view of strut analysis parameters 51 a) Load-displacement curve b) Stress-strain curve 53 a) Schematic diagram of three-point bending test b) Actual three-point bending test 54 a) Schematic diagram of vertebral compression test b) Macroscopic images for normalization.56 a) Schematic diagram of femoral neck fracture test b) Actual femoral neck fracture test..57 Fluorescent labeling (single and double) on undecalcified vertebral section 60 Topographical map displaying 8 field quantified using Bioquant morphometry system. 61 TRAP stained section of decalcified vertebra TRAP-positive osteoclast with hematoxylin-stained nuclei BSE image of vertebra with magnified subregion to illustrate varying grey levels x
11 Figure 3.17 Figure 4.1 Figure 4.2 Figure 4.3 Figure 4.4 Figure 4.5 Figure 4.6 Figure 4.7 Figure 4.8 Figure 4.9 Figure 4.10 Figure 4.11 Figure 4.12 Figure 4.13 Figure 4.14 Figure 4.15 Figure 4.16 Figure 4.17 Figure 4.18 Figure 4.19 Figure 4.20 Figure 4.21 Figure 4.22 Figure 4.23 Schematic mineralization distributions as determined by quantified BSE imaging 67 Colour difference due to Pioglitazone treatment...71 Histological slides (x10) for Pioglitazone-treated and control mice. 80 Percent of osteoclasts with number of nuclei for Pioglitazonetreated and control male mice Percent of osteoclasts with number of nuclei for Pioglitazonetreated and control female mice 83 Percent of osteoclasts with number of nuclei for Pioglitazonetreated and control OVX mice. 83 Total bone mineralization profiles for Pioglitazonetreated and control male mice Total bone mineralization profiles for Pioglitazonetreated and control female mice 85 Total bone mineralization profiles for Pioglitazonetreated and control female mice 86 Trabecular bone mineralization profiles for Pioglitazonetreated and control male mice Trabecular bone mineralization profiles for Pioglitazonetreated and control female mice 88 Trabecular bone mineralization profiles for Pioglitazonetreated and control OVX mice. 88 Cortical bone mineralization profiles for Pioglitazonetreated and control male mice Cortical bone mineralization profiles for Pioglitazonetreated and control female mice 90 Cortical bone mineralization profiles for Pioglitazonetreated and control OVX mice. 90 Percent of osteoclasts with number of nuclei for Sitagliptintreated and control male mice. 102 Percent of osteoclasts with number of nuclei for Sitagliptintreated and control female mice Percent of osteoclasts with number of nuclei for Sitagliptintreated and control OVX mice 103 Total mineralization profiles for Sitagliptin-treated and control male mice Total mineralization profiles for Sitagliptin-treated and control female mice 105 Total mineralization profiles for Sitagliptin-treated and control OVX mice Trabecular mineralization profiles for Sitagliptin-treated and control male mice 107 Trabecular mineralization profiles for Sitagliptin-treated and control female mice..108 Trabecular mineralization profiles for Sitagliptin-treated and control OVX mice xi
12 Figure 4.24 Figure 4.25 Figure 4.26 Figure 5.1 Figure 5.2 Figure 5.3 Figure 5.4 Figure 5.5 Figure 5.6 Figure 5.7 Figure 5.8 Figure 5.9 Figure 5.10 Figure 5.11 Figure 5.12 Cortical mineralization profiles for Sitagliptin-treated and control male mice 109 Cortical mineralization profiles for Sitagliptin-treated and control female mice. 110 Cortical mineralization profiles for Sitagliptin-treated and control OVX mice Percent of osteoclasts with number of nuclei for DPP-4 KO and WT male mice Percent of osteoclasts with number of nuclei for DPP-4 KO and WT female mice Percent of osteoclasts with number of nuclei for DPP-4 KO and WT OVX mice. 126 Total mineralization profiles for DPP-4 KO and WT male mice Total mineralization profiles for DPP-4 KO and WT female mice Total mineralization profiles for DPP-4 KO and WT OVX mice Trabecular mineralization profiles for DPP-4 KO and WT male mice Trabecular mineralization profiles for DPP-4 KO and WT female mice Trabecular mineralization profiles for DPP-4 KO and WT OVX mice 131 Cortical mineralization profiles for DPP-4 KO and WT male mice Cortical mineralization profiles for DPP-4 KO and WT female mice Cortical mineralization profiles for DPP-4 KO and WT OVX mice 133 xii
13 LIST OF ABBREVIATIONS abmd areal bone mineral density ADOPT A diabetes outcome progression trial AGE Advanced glycation end products BFR Bone formation rate BMC Bone mineral content BMD Bone mineral density BMI Body mass index BMU Basic multicellular unit BSE Back-scatter electron BV/TV Bone Volume/Tissue Volume AP Anterior-Posterior BSE Back-scattered electron Calcr Calcitonin receptor CAR2 Carbonic anhydrase 2 CTSK Cathepsin K CG Chromogranin CLIP Corticotropin-like intermediate lobe peptide CTSK Cathepsin K CTX Carboxyterminal cross-linking telopeptide of bone collagen Cdk5 Cyclin-dependent kinase-5 DEXA Dual energy X-ray absorptiometry DPP-4 Dipeptidyl peptidase-4 Fosl1 fos-like antigen-1 FWHMH Full width at half maximum height GCP-2 Granulocyte chemotactic protein-2 GIP Glucose-dependent insulinotropic peptide GIPR Glucose-dependent insulinotropic peptide receptor GLP-1 Glucagon-like peptide-1 GLP-1R Glucagon-like peptide-1 receptor GLP-2 Glucagon-like peptide-2 GLUT-2 Glucose Transporter 2 GRP Gastrin-releasing peptide IGT Impaired glucose tolerance IL-1#/2 Interleukin-1/2 IP-10 Interferon-inducible protein 10 JNK Jun-N terminal kinase KO Knockout MAR Mineral Aposition Rate MCP Monocyte chemotactic protein Micro-CT Micro-Computed Tomography MS Mineralized surface NFAT Nuclear Factor Activated T-cells NFATc1 NFAT calcineurin-dependent-1 xiii
14 NF-!B Nuclear Factor!B NPY Neuropeptide Y OVX Ovariectomized PHM Peptide histidine methionine PMMA Poly(methyl methacrylate) PPAR Peroxisome proliferators-activated receptors PTH Parathyroid hormone PYY Peptide YY RANK/RANKL Receptor activator of nuclear factor!b/ligand RANTES Regulated on activation of normal T-cell expressed and secreted s-ctx C-terminal telopeptide region of collagen type I SDF-1 $/# Stromal-cell derived factor-1 SEM Scanning Electron Microscope T1DM Type 1 Diabetes Mellitus T2DM Type 2 Diabetes Mellitus Tb.N. Trabecular Number Tb.Sp. Trabecular Separation Tb.Th. Trabecular Thickness TNF-$ Tumor Necrosis Factor alpha TRAP Tartrate-resistant acid phosphatase TRAF6 TNF receptor associated factor 6 TZD Thiazolidinedione vbmd volumetric bone mineral density WT Wildtype xiv
15 CHAPTER 1: INTRODUCTION AND BACKGROUND 1
16 1. Motivation and Rationale: The dramatic increase in overweight and obese Canadians has been deemed to constitute an epidemic. According to a 2004 national survey, 23.1% of Canadian adults aged 18 and over have a body mass index (BMI) over 30, classifying them as obese, and an additional 36.1% of Canadian adults are considered overweight [1]. Increasing rates of obesity can have tremendous negative health implications and is a risk factor for Type 2 Diabetes Mellitus (T2DM). In 2005, 1.8 million Canadians, aged 12 and older, reported having been diagnosed with diabetes mellitus and this number is projected to increase to 2.4 million Canadians by The Canadian Diabetes Association estimates that 90% of these cases are T2DM [2]. Burdens associated with the disease include shortened life expectancy, significant health complications, as well as health care costs for those afflicted [3]. Skeletal health is an important issue for an aging society since risk of fracture increases exponentially with age. Diabetes mellitus can further compromise skeletal integrity as studies have shown increased risk of fracture (hip, foot, proximal humerus) and increased morbidity associated with fractures due to slower fracture healing and diabetic complications [4]. Additionally, thiazolidinediones (TZD), a specific class of anti-diabetic drugs prescribed to improve glycemic control and insulin sensitivity, can increase fracture risk in patients [5-11], especially in postmenopausal women [4, 9]. Therefore, it is necessary to examine the effects of TZDs on the skeleton as well as other classes of anti-diabetic medications that may protect and/or positively affect the skeleton. One such class of anti-diabetic drug, Dipeptidyl Peptidase-4 (DPP-4) inhibitors, can improve glycemic control in patients with T2DM and may have a positive effect on bone via potentiation of incretin hormones [12-22]. A thorough examination on the effects of DPP-4 inhibition on bone quality is important in understanding the potential of DPP-4 inhibitors for the treatment of T2DM without negatively impacting the skeleton. 2
17 1.1 Bone Biology The skeletal system serves many physiological purposes. Bone is the main constituent of the skeletal system, which differs from connective tissue by its rigidity and hardness [23]. These main characteristics of bone allow it to fulfill many of its biomechanical functions such as providing structural support, protection of organs and movement. The skeletal system also serves many metabolic functions such as the production of red blood cells in the bone marrow and acting as a storage unit for inorganic salts (calcium, phosphate, magnesium) [24]. From a biomechanical standpoint, bone is a complex, porous, composite structure that possesses the ability to continually adapt to changes in its physiological or mechanical environment [23, 25]. The capacity of bone to meet its physiological purposes cannot be understood without a thorough understanding of basic bone biology including bone structure, composition and remodeling Bone Structure: There are two morphological types of bone found in mammalian skeletons: cortical and trabecular bone. The latter is composed of an interior meshwork of trabeculae that is porous and spongy with marrow-filled voids [26]. Trabecular bone is found in the epiphysis and metaphysis of long bones and in the cores of cuboid bone. Trabecular bone, possessing a much larger surface area, has a much higher turnover rate in comparison to cortical bone. This characteristic typically results in trabecular bone being more readily affected by dysfunctions in bone remodeling. Cortical bone is compact, highly organized and contains little porosity, providing the skeletal system with its strength and rigidity. It is found in the diaphysis of long bones and in the outer shell of cuboid bone. Cortical bone makes up approximately 80% of total bone mass in the adult skeleton [23]. Figure 1.1 a) and b) illustrate the physiology of long and cuboid bones respectively. 3
18 a) Long Bone: Femur; b)cuboid Bone: Vertebra; Figure 1.1: Physiology of (a) long bone [27] and (b) cuboid bone [28] Cortical and trabecular bone are made up of two types of bone, woven and lamellar. Woven bone serves as provisional material that is formed quickly during periods of rapid growth or extensive repair. It is less organized and eventually resorbed by lamellar bone. Lamellar bone is built up of unit layers of lamellae and is formed during normal periods of growth and repair [23]. Cortical bone is organized and formed around Haversian systems, which is the central vascular canal, into cylindrical units running parallel to the length of the bone (Figure 1.2). A single cylindrical unit is known as an osteon, and numerous osteons are organized to form cortical bone. Volkmann s canals run through the osteon at a perpendicular angle to the Haversian canal in order to connect the latter with the periosteum. Lacunae are small cavities containing osteocytes which are connected by microscopic canals called the canaliculi. The radiating processes of osteocytes project into the canaliculi where they may distribute materials picked up from the blood vessels to the bone matrix [23, 29, 30]. 4
19 Figure 1.2: Haversian system in cortical bone [31] Bone Composition: The inorganic material, making up approximately 65% of bone, consists of impure hydroxyapatite, Ca 10 (PO 4 ) 6 (OH) 2, which forms crystal structures that may incorporate other constituents including carbonate, citrate, magnesium, fluoride and strontium [23]. This material provides the bone with its rigidity and strength [32]. Bone is a composite material consisting of organic and inorganic material. The organic matrix, which makes up approximately 35% of bone, is comprised of collagen, proteins, lipids, and cells. Non-collagenous proteins include osteocalcin, osteonectin, osteopontin and bone sialoprotein. These proteins are thought to play an important role in the size, orientation and fixation of the hydroxyapatite crystals to the collagen framework [23]. Roughly 90% of the organic matrix of bone consists of type I collagen as well as trace amounts of type III and type V. Collagen type I is derived from procollagen type I, which is secreted by fibroblasts and osteoblasts. Collagen is composed of three left-handed polypeptide chains that are cross-linked to form a right-handed triple-helix molecule approximately 1.5 nm in diameter and 300 nm in length [33]. Collagen self-assembles into fibrils having a 67 nm periodicity and 40 nm gaps between the ends of the molecules. Collagen fibrils undergo further arrangement into a quarter-staggered pattern resulting in the collagen fiber. The organic matrix is essential for 5
20 bone growth and ductility as well as providing a structural framework for the inorganic matrix [23, 30]. Figure 1.3 illustrates the hierarchical structure of collagen and mineral. Figure 1.3: Hierarchical structure of collagen molecules and fibers [30] This figure illustrates the assembly of collagn fibrils/fibers and bone mineral crystals. A 67 nm periodic pattern results from the presence of adjacent hole (40 nm) and overlap (27 nm) regions of assembled molecules Bone Remodeling: Bone tissue consists of three types of cells, these are; osteocytes that are embedded in cavities within the matrix, osteoblasts that form bone, and osteoclasts that resorb bone [34]. Both osteoblasts and osteoclasts are derived from precursors originating in the bone marrow. The mesenchymal stem cell serves as the precursor to osteoblasts, as well as bone marrow stromal cells, chondrocytes, muscle cells and adipocytes. Osteoblast precursors are thought to reach bone from migration of progenitors from neighbouring connective tissues. Osteoclasts are derived from the hematopoietic cells of the monocyte/macrophage lineage and reach bone from the 6
21 circulation [35]. The bone cells responsible for bone remodeling each possess unique roles within bone but do so in a synergistic fashion. Bone resorption and formation are not separate processes. Osteoclasts and osteoblasts are components of a temporary structure known as the basic multicellular unit (BMU). In addition to a team of osteoclasts and osteoblasts, the BMU also comprises a central vascular capillary, a nerve supply, and associated connective tissue [35]. The BMU travels through bone, excavating and replacing a tunnel (cortical bone) or a trench (trabecular bone). The average lifespan of a BMU is approximately 6-9 months, which is much longer than the lifespan of its constituent cells [35]. It is therefore necessary that there be a continuous supply of new osteoclasts and osteoblasts from their respective progenitors. Bone remodeling or turnover occurs in five separate stages (Figure 1.4). The first stage of bone remodeling involves the resorption (1) of old or damaged bone and this occurs by the maturation of pre-osteoclasts into activated osteoclasts. During the resorption stage, the mineral phase of bone is dissolved by osteoclast-secreted hydrogen ions while the organic matrix is broken down by a multitude of protease enzymes [36]. A pit is formed where the old bone has been dissolved and this step signals the recruitment of osteoblasts or bone forming cells. Preosteoblasts or lining cells, line the interior of the pit during the reversal (2) stage of bone remodeling. Activated osteoblasts are recruited for the third remodeling stage, known as bone formation (3). These cells deposit unmineralized collageneous bone matrix, which is termed osteoid [36]. Bone-forming osteoblasts also synthesize a number of non-collagenous proteins that are incorporated into the matrix, including osteocalcin and osteonectin [35]. A later stage, known as mineralization (4), occurs when osteoblasts are signaled to deposit mineral. The final stage termed quiescence (5) occurs after a majority of the osteoid has been mineralized and the surface is at a resting stage. The remainder of the osteoid will be mineralized during a secondary 7
22 mineralization which is governed by mature osteoblasts [36]. This extensive remodeling process allows regions of bone to be varied in age, degree of mineralization and structure. The annual rate of turnover in cortical and trabecular bone is 4% and 28%, respectively [35]. (5) (1) (2) (3) (4) Figure 1.4: Bone remodeling cycle [37] Bone Diseases Bone remodeling is a homeostatic system which, when properly balanced, ensures that the amount of bone laid down by osteoblasts matches the amount removed by osteoclasts. As bone formation and bone resorption are coupled processes, bone diseases often occur when one of these processes occur at a greater rate than the other [35]. Osteoporosis is a well-known bone disease that currently affects 1 in 4 females and 1 in 8 males over the age of 50. It has been called the silent thief as the disease leads to a very gradual reduction in bone mass that usually occurs without symptoms. This condition results from increased bone resorption over formation, which leads to an increased susceptibility to fracture. Conversely, a bone disease termed osteopetrosis is a disease resulting from an increased bone formation over resorption, which leads to denser and more brittle bones due to impaired remodeling. Both skeletal diseases illustrate, through opposite mechanisms, the importance of homeostatic control of the bone remodeling process [23]. 8
23 1.1.5 Osteoporosis The medical definition of osteoporosis defines it as a systemic skeletal disorder that is characterized by low bone mass and microarchitectural deterioration of bone tissue. The decreased bone density impairs the mechanical strength of the bone, rendering it more susceptible to fracture [38, 39]. Osteoporosis may exist in either a primary or secondary form. Primary osteoporosis is the most common form and is classified into postmenopausal (type I) and agerelated (type II) osteoporosis. Secondary osteoporosis is attributed to other diseases or conditions that predispose the bone to increased deterioration and is of a type III classification [39]. Type I or postmenopausal osteoporosis affects women between the ages of 50 to 75 due to a depletion of estrogen levels from menopausal loss of ovarian function. Bone mineral density (BMD) remains relatively constant in both genders from young adulthood when peak bone mass is achieved up until middle life [40]. Arrest of ovarian function marks the initiation of the accelerated phase of bone loss seen in postmenopausal women. This accelerated phase of osteoporosis is most noticeably observed over 5-10 years following menopause and accounts for 20-30% losses in trabecular bone and 5-10% losses in cortical bone [35, 41]. Although the effects of estrogen depletion on postmenopausal osteoporosis are not fully understood, it is accepted that bone loss is mediated mainly by loss of the direct restraining effects of estrogen on bone cell functions. Estrogen acts through high affinity estrogen receptors in osteoblasts and osteoclasts to restrain bone remodeling [41]. In a population-based study by Garnero et al. it was found that menopause increased bone formation and resorption markers by approximately 45% and 90% respectfully [42, 43]. The dramatic increases in bone turnover rate coupled with a greater overall bone resorption are responsible for the accelerated rate of postmenopausal bone loss. Type I osteoporosis is characterized by low-trauma fractures which include compression and collapse of the spine as well as fractures of the hip, wrist and forearm caused by minor falls or accidents. 9
24 Over time, the accelerated phase of type I osteoporosis merges asymptomatically with the late phase of slow bone loss which continues indefinitely with age (type II osteoporosis) [35, 39, 41]. Type II or senile osteoporosis affects both men and women beyond the age of 70. This bone disease occurs when the bone resorption and formation processes are no longer coordinated with an increase in bone resorption. There is evidence of an age-related decrease in intestinal responsiveness to vitamin D as well as impaired ability of the aging kidney to synthesize 1,25- dihydroxyvitamin D that may contribute to this form of osteoporosis [44, 45]. It is also thought that andropause, or the gradual decline of exo- and endocrine testicular function that results in lower androgen levels with age might increase bone resorption in aged men [23, 46]. Senile osteoporosis affects both trabecular and cortical bone with typical fractures seen in the femoral neck, vertebrae, proximal humerus, proximal tibia, and pelvis. A secondary contributor to both type I and type II osteoporosis is a lower bone production during adolescence and a decrease peak bone mass [23, 47]. Secondary or type III osteoporosis is caused by other conditions, illnesses or medication/drug use. This type of osteoporosis accounts for less than 5% of osteoporosis cases. Endocrine diseases such as hyperparathyroidism, hypogonadism and diabetes mellitus can lead to the development of secondary osteoporosis. Other miscellaneous conditions that may also lead to secondary osteoporosis includes chronic renal failure, liver disease, immobilization, malabsorption syndromes and the use of drugs such as glucocorticosteroids and barbiturates [48]. Figure 1.5 illustrates the thinning of trabeculae that typically occurs in patients with osteoporosis. Reduced trabecular connectivity and decreases in trabecular bone volume result in reduced structural integrity of the bone. These changes lead to an increase in fracture risk among patients with osteoporosis [49]. The assessment of bone quality involves understanding the numerous parameters that can lead to increased fracture risk in bone diseases like osteoporosis. 10
25 Figure 1.5: Normal trabecular bone architecture versus osteoporotic trabecular bone architecture [50] 1.2 Bone Quality Bone quality can be broadly defined as a bone s ability to resist fracture [51]. By architectural design, bone is fashioned to achieve strength, flexibility and lightness. Bones must have strength to carry loads and resist deformation but be flexible in order to change shape and absorb energy from the varying forces exerted on them. This includes shortening and widening during compression as well as lengthening and narrowing during tension. Additionally, bones must be light to allow for rapid and easy movement [52]. In order to fulfill such physiological purposes, bone is a complex, composite material that holds several skeletal characteristics and mechanical properties [53]. The summation of these features allow bone to resist fracture and no single property alone can predict overall bone quality. Therefore, the assessment of bone quality should be measured based on the multiple skeletal characteristics that allow it to meet these functions. Currently, the most common diagnostic tool used to assess a patient s susceptibility to fracture is the measurement of bone mineral density (BMD) by Dual Energy X-ray Absorptiometry (DEXA) [39, 40, 47, 54]. However, BMD represents one parameter of several that provide bone with the ability to resist fracture. Additionally, the BMD measured by DEXA does not represent a true measure of density as it is calculated from a two-dimensional scan [46, 54]. In order to better assess bone quality, it is important to evaluate the material properties of 11
26 bone, such as mineralization and density, as they contribute to the intrinsic strength of bone. However, it is also crucial to evaluate a bone s structural properties including size, shape and the architecture and connectivity of trabecular bone [53]. Finally, both the structural and material properties of bone are greatly influenced by the balance of bone formation and resorption. For these reasons, a thorough evaluation of bone quality would include measuring the structural and material properties of bone and bone remodeling in addition to density alone. A flowchart summarizing the characteristics relative to fracture risk and bone quality can be found in Figure 1.6. Fracture Risk Mechanical Properties Material Properties 1- Mineralization Bone Mineral Density Structural Properties 1- Architecture 2- Connectivity Bone Remodeling 1- Formation 2- Resorption Figure 1.6: Relationship between bone fragility and various properties of bone Bone Mineral Density Currently, the major noninvasive method available for the early diagnosis of osteoporosis is the measurement of areal BMD (abmd) by DEXA. abmd is considered a major determinant of bone strength and is used to diagnose osteoporosis, applying criteria established by a working group commissioned by the World Health Organization [55]. Additionally, research studies have 12
27 shown that abmd appears to be a good predictor of bone strength[56-58]. Clinical studies on inhibitors of bone resorption, such as bisphosphonates or estrogens, have shown an association between an increase in abmd and a decreased risk of fracture, although changes are not always commensurate [59-61]. In preclinical studies, abmd predicted 50-75% of the variation in ultimate strength, independently of laboratories performing the studies or of the mammalian species used (pig, monkey, rat, mouse)[55, 62, 63]. These findings underline that abmd has a high level of prediction of bone strength but is not completely sufficient in assessing overall bone quality. It is important to understand the limitations of this method since abmd is a widely used parameter in clinical practice and research. abmd is calculated based on a scanned area, not volume, and is therefore not sensitive to density changes in all bone dimensions. The length and width of the scanned bone is known, but not its depth [40, 52]. As bone ages, bone resorption on the endocortical, intracortical, and trabecular surfaces reduces the amount of bone within the periosteal envelope. Simultaneously, bone formation of the periosteal surface partly offsets and compensates for bone resorption of the inner surface. Areal densitometry cannot detect these surface-specific changes. For example, DEXA incorrectly suggests that bone density increases during growth. However, this is mainly caused by increased bone size and does not reflect the mineral changes occurring within the periosteal envelope. DEXA also cannot distinguish sexspecific changes as male abmd is generally higher than female abmd because of a greater periosteal apposition rate and larger bone size. On the other hand, volumetric BMD (vbmd) of the whole bone remains constant or increases slightly during growth and is no different in either sex [52]. 13
28 1.2.2 Bone Mechanical Properties Bone fragility can be defined as bone s susceptibility to fracture. Therefore, the evaluation of mechanical properties is greatly important for the assessment of bone quality as they are directly related to fracture risk. Fractures occur for multiple reasons not solely defined by bone strength. Fractures occur when an applied load exceeds the strength of a bone but can also occur when bone is unable to absorb the energy applied by exerting forces. Ductility is described as the ability of bone to efficiently absorb energy and resist deformation from a load [64, 65]. Testing the mechanical properties of bone can assess a number of biomechanical parameters used to characterize skeletal integrity. Bone is considered an anisotropic material, meaning that its mechanical properties vary according to the direction of the load [25, 53, 65]. For this reason, several types of mechanical tests are performed on various bone sites in order to assess bone quality. Three-point bending of femora is used to test the mechanical properties of cortical bone undergoing bending until failure. This is considered a combined failure mode test because bone is tested both in tension and in compression. Vertebral compression testing is performed on the lumbar vertebrae and mainly tests the mechanical properties of trabecular bone undergoing compression. Finally, femoral neck fracture is used as a clinically relevant test because it mimics hip fractures, a common fracture seen in patients with osteoporosis [39]. From these mechanical tests, important structural and material properties are evaluated in order to thoroughly assess skeletal integrity and susceptibility to fracture [64]. 14
29 1.2.3 Structural Properties When discussing bone mechanics, it is important to distinguish between the structural and material properties of bone. The relationship between the forces applied on bone and the resulting deformations characterizes the structural behaviour or structural properties of the whole bone. Structural properties are measurements of extrinsic mechanical properties, meaning they are influenced by the size and shape of the bone as well as properties of bone tissue [53]. All mechanical tests produce load-displacement curves from which the structural properties are assessed (Figure 3.8a). The biomechanical definition of bone fragility includes four components that are evaluated from load-displacement curves. These include strength, work to failure, ductility and stiffness [51]. Extrinsic bone strength (ultimate load) is defined as the height of the curve and work to failure (energy to failure) is the area under the curve. Ductility is estimated from the width of the curve (failure displacement). Stiffness is the slope of the elastic region of the loaddisplacement curve and represents the rigidity of the structure [51, 53]. The structural properties of cortical bone include cortical thickness, cross-sectional area and moment of inertia. Typically, larger bones with a thicker cross-sectional area absorb greater loads before fracture [51]. Structural properties of trabecular bone relate to trabecular architecture: the number, thickness and separation of individual trabeculae, as well as trabecular connectivity. Predictably, a greater number of thicker trabeculae with less separation and high connectivity are associated with greater trabecular strength [66-69] Material Properties In addition to bone size and shape, a bone s ability to resist fracture is also dependent on its intrinsic or material properties. Stress-strain curves are evaluated in order to assess the intrinsic mechanical properties of bone including strength (ultimate stress), work to failure (toughness), ductility (failure strain), and stiffness (Young s modulus) [51, 64]. The stress-strain curves are 15
30 converted by use of engineering formulae and specimen-specific geometrical information (Figure 3.8b). The resulting biomechanical properties are reflective of the material properties of bone and are independent of bone size and shape [53]. As previously mentioned, bone is primarily comprised of an organic and mineral phase, both of which contribute to its mechanical properties. The mineral phase confers bone with its strength and stiffness but can lead to reduced ductility, or brittleness, at hypermineralized levels. The organic phase confers bone with its ductility, playing a greater role in affecting overall bone toughness [70]. The quality of the mineral phase is assessed by areal and volumetric densitometry and the degree of mineralization. Changes in the quality of the organic phase can also have profound effects on bone fragility. Reduced collagen cross-linking and disordered arrangement of collagen fibers has been correlated with reduced mechanical properties, specifically increased brittleness [65, 70-72]. Additionally, reduced concentrations of collagen cross-links, obtained from treating rats with a collagen cross-link inhibitor (#-amino-propionitrile) resulted in decreased bone strength and stiffness [73]. Age-related changes to the collagen network largely correlate with the deterioration of aged bone. Such age-related changes include the formation of nonenzymatic cross-links in collagen, termed advanced glycation end-products (AGEs). The accumulation of AGEs over time result in the stiffening of the collagen network, leading to increased skeletal fragility and fracture risk [25, 72, 74, 75] Bone Remodeling The process by which bone is remodeled has been explained and is illustrated in Figure 3. Skeletal diseases are often the result of an imbalance or altered rate of bone remodeling and result in increased skeletal fragility [23]. Bone resorption is assessed by quantifying tartrate resistant acid phosphatase (TRAP) positive osteoclasts from thin sections of bone [76]. Bone formation is 16
31 assessed by measuring the distance between fluorescent calcein green mineralization markers given before and after a specific period, a technique termed dynamic histomorphometry [77]. 1.3 Type 2 Diabetes Mellitus (T2DM): Diabetes mellitus refers to a metabolic disorder that is characterized by chronic hyperglycemia and disturbances in carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, action or both [78]. More specifically, Type 2 diabetes mellitus (T2DM) is characterized by peripheral insulin resistance, impaired regulation of hepatic glucose production, and declining!-cell function [79]. This form of diabetes differs from Type 1 diabetes mellitus (T1DM) because autoimmune destruction of pancreatic islets does not occur. T2DM is multigenic, meaning that many different combinations of gene defects may exist among patients with the disease [80]. It is accepted that the development of T2DM results from an interplay of genetic and environmental factors that stress the glucose homeostasis system by promoting insulin resistance or worsening!-cell function (Figure 1.7) [80-82]. The risk of developing T2DM increases with age, obesity, and lack of physical activity [78]. When left undiagnosed or poorly controlled, the effects of diabetes include a reduced life expectancy and quality of life, as well as a greater risk of blindness, heart disease, stroke, peripheral neuropathy, renal disease and amputation [83, 84].. Figure 1.7: Representation of combined features in the development of T2DM [82] 17
32 1.3.1 T2DM and Bone: Diabetes mellitus is considered a heterogenous multi-organ disorder because it can cause severe effects on various systems and tissues of the body. Perturbations of the skeletal system can also result from diabetes including diminished bone formation, reduced fracture healing and osteoporosis [85]. T1DM has been largely associated with lower BMD [86-88]and increased fracture risk when compared to non-diabetic controls [89, 90]. The relationship between T2DM and bone quality is still controversial as studies have shown conflicting evidence regarding its association with BMD and fracture risk [91]. In general, the majority of studies have found average or elevated BMD levels in patients with T2DM as well as an increased risk of fracture despite increased BMD levels [92-95]. This includes the Rotterdam Study which found an increased risk of non-vertebral fractures even when T2DM patients had a higher BMD at baseline [95]. A study by Nicodemus et al., revealed that postmenopausal women with T2DM had a 1.7- fold higher risk of hip fracture than women without the disease. Additionally, the risk of hipfracture in these patients increased with increasing duration of the disease [96]. Nevertheless, T2DM has a considerably lower fracture risk than T1DM, suggesting increased BMD has a protective effect against fracture that is lost with increasing duration of the disease [91] T2DM and Bone Quality: T2DM may largely impact bone quality through numerous and sometimes contradictory mechanisms. In general, the potential adverse effects of T2DM on bone could be mediated by insulin deficiency or resistance, hyperglycemia, advanced glycation end products (AGEs), abnormal cytokine and adipokine production, and impaired neuromuscular/skeletal interactions [3]. Patients with T2DM also have a greater risk of falling due to complications from the disease including poor vision and peripheral neuropathy [10]. T2DM may indirectly affect the skeleton through obesity, altered insulin levels and/or hyperglycemia. 18
33 Obesity: Numerous studies suggest that obesity protects against bone loss since body mass index (BMI) is positively correlated with BMD and negatively associated with the presence of osteoporosis in patients with T2DM [97-99]. The protective effect of obesity against fracture is even seen in post-menopausal women, as shown by lower rates of bone resorption [43] and higher bone density of the axial skeleton when compared to thinner post-menopausal women [100, 101]. However, the exact mechanisms explaining the effects of obesity on the skeleton are not fully understood. Possible mechanisms include increased mechanical loading on the skeleton from higher body weight, increased capacity of estradiol generation by aromatase in peripheral fat tissue as well as association of fat mass with the secretion of other hormones or adipocytokines with potential skeletal effects (amylin, leptin, adiponectin)[10, 101, 102]. Nevertheless, evidence suggests that obesity plays a positive role on BMD and may provide initial protection against fractures that does not persist as the duration and complications from T2DM increase Insulin: Major debate exists whether insulin has an anabolic effect on bone. This is of importance in the association between diabetes and bone as T2DM may be characterized by a state of hyperinsulinemia and insulin resistance [78]. Studies have demonstrated detrimental effects of insulin deficiency on the biomechanical properties of bone. Decreases in bone strength, the rate of mineral apposition, osteoid surface, osteoblastic activity as well as deficits in mineralized surface area and fewer osteoclasts have been observed in an untreated insulindeficient diabetic rat model [103, 104]. Hyperinsulinemia may initially improve BMD in patients with T2DM but may negatively affect the skeleton as!-cell function and insulin production worsens with progression of the disease [10]. Conversely, patients with T1DM are insulinopenic and have reduced BMD [105]. A more recent study examined the effects of removal of the insulin receptor in osteoblasts and reported reduced bone formation and acquisition in these mice [106]. 19
34 Research largely suggests that insulin plays a crucial role in bone metabolism however the effects of insulin resistance on bone, despite hyperinsulinema, are still not fully understood [91] Hyperglycemia: T2DM is characterized by chronic hyperglycemia, which can have several adverse effects on the skeleton. Hyperglycemia is known to generate a higher concentration of advanced glycation end-products (AGE) in collagen that can reduce bone strength [75, 107]. AGEs occur at a greater rate in diabetics since more sugar is present in the extracellular space. These sugars react nonenzymatically with amino groups and can create covalently linked collagen cross-links that change the mechanical properties of the matrix and bone [75]. Interestingly, a spontaneous diabetic rat study revealed that high pentosidine content, an AGE biomarker, was associated with reduced biomechanical properties when compared to non-diabetic control rats, despite similar BMD [107]. AGE accumulation can also lead to deficient bone formation by inhibiting the phenotypic expression of osteoblasts, promote osteoblast apoptosis and increase osteoclast-induced bone resorption [91]. Hyperglycemia can indirectly lead to increased bone loss because an excessive loss of calcium in the urine is associated with increased glycosuria. This resulting hypercalciuria can negatively affect the skeleton as it results from increased calcium loss from bone [3, 10, 108]. Hyperglycemia is also associated with defects in vitamin D levels. Vitamin D mediates bone health through its active form, calcitriol, and is needed for optimal calcium absorption, acting in concert with parathyroid hormone (PTH) as a major physiological regulator for calcium absorption [109]. A population-based study by Baynes et al found that glucose levels and total insulin concentrations were inversely associated with the serum concentration of 25-hydroxyvitamin D 3 during a standard 75-g oral glucose tolerance test [110]. 20
35 Figure 1.8: Multitude of diabetes mechanisms thought to reduce bone quality This figure represents a suggested model of potential deleterious effects of diabetes and the skeleton. A) Amylin: peptide hormone co-secreted with insulin by pancreatic #-cells. B)IGF-1: Insulin-like growth factor-1 C)RANK/RANKL: Receptor Activator for the Nuclear Factor kb and Ligand[3] Overall, the literature suggests that hyperglycemia has a largely detrimental effect on bone quality that can be perpetuated by a multitude of pathways (Figure 1.8). The skeletal effects of hyperglycemia may be initially compensated by hyperinsulinemia and obesity in individuals with T2DM. Despite the discrepancies that exist regarding possible mechanisms, it is largely accepted that T2DM can lead to altered bone quality and increased fracture risk. Studies of T2DM serve to highlight that BMD may not be representative of bone quality and stress the importance of adequate glycemic control and prevention of diabetic complications. Several forms of anti-diabetic drugs are used to improve glucose homeostasis in patients with T2DM and their effects on the skeleton warrant investigation. The effects of two types of anti-diabetic drugs on bone quality are evaluated in this study: thiazolidinediones (TZD) and DPP-4 inhibitors. 1.4 Thiazolidinediones (TZD) TZDs are a class of anti-diabetic drug widely prescribed for T2DM because of their ability to improve insulin sensitivity. TZDs bind to and activate peroxisome proliferators-activated 21
36 receptors (PPAR), including PPAR-! and -$ subtypes. PPARs are nuclear hormone receptors implicated in a number of metabolic processes including roles in cell proliferation, differentiation and survival [111]. Activation of these receptors leads to increased glucose utilization and insulin sensitivity as well as decreased hepatic glucose output [4, 112]. More specifically, PPAR-! activation in adipocytes is thought to improve metabolic parameters by increasing subcutaneous fat deposition at the expense of visceral fat and decreasing serum levels of fatty acids that may have inhibitory effects on insulin signaling [113]. Additionally, PPAR-! activation in adipocytes also promotes the production of adipocytokines (i.e., adiponectin) that are thought, although research is still controversial, to promote insulin action. PPAR-! activation is also thought to decrease the expression of leptin, tumor necrosis factor (TNF)-$ and resistin, which are thought to have inhibitory effects on insulin (Figure 1.9) [113]. Furthermore, TZDs may block the phosphorylation of PPAR-! by cyclin-dependent kinase-5 (Cdk5) which demonstrated a tight association with anti-diabetic effects of the TZD Rosiglitazone in obese patients [114]. Figure 1.9: The effects of PPAR! agonist in muscle, liver and adipose tissue [115] 22
37 1.4.1 TZDs and Bone: A Diabetes Outcome Progression Trial (ADOPT) revealed that TZD usage is associated with increased fracture risk. ADOPT was a randomized, controlled clinical trial with the purpose of comparing the effects of the TZD Rosiglitazone (Avandia, GlaxoSmithKline, Canada) and two other classes of anti-diabetic drugs on glucose control in drug-naïve patients recently diagnosed with diabetes [7]. It was found that Rosiglitazone doubled the risk of bone fractures in females with T2DM compared with those taking metformin or glyburide. Interestingly, these fractures were predominantly found among the upper and lower limbs but not among typical osteoporotic fracture sites such as the hip or vertebrae. The increased risk of fracture occurred in pre- and postmenopausal women, manifested after a year of treatment and did not appear to be the result of increased falls. Although increased fracture risk was reported in both pre- and postmenopausal women, the latter group was reported to have the greater susceptibility to fracture. No increased risk of fracture among men taking Rosiglitazone was reported, even after 5 years of follow-up [7]. Following initial publication of the ADOPT results, it was then reported that Pioglitazone (Actos, Takeda Pharmaceuticals, Japan) was associated with a ~70% increase in fracture risk in women [11]. Rosiglitazone constitutes the most extensively studied PPAR ligand relative to bone metabolism since it possesses the greatest PPAR-! affinity among the most used TZDs. Rodent studies involving the effects of Rosiglitazone treatment on bone have revealed reductions in BMD, bone volume and reduced trabecular architecture [ ]. On the other hand, the effects of Pioglitazone on bone metabolism are much less understood. It is important to assess the effects of Pioglitazone on the skeleton as different TZDs can produce varying clinical responses in an organism. Differences seen in the physiological response to Rosiglitazone or Pioglitazone have been attributed to the in vitro observation that Pioglitazone activates both PPAR-! and -$ 23
38 while Rosiglitazone predominantly activates PPAR-! [121, 122]. Nevertheless, controlled trials did reveal an increased fracture rate in patients receiving Pioglitazone and rodents treated with Pioglitazone exhibited bone loss as noted by reduced BMD and cortical mechanical strength [9, 11, 123, 124] Mechanism of Action: TZDs are thought to negatively affect bone quality through inhibitory effects on bone formation and positive effects on bone resorption. PPAR-! receptors are expressed on mesenchymal stem cells that serve as progenitor cells to many cell types including adipocytes and bone-forming osteoblasts. In vitro studies have revealed that TZD activation of PPAR-! receptors can lead to preferential differentiation of mesenchymal stem cells into marrow adipocytes at the expense of osteoblasts as well as suppress osteogenic transcription factors (Figure 1.10a) [117, ]. Simultaneously, a study by Wan et al., revealed that PPAR-! receptors play an important role in osteoclast differentiation and subsequent bone resorption. The effect of PPAR-! activation on osteoclastogenesis is mediated by direct stimulatory properties of c-fos expression, a factor in the RANKL signaling pathway in osteoclasts (Figure 1.10b). Localized deletion of PPAR-! in the hematopoietic lineages resulted in osteopetrotic mice with significantly reduced osteoclasts. Osteoblasts and bone formation were unaffected because deletion of PPAR-! was localized only in osteoclast progenitors cells. PPAR-! activation by Rosiglitazone also promoted osteoclast differentiation and bone resorption in a dose-dependant manner [129]. These studies suggest that TZDs negatively affect bone by negatively altering bone formation and positively altering bone resorption. 24
39 Figure 1.10: Proposed TZD mechanisms in (a) bone formation (b) bone resorption [129] a) Demonstrates that osteoblasts and adipocytes are derived from the mesenchymal stem cell lineage which make osteoblasts and bone formation susceptible to inhibitory effects if adipocytes are preferentially differentiated by PPAR-! activation. b) Demonstrates the role of PPAR-! in c-fos expression, which is important in osteoclastogenesis. Mutants lacking PPAR-! receptors in the hematopoetic stem cell lineage demonstrated reduced c- Fos expression as well as Fosl1 and NFATc1, two downstream elements of c-fos. 1.5 DPP-4 Inhibition: DPP-4 inhibitors are a novel treatment for T2DM and function by inhibiting the enzyme dipeptidyl peptidase-4 (DPP-4). The therapeutic benefits of DPP-4 inhibitors as anti-diabetic drugs are attributed to their ability to extend the biological activity of incretin hormones [ ]. DPP-4 inhibition is a potent treatment for T2DM because incretins play a large role in postprandial insulin secretion and blood glucose homeostasis. DPP-4 inhibitors are beneficial because they do not lead to side effects noted with other T2DM treatments such as weight gain and/or hypoglycemic episodes [134]. Positive improvements in islet survival and function as well as!-cell mass maintenance have also been noted from DPP-4 inhibitor treatment in diabetic rodents [135]. DPP-4, also referred to as CD26, exists in two forms: 1) a soluble enzyme found in circulation and 2) a membrane-anchored, largely extracellular form, with implications in intracellular signal transduction pathways [136]. DPP-4 is a widely expressed, multifunctional glycoprotein present in various tissues including the liver, lung, kidney, and intestine as well as on endothelial cells, lymphocytes, T-cells, B-cells and natural killer cells [ ]. DPP-4 cleaves off 25
40 N-terminal dipeptides from proteins having proline or alanine in amino acid position two. DPP-4 belongs to a complex gene family containing similarly capable members that cleave structurally related proteins. The existence of similar DPP-4 like enzymes implicates a need for highly selective DPP-4 inhibitors since inhibition of the similar DPP-8 and 9 enzymes produce serious toxicity in animal models [137, 140]. The DPP-4 inhibitor used in this study, Sitagliptin (Januvia, Merck Frosst, Canada), is a highly selective DPP-4 inhibitor. A large number of DPP-4 substrates have been discovered in vitro but comparatively fewer appear to exist as in vivo endogenous physiological substrates. A peptide is considered an endogenous physiological DPP-4 substrate if intact to cleaved forms are significantly altered following genetic inactivation or chemical inhibition of DPP-4 activity in vivo. Table 1.1 summarizes peptides that act as DPP-4 substrates in vitro as well as endogenous physiological DPP-4 substrates cleaved in vivo [131]. Table 1.1: in vitro and in vivo peptides cleaved by DPP-4 [131] DPP-4 Peptide Substrates in vitro in vivo Aprotinin IP-10 GLP-1 BNP MDC GLP-2 Bradykinin MCP-1 GIP!-Casomorphin MCP-2 SDF-1" CG MCP-3 SDF-1! CLIP Tyr-melanostatin Substance P Endomorphin-2 "1-Microglobulin Enterostatin NPY Eotaxin PHM GCP-2 Prolactin GHRH PYY GRP RANTES IGF-1 Trypsinogen IL-2 Trypsinogen propeptide colipase IL-1! CG, chromogranin;clip, corticotropin-like intermediate lobe peptide; GCP-2, granulocyte chemotactic protein-2; GRP, gastrin-releasing peptide; IL-1, interleukin-1!; IL-2, interleukin-2; IP-10, interferon inducible protein 10, also known as CXCL10 or chemokine (C-X-C motif) ligand 10; MCP, monocyte chemotactic protein; MDC, macrophage-derived chemokine; PHM, peptide histidine methionine; RANTES, regulated on activation normal T-cell expressed and secreted. 26
41 It is crucial to distinguish between in vitro and in vivo substrates since small changes in the ratio of intact to cleaved forms may not be sufficient to produce a biological effect. However, DPP-4 substrates classified in vitro could also exist as in vivo forms but have not been classified as so due to limitations in their detection and measurement. Of the in vivo forms, Substance P is a neuropeptide with vasodilatory effects that are attenuated by DPP-4 in vivo. Conversely, these vasodilatory effects are potentiated by DPP-4 inhibition that are consistent with reports of nasopharyngitis in patients treated with DPP-4 inhibitors [134]. Stromal cell-derived factor-1 (SDF-1) " and! are chemokines that play crucial roles in lymphocyte chemotaxis and immune function. Finally, glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) and -2 (GLP-2) are enteric hormones that play important roles in glucose homeostasis [136]. Incretins are gastrointestinal hormones with insulinotropic effects that are secreted in response to food ingestion [136]. Currently, GIP and GLP-1 are the only peptides classified as incretin hormones in humans. Incretin-receptor activation leads to glucose-dependent insulin secretion, induction of ß-cell proliferation, and enhanced resistance to apoptosis [134]. Figure 1.11 illustrates the amino acid sequences of GIP and GLP-1 and highlights the alanine residue located in position two that constitutes the cleavage site for DPP-4. 27
42 Figure 1.11: Amino acid sequence of GIP and GLP-1 with point of cleavage by DPP-4 GIP was the first incretin polypeptide identified and is secreted from duodenal K-cells in the proximal small intestine. GLP-1 is secreted from the L-cells of the small intestine [134]. Levels of GIP and GLP-1 are low in the fasted state and rise within minutes of food ingestion. Both GIP and GLP-1 stimulate glucose-dependent insulin secretion via activation of their specific G protein-coupled receptors expressed directly on islet #-cells [141]. Intact GIP (1-42), but not GLP-1, also promotes energy storage via direct actions on adipose tissue. Intact GLP-1 (7-36) exerts important actions for glucose homeostasis such as inhibition of glucagon secretion, fasting glucose control, satiety, and gastric emptying [142]. DPP-4 cleaves these incretin hormones after their secretion and inactive forms of GIP (3-42) and GLP-1 (9-36) have no insulinotropic effects in vivo. The half-life of intact biologically active GIP is less than two minutes in rodents and seven 28
43 minutes in healthy subjects. GLP-1 has a shorter half-life of approximately two minutes in humans because DPP-4 expression occurs in the endothelium directly adjacent to the sites of GLP-1 release. GIP stimulates GLP-1 secretion in animal studies but does not do so in humans. The incretin effect is estimated to account for approximately 50%-70% of the total insulin secreted after oral glucose administration [134]. Intact GLP-2 (1-33) is co-secreted with GLP-1 from the L-cells of the small intestine. It is not classified as an incretin hormone because it does not have insulinotropic effects. However, GLP-2 has trophic effects on the small and large intestine largely mediated by stimulation of cell proliferation and inhibition of apoptosis and proteolysis. GLP-2 also plays a role in gastric motility and acid secretion, suppression of food intake, stimulation of intestinal blood flow as well as enterocyte glucose transport and Glucose Transporter 2(GLUT-2) expression [130, 136, 143, 144]. Similarly to GIP and GLP-1, the levels of GLP-2 increase within minutes of nutrient ingestion and GLP-2 possesses an alanine in position 2 allowing its inactivation (3-33) by DPP-4. GLP-1 and GLP-2 are termed glucagon-like peptides because they are both derived from tissue-specific post-translational modifications of the proglucagon gene located on the long arm of human chromosome 2 (Figure 1.12). It is comprised of 6 exons and 5 introns with the entire coding sequences for GLP-1 and GLP-2 located on exons 4 and 5, respectively. The proglucagon gene is expressed in the $-cells of the endocrine pancreas, the L-cells of the intestine and neurons of the brainstem and hypothalamus [134]. 29
44 Gene mrna Protein Tissue-specific posttranslational processing Figure 1.12: Posttranslational processing of proglucagon [134] DPP-4 Knockout Mice: Evaluating the effects of complete genetic inactivation of DPP-4 activity using DPP-4 knockout mice is important in further understanding the role DPP-4 may play in important physiological processes. Additionally, characterizing phenotypes in a DPP-4 knockout model may aid in understanding potential side effects of DPP-4 inhibitor use. DPP-4 knockout mice have been generated by homologous recombination in C57BL/6 derived embryonic stem cells using a DPP-4 specific targeting construct. The targeting construct was designed to excise part of the promoter and exons 1 and 2, encoding both the cytoplasmic and transmembrane forms of DPP-4. This resulted in homozygous DPP-4 -/- mice on a C57BL/6 background with complete inactivation of both DPP-4 forms, soluble and membrane-bound [139]. Complete inactivation of DPP-4 activity produces relatively healthy mice, with a modest immune phenotype, despite the putative role for DPP-4 in immune function. Plasma from homozygous knockout mice (-/-) revealed no significant DPP-4 activity as well as no significant levels of N-terminal truncation of the peptides GLP-1 or GIP. However it is important to note that, despite complete absence of DPP-4, plasma from -/- mice contained residual enzyme activity 30
45 [139]. This finding is consistent with the existence of distinct enzymes with DPP-4-like proteolytic activity [131]. DPP-4 -/- mice also had significantly lower blood glucose concentrations after glucose challenge and significantly higher glucose stimulated insulin levels when compared to wildtype mice [139]. Accordingly, DPP-4 -/- mice fail to become obese on a high fat diet partially due to reduced food intake and enhanced metabolic energy expenditure. These mice are also resistant to high fat diet-induced glucose intolerance or diabetes as well as diabetogenic doses of streptozotocin [145]. Overall, DPP-4 knockout mice have enhanced glucose homeostasis attributed to increased levels of bioactive incretin hormones [139]. Currently, no studies exist regarding the effects of genetic DPP-4 inactivation on bone quality or whether a significant bone phenotype exists in these mice DPP-4 Inhibition and Bone: The purpose of this study was to examine the effects of DPP-4 inhibition on bone quality by use of chemical inhibition and genetic ablation of DPP-4 activity. Both forms of DPP-4 inhibition potentiate the enteric hormones GIP, GLP-1 and GLP-2 in vivo which have known effects in metabolism and digestion [134]. Addtionally, GIP, GLP-1 and GLP-2 receptors are expressed in other tissues, motivating the study of other biological processes that may be affected from their potentiation [130, 136, 138]. There is increasing evidence suggesting that GIP, GLP-1 and GLP-2 may play important roles as physiological regulators of bone turnover and metabolism. The GIP receptor is expressed in osteoblasts [13] and GIP increased collagen type-1 expression and alkaline phosphatase activity in osteoblast-like cells [12, 13] as well as protected osteoblasts from apoptosis [19]. GIP receptors have also been found on osteoclasts and GIP was found to inhibit bone resorption in vitro [22]. Additionally, a GIP receptor knockout mouse model (GIPR-/-) exhibited a low bone mass 31
46 phenotype due to decreased bone formation and increased bone resorption [19, 20]. Intravenous administration of GIP also prevented ovariectomy-induced bone loss in a rat model [12]. On the other hand, the effects of GLP-1 on bone are less defined because GLP-1 appears to have no direct effect on osteoblasts or osteoclasts. Nevertheless, GLP-1 receptor knockout mice (GLP-1R -/-) exhibited cortical osteopenia, reduced bone density and increased histomorphometric bone resorption parameters [21]. These effects are thought to occur indirectly through a calcitonin-dependent pathway since GLP-1R -/- mice exhibited reduced levels of calcitonin mrna transcripts in the thyroid. Additionally, a GLP-1R agonist was found to increase calcitonin mrna transcripts in the thyroid of wild-type mice [18, 21, 146]. GLP-2 is thought to have a positive effect on bone based on GLP-2 administration studies. One study involved the administration of three varying doses of GLP-2 over four months in postmenopausal women with low BMD. Treatment with GLP-2 resulted in significant dosedependent increases in total hip BMD as well as reduction of serum concentrations of fragments derived from the degradation of the C-terminal telopeptide region of collagen type I (s-ctx), a marker of bone resorption [16, 17, 128]. Another study involved a five-week treatment of GLP-2 in short-bowel patients with no colon and resulted in significantly increased spinal BMD [15]. However, the role of GLP-2 on bone may, in part, relate to increased nutrient absorption rather than a direct effect on bone turnover [14]. It is important to note that the doses of GLP-2 administered to produce these effects are significantly higher than normal postprandial levels [17]. Overall, there is increasing evidence to support a positive role for enteric hormones GIP, GLP-1 and GLP-2 on bone metabolism and homeostasis. These studies include receptor knockout mouse models as well as directly administered peptide studies. However, little evidence exists regarding the skeletal effects of potentiating enteric hormone action by DPP-4 inhibition. 32
47 The effects of DPP-4 inhibition on the skeleton is an important area of investigation since it is a current T2DM treatment and patients with this disease experience increased skeletal fragility. 1.6 Mouse Model: Animal models play a crucial role in research as they provide a more uniform experimental material to study the effects of therapies and manipulated genetic conditions on bone quality. Mice are often considered the experimental model of choice because of their short life-span, ease of breeding and reproductive capacity, homology between human and mouse genomes and availability of genomically identical mouse strains [147]. Additionally, among all mammals, mice are the most promising animals for genetic experimental work [148]. A major disadvantage to using mice as a model for bone quality is the differences in skeletal systems between mice and humans. Most notably, mice do not have haversian systems and therefore lack substantial intracortical remodeling [149]. Also, mice have a relatively small skeleton, a disadvantage as the majority of techniques used to assess bone mechanical properties were designed for the testing of larger bones. The small size of mouse bones present technical challenges in terms of generating precise and repeatable results. Despite these disadvantages, mice serve as a good model for preliminary experimental investigations since they meet the four main requirements of a good animal model: convenience, relevance, predictability and appropriateness [150]. The advent of inbred strains of mice, such as the C57BL/6 strain used in this study, has allowed researchers to limit genetic variation among specimens thereby, rendering experiments more controlled. The use of inbred mouse strains has become common practice and there are several inbred mouse strains available with well-defined differences in peak bone mass and susceptibility to bone loss [151]. More specifically, there have been three main skeletal phases noted in the C57BL/6 mouse strain; the rapid growth phase (>5 months), peak bone mass phase (5-9 months) and age-related bone loss phase (>12 months) [152]. Epiphyseal closure, a measure 33
48 of adulthood, has been reported by 6-8 months of age [153]. Seven months was the designated age of sacrifice used for this study as it provides sufficient time for adequate drug treatment as well as adult mice in the peak bone mass phase. The ovariectomized (OVX) rodent is the most commonly used animal model for osteoporosis studies [154]. In mice, ovariectomy is followed by accelerated bone loss mediated through estrogen deficiency that parallels the situation seen in women after menopause. This ovariectomy-induced bone loss is prevented by replacement with estrogen and its ability to reverse bone loss is dose-dependent. It requires four weeks following OVX to allow for a new steadystate to develop in the skeleton and eight weeks are recommended before bones are subjected to structural or histomorphometric analysis [153]. For the purpose of this study, three months was the selected age for OVX in order to provide the four weeks necessary for steady-state development prior to drug treatment in selected mice. Additionally, OVX at three months of age allowed sufficient time to produce a positmenopausal-like bone loss phenotype before sacrifice at seven months of age. The drug portion of this study involved the use of a high-fat fed mouse model for impaired glucose tolerance (IGT) and early T2DM. This model was initially described by Surwit et al., and has been shown to be most efficient in the C57BL/6 mouse strain [ ]. The B6 mouse is a good strain for this model because it develops obesity, hyperinsulinemia, hyperglycemia and hypertension when fed a high-fat diet. The development of insulin resistance, hyperglycemia and obesity in this mouse strain parallels that seen in humans [155]. Additionally, diet-induced obesity and IGT in this mouse is associated with the selective deposition of fat to the abdominal region which is an independent risk factor for diabetes in humans [155]. It is accepted that these mice develop hyperglycemia after one month of high fat feeding however hyperglycemia and hyperinsulinemia can occur as early as one week following introduction of high 34
49 fat diet [ ]. Mice of the anti-diabetic drug study were fed a high fat diet at three months of age in order ensure adequate development of glucose intolerance before drug treatment at four months of age. Overall, exposure to high fat diet leads to the development of glucose intolerance but not frank diabetes in these mice [155, 158]. The mice involved in the genetic portion of this study were not fed a high fat diet because DPP-4 -/- mice are resistant to diet induced obesity and diabetes [145]. DPP-4 -/- mice and their wildtype controls (+/+) were fed regular chow in order to prevent the disparity in phenotype that would form between them if both were fed a high fat diet. 35
50 CHAPTER 2: HYPOTHESIS AND OBJECTIVES 36
51 2. Introduction: The enteric hormones GIP, GLP-1 and GLP-2 are suspected of having positive effects on bone by either direct or indirect mechanisms [12-22]. However, the physiological effects of these hormones are short-lived due to their cleavage and subsequent inactivation by the DPP-4 enzyme. DPP-4 inhibition is a beneficial treatment method for T2DM because it results in enhanced bioactivity of the above mentionned enteric hormones. Given the possible role of these enteric hormones in bone metabolism, the increased susceptibility of fracture in patients with T2DM and the role of DPP-4 inhibition in enteric hormone potentiation and T2DM treatment, these features provide the motivation to evaluate the effects that DPP-4 inhibition may have on bone quality. The primary goal of this project is to examine the effects of DPP-4 inhibition on bone quality. Two models were used for this purpose: a chemical inhibition model, by use of the highly selective DPP-4 inhibitor Sitagliptin, and a genetic inactivation model, by use of DPP-4 -/- mice. The study was further divided into two parts: an anti-diabetic drug study and the genetic inactivation study. These are regarded as two separate parts because the mice used for each are not identical strains and diets are significantly different. 2.1 Anti-Diabetic Drug Study Mice treated with Sitagliptin were given a high fat diet because a glucose intolerant mouse was more representative of the conditions warranting this type of treatment in humans. However, Sitagliptin treatment in high fat fed mice improves their glucose homeostasis and insulin levels [158], two factors that are thought to significantly affect bone in patients with T2DM [3]. In order to more precisely account for DPP-4 inhibition, another group of mice were treated with a second anti-diabetic drug, expected to stabilize glucose and insulin levels but through a different mechanism from DPP-4 inhibition. Pioglitazone, a TZD, was chosen because less evidence exists regarding its role in bone quality in comparison to Rosliglitazone, a more extensively studied 37
52 TZD. The differences seen in skeletal effects between mice treated with Sitagliptin, the DPP-4 inhibitor, and those treated with Pioglitazone, the TZD, to a vehicle control would be more reflective of their respective mechanisms of action since both effectively stabilize glucose and insulin levels in mice. Hypothesis: TZD treatment will result in bone loss and compromised bone quality in a glucose intolerant mouse model. Conversely, chemical DPP-4 inhibition will positively affect bone quality in a glucose intolerant mouse model. The effects of ovariectomy will further emphasize the effects seen from TZD and DPP-4 inhibitor treatment. Objective 1: Evaluate the effects of TZD treatment, by Pioglitazone, on murine bone quality in glucose intolerant male, female and OVX mice. Objective 2: Evaluate the effects of DPP-4 inhibitor treatment, by Sitagliptin, on bone quality in glucose intolerant male, female and OVX mice. All mice were sacrificed at seven months of age and treated mice are compared to untreated vehicle controls. Bone quality is assessed through densitometry, mechanical, structural, material and remodelling properties. 2.2 Genetic Inactivation Study The genetic inactivation study involved the use of DPP-4 -/- mice that are an inbred strain of mice generated from an initial stock of C57BL/6 mice purchased from a different breeder source than those used in the anti-diabetic drug study. For these reasons, the mice used in both the genetic inactivation and anti-diabetic drug studies are not considered directly comparable. Additionally, DPP-4 -/- mice are inherently resistant to obesity and diet-induced glucose intolerance but their wildtype controls are not [145]. Subjecting both to a high fat diet would have produced a large disparity in glucose and insulin levels making any skeletal differences seen between them less likely attributable to DPP-4 inhibition. Therefore, unlike the anti-diabetic drug 38
53 study, DPP-4 -/- and wildtype are given regular rodent chow until they are sacrificed. As such, the project can be divided into two primary studies using different mouse models. Hypothesis: Complete genetic inactivation of DPP-4 will result in an enhanced bone phenotype that is further emphasized with ovariectomy. Objective 3: Evaluate the effects of genetic inactivation of DPP-4 on bone quality in male, female and OVX DPP-4 -/- mice. All mice were sacrificed at seven months of age and DPP-4 -/- mice are compared to DPP-4 +/+ littermate controls (wildtype). Bone quality is assessed through densitometry, mechanical, structural, material and remodelling properties. 39
54 CHAPTER 3: MATERIALS AND METHODS 40
55 3. Introduction This chapter outlines the various techniques performed in order to evaluate murine bone quality. Each technique includes a brief technical background, details regarding its experimental setup and important parameters derived from each test. 3.1 Mice and Experimental Design: Anti-Diabetic Drug Study: The mice used for the anti-diabetic drug study were purchased from Taconic Laboratories (Hudson, NY) and housed in the animal colony at the Toronto Centre of Phenogenomics (Toronto, Ontario). Mice for both perspective studies were divided into three groups. The first group (male mice) includes vehicle controls (S and P) and treatment with either Sitagliptin or Pioglitazone. Sitagliptin and Pioglitazone-treated male groups were bred and housed at different times with respective and independent control groups. Vehicle S and Vehicle P groups correspond to the littermate controls bred and housed with Sitagliptin and Pioglitazone-treated male mice respectively. The second group (female mice) consists of a single vehicle control and treatments with either Sitagliptin or Pioglitazone. The third group (OVX mice) was ovariectomized in order to establish a significant reduction in bone density mimicking the reduction seen in postmenopausal women. Female mice that underwent ovariectomy were bred and housed independently of unovariectomized female mice. Similarly to the female group, the third group includes a single vehicle control and treatments with either Sitagliptin or Pioglitazone. Group sample sizes (n) for all mice in the anti-diabetic drug study are summarized in the Table
56 Table 3.1 Sample sizes for anti-diabetic drug study Group n Model Strain Treatment Male Mice Sitagliptin 1- Vehicle S 11 Male C57BL/6 No Treatment 2- Sitagliptin 11 Male C57BL/6 4g/kg rodent chow Sitagliptin Male Mice Pioglitazone 1- Vehicle P 9 Male C57BL/6 No Treatment 2- Pioglitazone 10 Male C57BL/6 0.28g/kg rodent chow Pioglitazone Male Total 41 Female Mice Sitagliptin + Pioglitazone 1- Vehicle 8 Female C57BL/6 Saline Vehicle 2- Sitagliptin 8 Female C57BL/6 3- Pioglitazone 12 Female C57BL/6 4g/kg rodent chow Sitagliptin 0.28 g/kg rodent chow Pioglitazone Female Total 28 OVX Mice Sitagliptin + Pioglitazone 1- Vehicle 9 OVX C57BL/6 Saline Vehicle 2- Sitagliptin 12 OVX C57BL/6 3- Pioglitazone 11 OVX C57BL/6 OVX Total 32 Study Total 101 4g/kg rodent chow Sitagliptin 0.28g/kg rodent chow Pioglitazone C57BL/6 mice were fed a standard rodent diet until three months of age and then a highfat diet (40% kcal from fat; Research Diets, New Brunswick, NJ) from three months of age until sacrifice (seven months of age). C57BL/6 mice fed a high-fat diet were used as a model because it has been shown to produce modest glucose intolerance in these mice but not frank diabetes [158]. OVX was performed at three months of age after which these mice were immediately placed on a high fat diet for one month followed by identical treatment with either Sitagliptin (Januvia, Merck Frosst, Canada) or Pioglitazone (Actos, Takeda Pharmaceuticals, Japan). Sitagliptin was mixed in the diet at a concentration of 4g/kg rodent chow [132] and Pioglitazone was mixed in the diet at a concentration of 0.28g/kg rodent chow as described [116, 117] at four months of age for all mice. Drug treatment continued for three months as several studies have shown that TZDs produce a 42
57 significant reduction in bone mass after 1-2 months of therapy in mice [116, 117]. Littermate controls were labeled as Vehicle groups and underwent the same dietary changes (high fat diet) as treated groups but without treatment. A timeline detailing the times of treatment, OVX and dietary changes can be found in Figure 3.1. Figure 3.1 Anti-diabetic drug study timeline Genetic Inactivation Study: The mice used for the genetic inactivation study were supplied from the Toronto Centre of Phenogenomics (Toronto, Ontario) in-bred colony originally purchased from Charles Rivers Laboratories (Massachusetts, USA). DPP-4 knockout mice were generated by homologous recombination in C57BL/6 derived embryonic stem cells using a DPP-4 specific targeting construct. The targeting construct was designed to excise part of the promoter and exons 1 and 2, encoding both the cytoplasmic and transmembrane forms of DPP-4. This resulted in homozygous DPP-4 -/- mice on a C57BL/6 background with complete inactivation of both DPP-4 forms, soluble and membrane-bound [139]. The mice were fed a standard rodent diet until sacrifice at seven months of age. A subset of female DPP4-/- (knockout, KO) and DPP4+/+ (wildtype, WT) mice were ovariectomized at three months of age, fed a standard rodent diet and sacrificed at seven months of age. A timeline detailing the genetic inactivation study can be found in Figure 3.2. Table 3.2 shows the sample size (n) for each group in the genetic inactivation study. 43
58 Figure 3.2 Genetic inactivation study timeline Table 3.2 Sample sizes Group n Model Strain Background Male Mice 1- WildType 14 Male C57BL/6 DPP4 +/+ 2- KnockOut 22 Male C57BL/6 DPP4 -/- Male Total 36 Female Mice 1- WildType 13 Female C57BL/6 DPP4 +/+ 2- KnockOut 19 Female C57BL/6 DPP4 -/- Female Total 32 OVX Female Mice 1- WildType 10 OVX Female C57BL/6 DPP4 +/+ 2- KnockOut 13 OVX Female C57BL/6 DPP4 -/- OVX Total 23 Study Total Limitations of Mice: It is important to note that Pioglitazone and Sitagliptin-treated mice are solely compared to their respective vehicle control groups. No statistical comparisons were made between Pioglitazone and Sitagliptin-treated mice directly. This study aims to evaluate the effects of DPP-4 inhibition on bone quality and Pioglitazone, being a TZD, is used as a control for agents that lower blood glucose but using an alternate mechanism. This was done to allow the changes seen due to Sitagliptin treatment to be more largely attributable to DPP-4 inhibition and not simply to its blood glucose lowering effects, which also result from Pioglitazone treatment. Treatment groups and their corresponding vehicle control groups are composed of mice bred and housed together at a specific time. As previously mentioned, the male treatment groups were created at different times and therefore remain separate with respective vehicle control groups. Additionally, 44
59 no direct comparisons were made between Sitagliptin-treated mice and DPP-4 -/- mice. Although these mice do share the C57BL/6 background, they are not genetically comparable Sacrifice and Dissection: At seven months of age, each mouse was euthanized in a carbon dioxide (CO 2 ) chamber. A final bodyweight was recorded after euthanization. The right and left femora and L5 and L6 vertebrae were excised, cleaned of adherent soft tissue and stored at -20 C in saline soaked gauze. Processes were carefully removed from all vertebrae. Bones were thawed at room temperature prior to any experimental testing. The L3 and L4 vertebrae were excised and immediately fixed in 10% neutral buffered formalin or 70% ethanol respectively, for histomorphometry. The L3 and L4 vertebrae were allowed to fix in at room temperature for five days prior to further processing for embedding purposes. 45
60 3.2 Experimental Assessment of Bone Quality: The experimental techniques performed in order to assess murine bone quality are summarized in Figure 3.3. All samples were similarly handled and evaluated. Bone Quality Mechanical Properties 1- Cortical: Three-Point Bending 2- Trabecular: Vertebral Compression 3- Both: Femoral Neck Fracture Material Properties Normalized: Digital Macroscopy, MicroCT 1- Mineralization: Back Scattered Electron (BSE) imaging Bone Mineral Density 1- abmd: DEXA 2- vbmd: Micro-CT Bone Remodeling 1- Formation: Dynamic Histomorphometry 2- Resorption: Osteoclast Staining Structural Properties 1- Architecture: Micro-CT 2- Connectivity: Strut Analysis Figure 3.3 Summary of experimental techniques used to assess bone quality 46
61 3.3 Areal Bone Mineral Density: Dual Energy X-ray Absorptiometry: Dual energy x-ray absorptiometry (DEXA) is a highly used scientific research and clinical tool used to measure bone and soft-tissue composition in vivo. DEXA involves the use of two different radiation x-ray beams with different energy peaks: 35 (low) and 80 (high) kev. One x-ray beam is absorbed mainly by soft tissue, the other by bone. As photons transverse the specimen, they are absorbed or scattered based on their attenuation properties. These x-ray attenuation properties allow the detector to distinguish the bone mineral from fat and lean soft tissue. Bone mineral content (BMC) is assessed by the amount of x-ray absorbed by bone and is divided by the area measured to determine the bone mineral density (BMD) [40]. Since BMD is obtained from a scanned two-dimensional area, it is therefore defined as an aerial measurement (g/cm 2 ) as opposed to a true measurement of volumetric density (g/cm 3 ) [159, 160]. DEXA was performed on excised left and right femora and lumbar 5-6 vertebrae using the Lunar PIXImus Bone Densitometer (Lunar GE Corp.) designed for small animal bone analysis. The PIXImus was calibrated by running a calibration scan on an aluminum/leucite phantom plate prior to each session. The bones were consistently placed in the same orientation on a polystyrene plate that simulates soft tissue thickness (Figure 3.4). Following the scan, the femora and vertebrae were re-wrapped in saline soaked gauge and stored at -20 C. Scans were analyzed using the PIXImus software. The results for the femora and vertebrae were averaged to provide femoral and vertebral measurements, respectively. 47
62 Figure 3.4 PIXImus scan of femora and vertebrae 3.4 Volumetric BMD and Bone Structural Properties: Micro-Computed Tomography: Micro-computed tomography (Micro-CT) is a clinical tool that uses x-ray beams to create high resolution (~6 microns) three-dimensional images for the calculation of volumetric BMD (vbmd) and to evaluate changes in trabecular bone microarchitecture [161]. Trabecular bone consists of a three-dimensional network of plates and rods. Factors such as age and disease can affect the connectivity, thickness and number of elements in this network, causing dramatic changes in bone quality [54, 161]. Micro-CT enables the analysis of these parameters to allow for numerical results. Right femora and L5 vertebrae were scanned using the Skyscan1174 compact Micro-CT (Skyscan; Kontich, Belgium). Pressure-sensitive adhesive was used to secure femora in an upright position within a saline-filled microfuge tube whereas vertebrae were glued to an upright scaffold at the center of a saline-filled microfuge tube. Tubes were securely fastened to the scanning platform in order to prevent any movement during scanning. All images were obtained at an x-ray voltage of 50 kv and current of 800 %A with a 0.25 mm aluminum filter to ensure a uniform beam. All scans were reconstructed and calibrated with the use of two hydroxyapatite standards that were provided by the manufacturer. Reconstructed images obtained from scanning were analyzed using the Skyscan CT-Analyzer software (Version 1.5.0). 48
63 Femoral scans were reconstructed with consistent settings (smoothing = 3, beam hardening correction = 31%, ring reduction correction = 6 and defect pixel mask = 10%). Femoral geometry and vbmd were assessed from the analysis of a region of interest created 0.25 mm above and below the midpoint (Figure 3.5). Femoral midpoints were measured by use of digital calipers and serve as the points of fracture for three-point bending tests. The parameters obtained from femoral analysis were vbmd (g/cm 3 ), anterior-posterior (AP) diameter (mm), principal moments of inertia (Imin and Imax; mm 4 ), cross-sectional bone area (mm 2 ) and cortical thickness (mm). Medio-lateral Anterior-Posterior Figure 3.5 (a) Cross-section of midpoint with labeled medio-lateral and anteriorposterior axis (b) Micro-CT scan of femur Vertebral scans were reconstructed with consistent settings (smoothing = 6, beam hardening correction = 31%, ring reduction correction = 12 and defect pixel mask = 10%). Vertebral trabecular architecture and vbmd were assessed by creating a region of interest along the length of the vertebrae but with exclusion of vertebral growth plates (Figure 3.6). The parameters obtained from analysis of vertebral scans include vbmd (g/cm 3 ), percent bone 49
64 volume (%), trabecular thickness (%m), trabecular number (mm -1 ) and trabecular separation (%m). These parameters were used to evaluate the structural quality and architecture of the vertebrae. Figure 3.6 (a) Cross-sectional view of scanned vertebra (b) Micro-CT scan of vertebra Strut Analysis The evaluation of trabecular connectivity is performed by strut analysis of 2-dimensional back-scattered electron (BSE) images of Spurr-embedded samples. Details regarding the processing required for BSE imaging are described in section The trabecular bone area internal to growth plates and cortex of L4 vertebral coronal sections was analyzed. These images were then thresholded in order to obtain binary images, which were analyzed on a Quantimet 500 IW system using the program Quips, written by Dr. Mircea Dumitriu to evaluate individual trabeculae as struts. The point of connection for three or more struts was defined as a node. The parameters evaluated are divided based on their ability to indicate connectivity or disconnectivity between trabecular struts. Measures of connectivity include total strut length (mm/mm 2 ), number of nodes (mm -2 ), length of node-to-node struts (mm/mm 2 ) and length of node-to-free struts (mm/mm 2 ). Measures of disconnectivity include number of free-to-free ends (mm -2 ) as well as length of free-to-free struts (mm/mm 2 ) [162](Figure 3.7). 50
65 FE node-node strut free-end (FE) FE node node-free FE free-free strut Figure 3.7 Thresholded image with schematic view of strut analysis parameters 3.5 Bone Mechanical Properties Destructive mechanical testing of whole bone is an effective way to determine overall skeletal fragility. A load-displacement curve is generated for every sample tested (Figure 3.8a). From this curve, several biomechanical parameters can be used to characterize the mechanical integrity of bone. The linear region of the curve represents elastic behaviour, meaning the specimen should return to its original shape if the applied load is removed while in this region. The slope of this region represents the bone s extrinsic stiffness (S; N/m) and is reflective of a bone s rigidity. The yield point defines the transition between the elastic and plastic regions of the curve. Unlike the elastic region, the plastic region is defined by permanent deformation of the specimen when the applied load is removed. The area under the load-displacement curve is termed energy to failure (U f ; mj) and is inversely proportional to the brittleness of bone. The maximum load applied to the specimen is termed ultimate load (F u ; N). Failure load (F f ; N) and failure displacement (d f ; mm), represent the load and deformation reached respectively, when the specimen has failed or fractured. The mechanical properties obtained from load-displacement curves are 51
66 influenced by differences in structural or geometrical properties and are therefore termed structural properties. Material properties are obtained when load-displacement curves are normalized to eliminate geometrical influences between specimens. This is achieved by using standard engineering formulae and geometric data to convert load-displacement curves into stress-strain curves (Figure 3.8a). It is important to note that the engineering formulae used to convert load-displacement curves into stress-strain curves do so on the assumption that the specimen is homogeneous along it length. This is not entirely accurate because bone is a composite material consisting of several hierarchical levels that are not uniform along its length. Therefore, conversion of loaddisplacement curves into stress-strain curves using these formulae serves only as an approximation of the true stress-strain behaviours of the samples tested in this study. Upon normalization, the slope of the linear elastic region is termed Young s modulus (E; MPa) and represents the intrinsic stiffness of the material. The intersection point between the stress-strain curve and a 0.2% strain offset parallel line to the linear portion of the curve defines the yield point. The area under the stress-strain curve represents the amount of energy required to cause fracture and is termed toughness (U f ; J/mm 3 ). Ultimate load, failure load and failure displacement are converted into and termed ultimate stress (& u ; MPa), failure stress (& f ; MPa) and failure strain ('; %) respectively. Ultimate stress as evaluated from the normalized stress-strain curve is also termed ultimate strength and represents an intrinsic property of bone [64, 65]. 52
67 a) Failure Load (N) Ultimate Load (N) Load (N) Stiffness (N/mm) Yield Point Energy to Failure (mj) Displacement (mm) Failure Displacement (mm) b) Failure Stress (Mpa) Ultimate Stress (MPa) Stress (MPa) Young s Modulus (MPa/%) Yield Point Yield Point Toughness (J/mm 3 ) Percent Strain (%) Failure Strain (%) Figure 3.8. a) Load-displacement curve b) Stress-strain curve Bone is defined as an anisotropic composite material and its structural properties vary with respect to loading direction [53]. Several different mechanical tests are performed in order to assess the anisotropic nature of bone as well as the contribution of either cortical bone, trabecular bone or the effect of both on bone mechanical strength. The following section provides a detailed description of the mechanical tests performed in this study. 53
68 3.5.1 Three-Point Bending Three-point bending is a method used to test the mechanical properties of cortical bone. During the test, the force being exerted on the bone creates a compressive stress where the force is loaded, and a tensile stress on the opposite side. A schematic diagram of three-point bending can be found in Figure 3.9a. Figure 3.9 a) Schematic diagram of three-point bending test b) Actual three-point bending test Three-point bending was performed on the excised right femora of all mice. All femora were subjected to micro-ct scanning prior to testing in order to obtain accurate measurements of intact femoral geometrical properties. Femoral midpoints were calculated based on femoral lengths measured using digital calipers. The Instron 4465 mechanical testing machine (Instron Canada Inc.,) with a 100 N load cell was used for all mechanical tests. The femora were positioned between two supports, 6 mm apart, with the posterior side facing downwards. A pre-load of approximately 1 N was applied to the midpoint of the femur, and then it was loaded to failure at a rate of 1 mm/min. Load versus time data was collected every 0.1 seconds by the LabView 5.0 data acquisition software (National Instruments Corp.; Austin, TX) as load-displacement curves. Ultimate load (N), stiffness (N/mm), failure displacement (mm) and energy to failure (mj) were determined from individual analysis of each load-displacement curve. The failure point was determined as the point of a sharp vertical drop and significantly reduced load. Load-displacement data were normalized to account for geometrical variations between samples using the AP 54
69 diameter (mm) and Imin (mm 4 ) generated per sample from micro-ct analysis. Stress (& ; MPa) and percent strain (' ; %) were then calculated using equations 1 and 2 respectively [64, 65]. Formula used to convert load (F) into stress (&): & * & : Stress (MPa) F : Measured Load (N) L : Gauge Length (6 mm) ( AP : AP Diameter (mm) I xx : Moment of Inertia (mm 4 ) F ) L )( 8I xx AP (Equation 1) Formula used to convert displacement into strain: 6 ' * L ' : Strain (%) ( AP : AP Diameter (mm) D : Measured Displacement (mm) L : Gauge Length (6 mm) D( AP (Equation 2) The material properties ultimate stress (MPa), Young s modulus (MPa/%), failure strain (%), and toughness (MPa.%) were determined from individual analysis of each stress-strain curve. 55
70 3.5.2 Vertebral Compression Vertebral compression is a mechanical test that is representative of the forces being exerted on vertebrae in every day life. This method tests the mechanical properties of trabecular bone undergoing a constant compressive stress. A schematic diagram of vertebral compression is shown in Figure 3.10a. Figure 3.10 a) Schematic diagram of vertebral compression test b) Macroscopic images for normalization The test was performed on the excised sixth lumbar vertebrae of all mice. The Instron 4465 mechanical testing machine (Instron Canada Inc.,) with a 100 N load cell was used. Prior to testing, digital pictures of the proximal vertebrae were taken using an optical microscope (Leica Q Win, Leica Microsystems Canada Inc., Richmond Hill, Ontario). From these images, vertebral body height and area were measured for data normalization. All vertebrae were carefully removed of any adherent soft tissue that may remain from the intervertebral discs. This allows the proximal and distal ends of the vertebra to be as flat as possible for testing purposes. A fine layer of cyanoacrylate-based adhesive was then applied to a metal plate to securely adhere the distal vertebrae so that its length is perpendicular to the plate. A preload of approximately 1 N was applied to the vertebra, and then loaded to failure at a rate of 0.5 mm/min. Load versus time data was collected every 0.1 seconds using LabView 5.0 data acquisition software (National Instruments Corp.; Austin, TX), and analyzed to obtain trabecular bone structural properties. These curves were then normalized into stress-strain curves in order to determine material 56
71 properties. Equation 3 was used to calculate stress (&; MPa) and equation 4 was used to calculate percent strain ('; %) [64, 65]. Formula used to convert load into stress: &: Stress (MPa) F: Measured Load (N) & * A: Cross-sectional area of vertebral body (mm 2 ) Formula used to convert displacement into strain: F A (Equation 3) ': Strain (%) D: Displacement (mm) l: Vertebral body height (mm) D ' * +100 l (Equation 4) Femoral Neck Fracture Testing Femoral neck fracture testing represents a clinically relevant test that attempts to mimic the hip fracture, which is a common fracture seen in individuals with increased fracture risk and osteoporosis [163]. Femoral necks possess a complex geometry and are composed of both cortical and trabecular bone. This type of testing causes the bone to undergo three types of stresses: compressive, tensile and shear. A schematic diagram of the femoral neck fracture test is shown below in Figure Figure a) Schematic diagram of femoral neck fracture b) Actual femoral neck fracture 57
72 Femoral neck fracture testing was performed on the proximal half of the right femora previously fractured by three-point bending. The midshaft of each femur was secured and mounted into the cavity of a custom-made jig using poly(methyl methracrylate) (PMMA). The midshaft of all samples were mounted at a 90, angle to the surface of the jig with an exposed bone height of approximately 3.5 mm. The mounted samples were left covered in saline-soaked gauze for 10 minutes with limited handling to ensure adequate hardening of the PMMA. The jig was attached to the crosshead of a 100 N load cell on the Instron 4465 mechanical testing machine (Instron Canada Inc.,). The crosshead was lowered so as to rest the femoral head on a custom-made plate possessing a passage for the greater trochanter. A preload of approximately 1 N was applied after which the crosshead speed was set at a rate of 1 mm/min until failure was achieved. Load versus time data was collected every 0.1 seconds from the LabView 5.0 data acquisition software (National Instruments Corp.; Austin, TX). Load-displacement curves were generated for each sample and used to calculate ultimate load (N), stiffness (N/mm), failure displacement (mm) and energy to failure (mj). Normalized material properties were not generated from femoral neck fracture testing due to the irregular geometry and angle of the femoral neck as well as the variability in precise sites of fracture. Comparing only the structural properties is currently the accepted approach [164]. 58
73 3.6 Evaluation of Bone Remodeling: Bone remodeling is a homeostatic system consisting of bone formation and resorption as coupled processes that exist to renew old or damaged bone. Uncoupling or imbalance of these processes can lead to increased skeletal fragility [23]. Bone histomorphometry is the means by which bone remodeling, modeling and structure can be quantitatively assessed. It is invaluable in determining cellular pathophysiology of osteoporosis and the mechanisms by which drugs affect bone [162]. Histomorphometry is an ex vivo technique whereby computer software is used to quantitatively analyze stained sections of bone by use of a microscope. Dynamic histomorphometry is used to define the amount of bone that has been formed within a specific period of time and is a direct measurement of bone formation. Specific staining of osteoclasts is used to quantitatively assess the level of bone resorption. Furthermore, osteoclast stained sections are analyzed at a higher magnification to differentiate the osteoclasts based on the amount of nuclei they contain because it has been established that number of osteoclast nuclei and bone resorptive rate are positively correlated [165, 166] Dynamic Histomorphometry: Dynamic Histomorphometry uses fluorochromes, such as calcein green, in order to measure bone formation rate parameters. Fluorochromes are calcium-binding substances that are preferentially incorporated at the active site of mineralization thereby labeling sites of new bone formation. Calcein green is often the fluorochrome of choice because it is nontoxic, can be administered at low doses and provides distinct and bright labels with resistance to fading [77]. Two injections of fluorochromes are given so that the width between each label can denote the amount of bone formation that occurred within a given time (mineral apposition rate) (Figure 3.12). All mice in this study were given two single intravenous injections of calcein green (0.6% calcein green; 30 mg/kg rodent) at 12 and 2 days before animal sacrifice. The interlabel width would therefore correspond to the bone formation that occurred during a 10-day period [77]. 59
74 25x Double Label 10x Single Label Interlabel Width Figure 3.12 Fluorecent labeling (single and double) on undecalcified vertebral section L4 vertebrae were excised, processes removed and immediately fixed in 70% ethanol for a minimum of 5 days to ensure adequate fixation [77]. All samples were then dehydrated in ascending concentrations of acetone and subsequently infiltrated in ascending ratios of unpolymerized Spurr resin and acetone. The samples were embedded in pure Spurr resin that was polymerized in an oven at 60,C for 48 hours. Coronal sections, 7-microns thick, were taken for each sample using a Reicher-Jung 2050 rotary microtome (Leica Microsystems Canada Inc., Richmond Hill, Ontario). Sections were placed on gelatinized slides, incubated in a 60,C oven for 48 hours and cover-slipped. The Leitz Bioquant morphometry system was used to quantify the bone formation parameters listed in Table 3.3. Only the trabecular bone in the area below the growth plate and within the cortex was analyzed. Eight serial fields were analyzed for each vertebral section using a 25x objective lens (Figure 3.13) 60
75 Table 3.3 Bone formation parameters Parameter Abbreviation Formula Unit Mineralizing Surface MS mm Percent Mineralizing %MS (MS/BS) x 100 % Surface Mineral Apposition Rate MAR Interlabel distance width/10 days µm/day Bone Formation Rate BFR/BS (MAR x MS)/BS µm/day Figure 3.13 Topographical map displaying 8 fields quantified using Bioquant morphometry system Osteoclast Staining: In bone tissue sections, tartrate-resistant acid phosphatase (TRAP) is a reliable marker for osteoclasts [76]. The number of TRAP-positive osteoclasts is correlated with osteoclastic activity and is therefore a measurement of bone resorption. TRAP-staining for osteoclasts was performed on L3 vertebrae in order to quantify osteoclast numbers and surface parameters. Excised L3 vertebrae were immediately fixed in 10% neutral buffered formalin and stored at room temperature for a minimum of 5 days. Samples were then decalcified using ethylenediaminetetraacetic acid (EDTA, 0.5 M, ph 7.4) at 4,C with daily solution changes. Complete decalcification was confirmed by faxitron imaging. Decalcified samples were then 61
76 processed (series of formalin, 70% ethanol, 90% ethanol, 100% ethanol, 100% xylene, and paraffin) and embedded in bone-specific paraffin. 5-micron thick coronal sections were cut using a Leica Reichert Jung 2030 microtome (Leica Microsystems Canada Inc., Richmond Hill, Ontario) and mounted on Superfrost Plus (high section adhesion) glass slides. The Acid Phosphatase Leukocyte kit and protcol (Procedure No. 386, Sigma-Aldrich Canada Ltd., Oakville, Ontario) were used to prepare and perform TRAP staining. Slides were incubated in the TRAP stain at 37,C for 1 hour with periodic shaking. Following incubation, slides were washed and counterstained with Acid Hematoxylin. Slides were cover-slipped using a water-soluble mounting media (Aqua Perm) and were allowed to dry overnight in a 37,C oven prior to analysis. 25x 10x Figure 3.14 TRAP stained section of decalcified vertebra (osteoclasts are stained in red) 62
77 The Leitz Bioquant morphometry system was used to quantify the osteoclast parameters for TRAP stained slides listed in Table 3.4. Eight serial fields were analyzed for each vertebral section using a 25x objective lens. See Figure 3.14 for example TRAP-stained section. Table 3.4 Osteoclast staining parameters Parameters Abbreviation Formula Unit Number of Osteoclasts #Oc N.Oc Osteoclast Surface Oc.S Oc.S mm Percent Osteoclast Surface %Oc.S (Oc.S/BS) x100 % Number of Osteoclasts per Bone Surface N.Oc/BS N.Oc/BS mm -1 Number of Osteoclasts per Osteoclast Surface N.Oc/Oc.S N.Oc/Oc.S mm Osteoclast Nuclei Counting: Bioquant analysis of TRAP-positive osteoclasts does not account for differences in cellular size. Large osteoclasts can form larger resorptive pits and have greater resorptive abilities than smaller osteoclasts. Cellular size can therefore provide a greater indication regarding the rate of bone resorption occurring at a given time. Osteoclasts increase in size by fusion with other osteoclasts, which result in multinuclear cells. The number of nuclei that are present in an osteoclast is often taken as an indication of total cell size [166]. TRAP-stained slides were evaluated at higher magnification (100x) in order to differentiate osteoclasts by the number of nuclei they contain (Figure 3.15). Only osteoclasts found on trabeculae beneath the growth plates and within the cortex were observed for nuclei content. Data is presented as the percent average number of osteoclasts with a specific number of nuclei (1,2,3,4,5+) divided by the total number of osteoclasts containing any nuclei. 63
78 Nuclei Trabeculae Trap positive osteoclast Figure 3.15 TRAP-postive osteoclast with hematoxylin-stained nuclei 3.7 Evaluation of Bone Mineral Properties The mineral quality and distribution can have a large effect on bone quality. Bone is in constant flux with variations in its mineral properties that can reflect parameters such as age, diet, disease and healthy states. Back-scattered electron (BSE) imaging generates a distribution of the mineralization values over a cross-section of local bone area. Analysis of mineralization profiles can provide a snapshot with respect to the age of the mineral distribution as well as its level of homogeneity [167] Quantitative Backscattered Electron Imaging Scanning electron microscopy (SEM) images are created by the striking of a beam of electrons onto a sample causing a change in electron momentum that results from the scattering between electrons and the elements they encounter. Heavy atoms with high atomic numbers scatter more strongly than lighter ones. Therefore, the collection of backscattered electrons, by BSE imaging, can be used to deduce compositional information. This is done indirectly by quantifying the differences in contrast levels within a bone area that correspond to varying 64
79 chemical compositions [32]. Regions containing higher atomic numbers have an increased probability of collisions with electrons. The integrity of electron collisions is not only related to atomic number, but also to the density of the atomic nuclei. Therefore an area of bone with a greater density of calcium atomic nuclei will show a higher number of electron collisions and result in higher contrast levels [32, 168, 169] (Figure 3.16). Less Mineralized More Mineralized Figure 3.16 BSE image of vertebra with magnified subregion to illustrate varying grey levels The Spurr blocks of embedded L4 vertebrae used for histomorphometry were used for the evaluation of mineralization distribution using BSE imaging. All Spurr-embedded samples were polished to a 1 %m diamond finish using a Phoenix BETA Grinder/Polisher. Polished blocks were carefully secured, bone surface side up, on 8.7 cm x 8.7 cm plexiglass plates with the use of Fimo polymer clay (Fimo Classic, Eberhard Faber). Care was taken to ensure all blocks on a plate were level with one another. Plated blocks were rendered conductive with carbon tape and 65
80 an evenly laid carbon-coating. Specimens were imaged on a Philips XL300ESEM system (Solid state BSE detector, FEI company, Hillsboro, OR, USA). Beam conditions were set at 20 kv with a working distance of 15 mm. All images were taken at a magnification of 150x. Spot size varied from in order to maintain a consistent beam current of -4 nanoamps (Standard operating procedure, Advanced Bioimaging Centre, Mount Sinai Hospital, Toronto, Ontario). A joint silicon dioxide (SiO 2 ) and magnesium fluoride (MgF 2 ) standard was used to calibrate and maintain contrast levels between imaging of every sample. This standard, in addition to a dentin control, were added to the plate prior to carbon taping and coating. Their respective heights were also adjusted to ensure they were level with plated blocks. The dual silicon dioxidemagnesium fluoride standard measurements are important to minimize potential variation and/or drift in contrast levels. An average of 4 fields was taken, stitched together and analyzed for total bone, trabecular bone and cortical bone by manually drawing masks in order to create separate profiles for each specimen. Mineralization profiles of total bone included the external cortical shell and internal trabeculae but excluded the vertebral growth plates. Mineralization profiles of trabecular bone solely included trabeculae below the growth plate and within the cortex. Finally, cortical bone mineralization profiles excluded all regions except for the external cortical shell. Histograms of the grey level distribution of the mineralization images were created where increasing grey levels represent a higher degree of mineralization. A cumulative log ratio known as the logit function was used to evaluate shifts in the mineralization profiles [170]. The logit function is calculated using Equation 5. / area 4 cutoff logit = In -. area 3 cutoff (Equation 5) 66
81 In relation to Equation 5, area refers to a specific mineralization range and cutoff values were chosen based on the average grey level mineralization peak for corresponding control groups (Vehicle or WildType). The closer the logit fuction is to zero, the more normally distributed the mineralization profile appears. Negative logit functions represent hypermineralized distributions while positive logit functions represent hypomineralized distributions (Figure 3.17). Heterogeneity of the distribution was represented by the full width at half the maximum height (FWHMH). Figure 3.17 Schematic mineralization distributions as determined by quantified BSE imaging 67
82 3.8 Statistical Analysis Anti-Diabetic Drug Study: All results were analyzed using SPSS 17.0 statistical analysis software. Independent t-tests were performed separately for Sitagliptin-treated Males (Sitagliptin vs Vehicle S) and Pioglitazonetreated Males (Pioglitazone vs Vehicle P). A one-way analysis of variance (ANOVA) was used separately for Female (Vehicle, Sitagliptin, Pioglitazone) and OVX Female (Vehicle, Sitagliptin, Pioglitazone) mice. Pairwise comparisons between groups were carried out using a Fisher Least Significant Difference (LSD) post-hoc test if group populations passed the homogeneity of variance testing. The non-parametric Dunnet s T3 post hoc test was performed if variances were not homogeneous. The data was considered to be statistically significant at a confidence level of 95% (p-value of 0.05). Data are presented as mean ± standard error (SE) Genetic Inactivation Study: All results were analyzed using SPSS 17.0 statistical analysis software. Independent t-tests were performed separately for Male, Female and OVX Female (KnockOut vs WildType) mice. The data was considered to be statistically significant at a confidence level of 95% (p-value of 0.05). Data are presented as mean ± standard error (SE). 68
83 CHAPTER 4: RESULTS FROM ANTI-DIABETIC DRUG STUDY 69
84 4. Introduction: Investigation into the skeletal effects from anti-diabetic drugs is important given the increased risk of fracture associated with T2DM. Specific forms of anti-diabetic drug treatments have been associated with reduced bone quality even though they improve bone-related mechanisms like glucose and insulin homeostasis. Two anti-diabetic drugs and their effects on bone quality in male, female and OVX female mice are evaluated given the need for T2DM treatments that do not exacerbate skeletal fragility. As such, the first two objectives of this thesis are addressed in this chapter. This chapter is divided into two parts: (1) Objective 1 effects of TZD treatment, Pioglitazone, on bone quality, and (2) Objective 2 effects of DPP-4 inhibitor treatment, Sitagliptin, on bone quality. 4.1 Objective 1: Effects of TZD Treatment, Pioglitazone, on Bone Quality A total of six groups were generated for Objective 1 of this study: A vehicle control and Pioglitazone-treated mice for male, female and OVX female mice. Bone densitometry, bone mechanics, structural properties, bone remodeling and mineral properties were used in order to evaluate bone quality. All techniques (Figure 3.3) were performed for all groups Weight: The weight of each mouse was recorded after sacrifice and can be viewed in Table 4.1. Increases in weight were seen due to Pioglitazone treatment in all mice but was only statistically significant in male mice. Table 4.1 Weight for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Weight (g) 42.2 ± ± 2.0* 33.4 ± ± ± ± 1.4 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 70
85 4.1.2 Observed Phenotype Bone Colour: The femora and vertebrae excised from Pioglitazone-treated mice appeared distinguishably more yellow in colour when compared to respective vehicle control groups. Figure 4.1 Colour difference due to Pioglitazone treatment Bone Densitometry: Areal bone mineral density (abmd) was evaluated using Dual Energy X-ray Absorptiometry (DEXA) and volumetric bone mineral density (vbmd) was determined using Micro-Computed Tomography (Micro-CT) Areal Bone Mineral Density: DEXA was performed on excised left and right femora and lumbar vertebrae (L5 and L6) for all mice. These values were averaged to provide femoral and vertebral abmd, respectively and are shown in Table 4.2. Significant decreases were seen in the femoral abmd of Pioglitazonetreated male, female and OVX female mice. Significant decreases were also noted in the femoral BMC of Pioglitazone-treated male and female mice. Pioglitazone-treated OVX mice exhibited significantly reduced vertebral abmd and BMC. 71
86 Table 4.2 DEXA results for Pioglitazone-treated and control mice Male Female OVX Vehicle P Pio Vehicle Pio Vehicle Pio n Femoral abmd (g/cm 2 ) Femoral BMC (g) Vertebral abmd (g/cm 2 ) Vertebral BMC (g) ± ± ± ± ± * ± * ± ± ± ± ± ± ± * ± * ± ± Values reported as mean ± standard error. * Significant (p ) compared to vehicle control Volumetric Bone Mineral Density: ± ± ± ± ± * ± ± * ± * Micro-CT was performed on the right femora and L5 vertebrae of all mice in order to assess femoral and vertebral vbmd, respectively. No significant changes were seen in the femoral vbmd for male, female or OVX mice treated with Pioglitazone. Vertebral vbmd was significantly reduced in Pioglitazone-treated male mice only (Table 4.3). Table 4.3 Femoral and vertebral vbmd for Pioglitazone-treated and control mice Male Female OVX Vehicle P Pio Vehicle Pio Vehicle Pio n Femoral vbmd (g/cm 3 ) ± ± ± ± ± ± n Vertebral vbmd (g/cm 3 ) ± ± 0.019* ± ± Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ±
87 4.1.4 Bone Mechanical Properties: Three-point bending, vertebral compression and femoral neck fracture testing were performed in order to evaluate the mechanical properties of bone in Pioglitazone treated and untreated mice. Results from both un-normalized and normalized data are presented. The unnormalized data are termed bone structural properties since they represent the failure behaviour at the whole bone level. On the other hand, normalized data represents the failure behaviour at the tissue level, and are termed bone material properties. Geometric data are also presented on the right femora and sixth lumbar vertebrae, as these were used for data normalization purposes Femoral Geometry: Micro-CT was used to assess the geometry of the right femora of all mice. No significant structural changes were observed due to Pioglitazone treatment in male, female or OVX mice (Table 4.4). Table 4.4 Geometrical properties of right femora for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n A-P Diameter 1.37 ± ± ± ± ± ± 0.01 (mm) Moment of Inertia (mm 4 ) Crosssectional bone area (mm 2 ) Cortical Thickness (mm) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ±
88 Three-Point Bending: Three-point bending was performed on right femora of all mice. The results from threepoint bending are shown in Table 4.5. No significant changes in mechanical properties were observed due to Pioglitazone treatment in male, female or OVX mice for this test. Table 4.5 Three-point bending results for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Structural Properties Ultimate Load 20.3 ± ± ± ± ± ± 1.0 (N) Failure Displacement (mm) 0.55 ± ± ± ± ± ± 0.08 Energy to Failure (mj) 8.2 ± ± ± ± ± ± 0.8 Stiffness ± ± ± ± ± 8.1 (N/mm) ± 5.2 Material Properties Ultimate ± 87.4 ± ± 4.2 Stress (MPa) ± ± ± 7.7 Failure Strain (%) 12.5 ± ± ± ± ± ± 1.7 Toughness (MPa) 7.9 ± ± ± ± ± ± 1.1 Young s ± ± ± ± ± ± Modulus (MPa) Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 74
89 Vertebral Geometry: Vertebral height and area were used to normalize vertebral compression data. The geometrical properties of the sixth lumbar vertebrae are shown in Table 4.6. No significant changes were observed due to Pioglitazone treatment in male, female or OVX mice. Table 4.6 Geometrical properties of L6 vertebrae for Pioglitazone-treated and control mice Male Female OVX Vehicle P Pio Vehicle Pio Vehicle Pio n Height (mm) 3.27 ± ± ± ± ± ± 0.08 Area (mm 2 ) 2.34 ± ± ± ± ± ± 0.09 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control Vertebral Compression: Vertebral compression was performed on the L6 vertebrae for all mice and the results are shown in Table 4.7. Pioglitazone-treated male mice exhibited significantly reduced stiffness, ultimate stress and modulus. Pioglitazone-treated female mice exhibited significantly reduced energy to failure and toughness. Finally, Pioglitazone-treated OVX mice experienced significantly reduced ultimate load, ultimate stress, and Young s modulus. 75
90 Table 4.7 Vertebral compression results for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Structural Properties Ultimate Load 24.9 ± ± ± ± ± ± 0.8* (N) Failure Displacement (mm) Energy to Failure (mj) 0.31 ± 0.03 Stiffness ± (N/mm) 8.8 Material Properties Ultimate Stress (MPa) Failure Strain (%) Toughness (MPa) Young s Modulus (MPa) 0.38 ± ± ± ± ± ± ± ± ± 0.3* 4.3 ± ± ± 9.7* ± ± ± ± ± ± 0.8* 10.3 ± ± ± ± 0.2* 9.5 ± ± ± ± ± ± ± ± ± ± 15.1* 0.87 ± ± ± 0.05* ± 31.5 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 0.64 ± ± ± ± 8.5* 76
91 Femoral Neck Fracture: Femoral neck fracture was performed on the proximal head of the right femora following three-point bending testing. The results from femoral neck fracture testing are shown in Table 4.8. No significant changes in mechanical properties from Pioglitazone treatment were observed in male, female or OVX mice for this test. Table 4.8 Femoral neck fracture results for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Structural Properties Ultimate Load 26.3 ± ± ± ± ± ± 0.9 (N) Failure Displacement (mm) Energy to Failure (mj) Stiffness (N/mm) 0.26 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± 8.3 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ±
92 4.1.5 Structural Properties: Vertebral structural properties were further assessed using Micro-CT and BSE imaging. Micro-CT was used to evaluate the 3D trabecular bone structural properties of the fifth lumbar vertebrae for all mice. These three-dimensional structural properties are shown in Table 4.9. Pioglitazone-treatment resulted in significantly reduced trabecular bone volume (BV/TV), trabecular thickness (Tb.Th.) and trabecular number (Tb. N.) in male mice. No significant changes were seen due to Pioglitazone treatment in female or OVX mice. Table 4.9 3D Trabecular bone structural properties for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n BV/TV (%) 28.0 ± ± 0.6* 19.6 ± ± ± ± 1.7 Tb.Th. (%m) 65.1 ± ± 0.9* 69.7 ± ± ± ± 1.4 Tb.N. 4.3 ± ± 0.1* 2.8 ± ± ± ± 0.3 (mm -1 ) Tb.Sp. (%m) ± ± ± ± 16.5 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ±
93 Strut analysis was evaluated by BSE imaging on the fourth lumbar vertebrae for all mice (Table 4.10). No significant changes in the 2D trabecular bone structural properties were observed due to Pioglitazone treatment in female or OVX mice. Pioglitazone-treated male mice experienced significantly reduced total strut length, number of nodes and length of node-to-node struts, three measures of connectivity. Table D Trabecular bone structural properties for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Measures of Connectivity Measures of Disconnectivity Total Strut Length (mm/mm 2 ) Number of Nodes (mm -2 ) Length of Node-Node Struts (mm/mm 2 ) Length of Node-Free Struts (mm/mm 2 ) Number of Free Ends (mm -2 ) Length of Free-Free Struts (mm/mm 2 ) 6.23 ± ± ± ± ± ± ± 0.52* 10.7 ± 0.9* 1.62 ± 0.17* 1.92 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± 0.08 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 4.19 ± ± ± ± ± ± ± ± ± ± ± ±
94 4.1.6 Observed Phenotype Bone Marrow: Histomorphometry was used to assess bone formation and resorption properties. Histological slides revealed a severe phenotype from Pioglitazone treatment not found in vehicle controls (Figure 4.2). The bone marrow of Pioglitazone-treated mice appears more heterogenous than vehicle controls. It is assumed that the appearance of the bone marrow in Pioglitazonetreated mice is due to increased adipogenesis. Male Vehicle Male Pioglitazone Female Vehicle Female Pioglitazone OVX Vehicle OVX Pioglitazone Figure 4.2 Histological slides (x10) for Pioglitazone-treated and control mice 80
95 4.1.7 Bone Formation: Bone formation was evaluated by dynamic histomorphometry on undecalcified sections of L4 vertebrae. No significant changes in bone formation parameters were observed due to Pioglitazone treatment in female or OVX mice. Pioglitazone-treated male mice did exhibit a significantly lower mineral apposition rate (Table 4.11). Table 4.11 Bone formation parameters for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Mineralizing Surface (%) 18.3 ± ± ± ± ± ± 3.6 Mineral Apposition 0.87 ± 0.73 ± 0.93 ± 0.93 ± 0.76 ± 0.06 Rate * ± 0.04 (%m/day) Bone Formation Rate (%m/day) ± ± ± ± ± ± Values reported as mean ± standard error. * Significant (p ) compared to vehicle control Bone Resorption: Bone resorption was assessed from osteoclast staining of Tartrate-Resistant Acid Phosphatase (TRAP) on decalcified sections of L3 vertebrae for all mice (Table 4.12). No significant changes in bone resorption parameters were observed in Pioglitazone-treated male or OVX mice. Pioglitazone-treated female mice did exhibit a significantly lower number of osteoclasts per osteoclast surface. Additionally, osteoclast nuclei were counted and the percentage of osteoclasts as a function of the number of nuclei for male, female and OVX mice are presented in Figures 4.3, 4.4 and 4.5, respectively. No differences in the amount of nuclei were seen when comparing Pioglitazone-treated mice to vehicle controls. 81
96 Table 4.12 Osteoclast staining results for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Number of Osteoclasts 43 ± 5 56 ± 5 64 ± 6 64 ± 5 45 ± 4 36 ± 4 (-) Osteoclast Surface (mm) Percent Osteoclast Surface (%) Number of Osteoclasts per Bone Surface (mm -1 ) Number of Osteoclasts per Osteoclast Surface 0.99 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± 1.6* 52.5 ± ± 3.9 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control Nuclei Count of Male Osteoclasts 70% 60% 50% 40% Male Vehicle Male Pioglitazone 30% 20% 10% 0% Amout of Nuclei Figure 4.3 Percent of osteoclasts with number of nuclei for Pioglitazone-treated and control male mice 82
97 Nuclei Count of Female Osteoclasts 60% 50% 40% Female Vehicle Female Pioglitazone 30% 20% 10% 0% Amout of Nuclei Figure 4.4 Percent of osteoclasts with number of nuclei for Pioglitazone-treated and control female mice Nuclei Count of OVX Osteoclasts 60% 50% 40% OVX Vehicle OVX Pioglitazone 30% 20% 10% 0% Amout of Nuclei Figure 4.5 Percent of osteoclasts with number of nuclei for Pioglitazone-treated and control OVX mice 83
98 4.1.9 Bone Mineral Properties: Bone mineral properties were evaluated by quantitative BSE imaging on sectioned polished Spurr blocks of L4 vertebrae for all mice. Mineralization distributions of three defined bone areas directly below the growth plate were determined: Total (trabecular + cortical), Trabecular, and Cortical TOTAL: Table 4.13 summarizes the parameters obtained from total bone mineralization profile analysis. The average mineralization profiles for total bone area are shown for male, female and OVX mice in figures 4.6, 4.7 and 4.8, respectively. No significant changes were noted in mineralization parameters when comparing Pioglitazone-treated female and OVX mice to respective vehicle controls. Pioglitazone treatment in male mice led to a significant increase in FWHMH (a measure of heterogeneity). Table 4.13 Results from BSE imaging of total bone for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Logit (-) Mineralization Peak (pixels) FWHMH (pixels) 0.73 ± ± ± ± ± ± ± ± ± ± ± ± 2 25 ± 1 28 ± 1* 24 ± 1 24 ± 1 23 ± 1 26 ± 1 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 84
99 Total Mineralization Profiles of Male Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.6 Total bone mineralization profiles for Pioglitazone-treated and control male mice 3.5 Total Mineralization Profiles of Female Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.7 Total bone mineralization profiles for Pioglitazone-treated and control female mice 85
100 Total Mineralization Profiles of OVX Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.8 Total bone mineralization profiles for Pioglitazone-treated and control OVX mice TRABECULAR: Table 4.14 summarizes the parameters obtained from trabecular bone mineralization profile analysis. The average mineralization profiles for trabecular bone area are shown for male, female and OVX mice in figures 4.9, 4.10 and 4.11, respectively. No significant changes in mineralization parameters were observed due to Pioglitazone treatment in female or OVX mice. Pioglitazone treatment in male mice did produce a significant increase in FWHMH (a measure of heterogeneity). 86
101 Table 4.14 Results from BSE imaging of trabecular bone for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Logit (-) Mineralization Peak (pixels) FWHMH (pixels) 0.87 ± ± ± ± ± ± ± ± ± ± ± ± 2 24 ± 1 26 ± 1* 25 ± 1 26 ± 1 26 ± 1 28 ± 1 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 3.5 Trabecular Mineralization Profiles of Male Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.9 Trabecular bone mineralization profiles for Pioglitazone-treated and control male mice 87
102 Trabecular Mineralization Profiles of Female Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.10 Trabecular bone mineralization profiles for Pioglitazone-treated and control female mice 3.5 Trabecular Mineralization Profiles of OVX Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.11 Trabecular bone mineralization profiles for Pioglitazone-treated and control OVX mice 88
103 CORTICAL: Table 4.15 summarizes the parameters obtained from cortical bone mineralization profile analysis. The average mineralization profiles for cortical bone area are shown for male, female and OVX mice in figures 4.12, 4.13 and 4.14, respectively. No significant changes were noted in these parameters when comparing Pioglitazone-treated mice to vehicle controls. Table 4.15 Results of BSE imaging of cortical bone for Pioglitazone-treated and control mice Male Female OVX Vehicle Pio Vehicle Pio Vehicle Pio n Logit (-) Mineralization Peak (pixels) Levels of Heterogeneity (pixels) 1.09 ± ± ± ± ± ± ± ± ± ± ± ± 2 23 ± 1 26 ± 2 21 ± 1 24 ± 1 22 ± 1 22 ± 1 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 3.5 Cortical Mineralization Profiles of Male Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.12 Cortical bone mineralization profiles for Pioglitazone-treated and control male mice 89
104 Cortical Mineralization Profiles of Female Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.13 Cortical bone mineralization profiles for Pioglitazone-treated and control female mice 3.5 Cortical Mineralization Profiles of OVX Mice Vehicle Pioglitazone % Pixel Intensity Grey Level Figure 4.14 Cortical bone mineralization profiles for Pioglitazone-treated and control OVX mice 90
105 Table 4.16 Summary of results from Pioglitazone treatment in mice Males Females OVX Bone Densitometry Femoral Vertebral 5aBMD (p=0.007) 5BMC (p=0.028) 5vBMD (p=0.011) 5aBMD (p=0.002) 5BMC (p=0.010) 5aBMD (p=0.002) 5aBMD (p=0.008) BMC (p=0.006) Geometrical Properties Femoral Vertebral Bone Mechanical Properties Three-Point Bending Femoral Neck Fracture Vertebral Compression 5Stiffness (p=0.034) 5Ultimate Stress (p=0.040) 5Young s Modulus (p=0.020) Trabecular Structural Properties 3D Micro-CT 2D Strut Analysis Bone Remodeling Bone Formation 5BV/TV (p=0.001) 5Tb.Th. (p=0.004) 5Tb. N. (p=0.025) 5Strut Length (p=0.021) 5Number of Nodes (p=0.026) 5Length Node-Node (p=0.021) 5Mineral Apposition Rate (p=0.030) 5Energy to Failure (p=0.014) 5Toughness (p=0.024) 5Ultimate Load (p=0.005) 5Ultimate Stress (p=0.010) 5Young s Modulus (p=0.014) Bone Resorption - 5# Os./Os Surface (p=0.010) - Mineral Properties Mineralization Profiles Total Bone 6Heterogeneity (p=0.047) - - Trabecular Bone 6Heterogeneity (p=0.036) Cortical Bone
106 4.2 Objective 2: Effects of DPP-4 inhibitor Treatment, Sitagliptin, on Bone Quality A total of six groups were generated for Objective 2 of this study: a vehicle control and Sitagliptin-treated mice for male, female and OVX mice. Bone densitometry, bone mechanics, structural properties, bone remodeling and mineral properties were used in order to evaluate bone quality. All techniques (Figure 3.3) were performed for all groups Weight: The weight of each mouse was recorded after sacrifice and can be viewed in Table No significant changes were observed in weight for Sitagliptin-treated mice when compared to vehicle controls. Table 4.17 Weight for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Weight (g) 38.4 ± ± ± ± ± ± 0.8 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control Bone Densitometry: Areal bone mineral density (abmd) was evaluated using Dual Energy X-ray Absorptiometry (DEXA) and volumetric bone mineral density (vbmd) was evaluated using Micro-Computed Tomography (Micro-CT) Areal Bone Mineral Density: DEXA was performed on excised left and right femora and lumber vertebrae (L5 and L6) for all mice. These values were averaged to provide femoral and vertebral abmd, respectively and are shown in Table A significant increase in vertebral abmd and BMC was observed in male mice treated with Sitagliptin. 92
107 Table 4.18 DEXA results for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Femoral abmd (g/cm 2 ) ± ± ± ± ± ± Femoral BMC (g) ± ± ± ± ± ± Vertebral abmd (g/cm 2 ) ± ± * ± ± ± ± Vertebral BMC (g) ± ± * ± ± Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ± Vertebral Bone Mineral Density: Micro-CT was performed on the right femora and L5 vertebrae of all mice providing a measurement of femoral and vertebral vbmd, respectively and results are shown in Table No significant changes were observed in femoral vbmd for Sitagliptin-treated male, female or OVX mice. Vertebral vbmd was not affected in male or OVX mice due to Sitagliptin treatment. Female mice treated with Sitagliptin experienced an increase in vertebral vbmd. Table 4.19 Femoral and vertebral vbmd for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Femoral vbmd (g/cm 3 ) ± ± ± ± ± ± n Vertebral vbmd (g/cm 3 ) ± ± ± ± 0.022* Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ±
108 4.2.3 Bone Mechanical Properties: Three-point bending, vertebral compression and femoral neck fracture testing were performed in order to evaluate the mechanical properties of bone in Sitagliptin-treated mice. Results from both un-normalized and normalized data are presented. The un-normalized data are termed bone structural properties since they represent the failure behaviour at the whole bone level. On the other hand, normalized data represents the failure behaviour at the tissue level, and are termed bone material properties. Geometric data are also presented on the right femora and sixth lumbar vertebrae, as these were used for data normalization purposes Femoral Geometry: Micro-CT was used to assess the geometry of the right femora of all mice. No significant structural changes were observed due to Sitagliptin treatment in male, female or OVX mice (Table 4.20). Table 4.20 Geometrical properties of right femora for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n A-P Diameter (mm) Moment of Inertia (mm 4 ) Crosssectional bone area (mm 2 ) Cortical Thickness (mm) 1.30 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 1.32 ± ± ± ± ± ± ± ±
109 Three-Point Bending: Three-point bending was performed on the right femora of all mice. The results from three-point bending are shown in Table No significant changes were observed in three-point bending mechanical properties due to Sitagliptin treatment in male, female or OVX mice. Table 4.21 Three-point bending results for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Structural Properties Ultimate Load 17.7 ± ± ± ± ± ± 0.5 (N) Failure Displacement (mm) 0.51 ± ± ± ± ± ± 0.04 Energy to Failure (mj) 6.1 ± ± ± ± ± ± 0.5 Stiffness ± ± ± ± ± 3.7 (N/mm) ± 4.2 Material Properties Ultimate ± Stress (MPa) ± ± ± ± ± 3.3 Failure Strain (%) 10.6 ± ± ± ± ± ± 0.8 Toughness (MPa) 9.2 ± ± ± ± ± ± 0.6 Young s ± ± ± ± ± ± Modulus (MPa) Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 95
110 Vertebral Geometry: Vertebral height and area were used to normalize vertebral compression data. The geometrical properties of the sixth lumbar vertebrae are shown in Table Sitagliptin treatment did not result in significant differences in vertebral geometry for female or OVX mice. However, a significant increase in vertebral body area was observed in Sitagliptin-treated male mice. Table 4.22 Geometry of the L6 vertebrae for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Height (mm) 2.98 ± 3.08 ± ± 3.33 ± ± 3.19 ± 0.07 Area (mm 2 ) ± ± 0.05* ± ± 0.12 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ± Vertebral Compression: Vertebral compression was performed on the L6 vertebrae for all mice and the results are shown in Table No significant changes in the vertebral mechanical properties were seen due to Sitagliptin-treatment for male or female mice. However, a significant decrease was noted in Young s modulus in Sitagliptin-treated OVX mice when compared to OVX vehicle controls. 96
111 Table 4.23 Vertebral compression results for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Structural Properties Ultimate Load 30.7 ± ± ± ± ± ± 1.8 (N) Failure Displacement (mm) 0.31 ± ± ± ± ± ± 0.05 Energy to Failure (mj) 5.4 ± ± ± ± ± ± 0.6 Stiffness ± ± ± ± 87.4 ± 13.1 (N/mm) ± 13.0 Material Properties Ultimate Stress (MPa) 13.7 ± ± ± ± ± ± 0.8 Failure Strain (%) 10.4 ± ± ± ± ± ± 1.8 Toughness 0.81 ± 0.87 ± 0.64 ± 0.83 ± ± 0.18 (MPa) ± 0.08 Young s ± ± ± ± ± ± Modulus * (MPa) Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 97
112 Femoral Neck Fracture: Femoral neck fracture was performed on the proximal head of the right femora following three-point bending testing. The results from femoral neck fracture testing are shown in Table No significant changes in mechanical properties were observed in male, female or OVX mice due to Sitagliptin treatment. Table 4.24 Femoral neck fracture results for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Structural Properties Ultimate Load 20.4 ± ± ± ± ± ± 1.0 (N) Failure Displacement (mm) Energy to Failure (mj) Stiffness (N/mm) 0.31 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± 11.2 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ±
113 4.2.4 Structural Properties: Vertebral structural properties were further assessed using Micro-CT and BSE imaging. Micro-CT was used to evaluated the trabecular architecture of the fifth lumbar vertebrae for all mice. No significant differences in the three-dimensional (3D) structural properties were observed in male or OVX mice treated with Sitagliptin (Table 4.25). Female mice treated with Sitagliptin experienced significant increases in trabecular bone volume (BV/TV), trabecular thickness (Tb. Th.), trabecular number (Tb. N.) and a significantly reduced trabecular separation (Tb. Sp.). Table D trabecular bone structural properties for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n BV/TV (%) 28.2 ± ± ± ± 1.9* 20.5 ± ± 1.4 Tb.Th. (%m) 71.4 ± ± ± ± 1.4* 64.7 ± ± 0.8 Tb.N. 3.9 ± ± ± ± 0.2* 3.2 ± ± 0.2 (mm -1 ) Tb.Sp. (%m) ± ± ± ± 20.6* Values reported as mean ± standard error. * Significant (p ) compared to vehicle control ± ±
114 BSE was used for strut analysis on the fourth lumbar vertebrae for all mice. No significant changes in the 2D trabecular bone structural properties were seen when comparing Sitagliptintreated female or OVX mice to their respective vehicle controls. Male mice treated with Sitagliptin exhibited a reduced total strut length and length of node-to-free struts, two measures of connectivity. Table D Trabecular bone structural properties for Sitagliptin-treated and control mice Male Female OVX Vehicle Sita Vehicle Sita Vehicle Sita n Measures of Connectivity Measures of Disconnectivity Total Strut Length (mm/mm 2 ) Number of Nodes (mm -2 ) Length of Node-Node Struts (mm/mm 2 ) Length of Node-Free Struts (mm/mm 2 ) Number of Free Ends (mm -2 ) Length of Free-Free Struts (mm/mm 2 ) 5.11 ± ± ± ± ± ± ± 0.21* 8.8 ± ± ± 0.14* 17.3 ± ± ± ± ± ± ± ± ± ± ± ± ± ± 0.07 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 4.19 ± ± ± ± ± ± ± ± ± ± ± ±
115 4.2.5 Bone Formation: Bone formation was evaluated by dynamic histomorphometry on undecalcified sections of L4 vertebrae. No significant changes were seen in bone formation parameters for male, female or OVX mice treated with Sitagliptin (Table 4.27). Table 4.27 Bone formation parameters for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Mineralizing Surface (%) 16.6 ± ± ± ± ± ± 2.0 Mineral Apposition 0.80 ± 0.93 ± 0.93 ± 0.73 ± ± 0.03 Rate ± 0.05 (%m/day) Bone Formation Rate (%m/day) ± ± ± ± ± ± Values reported as mean ± standard error. * Significant (p ) compared to vehicle control Bone Resorption: Bone resorption was assessed from osteoclast staining for Tartrate-Resistant Acid Phosphatase (TRAP) on decalcified sections of L3 vertebrae for all mice (Table 4.28). No significant changes were seen in any osteoclast staining parameter when comparing Sitagliptintreated male, female or OVX mice to respective vehicle controls. Additionally, osteoclast nuclei were counted and the percentage of osteoclasts as a function of the number of nuclei for male, female and OVX mice are presented in Figure 4.15, 4.16, and 4.17, respectively. No major differences in the amount of nuclei were seen when comparing Sitagliptin-treated mice to vehicle controls. 101
116 Table 4.28 Osteoclast staining results for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Number of Osteoclasts 66 ± 6 61 ± 6 64 ± 6 53 ± 7 45 ± 4 52 ± 6 (-) Osteoclast 0.93 ± Surface 1.2 ± ± ± ± (mm) 1.5 ± 0.3 Percent Osteoclast Surface 7.1 ± ± ± ± ± ± 1.9 (%) Number of Osteoclasts per Bone 3.9 ± ± ± ± ± ± 0.5 Surface (mm -1 ) Number of Osteoclasts per Osteoclast Surface (mm -1 ) 56.7 ± ± ± ± ± ± 3.8 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 70% 60% 50% 40% 30% 20% 10% 0% Nuclei Count of Male Osteoclasts Amout of Nuclei Male Vehicle Male Sitagliptin Figure 4.15 Percent of osteoclasts with number of nuclei for Sitagliptin-treated and control male mice 102
117 Nuclei Count of Female Osteoclasts 70% 60% 50% 40% Female Vehicle Female Sitagliptin 30% 20% 10% 0% Amout of Nuclei Figure 4.16 Percent of osteoclasts with number of nuclei for Sitagliptin-treated and control female mice Nuclei Count of OVX Osteoclasts 60% 50% 40% OVX Vehicle OVX Sitagliptin 30% 20% 10% 0% Amout of Nuclei Figure 4.17 Percent of osteoclasts with number of nuclei for Sitagliptin-treated and control OVX mice 103
118 4.2.7 Bone Mineral Properties: Bone mineral properties were evaluated by quantitative BSE imaging on sectioned polished Spurr blocks of L4 vertebrae for all mice. Mineralization distributions of three-defined bone areas directly below the growth plate were determined: Total (trabecular + cortical), Trabecular and Cortical TOTAL: Table 4.29 summarizes the parameters obtained from total bone mineralization profile analysis. The average mineralization profiles for total bone area are shown for male, female and OVX mice in figures 4.18, 4.19 and 4.20 respectively. The logit of all Sitagliptin-treated mice exhibited a significantly increased degree of mineralization. The total bone mineralization profiles of Sitagliptin-treated female and OVX mice exhibited a significant grey level shift towards higher mineralization. Table 4.29 Results from BSE imaging of total bone for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Logit (-) Mineralization Peak (pixels) FWHM (pixels) 1.06 ± ± 0.15* 0.90 ± ± 0.26* 1.06 ± ± 0.15* 173 ± ± ± ± 2* 173 ± ± 2* 27 ± 1 28 ± 1 24 ± 1 22 ± 1 24 ± 1 23 ± 1 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 104
119 Total Mineralization Profiles of Male Mice Vehicle Sitagliptin % Pixel Intensity % Grey Level Figure 4.18 Total mineralization profiles for Sitagliptin-treated and control male mice 3.5 Total Mineralization Profiles of Female Mice Vehicle Sitagliptin % Pixel Intensity %* Grey Level Figure 4.19 Total mineralization profiles for Sitagliptin-treated and control female mice 105
120 Total Mineralization Profiles of OVX Mice Vehicle Sitagliptin % Pixel Intensity %* Grey Level Figure 4.20 Total mineralization profiles for Sitagliptin-treated and control OVX mice TRABECULAR: Table 4.30 summarizes the parameters obtained from trabecular bone mineralization profile analysis. The average mineralization profiles for trabecular bone area are shown for male, female and OVX mice in figures 4.21, 4.22 and 4.23, respectively. The total bone mineralization profiles of all Sitagliptin-treated mice exhibited a significant grey level shift towards higher mineralization and increased degree of mineralization (reduced logit). 106
121 Table 4.30 Results from BSE imaging of trabecular bone for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Logit (-) Mineralization Peak (pixels) FWHMH (pixels) 1.04 ± ± 0.13* 1.05 ± ± 0.27* 1.00 ± ± 0.12* 170 ± ± 1* 169 ± ± 2* 170 ± ± 2* 29 ± 1 28 ± 1 25 ± 1 24 ± 1 26 ± 1 25 ± 1 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 3.5 Trabecular Mineralization Profiles of Male Mice Vehicle Sitagliptin % Pixel Intensity %* Grey Level Figure 4.21 Trabecular mineralization profiles for Sitagliptin-treated and control male mice 107
122 Trabecular Mineralization Profiles of Female Mice Vehicle Sitagliptin % Pixel Intensity %* Grey Level Figure 4.22 Trabecular mineralization profiles for Sitagliptin-treated and control female mice 3.5 Trabecular Mineralization Profiles of OVX Mice Vehicle Sitagliptin % Pixel Intensity %* Grey Level Figure 4.23 Trabecular mineralization profiles for Sitagliptin-treated and control OVX mice 108
123 CORTICAL: Table 4.31 summarizes the parameters obtained from cortical bone mineralization profile analysis. The average mineralization profiles for cortical bone area are shown for male, female and OVX mice in figures 4.24, 4.25 and 4.26, respectively. No significant changes were noted in these parameters when comparing Sitagliptin-treated male and female mice with respective vehicle controls. OVX mice treated with Sitagliptin exhibiteded an increased degree of mineralization (reduced logit). Table 4.31 Results from BSE imaging of cortical bone for Sitagliptin-treated and control mice Male Female OVX Vehicle Sitagliptin Vehicle Sitagliptin Vehicle Sitagliptin n Logit (-) Mineralization Peak (pixels) FWHM (pixels) 1.02± ± ± ± ± ± 0.23* 176 ± ± ± ± ± ± 2 22 ± 1 24 ± 1 21 ± 1 20 ± 1 22 ± 1 20 ± 1 Values reported as mean ± standard error. * Significant (p ) compared to vehicle control 3.5 Cortical Mineralization Profiles of Male Mice Vehicle Sitagliptin % Pixel Intensity Grey Level Figure 4.24 Cortical mineralization profiles for Sitagliptin-treated and control male mice 109
124 Cortical Mineralization Profiles of Female Mice Vehicle Sitagliptin % Pixel Intensity % Grey Level Figure 4.25 Cortical mineralization profiles for Sitagliptin-treated and control female mice 3.5 Cortical Mineralization Profiles of OVX Mice Vehicle Sitagliptin % Pixel Intensity % Grey Level Figure 4.26 Cortical mineralization profiles for Sitagliptin-treated and control OVX mice 110
125 Table 4.32 Summary of results from Sitagliptin treatment mice Males Females OVX Bone Densitometry Femoral Vertebral 6aBMD (p=0.004) 6BMC (p=0.004) 6vBMD (p=0.002) Geometrical Properties Femoral Vertebral 6Area (p=0.043) - - Bone Mechanical Properties Three-Point Bending Femoral Neck Fracture Vertebral Compression Trabecular Structural Properties 3D Micro-CT 2D Strut Analysis Strut Length (p=0.039) 5Length Node-Free (p=0.022) 6BV/TV (p=0.002) 6Tb. Th. (p=0.005) 6Tb. N. (p=0.008) 5Tb. Sp. (p=0.015) - 5Young s Modulus (p=0.05) - - Bone Remodeling Bone Formation Bone Resorption Mineral Properties Mineralization Profiles Total Bone 5Logit (p=0.044) Trabecular Bone Cortical Bone 5Logit (p=0.026) 7Shift (p=0.036) 5Logit (p=0.012) 7Shift (p=0.018) Logit (p=0.012) 7Shift (p=0.005) Logit (p=0.002) 7Shift (p=0.005) 5Logit (p=0.002) 7Shift (p=0.002) 5Logit (p=0.012) 111
126 4.3 Summary of Anti-Diabetic Drug Study: The following section provides a summary of the significant findings from the antidiabetic drug study. The evaluation of the effects of Pioglitazone on bone quality in male, female and OVX mice has provided insight into the role of other TZDs on the skeleton. Additionally, investigating the effects of Sitagliptin on bone quality in male, female and OVX mice has provided insight into the role of DPP-4 inhibition on the skeleton Objective 1: Effects of TZD Treatment, Pioglitazone, on Bone Quality 8 Pioglitazone treatment did not significantly alter the weight of female or OVX mice with respect to vehicle controls but did result in significantly heavier male mice. Bones excised from all Pioglitazone-treated mice appear distinctly more yellow in colour. 8 Femoral abmd was significantly reduced due to Pioglitazone treatment in all mice with a significantly lower vertebral abmd in OVX mice as well. On the other hand, no significant changes were noted in femoral vbmd due to Pioglitazone treatment but treated males exhibited significantly reduced vertebral vbmd. 8 Vertebral compression showed significantly reduced mechanics due to Pioglitazone treatment in all mice. Pioglitazone-treated males had reduced stiffness, ultimate stress, and Young s modulus. Pioglitazone-treated females had reduced energy to failure and toughness. Finally, Pioglitazone-treated OVX mice had reduced ultimate load, ultimate stress and Young s modulus. 8 Pioglitazone-treated male mice experienced significantly reduced 3D trabecular architecture as well as total strut length, number of nodes and length of node-node strut (measures of connectivity). Additionally, treated male mice had a lower mineral apposition rate, an indication of reduced bone formation. 112
127 8 The vertebral total and trabecular bone mineralization profile of Pioglitazone-treated male mice was significantly more heterogenous than vehicle controls. 8 Histological slides revealed that Pioglitazone treatment led to a less uniform bone marrow in all mice with what appears to be increased adipogenesis Objective 2: Effects of DPP-4 inhibitor Treatment, Sitagliptin, on Bone Quality 8 Sitagliptin treatment did not significantly alter the weight in male, female or OVX mice when compared to respective vehicle controls. 8 Sitagliptin treatment resulted in a significantly increased vertebral abmd and BMC in male mice but this increase did not carry over in vbmd for male mice. 8 Sitagtliptin-treated female mice exhibited significantly increased vertebral vbmd and enhanced trabecular architecture. 8 Vertebral compression revealed that Sitagliptin-treated OVX mice experienced a significantly lower Young s modulus. 8 Analysis of the mineralization profiles of treated mice revealed that Sitagliptin treatment led to increased mineralization in total and trabecular bone area for all mice. This increased degree of mineralization continued in Sitagliptin-treated OVX mice when cortical bone mineralization profiles were analyzed. 113
128 CHAPTER 5: RESULTS FROM GENETIC INACTIVATION STUDY 114
129 5. Introduction: The clinical role of DPP-4 inhibitors in the treatment of T2DM provided motivation for characterizing the bone phenotype of DPP-4 -/- mice. Characterizing the bone phenotype in DPP-4 -/- mice is important in gaining a thorough understanding of the potential role of DPP-4 on bone as well as potential side-effects from DPP-4 inhibitor treatment. This chapter focuses on results from Objective 3 Effects of genetic inactivation of DPP-4 on bone quality in DPP-4 -/- mice. 5.1 Objective 3: Effects of Genetic Inactivation of DPP-4 on Bone Quality A total of six groups were generated for Objective 3 of this study. DPP-4 -/- mice and littermate +/+ controls were generated respectively for male, female, and OVX female mice. Bone densitometry, bone mechanics, structural properties, bone remodeling and mineral properties were used in order to evaluate bone quality. All techniques (Figure 3.3) were performed for all groups Weight: The weight of each mouse was recorded after sacrifice and can be viewed in Table 5.1. No significant differences were noted in the weight of male and female knockout mice when compared to respective wildtype controls. OVX knockout mice exhibited a significant decrease in weight when compared to OVX wildtype mice. Table 5.1 Weight for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Weight (g) 30.7 ± ± ± ± ± ± 1.2* Values reported as mean ± standard error. * Significant (p ) compared to wildtype control 115
130 5.1.2 Bone Densitometry: Areal bone mineral density (abmd) was evaluated using by Dual Energy X-ray Absorptiometry (DEXA) and volumetric bone mineral density (vbmd) was evaluated using Micro-Computed Tomography (Micro-CT) Areal Bone Mineral Density: DEXA was performed on excised left and right femora and lumbar vertebrae (L5 and L6). These values were averaged to provide femoral and vertebral abmd, respectively and results are shown in Table 5.2. No effect was seen due to genetic inactivation of DPP-4 in male or female mice however, significant decreases were seen in the femoral and vertebral abmd of OVX knockout mice. Femoral and vertebral BMC was also significantly lower in OVX knockout mice when compared to OVX wildtype mice. Table 5.2 DEXA results for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Femoral abmd (g/cm 2 ) Femoral BMC (g) Vertebral abmd (g/cm 2 ) Vertebral BMC (g) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± Values reported as mean ± standard error. * Significant (p ) compared to wildtype control ± ± ± ± ± * ± * ± * ± * 116
131 Volumetric Bone Mineral Density: Micro-CT was performed on the right femora and L5 vertebrae of all mice providing a measurement of femoral and vertebral vbmd, respectively (Table 5.3). No significant differences were seen in femoral or vertebral vbmd due to genetic inactivation of DPP-4 in male, female or OVX mice. Table 5.3 Femoral and vertebral vbmd for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Femoral vbmd (g/cm 3 ) ± ± ± ± ± ± n Vertebral vbmd (g/cm 3 ) ± ± ± ± Values reported as mean ± standard error. * Significant (p ) compared to wildtype control Bone Mechanical Properties: ± ± Three-point bending, vertebral compression and femoral neck fracture were performed in order to evaluate the mechanical properties of bone in DPP-4 knockout and wildtype mice. Results from both un-normalized and normalized data are presented. The un-normalized data are termed bone structural properties since they represent the failure behaviour at the whole bone level. On the other hand, normalized data represents the failure behaviour at the tissue level, and are termed bone material properties. Geometric data are also presented on the right femora and sixth lumbar vertebrae, as these were used for data normalization purposes. 117
132 Femoral Geometry: Micro-CT was used to assess the geometry of the right femora of all mice (Table 5.4). No significant structural changes were observed due to DPP-4 inactivation in male or female mice. However, the femora of knockout OVX mice appear to be significantly thinner and smaller with decreases observed in the anterior-posterior (A-P) diameter, moment of inertia, cross-sectional bone area, and cortical thickness. Table 5.4 Geometrical properties of right femora for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n A-P Diameter (mm) Moment of Inertia (mm 4 ) Crosssectional bone area (mm 2 ) Cortical Thickness (mm) 1.38 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± Values reported as mean ± standard error. * Significant (p ) compared to wildtype control 1.33 ± 0.01* ± 0.003* ± 0.016* ± 0.004* Three-Point Bending: Three-point bending was performed on the right femora of all mice. The results from three-point bending are shown in Table 5.5. DPP-4 knockout male and female mice did not experience any changes in cortical mechanical properties assessed from three-point bending. On the other hand, OVX knockout mice exhibited significantly reduced stiffness. 118
133 Table 5.5 Three-point bending results for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Structural Properties Ultimate Load 17.0 ± ± ± ± ± ± 0.7 (N) Failure Displacement (mm) 0.70 ± ± ± ± ± ± 0.04 Energy to Failure (mj) 7.9 ± ± ± ± ± ± 0.5 Stiffness ± ± ± ± ± ± 6.8 (N/mm) * Material Properties Ultimate ± ± ± 99.4 ± ± 4.8 Stress (MPa) ± 7.3 Failure Strain (%) 14.7 ± ± ± ± ± ± 1.0 Toughness (Mpa) 10.9 ± ± ± ± ± ± 1.0 Young s ± ± ± ± ± ± Modulus (Mpa) Values reported as mean ± standard error. * Significant (p ) compared to wildtype control Vertebral Geometry: Vertebral height and area were used to normalize vertebral compression data. The geometrical properties of the sixth lumbar vertebrae are shown in Table 5.6. No significant differences in vertebral geometry were seen from genetic inactivation of DPP-4 in male, female or OVX mice. Table 5.6 Geometrical properties of L6 vertebrae for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Height (mm) Area (mm 2 ) 3.24 ± ± ± ± ± ± ± ± 0.04 Values reported as mean ± standard error. * Significant (p ) compared to wildtype control 3.04 ± ± ± ±
134 Vertebral Compression: Vertebral compression was performed on the L6 vertebrae for all mice and the results are shown in Table 5.7. Male knockout mice exhibited significantly reduced ultimate load whereas female knockout mice experienced a significantly increased Young s modulus. No significant differences were seen when comparing OVX knockout mice to OVX wildtype controls. Table 5.7 Vertebral compression results for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Structural Properties Ultimate Load 24.1 ± ± 1.3* 17.7 ± ± ± ± 2.2 (N) Failure Displacement (mm) 0.34 ± ± ± ± ± ± 0.05 Energy to Failure (mj) 5.0 ± ± ± ± ± ± 0.9 Stiffness ± 83.8 ± ± 89.1 ± 83.8 ± 7.4 (N/mm) ± 9.7 Material Properties Ultimate Stress (Mpa) 9.8 ± ± ± ± ± ± 1.0 Failure Strain (%) 10.4 ± ± ± ± ± ± 1.5 Toughness 0.62 ± 0.54 ± 0.68 ± 0.49 ± ± 0.09 (Mpa) ± 0.13 Young s ± ± ± ± ± ± Modulus * (Mpa) Values reported as mean ± standard error. * Significant (p ) compared to wildtype control 120
135 Femoral Neck Fracture: Femoral neck fracture was performed on the proximal head of the right femora following three-point bending testing. The results from femoral neck fracture testing are shown in Table 5.8. No significant changes in femoral neck mechanical properties were observed in male or female knockout mice when compared to wildtype controls. Knockout OVX mice exhibited significantly reduced femoral neck mechanical properties with decreases in ultimate load, energy to failure and stiffness. Table 5.8 Femoral neck fracture results for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Structural Properties Ultimate Load 22.5 ± ± ± ± ± ± 0.5* (N) Failure Displacement (mm) Energy to Failure (mj) Stiffness (N/mm) 0.27 ± ± ± ± ± ± ± ± ± ± ± ± 0.2* ± ± ± ± 8.8 Values reported as mean ± standard error. * Significant (p ) compared to wildtype control ± ± 4.7* 121
136 5.1.4 Structural Properties: Vertebral structural properties were further assessed using Micro-CT and BSE imaging. Micro-CT was used to evaluate the trabecular architecture of the fifth lumbar vertebrae for all mice. These three-dimensional structural properties are shown in Table 5.9. Male knockout mice experienced significantly reduced trabecular thickness and female knockout mice experienced a significantly lower trabecular number. No changes were seen in the trabecular architecture of the L5 vertebrae of OVX knockout mice. Table 5.9 3D Trabecular bone structural properties for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n BV/TV (%) 26.2 ± ± ± ± ± ± 1.1 Tb.Th. (%m) 68.2 ± ± 1.0* 66.7 ± ± ± ± 0.7 Tb.N. 3.8 ± ± ± ± 0.2* 3.1 ± ± 0.2 (mm -1 ) Tb.Sp. (%m) ± ± ± ± 17.3 Values reported as mean ± standard error. * Significant (p ) compared to wildtype control ± ±
137 BSE was used for strut analysis on the fourth lumbar vertebrae for all mice (Table 5.10). No significant changes in 2D trabecular bone structural properties were seen when comparing male or OVX knockout mice to corresponding wildtype mice. Female knockout mice experienced a significant reduction in length of node-to-free struts, a measure of connectivity. Table D Trabecular bone structural properties for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Measures of Connectivity Measures of Disconnectivity Total Strut Length (mm/mm 2 ) Number of Nodes (mm -2 ) Length of Node-Node Struts (mm/mm 2 ) Length of Node-Free Struts (mm/mm 2 ) Number of Free Ends (mm -2 ) Length of Free-Free Struts (mm/mm 2 ) 4.70 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± 0.07* 12.9 ± ± 0.07 Values reported as mean ± standard error. * Significant (p ) compared to wildtype control 3.21 ± ± ± ± ± ± ± ± ± ± ± ±
138 5.1.5 Bone Formation: Bone formation was evaluated by dynamic histomorphometry on undecalcified sections of L4 vertebrae. No significant changes in bone formation parameters were seen in female or OVX knockout mice. A significant decrease was seen in mineral apposition rate when comparing male knockout mice to wildtype mice. Table 5.11 Bone formation parameters for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Mineralizing Surface (%) 25.5 ± ± ± ± ± ± 1.2 Mineral 0.60 ± 0.52 ± 0.65 ± 0.49 ± Apposition 0.61 ± 0.03 Rate * ± 0.01 (um/day) Bone Formation Rate (um/day) ± ± ± ± ± ± Values reported as mean ± standard error. * Significant (p ) compared to wildtype control Bone Resorption: Bone resorption was assessed from osteoclast staining for Tartrate-Resistant Acid Phosphatase (TRAP) on decalcified sections of L3 vertebrae for all mice (Table 5.12). No significant changes were seen in osteoclast staining parameters when comparing knockout to wildtype for male, female or OVX mice. Additionally, osteoclast nuclei were counted and the percentage of osteoclasts as a function of the number for male, female and OVX mice are presented in Figure 5.1, 5.2, 5.3, respectively. No major differences in the amount of nuclei were seen when comparing knockout to wildtype. 124
139 Table 5.12 Osteoclast staining results for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Number of Osteoclasts 55 ± 6 43 ± 5 74 ± ± 4 51 ± 5 55 ± 5 (-) Osteoclast Surface (mm) Percent Osteoclast Surface (%) Number of Osteoclasts per Bone Surface (mm -1 ) Number of Osteoclasts per Osteoclast Surface 0.89 ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± 2.0 Values reported as mean ± standard error. * Significant (p ) compared to wildtype control Nuclei Count of Male Osteoclasts 80% 70% 60% 50% Male WildType Male KnockOut 40% 30% 20% 10% 0% Amout of Nuclei Figure 5.1 Percent of osteoclasts with number of nuclei for DPP-4 KO and WT male mice 125
140 Nuclei Count of Female Osteoclasts 80% 70% 60% 50% 40% Female WildType Female KnockOut 30% 20% 10% 0% Amount of Nuclei Figure 5.2 Percent of osteoclasts with number of nuclei for DPP-4 KO and WT female mice 70% Nuclei Count of OVX Osteoclasts 60% 50% 40% OVX WildType OVX KnockOut 30% 20% 10% 0% Amount of Nuclei Figure 5.3 Percent of osteoclasts with number of nuclei for DPP-4 KO and WT OVX mice 126
141 5.1.7 Bone Mineral Properties: Bone mineral properties were evaluated by quantitative BSE imaging on sectioned polished Spurr blocks of L4 vertebrae for all mice. Mineralization distributions of three defined bone areas directly below the growth plate were determined: Total (trabecular + cortical), Trabecular, and Cortical TOTAL: Table 5.13 summarizes the parameters obtained from total bone mineralization profile analysis. The average mineralization profiles for total bone area are shown for male, female and OVX mice in figures 5.4, 5.5 and 5.6 respectively. No significant changes were noted in these parameters when comparing knockout to wildtype for male, female or OVX mice. Total mineralization profiles of female and OVX knockout mice did experience a slight shift in mineralization but this was not significant. Table 5.13 Results from BSE imaging of total bone for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Logit (-) Mineralization Peak (pixels) FWHM (pixels) 1.08 ± ± ± ± ± ± ± ± ± ± ± ± 1 28 ± 1 27 ± 1 27 ± 1 26 ± 1 29 ± 2 28 ± 1 Values reported as mean ± standard error. * Significant (p ) compared to wildtype control 127
142 2.5 Total Mineralization Profiles of Male 2 WildType KnockOut Grey Level Figure 5.4 Total mineralization profiles for DPP-4 KO and WT male mice 2.5 Total Mineralization Profiles of Female Mice 2 WildType KnockOut % GreyLevel Figure 5.5 Total mineralization profiles for DPP-4 KO and WT female mice 128
143 Total Mineralization Profiles of OVX Female Mice WildType KnockOut % GreyLevel Figure 5.6 Total mineralization profiles for DPP-4 KO and WT OVX mice TRABECULAR: Table 5.14 summarizes the parameters obtained from total bone mineralization profile analysis. The average mineralization profiles for trabecular bone area are shown for male, female and OVX mice in figures 5.7, 5.8 and 5.9, respectively. No significant changes were noted in these parameters when comparing knockout to wildtype for male, female or OVX mice. Trabecular mineralization profiles of female and OVX knockout mice did experience a slight shift in mineralization but this was not significant. 129
144 Table 5.14 Results from BSE imaging of trabecular bone for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Logit (-) Mineralization Peak (pixels) FWHMH (pixels) 0.95 ± ± ± ± ± ± ± ± ± ± ± ± 2 29 ± 1 30 ± 1 30 ± 1 30 ± 1 30 ± 2 29 ± 2 Values reported as mean ± standard error. * Significant (p ) compared to wildtype control 2.5 Trabecular Mineralization Profiles of Male Mice 2 WildType KnockOut Grey Level Figure 5.7 Trabecular mineralization profiles for DPP-4 KO and WT male mice 130
145 Trabecular Mineralization Profiles of Female WildType KnockOut % GreyLevel Figure 5.8 Trabecular mineralization profiles for DPP-4 KO and WT female mice 2.5 Trabecular Mineralization Profiles of OVX Female Mice 2 WildType KnockOut % GreyLevel Figure 5.9 Trabecular mineralization profiles of DPP-4 KO and WT OVX mice 131
146 CORTICAL: Table 5.15 summarizes the parameters obtained from total bone mineralization profile analysis. The average mineralization profiles for cortical bone area are shown for male, female and OVX mice in figures 5.10, 5.11 and 5.12 respectively. No significant changes were noted in these parameters when comparing knockout to wildtype for male, female or OVX mice. Female and OVX knockout mice did experience a slight increase in mineralization when compared to corresponding wildtype controls however, this was not significant. Table 5.15 Results from BSE imaging of cortical bone for DPP-4 KO and WT mice Male Female OVX WT KO WT KO WT KO n Logit (-) Mineralization Peak (pixels) FWHMH (pixels) 0.92 ± ± ± ± ± ± ± ± ± ± ± ± 1 23 ± 1 22 ± 1 24 ± 1 24 ± 1 21 ± 1 21 ± 1 Values reported as mean ± standard error. * Significant (p ) compared to wildtype control 3.5 Cortical Mineralization Profiles of Male Mice WildType KnockOut Grey Level Figure 5.10 Cortical mineralization profiles for DPP-4 KO and WT male mice 132
147 Cortical Mineralization Profiles of Female Mice WildType KnockOut % GreyLevel Figure 5.11 Cortical mineralization profiles for DPP-4 KO and WT female mice 3.5 Cortical Mineralization Profiles of OVX Female Mice WildType KnockOut % GreyLevel Figure 5.12 Cortical bone mineralization profiles for DPP-4 KO and WT OVX mice 133
148 Table 5.16 Summary of results for DPP-4 KO and WT mice Males Females OVX Bone Densitometry Femoral Vertebral Geometrical Properties Femoral aBMD (p<0.001) 5BMC (p<0.001) 5aBMD (p=0.012) 5BMC (p<0.001) 5A-P Diameter (p<0.001) 5Moment of Inertia (p<0.001) 5Cross-sectional Area (p=0.001) 5Cortical Thickness (p=0.011) Vertebral Bone Mechanical Properties Three-Point Bending Femoral Neck Fracture Vertebral 5Ultimate Load Compression (p=0.042) Trabecular Structural Properties 3D Micro-CT 5Tb. Th. (p=0.017) 2D Strut Analysis - Bone Remodeling Bone Formation Mineral Apposition Rate (p=0.037) 6Young s modulus (p=0.036) 5Tb. N. (p=0.019) 5Length Node-Free (p=0.013) 5Stiffness (p=0.005) 5Ultimate Load (p<0.001) 5Energy to Failure (p=0.014) 5Stiffness (p<0.001) - - Bone Resorption Mineral Properties Mineralization Profiles Total Bone Trabecular Bone Cortical Bone
149 5.2 Summary of Genetic Inactivation Study: The following section provides a summary of the significant findings from the genetic inactivation study. The evaluation of bone quality in male, female and OVX DPP-4 -/- mice has provided insight into the role of DPP-4 in bone mineral homeostasis and metabolism. 8 Genetic inactivation of DPP-4 did not produce a significant change in femoral or vertebral BMD, areal or volumetric, in male or female mice. However, noted decreases in femoral and vertebral abmd were noted in OVX knockout mice. 8 OVX knockout mice had significantly smaller and thinner femora but no changes in femoral geometry were noted in male or female knockout mice. 8 OVX knockout mice experienced significantly reduced stiffness by three-point bending and ultimate load, energy to failure, and stiffness by femoral neck fracture. 8 Male knockout mice exhibited a significantly reduced ultimate load by vertebral compression as well as reduced trabecular thickness and mineral apposition rate. 8 Female knockout mice experienced an increased Young s modulus by vertebral compression. However, female knockout mice also experienced reduced trabecular number and a lower length of node-free strut, a measure of connectivity. 8 Mineralization profiles revealed slightly increased mineralization in female and OVX knockout mice but this was not significant. 135
150 CHAPTER 6: DISCUSSION 136
151 6. Introduction: The major goal of this study was to investigate the effects of two anti-diabetic drugs, Pioglitazone and Sitagliptin, on bone quality in a glucose intolerant mouse model. Both drugs restore glucose and insulin homeostasis in a challenged mouse model but do so from different physiological mechanisms. Pioglitazone is a TZD that binds to and activates PPAR-! receptors [122] whereas Sitagliptin is a DPP-4 inhibitor that inhibits the DPP-4 enzyme from cleaving incretin hormones [ , 135, 137]. The effects of these two anti-diabetic drugs were evaluated in glucose intolerant male, female and OVX female mice. Another goal of this study was to further characterize the effects of DPP-4 inhibition on bone quality using a DPP-4 knockout mouse model. The effects of genetic inactivation of DPP-4 were evaluated in euglycemic male, female and OVX female mice. Bone quality was evaluated through densitometry, mechanical testing and techniques used to evaluate bone remodeling, bone structure and mineral properties. The discussion is divided into three main components that serve to explain the findings from all three objectives of this study. Objective 1) Effects of TZD treatment, Pioglitazone, on bone quality and Objective 2) Effects of DPP-4 inhibitor treatment, Sitagliptin, on bone quality are both classified under the anti-diabetic drug study. Objective 3) Effects of genetic inactivation of DPP-4 on bone quality is part of the genetic inactivation study. 6.1 Anti-diabetic drug study: The motivation for the anti-diabetic drug study stems from the increased fracture risk associated with T2DM. The characterization of anti-diabetic drugs that exacerbate the skeleton in these patients is important as well as the search for skeletally neutral or positive anti-diabetic drugs. This study aims to evaluate if the TZD Pioglitazone has negative effects on the skeleton as well as determining if the DPP-4 inhibitor Sitagliptin could be a skeletally positive anti-diabetic drug. The results of this study are discussed by individual groups (male, female, OVX). 137
152 6.1.1 Objective 1: Effects of TZD treatment, Pioglitazone, on bone quality Pioglitazone treatment led to increased weight gain in male (619%), female (611%) and OVX (68%) mice when compared to respective vehicle controls however, the increase was only significant in male mice (p=0.001). Weight gain is associated with Pioglitazone treatment, and appears to be greater than that seen from other TZDs [171, 172]. Another side-effect from Pioglitazone treatment is increased total body adiposity [172]. Femora and vertebrae that were excised from Pioglitazone-treated mice were distinctly more yellow in colour when compared to vehicle controls. Accordingly, histological slides revealed substantially more fat deposits in the bone marrow of Pioglitazone-treated mice than in vehicle controls. It is assumed that the accumulation of fat in the Pioglitazone samples resulted in the noticeably different bone colour. This finding coincides with other studies concluding that TZDs led to increased bone marrow fat volume in rodents [ , 173]. The increase in bone marrow fat volume is largely thought to occur because of preferential differentiation of mesenchymal progenitor cells into adipocytes over osteoblasts [125]. It is assumed that increased bone marrow adiposity should lead to reduced osteoblastogenesis and subsequent bone formation. However, two other TZDs, Troglitazone and Netoglitazone, produced increased bone marrow adiposity when given at anti-hyperglycemic doses but had no effect on trabecular bone volume and was not associated with bone loss in mice [126, 173]. Therefore, it is important to note that increased bone marrow adiposity may be indicative of events that could lead to reduced bone quality but is not consistently associated with it. Nevertheless, histological slides reveal that the dose of Pioglitazone given to mice in this study was enough to produce this noted TZD phenotype. Male mice exhibited the greatest degree of changes due to Pioglitazone treatment. DEXA revealed a significant decrease in femoral abmd (511%, p=0.007) and BMC (516%, p=0.028) in Pioglitazone-treated male mice. The reductions seen from DEXA coincide with a study that 138
153 found a significant reduction in tibial abmd in Wistar rats treated with Pioglitazone [123]. However, the decrease in femoral abmd was not seen in femoral vbmd assessed by Micro-CT and serve to highlight the differences in these two techniques. DEXA measures abmd from a 2D scanned image and does not take whole bone structure into account. Therefore, the vbmd assessed by Micro-CT is considered a more valid measurement of density because it is calculated from a 3D scan. Micro-CT did reveal a significant decrease in vertebral vbmd (521%, p=0.011) for Pioglitazone-treated male mice suggesting an adverse effect on trabecular bone. Accordingly, no significant changes were seen in the femoral geometry or mechanics of Pioglitazone-treated male mice. Evaluation of the 3D trabecular bone structural properties revealed significant reductions in trabecular bone volume (514%, p=0.001), trabecular thickness (56%, 0.004), and trabecular number (59%, p=0.025) for Pioglitazone-treated male mice. Similarly, strut analysis also revealed significant reductions in total strut length (510%, p=0.021), number of nodes (522%, p=0.026) and length of node-node struts (535%, p=0.006) in Pioglitazone-treated male mice, indicative of reduced trabecular connectivity. These results further emphasize an adverse effect due to Pioglitazone treatment on trabecular bone in male mice and similar reductions were seen in 3D trabecular bone structural properties in the Lazarenko et al., study in Rosiglitazone-treated male mice [116]. Results from vertebral compression coincide to the results seen thus far suggesting an adverse effect due to Pioglitazone treatment in male mice. More specifically, treated male mice experienced significantly reduced stiffness (524%, p=0.034), ultimate stress (526%, p=0.040) and Young s modulus (529%, p=0.020) and suggest that Pioglitazone treatment reduced vertebral 139
154 strength in male mice. These findings corresponds to the Lazarenko et al., study that found reduced vertebral strength from Rosiglitazone treatment in mice [116]. The assessment of bone formation parameters by dynamic histomorphometry revealed a significant decrease in the mineral apposition rate (516%, p=0.03) in Pioglitazone-treated male mice. This finding corresponds to the Lazarenko et al., study that found reduced mineral apposition due to Rosiglitazone treatment in C57BL/6 male mice [116]. Mineral apposition rate is calculated from the width between incorporated fluorochrome labels divided by the 10-day period between injections. This provides a quantitative assessment of the extent of bone formation over a specific period of time. Based on these results, Pioglitazone treatment appears to reduce bone formation in male mice. The suggested reason for reduced bone formation from TZD treatment is due to PPAR-! activation on mesenchymal progenitor cells causing them to preferentially differentiate into adipocytes at the expense of osteoblasts [117, 125, 127, 128]. Finally, mineralization profile analysis revealed a significant increase in the heterogeneity for total bone (611%, p=0.047) and trabecular bone area (68%, p=0.036) of Pioglitazone-treated male mice. The decrease in mineral apposition rate as well as increased heterogeneity of total and trabecular mineralization profiles could potentially suggest that Pioglitazone treatment delays mineralization of new bone [174]. Additionally, the loss of a significant increase in heterogeneity in the cortical bone mineralization profiles reflect the results of this study that show the effects of Pioglitazone treatment being largely localized to trabecular regions. Pioglitazone treatment in female and OVX female mice did not produce the same severity of changes as was seen in male mice. Pioglitazone-treated female mice exhibited a significantly reduced femoral abmd (55%, p=0.002) and BMC (57%, p=0.010) but femoral vbmd, as evaluated by Micro-CT, remained unchanged. Similarly to male mice, no changes were seen in the femoral mechanics of Pioglitazone-treated female mice suggesting that Pioglitazone-treatment is 140
155 not adversely affecting cortical bone quality. Changes were seen in the vertebral mechanics of Pioglitazone-treated female mice with reductions in energy to failure (551%, p=0.014) and toughness (537%, p=0.024). 3D and 2D trabecular bone structural properties did not reveal any changes due to Pioglitazone treatment in female mice, which suggests that the changes seen in the vertebral mechanics are not likely due to adverse effects on the mineral. Energy to failure and toughness are largely influenced by collagen content and not necessarily to changes in the mineral phase [71, 72]. Furthermore, vertebral vbmd of treated female mice remained unchanged and it has been shown that changes in these mechanical parameters are not always reflected in a change in density [175]. No significant changes were seen in the bone formation parameters or mineralization profiles for Pioglitazone-treated female mice. A lack of changes in these tests further confirm a less severe phenotype in treated female mice than was seen in treated male mice. Osteoclast staining did reveal a significantly reduced number of osteoclast per osteoclast surface (522%, p=0.010) in Pioglitazone-treated female mice. This parameter suggests that the osteoclasts present in Pioglitazone-treated female mice have a larger surface area than in vehicle control mice and highlight a noted osteoclast phenotype from TZD treatment. The increased bone marrow adiposity due to TZD treatment appears to push the osteoclasts against the trabeculae, giving them a stretched out appearance and explains the significant change in the above mentioned parameter. Osteoclasts with a larger surface area should contain more nuclei and have a greater resorptive ability, which is why the quantification of the degree of nucleation in osteoclasts was performed [165, 166]. However, no major differences were found from osteoclast nuclei counting but it was observed that osteoclasts in Pioglitazone-treated mice appeared pressed against the trabecular surface from bone marrow adipocity (Appendix Figure A1). Rarely could the degree of nucleation be deduced from these pressed osteoclasts suggesting either a treatment-induced 141
156 defect of osteoclasts or under representation of the true nuclei numbers. This pressed osteoclast phenotype was noted in all Pioglitazone-treated mice. As previously mentioned, Pioglitazone treatment does not produce as severe of an effect in OVX mice as was seen in male mice. DEXA revealed a significantly reduced femoral abmd (p=0.002) and vertebral abmd (p=0.008) and BMC (p=0.006) due to Pioglitazone treatment in OVX mice. However, these reductions were not seen in either the femoral or vertebral vbmd as assessed by Micro-CT, which is considered a more valid method for BMD measurements. Similarly to all Pioglitazone-treated mice of this study, no significant changes were seen in the femoral geometry or mechanics in OVX mice. The lack of changes in cortical bone mechanics was unexpected but may suggest that a longer treatment period or larger dose may be required. Vertebral compression did reveal reduced vertebral strength due to Pioglitazone treatment in OVX mice with significant decreases seen in ultimate load (537%, p=0.005), ultimate stress (543%, p=0.01) and Young s modulus (546%, p=0.014). This finding corresponds to the Lazarenko et al., study that found reduced vertebral strength from Rosiglitazone treatment in mice [116]. However, no significant changes were seen in vertebral vbmd or 3D/2D trabecular bone structural properties which were unexpected given the reduced vertebral strength seen from treatment in these mice. Inconsistencies between the vertebral compression data, which showed reductions, and the Micro-CT and strut analysis data, which showed no changes, may be due to differences with respect to different anatomical location. Vertebral compression was performed on the L6 vertebrae whereas Micro-CT and strut analysis were performed on the L5 and L4 vertebrae, respectively. No significant changes were seen in the mineralization profiles, or bone formation and resorption parameters for Pioglitazone-treated OVX mice. A lack of changes in these tests further confirm a less severe phenotype in treated OVX mice than was seen in treated male mice. 142
157 There were no significant changes due to Pioglitazone treatment in the femoral geometry, mechanics or bone resorption parameters for male, female or OVX mice. The lack of changes seen in femoral mechanics were unexpected given reports that TZD treatment resulted in fractures of the distal upper and lower limbs and not of the spine [4, 5, 9, 11, 81]. However, cortical bone has a lower surface area and overall slower turnover rate than trabecular bone and may suggest that a longer treatment period is required before changes due to Pioglitazone treatment are seen there [23]. The Syvenson et al., study that found reductions in three-point bending mechanics used a longer treatment time and larger dose of Pioglitazone [124]. Still, no changes were seen in femoral neck fracture data despite longer and larger treatment dose for that study. The lack of major significant changes in the osteoclast staining data was surprising given the information regarding the role of PPAR-! activation and Rosiglitazone on osteoclastogenesis [129]. However, an in vivo study using Rosiglitazone in growing (1 month), adult (6 month), and aged (24 month) C57BL/6 male mice only found changes in the TRAP-positive osteoclast data in aged mice [116]. The adult group, which is closer to the age group of this study, showed no changes. Aging was identified as a confounding factor for Rosiglitazone-induced bone loss that correlated with increased expression of PPAR-! in bone marrow mesenchymal progenitor cells. The authors suggest that the increased osteoclast numbers of aged mice from Rosiglitazone treatment was associated with increased mesenchymal cell support for osteoclast development [116]. A study by Soroceanu et al also did not find differences in osteoclast staining data due to Rosiglitazone treatment in 4-month old C57BL/6 male mice [118]. It was largely unexpected that the adverse effects observed from Pioglitazone-treatment were largely in the male group, with limited changes seen in the female or OVX groups. This was surprising because the ADOPT study and studies observed from Takeda Pharmaceuticals found 143
158 no association between Rosiglitazone or Pioglitazone treatment and increased fracture risk in male subjects [4, 5, 7, 11]. These studies revealed that postmenopausal women were the group at the greatest fracture risk from TZD treatment with pre-menopausal women being at a lesser risk but an increased risk nonetheless. It was expected based on the results from these studies that adverse skeletal effects due to Pioglitazone treatment would increase in severity from male to female mice and to the greatest degree in OVX female mice. This sexually dimorphic effect on bone quality seen from TZD treatment in human studies could potentially relate to a gender difference in drug response. It has been observed that women demonstrate a greater response to Pioglitazone treatment [176]. Postmenopausal women are likely even more susceptible to increased fracture risk from TZD treatment because they are of older age and are already challenged skeletally from the arrest in ovarian production of estrogen. However, despite the lack of an association between TZD induced fracture risk in male subjects from the ADOPT or Takeda Pharmaceutical studies, rodent studies have demonstrated an effect in male animals [ ] and these effects are reflective of those seen in this study. Moreover, a study by Yaturu et al., also found a decrease in BMD associated with Rosiglitazone in men with T2DM [177]. Therefore, the large effect seen from Pioglitazone treatment in male mice, although unexpected, is still validated from previous literature. However, the small effects seen in Pioglitazone-treated female or OVX mice are not validated from the literature. Firstly, it is important to note that decreases were seen in the vertebral mechanics of these mice as well as an apparent increase in bone marrow adiposity. However, these results are not further exemplified by the other techniques used to assess bone quality and do not compare in terms of severity to the effects seen in treated male mice. The lack of major changes seen in these mice may be indicative of a failure to reach doses high enough to produce as intense of a skeletal effect in female or OVX mice as was seen in male mice. Insulin tolerance testing was performed to monitor the effects of Pioglitazone treatment in mice and 144
159 further suggest that males showed a greater response to treatment than female and OVX mice. Treated male mice were more insulin sensitive than vehicle control mice whereas treated female mice had a similar degree of insulin sensitivity to vehicle control mice and treated OVX mice were less insulin sensitive than vehicle control mice. Similarly, treated male mice were the only group to experience a significant increase in weight, which is a side-effect from Pioglitazone treatment [171, 172]. These factors may suggest that the effectiveness of the drug was greatest in male mice, which could explain why male mice experienced more adverse skeletal effects from treatment than female or OVX mice. Additionally, Sottile et al., did not find significant differences in BMD and histomorphometric parameters for intact female mice, despite major adverse changes in OVX mice, treated with a similar dose of Rosiglitazone [119]. A lack of major effects from TZD treatment in intact females in the Sottile et al study and our study serve to highlight the complexities of PPAR-! activation and bone quality. Finally, the lack of changes in structural and mineral parameters of female and OVX mice, despite reductions in their vertebral mechanical properties, suggest potential adverse effects to the collagen phase of bone due to Pioglitazone treatment. Reductions in the integrity of collagen can negatively affect the toughness and strength of bone [71, 72]. One study found that TZD activation of PPAR-! resulted in suppression of Type 1 collagen in a stromal cell line, implying that PPAR-! activation may affect collagen as well as bone remodeling [127]. The reductions seen in vertebral energy to failure and toughness of Pioglitazone-treated female mice largely suggest effects to the collagen phase of bone. Interestingly, the reduction of collagen biosynthesis of PPAR-! was found to be dependent on estrogen in endrometrial adenocarcinoma and breast cancer cells [178, 179]. It is also important to note that skeletal effects appear to be distinctive to specific TZD treatment. Rosiglitazone is the most extensively studied TZD in terms of its effects on bone 145
160 quality and it is largely accepted that these effects are negative [4-6, 9, 81, , 127, 129].However, evidence regarding other TZDs and their effects on the skeleton are less conclusive. As previously mentioned, Troglitazone and Netoglitazone produce increased bone marrow adiposity but do not appear to result in increased bone loss in mice [126, 173]. Another study found that Troglitazone prevented the development of diabetic osteopenia in Zucker diabetic fatty (ZDF) rats [180]. Differences in the action of TZDs on bone may be attributable to their affinities towards PPAR subtypes [181]. The PPAR family consists of three subtypes, PPAR- $, -#/9 and -! and have each been identified in osteoblasts and osteoclasts [124]. Rosiglitazone largely binds the PPAR-! subtype [182, 183] while Pioglitazone has been shown to bind PPAR! with some affinity for PPAR-$ [122]. PPAR-$ agonists do not appear to negatively affect bone to the same degree as PPAR-! agonists. Troglitazone, like Pioglitazone, binds to PPAR-! and less strongly to PPAR-$ and its effects on bone are still conflicting [173, 180]. Syvenson et al., also found that the PPAR-$ agonist fenofibrate actually improved femoral BMD in mice [124]. Therefore, the effects of one TZD on bone cannot be used to fully predict the effects of another. Pioglitazone has been observed to produce negative effects on bone quality [11, 123, 124] but differences in the degree of its effects to other TZDs, like Rosiglitazone, could be attributable to its combined affinity to PPAR subtypes. In summary, Pioglitazone treatment in high-fat fed male, female and OVX female mice led to adverse effects on trabecular bone quality. Male mice experienced the greatest effects from Pioglitazone treatment with reductions seen in vertebral vbmd, 3D and 2D trabecular bone structural properties, vertebral strength and bone formation. Female mice experienced reductions in vertebral energy to failure and toughness whereas OVX female mice experienced reduced vertebral strength due to Pioglitazone treatment. These results suggest that Pioglitazone has a 146
161 largely negative effect on bone despite its improvements on insulin sensitivity and glucose control in a high-fat fed, glucose intolerant mouse model Objective 2: Effects of DPP-4 inhibitor treatment, Sitagliptin, on bone quality Sitagliptin treatment appears to be weight neutral in mice, producing only a slight decrease in the weight of OVX female mice (59%, p=0.06) although this was not significant. Additionally, Sitagliptin treatment did not produce a significant phenotype on bone colour or bone marrow adiposity as seen from Pioglitazone treatment. These findings correspond to studies that have shown a neutral effect on body weight and no effect on total fat mass from Sitagliptin treatment [184, 185]. Female mice exhibited the most beneficial effect from Sitagliptin treatment. A significant increase was seen in vertebral vbmd (631%, p=0.002) in Sitagliptin-treated female mice. It is assumed that the positive effect on the vertebral vbmd in Sitagliptin-treated female mice may be attributable to sustained enteric hormone action. A 5-week GLP-2 administration study revealed significant increases in the spinal BMD of short-bowel patients with no colon [15]. Accordingly, a 4-month GLP-2 administration study in post-menopausal women with low BMD revealed a dosedependent increase in hip BMD from treatment [17]. An in vivo study administering GIP to OVX rats revealed an increase in BMD when compared to untreated OVX rats [12]. However, a study on Sitagliptin treatment in euglycemic OVX rats demonstrated no effect on vertebral or femoral abmd. for all treatment doses [186]. Rats of that study were treated by oral gavage (100, 300 and 500 mg/kg) for a total of three months. Sitagliptin-treatment appears to enhance the 3D trabecular structural properties in female mice with increases in trabecular bone volume (629%, p = 0.002), trabecular thickness (68%, p = 0.005), trabecular number (622%, p=0.008) and a significant decrease in trabecular separation (529%, p=0.015). The positive effects from Sitagliptin treatment on trabecular structural 147
162 properties in female mice is also thought to be due to potentiation of enteric hormones GIP, GLP-1 and GLP-2. The positive changes in 3D trabecular bone structural properties do not coincide with vertebral compression data that showed no differences in Sitagliptin-treated female mice. Moreover, no changes were seen in the 2D trabecular structural properties for Sitagliptintreated female mice. Micro-CT is a more reliable method than strut analysis in evaluating trabecular structural properties because they are evaluated in 3D. However, data between Micro- CT and vertebral compression have been found to be largely correlated [187, 188] but was not found in this study. The inconsistencies between Micro-CT, strut analysis and vertebral compression data could be due to differences in anatomical location (L5, L4, L6). Another possibility could be a compromised external cortical shell that could minimize the positive trabecular structure with respect to overall vertebral mechanical integrity [189]. Finally, total and trabecular bone area mineralization profiles exhibited a significant shift towards increased mineralization in Sitagliptin-treated female mice. The increases in the degree of mineralization from Sitagliptin treatment may suggest that DPP-4 inhibition slows down the resorptive rate of bone. GLP-2 administration has been associated with an acute suppression in bone resorption based on bone marker evaluation [15, 16]. Suppressed bone resorption could allow more time for secondary mineralization to occur, resulting in a more mineralized bone. Another explanation for the increase in the degree of mineralization could be improved calcium deposition on bone, which was found to be directly linked to GIP by evaluation of GIPR-/- mice [19]. Greater calcium deposition on bone could produce a denser, more mineralized bone. Sitagliptin-treated male mice exhibited a significantly increased vertebral abmd (613%, p=0.004) and BMC (629%, p=0.004) but no changes were seen in the vertebral vbmd of these mice. Vertebral compression and the 3D trabecular structural properties remained unchanged in Sitagliptin-treated male mice, despite the increase in vertebral abmd. These findings suggest that 148
163 the increase seen in vertebral abmd and BMC may be due to DEXA measuring density from a 2D scan, not taking the whole bone structure into account. An unexpected finding in terms of trabecular connectivity was seen with a significantly reduced total strut length (514%, p=0.039) and length of node-free strut (523%, p=0.022) in Sitagliptin-treated male mice. This finding was unexpected given that Sitagliptin was expected to produce either a skeletally positive or neutral effect. However, given the limitations of the strut analysis technique and that no other changes were noted in other measures of connectivity, these changes do not largely indicate an adverse effect from Sitagliptin treatment on 2D trabecular connectivity in male mice. Similarly to female mice, total and trabecular bone mineralization profiles exhibited a significantly higher degree of mineralization in Sitagliptin-treated male mice. This suggests that Sitagliptin treatment affects the mineral phase of bone, possibly due to enteric hormone-reduced bone resorption or improved calcium deposition [16, 19], but these changes did not translate into increases in vbmd or enhanced mechanical properties. Sitagliptin-treatment in OVX mice produced no significant effects on femoral or vertebral abmd or vbmd. Accordingly, no significant changes were seen in 3D or 2D trabecular structural properties. Unexpectedly, Sitagliptin-treated OVX mice exhibited a barely significant decrease in vertebral Young s modulus (57%, p=0.05). The decrease in this parameter was unexpected given that Sitagliptin treatment was expected to have a skeletally neutral or positive effect. A possible reason for this decrease may relate to the protective effects of increased weight in OVX-induced bone loss since Sitagliptin treatment appears to minimize the weight gain that occurs post-ovx [190]. However, the overall decrease was small and does not suggest a major adverse effect from Sitagliptin treatment on vertebral mechanics in an OVX model. Similarly to Sitagliptin treated male and female mice, total and trabecular bone mineralization profiles show a significant increase in the degree of mineralization. A significant increase in the degree of mineralization was also 149
164 exhibited in the cortical bone mineralization profiles for Sitagliptin-treated OVX mice. These mineralization profile changes, which were found in all Sitagliptin-treated groups, suggest an effect of DPP-4 inhibition on the mineral phase of bone. As previously mentioned, these changes may be due to enhanced bioactivity of enteric hormones GIP, GLP-1 and GLP-2 which have effects on reduced resorption and improved calcium deposition [16, 19]. The increases seen in the degree of mineralization did not translate to improved mechanical properties. No significant changes were seen from Sitagliptin treatment in histomorphometric analysis for any group. Expectations regarding the effects of Sitagliptin treatment on histomorphometric data are based on two receptor knockout studies. A GIPR -/- study revealed a significantly lower mineral apposition rate and significantly higher number of osteoclasts in GIPR -/- mice [19]. The results from the Tsukiyama et al., study suggest that lack of GIP activity, because of deletion of its receptor, negatively affects bone formation and increases bone-resorbing osteoclasts. Based on those findings, an increase in mineral apposition rate as well as reductions in the number of osteoclasts would be expected given that DPP-4 inhibition, by Sitagliptin, produces increased postprandial GIP activity [133]. However, these parameters remained unchanged in this study, suggesting that increased GIP bioactivity from DPP-4 inhibition may not be large enough to significantly alter bone formation or resorption in these mice. A GLP-1R -/- study revealed significant increases in osteoclast numbers of the tibia but not in the spine of GLP-1R -/- mice. The authors also did not find any significant changes in the tibial or spinal mineral apposition rate of these KO mice [21]. In our study, TRAP staining and analysis was performed on the trabecular bone of L3 vertebrae. Based on the Yamada et al., study, TRAP staining of cortical bone, such as the tibia, may have been required to detect changes in osteoclasts numbers due to increased bioactivity of GLP-1 from Sitagliptin treatment. In general, the histomorphometric effects seen from the GIP and GLP-1 receptor KO studies [19, 21] may suggest that a basal level of these 150
165 hormones is required for bone maintenance but enhancement of bone may require a greater increase in intact GIP and GLP-1 levels than is produced from Sitagliptin treatment. There were also no significant changes in the mechanical properties of Sitagliptin-treated mice with the exception of the barely significant decrease seen in vertebral Young s modulus for treated OVX mice. No studies currently exist regarding the direct effects of DPP-4 inhibition or enteric hormones on bone mechanics. However, the positive effects seen from enteric hormones on bone quality would loosely suggest that positive mechanical effects should occur from Sitagliptin-treatment [12-22]. However, the results of this study suggest that Sitagliptin has a skeletally neutral effect on cortical bone mechanics that is independent of gender or OVX. Vertebral mechanics are also largely unaffected from Sitagliptin-treatment. The lack of major significant changes from Sitagliptin treatment was unexpected given several studies implicating a positive role for the enteric hormones GIP, GLP-1, GLP-2 on bone [12-22]. However, Kimmel et al., found no effect from Sitagliptin on BMD in OVX rats suggesting a largely neutral role on the skeleton [186]. With respect to our study, the dose of Sitagliptin given to mice should provide an :80% inactivation of plasma DPP-4 levels [132]. That level of DPP-4 inactivation should result in an approximate 2-4 fold increase in postprandial levels of active enteric hormones [184, 191]. These values have been previously measured in the Drucker laboratory with respect to a similar dose of Sitagliptin used to treat the mice in our study [192]. It is possible that the increase in enteric hormone levels attained by Sitagliptin treatment were not high enough to produce a large effect on bone. One study involving the administration of GLP-2 concluded that the amount needed to produce a significant reduction in bone resorption was 100 times higher than postprandial physiological conditions [17]. On the other hand, large negative phenotypic effects on bone have been reported in GIP and GLP-1 receptor knockout studies [19, 21]. These studies involved preventing the action of normal physiological 151
166 levels of GIP and GLP-1, via removal of the receptor, and were enough to produce large negative effects on bone [19, 21]. This may suggest that a basal physiological level of incretin action is required for the maintenance of bone but skeletal enhancement may require significantly higher levels not attained by chemical inhibition of DPP-4. It is also important to note that our study did not involve the direct manipulation of enteric hormones GIP, GLP-1 or GLP-2 but of the enzyme that quickly inactivates them. This results in an indirect manipulation of these hormones through DPP-4 inhibition that predisposes this study to the implications of compensatory mechanisms or feedback inhibition. Compensatory mechanisms include rapid enteric hormone clearing by the kidneys or reduced enteric hormone secretion from the intestinal lining. A study in dogs revealed a reduction in total incretin levels after DPP-4 inhibition which was reflective of reduced incretin secretion [193]. Another study demonstrated this feedback mechanism in humans where exogenous GLP-1 infusion led to a reduction in levels of endogenous amidated GLP-1 [194]. In a study using Sitagliptin, it was found that Sitagliptin therapy increased meal-stimulated plasma active GLP-1 levels 2-fold in male volunteers however, values of GLP-1 levels were reduced after 10 days of therapy [191]. Furthermore, other DPP-4 like enzymes exist with the ability to cleave the enteric hormones implicated in this study as well as minimize their effects on bone [195]. A study using the DPP-4 inhibitor valine-pyrrolidide noted reduced degradation of GIP but not complete protection, suggesting that DPP-4 is not the sole enzyme responsible for cleaving GIP and similar peptides [193]. It is possible that secretion of similarly capable enzymes could be increased to compensate for loss of DPP-4 activity. The effects of these compensatory mechanisms over the 3-month period of treatment in these mice may have reduced available levels of active GIP, GLP-1 and GLP-2 subsequently reducing their effects on the skeletal system. 152
167 Overall, Sitagliptin treatment produced some positive effects in male and female mice. These included increased vertebral abmd and BMC in treated male mice. Sitagliptin treatment in female mice led to significantly increased vertebral vbmd as well as enhanced trabecular architecture but these improvements were lost in females following OVX. Quantitative BSE imaging revealed that male, female and OVX mice exhibited significantly increased mineralization of vertebral total and trabecular bone due to Sitagliptin treatment. Anti-diabetic Drug Study: Concluding Summary: The goal of the anti-diabetic drug study was to characterize the effects of Pioglitazone and Sitagliptin treatment on the skeleton. It was expected that Pioglitazone treatment would adversely affect the skeleton and that Sitagliptin treatment would either produce a positive or neutral skeletal effect. The results of this study confirmed that Pioglitazone treatment did produce adverse effects on trabecular bone in male, female and OVX mice. Sitagliptin treatment did not improve the mechanical integrity of bone but did produce positive effects on the vertebral vbmd and trabecular architecture in female mice as well as increased the degree of mineralization in total and trabecular bone areas of all mice. This study emphasizes the potential risk of Pioglitazone treatment to exacerbate fracture risk in patients with T2DM. Additionally, Sitagliptin treatment does not appear to compromise mechanical integrity and would therefore be an alternative treatment method for T2DM that does not increase fracture risk. Interestingly, both treatment methods aimed to improve similar physiological conditions in a challenged high fat fed mouse model but produced opposing effects on the skeleton. The opposing skeletal effects from both anti-diabetic drugs highlight the importance of proper investigation into the mechanisms (PPAR-! activation or DPP-4 inhibition) by which drugs produce similar physiological outcomes (insulin sensitivity and glucose control). 153
168 6.2 Genetic inactivation study: The genetic inactivation study was performed in order to gain a further understanding of the role of DPP-4 inhibition on bone quality. This is the first study to characterize a bone phenotype in DPP-4 knockout mice and literature that may aid in the interpretation of results in this study is fairly limited. The results of this study will be discussed by group (male, female, OVX) Objective 3: Effects of genetic inactivation of DPP-4 on bone quality Overall, genetic inactivation of DPP-4 produced its greatest effect on bone quality in OVX female mice. This group saw the most significant changes, which were reductions in femoral and vertebral abmd, femoral geometry and the structural properties of three-point bending and femoral neck fracture. More specifically, OVX knockout mice had significantly smaller femora with decreases seen in the AP diameter (54%, p<0.001), moment of inertia (520%, p<0.001), cross-sectional bone area (515%, p=0.001) and cortical thickness (512%, p=0.011). Three-point bending revealed a significantly reduced stiffness (528%, p<0.001) and femoral neck fracture revealed significantly lower ultimate load (526%, p<0.001), energy to failure (528%, p=0.014) and stiffness (528%, p<0.001). These parameters each correspond to reduced structural mechanical properties in OVX knockout mice. The reductions seen in DEXA, femoral geometry and structural mechanical properties are either a direct measurement of structure or are structuredependent parameters. The reduced geometry and structural properties of the OVX knockout skeleton may be due to DPP-4 inactivation producing smaller mice following OVX as no major changes in femoral size or structural properties were observed in intact female knockout mice. Genetic inactivation of DPP-4 significantly reduced the weight (516% p=0.011) of OVX knockout mice when compared to OVX wildtype controls. Weight gain is an undesirable effect of 154
169 OVX in rodent models because it provides partial protection against osteopenia [190]. Genetic inactivation of DPP-4 in the OVX group significantly prevented this weight gain, which may have hindered its protective effects on the skeleton. OVX has also been shown to result in increased modeling-dependent bone gain on the periosteal envelope, which is a compensatory attempt to offset an increase in endocortical resorption [196, 197]. The significant reductions in femoral geometry, which resulted in the reduced cortical structural properties, may be due to suppression of this OVX-induced periosteal modeling by genetic inactivation of DPP-4. Male and female mice exhibited fewer changes than were seen in OVX mice. Male knockout mice exhibited a reduced vertebral ultimate load (529%, p=0.042), trabecular thickness (55%, p=0.017) and mineral apposition rate (513%, p=0.037). These parameters suggest that genetic inactivation of DPP-4 led to reduced structural properties as well as reduced bone formation in male mice. However, it is important to note that the changes seen from DPP-4 knockout in males are minimal, given the wide range of tests performed to quantify bone quality. A majority of techniques found no significant differences between male knockout to wildtype controls and those that were reduced were one of several parameters that remained unchanged within a given test. The effect of genetic inactivation of DPP-4 in male mice is difficult to estimate since only three parameters were affected from a multitude of tests. However, most of the changes seen were reductions, suggestion that genetic inactivation of DPP-4 does not produce an enhanced bone phenotype and that the enzyme may be important to bone metabolism. On the other hand, female knockout mice experienced an increased vertebral Young s modulus (629%, p=0.036), suggesting a more rigid bone, but had reduced trabecular number (519%, p=0.019) and length of node-to-free strut (537%, p=0.006). The reductions seen in 3D and 2D trabecular structural properties suggest that DPP-4 inactivation may have a negative effect on the structural integrity of trabecular bone. As mentioned for male mice, these are two 155
170 structural parameters from a multitude of tests performed, and do not largely suggest that DPP-4 inactivation has a major effect on bone quality. The increase in vertebral Young s modulus is an intrinsic measurement of stiffness and is a structure-independent property. This loosely suggests that genetic inactivation of DPP-4 produced a positive effect on the material content of vertebrae in female mice. Interestingly, mineralization profiles for female and OVX mice appear slightly more shifted towards increased mineralization. These shifts were not significant but are notable given the significant shifts towards increased mineralization observed from Sitagliptin treatment. The increase in vertebral Young s modulus may correspond to the slight hypermineralization seen in the mineralization profiles or may correspond to an enhanced vertebral cortical shell. However, the lack of significant changes in the majority of tests performed in this study do not suggest that genetic inactivation of DPP-4 largely affected bone quality in male or female mice. The purpose of the genetic inactivation study was to further characterize the effects of DPP-4 inhibition on bone quality in mice. It was expected that the phenotype seen from Sitagliptin treatment would be more enhanced in DPP-4 knockout mice because the enzyme has been completely inactivated as opposed to partially inactivated (~>80%) by chemical inhibition at four months of age. The effects seen due to Sitagliptin treatment were positive effects on vertebral vbmd and trabecular architecture in female mice as well as increases in the degree of mineralization of total and trabecular bone areas of the vertebrae in all treated mice. However, the results of the genetic inactivation study do not suggest a positive effect from genetic inactivation of the DPP-4 gene. The genetic inactivation study observed slight changes in single parameters in a few tests for male and female knockout mice with a majority of these changes leaning towards reductions in bone parameters. Overall, the lack of major changes in male and female knockout mice suggest that genetic inactivation of DPP-4 does not produce a major bone phenotype in these mice. However, the changes that were seen in male and female mice were mostly negative 156
171 changes (reduced trabecular number, thickness etc), which suggests that DPP-4 may play a small role in bone quality. Significant changes were seen in the OVX knockout mice but these reflected size-dependent properties. OVX knockout mice did significantly weigh less than their OVX wildtype controls and weight gain has a known protective effect against OVX-induced bone loss [190]. It is difficult to speculate why structure and structure-dependent properties were significantly reduced in OVX knockout mice. OVX knockout mice significantly weighed less than OVX wildtype control, which is known to have protective skeletal effects in an OVX model [190]. The resistance to OVX-induced weight gain in DPP-4 knockout mice may occur similarly to the resistance to high-fat diet induced weight gain noted in these mice. DPP-4 knockout mice exhibit significantly reduced adiposity and plasma leptin levels when challenged with a high-fat diet [145]. Increased adiposity can have protective effects against OVX-induced bone loss because of increased weight-bearing (inducing bone formation) and peripheral aromatization of adrenal androgens to estradiol that occurs in adipose tissue [198]. Additionally, leptin is largely secreted by adipocytes and is thought to mediate at least part of the protective effect of fat mass on the skeleton and reduces OVX-induced bone loss in rats [198, 199]. Therefore, a resistance to OVXinduced weight gain, possibly resulting in lower adiposity and plasma leptin levels in DPP-4 knockout mice, may evade their protective effects on the skeleton. It has also been shown that OVX in rats produces an over-accumulation of neuropeptide Y (NPY), which is a substrate for DPP-4 and has been shown to regulate bone growth [131, 200]. NPY has been shown to regulate bone by increasing bone mass during times of obesity when hypothalamic NPY expression levels are low and reducing bone mass to conserve energy, when hypothalamic NPY expression levels are high [201]. Over-accumulation of NPY following OVX could signal a reduction in bone mass and could lead to adverse effects on bone when coupled to further NPY accumulation in 157
172 knockout mice because of no DPP-4 activity. Furthermore, infusion of NPY inhibited bone mass in obese mice and deletion of the NPY Y2R receptor in the hypothalamic neurons of normal mice significantly promoted bone growth, highlighting the inverse relationship between NPY levels and bone mass [ ]. Additionally, GIPR-/- mice exhibited altered NPY levels following OVX [204]. These potential mechanisms could potentially lead to the reductions seen in the OVX knockout mice. It is important to exercise caution when comparing the skeletal effects seen from DPP-4 inhibition in the anti-diabetic drug and genetic inactivation studies. Sitagliptin-treated mice were compared to high-fat fed control mice whereas DPP-4 knockout mice were compared to healthy wildtype mice. This may create a greater physiological disparity between treated and control mice in the anti-diabetic drug study than for knockout and wildtype mice in the genetic inactivation study. More thoroughly, the physiological differences between high-fat fed mice with hyperinsulinemia and hyperglycemia and Sitagliptin-treated high-fat fed mice with stabilized insulin and glucose control could induce a greater skeletal effect than DPP-4 inhibition. Studying the effects of another anti-diabetic drug, Pioglitazone, was expected to serve as a control for insulin and glucose stabilization from treatment. However, the known skeletal effects of PPAR-! activation [4-9, , , 177] may invalidate the use of Pioglitazone as a proper control for improvements in insulin and glucose homeostasis. Therefore, the differences in skeletal effects between DPP-4 inhibition for the anti-diabetic drug and genetic inactivation study may not solely be due to differences in the form of inhibition (chemical or genetic) but due to the model used to assess differences (glucose intolerant or euglycemic). It was originally expected that DPP-4 knockout mice would have enhanced bone quality because of their improved metabolic control and sustained bioactivity of GIP, GLP-1 and GLP-2. However, this effect was not seen as the knockout phenotype appears to be largely neutral in male 158
173 and female mice with reductions in size-dependent and/or structural properties in OVX mice. It is important to note that compensatory mechanisms may still play a major role in minimizing the effects of enteric hormone action on the skeleton. These include rapid clearing of enteric hormones by the kidneys as well as reduced secretion from the intestinal lining. Similarly, studies with DPP-4 -/- mice have noted levels of DPP-4-like activity in those animals, which suggests the presence of similarly capable enzymes [139, 205]. It is possible that secretion of similarly capable enzymes could be increased to compensate for a lack of DPP-4 activity. Direct comparison of the phenotypes arising in mice treated with Sitagliptin versus DPP-4 knockout mice is difficult for several reasons. First, Sitagliptin-treated mice were studied on a high fat diet known to produce multiple changes in insulin secretion, insulin action and levels of circulating adipokines, independent metabolic parameters that may influence the skeleton. Furthermore, Sitagliptin produces partial but incomplete reduction of DPP-4 activity, whereas DPP-4 knockout mice exhibit complete disruption of enzyme activity. Additionally, Sitagliptin-treated mice were subjected to the effects of partial DPP-4 inhibition at four months of age whereas DPP-4 knockout mice have never possessed the enzyme. It could be assumed that the degree of compensatory mechanisms is likely more developed in knockout mice than in treated mice. In short, it appears that genetic DPP-4 inactivation does not appear to produce a significant bone phenotype in male and female mice but does produce significant structural effects in female knockout mice after OVX. OVX knockout mice experienced reductions in parameters largely affected by size, such as femoral and vertebral abmd evaluated by DEXA and structural properties of three-point bending and femoral neck fracture. These changes could be reflective of smaller DPP-4 knockout mice following OVX. 159
174 6.3 DPP-4 Inhibition on Bone The motivation for examining the effects of DPP-4 inhibition on the skeleton was largely based on studies that have suggested positive skeletal effects from the enteric hormones GIP, GLP-1 and GLP-2 [14-22]. The effects seen from both Sitagliptin treatment and DPP-4 knockout have been interpreted based on the above mentioned enteric hormone studies. However, several DPP-4 substrates exist with the potential to directly or indirectly affect bone quality. Unexpected effects in this study may be attributable to the many DPP-4 substrates and downstream signaling pathways that might be affected from DPP-4 inhibition. For example, DPP-4 cleaves the proinflammatory chemokine SDF-1 which has been shown to increase matrix metallopeptidase-9 (MMP-9), a matrix-degrading enzyme essential for osteoclast precursor cells. A study by Yu et al., suggests that SDF-1, through increases in MMP-9, may be a key signal for the selective attraction of circulating osteoclast precursors into bone as well as their development and function [206]. It could be possible that increased levels of intact SDF-1, due to DPP-4 inhibition, could destabilize its role in osteoclast precursor recruitment, development, and function. Additionally, DPP-4 -/- mice have been shown to have higher levels of circulating substance P (~2 fold) [205] and addition of substance P to cultured osteoclasts has been shown to enhance their bone resorptive activity [207]. Furthermore, DPP-4 has been shown to preferentially bind collagen, which may be important for its function [208]. A study by Jost et al., indicates that collagen trimming by DPP-4 is prevented following chemical DPP-4 inhibition, resulting in alterations in collagen metabolism in vivo. The authors encourage monitoring patients receiving DPP-4 inhibitor treatment to ensure no adverse effects from altered collagen metabolism [209]. Altered collagen metabolism could negatively affect bone by reducing the quality of the collagen network, which can affect toughness and bone strength [71, 72]. Finally, peptide YY (PYY) is a known physiological substrate of DPP- 4 and intact levels of PYY were increased due to Sitagliptin treatment in T2DM patients [210]. 160
175 PYY plays a critical role in regulating bone mass as PYY deficient mice displayed reductions in trabecular bone mass and bone strength [211]. Improved levels of intact PYY due to inhibition or inactivation of DPP-4 may also play a role in any skeletal phenotypes noted in this study. These studies serve to highlight the multitude of pathways though which DPP-4 can affect bone in addition to potentiated enteric hormones GIP, GLP-1 and GLP-2. It is also difficult to predict changes that should theoretically occur from DPP-4 inhibition. For instance, growth hormone-releasing hormone (GHRH) was one of the first peptides demonstrated as a substrate for DPP-4. It was predicted that increased levels of intact GHRH would stimulate growth hormone secretion and increase circulating levels of insulin-like growth factor (IGF)-1 and somatic growth. However, DPP-4 -/- mice do not exhibit increased body size or organ growth and Sitagliptin treatment in pigs that produced 90% inhibition of plasma DPP-4 activity was not associated with alterations in the circulating concentrations of IGF-1 [131]. Additionally, a study by Aaboe et al., found no changes in intact GLP-2 levels from 12 weeks of Sitagliptin treatment despite GLP-2 being a known substrate for DPP-4 [210]. These factors make it difficult to interpret or predict skeletal changes from DPP-4 inhibition based on downstream effects or potential DPP-4 substrates. 161
176 6.4 Methodological Limitations There are several limitations to the experimental techniques used for the evaluation of bone quality. The limitations of these techniques should be taken into consideration when interpreting the results. Understanding some of these methodological issues may help to explain unexpected findings and to help limit any shortcomings when discussing future work. Despite the limitations, the techniques used in the evaluation of bone quality are commonly accepted techniques in this field of study Mice: In the anti-diabetic drug study, Pioglitazone and Sitagliptin were given at specific doses in animal chow and mice were under ad libitum (free-feeding) conditions. This leads to variability in the amount of drug each mouse is ingesting because of differences in food intake which are affected by a multitude of variables including the social environment, the position of the cage on the rack, temperature, and light:dark cycles [ ]. Because the drug is delivered to mice via supplemented food, drug ingestion will occur multiple times a day based on the grazing feeding patterns of mice. A review of feeding patterns in mice fed ad libitum found that the number of meals ingested in a 24-hour period varied from 2 to 50 [213]. This means that drugs are not given in a once-a-day manner as treatment usually occurs in humans and the amount of exposure to the drug will vary slightly from mouse to mouse. Additionally, it has been noted that C57BL/6 mice exhibit varying feeding stages over time on a high fat diet. Specifically, high-fat fed C57BL/6 mice exhibit a reduced food intake stage after 5 weeks of high-fat feeding followed by an increased food intake stage after 8 weeks of high-fat feeding [215]. This means that the drug doses the mice are ingesting will be slightly above or below therapeutic doses based on feeding stages and 162
177 patterns. Changes in food intake result in increased variability in drug intake over time and with respect to each mouse. Because of the number of mice involved in this study, mice were bred, aged and housed at various time points, making it necessary to collect mice at different intervals. Although housing conditions are strictly monitored and maintained at consistent conditions, slight variability can occur on a microenvironmental scale throughout the year. Variation can exist in factors that can have physiological effects such as temperature, sound and chow. This variability was limited in the anti-diabetic drug study since treated mice and their vehicle controls were ensured to be bred and housed within similar periods to one another. However, the genetic inactivation study involved breeding and rearing small batches of knockout and wildtype mice over the course of two years in order to attain full group sizes. Mice were sacrificed in small groups at a time (6-15 mice) and bones were excised and stored in either fixative (ethanol or formalin) or in saline-soaked gauze in a -20,C freezer. As such, not all samples within and between groups were necessarily stored for the same period of time before testing. Nevertheless, research has shown that freezer storage of well-hydrated bone specimens does not adversely affect mechanical properties [65, 216, 217]. Ovariectomy (OVX) in rodents is a commonly used animal model for studying osteoporosis [153, 154]. This involves surgical removal of the ovaries, the estrogen-producing gonads and primary sex organ of female mice. Removal of the ovaries by OVX results in a steep decline in estrogen production, producing the skeletal effects typically seen from menopause in women [64]. Typically, OVX mice are compared to sham-control mice, which are mice that underwent the similar conditions of surgery but without removal of the ovaries. Shams are used in OVX studies in order to control for the effects of surgery (anesthesia, stress, recovery etc) on the 163
178 mouse. However, shams were not used in either the anti-diabetic drug or genetic inactivation study but the effects of OVX on bone were validated by comparison with intact female mice Dual Energy X-Ray Absorptiometry (DEXA) The most obvious limitation to this technique is that its density measurement is based on area rather than volume when calculating BMD. As such, areal BMD (abmd) is not sensitive to changes in the axis parallel to the direction of x-ray projection (specimen thickness). An areal measurement of BMD makes it susceptible to alterations in sample geometry and orientation during the scan. It is therefore critical to position the bones in a consistent manner from scan to scan which can be difficult because of the complex geometry of excised bones. However, care was taken to consistently position specimens and a plate with a grid pattern was used to ensure their position relative to one another in the machine was always consistent. Additionally, there is an inverse relationship between bone size and precision and accuracy of DEXA measurements where smaller bones limit the use of DEXA [218]. It is important to note that the manufacturer recommends the use of whole body BMD in intact animals because greater variability occurs when measuring ex vivo specimens. The variability noted when measuring abmd and BMC of ex vivo specimens may be due to a greater variability in excision and cleaning of bone specimens, resulting in a variable amount of soft tissue which may influence BMC values [219]. However, excised bones are often measured by DEXA and have been shown to be comparable to BMD measurements in in vivo specimens [219]. Overall, DEXA is a widely used and accepted technique [220]. 164
179 6.4.3 Three-Point Bending Three-point bending of murine femora is performed despite major issues due to inherent femoral geometry and size. Firstly, an ideal gauge length should be 16 times the thickness of the specimen in order to ensure failure is due to bending (tensile/compressive) and not a result of shear forces. However, this gauge-length-to-specimen-width ratio is not possible in bending of whole mouse femora. Even with this limitation, mechanical testing is performed similarly for all specimens and results are always compared between two experimental groups. Secondly, the assumption that the cortical bone shaft is homogenous in geometry and intrinsic material properties is made when applying normalization formulae (Equations 1 and 2). However, bone is a natural composite material with variations in the material properties and cross-sectional area along the shaft. This heterogeneity cannot be avoided but the segment between the 6 mm gauges has been shown to have the minimal amount of variation in cross-sectional area. Nonetheless, mechanical testing and normalization techniques are consistent and our statistical comparisons are relative between groups making these differences less critical. Despite these limitations, threepoint bending of whole mouse femora is an acceptable and widespread technique used to assess skeletal fragility [64, 65] Vertebral Compression Vertebral compression is a clinically relevant mechanical test as it simulates the common vertebral fractures seen in individuals with osteoporosis. However, the small size of mouse vertebrae presents a difficult challenge in handling and mechanical testing. Firstly, vertebral processes must be removed prior to testing which involves manually cutting them from the vertebral body. Their removal can result in microcracks and defects in the vertebral body that can lead to potentially inferior mechanical integrity. Furthermore, the precise set-up of vertebrae can 165
180 be difficult to achieve. It is important that the top and bottom of the vertebral body are parallel to one another and that the vertebra is upright and perpendicular to the upper and lower mechanical plates. The intervertebral discs are removed as close to the bone as possible to ensure the top and bottom of the vertebral body are flat to one another. Vertebrae are held upright on a plate by use of a cyanoacrylate-based adhesive. However, difficulties in bonding between the moist vertebral body and the adhesive can occur leading to shifting of the sample once in contact with the upper mechanical plate. Once the vertebral body is in an upright position, a specific amount of time is required to ensure the adhesive is dry in order to prevent movement during the test. As such, it was difficult to keep the vertebral body moist between the time after setting the specimen in adhesive and running the test. Care was taken to ensure the set-up and testing was run as consistently as possible and any unexpected occurrences were noted. Moreover, the use of a sufficient sample size helped to decrease the variation in measurements. Additionally, the assumption is made that the vertebral body is a solid structure when applying normalization formulae. Since the vertebral body is porous, the actual vertebral body volume is less than is estimated from the geometrical properties assessed per sample. Therefore, the normalized properties from vertebral compression are underestimations of the true properties. Despite these limitations, vertebral compression is an accepted method in this field of study [164, 187, 221, 222] Femoral Neck Fracture: The femoral neck fracture test involves embedding the proximal femur in a polymethyl methacrylate (PMMA) material. Methodological issues with this test can arise from inadequate bonding between the potting material and the moist bone sample, which could result in shifting upon initial application of the load. Moreover, it is difficult to ensure the femoral shaft is properly aligned to the direction of the applied load. Also, the complex geometry of the femoral neck 166
181 prevent this test from being normalized. Nevertheless, care was taken to minimize discrepancies and the femoral neck fracture is still a widely accepted, clinically-relevant mechanical test [164, 187, 223, 224] Histomorphometry and Strut Analysis: A major limitation to histomorphometry and strut analysis is that these techniques measure bone properties in only two dimensions. Nevertheless, these approaches are widely used and strong correlations have been found between 2D assessment tools and newer 3D tools [49, 225]. The most challenging issue with these techniques is ensuring that the location for 2D analysis is taken at equivalent depths within the bone from sample to sample. This is often difficult to achieve during the sectioning process and is only validated by visual inspection. However, care was taken to ensure relative consistencies and an appropriate sample size is always used to decrease variability. Additionally, the area of measurement for histomorphometry and strut analysis is user defined and is therefore subject to some variation between samples. To maintain consistency, analysis of histomorphometric sections was always limited to eight fields within the outer cortex. For strut analysis, a mask was manually drawn to always exclude the outer cortex and growth plates Micro-Computed Tomography: Some methodological issues with respect to Micro-CT should be taken into account when interpreting results. Firstly, the location of the specimen within the microfuge tube could potentially influence BMD measurements. This is mostly due to curvature near the tip of the tube that could irregularly diffract X-ray projections so care was taken to ensure that specimens were not located near that region. Secondly, a contoured region of interest (ROI) is created by the user 167
182 in order to exclude the outer cortical shell when analyzing trabecular structure and vertebral BMD. Variations in the positioning of the ROI may create some variation between samples. Care was taken to ensure that each ROI was consistently drawn with a similar distance from the outer cortical shell and growth plates. The values from Micro-CT are often used to validate results obtained from vertebral compression. It should be taken into account that the L5 vertebra was scanned for Micro-CT but the L6 vertebra was used for mechanical testing. Additionally, vertebral compression is largely thought to test trabecular bone despite the outer cortical shell which can largely influence results from the test. Vertebral vbmd from Micro-CT is calculated from the assigned ROI and therefore does not include mineral from the outer cortical shell. One study found the total vertebral BMD (trabecular and cortical shell) was a significantly better determinant of mechanical integrity than trabecular or cortical vertebral BMD [226]. 168
183 CHAPTER 7: CONCLUSIONS 169
184 7. Introduction: This thesis represents the first study to examine the effects of TZD treatment, using Pioglitazone, and DPP-4 inhibitor treatment, using Sitagliptin, in a glucose intolerant mouse model. Additionally, this thesis was the first to characterize the effects of genetic DPP-4 inactivation on bone quality using a DPP-4 knockout mouse model. Bone quality was evaluated through densitometry, mechanical testing and techniques used to assess remodeling, structural and material properties. 7.1 Conclusions from the Anti-Diabetic Drug Study Pioglitazone treatment on Bone Quality Pioglitazone treatment led to adverse effects on the vertebral mechanics of glucose intolerant male, female and OVX mice. Male mice exhibited the greatest effect due to Pioglitazone treatment with reductions seen in vertebral vbmd, trabecular architecture, trabecular connectivity and bone formation. Additionally, Pioglitazone treatment resulted in increased bone marrow adiposity for all treated mice. These results indicate that TZD treatment by Pioglitazone adversely affects trabecular bone quality in male, female and OVX mice Sitagliptin treatment on Bone Quality Sitagliptin treatment resulted in improvements in the vertebral vbmd and trabecular architecture of female mice as well as increased mineralization in male, female and OVX mice. However, Sitagliptin treatment had a largely neutral effect on mechanical properties in all mice. These results indicate that DPP-4 inhibitor treatment has a slight positive effect in female mice that was lost following ovariectomy. 170
185 7.2 Conclusions from the Genetic Inactivation Study Genetic inactivation of DPP-4 had a minimal effect in male and female mice but produced negative effects on the femoral geometry and mechanical properties in OVX female mice. The negative changes seen in OVX KO mice relate to reduced femoral size and suggest that genetic inactivation of DPP-4 may affect bone growth in female mice in the absence of estrogen. 171
186 CHAPTER 8: FUTURE WORK 172
187 8. Introduction: The purpose of this project was to evaluate the effects of two anti-diabetic drugs on bone quality and characterizing a bone phenotype in a DPP-4 knockout mouse model. Several other bone properties, as well as variations in the mouse model, can be investigated in order to help further define the skeletal effects due to TZD or DPP-4 inhibitor treatment and genetic inactivation of DPP Future Areas of Investigation: the Effects of Pioglitazone and Sitagliptin on Bone Quality: The anti-diabetic drug study found adverse effects on the trabecular bone in mice treated with Pioglitazone. Adverse effects due to Pioglitazone treatment were expected given the research regarding TZD use and the skeleton [5-11, 111, 116, 118, 119, 123]. However, the intensity of skeletal effects was greater in male mice than in female and OVX female mice which does not agree with previous literature [5, 9-11]. This finding could be an issue with the drug-supplemented chow used to treat mice in this study (see methodological limitations). This form of treatment is largely performed in research studies however, it does produce variability in terms of the feeding patterns from mouse to mouse. The grazing feeding patterns of mice also subject them to small portions of drug over the course of their feeding period instead of a specific therapeutic dose once a day as seen in humans. Oral gavage as a treatment method provides the advantage of treating animals with a guaranteed dose at a standardized time each day. However, several disadvantages with the gavage method include oesophageal or stomach damage, intubation of the lungs and added stress to the animal. Additionally, the gavage dosing of animals is also very labour-intensive [227]. The Jello dosing method was first examined as a vehicle for the effective dosing of rats with buprenorphine [228]. This method would involve dissolving a specific quantity of drug in a small amount of jello that would solidify and be given to each animal on a 173
188 daily basis. Its major advantage is that an effective dose can be guaranteed in each animal as well as at a standardized time each day. It is also considered a stress-free means of administering compounds to animals [229]. It might be advantageous to include this type of dosing in future studies that examine the effects of Pioglitazone and Sitagliptin on bone quality. The effects due to TZD treatment and to a lesser degree, Pioglitazone treatment, on the skeleton have been previously investigated in glucose homeostatic animals [ , 124]. More specifically, these studies did not use diabetic models to assess the effects of TZD or Pioglitazone treatment on the skeleton. The use of non-diabetic animals is important when initially examining the skeletal effects of these drugs because changes seen from treatment can be more likely attributed to the drug mechanism and not secondary effects (insulin and glucose control) from treatment. However, it is also important to assess the skeletal effects of these drugs in a diabetic model because this is more representative of their usage in patients. Pioglitazone treatment in the glucose intolerant mice of this study is important since it is a better representation of the physiological condition occurring in humans receiving Pioglitazone treatment. On the other hand, investigations into the skeletal effects of Sitagliptin treatment in non-diabetic models are fairly limited [186]. Examining the effects of Sitagliptin treatment in non-diabetic animals may be an important step in further understanding the role that chemical DPP-4 inhibition may play on the skeleton. Another route for future work would be to study the longitudinal effects of Pioglitazone and Sitagliptin on bone quality. Lazarenko et al found differences in the effects of Rosiglitazone in young (1 month), adult (6 month), and aged (24 months) mouse models [116]. It would be interesting to examine the effects of Pioglitazone treatment on various age groups since each 174
189 TZD has a distinctive effect on the skeleton. This may also be beneficial for Sitagliptin treatment to determine if age or bone phase plays a role in the skeletal effects of treatment. 8.2 Future Areas of Investigation: The Effects of Genetic Inactivation of DPP-4 on Bone Quality: The genetic inactivation study did not observe a major bone phenotype from inactivation of DPP-4 in the knockout mouse model. It would be important to investigate whether a bone phenotype might develop due to genetic DPP-4 inactivation if a challenged mouse model was used. DPP-4 knockout mice are resistant to high-fat diet and/or diabetogenic doses of streptozotocin [139, 145] but higher streptozotocin doses could produce a pre-diabetic phenotype. Such a study may be important in understanding the skeletal effects DPP-4 inhibition might have in diabetic patients as it would better mimic the multiple physiological conditions from the disease in these individuals. Additionally, it would be beneficial to perform a longitudinal study investigating the bone phenotype in young, adult and aged DPP-4 knockout mice. A bone phenotype from DPP-4 inactivation may vary with respect to murine bone phases (rapid growth phase, peak bone phase, bone loss phase). 175
190 References: [1] "Canadian Community Health Survey: Nutrition (CCHS) " Cycle 2.2, [2] "Clinical practice guidelines for the prevention and management of diabetes in Canada," 2008 Canadian Journal of Diabetes, 38. [3] L. C. Hofbauer, C. C. Brueck, S. K. Singh et al., Osteoporosis in patients with diabetes mellitus, J Bone Miner Res, vol. 22, no. 9, pp , Sep, [4] A. V. Schwartz, D. E. Sellmeyer, E. Vittinghoff et al., Thiazolidinedione use and bone loss in older diabetic adults, J Clin Endocrinol Metab, vol. 91, no. 9, pp , Sep, [5] A. Grey, Skeletal consequences of thiazolidinedione therapy, Osteoporos Int, vol. 19, no. 2, pp , Feb, [6] A. Grey, M. Bolland, G. Gamble et al., The peroxisome proliferator-activated receptorgamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial, J Clin Endocrinol Metab, vol. 92, no. 4, pp , Apr, [7] S. E. Kahn, B. Zinman, J. M. Lachin et al., Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT), Diabetes Care, vol. 31, no. 5, pp , May, [8] A. Lau, and W. Harper, Thiazolidinediones and their effect on bone metabolism: A Review, Canadian Journal of Diabetes, vol. 31, no. 4, pp , [9] C. Meier, M. E. Kraenzlin, M. Bodmer et al., Use of thiazolidinediones and fracture risk, Arch Intern Med, vol. 168, no. 8, pp , Apr 28, [10] A. V. Schwartz, Diabetes Mellitus: Does it Affect Bone?, Calcif Tissue Int, vol. 73, no. 6, pp , Dec, [11] Takeda, "Observation of an increased incidence of fractures in female patients who received long-term treatment with ACTOS (Pioglitazone HCL) tablets for type 2 diabetes mellitus (Letter to Health Care Providers)," Accessed , 2007]. [12] R. J. Bollag, Q. Zhong, K. H. Ding et al., Glucose-dependent insulinotropic peptide is an integrative hormone with osteotropic effects, Mol Cell Endocrinol, vol. 177, no. 1-2, pp , May 25,
191 [13] R. J. Bollag, Q. Zhong, P. Phillips et al., Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors, Endocrinology, vol. 141, no. 3, pp , Mar, [14] J. A. Clowes, S. Khosla, and R. Eastell, Potential role of pancreatic and enteric hormones in regulating bone turnover, J Bone Miner Res, vol. 20, no. 9, pp , Sep, [15] K. V. Haderslev, P. B. Jeppesen, B. Hartmann et al., Short-term administration of glucagon-like peptide-2. Effects on bone mineral density and markers of bone turnover in short-bowel patients with no colon, Scand J Gastroenterol, vol. 37, no. 4, pp , Apr, [16] D. B. Henriksen, P. Alexandersen, N. H. Bjarnason et al., Role of gastrointestinal hormones in postprandial reduction of bone resorption, J Bone Miner Res, vol. 18, no. 12, pp , Dec, [17] D. B. Henriksen, P. Alexandersen, B. Hartmann et al., Four-month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD, Bone, vol. 45, no. 5, pp , Nov, [18] Y. Lamari, C. Boissard, M. S. Moukhtar et al., Expression of glucagon-like peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagon-like peptide 1, FEBS Lett, vol. 393, no. 2-3, pp , Sep 16, [19] K. Tsukiyama, Y. Yamada, C. Yamada et al., Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion, Mol Endocrinol, vol. 20, no. 7, pp , Jul, [20] D. Xie, H. Cheng, M. Hamrick et al., Glucose-dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover, Bone, vol. 37, no. 6, pp , Dec, [21] C. Yamada, Y. Yamada, K. Tsukiyama et al., The murine glucagon-like peptide-1 receptor is essential for control of bone resorption, Endocrinology, vol. 149, no. 2, pp , Feb, [22] Q. Zhong, T. Itokawa, S. Sridhar et al., Effects of glucose-dependent insulinotropic peptide on osteoclast function, Am J Physiol Endocrinol Metab, vol. 292, no. 2, pp. E543-8, Feb, [23] W. S. Jee, "Integrated bone tissue physiology: Anatomy and physiology," Bone Mechanics Handbook, S. Cowin, ed., pp , Boca Raton, Florida: CRC Press LLC,
192 [24] P. Shipman, A. Walker, and D. Bichell, The Human Skeleton, Massachusetts, USA Harvard University Press, [25] S. Viguet-Carrin, P. Garnero, and P. D. Delmas, The role of collagen in bone strength, Osteoporos Int, vol. 17, no. 3, pp , [26] E. Bonucci, "Basic Composition and Structure of Bone," Mechanical Testing of Bone and the Bone - Implant Interface, Y. An and R. Draughn, eds., p. 624, Boca Raton, Florida: CRC Press LLC, [27] F. Lee, "Free e-books Eduction Website: Web Books Publishing," Medicine - Physiology, [28] T. Tramelle, "IndyspineMD Lumabr Spinal Anatomy:," 2006]. [29] J. M. Crolet, B. Aoubiza, and A. Meunier, Compact bone: numerical simulation of mechanical characteristics, J Biomech, vol. 26, no. 6, pp , Jun, [30] J. Y. Rho, L. Kuhn-Spearing, and P. Zioupos, Mechanical properties and the hierarchical structure of bone, Med Eng Phys, vol. 20, no. 2, pp , Mar, [31] A. Singh, Bone and Spine Education Resource, [32] A. Boskey, "Bone Mineralization," Bone Mechanics Handbook, S. Cowen, ed., pp , Boca Raton, Florida: CRC Press LLC, [33] K. von der Mark, "Components of the organic extracellular matrix of bone and cartilage: Structure and biosynthesis of colalgens," Dynamics of Bone and Cartilage Metabolism, M. Seibel, S. Robins and J. Bilezikian, eds., pp. 3-18: Elsevier Inc., [34] S. Jr Marks, and D. Hermey, "The structure and development of bone," Principles of Bone Biology, J. R. Bilezikian, L. and C. Rosen, eds., pp. 3-16, California, USA: Academic Press, [35] S. C. Manolagas, Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis, Endocr Rev, vol. 21, no. 2, pp , Apr, [36] R. Majeska, "Cell biology of bone," Bone Mechanics Handbook, S. Cowin, ed., pp , Boca Raton, Florida: CRC Press LLC, [37] C. Newvine, "Bone Remodeling Cycle," 2005]. 178
193 [38] C. Cooper, Epidemiology of Osteoporosis, Osteoporosis International, vol. 9, no. 8, pp. S2- S8, [39] D. L. Glaser, and F. S. Kaplan, Osteoporosis. Definition and clinical presentation, Spine (Phila Pa 1976), vol. 22, no. 24 Suppl, pp. 12S-16S, Dec 15, [40] J. Kanis, Diagnosis of Osteoporosis and Fracture Risk, Lancet: Osteoporosis III, vol. 359, pp , [41] B. L. Riggs, S. Khosla, and L. J. Melton, 3rd, A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men, J Bone Miner Res, vol. 13, no. 5, pp , May, [42] P. Garnero, E. Sornay-Rendu, M. C. Chapuy et al., Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis, J Bone Miner Res, vol. 11, no. 3, pp , Mar, [43] P. Garnero, E. Sornay-Rendu, B. Claustrat et al., Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study, J Bone Miner Res, vol. 15, no. 8, pp , Aug, [44] P. R. Ebeling, M. E. Sandgren, E. P. DiMagno et al., Evidence of an age-related decrease in intestinal responsiveness to vitamin D: relationship between serum 1,25- dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women, J Clin Endocrinol Metab, vol. 75, no. 1, pp , Jul, [45] K. S. Tsai, H. Heath, 3rd, R. Kumar et al., Impaired vitamin D metabolism with aging in women. Possible role in pathogenesis of senile osteoporosis, J Clin Invest, vol. 73, no. 6, pp , Jun, [46] S. Boonen, D. Vanderschueren, X. G. Cheng et al., Age-related (type II) femoral neck osteoporosis in men: biochemical evidence for both hypovitaminosis D- and androgen deficiency-induced bone resorption, J Bone Miner Res, vol. 12, no. 12, pp , Dec, [47] S. R. Cummings, and L. J. Melton, Epidemiology and outcomes of osteoporotic fractures, Lancet, vol. 359, no. 9319, pp , May 18, [48] Z. Orlic, and L. Raisz, Causes of secondary osteoporosis, Journal of Clinical Densitometry, vol. 2, no. 1, pp , [49] A. M. Parfitt, M. K. Drezner, F. H. Glorieux et al., Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR 179
194 Histomorphometry Nomenclature Committee, J Bone Miner Res, vol. 2, no. 6, pp , Dec, [50] "Centers for Disease Control and Prevention (CDCP)," [51] C. H. Turner, Determinants of skeletal fragility and bone quality, J Musculoskelet Neuronal Interact, vol. 2, no. 6, pp , Dec, [52] E. Seeman, Pathogenesis of bone fragility in women and men, Lancet, vol. 359, no. 9320, pp , May 25, [53] S. Cowin, "Mechanics of Materials," Bone Mechanics Handbook, S. Cowin, ed., pp , Boca Raton, Florida: CRC Press LLC, [54] D. Felsenberg, and S. Boonen, The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management, Clin Ther, vol. 27, no. 1, pp. 1-11, Jan, [55] P. Ammann, R. Rizzoli, J. M. Meyer et al., Bone density and shape as determinants of bone strength in IGF-I and/or pamidronate-treated ovariectomized rats, Osteoporos Int, vol. 6, no. 3, pp , [56] T. Hansson, B. Roos, and A. Nachemson, The bone mineral content and ultimate compressive strength of lumbar vertebrae, Spine (Phila Pa 1976), vol. 5, no. 1, pp , Jan-Feb, [57] I. Leichter, J. Y. Margulies, A. Weinreb et al., The relationship between bone density, mineral content, and mechanical strength in the femoral neck, Clin Orthop Relat Res, vol. 163, no. 163, pp , Mar, [58] D. Marshall, O. Johnell, and H. Wedel, Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures, Bmj, vol. 312, no. 7041, pp , May 18, [59] B. Ettinger, D. M. Black, B. H. Mitlak et al., Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators, Jama, vol. 282, no. 7, pp , Aug 18, [60] M. C. Hochberg, S. Greenspan, R. D. Wasnich et al., Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents, J Clin Endocrinol Metab, vol. 87, no. 4, pp , Apr,
195 [61] B. L. Riggs, and L. J. Melton, 3rd, Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density, J Bone Miner Res, vol. 17, no. 1, pp. 11-4, Jan, [62] P. Ammann, R. Rizzoli, and J. P. Bonjour, "Preclinical evaluation of new therapeutic agents for osteoporosis," Osteoporosis: Diagnosis and Management, P. J. Meunier, ed., pp , London: Muntin Dunitz, [63] R. Balena, B. C. Toolan, M. Shea et al., The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates, J Clin Invest, vol. 92, no. 6, pp , Dec, [64] C. Turner, and D. Burr, "Experimental techniques for bone mechanics," Bone Mechanics Handbook, S. Cowin, ed., pp , Boca Raton, Florida: CRC Press LLC, [65] C. H. Turner, and D. B. Burr, Basic biomechanical measurements of bone: a tutorial, Bone, vol. 14, no. 4, pp , Jul-Aug, [66] B. Cortet, and X. Marchandise, Bone microarchitecture and mechanical resistance, Joint Bone Spine, vol. 68, pp , [67] R. W. McCalden, J. A. McGeough, and C. M. Court-Brown, Age-related changes in the compressive strength of cancellous bone. The relative importance of changes in density and trabecular architecture, J Bone Joint Surg Am, vol. 79, no. 3, pp , Mar, [68] L. Mosekilde, C. C. Danielsen, and J. Gasser, The effect on vertebral bone mass and strength of long term treatment with antiresorptive agents (estrogen and calcitonin), human parathyroid hormone-(1-38), and combination therapy, assessed in aged ovariectomized rats, Endocrinology, vol. 134, no. 5, pp , May, [69] J. S. Thomsen, E. N. Ebbesen, and L. Mosekilde, Predicting human vertebral bone strength by vertebral static histomorphometry, Bone, vol. 30, no. 3, pp , Mar, [70] D. B. Burr, The contribution of the organic matrix to bone's material properties, Bone, vol. 31, no. 1, pp. 8-11, Jul, [71] X. Wang, R. A. Bank, J. M. TeKoppele et al., Effect of collagen denaturation on the toughness of bone, Clin Orthop Relat Res, vol. 371, no. 371, pp , Feb, [72] X. Wang, X. Shen, X. Li et al., Age-related changes in the collagen network and toughness of bone, Bone, vol. 31, no. 1, pp. 1-7, Jul,
196 [73] H. Oxlund, M. Barckman, G. Ortoft et al., Reduced concentrations of collagen crosslinks are associated with reduced strength of bone, Bone, vol. 17, no. 4 Suppl, pp. 365S- 371S, Oct, [74] J. J. Tomasek, S. W. Meyers, J. B. Basinger et al., Diabetic and age-related enhancement of collagen-linked fluorescence in cortical bones of rats, Life Sci, vol. 55, no. 11, pp , [75] D. Vashishth, G. J. Gibson, J. I. Khoury et al., Influence of nonenzymatic glycation on biomechanical properties of cortical bone, Bone, vol. 28, no. 2, pp , Feb, [76] A. R. Hayman, A. J. Bune, J. R. Bradley et al., Osteoclastic tartrate-resistant acid phosphatase (Acp 5): its localization to dendritic cells and diverse murine tissues, J Histochem Cytochem, vol. 48, no. 2, pp , Feb, [77] R. Erben, "Bone-labeling techniques," Handbook of Histology Methods for Bone and Cartilage, Y. M. An, Kylie, ed., pp , Totowa, New Jersey: Humana Press, [78] K. G. Alberti, and P. Z. Zimmet, Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation, Diabet Med, vol. 15, no. 7, pp , Jul, [79] R. J. Mahler, and M. L. Adler, Clinical review 102: Type 2 diabetes mellitus: update on diagnosis, pathophysiology, and treatment, J Clin Endocrinol Metab, vol. 84, no. 4, pp , Apr, [80] P. Froguel, and G. Velho, Genetic determinants of type 2 diabetes, Recent Prog Horm Res, vol. 56, pp , [81] S. E. Kahn, The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes, Diabetologia, vol. 46, no. 1, pp. 3-19, Jan, [82] J. Leahy, "Pathogenesis of type 2 diabetes mellitus," Type 2 Diabetes Mellitus, M. Feinglos and M. A. Bethel, eds., pp , Totowa, New Jersey: Herman Press. Springer Science and Business, [83] H. Freeman, and R. D. Cox, Type-2 diabetes: a cocktail of genetic discovery, Hum Mol Genet, vol. 15 Spec No 2, no. 2, pp. R202-9, Oct 15, [84] M. T. Malecki, Genetics of type 2 diabetes mellitus, Diabetes Res Clin Pract, vol. 68 Suppl1, no. 21, pp. S10-21, Jun,
197 [85] G. K. Reddy, L. Stehno-Bittel, S. Hamade et al., The biomechanical integrity of bone in experimental diabetes, Diabetes Res Clin Pract, vol. 54, no. 1, pp. 1-8, Oct, [86] J. C. Krakauer, M. J. McKenna, N. F. Buderer et al., Bone loss and bone turnover in diabetes, Diabetes, vol. 44, no. 7, pp , Jul, [87] M. Munoz-Torres, E. Jodar, F. Escobar-Jimenez et al., Bone mineral density measured by dual X-ray absorptiometry in Spanish patients with insulin-dependent diabetes mellitus, Calcif Tissue Int, vol. 58, no. 5, pp , May, [88] A. Rozadilla, J. M. Nolla, E. Montana et al., Bone mineral density in patients with type 1 diabetes mellitus, Joint Bone Spine, vol. 67, no. 3, pp , [89] L. Forsen, H. E. Meyer, K. Midthjell et al., Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trondelag Health Survey, Diabetologia, vol. 42, no. 8, pp , Aug, [90] J. Miao, K. Brismar, O. Nyren et al., Elevated hip fracture risk in type 1 diabetic patients: a population-based cohort study in Sweden, Diabetes Care, vol. 28, no. 12, pp , Dec, [91] A. Rakel, O. Sheehy, E. Rahme et al., Osteoporosis among patients with type 1 and type 2 diabetes, Diabetes Metab, vol. 34, no. 3, pp , Jun, [92] E. M. Dennison, H. E. Syddall, A. Aihie Sayer et al., Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: evidence for an indirect effect of insulin resistance?, Diabetologia, vol. 47, no. 11, pp , Nov, [93] K. J. Ottenbacher, G. V. Ostir, M. K. Peek et al., Diabetes mellitus as a risk factor for stroke incidence and mortality in Mexican American older adults, J Gerontol A Biol Sci Med Sci, vol. 59, no. 6, pp. M640-5, Jun, [94] E. S. Strotmeyer, J. A. Cauley, A. V. Schwartz et al., Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study, Arch Intern Med, vol. 165, no. 14, pp , Jul 25, [95] P. L. van Daele, R. P. Stolk, H. Burger et al., Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study, Ann Intern Med, vol. 122, no. 6, pp , Mar 15, [96] K. K. Nicodemus, and A. R. Folsom, Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women, Diabetes Care, vol. 24, no. 7, pp , Jul,
198 [97] M. J. Bridges, S. H. Moochhala, J. Barbour et al., Influence of diabetes on peripheral bone mineral density in men: a controlled study, Acta Diabetol, vol. 42, no. 2, pp. 82-6, Jun, [98] J. L. Perez-Castrillon, D. De Luis, J. C. Martin-Escudero et al., Non-insulin-dependent diabetes, bone mineral density, and cardiovascular risk factors, J Diabetes Complications, vol. 18, no. 6, pp , Nov-Dec, [99] M. Wakasugi, R. Wakao, M. Tawata et al., Bone mineral density measured by dual energy x-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus, Bone, vol. 14, no. 1, pp , [100] S. M. Haffner, and R. L. Bauer, The association of obesity and glucose and insulin concentrations with bone density in premenopausal and postmenopausal women, Metabolism, vol. 42, no. 6, pp , Jun, [101] C. Ribot, F. Tremollieres, J. M. Pouilles et al., Obesity and postmenopausal bone loss: the influence of obesity on vertebral density and bone turnover in postmenopausal women, Bone, vol. 8, no. 6, pp , [102] C. J. Rosen, and M. L. Bouxsein, Mechanisms of disease: is osteoporosis the obesity of bone?, Nat Clin Pract Rheumatol, vol. 2, no. 1, pp , Jan, [103] P. K. Dixit, and R. A. Ekstrom, Decreased breaking strength of diabetic rat bone and its improvement by insulin treatment, Calcif Tissue Int, vol. 32, no. 3, pp , [104] W. G. Goodman, and M. T. Hori, Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization, Diabetes, vol. 33, no. 9, pp , Sep, [105] K. M. Thrailkill, C. K. Lumpkin, Jr., R. C. Bunn et al., Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues, Am J Physiol Endocrinol Metab, vol. 289, no. 5, pp. E735-45, Nov, [106] K. Fulzele, R. C. Riddle, D. J. DiGirolamo et al., Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition, Cell, vol. 142, no. 2, pp , Jul 23, [107] M. Saito, K. Fujii, Y. Mori et al., Role of collagen enzymatic and glycation induced crosslinks as a determinant of bone quality in spontaneously diabetic WBN/Kob rats, Osteoporos Int, vol. 17, no. 10, pp , Oct, [108] E. D'Erasmo, D. Pisani, A. Ragno et al., Calcium homeostasis during oral glucose load in healthy women, Horm Metab Res, vol. 31, no. 4, pp , Apr,
199 [109] A. M. Parfitt, J. C. Gallagher, R. P. Heaney et al., Vitamin D and bone health in the elderly, Am J Clin Nutr, vol. 36, no. 5 Suppl, pp , Nov, [110] K. C. Baynes, B. J. Boucher, E. J. Feskens et al., Vitamin D, glucose tolerance and insulinaemia in elderly men, Diabetologia, vol. 40, no. 3, pp , Mar, [111] C. Giaginis, A. Tsantili-Kakoulidou, and S. Theocharis, Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism, Fundam Clin Pharmacol, vol. 21, no. 3, pp , Jun, [112] C. E. Murphy, and P. T. Rodgers, Effects of thiazolidinediones on bone loss and fracture, Ann Pharmacother, vol. 41, no. 12, pp , Dec, [113] D. D. Moore, 'No, really, how do they work?', Genes Dev, vol. 19, no. 4, pp , Feb 15, [114] J. H. Choi, A. S. Banks, J. L. Estall et al., Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5, Nature, vol. 466, no. 7305, pp , Jul 22, [115] A. Kumar, R. Nair, J. Kamath et al., Oral anti-glycemic agents for the treatment of type II diabetes mellitus: a review, PharmaInfo.net, vol [116] O. P. Lazarenko, S. O. Rzonca, W. R. Hogue et al., Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone, Endocrinology, vol. 148, no. 6, pp , Jun, [117] S. O. Rzonca, L. J. Suva, D. Gaddy et al., Bone is a target for the antidiabetic compound rosiglitazone, Endocrinology, vol. 145, no. 1, pp , Jan, [118] M. A. Soroceanu, D. Miao, X. Y. Bai et al., Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis, J Endocrinol, vol. 183, no. 1, pp , Oct, [119] V. Sottile, K. Seuwen, and M. Kneissel, Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone), Calcif Tissue Int, vol. 75, no. 4, pp , Oct, [120] A. L. Werner, and M. T. Travaglini, A review of rosiglitazone in type 2 diabetes mellitus, Pharmacotherapy, vol. 21, no. 9, pp , Sep, [121] M. Diamant, and R. J. Heine, Thiazolidinediones in type 2 diabetes mellitus: current clinical evidence, Drugs, vol. 63, no. 13, pp ,
200 [122] J. Sakamoto, H. Kimura, S. Moriyama et al., Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone, Biochem Biophys Res Commun, vol. 278, no. 3, pp , Nov 30, [123] C. T. Jennermann, J., D. Cowan, B. C. Pennink, K. et al., Effects of thiazolidinediones on bone turnover in the rat, Journal of Bone and Mineral Research, vol. 10, pp. S241, [124] U. Syversen, A. K. Stunes, B. I. Gustafsson et al., Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone, BMC Endocr Disord, vol. 9, no. 10, pp. 10, [125] A. A. Ali, R. S. Weinstein, S. A. Stewart et al., Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation, Endocrinology, vol. 146, no. 3, pp , Mar, [126] O. P. Lazarenko, S. O. Rzonca, L. J. Suva et al., Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat, Bone, vol. 38, no. 1, pp , Jan, [127] B. Lecka-Czernik, I. Gubrij, E. J. Moerman et al., Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2, J Cell Biochem, vol. 74, no. 3, pp , Sep 1, [128] B. Lecka-Czernik, E. J. Moerman, D. F. Grant et al., Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation, Endocrinology, vol. 143, no. 6, pp , Jun, [129] Y. Wan, L. W. Chong, and R. M. Evans, PPAR-gamma regulates osteoclastogenesis in mice, Nat Med, vol. 13, no. 12, pp , Dec, [130] D. J. Drucker, Biological actions and therapeutic potential of the glucagon-like peptides, Gastroenterology, vol. 122, no. 2, pp , Feb, [131] D. J. Drucker, Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action, Diabetes Care, vol. 30, no. 6, pp , Jun, [132] J. Mu, J. Woods, Y. P. Zhou et al., Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes, Diabetes, vol. 55, no. 6, pp , Jun, [133] E. J. Verspohl, Novel therapeutics for type 2 diabetes: incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors, Pharmacol Ther, vol. 124, no. 1, pp , Oct,
201 [134] L. L. Baggio, and D. J. Drucker, Biology of incretins: GLP-1 and GIP, Gastroenterology, vol. 132, no. 6, pp , May, [135] R. Gadsby, New treatments for type 2 diabetes--the DPP4 inhibitors, Prim Care Diabetes, vol. 1, no. 4, pp , Dec, [136] D. J. Drucker, The biology of incretin hormones, Cell Metab, vol. 3, no. 3, pp , Mar, [137] R. Gadsby, Efficacy and safety of Sitagliptin in the treatment of type 2 diabetes, Clinical Medicine: Therapeutics, vol. 1, pp , [138] S. Iwata, and C. Morimoto, CD26/dipeptidyl peptidase IV in context. The different roles of a multifunctional ectoenzyme in malignant transformation, J Exp Med, vol. 190, no. 3, pp , Aug 2, [139] D. Marguet, L. Baggio, T. Kobayashi et al., Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26, Proc Natl Acad Sci U S A, vol. 97, no. 12, pp , Jun 6, [140] J. J. Wu, H. K. Tang, T. K. Yeh et al., Biochemistry, pharmacokinetics, and toxicology of a potent and selective DPP8/9 inhibitor, Biochem Pharmacol, vol. 78, no. 2, pp , Jul 15, [141] D. Drucker, Physiology of the Gastrointestinal Tract, 4th ed.: Academic Press, [142] D. J. Drucker, The role of gut hormones in glucose homeostasis, J Clin Invest, vol. 117, no. 1, pp , Jan, [143] A. Au, A. Gupta, P. Schembri et al., Rapid insertion of GLUT2 into the rat jejunal brushborder membrane promoted by glucagon-like peptide 2, Biochem J, vol. 367, no. Pt 1, pp , Oct 1, [144] D. G. Burrin, Y. Petersen, B. Stoll et al., Glucagon-like peptide 2: a nutrient-responsive gut growth factor, J Nutr, vol. 131, no. 3, pp , Mar, [145] S. L. Conarello, Z. Li, J. Ronan et al., Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance, Proc Natl Acad Sci U S A, vol. 100, no. 11, pp , May 27, [146] B. Nuche-Berenguer, P. Moreno, P. Esbrit et al., Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistance states, Calcified Tissue International, vol. 84, pp ,
202 [147] W. G. Beamer, L. R. Donahue, and C. J. Rosen, Genetics and bone. Using the mouse to understand man, J Musculoskelet Neuronal Interact, vol. 2, no. 3, pp , Mar, [148] M. Priemel, A. F. Schilling, M. Haberland et al., Osteopenic mice: animal models of the aging skeleton, J Musculoskelet Neuronal Interact, vol. 2, no. 3, pp , Mar, [149] W. S. Jee, and W. Yao, Overview: animal models of osteopenia and osteoporosis, J Musculoskelet Neuronal Interact, vol. 1, no. 3, pp , Mar, [150] D. Kalu, "Animal models in the aging skeleton," The Aging Skeleton, C. J. Rosen, J. Glowacki and J. P. Bilezikian, eds., pp , San Diege, USA: Academic Press, [151] C. J. Rosen, W. G. Beamer, and L. R. Donahue, Defining the genetics of osteoporosis: using the mouse to understand man, Osteoporos Int, vol. 12, no. 10, pp , [152] H. Z. Ke, In vivo characterization of skeletal phenotype of genetically modified mice, J Bone Miner Metab, vol. 23 Suppl, pp. 84-9, [153] P. Pogoda, M. Priemel, A. F. Schilling et al., Mouse models in skeletal physiology and osteoporosis: experiences and data on 14,839 cases from the Hamburg Mouse Archives, J Bone Miner Metab, vol. 23 Suppl, pp , [154] A. S. Turner, Animal models of osteoporosis--necessity and limitations, Eur Cell Mater, vol. 1, pp , Jun 22, [155] S. Collins, T. L. Martin, R. S. Surwit et al., Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics, Physiol Behav, vol. 81, no. 2, pp , Apr, [156] R. S. Surwit, M. N. Feinglos, J. Rodin et al., Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice, Metabolism, vol. 44, no. 5, pp , May, [157] R. S. Surwit, C. M. Kuhn, C. Cochrane et al., Diet-induced type II diabetes in C57BL/6J mice, Diabetes, vol. 37, no. 9, pp , Sep, [158] M. S. Winzell, and B. Ahren, The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes, Diabetes, vol. 53 Suppl 3, no. 3, pp. S215-9, Dec, [159] J. E. Adams, Single and dual energy X-ray absorptiometry, Eur Radiol, vol. 7 Suppl 2, no. 2, pp. S20-31,
203 [160] R. B. Mazess, H. S. Barden, J. P. Bisek et al., Dual-energy x-ray absorptiometry for totalbody and regional bone-mineral and soft-tissue composition, Am J Clin Nutr, vol. 51, no. 6, pp , Jun, [161] J. C. van der Linden, J. H. Waarsing, and H. Weinans, The use of micro-ct to study bone architecture dynamics noninvasively, Drug Discovery Today: Technologies, vol. 3, no. 2, pp , [162] J. E. Compston, N. J. Garrahan, P. I. Croucher et al., Quantitative analysis of trabecular bone structure, Bone, vol. 14, no. 3, pp , May-Jun, [163] T. Jamsa, A. Koivukangas, J. Ryhanen et al., Femoral neck is a sensitive indicator of bone loss in immobilized hind limb of mouse, J Bone Miner Res, vol. 14, no. 10, pp , Oct, [164] M. P. Akhter, D. M. Cullen, G. Gong et al., Bone biomechanical properties in prostaglandin EP1 and EP2 knockout mice, Bone, vol. 29, no. 2, pp , Aug, [165] G. P. Makris, and J. L. Saffar, Quantitative relationship between osteoclasts, osteoclast nuclei and the extent of the resorbing surface in hamster periodontal disease, Arch Oral Biol, vol. 27, no. 11, pp , [166] K. Piper, A. Boyde, and S. J. Jones, The relationship between the number of nuclei of an osteoclast and its resorptive capability in vitro, Anat Embryol (Berl), vol. 186, no. 4, pp , Sep, [167] M. Grynpas, Age and disease-related changes in the mineral of bone, Calcif Tissue Int, vol. 53 Suppl 1, no. 1, pp. S57-64, [168] A. L. Boskey, Assessment of bone mineral and matrix using backscatter electron imaging and FTIR imaging, Curr Osteoporos Rep, vol. 4, no. 2, pp. 71-5, Jun, [169] R. Wang, and Y. An, "Observation of Material Failure Mode Using a SEM with a Built-In Mechanical Testing Device," Mechanical Testing of Bone and the Bone-Implant Interface, Y. An and R. Draughn, eds., p. 624, Boca Raton, Florida: CRC Press LLC, [170] K. Lundon, M. Dumitriu, and M. Grynpas, The long-term effect of ovariectomy on the quality and quantity of cancellous bone in young macaques, Bone Miner, vol. 24, no. 2, pp , Feb, [171] J. Chilcott, J. Wight, M. Lloyd Jones et al., The clinical effectiveness and costeffectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review, Health Technol Assess, vol. 5, no. 19, pp. 1-61,
204 [172] K. Promrat, G. Lutchman, G. I. Uwaifo et al., A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis, Hepatology, vol. 39, no. 1, pp , Jan, [173] L. Tornvig, L. I. Mosekilde, J. Justesen et al., Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice, Calcif Tissue Int, vol. 69, no. 1, pp , Jul, [174] M. Mousny, S. Omelon, L. Wise et al., Fluoride effects on bone formation and mineralization are influenced by genetics, Bone, vol. 43, no. 6, pp , Dec, [175] X. D. Wang, N. S. Masilamani, J. D. Mabrey et al., Changes in the fracture toughness of bone may not be reflected in its mineral density, porosity, and tensile properties, Bone, vol. 23, no. 1, pp , Jul, [176] L. D. Grossman, New solutions for type 2 diabetes mellitus: the role of pioglitazone, Pharmacoeconomics, vol. 20 Suppl 1, pp. 1-9, [177] S. Yaturu, B. Bryant, and S. K. Jain, Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men, Diabetes Care, vol. 30, no. 6, pp , Jun, [178] B. Kociecka, A. Surazynski, W. Miltyk et al., The effect of Telmisartan on collagen biosynthesis depends on the status of estrogen activation in breast cancer cells, Eur J Pharmacol, vol. 628, no. 1-3, pp. 51-6, Feb 25, [179] A. Surazynski, K. Jarzabek, W. Miltyk et al., Estrogen-dependent regulation of PPARgamma signaling on collagen biosynthesis in adenocarcinoma endometrial cells, Neoplasma, vol. 56, no. 5, pp , [180] T. Shibata, S. Takeuchi, S. Yokota et al., Effects of peroxisome proliferator-activated receptor-alpha and -gamma agonist, JTT-501, on diabetic complications in Zucker diabetic fatty rats, Br J Pharmacol, vol. 130, no. 3, pp , Jun, [181] J. M. Gimble, S. Zvonic, Z. E. Floyd et al., Playing with bone and fat, J Cell Biochem, vol. 98, no. 2, pp , May 15, [182] M. J. Reginato, S. T. Bailey, S. L. Krakow et al., A potent antidiabetic thiazolidinedione with unique peroxisome proliferator-activated receptor gamma-activating properties, J Biol Chem, vol. 273, no. 49, pp , Dec 4, [183] T. M. Willson, J. E. Cobb, D. J. Cowan et al., The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones, J Med Chem, vol. 39, no. 3, pp , Feb 2,
205 [184] P. Aschner, M. S. Kipnes, J. K. Lunceford et al., Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes, Diabetes Care, vol. 29, no. 12, pp , Dec, [185] B. Charbonnel, A. Karasik, J. Liu et al., Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone, Diabetes Care, vol. 29, no. 12, pp , Dec, [186] D. Kimmel, T. Cusick, J. Mu et al., Thiazolidinediones, but not sitagliptin, exacerbate ovariectomy induced bone loss in rats (Abstract), Diabetes, vol. 58, no. Supplement 1, pp. A374, [187] R. F. Klein, M. Shea, M. E. Gunness et al., Phenotypic characterization of mice bred for high and low peak bone mass, J Bone Miner Res, vol. 16, no. 1, pp , Jan, [188] S. M. Tommasini, T. G. Morgan, M. van der Meulen et al., Genetic variation in structurefunction relationships for the inbred mouse lumbar vertebral body, J Bone Miner Res, vol. 20, no. 5, pp , May, [189] M. A. Haidekker, R. Andresen, and H. J. Werner, Relationship between structural parameters, bone mineral density and fracture load in lumbar vertebrae, based on highresolution computed tomography, quantitative computed tomography and compression tests, Osteoporos Int, vol. 9, no. 5, pp , [190] T. J. Wronski, P. A. Schenck, M. Cintron et al., Effect of body weight on osteopenia in ovariectomized rats, Calcif Tissue Int, vol. 40, no. 3, pp , Mar, [191] G. A. Herman, A. Bergman, C. Stevens et al., Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes, J Clin Endocrinol Metab, vol. 91, no. 11, pp , Nov, [192] M. Sauve, K. Ban, M. A. Momen et al., Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice, Diabetes, vol. 59, no. 4, pp , Apr, [193] C. F. Deacon, S. Wamberg, P. Bie et al., Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal-induced incretin secretion in dogs, J Endocrinol, vol. 172, no. 2, pp , Feb, [194] M. B. Toft-Nielsen, S. Madsbad, and J. J. Holst, Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients, Diabetes Care, vol. 22, no. 7, pp , Jul,
206 [195] M. S. Geier, D. Tenikoff, R. Yazbeck et al., Development and resolution of experimental colitis in mice with targeted deletion of dipeptidyl peptidase IV, J Cell Physiol, vol. 204, no. 2, pp , Aug, [196] Y. Jiang, J. Zhao, H. K. Genant et al., Long-term changes in bone mineral and biomechanical properties of vertebrae and femur in aging, dietary calcium restricted, and/or estrogen-deprived/-replaced rats, J Bone Miner Res, vol. 12, no. 5, pp , May, [197] R. T. Turner, J. J. Vandersteenhoven, and N. H. Bell, The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats, J Bone Miner Res, vol. 2, no. 2, pp , Apr, [198] B. Burguera, L. C. Hofbauer, T. Thomas et al., Leptin reduces ovariectomy-induced bone loss in rats, Endocrinology, vol. 142, no. 8, pp , Aug, [199] T. Thomas, B. Burguera, L. J. Melton, 3rd et al., Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women, Bone, vol. 29, no. 2, pp , Aug, [200] D. A. Ainslie, M. J. Morris, G. Wittert et al., Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y, Int J Obes Relat Metab Disord, vol. 25, no. 11, pp , Nov, [201] P. A. Baldock, N. J. Lee, F. Driessler et al., Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight, PLoS One, vol. 4, no. 12, pp. e8415, [202] P. A. Baldock, A. Sainsbury, M. Couzens et al., Hypothalamic Y2 receptors regulate bone formation, J Clin Invest, vol. 109, no. 7, pp , Apr, [203] P. Ducy, M. Amling, S. Takeda et al., Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass, Cell, vol. 100, no. 2, pp , Jan 21, [204] F. Isken, A. F. Pfeiffer, R. Nogueiras et al., Deficiency of glucose-dependent insulinotropic polypeptide receptor prevents ovariectomy-induced obesity in mice, Am J Physiol Endocrinol Metab, vol. 295, no. 2, pp. E350-5, Aug, [205] R. Guieu, E. Fenouillet, C. Devaux et al., CD26 modulated nociception in mice via its dipeptidyl-peptidase IV activity, Behavioural Brain Research, vol. 166, no. 2, pp ,
207 [206] X. Yu, Y. Huang, P. Collin-Osdoby et al., Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration, J Bone Miner Res, vol. 18, no. 8, pp , Aug, [207] T. Mori, T. Ogata, H. Okumura et al., Substance P regulates the function of rabbit cultured osteoclast; increase of intracellular free calcium concentration and enhancement of bone resorption, Biochem Biophys Res Commun, vol. 262, no. 2, pp , Aug 27, [208] K. Loster, K. Zeilinger, D. Schuppan et al., The cysteine-rich region of dipeptidyl peptidase IV (CD 26) is the collagen-binding site, Biochem Biophys Res Commun, vol. 217, no. 1, pp , Dec 5, [209] M. M. Jost, J. Lamerz, H. Tammen et al., In vivo profiling of DPP4 inhibitors reveals alterations in collagen metabolism and accumulation of an amyloid peptide in rat plasma, Biochem Pharmacol, vol. 77, no. 2, pp , Jan 15, [210] K. Aaboe, F. K. Knop, T. Vilsboll et al., Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and nonglucose induced insulin secretion in patients with type 2 diabetes mellitus, Diabetes Obes Metab, vol. 12, no. 4, pp , Apr, [211] K. E. Wortley, K. Garcia, H. Okamoto et al., Peptide YY regulates bone turnover in rodents, Gastroenterology, vol. 133, no. 5, pp , Nov, [212] D. L. Greenman, P. Bryant, R. L. Kodell et al., Relationship of mouse body weight and food consumption/wastage to cage shelf level, Lab Anim Sci, vol. 33, no. 6, pp , Dec, [213] N. Rowland, M. Chaney, and C. Vaughan, Food intake and meal patterns of mice under various schedules of food reinforcement, Appetite, vol. 49, no. 1, pp. 324, [214] M. Weihe, "The laboratory rat," Universities Federation for Nimal Welfare handbook on the cost and management of laboratory animals, T. Poole, ed., pp , New York, New York: Churchill Lingstone, Inc, [215] S. Lin, T. C. Thomas, L. H. Storlien et al., Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice, Int J Obes Relat Metab Disord, vol. 24, no. 5, pp , May, [216] R. E. Borchers, L. J. Gibson, H. Burchardt et al., Effects of selected thermal variables on the mechanical properties of trabecular bone, Biomaterials, vol. 16, no. 7, pp , May,
208 [217] R. R. Pelker, G. E. Friedlaender, T. C. Markham et al., Effects of freezing and freezedrying on the biomechanical properties of rat bone, J Orthop Res, vol. 1, no. 4, pp , [218] G. E. Lopez Franco, T. K. O'Neil, S. J. Litscher et al., Accuracy and precision of PIXImus densitometry for ex vivo mouse long bones: comparison of technique and software version, J Clin Densitom, vol. 7, no. 3, pp , Fall, [219] A. Iida-Klein, S. S. Lu, K. Yokoyama et al., Precision, accuracy, and reproducibility of dual X-ray absorptiometry measurements in mice in vivo, J Clin Densitom, vol. 6, no. 1, pp , Spring, [220] S. Kolta, M. C. De Vernejoul, P. Meneton et al., Bone mineral measurements in mice: comparison of two devices, J Clin Densitom, vol. 6, no. 3, pp , Fall, [221] M. Bonyadi, S. D. Waldman, D. Liu et al., Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice, Proc Natl Acad Sci U S A, vol. 100, no. 10, pp , May 13, [222] W. E. Ward, S. Kim, D. Chan et al., Serum equol, bone mineral density and biomechanical bone strength differ among four mouse strains, J Nutr Biochem, vol. 16, no. 12, pp , Dec, [223] M. P. Akhter, D. J. Wells, S. J. Short et al., Bone biomechanical properties in LRP5 mutant mice, Bone, vol. 35, no. 1, pp , Jul, [224] T. Jamsa, J. Tuukkanen, and P. Jalovaara, Femoral neck strength of mouse in two loading configurations: method evaluation and fracture characteristics, J Biomech, vol. 31, no. 8, pp , Aug, [225] P. A. Revell, Histomorphometry of bone, J Clin Pathol, vol. 36, no. 12, pp , Dec, [226] J. M. Buckley, K. Loo, and J. Motherway, Comparison of quantitative computed tomography-based measures in predicting vertebral compressive strength, Bone, vol. 40, no. 3, pp , Mar, [227] J. P. Zimmer, S. M. Lewis, and J. L. Moyer, Comparison of gavage, water bottle, and a high-moisture diet bolus as dosing methods for quantitative D-xylose administration to B6D2F1 (Mus musculus) mice, Lab Anim, vol. 27, no. 2, pp , Apr, [228] C. Pekow, Buprenorphine, Jell-O recipe for rodent analgesia, Synapse, vol. 25, pp ,
209 [229] J. H. Liles, P. A. Flecknell, J. Roughan et al., Influence of oral buprenorphine, oral naltrexone or morphine on the effects of laparotomy in the rat, Lab Anim, vol. 32, no. 2, pp , Apr,
210 Appendix A1: a) Osteoclasts Fat cell Fat cell b) Osteoclasts Figure A1: a) Pioglitazone-treated female Fat cells pushing against osteoclasts b) Vehicle control female Normal osteoclasts and bone marrow 196
Chapter 6: Skeletal System: Bones and Bone Tissue
 Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
Chapter 6: Skeletal System: Bones and Bone Tissue I. Functions A. List and describe the five major functions of the skeletal system: 1. 2. 3.. 4. 5.. II. Cartilage A. What do chondroblasts do? B. When
Functions of the Skeletal System. Chapter 6: Osseous Tissue and Bone Structure. Classification of Bones. Bone Shapes
 Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
Chapter 6: Osseous Tissue and Bone Structure Functions of the Skeletal System 1. Support 2. Storage of minerals (calcium) 3. Storage of lipids (yellow marrow) 4. Blood cell production (red marrow) 5. Protection
BONE TISSUE. Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology
 BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
BONE TISSUE Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology BONE FUNCTION Support Protection (protect internal organs) Movement (provide leverage system for skeletal muscles, tendons, ligaments
The Skeletal System:Bone Tissue
 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
Biology. Dr. Khalida Ibrahim
 Biology Dr. Khalida Ibrahim BONE TISSUE Bone tissue is a specialized form of connective tissue and is the main element of the skeletal tissues. It is composed of cells and an extracellular matrix in which
Biology Dr. Khalida Ibrahim BONE TISSUE Bone tissue is a specialized form of connective tissue and is the main element of the skeletal tissues. It is composed of cells and an extracellular matrix in which
SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over 3 Weeks. A SEPARATE WORKSHEET WILL BE PROVIDED.
 BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
BIO 211; Anatomy and Physiology I REFERENCE: CHAPTER 07 1 Dr. Lawrence Altman Naugatuck Valley Community College LECTURE TOPICS OUTLINE SKELETAL SYSTEM I NOTE: LAB ASSIGNMENTS for this topic will run over
BIOLOGY and BIOMECHANICS OF NORMAL & OSTEOPOROTIC BONE
 BIOLOGY and BIOMECHANICS OF NORMAL & OSTEOPOROTIC BONE Andreas Panagopoulos, MD, PhD Assistant Professor in Orthopaedics University Hospital of Patras, Orthopaedic Clinic Objectives Bone structure and
BIOLOGY and BIOMECHANICS OF NORMAL & OSTEOPOROTIC BONE Andreas Panagopoulos, MD, PhD Assistant Professor in Orthopaedics University Hospital of Patras, Orthopaedic Clinic Objectives Bone structure and
OSSEOUS TISSUE & BONE STRUCTURE PART I: OVERVIEW & COMPONENTS
 OSSEOUS TISSUE & BONE STRUCTURE PART I: OVERVIEW & COMPONENTS The Skeletal System Skeletal system includes: bones of the skeleton, cartilages, ligaments, and connective tissues What are the functions of
OSSEOUS TISSUE & BONE STRUCTURE PART I: OVERVIEW & COMPONENTS The Skeletal System Skeletal system includes: bones of the skeleton, cartilages, ligaments, and connective tissues What are the functions of
What are the parts of the skeletal system? Chapter 6- Part I Bones and Skeletal Tissues. Growth of Cartilage. Bones come in many shapes
 Chapter 6- Part I Bones and Skeletal Tissues Components of the skeletal system Classification of Bone (bone shapes) Functions of bone Bone structure Microscopic structure of bone and bone cells What are
Chapter 6- Part I Bones and Skeletal Tissues Components of the skeletal system Classification of Bone (bone shapes) Functions of bone Bone structure Microscopic structure of bone and bone cells What are
BONE REMODELLING. Tim Arnett. University College London. Department of Anatomy and Developmental Biology
 BONE REMODELLING Tim Arnett Department of Anatomy and Developmental Biology University College London The skeleton, out of sight and often out of mind, is a formidable mass of tissue occupying about 9%
BONE REMODELLING Tim Arnett Department of Anatomy and Developmental Biology University College London The skeleton, out of sight and often out of mind, is a formidable mass of tissue occupying about 9%
Derived copy of Bone *
 OpenStax-CNX module: m57739 1 Derived copy of Bone * Shannon McDermott Based on Bone by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By
OpenStax-CNX module: m57739 1 Derived copy of Bone * Shannon McDermott Based on Bone by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By
Chapter 6: SKELETAL SYSTEM
 Chapter 6: SKELETAL SYSTEM I. FUNCTIONS A. Support B. Protection C. Movement D. Mineral storage E. Lipid storage (Fig. 6.8b) F. Blood cell production (Fig. 6.4) II. COMPONENTS A. Cartilage 1. Hyaline 2.
Chapter 6: SKELETAL SYSTEM I. FUNCTIONS A. Support B. Protection C. Movement D. Mineral storage E. Lipid storage (Fig. 6.8b) F. Blood cell production (Fig. 6.4) II. COMPONENTS A. Cartilage 1. Hyaline 2.
Chapter 39: Exercise prescription in those with osteoporosis
 Chapter 39: Exercise prescription in those with osteoporosis American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise testing and prescription (6th ed.). New York:
Chapter 39: Exercise prescription in those with osteoporosis American College of Sports Medicine. (2010). ACSM's resource manual for guidelines for exercise testing and prescription (6th ed.). New York:
The Skeletal System:Bone Tissue
 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose, and dense
Chapter 6 Bones and Bone Tissue Chapter Outline
 Chapter 6 Bones and Bone Tissue Chapter Outline Module 6.1: Introduction to Bones as Organs (Figures 6.1, 6.2, 6.3, 6.4) A. The skeletal system includes the bones, joints, and their associated supporting
Chapter 6 Bones and Bone Tissue Chapter Outline Module 6.1: Introduction to Bones as Organs (Figures 6.1, 6.2, 6.3, 6.4) A. The skeletal system includes the bones, joints, and their associated supporting
Chapter 6: Osseous Tissue and Bone Structure
 Chapter 6: Osseous Tissue and Bone Structure I. An Introduction to the Skeletal System, p. 180 Objective: Describe the functions of the skeletal system The skeletal system includes: - bones of the skeleton
Chapter 6: Osseous Tissue and Bone Structure I. An Introduction to the Skeletal System, p. 180 Objective: Describe the functions of the skeletal system The skeletal system includes: - bones of the skeleton
CHAPTER 6 LECTURE OUTLINE
 CHAPTER 6 LECTURE OUTLINE I. INTRODUCTION A. Bone is made up of several different tissues working together: bone, cartilage, dense connective tissue, epithelium, various blood forming tissues, adipose
CHAPTER 6 LECTURE OUTLINE I. INTRODUCTION A. Bone is made up of several different tissues working together: bone, cartilage, dense connective tissue, epithelium, various blood forming tissues, adipose
KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM
 ANATOMY & PHYSIOLOGY 1 (101-805 - AB) PAUL ANDERSON 2011 KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM A Overview of The Skeletal System 1. Definition: Anatomically the SKELETAL SYSTEM consists of bones, cartilages,
ANATOMY & PHYSIOLOGY 1 (101-805 - AB) PAUL ANDERSON 2011 KEY CONCEPTS Unit 6 THE SKELETAL SYSTEM A Overview of The Skeletal System 1. Definition: Anatomically the SKELETAL SYSTEM consists of bones, cartilages,
DISEASES WITH ABNORMAL MATRIX
 DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
DISEASES WITH ABNORMAL MATRIX MSK-1 FOR 2 ND YEAR MEDICAL STUDENTS Dr. Nisreen Abu Shahin CONGENITAL DISEASES WITH ABNORMAL MATRIX OSTEOGENESIS IMPERFECTA (OI): also known as "brittle bone disease" a group
An Introduction to the Skeletal System Skeletal system includes Bones of the skeleton Cartilages, ligaments, and connective tissues
 An Introduction to the Skeletal System Skeletal system includes Bones of the skeleton Cartilages, ligaments, and connective tissues Functions of the Skeletal System Support Storage of minerals (calcium)
An Introduction to the Skeletal System Skeletal system includes Bones of the skeleton Cartilages, ligaments, and connective tissues Functions of the Skeletal System Support Storage of minerals (calcium)
Skeletal System. The skeletal System... Components
 Skeletal System The skeletal System... What are the general components of the skeletal system? What does the skeletal system do for you & how does it achieve these functions? Components The skeletal system
Skeletal System The skeletal System... What are the general components of the skeletal system? What does the skeletal system do for you & how does it achieve these functions? Components The skeletal system
OpenStax-CNX module: m Bone Structure * Ildar Yakhin. Based on Bone Structure by OpenStax. Abstract
 OpenStax-CNX module: m63474 1 Bone Structure * Ildar Yakhin Based on Bone Structure by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By
OpenStax-CNX module: m63474 1 Bone Structure * Ildar Yakhin Based on Bone Structure by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By
SKELETAL SYSTEM CHAPTER 07. Bone Function BIO 211: ANATOMY & PHYSIOLOGY I. Body Movement interacts with muscles bones act as rigid bar of a lever
 Page 1 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 07 SKELETAL SYSTEM Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill. Some illustrations are courtesy of
Page 1 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 07 SKELETAL SYSTEM Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill. Some illustrations are courtesy of
SKELETAL SYSTEM CHAPTER 07 BIO 211: ANATOMY & PHYSIOLOGY I
 BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 07 SKELETAL SYSTEM Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill. Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 CHAPTER 07 SKELETAL SYSTEM Dr. Lawrence G. G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill. Some illustrations are courtesy of McGraw-Hill.
Skeletal Tissues. Skeletal tissues. Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs.
 Skeletal Tissues Functions 1) support 2) protection 3) movement Skeletal tissues Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs. Aids muscle contraction; generate
Skeletal Tissues Functions 1) support 2) protection 3) movement Skeletal tissues Frame; muscles, organs and CT attach. Brain, spinal cord, thoracic organs; heart and lungs. Aids muscle contraction; generate
SKELETAL TISSUES CHAPTER 7 INTRODUCTION TO THE SKELETAL SYSTEM TYPES OF BONES
 SKELETAL TISSUES CHAPTER 7 By John McGill Supplement Outlines: Beth Wyatt Original PowerPoint: Jack Bagwell INTRODUCTION TO THE SKELETAL SYSTEM STRUCTURE Organs: Bones Related Tissues: Cartilage and Ligaments
SKELETAL TISSUES CHAPTER 7 By John McGill Supplement Outlines: Beth Wyatt Original PowerPoint: Jack Bagwell INTRODUCTION TO THE SKELETAL SYSTEM STRUCTURE Organs: Bones Related Tissues: Cartilage and Ligaments
Osseous Tissue and Bone Structure
 C h a p t e r 6 Osseous Tissue and Bone Structure PowerPoint Lecture Slides prepared by Jason LaPres Lone Star College - North Harris Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin
C h a p t e r 6 Osseous Tissue and Bone Structure PowerPoint Lecture Slides prepared by Jason LaPres Lone Star College - North Harris Copyright 2009 Pearson Education, Inc., publishing as Pearson Benjamin
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
-the emphasis on this section is the structure and function of bone tissue and on the dynamics of its formation and remodeling throughout life.
 Biology 325 Fall 2004 BONES AND SKELETAL TISSUES Introduction -skeleton contains cartilage and bones -the emphasis on this section is the structure and function of bone tissue and on the dynamics of its
Biology 325 Fall 2004 BONES AND SKELETAL TISSUES Introduction -skeleton contains cartilage and bones -the emphasis on this section is the structure and function of bone tissue and on the dynamics of its
Skeletal System worksheet
 Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
ANATOMY & PHYSIOLOGY - CLUTCH CH. 8 - BONE AND CARTILAGE.
 !! www.clutchprep.com CONCEPT: BONE CLASSIFICATIONS There are four classifications of bones based on their 1. Long bones are greater in length than in width - Found in the upper and lower limbs (ex: arm,
!! www.clutchprep.com CONCEPT: BONE CLASSIFICATIONS There are four classifications of bones based on their 1. Long bones are greater in length than in width - Found in the upper and lower limbs (ex: arm,
Regulation of the skeletal mass through the life span
 Regulation of the skeletal mass through the life span Functions of the skeletal system Mechanical protection skull Movement leverage for muscles Mineral metabolism calcium store Erythropoiesis red blood
Regulation of the skeletal mass through the life span Functions of the skeletal system Mechanical protection skull Movement leverage for muscles Mineral metabolism calcium store Erythropoiesis red blood
Module 2:! Functional Musculoskeletal Anatomy A! Semester 1! !!! !!!! Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb!
 Functional Musculoskeletal Anatomy A Module 2: Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb Semester 1 1 18. Bone Tissue & Growth of Bones 18.1 Describe the structure of bone tissue
Functional Musculoskeletal Anatomy A Module 2: Hard Tissues, Distal Upper Limb & Neurovascular Supply of Upper Limb Semester 1 1 18. Bone Tissue & Growth of Bones 18.1 Describe the structure of bone tissue
Human Biology Chapter 15.3: Bone Structure *
 OpenStax-CNX module: m58082 1 Human Biology Chapter 15.3: Bone Structure * Willy Cushwa Based on Bone Structure by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons
OpenStax-CNX module: m58082 1 Human Biology Chapter 15.3: Bone Structure * Willy Cushwa Based on Bone Structure by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons
CT Imaging of skeleton in small animals. Massimo Marenzana
 CT Imaging of skeleton in small animals Massimo Marenzana Introduction Osteoporosis is a disease in which bones become fragile and more likely to break. It can be defined as a systemic skeletal disease
CT Imaging of skeleton in small animals Massimo Marenzana Introduction Osteoporosis is a disease in which bones become fragile and more likely to break. It can be defined as a systemic skeletal disease
Types of Bones. 5 basic types of bones: Sutural bones - in joint between skull bones
 The Skeletal System The Skeletal System Bone and their cartilage, ligaments & tendons. Dynamic and ever changing throughout life Skeleton contains all 4 tissue types; Epithelial, connective, muscle and
The Skeletal System The Skeletal System Bone and their cartilage, ligaments & tendons. Dynamic and ever changing throughout life Skeleton contains all 4 tissue types; Epithelial, connective, muscle and
Bone Tissue- Chapter 5 5-1
 Bone Tissue- Chapter 5 5-1 Bone Functions Support Protection Assistance in movement Mineral storage and release Blood cell production Triglyceride storage 5-2 Bone Chemistry Water (25%) Organic Constituent
Bone Tissue- Chapter 5 5-1 Bone Functions Support Protection Assistance in movement Mineral storage and release Blood cell production Triglyceride storage 5-2 Bone Chemistry Water (25%) Organic Constituent
Due in Lab. Due next week in lab - Scientific America Article Select one article to read and complete article summary
 Due in Lab 1. Skeletal System 33-34 2. Skeletal System 26 3. PreLab 6 Due next week in lab - Scientific America Article Select one article to read and complete article summary Cell Defenses and the Sunshine
Due in Lab 1. Skeletal System 33-34 2. Skeletal System 26 3. PreLab 6 Due next week in lab - Scientific America Article Select one article to read and complete article summary Cell Defenses and the Sunshine
Beyond BMD: Bone Quality and Bone Strength
 Beyond BMD: Bone Quality and Bone Strength Consultant / advisor: Amgen, Eli Lilly, Merck Disclosures Mary L. Bouxsein, PhD Beth Israel Deaconess Medical Center Harvard Medical School, Boston, MA mbouxsei@bidmc.harvard.edu
Beyond BMD: Bone Quality and Bone Strength Consultant / advisor: Amgen, Eli Lilly, Merck Disclosures Mary L. Bouxsein, PhD Beth Israel Deaconess Medical Center Harvard Medical School, Boston, MA mbouxsei@bidmc.harvard.edu
Deposition of Bone by the Osteoblasts. Bone is continually being deposited by osteoblasts, and it is continually being resorbed where osteoclasts are
 Bone remodeling Deposition of Bone by the Osteoblasts. Bone is continually being deposited by osteoblasts, and it is continually being resorbed where osteoclasts are active. This mechanism is always is
Bone remodeling Deposition of Bone by the Osteoblasts. Bone is continually being deposited by osteoblasts, and it is continually being resorbed where osteoclasts are active. This mechanism is always is
For more information about how to cite these materials visit
 Author(s): University of Michigan Medical School, Department of Cell and Developmental Biology License: Unless otherwise noted, the content of this course material is licensed under a Creative Commons
Author(s): University of Michigan Medical School, Department of Cell and Developmental Biology License: Unless otherwise noted, the content of this course material is licensed under a Creative Commons
Fig Articular cartilage. Epiphysis. Red bone marrow Epiphyseal line. Marrow cavity. Yellow bone marrow. Periosteum. Nutrient foramen Diaphysis
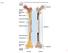 Fig. 7.1 Articular cartilage Epiphysis Red bone marrow Epiphyseal line Marrow cavity Yellow bone marrow Nutrient foramen Diaphysis Site of endosteum Compact bone Spongy bone Epiphyseal line Epiphysis Articular
Fig. 7.1 Articular cartilage Epiphysis Red bone marrow Epiphyseal line Marrow cavity Yellow bone marrow Nutrient foramen Diaphysis Site of endosteum Compact bone Spongy bone Epiphyseal line Epiphysis Articular
Outline. Skeletal System. Functions of Bone. Bio 105: Skeletal System 3/17/2016. The material from this lecture packet will be on the lecture exam
 Bio 105: Skeletal System Lecture 8 Chapter 5 The material from this lecture packet will be on the lecture exam The identification that you do after this lecture will be on the lab exam Outline I. Overview
Bio 105: Skeletal System Lecture 8 Chapter 5 The material from this lecture packet will be on the lecture exam The identification that you do after this lecture will be on the lab exam Outline I. Overview
Peggers Super Summaries Basic Sciences Bone
 Bone Overview & Turnover BONES Function o Support o Protection o Assisting movement o Storage of minerals o Production of red blood cells from marrow Types o Cancellous o Compact with Haversian systems
Bone Overview & Turnover BONES Function o Support o Protection o Assisting movement o Storage of minerals o Production of red blood cells from marrow Types o Cancellous o Compact with Haversian systems
The Skeletal System. Chapter 7a. Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life
 The Skeletal System Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life Chapter 7a Support Protection Movement Storage areas Minerals Lipids Hemopoiesis
The Skeletal System Skeletal System Introduction Functions of the skeleton Framework of bones The skeleton through life Chapter 7a Support Protection Movement Storage areas Minerals Lipids Hemopoiesis
Osseous Tissue and Bone Structure
 6 Osseous Tissue and Bone Structure PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to the Skeletal System Learning Outcomes 6-1 Describe the primary
6 Osseous Tissue and Bone Structure PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to the Skeletal System Learning Outcomes 6-1 Describe the primary
Chapter 6 Skeletal System
 Chapter 6 Skeletal System Functions of the skeletal system/bone 1. Support skeletal system is the internal framework of the body 2. Protection protects internal organs 3. Movement muscles & bones work
Chapter 6 Skeletal System Functions of the skeletal system/bone 1. Support skeletal system is the internal framework of the body 2. Protection protects internal organs 3. Movement muscles & bones work
Chapter 7. Skeletal System
 Chapter 7 Skeletal System 1 Introduction: A. Bones are very active, living tissues B. Each bone is made up of several types of tissues and so is an organ. C. Bone functions include: muscle attachment,
Chapter 7 Skeletal System 1 Introduction: A. Bones are very active, living tissues B. Each bone is made up of several types of tissues and so is an organ. C. Bone functions include: muscle attachment,
FORMATION OF BONE. Intramembranous Ossification. Bone-Lec-10-Prof.Dr.Adnan Albideri
 FORMATION OF BONE All bones are of mesodermal origin. The process of bone formation is called ossification. We have seen that formation of most bones is preceded by the formation of a cartilaginous model,
FORMATION OF BONE All bones are of mesodermal origin. The process of bone formation is called ossification. We have seen that formation of most bones is preceded by the formation of a cartilaginous model,
Dr. Heba Kalbouneh. Dr. Heba Kalbouneh. Dr. Heba Kalbouneh
 Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Bone Special connective tissue. Cartilages + Bones = Skeleton. Osseous = refers to bone. Functions of the bone: 1. Support: It forms the framework
Dr. Heba Kalbouneh Dr. Heba Kalbouneh Dr. Heba Kalbouneh Bone Special connective tissue. Cartilages + Bones = Skeleton. Osseous = refers to bone. Functions of the bone: 1. Support: It forms the framework
LAB: The Skeletal System System
 WLHS/A&P/Oppelt Name LAB: The Skeletal System System Background: The skeletal system is primarily responsible for supporting the body and protecting vital organs. We are bone with more than 270 bones that
WLHS/A&P/Oppelt Name LAB: The Skeletal System System Background: The skeletal system is primarily responsible for supporting the body and protecting vital organs. We are bone with more than 270 bones that
b. Adult bones produce 2.5 million RBCs each second.
 Ch 6 Skeletal System I. Functions of the Skeletal System A. The skeletal system consists of: 1. bones, cartilage, tendons and ligaments B. Living bone is not Gr. dried up 1. It is dynamic and adaptable
Ch 6 Skeletal System I. Functions of the Skeletal System A. The skeletal system consists of: 1. bones, cartilage, tendons and ligaments B. Living bone is not Gr. dried up 1. It is dynamic and adaptable
Skeletal System worksheet
 Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
Skeletal System worksheet Name Section A: Intro to Skeletal System The skeletal system performs vital functions that enable us to move through our daily lives. Support - The skeleton provides support and
BIOL 2457 CHAPTER 6 SI 1. irregular ectopic: sutural (Wormian) The is between the shaft and end. It contains cartilage that is
 BIOL 2457 CHAPTER 6 SI 1 1. List 5 functions of bones: 2. Classify bones according to shape: give descriptions and examples: long short flat irregular ectopic: sutural (Wormian) ectopic: sesamoid 3. The
BIOL 2457 CHAPTER 6 SI 1 1. List 5 functions of bones: 2. Classify bones according to shape: give descriptions and examples: long short flat irregular ectopic: sutural (Wormian) ectopic: sesamoid 3. The
The Skeletal System PART A. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Skeletal System 5 PART A The Skeletal System Parts of the skeletal system Bones (skeleton) Joints
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Skeletal System 5 PART A The Skeletal System Parts of the skeletal system Bones (skeleton) Joints
Section 1. What is osteoporosis? Your bones. Bones and osteoporosis. Who is affected by osteoporosis? Consequences of osteoporosis
 4 Section 1 What is osteoporosis? Your bones Bones and osteoporosis Who is affected by osteoporosis? Consequences of osteoporosis Less common types of osteoporosis Other bone conditions 5 Osteoporosis
4 Section 1 What is osteoporosis? Your bones Bones and osteoporosis Who is affected by osteoporosis? Consequences of osteoporosis Less common types of osteoporosis Other bone conditions 5 Osteoporosis
The Skeletal System. Chapter 4
 The Skeletal System Chapter 4 FUNCTIONS OF THE SKELETAL SYSTEM Support o Provides shape Protection o Internal organs Movement o Provides structure for muscle to act upon Storage o Minerals & fat Blood
The Skeletal System Chapter 4 FUNCTIONS OF THE SKELETAL SYSTEM Support o Provides shape Protection o Internal organs Movement o Provides structure for muscle to act upon Storage o Minerals & fat Blood
Osteoporosis. When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of.
 Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
The Skeletal Response to Aging: There s No Bones About It!
 The Skeletal Response to Aging: There s No Bones About It! April 7, 2001 Joseph E. Zerwekh, Ph.D. Interrelationship of Intestinal, Skeletal, and Renal Systems to the Overall Maintenance of Normal Calcium
The Skeletal Response to Aging: There s No Bones About It! April 7, 2001 Joseph E. Zerwekh, Ph.D. Interrelationship of Intestinal, Skeletal, and Renal Systems to the Overall Maintenance of Normal Calcium
A Novel Murine Model Of Adynamic Bone Disease (ABD)
 A Novel Murine Model Of Adynamic Bone Disease (ABD) Adeline H. Ng 1,2, Thomas L. Willett, PhD 2,1, Benjamin A. Alman 3,1, Marc D. Grynpas 1,2. 1 University of Toronto, Toronto, ON, Canada, 2 Samuel Lunenfeld
A Novel Murine Model Of Adynamic Bone Disease (ABD) Adeline H. Ng 1,2, Thomas L. Willett, PhD 2,1, Benjamin A. Alman 3,1, Marc D. Grynpas 1,2. 1 University of Toronto, Toronto, ON, Canada, 2 Samuel Lunenfeld
Gross Anatomy. Landmarks on a typical long bone. Membranes. Diaphysis Epiphysis Membranes. Periosteum Endosteum
 BONE STRUCTURE Gross Anatomy Landmarks on a typical long bone Diaphysis Epiphysis Membranes Membranes Periosteum Endosteum Diaphysis Long tubular diaphysis is the shaft of the bone Collar of compact bone
BONE STRUCTURE Gross Anatomy Landmarks on a typical long bone Diaphysis Epiphysis Membranes Membranes Periosteum Endosteum Diaphysis Long tubular diaphysis is the shaft of the bone Collar of compact bone
Bone. OpenStax College
 OpenStax-CNX module: m44789 1 Bone OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able to:
OpenStax-CNX module: m44789 1 Bone OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able to:
Skeletal System. Bio 105
 Skeletal System Bio 105 Outline I. Overview of the skeletal system II. Function of bones III. Bone structure IV. Bone cells V. Cartilage VI. Tendons and Ligaments VII. Joints VIII. Bone development IX.
Skeletal System Bio 105 Outline I. Overview of the skeletal system II. Function of bones III. Bone structure IV. Bone cells V. Cartilage VI. Tendons and Ligaments VII. Joints VIII. Bone development IX.
Outline. Skeletal System. Tendons link the skeletal and the muscular systems.
 Outline Skeletal System Bio 105 I. Overview of the skeletal system II. Function of bones III. Bone structure IV. Bone cells V. Cartilage VI. Tendons and Ligaments VII. Joints VIII. Bone development IX.
Outline Skeletal System Bio 105 I. Overview of the skeletal system II. Function of bones III. Bone structure IV. Bone cells V. Cartilage VI. Tendons and Ligaments VII. Joints VIII. Bone development IX.
Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis
 Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis Andreas Panagopoulos MD, PhD Upper Limb & Sports Medicine Orthopaedic Surgeon Assistant Professor, University of Patras Outline Teriparatide
Anabolic Therapy With Teriparatide Indications Beyond Osteoporosis Andreas Panagopoulos MD, PhD Upper Limb & Sports Medicine Orthopaedic Surgeon Assistant Professor, University of Patras Outline Teriparatide
What is bone? Specialized form of connective tissue: mineralized collagen matrix, therefore very rigid and strong while still retaining some degree of
 Bone What is bone? Specialized form of connective tissue: mineralized collagen matrix, therefore very rigid and strong while still retaining some degree of flexibility Other types of connective tissue:
Bone What is bone? Specialized form of connective tissue: mineralized collagen matrix, therefore very rigid and strong while still retaining some degree of flexibility Other types of connective tissue:
The Skeletal System PART A
 5 The Skeletal System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Skeletal System
5 The Skeletal System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Skeletal System
Copyright 2004 Lippincott Williams & Wilkins. 2. Bone Structure. Copyright 2004 Lippincott Williams & Wilkins
 Chapter 7 The Skeleton: Bones and Joints The Skeleton Skeletal system is made up of bones and joints and supporting connective tissue. 1. Bone Functions 1. To store calcium salts 2. To protect delicate
Chapter 7 The Skeleton: Bones and Joints The Skeleton Skeletal system is made up of bones and joints and supporting connective tissue. 1. Bone Functions 1. To store calcium salts 2. To protect delicate
Pathophysiology of Postmenopausal & Glucocorticoid Induced Osteoporosis. March 15, 2016 Bone ECHO Kate T Queen, MD
 Pathophysiology of Postmenopausal & Glucocorticoid Induced Osteoporosis March 15, 2016 Bone ECHO Kate T Queen, MD Review: normal bone formation Bone Modeling Remodeling Peak Bone Mass Maximum bone mass
Pathophysiology of Postmenopausal & Glucocorticoid Induced Osteoporosis March 15, 2016 Bone ECHO Kate T Queen, MD Review: normal bone formation Bone Modeling Remodeling Peak Bone Mass Maximum bone mass
Introduction to Biomedical Engineering: Basic concepts and Bone Biomechanics
 Introduction to Biomedical Engineering: Basic concepts and Bone Biomechanics Fall 2016, AKUT G. Rouhi Biomechanics The field of biomechanics applies principles of mechanics to study the structure and function
Introduction to Biomedical Engineering: Basic concepts and Bone Biomechanics Fall 2016, AKUT G. Rouhi Biomechanics The field of biomechanics applies principles of mechanics to study the structure and function
Nutritional Aspects of Osteoporosis Care and Treatment Cynthia Smith, FNP-BC, RN, MSN, CCD Pars Osteoporosis Clinic, Belpre, Ohio
 Osteoporosis 1 Nutritional Aspects of Osteoporosis Care and Treatment Cynthia Smith, FNP-BC, RN, MSN, CCD Pars Osteoporosis Clinic, Belpre, Ohio 1) Objectives: a) To understand bone growth and development
Osteoporosis 1 Nutritional Aspects of Osteoporosis Care and Treatment Cynthia Smith, FNP-BC, RN, MSN, CCD Pars Osteoporosis Clinic, Belpre, Ohio 1) Objectives: a) To understand bone growth and development
Ossification and Bone Remodeling
 Ossification and Bone Remodeling Pre-natal Ossification Embryonic skeleton: fashioned from fibrous membranes or cartilage to accommodate mitosis. 2 types of pre-natal ossification (bone formation) 1.
Ossification and Bone Remodeling Pre-natal Ossification Embryonic skeleton: fashioned from fibrous membranes or cartilage to accommodate mitosis. 2 types of pre-natal ossification (bone formation) 1.
Skeletal System. Chapter 6.1 Human Anatomy & Physiology
 Skeletal System Chapter 6.1 Human Anatomy & Physiology Overview of Skeletal System Bones Joints Skeletal System Cartilage Tendons (bone to muscle) Ligaments (bone to bone) Function of the Skeletal System
Skeletal System Chapter 6.1 Human Anatomy & Physiology Overview of Skeletal System Bones Joints Skeletal System Cartilage Tendons (bone to muscle) Ligaments (bone to bone) Function of the Skeletal System
Osteoporosis. Treatment of a Silently Developing Disease
 Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
36 1 The Skeletal System Slide 1 of 40
 1 of 40 The Skeleton All organisms need structural support. Unicellular organisms have a cytoskeleton. Multicellular animals have either an exoskeleton (arthropods) or an endoskeleton (vertebrates). 2
1 of 40 The Skeleton All organisms need structural support. Unicellular organisms have a cytoskeleton. Multicellular animals have either an exoskeleton (arthropods) or an endoskeleton (vertebrates). 2
CIC EDIZIONI INTERNAZIONALI. Bone fragility: current reviews and clinical features. Mini review
 Mini review Paolo Tranquilli Leali, MD, FBSE Carlo Doria, MD, PhD Alexandros Zachos, MD Adriano Ruggiu, MD Fabio Milia, MD Francesca Barca, MD Orthopaedic Department, University of Sassari, Sassari, Italy
Mini review Paolo Tranquilli Leali, MD, FBSE Carlo Doria, MD, PhD Alexandros Zachos, MD Adriano Ruggiu, MD Fabio Milia, MD Francesca Barca, MD Orthopaedic Department, University of Sassari, Sassari, Italy
Understanding Osteoporosis
 Understanding Osteoporosis Professor Juliet E. Compston Published by Family Doctor Publications Limited in association with the British Medical Association IMPORTANT NOTICE This book is intended not as
Understanding Osteoporosis Professor Juliet E. Compston Published by Family Doctor Publications Limited in association with the British Medical Association IMPORTANT NOTICE This book is intended not as
Beyond BMD: Bone Quality and Bone Strength
 Beyond BMD: Bone Quality and Bone Strength Mary L. Bouxsein, PhD Center for Advanced Orthopedic Studies Department of Orthopedic Surgery, Harvard Medical School MIT-Harvard Health Sciences and Technology
Beyond BMD: Bone Quality and Bone Strength Mary L. Bouxsein, PhD Center for Advanced Orthopedic Studies Department of Orthopedic Surgery, Harvard Medical School MIT-Harvard Health Sciences and Technology
Bone mechanics bone strength and beyond
 Bone mechanics bone strength and beyond Tim Arnett Bone Curriculum Symposium 2018 Department of Cell & Developmental Biology University College London Factors affecting bone strength Mass Architectural
Bone mechanics bone strength and beyond Tim Arnett Bone Curriculum Symposium 2018 Department of Cell & Developmental Biology University College London Factors affecting bone strength Mass Architectural
Anatomy & Physiology
 Anatomy & Physiology 101-805 Unit 6 The Skeletal System Paul Anderson 2011 Skeletal System: Components Bones major organs of system, have all functions of system. Cartilages connect & protect bones at
Anatomy & Physiology 101-805 Unit 6 The Skeletal System Paul Anderson 2011 Skeletal System: Components Bones major organs of system, have all functions of system. Cartilages connect & protect bones at
BONE LABORATORY DEMONSTRATIONS. These demonstrations are found on the bulletin boards outside the MCO Bookstore.
 BONE LABORATORY DEMONSTRATIONS These demonstrations are found on the bulletin boards outside the MCO Bookstore. COMPACT & TRABECULAR BONE - LM When viewed under the polarizing light microscope, the layering
BONE LABORATORY DEMONSTRATIONS These demonstrations are found on the bulletin boards outside the MCO Bookstore. COMPACT & TRABECULAR BONE - LM When viewed under the polarizing light microscope, the layering
Chapter 6 Part B Bones and Skeletal Tissue
 Chapter 6 Part B Bones and Skeletal Tissue 6.5 Bone Development Ossification (osteogenesis) is the process of bone tissue formation Formation of bony skeleton begins in month 2 of development Postnatal
Chapter 6 Part B Bones and Skeletal Tissue 6.5 Bone Development Ossification (osteogenesis) is the process of bone tissue formation Formation of bony skeleton begins in month 2 of development Postnatal
Chapter 5 The Skeletal System:Bone Tissue. Functions of Bone. Bones
 Chapter 5 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose,
Chapter 5 The Skeletal System:Bone Tissue Dynamic and ever-changing throughout life Skeleton composed of many different tissues cartilage, bone tissue, epithelium, nerve, blood forming tissue, adipose,
HASPI Medical Anatomy & Physiology 08a Lab Activity
 HASPI Medical Anatomy & Physiology 08a Lab Activity Name(s): Period: Date: The Skeletal System The skeletal system is primarily responsible for supporting the body and protecting vital organs. We are born
HASPI Medical Anatomy & Physiology 08a Lab Activity Name(s): Period: Date: The Skeletal System The skeletal system is primarily responsible for supporting the body and protecting vital organs. We are born
Pathophysiology of fracture healing
 Pathophysiology of fracture healing Bone anatomy and biomechanics Fracture patterns Bone healing and blood supply Influence of implants 1 What is the structure of bone? 2 Bone structure Four levels: Chemical
Pathophysiology of fracture healing Bone anatomy and biomechanics Fracture patterns Bone healing and blood supply Influence of implants 1 What is the structure of bone? 2 Bone structure Four levels: Chemical
Osseous Tissue and Bone Structure
 6 Osseous Tissue and Bone Structure PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to the Skeletal System Learning Outcomes 6-1 Describe the primary
6 Osseous Tissue and Bone Structure PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to the Skeletal System Learning Outcomes 6-1 Describe the primary
BONE HISTOLOGY SLIDE PRESENTATION
 BONE HISTOLOGY SLIDE PRESENTATION PRESENTED BY: SKELETECH, INC. Clients and Friends: SkeleTech invites you to use these complimentary images for your own presentations or as teaching slides for bone biology.
BONE HISTOLOGY SLIDE PRESENTATION PRESENTED BY: SKELETECH, INC. Clients and Friends: SkeleTech invites you to use these complimentary images for your own presentations or as teaching slides for bone biology.
Chapter 4. Cartilage and Bone. Li Shu-Lei instructor. Dept. Histology and Embryology, School of Basic Medical Sciences, Jilin University
 Chapter 4 Cartilage and Bone Li Shu-Lei instructor Dept. Histology and Embryology, School of Basic Medical Sciences, Jilin University I Cartilage a specialized connective tissue Characterizers: Cartilage
Chapter 4 Cartilage and Bone Li Shu-Lei instructor Dept. Histology and Embryology, School of Basic Medical Sciences, Jilin University I Cartilage a specialized connective tissue Characterizers: Cartilage
Rama Nada. - Mousa Al-Abbadi. 1 P a g e
 - 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
- 1 - Rama Nada - - Mousa Al-Abbadi 1 P a g e Bones, Joints and Soft tissue tumors Before we start: the first 8 minutes was recalling to Dr.Mousa s duties, go over them in the slides. Wherever you see
Bone Development. Two Types of OssificaDon 10/18/14. Osteogenesis ( ) bone Dssue formadon Stages. Bones and Skeletal Tissues: Part B
 Bone Development 6 Bones and Skeletal Tissues: Part B Osteogenesis ( ) bone Dssue formadon Stages Bone formadon begins in the 2nd month of development Postnatal bone growth undl early adulthood Bone remodeling
Bone Development 6 Bones and Skeletal Tissues: Part B Osteogenesis ( ) bone Dssue formadon Stages Bone formadon begins in the 2nd month of development Postnatal bone growth undl early adulthood Bone remodeling
Functions of the Skeletal System
 SKELETAL SYSTEM Functions of the Skeletal System Support: Internal framework that supports and anchors all soft organs. Protection: Bones protect soft body organs Body movement skeletal muscle attached
SKELETAL SYSTEM Functions of the Skeletal System Support: Internal framework that supports and anchors all soft organs. Protection: Bones protect soft body organs Body movement skeletal muscle attached
SKELETAL SYSTEM. Introduction Notes (pt 1)
 SKELETAL SYSTEM Introduction Notes (pt 1) I. INTRODUCTION 1. Bones include active, living tissues: bone tissue, cartilage, dense connective tissue, blood, and nervous tissue. 2. Bones: support and protect
SKELETAL SYSTEM Introduction Notes (pt 1) I. INTRODUCTION 1. Bones include active, living tissues: bone tissue, cartilage, dense connective tissue, blood, and nervous tissue. 2. Bones: support and protect
Skeletal Tissues Dr. Ali Ebneshahidi
 Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection: Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Skeletal Tissues Dr. Ali Ebneshahidi Functions of Bones 1. Support and protection: Bones give shape to body structure. Bones provide support to body weight. Certain bones protect vital internal organs
Osteology. Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College
 Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
Osteology Dr. Carmen E. Rexach Anatomy 35 Mt San Antonio College Functions of the Skeletal System: Support Movement Protection Hemopoiesis Electrolyte balance (Ca ++ /PO -3 4 ) Acid-base balance Storage
Lab Animal Tissue. LEARNING OBJECTIVES: To understand the relationship between the structure and function of different animal tissues
 Name: Bio A.P. PURPOSE: HYPOTHESIS: NONE Lab Animal Tissue BACKGROUND: In animals, groups of closely related cells specialized to perform the same function are called tissues. There are four general classes
Name: Bio A.P. PURPOSE: HYPOTHESIS: NONE Lab Animal Tissue BACKGROUND: In animals, groups of closely related cells specialized to perform the same function are called tissues. There are four general classes
The Skeletal System Vertebral column Sacrum. Osseous tissue For the body and soft organs. Magnesium, sodium, fluoride Levers for muscle action
 10/1/2016 Cranium Facial s Skull Clavicle Scapula Sternum Rib Humerus Vertebra Radius Ulna Carpals Thoracic cage (ribs and sternum) The Skeletal System Vertebral column Sacrum Phalanges Metacarpals Femur
10/1/2016 Cranium Facial s Skull Clavicle Scapula Sternum Rib Humerus Vertebra Radius Ulna Carpals Thoracic cage (ribs and sternum) The Skeletal System Vertebral column Sacrum Phalanges Metacarpals Femur
Hierarchical Microimaging of Bone Structure and Function
 Hierarchical Microimaging of Bone Structure and Function Prof. Ralph Müller, PhD Institute for Biomechanics, ETH Zürich, Zürich, Switzerland 1 Motivation and aims Engineering endpoints have become an important
Hierarchical Microimaging of Bone Structure and Function Prof. Ralph Müller, PhD Institute for Biomechanics, ETH Zürich, Zürich, Switzerland 1 Motivation and aims Engineering endpoints have become an important
ANATOMY & PHYSIOLOGY 1 ( ) For Intensive Nursing PAUL ANDERSON SAMPLE TEST
 ANATOMY & PHYSIOLOGY 1 (101-805) For Intensive Nursing PAUL ANDERSON SAMPLE TEST 3 2011 1. If calcium levels in the extracellular fluid are too low, parathyroid hormone secretion would and osteoclast activity
ANATOMY & PHYSIOLOGY 1 (101-805) For Intensive Nursing PAUL ANDERSON SAMPLE TEST 3 2011 1. If calcium levels in the extracellular fluid are too low, parathyroid hormone secretion would and osteoclast activity
Musculoskeletal Clinical Correlates: Osseous Conditions in Dental Patients
 Musculoskeletal Clinical Correlates: Osseous Conditions in Dental Patients Learning Objectives Define osteoporosis and explain how it is diagnosed. Describe the main risk factors for developing osteoporosis.
Musculoskeletal Clinical Correlates: Osseous Conditions in Dental Patients Learning Objectives Define osteoporosis and explain how it is diagnosed. Describe the main risk factors for developing osteoporosis.
