Instructions for Use
|
|
|
- Cora Manning
- 6 years ago
- Views:
Transcription
1 CONTINENCE RESTORATION SYSTEM Instructions for Use Humanitarian Device Authorized by Federal (USA) Law for use in the treatment of fecal incontinence in patients who are not candidates for or have previously failed conservative treatment and less invasive therapy options (e.g., injectable bulking agents, radiofrequency ablation, sacral nerve stimulation). The effectiveness of this device for this use has not been demonstrated. Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician.
2 1. SYSTEM DESCRIPTION The FENIX Continence Restoration System (FENIX System) is comprised of the following components: FENIX Implant FENIX Sizing Tool (packaged separately) FENIX Introducer Tool (packaged separately) The FENIX Implant consists of a series of titanium beads with magnetic cores that are connected with independent titanium wires to form an annular shape (Figure 1). The attractive force of the magnetic beads is designed to prevent involuntary opening of the anal canal (Figure 2). The implant is offered in multiple sizes to accommodate variation in sphincter size. The sizes are denoted by the model number (e.g., FXS18 = 18 Bead Implant). The FENIX Sizing Tool is utilized to associate the anal sphincter size to an appropriate FENIX Implant. The FENIX Introducer Tool is used to assist in placing the sizing tool and the implant, if desired. Figure 1 Illustration of Implant Figure 2 Device Implanted 2. INDICATION FOR USE he FENIX Continence Restoration System is indicated for the treatment of fecal incontinence in patients who are not candidates for or have previously failed conservative treatment and less invasive therapy options (e.g., injectable bulking agents, radiofrequency ablation, sacral nerve stimulation). 3. CONTRAINDICATIONS 3.1. Do not implant the FENIX device in patients with suspected or known allergies to titanium. 4. WARNINGS 4.1. Patients with diabetes, other immunocompromised disease, or open sores near the site of surgery may have increased risk of infection associated with a prosthesis. Infection that fails to respond to antibiotic therapy may result in removal of the implant The FENIX Implant is MR Unsafe. After implantation, the patient should not be exposed to an MRI environment. The MRI environment could cause serious injury to the patient and/or interfere with the magnetic strength and the function of the implant. A recommendation should be made to patients receiving the FENIX Implant to register their implant with the MedicAlert Foundation ( or equivalent organization. In the event alternative diagnostic procedures cannot be used and MRI is required, the FENIX Implant can be removed The implant should not be exposed to temperatures above 60 C (140 F) as this could adversely affect the magnets and the function of the device. Page 2 of 12
3 4.4. Erosion may be caused by infection, improper sizing, or tissue damage. The implant may erode through adjacent tissue (e.g., the anal wall or through the perineal skin). 5. PRECAUTIONS 5.1. The safety and effectiveness of the FENIX Implant has not been established for the following conditions: Anal sphincter sizes smaller or larger than offered FENIX Implant size range Congenital anorectal/pelvic malformation Suspected or confirmed anal or rectal cancer History of pelvic radiation No rectal reservoir due to resection External full thickness rectal prolapse Significant obstructed defecation or other significant chronic defecatory motility disorders Inflammatory Bowel Disease (Crohn s and ulcerative colitis) Watery diarrhea that is unmanageable by medication or diet changes Active pelvic infection Systemic disease as source of FI (e.g., scleroderma, dementia) Pregnant or plan to become pregnant 5.2. Implantation of the FENIX device should only be performed by physicians who have received product specific training The sterile package and implant should be inspected prior to use. If sterility or performance of the device is suspect or compromised, it should not be used The device is intended for single use only. Do NOT re-sterilize the device. Functionality and sterility of the device cannot be assured if re-used The implant is magnetic and will be attracted to ferrous objects in the surgical field and other surgical instruments that are ferromagnetic Patients should undergo a monthly rectal examination for 3 months after placement of the implant or if new onset or worsening of pain, wound drainage, or bleeding occurs to further mitigate the risk of infection or device erosion. 6. ADVERSE EVENTS 6.1. Adverse events that may result from the use of the FENIX Implant are those commonly associated with general surgical procedures as well as those associated with the device specifically Potential adverse events associated with general surgery and anesthesia include adverse reaction to anesthesia (headache, muscle pain, nausea), anaphylaxis, cardiac arrest, death, fever, hypotension, hypoxemia, infection, myocardial infarction, pneumonia, pulmonary embolism, respiratory distress, thrombophlebitis, and vomiting Potential adverse events associated specifically with the FENIX implant include bleeding, death, device erosion, device explant/re-operation, device failure, device migration (device does not appear to be at implant site), impaction or defecatory disorder, impaired colonic motility, inability to pass gas, infection, injury to the anus, rectum, or vagina, pain, pruritus ani, recto-vaginal fistula, constipation, and worsening of pre-operative symptoms. Additional unknown risks may exist Safety results follow for a completed clinical study that enrolled 35 subjects. Adverse events were assessed and documented from the time of implant and throughout study participation. The reported adverse events are based on the investigator s reporting of onset, relatedness to device and/or procedure, severity and status. All data collected in the study is presented for adverse events (AEs), serious adverse events (SAEs), explants, and other surgical interventions. Table 1 below provides an accounting of the number of subjects that completed each follow-up visit. The events reported in Tables 2-5 include data from all follow-up visits completed during the study. Page 3 of 12
4 Table 1 Subject Accountability Through Study Completion Subject Status Month 12 Month 24 Month 36 Month 48 Month 60 Follow-up Visit Completed Missed Visit Completed Requirements Explanted Deceased* Lost to Follow-up/Withdrawal Total Subjects *Patient death caused by cirrhosis of the liver, unrelated to device or procedure. One patient completed the study requirements, and exited at 12 months No deaths, life-threatening conditions or unanticipated adverse effects due to the device were reported in the clinical study. One patient death was reported as caused by cirrhosis of the liver and unrelated to the device or procedure A total of 30 adverse events in 20 subjects were reported as related to the device and/or procedure (Table 2). The most frequently reported adverse events related to the device and/or procedure included impaction or defecatory disorder, pain, device erosion, infection, and bleeding. Table 2 Adverse Events Related to Device and/or Procedure or Relationship Unknown Adverse Event Impaction or defecatory disorder AE (n) Related or Unknown Mild 2 Moderate 2 Severe 2 Subj. AE % (n) 1 (n) Subj. % (n) AE (n) Subj. % (n) AE (n) Subj. % (n) % (7) 3 8.6% (3) % (4) 1 2.9% (1) Pain % (7) % (5) 2 5.7% (2) 0 0% Device Erosion % (4) 1 2.9% (1) 2 5.7% (2) 1 2.9% (1) Infection % (4) 0 0% 0 0% % (4) Bleeding % (4) 3 8.6% (3) 1 2.9% (1) 0 0% Constipation 2 5.7% (2) 1 2.9% (1) 1 2.9% (1) 0 0% Allergy, inflammation reaction 1 2.9% (1) 0 0% 0 0% 0 0% Total % (20) % (11) % (9) % (6) 1 Subjects may have had more than one type of event. Subjects may have had more than one event of the same type. 2 The rating of severity (mild, moderate or severe) describes the extent for which the adverse event impacts the subject s ability to perform usual activities. An AE is considered to be severe if found to be incapacitating with inability to do work or usual activities. Page 4 of 12
5 There were seven (7) events in six (6) subjects reported as serious adverse events (SAEs) related or unknown to the device and/or procedure. Adverse events are considered serious if the event results in death, is life-threatening, requires subject hospitalization (>24 hours), requires prolongation of existing hospitalization, results in persistent or significant disability, or requires intervention to prevent impairment. All six severe adverse events listed in Table 2 were also classified as serious adverse events in Table 3. The serious adverse event rate was 17.1% (6/35) (Table 3). Four (4) subjects had the device explanted related to the SAE. Nearly all SAEs occurred <30 days after the implant procedure. The SAEs resolved with no residual effects in all cases. Table 3 Serious Adverse Events (Related or Unknown) Device Events Subjects Serious Adverse Event Explant (n) %(n) 1 (n) Days from Onset to Implant Infection % (4) 3 7, 9, 15, and 251 Device erosion 1 2.9% (1) 1 28 Allergy, inflammation reaction 1 2.9% (1) 0 2 Impaction or defecatory disorder 1 2.9% (1) 0 23 Total % (6) 4 Not Applicable 1 Subjects may have had more than one type of event. Subject The device explant rate was 20% (7/35). Reasons for device explant included device erosion, infection and lack of effect (Table 4). All device removals required a surgical intervention, with the exception of one that was reported as passing during defecation. Surgical removal was performed safely in all cases with no complications or clinical sequelae. Table 4 Device Explant by Reason and Number of Events Reason for Device Explant Number of Events Infection 3 Device erosion 3 Lack of effect 1 Total A total of eight (8) subjects underwent device explant, other surgical intervention or a combination of device explant and other surgical intervention (Table 5) related to the device or the condition of FI. Other surgical interventions included stoma creation in three (3) subjects and placement of sacral nerve stimulation in one (1) subject. Device Explant Table 5 Device Explants and Other Surgical Intervention Reason for Device Explant Other Surgical Intervention Number of Surgical Interventions 01 Yes Device Erosion No None * 02 Yes Device Erosion No 1 03 Yes Infection No 1 04 Yes Infection No 1 05 Yes Continuing FI Stoma, lack of effect 1 06 Yes Infection Stoma, lack of effect 2 07 Yes Device Erosion Sacral nerve stimulation, lack of effect 2 08 No NA Stoma, impaction/defecatory disorder 1 * Subject 01: Device eroded through mucosa and is suspected to have passed through the anus; no intervention required. Subject 05: Device explant and stoma performed during the same surgical procedure. Page 5 of 12
6 Details of the device explants and other surgical interventions are provided below: One subject upon pelvic x-ray was found to have the device separated at the suture. No intervention was performed. Subsequently, it was presumed that the device passed through the anal mucosa during toileting at 47 days post-implant. The event resolved without sequelae. One subject was noted to have device erosion during a rectal exam 27 days post-implant. The device was explanted 28 days post-implant. One subject developed an infection that was not responsive to antibiotics. The device was explanted 47 days post-implant due to the infection. One subject continued to have fecal incontinence symptoms and at 69 days post-implant elected to have the device removed and underwent reoperation for a stoma creation. One subject experienced an infection, which resulted in device explant 41 days postimplant. After removal, the subject underwent reoperation for the creation of a stoma. One subject elected to have the device explanted and sacral nerve stimulation implanted 906 days post-implant due to reoccurring vaginal discharge secondary to device erosion. One subject experienced an infection that resulted in device explant 261 days postimplant. One subject underwent stoma creation for defecatory disorder (constipation) 154 days post-implant. The FENIX Device was not explanted. 7. CLINICAL STUDY A multicenter, prospective, non-randomized clinical study was conducted to demonstrate the safety and probable benefit of the FENIX implant. A total of 35 subjects were enrolled. Fifteen (15) subjects were enrolled at two (2) institutions in the U.S and 20 subjects were enrolled at two (2) institutions outside the U.S. Subjects enrolled in the study had severe fecal incontinence (FI), and had previously failed conservative and/or less invasive therapies. The primary safety parameter was the rate of all adverse events (AEs) monitored through the entire course of the clinical study from implant through subject withdrawal. AE reporting was based on the investigator s reporting of onset, relatedness to device and/or procedure, severity and status. Device and/or procedure-related events were summarized separately (Please see Section 6.0 for safety results of this study). Probable benefit of the FENIX implant was characterized as the reduction of FI symptoms evaluated by a subject-completed bowel diary and improved quality of life using a selfadministered questionnaire, the Fecal Incontinence Quality of Life (FIQOL) Eligibility Inclusion Criteria Enrollment was limited to subjects that met the following inclusion criteria: Age > 19 years, < 85 years, life expectancy >3 years Documented history of severe fecal incontinence for at least 6 months Subject diary documents > 2 episodes per week on average over diary period; leakage greater than seepage Subject had failed standard conservative and medical therapy Subject was a surgical candidate Subject was willing and able to cooperate with follow-up examinations Subject has been informed of the study procedures and the treatment and has signed an informed consent form and provided authorization to use and disclose information for research purposes. Page 6 of 12
7 Exclusion Criteria Subjects were not permitted to enroll in the FENIX study if they met any of the following exclusion criteria: Patient had a history of significant obstructed defecation or other significant chronic defecatory motility disorders Patient had current, external full thickness rectal or vaginal prolapse Patient had an electric or metallic implant within 10cm of the area of device placement Patient had Inflammatory Bowel Disease Patient had Irritable Bowel Syndrome Patient has systemic disease as source of FI (scleroderma, neurologic disorders, Crohn s) Patient had active pelvic infection Patient had chronic diarrhea Patient diagnosed with anal, rectal, or colon cancer within 2 years Patient had prior anterior resection of the rectum Patient had undergone pelvic radiation therapy Patient had significant scarring of the recto-vaginal septum, or a history of recto-vaginal fistula Patient had previous anorectal posterior compartment surgery The FENIX procedure was an emergency procedure Patient was being treated with another investigational drug or device Patient couldn t understand trial requirements or was unable to comply with follow-up schedule Patient was pregnant, nursing, or planned to become pregnant Patient had a history of complex anal fistula Baseline Demographics and Characteristics The baseline demographics are summarized below: There were 34 females (97.1%) and 1 male (2.9%) Mean age at the time of enrollment was 64.1 years (range 41.5 to 77.7 years) 100% were Caucasian Mean body mass index was 26.8 (range 18.1 to 48.5) Etiology of FI by percentage of subjects was 53.1% obstetric trauma, 25.0% neuropathic and 18.8% idiopathic FI incontinence type by percentage of subjects was 12.1% passive incontinence (no awareness of stool loss), 24.2% urge incontinence (inability to deter defecation), and 63.6% both Probable Benefit Bowel Diary Subjects completed a 21-day bowel diary to collect and evaluate the number of FI episodes per week, FI days per week, and number of urgent episodes per week. FI symptoms were considered improved if the bowel diary parameters showed at a least 50% reduction at follow-up compared to baseline. Success criteria applied to the bowel diary data were as follows: Proportion of subjects achieving at least a 50% reduction in FI episodes per week when compared to baseline Proportion of subjects achieving at least a 50% reduction in FI days per week when compared to baseline Proportion of subjects achieving at least a 50% reduction in urgent episodes per week when compared to baseline Page 7 of 12
8 For evaluable subjects (subjects completing a bowel diary at follow-up), a reduction of at least 50% was achieved in a clinically significant number of evaluated subjects for FI episodes, FI days, and urgent episodes (Table 6). The treatment group (all subjects implanted) showed the majority of subjects had a reduction of at least 50% in FI episodes per week at all but one follow-up interval (Table 7). Outcome (per week) >50% reduction in FI episodes Table 6 Reduction in FI from Baseline (Evaluable Subjects) 6 Months 82.1% (23/28) >50% reduction in FI days 71.4% (20/28) >50% reduction in urgent episodes 84.6% (22/26)* 12 Months 78.6% (22/28) 67.9% (19/28) 50.0% (13/26)* 24 Months 70.4% (19/27) 59.3% (16/27) 48.0% (12/25)* *Two patients at each follow-up were not evaluable for this specific criterion. 36 Months 90.9% (20/22) 77.3% (17/22) 65.0% (13/20)* 48 Months 81.8% (18/22) 68.2% (15/22) 75.0% (15/20)* 60 Months 72.7% (16/22) 72.7% (16/22) 60.0% (12/20)* Outcome (per week) >50% reduction in FI episodes >50% reduction in FI days >50% reduction in urgent episodes Table 7 Reduction in FI from Baseline (Treatment Group) 6 Months 12 Months 24 Months 36 Months 65.7% (23/35) 57.1% (20/35) 62.9% (22/35) 62.9% (22/35) 54.3% (19/35) 37.1% (13/35) 54.3% (19/35) 45.7% (16/35) 34.3% (12/35) 57.1% (20/35) 48.6% (17/35) 37.1% (13/35) 48 Months 51.4% (18/35) 42.9% (15/35) 42.9% (15/35) 60 Months 45.7% (16/35) 45.7% (16/35) 34.3% (12/35) Improvement in bowel diary parameters reported as number of FI episodes and urgent episodes, or days per week are presented in Table 8. The reduction in number of FI days per week, FI episodes per week, and urgency episodes per week as reported by subjects reduced significantly. FI episodes decreased from 13.9 episodes at baseline, to 2.9 episodes per week at 60 months. The mean number of FI days per week decreased from 6.0 days per week at baseline to 1.9 at 60 months, and urgency episodes decreased from 6.7 episodes per week to just 2.6 at 60 months post-implant. Table 8 Bowel Diary Parameters Reported as Mean FI Episodes or Days per Week Bowel Diary Baseline 6 Months 12Months Months Months Months Parameters N=35 N=28 N=28 N=27 N=22 N=22 60 Months N=22 FI Episodes per Week 13.9± ± ± ± ± ± ±3.1 FI Days per Week 6.0± ± ± ± ± ± ±1.8 Urgency Episodes per Week 1 Values are means ±SD 6.7± ± ± ± ± ± ± Fecal Incontinence Quality of Life (FIQOL) Questionnaire The FIQOL questionnaire is a patient-completed questionnaire designed to assess the impact of FI on various aspects of a patient s life. The FIQOL questionnaire is comprised of four scales: Lifestyle, Coping/Behavior, Depression/Self-Perception, and Embarrassment. Scores range from 1 to 4, with a score of 1 indicating the lowest functional status of quality-of-life. Figure 3 Page 8 of 12
9 presents the mean FIQOL data for subjects that fully completed the questionnaire. In some instances, subjects completed only a portion of the questionnaire, not providing sufficient data to calculate a score for that particular scale. This results in a varying number of respondents at each time point for each of the four scales. The total subjects providing complete responses for each of the four scales at all intervals included in the mean FIQOL Score calculation are provided below. Improvements from baseline are seen in all four scales at all follow-up intervals. Lifestyle: Baseline (n=34), 12 months (n=28), 24 months (n= 25), 36 months (n=24), 48 months (n=22), 60 months (n=23) Coping/Behavior: Baseline (n=35), 12 months (n=28), 24 months (n=26), 36 months (n=24), 48 months (n=22), 60 months (n=23) Depression/Self Perception: Baseline (n=33), 12 months (n=27), 24 months (n=26), 36 months (n= 23), 48 months (n=22), 60 months (n=22) Embarrassment: Baseline (n=34), 12 months (n=28), 24 months (n=26), 36 months (n=24), 48 months (n=22), 60 months (n=23) Figure 3 Mean FIQOL Scores at Baseline through 60 Months Page 9 of 12
10 8. DIRECTIONS FOR USE 8.1. Surgical Access Gain appropriate surgical access to the anal canal at the region of the external anal sphincter Dissect the soft tissues away from the outside of the external anal sphincter. Tissue should be removed to expose the outer muscle of the anal sphincter. Create a tunnel circumferentially around the external anal sphincter. Care should be taken to avoid injuring the pudendal nerve bundles Sizing of the Anal Canal Use the FENIX Sizing Tool to determine the FENIX Implant size. The FENIX Implant sizes are denoted by the model number (e.g., FXS18 = 18 Bead Implant) Guide the FENIX Introducer Tool around the anal canal tunnel per the Introducer Tool instructions for use Bring the FENIX Sizing Tool into the surgical field Thread the FENIX Sizing Tool suture through the cross-hole feature of the FENIX Introducer Tool Rotate the FENIX Introducer Tool to place the FENIX Sizing Tool around the anal canal in the surgically created tunnel Perform sizing per the appropriate FENIX Sizing Tool instructions for use and subsequently remove the Sizing Tool Placement of the FENIX Implant Guide the FENIX Introducer Tool around the anal canal tunnel per the Introducer Tool instructions for use Bring the chosen FENIX Implant into the surgical field Thread the FENIX Implant suture through the cross-hole feature of the FENIX Introducer Tool Rotate the FENIX Introducer Tool to place the FENIX Implant around the anal canal in the surgically created tunnel Remove the FENIX Introducer Tool, leaving the FENIX Implant in place around the external anal sphincter with the ends of the implant sutures exposed Locate the implant around the dissected space in the same location that was measured Verify chosen FENIX Implant size is appropriate via visual examination or fluoroscopy Using the suture provided, secure the ends of the implant with hand tied knots incorporating the green and white sutures such that the eyelets of the implant are touching or overlapping. Once secured, trim sutures above the knot and rotate the device to move the suture knot away from the surgical access wound. 9. IMPLANT REMOVAL If implant removal is necessary, carefully free the implant from surrounding tissue by dissecting along the outside of the beads, moving from anterior to posterior on each side of the anal canal. Bead links (wires) may be cut to facilitate the removal of individual segments. Particular care should be taken to avoid injury to the pudendal nerve bundles. The implant should be returned to Torax Medical Inc. to allow for technical device analysis. Page 10 of 12
11 10. PACKAGING/STORAGE The FENIX Implant is provided sterile and designed to remain sterile unless the primary product pouch has been opened or damaged. Store in a cool, dry place. If opened and not used, discard implant or return implant to Torax Medical Inc. Do Not Resterilize. 11. LIMITED WARRANTY (a) Torax warrants that the product shall be free from material defects in materials and/or workmanship, and shall perform substantially in accordance with the written specifications, through the earlier of (i) the expiration of the shelf-life as specified on the applicable product labeling or (ii) the date on which the products are used or implanted. (b) This limited warranty does not extend to damage caused by (i) abuse or misuse of any product, (ii) accident or neglect by you or a third party; (iii) use of the product other than in accordance with Torax s instructions or specifications; or (iv) any alterations made to the product after shipment. (c) Torax s entire liability and your exclusive remedies under this limited warranty are, at Torax s option, for Torax to use commercially reasonable efforts to fix or replace the defective product. (d) EXCEPT AS EXPRESSLY STATED ABOVE, TORAX MAKES NO WARRANTIES, EXPRESS OR IMPLIED, WRITTEN OR ORAL, BY OPERATION OF LAW OR OTHERWISE, OF ANY PRODUCTS OR SERVICES FURNISHED UNDER OR IN CONNECTION WITH THIS AGREEMENT. TORAX DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, AND THOSE WARRANTIES ARISING BY STATUTE OR OPERATION OF LAW, OR FROM A COURSE OF DEALING OR USAGE OR TRADE. Page 11 of 12
12 Manufacturer 4188 Lexington Avenue North Shoreview, Minnesota USA Phone: (651) Fax: (651) Torax Medical, Inc. and the FENIX Continence Restoration System are registered trademarks of Torax Medical Incorporated Rev 2 November 2016 Page 12 of 12
Fecal Incontinence. What is fecal incontinence?
 Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
Instructions for Use Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician.
 Instructions for Use Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. LINX Reflux Management System Instructions for Use Page 1 of 16 1. SYSTEM DESCRIPTION The
Instructions for Use Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. LINX Reflux Management System Instructions for Use Page 1 of 16 1. SYSTEM DESCRIPTION The
An effective and minimally invasive bridge between conservative therapy and invasive surgery for BCD (bowel control disorder).
 An effective and minimally invasive bridge between conservative therapy and invasive surgery for BCD (bowel control disorder). Mederi Therapeutics has developed this kit to help you raise awareness of
An effective and minimally invasive bridge between conservative therapy and invasive surgery for BCD (bowel control disorder). Mederi Therapeutics has developed this kit to help you raise awareness of
LINX Reflux Management System. Patient Information. Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician.
 LINX Reflux Management System Patient Information Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. 2 Table of Contents What is the LINX System? 3 Why doctors
LINX Reflux Management System Patient Information Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. 2 Table of Contents What is the LINX System? 3 Why doctors
LINX. A new, FDA approved treatment for GERD
 LINX A new, FDA approved treatment for GERD What Causes Reflux? Gastroesophageal reflux disease (GERD), also called reflux, is a chronic, often progressive disease caused by a weak lower esophageal sphincter
LINX A new, FDA approved treatment for GERD What Causes Reflux? Gastroesophageal reflux disease (GERD), also called reflux, is a chronic, often progressive disease caused by a weak lower esophageal sphincter
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
LINX Reflux Management System
 LINX Reflux Management System Patient Information Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. LINX Reflux Management System 2 Table of Contents What is the
LINX Reflux Management System Patient Information Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. LINX Reflux Management System 2 Table of Contents What is the
Accidental Bowel Leakage (Fecal Incontinence)
 Accidental Bowel Leakage (Fecal Incontinence) What is Accidental Bowel Leakage (ABL)? Accidental bowel leakage is the inability to control solid or liquid stool. This is the inability to control gas and
Accidental Bowel Leakage (Fecal Incontinence) What is Accidental Bowel Leakage (ABL)? Accidental bowel leakage is the inability to control solid or liquid stool. This is the inability to control gas and
q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE
 493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
A PATIENT GUIDE TO Understanding Stress Urinary Incontinence
 A PATIENT GUIDE TO Understanding Stress Urinary Incontinence Q: What is SUI? A: Stress urinary incontinence is defined as the involuntary leakage of urine. The problem afflicts approximately 18 million
A PATIENT GUIDE TO Understanding Stress Urinary Incontinence Q: What is SUI? A: Stress urinary incontinence is defined as the involuntary leakage of urine. The problem afflicts approximately 18 million
Duc M. Vo, MD, FACS Northwest Surgical Specialists
 Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
Hemorrhoids. Carlos R. Alvarez-Allende PGY-III Colorectal Surgery
 Hemorrhoids Carlos R. Alvarez-Allende PGY-III Colorectal Surgery Overview Anatomy Classification Etiology Incidence Symptoms Differential Diagnosis Medical Management Surgical Management Anatomy Anal canal
Hemorrhoids Carlos R. Alvarez-Allende PGY-III Colorectal Surgery Overview Anatomy Classification Etiology Incidence Symptoms Differential Diagnosis Medical Management Surgical Management Anatomy Anal canal
Treatments for Fecal Incontinence A Review of the Research for Adults
 Treatments for Fecal Incontinence A Review of the Research for Adults e Is This Information Right for Me? This information is right for you if: Your health care professional* said you or your loved one
Treatments for Fecal Incontinence A Review of the Research for Adults e Is This Information Right for Me? This information is right for you if: Your health care professional* said you or your loved one
Novel Options for the Management of Fecal Incontinence
 Novel Options for the Management of Fecal Incontinence Arnold Wald, MD, MACG University of Wisconsin School of Medicine and Public Health, Madison WI ANORECTAL CONTINENCE MECHANISMS Reservoir Elements
Novel Options for the Management of Fecal Incontinence Arnold Wald, MD, MACG University of Wisconsin School of Medicine and Public Health, Madison WI ANORECTAL CONTINENCE MECHANISMS Reservoir Elements
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
PREPARING FOR ANORECTOAL MANOMETRY. ManoScan Anorectal Manometry System
 PREPARING FOR ANORECTOAL MANOMETRY ManoScan Anorectal Manometry System WHAT IS ANORECTAL MANOMETRY? Anorectal manometry is a test used to evaluate the function and coordination of the sphincter and pelvic
PREPARING FOR ANORECTOAL MANOMETRY ManoScan Anorectal Manometry System WHAT IS ANORECTAL MANOMETRY? Anorectal manometry is a test used to evaluate the function and coordination of the sphincter and pelvic
Pelvic Floor Disorders. Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon
 Pelvic Floor Disorders Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon What is Pelvic Floor Disorder Surgical perspective symptoms of RED, FI or prolapse on the background
Pelvic Floor Disorders Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon What is Pelvic Floor Disorder Surgical perspective symptoms of RED, FI or prolapse on the background
A Case of Fecal Incontinence: Medical and Interventional Treatment Options
 A Case of Fecal Incontinence: Medical and Interventional Treatment Options HPI JP is a 69 year-old F with a 12-month history of FI. Her symptoms began after a colonoscopy She has been experiencing passive
A Case of Fecal Incontinence: Medical and Interventional Treatment Options HPI JP is a 69 year-old F with a 12-month history of FI. Her symptoms began after a colonoscopy She has been experiencing passive
Rectal Cancer. About the Colon and Rectum. Symptoms. Colorectal Cancer Screening
 Patient information regarding care and surgery associated with RECTAL CANCER by Robert K. Cleary, M.D., John C. Eggenberger, M.D., Amalia J. Stefanou., M.D. location: Michigan Heart and Vascular Institute,
Patient information regarding care and surgery associated with RECTAL CANCER by Robert K. Cleary, M.D., John C. Eggenberger, M.D., Amalia J. Stefanou., M.D. location: Michigan Heart and Vascular Institute,
2/5/2016. ABS Complications. Anal Slings-investigational
 ABS Complications Anal Slings-investigational Similar to transvaginal tape or transobturator tape for UI Dacron, mersilene, polyester, and teflon mesh, fascia lata Wound infections, sinus tract, t ulcer
ABS Complications Anal Slings-investigational Similar to transvaginal tape or transobturator tape for UI Dacron, mersilene, polyester, and teflon mesh, fascia lata Wound infections, sinus tract, t ulcer
Stapled transanal rectal resection for obstructed defaecation syndrome
 Stapled transanal rectal resection for obstructed Issued: June 2010 www.nice.org.uk/ipg351 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Stapled transanal rectal resection for obstructed Issued: June 2010 www.nice.org.uk/ipg351 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Sacral Nerve Stimulation for Faecal Incontinence
 Sacral Nerve Stimulation for Faecal Incontinence Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital
Sacral Nerve Stimulation for Faecal Incontinence Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida
 Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida Coloplast develops products and services that make life easier for people with very personal and private
Peristeen and the Neurogenic Bowel Dysfunction Score (NBD) for pediatric patients with spina bifida Coloplast develops products and services that make life easier for people with very personal and private
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
SACRAL NERVE STIMULATION FOR EXPERIENCE IN CHILDREN
 SACRAL NERVE STIMULATION FOR COLORECTAL DISEASES: EXPERIENCE IN CHILDREN C. LOUIS-BORRIONE - JM. GUYS TIMONE-ENFANTS MARSEILLE SACRAL NEUROMODULATION IN CHILDREN 26 : Humphreys et al - 23 children with
SACRAL NERVE STIMULATION FOR COLORECTAL DISEASES: EXPERIENCE IN CHILDREN C. LOUIS-BORRIONE - JM. GUYS TIMONE-ENFANTS MARSEILLE SACRAL NEUROMODULATION IN CHILDREN 26 : Humphreys et al - 23 children with
Surgery and Crohn s. Crohn s Disease 70 % Why Operate? Complications of Disease. The Gastrointestinal Tract. Surgery for Inflammatory Bowel Disease
 The Gastrointestinal Tract Surgery for Inflammatory Bowel Disease Jonathan Chun, MD The regon Clinic Gastrointestinal and Minimally Invasive Surgery Crohn s Disease Can affect anywhere in the GI tract,
The Gastrointestinal Tract Surgery for Inflammatory Bowel Disease Jonathan Chun, MD The regon Clinic Gastrointestinal and Minimally Invasive Surgery Crohn s Disease Can affect anywhere in the GI tract,
2. Have your symptoms affected your ability to carry out your daily activities? YES NO
 QUESTIONNAIRE Page 1 of 5 Date: Referring MD (Name, Address, Phone Number): Primary Care Physician (Name and Address, Phone Number): Reason for visit: 1. How long have you had symptoms? Describe your symptoms?
QUESTIONNAIRE Page 1 of 5 Date: Referring MD (Name, Address, Phone Number): Primary Care Physician (Name and Address, Phone Number): Reason for visit: 1. How long have you had symptoms? Describe your symptoms?
GI Physiology - Investigating and treating patients with pelvic floor dysfunction. Lynne Smith Department of GI Physiology NGH Sheffield
 GI Physiology - Investigating and treating patients with pelvic floor dysfunction Lynne Smith Department of GI Physiology NGH Sheffield Aims o o o To give an overview of lower GI investigations To demonstrate
GI Physiology - Investigating and treating patients with pelvic floor dysfunction Lynne Smith Department of GI Physiology NGH Sheffield Aims o o o To give an overview of lower GI investigations To demonstrate
Colorectal procedure guide
 Colorectal procedure guide Illustrations by Lisa Clark Biodesign ADVANCED TISSUE REPAIR cookmedical.com 2 INDEX Anal fistula repair Using the Biodesign plug with no button.... 4 Anal fistula repair Using
Colorectal procedure guide Illustrations by Lisa Clark Biodesign ADVANCED TISSUE REPAIR cookmedical.com 2 INDEX Anal fistula repair Using the Biodesign plug with no button.... 4 Anal fistula repair Using
ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION. Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital
 ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital Accidental Bowel Leakage What Gets the Woman into Your Office 67%
ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital Accidental Bowel Leakage What Gets the Woman into Your Office 67%
LOTRONEX and its authorized generic alosetron hydrochloride:
 LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The Prescribing Program for LOTRONEX TM Prescriber Education Slide Deck PROMETHEUS and the Link Design
LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The Prescribing Program for LOTRONEX TM Prescriber Education Slide Deck PROMETHEUS and the Link Design
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
Bard: Continence Therapy. Stress Urinary Incontinence. Regaining Control. Restoring Your Lifestyle.
 Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Urinary incontinence is a common problem and one that can be resolved by working
Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Urinary incontinence is a common problem and one that can be resolved by working
SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
Pelvic Prolapse. A Patient Guide to Pelvic Floor Reconstruction
 Pelvic Prolapse A Patient Guide to Pelvic Floor Reconstruction Pelvic Prolapse When an organ becomes displaced, or slips down in the body, it is referred to as a prolapse. Your physician has diagnosed
Pelvic Prolapse A Patient Guide to Pelvic Floor Reconstruction Pelvic Prolapse When an organ becomes displaced, or slips down in the body, it is referred to as a prolapse. Your physician has diagnosed
Voiding Diary. Begin recording upon rising in the morning and continue for a full 24 hours.
 Urodvnamics Your physician has scheduled you for a test called URODYNAMICS. This test is a series of different measurements of bladder function and can be used to determine the cause of a variety of bladder
Urodvnamics Your physician has scheduled you for a test called URODYNAMICS. This test is a series of different measurements of bladder function and can be used to determine the cause of a variety of bladder
Understanding the Benefits and Risks
 LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The LOTRONEX REMS Program Prescriber Education Slide Deck LOTRONEX is a registered trademark of Prometheus
LOTRONEX and its authorized generic alosetron hydrochloride: Understanding the Benefits and Risks The LOTRONEX REMS Program Prescriber Education Slide Deck LOTRONEX is a registered trademark of Prometheus
2/5/2016. Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach
 Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach Holly E Richter, PhD, MD, FACOG, FACS J Marion Sims Professor Obstetrics and Gynecology Professor
Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach Holly E Richter, PhD, MD, FACOG, FACS J Marion Sims Professor Obstetrics and Gynecology Professor
FISTULA CARE. FISTULA TREATMENT COMPLICATIONS: Reporting Guidelines. Updated 12/12/12
 FISTULA CARE FISTULA TREATMENT COMPLICATIONS: Reporting Guidelines Updated 12/12/12 EngenderHealth, 440 Ninth Avenue, New York, NY 10001, USA Telephone: 212-561-8000; Fax: 212-561-8067; E-mail: fistulacare@engenderhealth.org
FISTULA CARE FISTULA TREATMENT COMPLICATIONS: Reporting Guidelines Updated 12/12/12 EngenderHealth, 440 Ninth Avenue, New York, NY 10001, USA Telephone: 212-561-8000; Fax: 212-561-8067; E-mail: fistulacare@engenderhealth.org
Surgical Management of IBD in the Age of Biologics
 Surgical Management of IBD in the Age of Biologics Lisa S. Poritz, M.D Associate Professor of Surgery Division of Colon and Rectal Surgery Objectives Discuss surgical management of IBD When to operate
Surgical Management of IBD in the Age of Biologics Lisa S. Poritz, M.D Associate Professor of Surgery Division of Colon and Rectal Surgery Objectives Discuss surgical management of IBD When to operate
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
3. Guarantee conditions
 Straumann Guarantee Straumann Guarantee (Valid as of April 1, 2012) 1. Guarantee beneficiary and scope This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland
Straumann Guarantee Straumann Guarantee (Valid as of April 1, 2012) 1. Guarantee beneficiary and scope This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Small Bowel and Colon Surgery
 Small Bowel and Colon Surgery Why Do I Need a Small Bowel Resection? A variety of conditions can damage your small bowel. In severe cases, your doctor may recommend removing part of your small bowel. Conditions
Small Bowel and Colon Surgery Why Do I Need a Small Bowel Resection? A variety of conditions can damage your small bowel. In severe cases, your doctor may recommend removing part of your small bowel. Conditions
The Role of Surgery in Inflammatory Bowel Disease. Cory D Barrat, MD Colon and Rectal Surgeon Mercy Health
 The Role of Surgery in Inflammatory Bowel Disease Cory D Barrat, MD Colon and Rectal Surgeon Mercy Health THANKS FOR INVITING ME! I have no financial disclosures Outline - Who am I and what do I do? -
The Role of Surgery in Inflammatory Bowel Disease Cory D Barrat, MD Colon and Rectal Surgeon Mercy Health THANKS FOR INVITING ME! I have no financial disclosures Outline - Who am I and what do I do? -
2. STRAUMANN PRODUCTS COVERED BY THE STRAUMANN GUARANTEE. Abutment attached to an implant* Replacement with equivalent ceramic abutment**
 Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
Fecal Incontinence. Inability to retain feces or bowel movements, resulting in involuntary passage of feces or bowel movements
 Fecal Incontinence (Involuntary Passage of Feces or Bowel Movements) Basics OVERVIEW Inability to retain feces or bowel movements, resulting in involuntary passage of feces or bowel movements GENETICS
Fecal Incontinence (Involuntary Passage of Feces or Bowel Movements) Basics OVERVIEW Inability to retain feces or bowel movements, resulting in involuntary passage of feces or bowel movements GENETICS
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use. Polyurethane Dual Lumen Occlusion Catheter
 Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS. A08/S/d Colorectal: Faecal Incontinence (Adult)
 A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
A Nursing Assessment Tool for Adults With Fecal Incontinence
 Journal of Wound, Ostomy and Continence Nursing 2000, 279- A Nursing Assessment Tool for Adults With Fecal Incontinence Christine Norton, MA, RN, and Sonya Chelvanayagam, MSc, RN Abstract Fecal incontinence
Journal of Wound, Ostomy and Continence Nursing 2000, 279- A Nursing Assessment Tool for Adults With Fecal Incontinence Christine Norton, MA, RN, and Sonya Chelvanayagam, MSc, RN Abstract Fecal incontinence
Incontinence; Lets talk about it. Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery
 Incontinence; Lets talk about it Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery Select the most appropriate subtitle for this talk A: Bladders gone wild! B: There s no such thing
Incontinence; Lets talk about it Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery Select the most appropriate subtitle for this talk A: Bladders gone wild! B: There s no such thing
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Robotic Ventral Rectopexy
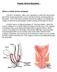 Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
03/13/18. A. Symptoms lasting for greater than or equal to 12 months that have resulted to significant impairment in activities of daily living; and
 Reference #: MC/I008 Page: 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/I008 Page: 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
ACG Clinical Guideline: Management of Benign Anorectal Disorders
 ACG Clinical Guideline: Management of Benign Anorectal Disorders Arnold Wald, MD, MACG 1, Adil E. Bharucha, MBBS, MD 2, Bard C. Cosman, MD, MPH, FASCRS 3 and William E. Whitehead, PhD, MACG 4 1 Division
ACG Clinical Guideline: Management of Benign Anorectal Disorders Arnold Wald, MD, MACG 1, Adil E. Bharucha, MBBS, MD 2, Bard C. Cosman, MD, MPH, FASCRS 3 and William E. Whitehead, PhD, MACG 4 1 Division
Management of Neurogenic Bowel Dysfunction. Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders
 Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Current Perspectives in Fecal Incontinence Treatment: The Use of Devices for the Management of Fecal Incontinence-An Evidence-Based Discussion
 Current Perspectives in Fecal Incontinence Treatment: The Use of Devices for the Management of Fecal Incontinence-An Evidence-Based Discussion Holly E. Richter, PhD, MD, FACOG, FACS J Marion Sims Professor
Current Perspectives in Fecal Incontinence Treatment: The Use of Devices for the Management of Fecal Incontinence-An Evidence-Based Discussion Holly E. Richter, PhD, MD, FACOG, FACS J Marion Sims Professor
PATIENT INFORMATION REMEDY ACETABULAR CUP. Overview
 PATIENT INFORMATION REMEDY ACETABULAR CUP 1 Overview The REMEDY Hip Spacer is a temporary hip implant that is used as part of a two-stage procedure. If you are receiving the REMEDY Hip Spacer, your surgeon
PATIENT INFORMATION REMEDY ACETABULAR CUP 1 Overview The REMEDY Hip Spacer is a temporary hip implant that is used as part of a two-stage procedure. If you are receiving the REMEDY Hip Spacer, your surgeon
Sacral Nerve Neuromodulation / Stimulation
 Sacral Nerve Neuromodulation / Stimulation Policy Number: 7.01.69 Last Review: 2/2018 Origination: 2/2001 Next Review: 2/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Sacral Nerve Neuromodulation / Stimulation Policy Number: 7.01.69 Last Review: 2/2018 Origination: 2/2001 Next Review: 2/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Section H Bladder and Bowel
 Instructor Guide Section H Bladder and Bowel Objectives State the intent of Section H Bladder and Bowel. Describe how to conduct the assessment for urinary incontinence. Describe how to conduct the assessment
Instructor Guide Section H Bladder and Bowel Objectives State the intent of Section H Bladder and Bowel. Describe how to conduct the assessment for urinary incontinence. Describe how to conduct the assessment
Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters
 Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
This information is intended as an overview only
 This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
Sacral Nerve Neuromodulation/Stimulation. Description
 Subject: Sacral Nerve Neuromodulation/Stimulation Page: 1 of 17 Last Review Status/Date: September 2015 Sacral Nerve Neuromodulation/Stimulation Description Sacral nerve neuromodulation (SNM), also referred
Subject: Sacral Nerve Neuromodulation/Stimulation Page: 1 of 17 Last Review Status/Date: September 2015 Sacral Nerve Neuromodulation/Stimulation Description Sacral nerve neuromodulation (SNM), also referred
BACTISEAL Endoscopic Ventricular Catheter (REF & )
 BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
EPISIOTOMY & PERINEAL TEARS Anatomy &Functionality May Dr. Annie Leong MBBS, FRANZCOG, CU
 EPISIOTOMY & PERINEAL TEARS Anatomy &Functionality May 2011 Dr. Annie Leong MBBS, FRANZCOG, CU Restore normal perineal anatomy Achieve good haemostasis Avoid infection and wound breakdown Avoid coital
EPISIOTOMY & PERINEAL TEARS Anatomy &Functionality May 2011 Dr. Annie Leong MBBS, FRANZCOG, CU Restore normal perineal anatomy Achieve good haemostasis Avoid infection and wound breakdown Avoid coital
Chapter 19. Assisting With Bowel Elimination. Elsevier items and derived items 2014, 2010 by Mosby, an imprint of Elsevier Inc. All rights reserved.
 Chapter 19 Assisting With Bowel Elimination Normal Bowel Elimination Time and frequency of bowel movements (BMs) vary. To assist with bowel elimination, you need to know these terms: Defecation is the
Chapter 19 Assisting With Bowel Elimination Normal Bowel Elimination Time and frequency of bowel movements (BMs) vary. To assist with bowel elimination, you need to know these terms: Defecation is the
Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas. Reprocessed Device for Single Use.
 Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
TRANSCUTANEOUS MECHANICAL NERVE STIMULATOR (TMNS) User s Guide
 TRANSCUTANEOUS MECHANICAL NERVE STIMULATOR (TMNS) User s Guide TMNS is a therapeutic genital vibrator to treat sexual dysfunction or as adjunct to Kegel s exercise device. (Tightening of the muscles of
TRANSCUTANEOUS MECHANICAL NERVE STIMULATOR (TMNS) User s Guide TMNS is a therapeutic genital vibrator to treat sexual dysfunction or as adjunct to Kegel s exercise device. (Tightening of the muscles of
Lets talk about Faecal incontinence (FI) in Scleroderma
 Lets talk about Faecal incontinence (FI) in Scleroderma Dr. Shamaila Butt Gastroenterology Research Registrar GI Physiology unit University College Hospital London GI manifestations in Scleroderma Oesophagus
Lets talk about Faecal incontinence (FI) in Scleroderma Dr. Shamaila Butt Gastroenterology Research Registrar GI Physiology unit University College Hospital London GI manifestations in Scleroderma Oesophagus
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Sacral Nerve Neuromodulation / Stimulation Page 1 of 23 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Sacral Nerve Neuromodulation / Stimulation Professional Institutional
Sacral Nerve Neuromodulation / Stimulation Page 1 of 23 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Sacral Nerve Neuromodulation / Stimulation Professional Institutional
Anorectal Diagnostic Overview
 Anorectal Diagnostic Overview 11-25-09 3.11.2010 2009 2010 Anorectal Manometry Overview Measurement of pressures and the annotation of rectal sensation throughout the rectum and anal canal to determine:
Anorectal Diagnostic Overview 11-25-09 3.11.2010 2009 2010 Anorectal Manometry Overview Measurement of pressures and the annotation of rectal sensation throughout the rectum and anal canal to determine:
Discharge information for patients Fistula plug for anal fistula
 Discharge information for patients Fistula plug for anal fistula Clinical Sciences Building Colorectal Surgery 0161 206 1249 All Rights Reserved 2017. Document for issue as handout.. What is an anal fistula?
Discharge information for patients Fistula plug for anal fistula Clinical Sciences Building Colorectal Surgery 0161 206 1249 All Rights Reserved 2017. Document for issue as handout.. What is an anal fistula?
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Motility Disorders. Pelvic Floor. Colorectal Center for Functional Bowel Disorders (N = 701) January 2010 November 2011
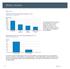 Motility Disorders Pelvic Floor Colorectal Center for Functional Bowel Disorders (N = 71) January 21 November 211 New Patients 35 3 25 2 15 1 5 Constipation Fecal Incontinence Rectal Prolapse Digestive-Genital
Motility Disorders Pelvic Floor Colorectal Center for Functional Bowel Disorders (N = 71) January 21 November 211 New Patients 35 3 25 2 15 1 5 Constipation Fecal Incontinence Rectal Prolapse Digestive-Genital
Chapter 22. Bowel Needs. Copyright 2019 by Elsevier, Inc. All rights reserved.
 Chapter 22 Bowel Needs Copyright 2019 by Elsevier, Inc. All rights reserved. Lesson 22.1 Define the key terms and key abbreviations in this chapter. Describe normal defecation and the observations to report.
Chapter 22 Bowel Needs Copyright 2019 by Elsevier, Inc. All rights reserved. Lesson 22.1 Define the key terms and key abbreviations in this chapter. Describe normal defecation and the observations to report.
Colorectal Surgery. Patient Care. Goals and Objectives
 Colorectal Surgery Patient Care 1) Interpret the results of clinical evaluations (history, physical examination) performed on patients with a) Hemorrhoids b) Perianal abscess/fistula c) Anal fissure d)
Colorectal Surgery Patient Care 1) Interpret the results of clinical evaluations (history, physical examination) performed on patients with a) Hemorrhoids b) Perianal abscess/fistula c) Anal fissure d)
Bowel Management for Children with Anorectal Malformations by Kathleen Guardino, RN, MSN
 Bowel Management for Children with Anorectal Malformations by Kathleen Guardino, RN, MSN Dr. Alberto Peña, Chief of Surgery at Schneider Children's Hospital created the posterior sagittal anorectoplasty
Bowel Management for Children with Anorectal Malformations by Kathleen Guardino, RN, MSN Dr. Alberto Peña, Chief of Surgery at Schneider Children's Hospital created the posterior sagittal anorectoplasty
Management of Urinary Incontinence in Older Women. Dr. Cecilia Cheon Department of Obs. & Gyn. Queen Elizabeth Hospital
 Management of Urinary Incontinence in Older Women Dr. Cecilia Cheon Department of Obs. & Gyn. Queen Elizabeth Hospital Epidemiology Causes Investigation Treatment Conclusion Elderly Women High prevalence
Management of Urinary Incontinence in Older Women Dr. Cecilia Cheon Department of Obs. & Gyn. Queen Elizabeth Hospital Epidemiology Causes Investigation Treatment Conclusion Elderly Women High prevalence
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
AXIOS Stent and Delivery System
 PRESCRIPTIVE INFORMATION AXIOS Stent and Delivery System Warning REFER TO THE DEVICE DIRECTIONS FOR USE FOR COMPLETE INSTRUCTIONS ON DEVICE USE. RX ONLY. CAUTION: FEDERAL LAW (USA) RESTRICTS THIS DEVICE
PRESCRIPTIVE INFORMATION AXIOS Stent and Delivery System Warning REFER TO THE DEVICE DIRECTIONS FOR USE FOR COMPLETE INSTRUCTIONS ON DEVICE USE. RX ONLY. CAUTION: FEDERAL LAW (USA) RESTRICTS THIS DEVICE
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Summary and conclusion. Summary And Conclusion
 Summary And Conclusion Summary and conclusion Rectal prolapse remain a disorder for which no single ideal treatment was approved for all cases. Complete rectal prolapse (procidentia) is the circumferential
Summary And Conclusion Summary and conclusion Rectal prolapse remain a disorder for which no single ideal treatment was approved for all cases. Complete rectal prolapse (procidentia) is the circumferential
Sacral Nerve Neuromodulation/Stimulation for Pelvic Floor Dysfunction
 Medical Policy Manual Surgery, Policy No. 134 Sacral Nerve Neuromodulation/Stimulation for Pelvic Floor Dysfunction Next Review: December 2018 Last Review: June 2018 Effective: July 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Surgery, Policy No. 134 Sacral Nerve Neuromodulation/Stimulation for Pelvic Floor Dysfunction Next Review: December 2018 Last Review: June 2018 Effective: July 1, 2018 IMPORTANT REMINDER
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
