acetylation17 or phosphorylationl8 may influence DNA-histone interactions and
|
|
|
- Hester West
- 6 years ago
- Views:
Transcription
1 PHOSPHORYLATION OF LIVER HISTONE FOLLOWING THE ADMINISTRATION OF GLUCAGON AND INSULIN* By THOMAS A. LANGAN CHARLES F. KETTERING RESEARCH LABORATORY, YELLOW SPRINGS, OHIO Communicated by Martin D. Kamen, August 25, 1969 Abstract.-The administration of glucagon to rats causes a marked increase in the phosphorylation of a specific serine residue in lysine-rich (f1) histone of liver during a one-hour period following the administration of the hormone. It is proposed that histone phosphorylation is the mechanism by which glucagon, and perhaps other hormones whose actions are mediated by adenosine 3',5'-cyclic phosphate (cyclic AMIP), induce RNA synthesis in target tissues. The incorporation of 32P-phosphate into lysine-rich histone is determined by isolation of a tryptic peptide which contains the phosphorylated serine residue. This peptide is identical to the major tryptic phosphopeptide obtained from lysine-rich histone after phosphorylation in vitro by a purified cyclic AMIP-dependent liver histone kinase preparation; the partial sequence Lys-Ala-SerPO4(Thr,Ser,Glu,Pro2,Gly, Val,Ile,Leu)Lys has been determined for the peptide. Hydrocortisone and adrenocorticotrophic hormone do not cause a detectable increase in histone phosphorylation in liver. However, insulin, which like glucagon induces an actinomycin sensitive synthesis of liver enzymes, also causes increased histone phosphorylation. Phosphorylation of histones, catalyzed by a specific protein kinase present in liver, is markedly increased by adenosine 3',5'-cyclic phosphate (cyclic AMP) both in vitro' and in vivo.2 Cyclic AMP acts as the mediator of many hormone responses3 and, in certain cases, these responses involve the synthesis of new protein4 I or are blocked by inhibitors of protein4' 6-9 and RNA synthesis. Thus, a role for the cyclic nucleotide in regulating transcription or translation of genetic information is implied. Histones may act as regulators of transcription from DNA templates in eukaryotic cells There is also evidence that modification of histones by acetylation17 or phosphorylationl8 may influence DNA-histone interactions and cause derepression of template activity. To test whether histone phosphorylation occurs during the induction of enzyme synthesis by hormones which act via cyclic AMP, the response of liver to glucagon administration was studied. Extensive evidence from the laboratories of Sutherland3' 19 and Park20 indicates that cyclic AMP mediates the effects of glucagon in liver. Administration of glucagon to rats causes de novo synthesis of a number of liver enzymes4' 5 and the induction of these enzymes is blocked by actinomycin D. It has been demonstrated recently that enzyme synthesis in liver can be induced directly by cyclic AMP.21, 22, 12 In this paper it is shown that glucagon, given in doses which are effective in inducing enzyme synthesis, causes a 15-to 25-fold increase in phosphorylation of a specific serine residue in rat liver lysinie-rich (f1) histone shortly after the ad- 1276
2 VOL. 64, 1969 BIOCHEMISTRY: T. A. LANGAN 1277 ministration of the hormone. Insulin, which like glucagon induces an actinomycin sensitive synthesis of enzymes in liver,4 also causes increased histone phosphorylation. On the basis of these results, a mechanism for the induction of RNA synthesis by hormones is proposed. A preliminary report of this work has appeared.23 Materials and Methods.-Electrophoresis and chromatography: Electrophoresis was carried out on a water-cooled flat plate; wicks leading to buffer vessels were isolated from electrophoresis papers by four layers of cellophane dialysis membrane. The buffers used were: ph 7.9, 0.06 Ml NH4HCO3;24 ph 4.7, 25 ml glacial acetic acid plus 25 ml pyridine per liter; ph 1.9, 25 ml 88% formic acid plus 87 ml glacial acetic acid per liter; ph 1.58, 100 ml 88% formic acid per liter, adjusted to ph 1.58 by further addition of formic acid. The chromatographic solvents used were: solvent A, pyridine/n-butanol/glacial acetic acid/h20 = 10/15/3/12; solvent B, n-butanol/glacial acetic acid/h20 = 4/1/2. Except where indicated, Eastman no cellulose thin layers were used. Purification and characterization of the major tryptic phosphopeptide of enzymatically phosphorylated lysine-rich histone: Calf thymus lysine-rich (fl) histone prepared by the methods of Johns26 was phosphorylated by incubation with purified liver histone kinase and 32P-ATP as previously described.26 Tryptic digestion and preparative scale paper electrophoresis were carried out as described in the legend to Figure 1. The major radioi. FIG. 1.-Tryptic peptides of enzymatically phosphorylated, 32p_ labeled calf thymus lysine-rich histone. (a), ninhydrin staining; and (b), radioautograph. Phosphorylated histone was digested at ph 8.0 for 4 hr at 370 with trypsin (trypsin:histone = 1:100). The ph was maintained by automatic titration with NH40H. The digest was subjected to paper electrophoresis in ph 7.9 buffer at 40 ORIGIN v/cm for3 hr. active band was eluted and further purified by chromatography with solvent A on cellulose thin layers, or by treatment with E. coli alkaline phosphatase (0.05 mg/ml in 0.04 M NH4HC03) followed by a second electrophoresis at ph 7.9. Samples purified by both methods had closely similar amino acid compositions, as determined with an automatic analyzer. (These analyses were kindly performed by Dr. K. Fry.) Conditions for the enzymatic digestions used to establish the partial sequence of the peptide were: leucine amino peptidase (Worthington) 0.5 mg/ml in M NH4HCO3, 2.5 mm MgCl2; trypsin (Sigma, twice crystallized), 0.15 mg/ml in 0.02 MI NH4HCO3. Amino acids released were identified by thin-layer electrophoresis for 40 min at 75 v/cm on 10 X 40 cm cellulose thin layers (Brinkmann) in formic acid buffer, ph l.58,27 followed by chromatography in the second dimension with solvent B. Hormones: Crystalline glucagon and bovine insulin, 25 units/mg, containing 0.024% glucagon, were gifts from Drs. B. H. Frank and W. Bromer, Eli Lilly Co. ACTH (Acthar) was purchased from Armour Company and hydrocortisone from Calbiochem. Determination of liver lysine-rich histone phosphorylation in vivo: Rats, 180 to 250 gin, a b
3 1278 BIOCHEMISTRY: T. A. LANGAN PROC. N. A. S. obtained from Holtzman Co. or Charles River Laboratories, were fasted overnight and injected intraperitoneally with the indicated dose of hormone in 0.5 ml of 0.14 M NaCl. Except where indicated, 2 mci of 32P phosphate (New England Nuclear) in 0.5 ml of 0.14 Ml NaCl containing 10-4 M carrier inorganic phosphate was injected immediately after hormone administration. Control animals received 32P-phosphate only. At the designated times, animals were killed and the livers quickly frozen in liquid nitrogen. The method of de Nooij and Westenbrink,28 slightly modified, was used to prepare lysine rich histone. Pooled livers from two rats were homogenized at 40C in a Waring blender for 1 min. at 85 v and 2 min at 45 y in 325 ml of 0.14 M NaCl, 0.01 M sodium citrate, ph 6.0. A 1-ml aliquot of the homogenate was frozen immediately for subsequent determination of the specific activity of liver phosphate pools by the method of Martin and Doty.29 The homogenate was filtered through a single layer of Miracloth (Chicopee Mills, Inc.) and centrifuged 15 min at 3300 g. The sediment was suspended in 280 ml of homogenizing medium and recentrifuged. To extract lysine-rich histone, the combined pellets were blended in 47.5 ml of H20 for 1 min at top speed and 2.5 ml of 100% (1 gm/ml) trichloroacetic acid was added during the next minute of blending. Blending was continued for an additional 30 sec and the suspension centrifuged 5 min at 3300g. The trichloroacetic acid concentratiqn of the supernatant was adjusted to 18% and the suspension allowed to stand in ice for 20 min before centrifuging for 20 min at 13,000 g. The precipitated lysine-rich histone was dissolved in water and reprecipitated twice with 25% trichloroacetic acid. It was then washed once with acetone containing 0.5 ml HCl/ 100 ml, once with acetone, and dried in vacuo. The dry histone was dissolved in 0.15 ml of H20 and insoluble material was removed by centrifugation. An aliquot was removed for protein determination by the biuret method84 in a final volume of 0.25 ml. The yield from 10 gm of liver was approximately 2 mg. Unlabeled enzymatically phosphorylated calf thymus lysine-rich histone containing 10 to 15 m,4moles of phosphate was then added as carrier, followed by 10 Mmoles of NH4HC03 and 50 jig of trypsin. After incubation for 1 hr at 370C, the digest was subjected to electrophoresis on Schleicher and Schuell no. 589 green ribbon paper in ph 7.9 buffer for 45 min at 100 v/cm. In order to remove material that is frequently present in this paper and that interferes with subsequent thinlayer chromatography of eluates, it was autoclaved before use for 90 min in 0.05 Ml NH40H, washed thoroughly with water, and soaked overnight in 95% ethanol. Tryptic digests of 32P-labeled enzymatically phosphorylated thymus lysine-rich histone were used as markers in the electrophoresis. Radioactive bands were located by radioautography (60 hr exposure to Kodak blue brand X-ray film) and eluted. The eluates were spotted on cellulose thin layers and chromatographed in solvent A. Radioactive peptides were located by radioautography for 12 to 16 hr, cut out, and counted in a low background GM counter. The observed counts were corrected to account for individual variations in the labeling of phosphate pools with the following formula: Relative cpm = observed cpm in phosphopeptide/cpm per pmole in liver inorganic phosphate. In some experiments, the specific activity of acid-labile phosphate in liver nucleotides adsorbable on charcoal"' was also measured and found not to differ significantly from the values for inorganic phosphate. None of the hormone treatments produced significant changes in the specific activity of liver phosphate pools. Results.-Characterization of phosphorylation site in lysine-rich histone: Previous studies32 of the phosphorylation of lysine-rich (f1) histone by liver histone kinase indicated that the maximum amount of phosphate which can be transferred to this histone is close to 1 mole per mole of protein. In order to characterize the phosphorylation site in lysine-rich histone, the distribution of phosphate in tryptic peptides of enzymatically phosphorylated, 32P-labeled calf thymus lysinerich histone was examined by paper electrophoresis at ph 7.9 (Fig. 1). One major radioactive peptide was found which represented 65 per cent of the total 32p in the digest shown, but which has been obtained in yields up to 80 per
4 VOL. 64, 1969 BIOCHEMISTRY: T. A. LANGAN 1279 TABLE 1. Composition of major tryptic phosphopeptide of enzymatically phosphorylated lysine-rich histone. Molar ratio Thr* 0.91 Ser* 1.78 Glu 1.27 Pro 1.89 Gly 1.03 Ala 1.23 Val 0.89 Ile 0.83 Leu 0.79 Lys 2.00 P * Corrected for 10% loss during hydrolysis. cent. A representative analysis of the major phosphopeptide, determined after purification from nonradioactive peptides which migrate with it at ph 7.9, is shown in Table 1. The analyses indicate that the peptide contains 13 amino acid residues, many of them neutral or hydrophobic. There are two serine residues and a single phosphate group. The amino acid sequence at the N-terminal end of the peptide has been investigated by enzymatic degradation (see Materials and Methods). Treatment with E. coli alkaline phosphatase followed by leucine amino peptidase releases 32P-inorganic phosphate, lysine, alanine, and serine, plus a peptide which is not degraded further. Treatment with leucine amino peptidase alone releases lysine and alanine plus a radioactive peptide. Phosphoserine and a nonradioactive peptide corresponding to the previous peptide product are then released slowly. Finally, treatment with high concentrations of trypsin releases free lysine, demonstrating the N-terminal location of one lysine residue. The partial sequence: Lys-Ala-SerPO4(Thr,Ser,Glu,Pro2,Gly,ValIle, Leu)Lys is indicated for the peptide by these data. In contrast, Dixon et al.33 have found that phosphorylation of trout testis histones II and IV occurs in the N-terminal sequence N-acetyl-SerPO4-Gly-Arg. The isolation in good yield of a single major tryptic phosphopeptide from enzymatically phosphorylated lysinerich histone provides conclusive evidence that liver histone kinase acts mainly on a single specific serine residue in the histone molecule, and not at many sites in a minor component or contaminant of the preparation. The isolation of this peptide also provided a specific method for demonstrating the action of histone kinase in ViVo2 and for studying the effects of hormones on histone phosphorylation, as described below. Phosphorylation of lysine-rich histone after hormone administration: Rats were injected with 32P-phosphate or 32P-phosphate plus glucagon and liver lysine-rich histone isolated, digested with trypsin, and subjected to paper electrophoresis as described in Materials and Methods. The tryptic digest of lysine-rich histone from glucagon treated animals showed a clear band of radioactivity corresponding to the major tryptic phosphopeptide from enzymatically phosphorylated lysine-rich histone (Fig. 2a). This band and the corresponding area from the control sample were eluted and chromatographed on cellulose thin layers, de-
5 : ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ BIOCHEMISTRY: T'. A. LANGAN PRoc. N. A. S....0 FIG. 2.-Radioactive phosphopeptides in tryptic digests of liver lysine-rich histone (Left) from control and hormone-treated rats. Paper electrophoresis was carried out at ph 7.9 and bands revealed by radioautography. Marker digests of enzymatically phosphorylated thymus lysine-rich histone are designated by the letter m. Glucagon (300 Mug/rat) was given 60 minand insulin (1.25 units/rat) and ACTH (5 units/rat) 70 min before animals were killed. 32p_ phosphate was given at the time of hormone administration, or 60 min-before killing control animals. (Right) FIG. 3.-Thin-layer chromatography of tryptic phosphopeptides isolated from liver lysine-rich histone of control and glucagon-treated rats. Lane 1, control; lane 2, glucagon; m designates marker peptide isolated from enzymatically phosphorylated lysine-rich histone. 32P-phosphate and glucagon (400,ug) injections were given 2 hr before animals were killed. Tryptic digests were subjected to electrophoresis at ph 7.9 and radioactive bands with mobilities corresponding to the major tryptic phosphopeptide of enzymatically phosphorylated lysine-rich histone (cf. Fig. 2) were eluted and chromatographed on cellulose with solvent A. Radioautographic exposure was continued for four days to visualize minor components. veloping with solvent A. The migration of the band from hormone-treated animals again matched that of the marker peptide from enzymatically phosphorylated histone, and a faint matching band in the control sample was also visible (Fig. 3). Note that glucagon causes a specific increase in the phosphorylation of the site contained in this peptide; whether other radioactive bands represent phosphorylation sites not affected by glucagon or contaminants of the histone preparation has not been determined. The identity of the major phosphopeptides isolated from liver lysine-rich histone of hormone-treated animals and from enzymatically phosphorylated lysine-rich histone was confirmed by further comparisons of chromatographic mobility in solvent B and electrophoretic migration at ph 4.7 and 1.9. In each of these systems, the mobility of the phosphopeptides derived from histone phosphorylated in vivo and in vitro was identical. In addition, radioactive phosphoserine was identified by electrophoresis at ph 1.9 as the sole phosphoamino acid in partial acid hydrolysates (2 N HC1, 16 hr, 100 )34 of phosphopeptide derived from histone phosphorylated 'n VivO.
6 VOL. 64, 1969 BIOCHEMISTRY: T'. A. LANGAN 1281 TABLE 2. Phosphorylation of liver lysine-rich hi-stone following administration of glucagon. Time after Relative cpin in glucagon Hormone and phosphopeptide/mg Expt. administration antibiotic injections lysine-rich histone A 15 min Glucagon min Glucagon + cycloheximide 141 None 26 B 1 hr Glucagon hr Glucagon + actinomycin D cycloheximide None 50 2 hr Glucagon 960 None 46 Expt. A-32P-phosphate and cycloheximide (10 mg) injections were given 1 hr and glucagon (300 IAg) 15 min before animals were killed. Rats weighed 180 to 200 gm. Expt. B-32P-phosphate and glucagon (400 Mg) injections were given at the indicated times before animals were killed. Actinomycin D (500 jug in 0.25 ml of propylene glycol) and cycloheximide (10 mg) were given 30 min before glucagon. Rats weighed 230 to 250 gm. For quantitative assay of histone phosphorylation in vivo, the peptide spots obtained after electrophoresis at ph 7.9 and subsequent thin-layer chromatography were cut out and counted. As shown in Table 2 (expt. A), the response to glucagon is readily detectable 15 minutes after intraperitoneal injection of the hormone. During the next two hours (expt. B), the level of histone phosphorylation rises to a level approximately 20-fold higher than that found in control animals. Phosphorylation is not blocked by actinomycin D or cycloheximide, indicating (1) that phosphorylation takes place on intact histone molecules and (2) that it is a primary effect of the hormone and not dependent on increased synthesis of histone kinase or other enzymes. The increased phosphorylation in response to glucagon administration is consistent with the known ability of glucagon to increase the concentration of cyclic AMP in liver'9 5 and with the previously observed affects of cyclic AMP on the activity of purified liver histone kinase,' and on the phosphorylation of liver histone in vivo.2 Histone phosphorylation in response to various doses of glucagon was studied. Clear increases in phosphorylation occur after administration of 10 Jg per rat, and the maximum response is obtained with doses of 100 tig or larger. Holten and Kenney4 have shown that doses of glucagon from 100 to 600 /Ag per rat produce four-to sixfold elevations in the level of liver tyrosine transaminase. Thus, the doses of glucagon which induce enzyme synthesis correspond to those which cause increased histone phosphorylation. The maximum levels of histone phosphorylation reached in the dose and time studies indicate that glucagon causes the phosphorylation of only a limited fraction of liver lysine-rich histone. The size of this fraction is difficult to determine accurately, but rough estimates indicate that it is on the order of 1 per cent of the total lysine-rich histone. The effects of ACTH, hydrocortisone, and insulin on liver histone phosphorylation were also examined (Fig. 2b, Table 3). ACTH, which acts via cyclic AMP to induce protein synthesis in adrenal cortex,9' 36 is without effect on histone phosphorylation in liver. Hydrocortisone, which induces enzyme synthesis in liver by a mechanism which does not involve cyclic AMP,37 also has no effect on liver histone phosphorylation. However, insulin does cause increased histone
7 1282 BIOCHEMISTRY: T. A. LANGAN PRoc. N. A. S. TABLE 3. Phosphorylation of liver lysine-rich histone following administration of various hormones. Relative cpm in phosphopeptide/mg Hormone and antibiotic injections lysine-rich histone None 29 Glucagon 636 Hydrocortisone 31 ACTH 24 Insulin 378 Insulin + actinomycin D + cycloheximide 338 Hydrocortisone (10 mg) was given 30 min before 32P-phosphate and the animals killed 70 min later. Actinomycin D (400 Mg) and cycloheximide (10 mg) were given 30 min before insulin. Other injections given as described in Figure 2. Rats weighed 180 to 200 gm. phosphorylation, as is clearly shown by the band of radioactive peptide in Figure 2b, and by the data presented in Table 3. Phosphorylation induced by insulin is also insensitive to actinomycin D and cycloheximide. The effect of insulin is not readily explained, since insulin appears to lower slightly the level of cyclic AMP in liver.38 However, as shown in Kenney's laboratory, insulin induces an actinomycin-sensitive synthesis of tyrosine transaminase in rat liver which is identical in all respects examined to the synthesis induced by glucagon.4 3 In both cases then, hormonal induction of enzyme synthesis is associated with an increase in histone phosphorylation. However, experiments with simpler systems, such as perfused livers, are needed to establish whether insulin acts directly on the liver to cause histone phosphorylation. Discussion.-Together with the evidence that histones probably function in some way in regulating the activity of genes,'3-16 the present findings make it possible to formulate a mechanism for the induction of RNA synthesis by glucagon, and perhaps by other hormones whose actions are mediated by cyclic AMP, which has some detail at the molecular level. The primary site of action of this type of hormone, as shown by the extensive studies of Sutherland and collaborators,3 is the enzyme adenyl cyclase. This is activated by the hormone to catalyze the formation of cyclic AMP which in turn activates histone kinase, resulting in the phosphorylation of histone. It is proposed that the induction of RNA synthesis might be brought about by a change in DNA-histone interaction resulting from histone phosphorylation; presumably this would involve a change in configuration of the histone with consequent derepression of the DNA template, followed by RNA and protein synthesis. The limited amount of histone phosphorylation observed in our studies is consistent with the specific effect of glucagon on the synthesis of a small number of enzymes in liver, but the basis for this specificity is not known. In addition, the role of cyclic AMP in regulating protein synthesis may be more complex than indicated here, since there is evidence that cyclic AMP stimulates the translations as well as the synthesis4' of RNA in bacteria and that glucagon can act at a post-transcriptional level in cultured liver cells.42 Note added in proof: Protein phosphorylation in response to insulin treatment has also been observed recently by A. E. Voytovitch, I. S. Owens, and Y. J. Topper (these PROCEEDINGS, 63, 213 (1969) in mammary tissue. In these experiments, isolated explants of mammary gland were used, excluding the possibility that insulin acted indirectly.
8 VOL. 64, 1969 BIOCHEMISTRY: T. A. LANGAN 1283 The author would like to thank Drs. F. T. Kenney, W. Wicks, P. Varandani, K. Fry, and G. von Ehrenstein for many helpful discussions and suggestions, and Mr. Ivan Filmore for his very capable and invaluable assistance in this work. Mr. B. Schaffhausen provided excellent help in performing preliminary investigations of histone phosphorylation in liver. * This research was supported in part by the U.S. Public Health Service, National Cancer Institute grant CA Publication 362 of the C. F. Kettering Research Laboratory. 1 Langan, T. A., Science, 162, 579 (1968). 2 Langan, T. A., J. Biol. Chem., 244, 5763 (1969). Sutherland, E. W., G. A. Robison, and R. W. Butcher, Circulation, 37, 279 (1968). 4Holten, D., and F. T. Kenney, J. Biol. Chem., 242, 4372 (1967). 6 Jost, J.-P., E. A. Khairallah, and H. C. Pitot, J. Biol. Chem., 243, 3057 (1968). 6 Ferguson, J. J., Jr., J. Biol. Chem., 238, 2754 (1963). 7 Garren, L. D., R. L. Ney, and W. W. Davis, these PROCEEDINGS, 53, 1443 (1965). 8 Marsh, J. M., R. W. Butcher, K. Savard, and E. W. Sutherland, J. Biol. Chem., 241, 5436 (1966). 9 Grahame-Smith, D. G., R. W. Butcher, R. L. Ney, and E. W. Sutherland, J. Biol. Chem., 242, 5535 (1967). 10Savard, K., J. M. Marsh, and B. F. Rice, Recent Progr. Hormone Res., 21, 285 (1965). 11 Farese, R. V., Endocrinology, 78, 929 (1966). 12 Jost, J.-P., A. W. Hsie, and H. V. Rickenberg, Biochem. Biophyt. Res. Commun., 34, 748 (1969). 13 Stedman, E., and E. Stedman, Nature, 166, 780 (1950). 14 Huang, R. C., and J. Bonner, these PROCEEDINGS, 48, 1216 (1962). 15Allfrey, V. G., V. C. Littau, and A. E. Mirsky, these PROCEEDINGS, 49, 414 (1963). 16Bekor, I., G. M. Kung, and J. Bonner, J. Mol. Biol., 39, 351 (1969). 17Pogo, B. G. T., A. 0. Pogo, V. G. Allfrey, and A. E. Mirsky, these PROCEEDINGS, 59, 1337 (1968). 18 Ord, M. G., and L. A. Stocken, Biochem. J., 107, 403 (1968). 19 Sutherland, E. W., and T. W. Rall, Pharmacol. Rev., 12, 265 (1960). 20Exton, J. H., and C. R. Park, Pharmacol. Rev., 18, 181 (1966). 21 Wicks, W. D., Science, 160, 997 (1968). 22Yeung, D., and I. T. Oliver, Biochemistry, 7, 3231 (1968). 23 Langan, T. A., Fed. Proc., 28, 600 (1969). 24Schwartz, J. H., Ph.D. thesis, Rockefeller University (1963). 25Johns, E. W., Biochem. J., 92, 55 (1964). 28Meisler, M. H., and T. A. Langan, J. Biol. Chem., 244, 4961 (1969). 27 Dreyer, W. J., and Bynum, E., in Methods in Enzymology (New York: Academic Press, 1967), vol. 11, p De Nooij, E. H., and Westenbrink, H. G. K., Biochim. Biophys. Acta, 62, 608 (1962). 29 Martin, J. B., and D. M. Doty, Anal. Chem., 21, 965 (1949). 10 Layne, E., in Methods in Enzymology (New York: Academic Press, 1957), vol. 3, p Crane, R. C., and F. Lipmann, J. Biol. Chem., 201, 235 (1953). 32 Langan, T. A., in Regulatory Mechanisms for Protein Synthesis in Mammalian Cells, eds. A. San Pietro, M. Lamborg, and F. T. Kenney, (New York: Academic Press, 1968), p Dixon, G. H., K. Marushige, V. Ling, M. Sung, and D. T. Wigle, Fed. Proc., 28, 599 (1969). 34 Lipmann, F., and P. Levene, J. Biol. Chem., 98, 109 (1932). 35 Robison, G. A., J. H. Exton, C. R. Park, and E. W. Sutherland, Fed. Proc., 26, 257 (1967). 36 Ney, R. L., R. N. Dexter, W. W. Davis, and L. D. Garren, J. Clin. Invest., 46, 1916 (1967). 37 Granner, D., L. R. Chase, G. D. Aurbach, and G. M. Tomkins, Science, 162, 1018 (1968). 38 Jefferson, L. S., J. J. Exton, R. W. Butcher, E. W. Sutherland, and C. R. Park, J. Biol. Chem., 243, 1031 (1968). 39 Hager, C. B., and F. T. Kenney, J. Biol. Chem., 243, 3296 (1968). 40 Pastan,'I.,'and R. L. Perlman, Fed. Proc., 28, 730 (1969). 41 Perlman,1R. L., and I. Pastan, J. Biol. Chem., 243, 5420 (1968). 42 Lee, K-L., Fed. Proc., 28, 729 (1969).
Blocking by Histones of Accessibility to DNA in Chromatin (DNase/RNA polymerase/dna polymerase)
 Proc. Nat. Acad. Sci. USA Vol. 69, No. 8, pp. 2115-2119, August 1972 Blocking by Histones of Accessibility to in Chromatin (/RNA polymerase/ polymerase) ALFRED E. MIRSKY AND BERT SILVERMAN The Rockefeller
Proc. Nat. Acad. Sci. USA Vol. 69, No. 8, pp. 2115-2119, August 1972 Blocking by Histones of Accessibility to in Chromatin (/RNA polymerase/ polymerase) ALFRED E. MIRSKY AND BERT SILVERMAN The Rockefeller
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Trypsin digestion: The lyophilized powder of the reduced S-carboxymethylated ACID) BETWEEN NORMAL (B+) AND THE COMMON NEGRO VARIANT
 A SINGLE AMINO ACID SUBSTITUTION (ASPARAGINE TO ASPARTIC ACID) BETWEEN NORMAL (B+) AND THE COMMON NEGRO VARIANT (A+) OF HUMAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE* BY AKIRA YOSHIDA DIVISION OF MEDICAL GENETICS,
A SINGLE AMINO ACID SUBSTITUTION (ASPARAGINE TO ASPARTIC ACID) BETWEEN NORMAL (B+) AND THE COMMON NEGRO VARIANT (A+) OF HUMAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE* BY AKIRA YOSHIDA DIVISION OF MEDICAL GENETICS,
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
Localization of Methylated Arginine in the Al Protein from Myelin
 Proc. Nat. Acad. Sci. USA Vol. 68, No. 4, pp. 765-769, April 1971 Localization of Methylated Arginine in the Al Protein from Myelin STEVEN BROSTOFF AND E. H. EYLAR The Salk Institute, San Diego, California
Proc. Nat. Acad. Sci. USA Vol. 68, No. 4, pp. 765-769, April 1971 Localization of Methylated Arginine in the Al Protein from Myelin STEVEN BROSTOFF AND E. H. EYLAR The Salk Institute, San Diego, California
Previous Class. Today. Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism. Protein Kinase Catalytic Properties
 Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Saccharomyces cerevisiae*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY 1988 by The American Society for Biochemistry and Molecular Biology, Inc. Vol. 263, No. 29, Issue of October 15, pp. 14948-14955, 1988 Printed in U.S.A. Purification
THE JOURNAL OF BIOLOGICAL CHEMISTRY 1988 by The American Society for Biochemistry and Molecular Biology, Inc. Vol. 263, No. 29, Issue of October 15, pp. 14948-14955, 1988 Printed in U.S.A. Purification
"Fritz, I. B., and S. K. Schultz, J. Biol. Chem., 240, 2188 (1965).
 VOL. 54, 1965 BIOCHEMISTRY: R. W. HENDLER 1233 'Fritz, I. B., Am. J. Physiol., 197, 297 (1959). 2 Fritz, I. B., and B. McEwen, Science, 129, 334 (1959). 3 Fritz, I. B., E. Kaplan, and K. T. N. Yue, Am.
VOL. 54, 1965 BIOCHEMISTRY: R. W. HENDLER 1233 'Fritz, I. B., Am. J. Physiol., 197, 297 (1959). 2 Fritz, I. B., and B. McEwen, Science, 129, 334 (1959). 3 Fritz, I. B., E. Kaplan, and K. T. N. Yue, Am.
Student Number: To form the polar phase when adsorption chromatography was used.
 Name: Student Number: April 14, 2001, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Lab Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided.. 2. The last page
Name: Student Number: April 14, 2001, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Lab Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided.. 2. The last page
ON THE NATURE OF THE TRANSALDOLASE-DIHYDROXYACETONE
 VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
The incorporation of labeled amino acids into lens protein. Abraham Speclor and Jin H. Kinoshita
 The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP
 CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
Student Number: THE UNIVERSITY OF MANITOBA April 16, 2007, 9:00 AM -12:00 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination
 Name: Student Number: THE UNIVERSITY OF MANITOBA April 16, 2007, 9:00 AM -12:00 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination MBIO / CHEM.2370 Examiner: Dr. A. Scoot 1. Answer ALL
Name: Student Number: THE UNIVERSITY OF MANITOBA April 16, 2007, 9:00 AM -12:00 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination MBIO / CHEM.2370 Examiner: Dr. A. Scoot 1. Answer ALL
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Synopsis. Received March 2, adrenaline. Mosinger and Kujalova (1964) reported that adrenaline-induced lipolysis
 Studies on Reduction of Lipolysis in Adipose Tissue on Freezing and Thawing YASUSHI SAITO1, NoBUO MATSUOKA1, AKIRA KUMAGAI1, HIROMICHI OKUDA2, AND SETSURO FUJII3 Chiba University, Chiba 280, Japan, 2Department
Studies on Reduction of Lipolysis in Adipose Tissue on Freezing and Thawing YASUSHI SAITO1, NoBUO MATSUOKA1, AKIRA KUMAGAI1, HIROMICHI OKUDA2, AND SETSURO FUJII3 Chiba University, Chiba 280, Japan, 2Department
HPLC '88. Poster Presentation. Isolation of Thymosin B4 from Thymosin Fraction 5 by Reverse Phase HPLC
 Essentials in HPLC '88 Poster Presentation Isolation of Thymosin B4 from Thymosin Fraction 5 by Reverse Phase HPLC M. Badamchian, M.P. Strickler, M.J. Stone, A.L. Goldstein for Waters.bioresearchThe absolute,
Essentials in HPLC '88 Poster Presentation Isolation of Thymosin B4 from Thymosin Fraction 5 by Reverse Phase HPLC M. Badamchian, M.P. Strickler, M.J. Stone, A.L. Goldstein for Waters.bioresearchThe absolute,
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN
 THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Acta Medica Okayama. Masana Ogata FEBRUARY Volume 16, Issue Article 2. Okayama University,
 Acta Medica Okayama Volume 16, Issue 1 1962 Article 2 FEBRUARY 1962 Studies on the protein synthesis in poisoning. III. Labeling of ph-5 enzyme with C14-glycine and the inhibition by para chloromercuribenzoate
Acta Medica Okayama Volume 16, Issue 1 1962 Article 2 FEBRUARY 1962 Studies on the protein synthesis in poisoning. III. Labeling of ph-5 enzyme with C14-glycine and the inhibition by para chloromercuribenzoate
2. Which of the following amino acids is most likely to be found on the outer surface of a properly folded protein?
 Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Biochemistry 2 Recita0on Amino Acid Metabolism
 Biochemistry 2 Recita0on Amino Acid Metabolism 04-20- 2015 Glutamine and Glutamate as key entry points for NH 4 + Amino acid catabolism Glutamine synthetase enables toxic NH 4 + to combine with glutamate
Biochemistry 2 Recita0on Amino Acid Metabolism 04-20- 2015 Glutamine and Glutamate as key entry points for NH 4 + Amino acid catabolism Glutamine synthetase enables toxic NH 4 + to combine with glutamate
BIL 256 Cell and Molecular Biology Lab Spring, Tissue-Specific Isoenzymes
 BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
Amino acids. Side chain. -Carbon atom. Carboxyl group. Amino group
 PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
Manual (Second edition)
 Reagent for RNA Extraction ISOGENⅡ Manual (Second edition) Code No. 311-07361 Code No. 317-07363 NIPPON GENE CO., LTD. Table of contents I Product description 1 II Product content 1 III Storage 1 IV Precautions
Reagent for RNA Extraction ISOGENⅡ Manual (Second edition) Code No. 311-07361 Code No. 317-07363 NIPPON GENE CO., LTD. Table of contents I Product description 1 II Product content 1 III Storage 1 IV Precautions
10 mm KCl in a Ti-15 zonal rotor at 35,000 rpm for 16 hr at
 Proc. Nat. Acad. SCi. USA Vol. 68, No. 11, pp. 2752-2756, November 1971 Translation of Exogenous Messenger RNA for Hemoglobin on Reticulocyte and Liver Ribosomes (initiation factors/9s RNA/liver factors/reticulocyte
Proc. Nat. Acad. SCi. USA Vol. 68, No. 11, pp. 2752-2756, November 1971 Translation of Exogenous Messenger RNA for Hemoglobin on Reticulocyte and Liver Ribosomes (initiation factors/9s RNA/liver factors/reticulocyte
TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
 Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
PTM Discovery Method for Automated Identification and Sequencing of Phosphopeptides Using the Q TRAP LC/MS/MS System
 Application Note LC/MS PTM Discovery Method for Automated Identification and Sequencing of Phosphopeptides Using the Q TRAP LC/MS/MS System Purpose This application note describes an automated workflow
Application Note LC/MS PTM Discovery Method for Automated Identification and Sequencing of Phosphopeptides Using the Q TRAP LC/MS/MS System Purpose This application note describes an automated workflow
STUDIES ON NUCLEAR STRUCTURE AND FUNCTION IN TETRAHYMENA PYRIFORMIS
 STUDIES ON NUCLEAR STRUCTURE AND FUNCTION IN TETRAHYMENA PYRIFORMIS III. Comparison of the Histones of Macro- and Micronuclei by Quantitative Polyacrylamide Gel Electrophoresis MARTIN A. GOROVSKY From
STUDIES ON NUCLEAR STRUCTURE AND FUNCTION IN TETRAHYMENA PYRIFORMIS III. Comparison of the Histones of Macro- and Micronuclei by Quantitative Polyacrylamide Gel Electrophoresis MARTIN A. GOROVSKY From
MEK1 Assay Kit 1 Catalog # Lot # 16875
 MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH
 Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
Communication. Identification of Methionine N -Acetyltransferase from Saccharomyces cerevisiae
 Communication THE JOURNAL OP BIOLOGICAL CHEMISTRY Vol. 265, No. 7, Issue of March 5, pp. 3603-3606,lSSO 0 1990 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U. S. A. Identification
Communication THE JOURNAL OP BIOLOGICAL CHEMISTRY Vol. 265, No. 7, Issue of March 5, pp. 3603-3606,lSSO 0 1990 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U. S. A. Identification
(From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New Jersey)
 CRYSTALLIZATION OF SALT-FREE CHYMOTRYPSINOGEN AND CHYMOTRYPSIN FROM SOLUTION IN DILUTE ETHYL ALCOHOL BY M. KUNITZ (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New
CRYSTALLIZATION OF SALT-FREE CHYMOTRYPSINOGEN AND CHYMOTRYPSIN FROM SOLUTION IN DILUTE ETHYL ALCOHOL BY M. KUNITZ (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New
(1373 Aspartic Acid >Asparagine)
 J. med. Genet. (1968). 5, 107. Haemoglobin Korle-Bu (1373 Aspartic Acid >Asparagine) Showinga One of the Two Amino Acid Substitutions of Haemoglobin C Harlem F. I. D. KONOTEY-AHULU, E. GALLO*, H. LEHMANN,
J. med. Genet. (1968). 5, 107. Haemoglobin Korle-Bu (1373 Aspartic Acid >Asparagine) Showinga One of the Two Amino Acid Substitutions of Haemoglobin C Harlem F. I. D. KONOTEY-AHULU, E. GALLO*, H. LEHMANN,
Problem-solving Test: The Mechanism of Protein Synthesis
 Q 2009 by The International Union of Biochemistry and Molecular Biology BIOCHEMISTRY AND MOLECULAR BIOLOGY EDUCATION Vol. 37, No. 1, pp. 58 62, 2009 Problem-based Learning Problem-solving Test: The Mechanism
Q 2009 by The International Union of Biochemistry and Molecular Biology BIOCHEMISTRY AND MOLECULAR BIOLOGY EDUCATION Vol. 37, No. 1, pp. 58 62, 2009 Problem-based Learning Problem-solving Test: The Mechanism
Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
 Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Catalog Number KA1538 48 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use...
Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Catalog Number KA1538 48 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use...
THE isolation and availability of crystalline
 Unidentified Factors in Poultry Nutrition. PROPERTIES AND PRELIMINARY FRACTIONATION OF A GROWTH FACTOR IN CONDENSED FISH SOLUBLES H. MENGE, C. A. DENTON, J. R. SIZEMORE, R. J. LILLIE AND H. R. BIRD Bureau
Unidentified Factors in Poultry Nutrition. PROPERTIES AND PRELIMINARY FRACTIONATION OF A GROWTH FACTOR IN CONDENSED FISH SOLUBLES H. MENGE, C. A. DENTON, J. R. SIZEMORE, R. J. LILLIE AND H. R. BIRD Bureau
Experiment 1. Isolation of Glycogen from rat Liver
 Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Biology. Lectures winter term st year of Pharmacy study
 Biology Lectures winter term 2008 1 st year of Pharmacy study 3 rd Lecture Chemical composition of living matter chemical basis of life. Atoms, molecules, organic compounds carbohydrates, lipids, proteins,
Biology Lectures winter term 2008 1 st year of Pharmacy study 3 rd Lecture Chemical composition of living matter chemical basis of life. Atoms, molecules, organic compounds carbohydrates, lipids, proteins,
Chapter PURIFICATION OF ALKALINE PROTEASES
 Chapter PURIFICATION OF ALKALINE PROTEASES E /xtracellular alkaline proteases produced by Bacillus sp. K 25 and bacillus pumilus K 242, were purified and the homogeneity was examined by electrophoresis.
Chapter PURIFICATION OF ALKALINE PROTEASES E /xtracellular alkaline proteases produced by Bacillus sp. K 25 and bacillus pumilus K 242, were purified and the homogeneity was examined by electrophoresis.
OF TRANSAMINASE IN RAT TISUES
 OF TRANSAMINASE IN RAT TISUES KOZO YAMADA, SHUNJI SAWAKI, AKIRA FUKUMURA AND MASARU HAYASHID epartment of Internal Mcdicine, Faculty of Medicine, Nagoya University, agoya Showa-ku, N (Received July 30,
OF TRANSAMINASE IN RAT TISUES KOZO YAMADA, SHUNJI SAWAKI, AKIRA FUKUMURA AND MASARU HAYASHID epartment of Internal Mcdicine, Faculty of Medicine, Nagoya University, agoya Showa-ku, N (Received July 30,
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
 EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
COLLOID DROPLET FORMATION IN DOG THYROID IN VITRO
 COLLOID DROPLET FORMATION IN DOG THYROID IN VITRO Induction by Dibutyryl Cyclic-AMP I. PASTAN and S. HI. WOLLMAN. Froml the National Institute of Arthritis and Metabolic Diseases and the National Cancer
COLLOID DROPLET FORMATION IN DOG THYROID IN VITRO Induction by Dibutyryl Cyclic-AMP I. PASTAN and S. HI. WOLLMAN. Froml the National Institute of Arthritis and Metabolic Diseases and the National Cancer
SensoLyte pnpp Alkaline Phosphatase Assay Kit *Colorimetric*
 SensoLyte pnpp Alkaline Phosphatase Assay Kit *Colorimetric* Catalog # 72146 Kit Size 500 Assays (96-well plate) Optimized Performance: This kit is optimized to detect alkaline phosphatase activity Enhanced
SensoLyte pnpp Alkaline Phosphatase Assay Kit *Colorimetric* Catalog # 72146 Kit Size 500 Assays (96-well plate) Optimized Performance: This kit is optimized to detect alkaline phosphatase activity Enhanced
Glucocorticoid Regulation of ACTH Sensitivity of
 Proceedings of the National Academy of Sciences Vol. 66, No. 3, pp. 995-1001, July 1970 Glucocorticoid Regulation of ACTH Sensitivity of Adenyl Cyclase in Rat Fat Cell Membranes T. Braun* and 0. Hechter
Proceedings of the National Academy of Sciences Vol. 66, No. 3, pp. 995-1001, July 1970 Glucocorticoid Regulation of ACTH Sensitivity of Adenyl Cyclase in Rat Fat Cell Membranes T. Braun* and 0. Hechter
This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth 2 points.
 MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
Characterization of the amino acids phosphorylated in E. coli proteins
 FEMS Microbiology Letters 17 (1983) 87-91 87 Published by Elsevier Biomedical Press Characterization of the amino acids phosphorylated in E. coli proteins 1. INTRODUCTION M. Manai and A.J. Cozzone Laboratory
FEMS Microbiology Letters 17 (1983) 87-91 87 Published by Elsevier Biomedical Press Characterization of the amino acids phosphorylated in E. coli proteins 1. INTRODUCTION M. Manai and A.J. Cozzone Laboratory
OF MELANOPHORE-EXPANDING, PRESSOR, AND OXYTOCIC
 Brit. J. Pharmacol. (1953), 8, 435. THE PURIFICATION. POTENCY, AND AMINO ACID CONTENT OF MELANOPHORE-EXPANDING, PRESSOR, AND OXYTOCIC PREPARATIONS FROM BEEF PITUITARY GLAND BY B. G. BENFEY From the Department
Brit. J. Pharmacol. (1953), 8, 435. THE PURIFICATION. POTENCY, AND AMINO ACID CONTENT OF MELANOPHORE-EXPANDING, PRESSOR, AND OXYTOCIC PREPARATIONS FROM BEEF PITUITARY GLAND BY B. G. BENFEY From the Department
Midi Plant Genomic DNA Purification Kit
 Midi Plant Genomic DNA Purification Kit Cat #:DP022MD/ DP022MD-50 Size:10/50 reactions Store at RT For research use only 1 Description: The Midi Plant Genomic DNA Purification Kit provides a rapid, simple
Midi Plant Genomic DNA Purification Kit Cat #:DP022MD/ DP022MD-50 Size:10/50 reactions Store at RT For research use only 1 Description: The Midi Plant Genomic DNA Purification Kit provides a rapid, simple
Edman Sample Preparation Part 2. Bill Henzel Genentech Inc.
 Edman Sample Preparation Part 2 Bill Henzel Genentech Inc. Determining a Protein is Blocked Estimate the amount of protein applied to the protein sequencer. Compare the staining intensity of the band of
Edman Sample Preparation Part 2 Bill Henzel Genentech Inc. Determining a Protein is Blocked Estimate the amount of protein applied to the protein sequencer. Compare the staining intensity of the band of
biotin per 409,000 gm of protein.4 Ryder et al.4 have reported preliminary investigations
 ACETYL COA CARBOXYLASE, I. REQUIREMENT FOR TWO PROTEIN FRACTIONS* BY ALFRED W. ALBERTS AND P. R. VAGELOS DEPARTMENT OF BIOLOGICAL CHEMISTRY, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE, ST. LOUIS, MISSOURI
ACETYL COA CARBOXYLASE, I. REQUIREMENT FOR TWO PROTEIN FRACTIONS* BY ALFRED W. ALBERTS AND P. R. VAGELOS DEPARTMENT OF BIOLOGICAL CHEMISTRY, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE, ST. LOUIS, MISSOURI
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
BIOCHEMICAL STUDIES ON CARBOHYDRATES. XL. Preparation of Mucoitin* from Umbilical Cords.
 The Journal of Biochemistry, Vol. 28, No. 3. BIOCHEMICAL STUDIES ON CARBOHYDRATES. XL. Preparation of Mucoitin* from Umbilical Cords. MASAMI BY SUZUKI. (From the Medico-Chemical Institute, Hokkaido Imperial
The Journal of Biochemistry, Vol. 28, No. 3. BIOCHEMICAL STUDIES ON CARBOHYDRATES. XL. Preparation of Mucoitin* from Umbilical Cords. MASAMI BY SUZUKI. (From the Medico-Chemical Institute, Hokkaido Imperial
Wheat Amino acids & Peptides for Hair Care. INCI Name EU/USA CAS # EINECS # Hydrolyzed wheat protein
 KELYAMIN Wheat Amino acids & Peptides for Hair Care Identification INCI Name EU/USA CAS # EINECS # Hydrolyzed wheat protein 70084-87-6 305-225-0 Composition % Liquid Powder Aqua Hydrolyzed wheat protein
KELYAMIN Wheat Amino acids & Peptides for Hair Care Identification INCI Name EU/USA CAS # EINECS # Hydrolyzed wheat protein 70084-87-6 305-225-0 Composition % Liquid Powder Aqua Hydrolyzed wheat protein
Consequently, lipoprotein fractions have been analyzed
 THE PHOSPHOLIPID COMPOSITION OF HUMAN SERUM LIPOPROTEIN FRACTIONS SEPARATED BY ULTRACENTRIFUGATION * BY GERALD B. PHILLIPS (From the Departments of Biochemistry and Medicine, College of Physicians and
THE PHOSPHOLIPID COMPOSITION OF HUMAN SERUM LIPOPROTEIN FRACTIONS SEPARATED BY ULTRACENTRIFUGATION * BY GERALD B. PHILLIPS (From the Departments of Biochemistry and Medicine, College of Physicians and
TLC SEPARATION OF AMINO ACIDS
 TLC SEPARATION OF AMINO ACIDS LAB CHROM 7 Adapted from Laboratory Experiments for Organic and Biochemistry. Bettelheim & Landesberg (PA Standards for Sci & Tech 3.1.12.D; 3.4.10.A; 3.7.12.B) INTRODUCTION
TLC SEPARATION OF AMINO ACIDS LAB CHROM 7 Adapted from Laboratory Experiments for Organic and Biochemistry. Bettelheim & Landesberg (PA Standards for Sci & Tech 3.1.12.D; 3.4.10.A; 3.7.12.B) INTRODUCTION
1. to understand how proteins find their destination in prokaryotic and eukaryotic cells 2. to know how proteins are bio-recycled
 Protein Targeting Objectives 1. to understand how proteins find their destination in prokaryotic and eukaryotic cells 2. to know how proteins are bio-recycled As a protein is being synthesized, decisions
Protein Targeting Objectives 1. to understand how proteins find their destination in prokaryotic and eukaryotic cells 2. to know how proteins are bio-recycled As a protein is being synthesized, decisions
quantitatively reduced by sodium borohydride in aqueous solution while glycosidically
 ENZYMATIC SYNTHESIS OF CYTIDINE 5'-MONOPHOSPHO-SIALIC ACIDS* BY SAUL ROSEMAN RACKHAM ARTHRITIS RESEARCH UNIT AND DEPARTMENT OF BIOLOGICAL CHEMISTRY, THE UNIVERSITY OF MICHIGAN Communicated by Henry Lardy,
ENZYMATIC SYNTHESIS OF CYTIDINE 5'-MONOPHOSPHO-SIALIC ACIDS* BY SAUL ROSEMAN RACKHAM ARTHRITIS RESEARCH UNIT AND DEPARTMENT OF BIOLOGICAL CHEMISTRY, THE UNIVERSITY OF MICHIGAN Communicated by Henry Lardy,
Steps at which eukaryotic gene expression can be controlled. Cell 7.5
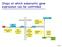 Steps at which eukaryotic gene expression can be controlled Cell 7.5 Protein Variability and Protein Activity Control Aminoacid sequence Three-dimensional shape (conformation) Function Protein processing
Steps at which eukaryotic gene expression can be controlled Cell 7.5 Protein Variability and Protein Activity Control Aminoacid sequence Three-dimensional shape (conformation) Function Protein processing
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
9/16/15. Properties of Water. Benefits of Water. More properties of water
 Properties of Water Solid/Liquid Density Water is densest at 4⁰C Ice floats Allows life under the ice Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form Benefits
Properties of Water Solid/Liquid Density Water is densest at 4⁰C Ice floats Allows life under the ice Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form Benefits
synthesis. Although such data strongly suggest that a protein
 Proc. Nat. Acad. Sci. USA Vol. 68, No. 7, pp. 1653-1657, July 1971 Mechanism of Action of Vitamin K: Evidence for the Conversion of a Precursor Protein to Prothrombin in the Rat (phylloquinone/baso4 adsorption/polyacrylamide
Proc. Nat. Acad. Sci. USA Vol. 68, No. 7, pp. 1653-1657, July 1971 Mechanism of Action of Vitamin K: Evidence for the Conversion of a Precursor Protein to Prothrombin in the Rat (phylloquinone/baso4 adsorption/polyacrylamide
Prerequisites Protein purification techniques and protein analytical methods. Basic enzyme kinetics.
 Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
EPIGENTEK. EpiQuik Global Histone H4 Acetylation Assay Kit. Base Catalog # P-4009 PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE
 EpiQuik Global Histone H4 Acetylation Assay Kit Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Global Histone H4 Acetylation Assay Kit is suitable for specifically measuring global
EpiQuik Global Histone H4 Acetylation Assay Kit Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Global Histone H4 Acetylation Assay Kit is suitable for specifically measuring global
Vets 111/Biov 111 Cell Signalling-2. Secondary messengers the cyclic AMP intracellular signalling system
 Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES
 1 SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES Proteins are important in food processing and food product development, as they are
1 SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES Proteins are important in food processing and food product development, as they are
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Global Histone H3 Acetylation Assay Kit
 Global Histone H3 Acetylation Assay Kit Catalog Number KA0633 96 assays Version: 06 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Global Histone H3 Acetylation Assay Kit Catalog Number KA0633 96 assays Version: 06 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Diabetologia 9 by Springer-Verlag 1977
 Diabetologia 13, 153-157 (1977) Diabetologia 9 by Springer-Verlag 1977 Effect of Experimental Diabetes and Glucagon on camp-dependent Protein Kinase in Rat Liver H. E. Weber 1, L. A. Menahan 2, S. N. Chaudhuri
Diabetologia 13, 153-157 (1977) Diabetologia 9 by Springer-Verlag 1977 Effect of Experimental Diabetes and Glucagon on camp-dependent Protein Kinase in Rat Liver H. E. Weber 1, L. A. Menahan 2, S. N. Chaudhuri
Mammalian Tissue Protein Extraction Reagent
 Mammalian Tissue Protein Extraction Reagent Catalog number: AR0101 Boster s Mammalian Tissue Protein Extraction Reagent is a ready-to-use Western blot related reagent solution used for efficient extraction
Mammalian Tissue Protein Extraction Reagent Catalog number: AR0101 Boster s Mammalian Tissue Protein Extraction Reagent is a ready-to-use Western blot related reagent solution used for efficient extraction
CRYSTALLINE PEPSIN BY JOHN H. NORTHROP. (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, iv. J.
 CRYSTALLINE PEPSIN III. PREPARATION OF ACTIVE CRYSTALLINE PEPSIN FROM INACTIVE DENATURED PEPSIN BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton,
CRYSTALLINE PEPSIN III. PREPARATION OF ACTIVE CRYSTALLINE PEPSIN FROM INACTIVE DENATURED PEPSIN BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton,
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Acetyl CoA Carboxylase: The Purified Transcarboxylase Component
 Proc. Nat. Acad. Sci. USA Vol. 68, No. 6, pp. 12591263, June 1971 Acetyl CoA Carboxylase: The Purified Transcarboxylase Component (acyl CoA binding/carboxylation/exchange reactions/biotin) ALFRED W. ALBERTS,
Proc. Nat. Acad. Sci. USA Vol. 68, No. 6, pp. 12591263, June 1971 Acetyl CoA Carboxylase: The Purified Transcarboxylase Component (acyl CoA binding/carboxylation/exchange reactions/biotin) ALFRED W. ALBERTS,
RELATIONS BETWEEN INSULIN AND PITUITARY HORMONES IN AMINO ACID METABOLISM
 RELATIONS BETWEEN INSULIN AND PITUITARY HORMONES IN AMINO ACID METABOLISM BY WILLIAM D. LOTSPEICH* WITH THE TECHNICAL ASSISTANCE OF JOAN B. SHELTON (From the Department of Physiology, Syracuse University
RELATIONS BETWEEN INSULIN AND PITUITARY HORMONES IN AMINO ACID METABOLISM BY WILLIAM D. LOTSPEICH* WITH THE TECHNICAL ASSISTANCE OF JOAN B. SHELTON (From the Department of Physiology, Syracuse University
Chapter 10. Regulatory Strategy
 Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED TRYPSIN
 Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
Trypsin Mass Spectrometry Grade
 058PR-03 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Trypsin Mass Spectrometry Grade A Chemically Modified, TPCK treated, Affinity Purified
058PR-03 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Trypsin Mass Spectrometry Grade A Chemically Modified, TPCK treated, Affinity Purified
EPIGENTEK. EpiQuik Global Acetyl Histone H3K27 Quantification Kit (Colorimetric) Base Catalog # P-4059 PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE
 EpiQuik Global Acetyl Histone H3K27 Quantification Kit (Colorimetric) Base Catalog # P-4059 PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Global Acetyl Histone H3K27 Quantification Kit (Colorimetric)
EpiQuik Global Acetyl Histone H3K27 Quantification Kit (Colorimetric) Base Catalog # P-4059 PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Global Acetyl Histone H3K27 Quantification Kit (Colorimetric)
Synthesis and Degradation of Liver Acetyl Coenzyme A Carboxylase
 Proc. Nat. Acad. Sci. USA Vol. 68, No. 9, pp. 2288-2292, September 1971 Synthesis and Degradation of Liver Acetyl Coenzyme A Carboxylase in Genetically Obese Mice (increased hepatic lipogenesis/immunochemical
Proc. Nat. Acad. Sci. USA Vol. 68, No. 9, pp. 2288-2292, September 1971 Synthesis and Degradation of Liver Acetyl Coenzyme A Carboxylase in Genetically Obese Mice (increased hepatic lipogenesis/immunochemical
Cyclic AMP-Mediated Induction of the Cyclic AMP Phosphodiesterase
 Proc. Nat. Acad. Sci. USA Vol. 71, No. 1, pp. 3844-3848, October 1974 Cyclic AMP-Mediated nduction of the Cyclic AMP Phosphodiesterase of C-6 Glioma Cells (dibutyryl cyclic AMP/norepinephrine/norepinephrine
Proc. Nat. Acad. Sci. USA Vol. 71, No. 1, pp. 3844-3848, October 1974 Cyclic AMP-Mediated nduction of the Cyclic AMP Phosphodiesterase of C-6 Glioma Cells (dibutyryl cyclic AMP/norepinephrine/norepinephrine
Identification of free amino acids in several crude extracts of two legumes
 1 2 Identification of free amino acids in several crude extracts of two legumes using Thin Layer Chromatography 3 Authors 4 5 6 7 8 9 Taghread Hudaib Key words 10 11 12 13 14 15 16 17 18 19 20 Amino acids;
1 2 Identification of free amino acids in several crude extracts of two legumes using Thin Layer Chromatography 3 Authors 4 5 6 7 8 9 Taghread Hudaib Key words 10 11 12 13 14 15 16 17 18 19 20 Amino acids;
demonstrated that, when protein synthesis was blocked by puromycin, the increases
 250 BIOCHEMISTRY: NOTEBOOM AND GORS&I PROC. N. A. S. I Buhler, D. R., Anal. Biochem., 4, 413 (1962). 8 Slnger, H. L., personal communication. 9 Britten, R. J., and R. D. Roberts, Science, 131, 32 (1960).
250 BIOCHEMISTRY: NOTEBOOM AND GORS&I PROC. N. A. S. I Buhler, D. R., Anal. Biochem., 4, 413 (1962). 8 Slnger, H. L., personal communication. 9 Britten, R. J., and R. D. Roberts, Science, 131, 32 (1960).
THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION
 THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION BY ROBERT K. CRANE* AND FRITZ LIPMANN (From the Biochemical Research Laboratory, Massachusetts General Hospital, and the Department of Biological Chemistry,
THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION BY ROBERT K. CRANE* AND FRITZ LIPMANN (From the Biochemical Research Laboratory, Massachusetts General Hospital, and the Department of Biological Chemistry,
Functional changes in rat liver trna following aflatoxin Β 1 administration
 J. Biosci., Vol. 3 Number 3, September 1981, pp. 215-219 Printed in India. Functional changes in rat liver trna following aflatoxin Β 1 administration R. K. BHATTACHARYA and V.S. ABOOBAKER Biochemistry
J. Biosci., Vol. 3 Number 3, September 1981, pp. 215-219 Printed in India. Functional changes in rat liver trna following aflatoxin Β 1 administration R. K. BHATTACHARYA and V.S. ABOOBAKER Biochemistry
BIO th September, 1997
 BIO 451 26th September, 1997 EXAM I This exam will be taken apart for grading. Please PRINT your name on each page. If you do not have sufficient room for your answer in the space provided, please continue
BIO 451 26th September, 1997 EXAM I This exam will be taken apart for grading. Please PRINT your name on each page. If you do not have sufficient room for your answer in the space provided, please continue
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Supplementary Figure 1. Method development.
 Supplementary Figure 1 Method development. Titration experiments to determine standard antibody:lysate concentration. Lysates (~2 mg of total proteins) were prepared from cells expressing FLAG- tagged
Supplementary Figure 1 Method development. Titration experiments to determine standard antibody:lysate concentration. Lysates (~2 mg of total proteins) were prepared from cells expressing FLAG- tagged
Supplementary Figure-1. SDS PAGE analysis of purified designed carbonic anhydrase enzymes. M1-M4 shown in lanes 1-4, respectively, with molecular
 Supplementary Figure-1. SDS PAGE analysis of purified designed carbonic anhydrase enzymes. M1-M4 shown in lanes 1-4, respectively, with molecular weight markers (M). Supplementary Figure-2. Overlay of
Supplementary Figure-1. SDS PAGE analysis of purified designed carbonic anhydrase enzymes. M1-M4 shown in lanes 1-4, respectively, with molecular weight markers (M). Supplementary Figure-2. Overlay of
Electronic Supplementary Information. Table of Contents
 Electronic Supplementary Information Examination of native chemical ligation using peptidyl prolyl thioester Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto, and Akira Otaka*
Electronic Supplementary Information Examination of native chemical ligation using peptidyl prolyl thioester Takahiro Nakamura, Akira Shigenaga, Kohei Sato, Yusuke Tsuda, Ken Sakamoto, and Akira Otaka*
On pages 131, 132, 136, and 137, the word conformational should be set off by quotation marks, rather than by parentheses.
 536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the
536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the
Protein Modification Overview DEFINITION The modification of selected residues in a protein and not as a component of synthesis
 Lecture Four: Protein Modification & Cleavage [Based on Chapters 2, 9, 10 & 11 Berg, Tymoczko & Stryer] (Figures in red are for the 7th Edition) (Figures in Blue are for the 8th Edition) Protein Modification
Lecture Four: Protein Modification & Cleavage [Based on Chapters 2, 9, 10 & 11 Berg, Tymoczko & Stryer] (Figures in red are for the 7th Edition) (Figures in Blue are for the 8th Edition) Protein Modification
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
 Acta Univ. Sapientiae, Alimentaria, 1 (2008) 49 60 Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
Acta Univ. Sapientiae, Alimentaria, 1 (2008) 49 60 Mercaptoethanesulfonic acid as the reductive thiol-containing reagent employed for the derivatization of amino acids with o-phthaldialdehyde analysis
PROTEINS. Building blocks, structure and function. Aim: You will have a clear picture of protein construction and their general properties
 PROTEINS Building blocks, structure and function Aim: You will have a clear picture of protein construction and their general properties Reading materials: Compendium in Biochemistry, page 13-49. Microbiology,
PROTEINS Building blocks, structure and function Aim: You will have a clear picture of protein construction and their general properties Reading materials: Compendium in Biochemistry, page 13-49. Microbiology,
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
