AMS Ambicor. Penile Prosthesis. Operating Room Manual. English
|
|
|
- Brice Johnston
- 6 years ago
- Views:
Transcription
1 AMS Ambicor Penile Prosthesis Operating Room Manual English
2 This page intentionally left blank.
3 Table of Contents General Information Detailed Device Description... 4 Device Characteristics... 4 Two-piece Device...4 Easy to Inflate and Deflate...4 Device Function... 4 Inflation...4 Deflation...4 Device Components... 5 Cylinder Sizes...5 AMS Ambicor Packaging...5 Sterilization... 5 Sterilization of Components...5 AMS Tools...6 Operating Room Instructions Operating Room Instructions... 7 Preoperative Set-up Materials...7 Documentation...7 Patient Preparation...7 Unpacking the Device...7 Intraoperative Procedures...8 Sizing...10 Method...10 Inflate/Deflate Test...10 Postoperative Procedures Postoperative Procedures Immediately Postoperative...11 After the Patient is Released from the Hospital...11 Evaluating Long-Term Function and Placement...11 Procedure Illustrations Procedure Illustrations Brief Summary Brief Summary
4 General Information Detailed Device Description The AMS Ambicor Penile Prosthesis is a closed, fluid-filled system consisting of a pair of cylinders, which are implanted in the corpora cavernosa, and a pump which is implanted in the scrotum. All components are connected by kink-resistant tubing. The device is delivered prefilled with normal saline and preconnected. The cylinders are inflated as fluid is pumped from the reservoirs, which are located in the proximal ends of the cylinders, into the main cylinder body, creating an erection. They are deflated as fluid is transferred back to the reservoirs, making the penis flaccid once again. Device Characteristics Two-piece Device This prefilled and preconnected two-piece (pump and cylinders) device eliminates the need to fill and connect the device during surgery. Easy to Inflate and Deflate The inflation pump in the scrotum is easy for the patient to locate and requires few squeezes to inflate the device, because each squeeze displaces a large amount of fluid. To deflate the device, the patient simply bends the penis towards the scrotum, holds the position for 6-12 seconds, and then releases. Inflation Device Function Inflation The inflation pump is placed in the scrotum. To inflate the cylinders, the patient should stabilize the pump with one hand and use the other hand to squeeze and release the pump bulb several times to make the cylinders stiff. When the cylinders are fully inflated, the pump bulb will be hard and can no longer be compressed. Deflation To deflate the device, the patient places his thumb under the shaft of the penis to act as a fulcrum and his fingers on top of the shaft of the penis. Using the fingers of the same or opposite hand, the patient should bend his penis down over his thumb (fulcrum) towards his scrotum to a degree angle, making sure that both cylinders are bent. The cylinders should be held in this position for approximately 6-12 seconds and then released. This will open the valves which allow the fluid to flow back into the reservoirs and pump. The patient may also deflate the device by bending the cylinders upwards; this can be done by placing his thumb on top of the shaft of his penis and following the same instructions as above. Deflation 4
5 General Information (continued) Device Components Cylinder Sizes The AMS Ambicor penile prosthesis is provided in the following cylinder sizes: Sizes (diameter by length) 12.5 mm x 14 cm 12.5 mm x 16 cm 12.5 mm x 18 cm 14 mm x 16 cm 14 mm x 18 cm 14 mm x 20 cm 15.5 mm x 18 cm 15.5 mm x 20 cm 15.5 mm x 22 cm AMS Ambicor Packaging The prosthesis is packaged in a fluid-filled inner pouch. The prosthesis is preconnected, prefilled, and sterilized in a pouch within a pouch system which is placed in a dustcover box. The proximal cylinder ends are secured in a protective cushion to minimize movement within the pouch. Rear tip extenders (RTEs) are packaged in the fluidfilled pouch with the Ambicor device. These RTEs include one pair each of 0.5 cm, 1 cm, 2 cm, and 3 cm RTEs. Ambicor RTEs are also available in a separate RTE kit that includes one pair each of 0.5 cm, 1 cm, 2 cm, and 3 cm RTEs, which is provided sterile. Each Ambicor prosthesis package also contains: 2 Keith needles Patient Information Form (PIF) One mailing envelope to return completed PIF to AMS Patient Medical Identification Card Ambicor Instructions for Use Sterilization Sterilization of Components American Medical Systems sterilizes the Ambicor Penile Prosthesis and RTEs. Under normal storage conditions, the devices will remain sterile until the expiration date as long as the sterile barriers of the packaging remain intact. To protect the integrity of the packaging and the function of the prosthesis, store the sterilized devices on a protected shelf or in a cabinet. The environment should be clean, dry, and near room temperature. For maximum protection during storage, leave the Ambicor prosthesis and RTEs in their packaging. Inspect the packaging for damage and check the expiration date before use. CAUTION: Do not reuse, reprocess, or resterilize the AMS Ambicor Penile Prosthesis or AMS Ambicor RTEs. They are intended for single use only. CAUTION: Do not use product that is past its expiration date. NOTE: For warnings, precautions and contraindications please refer to the AMS Ambicor Instructions for Use provided in the product packaging. 5
6 General Information (continued) AMS Tools American Medical Systems has surgical instruments that can be used during the surgery to help facilitate the surgeon s implantation of the penile prosthesis. For sterilization information, refer to the instructions provided with the tools. The following tools can be ordered from AMS. Non-sterile. These tools may be resterilized and reused. AMS Sizer Furlow Insertion Tool AMS Closing Tool Sterile Proximal Tool (dual use for cylinder insertion and closing) Disposable Dilators SKW Retractor Kit CAUTION: Do not resterilize or reuse the sterile tools. They are intended for single use only. CAUTION: Do not use product that is past its expiration date. 6
7 Operating Room Instructions Operating Room Instructions The following instructions are intended as a guide for the surgeon. CAUTION: This device is to be used only by physicians who are knowledgeable and have received appropriate training regarding the use of inflatable penile prostheses. This Manual is not intended to be a complete reference. Preoperative Set-up Materials The hospital should provide those instruments normally required for a urological surgical procedure. In addition to the AMS Ambicor penile prosthesis, you will need the following sterile setup: Instruments: 2 Keith needles provided in Ambicor packaging for passing cylinder traction sutures through glans Corporal length measuring tool. Furlow Insertion Tool for passing traction sutures through glans. Corporal dilators (7mm-17mm) 1 sterile surgical stand (Mayo stand) or a stainless steel tray 1 basin of sterile normal saline Antibiotic solution for irrigation (optional) AMS Closing Tool (optional) Documentation One AMS Ambicor Penile Prosthesis Instructions for Use brochure One Patient Information Form (PIF) One mailing envelope for returning the completed PIF to AMS One AMS Ambicor Patient Medical Identification Card Patient Preparation Before the surgery, doctors should take adequate steps to limit the risk of post-operative infection. Once the patient is in the operating room, the abdominal and genital area should be shaved. Following the shave, the area should be scrubbed with povidone-iodine soap for ten minutes or in accordance with the approved hospital preoperative scrub procedure. Establish the sterile field, drape, and prepare the patient according to the physician s instructions. Throughout the procedure, the surgical site should be flushed with copious amounts of broadspectrum antibiotics. The patient should be positioned so that a penoscrotal incision can be made. Unpacking the Device NOTE: Do not unpackage the prosthesis until after the surgeon dilates and measures both corpora cavernosa intraoperatively. NOTE: The adhesive label at one end of the dustcover box and the small, peelable labels on the foil pouches show the part and serial/lot numbers and the size of the components. Record this information on and affix this label to the Patient Information Form (PIF). Keep the sterile pouches in the dustcover boxes until the components are in the operating room. Remove the pouches from the dustcover box in the operating room by opening the unlabeled end of the dustcover box. 7
8 Operating Room Instructions (continued) To remove the Ambicor device from its packaging: 1. Remove the inner fluid-filled foil pouch from the outer pouch. CAUTION: To avoid the possibility of cutting the Ambicor prosthesis or RTEs, do not cut the package open with scissors. 2. Place the sterile, fluid-filled foil pouch onto a sterile, lint free Mayo stand. CAUTION: Cloth towels placed on the Mayo stand may transfer lint to the AMS components. 3. Hold the foil pouch over a kidney basin containing sterile normal saline. Carefully tear the foil pouch at the side notch. Empty the Ambicor device, its RTEs, and the fluid into the kidney basin. Add sterile normal saline as needed to cover the components in the basin. NOTE: Do NOT leave the prosthesis exposed to air. Submerge the prosthesis in the fluid from the foil pouch or in sterile normal saline immediately after removing it from the foil pouch. Keep the prosthesis submerged until implantation to prevent air from entering the device. 4. Remove the tips of the Ambicor cylinders from the protective end cushion. 5. Discard the end cushion. 6. Leave the device and RTEs in the basin until ready to implant. Intraoperative Procedures Surgical Procedures Assemble the necessary equipment and position the patient for a penoscrotal surgical approach. The penoscrotal approach leaves the incision well hidden and provides convenient access to the corpora cavernosa. Establish the sterile field, drape and prepare the patient according to the physician s instruction. Throughout the procedure the surgical site may be flushed with copious amounts of broad-spectrum antibiotic. The following description is an overview of the penoscrotal surgical approach. Penoscrotal Approach 1. To begin, place a Foley catheter to facilitate identification of the urethra. 2. Some physicians use the SKW Retractor System to place the penis on stretch to provide exposure of the corpora. 3. Make a 2 cm to 3 cm incision through the median raphe of the scrotum at the penoscrotal angle. Some physicians may prefer a high scrotal incision for better proximal corporal access. Then, laterally retract the corpus spongiosum to avoid damaging the urethra. 4. Dissect through to Buck s fascia to expose the tunica albuginea. Place stay sutures to use as a reference point when measuring the corpora. Then, make an incision into one of the corpora cavernosa. 5. Dilate the proximal corpus (crus) and the distal corpus to create a space for inserting a penile cylinder. Dilate the corpus cavernosum to mm. Note: the available Ambicor cylinders diameters are 12.5 mm, 14 mm, and 15 mm. 8
9 Operating Room Instructions (continued) After dilating one corpus cavernosum, incise and dilate the adjacent corpus cavernosum following the same procedure. 6. To select the cylinders and rear tip extenders that will fit the patient s anatomy, measure each corpus proximally and distally using the Furlow Insertion Tool or appropriate measuring device (See Sizing section for choosing cylinder size.). As a general rule, the corporotomy is best placed when two-thirds of the total corporal measurement is distal to the incision and onethird is proximal. This facilitates the placement of the cylinders, and may avoid the need to extend the corporotomy during the procedure. 7. Select the appropriate size cylinders, apply rear tip extenders (if necessary), and use the insertion tool(s) to introduce the cylinders into the corpora cavernosa, as follows: a. When inserting the cylinder distally, use the Furlow Insertion Tool to put the penis on mild stretch. The tool should be palpable beneath the glans. b. First thread the pulling suture at the front of the cylinder through a Keith needle, then place the Keith needle in the insertion tool. c. Insert the Furlow insertion tool distally into the corpora cavernosa and pass the needle through the glans. d. Inflate the cylinders fully, then insert the proximal end of the cylinder. e. Deflate the cylinders by bending both evenly to degrees and holding them for 6-12 seconds. f. Position the distal ends of the cylinders by pulling the traction sutures. g. Use the Proximal Tool to place the proximal cylinder ends into the corpora. During insertion, be sure that the Furlow Insertion Tool is in the ipsilateral corpora at the distal penis. Because the intracavernosal septum may be inconsistent distally, it is easy to cross over to the contralateral side. If you think you may have crossed over, place a dilator into the other side. If the cylinder does cross over, no repair is necessary. Simply remove the cylinder and place it correctly. NOTE: The corporal incision may need to be extended to ensure the input tube exits directly from the corporotomy. Once both cylinders are implanted, close the tunica albuginea. Place the AMS Closing Tool or the closing end of the Proximal Tool (or other suitable instruments) over the cylinder to protect it from inadvertant needle injury, so that the corporal body can be closed. This should be done with meticulous attention to hemostasis. 8. Use blunt dissection to form a pocket in the most lateral and dependent portion of the scrotum. 9. Insert the pump into the scrotal pocket. The tubing between the pump and cylinders should not be palpable to the patient. 10. After both the cylinders and pump are implanted, check the function of the prosthesis by inflating and deflating the device. 11. Some physicians close the dartos in two layers with a running 2-0 absorbable suture. Close the skin. 12. Apply a wound dressing. Leave the device partially inflated. Some choose to tape the penis to the abdomen overnight. In addition, a drain may be placed for 12 to 24 hours. For postoperative instructions, see the section entitled Postoperative Procedures. 9
10 Operating Room Instructions (continued) Sizing Below is the recommended method of selecting cylinder sizes for the AMS Ambicor penile prostheses. Method This method allows the tubing to exit directly from the corporotomy. Follow the formula described below to select the appropriate cylinder length and number of rear tip extenders. If necessary, extend the length of the corporotomy. Inflate/Deflate Test After all components are implanted, inflate the device to check the quality of the erection and deflate to evaluate flaccidity. The penis should lie down close to the body when deflated. There may be some swelling that precludes a good flaccid result. 1. Calculate the Total Corporal Length (Distal + Proximal) Example Distal Corporal Length 11 cm Proximal Corporal Length +8 cm Total Corporal Length 19 cm 2. Subtract 2 cm from the Total Corporal Length to obtain an Adjusted Measurement. Example Total Corporal Length 19 cm -2 cm Adjusted Measurement 17 cm 3. Select the closest cylinder size that is shorter than or equal to the Adjusted Measurement. Example Adjusted Measurement 17 cm Selected Cylinder Length 16 cm 4. Subtract the Selected Cylinder Length from the Total Corporal Length to determine the length of rear tip extenders required to fit the patient. Example Total Corporal Length 19 cm Selected Cylinder Length -16 cm Rear Tip Extender Length 3 cm 10
11 Postoperative Procedures Postoperative Procedures Immediately Postoperative After the surgery, some physicians partially inflate the cylinders for the first 24 hours. This will aid hemostasis. The physician may place a closed system drain in the abdomen to drain excess fluid from the incision site. After 24 hours, remove the dressing and completely deflate the cylinders. Support the penis on the abdomen for four to six weeks to obtain a straight erection. Taping the penis to the abdomen is the usual process. After the Patient is Released from the Hospital After the patient has returned home and the swelling from the surgery has subsided, the physician may ask the patient to pull down on the pump located in the scrotum to properly position it. Positioning the pump makes it easier for the patient to locate the pump. The frequency of positioning the pump is the physician s decision. Some physicians have their patients position the pump several times daily. To position the pump in the scrotum, a patient should: 1. Locate the pump in the scrotum. 2. Grasp the pump firmly and carefully pull the pump down in the scrotum. The patient should gently pull the pump into a position close to the outer scrotal wall. After three to six weeks, the physician may instruct the patient to begin cycling the device for the first time. To cycle the device, the patient inflates and deflates the prosthesis several times. The cylinders must be fully inflated before they can be deflated. It may be painful for the patient the first few times that he inflates and deflates the device. However, after the postoperative healing period, the pain should subside. Instruct the patient to inflate and deflate the prosthesis several times daily. Four to six weeks postoperatively, instruct the patient that it is possible to begin using the prosthesis to have gentle intercourse. To determine if the patient is ready to use the device: 1. Check the incision site to be sure that it has healed properly. There should be no redness, swelling, or drainage. Any of these things may indicate that an infection is present and the infection should be treated promptly with antibiotics. 2. Ask the patient about pain when cycling the device and observe the patient inflating and deflating the device. After determining that the patient knows how to operate the device and that the device is functioning correctly, inform the patient that it is possible to begin intercourse. Evaluating Long-Term Function and Placement After the postoperative healing period, the physician should continue to have contact with the patient at least on an annual basis to evaluate the function of the device. During the annual evaluation, ask the patient how the device is functioning and if he has noticed any changes in the function, for example, the cylinders losing rigidity. Also check the patient for signs of infection or erosion. If the patient is having mechanical difficulty with the device, or if there is infection or erosion present, revision surgery may be necessary. 11
12 Procedure Illustrations Procedure Illustrations
13 Procedure Illustrations (continued)
14 Brief Summary Brief Summary The AMS Ambicor Penile Prosthesis is intended for use in the treatment of chronic, organic, male erectile dysfunction (impotence). These devices are contraindicated in patients who have active urogenital infections or active skin infections in the region of surgery. Implantation will make latent natural or spontaneous erections, as well as other interventional treatment options, impossible. Men with diabetes, spinal cord injuries, or open sores may have an increased risk of infection. Failure to evaluate and treat device erosion may result in infection and loss of tissue. Implantation may result in penile shortening, curvature, or scarring. Possible adverse events include, but are not limited to, urogenital pain (usually associated with healing), patient dissatisfaction, mechanical malfunction, auto-inflation, penile curvature or sensation change, urogenital hematoma, urogenital edema, and infection. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, and potential adverse events. Rx Only. 14
15 This page intentionally left blank. 15
16 Australian Sponsor Address Boston Scientific (Australia) Pty Ltd PO Box 332 BOTANY NSW 1455 Australia Free Phone Free Fax Brazil Local Contact Para informações de contato da Boston Scientific do Brasil Ltda, por favor, acesse o link American Medical Systems, Inc Bren Road West Minnetonka, MN U.S.A. US toll-free: Tel: Tel: American Medical Systems Europe B.V. Haarlerbergweg 23 G 1101 CH Amsterdam Zuid-Oost The Netherlands Boston Scientific Corporation or its affiliates. All rights reserved. All trademarks are the property of the respective owners (A/W Rev J) ( )
Operating Room Manual. AMS 700 with MS Pump. Penile Prosthesis. English
 AMS 700 with MS Pump Penile Prosthesis Operating Room Manual English This page intentionally left blank. Table of Contents Introduction................... 5 Overview.... 5 Device Description..............
AMS 700 with MS Pump Penile Prosthesis Operating Room Manual English This page intentionally left blank. Table of Contents Introduction................... 5 Overview.... 5 Device Description..............
Operating Room Manual. AMS 700 with MS Pump. Penile Prosthesis. English
 AMS 700 with MS Pump Penile Prosthesis Operating Room Manual English Contact List American Medical Systems, Inc. U.S. Headquarters 10700 Bren Road West Minnetonka, MN 55343 U.S.A. U.S. Toll Free: 800 328
AMS 700 with MS Pump Penile Prosthesis Operating Room Manual English Contact List American Medical Systems, Inc. U.S. Headquarters 10700 Bren Road West Minnetonka, MN 55343 U.S.A. U.S. Toll Free: 800 328
AMS 800 Urinary Control System ACTIVATING:
 AMS 800 Urinary Control System 1 ACTIVATING: Begin by stabilizing the control pump in the proper place by grasping the tubing above the control pump. Use your other hand to firmly squeeze the sides of
AMS 800 Urinary Control System 1 ACTIVATING: Begin by stabilizing the control pump in the proper place by grasping the tubing above the control pump. Use your other hand to firmly squeeze the sides of
Penile implants What to expect and how to prepare
 Penile implants What to expect and how to prepare Penile implants can restore erectile function. Explore your choices and find out what to expect from this procedure. Penile implants are artificial devices
Penile implants What to expect and how to prepare Penile implants can restore erectile function. Explore your choices and find out what to expect from this procedure. Penile implants are artificial devices
SHAH PENILE PROSTHESIS (Product Brochure and Instructions for use)
 SHAH PENILE PROSTHESIS (Product Brochure and Instructions for use) Sterile, EO Sterilized The Shah Penile Prosthesis is a solid silicone, semi rigid, flexible implant for the management of erectile dysfunction.
SHAH PENILE PROSTHESIS (Product Brochure and Instructions for use) Sterile, EO Sterilized The Shah Penile Prosthesis is a solid silicone, semi rigid, flexible implant for the management of erectile dysfunction.
AMS 800 Urinary Control System
 AMS 800 Urinary Control System 1 DAILY USE: While the cuff is closed, urine stays inside the bladder. To urinate, open the cuff as follows: Feel for the control pump in your scrotum. Stabilize the control
AMS 800 Urinary Control System 1 DAILY USE: While the cuff is closed, urine stays inside the bladder. To urinate, open the cuff as follows: Feel for the control pump in your scrotum. Stabilize the control
PROBLEMS AND VARIATIONS THREE-COMPONENT INFLATABLE PENILE PROSTHESIS IN THE PLACEMENT OF THE. Troubleshooting for the Malfunctioning Prosthesis
 PROBLEMS AND VARIATIONS IN THE PLACEMENT OF THE 24 THREE-COMPONENT INFLATABLE PENILE PROSTHESIS Troubleshooting for the Malfunctioning Prosthesis Although there are ongoing changes in the design of penile
PROBLEMS AND VARIATIONS IN THE PLACEMENT OF THE 24 THREE-COMPONENT INFLATABLE PENILE PROSTHESIS Troubleshooting for the Malfunctioning Prosthesis Although there are ongoing changes in the design of penile
ZEPHYR SURGICAL IMPLANTS SÀRL
 For Operating Room NOTE This document is a complementary checklist to the two training videos, which you can be accessed and download from our website: ZSI 375 Training Video, ZSI 375 Functioning Protocol.
For Operating Room NOTE This document is a complementary checklist to the two training videos, which you can be accessed and download from our website: ZSI 375 Training Video, ZSI 375 Functioning Protocol.
AMS 800. Urinary Control System For Male Patients. Operating Room Manual
 AMS 800 Urinary Control System For Male Patients Operating Room Manual Table of Contents Introduction Overview... 1 Indications for Use... 1 Additional Information... 1 System Description... 1 System
AMS 800 Urinary Control System For Male Patients Operating Room Manual Table of Contents Introduction Overview... 1 Indications for Use... 1 Additional Information... 1 System Description... 1 System
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
AMS Spectra Concealable Penile Prosthesis
 Information and Instructions for Patients Considering an AMS Spectra Concealable Penile Prosthesis Introduction By now, you have probably learned about the various causes of impotence (also known as erectile
Information and Instructions for Patients Considering an AMS Spectra Concealable Penile Prosthesis Introduction By now, you have probably learned about the various causes of impotence (also known as erectile
MANUALFor Operating Room
 DOCUMENT OPERATING ROOM FOR MANUALFor Operating Room NOTE This document is a complementary checklist to the two training videos, which you can access and download from our website: ZSI 375 Training Video,
DOCUMENT OPERATING ROOM FOR MANUALFor Operating Room NOTE This document is a complementary checklist to the two training videos, which you can access and download from our website: ZSI 375 Training Video,
Aus Artificial Uretheral Sphincter Port System
 NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Continence Control System USER S MANUAL
 Continence Control System USER S MANUAL Continence Control System User's Manual Caution: Read this user s manual completely before using this system Causes of Incontinence Causes are many and varied. Most
Continence Control System USER S MANUAL Continence Control System User's Manual Caution: Read this user s manual completely before using this system Causes of Incontinence Causes are many and varied. Most
AMS 800. Operating Room Manual. Urinary Control System For Male, Female, and Pediatric Patients. English
 AMS 800 Urinary Control System For Male, Female, and Pediatric Patients Operating Room Manual English Table of Contents Contents Disclosures Indications For Use... 1 Contraindications... 1 Warnings...
AMS 800 Urinary Control System For Male, Female, and Pediatric Patients Operating Room Manual English Table of Contents Contents Disclosures Indications For Use... 1 Contraindications... 1 Warnings...
with Blom-Singer Gel Cap Insertion System
 with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
The recent popularity of the drug sildenafil citrate, known as Viagra, has helped publicize the problem of erectile dysfunction (ED).
 PENILE IMPLA ERECTILE M A R I B E T H M I L L S The recent popularity of the drug sildenafil citrate, known as Viagra, has helped publicize the problem of erectile dysfunction (ED). Because of Viagra,
PENILE IMPLA ERECTILE M A R I B E T H M I L L S The recent popularity of the drug sildenafil citrate, known as Viagra, has helped publicize the problem of erectile dysfunction (ED). Because of Viagra,
INFORMED CONSENT FOR INSERTION OF PENILE PROSTHESIS
 INFORMED CONSENT FOR INSERTION OF PENILE PROSTHESIS 1) I hereby request and authorize Dr. Harold M. Reed assisted by his designated urological associates and surgical technicians, to perform the urological
INFORMED CONSENT FOR INSERTION OF PENILE PROSTHESIS 1) I hereby request and authorize Dr. Harold M. Reed assisted by his designated urological associates and surgical technicians, to perform the urological
This information is intended as an overview only
 This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
SURGERY FOR PEYRONIE S DISEASE. PEYRONIE S DISEASE WITHOUT IMPOTENCE Exposure and Mobilization of Dorsal Nerves and Vessels
 SURGERY FOR 25 PEYRONIE S DISEASE PEYRONIE S DISEASE WITHOUT Exposure and Mobilization of Dorsal Nerves and Vessels FIG. 25-1. Most surgeons use a degloving procedure via a circumferential skin incision
SURGERY FOR 25 PEYRONIE S DISEASE PEYRONIE S DISEASE WITHOUT Exposure and Mobilization of Dorsal Nerves and Vessels FIG. 25-1. Most surgeons use a degloving procedure via a circumferential skin incision
Desara and Desara Blue
 Desara and Desara Blue Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc.
Desara and Desara Blue Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc.
Surgical Leads. Directions for Use. CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician.
 Surgical Leads Directions for Use CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91078747-08 Content: 92190987 REV A Guarantees Boston Scientific
Surgical Leads Directions for Use CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician. 91078747-08 Content: 92190987 REV A Guarantees Boston Scientific
Better Post-Op Pain Control Starts Here
 Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
limbsandthings.com Advanced Catheterisation Trainer User Guide For more skills training products visit Limbs & Things Ltd.
 Advanced Catheterisation Trainer Product No: 60150 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Advanced Catheterisation Trainer Product No: 60150 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Instruction For Use for All Silicon Foley Catheter
 General Description: All Silicone Foley Catheter for single use is a thin, is a flexible tube passed through the urethra and into the bladder to drain urine. It is the most common type of indwelling urinary
General Description: All Silicone Foley Catheter for single use is a thin, is a flexible tube passed through the urethra and into the bladder to drain urine. It is the most common type of indwelling urinary
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
Achieving Independence. A Guide to Self-Catheterization with the Bard Touchless Plus Intermittent Catheter System
 Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization with the Bard Touchless Plus Intermittent Catheter System Achieving Independence Introducing the Bard Touchless Plus Catheter One
Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization with the Bard Touchless Plus Intermittent Catheter System Achieving Independence Introducing the Bard Touchless Plus Catheter One
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
LESSON ASSIGNMENT. Urinary System Diseases/Disorders. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 4 Urinary System Diseases/Disorders LESSON ASSIGNMENT Paragraphs 4-1 through 4-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Identify the purposes
LESSON ASSIGNMENT LESSON 4 Urinary System Diseases/Disorders LESSON ASSIGNMENT Paragraphs 4-1 through 4-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Identify the purposes
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
The Urologist, volume 2, number 3, 2014
 The Urologist, volume 2, number 3, 2014 Three-pieces inflatable penile prosthesis implantation with penoscrotal approach and Scrotal Septum Sparing technique: description and early experience Enrico Conti,
The Urologist, volume 2, number 3, 2014 Three-pieces inflatable penile prosthesis implantation with penoscrotal approach and Scrotal Septum Sparing technique: description and early experience Enrico Conti,
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
Instructions for Use io-flex Probe
 DEVICE DESCRIPTION: The is an accessory of the Amendia io-flex System, which includes the MicroBlade Shaver device. The io-flex Probe is designed to access the neural foramen. It is comprised of an outer
DEVICE DESCRIPTION: The is an accessory of the Amendia io-flex System, which includes the MicroBlade Shaver device. The io-flex Probe is designed to access the neural foramen. It is comprised of an outer
AMS 800. Urinary Control System. User s Guide. Table of Contents. The AMS 800 Urinary Control System...1. Using the AMS 800 Urinary Control System...
 AMS 800 Urinary Control System User s Guide Table of Contents The AMS 800 Urinary Control System...1 Using the AMS 800 Urinary Control System...2 Special precautions to take with your 800 before undergoing
AMS 800 Urinary Control System User s Guide Table of Contents The AMS 800 Urinary Control System...1 Using the AMS 800 Urinary Control System...2 Special precautions to take with your 800 before undergoing
CAVERJECT Impulse Alprostadil, Prostaglandin E1 (PGE1) 10 and 20 micrograms
 CAVERJECT Impulse Alprostadil, Prostaglandin E1 (PGE1) 10 and 20 micrograms Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about CAVERJECT Impulse. It
CAVERJECT Impulse Alprostadil, Prostaglandin E1 (PGE1) 10 and 20 micrograms Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about CAVERJECT Impulse. It
Before you use. CAVERJECT Impulse. When you must not use. CAVERJECT Impulse
 Alprostadil, Prostaglandin E1 (PGE1) 10 and 20 micrograms Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about CAVERJECT Impulse. It does not contain all
Alprostadil, Prostaglandin E1 (PGE1) 10 and 20 micrograms Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about CAVERJECT Impulse. It does not contain all
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE
 RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
Desara Blue OV D I. Sling for Female Stress Urinary Incontinence. Instructions For Use
 Desara Blue OV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc. 5171 Clareton
Desara Blue OV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc. 5171 Clareton
Information for Patients. Priapism. English
 Information for Patients Priapism English Table of contents What is priapism?... 3 What causes priapism?... 3 Diagnosing priapism... 3 Treating priapism... 4 Conservative, first- and second-line treatments...
Information for Patients Priapism English Table of contents What is priapism?... 3 What causes priapism?... 3 Diagnosing priapism... 3 Treating priapism... 4 Conservative, first- and second-line treatments...
Procedures to address priapism
 Procedures to address priapism INTRODUCTION Priapism is a urologic emergency that can lead to penile ischemia if not promptly addressed. It is generally defined as an erection lasting longer than 4 hours
Procedures to address priapism INTRODUCTION Priapism is a urologic emergency that can lead to penile ischemia if not promptly addressed. It is generally defined as an erection lasting longer than 4 hours
Instruction Guide to Sterile Self-Intermittent Catheterization For Men Using the Cure Catheter Closed System
 Cure Medical donates 10% of net income to medical research in pursuit of a cure for spinal cord injuries and central nervous system disorders. For information on scientific advancements, visit www.curemedical.com.
Cure Medical donates 10% of net income to medical research in pursuit of a cure for spinal cord injuries and central nervous system disorders. For information on scientific advancements, visit www.curemedical.com.
Free Flap Phalloplasty For Female To Male Gender Dysphoria
 SURGICAL TECHNIQUES Free Flap Phalloplasty For Female To Male Gender Dysphoria Giulio Garaffa, MD, PhD, FECSM, FRCS (Eng), David J. Ralph, BSc, MS, FRCS (Urol) St Peter s Andrology and the Institute of
SURGICAL TECHNIQUES Free Flap Phalloplasty For Female To Male Gender Dysphoria Giulio Garaffa, MD, PhD, FECSM, FRCS (Eng), David J. Ralph, BSc, MS, FRCS (Urol) St Peter s Andrology and the Institute of
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5575 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5575 Entity: Fairview Pharmacy Services
BP and Heart Rate by Telemetry
 BP and Heart Rate by Telemetry Version: 1 Modified from: Butz et al. Physiol Genomics. 2001 Mar 8;5(2):89-97. Edited by: Dr. Lynette Bower, UC Davis Summary Reagents and Materials Protocol Reagent Preparation
BP and Heart Rate by Telemetry Version: 1 Modified from: Butz et al. Physiol Genomics. 2001 Mar 8;5(2):89-97. Edited by: Dr. Lynette Bower, UC Davis Summary Reagents and Materials Protocol Reagent Preparation
How to Change a. Foley catheter. Patient Education Rehabilitation Nursing. Step-by-step instructions for the caregiver
 Patient Education How to Change a Foley Catheter Step-by-step instructions for the caregiver This handout gives step-bystep instructions for changing a Foley catheter, which is a tube in your bladder to
Patient Education How to Change a Foley Catheter Step-by-step instructions for the caregiver This handout gives step-bystep instructions for changing a Foley catheter, which is a tube in your bladder to
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
BACTISEAL Endoscopic Ventricular Catheter (REF & )
 BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
Primary Coverage. The Benefits of Circumcision Operation Principle Academic Article Circumstapler Advantages Operating Procedure Post Operation Caring
 Circumstapler Primary Coverage The Benefits of Circumcision Operation Principle Academic Article Circumstapler Advantages Operating Procedure Post Operation Caring The Benefits of Circumcision? There is
Circumstapler Primary Coverage The Benefits of Circumcision Operation Principle Academic Article Circumstapler Advantages Operating Procedure Post Operation Caring The Benefits of Circumcision? There is
3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used
 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used to cover and protect catheter sites and to secure devices
3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Description 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is used to cover and protect catheter sites and to secure devices
Technique Guide. SynPOR HD Ocular Spheres. For augmentation or reconstruction.
 Technique Guide SynPOR HD Ocular Spheres. For augmentation or reconstruction. Table of Contents Introduction SynPOR HD Ocular Spheres 2 Indications and Contraindications 3 Surgical Technique Sizing and
Technique Guide SynPOR HD Ocular Spheres. For augmentation or reconstruction. Table of Contents Introduction SynPOR HD Ocular Spheres 2 Indications and Contraindications 3 Surgical Technique Sizing and
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide)
 Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Drainage Frequency: PATIENT GUIDE. Dressing Frequency: Every Drainage Weekly Drainage. Physician Contact Information. Dr. Phone:
 Drainage Frequency: PATIENT GUIDE Dressing Frequency: Every Drainage Weekly Drainage Physician Contact Information Dr. Phone: CHEST DRAINAGE Pleural Space Insertion Site Cuff Exit Site Catheter Valve Connector
Drainage Frequency: PATIENT GUIDE Dressing Frequency: Every Drainage Weekly Drainage Physician Contact Information Dr. Phone: CHEST DRAINAGE Pleural Space Insertion Site Cuff Exit Site Catheter Valve Connector
Advanced Catheterisation Trainer User Guide
 Advanced Catheterisation Trainer User Guide Also for Advanced Male Catheterisation Trainer Part No: 605 Advanced Female Catheterisation Trainer Part No: 6055 Designed and manufactured by Limbs & Things
Advanced Catheterisation Trainer User Guide Also for Advanced Male Catheterisation Trainer Part No: 605 Advanced Female Catheterisation Trainer Part No: 6055 Designed and manufactured by Limbs & Things
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
for the ideal insertion of peritoneal dialysis catheter (patent pending)
 for the ideal insertion of peritoneal dialysis catheter (patent pending) CAUTION: U.S. federal law restricts this device to sale by or on the order of a properly licensed practitioner. DEVICE DESCRIPTION
for the ideal insertion of peritoneal dialysis catheter (patent pending) CAUTION: U.S. federal law restricts this device to sale by or on the order of a properly licensed practitioner. DEVICE DESCRIPTION
Bard CapSure. Technique Guide. Permanent Fixation System SOFT TISSUE REPAIR. Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair
 Bard CapSure Permanent Fixation System Technique Guide Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
Bard CapSure Permanent Fixation System Technique Guide Laparoscopic Inguinal, Open and Laparoscopic Ventral Hernia Repair SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. The opinions
01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation.
 Intraosseous Infusion System Directions for Use 01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation. EC REP Emerge
Intraosseous Infusion System Directions for Use 01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation. EC REP Emerge
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
The A-Stretch penis enlargement and male enhancement exercise benefits: erect penis length flaccid penis length
 The A-Stretch The A-Stretch exercise pushes the penis up, while pulling it forward. This penis enlargement exercise mainly stretches the upper part of the shaft. It is the opposite exercise of the V-Stretch.
The A-Stretch The A-Stretch exercise pushes the penis up, while pulling it forward. This penis enlargement exercise mainly stretches the upper part of the shaft. It is the opposite exercise of the V-Stretch.
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Correction System. Surgical Technique
 Nextra Hammertoe Correction System Surgical Technique Maximized Bone Purchase* Stable and Secure Phalanx Optimized Screw Design Adjustable Bone-to-Bone Apposition Progressive Ratchet Tightening Mechanism
Nextra Hammertoe Correction System Surgical Technique Maximized Bone Purchase* Stable and Secure Phalanx Optimized Screw Design Adjustable Bone-to-Bone Apposition Progressive Ratchet Tightening Mechanism
Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh
 Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size 5955450 Circle 4.5" (11.4 cm) 5955460 Ellipse 4" x 6" (10.2 cm x 15.2 cm) 5955600 Circle 6" (15.2 cm) 5955680 Ellipse
Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size 5955450 Circle 4.5" (11.4 cm) 5955460 Ellipse 4" x 6" (10.2 cm x 15.2 cm) 5955600 Circle 6" (15.2 cm) 5955680 Ellipse
ARROW ENDURANCE. Extended Dwell Peripheral Catheter System. Rx only.
 ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Find your ED cure End your frustration. Renew your confidence. Feel complete. Take the next steps. Erectile dysfunction and heart disease
 Take the next steps Visit your general practitioner or cardiologist to learn more about your risk for cardiovascular disease. Visit EDCure.org to: Take the online ED quiz and get your customized treatment
Take the next steps Visit your general practitioner or cardiologist to learn more about your risk for cardiovascular disease. Visit EDCure.org to: Take the online ED quiz and get your customized treatment
Trigger Finger Release
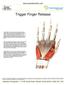 Trigger Finger Release Trigger finger, also known as stenosing tenosynovitis, occurs when one of the tendons responsible for bending a finger or the thumb develops a thickening, known as a nodule, and
Trigger Finger Release Trigger finger, also known as stenosing tenosynovitis, occurs when one of the tendons responsible for bending a finger or the thumb develops a thickening, known as a nodule, and
Uncompromised Exposure
 Uncompromised Exposure Thompson Techniques + User Manual: HIP SYSTEM The Thompson Hip System has an elegant design that optimizes the synergy between instrumentation and surgical technique allowing unimpeded
Uncompromised Exposure Thompson Techniques + User Manual: HIP SYSTEM The Thompson Hip System has an elegant design that optimizes the synergy between instrumentation and surgical technique allowing unimpeded
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Biomechanics. and Functional Anatomy. of Human Male Genitalia. For designers and creators of biomimetic androids, dolls and robots
 Biomechanics and Functional Anatomy of Human Male Genitalia For designers and creators of biomimetic androids, dolls and robots The Penis The shaft or body of the penis is formed principally by a fused
Biomechanics and Functional Anatomy of Human Male Genitalia For designers and creators of biomimetic androids, dolls and robots The Penis The shaft or body of the penis is formed principally by a fused
UNDERSTANDING PEYRONIE S DISEASE
 Learn more about Peyronie s disease and how Chesapeake Urology s Men s Sexual Health specialists can help restore your quality of life. Call 877-422-8237 to schedule an appointment with a urologist or
Learn more about Peyronie s disease and how Chesapeake Urology s Men s Sexual Health specialists can help restore your quality of life. Call 877-422-8237 to schedule an appointment with a urologist or
MEDICAL CORPORATION Asbury Rd., P.O. Box 758, Farmingdale, NJ USA Fax
 MEDICAL CORPORATION 5206 Asbury Rd., P.O. Box 758, Farmingdale, NJ 07727 USA 732-938-2266 800-323-4035 Fax 732-938-2399 MYO/WIRE Temporary Cardiac Pacing Wire System MYO/WIRE temporary cardiac pacing wires.
MEDICAL CORPORATION 5206 Asbury Rd., P.O. Box 758, Farmingdale, NJ 07727 USA 732-938-2266 800-323-4035 Fax 732-938-2399 MYO/WIRE Temporary Cardiac Pacing Wire System MYO/WIRE temporary cardiac pacing wires.
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
CATHETERIZATION TRAINER ON A STAND
 CATHETERIZATION TRAINER ON A STAND Parts identification: Clamp Prostate velcro strap (male genitalia only) Genitalia stand male female Urethra channel Bladder tubing and luer locks Female genitalia Male
CATHETERIZATION TRAINER ON A STAND Parts identification: Clamp Prostate velcro strap (male genitalia only) Genitalia stand male female Urethra channel Bladder tubing and luer locks Female genitalia Male
STANDARDIZED PROCEDURE REPROGRAMMING AND REFILLING INTRATHECAL BACLOFEN PUMPS and ACCESSING THE CATHETER ACCESS PORT (Adult,Peds)
 I. Definition The purpose of this procedure is to allow the Advanced Health Practitioner (AHP) to reprogram and refill intrathecal Baclofen pumps, as well as access the catheter access port for those AHPs
I. Definition The purpose of this procedure is to allow the Advanced Health Practitioner (AHP) to reprogram and refill intrathecal Baclofen pumps, as well as access the catheter access port for those AHPs
GUIDELINES FOR THE USE OF INVICORP INJECTION FOR MEN WITH ERECTILE DYSFUNCTION.
 GUIDELINES FOR THE USE OF INVICORP INJECTION FOR MEN WITH ERECTILE DYSFUNCTION. You should carry out the first Invicorp injection under a doctor s/nurse s supervision. Preparation of Invicorp injection
GUIDELINES FOR THE USE OF INVICORP INJECTION FOR MEN WITH ERECTILE DYSFUNCTION. You should carry out the first Invicorp injection under a doctor s/nurse s supervision. Preparation of Invicorp injection
CableFIX Xpress Carpometacarpal Fixation System. Operative technique
 CableFIX Xpress Carpometacarpal Fixation System Operative technique CableFIX Xpress Carpometacarpal Fixation System CableFIX Xpress Carpometacarpal Fixation System Contents 1. Indications and contraindications...
CableFIX Xpress Carpometacarpal Fixation System Operative technique CableFIX Xpress Carpometacarpal Fixation System CableFIX Xpress Carpometacarpal Fixation System Contents 1. Indications and contraindications...
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
DMF Page 63. Description
 DMF Page 60 DMF Page 61 DMF Page 62 4 GB Description The SPONGOSTAN Absorbable Haemostatic Gelatin Powder is a sterile, water-insoluble, porcine gelatin absorbable powder intended for haemostatic use by
DMF Page 60 DMF Page 61 DMF Page 62 4 GB Description The SPONGOSTAN Absorbable Haemostatic Gelatin Powder is a sterile, water-insoluble, porcine gelatin absorbable powder intended for haemostatic use by
EVOGAM. Information for patients Evogam 2014 NZ Patient Brochure Update v11
 EVOGAM Information for patients 11881 Evogam 2014 NZ Patient Brochure Update v11 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have
EVOGAM Information for patients 11881 Evogam 2014 NZ Patient Brochure Update v11 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have
limbsandthings.com Advanced Female Catheterisation Trainer User Guide For more skills training products visit Limbs & Things Ltd.
 Advanced Female Catheterisation Trainer Product No: 60155 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Advanced Female Catheterisation Trainer Product No: 60155 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Parts identification:
 TRANSPARENT CATHETERIZATION SIMULATOR Parts identification: Clamp Urethra channel Prostate velcro strap (male genitalia only) Transparent Simulator Bladder tubing and luer locks Female genitalia Male genitalia
TRANSPARENT CATHETERIZATION SIMULATOR Parts identification: Clamp Urethra channel Prostate velcro strap (male genitalia only) Transparent Simulator Bladder tubing and luer locks Female genitalia Male genitalia
HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE
 HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For Single Patient Use Only www.lilly.ca INTRODUCTION HumaPen SAVVIO is designed for ease of use. You can give yourself multiple doses from one
HumaPen SAVVIO INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For Single Patient Use Only www.lilly.ca INTRODUCTION HumaPen SAVVIO is designed for ease of use. You can give yourself multiple doses from one
METHOD OF USE READ BEFORE USE
 Gold Mini METHOD OF USE READ BEFORE USE Table of Contents Page 1. DEFINITION 2. DESCRIPTION 3. INDICATIONS 4. PERSONALIZE Andropenis 5. FITTING Andropenis 6. PENILE CURVATURE AND PEYRONIE S DISEASE 7.
Gold Mini METHOD OF USE READ BEFORE USE Table of Contents Page 1. DEFINITION 2. DESCRIPTION 3. INDICATIONS 4. PERSONALIZE Andropenis 5. FITTING Andropenis 6. PENILE CURVATURE AND PEYRONIE S DISEASE 7.
Instructions for Use. For use with. 10 mg vial
 Instructions for Use For use with 10 mg vial Table of Contents Parts of the ZOMA-Jet 10... 1 Supplies you will need to mix a ZOMACTON 10 mg Vial... 2 Mix a ZOMACTON 10 mg vial... 3 Reset the ZOMA-Jet 10...
Instructions for Use For use with 10 mg vial Table of Contents Parts of the ZOMA-Jet 10... 1 Supplies you will need to mix a ZOMACTON 10 mg Vial... 2 Mix a ZOMACTON 10 mg vial... 3 Reset the ZOMA-Jet 10...
