PHOSPHOROLYSIS AND SYNTHESIS OF SUCROSE WITH A BACTERIAL PREPARATION
|
|
|
- Philip Weaver
- 5 years ago
- Views:
Transcription
1 PHOSPHOROLYSIS AND SYNTHESIS OF SUCROSE WITH A BACTERIAL PREPARATION BY M. DOUDOROFF, N. KAPLAN, AND W. Z. HASSID (From the Department of Bacteriology, Division of Biochemistry of the Medical School, and the Division of Plant Nutrition of the College of Agriculture, University of California, Berkeley) (Received for publication, January 4, 1943) The recent discovery of the reversible phosphorolysis of polysaccharides by Cori and Cori (1, 2)) Kiessling (3), and Hanes (4) has shed considerable light on the mechanism of synthesis of glycogen and &arch in animal and plant tissues. On the other hand, very little is known about the mechanism of sucrose formation in plants. It has been suggested previously (5-S) that phosphorylated sugars may play a r&e in the synthesis of sucrose, but no direct evidence has been brought forth thus far in support of this view. Since invertase is widely distributed in plants, it has been held by some investigators (5, 8) that this enzyme is responsible not only for hydrolysis of sucrose, but also for its formation. This view, however, could not be substantiated (9, 10). It is of interest to point out in this connection that attempts to synthesize sucrose by chemical methods have not met with success to date (11,12). The occurrence of such an enzymic phosphorolysis in bacteria gives promise of helping to explain not only the mechanism of sucrose synthesis, but also the so called direct utilization of disaccharides claimed to occur in some microorganisms (13, 14). EXPERIMENTAL The organism Pseudomonas sacchar~phila Doudoroff (15) was selected for the present studies. This organism exhibits the unusual ability of developing more rapidly with certain complex sugars than with monosaccharides. Under certain conditions, it oxidizes such sugars as sucrose, trehalose, maltose, melibiose, and raffinose at a much higher rate than the constituent hexoses. The bacteria were grown in a liquid medium containing M/30 KH,POh- Na2HP04, Sijrensen phosphate buffer at ph 6.64, 0.1 per cent NH&l, 0.05 per cent MgS04, per cent FeCls, per cent CaC12, and 0.3 per cent sucrose at 29 with constant agitation to provide ample aeration. Under such conditions, almost 50 per cent of the carbon content of sucrose is converted into cell material, the remainder being oxidized to COZ. Traces of reducing sugar appear in the medium, as well as occasionally small amounts of pyruvic acid, which disappears in the later stages of develop- 67
2 68 SUCHOSE ment. The cells were harvested by centrifugation, washed twice with distilled water, and dried at room temperature in vacua over PZ05. For experimental purposes, preparations were made by grinding a sufficient amount of dry bacteria with wa.t er to result, after various further additions, in a final suspension containing 2 per cent of the dried cells by weight. Such suspensions were found to possess a negligible endogencus respiration (5 to 12 c.mm. of O2 per 2 cc. per hour) which was practically unaffected by t]hc addition of sugars as substrates or by treatment with fluoride and iodoacetat,e. An equally negligible production of acid (10 to 15 c.mm. of COZ liberated from bicarbonate per 2 cc. per hour) was also observed both under aerobic and anaerobic conditions in the absence of substrate. Since the organism is an obligate aerobe, incapable of fermenting sugars, it is not surprising that no fermentat,ion of sucrose or glucose could be detected with the dry cell preparations. Phosphorolysis of Sucrose-When dry bacterial preparations were incubated with phosphate or phosphate-bicarbonate-coz buffers at ph 6.3 to 7.0 in the presence of sucrose, a rapid esterification of inorganic phosphate was observed. The disappearance of the phosphate could be measured either with the aid of a modified Fiske-Subbarow method or manometrically in a Warburg respirometcr (as COZ liberated from bicarbonate by the formation of a strongly acidic ester). No esterification what,ever could be observed with glucose, fructose, or a mixture of the two by such cells, nor by preparations made with bacteria grown with glucose as sole carbon source. The esterification of phosphate in the presence of sucrose was not inhibited by either 0.05 M fluoride, 0.01 M iodoacetate, or both, and proceeded equally well under aerobic and anaerobic conditions. All of the esterified phosphate could be shown to be in the bariumsoluble, 7 minute-hydrolyzable fract,ion of t,he suspensions. The ester was separated and identified as glucose-l-phosphate as follows: The reaction mixture was treated with trichloroacetic acid, centrifuged, and then ba,rium acet)ate added. After neutralization and removal of the inorganic phosphate, the barium salt of the ester was precipit,abed by the addition of 3 volumes of ethanol. The material was extracted t>wice with water, the insoluble residue discarded, and the supernat,ant liquid precipitated with 1.5 volumes of alcohol. After this operation was repeated, the material obtained was almost completely soluble in water. The substance did not reduce alkaline copper or ferricyanide solutions. However, on hydrolysis with 1 N sulfuric acid for 7 minutes at IOO, and neutralization of the solution, aft.er removal of the barium sulfate precipitate, an osazone was formed. This was identified as glucosazone. That it was the osazone of glucose and not of fructose is indicated by the negative Roe test, (16) and by the hypoiodidc rcact,ion given by the hydrolyzed solution.
3 DOUDOIZOFF, KAPh4N, AND HASRID 69 The barium salt of the ester was converted into the dipotassium salt as follows: The salt was dissolved, and a sufficient amount of sulfuric, acid was added to make the ph of the solution I.8. The precipitated barium sulfate was removed by cent,rifugation, the solution was brought to ph 8.3 with potassium hydroxide, and 1.5 volumes of ethanol were added. After 24 hours bhe ester separated out in the form of a crystalline salt The crystals were filtered off, washed with alcohol, and drir>d in vucuo at 40. A few of t he crystals were dissolved in water and treated with potato phosphorylase at ph 6.0. Starch, which was identified by the brilliant, blue color when treated w&h iodine, rapidly appeared. This reacgon is characteristic of glucose-l-phosphate (4). The following analyses were omainrd. CeHuOs. 0 Pod. KI. 2HzO Calculated P.,......,..,.., 8.33 Aldose _ Specific Relation-[a], = +78 (in water, c = 1) It might be expected that the phosphorolysis of sucrose would lead to the production of 1 mole of fructose per mole of Cori ester in accordance with the equation, sucrose + HJ O4 + glucose-1-phosphat c + fructose. This, indeed, was found t,o be t)he case. In addition to the phosphorolytic propert,y, the suspensions showed a rather strong invcrtasc activity resulting in the formation of glucose and fructose in equimolar ratio. That this was not due to a phosphorolysis followed by a hydrolysis of the Cori ester to glucose and phosphate is indicated by the fact that glucose-s-phosphate remained practically unattacked when supplied as substrate under similar conditions, except for a slight initial dephosphorylat,ion to be discussed further. To show the quantitative relationship between the various products of sucrose breakdown, 10 cc. of a 2 per cent suspension of dry bacteria were prepared in approximately M/GO phosphate buffer solution at ph 6.64 containing approximately 0.05 M sucrose, O..l M NaHC03, 0.05 M NaF, and 0.01 M iodoacetate. The suspension was shaken for 1 hour at 29 in an atmosphere of Cog, whereupon trichloroacetic acid was added and the precipitate centrifuged off. A control experiment was carried out under similar conditions, except that sucrose was not added until after precipitation with trichloroacetic acid. An aliquot of t he neutralized supernatant liquid was analyzed for inorganic phosphate, while, in another, the phosphate ester was removed as the lead salt. Total reducing sugars were determined after removal of the ester by the method of Hassid (17) and glucose by the reduction of hypoiodite (18). Fructose was estimated by difference, after correction for the reduction Found
4 70 SUCROSE of hypoiodite by fructose and sucrose in solution had been applied. Sucrose was determined by the increase in reducing sugar after acid hydrolysis. The lead salt of the ester was hydrolyzed and the glucose content determined as above, with a small correction for the ester remaining in solution, determined as hydrolyzable phosphate. The results are presented in Table I. To show the interrelation of phosphorolytic and hydrolytic activities carried out by the preparations, two aliquots of a suspension were allowed t,o act on approximately M/30 sucrose in M/15 NaHCO$ in an atmosphere of coz. One cont,ained only the inorganic phosphate introduced with the dry cells ( M), while the other was made up with ph 6.5 phosphate buffer to a concentration of M. Samples were removed, after various periods of incubation with constant shaking at 29, and analyzed for reducing sugar and for inorganic phos- Sucrose Breakdown TABLE I by Dry Cell Preparation m.ep. (a) Sucrose utilized (as hexose) (b) P esterified (Fiske-Subbarow method) (c) Glucose esterified (hydrolysis of ester) (4 found as free sugar (e) Fructose (.f) in excess of free glucose (e) - (a) (g) Products recovered (c) + (d) + (e) Recovery of sucrose products (g/a), %....,103 Ratio, fructose to Cori ester in phosphorolysis (j/e) phate. The results are presented in Table II, together with the computed amount of sucrose utilized, obtained by the summation of esterified and reducing sugars found. It may be seen from Table II that the phosphorolytic and hydrolytic enzymes apparently compete for the sucrose. The nature of the data makes it impossible to compute an equilibrium constant for the phosphorolysis. The possibility of separating the two functions of the preparations will be investigated in the future. It is interesting to note that following the initial increase in esterified phosphate, there is a gradual decrease, strongly indicating the reversible nature of the phosphorolysis, which was proved by further experiments. The irreversible hydrolysis of sucrose would force the phosphorolytic reaction to the left, resulting in the disappearance of the ester. Synthesis of Sucrose-As stated earlier, dry cell preparations were found
5 I DOUDOROFF, KAPLAN, AND HASSID 71 to have very little action on glucose-l-phosphate under conditions favoring phosphorolysis. Thus when M/9 Cori ester (K salt acidified to ph 6.3 to 6.5 with acetic acid) was added to a preparation in ~/15 NaHC03 in an atmosphere of COZ, only a small fraction (about 2 per cent) of the phosphate was split off in 40 minutes at 29, after which time no further dephosphorylation occurred. Measurements of the reducing sugar value indicated a somewhat greater amount of reducing sugar formed than would be expect,ed if the process were merely hydrolytic (phosphatase) activity, and suggested that, besides hydrolysis, the formation of a small amount of reducing ester (such as glucose-6-phosphate) might t,ake place (1). No starch or glycogen could be detected with iodine. When, however, M/9 fructose was added together with glucose-l-phosphate under similar conditions, a fairly rapid and constant liberation of TABLE Course of Sucrose Breakdown -.~- I. (Initial phosphate M) (a) Total P esterified.. (b) Reducing sugar produced.. (c) Sucrose used (as hexose) (a) + (b) II. (Initial phosphate M) (a) Total P esterified.. (b) Reducing sugar produced.. (c) Sucrose used (as hexose) (a) + (b) -- -l-. II I- M.eq. per 10 cc. after various periods of incubation at min. TI 60 min. T 120 min. 180 min inorganic phosphate was observed, accompanied at first by a decrease in reducing value, and later by an increase paralleling the liberation of phosphate. This was taken as evidence for the formation of a non-reducing sugar from glucose-l-phosphate and fructose, followed by its hydrolysis to hexose constituents. The non-reducing sugar was identified as sucrose by the simultaneous determination of increase in reducing value and change in optical rotation of the deproteinized and neutralized solutions before and after inversion with yeast invertase. (Small corrections for the reducing and optical properties of the invertase preparations obtained from the Wallerstein Laboratories were made. Inorganic phosphate determinations before and after inversion indicated no effect of invertase on the Cori ester present.) In one experiment, the total amount of sucrose was calculated as 9.05 mg. (ho.28 mg.) on the basis of reducing value, and as 9.55 mg. (ho.6 mg.)
6 72 SUCROSE on the basis of polarimetric readings. In another case, mg. (ho.5 mg.) were found by the former met.hod as compared with 11.l mg. (f 1 mg.) by the latter. No sucrose could be detected in the controls, to which trichloroacetic acid was added before glucose-l-phosphate and fructose, nor in preparations with fructose alone, the Cori eskr alone, or glucose and fructose together. Table III shows the relation of synthesis of sucrose to the utilization of glucose-l-phosphate and fructose by 5 cc. of a bacterial preparation with ~/15 XaHCO~, M/9 Cori s cst,er, and M fructose incubated at 29 in an atmosphere of COZ. Both the theoretical values for sucrose, computed as reducing sugar unaccounted for, and the actual values, found as increase in reducing sugar after hydrolysis with invertase, are given. From Table III it is evident that, under the conditions of the experiment, the sucrose concentration rapidly reaches a value approximating 6 per TABLE Sucrose Formation from Glucose-l-phosphate and Fructose. - (a) Reducing sugar...i (b) Total change in reducing sugar.. (c) P liberated from Cori ester.. (d) Sucrose calculated (as hexose) (c) - (b). 0 (e) Sucrose found (as hexose) with invertase.. i 0 - III min / M.eq. per 5 cc. 60min. / 120min. 180 min I I cent of the sum of the constituents, and remains constant at this level, at which the rate of hydrolysis must equal that of synthesis. Other experiments gave as good or even better agreement between the theoretical sucrose values and those actually determined with invertase. Treating with iodoacetate and fluoride did not materially affect the reaction. The addition of M/70 sucrose was shown to depress the liberation of inorganic phosphate by preparations with the Cori ester and fructose, but the addition of ~/60 phosphate buffer or of yeast invertase to the suspensions did not appreciably affect the rate. Experiments with Other Sugars-It has already been stated that no esterification of inorganic phosphate could be observed with glucose as substrate even by preparations of bacteria grown with glucose as sole carbon source. It might be expected that a phosphorolysis of trehalose and maltose would be observed with dried bacteria obtained from cultures
7 DOUDOROFF, KAPLAN, AND HASSID 73 with these sugars. However, no esterification could be detected in the presence of these sugars under conditions similar to those employed in experiments with sucrose. Either t he phosphorolytic enzymes were inactive in these preparations, or the breakdown of these sugars follows a different course than that for sucrose. This observation was disturbing, in view of the fact that, especially with trehalose, the metabolism of the bacteria, both in growing cultures and in resting cell suspensions, is remarkably similar to that with sucrose. An interesting observation was made with trehalose-grown bacteria; namely, that the dry cell preparations were capable of hydrolyzing this sugar fairly rapidly, although the int,act cells have very little act,ivity, recalling the findings of Deere et al. (19, 20). That this hydrolysis was not due to a phosphorolysis coupled wit(h phosphatase activity seemed almost certain from the findings that it proceeded at exactly the same rate both in the presence and in the absence of added phosphat,e, and that glucose-l-phosphate was att,ackcd at a very low rate as compared with t)hat, for the hydrolysis of trchalose. Of further int,erest was the observation that preparations from cult,ures grown with trehalcse showed neither an appreciable hydrolysis nor phosphorolysis of sucrose. This was not unexpected, in view of the extremely adaptive character of the enzymes reported previously for this bacterium. DISCUSSION Although the discovery of the phosphorolytic breakdown and synthesis of sucrose by preparations of Pseudomonas saccharophila is of general interest, it falls short of explaining the behavior of the intact organisms with respect to this sugar as well as ot,her disaccharides. The living cells are capable of oxidizing the entire sucrose molecule at a very high rate (with a synthesis of about two-thirds of the sugar to cell material by starved resting cell suspensions). Glucose, on the other hand, is oxidized very slowly by bacteria accustomed to sucrose, while fructose supplied in equivalent concentrations is practically unat,tacked. Yet fructose is the product of both the phosphorolysis and hydrolysis of sucrose by the bacterial preparations. Three theories may be suggested in possible explanation of this behavior: (1) That the permeability of the cell membrane prevents the penetration of the free hexose. (2) That the hexose constituent, during or immediately after phosphorolysis or hydrolysis, is, for some reason, more readily attacked than a free hexose molecule. That the y form of fructose would fulfil such a requirement does not seem very likely, since the preparations are capable of using the normal form of fructose in the synthesis of sucrose. (3) That, in living cells, the disaccharide acts first as a phosphate acceptor and is then phosphorolyzed
8 74 SUCROSE to 2 molecules of hexose phosphate. Since no respiratory or fermentative ability was shown by the preparations, there could be no generation of suitable phosphate donors for the phosphorylation of either the hexose or the disaccharide, and the normal course of events may have been altered by this deficiency. It is even possible that the inability of dry cells to phosphorolyze trehalose and maltose may have been due to the inavailability of such donors. Experiments designed to test the above possibilities are now in progress. SUMMARY 1. A dry preparation of the bacterium Pseudomonas saccharophila has been found capable of phosphorolyzing sucrose to glucose-l-phosphate and fructose. 2. With glucose-l-phosphate and fructose as substrates, the formation of sucrose could be demonstrated by the reversal of the above reaction. 3. The competing hydrolytic and phosphorolytic properties of the preparation could be summarized as follows: Sucrose + H3P01 + glucose-l-phosphate glucose + fructose + fructose The authors wish to express their gratitude to Professor H. A. Barker for his valuable suggestions. Addendum-Since the preparation of this manuscript, an article by Kagan, Latker, and Zfasman (21) has come to our attention. These authors present evidence for the phosphorolysis of sucrose with the production of glucose-l-phosphate by intact cell suspensions of the bacterium Leuconostoc mesenteroides. BIBLIOGRAPHY 1. Cori, C. F., and Cori, G. T., Proc. Sot. Exp. Biol. and Med., 34, 702 (1936). 2. Cori, C. F., Endocrinology, 26, 285 (1940); Biological symposia, Lancaster, 6, 131 (1941). 3. Kiessling, W., Biochem. Z., 302, 50 (1939). 4. Hanes, C. S., Proc. Roy. Sot. London, Series B, 128, 421 (1940); 129, 174 (1940). 5. Oparin, A., and Kursanov, A., Biochem. Z., 239, 1 (1931). 6. Burkard, J., and Neuberg, C., Biochem. Z., 270, 229 (1934). 7. Hassid, W. Z., Plant Physiol., 13, 641 (1938). 8. Kursanov, A., and Kryukova, N., Biokhimiya, 4, 229 (1939). 9. Lebedew, A., and Dikanowa, A., 2. physiol. Chem., 231, 271 (1935). 10. Leonard, 0. A., Am. J. Bot., 26, 78 (1938). 11. Irvine, J. C., Oldham, J. W. H., and Skinner, A. F., J. Am. Chem. Sot., 61, 1279 (1929).
9 DOUDOROFF, KAPLAN, AND HASSID Irvine, J. C., and Oldham, J. W. H., J. Am. Chem. Sot., 61, 3609 (1929). 13. Wright, H. D., J. Path. and Bact., 43, 487 (1936); 46, 117 (1937); 46, 261 (1938). 14. Leibowitz, J., and Hestrin, S., Enzymologia, 6, 15 (1939). 15. Doudoroff, M., Enzymologia, 9, 59 (1940). 16. Roe, J. H., J. Biol. Chem., 10 7, 15 (1934). 17. Hassid, W. Z., Ind. and Eng. Chem., Anal. Ed., 9, 228 (1937). 18. Yemm, E. W., Proc. Roy. Sot. London, Series B, 117, 485 (1935). 19. Deere, C. J., Dulaney, A. D., and Michelson, I. D., J. Bad., 37, 355 (1939). 20. Deere, C. J., -1. Bad., 37, 473 (1939). 21. Kagan, B. O., Latker, S. N., and Zfasman, E. M., Biokhimiya, 7, 92 (1942).
10 PHOSPHOROLYSIS AND SYNTHESIS OF SUCROSE WITH A BACTERIAL PREPARATION M. Doudoroff, N. Kaplan and W. Z. Hassid J. Biol. Chem. 1943, 148: Access the most updated version of this article at Alerts: When this article is cited When a correction for this article is posted Click here to choose from all of JBC's alerts This article cites 0 references, 0 of which can be accessed free at ml#ref-list-1
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE
 METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE*
 THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE* BY W. R. BROWN (From Ihe Laboratory of Biochemistry, University of Cincinnati, Cincinnati) (Received for publication, November 21, 1935)
THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE* BY W. R. BROWN (From Ihe Laboratory of Biochemistry, University of Cincinnati, Cincinnati) (Received for publication, November 21, 1935)
OXIDATIVE FERMENTATION OF D-RIBOSE BY LACTOBACILLUS PLANTARUM NO. 11 (Preliminary Report)
 J. Gen. Appl. Microbiol. Vol. 4, No. 2, 1958 OXIDATIVE FERMENTATION OF D-RIBOSE BY LACTOBACILLUS PLANTARUM NO. 11 (Preliminary Report) SAKUZO FUKUI and AKIRA OI Division of 7ymomycology, The Institute
J. Gen. Appl. Microbiol. Vol. 4, No. 2, 1958 OXIDATIVE FERMENTATION OF D-RIBOSE BY LACTOBACILLUS PLANTARUM NO. 11 (Preliminary Report) SAKUZO FUKUI and AKIRA OI Division of 7ymomycology, The Institute
THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES
 THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM (From the Departments oj Physiology and Pharmacology and Biochemistry, Duke
THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM (From the Departments oj Physiology and Pharmacology and Biochemistry, Duke
A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT
 A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT BY M. L. CALDWELL AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for publication,
A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT BY M. L. CALDWELL AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for publication,
PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS
 PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM* (From the Departments of Physiology and Biochemistry, Duke University School of Medicine, Durham)
PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM* (From the Departments of Physiology and Biochemistry, Duke University School of Medicine, Durham)
Carbohydrates- Disaccharides. By Dr. Bhushan R. Kavimandan
 Carbohydrates- Disaccharides By Dr. Bhushan R. Kavimandan Disaccharides ofbiological importance: Disaccharides consist of two monosaccharides joined by glycosidic linkages. They are crystalline, water-soluble
Carbohydrates- Disaccharides By Dr. Bhushan R. Kavimandan Disaccharides ofbiological importance: Disaccharides consist of two monosaccharides joined by glycosidic linkages. They are crystalline, water-soluble
possibilities occurs. It has been found that the organism acquires addition of vitamin B1 to cells of P. pentosaceum which had
 ADAPTATION OF THE PROPIONIC-ACID BACTERIA TO VITAMIN B1 SYNTHESIS INCLUDING A METHOD OF ASSAY M. SILVERMAN AND C. H. WERKMAN Bacteriology Section, Industrial Science Research Institute, Iowa State College,
ADAPTATION OF THE PROPIONIC-ACID BACTERIA TO VITAMIN B1 SYNTHESIS INCLUDING A METHOD OF ASSAY M. SILVERMAN AND C. H. WERKMAN Bacteriology Section, Industrial Science Research Institute, Iowa State College,
ION ANTAGONISMS AFFECTING GLYCOLYSIS BY BACTERIAL SUSPENSIONS*
 ION ANTAGONISMS AFFECTING GLYCOLYSIS BY BACTERIAL SUSPENSIONS* BY HIROSHI TSUYUKIt AND ROBERT A. MAcLEOD (From the Department of Biochemistry, Queen s University, Kingston, Ontario, Canada) (Received for
ION ANTAGONISMS AFFECTING GLYCOLYSIS BY BACTERIAL SUSPENSIONS* BY HIROSHI TSUYUKIt AND ROBERT A. MAcLEOD (From the Department of Biochemistry, Queen s University, Kingston, Ontario, Canada) (Received for
THE EFFECT OF ANTICOAGULANTS ON DETERMINA- TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER
 THE EFFECT OF ANTICOAGULANTS ON DETERMINA TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER (From the Department of Laboratories, Henry Ford Hospital, Detroit) (Received for publication,
THE EFFECT OF ANTICOAGULANTS ON DETERMINA TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER (From the Department of Laboratories, Henry Ford Hospital, Detroit) (Received for publication,
BIOCHEMICAL STUDIES ON CARBOHYDRATES. XL. Preparation of Mucoitin* from Umbilical Cords.
 The Journal of Biochemistry, Vol. 28, No. 3. BIOCHEMICAL STUDIES ON CARBOHYDRATES. XL. Preparation of Mucoitin* from Umbilical Cords. MASAMI BY SUZUKI. (From the Medico-Chemical Institute, Hokkaido Imperial
The Journal of Biochemistry, Vol. 28, No. 3. BIOCHEMICAL STUDIES ON CARBOHYDRATES. XL. Preparation of Mucoitin* from Umbilical Cords. MASAMI BY SUZUKI. (From the Medico-Chemical Institute, Hokkaido Imperial
ESCHERICHIA COLI-MUTABILE1. antiseptics employed "activated" the lactase which was present, "activate" the lactase.
 ON THE "ACTIVATION" OF THE LACTASE OF ESCHERICHIA COLI-MUTABILE1 CHARLES J. DEERE Department of Chemistry, University of Tennessee School of Biological Sciences, Memphis Received for publication August
ON THE "ACTIVATION" OF THE LACTASE OF ESCHERICHIA COLI-MUTABILE1 CHARLES J. DEERE Department of Chemistry, University of Tennessee School of Biological Sciences, Memphis Received for publication August
lactose-fermenting variants (reds). Appreciable lactose utilization variants. Hershey and Bronfenbrenner (1936) found the non-lactosefermenting
 THE LACTASE ACTIVITY OF ESCHERICHIA COLI- MUTABILE' CHARLES J. DEERE, ANNA DEAN DULANEY AND I. D. MICHELSON Department of Chemistry and Department of Bacteriology, University of Tennessee School of Biological
THE LACTASE ACTIVITY OF ESCHERICHIA COLI- MUTABILE' CHARLES J. DEERE, ANNA DEAN DULANEY AND I. D. MICHELSON Department of Chemistry and Department of Bacteriology, University of Tennessee School of Biological
STUDIES ON THE ENZYMES OF PNEUMOCOCCUS.
 Published Online: 1 November, 1920 Supp Info: http://doi.org/10.1084/jem.32.5.583 Downloaded from jem.rupress.org on September 29, 2018 STUDIES ON THE ENZYMES OF PNEUMOCOCCUS. III. CARBOHYDRATE-SPLITTING
Published Online: 1 November, 1920 Supp Info: http://doi.org/10.1084/jem.32.5.583 Downloaded from jem.rupress.org on September 29, 2018 STUDIES ON THE ENZYMES OF PNEUMOCOCCUS. III. CARBOHYDRATE-SPLITTING
CRYSTALLINE PEPSIN BY JOHN H. NORTHROP. (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, iv. J.
 CRYSTALLINE PEPSIN III. PREPARATION OF ACTIVE CRYSTALLINE PEPSIN FROM INACTIVE DENATURED PEPSIN BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton,
CRYSTALLINE PEPSIN III. PREPARATION OF ACTIVE CRYSTALLINE PEPSIN FROM INACTIVE DENATURED PEPSIN BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton,
THE RESPIRATION MECHANISM OF PNEUMOCOCCUS. III*
 THE RESPIRATION MECHANISM OF PNEUMOCOCCUS. III* BY M. G. SEVAG A~rD LORE MAIWEG (From the Robert Koch Institute, Berlin, Germany) (Received for publication, April 11, 1934) In two previous communications
THE RESPIRATION MECHANISM OF PNEUMOCOCCUS. III* BY M. G. SEVAG A~rD LORE MAIWEG (From the Robert Koch Institute, Berlin, Germany) (Received for publication, April 11, 1934) In two previous communications
EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH
 Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
GLYCOGEN BEFORE THE LAB YOU HAVE TO READ ABOUT:
 GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
6 The chemistry of living organisms
 Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
STUDIES ON CHOLINESTERASE*
 STUDIES ON CHOLINESTERASE* III. PURIFICATION OF THE ENZYME FROM ELECTRIC TISSUE BY FRACTIONAL AMMONIUM SULFATE PRECIPITATION BY MORTIMER A. ROTHENBERG AND DAVID NACHMANSOHN (From the Departments of Neurology
STUDIES ON CHOLINESTERASE* III. PURIFICATION OF THE ENZYME FROM ELECTRIC TISSUE BY FRACTIONAL AMMONIUM SULFATE PRECIPITATION BY MORTIMER A. ROTHENBERG AND DAVID NACHMANSOHN (From the Departments of Neurology
THE EFFECT OF FLUORINE UPON THE PHOSPHATASE CONTENT OF PLASMA, BONES, AND TEETH OF ALBINO RATS
 THE EFFECT OF FLUORINE UPON THE PHOSPHATASE CONTENT OF PLASMA, BONES, AND TEETH OF ALBINO RATS BY MARGARET CAMMACK SMITH AND EDITH M. LANTZ (From the Department oj Nutrition, Agricultural Experiment Station,
THE EFFECT OF FLUORINE UPON THE PHOSPHATASE CONTENT OF PLASMA, BONES, AND TEETH OF ALBINO RATS BY MARGARET CAMMACK SMITH AND EDITH M. LANTZ (From the Department oj Nutrition, Agricultural Experiment Station,
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation
 EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation Materials Needed About 3-5 g each of Glucose, Fructose, Maltose, Sucrose, Starch sodium bicarbonate, NaC 3 (s) 15
EXPERIMENT 6 Properties of Carbohydrates: Solubility, Reactivity, and Specific Rotation Materials Needed About 3-5 g each of Glucose, Fructose, Maltose, Sucrose, Starch sodium bicarbonate, NaC 3 (s) 15
decarboxylation. Further work with the enzyme systems involved has shown
 THE BACTERIAL OXIDATION OF AROMATIC COMPOUNDS IV. STITDIES ON THE MECHANISM OF ENZYMATC DEGRADATION OF PROTOCATECHuiC ACID' R. Y. STANIER Department of Bacteriology, University of California, Berkeley,
THE BACTERIAL OXIDATION OF AROMATIC COMPOUNDS IV. STITDIES ON THE MECHANISM OF ENZYMATC DEGRADATION OF PROTOCATECHuiC ACID' R. Y. STANIER Department of Bacteriology, University of California, Berkeley,
METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI
 METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI I. L- RHAMNOSE ISOMERASE DOROTHY M. WILSON1 AND SAM AJL Department of Bacteriology, Walter Reed Army Institute of Research, Washington, D. C. The methyl pentose,
METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI I. L- RHAMNOSE ISOMERASE DOROTHY M. WILSON1 AND SAM AJL Department of Bacteriology, Walter Reed Army Institute of Research, Washington, D. C. The methyl pentose,
and the cells removed by centrifugation. These were resuspended in sterile 1949a), growth was measured in terms of acid production while dextran was
 THE NUTRITIONAL REQUIREMENTS OF LEUCONOSTOC DEXTRANICUM FOR GROWTH AND DEXTRAN SYNTHESIS1 VIRGINIA WHITESIDE-CARLSON AND CARMEN L. ROSANO Biochemistry Department, Medical College of Alabama, Birmingham,
THE NUTRITIONAL REQUIREMENTS OF LEUCONOSTOC DEXTRANICUM FOR GROWTH AND DEXTRAN SYNTHESIS1 VIRGINIA WHITESIDE-CARLSON AND CARMEN L. ROSANO Biochemistry Department, Medical College of Alabama, Birmingham,
ACETONE DERIVATIVES OF d-ribose. II.
 ACETONE DERIVATIVES OF d-ribose. II. BY P. A. LEVENE AND ERIC T. STILLER* (From the Laboratories of The Rockefeller Institute for Medical Research, New York) (Received for publication, June 14, 1934) The
ACETONE DERIVATIVES OF d-ribose. II. BY P. A. LEVENE AND ERIC T. STILLER* (From the Laboratories of The Rockefeller Institute for Medical Research, New York) (Received for publication, June 14, 1934) The
Experiment 1. Isolation of Glycogen from rat Liver
 Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Polysaccharide phosphorylase
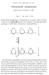 CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
Carbohydrates. Organic compounds which comprise of only C, H and O. C x (H 2 O) y
 Carbohydrates Organic compounds which comprise of only C, H and O C x (H 2 O) y Carbohydrates Monosaccharides Simple sugar Soluble in water Precursors in synthesis triose sugars of other (C3) molecules
Carbohydrates Organic compounds which comprise of only C, H and O C x (H 2 O) y Carbohydrates Monosaccharides Simple sugar Soluble in water Precursors in synthesis triose sugars of other (C3) molecules
CHEM121 Unit 2: Carbohydrate Metabolism
 CHEM121 Unit 2: Carbohydrate Metabolism Lecture 3 At the end of the lecture, students should be able to: Define metabolism Discuss the structure and function of ATP in metabolism Discuss glycolysis in
CHEM121 Unit 2: Carbohydrate Metabolism Lecture 3 At the end of the lecture, students should be able to: Define metabolism Discuss the structure and function of ATP in metabolism Discuss glycolysis in
PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS*
 PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS* BY WALTER H. SEEGERS (Prom the Department of Pathology, State University of Zowa, Iowa City) (Received for publication,
PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS* BY WALTER H. SEEGERS (Prom the Department of Pathology, State University of Zowa, Iowa City) (Received for publication,
Topic 3: The chemistry of life (15 hours)
 Topic : The chemistry of life (5 hours). Chemical elements and water.. State that the most frequently occurring chemical elements in living things are carbon, hydrogen, oxygen and nitrogen...2 State that
Topic : The chemistry of life (5 hours). Chemical elements and water.. State that the most frequently occurring chemical elements in living things are carbon, hydrogen, oxygen and nitrogen...2 State that
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19
 TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
THE following account of the carbohydrate metabolism constitutes one phase
 [ 133 ] THE CARBOHYDRATE METABOLISM OF GERMINATING BARLEY BY A. L. JAMES Department of Botany, Oxford (With 3 figures in the text) INTRODUCTION THE following account of the carbohydrate metabolism constitutes
[ 133 ] THE CARBOHYDRATE METABOLISM OF GERMINATING BARLEY BY A. L. JAMES Department of Botany, Oxford (With 3 figures in the text) INTRODUCTION THE following account of the carbohydrate metabolism constitutes
HAGEDORN AND JENSEN TO THE DETER- REDUCING SUGARS. MINATION OF LARGER QUANTITIES OF XIV. AN APPLICATION OF THE METHOD OF
 XIV. AN APPLICATION OF THE METHOD OF HAGEDORN AND JENSEN TO THE DETER- MINATION OF LARGER QUANTITIES OF REDUCING SUGARS. By CHARLES SAMUEL HANES (Junior Scholar of the Exhibition of 1851). From the Botany
XIV. AN APPLICATION OF THE METHOD OF HAGEDORN AND JENSEN TO THE DETER- MINATION OF LARGER QUANTITIES OF REDUCING SUGARS. By CHARLES SAMUEL HANES (Junior Scholar of the Exhibition of 1851). From the Botany
THE ISOLATION OF A MUCOPOLYSACCHARIDE FROM SYNOVIAL FLUID*
 THE ISOLATION OF A MUCOPOLYSACCHARIDE FROM SYNOVIAL FLUID* BY KARL MEYER, ELIZABETH M. SMYTH, AND MARTIN H. DAWSON (From the Department of Ophthalmology, College of Physicians and Surgeons, Columbia University,
THE ISOLATION OF A MUCOPOLYSACCHARIDE FROM SYNOVIAL FLUID* BY KARL MEYER, ELIZABETH M. SMYTH, AND MARTIN H. DAWSON (From the Department of Ophthalmology, College of Physicians and Surgeons, Columbia University,
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic
 Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
THE ENZYMATIC HYDROLYSIS OF GLUTATHIONE BY RAT KIDNEY
 THE ENZYMATIC HYDROLYSIS OF GLUTATHIONE BY RAT KIDNEY BY E. F. SCHROEDER AND GLADYS E. WOODWARD (From The Biochemical Research Foundation of the Franklin Institute, Philadelphia) (Received for publication,
THE ENZYMATIC HYDROLYSIS OF GLUTATHIONE BY RAT KIDNEY BY E. F. SCHROEDER AND GLADYS E. WOODWARD (From The Biochemical Research Foundation of the Franklin Institute, Philadelphia) (Received for publication,
Phases of the bacterial growth:
 L3: Physiology of Bacteria: Bacterial growth Growth is the orderly increase in the sum of all the components of an organism. Cell multiplication is a consequence of growth, in unicellular organism, growth
L3: Physiology of Bacteria: Bacterial growth Growth is the orderly increase in the sum of all the components of an organism. Cell multiplication is a consequence of growth, in unicellular organism, growth
Biochemistry Name: Practice Questions
 Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Biochemistry - I SPRING Mondays and Wednesdays 9:30-10:45 AM (MR-1307) Lecture 16. Based on Profs. Kevin Gardner & Reza Khayat
 Biochemistry - I Mondays and Wednesdays 9:30-10:45 AM (MR-1307) SPRING 2017 Lecture 16 Based on Profs. Kevin Gardner & Reza Khayat 1 Catabolism of Di- and Polysaccharides Catabolism (digestion) begins
Biochemistry - I Mondays and Wednesdays 9:30-10:45 AM (MR-1307) SPRING 2017 Lecture 16 Based on Profs. Kevin Gardner & Reza Khayat 1 Catabolism of Di- and Polysaccharides Catabolism (digestion) begins
MECHANISM OF INHIBITION OF PHOSPHATASE ACTIVITY BY GLYCINE
 MECHANISM OF INHIBITION OF PHOSPHATASE ACTIVIT B GLCINE B OSCAR BODANSK (From the Department of Pharmacology, Cornell University Medical College, New ork City) (Received for publication, July 11, 1946)
MECHANISM OF INHIBITION OF PHOSPHATASE ACTIVIT B GLCINE B OSCAR BODANSK (From the Department of Pharmacology, Cornell University Medical College, New ork City) (Received for publication, July 11, 1946)
III. Metabolism Glucose Catabolism Part II
 Department of Chemistry and Biochemistry University of Lethbridge III. Metabolism Glucose Catabolism Part II Slide 1 Metabolic Fates of NADH and Pyruvate Cartoon: Fate of pyruvate, the product of glycolysis.
Department of Chemistry and Biochemistry University of Lethbridge III. Metabolism Glucose Catabolism Part II Slide 1 Metabolic Fates of NADH and Pyruvate Cartoon: Fate of pyruvate, the product of glycolysis.
f e c-o- ;,.I ~ 0- ~. /,/ II 4.'" c-o- a::.. ~ \I 1 H~c,-c-c-o- ~
 C) When oxygen is no longer limiting, the lactate that results from lactic acid fermentation is0 cleared from the body. Actually, most ofit is converted back into glucose at the liver. i) n general terms
C) When oxygen is no longer limiting, the lactate that results from lactic acid fermentation is0 cleared from the body. Actually, most ofit is converted back into glucose at the liver. i) n general terms
THE MILK-CLOTTING ACTION OF PAPAIN*
 THE MILK-CLOTTING ACTION OF PAPAIN* BY A. K. BALLS.4ND SAM R. HOOVER (From the Food Research Division, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington) (Received for
THE MILK-CLOTTING ACTION OF PAPAIN* BY A. K. BALLS.4ND SAM R. HOOVER (From the Food Research Division, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington) (Received for
by both esterification and acetylation of the liver concentrate inorganic salts and a source of energy such as glycerol or
 BETA ALANINE AS A GROWTH ACCESSORY FOR THE DIPHTHERIA BACILLUS J. HOWARD MUELLER AND SIDNEY COHEN Department of Bacteriology and Immunology, Harvard University Medical School, Boston, Massachusetts Received
BETA ALANINE AS A GROWTH ACCESSORY FOR THE DIPHTHERIA BACILLUS J. HOWARD MUELLER AND SIDNEY COHEN Department of Bacteriology and Immunology, Harvard University Medical School, Boston, Massachusetts Received
THE SOLUBLE SPECIFIC SUBSTANCE OF A STRAIN OF FRIEDLANDER'S BACILLUS. (From the Hospital of The Rockefeller Institute for Medical Research.
 Published Online: 1 November, 1925 Supp Info: http://doi.org/10.1084/jem.42.5.701 Downloaded from jem.rupress.org on November 20, 2018 THE SOLUBLE SPECIFIC SUBSTANCE OF A STRAIN OF FRIEDLANDER'S BACILLUS.
Published Online: 1 November, 1925 Supp Info: http://doi.org/10.1084/jem.42.5.701 Downloaded from jem.rupress.org on November 20, 2018 THE SOLUBLE SPECIFIC SUBSTANCE OF A STRAIN OF FRIEDLANDER'S BACILLUS.
Soil organic matter composition, decomposition, mineralization and immobilization
 Soil organic matter composition, decomposition, mineralization and immobilization SOIL ORGANIC MATTER Substances containing carbon are organic matter. Soil organic matter consists of decomposing plant
Soil organic matter composition, decomposition, mineralization and immobilization SOIL ORGANIC MATTER Substances containing carbon are organic matter. Soil organic matter consists of decomposing plant
THE COMPOSITION OF FLAXSEED MUCILAGE*
 THE COMPOSITION OF FLAXSEED MUCILAGE* BY ERNEST ANDERSON AND HARRY J. LOWE (From the University of Arizona, Tucson) (Received for publication, January 20,1947) Flaxseed mucilage contains d-galacturonic
THE COMPOSITION OF FLAXSEED MUCILAGE* BY ERNEST ANDERSON AND HARRY J. LOWE (From the University of Arizona, Tucson) (Received for publication, January 20,1947) Flaxseed mucilage contains d-galacturonic
THE SPARING ACTION OF FAT ON VITAMIN B
 THE SPARING ACTION OF FAT ON VITAMIN B VI. THE INFLUENCE OF THE LEVELS OF PROTEIN AND VITAMIN G BY HERBERT M. EVANS, SAMUEL LEPKOVSKY, AND ELIZABETH A. MURPHY (From the Institute of Experimental Biology,
THE SPARING ACTION OF FAT ON VITAMIN B VI. THE INFLUENCE OF THE LEVELS OF PROTEIN AND VITAMIN G BY HERBERT M. EVANS, SAMUEL LEPKOVSKY, AND ELIZABETH A. MURPHY (From the Institute of Experimental Biology,
NATIONAL ACADEMY OF SCIENCES Volume 31 December Number 12
 PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES Volume 31 December 15. 1945 Number 12 Copyright 1946 by the National Academy of Sciences THE SYNTHESIS OF BUTYRIC AND CAPROIC ACIDS FROM ETHANOL AND ACETIC
PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES Volume 31 December 15. 1945 Number 12 Copyright 1946 by the National Academy of Sciences THE SYNTHESIS OF BUTYRIC AND CAPROIC ACIDS FROM ETHANOL AND ACETIC
THE OXIDATION OF LACTOSE AND MALTOSE TO BIONIC ACIDS BY PSEUDOMONAS*
 THE OXIDATION OF LACTOSE AND MALTOSE TO BIONIC ACIDS BY PSEUDOMONAS* BY FRANK H. STODOLA AND LEWIS B. LOCKWOOD (From the Fermentation Division, Northern Regional Research Laboratory,t Peoria, Illinois)
THE OXIDATION OF LACTOSE AND MALTOSE TO BIONIC ACIDS BY PSEUDOMONAS* BY FRANK H. STODOLA AND LEWIS B. LOCKWOOD (From the Fermentation Division, Northern Regional Research Laboratory,t Peoria, Illinois)
A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* Previous studies (1, 2) have shown that after the ingestion of caffeine
 A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* BY HERBERT H. CORNISH AND A. A. CHRISTMAN (From the Department of Biological Chemistry, Medical School, University of Michigan,
A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* BY HERBERT H. CORNISH AND A. A. CHRISTMAN (From the Department of Biological Chemistry, Medical School, University of Michigan,
CHEMISTRY OF LIFE 05 FEBRUARY 2014
 CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED TRYPSIN
 Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
CHARACTERISTICS OF CELLOBIOSE PHOSPHORYLASE
 CHARACTERISTICS OF CELLOBIOSE PHOSPHORYLASE JAMES K. ALEXANDER' Department of Botany and Bacteriology, Montana State College, Bozemian, Montana Received for publication November 22, 1960 The phosphorolysis
CHARACTERISTICS OF CELLOBIOSE PHOSPHORYLASE JAMES K. ALEXANDER' Department of Botany and Bacteriology, Montana State College, Bozemian, Montana Received for publication November 22, 1960 The phosphorolysis
THE CARBOHYDRATE METABOLISM OF TUMORS.
 THE CARBOHYDRATE METABOLISM OF TUMORS. II. CHANGES IN THE SUGAR, LACTIC ACID, AND CO COMBINING POWER OF BLOOD PASSING THROUGH A TUMOR. BY CARL F. CORI AND GERTY T. CORI. (From the State Institute for ihe
THE CARBOHYDRATE METABOLISM OF TUMORS. II. CHANGES IN THE SUGAR, LACTIC ACID, AND CO COMBINING POWER OF BLOOD PASSING THROUGH A TUMOR. BY CARL F. CORI AND GERTY T. CORI. (From the State Institute for ihe
ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA
 J. Gen. App!. Microbiol., 34, 213-219 (1988) ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA TOSHIRO HAYASHI, RYO IOROI,*
J. Gen. App!. Microbiol., 34, 213-219 (1988) ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA TOSHIRO HAYASHI, RYO IOROI,*
GLUCONATE OXIDATION BY PSEUDOMONAS FLUORESCENS
 GLUCONATE OXIDATION BY PSEUDOMONAS FLUORESCENS BY H. J. KOEPSELL (89om the Northern Regional Research Laboratory,t Peoria, Illinois) (Received for publication, March 25, 1950) The oxidation of glucose
GLUCONATE OXIDATION BY PSEUDOMONAS FLUORESCENS BY H. J. KOEPSELL (89om the Northern Regional Research Laboratory,t Peoria, Illinois) (Received for publication, March 25, 1950) The oxidation of glucose
THE ASSIMILATION OF AMMONIA NITROGEN BY THE TOBACCO PLANT: A PRELIMINARY STUDY WITH ISOTOPIC NITROGEN. (Received for publication, July 3, 1940)
 THE ASSIMILATION OF AMMONIA NITROGEN BY THE TOBACCO PLANT: A PRELIMINARY STUDY WITH ISOTOPIC NITROGEN BY HUBERT BRADFORD VICKERY AND GEORGE W. PUCHER (Prom the Biochemical Laboratory of the Connecticut
THE ASSIMILATION OF AMMONIA NITROGEN BY THE TOBACCO PLANT: A PRELIMINARY STUDY WITH ISOTOPIC NITROGEN BY HUBERT BRADFORD VICKERY AND GEORGE W. PUCHER (Prom the Biochemical Laboratory of the Connecticut
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Which of the following statements concerning anabolic reactions is FALSE? A. They are generally endergonic. B. They usually require ATP. C. They are part of metabolism. D.
MULTIPLE CHOICE QUESTIONS 1. Which of the following statements concerning anabolic reactions is FALSE? A. They are generally endergonic. B. They usually require ATP. C. They are part of metabolism. D.
I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy
 Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Mechanism of Salivary Amylase Action
 Proceedings of the Iowa Academy of Science Volume 57 Annual Issue Article 22 1950 Mechanism of Salivary Amylase Action John H. Pazur Iowa State College Dexter French Iowa State College Doris W. Knapp Iowa
Proceedings of the Iowa Academy of Science Volume 57 Annual Issue Article 22 1950 Mechanism of Salivary Amylase Action John H. Pazur Iowa State College Dexter French Iowa State College Doris W. Knapp Iowa
RICINOLEATE UPON BACTERIA
 A COMPARATIVE STUDY OF THE ACTION OF SODIUM RICINOLEATE UPON BACTERIA From the Division of Laboratories and Research, New York State Department of Health, Albany Received for publication, May 14, 1928
A COMPARATIVE STUDY OF THE ACTION OF SODIUM RICINOLEATE UPON BACTERIA From the Division of Laboratories and Research, New York State Department of Health, Albany Received for publication, May 14, 1928
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
colorimetrically by the methylene blue method according to Fogo and manometrically. In the presence of excess sulfur the amount of oxygen taken up
 GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
Marah Bitar. Bayan Abusheikha ... Anas Abu-Humaidan
 5 Marah Bitar Bayan Abusheikha... Anas Abu-Humaidan Bacterial Metabolism -Metabolism has two components, catabolism and anabolism. -Catabolism encompasses processes that harvest energy released from the
5 Marah Bitar Bayan Abusheikha... Anas Abu-Humaidan Bacterial Metabolism -Metabolism has two components, catabolism and anabolism. -Catabolism encompasses processes that harvest energy released from the
FATTY ACID METABOLISM
 FATTY ACID METABOLISM III. REACTIONS OF CARBOXYL-LABELED ACETIC ACID IN LIVER AND KIDKEY* BY SIDNEY WEINHOUSE, GRACE MEDES, AND NORMAN F. FLOYD (From the Lankenau Hospital Research Institute, Philadelphia)
FATTY ACID METABOLISM III. REACTIONS OF CARBOXYL-LABELED ACETIC ACID IN LIVER AND KIDKEY* BY SIDNEY WEINHOUSE, GRACE MEDES, AND NORMAN F. FLOYD (From the Lankenau Hospital Research Institute, Philadelphia)
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
FUNCTION OF PYRIDOXAL PHOSPHATE: RESOLUTION AND PURIFICATION OF THE TRYPTOPHANASE ENZYME OF ESCHERICHIA COLI
 FUNCTION OF PYRIDOXAL PHOSPHATE: RESOLUTION AND PURIFICATION OF THE TRYPTOPHANASE ENZYME OF ESCHERICHIA COLI BY W. A. WOOD,* I. c. GUNSALUS, AND W. W. UMBREIT (From the Laboratory of Bacteriology, College
FUNCTION OF PYRIDOXAL PHOSPHATE: RESOLUTION AND PURIFICATION OF THE TRYPTOPHANASE ENZYME OF ESCHERICHIA COLI BY W. A. WOOD,* I. c. GUNSALUS, AND W. W. UMBREIT (From the Laboratory of Bacteriology, College
rotary shaker at approximately 25 C. When incubation at 30 C was desired, the flasks were at 10,000 RPM for 5 min, washing the cells
 SOME ASPECTS OF THE INDUCED BIOSYNTHESIS OF ALPHA-AMYLASE OF PSEUDOMONAS SACCHAROPHILA1 ALVIN MARKOVITZ2 AND HAROLD P. KLEIN' Department of Microbiology, School of Medicine, University of Washington, Seattle,
SOME ASPECTS OF THE INDUCED BIOSYNTHESIS OF ALPHA-AMYLASE OF PSEUDOMONAS SACCHAROPHILA1 ALVIN MARKOVITZ2 AND HAROLD P. KLEIN' Department of Microbiology, School of Medicine, University of Washington, Seattle,
Metabolism. Learning objectives are to gain an appreciation of: Part II: Respiration
 Metabolism Part I: Fermentations ti Part II: Respiration Learning objectives are to gain an appreciation of: Catabolism and anabolism ATP Generation and energy conservation Fermentation 1 Importance of
Metabolism Part I: Fermentations ti Part II: Respiration Learning objectives are to gain an appreciation of: Catabolism and anabolism ATP Generation and energy conservation Fermentation 1 Importance of
STUDIES ON GLUTELINS. (Received for publication, March 2, 1927.)
 STUDIES ON GLUTELINS. I. THE 01- AND,8-GLUTELINS OF WHEAT (TRITICUM VULGARE).* BY FRANK A. CSONKA AND D. BREESE JONES. (From the Protein Investigation Laboratory, Bureau of Chemistry, United States Department
STUDIES ON GLUTELINS. I. THE 01- AND,8-GLUTELINS OF WHEAT (TRITICUM VULGARE).* BY FRANK A. CSONKA AND D. BREESE JONES. (From the Protein Investigation Laboratory, Bureau of Chemistry, United States Department
What are the most common elements in living organisms? What is the difference between monomers, dimers and polymers?
 What do each of these terms mean? Atom Molecule Element Compound Organic Inorganic What are the most common elements in living organisms? What are the roles of magnesium, iron, phosphate and calcium in
What do each of these terms mean? Atom Molecule Element Compound Organic Inorganic What are the most common elements in living organisms? What are the roles of magnesium, iron, phosphate and calcium in
FURTHER STUDIES UPON THE PURIFICATION AND PROPERTIES OF MALT AMYLASE
 FURTHER STUDIES UPON THE PURIFICATION AND PROPERTIES OF MALT AMYLASE BY H. C. SHERMAN, M. L. CALDWELL, AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for
FURTHER STUDIES UPON THE PURIFICATION AND PROPERTIES OF MALT AMYLASE BY H. C. SHERMAN, M. L. CALDWELL, AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for
Introduction to Microbiology BIOL 220, Summer Session 1, 1996 Exam # 2
 Name I. Multiple Choice (1 point each) Introduction to Microbiology BIOL 220, Summer Session 1, 1996 Exam # 2 D 1. Which transport process requires energy? A. Osmosis C. Diffusion B. Facilitated diffusion
Name I. Multiple Choice (1 point each) Introduction to Microbiology BIOL 220, Summer Session 1, 1996 Exam # 2 D 1. Which transport process requires energy? A. Osmosis C. Diffusion B. Facilitated diffusion
A modification of Fiske and Subbarow's method for determination of phosphocreatine 1,
 A modification of Fiske and Subbarow's method for determination of phosphocreatine 1, By N.-O. Abdon (Lund) and Erik Jacobsen (Copenhagen). (From the Pharmacological Department, University of Lund, Sweden.)
A modification of Fiske and Subbarow's method for determination of phosphocreatine 1, By N.-O. Abdon (Lund) and Erik Jacobsen (Copenhagen). (From the Pharmacological Department, University of Lund, Sweden.)
points raised, and the following is an account of what I have done under touched, but my work has fallen under two main heads:
 NOTES ON CREATININE. BY P. C. COLLS, late Assistant Demonstrator in Physiology, King's College, London. (From the Physiological Laboratory, King's College, London.) ABOUT two years ago, a lengthy correspondence
NOTES ON CREATININE. BY P. C. COLLS, late Assistant Demonstrator in Physiology, King's College, London. (From the Physiological Laboratory, King's College, London.) ABOUT two years ago, a lengthy correspondence
Chemical Formulas. Chemical Formula CH 3 COCHCHOCHClCHNH Lewis Dot Structure
 Biochemistry . Chemical Formulas A chemical formula represents the chemical makeup of a compound. It shows the numbers and kinds of atoms present in a compound. It is a kind of shorthand that scientists
Biochemistry . Chemical Formulas A chemical formula represents the chemical makeup of a compound. It shows the numbers and kinds of atoms present in a compound. It is a kind of shorthand that scientists
ELECTROPHORETIC STUDIES OF SONIC EXTRACTS OF PROTEUS VULGARIS
 ELECTROPHORETIC STUDIES OF SONIC EXTRACTS OF PROTEUS VULGARIS I. EFFECT OF GROWTH ENVIRONMENT ON ELECTROPHORETIC PATTERNS' SIDNEY D. RODENBERG Laboratory of Microbiology, Division of Biology, University
ELECTROPHORETIC STUDIES OF SONIC EXTRACTS OF PROTEUS VULGARIS I. EFFECT OF GROWTH ENVIRONMENT ON ELECTROPHORETIC PATTERNS' SIDNEY D. RODENBERG Laboratory of Microbiology, Division of Biology, University
Consequently, the authors decided to investigate the various A STUDY OF METHODS FOR THE DETERMINATION OF
 A STUDY OF METHODS FOR THE DETERMINATION OF REDUCING SUGARS IN BACTERIAL CULTURES COLORIMETRIC METHODS DOROTHEA KLEMME AND CHARLES F. POE Division of Sanitary Chemistry, Department of Chemistry, University
A STUDY OF METHODS FOR THE DETERMINATION OF REDUCING SUGARS IN BACTERIAL CULTURES COLORIMETRIC METHODS DOROTHEA KLEMME AND CHARLES F. POE Division of Sanitary Chemistry, Department of Chemistry, University
LAB 6 Fermentation & Cellular Respiration
 LAB 6 Fermentation & Cellular Respiration INTRODUCTION The cells of all living organisms require energy to keep themselves alive and fulfilling their roles. Where does this energy come from? The answer
LAB 6 Fermentation & Cellular Respiration INTRODUCTION The cells of all living organisms require energy to keep themselves alive and fulfilling their roles. Where does this energy come from? The answer
Ch13. Sugars. What biology does with monosaccharides disaccharides and polysaccharides. version 1.0
 Ch13 Sugars What biology does with monosaccharides disaccharides and polysaccharides. version 1.0 Nick DeMello, PhD. 2007-2015 Ch13 Sugars Haworth Structures Saccharides can form rings. That creates a
Ch13 Sugars What biology does with monosaccharides disaccharides and polysaccharides. version 1.0 Nick DeMello, PhD. 2007-2015 Ch13 Sugars Haworth Structures Saccharides can form rings. That creates a
Experiment 20 Identification of Some Carbohydrates
 Experiment 20 Identification of Some arbohydrates arbohydrates are the direct product of the photosynthetic combination of carbon dioxide and water. By weight, they are the most common organic compounds
Experiment 20 Identification of Some arbohydrates arbohydrates are the direct product of the photosynthetic combination of carbon dioxide and water. By weight, they are the most common organic compounds
Carbohydrate Metabolism I
 Carbohydrate Metabolism I Outline Glycolysis Stages of glycolysis Regulation of Glycolysis Carbohydrate Metabolism Overview Enzyme Classification Dehydrogenase - oxidizes substrate using cofactors as
Carbohydrate Metabolism I Outline Glycolysis Stages of glycolysis Regulation of Glycolysis Carbohydrate Metabolism Overview Enzyme Classification Dehydrogenase - oxidizes substrate using cofactors as
THE ISOLATION AND CHARACTERIZATION OF A STARCH POLYSACCHARIDE FROM THE LEAF TISSUE OF THE APPLE TREE (MALUS MALUS)
 THE ISOLATION AND CHARACTERIZATION OF A STARCH POLYSACCHARIDE FROM THE LEAF TISSUE OF THE APPLE TREE (MALUS MALUS) BY CARL NIEMANN, ARTHUR B. ANDERSON, AND KARL PAUL LINK (From the Biochemistry Research
THE ISOLATION AND CHARACTERIZATION OF A STARCH POLYSACCHARIDE FROM THE LEAF TISSUE OF THE APPLE TREE (MALUS MALUS) BY CARL NIEMANN, ARTHUR B. ANDERSON, AND KARL PAUL LINK (From the Biochemistry Research
4.1. Components of sweet sorghum stem juice
 4.1. Components of sweet sorghum stem juice The stem juice of sweet sorghum is rich in fermentative sugar and is a desirable alcoholic fermentation material. It is difficult to measure the juice Sugar
4.1. Components of sweet sorghum stem juice The stem juice of sweet sorghum is rich in fermentative sugar and is a desirable alcoholic fermentation material. It is difficult to measure the juice Sugar
Chapter 5 MITOCHONDRIA AND RESPIRATION 5-1
 Chapter 5 MITOCHONDRIA AND RESPIRATION All organisms must transform energy. This energy is required to maintain a dynamic steady state, homeostasis, and to insure continued survival. As will be discussed
Chapter 5 MITOCHONDRIA AND RESPIRATION All organisms must transform energy. This energy is required to maintain a dynamic steady state, homeostasis, and to insure continued survival. As will be discussed
9. At about 0 C., most enzymes are (1.) inactive (2.) active (3.) destroyed (4.) replicated
 Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION
 THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION BY ROBERT K. CRANE* AND FRITZ LIPMANN (From the Biochemical Research Laboratory, Massachusetts General Hospital, and the Department of Biological Chemistry,
THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION BY ROBERT K. CRANE* AND FRITZ LIPMANN (From the Biochemical Research Laboratory, Massachusetts General Hospital, and the Department of Biological Chemistry,
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP
 CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
Anaerobic Respiration
 Anaerobic Respiration Inquire: Fermentation Overview As was previously stated, cellular respiration can yield 36-38 ATP molecules under aerobic conditions. If oxygen is not present, ATP is only produced
Anaerobic Respiration Inquire: Fermentation Overview As was previously stated, cellular respiration can yield 36-38 ATP molecules under aerobic conditions. If oxygen is not present, ATP is only produced
FORMATION OF LACTIC ACID, AN INITIAL PROCESS IN WORKING MUSCLE*
 FORMATION OF LACTIC ACID, AN INITIAL PROCESS IN WORKING MUSCLE* BY EUNICE V FLOCK, DWIGHT J INGLE, AND JESSE L BOLLMAN (From the Division of Experimental Medicine, The Mayo Foundation, Rochester, Minnesota)
FORMATION OF LACTIC ACID, AN INITIAL PROCESS IN WORKING MUSCLE* BY EUNICE V FLOCK, DWIGHT J INGLE, AND JESSE L BOLLMAN (From the Division of Experimental Medicine, The Mayo Foundation, Rochester, Minnesota)
CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS IV. Tm~ SX~TI~SlS of Tm~ p-amn~obenzx~ ETm~R OF THE SOLUBLE
 Published Online: 1 September, 1931 Supp Info: http://doi.org/10.1084/jem.54.3.431 Downloaded from jem.rupress.org on October 31, 2018 CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS IV.
Published Online: 1 September, 1931 Supp Info: http://doi.org/10.1084/jem.54.3.431 Downloaded from jem.rupress.org on October 31, 2018 CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS IV.
EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY
 J. Gen. App!. Microbiol,, 21, 51-59 (1975) EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY CLOSTRIDIUM ACETOBUTYLICUM BURT ENSLEY, JOHN J. McHUGH, AND LARRY L. BARTON Department
J. Gen. App!. Microbiol,, 21, 51-59 (1975) EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY CLOSTRIDIUM ACETOBUTYLICUM BURT ENSLEY, JOHN J. McHUGH, AND LARRY L. BARTON Department
Chapter 18: Carbohydrate Metabolism
 Vocabulary Biotin: a CO2 carrier molecule Cori Cycle: a pathway in carbohydrate metabolism that links glycolysis in the liver with gluconeogenesis in the liver Debranching Enzyme: an enzyme that hydrolyzes
Vocabulary Biotin: a CO2 carrier molecule Cori Cycle: a pathway in carbohydrate metabolism that links glycolysis in the liver with gluconeogenesis in the liver Debranching Enzyme: an enzyme that hydrolyzes
This place covers: Reducing the size of material from which sugars are to be extracted; Presses and knives therefor,
 CPC - C13B - 2017.08 C13B PRODUCTION OF SUCROSE; APPARATUS SPECIALLY ADAPTED THEREFOR (chemically synthesised sugars or sugar derivatives C07H; fermentation or enzyme-using processes for preparing compounds
CPC - C13B - 2017.08 C13B PRODUCTION OF SUCROSE; APPARATUS SPECIALLY ADAPTED THEREFOR (chemically synthesised sugars or sugar derivatives C07H; fermentation or enzyme-using processes for preparing compounds
LOCATION AND POSSIBLE ROLE OF ESTERIFIED PHOSPHORUS IN STARCH FRACTIONS 1
 LOCATION AND POSSIBLE ROLE OF ESTERIFIED PHOSPHORUS IN STARCH FRACTIONS 1 ABSTRACT Phosphorus determinations were made on various amelose and pectin fractions by a modified technique. Results were reproducible
LOCATION AND POSSIBLE ROLE OF ESTERIFIED PHOSPHORUS IN STARCH FRACTIONS 1 ABSTRACT Phosphorus determinations were made on various amelose and pectin fractions by a modified technique. Results were reproducible
