CHARACTERISTICS OF CELLOBIOSE PHOSPHORYLASE
|
|
|
- Belinda Cummings
- 5 years ago
- Views:
Transcription
1 CHARACTERISTICS OF CELLOBIOSE PHOSPHORYLASE JAMES K. ALEXANDER' Department of Botany and Bacteriology, Montana State College, Bozemian, Montana Received for publication November 22, 1960 The phosphorolysis of cellobiose was first demonstrated by Sih and McBee (1955a, b). These workers found that cellobiose phosphorylase was present in cell-free extracts of Clostridium thermocellum. Recently cellobiose phosphorylase has been reported from two other bacteria, Ruminococcus flavefaciens (Ayers, 1958, 1959), and Cellvibrio gilvus (Hulcher and King, 1958). This enzyme also catalyzes the synthesis of cellobiose froin a-d-glucose 1-phosphate and glucose (Sih, Nelson, and McBee, 1957; Ayers, 1959). In the present work, the stoichiometry of the reaction and the effect of physical and chemical agents on the activity of the enzyme were studied. Studies on the specificity of the enzyme revealed that D-glucose, D-xylose, L-xylose, 2-deoxyglucose, D-glucosamine, arsenate, and inorganic phosphate can act as substrates. Evidence has been obtained which indicates that the reaction proceeds by a single displacement mechanism. A preliminary report of this work has been published (Alexander, 1958). MATERIALS AND METHODS Glucose 6-phosphate, a-glucose 1-phosphate, 2-deoxyglucose, D-ribose, D-gluconate, A-glueonolactone, N-acetylglucosamine, D-glucosamine, L-arabinose, and lactose were obtained from Nutritional Biochemicals Corporation. Cellobiose was a product of Krishell Laboratories. D-Xylose, L-xylose, D-mannose, L-fructose, D- sorbitol, D-galactose, salicin, and trehalose were obtained from Difco Laboratories. Glucose was obtained from Allied Chemical and Dye Corporation; glueuronate from Sigma Chemical Company; gentiobiose from General Biochemicals Incorporated; and maltose from Pfanstiehl Chemical Company. 3-Glucose 1-phosphate was generously supplied by Dr. Charlotte Fitting. 1 Present address: Department of Biological Chemistry, Hahnemann Medical College, Philadelphia, Pennsylvania. Glucose oxidase was obtained from the Takamine Company. C. thermocellum strain 651 was used in this work. The organism was grown in a modified medium of McBee (1948) containing the following percentage composition: NaCl 0.3; (NH4)2- SO4, 0.1; K2HPO4, 0.05; KH2PO4, 0.05; MgSO4. 7H20, 0.01; CaCl2-2H20, 0.001; sodium thioglycolate, 0.02; yeast extract, 0.1; cellulose, 0.25; and NaHCO3, 0.5. To satisfy the anaerobic requirements of the organism, oxygen-free conditions were obtained by flushing the media with an inert gas (Hungate, 1950). Approximately 1 g (wet weight) of cells per liter was obtained in this medium. Cell-free extracts were prepared by grinding cells with glass beads in a Waring Blendor which was jacketed with an ice bath (Lamanna and Mallette, 1954). About 15 to 30 g of wet cells, suspended in 7 to 15 ml of water, and 20 to 40 g of glass beads were ground in a blender for 15 to 30 min. After grinding, 20 to 40 ml of water were added. Mixing was resumed until an even suspension was obtained and then the material was centrifuged for 1 hr at approximately 6,000 X g. The supernatant fluid served as the crude enzyme. The crude enzyme preparation was partially purified by (NH4)2S04 fractionation. To 8 ml of the crude preparation 3 ml of cold saturated (NH4)2S04 solution were added and this portion was allowed to stand 1 hr. After centrifugation at 6,000 X g for 1 hr the precipitate was discarded and 11 ml of saturated (NH4)2S04 solution were added to the supernatant. This portion was allowed to stand 1 hr and then it was centrifuged for 1 hr at 6,000 X g. The precipitate, containing cellobiose phosphorylase, was dissolved in water and diluted to 8 ml. This entire purification procedure was repeated two times. All steps in the purification were carried out at 5 C without adjusting the ph of the ammonium sulfate solution. Phosphoglucomutase, 903
2 904 ALEXANDER [VOL. 81 the only known interfering enzyme, was eliminated by this procedure (phosphoglucomutase activity was determined by measuring the disappearance in acid labile phosphate). Cellobiose or glucose 1-phosphatase activity could not be detected in either the crude or the purified preparations. Thus, the purified preparations could be used to study the cellobiose phosphorylase reaction in the absence of known interfering reactions. The purified enzyme preparations were used throughout this study. The ph of the reaction mixtures was 7.0; the incubation temperature 37 C. The reaction mixtures were buffered with barbital-acetate buffer (Michaelis, 1931). Analytical procedures. Cellobiose phosphorylase activity was determined by measuring the change in inorganic phosphate when the enzyme was incubated in the presence of either cellobiose and phosphate, or glucose and glucose 1-phosphate. Samples of the reaction mixtures were removed at various time intervals and the enzyme was inactivated by boiling for 3 min. After boiling, the protein was precipitated with either 5% trichloroacetic acid or N HCl. Phosphate was determined by the method of Fiske and SubbaRow (1925). Glucose 1- phosphate was estimated by measuring the increase in inorganic phosphate after hydrolysis in N HCI for 7 min at 100 C. Glucose was determined manometrically as described by Keilin and Hartree (1948). The crude glucose oxidase preparations used in the glucose determinations exhibited a small amount of f3-glucosidase activity. The error introduced in these determinations due to the hydrolysis of cellobiose was minimized by including two standards in each manometric analysis. One standard consisted of a known amount of glucose and the other contained glucose, cellobiose, glucose 1-phosphate, and phosphate in approximately the same amounts as the unknown sample. Cellobiose was hydrolyzed with,3-glucosidase and the increase in free glucose was determined manometrically. An active,b-glucosidase was precipitated from the crude glucose oxidase preparation with 50% acetone in the cold (a heat stable g-glucosidase inhibitor was recovered in the supernatant). The acetone-precipitated preparations retained glucose oxidase activity and hydrolyzed cellobiose about five times as fast as did the crude preparations. The hydrolysis of cellobiose proceeded at a slower rate than the oxidation of glucose. Extending the determination period to 3 to 4 hr, however, permitted complete hydrolysis of cellobiose. Standards similar to those described above were included in each analysis. Chromatography of sugars was carried out in the manner described by Sih et al. (1957). The amount of protein in the enzyme preparations was estimated by biuret method of Levin and Brauer (1951). RESULTS Stoichiometry and equilibrium of reaction. When cellobiose phosphorylase was incubated with cellobiose and phosphate, equimolar quantities of glucose and glucose 1-phosphate were formed (Table 1). The molar quantities of glucose and glucose 1-phosphate formed were approximately equal to the amounts of cellobiose and phosphate TABLE 1 Analysis of products and reactants phosphorylase reaction of cellobiose Expt No. 1 Expt No. 2 Expt No. 3 Initial Final Initial Final Initial Final,umoles/ml umoles/nl pimoles/ml Cellobiose Phosphate Glucose 1- phosphate Glucose K The reaction mixtures were incubated at 37 C. The ph of the reaction mixtures was 7.0. In experiment no. 1, the reaction mixture contained 4.0 ml enzyme (28 mg protein), 1.2 ml 0.1 M cellobiose, 0.8 ml 0.1 M phosphate, 2.0 ml 0.03 M barbital-acetate buffer, and was incubated for 80 min. In experiment no. 2, the reaction mixture contained 2.0 ml enzyme (20 mg protein), 0.8 ml M glucose 1-phosphate, 0.8 ml 0.1 M glucose, 4.0 ml 0.03 M barbital-acetate buffer, 0.4 ml water, and was incubated for 75 min. In experiment no. 3, the reaction mixture contained 1.0 ml enzyme (10 mg protein), 0.6 ml 0.1 M cellobiose, 0.6 ml 0.1 M phosphate, 0.2 ml M glucose 1-phosphate, 0.6 ml 0.1 M glucose, 1.0 ml 0.03 M barbitalacetate buffer, 4.0 ml water, and was incubated for 30 min.
3 19611 CELLOBIOSE PHOSPHORYLASE 905 utilized. When cellobiose phosphorylase was incubated with glucose and glucose 1-phosphate, the molar quantities of cellobiose and phosphate formed were equal to the molar quantities of reactants utilized. The equilibrium constant, K = (cellobiose)- (phosphate)/(glucose)(glucose 1-phosphate), is about 4.3 (Table 1). In these experiments, the activity was followed by measuring the change in phosphate at 10- to 20-min intervals, until no further change was detected. Since nearly the same values were obtained from either side, it appears that these values approximate the true equilibrium constant of the reaction. Effect of hydrogen ion concentration. The optimal ph for the activity of cellobiose phosphorylase is about 7.0, in M barbital-acetate buffer. The enzyme is active over quite a wide range; activity has been detected at ph 4.6 and at ph 8.1. No activity was observed, however, at ph 4.1 or at ph 8.7. TABLE 2 Effect of inhibitors on the activity phosphorylase of cellobiose Addition Per Cent Inhibition p-chloromercuribenzoate, 1 X 10-M.42 p-chloromercuribenzoate, 2 X 10l- 100 p-chloromercuribenzoate, 1 X 10-6 M; cysteine, 1 X 10-4 M... 7 p-chloromercuribenzoate, 2 X 10-5 M; cysteine, 1 X 10-4 M AgNO3, 1 X AgNO3, 1 X 10-4 M; cysteine, 1 X 104 M. 91 AgNO3, 1 X 104 M; cysteine, 1 X 10-2 M.. 0 Phlorizin, 1.25 X 10-3 M... 8 Phlorizin, 2.5 X 10-3 M Phlorizin, 5.0 X 103 M NaF, 0.1 M 21 NaF, The reaction mixtures were incubated at 37 C for 1 hr. Each of the reaction mixtures contained 0.5 ml enzyme (4 mg protein), 0.3 ml 0.1 M cellobiose, 0.2 ml 0.1 M phosphate, 0.2 ml 0.03 M barbital-acetate buffer. The total volume was made up to 2.0 ml with water, cysteine (0.02 ml (0.1 M) or 0.2 ml (0.001 M)), or inhibitor. The amounts of the various inhibitors added were: 0.2 or 0.4 ml 1 X 10-4 M p-chloromercuribenzoate; 0.2 ml M AgNO3; 0.2, 0.4, or 0.8 ml M phlorizin; and 0.4 or 0.8 ml 0.5 M NaF. TABLE 3 Effect of glucose on cellobiose phosphorolysis Cellobiose Additions Glucose Phosphate Esterified Per Cent Inhibition jsmoles/ml pmoles/ml The reaction mixtures were incubated for 20 min at 37 C. The reaction mixtures contained 0.2 ml enzyme (3 mg protein); 0.075, 0.15, or 0.3 ml 0.1 M cellobiose; ml 0.1 M phosphate; or 0.15 ml 0.1 M glucose; ml 0.01 M ethylenediamine-tetraacetic acid (EDTA), and were diluted to 1.0 ml with water. EDTA was used in certain experiments after it was learned that the enzyme was sensitive to sulfhydryl reagents. Since no increased activity was observed with EDTA, it was omitted from some of the experiments. Inhibitors. Cellobiose phosphorylase activity was completely inhibited by 2 X 10-5 M p- chloromercuribenzoate, whereas 1 X 10-5 M p-chloromercuribenzoate caused about 40% inhibition (Table 2). The inhibition could be partially reversed by 1 X 10-4 M cysteine. Silver nitrate (1 X 10-4 M) also inhibited cellobiose phosphorylase activity. This inhibition was completely reversed by 1 X 102 M cysteine. These data suggest that the enzyme has an essential sulfhydryl group. Inhibition of cellobiose phosphorylase by phlorizin was directly proportional to the concentration of phlorizin between and M. About 20% of the activity is inhibited by 0.1 M NaF, whereas 0.2 M NaF almost completely inhibited activity. The same concentrations (0.1 and 0.2 M) of NaCl or (NH4)2SO4 caused little or no inhibition. Magnesium sulfate in concentrations of 0.01 or 0.02 M had no significant effect on the activity. The inhibition of phosphorolysis by glucose is shown in Table 3. Further study will be needed to determine the nature of glucose inhibition.
4 906 ALEXANDER [VOL. 81 Specificity of cellobiose phosphorylase. When cellobiose phosphorylase was incubated with glucose 1-phosphate and D-xylose, L-xylose, D-glucosamine, 2-deoxyglucose, or glucose, phosphate was liberated as shown in Table 4. The liberation of phospnate suggests that di- TABLE 4 Liberation of inorganic phosphate when cellobiose phosphorylase was incubated with glucose 1-phosphate and various other compounds Expt No. Compound Added Phosphate Liberated I hr 2 hr Amoles/ml 1 None 0 D-Glucose D-Xylose L-Xylose Deoxyglucose D-Gluconate Glucuronate Glucose 6-phosphate D-Glucose D-Ribose D-Sorbitol D-Glucose A-gluconolactone D-Glucosamine N-Acetylglucosamine D-Glucose L-Arabinose 0 0 L-Fructose 0 0 D-Galactose 0 0 D-Mannose The reaction mixtures contained 0.5 ml enzyme (6 to 8 mg protein) 0.3 ml 0.1 M monosaccharide, 0.2 ml M glucose 1-phosphate, 0.2 ml 0.01 M EDTA, and 0.8 ml water. saccharides are synthesized according to reactions 1 to 5 (see below). The identity of these compounds has not been proved. The argument for suggesting a 3-1,2- glucosidic linkage in the L-xylose-containing disaccharide is presented below. Paper chromatograms of the reaction mixtures containing D-xylose or L-xylose likewise suggest that pentose-containing disaccharides had been synthesized (Fig. 1). After the chromatograms were dipped into an aniline-phthalic acid color developer and allowed to stand at room temperature, red spots developed at the positions of the pentoses and the presumed pentose-containing disaccharides, but no color was present in the cellobiose position. Cellobiose did not develop a color until after the chromatograms were heated. The color of the pentoses and pentosecontaining disaccharides turned reddish-brown upon heating, in contrast to the plain brown color of cellobiose. No attempts were made to obtain chromatographic separation of the disaccharides synthesized with D-glucosamine or 2- deoxyglucose. No significant amount of phosphate was liberated when cellobiose phosphorylase was incubated with glucose 1-phosphate and D- gluconate, D-glucuronate, D-glucose 6-phosphate, D-ribose, D-sorbitol, 6-gluconolactone, N-acetylglucosamine, L-arabinose, L-fructose, D-galactose, or D-mannose (Table 4). These compounds apparently are unable to act as glucosyl acceptors. Phosphorolysis of disaccharides. Cellobiosephosphorylase was unable to catalyze the phosphorolysis of gentiobiose, salicin, lactose, or maltose, for no significant amount of phosphate disappeared when the enzyme was incubated with phosphate and these sugars. In contrast to the results with these sugars, relatively large amounts of phosphate disappeared when the enzyme was incubated with cellobiose and phosphate. The enzyme presumably would D-Glucose + glucose 1 phosphate = 4-0-,3-D-glucopyranosyl-D-glucopyranose (cellobiose) + phosphate (1) D-Xylose + glucose 1 phosphate = D-glucopyranosyl-D-xylopyranose + phosphate (2) L-Xylose + glucose 1 phosphate = 2-0-f3-D-glucopyranosyl-L-xylopyranose + phosphate (3) 2-Deoxyglucose + glucose 1 phosphate = 4-O-$-D-glucopyranosyl-D-deoxyglucose + phosphate (4) D-Glucosamine + glucose 1 phosphate -, 4-0-,B-D-glucopyranosyl-D-glucosamine + phosphate (5)
5 w... ^....P': :;.0. :.. '' : 1961] CELLOBIOSE PHOSPHORYLASE 907 that an inversion occurred in the reaction (Sih et al., 1957; Ayers, 1959). The occurrence of an inversion is further supported by the finding that the enzyme is specific for a-glucose 1- phosphate and it is unable to act on,8-glucose 1-phosphate as shown in Table 5. - Arsenolysis of cellobiose. Arsenate is able to substitute for phosphate in the reaction resulting A i. in the arsenolysis of cellobiose. When the enzyme *f::* *.: was incubated with 100,umoles of cellobiose and 10,umoles of arsenate, nearly 46,umoles of :.:<.;. glucose were formed (Table 6). Since the amount of glucose formed was more than 4 times as great as the amount of arsenate present, it is apparent that the enzyme catalyzed the arsenolysis of cellobiose....i. Mechanism of the reaction. The occurrence of an inversion in both the cellobiose and maltose phosphorylase (Fitting and Doudoroff, 1952) reactions suggests that the mechanism of these reactions is similar. Additional information on A_:F- s the mechanism of the cellobiose phosphorylase reaction is shown in the following experiments. An experiment was designed to determine the ability of cellobiose phosphorylase to catalyze the exchange between glucose 1-phosphate and arsenate. If this exchange occurred, glucose 1- arsenate would be formed and this product would be expected to undergo spontaneous decomposition, resulting in the formation of free glucose and arsenate. No significant amount of free glucose was formed when the enzyme was incubated with glucose 1-phosphate and arsenate :.: D... ^ a c D Fig. 1. Chromatogram on the left shows a compound tentatively identified as 4-0-#-Dglucopyranosyl-D-xylopyranose in the lower spots of rows A and B. Row A is from 0.2 ml of the reaction mixture described in Table 4; row B is from 0.1 ml of the same reaction mixture. Row C shows cellobiose and row D shows D-xylose. The chromatogram on the right is the same as the other chromatogram except that L-xylose was used instead of D-xylose. The solvent was applied to the paper for about 20 hr at room temperature. catalyze the phosphorolysis of the four new compounds which appear to have been synthesized. f-d-glucose 1-phosphate. The synthesis of cellobiose from a-glucose 1-phosphate and glucose with cellobiose phosphorylase suggested TABLE 5 Liberation of phosphate when cellobiose phosphorylase was incubated with glucose and,- or a- glucose 1 -phosphate Reaction Mixtures Compound Added Phosphate ~~~~~~~Liberated 30 min 60 miii,umoles/ml 1 a-glucose 1-phosphate ,B-Glucose 1-phosphate (boiled) f,-glucose 1-phosphate The reaction mixtures contained 0.15 ml enzyme (2 mg protein), 0.2 ml 0.1 M glucose, 0.08 ml M a-glucose 1-phosphate or 0.6 ml 0.01 M,B-glucose 1-phosphate, 0.05 ml 0.14 M barbitalacetate buffer and were diluted to 1.0 ml with water.
6 9708 ALEXANDER [VOL. 81 TABLE 6 Arsenolysis of cellobiose and the lack of exchange between glucose 1-phosphate and arsenate Reaction Mixture* No. with Substrate Addition Hus GlucoseGlcs Cello- Cellobiose Cellobiose Gluhose 1-phosbiose arsenate phosphate phates- phate arsenate,moles glucose formed/ml Reaction mixtures 1, 2, and 3 contained 1.0 ml 0.1 M cellobiose; reaction mixtures 2 and 5 contained 0.1 ml 0.1 M arsenate; reaction mixture 3 contained 0.1 ml 0.1 M phosphate; reaction mixtures 4 and 5 contained 1.0 ml M glucose 1- phosphate; each reaction mixture contained 0.5 ml enzyme (5 mg protein) and 0.4 ml of 0.03 M barbital-acetate buffer (total volume 2.0 ml). The reaction mixtures were buffered with barbital-acetate buffer. * In reaction mixtures 1, 2, and 3, glucose was determined manometrically. In reaction mixtures 4 and 5, glucose was determined by the method of Saifer, Valenstein, and Hughes (1941). (Table 6). It has been shown that arsenate is able to substitute for phosphate in the decomposition of cellobiose. Therefore, it might be inferred that the exchange between glucose 1-phosphate and phosphate does not occur because the exchange between glucose 1-phosphate and arsenate does not occur. The following experiment was designed to determine the ability of cellobiose phosphorylase to catalyze the exchange between cellobiose and and D-xylose. If this exchange occurred, 4-0-,B-D-glucopyranosyl-D-xylopyranose and free glucose would be expected to be formed in the absence of phosphate. No spot corresponding to 4-0-/3-D-glucopyranosyl-D-xylopyranose2 was present when this reaction mixture was chromatographed i-D-Glucopyranosyl -D-xylopyranose could be distinguished from cellobiose in that it formed a red color with the aniline-phthalic acid color developer. As a control, another reaction mixture was included in this experiment which differed from the above in that it contained a small amount of phosphate. The following reactions were expected to occur in this reaction mixture: Cellobiose + phosphate glucose 1-phosphate + glucose D-xylose + glucose 1-phosphate = Dglucopyranosyl-D-xylopyranose + phosphate The phosphate liberated in the synthesis of 4-0- g-d-glucopyranosyl-d-xylopyranose would be able to react again in the phosphorolysis of cellobiose. The detection of a compound giving the reaction expected of the pentose-containing disaccharide in the chromatogram is evidence that these reactions did occur. The results indicate that 4-0-,3-D-glucopyranosyl-D-xylopyranose is formed only in the presence of phosphate. It appears, therefore, that the direct exchange of cellobiose and D-xylose is not catalyzed by cellobiose phosphorylase. Likewise, the enzyme would not be expected to catalyze the exchange between cellobiose and glucose. The inability of cellobiose phosphorylase to catalyze the exchange reactions between glucose 1-phosphate and arsenate, and cellobiose and D- xylose is analogous to the results obtained with maltose phosphorylase (Fitting and Doudoroff, 1952). It seems likely, therefore, that the cellobiose and maltose phosphorylase reactions have similar mechanisms. DISCUSSION Thermodynamic considerations. On the basis of the equilibrium constant of the cellobiose phosphorylase reaction the free energy change for the synthesis of cellobiose from glucose and glucose 1-phosphate is about -900 cal. The free energy of hydrolysis of cellobiose is estimated to be -3,900 cal. The equilibrium of the maltose phosphorylase reaction (Fitting and Doudoroff, 1952) appears to be identical to the equilibrium of the cellobiose phosphorylase reaction. From the equilibrium of the maltose phosphorylase reaction the free energy of hydrolysis of maltose has been estimated to be about -4,000 cal (Kalckar, 1954). For this value the energy of,b-glucose 1-phosphate, the product of maltose phosphorolysis, was assumed to be nearly the same as the energy of a-glucose 1-phosphate. This assumption
7 19611 CELLOBIOSE PHOSPHORYLASE 909 is supported by the information obtained in this study. Any significant influence by the configuration of the phosphate or glucosidic bonds on the energy level should be reflected in the equilibrium constants of these phosphorylase reactions. It seems likely, therefore, that the configuration of these bonds has little or no influence on the energy level. Specificity of cellobiose phosphorylase. The ability of cellobiose phosphorylase to catalyze the condensation of glucose 1-phosphate with D-glucose, D-xylose, 2-deoxyglucose, or glucosamine can be explained by assuming that the enzyme is relatively nonspecific for the C-2 and C-5 groups of these substrates. It seems reasonable to assume further that these compounds are attached to a glucosyl radical through a f3-1, 4-glucosidic linkage as in cellobiose. On the basis of these assumptions, certain interpretations can be made with regard to the specificity of cellobiose phosphorylase. The H and OH groups of C-2 cannot be reversed because mannose did not react. However, 2-deoxyglucose did react, although the reaction was not as rapid as with glucose. Thus, the OH group cannot replace the H group of C-2, but the OH group of C-2 can be replaced by H. Likewise, this OH group can be replaced by NH2 for glucosamine was able to react. The lack of specificity at this position is limited, however, for the OH group could not be replaced by the acetylamine group of N-acetylglucosamine. The inability of D-ribose to act as a glucosyl acceptor suggests that the H and OH groups attached to C-3 cannot be reversed. Likewise, the position of the H and OH groups attached to C-4 cannot be reversed for D-galactose did not react. The ability of both D-glucose and D-xylose to react indicates that CH20H group can be replaced by an H group. Therefore, the enzyme appears to be somewhat nonspecific for the groups attached to C-5. In view of these specific requirements of the enzyme, it was surprising to find that L-xylose was able to act as a glucosyl acceptor. L-Xylose would not be expected to combine with a glucosyl group through C-4 because the H and OH groups on C-2, C-3, and C-4 would be reversed from the position in which they occur in other compounds which react. If, however, L-xylose were to attach to the glucosyl group through C-2 in such a way that C-4 of L-xylose would occupy the same position as C-2 of glucose, then the sequence of H and OH groups in L-xylose would be the same as in D-glucose. Thus, on the basis of these data a 3-1,2-glucosidic linkage in the L- xylose-containing disaccharide would seem possible. Mechanism of reaction. The occurrence of an inversion in the cellobiose phosphorylase reaction and the inability of the enzyme to catalyze the exchange between either cellobiose and D-xylose or glucose 1-phosphate and arsenate indicates a single displacement mechanism for the reaction as described by Koshland (1954a, b). Koshland's discussion of the mechanism of the maltose phosphorylase reaction apparently can be extended to the cellobiose phosphorylase reaction. The only significant difference in maltose and cellobiose phosphorylase appears to be the specificity for the configuration of the glucosidic and phosphoglucose bonds. That the active surface of the two enzymes is similar is suggested by the similarity in specificity for various substrates. D-glucose, D-xylose, phosphate, and arsenate were able to act as substrates for both enzymes, whereas D-mannose-, D-ribose, D- galactose, L-arabinose, and D-gluconate were inactive (Fitting and Doudoroff, 1952). It seems possible that the active surface of cellobiose phosphorylase is essentially a mirror image of the surface of maltose phosphorylase. ACKNOWLEDGMENTS The author wishes to thank R. H. McBee and N. M. Nelson for their advice in this work. This investigation was supported in part by a research grant (A-1088) from the National Institute of Arthritis and Metabolic Diseases. SUMMARY Cellobiose phosphorylase, obtained from cellfree extracts of Clostridium thermocellum, was purified sufficiently to remove known interfering enzymes. The enzyme catalyzes the phosphorolysis of cellobiose with the formation of equimolar quantitites of glucose and glucose 1-phosphate. The reaction is reversible, for equimolar quantities of cellobiose and inorganic phosphate are formed from glucose and a-d-glucose 1-phosphate. The equilibrium constant, K = (cellobiose) (phosphate)/(glucose)(glucose 1-phosphate), at ph 7 and 37 C, is about 4.3.
8 '910 ALEXANDER [VOL. 81 D-Glucose, D-xylose, L-xylose, 2-deoxyglucose, D-glucosamine, phosphate, and arsenate can serve as substrates for the enzyme. (-Glucose 1- phosphate was unable to replace a-glucose 1- phosphate indicating that an inversion occurs in the reaction. On the basis of the inversion and the inability of the enzyme to catalyze the exchange between either cellobiose and D-xylose or glucose 1-phosphate and arsenate, a single displacement mechanism is proposed for the reaction. REFERENCES ALEXANDER, J. K Studies on a purified cellobiose phosphorylase. Bacteriol. Proc., 1958, 125. AYERS, W. A Phosphorylation of cellobiose and glucose by Ruminococcus flave faciens. J. Bacteriol., 76, AYERS, W. A Phosphorolysis and synthesis of cellobiose by cell extracts from Ruminococcus flavefaciens. J. Biol. Chem., 233, FISKE, C. H., AND Y. SUBBARow 1925 The colorimetric determination of phosphorus. J. Biol. Chem., 66, FIrrING, C., AND M. DOUDOROFF 1952 Phosphorolysis of maltose by enzyme preparations from Neisseria meningitidis. J. Biol. Chem., 199, HU1,CHER, F. H., AND K. W. KING 1958 Metabolic basis for disaccharide preference in a Cellvibrio. J. Bacteriol. 76, HUNGATE, R. E The anaerobic mesophilic cellulolytic bacteria. Bacteriol. Rev., 14, KALCKAR, H. M The mechanism of transglycosidation. In The mechanism of enzyme action, pp Edited by W. D. McElroy and B. Glass. The Johns Hopkins Press, Baltimore. KEILIN, D., AND E. F. HARTREE 1948 The use of glucose oxidase (notatin) for the determination of glucose in biological material and for the study of glucose-producing systems by manometric methods. Biochem. J., 42, KOSHLAND, D. E. 1954a Group transfer as a substitution mechanism. In The mechanism of enzyme action, pp Edited by W. D. McElroy and B. Glass. The Johns Hopkins Press, Baltimore. KOSHLAND, D. E. 1954b A physical organic approach to enzymatic mechanisms. Trans. N. Y. Acad. Sci., 16, LAMANNA, C., AND M. F. MALLETTE 1954 Use of glass beads for the mechanical rupture of microorganisms in concentrated suspensions. J. Bacteriol., 67, LEVIN, R., AND R. W. BRAUER 1951 The Biuret reaction for determiination of proteins-an improved reagent and its application. J. Lab. Clin. Med., 38, McBEE, R. H The culture and physiology of a thermophilic cellulose-fermenting bacterium. J. Bacteriol., 56, MICHAELIS, L Der acetat-veronal puffer. Biochem. Z., 234, SAIFER, A., F. VALENSTEIN, AND J. P. HUGHES 1941 The photometric determination of sugar in biological fluids by ferricyanide reduction. J. Lab. Clin. Med., 26, SIH, C. J., AND R. H. McBEE 1955a A phosphorylase active on cellobiose. Bacteriol. Proc., 1955, 126. SIH, C. J., AND R. H. McBEE 1955b A cellobiose phosphorylase in Clostridium thermocellum. Proc. Montana Acad. Sci., 15, SIH, C. J., N. M. NELSON, AND R. H. McBEE 1957 Biological synthesis of cellobiose. Science, 126,
METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI
 METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI I. L- RHAMNOSE ISOMERASE DOROTHY M. WILSON1 AND SAM AJL Department of Bacteriology, Walter Reed Army Institute of Research, Washington, D. C. The methyl pentose,
METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI I. L- RHAMNOSE ISOMERASE DOROTHY M. WILSON1 AND SAM AJL Department of Bacteriology, Walter Reed Army Institute of Research, Washington, D. C. The methyl pentose,
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE
 METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
Carbohydrates. Objectives. Background. Experiment 6
 1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
1 of 6 3/15/2011 7:27 PM Experiment 6 Carbohydrates Objectives During this experiment you will look at some of the physical and chemical properties of carbohydrates. Many of the carbohydrates, especially
B. 1% (w/v) Salicin Substrate Solution (Salicin) (Prepare 50 ml in Reagent A using Salicin, Sigma Prod. No. S-0625.)
 SIGMA QUALITY CONTROL TEST PROCEDURE (Q]\PDWLFÃ$VVD\ÃRIÃ */8&26,'$6( PRINCIPLE: 'Glucoside + H 2 O Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD:
SIGMA QUALITY CONTROL TEST PROCEDURE (Q]\PDWLFÃ$VVD\ÃRIÃ */8&26,'$6( PRINCIPLE: 'Glucoside + H 2 O Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD:
Enzymatic Assay of ß-GLUCOSIDASE (EC )
 PRINCIPLE: ß-D-Glucoside + H 2 O ß-Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric 1 REAGENTS: A. 100 mm Sodium Acetate Buffer, ph 5.0
PRINCIPLE: ß-D-Glucoside + H 2 O ß-Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric 1 REAGENTS: A. 100 mm Sodium Acetate Buffer, ph 5.0
colorimetrically by the methylene blue method according to Fogo and manometrically. In the presence of excess sulfur the amount of oxygen taken up
 GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
Farah Al-Khaled. Razi Kittaneh. Mohammad Omari
 7 Farah Al-Khaled Razi Kittaneh Mohammad Omari Dr. Mamoun Ahram In this lecture we are going to talk about modified sugars. Remember: The Fischer projection can be turned into a ring structure (which is
7 Farah Al-Khaled Razi Kittaneh Mohammad Omari Dr. Mamoun Ahram In this lecture we are going to talk about modified sugars. Remember: The Fischer projection can be turned into a ring structure (which is
OXIDATIVE FERMENTATION OF D-RIBOSE BY LACTOBACILLUS PLANTARUM NO. 11 (Preliminary Report)
 J. Gen. Appl. Microbiol. Vol. 4, No. 2, 1958 OXIDATIVE FERMENTATION OF D-RIBOSE BY LACTOBACILLUS PLANTARUM NO. 11 (Preliminary Report) SAKUZO FUKUI and AKIRA OI Division of 7ymomycology, The Institute
J. Gen. Appl. Microbiol. Vol. 4, No. 2, 1958 OXIDATIVE FERMENTATION OF D-RIBOSE BY LACTOBACILLUS PLANTARUM NO. 11 (Preliminary Report) SAKUZO FUKUI and AKIRA OI Division of 7ymomycology, The Institute
Sheet #10 Dr. Mamoun Ahram Sec 1,2,3 15/07/2014. Carbohydrates 2
 Carbohydrates 2 A study Guide: Kindly,refer to the slide number,look at the structures and read the sheet notes well,most of the slides content besides all what the doctor said are mentioned here,good
Carbohydrates 2 A study Guide: Kindly,refer to the slide number,look at the structures and read the sheet notes well,most of the slides content besides all what the doctor said are mentioned here,good
PHOSPHOROLYSIS AND SYNTHESIS OF SUCROSE WITH A BACTERIAL PREPARATION
 PHOSPHOROLYSIS AND SYNTHESIS OF SUCROSE WITH A BACTERIAL PREPARATION BY M. DOUDOROFF, N. KAPLAN, AND W. Z. HASSID (From the Department of Bacteriology, Division of Biochemistry of the Medical School, and
PHOSPHOROLYSIS AND SYNTHESIS OF SUCROSE WITH A BACTERIAL PREPARATION BY M. DOUDOROFF, N. KAPLAN, AND W. Z. HASSID (From the Department of Bacteriology, Division of Biochemistry of the Medical School, and
Glycosides. Carbohydrates
 Carbohydrates A carbohydrate is a large biological molecule consisting of carbon, hydrogen, and oxygen atoms, usually with a hydrogen:oxygen atom ratio of 2:1. Glycosides Acetal derivatives formed when
Carbohydrates A carbohydrate is a large biological molecule consisting of carbon, hydrogen, and oxygen atoms, usually with a hydrogen:oxygen atom ratio of 2:1. Glycosides Acetal derivatives formed when
24.1 Introduction to Carbohydrates
 24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
CELLULASE from PENICILLIUM FUNICULOSUM
 CELLULASE from PENICILLIUM FUNICULOSUM Prepared at the 55th JECFA (2000) and published in FNP 52 Add 8 (2000), superseding tentative specifications prepared at the 31st JECFA (1987) and published in FNP
CELLULASE from PENICILLIUM FUNICULOSUM Prepared at the 55th JECFA (2000) and published in FNP 52 Add 8 (2000), superseding tentative specifications prepared at the 31st JECFA (1987) and published in FNP
GENERAL TESTS FOR CARBOHYDRATE. By Sandip Kanazariya
 GENERAL TESTS FOR CARBOHYDRATE By Sandip Kanazariya Introduction Carbohydrates are of great importance to human beings. They are major part of our diet, providing 60-70% of total energy required by the
GENERAL TESTS FOR CARBOHYDRATE By Sandip Kanazariya Introduction Carbohydrates are of great importance to human beings. They are major part of our diet, providing 60-70% of total energy required by the
Carbon. p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms
 Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
ON THE NATURE OF THE TRANSALDOLASE-DIHYDROXYACETONE
 VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
Experiment 20 Identification of Some Carbohydrates
 Experiment 20 Identification of Some arbohydrates arbohydrates are the direct product of the photosynthetic combination of carbon dioxide and water. By weight, they are the most common organic compounds
Experiment 20 Identification of Some arbohydrates arbohydrates are the direct product of the photosynthetic combination of carbon dioxide and water. By weight, they are the most common organic compounds
Welcome to Class 7. Class 7: Outline and Objectives. Introductory Biochemistry
 Welcome to Class 7 Introductory Biochemistry Class 7: Outline and Objectives l Monosaccharides l Aldoses, ketoses; hemiacetals; epimers l Pyranoses, furanoses l Mutarotation, anomers l Disaccharides and
Welcome to Class 7 Introductory Biochemistry Class 7: Outline and Objectives l Monosaccharides l Aldoses, ketoses; hemiacetals; epimers l Pyranoses, furanoses l Mutarotation, anomers l Disaccharides and
Qualitative chemical reaction of functional group in protein
 Qualitative chemical reaction of functional group in protein Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product formed by
Qualitative chemical reaction of functional group in protein Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product formed by
Carbon. Has four valence electrons Can bond with many elements. Can bond to other carbon atoms. Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
 Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
MahaAbuAjamieh. BahaaNajjar. MamoonAhram
 7 MahaAbuAjamieh BahaaNajjar MamoonAhram Carbohydrates (saccharides) can be classified into these main categories: 1. Monosaccharides, they are simplesugars (the simplest units), such as glucose, galactose
7 MahaAbuAjamieh BahaaNajjar MamoonAhram Carbohydrates (saccharides) can be classified into these main categories: 1. Monosaccharides, they are simplesugars (the simplest units), such as glucose, galactose
Qualitative test of protein-lab2
 1- Qualitative chemical reactions of amino acid protein functional groups: Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product
1- Qualitative chemical reactions of amino acid protein functional groups: Certain functional groups in proteins can react to produce characteristically colored products. The color intensity of the product
Carbohydrates. Chapter 18
 Carbohydrates Chapter 18 Biochemistry an overview Biochemistry is the study of chemical substances in living organisms and the chemical interactions of these substances with each other. Biochemical substances
Carbohydrates Chapter 18 Biochemistry an overview Biochemistry is the study of chemical substances in living organisms and the chemical interactions of these substances with each other. Biochemical substances
For example, monosaccharides such as glucose are polar and soluble in water, whereas lipids are nonpolar and insoluble in water.
 Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Module-04: Food carbohydrates: Monosaccharides and Oligosaccharides
 Paper No. 01 Paper Title: Food Chemistry Module-04: Food carbohydrates: Monosaccharides and Oligosaccharides Monosaccharides The simplest form of carbohydrates is the monosaccharide. Monosaccharides are
Paper No. 01 Paper Title: Food Chemistry Module-04: Food carbohydrates: Monosaccharides and Oligosaccharides Monosaccharides The simplest form of carbohydrates is the monosaccharide. Monosaccharides are
Tests for Carbohydrates
 Goals bserve physical and chemical properties of some common carbohydrates. Use physical and chemical tests to distinguish between monosaccharides, disaccharides, and polysaccharides. Identify an unknown
Goals bserve physical and chemical properties of some common carbohydrates. Use physical and chemical tests to distinguish between monosaccharides, disaccharides, and polysaccharides. Identify an unknown
2-2 Properties of Water
 2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
1. Which of the following contributes to the tertiary structure of proteins?
 Chemistry 11 Spring 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free
Chemistry 11 Spring 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free
STUDIES OF AN ENZYME PRODUCED BY BACILLUS FULMINANS
 STUDIES OF AN ENZYME PRODUCED BY BACILLUS FULMINANS THAT INACTIVATES BLOOD GROUP 0 SUBSTANCE1 INGEBORG NAYLOR AND HAROLD BAER Department of Microbiology, Tulane University School of Medicine, New Orleans,
STUDIES OF AN ENZYME PRODUCED BY BACILLUS FULMINANS THAT INACTIVATES BLOOD GROUP 0 SUBSTANCE1 INGEBORG NAYLOR AND HAROLD BAER Department of Microbiology, Tulane University School of Medicine, New Orleans,
QUALITATIVE TESTS OF CARBOHYDRATE
 QUALITATIVE TESTS OF CARBOHYDRATE MACROMOLECULE CARBOHYDRATES Are the key source of energy used by living things. Also serve as extracellular structural elements as in cell wall of bacteria and plant.
QUALITATIVE TESTS OF CARBOHYDRATE MACROMOLECULE CARBOHYDRATES Are the key source of energy used by living things. Also serve as extracellular structural elements as in cell wall of bacteria and plant.
Experiment 2 Introduction
 Characterization of Invertase from Saccharomyces cerevisiae Experiment 2 Introduction The method we used in A Manual for Biochemistry I Laboratory: Experiment 7 worked well to detect any created reducing
Characterization of Invertase from Saccharomyces cerevisiae Experiment 2 Introduction The method we used in A Manual for Biochemistry I Laboratory: Experiment 7 worked well to detect any created reducing
Experiment 1. Isolation of Glycogen from rat Liver
 Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Name: Period: Date: Testing for Biological Macromolecules Lab
 Testing for Biological Macromolecules Lab Introduction: All living organisms are composed of various types of organic molecules, such as carbohydrates, starches, proteins, lipids and nucleic acids. These
Testing for Biological Macromolecules Lab Introduction: All living organisms are composed of various types of organic molecules, such as carbohydrates, starches, proteins, lipids and nucleic acids. These
Biological molecules = Biomolecules = Compounds of life
 Biological molecules = Biomolecules = Compounds of life Carbohydrates Proteins & Amino Acids Mono-saccharides Olego-saccharides Di-saccharides Poly-saccharides Lipids Oils & Fats Amino acids Proteins Enzymes
Biological molecules = Biomolecules = Compounds of life Carbohydrates Proteins & Amino Acids Mono-saccharides Olego-saccharides Di-saccharides Poly-saccharides Lipids Oils & Fats Amino acids Proteins Enzymes
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/81/83984357.jpg) BCH302 [Practical] 1 Carbohydrates are defined as the polyhydroxy aldehydes or polyhydroxy ketones. Most, but not all carbohydrate have a formula (CH 2 O)n (hence the name hydrate of carbon). Sugars ends
BCH302 [Practical] 1 Carbohydrates are defined as the polyhydroxy aldehydes or polyhydroxy ketones. Most, but not all carbohydrate have a formula (CH 2 O)n (hence the name hydrate of carbon). Sugars ends
Ch 2 Molecules of life
 Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Lab 2. The Chemistry of Life
 Lab 2 Learning Objectives Compare and contrast organic and inorganic molecules Relate hydrogen bonding to macromolecules found in living things Compare and contrast the four major organic macromolecules:
Lab 2 Learning Objectives Compare and contrast organic and inorganic molecules Relate hydrogen bonding to macromolecules found in living things Compare and contrast the four major organic macromolecules:
Biomolecules. Unit 3
 Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Biomolecules Unit 3 Atoms Elements Compounds Periodic Table What are biomolecules? Monomers vs Polymers Carbohydrates Lipids Proteins Nucleic Acids Minerals Vitamins Enzymes Triglycerides Chemical Reactions
Figure 2. Figure 1. Name: Bio AP Lab Organic Molecules
 Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
I (CH 2 O) n or H - C - OH I
 V. ARBYDRATE arbohydrates (glycans) have the following basic composition: I ( ) n or - - I Many carbohydrates are soluble in water. The usual chemical test for the simpler carbohydrates is heating with
V. ARBYDRATE arbohydrates (glycans) have the following basic composition: I ( ) n or - - I Many carbohydrates are soluble in water. The usual chemical test for the simpler carbohydrates is heating with
Dr. Nafith Abu Tarboush. Rana N. Talj
 2 Dr. Nafith Abu Tarboush June 19 th 2013 Rana N. Talj Review: Fischer suggested a projection in which the horizontal bonds are projecting towards the viewer and the vertical ones project away from the
2 Dr. Nafith Abu Tarboush June 19 th 2013 Rana N. Talj Review: Fischer suggested a projection in which the horizontal bonds are projecting towards the viewer and the vertical ones project away from the
ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA
 J. Gen. App!. Microbiol., 34, 213-219 (1988) ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA TOSHIRO HAYASHI, RYO IOROI,*
J. Gen. App!. Microbiol., 34, 213-219 (1988) ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA TOSHIRO HAYASHI, RYO IOROI,*
GLYCOGEN BEFORE THE LAB YOU HAVE TO READ ABOUT:
 GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
Kinetics analysis of β-fructofuranosidase enzyme. 1-Effect of Time Incubation On The Rate Of An Enzymatic Reaction
 Kinetics analysis of β-fructofuranosidase enzyme 1-Effect of Time Incubation On The Rate Of An Enzymatic Reaction Enzyme kinetics It is the study of the chemical reactions that are catalyzed by enzymes.
Kinetics analysis of β-fructofuranosidase enzyme 1-Effect of Time Incubation On The Rate Of An Enzymatic Reaction Enzyme kinetics It is the study of the chemical reactions that are catalyzed by enzymes.
Fundamentals of Organic Chemistry. CHAPTER 6: Carbohydrates
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Carbohydrates 1. Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras
 Carbohydrates 1 Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail: stevenemassey@gmail.com
Carbohydrates 1 Steven E. Massey, Ph.D. Assistant Professor Bioinformatics Department of Biology University of Puerto Rico Río Piedras Office & Lab: NCN#343B Tel: 787-764-0000 ext. 7798 E-mail: stevenemassey@gmail.com
THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION
 THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION BY ROBERT K. CRANE* AND FRITZ LIPMANN (From the Biochemical Research Laboratory, Massachusetts General Hospital, and the Department of Biological Chemistry,
THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION BY ROBERT K. CRANE* AND FRITZ LIPMANN (From the Biochemical Research Laboratory, Massachusetts General Hospital, and the Department of Biological Chemistry,
CLASS 11th. Biomolecules
 CLASS 11th 01. Carbohydrates These are the compound of carbon, hydrogen and oxygen having hydrogen and oxygen in the same ratio as that of water, i.e. 2 : 1. They are among the most widely distributed
CLASS 11th 01. Carbohydrates These are the compound of carbon, hydrogen and oxygen having hydrogen and oxygen in the same ratio as that of water, i.e. 2 : 1. They are among the most widely distributed
½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = X X= 325 g
 BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
EXTRACELLULAR PROTEINASE OF STREPTOCOCCUS LACTIS'
 JOURnNAL OF BACTERIOLOGY Vol. 87, No. 1, pp. 49-53 January, 1964,Copyright 1964 by the American Society for Microbiology Printed in U.S.A. EXTRACELLULAR PROTEINASE OF STREPTOCOCCUS LACTIS' W. T. WILLIAMSON,
JOURnNAL OF BACTERIOLOGY Vol. 87, No. 1, pp. 49-53 January, 1964,Copyright 1964 by the American Society for Microbiology Printed in U.S.A. EXTRACELLULAR PROTEINASE OF STREPTOCOCCUS LACTIS' W. T. WILLIAMSON,
Chapter 23 Carbohydrates and Nucleic Acids. Carbohydrates
 Chapter 23 Carbohydrates and Nucleic Acids Carbohydrates Synthesized by plants using sunlight to convert CO 2 and H 2 O to glucose and O 2. Polymers include starch and cellulose. Starch is storage unit
Chapter 23 Carbohydrates and Nucleic Acids Carbohydrates Synthesized by plants using sunlight to convert CO 2 and H 2 O to glucose and O 2. Polymers include starch and cellulose. Starch is storage unit
Ch13. Sugars. What biology does with monosaccharides disaccharides and polysaccharides. version 1.0
 Ch13 Sugars What biology does with monosaccharides disaccharides and polysaccharides. version 1.0 Nick DeMello, PhD. 2007-2015 Ch13 Sugars Haworth Structures Saccharides can form rings. That creates a
Ch13 Sugars What biology does with monosaccharides disaccharides and polysaccharides. version 1.0 Nick DeMello, PhD. 2007-2015 Ch13 Sugars Haworth Structures Saccharides can form rings. That creates a
Phases Available Description Applications Additional Notes RCM-Monosaccharide (L19 packing)*
 Carbohydrate and Organic Acid Analysis Excellent resolution Wide range of selectivities Excellent column-to-column reproducibility Recommended alternative to Bio-Rad, Supelco Supelcogel and Waters Sugar-Pak
Carbohydrate and Organic Acid Analysis Excellent resolution Wide range of selectivities Excellent column-to-column reproducibility Recommended alternative to Bio-Rad, Supelco Supelcogel and Waters Sugar-Pak
Carbohydrates. Haworth Projections
 Carbohydrates There are, for all intents and purposes, 2 classes of carbohydrates: pyranoses and furanoses ( ose is the suffix for a sugar). These two classes are so called because their carbon skeletons
Carbohydrates There are, for all intents and purposes, 2 classes of carbohydrates: pyranoses and furanoses ( ose is the suffix for a sugar). These two classes are so called because their carbon skeletons
possibilities occurs. It has been found that the organism acquires addition of vitamin B1 to cells of P. pentosaceum which had
 ADAPTATION OF THE PROPIONIC-ACID BACTERIA TO VITAMIN B1 SYNTHESIS INCLUDING A METHOD OF ASSAY M. SILVERMAN AND C. H. WERKMAN Bacteriology Section, Industrial Science Research Institute, Iowa State College,
ADAPTATION OF THE PROPIONIC-ACID BACTERIA TO VITAMIN B1 SYNTHESIS INCLUDING A METHOD OF ASSAY M. SILVERMAN AND C. H. WERKMAN Bacteriology Section, Industrial Science Research Institute, Iowa State College,
202 S. IsExi and T. IKEDA [Vol. 32,
 No. 3] 201 47. On Bacterial Enzyme Specifically Decomposing Group B Substance By Shoei ISEKI and Tsukasa IKEDA Department of Legal Medicine, School of Medicine, Gunma University, Maebashi, Japan (Comm.
No. 3] 201 47. On Bacterial Enzyme Specifically Decomposing Group B Substance By Shoei ISEKI and Tsukasa IKEDA Department of Legal Medicine, School of Medicine, Gunma University, Maebashi, Japan (Comm.
Carbohydrates- Disaccharides. By Dr. Bhushan R. Kavimandan
 Carbohydrates- Disaccharides By Dr. Bhushan R. Kavimandan Disaccharides ofbiological importance: Disaccharides consist of two monosaccharides joined by glycosidic linkages. They are crystalline, water-soluble
Carbohydrates- Disaccharides By Dr. Bhushan R. Kavimandan Disaccharides ofbiological importance: Disaccharides consist of two monosaccharides joined by glycosidic linkages. They are crystalline, water-soluble
BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. i) Carbohydrates B3. ii) Proteins & Nucleic Acids.
 BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. 1. Biomolecules i) Carbohydrates B3 ii) Proteins & Nucleic Acids iii) Steroids iv) Terpenes & Cartenoids B27 B61 B65 2. Spectroscopy v)
BIOMOLECULES & SPECTROSCOPY TABLE OF CONTENTS S.NO. TOPIC PAGE NO. 1. Biomolecules i) Carbohydrates B3 ii) Proteins & Nucleic Acids iii) Steroids iv) Terpenes & Cartenoids B27 B61 B65 2. Spectroscopy v)
QUANTITATIVE TEST (CHEMICAL) FOR SUGARS IN SUGARCANE. Talha Saeed. Faisal Iftikhar. Mam AMMARA AINEE
 Assignment title QUANTITATIVE TEST (CHEMICAL) FOR SUGARS IN SUGARCANE Submitted by Subject Talha Saeed Roll # 37 Faisal Iftikhar Roll # 18 B.Sc. (Hons) Food Science and Technology 6 th Semester (Regular)
Assignment title QUANTITATIVE TEST (CHEMICAL) FOR SUGARS IN SUGARCANE Submitted by Subject Talha Saeed Roll # 37 Faisal Iftikhar Roll # 18 B.Sc. (Hons) Food Science and Technology 6 th Semester (Regular)
Chapter 1. Chemistry of Life - Advanced TABLE 1.2: title
 Condensation and Hydrolysis Condensation reactions are the chemical processes by which large organic compounds are synthesized from their monomeric units. Hydrolysis reactions are the reverse process.
Condensation and Hydrolysis Condensation reactions are the chemical processes by which large organic compounds are synthesized from their monomeric units. Hydrolysis reactions are the reverse process.
BCH 4053 Spring 2001 Chapter 7 Lecture Notes
 BC 4053 Spring 2001 Chapter 7 Lecture Notes 1 Chapter 7 Carbohydrates 2 Carbohydrates: Nomenclature ydrates of carbon General formula (C 2 ) n (simple sugars) or C x ( 2 0) y Monosaccharides (simple sugars)
BC 4053 Spring 2001 Chapter 7 Lecture Notes 1 Chapter 7 Carbohydrates 2 Carbohydrates: Nomenclature ydrates of carbon General formula (C 2 ) n (simple sugars) or C x ( 2 0) y Monosaccharides (simple sugars)
6 The chemistry of living organisms
 Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic
 Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
Name a property of. water why is it necessary for life?
 02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
02.09.18 Name a property of + water why is it necessary for life? n Cohesion n Adhesion n Transparency n Density n Solvent n Heat capacity + Macromolecules (2.3 & some of 2.4) + Organic Molecules All molecules
I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy
 Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Chapter 22 Carbohydrates Chem 306 Roper I. Carbohydrates Overview A. Carbohydrates are a class of biomolecules which have a variety of functions. 1. energy 2. energy storage 3. structure 4. other functions!
Enzymatic Assay of POLYGALACTURONASE (EC )
 PRINCIPLE: Polygalacturonic Acid + H 2 O PG > Reducing Sugars Abbreviations: PG = Polygalacturonase CONDITIONS: T = 30 C, ph 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric REAGENTS: A. 50 mm Sodium
PRINCIPLE: Polygalacturonic Acid + H 2 O PG > Reducing Sugars Abbreviations: PG = Polygalacturonase CONDITIONS: T = 30 C, ph 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric REAGENTS: A. 50 mm Sodium
Enzymes - Exercise 3 - Rockville
 Enzymes - Exercise 3 - Rockville Objectives -Understand the function of an enzyme. -Know what the substrate, enzyme, and the product of the reaction for this lab. -Understand how at various environments
Enzymes - Exercise 3 - Rockville Objectives -Understand the function of an enzyme. -Know what the substrate, enzyme, and the product of the reaction for this lab. -Understand how at various environments
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Aim: To study the effect of ph on the action of salivary amylase. NCERT
 Exercise 28 Aim: To study the effect of ph on the action of salivary amylase. Principle: Optimal activity for most of the enzymes is generally observed between ph 5.0 and 9.0. However, a few enzymes, e.g.,
Exercise 28 Aim: To study the effect of ph on the action of salivary amylase. Principle: Optimal activity for most of the enzymes is generally observed between ph 5.0 and 9.0. However, a few enzymes, e.g.,
Enzyme activity Page 1 of 8
 Enzyme activity Page 1 of 8 Contains: - protease - L-glutaminase - Leucine aminopeptidase - sucrase - amylase - cellulase - lipase - catalase - carboxypeptidase Protease Assay Based on: Frankena J., van
Enzyme activity Page 1 of 8 Contains: - protease - L-glutaminase - Leucine aminopeptidase - sucrase - amylase - cellulase - lipase - catalase - carboxypeptidase Protease Assay Based on: Frankena J., van
ESCHERICHIA COLI-MUTABILE1. antiseptics employed "activated" the lactase which was present, "activate" the lactase.
 ON THE "ACTIVATION" OF THE LACTASE OF ESCHERICHIA COLI-MUTABILE1 CHARLES J. DEERE Department of Chemistry, University of Tennessee School of Biological Sciences, Memphis Received for publication August
ON THE "ACTIVATION" OF THE LACTASE OF ESCHERICHIA COLI-MUTABILE1 CHARLES J. DEERE Department of Chemistry, University of Tennessee School of Biological Sciences, Memphis Received for publication August
Analysis - Carbohydrate analysis
 employ a technique called ligand exchange chromatography for the separation of monosaccharides, disaccharides and oligosaccharides up to 15 glucose units long. Ligand exchange resins are highly sulfonated
employ a technique called ligand exchange chromatography for the separation of monosaccharides, disaccharides and oligosaccharides up to 15 glucose units long. Ligand exchange resins are highly sulfonated
BCM 101 BIOCHEMISTRY Week 4 Practical Chemistry of proteins
 BCM 101 BIOCHEMISTRY Week 4 Practical Chemistry of proteins The word protein is derived from the Greek word proteios, which means of primary importance. In fact, proteins plays an important role in all
BCM 101 BIOCHEMISTRY Week 4 Practical Chemistry of proteins The word protein is derived from the Greek word proteios, which means of primary importance. In fact, proteins plays an important role in all
GLUTAMIC ACID DEHYDROGENASE OF PASTEURELLA TULARENSIS1
 GLUTAMIC ACID DEHYDROGENASE OF PASTEURELLA TULARENSIS1 GEORGE RENDINA2 AND R. C. MILLS Department of Biochemistry, University of Kansas, Lawrence, Kansas Received for publication April 16, 1957 As part
GLUTAMIC ACID DEHYDROGENASE OF PASTEURELLA TULARENSIS1 GEORGE RENDINA2 AND R. C. MILLS Department of Biochemistry, University of Kansas, Lawrence, Kansas Received for publication April 16, 1957 As part
3. Hydrogen bonds form between which atoms? Between an electropositive hydrogen and an electronegative N, O or F.
 Chemistry of Life Answers 1. Differentiate between an ionic and covalent bond. Provide an example for each. Ionic: occurs between metals and non-metals, e.g., NaCl Covalent: occurs between two non-metals;
Chemistry of Life Answers 1. Differentiate between an ionic and covalent bond. Provide an example for each. Ionic: occurs between metals and non-metals, e.g., NaCl Covalent: occurs between two non-metals;
For more info visit
 Carbohydrates Classification of carbohydrates: Monosaccharides: Polyhydroxy aldehydes or polyhydroxy ketones which cannot be decomposed by hydrolysis to give simpler carbohydrates.examples: Glucose, Fructose,
Carbohydrates Classification of carbohydrates: Monosaccharides: Polyhydroxy aldehydes or polyhydroxy ketones which cannot be decomposed by hydrolysis to give simpler carbohydrates.examples: Glucose, Fructose,
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
Scholars Research Library. Purification and characterization of neutral protease enzyme from Bacillus Subtilis
 Journal of Microbiology and Biotechnology Research Scholars Research Library J. Microbiol. Biotech. Res., 2012, 2 (4):612-618 (http://scholarsresearchlibrary.com/archive.html) Purification and characterization
Journal of Microbiology and Biotechnology Research Scholars Research Library J. Microbiol. Biotech. Res., 2012, 2 (4):612-618 (http://scholarsresearchlibrary.com/archive.html) Purification and characterization
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
Carbohydrates. b. What do you notice about the orientation of the OH and H groups in glucose? Are they in the axial or equatorial position?
 1. The 3D structure of glucose and galactose are shown. Carbohydrates D-glucose D-galactose a. Is the axial or equatorial position more stable in the chair conformation? b. What do you notice about the
1. The 3D structure of glucose and galactose are shown. Carbohydrates D-glucose D-galactose a. Is the axial or equatorial position more stable in the chair conformation? b. What do you notice about the
Screening of Rice Straw Degrading Microorganisms and Their Cellulase Activities
 Research 83 KKU Sci. J.37 (Supplement) 83-88 (2009) Screening of Rice Straw Degrading Microorganisms and Their Cellulase Activities Abstract Atcha Boonmee 1,2* Rice straw is one of the most abundant agricultural
Research 83 KKU Sci. J.37 (Supplement) 83-88 (2009) Screening of Rice Straw Degrading Microorganisms and Their Cellulase Activities Abstract Atcha Boonmee 1,2* Rice straw is one of the most abundant agricultural
Enzymatic Assay of ß-GALACTOSIDASE (EC ) from E. coli ß-Lactose as Substrate
 PRINCIPLE: ß-Lactose + H 2 O ß-Galactosidase > D-Glucose + D-Galactose D-Glucose + O 2 + H 2 O Glucose Oxidase > D-Gluconic Acid + H 2 O 2 H 2 O 2 + o-dianisidine(reduced) Peroxidase > H 2 O + o-dianisidine(oxidized)
PRINCIPLE: ß-Lactose + H 2 O ß-Galactosidase > D-Glucose + D-Galactose D-Glucose + O 2 + H 2 O Glucose Oxidase > D-Gluconic Acid + H 2 O 2 H 2 O 2 + o-dianisidine(reduced) Peroxidase > H 2 O + o-dianisidine(oxidized)
Polysaccharide phosphorylase
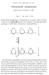 CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
CHEMISTRY OF LIFE 05 FEBRUARY 2014
 CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
Biochemistry. Chapter 6
 Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Chapter 2 Part 3: Organic and Inorganic Compounds
 Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY
 J. Gen. App!. Microbiol,, 21, 51-59 (1975) EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY CLOSTRIDIUM ACETOBUTYLICUM BURT ENSLEY, JOHN J. McHUGH, AND LARRY L. BARTON Department
J. Gen. App!. Microbiol,, 21, 51-59 (1975) EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY CLOSTRIDIUM ACETOBUTYLICUM BURT ENSLEY, JOHN J. McHUGH, AND LARRY L. BARTON Department
lactose-fermenting variants (reds). Appreciable lactose utilization variants. Hershey and Bronfenbrenner (1936) found the non-lactosefermenting
 THE LACTASE ACTIVITY OF ESCHERICHIA COLI- MUTABILE' CHARLES J. DEERE, ANNA DEAN DULANEY AND I. D. MICHELSON Department of Chemistry and Department of Bacteriology, University of Tennessee School of Biological
THE LACTASE ACTIVITY OF ESCHERICHIA COLI- MUTABILE' CHARLES J. DEERE, ANNA DEAN DULANEY AND I. D. MICHELSON Department of Chemistry and Department of Bacteriology, University of Tennessee School of Biological
HEMICELLULASE from ASPERGILLUS NIGER, var.
 HEMICELLULASE from ASPERGILLUS NIGER, var. Prepared at the 55th JECFA (2000) and published in FNP 52 Add 8 (2000), superseding tentative specifications prepared at the 31st JECFA (1987) and published in
HEMICELLULASE from ASPERGILLUS NIGER, var. Prepared at the 55th JECFA (2000) and published in FNP 52 Add 8 (2000), superseding tentative specifications prepared at the 31st JECFA (1987) and published in
THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE*
 THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE* BY W. R. BROWN (From Ihe Laboratory of Biochemistry, University of Cincinnati, Cincinnati) (Received for publication, November 21, 1935)
THE HYDROLYSIS OF STARCH BY HYDROGEN PEROXIDE AND FERROUS SULFATE* BY W. R. BROWN (From Ihe Laboratory of Biochemistry, University of Cincinnati, Cincinnati) (Received for publication, November 21, 1935)
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
Experiment 3: Activity Determination
 Experiment 3: Activity Determination Introduction: Specific activity is a method for measuring enzymatic activity and the enzyme purity in a mixture. In order to determine the specific activity of an enzyme,
Experiment 3: Activity Determination Introduction: Specific activity is a method for measuring enzymatic activity and the enzyme purity in a mixture. In order to determine the specific activity of an enzyme,
Carbohydrate Chemistry
 Carbohydrate Chemistry The term carbohydrate is derived from the Cn(2O)n general chemical formula Carbohydrates are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis
Carbohydrate Chemistry The term carbohydrate is derived from the Cn(2O)n general chemical formula Carbohydrates are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis
Name LastName Student ID
 Name LastName Student ID 1) (12 points) Imidazopyridine derivatives such as 1-deaza-9H-purines (like 1) and 3- deaza-9h-purines (like 2) represent privileged structures in medicinal chemistry and they
Name LastName Student ID 1) (12 points) Imidazopyridine derivatives such as 1-deaza-9H-purines (like 1) and 3- deaza-9h-purines (like 2) represent privileged structures in medicinal chemistry and they
CHAPTER 27 CARBOHYDRATES SOLUTIONS TO REVIEW QUESTIONS
 27 09/17/2013 11:12:35 Page 397 APTER 27 ARBYDRATES SLUTINS T REVIEW QUESTINS 1. In general, the carbohydrate carbon oxidation state determines the carbon s metabolic energy content. The more oxidized
27 09/17/2013 11:12:35 Page 397 APTER 27 ARBYDRATES SLUTINS T REVIEW QUESTINS 1. In general, the carbohydrate carbon oxidation state determines the carbon s metabolic energy content. The more oxidized
MOTILE ENTEROCOCCI (STREPTOCOCCUS FAECIUM VAR. MOBILIS VAR. N.) ISOLATED FROM GRASS SILAGE
 MOTILE ENTEROCOCCI (STREPTOCOCCUS FAECIUM VAR. MOBILIS VAR. N.) ISOLATED FROM GRASS SILAGE C. W. LANGSTON, JOYCE GUTIERREZ, AND CECELIA BOUMA Dairy Cattle Research Branch, Agricultural Research Center,
MOTILE ENTEROCOCCI (STREPTOCOCCUS FAECIUM VAR. MOBILIS VAR. N.) ISOLATED FROM GRASS SILAGE C. W. LANGSTON, JOYCE GUTIERREZ, AND CECELIA BOUMA Dairy Cattle Research Branch, Agricultural Research Center,
Carbohydrates. Chapter 12
 Carbohydrates Chapter 12 Educational Goals 1. Given a Fischer projection of a monosaccharide, classify it as either aldoses or ketoses. 2. Given a Fischer projection of a monosaccharide, classify it by
Carbohydrates Chapter 12 Educational Goals 1. Given a Fischer projection of a monosaccharide, classify it as either aldoses or ketoses. 2. Given a Fischer projection of a monosaccharide, classify it by
SUPPLEMENTARY INFORMATION. Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was
 SUPPLEMENTARY INFORMATION Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was grown in a casein-based semisynthetic medium (C+Y) supplemented with yeast extract (1 mg/ml of
SUPPLEMENTARY INFORMATION Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was grown in a casein-based semisynthetic medium (C+Y) supplemented with yeast extract (1 mg/ml of
fossum/files/2012/01/10 Enzymes.pdf
 http://www.laney.edu/wp/cheli fossum/files/2012/01/10 Enzymes.pdf Enzyme Catalysis Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without
http://www.laney.edu/wp/cheli fossum/files/2012/01/10 Enzymes.pdf Enzyme Catalysis Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without
