The makings of maleness: towards an integrated view of male sexual development
|
|
|
- Maximilian Armstrong
- 6 years ago
- Views:
Transcription
1 The makings of maleness: towards an integrated view of male sexual development Dagmar Wilhelm* and Peter Koopman* Abstract As the mammalian embryo develops, it must engage one of the two distinct programmes of gene activity, morphogenesis and organogenesis that characterize males and females. In males, sexual development hinges on testis determination and differentiation, but also involves many coordinated transcriptional, signalling and endocrine networks that underpin the masculinization of other organs and tissues, including the brain. Here we bring together current knowledge about these networks, identify gaps in the overall picture, and highlight the known defects that lead to disorders of male sexual development. Hypospadias Incorrect placement of the urethral opening in males, not at the tip of the penis. *Division of Molecular Genetics and Development, Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia. ARC Centre of Excellence in Biotechnology and Development, Institute for Molecular Bioscience, The University of Queensland. Correspondence to P.K. doi: /nrg1903 Published online 11 July 2006 The differences between males and females are a source of enduring fascination. This is hardly surprising, given the mysteries surrounding these differences and their profound effect on our daily lives. For the developmental biologist, the morphological differences between the sexes are particularly intriguing given that they arise through dichotomous differentiation of a common set of precursor tissues, a unique situation in embryonic development. For any given species, there are not one but two developmental biologies. In simple terms, the development of mammalian maleness or femaleness hinges on whether testes or ovaries form in the embryo from the paired, ambipotent structures known as genital ridges 1. This decision depends on the presence and correct function of a maledetermining gene from the Y chromosome, Sry, which functions in a specific subset of genital ridge cells to stimulate them to differentiate as Sertoli cells the cells that interact with and nurture the germ cells. The Sertoli cells then seem to orchestrate the differentiation of other cell types required for testis formation, such as the germ cells and steroid-producing cells. If Sry is absent or does not function correctly, other regulatory cascades lead to ovary development and female characteristics. In reality, sexual development is vastly more complex, hinging on delicate networks of molecular signals that specify sex-specific differentiation, organogenesis and endocrine function. The fragility of these networks is clear: disorders of sexual development are among the most common human birth defects. They range in frequency and also in severity, from hypospadias to complete sexual ambiguity and sex reversal (TABLE 1), and are often associated with secondary effects such as infertility and gonadal tumours. Sexual dysgenesis is often traumatic, stigmatized and under-recognized as a medical issue in our society, and identifying responsible genes will be increasingly important for diagnosing these disorders, counselling affected patients, and making a prognosis that will inform gender-assignment options. However, genes responsible for proper sexual development are often difficult to find by conventional genetic studies, which require fertility. The discovery of Sry in 1990 (REFS 2,3) opened the way for genetic dissection of the cascade of events leading to male development. There is a growing molecular and cellular understanding of testis determination and the early events in testis differentiation, mainly from analyses of mouse mutants and genotype phenotype correlations in humans who are affected by disorders of sexual development. In addition, technologies such as microarray analysis have identified many genes that show sex-specific expression during gonad development and might be important in testis development. However, key questions still remain. How does SRY induce Sertoli cell differentiation? How do Sertoli cells orchestrate the differentiation of the other testicular cell lineages, including the germ line? What factors, gonadal or non-gonadal, are necessary to induce secondary sexual characteristics such as testicular descent and differentiation of the external genitalia? What are the phenotypic consequences of mutations in genes that are important in these processes? We focus on these questions in an effort to summarize the knowns and unknowns of male sexual development, from testis specification, to the development of the genital tract, male accessory sex 620 AUGUST 2006 VOLUME 7
2 Table 1 Disorders of human sexual development Syndrome Phenotype Genes or other alterations known to be involved Hypospadias Incorrect placement of the urethral opening in males HOXA13, HOXD13 Cryptorchidism Failure of testicular descent INSL3, LGR8 XY true hermaphroditism Ovotestes, ambiguous genitalia Trisomy of chromosome 22 XY sex reversal Ovaries, female genitalia and secondary characteristics SRY, SOX9 Swyer syndrome XY females with complete gonadal dysgenesis SRY Denys Drash syndrome Pseudohermaphroditism, nephropathy, predisposition to Wilms tumour WT1 Frasier syndrome Pseudohermaphroditism, progressive glomerulopathy WT1 WAGR syndrome Wilms tumour, aniridia, genito-urinary malformations, mental retardation WT1 Adrenal hypoplasia congenita Adrenal hypoplasia, hypogonadotropic hypogonadism DAX1 Campomelic dysplasia Skeletal dysmorphology and XY sex reversal SOX9 Persistent Müllerian duct Normally virilized men with a uterus and fallopian tubes, often with AMH, AMHR syndrome unilateral inguinal hernia or cryptorchidism Congenital bilateral aplasia of vas deferens Absence of vasa deferentia, infertility CFTR X-linked lissencephaly with abnormal genitalia syndrome α-thalassaemia/mental retardation syndrome, X-linked Congenital adrenal hyperplasia Microcephaly, lissencephaly, agenesis of the corpus callosum, epilepsy, poor temperature regulation, chronic diarrhoea, ambiguous genitalia Severe mental retardation, α-thalassaemia, genital abnormalities, facial anomalies, lung, kidney and digestive problems Androgen excess produced by adrenal glands, XX with varying degrees of virilization ARX ATRX Enzymes that are necessary for hormone production (21-hydroxylase in 95% of patients) Androgen insensitivity syndrome Varying from nearly normal male to nearly normal female AR Hand-foot-genital syndrome Short first metacarpals and metatarsals, carpal and tarsal fusion, fifth-finger clinodactyly, genital abnormalities in both sexes, hypospadias in the male HOXA13, HOXD13 This table summarizes the sexual disorders mentioned in the text. For references and further reading see REF AMH, anti-müllerian hormone; AMHR, anti- Müllerian hormone receptor; AR, androgen receptor; ARX, aristaless-related homeobox; ATRX, α-thalassaemia/mental retardation syndrome, X-linked; CFTR, cystic fibrosis transmembrane conductance regulator; HOXA13, homeobox A13; INSL3, insulin-like 3; LGR8, the INSL3 receptor; SOX9, SRY-box containing gene 9; WT1, Wilms tumour 1. Eutherian mammals Mammals that have a placenta; includes all mammals except monotremes and marsupials. organs and external genitalia, to male-specific brain features (FIG. 1). We aim to move beyond studies of testis development in order to build up an integrated picture of the breadth of issues in male sexual development. Development of the testes In eutherian mammals, the first morphological sign of male differentiation is the formation of cords in nascent testes. Before this, both male and female embryos develop paired, ambipotent genital ridges that are indistinguishable between sexes. The indifferent gonad. The genital ridges form as linear swellings on the ventromedial surfaces of the mesonephroi. Each mesonephros is derived from intermediate mesoderm in the aorta-gonad-mesonephros region of the trunk, flanking the dorsal aorta and bounded ventrally by epithelium at its coelomic surface (FIG. 1a). In both sexes, mesonephric mesenchyme and thickening coelomic epithelium contribute to the evagination of the genital ridge from the mesonephros as development proceeds. In mice, the genital ridges start to appear around 10 days post coitum (dpc), and remain morphologically undifferentiated until about 12 dpc, despite different programmes of genetic activity in males and females beginning around 10.5 dpc (see below). Mutational analyses in mice have shown that several transcription-factor genes are required for the early formation of the indifferent genital ridges, including empty spiracles homologue 2 (Emx2) (REF. 4), GATA-binding protein 4 (Gata4) (REF. 5), Lim homeobox protein 9 (Lhx9) (REF. 6), steroidogenic factor 1 (Sf1; also known as NR5A1) (REFS 7,8), and Wilms tumour 1 (Wt1) (REF. 4). Two of these, WT1 and SF1, are also crucial for the formation of the genital ridges in humans. Mutations in these genes result in malformed gonads and ambiguous genitalia 9,10. Intriguingly, Wt1 and Sf1 have additional, sex-specific roles later in gonadal development. One enigmatic gene, Dax1 (also known as Nr0b1), is expressed in the indifferent gonad of both sexes, and gain-of-function and loss-of-function mutations in mice affect both testis and ovary determination. It may be that Dax1 levels and thresholds are extremely critical for its male versus female function 11. Sry the male determinant. The bifurcation in the development of testes and ovaries is triggered by the expression of Sry. Loss-of-function and gain-of-function mutations in mice and humans indicate that this gene is necessary and sufficient for testis development in mammals Its expression is tightly regulated in mice, occurring in a wave that starts in the centre of the genital NATURE REVIEWS GENETICS VOLUME 7 AUGUST
3 a Development of external genitalia Allantois Cloacal membrane Cloaca Hindgut Urorectal septum 10.5 dpc onwards 11.5 dpc b Formation of the aorta-genital-ridgemesonephros region Mesonephros Genital ridge Coelomic epithelium Aorta Wolffian duct Müllerian duct Genital tubercule Urogenital sinus dpc c Testis and genital tract differentiation Seminal vesicle Prostatic bud Urethra Distal urethral epithelium Penile urethra Vas deferens Epididymis Testis Testicular cords Efferent ducts Rete testes dpc e Development of brain dimorphisms d Testicular descent 1st phase 2nd phase Kidney Cranial ligament Gubernaculum Gubernaculum Scrotum Gential tubercle Figure 1 Major steps in male sexual differentiation. The external genitalia appear as a mesenchymal swelling between the two layers of the cloacal membrane at around 10.5 dpc and develop further under the influence of testicular androgens (a). A transverse section through a mouse embryo at 11.5 days post coitum (dpc) shows the developing urogenital ridge, which develops out of the intermediate mesoderm on either side of the aorta and is composed of the mesonephros and genital ridge. Within the mesonephros the Wolffian (male) and Müllerian (female) ducts form. At 11.5 dpc males cannot be distinguished morphologically from females (b). However, expression of the male-determining gene Sry induces a cascade of gene expression that results in the differentiation of the genital ridge and the Wolffian duct into the testis and male genital tract (rete testes, epididymis, vas deferens and seminal vesicle), respectively (c). At 13 dpc the bipotential urogenital sinus develops and at 17.5 dpc the prostatic bud is formed by the urogenital sinus under the influence of testicular androgens. Between 14.5 and 18 dpc the testis migrates into the developing scrotum in two phases (d). The first phase is due to an enlargement of the gubernaculum, whereas during the second phase the gubernaculum migrates into the scrotum, guided by calcitonin-related peptide that is released by the genito-femoral nerve. The brain develops sexual dimorphisms, some of which are due to direct genetic effects, although most are caused by sex hormones (e). Ovotestes Gonads in which ovarian and testicular tissue are present together. XY true hermaphroditism This condition comprises the presence of both ovarian and testicular tissue either in the same gonad as an ovotestis, or an ovary and a testis. ridges around 10.5 dpc, reaching peak levels and encompassing the whole gonad at 11.5 dpc, before declining, first in the centre then later at the poles, to undetectable levels around 12.5 dpc (REFS 15,16). However, the molecular mechanism of this regulation remains a mystery. Several factors have been implicated by virtue of reduced Sry expression in targeted mouse mutants; these include a splice variant of WT1, GATA4 and its cofactor FOG2 (also known as ZFPM2), and the insulin receptor family 5,17,18. It is not clear in these mutants whether the level of Sry expression per cell is affected, or the number of cells that express Sry is reduced, and none of these genes is expressed in a wave pattern similar to Sry. Differences between species imply that it is immaterial how quickly Sry expression is shut down. However, the time at which peak levels of expression are attained is crucial. In a phenomenon known as B6-Y DOM sex reversal 22, Y chromosomes from several Mus domesticus variants differ in their ability to induce testis formation on a C57BL/6J genetic background, resulting in phenotypes that range from complete XY sex reversal, to unilateral or bilateral ovotestes, to delayed testis formation. This has been ascribed to delayed expression of Sry from these Y chromosomes 23,24, adding to previous evidence that levels of Sry expression are important for male sex-determining function 25. Similar regulatory phenomena might explain the occurrence of ovotestes in some cases of XY true hermaphroditism in humans. The current belief is that Sry expression must reach a certain threshold level within a definite temporal window of competence 622 AUGUST 2006 VOLUME 7
4 XY gonadal dysgenesis This can lead to pure gonadal dysgenesis, in which patients have streak gonads (undeveloped gonadal structures), Müllerian structures (owing to insufficient AMH secretion) and a complete absence of virilization. Alternatively, patients can have dysgenetic testes. In this case, enough AMH is produced to regress the Müllerian duct and there might be enough testosterone for partial virilization. Campomelic dysplasia A syndrome that is characterized by skeletal abnormalities and sex reversal, caused by mutations in SOX9. in the precursors of supporting cells, otherwise the ovary-determining pathway will initiate in these cells. Sry encodes a nuclear, high-mobility group (HMG) domain protein that binds and bends DNA. Outside the HMG domain, SRY exhibits little sequence or structural conservation between species, and almost all mutations that have a clinical phenotype (for example, XY females with complete gonadal dysgenesis in Swyer syndrome) reside within this motif, underscoring the importance of this domain 26. These mutations generally impair SRY DNA binding and/or bending, or nuclear translocation The SRY protein in humans and mice has other domains that mediate protein protein interaction and transcriptional transactivation in vitro. The significance of the former is suggested by some missense and frameshift mutations causing XY sex reversal in humans However, it is not clear whether SRY functions as a transcriptional activator in vivo; the frequency of SRY-negative XX maleness in the human population has been taken as evidence that SRY might repress a repressor of the male programme rather than directly activate a sex-determining cascade 34. Either way, it is curious that no target genes for SRY have been identified, and the molecular cascade of events triggered by SRY activity still remains obscure. Differentiation of Sertoli cells. The genital ridges consist of several cell lineages (FIG. 2), each of which is thought to be bipotential, generating different cell types in testes and ovaries depending on the signals received. Among these is a progenitor known as the supporting-cell precursor lineage, which gives rise to both the Sertoli cells and the ovarian granulosa cells that support the development of germ cells in males and females, respectively. Landmark experiments by Burgoyne and colleagues 35 that involve mouse XX XY chimaeras showed that all gonadal lineages were composed of similar numbers of XX and XY cells, but Sertoli cells were overwhelmingly XY. This indicates that SRY functions cell autonomously to trigger the differentiation of Sertoli cells, and that pre-sertoli cells (defined as supporting cells that express Sry and/or Sox9 (SRY-box containing gene 9) but are not yet arranged into cords) can signal to other lineages to induce their male-specific differentiation and so orchestrate testis development. However, up to 10% of Sertoli cells in these chimaeras were found to be XX. This implies the existence of a mechanism by which supporting cell precursors that do not express Sry can be recruited to differentiate into Sertoli cells through a secreted signal. Recent work has shown that pre-sertoli cells produce prostaglandin D2, which, by binding to its receptor and upregulating Sox9 expression, recruits other cells to the Sertoli cell fate 15,36,37. This might act as a backup mechanism, reinforcing male sex-determination when Sry expression or function is impaired. Downstream of Sry, other factors such as SOX9, SOX8, DAX1 and FGF9 (fibroblast growth factor 9) have a role in Sertoli cell differentiation and function. Sox9 is the best candidate for a direct SRY target gene, although conclusive proof has yet to be produced. In mice, Sox9 is expressed shortly after the onset of Sry expression and in the same cell type, the pre-sertoli cell It is clear that Sox9 represents an early acting and crucial component of the male sex-determining pathway: loss of function of SOX9 results in XY gonadal dysgenesis in mice and also in the human disorder campomelic dysplasia, whereas gain of function in transgenic mice induces XX maleness Sox9 is present in a wide diversity of metazoans, possibly fulfilling a similar role even in Drosophila melanogaster 48, suggesting that it is an ancient and conserved effector Endothelial cells Pecam1 Germ cells Oct4, Pecam1, E cadherin Sertoli cells Sox9, Amh, Dhh, Dmrt1 Peritubular myoid cells Ptc Leydig cells Scc, HSD3B, Ptc Figure 2 Histological and gene-expression map of an early embryonic testis. A haematoxylin and eosin stained section that illustrates the different cell types of the testis and the genes that are specifically expressed in these cells: endothelial cells (red) form the male-specific vasculature, germ cells (orange) that later develop into sperm are enclosed by a layer of Sertoli cells (green), which support and nourish the germ cells, and peritubular myoid cells (black), which help to maintain cord integrity and are later responsible for pulsatory contractions that are required for export of sperm. The steroid-producing Leydig cells (purple) reside in the interstitium together with other cells such as macrophages and mesenchymal cells. Amh, anti-müllerian hormone; Dhh, desert hedgehog; Dmrt1, doublesex and mab-3 related transcription factor 1; Pecam1, platelet/endothelial cell-adhesion molecule 1; Ptc, patched; Scc, side-chain cleavage (also known as Cyp11a1); Sox9, SRY-box containing gene 9; HSD3B, hydroxy-δ-5- steroid dehydrogenase-3β. NATURE REVIEWS GENETICS VOLUME 7 AUGUST
5 Lissencephaly A brain malformation that is characterized by the incomplete development of the folds of the outer region of the brain (the cerebral cortex), which causes the surface of the brain to appear abnormally thickened and unusually smooth. Ovarian follicle A cyst in which the oocyte matures. of male sex determination, in contrast to Sry, which is exclusively mammalian. The elusive relationship between SRY and Sox9, and the probable nature of the transcriptional cascade downstream of SRY, have been reviewed extensively elsewhere 49,50. Besides the differentiation of Sertoli cells, testis development immediately after the onset of Sry expression differs from female gonad development in its increased cell proliferation 51 and the induced immigration of mesonephric cells 52,53. Although several factors such as neurotropin 3 (NT3; also known as NTF3) (REF. 54), hepatocyte growth factor (HGF) 55, and platelet-derived growth factor-α (PDGFA) 56 are able to induce this cell migration in vitro, none has been shown to have a role in vivo. Presumably, because this migration and increased proliferation are male-specific events, the factors that mediate them are regulated either by Sry or one of its early downstream genes such as Sox9. The immigrant mesonephric cells are thought to differentiate into Leydig, endothelial and peritubular myoid cells (FIG. 2), depending on interactions with somatic cells within the gonad. This raises the question of the nature of these interactions and how these other testicular cell types differentiate. Testis differentiation peritubular myoid cells. Peritubular myoid (PM) cells are flat, smooth-muscle-like cells that ensheath testis cords and are necessary for cord development and structural integrity 53,57. Little is known about them, partly owing to the lack of a specific marker 58. One factor that is correlated with differentiation of PM cells is the Sertoli cell-specific, secreted factor desert hedgehog (DHH). Its receptor patched (PTC; also known as PTCH1) is expressed on PM and Leydig cells. Null mutation of Dhh in mice led to impaired differentiation of PM and Leydig cells and subsequently to feminized males Similarly, mutations in human DHH have been associated with partial and pure XY gonadal dysgenesis accompanied by impaired cord formation and reduced testosterone levels Other unknown Sertoli cell-derived factors might be involved in directing PM cell differentiation. Testis differentiation endothelial cells. Although gonads of both sexes are heavily vascularized from an early stage 65,66, endothelial cells in the testis form a characteristic vasculature with a prominent coelomic vessel on the anti-mesonephric surface and branches between the testis cords. The formation of the coelomic vessel, but not the side branches, is suppressed by the secreted signalling molecule WNT4. Null mutation of Wnt4 in mice resulted in the ectopic formation of a coelomic vessel in XX animals 67. Because blood vessels and testis cords occupy complementary domains, there is likely to be an interplay between testis cord formation and vascular patterning, although is not clear which directs which. Testis differentiation Leydig cells. Fetal Leydig cells synthesize crucial hormones for male sex differentiation. The aristaless-related homeobox gene ARX, identified in X-linked lissencephaly with abnormal genitalia, is associated with a block in Leydig cell differentiation 68,69. Additionally, ATRX (α-thalassaemia/mental retardation syndrome, X-linked), also known as XNP or XH2, is mutated in the ATRX syndrome, which is characterized by severe mental retardation, α-thalassaemia and a range of genital abnormalities that suggest that ATRX could be involved in Leydig cell development 70. For both genes, however, the molecular role during Leydig cell differentiation is unknown. In addition, signalling through PDGFs and their receptor PDGFRA have been identified in knockout mice to be essential for fetal 71 and adult 72 Leydig cell differentiation. Testis differentiation germ cells. Primordial germ cells originate neither in the gonad nor the mesonephros, but instead migrate from their origin at the posterior extremity of the embryo through the hindgut to populate the genital ridges at around 10.5 dpc in the mouse. Here they associate with somatic cells to form primitive sex cords, the precursors of testis cords and ovarian follicles. Important factors in germ-cell migration include the fragilis proteins IFITM1 and IFITM3 (interferon-induced transmembrane proteins 1 and 3) (REF. 73), whereas stromal cell-derived factor 1 (SDF1; also known as CXCL12) and its receptor CXCR4 are involved in the colonization of the genital ridges 74. Like other cell types in the gonad, germ cells are influenced by Sertoli cells to differentiate in a malespecific fashion regardless of their sex-chromosome genotype. Germ cells in a testis enter a state of mitotic arrest around 12.5 dpc (REFS 75,76), a state in which they remain until after birth, whereas germ cells in an ovarian environment enter meiosis around 13.5 dpc, signalling the onset of oogenesis 77. Interestingly, the long-standing dogma that germ cells enter meiosis cell autonomously is questioned by recent data showing that retinoic acid, produced by the mesonephros, induces entry into meiosis. Male germ cells are protected from the effects of retinoic acid by their enclosure within the testis cords. Sertoli cells, which surround primordial germ cells in the testis cords, express CYP26B1, an enzyme that breaks down retinoic acid 78,79. However, the mechanism by which male germ cells arrest mitotically has not been determined. The fact that after 12.5 dpc male germ cells are committed to develop as spermatogonia suggests that either mitotic arrest prevents entry into meiosis, or that a temporal window of competence to respond to retinoic acid signalling exists within the germ cells. Germ cells are crucial for the differentiation of ovarian follicles and the maintenance of the ovary, presumably secreting factors that are required for these processes. This signalling might be a consequence of entry into meiosis. By contrast, germ cells are not required for testicular development or maintenance: fetal testes in which the germ cells are chemically or genetically depleted develop normally 80. Testicular descent. An important aspect of sexual development is that testes and ovaries end up in different locations in the body. The testes migrate to their final 624 AUGUST 2006 VOLUME 7
6 Cryptorchidism The condition of having undescended testes. Persistent Müllerian duct syndrome A rare form of male pseudohermaphroditism that is most commonly characterized by bilateral fallopian tubes and a uterus combined with an otherwise more or less normal male phenotype. location in two phases. The transabdominal phase of testicular descent occurs in mice between 14.5 and 18 dpc (in humans at 8 to 15 weeks of gestation), and is controlled by the enlargement of the caudal genito-inguinal ligament and the gubernaculum, and by the regression of the cranial ligament (FIG. 1c). Mutation studies in mice demonstrated that the insulin-like 3 (INSL3) hormone, produced by Leydig cells, using its receptor LGR8 (also named GREAT), is necessary and sufficient to mediate this phase of testicular descent 81. Mutations in these genes have been found in male patients with cryptorchidism, but these account for only a small proportion of all cases of this common disorder 82. The second, inguinoscrotal phase is usually completed by 20 days after birth in mice, and by the thirty-fifth week in humans. In contrast to the first phase, which is dependent on the growth of the gubernaculum, this phase requires the migration of the gubernaculum from the groin into the scrotum (FIG. 1c). This is guided by the neurotransmitter calcitonin gene-related peptide (CGRP; also known as CALCA), which is released by the genitofemoral nerve under the control of androgens. Mutations in genes that are involved in androgen signalling and those that encode transcription factors, such as homeobox A10 (Hoxa10), Hoxa11 and Desrt (developmentally and sexually retarded with transient immune abnormalities), cause disruption of the second stage of descent in mice, leaving the testes at the level of the bladder, which is in contrast to Insl3 and Lgr8 mutations that lead to a high intra-abdominal location 83,84. Clearly the regulatory mechanisms that drive testicular descent are sensitive to imbalance and malfunction and are often secondary to other disorders factors that might contribute to the high frequency of undescended testes in newborn boys. Development of the male genital tract Early stages the genital ducts. Both male and female embryos initially have two pairs of genital ducts. In males, one pair, the Wolffian (or mesonephric) ducts, generate the mature genital tract, whereas the other, the Müllerian (or paramesonephric) ducts, disappear. In females, the Müllerian ducts survive and the Wolffian ducts disappear. This represents a different strategy to that used in gonad development, which involves dichotomous differentiation of a bipotential precursor tissue. However, a bipotential structure, the urogenital sinus, contributes to the genital tract by forming the prostate in males and the lower vagina in females (FIG. 1b,d). In an XY embryo, the Müllerian ducts degenerate in an active process, involving a TGFB (transforming growth factor-β)-family molecule, anti-müllerian hormone (AMH; also known as Müllerian-inhibiting substance or MIS). AMH is secreted by the Sertoli cells of differentiating testes and binds to its receptor, MISRII (also known as AMHR2), on the surface of Müllerian duct mesenchymal cells. This induces a signalling cascade that results in the production and secretion of matrix metalloproteinase 2 (MMP2), which induces apoptosis in the Müllerian duct epithelial cells 85. Failure in these processes in humans can lead to persistent Müllerian duct syndrome (PMDS) Epididymis Interstitium Testis cords Vas deferens Seminal vesicle Initial segment Caput Corpus Cauda Bmp4 Ros1 Bmp7,8 Bmp7,8 Hoxa9,10,11 Hoxd9,10 Fgf10 Gdf7 Figure 3 Schematic representation of the differentiation of the male genital tract. The Wolffian duct differentiates under the influence of testicular androgens into the epididymis, vas deferens and seminal vesicle. The epididymis can be further divided, morphologically and functionally, into the initial segment, caput, corpus and cauda. The sperm, which are produced in the testes, mature during their passage through the caput and corpus, whereas the cauda functions predominantly for storage. The different segments of the male genital tract are marked by specific gene expression. Null mutations of these genes result in phenotypes that are restricted to these segments. Homeobox A10 (Hoxa10) and Hoxa11 knockout mice are sterile and the epididymis shows homeotic transformation. In Hoxa10 null mice the cauda epididymis seems to have transformed into the corpus, whereas in Hoxa11 knockout mice the vas deferens shows partial transformation into the cauda. Mutations in bone morphogenetic protein 4 (Bmp4) result in extensive degeneration of the epididymal epithelium of the corpus region, rather than in the caput and cauda regions as for Bmp7 and Bmp8 knockout mice, whereas mice that are null for Ros1 show defects in the differentiation of the initial segment. For the proper development of the seminal vesicle, fibroblast growth factor 10 (Fgf10) and growth differentiation factor 7 (Gdf7) are required. Later stages differentiation of the Wolffian duct. In males, the Wolffian ducts further develop under the influence of testosterone, produced by Leydig cells, into a system of organs the epididymis, vas deferens and seminal vesicle (FIGS 1b,3) that connect the testes with the urethra. These Wolffian duct derivatives can be distinguished by their morphologies, specific NATURE REVIEWS GENETICS VOLUME 7 AUGUST
7 a Ventral Dorsal b Ventral Dorsal Genital tubercle Cloacal membrane Urethral fold Labioscrotal swellings Genital tubercle Distal urethral epithelium Mesenchyme Urethral groove Fusion of urethral folds Anlage A group of cells that are destined to become a specific structure or tissue in the adult, but have not yet differentiated. Outgrowth Apoptosis Hoxa13, Hoxd13 Bmp7 Fgf8 Wnt5a Bmp4, Hoxa13, Hoxd13, Ptc Shh Proliferation Fgf10 Figure 4 Development of the external genitalia. a Caudal view of the indifferent anlage. The cloaca is closed off from the exterior by the cloacal membrane, which is bordered anteriorly by the genital tubercle and laterally by the urethral and labioscrotal swellings. Sonic hedgehog (SHH) signalling from the epithelium results in the upregulation of bone morphogenetic protein (Bmp4), homeobox A13 (Hoxa13), Hoxd13 and patched (Ptc) expression in the mesenchyme. This expression pattern exhibits a balance of apoptosis that is induced by Bmp4 and proliferation, which is indirectly controlled by Hoxa13 and Hoxd13 through the upregulation of fibroblast growth factor 8 (Fgf8). This balance is necessary for correct development of the genital tubercle with misregulation resulting in hypospadias. b The genital tubercle elongates to form the penis, while the urethral groove forms on the ventral aspect of the genital tubercle, extending distally in a solid epithelial plate (distal urethral epithelium). The penile urethra subsequently forms by proximal to distal fusion of the urethral folds. The labioscrotal swellings migrate caudally and fuse at the midline to form the scrotum. SHH from the epithelium and FGF10 from the mesenchyme maintain their own expression in a positivefeedback loop. HOXA13 and HOXD13 continue to signal back to the distal urethral epithelium to induce Bmp7 and Fgf8 expression. Shh gene-expression patterns and functions, although they are contiguous and collaborate with each other in supporting male gamete development. Similar to other testosterone-induced, male-specific organs such as the prostate and external genitalia, their development depends on epithelial mesenchymal interactions. It is believed that region-specific, inductive signals from the surrounding mesenchyme specify the characteristic differentiation along the Wolffian duct. Knockout mouse models of Gdf7, Bmp4, Bmp7, Bmp8a and Bmp8b (which encode bone morphogenic proteins) and the homeo box genes Hoxa10 and Hoxa11 (REFS 93,94) result in defects in specific areas of the epididymis and seminal vesicle, confirming the region-specific requirement for signalling molecules and transcription factors (FIG. 3). Furthermore, the phenotypic effects of a null mutation of Ros1 implicates the encoded tyrosine kinase receptor in the regionalization and terminal differentiation of the epididymal epithelium 95. Studies of mouse mutants have also identified two genes that are necessary for the proliferation of epithelial cells, and therefore tube elongation: those that encode the orphan G-protein coupled receptor LGR4 (REF. 96) and relaxin, a naturally occurring inhibitor of collagen deposition 97. Subsequently, the growth factor PDGFA, expressed in the epithelium and its receptor PDGFRA in the surrounding mesenchyme maintain the structural integrity of the tube 98. Studies of the human disorder congenital bilateral aplasia of vas deferens (CBAVD) have implicated the cystic fibrosis transmembrane conductance regulator (CFTR) in the development of the male reproductive tract. A high proportion of males with cystic fibrosis also display CBAVD and are therefore infertile. However, many CBAVD patients with CFTR mutations do not show the cystic fibrosis lung phenotype. This is probably explained by differences in the tissue-specific alternative splicing of CFTR between the vas deferens and the lung 99. However, it is not yet known how mutation of this membrane-bound chloride channel leads to the loss of vasa deferentia during development. Development of the external genitalia A second organ system that differentiates under the influence of testosterone is the external genitalia. The specialized male and female genital anatomy that has evolved in mammals provides greatly increased reproductive efficiency compared with the system of external fertilization found in many birds and fish 100. Perhaps even more so than the gonads and the duct systems, the vastly different morphologies of the male and female external genitalia belie their origins from a common set of ambiguous embryonic structures. The indifferent stage. In mice, at around 10.5 dpc, the external genitalia first become visible as a small mesenchymal swelling between the two layers of the cloacal membrane (FIG. 1d). Subsequently, the ventrally located urethral groove appears, which has a solid plate of epithelial cells at the distal end. This distal urethal epithelium, which is comparable to the apical ectodermal ridge of the limb bud, functions as a signalling centre to stimulate the outgrowth and differentiation of the genital tubercle through epithelial mesenchymal interactions. The expression and relationship of the key molecules involved in this outgrowth Fgfs, SHH (sonic hedgehog), Wnts, HOXA13 and HOXD13, Bmps and their antagonist noggin (FIG. 4; Supplementary information S1 (table)) are remarkably similar to those involved in limb 626 AUGUST 2006 VOLUME 7
8 Branchial arches A series of paired segmental structures that are composed of ectoderm, mesoderm and neural crest cells that are positioned on each side of the developing pharynx. In mammals, the branchial arches contribute to pharyngeal organs and to the connective, skeletal, neural and vascular tissues of the head and neck. Congenital adrenal hyperplasia A condition that is in most cases due to CYP21 deficiency, and is characterized by the deficiency in the hormones cortisol and aldosterone and an overproduction of androgens, which results in ambiguous genitalia in females. bud development and branchial arch outgrowth during craniofacial development 101. Differentiation of male external genitalia. After this initial, bipotential phase, at around 16 dpc in mice and 12 weeks of gestation in humans, the development of the external genitalia becomes sex-specific. 5α-Reductase is expressed in the genital tubercle mesenchyme, and in males it converts testosterone into 5α-dihydrotestosterone (DHT), the biologically more potent androgen. DHT signals through the androgen receptor (AR) that is present in cells of the developing external genitalia, which leads to a series of morphological changes. The genital tubercle elongates further with the urethral folds approaching each other, finally fusing, from proximal to distal, to form the tubular penile urethra (FIG. 4b). The scrotum is formed by the genital swellings, which move caudally and fuse in the midline. 5α-Reductase and AR are also expressed in the female external genitalia, but do not lead to male differentiation owing to the lack of testosterone. However, in females with congenital adrenal hyperplasia, the adrenal glands produce abnormally high levels of androgens, which lead to varying degrees of virilization of the female external genitalia 102. In males, mutations resulting in a defective AR, or low levels of AR, cause androgen insensitivity syndrome, the most common form of XY sex reversal. This disorder is characterized by deficient or absent virilization of 46,XY individuals despite normal or even elevated androgen levels. More than 300 mutations in the X-linked, single-copy AR gene have been described, leading to phenotypes that range from complete androgen insensitivity and female phenotype, to partial insensitivity and ambiguous genitalia, to mild forms with a male phenotype but that are infertile. The testes do not usually descend; however, there is no uterus because the degeneration of the Müllerian ducts is androgen-independent. Mutations in the gene encoding 5α-reductase are another common cause of male pseudohermaphroditism. Such individuals are deficient in DHT, which is required for the full masculinization of the external genitalia and development of the prostate, but not for the normal differentiation of the Wolffian ducts, epididymides, vasa deferentia and seminal vesicles 103. The high prevalence of hypospadias in humans suggests that urethral fusion is a delicate and finely regulated process. The cell-surface molecules ephrins, and their receptors (Ephs), have been implicated in this process: mice in which ephrin B2 and EphB2/EphB3 signalling is disrupted show variable levels of incomplete urethral tubularization 104. In addition, mutations in many of the above-mentioned genes that control the initial phase of genital tubercle outgrowth result in hypospadias, indicating a requirement for coordinated control of urethral fusion and genital tubercle outgrowth. For example, mutations in HOXA13 and HOXD13 lead to hand-foot-genital syndrome, an autosomal dominant disorder, which is characterized by malformation of the distal limbs, accompanied by hypospadias Furthermore, experiments in rodents have suggested a Initiation b Growth c Branching morphogenesis FGF7 d Differentiation and maturation HOXA13 HOXD13 FGF10 FGFR2 SHH AR? SHH Urethra SHH NKX3.1 Urethra TGFB1 BMP4 BMP7 p63 FOXA1 Urethra Notch Progression of ductal lumen, differentiation of basal and luminal cell types, and formation of smooth muscle cells Figure 5 Morphological and gene-expression changes during prostate development. a Circulating androgens initiate the development of the prostate from the urogenital sinus; the androgen receptor (AR), which is necessary for budding of the urethral epithelium (green), is expressed in the mesenchyme (pink). An unknown factor, which could be an activator or repressor, mediates a signal to the epithelium, which causes the upregulation of Shh expression. b Sonic hedgehog (SHH), at least in part by upregulating the transcription factor NKX3.1, and the mesenchymal homeobox genes Hoxa13 and Hoxd13 promote further growth of the prostatic ducts. c Most of these ducts remain unbranched until birth in rodents but, subsequently, epithelial mesenchymal interactions result in further elongation and branching morphogenesis. Ductal branching and budding are inhibited by the mesenchymal signalling factors BMP4 (bone morphogenetic protein 4) and BMP7, and stimulated by the antagonist of these Bmps, Notch. FGF7 (fibroblast growth factor 7) and FGF10, expressed in the mesenchyme, bind to FGFR2 on epithelial cells, which leads to the maintenance of SHH expression. This positive regulation is limited by the negative-feedback loop of downregulation of Fgf expression by SHH signalling. d Finally, in a proximal to distal direction, the epithelial cell types differentiate under the control of transcription factors such as p63 and forkhead box A1 (FOXA1), smooth muscle cells form around the epithelium and the ductal lumen advances. NATURE REVIEWS GENETICS VOLUME 7 AUGUST
9 Genital tubercle Penis Brain Wolffian duct Vas deferens, epididymis, seminal vesicle Androgen receptor, Shh, Wnts, Fgfs, HoxA13, HoxD13, Bmps, noggin, Ephs, ephrins Sry? Other genes? Androgen receptor, Bmps, Hox, Cftr, Hoxa10, Hoxa11, Lgr4, Pdgfa, Pdgfra External genitalia Androgens Androgens Androgens Male genital tract Testicular descent Insl3, Lgr8, Hoxa10, Hoxa11, Desrt Genital ridge Sry, Sox9, Sox8, Fgf9, Sf1, Dhh, Ptc, Wt1, Dax1, Atrx, Arx, Pod1, Pdgfa, Pdgs Testis Urogenital sinus Prostate Androgens Androgen receptor, Shh, Fgfs, Hoxa13, Hoxd13, CD44, follistatin Figure 6 Overview of genetic pathways that drive male sexual differentiation in mammals. The Y-linked gene Sry is the master switch that determines the differentiation of the bipotential genital ridge into a testis (bottom middle box). Expression of Sry sets in motion a cascade of male-specific gene expression such as Sox9, Sox8 and Fgf9. Later, the testis has to descend (depicted by a broad arrow) into the scrotum for full functionality. Subsequently, testicular androgens initiate the differentiation of secondary male sexual characteristics such as the male genital tract, external genitalia and brain, which involves organ-specific, regulatory gene networks. that some industrial chemicals, pharmaceuticals, environmental pollutants and natural products have anti-androgenic properties that can result in the feminization of male external genitalia, which might explain the alarming increase in the occurrence of hypospadias in recent decades 108. Development of the prostate The prostate is an essential mammalian-specific, male accessory sex gland that contributes to the seminal plasma fluid. This important role in mammalian reproduction and the high incidence in humans of prostatic diseases, including benign and malignant tumours, make it necessary to understand prostate development and biology. The prostate develops from the urogenital sinus, which is derived from the cloaca that is a caudal extension of the hindgut (FIG. 1b,d). Interestingly, its development is similar to that of the external genitalia. The indifferent urogenital sinus forms in both male and female mice at approximately 13 dpc (7 weeks of gestation in humans), and remains morphologically indistinguishable until 17.5 dpc, when testicular androgens induce the outgrowth of solid buds from the urogenital sinus epithelium into the urogenital sinus mesenchyme 109,110. Curiously, the androgen receptor is expressed on the mesenchyme and induces prostatic epithelial development, which implies that there is an unidentified, secreted, mesenchymal factor that mediates the action of androgens. After this initial hormone-dependent stage, the development of the prostate is characterized by epithelial mesenchymal interactions, resulting in cell differentiation and branching morphogenesis, that involve the same key molecules (FGFs, SHH, BMPs, HOXA13 and HOXD13) as the development of the external genitalia, in addition to a few others (for example, CD44 and follistatin) 111,112 (FIG. 5; Supplementary information S2 (table)). How do the same set of control genes lead to the formation of these distinct structures? This remains unknown, but interacting partners might be expressed differentially in these structures, or different thresholds or temporal and spatial combinations of expression of these key genes might occur in different tissues. Sexual differences in the brain Males and females have clear behavioural differences and are differentially susceptible to many behavioural, emotional and personality disorders. Sex-specific differences in brain morphology traditionally have been attributed to the action of steroid hormones that are produced by the gonads. Although these are still seen as the main factors, recent data suggest that genetic differences could also have a direct role. Microarray analysis identified more than 50 genes that show a sex-specific expression pattern in the brain of 10.5 dpc mice, a stage that is too early for any gonadal hormone influence, which supports the hypothesis that chromosomal constitution has a role in mammalian brain differentiation 113. These genes encode proteins that range from those that regulate the cell cycle and control transcription, to enzymes and structural proteins. It remains unclear whether the sex-specific expression of these genes is a cause or consequence of brain sexual dimorphism, and, if they do contribute to neural differentiation, what their functions might be. A more restricted screen was used to test the hypothesis that genes encoded on the sex chromosomes have a direct role in sexual differentiation of brain and sexspecific behaviours 114. This study used a fully fertile mouse model in which Sry was moved from the Y chromosome to an autosome. By breeding these mice with wild-type XX females four genotypes were produced: XX females, XY Sry females, XY Sry (Sry + ) males, and XX (Sry + ) males, a system in which sex determination is 628 AUGUST 2006 VOLUME 7
10 Substantia nigra A region of the ventral midbrain that contains pigment and sends afferent dopaminereleasing neurons to the striatum. independent of the Y chromosome. One sexual dimorphism, the density of vasopressin-immunoreactive fibres, was identified that is due to direct effects of sex chromosomal genes 114. However, this mouse model did not allow the effects of the male-determining gene Sry to be investigated. Expression of Sry mrna has been reported in the mouse and human brain , but the biological significance has remained obscure. Recently, Dewing and co-workers demonstrated that not only the mrna but also SRY protein is expressed in tyrosine-hydroxylaseexpressing neurons of the substantia nigra, and that experimentally induced knock-down of Sry expression results in a decrease of tyrosine hydroxylase, suggesting that the expression of this enzyme might be directly regulated by SRY. Tyrosine hydroxylase is the rate-limiting enzyme in the synthesis of dopamine in the dopaminergic neurons of the nigrostratial system that controls specific motor behaviours. Interestingly, experimental, unilateral downregulation of Sry has led to quantifiable sensorimotor deficits in male mice, assessed by akinesia and limb-use asymmetry tests, which implicates the direct control of specific motor behaviour by Sry independent of hormonal influences 119. Although these experiments provide evidence that Sry and/or other genes are directly involved in sexual dimorphism of the brain, more work is needed to elucidate the precise molecular mechanisms, and how these are modified by different androgen dosages. Challenges for the future The mammalian embryo develops for a significant period in a sexually ambiguous manner, until the sexdetermining switch gene Sry is activated, testes differentiate and other organs and tissues that differ between males and females including the brain set off on their sex-specific developmental trajectories. Therefore, in both male and female embryos, the complete set of genes, cell types and precursor structures must exist to equip the embryo for each of two possible outcomes. A remarkable set of strategies is used in different parts of the embryo to achieve this plasticity. For example, cell lineages inhabit the early genital ridges that are capable of male-specific or female-specific differentiation, depending on secreted signals from nascent Sertoli cells. In the developing duct system there is no bipotential precursor, but instead both sets of ducts are laid down initially, with only one surviving at the expense of the other. The external genitalia, on the other hand, are constructed from basically the same cell types in males and females, but are moulded into strikingly different forms through the action of elaborate endocrine and paracrine cascades. Significant progress has been made in identifying genes and regulatory networks that drive male-specific differentiation (FIG. 6). The picture that emerges is one of a complex interplay between transcription factors, secreted signalling molecules, hormones and receptors that, if disturbed, can result in various phenotypes. Interestingly, a similar network of hub genes including members of the Hox, Fgf, hedgehog and Wnt families has an important role in each system (the testes, prostate and external genitalia). The challenge is to find the specific members of each family that are unique to a particular system, and the tissue-specific target genes that are regulated by this common network. Despite the advances described above, most cases of XY sex reversal, SRY-negative XX sex reversal and true hermaphroditism remain unexplained at the molecular level. Either a large number of key sex-determining genes await identification, or mutations outside the coding regions of crucial known genes such as Sry and Sox9 (for example, regulatory mutations or mutations that affect post-transcriptional processing) are more prevalent than previously suspected or both. Approaches such as microarray profiling, comparative genomic hybridization and mutagenesis screening will no doubt have an important role in efforts to understand human disorders of sexual development. In addition to explaining testis development, these methods will contribute to the growing picture of how other parts of the male reproductive system develop. This, in turn, will provide a clearer picture of how development of the seemingly disparate elements of male ontogeny is coordinated. Until then, we can only wonder at the complexity of events between the activation of Sry and the characteristics that make men well, men. 1. Jost, A. Recherches sur la differentiation sexuelle de l embryon de lapin. Arch. Anat. Microsc. Morphol. Exp. 36, (1947) (in French). 2. Gubbay, J. et al. A gene mapping to the sexdetermining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346, (1990). 3. Sinclair, A. H. et al. A gene from the human sexdetermining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, (1990). 4. Miyamoto, N., Yoshida, M., Kuratani, S., Matsuo, I. & Aizawa, S. Defects of urogenital development in mice lacking Emx2. Development 124, (1997). 5. Tevosian, S. G. et al. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129, (2002). 6. Birk, O. et al. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403, (2000). 7. Achermann, J. C., Ito, M., Ito, M., Hindmarsh, P. C. & Jameson, J. L. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nature Genet. 22, (1999). 8. Achermann, J. et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose dependent manner. J. Clin. Endocrinol. Metab. 87, (2002). 9. Ozisik, G., Achermann, J. C. & Jameson, J. L. The role of SF1 in adrenal and reproductive function: insight from naturally occurring mutations in humans. Mol. Genet. Metab. 76, (2002). 10. Englert, C. WT1 more than a transcription factor? Trends Biochem. Sci. 23, (1998). 11. Ludbrook, L. M. & Harley, V. R. Sex determination: a window of DAX1 activity. Trends Endocrinol. Metab. 15, (2004). 12. Berta, P. et al. Genetic evidence equating SRY and the male sex determining gene. Nature 348, (1990). 13. Jäger, R. J., Anvret, M., Hall, K. & Scherer, G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature 348, (1990). 14. Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. & Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 351, (1991). 15. Wilhelm, D. et al. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev. Biol. 287, (2005). This work includes the generation and characterization of the first antibody to mouse SRY. 16. Bullejos, M. & Koopman, P. Spatially dynamic expression of Sry in mouse genital ridges. Dev. Dyn. 221, (2001). 17. Nef, S. et al. Testis determination requires insulin receptor family function in mice. Nature 426, (2003). 18. Hammes, A. et al. Two splice variants of the Wilms tumor 1 gene have distinct functions during sex NATURE REVIEWS GENETICS VOLUME 7 AUGUST
W.S. O University of Hong Kong
 W.S. O University of Hong Kong Development of the Genital System 1. Sexual differentiation 2. Differentiation of the gonads a. Germ cells extragonadal in origin b. Genital ridge intermediate mesoderm consisting
W.S. O University of Hong Kong Development of the Genital System 1. Sexual differentiation 2. Differentiation of the gonads a. Germ cells extragonadal in origin b. Genital ridge intermediate mesoderm consisting
Development of the Genital System
 Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
11. SEXUAL DIFFERENTIATION. Germinal cells, gonocytes. Indifferent stage INDIFFERENT STAGE
 11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
IN SUMMARY HST 071 NORMAL & ABNORMAL SEXUAL DIFFERENTIATION Fetal Sex Differentiation Postnatal Diagnosis and Management of Intersex Abnormalities
 Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz IN SUMMARY HST 071 Title: Fetal Sex Differentiation Postnatal Diagnosis
Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz IN SUMMARY HST 071 Title: Fetal Sex Differentiation Postnatal Diagnosis
Sex Determination and Development of Reproductive Organs
 Sex Determination and Development of Reproductive Organs Sex determination The SRY + gene is necessary and probably sufficient for testis development The earliest sexual difference appears in the gonad
Sex Determination and Development of Reproductive Organs Sex determination The SRY + gene is necessary and probably sufficient for testis development The earliest sexual difference appears in the gonad
Development of the urogenital system
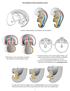 Development of the urogenital system Location of the pronephros, mesonephros and metanephros Differentiation of the intermedierm mesoderm into nephrotome and mesonephric tubules Connection between aorta
Development of the urogenital system Location of the pronephros, mesonephros and metanephros Differentiation of the intermedierm mesoderm into nephrotome and mesonephric tubules Connection between aorta
PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION
 PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION Prof. Dr med. Jolanta Słowikowska-Hilczer Department of Andrology and Reproductive Endocrinology Medical University of Łódź, Poland Sexual determination
PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION Prof. Dr med. Jolanta Słowikowska-Hilczer Department of Andrology and Reproductive Endocrinology Medical University of Łódź, Poland Sexual determination
under its influence, male development occurs; in its absence, female development is established.
 Sex differentiation is a complex process that involves many genes, including some that are autosomal. The key to sexual dimorphism is the Y chromosome, which contains the testis determining gene called
Sex differentiation is a complex process that involves many genes, including some that are autosomal. The key to sexual dimorphism is the Y chromosome, which contains the testis determining gene called
Animal Science 434 Reproductive Physiology"
 Animal Science 434 Reproductive Physiology" Embryogenesis of the Pituitary and Sexual Development: Part A Development of the Pituitary Gland" Infundibulum" Brain" Rathke s Pouch" Stomodeum" Germ Cell Migration"
Animal Science 434 Reproductive Physiology" Embryogenesis of the Pituitary and Sexual Development: Part A Development of the Pituitary Gland" Infundibulum" Brain" Rathke s Pouch" Stomodeum" Germ Cell Migration"
Urinary system development. Male ( ) and Female ( ) Reproductive Systems Development
 Urinary system development Male ( ) and Female ( ) Reproductive Systems Development Urogenital system develops from mesodermal uro-genital ridge (intermediate mesoderm) development of male and female genital
Urinary system development Male ( ) and Female ( ) Reproductive Systems Development Urogenital system develops from mesodermal uro-genital ridge (intermediate mesoderm) development of male and female genital
10. Development of genital system. Gonads. Genital ducts. External genitalia.
 10. Development of genital system. Gonads. Genital ducts. External genitalia. Gonads, genital ducts and the external genital organs initially pass through an indifferent period of development, which is
10. Development of genital system. Gonads. Genital ducts. External genitalia. Gonads, genital ducts and the external genital organs initially pass through an indifferent period of development, which is
Animal Science 434 Reproductive Physiology
 Animal Science 434 Reproductive Physiology Development of the Pituitary Gland Lec 5: Embryogenesis of the Pituitary and Sexual Development Stomodeum Brain Infundibulum Rathke s Pouch Germ Cell Migration
Animal Science 434 Reproductive Physiology Development of the Pituitary Gland Lec 5: Embryogenesis of the Pituitary and Sexual Development Stomodeum Brain Infundibulum Rathke s Pouch Germ Cell Migration
Approach to Disorders of Sex Development (DSD)
 Approach to Disorders of Sex Development (DSD) Old name: The Approach to Intersex Disorders Dr. Abdulmoein Al-Agha, FRCP Ass. Professor & Consultant Pediatric Endocrinologist, KAUH, Erfan Hospital & Ibn
Approach to Disorders of Sex Development (DSD) Old name: The Approach to Intersex Disorders Dr. Abdulmoein Al-Agha, FRCP Ass. Professor & Consultant Pediatric Endocrinologist, KAUH, Erfan Hospital & Ibn
Urogenital System. PUMC Dept. of Anat. Hist. & Embry. 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College
 Urogenital System 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College intermediate mesoderm urogenital ridge mesonephric ridge genital ridge I. Urinary System 1. kidney
Urogenital System 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College intermediate mesoderm urogenital ridge mesonephric ridge genital ridge I. Urinary System 1. kidney
DEVELOPMENT (DSD) 1 4 DISORDERS OF SEX
 Wit JM, Ranke MB, Kelnar CJH (eds): ESPE classification of paediatric endocrine diagnosis. 4. Disorders of sex development (DSD). Horm Res 2007;68(suppl 2):21 24 ESPE Code Diagnosis OMIM ICD 10 4 DISORDERS
Wit JM, Ranke MB, Kelnar CJH (eds): ESPE classification of paediatric endocrine diagnosis. 4. Disorders of sex development (DSD). Horm Res 2007;68(suppl 2):21 24 ESPE Code Diagnosis OMIM ICD 10 4 DISORDERS
- production of two types of gametes -- fused at fertilization to form zygote
 Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Chapter 18 Development. Sexual Differentiation
 Chapter 18 Development Sexual Differentiation There Are Many Levels of Sex Determination Chromosomal Sex Gonadal Sex Internal Sex Organs External Sex Organs Brain Sex Gender Identity Gender Preference
Chapter 18 Development Sexual Differentiation There Are Many Levels of Sex Determination Chromosomal Sex Gonadal Sex Internal Sex Organs External Sex Organs Brain Sex Gender Identity Gender Preference
Chapter 16: Steroid Hormones (Lecture 17)
 Chapter 16: Steroid Hormones (Lecture 17) A) 21 or fewer carbon atoms B) Precursor: 27 carbon cholesterol C) major classes of steroid hormones 1) progestagens a) progesterone- prepares lining of uterus
Chapter 16: Steroid Hormones (Lecture 17) A) 21 or fewer carbon atoms B) Precursor: 27 carbon cholesterol C) major classes of steroid hormones 1) progestagens a) progesterone- prepares lining of uterus
Embryology /organogenesis/ Week 4 Development and teratology of reproductive system.
 Embryology /organogenesis/ Week 4 Development and teratology of reproductive system. Male or female sex is determined by spermatozoon Y in the moment of fertilization SRY gene, on the short arm of the
Embryology /organogenesis/ Week 4 Development and teratology of reproductive system. Male or female sex is determined by spermatozoon Y in the moment of fertilization SRY gene, on the short arm of the
DAX1, testes development role 7, 8 DFFRY, spermatogenesis role 49 DMRT genes, male sex differentiation role 15
 Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Subject Index N-Acetylcysteine, sperm quality effects 71 Ambiguous genitalia, origins 1, 2 Anti-Müllerian hormone function 13 receptors 13 Sertoli cell secretion 10, 38 Apoptosis assays in testes 73, 74
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Biology of Reproduction-Biol 326
 Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
Please Take Seats by Gender as Shown Leave Three Seats Empty in the Middle
 Please Take Seats by Gender as Shown Leave Three Seats Empty in the Middle Women Men Sexual Differentiation & Development Neal G. Simon, Ph.D. Professor Dept. of Biological Sciences Signaling Cascade &
Please Take Seats by Gender as Shown Leave Three Seats Empty in the Middle Women Men Sexual Differentiation & Development Neal G. Simon, Ph.D. Professor Dept. of Biological Sciences Signaling Cascade &
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
Normal and Abnormal Development of the Genital Tract. Dr.Raghad Abdul-Halim
 Normal and Abnormal Development of the Genital Tract Dr.Raghad Abdul-Halim objectives: Revision of embryology. Clinical presentation, investigations and clinical significance of most common developmental
Normal and Abnormal Development of the Genital Tract Dr.Raghad Abdul-Halim objectives: Revision of embryology. Clinical presentation, investigations and clinical significance of most common developmental
Bio Section IV Late Development. Development of the Tetrapod Limb. Pattern Formation. Skeletal pattern formation. Gilbert 9e Chapter 13
 Bio 127 - Section IV Late Development Development of the Tetrapod Limb Gilbert 9e Chapter 13 Pattern Formation Limbs show an amazing aspect of development Similarities: Architectural symmetry in all four
Bio 127 - Section IV Late Development Development of the Tetrapod Limb Gilbert 9e Chapter 13 Pattern Formation Limbs show an amazing aspect of development Similarities: Architectural symmetry in all four
DEFINITION: Masculinization of external genitalia in patients with normal 46XX karyotype.
 INTERSEX DISORDERS DEFINITION: Masculinization of external genitalia in patients with normal 46XX karyotype. - Degree of masculinization variable: - mild clitoromegaly - complete fusion of labia folds
INTERSEX DISORDERS DEFINITION: Masculinization of external genitalia in patients with normal 46XX karyotype. - Degree of masculinization variable: - mild clitoromegaly - complete fusion of labia folds
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Sexual differentiation is sequential process:
 Genital lsystem J. H. Lue Sexual differentiation is sequential process:.genetic (chromosomal) sex -- determined at fertilization.gonad sex -- is differentiated after 7th week.phenotypic sex -- under normal
Genital lsystem J. H. Lue Sexual differentiation is sequential process:.genetic (chromosomal) sex -- determined at fertilization.gonad sex -- is differentiated after 7th week.phenotypic sex -- under normal
Development of the female Reproductive System. Dr. Susheela Rani
 Development of the female Reproductive System Dr. Susheela Rani Genital System Gonads Internal genitals External genitals Determining sex chronology of events Genetic sex Determined at fertilization Gonadal
Development of the female Reproductive System Dr. Susheela Rani Genital System Gonads Internal genitals External genitals Determining sex chronology of events Genetic sex Determined at fertilization Gonadal
Intersex Genital Mutilations in ICD-10 Zwischengeschlecht.org / StopIGM.org 2014 (v2.1)
 Intersex Genital Mutilations in ICD-10 Zwischengeschlecht.org / StopIGM.org 2014 (v2.1) ICD-10 Codes and Descriptions: http://apps.who.int/classifications/icd10/browse/2010/en 1. Reference: 17 Most Common
Intersex Genital Mutilations in ICD-10 Zwischengeschlecht.org / StopIGM.org 2014 (v2.1) ICD-10 Codes and Descriptions: http://apps.who.int/classifications/icd10/browse/2010/en 1. Reference: 17 Most Common
Sexual Development. 6 Stages of Development
 6 Sexual Development 6 Stages of Development Development passes through distinct stages, the first of which is fertilization, when one sperm enters one ovum. To enter an ovum, a sperm must undergo the
6 Sexual Development 6 Stages of Development Development passes through distinct stages, the first of which is fertilization, when one sperm enters one ovum. To enter an ovum, a sperm must undergo the
Goals. Disorders of Sex Development (Intersex): An Overview. Joshua May, MD Pediatric Endocrinology
 Disorders of Sex Development (Intersex): An Overview Joshua May, MD Pediatric Endocrinology Murphy, et al., J Ped Adol Gynecol, 2011 Goals Objectives: Participants will be able to: 1. Apply the medical
Disorders of Sex Development (Intersex): An Overview Joshua May, MD Pediatric Endocrinology Murphy, et al., J Ped Adol Gynecol, 2011 Goals Objectives: Participants will be able to: 1. Apply the medical
Growth pattern of the sex ducts in foetal mouse hermaphrodites
 /. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
/. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
Topics for this lecture: Sex determination Sexual differentiation Sex differences in behavior and CNS development. 1) organizational effects of
 Topics for this lecture: Sex determination Sexual differentiation Sex differences in behavior and CNS development. 1) organizational effects of gonadal steroids on CNS development 2) our model system:
Topics for this lecture: Sex determination Sexual differentiation Sex differences in behavior and CNS development. 1) organizational effects of gonadal steroids on CNS development 2) our model system:
Development of the Urinary System. 3 Distinct Embryonic Kidney Structures
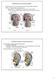 Development of the Urinary System Excretory portion of urinary system derived from intermediate mesoderm Week 4: 1 st nephrons/renal corpuscles form Nephrotomes form and develop hollow lumens to form nephric
Development of the Urinary System Excretory portion of urinary system derived from intermediate mesoderm Week 4: 1 st nephrons/renal corpuscles form Nephrotomes form and develop hollow lumens to form nephric
Bi-potent Gonads. Sex Determination
 יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase
 Human Sexuality Exam II Review Material Gametogenesis: Oogenesis 1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase 2. Know the relative
Human Sexuality Exam II Review Material Gametogenesis: Oogenesis 1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase 2. Know the relative
Disorders of gonadal and sexual development
 Disorders of gonadal and sexual development gonadal embryogenesis, cytogenetics/molecular abnormalities, and clinical aspects Pr I.Maystadt 08/01/2016 IPG Male Genitalia bladder prostate penis Seminal
Disorders of gonadal and sexual development gonadal embryogenesis, cytogenetics/molecular abnormalities, and clinical aspects Pr I.Maystadt 08/01/2016 IPG Male Genitalia bladder prostate penis Seminal
Disordered Sex Differentiation Mixed gonadal dysgenesis Congenital adrenal hyperplasia Mixed gonadal dysgenesis
 Disordered Sex Differentiation DSD has superceded intersex in describing genital anomalies in childhood DSD results from hormonal imbalances due to (i) abnormal genetic status, (ii) enzyme defects, or
Disordered Sex Differentiation DSD has superceded intersex in describing genital anomalies in childhood DSD results from hormonal imbalances due to (i) abnormal genetic status, (ii) enzyme defects, or
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Indifferent ducts of embryo Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Y chromosome present Y chromosome absent Phenotypic sex is depends on development of external
Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor)
 Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Indifferent ducts of embryo Y chromosome present Y chromosome absent Male Female penis ovary uterus vagina testis Biology of gender Sex chromosomes determine gonadal sex (testis-determining factor) Phenotypic
Sexual differentiation:
 Abnormal Development of Female Genitalia Dr. Maryam Fetal development of gonads, external genitalia, Mullerian ducts and Wolffian ducts can be disrupted at a variety of points, leading to a wide range
Abnormal Development of Female Genitalia Dr. Maryam Fetal development of gonads, external genitalia, Mullerian ducts and Wolffian ducts can be disrupted at a variety of points, leading to a wide range
Efferent Ducts and Epididymis
 increase) the secretion of each of the androgen regulated proteins. Regulation of spermatogenesis is therefore an extremely complex cascade of cell-cell interactions with the Leydig cells supporting germ
increase) the secretion of each of the androgen regulated proteins. Regulation of spermatogenesis is therefore an extremely complex cascade of cell-cell interactions with the Leydig cells supporting germ
Let s Talk About Hormones!
 Let s Talk About Hormones! This lesson was created by Serena Reves and Nichelle Penney, with materials from the BCTF and The Pride Education Network. Hormones are responsible for the regulation of many
Let s Talk About Hormones! This lesson was created by Serena Reves and Nichelle Penney, with materials from the BCTF and The Pride Education Network. Hormones are responsible for the regulation of many
Mohammad Sha ban. Basheq Jehad. Hamzah Nakhleh
 11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
Sexual Differentiation Fall 2008 Bios 90
 Sexual Differentiation Fall 2008 Bios 90 Jennifer Swann, Professor Dept Biol Sci, Lehigh University Asexual reproduction Budding : offspring develop as a growth on the body of the parent jellyfishes, echinoderms,
Sexual Differentiation Fall 2008 Bios 90 Jennifer Swann, Professor Dept Biol Sci, Lehigh University Asexual reproduction Budding : offspring develop as a growth on the body of the parent jellyfishes, echinoderms,
Biology of Reproduction- Zool 346 Exam 2
 Biology of Reproduction- Zool 346 Exam 2 ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER. Some critical words are boldfaced. This exam is 7 pages long.
Biology of Reproduction- Zool 346 Exam 2 ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER. Some critical words are boldfaced. This exam is 7 pages long.
Development of the Urinary System
 Development of the Urinary System Lecture Objectives Understand the development of the kidney and related organs of the urinary system. Define the pronephrons, mesonephrons and metanephrons. Understand
Development of the Urinary System Lecture Objectives Understand the development of the kidney and related organs of the urinary system. Define the pronephrons, mesonephrons and metanephrons. Understand
Sexual Reproduction. For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species.
 Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Sex Determination and Gonadal Sex Differentiation in Fish
 Sex Determination and Gonadal Sex Differentiation in Fish Yoshitaka Nagahama Okazaki National Research Institutes, Japan This first slide shows the processes of gonadal sex differentiation and gametogenesis
Sex Determination and Gonadal Sex Differentiation in Fish Yoshitaka Nagahama Okazaki National Research Institutes, Japan This first slide shows the processes of gonadal sex differentiation and gametogenesis
Sex chromosomes and sex determination
 Sex chromosomes and sex determination History (1) 1940-ties Alfred Jost embryo-surgical experiments on gonads gonadal sex; male gonadal sex presence of testes; female gonadal sex lack of testes. History
Sex chromosomes and sex determination History (1) 1940-ties Alfred Jost embryo-surgical experiments on gonads gonadal sex; male gonadal sex presence of testes; female gonadal sex lack of testes. History
DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT. Reading Assignment: The Developing Human, Clinically Oriented Embryology pp
 Developmental Anatomy Steven M. Hill, Ph.D. 9/16/13 DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT Reading Assignment: The Developing Human, Clinically Oriented Embryology pp. 264-273. Objectives:
Developmental Anatomy Steven M. Hill, Ph.D. 9/16/13 DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT Reading Assignment: The Developing Human, Clinically Oriented Embryology pp. 264-273. Objectives:
BIOL 2402 Reproductive Systems!
 Dr. Chris Doumen! Female Reproductive Anatomy BIOL 2402 Reproductive Systems! Establishing the Ovarian Cycle During childhood, until puberty Ovaries grow and secrete small amounts of estrogens Estrogen
Dr. Chris Doumen! Female Reproductive Anatomy BIOL 2402 Reproductive Systems! Establishing the Ovarian Cycle During childhood, until puberty Ovaries grow and secrete small amounts of estrogens Estrogen
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
C. Patrick Shahan, MD University of Tennessee Health Science Center Department of Surgery
 C. Patrick Shahan, MD University of Tennessee Health Science Center Department of Surgery Drop use of hermaphrodite and derivatives 1 in 15,000 live births Congenital Adrenal Hyperplasia Mixed Gonadal
C. Patrick Shahan, MD University of Tennessee Health Science Center Department of Surgery Drop use of hermaphrodite and derivatives 1 in 15,000 live births Congenital Adrenal Hyperplasia Mixed Gonadal
Midgut. Over its entire length the midgut is supplied by the superior mesenteric artery
 Gi Embryology 3 Midgut the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct or yolk stalk Over its entire length the
Gi Embryology 3 Midgut the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct or yolk stalk Over its entire length the
Development of the urinary system
 Development of the urinary system WSO School of Biomedical Sciences, University of Hong Kong. 3 sets of kidneys developing in succession (temporally and spatially) : Pronephros ] Mesonephros ]- Intermediate
Development of the urinary system WSO School of Biomedical Sciences, University of Hong Kong. 3 sets of kidneys developing in succession (temporally and spatially) : Pronephros ] Mesonephros ]- Intermediate
Bios 90/95. Jennifer Swann, PhD
 Sexual Differentiation Fall 2007 Bios 90/95 Jennifer Swann, PhD Dept Biol Sci, Lehigh University Why have sexes? What determines sex? Environment Genetics Hormones What causes these differences? The true
Sexual Differentiation Fall 2007 Bios 90/95 Jennifer Swann, PhD Dept Biol Sci, Lehigh University Why have sexes? What determines sex? Environment Genetics Hormones What causes these differences? The true
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Urinary System. ectoderm. notochord. mesonephric tubules. Nephrogenic Cord (left)
 Urinary System NOTE: Urine proion requires an increased capillary surface area (glomeruli), epithelial s to collect plasma filtrate and extract desirable constituents, and a system to convey urine away
Urinary System NOTE: Urine proion requires an increased capillary surface area (glomeruli), epithelial s to collect plasma filtrate and extract desirable constituents, and a system to convey urine away
2. Which of the following factors does not contribute to ion selectivity?
 General Biology Summer 2014 Exam II Sample Answers 1. Which of the following is TRUE about a neuron at rest? A. The cytosol is positive relative to the outside B. Na+ concentrations are higher inside C.
General Biology Summer 2014 Exam II Sample Answers 1. Which of the following is TRUE about a neuron at rest? A. The cytosol is positive relative to the outside B. Na+ concentrations are higher inside C.
1) Intersexuality - Dr. Huda
 1) Intersexuality - Dr. Huda DSD (Disorders of sex development) occur when there is disruption of either: Gonadal differentiation Fetal sex steroid production or action Mullerian abnormalities and Wolffian
1) Intersexuality - Dr. Huda DSD (Disorders of sex development) occur when there is disruption of either: Gonadal differentiation Fetal sex steroid production or action Mullerian abnormalities and Wolffian
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Human Anatomy Unit 3 REPRODUCTIVE SYSTEM
 Human Anatomy Unit 3 REPRODUCTIVE SYSTEM In Anatomy Today Male Reproductive System Gonads = testes primary organ responsible for sperm production development/maintenan ce of secondary sex characteristics
Human Anatomy Unit 3 REPRODUCTIVE SYSTEM In Anatomy Today Male Reproductive System Gonads = testes primary organ responsible for sperm production development/maintenan ce of secondary sex characteristics
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
1. Both asexual and sexual reproduction occur in the animal kingdom
 1. Both asexual and sexual reproduction occur in the animal kingdom Asexual reproduction involves the formation of individuals whose genes all come from one parent. There is no fusion of sperm and egg.
1. Both asexual and sexual reproduction occur in the animal kingdom Asexual reproduction involves the formation of individuals whose genes all come from one parent. There is no fusion of sperm and egg.
When testes make no testosterone: Identifying a rare cause of 46, XY female phenotype in adulthood
 When testes make no testosterone: Identifying a rare cause of 46, XY female phenotype in adulthood Gardner DG, Shoback D. Greenspan's Basic & Clinical Endocrinology, 10e; 2017 Sira Korpaisarn, MD Endocrinology
When testes make no testosterone: Identifying a rare cause of 46, XY female phenotype in adulthood Gardner DG, Shoback D. Greenspan's Basic & Clinical Endocrinology, 10e; 2017 Sira Korpaisarn, MD Endocrinology
Urogenital system - Development. Aleš Hampl
 Urogenital system - Development Aleš Hampl cloaca Urogenital system Overall picture Urogenital system Reminder Urogenital system Intermediate mesoderm Urogenital system Early forms of kidneys - Pronephros
Urogenital system - Development Aleš Hampl cloaca Urogenital system Overall picture Urogenital system Reminder Urogenital system Intermediate mesoderm Urogenital system Early forms of kidneys - Pronephros
Obstetrics Content Outline Obstetrics - Fetal Abnormalities
 Obstetrics Content Outline Obstetrics - Fetal Abnormalities Effective February 2007 10 16% renal agenesis complete absence of the kidneys occurs when ureteric buds fail to develop Or degenerate before
Obstetrics Content Outline Obstetrics - Fetal Abnormalities Effective February 2007 10 16% renal agenesis complete absence of the kidneys occurs when ureteric buds fail to develop Or degenerate before
Reproductive Endocrinology. Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007
 Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
Reproductive Endocrinology Isabel Hwang Department of Physiology Faculty of Medicine University of Hong Kong Hong Kong May2007 isabelss@hkucc.hku.hk A 3-hormone chain of command controls reproduction with
FLASH CARDS. Kalat s Book Chapter 11 Alphabetical
 FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 11 Alphabetical alpha-fetoprotein alpha-fetoprotein Alpha-Fetal Protein (AFP) or alpha-1- fetoprotein. During a prenatal sensitive period, estradiol
FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 11 Alphabetical alpha-fetoprotein alpha-fetoprotein Alpha-Fetal Protein (AFP) or alpha-1- fetoprotein. During a prenatal sensitive period, estradiol
Genetic Sex Determination
 Genetic Sex Determination Sex is determined by the heat of male partner during intercourse.. Courtesy of Humphrey Yao Aristotle (384-322 B.C.) Sex Differentiation: a favorite topic for philosophers and
Genetic Sex Determination Sex is determined by the heat of male partner during intercourse.. Courtesy of Humphrey Yao Aristotle (384-322 B.C.) Sex Differentiation: a favorite topic for philosophers and
Martin Ritzén. bioscience explained Vol 7 No 2. Girl or boy: What guides gender development and how can this be a problem within
 Martin Ritzén Professor emeritus, Karolinska Institutet, Stockholm, Sweden Girl or boy: What guides gender development and how can this be a problem within sport? Introduction During the 2009 athletics
Martin Ritzén Professor emeritus, Karolinska Institutet, Stockholm, Sweden Girl or boy: What guides gender development and how can this be a problem within sport? Introduction During the 2009 athletics
PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception
 PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception REVIEW OF PELVIS I Pelvic brim, inlet Pelvic outlet True pelvis-- --viscera Tilt forward Mid-sagital views-- --how the
PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception REVIEW OF PELVIS I Pelvic brim, inlet Pelvic outlet True pelvis-- --viscera Tilt forward Mid-sagital views-- --how the
Animal Reproduction. Hypothalamic-pituitary-gonadal axis. # lectures for cumulative test # 01 book 01
 Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 05 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 05 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
DISORDERS OF MALE GENITALS
 Wit JM, Ranke MB, Kelnar CJH (eds): ESPE classification of paediatric endocrine diagnosis. 9. Testicular disorders/disorders of male genitals. Horm Res 2007;68(suppl 2):63 66 ESPE Code Diagnosis OMIM ICD10
Wit JM, Ranke MB, Kelnar CJH (eds): ESPE classification of paediatric endocrine diagnosis. 9. Testicular disorders/disorders of male genitals. Horm Res 2007;68(suppl 2):63 66 ESPE Code Diagnosis OMIM ICD10
The Reproductive System
 16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5)
 IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5) For all the questions on this exam, the correct answer is the single best answer that is available in the answer key. 1) Which of the following is NOT
IB 140 Midterm #1 PRACTICE EXAM (lecture topics 1-5) For all the questions on this exam, the correct answer is the single best answer that is available in the answer key. 1) Which of the following is NOT
The Reproductive System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
The Reproductive System
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Reproductive System 16PART A The Reproductive System Gonads primary sex organs Testes in males
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
AMBIGUOUS GENITALIA. Dr. HAKIMI, SpAK. Dr. MELDA DELIANA, SpAK
 AMBIGUOUS GENITALIA (DISORDERS OF SEXUAL DEVELOPMENT) Dr. HAKIMI, SpAK Dr. MELDA DELIANA, SpAK Dr. SISKA MAYASARI LUBIS, SpA Pediatric Endocrinology division USU/H. ADAM MALIK HOSPITAL 1 INTRODUCTION Normal
AMBIGUOUS GENITALIA (DISORDERS OF SEXUAL DEVELOPMENT) Dr. HAKIMI, SpAK Dr. MELDA DELIANA, SpAK Dr. SISKA MAYASARI LUBIS, SpA Pediatric Endocrinology division USU/H. ADAM MALIK HOSPITAL 1 INTRODUCTION Normal
18 Urinary system. 19 Male reproductive system. Female reproductive system. Blok 11: Genital and Urinary Tract Diseases
 Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Embryology Relevant to Ultrasound Imaging of the Male Genitalia
 Embryology Relevant to Ultrasound Imaging of the Male Genitalia Gideon Richards and Bruce R. Gilbert A basic understanding of the embryologic development of the male genitalia and the male genital blood
Embryology Relevant to Ultrasound Imaging of the Male Genitalia Gideon Richards and Bruce R. Gilbert A basic understanding of the embryologic development of the male genitalia and the male genital blood
Developmental Changes of Müllerian and Wolffian Ducts in Domestic Cat Fetuses
 Exp. Anim. 58(1), 41 45, 2009 Note Developmental Changes of Müllerian and Wolffian Ducts in Domestic Cat Fetuses Tomo INOMATA 1), Hiroyoshi NINOMIYA 1), Katsuyasu SAKITA 1), Naomi KASHIWAZAKI 2), Junya
Exp. Anim. 58(1), 41 45, 2009 Note Developmental Changes of Müllerian and Wolffian Ducts in Domestic Cat Fetuses Tomo INOMATA 1), Hiroyoshi NINOMIYA 1), Katsuyasu SAKITA 1), Naomi KASHIWAZAKI 2), Junya
Ch 20: Reproduction. Keypoints: Human Chromosomes Gametogenesis Fertilization Early development Parturition
 Ch 20: Reproduction Keypoints: Human Chromosomes Gametogenesis Fertilization Early development Parturition SLOs Contrast mitosis/meiosis, haploid/diploid, autosomes/sex chromosomes. Outline the hormonal
Ch 20: Reproduction Keypoints: Human Chromosomes Gametogenesis Fertilization Early development Parturition SLOs Contrast mitosis/meiosis, haploid/diploid, autosomes/sex chromosomes. Outline the hormonal
Maldescended testis in Adults. Dr. BG GAUDJI Urologist STEVE BIKO ACADEMIC HOSPITAL
 Maldescended testis in Adults Dr. BG GAUDJI Urologist STEVE BIKO ACADEMIC HOSPITAL Definitions Cryptorchid: testis neither resides nor can be manipulated into the scrotum Ectopic: aberrant course Retractile:
Maldescended testis in Adults Dr. BG GAUDJI Urologist STEVE BIKO ACADEMIC HOSPITAL Definitions Cryptorchid: testis neither resides nor can be manipulated into the scrotum Ectopic: aberrant course Retractile:
Urinary System Chapter 16
 Urinary System Chapter 16 1 Urology- the branch of medicine that treats male and female urinary systems as well as the male reproductive system. Nephrology- the scientific study of the anatomy, physiology,
Urinary System Chapter 16 1 Urology- the branch of medicine that treats male and female urinary systems as well as the male reproductive system. Nephrology- the scientific study of the anatomy, physiology,
Formation of Urine: Formation of Urine
 The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
2. Which male target tissues respond to testosterone, and which require dihydrotestosterone?
 308 PHYSIOLOGY CASES AND PROBLEMS Case 56 Male Pseudohermaphroditism: Sa-Reductase Deficiency Fourteen years ago, Wally and Wanda Garvey, who live in rural North Carolina, had their first child. The baby
308 PHYSIOLOGY CASES AND PROBLEMS Case 56 Male Pseudohermaphroditism: Sa-Reductase Deficiency Fourteen years ago, Wally and Wanda Garvey, who live in rural North Carolina, had their first child. The baby
Sex differentiation to puberty. Introduction
 ex to puberty exual in general: chromosomal, gonadal and phenotypic sex, endocrine control of phenotypic, role of testicular hormones in male development, disorders of sexual 30 Hypothalamic sexual : sexual
ex to puberty exual in general: chromosomal, gonadal and phenotypic sex, endocrine control of phenotypic, role of testicular hormones in male development, disorders of sexual 30 Hypothalamic sexual : sexual
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE
 ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
ESUR SCROTAL AND PENILE IMAGING WORKING GROUP MULTIMODALITY IMAGING APPROACH TO SCROTAL AND PENILE PATHOLOGIES 2ND ESUR TEACHING COURSE NORMAL ANATOMY OF THE SCROTUM MICHAEL NOMIKOS M.D. F.E.B.U. UROLOGICAL
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
a) They are the most common cause of pediatric kidney failure. b) They are always symptomatic. c) They can be asymmetric.
 Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems
 CH. 15 - REPRODUCTIVE SYSTEM Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems 3. Male Reproductive anatomy and physiology. Testes = paired
CH. 15 - REPRODUCTIVE SYSTEM Objectives: 1. Review male & female reproductive anatomy 2. Gametogenesis & steroidogenesis 3. Reproductive problems 3. Male Reproductive anatomy and physiology. Testes = paired
MALE REPRODUCTIVE SYSTEM
 MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
MALE REPRODUCTIVE SYSTEM 1. The male reproductive system is made up of the following structures, EXCEPT: a. prostate; b. testicle; c. spermatic ducts; d. vestibular bulbs; e. seminal vesicles. 2.The testicle:
