Release of Adenosine by the Normal Myocardium in Dogs and Its Relationship to the Regulation of Coronary Resistance
|
|
|
- Susanna Riley
- 6 years ago
- Views:
Transcription
1 Release of Adenosine by the Normal Myocardium in Dogs and Its Relationship to the Regulation of Coronary Resistance By Rafael Rubio, Ph.D., and Robert M. Berne, M.D. ABSTRACT The evidence supporting the hypothesis that adenosine is the mediator of metabolic regulation of coronary blood flow was obtained from experiments characterized by myocardial hypoxia. If adenosine serves the role of physiological regulator of coronary blood flow, it must also be released by the normal heart. Experiments designed to study this question were performed on 15 open-chest dogs in which adenosine was sought in perfusates of the epicardial surface of the well-oxygenated heart. The pericardial space was perfused with warm (37 C) Tyrode's or Krebs-Henseleit solutions (400 to 1200 ml over 1 to 3 hours), and the perfusates were analyzed for adenosine. With a normal myocardial oxygen supply, adenosine was present in the perfusates in a concentration of 3.1 ± 0.5 X 10~ 8 M. Partial asphyxia, induced by reducing pulmonary ventilation, significantly (P < 0.02) increased the adenosine concentration of the perfusates to 5.4 ± 0.8 X 10~ S M. In four dogs the normal pericardial fluid was found to contain adenosine in a concentration of 10.9 ± 2.9 X 10~ 7 M, which probably represents the basal extracellular adenosine concentration in the myocardium. The results indicate that the normal myocardial cells release adenosine continuously into the surrounding interstitial fluid, and it is suggested that the level of the interstitial fluid concentration of adenosine probably regulates coronary blood flow to maintain the oxygen balance of the myocardium. ADDITIONAL KEY WORDS coronary blood flow myocardial metabolites myocardial nucleotides autoregulaion of coronary blood flow adenine nucleotide degradation in heart pericardial fluid vascular smooth muscle relaxation superfusion of cardiac surface control of arterioles in the heart myocardial oxygen balance 9 Coronary blood flow normally correlates well with myocardial oxygen balance (1-5), and it has been suggested that the tone of the coronary vascular bed is significantly affected by the metabolism of the myocardial cells (2, 6-8). However, the precise link between coronary vascular tone and the metabolic activity of the myocardium is unknown (9). One possible mediator of metabolic regulation of coronary blood flow is adenosine (10), a From the Department of Physiology, School of Medicine, University of Virginia, Charlottesville, Virginia This work was supported in part by U. S. Public Health Service Grant HE10384 from the National Heart Institute. Received June 2, Accepted for publication August 12, powerful coronary vasodilator. The adenosine hypothesis proposes that adenosine is continuously released by the myocardial cells into the interstitial fluid, and its release is enhanced in response to either an increase in cardiac oxygen consumption or a reduction in the arterial oxygen supply. An increase in the concentration of adenosine in the interstitial fluid would cause the coronary resistance vessels to dilate and the coronary blood flow to increase to the point at which the original myocardial oxygen balance would be restored. In this fashion, adenosine may function as a signal to indicate the amount of oxygen required by the myocardium. The adenosine hypothesis is based on data obtained under experimental conditions of myocardial hypoxia, and hence it is inconclu- Circulaiion Research, Vol. XXV, October
2 408 RUBIO, BERNE sive as to its relevance to the normal physiological regulation of coronary blood flow. Recently, it has been possible to isolate adenosine from coronary sinus blood during the periods of reactive hyperemia following brief periods of coronary occlusion (11). The concentration of adenosine that was calculated to be present in the myocardial interstitial fluid during reactive hyperemia exceeds the concentration required to elicit maximal coronary dilation (11). Whether adenosine is involved in the regulation of coronary blood flow in the absence of myocardial hypoxia has not been evaluated because of the difficulties in detecting the minute concentrations of the nucleoside required to induce small degrees of coronary dilation (11). To accept the applicability of the adenosine hypothesis to the physiological regulation of coronary blood flow, it must be demonstrated that: (1) there is a continuous release of adenosine by the normal myocardial cells into the interstitial fluid, (2) the actual product released is not an adenosine precursor but is adenosine itself, and (3) there is a precise quantitative causeand-effect relationship between the interstitial fluid concentration of adenosine and the diameter of the coronary resistance vessels. The present report represents an attempt to satisfy the first criterion. Methods Dogs weighing between 16 and 20 kg were anesthetized with pentobarbital, 30 mg/kg, and after starting artificial respiration their chests were opened by a midstemal incision. The fat and small vessels surrounding the pericardium were removed, and one or two silastic tubes (4 mm diameter) were tied into the pericardial sac for superfusion of the surface of the heart. When two tubes were used, the opening of one was placed anteriorly near the apex of the heart and the second tube was placed posteriorly at the base of the heart. The apical tube served as an inlet and the basal tube as an outlet for the perfusing fluid. When just one tube was used, its opening was placed at the base of the heart, and it served as an inlet and outlet for fluid left in contact with the epicardial surface for varying periods of time. Either Tyrode's or Krebs-Henseleit solutions (37 C, ph 7.4, and containing 5% dextran) served as the perfusion fluid. The solutions were equilibrated with mixtures of either 95% 0 2 and 5% CO 2 or 10% O 2, 85% N 2, and 5% CO 2. In some experiments, heparin was administered and an extracorporeal dialyzing system was placed in series with a femoral artery. The cellulose dialysis tubing through which the blood flowed had a mean pore radius of 24 A which permitted solutes below a molecular weight of 30,000 to move freely across the membrane. The dialysis tube was 3 cm in diameter, 24 to 25 cm in length, and had a surface area of 230 cm 2. This surface area is approximately equal to that of the epicardium, estimated by dividing the mean weight of a pericardial sac (averaged from six different animals) by the mean weight of 1 cm 2 of sac. The perfusion fluid used for dialysis of the femoral arterial blood was the same with respect to composition and volume as that used for superfusion of the epicardial surface of the heart. The pericardial perfusates and blood dialysates were collected by two different procedures. In experiments 1 through 8 the perfusion or dialyzing fluid was infused continuously at a rate between 5 and 8 ml/min, whereas in experiments 9 through 15, 75 to 85 ml of fluid was introduced into the pericardial sac or dialyzing system and left there for specified periods of time. The volume used was determined by the size of the heart. The fluid in the pericardial sac and outside the dialysis tubing was simultaneously replaced by equal volumes of fresh fluid every 10 minutes. In the case of the blood dialysates, the fluid was stirred by rapid withdrawal and reinjection with a large syringe every 2 or 3 minutes. Each aliquot of pericardial and dialysis fluid was immediately placed in boiling water for 10 minutes to destroy any enzymatic activity. They were then pooled and processed for adenosine determination as previously described (11). The total volume of fluid collected in each experiment was between 400 and 1,200 ml and was collected over a period of 1 to 3 hours. Protocol l. In the first series of experiments (1 through 7) samples of pericardial perfusates were collected under control conditions and during partial asphyxia. Asphyxia was produced by interrupting the artificial respiration every fifth minute for 1 minute. The perfusion fluid used during the periods of partial asphyxia was equilibrated with 10% O 2, 5% CO 2 and 85% N 2. Protocols 2 and 3. In the second series of experiments, two different protocols were followed. In one group, (8 through 11, protocol 2), the control period of pericardial perfusion was followed by a period of asphyxia, and that, in turn, by a recovery period. In all of these experiments, a blood dialysate sample was obtained simultaneously with each pericardial
3 ADENOSINE RELEASE BY THE NORMAL HEART 409 perfusate sample. In the second group of experiments (12 through 15, protocol 3) 75 to 85 ml of perfusion fluid was introduced into the pericardial sac and removed 2.5 minutes later and kept at 37 C in a water bath. This sample was designated "control." Immediately after collection of the control sample, artificial respiration was stopped for a period of 75 seconds, and 15 seconds after the start of asphyxia an equal volume of fresh perfusion fluid was injected into the pericardial sac. It was removed 2.5 minutes later and kept at 37 C; it was designated "asphyxia." Immediately after collection of the first asphyxia sample, the first control sample was reintroduced into the pericardial sac and the cycle repeated. Each control and asphyxia sample was exposed to the heart four times for a total contact time with the epicardial surface of 10 minutes. After the fourth exposure, each sample was immersed in boiling water for 10 minutes. The cycle was then repeated with an equal volume of fresh perfusion fluid. From 24 to 32 cycles were earned out in each experiment. All control samples were pooled, as were all asphyxia samples. The blood dialysates were cycled in a similar manner, but the control samples were pooled with the asphyxia samples. The volume (V) of the pericardial fluid normally existing in the pericardial sac was also TABLE 1 measured in four dogs and its adenosine content determined. This volume was usually very small and since it is impossible to withdraw all of it for quantitation, its volume was estimated by the dilution of an inulin solution introduced into the sac. The following procedure was used. Five milliliters of perfusion fluid was introduced into the pericardial sac (total volume of sac = 5 + V ml), and 1 minute later 0.5 ml was removed and used in the inulin blank determination. Immediately thereafter, 1 ml of inulin solution (Cj = 2 mg/ml) was introduced into the pericardial sac (total volume = V ml), and 30 seconds later 0.5 ml of pericardial fluid was removed for measurement of the inulin concentration (C 2 ). Then, 80 ml of perfusion fluid was introduced into the sac, and 1 minute later all the fluid that could be withdrawn from the sac was removed and another 80 ml of fresh perfusion fluid was introduced. The second 80 ml was removed 1 minute later, and the two pericardial washes were pooled. The combined sample was analyzed for adenosine, and the amount found was taken to represent the total present in the undiluted pericardial fluid. The inulin determination was made by the method of Roe et al. (12), and the pericardial fluid volume was determined by applying the following formula: C x Xl ml = C 2 (5.5 + V) and solving for V. Concentration of Adenosine (X 10~ S M) in Pericardial Perfusutcs and Blood Dialysates Control Asphyxia* Recovery Expt. Pericardial Blood Pericardial Blood Pericardial Blood no. perfusate dialysate perfusate dialysate perfusate dialysate G G G J.i) O.G S.O Protocol Protocol Protocol S.O f O.Gf l.of G 2.G O.G 1.9 * Respirator was interrupted for I minute every 5 minutes for protocols 1 and 2, and for 75 seconds every 5 minutes for protocol 3. f The control samples were pooled with these.
4 410 RUBIO, BERNE TABLE 2 Adenosine in Pericanlial Perfusates and Arterial Blood Dialysates for All Experiments Control Asphyxia No. expts. No. determinations Adenosine (XlCT'.u) Pericardial P erf \i sales ±0.5 (si-:)* ±0.8 (SK)* Blood Dialysalcs 13 0.G ± 0.2 (SK) * The difference between the adeno.sine concentralion in the pericardial perfusate of the control and asphyxia samples is significant (P < 0.02). Results AOENOSINE IN PERICARDIAL PERFUSATES Adenosine was found in each of 31 samples of pericardial perfusate obtained in 15 experiments. Table 1 (protocol 1) presents the results of the first seven experiments. In experiments 4 through 7, the concentration of adenosine was found to be increased in the pericardial perfusates collected during asphyxia following a control period. It seemed possible that the adenosine in the pericardial perfusates may have originated in the coronary blood and not in the myocardium, even though we had previously been unable to find adenosine in plasma samples of arterial blood. Even with arterial blood concentrations of adenosine below detectable levels, the large volume of perfusion fluid could pick up enough of the nucleoside by dialysis of the coronary blood on or near the surface of the heart to yield measurable quantities. Therefore, the arterial blood contribution of adenosine to the pericardial perfusates was determined, and the results are reported in Table 1 (protocols 2 and 3). Most of the blood dialysates showed the presence of adenosine, but the concentration was lower than that of the corresponding pericardial perfusates. Furthermore, adenosine was absent in some of the samples. To avoid possible artifacts introduced by the order of sampling, the second series of experiments was undertaken according to protocols 2 and 3. These experiments eliminate the possibility of sampling as the cause of the increased adenosine concentration in the pericardial perfusates collected during the asphyxia period. The results are presented in Table 1. Although the differences were small, the adenosine concentrations of the pericardial perfusates in the asphyxia samples were, in general, higher than the corresponding control and recovery samples. The only exceptions were experiments 10 and 13. Table 2 summarizes all the data on the adenosine concentration of pericardial perfusates for the experiments presented in Table 1, as well as all the data on the adenosine concentration of the blood dialysates. The adenosine concentration of the control pericardial perfusate was fivefold greater than that of the blood dialysates (3.1 vs. 0.6). ADENOSINE IN PERICARDIAL FLUID The pericardial fluid volume, the total amount and concentration of adenosine present in pericardial fluid, and the calculated amount of adenosine per gram of myocardium are given in Table 3. The calculated total amount of adeno.sine in normal pericardial fluid is very small, and its precise quantitation TABLE 3 Volume and Adenosine Content of Normal Pericardial Fluid 1 Experiment no Means ; t SK Volume (ml) Total amount of adenosine (m/iinolcs) Concentration of adenosine (X IO~".\i) Calculated amount of adenosine (X IO~ 10 moles) per gram of tissue 2.G l.s G l.s 3.4 1S.0 3.S 3.4 ± ± ± ± 0.G
5 ADENOSINE RELEASE BY THE NORMAL HEART 411 basal extracellular adenosine concentration in the myocardium. This, of course, only holds true for normal pericardial fluid collected under steady-state conditions. With hypoxia or during pericardial perfusion there is in all likelihood a steep gradient in the adenosine concentration from interstitial fluid to pericardial fluid (or perfusate), making calculations of myocardial adenosine concentrations and any attempts at correlation of adenosine release with coronary blood flow meaningless. For this reason coronary flow was not measured in the present study. The restriction of adenosine to the interstitial spaces is a reasonable assumption based on the cellular localization and properties of the enzymes 5'-nucleotidase, adenylic acid deaminase (AMP-deaminase), and adenosine deaminase, as revealed by histochemical and cell fractionation studies (17-19). The localization of these enzymes is illustrated in Figure 1 which represents a myocardial cell surrounded by interstitial fluid, a capillary, and an arteriole. AMP is hydrolyzed to adenosine by the 5'-nucleotidase which, according to histochemical (19) and cell fractionation studies (17, 18) is primarily bound to the cell membrane. However, some intracellular AMP is evidently deaminated to inosinic acid (20) by the AMP-deaminase (17, 18). Hence, there is probably no free adenosine within the myocardial cell because of the high protoplasmic concentration of adenosine deaminase (17,18). Some of the adenosine moving back from the interstitial fluid into the myocardial cell is rephosphorylated to AMP (21) by action of adenosine kinase (22). Phosphorylation of adenosine occurs by some unknown mechanism which circumvents the action of the adenosine deaminase, as indicated by the fact that 14 C-adenosine infused into the coronary arteries at low concentrations is readily incorporated into the adenine nucleotide pool with a negligible degree of deamination (21). It is possible that this enzyme is also membrane bound and, therefore, has first access to the adenosine that enters the myocardial cell. As illustrated in Figure 1, the intracellular inosine may arise from dephos- is beyond the limits of our methods. Nevertheless, the approximations reported here are reasonable since the enzymatic assays can detect as low as 1 m/^mole and can quantitate 3 m/iimoles with precision. For calculation of the amount of adenosine per gram of myocardium, it was assumed that the adenosine is restricted to the extracellular space (see discussion) and that the extracellular fluid volume comprises 0.2 ml/g of wet myocardium (13-15). Hence, 10.9 X Kh 10 moles/ml of interstitial fluid X 0.2 = 2.2 X 10" 10 moles/g of myocardium. Discussion The fact that adenosine is found in pericardial fluid and pericardial perfusates demonstrates that the normal myocardium releases adenosine into the surrounding fluid. This release is accelerated by asphyxia. The possibility of pericardial adenosine originating in blood can be eliminated because the concentration of adenosine in blood dialysates was consistently lower than that of the corresponding pericardial perfusates. The normal pericardial fluid is probably representative of the myocardial interstitial fluid (16). Therefore, the concentration of adenosine in normal pericardial fluid should represent the CAPILLARY ARTERIOLE "o Adenosine Deaminase Adenylic Acid Deaminase 5'Nucleotidase Adenosine Kinase FIGURE 1 Schematic diagram of myocardial tissue illustrating the formation, fate and site of action of adenosine arising from intracellular ATP (discussion in text).
6 412 RUBIO, BERNE phorylation of inosinic acid or deamination of adenosine moving back into the cell. The concentration and tissue distribution of inosine and inosinic acid in the normal heart are unknown. As illustrated in Figure 1, adenosine present in the interstitial fluid would dilate the coronary arterioles, and the degree of dilation would be proportional to the adenosine concentration. As previously discussed (11), the adenosine concentration of the interstitial fluid is determined by its rate of release, uptake by the myocardial cell, washout by the perfusing blood, and uptake and inactivation by the red cells (11). During myocardial hypoxia, there is an increased rate of adenosine release (Table 1), a rise in the tissue levels of adenosine (20, 23), a washout of a greater amount of adenosine by the perfusing blood (11) or saline perfusates (24, 25), and a decrease in coronary resistance (10, 11, 23, 25). The amount of adenosine per gram of myocardium (Table 3) was calculated making two basic assumptions. First, adenosine is found only in the extracellular spaces, and second, the normal pericardial fluid is representative of normal myocardial interstitial fluid. The calculated average value of 2.2 ±0.6 (SE)XIO" 10 moles/g is in close agreement with the value of 2.8 ± 1.3 (SD) X 10" 10 moles/g obtained by direct measurement of tissue adenosine content (23). In a previous study (11), a normal adenosine FIGURE 2 Schematic representation of the relationship between cell metabolism and adenosine formation in the regulation of coronary blood flow (discussion in text). tissue content of the order of 10~ n moles/g was predicted. The rate of adenosine release is probably ultimately controlled by the cell concentrations of adenine nucleotides and inorganic phosphate (Pi). The rate of hydrolysis of AMP compared to its rate of deamination appears to be controlled by the balance of the concentrations of these substances (17, 18, 26). In-vitro studies with 5'-nucleotidase and AMP-deaminase demonstrate that the quantities of adenosine and inosinic acid produced by these reactions is proportional to the amount of AMP available to these two enzymes which compete for the same substrate. However, ATP exerts an inhibitory effect on 5'-nucleotidase (17, 18) and a stimulatory effect on the AMP-deaminase (18), whereas Pi inhibits AMP-deaminase (26). How the adenosine-generating system may respond to myocardial hypoxia is illustrated in Figure 2 (right side). The AMP concentration increases with myocardial hypoxia, thereby providing more substrate for 5'- nucleotidase whereas the ATP level decreases (27). A decrease in ATP concentration increases 5'-nucleotidase activity and decreases the activity of AMP-deaminase, thereby favoring AMP degradation to adenosine. Pi levels are also increased in myocardial hypoxia (27, 28) providing additional inhibition of AMP-deaminase activity; this will also favor the degradation of AMP to adenosine. With an increase in adenosine formation, the concentration of adenosine in the vicinity of the arterioles should increase and induce vasodilation which in turn would enhance coronary blood flow and the myocardial oxygen supply. The restoration of oxygen balance will accelerate the regeneration of ATP, from AMP, ADP, and Pi. Thus, the process is self-regulatory, adenosine functioning as an error signal to indicate the amount of oxygen required by the myocardium. The coronary blood flow is critically adjusted to a level where the oxygen demands of the myocardium are just met by the supply (2, 3, 5-7). The mechanism that controls myocardial oxygen supply and which may be
7 ADENOSINE RELEASE BY THE NORMAL HEART 413 mediated by the precise release of adenosine has a parallelism in the mechanism that controls the supply of electrons utilized in oxidative phosphorylation by regulation of acetyl-coa metabolism (27-35) (left side of Fig. 2). Several of the enzymes of glycolysis, the tricarboxylic acid cycle, fatty acid metabolism, and respiratory electron transport (36) are either inhibited by ATP or stimulated by AMP, ADP, and Pj. Consequently, the low ATP levels associated with high AMP, ADP, and Pi levels that may prevail in myocardial hypoxia will increase the supply of acetyl-coa to the enzymes of the tricarboxylic acid cycle by augmenting glycolysis (27, 28, 30-35) and possibly by decreasing fatty acid synthesis (32). In addition, the rate of oxidation of acetyl-coa will be accelerated by activation of tricarboxylic acid cycle enzymes (30, 32) and by an increase in the mitochondrial respiratory rate (36). However, these processes can only proceed if the supply of electrons (left side of Fig. 2) to the respiratory chain is matched by an equivalent supply of oxygen (right side of Fig. 2). In this manner, the regeneration of ATP from AMP, ADP, and P, would be accelerated and would constitute a self-regulating mechanism. This scheme could explain the close parallelism between myocardial oxygen balance and coronary blood flow, as observed in reactive hyperemia, arterial hypoxemia, and autoregulation of blood flow. During reactive hyperemia, myocardial oxygen consumption (1), coronary blood flow (1, 11, 23), myocardial release of adenosine (11, 23), and the levels of pyruvate and lactate in the coronary venous blood (1) are increased. The elevated AMP, ADP, and Pi levels and the low ATP levels reached during the preceding ischemic period (27) would stimulate mitochondrial respiration (36) and the release of adenosine (17, 18). Thus, when the circulation is reestablished, the coronary blood flow is increased and oxygen is extracted from the blood at a faster rate. Similarly, during moderate arterial hypoxemia, myocardial oxygen consumption remains constant, and there is an increase in coronary blood flow roughly proportional to the degree of hypoxemia (2, 4, 5). The increased AMP, ADP, and P) levels and the low ATP, possibly associated with this condition, would increase the mitochondrial respiratory rate (36), thus maintaining a constant rate of extraction of oxygen from extracellular fluid with a lower oxygen concentration (as reflected by the reduced venous oxygen tension) (5). For the same reason, the release of adenosine would be increased, thereby providing the myocardium with a larger flow of blood containing less oxygen. That is, the reciprocal relationship between the effects of AMP, ADP, and Pj with respect to ATP provides a link between myocardial oxygen consumption and coronary blood flow and can account for the parallelism observed between these two variables. In this view, coronary blood flow and oxygen consumption are controlled by a common factor, which is different from the frequently stated view that oxygen consumption is the main determinant of coronary blood flow (2, 6, 8). This hypothesis for the regulation of coronary blood flow does not imply that adenosine is the only determinant of vascular resistance, since there are others known to be involved (9). However, it is suggested tiiat adenosine modulates the effects of all these other determinants and adjusts coronary blood flow to maintain the myocardium in oxygen balance. If, for example, coronary perfusion pressure were elevated so that the myocardium was overperfused, the increased oxygen supply would produce a decrease in the interstitial fluid adenosine concentration and thereby lower coronary blood flow (autoregulation) toward the level where myocardial oxygen balance would be maintained. Heretofore, only myocardial hypoxia was known to cause release of adenosine from the heart, and there was no evidence to support the concept that autoregulation of coronary blood flow in response to elevation of coronary perfusion pressure could be due to a reduction in myocardial adenosine concentration. However, the finding of adenosine release from the normal heart makes this aspect of regulation of coronary blood flow feasible, although it
8 414 RUBIO, BERNE remains to be demonstrated that the basal cardiac adenosine concentration is indeed reduced by excess coronary blood flow. References 1. CoFFMAisr, J. D., AND GRECG, D. E.: Oxygen metabolism and oxygen debt repayment after myocardial ischemia. Am. J. Physiol. 201: 88, ECKENHOFF, J. E., HAFKENSCHIEL, J. H., LANDMESSER, C. M., AND HABMEL, M.: Cardiac oxygen metabolism and control of the coronary circulation. Am. J. Physiol. 149: 634, GOODALE, W. T., OLSON, R. E., AND HACKEL, D. B.: Effects of fasting and diabetes mellitus on myocardial metabolism in man. Am. J. Med. 27: 212, HILTON, R., AND EICHHOLTZ, F.: Influence of chemical factors on the coronary circulation. J. Physiol. (London) 59: 413, SCOTT, J. C: Myocardial coefficient of oxygen utilization. Circulation Res. 9: 906, ALLELA, A., WILLIAMS, F. L., BOLENE- WILLIAMS, C, AND KATZ, L. N.: Interrelation between cardiac oxygen consumption and coronary blood flow. Am. J. Physiol. 183: 570, BEBNE, R. M., BLACKMON, J. R., AND GARDNER, T. H.: Hypoxemia and coronary flow. J. Clin. Invest. 36: 1101, BHAUNWALD, E., SARNOFF, S. J., CASE, R. B., STAINSBY, W. N., AND WELCH, G. H., JR.: Hemodynamic determinants of coronary flow: Effects of changes in aortic pressure and cardiac output on the relationship between myocardial oxygen consumption and coronary flow. Am. J. Physiol. 192: 157, BERNE, R. M.: Regulation of coronary blood flow. Physiol. Rev. 44: 1, BERNE, R. M.: Cardiac nucleotides in hypoxia: Possible role in regulation of coronary blood flow. Am. J. Physiol. 204: 317, RUBIO, R., BERNE, R. M., AND KATORI, M.: Release of adenosine in reactive hyperemia of the dog heart. Am. J. Physiol. 216: 56, ROE, J. H., EPSTEIN, J. H., AND GOLDSTEIN, N. P.: Photometric method for the determination of inulin in plasma and urine. J. Biol. Chem. 178: 839, ADDANKI, S., CAHILL, F. D., AND SOTOS, J. F.: Rapid determination of extracellular space with inulin-h 3. Atomlight 61: 6, PAGE, E.: Cat heart muscle in vitro: III. Extracellular space. J. Cen. Physiol. 46: 201, SPERELAKIS, N., AND HOSHIKO, T.: Electrical impedance of cardiac muscle. Circulation Res. 9: 1280, MAUKER, F. W., WARREN, M. F., AND DRINKER, C. K.: Composition of mammalian pericardia] and peritoneal fluids. Am. J. Physiol. 129: 635, BAER, H. P., DRUMMOXD, G. I., AND DUNCAN, E. L.: Formation and deamination of adenosine by cardiac muscle enzymes. Mol. Pharmacol. 2: 67, BURGER, R., AND LOWENSTEIN, J. M.: Adenylate Deaminase: III. Regulation of deamination pathways in extracts of rat heart and lung. J. Biol. Chem. 242: 5281, ROSTGAARD, J., AND BEHNKE, O.: Fine structural localization of adenine nucleoside phosphatase activity in the sarcoplasmic reticulum and the T system of rat myocardium. J. Ultrastruct. Res. 12: 576, IMAI, S., RILEY, A. L., AND BERNE, R. M.: Effect of ischemia on adenine nucleotides in cardiac and skeletal muscle. Circulation Res. 15: 443, JACOB, M. I., AND BERNE, R. M.: Metabolism of purine derivatives by the isolated cat heart. Am. J. Physio]. 198: 322, GOLDTHWAIT, D. A.: Mechanisms of synthesis of purine nucleotides in heart muscle extracts. J. Clin. Invest. 36: 1572, OLSSON, R. A.: Adenosine metabolism during myocardial reactive hyperemia. Federation Proc. 28: 779, RICHMAN, H. C, AND WYBORNY, L.: Adenine nucleotide degradation in the rabbit heart. Am. J. Physiol. 207: 1139, KATORI, M., AND BERNE, R. M.: Release of adenosine from anoxic hearts: Relationship to coronary flow. Circulation Res. 19: 420, LEE, Y. P., AND WANG, M. H.: Studies of the nature of the inhibitory action of inorganic phosphate, fluoride, and detergents on adenylic acid deaminase activity and on the activation by adenosine triphosphate. J. Biol. Chem. 243: 2260, WILLIAMSON, J. R.: Clycolytic control mechanisms: II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J. Biol. Chem. 241: 5026, WOLLENBERGER, A., AND KRAUSE, E. G.: Metabolic control characteristics of the acutely ischemic myocardium. Am. J. Cardiol. 22: 349, ATKINSON, D. E.: Biological feedback control at the molecular level. Science 150: 851, ATKINSON, D. E.: Energy charge of the adenylate pool as a regulatory parameter: Interaction with feedback modifiers. Biochemistry 7: 4030, 1968.
9 ADENOSINE RELEASE BY THE NORMAL HEART AXELROD, B.: Glycolysis. In Metabolic Pathways, 3d ed., edited by D. M. Creenberg. New York, Academic Press, 1967, vol. 1, pp LOWENSTEINT, J. M.: Tricarboxylic acid cycle. In Metabolic Pathways, 3d ed., edited by D. M. Creenberg. New York, Academic Press, 1967, vol. 1, pp MANSOUH, T. E.: Studies on heart phosphofructokinase: Purification, inhibition, and activation. J. Biol. Chem. 238: 2285, PARMECCTANI, A., AND MORGAN, H. E.: Effect of adenine nucleotides and inorganic phosphate on muscle phosphorylase activity. Biochem. Biophys. Res. Commun. 9: 252, WILLIAMSON, J. R.: Metabolic control in the perfused rat heart. In Control of Energy Metabolism, edited by B. Chance, R. W. Estabrook, and J. R. Williamson. New York, Academic Press, 1965, pp LEHXINGER, A. L.: Mitochondrion, 2d ed. New York, W. A. Benjamin, Inc., 1965, pp
10 Release of Adenosine by the Normal Myocardium in Dogs and Its Relationship to the Regulation of Coronary Resistance Rafael Rubio and Robert M. Berne Circ Res. 1969;25: doi: /01.RES Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX Copyright 1969 American Heart Association, Inc. All rights reserved. Print ISSN: Online ISSN: The online version of this article, along with updated information and services, is located on the World Wide Web at: Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: Subscriptions: Information about subscribing to Circulation Research is online at:
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Pyruvate + NADH + H + ==== Lactate + NAD +
 1 UNIVERSITY OF PAPUA NEW GUINEA SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PBL SEMINAR ANAEROBIC METABOLISM - An Overview
1 UNIVERSITY OF PAPUA NEW GUINEA SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PBL SEMINAR ANAEROBIC METABOLISM - An Overview
Medical Biochemistry and Molecular Biology department
 Medical Biochemistry and Molecular Biology department Cardiac Fuels [Sources of energy for the Cardiac muscle] Intended learning outcomes of the lecture: By the end of this lecture you would be able to:-
Medical Biochemistry and Molecular Biology department Cardiac Fuels [Sources of energy for the Cardiac muscle] Intended learning outcomes of the lecture: By the end of this lecture you would be able to:-
4. Which step shows a split of one molecule into two smaller molecules? a. 2. d. 5
 1. Which of the following statements about NAD + is false? a. NAD + is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD + has more chemical energy than NADH. c. NAD + is reduced
1. Which of the following statements about NAD + is false? a. NAD + is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD + has more chemical energy than NADH. c. NAD + is reduced
THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals
 Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
Br. J. Anaesth. (1981), 53, 131 THE GLUCOSE-FATTY ACID-KETONE BODY CYCLE Role of ketone bodies as respiratory substrates and metabolic signals J. C. STANLEY In this paper, the glucose-fatty acid cycle
In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic
 Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Respiration. Respiration. How Cells Harvest Energy. Chapter 7
 How Cells Harvest Energy Chapter 7 Respiration Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs:
How Cells Harvest Energy Chapter 7 Respiration Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs:
This is an example outline of 3 lectures in BSC (Thanks to Dr. Ellington for sharing this information.)
 This is an example outline of 3 lectures in BSC 2010. (Thanks to Dr. Ellington for sharing this information.) Topic 10: CELLULAR RESPIRATION (lectures 14-16) OBJECTIVES: 1. Know the basic reactions that
This is an example outline of 3 lectures in BSC 2010. (Thanks to Dr. Ellington for sharing this information.) Topic 10: CELLULAR RESPIRATION (lectures 14-16) OBJECTIVES: 1. Know the basic reactions that
Adenosine triphosphate (ATP)
 Adenosine triphosphate (ATP) 1 High energy bonds ATP adenosine triphosphate N NH 2 N -O O P O O P O- O- O O P O- O CH 2 H O H N N adenine phosphoanhydride bonds (~) H OH ribose H OH Phosphoanhydride bonds
Adenosine triphosphate (ATP) 1 High energy bonds ATP adenosine triphosphate N NH 2 N -O O P O O P O- O- O O P O- O CH 2 H O H N N adenine phosphoanhydride bonds (~) H OH ribose H OH Phosphoanhydride bonds
g) Cellular Respiration Higher Human Biology
 g) Cellular Respiration Higher Human Biology What can you remember about respiration? 1. What is respiration? 2. What are the raw materials? 3. What are the products? 4. Where does it occur? 5. Why does
g) Cellular Respiration Higher Human Biology What can you remember about respiration? 1. What is respiration? 2. What are the raw materials? 3. What are the products? 4. Where does it occur? 5. Why does
Introduction to Carbohydrate metabolism
 Introduction to Carbohydrate metabolism Some metabolic pathways of carbohydrates 1- Glycolysis 2- Krebs cycle 3- Glycogenesis 4- Glycogenolysis 5- Glyconeogenesis - Pentose Phosphate Pathway (PPP) - Curi
Introduction to Carbohydrate metabolism Some metabolic pathways of carbohydrates 1- Glycolysis 2- Krebs cycle 3- Glycogenesis 4- Glycogenolysis 5- Glyconeogenesis - Pentose Phosphate Pathway (PPP) - Curi
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy
 Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
A cell has enough ATP to last for about three seconds.
 Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]
![3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]](/thumbs/72/66350985.jpg) 3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
Chemical Energy. Valencia College
 9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
Class XI Chapter 14 Respiration in Plants Biology. 1. It is a biochemical process. 1. It is a physiochemical process.
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Unit 2: Metabolic Processes
 How is energy obtained biologically? Recall: Red Ox Reactions Unit 2: Metabolic Processes Oxidation Is the chief mechanism by which chemical potential energy is released This energy comes from reduced
How is energy obtained biologically? Recall: Red Ox Reactions Unit 2: Metabolic Processes Oxidation Is the chief mechanism by which chemical potential energy is released This energy comes from reduced
Carbohydrate Metabolism
 Chapter 34 Carbohydrate Metabolism Carbohydrate metabolism is important for both plants and animals. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison,
Chapter 34 Carbohydrate Metabolism Carbohydrate metabolism is important for both plants and animals. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison,
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
RESPIRATION Worksheet
 A.P. Bio L.C. RESPIRATION Worksheet 1. In the conversion of glucose and oxygen to carbon dioxide and water a) which molecule becomes reduced? b) which molecule becomes oxidized? c) what happens to the
A.P. Bio L.C. RESPIRATION Worksheet 1. In the conversion of glucose and oxygen to carbon dioxide and water a) which molecule becomes reduced? b) which molecule becomes oxidized? c) what happens to the
16. Exercise Energetics
 16. Exercise The performance of muscular exercise not only throws a strain on the musculoskeletal system itself but it also tests the reserves of virtually every system in the body. Exercising muscles
16. Exercise The performance of muscular exercise not only throws a strain on the musculoskeletal system itself but it also tests the reserves of virtually every system in the body. Exercising muscles
Cellular Respiration
 Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY
 MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY BOOKLET 10 NAME: CLASS: 1 S.Tagore Middletown South High School March 2013 LEARNING OUTCOMES The role and production of ATP (a) Importance, role and structure of ATP
MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY BOOKLET 10 NAME: CLASS: 1 S.Tagore Middletown South High School March 2013 LEARNING OUTCOMES The role and production of ATP (a) Importance, role and structure of ATP
PHYSIOLOGY MeQ'S (Morgan) All the following statements related to blood volume are correct except for: 5 A. Blood volume is about 5 litres. B.
 PHYSIOLOGY MeQ'S (Morgan) Chapter 5 All the following statements related to capillary Starling's forces are correct except for: 1 A. Hydrostatic pressure at arterial end is greater than at venous end.
PHYSIOLOGY MeQ'S (Morgan) Chapter 5 All the following statements related to capillary Starling's forces are correct except for: 1 A. Hydrostatic pressure at arterial end is greater than at venous end.
Background knowledge
 Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
Aerobic vs Anaerobic Respiration. 1. Glycolysis 2. Oxidation of Pyruvate and Krebs Cycle
 CELLULAR RESPIRATION Student Packet SUMMARY ALL LIVING SYSTEMS REQUIRE CONSTANT INPUT OF FREE ENERGY Cellular respiration is a catabolic pathway in which glucose and other organic fuels (such as starch,
CELLULAR RESPIRATION Student Packet SUMMARY ALL LIVING SYSTEMS REQUIRE CONSTANT INPUT OF FREE ENERGY Cellular respiration is a catabolic pathway in which glucose and other organic fuels (such as starch,
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Which of the following statements concerning anabolic reactions is FALSE? A. They are generally endergonic. B. They usually require ATP. C. They are part of metabolism. D.
MULTIPLE CHOICE QUESTIONS 1. Which of the following statements concerning anabolic reactions is FALSE? A. They are generally endergonic. B. They usually require ATP. C. They are part of metabolism. D.
Chapter 7 Cellular Respiration and Fermentation*
 Chapter 7 Cellular Respiration and Fermentation* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Life Is Work
Chapter 7 Cellular Respiration and Fermentation* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Life Is Work
How Cells Harvest Energy. Chapter 7. Respiration
 How Cells Harvest Energy Chapter 7 Respiration Organisms classified on how they obtain energy: autotrophs: produce their own organic molecules through photosynthesis heterotrophs: live on organic compounds
How Cells Harvest Energy Chapter 7 Respiration Organisms classified on how they obtain energy: autotrophs: produce their own organic molecules through photosynthesis heterotrophs: live on organic compounds
Coronary Circulation Under normal conditions cardiac muscle metabolism is almost exclusively aerobic depending on oxidative phosorylation to
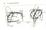 Coronary Circulation Under normal conditions cardiac muscle metabolism is almost exclusively aerobic depending on oxidative phosorylation to resynthesis the ATP continuously utilized by repetitive, excitation,
Coronary Circulation Under normal conditions cardiac muscle metabolism is almost exclusively aerobic depending on oxidative phosorylation to resynthesis the ATP continuously utilized by repetitive, excitation,
Name Class Date. 1. Cellular respiration is the process by which the of "food"
 Name Class Date Cell Respiration Introduction Cellular respiration is the process by which the chemical energy of "food" molecules is released and partially captured in the form of ATP. Carbohydrates,
Name Class Date Cell Respiration Introduction Cellular respiration is the process by which the chemical energy of "food" molecules is released and partially captured in the form of ATP. Carbohydrates,
5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM
 5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM Introduction: Variety of hormones and other molecules regulate the carbohydrates metabolism. Some of these have already been cited in previous sections.
5.0 HORMONAL CONTROL OF CARBOHYDRATE METABOLISM Introduction: Variety of hormones and other molecules regulate the carbohydrates metabolism. Some of these have already been cited in previous sections.
Effect of Atrial and Ventricular Tachycardia on Cardiac Oxygen Consumption
 Effect of Atrial and Ventricular Tachycardia on Cardiac Oxygen Consumption fly Henry S. Badeer, M.D., and Kholil A. Feisol, M.D. Clinical experience has shown that ventricular is a more serious arrhythmia
Effect of Atrial and Ventricular Tachycardia on Cardiac Oxygen Consumption fly Henry S. Badeer, M.D., and Kholil A. Feisol, M.D. Clinical experience has shown that ventricular is a more serious arrhythmia
anabolic pathways- Catabolic Amphibolic
 METABOLISM Introduction The fate of dietary components after digestion and absorption constitute metabolism regulated by metabolic pathway 3 types: anabolic pathways- Synthesis of compound e.g. synthesis
METABOLISM Introduction The fate of dietary components after digestion and absorption constitute metabolism regulated by metabolic pathway 3 types: anabolic pathways- Synthesis of compound e.g. synthesis
Chemistry 1120 Exam 4 Study Guide
 Chemistry 1120 Exam 4 Study Guide Chapter 12 12.1 Identify and differentiate between macronutrients (lipids, amino acids and saccharides) and micronutrients (vitamins and minerals). Master Tutor Section
Chemistry 1120 Exam 4 Study Guide Chapter 12 12.1 Identify and differentiate between macronutrients (lipids, amino acids and saccharides) and micronutrients (vitamins and minerals). Master Tutor Section
Fig In the space below, indicate how these sub-units are joined in a molecule of ATP.
 1 (a) Adenosine tri-phosphate (ATP) is an important product of respiration. The ATP molecule is made up of five sub-units, as shown in Fig. 5.1. adenine phosphates O ribose Fig. 5.1 (i) In the space below,
1 (a) Adenosine tri-phosphate (ATP) is an important product of respiration. The ATP molecule is made up of five sub-units, as shown in Fig. 5.1. adenine phosphates O ribose Fig. 5.1 (i) In the space below,
Pressure-Flow Relationships in the Coronary Circulation
 Pressure-Flow Relationships in the Coronary Circulation By Wadie M. Fam, M.D., Ph.D., and Maurice McGregor, M.D. ABSTRACT This study was designed to define more accurately the respective influences of
Pressure-Flow Relationships in the Coronary Circulation By Wadie M. Fam, M.D., Ph.D., and Maurice McGregor, M.D. ABSTRACT This study was designed to define more accurately the respective influences of
Metabolism Energy Pathways Biosynthesis. Catabolism Anabolism Enzymes
 Topics Microbial Metabolism Metabolism Energy Pathways Biosynthesis 2 Metabolism Catabolism Catabolism Anabolism Enzymes Breakdown of complex organic molecules in order to extract energy and dform simpler
Topics Microbial Metabolism Metabolism Energy Pathways Biosynthesis 2 Metabolism Catabolism Catabolism Anabolism Enzymes Breakdown of complex organic molecules in order to extract energy and dform simpler
OCR. Respiration Questions
 OCR Respiration Questions 12 4 (a) The first stage in respiration involves the conversion of one molecule of glucose into two molecules of a 3C compound that can enter mitochondria when oxygen is
OCR Respiration Questions 12 4 (a) The first stage in respiration involves the conversion of one molecule of glucose into two molecules of a 3C compound that can enter mitochondria when oxygen is
III. 6. Test. Respiració cel lular
 III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
Cellular Respiration
 Cellular I can describe cellular respiration Cellular respiration is a series of metabolic pathways releasing energy from a foodstuff e.g. glucose. This yields energy in the form of ATP adenosine P i P
Cellular I can describe cellular respiration Cellular respiration is a series of metabolic pathways releasing energy from a foodstuff e.g. glucose. This yields energy in the form of ATP adenosine P i P
Cellular Pathways That Harvest Chemical Energy. Cellular Pathways That Harvest Chemical Energy. Cellular Pathways In General
 Cellular Pathways That Harvest Chemical Energy A. Obtaining Energy and Electrons from Glucose Lecture Series 12 Cellular Pathways That Harvest Chemical Energy B. An Overview: Releasing Energy from Glucose
Cellular Pathways That Harvest Chemical Energy A. Obtaining Energy and Electrons from Glucose Lecture Series 12 Cellular Pathways That Harvest Chemical Energy B. An Overview: Releasing Energy from Glucose
How Cells Release Chemical Energy. Chapter 7
 How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
MUSCLE METABOLISM. Honors Anatomy & Physiology
 MUSCLE METABOLISM Honors Anatomy & Physiology ROLE OF ATP ATP binds to myosin heads and upon hydrolysis into ADP and Pi, transfers its energy to the cross bridge, energizing it. ATP is responsible for
MUSCLE METABOLISM Honors Anatomy & Physiology ROLE OF ATP ATP binds to myosin heads and upon hydrolysis into ADP and Pi, transfers its energy to the cross bridge, energizing it. ATP is responsible for
Glucose is the only source of energy in red blood cells. Under starvation conditions ketone bodies become a source of energy for the brain
 Glycolysis 4 / The Text :- Some Points About Glucose Glucose is very soluble source of quick and ready energy. It is a relatively stable and easily transported. In mammals, the brain uses only glucose
Glycolysis 4 / The Text :- Some Points About Glucose Glucose is very soluble source of quick and ready energy. It is a relatively stable and easily transported. In mammals, the brain uses only glucose
A New Technique for Repeated Measurement of Cardiac Output During Cardiopulmonary Resuscitation
 Purdue University Purdue e-pubs Weldon School of Biomedical Engineering Faculty Publications Weldon School of Biomedical Engineering 1980 A New Technique for Repeated Measurement of Cardiac Output During
Purdue University Purdue e-pubs Weldon School of Biomedical Engineering Faculty Publications Weldon School of Biomedical Engineering 1980 A New Technique for Repeated Measurement of Cardiac Output During
Respiration. Respiration. Respiration. How Cells Harvest Energy. Chapter 7
 How Cells Harvest Energy Chapter 7 Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs: live on
How Cells Harvest Energy Chapter 7 Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs: live on
UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017
 LH14 UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017 INTRODUCTION TO SPORT AND EXERCISE PHYSIOLOGY MODULE NO: SPS4002 Date: Thursday
LH14 UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017 INTRODUCTION TO SPORT AND EXERCISE PHYSIOLOGY MODULE NO: SPS4002 Date: Thursday
Chapter 9, Part 2. Cardiocirculatory Adjustments to Exercise
 Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
Lecture 5: Cell Metabolism. Biology 219 Dr. Adam Ross
 Lecture 5: Cell Metabolism Biology 219 Dr. Adam Ross Cellular Respiration Set of reactions that take place during the conversion of nutrients into ATP Intricate regulatory relationship between several
Lecture 5: Cell Metabolism Biology 219 Dr. Adam Ross Cellular Respiration Set of reactions that take place during the conversion of nutrients into ATP Intricate regulatory relationship between several
Downloaded from by guest on July 15, 2018
 33 Consistent Parallel Relationships among Myocardial Oxygen Consumption, Coronary Blood Flow, and Pericardial Infusate Adenosine Concentration with Various Interventions and ^-Blockade in the Dog Robert
33 Consistent Parallel Relationships among Myocardial Oxygen Consumption, Coronary Blood Flow, and Pericardial Infusate Adenosine Concentration with Various Interventions and ^-Blockade in the Dog Robert
1. The ultimate electron acceptor of respiration in an aerobic organisms is: Cytochrome Oxygen Hydrogen Glucose
 7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a b c d Cytochrome Oxygen Hydrogen Glucose 2. Phosphorylation
7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a b c d Cytochrome Oxygen Hydrogen Glucose 2. Phosphorylation
Under aerobic conditions, pyruvate enters the mitochondria where it is converted into acetyl CoA.
 Under aerobic conditions, pyruvate enters the mitochondria where it is converted into acetyl CoA. Acetyl CoA is the fuel for the citric acid cycle, which processes the two carbon acetyl unit to two molecules
Under aerobic conditions, pyruvate enters the mitochondria where it is converted into acetyl CoA. Acetyl CoA is the fuel for the citric acid cycle, which processes the two carbon acetyl unit to two molecules
Metabolism. Metabolic pathways. BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Metabolism Metabolism is the chemical change of
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 11: Metabolic Pathways http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Metabolism Metabolism is the chemical change of
Chapter 9: Cellular Respiration
 Chapter 9: Cellular Respiration To perform their many tasks, living cells require energy from outside sources. Energy stored in food utimately comes from the sun. Photosynthesis makes the raw materials
Chapter 9: Cellular Respiration To perform their many tasks, living cells require energy from outside sources. Energy stored in food utimately comes from the sun. Photosynthesis makes the raw materials
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Respiration Practice Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements describes NAD+? A) NAD+ can donate
Respiration Practice Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements describes NAD+? A) NAD+ can donate
Chap 3 Metabolism and Growth
 Chap 3 Metabolism and Growth I. Metabolism Definitions: Metabolism includes two parts: anabolism and catabolism Catabolism: Anabolism: Aerobic metabolism: catabolism anabolis m catabolis anabolis m Anaerobic
Chap 3 Metabolism and Growth I. Metabolism Definitions: Metabolism includes two parts: anabolism and catabolism Catabolism: Anabolism: Aerobic metabolism: catabolism anabolis m catabolis anabolis m Anaerobic
Citation Acta medica Nagasakiensia. 1984, 29
 NAOSITE: Nagasaki University's Ac Title Author(s) Efficacy of Coenzyme Q10 Administra Aortic Stenosis and Pacemaker Induc Igarashi, Katsuro Citation Acta medica Nagasakiensia. 1984, 29 Issue Date 1984-10-25
NAOSITE: Nagasaki University's Ac Title Author(s) Efficacy of Coenzyme Q10 Administra Aortic Stenosis and Pacemaker Induc Igarashi, Katsuro Citation Acta medica Nagasakiensia. 1984, 29 Issue Date 1984-10-25
Chapter 5. Microbial Metabolism
 Chapter 5 Microbial Metabolism Metabolism Collection of controlled biochemical reactions that take place within a microbe Ultimate function of metabolism is to reproduce the organism Metabolic Processes
Chapter 5 Microbial Metabolism Metabolism Collection of controlled biochemical reactions that take place within a microbe Ultimate function of metabolism is to reproduce the organism Metabolic Processes
Lecture Sixteen: METABOLIC ENERGY: [Based on GENERATION Chapter 15
 Lecture Sixteen: METABOLIC ENERGY: [Based on GENERATION Chapter 15 AND STORAGE Berg, (Figures in red are for the 7th Edition) Tymoczko (Figures in Blue are for the 8th Edition) & Stryer] Two major questions
Lecture Sixteen: METABOLIC ENERGY: [Based on GENERATION Chapter 15 AND STORAGE Berg, (Figures in red are for the 7th Edition) Tymoczko (Figures in Blue are for the 8th Edition) & Stryer] Two major questions
AP Bio Photosynthesis & Respiration
 AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
Chapter 5 Microbial Metabolism: The Chemical Crossroads of Life
 Chapter 5 Microbial Metabolism: The Chemical Crossroads of Life Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. The Metabolism of Microbes metabolism all chemical
Chapter 5 Microbial Metabolism: The Chemical Crossroads of Life Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. The Metabolism of Microbes metabolism all chemical
Reading Assignments. A. Energy and Energy Conversions. Lecture Series 9 Cellular Pathways That Harvest Chemical Energy. gasoline) or elevated mass.
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Syllabus for BASIC METABOLIC PRINCIPLES
 Syllabus for BASIC METABOLIC PRINCIPLES The video lecture covers basic principles you will need to know for the lectures covering enzymes and metabolism in Principles of Metabolism and elsewhere in the
Syllabus for BASIC METABOLIC PRINCIPLES The video lecture covers basic principles you will need to know for the lectures covering enzymes and metabolism in Principles of Metabolism and elsewhere in the
Bioenergetics. Chapter 3. Objectives. Objectives. Introduction. Photosynthesis. Energy Forms
 Objectives Chapter 3 Bioenergetics Discuss the function of cell membrane, nucleus, & mitochondria Define: endergonic, exergonic, coupled reactions & bioenergetics Describe how enzymes work Discuss nutrients
Objectives Chapter 3 Bioenergetics Discuss the function of cell membrane, nucleus, & mitochondria Define: endergonic, exergonic, coupled reactions & bioenergetics Describe how enzymes work Discuss nutrients
7 Pathways That Harvest Chemical Energy
 7 Pathways That Harvest Chemical Energy Pathways That Harvest Chemical Energy How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of Glucose Metabolism? How Is Energy Harvested
7 Pathways That Harvest Chemical Energy Pathways That Harvest Chemical Energy How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of Glucose Metabolism? How Is Energy Harvested
Enzymes Part III: regulation II. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
(Received 22 July 1957) It is now generally accepted that the unequal distribution of ions between cells
 190 J. Physiol. (I958) I40, I90-200 THE EFFECT OF ALTERATIONS OF PLASMA SODIUM ON THE SODIUM AND POTASSIUM CONTENT OF MUSCLE IN THE RAT By F. 0. DOSEKUN AND D. MENDEL From the Department of Physiology,
190 J. Physiol. (I958) I40, I90-200 THE EFFECT OF ALTERATIONS OF PLASMA SODIUM ON THE SODIUM AND POTASSIUM CONTENT OF MUSCLE IN THE RAT By F. 0. DOSEKUN AND D. MENDEL From the Department of Physiology,
Ch. 9 Cell Respiration. Title: Oct 15 3:24 PM (1 of 53)
 Ch. 9 Cell Respiration Title: Oct 15 3:24 PM (1 of 53) Essential question: How do cells use stored chemical energy in organic molecules and to generate ATP? Title: Oct 15 3:28 PM (2 of 53) Title: Oct 19
Ch. 9 Cell Respiration Title: Oct 15 3:24 PM (1 of 53) Essential question: How do cells use stored chemical energy in organic molecules and to generate ATP? Title: Oct 15 3:28 PM (2 of 53) Title: Oct 19
Enzymes what are they?
 Topic 11 (ch8) Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis 1 Catabolism Anabolism Enzymes Metabolism 2 Metabolic balancing act Catabolism Enzymes involved in breakdown of complex
Topic 11 (ch8) Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis 1 Catabolism Anabolism Enzymes Metabolism 2 Metabolic balancing act Catabolism Enzymes involved in breakdown of complex
CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM
 CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM Metabolism Bioenergetics is the transfer and utilization of energy in biological systems The direction and extent to which a chemical reaction
CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM Metabolism Bioenergetics is the transfer and utilization of energy in biological systems The direction and extent to which a chemical reaction
Metabolism. Chapter 5. Catabolism Drives Anabolism 8/29/11. Complete Catabolism of Glucose
 8/29/11 Metabolism Chapter 5 All of the reactions in the body that require energy transfer. Can be divided into: Cell Respiration and Metabolism Anabolism: requires the input of energy to synthesize large
8/29/11 Metabolism Chapter 5 All of the reactions in the body that require energy transfer. Can be divided into: Cell Respiration and Metabolism Anabolism: requires the input of energy to synthesize large
Integration of Metabolism
 Integration of Metabolism Metabolism is a continuous process. Thousands of reactions occur simultaneously in order to maintain homeostasis. It ensures a supply of fuel, to tissues at all times, in fed
Integration of Metabolism Metabolism is a continuous process. Thousands of reactions occur simultaneously in order to maintain homeostasis. It ensures a supply of fuel, to tissues at all times, in fed
DIAGNOTICS QUESTION CLUSTERS: Defining and assessing understanding
 DIAGNOTICS QUESTION CLUSTERS: Defining and assessing understanding Introduction to the Project NSF funded Personnel scientists, science educators Biology and geology cycles o Photosynthesis o Respiration
DIAGNOTICS QUESTION CLUSTERS: Defining and assessing understanding Introduction to the Project NSF funded Personnel scientists, science educators Biology and geology cycles o Photosynthesis o Respiration
Ch 9: Cellular Respiration
 Ch 9: Cellular Respiration Cellular Respiration An overview Exergonic reactions and catabolic pathway Energy stored in bonds of food molecules is transferred to ATP Cellular respiration provides the energy
Ch 9: Cellular Respiration Cellular Respiration An overview Exergonic reactions and catabolic pathway Energy stored in bonds of food molecules is transferred to ATP Cellular respiration provides the energy
Control of blood tissue blood flow. Faisal I. Mohammed, MD,PhD
 Control of blood tissue blood flow Faisal I. Mohammed, MD,PhD 1 Objectives List factors that affect tissue blood flow. Describe the vasodilator and oxygen demand theories. Point out the mechanisms of autoregulation.
Control of blood tissue blood flow Faisal I. Mohammed, MD,PhD 1 Objectives List factors that affect tissue blood flow. Describe the vasodilator and oxygen demand theories. Point out the mechanisms of autoregulation.
Page 2 of 51 WJEC/CBAC 2016 pdfcrowd.com
 1. Page 2 of 51 WJEC/CBAC 2016 Page 3 of 51 WJEC/CBAC 2016 2. Page 4 of 51 WJEC/CBAC 2016 Page 5 of 51 WJEC/CBAC 2016 3. Page 6 of 51 WJEC/CBAC 2016 Page 7 of 51 WJEC/CBAC 2016 4. Page 8 of 51 WJEC/CBAC
1. Page 2 of 51 WJEC/CBAC 2016 Page 3 of 51 WJEC/CBAC 2016 2. Page 4 of 51 WJEC/CBAC 2016 Page 5 of 51 WJEC/CBAC 2016 3. Page 6 of 51 WJEC/CBAC 2016 Page 7 of 51 WJEC/CBAC 2016 4. Page 8 of 51 WJEC/CBAC
RESPIRATION IN PLANTS
 7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a Cytochrome b Oxygen c Hydrogen d Glucose 2. Phosphorylation
7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a Cytochrome b Oxygen c Hydrogen d Glucose 2. Phosphorylation
Major Pathways in Carbohydrate Metabolism
 Major Pathways in Carbohydrate Metabolism 70 Stage 1: Digestion of Carbohydrates In Stage 1, the digestion of carbohydrates Begins in the mouth where salivary amylase breaks down polysaccharides to smaller
Major Pathways in Carbohydrate Metabolism 70 Stage 1: Digestion of Carbohydrates In Stage 1, the digestion of carbohydrates Begins in the mouth where salivary amylase breaks down polysaccharides to smaller
WHY IS THIS IMPORTANT?
 CHAPTER 3 ESSENTIALS OF METABOLISM WHY IS THIS IMPORTANT? It is important to have a basic understanding of metabolism because it governs the survival and growth of microorganisms The growth of microorganisms
CHAPTER 3 ESSENTIALS OF METABOLISM WHY IS THIS IMPORTANT? It is important to have a basic understanding of metabolism because it governs the survival and growth of microorganisms The growth of microorganisms
Energy sources in skeletal muscle
 Energy sources in skeletal muscle Pathway Rate Extent ATP/glucose 1. Direct phosphorylation Extremely fast Very limited - 2. Glycolisis Very fast limited 2-3 3. Oxidative phosphorylation Slow Unlimited
Energy sources in skeletal muscle Pathway Rate Extent ATP/glucose 1. Direct phosphorylation Extremely fast Very limited - 2. Glycolisis Very fast limited 2-3 3. Oxidative phosphorylation Slow Unlimited
1. Draw and annotate a molecule of ATP to show how it stores and releases energy. 2. List six cellular process that use ATP as a source of energy.
 ATP 1. Draw and annotate a molecule of ATP to show how it stores and releases energy. 2. List six cellular process that use ATP as a source of energy. 3.7 Cell Respiration 3. Define cell respiration. The
ATP 1. Draw and annotate a molecule of ATP to show how it stores and releases energy. 2. List six cellular process that use ATP as a source of energy. 3.7 Cell Respiration 3. Define cell respiration. The
Active Learning Exercise 5. Cellular Respiration
 Name Biol 211 - Group Number Active Learning Exercise 5. Cellular Respiration Reference: Chapter 9 (Biology by Campbell/Reece, 8 th ed.) 1. Give the overall balanced chemical equation for aerobic cellular
Name Biol 211 - Group Number Active Learning Exercise 5. Cellular Respiration Reference: Chapter 9 (Biology by Campbell/Reece, 8 th ed.) 1. Give the overall balanced chemical equation for aerobic cellular
0.40. Biochemistry of Carbohydrates
 0.40 Biochemistry of Carbohydrates Biochemistry of Carbohydrates ATP ADP Glycolysis The Breakdown of Glucose Primary Energy Source of Cells Central Metabolic Pathway All Reactions Occur in Cytoplasm Two
0.40 Biochemistry of Carbohydrates Biochemistry of Carbohydrates ATP ADP Glycolysis The Breakdown of Glucose Primary Energy Source of Cells Central Metabolic Pathway All Reactions Occur in Cytoplasm Two
What is Glycolysis? Breaking down glucose: glyco lysis (splitting sugar)
 What is Glycolysis? Breaking down glucose: glyco lysis (splitting sugar) Most ancient form of energy capture. Starting point for all cellular respiration. Inefficient: generates only 2 ATP for every 1
What is Glycolysis? Breaking down glucose: glyco lysis (splitting sugar) Most ancient form of energy capture. Starting point for all cellular respiration. Inefficient: generates only 2 ATP for every 1
number Done by Corrected by Doctor Nayef Karadsheh
 number 13 Done by Asma Karameh Corrected by Saad hayek Doctor Nayef Karadsheh Gluconeogenesis This lecture covers gluconeogenesis with aspects of: 1) Introduction to glucose distribution through tissues.
number 13 Done by Asma Karameh Corrected by Saad hayek Doctor Nayef Karadsheh Gluconeogenesis This lecture covers gluconeogenesis with aspects of: 1) Introduction to glucose distribution through tissues.
Plant Respiration. Exchange of Gases in Plants:
 Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
Section B: The Process of Cellular Respiration
 CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section B: The Process of Cellular Respiration 1. Respiration involves glycolysis, the Krebs cycle, and electron transport: an overview 2. Glycolysis
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section B: The Process of Cellular Respiration 1. Respiration involves glycolysis, the Krebs cycle, and electron transport: an overview 2. Glycolysis
Biol 219 Lec 7 Fall 2016
 Cellular Respiration: Harvesting Energy to form ATP Cellular Respiration and Metabolism Glucose ATP Pyruvate Lactate Acetyl CoA NAD + Introducing The Players primary substrate for cellular respiration
Cellular Respiration: Harvesting Energy to form ATP Cellular Respiration and Metabolism Glucose ATP Pyruvate Lactate Acetyl CoA NAD + Introducing The Players primary substrate for cellular respiration
1 Respiration is a vital process in living organisms. All organisms carry out glycolysis. The Krebs cycle also occurs in some organisms.
 1 Respiration is a vital process in living organisms. All organisms carry out glycolysis. The Krebs cycle also occurs in some organisms. (a) The diagram below shows some of the stages in glycolysis, using
1 Respiration is a vital process in living organisms. All organisms carry out glycolysis. The Krebs cycle also occurs in some organisms. (a) The diagram below shows some of the stages in glycolysis, using
Cellular Respiration: Harvesting Chemical Energy CHAPTER 9
 Cellular Respiration: Harvesting Chemical Energy CHAPTER 9 9.1 Metabolic pathways that release energy are exergonic and considered catabolic pathways. Fermentation: partial degradation of sugars that occurs
Cellular Respiration: Harvesting Chemical Energy CHAPTER 9 9.1 Metabolic pathways that release energy are exergonic and considered catabolic pathways. Fermentation: partial degradation of sugars that occurs
Glycolysis Part 2. BCH 340 lecture 4
 Glycolysis Part 2 BCH 340 lecture 4 Regulation of Glycolysis There are three steps in glycolysis that have enzymes which regulate the flux of glycolysis These enzymes catalyzes irreversible reactions of
Glycolysis Part 2 BCH 340 lecture 4 Regulation of Glycolysis There are three steps in glycolysis that have enzymes which regulate the flux of glycolysis These enzymes catalyzes irreversible reactions of
Chapter 8. An Introduction to Microbial Metabolism
 Chapter 8 An Introduction to Microbial Metabolism The metabolism of microbes Metabolism sum of all chemical reactions that help cells function Two types of chemical reactions: Catabolism -degradative;
Chapter 8 An Introduction to Microbial Metabolism The metabolism of microbes Metabolism sum of all chemical reactions that help cells function Two types of chemical reactions: Catabolism -degradative;
Chapter 10! Chapter 10, Part 2 Muscle. Muscle Tissue - Part 2! Pages !
 ! Chapter 10, Part 2 Muscle Chapter 10! Muscle Tissue - Part 2! Pages 308-324! SECTION 10-5! Sarcomere shortening and muscle fiber stimulation produce tension! 2! Tension Production - Muscle FIBER! All-or-none
! Chapter 10, Part 2 Muscle Chapter 10! Muscle Tissue - Part 2! Pages 308-324! SECTION 10-5! Sarcomere shortening and muscle fiber stimulation produce tension! 2! Tension Production - Muscle FIBER! All-or-none
BCH 4054 Chapter 19 Lecture Notes
 BCH 4054 Chapter 19 Lecture Notes 1 Chapter 19 Glycolysis 2 aka = also known as verview of Glycolysis aka The Embden-Meyerhoff Pathway First pathway discovered Common to almost all living cells ccurs in
BCH 4054 Chapter 19 Lecture Notes 1 Chapter 19 Glycolysis 2 aka = also known as verview of Glycolysis aka The Embden-Meyerhoff Pathway First pathway discovered Common to almost all living cells ccurs in
Cellular Respiration Other Metabolites & Control of Respiration. AP Biology
 Cellular Respiration Other Metabolites & Control of Respiration Cellular respiration: Beyond glucose: Other carbohydrates: Glycolysis accepts a wide range of carbohydrates fuels. polysaccharides glucose
Cellular Respiration Other Metabolites & Control of Respiration Cellular respiration: Beyond glucose: Other carbohydrates: Glycolysis accepts a wide range of carbohydrates fuels. polysaccharides glucose
Bromeliads are CAM plants. Pineapple is a bromeliad. CAM was discovered in the Crassulaceae, a family of plants that includes jade plant.
 Bromeliads are CAM plants Pineapple is a bromeliad CAM was discovered in the Crassulaceae, a family of plants that includes jade plant. Developing and non-photosynthetic sink tissues depend on a supply
Bromeliads are CAM plants Pineapple is a bromeliad CAM was discovered in the Crassulaceae, a family of plants that includes jade plant. Developing and non-photosynthetic sink tissues depend on a supply
2) The molecule that functions as the reducing agent (electron donor) in a redox or oxidationreduction
 Campbell Biology in Focus (Urry) Chapter 7 Cellular Respiration and Fermentation 7.1 Multiple-Choice Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex
Campbell Biology in Focus (Urry) Chapter 7 Cellular Respiration and Fermentation 7.1 Multiple-Choice Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex
Asmusssen, Hald & Larsen (1948) observed that the infusion of acetaldehyde
 234 J. Physiol. (1963), 168, pp. 234-237 With 2 plates and 1 text-figure Printed in Great Britain THE ACTION OF ACETALDEHYDE ON THE CHEMO- RECEPTORS OF THE CAROTID GLOMUS BY N. JOELS AND E. NEIL From the
234 J. Physiol. (1963), 168, pp. 234-237 With 2 plates and 1 text-figure Printed in Great Britain THE ACTION OF ACETALDEHYDE ON THE CHEMO- RECEPTORS OF THE CAROTID GLOMUS BY N. JOELS AND E. NEIL From the
