Basic knowledge of POCT instuments. The Tide Resort Bangsan Beach 18 June 2009
|
|
|
- Warren Banks
- 5 years ago
- Views:
Transcription
1 Basic knowledge of POCT instuments The Tide Resort Bangsan Beach 18 June
2 POCT instruments concepts Point of care testing Diagnostic medical devices used at or near the site of patient care Bed site, OR, ED, ICU, field, home. Provide near real time analysis and rapid test results Reduce therapeutic turnaround time Shorten length of hospital stay User friendly, Reducing dependence on operator technique. Potentially improve patient outcomes Example :Electrolyte, Hct, Blood gas and metabolites, Cardiac injury markers, Hemostatis indicator, Drugs of abuse, Infectious disease markers, Molecular diagnostics 2
3 Classification 3
4 Classification Characteristic Transportable Portable Handheld Immuno detection device Size Power source Test menu Sampling method Large and heavy On carts for mobile use AC, Batteries back up for short term use Broad test menu, Customize the tests Automatic for reducing dependence on operator technical /manual Minimize the volume of blood need per analysis. WB, P, S, U, CSF. Medium easier access to AC patients or Batteries Fixed, Single to multi test Automatic/m anual W, P, S, U Small or miniature Batteries Fixed / limited Manual WB Medium to miniature Variable to none Fixed, Single to multi test Manual WB,P, U, Saliva 4
5 Classification Characteris tic Instrument Electrode/se nsor, Reagent Calibration Transportable Portable Handheld Immuno detection device Blood gas system. The electrode/sensor, reagents, cartrige/cuvette are separated and removable. Automatic internal one and two point calibration that is performed periodically Blood gas, PT,aPTT, HbA1c, Chemistry, The electrode /sensors and reagents are built and packaged within the disposable cartridge. Periodic or prior to testing depend on multiple or single use cartridges Blood gas, Glucose meter The electrode and biosensor are constructed in to the test strips, Dryreagent Don t chemistry have internal calibration but have electronic verification steps Cardiac testing, Drug abuse, D-Dimer No calibration is needed 5
6 Classification Characteristic Transportable Portable Handheld Immuno detection device Biohazard LIS connectivity Password security Build in biohazard container Available Available Build in biohazard container Possibly available Possibly available Lack of waste storage, Exposed to biohazards Limited Limited Biohazard storage none none 6
7 Principle Amperometer CoaguChek XS 7
8 Reflectance photometry 8
9 Reflectance of u 411 9
10 Reflectance of h232 with CCD (Charge couple devices) Digital camera chip 10
11 11
12 QC Conventional method Manual external introduction Automatic QC testing Omni system Automatic draw QC materials which are onboard at operator defined schedule Intelligent quality control ( iqc ) Piccolo system (Abaxis) USA Verifies the chemistry, Optics and Electronics function of the analyzer during each run. No operator interaction 12
13 QC The integrated Quality Control (QC) function The fill detect system will indicate that an adequate sample has been applied to the test strip. Checks each test strip. Misuse, such as improper storage of the test strip in hot and humid environment as well as exposure to extended ambient light, can be detected by the system. In the case of misused test strips a QC error will be displayed on the device. 13
14 QC Electronic quality control Available portable and handheld instruments Supplements the traditional liquid QC procedures Evaluation of the optics, electronic and computational component of the system Does not validate the performance and functionality of the cartridge. Is not designed to replace liquid QC testing 14
15 Potential sources of errors in POCT Pre-analytic Patient identity Specimen collection Incorrect, mishandled, expired, unlabeled or unstable of test strips, cartridges, cassettes, or reagents Leukocytosis > 50,000 /ul, Thrombocytosis >600,000/ul, anemia (Hb < 7.5 g/dl), ph, Osmolarity, protein, lipid, heterophile Ab, Autoimmune Direct or indirect effects of transfused blood products or additives Analytic Physical (Temp, altitude, vibration, humidity) Sample (bubbles,viscosity, thrombi Calibration protocol not followed, calibration code wrong Sensor drift, aging Newborn glucose limits Lack of IQC, EQA, PT Operator not qualified, certified Maintenance Unstable power source 15
16 Potential sources of errors in POCT Post-analytic Critical values omitted, not recognized Wrong units, No results documentation No LIS No patient demographics 16
17 Selection and Evaluation of POCT System performance Broad test menu Accuracy, precision, resolution, reproducibility, stability, and response time Linearity Relationship to tests performed in the main lab Compatibility with other in vitro, ex vivo, in vivo Artifact elimination, error detection, interference warning and specimen flagging Technical and quality features Throughput, automated calibration, interrupt capability, duration of analysis cycle Compact, reliable, durable, light weight, mobile, and power efficient with battery operation Reagent stability, shelf life, lot size Continuous quality improvement (quality control, quality monitors, proficiency testing) Compliance with federal, state and accreditation regulation 17
18 Selection and Evaluation of POCT Conservation of patient blood volume Specimen volume type and matrix (eg.,whole blood, plasma, or serum) accepted Safety, ergonomics, security, and risk Biohazard control, containment, and disposal Speed, ease, simplicity and user friendly operation Economic efficacy Flexibility, Modularity, Exchangeability, expandability and upgrade capability Cost of instruments, consumables, and maintenance 18
19 Selection and Evaluation of POCT Information interpretation and integration Remote review, remote control, and data management system Networking interfacing and wireless communications and critical results notification 19
20 Top 5 requirements for POCT Accurate and precise results that correlate with the laboratory Easy to use Customer support Low cost solutions Connectivity including data management 20
21 Method validation Moderately complex test methodology Comparison of method (accuracy) Replication experiment (precision) Linearity experiment (reportable range) Verification of reference range Highly complex test methodology A waived or moderately complex test methodology, if modified by the user, automatically become high complexity Comparison of method (accuracy) Replication experiment (precision) Linearity experiment (reportable range) Detection limit experiment (Sensitivity) Interference experiment Recovery experiment Extensive reference range 21
22 Imprecision Glucose No. L1 L MEAN SD %CV Criteria of acceptant Imprecision N 20 Level 2 Within run or between run 0.25*TE Between run 0.33*TE Example TE Glucose = 10% Criteria of within run 2.5% L1 = 1.22 PASS L2 = 0.72 PASS 22
23 Comparison chart No RXL GLUCOSE (mg/dl) c Correlation ( r ) Slope Y-Intercept T-TEST c C501 & RXL y = x R 2 = Minimum criteria N = 20, 40 RXL Duration = with 2 hrs / 5 days r < 0.99 r 2 <
24 Criteria of acceptant Inaccuracy SE + RE = % Bias + (3*% CV ) Bias = ( (x c * b) + a ) x c = (100 * ) = = 8.04 %Bias = Bias * 100 / x c = 8.04 *100/100 = 8.04 %CV = 3*1.22 = 3.66 %Bias + (3* % CV) = = 11.7 FAIL TE Parameter Within Run Precision Acceptable %CV Comparison a b Xc SE %bias %RE TEcal %TEa conc. %CV 1/4 of TEa between intercept slope Yc-Xc value Absol ute (CV) % C1 &RXL Glucose C1 & C
25 Precision chart 25
26 Comparison chart 26
27 The linearity or Reportable range Experiment Clinical and Laboratory Standards Institute (CLSI) recommends a minimum of at least 4 and preferably 5 different levels or concentration Materials standard solutions linearity set from manufacturers proficiency testing agencies pools of patient specimen when high values are available in some cases e.g.tdm spike a pool with the analyte to be measured Selection of the diluent General chemistry tests; water and saline will be OK For other tests; better to use bovine or serum albumin preparations; specimen with low concentrations; drug free serum Follow the manufacturer s recommendation of the diluent to use 27
28 Linearity Procedure for preparation specimen 2 pools; one near the zero level or close to detection limit. The other near or slightly above the expected upper limit of the working range Pool1 : low pool Mixture2 : 3 parts pool1+1 part pool5 Mixture3 : 2 parts pool1+2 part pool5 Mixture4 : 1 part pool1+3 part pools5 Pool5 : high pool Mix 1 Mix 2 Mix 3 Mix 4 28
29 Linearity Number of replicate measurements NCCLS recommends 4 measurements on each specimens 3 replicates are generally sufficient. Pool or Mixture Mean (Y) Theoretical (X) % Recovery Pool 1 Mean value Mean pool 1 Y1*100/X1 Mixture 2 Mean value (Mean pool1 * 3) + (Mean pool5 * 1)/4 Y2*100/X2 Pool1 : Pool5 3 : 1 Mixture 3 Mean value (Mean pool1 * 2) + (Mean pool5 * 2)/4 Y3*100/X3 Pool1 : Pool5 2 : 2 Mixture 4 Mean value (Mean pool1 * 1) + (Mean pool5 * 3)/4 Y4*100/X4 Pool1 : Pool5 1 : 3 Pool 5 Mean value Mean pool 5 Y5*100/X5 29
30 Linearity Theoretical value Measment value % Recovery Pool #DIV/0! Mixture Mixture Mixture Pool Linearity experiment %Recovery Linearity experiment Mix 2 Mix 3 Mix 4 Pool Dilution 30
31 Verify reference interval Criteria Transference technique Reference population N= 20 Blood donor Hospitalization Alcohol consumption Blood pressure abnormal Drug abuse Tobacco use Pregnancy Oral contraceptives etc,. 95% percentile acceptable N = 20 criteria acceptance 1 that out of reference range 31
32 Sensitivity or Detection limit experiment Analytical sensitivity (lower detection limit) Immunology The detection limit represents the lowest analyte level that can be distinguished from zero. It is calculated as the value lying two standard deviations above that of the lowest standard (master calibrator, (standard SD, within-run precision, n = 21). Chemistry The lower detection limit represents the lowest measurable analyte level that can be distinguished from zero. It is calculated as the value lying three standard deviations above that of the lowest standard (standard SD, within-run precision, n = 21). 32
33 Sensitivity or Detection limit experiment The limit of blank (LoB).. The limit of blank corresponds to the concentration below which analyte free samples are found with a probability of 95%. LoB = mean blank SD blank The limit of detection (LoD). The limit of detection corresponds to the sample concentration which leads with a probability of 95% to a measurement result above the limit of blank. LoD = LoB SD blank The limit of quantitation (LoQ) is the lowest concentration that can be reproducibly measured with the between run coefficient of variation of < 20%. LoQ = Bias spike + 2SD spike 33
34 Criteria of acceptance 34
35 Waived tests requirements by accrediting agency Requirement CLIA JCAHO CAP Daily QC requirement Participation in regulatory proficiency testing Establishment of accuracy (twice each year) for analytes not in proficiency testing Method verification before implementation Method verification before implementation for multiple instrument, same model Follow test manufacturer s direction Only for CLSI regulated analytes Follow test manufacturer s direction Only for CLSI regulated analytes Yes Yes Yes No Accuracy, precision, reportable range, and appropriateness of reference ranges No No Yes Doesn t recognize waived testing. All tests must meet the same standards For all analytes when proficiency test is available Accuracy, precision, reportable range, sensitivity and appropriateness of reference ranges 35
36 Selected POCT requirements Requirement CLIA JCAHO CAP Assessment of accuracy at least every 6 months Assessment of reportable range every 6 months No Yes Yes No No Yes Method correlation at least every 6 months For test results from different methodologies under the same CLIA certificate For test results from different methodologies across entire JCAHO For test resutls from different methodologies under the same CLIA certificate Moderately complex testing QC for most analytes 2 levels of QC per day. More frequent evaluation is required for some analytes 2 levels of QC per day. More frequent evaluation is required for some analytes 2 levels of QC per day. More frequent evaluation is required for some analytes 36
37 Selected POCT requirements Requirement CLIA JCAHO CAP Accepts electronic controls Yes, follow manufacturer s recommendation Yes, with verification of manufacturer s claims and periodic assessment with liquid controls Yes, with verification of manufacturer s claims and periodic assessment with liquid controls CAP,College of American Pathologists, CLIA, Clinical Laboratory Improvement Amendments, JCAHO, Joint Commission on Accreditation of Healthcare Organizations. 37
38 Selected POCT requirements Requirement CLIA JCAHO CAP Non waived test Unmodified Moderate and high complexity Accuracy Precision Reportable range Verify reference range Accuracy Precision Reportable range Verify reference range Accuracy Precision Reportable range Non waived test Modified or developed in house Accuracy Precision Reportable range Verify reference range Analytical sensitivity Analytical specificity (interfering substances) Recovery Accuracy Precision Reportable range Verify reference range Analytical sensitivity Analytical specificity (interfering substances) Accuracy Precision Reportable range Analytical sensitivity Analytical specificity (interfering substances 38
39 We Innovate Healthcare
The Virtues and Pitfalls of Implementing a New Test
 The Virtues and Pitfalls of Implementing a New Test James H. Nichols, Ph.D., DABCC, FACB Professor of Clinical Pathology, Microbiology and Immunology Associate Medical Director for Clinical Operations
The Virtues and Pitfalls of Implementing a New Test James H. Nichols, Ph.D., DABCC, FACB Professor of Clinical Pathology, Microbiology and Immunology Associate Medical Director for Clinical Operations
USING THE ACCESS AMH ASSAY IN YOUR LABORATORY
 INFORMATION BULLETIN USING THE ACCESS AMH ASSAY IN YOUR LABORATORY ///////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
INFORMATION BULLETIN USING THE ACCESS AMH ASSAY IN YOUR LABORATORY ///////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
Polymer Technology Systems, Inc. CardioChek PA Comparison Study
 Polymer Technology Systems, Inc. CardioChek PA Comparison Study Evaluation Protocol: Accuracy Precision Clinical Correlation PTS Panels Lipid Panel Test Strips For Use in Comparisons to a Reference Laboratory
Polymer Technology Systems, Inc. CardioChek PA Comparison Study Evaluation Protocol: Accuracy Precision Clinical Correlation PTS Panels Lipid Panel Test Strips For Use in Comparisons to a Reference Laboratory
METHOD VALIDATION CASE
 METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION: WHY, HOW AND WHEN?
 METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
Method Comparison Report Semi-Annual 1/5/2018
 Method Comparison Report Semi-Annual 1/5/2018 Prepared for Carl Commissioner Regularatory Commission 123 Commission Drive Anytown, XX, 12345 Prepared by Dr. Mark Mainstay Clinical Laboratory Kennett Community
Method Comparison Report Semi-Annual 1/5/2018 Prepared for Carl Commissioner Regularatory Commission 123 Commission Drive Anytown, XX, 12345 Prepared by Dr. Mark Mainstay Clinical Laboratory Kennett Community
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE
 A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
INTERNAL QUALITY CONTROL ( Q I C) QC)
 EXTERNAL QUALITY ASSESSMENT PROGRAM (EQAP) BIOCHEMISTRY DEPARTMENT R. Mohammadi Biochemist (Ph.D.) Faculty member of Medical Faculty TWO COMPLEMENTARY COMPONENETS OF TQM ARE Internal Quality Control (IQC)
EXTERNAL QUALITY ASSESSMENT PROGRAM (EQAP) BIOCHEMISTRY DEPARTMENT R. Mohammadi Biochemist (Ph.D.) Faculty member of Medical Faculty TWO COMPLEMENTARY COMPONENETS OF TQM ARE Internal Quality Control (IQC)
2014 Continuing Compliance Master Series Best Practices in Alternative Assessment of Performance
 2014 Continuing Compliance Master Series Best Practices in Alternative Assessment of Performance Brad S. Karon, MD, PhD, FCAP November 19, 2014 www.cap.org Today s Presenter Brad S. Karon, MD, PhD, FCAP
2014 Continuing Compliance Master Series Best Practices in Alternative Assessment of Performance Brad S. Karon, MD, PhD, FCAP November 19, 2014 www.cap.org Today s Presenter Brad S. Karon, MD, PhD, FCAP
QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800
 QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800 Beckman Coulter Reagent REF A53727 The QMS Tacrolimus Immunoassay is intended for the quantitative determination of tacrolimus in human whole
QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800 Beckman Coulter Reagent REF A53727 The QMS Tacrolimus Immunoassay is intended for the quantitative determination of tacrolimus in human whole
BLOOD IS COMPLEX ANALYZING IT SHOULDN T BE DxH 500 * *Not available for sale in the U.S.
 BLOOD IS COMPLEX ANALYZING IT SHOULDN T BE DxH 500 * *Not available for sale in the U.S. Introducing the DxH 500, an Open-vial Hematology System *. Quality 5-part Differential in a Compact Design The first
BLOOD IS COMPLEX ANALYZING IT SHOULDN T BE DxH 500 * *Not available for sale in the U.S. Introducing the DxH 500, an Open-vial Hematology System *. Quality 5-part Differential in a Compact Design The first
Performance Characteristics of a New Single-Sample Freezing Point Depression Osmometer
 AACC, July 2007 Performance Characteristics of a New Single-Sample Freezing Point Depression Osmometer E. Garry, M. Pesta, N. Zampa Advanced Instruments, Inc., Norwood, MA 02062 AbstracT The Advanced Model
AACC, July 2007 Performance Characteristics of a New Single-Sample Freezing Point Depression Osmometer E. Garry, M. Pesta, N. Zampa Advanced Instruments, Inc., Norwood, MA 02062 AbstracT The Advanced Model
Why do we need SD LipidoCare?
 SD Biosensor, Inc. Innovative Global Leader of In Vitro Diagnostics Total supplier of Blood Glucose Monitoring System and Lipid Analyzer System in vitro diagnostics test kits for Point of Care Testing
SD Biosensor, Inc. Innovative Global Leader of In Vitro Diagnostics Total supplier of Blood Glucose Monitoring System and Lipid Analyzer System in vitro diagnostics test kits for Point of Care Testing
Hemostasis Test Validation, Performance and Reference Intervals
 Hemostasis Test Validation, Performance and Reference Intervals Richard A. Marlar, Ph.D. Pathology and Laboratory Medicine Oklahoma City VA Medical Center University of Oklahoma Health Sciences Center
Hemostasis Test Validation, Performance and Reference Intervals Richard A. Marlar, Ph.D. Pathology and Laboratory Medicine Oklahoma City VA Medical Center University of Oklahoma Health Sciences Center
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
METHOD VALIDATION CASE
 METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
Quality Specifications for POCT
 Quality Specifications for POCT the patient journey perspective Christopher P Price Department of Clinical Biochemistry, University of Oxford, Oxford, UK Quality Specifications fit-for-purpose QUALITY
Quality Specifications for POCT the patient journey perspective Christopher P Price Department of Clinical Biochemistry, University of Oxford, Oxford, UK Quality Specifications fit-for-purpose QUALITY
Manual. Transfer step. Figure 1. Workflow comparison of manual phenylalanine assay and automated GSP Neonatal Phenylalanine assay
 TECHNICAL NOTE GSP Neonatal Phenylalanine kit 3308-0010/3308-001B GSP Neonatal Phenylalanine kit (3308-0010/3308-001B) is a fully Introduction automated enzymatic assay for the GSP system. Optimized for
TECHNICAL NOTE GSP Neonatal Phenylalanine kit 3308-0010/3308-001B GSP Neonatal Phenylalanine kit (3308-0010/3308-001B) is a fully Introduction automated enzymatic assay for the GSP system. Optimized for
Mass Spectrometry Made Simple for Clinical Diagnostic Labs
 Mass Spectrometry Made Simple for Clinical Diagnostic Labs The SCIEX Topaz System with FDA-Cleared Topaz Vitamin D 200M Assay Kit Mass Spectrometry Made Simple SCIEX Topaz System for Clinical Diagnostics
Mass Spectrometry Made Simple for Clinical Diagnostic Labs The SCIEX Topaz System with FDA-Cleared Topaz Vitamin D 200M Assay Kit Mass Spectrometry Made Simple SCIEX Topaz System for Clinical Diagnostics
Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007
 Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007 Thomas J. Spira, M.D. International Laboratory Branch Global AIDS Program Centers for Disease
Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007 Thomas J. Spira, M.D. International Laboratory Branch Global AIDS Program Centers for Disease
Quality Control for Point-of-Care Testing
 Quality Control for Point-of-Care Testing James H. Nichols, PhD, DABCC, FACB Professor of Pathology, Microbiology and Immunology Medical Director of Clinical Chemistry and Point-of-Care Testing Vanderbilt
Quality Control for Point-of-Care Testing James H. Nichols, PhD, DABCC, FACB Professor of Pathology, Microbiology and Immunology Medical Director of Clinical Chemistry and Point-of-Care Testing Vanderbilt
Rapid Detection FAQs
 Rapid Detection FAQs 3M Rapid Detection Reader What are the dimensions of the Rapid Detection Reader? The Rapid Detection Reader includes the Control Module and the Test Module. The Control Module is 185
Rapid Detection FAQs 3M Rapid Detection Reader What are the dimensions of the Rapid Detection Reader? The Rapid Detection Reader includes the Control Module and the Test Module. The Control Module is 185
Patient Safety: A Quality System Approach To POCT QC/QA
 Patient Safety: A Quality System Approach To POCT QC/QA Ellis Jacobs, Ph.D., DABCC New York University School of Medicine Coler-Goldwater Specialty Hospital & Nursing Facility New York, New York Point-of-Care
Patient Safety: A Quality System Approach To POCT QC/QA Ellis Jacobs, Ph.D., DABCC New York University School of Medicine Coler-Goldwater Specialty Hospital & Nursing Facility New York, New York Point-of-Care
BÜHLMANN fcal turbo. Calprotectin turbidimetric assay for professional use. Reagent Kit B-KCAL-RSET. Revision date:
 BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
cobas u 601 urine analyzer Providing precise and secure test results
 Providing precise and secure test results 1 of 6 Providing precise and secure test results cobas u 601 urine analyzer is a modular, analytical system platform for urine strip testing and features a fully
Providing precise and secure test results 1 of 6 Providing precise and secure test results cobas u 601 urine analyzer is a modular, analytical system platform for urine strip testing and features a fully
Mission Hb accurately detects both
 Mission Digital Hb-Testing System: Save Cost by testing 2- Parameters with Single Strip real time ie: i.e: Hemoglobin & Hematocrit-[PCV]. Presented By : Remedy Healthcare Private Limited, New Delhi, [INDIA].
Mission Digital Hb-Testing System: Save Cost by testing 2- Parameters with Single Strip real time ie: i.e: Hemoglobin & Hematocrit-[PCV]. Presented By : Remedy Healthcare Private Limited, New Delhi, [INDIA].
Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4000
 v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
Individual Quality Control Plans (IQCP) Risk Assessment
 Individual Quality Control Plans (IQCP) Risk Assessment James H. Nichols, PhD, DABCC, FACB Professor of Clinical Pathology, Microbiology and Immunology Medical Director of Clinical Chemistry and Point-of-Care
Individual Quality Control Plans (IQCP) Risk Assessment James H. Nichols, PhD, DABCC, FACB Professor of Clinical Pathology, Microbiology and Immunology Medical Director of Clinical Chemistry and Point-of-Care
What I will talk about
 EQA of POCT methods: How to interpret results? Anne Stavelin Labquality Days, Helsinki 12 February 2016 www.noklus.no Norwegian quality improvement of primary care laboratories What I will talk about What
EQA of POCT methods: How to interpret results? Anne Stavelin Labquality Days, Helsinki 12 February 2016 www.noklus.no Norwegian quality improvement of primary care laboratories What I will talk about What
Patient Safety: A Quality System Approach To POCT QC/QA
 Patient Safety: A Quality System Approach To POCT QC/QA Ellis Jacobs, Ph.D., DABCC New York University School of Medicine Coler-Goldwater Specialty Hospital & Nursing Facility New York, New York Point-of-Care
Patient Safety: A Quality System Approach To POCT QC/QA Ellis Jacobs, Ph.D., DABCC New York University School of Medicine Coler-Goldwater Specialty Hospital & Nursing Facility New York, New York Point-of-Care
GLUH PRINCIPLE REF B ANNUAL REVIEW Reviewed by. Date. Date INTENDED USE
 SYNCHRON System(s) Chemistry Information Sheet Copyright 2014 Beckman Coulter, Inc. GLUH Glucose REF B24985 For In Vitro Diagnostic Use Rx Only ANNUAL REVIEW Reviewed by Date Reviewed by Date PRINCIPLE
SYNCHRON System(s) Chemistry Information Sheet Copyright 2014 Beckman Coulter, Inc. GLUH Glucose REF B24985 For In Vitro Diagnostic Use Rx Only ANNUAL REVIEW Reviewed by Date Reviewed by Date PRINCIPLE
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa
 MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on. CUBE analyser (CA0100)
 Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on CUBE analyser (CA0100) Location Location: Eurolyser Diagnostica GmbH Operators: Simone Wieser; Franz Helminger; Michael Gruber Date: July-November
Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on CUBE analyser (CA0100) Location Location: Eurolyser Diagnostica GmbH Operators: Simone Wieser; Franz Helminger; Michael Gruber Date: July-November
1. PROTOCOL. Comparison Study Summary. Tuality Healthcare 324 SE 9 th Ave. Suite E Hillsboro, OR July 30, 2014
 Comparison Study Summary Tuality Healthcare 324 SE 9 th Ave. Suite E Hillsboro, OR 97123 July 30, 2014 1. PROTOCOL This study was conducted on July 24 th, 2014 at Tuality Healthcare, Hillsboro, OR. The
Comparison Study Summary Tuality Healthcare 324 SE 9 th Ave. Suite E Hillsboro, OR 97123 July 30, 2014 1. PROTOCOL This study was conducted on July 24 th, 2014 at Tuality Healthcare, Hillsboro, OR. The
GB/T Translated English of Chinese Standard: GB/T
 Translated English of Chinese Standard: GB/T 19634-2005 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 11.100 C 44 National Standard of the People s Republic of China GB/T 19634-2005 In vitro
Translated English of Chinese Standard: GB/T 19634-2005 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 11.100 C 44 National Standard of the People s Republic of China GB/T 19634-2005 In vitro
It s Just a Waived Glucose, Isn t It?
 It s Just a Waived Glucose, Isn t It? What Is the Next Step? Becky Damiani, MT (ASCP), Senior Inspection Specialist Laboratory Accreditation Program Objectives Understand the CLIA requirements surrounding
It s Just a Waived Glucose, Isn t It? What Is the Next Step? Becky Damiani, MT (ASCP), Senior Inspection Specialist Laboratory Accreditation Program Objectives Understand the CLIA requirements surrounding
SCIEX Vitamin D 200M Assay for the Topaz System
 The First FDA-Cleared LC-MS/MS Assay for Vitamin D SCIEX Vitamin D 200M Assay for the Topaz System The first FDA-cleared LC-MS/MS assay for Vitamin D Vitamin D is an important building block for human
The First FDA-Cleared LC-MS/MS Assay for Vitamin D SCIEX Vitamin D 200M Assay for the Topaz System The first FDA-cleared LC-MS/MS assay for Vitamin D Vitamin D is an important building block for human
Cardiac Assessment Controls
 Bio-Rad Laboratories CARDIAC ASSESSMENT CONTROLS Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
Bio-Rad Laboratories CARDIAC ASSESSMENT CONTROLS Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
25-Hydroxy Vitamin D TOTAL (25HD) on Liaison XL
 25-Hydroxy Vitamin D TOTAL (25HD) on Liaison XL I. PRINCIPLE The role of vitamin D in bone and mineral metabolism was recognized from its first identification as a factor that could cure rickets. However,
25-Hydroxy Vitamin D TOTAL (25HD) on Liaison XL I. PRINCIPLE The role of vitamin D in bone and mineral metabolism was recognized from its first identification as a factor that could cure rickets. However,
Workshop on Proficiency Testing for Medical Laboratories. Daniel W. Tholen, M.S. March 13, 2013
 Workshop on Proficiency Testing for Medical Laboratories Daniel W. Tholen, M.S. March 13, 2013 Workshop Schedule It s a Workshop! not a lecture Discussion expected My background in PT for medical laboratories
Workshop on Proficiency Testing for Medical Laboratories Daniel W. Tholen, M.S. March 13, 2013 Workshop Schedule It s a Workshop! not a lecture Discussion expected My background in PT for medical laboratories
Determination of hemoglobin is one of the most commonly
 ORIGINAL ARTICLE Multiple-Site Analytic Evaluation of a New Portable Analyzer, HemoCue Hb 201+, for Point-of-Care Testing Sten-Erik Bäck, PhD,* Carl G. M. Magnusson, PhD, Lena K. Norlund, MD, PhD, Henning
ORIGINAL ARTICLE Multiple-Site Analytic Evaluation of a New Portable Analyzer, HemoCue Hb 201+, for Point-of-Care Testing Sten-Erik Bäck, PhD,* Carl G. M. Magnusson, PhD, Lena K. Norlund, MD, PhD, Henning
Alere Technologies GmbH
 Alere Technologies GmbH Mobile Smart Instruments for Automated Image Analysis and Point of Care Diagnosis April 2011 Alere Technologies GmbH Alere Technologies GmbH Based in Jena, Thuringia, Germany ~300
Alere Technologies GmbH Mobile Smart Instruments for Automated Image Analysis and Point of Care Diagnosis April 2011 Alere Technologies GmbH Alere Technologies GmbH Based in Jena, Thuringia, Germany ~300
LIPASE liquicolor. Design Verification. Multipurpose Reagent
 Design Verification LIPASE liquicolor Multipurpose Reagent CONTENTS 1 Introduction... 2 2 Imprecision... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 3 4 Recovery of
Design Verification LIPASE liquicolor Multipurpose Reagent CONTENTS 1 Introduction... 2 2 Imprecision... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 3 4 Recovery of
HM5. Hematology Analyzer BETTER. ACTUALLY.
 HM5 Hematology Analyzer BETTER. ACTUALLY. Advanced Hematology Five-Part Differential The VetScan HM5 is a fully automated five-part differential hematology analyzer displaying a comprehensive 24-parameter
HM5 Hematology Analyzer BETTER. ACTUALLY. Advanced Hematology Five-Part Differential The VetScan HM5 is a fully automated five-part differential hematology analyzer displaying a comprehensive 24-parameter
FreeStyle Lite A Blood Glucose Meter That Requires No Coding
 Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
A validation study of after reconstitution stability of diabetes: level 1 and diabetes level 2 controls
 A validation study of after reconstitution stability of diabetes: level 1 and diabetes level 2 controls Shyamali Pal R B Diagnostic Private Limited, Lake Town, Kolkata, India INFO ABSTRACT Corresponding
A validation study of after reconstitution stability of diabetes: level 1 and diabetes level 2 controls Shyamali Pal R B Diagnostic Private Limited, Lake Town, Kolkata, India INFO ABSTRACT Corresponding
Disclosures. Relevant Financial Relationship(s): Nothing to Disclose. Off Label Usage: Nothing to Disclose 9/19/2017. Proficiency Testing
 September 11, 2017 Teri Ross, Quality Specialist II Doreen Ryan, MA, MT; Quality Operations Supervisor Deborah J. Wells, MPA, MT(ASCP)SH, Quality Management Coordinator Disclosures Relevant Financial Relationship(s):
September 11, 2017 Teri Ross, Quality Specialist II Doreen Ryan, MA, MT; Quality Operations Supervisor Deborah J. Wells, MPA, MT(ASCP)SH, Quality Management Coordinator Disclosures Relevant Financial Relationship(s):
Worksheet. Worksheet. Worksheet. Worksheet. Student Performance Guide. Student Performance Guide
 LESSON 6-3 Laboratory Reagent Preparation and Calculations Worksheet LESSON 6-4 Chemistry Instrumentation in the Physician Office Laboratory Worksheet LESSON 6-6 Measuring Blood Glucose Worksheet LESSON
LESSON 6-3 Laboratory Reagent Preparation and Calculations Worksheet LESSON 6-4 Chemistry Instrumentation in the Physician Office Laboratory Worksheet LESSON 6-6 Measuring Blood Glucose Worksheet LESSON
25-Hydroxyvitamin D2-D3 Serum VD-200 UHPLC Analysis Kit User Manual
 Page 1 / 12 25-Hydroxyvitamin D2-D3 Serum VD-200 UHPLC Analysis Kit User Manual ZV-4027-0500-10 500 2-8 C Page 2 / 12 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. IVD SYMBOLS...
Page 1 / 12 25-Hydroxyvitamin D2-D3 Serum VD-200 UHPLC Analysis Kit User Manual ZV-4027-0500-10 500 2-8 C Page 2 / 12 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. IVD SYMBOLS...
Advanced Hematology Five-Part Differential. Simple Operation
 HematologyAnalyzer Advanced Hematology Five-Part Differential analyzer displaying a comprehensive 22-parameter complete blood count (CBC) with cellular histograms on an easy-to-read touch-screen. Its superior
HematologyAnalyzer Advanced Hematology Five-Part Differential analyzer displaying a comprehensive 22-parameter complete blood count (CBC) with cellular histograms on an easy-to-read touch-screen. Its superior
GlucCell TM SYSTEM USER S GUIDE Ver 2.1 CELL CULTURE GLUCOSE METER. Important Information. Intended Use. Caution. About the System
 GlucCell TM SYSTEM USER S GUIDE Ver 2.1 Intended Use The GlucCell TM Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during cell culture.
GlucCell TM SYSTEM USER S GUIDE Ver 2.1 Intended Use The GlucCell TM Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during cell culture.
The analytical phase
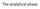 The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
liquicolor (AMP Buffer, IFCC) Design Verification
 Design Verification (AMP Buffer, IFCC) liquicolor 1 Introduction... 2 2 Imprecision... 2 3 arity and Detection Limit... 2 3.1 arity... 2 3.2 Detection Limit... 3 4 Comparison of Methods... 3 5 Stability...
Design Verification (AMP Buffer, IFCC) liquicolor 1 Introduction... 2 2 Imprecision... 2 3 arity and Detection Limit... 2 3.1 arity... 2 3.2 Detection Limit... 3 4 Comparison of Methods... 3 5 Stability...
Standard Operating Procedure
 Subject Free Thyroxine (FT4-II) cobas e411 Index Number Lab-1594 Section Regional/Affiliate Subsection Laboratory Category Departmental Contact Goplin, Darcy Last Revised 2/2/2017 References Required document
Subject Free Thyroxine (FT4-II) cobas e411 Index Number Lab-1594 Section Regional/Affiliate Subsection Laboratory Category Departmental Contact Goplin, Darcy Last Revised 2/2/2017 References Required document
Bovine Insulin ELISA
 Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
ImmunoCAP Systems. Optimized for allergy testing - Precision - Accuracy - Consistency
 ImmunoCAP Systems Optimized for allergy testing - Precision - Accuracy - Consistency 1 The ImmunoCAP systems from Phadia offer an optimal allergy testing solution for every laboratory, regardless of testing
ImmunoCAP Systems Optimized for allergy testing - Precision - Accuracy - Consistency 1 The ImmunoCAP systems from Phadia offer an optimal allergy testing solution for every laboratory, regardless of testing
Validation Case studies: the good, the bad and the molecular
 Validation Case studies: the good, the bad and the molecular Patti Jones PhD Professor of Pathology UT Southwestern Medical Center Director of Chemistry Children s Medical Center Dallas Presented by AACC
Validation Case studies: the good, the bad and the molecular Patti Jones PhD Professor of Pathology UT Southwestern Medical Center Director of Chemistry Children s Medical Center Dallas Presented by AACC
Abaxis Piccolo xpress TM
 Abaxis Piccolo xpress TM A breakthrough in point-of care diagnostics A breakthrough in point of care diagnostics Fast, easy, accurate on-site testing The Piccolo xpress TM is a fully automated system,
Abaxis Piccolo xpress TM A breakthrough in point-of care diagnostics A breakthrough in point of care diagnostics Fast, easy, accurate on-site testing The Piccolo xpress TM is a fully automated system,
Point of Care testing refers to all laboratory testing that is done outside of the walls of the clinical laboratory in the proximity of the patient.
 1 2 Point of Care testing refers to all laboratory testing that is done outside of the walls of the clinical laboratory in the proximity of the patient. All such tests are considered lab tests and are
1 2 Point of Care testing refers to all laboratory testing that is done outside of the walls of the clinical laboratory in the proximity of the patient. All such tests are considered lab tests and are
(a) y = 1.0x + 0.0; r = ; N = 60 (b) y = 1.0x + 0.0; r = ; N = Lot 1, Li-heparin whole blood, HbA1c (%)
 cobas b system - performance evaluation Study report from a multicenter evaluation of the new cobas b system for the measurement of HbAc and lipid panel Introduction The new cobas b system provides a point-of-care
cobas b system - performance evaluation Study report from a multicenter evaluation of the new cobas b system for the measurement of HbAc and lipid panel Introduction The new cobas b system provides a point-of-care
Solutions at GE Inspection Technologies
 Solutions at GE Inspection Technologies Business Overview Paul A. Meyer Our Company A global leader in technology-driven inspection solutions that deliver productivity, quality, and safety to our customers
Solutions at GE Inspection Technologies Business Overview Paul A. Meyer Our Company A global leader in technology-driven inspection solutions that deliver productivity, quality, and safety to our customers
Individual Lab Report Ci-Trol Nov,2016. Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC Your Lab
 Individual Lab Report Ci-Trol Nov,2016 ST VINCENT MEDICAL CENTER LABORATORY(LAB# 7300 ) 2131 WEST THIRD STREET LOS ANGELES CA USA 90057 Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC SYSMEX
Individual Lab Report Ci-Trol Nov,2016 ST VINCENT MEDICAL CENTER LABORATORY(LAB# 7300 ) 2131 WEST THIRD STREET LOS ANGELES CA USA 90057 Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC SYSMEX
Rat C-peptide ELISA. For the quantitative determination of C-peptide in rat serum
 Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum Please read carefully due to Critical Changes, e.g., see Calculation of Results. For Research Use Only. Not For Use In Diagnostic
Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum Please read carefully due to Critical Changes, e.g., see Calculation of Results. For Research Use Only. Not For Use In Diagnostic
Analysis of Micronutrients in Milk by Flame Atomic Absorption Using FAST Flame Sample Automation for Increased Sample Throughput
 APPLICATION NOTE Atomic Absorption Author: Nick Spivey PerkinElmer, Inc. Shelton, CT Analysis of Micronutrients in Milk by Flame Atomic Absorption Using FAST Flame Sample Automation for Increased Sample
APPLICATION NOTE Atomic Absorption Author: Nick Spivey PerkinElmer, Inc. Shelton, CT Analysis of Micronutrients in Milk by Flame Atomic Absorption Using FAST Flame Sample Automation for Increased Sample
GlucCell TM SYSTEM USER S GUIDE ver 2.3 CELL CULTURE GLUCOSE METER. Important Information. Intended Use. Caution. About the System
 GlucCell TM SYSTEM USER S GUIDE ver 2.3 Intended Use The GlucCell TM Cell Culture Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during
GlucCell TM SYSTEM USER S GUIDE ver 2.3 Intended Use The GlucCell TM Cell Culture Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during
Auto HbA1C ANALYZER HbA1LC HPLC 3660
 Turnkey Laboratories Solutions Auto HbA1C ANALYZER HbA1LC HPLC 3660 Viewable Accuracy Real Time Summarized Chromatogram Authentic Results EPC / PRODUCTS / APPLICATION / SOFTWARE / ACCESSORIES / CONSUMABLES
Turnkey Laboratories Solutions Auto HbA1C ANALYZER HbA1LC HPLC 3660 Viewable Accuracy Real Time Summarized Chromatogram Authentic Results EPC / PRODUCTS / APPLICATION / SOFTWARE / ACCESSORIES / CONSUMABLES
For In Vitro Diagnostic Use
 SYNCHRON System(s) Chemistry Information Sheet Lactate REF A95550 For In Vitro Diagnostic Use ANNUAL REVIEW Refer to Review and Revision Coversheet at the front of each method. PRINCIPLE INTENDED USE reagent,
SYNCHRON System(s) Chemistry Information Sheet Lactate REF A95550 For In Vitro Diagnostic Use ANNUAL REVIEW Refer to Review and Revision Coversheet at the front of each method. PRINCIPLE INTENDED USE reagent,
The performance of Microdot Test Strips has been evaluated both in laboratory and in clinical tests.
 Chemical Composition Each Microdot strip contains the enzyme glucose dehydrogenase (Bacillus Sp.) = 1 IU and other ingredients (mediator, NAD, lysing agents etc.) = 200 µg. Test Strips When blood is added
Chemical Composition Each Microdot strip contains the enzyme glucose dehydrogenase (Bacillus Sp.) = 1 IU and other ingredients (mediator, NAD, lysing agents etc.) = 200 µg. Test Strips When blood is added
Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry.
 2013/07/30 A93A01315AUS A11A01641 60 ml 15 ml Pentra C400 Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry. QUAL-QA-TEMP-0846
2013/07/30 A93A01315AUS A11A01641 60 ml 15 ml Pentra C400 Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry. QUAL-QA-TEMP-0846
SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
 MEDICAL ELECTRONIC SYSTEMS 5757 W. Century Blvd. Suite 805 Los Angeles, CA. 90045 Remember, it ALL Started with a Sperm! www.mes global.com SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
MEDICAL ELECTRONIC SYSTEMS 5757 W. Century Blvd. Suite 805 Los Angeles, CA. 90045 Remember, it ALL Started with a Sperm! www.mes global.com SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
Glucose Analytical Comparability Evaluation of the YSI 2300 STAT Plus and YSI 2900D Biochemistry Analyzers
 Glucose Analytical Comparability Evaluation of the YSI 2300 STAT Plus and YSI 2900D iochemistry Analyzers Life Sciences Data for Life. TM YSI Life Sciences White Paper 91 Authors: Kevin Schlueter, PhD,
Glucose Analytical Comparability Evaluation of the YSI 2300 STAT Plus and YSI 2900D iochemistry Analyzers Life Sciences Data for Life. TM YSI Life Sciences White Paper 91 Authors: Kevin Schlueter, PhD,
Interference and Point-of-Care Testing Devices
 Interference and Point-of-Care Testing Devices Nam K. Tran, PhD, HCLD, (ABB), FACB Associate Clinical Professor Director of Clinical Chemistry, Special Chemistry/Toxicology and POCT 1 Learning Objectives
Interference and Point-of-Care Testing Devices Nam K. Tran, PhD, HCLD, (ABB), FACB Associate Clinical Professor Director of Clinical Chemistry, Special Chemistry/Toxicology and POCT 1 Learning Objectives
Next Generation Rapid Diagnostics
 Next Generation Rapid Diagnostics INTERNATIONAL PRODUCT CATALOG Quidel is a leading manufacturer of diagnostic healthcare solutions serving to enhance the health and well-being of people around the globe
Next Generation Rapid Diagnostics INTERNATIONAL PRODUCT CATALOG Quidel is a leading manufacturer of diagnostic healthcare solutions serving to enhance the health and well-being of people around the globe
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System
 Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Insulin ELISA. For the quantitative determination of insulin in serum and plasma.
 Insulin ELISA For the quantitative determination of insulin in serum and plasma. For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Insulin ELISA For the quantitative determination of insulin in serum and plasma. For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
AIA-600II Assay Specifications TgAb Test Code 048
 AIA-600II Assay Specifications TgAb Test Code 048 Screen Item Data Screen 1 Lot ***Enter current cal. lot no. Cal 1 0 IU/mL Cal 2 2 IU/mL (example) Cal 3 4 IU/mL (example) Cal 4 10 IU/mL (example) Cal
AIA-600II Assay Specifications TgAb Test Code 048 Screen Item Data Screen 1 Lot ***Enter current cal. lot no. Cal 1 0 IU/mL Cal 2 2 IU/mL (example) Cal 3 4 IU/mL (example) Cal 4 10 IU/mL (example) Cal
Quantitative Measurement of Emergency Biomarkers in Parallel
 Point-of-Care Immunoassay Analyzer Quantitative Measurement of Emergency Biomarkers in Parallel Troponin I, Myoglobin, CK-MB, D-Dimer, NTproBNP, hscrp Results in 17 minutes Direct measurement from whole
Point-of-Care Immunoassay Analyzer Quantitative Measurement of Emergency Biomarkers in Parallel Troponin I, Myoglobin, CK-MB, D-Dimer, NTproBNP, hscrp Results in 17 minutes Direct measurement from whole
The challenges of quality assessment for point-of-care testing. Anne Stavelin. EQALM Symposium, Bergen 8 October General challenges
 The challenges of quality assessment for point-of-care testing Anne Stavelin EQALM Symposium, Bergen 8 October 2015 www.noklus.no Norwegian quality improvement of primary care laboratories General challenges
The challenges of quality assessment for point-of-care testing Anne Stavelin EQALM Symposium, Bergen 8 October 2015 www.noklus.no Norwegian quality improvement of primary care laboratories General challenges
POC Testing: The New Standard. InnovaStar From DiaSys.
 POC Testing: The New Standard. InnovaStar From DiaSys. Easy to use, superior precision, rapid results: InnovaStar from DiaSys brings POC testing to a whole new level. A fully automatic, compact analyzer
POC Testing: The New Standard. InnovaStar From DiaSys. Easy to use, superior precision, rapid results: InnovaStar from DiaSys brings POC testing to a whole new level. A fully automatic, compact analyzer
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
Cardiac Assessment Controls A critical element of reliable cardiac testing
 Cardiac Assessment Controls Cardiac Assessment Controls A critical element of reliable cardiac testing Choose the products best suited for your cardiac assessment needs Each year, 17.7 million people on
Cardiac Assessment Controls Cardiac Assessment Controls A critical element of reliable cardiac testing Choose the products best suited for your cardiac assessment needs Each year, 17.7 million people on
Tips for Training New Customers
 Tips for Training New Customers Good Habits: Test Strips Recap vial immediately and tightly after removing a single test strip. Never remove the desiccant from the vial. Do not lay test strips out ahead
Tips for Training New Customers Good Habits: Test Strips Recap vial immediately and tightly after removing a single test strip. Never remove the desiccant from the vial. Do not lay test strips out ahead
I know my value CoaguChek XS Pro system
 I know my value CoaguChek XS Pro system The smart way to manage your anticoagulation clinic CoaguChek XS Pro system A barcode scanner can make a difference There has been an extraordinary increase in the
I know my value CoaguChek XS Pro system The smart way to manage your anticoagulation clinic CoaguChek XS Pro system A barcode scanner can make a difference There has been an extraordinary increase in the
PROCEDURE. TITLE: Bedside Glucose Monitoring PC Laboratory. Issuing Department: Clinical Director Signature: Departments Involved:
 PROCEDURE TITLE: Bedside Glucose Monitoring Issuing Department: Clinical Director Signature: Departments Involved: Laboratory Nursing Effective Date: 10/97 Review Dates: 09/01, 07/02, 05/13 Revision Dates:
PROCEDURE TITLE: Bedside Glucose Monitoring Issuing Department: Clinical Director Signature: Departments Involved: Laboratory Nursing Effective Date: 10/97 Review Dates: 09/01, 07/02, 05/13 Revision Dates:
Case Studies, or Verification Vignettes
 Case Studies, or Verification Vignettes 1 Vignette #1 Change from One Automated AST to Another Your lab is changing from one FDA-cleared automated AST to another Is a verification study required? 2 Yes
Case Studies, or Verification Vignettes 1 Vignette #1 Change from One Automated AST to Another Your lab is changing from one FDA-cleared automated AST to another Is a verification study required? 2 Yes
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE. Quantitative (Oxide Semiconductor Alcohol Sensor)
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k060343 B. Purpose for Submission: New Device for the US market. C. Measurand: Breath Alcohol D. Type
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k060343 B. Purpose for Submission: New Device for the US market. C. Measurand: Breath Alcohol D. Type
Customer Bulletin. Questions/Comments? Please call Customer Service at (Direct) or (Toll-free inside USA).
 Customer Bulletin Assignment of Ranges for PTS Panels Multi-Chemistry Controls (Cat. No./REF 721) and PTS Panels Controls (Cat. No./REF 722) Background: PTS Panels Multi-Chemistry Controls and Controls
Customer Bulletin Assignment of Ranges for PTS Panels Multi-Chemistry Controls (Cat. No./REF 721) and PTS Panels Controls (Cat. No./REF 722) Background: PTS Panels Multi-Chemistry Controls and Controls
CLINITEK Novus Automated Urine Chemistry Analyzer
 CLINITEK, Atlas, Novus, Status+, and all associated marks are trademarks of Siemens Healthcare Diagnostics Inc. or its affiliates. All other trademarks are properties of their respective owners. Product
CLINITEK, Atlas, Novus, Status+, and all associated marks are trademarks of Siemens Healthcare Diagnostics Inc. or its affiliates. All other trademarks are properties of their respective owners. Product
Gentian Canine CRP Immunoassay Application Note for scil VitroVet*
 v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
In Vitro Diagnostic Glucose Test System
 CDRH Final Guidance 2-Page Cover Sheet Guidance for Industry In Vitro Diagnostic Glucose Test System Document issued on: July 6, 1998 U.S. Department Of Health and Human Services Food and Drug Administration
CDRH Final Guidance 2-Page Cover Sheet Guidance for Industry In Vitro Diagnostic Glucose Test System Document issued on: July 6, 1998 U.S. Department Of Health and Human Services Food and Drug Administration
Accuracy and Precision in Point-of-Care Lipid Testing: CardioChek P A Point-of-Care Test System and PTS Panels Test Strips
 Accuracy and Precision in Point-of-Care Lipid Testing: CardioChek P A Point-of-Care Test System and PTS Panels Test Strips Sponsored by Arthur Roberts, MD of The Living Heart Foundation The Living Heart
Accuracy and Precision in Point-of-Care Lipid Testing: CardioChek P A Point-of-Care Test System and PTS Panels Test Strips Sponsored by Arthur Roberts, MD of The Living Heart Foundation The Living Heart
It s a Gas! Issues in the Blood Gas Laboratory. D. Robert Dufour, MD, FCAP Consultant Pathologist VA Medical Center, Washington DC
 It s a Gas! Issues in the Blood Gas Laboratory D. Robert Dufour, MD, FCAP Consultant Pathologist VA Medical Center, Washington DC Learning Objectives After participating in this session, you will be able
It s a Gas! Issues in the Blood Gas Laboratory D. Robert Dufour, MD, FCAP Consultant Pathologist VA Medical Center, Washington DC Learning Objectives After participating in this session, you will be able
The NEPHROCHECK Test System Training Program. The NEPHROCHECK Training Program US: Astute Medical, Inc PN Rev D 2014/09/16
 The NEPHROCHECK Test System Training Program The NEPHROCHECK Training Program US: Astute Medical, Inc. 2014 PN 700001 Rev D 2014/09/16 Learning Objectives At the conclusion of this training, participants
The NEPHROCHECK Test System Training Program The NEPHROCHECK Training Program US: Astute Medical, Inc. 2014 PN 700001 Rev D 2014/09/16 Learning Objectives At the conclusion of this training, participants
Clinical Chemistry Specific Proteins Presentation
 Clinical Chemistry Specific Proteins Presentation November 2004 AEROSET and c8000 are registered trademarks of Abbott Laboratories. All other trademarks, brands, trade names and product names are the property
Clinical Chemistry Specific Proteins Presentation November 2004 AEROSET and c8000 are registered trademarks of Abbott Laboratories. All other trademarks, brands, trade names and product names are the property
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
STELLUX C-peptide Chemiluminescence ELISA
 STELLUX C-peptide Chemiluminescence ELISA For the quantitative determination of Human C-peptide in Human Serum, Heparin Plasma, EDTA plasma, and Tissue Culture Supernatants. For In Vitro Diagnostic use
STELLUX C-peptide Chemiluminescence ELISA For the quantitative determination of Human C-peptide in Human Serum, Heparin Plasma, EDTA plasma, and Tissue Culture Supernatants. For In Vitro Diagnostic use
Management of Central Venous Access Devices. Blood Glucose Monitoring
 Management of Central Venous Access Devices Blood Glucose Monitoring Purpose To provide education on the standard of care regarding the use and monitoring of the Accu- Chek Blood glucose machine, including
Management of Central Venous Access Devices Blood Glucose Monitoring Purpose To provide education on the standard of care regarding the use and monitoring of the Accu- Chek Blood glucose machine, including
Patient: 55 y female (ambulatory)
 Disclosures Speaking Honoraria Radiometer (Canada) Nova Biomedical, Draeger Roche Diagnostics (Canada) Research Support (Reagents, Instrumentation, Travel) Nova Biomedical Abbott Laboratories (Canada)
Disclosures Speaking Honoraria Radiometer (Canada) Nova Biomedical, Draeger Roche Diagnostics (Canada) Research Support (Reagents, Instrumentation, Travel) Nova Biomedical Abbott Laboratories (Canada)
