PROSTIN VR STERILE SOLUTION
|
|
|
- Buck Francis
- 5 years ago
- Views:
Transcription
1 PROSTIN VR STERILE SOLUTION PRODUCT INFORMATION PROSTIN VR STERILE SOLUTION (alprostadil; prostaglandin E 1 ) DESCRIPTION PROSTIN VR Sterile Solution contains 500 micrograms alprostadil in 1.0 ml of dehydrated alcohol. It is white to off-white crystalline powder with a melting point between 110 C and 116 C. Its solubility at 35 C is 8000 micrograms per 100 ml double distilled water. The chemical formula for PROSTIN VR is (11-a, 13E, 15S)-11, 15 dihydroxy-9-oxoprost-13-en- 1-oic acid, and its structural formula is as follows. The molecular weight of alprostadil is PHARMACOLOGY Alprostadil (also known as prostaglandin E 1 ) relaxes the ductus arteriosus in early post-natal life and supports its patency when continuously infused intravenously or intra-arterially in neonates with congenital heart defects who depend on a patent ductus for survival. The desired pharmacological effects are obtained with an initial dosage of 0.1 micrograms per kilogram per minute. Higher doses do not offer added benefit. Postnatally, the ductus arteriosus rapidly loses its responsiveness to alprostadil and consequently alprostadil appears to be most effective within 96 hours after birth, particularly when the pre-infusion arterial po 2 is less than 40 mm Hg. The estimated half-life of alprostadil is 5 to 10 minutes. Intravenously administered alprostadil is rapidly distributed and metabolised and the pulmonary vascular bed removes about 68% of the drug in a single pass. Alprostadil is weakly bound to serum albumin. The major route of elimination of alprostadil and its metabolites is via the kidneys. In laboratory animals and humans, alprostadil can lower blood pressure, probably by relaxing the smooth Commercial/Non-Commercial Page 1 of 6
2 muscle of the cardiovascular system. Alprostadil can elevate body temperature and this effect has been observed in some neonates receiving the drug. The mechanism of action of alprostadil is unknown but an active role for prostaglandin in maintaining ductus patency during foetal life is supported by the presence of biosynthetic pathways in the ductus, the constrictor effect of prostaglandin synthetase inhibitors and the relaxant action of PGE 2 and related agents. INDICATIONS PROSTIN VR Sterile Solution (alprostadil) is indicated for palliative, not definitive, therapy to temporarily maintain the patency of the ductus arteriosus until corrective or palliative surgery can be performed in neonates who have congenital heart defects and who depend upon a patent ductus for survival. Such congenital heart defects include pulmonary atresia, pulmonary stenosis, tricuspid atresia, tetralogy of Fallot, interruption of the aortic arch, coarctation of the aorta, mitral atresia, or transposition of the great vessels with or without other defects. PROSTIN VR should be administered only by medically trained personnel in facilities in which paediatric patients can receive or have access to paediatric intensive care. CONTRAINDICATIONS PROSTIN VR is contraindicated in the following patients: 1. Cyanotic neonates with persistent foetal circulation. 2. Neonates with total anomalous pulmonary venous return below the diaphragm, neonates with polysplenia or asplenia in whom pulmonary atresia is combined with anomalous pulmonary venous return which may be obstructed. In such patients PROSTIN VR may precipitate pulmonary oedema because of increased pulmonary blood flow. PRECAUTIONS Approximately 10% to 12% of neonates treated with PROSTIN VR experienced apnoea. Apnoea is seen most often in neonates weighing less than 2 kg at birth and usually appears during the first hour of drug infusion. Therefore PROSTIN VR should be used where facilities for ventilatory assistance and intubation are immediately available. Some studies suggest that PGE 1 administration causes a weakening effect on the structure of the wall of the ductus arteriosus rendering the vessels prone to laceration. These effects may extend into the wall of the aorta and may cause problems in surgical procedures. Cortical proliferation of the long bones has followed long-term infusions of alprostadil in infants and dogs. The proliferation in infants regressed after withdrawal of the drug. Commercial/Non-Commercial Page 2 of 6
3 The administration of PROSTIN VR to neonates may result in gastric outlet obstruction secondary to antral hyperplasia. This effect appears to be related to duration of therapy and cumulative dose of the drug. Neonates receiving PROSTIN VR at recommended doses for more than 120 hours should be closely monitored for evidence of antral hyperplasia and gastric outlet obstruction. PROSTIN VR should be infused for the shortest time and at the lowest dose which will produce the desired effects. The risk of long-term infusion of PROSTIN VR should be weighed against the possible benefits that critically ill infants may derive from its administration. In general, it is recommended that the preparation should not be administered for more than 2 or 3 days at a time. Since PROSTIN VR appears most effective within 96 hours after birth every effort should be made to start infusion of the drug during this period. Use PROSTIN VR cautiously in neonates with histories of bleeding tendencies. Care should be taken to avoid the use of PROSTIN VR in neonates with respiratory distress syndrome (hyaline membrane disease), which sometimes can be confused with cyanotic heart disease. If full diagnostic facilities are not immediately available, cyanosis (po 2 less than 40 mm Hg) and restricted pulmonary blood flow apparent on X-ray are good indicators of congenital heart defects. In all neonates, intermittently monitor arterial pressure by umbilical artery catheter, auscultation, or with a Doppler transducer. Should arterial pressure fall significantly, decrease the rate of infusion immediately. Carcinogenesis, Mutagenesis and Impairment of Fertility Long term carcinogenicity and fertility studies have not been done. No potential for mutagenic activity was revealed in assays of gene mutation in bacterial and mammalian cells, or in DNA damage assays; however, alprostadil has not been tested in assays for chromosomal damage. Testicular atrophy and/or degeneration has been observed in rats receiving high doses (10 mg/day for 35 days or longer) of PGE 1. The relevance of this to the human neonate is not known. ADVERSE EFFECTS Cardiovascular System: The most common adverse reactions reported were flushing in 10.1% of patients, bradycardia in 6.7%, hypotension in 3.9%, tachycardia in 2.8%, cardiac arrest in 1.1% and oedema in 1.1%. The following reactions were reported in less than 1% of the patients: congestive heart failure, hyperaemia, pneumopericardium, second degree heart block, shock, spasm of the right ventricle infundibulum, supraventricular tachycardia, ventricular fibrillation, ventricular hypertrophy and tachyphylaxis. Central Nervous System: Apnoea was reported in 11.5% of neonates treated. The other most common adverse reactions reported were fever in 13.8% of patients treated, and seizures in 4.1%. The following reactions were reported in less than 1% of the patients: cerebral Commercial/Non-Commercial Page 3 of 6
4 bleeding with recorded fatalities, hyperextension of the neck, hyperirritability, hypothermia, jitteriness, lethargy, microcephaly and stiffness. Respiratory System: The following reactions were reported in less than 1% of the patients: bradypnoea, bronchial wheezing, hypercapnia, hypoplastic lungs, pneumothorax, respiratory depression, respiratory distress and tachypnoea. Gastrointestinal System: The most common adverse reaction reported was diarrhoea in 2.6% of the patients. The following reactions were reported in less than 1% of the patients: biliary atresia, gastric regurgitation and hyperbilirubinaemia. Haematological: The most common adverse reaction reported was disseminated intravascular coagulation in 1.1% of patients. The following events were reported in less than 1% of the patients: anaemia, bleeding, thrombocytopenia and hypochromic anaemia. Excretory System: No adverse reactions were reported in more than 1% of the patients. Those reported in less than 1% of the patients were anuria, haematuria, polycystic kidneys and renal failure. Infection: Sepsis was reported in 1.6% of the patients. Peritonitis was reported in less than 1% of the patients. Metabolic: The most common adverse reaction reported was hypokalaemia in 1.1% of the patients. Hypoglycaemia and hyperkalaemia were reported in less than 1% of the patients. Ductus Arteriosus Histological Changes: One group of investigators reported "oedema of the media, separation of the medial components by clear spaces, pathological interruption of the internal elastic lamina and intimal laceration some of which extended into the media" in the ducti arteriosi of four patients. Cortical Proliferation of the Long Bones: Following long-term infusion of PROSTIN VR, cortical proliferation of long bones has been reported. DOSAGE AND ADMINISTRATION Infusion should begin with 0.1 micrograms alprostadil per kilogram of body weight per minute. Doses above 0.1 micrograms per kilogram per minute, do not appear to offer additional benefits. When an effect is achieved, decrease the infusion to the lowest possible dose while maintaining the desired effects. The preferable route of administration for PROSTIN VR (alprostadil) is by continuous intravenous infusion into a large vein. Alternatively, PROSTIN VR may be administered through an umbilical artery catheter placed at the ductal opening. Adverse effects have occurred with both routes of administration, but the types of reactions are different. A higher incidence of flushing has been associated with intra-arterial than with intravenous administration. Commercial/Non-Commercial Page 4 of 6
5 Dilution Instructions Withdraw the appropriate volume of PROSTIN VR (alprostadil) from the ampoule and dissolve in sterile sodium chloride injection USP. Dilute to volumes appropriate for the pump delivery system available. Prepare fresh infusion solutions every 24 hours. Discard any solution more than 24 hours old. Neither PROSTIN VR nor the further diluted solutions contain an antimicrobial agent. To avoid microbial contamination hazards, the further diluted solutions should be used as soon as practicable. Any solution not used within 24 hours should be discarded. The following alprostadil concentrations (μg/ml) are achieved by adding 500 μg of alprostadil to various volumes of diluent: Micrograms Alprostadil Added Total Volume 500 μg (1 ml)** 250 ml 2.0 μg/ml 100 ml 5.0 μg/ml 50 ml 10.0 μg/ml 25 ml 20.0 μg/ml ** Volume of alprostadil withdrawn from ampoule Infusion Rate (ml/hr)= Dosage (μg/kg/min x patient weight (kg)x60 min/hr Final Concentration to be used (μg/ml) Example: To provide 0.1 μg/kg/min to a 2.8 kg neonate, using a final alprostadil concentration of 5 μg/ml Infusion Rate = 0.1 μg/kg/min x 2.8 kg x 60 min/hr = 3.36 ml/hr 5 μg/ml With an infusion pump limited to discrete infusion rates, infuse 2 or 4 ml per hour. The infusion solution may be mixed conveniently in a graduated mixing chamber inserted between the IV bottle and the pump. Change the dosage from 0.1 micrograms per kilogram of body weight per minute to 0.05 micrograms per kilogram of body weight per minute by reducing the pump rate to one-half the original rate. If undiluted PROSTIN VR comes in direct contact with a plastic container the solution may turn hazy. Should this occur the solution should be discarded. Commercial/Non-Commercial Page 5 of 6
6 OVERDOSAGE Overdose data is limited. Apnoea, bradycardia, pyrexia, hypotension and flushing may be signs of drug overdose. If apnoea or bradycardia occur, the infusion should be discontinued and the appropriate medical treatment initiated. There is no antidote for alprostadil overdose. Treatment is symptomatic and supportive. Support respiratory and cardiac function. Monitor pulmonary function, vital signs, ECG and pulse oximetry, and fluid and electrolyte status in patients with significant diarrhoea. Caution should be used if the infusion is restarted. If pyrexia or hypotension occur, the infusion rate should be reduced until these symptoms subside. Flushing is usually attributed to incorrect intra-arterial catheter placement and is usually alleviated by repositioning the tip of the catheter. Contact the Poisons Information Centre for advice on the management of an overdose. PRESENTATION PROSTIN VR (alprostadil) Sterile Solution is available in ampoules containing: 1 ml of 500 μg/ml alprostadil in ethanol: 5's. 0.5 ml of 250 μg/ml alprostadil in ethanol: 5 s (not marketed) 0.2 ml of 100 μg/ml alprostadil in ethanol: 5 s (not marketed) STORAGE CONDITIONS Store at 2 C to 8 C (Refrigerate. Do not freeze) Pfizer Australia Pty Ltd ABN Wharf Road West Ryde NSW 2114 POISON SCHEDULE OF THE MEDICINE Prescription only medicine (S4) Approved by TGA 14 January 1994 Date of most recent amendment: 15 November 2006 Registered Trademark Commercial/Non-Commercial Page 6 of 6
Duct Dependant Congenital Heart Disease
 Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease This guideline has been agreed by both NTS & CATS Document Control Information Author CATS/NTS Author Position
Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease This guideline has been agreed by both NTS & CATS Document Control Information Author CATS/NTS Author Position
Duct Dependant Congenital Heart Disease
 Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease Document Control Information Author CATS/NTS Author Position CC Transport Services Document Owner E. Polke
Children s Acute Transport Service Clinical Guidelines Duct Dependant Congenital Heart Disease Document Control Information Author CATS/NTS Author Position CC Transport Services Document Owner E. Polke
When is Risky to Apply Oxygen for Congenital Heart Disease 부천세종병원 소아청소년과최은영
 When is Risky to Apply Oxygen for Congenital Heart Disease 부천세종병원 소아청소년과최은영 The Korean Society of Cardiology COI Disclosure Eun-Young Choi The author have no financial conflicts of interest to disclose
When is Risky to Apply Oxygen for Congenital Heart Disease 부천세종병원 소아청소년과최은영 The Korean Society of Cardiology COI Disclosure Eun-Young Choi The author have no financial conflicts of interest to disclose
The Blue Baby. Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin
 The Blue Baby Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin Session Structure Definitions and assessment of cyanosis Causes of blue baby Structured approach to assessing
The Blue Baby Network Stabilisation of the Term Infant Study Day 15 th March 2017 Joanna Behrsin Session Structure Definitions and assessment of cyanosis Causes of blue baby Structured approach to assessing
Congenital Heart Defects
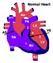
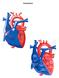 Normal Heart Congenital Heart Defects 1. Patent Ductus Arteriosus The ductus arteriosus connects the main pulmonary artery to the aorta. In utero, it allows the blood leaving the right ventricle to bypass
Normal Heart Congenital Heart Defects 1. Patent Ductus Arteriosus The ductus arteriosus connects the main pulmonary artery to the aorta. In utero, it allows the blood leaving the right ventricle to bypass
Screening for Critical Congenital Heart Disease
 Screening for Critical Congenital Heart Disease Caroline K. Lee, MD Pediatric Cardiology Disclosures I have no relevant financial relationships or conflicts of interest 1 Most Common Birth Defect Most
Screening for Critical Congenital Heart Disease Caroline K. Lee, MD Pediatric Cardiology Disclosures I have no relevant financial relationships or conflicts of interest 1 Most Common Birth Defect Most
How to Recognize a Suspected Cardiac Defect in the Neonate
 Neonatal Nursing Education Brief: How to Recognize a Suspected Cardiac Defect in the Neonate https://www.seattlechildrens.org/healthcareprofessionals/education/continuing-medical-nursing-education/neonatalnursing-education-briefs/
Neonatal Nursing Education Brief: How to Recognize a Suspected Cardiac Defect in the Neonate https://www.seattlechildrens.org/healthcareprofessionals/education/continuing-medical-nursing-education/neonatalnursing-education-briefs/
3/14/2011 MANAGEMENT OF NEWBORNS CARDIAC INTENSIVE CARE CONFERENCE FOR HEALTH PROFESSIONALS IRVINE, CA. MARCH 7, 2011 WITH HEART DEFECTS
 CONFERENCE FOR HEALTH PROFESSIONALS IRVINE, CA. MARCH 7, 2011 MANAGEMENT OF NEWBORNS WITH HEART DEFECTS A NTHONY C. CHANG, MD, MBA, MPH M E D I C AL D I RE C T OR, HEART I N S T I T U T E C H I LDRE N
CONFERENCE FOR HEALTH PROFESSIONALS IRVINE, CA. MARCH 7, 2011 MANAGEMENT OF NEWBORNS WITH HEART DEFECTS A NTHONY C. CHANG, MD, MBA, MPH M E D I C AL D I RE C T OR, HEART I N S T I T U T E C H I LDRE N
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Cardiac Emergencies in Infants. Michael Luceri, DO
 Cardiac Emergencies in Infants Michael Luceri, DO October 7, 2017 I have no financial obligations or conflicts of interest to disclose. Objectives Understand the scope of congenital heart disease Recognize
Cardiac Emergencies in Infants Michael Luceri, DO October 7, 2017 I have no financial obligations or conflicts of interest to disclose. Objectives Understand the scope of congenital heart disease Recognize
Pfizer Laboratories (Pty) Ltd P&U Etoposide CSV Range Approved PI: 12 Sep 2005 Page 1 of 5
 Approved PI: 12 Sep 2005 Page 1 of 5 SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): P&U ETOPOSIDE CSV 100 mg/5 ml INJECTION (INJECTION CONCENTRATE FOR INTRAVENOUS INFUSION) P&U ETOPOSIDE CSV
Approved PI: 12 Sep 2005 Page 1 of 5 SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): P&U ETOPOSIDE CSV 100 mg/5 ml INJECTION (INJECTION CONCENTRATE FOR INTRAVENOUS INFUSION) P&U ETOPOSIDE CSV
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH)
 COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
ACETYLCYSTEINE INJECTION
 ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
Cardiology Competency Based Goals and Objectives
 Cardiology Competency Based Goals and Objectives COMPETENCY 1. Patient Care. Provide family centered patient care that is developmentally and age appropriate, compassionate, and effective for the treatment
Cardiology Competency Based Goals and Objectives COMPETENCY 1. Patient Care. Provide family centered patient care that is developmentally and age appropriate, compassionate, and effective for the treatment
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
Cardiovascular Pathophysiology: Right to Left Shunts aka Cyanotic Lesions
 Cardiovascular Pathophysiology: Right to Left Shunts aka Cyanotic Lesions Ismee A. Williams, MD, MS iib6@columbia.edu Pediatric Cardiology Learning Objectives To discuss the hemodynamic significance of
Cardiovascular Pathophysiology: Right to Left Shunts aka Cyanotic Lesions Ismee A. Williams, MD, MS iib6@columbia.edu Pediatric Cardiology Learning Objectives To discuss the hemodynamic significance of
Cardiovascular Pathophysiology: Right to Left Shunts aka Cyanotic Lesions Ismee A. Williams, MD, MS Pediatric Cardiology
 Cardiovascular Pathophysiology: Right to Left Shunts aka Cyanotic Lesions Ismee A. Williams, MD, MS iib6@columbia.edu Pediatric Cardiology Learning Objectives To discuss the hemodynamic significance of
Cardiovascular Pathophysiology: Right to Left Shunts aka Cyanotic Lesions Ismee A. Williams, MD, MS iib6@columbia.edu Pediatric Cardiology Learning Objectives To discuss the hemodynamic significance of
PRODUCT INFORMATION. NAME OF THE MEDICINE Compound Sodium Lactate (Hartmann's) Solution for Injection
 PRODUCT INFORMATION NAME OF THE MEDICINE Compound Sodium Lactate (Hartmann's) Solution for Injection DESCRIPTION Molecular formulae. Potassium chloride: KCl; sodium chloride: NaCl; calcium chloride dihydrate:
PRODUCT INFORMATION NAME OF THE MEDICINE Compound Sodium Lactate (Hartmann's) Solution for Injection DESCRIPTION Molecular formulae. Potassium chloride: KCl; sodium chloride: NaCl; calcium chloride dihydrate:
CONGENITAL HEART DISEASE (CHD)
 CONGENITAL HEART DISEASE (CHD) DEFINITION It is the result of a structural or functional abnormality of the cardiovascular system at birth GENERAL FEATURES OF CHD Structural defects due to specific disturbance
CONGENITAL HEART DISEASE (CHD) DEFINITION It is the result of a structural or functional abnormality of the cardiovascular system at birth GENERAL FEATURES OF CHD Structural defects due to specific disturbance
Heart and Soul Evaluation of the Fetal Heart
 Heart and Soul Evaluation of the Fetal Heart Ivana M. Vettraino, M.D., M.B.A. Clinical Associate Professor, Michigan State University College of Human Medicine Objectives Review the embryology of the formation
Heart and Soul Evaluation of the Fetal Heart Ivana M. Vettraino, M.D., M.B.A. Clinical Associate Professor, Michigan State University College of Human Medicine Objectives Review the embryology of the formation
PFIZER INC. Study Center(s): A total of 6 centers took part in the study, including 2 in France and 4 in the United States.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Slide 1. Slide 2. Slide 3 CONGENITAL HEART DISEASE. Papworth Hospital NHS Trust INTRODUCTION. Jakub Kadlec/Catherine Sudarshan INTRODUCTION
 Slide 1 CONGENITAL HEART DISEASE Jakub Kadlec/Catherine Sudarshan NHS Trust Slide 2 INTRODUCTION Most common congenital illness in the newborn Affects about 4 9 / 1000 full-term live births in the UK 1.5
Slide 1 CONGENITAL HEART DISEASE Jakub Kadlec/Catherine Sudarshan NHS Trust Slide 2 INTRODUCTION Most common congenital illness in the newborn Affects about 4 9 / 1000 full-term live births in the UK 1.5
CongHeartDis.doc. Андрій Миколайович Лобода
 CongHeartDis.doc Андрій Миколайович Лобода 2015 Зміст 3 Зміст Зміст 4 A child with tetralogy of Fallot is most likely to exhibit: -Increased pulmonary blood flow -Increased pressure in the right ventricle
CongHeartDis.doc Андрій Миколайович Лобода 2015 Зміст 3 Зміст Зміст 4 A child with tetralogy of Fallot is most likely to exhibit: -Increased pulmonary blood flow -Increased pressure in the right ventricle
9/8/2009 < 1 1,2 3,4 5,6 7,8 9,10 11,12 13,14 15,16 17,18 > 18. Tetralogy of Fallot. Complex Congenital Heart Disease.
 Current Indications for Pediatric CTA S Bruce Greenberg Professor of Radiology Arkansas Children s Hospital University of Arkansas for Medical Sciences greenbergsbruce@uams.edu 45 40 35 30 25 20 15 10
Current Indications for Pediatric CTA S Bruce Greenberg Professor of Radiology Arkansas Children s Hospital University of Arkansas for Medical Sciences greenbergsbruce@uams.edu 45 40 35 30 25 20 15 10
Diuretics: Increased risk of renal dysfunction. (7) See 17 for PATIENT COUNSELING INFORMATION. Revised: 10/2017 FULL PRESCRIBING INFORMATION: CONTENTS
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEOPROFEN safely and effectively. See full prescribing information for NEOPROFEN. NEOPROFEN (ibuprofen
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEOPROFEN safely and effectively. See full prescribing information for NEOPROFEN. NEOPROFEN (ibuprofen
Anatomy & Physiology
 1 Anatomy & Physiology Heart is divided into four chambers, two atrias & two ventricles. Atrioventricular valves (tricuspid & mitral) separate the atria from ventricles. they open & close to control flow
1 Anatomy & Physiology Heart is divided into four chambers, two atrias & two ventricles. Atrioventricular valves (tricuspid & mitral) separate the atria from ventricles. they open & close to control flow
Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein of which at least 95% is human albumin.
 1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
Introduction. Pediatric Cardiology. General Appearance. Tools of Assessment. Auscultation. Vital Signs
 Introduction Pediatric Cardiology An introduction to the pediatric patient with heart disease: M-III Lecture Douglas R. Allen, M.D. Assistant Professor and Director of Community Pediatric Cardiology at
Introduction Pediatric Cardiology An introduction to the pediatric patient with heart disease: M-III Lecture Douglas R. Allen, M.D. Assistant Professor and Director of Community Pediatric Cardiology at
SUMMARY OF PRODUCT CHARACTERISTICS. Flexbumin 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
Calcium folinate as equivalent to folinic acid 50 mg/5 ml, 100 mg/10 ml COO - Ca2+, xh2o CH 2
 Product Information LEUCOVORIN CALCIUM INJECTION Calcium folinate as equivalent to folinic acid 50 mg/5 ml, 100 mg/10 ml NAME OF THE MEDICINE Non-proprietary name: calcium folinate Chemical name: calcium
Product Information LEUCOVORIN CALCIUM INJECTION Calcium folinate as equivalent to folinic acid 50 mg/5 ml, 100 mg/10 ml NAME OF THE MEDICINE Non-proprietary name: calcium folinate Chemical name: calcium
Foetal Cardiology: How to predict perinatal problems. Prof. I.Witters Prof.M.Gewillig UZ Leuven
 Foetal Cardiology: How to predict perinatal problems Prof. I.Witters Prof.M.Gewillig UZ Leuven Cardiopathies Incidence : 8-12 / 1000 births ( 1% ) Most frequent - Ventricle Septum Defect 20% - Atrium Septum
Foetal Cardiology: How to predict perinatal problems Prof. I.Witters Prof.M.Gewillig UZ Leuven Cardiopathies Incidence : 8-12 / 1000 births ( 1% ) Most frequent - Ventricle Septum Defect 20% - Atrium Septum
PRODUCT INFORMATION. DESCRIPTION Addaven is a concentrated trace element solution for infusion which is clear and colourless to slightly yellow.
 PRODUCT INFORMATION Addaven NAME OF MEDICINE Addaven is a concentrated trace element solution containing the following simple salts: chromic chloride hexahydrate (CAS No.:10060-12-5), cupric chloride dihydrate
PRODUCT INFORMATION Addaven NAME OF MEDICINE Addaven is a concentrated trace element solution containing the following simple salts: chromic chloride hexahydrate (CAS No.:10060-12-5), cupric chloride dihydrate
: Provide cardiovascular preventive counseling to parents and patients with specific cardiac diseases about:
 Children s Hospital & Research Center Oakland Cardiology Primary Goals for this Rotation 5.13 GOAL: Prevention, Counseling and Screening (Cardiovascular). Understand the role of the pediatrician in preventing
Children s Hospital & Research Center Oakland Cardiology Primary Goals for this Rotation 5.13 GOAL: Prevention, Counseling and Screening (Cardiovascular). Understand the role of the pediatrician in preventing
SUMMARY OF PRODUCT CHARACTERISTICS. Albuman 40 g/l is a solution containing 40 g/l (4%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 40 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 40 g/l is a solution containing 40 g/l (4%)
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 40 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 40 g/l is a solution containing 40 g/l (4%)
PRODUCT INFORMATION. BiCNU (Carmustine)
 PRODUCT INFORMATION BiCNU (Carmustine) APPROVED NAME Carmustine. DESCRIPTION Carmustine is one of the nitrosoureas. The chemical name is 1, 3-bis(2-chloroethyl)-1-nitrosourea; the structural formula is:
PRODUCT INFORMATION BiCNU (Carmustine) APPROVED NAME Carmustine. DESCRIPTION Carmustine is one of the nitrosoureas. The chemical name is 1, 3-bis(2-chloroethyl)-1-nitrosourea; the structural formula is:
SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS PRODUCT SUMMARY 1. NAME OF THE MEDICINAL PRODUCT Sterile Potassium Chloride Concentrate 15%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 15% of Potassium Chloride in
SUMMARY OF PRODUCT CHARACTERISTICS PRODUCT SUMMARY 1. NAME OF THE MEDICINAL PRODUCT Sterile Potassium Chloride Concentrate 15%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 15% of Potassium Chloride in
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 5%, 50 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 5% is a solution containing 50 g/l of total
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 5%, 50 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 5% is a solution containing 50 g/l of total
Adult Echocardiography Examination Content Outline
 Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
Adult Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 Anatomy and Physiology Pathology Clinical Care and Safety Measurement Techniques, Maneuvers,
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
LONITEN PRODUCT INFORMATION LONITEN DESCRIPTION CLINICAL PHARMACOLOGY AI brand of minoxidil tablets
 LONITEN PRODUCT INFORMATION AI.082-1 LONITEN brand of minoxidil tablets DESCRIPTION LONITEN (minoxidil) is an orally active peripheral vasodilator. The pure compound is soluble in water to the extent of
LONITEN PRODUCT INFORMATION AI.082-1 LONITEN brand of minoxidil tablets DESCRIPTION LONITEN (minoxidil) is an orally active peripheral vasodilator. The pure compound is soluble in water to the extent of
Congenital Heart Disease: Physiology and Common Defects
 Congenital Heart Disease: Physiology and Common Defects Jamie S. Sutherell, M.D, M.Ed. Associate Professor, Pediatrics Division of Cardiology Director, Medical Student Education in Pediatrics Director,
Congenital Heart Disease: Physiology and Common Defects Jamie S. Sutherell, M.D, M.Ed. Associate Professor, Pediatrics Division of Cardiology Director, Medical Student Education in Pediatrics Director,
50% Concentrated Injection
 NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
PACKAGE LEAFLET: INFORMATION FOR THE USER. Active substance: enoximone
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
Neosynephrine. Name of the Medicine
 Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
NARCAN Injection. 4,5 α Epoxy-3,14 dihydroxy 17 (prop-2-enyl)morphinan-6-one hydrochloride
 NAME OF MEDICINE Naloxone hydrochloride The molecular weight of naloxone hydrochloride is 363.84 and the CAS registry number for the drug substance (naloxone hydrochloride) is 357-08-4. The molecular formula
NAME OF MEDICINE Naloxone hydrochloride The molecular weight of naloxone hydrochloride is 363.84 and the CAS registry number for the drug substance (naloxone hydrochloride) is 357-08-4. The molecular formula
DRUG GUIDELINE. HYDRALAZINE (Intravenous severe hypertension in pregnancy)
 DRUG GUIDELINE HYDRALAZINE (Intravenous severe hypertension SCOPE (Area): FOR USE IN: Labour Ward, HDU, Theatre and ED EXCLUSIONS: Paediatrics (seek Paediatrician advice) and other general wards. SCOPE
DRUG GUIDELINE HYDRALAZINE (Intravenous severe hypertension SCOPE (Area): FOR USE IN: Labour Ward, HDU, Theatre and ED EXCLUSIONS: Paediatrics (seek Paediatrician advice) and other general wards. SCOPE
Supplemental Information
 ARTICLE Supplemental Information SUPPLEMENTAL TABLE 6 Mosaic and Partial Trisomies Thirty-eight VLBW infants were identified with T13, of whom 2 had mosaic T13. T18 was reported for 128 infants, of whom
ARTICLE Supplemental Information SUPPLEMENTAL TABLE 6 Mosaic and Partial Trisomies Thirty-eight VLBW infants were identified with T13, of whom 2 had mosaic T13. T18 was reported for 128 infants, of whom
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
Index Note: Page numbers of article titles are in boldface type. A Acute coronary syndrome(s), anticoagulant therapy in, 706, 707 antiplatelet therapy in, 702 ß-blockers in, 703 cardiac biomarkers in,
PRODUCT INFORMATION. IPRAVENT Inhalation Solution Unit Dose NAME OF THE MEDICINE DESCRIPTION. Ipratropium Bromide BP
 PRODUCT INFORMATION IPRAVENT Inhalation Solution Unit Dose NAME OF THE MEDICINE Ipratropium Bromide BP Ipratropium bromide is a quaternary isopropyl derivative of atropine. Its chemical name is (1R, 3r,
PRODUCT INFORMATION IPRAVENT Inhalation Solution Unit Dose NAME OF THE MEDICINE Ipratropium Bromide BP Ipratropium bromide is a quaternary isopropyl derivative of atropine. Its chemical name is (1R, 3r,
By Dickens ATURWANAHO & ORIBA DAN LANGOYA MAKchs, MBchB CONGENTAL HEART DISEASE
 By Dickens ATURWANAHO & ORIBA DAN LANGOYA MAKchs, MBchB CONGENTAL HEART DISEASE Introduction CHDs are abnormalities of the heart or great vessels that are present at birth. Common type of heart disease
By Dickens ATURWANAHO & ORIBA DAN LANGOYA MAKchs, MBchB CONGENTAL HEART DISEASE Introduction CHDs are abnormalities of the heart or great vessels that are present at birth. Common type of heart disease
SURGICAL TREATMENT AND OUTCOME OF CONGENITAL HEART DISEASE
 SURGICAL TREATMENT AND OUTCOME OF CONGENITAL HEART DISEASE Mr. W. Brawn Birmingham Children s Hospital. Aims of surgery The aim of surgery in congenital heart disease is to correct or palliate the heart
SURGICAL TREATMENT AND OUTCOME OF CONGENITAL HEART DISEASE Mr. W. Brawn Birmingham Children s Hospital. Aims of surgery The aim of surgery in congenital heart disease is to correct or palliate the heart
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
DRUG GUIDELINE SODIUM NITROPRUSSIDE
 DRUG GUIDELINE SODIUM NITROPRUSSIDE SCOPE (Area): FOR USE IN: Critical Care Unit, ED and Theatre EXCLUSIONS: Paediatrics (seek Paediatrician advice) and General Wards SCOPE (Staff): Medical, Nursing and
DRUG GUIDELINE SODIUM NITROPRUSSIDE SCOPE (Area): FOR USE IN: Critical Care Unit, ED and Theatre EXCLUSIONS: Paediatrics (seek Paediatrician advice) and General Wards SCOPE (Staff): Medical, Nursing and
(human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS
 albunorm TM 5% (human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS UK IRELAND and Ireland Octapharma Limited The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom 1. Name
albunorm TM 5% (human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS UK IRELAND and Ireland Octapharma Limited The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom 1. Name
MINIMS AMETHOCAINE EYE DROPS
 MINIMS AMETHOCAINE EYE DROPS NAME OF THE MEDICINE Amethocaine hydrochloride Synonyms: Tetracaine hydrochloride Structural formula: Chemical name: 2-(dimethylamino)ethyl 4-(butylamino)benzoate hydrochloride
MINIMS AMETHOCAINE EYE DROPS NAME OF THE MEDICINE Amethocaine hydrochloride Synonyms: Tetracaine hydrochloride Structural formula: Chemical name: 2-(dimethylamino)ethyl 4-(butylamino)benzoate hydrochloride
Notes: 1)Membranous part contribute in the formation of small portion in the septal cusp.
 Embryology 9 : Slide 16 : There is a sulcus between primitive ventricular and bulbis cordis that will disappear gradually and lead to the formation of one chamber which is called bulboventricular chamber.
Embryology 9 : Slide 16 : There is a sulcus between primitive ventricular and bulbis cordis that will disappear gradually and lead to the formation of one chamber which is called bulboventricular chamber.
PRODUCT INFORMATION. Colistin Link. Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial
 PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
Patent ductus arteriosus PDA
 Patent ductus arteriosus PDA Is connecting between the aortic end just distal to the origin of the LT sub clavian artery& the pulmonary artery at its bifurcation. Female/male ratio is 2:1 and it is more
Patent ductus arteriosus PDA Is connecting between the aortic end just distal to the origin of the LT sub clavian artery& the pulmonary artery at its bifurcation. Female/male ratio is 2:1 and it is more
Hypoglycaemia of the neonate. Dr. L.G. Lloyd Dept. Paediatrics
 Hypoglycaemia of the neonate Dr. L.G. Lloyd Dept. Paediatrics Why is glucose important? It provides 60-70% of energy needs Utilization obligatory by red blood cells, brain and kidney as major source of
Hypoglycaemia of the neonate Dr. L.G. Lloyd Dept. Paediatrics Why is glucose important? It provides 60-70% of energy needs Utilization obligatory by red blood cells, brain and kidney as major source of
Dear Parent/Guardian,
 Dear Parent/Guardian, You have indicated on school records that your child has an ongoing health problem that may require medication and/or treatment during the school day with rescue medication. Attached
Dear Parent/Guardian, You have indicated on school records that your child has an ongoing health problem that may require medication and/or treatment during the school day with rescue medication. Attached
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
SPECIFIC HEART DEFECTS
 A. Acyanotic Defects 1. Ventricular Septal Defect (VSD): SPECIFIC HEART DEFECTS Which side of the heart is stronger? Left This is when there is an opening between the left and right ventricle (in the septum)
A. Acyanotic Defects 1. Ventricular Septal Defect (VSD): SPECIFIC HEART DEFECTS Which side of the heart is stronger? Left This is when there is an opening between the left and right ventricle (in the septum)
Amino Acids and Sorbitol injection with/without Electrolytes NIRMIN *
 For the use of a registered medical practitioner or a Hospital or a Laboratory only Amino Acids and Sorbitol injection with/without Electrolytes NIRMIN * DESCRIPTION: NIRMIN * is a clear, colourless injection
For the use of a registered medical practitioner or a Hospital or a Laboratory only Amino Acids and Sorbitol injection with/without Electrolytes NIRMIN * DESCRIPTION: NIRMIN * is a clear, colourless injection
New Zealand Data Sheet. Poractant alfa (Phospholipid fraction of porcine lung) 80 mg/ml
 CUROSURF New Zealand Data Sheet Poractant alfa (Phospholipid fraction of porcine lung) 80 mg/ml Presentation Sterile suspension in single-dose vials for intratracheal or intrabronchial administration.
CUROSURF New Zealand Data Sheet Poractant alfa (Phospholipid fraction of porcine lung) 80 mg/ml Presentation Sterile suspension in single-dose vials for intratracheal or intrabronchial administration.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Acetylcysteine 200 mg/ml Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml. Each 10ml ampoule contains
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Acetylcysteine 200 mg/ml Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml. Each 10ml ampoule contains
Chapter 8 ADMINISTRATION OF BLOOD COMPONENTS
 Chapter 8 ADMINISTRATION OF BLOOD COMPONENTS PRACTICE POINTS Give the right blood product to the right patient at the right time. Failure to correctly check the patient or the pack can be fatal. At the
Chapter 8 ADMINISTRATION OF BLOOD COMPONENTS PRACTICE POINTS Give the right blood product to the right patient at the right time. Failure to correctly check the patient or the pack can be fatal. At the
CYANOTIC CONGENITAL HEART DISEASES. PRESENTER: DR. Myra M. Koech Pediatric cardiologist MTRH/MU
 CYANOTIC CONGENITAL HEART DISEASES PRESENTER: DR. Myra M. Koech Pediatric cardiologist MTRH/MU DEFINITION Congenital heart diseases are defined as structural and functional problems of the heart that are
CYANOTIC CONGENITAL HEART DISEASES PRESENTER: DR. Myra M. Koech Pediatric cardiologist MTRH/MU DEFINITION Congenital heart diseases are defined as structural and functional problems of the heart that are
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
(angiotensin II) injection for intravenous infusion
 ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
PATENT DUCTUS ARTERIOSUS (PDA)
 PATENT DUCTUS ARTERIOSUS (PDA) It is a channel that connect the pulmonary artery with the descending aorta (isthumus part). It results from the persistence of patency of the fetal ductus arteriosus after
PATENT DUCTUS ARTERIOSUS (PDA) It is a channel that connect the pulmonary artery with the descending aorta (isthumus part). It results from the persistence of patency of the fetal ductus arteriosus after
Saturation With Oxygen for Ductal Dependent Congenital Heart Diseases Before and After the Prostaglandin Therapy
 & Saturation With Oxygen for Ductal Dependent Congenital Heart Diseases Before and After the Prostaglandin Therapy Senka Dinarević*, Hajrija Maksić, Majda Haznadar Paediatric clinic of the Clinical Centre
& Saturation With Oxygen for Ductal Dependent Congenital Heart Diseases Before and After the Prostaglandin Therapy Senka Dinarević*, Hajrija Maksić, Majda Haznadar Paediatric clinic of the Clinical Centre
VENTOLIN RESPIRATOR SOLUTION
 VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
MICHIGAN. State Protocols. Pediatric Cardiac Table of Contents 6.1 General Pediatric Cardiac Arrest 6.2 Bradycardia 6.
 MICHIGAN State Protocols Protocol Number Protocol Name Pediatric Cardiac Table of Contents 6.1 General Pediatric Cardiac Arrest 6.2 Bradycardia 6.3 Tachycardia PEDIATRIC CARDIAC PEDIATRIC CARDIAC ARREST
MICHIGAN State Protocols Protocol Number Protocol Name Pediatric Cardiac Table of Contents 6.1 General Pediatric Cardiac Arrest 6.2 Bradycardia 6.3 Tachycardia PEDIATRIC CARDIAC PEDIATRIC CARDIAC ARREST
MINIMS OXYBUPROCAINE EYE DROPS
 MINIMS OXYBUPROCAINE EYE DROPS Name of Medicine: Oxybuprocaine Hydrochloride Description: Composition: Active. Benoxinate (oxybuprocaine) HCI USP. Inactive. Water Purified and Hydrochloric acid. No preservatives
MINIMS OXYBUPROCAINE EYE DROPS Name of Medicine: Oxybuprocaine Hydrochloride Description: Composition: Active. Benoxinate (oxybuprocaine) HCI USP. Inactive. Water Purified and Hydrochloric acid. No preservatives
BRICANYL INJECTION. terbutaline sulfate PRODUCT INFORMATION
 BRICANYL INJECTION terbutaline sulfate PRODUCT INFORMATION NAME OF THE MEDICINE Terbutaline sulfate, 2-(tert-butylamino)-1-(3,5-dihydroxyphenyl) ethanol sulfate, a sympathomimetic bronchodilator with a
BRICANYL INJECTION terbutaline sulfate PRODUCT INFORMATION NAME OF THE MEDICINE Terbutaline sulfate, 2-(tert-butylamino)-1-(3,5-dihydroxyphenyl) ethanol sulfate, a sympathomimetic bronchodilator with a
Pediatric Echocardiography Examination Content Outline
 Pediatric Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 Anatomy and Physiology Normal Anatomy and Physiology 10% 2 Abnormal Pathology and Pathophysiology
Pediatric Echocardiography Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 Anatomy and Physiology Normal Anatomy and Physiology 10% 2 Abnormal Pathology and Pathophysiology
(human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS
 albunorm TM 20% (human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS UK IRELAND and Ireland Octapharma Limited The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom 1. Name
albunorm TM 20% (human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS UK IRELAND and Ireland Octapharma Limited The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom 1. Name
PRODUCT INFORMATION LOFENOXAL
 PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
Solution for cardiac perfusion in viaflex plastic container
 CARDIOPLEGIA SOLUTION A Solution for cardiac perfusion in viaflex plastic container DESCRIPTION Cardioplegia Solution A is a sterile, non-pyrogenic solution in a Viaflex bag. It is used to induce cardiac
CARDIOPLEGIA SOLUTION A Solution for cardiac perfusion in viaflex plastic container DESCRIPTION Cardioplegia Solution A is a sterile, non-pyrogenic solution in a Viaflex bag. It is used to induce cardiac
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
Echocardiography in Adult Congenital Heart Disease
 Echocardiography in Adult Congenital Heart Disease Michael Vogel Kinderherz-Praxis München CHD missed in childhood Subsequent lesions after repaired CHD Follow-up of cyanotic heart disease CHD missed in
Echocardiography in Adult Congenital Heart Disease Michael Vogel Kinderherz-Praxis München CHD missed in childhood Subsequent lesions after repaired CHD Follow-up of cyanotic heart disease CHD missed in
The blue baby. Case 4
 Case 4 The blue baby Mrs Smith has brought her baby to A&E because she says he has started turning blue. What are your immediate differential diagnoses? 1 Respiratory causes: Congenital respiratory disorder.
Case 4 The blue baby Mrs Smith has brought her baby to A&E because she says he has started turning blue. What are your immediate differential diagnoses? 1 Respiratory causes: Congenital respiratory disorder.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200g/l, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200g/l, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
INTRAVENOUS FLUID THERAPY
 INTRAVENOUS FLUID THERAPY PRINCIPLES Postnatal physiological weight loss is approximately 5 10% in first week of life Preterm neonates have more total body water and may lose 10 15% of their weight in
INTRAVENOUS FLUID THERAPY PRINCIPLES Postnatal physiological weight loss is approximately 5 10% in first week of life Preterm neonates have more total body water and may lose 10 15% of their weight in
MIACALCIC (salcatonin)
 1 MIACALCIC (salcatonin) DESCRIPTION A synthesised polypeptide hormone structurally identical with salmon calcitonin. It contains 32 amino-acids in linear sequence with a disulphide bridge at position
1 MIACALCIC (salcatonin) DESCRIPTION A synthesised polypeptide hormone structurally identical with salmon calcitonin. It contains 32 amino-acids in linear sequence with a disulphide bridge at position
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
Congenital Heart Disease
 Congenital Heart Disease Mohammed Alghamdi, MD, FRCPC, FAAP, FACC Associate Professor and Consultant Pediatric Cardiology, Cardiac Science King Fahad Cardiac Centre King Saud University INTRODUCTION CHD
Congenital Heart Disease Mohammed Alghamdi, MD, FRCPC, FAAP, FACC Associate Professor and Consultant Pediatric Cardiology, Cardiac Science King Fahad Cardiac Centre King Saud University INTRODUCTION CHD
The Fetal Cardiology Program
 The Fetal Cardiology Program at Texas Children s Fetal Center About the program Since the 1980s, Texas Children s Fetal Cardiology Program has provided comprehensive fetal cardiac care to expecting families
The Fetal Cardiology Program at Texas Children s Fetal Center About the program Since the 1980s, Texas Children s Fetal Cardiology Program has provided comprehensive fetal cardiac care to expecting families
Survival Rates of Children with Congenital Heart Disease continue to improve.
 DOROTHY RADFORD Survival Rates of Children with Congenital Heart Disease continue to improve. 1940-20% 1960-40% 1980-70% 2010->90% Percentage of children with CHD reaching age of 18 years 1938 First Patent
DOROTHY RADFORD Survival Rates of Children with Congenital Heart Disease continue to improve. 1940-20% 1960-40% 1980-70% 2010->90% Percentage of children with CHD reaching age of 18 years 1938 First Patent
Congenital heart disease: When to act and what to do?
 Leading Article Congenital heart disease: When to act and what to do? Duminda Samarasinghe 1 Sri Lanka Journal of Child Health, 2010; 39: 39-43 (Key words: Congenital heart disease) Congenital heart disease
Leading Article Congenital heart disease: When to act and what to do? Duminda Samarasinghe 1 Sri Lanka Journal of Child Health, 2010; 39: 39-43 (Key words: Congenital heart disease) Congenital heart disease
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
CUROSURF (poractant alfa) intratracheal suspension Initial U.S. Approval: 1999
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUROSURF safely and effectively. See full prescribing information for CUROSURF. CUROSURF (poractant
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUROSURF safely and effectively. See full prescribing information for CUROSURF. CUROSURF (poractant
IMOGAM RABIES PASTEURIZED - HUMAN RABIES IMMUNOGLOBULIN. Active ingredient: Human proteins mg
 IMOGAM RABIES PASTEURIZED - HUMAN RABIES IMMUNOGLOBULIN COMPOSITION 1 ml of human rabies immunoglobulin contains: Active ingredient: Human proteins 100 180 mg containing (IgG class) human rabies immunoglobulins
IMOGAM RABIES PASTEURIZED - HUMAN RABIES IMMUNOGLOBULIN COMPOSITION 1 ml of human rabies immunoglobulin contains: Active ingredient: Human proteins 100 180 mg containing (IgG class) human rabies immunoglobulins
Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating)
 Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating) Table 1: Appropriate indications (median score 7-9) Indication
Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating) Table 1: Appropriate indications (median score 7-9) Indication
Absent Pulmonary Valve Syndrome
 Absent Pulmonary Valve Syndrome Fact sheet on Absent Pulmonary Valve Syndrome In this condition, which has some similarities to Fallot's Tetralogy, there is a VSD with narrowing at the pulmonary valve.
Absent Pulmonary Valve Syndrome Fact sheet on Absent Pulmonary Valve Syndrome In this condition, which has some similarities to Fallot's Tetralogy, there is a VSD with narrowing at the pulmonary valve.
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
3. PHARMACEUTICAL FORM Solution for infusion. A clear, slightly viscous liquid; it is almost colourless, yellow, amber or green.
 1. NAME OF THE MEDICINAL PRODUCT Albutein 250 g/l, solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human albumin Albutein 250 g/l is a solution containing 250 g/l of total protein of
1. NAME OF THE MEDICINAL PRODUCT Albutein 250 g/l, solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human albumin Albutein 250 g/l is a solution containing 250 g/l of total protein of
Critical Heart Disease in the Newborn. What you need to know
 Critical Heart Disease in the Newborn What you need to know DISCLOSURES Nothing to report OBJECTIVES DESCRIBE NEONATAL CARDIOVASCULAR PHYSIOLOGY RECOGNIZE NEONATAL CARDIAC EMERGENCIES FORMULATE TREATMENT
Critical Heart Disease in the Newborn What you need to know DISCLOSURES Nothing to report OBJECTIVES DESCRIBE NEONATAL CARDIOVASCULAR PHYSIOLOGY RECOGNIZE NEONATAL CARDIAC EMERGENCIES FORMULATE TREATMENT
PRODUCT INFORMATION. IPRAVENT Inhalation Solution Multi Dose NAME OF THE MEDICINE DESCRIPTION PHARMACOLOGY. Ipratropium Bromide BP
 PRODUCT INFORMATION IPRAVENT Inhalation Solution Multi Dose NAME OF THE MEDICINE Ipratropium Bromide BP Ipratropium bromide is a quaternary isopropyl derivative of atropine. Its chemical name is (1R, 3r,
PRODUCT INFORMATION IPRAVENT Inhalation Solution Multi Dose NAME OF THE MEDICINE Ipratropium Bromide BP Ipratropium bromide is a quaternary isopropyl derivative of atropine. Its chemical name is (1R, 3r,
