200 to 400 mg. 6 mg/kg to 10 mg/kg (maximum 400 mg per dose) 10 mg/kg (maximum 400 mg per dose) 10 mg/kg (maximum 400 mg per dose) Every 8 hours
|
|
|
- Samantha York
- 6 years ago
- Views:
Transcription
1 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights d nt include all the infrmatin needed t use CIPRO IV safely and effectively. See full prescribing infrmatin fr CIPRO IV. CIPRO IV (ciprflxacin) injectin, fr intravenus use Initial U.S. Apprval: 1987 WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS See full prescribing infrmatin fr cmplete bxed warning. Flurquinlnes, including CIPRO IV, have been assciated with disabling and ptentially irreversible serius adverse reactins that have ccurred tgether (5.1), including: Tendinitis and tendn rupture (5.2) Peripheral neurpathy (5.3) Central nervus system effects (5.4) Discntinue CIPRO immediately and avid the use f flurquinlnes, including CIPRO, in patients wh experience any f these serius adverse reactins (5.1) Flurquinlnes, including CIPRO IV, may exacerbate muscle weakness in patients with myasthenia gravis. Avid CIPRO IV in patients with knwn histry f myasthenia gravis. (5.5) Because flurquinlnes, including CIPRO, have been assciated with serius adverse reactins ( ), reserve CIPRO fr use in patients wh have n alternative treatment ptins fr the fllwing indicatins: Acute exacerbatin f chrnic brnchitis (1.9) Acute sinusitis (1.11) RECENT MAJOR CHANGES Warnings and Precautins (5.15) 7/ INDICATIONS AND USAGE CIPRO IV is a flurquinlne antibacterial indicated in adults ( 18 years f age) with the fllwing infectins caused by designated, susceptible bacteria and in pediatric patients where indicated: Skin and Skin structure Infectins (1.1) Bne and Jint infectins (1.2) Cmplicated Intra-Abdminal infectins (1.3) Nscmial Pneumnia (1.4) Empirical Therapy fr Febrile Neutrpenic Patients (1.5) Inhalatinal Anthrax Pst-Expsure in Adult and Pediatric Patients (1.6) Plague in adult and pediatric patients (1.7) Chrnic Bacterial Prstatitis (1.8) Lwer respiratry tract infectins (1.9) Acute Exacerbatin f Chrnic Brnchitis Urinary Tract Infectins (1.10) Urinary Tract Infectins (UTI) Cmplicated UTI and Pyelnephritis in Pediatric Patients Acute Sinusitis (1.11) Usage T reduce the develpment f drug-resistant bacteria and maintain the effectiveness f CIPRO IV and ther antibacterial drugs, CIPRO IV shuld be used nly t treat r prevent infectins that are prven r strngly suspected t be caused by bacteria. (1.12) DOSAGE AND ADMINISTRATION Adult Dsage Guidelines Infectin Dse Frequency Duratin Skin and Skin Structure 400 mg every 8 t 12 hurs 7 14 days Bne and Jint 400 mg every 8 t 12 hurs 4 t 8 weeks Cmplicated Intra-Abdminal 400 mg every 12 hurs 7 14 days Nscmial Pneumnia 400 mg every 8 hurs days 400 mg Empirical Therapy In Febrile and every 8 hurs Neutrpenic Patients Piperacillin 50 mg/kg every 4 hurs 7 14 days Inhalatinal anthrax(pst- 400 mg every 12 hurs 60 days expsure) Plague 400 mg every 8 t 12 hurs 14 days Chrnic Bacterial prstatitis 400 mg every 12 hurs 28 days Lwer Respiratry Tract 400 mg every 8 t 12 hurs 7 14 days Urinary Tract 200 t 400 mg every 8 t 12 hurs 7 14 days Acute Sinusitis 400 mg every 12 hurs 10 days Adults with creatinine clearance 5 29 ml/min mg q 18 h (2.3) Pediatric Intravenus Dsing Guidelines Infectin Dse Frequency Duratin Cmplicated UTI and Pyelnephritis (patients frm 1 t 17 years f age) Inhalatinal Anthrax (Pst-Expsure) Plague 6 mg/kg t 10 mg/kg (maximum 400 mg per dse) 10 mg/kg (maximum 400 mg per dse) 10 mg/kg (maximum 400 mg per dse) Every 8 hurs Every 12 hurs Every 8 t 12 hurs days 1 60 days days DOSAGE FORMS AND STRENGTHS Injectin: 400 mg/200 ml CONTRAINDICATIONS Knwn hypersensitivity t CIPRO r ther quinlnes (4.1, 5.7) Cncmitant administratin with tizanidine (4.2) WARNINGS AND PRECAUTIONS Hypersensitivity and ther serius reactins: Serius and smetimes fatal reactins (fr example, anaphylactic reactins) may ccur after first r subsequent dses f CIPRO IV. Discntinue CIPRO IV at the first sign f skin rash, jaundice r any sign f hypersensitivity. (4.1, 5.6, 5.7) Hepattxicity: Discntinue immediately if signs and symptms f hepatitis ccur. (5.8) Clstridium difficile-assciated Diarrhea: Evaluate if clitis ccurs. (5.10) QT Prlngatin: Prlngatin f the QT interval and islated cases f trsade de pintes have been reprted. Avid use in patients with knwn prlngatin, thse with hypkalemia, and with ther drugs that prlng the QT interval. (5.11, 7, 8.5) ADVERSE REACTIONS The mst cmmn adverse reactins 1% were nausea, diarrhea, liver functin tests abnrmal, vmiting, central nervus system disturbance, lcal intravenus site reactins esinphilia, headache, restlessness, and rash. (6) TO REPORT SUSPECTED ADVERSE REACTIONS, CONTACT BAYER HealthCare Pharmaceuticals Inc. at r FDA at FDA-1088 r DRUG INTERACTIONS Interacting Drug Interactin Thephylline Serius and fatal reactins. Avid cncmitant use. Mnitr serum level (7) Warfarin Anticagulant effect enhanced. Mnitr prthrmbin time, INR, and bleeding (7) Antidiabetic agents Hypglycemia including fatal utcmes have been reprted. Mnitr bld glucse (7) Phenytin Mnitr phenytin level (7) Methtrexate Mnitr fr methtrexate txicity (7) Cyclsprine May increase serum creatinine. Mnitr serum creatinine (7) USE IN SPECIFIC POPULATIONS See full prescribing infrmatin fr pediatric patients (8.4) and use in geriatric (8.5) See 17 fr PATIENT COUNSELING INFORMATION and Medicatin Guide Revised: 7/2017 NDA CIPRO IV FDA Apprved 26 Jul 2017
2 FULL PRESCRIBING INFORMATION: CONTENTS WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS 1 INDICATIONS AND USAGE 1.1 Skin and Skin Structure Infectins 1.2 Bne and Jint Infectins 1.3 Cmplicated Intra-Abdminal Infectins 1.4 Nscmial Pneumnia 1.5 Empirical Therapy fr Febrile Neutrpenia Patients 1.6 Inhalatinal Anthrax (Pst-Expsure) 1.7 Plague 1.8 Chrnic Bacterial Prstatitis 1.9 Lwer Respiratry Tract Infectins 1.10 Urinary Tract Infectins 1.11 Acute Sinusitis 1.12 Usage 2 DOSAGE AND ADMINISTRATION 2.1 Dsage in Adults 2.2 Dsage in Pediatric Patients 2.3 Dsage Mdificatins in Patients with Renal Impairment 2.4 Preparatin f CIPRO IV fr Administratin 2.5 Imprtant Administratin Instructins 3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS 4.1 Hypersensitivity 4.2 Tizanidine 5 WARNINGS AND PRECAUTIONS 5.1 Disabling and Ptentially Irreversible Serius Adverse Reactins Including Tendinitis and Tendn Rupture, Peripheral Neurpathy, and Central Nervus System Effects 5.2 Tenditis and Tendn Rupture 5.3 Peripheral Neurpathy 5.4 Central Nervus System Effects 5.5 Exacerbatin f Myasthenia Gravis 5.6 Other Serius and Smetimes Fatal Reactins 5.7 Hypersensitivity Reactins 5.8 Hepattxicity 5.9 Serius Adverse Reactins with Cncmitant Thephylline 5.10 Clstridium difficile-assciated Diarrhea 5.11 Prlngatin f the QT Interval 5.12 Musculskeletal Disrders in Pediatric Patients and Arthrpathic Effects in Animals 5.13 Phtsensitivity/Phttxicity 5.14 Develpment f Drug Resistant Bacteria 5.15 Ptential Risks With Cncmitant Use f Drugs Metablized by Cytchrme P450 1A2 Enzymes 5.17 Crystalluria 5.16 Peridic Assessment f Organ System Functins 6 ADVERSE REACTIONS 6.1 Clinical Trials Experience 6.2 Pstmarketing Experience 6.3 Adverse Labratry Changes 7 DRUG INTERACTIONS 8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy 8.3 Nursing Mthers 8.4 Pediatric Use 8.5 Geriatric Use 8.6 Renal Impairment 8.7 Hepatic Impairment 10 OVERDOSAGE 11 DESCRIPTION 12 CLINICAL PHARMACOLOGY 12.1 Mechanism f Actin 12.3 Pharmackinetics 12.4 Micrbilgy 13 NONCLINICAL TOXICOLOGY 13.1 Carcingenesis, Mutagenesis, Impairment f Fertility 13.2 Animal Txiclgy and/r Pharmaclgy 14 CLINICAL STUDIES 14.1 Empirical Therapy In Adult Febrile Neutrpenic Patients 14.2 Cmplicated Urinary Tract Infectin and Pyelnephritis Efficacy in Pediatric Patients 14.3 Inhalatinal Anthrax in Adults and Pediatrics 14.4 Plague 15 REFERENCES 16 HOW SUPPLIED/STORAGE AND HANDLING 17 PATIENT COUNSELING INFORMATION *Sectins r subsectins mitted frm the full prescribing infrmatin are nt listed. NDA CIPRO IV FDA Apprved 26 Jul 2017
3 FULL PRESCRIBING INFORMATION WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS Flurquinlnes, including CIPRO IV, have been assciated with disabling and ptentially irreversible serius adverse reactins that have ccurred tgether [see Warnings and Precautins (5.1)] including: Tendinitis and tendn rupture [see Warnings and Precautins (5.2)] Peripheral neurpathy [see Warnings and Precautins (5.3)] Central nervus system effects [see Warnings and Precautins (5.4)] Discntinue CIPRO immediately and avid the use f flurquinlnes, including CIPRO, in patients wh experience any f these serius adverse reactins [see Warnings and Precautins (5.1)]. Flurquinlnes, including CIPRO IV, may exacerbate muscle weakness in patients with myasthenia gravis. Avid CIPRO IV in patients with knwn histry f myasthenia gravis [see Warnings and Precautins (5.5)]. Because flurquinlnes, including CIPRO, have been assciated with serius adverse reactins [see Warnings and Precautins ( )], reserve CIPRO fr use in patients wh have n alternative treatment ptins fr the fllwing indicatins: Acute exacerbatin f chrnic brnchitis [see Indicatins and Usage (1.9)] Acute Sinusitis [see Indicatins and Usage (1.11)] 1 INDICATIONS AND USAGE 1.1 Skin and Skin Structure Infectins CIPRO IV is indicated in adult patients fr treatment f skin and skin structure infectins caused by Escherichia cli, Klebsiella pneumniae, Enterbacter clacae, Prteus mirabilis, Prteus vulgaris, Prvidencia stuartii, Mrganella mrganii, Citrbacter freundii, Pseudmnas aeruginsa, methicillin-susceptible Staphylcccus aureus, methicillinsusceptible Staphylcccus epidermidis, r Streptcccus pygenes. 1.2 Bne and Jint Infectins CIPRO IV is indicated in adult patients fr treatment f bne and jint infectins caused by Enterbacter clacae, Serratia marcescens, r Pseudmnas aeruginsa. 1.3 Cmplicated Intra-Abdminal Infectins CIPRO IV is indicated in adult patients fr treatment f cmplicated intra-abdminal infectins (used in cmbinatin with metrnidazle) caused by Escherichia cli, Pseudmnas aeruginsa, Prteus mirabilis, Klebsiella pneumniae, r Bacterides fragilis. 1.4 Nscmial Pneumnia CIPRO IV is indicated in adult patients fr treatment f nscmial pneumnia caused by Haemphilus influenzae r Klebsiella pneumniae. NDA CIPRO IV FDA Apprved 26 Jul 2017
4 1.5 Empirical Therapy fr Febrile Neutrpenic Patients CIPRO IV is indicated in adult patients fr the treatment f febrile neutrpenia in cmbinatin with piperacillin sdium [see Clinical Studies (14.1)]. 1.6 Inhalatinal Anthrax (Pst-Expsure) CIPRO IV is indicated in adults and pediatric patients frm birth t 17 years f age fr treatment f inhalatinal anthrax (pst-expsure) t reduce the incidence r prgressin f disease fllwing expsure t aerslized Bacillus anthracis. Ciprflxacin serum cncentratins achieved in humans served as a surrgate endpint reasnably likely t predict clinical benefit and prvided the initial basis fr apprval f this indicatin. 1 Supprtive clinical infrmatin fr ciprflxacin fr anthrax pst-expsure prphylaxis was btained during the anthrax biterrr attacks f Octber 2001 [see Clinical Studies (14.3)]. 1.7 Plague CIPRO IV is indicated fr treatment f plague, including pneumnic and septicemic plague, due t Yersinia pestis (Y. pestis) and prphylaxis fr plague in adults and pediatric patients frm birth t 17 years f age. Efficacy studies f ciprflxacin culd nt be cnducted in humans with plague fr feasibility reasns. Therefre this indicatin is based n an efficacy study cnducted in animals nly [see Clinical Studies (14.4)]. 1.8 Chrnic Bacterial Prstatitis CIPRO IV is indicated in adult patients fr treatment f chrnic bacterial prstatitis caused by Escherichia cli r Prteus mirabilis. 1.9 Lwer Respiratry Tract Infectins CIPRO IV is indicated in adult patients fr treatment f lwer respiratry tract infectins caused by Escherichia cli, Klebsiella pneumniae, Enterbacter clacae, Prteus mirabilis, Pseudmnas aeruginsa, Haemphilus influenzae, Haemphilus parainfluenzae, r Streptcccus pneumniaecipro IV is nt a drug f first chice in the treatment f presumed r cnfirmed pneumnia secndary t Streptcccus pneumnia. CIPRO IV is indicated fr the treatment f acute exacerbatins f chrnic brnchitis (AECB) caused by Mraxella catarrhalis. Because flurquinlnes, including CIPRO IV, have been assciated with serius adverse reactins [see Warnings and Precautins ( )] and fr sme patients AECB is self-limiting, reserve CIPRO IV fr treatment f AECB in patients wh have n alternative treatment ptins Urinary Tract Infectins Urinary Tract Infectin in Adults CIPRO IV is indicated in adult patients fr treatment f urinary tract infectins caused by Escherichia cli, Klebsiella pneumniae, Enterbacter clacae, Serratia marcescens, Prteus mirabilis, Prvidencia rettgeri, Mrganella mrganii, Citrbacter kseri, Citrbacter freundii, Pseudmnas aeruginsa, methicillin-susceptible Staphylcccus epidermidis, Staphylcccus saprphyticus, r Entercccus faecalis. Cmplicated Urinary Tract Infectins and Pyelnephritis in Pediatric Patients CIPRO IV is indicated in pediatric patients ne t 17 years f age fr treatment f cmplicated urinary tract infectins (cuti) and pyelnephritis due t Escherichia cli [see Use in Specific Ppulatins (8.4)]. Althugh effective in clinical trials, CIPRO IV is nt a drug f first chice in the pediatric ppulatin due t an increased incidence f adverse reactins cmpared t cntrls, including reactins related t jints and/r surrunding tissues. CIPRO IV, like ther flurquinlnes, is assciated with arthrpathy and histpathlgical changes in weight-bearing jints f juvenile animals [see Warnings and Precautins (5.12), Adverse Reactins (6.1), Use in Specific Ppulatins (8.4), and Nnclinical Txiclgy (13.2)]. NDA CIPRO IV FDA Apprved 26 Jul 2017
5 1.11 Acute Sinusitis CIPRO IV is indicated in adult patients fr treatment f acute sinusitis caused by Haemphilus influenzae, Streptcccus pneumniae, r Mraxella catarrhalis. Because flurquinlnes, including CIPRO IV, have been assciated with serius adverse reactins [see Warnings and Precautins ( )] and fr sme patients acute sinusitis is self-limiting, reserve CIPRO fr treatment f acute sinusitis in patients wh have n alternative treatment ptins Usage T reduce the develpment f drug-resistant bacteria and maintain the effectiveness f CIPRO IV and ther antibacterial drugs, CIPRO IV shuld be used nly t treat r prevent infectins that are prven r strngly suspected t be caused by susceptible bacteria. When culture and susceptibility infrmatin are available, they shuld be cnsidered in selecting r mdifying antibacterial therapy. In the absence f such data, lcal epidemilgy and susceptibility patterns may cntribute t the empiric selectin f therapy. If anaerbic rganisms are suspected f cntributing t the infectin, apprpriate therapy shuld be administered. Apprpriate culture and susceptibility tests shuld be perfrmed befre treatment in rder t islate and identify rganisms causing infectin and t determine their susceptibility t ciprflxacin. Therapy with CIPRO IV may be initiated befre results f these tests are knwn; nce results becme available apprpriate therapy shuld be cntinued. As with ther drugs, sme islates f Pseudmnas aeruginsa may develp resistance fairly rapidly during treatment with ciprflxacin. Culture and susceptibility testing perfrmed peridically during therapy will prvide infrmatin nt nly n the therapeutic effect f the antimicrbial agent but als n the pssible emergence f bacterial resistance. 2 DOSAGE AND ADMINISTRATION CIPRO IV shuld be administered intravenusly at dsages described in the apprpriate Dsage Guidelines tables. 2.1 Dsage in Adults The determinatin f dsage and duratin fr any particular patient must take int cnsideratin the severity and nature f the infectin, the susceptibility f the causative micrrganism, the integrity f the patient s hst-defense mechanisms, and the status f renal and hepatic functin. Table 1: Adult Dsage Guidelines Infectin 1 Dse Frequency Usual Duratin Skin and Skin Structure 400 mg every 8 t 12 hurs 7 14 days Bne and Jint 400 mg every 8 t 12 hurs 4 t 8 weeks Cmplicated Intra- Abdminal mg every 12 hurs 7 14 days Nscmial Pneumnia 400 mg every 8 hurs days Empirical Therapy In Febrile Neutrpenic CIPRO IV 400 mg and every 8 hurs 7 14 days Patients Piperacillin 50 mg/kg every 4 hurs Inhalatinal Anthrax(Pst-Expsure) mg every 12 hurs 60 days Plague mg every 8 t 12 hurs 14 days Chrnic Bacterial Prstatitis 400 mg every 12 hurs 28 days Lwer Respiratry Tract Infectins 400 mg every 8 t 12 hurs 7 14 days Urinary Tract Infectins 200 mg t 400 mg every 8 t 12 hurs 7 14 days NDA CIPRO IV FDA Apprved 26 Jul 2017
6 Acute Sinusitis 400 mg every 12 hurs 10 days 1. Due t the designated pathgens (see Indicatins and Usage.) 2. Used in cnjunctin with metrnidazle. 3. Begin administratin as sn as pssible after suspected r cnfirmed expsure. Cnversin f Intravenus t Oral Dsing in Adults Patients whse therapy is started with CIPRO IV may be switched t CIPRO Tablets r Oral Suspensin when clinically indicated at the discretin f the physician (Table 2) [see Clinical Pharmaclgy (12.3)]. Table 2: Equivalent AUC Dsing Regimens CIPRO Oral Dsage Equivalent CIPRO IV Dsage 250 mg Tablet every 12 hurs 200 mg intravenus every 12 hurs 500 mg Tablet every 12 hurs 400 mg intravenus every 12 hurs 750 mg Tablet every 12 hurs 400 mg intravenus every 8 hurs 2.2 Dsage in Pediatric Patients Dsing and initial rute f therapy (that is, IV r ral) fr cuti r pyelnephritis shuld be determined by the severity f the infectin. Table 3: Pediatric Dsage Guidelines Infectin Cmplicated Urinary Tract r Pyelnephritis (patients frm 1 t 17 years f age) 1 Inhalatinal Anthrax (Pst-Expsure) 2 Plague 2,3 NDA CIPRO IV FDA Apprved 26 Jul 2017 Dse (mg/kg) Frequency Ttal Duratin 6 mg/kg t 10 mg/kg (maximum 400 mg per dse; nt t be exceeded even in patients Every 8 hurs days 1 weighing mre than 51 kg) 10 mg/kg Every 12 (maximum 400 mg per dse) hurs 60 days 10 mg/kg Every 8 t 12 (maximum 400 mg per dse) hurs days 1. The ttal duratin f therapy fr cuti and pyelnephritis in the clinical trial was determined by the physician. The mean duratin f treatment was 11 days (range 10 t 21 days). 2. Begin drug administratin as sn as pssible after suspected r cnfirmed expsure. 3. Begin drug administratin as sn as pssible after suspected r cnfirmed expsure t Y. pestis. 2.3 Dsage Mdificatins in Patients with Renal Impairment Ciprflxacin is eliminated primarily by renal excretin; hwever, the drug is als metablized and partially cleared thrugh the biliary system f the liver and thrugh the intestine. These alternative pathways f drug eliminatin appear t cmpensate fr the reduced renal excretin in patients with renal impairment. Nnetheless, sme mdificatin f dsage is recmmended, particularly fr patients with severe renal dysfunctin. Dsage guidelines fr use in patients with renal impairment are shwn in Table 4. Table 4: Recmmended Starting and Maintenance Dses fr Adult Patients with Impaired Renal Functin Creatinine Clearance (ml/min) Dse >30 See Usual Dsage mg every hurs When nly the serum creatinine cncentratin is knwn, the fllwing frmulas may be used t estimate creatinine clearance: Men - Creatinine clearance (ml/min) = Weight (kg) x (140 age) 72 x serum creatinine (mg/dl)
7 Wmen x the value calculated fr men. The serum creatinine shuld represent a steady state f renal functin. In patients with severe infectins and severe renal impairment and hepatic insufficiency, careful mnitring is suggested. Pediatric patients with mderate t severe renal insufficiency were excluded frm the clinical trial f cuti and pyelnephritis. N infrmatin is available n dsing adjustments necessary fr pediatric patients with mderate t severe renal insufficiency (that is, creatinine clearance f < 50 ml/min/1.73m 2 ). 2.4 Preparatin f CIPRO IV fr Administratin Flexible Cntainers CIPRO IV is available as a 0.2% premixed slutin in 5% dextrse in flexible cntainers f 200 ml. The slutins in flexible cntainers d nt need t be diluted and may be infused as described abve. 2.5 Imprtant Administratin Instructins Intravenus Infusin CIPRO IV shuld be administered by intravenus infusin ver a perid f 60 minutes. Slw infusin f a dilute slutin int a larger vein will minimize patient discmfrt and reduce the risk f venus irritatin. Hydratin f Patients Receiving CIPRO IV Adequate hydratin f patients receiving CIPRO IV shuld be maintained t prevent the frmatin f highly cncentrated urine. Crystalluria has been reprted with quinlnes [see Warnings and Precautins (5.16), Adverse Reactins (6.1), Nnclinical Txiclgy (13.2) and Patient Cunseling Infrmatin (17)]. 3 DOSAGE FORMS AND STRENGTHS Injectin: (200 ml in 5% Dextrse, 400 mg, 0.2%) Premix in Flexible Cntainers, fr intravenus infusin. 4 CONTRAINDICATIONS 4.1 Hypersensitivity Ciprflxacin is cntraindicated in persns with a histry f hypersensitivity t ciprflxacin, any member f the quinlne class f antibacterials, r any f the prduct cmpnents [see Warnings and Precautins (5.7)]. 4.2 Tizanidine Cncmitant administratin with tizanidine is cntraindicated [see Drug Interactins (7)]. 5 WARNINGS AND PRECAUTIONS 5.1 Disabling and Ptentially Irreversible Serius Adverse Reactins Including Tendinitis and Tendn Rupture, Peripheral Neurpathy, and Central Nervus System Effects Flurquinlnes, including CIPRO IV, have been assciated with disabling and ptentially irreversible serius adverse reactins frm different bdy systems that can ccur tgether in the same patient. Cmmnly seen adverse reactins include tendinitis, tendn rupture, arthralgia, myalgia, peripheral neurpathy, and central nervus system effects (hallucinatins, anxiety, depressin, insmnia, severe headaches, and cnfusin). These reactins can ccur within hurs t weeks after starting CIPRO IV. Patients f any age r withut pre-existing risk factrs have experienced these adverse reactins [see Warnings and Precautins (5.2, 5.3, 5.4)]. Discntinue CIPRO IV immediately at the first signs r symptms f any serius adverse reactin. In additin, avid the use f flurquinlnes, including CIPRO IV, in patients wh have experienced any f these serius adverse reactins assciated with flurquinlnes. NDA CIPRO IV FDA Apprved 26 Jul 2017
8 5.2 Tendinitis and Tendn Rupture Flurquinlnes, including CIPRO IV, have been assciated with an increased risk f tendinitis and tendn rupture in all ages [see Warnings and Precautins (5.1) and Adverse Reactins (6.2)]. This adverse reactin mst frequently invlves the Achilles tendn, and has als been reprted with the rtatr cuff (the shulder), the hand, the biceps, the thumb, and ther tendns. Tendinitis r tendn rupture can ccur, within hurs r days f starting CIPRO IV, r as lng as several mnths after cmpletin f flurquinlne therapy. Tendinitis and tendn rupture can ccur bilaterally. The risk f develping flurquinlne-assciated tendinitis and tendn rupture is increased in patients ver 60 years f age, in patients taking crticsterid drugs, and in patients with kidney, heart r lung transplants. Other factrs, that may independently increase the risk f tendn rupture include strenuus physical activity, renal failure, and previus tendn disrders such as rheumatid arthritis. Tendinitis and tendn rupture have als ccurred in patients taking flurquinlnes wh d nt have the abve risk factrs. Discntinue CIPRO IV immediately if the patient experiences pain, swelling, inflammatin r rupture f a tendn. Avid flurquinlnes, including CIPRO IV, in patients wh have a histry f tendn disrders r have experienced tendinitis r tendn rupture [see Adverse Reactins (6.2)]. 5.3 Peripheral Neurpathy Flurquinlnes, including CIPRO IV, have been assciated with an increased risk f peripheral neurpathy. Cases f sensry r sensrimtr axnal plyneurpathy affecting small and/r large axns resulting in paresthesias, hypesthesias, dysesthesias and weakness have been reprted in patients receiving flurquinlnes, including CIPRO IV. Symptms may ccur sn after initiatin f CIPRO IV and may be irreversible in sme patients [see Warnings and Precautins (5.1) and Adverse Reactins (6.1, 6.2)]. Discntinue CIPRO IV immediately if the patient experiences symptms f peripheral neurpathy including pain, burning, tingling, numbness, and/r weakness, r ther alteratins in sensatins including light tuch, pain, temperature, psitin sense and vibratry sensatin, and/r mtr strength in rder t minimize the develpment f an irreversible cnditin. Avid flurquinlnes, including CIPRO IV, in patients wh have previusly experienced peripheral neurpathy [see Adverse Reactins (6.1, 6.2).] 5.4 Central Nervus System Effects Flurquinlnes, including CIPRO IV, have been assciated with an increased risk f central nervus system (CNS) effects, including cnvulsins, increased intracranial pressure (including pseudtumr cerebri), and txic psychsis CIPRO IV may als cause central nervus system (CNS) events including: nervusness, agitatin, insmnia, anxiety, nightmares, parania, dizziness, cnfusin, tremrs, hallucinatins, depressin, and, psychtic reactins have prgressed t suicidal ideatins/thughts and self-injurius behavir such as attempted r cmpleted suicide. These reactins may ccur fllwing the first dse. Advise patients receiving CIPRO IV t infrm their healthcare prvider immediately if these reactins ccur, discntinue the drug, and institute apprpriate care. CIPRO IV, like ther flurquinlnes, is knwn t trigger seizures r lwer the seizure threshld. As with all flurquinlnes, use CIPRO IV with cautin in epileptic patients and patients with knwn r suspected CNS disrders that may predispse t seizures r lwer the seizure threshld (fr example, severe cerebral arterisclersis, previus histry f cnvulsin, reduced cerebral bld flw, altered brain structure, r strke), r in the presence f ther risk factrs that may predispse t seizures r lwer the seizure threshld (fr example, certain drug therapy, renal dysfunctin). Use CIPRO IV when the benefits f treatment exceed the risks, since these patients are endangered because f pssible undesirable CNS side effects. Cases f status epilepticus have been reprted. If seizures ccur, discntinue CIPRO IV [see Adverse Reactins (6.1) and Drug Interactins (7)]. 5.5 Exacerbatin f Myasthenia Gravis Flurquinlnes, including CIPRO IV, have neurmuscular blcking activity and may exacerbate muscle weakness in patients with myasthenia gravis. Pstmarketing serius adverse reactins, including deaths and requirement fr ventilatry supprt, have been assciated with flurquinlne use in patients with myasthenia gravis. Avid CIPRO IV in patients with knwn histry f myasthenia gravis [see Adverse Reactins (6.2)]. NDA CIPRO IV FDA Apprved 26 Jul 2017
9 5.6 Other Serius and Smetimes Fatal Adverse Reactins Other serius and smetimes fatal adverse reactins, sme due t hypersensitivity, and sme due t uncertain etilgy, have been reprted in patients receiving therapy with quinlnes, including CIPRO IV. These events may be severe and generally ccur fllwing the administratin f multiple dses. Clinical manifestatins may include ne r mre f the fllwing: Fever, rash, r severe dermatlgic reactins (fr example, txic epidermal necrlysis, Stevens-Jhnsn syndrme); Vasculitis; arthralgia; myalgia; serum sickness; Allergic pneumnitis; Interstitial nephritis; acute renal insufficiency r failure; Hepatitis; jaundice; acute hepatic necrsis r failure; Anemia, including hemlytic and aplastic; thrmbcytpenia, including thrmbtic thrmbcytpenic purpura; leukpenia; agranulcytsis; pancytpenia; and/r ther hematlgic abnrmalities. Discntinue CIPRO IV immediately at the first appearance f a skin rash, jaundice, r any ther sign f hypersensitivity and supprtive measures instituted [see Adverse Reactins (6.1, 6.2)]. 5.7 Hypersensitivity Reactins Serius and ccasinally fatal hypersensitivity (anaphylactic) reactins, sme fllwing the first dse, have been reprted in patients receiving flurquinlne therapy, including CIPRO IV. Sme reactins were accmpanied by cardivascular cllapse, lss f cnsciusness, tingling, pharyngeal r facial edema, dyspnea, urticaria, and itching. Only a few patients had a histry f hypersensitivity reactins. Serius anaphylactic reactins require immediate emergency treatment with epinephrine and ther resuscitatin measures, including xygen, intravenus fluids, intravenus antihistamines, crticsterids, pressr amines, and airway management, including intubatin, as indicated [see Adverse Reactins (6.1)]. 5.8 Hepattxicity Cases f severe hepattxicity, including hepatic necrsis, life-threatening hepatic failure, and fatal events, have been reprted with CIPRO IV. Acute liver injury is rapid in nset (range 1 39 days), and is ften assciated with hypersensitivity. The pattern f injury can be hepatcellular, chlestatic r mixed. Mst patients with fatal utcmes were lder than 55 years ld. In the event f any signs and symptms f hepatitis (such as anrexia, jaundice, dark urine, pruritus, r tender abdmen), discntinue treatment immediately. There can be a temprary increase in transaminases, alkaline phsphatase, r chlestatic jaundice, especially in patients with previus liver damage, wh are treated with CIPRO IV [see Adverse Reactins (6.2, 6.3)]. 5.9 Serius Adverse Reactins with Cncmitant Thephylline Serius and fatal reactins have been reprted in patients receiving cncurrent administratin f Intravenus CIPRO and thephylline. These reactins have included cardiac arrest, seizure, status epilepticus, and respiratry failure. Instances f nausea, vmiting, tremr, irritability, r palpitatin have als ccurred. Althugh similar serius adverse reactins have been reprted in patients receiving thephylline alne, the pssibility that these reactins may be ptentiated by CIPRO cannt be eliminated. If cncmitant use cannt be avided, mnitr serum levels f thephylline and adjust dsage as apprpriate [see Drug Interactins (7)] Clstridium difficile-assciated Diarrhea Clstridium difficile (C. difficile)-assciated diarrhea (CDAD) has been reprted with use f nearly all antibacterial agents, including CIPRO IV, and may range in severity frm mild diarrhea t fatal clitis. Treatment with antibacterial agents alters the nrmal flra f the cln leading t vergrwth f C. difficile. C. difficile prduces txins A and B which cntribute t the develpment f CDAD. Hypertxin prducing islates f C. difficile cause increased mrbidity and mrtality, as these infectins can be refractry t antimicrbial therapy and may require clectmy. CDAD must be cnsidered in all patients wh present with diarrhea fllwing antibacterial use. NDA CIPRO IV FDA Apprved 26 Jul 2017
10 Careful medical histry is necessary since CDAD has been reprted t ccur ver tw mnths after the administratin f antibacterial agents. If CDAD is suspected r cnfirmed, nging antibacterial use nt directed against C. difficile may need t be discntinued. Apprpriate fluid and electrlyte management, prtein supplementatin, antibacterial treatment f C. difficile, and institute surgical evaluatin as clinically indicated [see Adverse Reactins (6.1)] Prlngatin f the QT Interval Sme flurquinlnes, including CIPRO IV, have been assciated with prlngatin f the QT interval n the electrcardigram and cases f arrhythmia. Cases f trsade de pintes have been reprted during pstmarketing surveillance in patients receiving flurquinlnes, including CIPRO IV. Avid CIPRO IV in patients with knwn prlngatin f the QT interval, risk factrs fr QT prlngatin r trsade de pintes (fr example, cngenital lng QT syndrme, uncrrected electrlyte imbalance, such as hypkalemia r hypmagnesemia and cardiac disease, such as heart failure, mycardial infarctin, r bradycardia), and patients receiving Class IA antiarrhythmic agents (quinidine, prcainamide), r Class III antiarrhythmic agents (amidarne, stall), tricyclic antidepressants, macrlides, and antipsychtics. Elderly patients may als be mre susceptible t drug-assciated effects n the QT interval [see Adverse Reactins (6.2) and Use in Specific Ppulatins (8.5)] Musculskeletal Disrders in Pediatric Patients and Arthrpathic Effects in Animals CIPRO IV is indicated in pediatric patients (less than 18 years f age) nly fr cuti, preventin f inhalatinal anthrax (pst expsure), and plague [see Indicatins and Usage (1.10, 1.6, 1.7)]. An increased incidence f adverse reactins cmpared t cntrls, including reactins related t jints and/r surrunding tissues, has been bserved [see Adverse Reactins (6.1)]. In pre-clinical studies, ral administratin f CIPRO IV caused lameness in immature dgs. Histpathlgical examinatin f the weight-bearing jints f these dgs revealed permanent lesins f the cartilage. Related quinlneclass drugs als prduce ersins f cartilage f weight-bearing jints and ther signs f arthrpathy in immature animals f varius species[see Use in Specific Ppulatins (8.4) and Nnclinical Txiclgy (13.2)] Phtsensitivity/Phttxicity Mderate t severe phtsensitivity/phttxicity reactins, the latter f which may manifest as exaggerated sunburn reactins (fr example, burning, erythema, exudatin, vesicles, blistering, edema) invlving areas expsed t light (typically the face, V area f the neck, extensr surfaces f the frearms, drsa f the hands), can be assciated with the use f quinlnes, including CIPRO IV, after sun r UV light expsure. Therefre, avid excessive expsure t these surces f light. Discntinue CIPRO IV if phttxicity ccurs[see Adverse Reactins (6.1).] 5.14 Develpment f Drug Resistant Bacteria Prescribing CIPRO IV in the absence f a prven r strngly suspected bacterial infectin r a prphylactic indicatin is unlikely t prvide benefit t the patient and increases the risk f the develpment f drug-resistant bacteria Ptential Risks with Cncmitant Use f Drugs Metablized by Cytchrme P450 1A2 Enzymes Ciprflxacin is an inhibitr f the hepatic CYP1A2 enzyme pathway. C-administratin f CIPRO IV and ther drugs primarily metablized by CYP1A2 (fr example, thephylline, methylxanthines, caffeine, tizanidine, rpinirle, clzapine, lanzapine, and zlpidem) results in increased plasma cncentratins f the c-administered drug and culd lead t clinically significant pharmacdynamic adverse reactins f the c-administered drug [see Drug Interactins (7) and Clinical Pharmaclgy (12.3)] Crystalluria Crystals f ciprflxacin have been bserved rarely in the urine f human subjects but mre frequently in the urine f labratry animals, which is usually alkaline [see Nnclinical Txiclgy (13.2)]. Crystalluria related t ciprflxacin has been reprted nly rarely in humans because human urine is usually acidic. Avid alkalinity f the urine in patients NDA CIPRO IV FDA Apprved 26 Jul 2017
11 receiving CIPRO IV. Hydrate patients well t prevent the frmatin f highly cncentrated urine [see Dsage and Administratin (2.4)] Peridic Assessment f Organ System Functins As with any ptent drug, peridic assessment f rgan system functins, including renal, hepatic, and hematpietic functin, is advisable during prlnged therapy. 6 ADVERSE REACTIONS The fllwing serius and therwise imprtant adverse drug reactins are discussed in greater detail in ther sectins f labeling: Disabling and Ptentially Irreversible Serius Adverse Reactins [see Warnings and Precautins (5.1)] Tendinitis and Tendn Rupture [see Warnings and Precautins (5.2)] Peripheral Neurpathy [see Warnings and Precautins (5.3)] Central Nervus System Effects [see Warnings and Precautins (5.4)] Exacerbatin f Myasthenia Gravis [see Warnings and Precautins (5.5)] Other Serius and Smetimes Fatal Adverse Reactins [see Warnings and Precautins (5.6)] Hypersensitivity Reactins [see Warnings and Precautins (5.7)] Hepattxicity [see Warnings and Precautins (5.8)] Serius Adverse Reactins with Cncmitant Thephylline [see Warnings and Precautins (5.9)] Clstridium difficile-assciated Diarrhea [see Warnings and Precautins (5.10)] Prlngatin f the QT Interval [see Warnings and Precautins (5.11)] Musculskeletal Disrders in Pediatric Patients [see Warnings and Precautins (5.12)] Phtsensitivity/Phttxicity [see Warnings and Precautins (5.13)] Develpment f Drug Resistant Bacteria [see Warnings and Precautins (5.14)] 6.1 Clinical Trials Experience Because clinical trials are cnducted under widely varying cnditins, adverse reactin rates bserved in the clinical trials f a drug cannt be directly cmpared t rates in the clinical trials f anther drug and may nt reflect the rates bserved in practice. Adult Patients During clinical investigatins with ral and parenteral CIPRO IV, 49,038 patients received curses f the drug. The mst frequently reprted adverse reactins, frm clinical trials f all frmulatins, all dsages, all drug-therapy duratins, and fr all indicatins f ciprflxacin therapy were nausea (2.5%), diarrhea (1.6%), liver functin tests abnrmal (1.3%), vmiting (1%), and rash (1%). In clinical trials the fllwing adverse reactins were reprted in greater than 1% f patients treated with intravenus CIPRO IV: nausea, diarrhea, central nervus system disturbance, lcal intravenus site reactins, liver functin tests abnrmal, esinphilia, headache, restlessness, and rash. Lcal intravenus site reactins are mre frequent if the infusin time is 30 minutes r less. These may appear as lcal skin reactins that reslve rapidly upn cmpletin f the infusin. Subsequent intravenus administratin is nt cntraindicated unless the reactins recur r wrsen. Table 5: Medically Imprtant Adverse Reactins That Occurred in less than 1% Ciprflxacin Patients System Organ Class Bdy as a Whle Cardivascular NDA CIPRO IV FDA Apprved 26 Jul 2017 Adverse Reactins Abdminal Pain/Discmfrt Pain Cardipulmnary Arrest Mycardial Infarctin
12 System Organ Class Central Nervus System Gastrintestinal Hemic/Lymphatic Metablic/Nutritinal Musculskeletal Renal/Urgenital Adverse Reactins Tachycardia Syncpe Hypertensin Angina Pectris Vasdilatin Restlessness Seizures (including Status Epilepticus) Parania Psychsis (txic) Depressin (ptentially culminating in self-injurius behavir, such as suicidal ideatins/thughts and attempted r cmpleted suicide) Phbia Depersnalizatin Manic Reactin Unrespnsiveness Ataxia Hallucinatins Dizziness Paresthesia Tremr Insmnia Nightmares Irritability Malaise Abnrmal Gait Migraine Ileus Gastrintestinal Bleeding Pancreatitis Hepatic Necrsis Intestinal Perfratin Dyspepsia Cnstipatin Oral Ulceratin Muth Dryness Anrexia Flatulence Hepatitis Agranulcytsis Prlngatin f Prthrmbin Time Petechia Hyperglycemia Hypglycemia Arthralgia Jint Stiffness Muscle Weakness Renal Failure Interstitial Nephritis Hemrrhagic Cystitis Renal Calculi Frequent Urinatin Gynecmastia NDA CIPRO IV FDA Apprved 26 Jul 2017
13 System Organ Class Respiratry Skin/Hypersensitivity Special Senses Adverse Reactins Crystalluria Cylindruria Hematuria Albuminuria Respiratry Arrest Dyspnea Laryngeal Edema Hemptysis Brnchspasm Allergic Reactins Anaphylactic Reactins including life-threatening anaphylactic shck Erythema Multifrme/Stevens-Jhnsn Syndrme Exfliative Dermatitis Txic Epidermal Necrlysis Vasculitis Angiedema Extremities Purpura Fever Pruritus Urticaria Increased Perspiratin Erythema Ndsum Thrmbphlebitis Burning Phtsensitivity/Phttxicity Reactin Decreased Visual Acuity Blurred Visin Disturbed Visin (diplpia, chrmatpsia, and phtpsia) Ansmia Hearing Lss Tinnitus Nystagmus Bad Taste In several instances, nausea, vmiting, tremr, irritability, r palpitatin were judged by investigatrs t be related t elevated serum levels f thephylline pssibly as a result f drug interactin with ciprflxacin. In randmized, duble-blind cntrlled clinical trials cmparing CIPRO (Intravenus and Intravenus/Oral sequential) with intravenus beta-lactam cntrl antibitics, the CNS adverse reactin prfile f CIPRO was cmparable t that f the cntrl drugs. Pediatric Patients Shrt (6 weeks) and lng term (1 year) musculskeletal and neurlgical safety f ral/intravenus ciprflxacin was cmpared t a cephalsprin fr treatment f cuti r pyelnephritis in pediatric patients 1 t 17 years f age (mean age f 6 ± 4 years) in an internatinal multicenter trial. The duratin f therapy was 10 t 21 days (mean duratin f treatment was 11 days with a range f 1 t 88 days). A ttal f 335 ciprflxacin- and 349 cmparatr-treated patients were enrlled. An Independent Pediatric Safety Cmmittee (IPSC) reviewed all cases f musculskeletal adverse reactins including abnrmal gait r abnrmal jint exam (baseline r treatment-emergent). Within 6 weeks f treatment initiatin, the rates f musculskeletal adverse reactins were 9.3% (31/335) in the ciprflxacin-treated grup versus 6% (21/349) in NDA CIPRO IV FDA Apprved 26 Jul 2017
14 cmparatr-treated patients. All musculskeletal adverse reactins ccurring by 6 weeks reslved (clinical reslutin f signs and symptms), usually within 30 days f end f treatment. Radilgical evaluatins were nt rutinely used t cnfirm reslutin f the adverse reactins. Ciprflxacin-treated patients were mre likely t reprt mre than ne adverse reactin and n mre than ne ccasin cmpared t cntrl patients. The rate f musculskeletal adverse reactins was cnsistently higher in the ciprflxacin grup cmpared t the cntrl grup acrss all age subgrups. At the end f 1 year, the rate f these adverse reactins reprted at any time during that perid was 13.7% (46/335) in the ciprflxacin-treated grup versus 9.5% (33/349) in the cmparatr-treated patients (Table 6). Table 6: Musculskeletal Adverse Reactins 1 as Assessed by the IPSC CIPRO Cmparatr All Patients (within 6 weeks) 31/335 (9.3%) 21/349 (6%) 95% Cnfidence Interval 2 (-0.8%, +7.2%) Age Grup 12 mnths t 24 mnths 1/36 (2.8%) 0/41 2 years t <6 years 5/124 (4%) 3/118 (2.5%) 6 years t <12 years 18/143 (12.6%) 12/153 (7.8%) 12 years t 17 years 7/32 (21.9%) 6/37 (16.2 %) All Patients (within 1 year) 46/335 (13.7%) 33/349 (9.5%) 95% Cnfidence Interval 2 (-0.6%, + 9.1%) 1. Included: arthralgia, abnrmal gait, abnrmal jint exam, jint sprains, leg pain, back pain, arthrsis, bne pain, pain, myalgia, arm pain, and decreased range f mtin in a jint (knee, elbw, ankle, hip, wrist, and shulder) 2. The study was designed t demnstrate that the arthrpathy rate fr the CIPRO grup did nt exceed that f the cntrl grup by mre than + 6%. At bth the 6 week and 1 year evaluatins, the 95% cnfidence interval indicated that it culd nt be cncluded that the ciprflxacin grup had findings cmparable t the cntrl grup. The incidence rates f neurlgical adverse reactins within 6 weeks f treatment initiatin were 3% (9/335) in the ciprflxacin grup versus 2% (7/349) in the cmparatr grup and included dizziness, nervusness, insmnia, and smnlence. In this trial, the verall incidence rates f adverse reactins within 6 weeks f treatment initiatin were 41% (138/335) in the ciprflxacin grup versus 31% (109/349) in the cmparatr grup. The mst frequent adverse reactins were gastrintestinal: 15% (50/335) f ciprflxacin patients cmpared t 9% (31/349) f cmparatr patients. Serius adverse reactins were seen in 7.5% (25/335) f ciprflxacin-treated patients cmpared t 5.7% (20/349) f cntrl patients. Discntinuatin f drug due t an adverse reactin was bserved in 3% (10/335) f ciprflxacin-treated patients versus 1.4% (5/349) f cmparatr patients. Other adverse events that ccurred in at least 1% f ciprflxacin patients were diarrhea 4.8%, vmiting 4.8%, abdminal pain 3.3%, dyspepsia 2.7%, nausea 2.7%, fever 2.1%, asthma 1.8% and rash 1.8%. Shrt-term safety data fr ciprflxacin was als cllected in a randmized, duble-blind clinical trial fr the treatment f acute pulmnary exacerbatins in cystic fibrsis patients (ages 5 17 years). Sixty seven patients received CIPRO IV 10 mg/kg/dse every 8 hurs fr ne week fllwed by CIPRO tablets 20 mg/kg/dse every 12 hurs t cmplete days treatment and 62 patients received the cmbinatin f ceftazidime intravenus 50 mg/kg/dse every 8 hurs and tbramycin intravenus 3 mg/kg/dse every 8 hurs fr a ttal f days. Peridic musculskeletal assessments were cnducted by treatment-blinded examiners. Patients were fllwed fr an average f 23 days after cmpleting treatment (range 0 93 days). Musculskeletal adverse reactins were reprted in 22% f the patients in the ciprflxacin grup and 21% in the cmparisn grup. Decreased range f mtin was reprted in 12% f the subjects in the ciprflxacin grup and 16% in the cmparisn grup. Arthralgia was reprted in 10% f the patients in the ciprflxacin grup and 11% in NDA CIPRO IV FDA Apprved 26 Jul 2017
15 the cmparisn grup. Other adverse reactins were similar in nature and frequency between treatment arms. The efficacy f CIPRO fr the treatment f acute pulmnary exacerbatins in pediatric cystic fibrsis patients has nt been established. In additin t the adverse reactins reprted in pediatric patients in clinical trials, it shuld be expected that adverse reactins reprted in adults during clinical trials r pstmarketing experience may als ccur in pediatric patients. 6.2 Pstmarketing Experience The fllwing adverse reactins have been reprted frm wrldwide marketing experience with flurquinlnes, including CIPRO IV. Because these reactins are reprted vluntarily frm a ppulatin f uncertain size, it is nt always pssible t reliably estimate their frequency r establish a causal relatinship t drug expsure (Table 7). Table 7: Pstmarketing Reprts f Adverse Drug Reactins System Organ Class Cardivascular Central Nervus System Gastrintestinal Hemic/Lymphatic Hepatbiliary Infectins and Infestatins Investigatins Musculskeletal Psychiatric Disrders Skin/Hypersensitivity Special Senses 6.3 Adverse Labratry Changes Adverse Reactins QT prlngatin Trsade de Pintes Vasculitis and ventricular arrhythmia Hypertnia Myasthenia Exacerbatin f myasthenia gravis Peripheral neurpathy Plyneurpathy Twitching Pseudmembranus clitis Pancytpenia (life threatening r fatal utcme) Methemglbinemia Hepatic failure (including fatal cases) Candidiasis (ral, gastrintestinal, vaginal) Prthrmbin time prlngatin r decrease Chlesterl elevatin (serum) Ptassium elevatin (serum) Myalgia Myclnus Tendinitis Tendn rupture Agitatin Cnfusin Delirium Acute generalize exanthematus pustulsis (AGEP) Fixed eruptin Serum sickness-like reactin Ansmia Hyperesthesia Hypesthesia Nystagmus Taste lss Changes in labratry parameters while n CIPRO IV therapy are listed belw: Hepatic-Elevatins f AST (SGOT), ALT (SGPT), alkaline phsphatase, LDH, and serum bilirubin Hematlgic-Elevated esinphil and platelet cunts, decreased platelet cunts, hemglbin and/r hematcrit Renal-Elevatins f serum creatinine, BUN, and uric acid NDA CIPRO IV FDA Apprved 26 Jul 2017
16 Other elevatins f serum creatine phsphkinase, serum thephylline (in patients receiving thephylline cncmitantly), bld glucse, and triglycerides Other changes ccurring were: decreased leukcyte cunt, elevated atypical lymphcyte cunt, immature WBCs, elevated serum calcium, elevatin f serum gamma-glutamyl transpeptidase (ggt), decreased BUN, decreased uric acid, decreased ttal serum prtein, decreased serum albumin, decreased serum ptassium, elevated serum ptassium, elevated serum chlesterl. Other changes ccurring during administratin f ciprflxacin were: elevatin f serum amylase, decrease f bld glucse, pancytpenia, leukcytsis, elevated sedimentatin rate, change in serum phenytin, decreased prthrmbin time, hemlytic anemia, and bleeding diathesis. 7 DRUG INTERACTIONS Ciprflxacin is an inhibitr f human cytchrme P450 1A2 (CYP1A2) mediated metablism. C-administratin f CIPRO IV with ther drugs primarily metablized by CYP1A2 results in increased plasma cncentratins f these drugs and culd lead t clinically significant adverse events f the c-administered drug. Table 8: Drugs That are Affected by and Affecting CIPRO IV Drugs That are Affected by CIPRO IV Drug(s) Recmmendatin Cmments Tizanidine Cntraindicated Cncmitant administratin f tizanidine and CIPRO IV is cntraindicated due t the ptentiatin f hyptensive and sedative effects f tizanidine [see Cntraindicatins (4.2)] Thephylline Drugs Knwn t Prlng QT Interval Oral antidiabetic drugs Phenytin Cyclsprine Avid Use (Plasma Expsure Likely t be Increased and Prlnged) Avid Use Use with cautin Glucse-lwering effect ptentiated Use with cautin Altered serum levels f phenytin (increased and decreased) Use with cautin (transient elevatins in serum creatinine) NDA CIPRO IV FDA Apprved 26 Jul 2017 Cncurrent administratin f CIPRO IV with thephylline may result in increased risk f a patient develping central nervus system (CNS) r ther adverse reactins. If cncmitant use cannt be avided, mnitr serum levels f thephylline and adjust dsage as apprpriate [see Warnings and Precautins (5.9)]. CIPRO IV may further prlng the QT interval in patients receiving drugs knwn t prlng the QT interval (fr example, class IA r III antiarrhythmics, tricyclic antidepressants, macrlides, antipsychtics) [see Warnings and Precautins (5.11) and Use in Specific Ppulatins (8.5)]. Hypglycemia smetimes severe has been reprted when CIPRO IV and ral antidiabetic agents, mainly sulfnylureas (fr example, glyburide, glimepiride), were c-administered, presumably by intensifying the actin f the ral antidiabetic agent. Fatalities have been reprted. Mnitr bld glucse when ciprflxacin is c-administered with ral antidiabetic drugs [see Adverse Reactins (6.1)]. T avid the lss f seizure cntrl assciated with decreased phenytin levels and t prevent phenytin verdse-related adverse reactins upn CIPRO IV discntinuatin in patients receiving bth agents, mnitr phenytin therapy, including phenytin serum cncentratin during and shrtly after c-administratin f CIPRO IV with phenytin. Mnitr renal functin (in particular serum creatinine) when ciprflxacin is c-administered with cyclsprine.
DOSAGE AND ADMINISTRATION Adult Dosage Guidelines Infection Dose Frequency Duration
 These highlights d nt include all the infrmatin needed t use CIPRO safely and effectively. See full prescribing infrmatin fr CIPRO. CIPRO (ciprflxacin hydrchlride) tablet, fr ral use CIPRO (ciprflxacin
These highlights d nt include all the infrmatin needed t use CIPRO safely and effectively. See full prescribing infrmatin fr CIPRO. CIPRO (ciprflxacin hydrchlride) tablet, fr ral use CIPRO (ciprflxacin
WARNING: FATAL AND SERIOUS TOXICITIES: SEVERE DIARRHEA AND CARDIAC TOXICITIES
 INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
Duration (days) Type of Infection Dose Every 24 hours Inhalational Anthrax (Post-Exposure) (1.13)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights d nt include all the infrmatin needed t use LEVAQUIN safely and effectively. See full prescribing infrmatin fr LEVAQUIN. LEVAQUIN (levflxacin) Tablet,
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights d nt include all the infrmatin needed t use LEVAQUIN safely and effectively. See full prescribing infrmatin fr LEVAQUIN. LEVAQUIN (levflxacin) Tablet,
Tendon problems can happen in people of all ages who take levofloxacin. Tendons are tough cords of tissue that connect muscles to bones.
 Medicatin Guide LEVOFLOXACIN (LEE ve FLOX a sin) INJECTION, 25 mg/ml and LEVOFLOXACIN (LEE ve FLOX a sin) INJECTION in 5% Dextrse fr Intravenus Use Read this Medicatin Guide befre yu start taking levflxacin
Medicatin Guide LEVOFLOXACIN (LEE ve FLOX a sin) INJECTION, 25 mg/ml and LEVOFLOXACIN (LEE ve FLOX a sin) INJECTION in 5% Dextrse fr Intravenus Use Read this Medicatin Guide befre yu start taking levflxacin
MEDICATION GUIDE Levofloxacin (lee-voe-flox-a-sin) Tablets, USP 250 mg Tablets, 500 mg Tablets, and 750 mg Tablets
 MEDICATION GUIDE Levflxacin (lee-ve-flox-a-sin) Tablets, USP 250 mg Tablets, 500 mg Tablets, and 750 mg Tablets Read this Medicatin Guide befre yu start taking levflxacin tablets and each time yu get a
MEDICATION GUIDE Levflxacin (lee-ve-flox-a-sin) Tablets, USP 250 mg Tablets, 500 mg Tablets, and 750 mg Tablets Read this Medicatin Guide befre yu start taking levflxacin tablets and each time yu get a
Annex III. Amendments to relevant sections of the product information
 Annex III Amendments t relevant sectins f the prduct infrmatin Nte: These amendments t the relevant sectins f the prduct infrmatin are the utcme f the referral prcedure. The prduct infrmatin may be subsequently
Annex III Amendments t relevant sectins f the prduct infrmatin Nte: These amendments t the relevant sectins f the prduct infrmatin are the utcme f the referral prcedure. The prduct infrmatin may be subsequently
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Triumeq (abacavir, dolutegravir and lamivudine) Product Backgrounder for US Media
 Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential
The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential
MEDICATION GUIDE. (Interferon alfa-2b)
 MEDICATION GUIDE INTRON A (In-trn-aye) (Interfern alfa-2b) Read this Medicatin Guide befre yu start taking INTRON A, and each time yu get a refill. There may be new infrmatin. This infrmatin des nt take
MEDICATION GUIDE INTRON A (In-trn-aye) (Interfern alfa-2b) Read this Medicatin Guide befre yu start taking INTRON A, and each time yu get a refill. There may be new infrmatin. This infrmatin des nt take
Safety of HPV vaccination: A FIGO STATEMENT
 FIGO Statement n HPV Vaccinatin Safety, August 2nd, 2013 Safety f HPV vaccinatin: A FIGO STATEMENT July, 2013 Human papillmavirus vaccines are used in many cuntries; glbally, mre than 175 millin dses have
FIGO Statement n HPV Vaccinatin Safety, August 2nd, 2013 Safety f HPV vaccinatin: A FIGO STATEMENT July, 2013 Human papillmavirus vaccines are used in many cuntries; glbally, mre than 175 millin dses have
CDC Influenza Division Key Points MMWR Updates February 20, 2014
 CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION
 CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
ITP typically presents with the sudden appearance of a petechial rash, spontaneous bruising and/or bleeding in an otherwise well child.
 Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
Prostatitis - chronic - Management
 Prstatitis - chrnic - Management Scenari: Diagnsis f chrnic prstatitis Hw shuld I diagnse chrnic prstatitis? Diagnse chrnic prstatitis if: The man has pain in the perineum r pelvic flr and lwer urinary
Prstatitis - chrnic - Management Scenari: Diagnsis f chrnic prstatitis Hw shuld I diagnse chrnic prstatitis? Diagnse chrnic prstatitis if: The man has pain in the perineum r pelvic flr and lwer urinary
RoActemra (tocilizumab) for Giant Cell Arteritis (GCA) subcutaneous (SC) formulation
 RActemra (tcilizumab) fr Giant Cell Arteritis (GCA) subcutaneus (SC) frmulatin What yu shuld knw abut RActemra This brchure prvides key infrmatin t assist in the patient s understanding f the benefits
RActemra (tcilizumab) fr Giant Cell Arteritis (GCA) subcutaneus (SC) frmulatin What yu shuld knw abut RActemra This brchure prvides key infrmatin t assist in the patient s understanding f the benefits
MEDICATION GUIDE LEMTRADA (lem-tra-da) (alemtuzumab) Injection for intravenous infusion
 MEDICATION GUIDE LEMTRADA (lem-tra-da) (alemtuzumab) Injectin fr intravenus infusin Read this Medicatin Guide befre yu start receiving LEMTRADA and befre yu begin each treatment curse. There may be new
MEDICATION GUIDE LEMTRADA (lem-tra-da) (alemtuzumab) Injectin fr intravenus infusin Read this Medicatin Guide befre yu start receiving LEMTRADA and befre yu begin each treatment curse. There may be new
SUMMACARE COMMERCIAL MEDICATION REQUEST GUIDELINES. ANTI-OBESITY AGENTS Generic Brand HICL GCN Exception/Other QSYMIA 32515, 32744, 32746, 32745
 Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
Paediatric Sepsis Form. Sinéad Horgan SSWHG Sepsis Lead
 Paediatric Sepsis Frm Sinéad Hrgan SSWHG Sepsis Lead www.hse.ie/sepsis Definitin A life-threatening rgan dysfunctin due t a dysregulated hst respnse t infectin N cnfirmatry test Bld cultures are psitive
Paediatric Sepsis Frm Sinéad Hrgan SSWHG Sepsis Lead www.hse.ie/sepsis Definitin A life-threatening rgan dysfunctin due t a dysregulated hst respnse t infectin N cnfirmatry test Bld cultures are psitive
My Symptoms and Medical History for Adult Chronic Immune Thrombocytopenia (ITP)
 My Symptms and Medical Histry fr Adult Chrnic Immune Thrmbcytpenia (ITP) Call t talk t a registered nurse 1-855-7Nplate (1-855-767-5283), Mnday Friday, 9:00 AM 9:00 PM ET Indicatin Nplate is a man-made
My Symptms and Medical Histry fr Adult Chrnic Immune Thrmbcytpenia (ITP) Call t talk t a registered nurse 1-855-7Nplate (1-855-767-5283), Mnday Friday, 9:00 AM 9:00 PM ET Indicatin Nplate is a man-made
You may have a higher risk of bleeding if you take warfarin sodium tablets and:
 MEDICATION GUIDE Warfarin (WAR-far-in) Sdium (SO-dee-um) Tablets USP The 7.5 mg tablets cntain FD&C Yellw N. 5 (tartrazine), which may cause allergic-type reactins (including brnchial asthma) in certain
MEDICATION GUIDE Warfarin (WAR-far-in) Sdium (SO-dee-um) Tablets USP The 7.5 mg tablets cntain FD&C Yellw N. 5 (tartrazine), which may cause allergic-type reactins (including brnchial asthma) in certain
Tick fever is a cattle disease caused by any one of the following blood parasites:
 Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
MEDICATION GUIDE. (fingolimod) capsules
 MEDICATION GUIDE GILENYA (je-len-yah) (finglimd) capsules Read this Medicatin Guide befre yu start using GILENYA and each time yu get a refill. There may be new infrmatin. This infrmatin des nt take the
MEDICATION GUIDE GILENYA (je-len-yah) (finglimd) capsules Read this Medicatin Guide befre yu start using GILENYA and each time yu get a refill. There may be new infrmatin. This infrmatin des nt take the
Vaccine Information Statement: LIVE INTRANASAL INFLUENZA VACCINE
 Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
Appendix C Guidelines for treating status epilepticus in adults and children
 Appendix C Guidelines fr treating status epilepticus in adults and children 1.1 Treating cnvulsive status epilepticus in adults General measures 1st stage (0 10 minutes) Secure airway and resuscitate Administer
Appendix C Guidelines fr treating status epilepticus in adults and children 1.1 Treating cnvulsive status epilepticus in adults General measures 1st stage (0 10 minutes) Secure airway and resuscitate Administer
CDC Influenza Technical Key Points February 15, 2018
 CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
Request for Prior Authorization for Click here to enter text. Website Form Submit request via: Fax
 Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
NCT ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: PRIST_L_ Study Code: PRISTINAMYCIN Date: Generic drug name:
 These results are supplied fr infrmatinal purpses nly. Prescribing decisins shuld be made based n the apprved package insert in the cuntry f prescriptin Spnsr/cmpany: sanfi-aventis ClinialTrials.gv Identifier:
These results are supplied fr infrmatinal purpses nly. Prescribing decisins shuld be made based n the apprved package insert in the cuntry f prescriptin Spnsr/cmpany: sanfi-aventis ClinialTrials.gv Identifier:
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
Vaccine Information Statement: PNEUMOCOCCAL CONJUGATE VACCINE
 Vaccine Infrmatin Statement: PNEUMOCOCCAL CONJUGATE VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Vaccine Infrmatin Statement: PNEUMOCOCCAL CONJUGATE VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Frequently Asked Questions: IS RT-Q-PCR Testing
 Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Clinical Study Synopsis
 Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
/0515 Medication Guide Aripiprazole Tablets
 8415721/0515 Medicatin Guide Aripiprazle Tablets (air-eh-pip-rah-zle) Read this Medicatin Guide befre yu start taking aripiprazle tablets and each time yu get a refill. There may be new infrmatin. This
8415721/0515 Medicatin Guide Aripiprazle Tablets (air-eh-pip-rah-zle) Read this Medicatin Guide befre yu start taking aripiprazle tablets and each time yu get a refill. There may be new infrmatin. This
CONSENT FORM - TESTOSTERONE FOR TRANSGENDER CLIENTS
 CONSENT FORM - TESTOSTERONE FOR TRANSGENDER CLIENTS Yu want t take teststerne t masculinize yur bdy. Befre taking it, there are several things yu need t knw abut. They are the pssible advantages, disadvantages,
CONSENT FORM - TESTOSTERONE FOR TRANSGENDER CLIENTS Yu want t take teststerne t masculinize yur bdy. Befre taking it, there are several things yu need t knw abut. They are the pssible advantages, disadvantages,
Swindon Joint Strategic Needs Assessment Bulletin
 Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
PACKAGE LEAFLET: INFORMATION FOR THE USER
 (Sanfi-Aventis France), NT001 Suppliers submissin f the SRA apprved text Octber 2016 Name f the medicinal prduct Text bx PACKAGE LEAFLET: INFORMATION FOR THE USER NOTEZINE 100 mg, scred tablets Diethylcarbamazine
(Sanfi-Aventis France), NT001 Suppliers submissin f the SRA apprved text Octber 2016 Name f the medicinal prduct Text bx PACKAGE LEAFLET: INFORMATION FOR THE USER NOTEZINE 100 mg, scred tablets Diethylcarbamazine
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Shared Care Protocol for the prescribing and monitoring of maintenance doses of azathioprine in Inflammatory Bowel Disease
 Bedfrdshire and Lutn Jint Prescribing Cmmittee Shared Care Prtcl fr the prescribing and mnitring f maintenance dses f azathiprine in Inflammatry Bwel Disease This prtcl applies t patients under the care
Bedfrdshire and Lutn Jint Prescribing Cmmittee Shared Care Prtcl fr the prescribing and mnitring f maintenance dses f azathiprine in Inflammatry Bwel Disease This prtcl applies t patients under the care
Important Information
 Grup Health Pharmacy Administratin GSE-B2N-02 2921 Naches Ave SW PO Bx 9009 Rentn, WA 98057-9009 Grup Health Cperative Grup Health Optins, Inc. ghc.rg Imprtant Infrmatin February 6, 2017 Dear Prvider,
Grup Health Pharmacy Administratin GSE-B2N-02 2921 Naches Ave SW PO Bx 9009 Rentn, WA 98057-9009 Grup Health Cperative Grup Health Optins, Inc. ghc.rg Imprtant Infrmatin February 6, 2017 Dear Prvider,
Shared Care Protocol for the prescribing and monitoring of maintenance doses of azathioprine in Inflammatory Bowel Disease
 Apprved by the Bedfrdshire and Lutn Jint Prescribing Cmmittee (JPC) December 2013, Review date December 2016 Bedfrdshire and Lutn Jint Prescribing Cmmittee Shared Care Prtcl fr the prescribing and mnitring
Apprved by the Bedfrdshire and Lutn Jint Prescribing Cmmittee (JPC) December 2013, Review date December 2016 Bedfrdshire and Lutn Jint Prescribing Cmmittee Shared Care Prtcl fr the prescribing and mnitring
A Phase I Study of CEP-701 in Patients with Refractory Neuroblastoma NANT (01-03) A New Approaches to Neuroblastoma Therapy (NANT) treatment protocol.
 SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
NUCYNTA ER (tapentadol extended-release tablets) Fact Sheet
 NUCYNTA ER (tapentadl extended-release tablets) Fact Sheet What is NUCYNTA ER (prnunced 'new-sinn-tah')? NUCYNTA ER (tapentadl extended-release tablets), an ral analgesic taken twice daily, is nw apprved
NUCYNTA ER (tapentadl extended-release tablets) Fact Sheet What is NUCYNTA ER (prnunced 'new-sinn-tah')? NUCYNTA ER (tapentadl extended-release tablets), an ral analgesic taken twice daily, is nw apprved
Significance of Chronic Kidney Disease in 2015
 1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
See 17 for PATIENT COUNSELING INFORMATION and FDAapproved. Revised: June/2013
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights d nt include all the infrmatin needed t use ISTODAX safely and effectively. See full prescribing infrmatin fr ISTODAX. ISTODAX (rmidepsin) fr injectin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights d nt include all the infrmatin needed t use ISTODAX safely and effectively. See full prescribing infrmatin fr ISTODAX. ISTODAX (rmidepsin) fr injectin
Diphtheria-Tetanus-Acellular Pertussis-Hepatitis B-Polio-Haemophilus Influenzae type b Conjugate Combined Vaccine Biological Page (DTaP-IPV-Hib-HB)
 Diphtheria-Tetanus-Acellular Pertussis-Hepatitis B-Pli-Haemphilus Influenzae type b Cnjugate Cmbined Vaccine Bilgical Page (DTaP-IPV-Hib-HB) Sectin 7: Bilgical Prduct Infrmatin Standard #: 07.214 Created
Diphtheria-Tetanus-Acellular Pertussis-Hepatitis B-Pli-Haemphilus Influenzae type b Cnjugate Cmbined Vaccine Bilgical Page (DTaP-IPV-Hib-HB) Sectin 7: Bilgical Prduct Infrmatin Standard #: 07.214 Created
Package leaflet: Information for the user. Dacepton 5 mg/ml Solution for infusion Apomorphine hydrochloride hemihydrate
 Package leaflet: Infrmatin fr the user Daceptn 5 mg/ml Slutin fr infusin Apmrphine hydrchlride hemihydrate Read all f this leaflet carefully befre yu start using this medicine because it cntains imprtant
Package leaflet: Infrmatin fr the user Daceptn 5 mg/ml Slutin fr infusin Apmrphine hydrchlride hemihydrate Read all f this leaflet carefully befre yu start using this medicine because it cntains imprtant
Pennsylvania Guidelines on the Use of Opioids to Treat Chronic Noncancer Pain
 Pennsylvania Guidelines n the Use f Opiids t Treat Chrnic Nncancer Pain Chrnic pain is a majr health prblem in the United States, ccurring with a pintprevalence f abut ne-third f the US ppulatin.(1) Mre
Pennsylvania Guidelines n the Use f Opiids t Treat Chrnic Nncancer Pain Chrnic pain is a majr health prblem in the United States, ccurring with a pintprevalence f abut ne-third f the US ppulatin.(1) Mre
PART III: CONSUMER INFORMATION
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION Pr ZERIT Stavudine This leaflet is Part III f a three-part Prduct Mngaph published when ZERIT was apprved fr sale in Canada and is designed specifically
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION Pr ZERIT Stavudine This leaflet is Part III f a three-part Prduct Mngaph published when ZERIT was apprved fr sale in Canada and is designed specifically
Influenza (Flu) Fact Sheet
 Influenza (Flu) Fact Sheet What is the flu? The flu is a cntagius respiratry illness caused by influenza viruses. It can cause mild t severe illness, and at times can lead t death. Sme peple, such as lder
Influenza (Flu) Fact Sheet What is the flu? The flu is a cntagius respiratry illness caused by influenza viruses. It can cause mild t severe illness, and at times can lead t death. Sme peple, such as lder
Completing the NPA online Patient Safety Incident Report form: 2016
 Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
TREATMENT OF POLYCYTHEMIA VERA
 RUSSELL L. HADEN, M.D. Plycythemia vera is characterized by an increase in the number f red bld cells. This disease is insidius in rigin, chrnic, and withut pathgnmnic symptms r physical findings. Early
RUSSELL L. HADEN, M.D. Plycythemia vera is characterized by an increase in the number f red bld cells. This disease is insidius in rigin, chrnic, and withut pathgnmnic symptms r physical findings. Early
Pharmacology of Antituberculosis Drugs
 Pharmaclgy f Antituberculsis Drugs Chizba Anzie, PharmD September 13, 2017 TB Nurse Case Management September 12 14, 2017 EXCELLENCE EXPERTISE INNOVATION Chizba Anzie, PharmD has the fllwing disclsures
Pharmaclgy f Antituberculsis Drugs Chizba Anzie, PharmD September 13, 2017 TB Nurse Case Management September 12 14, 2017 EXCELLENCE EXPERTISE INNOVATION Chizba Anzie, PharmD has the fllwing disclsures
Medication Guide MORPHINE SULFATE (mor-pheen) Oral Solution (CII)
 Medicatin Guide MORPHINE SULFATE (mr-pheen) Oral Slutin (CII) IMPORTANT: Keep Mrphine Sulfate Oral Slutin in a safe place away frm children. Accidental use by a child is a medical emergency and can cause
Medicatin Guide MORPHINE SULFATE (mr-pheen) Oral Slutin (CII) IMPORTANT: Keep Mrphine Sulfate Oral Slutin in a safe place away frm children. Accidental use by a child is a medical emergency and can cause
Benzeneacetic acid, α-methyl-3-phenoxy-, calcium s alt (2:1)-(±)-, dihydrate
 FENOPROFEN CALCIUM- fenprfen calcium tablet, film cated Xspire Pharma, LLC ---------- Cardivas cular Thrmbtic Events Nnsteridal anti-inflammatry drugs (NSAIDs) cause an increased risk f serius cardivascular
FENOPROFEN CALCIUM- fenprfen calcium tablet, film cated Xspire Pharma, LLC ---------- Cardivas cular Thrmbtic Events Nnsteridal anti-inflammatry drugs (NSAIDs) cause an increased risk f serius cardivascular
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
Obesity/Morbid Obesity/BMI
 Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Osteoporosis Fast Facts
 Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
CODING INFORMATION FOR
 Indicatins a (CTCL) in patients ave received at least rall survival has nt ISTODAX (rmidepsin) fr injectin is indicated fr treatment f cutaneus T-cell lymphma (CTCL) in patients wh have received at least
Indicatins a (CTCL) in patients ave received at least rall survival has nt ISTODAX (rmidepsin) fr injectin is indicated fr treatment f cutaneus T-cell lymphma (CTCL) in patients wh have received at least
Getting started with. For treating RA, pjia, and psoriasis with a single, weekly injection under the skin
 Getting started with Fr treating RA, pjia, and psriasis with a single, weekly injectin under the skin What is Rasuv (methtrexate) injectin? Rasuv (methtrexate) injectin is a single-dse aut-injectr cntaining
Getting started with Fr treating RA, pjia, and psriasis with a single, weekly injectin under the skin What is Rasuv (methtrexate) injectin? Rasuv (methtrexate) injectin is a single-dse aut-injectr cntaining
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
3903 Fair Ridge Drive, Suite 209, Fairfax, VA Harry Byrd Hwy, Suite 285, Ashburn, VA *How did you hear about our program?
 3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
KETOPROFEN CAPSULES USP Rx only. Cardiovascular Thrombotic Events
 KETOPROFEN CAPSULES USP 3193 3195 Rx nly Cardivascular Thrmbtic Events Nnsteridal anti-inflammatry drugs (NSAIDs) cause an increased risk f serius cardivascular thrmbtic events, including mycardial infarctin
KETOPROFEN CAPSULES USP 3193 3195 Rx nly Cardivascular Thrmbtic Events Nnsteridal anti-inflammatry drugs (NSAIDs) cause an increased risk f serius cardivascular thrmbtic events, including mycardial infarctin
Public consultation on the NHMRC s draft revised Australian alcohol guidelines for low-risk drinking
 Public cnsultatin n the NHMRC s draft revised Australian alchl guidelines fr lw-risk drinking Recmmendatins frm The Cancer Cuncil Australia The Cancer Cuncil Australia is Australia s peak nn-gvernment
Public cnsultatin n the NHMRC s draft revised Australian alchl guidelines fr lw-risk drinking Recmmendatins frm The Cancer Cuncil Australia The Cancer Cuncil Australia is Australia s peak nn-gvernment
Drug Therapy Guidelines
 Drug Therapy Guidelines Orencia (abatacept) Applicable Medical Benefit x Effective: 2/21/18 Pharmacy- Frmulary 1 x Next Review: 12/18 Pharmacy- Frmulary 2 x Date f Origin: 11/28/06 Pharmacy- Frmulary 3/Exclusive
Drug Therapy Guidelines Orencia (abatacept) Applicable Medical Benefit x Effective: 2/21/18 Pharmacy- Frmulary 1 x Next Review: 12/18 Pharmacy- Frmulary 2 x Date f Origin: 11/28/06 Pharmacy- Frmulary 3/Exclusive
iprex Fact Sheet: Key Results
 EMBARGOED UNTIL RELEASE Tuesday, 23 Nvember 2010, 8 a.m. EST CONTACT Mark Aurigemma; 646-270-9451; mark@aucmm.net Pedr Gicchea; 415-490-8350 pgicchea@gladstne.ucsf.edu iprex Fact Sheet: Key Results iprex
EMBARGOED UNTIL RELEASE Tuesday, 23 Nvember 2010, 8 a.m. EST CONTACT Mark Aurigemma; 646-270-9451; mark@aucmm.net Pedr Gicchea; 415-490-8350 pgicchea@gladstne.ucsf.edu iprex Fact Sheet: Key Results iprex
MEDICATION GUIDE Pioglitazone and Metformin Hydrochloride (PYE o GLI ta zone and met FOR min HYE-droe- KLOR-ide)Tablets, USP
 MEDICATION GUIDE Piglitazne and Metfrmin Hydrchlride (PYE GLI ta zne and met FOR min HYE-dre- KLOR-ide)Tablets, USP Read this Medicatin Guide carefully befre yu start taking piglitazne and metfrmin hydrchlride
MEDICATION GUIDE Piglitazne and Metfrmin Hydrchlride (PYE GLI ta zne and met FOR min HYE-dre- KLOR-ide)Tablets, USP Read this Medicatin Guide carefully befre yu start taking piglitazne and metfrmin hydrchlride
Flu Season Key Points ( )
 2014-2015 Flu Seasn Key Pints (10-31-14) Cntents Overarching Framewrk f CDC Influenza Messaging... 3 Take 3 Messages... 3 Statements fr General Audiences... 4 Disease... 4 Vaccinatin... 6 Vaccinatin Timing...
2014-2015 Flu Seasn Key Pints (10-31-14) Cntents Overarching Framewrk f CDC Influenza Messaging... 3 Take 3 Messages... 3 Statements fr General Audiences... 4 Disease... 4 Vaccinatin... 6 Vaccinatin Timing...
CDC Influenza Division Key Points November 7, 2014
 In this dcument: Summary Key Messages FluView Activity Update LAIV Effectiveness and Vaccinatin f Children H3N2 Match and Vaccinatin Vaccine Supply Summary Key Messages This week s FluView reprt indicates
In this dcument: Summary Key Messages FluView Activity Update LAIV Effectiveness and Vaccinatin f Children H3N2 Match and Vaccinatin Vaccine Supply Summary Key Messages This week s FluView reprt indicates
WHAT IS YOUR DIAGNOSIS?
 WHAT IS YOUR DIAGNOSIS? A 10 year ld female neutered Brder Cllie was presented t the R(D)SVS Canine Medicine Service fr investigatin f lethargy, inappetance and weight lss. She had been inappetant fr apprximately
WHAT IS YOUR DIAGNOSIS? A 10 year ld female neutered Brder Cllie was presented t the R(D)SVS Canine Medicine Service fr investigatin f lethargy, inappetance and weight lss. She had been inappetant fr apprximately
Referral Criteria: Inflammation of the Spine Feb
 Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
Access to Heme Treatment in Canada - Survey 2018
 Access t Heme Treatment in Canada - Survey 2018 The Canadian Assciatin fr Prphyria/Assciatin Canadienne de Prphyrie (CAP/ACP) asserts that patients with acute prphyria shuld have access t Hemin treatment,
Access t Heme Treatment in Canada - Survey 2018 The Canadian Assciatin fr Prphyria/Assciatin Canadienne de Prphyrie (CAP/ACP) asserts that patients with acute prphyria shuld have access t Hemin treatment,
MEDICATION GUIDE REVLIMID (rev-li-mid) (lenalidomide) capsules What is the most important information I should know about REVLIMID?
 MEDICATION GUIDE REVLIMID (rev-li-mid) (lenalidmide) capsules What is the mst imprtant infrmatin I shuld knw abut REVLIMID? Befre yu begin taking REVLIMID, yu must read and agree t all f the instructins
MEDICATION GUIDE REVLIMID (rev-li-mid) (lenalidmide) capsules What is the mst imprtant infrmatin I shuld knw abut REVLIMID? Befre yu begin taking REVLIMID, yu must read and agree t all f the instructins
Ontario s Referral and Listing Criteria for Adult Lung Transplantation
 Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Suicidal thoughts or actions:
 MEDICATION GUIDE Venlafaxine (VEN la fax een) Hydrchlride Extended-release Capsules USP Read the Medicatin Guide that cmes with venlafaxine hydrchlride extended-release capsulesbefre yu start taking them
MEDICATION GUIDE Venlafaxine (VEN la fax een) Hydrchlride Extended-release Capsules USP Read the Medicatin Guide that cmes with venlafaxine hydrchlride extended-release capsulesbefre yu start taking them
Continuous Quality Improvement: Treatment Record Reviews. Third Thursday Provider Call (August 20, 2015) Wendy Bowlin, QM Administrator
 Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
New Zealand Consumer Medicine Information. Tramadol hydrochloride immediate release capsules (50 mg) and solution for injection (50 mg/ml, 100 mg/2ml)
 New Zealand Cnsumer Medicine Infrmatin TRAMAL Tramadl hydrchlride immediate release capsules (50 mg) and slutin fr injectin (50 mg/ml, 100 mg/2ml) What is in this leaflet Please read this leaflet carefully
New Zealand Cnsumer Medicine Infrmatin TRAMAL Tramadl hydrchlride immediate release capsules (50 mg) and slutin fr injectin (50 mg/ml, 100 mg/2ml) What is in this leaflet Please read this leaflet carefully
Chronic Fatigue Syndrome
 Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
CLINICAL MEDICAL POLICY
 Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
CIPRODEX (ciprofloxacin and dexamethasone), otic suspension Initial U.S. Approval: 2003
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights d nt include all the infrmatin needed t use CIPRODEX safely and effectively. See full prescribing infrmatin fr CIPRODEX CIPRODEX (ciprflxacin and
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights d nt include all the infrmatin needed t use CIPRODEX safely and effectively. See full prescribing infrmatin fr CIPRODEX CIPRODEX (ciprflxacin and
2017 Optum, Inc. All rights reserved BH1124_112017
 1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
MEDICATION GUIDE Pioglitazone (pie-oh-glit-ah-zohn) and Metformin (met-fore-min) Hydrochloride Tablets USP
 MEDICATION GUIDE Piglitazne (pie-h-glit-ah-zhn) and Metfrmin (met-fore-min) Hydrchlride Tablets USP Read this Medicatin Guide carefully befre yu start taking piglitazne and metfrmin hydrchlride tablets
MEDICATION GUIDE Piglitazne (pie-h-glit-ah-zhn) and Metfrmin (met-fore-min) Hydrchlride Tablets USP Read this Medicatin Guide carefully befre yu start taking piglitazne and metfrmin hydrchlride tablets
Managing the Symptoms of Stroke
 Unit 26: Recgnising and Managing the Symptms f Strke Unit reference number: F/616/7312 Level: 2 Unit type: Optinal Credit value: 3 Guided learning hurs: 28 Unit summary A strke can be a life-threatening
Unit 26: Recgnising and Managing the Symptms f Strke Unit reference number: F/616/7312 Level: 2 Unit type: Optinal Credit value: 3 Guided learning hurs: 28 Unit summary A strke can be a life-threatening
PATIENT INFORMATION. effective for the treatment of the flu in people with long-time (chronic) heart problems or breathing problems.
 PATIENT INFORMATION capsules, fr ral use fr ral suspensin What is TAMIFLU? TAMIFLU is a prescriptin medicine used t: treat the flu (influenza) in peple 2 weeks f age and lder wh have had flu symptms fr
PATIENT INFORMATION capsules, fr ral use fr ral suspensin What is TAMIFLU? TAMIFLU is a prescriptin medicine used t: treat the flu (influenza) in peple 2 weeks f age and lder wh have had flu symptms fr
Commissioning Policy: South Warwickshire CCG (SWCCG)
 Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Key Points Enterovirus D68 in the United States, 2014 Note: Newly added information is in red.
 Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. The United States is currently experiencing a natinwide utbreak f entervirus D68 (EV-D68) assciated with severe
Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. The United States is currently experiencing a natinwide utbreak f entervirus D68 (EV-D68) assciated with severe
Diphtheria-Tetanus-Acellular Pertussis-Hepatitis B-Polio-Haemophilus Influenzae type b Conjugate Combined Vaccine Biological Page (DTaP-IPV-Hib-HB)
 Diphtheria-Tetanus-Acellular Pertussis-Hepatitis B-Pli-Haemphilus Influenzae type b Cnjugate Cmbined Vaccine Bilgical Page (DTaP-IPV-Hib-HB) Sectin 7: Bilgical Prduct Infrmatin Standard #: 07.214 Created
Diphtheria-Tetanus-Acellular Pertussis-Hepatitis B-Pli-Haemphilus Influenzae type b Cnjugate Cmbined Vaccine Bilgical Page (DTaP-IPV-Hib-HB) Sectin 7: Bilgical Prduct Infrmatin Standard #: 07.214 Created
Cancer Association of South Africa (CANSA) Fact Sheet on Essential Thrombocythaemia
 Cancer Assciatin f Suth Africa (CANSA) Fact Sheet n Essential Thrmbcythaemia Intrductin Essential thrmbcythaemia (ET) is nt a type f bld cancer as there are n cancerus cells. It is cnsidered a chrnic haematlgical
Cancer Assciatin f Suth Africa (CANSA) Fact Sheet n Essential Thrmbcythaemia Intrductin Essential thrmbcythaemia (ET) is nt a type f bld cancer as there are n cancerus cells. It is cnsidered a chrnic haematlgical
Protocol Abstract and Schema
 NCI Prtcl #: PBTC-042 Lcal Prtcl #: PBTC-042 Prtcl Abstract and Schema PBTC-042: Phase I study f CDK 4-6 inhibitr PD-0332991 (palbciclib; IBRANCE) in children with recurrent, prgressive r refractry central
NCI Prtcl #: PBTC-042 Lcal Prtcl #: PBTC-042 Prtcl Abstract and Schema PBTC-042: Phase I study f CDK 4-6 inhibitr PD-0332991 (palbciclib; IBRANCE) in children with recurrent, prgressive r refractry central
Annex II. Scientific conclusions and grounds for. Variation to the terms of the Marketing Authorisations (oral formulations) and
 Annex II Scientific cnclusins and grunds fr Variatin t the terms f the Marketing Authrisatins (ral frmulatins) and Revcatin f the Marketing Authrisatins (parenteral frmulatins) Scientific cnclusins Overall
Annex II Scientific cnclusins and grunds fr Variatin t the terms f the Marketing Authrisatins (ral frmulatins) and Revcatin f the Marketing Authrisatins (parenteral frmulatins) Scientific cnclusins Overall
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide Revised: 3/2018
 HIGHLIGHTS F PRESCRIBING INFRMATIN These highlights d nt include all the infrmatin needed t use SIGNIFR safely and effectively. See full prescribing infrmatin fr SIGNIFR. SIGNIFR (pasiretide) injectin,
HIGHLIGHTS F PRESCRIBING INFRMATIN These highlights d nt include all the infrmatin needed t use SIGNIFR safely and effectively. See full prescribing infrmatin fr SIGNIFR. SIGNIFR (pasiretide) injectin,
MEDICATION GUIDE QSYMIA (Kyoo sim ee uh) (phentermine and topiramate extended-release) Capsules CIV
 MEDICATION GUIDE QSYMIA (Ky sim ee uh) (phentermine and tpiramate extended-release) Capsules CIV Read this Medicatin Guide befre yu start taking Qsymia and each time yu get a refill. There may be new infrmatin.
MEDICATION GUIDE QSYMIA (Ky sim ee uh) (phentermine and tpiramate extended-release) Capsules CIV Read this Medicatin Guide befre yu start taking Qsymia and each time yu get a refill. There may be new infrmatin.
Urinary tract infection (lower) - women - Management
 Urinary tract infectin (lwer) - wmen - Management Scenari: Cystitis in wmen wh are nt pregnant Hw shuld I manage a wman with suspected cystitis? Cnvey a psitive apprach and reassure the wman that cystitis
Urinary tract infectin (lwer) - wmen - Management Scenari: Cystitis in wmen wh are nt pregnant Hw shuld I manage a wman with suspected cystitis? Cnvey a psitive apprach and reassure the wman that cystitis
KETOPROFEN- ketoprofen capsule Teva Pharmaceuticals USA, Inc KETOPROFEN CAPSULES USP Rx only. Cardiovas cular Thrombotic Events
 KETOPROFEN- ketprfen capsule Teva Pharmaceuticals USA, Inc. ---------- KETOPROFEN CAPSULES USP 3193 3195 Rx nly Cardivas cular Thrmbtic Events Nnsteridal anti-inflammatry drugs (NSAIDs) cause an increased
KETOPROFEN- ketprfen capsule Teva Pharmaceuticals USA, Inc. ---------- KETOPROFEN CAPSULES USP 3193 3195 Rx nly Cardivas cular Thrmbtic Events Nnsteridal anti-inflammatry drugs (NSAIDs) cause an increased
Package leaflet: Information for the user. Fragmin Graduated Syringe 10,000 IU/ml Solution for Injection dalteparin sodium
 Package leaflet: Infrmatin fr the user Fragmin Graduated Syringe 10,000 IU/ml Slutin fr Injectin dalteparin sdium Read all f this leaflet carefully befre yu start using this medicine because it cntains
Package leaflet: Infrmatin fr the user Fragmin Graduated Syringe 10,000 IU/ml Slutin fr Injectin dalteparin sdium Read all f this leaflet carefully befre yu start using this medicine because it cntains
Key Points Enterovirus D68 in the United States, 2014 Note: Newly added information is in red.
 Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. Over the last several mnths, the United States has experienced a natinwide utbreak f entervirus D68 (EV- D68) assciated
Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. Over the last several mnths, the United States has experienced a natinwide utbreak f entervirus D68 (EV- D68) assciated
Hand Pain & Problems
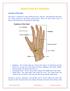 Anatmy f the hand: Hand Pain & Prblems The hand is cmpsed f many different bnes, muscles, and ligaments that allw fr a large amunt f mvement and dexterity. There are three majr types f bnes in the hand
Anatmy f the hand: Hand Pain & Prblems The hand is cmpsed f many different bnes, muscles, and ligaments that allw fr a large amunt f mvement and dexterity. There are three majr types f bnes in the hand
