Part 1 The Cell and the Cellular Environment
|
|
|
- Angelica Wilkinson
- 6 years ago
- Views:
Transcription
1 1 Chapter 3 Anatomy and Physiology Part 1 The Cell and the Cellular Environment 2 The Human Cell The is the fundamental unit of the human body. Cells contain all the necessary for life functions. 3 Cell Structure The cell membrane is the outer covering that encircles and protects the cell. It is semipermeable. is the thick, viscous fluid that fills and gives shape to the cell. are structures that perform specific functions within a cell. 4 Organelles : Contains the DNA Endoplasmic reticulum: synthesis functions Golgi apparatus: synthesis and secretion functions : energy factories Lysosomes: contains digestive enzymes : neutralize toxins 5 The Normal Cell 6 Cell Function All human cells have the same general structure and material. Differentiation causes cells to become. There are major functions of cells. 7 Major Functions of Cells (muscle cells) Conductivity (nerve cells) Metabolic absorption (intestines and kidneys) (produces hormones, mucus, sweat, saliva) Excretion (all cells break down nutrients) Respiration (take in oxygen) (enlarge, divide, and reproduce themselves) 8 Tissues Tissue refers to a of cells that perform a similar function. 9 Tissue Types 1
2 10 Epithelial Tissue Lines internal and body surfaces and protects the body. Some forms perform specialized functions: -Secretion -Absorption - -Filtration, mucous membranes, lining of intestinal tract. 11 Muscle Tissue Has the capability of contraction when stimulated. Muscle Muscle Muscle 12 Connective Tissue Most tissue in the body. Provides support, connection, and insulation. Examples include, cartilage, and fat. is classified as connective tissue. 13 Nerve Tissue Specialized tissue that transmits impulses throughout the body. Examples include the, spinal cord, and peripheral nerves. 14 Organs, Organ Systems, and the Organism An organ is a group of functioning together. A group of organs working together is an organ. The sum of all cells, tissues, organs, and organ systems makes up an organism. 15 Organ Systems 1 Cardiovascular Gastrointestinal Genitourinary Reproductive 2 Endocrine Muscular 2
3 16 System Integration 17 Homeostasis Homeostasis is the term for the body s natural tendency to keep the internal environment and metabolism and normal. A significant amount of is required to maintain the anatomy and physiology of the body. 18 Metabolism Metabolism is the term used to refer to the building up ( ) and breaking down ( ) of biochemical substances to produce energy. 19 The body s cells interact and intercommunicate with substances secreted by various body. If these messages are interrupted, a disease can develop or advance 20 Endocrine Glands Sometimes called glands. Secrete directly into the circulatory system. Some endocrine glands include: pituitary,, parathyroid, adrenal glands, Islets of Langerhans in the pancreas, testes, and ovaries. 21 Exocrine Glands 22 Water Secrete substances such as, saliva, tears, mucus,and digestive juices onto the epithelial surfaces via ducts. 23 Total Body Water (TBW) Water is the most substance in the human body Total Body Water (TBW): The total amount of water in the body at a given time. Average adult weighing 70kg, TBW is about liters (11 gallons) 24 Fluids Intracellular fluid (ICF): the fluid the cells Extracellular fluid (ECF): the fluid the body cells 25 Extracellular Fluid Two types of ECF: fluid: fluid within the circulatory system (blood plasma) fluid: fluid within body tissues that is outside the cells and outside the vascular system Synovial fluid, aqueous humor, secretions such as saliva, tears, etc. 3
4 26 Body Fluid Compartments Intracellular fluids make up % of total body water Liters in a 70kg person Extracellular fluid make up % of total body water 10.5 Liters in a 70kg person Interstitial fluids % of TBW Intravascular fluids % of TBW 27 Dehydration The body requires a proper balance of input and % to function properly Dehydration is an abnormal % in body fluids 28 Causes of Dehydration losses vomiting, diarrhea Increased loss: through normal mechanisms sweat, water vapor from lungs, saliva Increased 29 Causes of Dehydration losses (3 rd space): losses into fluid compartments peritonitus, pancreatitis, bowel obstruction losses burns, surgical drains, open wounds 30 S/S of Dehydration Dry mucous membranes Poor skin Excessive If severe, S/S of hypovolemic shock Treatment is fluid replacement Positive Test 10% increase in rate OR 10% decrease in BP between lying and sitting 31 Overhydration Overhydration is the presence of an abnormally high amount of body May indicate presence of heart Treatment is of excess fluids 32 Electrolytes 33 Electrolytes Substances that, in water, separate into electrically particles called ions : charged particles 4
5 34 Cations have a positive charge. have a negative charge. 35 Sodium (Na + ) Most prevalent cation in fluid. follows sodium. Important in transmission of nervous impulses. Hypernatremia is an abnormal increase in sodium. is an abnormal decrease in sodium. 36 Potassium (K + ) Most prevalent cation in the fluid. Important in transmission of electrical impulses. is an abnormally high potassium level. Hypokalemia is an abnormally potassium level. 37 Calcium (Ca ++ ) Plays a major role in contraction as well as nervous impulse transmission. Hypercalcemia is an abnormally increased calcium level. is an abnormally low calcium level. 38 Magnesium (Mg ++ ) 39 Anions Necessary for several biochemical processes. Closely associated with. is an abnormally increased level of magnesium. Hypomagnesemia is an abnormally decreased level of magnesium. 40 Chloride (Cl - ) charge balances the positive charge of cations. Major role in fluid balance and function. Associated with. 41 Bicarbonate (HCO 3 - ) Principle of the body. Buffer: preserves or restores normal acid-base Neutralizes the ion and other organic acids. 42 Phosphate (HPO 4 - ) Important in body storage Closely associated with magnesium in 5
6 function. Acts as a, primarily in the intracellular space. 43 Osmosis and Diffusion 44 Osmosis Osmosis is the passage of a solvent through a membrane; movement of solvent in a solution from an area of solute concentration to an area of concentration : a liquid substance that is capable of dissolving other substance : a dissolved substance 45 Osmosis 46 Diffusion Diffusion the movement of molecules through a semipermeable membrane from an area of concentration to an area of concentration Usually an Requires no energy 47 Diffusion 48 Types of Solutions (1 of 2) solutions on opposite sides of a membrane are equal in concentration. Results in fluid shift LR, NS the concentration of a given solute is greater on one side of a membrane than the other. Results in of fluids D50W, D25W 49 Types of Solutions (2 of 2) the concentration of a given solute is less on one side of a membrane than the other. Fluid will to a higher concentration D5W 50 Active Transport The movement of a substance across the cell membrane against the osmotic (toward the side that already has more of the substance). Opposite to the normal direction of diffusion Faster than diffusion. Requires. 51 Facilitated Diffusion 6
7 Certain molecules can move across the cell membrane with the assistance of helper. is one example. Depending on the substance, this movement may or may not require. 52 Osmotic vs. Oncotic Osmotic pressure exerted by the concentration of solutes on one side of a semipermeable membrane. force (colloid osmotic pressure): osmotic pressure exerted by large protein particles (colloids) 53 Acid-Base Balance 54 Acid-Base Balance Acid-base balance is a dynamic relationship that reflects the relative concentration of ions in the body. Hydrogen ions are acidic and the concentration of those in the body must be maintained within fairly strict limits. Any adversely affects the body Changes from to second 55 The ph Scale ph=potential of The ph scale is inversely related to hydrogen ion concentration The greater the hydrogen concentration, the lower the ph The ph scale ranges from to Normal body ph is to ph below 7.35= ph above 7.45= Variance of can be lethal 56 Regulation of Acid-Base Balance The body is constantly producing hydrogen ions (acids) through and other biochemical processes To maintain the correct acid-base balance, these hydrogen ions must be 57 3 Mechanisms to Remove Hydrogen system (bicarbonate buffer system) function 58 Buffer System (Bicarbonate Buffer System) The mechanism. Two components of this system are bicarbonate ion (HCO 3 - ) and carbonic (H 2 CO 3 ) and are normally in equilibrium with hydrogen (H + ). 7
8 59 H + + HCO 3 - H 2 CO 3 Hydrogen may combine with to produce carbonic acid. In other circumstances carbonic acid will into bicarbonate and hydrogen. Normally, for every molecule of carbonic acid, there are molecules of bicarbonate ion. (Ratio of 20:1) 60 H + + HCO 3 - H 2 CO 3 An increase in hydrogen ion ( ) is corrected as the excess hydrogen ions combine with bicarbonate ions to form carbonic acid Increase in hydrogen ion leads to an increase in acid A decrease in hydrogen ions ( ) will cause carbonic acid to dissociate into bicarbonate ion and hydrogen ion 61 H + + HCO 3 - H 2 CO 3 Acid: H + + HCO 3 - H 2 CO 3 Acid: H + + HCO 3 - H 2 CO 3 62 Acid-base Relations Relevant to ph 63 Respiratory and Kidney Mechanisms Increased respirations cause increased elimination of which causes a decrease in hydrogen ions and an increase in ph. The kidneys regulate the ph by altering the concentration of ions in the blood. 64 Acid Base Balance Disorders Respiratory Respiratory Metabolic Metabolic 65 The Respiratory Component of Acid-Base Balance Decreased ventilations lead to PaCO 2 and carbonic acid which lowers the ph ventilations = PaCO 2 + H 2 CO 3 = Respiratory Increased ventilations lead to PaCO 2 and carbonic acid which increases the ph ventilations = PaCO 2 + H 2 CO 3 = Respiratory 66 The Respiratory Component of Acid-Base Balance 67 Respiratory Acidosis CO 2 + H 2 0 H 2 CO 3 H + + HCO 3 8
9 Simplified: CO 2 H + As CO 2, ph should decrease producing acidosis Commonly seen on patients in respiratory or breathing very slowly 68 Respiratory Alkalosis CO 2 + H 2 0 H 2 CO 3 H + + HCO 3 Simplified: CO 2 H + As CO 2 decreases, ph should producing alkalosis Caused by 69 Normal Values ph: to PaCO 2 : to torr PO 2 : to torr 70 Abnormal Values Respiratory : ph and CO 2 Respiratory : ph and CO 2 71 Example: ph = 7.25 PaCO 2 = 60 What is the acid/base imbalance? 72 Example: A patient is found unconscious and breathing 6 times per minute Should the PaCO 2 be high or low? Should the ph be high or low? What is the respiratory ph imbalance? 73 Example: A patient is found hyperventilating and breathing 46 times per minute Should the PaCO 2 be high or low? Should the ph be high or low? What is the respiratory ph imbalance? 9
10 74 Example: ph = 7.60 PaCO 2 = 20 What is the acid/base imbalance? 75 Simplifying Acid/Base Imbalances Look at the ph If it is below 7.35 then it is If it is above 7.45 then it is Look at the PaCO 2 If it is below torr or above torr, then it is respiratory If the PaCO 2 is within torr, then the imbalance is 76 Simplifying Acid/Base Imbalances There can be combination of metabolic and respiratory Respiratory problems will normally affect changes Metabolic problems will normally affect changes Metabolic imbalances will be discussed later 10
Principles of Fluid Balance
 Principles of Fluid Balance I. The Cellular Environment: Fluids and Electrolytes A. Water 1. Total body water (TBW) = 60% of total body weight 2. Fluid Compartments in the Body a. Intracellular Compartment
Principles of Fluid Balance I. The Cellular Environment: Fluids and Electrolytes A. Water 1. Total body water (TBW) = 60% of total body weight 2. Fluid Compartments in the Body a. Intracellular Compartment
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE
 Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
Chapter 27: WATER, ELECTROLYTES, AND ACID-BASE BALANCE I. RELATED TOPICS Integumentary system Cerebrospinal fluid Aqueous humor Digestive juices Feces Capillary dynamics Lymph circulation Edema Osmosis
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Principles of Anatomy and Physiology 14 th Edition CHAPTER 27 Fluid, Electrolyte, and Acid Base Fluid Compartments and Fluid In adults, body fluids make up between 55% and 65% of total body mass. Body
Water, Electrolytes, and Acid-Base Balance
 Chapter 27 Water, Electrolytes, and Acid-Base Balance 1 Body Fluids Intracellular fluid compartment All fluids inside cells of body About 40% of total body weight Extracellular fluid compartment All fluids
Chapter 27 Water, Electrolytes, and Acid-Base Balance 1 Body Fluids Intracellular fluid compartment All fluids inside cells of body About 40% of total body weight Extracellular fluid compartment All fluids
CHAPTER 27 LECTURE OUTLINE
 CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
CHAPTER 27 LECTURE OUTLINE I. INTRODUCTION A. Body fluid refers to body water and its dissolved substances. B. Regulatory mechanisms insure homeostasis of body fluids since their malfunction may seriously
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013
 Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013 Cells form 4 basic tissue groups: 1. Epithelial 2. Connective
Emergency Medical Training Services Emergency Medical Technician Paramedic Program Outlines Outline Topic: Patho Instructor Notes Revised: 11/2013 Cells form 4 basic tissue groups: 1. Epithelial 2. Connective
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES
 Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES Body Water Content Water Balance: Normal 2500 2000 1500 1000 500 Metab Food Fluids Stool Breath Sweat Urine
Amjad Bani Hani Ass.Prof. of Cardiac Surgery & Intensive Care FLUIDS AND ELECTROLYTES Body Water Content Water Balance: Normal 2500 2000 1500 1000 500 Metab Food Fluids Stool Breath Sweat Urine
Chapter 20 8/23/2016. Fluids and Electrolytes. Fluid (Water) Fluid (Water) (Cont.) Functions
 Chapter 20 Fluids and Electrolytes All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Fluid (Water) Functions Provides an extracellular transportation
Chapter 20 Fluids and Electrolytes All items and derived items 2015, 2011, 2006 by Mosby, Inc., an imprint of Elsevier Inc. All rights reserved. Fluid (Water) Functions Provides an extracellular transportation
Water compartments inside and outside cells maintain a balanced distribution of total body water.
 Chapter 9 Water Balance Chapter 9 Lesson 9.1 Key Concepts Water compartments inside and outside cells maintain a balanced distribution of total body water. The concentration of various solute particles
Chapter 9 Water Balance Chapter 9 Lesson 9.1 Key Concepts Water compartments inside and outside cells maintain a balanced distribution of total body water. The concentration of various solute particles
Fluid, Electrolyte, and Acid Base Balance
 25 Fluid, Electrolyte, and Acid Base Balance Lecture Presentation by Lori Garrett Note to the Instructor: For the third edition of Visual Anatomy & Physiology, we have updated our PowerPoints to fully
25 Fluid, Electrolyte, and Acid Base Balance Lecture Presentation by Lori Garrett Note to the Instructor: For the third edition of Visual Anatomy & Physiology, we have updated our PowerPoints to fully
WATER, SODIUM AND POTASSIUM
 WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
WATER, SODIUM AND POTASSIUM Attila Miseta Tamás Kőszegi Department of Laboratory Medicine, 2016 1 Average daily water intake and output of a normal adult 2 Approximate contributions to plasma osmolality
Major intra and extracellular ions Lec: 1
 Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Acid-Base Balance 11/18/2011. Regulation of Potassium Balance. Regulation of Potassium Balance. Regulatory Site: Cortical Collecting Ducts.
 Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
Influence of Other Hormones on Sodium Balance Acid-Base Balance Estrogens: Enhance NaCl reabsorption by renal tubules May cause water retention during menstrual cycles Are responsible for edema during
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
H 2 O, Electrolytes and Acid-Base Balance
 H 2 O, Electrolytes and Acid-Base Balance Body Fluids Intracellular Fluid Compartment All fluid inside the cells 40% of body weight Extracellular Fluid Compartment All fluid outside of cells 20% of body
H 2 O, Electrolytes and Acid-Base Balance Body Fluids Intracellular Fluid Compartment All fluid inside the cells 40% of body weight Extracellular Fluid Compartment All fluid outside of cells 20% of body
1. 09/07/16 Ch 1: Intro to Human A & P 1
 Table of Contents # Date Title Page # 1. 09/07/16 Ch 1: Intro to Human A & P 1 2. 09/19/16 Ch 18: Water, Electrolyte, and Acid-Base Balance 5 i 1 09/19/16 Chapter 18: Water, Electrolyte, and Acid-Base
Table of Contents # Date Title Page # 1. 09/07/16 Ch 1: Intro to Human A & P 1 2. 09/19/16 Ch 18: Water, Electrolyte, and Acid-Base Balance 5 i 1 09/19/16 Chapter 18: Water, Electrolyte, and Acid-Base
BIO132 Chapter 27 Fluid, Electrolyte and Acid Base Balance Lecture Outline
 BIO132 Chapter 27 Fluid, Electrolyte and Acid Base Balance Lecture Outline Fluid divisions 1. Extracellular fluid (ECF) 2. Intracellular fluid (ICF) Stabilization 1. Fluid balance 2. Electrolyte balance
BIO132 Chapter 27 Fluid, Electrolyte and Acid Base Balance Lecture Outline Fluid divisions 1. Extracellular fluid (ECF) 2. Intracellular fluid (ICF) Stabilization 1. Fluid balance 2. Electrolyte balance
Body Fluids and Fluid Compartments
 Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Fluids and electrolytes
 Body Water Content Fluids and electrolytes Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; healthy females
Body Water Content Fluids and electrolytes Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; healthy females
Chapter 1 Introduction to Physiology and Homeostasis
 Chapter 1 Introduction to Physiology and Homeostasis MULTIPLE CHOICE 1. Select the incorrect association. a. anatomy/function b. human body/multicellular. c. carbon dioxide/cell waste product. d. physiology/body
Chapter 1 Introduction to Physiology and Homeostasis MULTIPLE CHOICE 1. Select the incorrect association. a. anatomy/function b. human body/multicellular. c. carbon dioxide/cell waste product. d. physiology/body
Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are
 Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Fluid, Electrolyte, and Acid-Base Balance Body Water Content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60%
Instrumental determination of electrolytes in urine. Amal Alamri
 Instrumental determination of electrolytes in urine Amal Alamri What is the Electrolytes? Electrolytes are positively and negatively chargedions, Found in Within body's cells extracellular fluids, including
Instrumental determination of electrolytes in urine Amal Alamri What is the Electrolytes? Electrolytes are positively and negatively chargedions, Found in Within body's cells extracellular fluids, including
1/3/2008. Karen Burke Priscilla LeMone Elaine Mohn-Brown. Medical-Surgical Nursing Care, 2e Karen Burke, Priscilla LeMone, and Elaine Mohn-Brown
 Medical-Surgical Nursing Care Second Edition Karen Burke Priscilla LeMone Elaine Mohn-Brown Chapter 7 Caring for Clients with Altered Fluid, Electrolyte, or Acid-Base Balance Water Primary component of
Medical-Surgical Nursing Care Second Edition Karen Burke Priscilla LeMone Elaine Mohn-Brown Chapter 7 Caring for Clients with Altered Fluid, Electrolyte, or Acid-Base Balance Water Primary component of
Acid Base Balance. Chapter 26 Balance. ph Imbalances. Acid Base Balance. CO 2 and ph. Carbonic Acid. Part 2. Acid/Base Balance
 Acid Base Balance Chapter 26 Balance Part 2. Acid/Base Balance Precisely balances production and loss of hydrogen ions (ph) The body generates acids during normal metabolism, tends to reduce ph Kidneys:
Acid Base Balance Chapter 26 Balance Part 2. Acid/Base Balance Precisely balances production and loss of hydrogen ions (ph) The body generates acids during normal metabolism, tends to reduce ph Kidneys:
Fluid and Electrolytes P A R T 4
 Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Electrolytes Solution
 Electrolytes Solution Substances that are not dissociated in solution are called nonelectrolytes, and those with varying degrees of dissociation are called electrolytes. Urea and dextrose are examples
Electrolytes Solution Substances that are not dissociated in solution are called nonelectrolytes, and those with varying degrees of dissociation are called electrolytes. Urea and dextrose are examples
The Urinary System 15PART B. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Urinary System 15PART B Ureters Slender tubes attaching the kidney to the bladder Continuous with
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Urinary System 15PART B Ureters Slender tubes attaching the kidney to the bladder Continuous with
Body Water ANS 215 Physiology and Anatomy of Domesticated Animals
 Body Water ANS 215 Physiology and Anatomy of Domesticated Animals I. Body Water A. Water is the most abundant constituent comprising 60% of total body weight. 1. Solvent for many chemicals of the body
Body Water ANS 215 Physiology and Anatomy of Domesticated Animals I. Body Water A. Water is the most abundant constituent comprising 60% of total body weight. 1. Solvent for many chemicals of the body
UNIVERSITY OF MEDICAL SCIENCES, ONDO DEPARTMENT OF PHYSIOLOGY PHS 211 TRANSPORT MECHANISM LECTURER: MR A.O. AKINOLA
 UNIVERSITY OF MEDICAL SCIENCES, ONDO DEPARTMENT OF PHYSIOLOGY PHS 211 TRANSPORT MECHANISM LECTURER: MR A.O. AKINOLA OUTLINE Introduction Basic mechanisms Passive transport Active transport INTRODUCTION
UNIVERSITY OF MEDICAL SCIENCES, ONDO DEPARTMENT OF PHYSIOLOGY PHS 211 TRANSPORT MECHANISM LECTURER: MR A.O. AKINOLA OUTLINE Introduction Basic mechanisms Passive transport Active transport INTRODUCTION
EH1008 Biomolecules. Inorganic & Organic Chemistry. Water. Lecture 2: Inorganic and organic chemistry.
 EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
Renal physiology D.HAMMOUDI.MD
 Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Renal physiology D.HAMMOUDI.MD Functions Regulating blood ionic composition Regulating blood ph Regulating blood volume Regulating blood pressure Produce calcitrol and erythropoietin Regulating blood glucose
Technical University of Mombasa Faculty of Applied and Health Sciences
 Technical University of Mombasa Faculty of Applied and Health Sciences DEPARTMENT OF MEDICAL SCIENCES UNIVERSITY EXAMINATION FOR THE DEGREE OF BACHELOR OF MEDICAL LABORATORY SCIENCES BMLS 12S -Regular
Technical University of Mombasa Faculty of Applied and Health Sciences DEPARTMENT OF MEDICAL SCIENCES UNIVERSITY EXAMINATION FOR THE DEGREE OF BACHELOR OF MEDICAL LABORATORY SCIENCES BMLS 12S -Regular
Chapter 24 Water, Electrolyte and Acid-Base Balance
 Chapter 24 Water, Electrolyte and Acid-Base Balance Total body water for 150 lb. male = 40L 65% ICF 35% ECF 25% tissue fluid 8% blood plasma, lymph 2% transcellular fluid (CSF, synovial fluid) Water Movement
Chapter 24 Water, Electrolyte and Acid-Base Balance Total body water for 150 lb. male = 40L 65% ICF 35% ECF 25% tissue fluid 8% blood plasma, lymph 2% transcellular fluid (CSF, synovial fluid) Water Movement
Acids and Bases their definitions and meanings
 Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Acids and Bases their definitions and meanings Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. (HCl H + Cl ) (H 2 CO 3 H + HCO 3 ) A base is an
Fluid and electrolyte balance, imbalance
 Fluid and electrolyte balance, imbalance Body fluid The fluids are distributed throughout the body in various compartments. Body fluid is composed primarily of water Water is the solvent in which all solutes
Fluid and electrolyte balance, imbalance Body fluid The fluids are distributed throughout the body in various compartments. Body fluid is composed primarily of water Water is the solvent in which all solutes
human cell Mader s Understanding Human Anatomy and Physiology Chapters 1, 3 and 4
 1 The human cell INTRODUCTION All living things are composed of cells, which are the smallest units of life and are so small they can only be viewed through a microscope. Cells are made from pre-existing
1 The human cell INTRODUCTION All living things are composed of cells, which are the smallest units of life and are so small they can only be viewed through a microscope. Cells are made from pre-existing
RCPS Curriculum Pacing Guide Subject: Anatomy and Physiology
 RCPS Curriculum Pacing Guide 2013 2014 Subject: Anatomy and Physiology Week of: SOL # Unit Bloom s Objectives Throughout All units the course During field trip Throughout the course A+P1 Collecting, analyzing,
RCPS Curriculum Pacing Guide 2013 2014 Subject: Anatomy and Physiology Week of: SOL # Unit Bloom s Objectives Throughout All units the course During field trip Throughout the course A+P1 Collecting, analyzing,
Body water content. Fluid compartments. Regulation of water output. Water balance and ECF osmolallty. Regulation of water intake
 Body water content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; females 50% This difference reflects
Body water content Infants have low body fat, low bone mass, and are 73% or more water Total water content declines throughout life Healthy males are about 60% water; females 50% This difference reflects
Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU. Dr. Mohamed Saad Daoud
 Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU 1 Course symbol: BCH 472 Course Title: Biochemistry of biological fluids Credit hours: 3(2+1) 2 Clinical Biochemistry is one of the
Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU 1 Course symbol: BCH 472 Course Title: Biochemistry of biological fluids Credit hours: 3(2+1) 2 Clinical Biochemistry is one of the
BIOL 221 Chapter 26 Fluids & Electrolytes. 35 slides
 BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides 1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides 1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender
 BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides 1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
BIOL 221 Chapter 26 Fluids & Electrolytes 35 slides 1 Body Water Content Total Body Water is the percentage of a person s weight that is water. TBW can easily vary due to: gender males have higher TBW
Acid/Base Balance. the concentrations of these two ions affect the acidity or alkalinity of body fluids
 Acid/Base Balance some of most critical ions in body fluids are H + (hydrogen) and OH - (hydroxyl) ions the concentrations of these two ions affect the acidity or alkalinity of body fluids acidity/alkalinity
Acid/Base Balance some of most critical ions in body fluids are H + (hydrogen) and OH - (hydroxyl) ions the concentrations of these two ions affect the acidity or alkalinity of body fluids acidity/alkalinity
Epithelial Tissues. Types of Epithelial Tissues: Lining of Kidney
 Epithelial Tissues Covers the entire body surface and most of the body s inner cavities Outer epidermis (skin) protects from injury and drying out Inner epidermal tissue (on internal surfaces) often serves
Epithelial Tissues Covers the entire body surface and most of the body s inner cavities Outer epidermis (skin) protects from injury and drying out Inner epidermal tissue (on internal surfaces) often serves
FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTASIS
 Chapter 27 1 BIOLOGY 2402 Anatomy and Physiology Lecture Chapter 27 FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTASIS Chapter 27 2 FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTAIS Body fluid refers to the body
Chapter 27 1 BIOLOGY 2402 Anatomy and Physiology Lecture Chapter 27 FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTASIS Chapter 27 2 FLUID, ELECTROLYTES, AND ACID-BASE HOMEOSTAIS Body fluid refers to the body
Acid and Base Balance
 Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
Acid and Base Balance 1 2 The Body and ph Homeostasis of ph is tightly controlled Extracellular fluid = 7.4 Blood = 7.35 7.45 < 7.35: Acidosis (acidemia) > 7.45: Alkalosis (alkalemia) < 6.8 or > 8.0: death
SOCM Fluids Electrolytes and Replacement Products PFN: SOMRXL09. Terminal Learning Objective. References. Hours: 2.0 Last updated: November 2015
 SOCM Fluids Electrolytes and Replacement Products PFN: SOMRXL09 Hours: 2.0 Last updated: November 2015 Slide 1 Terminal Learning Objective Action: Communicate knowledge of Fluid, Electrolyte, and Acid
SOCM Fluids Electrolytes and Replacement Products PFN: SOMRXL09 Hours: 2.0 Last updated: November 2015 Slide 1 Terminal Learning Objective Action: Communicate knowledge of Fluid, Electrolyte, and Acid
Carbon Dioxide Transport. Carbon Dioxide. Carbon Dioxide Transport. Carbon Dioxide Transport - Plasma. Hydrolysis of Water
 Module H: Carbon Dioxide Transport Beachey Ch 9 & 10 Egan pp. 244-246, 281-284 Carbon Dioxide Transport At the end of today s session you will be able to : Describe the relationship free hydrogen ions
Module H: Carbon Dioxide Transport Beachey Ch 9 & 10 Egan pp. 244-246, 281-284 Carbon Dioxide Transport At the end of today s session you will be able to : Describe the relationship free hydrogen ions
UNIT VI: ACID BASE IMBALANCE
 UNIT VI: ACID BASE IMBALANCE 1 Objectives: Review the physiological mechanism responsible to regulate acid base balance in the body i.e.: Buffers (phosphate, hemoglobin, carbonate) Renal mechanism Respiratory
UNIT VI: ACID BASE IMBALANCE 1 Objectives: Review the physiological mechanism responsible to regulate acid base balance in the body i.e.: Buffers (phosphate, hemoglobin, carbonate) Renal mechanism Respiratory
Cells & Tissues. Chapter 3
 Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Acids, Bases, and Salts
 Acid / Base Balance Objectives Define an acid, a base, and the measure of ph. Discuss acid/base balance, the effects of acidosis or alkalosis on the body, and the mechanisms in place to maintain balance
Acid / Base Balance Objectives Define an acid, a base, and the measure of ph. Discuss acid/base balance, the effects of acidosis or alkalosis on the body, and the mechanisms in place to maintain balance
Chapter 15 Fluid and Acid-Base Balance
 Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Fluid, electrolyte, and acid-base balance
 Fluid, electrolyte, and acid-base balance Chapter 50 Ra'eda Almashaqba 1 Fluid, electrolyte, and acid-base balance About 46% to 60%of the average adult's weight is water, which is vital to health and normal
Fluid, electrolyte, and acid-base balance Chapter 50 Ra'eda Almashaqba 1 Fluid, electrolyte, and acid-base balance About 46% to 60%of the average adult's weight is water, which is vital to health and normal
download instant at
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The smallest living units capable of carrying out their own basic life functions are
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The smallest living units capable of carrying out their own basic life functions are
6. The diagram below represents an interaction between parts of an organism.
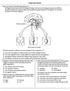 Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Endocrine Review 1. Base your answer to the following question on the diagram below and on your knowledge of biology. Each arrow in the diagram represents a different hormone released by the pituitary
Fluid, Electrolyte, and Acid-Base Balance. Maintaining Water and Electrolyte Balance of Blood
 514 Essentials of Human Anatomy and Physiology Intracellular fluid volume = 25 L, 40% body weight Total body water volume = 40 L, 60% body weight Extracellular fluid (ECF) volume =15 L, 20% body weight
514 Essentials of Human Anatomy and Physiology Intracellular fluid volume = 25 L, 40% body weight Total body water volume = 40 L, 60% body weight Extracellular fluid (ECF) volume =15 L, 20% body weight
Know your Ingredients: Triclosan, SD Alcohol 40, Propylene Glycol, Propylene Glycol, Tetrasodium EDTA, D&C Green 5, Sodium Stearate, and water.
 Urinary System There are four eliminative channels comprised of organs which help keep our bodies free from toxins, the intestinal, respiratory, urinary and integumentary systems are all responsible for
Urinary System There are four eliminative channels comprised of organs which help keep our bodies free from toxins, the intestinal, respiratory, urinary and integumentary systems are all responsible for
Langara College Spring archived
 COURSE DETAILS Department of Human Kinetics and Recreation Human Kinetics 1191 Anatomy & Physiology II Term: 2007-10 Sections: 001, 002, 003 Lecture/Seminar/Lab Hrs: 2: 0: 2 Credits: 3 Schedule: Section
COURSE DETAILS Department of Human Kinetics and Recreation Human Kinetics 1191 Anatomy & Physiology II Term: 2007-10 Sections: 001, 002, 003 Lecture/Seminar/Lab Hrs: 2: 0: 2 Credits: 3 Schedule: Section
ACID BASE BALANCE & BODY FLUID. Ani Retno Prijanti Renal and Body Fluids Module Juni 2008
 ACID BASE BALANCE & BODY FLUID Ani Retno Prijanti Renal and Body Fluids Module Juni 2008 1 Continuous Mixing of Body Fluids 2 Water Balance and ECF Osmolality To remain properly hydrated, water intake
ACID BASE BALANCE & BODY FLUID Ani Retno Prijanti Renal and Body Fluids Module Juni 2008 1 Continuous Mixing of Body Fluids 2 Water Balance and ECF Osmolality To remain properly hydrated, water intake
System Name: INTEGUMENTARY (cell wall) (Lysosomes) Main Organs: Main Organs: SKIN HAIR NAILS KIDNEYS URETERS BLADDER URETHRA
 URINARY System Name: (Lysosomes) KIDNEYS URETERS BLADDER URETHRA LUNGS SKIN EXCRETORY System Name: INTEGUMENTARY (cell wall) SKIN HAIR NAILS Skin is the largest Organ. The excretory system collects and
URINARY System Name: (Lysosomes) KIDNEYS URETERS BLADDER URETHRA LUNGS SKIN EXCRETORY System Name: INTEGUMENTARY (cell wall) SKIN HAIR NAILS Skin is the largest Organ. The excretory system collects and
Chapter 2. Fluid, Electrolyte, and Acid-Base Imbalances
 Chapter 2 Fluid, Electrolyte, and Acid-Base Imbalances Review of Concepts and Processes The major component of the body is water. Water is located in these compartments: Intracellular fluid (ICF) compartment
Chapter 2 Fluid, Electrolyte, and Acid-Base Imbalances Review of Concepts and Processes The major component of the body is water. Water is located in these compartments: Intracellular fluid (ICF) compartment
Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and
 CHAPTER 4 Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and recognize normal tissues under the microscope
CHAPTER 4 Study of different tissues Abnormal cells and tissues can be compared to normal tissues to identify disease, such as cancer Being able to know and recognize normal tissues under the microscope
Metabolic Alkalosis: Vomiting
 RENAL ANL) ACID-BASE PHYSIOLOGY 213 Case 37 Metabolic Alkalosis: Vomiting Maria Cuervo is a 20-year-old philosophy major at a state university. When the "24-hour" stomach flu went around campus during
RENAL ANL) ACID-BASE PHYSIOLOGY 213 Case 37 Metabolic Alkalosis: Vomiting Maria Cuervo is a 20-year-old philosophy major at a state university. When the "24-hour" stomach flu went around campus during
4. A phospholipid is an example of organization at the level.
 1. Physiology is the study of a. the structures of anatomical features. b. cellular metabolism. c. processes that allow organisms to function. d. how organ systems develop from the embryo. 2. Mary spends
1. Physiology is the study of a. the structures of anatomical features. b. cellular metabolism. c. processes that allow organisms to function. d. how organ systems develop from the embryo. 2. Mary spends
5/18/2017. Specific Electrolytes. Sodium. Sodium. Sodium. Sodium. Sodium
 Specific Electrolytes Hyponatremia Hypervolemic Replacing water (not electrolytes) after perspiration Freshwater near-drowning Syndrome of Inappropriate ADH Secretion (SIADH) Hypovolemic GI disease (decreased
Specific Electrolytes Hyponatremia Hypervolemic Replacing water (not electrolytes) after perspiration Freshwater near-drowning Syndrome of Inappropriate ADH Secretion (SIADH) Hypovolemic GI disease (decreased
Osmoregulation and Osmotic Balance
 OpenStax-CNX module: m44808 1 Osmoregulation and Osmotic Balance OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this
OpenStax-CNX module: m44808 1 Osmoregulation and Osmotic Balance OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this
Chapter 1: Cells and Tissues
 Chapter 1: Cells and Tissues Cells and Tissues Carry out all chemical activities needed to sustain life Cells are the building blocks of all living things Tissues are groups of cells that are similar in
Chapter 1: Cells and Tissues Cells and Tissues Carry out all chemical activities needed to sustain life Cells are the building blocks of all living things Tissues are groups of cells that are similar in
Water in the Body. Page Water = ~50 to 70% of body weight, depending on body fat % (higher body fat=lower water content)
 Water in the Body Page 373-375 Water = ~50 to 70% of body weight, depending on body fat % (higher body fat=lower water content) Major fluid compartments: Intracellular fluid: cytoplasm of cells is an aqueous
Water in the Body Page 373-375 Water = ~50 to 70% of body weight, depending on body fat % (higher body fat=lower water content) Major fluid compartments: Intracellular fluid: cytoplasm of cells is an aqueous
Anatomy & Homeostasis. Unit 5
 Anatomy & Homeostasis Unit 5 Main Ideas discuss with a buddy 2 What is Homeostasis? How is homeostasis different in single-celled organisms vs. multicellular organisms? What unique challenges to maintaining
Anatomy & Homeostasis Unit 5 Main Ideas discuss with a buddy 2 What is Homeostasis? How is homeostasis different in single-celled organisms vs. multicellular organisms? What unique challenges to maintaining
Homeostatic Regulation
 Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Slide 1. Slide 2. Slide 3. Learning Outcomes. Acid base terminology ARTERIAL BLOOD GAS INTERPRETATION
 Slide 1 ARTERIAL BLOOD GAS INTERPRETATION David O Neill MSc BSc RN NMP FHEA Associate Lecturer (Non Medical Prescribing) Cardiff University Advanced Nurse Practitioner Respiratory Medicine Slide 2 Learning
Slide 1 ARTERIAL BLOOD GAS INTERPRETATION David O Neill MSc BSc RN NMP FHEA Associate Lecturer (Non Medical Prescribing) Cardiff University Advanced Nurse Practitioner Respiratory Medicine Slide 2 Learning
Chapter 27: Fluid, Electrolyte, and Acid Base Balance
 Chapter 27: Fluid, Electrolyte, and Acid Base Balance Fluid, Electrolyte, and Acid Base Balance: An Overview, p. 995 Most of your body weight is water. Water accounts for up to 99 percent of the volume
Chapter 27: Fluid, Electrolyte, and Acid Base Balance Fluid, Electrolyte, and Acid Base Balance: An Overview, p. 995 Most of your body weight is water. Water accounts for up to 99 percent of the volume
The Urinary System PART B
 15 The Urinary System PART B PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Urinary Bladder Smooth,
15 The Urinary System PART B PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Urinary Bladder Smooth,
Interactions Among Animal Systems. Biology 10(A)
 Interactions Among Animal Systems Biology 10(A) Interactions Among Animal Systems Learning Objectives Identify major organ systems in animals Describe the interactions that occur among systems to carry
Interactions Among Animal Systems Biology 10(A) Interactions Among Animal Systems Learning Objectives Identify major organ systems in animals Describe the interactions that occur among systems to carry
Organ Systems (ch21-26) Practice Questions. Name:
 1. Which one of the following types of tissue stores fat in the body? A) blood B) cartilage C) bone D) adipose tissue E) fibrous connective tissue 2. Which of the following tissues does not match its function?
1. Which one of the following types of tissue stores fat in the body? A) blood B) cartilage C) bone D) adipose tissue E) fibrous connective tissue 2. Which of the following tissues does not match its function?
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + )
 Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration in body fluids Precise regulation of ph at
Chapter 20 UNIFYING CONCEPTS OF ANIMAL STRUCTURE AND FUNCTION
 Chapter 20 UNIFYING CONCEPTS OF ANIMAL STRUCTURE AND FUNCTION I. Life is based on many structural levels Levels of animal structure: Atoms and molecules Cells Tissues Organs Organ systems Organism: May
Chapter 20 UNIFYING CONCEPTS OF ANIMAL STRUCTURE AND FUNCTION I. Life is based on many structural levels Levels of animal structure: Atoms and molecules Cells Tissues Organs Organ systems Organism: May
Chapter 24 Lecture Outline
 Chapter 24 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright McGraw-Hill Education. Permission required for reproduction or
Chapter 24 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright McGraw-Hill Education. Permission required for reproduction or
Organs and Systems Organ: System:
 3.2 Organ Systems Organs and Systems Organ: a combination of several types of tissues working together to perform a specific function System: a group of tissues and organs that perform specific functions
3.2 Organ Systems Organs and Systems Organ: a combination of several types of tissues working together to perform a specific function System: a group of tissues and organs that perform specific functions
Advanced Anatomy and Physiology
 Wisconsin Indianhead Technical College 10806179 Advanced Anatomy and Physiology Course Outcome Summary Course Information Description Instructional Level Total Credits 4.00 Total Hours 80.00 Advanced Anatomy
Wisconsin Indianhead Technical College 10806179 Advanced Anatomy and Physiology Course Outcome Summary Course Information Description Instructional Level Total Credits 4.00 Total Hours 80.00 Advanced Anatomy
Chapter 16. Human Anatomy
 Chapter 16 Human Anatomy Each System we will examine: Structures types Problems or health concerns How to care for that system Skeletal System Made up of bones, joints, connective tissue Is the frame for
Chapter 16 Human Anatomy Each System we will examine: Structures types Problems or health concerns How to care for that system Skeletal System Made up of bones, joints, connective tissue Is the frame for
Name: Date: Block: Biology 12
 Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Acid - base equilibrium
 Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Laughter and the Body Systems
 Laughter and the Body Systems Laughter causes the lungs to pump out carbon dioxide, the eyes to cleanse themselves with tears, the muscles to relax, the flow of adrenaline to increase, and the cardiovascular
Laughter and the Body Systems Laughter causes the lungs to pump out carbon dioxide, the eyes to cleanse themselves with tears, the muscles to relax, the flow of adrenaline to increase, and the cardiovascular
HOMEOSTASIS & IMMUNITY Week Two Packet
 Ms. Scott HOMEOSTASIS & IMMUNITY Week Two Packet Packet Grade: / 9 Completed notes / 30 Completed Classwork / 30 Completed Homework / 10 Packet turned in on time / 1 Name and Class are filled in / 80 Total
Ms. Scott HOMEOSTASIS & IMMUNITY Week Two Packet Packet Grade: / 9 Completed notes / 30 Completed Classwork / 30 Completed Homework / 10 Packet turned in on time / 1 Name and Class are filled in / 80 Total
Chapter 20 Endocrine System
 Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Acid-Base Balance Dr. Gary Mumaugh
 Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Acid-Base Balance Dr. Gary Mumaugh Introduction Acid-base balance is one of the most important of the body s homeostatic mechanisms Acid-base balance refers to regulation of hydrogen ion (H + ) concentration
Cell membrane & Transport. Dr. Ali Ebneshahidi Ebneshahidi
 Cell membrane & Transport Dr. Ali Ebneshahidi Cell Membrane To enclose organelles and other contents in cytoplasm. To protect the cell. To allow substances into and out of the cell. To have metabolic reactions
Cell membrane & Transport Dr. Ali Ebneshahidi Cell Membrane To enclose organelles and other contents in cytoplasm. To protect the cell. To allow substances into and out of the cell. To have metabolic reactions
Body Systems Overview
 Body Systems Overview Body Systems work together: If you damage one system, you may damage several for example, smoking irritates the lungs and destroys the cells of the immune system Levels of Organization
Body Systems Overview Body Systems work together: If you damage one system, you may damage several for example, smoking irritates the lungs and destroys the cells of the immune system Levels of Organization
Fluid, Electrolyte, and Acid Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid Base Balance Bi 233 Body Water Content Largest component of the body Infants have low body fat, low bone mass, and are 73% or more water Healthy males are about
Chapter 26 Fluid, Electrolyte, and Acid Base Balance Bi 233 Body Water Content Largest component of the body Infants have low body fat, low bone mass, and are 73% or more water Healthy males are about
Transport across the cell membrane
 Transport across the cell membrane Learning objectives Body compartments ECF and ICF Constituents Lipid Bilayer: Barrier to water and water-soluble substances ions glucose H 2 O urea CO 2 O 2 N 2 halothane
Transport across the cell membrane Learning objectives Body compartments ECF and ICF Constituents Lipid Bilayer: Barrier to water and water-soluble substances ions glucose H 2 O urea CO 2 O 2 N 2 halothane
REVIEW: Section 1: Human Organization and the chemistry of life A) Chemistry of life I. Elements II. Atoms III. Matter Matter
 REVIEW: Section 1: Human Organization and the chemistry of life A) Chemistry of life I. Elements Cannot be broken down by chemical means and still retain the same chemical and physical characteristics
REVIEW: Section 1: Human Organization and the chemistry of life A) Chemistry of life I. Elements Cannot be broken down by chemical means and still retain the same chemical and physical characteristics
Tissue = groups of cells that are similar in structure and function
 Tissue = groups of cells that are similar in structure and function Types Epithelial - covering Connective - support Muscle - movement Nervous - control Membranes line body cavities and hold organs together
Tissue = groups of cells that are similar in structure and function Types Epithelial - covering Connective - support Muscle - movement Nervous - control Membranes line body cavities and hold organs together
Chapter 11 - Endocrine System
 Chapter 11 - Endocrine System 11.1 Introduction A. The endocrine system is made up of the cells, tissues, and organs that secrete hormones into body fluids. B. The body has two kinds of glands, exocrine
Chapter 11 - Endocrine System 11.1 Introduction A. The endocrine system is made up of the cells, tissues, and organs that secrete hormones into body fluids. B. The body has two kinds of glands, exocrine
Unit 2 Warm Ups. Equilibrium
 Unit 2 Warm Ups Equilibrium 1. Cell wall 2. Mitochondria 3. Chloroplast 4. Vesicle 5. Vacuole 6. Rough Endoplasmic Reticulum 7. Smooth Endoplasmic Reticulum 8. Cytoskeleton 9. Lysosomes 10.Cell Membrane
Unit 2 Warm Ups Equilibrium 1. Cell wall 2. Mitochondria 3. Chloroplast 4. Vesicle 5. Vacuole 6. Rough Endoplasmic Reticulum 7. Smooth Endoplasmic Reticulum 8. Cytoskeleton 9. Lysosomes 10.Cell Membrane
Subject: Biology for Biomedical Engineering Unit Sub Unit Course Content No of Marks Questions Introduction to Biology Animal Tissues Human Biology
 Question Pattern for Entrance Examinations Subject: Biology for Biomedical Engineering Unit Sub Unit Course Content No of Marks Questions A Introduction to Biology 1 1 Nature and Scope of Biology 1 1 2
Question Pattern for Entrance Examinations Subject: Biology for Biomedical Engineering Unit Sub Unit Course Content No of Marks Questions A Introduction to Biology 1 1 Nature and Scope of Biology 1 1 2
How Normal Body Processes Are Altered By Disease and Injury
 1 Chapter 4, General Principles of Pathophysiology Part 1 How Normal Body Processes Are Altered By Disease and Injury 2 How Cells Respond to Change and Injury 3 Pathology & Pathophysiology : the study
1 Chapter 4, General Principles of Pathophysiology Part 1 How Normal Body Processes Are Altered By Disease and Injury 2 How Cells Respond to Change and Injury 3 Pathology & Pathophysiology : the study
