On pages 131, 132, 136, and 137, the word conformational should be set off by quotation marks, rather than by parentheses.
|
|
|
- Tamsyn Robbins
- 5 years ago
- Views:
Transcription
1 536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the January issue of volume 51 (1964), pp , the concentration of glycogen given in Tables 4 and 5 should be M X 10-4 instead of M X On pages 131, 132, 136, and 137, the word conformational should be set off by quotation marks, rather than by parentheses.
2 THE ROLE OF ADENYLIC ACID IN THE ACTIVATION OF PHOSPHORYLASE* BY ERNST HELMREICH AND CARL F. CORI DEPARTMENT OF BIOLOGICAL CHEMISTRY, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE, ST. LOUIS, MISSOURI Communicated November 12, 1963 The question of how 5'-AMIP' increases phosphorylase activity has long been a puzzle.2 The conversion of muscle phosphorylase a to b by a specific phosphatase results in an absolute dependence of the enzyme on 5'-AMP for activity.3 Measurements of light scattering indicate that the molecular dissociation which accompanies the a--b transformation of muscle phosphorylase is not reversed by 5'- AMP.4 Phosphorylase b differs from a by the absence of phosphate groups bound to serine residues, but potato phosphorylase which also lacks these phosphate groups does not require 5'-ANIP for activity.5 More surprising still is the observation that 5'-AMP can substitute for phosphohexapeptide residues6 split off from phosphorylase a by trypsin.7 Muscle phosphorylase has a specific binding site for 5'-AMP,5 but no evidence could be obtained that the nucleotide participates in the reaction catalyzed by the enzyme.9 From the temperature dependence of the phosphorylase a reaction in the presence and absence of 5'-AMP it was calculated that the binding of 5'-AMP to the enzyme lowered the energy of activation of the catalytic system.10 Monod et al.ii have recently summarized evidence which emphasizes the importance of (conformational) changes of enzymes for their catalytic activity and have used phosphorylase as a specific example. The present results indicate that inorganic P and glycogen are bound more tightly in the presence of 5'-AMP and that the concentration of the substrates in turn influences the affinity of the enzyme for 5'-AMP. These effects express themselves kinetically in large changes in Km under conditions where there is little change in Vmax. Experimental Procedure.-Most of the measurements of the phosphorylase reaction were carried out in the direction, glycogen + inorganic P - glucose-l-p. In the past it had been difficult to determine initial rates, because at ph 6.7 equilibrium is reached when only 20% of the inorganic P has disappeared. These difficulties were overcome in a coupled assay system containing phosphoglucomutase, glucose-6-p dehydrogenase, and TPN. The auxiliary enzymes were added in large excess, so that there was only a short lag period before linear rates, proportional to the phosphorylase concentration, were attained. The concentrations of phosphorylase were so chosen that the rate of the reaction could conveniently be followed by taking readings at 340 mu every 2-3 min up to 20 min. The temperature was kept constant at 240 by means of a jacketed cellholder and a circulating waterbath. Reactions were started by addition of phosphate or, in the case of phosphorylase b, by addition of 5'-AMP. No reaction was observed with phosphorylase b, unless 5'-AMP was added. The concentration of the reactants in 1 ml of reaction mixture in cuvettes of 1 cm light path was: Tris-acetate, 45 mm; EDTA-Na2, 2 mm; Mgacetate, 10 mm; 2-mercaptoethanol, 1 mm; TPN 0.6 mm; glucose, 1,6-diP12 5 X 10-8 M; phosphoglucomutase 10 jig; glucose-6-p dehydrogenase 2 jig; phosphorylase jig, ph 7.5. Enzymes: Crystalline muscle phosphoglucomutase and crystalline yeast glucose-6-p dehydrogenase were from Boehringer and Sons. Excess (NH4)2S04 was removed by dialysis. Phosphorylase b and a were prepared from frozen rabbit skeletal muscle The phosphorylase preparations were recrystallized at least 4 times. In order to ensure absence of nucleotides, the preparations were treated as follows. Crystals were redissolved in 1-2 ml of a 0.1 M glycerophosphate, 0.1 mm EDTA solution, ph 6.8 at 300 and layered on a pellet of repeatedly washed Dowex-l-X-8 acetate. After gentle mixing for 10 min, the resin was removed by centrifugation. 131
3 132 BIOCHEMISTRY: HELMREICH AND CORI PROC. N. A. S. The supernatant solution (1-2 ml) was added to 0.30 ml of a 30% Norit A suspension in water. After again mixing for 5 min, the mixture was centrifuged, and the supernatant solution treated once more with Norit. The ratio E260/E280 was 0.53 after Norit treatment. The enzyme solution was stored under toluene. Dilutions 1: 100-1:300 were made with a solution of 0.01 Al tris-acetate (ph 7), 0.1 mm EDTA, 0.1 mm 2-mercaptoethanol, and 1% egg albumin immediately preceding kinetic measurements. Dilute enzyme solutions were kept on ice, usually no longer than 1 day. For some experiments phosphorylase a was prepared enzymatically by incubating charcoal-treated phosphorylase b with a 100-fold purified rabbit muscle phosphorylase b kinase preparation'5 in the presence of 3 X 10-3 M ATP and 1 X 10-2 M Mg-acetate. These preparations were diluted 1: 22,500 for kinetic measurements. Glycogen: Rabbit liver glycogen was purified as follows: to 100 ml of 5% aqueous glycogen solution were added 200 mg Norit A, and the suspension was mixed for 10 min at room temperature. The Norit was removed by centrifugation or by filtration, and the treatment with Norit was repeated once or twice. The glycogen was then reprecipitated twice with alcohol and dried. It has been reported that commercial samples of glycogen contain 5'-AMP.l6 In order to test the effectiveness of the charcoal treatment in removing nucleotides, 1 X 10-5 M C14-labeled 5'- AMP was added to the glycogen solution. No radioactivity was recovered after Norit treatment. If there had remained 1 X 10-8 M C14-AMP in the glycogen after Norit treatment, it would have been detected. Materials: TPN-Na was a product of Boehringer and Sons, Germany; we are indebted to Dr. H. U. Bergmeyer for a sample of TPN-Na (Lot no ) which was stated to be free of 5'-AMP by enzymatic tests. We have also tested this TPN preparation in our assay system, using 0.36 jg charcoal-treated phosphorylase a. With 0.24 umoles TPN per ml, the rate was O.D.340 units X min-'. At 5 times this concentration the rate was O.D. units X min-'. With 0.24,moles TPN, but in the presence of 5 X 10-4 M 5'-AMP, the rate was Hence, concentrations of 5'-AMP which could activate phosphorylase a, were not detected in this TPN preparation. Rabbit liver glycogen was purchased from Mann Research Laboratories, Inc. 5'-AMP-8-C14, specific activity 1.5 mc per mmole, was obtained from Tracerlab, Inc. Dowex-1, 5'-AMP, ATP, sodium salts of glucose-i-p and glucose-6-p, Tris-acetate, and 2 times recrystallized egg albumin were purchased from the Sigma Chemical Co. EDTA-Na2 was a product of Geigy Chemical Co., and 2-mercaptoethanol of Eastman Kodak Co. Norit A was from Pfanstiehl Laboratories, Inc. The Norit was washed successively with HCl and then with water until neutral. All other chemicals were ACS grade reagents. Double distilled or deionized H20 was used throughout. Results.-Kinetic measurements with phosphorylase a: Very few values for the Km of inorganic P for phosphorylase are recorded in the literature.2 Preliminary experiments with the coupled assay system were carried out with phosphorylase a prepared by the action of kinase on phosphorylase b. Two such preparations gave Km values for inorganic P of 15 and 9 X 10-3 M (Fig. 1, curves I and II). On addition of 5'-AMP to one of these preparations, the Km value decreased to 5 X 10-3 M (Fig. 1, curve III). A similar value was obtained with phosphorylase a crystallized directly from rabbit muscle (Fig. 1, curve IV). The experiment was repeated with another preparation of phosphorylase a crystallized from rabbit muscle with similar results. On addition of 5'-AMP the Km for inorganic P decreased from 15 to 5 X 10-3 M (Table 2). The fact that the values of Vmax for the curves in Figure 1 are nearly the same would indicate that at high phosphate concentration 5'-AMP is not needed for activity. Conversely, the increasing stimulation by 5'-AMP with decreasing phosphate concentration would indicate that the enzyme becomes more and more dependent on 5'-AMP for activity. These observations suggested that (conformational) changes of the enzyme at low substrate concentration were counteracted by 5'-AMP. That 5'-AMP can effect a reversal in the case of enzyme in-
4 VOL. 51, 1964 BIOCHEMISTRY: HELMREICH AND CORI 133 activation by dilution is indicated by 600 the experiment in Table 1. It can be / seen that dilution of the enzyme with- l out protective protein resulted in a 40 / per cent loss of activity (a rate of versus 0.03 per min). Addition of 5'- / AMP restored activity, as shown by 420/ nearly equal rates whether or not the / dilution of the enzyme had been carried 360/ out with egg albumin as protective "/v / protein. Rate measurements with the / unprotected enzyme made 24 hr later 2 AlX miv. indicated that the enzyme, after the / rapid initial change, was relatively sta- 80 ble. It is also shown that egg albumin / added to the cuvette did not reactivate 120 / the enzyme. Other data in Table 1 relate to the 60A sensitivity of the enzyme to 5'-AMP. Based on the increment over the rate C 0'5 It 1.5 2'0 25 without 5'-AMP, a concentration of 1 I/ P X I0-3 X 10-6 M 5'-AMP gave gave about 1/2 FIG. 1.Km of inorganic P for phosphorylase. a. Curves I and II refer to two batches of maximal stimulation of activity. At phosphorylase a prepared from the same phos- 0.5 mm inorganic P as compared to 5 phorylase b stock solution with kinase, ATP, and Mg++ and tested without added 5'-AMP. ma\j, the rate without 5'-AMP had Curve III (0) refers to enzyme preparation II decreased 90 per cent and that with tested in the presence of 5 X 10-4 M 5'-AMP. Curve IV (U) refers to phosphorylase a crystal- 5'-AMP 57 per cent. Consequently, lized directly from rabbit muscle and tested in the stimulation by 5'-AMP becomes the presence of 5 X 10-4 M 5'-AMP, after appropriate dilution so as to give the same ormuch greater at the low substrate dinate intercept as the other curves. The concentration. Similar observations glycogen concentration was 0.1%. The rate v represents the linear part of the curve and is exwere made recently by Dr. 0. H. pressed as optical density change per minute. Lowry 17 Previous observations were made with a standard test system with 16 mm natural or synthetic glucose-i-p as substrate. Under these conditions most of the TABLE 1 ACTIVATION OF PHOSPHORYLASE a BY 5'-AMP --- Without Egg Albumin - - With Egg Albumin (0.1%) -_ Hr after % activity % activity dilution no AMP + AMP without AMP no AMP + AMP without AMP * (1 X 10-6M) "t (1 X 10-7 M) it (1 X 10-8 M) cc 0.022t * At 5 X 10-4 M inorganic P this value was 17, based on rates of and 0.018, without and with 5'-AMP respectively. t Egg albumin added to the cuvette. One part of a charcoal-treated phosphorylase a solution was diluted with egg albumin, as described under Experimental Procedure. Another part was diluted identically, except that egg albumin was omitted. Both samples were kept on ice. For enzymatic tests 0.1 ml aliquots containing 2.7 erg of enzyme were added to the cuvettes. Inorganic P, 5'-AMP, and glycogen were 5 X 10-3 M, 5 X 10-5 M, and 0.1%, respectively, except when stated otherwise. A TPN preparation shown to be free of 5'-AMP was used at a concentration of 0.20 mm. The rates are given es optical density changes per minute at 340 my.
5 134 BIOCHEMISTRY: HELMREICH AND CORI PROC. N. A. S. phosphorylase a preparations showed an activity without 5'-AMIP which was per cent of that in the presence of 5'-AMP. Recently, fractions of very high specific activity were obtained by gradient elution of phosphorylase a from DEAE cellulose columns. The peak fractions were not stimulated at all by 5'-AMP in the above test, or stimulated only 5-10 per cent. On aging, these preparations became increasingly responsive to stimulation by 5'-AMP.'5 Phosphorylase a preparations freshly made from phosphorylase b by kinase often show little stimulation by 5'-AMP (cf. curves II and III, Fig. 1). This applies also to phosphorylase formed in vivo. Thus, the phosphorylase present in diluted, charcoal-treated extracts of tetanized frog sartorius muscle often was stimulated no more than 5 per cent when 5'-AMP was added.'9 In de novo synthesis,20 polysaccharide chains are formed when the only reactants are phosphorylase a and glucose-i-p. Recently, Drs. Jllingworth and Brown kindly repeated this experiment, using Norit-treated phosphorylase a and chemically synthesized glucose-1-p. Formation of polysaccharide chains set in after the usual lag period. Addition of 5'-AMP greatly shortened the lag period. When the reaction became linear with time, the sample without 5'-AMP formed about 1.4 Mumoles inorganic P per ml per hr, as compared to 3.3.omoles for the sample with 5'-AMP. The same phosphorylase a preparation tested either with glucose-i-p (16 mm) or inorganic P (10 mm) in the presence of glycogen showed an activity of 68 and 62 per cent, respectively, of that obtained in the presence of 5'-AMP. These observations show that phosphorylase a has enzymatic activity in the absence of 5'-AMP. It then seems possible that the absolute requirement of phosphorylase b for 5'-AMP could be the result of a less rigid structure of catalytic site. The changes in molecular weight and in charges which are associated with the a-.~b transformation could be responsible for the differences between the two enzymes. Determination of Km with phosphorylase b: Preliminary data on the effect of 90 5'-AM\'IP on the velocity of the phos- 80 phorylase b reaction are shown in 70 /l C Figure 2 in the form of a double recip- 60 / / rocal plot. Within the range of 5'- AMP concentrations of 1 X 10-4 M /1V 50 - / /to 1 X 10-' M, the experimental 40 / points extrapolate to the ordinate, and 30 these were the concentrations chosen 20 /SSfor the final experiments. At lower I 0 concentrations the experimental points 0' ' do not yield an ordinate intercept. The I/AMP x 10-5 reason for this falling off in rate has FIG. 2.-Km of 5'-AMP for phosphorylase b. not been ascertained. It was also ob- The enzyme dilution was made with buffer served that the same enzyme solution containing glycogen so as to give a final concentration of 0.2%. Curves I and II refer on aging, as well as different preparato enzyme tested at concentrations of 1 X tions made from the same stock solu M and 1 X 10-2 M inorganic P, respectively. Km calculated from Curve I was tion, may give large variations in the 2.6 X 104 M. The portion of Curve II which Km value. For this reason it was intercepts the ordinate yields a Km value of 2.2 X 10-5 M. found necessary to complete all meas- its
6 VOL. 51, 1964 BIOCHEMISTRY: HELMREICH AND CORI 135 urements of a series on the same day and with the same enzyme solution. In the final series, 4 concentrations of inorganic P (1-10 X 10-3 M) were used at each of 4 concentrations of 5'-AMP (1.5-9 X 10-5 M), a total of 16 measurements of initial rate. From a plot of 1/v versus 1/S, depending on whether S represents the concentration of inorganic P or of 5'-AMP, one obtains the respective Km values. The data in Tables 2 and 3 represent such a series. As a measure of the consistency of the data, the 1/v versus 1/S plots were found to extrapolate to the same Vmax within close limits. Table 2 shows that the Km for inorganic P decreases progressively from 23 TABLE 2 Km OF ORTHOPHOSPHATE (P) AND Ki OF ARSENATE (As) AT DIFFERENT CONCENTRATIONS OF 5'-AMP AND 0.1% GLYCOGEN Phosphorylase b Phosphorylase a of 5'-AMP Km (P) Ki (As) Km (P) M X 1O05 M X 10-5 M X 10-3 M X to 1.5 X 10-3 M as the concentration of 5'-AMP is increased over its effective range. This result could be expressed by saying that the essential cofactor 5'-AMP has the effect of increasing the affinity of the enzyme for inorganic P. Further insight is provided by the experiments with arsenate (Table 2). Arsenate acts as a competitive inhibitor of phosphate. When the enzyme concentration is of the order of a few,ug per ml, the reaction leading to the formation of free glucose (presumably via glucose-l-arsenate) is too slow to be measured. Table 2 shows that arsenate has an affinity for the enzyme which is similar to that of phosphate and that the inhibitor constant (Ki) is influenced in a similar manner by the concentration of 5'-AMP, as is the Km of phosphate. In this case it is clearly the binding affinity of the competitor for the active site which is influenced by the concentration of 5'-AMP. The experiment in Figure 2 anticipates the results shown in greater detail in Table 3, namely, that the Km value for 5'-AMP decreases as the concentration of inorganic P is increased, without a significant change in Vax. Determinations of the Km of 5'-AMP with glucose-i-p as substrate are included in Table 3. At TABLE 3 Km OF 5'-AMP AND Ki OF ATP FOR PHOSPHORYLASE b AT DIFFERENT CONCENTRATIONS OF PHOSPHATE AND 0.1% GLYCOGEN of phosphate Km (S-AMP) Ki (ATP) Ratio Substrate M X 10-3 M X 10-5 M X 10-3 Ki/Km Orthophosphate "I Glucose-i-P * it IC * Values reported in the literatures
7 136 BIOCHEMISTRY: HELMREICH AND CORI PROC. N. A. S. high substrate concentrations the Km values for 5'-AMP approach the apparent dissociation constant of 6.6 X 10-5 M reported for phosphorylase b.8 Parmeggiani and Morgan 21 have reported that ATP inhibits phosphorylase b by competing with 5'-AMP. In Table 3 the inhibitor constant, K,, has been calculated at 3 different concentrations of inorganic P. The appropriate Km values for 5'-AMP at these phosphate concentrations were used in this calculation. From the relative constancy of the ratio, Ki/Km, it can be concluded that the concentration of substrate influences the binding affinity of the enzyme for 5'-AMP and for its competitor in a similar manner. The numerical value of this ratio shows that ATP is a rather weak competitive inhibitor. Kmfor glycogen: The (conformational) changes of the enzyme induced by 5'-AMP at one substrate binding site raised the question as to what effects 5'-AMP would have on the second substrate binding site, that for glycogen. The Km value for glycogen in the direction of degradation could be determined with the coupled assay system. The range of concentrations of glycogen ( mg per 100 ml corresponding to 1 X 10-4 to 10 X 10-4 M end group) was so chosen that initial linear rates were measured even at the lowest concentration. 22 TABLE 4 Km OF GLYCOGEN AND Km OF 5'-AMP FOR PHOSPHORYLASE b AT 1 X 10-2 M INORGANIC P of 5'-AMP Km (glycogen) of glycogen Km (5'-AMP) M X 105- M X 10-5 M X 10-5 M X * * From Fig. 2, curve II. The concentration of glycogen is expressed as moles of terminal glucose units at the nonreducing end of the chains. In Table 4, columns 1 and 2, the Km value for glycogen decreased from 14 to 4.3 X 10-5 M as the concentration of 5'-AMP was increased from 3 to 9 X 10-5 M. This can be compared with a similar decrease in the Km value for inorganic P within the same range of 5'-AMP concentrations (cf. Table 2). Also shown in Table 4 is the reverse experiment, i.e., the influence of the concentration of glycogen on the Km of 5'-AMP. Analogous effects were obtained by changing the concentration of inorganic P (cf. Table 3). These results indicate that the same reciprocal relationship exists between the binding sites for glycogen and 5'-AMP as between the binding sites for inorganic P (or glucose-1-p) and 5'-AMP. Interaction of substrate binding sites: Two possible variations in the combination of inorganic P, glycogen, and 5'-AMP have so far been examined. In Tables 2 and 3 the concentration of glycogen was kept constant and in Table 4 that of inorganic P was kept constant, while the concentration of the other 2 substances was varied. It remained to keep the concentration of 5'-AMP constant and to vary the concentration of the 2 substrates. The experiments are shown in Table 5. At 1 X 10-4 M and 6 X 10-5 M 5'-AMP, neither the Km for glycogen nor that for inorganic P was influenced significantly by varying the concentration of the respective substrate partner. Moreover, the Km values were close to those predicted from Tables 4 and 2, respectively. In these experiments Vmax did not remain constant (Table 5). Thus, within the range of substrate concentrations
8 VOL. 51, 1964 BIOCHEMISTRY: HELMREICH AND CORI 137 TABLE 5 Km OF INORGANIC P AND Km OF GLYCOGEN FOR PHOSPHORYLASE b AT FIXED 5'-AMP CONCENTRATIONS of inorganic P Km (glycogen) Vmas of glycogen Km (P) Vma. M X 10-3 M X 10-6 min-' M X 1O-5 M X 10-' min' 1 X 1O-4 M 5-AMP 1 X 10-4 M 5'-AMP X 10-5 M5'-AMP 6 X 10-5 M5-AMP 'I Different enzyme preparations were used for the 2 experiments. chosen, no interaction between the 2 substrate binding sites could be demonstrated. Discussion.-Phosphorylase b was one of the first of a class of enzymes which were shown to need a cofactor for activity. The function of the cofactor, where it has been investigated, seems to be similar to that described here, that is, the cofactor changes the affinity of the enzyme for its substrate From the data in Tables 2 and 3 it can be inferred that, as the concentration of 5'-AMP is increased, the Km for inorganic P becomes very small, and vice versa. It has not been possible, however, to replace the requirement of phosphorylase b for 5'-AMP for activity by very high substrate concentration. Phosphorylase a, on the other hand, is catalytically active in the absence of 5'-AMP. The stimulation of phosphorylase a activity by 5'-AMP increases as the concentration of substrate is decreased (cf. Fig. 1 and Table 1). This is a consequence of a change in Km for substrate under the influence of 5'-AMP and would lead to the prediction that at still lower substrate concentration phosphorylase a would appear to be inactive unless 5'-AMP is added. The two forms of the enzyme, a and b, could still be distinguished under these conditions, because the former is stimulated by much lower concentrations of 5'-AMP than the latter. It would be of great importance to know to what extent these findings based on experiments in vitro apply to intact muscle. The chief difficulty is lack of knowledge of the actual concentration of substrates in contact with the enzyme.26 If the concentration of inorganic P (about 2 mm in the intracellular water) and that of 5'-AMP (about 3 X 10-4 M)27 in resting frog muscle were taken at face value, then, based on the Km values in Tables 2 and 3, phosphorylase b should be more than 50 per cent active, but this is obviously not true because stimulation may result in a 100-fold increase in enzyme activity.26 Another factor to be considered is that phosphorylase as part of the structure of intact muscle may be less subject to conformational changes than under in vitro conditions. Summary.-The Km of muscle phosphorylase b for inorganic P and for glycogen decreased progressively as the concentration of 5'-AMP was increased. Conversely, the addition of either substrate in increasing concentration caused a decrease in the Km for 5'-AMP. These mutual interactions are presumably the result of (conformational) alterations of the enzyme protein. No interaction between the two substrate binding sites could be demonstrated when the concentration of 5'-AMP was held constant and that of the substrates was varied,
9 138 BIOCHEMISTRY: HELMREICH AND CORI PROC. N. A. S. Muscle phosphorylase a differs from phosphorylase b in being active in the absence of 5'-AMP and in being stimulated by much smaller concentrations of 5'-AMP. The degree of stimulation of phosphorylase a by 5'-AMP depends on the substrate concentration. This is a consequence of a decrease in Km for substrate when 5'-AMP is added while there is no significant change in Vmax. The dependence of muscle phosphorylase on 5'-AMP for activity, absolute in the case of phosphorylase b and relative in the case of phosphorylase a, is explained in either case by increased affinity of the enzyme for substrate. We wish to thank Dr. Maria Michaelides for the preliminary results, and Mr. Roger Sherman for excellent technical assistance. * Supported in part by the Nutrition Foundation and by research grants Al and AM from the National Institutes of Health, USPHS. Results of this work have been reported in part at the Ciba Symposium on Glycogen Metabolism, July 23, 1963, London, England. 1 Abbreviations are as follows: 5'-AMP, 5'-adenosine monophosphate; inorganic P, orthophosphate; Tris, Tris(hydroxymethyl) aminomethane; EDTA, ethylenediamine-tetraacetate. 2 Brown, D. H., and C. F. Cori, in The Enzymes, ed. P. D. Boyer, H. A. Lardy, and K. Myrback (New York: Academic Press, Inc., 1961), 2nd ed., vol. 5, pp Cori, G. T., and C. F. Cori, J. Biol. Chem., 158, 321 (1945). 4We are indebted to Dr. Carl Frieden for these measurements. 6 Lee, Y. P., Biochim. Biophys. Acta, 43, 18, 25 (1960). 6Fischer, E. H., D. J. Graves, E. S. Crittenden, and E. G. Krebs, J. Biol. Chem., 234, 1698 (1959). 7Keller, P. J., J. Biol. Chem., 214, 135 (1955). 8 Madsen, N. B., and C. F. Cori, J. Biol. Chem., 224, 899 (1957). 9 Cohn, M., and G. T. Cori, J. Biol. Chem., 175, 89 (1948). 10 Madsen, N. B., and C. F. Cori, Biochim. Biophys. Acta, 15, 516 (1954). "Monod, J., J. P. Changeux, and F. Jacob, J. Mol. Biol., 6, 306 (1963). 12 We are indebted to Dr. H. T. Narahara for this preparation. 13 Fischer, E. H., and E. G. Krebs, J. Biol. Chem., 231, 65 (1958). 14 Illingworth, B., and G. T. Cori, Biochem. Preparations, 3, 1 (1953). 15 Danforth, W. H., and E. Helmreich, in preparation. 16 Fischer, E. H., and E. G. Krebs, in Methods in Enzymology, ed. S. P. Colowick and N. 0. Kaplan (Academic Press, 1962), vol. 5, pp When glycogen is treated with strong alkali, as in the Pflueger method, most of the 5'-AMP would be expected to be destroyed. 17 Private communication from Dr. 0. H. Lowry. 18 Private communication from Dr. B. Illingworth. '9 Danforth, W. H., E. Helmreich, and C. F. Cori, these PROCEEDINGS, 48, 1191 (1962). 20 Illingworth, B., D. H. Brown, and C. F. Cori, these PROCEEDINGS, 47, 469 (1961). 21 Parmeggiani, A., and H. E. Morgan, Biochem. Biophys. Res. Comm., 9, 252 (1962). 22 It is difficult to determine a Km value for glycogen because the reaction rate with either glucose-l-p or inorganic P remains constant only within certain limits of chain elongation or chain shortening. As the glycogen concentration is decreased, this limit is quickly exceeded, and the rate of the reaction falls off. An apparent Km value corresponding to 1 X 10-4 M end group was previously obtained with glucose-i-p as substrate. The true Km value would be lower to the extent to which some of the end groups failed to react. 23 Kornfeld, R., and D. H. Brown, J. Biol. Chem., 237, 1772 (1962). 24 Hathaway, J. A., and D. E. Atkinson, J. Biol. Chem., 238, 2875 (1963). 26 Frieden, C., J. Biol. Chem., 238, 3286 (1963). 26 Helmreich, E., S. Karpatkin, and C. F. Cori, in Ciba Symposium on Glycogen Metabolism (1963), in press. 27 Lange, G., Biochem. Z., 326, 172 (1955).
Enzymatic Assay of PHOSPHORYLASE KINASE (EC )
 PRINCIPLE: Enzymatic Assay of PHOSPHORYLASE KINASE 2 Phosphorylase b + 4 ATP Phosphorylase Kinase > Phosphorylase a + 4 ADP Glycogen n + P i Phosphorylase a > Glycogen n-1 + a-d-glucose 1-Phosphate a-d-glucose
PRINCIPLE: Enzymatic Assay of PHOSPHORYLASE KINASE 2 Phosphorylase b + 4 ATP Phosphorylase Kinase > Phosphorylase a + 4 ADP Glycogen n + P i Phosphorylase a > Glycogen n-1 + a-d-glucose 1-Phosphate a-d-glucose
CO?IU VERSIOS OF PHOSPHORYLASE b TO PHOSPHORYLASE a IS MT SCLE EXTRACTS*
 CO?IU VERSIOS OF PHOSPHORYLASE b TO PHOSPHORYLASE a IS MT SCLE EXTRACTS* BY EDMOND H. FISCHER AND EDWIN G. KREBS (From the Department of Biochemistry, University of Washington, Seattle, Washington) (Received
CO?IU VERSIOS OF PHOSPHORYLASE b TO PHOSPHORYLASE a IS MT SCLE EXTRACTS* BY EDMOND H. FISCHER AND EDWIN G. KREBS (From the Department of Biochemistry, University of Washington, Seattle, Washington) (Received
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/ MCQs. Location : 102, 105, 106, 301, 302
 FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
preparation which is available contains an oligo-1,4-0 1,4-glucantransferase
 THE PROPERTIES OF AN OLIGO-1,4 -- 1,4-GLUCANTRANSFERASE FROM ANIMAL. TISSUES* BY DAVID H. BROWN AND BARBARA ILLINGWORTH DEPARTMENT OF BIOLOGICAL CHEMISTRY, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE, SAINT
THE PROPERTIES OF AN OLIGO-1,4 -- 1,4-GLUCANTRANSFERASE FROM ANIMAL. TISSUES* BY DAVID H. BROWN AND BARBARA ILLINGWORTH DEPARTMENT OF BIOLOGICAL CHEMISTRY, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE, SAINT
Enzymes: The Catalysts of Life
 Chapter 6 Enzymes: The Catalysts of Life Lectures by Kathleen Fitzpatrick Simon Fraser University Activation Energy and the Metastable State Many thermodynamically feasible reactions in a cell that could
Chapter 6 Enzymes: The Catalysts of Life Lectures by Kathleen Fitzpatrick Simon Fraser University Activation Energy and the Metastable State Many thermodynamically feasible reactions in a cell that could
Biochem sheet (5) done by: razan krishan corrected by: Shatha Khtoum DATE :4/10/2016
 Biochem sheet (5) done by: razan krishan corrected by: Shatha Khtoum DATE :4/10/2016 Note about the last lecture: you must know the classification of enzyme Sequentially. * We know that a substrate binds
Biochem sheet (5) done by: razan krishan corrected by: Shatha Khtoum DATE :4/10/2016 Note about the last lecture: you must know the classification of enzyme Sequentially. * We know that a substrate binds
TEMPORARY INHIBITION OF TRYPSIN*
 TEMPORARY INHIBITION OF TRYPSIN* BY M. LASKOWSKI AND FENG CHI WU (From the Department oj Biochemistry, Marquette University School of Medicine, Milwaukee, Wisconsin) (Received for publication, April 30,
TEMPORARY INHIBITION OF TRYPSIN* BY M. LASKOWSKI AND FENG CHI WU (From the Department oj Biochemistry, Marquette University School of Medicine, Milwaukee, Wisconsin) (Received for publication, April 30,
Name: Student Number
 UNIVERSITY OF GUELPH CHEM 454 ENZYMOLOGY Winter 2003 Quiz #1: February 13, 2003, 11:30 13:00 Instructor: Prof R. Merrill Instructions: Time allowed = 80 minutes. Total marks = 34. This quiz represents
UNIVERSITY OF GUELPH CHEM 454 ENZYMOLOGY Winter 2003 Quiz #1: February 13, 2003, 11:30 13:00 Instructor: Prof R. Merrill Instructions: Time allowed = 80 minutes. Total marks = 34. This quiz represents
colorimetrically by the methylene blue method according to Fogo and manometrically. In the presence of excess sulfur the amount of oxygen taken up
 GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
Polysaccharide phosphorylase
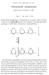 CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
Prerequisites Protein purification techniques and protein analytical methods. Basic enzyme kinetics.
 Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
J. Biosci., Vol. 7, Number 2, March 1985, pp Printed in India.
 J. Biosci., Vol. 7, Number 2, March 1985, pp. 123 133. Printed in India. Irreversibility of the interaction of human growth hormone with its receptor and analysis of irreversible reactions in radioreceptor
J. Biosci., Vol. 7, Number 2, March 1985, pp. 123 133. Printed in India. Irreversibility of the interaction of human growth hormone with its receptor and analysis of irreversible reactions in radioreceptor
Case 19 Purification of Rat Kidney Sphingosine Kinase
 Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
 Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Biology 2180 Laboratory #3. Enzyme Kinetics and Quantitative Analysis
 Biology 2180 Laboratory #3 Name Introduction Enzyme Kinetics and Quantitative Analysis Catalysts are agents that speed up chemical processes and the catalysts produced by living cells are called enzymes.
Biology 2180 Laboratory #3 Name Introduction Enzyme Kinetics and Quantitative Analysis Catalysts are agents that speed up chemical processes and the catalysts produced by living cells are called enzymes.
A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE*
 A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE* BY SEYMOUR KAUFMAN (From the Laboratory of Cellular Pharmacology, National Institute of Mental Health, United States Department
A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE* BY SEYMOUR KAUFMAN (From the Laboratory of Cellular Pharmacology, National Institute of Mental Health, United States Department
The effects of ph on Type VII-NA Bovine Intestinal Mucosal Alkaline Phosphatase Activity
 The effects of ph on Type VII-NA Bovine Intestinal Mucosal Alkaline Phosphatase Activity ANDREW FLYNN, DYLAN JONES, ERIC MAN, STEPHEN SHIPMAN, AND SHERMAN TUNG Department of Microbiology and Immunology,
The effects of ph on Type VII-NA Bovine Intestinal Mucosal Alkaline Phosphatase Activity ANDREW FLYNN, DYLAN JONES, ERIC MAN, STEPHEN SHIPMAN, AND SHERMAN TUNG Department of Microbiology and Immunology,
MECHANISM OF INHIBITION OF PHOSPHATASE ACTIVITY BY GLYCINE
 MECHANISM OF INHIBITION OF PHOSPHATASE ACTIVIT B GLCINE B OSCAR BODANSK (From the Department of Pharmacology, Cornell University Medical College, New ork City) (Received for publication, July 11, 1946)
MECHANISM OF INHIBITION OF PHOSPHATASE ACTIVIT B GLCINE B OSCAR BODANSK (From the Department of Pharmacology, Cornell University Medical College, New ork City) (Received for publication, July 11, 1946)
Dual nucleotide specificity of bovine glutamate dehydrogenase
 Biochem J. (1980) 191, 299-304 Printed in Great Britain 299 Dual nucleotide specificity of bovine glutamate dehydrogenase The role of negative co-operativity Stephen ALX and J. llis BLL Department ofbiochemistry,
Biochem J. (1980) 191, 299-304 Printed in Great Britain 299 Dual nucleotide specificity of bovine glutamate dehydrogenase The role of negative co-operativity Stephen ALX and J. llis BLL Department ofbiochemistry,
BASIC ENZYMOLOGY 1.1
 BASIC ENZYMOLOGY 1.1 1.2 BASIC ENZYMOLOGY INTRODUCTION Enzymes are synthesized by all living organisms including man. These life essential substances accelerate the numerous metabolic reactions upon which
BASIC ENZYMOLOGY 1.1 1.2 BASIC ENZYMOLOGY INTRODUCTION Enzymes are synthesized by all living organisms including man. These life essential substances accelerate the numerous metabolic reactions upon which
Margaret A. Daugherty Fall 2003
 Enzymes & Kinetics IV Regulation and Allostery ENZYME-SUBSTRATE INTERACTIONS THE LOCK & KEY MODEL Margaret A. Daugherty Fall 2003 A perfect match between enzyme and substrate can explain enzyme specificity
Enzymes & Kinetics IV Regulation and Allostery ENZYME-SUBSTRATE INTERACTIONS THE LOCK & KEY MODEL Margaret A. Daugherty Fall 2003 A perfect match between enzyme and substrate can explain enzyme specificity
CHEMICAL STUDIES ON BACTERIAL AGGLUTINATION II. THE IDENTITY OF PRECIPITIN AND AGGLUTININ* BY MICHAEL HEIDELBERGER, PH.D., AND ELVIN A.
 CHEMICAL STUDIES ON BACTERIAL AGGLUTINATION II. THE IDENTITY OF PRECIPITIN AND AGGLUTININ* BY MICHAEL HEIDELBERGER, PH.D., AND ELVIN A. KABAT (From the Laboratories of the Departments of Medicine and Biological
CHEMICAL STUDIES ON BACTERIAL AGGLUTINATION II. THE IDENTITY OF PRECIPITIN AND AGGLUTININ* BY MICHAEL HEIDELBERGER, PH.D., AND ELVIN A. KABAT (From the Laboratories of the Departments of Medicine and Biological
Biochemistry Department. Level 1 Lecture No : 3 Date : 1 / 10 / Enzymes kinetics
 Biochemistry Department Level 1 Lecture No : 3 Date : 1 / 10 / 2017 Enzymes kinetics 1 Intended Learning Outcomes By the end of this lecture, the student will be able to: 1.Understand what is meant by
Biochemistry Department Level 1 Lecture No : 3 Date : 1 / 10 / 2017 Enzymes kinetics 1 Intended Learning Outcomes By the end of this lecture, the student will be able to: 1.Understand what is meant by
Tala Saleh. Ahmad Attari. Mamoun Ahram
 23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
Lecture 34. Carbohydrate Metabolism 2. Glycogen. Key Concepts. Biochemistry and regulation of glycogen degradation
 Lecture 34 Carbohydrate Metabolism 2 Glycogen Key Concepts Overview of Glycogen Metabolism Biochemistry and regulation of glycogen degradation Biochemistry and regulation of glycogen synthesis What mechanisms
Lecture 34 Carbohydrate Metabolism 2 Glycogen Key Concepts Overview of Glycogen Metabolism Biochemistry and regulation of glycogen degradation Biochemistry and regulation of glycogen synthesis What mechanisms
B. 50 mm Calcium Chloride Solution (CaCl 2 ) (Prepare 25 ml in Reagent A using Calcium Chloride, Dihydrate, Sigma Prod. No. C-3881.
 SIGMA QUALITY CONTROL TEST PROCEDURE ProductInformation Enzymatic Assay of PHOSPHOLIPASE C PRINCIPLE: L-α-Lecithin + H 2 O Phospholipase C > 1,2-Diglyceride + Choline Phosphate Choline phosphate + H 2
SIGMA QUALITY CONTROL TEST PROCEDURE ProductInformation Enzymatic Assay of PHOSPHOLIPASE C PRINCIPLE: L-α-Lecithin + H 2 O Phospholipase C > 1,2-Diglyceride + Choline Phosphate Choline phosphate + H 2
A GLUCOSEPHOSPHATE ISOMERASE INHIBITOR OF SEASONAL OCCURRENCE IN COD (GADUS MORHUA) AND OTHER FISH
 J. mar. biol. Ass. U.K. (969) 49, 447-453 447 Printed in Great Britain A GLUCOSEPHOSPHATE ISOMERASE INHIBITOR OF SEASONAL OCCURRENCE IN COD (GADUS MORHUA) AND OTHER FISH By P. R. DANDO The Plymouth Laboratory
J. mar. biol. Ass. U.K. (969) 49, 447-453 447 Printed in Great Britain A GLUCOSEPHOSPHATE ISOMERASE INHIBITOR OF SEASONAL OCCURRENCE IN COD (GADUS MORHUA) AND OTHER FISH By P. R. DANDO The Plymouth Laboratory
Past Years Questions Chpater 6
 Past Years Questions Chpater 6 **************************************** 1) Which of the following about enzymes is Incorrect? A) Most enzymes are proteins. B) Enzymes are biological catalysts. C) Enzymes
Past Years Questions Chpater 6 **************************************** 1) Which of the following about enzymes is Incorrect? A) Most enzymes are proteins. B) Enzymes are biological catalysts. C) Enzymes
GENERAL THOUGHTS ON REGULATION. Lecture 16: Enzymes & Kinetics IV Regulation and Allostery REGULATION IS KEY TO VIABILITY
 GENERAL THOUGHTS ON REGULATION Lecture 16: Enzymes & Kinetics IV Regulation and Allostery Margaret A. Daugherty Fall 2004 1). Enzymes slow down as product accumulates 2). Availability of substrates determines
GENERAL THOUGHTS ON REGULATION Lecture 16: Enzymes & Kinetics IV Regulation and Allostery Margaret A. Daugherty Fall 2004 1). Enzymes slow down as product accumulates 2). Availability of substrates determines
PhosFree TM Phosphate Assay Biochem Kit
 PhosFree TM Phosphate Assay Biochem Kit (Cat. # BK050) ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 To order by e-mail: cservice@cytoskeleton.com Technical
PhosFree TM Phosphate Assay Biochem Kit (Cat. # BK050) ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 To order by e-mail: cservice@cytoskeleton.com Technical
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
liberated in the body is probably less than 1 part in a million. The
 547.435-292: 577.153 KINETICS OF CHOLINE ESTERASE. By A. J. CLARK, J. RAVENT6S, E. STEDMAN, and ELLEN STEDMAN. From the Departments of Pharmacology and Medical Chemistry, University of Edinburgh. (Received
547.435-292: 577.153 KINETICS OF CHOLINE ESTERASE. By A. J. CLARK, J. RAVENT6S, E. STEDMAN, and ELLEN STEDMAN. From the Departments of Pharmacology and Medical Chemistry, University of Edinburgh. (Received
ON THE NATURE OF THE TRANSALDOLASE-DIHYDROXYACETONE
 VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
Self-association of α-chymotrypsin: Effect of amino acids
 J. Biosci., Vol. 13, Number 3, September 1988, pp. 215 222. Printed in India. Self-association of α-chymotrypsin: Effect of amino acids T. RAMAKRISHNA and M. W. PANDIT* Centre for Cellular and Molecular
J. Biosci., Vol. 13, Number 3, September 1988, pp. 215 222. Printed in India. Self-association of α-chymotrypsin: Effect of amino acids T. RAMAKRISHNA and M. W. PANDIT* Centre for Cellular and Molecular
Activation of Cardiac Phosphorylase b Kinase*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 241, No. 24, Issue of December 25, PP. 5893-5898, 1966 Printed in U.S.A. Activation of Cardiac Phosphorylase b Kinase* GEORGE I. DRUMMOND AND LOVERNE DUNCAN (Received
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 241, No. 24, Issue of December 25, PP. 5893-5898, 1966 Printed in U.S.A. Activation of Cardiac Phosphorylase b Kinase* GEORGE I. DRUMMOND AND LOVERNE DUNCAN (Received
The Behaviour of Lactobacillus arabinosus towards Nicotinic Acid
 Vol. 44 153 The Behaviour of Lactobacillus arabinosus towards Nicotinic Acid and its Derivatives By H. McILWAIN, D. A. STANLEY AND D. E. HUGHES Unit for Cell Metabolism (Medical Research, Council), Department
Vol. 44 153 The Behaviour of Lactobacillus arabinosus towards Nicotinic Acid and its Derivatives By H. McILWAIN, D. A. STANLEY AND D. E. HUGHES Unit for Cell Metabolism (Medical Research, Council), Department
The incorporation of labeled amino acids into lens protein. Abraham Speclor and Jin H. Kinoshita
 The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
Phosphatase Activity of Drosophila Salivary Glands
 Phosphatase Activity of Drosophila Salivary Glands BY W. L. DOYLE (From the Department of Anatomy, University of Chicago) THE presence of alkaline phosphatase in chromosomes has been demonstrated by means
Phosphatase Activity of Drosophila Salivary Glands BY W. L. DOYLE (From the Department of Anatomy, University of Chicago) THE presence of alkaline phosphatase in chromosomes has been demonstrated by means
2. Which of the following amino acids is most likely to be found on the outer surface of a properly folded protein?
 Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
STUDIES OF THE MECHANISM OF ACTION OF COBAMIDE COENZYMES
 STUDIES OF THE MECHANISM OF ACTION OF COBAMIDE COENZYMES R. H. Abeles and H. A. Lee, Jr. University of Michigan Medical School, Ann Arbor, Mich. Aerobacter aerogenes converts propanediol to propionaldehyde,
STUDIES OF THE MECHANISM OF ACTION OF COBAMIDE COENZYMES R. H. Abeles and H. A. Lee, Jr. University of Michigan Medical School, Ann Arbor, Mich. Aerobacter aerogenes converts propanediol to propionaldehyde,
Effects of Amino Acids and Glutathione on Rat Liver Histidase Activity in vitro
 [Agr. Biol. Chem., Vol. 34, No. 5, p. 710-714, 1970] Effects of Amino Acids and Glutathione on Rat Liver Histidase Activity in vitro By Katuhiko NODA Department of Nutrition, School of Medicine, Tokushima
[Agr. Biol. Chem., Vol. 34, No. 5, p. 710-714, 1970] Effects of Amino Acids and Glutathione on Rat Liver Histidase Activity in vitro By Katuhiko NODA Department of Nutrition, School of Medicine, Tokushima
Enzymes Part III: regulation II. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
CYTIDINE. Enzymatic synthesis of cytidine diphosphate diglyceride
 Enzymatic synthesis of cytidine diphosphate diglyceride JAMES R. CARTER* and EUGENE P. KENNEDY Department of Biological Chemistry, Harvard Medical School, Boston, Massachusetts ABSTRACT Evidence is presented
Enzymatic synthesis of cytidine diphosphate diglyceride JAMES R. CARTER* and EUGENE P. KENNEDY Department of Biological Chemistry, Harvard Medical School, Boston, Massachusetts ABSTRACT Evidence is presented
Previous Class. Today. Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism. Protein Kinase Catalytic Properties
 Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Previous Class Detection of enzymatic intermediates: Protein tyrosine phosphatase mechanism Today Protein Kinase Catalytic Properties Protein Phosphorylation Phosphorylation: key protein modification
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE
 METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIDS. Enzyme Kinetics and Control Reactions
 Handout Enzyme Kinetics and Control Reactions ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIDS Enzyme Kinetics and Control Reactions I. Kinetics A. Reaction rates 1. First order (reaction rate is
Handout Enzyme Kinetics and Control Reactions ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIDS Enzyme Kinetics and Control Reactions I. Kinetics A. Reaction rates 1. First order (reaction rate is
Experiment 1. Isolation of Glycogen from rat Liver
 Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Quiz 4 Review Guide Fall 2018
 Quiz 4 Review Guide Fall 2018 Major Topics: Enzyme Kinetics: o reaction rates and catalysis; transition state binding theory o Michaelis-Menten equation and interpretation o Inhibitors types and explanations
Quiz 4 Review Guide Fall 2018 Major Topics: Enzyme Kinetics: o reaction rates and catalysis; transition state binding theory o Michaelis-Menten equation and interpretation o Inhibitors types and explanations
A Kinetic Study of Glucose-6-phosphate Dehydrogenase
 A Kinetic Study of Glucose-6-phosphate Dehydrogenase (Received for publication, September 10, 1975) MOHAMMED. KANJ, MYRON L. TOEWS, AND W. ROBERT CARPER* From the Department of Chemistry, Wichita State
A Kinetic Study of Glucose-6-phosphate Dehydrogenase (Received for publication, September 10, 1975) MOHAMMED. KANJ, MYRON L. TOEWS, AND W. ROBERT CARPER* From the Department of Chemistry, Wichita State
B. 1% (w/v) Salicin Substrate Solution (Salicin) (Prepare 50 ml in Reagent A using Salicin, Sigma Prod. No. S-0625.)
 SIGMA QUALITY CONTROL TEST PROCEDURE (Q]\PDWLFÃ$VVD\ÃRIÃ */8&26,'$6( PRINCIPLE: 'Glucoside + H 2 O Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD:
SIGMA QUALITY CONTROL TEST PROCEDURE (Q]\PDWLFÃ$VVD\ÃRIÃ */8&26,'$6( PRINCIPLE: 'Glucoside + H 2 O Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD:
Enzymatic Assay of CHOLINE KINASE (EC )
 Enzymatic Assay of CHOLINE KINASE PRINCIPLE: Choline + ATP CK > o-phosphocholine + ADP ADP + PEP PK > ATP + Pyruvate Pyruvate + ß-NADH LDH > Lactate + ß-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate
Enzymatic Assay of CHOLINE KINASE PRINCIPLE: Choline + ATP CK > o-phosphocholine + ADP ADP + PEP PK > ATP + Pyruvate Pyruvate + ß-NADH LDH > Lactate + ß-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate
Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds
 Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds By Edward J. Leonard, M.D., and Stephen Hajdu, M.D. A plasma protein system of mammalian origin which increases the contractile
Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds By Edward J. Leonard, M.D., and Stephen Hajdu, M.D. A plasma protein system of mammalian origin which increases the contractile
10 mm KCl in a Ti-15 zonal rotor at 35,000 rpm for 16 hr at
 Proc. Nat. Acad. SCi. USA Vol. 68, No. 11, pp. 2752-2756, November 1971 Translation of Exogenous Messenger RNA for Hemoglobin on Reticulocyte and Liver Ribosomes (initiation factors/9s RNA/liver factors/reticulocyte
Proc. Nat. Acad. SCi. USA Vol. 68, No. 11, pp. 2752-2756, November 1971 Translation of Exogenous Messenger RNA for Hemoglobin on Reticulocyte and Liver Ribosomes (initiation factors/9s RNA/liver factors/reticulocyte
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
Serrata) Alkaline Phosphatase
 Vol. 41, No. 5, April 1997 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 951-959 An Essential Tryptophan Residue of Green Crab (Syclla Serrata) Alkaline Phosphatase Wen-Zhu Zheng 1, Qing-Xi Chen
Vol. 41, No. 5, April 1997 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 951-959 An Essential Tryptophan Residue of Green Crab (Syclla Serrata) Alkaline Phosphatase Wen-Zhu Zheng 1, Qing-Xi Chen
Enzymatic Assay of RIBONUCLEIC ACID POLYMERASE 1 (EC )
 PRINCIPLE: Enzymatic Assay of RIBONUCLEIC ACID POLYMERASE 1 DNA + NTP RNA Polymerase > DNA + RNA + PP i PP i + UDPG UDPG Pyrophosphorylase > UTP + Glucose 1-Phosphate Glucose 1-Phosphate Phosphoglucomutase
PRINCIPLE: Enzymatic Assay of RIBONUCLEIC ACID POLYMERASE 1 DNA + NTP RNA Polymerase > DNA + RNA + PP i PP i + UDPG UDPG Pyrophosphorylase > UTP + Glucose 1-Phosphate Glucose 1-Phosphate Phosphoglucomutase
ENZYMES: CLASSIFICATION, STRUCTURE
 ENZYMES: CLASSIFICATION, STRUCTURE Enzymes - catalysts of biological reactions Accelerate reactions by a millions fold Common features for enzymes and inorganic catalysts: 1. Catalyze only thermodynamically
ENZYMES: CLASSIFICATION, STRUCTURE Enzymes - catalysts of biological reactions Accelerate reactions by a millions fold Common features for enzymes and inorganic catalysts: 1. Catalyze only thermodynamically
Six Types of Enzyme Catalysts
 Six Types of Enzyme Catalysts Although a huge number of reactions occur in living systems, these reactions fall into only half a dozen types. The reactions are: 1. Oxidation and reduction. Enzymes that
Six Types of Enzyme Catalysts Although a huge number of reactions occur in living systems, these reactions fall into only half a dozen types. The reactions are: 1. Oxidation and reduction. Enzymes that
Vets 111/Biov 111 Cell Signalling-2. Secondary messengers the cyclic AMP intracellular signalling system
 Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
B. 15 mm Ouabain Solution (Ouabain) (Prepare 10 ml in Reagent A using Ouabain Octahydrate, Sigma Prod. No. O3125.)
 SIGMA QUALITY CONTROL TEST PROCEDURE Sigma Prod. No. A7510 PRINCIPLE: ATP + H 2 O ATPase > ADP + P i Abbreviations used: ATPase = Adenosine 5'-Triphosphatase ATP = Adenosine 5'-Triphosphate ADP = Adenosine
SIGMA QUALITY CONTROL TEST PROCEDURE Sigma Prod. No. A7510 PRINCIPLE: ATP + H 2 O ATPase > ADP + P i Abbreviations used: ATPase = Adenosine 5'-Triphosphatase ATP = Adenosine 5'-Triphosphate ADP = Adenosine
The University of ~ukurova, Art & Science Faculty, Department of Chemistry, BaIcali, Adana-TURKEY
 BIOCHEMISTRY andmolecular BIOLOGY INTERNATIONAL pages 227-232 EFFECTS OF SULFHYDRYL COMPOUNDS ON THE INHIBITION OF ERYTHROCYTE MEMBRANE Na+-K + ATPase BY OZONE Rmnazan Bilgin, Sermin Gill, S. Seyhan Ttikel
BIOCHEMISTRY andmolecular BIOLOGY INTERNATIONAL pages 227-232 EFFECTS OF SULFHYDRYL COMPOUNDS ON THE INHIBITION OF ERYTHROCYTE MEMBRANE Na+-K + ATPase BY OZONE Rmnazan Bilgin, Sermin Gill, S. Seyhan Ttikel
Figure 1 Original Advantages of biological reactions being catalyzed by enzymes:
 Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Kinetics of Regulatory Enzymes
 THE JOURNAL OF BIOLOGICAL C~E~~ISTRY Vol 240, No 6, June 1965 f+ rinka in USA Kinetics of Regulatory Enzymes KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND
THE JOURNAL OF BIOLOGICAL C~E~~ISTRY Vol 240, No 6, June 1965 f+ rinka in USA Kinetics of Regulatory Enzymes KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND
Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2011
 Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2011 I. (25 points) Fill in all of the enzyme catalyzed reactions which convert glycogen to lactate. Draw the correct structure for each intermediate
Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2011 I. (25 points) Fill in all of the enzyme catalyzed reactions which convert glycogen to lactate. Draw the correct structure for each intermediate
االمتحان النهائي لعام 1122
 االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS
 PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM* (From the Departments of Physiology and Biochemistry, Duke University School of Medicine, Durham)
PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM* (From the Departments of Physiology and Biochemistry, Duke University School of Medicine, Durham)
[GANN, 59, ; October, 1968] CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES
![[GANN, 59, ; October, 1968] CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES [GANN, 59, ; October, 1968] CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES](/thumbs/87/96205960.jpg) [GANN, 59, 415-419; October, 1968] UDC 616-006-092.18 CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES Kiyoshi TSUNEMATSU, Shin-ichi YOKOTA, and Tadao SHIRAISHI (Third Department of Internal
[GANN, 59, 415-419; October, 1968] UDC 616-006-092.18 CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES Kiyoshi TSUNEMATSU, Shin-ichi YOKOTA, and Tadao SHIRAISHI (Third Department of Internal
(Adams 8c Purves 1958), or LATS-protector (LATS-P) (Adams 8c Kennedy. 1967). The failure of the McKenzie (1958) mouse bioassay to detect LATS in
 Department of Endocrinology, Royal Prince Alfred Hospital, and Department of Medicine, University of Sydney, Sydney, Australia THE THYROTROPHIN RECEPTOR IN HUMAN THYROID PLASMA MEMBRANES: EFFECT OF SERUM
Department of Endocrinology, Royal Prince Alfred Hospital, and Department of Medicine, University of Sydney, Sydney, Australia THE THYROTROPHIN RECEPTOR IN HUMAN THYROID PLASMA MEMBRANES: EFFECT OF SERUM
Enzymatic Assay of PHOSPHODIESTERASE, 3':5'-CYCLIC NUCLEOTIDE Crude Complex
 PRINCIPLE: 3':5'-cAMP + H 2 O PDE-3':5'-CN > AMP AMP + ATP Myokinase > 2 ADP 2 ADP + 2 PEP Pyruvate Kinase > 2 ATP + 2 Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD Abbreviations
PRINCIPLE: 3':5'-cAMP + H 2 O PDE-3':5'-CN > AMP AMP + ATP Myokinase > 2 ADP 2 ADP + 2 PEP Pyruvate Kinase > 2 ATP + 2 Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD Abbreviations
Chapter 10. Regulatory Strategy
 Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Mannose Metabolism in the Human Erythrocyte
 Mannose Metabolism in the Human Erythrocyte ERNEST BEUTLER and LESLIE TEEPLE From the City of Hope Medical Center, Duarte, California 911 A B S T R A C T The metabolism of mannose by human erythrocytes
Mannose Metabolism in the Human Erythrocyte ERNEST BEUTLER and LESLIE TEEPLE From the City of Hope Medical Center, Duarte, California 911 A B S T R A C T The metabolism of mannose by human erythrocytes
GLYCOGEN BEFORE THE LAB YOU HAVE TO READ ABOUT:
 GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
OF TRANSAMINASE IN RAT TISUES
 OF TRANSAMINASE IN RAT TISUES KOZO YAMADA, SHUNJI SAWAKI, AKIRA FUKUMURA AND MASARU HAYASHID epartment of Internal Mcdicine, Faculty of Medicine, Nagoya University, agoya Showa-ku, N (Received July 30,
OF TRANSAMINASE IN RAT TISUES KOZO YAMADA, SHUNJI SAWAKI, AKIRA FUKUMURA AND MASARU HAYASHID epartment of Internal Mcdicine, Faculty of Medicine, Nagoya University, agoya Showa-ku, N (Received July 30,
A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT
 A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT BY M. L. CALDWELL AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for publication,
A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT BY M. L. CALDWELL AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for publication,
3-P dehydrogenase. They concluded that each subunit expressed
 Proc. Nat. Acad. Sci. USA Vol. 69, No. 8, pp. 2278-2282, August 1972 Interactions between Native and Chemically Modified Subunits of Matrix-Bound Glycogen Phosphorylase* (hybrid enzyme/monomers/dimers/pyridoxal-phosphate)
Proc. Nat. Acad. Sci. USA Vol. 69, No. 8, pp. 2278-2282, August 1972 Interactions between Native and Chemically Modified Subunits of Matrix-Bound Glycogen Phosphorylase* (hybrid enzyme/monomers/dimers/pyridoxal-phosphate)
Chapter 2 Transport Systems
 Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Chapter 2 Transport Systems The plasma membrane is a selectively permeable barrier between the cell and the extracellular environment. It permeability properties ensure that essential molecules such as
Regulation of glycolysis/fructolysis in buffalo
 Regulation of glycolysis/fructolysis in buffalo spermatozoa K. K. Gandhi and S. R. Anand National Dairy Research Institute, Karnal-132001, India Summary. Assay of maximal activities of 11 glycolytic enzymes
Regulation of glycolysis/fructolysis in buffalo spermatozoa K. K. Gandhi and S. R. Anand National Dairy Research Institute, Karnal-132001, India Summary. Assay of maximal activities of 11 glycolytic enzymes
NOVEL SUBSTRATES OF YEAST ALCOHOL DEHYDROGENASE--4. ALLYL ALCOHOL AND ETHYLENE GLYCOL
 pages 1-8 Received lune 15, 1998. Accepted July 6, 1998. NOVEL SUBSTRATES OF YEAST ALCOHOL DEHYDROGENASE--4. ALLYL ALCOHOL AND ETHYLENE GLYCOL Svetlana Trivid 1 and Vladimir Leskovac 2. I Faculty of Science
pages 1-8 Received lune 15, 1998. Accepted July 6, 1998. NOVEL SUBSTRATES OF YEAST ALCOHOL DEHYDROGENASE--4. ALLYL ALCOHOL AND ETHYLENE GLYCOL Svetlana Trivid 1 and Vladimir Leskovac 2. I Faculty of Science
REGULA TION OF GLUTAMINE SYNTHETASE, VIII.
 REGULA TION OF GLUTAMINE SYNTHETASE, VIII. A TP: GLUTAMINE SYNTHETASE ADENYLYLTRANSFERASE, AN ENZYME THAT CATALYZES ALTERATIONS IN THE REGULATORY PROPERTIES OF GLUTAMINE SYNTHETASE BY HENRY S. KINGDON,*
REGULA TION OF GLUTAMINE SYNTHETASE, VIII. A TP: GLUTAMINE SYNTHETASE ADENYLYLTRANSFERASE, AN ENZYME THAT CATALYZES ALTERATIONS IN THE REGULATORY PROPERTIES OF GLUTAMINE SYNTHETASE BY HENRY S. KINGDON,*
R.'ecent evidence strongly suggests that
 Activators and inhibitors of lens aldose reductase /. A. Jedziniak and J. H. Kinoshita Aldose reductase in a highly purified state is unstable. It requires the presence of thiol groups to maintain it in
Activators and inhibitors of lens aldose reductase /. A. Jedziniak and J. H. Kinoshita Aldose reductase in a highly purified state is unstable. It requires the presence of thiol groups to maintain it in
Enzymes. Enzymes : are protein catalysts that increase the rate of reactions without being changed in the overall process.
 Enzymes Enzymes Enzymes : are protein catalysts that increase the rate of reactions without being changed in the overall process. All reactions in the body are mediated by enzymes A + B E C A, B: substrate
Enzymes Enzymes Enzymes : are protein catalysts that increase the rate of reactions without being changed in the overall process. All reactions in the body are mediated by enzymes A + B E C A, B: substrate
decarboxylation. Further work with the enzyme systems involved has shown
 THE BACTERIAL OXIDATION OF AROMATIC COMPOUNDS IV. STITDIES ON THE MECHANISM OF ENZYMATC DEGRADATION OF PROTOCATECHuiC ACID' R. Y. STANIER Department of Bacteriology, University of California, Berkeley,
THE BACTERIAL OXIDATION OF AROMATIC COMPOUNDS IV. STITDIES ON THE MECHANISM OF ENZYMATC DEGRADATION OF PROTOCATECHuiC ACID' R. Y. STANIER Department of Bacteriology, University of California, Berkeley,
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19
 TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
Communication. Identification of Methionine N -Acetyltransferase from Saccharomyces cerevisiae
 Communication THE JOURNAL OP BIOLOGICAL CHEMISTRY Vol. 265, No. 7, Issue of March 5, pp. 3603-3606,lSSO 0 1990 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U. S. A. Identification
Communication THE JOURNAL OP BIOLOGICAL CHEMISTRY Vol. 265, No. 7, Issue of March 5, pp. 3603-3606,lSSO 0 1990 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U. S. A. Identification
Problem-solving Test: The Mechanism of Protein Synthesis
 Q 2009 by The International Union of Biochemistry and Molecular Biology BIOCHEMISTRY AND MOLECULAR BIOLOGY EDUCATION Vol. 37, No. 1, pp. 58 62, 2009 Problem-based Learning Problem-solving Test: The Mechanism
Q 2009 by The International Union of Biochemistry and Molecular Biology BIOCHEMISTRY AND MOLECULAR BIOLOGY EDUCATION Vol. 37, No. 1, pp. 58 62, 2009 Problem-based Learning Problem-solving Test: The Mechanism
Studies on the Glucanase of Sclerotinia libertiana. EBATA and Yukio SATOMURA
 Studies on the Glucanase of Sclerotinia libertiana By Junko EBATA and Yukio SATOMURA Faculty of Science, Osaka City University, Osaka Received December 13, 1962 The digestion of yeast cells with the glucanase
Studies on the Glucanase of Sclerotinia libertiana By Junko EBATA and Yukio SATOMURA Faculty of Science, Osaka City University, Osaka Received December 13, 1962 The digestion of yeast cells with the glucanase
PFK Activity Assay Kit (Colorimetric)
 PFK Activity Assay Kit (Colorimetric) Catalog Number KA3761 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
PFK Activity Assay Kit (Colorimetric) Catalog Number KA3761 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
DIHYDROSTREPTOMYCIN, VITAMIN K2-COUPLED
 JOURNAL OF BACTERIOLOGY Vol. 88, No. 4, p. 1019-1023 October, 1964 Copyright 1964 American Society for Microbiology Printed in U.S.A. DIHYDROSTREPTOMYCIN, VITAMIN K2-COUPLED TETRAZOLIUM REDUCTION, AND
JOURNAL OF BACTERIOLOGY Vol. 88, No. 4, p. 1019-1023 October, 1964 Copyright 1964 American Society for Microbiology Printed in U.S.A. DIHYDROSTREPTOMYCIN, VITAMIN K2-COUPLED TETRAZOLIUM REDUCTION, AND
Enzymatic Assay of ß-GLUCOSIDASE (EC )
 PRINCIPLE: ß-D-Glucoside + H 2 O ß-Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric 1 REAGENTS: A. 100 mm Sodium Acetate Buffer, ph 5.0
PRINCIPLE: ß-D-Glucoside + H 2 O ß-Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric 1 REAGENTS: A. 100 mm Sodium Acetate Buffer, ph 5.0
THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION
 THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION BY ROBERT K. CRANE* AND FRITZ LIPMANN (From the Biochemical Research Laboratory, Massachusetts General Hospital, and the Department of Biological Chemistry,
THE EFFECT OF ARSENATE ON AEROBIC PHOSPHORYLATION BY ROBERT K. CRANE* AND FRITZ LIPMANN (From the Biochemical Research Laboratory, Massachusetts General Hospital, and the Department of Biological Chemistry,
THE MILK-CLOTTING ACTION OF PAPAIN*
 THE MILK-CLOTTING ACTION OF PAPAIN* BY A. K. BALLS.4ND SAM R. HOOVER (From the Food Research Division, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington) (Received for
THE MILK-CLOTTING ACTION OF PAPAIN* BY A. K. BALLS.4ND SAM R. HOOVER (From the Food Research Division, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington) (Received for
A Protein Kinase Inhibitor in Brown Adipose Tissue of Developing Rats
 Biochem. J. (1974) 138, 195-199 Printed in Great Britain 195 A Protein Kinase Inhibitor in Brown Adipose Tissue of Developing Rats By JOSEF P. SKALA, GEORGE I. DRUMMOND and PETER HAHN Departments ofpaediatrics,
Biochem. J. (1974) 138, 195-199 Printed in Great Britain 195 A Protein Kinase Inhibitor in Brown Adipose Tissue of Developing Rats By JOSEF P. SKALA, GEORGE I. DRUMMOND and PETER HAHN Departments ofpaediatrics,
FUNDAMENTALS OF BIOCHEMISTRY, CELL BIOLOGY AND BIOPHYSICS Vol. I - Biochemistry of Vitamins, Hormones and Other Messenger Molecules - Chris Whiteley
 BIOCHEMISTRY OF VITAMINS, HORMONES AND OTHER MESSENGER MOLECULES Chris Whiteley Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa Keywords: phosphorylation, phosphorylase,
BIOCHEMISTRY OF VITAMINS, HORMONES AND OTHER MESSENGER MOLECULES Chris Whiteley Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa Keywords: phosphorylation, phosphorylase,
5-Aminolevulinic-Acid Synthetase of Rhodopseudomonas sp heroides Y
 Eur. J. Biochem. 40, 19-24 (1973) 5-Aminolevulinic-Acid Synthetase of Rhodopseudomonas sp heroides Y Kinetic Mechanism and nhibition by ATP Michitle FANCA-GAGNER and Jenny CLEMENT-METRAL Laboratoire de
Eur. J. Biochem. 40, 19-24 (1973) 5-Aminolevulinic-Acid Synthetase of Rhodopseudomonas sp heroides Y Kinetic Mechanism and nhibition by ATP Michitle FANCA-GAGNER and Jenny CLEMENT-METRAL Laboratoire de
KE-SIALIQ Sialic Acid Quantitation Kit. SialiQuant Sialic Acid Quantitation Kit
 SialiQuant Sialic Acid Quantitation Kit Part Number KE-SIALIQ Certification of Analysis Lot Number 706.1A Kit Storage Kits should be stored at 4 C. Kit Contents Kit contains all the reagents to quickly
SialiQuant Sialic Acid Quantitation Kit Part Number KE-SIALIQ Certification of Analysis Lot Number 706.1A Kit Storage Kits should be stored at 4 C. Kit Contents Kit contains all the reagents to quickly
ENZYMES QUESTIONSHEET 1
 QUESTIONSHEET 1 The apparatus illustrated below can be used to investigate the activity of the enzyme catalase, which is found in liver. The liver tissue has been ground up and mixed with a buffer solution.
QUESTIONSHEET 1 The apparatus illustrated below can be used to investigate the activity of the enzyme catalase, which is found in liver. The liver tissue has been ground up and mixed with a buffer solution.
