Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury
|
|
|
- Darcy Farmer
- 6 years ago
- Views:
Transcription
1 Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury Publication July 2016
2 Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury Review date February 2018 Table of Contents Subject Page No 1. Introduction 3 2. Acute kidney injury Medication Optimisation Pro forma 4 3. High risk medications and actions 5 4. Conclusion Acknowledgements 14 To the best of our knowledge, the contents of this publication are in line with National Institute for Health and Care Excellence guidance relating to the management and treatment of acute kidney injury. Professional advice should be sought before taking, or refraining from taking, any action on the basis of the content of this publication. We cannot be held responsible for any errors or omissions therein, nor for the consequences of these or for any loss or damage suffered by readers or any third party informed of its contents. The UK Renal Registry disclaims all liability and responsibility arising from any reliance placed on the information contained in this publication by you or any third party who may be informed of its contents. Guidelines for medicines optimisation in patients with acute kidney injury 2
3 1. Introduction Acute kidney injury (AKI) is the sudden loss of kidney function over a period of hours or days. Since the kidneys are one of the major excretory pathways for the removal of drugs from the body, this sudden loss of kidney function can have major implications for a patient s prescribed medication regime. The term nephrotoxic should be used with caution. Few medications truly have direct toxic effects on the kidneys, but several have the potential to impair renal function if used under certain circumstances, such as where the patient has a degree of chronic kidney disease in conjunction with hypovolaemia and acute illness. Under these circumstances, continued use of these medications may further exacerbate an episode of AKI. The Think Kidneys Programme has taken the decision to avoid the use of the term nephrotoxic. In addition, many medications are cleared via the kidneys, so have the potential to accumulate during an episode of AKI. The result of this may be a further deterioration in kidney function, or there may be other adverse effects such a bone marrow or CNS toxicity. Hence it is necessary to review the use of these medications, and amend the doses appropriate to the level of the patient s renal function. When a patient is either admitted with AKI, or develops AKI during an admission episode, a thorough review of medication is required in order to: Eliminate the potential cause/risk/contributory factor for AKI Avoid inappropriate combinations of medications in the context of AKI Reduce adverse events Ensure that doses of prescribed medication are appropriate for the patient s level of renal function Ensure that all medicines prescribed are clinically appropriate. Points to note and questions to ask in the medicines management of these patients include: Which medications should be suspended? Which medications should not be suspended? Which medications may be used with caution? Are there any alternative therapeutic options? If a medication must be used, in order to minimise harm: Amend doses appropriate to the patient s level of renal function Monitor blood levels of drugs wherever possible Keep course of treatment as short as possible Discuss treatment with pharmacist/microbiologist Guidelines for medicines optimisation in patients with acute kidney injury 3
4 Ensure appropriate information and advice is given on discharge: From the ICU to the ward From the ward to the GP (and care home if required) From the ward to the patient and their family/carers 2. Acute kidney injury Medication Optimisation Pro forma In order to optimise the prescribing of medications to a patient with AKI, the following points should be considered: 1. Is the patient receiving medication which may impair renal function? Contrast media ACE Inhibitor NSAIDs Diuretics Angiotensin receptor blocker Consider withholding these agents during an episode of AKI. 2. Medication Is the patient taking any other medications which could exacerbate AKI? Consider withholding them. Is the patient prescribed any medications where the dose needs to be amended in renal impairment? Amend medication doses appropriate to the patient s degree of renal impairment. In house guidelines for drug use in AKI are recommended for example for. antibiotics, analgesia, contrast media, chemotherapy. 3. Educate the patient before discharge about which medications to restart and when, which medicines to avoid etc. 4. Ensure comprehensive information on which medications to restart and when, is communicated to the GP or next care setting. Guidelines for medicines optimisation in patients with acute kidney injury 4
5 Other useful reference sources to facilitate dose adjustment in AKI include: Group of medicines Suggested guidelines Anti-retrovirals /HAART National Institute of Health HIV/AIDS Treatment Guidelines Chemotherapy North London Cancer Network Guidelines Mental Health The Maudsley Prescribing Guidelines General medications The Renal Drug Database General medications Manufacturers Summary of Product Characteristics 3. High risk medicines and actions The following list of medications is not exhaustive. Remember to consider ALL medications including any usual long term medications. Remember to check medication history thoroughly and ask about over the counter preparations, herbal remedies/teas and alternative therapies. Check recreational use of drugs (cocaine, ketamine etc) as these have been implicated in rhabdomyolysis. With reference to the table below, the three types of problem associated with the use of drugs in AKI are:- 1) effects on renal/fluid/electrolyte physiology 2) change in the side effect profile when renal function is reduced 3) direct action on the kidneys This format is therefore adopted in the following table. Guidelines for medicines optimisation in patients with acute kidney injury 5
6 Drug NSAIDs / COX II inhibitors Effects on renal/fluid/electrolyte physiology Altered haemodynamics within the kidney leading to underperfusion and reduced glomerular filtration Change in the side effect profile when renal function is reduced Analgesics Direct action on the kidneys Acute interstitial nephritis (rare) Action in presence of AKI Avoid Opioid analgesics Tramadol Benzodiazepines Aciclovir Accumulation of active metabolites (especially morphine, pethidine and codeine) increased incidence of CNS side effects & respiratory depression May accumulate leading to increased sedation, mental confusion and respiratory depression Accumulation of drug & active metabolites leading to increased sedation & mental confusion Antibiotics / Antifungals / Antivirals Accumulation leading to mental Crystal nephropathy. confusion, seizures. Avoid XL / SR preparations. and frequency. Use short acting preparations wherever possible. Use opiates with minimal renal excretion e.g. fentanyl, oxycodone, hydromorphone, tramadol. Avoid XL preparations & monitor for excessive sedation Beware if patient is at risk of dehydration - Encourage patient to Guidelines for medicines optimisation in patients with acute kidney injury 6
7 drink plenty Aminoglycosides Amphotericin IV Fungizone Co-trimoxazole Ototoxicity Tubular cell toxicity Avoid if possible. If use is unavoidable, reduce dose &/or increase dosing interval Monitor drug levels and renal function 2 3 times per week Hypokalaemia Tubular cell toxicity Avoid rapid infusion Consider Ambisome preparation Hyperkalaemia Crystal nephropathy. Beware if patient is at risk of dehydration - Encourage patient to drink plenty Fluconazole Ganciclovir IV Penicillins Teicoplanin Accumulation leading to acute mental confusion, coma, seizures Accumulation leading to neutropenia, anaemia and thrombocytopenia Accumulation leading to CNS side effects including seizures Accumulation leading to CNS excitation, seizures, & blood dyscrasias Crystal nephropathy Acute interstitial nephritis (rare) Glomerulonephritis. Check for drug interactions that may be contributing to AKI, eg. consider withholding statins due to risk of rhabdomyolysis Monitor renal function and full blood count Avoid rapid infusions Monitor levels Guidelines for medicines optimisation in patients with acute kidney injury 7
8 Tetracycline Trimethoprim Valganciclovir Vancomycin Phenytoin Increased risk of hyperkalaemia(especially in combination with spironolactone or ACEI/ARB). Interferes with tubular secretion of creatinine leading to a rise in serum creatinine (without a true change in GFR). Accumulation leading to renal dysfunction, benign cranial hypertension, jaundice, hepatitis Accumulation leading to hyperkalaemia (particularly with high doses), nausea and vomiting Accumulation leading to neutropenia, anaemia and thrombocytopenia Accumulation leading to renal toxicity, ototoxicity Acute interstitial nephritis (rare) Acute interstitial nephritis (rare) Acute interstitial nephritis (rare) Antiepileptics (including drugs used for neuropathic pain) Risk of phenytoin toxicity if Acute interstitial nephritis patient has low serum albumin (rare) levels Avoid Avoid or reduce dose (particularly if patient is already taking an ACEI, ARB or spironolactone) Studies have shown that elderly patients prescribed trimethoprim have a 12 x greater risk of developing life-threatening hyperkalaemia if already taking spironolactone, and a 7-fold increased risk of life-threatening hyperkalaemia, and a 1.5 x increased risk of sudden death if already taking an ACEI or ARB. Monitor renal function and full blood count / increase dose interval Monitor levels Monitor levels Correct phenytoin levels for uraemia and low serum albumin or measure salivary phenytoin (if assay available) Guidelines for medicines optimisation in patients with acute kidney injury 8
9 Pregabalin & Gabapentin Levetiracetam Antihypertensives (including Cachannel blockers, -blockers, - blockers, etc) ACEI / ARBs / Aliskiren Thiazide & Loop Diuretics Hyperkalaemia Hypokalaemia, hypocalcaemia, hypomagnesaemia, hyponatraemia, hyperuricaemia (rare) Volume depletion Accumulation leading to increase in CNS side effects Accumulation leading to increase in CNS side effects Antihypertensives Hypotension. May exacerbate renal hypoperfusion. Risk of bradycardia with - blockers. Tinnitus & deafness (usually with high doses and rapid IV administration), Altered haemodynamics. Can impair the kidneys ability to maintain GFR when perfusion is compromised. Overdiuresis leading to hypoperfusion of the kidneys can cause or exacerbate AKI. Consider withholding / reduce dose depending on clinical signs Some patients who continue taking -blockers during an episode of AKI have developed complete heart block and required temporary pacing. In some situations, e.g. heart failure with a decent blood pressure, continuing these agents might actually be helpful. If patient is hypertensive, consider alternative antihypertensive agents, eg, calcium channel blockers, thiazide-type diuretics, alfa-blockers, beta-blockers if appropriate Loop diuretics (furosemide & bumetanide) preferred as thiazides less effective if GFR < 25ml/min. However thiazides can potentiate the effects of loop diuretics. Higher doses may be needed to achieve a diuresis in patients with Guidelines for medicines optimisation in patients with acute kidney injury 9
10 fluid overload. Potassium sparing Diuretics (Amiloride, spironolactone, eplerenone) Hypoglycaemic agents Metformin Contrast Media Calcineurin inhibitors e.g. ciclosporin, tacrolimus Methotrexate Hyperkalaemia Increased risk of hyperkalaemia Accumulation leading to hypoglycaemia Lactic acidosis. Accumulation leading to hypoglycaemia Hypoperfusion of the kidneys Direct tubular toxic effect. Incidence of CIN higher with high- & low-osmolar contrast media, and lower with iso-osmolar, non-ionic contrast media Immunosuppressants (DMARDs, chemotherapy) Increased risk of neurotoxicity Increased risk of nephrotoxicity Accumulation leading to e.g. excessive bone marrow Crystal nephropathy Avoid Avoid long-acting preparations. Monitor blood glucose levels & if necessary Avoid if GFR < 30 ml/min Seek nephrologist advice if undergoing contrast procedure or at risk of AKI Ensure patient is well hydrated preexposure to contrast, PROVIDED the patient is able to tolerate IV fluids. This is NOT recommended for patients with congestive heart failure pre-coronary angiogram. IV sodium chloride or sodium bicarbonate are most effective Seek advice of transplant centre regarding monitoring levels and dose adjustment Avoid especially if patient at risk of hyperkalaemia Guidelines for medicines optimisation in patients with acute kidney injury 10
11 Allopurinol 5 aminosalicylates Antihistamines, Anti-psychotics, Antispasmodics Ayurvedic medicines Bisphosphonates IV Colchicine suppression, mucositis, acute hepatic toxicity, acute interstitial pneumonitis Others Accumulation of allopurinol and its metabolites leading to agranulocytosis, aplastic anaemia, thrombocytopenia Anticholinergic side effects. Urinary retention. Some ayurvedic medicines also contain heavy metals Diarrhoea / vomiting causing hypovolaemia Acute interstitial nephritis (rare) Tubular and glomerular damage. Acute interstitial nephritis (rare) Cases of renal impairment have been reported Can cause impaired renal function especially when given in high doses and short duration infusions Exacerbates hypoperfusion if also taking a NSAID Monitor levels and consider folinic acid rescue Correct fluid balance Start at a low dose to avoid severe rash, but can then usually safely be titrated up against serum urate Avoid Avoid XL preparations Digoxin 1. Aggravates hyperkalaemia May accumulate leading to Avoid Check drug history thoroughly Patients may not consider herbal preparations / teas as medicines and infuse at correct rate Advantages of correction of severe hypercalcaemia may outweigh risks: seek specialist advice Low doses e.g. 500mcg bd or tds are effective. Do not use NSAIDs for gout; if Colchicine causes unacceptable adverse effects, consider a short course of corticosteroids Guidelines for medicines optimisation in patients with acute kidney injury 11
12 bradycardia, visual disturbances, mental confusion Monitor potassium & drug levels Herbal preparations Lipid-lowering agents e.g. fibrates, statins Lithium May cause AKI if rhabdomyolysis present Hypernatraemia. AKI exacerbated in hypovolaemia and in combination with ACE inhibitors / ARB / NSAIDs Cat s Claw has antiinflammatory properties and has been implicated in causing AKI and hypotension with antihypertensives Possible increased risk of rhabdomyolysis Accumulation leading to nausea, diarrhoea, blurred vision, fine resting tremor, muscular weakness and drowsiness, increasing confusion, blackouts, fasciculation and increased deep tendon reflexes, myoclonic twitches and jerks, choreoathetoid movements, urinary or faecal incontinence, The toxic effects of herbal remedies to the kidneys may be exacerbated when used with concomitant medicines which can affect kidney function. Chinese herbal medicines with aristocholic acid have been implicated in interstitial nephritis. Chronic interstitial nephropathy (rare) Some herbal medicines also interact with prescribed medicines, eg. St. John s Wort potentiates the effects of ciclosporin & tacrolimus. Check drug history thoroughly. Patients may not consider herbal preparations / teas as medicines Stop if AKI due to rhabdomyolysis. Otherwise, continue therapy but monitor. Stop if patient develops unexplained / persistent muscle pain Avoid where possible Monitor lithium levels Seek advice for alternative Encourage patient to drink plenty. Patients on long-term lithium nearly always have a degree of diabetes insipidus, and are therefore at serious risk of developing hypernatraemia due to true dehydration when unwell without adequate water intake. Guidelines for medicines optimisation in patients with acute kidney injury 12
13 Nitrates / Nicorandil Low molecular weight heparins Warfarin increasing restlessness followed by stupor. Very rarely, it is associated with neuroleptic malignant syndrome. Hypotension Anticoagulants Risk of accumulation leading to increased risk of bleeding INR may be raised due to acute rise in urea and warfarin displacement from binding sites May exacerbate hypoperfusion Monitor serum sodium concentration Consider withholding / reduce dose depending on clinical signs Monitor anti-xa levels and consider reducing dose or switching to an alternative agent as per local guidelines Monitor INR and consider reducing dose or withholding depending on indication for use Direct Oral Anticoagulants May accumulate leading to increased risk of bleeding. Consider withholding, particularly agents with high renal clearance. Routine blood testing does not detect those people at high risk of bleeding Guidelines for medicines optimisation in patients with acute kidney injury 13
14 1. Conclusion These guidelines are not exhaustive and are only intended to act as an aide memoire to the medicines optimisation of patients with AKI. For further advice, please contact a renal pharmacist or nephrologist. 2. Acknowledgements Acknowledgements and thanks go to the following members of the Think Kidneys Intervention Workstream for their contribution to this publication: Caroline Ashley, Renal Pharmacist, Royal Free London NHS Foundation Trust Marlies Ostermann, Consultant in Nephrology and Critical Care, Guys and St Thomas NHS Foundation Trust Sue Shaw, Renal Pharmacist, Derby Teaching Hospitals, NHS Foundation Trust Thanks also go to the UK Renal Pharmacy Group, who developed the original AKI pharmacists toolkit and allowed us to tailor this specifically for the Think Kidneys programme. Guidelines for medicines optimisation in patients with acute kidney injury 14
15 Checklist for medicines optimisation in patients with acute kidney injury (AKI) 1. Is the patient on any of the following medications? ACEI ARB Diuretics NSAIDs Metformin Aminoglycosides Consider withholding them discuss with the medical team 2. Is the patient taking any other medications which could exacerbate AKI? Consider withholding them 3. Is the patient prescribed any medications where the dose needs to be amended in renal impairment? Amend doses appropriate to level of renal function 4. Monitor U&Es & re-assess renal function daily 5. Monitor blood levels of relevant drugs e.g. Aminoglycosides 6. Ensure the patient is counselled before discharge in regards to which medications to restart and when, and which medications to avoid 7. Ensure comprehensive information on which medications to restart and when is communicated via the discharge summary to the GP and/or next care setting Guidelines for medicines optimisation in patients with acute kidney injury 15
Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury in Secondary Care
 Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury in Secondary Care Publication date 01.06.2015 Guidelines for Medicines Optimisation in Patients with Acute Kidney injury in Secondary
Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury in Secondary Care Publication date 01.06.2015 Guidelines for Medicines Optimisation in Patients with Acute Kidney injury in Secondary
Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury in Secondary Care
 Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury in Secondary Care Caroline Ashley Renal Pharmacist, Royal Free London NHS Foundation Trust Marlies Ostermann Consultant in Nephrology
Guidelines for Medicines Optimisation in Patients with Acute Kidney Injury in Secondary Care Caroline Ashley Renal Pharmacist, Royal Free London NHS Foundation Trust Marlies Ostermann Consultant in Nephrology
Southern Derbyshire Shared Care Pathology Guidelines. AKI guidelines for primary care
 Southern Derbyshire Shared Care Pathology Guidelines AKI guidelines for primary care Contents: Flow Diagram: Recommended response time to AKI warning stage test results for adults in primary care 2 Table
Southern Derbyshire Shared Care Pathology Guidelines AKI guidelines for primary care Contents: Flow Diagram: Recommended response time to AKI warning stage test results for adults in primary care 2 Table
Doncaster & Bassetlaw. AKI guidelines for primary care
 Doncaster & Bassetlaw AKI guidelines for primary care Contents: FLOW DIAGRAM: MANAGEMENT OF PATIENTS WITH AKI DETECTED IN PRIMARY CARE... 2 FLOW DIAGRAM: MANAGEMENT OF HYPERKALAEMIA.... 3 FLOW DIAGRAM:
Doncaster & Bassetlaw AKI guidelines for primary care Contents: FLOW DIAGRAM: MANAGEMENT OF PATIENTS WITH AKI DETECTED IN PRIMARY CARE... 2 FLOW DIAGRAM: MANAGEMENT OF HYPERKALAEMIA.... 3 FLOW DIAGRAM:
keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests
 www.bpac.org.nz keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests Why do we monitor patients taking diuretics and what do we monitor? Monitoring a person on
www.bpac.org.nz keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests Why do we monitor patients taking diuretics and what do we monitor? Monitoring a person on
STOPP START Toolkit Supporting Medication Review in the Older Person
 STOPP START Toolkit Supporting Medication Review in the Older Person STOPP: Screening Tool of Older People s potentially inappropriate Prescriptions START: Screening Tool to Alert doctors to Right (appropriate,
STOPP START Toolkit Supporting Medication Review in the Older Person STOPP: Screening Tool of Older People s potentially inappropriate Prescriptions START: Screening Tool to Alert doctors to Right (appropriate,
Acute Kidney Injury (AKI) Undergraduate nurse education
 Acute Kidney Injury (AKI) Undergraduate nurse education Year Three Developed Summer 2017 Objectives Understand Acute Kidney Injury and its relevance to patient care. Brief revision of the Anatomy and physiology
Acute Kidney Injury (AKI) Undergraduate nurse education Year Three Developed Summer 2017 Objectives Understand Acute Kidney Injury and its relevance to patient care. Brief revision of the Anatomy and physiology
Acute Renal Failure. Dr Kawa Ahmad
 62 Acute Renal Failure Dr Kawa Ahmad Acute Renal Failure It is characterised by an abrupt reduction (usually within a 48- h period) in kidney function. This results in an accumulation of nitrogenous waste
62 Acute Renal Failure Dr Kawa Ahmad Acute Renal Failure It is characterised by an abrupt reduction (usually within a 48- h period) in kidney function. This results in an accumulation of nitrogenous waste
Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE. Drug: AZATHIOPRINE Protocol number: CV 04
 Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE Drug: AZATHIOPRINE Protocol number: CV 04 Indication: RENAL, PANCREAS OR COMBINED RENAL PANCREAS TRANSPLANTATION LIVER
Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE Drug: AZATHIOPRINE Protocol number: CV 04 Indication: RENAL, PANCREAS OR COMBINED RENAL PANCREAS TRANSPLANTATION LIVER
Factsheet Sacubitril valsartan (Entresto ) for chronic heart failure with reduced ejection fraction
 North Central London Joint Formulary Committee Factsheet Sacubitril valsartan (Entresto ) for chronic heart failure with reduced ejection fraction Start date: July 2016 Review date: July 2019 Document
North Central London Joint Formulary Committee Factsheet Sacubitril valsartan (Entresto ) for chronic heart failure with reduced ejection fraction Start date: July 2016 Review date: July 2019 Document
Patient details GP details Specialist details Name GP Name Dr Specialist Name Dr R. Horton
 Rationale for Initiation, Continuation and Discontinuation (RICaD) Sacubitril/Valsartan (Entresto) For the treatment of symptomatic heart failure with reduced ejection fraction (NICE TA388) This document
Rationale for Initiation, Continuation and Discontinuation (RICaD) Sacubitril/Valsartan (Entresto) For the treatment of symptomatic heart failure with reduced ejection fraction (NICE TA388) This document
MATRIX (Methotrexate, Cytarabine, Thiotepa and Rituximab)
 MATRIX (Methotrexate, Cytarabine, Thiotepa and Rituximab) Indication First line treatment of primary CNS lymphoma. ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 and 6 Rituximab
MATRIX (Methotrexate, Cytarabine, Thiotepa and Rituximab) Indication First line treatment of primary CNS lymphoma. ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 and 6 Rituximab
Shared Care Guideline Metolazone for fluid management in CKD (Adults)
 Shared Care Guideline Metolazone for fluid management in CKD (Adults) It is vital for safe and appropriate patient care that there is a clear understanding of where clinical and prescribing responsibility
Shared Care Guideline Metolazone for fluid management in CKD (Adults) It is vital for safe and appropriate patient care that there is a clear understanding of where clinical and prescribing responsibility
Cisplatin and Gemcitabine (bladder)
 Cisplatin and Gemcitabine (bladder) Indication Palliative therapy for locally advanced or metastatic bladder cancer in patients with good renal function. Palliative therapy for urothelial transitional
Cisplatin and Gemcitabine (bladder) Indication Palliative therapy for locally advanced or metastatic bladder cancer in patients with good renal function. Palliative therapy for urothelial transitional
Frimley Health Area Prescribing Committee
 Frimley Health Area Prescribing Committee Frimley Health NHS Foundation Trust North East Hampshire and Farnham CCG East Berkshire CCG Surrey Heath CCG Buckinghamshire CCG SHARED CARE Guideline Amber Traffic
Frimley Health Area Prescribing Committee Frimley Health NHS Foundation Trust North East Hampshire and Farnham CCG East Berkshire CCG Surrey Heath CCG Buckinghamshire CCG SHARED CARE Guideline Amber Traffic
BACKGROUND Measuring renal function :
 A GUIDE TO USE OF COMMON PALLIATIVE CARE DRUGS IN RENAL IMPAIRMENT These guidelines bring together information and recommendations from the Palliative Care formulary (PCF5 ) BACKGROUND Measuring renal
A GUIDE TO USE OF COMMON PALLIATIVE CARE DRUGS IN RENAL IMPAIRMENT These guidelines bring together information and recommendations from the Palliative Care formulary (PCF5 ) BACKGROUND Measuring renal
Dorset Health Technologies Forum SHARED CARE GUIDELINE FOR PRESCRIBING EPLERENONE (INSPRA )
 INDICATION SHARED CARE GUIDELINE FOR PRESCRIBING EPLERENONE (INSPRA ) Eplerenone is an aldosterone antagonist licensed to be used in addition to standard therapy including beta-blockers, to reduce the
INDICATION SHARED CARE GUIDELINE FOR PRESCRIBING EPLERENONE (INSPRA ) Eplerenone is an aldosterone antagonist licensed to be used in addition to standard therapy including beta-blockers, to reduce the
WEEK. MPharm Programme. Acute Kidney Injury. Alan M. Green MPHM13: Acute Kidney Injury. Slide 1 of 47
 MPharm Programme Acute Kidney Injury Alan M. Green 2017 Slide 1 of 47 Overview Renal Function What is it? Why does it matter? What causes it? Who is at risk? What can we (Pharmacists) do? How do you recognise
MPharm Programme Acute Kidney Injury Alan M. Green 2017 Slide 1 of 47 Overview Renal Function What is it? Why does it matter? What causes it? Who is at risk? What can we (Pharmacists) do? How do you recognise
Composition: Each Tablet contains. Pharmacokinetic properties:
 Composition: Each Tablet contains Torsemide 5/10/20/40/100mg Pharmacokinetic properties: Torsemide is well absorbed from the gastrointestinal tract. Peak serum concentrations are achieved within 1 hour
Composition: Each Tablet contains Torsemide 5/10/20/40/100mg Pharmacokinetic properties: Torsemide is well absorbed from the gastrointestinal tract. Peak serum concentrations are achieved within 1 hour
Renal Transporters- pathophysiology of drug - induced renal disorders. Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November
 Renal Transporters- pathophysiology of drug - induced renal disorders Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November Renal Failure Up to 25% of acute renal failure is drug induced
Renal Transporters- pathophysiology of drug - induced renal disorders Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November Renal Failure Up to 25% of acute renal failure is drug induced
Chronic Kidney Disease in Primary Care
 Clinical Stream Chronic Kidney Disease in Primary Care Dr Gerald Waters Dr Gerald Waters Renal Physician Chronic Kidney Disease Chronic Kidney Disease Normal functions of Kidneys Management of CKD Drugs
Clinical Stream Chronic Kidney Disease in Primary Care Dr Gerald Waters Dr Gerald Waters Renal Physician Chronic Kidney Disease Chronic Kidney Disease Normal functions of Kidneys Management of CKD Drugs
CKD FOR INTERNISTS. Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College
 CKD FOR INTERNISTS Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College INTRODUCTION In 2002, the National Kidney Foundation s Kidney Disease Outcomes Quality Initiative(KDOQI)
CKD FOR INTERNISTS Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College INTRODUCTION In 2002, the National Kidney Foundation s Kidney Disease Outcomes Quality Initiative(KDOQI)
StRs and CT doctors in haematology. September Folinic acid dose modified.
 High dose Methotrexate and folinic acid rescue Full Title of Guideline: Author (include email and role): Division & Speciality: Clinical Guideline Review Date September 2018 GUIDELINE FOR THE USE OF HIGH
High dose Methotrexate and folinic acid rescue Full Title of Guideline: Author (include email and role): Division & Speciality: Clinical Guideline Review Date September 2018 GUIDELINE FOR THE USE OF HIGH
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck
 Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
Management of early chronic kidney disease
 Management of early chronic kidney disease GREENLANE SUMMER GP SYMPOSIUM 2018 Jonathan Hsiao Renal and General Physician Introduction A growing public health problem in NZ and throughout the world. Unknown
Management of early chronic kidney disease GREENLANE SUMMER GP SYMPOSIUM 2018 Jonathan Hsiao Renal and General Physician Introduction A growing public health problem in NZ and throughout the world. Unknown
New Zealand Data Sheet. Arrow-Bendrofluazide Bendroflumethiazide (also known as Bendrofluazide) Tablets 2.5mg and 5mg
 New Zealand Data Sheet Arrow-Bendrofluazide Bendroflumethiazide (also known as Bendrofluazide) Tablets 2.5mg and 5mg Description Arrow-Bendrofluazide Tablets contain 2.5 and 5 mg of the active ingredient
New Zealand Data Sheet Arrow-Bendrofluazide Bendroflumethiazide (also known as Bendrofluazide) Tablets 2.5mg and 5mg Description Arrow-Bendrofluazide Tablets contain 2.5 and 5 mg of the active ingredient
Prescribing Guidelines Prescribing arrangement for the management of patients transferring from Secondary Care to Primary Care
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Chronic heart failure: management of chronic heart failure in adults in primary and secondary care (partial update)
 Chronic heart failure: management of chronic heart failure in adults in primary and secondary care (partial update) NICE guideline Apendix C The algorithms Draft for consultation, January 2010 Chronic
Chronic heart failure: management of chronic heart failure in adults in primary and secondary care (partial update) NICE guideline Apendix C The algorithms Draft for consultation, January 2010 Chronic
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix
 Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer
 Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer Page 1 of 5 Indication: Confirmed HER2-positive (3+ or FISH+) metastatic adenocarcinoma of the stomach or gastrooesophageal junction. Patient
Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer Page 1 of 5 Indication: Confirmed HER2-positive (3+ or FISH+) metastatic adenocarcinoma of the stomach or gastrooesophageal junction. Patient
PRESCRIBING GUIDANCE METHOTREXATE for the treatment of vasculitis
 PRESCRIBING GUIDANCE METHOTREXATE for the treatment of vasculitis For the latest information on interactions and adverse effects, always consult the latest version of the Summary of Product Characteristics
PRESCRIBING GUIDANCE METHOTREXATE for the treatment of vasculitis For the latest information on interactions and adverse effects, always consult the latest version of the Summary of Product Characteristics
Mycophenolate Mofetil (MMF)
 SCG: For Transplant patients The following guidelines are designed to provide information relating to mycophenolate mofetil and to outline the responsibilities of the primary and secondary care teams in
SCG: For Transplant patients The following guidelines are designed to provide information relating to mycophenolate mofetil and to outline the responsibilities of the primary and secondary care teams in
Ciclosporin for Rheumatology and Dermatology use (Adults)
 Shared Care Guideline Ciclosporin for Rheumatology and Dermatology use (Adults) Introduction This shared care agreement outlines the responsibilities between the specialist and the generalist for managing
Shared Care Guideline Ciclosporin for Rheumatology and Dermatology use (Adults) Introduction This shared care agreement outlines the responsibilities between the specialist and the generalist for managing
CLINICAL GUIDELINES ID TAG
 CLINICAL GUIDELINES ID TAG Title: Treatment of Hypomagnesaemia in adults Author: Speciality / Division: Directorate: Dr Peter Sharpe, Dr Neal Morgan, Jillian Redpath Chemical Pathology/Nephrology/Pharmacy
CLINICAL GUIDELINES ID TAG Title: Treatment of Hypomagnesaemia in adults Author: Speciality / Division: Directorate: Dr Peter Sharpe, Dr Neal Morgan, Jillian Redpath Chemical Pathology/Nephrology/Pharmacy
SCG: For Transplant patients AREAS OF RESPONSIBILITY FOR THE SHARING OF CARE
 SCG: For Transplant patients The following guidelines are designed to provide information relating to tacrolimus and to outline the responsibilities of the primary and secondary care teams in the prescribing
SCG: For Transplant patients The following guidelines are designed to provide information relating to tacrolimus and to outline the responsibilities of the primary and secondary care teams in the prescribing
R-IDARAM. Dexamethasone is administered as an IV infusion in 100mL sodium chloride 0.9% over 30 minutes.
 R-IDARAM Indication Secondary CNS lymphoma ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 Rituximab 375mg/m 2 IV infusion 1 Methotrexate 12.5mg Intrathecal 1 Cytarabine 70mg
R-IDARAM Indication Secondary CNS lymphoma ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 Rituximab 375mg/m 2 IV infusion 1 Methotrexate 12.5mg Intrathecal 1 Cytarabine 70mg
0BCore Safety Profile. Pharmaceutical form(s)/strength: Film-coated tablet 40, 80, 160, 320 mg SE/H/PSUR/0024/003 Date of FAR:
 0BCore Safety Profile Active substance: Valsartan Pharmaceutical form(s)/strength: Film-coated tablet 40, 80, 160, 320 mg P-RMS: SE/H/PSUR/0024/003 Date of FAR: 28.02.2013 4.2 Posology and method of administration
0BCore Safety Profile Active substance: Valsartan Pharmaceutical form(s)/strength: Film-coated tablet 40, 80, 160, 320 mg P-RMS: SE/H/PSUR/0024/003 Date of FAR: 28.02.2013 4.2 Posology and method of administration
NHS GG&C -Mental Health Services. Lithium Ward Bundle
 Lithium Ward Bundle Lithium is a useful drug, particularly in the maintenance treatment of bipolar affective disorder, recurrent depression and self injurious behaviour. It is widely used and most patients
Lithium Ward Bundle Lithium is a useful drug, particularly in the maintenance treatment of bipolar affective disorder, recurrent depression and self injurious behaviour. It is widely used and most patients
Cisplatin / 5-Fluorouracil for Vulval Cancer
 Cisplatin / 5-Fluorouracil for Vulval Cancer Indication: Palliative therapy in patients with Vulval Cancer Regimen details: Cisplatin 75mg/m 2 (*) IV 5-Fluorouracil (5-FU) 1000mg/m 2 /24 hours IV D4 (*)Consider
Cisplatin / 5-Fluorouracil for Vulval Cancer Indication: Palliative therapy in patients with Vulval Cancer Regimen details: Cisplatin 75mg/m 2 (*) IV 5-Fluorouracil (5-FU) 1000mg/m 2 /24 hours IV D4 (*)Consider
Oxaliplatin and Gemcitabine
 Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Policy on Pharmacological Therapies Practice Guidance Note Reducing Dosing Errors with Opioid Medicines V04
 Policy on Pharmacological Therapies Practice Guidance Note Reducing Dosing Errors with Opioid Medicines V04 Date issued Issue 1 Nov 2018 Planned review Nov 2021 PPT-PGN 18 part of NTW(C)38 Pharmaceutical
Policy on Pharmacological Therapies Practice Guidance Note Reducing Dosing Errors with Opioid Medicines V04 Date issued Issue 1 Nov 2018 Planned review Nov 2021 PPT-PGN 18 part of NTW(C)38 Pharmaceutical
Low Efficacy Diuretics. Potassium sparing diuretics. Carbonic anhydrase inhibitors. Osmotic diuretics. Miscellaneous
 University of Al Qadisiyah College of Pharmacy Dr. Bassim I Mohammad, MBChB, MSc, Ph.D Low Efficacy Diuretics 1. Potassium sparing diuretics 2. Carbonic anhydrase inhibitors 3. Osmotic diuretics 4. Miscellaneous
University of Al Qadisiyah College of Pharmacy Dr. Bassim I Mohammad, MBChB, MSc, Ph.D Low Efficacy Diuretics 1. Potassium sparing diuretics 2. Carbonic anhydrase inhibitors 3. Osmotic diuretics 4. Miscellaneous
CHRONIC KIDNEY DISEASE DIAGNOSIS
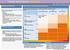 CHRONIC KIDNEY DISEASE DIAGSIS GFR categories, description and range WHO SHOULD BE TESTED FOR CKD CLASSIFICATION OF CKD USING egfr AND ACR CATEGORIES Offer testing for CKD using egfr, creatinine and ACR
CHRONIC KIDNEY DISEASE DIAGSIS GFR categories, description and range WHO SHOULD BE TESTED FOR CKD CLASSIFICATION OF CKD USING egfr AND ACR CATEGORIES Offer testing for CKD using egfr, creatinine and ACR
Appendix 4: Renal impairment
 Appendix 4: Renal impairment Reduced renal function may cause problems with drug therapy for the following reasons: 1. The failure to excrete a drug or its metabolites may produce toxicity. 2. The to some
Appendix 4: Renal impairment Reduced renal function may cause problems with drug therapy for the following reasons: 1. The failure to excrete a drug or its metabolites may produce toxicity. 2. The to some
Carboplatin and Fluorouracil
 Carboplatin and Fluorouracil Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer for patients where cisplatin and / or cetuximab are not appropriate. Performance
Carboplatin and Fluorouracil Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer for patients where cisplatin and / or cetuximab are not appropriate. Performance
PACKAGE LEAFLET: Information for the patient FUROSEMID Tablets - 40 mg Solution for injection - 20 mg / 2 ml (Furosemide)
 PACKAGE LEAFLET: Information for the patient FUROSEMID Tablets - 40 mg Solution for injection - 20 mg / 2 ml (Furosemide) Read this leaflet carefully before you start taking this medicine. - Keep this
PACKAGE LEAFLET: Information for the patient FUROSEMID Tablets - 40 mg Solution for injection - 20 mg / 2 ml (Furosemide) Read this leaflet carefully before you start taking this medicine. - Keep this
Analgesia in patients with impaired renal function Formulary Guidance
 Analgesia in patients with impaired renal function Formulary Guidance Approved by Trust D&TC: January 2010 Revised March 2017 Contents Paragraph Page 1 Aim 4 2 Introduction 4 3 Assessment of renal function
Analgesia in patients with impaired renal function Formulary Guidance Approved by Trust D&TC: January 2010 Revised March 2017 Contents Paragraph Page 1 Aim 4 2 Introduction 4 3 Assessment of renal function
PRESCRIBING IN THE ELDERLY. CARE HOME PHARMACY TEAM Bhavini Shah, Eleesha Pentiah & Puja Vyas
 PRESCRIBING IN THE ELDERLY CARE HOME PHARMACY TEAM Bhavini Shah, Eleesha Pentiah & Puja Vyas LEARNING OUTCOMES Medicines Optimisation The effects of aging on health and medicines. Polypharmacy Acute Kidney
PRESCRIBING IN THE ELDERLY CARE HOME PHARMACY TEAM Bhavini Shah, Eleesha Pentiah & Puja Vyas LEARNING OUTCOMES Medicines Optimisation The effects of aging on health and medicines. Polypharmacy Acute Kidney
Diabetes in Renal Patients. Contents. Understanding Diabetic Nephropathy
 Diabetes in Renal Patients Contents Understanding Diabetic Nephropathy What effect does CKD have on a patient s diabetic control? Diabetic Drugs in CKD and Dialysis Patients Hyper and Hypoglycaemia in
Diabetes in Renal Patients Contents Understanding Diabetic Nephropathy What effect does CKD have on a patient s diabetic control? Diabetic Drugs in CKD and Dialysis Patients Hyper and Hypoglycaemia in
STOPP and START criteria October 2011
 # START and STOPP are newer criteria to identify potentially inappropriate medications in elderly, including drug drug and drug disease interactions, drugs which increase risk of falls and drugs which
# START and STOPP are newer criteria to identify potentially inappropriate medications in elderly, including drug drug and drug disease interactions, drugs which increase risk of falls and drugs which
Nephrology. 3 rd Year Revision Session 06/05/17 Cathal Hannan
 Nephrology 3 rd Year Revision Session 06/05/17 Cathal Hannan Aims Acute Kidney Injury-recognition and management Sample OSCE Station Clinically relevant renal physiology Aetiology of Chronic Kidney Disease
Nephrology 3 rd Year Revision Session 06/05/17 Cathal Hannan Aims Acute Kidney Injury-recognition and management Sample OSCE Station Clinically relevant renal physiology Aetiology of Chronic Kidney Disease
Acute Kidney Injury shared guidance
 Acute Kidney Injury shared guidance Acute Kidney Injury (AKI) Fluid balance assessment (NICE CG 169) Assess the patient s likely fluid and electrolyte needs 1.History previous limited intake, thirst, abnormal
Acute Kidney Injury shared guidance Acute Kidney Injury (AKI) Fluid balance assessment (NICE CG 169) Assess the patient s likely fluid and electrolyte needs 1.History previous limited intake, thirst, abnormal
Swindon Diabetes Guidelines: Management of Chronic Kidney Disease Associated with Diabetes Mellitus
 Swindon Diabetes Guidelines: Management of Chronic Kidney Disease Associated with Diabetes Mellitus 1 Contents Executive Summary... 3 How to Screen for Diabetic Nephropathy... 4 What to Measure... 4 Frequency
Swindon Diabetes Guidelines: Management of Chronic Kidney Disease Associated with Diabetes Mellitus 1 Contents Executive Summary... 3 How to Screen for Diabetic Nephropathy... 4 What to Measure... 4 Frequency
Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors:
 INDICATION High grade lymphoma with high risk of CNS involvement Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors: Age > 60 Raised serum
INDICATION High grade lymphoma with high risk of CNS involvement Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors: Age > 60 Raised serum
Cisplatin / 5-Fluorouracil (+ Trastuzumab) in Gastric Cancer
 Cisplatin / 5-Fluorouracil (+ Trastuzumab) in Gastric Cancer Indication: Confirmed HER2-positive (3+ or FISH+) metastatic adenocarcinoma of the stomach or gastro-oesophageal junction. For Trastuzumab doses
Cisplatin / 5-Fluorouracil (+ Trastuzumab) in Gastric Cancer Indication: Confirmed HER2-positive (3+ or FISH+) metastatic adenocarcinoma of the stomach or gastro-oesophageal junction. For Trastuzumab doses
ZAROXOLYN 5mg tablets ZAROXOLYN 10 mg tablets Metolazone. PHARMACOTHERAPEUTIC CATEGORY Diuretic with minor diuretic action, non-associated.
 ZAROXOLYN 5mg tablets ZAROXOLYN 10 mg tablets Metolazone PHARMACOTHERAPEUTIC CATEGORY Diuretic with minor diuretic action, non-associated. THERAPEUTIC INDICATIONS Zaroxolyn is indicated in the treatment
ZAROXOLYN 5mg tablets ZAROXOLYN 10 mg tablets Metolazone PHARMACOTHERAPEUTIC CATEGORY Diuretic with minor diuretic action, non-associated. THERAPEUTIC INDICATIONS Zaroxolyn is indicated in the treatment
Prescribing Framework for Ciclosporin Post Solid Organ Transplant
 Hull & East Riding Prescribing Committee Prescribing Framework for Ciclosporin Post Solid Organ Transplant Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker) GP s Name:...
Hull & East Riding Prescribing Committee Prescribing Framework for Ciclosporin Post Solid Organ Transplant Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker) GP s Name:...
Gemcitabine, Carboplatin and Bevacizumab (gynae)
 Gemcitabine, Carboplatin and Bevacizumab (gynae) Indication Second line advanced epithelial ovarian, fallopian tube or primary peritoneal cancer. WHO performance status 0 or 1. (Funding via the CDF) ICD-10
Gemcitabine, Carboplatin and Bevacizumab (gynae) Indication Second line advanced epithelial ovarian, fallopian tube or primary peritoneal cancer. WHO performance status 0 or 1. (Funding via the CDF) ICD-10
Bulletin Independent prescribing information for NHS Wales
 Bulletin Independent prescribing information for NHS Wales October 2017 Chronic kidney disease Chronic kidney disease (CKD) is a long-term irreversible deterioration in the function of the kidneys, often
Bulletin Independent prescribing information for NHS Wales October 2017 Chronic kidney disease Chronic kidney disease (CKD) is a long-term irreversible deterioration in the function of the kidneys, often
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES
 GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
Cisplatin and Vinorelbine and radiotherapy (NSCLC)
 Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Cisplatin and Vinorelbine and radiotherapy (NSCLC)
 Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults. Consultation Group: See Page 4. Review Date: June 2021
 NHS...... Grampian NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Consultation Group: Approver:. Senior Medicines Information Pharmacist See Page 4 Medicine
NHS...... Grampian NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Consultation Group: Approver:. Senior Medicines Information Pharmacist See Page 4 Medicine
Polypharmacy: Guidance for Prescribing in Frail Adults
 Polypharmacy: Guidance for Prescribing in Frail Adults Why is reviewing polypharmacy important? Medication is by far the most common form of medical intervention. Four out of five people aged over 75 years
Polypharmacy: Guidance for Prescribing in Frail Adults Why is reviewing polypharmacy important? Medication is by far the most common form of medical intervention. Four out of five people aged over 75 years
ECX. Anti-emetics: Day 1: highly emetogenic Days 2 21: mildly emetogenic
 Page 1 of 5 As an alternative to ECF: For locally advanced (inoperable) or metastatic oesophageal or gastric cancer; peri-operative use in oesophageal or gastric cancer; adenocarcinoma of unknown primary
Page 1 of 5 As an alternative to ECF: For locally advanced (inoperable) or metastatic oesophageal or gastric cancer; peri-operative use in oesophageal or gastric cancer; adenocarcinoma of unknown primary
ELECTROLYTES RENAL SHO TEACHING
 ELECTROLYTES RENAL SHO TEACHING Metabolic Alkalosis 2 factors are responsible for generation and maintenance of metabolic alkalosis this includes a process that raises serum bicarbonate and a process that
ELECTROLYTES RENAL SHO TEACHING Metabolic Alkalosis 2 factors are responsible for generation and maintenance of metabolic alkalosis this includes a process that raises serum bicarbonate and a process that
Rituximab, Gemcitabine, Dexamethasone and Cisplatin RGDP Regimen
 Rituximab, Gemcitabine, Dexamethasone and Cisplatin RGDP Regimen Available for Routine Use in Burton in-patient N/A Derby in-patient Burton day-case Derby day-case Burton outreach chemotherapy clinic N/A
Rituximab, Gemcitabine, Dexamethasone and Cisplatin RGDP Regimen Available for Routine Use in Burton in-patient N/A Derby in-patient Burton day-case Derby day-case Burton outreach chemotherapy clinic N/A
CHEMOTHERAPY PROTOCOL FOR ADMINISTRATION OF VENETOCLAX
 CHEMOTHERAPY PROTOCOL FOR ADMINISTRATION OF VENETOCLAX Therapeutic Indications: Venetoclax monotherapy is indicated for the treatment of chronic lymphocytic leukaemia (CLL) in the presence of 17p deletion
CHEMOTHERAPY PROTOCOL FOR ADMINISTRATION OF VENETOCLAX Therapeutic Indications: Venetoclax monotherapy is indicated for the treatment of chronic lymphocytic leukaemia (CLL) in the presence of 17p deletion
TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour
 TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour Indication: Second line therapy in Relapsed Germ Cell Tumours Regimen details: Paclitaxel 175mg/m 2 IV Cisplatin 20mg/m 2 IV - D5 Ifosfamide
TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour Indication: Second line therapy in Relapsed Germ Cell Tumours Regimen details: Paclitaxel 175mg/m 2 IV Cisplatin 20mg/m 2 IV - D5 Ifosfamide
Tacrolimus. Information for patients about using the drug Tacrolimus.
 Tacrolimus Information for patients about using the drug Tacrolimus. Your consultant has recommended you start treatment with tacrolimus. This leaflet has been developed to provide you with additional
Tacrolimus Information for patients about using the drug Tacrolimus. Your consultant has recommended you start treatment with tacrolimus. This leaflet has been developed to provide you with additional
CHRONIC KIDNEY DISEASE DIAGNOSIS
 CHRONIC KIDNEY DISEASE DIAGSIS WHO SHOULD BE TESTED FOR CKD Offer testing for CKD using egfr, serum creatinine and urinary ACR to people with any of the following risk factors: diabetes hypertension acute
CHRONIC KIDNEY DISEASE DIAGSIS WHO SHOULD BE TESTED FOR CKD Offer testing for CKD using egfr, serum creatinine and urinary ACR to people with any of the following risk factors: diabetes hypertension acute
Prescribing Dilemmas. Sue Mulvenna Head of Pharmacy and CD Accountable Officer South Region SW 19 th May
 Prescribing Dilemmas Sue Mulvenna Head of Pharmacy and CD Accountable Officer South Region SW 19 th May 2016 The four principles of medicines optimisation Aim to understand the patient s experience Evidence
Prescribing Dilemmas Sue Mulvenna Head of Pharmacy and CD Accountable Officer South Region SW 19 th May 2016 The four principles of medicines optimisation Aim to understand the patient s experience Evidence
COMMON DRUG RELATED PROBLEMS SEEN IN PACE AND MECHANISMS TO MITIGATE RISK
 COMMON DRUG RELATED PROBLEMS SEEN IN PACE AND MECHANISMS TO MITIGATE RISK Robert L Alesiani, PharmD, CGP Chief Pharmacotherapy Officer CareKinesis, Inc. (a Tabula Rasa Healthcare Company) 2 3 4 5 Pharmacogenomics
COMMON DRUG RELATED PROBLEMS SEEN IN PACE AND MECHANISMS TO MITIGATE RISK Robert L Alesiani, PharmD, CGP Chief Pharmacotherapy Officer CareKinesis, Inc. (a Tabula Rasa Healthcare Company) 2 3 4 5 Pharmacogenomics
Acute Kidney Injury in The Acute Oncology Patient
 Acute Kidney Injury in The Acute Oncology Patient Dr Andrew Lewington BSc MEd MD FRCP Consultant Renal Physician/Honorary Senior Lecturer Leeds Teaching Hospitals Definition Definitions and terminology
Acute Kidney Injury in The Acute Oncology Patient Dr Andrew Lewington BSc MEd MD FRCP Consultant Renal Physician/Honorary Senior Lecturer Leeds Teaching Hospitals Definition Definitions and terminology
Heart Failure (HF) - Primary Care Flow Charts. Pre diagnosis Symptoms or signs suggestive of HF
 Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts. Symptoms or signs suggestive of HF. Pre diagnosis. Refer to the Heart Failure Clinic at VHK for
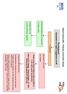 Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
GENERAL PRACTITIONER (GP) SUPPORT PACK
 Document Reference MM 050 Integrated Care Pathway (ICP) for the Management of clozapine GENERAL PRACTITIONER (GP) SUPPORT PACK One pack to be printed for each patient initiated on clozapine treatment,
Document Reference MM 050 Integrated Care Pathway (ICP) for the Management of clozapine GENERAL PRACTITIONER (GP) SUPPORT PACK One pack to be printed for each patient initiated on clozapine treatment,
BURINEX PRODUCT INFORMATION NAME OF THE MEDICINE DESCRIPTION PHARMACOLOGY. Pharmacodynamics. (bumetanide)
 NAME OF THE MEDICINE BURINEX PRODUCT INFORMATION (bumetanide) Burinex 1 mg tablets contain bumetanide i.e. 3-n-(butylamino)-4-phenoxy-5-sulphamoyl-benzoic acid. The structural formula of bumetanide is:
NAME OF THE MEDICINE BURINEX PRODUCT INFORMATION (bumetanide) Burinex 1 mg tablets contain bumetanide i.e. 3-n-(butylamino)-4-phenoxy-5-sulphamoyl-benzoic acid. The structural formula of bumetanide is:
Cisplatin and Pemetrexed (NSCLC, mesothelioma)
 Cisplatin and Pemetrexed (NSCLC, mesothelioma) Indication First-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) if the histology of the tumour has been confirmed as
Cisplatin and Pemetrexed (NSCLC, mesothelioma) Indication First-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) if the histology of the tumour has been confirmed as
Ketorolac injection. Supportive care
 Supportive care Ketorolac injection Supportive care: specialist medicines This leaflet provides information on a medicine called ketorolac which is used to treat pain that is difficult to control. It is
Supportive care Ketorolac injection Supportive care: specialist medicines This leaflet provides information on a medicine called ketorolac which is used to treat pain that is difficult to control. It is
Medical therapy of AKI complications. Refik Gökmen AKI Academy 18 October 2014
 Medical therapy of AKI complications Refik Gökmen AKI Academy 18 October 2014 Medical therapy of AKI complications Hyperkalaemia Volume status, fluid therapy Acidosis Calcium & phosphate Bleeding risk
Medical therapy of AKI complications Refik Gökmen AKI Academy 18 October 2014 Medical therapy of AKI complications Hyperkalaemia Volume status, fluid therapy Acidosis Calcium & phosphate Bleeding risk
Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x 4 (3-weekly) in Early Breast Cancer
 Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x Indication: Neoadjuvant or adjuvant therapy for moderate to high risk node positive breast
Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x Indication: Neoadjuvant or adjuvant therapy for moderate to high risk node positive breast
1/21/2016 UPDATE ON THE AMERICAN GERIATRICS SOCIETY 2015 BEERS CRITERIA DISCLOSURE OBJECTIVES AGING GOALS BEERS CRITERIA
 UPDATE ON THE AMERICAN GERIATRICS SOCIETY 2015 BEERS CRITERIA DISCLOSURE I have no financial conflict of interest to disclose. Lacey Charbonneau, Pharm.D. PGY-1 Community Practice Resident Baptist Medical
UPDATE ON THE AMERICAN GERIATRICS SOCIETY 2015 BEERS CRITERIA DISCLOSURE I have no financial conflict of interest to disclose. Lacey Charbonneau, Pharm.D. PGY-1 Community Practice Resident Baptist Medical
NPSA SAFER LITHIUM THERAPY GUIDELINES FOR NHS LANARKSHIRE
 NPSA SAFER LITHIUM THERAPY GUIDELINES FOR NHS LANARKSHIRE Implementation Date Spring 2013 Review Date Spring 2016 NPSA Safer Lithium Therapy Guidelines for NHS Lanarkshire BACKGROUND On 1 st December 2009
NPSA SAFER LITHIUM THERAPY GUIDELINES FOR NHS LANARKSHIRE Implementation Date Spring 2013 Review Date Spring 2016 NPSA Safer Lithium Therapy Guidelines for NHS Lanarkshire BACKGROUND On 1 st December 2009
TIP Paclitaxel, Ifosfamide and Cisplatin
 Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
INTRAVENOUS FLUIDS PRINCIPLES
 INTRAVENOUS FLUIDS PRINCIPLES Postnatal physiological weight loss is approximately 5-10% Postnatal diuresis is delayed in Respiratory Distress Syndrome (RDS) Preterm babies have limited capacity to excrete
INTRAVENOUS FLUIDS PRINCIPLES Postnatal physiological weight loss is approximately 5-10% Postnatal diuresis is delayed in Respiratory Distress Syndrome (RDS) Preterm babies have limited capacity to excrete
Specialist Palliative Care Audit and Guidelines Group (SPAGG)
 Specialist Palliative Care Audit and Guidelines Group (SPAGG) Clinical Guideline for the Prescribing and Administration of Furosemide via continuous subcutaneous infusion (CSCI) for Heart Failure Patients
Specialist Palliative Care Audit and Guidelines Group (SPAGG) Clinical Guideline for the Prescribing and Administration of Furosemide via continuous subcutaneous infusion (CSCI) for Heart Failure Patients
DERBY-BURTON CANCER NETWORK CONTROLLED DOC NO:
 OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST. Systemic Anti Cancer Treatment Protocol. EDP + mitotane
 Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHAHANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical
Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHAHANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical
Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE. Drug: MYCOPHENOLATE MOFETIL/SODIUM Protocol number: CV 15
 Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE Drug: MYCOPHENOLATE MOFETIL/SODIUM Protocol number: CV 15 Indication: RENAL, PANCREAS OR COMBINED RENAL PANCREAS TRANSPLANTATION
Cardiff & Vale (C&V) UHB Corporate Medicines Management Group (c MMG) SHARED CARE Drug: MYCOPHENOLATE MOFETIL/SODIUM Protocol number: CV 15 Indication: RENAL, PANCREAS OR COMBINED RENAL PANCREAS TRANSPLANTATION
Breast Pathway Group EC x 4: Epirubicin & Cyclophosphamide in Early Breast Cancer
 Breast Pathway Group EC x 4: & in Early Breast Cancer Indication: Neoadjuvant or adjuvant treatment for moderate to high risk breast cancer Regimen details: 90 mg/m 2 IV Day 1 600 mg/m 2 IV Day 1 Administration:
Breast Pathway Group EC x 4: & in Early Breast Cancer Indication: Neoadjuvant or adjuvant treatment for moderate to high risk breast cancer Regimen details: 90 mg/m 2 IV Day 1 600 mg/m 2 IV Day 1 Administration:
Carboplatin / Liposomal Doxorubicin CARBO/CAELYX Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Carfilzomib and Dexamethasone (CarDex)
 Carfilzomib and Dexamethasone (CarDex) Indication Relapsed multiple myeloma for patients who have had only one previous line of therapy (that did not include bortezomib). (NICE TA457) ICD-10 codes Codes
Carfilzomib and Dexamethasone (CarDex) Indication Relapsed multiple myeloma for patients who have had only one previous line of therapy (that did not include bortezomib). (NICE TA457) ICD-10 codes Codes
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
PROFESSIONAL INFORMATION
 SCHEDULING STATUS: S1 PROPRIETARY NAME AND DOSAGE FORM: Aleve Tablets COMPOSITION: Each tablet contains naproxen sodium 220 mg (equivalent to 200 mg naproxen) CATEGORY AND CLASS: A / 2.7 Antipyretic or
SCHEDULING STATUS: S1 PROPRIETARY NAME AND DOSAGE FORM: Aleve Tablets COMPOSITION: Each tablet contains naproxen sodium 220 mg (equivalent to 200 mg naproxen) CATEGORY AND CLASS: A / 2.7 Antipyretic or
Renal Palliative Care Last Days of Life
 Renal Palliative Care Last Days of Life Introduction This guideline is an aid to clinical decision-making and good practice for patients with stage 4-5 chronic kidney disease (egfr
Renal Palliative Care Last Days of Life Introduction This guideline is an aid to clinical decision-making and good practice for patients with stage 4-5 chronic kidney disease (egfr
Paediatric Renal Disease
 Pharmacy Department Paediatric Renal Disease Andrew.wignell@nuh.nhs.uk NUH Pharmacy At the end of the session you should be able to: Determine the level/type of renal dysfunction in a child and what the
Pharmacy Department Paediatric Renal Disease Andrew.wignell@nuh.nhs.uk NUH Pharmacy At the end of the session you should be able to: Determine the level/type of renal dysfunction in a child and what the
Back to Basics: The Basics of Medication Monitoring
 DIAMOND PHARMACY SERVICES Back to Basics: The Basics of Medication Monitoring Presented by: Dr. Deborah Milito, Pharm. D., CGP. Director of Clinical and Consultant Services Skilled Division Annual Educational
DIAMOND PHARMACY SERVICES Back to Basics: The Basics of Medication Monitoring Presented by: Dr. Deborah Milito, Pharm. D., CGP. Director of Clinical and Consultant Services Skilled Division Annual Educational
Younger adults with a family history of premature artherosclerotic disease should have their cardiovascular risk factors measured.
 Appendix 2A - Guidance on Management of Hypertension Measurement of blood pressure All adults from 40 years should have blood pressure measured as part of opportunistic cardiovascular risk assessment.
Appendix 2A - Guidance on Management of Hypertension Measurement of blood pressure All adults from 40 years should have blood pressure measured as part of opportunistic cardiovascular risk assessment.
Ixazomib with Lenalidomide and Dexamethasone (IRd)
 Indication Ixazomib, with lenalidomide and dexamethasone, is recommended for use within the Cancer Drugs Fund as an option for treating multiple myeloma for patients who have already had 2 or 3 lines of
Indication Ixazomib, with lenalidomide and dexamethasone, is recommended for use within the Cancer Drugs Fund as an option for treating multiple myeloma for patients who have already had 2 or 3 lines of
