The battle of the sexes
|
|
|
- Juliana Stafford
- 5 years ago
- Views:
Transcription
1 Mechanisms of Development 92 (2000) 89±103 Review paper The battle of the sexes Blanche Capel* Department of Cell Biology, Duke University Medical Center, Durham, NC 27710, USA Received 28 July 1999; accepted 15 October 1999 Abstract The sex determining gene, Sry, determines the sex of the organism by initiating development of a testis rather than an ovary from the cells of the bipotential gonad. In the 10 years since the discovery of Sry, new genes and cellular pathways that operate in the establishment of the gonadal primordium and the initiation of testis development have been discovered. Experiments de ning mechanisms downstream of Sry are providing clear examples of how a regulatory transcription factor initiates cellular processes including proliferation and cell migration, which in turn in uence architectural patterning, fate commitment, and differentiation of cells within an organ. q 2000 Elsevier Science Ireland Ltd. All rights reserved. Keywords: Sex determination; Sry; Sertoli cell; Leydig cell; Peritubular myoid cell; Cell migration; Vascular formation; Proliferation; Sox9; Mis; Wt1; Dax1; Sf1; M33; ATRX; Wnt4; XH2; Dmrt1; Mouse; Chicken; Alligator 1. Introduction Recently there has been a resurgence of interest in the organogenesis of the gonad due in part to the discovery of the sex determining gene, Sry (Gubbay et al., 1990; Sinclair et al., 1990). In mammals, this single gene on the Y chromosome determines the sex of the organism by initiating the development of a testis instead of an ovary from the gonadal primordium (Koopman et al., 1991). Renewed interest in the problem of gonadal development has resulted in new information about the genes and cellular pathways involved in the divergent development of this unique organ system. The gonad arises as an identical primordium in all embryos, poised in a precarious balance between male and female developmental pathways. Depending on whether or not a Y chromosome is present, this primordium follows a testis or ovarian fate. It has been recognized for some time that the study of stepwise changes in cell organization as the bipotential gonad diverges toward testis or ovary might provide important information about how the cellular architecture of organs is achieved. Much comparative descriptive work has been done on the morphological development of the testis and ovary over the last 50 years (for example see Pelliniemi, 1975; Paranko, 1987; Wartenberg et al., 1991). Against this backdrop of information, the discovery of Sry * Tel.: ; fax: address: b.capel@cellbio.duke.edu (B. Capel) provided a clear molecular anchor point for the divergence of gonad development between male and female pathways. Sry encodes a transcription factor believed to activate and/or repress target genes to initiate the male pathway (Lovell-Badge, 1992). Under the in uence of this genetic switch, the cells of the early gonad are induced to differentiate into testis-speci c cell types and to organize into testis-speci c morphology (Fig. 1). To identify the genes and cellular pathways downstream of Sry, it has been informative to compare the earliest differences between XX and XY gonads at the molecular and cellular levels. Subtractive screens to identify early molecular differences between the testis and ovary, efforts to study changes in cell organization, adhesion, and structure, and assays to identify differences in cellular pathways between testis and ovary are all providing new information. A useful feature of the gonadogenesis system is that, in contrast to other organs such as the kidney, lung or heart, the gonad is non-essential for viability. Because of this, adult carriers of mutations in genes central to gonad development often survive and are recognized because they are infertile or sex-reversed. From this important resource several genes in the sex determination cascade have been identi ed through studies of humans (for example see Pelletier et al., 1991; Muscatelli et al., 1994; Wagner et al., 1994; Ion et al., 1996) and mice (Kriedberg et al., 1993; Luo et al., 1994; Shawlot and Behringer, 1995; Miyamoto et al., 1997; Katoh-Fukui et al., 1998). Given the high conservation of the molecular players in /00/$ - see front matter q 2000 Elsevier Science Ireland Ltd. All rights reserved. PII: S (99)
2 90 B. Capel / Mechanisms of Development 92 (2000) 89± Establishment of the gonad primordium 2.1. The origin of the urogenital ridge Fig. 1. Confocal images of 11.5 and 12.5 d.p.c. gonads stained with an antibody against laminin1. The gonad (g) forms as a bipotential primordium on the ventro-medial surface of the mesonephros in XX and XY embryos. Within the mesonephros is the mesonephric duct (md) and mesonephric tubules (mt). Sry expression peaks at 11.5 d.p.c. in XY gonadal cells, inducing growth of the gonadal primordium, differentiation, and epithelialization of Sertoli cells to enclose germ cells into testis cords (tc), surrounded by a basal lamina (bl) by 12.5 d.p.c. These distinct morphological changes are not seen in XX gonads. other developmental pathways, it has been surprising to nd no conservation of the sex determining switch between species. In other animals, sex is determined by the X to autosome ratio (Cline and Meyer, 1996), environmental factors (Ferguson and Joanen, 1982; Bull, 1983), or social cues (Francis et al., 1993). To date, no Sry ortholog has been discovered beyond mammals; however, a number of other genes in the gonadogenesis pathway have been found to be expressed in a similar manner in other vertebrates. The basic structure of the testis is highly conserved among vertebrates, and there are striking parallels with testis structure in Drosophila. This suggests that the mechanistic pathways that control the architecture and cellular development of the organ also are conserved. For these reasons, information from the study of molecular pathways of gonadogenesis in other animals may provide valuable insights into the mammalian system. Although no direct target of SRY is yet known, it is hoped that investigators working backwards from molecular and cellular pathways that generate testis-speci c structures, and investigators working to identify targets downstream of Sry, eventually can link gene expression with the control of cell and organ architecture. I will focus this review on the genes and cellular pathways that have been discovered, distinguishing three overlapping phases of organogenesis of the gonad: during the rst phase, the bipotential gonad primordium is established; during the second phase, the fate of the gonad is decided; and during the third phase, pathways downstream of Sry initiate testis organogenesis. Where information from other systems seems instructive, I will compare gonad organogenesis in several other vertebrates and Drosophila. In vertebrates, the gonads arise as paired structures within the intermediate mesoderm which lies on either side of the embryo lling much of the coelomic cavity between the limb buds during the rst half of development (Fig. 2). Within this region, three segments comprising the urogenital ridge are distinguished from anterior to posterior: (1) the pronephros, which includes the adrenal primordium near its caudal end; (2) the mesonephros, the central region from which the gonad arises; and (3) the metanephros, the most posterior region in which the kidney forms. The mesonephric duct rst appears in mouse in short, transient segments within the pronephros, then as a stable continuous tube along the length of the urogenital ridge, adjoining the cloaca at its caudal end. In mammals, the primordia for both male and female ductal systems are initially present in the mesonephroi. The mesonephric ducts are the progenitors of the male ductal system. The antecedent of the female ductal system, the MuÈllerian duct, appears between 11.5 and 12.5 days post-coitum (d.p.c.) by invagination of a tube from the surface epithelium of the mesonephros. This tube parallels the mesonephric duct in both male and female embryos. Fig. 2. Scanning electron micrographs of the urogenital ridge. (A) At 10.5 d.p.c. the urogenital ridge lls most of the body cavity between the limb buds. m, mesonephros; g, genital ridge region; the arrow indicates a pore on the coelomic surface of the genital ridge region. (B) By 11.5 d.p.c. the gonad (g) in the mesonephric region of the ridge and kidney (k) in the metanephric region of the ridge are distinct from the mesonephros. (m) No sexual dimorphisms are apparent. Modi ed from Fig. 8, Capel and Lovell-Badge (1993). Reproduced by permission from the Publishers (JAI Press).
3 B. Capel / Mechanisms of Development 92 (2000) 89± Only one of the two ductal systems will normally develop further in mammals, depending on whether differentiation of a testis or ovary has begun. Under the in uence of several products of the early testis, MuÈllerian inhibiting substance (MIS, also known as anti-muèllerian hormone, AMH) and testosterone, the MuÈllerian duct regresses and the mesonephric duct (often referred to as the WoÈlf an duct at later stages) gives rise to the epididymis and vas deferens (Josso, 1970). In the absence of MIS and testosterone, the MuÈllerian ductal system differentiates further into oviduct, uterus, and upper vagina, while the mesonephric (WoÈlf an) ductal system regresses (Behringer et al., 1994). At a rst glance, the mammalian system seems different from the Drosophila system. In Drosophila the genital ducts arise from the genital disk, an imaginal disk at the posterior end of the embryo. The genital disk in Drosophila is a bipotential primordium: its cells have regional identity such that cells in one half of the disk develop into the female genital ducts in the case where the X to autosome ratio is greater than or equal to 1, whereas cells in the reciprocal half of the disk develop as male genital ducts when the X to autosome ratio is less than 1 (Stern, 1941; Chen and Baker, 1997; Kozopas et al., 1998). The mammalian mesonephros can be viewed similarly as a regionalized primordium in which the two genital duct primordia are arrested at a more advanced developmental stage. Further development of one primordium or the other in mammals is elicited by signals from the developing testis or ovary. During early development, it is likely that the mesonephric ductal system plays a critical signaling role in development of the entire urogenital system. Posteriorly, the ureteric bud branches from the mesonephric duct into the metanephric mesenchyme to found the kidney. Anteriorly, it has been suggested that the mesonephric duct acts similarly as an inducer or cell contributor to in uence gonad and adrenal development. The mesonephric tubules form shortly after the appearance of the mesonephric duct and extend through the mesenchyme of the mesonephros toward the coelomic surface by a process of condensation that resembles branching morphogenesis in the kidney. At the anterior end of the mesonephric duct, a complex group of tubules form a structure known as the giant nephron. This structure closely resembles a kidney nephron, consisting of tubules in close association with an elaborate vascular network. Near this region of the mesonephros, the cortical cells of the adrenal gland arise (Morley et al., 1996). EM studies reveal a region of condensing mesenchyme ventral to the posterior cardinal vein believed to give rise to both cortical cells of the adrenal and a population of cells extending toward the gonad (Smith and Mackay, 1991). The mesonephric tubules may play a role in the development of both the adrenal gland and the gonad either through signaling to the surrounding regions or by the direct contribution of cells to the forming organs. Several genes characterized in kidney morphogenesis are important in the development of the mesonephric duct, tubules, and surrounding mesenchyme, including Wt1, Pax2, and several Wnt genes. Wt1 is expressed widely throughout the urogenital ridge, in the mesonephros, the kidney, and the gonad (Armstrong et al., 1992). It is believed to mediate both the outgrowth of the ureteric bud, and the response of the metanephric mesenchyme to the growth of the ureteric bud during kidney development (Moore et al., 1999). In Wt1 2/2 mutants, the cranial mesonephric tubules appear to form normally, but tubules are absent from caudal regions, suggesting that mesonephric tubules are a heterogeneous population that form by at least two different mechanisms (Sainio et al., 1997). Pax2 is expressed in condensing mesenchyme and epithelial derivatives during kidney tubulogenesis (Dressler and Douglass, 1992). Expression of Pax2 within the mesonephros is limited to the mesonephric ducts and tubules. In Pax2 2/2 mice, the mesonephric duct is normal, but, with the exception of the most anterior cluster, the tubules form only as vestigial nubs on the mesonephric duct (J. Karl and B. Capel, unpublished data). No defects in gonadal development have been described in either sex in Pax2 2/2 mice (Torres et al., 1995; J. Karl and B. Capel, unpublished data), showing that formation of the mesonephric tubules is not a requisite for gonadal development. Several Wnt genes are expressed in or around the WoÈlf- an and MuÈllerian ductal systems and play a role in their development. Wnt7a is expressed at the anterior end of the mesonephros at 11.5 d.p.c., then throughout the MuÈllerian duct by 12.5 d.p.c. In Wnt7a 2/2 mice, the MuÈllerian duct is poorly developed in females and fails to regress in males (Parr and McMahon, 1998). Wnt4, which is involved in the formation of pre-tubular cell aggregates in kidney morphogenesis (Stark et al., 1994), is expressed in the mesenchyme surrounding the ducts, especially concentrated around the MuÈllerian ducts. In Wnt4 2/2 mice, the MuÈllerian duct is absent in both females and males (Vanio et al., 1999). Wnt5a is also expressed as early as 10.5 d.p.c. in the mesenchyme surrounding the mesonephric duct (Danielson et al., 1995), but data from null mutants have not yet been reported Emergence of the gonad The sub-region of the urogenital ridge where the gonad arises is called the genital ridge. The speci cation of the position of the genital ridge along the anterior posterior axis has not been examined molecularly in mammals. In Drosophila, the axial position of the gonads is determined by abda and AbdB. Mutations in these genes shift the position where the gonad condenses along the body axis (Brookman et al., 1992). Homologs of abda and AbdB from the 5 0 ends of the Hox3 and Hox4 clusters in mammals are expressed in the mesonephros and genital ridge. The most interesting expression pattern that has been reported is for Hox4.3, expressed at 10.5 d.p.c. in the mesonephric ducts, tubules, and in the coelomic epithelium and its subjacent
4 92 B. Capel / Mechanisms of Development 92 (2000) 89±103 cells (Izpisua-Belmonte et al., 1990). The axial position of the mesonephric ductal system, or of the genital ridge, may be speci ed in mammals by combinatorial expression of Hox genes. The gonads emerge on the ventro-medial surface of the mesonephros by thickening of the coelomic epithelium of this region in both males and females between 10.5 and 11.5 d.p.c. (8±18 tail somites (t.s.), counted according to Hacker et al., 1995). Proliferation studies in our laboratory reveal that this occurs at least partially by proliferation of the coelomic epithelial cells (Schmahl et al., 2000). Scanning electron micrographs detect pores in the coelomic surface as early at 10.5 d.p.c. when the region where the gonad is forming is rst distinguishable from the mesonephros (Fig. 2, arrow). During early development of the gonad, the coelomic epithelium serves as a source of multiple gonadal cell lineages that delaminate from the coelomic surface and move to the interior of the gonad in both XY and XX embryos (Karl and Capel, 1998; Schmahl et al., 2000). At this stage the gonad primordium resembles a strati ed epithelium on the surface of the mesonephros. The recruitment of underlying cells from the mesonephros to the epithelial population may also augment the cell population in the gonadal primordium. From the earliest stages of gonad development, the mesonephric tubules can be seen to form continuous bridges to the epithelial cells of the gonad in male and female genital ridges (Karl and Capel, 1995). These structures, which have been reported previously in EM studies (Upadhyay et al., 1979), are obvious by confocal imaging of whole gonads stained with antibodies against laminin1 (Fig. 1). No function for these tubule connections has yet been de ned Genes that disrupt early formation of the gonad in both sexes The formation of the gonad can be blocked at early steps by null mutations in Lim1 (Shawlot and Behringer, 1995), steroidogenic factor 1 (Sf1) (Luo et al., 1994), the Wilms tumor gene (Wt1) (Kriedberg et al., 1993), or Emx2 (Miyamoto et al., 1997). Of these genes, the role of Lim1 is the least well de ned. Lim1 is a homeodomain protein expressed throughout the mesonephric ductal system. Rare Lim1 2/2 mutants that survive do not have kidneys or gonads, but the stage of disruption of gonadal development has not been characterized (Shawlot and Behringer, 1995). In the cases of Sf1, Wt1, and Emx2 null mutants, the gonad begins to form and then regresses. All three of these genes may be involved in initial signals that lead to the expansion of the genital ridge. Expression of SF1 has been reported between 10.5 and 11.5 d.p.c. in a group of cells that arise at the anterior end of the mesonephros and separate into two populations, one of which moves with germ cells toward the gonad, and the other of which condenses to form the adrenal cortex. Sf1/ SF1 is also expressed from the earliest stages in the coelomic epithelium of the genital ridge (Ikeda et al., 1994; Morohashi, 1997; Schmahl et al., 2000). Sf1 2/2 mice initiate development of a distinct gonadal primordium at 11.5 d.p.c., which regresses by apoptosis before 12.5 d.p.c. The adrenal is also absent (Luo et al., 1994). In addition to its role in the formation of a subset of mesonephric tubules,wt1 is also expressed in the gonadal primordium itself where it may have additional roles (Armstrong et al., 1992). InWt1 2/2 mutants, kidney development is arrested, the coelomic epithelium fails to thicken, and the gonad is completely regressed by 14.5 d.p.c. (Kriedberg et al., 1993). Emx2 is a mammalian homolog of a Drosophila head gap gene that is expressed in the mesonephric ducts, tubules and coelomic epithelium. During nephrogenesis, it is believed to be involved in the response of the ureteric bud to the metanephric mesenchyme. Its role in gonad development is less clear. In Emx2 2/2 mutants, the mesonephric ducts and tubules degenerate and the coelomic epithelium at the surface of the genital ridge fails to expand (Miyamoto et al., 1997) Founding cell lineages in the gonad There are two different types of bipotential primordia: (1) primordia in which cells located in different regions have different fate potentials; and (2) primordia in which the same cells have two different possible fates. The Drosophila genital disk has been shown to be a compartmentalized bipotential primordium. There are two different cell populations, each with only one possible fate. One of two regions is induced to develop while the other region is lost (Stern, 1941; Chen and Baker, 1997). In the mammalian gonad, the founding cell populations have the capacity to differentiate as testicular or ovarian cell types. Emil Witschi proposed that, like the Drosophila genital disk, the bipotential gonad is regionalized. Witschi proposed that the ovary develops from the coelomic epithelium while the testis develops from the medulla (Witschi, 1951). Available evidence currently refutes this model and favors the hypothesis that the early mammalian gonad is a bipotential primordium in which single cells can follow one of two possible fates. DiI lineage tracing experiments show that cells arise from the coelomic epithelium in both testis and ovary (Fig. 3) (Karl and Capel, 1998). In addition, neither the epithelium in males nor the medulla in females has been seen to undergo extensive apoptosis as might be expected according to Witschi's model (J. Schmahl and K. Tanaka, unpublished data). Precursors for both supporting cell types and steroid-secreting cell types are believed to be present in the early gonad (Merchant-Larios et al., 1993). Several lines of evidence indicate that the supporting cell precursor, so named for its role in sustaining and nourishing germ cells in both sexes, can develop into either testis-speci- c (Sertoli) or ovary-speci c (granulosa) cell types. In mosaic gonads in vivo, consisting of a mixture of XX and
5 B. Capel / Mechanisms of Development 92 (2000) 89± Fig. 3. Model of DiI lineage experiments. The coelomic epithelium contributes multiple cell types to the XX and XY gonad from the earliest stages of gonadal development. When single coelomic epithelial cells are labeled between 15 and 18 t.s. (just prior to 11.5 d.p.c.) with DiI (red), they give rise to supporting cell precursors and other lineages within XX and XY gonads. In the XY gonad some cells labeled at 15±18 t.s. differentiate as Sertoli cells enclosing germ cells (black) inside testis cords by 28 t.s. (12.5 d.p.c.). Other labeled cells reside in the interstitial space. XY cells, XX cells have been seen to develop as Sertoli cells (Palmer and Burgoyne, 1991a), and XY cells have been seen to develop as granulosa cells (Burgoyne et al., 1988). In organ culture assays, when the XX gonad is induced to form testis cords, XX cells acquire Sertoli-like morphology and express male-speci c markers (Tilmann and Capel, 1999). Although evidence is less clear at present, steroid secreting Leydig cells in the testis and theca cells in the ovary, which engage many common steroid pathways, may also derive from a single steroidogenic precursor. There is evidence that other characteristic cell types in the gonad (which will be considered later) are recruited differently in the testis versus the ovary Sertoli cells Sertoli cells are believed to act as the organizing center of the male gonad. They are the rst cell type known to differentiate in the gonad. In situ hybridization (Koopman et al., 1990) and RNAse protection studies (Hacker et al., 1995) show that Sry is expressed in the gonad at 11.5 d.p.c. In the absence of an antibody against SRY, or a de nitive reporter construct, no direct evidence exists concerning its cellspeci c expression pattern. Nevertheless, there is strong genetic evidence that it is the pre-sertoli cell that expresses Sry. In mosaic mice generated between XX and XY cells, the proportion of XX to XY cells is usually 50:50 in the absence of selection for one or the other genotype. Within the testis of chimeric mice, all lineages with the exception of Sertoli cells were found in a 50:50 ratio. However, Sertoli cells were.90% XY, indicating a strong bias in this cell lineage for the presence of the Y chromosome (Palmer and Burgoyne, 1991a). These experiments have been interpreted to mean that the pre-sertoli cell is the only cell in the testis in which Sry exerts a critical effect. The origin of Sertoli cells has fostered a long-standing debate in the eld. Sertoli cells appear to be present in the gonad by 11.5 d.p.c. In experiments where the gonad was separated from the mesonephros at 11.5 d.p.c. and cultured alone, anti-muèllerian hormone (MIS), a marker speci c to Sertoli cells, could be detected in the medium, indicating that Sertoli cells must have arrived prior to the time of separation from the mesonephros, 11.5 d.p.c. (Merchant-Larios et al., 1993). Over the years, Sertoli cells have been proposed to arise from the mesonephros, its ducts and tubules, the coelomic epithelium, or both (Byskov, 1986 and references therein). In experiments where cells at the coelomic epithelium were labeled with DiI prior to 18 t.s. (,11.5 d.p.c.) single cells gave rise to Sertoli cells, additional labeled cells that reside outside cords, and some labeled cells that remain in the coelomic epithelium (shown diagrammatically in Fig. 3). In XX gonads, single labeled cells gave rise to WT1 positive and negative cells ± also likely to include at least two distinct populations. It is not known whether this asymmetric fate decision takes place during cell division, or arises thereafter through cellular inductions. Although DiIlabeled coelomic epithelial cells continue to move into the XY gonad until the tunica albuginea is formed (,12.5 d.p.c.), they do not give rise to Sertoli cells after 18 t.s. These results were supported by proliferation studies during the formation of the gonad. Using bromodeoxyuridine (BrdU) labeling, the coelomic epithelium was found to be a highly proliferative region from the earliest stages of gonadogenesis. Pulse-labeling experiments indicate that pre-sertoli cells divide at all stages between 8 and 18 t.s. (,10.5±11.5 d.p.c.). Sertoli cells cease division between 18 and 24 t.s. and resume thereafter as testis cords are forming (Schmahl et al., 2000). These results do not rule out the possibility that a second source of Sertoli cells exists. It has been suggested that this is the case in mammals (Wartenberg et al., 1986). If this is true, they must migrate into the gonad prior to 11.5 d.p.c. or arise in situ because they are not detected in recombinant
6 94 B. Capel / Mechanisms of Development 92 (2000) 89±103 organ culture experiments assembled at 11.5 d.p.c. and after using marked mesonephroi (Martineau et al., 1997). In Drosophila, there are two populations of supporting cells in the testis with different functions and distinct origins (Boyle and DiNardo, 1995). Lineage tracing experiments and proliferation studies in the XY gonad have bene ted from the rapid differentiation of testis architecture and testis-speci c expression markers between 11.5 and 12.5 d.p.c. in mouse (Karl and Capel, 1998; Schmahl et al., 2000). In contrast, neither distinctive morphology nor cell markers are available to distinguish ovarian cell types during this period. As a consequence, it has been possible only to trace the origin of testis-speci c cell types; however, if our model is correct, the origin of the supporting and steroid lineages in the ovary will be identical to that in the testis Leydig cells Separation and culture of gonads apart from the mesonephros indicates that in addition to pre-sertoli cells, the Leydig precursor is already present in the gonad by 11.5 d.p.c. because testosterone also can be detected in the culture medium (Merchant-Larios et al., 1993). Since the adrenal and the gonad both contain cells of the steroid lineage and arise close together in the urogenital ridge, the possibility of a common population of steroid precursors has been investigated. A group of cells detected with NCAM antibody staining has been seen to stream toward the gonad from a region at the anterior of the mesonephros where the adrenal gland also arises (Mayerhofer et al., 1996). The expression of two steroid cell markers common to the adrenal and the gonad, Sf1 and Dax1, has been seen in the anterior mesonephros at early stages by RNA in situ experiments (A. Swain, K. Parker, pers. commun.). Using an antibody detection approach, it has been reported that part of the SF1 expressing population is sequestered in the adrenal, and part is associated with germ cells moving toward the gonad (Morohashi, 1997). A recent report indicated that several Leydig cells were detected by electron microscopy techniques among marked cells that had migrated from the mesonephros (Merchant-Larios and Moreno-Mendoza, 1998). Although these experiments are not de nitive, the sum of this evidence suggests that an origin in the mesonephros is most likely. 3. The battle of the sexes In mammals, the decision to form a testis or an ovary from the gonadal primordium is the primary sex determining step. All secondary sex characteristics arise as a result of the presence or absence of hormones produced by the testis or ovary. This basic tenant of mammalian sex determination was established by Alfred Jost in Jost removed the testis or ovary from developing rabbit fetuses and discovered that in the absence of a testis or an ovary, the fetus develops as a female (Jost, 1947). These experiments clearly demonstrated that in the absence of a testis, no other region in the embryo is capable of superimposing the male pattern on development. It follows that in mammals we can expect the sex determining switch to act on the cells of the gonad. While expression of this gene may occur in other regions of the embryo, its effect there does not exert a de nitive in uence on sex determination in the absence of a testis Sry versus `Z' Sry, the mammalian sex determining switch, is a Y-linked gene that is expressed in the XY gonad between 10.5 and 12.5 d.p.c. When Sry is deleted from the Y chromosome (Lovell-Badge and Robertson, 1990; Gubbay et al., 1992) or carries mutations within the gene (Hawkins et al., 1992) an ovary forms. On the other hand, the expression of Sry in the gonad of an XX embryo leads to the organization of gonadal cells into testis cord structures (Koopman et al., 1991; Eicher et al., 1995). These experiments proved that Sry is the only gene from the Y chromosome required to initiate testis organogenesis among the cells of the gonad primordium. Sry encodes a DNA binding protein of the HMG-box family that recognizes both chromatin structure and a speci- c binding sequence (Ferrari et al., 1992; Rimini et al., 1995). Members of this family are known to bend DNA and are thought to activate or repress targets through their effect on chromatin structure (Giese et al., 1992; Grosschedl et al., 1994). While the DNA binding domain of all mammalian SRY proteins is highly conserved, regions outside this domain have diverged rapidly, among mouse, human, and other primates (Whit eld et al., 1993). The mouse SRY protein encodes an activation domain at its 3 0 end which is absent in the human protein (Dubin and Ostrer, 1994). The human protein has been shown to associate with a partner containing two PDZ domains, speculated to link SRY to another protein with a trans-activation function (Poulat et al., 1997). This divergent specialization may explain why the human SRY gene does not cause sex reversal when introduced as a transgene into mouse (R. Lovell-Badge, pers. commun.). Genetic arguments to explain the occurrence of males on XX backgrounds in the absence of the Sry gene hypothesize that Sry normally acts as a repressor of the female pathway in mammals (Eicher and Washburn, 1986; Goodfellow and Lovell-Badge, 1993; McElreavey et al., 1993; Jimenez et al., 1996). It has been hypothesized that there is a gene `Z' that represses male development and/or activates female development. The function of Sry is postulated to be the repression of `Z' which, in turn, relieves repression of the male pathway. Mutations that inactivate `Z' could lead to the de-repression of the male pathway in XX females in the absence of Sry. Alternatively, over-expression of `Z' could block male development in the presence of Sry. Such a model is predicted to be sensitive to threshold effects relat-
7 B. Capel / Mechanisms of Development 92 (2000) 89± ing to the dosage and timing of gene expression. In fact Sry and other genes in the pathway show strong dosage and timing effects. In humans, a region of the X chromosome, Xp21, is responsible for a syndrome called dosage-sensitive sex reversal (DSS). Duplication or translocation of this region to an autosome where it is thought to escape X inactivation is known to cause male to female sex reversal. An unusual member of the nuclear hormone receptor family, DAX1, was isolated from Xp21 (Bardoni et al., 1994). Human mutations in this gene were shown to lead to adrenal hypoplasia and hypogonadotropic hypogonadism (Muscatelli et al., 1994). When mouse Dax1 was expressed in combination with weak or late-acting alleles of Sry in transgenic mice, male to female sex reversal was seen, suggesting that Dax1 acts antagonistically to Sry in a dosage-sensitive manner to regulate sex determination (Swain et al., 1998). These data suggested that DAX1 could be the hypothetical gene `Z'. However, a reported null mutation in mouse Dax1 affected spermatogenesis, but did not affect ovary or testis development, indicating that Dax1 cannot be the sole regulator of the female pathway or the sole repressor of the male pathway in mouse (Yu et al., 1998). Explanations for this discrepancy include the possibility that the Dax1 2/2 mouse is not a complete null mutant, that the heterologous expression of Dax1 in transgenic mice does not re ect the normal role of Dax1 normally does in vivo, or that Dax1 acts somewhat differently in the mouse than in the human. Sox3 (Sry-like HMG-box protein 3) is another X-linked gene that has been proposed as an alternative candidate for `Z'. There is no direct evidence yet that Sox3 is involved in the sex determination affair; however, theoretical evolutionary arguments have been presented that this gene could have evolved as an X-linked antagonist of Sry (Graves, 1998). Sox3 is the member of the Sry-like HMG protein family believed to be ancestral to Sry itself (Collignon et al., 1996). Sox3 is X-linked in all mammals, whereas DAX1 is not X-linked in marsupials (Pask et al., 1997), although X- chromosome linkage is not a requisite for Z. Sox3 does not lie in the Xp21 interval in humans, nor is there any clear evidence that human mutations lead to primary defects in testis or ovarian development (StevanovicË et al., 1993). Because null mutations in Sox3 cause early embryonic lethality in mouse as a result of early embryonic functions of the Sox3 gene (R. Lovell-Badge, pers. commun.), a test of this theory will require a conditional mutant Ovotestes: a stalemate The toggle between the switches to the male or female pathways suggested by these models is in accord with the biology of the sex determination system in general. The gonad is poised for the battle of the sexes before differentiation begins. In temperature-sensitive systems where Sry is not involved, for example, in alligators and turtles, the ratio of male to female development is 50:50 at one or two critical temperatures (Bull, 1983). In other words, there is a point on the curve where the decision goes either way with equal probability, but development of ovotestes is rare. Normally the program is set before it is initiated: the window of susceptibility to temperature shifts begins well in advance of the stage at which morphological changes have been observed (Wibbels et al., 1991; Smith and Joss, 1993). In mammals, the occurrence of ovotestes is also quite rare: development of the ovary or testes is strongly canalized, and once the balance is shifted in a given direction, the entire cell population in the gonad is usually recruited to whichever program is initiated. An exception is the formation of ovotestes in hermaphrodites where the Y POS chromosome from Mus domesticus poschiavinus is crossed onto certain Mus musculus background strains, notably, C57BL/6 (B6). B6 XY POS individuals develop either ovaries or ovotestes that persist into adult life. Because the Y POS chromosome induces testes formation within XY Mus domesticus poschiavinus mice, it has been proposed that B6 variants of testis determining autosomal genes (Tda's) are responsible for failure of testis development in B6 XY POS mice (Eicher, 1988). Regions on mouse chromosomes 2, 4 and 5 are associated with ovotestis development in backcross mapping studies, but no speci c genes have been identi ed (Eicher et al., 1996). Identi cation of the Tda's is expected to provide important genetic players involved in organogenesis of the testis versus the ovary. The organization of ovotestes in B6 XY POS (and other known cases) typically consists of central cords that may be somewhat asymmetric and polar regions of ovarian follicles (Bradbury, 1987; Eicher et al., 1995, 1996; Nagamine et al., 1998). In a less extreme case, the Y chromosome from the Mus domesticus strain AKR causes a delay in testis cord formation in B6 mice (Washburn and Eicher, 1989). In this instance, testis cords form,24 h late in the central region of the gonad, then gradually extend to the ends of the gonad over the next 2±3 days. A theory was advanced to account for the formation of ovotestes in B6 XY POS mice suggesting that there is a narrow window of time during gonad development when testis determination must occur in order to forestall the female pathway. It was suggested that the male sex determining gene normally must act prior to female determining genes to initiate the male pathway and repress the female pathway (Eicher and Washburn, 1986). The formation of ovotestes might result from a late-acting or lower-expressing allele of the male sex determining gene, creating a mismatch between the timing or level of Sry expression and the timing of entry into the female pathway (Palmer and Burgoyne, 1991b). Evidence suggests that the timing and level of Sry expression is critical in transgenic mice (Goodfellow and Lovell-Badge, 1993), and mice carrying Y chromosome deletions that affect the level of Sry expression (Capel et al., 1993). New evidence from organ culture experiments using XY gonads to induce male differentiation in XX gonads indicates that induction can occur only prior
8 96 B. Capel / Mechanisms of Development 92 (2000) 89±103 to 12.5 d.p.c., also supporting the idea that the testis pathway must initiate during a narrow window of development (Tilmann and Capel, 1999; see below). This is similar in principle to the temperature-sensitive sex determination systems in which a clear window of sensitivity can be de ned experimentally by temperature shift experiments. We must still explain why an ovotestes persists in B6 XY POS mice, but not in B6 XY AKR. Presumably a difference in the timing or expression level of Sry between Y POS and Y AKR relative to the ovary determining genes in B6 traps B6 XY POS in an ovotestes state War babies The role of germ cells at this switch in development is different between male and female pathways. Germ cells migrate to the gonad through the gut mesentery and the mesonephros, colonizing the gonad between 9.5 and 11.0 d.p.c. (Ginsburg et al., 1990; Gomperts et al., 1994). Germ cells are not required for testis organogenesis. There is some evidence that cord formation is slightly delayed in sterile mutants; however, cords do form and the structure of the testis is normal (McLaren, 1988). Germ cells in XX and XY gonads proliferate similarly until 12.5 d.p.c. (Schmahl et al., 2000). At that stage, germ cells in the XY gonad are sequestered inside the forming testis cords where they arrest division for approximately 1 week and resume mitosis after birth. In XX gonads, germ cells are required for the initial organization of the ovary into characteristic follicles and for the maintenance of follicles thereafter. In sterile mutants, or in cases where germ cells are lost, the follicular structure of the ovary either never forms or rapidly degenerates (McLaren, 1995). Germ cells in the XX gonad undergo one nal mitosis at 13.5 d.p.c. then enter meiosis (McLaren and Southee, 1997). In experimental cases where germ cells are mislocated outside testis cords, for example, in the ovarian regions of ovotestes, or in cases where germ cells erroneously migrate to the adrenal, both XX and XY germ cells enter meiosis with a timing similar to normal XX germ cells in the gonad (McLaren and Southee, 1997). Therefore, the progression of germ cell development to meiosis is a cell autonomous property of XX germ cells that operates in a clock-like manner. The in uence of pre-sertoli cells, and perhaps the organization of testis cords, is required to inhibit this step. The genes that mediate these interactions between somatic cells and germ cells are not yet known. Homozygous null mutations in Bmp8a (Zhao et al., 1996) and Dhh (Bitgood et al., 1996) both lead to fertility defects and the loss of germ cells in XY mice. These genes may be involved in the interactions that transpire between somatic cells and germ cells in the early XY gonad. The structural organization of the testis has not been carefully analyzed in either of these cases. Mutations that present as fertility defects may actually impair testis cord formation and affect the control of germ cells indirectly. 4. Diverging development Approximately 36 h after Sry expression is initiated in XY gonads, changes in the organization of cells are obvious (Fig. 1). The most striking of these male-speci c changes are: (1) the organization of the somatic supporting cell lineage, the pre-sertoli cell, to surround germ cells in testis cord structures; (2) a dramatic increase in the size of XY versus XX gonads; and (3) the appearance of a characteristic blood vessel on the surface of the XY gonad. Although changes must also be occurring in the XX gonad during this 48 h of development, no obvious signs of ovarian differentiation have been described. While no direct targets of SRY are yet known, a number of important molecular players have been identi ed by various approaches. Although the gene that operates the switch to the male pathway is different in non-mammalian species, downstream of this switch, many of the genes that control morphogenesis of the testis and differentiation of Sertoli and other cell types of the gonad are the same Known molecular players in the testis determination pathway SOX9 (Sry-like HMG-box protein 9) was rst identi ed in humans as the gene responsible for campomelic dysplasia, a bone disease often associated with male to female sex reversal in heterozygous carriers (Schafer et al., 1995). Isolation and characterization of this gene revealed its relation to Sry through a familial HMG-box domain. Subsequently, expression of Sox9 was found to be sexually dimorphic in gonads of many species, including mouse (Wright et al., 1995; da Silva et al., 1996), chicken (Wright et al., 1995; da Silva et al., 1996), turtle (Spotila et al., 1998), and alligator (Western et al., 1999). In mouse, Sox9 is rst expressed at low levels in both male and female gonads. By 11.5 d.p.c., expression is up-regulated in XY gonads and down-regulated in XX gonads. Once cord formation has occurred, Sox9 expression is speci c to Sertoli cells where the protein is seen to move to the nucleus between 11.5 and 12.5 d.p.c. (da Silva et al., 1996). Because of its clear association with sex reversal in humans, its early dimorphic expression in all vertebrate systems in which it has been examined, and its Sertoli cell-speci c expression pro le, Sox9 is considered a critical gene in the early differentiation of Sertoli cells. MIS, which is a TGF-b family member, is also one of the earliest known products of Sertoli cells. It induces complete regression of the MuÈllerian ductal system (for review see Teixeira and Donahoe, 1996). Male mice carrying null mutations in Mis (Behringer et al., 1994), or the Mis receptor (Mishina et al., 1996) fail to undergo regression of the MuÈllerian duct. MIS was rst characterized as the factor in serum that induces the masculinization of the female twin in freemartin cattle (Jost et al., 1975), and later shown to have a masculinizing effect on ovaries in culture (Vigier et al.,
9 B. Capel / Mechanisms of Development 92 (2000) 89± ). It has been hypothesized to act by inducing the degeneration of germ cells which leads to a loss of ovarian structure and the appearance of testis cord-like structures as a secondary effect (McLaren, 1990). Mis 2/2 mutant mice, although they retain MuÈllerian ducts, develop normal testes; therefore, Mis appears to have no essential role in early testis organ development in mouse (Behringer et al., 1994). Mis expression is rst detected in the XY mouse at the peak of Sry expression (11.5 d.p.c.), according to sensitive RNAse protection methods (Hacker et al., 1995). Sexually dimorphic expression of Mis has been characterized in many vertebrates including mouse (MuÈnsterberg and Lovell-Badge, 1991), chicken (Oreal et al., 1998), and alligator (Western et al., 1999). Although a putative SRY binding site is present in the Mis promoter, in vitro assays showed that Mis transcription is not directly activated by SRY (Haqq et al., 1994). Sox9 expression clearly precedes Mis expression in the mouse. In vitro experiments were conducted that strongly implicated SOX9 and SF1 in the transcriptional regulation of Mis (Shen et al., 1994; De Santa Barbara et al., 1998). Recently, a homologous recombination-mediated deletion of the HMG-type SOX9 binding site 5 0 of the Mis gene resulted in a failure to activate the gene in vivo (Arango et al., 1999). In mouse, SOX9 begins to localize to the nucleus of XY gonadal cells around 11.5 d.p.c. (da Silva et al., 1996), almost exactly the time that transcriptional activation of Mis begins according to RNAse protection studies (Hacker et al., 1995). The story has become more complicated as data concerning MIS versus SOX9 RNA expression have accumulated from other vertebrates. In chicken MIS expression precedes SOX9 expression by 1 day (Oreal et al., 1998), while in alligator, expression of MIS precedes SOX9 by more than 3 days (Western et al., 1999), indicating that SOX9 cannot be the regulator of MIS in these systems. Indeed the organization of the 5 0 regulatory region of the chicken MIS gene is highly diverged from the mouse (Oreal et al., 1998). The explanation for this difference from the mammalian system is not clear at present although it has been suggested that it may result from the greater in uence of gonadal hormones on sexual development in non-eutherian mammals (Arango et al., 1999). These results raise questions about the position and role of SOX9 in the sex determination hierarchy. In alligator, expression of MIS is thought to initiate at the earliest time of commitment to Sertoli cell fate, whereas SOX9 is expressed at the time when pre-sertoli cells organize into cords (Western et al., 1999). In chicken and alligator, these processes occur over a period of 2±4 days which makes it easier to distinguish their sequence (Fig. 3). In mouse, commitment to Sertoli cell fate and organization into cords occur within 24±36 h, which makes it more dif cult to determine the order of events and to identify a role for SOX9 in the context of one or the other of these processes (Fig. 4). In addition to their roles in early gonadogenesis, Sf1, Wt1, and Wnt4 probably have roles in sex-speci c differentiation of the testis. Sf1 encodes an orphan steroid hormone receptor essential to the regulation of genes involved in steroid synthesis in both the gonad and the adrenal gland. It is expressed initially in Sertoli, and probably Leydig precursor cells, then is gradually down-regulated in Sertoli and upregulated in Leydig cells after testis cord formation. SF1 has been implicated in the regulation of MIS (Giuili et al., 1997; Arango et al., 1999). The Wt1 locus is an extremely complex locus encoding 16 known variants of the WT1 protein. In Frasier and Denys Drash Syndromes in humans, heterozygous mutations in Wt1 lead to decreased dosage of particular splicoforms. In both cases, more severe disruption of genital development occurs in XY than in XX carriers (for reviews see Hastie, 1992; Schedl and Hastie, 1998), suggesting that, in addition to the role of WT1 in early gonad development in both sexes, there are auxiliary effects on the testis pathway. An in vitro study indicated that Wt1 is up-regulated by the expression of SRY (Toyooka et al., 1998). WT1 itself has been implicated in the regulation of Sf1 in in vitro assays (Nachtigal et al., 1998). Wnt4 recently has been implicated in the pathway leading to steroid cell development. In Wnt4 2/2 mutants, cells develop in the ovary that expresses steroid enzymes char- Fig. 4. The order of initiation of Sox9 and Mis transcription is reversed between mouse and two other vertebrates, chicken and alligator. dark blue arrow, SOX9 expression; light blue arrow, MIS expression. Days post-fertilization is indicated on the horizontal axis. In mouse, the known periods of Sertoli cell commitment (S, green bracket) and cord formation (CF, red bracket) are compressed within,36 h. In alligator the period over which these events occur is,9 days.
10 98 B. Capel / Mechanisms of Development 92 (2000) 89±103 acteristic of the male-speci c Leydig cell. Adult testes in Wnt4 2/2 mice show disruption of testis architecture as well as disruption of ductal development described earlier (Vanio et al., 1999). This nding has led to the genetic hypothesis that Wnt4 is required to suppress the development of Leydig cells in the ovary. Two genes shown to affect gonadogenesis, XH2 and M33, are associated with chromatin domain organization. X-Linked alpha thalassaemia (ATR-X) is a human syndrome characterized by severe mental retardation and male to female sex reversal. This syndrome has been mapped to the XH2 gene on Xq13 (Ion et al., 1996). No null mutations in XH2 have been reported in mouse. XH2 is a relative of the Drosophila gene brahma, a member of the helicase superfamily implicated in general transcriptional domain activation (Tamkun et al., 1992). M33 is a mouse gene related to a Drosophila polycomb group gene. In mouse, homozygous mutants in M33 show retarded growth and development and variable levels of sex reversal (Katoh- Fukui et al., 1998). In Drosophila, the polycomb genes form multimeric complexes to repress chromatin domains. Mutations in either of these gene families lead to homeotic transformations in Drosophila. Loss of function brahma mutations suppress loss of function mutations in polycomb (Tamkun et al., 1992). It is not yet clear at which step M33 or XH2 exerts effects on gonad formation. Because SRY is also believed to act as a regulator of chromatin structure, there may be interactions between Sry and one or more of these genes that in uence the battle of the sexes at the level of chromatin organization. Another gene, DMRT1, was identi ed recently from a region on human chromosome 9p that is associated with male to female sex reversal (Raymond et al., 1998). This gene was identi ed through its DM-DNA binding domain homology to mab3 in C. elegans and dsx in Drosophila. Heretofore, there have been no common elements in sex determination pathways between phyla. mab3 and dsx are the rst orthologous elements known to act downstream in both the sex determination hierarchies in C. elegans and Drosophila. Dmrt1 has been isolated from mice and shown to be expressed early in both sexes, becoming male-speci c between 13.5 and 14.5 d.p.c. In chicken, the gene is located on the Z chromosome and is more highly expressed in males from stage 25 (Raymond et al., 1999). Efforts to generate null mutations in the mouse gene are underway in several laboratories. Numerous differential screens have been undertaken between XX and XY gonads soon after differentiation is initiated. Many of the genes isolated have represented legitimate differences (for example, differences in steroid enzymes), but have not uncovered new pathways. Perhaps the most informative gene yet reported from such a screen is testatin (Tohonen et al., 1998). This gene was isolated from a reverse transcription polymerase chain reaction (RT-PCR) differential screen designed to detect secreted proteins. Testatin is expressed speci cally in XY gonads as early as 11.5 d.p.c. It belongs to the cystatin family of proteinases known to play a role in tissue remodeling. Its natural role in testis organogenesis is currently under investigation; however, it has exciting possibilities as the rst isolated example of a predicted large group of proteins that regulate the structural reorganization of gonadal cells Sry induces changes in proliferation in gonadal cells The earliest effect of Sry expression yet detected occurs between 16 and 18 t.s. (just before 11.5 d.p.c.), when proliferation in SF1 positive cells within the XY coelomic epithelium increases at least two-fold in the population of cells that give rise to Sertoli cell precursors. This difference in the proliferation pathway is induced directly or indirectly by Sry, however, it is not yet clear exactly how it is related to Sry expression and to Sertoli cell fate commitment. After 18 t.s. (11.5 d.p.c.), proliferation drastically increases in the XY coelomic epithelium in a second phase distinguished by two features: (1) proliferating cells are negative for SF1; and (2) they do not give rise to Sertoli cells. This second phase of proliferation also is dependent on Sry (Schmahl et al., 2000). An increase in proliferation and hypertrophy of cells believed to be Sertoli precursors has been reported as the rst sign of diverging male development in alligators where this change precedes cord formation by 1±4 days (Smith and Joss, 1993). The increase in the size of the male gonad is common to many species and led to the theory that an increase in the growth rate of XY embryos results in testis determination (Mittwoch, 1989). Proliferation may be a critical step that marks the entry into the male pathway in many sex determination systems. However, rather than the cause of sex determination, this effect is a consequence of the decision to enter the testis pathway, initiated by the switch that controls testis determination Sry induces the recruitment of other lineages to the gonad: peritubular and endothelial cells The cell types present in the early testis, in addition to Sertoli and Leydig cells, include peritubular myoid cells, endothelial cells, and other uncharacterized cell types in the interstitial space. Sertoli cells surround germ cells. Peritubular myoid cells surround Sertoli cells, depositing a basal lamina in collaboration with Sertoli cells to enclose germ cells inside testis cords. This organization relegates Leydig cells to the interstitial region between cords in close association with arterial and lymphatic elements of the vasculature. This basic structure (with minor variations) is characteristic of all vertebrate testes. Early organ culture experiments suggested that cells move into the gonad from the mesonephros (Buehr et al., 1993). More recent experiments showed that cell migration from the mesonephros is a male-speci c mechanism of development, dependent on the presence of Sry in the gonad (Martineau et al., 1997; Capel et al., 1999). The
11 B. Capel / Mechanisms of Development 92 (2000) 89± migrating cell types include cells surrounding Sertoli cells in positions characteristic of peritubular myoid cells, cells that are positive for the endothelial marker, platelet endothelial cell adhesion molecule (PECAM), and other cells that are closely associated with the vasculature, but negative for PECAM (Martineau et al., 1997) (Fig. 5). This analysis has been hampered by the absence of early markers that clearly de ne the peritubular myoid and other interstitial lineages. In Drosophila a similar phenomenon occurs: when the male genital duct grows to reach the gonadal primordium, it donates cells to the developing testis. The migration of cells from the male genital duct is dependent on signals from DWnt2. In Drosophila, as in mammals, the germ cells are enclosed by the supporting cell lineage. The migrating cells include a myoid cell lineage that surrounds the supporting cells of the Drosophila gonad, and pigment cells that lie on the outside of the myoid lineage (Kozopas et al., 1998). This organization shows striking similarity to the migrating cell types and to the basic architecture of the testis cord in mammals Migration of cells from the mesonephros is critical for testis cord formation in mouse An early report indicated that cord formation was impaired in gonads separated from their mesonephroi (Buehr et al., 1993). Studies using carefully staged gonads show that separation of the mesonephros must occur by 16± 17 t.s. to disrupt cord formation, indicating that cell migration must begin by this time (Tilmann and Capel, 1999). Migration of cells from the mesonephros occurs at least until 16.5 d.p.c. into XY gonads, but not at any stage during this period into XX gonads (Martineau et al., 1997). Fig. 5. Between 16 and 28 t.s. the rst dimorphisms are seen between XX and XY gonads. Sry induces migration of mesonephric cells into the XY gonad. Precursors of peritubular myoid cells in the mesonephros (light blue) move into the gonad and induce supporting cells (green) to surround germ cells (black) inside testis cords. Vascular tissue (dark blue) also arises from the mesonephros in XY gonads. Because markers are not available, it is not yet clear whether signals for the migration of different cell types are distinct but overlapping in time; however, this seems likely (see below). Attempts to characterize the signals revealed that migration can be induced into XX gonads by factors present in XY gonads. These experiments indicated that the factor is a chemoattractant signal that operates over a long distance (at least 100 mm) either by diffusion through the tissue, or by a cell to cell relay system. Cell migration is delayed in B6 XY AKR mice in which cord formation is delayed, and nearly absent in B6 XY POS mice that form ovaries or ovotestes. In a recombinant inbred strain derived from B6 DBA2, BXD21, ovotestes always form in the presence of the Y POS chromosome. Using this strain as the gonad donor, migrating cells were exclusively associated with regions where testis cords formed and not with ovarian regions (K.H. Albrecht, B.C. Capel, L.L. Washburn, E.M. Eicher, submitted). These experiments demonstrated a tight correlation between mesonephric cell migration and testis cord formation. Migrating cells from the mesonephros into the gonad induce male-type tissue organization (shown diagrammatically in Fig. 5). Germ cells aggregate inside cord-like structures, a basal lamina is deposited between supporting cells and peritubular myoid cells, and the supporting cells inside cords down-regulate Dax1 and up-regulate Sox9 in a manner typical of male differentiation. These effects are completely dependent on cell migration from the mesonephros (Tilmann and Capel, 1999). Both cord formation and Sox9 expression, considered to be the rst known indication of Sertoli differentiation, can be induced in the XX gonad by migrating mesonephric cells in the complete absence of Sry in the responding gonad. The processes of polarizing pre- Sertoli cells and inducing the expression of Sox9 may be codependent. Precedents for a similar effect occur in the mammary gland (Strueli et al., 1991). This might suggest that the induction of Sertoli cell differentiation depends only on mesonephric cell migration. However, there are probably other factors involved. In XX $ XY mosaic gonads, the signal for cell migration would be expected to arise from XY cells, randomly scattered throughout the bed of the gonad. Cells responding to the signal would move from the mesonephros into the gonad, randomly encounter a supporting cell precursor (of XX or XY genotype), and induce Sertoli differentiation. However, when XX $ XY mosaics are made, Sertoli cells are.90% XY, indicating a strong bias for the XY cells to respond to induction (Palmer and Burgoyne, 1991a). Possible explanations for this include: (1) the XY supporting cell has a predisposition to differentiate as a Sertoli cell as the result of prior Sry expression; (2) this bias results from the more rapid proliferation of the XY supporting cells; or (3) XY Sertoli precursors produce the signal for cell migration, and therefore may have a chemoattractive effect on the migrating cells (Tilmann and Capel, 1999).
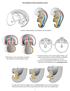
11. SEXUAL DIFFERENTIATION. Germinal cells, gonocytes. Indifferent stage INDIFFERENT STAGE
 11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
Development of the Genital System
 Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Wnt4 is required for proper male as well as female sexual development
 Developmental Biology 276 (2004) 431 440 www.elsevier.com/locate/ydbio Wnt4 is required for proper male as well as female sexual development Katherine Jeays-Ward 1, Mathieu Dandonneau 1, Amanda Swain*
Developmental Biology 276 (2004) 431 440 www.elsevier.com/locate/ydbio Wnt4 is required for proper male as well as female sexual development Katherine Jeays-Ward 1, Mathieu Dandonneau 1, Amanda Swain*
Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad
 Development 126, 2883-2890 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV4174 2883 Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation
Development 126, 2883-2890 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV4174 2883 Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation
Animal Science 434 Reproductive Physiology
 Animal Science 434 Reproductive Physiology Development of the Pituitary Gland Lec 5: Embryogenesis of the Pituitary and Sexual Development Stomodeum Brain Infundibulum Rathke s Pouch Germ Cell Migration
Animal Science 434 Reproductive Physiology Development of the Pituitary Gland Lec 5: Embryogenesis of the Pituitary and Sexual Development Stomodeum Brain Infundibulum Rathke s Pouch Germ Cell Migration
Animal Science 434 Reproductive Physiology"
 Animal Science 434 Reproductive Physiology" Embryogenesis of the Pituitary and Sexual Development: Part A Development of the Pituitary Gland" Infundibulum" Brain" Rathke s Pouch" Stomodeum" Germ Cell Migration"
Animal Science 434 Reproductive Physiology" Embryogenesis of the Pituitary and Sexual Development: Part A Development of the Pituitary Gland" Infundibulum" Brain" Rathke s Pouch" Stomodeum" Germ Cell Migration"
Urinary system development. Male ( ) and Female ( ) Reproductive Systems Development
 Urinary system development Male ( ) and Female ( ) Reproductive Systems Development Urogenital system develops from mesodermal uro-genital ridge (intermediate mesoderm) development of male and female genital
Urinary system development Male ( ) and Female ( ) Reproductive Systems Development Urogenital system develops from mesodermal uro-genital ridge (intermediate mesoderm) development of male and female genital
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
W.S. O University of Hong Kong
 W.S. O University of Hong Kong Development of the Genital System 1. Sexual differentiation 2. Differentiation of the gonads a. Germ cells extragonadal in origin b. Genital ridge intermediate mesoderm consisting
W.S. O University of Hong Kong Development of the Genital System 1. Sexual differentiation 2. Differentiation of the gonads a. Germ cells extragonadal in origin b. Genital ridge intermediate mesoderm consisting
under its influence, male development occurs; in its absence, female development is established.
 Sex differentiation is a complex process that involves many genes, including some that are autosomal. The key to sexual dimorphism is the Y chromosome, which contains the testis determining gene called
Sex differentiation is a complex process that involves many genes, including some that are autosomal. The key to sexual dimorphism is the Y chromosome, which contains the testis determining gene called
Growth pattern of the sex ducts in foetal mouse hermaphrodites
 /. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
/. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
Sex chromosomes and sex determination
 Sex chromosomes and sex determination History (1) 1940-ties Alfred Jost embryo-surgical experiments on gonads gonadal sex; male gonadal sex presence of testes; female gonadal sex lack of testes. History
Sex chromosomes and sex determination History (1) 1940-ties Alfred Jost embryo-surgical experiments on gonads gonadal sex; male gonadal sex presence of testes; female gonadal sex lack of testes. History
Sex Determination and Gonadal Sex Differentiation in Fish
 Sex Determination and Gonadal Sex Differentiation in Fish Yoshitaka Nagahama Okazaki National Research Institutes, Japan This first slide shows the processes of gonadal sex differentiation and gametogenesis
Sex Determination and Gonadal Sex Differentiation in Fish Yoshitaka Nagahama Okazaki National Research Institutes, Japan This first slide shows the processes of gonadal sex differentiation and gametogenesis
Topics for this lecture: Sex determination Sexual differentiation Sex differences in behavior and CNS development. 1) organizational effects of
 Topics for this lecture: Sex determination Sexual differentiation Sex differences in behavior and CNS development. 1) organizational effects of gonadal steroids on CNS development 2) our model system:
Topics for this lecture: Sex determination Sexual differentiation Sex differences in behavior and CNS development. 1) organizational effects of gonadal steroids on CNS development 2) our model system:
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Development of the urogenital system
 Development of the urogenital system Location of the pronephros, mesonephros and metanephros Differentiation of the intermedierm mesoderm into nephrotome and mesonephric tubules Connection between aorta
Development of the urogenital system Location of the pronephros, mesonephros and metanephros Differentiation of the intermedierm mesoderm into nephrotome and mesonephric tubules Connection between aorta
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Chapter 16: Steroid Hormones (Lecture 17)
 Chapter 16: Steroid Hormones (Lecture 17) A) 21 or fewer carbon atoms B) Precursor: 27 carbon cholesterol C) major classes of steroid hormones 1) progestagens a) progesterone- prepares lining of uterus
Chapter 16: Steroid Hormones (Lecture 17) A) 21 or fewer carbon atoms B) Precursor: 27 carbon cholesterol C) major classes of steroid hormones 1) progestagens a) progesterone- prepares lining of uterus
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
Embryology /organogenesis/ Week 4 Development and teratology of reproductive system.
 Embryology /organogenesis/ Week 4 Development and teratology of reproductive system. Male or female sex is determined by spermatozoon Y in the moment of fertilization SRY gene, on the short arm of the
Embryology /organogenesis/ Week 4 Development and teratology of reproductive system. Male or female sex is determined by spermatozoon Y in the moment of fertilization SRY gene, on the short arm of the
Bio Section IV Late Development. Development of the Tetrapod Limb. Pattern Formation. Skeletal pattern formation. Gilbert 9e Chapter 13
 Bio 127 - Section IV Late Development Development of the Tetrapod Limb Gilbert 9e Chapter 13 Pattern Formation Limbs show an amazing aspect of development Similarities: Architectural symmetry in all four
Bio 127 - Section IV Late Development Development of the Tetrapod Limb Gilbert 9e Chapter 13 Pattern Formation Limbs show an amazing aspect of development Similarities: Architectural symmetry in all four
Sexual Reproduction. For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species.
 Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
DAX1, testes development role 7, 8 DFFRY, spermatogenesis role 49 DMRT genes, male sex differentiation role 15
 Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Comparative Anatomy Urogenital System. Note Set 11 Chapter 15
 Comparative Anatomy Urogenital System Note Set 11 Chapter 15 Urogenital System Ducts of excretory and reproductive systems are intimately associated Figure 14.1: Embryonic and evolutionary development
Comparative Anatomy Urogenital System Note Set 11 Chapter 15 Urogenital System Ducts of excretory and reproductive systems are intimately associated Figure 14.1: Embryonic and evolutionary development
Sex Determination and Development of Reproductive Organs
 Sex Determination and Development of Reproductive Organs Sex determination The SRY + gene is necessary and probably sufficient for testis development The earliest sexual difference appears in the gonad
Sex Determination and Development of Reproductive Organs Sex determination The SRY + gene is necessary and probably sufficient for testis development The earliest sexual difference appears in the gonad
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Genetic Sex Determination
 Genetic Sex Determination Sex is determined by the heat of male partner during intercourse.. Courtesy of Humphrey Yao Aristotle (384-322 B.C.) Sex Differentiation: a favorite topic for philosophers and
Genetic Sex Determination Sex is determined by the heat of male partner during intercourse.. Courtesy of Humphrey Yao Aristotle (384-322 B.C.) Sex Differentiation: a favorite topic for philosophers and
Bi-potent Gonads. Sex Determination
 יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
Regionally Distinct Potencies of Mouse XY Genital Ridge to Initiate Testis Differentiation Dependent on Anteroposterior Axis
 DEVELOPMENTAL DYNAMICS 228:247 253, 2003 ARTICLE Regionally Distinct Potencies of Mouse XY Genital Ridge to Initiate Testis Differentiation Dependent on Anteroposterior Axis Ryuji Hiramatsu, 1 Yoshiakira
DEVELOPMENTAL DYNAMICS 228:247 253, 2003 ARTICLE Regionally Distinct Potencies of Mouse XY Genital Ridge to Initiate Testis Differentiation Dependent on Anteroposterior Axis Ryuji Hiramatsu, 1 Yoshiakira
Loss of Mitogen-Activated Protein Kinase Kinase Kinase 4 (MAP3K4) Reveals a Requirement for MAPK Signalling in Mouse Sex Determination
 Loss of Mitogen-Activated Protein Kinase Kinase Kinase 4 (MAP3K4) Reveals a Requirement for MAPK Signalling in Mouse Sex Determination Debora Bogani 1, Pam Siggers 1, Rachel Brixey 1, Nick Warr 1, Sarah
Loss of Mitogen-Activated Protein Kinase Kinase Kinase 4 (MAP3K4) Reveals a Requirement for MAPK Signalling in Mouse Sex Determination Debora Bogani 1, Pam Siggers 1, Rachel Brixey 1, Nick Warr 1, Sarah
Please Take Seats by Gender as Shown Leave Three Seats Empty in the Middle
 Please Take Seats by Gender as Shown Leave Three Seats Empty in the Middle Women Men Sexual Differentiation & Development Neal G. Simon, Ph.D. Professor Dept. of Biological Sciences Signaling Cascade &
Please Take Seats by Gender as Shown Leave Three Seats Empty in the Middle Women Men Sexual Differentiation & Development Neal G. Simon, Ph.D. Professor Dept. of Biological Sciences Signaling Cascade &
Chapter 18 Development. Sexual Differentiation
 Chapter 18 Development Sexual Differentiation There Are Many Levels of Sex Determination Chromosomal Sex Gonadal Sex Internal Sex Organs External Sex Organs Brain Sex Gender Identity Gender Preference
Chapter 18 Development Sexual Differentiation There Are Many Levels of Sex Determination Chromosomal Sex Gonadal Sex Internal Sex Organs External Sex Organs Brain Sex Gender Identity Gender Preference
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Cellular mechanisms of sex determination in the red-eared slider turtle, Trachemys scripta
 Mechanisms of Development 121 (2004) 1393 1401 www.elsevier.com/locate/modo Cellular mechanisms of sex determination in the red-eared slider turtle, Trachemys scripta Humphrey H.-C. Yao 1, Leo DiNapoli,
Mechanisms of Development 121 (2004) 1393 1401 www.elsevier.com/locate/modo Cellular mechanisms of sex determination in the red-eared slider turtle, Trachemys scripta Humphrey H.-C. Yao 1, Leo DiNapoli,
Testis cord morphogenesis: determination, establishment, and maintenance 1
 Anim. Reprod., v.3, n.2, p.92-97, April/June. 2006. Testis cord morphogenesis: determination, establishment, and maintenance 1 C-F. Liu, D.R. Archambeault, H H-C. Yao 2 Department of Veterinary Biosciences,
Anim. Reprod., v.3, n.2, p.92-97, April/June. 2006. Testis cord morphogenesis: determination, establishment, and maintenance 1 C-F. Liu, D.R. Archambeault, H H-C. Yao 2 Department of Veterinary Biosciences,
SUMMARY. Keywords: quail, Coturnix japonica, morphology, ovary, oviduct, neurotrophins, immunohistochemistry
 SUMMARY Keywords: quail, Coturnix japonica, morphology, ovary, oviduct, neurotrophins, immunohistochemistry Studies on the development of biological systems have expanded using animal models, always to
SUMMARY Keywords: quail, Coturnix japonica, morphology, ovary, oviduct, neurotrophins, immunohistochemistry Studies on the development of biological systems have expanded using animal models, always to
IN SUMMARY HST 071 NORMAL & ABNORMAL SEXUAL DIFFERENTIATION Fetal Sex Differentiation Postnatal Diagnosis and Management of Intersex Abnormalities
 Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz IN SUMMARY HST 071 Title: Fetal Sex Differentiation Postnatal Diagnosis
Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz IN SUMMARY HST 071 Title: Fetal Sex Differentiation Postnatal Diagnosis
Mesonephric contribution to testis differentiation in the fetal mouse
 Development 117, 273-281 (1993) Printed in Great Britain The Company of Biologists Limited 1993 273 Mesonephric contribution to testis differentiation in the fetal mouse Mia Buehr, Subin Gu* and Anne McLaren
Development 117, 273-281 (1993) Printed in Great Britain The Company of Biologists Limited 1993 273 Mesonephric contribution to testis differentiation in the fetal mouse Mia Buehr, Subin Gu* and Anne McLaren
CHARA CTERS* nearly sterile. As in other similar combinations the sex glands are reduced ZOOLOGY: E. WITSCHI
 ZOOLOGY: E. WITSCHI VOL. 23, 1937 35 STIMULATIVE AND INHIBITIVE INDUCTION IN THE DEVELOPMENT OF PRIMARY AND SECONDARY SEX CHARA CTERS* By EMIL WITSCHI DEPARTMENT OF ZOOLOGY, STATE UNIVERSITY OF IOWA Read
ZOOLOGY: E. WITSCHI VOL. 23, 1937 35 STIMULATIVE AND INHIBITIVE INDUCTION IN THE DEVELOPMENT OF PRIMARY AND SECONDARY SEX CHARA CTERS* By EMIL WITSCHI DEPARTMENT OF ZOOLOGY, STATE UNIVERSITY OF IOWA Read
Sex determination and SRY: down to a wink and a nudge?
 Review Feature Review Sex determination and SRY: down to a wink and a nudge? Ryohei Sekido and Robin Lovell-Badge Division of Developmental Genetics, Medical Research Council National Institute for Medical
Review Feature Review Sex determination and SRY: down to a wink and a nudge? Ryohei Sekido and Robin Lovell-Badge Division of Developmental Genetics, Medical Research Council National Institute for Medical
Urinary System Chapter 16
 Urinary System Chapter 16 1 Urology- the branch of medicine that treats male and female urinary systems as well as the male reproductive system. Nephrology- the scientific study of the anatomy, physiology,
Urinary System Chapter 16 1 Urology- the branch of medicine that treats male and female urinary systems as well as the male reproductive system. Nephrology- the scientific study of the anatomy, physiology,
Chapter 7 DEVELOPMENT AND SEX DETERMINATION
 Chapter 7 DEVELOPMENT AND SEX DETERMINATION Chapter Summary The male and female reproductive systems produce the sperm and eggs, and promote their meeting and fusion, which results in a fertilized egg.
Chapter 7 DEVELOPMENT AND SEX DETERMINATION Chapter Summary The male and female reproductive systems produce the sperm and eggs, and promote their meeting and fusion, which results in a fertilized egg.
Urogenital System. PUMC Dept. of Anat. Hist. & Embry. 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College
 Urogenital System 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College intermediate mesoderm urogenital ridge mesonephric ridge genital ridge I. Urinary System 1. kidney
Urogenital System 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College intermediate mesoderm urogenital ridge mesonephric ridge genital ridge I. Urinary System 1. kidney
Formation of Urine: Formation of Urine
 The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
Biology of Reproduction-Biol 326
 Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
SALSA MLPA probemix P185-C2 Intersex Lot C2-1015: As compared to the previous version C1 (lot C1-0611), the lengths of four probes have been adjusted.
 mix P185-C2 Intersex Lot C2-1015: As compared to the previous version C1 (lot C1-0611), the lengths of four s have been adjusted. The sex-determining region on chromosome Y (SRY) is the most important
mix P185-C2 Intersex Lot C2-1015: As compared to the previous version C1 (lot C1-0611), the lengths of four s have been adjusted. The sex-determining region on chromosome Y (SRY) is the most important
Disorders of gonadal and sexual development
 Disorders of gonadal and sexual development gonadal embryogenesis, cytogenetics/molecular abnormalities, and clinical aspects Pr I.Maystadt 08/01/2016 IPG Male Genitalia bladder prostate penis Seminal
Disorders of gonadal and sexual development gonadal embryogenesis, cytogenetics/molecular abnormalities, and clinical aspects Pr I.Maystadt 08/01/2016 IPG Male Genitalia bladder prostate penis Seminal
10. Development of genital system. Gonads. Genital ducts. External genitalia.
 10. Development of genital system. Gonads. Genital ducts. External genitalia. Gonads, genital ducts and the external genital organs initially pass through an indifferent period of development, which is
10. Development of genital system. Gonads. Genital ducts. External genitalia. Gonads, genital ducts and the external genital organs initially pass through an indifferent period of development, which is
Lesson 31. Lesson Outline:
 Lesson 31 Lesson Outline: The Urogenital System Urinary System o Embryonic Origins o Phylogenetic Trends Function Urinary Bladder Implications for the Evolution of the Vertebrates o Freshwater or Seawater
Lesson 31 Lesson Outline: The Urogenital System Urinary System o Embryonic Origins o Phylogenetic Trends Function Urinary Bladder Implications for the Evolution of the Vertebrates o Freshwater or Seawater
- production of two types of gametes -- fused at fertilization to form zygote
 Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Regulation of Male Sexual Development by Sry and Sox9
 JOURNAL OF EXPERIMENTAL ZOOLOGY 290:463 474 (2001) Regulation of Male Sexual Development by Sry and Sox9 PETER KOOPMAN,* MONICA BULLEJOS, AND JO BOWLES Centre for Molecular and Cellular Biology, Institute
JOURNAL OF EXPERIMENTAL ZOOLOGY 290:463 474 (2001) Regulation of Male Sexual Development by Sry and Sox9 PETER KOOPMAN,* MONICA BULLEJOS, AND JO BOWLES Centre for Molecular and Cellular Biology, Institute
BIOL 2402 Reproductive Systems!
 Dr. Chris Doumen! Female Reproductive Anatomy BIOL 2402 Reproductive Systems! Establishing the Ovarian Cycle During childhood, until puberty Ovaries grow and secrete small amounts of estrogens Estrogen
Dr. Chris Doumen! Female Reproductive Anatomy BIOL 2402 Reproductive Systems! Establishing the Ovarian Cycle During childhood, until puberty Ovaries grow and secrete small amounts of estrogens Estrogen
Development of the Urinary System. 3 Distinct Embryonic Kidney Structures
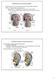 Development of the Urinary System Excretory portion of urinary system derived from intermediate mesoderm Week 4: 1 st nephrons/renal corpuscles form Nephrotomes form and develop hollow lumens to form nephric
Development of the Urinary System Excretory portion of urinary system derived from intermediate mesoderm Week 4: 1 st nephrons/renal corpuscles form Nephrotomes form and develop hollow lumens to form nephric
Analysis of the Sex-determining Region of the Y Chromosome (SRY) in a Case of 46, XX True Hermaphrodite
 Clin Pediatr Endocrinol 1994; 3(2): 91-95 Copyright (C) 1994 by The Japanese Society for Pediatric Endocrinology Analysis of the Sex-determining Region of the Y Chromosome (SRY) in a Case of 46, XX True
Clin Pediatr Endocrinol 1994; 3(2): 91-95 Copyright (C) 1994 by The Japanese Society for Pediatric Endocrinology Analysis of the Sex-determining Region of the Y Chromosome (SRY) in a Case of 46, XX True
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats
 Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Mohammad Sha ban. Basheq Jehad. Hamzah Nakhleh
 11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
Development of the female Reproductive System. Dr. Susheela Rani
 Development of the female Reproductive System Dr. Susheela Rani Genital System Gonads Internal genitals External genitals Determining sex chronology of events Genetic sex Determined at fertilization Gonadal
Development of the female Reproductive System Dr. Susheela Rani Genital System Gonads Internal genitals External genitals Determining sex chronology of events Genetic sex Determined at fertilization Gonadal
I) Development: tissue differentiation and timing II) Whole Chromosome Regulation
 Epigenesis: Gene Regulation Epigenesis : Gene Regulation I) Development: tissue differentiation and timing II) Whole Chromosome Regulation (X chromosome inactivation or Lyonization) III) Regulation during
Epigenesis: Gene Regulation Epigenesis : Gene Regulation I) Development: tissue differentiation and timing II) Whole Chromosome Regulation (X chromosome inactivation or Lyonization) III) Regulation during
SEX DETERMINATION AND SEX CHROMOSOMES
 Klug et al. 2006, 2009 Concepts of Genetics Chapter 7 STUDY UNIT 5 SEX DETERMINATION AND SEX CHROMOSOMES Some species reproduce asexually Most diploid eukaryotes reproduce sexually Parent (2n) Parent (2n)
Klug et al. 2006, 2009 Concepts of Genetics Chapter 7 STUDY UNIT 5 SEX DETERMINATION AND SEX CHROMOSOMES Some species reproduce asexually Most diploid eukaryotes reproduce sexually Parent (2n) Parent (2n)
1. Both asexual and sexual reproduction occur in the animal kingdom
 1. Both asexual and sexual reproduction occur in the animal kingdom Asexual reproduction involves the formation of individuals whose genes all come from one parent. There is no fusion of sperm and egg.
1. Both asexual and sexual reproduction occur in the animal kingdom Asexual reproduction involves the formation of individuals whose genes all come from one parent. There is no fusion of sperm and egg.
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT. Reading Assignment: The Developing Human, Clinically Oriented Embryology pp
 Developmental Anatomy Steven M. Hill, Ph.D. 9/16/13 DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT Reading Assignment: The Developing Human, Clinically Oriented Embryology pp. 264-273. Objectives:
Developmental Anatomy Steven M. Hill, Ph.D. 9/16/13 DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT Reading Assignment: The Developing Human, Clinically Oriented Embryology pp. 264-273. Objectives:
Nature Genetics: doi: /ng Supplementary Figure 1. Assessment of sample purity and quality.
 Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
The makings of maleness: towards an integrated view of male sexual development
 The makings of maleness: towards an integrated view of male sexual development Dagmar Wilhelm* and Peter Koopman* Abstract As the mammalian embryo develops, it must engage one of the two distinct programmes
The makings of maleness: towards an integrated view of male sexual development Dagmar Wilhelm* and Peter Koopman* Abstract As the mammalian embryo develops, it must engage one of the two distinct programmes
Development of the Urinary System
 Development of the Urinary System Lecture Objectives Understand the development of the kidney and related organs of the urinary system. Define the pronephrons, mesonephrons and metanephrons. Understand
Development of the Urinary System Lecture Objectives Understand the development of the kidney and related organs of the urinary system. Define the pronephrons, mesonephrons and metanephrons. Understand
Reproductive physiology. About this Chapter. Case introduction. The brain directs reproduction 2010/6/29. The Male Reproductive System
 Section Ⅻ Reproductive physiology Ming-jie Wang E-Mail: mjwang@shmu.edu.cn About this Chapter The reproductive organs and how they work the major endocrine functions of sexual glands actions of sex hormones
Section Ⅻ Reproductive physiology Ming-jie Wang E-Mail: mjwang@shmu.edu.cn About this Chapter The reproductive organs and how they work the major endocrine functions of sexual glands actions of sex hormones
Sexual Development. 6 Stages of Development
 6 Sexual Development 6 Stages of Development Development passes through distinct stages, the first of which is fertilization, when one sperm enters one ovum. To enter an ovum, a sperm must undergo the
6 Sexual Development 6 Stages of Development Development passes through distinct stages, the first of which is fertilization, when one sperm enters one ovum. To enter an ovum, a sperm must undergo the
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Chapter 11 Gene Expression
 Chapter 11 Gene Expression 11-1 Control of Gene Expression Gene Expression- the activation of a gene to form a protein -a gene is on or expressed when it is transcribed. -cells do not always need to produce
Chapter 11 Gene Expression 11-1 Control of Gene Expression Gene Expression- the activation of a gene to form a protein -a gene is on or expressed when it is transcribed. -cells do not always need to produce
PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION
 PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION Prof. Dr med. Jolanta Słowikowska-Hilczer Department of Andrology and Reproductive Endocrinology Medical University of Łódź, Poland Sexual determination
PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION Prof. Dr med. Jolanta Słowikowska-Hilczer Department of Andrology and Reproductive Endocrinology Medical University of Łódź, Poland Sexual determination
Today. Genomic Imprinting & X-Inactivation
 Today 1. Quiz (~12 min) 2. Genomic imprinting in mammals 3. X-chromosome inactivation in mammals Note that readings on Dosage Compensation and Genomic Imprinting in Mammals are on our web site. Genomic
Today 1. Quiz (~12 min) 2. Genomic imprinting in mammals 3. X-chromosome inactivation in mammals Note that readings on Dosage Compensation and Genomic Imprinting in Mammals are on our web site. Genomic
DEVELOPMENT (DSD) 1 4 DISORDERS OF SEX
 Wit JM, Ranke MB, Kelnar CJH (eds): ESPE classification of paediatric endocrine diagnosis. 4. Disorders of sex development (DSD). Horm Res 2007;68(suppl 2):21 24 ESPE Code Diagnosis OMIM ICD 10 4 DISORDERS
Wit JM, Ranke MB, Kelnar CJH (eds): ESPE classification of paediatric endocrine diagnosis. 4. Disorders of sex development (DSD). Horm Res 2007;68(suppl 2):21 24 ESPE Code Diagnosis OMIM ICD 10 4 DISORDERS
.Protein LYONIZATION. The process by which all X chromosomes in excess of one are made genetically inactive and heterochromatic.
 + Electrical field - LYONIZATION Colleen Jackson-Cook, Ph.D, FACMG Sanger Hall, Room 5-7 ccook@mcvh-vcu.edu The process by which all X chromosomes in excess of one are made genetically inactive and heterochromatic.
+ Electrical field - LYONIZATION Colleen Jackson-Cook, Ph.D, FACMG Sanger Hall, Room 5-7 ccook@mcvh-vcu.edu The process by which all X chromosomes in excess of one are made genetically inactive and heterochromatic.
Temperature-Dependent Sex Determination in the American Alligator: AMH Precedes SOX9 Expression
 DEVELOPMENTAL DYNAMICS 216:411 419 (1999) Temperature-Dependent Sex Determination in the American Alligator: AMH Precedes SOX9 Expression PATRICK S. WESTERN, 1,2 JENNY L. HARRY, 3 JENNIFER A. MARSHALL
DEVELOPMENTAL DYNAMICS 216:411 419 (1999) Temperature-Dependent Sex Determination in the American Alligator: AMH Precedes SOX9 Expression PATRICK S. WESTERN, 1,2 JENNY L. HARRY, 3 JENNIFER A. MARSHALL
Supplemental Figure 1: Leydig cells are reduced at multiple stages in both male sterile mutants
 SUPPLEMENTAL FIGURE LEGENDS: Supplemental Figure 1: Leydig cells are reduced at multiple stages in both male sterile mutants (Sgpl1 -/- and Plekha1 -/- ). Using an antibody against CYP11a1 to label Leydig
SUPPLEMENTAL FIGURE LEGENDS: Supplemental Figure 1: Leydig cells are reduced at multiple stages in both male sterile mutants (Sgpl1 -/- and Plekha1 -/- ). Using an antibody against CYP11a1 to label Leydig
Reproductive Endocrinology. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007
 Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
18 Urinary system. 19 Male reproductive system. Female reproductive system. Blok 11: Genital and Urinary Tract Diseases
 Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Urinary System. ectoderm. notochord. mesonephric tubules. Nephrogenic Cord (left)
 Urinary System NOTE: Urine proion requires an increased capillary surface area (glomeruli), epithelial s to collect plasma filtrate and extract desirable constituents, and a system to convey urine away
Urinary System NOTE: Urine proion requires an increased capillary surface area (glomeruli), epithelial s to collect plasma filtrate and extract desirable constituents, and a system to convey urine away
Sex Differentiation. Course Outline. Topic #! Topic lecture! Silverthorn! Membranes (pre-requisite material)!!
 Sex Differentiation The goal of these lectures is to discuss how a control system is formed. For this, we will use basic physiology associated with the control of reproduction (from sexual differentiation
Sex Differentiation The goal of these lectures is to discuss how a control system is formed. For this, we will use basic physiology associated with the control of reproduction (from sexual differentiation
a) They are the most common cause of pediatric kidney failure. b) They are always symptomatic. c) They can be asymmetric.
 Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following hormones controls the release of anterior pituitary gonadotropins? A) LH
Reproduction Review YOU ARE EXPECTED TO KNOW THE MEANING OF ALL THE FOLLOWING TERMS:
 Reproduction Review YOU ARE EXPECTED TO KNOW THE MEANING OF ALL THE FOLLOWING TERMS: CHROMOSOME GENE DNA TRAIT HEREDITY INTERPHASE MITOSIS CYTOKINESIS ASEXUAL BINARY FISSION CELL CYCLE GENETIC DIVERSITY
Reproduction Review YOU ARE EXPECTED TO KNOW THE MEANING OF ALL THE FOLLOWING TERMS: CHROMOSOME GENE DNA TRAIT HEREDITY INTERPHASE MITOSIS CYTOKINESIS ASEXUAL BINARY FISSION CELL CYCLE GENETIC DIVERSITY
The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development in an XX/XY mouse
 Development 101 Supplement. 143 149 (1987) Printed in Great Britain The Company of Biologists Limited 1987 143 The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development
Development 101 Supplement. 143 149 (1987) Printed in Great Britain The Company of Biologists Limited 1987 143 The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development
GENDER James Bier
 GENDER 2005-2008 James Bier Objectives 1. State the method of determining gender in several genetic systems. 2. List the three regions of the Y chromosome. 3. Describe the events that promote sexual development
GENDER 2005-2008 James Bier Objectives 1. State the method of determining gender in several genetic systems. 2. List the three regions of the Y chromosome. 3. Describe the events that promote sexual development
A Genetic Program for Embryonic Development
 Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Concept 18.4: A program of differential gene expression leads to the different cell types in a multicellular organism During embryonic development, a fertilized egg gives rise to many different cell types
Animal Reproduction. Hypothalamic-pituitary-gonadal axis. # lectures for cumulative test # 01 book 01
 Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 05 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 05 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Sexual differentiation:
 Abnormal Development of Female Genitalia Dr. Maryam Fetal development of gonads, external genitalia, Mullerian ducts and Wolffian ducts can be disrupted at a variety of points, leading to a wide range
Abnormal Development of Female Genitalia Dr. Maryam Fetal development of gonads, external genitalia, Mullerian ducts and Wolffian ducts can be disrupted at a variety of points, leading to a wide range
Results and Problems in Cell Differentiation
 Results and Problems in Cell Differentiation Volume 58 Series editors Jacek Z. Kubiak, Rennes CX, France Malgorzata Kloc, Houston, TX, USA More information about this series at http://www.springer.com/series/400
Results and Problems in Cell Differentiation Volume 58 Series editors Jacek Z. Kubiak, Rennes CX, France Malgorzata Kloc, Houston, TX, USA More information about this series at http://www.springer.com/series/400
Testicular stem cells
 Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Chapter 26: Reproductive Systems. Male 11/29/2015. Male reproductive system is composed of... BIO 218 Fall Gonads (testes)
 Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Chapter 26: Reproductive Systems BIO 218 Fall 2015 Male Male reproductive system is composed of... Gonads (testes) Duct system (epididymis, ductus deferens, ejaculatory ducts, urethra) Accessory sex glands
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
MRC-Holland MLPA. Description version 08; 07 May 2015
 mix P185-C1 Intersex Lot C1-0611: As compared to the previous version B2 (lot B2-0311), s for CYP21A2 have been removed and s for the CXorf21 gene as well as additional s for NR0B1, NR5A1 and the Y chromosome
mix P185-C1 Intersex Lot C1-0611: As compared to the previous version B2 (lot B2-0311), s for CYP21A2 have been removed and s for the CXorf21 gene as well as additional s for NR0B1, NR5A1 and the Y chromosome
Cell Divisions. The autosomes represent the whole body. * Male Sex Chromosomes: XY * Female Sex Chromosomes: XX
 Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
