Urgent Notice September 16, 2014
|
|
|
- Mildred Phelps
- 6 years ago
- Views:
Transcription
1 Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager, ICU/Neuro ICU Manager: Please be aware that Codman Neuro is initiating a voluntary recall of all lots of EDS 3 CSF External Drainage Systems, with expiration on or before August 2017 ( , please see attachment). The system is indicated for draining cerebrospinal fluid (CSF) and other fluids of similar characteristics as a means of reducing intracranial pressure and CSF volume when the insertion of a permanent, internal shunt is not indicated. Please be aware that products in your possession may be affected. Affected Product (all lots) Code GTIN Description EDS 3CSF External Drainage System with Ventricular Catheter EDS 3CSF External Drainage System (no Ventricular Catheter) Lumbar Drainage Catheter Kit II with EDS 3System This recall is being initiated because the tubing within the system that drains CSF may leak or disconnect from the joints. Leakage and tube separations may result in over- or under-drainage of CSF from the ventricular system or introduction of air into the ventricular system (pneumocephalus). This may result in collapsed ventricles, subdural bleeding, or an inability to properly control elevated intracranial pressure. The tubing disconnection or leakage may also increase the risk of ventriculitis. If undetected or untreated each of these events may cause severe brain injury, which may lead to coma, stroke or death. Actions for Clinicians Treating Patients with the EDS 3 System These systems are most often used on neurocritical care floors and these issues are likely to be detected immediately. For patients currently being managed with the EDS 3 System, the system should be replaced immediately. In the case where no substitute drainage system is immediately available, the EDS 3 System may continue to be used until an alternative product can be obtained.
2 Manipulation of tubing should be minimized and extra vigilance is required for early detection of leakage and/or disconnection. As of September 9, 2014, approximately percent of complaints reported a failed connection from the joint and percent reported leakage according to the most recent complaint data, but some customers complaint rates have been as high as 2.6 percent. Please provide this notice to any neurosurgeons or other clinicians at your facility who are managing patients with the EDS 3 System. We request that you immediately check all inventory to determine if you have affected product. Please use the included instructions to report your inventory status and return affected product. We apologize for the inconvenience this recall may cause. At Codman Neuro, we are dedicated to delivering products that meet the highest quality standards. We regret the need to undertake this voluntary recall and we are committed to resolving this issue. If you have any questions or concerns regarding this notification, please contact your local Codman Neuro Representative or Scientific and Medical Affairs at SciMedAffairs@its.jnj.com or (866) Thank you for your cooperation. Sincerely, J. Thomas Megerian MD, PhD Vice President - Strategic Medical Affairs and Medical Sciences
3 Instructions for Reporting Inventory Status, Returning Product and Obtaining Credit You are receiving this voluntary recall notification because our records indicate that you are the recipient of one or more products affected by this recall. Please complete the enclosed U.S Acknowledgement Form and fax the completed form to It is important that we receive this acknowledgement form, even if you have no affected product in your inventory. You may also the form to us at Product Returns: Please isolate all inventory of the affected product and return to Stericycle at the following address: Stericycle 2670 Executive Drive, Suite A Attn: Event #7229 Indianapolis, IN Enclosed is a prepaid UPS shipping label to be used for the return of affected product. If you need additional return labels, please contact Stericycle directly at A photocopy of the completed U.S. Acknowledgement Form should be used as a packing slip and inserted into the box along with the units you are returning. Full credit will be issued against your original invoice for any recalled product being returned.
4 U.S. ACKNOWLEDGEMENT FORM This signed form acknowledges receipt of the recall letter issued by Codman Neuro regarding the EDS 3 CSF External Drainage System. Not completing this form is in its entirety can cause a delay in processing your credit. We have checked our current inventory for affected products listed in the recall announcement and indicated our inventory levels below: We acknowledge the receipt of this information but do not have the affected EDS 3 CSF External Drainage System products in stock. We acknowledge the receipt of this information and the products listed below are in stock and will be returned to Codman Neuro for dollar credit. We have affected product, but it is currently in use with no available alternative, so we are retaining product to address emergent patient need. We will return affected product once an alternative product is available. Product Code Lot Number Quantity Hospital Name/Location: Print Name: Phone Number: Authorized Signature/Date
5 ATTACHMENT 1 EDS 3 CSF External Drainage System (Sample) Product Label Expiration date information located here. All product affected expires on or before
URGENT: VOLUNTARY DRUG RECALL 07/05/2016
 9075 Centre Pointe Drive, Suite 140 West Chester, OH 45069 Phone: 800.543.2111 URGENT: VOLUNTARY DRUG RECALL 07/05/2016 GLUCOSE TEST STRIPS, TRUETRACK, 50/BOX NDC# 56151-0850-50 Besse# 31330 TRUETRACK
9075 Centre Pointe Drive, Suite 140 West Chester, OH 45069 Phone: 800.543.2111 URGENT: VOLUNTARY DRUG RECALL 07/05/2016 GLUCOSE TEST STRIPS, TRUETRACK, 50/BOX NDC# 56151-0850-50 Besse# 31330 TRUETRACK
URGENT FIELD SAFETY NOTICE/ DEVICE RECALL
 URGENT FIELD SAFETY NOTICE/ DEVICE RECALL COMMERCIAL NAME: NC Trek RX Coronary Dilatation Catheter NC Traveler RX Coronary Dilatation Catheter NC Tenku RX PTCA Balloon Catheter FSCA-Identifier: Type of
URGENT FIELD SAFETY NOTICE/ DEVICE RECALL COMMERCIAL NAME: NC Trek RX Coronary Dilatation Catheter NC Traveler RX Coronary Dilatation Catheter NC Tenku RX PTCA Balloon Catheter FSCA-Identifier: Type of
URGENT FIELD SAFETY NOTICE
 AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
URGENT MEDICINE RECALL
 Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
Wednesday 7 th September 2016 URGENT MEDICINE RECALL GlucaGen HypoKit (glucagon (rys) as HCl 1 mg (1 IU) powder vial with 1 ml, prefilled solvent syringe); AUST R 47105 Batch numbers: FS6X465, FS6X536,
URGENT FIELD SAFETY NOTICE
 August X, 2018 Olympus reference: QIL 151-005 URGENT FIELD SAFETY NOTICE IMPORTANT UPDATED LABELING INFORMATION NEW REPROCESSING INSTRUCTIONS FOR THE OLYMPUS PJF-160 DUODENOSCOPE ATTENTION: Endoscopy Department,
August X, 2018 Olympus reference: QIL 151-005 URGENT FIELD SAFETY NOTICE IMPORTANT UPDATED LABELING INFORMATION NEW REPROCESSING INSTRUCTIONS FOR THE OLYMPUS PJF-160 DUODENOSCOPE ATTENTION: Endoscopy Department,
URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP)
 May 4, 2018 via [INSERT METHOD] URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP) AFFECTED PRODUCT PART NUMBER DISTRIBUTION DATE Cardiosave
May 4, 2018 via [INSERT METHOD] URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP) AFFECTED PRODUCT PART NUMBER DISTRIBUTION DATE Cardiosave
New York State Department of Health. Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine
 New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
New York State Department of Health Notice of Voluntary Recall of Influenza A (H1N1) 2009 Monovalent Vaccine The U.S. Centers for Disease Control and Prevention have announced a Non safety Related Voluntary
URGENT MEDICAL DEVICE CORRECTION. Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps
 URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER
 URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER Commercial Name: Unomedical Sterile Urine Drainage Bags LOT No: Please see attached sheet for reference numbers & affected LOT numbers FSCA Id: 2010/11
URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER Commercial Name: Unomedical Sterile Urine Drainage Bags LOT No: Please see attached sheet for reference numbers & affected LOT numbers FSCA Id: 2010/11
Manometer Tray, Jamshidi Needle Biopsy, Illinois (TJ) Needle Aspiration, Jamshidi (TJ) Needle Bone Marrow, Thoracentesis/Paracentesis Kit
 URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number:
URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number:
NovoPen Echo URGENT MEDICAL DEVICE RECALL
 NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
Urgent Patient Notification
 Urgent Patient Notification Comfort, Comfort Short, and Contact Detach Infusion Sets - Tubing May Detach From the Connect/Disconnect Location November 24, 2014 Dear Valued Customer: At Animas, we hold
Urgent Patient Notification Comfort, Comfort Short, and Contact Detach Infusion Sets - Tubing May Detach From the Connect/Disconnect Location November 24, 2014 Dear Valued Customer: At Animas, we hold
Important Information about your 2.0 ml Animas Insulin Pump Cartridges
 URGENT: INSULIN PUMP 2.0 ml CARTRIDGE RECALL February 24, 2011 Dear Animas Pumper: Important Information about your 2.0 ml Animas Insulin Pump Cartridges Animas Corporation is dedicated to supporting pumpers
URGENT: INSULIN PUMP 2.0 ml CARTRIDGE RECALL February 24, 2011 Dear Animas Pumper: Important Information about your 2.0 ml Animas Insulin Pump Cartridges Animas Corporation is dedicated to supporting pumpers
Ref: PFSN18 Deviations of high (>4.5) CoaguChek INR values due to calibration with WHO reference standard rtf/16 SBN-CPS
 Consumer Letter (CoaguChek XS PT Test PST, CoaguChek XS PT Test, CoaguChek PT Test) Urgent Field Safety Notice Limited, Ref: PFSN18 Deviations of high (>4.5) CoaguChek INR values due
Consumer Letter (CoaguChek XS PT Test PST, CoaguChek XS PT Test, CoaguChek PT Test) Urgent Field Safety Notice Limited, Ref: PFSN18 Deviations of high (>4.5) CoaguChek INR values due
URGENT: DIETARY SUPPLEMENT RECALL
 URGENT: DIETARY SUPPLEMENT RECALL May 26, 2011 Re: NOW Calcium & Magnesium Softgels Product Codes 1251, 1252 Dear Valued Customer: NOW Foods is conducting a voluntary recall of selected lots of all sizes
URGENT: DIETARY SUPPLEMENT RECALL May 26, 2011 Re: NOW Calcium & Magnesium Softgels Product Codes 1251, 1252 Dear Valued Customer: NOW Foods is conducting a voluntary recall of selected lots of all sizes
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan
 URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
Urgent Field Corrective Action
 Siemens Healthcare Diagnostics Products GmbH N Latex CDT Urgent Field Corrective Action PP-18-002.A.OUS May 2018 Negative bias of %CDT values Our records indicate that your facility may have received the
Siemens Healthcare Diagnostics Products GmbH N Latex CDT Urgent Field Corrective Action PP-18-002.A.OUS May 2018 Negative bias of %CDT values Our records indicate that your facility may have received the
HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3
 URGENT MEDICAL DEVICE RECALL HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3 May 21, 2018 Dear Physician, We are providing additional information to the letter we recently issued
URGENT MEDICAL DEVICE RECALL HeartMate 3 Left Ventricular Assist System Catalog #106524US LVAS KIT, HM3 May 21, 2018 Dear Physician, We are providing additional information to the letter we recently issued
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
If you have any questions, please contact Jerry Webb at ext or
 1. On May 3, 2013, Health & Life, Co., LTD, initiated a nationwide recall of 242,892 EZ Breathe Atomizers, Model #EZ-100, after becoming aware of an increase in the number of complaints. The Atomizers
1. On May 3, 2013, Health & Life, Co., LTD, initiated a nationwide recall of 242,892 EZ Breathe Atomizers, Model #EZ-100, after becoming aware of an increase in the number of complaints. The Atomizers
Urgent Field Safety Notice
 ADVIA Centaur Systems Dimension Vista Systems IMMULITE Systems Urgent Field Safety Notice CC 17-06.A.OUS Elevated Results in Patient s Due to Cross of with Our records indicate that your facility may have
ADVIA Centaur Systems Dimension Vista Systems IMMULITE Systems Urgent Field Safety Notice CC 17-06.A.OUS Elevated Results in Patient s Due to Cross of with Our records indicate that your facility may have
Urgent field safety notice
 Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Perioperative Management Of Extra-Ventricular Drains (EVD)
 Perioperative Management Of Extra-Ventricular Drains (EVD) Dr. Vijay Tarnal MBBS, FRCA Clinical Assistant Professor Division of Neuroanesthesiology Division of Head & Neck Anesthesiology Michigan Medicine
Perioperative Management Of Extra-Ventricular Drains (EVD) Dr. Vijay Tarnal MBBS, FRCA Clinical Assistant Professor Division of Neuroanesthesiology Division of Head & Neck Anesthesiology Michigan Medicine
URGENT FIELD SAFETY NOTICE - EXPANSION
 URGENT FIELD SAFETY NOTICE - EXPANSION August 18, 2017 Product Name Catalog Number BD MAX TM Vaginal Panel 443710 BD MAX UVE Specimen Collection Kit 443376 BD MAX CT/GC/TV 442970 BD MAX CT/GC 442969 Dear
URGENT FIELD SAFETY NOTICE - EXPANSION August 18, 2017 Product Name Catalog Number BD MAX TM Vaginal Panel 443710 BD MAX UVE Specimen Collection Kit 443376 BD MAX CT/GC/TV 442970 BD MAX CT/GC 442969 Dear
Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193. ETEST Ceftriaxone TXL32 (Ref , ) FOAM packaging
 16 January 2017 Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193 ETEST Ceftriaxone TXL32 (Ref. 507058, 507018) FOAM packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 507058,
16 January 2017 Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193 ETEST Ceftriaxone TXL32 (Ref. 507058, 507018) FOAM packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 507058,
Procedures commonly seen at Vanderbilt Medical Center PACU s: Cervical, thoracic, lumbar, and sacral spine surgeries. Goes to 6N
 Procedures commonly seen at Vanderbilt Medical Center PACU s: Cervical, thoracic, lumbar, and sacral spine surgeries Goes to 6N Burr holes and Craniotomies for hemorrhage, tumors, trauma, debulking Goes
Procedures commonly seen at Vanderbilt Medical Center PACU s: Cervical, thoracic, lumbar, and sacral spine surgeries Goes to 6N Burr holes and Craniotomies for hemorrhage, tumors, trauma, debulking Goes
PRODUCT RECALL NOTICE URGENT
 PRODUCT RECALL NOTICE URGENT Date: July 21, 2018 To: Re: Mondelēz Global LLC Customers Recall Notice Certain Ritz Cracker Sandwiches and Ritz Bits product in the U.S., including Puerto Rico & U.S. Virgin
PRODUCT RECALL NOTICE URGENT Date: July 21, 2018 To: Re: Mondelēz Global LLC Customers Recall Notice Certain Ritz Cracker Sandwiches and Ritz Bits product in the U.S., including Puerto Rico & U.S. Virgin
Urgent Medical Device Correction
 Urgent Medical Device Correction Contact TM Detach Infusion Sets: Needle May Fracture From the Connect/Disconnect Location August 10, 2015 Dear Valued Customer: Unomedical a/s, a ConvaTec Company has initiated
Urgent Medical Device Correction Contact TM Detach Infusion Sets: Needle May Fracture From the Connect/Disconnect Location August 10, 2015 Dear Valued Customer: Unomedical a/s, a ConvaTec Company has initiated
NEUROSURGERY LiquoGuard
 NEUROSURGERY LiquoGuard Member of CENTROTEC Group a new InTRODUCTION TO LiquoGuard CSF Management LiquoGuard (Liquor = German for CSF) represents a revolutionary step in CSF drainage, maximizing patient
NEUROSURGERY LiquoGuard Member of CENTROTEC Group a new InTRODUCTION TO LiquoGuard CSF Management LiquoGuard (Liquor = German for CSF) represents a revolutionary step in CSF drainage, maximizing patient
Urgent Medical Device Recall
 PENTAX Medical 3 Paragon Drive Montvale, New Jersey 07645 Toll-free: 800-431-5880 Tel: 201-571-2300 Fax: 201-799-4032 June 12, 2014 Urgent Medical Device Recall Field Safety Corrective Action IFU Addendum
PENTAX Medical 3 Paragon Drive Montvale, New Jersey 07645 Toll-free: 800-431-5880 Tel: 201-571-2300 Fax: 201-799-4032 June 12, 2014 Urgent Medical Device Recall Field Safety Corrective Action IFU Addendum
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE
 SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: INTRACRANIAL PRESSURE MONITORING USING ICP CODMAN DEVICE MONITORING BOLT AND INTRACRANIAL PRESSURE MONITORING DRAINAGE OF CEREBRAL SPINAL
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: INTRACRANIAL PRESSURE MONITORING USING ICP CODMAN DEVICE MONITORING BOLT AND INTRACRANIAL PRESSURE MONITORING DRAINAGE OF CEREBRAL SPINAL
Quality Metrics. Stroke Related Procedure Outcomes
 Quality Metrics Stroke Related Procedure Outcomes Below is a description of some of the stroke-related procedures performed at St. Dominic Hospital in Jackson, with quality information on the complication
Quality Metrics Stroke Related Procedure Outcomes Below is a description of some of the stroke-related procedures performed at St. Dominic Hospital in Jackson, with quality information on the complication
Urgent Field Safety Notice
 Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Ventriculo-Peritoneal/ Lumbo-Peritoneal Shunts
 Ventriculo-Peritoneal/ Lumbo-Peritoneal Shunts Exceptional healthcare, personally delivered Ventriculo-Peritoneal/ Lumbo-Peritoneal Shunts What is hydrocephalus? Hydrocephalus is the build up of an excess
Ventriculo-Peritoneal/ Lumbo-Peritoneal Shunts Exceptional healthcare, personally delivered Ventriculo-Peritoneal/ Lumbo-Peritoneal Shunts What is hydrocephalus? Hydrocephalus is the build up of an excess
FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV REV.8 Guideline Vigilance)
 FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV 2.12-1 REV.8 Guideline Vigilance) In Vitro Diagnostics Medical Devices : Type: Instruments for professional use. Attention :
FIELD SAFETY NOTICE (FSN) (Please refer to Annex 5 of the Guideline: MEDDEV 2.12-1 REV.8 Guideline Vigilance) In Vitro Diagnostics Medical Devices : Type: Instruments for professional use. Attention :
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST
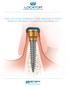 THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
Flexi-Needles. BMI_FLEXINEEDLES_v2 web
 Flexi-Needles BMI_FLEXINEEDLES_v2 _ _web Best Medical can provide any standard or customized configuration made to your exact specifications, sterile or non-sterile, shipped within 24 hours worldwide!
Flexi-Needles BMI_FLEXINEEDLES_v2 _ _web Best Medical can provide any standard or customized configuration made to your exact specifications, sterile or non-sterile, shipped within 24 hours worldwide!
Health Services. Procedure Manual
 Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
UNDERSTANDING HYDROCEPHALUS
 UNDERSTANDING HYDROCEPHALUS Offering solutions for patients. 1 UNDERSTANDING HYDROCEPHALUS Dear Reader: Your doctor has either recommended the CODMAN HAKIM Precision Fixed Pressure Valve, the CODMAN HAKIM
UNDERSTANDING HYDROCEPHALUS Offering solutions for patients. 1 UNDERSTANDING HYDROCEPHALUS Dear Reader: Your doctor has either recommended the CODMAN HAKIM Precision Fixed Pressure Valve, the CODMAN HAKIM
National Hospital for Neurology and Neurosurgery
 National Hospital for Neurology and Neurosurgery Venous sinus stents (for the treatment of venous sinus stenosis and idiopathic intracranial hypertension) Lysholm Department of Neuroradiology If you would
National Hospital for Neurology and Neurosurgery Venous sinus stents (for the treatment of venous sinus stenosis and idiopathic intracranial hypertension) Lysholm Department of Neuroradiology If you would
URGENT FIELD SAFETY NOTICE FSCA3193. Etest Ceftriaxone TXL32 (Ref , ) SPB Packaging
 16 January 2017 Attachment 3 URGENT FIELD SAFETY NOTICE FSCA3193 Etest Ceftriaxone TXL32 (Ref. 412302, 412303) SPB Packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 412302, 412303)
16 January 2017 Attachment 3 URGENT FIELD SAFETY NOTICE FSCA3193 Etest Ceftriaxone TXL32 (Ref. 412302, 412303) SPB Packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 412302, 412303)
Product NDC Code Lot Number Expiry Dates Distribution Date VALSARTAN TABLETS 40MG 30CT
 Prinston Pharmaceutical Inc Issues Voluntary Nationwide Recall of Valsartan and Valsartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodimethylamine (NDMA) in the Products
Prinston Pharmaceutical Inc Issues Voluntary Nationwide Recall of Valsartan and Valsartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodimethylamine (NDMA) in the Products
Product Codes: 5C4474, R5C8320
 12 th November 2014 Field Safety Notice Dear Baxter Healthcare is sending this communication to inform you of additional and updated warnings and cautions being implemented in HomeChoice/HomeChoice
12 th November 2014 Field Safety Notice Dear Baxter Healthcare is sending this communication to inform you of additional and updated warnings and cautions being implemented in HomeChoice/HomeChoice
ID Number: To maintain consistent standards for monitoring CSF drainage and the patients neurological status.
 Policies & Procedures Title: Cerebrospinal Fluid (CSF) Drainage LUMBAR - Post Thoraco-aortic Aneurysm - Management of drainage system - Spinal pressure monitoring - Care of patient - Assisting with removal
Policies & Procedures Title: Cerebrospinal Fluid (CSF) Drainage LUMBAR - Post Thoraco-aortic Aneurysm - Management of drainage system - Spinal pressure monitoring - Care of patient - Assisting with removal
3908 ½ River Ave. Newport Beach, California contact[at]dollecommunications[dot]com. Hydrocephalus Consult Report Published On-Line
![3908 ½ River Ave. Newport Beach, California contact[at]dollecommunications[dot]com. Hydrocephalus Consult Report Published On-Line 3908 ½ River Ave. Newport Beach, California contact[at]dollecommunications[dot]com. Hydrocephalus Consult Report Published On-Line](/thumbs/96/127081733.jpg) STEPHEN M. DOLLE Hydrocephalus Monitoring & Consults 3908 ½ River Ave. Newport Beach, California 92663 contact[at]dollecommunications[dot]com Hydrocephalus Consult Report Published On-Line March 4, 2014
STEPHEN M. DOLLE Hydrocephalus Monitoring & Consults 3908 ½ River Ave. Newport Beach, California 92663 contact[at]dollecommunications[dot]com Hydrocephalus Consult Report Published On-Line March 4, 2014
URGENT: MEDICAL DEVICE CORRECTION
 December 5, 2014 Dear Healthcare Professional, URGENT: MEDICAL DEVICE CORRECTION Alere INRatio PT/INR Monitor system This letter contains important information co ncerning the Alere IN Ratio PT/I NR Monitor
December 5, 2014 Dear Healthcare Professional, URGENT: MEDICAL DEVICE CORRECTION Alere INRatio PT/INR Monitor system This letter contains important information co ncerning the Alere IN Ratio PT/I NR Monitor
BRINEURA (cerliponase alfa)
 BRINEURA (cerliponase alfa) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BRINEURA (cerliponase alfa) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
URGENT FIELD SAFEY NOTICE
 April x, 2015 URGENT FIELD SAFETY NOTICE Software Anomaly using VITROS 5,1 FS Chemistry Systems Dear Customer, As part of a Field Safety Corrective Action, the purpose of this notification is to inform
April x, 2015 URGENT FIELD SAFETY NOTICE Software Anomaly using VITROS 5,1 FS Chemistry Systems Dear Customer, As part of a Field Safety Corrective Action, the purpose of this notification is to inform
SPONSORSHIP OPPORTUNITIES
 SPONSORSHIP OPPORTUNITIES 2018 WHAT IS HYDROCEPHALUS? Hydrocephalus is caused by an imbalance in the production and absorption of cerebral spinal fluid in the brain which causes the ventricles to enlarge.
SPONSORSHIP OPPORTUNITIES 2018 WHAT IS HYDROCEPHALUS? Hydrocephalus is caused by an imbalance in the production and absorption of cerebral spinal fluid in the brain which causes the ventricles to enlarge.
Raritan Pharmaceuticals, Inc. 6/20/17
 Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Magnesium Sulfate in Water for Injection is indicated for the prevention and control of seizures in preeclampsia and eclampsia, respectively.
 a HOSPIRA ISSUES A VOLUNTARY NATIONWIDE RECALL OF ONE LOT OF MAGNESIUM SULFATE IN WATER FOR INJECTION DUE TO INCORRECT BARCODE LABELING ON THE PRIMARY CONTAINER Consumers: 1-888-345-4680 Media: 610-329-1340
a HOSPIRA ISSUES A VOLUNTARY NATIONWIDE RECALL OF ONE LOT OF MAGNESIUM SULFATE IN WATER FOR INJECTION DUE TO INCORRECT BARCODE LABELING ON THE PRIMARY CONTAINER Consumers: 1-888-345-4680 Media: 610-329-1340
BACTISEAL Endoscopic Ventricular Catheter (REF & )
 BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
Important information on the Accu-Chek Insight insulin pump system: Update of the handling instructions for changing an insulin cartridge
 Consumer Letter (Accu-Chek Insight PFC) Urgent field safety notice Location, Date Important information on the Accu-Chek Insight insulin pump system: Update of the handling instructions
Consumer Letter (Accu-Chek Insight PFC) Urgent field safety notice Location, Date Important information on the Accu-Chek Insight insulin pump system: Update of the handling instructions
Indwelling pleural drainage system explained
 Indwelling pleural drainage system explained Respiratory Medicine Patient Information Leaflet Introduction This leaflet is about the procedure to fit an indwelling drainage system. It tells you what the
Indwelling pleural drainage system explained Respiratory Medicine Patient Information Leaflet Introduction This leaflet is about the procedure to fit an indwelling drainage system. It tells you what the
Subarachnoid Haemorrhage
 2011 Subarachnoid Haemorrhage Subarachnoid Haemorrhage This pamphlet will briefly describe what may happen to a person who has a subarachnoid haemorrhage (SAH). We would like to encourage you to read this
2011 Subarachnoid Haemorrhage Subarachnoid Haemorrhage This pamphlet will briefly describe what may happen to a person who has a subarachnoid haemorrhage (SAH). We would like to encourage you to read this
Contacts Consumers:
 Prinston Pharmaceutical Inc. issues Voluntary Nationwide Recall of Irbesartan and Irbesartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodiethylamine (NDEA) in the
Prinston Pharmaceutical Inc. issues Voluntary Nationwide Recall of Irbesartan and Irbesartan HCTZ Tablets Due to detection of a Trace Amount of Unexpected Impurity, N- nitrosodiethylamine (NDEA) in the
URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL
 January 15, 2018 To: Subject: Reference: Surgeons / Hospitals URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL ZFA2017-00006 Affected Product: ACE Trochanteric Nail (ATN) System See Attachment 2 Affected
January 15, 2018 To: Subject: Reference: Surgeons / Hospitals URGENT MEDICAL DEVICE FIELD SAFETY NOTICE- REMOVAL ZFA2017-00006 Affected Product: ACE Trochanteric Nail (ATN) System See Attachment 2 Affected
Recall Guidelines. for Chinese Medicine Products
 Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
Recall Guidelines for Chinese Medicine Products April 2018 Recall Guidelines for Chinese Medicine Products Chinese Medicines Board Chinese Medicine Council of Hong Kong Compiled in September 2005 1 st
We are writing to inform you about an issue concerning your CoaguChek system and test strips. Please read this information carefully.
 Consumer Letter (CoaguChek XS PT Test PST, CoaguChek XS PT Test, CoaguChek PT Test) Urgent Field Safety Notice Location, Date We are writing to inform you about an issue concerning
Consumer Letter (CoaguChek XS PT Test PST, CoaguChek XS PT Test, CoaguChek PT Test) Urgent Field Safety Notice Location, Date We are writing to inform you about an issue concerning
URGENT: Important Safety Information
 October 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Crystallization, Cracked Needle Hubs and Particulate in Diazepam Injection, USP,
October 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Crystallization, Cracked Needle Hubs and Particulate in Diazepam Injection, USP,
Inaccuracies in CMS Interim Final RVUs for Intracranial Endovascular Intervention Codes
 Andy Slavitt, Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW Washington,
Andy Slavitt, Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW Washington,
Harvard Pilgrim Spine Management Provider Training
 Harvard Pilgrim Spine Management Provider Training NIA Training Program 2 NIA Program Agenda Our Program 1. Authorization Process 2. Other Program Components 3. Provider Tools and Contact Information RadMD
Harvard Pilgrim Spine Management Provider Training NIA Training Program 2 NIA Program Agenda Our Program 1. Authorization Process 2. Other Program Components 3. Provider Tools and Contact Information RadMD
BMI Medical. Ventricular Catheter T
 Shunting System Ventricular Catheter 01101 01101T Barium-impregnated silicone catheter provides resistance to kinking and compression. Stainless steel stylet allows catheter to be directed during catheter
Shunting System Ventricular Catheter 01101 01101T Barium-impregnated silicone catheter provides resistance to kinking and compression. Stainless steel stylet allows catheter to be directed during catheter
URGENT FIELD SAFETY NOTICE IMMEDIATE ACTION REQUIRED
 Ref No: Date: 22.08.2018 Type of Action: PFSN18 Deviations of high (>4.5) CoaguChek INR values due to calibration with WHO reference standard rtf/16 SBN-CPS-2018-014 Field Safety Corrective Action (FSCA)
Ref No: Date: 22.08.2018 Type of Action: PFSN18 Deviations of high (>4.5) CoaguChek INR values due to calibration with WHO reference standard rtf/16 SBN-CPS-2018-014 Field Safety Corrective Action (FSCA)
Muscular Dystrophy Canada s Safeway Mobility Grant
 What are Safeway Mobility Grants? Muscular Dystrophy Canada s Safeway Mobility Grant Muscular Dystrophy Canada has teamed up with Canada Safeway to raise much needed funds to support those living with
What are Safeway Mobility Grants? Muscular Dystrophy Canada s Safeway Mobility Grant Muscular Dystrophy Canada has teamed up with Canada Safeway to raise much needed funds to support those living with
URGENT: Important Safety Information
 July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
Accommodations. Neurosurgeons, Spine Surgeons. Spine and Neurosurgery clinicians. Spine and Neurosurgery Residents and Fellows.
 This symposium is designed for practicing physicians in Neurosurgery, Neurology, Spine Surgery, nurses/physician assistants who are interested in the most recent treatment options, emerging therapies and
This symposium is designed for practicing physicians in Neurosurgery, Neurology, Spine Surgery, nurses/physician assistants who are interested in the most recent treatment options, emerging therapies and
BERKELEY DAVIS IRVINE LOS ANGELES MERCED RIVERSIDE SAN DIEGO SAN FRANCISCO
 UNIVERSITY OF CALIFORNIA SAN FRANCISCO BERKELEY DAVIS IRVINE LOS ANGELES MERCED RIVERSIDE SAN DIEGO SAN FRANCISCO SANTA BARBARA SANTA CRUZ STANTON A. GLANTZ, PhD 530 Parnassus Suite 366 Professor of Medicine
UNIVERSITY OF CALIFORNIA SAN FRANCISCO BERKELEY DAVIS IRVINE LOS ANGELES MERCED RIVERSIDE SAN DIEGO SAN FRANCISCO SANTA BARBARA SANTA CRUZ STANTON A. GLANTZ, PhD 530 Parnassus Suite 366 Professor of Medicine
Product Correction Urgent Immediate Action Required
 Abbott Ireland Diagnostics Division Lisnamuck, Longford Co. Longford, Ireland DIAGNOSTICS Urgent Field Safety Notice Product Correction Urgent Immediate Action Required Date Issued October 12, 2018 Product
Abbott Ireland Diagnostics Division Lisnamuck, Longford Co. Longford, Ireland DIAGNOSTICS Urgent Field Safety Notice Product Correction Urgent Immediate Action Required Date Issued October 12, 2018 Product
Urgent Safety Notice. Recall. concerning the. sterile Trauma Plate Systems
 aap Implantate AG Lorenzweg 5 D-12099 Berlln Germanv CUSTOMER NAME STREET No. ZIP-CODE, PLACE Urgent Safety Notice Recall concerning the sterile Trauma Plate Systems Berlin, May 5 1 h, 2017 Reference-No.:
aap Implantate AG Lorenzweg 5 D-12099 Berlln Germanv CUSTOMER NAME STREET No. ZIP-CODE, PLACE Urgent Safety Notice Recall concerning the sterile Trauma Plate Systems Berlin, May 5 1 h, 2017 Reference-No.:
Blue Cross Blue Shield of Nebraska Spine Management Provider Training. Provider Training Presented by Leta Genasci
 Blue Cross Blue Shield of Nebraska Spine Management Provider Training Provider Training Presented by Leta Genasci NIA Magellan Training Program 2 NIA Magellan 1 Program Agenda Our Program 1. Authorization
Blue Cross Blue Shield of Nebraska Spine Management Provider Training Provider Training Presented by Leta Genasci NIA Magellan Training Program 2 NIA Magellan 1 Program Agenda Our Program 1. Authorization
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE
 SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: INTRACRANIAL PRESSURE MONITORING AND DRAINAGE OF CEREBROSPINAL FLUID VIA Nursing DATE: REVIEWED: PAGES: 7/88 1/18 1 of 9 RESPONSIBILITY:
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: INTRACRANIAL PRESSURE MONITORING AND DRAINAGE OF CEREBROSPINAL FLUID VIA Nursing DATE: REVIEWED: PAGES: 7/88 1/18 1 of 9 RESPONSIBILITY:
CODING SHEET HYDROCEPHALUS REIMBURSEMENT. All Medicare information is current as of the time of printing.
 CODING SHEET HYDROCEPHALUS REIMBURSEMENT All Medicare information is current as of the January 2014 Hydrocephalus ing Coding Options Commonly Billed Codes for Physicians, Hospitals, and Ambulatory Surgery
CODING SHEET HYDROCEPHALUS REIMBURSEMENT All Medicare information is current as of the January 2014 Hydrocephalus ing Coding Options Commonly Billed Codes for Physicians, Hospitals, and Ambulatory Surgery
Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration
 Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration Table of Contents: 12.1. Randomization...12-2 12.2. Treatment...12-2 12.3. Drug Supply and Storage...12-2 12.4.
Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration Table of Contents: 12.1. Randomization...12-2 12.2. Treatment...12-2 12.3. Drug Supply and Storage...12-2 12.4.
KNOWLEDGE BASE: 1. These patients will be cared for in the following nursing departments: critical care, 5ET (Neuro), PACU, Radiology or ECC.
 SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: LUMBAR INTRATHECAL SUBARACHNOID CEREBROSPINAL FLUID CATHETER (Set Up and Maintenance) - ADULTS (neu02) Nursing DATE: REVIEWED: PAGES: RESPONSIBILITY:
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE TITLE: ISSUED FOR: LUMBAR INTRATHECAL SUBARACHNOID CEREBROSPINAL FLUID CATHETER (Set Up and Maintenance) - ADULTS (neu02) Nursing DATE: REVIEWED: PAGES: RESPONSIBILITY:
CCC Bike Tour Fundraising Materials Guide
 2017 CCC Bike Tour Fundraising Materials Guide [Type the document subtitle] photo credit: Nick Agelidis CCC 17 Fundraising Materials Guide Version 1 11/21/2016 Sample Letter 1 Date Name Street City, State,
2017 CCC Bike Tour Fundraising Materials Guide [Type the document subtitle] photo credit: Nick Agelidis CCC 17 Fundraising Materials Guide Version 1 11/21/2016 Sample Letter 1 Date Name Street City, State,
Responding to a Food Recall
 Responding to a Food Recall Time: 4 hours National Food Service Management Institute The University of Mississippi 2013 Table of Contents Objectives... ii Lesson 1: Introduction to the Food Recall Process...
Responding to a Food Recall Time: 4 hours National Food Service Management Institute The University of Mississippi 2013 Table of Contents Objectives... ii Lesson 1: Introduction to the Food Recall Process...
Indwelling Urinary Catheters And Drainage systems
 If you require this leaflet in any other format, e.g., large print, please telephone 01935 384256 Indwelling Urinary Catheters And Drainage systems Useful organisations Ms Society Helpline Tel: 0808 800
If you require this leaflet in any other format, e.g., large print, please telephone 01935 384256 Indwelling Urinary Catheters And Drainage systems Useful organisations Ms Society Helpline Tel: 0808 800
Omaha Sports Complex Omaha, Nebraska
 2018 Sponsorship Opportunities RIVALZ Omaha, NE Friday, April 20 7:00pm Kick Off 9:00pm After Party Omaha Sports Complex Omaha, Nebraska Nebraska Chapter RIVALZ Blondes vs Brunettes Omaha Sports Complex
2018 Sponsorship Opportunities RIVALZ Omaha, NE Friday, April 20 7:00pm Kick Off 9:00pm After Party Omaha Sports Complex Omaha, Nebraska Nebraska Chapter RIVALZ Blondes vs Brunettes Omaha Sports Complex
Several internal issues and external causes resulted in our challenge to satisfactorily meet those time frames:
 December 2018 RE: Update to Supply Constraints to BD Hypodermic Systems Products Dear Valued Customer, We want to first recognize your patience as BD continues to drive towards rapidly improving the continuity
December 2018 RE: Update to Supply Constraints to BD Hypodermic Systems Products Dear Valued Customer, We want to first recognize your patience as BD continues to drive towards rapidly improving the continuity
US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... FDA Home Page CDRH Home Page Search A-Z Index
 US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... http://www.fda.gov/cdrh/safety/102008-surgicalmesh.html Page 1 of 3 FDA Home Page CDRH Home Page Search
US FDA/CDRH: Public Health Notification: Serious Complications Associated with Transvaginal Place... http://www.fda.gov/cdrh/safety/102008-surgicalmesh.html Page 1 of 3 FDA Home Page CDRH Home Page Search
Services provided beyond a Member s benefit limit are not covered unless a BLE is requested and approved by Avesis.
 April 1, 2012 Dear Provider: Avesis would like to thank you for your continued participation in the Avesis UPMC for You dental network. This notice is to inform you of some upcoming changes to benefits
April 1, 2012 Dear Provider: Avesis would like to thank you for your continued participation in the Avesis UPMC for You dental network. This notice is to inform you of some upcoming changes to benefits
Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA Telephone: 510/
 Department of Health and Human Services Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Telephone: 510/337-6700 WARNING LETTER December
Department of Health and Human Services Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Telephone: 510/337-6700 WARNING LETTER December
Appendix 1: search terms
 Appendix 1: search terms MEDLINE (PubMed) Abbreviated search strategy (full syntax available) 1. human 2. immunodeficiency 3. virus 4. 1 AND 2 AND 3 5. human immunodeficiency virus 6. HIV 7. acquired 8.
Appendix 1: search terms MEDLINE (PubMed) Abbreviated search strategy (full syntax available) 1. human 2. immunodeficiency 3. virus 4. 1 AND 2 AND 3 5. human immunodeficiency virus 6. HIV 7. acquired 8.
March 21, Subject: Availability of Blister-Packaged XELODA (capecitabine) Tablets
 March 21, 2011 Subject: Availability of Blister-Packaged XELODA (capecitabine) Tablets Dear Healthcare Professional: The purpose of this letter is to inform you of the temporary availability of an alternative
March 21, 2011 Subject: Availability of Blister-Packaged XELODA (capecitabine) Tablets Dear Healthcare Professional: The purpose of this letter is to inform you of the temporary availability of an alternative
NIA Magellan 1 Medical Specialty Solutions
 NIA Magellan 1 Medical Specialty Solutions Florida Blue Spine Management- Provider Training Presented by: Michele L. DeCaprio, MBA Manager, Provider Relations 1 NIA Magellan refers to National Imaging
NIA Magellan 1 Medical Specialty Solutions Florida Blue Spine Management- Provider Training Presented by: Michele L. DeCaprio, MBA Manager, Provider Relations 1 NIA Magellan refers to National Imaging
AMERICAN BOARD OF ADOLESCENT PSYCHIATRY
 AMERICAN BOARD OF ADOLESCENT PSYCHIATRY SUPPORTED BY THE AMERICAN SOCIETY FOR ADOLESCENT PSYCHIATRY Candidate Guide & Certification Examination Application American Society For Adolescent Psychiatry P.O.
AMERICAN BOARD OF ADOLESCENT PSYCHIATRY SUPPORTED BY THE AMERICAN SOCIETY FOR ADOLESCENT PSYCHIATRY Candidate Guide & Certification Examination Application American Society For Adolescent Psychiatry P.O.
INFORMATION FOR PATIENTS
 The British Association of Urological Surgeons 35-43 Lincoln s Inn Fields London WC2A 3PE Phone: Fax: Website: E- mail: +44 (0)20 7869 6950 +44 (0)20 7404 5048 www.baus.org.uk admin@baus.org.uk INFORMATION
The British Association of Urological Surgeons 35-43 Lincoln s Inn Fields London WC2A 3PE Phone: Fax: Website: E- mail: +44 (0)20 7869 6950 +44 (0)20 7404 5048 www.baus.org.uk admin@baus.org.uk INFORMATION
HAZARD ALERT. Important Medical Device Advisory. Premature Battery Depletion with Implantable Cardioverter Defibrillator
 HAZARD ALERT St. Jude Medical New Zealand Limited 54 Carbine Road Mt Wellington 1641 NEW ZEALAND Company Number 3432231 Tel 0800 756 269 Fax 0800 756 329 Important Medical Device Advisory Premature Battery
HAZARD ALERT St. Jude Medical New Zealand Limited 54 Carbine Road Mt Wellington 1641 NEW ZEALAND Company Number 3432231 Tel 0800 756 269 Fax 0800 756 329 Important Medical Device Advisory Premature Battery
PHILADELPHIA KIDNEY WALK Partnership Opportunities
 PHILADELPHIA KIDNEY WALK Partnership Opportunities Sunday, October 11, 2015 Philadelphia Museum of Art http://donate.kidney.org/philly Chairman: Robert J. Myers Partner, Ernst & Young Page 1 ing the Kidney
PHILADELPHIA KIDNEY WALK Partnership Opportunities Sunday, October 11, 2015 Philadelphia Museum of Art http://donate.kidney.org/philly Chairman: Robert J. Myers Partner, Ernst & Young Page 1 ing the Kidney
SPONSORSHIP OPPORTUNITIES
 2018 SPONSORSHIP OPPORTUNITIES Our region has led the nation in showing how collaboration can make us more effective as advocates, as funders, and as developers, and at the heart of that leadership is
2018 SPONSORSHIP OPPORTUNITIES Our region has led the nation in showing how collaboration can make us more effective as advocates, as funders, and as developers, and at the heart of that leadership is
got you covered. Codman s The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions.
 Codman s got you covered. The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions. AN INTEGRA LIFESCIENCES COMPANY The Leader in Dural
Codman s got you covered. The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions. AN INTEGRA LIFESCIENCES COMPANY The Leader in Dural
Oregon Immunization Program
 Oregon Immunization Program Standard Operating Procedures for Vaccine Management It is the direct responsibility of the staff person designated below to safeguard and ensure the maintenance of vaccines
Oregon Immunization Program Standard Operating Procedures for Vaccine Management It is the direct responsibility of the staff person designated below to safeguard and ensure the maintenance of vaccines
What to Expect When You Visit. The First Visit. Follow Up Visits. Laboratory Tests
 Vibrant Health Naturopathic Medical Center & Clinical Thermography Dr. Nicole Schertell 100 Shattuck Way, Newington, NH 03801 (603) 431-6677 or (603) 610-7718 1-888-796-2862 fax Welcome! Vibrant Health
Vibrant Health Naturopathic Medical Center & Clinical Thermography Dr. Nicole Schertell 100 Shattuck Way, Newington, NH 03801 (603) 431-6677 or (603) 610-7718 1-888-796-2862 fax Welcome! Vibrant Health
STUDY CLUB INFORMATION GUIDE
 THE COLLEGE OF DENTAL HYGIENISTS OF BRITISH COLUMBIA 600-3795 Carey Road Telephone (250) 383 4101 Victoria, British Columbia V8Z 6T8 Facsimile (250) 383 4144 STUDY CLUB INFORMATION GUIDE Beginning in 2016,
THE COLLEGE OF DENTAL HYGIENISTS OF BRITISH COLUMBIA 600-3795 Carey Road Telephone (250) 383 4101 Victoria, British Columbia V8Z 6T8 Facsimile (250) 383 4144 STUDY CLUB INFORMATION GUIDE Beginning in 2016,
Lumbar drains. Information for patients Neurosurgery
 Lumbar drains Information for patients Neurosurgery Why do I need drainage of my cerebrospinal fluid (CSF)? The brain and spinal cord are bathed in clear fluid like a baby in the womb. This cerebrospinal
Lumbar drains Information for patients Neurosurgery Why do I need drainage of my cerebrospinal fluid (CSF)? The brain and spinal cord are bathed in clear fluid like a baby in the womb. This cerebrospinal
Fundraising Information Packet
 Fundraising Information Packet Contact: Down Syndrome Association For Families of Nebraska Lincoln NE 68505 www.dsafnebraska.org Email: stepupwalk@dsafnebraska.org (402) 421-1338 Page 1 Dear Team Captain,
Fundraising Information Packet Contact: Down Syndrome Association For Families of Nebraska Lincoln NE 68505 www.dsafnebraska.org Email: stepupwalk@dsafnebraska.org (402) 421-1338 Page 1 Dear Team Captain,
RE: Temporary Changes to Aggregate Production Quotas for IV Opioid Products to Address Shortages
 February 27, 2018 [Submitted via U.S. Mail] Robert W. Patterson Acting Administrator Drug Enforcement Administration 700 Army Navy Drive Arlington, VA 22202 [Submitted electronically to ODLP@usdoj.gov]
February 27, 2018 [Submitted via U.S. Mail] Robert W. Patterson Acting Administrator Drug Enforcement Administration 700 Army Navy Drive Arlington, VA 22202 [Submitted electronically to ODLP@usdoj.gov]
THE IBECA SHOW PRESENTS HOW TO WIN! July Park Place Hotel, Traverse City, MI
 THE IBECA SHOW PRESENTS HOW TO WIN! July 13-16 2014 Park Place Hotel, Traverse City, MI Hello Industry Friends, Thank you for considering the IBECA Show, formerly known as the IBO Show. The dates of our
THE IBECA SHOW PRESENTS HOW TO WIN! July 13-16 2014 Park Place Hotel, Traverse City, MI Hello Industry Friends, Thank you for considering the IBECA Show, formerly known as the IBO Show. The dates of our
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS
 RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
RULES OF THE TENNESSEE BOARD OF PHARMACY CHAPTER 1140-17 DRUG DONATION REPOSITORY PROGRAM TABLE OF CONTENTS 1140-17-.01 Definitions 1140-17-.02 Purpose 1140-17-.03 Eligibility Criteria for Program Participation
