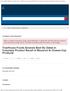
Product NDC Code Lot Number Expiry Dates Distribution Date VALSARTAN TABLETS 40MG 30CT
|
|
|
- Judith Anthony
- 5 years ago
- Views:
Transcription
1 Prinston Pharmaceutical Inc Issues Voluntary Nationwide Recall of Valsartan and Valsartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodimethylamine (NDMA) in the Products Contacts Consumers: Pharmacies, Distributors and Wholesalers: Solco Customer Service at , Option 5 or or fax to: customerservice@solcohealthcare.com; , for Product Return Press Release Valsartan and Valsartan HCTZ Tablets Recall FOR IMMEDIATE RELEASE - CRANBURY, NEW JERSEY, July 13, Prinston Pharmaceutical Inc., dba Solco Healthcare LLC., is voluntarily recalling all lots of Valsartan Tablets, 40 mg, 80mg, 160mg, and 320mg; and Valsartan-Hydrochlorothiazide Tablets, 80mg/12.5mg, 160mg/12.5mg, 160mg/25mg, 320mg/12.5mg, and 320mg/25mg to the consumer level. This product recall is due to the detection of a trace amount of an unexpected impurity, N-nitrosodimethylamine (NDMA), in an active pharmaceutical ingredient by the manufacturer Zhejiang Huahai Pharmaceutical Co. Ltd. -- in the manufacture of the subject product lots. This impurity has been classified as a probable human carcinogen as per International Agency for Research on Cancer (IARC) classification. However, at present Prinston is unaware of any evidence that NDMA has resulted in any harm to patients taking the drugs subject to this recall. To date, Prinston Pharmaceutical Inc. has not received any reports of adverse events related to this recall. The products are indicated for the treatment of hypertension. Consumers and Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication. Patients who are on valsartan should continue taking their medication, as the risk of harm to a patient s health may be higher if the treatment is stopped immediately without any alternative treatment. Product NDC Code Lot Number Expiry Dates Distribution Date VALSARTAN 40MG 30CT All lots From Jul 18 to Jan 20 Oct 2015 Jun 2018 VALSARTAN 80MG 90CT All lots From Jul 18 to Jan 20 Oct 2015 Jun 2018 VALSARTAN 160MG 90CT All lots From Jul 18 to Jan 20 Oct 2015 Jun 2018 VALSARTAN 320MG 90CT All lots From Jul 18 to Jan 20 Oct 2015 Jun MG/12.5MG 90CT All lots From Jul 18 to Jan 20 Jun 2016 Jun MG/12.5MG 90CT All lots From Jul 18 to Jan 20 Jun 2016 Jun 2018
2 160MG/25MG 90CT 320MG/12.5MG 90CT 320MG/25MG 90CT All lots From Jul 18 to Jan 20 Jun 2016 Jun All lots From Jul 18 to Jan 20 Jun 2016 Jun All lots From Jul 18 to Jan 20 Jun 2016 Jun 2018 The lot number and expiry date information can be found on the manufacturer's unit (see photographs below of packaged product bottle labels). Valsartan 40 mg strength, 30 ct bottle Valsartan 80 mg strength, 90 ct bottle Valsartan 160 mg strength, 90 ct bottle
3 Valsartan 320 mg strength, 90 ct bottle Valsartan HCTZ 80 mg/12.5mg strength, 90 ct bottle Valsartan HCTZ 160 mg/12.5mg strength, 90 ct bottle
4 Valsartan HCTZ 160 mg/25mg strength, 90 ct bottle Valsartan HCTZ 320 mg/12.5mg strength, 90 ct bottle Valsartan HCTZ 320 mg/25mg strength, 90 ct bottle Retail pharmacies in possession of any unused products: Valsartan Tablets, 40 mg, 80mg, 160mg, and 320mg; and Valsartan-HCTZ Tablets, 80mg/12.5mg, 160mg/12.5mg, 160mg/25mg, 320mg/12.5mg, and 320mg/25mg, within expiry dates from Jul 2018 to Jan 2020 should immediately return the product by following the instructions below:
5 Pharmacists and wholesalers are asked to check their inventories, segregate any impacted inventory. Immediate discontinue use and distribution of the identified lot numbers. A credit memo will be issued covering the quantity of your product returned. Return products to: DLSS (Dohmen Life Science Services) Attn: Returns Department 4580 S. Mendenhall, Memphis, TN Note: A call tag, a pre-printed, pre-paid return label will be provided to you for product return; return is free of charge. For call tag, please contact Solco Customer Service at , Option 5, Monday through Friday (9am to 5pm EST). Wholesalers: No call is necessary, just send debit memo via or fax to: customerservice@solcohealthcare.com; fax Solco is notifying its distributors and customers by letter and and is arranging for return of all recalled products. Pharmacies and wholesalers that received the impacted products will receive a letter as well as a copy of this press release with their recall notification information. Additional information regarding this recall s affected product lots and expiry dates can be found at or to download at Consumers and patients with medical-related questions regarding this recall, please contact Solco at between the hours of 9:00 a.m. to 5:00 p.m. EST Monday through Friday. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this product. Adverse reactions or quality problems associated with the use of this product may be reported to FDA's MedWatch Adverse Event Reporting program either by phone, on line, by regular mail or by fax. Complete and submit the report Online: Regular Mail or Fax: Download form or call to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to FDA This Product Recall is being made with the knowledge of the United States Food and Drug Administration (FDA).
Contacts Consumers:
 Prinston Pharmaceutical Inc. issues Voluntary Nationwide Recall of Irbesartan and Irbesartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodiethylamine (NDEA) in the
Prinston Pharmaceutical Inc. issues Voluntary Nationwide Recall of Irbesartan and Irbesartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodiethylamine (NDEA) in the
2 Mylan Valsartan/Combination Class 2 Recall
 Mylan Expands Its Voluntary Nationwide Recall of Valsartan Tablets, USP, Amlodipine and Valsartan Tablets, USP, and Valsartan and Hydrochlorothiazide Tablets, USP, to all Lots Within Expiry Due to The
Mylan Expands Its Voluntary Nationwide Recall of Valsartan Tablets, USP, Amlodipine and Valsartan Tablets, USP, and Valsartan and Hydrochlorothiazide Tablets, USP, to all Lots Within Expiry Due to The
Company Announcement. For Immediate Release. Contact. Announcement
 Company Announcement When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the
Company Announcement When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the
FOR IMMEDIATE RELEASE
 FOR IMMEDIATE RELEASE Media: Corey Kerr (614) 757-3383 Voluntary Nationwide Recall of All Liquid Products Manufactured by PharmaTech LLC and Distributed by Leader Brand, Major Pharmaceuticals, and Rugby
FOR IMMEDIATE RELEASE Media: Corey Kerr (614) 757-3383 Voluntary Nationwide Recall of All Liquid Products Manufactured by PharmaTech LLC and Distributed by Leader Brand, Major Pharmaceuticals, and Rugby
Magnesium Sulfate in Water for Injection is indicated for the prevention and control of seizures in preeclampsia and eclampsia, respectively.
 a HOSPIRA ISSUES A VOLUNTARY NATIONWIDE RECALL OF ONE LOT OF MAGNESIUM SULFATE IN WATER FOR INJECTION DUE TO INCORRECT BARCODE LABELING ON THE PRIMARY CONTAINER Consumers: 1-888-345-4680 Media: 610-329-1340
a HOSPIRA ISSUES A VOLUNTARY NATIONWIDE RECALL OF ONE LOT OF MAGNESIUM SULFATE IN WATER FOR INJECTION DUE TO INCORRECT BARCODE LABELING ON THE PRIMARY CONTAINER Consumers: 1-888-345-4680 Media: 610-329-1340
Detox Transforms Health and Nutrition Issues Voluntary Nationwide Recall of Dietary Supplements Due to the Presence of Undeclared Drug Ingredients
 Recalls, Market Withdrawals, & Safety Alerts > Detox Transforms Health and Nutrition Issues Voluntary Nationwide Recall of Dietary Supplements Due to the Pre... U.S. Food and Drug Administration back to
Recalls, Market Withdrawals, & Safety Alerts > Detox Transforms Health and Nutrition Issues Voluntary Nationwide Recall of Dietary Supplements Due to the Pre... U.S. Food and Drug Administration back to
Hospira Issues A Voluntary Nationwide Recall For 8.4% Sodium Bicarbonate Injection, USP,
 Hospira Issues A Voluntary Nationwide Recall For 8.4% Sodium Bicarbonate Injection, USP, Neut TM (Sodium Bicarbonate 4% additive solution), QUELICIN TM (Succinylcholine Chloride Injection, USP) and Potassium
Hospira Issues A Voluntary Nationwide Recall For 8.4% Sodium Bicarbonate Injection, USP, Neut TM (Sodium Bicarbonate 4% additive solution), QUELICIN TM (Succinylcholine Chloride Injection, USP) and Potassium
BAXTER INITIATES VOLUNTARY NATIONWIDE RECALL OF SELECT LOTS OF IV SOLUTIONS DUE TO THE POTENTIAL FOR LEAKING CONTAINERS AND PARTICULATE MATTER
 FOR IMMEDIATE RELEASE Media Contact: John O Malley (224) 948-5353 media@baxter.com Investor Contact: Clare Trachtman (224) 948-3085 BAXTER INITIATES VOLUNTARY NATIONWIDE RECALL OF SELECT LOTS OF IV SOLUTIONS
FOR IMMEDIATE RELEASE Media Contact: John O Malley (224) 948-5353 media@baxter.com Investor Contact: Clare Trachtman (224) 948-3085 BAXTER INITIATES VOLUNTARY NATIONWIDE RECALL OF SELECT LOTS OF IV SOLUTIONS
Allergan s Blephamide (sulfacetamide/prednisolone ophthalmic ointment) 10%/0.2%, 3.5Gm tube
 Allergan s Blephamide (sulfacetamide/prednisolone ophthalmic ointment) 10%/0.2%, 3.5Gm tube On Aug. 24, 2015, Allergan recalled three lots (86258 expires September 2017, 87189 December 2017 and 87514 February
Allergan s Blephamide (sulfacetamide/prednisolone ophthalmic ointment) 10%/0.2%, 3.5Gm tube On Aug. 24, 2015, Allergan recalled three lots (86258 expires September 2017, 87189 December 2017 and 87514 February
If you have any questions, please contact Jerry Webb at ext or
 1. On May 3, 2013, Health & Life, Co., LTD, initiated a nationwide recall of 242,892 EZ Breathe Atomizers, Model #EZ-100, after becoming aware of an increase in the number of complaints. The Atomizers
1. On May 3, 2013, Health & Life, Co., LTD, initiated a nationwide recall of 242,892 EZ Breathe Atomizers, Model #EZ-100, after becoming aware of an increase in the number of complaints. The Atomizers
IMPORTANT DRUG WARNING
 IMPORTANT DRUG INFORMATION UPDATE IMPORTANT DRUG WARNING Rifampin for Injection USP, 600 mg/vial See Page 4 for Lots Pfizer NDC: 0069-0112-01; NOVAPLUS NDC: 0069-0141-01 December 4, 2013 Attention: Product
IMPORTANT DRUG INFORMATION UPDATE IMPORTANT DRUG WARNING Rifampin for Injection USP, 600 mg/vial See Page 4 for Lots Pfizer NDC: 0069-0112-01; NOVAPLUS NDC: 0069-0141-01 December 4, 2013 Attention: Product
Compounds Update. Controlled Substance Safety Edits. DESI Products
 SUPPORTING OUR PROVIDER PARTNERS THROUGH COMMUNICATION AND COLLABORATION. DATE OCTOBER 2018 ISSUE 3 HELPFUL NUMBERS FOR PROVIDERS CVS: 1-888-512-8935 Primary: 004336 Secondary Commercial: 013089 Secondary
SUPPORTING OUR PROVIDER PARTNERS THROUGH COMMUNICATION AND COLLABORATION. DATE OCTOBER 2018 ISSUE 3 HELPFUL NUMBERS FOR PROVIDERS CVS: 1-888-512-8935 Primary: 004336 Secondary Commercial: 013089 Secondary
Effexor XR 150mg and one lot of Greenstone Venlafaxine 150mg extended-release capsules
 Effexor XR 150 and one lot of Greenstone Venlafaxine 150 extended-release capsules On March 6, 2014 Pfizer recalled two lots of Effexor XR 150 and one lot of Greenstone Venlafaxine 150 extended-release
Effexor XR 150 and one lot of Greenstone Venlafaxine 150 extended-release capsules On March 6, 2014 Pfizer recalled two lots of Effexor XR 150 and one lot of Greenstone Venlafaxine 150 extended-release
URGENT: Important Safety Information
 July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
URGENT: Important Safety Information
 October 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Crystallization, Cracked Needle Hubs and Particulate in Diazepam Injection, USP,
October 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Crystallization, Cracked Needle Hubs and Particulate in Diazepam Injection, USP,
Urgent field safety notice
 Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
URGENT MEDICINE RECALL
 Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
POLICY AND PROCEDURE DOCUMENT NAME: Drug Recall Notification Process
 PAGE: 1 of 6 SCOPE: Centene Corporate, Magnolia Health Plan Department, and Envolve Solutions. PURPOSE: To identify and notify prescribers and members affected by FDA-required or voluntary drug withdrawals
PAGE: 1 of 6 SCOPE: Centene Corporate, Magnolia Health Plan Department, and Envolve Solutions. PURPOSE: To identify and notify prescribers and members affected by FDA-required or voluntary drug withdrawals
URGENT FIELD SAFETY NOTICE
 AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
URGENT: DIETARY SUPPLEMENT RECALL
 URGENT: DIETARY SUPPLEMENT RECALL May 26, 2011 Re: NOW Calcium & Magnesium Softgels Product Codes 1251, 1252 Dear Valued Customer: NOW Foods is conducting a voluntary recall of selected lots of all sizes
URGENT: DIETARY SUPPLEMENT RECALL May 26, 2011 Re: NOW Calcium & Magnesium Softgels Product Codes 1251, 1252 Dear Valued Customer: NOW Foods is conducting a voluntary recall of selected lots of all sizes
Butala Emporium, Inc. Recalls Eleven Ayurvedic Dietary Supplements Because of Elevated Levels of Lead and Mercury
 Recalls, Market Withdrawals, & Safety Alerts > Butala Emporium, Inc. Recalls Eleven Ayurvedic Dietary Supplements Because of Elevated Levels of Lead and Me... U.S. Food and Drug Administration back to
Recalls, Market Withdrawals, & Safety Alerts > Butala Emporium, Inc. Recalls Eleven Ayurvedic Dietary Supplements Because of Elevated Levels of Lead and Me... U.S. Food and Drug Administration back to
Suspected Defective Product Report
 Defective Product Report Form European Medicines Agency Inspections Suspected Defective Product Report London, 9 February 2007 Doc. Ref. EMEA/INS/GMP/75464/2007 1. Origin of Report Shaded areas to be completed
Defective Product Report Form European Medicines Agency Inspections Suspected Defective Product Report London, 9 February 2007 Doc. Ref. EMEA/INS/GMP/75464/2007 1. Origin of Report Shaded areas to be completed
Black Widow Antivenin
 Drug Shortage information Action Plan Merck has low inventory of Antivenin Latrodectus Mactans. The company cannot estimate when regular distribution will resume. Merck announced that the expiration date
Drug Shortage information Action Plan Merck has low inventory of Antivenin Latrodectus Mactans. The company cannot estimate when regular distribution will resume. Merck announced that the expiration date
Guidelines for Product Recall or Withdrawal
 REPUBLIC OF KENYA PHARMACY AND POISONS BOARD Guidelines for Product Recall or Withdrawal Edition 1 Date of release for publication June 2006 Date of implementation June 2006 This document has been prepared
REPUBLIC OF KENYA PHARMACY AND POISONS BOARD Guidelines for Product Recall or Withdrawal Edition 1 Date of release for publication June 2006 Date of implementation June 2006 This document has been prepared
Case: 4:18-cv RLW Doc. #: 1 Filed: 09/11/18 Page: 1 of 44 PageID #: 1
 Case: 4:18-cv-01525-RLW Doc. #: 1 Filed: 09/11/18 Page: 1 of 44 PageID #: 1 UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF ISSOURI EASTERN DIVISION JAES JONES, individually and on behalf ) of
Case: 4:18-cv-01525-RLW Doc. #: 1 Filed: 09/11/18 Page: 1 of 44 PageID #: 1 UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF ISSOURI EASTERN DIVISION JAES JONES, individually and on behalf ) of
URGENT: VOLUNTARY DRUG RECALL 07/05/2016
 9075 Centre Pointe Drive, Suite 140 West Chester, OH 45069 Phone: 800.543.2111 URGENT: VOLUNTARY DRUG RECALL 07/05/2016 GLUCOSE TEST STRIPS, TRUETRACK, 50/BOX NDC# 56151-0850-50 Besse# 31330 TRUETRACK
9075 Centre Pointe Drive, Suite 140 West Chester, OH 45069 Phone: 800.543.2111 URGENT: VOLUNTARY DRUG RECALL 07/05/2016 GLUCOSE TEST STRIPS, TRUETRACK, 50/BOX NDC# 56151-0850-50 Besse# 31330 TRUETRACK
New York State Department of Health. Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine
 New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
URGENT FIELD SAFETY NOTICE/ DEVICE RECALL
 URGENT FIELD SAFETY NOTICE/ DEVICE RECALL COMMERCIAL NAME: NC Trek RX Coronary Dilatation Catheter NC Traveler RX Coronary Dilatation Catheter NC Tenku RX PTCA Balloon Catheter FSCA-Identifier: Type of
URGENT FIELD SAFETY NOTICE/ DEVICE RECALL COMMERCIAL NAME: NC Trek RX Coronary Dilatation Catheter NC Traveler RX Coronary Dilatation Catheter NC Tenku RX PTCA Balloon Catheter FSCA-Identifier: Type of
Class I Drugs Event Event ID: Product Type: Status: Date Terminated: Recall Initiation Date: Voluntary / Mandated: Center Classification Date:
 Class I Event 76678 12/29/2015 06/13/2017 E-Mail Complete Pharmacy and Medical Solutions LLC 5829 Nw 158th St Miami Lakes FL United States Distributed throughout Florida Human Chorionic Gonadotropin, 125
Class I Event 76678 12/29/2015 06/13/2017 E-Mail Complete Pharmacy and Medical Solutions LLC 5829 Nw 158th St Miami Lakes FL United States Distributed throughout Florida Human Chorionic Gonadotropin, 125
IMPAACT MOCHA More Options for Children and Adolescents
 IMPAACT 2017 Phase I/II Study of the Safety, Acceptability, Tolerability, and Pharmacokinetics of Oral and Long-Acting Injectable Cabotegravir and Long-Acting Injectable Rilpivirine in Virologically Suppressed
IMPAACT 2017 Phase I/II Study of the Safety, Acceptability, Tolerability, and Pharmacokinetics of Oral and Long-Acting Injectable Cabotegravir and Long-Acting Injectable Rilpivirine in Virologically Suppressed
Manometer Tray, Jamshidi Needle Biopsy, Illinois (TJ) Needle Aspiration, Jamshidi (TJ) Needle Bone Marrow, Thoracentesis/Paracentesis Kit
 URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number:
URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number:
UKALL14 DRUG SUPPLY GUIDELINES
 CONTACT DETAILS UKALL14 DRUG SUPPLY GUIDELINES For further information on trial drugs, trial protocol, dosing, supply drug and distribution queries please contact: UKALL14 Trial Team Phone: 0207 679 9860
CONTACT DETAILS UKALL14 DRUG SUPPLY GUIDELINES For further information on trial drugs, trial protocol, dosing, supply drug and distribution queries please contact: UKALL14 Trial Team Phone: 0207 679 9860
FlexRx 6-Tier. SM Pharmacy Benefit Guide
 FlexRx 6-Tier SM Pharmacy Benefit Guide Welcome to FlexRx The AllWays Health Partners FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many
FlexRx 6-Tier SM Pharmacy Benefit Guide Welcome to FlexRx The AllWays Health Partners FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS
 RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3
 URGENT MEDICAL DEVICE RECALL HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3 May 21, 2018 Dear Physician, We are providing additional information to the letter we recently issued
URGENT MEDICAL DEVICE RECALL HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3 May 21, 2018 Dear Physician, We are providing additional information to the letter we recently issued
Dispensing and administration of emergency opioid antagonist without a
 68-7-23. Dispensing and administration of emergency opioid antagonist without a prescription. (a) A pharmacist may dispense an FDA-approved emergency opioid antagonist and the necessary medical supplies
68-7-23. Dispensing and administration of emergency opioid antagonist without a prescription. (a) A pharmacist may dispense an FDA-approved emergency opioid antagonist and the necessary medical supplies
Important Information about your 2.0 ml Animas Insulin Pump Cartridges
 URGENT: INSULIN PUMP 2.0 ml CARTRIDGE RECALL February 24, 2011 Dear Animas Pumper: Important Information about your 2.0 ml Animas Insulin Pump Cartridges Animas Corporation is dedicated to supporting pumpers
URGENT: INSULIN PUMP 2.0 ml CARTRIDGE RECALL February 24, 2011 Dear Animas Pumper: Important Information about your 2.0 ml Animas Insulin Pump Cartridges Animas Corporation is dedicated to supporting pumpers
ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies
 ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies ISSUING BODY: The Ministry of Health published in: the Official
ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies ISSUING BODY: The Ministry of Health published in: the Official
Supporting our provider partners through communication and collaboration. Formulary Updates
 Supporting our provider partners through communication and collaboration. DATE DECEMBER 2014 ISSUE 4 HELPFUL NUMBERS FOR PROVIDERS Passport Health Plan Magellan: 1-800-846-7971 Bin: 016523 Processor control:
Supporting our provider partners through communication and collaboration. DATE DECEMBER 2014 ISSUE 4 HELPFUL NUMBERS FOR PROVIDERS Passport Health Plan Magellan: 1-800-846-7971 Bin: 016523 Processor control:
Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States
 Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States SOUTH SAN FRANCISCO, Calif. February 14, 2012 Roche and Genentech have been informed that a counterfeit product,
Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States SOUTH SAN FRANCISCO, Calif. February 14, 2012 Roche and Genentech have been informed that a counterfeit product,
Your Prescription Card. Your guide for savings.
 CVS Caremark 620 Epsilon Drive Pittsburgh, PA 15238 4750-_PM 9501 E. Shea Blvd SCOTTSDALE, AZ 85260 Your Prescription Card. Your guide for savings. Dear Plan Member, Welcome to your new benefits. Attached
CVS Caremark 620 Epsilon Drive Pittsburgh, PA 15238 4750-_PM 9501 E. Shea Blvd SCOTTSDALE, AZ 85260 Your Prescription Card. Your guide for savings. Dear Plan Member, Welcome to your new benefits. Attached
March 21, Subject: Availability of Blister-Packaged XELODA (capecitabine) Tablets
 March 21, 2011 Subject: Availability of Blister-Packaged XELODA (capecitabine) Tablets Dear Healthcare Professional: The purpose of this letter is to inform you of the temporary availability of an alternative
March 21, 2011 Subject: Availability of Blister-Packaged XELODA (capecitabine) Tablets Dear Healthcare Professional: The purpose of this letter is to inform you of the temporary availability of an alternative
Texas Vendor Drug Program Specialty Drug List Process. February 2019
 Texas Vendor Drug Program Specialty Drug List Process February 2019 Table of Contents Table of Contents...1 1 About the Specialty Drug List...2 1.1 Information for Pharmacies... 2 1.2 Information for Managed
Texas Vendor Drug Program Specialty Drug List Process February 2019 Table of Contents Table of Contents...1 1 About the Specialty Drug List...2 1.1 Information for Pharmacies... 2 1.2 Information for Managed
Urgent Notice September 16, 2014
 Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager,
Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager,
STATE OF NEW JERSEY Division of Gaming Enforcement CASINO HOTEL ALCOHOLIC BEVERAGE MERCHANDISING PERMIT APPLICATION
 STATE OF NEW JERSEY Division of Gaming Enforcement CASINO HOTEL ALCOHOLIC BEVERAGE MERCHANDISING PERMIT APPLICATION STATE OF NEW JERSEY Division of Gaming Enforcement MERCHANDISING PERMIT APPLICATION FOR
STATE OF NEW JERSEY Division of Gaming Enforcement CASINO HOTEL ALCOHOLIC BEVERAGE MERCHANDISING PERMIT APPLICATION STATE OF NEW JERSEY Division of Gaming Enforcement MERCHANDISING PERMIT APPLICATION FOR
NovoPen Echo URGENT MEDICAL DEVICE RECALL
 NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... FDA Home Page CDRH Home Page Search A-Z Index
 US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... http://www.fda.gov/cdrh/safety/102008-surgicalmesh.html Page 1 of 3 FDA Home Page CDRH Home Page Search
US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... http://www.fda.gov/cdrh/safety/102008-surgicalmesh.html Page 1 of 3 FDA Home Page CDRH Home Page Search
Recall -- Firm Press Release
 Recalls, Market Withdrawals, & Safety Alerts > Iowa Select Herbs, LLC Issues a Nationwide Recall of Its Products Pursuant to Consent Decree Issued by the Feder... U.S. Food and Drug Administration back
Recalls, Market Withdrawals, & Safety Alerts > Iowa Select Herbs, LLC Issues a Nationwide Recall of Its Products Pursuant to Consent Decree Issued by the Feder... U.S. Food and Drug Administration back
PRODUCT RECALL NOTICE URGENT
 PRODUCT RECALL NOTICE URGENT Date: July 21, 2018 To: Re: Mondelēz Global LLC Customers Recall Notice Certain Ritz Cracker Sandwiches and Ritz Bits product in the U.S., including Puerto Rico & U.S. Virgin
PRODUCT RECALL NOTICE URGENT Date: July 21, 2018 To: Re: Mondelēz Global LLC Customers Recall Notice Certain Ritz Cracker Sandwiches and Ritz Bits product in the U.S., including Puerto Rico & U.S. Virgin
News & Views. Antipsychotics on Maryland Medicaid PDL and Coverage of a 30-day Emergency Supply of Atypical Antipsychotics
 Maryland Medicaid Pharmacy Program News & Views February 2011 Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy Antipsychotics on Maryland Medicaid PDL and Coverage
Maryland Medicaid Pharmacy Program News & Views February 2011 Maryland Department of Health and Mental Hygiene /Office of Systems, Operations and Pharmacy Antipsychotics on Maryland Medicaid PDL and Coverage
IMPORTANT DRUG INFORMATION
 IMPORTANT DRUG INFORMATION 2 nd January 2019 Subject: ERWINAZE Batch 191K Notice of *New* Special Handling Instructions Use a 0.2-micron, low protein binding, in-line filter for IV administration of ERWINAZE
IMPORTANT DRUG INFORMATION 2 nd January 2019 Subject: ERWINAZE Batch 191K Notice of *New* Special Handling Instructions Use a 0.2-micron, low protein binding, in-line filter for IV administration of ERWINAZE
American Emu Association. Certified Emu Oil Program
 Table of Contents Part 1. Frequently Asked Questions... 3 Part 2. AEA Certified Fully Refined Seal & Verbiage Usage Requirements... 5 Section A. Program Requirements... 5 Section B. Product Labeling Requirements...
Table of Contents Part 1. Frequently Asked Questions... 3 Part 2. AEA Certified Fully Refined Seal & Verbiage Usage Requirements... 5 Section A. Program Requirements... 5 Section B. Product Labeling Requirements...
Acetylcysteine Inhalation Solution
 Drug Shortage information Action Plan delays and another manufacturer voluntarily suspending production. Hospira has a limited supply available and other manufacturers unable to estimate a release date.
Drug Shortage information Action Plan delays and another manufacturer voluntarily suspending production. Hospira has a limited supply available and other manufacturers unable to estimate a release date.
Pharmacy benefit guide
 FlexRx SM 5-Tier Pharmacy benefit guide 1 Welcome to FlexRx The NHP FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many common medications
FlexRx SM 5-Tier Pharmacy benefit guide 1 Welcome to FlexRx The NHP FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many common medications
PLEASE NOTE. For more information concerning the history of this Act, please see the Table of Public Acts.
 PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to July 1, 2012. It is intended for information and reference purposes only. This
PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to July 1, 2012. It is intended for information and reference purposes only. This
$250 (Deductible does not apply to Tier 1 and Tier 2) $500 (Deductible does not apply to Tier 1 and Tier 2)
 Benefit Summary Outpatient Prescription Drug Illinois 5/50/100/250 Plan 455 Your Co-payment and/or Co-insurance is determined by the tier to which the Prescription Drug List (PDL) Management Committee
Benefit Summary Outpatient Prescription Drug Illinois 5/50/100/250 Plan 455 Your Co-payment and/or Co-insurance is determined by the tier to which the Prescription Drug List (PDL) Management Committee
Food Service Industry Membership
 Coeliac Western Australia Food Service Industry Membership Page 1 About Who we are Coeliac Western Australia (CWA) is a not-for-profit organisation established in 1979 to assist and support people who
Coeliac Western Australia Food Service Industry Membership Page 1 About Who we are Coeliac Western Australia (CWA) is a not-for-profit organisation established in 1979 to assist and support people who
2016 Travelers Prescription Drug Plan Blue Cross Blue Shield Plan and United Healthcare Choice Plus Plan
 2016 Travelers Prescription Drug Plan Blue Cross Blue Shield Plan and United Healthcare Choice Plus Plan Plan Details, Programs, and Policies Table of Contents Click on the links below to be taken to that
2016 Travelers Prescription Drug Plan Blue Cross Blue Shield Plan and United Healthcare Choice Plus Plan Plan Details, Programs, and Policies Table of Contents Click on the links below to be taken to that
Case 3:15-cv PGS-TJB Document 1 Filed 08/04/15 Page 1 of 111 PageID: 1 ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) )
 Case 3:15-cv-05982-PGS-TJB Document 1 Filed 08/04/15 Page 1 of 111 PageID: 1 Liza M. Walsh Christine I. Gannon CONNELL FOLEY LLP One Newark Center 1085 Raymond Boulevard, 19th Floor Newark, New Jersey
Case 3:15-cv-05982-PGS-TJB Document 1 Filed 08/04/15 Page 1 of 111 PageID: 1 Liza M. Walsh Christine I. Gannon CONNELL FOLEY LLP One Newark Center 1085 Raymond Boulevard, 19th Floor Newark, New Jersey
Medicare Part D Overutilization Monitoring System
 Medicare Part D Overutilization Monitoring System Lisa Thorpe, Division of Part D Policy Gary Wirth, Division of Clinical and Operational Performance Medicare Drug Benefit and C&D Data Group July 17, 2013
Medicare Part D Overutilization Monitoring System Lisa Thorpe, Division of Part D Policy Gary Wirth, Division of Clinical and Operational Performance Medicare Drug Benefit and C&D Data Group July 17, 2013
Product Recalls. Protecting Public Health. Presented by William Stewart & Thomas DiBruno
 Product Recalls Protecting Public Health Presented by William Stewart & Thomas DiBruno Overview Agency s Mission Office of Field Operation (OFO) FSIS Directive 8080.1 Rev 5 Recall Effectiveness Checks
Product Recalls Protecting Public Health Presented by William Stewart & Thomas DiBruno Overview Agency s Mission Office of Field Operation (OFO) FSIS Directive 8080.1 Rev 5 Recall Effectiveness Checks
Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol
 Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team
Metformin MR to low cost branded generic (Sukkarto SR) Switch Protocol Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team
DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT
 c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
Access Points: Frequently Asked Questions 15 June 2016
 Access Points: Frequently Asked Questions 15 June 2016 Access Point Interim Agreement Q. Why have I received an Interim Agreement? The Commonwealth and Diabetes Australia have entered into a new NDSS Agreement
Access Points: Frequently Asked Questions 15 June 2016 Access Point Interim Agreement Q. Why have I received an Interim Agreement? The Commonwealth and Diabetes Australia have entered into a new NDSS Agreement
TreeHouse Foods Amends Best By Dates in Voluntary Product Recall of Macaroni & Cheese Cup Products
 Recalls, Market Withdrawals, & Safety Alerts > TreeHouse Foods Amends Best By Dates in Voluntary Product Recall of Macaroni & Cheese Cup Products back to Recalls, Market Withdrawals, & Safety Alerts Company
Recalls, Market Withdrawals, & Safety Alerts > TreeHouse Foods Amends Best By Dates in Voluntary Product Recall of Macaroni & Cheese Cup Products back to Recalls, Market Withdrawals, & Safety Alerts Company
WELCOME. To learn more or place an order, please call
 PRODUCT CATALOG WELCOME OUR VALUES American Regent is committed to providing you with a growing and diversified portfolio of specialty injectable products to help meet the needs of today s ever-changing
PRODUCT CATALOG WELCOME OUR VALUES American Regent is committed to providing you with a growing and diversified portfolio of specialty injectable products to help meet the needs of today s ever-changing
URGENT MEDICAL DEVICE CORRECTION. Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps
 URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
Enforcement Report - Week of November 1, 2017
 Enforcement Report - Week of November 1, 2017 Class I Event 77841 06/22/2017 10/24/2017 Press Release Vitility 7283 Florin Mall Dr Apt #3 Sacramento CA United States Nationwide in the USA MAN OF STEEL
Enforcement Report - Week of November 1, 2017 Class I Event 77841 06/22/2017 10/24/2017 Press Release Vitility 7283 Florin Mall Dr Apt #3 Sacramento CA United States Nationwide in the USA MAN OF STEEL
2018 Travelers Prescription Drug Plan High Deductible + HSA Plan
 2018 Travelers Prescription Drug Plan High Deductible + HSA Plan Plan Details, Programs, and Policies Table of Contents Click on the links below to be taken to that section National Preferred Formulary
2018 Travelers Prescription Drug Plan High Deductible + HSA Plan Plan Details, Programs, and Policies Table of Contents Click on the links below to be taken to that section National Preferred Formulary
Herbal medicines and supplements for cancer patients
 Herbal medicines and supplements for cancer patients Questions and answers Information for patients Pharmacy page 2 of 8 This leaflet is for all patients having treatment for cancer who are taking or thinking
Herbal medicines and supplements for cancer patients Questions and answers Information for patients Pharmacy page 2 of 8 This leaflet is for all patients having treatment for cancer who are taking or thinking
Pharmacy Manual. Cambridge University Hospitals NHS Foundation Trust & University of Cambridge
 Page 1 of 11 Pharmacy Manual Trial Title: PRedicting Outcomes For Crohn s disease using a molecular biomarker (PROFILE) trial EudraCT Number: N/A (non-ctimp study) ISRCTN: 11808228 REC Reference: 17/EE/0382
Page 1 of 11 Pharmacy Manual Trial Title: PRedicting Outcomes For Crohn s disease using a molecular biomarker (PROFILE) trial EudraCT Number: N/A (non-ctimp study) ISRCTN: 11808228 REC Reference: 17/EE/0382
September page 1 / 15
 September 2018 page 1 / 15 OTC Medicines Corner Advising on this article: Tara Whetsel September 4, 2018 One-dose-fits-all approach to aspirin therapy for primary prevention is not ideal Key Point The
September 2018 page 1 / 15 OTC Medicines Corner Advising on this article: Tara Whetsel September 4, 2018 One-dose-fits-all approach to aspirin therapy for primary prevention is not ideal Key Point The
The REDOXS Study. REducing Deaths due to OXidative Stress. A randomized trial of glutamine and antioxidant supplementation in critically ill patients
 The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
One MedImmune Way. Gaithersburg, MD 20878
 One MedImmune Way Gaithersburg, MD 20878 TO: RE: Immunization Provider or Awardee FluMist 2014-2015 Replacement Program This letter is to inform you of the FluMist Replacement Program for product purchased
One MedImmune Way Gaithersburg, MD 20878 TO: RE: Immunization Provider or Awardee FluMist 2014-2015 Replacement Program This letter is to inform you of the FluMist Replacement Program for product purchased
Dry Needling (DN) Registration
 Dry Needling (DN) Registration To register for any of our programs, please complete the enclosed registration form (next page) and mail of fax the form to us. Fees Cost of the DN 1-3 courses is US $895
Dry Needling (DN) Registration To register for any of our programs, please complete the enclosed registration form (next page) and mail of fax the form to us. Fees Cost of the DN 1-3 courses is US $895
Unit 204 Assist with the assembly of prescribed items
 Element 1 Assemble prescribed 171 172 Element 1 Assemble prescribed Background Assembling prescribed is a complex process. The two main components of this process are labelling and dispensing. It is essential
Element 1 Assemble prescribed 171 172 Element 1 Assemble prescribed Background Assembling prescribed is a complex process. The two main components of this process are labelling and dispensing. It is essential
Counterfeit Medicinal Products. FINLAND Roschier, Attorneys Ltd.
 Counterfeit Medicinal Products FINLAND Roschier, Attorneys Ltd. CONTACT INFORMATION Mikael Segercrantz Robert Hagelstam Roschier, Attorneys Ltd. Keskuskatu 7 A 00100 Helsinki, Finland 358.20.506.6000 mikael.segercrantz@roschier.com
Counterfeit Medicinal Products FINLAND Roschier, Attorneys Ltd. CONTACT INFORMATION Mikael Segercrantz Robert Hagelstam Roschier, Attorneys Ltd. Keskuskatu 7 A 00100 Helsinki, Finland 358.20.506.6000 mikael.segercrantz@roschier.com
Recall Guidelines. for Chinese Medicine Products
 Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
LIFESTYLE MEDICO SPECIAL OFFER TO HKICS MEMBERS
 LIFESTYLE MEDICO SPECIAL OFFER TO HKICS MEMBERS Lifestyle Medico aims to improve the lives of those we serve through the delivery of affordable, effective and efficient lifestyle health care solutions.
LIFESTYLE MEDICO SPECIAL OFFER TO HKICS MEMBERS Lifestyle Medico aims to improve the lives of those we serve through the delivery of affordable, effective and efficient lifestyle health care solutions.
Lamivudine Tablets 150 mg (Macleods Pharmaceuticals Ltd), HA424. WHOPAR Part 5 05/2011, version 1.0 LABELLING. Page 1 of 8
 LABELLING Page 1 of 8 PARTICULARS TO APPEAR ON THE OUTER PACKAGING Bottle carton label 1. NAME OF THE MEDICINAL PRODUCT Lamivudine 150 mg Tablets * 2. STATEMENT OF ACTIVE SUBSTANCE Each film-coated tablet
LABELLING Page 1 of 8 PARTICULARS TO APPEAR ON THE OUTER PACKAGING Bottle carton label 1. NAME OF THE MEDICINAL PRODUCT Lamivudine 150 mg Tablets * 2. STATEMENT OF ACTIVE SUBSTANCE Each film-coated tablet
Do not open the test booklet prior to being told to do so.
 Last Name: Pharmacy 431 Pharmacy Ethics and Law Quiz 5 Do not open the test booklet prior to being told to do so. I, the undersigned student, agree to do my best on the exam and that I have only used resources
Last Name: Pharmacy 431 Pharmacy Ethics and Law Quiz 5 Do not open the test booklet prior to being told to do so. I, the undersigned student, agree to do my best on the exam and that I have only used resources
AFDO Conference June 9, Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide
 AFDO Conference June 9, 2009 Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide Government Agencies 483 observations A violation of the FD&C Act involving
AFDO Conference June 9, 2009 Mercedes Mota, Director Medical Device, Surgical Care Johnson & Johnson Quality & Compliance Worldwide Government Agencies 483 observations A violation of the FD&C Act involving
Urgent Medical Device Recall
 PENTAX Medical 3 Paragon Drive Montvale, New Jersey 07645 Toll-free: 800-431-5880 Tel: 201-571-2300 Fax: 201-799-4032 June 12, 2014 Urgent Medical Device Recall Field Safety Corrective Action IFU Addendum
PENTAX Medical 3 Paragon Drive Montvale, New Jersey 07645 Toll-free: 800-431-5880 Tel: 201-571-2300 Fax: 201-799-4032 June 12, 2014 Urgent Medical Device Recall Field Safety Corrective Action IFU Addendum
Medical information Cook Children s
 Medical information Health care providers Primary care provider (PCP): City: State: ZIP code: Phone: Fax: Email: Preferred hospital: Phone: Fax: Email: Specialty hospital: Phone: Fax: Email: Lab: Phone:
Medical information Health care providers Primary care provider (PCP): City: State: ZIP code: Phone: Fax: Email: Preferred hospital: Phone: Fax: Email: Specialty hospital: Phone: Fax: Email: Lab: Phone:
PURCHASING MEMORANDUM
 NUMBER: CL-602 DATE: March 3, 2005 Pricing Clarification MMCAP has received several calls concerning the prices invoiced for vaccine received during the current flu season. All orders filled by Aventis
NUMBER: CL-602 DATE: March 3, 2005 Pricing Clarification MMCAP has received several calls concerning the prices invoiced for vaccine received during the current flu season. All orders filled by Aventis
Timeline of Major Events:
 Senate HELP Committee- Pharmacy Compounding: Implications of the 2012 Meningitis Outbreak November 15, 2012: Dr. Marion Kainer On behalf of the Tennessee Department of Health, I would like to thank Senator
Senate HELP Committee- Pharmacy Compounding: Implications of the 2012 Meningitis Outbreak November 15, 2012: Dr. Marion Kainer On behalf of the Tennessee Department of Health, I would like to thank Senator
Pathology Service User Guide Haematology
 Pathology Service User Guide Haematology St Richard s This section of the Pathology Service User Guide includes: Anticoagulant Therapy Information about the Anticoagulant Clinic Low Molecular Weight Heparin
Pathology Service User Guide Haematology St Richard s This section of the Pathology Service User Guide includes: Anticoagulant Therapy Information about the Anticoagulant Clinic Low Molecular Weight Heparin
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan
 URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
Furthermore, medications dispensed by physicians often cost more for the payer than the same medication dispensed by a retail pharmacy.
 Physician Dispensing Physician Dispensing RELEVANCE IN WORKERS COMP Physician dispensing is when prescribers give medications directly to patients, rather than giving patients prescriptions to fill at
Physician Dispensing Physician Dispensing RELEVANCE IN WORKERS COMP Physician dispensing is when prescribers give medications directly to patients, rather than giving patients prescriptions to fill at
Health Authority Abu Dhabi. Document Title: Policy for Recall of Drugs and Healthcare Products Document Ref. Number:
 Health Authority Abu Dhabi Document Title: Policy for Recall of Drugs and Healthcare Products Document Ref. Number: PPR/DMP/DR/P0001 Version 0.9 Approval Date: August 2007 Effective Date: August 2007 Last
Health Authority Abu Dhabi Document Title: Policy for Recall of Drugs and Healthcare Products Document Ref. Number: PPR/DMP/DR/P0001 Version 0.9 Approval Date: August 2007 Effective Date: August 2007 Last
ARCADIA Study Coordinator Training Investigator Meeting
 ARCADIA Study Coordinator Training Learning Goals 1. The coordinator will be able to identify the 3 screening tests required to meet the atrial cardiopathy criteria for ARCADIA. 2. The coordinator will
ARCADIA Study Coordinator Training Learning Goals 1. The coordinator will be able to identify the 3 screening tests required to meet the atrial cardiopathy criteria for ARCADIA. 2. The coordinator will
Influenza Update. Kelly L. Moore, MD, MPH Medical Director, Immunization Program TN Department of Health TPHA Epi Section September 3, 2009
 2009-2010 Influenza Update Kelly L. Moore, MD, MPH Medical Director, Immunization Program TN Department of Health TPHA Epi Section September 3, 2009 Outline Epidemiology to date What to do until vaccine
2009-2010 Influenza Update Kelly L. Moore, MD, MPH Medical Director, Immunization Program TN Department of Health TPHA Epi Section September 3, 2009 Outline Epidemiology to date What to do until vaccine
South Plains Emergency Medical Services, Inc. P.O. Box Lubbock, Texas 79453
 South Plains Emergency Medical Services, Inc. P.O. Box 53597 Lubbock, Texas 79453 June 28, 2017 TO: SPEMS EMS Services SUBJECT: SPEMS PARAMEDIC PROTOCOL VARIANCE With the ongoing medication shortages,
South Plains Emergency Medical Services, Inc. P.O. Box 53597 Lubbock, Texas 79453 June 28, 2017 TO: SPEMS EMS Services SUBJECT: SPEMS PARAMEDIC PROTOCOL VARIANCE With the ongoing medication shortages,
What is Quitline Iowa?
 CONTENTS: What is Quitline Iowa? 0 A telephone counseling helpline for tobacco-use cessation. Free to all residents of the state of Iowa Open Monday-Thursday 7:00am 12:00am / Friday 7:00am 9:00pm / Saturday
CONTENTS: What is Quitline Iowa? 0 A telephone counseling helpline for tobacco-use cessation. Free to all residents of the state of Iowa Open Monday-Thursday 7:00am 12:00am / Friday 7:00am 9:00pm / Saturday
Please see the attached drug alert for onward transmission as below.
 Chief Medical Officer Directorate Pharmacy and Medicines Division T: 0131-244 2528 E: irene.fazakerley@gov.scot IMMEDIATE MESSAGE TO: 1. Directors of Pharmacy 2. Medical Directors NHS Boards 18 April 2018
Chief Medical Officer Directorate Pharmacy and Medicines Division T: 0131-244 2528 E: irene.fazakerley@gov.scot IMMEDIATE MESSAGE TO: 1. Directors of Pharmacy 2. Medical Directors NHS Boards 18 April 2018
A helping hand when you need it most
 A helping hand when you need it most Priority Services Register Promise The Priority Services Register (PSR) is free to join. It helps energy companies * like us to look after customers who have extra
A helping hand when you need it most Priority Services Register Promise The Priority Services Register (PSR) is free to join. It helps energy companies * like us to look after customers who have extra
PHARMACY SERVICE ARRANGEMENTS FOR THE SUPPLY OF PALLIATIVE CARE SYRINGES AND MEDICINES FOR COMMUNITY PATIENTS
 PHARMACY SERVICE ARRANGEMENTS FOR THE SUPPLY OF PALLIATIVE CARE SYRINGES AND MEDICINES FOR COMMUNITY PATIENTS The benefits of prefilled syringes for palliative care from the hospital pharmacy service In
PHARMACY SERVICE ARRANGEMENTS FOR THE SUPPLY OF PALLIATIVE CARE SYRINGES AND MEDICINES FOR COMMUNITY PATIENTS The benefits of prefilled syringes for palliative care from the hospital pharmacy service In
