as an ATP regenerating system. 5-Oxoproline analogs were were purified from rat kidney. Rat kidney -y-glutamyl transpeptidase
|
|
|
- Shon Melton
- 5 years ago
- Views:
Transcription
1 Proc. Nati. Acad. Sci. USA Vol. 74, No. 8, pp , August 1977 Biochemistry Selective inhibition of y-glutamyl-cycle enzymes by substrate analogs (glutathione/glutamate and yglutamyl amino acid analogs/methionine sulfoximine/'y-glutamyl transpeptidase and cyclotransferase/5-oxoprolinase) OWEN W. GRIFFITH AND ALTON MEISTER Department of Biochemistry, Cornell University Medical College, New York, New York Contributed by Alton Meister, June 8,1977 Substrate analogs have been obtained that ABSTRACT selectively inhibit the reactions of the -glutamyl cycle or that are susceptible to only limited metabolism by the cycle. Thus, glutathione synthesis may be inhibited and analogs of glutathione may be synthesized that do not participate in transpeptidation. Specific inhibitors of "y-glutamylcyclotransferase and 5-oxoprolinase have been obtained. The findings offer new approaches to the in vivo study of the cycle and also to the design of more specifically directed analogs of inhibitors such as methionine sulfoximine and 6-diazo-5-oxonorleucine. The enzymic reactions of the y-glutamyl cycle [which accounts for the synthesis and degradation of glutathione (Fig. 1) (1)] include synthesis of the "y-glutamyl peptide bond, transfer of the y-glutamyl group to an acceptor, cyclization of the -y-glutamyl group to form 5-oxoproline, and energy-dependent decyclization of 5-oxoproline to glutamate. In an approach to further understanding of the function of the cycle, we have sought specific inhibitors of its individual reactions and also substrate analogs that would function in some but not all of the reactions. Such goals might be difficult to achieve because all of these reactions involve the y-carboxyl group of glutamate, and a given substrate analog might therefore be expected to interact with more than one enzyme. However, the several enzymic reaction pathways are clearly different and are presumably associated with differences in the enzyme-bound conformations of the glutamate carbon chain at the active sites of the several enzymes. In the present work, we have attempted to exploit such expected differences among the enzymes by manipulation of substrate structure.* We have found that suitable modification of the glutamyl moiety of the substrates yields the desired results: effective and specific inhibitors or nonmetabolizable analogs at each of the steps of the 'y-glutamyl cycle. The present findings thus offer several potentially new approaches to the study of the cycle in vvo and also provide information relevant to further investigations on the mechanisms of action of these metabolically related enzymes. The data reported here may be applied to the modification of potent (but relatively nonspecific) inhibitors such as methionine sulfoximine, azaserine, and 6-diazo-5-oxonorleucine so as to achieve one-enzyme specificity. The findings also make possible improved assay procedures for y-glutamyl-cysteine synthetase, glutathione synthetase, and y-glutamylcyclotransferase in which the substrates and products are not degraded by other enzymes present in unpurified tissue extracts. The costs of publication of this article were defrayed in part by the payment of page charges from funds made available to support the research which is the subject of the article. This article must therefore be hereby marked "advertisement" in accordance with 18 U. S. C solely to indicate this fact EXPERIMENTAL Materials. The various compounds were obtained or synthesized as follows: N-methyl-L-glutamic acid (Vega-Fox), a-methyl-dl-glutamic acid and L-a-aminobutyric acid (Sigma), D-glutamic acid (K and K), threo-13-hydroxyglutamic acid (Nutritional Biochemical Corp.), threo-y-hydroxy-lglutamic acid (Calbiochem),,3-methylglutamic acid (3), -ymethylglutamic acid (4), fl-aminoglutarate (13-glutamate) and fl-aminoadipate (5), and L-imidazolidone-4-carboxylate (6). DL-Aminomethylsuccinic acid was prepared from dimethylitaconate by the general procedure of Feuer and Swartz (5). D-y-Glutamyl-L-a-aminobutyric acid, 'y-(y-methyl)glutamyl-l-a-aminobutyric acid, and fl-aminoglutaryl-l-a-aminobutyric acid were prepared via, the corresponding N- phthaloyl glutamic anhydride analogs as described (7). L-'y- (N-Methyl)glutamyl-L-a-aminobutyricacid, L-'y-(a-methyl)- glutamyl-l-a-aminobutyric acid, y-(f-methyl)glutamyl-la-aminobutyric acid, and f3-aminoglutaryl-l-a-aminobutyric acid were prepared enzymatically with -y-glutamyl-cysteine synthetase by using phosphoenolpyruvate and pyruvate kinase as an ATP regenerating system. 5-Oxoproline analogs were prepared from the appropriate amino acid as described (8). 'y-glutamyl-cysteine synthetase (9) and 5-oxoprolinase (8) were purified from rat kidney. Rat kidney -y-glutamyl transpeptidase and sheep brain 7y-glutamylcyclotransferase were prepared by Suresh Tate and Vaira Wellner, respectively, of this laboratory. Glutathione synthetase was purified from rat kidney by a method to be described. Methods. The various enzyme activities were assayed as described in the tables. In the 'y-glutamyl-cysteine synthetase, glutathione synthetase, and -y-glutamyl transpeptidase assays "4C-labeled amino acids were separated from y-glutamyl 14C-labeled amino acids on small (0.5 X 7 cm) columns of Dowex 1 (acetate). Enzyme reactions were stopped by adding 100-,ul aliquots to 1 or 2 ml of 20 mm acetic acid; the solutions obtained were then added to small Dowex 1 columns. "4C- Labeled amino acids were washed through with 4-8 ml of 20 mm acetic acid, and y-glutamyl amino acids were eluted with 4 ml of 1.5 M ammonium acetate. RESULTS Glutathione synthesis The enzymes that catalyze the two-step synthesis of glutathione from glutamate, cysteine, and glycine (y-glutamyl-cysteine and glutathione synthetases) also catalyze the synthesis of the naturally occurring analogs, ophthalmic and norophthalmic acids (10-12), compounds in which the cysteine moiety is replaced * A preliminary account of some of these findings has appeared (2).
2 Biochemistry: Griffith and Meister ENZYMES Substrate y-glu-cysh GSH y-gujtranrs- y-gucylo- Analogs Synthetase Synthetase peptidase transease5-5 ase D-Glu O, a-methyl-glu (+) N-Methyl-Glu O.; _-MthA-Glu (+), +,j 0 + O, y-methyl-glu o + 0 (+), FIG. 1. Interaction of glutamate and glutamyl analogs with enzymes of the y-glutamyl cycle. CysH, cysteine; GSH, glutathione. Symbols are as follows: I, >506 inhibition; +, >10% as active as L-Glu; (+), 1-10% as active as L-Glu; and 0, <1% as active as L-Glu. by a-aminobutyrate and alanine, respectively. In many of the studies that have been carried out on these enzymes, including those reported here, L-a-aminobutyrate has been used in place of L-cysteine to avoid complications associated with the spontaneous oxidation of cysteine and y-glutamyl-cysteine. The activity of glutathione synthetase toward a series of y- glutamyl-a-aminobutyrate analogs is given in Table 1 together with a summary of data previously obtained (9) on y-glutamyl-cysteine synthetase. It is notable that glutathione synthetase exhibited much broader specificity toward substrates in which the glutamyl group was modified than did 'y-glutamyl-cysteine synthetase. Particularly striking was the finding that glutathione synthetase was active toward D-y-glutamyl- L-a-aminobutyrate. In contrast, although D-glutamate is activated by y-glutamyl-cysteine synthetase (9), it was not used for peptide formation. Thus, the glutathione synthesis system is protected in voo against introduction of a D-y-glutamyl residue only at the first step of its biosynthesis. It is also notable that,3-methylglutamate and y-methylglutamate were essentially inactive as substrates for y-glutamylcysteine synthetase; indeed, they inhibited this enzyme markedly. Whereas the mechanism of y-glutamyl-cysteine synthetase resembles that of glutamine synthetase in a number of respects (12, 13), the glutamate binding sites of these enzymes seem to differ significantly. Thus, N-methylglutamate was not a substrate of glutamine synthetase,t whereas glutamine synthetase was active toward fl-methylglutamate and y-methylglutamate. Enzymes that utilize glutathione y-glutamyl transpeptidase was significantly active with analogs containing D-glutamyl and a-methylglutamyl residues but not with those containing N-methylglutamyl, j-glutamyl, 6- methylglutamyl, or y-methylglutamyl moieties (Table 2). The corresponding analogs of glutathione would be expected to show similar substrate activity with transpeptidase. Previous t V. P. Wellner, unpublished studies in this laboratory. Proc. Nati. Acad. Sci. USA 74 (1977) 3331 studies in this laboratory showed that D-y-glutamyl-p-nitroanilide is a highly active substrate of y-glutamyl transpeptidase (14, 15); because D-amino acids are not acceptor substrates (16, 17), D-y-glutamyl-p-nitroanilide may be used in a convenient assay procedure that precludes autotranspeptidation (15, 18, 19). Similarly, a-substituted amino acids are not acceptors of the y-glutamyl group (17) and therefore autotranspeptidation does not occur with a-methyl-l-glutamyl-a-aminobutyrate (20). The ability of y-glutamyl transpeptidase to interact with D- and L-'y-glutamyl substrates is reminiscent of findings with glutamine synthetase that showed that both D- and L-glutamate and a-methyl-l-glutamate bind to the enzyme in an extended conformation (21, 22). However, the substrate-enzyme interactions involved in glutamine synthetase and y-glutamyl transpeptidase are clearly different because IB-aminoglutaryl-L-a-aminobutyrate, 0-methylglutamyl-La-aminobutyrate, and y-methylglutamyl-l-a-aminobutyrate are neither substrates nor inhibitors of the transpeptidase whereas the corresponding free glutamate analogs (a-glutamate, fl-methylglutamate, and oy-methylglutamate) are substrates of glutamine synthetase (22). In contrast to the results observed with 'y-glutamyl transpeptidase, only one of the analogs examined here, '-(,3- methyl)glutamyl-l-a-aminobutyrate, was a substrate of 'yglutamylcyclotransferase. The fact that the glutamyl derivatives with modifications at the a-carbon atom (i.e., D-glutamyl and a-methylglutamyl) do -not react presumably reflects the need in the cyclotransferase reaction to bring the a-carbon atom quite close to the catalytic center of the active site. The requirements for proper size and alignment in this region would be expected to be stringent.,6-aminoglutaryl-l-a-aminobutyrate was not expected to be a substrate of 'y-glutamylcyclotransferase because its reaction would require the formation of a 4-member ring. This analog is, however, an extremely potent inhibitor of the enzyme. As shown in Table 2, j8-aminoglutaryl-l-a-aminobutyrate almost completely inhibited the reaction of L-y-glutamyl-L-a-aminobutyrate at an equimolar concentration. In a similar experiment in which the concentration of inhibitor was reduced 10-fold, the inhibition was 67%. Whereas the inhibition is not accompanied by covalent attachment of the inhibitor, the inhibition is sufficiently strong to be useful in vwvo. Preliminary experiments have shown that the inhibitor is well transported. 5-Oxoprolinase Table 3 summarizes data on the interaction of 5-oxoprolinase with several 5-oxoproline analogs. As wi th the cyclotransferase reaction, the tolerance toward substitution by methyl groups was very limited. 5-Oxo-D-proline, N-methyl-5-oxoproline, and 2-methyl-5-oxoproline were neither substrates nor inhibitors. 4-Methyl-5-oxoproline was slightly but significantly active as a substrate; this compound and 3-methyl-5-oxoproline were potent inhibitors. Previous studies showed that the enzyme is active toward 2-piperidone-6-carboxylate and the 3- and 4- hydroxy derivatives of 5-oxo-L-proline (8). L-2-Imidazolidone-4-carboxylate is a potent competitive inhibitor in vitro (8, 23), and the effect of this compound has also been demonstrated in vlvo (6, 23). It is of interest that several of the compounds studied here, which are not decyclized by the enzyme, nevertheless induce ATP cleavage (8, 24, 25). DISCUSSION Data on the interaction of substrate analogs with the five enzymes of the -y-glutamyl cycle that catalyze reactions involving
3 3332 Biochemistry: Griffith and Meister Proc. Natl. Acad. Sci. USA 74 (1977) Table 1. Activity of glutathione synthesis enzymes toward substrate analogs y-glutamyl-cysteine synthetase* Glutathione synthetaset Substrate Relative % Relative % or analog activity inhibition activity inhibition L-Glutamate [100] [100] D-Glutamate fl-aminoglutarate N-Methyl-L-glutamate a-methyl-dl-glutamate fl-methyl-dl-glutamate y-methyl-dl-glutamate < * From ref. 9; based on Pi formation. No peptide was formed with D-glutamate. t Activity was determined by measurement of the incorporation of [14C]glycine into-tripeptide in the presence-of/y-glutamyl (or analog) L-aaminobutyrate in 1-ml reaction mixtures containing 150 mm imidazole.hcl (ph 7.2), 100 mm KCl, 10 mm [14C]glycine, 5 mm peptide (10 mm for -y-methyl derivative), 10 mm ATP, 10 mm phosphoenolpyruvate, 20 mm MgCl2, 0.5 mg of bovine serum albumin, and 2 international units of pyruvate kinase and glutathione synthetase. After min, aliquots were assayed for [14C]tripeptide formation (see Experimental). Inhibition of L-y-glutamyl-L-a-aminobutyrylglycine synthesis was determined in 250-,ul reaction mixtures containing 100 mm imidazole-hcl (ph 7.2), 100 mm KCl, 10 mm ATP, 10 mm phosphoenolpyruvate, 20 mm MgCl2, 10 mm L-y-glutamyl-L-a-aminobutyrate, 10 mm peptide analog (20 mm for -y-methyl derivative), 10 mm [14Cjglycine, 0.5 mg of bovine serum albumin, and 2 international units of pyruvate kinase and glutathione synthetase. After 15 and 30 min, aliquots were assayed for [14C]tripeptide formation. t y-glutamyl-cysteine synthetase was tested with a mixture of four isomers; the product of this enzymatic reaction was used for studies on glutathione synthetase. Mixture of four diastereoisomers. glutamate or glutamate derivatives are summarized in Fig. 1. It would be expected that the analogs studied here would be transported into cells and, indeed, this is supported by preliminary studies on a number of them. It is thus possible to consider several in uivo approaches in which selective inhibition of specific reactions might be achieved. Glutathione biosynthesis is normally controlled by feedback inhibition of Y-glutamyl-cysteine synthetase by glutathione and probably also by the tissue concentrations of cysteine (12, 13, 26). Depression of tissue levels of glutathione has been observed after administration of D-glutamate (27). The present data indicate that glutathione biosynthesis may be inhibited by administration of fl-methylglutamate and -y-methylglutamate. y-glutamyl-cysteine synthetase, like glutamine synthetase (1, 13, 28, 29), is inhibited by~methionine sulfoximine, an effect due to phosphorylation of this analog and its binding to the active site of the enzyme as methionine sulfoximine phosphate (29, 30). Administration of methionine sulfoximine to animals leads to markedly decreased levels of glutathione in the kidney and liver (31). The convulsant activity of methionine sulfoximine is probably due to inhibition of glutamine synthetase or,y-glutamyl-cysteine synthetase or both (32, 33). The possibility that the convulsant activity is due to metabolic products derived from methionine sulfoximine via the corresponding a-imino or a-keto derivatives (34) is excluded by recent work in this laboratory in which a-methylmethionine sulfoximine was prepared (0. W. Griffith and A. Meister, unpublished data). As expected from the present (Fig. 1) and previous (28, 29) Table 2. Activity of glutathione-utilizing enzymes toward substrate analogs Transpeptidaset Cyclotransferaset Substrate Relative % Relative % or analog* activity inhibition activity inhibition L-y-Glutamyl-L-Abat [100] [100] D--y-Glutamyl-L-Aba f3-aminoglutaryl-l-aba L-'y-(N-Methyl)glutamyl-L-Aba L--y-(a-Methyl)glutamyl-L-Aba y-(,b-methyl)glutamyl-l-aba y-(y-methyl)glutamyl-l-aba Glutathione * Aba, a-aminobutyrate. t Transpeptidase activity was determined in 600-,l reaction mixtures containing 175 mm Tris-HCl (ph 8.0), 10 mm donor analog [20 mm for -y-(y-methyl)glutamyl-l-abaj, 25 mm L-[14C]methionine, and 1.6 units of transpeptidase. Formation of -y-glutamyl-l-[14c]methionine (or analog) was assayed at 5-30 min (see Experimental). Inhibition of transpeptidation between L-y-glutamyl-L-Aba and L-[14C]methionine was measured in 250-Ml reaction mixtures containing 175 mm Tris-HCl (ph 8.0), 10 mm L--y-glutamyl-L-a-aminobutyrate, 10 mm analog [20 mm for y-(-y-methyl)glutamyl-l-aba], 25 mm L-[14C]methionine, and 08 unit of transpeptidase. Formation of y-glutamyl-l-[14c]methionine was assayed at min. The product formed presumably includes material derived both from L--y-glutamyl-L-a-aminobutyrate and from those analogs that are active donors. t Cyclotransferase activity was determined as enzyme-dependent release of L-a-aminobutyrate. The 200-,ul reaction mixtures contained 100 mm Tris-HCl (ph 8.0), 10 mm analog, and 10 units of cyclotransferase. After min, aliquots were removed, quenched in 5% sulfosalicyclic acid, and analyzed for a-aminobutyrate on a Durrum amino acid analyzer. Cyclotransferase inhibition of 5-oxo-L-[14C]proline formation from L--y-[4C]glutamyl-L-a-aminobutyrate was measured in 250-ul reaction mixtures containing 100 mm Tris-HCl (ph 8.0), 8 mm L--Y-['4C]glutamyl-L-a-aminobutyrate, 8 mm inhibitor, and 10 units of cyclotransferase. At 45 and 90 min, 100-,il aliquots were removed and quenched with 0.5 ml of 1% trichloroacetic acid. The resulting solution was loaded on columns (0.5 X 3 cm) of Dowex 5OW (H+), followed by 3 ml of H20. An aliquot of the total effluent was analyzed for 14C.
4 Table 3. Biochemistry: Griffith and Meister Activity of 5-oxo-L-prolinase toward substrate ainlos Relative % Analog activity inhibition 5-Oxo-L-proline [100] 5-Oxo-D-proline 0 1 N-Methyl-5-oxo-L-proline Methyl-5-oxo-DL-proline Methyl-5-oxoprolinet Methyl-5-oxoprolinet 4 66 threo-3-hydroxy-5-oxo-dl-proline 19 1 threo-4-hydroxy-5-oxo-dl-proline DL-2-Pyrrolidone-4-carboxylate 0 0 DL-2-Piperidone-6-carboxylate 7 6 DL-5-Carboxymethyl-2-pyrrolidone 0 3 L-Imidazolidone-4-carboxylate 0 86 * 5-Oxoprolinase was assayed as enzyme-dependent formation of amino acid. The 1-ml reaction mixtures contained 150 mm Tris.HCI, (ph 7.8), 150mM KCl, 10 mm analog (20 mm for DL-mixtures, 40 mm for four-isomer mixtures), 10 mm ATP, 5 mm phosphoenolpyruvate, 15 mm MgCl2, 0.15 mm EDTA, 5 mm dithiothreitol, 10 international units of pyruvate kinase, and 11 units of 5-oxoprolinase. After 30 min, aliquots of the reaction mixtures were analyzed for amino acid on a Durrum amino acid analyzer. Inhibition of 5- oxoprolinase was assayed in 500-gid reaction mixtures containing 100 mm Tris-HCl (ph 7.8), 150 mm KCl, 2 mm 5-oxo-L-[14CJproline, 2 mm 5-oxoproline analog (4 mm for DL-mixtures, 8 mm for fourisomer mixtures), 4 mm ATP, 2 mm phosphoenolpyruvate, 7.5 mm MgCl2, 0.1 mm EDTA, 5 mm dithiothreitol, TO international units of pyruvate kinase, and 1 unit of 5-oXo-L-prolinase. After 30 min, the reaction mixtures were analyzed for L-[14C~glutamate as described (8). t Mixture of four isomers. findings, a-methylmethionine sulfoximine (which has convulsant activity) inhibits both y-glutamyl-cysteine synthetase and glutamine synthetase. Additional studies on methionine sulfoximine analogs, based on the glutamate specificities observed here, are now needed with the aim of selectively inhibiting these synthetases. Glutathione biosynthesis may also be perturbed by administration of N-methyl-glutamate or fl-glutamate. These glutamate analogs can thus lead to synthesis of glutathione analogs that are not substrates for transpeptidation. It would be of interest to learn whether such modified glutathiones can be used by glutathione reductase or by glutathione S-transferases; this type of information might be helpful in elucidating the physiological role- of glutathione in relation to specific enzymic phenomena. Of the several y-glutamyl-l-a-aminobutyrate analogs that were examined as substrates and inhibitors of glutathione synthetase, all but two (those derived from D-glutamate and 7y-methylglutamate) would be expected to be synthesized in vivo. It might be possible to introduce in vivo a glutathione analog possessing a D-glutamyl moiety or a y-methylglutamyl moiety by administration of the corresponding COOH-terminal cysteine dipeptide. It would be expected that the D-glutamyl and a-methyl-l-glutamyl modifications of glutathione would be substrates -for y-glutamyl transpeptidase (in contrast to modified glutathiones in which the y-glutamyl moieties are replaced by N-methylglutamyl, #-glutamyl, flmethylglutamyl, or y-methylglutamyl groups). Although D-y-glutamyl and a-methylglutamyl derivatives are substrates of the transpeptidase, the'se compounds are not substrates of y-glutamylcyclotransferase and in fact D-'y-glutamyl amino acids inhibit this enzyme. Studies with D-'y-glutamyl- or L-a-methyl-glutamyl-L-amino acids might therefore -elucidate the extent to which such compounds are hydrolyzed-.4n-vo by the action Proc. Natl. Acad. Sci. USA 74 (1977) 3333 of -glutamyl transpeptidase. In a similar manner, -(i3- methyl)glutamyl-l-a-aminobutyrate may be used to measure in vio cyclotransferase activity without interference by transpeptidase. These studies have also led to the finding of two highly active inhibitors of y-glutamylcyclotransferase [D-yglutamyl-L-a-aminobutyrate and 'y-(,-glutamyl)-l-a-aminobutyrate]; preliminary studies indicate that these compounds are transported into cells and they, therefore, may be of value in elucidating the in vivo function of y-glutamylcyclotransferase. The present and earlier studies thus indicate that the 7-glutamyl cycle may be inhibited with respect to glutathione synthesis (D-glutamate, fl-methylglutamate, methionine sulfoximine), transpeptidation [7-glutamyl hydrazones of a-keto acids (17), azaserine, 6-diazo-5-oxonorleucine (35, 36)], cyclization (D-'y-glutamyl amino acids, fl-aminoglutaryl amino acids), and decyclization of 5-oxoproline. These studies have also revealed a number of analogs that are susceptible to only limited metabolism; finally, they suggest logical approaches to the design of new and more specific analogs of azaserine, 6-diazo-5-oxonorleucine, and methionine sulfoximine. We thank Mr. Lawrence Eckler for his skillful technical assistance. This research was supported in part by grants from the National Science Foundation and the National Institutes of Health, U.S. Public Health Service. O.W.G. is a Postdoctoral Research Fellow of the Natiorial Institutes of Health, U.S. Public Health Service (1 IF22 AM 01397). 1. Meister, A. & Tate, S. S. (1976) Annu. Rev. Biochem. 45, Griffith, 0. W. (1977) Fed. Proc. 36, Morrison, D. C. (1955) J. Am. Chem. Soc. 77, Done, J. & Fowden, L. (1952) Biochem. J. 51, Feuer, H. & Swartz, W. A. (1955) J. Am. Chem. Soc. 77, Van Der Werf, P., Stephani, R. A. & Meister, A. (1974) Proc. Nati. Acad. Sci. USA, 71, Orlowski, M. & Meister, A. (1973) J. Biol. Chem. 248, Van Der Werf, P., Griffith, 0. & Meister, A. (1975) J. Blol. Chem. 250, Sekura, R. & Meister, A. (1977) J. Biol. Chem. 252, Waley, S. G. (1956) Biochem. J. 64, Waley, S. G. (1958) Biochem. J. 68, Meister, A. (1975) in Metabolism of Sulfur Compounds, Metabolic Pathways, ed. Greenberg, D. M. (Academic Press, New York), Vol. 7, 3rd ed., pp Meister, A. (1974) The Enzymes (Academic Press, New York), Vol. 10, 3rd ed., pp , Orlowski, M. & Meister, A. (1963) Biochim. Biophys. Acta 73, Thompson, G. A. & Meister, A. (1976) Biochem. Biophys. Res. Commun. 71, Fodor, P. J., Miller, A. & Waelsch, H. (1953) J. Biol. Chem. 202, Tate, S. S. & Meister, A. (1974) J. Biol. Chem. 249, Meister, A., Tate, S. S. & Thompson, G. A. (1977) CIBA Symp., in press. 19. Thompson, G. A. & Meister, A. (1977) J. Biol. Chem., in press. 20. Karkowski, A. M., Bergamini, M. V. W. & Orlowski, M. (1976) J. Biol. Chem. 251, Kagan, H. M., Manning, L. R. & Meister, A. (1965) Biochemistry 4, Meister, A. (1968) Adv. Enzymol. 31, Van Der Werf, P., Stephani, R. A., Orlowski, M. & Meister, A. (1973) Proc. Nati. Acad. Sci. USA 70, Griffith, 0. W., Van Der Werf, P. & Meister, A. (1976) in Glutathione: Metabolism and Function, eds. Arias, I. M. & Jakoby,
5 3334 Biochemistry: Griffith and Meister W. B., Krok Foundation Symposium, Santa Barbara, Calif., June 1-3, 1975 (Raven Press, New York), pp Griffith, 0. W. & Meister, A. (1976) Biochem. Blophys. Res. Commun. 70, Richman, P. & Meister, A. (1975) J. Blol. Chem. 250, Sekura, R., Van Der Werf, P. & Meister, A. (1976) Blochem. Blophys. Res. Commun. 71, Ronzio, R. A., Rowe, W. B. & Meister, A. (1969) Biochemistry 8, Manning, J. M., Moore, S., Rowe, W. B. & Meister, A. (1969) Biochemistry 8, Richman, P. G., Orlowski, M. & Meister, A. (1973) J. Blol. Chem. 248, Proc. Nati. Acad. Sci. USA 74 (1977) 31. Palekar, A. G., Tate, S. S. & Meister, A. (1975) Biochem. Biophys. Res. Commun. 62, Rowe, W. B. & Meister, A. (1970) Proc. Nati. Acad. Sci. USA, 66, Meister, A. (1974) in Symposium on Brain Dysfunction in Metabolic Disorders, Research Publications of the Association for Research in Nervous and Mental Disease (Raven Press, New York), Vol. 53, pp Cooper, A. J. L., Stephani, R. A. & Meister, A. (1976) J. Biol. Chem. 251, Tate, S. S. & Meister, A. (1977) Proc. Natl. Acad. Sci. USA 74, Inoue, M., Horiuchi, S. & Morino, Y. (1966) Eur. J. Biochem. 73,
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Previous Class. Today. Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism. Protein Kinase Catalytic Properties
 Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Significance of glutathione S-conjugate for glutathione metabolism in human erythrocytes
 Eur. J. Biochem. 145,131-136 (1984) 0 FEBS 1984 Significance of glutathione S-conjugate for glutathione metabolism in human erythrocytes Takahito KONDO, Naoyuki TANIGUCHI, and Yoshikazu KAWAKAMI First
Eur. J. Biochem. 145,131-136 (1984) 0 FEBS 1984 Significance of glutathione S-conjugate for glutathione metabolism in human erythrocytes Takahito KONDO, Naoyuki TANIGUCHI, and Yoshikazu KAWAKAMI First
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Functional changes in rat liver trna following aflatoxin Β 1 administration
 J. Biosci., Vol. 3 Number 3, September 1981, pp. 215-219 Printed in India. Functional changes in rat liver trna following aflatoxin Β 1 administration R. K. BHATTACHARYA and V.S. ABOOBAKER Biochemistry
J. Biosci., Vol. 3 Number 3, September 1981, pp. 215-219 Printed in India. Functional changes in rat liver trna following aflatoxin Β 1 administration R. K. BHATTACHARYA and V.S. ABOOBAKER Biochemistry
Biochemistry 2 Recita0on Amino Acid Metabolism
 Biochemistry 2 Recita0on Amino Acid Metabolism 04-20- 2015 Glutamine and Glutamate as key entry points for NH 4 + Amino acid catabolism Glutamine synthetase enables toxic NH 4 + to combine with glutamate
Biochemistry 2 Recita0on Amino Acid Metabolism 04-20- 2015 Glutamine and Glutamate as key entry points for NH 4 + Amino acid catabolism Glutamine synthetase enables toxic NH 4 + to combine with glutamate
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/ MCQs. Location : 102, 105, 106, 301, 302
 FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
PAPER No. : 16 Bioorganic and biophysical chemistry MODULE No. : 25 Coenzyme-I Coenzyme A, TPP, B12 and biotin
 Subject Paper No and Title Module No and Title Module Tag 16, Bio organic and Bio physical chemistry 25, Coenzyme-I : Coenzyme A, TPP, B12 and CHE_P16_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
Subject Paper No and Title Module No and Title Module Tag 16, Bio organic and Bio physical chemistry 25, Coenzyme-I : Coenzyme A, TPP, B12 and CHE_P16_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Chapter 23 Enzymes 1
 Chapter 23 Enzymes 1 Enzymes Ribbon diagram of cytochrome c oxidase, the enzyme that directly uses oxygen during respiration. 2 Enzyme Catalysis Enzyme: A biological catalyst. With the exception of some
Chapter 23 Enzymes 1 Enzymes Ribbon diagram of cytochrome c oxidase, the enzyme that directly uses oxygen during respiration. 2 Enzyme Catalysis Enzyme: A biological catalyst. With the exception of some
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 31 Amino Acid Synthesis 2013 W. H. Freeman and Company Chapter 31 Outline Although the atmosphere is approximately 80% nitrogen,
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 31 Amino Acid Synthesis 2013 W. H. Freeman and Company Chapter 31 Outline Although the atmosphere is approximately 80% nitrogen,
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Lecture 10 - Protein Turnover and Amino Acid Catabolism
 Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):
 Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 A schematic representation of the most relevant
Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 A schematic representation of the most relevant
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Lecture 11 - Biosynthesis of Amino Acids
 Lecture 11 - Biosynthesis of Amino Acids Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction Biosynthetic pathways for amino acids, nucleotides and lipids
Lecture 11 - Biosynthesis of Amino Acids Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction Biosynthetic pathways for amino acids, nucleotides and lipids
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
MBB 694:407, 115:511. Please use BLOCK CAPITAL letters like this --- A, B, C, D, E. Not lowercase!
 MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
Carbon and the Molecular Diversity of Life. Chapter 4
 Carbon and the Molecular Diversity of Life Chapter 4 CARBON Carbon is has ability to form large and complex, molecules Aspirin molecular formula? A triglyceride Organic chemistry is study of carbon compounds
Carbon and the Molecular Diversity of Life Chapter 4 CARBON Carbon is has ability to form large and complex, molecules Aspirin molecular formula? A triglyceride Organic chemistry is study of carbon compounds
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
From the Department of Biochemistry, Cornell University Medical College, New York, New York 10091
 THE Jomwn~ or BIO~GICAL CHEMIBTRY Vol. 248, No. 8, Issue of April 25, pp. 2836-2844, 1973 Printed in U.S.A. y-glutamyl Cyclotransferase DISTRIBUTION, ISOZYMIC FORMS, AND SPECIFICITY * MARIAN ORLOWSKI AND
THE Jomwn~ or BIO~GICAL CHEMIBTRY Vol. 248, No. 8, Issue of April 25, pp. 2836-2844, 1973 Printed in U.S.A. y-glutamyl Cyclotransferase DISTRIBUTION, ISOZYMIC FORMS, AND SPECIFICITY * MARIAN ORLOWSKI AND
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture -02 Amino Acids II
 Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
UNIVERSITY OF GUELPH CHEM 4540 ENZYMOLOGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS
 UNIVERSITY F GUELPH CHEM 4540 ENZYMLGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS Instructions: Time allowed = 80 minutes. Total marks = 30. This quiz represents
UNIVERSITY F GUELPH CHEM 4540 ENZYMLGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS Instructions: Time allowed = 80 minutes. Total marks = 30. This quiz represents
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
Six Types of Enzyme Catalysts
 Six Types of Enzyme Catalysts Although a huge number of reactions occur in living systems, these reactions fall into only half a dozen types. The reactions are: 1. Oxidation and reduction. Enzymes that
Six Types of Enzyme Catalysts Although a huge number of reactions occur in living systems, these reactions fall into only half a dozen types. The reactions are: 1. Oxidation and reduction. Enzymes that
Student Number: To form the polar phase when adsorption chromatography was used.
 Name: Student Number: April 14, 2001, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Lab Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided.. 2. The last page
Name: Student Number: April 14, 2001, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Lab Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided.. 2. The last page
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
Mechanisms of Enzymes
 Mechanisms of Enzymes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy How enzymes work * Chemical reactions have an energy
Mechanisms of Enzymes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy How enzymes work * Chemical reactions have an energy
Amino acid metabolism
 Amino acid metabolism The important reaction commonly employed in the breakdown of an amino acid is always the removal of its -amino group. The product ammonia is excreted after conversion to urea or other
Amino acid metabolism The important reaction commonly employed in the breakdown of an amino acid is always the removal of its -amino group. The product ammonia is excreted after conversion to urea or other
Adenosine triphosphate (ATP)
 Adenosine triphosphate (ATP) 1 High energy bonds ATP adenosine triphosphate N NH 2 N -O O P O O P O- O- O O P O- O CH 2 H O H N N adenine phosphoanhydride bonds (~) H OH ribose H OH Phosphoanhydride bonds
Adenosine triphosphate (ATP) 1 High energy bonds ATP adenosine triphosphate N NH 2 N -O O P O O P O- O- O O P O- O CH 2 H O H N N adenine phosphoanhydride bonds (~) H OH ribose H OH Phosphoanhydride bonds
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
(5) 1. List five unusual properties of water resulting from its hydrogen bonded structure
 BCH 4053 June 1, 2001 Points HOUR TEST 1 NAME (5) 1. List five unusual properties of water resulting from its hydrogen bonded structure. Page Points 1 2 3 4 5 Total (5) 2. Draw a diagram to show how water
BCH 4053 June 1, 2001 Points HOUR TEST 1 NAME (5) 1. List five unusual properties of water resulting from its hydrogen bonded structure. Page Points 1 2 3 4 5 Total (5) 2. Draw a diagram to show how water
CELLULAR METABOLISM. Metabolic pathways can be linear, branched, cyclic or spiral
 CHM333 LECTURE 24 & 25: 3/27 29/13 SPRING 2013 Professor Christine Hrycyna CELLULAR METABOLISM What is metabolism? - How cells acquire, transform, store and use energy - Study reactions in a cell and how
CHM333 LECTURE 24 & 25: 3/27 29/13 SPRING 2013 Professor Christine Hrycyna CELLULAR METABOLISM What is metabolism? - How cells acquire, transform, store and use energy - Study reactions in a cell and how
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
The Conservation of Homochirality and Prebiotic Synthesis of Amino Acids
 The Conservation of Homochirality and Prebiotic Synthesis of Amino Acids Harold J. Morowitz SFI WORKING PAPER: 2001-03-017 SFI Working Papers contain accounts of scientific work of the author(s) and do
The Conservation of Homochirality and Prebiotic Synthesis of Amino Acids Harold J. Morowitz SFI WORKING PAPER: 2001-03-017 SFI Working Papers contain accounts of scientific work of the author(s) and do
AMINO ACIDS NON-ESSENTIAL ESSENTIAL
 Edith Frederika Introduction A major component of food is PROTEIN The protein ingested as part of our diet are not the same protein required by the body Only 40 to 50 gr of protein is required by a normal
Edith Frederika Introduction A major component of food is PROTEIN The protein ingested as part of our diet are not the same protein required by the body Only 40 to 50 gr of protein is required by a normal
Catabolism of Carbon skeletons of Amino acids. Amino acid metabolism
 Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
Catabolism of Carbon skeletons of Amino acids Amino acid metabolism Carbon skeleton Carbon Skeleton a carbon skeleton is the internal structure of organic molecules. Carbon Arrangements The arrangement
Metabolism of Nucleotides
 Metabolism of Nucleotides Outline Nucleotide degradation Components of Nucleobases Purine and pyrimidine biosynthesis Hyperuricemia Sources Nucleotide degradation The nucleotides are among the most complex
Metabolism of Nucleotides Outline Nucleotide degradation Components of Nucleobases Purine and pyrimidine biosynthesis Hyperuricemia Sources Nucleotide degradation The nucleotides are among the most complex
Chapter 11: Enzyme Catalysis
 Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
THE UNIVERSITY OF MANITOBA. DATE: Oct. 22, 2002 Midterm EXAMINATION. PAPER NO.: PAGE NO.: 1of 6 DEPARTMENT & COURSE NO.: 2.277/60.
 PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
االمتحان النهائي لعام 1122
 االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
Glutathione Synthesis in Human Erythrocytes
 Glutathione Synthesis in Human Erythrocytes II. PURIFICATION AND PROPERTIES OF THE ENZYMES OF GLUTATHIONE BIOSYNTHESIS PHILI W. MAjEUS, M. J. BRAUNER, M. B. SMITH, and VIRGINIA MINNICH From the Departments
Glutathione Synthesis in Human Erythrocytes II. PURIFICATION AND PROPERTIES OF THE ENZYMES OF GLUTATHIONE BIOSYNTHESIS PHILI W. MAjEUS, M. J. BRAUNER, M. B. SMITH, and VIRGINIA MINNICH From the Departments
Calpain Assays, Nerve injury and Repair
 Calpain Assays, Nerve injury and Repair Annual Progress Report (G-3 3-Y44) April 2001 James C. Powers School of Chemistry and Biochemistry Georgia Institute of Technology Atlanta, GA 30332-0400 (404) 894-4038
Calpain Assays, Nerve injury and Repair Annual Progress Report (G-3 3-Y44) April 2001 James C. Powers School of Chemistry and Biochemistry Georgia Institute of Technology Atlanta, GA 30332-0400 (404) 894-4038
Vocabulary. Chapter 20: Electron Transport and Oxidative Phosphorylation
 Vocabulary ATP Synthase: the enzyme responsible for production of ATP in mitochondria Chemiosmotic Coupling: the mechanism for coupling electron transport to oxidative phosphorylation; it requires a proton
Vocabulary ATP Synthase: the enzyme responsible for production of ATP in mitochondria Chemiosmotic Coupling: the mechanism for coupling electron transport to oxidative phosphorylation; it requires a proton
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
Metabolism of amino acids. Vladimíra Kvasnicová
 Metabolism of amino acids Vladimíra Kvasnicová Classification of proteinogenic AAs -metabolic point of view 1) biosynthesis in a human body nonessential (are synthesized) essential (must be present in
Metabolism of amino acids Vladimíra Kvasnicová Classification of proteinogenic AAs -metabolic point of view 1) biosynthesis in a human body nonessential (are synthesized) essential (must be present in
PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77
 PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77 DIGESTION & ABSORPTION DIETARY PROTEINS SERVE 3 FUNCTIONS 1. THEIR CONSTITUTENT AMINOACIDS ARE USED FOR SYNTHESIS OF BODY PROTEINS
PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77 DIGESTION & ABSORPTION DIETARY PROTEINS SERVE 3 FUNCTIONS 1. THEIR CONSTITUTENT AMINOACIDS ARE USED FOR SYNTHESIS OF BODY PROTEINS
Intrahepatic transport and utilization of biliary glutathione and
 Proc. Natl. Acad. Sci. USA Vol. 83, pp. 124625, March 1986 Biochemistry ntrahepatic transport and utilization of biliary glutathione and its metabolites (developmental changesy-glutamylglutathione-glutamylcyst(e)inecyst(e)inylglyciney-glutamyl
Proc. Natl. Acad. Sci. USA Vol. 83, pp. 124625, March 1986 Biochemistry ntrahepatic transport and utilization of biliary glutathione and its metabolites (developmental changesy-glutamylglutathione-glutamylcyst(e)inecyst(e)inylglyciney-glutamyl
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Review of Biochemistry
 Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Enzymes Part III: regulation II. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Figure 1 Original Advantages of biological reactions being catalyzed by enzymes:
 Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Metabolic Changes of Drugs and Related Organic Compounds
 Metabolic Changes of Drugs and Related Organic Compounds 3 rd stage/ 1 st course Lecture 7 Shokhan J. Hamid 1 Phase II or Conjugation Reactions Phase I or functionalization reactions do not always produce
Metabolic Changes of Drugs and Related Organic Compounds 3 rd stage/ 1 st course Lecture 7 Shokhan J. Hamid 1 Phase II or Conjugation Reactions Phase I or functionalization reactions do not always produce
Drug Metabolism Phase 2 conjugation reactions. Medicinal chemistry 3 rd stage
 Drug Metabolism Phase 2 conjugation reactions Medicinal chemistry 3 rd stage 1 Phase II or Conjugation reactions 1. Glucuronic acid conjugation 2. Sulfate conjugation 3. Glycine and Glutamine conjugation
Drug Metabolism Phase 2 conjugation reactions Medicinal chemistry 3 rd stage 1 Phase II or Conjugation reactions 1. Glucuronic acid conjugation 2. Sulfate conjugation 3. Glycine and Glutamine conjugation
erythrocyte membranes (transport/inhibition/isozyme)
 Proc. Nad. Acad. Sci. USA Vol. 84, pp. 7373-7377, November 1987 Biochemistry Glutathione disulfide-stimulated Mg2+-ATPase of human erythrocyte membranes (transport/inhibition/isozyme) TAKAHITO KONDO*,
Proc. Nad. Acad. Sci. USA Vol. 84, pp. 7373-7377, November 1987 Biochemistry Glutathione disulfide-stimulated Mg2+-ATPase of human erythrocyte membranes (transport/inhibition/isozyme) TAKAHITO KONDO*,
Citrate Cycle. Lecture 28. Key Concepts. The Citrate Cycle captures energy using redox reactions
 Citrate Cycle Lecture 28 Key Concepts The Citrate Cycle captures energy using redox reactions Eight reactions of the Citrate Cycle Key control points in the Citrate Cycle regulate metabolic flux What role
Citrate Cycle Lecture 28 Key Concepts The Citrate Cycle captures energy using redox reactions Eight reactions of the Citrate Cycle Key control points in the Citrate Cycle regulate metabolic flux What role
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Marah Bitar. Faisal Nimri ... Nafeth Abu Tarboosh
 8 Marah Bitar Faisal Nimri... Nafeth Abu Tarboosh Summary of the 8 steps of citric acid cycle Step 1. Acetyl CoA joins with a four-carbon molecule, oxaloacetate, releasing the CoA group and forming a six-carbon
8 Marah Bitar Faisal Nimri... Nafeth Abu Tarboosh Summary of the 8 steps of citric acid cycle Step 1. Acetyl CoA joins with a four-carbon molecule, oxaloacetate, releasing the CoA group and forming a six-carbon
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Lecture 34. Carbohydrate Metabolism 2. Glycogen. Key Concepts. Biochemistry and regulation of glycogen degradation
 Lecture 34 Carbohydrate Metabolism 2 Glycogen Key Concepts Overview of Glycogen Metabolism Biochemistry and regulation of glycogen degradation Biochemistry and regulation of glycogen synthesis What mechanisms
Lecture 34 Carbohydrate Metabolism 2 Glycogen Key Concepts Overview of Glycogen Metabolism Biochemistry and regulation of glycogen degradation Biochemistry and regulation of glycogen synthesis What mechanisms
Chapter 10. Regulatory Strategy
 Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Division of Applied Life Sciences (Institute for Chemical R
 Chair Division of Applied Life Sciences (Institute for Chemical R 2.3.12 Laboratory:Chemistry of Molecular Biocatalysts Member: Professor Hiratake, Jun, Dr. Agric. Sci. Assistant Professor Watanabe, bunta,
Chair Division of Applied Life Sciences (Institute for Chemical R 2.3.12 Laboratory:Chemistry of Molecular Biocatalysts Member: Professor Hiratake, Jun, Dr. Agric. Sci. Assistant Professor Watanabe, bunta,
Syllabus for BASIC METABOLIC PRINCIPLES
 Syllabus for BASIC METABOLIC PRINCIPLES The video lecture covers basic principles you will need to know for the lectures covering enzymes and metabolism in Principles of Metabolism and elsewhere in the
Syllabus for BASIC METABOLIC PRINCIPLES The video lecture covers basic principles you will need to know for the lectures covering enzymes and metabolism in Principles of Metabolism and elsewhere in the
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 30 Amino Acid Degradation and the Urea Cycle 2013 W. H. Freeman and Company Chapter 30 Outline Amino acids are obtained from the
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 30 Amino Acid Degradation and the Urea Cycle 2013 W. H. Freeman and Company Chapter 30 Outline Amino acids are obtained from the
Nitrogen Metabolism. Pratt and Cornely Chapter 18
 Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Module No. # 01 Lecture No. # 19 TCA Cycle
 Biochemical Engineering Prof. Dr. Rintu Banerjee Department of Agricultural and Food Engineering Asst. Prof. Dr. Saikat Chakraborty Department of Chemical Engineering Indian Institute of Technology, Kharagpur
Biochemical Engineering Prof. Dr. Rintu Banerjee Department of Agricultural and Food Engineering Asst. Prof. Dr. Saikat Chakraborty Department of Chemical Engineering Indian Institute of Technology, Kharagpur
Structure of -amino acids. Stereoisomers of -amino acids. All amino acids in proteins are L-amino acids, except for glycine, which is achiral.
 amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
Tala Saleh. Ahmad Attari. Mamoun Ahram
 23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
TRANSLATION. Translation is a process where proteins are made by the ribosomes on the mrna strand.
 TRANSLATION Dr. Mahesha H B, Yuvaraja s College, University of Mysore, Mysuru. Translation is a process where proteins are made by the ribosomes on the mrna strand. Or The process in the ribosomes of a
TRANSLATION Dr. Mahesha H B, Yuvaraja s College, University of Mysore, Mysuru. Translation is a process where proteins are made by the ribosomes on the mrna strand. Or The process in the ribosomes of a
number Done by Corrected by Doctor Nayef Karadsheh
 number 13 Done by Asma Karameh Corrected by Saad hayek Doctor Nayef Karadsheh Gluconeogenesis This lecture covers gluconeogenesis with aspects of: 1) Introduction to glucose distribution through tissues.
number 13 Done by Asma Karameh Corrected by Saad hayek Doctor Nayef Karadsheh Gluconeogenesis This lecture covers gluconeogenesis with aspects of: 1) Introduction to glucose distribution through tissues.
III. 6. Test. Respiració cel lular
 III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
Coenzymes, vitamins and trace elements 209. Petr Tůma Eva Samcová
 Coenzymes, vitamins and trace elements 209 Petr Tůma Eva Samcová History and nomenclature of enzymes 1810, Gay-Lussac made an experiment with yeats alter saccharide to ethanol and CO 2 Fermentation From
Coenzymes, vitamins and trace elements 209 Petr Tůma Eva Samcová History and nomenclature of enzymes 1810, Gay-Lussac made an experiment with yeats alter saccharide to ethanol and CO 2 Fermentation From
CHAPTER 10: REGULATORY STRATEGIES. Traffic signals control the flow of traffic
 CHAPTER 10: REGULATORY STRATEGIES Traffic signals control the flow of traffic INTRODUCTION CHAPTER 10 The activity of enzymes must often be regulated so that they function at the proper time and place.
CHAPTER 10: REGULATORY STRATEGIES Traffic signals control the flow of traffic INTRODUCTION CHAPTER 10 The activity of enzymes must often be regulated so that they function at the proper time and place.
This student paper was written as an assignment in the graduate course
 77:222 Spring 2003 Free Radicals in Biology and Medicine Page 0 This student paper was written as an assignment in the graduate course Free Radicals in Biology and Medicine (77:222, Spring 2003) offered
77:222 Spring 2003 Free Radicals in Biology and Medicine Page 0 This student paper was written as an assignment in the graduate course Free Radicals in Biology and Medicine (77:222, Spring 2003) offered
Amino Acid Metabolism
 Amino Acid Metabolism Last Week Most of the Animal Kingdom = Lazy - Most higher organisms in the animal kindom don t bother to make all of the amino acids. - Instead, we eat things that make the essential
Amino Acid Metabolism Last Week Most of the Animal Kingdom = Lazy - Most higher organisms in the animal kindom don t bother to make all of the amino acids. - Instead, we eat things that make the essential
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
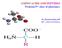 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
MBioS 303 Recitation Introductory Biochemistry, Summer 2008 Practice Problem Set #7: General Metabolism Concepts, Glycolysis and the TCA Cycle
 MBioS 303 Recitation Introductory Biochemistry, Summer 2008 Practice Problem Set #7: General Metabolism Concepts, Glycolysis and the TCA Cycle (1) Glucose 1-pohsphate is converted to fructose 6-phosphate
MBioS 303 Recitation Introductory Biochemistry, Summer 2008 Practice Problem Set #7: General Metabolism Concepts, Glycolysis and the TCA Cycle (1) Glucose 1-pohsphate is converted to fructose 6-phosphate
PEPTIDES and PROTEINS
 PEPTIDES and PROTEINS - Learned basic chemistry of amino acids structure and charges - Chemical nature/charges of amino acids is CRUCIAL to the structure and function of proteins - Amino acids can assemble
PEPTIDES and PROTEINS - Learned basic chemistry of amino acids structure and charges - Chemical nature/charges of amino acids is CRUCIAL to the structure and function of proteins - Amino acids can assemble
Nitrogen Metabolism. Overview
 Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Nitrogen Metabolism Pratt and Cornely Chapter 18 Overview Nitrogen assimilation Amino acid biosynthesis Nonessential aa Essential aa Nucleotide biosynthesis Amino Acid Catabolism Urea Cycle Juicy Steak
Polar bodies are either introduced or unmasked, which results in more polar metabolites Phase I reactions can lead either to activation or
 Polar bodies are either introduced or unmasked, which results in more polar metabolites Phase I reactions can lead either to activation or inactivation of the drug (i.e. therapeutic effects or toxicity)
Polar bodies are either introduced or unmasked, which results in more polar metabolites Phase I reactions can lead either to activation or inactivation of the drug (i.e. therapeutic effects or toxicity)
III. Metabolism - Gluconeogenesis
 Department of Chemistry and Biochemistry University of Lethbridge III. Metabolism - Gluconeogenesis Carl & Gertrude Cori Slide 1 Carbohydrate Synthesis Lactate, pyruvate and glycerol are the important
Department of Chemistry and Biochemistry University of Lethbridge III. Metabolism - Gluconeogenesis Carl & Gertrude Cori Slide 1 Carbohydrate Synthesis Lactate, pyruvate and glycerol are the important
Midterm 2. Low: 14 Mean: 61.3 High: 98. Standard Deviation: 17.7
 Midterm 2 Low: 14 Mean: 61.3 High: 98 Standard Deviation: 17.7 Lecture 17 Amino Acid Metabolism Review of Urea Cycle N and S assimilation Last cofactors: THF and SAM Synthesis of few amino acids Dietary
Midterm 2 Low: 14 Mean: 61.3 High: 98 Standard Deviation: 17.7 Lecture 17 Amino Acid Metabolism Review of Urea Cycle N and S assimilation Last cofactors: THF and SAM Synthesis of few amino acids Dietary
PII S (99) BIOLOGIC AND PHARMACOLOGIC REGULATION OF MAMMALIAN GLUTATHIONE SYNTHESIS OWEN W. GRIFFITH
 PII S0891-5849(99)00176-8 Free Radical Biology & Medicine, Vol. 27, Nos. 9/10, pp. 922 935, 1999 Copyright 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0891-5849/99/$ see front matter
PII S0891-5849(99)00176-8 Free Radical Biology & Medicine, Vol. 27, Nos. 9/10, pp. 922 935, 1999 Copyright 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0891-5849/99/$ see front matter
Aerobic Fate of Pyruvate. Chapter 16 Homework Assignment. Chapter 16 The Citric Acid Cycle
 Chapter 16 Homework Assignment The following problems will be due once we finish the chapter: 1, 3, 7, 10, 16, 19, 20 Additional Problem: Write out the eight reaction steps of the Citric Acid Cycle, using
Chapter 16 Homework Assignment The following problems will be due once we finish the chapter: 1, 3, 7, 10, 16, 19, 20 Additional Problem: Write out the eight reaction steps of the Citric Acid Cycle, using
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Respiration. Respiration. Respiration. How Cells Harvest Energy. Chapter 7
 How Cells Harvest Energy Chapter 7 Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs: live on
How Cells Harvest Energy Chapter 7 Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs: live on
An Introduction to Enzyme Structure and Function
 An Introduction to Enzyme Structure and Function Enzymes Many reactions in living systems are similar to laboratory reactions. 1. Reactions in living systems often occur with the aid of enzymes. 2. Enzymes
An Introduction to Enzyme Structure and Function Enzymes Many reactions in living systems are similar to laboratory reactions. 1. Reactions in living systems often occur with the aid of enzymes. 2. Enzymes
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
