VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE
|
|
|
- Alannah Dixon
- 5 years ago
- Views:
Transcription
1 VANDERBILT TUDENT VOLUNTEER FOR CIENCE Diffusion Fall 2018 oal: To understand diffusion, the process in which there is movement of a substance from an area of high concentration of that substance to an area of low concentration TN Curriculum Alignment: 7.L1.2 VVer Lesson Outline I. Introduction Discuss the motion of molecules using examples such as the smell of cooking from a distance or the smell of perfume in the air when someone wearing perfume walks by. A. Modeling emi-permeable Membranes One VV member will show students how to use a container with a wire-screen separating rye seeds and bean seeds as a model for a semi-permeable membrane. The rye seeds, representing small molecules, pass through the screen but the bean seeds, representing large molecules, do not pass through the screen. B. Dialysis tubing and Relative izes of Molecules how students the paper models of iodine, glucose, and starch. Discuss the relative sizes and point out that starch is a "polymer molecule made up of hundreds of glucose molecules joined together. II. Testing for lucose and tarch A. lucose Test tudent use glucose test strips to become familiar with the positive test for glucose. B. tarch Test tudents use iodine to test for starch. III. Diffusion of lucose and tarch A. lucose Diffusion A VV volunteer should distribute the dialysis tubing (containing glucose and starch) in the cup to each pair of students. B. Predicting Which Molecules Will Diffuse While students are waiting, show them the paper models of the molecules again and have the students try to predict which molecules will diffuse through the tubing. C. Testing for Diffusion of lucose roups test for glucose after 10 minutes. D. Testing for Diffusion of tarch After a positive test for glucose outside the dialysis tubing has been obtained, students can add ALL the rest of the iodine to the water in the cup. tudents should observe a purple/black color form inside the dialysis tubing. IV. Review ummarize the glucose and starch dialysis results for the whole class. As part of this review, show the models of iodine, glucose, and starch to make sure students understand the relationship of molecular size to their ability to diffuse through semi-permeable membranes. LOOK AT THE VIDEO BEFORE YOU O OUT TO YOUR CLAROOM UE THE PPT AND VIDEO TO VIUALIZE THE MATERIAL UED IN EACH ECTION.
2 Notes on solutions used: The glucose solution is made to be 30%. The starch solution is made from soluble starch (a handful of starch peanuts in 1 L. water plus 1 tsp cornstarch. The solution mixture inside the dialysis tubing is 80% glucose/20% starch. 1. Before the lesson: In the car ride, read through this quiz together as a team. Make sure each team member has read the lesson and has a fundamental understanding of the material. 1. What is diffusion? 2. What is a semi-permeable membrane, and what is the relationship between molecular size and ability to diffuse through a semi-permeable membrane? 3. Which molecule(s) is/are permeable through the dialysis tubing? Which is/are impermeable? 4. Which molecule is a polymer? 5. How can the presence of glucose be detected? How can the presence of starch be detected? 2. During the Lesson: Here are some Fun Facts for the lesson for VV members Diffusion is a passive process, meaning that it occurs spontaneously, without the input of energy. The rate of diffusion is affected by size of molecule, steepness of concentration gradient, and temperature (related to the speed at which the molecules are moving). Examples of diffusion in everyday life: the contents of a teabag diffuse into hot water, helium diffuses out of a balloon causing it to deflate, the smell of warm cookies diffuses throughout a room Water, oxygen, carbon dioxide, and various other essential molecules are constantly diffusing across the membranes of our cells. The diffusion of water is called osmosis. Water molecules move across a membrane trying to achieve equilibrium. Example: a carrot placed overnight in fresh water will swell; a carrot placed in salt water will shrivel. et-up VV members do this while one member starts Introduction Materials needed for set-up for 16 pairs: 16 6-oz plastic cups 16 pieces of dialysis tubing containing the glucose and starch mixture. 16 plastic plates 32 1oz cups water Count the number of students and remove enough dialysis tubes for each pair. Place the dialysis tubes into individual 6oz cups and place each cup on a plate. Pour enough water into the cup so that the water JUT covers the tubing. et aside do not give to students until Part III. Take 32 1-oz cups and pour a little water in the bottom of each cup. ave for ection II. Unpacking the Kit what you will need for each part While one team member starts the introduction, another should write the following vocabulary words on the board: diffusion, osmosis, dialysis tubing, glucose, starch, iodine, semi-permeable membrane Refer to vocabulary words throughout the lesson when you encounter them. _
3 For Part IA: Introduction. 16 clear plastic containers with wire screen and seeds, 32 Observation heets For Part IB: Relative izes of Molecules 1 dialysis tube containing glucose and starch 1 set of laminated paper models of iodine, glucose, and starch For Part II: Testing for lucose and tarch For Part IIA. lucose Test 16 Instruction heets, 16 plastic bags each containing 3 lucose Test trips (in a small bag) and 1 lucose Test Results Chart (laminated), 16 1-oz bottles of 30% glucose, 16 1-oz cups of water, 16 tweezers For Part IIB. tarch Test Above materials plus additional materials: 16 1 oz. cups of water, 16 dropper bottles of iodine (in a protective plastic container), 16 oz containers of starch suspension (shake well) For Part III: Diffusion of lucose and tarch IIIA Diffusion: 16 pieces of dialysis tubing placed in 6-oz plastic cups, (see set-up) 16 plastic plates, III B: Predicting Which Molecules Will Diffuse Paper models of the molecules from IB For Part IIIC. Testing for Diffusion of lucose lucose strips and tweezers from Part IIA Part III D. Testing for Diffusion of tarch Remaining iodine from Part IIB I. Introduction Learning oals: tudents define the term semi-permeable membrane, give realworld examples, and demonstrate how they can be used to separate different-sized molecules Note: Organize the students into pairs Discuss the motion of molecules using examples such as the smell of cooking from a distance or the smell of perfume in the air when someone wearing perfume walks by. o This happens because molecules are in constant motion and gas molecules (perfume, aroma of cooking) mix (diffuse) with the air in the vicinity. A. Modeling emi-permeable Membranes Materials oz. clear plastic containers with wire screen and seeds 32 Observation heets _
4 Ask students: What is a semi-permeable membrane? Include the following information in the discussion: o A semi-permeable membrane is a membrane in a cell that allows materials to pass into and out of a cell. o The openings in the membrane are large enough to allow some substances to move in and out of the cell, but are small enough to keep some substances from leaving or entering the cell. ive each student an observation sheet ive each pair one of the 16 oz. clear plastic containers with lids that contains a wire screen in the middle with rye seeds on one side and bean seeds on the other side. Rye and bean seeds are used to represent molecules of two different sizes. The wire grid screen represents a semi-permeable membrane (such as a cell membrane in plants or animals). The holes represent the pores or openings in the membrane. Ask one student to keep the container in view of all group members and shake the plastic container sideways, keeping the lid up and observe what happens. Ask students to explain what happened.. o The students should observe that the rye seeds can pass through the wire screen (both o ways) but the bean seeds cannot. After a few minutes, the levels of seeds will no longer be equal because the side with the bean seeds will have some of the rye seeds as well. B. Dialysis tubing and Relative izes of Molecules Materials: 1 dialysis tube containing glucose and starch 1 set of laminated paper models of iodine, glucose, and starch how students the paper models of the three molecules, and tell them the names of the molecules. Do not discuss anything about these molecules except to tell them that the solutions they are using today contain these molecules. The VV instructor should hold up a dialysis tube with glucose and starch so that the class can see it. o Have the students observe that there are no fluids leaking out of the tubing. o Tell the students that the dialysis tubing is similar to a cell membrane, and that the students are going to discover which of the three molecules are small enough the pass through the tubing. how the students the tubing in the prepared cups and point out the water just covering the tubing. Tell students to look at the diagram on the observation sheet and point out that the dialysis tubing contains starch and glucose molecules. o tarch molecules are represented by large s and glucose molecules are represented by s. o Iodine molecules, represented by s, are shown outside the dialysis tubing because they will be added to the outer solution during the experiment. o Water is H 2O Water, H 2O _
5 Tell students that they will work in pairs for the following experiments. II. Testing for lucose and tarch Learning oals: tudents identify different indicators that can be used to systematically test for the presence of various molecules Materials - distribute to each pair: 1 Instruction heet 1 plastic bag containing 3 lucose Test trips (in a small bag) and 1 lucose Test Results Chart (laminated) 1 1-oz bottle of 30% glucose 1 1-oz cup of water 1 tweezer Note: One VV volunteer will demonstrate the following procedure and will give the instructions; the other volunteers should monitor pairs to make sure procedures are being followed accurately and to give assistance as needed. tudents can refer to the instruction sheet as they are doing the experiments but you will still need to guide them through the procedures. Tell the students that they need to know how to prove which molecules have moved through the membrane. They need to know how to test for glucose and starch. A. lucose Test Ask students if they know about testing for glucose with glucose strips. o Diabetics use these strips to monitor their glucose levels. Tell the students to place the 1-oz cup of water and the 1-oz glucose bottle on the appropriate circles on the observation sheet. o Take the cap off of the 1-oz glucose bottle. Tell students not to touch the glucose test strip with their fingers - use the tweezers. Dip one end of the test strip into the 1 oz. plastic bottle labeled glucose. Hold the strip above the bottle to remove any excess solution. o Place this strip in the rectangle on the paper (below the 1 oz bottle). Then test the water cup with another glucose test strip, following the same procedure. Wait a few minutes before checking the results. Tell students to compare the color of glucose test strips with the lucose Results Color Chart, and record the values from the lucose Results Color Chart on their observation sheets. o Yellow indicates no glucose and shades of green indicate the presence of glucose. The darker the shade of green, the more glucose is present. o Test strips dipped in glucose should be dark green indicating the presence of lots of glucose. o Test strips dipped in water should remain yellow. Use these strips to verify the final test results later in the lesson. Note: The test strip dipped in water should be yellow indicating the absence of glucose. If anyone s strip did turn green, try to determine the reason the strip turned green. This could happen due to contamination if glucose was spilled in the water or if a student touched the pad of the strip after handling the glucose set-up. Tell students to replace cap on 1-oz bottle of glucose. _
6 B. tarch Test Distribute the following additional materials to each pair: 1 1 oz. cup to use for testing water 1 dropper bottle of iodine (in a protective plastic container) 1 1 oz container of starch suspension (shake well) Tell students to place the 1-oz cup of water and the 1-oz starch container on the appropriate circles on the observation sheet. They should shake the 1-oz starch container and then remove the cap. Tell students to add one squirt of iodine to both the 1 oz cup containing water and the 1-oz container of starch. Tell students to check for a color change and record the color, if any, on their observation sheet. A dark purple/black color indicates the presence of starch in the starch container. The water cup should be a light orange/yellow or amber color which indicates the presence of iodine only. Then have students put the cap back on the 1-oz starch container. Tell the students they must not disturb the cup and the dialysis tubing. Learning oals: tudents identify different indicators that can be used to systematically test for the presence of various molecules III. Diffusion of lucose and tarch Materials: Distribute the earlier prepared 6 oz. plastic cups containing a piece of dialysis tubing in water for each pair and plates. A. lucose Diffusion Tell students that diffusion of glucose takes time, but it has already been happening while they have been discussing diffusion. They need to leave the dialysis tubing for another 10 minutes to allow time for diffusion to occur. o on with section B while students wait. B. Predicting Which Molecules will Diffuse Materials: 1 set of laminated paper models of iodine, glucose, and starch (paper models are stored in binder) Review the relative size of the molecules by showing the students the paper models of the three molecules again. Discuss the relative sizes of the molecules, pointing out that the results of today s activities will be dependent upon the different sizes of iodine, glucose, and starch molecules. Point out that starch is a "polymer molecule made up of hundreds of glucose molecules joined together. Have the students refer back to their seed containers. o Tell the students that this is a good model for a semi-permeable membrane. _
7 o The small rye seeds represent small molecules, such as water, iodine, or glucose that can pass through a porous membrane both ways while larger molecules cannot. o The larger bean seeds represent large molecules such as starch molecules that cannot pass through the semi-permeable membrane. Ask the students if they can predict which way the different molecules will move. o The iodine is a small molecule and can move from the water outside the tubing, to inside it. o The starch is a large molecule and cannot get outside the tubing. o The glucose is small and should be able to move from inside the tubing to the water on the outside. Tell students that the molecules of substances have been diffusing in the experiments set up earlier in the lesson and it is time to check on these experiments and investigate what has been happening. Caution students to wait for instructions before they do the experiments. C. Testing for Diffusion of lucose After the tubing has been in the water for about 10 minutes: Ask students to dip a clean glucose test strip into the water close to the dialysis tubing (it may even touch the tubing) and place the test strip on the appropriate rectangle of the observation sheet. While students are waiting for the results of this test, ask them what the results of the glucose test strip will tell them. o If the test strip remains yellow, then no glucose was able to pass through the dialysis tubing. o If the test strip turns green, then glucose was able to pass through the dialysis tubing. Ask students to check the glucose test strip, compare its color with the lucose Results Color Chart, and record the value on their observation sheet. The glucose test strip should turn green within 1 minute, indicating the presence of glucose in the water. This shows that glucose molecules have passed through the dialysis tubing. If it did not turn green, test again (close to the tube) after several more minutes have passed Ask students to look at the plastic container of seeds. Ask them if this were a model of the glucose experiment, which seeds represent the glucose molecules. o The small seeds are the glucose molecules because they could travel through the dialysis tubing. Ask students to refer to the diagram on the observation sheet and use arrows to show the direction glucose molecules have moved. D. Testing for Diffusion of tarch Note: This part MUT be done after a positive test for glucose has been obtained. The glucose test strips will not work after iodine has been added to the water. Have students unscrew the lid on the iodine bottles and add all the rest of the iodine to the water in the cup that is holding the dialysis tubing. The solution should be a light orange/yellow or amber color. Note: If a positive test occurs when the iodine is added to the water around the dialysis tubing, the tubing has a leak. If this happens, empty their cup, rinse with water, and place a newly rinsed dialysis tubing in the cup and add iodine again. (Use the extra bottle of iodine that was provided.) If all else fails, have them observe the results of another group. _
8 Ask students to observe the solution inside the dialysis tubing and the water surrounding it for a few minutes. o If they observe a color change, they should record it on their observation sheet. o tudents should observe a purple/black color inside the dialysis tubing. Ask students what this purple/black color tells them. o The purple/black color indicates that iodine molecules have passed through the dialysis tubing and detected the presence of starch inside the dialysis tubing. ince the outside solution is not purple/black, starch molecules have not passed through the dialysis tubing into the water. Tell students to look at the plastic container of seeds. o Ask them if this were a model of the iodine and starch experiment, which seeds represent the iodine molecules and which represent the starch molecules. o The large seeds are the starch molecules because they could not get out of the dialysis tubing; the small seeds are the iodine molecules because they could travel through the dialysis tubing. Ask students to refer to the diagram on the observation sheet and use arrows to show the direction iodine molecules have moved. Learning oals: tudents define the term semi-permeable membrane, give real-world examples, and demonstrate how they can be used to separate different-sized molecules tudents identify different indicators that can be used to systematically test for the presence of various molecules IV. Review ummarize the glucose and starch dialysis results for the whole class. Refer to diagram on observation sheet during review. lucose gave a positive test in the water surrounding the dialysis tubing. Therefore, glucose molecules traveled through the dialysis tubing. The water in the cup remained yellow (the color of iodine), not the purple color found when starch is present. Therefore, starch molecules did not travel through the dialysis tubing into the water. However, there is a purple-black color inside the tubing. Therefore, iodine molecules traveled into the dialysis tubing and reacted with the starch molecules how the molecule models of iodine, glucose, and starch to the students again to emphasize the relationship between molecular size and the ability to diffuse through a semi-permeable membrane like dialysis tubing. Collect used dialysis tubing in a large ziploc bag or dispose of them at the school. Return all unused tubing. Pour contents of water in all cups down the drain. Return all cups to lab in plastic garbage bag. Please do not let glucose solutions leak into lesson box that makes for a very sticky mess to clean. Return used 1-oz bottles of glucose and starch and all solution containers to the VV lab for re-use. Reference for Part V: J.. Morse and E. Vitz, A imple Demonstration Model of Osmosis, J. Chem. Educ., Vol. 76, pp , January, Lesson written by Pat Tellinghuisen, Coordinator of VV, Vanderbilt University Dr. Melvin Joesten, Professor Emeritus, Chemistry Department, Vanderbilt University usan Clendenen, Teacher Consultant, Vanderbilt University We gratefully acknowledge the assistance of Ann Orman and Kay Boone, MNP teachers. _
9 Diffusion Instruction heet I. Introduction VV team will discuss the concept of diffusion. A. emi-permeable Membranes - Begins with a discussion. VV teams will hand out a bean container to each pair. 1. Keep the container in view of all group members; shake the container sideways, keeping the lid up; and observe what happens. 2. Be ready to explain what happened. B. Diffusion Through Dialysis Tubing 1. Observe the dialysis tubing held up by the VV instructor. 2. Look at the diagram on your observation sheet. tarch molecules are s, glucose molecules are s, and iodine molecules are I2. II. Testing for lucose and tarch A. lucose Test 1. Place the 1-oz cup of water and the 1-oz glucose container on the appropriate circles on the observation sheet. Remove the cap of the 1-oz glucose container. 2. Take one of the glucose strips, but do not to touch the glucose test strip with your fingers. Use tweezers. 3. Dip one end of one test strip into the 1 oz. container holding the glucose solution and remove from the container by pulling the strip past the edge of the container to remove any excess solution. 4. Place this strip in the rectangle on the paper (beside the 1 oz container). 5. Then test the cup containing water with another glucose test strip, following the same procedure. 6. Wait a few minutes before checking the results and then compare the color of the strips with the lucose Results Color Chart. Record the value on the observation sheet. 7. YELLOW indicates NO LUCOE and shades of REEN indicate the presence of LUCOE. The darker the shade of green, the more glucose is present 8. Replace the cap on the1-oz container of glucose. B. tarch Test 1. Place the 1-oz cup of water and the 1-oz starch container on the appropriate circles on the observation sheet. 2. hake the 1-oz starch container and then remove the cap. 3. Add one squirt of iodine to both the 1oz cup containing water and the 1-oz container of starch. 4. Record the color of starch solution on the observation sheet. Then put the cap back on the 1-oz starch container.
10 III. Diffusion of lucose and tarch A. lucose Diffusion 1. A VV volunteer will distribute to each pair a plate holding a cup with dialysis tubing and water. 2. Leave the cup undisturbed until later (wait at least 10 minutes) in the period to allow time for diffusion to occur. B. Predicting Which Molecules Will Diffuse 1. Think about the seed containers. Remember what size beans crossed the wire screen. 2. The dialysis tubing is a semi-permeable membrane like the wire screen. The larger beans are like large molecules such as starch molecules. The rye seeds are like small molecules such as water, iodine, and glucose. 3. Predict which molecules will diffuse in what direction in the experiment. Draw arrows next to a starch (), glucose () and iodine (I2) molecule to show the predicted diffusion direction. C. Testing for lucose Diffusion 1. Dip a clean glucose test strip into the water close to the dialysis tubing (it may even touch the tubing) and place the test strip on the appropriate rectangle of the observation sheet. 2. Compare the strip from glucose test with the lucose Results Color Chart and record the value on the observation sheet. 3. Look at the plastic container of seeds. Determine which seeds would represent the glucose molecules. 4. Look at the diagram on your observation sheet. Was your prediction for the movement of glucose molecules correct? 5. D. Testing for tarch Diffusion 1. Remove the cap from the iodine bottle and add ALL the rest of the iodine to the water in the cup holding the dialysis tubing. 2. Observe the solution and record any color change on the observation sheet. The starch /iodine change may take 2-5 minutes to occur. 3. Look at the plastic container of seeds. Determine which seeds would represent the starch molecules. 4. Look at the diagram on your observation sheet. Was your prediction for the movement of iodine and starch molecules correct? IV. REVIEW
11 Observation heet Name Vocabulary Words: diffusion, osmosis, dialysis tubing, glucose, starch, iodine, semi-permeable membrane LUCOE TET cup with water container with glucose What color is the glucose strip after it is dipped in water? cup with water What color is the glucose strip after it is dipped in glucose solution? TARCH TET container with starch What color is the water after iodine is added? What color is the starch solution after iodine is added? Predict the Direction of Movement of the Molecules: Remembering what size beans crossed the wire screen, predict which molecules will diffuse in what direction in the experiment. Draw arrows next to a starch (), glucose () and iodine (I2) molecule in the cup diagram below, to show the predicted diffusion direction. DIALYI TUBIN TET Water, H 2 O 10 minutes after dialysis tubing is added to water: What is the color of the glucose strip when it is dipped into the liquid closest to the tubing? _ 5 minutes after the iodine is added: What is the color of solution inside dialysis tubing _ Were your predictions for the movement of the molecules correct?
12 Observation heet - Answers Name Vocabulary Words: diffusion, osmosis, dialysis tubing, glucose, starch, iodine, semi-permeable membrane LUCOE TET cup with water container with glucose What color is the glucose strip after it is dipped in water? no color cup with water TARCH TET What color is the glucose strip after it is dipped in glucose solution? green container with starch What color is the water after iodine is added? pale yellow the color of dilute iodine What color is the starch solution after iodine is added? Blue/Black Predict the Direction of Movement of the Molecules: Remembering what size beans crossed the wire screen, predict which molecules will diffuse in what direction in the experiment. Draw arrows next to a starch (), glucose () and iodine (I2) molecule in the cup diagram below, to show the predicted diffusion direction. DIALYI TUBIN TET Water, H 2 O 10 minutes after dialysis tubing is added to water: What is the color of the glucose strip when it is dipped into the liquid closest to the tubing? _ 5 minutes after the iodine is added: What is the color of solution inside dialysis tubing _ Were your predictions for the movement of the molecules correct?
Cell Membranes: Diffusion and Osmosis
 STO-112 Cell Membranes: Diffusion and Osmosis Part 1: Diffusion Diffusion is a process by which molecules move into or out of cells. To diffuse into or out of a cell, molecules must pass through the cell
STO-112 Cell Membranes: Diffusion and Osmosis Part 1: Diffusion Diffusion is a process by which molecules move into or out of cells. To diffuse into or out of a cell, molecules must pass through the cell
LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE
 LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE NAME: PERIOD: DATE: Building Background Knowledge: 1) SELECTIVELY PERMEABLE MEMBRANE: Every cell is surrounded by a selectively permeable membrane
LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE NAME: PERIOD: DATE: Building Background Knowledge: 1) SELECTIVELY PERMEABLE MEMBRANE: Every cell is surrounded by a selectively permeable membrane
Osmosis in Potato Slices
 Osmosis in Potato Slices Vanderbilt Student Volunteers for Science Training Presentation 2018-2019 VINSE/VSVS Rural Important! Please use this resource to reinforce your understanding of the lesson! Make
Osmosis in Potato Slices Vanderbilt Student Volunteers for Science Training Presentation 2018-2019 VINSE/VSVS Rural Important! Please use this resource to reinforce your understanding of the lesson! Make
LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE
 LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE NAME: PERIOD: DATE: Building Background Knowledge: 1) SELECTIVELY PERMEABLE MEMBRANE: Every cell is surrounded by a selectively permeable membrane
LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE NAME: PERIOD: DATE: Building Background Knowledge: 1) SELECTIVELY PERMEABLE MEMBRANE: Every cell is surrounded by a selectively permeable membrane
BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION. READING: Please read pages & in your text prior to lab.
 BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION READING: Please read pages 27-31 & 83-86 in your text prior to lab. INTRODUCTION: All living things depend on water. A water molecule is made up of an oxygen atom
BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION READING: Please read pages 27-31 & 83-86 in your text prior to lab. INTRODUCTION: All living things depend on water. A water molecule is made up of an oxygen atom
DIFFUSON AND OSMOSIS INTRODUCTION diffusion concentration gradient. net osmosis water potential active transport
 DIFFUSON AND OSMOSIS NAME DATE INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. Raw materials such as oxygen and sugars needed
DIFFUSON AND OSMOSIS NAME DATE INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. Raw materials such as oxygen and sugars needed
To understand osmosis, we must focus on the behavior of the solvent, not the solute.
 GCC CHM 130LL Osmosis and Dialysis Purpose: The purpose of this experiment is to observe the closely related phenomena of osmosis and diffusion as it relates to dialysis. It is hoped that you will be able
GCC CHM 130LL Osmosis and Dialysis Purpose: The purpose of this experiment is to observe the closely related phenomena of osmosis and diffusion as it relates to dialysis. It is hoped that you will be able
Diffusion and Osmosis
 Diffusion and Osmosis Introduction: In this exercise you will measure diffusion of small molecules through dialysis tubing, an example of a semi permeable membrane. The movement of a solute through a semi
Diffusion and Osmosis Introduction: In this exercise you will measure diffusion of small molecules through dialysis tubing, an example of a semi permeable membrane. The movement of a solute through a semi
Diffusion across a Selectively Permeable Membrane
 Diffusion across a Selectively Permeable Membrane Each cell is surrounded by a selectively permeable cell membrane Cell Membrane which regulates what gets into and out of the cell. A selectively permeable
Diffusion across a Selectively Permeable Membrane Each cell is surrounded by a selectively permeable cell membrane Cell Membrane which regulates what gets into and out of the cell. A selectively permeable
The Role of the Cell Membrane in Transport
 The Role of the Cell Membrane in Transport diffusion: the spontaneous movement of particles from an area of higher concentration to an area of lower concentration Many people, young and old, enjoy a nice
The Role of the Cell Membrane in Transport diffusion: the spontaneous movement of particles from an area of higher concentration to an area of lower concentration Many people, young and old, enjoy a nice
Cell Diffusion & Permeability: See-Through Eggs Teacher Version
 Cell Diffusion & Permeability: See-Through Eggs Teacher Version In this lab, students will learn about the permeability of the cell membrane. By studying the ability of a shell-less egg to absorb various
Cell Diffusion & Permeability: See-Through Eggs Teacher Version In this lab, students will learn about the permeability of the cell membrane. By studying the ability of a shell-less egg to absorb various
Investigating Osmosis By Amy Dewees,Jenkintown.High School and Dr. Ingrid Waldron, Department of Biology, University of Pennsylvania, 20091
 Investigating Osmosis By Amy Dewees,Jenkintown.High School and Dr. Ingrid Waldron, Department of Biology, University of Pennsylvania, 20091 What is diffusion? What does it mean to say that a membrane is
Investigating Osmosis By Amy Dewees,Jenkintown.High School and Dr. Ingrid Waldron, Department of Biology, University of Pennsylvania, 20091 What is diffusion? What does it mean to say that a membrane is
Name: Date Block Selective Permeability
 LAB Name: Date Block Selective Permeability OBJECTIVES: Observe the selective permeability of an artificial membrane. Observe diffusion of substances across an artificial membrane. Devise a model for the
LAB Name: Date Block Selective Permeability OBJECTIVES: Observe the selective permeability of an artificial membrane. Observe diffusion of substances across an artificial membrane. Devise a model for the
Name: There are two things that will determine which particles will pass through and which will not:
 18 Diffusion and Osmosis in Living Systems Name: Problem: How do substances move into and out of cells? Introduction: In order for cells to carry on their life processes, they must take in materials and
18 Diffusion and Osmosis in Living Systems Name: Problem: How do substances move into and out of cells? Introduction: In order for cells to carry on their life processes, they must take in materials and
Passive Transport Lab: Diffusion and Osmosis
 Name Date Period Passive Transport Lab: Diffusion and Osmosis OBJECTIVE: Apply your understanding of the processes of diffusion and osmosis to explain observational data. PART A: Starch and Iodine MATERIALS
Name Date Period Passive Transport Lab: Diffusion and Osmosis OBJECTIVE: Apply your understanding of the processes of diffusion and osmosis to explain observational data. PART A: Starch and Iodine MATERIALS
Osmosis and Diffusion: How biological membranes are important This page is a lab preparation guide for instructors.
 Osmosis and Diffusion: How biological membranes are important This page is a lab preparation guide for instructors. **All solutions and dialysis bags can easily be prepared prior to lab start to maximize
Osmosis and Diffusion: How biological membranes are important This page is a lab preparation guide for instructors. **All solutions and dialysis bags can easily be prepared prior to lab start to maximize
Name: Bio A.P. Lab Diffusion & Osmosis
 Name: Bio A.P. Lab Diffusion & Osmosis BACKGROUND: Many aspects of the life of a cell depend on the fact that atoms and molecules are constantly in motion (kinetic energy). This kinetic energy results
Name: Bio A.P. Lab Diffusion & Osmosis BACKGROUND: Many aspects of the life of a cell depend on the fact that atoms and molecules are constantly in motion (kinetic energy). This kinetic energy results
Diffusion and Osmosis
 Diffusion and Osmosis OBJECTIVES: 1. To explore how different molecules move by diffusion and osmosis through semi-permeable membranes. 2. To understand how concentration affects the movement of substances
Diffusion and Osmosis OBJECTIVES: 1. To explore how different molecules move by diffusion and osmosis through semi-permeable membranes. 2. To understand how concentration affects the movement of substances
STATION 4: TONICITY due to OSMOSIS / Turgor Pressure in Plants
 STATION 4: TONICITY due to OSMOSIS / Turgor Pressure in Plants Tonicity is the concentration of solutions that determines the direction water will move across a semi-permeable membrane. A solution is a
STATION 4: TONICITY due to OSMOSIS / Turgor Pressure in Plants Tonicity is the concentration of solutions that determines the direction water will move across a semi-permeable membrane. A solution is a
Table of Contents Title Page Number Due Date Stamp
 1 Table of Contents Title Page Number Due Date Stamp Calendar 3 Warm - Ups 4 Carbon Based Molecules 5 02/20/2018 Notes Cell Membrane Notes 8 02/20/2018 Membrane Structure and Cell Signaling Worksheet Diffusion
1 Table of Contents Title Page Number Due Date Stamp Calendar 3 Warm - Ups 4 Carbon Based Molecules 5 02/20/2018 Notes Cell Membrane Notes 8 02/20/2018 Membrane Structure and Cell Signaling Worksheet Diffusion
Biology Cell Unit Homework Packet #3
 Biology Cell Unit Homework Packet #3 Name DUE: Hour HW #5 Egg Demo Drawings Analysis HW #6 Elodea Drawings lab Analysis HW #7 Cell Questions Membrane and Transport HW #8 Questions / 5 possible points Homework
Biology Cell Unit Homework Packet #3 Name DUE: Hour HW #5 Egg Demo Drawings Analysis HW #6 Elodea Drawings lab Analysis HW #7 Cell Questions Membrane and Transport HW #8 Questions / 5 possible points Homework
Biology Unit 5 Cancer, Lab Activity 5-2
 Biology Unit 5 Cancer, Lab Activity 5-2 The Plasma membrane serves as a barrier between the internal cell environment and the external world. The plasma membrane is a dynamic structure. It allows some
Biology Unit 5 Cancer, Lab Activity 5-2 The Plasma membrane serves as a barrier between the internal cell environment and the external world. The plasma membrane is a dynamic structure. It allows some
This hands-on workshop is designed to teach students about which foods contain starch and the properties of starch
 Aim: This hands-on workshop is designed to teach students about which foods contain starch and the properties of starch Curriculum Links: By contributing to investigations into familiar changes in substances
Aim: This hands-on workshop is designed to teach students about which foods contain starch and the properties of starch Curriculum Links: By contributing to investigations into familiar changes in substances
250-mL beakers. iodine solution metric ruler. 10-mL graduated cylinders pipettes. (Read the Procedure first to answer the Questions)
 Detecting Diffusion Introduction A cell membrane is a selectively permeable barrier. Some particles can pass through the cell membrane while other particles are held back. Solutes that can move across
Detecting Diffusion Introduction A cell membrane is a selectively permeable barrier. Some particles can pass through the cell membrane while other particles are held back. Solutes that can move across
Diffusion and Osmosis
 Diffusion and Osmosis During your first year of residency at Mountainside Hospital, you are treating a group of patients that exhibit signs of dehydration. You have to be sure to take note of all the solutes
Diffusion and Osmosis During your first year of residency at Mountainside Hospital, you are treating a group of patients that exhibit signs of dehydration. You have to be sure to take note of all the solutes
Cellular Transport Worksheet
 Cellular Transport Worksheet Name Section A: Cell Membrane Structure 1. Label the cell membrane diagram. You ll need to draw lines to some of the structures. **Draw cholesterol molecules in the membrane.**
Cellular Transport Worksheet Name Section A: Cell Membrane Structure 1. Label the cell membrane diagram. You ll need to draw lines to some of the structures. **Draw cholesterol molecules in the membrane.**
HUMAN BODY SECTION 4: DIGESTION From Hands on Science by Linda Poore, 2003.
 HUMAN BODY SECTION 4: DIGESTION From Hands on Science by Linda Poore, 2003. STANDARDS: Students know the sequential steps of digestion and the roles of teeth and the mouth, esophagus, stomach, small intestine,
HUMAN BODY SECTION 4: DIGESTION From Hands on Science by Linda Poore, 2003. STANDARDS: Students know the sequential steps of digestion and the roles of teeth and the mouth, esophagus, stomach, small intestine,
CELLS ARE A BAG OF GOO
 Fifth Grade, 2013 CELLS ARE A BAG OF GOO By Michael E. Knotts, Curt M. Peterson, and Diana Anderson 2013 by Michael E. Knotts, Curt M. Peterson, and Diana Anderson, All Rights Reserved Objective and Overview:
Fifth Grade, 2013 CELLS ARE A BAG OF GOO By Michael E. Knotts, Curt M. Peterson, and Diana Anderson 2013 by Michael E. Knotts, Curt M. Peterson, and Diana Anderson, All Rights Reserved Objective and Overview:
Egg-speriment (Osmosis Lab) 2009
 Purpose/Objectives: Osmosis can have important consequences for the cell. The purpose of this lab is to study the effects of osmosis on a cell that is submerged in different aqueous environments: vinegar,
Purpose/Objectives: Osmosis can have important consequences for the cell. The purpose of this lab is to study the effects of osmosis on a cell that is submerged in different aqueous environments: vinegar,
Cell Diffusion & Permeability: See-Through Eggs Student Advanced Version
 Cell Diffusion & Permeability: See-Through Eggs Student Advanced Version In this lab, students will learn about the permeability of the cell membrane. By studying the ability of a shell-less egg to absorb
Cell Diffusion & Permeability: See-Through Eggs Student Advanced Version In this lab, students will learn about the permeability of the cell membrane. By studying the ability of a shell-less egg to absorb
Biology Cell Unit Homework Packet #3
 Biology Cell Unit Homework Packet #3 Name DUE: Hour HW #5 Egg Demo Drawings Analysis HW #6 Elodea Drawings lab Analysis HW #7 Cell Questions Membrane and Transport HW #8 Questions / 5 possible points Homework
Biology Cell Unit Homework Packet #3 Name DUE: Hour HW #5 Egg Demo Drawings Analysis HW #6 Elodea Drawings lab Analysis HW #7 Cell Questions Membrane and Transport HW #8 Questions / 5 possible points Homework
Who Killed the Flowers? Teacher Information
 Who Killed the Flowers? Teacher Information Summary: Mrs. Powell, the science teacher, suspects that someone killed some flowers in the school s greenhouse by urinating on them. In this science lab, students
Who Killed the Flowers? Teacher Information Summary: Mrs. Powell, the science teacher, suspects that someone killed some flowers in the school s greenhouse by urinating on them. In this science lab, students
AP Lab Four: Water Potential and Osmosis
 AP Biology AP Lab Four: Water Potential and Osmosis Name Atoms and molecules are constantly in motion, bumping off of membranes, barriers, each other, without end. The results of this among other phenomena
AP Biology AP Lab Four: Water Potential and Osmosis Name Atoms and molecules are constantly in motion, bumping off of membranes, barriers, each other, without end. The results of this among other phenomena
= only some molecules can get in or out of the cell. allow substances (other than lipids) in and out
 Name: Cell Membrane and Cell Transport Notes I. Cell Membrane (cells need an inside and outside) a. separate cell from its environment b. cell membrane is the boundary c. cell membrane controls what gets
Name: Cell Membrane and Cell Transport Notes I. Cell Membrane (cells need an inside and outside) a. separate cell from its environment b. cell membrane is the boundary c. cell membrane controls what gets
Name: NYS DIFFUSION LAB REVIEW Date: PACKET 1: Difusion Through a Membrane
 Name: NYS DIFFUSION LAB REVIEW Date: PACKET 1: Difusion Through a Membrane 1. The diagram below represents a laboratory setup used to demonstrate the movement of molecules across a selectively permeable
Name: NYS DIFFUSION LAB REVIEW Date: PACKET 1: Difusion Through a Membrane 1. The diagram below represents a laboratory setup used to demonstrate the movement of molecules across a selectively permeable
Course Learning Outcomes for Unit III. Reading Assignment. Unit Lesson. UNIT III STUDY GUIDE Essential Parts: Cells
 UNIT III STUDY GUIDE Essential Parts: Cells Course Learning Outcomes for Unit III Upon completion of this unit, students should be able to: 1. Evaluate concepts and theories of basic biological sciences
UNIT III STUDY GUIDE Essential Parts: Cells Course Learning Outcomes for Unit III Upon completion of this unit, students should be able to: 1. Evaluate concepts and theories of basic biological sciences
Diffusion and Osmosis
 Diffusion and Osmosis Keywords Diffusion Osmosis Selectively permeable Turgor Pressure Keywords Visking Tubing Food preservation Selectively Permeable membranes Selectively permeable membrane allows some
Diffusion and Osmosis Keywords Diffusion Osmosis Selectively permeable Turgor Pressure Keywords Visking Tubing Food preservation Selectively Permeable membranes Selectively permeable membrane allows some
Table of Contents. Dialysis Port Care Chemotherapy Port Care G-Tube Care Colostomy Bags Wound Dressings
 Table of Contents Dialysis Port Care Chemotherapy Port Care G-Tube Care Colostomy Bags Wound Dressings Dialysis Port Care Know What Type of Vascular Access You Have. Fistula: An artery in your forearm
Table of Contents Dialysis Port Care Chemotherapy Port Care G-Tube Care Colostomy Bags Wound Dressings Dialysis Port Care Know What Type of Vascular Access You Have. Fistula: An artery in your forearm
In groups of 3, half the class will conduct factors affecting diffusion lab while the others are working on osmosis
 Factors Affecting Diffusion & Osmosis In groups of 3, half the class will conduct factors affecting diffusion lab while the others are working on osmosis 1 THE CELL IN ACTION! 2 Passive Transport The net
Factors Affecting Diffusion & Osmosis In groups of 3, half the class will conduct factors affecting diffusion lab while the others are working on osmosis 1 THE CELL IN ACTION! 2 Passive Transport The net
Name Date. In this lab investigation you will investigate the movement of water through a selectively permeable membrane.
 This lab will be hand-written in your data book AP Osmosis Labs Part A (was done in previous a previous class: Dialysis tube + Starch + Glucose) Part B: Osmosis Unknowns In this lab investigation you will
This lab will be hand-written in your data book AP Osmosis Labs Part A (was done in previous a previous class: Dialysis tube + Starch + Glucose) Part B: Osmosis Unknowns In this lab investigation you will
Activity 5.2 Using chemical change to identify an unknown
 Activity 5.2 Using chemical change to identify an unknown How can you identify an unknown powder? In this activity, students will develop a method to test five similar-looking powders with four test liquids.
Activity 5.2 Using chemical change to identify an unknown How can you identify an unknown powder? In this activity, students will develop a method to test five similar-looking powders with four test liquids.
Quotes from Next Generation Science Standards, available at
 Teacher Preparation Notes for Diffusion across a Selectively Permeable Membrane Drs. Jennifer Doherty and Ingrid Waldron, Department of Biology, University of Pennsylvania, 2015 1 Students investigate
Teacher Preparation Notes for Diffusion across a Selectively Permeable Membrane Drs. Jennifer Doherty and Ingrid Waldron, Department of Biology, University of Pennsylvania, 2015 1 Students investigate
Cells & Transport. Chapter 7.1, 7.2, & 7.4
 Cells & Transport Chapter 7.1, 7.2, & 7.4 Do Now How big is a cell? How many cells are we made of? How many cells is the smallest living organism made of? Objectives Describe how cells were discovered
Cells & Transport Chapter 7.1, 7.2, & 7.4 Do Now How big is a cell? How many cells are we made of? How many cells is the smallest living organism made of? Objectives Describe how cells were discovered
Lab #6: Cellular Transport Mechanisms Lab
 Lab #6: Cellular Transport Mechanisms Lab OVERVIEW One of the major functions of the plasma membrane is to regulate the movement of substances into and out of the cell. This process is essential in maintaining
Lab #6: Cellular Transport Mechanisms Lab OVERVIEW One of the major functions of the plasma membrane is to regulate the movement of substances into and out of the cell. This process is essential in maintaining
What is the function of the cell membrane?
 What is the function of the cell membrane? 1. DIFFUSION: The movement of molecules from an area of high concentration to an area of lower concentration. Why do molecules move from high concentration to
What is the function of the cell membrane? 1. DIFFUSION: The movement of molecules from an area of high concentration to an area of lower concentration. Why do molecules move from high concentration to
Name: Teacher: Ms. Petrakos. Lesson #21 - Cell Transport Diffusion Review
 Name: Teacher: Ms. Petrakos Lesson #21 - Cell Transport Diffusion Review Figure 1 Base your answer to the question on the diagram below and on your knowledge of biology. The diagram represents an experimental
Name: Teacher: Ms. Petrakos Lesson #21 - Cell Transport Diffusion Review Figure 1 Base your answer to the question on the diagram below and on your knowledge of biology. The diagram represents an experimental
Thurs Nov 3/Fri Nov 4
 Thurs Nov 3/Fri Nov 4 3.4 Quiz next Tuesday nov 8! (PER 2 & 3 YOU WILL BE AT REALITY TOWN!) Starter: No starter today! Read introduction for part A of the lab! Diffusion and Osmosis Lab Diffusion: Osmosis:
Thurs Nov 3/Fri Nov 4 3.4 Quiz next Tuesday nov 8! (PER 2 & 3 YOU WILL BE AT REALITY TOWN!) Starter: No starter today! Read introduction for part A of the lab! Diffusion and Osmosis Lab Diffusion: Osmosis:
Biology Movement across the Cell Membrane
 Biology 160 - Movement across the Cell Membrane Prelab Information Movement is one of the characteristics of life. The ability to control the movement of material across the cell membrane is an incredibly
Biology 160 - Movement across the Cell Membrane Prelab Information Movement is one of the characteristics of life. The ability to control the movement of material across the cell membrane is an incredibly
Cell Processes. Chapter 3. Learning Target 3/15/16. l I can. l 1)Explain the difference between diffusion and osmosis.
 Chapter 3 Cell Processes Learning Target l I can. l 1)Explain the difference between diffusion and osmosis. l 2)Predict the movement of particles into and out of a cell. 1 Section 3.2 MOVING CELLULAR MATERIAL
Chapter 3 Cell Processes Learning Target l I can. l 1)Explain the difference between diffusion and osmosis. l 2)Predict the movement of particles into and out of a cell. 1 Section 3.2 MOVING CELLULAR MATERIAL
Lab 4: Osmosis and Diffusion
 Page 4.1 Lab 4: Osmosis and Diffusion Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Page 4.1 Lab 4: Osmosis and Diffusion Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
LAB Potato Cores Honors Biology, Newton North High
 Name Date Block LAB Potato Cores Honors Biology, Newton North High BACKGROUND: Osmosis is a type of passive transport. No input of energy is needed in order for water to pass through a selectively permeable
Name Date Block LAB Potato Cores Honors Biology, Newton North High BACKGROUND: Osmosis is a type of passive transport. No input of energy is needed in order for water to pass through a selectively permeable
Chapter 3.4 & 3.5 Cell Transport (Osmosis and Diffusion) = only some molecules can get in or out of the cell
 Chapter 3.4 & 3.5 Cell Transport (Osmosis and Diffusion) I. Cell Membrane (cells need an inside and outside) a. separate cell from its environment b. cell membrane is the boundary c. cell membrane controls
Chapter 3.4 & 3.5 Cell Transport (Osmosis and Diffusion) I. Cell Membrane (cells need an inside and outside) a. separate cell from its environment b. cell membrane is the boundary c. cell membrane controls
You will test a sample of the patient s urine to determine if her kidneys are functioning normally.
 STO-118 A Kidney Problem? The Case Ten years ago, your patient was diagnosed with Type 2 diabetes. She has been careless about following the treatment needed to keep her blood glucose levels regulated.
STO-118 A Kidney Problem? The Case Ten years ago, your patient was diagnosed with Type 2 diabetes. She has been careless about following the treatment needed to keep her blood glucose levels regulated.
To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status.
 Module 9 Performing HIV Rapid Tests Purpose To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status. Pre-requisite Modules Module 3: Overview
Module 9 Performing HIV Rapid Tests Purpose To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status. Pre-requisite Modules Module 3: Overview
Diffusion, osmosis, transport mechanisms 43
 Diffusion, osmosis, transport mechanisms 43 DIFFUSION, OSMOSIS AND TRANSPORT MECHANISMS The cell membrane is a biological membrane that separates the interior of all cells from the outside environment
Diffusion, osmosis, transport mechanisms 43 DIFFUSION, OSMOSIS AND TRANSPORT MECHANISMS The cell membrane is a biological membrane that separates the interior of all cells from the outside environment
Lab 6: Cellular Respiration
 Lab 6: Cellular Respiration Metabolism is the sum of all chemical reactions in a living organism. These reactions can be catabolic or anabolic. Anabolic reactions use up energy to actually build complex
Lab 6: Cellular Respiration Metabolism is the sum of all chemical reactions in a living organism. These reactions can be catabolic or anabolic. Anabolic reactions use up energy to actually build complex
Passive Transport: Practice Problems PAP BIOLOGY
 Passive Transport: Practice Problems PAP BIOLOGY #1 Draw a diagram where the cell has low concentration of salt molecules and the environment it is in has a high concentration of salt molecules in a water
Passive Transport: Practice Problems PAP BIOLOGY #1 Draw a diagram where the cell has low concentration of salt molecules and the environment it is in has a high concentration of salt molecules in a water
Who took Kaleb s ipod? -- An organic compound mystery
 Who took Kaleb s ipod? -- An organic compound mystery Dr. Jennifer Doherty, Dr. Ingrid Waldron and Dr. Lori Spindler, Department of Biology, University of Pennsylvania, copyright 2009 Adapted from Identity
Who took Kaleb s ipod? -- An organic compound mystery Dr. Jennifer Doherty, Dr. Ingrid Waldron and Dr. Lori Spindler, Department of Biology, University of Pennsylvania, copyright 2009 Adapted from Identity
Cell Membrane (Transport) Notes
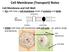 Cell Membrane (Transport) Notes Cell Membrane and Cell Wall: ALL cells have a cell membrane made of proteins and lipids protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump SOME cells
Cell Membrane (Transport) Notes Cell Membrane and Cell Wall: ALL cells have a cell membrane made of proteins and lipids protein channel Cell Membrane Layer 1 Layer 2 lipid bilayer protein pump SOME cells
WEAR GOGGLES, GLOVES AND A LAB APRON!!!!
 Organic Food Lab =) Problem: What test are used to discover if certain organic molecules are present in food? Could these tests be used to identify an unknown food? Background: We will be studying various
Organic Food Lab =) Problem: What test are used to discover if certain organic molecules are present in food? Could these tests be used to identify an unknown food? Background: We will be studying various
EXERCISE Transport Mechanisms in the Body
 EXERCISE Transport Mechanisms in the Body 2 OBJECTIVES After completing these activities, you should be able to: Understand the differences between passive and active processes of transport Define diffusion,
EXERCISE Transport Mechanisms in the Body 2 OBJECTIVES After completing these activities, you should be able to: Understand the differences between passive and active processes of transport Define diffusion,
Cellular Transport. 1. A potato core was placed in a beaker of water as shown in the figure below.
 Name: Date: 1. potato core was placed in a beaker of water as shown in the figure below. Which diagram best represents the net movement of molecules?.. C. D. page 1 2. The following question(s) is/are
Name: Date: 1. potato core was placed in a beaker of water as shown in the figure below. Which diagram best represents the net movement of molecules?.. C. D. page 1 2. The following question(s) is/are
The Cell Membrane and Homeostasis What is the cell membrane? A quick review A. Cell Membrane and Cell Transport. Unit 2: Cells and Cell Transport
 Unit 2: Cells and Cell Transport Cell Membrane and Cell Transport Name: Directions: Go to https://shimkoscience.weebly.com/ and on the Biology page, find the document labelled Cell Membrane and Cell Transport
Unit 2: Cells and Cell Transport Cell Membrane and Cell Transport Name: Directions: Go to https://shimkoscience.weebly.com/ and on the Biology page, find the document labelled Cell Membrane and Cell Transport
Organic Compounds in the Foods
 Organic Compounds in the Foods Purpose: This lab activity will help you understand the chemical composition (i.e., carbohydrates, proteins, and fats) of the foods that you eat. Materials we will be using:
Organic Compounds in the Foods Purpose: This lab activity will help you understand the chemical composition (i.e., carbohydrates, proteins, and fats) of the foods that you eat. Materials we will be using:
1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4
 Topic 3: Movement of substances across cell membrane 1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4 Directions: Questions 2 and 3 refer to
Topic 3: Movement of substances across cell membrane 1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4 Directions: Questions 2 and 3 refer to
Passive Transport. Does not expend cellular energy for the movement to take place. Ex-rolling down a hill
 Passive Transport Fluid Mosaic Model Passive Transport Does not expend cellular energy for the movement to take place Ex-rolling down a hill Parts of a Solution Solute: what gets dissolved Solvent: What
Passive Transport Fluid Mosaic Model Passive Transport Does not expend cellular energy for the movement to take place Ex-rolling down a hill Parts of a Solution Solute: what gets dissolved Solvent: What
Chapter MEMBRANE TRANSPORT
 Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
Who took Jerell s ipod? An organic compound mystery 1
 Who took Jerell s ipod? An organic compound mystery 1 Jerell is a 10 th grade student who works at McDonald s on the weekends. While on break, Jerell was studying for his biology test and listening to
Who took Jerell s ipod? An organic compound mystery 1 Jerell is a 10 th grade student who works at McDonald s on the weekends. While on break, Jerell was studying for his biology test and listening to
Cell Membrane Structure and Function. Hot Seat
 Cell Membrane Structure and Function Hot Seat Rules A. You are competing against classmates in your row (across the classroom). The hot seat is the seat in each row closest to the outside windows. B. Your
Cell Membrane Structure and Function Hot Seat Rules A. You are competing against classmates in your row (across the classroom). The hot seat is the seat in each row closest to the outside windows. B. Your
Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction
 Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction Most biological molecules fall into one of four varieties: proteins, carbohydrates, lipids and nucleic acids. These are sometimes
Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction Most biological molecules fall into one of four varieties: proteins, carbohydrates, lipids and nucleic acids. These are sometimes
Warm Up 12/06/2018. In a Solution of Salt Water, which substance acts as the Solvent and which substance acts as the Solute?
 Warm Up 12/06/2018 In a Solution of Salt Water, which substance acts as the Solvent and which substance acts as the Solute? Cell Membrane and Cell Transport How Nutrients move in and Wastes Move Out of
Warm Up 12/06/2018 In a Solution of Salt Water, which substance acts as the Solvent and which substance acts as the Solute? Cell Membrane and Cell Transport How Nutrients move in and Wastes Move Out of
4. 10/09/14 Ch. 5: Populations /22/14 Ch. 2: Chemistry of Life 55
 Table of Contents # Date Title Page # 1. 1 2. 09/02/14 Ch. 1: The Science of Biology 09/16/14 Ch. 4: Ecosystems and Communities 17 3. 09/23/14 Ch. 3: The Biosphere 26 4. 10/09/14 Ch. 5: Populations 45
Table of Contents # Date Title Page # 1. 1 2. 09/02/14 Ch. 1: The Science of Biology 09/16/14 Ch. 4: Ecosystems and Communities 17 3. 09/23/14 Ch. 3: The Biosphere 26 4. 10/09/14 Ch. 5: Populations 45
LAB 04 Diffusion and Osmosis
 LAB 04 Diffusion and Osmosis Objectives: Describe the physical mechanisms of diffusion and osmosis. Understand the relationship between surface area and rate of diffusion. Describe how molar concentration
LAB 04 Diffusion and Osmosis Objectives: Describe the physical mechanisms of diffusion and osmosis. Understand the relationship between surface area and rate of diffusion. Describe how molar concentration
Cell Diffusion and Osmosis Lab: Directions
 Cell Diffusion and Osmosis Lab: Directions Adapted from AP bio lab 4 http://media.collegeboard.com/digitalservices/pdf/ap/bio-manual/bio_lab4-diffusionandosmosis.pdf Please return Background: Most cells
Cell Diffusion and Osmosis Lab: Directions Adapted from AP bio lab 4 http://media.collegeboard.com/digitalservices/pdf/ap/bio-manual/bio_lab4-diffusionandosmosis.pdf Please return Background: Most cells
Carbohydrates Chemical Composition and Identification
 Carbohydrates Chemical Composition and Identification Introduction: Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding
Carbohydrates Chemical Composition and Identification Introduction: Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding
Macromolecule Virtual Lab
 Part A Macromolecule Virtual Lab Go to the website: http://faculty.kirkwood.edu/apeterk/learningobjects/biologylabs.htm CARBOHYDRATES Scroll down to the bottom and click on Carbohydrate 1. What do carbohydrates
Part A Macromolecule Virtual Lab Go to the website: http://faculty.kirkwood.edu/apeterk/learningobjects/biologylabs.htm CARBOHYDRATES Scroll down to the bottom and click on Carbohydrate 1. What do carbohydrates
Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules
 Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules Introduction: There are four broad classes of macromolecules that can be found in living systems. Each type of
Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules Introduction: There are four broad classes of macromolecules that can be found in living systems. Each type of
Biology Movement Across the Cell Membrane
 Biology 160 - Movement Across the Cell Membrane Prelab Information Movement is one of the characteristics of life. The ability to control the movement of material across the cell membrane is an incredibly
Biology 160 - Movement Across the Cell Membrane Prelab Information Movement is one of the characteristics of life. The ability to control the movement of material across the cell membrane is an incredibly
Ideas for investigations: 1. Prepare slime and investigate its properties. Equipment and consumables. Making the slime
 Introduction : Polymers are long molecules made up of many repeated subunits joined end-to-end. Their name comes from Greek: poly (many) and mer (parts). Polymers are found throughout nature: examples
Introduction : Polymers are long molecules made up of many repeated subunits joined end-to-end. Their name comes from Greek: poly (many) and mer (parts). Polymers are found throughout nature: examples
Introduction: Lab Safety: Student Name: Spring 2012 SC135. Laboratory Exercise #4: Biologically Important Molecules
 FMCC Student Name: Spring 2012 SC135 Introduction: Laboratory Exercise #4: Biologically Important Molecules The major groups of biologically important molecules are: Carbohydrates, Lipids, Proteins and
FMCC Student Name: Spring 2012 SC135 Introduction: Laboratory Exercise #4: Biologically Important Molecules The major groups of biologically important molecules are: Carbohydrates, Lipids, Proteins and
INVESTIGATION : Determining Osmolarity of Plant Tissue
 INVESTIGATION : Determining Osmolarity of Plant Tissue AP Biology This lab investigation has two main components. In the first component, you will learn about the osmolarity of plant tissues and the property
INVESTIGATION : Determining Osmolarity of Plant Tissue AP Biology This lab investigation has two main components. In the first component, you will learn about the osmolarity of plant tissues and the property
Activity 4.2 Dissolving a substance in different liquids
 Activity 4.2 Does colored sugar dissolve equally well in water, vegetable oil, and alcohol? In the introductory story on Activity sheet 4.1, the student added drink mix to different liquids. Many drink
Activity 4.2 Does colored sugar dissolve equally well in water, vegetable oil, and alcohol? In the introductory story on Activity sheet 4.1, the student added drink mix to different liquids. Many drink
fossum/files/2012/01/10 Enzymes.pdf
 http://www.laney.edu/wp/cheli fossum/files/2012/01/10 Enzymes.pdf Enzyme Catalysis Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without
http://www.laney.edu/wp/cheli fossum/files/2012/01/10 Enzymes.pdf Enzyme Catalysis Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without
Learning Outcomes. 2. Diffusion takes place through the cell membrane because it is selectively permeable.
 Diffusion Learning Outcomes 1. Diffusion is the movement of molecules from a high concentration to a low concentration down a concentration gradient until evenly spread. 2. Diffusion takes place through
Diffusion Learning Outcomes 1. Diffusion is the movement of molecules from a high concentration to a low concentration down a concentration gradient until evenly spread. 2. Diffusion takes place through
Figure 2. Figure 1. Name: Bio AP Lab Organic Molecules
 Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
Diffusion, Osmosis and Active Transport
 Diffusion, Osmosis and Active Transport Part A: Diffusion A living cell interacts constantly with the environmental medium that surrounds it. The plasma membrane surrounding a cell is a living, selectively
Diffusion, Osmosis and Active Transport Part A: Diffusion A living cell interacts constantly with the environmental medium that surrounds it. The plasma membrane surrounding a cell is a living, selectively
AGENDA for 01/09/14 AGENDA: HOMEWORK: Due end of period OBJECTIVES:
 AGENDA for 01/09/14 AGENDA: 1. 2.3.2: Diabetic Emergency! Blood Glucose Effects on Simulated Cellular Models Egg Demo Day 3 OBJECTIVES: 1. Design an experiment to simulate osmosis in body cells 2. Relate
AGENDA for 01/09/14 AGENDA: 1. 2.3.2: Diabetic Emergency! Blood Glucose Effects on Simulated Cellular Models Egg Demo Day 3 OBJECTIVES: 1. Design an experiment to simulate osmosis in body cells 2. Relate
Observing Osmosis Lab
 Observing Osmosis Lab Background Information: Molecules are in constant motion, and tend to move from areas of higher concentrations to areas of lower concentrations. Diffusion is defined as the movement
Observing Osmosis Lab Background Information: Molecules are in constant motion, and tend to move from areas of higher concentrations to areas of lower concentrations. Diffusion is defined as the movement
the contents of the cell from the environment.
 Name: Date: Period: Living Environment Unit 3: Cellular Processes Study Guide Due Date: Test Date: Unit 3 Important Topics: I. Aim # 14 Cell Membrane II. Aim # 15 NYS Diffusion Lab III. Aim # 16 Photosynthesis
Name: Date: Period: Living Environment Unit 3: Cellular Processes Study Guide Due Date: Test Date: Unit 3 Important Topics: I. Aim # 14 Cell Membrane II. Aim # 15 NYS Diffusion Lab III. Aim # 16 Photosynthesis
Chemistry Mr. O Sullivan Lab Report Experiment #11. Determination of techniques to prevent the Browning of Cut Produce
 Chemistry 101-292 Mr. O Sullivan Lab Report Experiment #11 Determination of techniques to prevent the Browning of Cut Produce Abstract The purpose of this experiment was to determine effective techniques
Chemistry 101-292 Mr. O Sullivan Lab Report Experiment #11 Determination of techniques to prevent the Browning of Cut Produce Abstract The purpose of this experiment was to determine effective techniques
EXERCISE 3 Carbon Compounds
 LEARNING OBJECTIVES EXERCISE 3 Carbon Compounds Perform diagnostic tests to detect the presence of reducing sugars (Benedict s), starch (Lugol s), protein (Biuret), lipid (SudanIV) and sodium chloride
LEARNING OBJECTIVES EXERCISE 3 Carbon Compounds Perform diagnostic tests to detect the presence of reducing sugars (Benedict s), starch (Lugol s), protein (Biuret), lipid (SudanIV) and sodium chloride
The Cell Membrane. Also known as the Plasma Membrane
 Student Objectives Know the different parts of the cell membrane Understand the role of the cell membrane in cellular transport Understand diffusion and osmosis Determine what will happen to plant and
Student Objectives Know the different parts of the cell membrane Understand the role of the cell membrane in cellular transport Understand diffusion and osmosis Determine what will happen to plant and
Organic Molecule Composition of Milk: Lab Investigation
 Name: Organic Molecule Composition of Milk: Lab Investigation Introduction & Background Milk & milk products have been a major food source from earliest recorded history. Milk is a natural, nutritionally
Name: Organic Molecule Composition of Milk: Lab Investigation Introduction & Background Milk & milk products have been a major food source from earliest recorded history. Milk is a natural, nutritionally
What is the major site of digestion? If you answered stomach, you missed it! The correct answer is small intestine.
 DIGESTION SIMULATION LAB PAP CLASS SET BACKGROUND INFORMATION What is digestion and where does it begin? Digestion is the breaking down of food into forms that our bodies can use. Your digestive system
DIGESTION SIMULATION LAB PAP CLASS SET BACKGROUND INFORMATION What is digestion and where does it begin? Digestion is the breaking down of food into forms that our bodies can use. Your digestive system
LAB 4 Macromolecules
 LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
Big. Cellular Processes: Idea. Energy and Communication DIFFUSION AND OSMOSIS. What causes my plants to wilt if I forget to water them?
 Big Cellular Processes: Idea 2 Energy and Communication INVESTIGATION 4 DIFFUSION AND OSMOSIS What causes my plants to wilt if I forget to water them? BACKGROUND Cells must move materials through membranes
Big Cellular Processes: Idea 2 Energy and Communication INVESTIGATION 4 DIFFUSION AND OSMOSIS What causes my plants to wilt if I forget to water them? BACKGROUND Cells must move materials through membranes
Observing Respiration
 Chapter 9 Cellular Respiration Design an Experiment Observing Respiration Introduction Cellular respiration occurs in all living things. During this process, animals take in oxygen and release carbon dioxide
Chapter 9 Cellular Respiration Design an Experiment Observing Respiration Introduction Cellular respiration occurs in all living things. During this process, animals take in oxygen and release carbon dioxide
