The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: Lessons learned from lesioning studies
|
|
|
- Magnus Bradford
- 6 years ago
- Views:
Transcription
1 Physiology & Behavior 76 (2002) The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: Lessons learned from lesioning studies Larry L. Bellinger a, *,1, Lee L. Bernardis b,c,d a Department of Biomedical Sciences, Baylor College of Dentistry, 3302 Gaston Avenue, Dallas, TX 75246, USA b VA Western New York Healthcare System, Buffalo NY 14215, USA c Department of Medicine, Faculty of Medicine and Nutrition Program, State University of New York at Buffalo, Buffalo, NY 14215, USA d Department of Exercise, Physiotherapy and Nutrition, Faculty of Health Related Professions, State University of New York at Buffalo, Buffalo, NY 14215, USA Accepted 16 January 2002 Abstract This review article discusses the well-established role of the dorsomedial hypothalamic nucleus (DMN) in feeding, drinking and body weight (BW) regulation. DMN lesions (L) in both weanling and mature rats of both sexes produce hypophagia, hypodipsia and reduced ponderal and linear growth in the presence of normal body composition. The growth reduction is not due to a deficient secretion of growth hormone, insulin-like growth factor-1, thyroxine, triiodothyronine or insulin. DMNL rats actively defend their lower BW (BW settling point) by becoming either hyper- or hypophagic, depending on the experimental manipulation, thereby defending both lean and fat mass. They also regulate their 24-h caloric intake, but they may overeat during the first hour of refeeding following a fast, possibly due to a reduced ability to monitor blood glucose or to respond to cholecystokinin (CCK). 2-Deoxy-D-glucose (2DG) increases c-fos expression in orexin-a neurons in the DMN, and DMNL eliminated the orexigenic effect of 2DG. DMNL rats on high-fat diets do not get as obese as controls, which may be due to a reduction of DMN neuropeptide Y (NPY). Rats lacking DMN CCK-A receptors are obese and have increased expression of NPY in the DMN, supporting earlier data that CCK may act at the DMN to suppress food intake. Excitotoxin studies showed that loss of DMN cell somata, and not fibers of passage, is important in the development of the DMNL syndrome. The DMN is a site where opioids increase food intake and knife-cut studies have shown that fibers traveling to/from the DMN are important in this response. An interaction of glucose and opioids in DMN may also be involved in the control of food intake. DMN knife cuts interrupting fibers in the posterior and ventral directions additively produce the hypophagia and reduced linear and ponderal growth observed after DMNL. Ventral cuts may interrupt important connections with the arcuate nucleus. Lateral and posterior DMN cuts additively produce the hypodipsic effect seen after DMNL, but DMNL rats respond normally to all water-regulatory challenges, i.e., the hypophagia is not due to a primary hypodipsia. The DMN has been shown to be involved in the rat s feeding response to an imbalanced amino acid diet. These data show the DMN has an important role in many processes that control both food intake and BW regulation. D 2002 Elsevier Science Inc. All rights reserved. Keywords: Food intake; Water intake; Hormones; Growth hormone; Cholecystokinin; Opioids 1. Introduction A number of laboratories using molecular biology approaches have recently presented data supporting an * Corresponding author. Tel.: ; fax: address: lbellinger@tambcd.edu (L.L. Bellinger). 1 A member of the Texas A&M University System Health Science Center. important role for the dorsomedial hypothalamic nucleus (DMN) in the control of ingestion and body weight (BW) regulation. However, the first experiment demonstrating a role for the DMN in influencing ingestive behavior goes back almost 60 years. Brugger [1] in 1943 reported a voracious drive to eat in cats that were electrically stimulated between the fornix and mamillothalamic tract, which is the area that corresponds to the DMN. Later, Larsson [2], using sheep, demonstrated that stimulation of the DMN did indeed produce hyperphagia and that this increased intake /02/$ see front matter D 2002 Elsevier Science Inc. All rights reserved. PII: S (02)
2 432 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) Early electrolytic lesion studies Fig. 1. Coronal section through the hypothalamus of a nonlesioned control rat. DMN, dorsomedial hypothalamic nucleus; MTT, mamillothalamic tract; Fx, fornix; III, 3rd ventricle. Magnification 25. Luxol fast blue and cresyl violet were used. was not due to current spread [3] to the lateral hypothalamic area (LHA). These findings set the stage for a large series of lesioning studies, including electrolytic, excitotoxin and knife cuts, which explored the role of the DMN in ingestive behavior and BW regulation. It was suggested to the authors that writing a historical perspective of what has been learned from DMN lesion (L) studies would provide a foundation of understanding of how the DMN influences ingestive behavior and BW regulation. With the fundamental insight of these earlier studies future studies of the DMN, employing newer techniques will be able to more fully comprehend the overall significance of their findings. In 1963 Bernardis et al. [7] asked the question: Do areas of the hypothalamus affect the control of growth? The investigators were particularly interested in how hypothalamic areas might control growth hormone (GH) secretion (Figs. 1 and 2). They reported that a rather large lesion (1 ma for 10 s) that included the DMN resulted in rats that were hypophagic and hypodipsic, had reduced ponderal and linear growth, but had normal oxygen consumption. The above study was confirmed by Bernardis [8] in 1970, where the lesion size was reduced to include only the DMN. The DMNL rats had normal percentage of fat and lean body mass when compared to control animals. This differentiated the DMNL rats from LHA-lesioned rats that had reduced fat stores (see Refs. [9,10]). In a pair-feeding study, it was shown [11] (but see Ref. [12]) that control rats pair-fed to the hypophagic DMNL rats had a significantly reduced percentage of lean body mass, whereas the DMNL rats had a normal percentage of lean body mass. It was suggested that within the framework of the DMNL rat s somatic physiological potential, it is better equipped to exist with smaller amounts of substrate for its metabolism than is the caloric-restricted sham-operated animal. The virtual normal intermediary metabolism of the hypophagic/hypodipsic DMNL rats has been shown repeatedly [13,14]. Still, it was uncertain as to whether the growth retardation of the DMNL rat was due to a primary decrease in food intake or whether it was secondary to some other cause such as alteration of a growth-promoting hormone. In a study published in 1974 [15], DMNL rats were shown in three separate trials, where single samples were taken, to have normal plasma GH concentrations. Nevertheless, GH is secreted with an ultradian rhythm [16] and single samples do not provide absolute evidence that the secretion is normal (see Section 5). 2. Electrolytic lesion-associated considerations Before addressing what has been learned from electrolytic DMNL studies, several considerations have to be taken into account. One has to consider: (a) the type, i.e., iron, stainless-steel or platinum iridium and size of the electrode used; (b) whether anodal or cathodal currents were used; (c) the amount of current applied and the length of time it was applied; (d) the fact that lesions destroy both cell somata and fibers of passage; (e) the lesion may damage a distant site if it destroys a primary blood supply to that region; (f) that there is always a need for good histology to determine the location of the lesion, however, the exact extent of the lesion is sometimes difficult to determine as it expands and then contracts with time; (g) acute physiological changes are different from chronic changes; and (h) that in the chronic situation, there may have been reinnervation, rerouting of signals or regenerative sprouting resulting in a resumed physiological response or alternately in a unphysiological reaction [4 6]. Fig. 2. Coronal section through hypothalamus of representative DMN electrolytic-lesioned rat. Note the increased volume of the ventricular space resulting from DMN tissue loss. MTT, mamillothalamic tract; Fx, fornix; III, 3rd ventricle. Magnification 25. Luxol fast blue and cresyl violet were used.
3 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) Additional questions to be answered Several lines of research were developed around 1974 to investigate the cause(s) of the DMNL-induced hypophagia, hypodipsia and growth retardation. It needed to be determined whether DMNL: (a) produced a reduction in growthpromoting hormone(s); (b) produced a primary hypophagia without BW regulation, that is, did the lesion simply remove a feeding system; (c) produced a primary hypodipsia with a resultant hypophagia and reduced BW; (d) produced a change in a BW settling point ; (e) produced an overresponsiveness to satiety factors, e.g., cholecystokinin (CCK), bombesin, glucose; (f) produced an underresponsiveness to mechanisms that would increase ingestion, e.g., glucoprivation; and (g) was the DMNL syndrome produced by damage to cell bodies in the DMN or fibers of passage or both. The direction of efferent/afferent fiber tracts involved in the DMNL syndrome was unknown. A more complete understanding of the neural connections involved in the DMNL syndrome would allow for a better integration of the DMN into our overall understanding of the mechanisms that control ingestion and BW regulation. Finally, it was also unknown whether the DMN had a role in other ingestive regulatory responses, such as the rat s response to ingesting an imbalanced amino acid (Imb) diet. 5. Endocrine findings It was necessary to determine whether the DMNL syndrome was caused by lesion-induced alterations of the endocrine system. One study [17] found that male rats with DMNL had elevated plasma prolactin concentrations, whereas other studies [18,19] showed that DMNL rats had a disrupted diurnal secretion of corticosterone. These two changes were thought not to be the causes of the DMNL syndrome, because it had been reported [20] that prolactin did not affect feeding behavior or BW gain in male rats and both DMNL rats and obese ventromedial hypothalamic nuclei (VMN) lesioned (L) rats have disrupted diurnal corticosterone secretion rhythms [19]. Plasma insulin concentration in DMNL rats were usually found to be normal [13], but at times were reported to be either higher and lower than control animals [18]. The reason for these differences in plasma insulin concentration was attributed to the fact that DMNL rats have a slightly disrupted diurnal food intake rhythm, where they take a little more food during the light phase than control animals [13]. Therefore, the relationship of when the rat had its last meal and the timing of the blood sample would account for the discrepancy in plasma insulin concentrations. GH and triiodothyronine were sampled every 4 h around the clock and total secretions were integrated and calculated in DMNL and control rats [18]. It was again found that the GH values were comparable to the control animals, but the DMNL rats had slightly elevated triiodothyronine concentrations [18]. Other experiments have shown that both plasma triiodothyronine and thyroxine were normal in DMNL rats [14], whereas thyroxine was suppressed in sham-operated rats that were pair-fed to DMNL rats [12]. Also, as mentioned above [7], DMNL rats have been found to have normal oxygen consumption. Bernardis and Tannenbaum [21] reported that DMNL rats sampled 39 days after lesioning had a normal ultradian secretion of GH. However, waiting such a long interval between lesioning and GH determination may have missed early changes in GH secretion. Therefore, Byrd and Bellinger [22] performed a second ultradian study, in which jugular cannulas were implanted and 5 days later DMNL were produced. Seven days later, blood samples were taken, GH assayed by radioimmunoassay and ultradian GH secretion analyzed using a computer program for the determination of episodic/ultradian hormone secretion, whereas total GH secretion was determined using a bioquantification system. The analyses showed that GH baseline and peak secretions, as well as interpeak intervals, were similar in DMNL and sham-operated rats. These findings were important, since differences in pulsatile secretion of GH have been reported to affect growth rates [23]. These data also showed that at a time when the BWs of the DMNL and sham-operated rats were still diverging the lesioned animals had normal GH secretion profiles. Also in support of a normal GH secretion in DMNL rats were the findings that the plasma concentration of insulin-like growth factor-1 was not attenuated in male and female lesioned rats [18,24]. Taken as a whole, these endocrine studies strongly indicated that the hypophagia and growth retardation of DMNL rats was not due to an endocrine deficiency. 6. BW regulation The next set of questions that were asked was whether the DMNL solely damaged a feeding/drinking system with resultant hypophagia, hypodipsia and reduced BW gain or whether the DMNL produced a more complex change, i.e., did the lesion damage a feeding system and also produce a reset in the hypothesized BW settling point [25]. If the latter was true, the DMNL rat should defend its new lower BW by utilizing other undamaged feeding and satiety systems. To test this, four groups of rats were utilized: Two groups were ad libitum-fed and two groups were food restricted, such that, at the time of lesioning or sham operations, their BWs were below the predicted BW that would be obtained after DMNL in an ad libitum-fed animal [26]. One ad libitum-fed group and one restricted group were subjected to DMNL, and the remaining two groups were sham operated. The ad libitum-fed DMNL rats showed the typical lesion-induced hypophagia and lost BW compared to the ad libitum-fed sham-operated group. After about 20 days, the BWs of the ad libitum-fed lesioned rats stabilized on a lower growth curve. In sharp contrast to this, the restricted rats
4 434 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) after DMNL lesioning showed an immediate hyperphagia and their food intake remained higher than that of the ad libitum-fed group for 10 days. After DMNL, the restricted group immediately began to gain BW and approached the attenuated BW of the ad libitum-fed DMNL rats and began to parallel their weight gain. At the end of the 28-day study, body composition of the DMNL groups was comparable to the control animals. These data demonstrated that the DMNL rats not only actively regulated their BW, but also did so with normal body composition. When comparing the settling point changes produced by LHA lesions with that occurring after DMNL a distinct difference becomes apparent, i.e., in the nature of the settling point reference. Most of the weight loss in LHAlesioned rats is due to loss of body fat, which brings about what appears to be a lowered body fat settling point. DMNL lowers the rats BW, but their body composition, as a percentage of BW, is normal. Thus, the DMNL rats are regulating their food intake around what was termed a BW settling point (see Ref. [26] for further discussion of this concept). It was not believed that the DMNL lowered the rat s BW settling point by removing all the available ingestive systems, because the rats regulate their lower-than-normal BW by: (a) responding with a normal increase in food consumption over a 24-h period following caloric deficits [13]; (b) decreasing their spontaneous intake when given additional calories by stomach tube [13]; and (c) becoming hypophagic after their BW had been artificially elevated by feeding them a palatable diet and then returning them to a chow diet [27]; and (d) having normal meal patterns for their size [28]. Next a series of experiments were performed that typically make normal rats obese. These studies were conducted to determine whether DMNL rats with their lowered BW settling point still have the necessary neural pathways to respond normally to challenges of the hypothesized body fat settling point. DMNL rats given high fat or junk food diets did become obese compared to chow fed DMNL rats [29 31]. However, DMNL rats given a high-fat diet do not become as obese as control animals [29,30], whereas when fed other palatable diets, they can become as obese or even more obese than similarly fed sham-operated rats [27,31]. These data suggest that DMNL rats are susceptible at varying degrees to dietary-induced obesity (DIO). Recently, it was observed that C57BL/6 mice presented with a highfat diet to induce DIO showed a profound increase in the expression of the orexigenic peptide neuropeptide Y (NPY) in the DMN and VMN with a concurrent reduction in arcuate nucleus (ARC) NPY [32]. The authors postulated that it was conceivable that NPY neurons originating in the DMN and VMN directly or indirectly participate in the maintenance of energy homeostasis. It was also reported that C57BL/6 mice subjected to DIO show enhanced c-fos expression in the DMN, LHA and perifornical area [33]. The authors suggested that enhanced neural activity might be responsible, at least partially, for increased diet-induced weight gain. Similarly, mice susceptible to DIO were shown to have increased c-fos-like immunoactivity expression in the DMN and LHA when fed a high-fat diet, whereas mice resistant to DIO did not [34]. When these animals were returned to a low-fat diet, they lost BW and decreased c-fos-like activity in the DMN and LHA. The authors [34] noted the earlier findings that DMNL rats fed high-fat diets [29,30] do not get as obese as controls on the high-fat diet and quote the earlier observation [29,30] that DMNL offers some protection against the development of obesity caused by ingestion of a high fat diet. Xin et al. [34] suggested that in normal animals, increased neural activity in the DMN may contribute to the development of DIO. The data taken as a whole suggest that DMNL may attenuate high-fat induced obesity but do not completely eliminate it, therefore, other undamaged system(s) that can promote DIO must still be functional. Additional support for a role of DMN NPY in feeding behavior comes from the findings that suckling and lactating rats have high levels of DMN NPY (see Ref. [35]). Intracerebroventricular injection of NPY induces c-fos expression in the DMN [36], suggesting that sites other than the perifornical region and PVN are important in NPY s orexigenic effects. Ovariectomy (OVX) has been reported to increase BW gain [37] until a new higher weight [38] or body fat set point [39] was obtained. However, some [40], but not all [41,42] of this weight gain was due to expansion of adipose tissue. Part of the weight gain was attributed to an increase in food intake, while others suggested that it might be due to hypoactivity or an increase in efficiency of food utilization [43,44]. As noted above, DMNL was suggested to lower the BW settling point, whereas OVX was suggested to increase the separate body fat set point. Different neural mechanism should control these two processes. Therefore, an experiment was conducted to determine [45] whether the DMNL rats would respond to OVX like control animals with regard to: (a) food intake; (b) efficiency of food utilization; (c) increase in linear growth in the normally growth-stunted DMNL rats; and (d) would OVX increase the body fat set point comparably in both groups? Two groups of rats were DMNL and two groups were sham operated. The DMNL groups showed their typical hypophagia, reduced BW gain, reduced linear growth, but normal body composition. Fifty-six days later, one DMNL and one sham-operated group were OVX and the remaining groups sham-ovx. The two OVX groups immediately started to gain a comparable amount of BW, i.e., an 18% increase in their BW over their weight at OVX. The OVX- DMNL and sham-operated OVX groups showed a significant increase in linear growth at the end of the study, which was 56 days after OVX. Both OVX groups also showed an expansion of their adipose stores. Notably, the food intake of the OVX groups did not differ from their respective
5 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) control group. Both OVX groups showed large comparable increases in efficiency of food utilization. These data [45] suggested that even though the DMNL lowered the rat s BW settling point, they appeared to be fully capable of responding to other regulatory challenges, i.e., the loss of estrogens that increased BW (fat and lean body mass) and linear growth. This suggested that multiple mechanisms were indeed involved in the regulation of fat and lean body mass. As a side bar to this experiment, it should be noted by young investigators that at the present time, Medline searches go back only to Several important papers pertinent to the above experiment and our present understanding of how OVX affects growth and body composition were published in the 1910s, 1930s and 1940s [37,41,42]. The young investigator should be cautioned that important literature exists that is not in computer databases. 7. Short-term controls of feeding (glucoprivic, CCK, bombesin, orexin-a) A series of studies were conducted to determine whether the hypophagia following DMNL was due to the lesioned rat not responding to mechanisms that would increase food intake, e.g., hypoglycemia or whether the DMNL rat was overresponding to mechanisms that would decrease food intake, e.g., hyperglycemia, CCK or bombesin. When rats were given electrolytic DMNL and challenged by the intracellular glucoprivic agent 2-deoxy-D-glucose (2DG), they did not increase their food intake [46], whereas control animals showed large increases in food consumption over 4 h. The 2DG response and general behavioral findings were confirmed later by Dalton et al. [47] in mature DMNL rats. When DMNL were produced using the excitotoxin kainic acid (KA), which destroys cell somata without damaging fibers of passage, and the rats challenged with 2DG, they again did not increase their food intake. This demonstrated that cell bodies in the DMN and not fibers of passage were important in the 2DG response. Also, unlike control animals, rats with DMNL showed no suppression of food intake following glucose infusion after a 24-h fast [46]. Furthermore, rats with DMNL also had attenuated responses to hypoglycemic-induced feeding following insulin injection [48]. Since the DMN has both glucose receptor neurons and glucose sensitive neurons [13], the suggestion was made that the DMN contained a feeding system that was responsive to glucopenia [49]. The recently discovered neuropeptide orexin-a has been shown to increase food intake when injected intracerebroventricularly [50]. Orexin-A cell bodies, fibers and receptors are located in both the LHA and the DMN [51 53]. Orexin- A increases food consumption when injected directly into the DMN, LHA, PVN and perifornical region [54]. Nicotine suppresses food intake, and it has been proposed that orexin in the DMN may have a role in this process [55]. Recently, Briski and Sylvester [56] reported that after giving 2DG, dual label cells for orexin-a and Fos were found in the DMN and LHA. The authors consider that these neurons may function within central pathways that govern adaptive processes to glucopenia. These data all support the original findings some 20 years ago [46,49] that the DMN contained neurons that responded to glucoprivation and that their destruction by electrolytic lesions or KA lesions removed a feeding system that may be partly responsible for the hypophagia observed in DMNL rats. However, it needs to be stressed that DMNL rats can competently regulate their, albeit lower than normal, 24-h caloric intake and, thus, their lack of response to glucoprivic feeding cannot be the sole mechanism responsible for their hypophagia [49]. The hypophagia present after DMNL could be caused by a lesion-induced overresponsiveness to certain satiety agents. Cholecystokinin-8 (CCK-8) and bombesin are peptides that are reported to produce physiological satiety [57]. CCK may work both peripherally or centrally to cause satiety. The DMN has both CCK cell bodies and fiber tracts [58] and could be a central site of CCK action, whereas the LHA is thought to be a central site where bombesin exerts its satiety effects [59]. When rats with electrolytic DMNL were given CCK-8 injection, they did not show the customary attenuation of feeding [60,61] that the control rats displayed. On the other hand, rats with DMNL did show the normal attenuation of feeding when injected with bombesin [60]. Therefore, rats with DMNL do not show a nonspecific lack of response to satiety peptides in general. Furthermore, the hypophagia shown by DMNL rats cannot be attributed to overresponsiveness to these satiety peptides, as their response was either attenuated or normal. In a later experiment, the DMNL syndrome was produced by ibotenic acid (IBO), which is an excitotoxin that destroys cell bodies (Fig. 3) but does not damage fibers of passage [62]. When the DMN IBO lesioned rats were tested with CCK-8, they showed a suppression of intake, but there was a trend for them to be less responsive than control animals. Two possibilities presented themselves. The first, which relates to a limitation of excitotoxin lesions, is that not all cell bodies are destroyed using this technique. While in this study, there was widespread loss of cell bodies, some cells remained, thus leading to the possibility that all cells responsive to CCK-8 may not have been destroyed. A second possibility was that the electrolytic DMNL destroyed fibers of passage involved in the CCK-8 response, which were spared by the IBO lesion. However, Blevins et al. [63] recently injected CCK-8 into a number of brain regions and the regions that were most responsive in suppressing feeding were the nucleus tractus solitarius and the DMN. Thus, it is possible that cell bodies that were spared by the IBO lesioning were responsive to CCK and the DMN is indeed one site where CCK can suppress feeding behavior. Further support for a direct role of CCK in the DMN comes from Bi et al. [64] who reported that Otsuka Long Evans Tokush-
6 436 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) Fig. 3. Coronal section through hypothalamus of representative rat infused with ibotenic acid into the DMN. Note the increased volume of the ventricular space resulting from DMN tissue loss. MTT, mamillothalamic tract; Fx, fornix; III, 3rd ventricle. Magnification 25. Luxol fast blue and cresyl violet were used. ima Fatty (OLETF) rats lacking CCK-A receptors are hyperphagic, obese and diabetic. The authors found that the expression of DMN NPY was increased in the OLETF rats. As noted above [58], the DMN contains CCK cell bodies and fibers. The DMN also contains CCK-A receptors [65,66] and expresses CCK-A receptor mrna [67]. The above authors [64] suggested that CCK acts on CCK-A receptors in the DMN to suppress NPY expression. The etiology of OLEFT hyperphagia and obesity may result from a lack of CCK-A receptors and an overexpression of the orexigenic peptide NPY. The above data suggest that the DMN may also contain a satiety system in addition to having feeding/drinking system(s) [49,60 62,68]. Again, it must be stressed that while the DMNL rat shows some deficits in its ability to regulate short-term food intake, it competently regulates its 24-h caloric intake [13,14]. However, the DMNL rats attenuated response to short term regulators, e.g., glucose and CCK, may explain why the DMNL rat eats more than control animals during the first hour following a 24-h fast [49,62]. 8. Opioids The DMN contains cell bodies and/or fiber tracts of a number of opioids including enkephalin, b-endorphin, dynorphin and others (see Ref. [13]). Injection of b-endorphin into the medial hypothalamic area (see Ref. [69]) has been reported to stimulate feeding. In two studies [61,68], DMNL and control rats were injected with the opioid antagonist naloxone. Naloxone suppressed food and water intake in sham-operated animals, whereas food intake was not suppressed at any dose in DMNL rats. The effect on water intake was more variable in DMNL rats, being either depressed or not affected. It was suggested that the DMN might well be a site of opioid receptors or pathways that make up a system that inhibits a satiety system with a resultant increase in ingestion [61]. Destruction of the DMN would remove the inhibition to the satiety system and result in the DMNL-induced hypophagia. As predicted earlier [26], since DMNL rats are not aphagic, other feeding system(s) must be left intact. In support of these naloxone data, Stanley et al. [69] reported increased feeding following direct infusion of opioids into the DMN. Recently, it was reported that rats with knife cuts posterior to the DMN, which were also hypophagic, did not show food intake suppression following naloxone injection [68]. This suggested that an efferent/afferent opioid system(s) traveled to/from that direction. Rats with knife cuts ventral to the DMN, which were also hypophagic, overresponded to the food intake-suppressive effects of naloxone. Carr et al. [70] have also suggested a role for DMN opioids in the control of feeding behavior. They reported that the orexigenic neurotransmitter NPY from the ARC may modulate DMN-induced feeding and that a feedback loop may exist. It is possible that the enhanced naloxone suppression of food intake in the DMN ventral knife-cut group was due to loss of afferent/efferent fibers between the ARC and the DMN. Finally, a link between glucoprivic-induced feeding in the DMN [46,49] and opioids [61] has been provided [71]. 2DG-induced Fos expression in DMN and PVN neurons was reduced when mu and kappa antagonist were given. The authors suggested that interaction of opioids with these two feeding related structures was necessary for maximal glucoprivic activation of the Fos signaling pathway. 9. Excitotoxin lesions It was unknown whether the effects induced by the electrolytic DMNL were due to loss of cell bodies in the DMN, or fibers of passage, or both. This question was addressed by first using the excitotoxin KA [49] and later IBO [62,72] to make DMNL. As noted above (Fig. 3), these excitotoxins destroy cell bodies while sparing afferent fibers of passage [73,74]. It should be recalled that one limitation of excitotoxin lesions is that the agents do not destroy all cell bodies and that KA and IBO can cause differential cell death [73,74]. Lastly, KA can diffuse from the site of injection and affect distant sites [73,74]. Young adult male rats with IBO lesions of the DMN [62,72] showed significant reduced food and water intake, BW and linear growth, although they had normal body composition. When the data [62] were analyzed for effect size, the r for food intake=2.05, water intake=3.98, BW=2.67 and change in linear growth=1.0. When fast-growing weanling rats were given DMNL [12], the effect size for linear growth was increased to 2.0. These findings indicate a large real lesion effect on the measured parameters. These changes following DMN excitotoxin lesions were reminiscent of
7 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) those observed after electrolytic DMNL and demonstrated that it was the loss of cell bodies that was producing these aspects of the DMNL syndrome. After a 24-h fast, both the IBO DMNL and sham-operated rats became hyperphagic, with the DMNL rats eating more than the control rats during the first hour of refeeding. Upon refeeding, the 24-h food intake of both groups was similar when expressed as a percentage of their prefasting intake. The intake of both groups returned to their baseline intakes over several days and both groups regained their lost BW in a comparable manner. These data showed that the normally hypophagic DMNL rat subjected to caloric restriction, which lowers its BW, had the capacity upon refeeding to become hyperphagic and defend its, albeit lower than normal, BW. To do this, the DMNL rats must have used undamaged feeding systems. Additionally, the finding that the IBO DMNL rats consumed more of the diet during the first hour of refeeding compared to the control animals may reflect the above noted deficits, e.g., CCK and glucose responsiveness, that the DMNL rats have in their short-term regulation of food intake. The observation that the IBO DMNL rats competently regulated their 24-h food intake supported the earlier findings [13] that the DMNL rat s lower intake is part of a stratagem to regulate their BW at new lower BW settling point [26]. Furthermore, the data showed that loss of cell bodies in the DMN is responsible for their new lower BW settling point. Rats with KA DMNL showed a more severe attenuation of food and water intake than that observed after electrolytic or IBO lesions. As noted above, it was suggested that the DMN contains both feeding drinking and satiety neuronal systems and KA and IBO can differentially destroy neurons [73,74]. It is possible [49] that the KA DMNL may have selectively removed the feeding drinking system(s) leaving the satiety system(s) unchecked, which produced a period of severe hypophagia and adipsia [49]. On the other hand, electrolytic DMNL would destroy both systems (it was hypothesized that the feeding drinking system(s) would have to be slightly more physiologically prominent than the satiety system), thus setting the ingestion at a lower level. Histology showed that IBO destroyed more DMNL neurons than that occurring after KA lesions. Therefore, it is possible that IBO destroyed both the feeding drinking system(s) and the satiety system(s), this being more reminiscent of electrolytic lesions [13]. It must be noted that after IBO DMNL, some neurons in the DMN still remained intact [62,72]. 10. Water intake regulation Rats with electrolytic or KA or IBO lesions are hypodipsic and consume less water per 100 g BW than shamoperated animals [49,62,75]. These data suggested that the loss of cell bodies in the DMN and not fibers of passage was responsible for the hypodipsia observed after DMNL. Despite the DMNL rats lower water intake per 100 g BW, they have normal plasma sodium, potassium and osmolality, which indicated that the DMNL rats were normally hydrated. It was previously suggested that the DMNL rats lower their water intake toward the minimum required for fluid balance by decreasing their secondary drinking, i.e., excess water intake above maintenance [62]. In support of this was a large study that investigated water intake regulation in DMNL rats [75]. DMNL rats were subjected to challenges of intracellular thirst, extracellular thirst, a combination of both and administration of angiotensin II. The DMNL rats responded to the thirst challenges either normally or more robustly than sham-operated animals. The latter findings may be due to the lack of secondary drinking in the DMNL rats that then would be more sensitive to any changes in body fluid regulation. In another study [62] during the first hour of rehydration, following 24 h of water deprivation, IBO DMNL rats consumed slightly more water than the sham-operated rats when intake was expressed as a percentage of baseline consumption (a percentage type of comparison was necessary, because the DMNL rats were hypodipsic in absolute terms compared to sham-operated rats). However, a similar comparison after 24 h of rehydration showed that the two groups consumed a normalized comparable amount of water. The above data as a whole demonstrated that the DMNL rat competently regulated its water intake. To further reiterate, the excitotoxin lesion studies [49,62,72] demonstrated that most aspects of the classical electrolytic DMNL (destroying cell bodies and afferent fibers of passage) could be attributed to loss of nerve somata in the DMN. 11. Fiber tracts, neurotransmitters and knife-cut studies The afferent and efferent neural connections of DMN somata have been explored in a number of studies [76 81]. However, nothing was known of the functional neural connections with other brain structures that may be involved in the DMNL syndrome. This was addressed in two recent knife-cut studies [68,72]. However, several items have to be considered when evaluating knife-cut studies. Many earlier studies used nonretractable knives that damaged tissue beyond the target area, and they used knives of a very large diameter that destroyed a great deal of tissue. Newer knives are retractable and are very small in width, which prevents unwanted tissue damage. If ascending and descending fiber tracts pass through the target area, one does not readily know if the resultant physiological changes are due to damage of the ascending, descending or both tracts. It is also possible that the knife cut could remove both stimulatory and inhibitory pathways with no resultant change in the measured parameter. With these considerations, knife cuts were made anterior, posterior, dorsal, ventral and lateral to the DMN [68,72]. Rats with posterior (Fig. 4) and ventral (Fig. 5) cuts showed
8 438 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) Fig. 4. Sagittal section through a representative rat that received a knife cut posterior to the DMN. The arrow shows the location of the cut, which is <240 mm from the midline. MTT, mamillothalamic tract; Fx, fornix; III, 3rd ventricle. Magnification 25. Luxol fast blue and cresyl violet were used. a reduction in food consumption, BW gain and linear growth. The magnitude of the reductions with the posterior and ventral cuts was about half that observed with electrolytic lesion or excitotoxin lesions, but the cumulative changes of both cuts approached that observed after destruction of the DMN. Despite the lower BWs of the posterior and ventral cut groups they had normal body composition. Rats with ventral and posterior cuts elevated their food intake following 24 h of food deprivation again showing they have the ability to regulate caloric intake and BW. Rats with posterior and lateral (Fig. 6) cuts were hypodipsic, but the magnitude was less than that observed after electrolytic or excitotoxin lesions. However, the cumulative changes were similar to that observed after electrolytic or excitotoxin lesioning. Ventral, posterior and lateral cuts would have Fig. 5. Coronal section through a representative rat that received knife cuts ventral to the DMN. The arrows show the location of the cuts. MTT, mamillothalamic tract; Fx, fornix; III, 3rd ventricle. Magnification 25. Luxol fast blue and cresyl violet were used. Fig. 6. Coronal section through a representative rat that received knife cuts lateral to the dorsomedial hypothalamic nucleus. The arrows show the location of the cuts. MTT, mamillothalamic tract; Fx, fornix; III, 3rd ventricle. Magnification 40. Luxol fast blue and cresyl violet were used. interrupted connections (see Refs. [68,72] for a complete description of neural connections) with many brain areas that have been shown to be involved in the control of feeding and drinking behavior and BW. One important area that ventral cuts deafferenated was the ARC. Our understanding of hypothalamic control of feeding behavior has recently been enhanced by a greater delineation of the neural circuitry involved in this process (see Ref. [82] for a review). The ARC has neurons that release a-melanocyte-stimulating hormone (a-msh), which is cleaved from the precursor proopiomelanocortin (POMC), at various brain sites including the PVN, LHA and perifornical region. Once released, a-msh attaches to two melanocortin receptors (MC3/MC4), where it inhibits feeding behavior. Colocalized with POMC is the peptide cocaine- and amphetamine-regulated transcript (CART), which also decreases feeding behavior. The ARC also has neurons that release NPY and agouti-related protein (AgRP) in the PVN, LHA and perifornical region. NPY interacts with Y1 and Y5 receptor types to promote an increase in feeding. AgRP is an antagonist to the MC3 and MC4 receptors, and in doing so, it promotes an increase in feeding. The above systems are influenced by leptin that is released from peripheral white adipose tissue and by pancreatic insulin. High plasma levels of leptin or insulin inhibit the NPY/AgRP systems, while stimulating the POMC/CART systems, whereas the reverse occurs during low plasma levels of leptin or insulin. Interestingly, the newly discovered orexigenic substance melanin-concentrating hormone (MCH) is colocalized with the anorectic peptide a-msh in the ARC and the orexigenic peptide orexin-a in the LHA [51,83,84]. Recently, the DMN has been shown to contain: a-msh fibers and MC4R; CART cell bodies and fiber tracts; MCH
9 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) cells and fiber; orexin cells and fibers; and AgRP fibers [51 53,83,85,86]. CART given intracerebroventricularly suppresses food intake and causes abnormal behavior, however, when CART was discretely injected into several brain areas including the DMN it increased feeding behavior [87]. The DMN has been shown to have the leptin long form receptor [88] and the anorectic compound leptin induces c-fos expression in CART neurons in the DMN, PVN and ARC [89]. The DMN cells also express the MC4R [90] and are innervated by ARC a-msh neurons. Food restriction has been shown to increase the MC4R in the DMN, ARC and VMN, due to a decrease in a-msh release. Fatty Zucker fa/fa rats also have increased MC4R expression in these same areas due to decreased a-msh release, whereas rats with DIO have decreased MC4R expression due to a-msh release trying to limit intake [90]. When a a-msh agonist was infused into the DMN and PVN, it suppressed food intake, whereas infusion of AgRP into the DMN and PVN increased food intake [91 93]. The agouti obese A(y) and the MC4R knockout mice have been reported to have high concentrations of the orexigenic NPY peptide in the DMN [94]. The DMN was also shown to contain prolactinreleasing peptide neurons, receptors and fibers [95]. Recently, it was demonstrated that injection of prolactinreleasing peptide into the DMN suppresses food intake, possibly by increasing the release of a-msh in the DMN [92]. Glucagon-like peptide 2 with cell bodies in the nucleus of the solitary tract has also been shown to decrease food intake and the DMN has been suggested to be its site of action in the hypothalamus [96]. The hypophagic-producing DMN ventral cuts would have disrupted some of these complex interactions, but it remains to be determined whether these pathways are contributing to the development of the DMNL syndrome Fig. 8. Coronal section through a representative rat that received knife cuts dorsal to the dorsomedial hypothalamic nucleus. The arrows show the location of the cuts. MTT, mamillothalamic tract; Fx, fornix; III, 3rd ventricle. Magnification 60. Luxol fast blue and cresyl violet were used. [68,72]. Additionally, since destruction of DMN nerve somata produced the DMNL syndrome, ventral and posterior knife cuts may have damaged efferent pathways [49,62]. It is of interest that anterior (Fig. 7) and dorsal (Fig. 8) cuts would have removed many, but not all, connections with the PVN, yet, these cuts did not affect food or water intake or BW gain, however, it is possible that a combination cut would have to be made to observe physiological changes. Fig. 7. Sagittal section through a representative rat that received a knife cut anterior to the DMN. The arrows show the location of the cut, which is <240 mm from the midline. VMN, ventromedial hypothalamic nucleus; PVN, paraventricular nucleus. Magnification 25. Luxol fast blue and cresyl violet were used. Fig. 9. Some agents thought to increase (solid lines) and decrease (broken lines) food intake (FI), while acting at the DMN. AgRp= agouti-related protein, a-msh= a-melanocyte-stimulating hormone, CCK=cholecystokinin, CART=cocaine- and amphetamine-regulated transcript, MCR4= melanocortin-4 receptor, NPY= neuropeptide Y, POMC=proopiomelanocortin cell and 2DG =2-deoxy-D-glucose. CART has been reported to both increase and decrease food intake (see text). Insulin, which is not shown, probably increases food intake indirectly by decreasing the concentration of blood glucose. Filled-in circles are neurons in the DMN that express the neurotransmitter noted.
10 440 L.L. Bellinger, L.L. Bernardis / Physiology & Behavior 76 (2002) Regulation of Imb diet intake Rats given an Imb diet go through three different response phases [97]. The first phase is recognition that they are eating an Imb diet and occurs during the first 3 h of eating the Imb diet. Recognition is followed by a decreased intake of the diet. The second phase is the development of a conditioned taste aversion, which occurs during the first day s consumption of the Imb diet. The third phase is an adaptation to the Imb diet over several days. The anterior piriform cortex has been shown to play an important role in the Phase 1 recognition of the Imb diet, the medial amygdala has a probable role in the Phase 2 development of the conditioned taste aversion and the hepatic vagus nerve has a role in the Phase 3 adaptation to the Imb diet [98]. Other brain areas involved in the rat s response to an Imb diet were explored using c-fos [99], and the results showed that the Imb diet increased c-fos expression in the DMN. It was subsequently shown that electrolytic DMNL initially prevented the rats recognition (Phase 1) that they were ingesting an Imb diet. The DMNL did not prevent a subsequent suppression of Imb diet intake during Phase 2. Clearly, different neural pathways were involved in the rats Phase 1 and Phase 2 responses. Lastly, the DMNL lesions attenuated Phase 3 adaptation to the Imb diet [100]. It was then shown [72] in a series of studies employing either electrolytic lesions, IBO lesions and knife cuts that transection of fibers of passage through the DMN are necessary for the early detection that the diet being consumed is an Imb diet. Multiple fiber pathways to/from the DMN are involved in the recognition phase as cuts anterior, dorsal, ventral and lateral, but not posterior, attenuated the rat s ability to detect that it was consuming an Imb diet. Cutting fibers entering/ leaving the DMN from the anterior direction attenuated somewhat the development of Phase 2. The study also showed [72] that DMN nerve somata were required for adaptation to the Imb diet. The available data show that the DMN directly or indirectly may have a role in all three phases of the rat s response to an Imb diet. 13. Summary The above foundational findings based on lesions produced by electrolytic, excitotoxin and knife-cut procedures clearly indicate that this technique was well suited to clarify many aspects of the functions of the DMN in the areas of food intake control, drinking and BW regulation. Subsequent and more recent studies (Fig. 9) have supported the earlier suggestions that this hypothalamic locus plays a crucial role in the homeostatic control of ingestive behavior and BW regulation. Finally, after the talk, Gerard Smith asked me how I got the DMN phoenix to fly again. I guess my reply would be that an important role of the DMN in the control of feeding behavior and BW regulation has been there for some time, but it is flying with a little less stealth these days. References [1] Brugger M. Fresstrieb als hypothalamisches symptom. Helv Physiol Acta 1943;1: [2] Larsson S. On the hypothalamic organization of the nervous mechanisms regulating food intake. Acta Physiol Scand 1954;32(Suppl 115):7 61. [3] Delgado JMR, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol 1953; 172: [4] Cox JR, Gisi JM, Wolf G. Deposit free electrolytic brain lesions: methodologic and histologic observations. Physiol Behav 1977;18: [5] Gold RM. Anodal electrolytic brain lesions: how current and electrode metal influence lesion size and hyperphagiosity. Physiol Behav 1975;14: [6] Moore RY. Surgical and chemical lesion techniques. In: Iversen L, Iversen S, Synder S, editors. Handbook of psychopharmacology. Chemical pathways in the brain, vol. 9. New York: Plenum, p [7] Bernardis LL, Box BM, Stevenson JAF. Growth following hypothalamic lesions in the weanling rat. Endocrinology 1963; 72: [8] Bernardis LL. Participation of the dorsomedial hypothalamic nucleus in the feeding center and water intake circuitry of the weanling rat. J Neuro-Visc Relat 1970;31: [9] Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci Biobehav Rev 1993;17: [10] Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev 1996;20: [11] Bernardis LL, Goldman JK. Growth and metabolic changes in weanling rats with lesions in the dorsomedial hypothalamic nuclei. Exp Brain Res 1972;15: [12] Bellinger LL, Castonguay TW, Bernardis LL. Hormone and somatic changes in rats pair-fed to growth retarded dorsomedial hypothalamic nuclei-lesioned rats. Brain Res Bull 1994;34: [13] Bernardis LL, Bellinger LL. The dorsomedial hypothalamic nucleus revisited: 1986 update. Brain Res Rev 1987;12: [14] Bernardis LL, Bellinger LL. The dorsomedial hypothalamic nucleus revisited: 1998 update. Proc Soc Exp Biol Med 1998;218: [15] Bernardis LL. Normal plasma growth hormone levels in growthretarded weanling rats with dorsomedial hypothalamic lesions. Exp Brain Res 1974;19: [16] Tannenbaum GS, Martin JB. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 1976;98: [17] Bernardis LL, Bellinger LL. Production of weanling rat ventromedial and dorsomedial hypothalamic syndromes by electrolytic lesions with platinum iridium electrodes. Neuroendocrinology 1976;22: [18] Bellinger LL, Bernardis LL, McCuster RH, Campion DR. Plasma hormone levels in growth-retarded rats with dorsomedial hypothalamic lesions. Physiol Behav 1985;34: [19] Bellinger LL, Bernardis LL, Mendel VE. Effect of ventromedial and dorsomedial hypothalamic lesions on circadian corticosterone rhythms. Neuroendocrinology 1976;22: [20] Pfaff D. Sex differences in food intake changes following pituitary growth hormone or prolactin injections. Proceedings, 77th Annual Convention of the American Psychology Association. Boston, MA; Washington, DC: American Psychology Association, p [21] Bernardis LL, Tannenbaum GS. Failure to demonstrate disruption of
CNS Control of Food Intake. Adena Zadourian & Andrea Shelton
 CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
Internal Regulation II Energy
 Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Chapter 12. Ingestive Behavior
 Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
Copyright 2017 The Guilford Press
 This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
Motivation 1 of 6. during the prandial state when the blood is filled
 Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
LESSON 3.3 WORKBOOK. How do we decide when and how much to eat?
 Appetite The psychological desire to eat, driven by feelings of pleasure from the brain. Hunger The biological or physiological need to eat, caused by a release of hormones from the digestive tract. LESSON
Appetite The psychological desire to eat, driven by feelings of pleasure from the brain. Hunger The biological or physiological need to eat, caused by a release of hormones from the digestive tract. LESSON
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction. Laura Gunter
 Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Ingestive Behavior: Feeding & Weight Regulation. Hypovolemic vs. Osmotic Thirst
 Ingestive Behavior: Feeding & Weight Regulation 1 Hypovolemic Thirst Receptors, CNS, Responses Salt Appetite Digestive components Glucose Homeostasis: Insulin & Glucagon Diabetes Mellitus 1 & 2 CNS Hypothalamic
Ingestive Behavior: Feeding & Weight Regulation 1 Hypovolemic Thirst Receptors, CNS, Responses Salt Appetite Digestive components Glucose Homeostasis: Insulin & Glucagon Diabetes Mellitus 1 & 2 CNS Hypothalamic
Ingestive Behaviors 33. Introduction. Page 1. control and story lines. (a review of general endocrinology) Integration (or the basic reflex arc model)
 Ingestive Behaviors 33 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Ingestive Behaviors 33 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Hypothalamus. Small, central, & essential.
 Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
1 Neuroregulation of Appetite
 Chapter 1 / Neuroregulation of Appetite 3 1 Neuroregulation of Appetite Ofer Reizes, PhD, Stephen C. Benoit, PhD, and Deborah J. Clegg, PhD CONTENTS INTRODUCTION THE DUAL-CENTERS HYPOTHESIS CONTROL OF
Chapter 1 / Neuroregulation of Appetite 3 1 Neuroregulation of Appetite Ofer Reizes, PhD, Stephen C. Benoit, PhD, and Deborah J. Clegg, PhD CONTENTS INTRODUCTION THE DUAL-CENTERS HYPOTHESIS CONTROL OF
Hormonal Regulation of Food Intake
 Physiol Rev 85: 1131 1158, 2005; doi:10.1152/physrev.00015.2004. Hormonal Regulation of Food Intake SARAH STANLEY, KATIE WYNNE, BARBARA McGOWAN, AND STEPHEN BLOOM Endocrine Unit, Imperial College Faculty
Physiol Rev 85: 1131 1158, 2005; doi:10.1152/physrev.00015.2004. Hormonal Regulation of Food Intake SARAH STANLEY, KATIE WYNNE, BARBARA McGOWAN, AND STEPHEN BLOOM Endocrine Unit, Imperial College Faculty
FLASH CARDS. Kalat s Book Chapter 10 Alphabetical
 FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
Chapter 1. General introduction. Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815
 Chapter 1 General introduction Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815 Chapter 1 B. Tiesjema, 2007 GENERAL INTRODUCTION NEURAL CIRCUITS INVOLVED IN ENERGY BALANCE
Chapter 1 General introduction Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815 Chapter 1 B. Tiesjema, 2007 GENERAL INTRODUCTION NEURAL CIRCUITS INVOLVED IN ENERGY BALANCE
Chapter 24 Cholesterol, Energy Balance and Body Temperature. 10/28/13 MDufilho
 Chapter 24 Cholesterol, Energy Balance and Body Temperature 10/28/13 MDufilho 1 Metabolic Role of the Liver Hepatocytes ~500 metabolic functions Process nearly every class of nutrient Play major role in
Chapter 24 Cholesterol, Energy Balance and Body Temperature 10/28/13 MDufilho 1 Metabolic Role of the Liver Hepatocytes ~500 metabolic functions Process nearly every class of nutrient Play major role in
Ingestive Behaviors 21. Introduction. Page 1. control and story lines. (a review of general endocrinology) Integration (or the basic reflex arc model)
 Ingestive Behaviors 21 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Ingestive Behaviors 21 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Neurobiology of food intake in health and disease
 Neurobiology of food intake in health and disease Gregory J. Morton, Thomas H. Meek and Michael W. Schwartz Abstract Under normal conditions, food intake and energy expenditure are balanced by a homeostatic
Neurobiology of food intake in health and disease Gregory J. Morton, Thomas H. Meek and Michael W. Schwartz Abstract Under normal conditions, food intake and energy expenditure are balanced by a homeostatic
Motility Conference Ghrelin
 Motility Conference Ghrelin Emori Bizer, M.D. Division of Gastroenterology/Hepatology November 21, 2007 Ghrelin: Basics Hormone produced by the A-like A endocrine cells in the oxyntic mucosa (stomach body
Motility Conference Ghrelin Emori Bizer, M.D. Division of Gastroenterology/Hepatology November 21, 2007 Ghrelin: Basics Hormone produced by the A-like A endocrine cells in the oxyntic mucosa (stomach body
Νευροφυσιολογία και Αισθήσεις
 Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 16 Κίνητρα Συμπεριφοράς ή Υποκίνηση (Motivation) Introduction Types of behavior Unconscious reflexes Voluntary
Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 16 Κίνητρα Συμπεριφοράς ή Υποκίνηση (Motivation) Introduction Types of behavior Unconscious reflexes Voluntary
Central nervous system control of food intake
 insight review article Central nervous system control of food intake Michael W. Schwartz*, Stephen C. Woods, Daniel Porte Jr*, Randy J. Seeley & Denis G. Baskin* Departments of Medicine* and Biological
insight review article Central nervous system control of food intake Michael W. Schwartz*, Stephen C. Woods, Daniel Porte Jr*, Randy J. Seeley & Denis G. Baskin* Departments of Medicine* and Biological
Temperature, Regulation, Thirst, and Hunger
 PSYB64 Lecture 6 Temperature, Regulation, Thirst, and Hunger 1. Homeostasis 2. Temperature 3. Thirst 4. Hunger 5. Obesity & Hunger Disorders HOMEOSTASIS Homeostasis: Physiological equilibrium Motivation:
PSYB64 Lecture 6 Temperature, Regulation, Thirst, and Hunger 1. Homeostasis 2. Temperature 3. Thirst 4. Hunger 5. Obesity & Hunger Disorders HOMEOSTASIS Homeostasis: Physiological equilibrium Motivation:
Metabolic Programming. Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 Metabolic Programming Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD nutritional stress/stimuli organogenesis of target tissues early period critical window consequence of stress/stimuli are
Metabolic Programming Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD nutritional stress/stimuli organogenesis of target tissues early period critical window consequence of stress/stimuli are
Leptin Intro/Signaling. ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph
 Leptin Intro/Signaling ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph Overview Intro to Leptin Definition & Sources Physiology Bound vs. Free Receptors Signaling JAK/STAT MAPK PI3K ACC Experimental findings
Leptin Intro/Signaling ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph Overview Intro to Leptin Definition & Sources Physiology Bound vs. Free Receptors Signaling JAK/STAT MAPK PI3K ACC Experimental findings
Figure 1: The leptin/melanocortin pathway Neuronal populations propagate the signaling of various molecules (leptin, insulin, ghrelin) to control
 Leptin Deficiency Introduction The leptin/melanocortin pathway plays a key role in the hypothalamic control of food intake. It is activated following the systemic release of the adipokine leptin (LEP)
Leptin Deficiency Introduction The leptin/melanocortin pathway plays a key role in the hypothalamic control of food intake. It is activated following the systemic release of the adipokine leptin (LEP)
Ghrelin mediates stressinduced. behavior in mice. Chuang et al 2011 L3: Love, Lust, Labor
 Ghrelin mediates stressinduced food-reward behavior in mice Chuang et al 2011 L3: Love, Lust, Labor Agenda Introduction What is Ghrelin? Previous Models New model Methods Results Discussion Conclusion
Ghrelin mediates stressinduced food-reward behavior in mice Chuang et al 2011 L3: Love, Lust, Labor Agenda Introduction What is Ghrelin? Previous Models New model Methods Results Discussion Conclusion
Insulin-Leptin Interactions
 Insulin-Leptin Interactions Ahmed S., Al-Azzam N., Cao B. Karshaleva B., Sriram S., Vu K. If you understand a system, you can predict it. Agenda - Energy homeostasis Overview of leptin and insulin Signaling
Insulin-Leptin Interactions Ahmed S., Al-Azzam N., Cao B. Karshaleva B., Sriram S., Vu K. If you understand a system, you can predict it. Agenda - Energy homeostasis Overview of leptin and insulin Signaling
Endocrine System. Modified by M. Myers
 Endocrine System Modified by M. Myers 1 The Endocrine System 2 Endocrine Glands The endocrine system is made of glands & tissues that secrete hormones. Hormones are chemicals messengers influencing a.
Endocrine System Modified by M. Myers 1 The Endocrine System 2 Endocrine Glands The endocrine system is made of glands & tissues that secrete hormones. Hormones are chemicals messengers influencing a.
Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Is obesity a brain disorder? What is the evidence to support obesity is a brain disorder? Environmental, biological, and behavioral issues Over
Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Is obesity a brain disorder? What is the evidence to support obesity is a brain disorder? Environmental, biological, and behavioral issues Over
Satiety induced by neuropeptide FF and gastrin in birds. Amanda Lynn Logan
 Satiety induced by neuropeptide FF and gastrin in birds Amanda Lynn Logan Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements
Satiety induced by neuropeptide FF and gastrin in birds Amanda Lynn Logan Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements
ENDOCRINOLOGY. Dr.AZZA SAJID ALKINANY 2 nd STAGE
 ENDOCRINOLOGY Dr.AZZA SAJID ALKINANY 2 nd STAGE THE RELATIONSHIP AMONG THE HYPOTHALMUS,POSTERIOR PITUITARY AND TARGET TISSUES. The posterior pituitary does not produce its own hormones, but stores and
ENDOCRINOLOGY Dr.AZZA SAJID ALKINANY 2 nd STAGE THE RELATIONSHIP AMONG THE HYPOTHALMUS,POSTERIOR PITUITARY AND TARGET TISSUES. The posterior pituitary does not produce its own hormones, but stores and
Understanding obesity by local overexpression of neuropeptides: a viral vector based approach
 Understanding obesity by local overexpression of neuropeptides: a viral vector based approach Cover photo: Chris Timmers Cover design: Marije Tiesjema Print: Gildeprint, Enschede ISBN: 978-90-39344675
Understanding obesity by local overexpression of neuropeptides: a viral vector based approach Cover photo: Chris Timmers Cover design: Marije Tiesjema Print: Gildeprint, Enschede ISBN: 978-90-39344675
Bi156 lecture 2, 1/6/12. Eating and weight regulation
 Bi156 lecture 2, 1/6/12 Eating and weight regulation Introduction: weight regulation in an affluent society In our society much effort and money is expended on regulation of weight. Failure to maintain
Bi156 lecture 2, 1/6/12 Eating and weight regulation Introduction: weight regulation in an affluent society In our society much effort and money is expended on regulation of weight. Failure to maintain
Peripubertal, leptin-deficient ob/ob female mice were used in an investigation of
 ESSICK-BROOKSHIRE, ELIZABETH ANN, M.S. The Effects of Peripherally Administered 17-β Estradiol and BIBP3226, a NPY Y1 Receptor Antagonist, on Food Intake, Body Mass, Reproductive Development and Behavior
ESSICK-BROOKSHIRE, ELIZABETH ANN, M.S. The Effects of Peripherally Administered 17-β Estradiol and BIBP3226, a NPY Y1 Receptor Antagonist, on Food Intake, Body Mass, Reproductive Development and Behavior
! acts via the autonomic nervous system. ! maintaining body weight within tight limits. ! ventromedial (VMN) ! arcuate (ARC) ! neuropeptide Y (NPY)
 Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Homeostasis Through Chemistry. The Endocrine System Topic 6.6
 Homeostasis Through Chemistry The Endocrine System Topic 6.6 Comparing NS & ES Animals have two systems of internal communication and regulation The nervous system Response time: Fast, quick Signals: electrical
Homeostasis Through Chemistry The Endocrine System Topic 6.6 Comparing NS & ES Animals have two systems of internal communication and regulation The nervous system Response time: Fast, quick Signals: electrical
Energy Balance. Applied Human Metabolism VII. Energy Out. Factors that effect BMR/RMR 17/03/2016
 Energy Balance Applied Human Metabolism VII Weight Regulation The balance of energy taken in or leaving the body determines body mass Energy In = Energy Out Weight Maintenance Energy In < Energy Out Weight
Energy Balance Applied Human Metabolism VII Weight Regulation The balance of energy taken in or leaving the body determines body mass Energy In = Energy Out Weight Maintenance Energy In < Energy Out Weight
Neuro-Physiology Kamal Mohammed Lecturer Of Physiology LECTURE NO (-) Hypothalamus. Faculty Of Medicine Dept.Of Physiology
 LECTURE NO (-) Neuro-Physiology Kamal Mohammed Lecturer Of Physiology Hypothalamus Faculty Of Medicine Dept.Of Physiology Hypothalamus Less than 1% of the brain mass Many connect the hypothalamus to the
LECTURE NO (-) Neuro-Physiology Kamal Mohammed Lecturer Of Physiology Hypothalamus Faculty Of Medicine Dept.Of Physiology Hypothalamus Less than 1% of the brain mass Many connect the hypothalamus to the
Chapter Nine. Temperature Regulation, Thirst, and Hunger
 Chapter Nine Temperature Regulation, Thirst, and Hunger Regulating Systems Homeostasis: Physiological equilibrium Epic FAIL with weight? Obesity rates 1980-2000 Motivation: Activating and directing behavior
Chapter Nine Temperature Regulation, Thirst, and Hunger Regulating Systems Homeostasis: Physiological equilibrium Epic FAIL with weight? Obesity rates 1980-2000 Motivation: Activating and directing behavior
Cholecystokinin antagonist, proglumide, stimulates growth hormone release in the rat
 J. Biosci., Vol. 15, Number 1, March 1990, pp. 17 21. Printed in India. Cholecystokinin antagonist, proglumide, stimulates growth hormone release in the rat E. VIJAYAN* and S. M. McCANN Department of Physiology,
J. Biosci., Vol. 15, Number 1, March 1990, pp. 17 21. Printed in India. Cholecystokinin antagonist, proglumide, stimulates growth hormone release in the rat E. VIJAYAN* and S. M. McCANN Department of Physiology,
THE ROLE OF DIETARY FAT IN HYPOTHALAMIC INSULIN AND LEPTIN RESISTANCE AND THE PATHOGENESIS OF OBESITY. Kelly Ann Posey.
 THE ROLE OF DIETARY FAT IN HYPOTHALAMIC INSULIN AND LEPTIN RESISTANCE AND THE PATHOGENESIS OF OBESITY By Kelly Ann Posey Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University
THE ROLE OF DIETARY FAT IN HYPOTHALAMIC INSULIN AND LEPTIN RESISTANCE AND THE PATHOGENESIS OF OBESITY By Kelly Ann Posey Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University
Investigation of the role of nesfatin-1/nucb2 in the central nervous system. Ph.D. thesis Katalin Könczöl
 Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Gut hormones KHATTAB
 Gut hormones PROF:ABD ALHAFIZ HASSAN KHATTAB Gut as an endocrine gland The talk will cover the following : Historical background. Why this subject is chosen. Gastro-intestinal hormones and their function.
Gut hormones PROF:ABD ALHAFIZ HASSAN KHATTAB Gut as an endocrine gland The talk will cover the following : Historical background. Why this subject is chosen. Gastro-intestinal hormones and their function.
Growth Hormone, Somatostatin, and Prolactin 1 & 2 Mohammed Y. Kalimi, Ph.D.
 Growth Hormone, Somatostatin, and Prolactin 1 & 2 Mohammed Y. Kalimi, Ph.D. I. Growth Hormone (somatotropin): Growth hormone (GH) is a 191 amino acid single chain polypeptide (MW 22,000 daltons). Growth
Growth Hormone, Somatostatin, and Prolactin 1 & 2 Mohammed Y. Kalimi, Ph.D. I. Growth Hormone (somatotropin): Growth hormone (GH) is a 191 amino acid single chain polypeptide (MW 22,000 daltons). Growth
Digestion: Endocrinology of Appetite
 Digestion: Endocrinology of Dr. Ritamarie Loscalzo Medical Disclaimer: The information in this presentation is not intended to replace a one on one relationship with a qualified health care professional
Digestion: Endocrinology of Dr. Ritamarie Loscalzo Medical Disclaimer: The information in this presentation is not intended to replace a one on one relationship with a qualified health care professional
Endocrine System. Endocrine vs. Exocrine. Bio 250 Human Anatomy & Physiology
 Endocrine System Bio 250 Human Anatomy & Physiology Endocrine vs. Exocrine Endocrine glands secrete their products called hormones into body fluids (the internal environment) Exocrine glands secrete their
Endocrine System Bio 250 Human Anatomy & Physiology Endocrine vs. Exocrine Endocrine glands secrete their products called hormones into body fluids (the internal environment) Exocrine glands secrete their
The Players. Liver Thyroid Adrenals Pancreas Reproductive System Pituitary Gut Bacteria
 The Players Part I Quick Review Understanding some of the key systems and their relationship to hormones is the best place to start It will help with some of the hormone interconnections Key to understanding
The Players Part I Quick Review Understanding some of the key systems and their relationship to hormones is the best place to start It will help with some of the hormone interconnections Key to understanding
INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS
 INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS 1 Introduction to the Biochemistry of Hormones and their Receptors Lectuctre1 Sunday 17/2/ Objectives: 1. To understand the biochemical nature
INTRODUCTION TO THE BIOCHEMISTRY OF HORMONES AND THEIR RECPTORS 1 Introduction to the Biochemistry of Hormones and their Receptors Lectuctre1 Sunday 17/2/ Objectives: 1. To understand the biochemical nature
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Chronic Stimulation of Leptin on Food Intake and Body Weight after Microinjection into the Ventromedial Hypothalamus of Conscious Rats
 TAJ December 2006; Volume 19 Number 2 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Original Article Chronic Stimulation of Leptin on Food Intake and Body Weight after Micro into the
TAJ December 2006; Volume 19 Number 2 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Original Article Chronic Stimulation of Leptin on Food Intake and Body Weight after Micro into the
What systems are involved in homeostatic regulation (give an example)?
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
An important function of the central nervous
 Perspectives in Diabetes Insulin Signaling in the Central Nervous System A Critical Role in Metabolic Homeostasis and Disease From C. elegans to Humans Daniel Porte, Jr., 1,2,3 Denis G. Baskin, 3 and Michael
Perspectives in Diabetes Insulin Signaling in the Central Nervous System A Critical Role in Metabolic Homeostasis and Disease From C. elegans to Humans Daniel Porte, Jr., 1,2,3 Denis G. Baskin, 3 and Michael
THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN. COGS 163 By: Pranav Singh Alexandra Villar
 THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN COGS 163 By: Pranav Singh Alexandra Villar INTRODUCTION Insulin is a hormone produced in the pancreas by the islets of Langerhans that regulates the
THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN COGS 163 By: Pranav Singh Alexandra Villar INTRODUCTION Insulin is a hormone produced in the pancreas by the islets of Langerhans that regulates the
Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone
 Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone II. mineralocorticoids i id aldosterone III. androgenic steroids dehydroepiandrosterone testosterone IV. estrogenic steroids
Adrenal Steroid Hormones (Chapter 15) I. glucocorticoids cortisol corticosterone II. mineralocorticoids i id aldosterone III. androgenic steroids dehydroepiandrosterone testosterone IV. estrogenic steroids
BIOLOGY - CLUTCH CH.45 - ENDOCRINE SYSTEM.
 !! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
!! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
Hypothalamus & pituitary gland
 Hypothalamus & pituitary gland Huiping Wang ( 王会平 ), PhD Department of Physiology Rm C541, Block C, Research Building, School of Medicine Tel: 88208292 Outline Hypothalamus Relationship between the hypothalamus
Hypothalamus & pituitary gland Huiping Wang ( 王会平 ), PhD Department of Physiology Rm C541, Block C, Research Building, School of Medicine Tel: 88208292 Outline Hypothalamus Relationship between the hypothalamus
Chapter 26. Hormones and the Endocrine System. Lecture by Edward J. Zalisko
 Chapter 26 Hormones and the Endocrine System PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture
Chapter 26 Hormones and the Endocrine System PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
Behavioral and Motivational mechanisms of Brain. Limbic system and the Hypothalamus
 Behavioral and Motivational mechanisms of Brain Limbic system and the Hypothalamus 1 General functions 1. Control of behavior 2. Control level of activities in different parts of brain 3. Motivational
Behavioral and Motivational mechanisms of Brain Limbic system and the Hypothalamus 1 General functions 1. Control of behavior 2. Control level of activities in different parts of brain 3. Motivational
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
Stressin with Ghrelin. Peptide Pods: Lara, Elijah, Karen, Brandon, Milena, Max & Lucile
 Stressin with Ghrelin Peptide Pods: Lara, Elijah, Karen, Brandon, Milena, Max & Lucile Agenda Ghrelin Essentials Review Associations of Ghrelin with eating, stress, metabolic factors, etc. High-fat diet
Stressin with Ghrelin Peptide Pods: Lara, Elijah, Karen, Brandon, Milena, Max & Lucile Agenda Ghrelin Essentials Review Associations of Ghrelin with eating, stress, metabolic factors, etc. High-fat diet
Chapter 20 Endocrine System
 Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Meccanismi fisiopatologici e trattamento dei disturbi metabolici in soggetti affetti da disturbo mentale grave
 Meccanismi fisiopatologici e trattamento dei disturbi metabolici in soggetti affetti da disturbo mentale grave Francesco Bartoli, MD, PhD Università degli Studi di Milano Bicocca Ospedale San Gerardo di
Meccanismi fisiopatologici e trattamento dei disturbi metabolici in soggetti affetti da disturbo mentale grave Francesco Bartoli, MD, PhD Università degli Studi di Milano Bicocca Ospedale San Gerardo di
A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants
 Am J Physiol Regul Integr Comp Physiol 286: R994 R1004, 2004; 10.1152/ajpregu.00727.2003. CALL FOR PAPERS Physiological Regulation of Appetite A mechanism underlying mature-onset obesity: evidence from
Am J Physiol Regul Integr Comp Physiol 286: R994 R1004, 2004; 10.1152/ajpregu.00727.2003. CALL FOR PAPERS Physiological Regulation of Appetite A mechanism underlying mature-onset obesity: evidence from
Homeostasis and Mechanisms of Weight Regulation
 Homeostasis and Mechanisms of Weight Regulation Purpose In this activity students will investigate how negative feedback mechanisms function to maintain homeostatic balance using a recently discovered
Homeostasis and Mechanisms of Weight Regulation Purpose In this activity students will investigate how negative feedback mechanisms function to maintain homeostatic balance using a recently discovered
Art labeling Activity: Figure 16.1
 ANP 1105D Winter 2013 Assignment 6 part I: The Endocrine Sy... Assignment 6 part I: The Endocrine System, Chapter 16 Due: 11:59pm on Monday, March 4, 2013 Note: To understand how points are awarded, read
ANP 1105D Winter 2013 Assignment 6 part I: The Endocrine Sy... Assignment 6 part I: The Endocrine System, Chapter 16 Due: 11:59pm on Monday, March 4, 2013 Note: To understand how points are awarded, read
Energy flow in the organism
 I. Parameters of energy metabolism, basal metabolic rate, measurements. II. Control of food intake, hunger and satiety Péter Sántha, 12.02. 2017. Energy flow in the organism NUTRIENTS PHYSICAL WORK HEAT
I. Parameters of energy metabolism, basal metabolic rate, measurements. II. Control of food intake, hunger and satiety Péter Sántha, 12.02. 2017. Energy flow in the organism NUTRIENTS PHYSICAL WORK HEAT
REGULATION OF FOOD INTAKE AND NUTRITIONAL STATE
 REGULATION OF FOOD INTAKE AND NUTRITIONAL STATE INTAKE OUTPUT CENTER OF SATIETY ncl. ventromedialis in hypothalamus - CENTER OF HUNGER (permanently active) lateral hypothalamus (nucleus under fasciculus
REGULATION OF FOOD INTAKE AND NUTRITIONAL STATE INTAKE OUTPUT CENTER OF SATIETY ncl. ventromedialis in hypothalamus - CENTER OF HUNGER (permanently active) lateral hypothalamus (nucleus under fasciculus
Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes
 Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes Dr Masud Haq Consultant Lead in Diabetes & Endocrinology Maidstone & Tunbridge Wells NHS Trust & The London Preventative
Injectable GLP 1 therapy: weight loss effects seen in obesity with and without diabetes Dr Masud Haq Consultant Lead in Diabetes & Endocrinology Maidstone & Tunbridge Wells NHS Trust & The London Preventative
Københavns Universitet. Feed intake and energy supply Tauson, Anne-Helene. Published in: Nutritional physiology of pigs. Publication date: 2012
 university of copenhagen Københavns Universitet Feed intake and energy supply Tauson, Anne-Helene Published in: Nutritional physiology of pigs Publication date: 2012 Document Version Publisher's PDF, also
university of copenhagen Københavns Universitet Feed intake and energy supply Tauson, Anne-Helene Published in: Nutritional physiology of pigs Publication date: 2012 Document Version Publisher's PDF, also
Chapter 45-Hormones and the Endocrine System. Simple Hormone Pathways
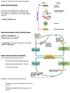 Chapter 45-Hormones and the Endocrine System Simple Hormone s Low ph in duodenum Hormones are released from an endocrine, travel through the bloodstream, and interact with the receptor or a target to cause
Chapter 45-Hormones and the Endocrine System Simple Hormone s Low ph in duodenum Hormones are released from an endocrine, travel through the bloodstream, and interact with the receptor or a target to cause
Chapter 20. Endocrine System Chemical signals coordinate body functions Chemical signals coordinate body functions. !
 26.1 Chemical signals coordinate body functions Chapter 20 Endocrine System! Hormones Chemical signals Secreted by endocrine glands Usually carried in the blood Cause specific changes in target cells Secretory
26.1 Chemical signals coordinate body functions Chapter 20 Endocrine System! Hormones Chemical signals Secreted by endocrine glands Usually carried in the blood Cause specific changes in target cells Secretory
The Role of Ghrelin and Leptin in Obesity: Is Exogenous Administration of These Hormones a Possible Drug Therapy?
 The Science Journal of the Lander College of Arts and Sciences Volume 4 Number 2 Spring 2011 Article 7 2011 The Role of Ghrelin and Leptin in Obesity: Is Exogenous Administration of These Hormones a Possible
The Science Journal of the Lander College of Arts and Sciences Volume 4 Number 2 Spring 2011 Article 7 2011 The Role of Ghrelin and Leptin in Obesity: Is Exogenous Administration of These Hormones a Possible
Food Intake Regulation & the Clock. Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 Food Intake Regulation & the Clock Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Circadian disruption affect multiple organ systems: The diagram provides examples of how circadian disruption
Food Intake Regulation & the Clock Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Circadian disruption affect multiple organ systems: The diagram provides examples of how circadian disruption
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control
 NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
Testosterone and other male hormones seem to be related to aggressive behavior in some species
 Testosterone and Male Aggression Testosterone and other male hormones seem to be related to aggressive behavior in some species In the fish species Oreochromis mossambicus, elevated levels have been found
Testosterone and Male Aggression Testosterone and other male hormones seem to be related to aggressive behavior in some species In the fish species Oreochromis mossambicus, elevated levels have been found
Monday, 17 April 2017 BODY FLUID HOMEOSTASIS
 Monday, 17 April 2017 BODY FLUID HOMEOSTASIS Phenomenon: shipwrecked sailor on raft in ocean ("water, water everywhere but not a drop to drink") Why are the sailors thirsty? (What stimulated thirst?) Why
Monday, 17 April 2017 BODY FLUID HOMEOSTASIS Phenomenon: shipwrecked sailor on raft in ocean ("water, water everywhere but not a drop to drink") Why are the sailors thirsty? (What stimulated thirst?) Why
THE PREPROGLUCAGON DERIVED PEPTIDES AND ENERGY HOMEOSTASIS
 THE PREPROGLUCAGON DERIVED PEPTIDES AND ENERGY HOMEOSTASIS A thesis submitted for the degree of Doctor of Philosophy Jennifer Parker 2013 Department of Medicine Imperial College London Abstract Obesity
THE PREPROGLUCAGON DERIVED PEPTIDES AND ENERGY HOMEOSTASIS A thesis submitted for the degree of Doctor of Philosophy Jennifer Parker 2013 Department of Medicine Imperial College London Abstract Obesity
Low ambient temperature lowers cholecystokinin and leptin plasma concentrations in adult men
 ISPUB.COM The Internet Journal of Gastroenterology Volume 7 Number 2 Low ambient temperature lowers cholecystokinin and leptin plasma concentrations in adult men M Pizon, P Tomasic, K Sztefko, Z Szafran
ISPUB.COM The Internet Journal of Gastroenterology Volume 7 Number 2 Low ambient temperature lowers cholecystokinin and leptin plasma concentrations in adult men M Pizon, P Tomasic, K Sztefko, Z Szafran
Orexin and Sleep. Team: A Little Bit of Leptin
 Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Chapter 16: Endocrine System 1
 Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Endocrine System. Chapter 20. Endocrine Glands and Hormones. The Endocrine System. Endocrine glands
 Chapter 20 Endocrine System Endocrine Glands and Hormones The endocrine system consists of glands and tissues that secrete hormones Hormones are chemicals that affect other glands or tissues, many times
Chapter 20 Endocrine System Endocrine Glands and Hormones The endocrine system consists of glands and tissues that secrete hormones Hormones are chemicals that affect other glands or tissues, many times
NOTES: ENDOCRINE SYSTEM (CH 9)
 NOTES: ENDOCRINE SYSTEM (CH 9) Endocrine System *The endocrine system consists of a range of glands and tissues throughout the body Functions of the Endocrine System: 1) Maintain balance within body (homeostasis)
NOTES: ENDOCRINE SYSTEM (CH 9) Endocrine System *The endocrine system consists of a range of glands and tissues throughout the body Functions of the Endocrine System: 1) Maintain balance within body (homeostasis)
Autonomic regulation of islet hormone secretion
 Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
WEIGHT GAIN DURING MENOPAUSE EMERGING RESEARCH
 MENOPAUSE WHEN DOES IT OCCUR? The cessation of the menstrual cycle for one year. WEIGHT GAIN DURING MENOPAUSE EMERGING RESEARCH Jan Schroeder, Ph.D. Chair of The Department of Kinesiology California State
MENOPAUSE WHEN DOES IT OCCUR? The cessation of the menstrual cycle for one year. WEIGHT GAIN DURING MENOPAUSE EMERGING RESEARCH Jan Schroeder, Ph.D. Chair of The Department of Kinesiology California State
BIOL 2458 A&P II CHAPTER 18 SI Both the system and the endocrine system affect all body cells.
 BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
A New Era of Migraine Management: The Challenging Landscape in Prevention
 Provided by MediCom Worldwide, Inc. Supported by an educational grant from Teva Pharmaceuticals What is a Neuropeptide? Small chains of amino acids released by neural cells (neurons or glial cells) to
Provided by MediCom Worldwide, Inc. Supported by an educational grant from Teva Pharmaceuticals What is a Neuropeptide? Small chains of amino acids released by neural cells (neurons or glial cells) to
Chemical Regulation. Chapter 26. Testosterone and Male Aggression: Is There a Link? THE NATURE OF CHEMICAL REGULATION
 Chapter 6 Chemical Regulation PowerPoint Lectures for Biology: Concepts and Connections, Fifth Edition Campbell, Reece, Taylor, and Simon Testosterone and Male Aggression: Is There a Link? Among male animals,
Chapter 6 Chemical Regulation PowerPoint Lectures for Biology: Concepts and Connections, Fifth Edition Campbell, Reece, Taylor, and Simon Testosterone and Male Aggression: Is There a Link? Among male animals,
Neuroendocrinology an integrative approach
 Neuroendocrinology an integrative approach JP Advis DVM, Ph.D. Bartlett Hall, Animal ciences, Cook, (848) 932-9240, advis@aesop.rutgers.edu 06 Course website: rci.rutgers.edu/~advis Material to be covered:
Neuroendocrinology an integrative approach JP Advis DVM, Ph.D. Bartlett Hall, Animal ciences, Cook, (848) 932-9240, advis@aesop.rutgers.edu 06 Course website: rci.rutgers.edu/~advis Material to be covered:
Human Biochemistry. Hormones
 Human Biochemistry Hormones THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM The ENDOCRINE SYSTEM = the organ system that regulates internal environment conditions by secreting hormones into
Human Biochemistry Hormones THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM The ENDOCRINE SYSTEM = the organ system that regulates internal environment conditions by secreting hormones into
Amylinergic control of food intake in lean and obese rodents
 Zurich Open Repository and Archive University of Zurich Main Library Winterthurerstr. 190 CH-8057 Zurich www.zora.uzh.ch Year: 2011 Amylinergic control of food intake in lean and obese rodents Boyle, C
Zurich Open Repository and Archive University of Zurich Main Library Winterthurerstr. 190 CH-8057 Zurich www.zora.uzh.ch Year: 2011 Amylinergic control of food intake in lean and obese rodents Boyle, C
Endocrine Glands: Hormone-secreting organs are called endocrine glands
 University of Jordan Department of Physiology and Biochemistry Nursing students, Academic year 2017/2018. ******************************************************************* Ref: Principles of Anatomy
University of Jordan Department of Physiology and Biochemistry Nursing students, Academic year 2017/2018. ******************************************************************* Ref: Principles of Anatomy
Hormones. Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6
 Hormones Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6 Tel. 030-8385-6920 (Sekret.) 030-8385-6922 (direkt) e-mail: vhaucke@chemie.fu-berlin.de http://userpage.chemie.fu-berlin.de/biochemie/aghaucke/teaching.html
Hormones Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6 Tel. 030-8385-6920 (Sekret.) 030-8385-6922 (direkt) e-mail: vhaucke@chemie.fu-berlin.de http://userpage.chemie.fu-berlin.de/biochemie/aghaucke/teaching.html
AND THE REGULATION OF ENERGY AND GLUCOSE HOMEOSTASIS. A thesis submitted in conformity with the requirements for the degree of
 CENTRAL NERVOUS SYSTEM NUTRIENT-SENSING AND THE REGULATION OF ENERGY AND GLUCOSE HOMEOSTASIS by CAROL KA-LO LAM A thesis submitted in conformity with the requirements for the degree of MASTER OF SCIENCE
CENTRAL NERVOUS SYSTEM NUTRIENT-SENSING AND THE REGULATION OF ENERGY AND GLUCOSE HOMEOSTASIS by CAROL KA-LO LAM A thesis submitted in conformity with the requirements for the degree of MASTER OF SCIENCE
Neural pathways controlling homeostatic and hedonic feeding in rats on free-choice diets van den Heuvel, J.K.
 UvA-DARE (Digital Academic Repository) Neural pathways controlling homeostatic and hedonic feeding in rats on free-choice diets van den Heuvel, J.K. Link to publication Citation for published version (APA):
UvA-DARE (Digital Academic Repository) Neural pathways controlling homeostatic and hedonic feeding in rats on free-choice diets van den Heuvel, J.K. Link to publication Citation for published version (APA):
Endocrine System Hormones (Ch. 45)
 Endocrine System Hormones (Ch. 45) Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body daily homeostasis & regulation of large
Endocrine System Hormones (Ch. 45) Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body daily homeostasis & regulation of large
Endocrine System Notes
 Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Early Life Effects on Hypothalamic Circuitry. By: The Mystery Gang
 Early Life Effects on Hypothalamic Circuitry By: The Mystery Gang Hypothalamic Circuits Controlling Energy Balance ARH (Arcuate nucleus of the Hypothalamus) Subgroup of Neurons 1: coexpress Neuropeptide
Early Life Effects on Hypothalamic Circuitry By: The Mystery Gang Hypothalamic Circuits Controlling Energy Balance ARH (Arcuate nucleus of the Hypothalamus) Subgroup of Neurons 1: coexpress Neuropeptide
Hypothalamus. To learn how the brain regulates neuroendocrine secretions NTA Ch 14, pgs Key Figs: 14-3; 14-4,
 Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Supplemental data Supplemental Figure Legends Supplemental Figure 1. Supplemental Figure 2.
 Supplemental data Supplemental Figure Legends Supplemental Figure 1. Analysis of deletion of AMPK!2 in POMC and AgRP neurons in control and POMC!2KO and AgRP!2KO mice. (A) mmunofluorescence analysis for
Supplemental data Supplemental Figure Legends Supplemental Figure 1. Analysis of deletion of AMPK!2 in POMC and AgRP neurons in control and POMC!2KO and AgRP!2KO mice. (A) mmunofluorescence analysis for
Obesity in aging: Hormonal contribution
 Obesity in aging: Hormonal contribution Hormonal issues in obesity and aging Hormonal role in regulation of energy balance Genetic component in hormonal regulation Life style contribution to hormonal changes
Obesity in aging: Hormonal contribution Hormonal issues in obesity and aging Hormonal role in regulation of energy balance Genetic component in hormonal regulation Life style contribution to hormonal changes
