METABOLISM OF THE PERFUSED SWTMBLADDER OF THE EUROPEAN EEL: OXYGEN, CARBON DIOXIDE, GLUCOSE AND LACTATE BALANCE
|
|
|
- Shawn Cook
- 6 years ago
- Views:
Transcription
1 J. exp. Biol. 144, (1989) 495 ^Printed in Great Britain The Company of Biologists Limited 1989 METABOLISM OF THE PERFUSED SWTMBLADDER OF THE EUROPEAN EEL: OXYGEN, CARBON DIOXIDE, GLUCOSE AND LACTATE BALANCE BY B. PELSTER, H. KOBAYASHI AND P. SCHEID Institut fiir Physiologie, Ruhr Universitat, Bochum, FRG Accepted 7 February 1989 Summary We have measured the metabolic activity in the vascularly isolated, salineperfused swimbladder of the eel (Anguilla anguilla) in order to investigate the pathways for CO2 formation in the gas gland tissue. Concentrations of O2, CO2, glucose and lactate were measured in the arterial inflow and venous outflow of the swimbladder, and metabolic rates were calculated by the direct Fick principle. 1. Total CO 2 production, averaging 55-Snmolmin" 1, was about 4-6 times the O2 consumption (mean 12-0nmolmin~ 1 ). This suggests that only about 22% of the CO2 is formed by aerobic glucose metabolism. 2. CO 2 formation from HCO 3 ~ or CO 2 washout does not appear to be significant in our experiments with steady perfusion of a saline containing a low level of HCO The ratio of lactate production to glucose uptake averaged 1-2, indicating that only 60% of the glucose is converted to lactate. Since only 1-2% of the glucose was found to be oxidized (2nmolmin~ 1 ), the extra glucose appears to be anoxidatively metabolized to CO2. 4. The anoxidative CO2 formation appears to be of functional importance for producing the high gas partial pressures of both CO 2 and O 2 which are required for secretion of these gases into the swimbladder. Introduction Many teleost fishes have developed a gas-filled swimbladder which enables them to keep neutral buoyancy at varying water depths. It is generally accepted that the gas enters the bladder from the blood by diffusion, and it is assumed that the high gas partial pressures in the blood perfusing the swimbladder are generated by acidification of the blood in the gas gland in conjunction with the countercurrent gas exchange in the rete mirabile (Steen, 1970; Fange, 1983). In fact, the gas gland is known to produce lactic acid even at high O 2 levels. This acid enhances, via the Bohr and Root effects, the O2 partial pressure and, by the conversion of HCO3" to CO2, the CO 2 partial pressure. The formation of lactate by gas gland cells has been demonstrated both in tissue preparations (Ball et al. 1955; D'Aoust, 1970; Deck, Key words: fish, gas secretion, metabolism, swimbladder.
2 496 B. PELSTER, H. KOBAYASHI AND P. SCHEID 1970) and in vivo (Kuhn et al. 1962; Steen, 1963ft; Enns et al. 1967). In this model, CO 2 in the swimbladder would derive either from oxidative metabolism or from HC(V. Oxidative metabolism has been estimated to contribute only 5 % of the total CO 2 formed (Wittenberg et al. 1964). Ball et al. (1955), Wittenberg et al. (1964) and D'Aoust (1970) found that most, if not all the CO 2 was liberated from bicarbonate, presumably by lactic acid. This assumption neglects, however, the presence of nonbicarbonate buffers, which would buffer some of the H + released from lactic acid, thus reducing the amount of H + available for conversion of HCO 3 ~ to CO 2. Therefore, a lactate/co 2 ratio of unity, found by Ball et al. (1955) and D'Aoust (1970), can only be explained if CO 2 derives from sources other than bicarbonate. Such a source could be the decarboxylation reaction of the pentose phosphate shunt, which yields CO 2 from carbohydrates without consuming O 2. Bostrdm etal. (1972) measured high activities of enzymes characteristic of the pentose phosphate shunt in the gas gland tissue of the cod, and increases in CO 2 content in blood passing through the gas gland have indeed been measured directly (Steen, 19636; Pelster et al ). The contribution of CO 2 via this route has not been estimated yet. In the present study, we have measured the metabolic activity in the salineperfused eel swimbladder to evaluate the contributions of the various pathways to CO 2 production. The saline-perfused preparation was preferred to the more physiological, blood-perfused swimbladder, as it more easily allows measurement of the perfusion rate and quantitative estimation of metabolic rates. Materials and methods Specimens of the freshwater-adapted European eel (Anguilla anguilla; body mass g) were purchased from a local supplier and kept in a freshwater aquarium at C until the experiment. Animal preparation and experimental set-up The animals were anaesthetized by adding MS 222 (0-1 g I" 1 ) to the water and were then placed into an 'eel holder', similar to that used by Steen (1963a). The gills were irrigated with well-oxygenated tap water (flow rate 4-51min~ 1 ), containing MS 222 at a concentration of approximately 0-05 gl" 1, a level sufficient to keep the animal anaesthetized with stable ventilatory activity. Preparation of animals was essentially the same as described elsewhere (H. Kobayashi, B. Pelster & P. Scheid, in preparation). Briefly, the body wall was opened ventrally, the swimbladder was exposed and carefully freed from connective tissue. The duct connecting the absorption and secretion part of the bladder was tied off, and small blood vessels and anastomoses bypassing the rete mirabile were carefully ligated without damaging rete vessels or the swimbladder wall. The gas secretion rate was too low to be measured accurately by a catheter inserted
3 CO2 production in swimbladder tissue 497 Heart pole Swimbladder pole Fig. 1. Diagram of the two retia in the eel swimbladder. The arterial inflow (ai) and venous outflow vessels (ve) at the heart pole were occlusively cannulated for perfusion. For serial perfusion (type S), ai of one and ve of the other rete were blocked (see Materials and methods). into the swimbladder (less than 0-1 ml h" 1 ). All experiments were carried out at C. The artery and vein at the heart pole (ai and ve in Fig. 1) were occlusively cannulated using PE50 and PE100 catheters, respectively, and the rete vessels were perfused with saline solution at ml min" 1 using an infusion pump (Precidor, Type 5003, Infers AG, Basel, Switzerland). This solution was a heparinized (100 i.u. mp 1 ) Ringer's solution which contained (inmmoll" 1 ) NaCl, 129; KC1, 5; MgSO 4, 0-9; CaCl 2, 1-1; glucose, 15; insulin, 8i.u. The colloid osmotic pressure was adjusted to that of eel plasma by adding 5 g I" 1 Dextran FP 70 (Serva, Heidelberg, FRG). The saline contained no bicarbonate and was equilibrated with air so as to remove nearly all CO 2. Experimental protocol Samples of the perfusate were collected at the venous outlet (ve), and their composition was compared with that of the perfusate at the inflow (ai). Venous collection was about every 30min for 3-4 h. For calculating metabolic rates from perfusion rate and concentrations in inflow and outflow, steady-state conditions have to prevail. Since countercurrent exchange in the rete during normal flow conditions (perfusion type C) retards attainment of steady state, a serial type of rete flow (type S) was achieved in some experiments by occluding the afferent artery of one and the efferent vein of the other rete at the heart pole (Fig. 1), this flow type enhanced attainment of steady state. Measurements were made in these experiments during both type C and type S flow. Analytical procedures Saline samples were analysed for oxygen content ; using the method of
4 498 B. PELSTER, H. KOBAYASHI AND P. SCHEID Tucker, 1967) and total CO 2 content (C CO2 ; using the method of Cameron, 1971) immediately after collection. For analysis of glucose and lactate concentrations, part of the sample was deproteinized with perchloric acid and neutralized with K 2 CO 3 or KOH. Employing this procedure, no extrusion of proteins from the tissue into the perfusate was detected. The assays were performed enzymatically as outlined by Bergmeyer (1974). Calculations The rates of production of CO 2 (McoJ ar >d lactate (M^) and the rates of consumption of O 2 (MoJ and glucose (Mciu) wer e calculated by mass balance from the perfusion rate (Q) and the concentration difference between inflow and outflow. Student's f-tests were used to test for statistical significance, and P< 0-01 was accepted as the limit of significance. Results At the onset of perfusion, there were high concentrations of lactate and CO 2 in the venous outflow (ve) which gradually decreased to a steady-state level. For both substances this was higher than the arterial level (ai; Fig. 2). For calculation of metabolic rates only steady-state values were taken into account. Table 1 shows concentrations of O 2, CO 2 and lactate in inflow (ai) and outflow (ve) of the rete during perfusion at two flow rates when there was no countercurrent flow (type S). The observed differences in these concentrations < Time from onset of perfusion (min) 240 Fig. 2. Concentrations of total CO2 (open circles, continuous line) and lactate (closed circles, dashed line) in the venous outflow (ve) of a saline-perfused swimbladder (perfusion type C) plotted against time from onset of perfusion. Concentrations in the perfusate at the entrance to the rete (ai) are presented as horizontal lines. Values of ve for t > 120 min were averaged in this experiment to yield the steady-state value from which metabolic activity was calculated.
5 Y Table 1. Arterial inflow (ai) and venous outjlow (ve) concentrations of O2 (C02), COZ (CC02) and lactate measured in saline- 2 perfused swimbladders at two perfusion rates during type S perfusion (serial flow, no countercurrent) r Q = 0.2 ml min-' (N = 2) Q = 0.4 ml min-' (N = 4) g. 0 ai ve ve-ai ai ve ve-ai 5. Co, (mmol I-') 0.22 f f k b q [Lactate] (mmol I-') 0.07 f f f f G.-- (mmol I-') 0.15 f f f f z a N, number of preparations. E Y g R
6 500 B. PELSTER, H. KOBAYASHI AND P. SCHEID Table 2. Rates of oxygen consumption {M Ol ) and CO2 excretion (M CO2 ) in salineperfused swimbladder tissue and the corresponding respiratory exchange ratio, R Expt no Mean ±S.D. Perfusion type C ct c ss c s c s c M O2 (nmolmin ') ±3-8* Mco, (nmol min ') ±32-1* R ±1-8** Perfusion type C, perfusion with countercurrent flow; type S, serial perfusion, no countercurrent flow. * Significantly different from zero (?< 0-01). ** Significantly different from unity (P < 0-01). t Only one rete mirabile perfused. between ai and ve were clearly dependent on the flow rate. Doubling the flow rate resulted in a reduction of the (ve-ai) differences by a factor of about 2. The rates of O 2 consumption (M O2 ) an^ CO 2 production (Mcoj)* calculated from concentration differences in individual experiments, are listed in Table 2. There were no differences between the flow types. In each experiment the CO2 production rate by far exceeded the O2 consumption rate. The resulting values of the respiratory exchange ratio, R = M C ojm Oz, are significantly above unity. The rates of glucose uptake (MGIU) an d lactate release (MLB) are presented in Table 3. On average, the rate of glucose consumption was higher than the rate of lactate production. Discussion For a quantitative evaluation of the metabolic activities of the gas gland its perfusion rate had to be known. Since blood flow rate cannot easily be obtained in the eel swimbladder without altering its function, we had to apply the technique of saline perfusion of the vascularly isolated tissue. The CO 2 and lactate values measured in this study, however, are similar to values that we obtained earlier by non-obstructive blood collection in intact eel swimbladder (Pelster et al ). We, therefore, assume the values measured in this study to be representative for eel swimbladder under physiological conditions.
7 CO2 production in swimbladder tissue 501 Table 3. Rates of lactate production (MLO) and glucose consumption (MGI U ) saline-perfused swimbladder tissue Expt no Mean ± S.D. Perfusion type C C c c ct c c s c sc c s c sc s (nmolmin" 1 ) ± 97* MGIU (nmolmin" 1 ) ± 110* Mi_a/M G iu ** Perfusion type C, perfusion with countercurrent flow; type S, perfusion without countercurrent flow. Significantly different from zero (P<0-01). ** Significantly different from 2 (F<0-01). fonly one rete mirabile perfused. Sources for CO2 formed in the gas gland The important results of this study are the demonstration of the high rate of CO2 production in the swimbladder tissue and the high respiratory exchange ratio, which significantly exceeded the value of unity expected for oxidative glucose breakdown. The values were calculated assuming no gas secretion into the swimbladder, which was indeed below the detection level. In our experiments the CO 2 may derive from three sources: washout from the tissue, oxidative metabolism and anaerobic decarboxylation reactions. Washout of HCO 3 ~ I CO 2 from the tissue As the CO 2 content in the perfusing solution was kept low, to ensure a high sensitivity for arteriovenous CO 2 differences, the swimbladder tissue had probably been cleansed of most of the CO2 and HCC>3~ during the perfusion period before
8 502 B. PELSTER, H. KOBAYASHI AND P. SCHEID measurements were performed. An enhanced conversion of HCO 3 ~ to CO 2 is to be expected as a result of lactic acid formation. The exact amount of HCO 3 ~/CO 2 washed out from the tissue is difficult to estimate. Let us consider first the conversion of HCC>3~ to CO 2 in the tissue, effected by the protons liberated from lactic acid, and its subsequent release into the perfusate. The amount of lactate produced (Table 1) would, indeed, suggest a large contribution to the CO 2 release into the perfusate, but there are two other possibilities for the H + apart from combining with HCO 3 ~. One is binding to nonbicarbonate buffers in the tissue, the other binding to any buffer in the perfusate and washout with it. The nominal buffer value of the perfusate was very low, and the ph change in the perfusate from ai to ve of 1-2 units suggests that the amount of H + washed out is probably low. We do not know the buffer value of the swimbladder tissue and, thus, cannot estimate the amount of CO 2 washed out from it by considering the H + liberated from lactic acid. There is another reason to suggest that the contribution of HCO 3 ~/CO 2 washout from tissue was probably small. Assuming a bicarbonate concentration of approximately 2mmoll~ 1, typical of muscle tissue, the tissue mass of the swimbladder (about 0-4g for an 800 g eel; Ball et al. 1955; Wittenberg et al. 1964) would contain less than l^mol of HCO 3 ~. Under steady-state conditions (see Fig. 2) this cannot contribute very much to the observed rate of CO 2 formation. CO 2 formation by aerobic glucose metabolism The rate of CO 2 production by glucose oxidation can best be estimated in our experiments from the O 2 consumption rate, which suggests that nnmolmin" 1 or about 20 % of the CO 2 derives from this pathway. It thus appears that the major fraction of the CO 2 formed must stem from the third source. CO 2 formation by anaerobic decarboxylation An example of anaerobic CO 2 formation is the pentose phosphate shunt. This pathway allows formation of CO 2 by decarboxylation of 6-phosphogluconate formed enzymatically from glucose. The pentose phosphate shunt is known to occur in ocular tissues of fish, where it contributes significantly to the glucose metabolism (Hoffert & Fromm, 1970). Bostrom et al. (1972) found that in the cod glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase, key enzymes of the pentose phosphate shunt, displayed higher activities in gas gland cells than in muscle cells. In preliminary measurements we found activities of these two enzymes in the eel gas gland similar in magnitude to those in liver tissue and about 10 times higher than their activities in muscle tissue. It is, thus, to be expected that part of the glucose should be diverted to the pentose phosphate shunt to form CO 2 by decarboxylation catalysed by 6-phosphogluconate dehydrogenase. In this case, an enzymatic reaction reoxidizing the NADPH produced in the pentose phosphate shunt is necessary. This reoxidation might occur by chain elongation of fatty acids. Another possibility for anaerobic CO 2 formation has been described for goldfish
9 CO2 production in swimbladder tissue 503 muscle (Van den Thillart & Verbeek, 1982; Mourik, 1982). This CO 2 formation, which is combined with ethanol formation (Shoubridge & Hochachka, 1980), is predominantly located in the mitochondria of red muscle cells. The observed activities of the pentose phosphate shunt enzymes, and the histological observation that gas gland tissue contains only few mitochondria (Dorn, 1961), lead us to favour the pentose phosphate shunt as a possible pathway for anaerobic CO 2 formation. Glucose as a source of CO 2 formation Since the pentose phosphate shunt requires glucose, more glucose should be degraded in our experiments than would be expected from lactate formation and oxidative metabolism. The balance of glucose metabolism is, therefore, of interest in our study. The glycogen stores in swimbladder tissue are too small to fuel glycolysis for more than a few minutes (D'Aoust, 1970), and blood (or saline) glucose is expected to be the main source for metabolism. Three pathways of glucose degradation have to be considered in our study: aerobic oxidation to CO 2, anaerobic fermentation to lactic acid and anaerobic decarboxylation reactions (for example, the pentose phosphate shunt). Aerobic metabolism does not seem to be of great importance in gas gland cells. In histological studies Dorn (1961) and Morris & Albright (1975) found only thin and irregularly distributed mitochondria. Bostrom et al. (1972) measured only a very low activity of cytochrome oxidase in gas gland tissue of the cod, Gadus morhua. This is in line with the low rate of aerobic glucose metabolism in our experiments, which was estimated from the O 2 consumption rate to be 2nmol min" 1 or only 1-2% of the total glucose metabolized. Swimbladder tissue is known to produce lactic acid even in the presence of oxygen (Ball et al. 1955; D'Aoust, 1970), and its rate of production allows us to estimate the rate of glucose metabolized by this route. Since two lactic acid molecules are formed from each glucose molecule, a MLa/Mciu ra tio of 2 would indicate complete degradation of glucose to lactate. The average value in our study of 1-2 indicates that only 60% of the glucose was used for anaerobic glycolysis. Considering also the oxidatively metabolized glucose, about 38% of the glucose metabolized could have entered the pentose phosphate shunt. It is not possible to predict the ratio of CO 2 to glucose in this pathway, but our data seem to indicate that most of the CO 2 was formed by the pentose phosphate shunt. Physiological significance of anaerobic CO 2 formation Anaerobic formation of CO2 appears to have several advantages for gas secretion into the swimbladder, which requires generation of high gas partial pressures, particularly of O 2 and CO 2, in blood (Fange, 1983). Let us first consider a situation in which high O 2 and CO 2 gas partial pressures are formed in a swimbladder by aerobic CO 2 and anaerobic lactic acid formation, but with no extra CO 2 production (Fig. 3). Pco? would mainly be increased by aerobic CO 2
10 504 B. PELSTER, H. KOBAYASHI AND P. SCHEID A Blood Gas gland Swimbladder Glucose - Glucose ^-Glycogen HbO 2 Pyruvate 1,. \ r?c>] v Tnose, CO2 Lactate, H + ',TCA Po,, Pco, Fig. 3. (A) Diagram of metabolic processes in gas gland cells contributing to the formation of CO2 and to the delivery of oxygen from the haemoglobin (Hb) in the capillaries. The anaerobic decarboxylation reaction was assumed to occur in the pentose phosphate shunt (PPS). Open arrows (<=) indicate an influence on haemoglobin oxygen-affinity. TCA, tricarboxylic acid cycle. (B) The diffusion gradients for the gases CO 2 and O 2 shown schematically. formation, and countercurrent enhancement in the rete (Kuhn et al. 1963) would further increase Pco?- Th e ensuing combined respiratory and metabolic acidosis (lactic acid formation) would release O 2 from the haemoglobin bond via the Bohr and Root effects (see Steen, 1970), and the increased P O2 would be further enhanced in the rete countercurrent system. It should, however, be noted that both CO2 and O 2 secretion into the swimbladder would counteract the increases in their partial pressure. Moreover, any CO 2 molecule formed would consume one O 2 molecule, thus also reducing Po 2 - And, even if some CO 2 were formed from HCO 3 ~, this would reduce the total salt concentration which is required for raising the partial pressures of inert gases (e.g. N 2 and Ar) by the salting-out effect (Gerth & Hemmingsen, 1982; Enns etal. 1967; Pelster et al. 1988a). In the presence of an anaerobic route for CO 2 formation (Fig. 3), the efficiency of raising all partial pressures would be significantly increased. Pco 2 itself cai
11 CO 2 production in swimbladder tissue 505 thereby attain higher values, and this would not reduce P^ levels. The highest Pcch values will be found in the gas gland cells, where CO 2 is formed, and CO 2 will diffuse from the tissue to the swimbladder and to the blood (Fig. 3). The concomitantly more pronounced reduction in ph would yield higher Pc^ levels in the blood by the Root and Bohr effect. Furthermore, HCO 3 ~ could be formed from CO 2 despite the lactacidosis, and this would increase the salt concentration for the salting-out effect. It is thus evident that anoxidative CO 2 formation, for example via the pentose phosphate shunt, may be of great significance for the formation of high gas partial pressures in the swimbladder. The authors wish to thank Mrs G. Ryfa and Mr S. Rohr for expert technical assistance. Financial support by the Ministerium fur Wissenschaft und Forschung des Landes Nordrhein-Westfalen (Grant no. IV B ) is gratefully acknowledged. References BALL, E. G., STRrrrMATTER, C. F. & COOPER, O. (1955). Metabolic studies on the gas gland of the swim bladder. Biol. Bull. mar. biol. Lab., Woods Hole 108, BERGMEYER, H. U. (ed.) (1974). Methoden der Enzymatischen Analyse. Weinheim: Verlag Chemie.Bd.Iu.II. BOSTROM, S. L., FANGE, R. & JOHANSSON, R. G. (1972). Enzyme activity patterns in gas gland tissue of the swimbladder of the cod (Gadus morhua). Comp. Biochem. Physiol. 43B, CAMERON, J. N. (1971). Rapid method for determination of total carbon dioxide in small blood samples. J. appl. Physiol. 31, D'AOUST, B. G. (1970). The role of lactic acid in gas secretion in the teleost swimbladder. Comp. Biochem. Physiol. 32, DECK, J. E. (1970). Lactic acid production by the swimbladder gas gland in vitro as influenced by glucagon and epinephrine. Comp. Biochem. Physiol. 34, DORN, E. (1961). Uber den Feinbau der Schwimmblase von Anguilla vulgaris L. Licht- und Elektronenmikroskopische Untersuchungen. Zellforsch. mikrosk. Anal. 55, ENNS, T., DOUGLAS, E. & SCHOLANDER, P. F. (1967). Role of the swimbladder rete of fish in secretion in inert gas and oxygen. Adv. Biol. Med. Phys. 11, FANGE, R. (1983). Gas exchange in fish swim bladder. Rev. Physiol. Biochem. Pharmac. 97, GERTH, W. A. & HEMMINGSEN, E. A. (1982). Limits of gas secretion by the salting-out effect in fish swimbladder rete. J. comp. Physiol. 146, HOFFERT, J. R. & FROMM, P. O. (1970). Quantitative aspects of glucose catabolism by rainbow and lake trout ocular tissues including alterations resulting from various pathological conditions. Expl Eye Res. 10, KUHN, H. J., MOSER, P. & KUHN, W. (1962). Haarnadelgegenstrom als Grundlage zur Erzeugung hoher Gasdriicke in der Schwimmblase von Tiefseefischen. Pfliigers Arch. ges. Physiol. 275, KUHN, W., RAMEL, A., KUHN, H. J. & MARTI, E. (1963). The filling mechanism of the swimbladder. Generation of high gas pressures through hairpin countercurrent multiplication. Experientia 19, MORRIS, S. M. & ALBRIGHT, J. T. (1975). The ultrastructure of the swimbladder of the toadfish, Opsanus tau L. Cell. Tissue Res. 164, MOURIK, J. (1982). Anaerobic metabolism in red skeletal muscle of goldfish (Carassius auratus L.). PhD thesis, University of Leiden, 163 pp.
12 506 B. PELSTER, H. KOBAYASHI AND P. SCHEID PELSTER, B., KOBAYASHI, H. & SCHEID, P. (1988a). Solubility of nitrogen and argon in eel whole blood and its relationship to ph. J. exp. Biol. 135, PELSTER, B., KOBAYASHI, H. & SCHEID, P. (19886). Production of acid by the gas gland of the swimbladder. Pflugers Arch. ges. Physiol. 412, R40. SHOUBRIDGE, E. A. & HOCHACHKA, P. W. (1980). Ethanol: novel endproduct of vertebrate anaerobic metabolism. Science 209, STEEN, J. B. (1963a). The physiology of the swimbladder in the eel Anguilla vulgaris. II. The reabsorption of gases. Acta physiol. Scand. 58, STEEN, J. B. (19636). The physiology of the swimbladder in the eel Anguilla vulgaris. III. The mechanism of gas secretion. Acta physiol. Scand. 59, STEEN, J. B. (1970). The swim bladder as a hydrostatic organ. In Fish Physiology, vol. IV (ed. W. S. Hoar & D. J. Randall), pp New York: Academic Press. TUCKER, V. A. (1967). Method for oxygen content and dissociation curves on microliter blood samples. /. appl. Physiol. 23, VAN DEN THILLART, G. & VERBEEK, R. (1982). Substrates for anaerobic CO2-production by the goldfish, Carassius auratus (L.): Decarboxylation of 14 C-labeled metabolites. /. comp. Physiol. 149, WITTENBERG, J. B., SCHWEND, M. J. & WITTENBERG, B. A. (1964). The secretion of oxygen into the swim-bladder of fish. III. The role of carbon dioxide. /. gen. Physiol. 48,
CONTRIBUTION OF THE PENTOSE PHOSPHATE SHUNT TO THE FORMATION OF CO 2 IN SWIMBLADDER TISSUE OF THE EEL
 J. exp. Biol. 197, 119 128 (1994) Printed in Great Britain The Company of Biologists Limited 1994 119 CONTRIBUTION OF THE PENTOSE PHOSPHATE SHUNT TO THE FORMATION OF CO 2 IN SWIMBLADDER TISSUE OF THE EEL
J. exp. Biol. 197, 119 128 (1994) Printed in Great Britain The Company of Biologists Limited 1994 119 CONTRIBUTION OF THE PENTOSE PHOSPHATE SHUNT TO THE FORMATION OF CO 2 IN SWIMBLADDER TISSUE OF THE EEL
GLUCOSE METABOLISM OF THE SWIMBLADDER TISSUE OF THE EUROPEAN EEL ANGUILLA ANGUILLA
 J. exp. Biol. 185, 169 178 (1993) Printed in Great Britain The Company of Biologists Limited 1993 169 GLUCOSE METABOLISM OF THE SWIMBLADDER TISSUE OF THE EUROPEAN EEL ANGUILLA ANGUILLA BERND PELSTER AND
J. exp. Biol. 185, 169 178 (1993) Printed in Great Britain The Company of Biologists Limited 1993 169 GLUCOSE METABOLISM OF THE SWIMBLADDER TISSUE OF THE EUROPEAN EEL ANGUILLA ANGUILLA BERND PELSTER AND
SIMULTANEOUS DIRECT AND INDIRECT CALORIMETRY ON NORMOXIC AND ANOXIC GOLDFISH
 J. exp. Biol. 142, 325-335 (1989) 325 Printed in Great Britain The Company of Biologists Limited 1989 SIMULTANEOUS DIRECT AND INDIRECT CALORIMETRY ON NORMOXIC AND ANOXIC GOLDFISH BY J. VAN WAVERSVELD,
J. exp. Biol. 142, 325-335 (1989) 325 Printed in Great Britain The Company of Biologists Limited 1989 SIMULTANEOUS DIRECT AND INDIRECT CALORIMETRY ON NORMOXIC AND ANOXIC GOLDFISH BY J. VAN WAVERSVELD,
Class XI Chapter 14 Respiration in Plants Biology. 1. It is a biochemical process. 1. It is a physiochemical process.
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
 Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Question 1: Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs cycle (c) Aerobic respiration and Fermentation (a) Respiration and combustion Respiration Combustion 1. It is a
Plant Respiration. Exchange of Gases in Plants:
 Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
Plant Respiration Exchange of Gases in Plants: Plants do not have great demands for gaseous exchange. The rate of respiration in plants is much lower than in animals. Large amounts of gases are exchanged
CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM
 CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM Metabolism Bioenergetics is the transfer and utilization of energy in biological systems The direction and extent to which a chemical reaction
CHY2026: General Biochemistry UNIT 7& 8: CARBOHYDRATE METABOLISM Metabolism Bioenergetics is the transfer and utilization of energy in biological systems The direction and extent to which a chemical reaction
3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]
![3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]](/thumbs/72/66350985.jpg) 3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
Chapter 5 MITOCHONDRIA AND RESPIRATION 5-1
 Chapter 5 MITOCHONDRIA AND RESPIRATION All organisms must transform energy. This energy is required to maintain a dynamic steady state, homeostasis, and to insure continued survival. As will be discussed
Chapter 5 MITOCHONDRIA AND RESPIRATION All organisms must transform energy. This energy is required to maintain a dynamic steady state, homeostasis, and to insure continued survival. As will be discussed
CARBOHYDRATE METABOLISM
 Note (Study Glycolysis, fermentation and their regulation, Gluconeogenesis and glycogenolysis, Metabolism of galactose, TCA cycle and Amphibolic role of the cycle, and Glyoxalic acid cycle, HMP shunt in
Note (Study Glycolysis, fermentation and their regulation, Gluconeogenesis and glycogenolysis, Metabolism of galactose, TCA cycle and Amphibolic role of the cycle, and Glyoxalic acid cycle, HMP shunt in
Biology Chapter-7 Cellular Respiration
 Biology-1406 Chapter-7 Cellular Respiration Energy is stored in Chemicals Catabolism- the breaking down of complex molecules, such as glucose, to release their stored energy. Catabolism may or may not
Biology-1406 Chapter-7 Cellular Respiration Energy is stored in Chemicals Catabolism- the breaking down of complex molecules, such as glucose, to release their stored energy. Catabolism may or may not
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
RESPIRATION IN PLANTS
 7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a Cytochrome b Oxygen c Hydrogen d Glucose 2. Phosphorylation
7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a Cytochrome b Oxygen c Hydrogen d Glucose 2. Phosphorylation
In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic
 Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
ALOYSIUS G.M. TIELENS, JOSEPHUS M. VAN DEN HEUVEL and SIMON G. VAN DEN BERGH
 Molecular and Biochemical Parasitology, 13 (1984) 301-307 Elsevier 301 MBP 00488 THE ENERGY METABOLISM OF FASCIOLA HEPATICA DURING ITS DEVELOPMENT IN THE FINAL HOST ALOYSIUS G.M. TIELENS, JOSEPHUS M. VAN
Molecular and Biochemical Parasitology, 13 (1984) 301-307 Elsevier 301 MBP 00488 THE ENERGY METABOLISM OF FASCIOLA HEPATICA DURING ITS DEVELOPMENT IN THE FINAL HOST ALOYSIUS G.M. TIELENS, JOSEPHUS M. VAN
1. The ultimate electron acceptor of respiration in an aerobic organisms is: Cytochrome Oxygen Hydrogen Glucose
 7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a b c d Cytochrome Oxygen Hydrogen Glucose 2. Phosphorylation
7777 CHAPTER 14 RESPIRATION IN PLANTS MULTIPLE CHOICE QUESTIONS 1. The ultimate electron acceptor of respiration in an aerobic organisms is: a b c d Cytochrome Oxygen Hydrogen Glucose 2. Phosphorylation
Review of Carbohydrate Digestion
 Review of Carbohydrate Digestion Glycolysis Glycolysis is a nine step biochemical pathway that oxidizes glucose into two molecules of pyruvic acid. During this process, energy is released and some of it
Review of Carbohydrate Digestion Glycolysis Glycolysis is a nine step biochemical pathway that oxidizes glucose into two molecules of pyruvic acid. During this process, energy is released and some of it
Chapter 7 Cellular Respiration and Fermentation*
 Chapter 7 Cellular Respiration and Fermentation* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Life Is Work
Chapter 7 Cellular Respiration and Fermentation* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. Life Is Work
MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY
 MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY BOOKLET 10 NAME: CLASS: 1 S.Tagore Middletown South High School March 2013 LEARNING OUTCOMES The role and production of ATP (a) Importance, role and structure of ATP
MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY BOOKLET 10 NAME: CLASS: 1 S.Tagore Middletown South High School March 2013 LEARNING OUTCOMES The role and production of ATP (a) Importance, role and structure of ATP
Chapter 9: Cellular Respiration: Harvesting Chemical Energy
 AP Biology Reading Guide Name: Date: Period Chapter 9: Cellular Respiration: Harvesting Chemical Energy Overview: Before getting involved with the details of cellular respiration and photosynthesis, take
AP Biology Reading Guide Name: Date: Period Chapter 9: Cellular Respiration: Harvesting Chemical Energy Overview: Before getting involved with the details of cellular respiration and photosynthesis, take
Chemical Energy. Valencia College
 9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
9 Pathways that Harvest Chemical Energy Valencia College 9 Pathways that Harvest Chemical Energy Chapter objectives: How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of
anabolic pathways- Catabolic Amphibolic
 METABOLISM Introduction The fate of dietary components after digestion and absorption constitute metabolism regulated by metabolic pathway 3 types: anabolic pathways- Synthesis of compound e.g. synthesis
METABOLISM Introduction The fate of dietary components after digestion and absorption constitute metabolism regulated by metabolic pathway 3 types: anabolic pathways- Synthesis of compound e.g. synthesis
Background knowledge
 Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
Integration of Metabolism
 Integration of Metabolism Metabolism is a continuous process. Thousands of reactions occur simultaneously in order to maintain homeostasis. It ensures a supply of fuel, to tissues at all times, in fed
Integration of Metabolism Metabolism is a continuous process. Thousands of reactions occur simultaneously in order to maintain homeostasis. It ensures a supply of fuel, to tissues at all times, in fed
R = Ribose / pentose (sugar). [3] (b) (i) Supplies energy; all reactions; in all cells; [Max 2] (not: produces)
![R = Ribose / pentose (sugar). [3] (b) (i) Supplies energy; all reactions; in all cells; [Max 2] (not: produces) R = Ribose / pentose (sugar). [3] (b) (i) Supplies energy; all reactions; in all cells; [Max 2] (not: produces)](/thumbs/93/114252105.jpg) . (a) P = phosphate (not: phosphoric acid) Q = nitrogenous base / organic base / adenine; R = Ribose / pentose (sugar). [3] (b) (i) Supplies energy; all reactions; in all cells; [Max ] (not: produces)
. (a) P = phosphate (not: phosphoric acid) Q = nitrogenous base / organic base / adenine; R = Ribose / pentose (sugar). [3] (b) (i) Supplies energy; all reactions; in all cells; [Max ] (not: produces)
Introduction. Living is work. To perform their many tasks, cells must bring in energy from outside sources.
 Introduction Living is work. To perform their many tasks, cells must bring in energy from outside sources. In most ecosystems, energy enters as sunlight. Light energy trapped in organic molecules is available
Introduction Living is work. To perform their many tasks, cells must bring in energy from outside sources. In most ecosystems, energy enters as sunlight. Light energy trapped in organic molecules is available
Chapter 8 Mitochondria and Cellular Respiration
 Chapter 8 Mitochondria and Cellular Respiration Cellular respiration is the process of oxidizing food molecules, like glucose, to carbon dioxide and water. The energy released is trapped in the form of
Chapter 8 Mitochondria and Cellular Respiration Cellular respiration is the process of oxidizing food molecules, like glucose, to carbon dioxide and water. The energy released is trapped in the form of
Gas Exchange in the Tissues
 Gas Exchange in the Tissues As the systemic arterial blood enters capillaries throughout the body, it is separated from the interstitial fluid by only the thin capillary wall, which is highly permeable
Gas Exchange in the Tissues As the systemic arterial blood enters capillaries throughout the body, it is separated from the interstitial fluid by only the thin capillary wall, which is highly permeable
Visit for Videos, Questions and Revision Notes. Describe how acetylcoenzyme A is formed in the link reaction
 Q1.(a) Describe how acetylcoenzyme A is formed in the link reaction. (b) In the Krebs cycle, acetylcoenzyme A combines with four-carbon oxaloacetate to form six-carbon citrate. This reaction is catalysed
Q1.(a) Describe how acetylcoenzyme A is formed in the link reaction. (b) In the Krebs cycle, acetylcoenzyme A combines with four-carbon oxaloacetate to form six-carbon citrate. This reaction is catalysed
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
How Cells Release Chemical Energy. Chapter 7
 How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
Cellular Respiration. Cellular Respiration. C 6 H 12 O 6 + 6O > 6CO 2 + 6H energy. Heat + ATP. You need to know this!
 Cellular Respiration LISA Biology Cellular Respiration C 6 H 12 O 6 + 6O 2 - - - - - > 6CO 2 + 6H 2 0 + energy You need to know this! Heat + ATP 1 Did that equation look familiar? * The equation for cellular
Cellular Respiration LISA Biology Cellular Respiration C 6 H 12 O 6 + 6O 2 - - - - - > 6CO 2 + 6H 2 0 + energy You need to know this! Heat + ATP 1 Did that equation look familiar? * The equation for cellular
CLASS 11 th. Respiration in Plants
 CLASS 11 th 01. Introduction All living cells require continuous supply of energy to perform various vital activities. This energy is released in controlled manner for cellular use via the process of respiration.
CLASS 11 th 01. Introduction All living cells require continuous supply of energy to perform various vital activities. This energy is released in controlled manner for cellular use via the process of respiration.
W ; Z ; X ; W ; 4 IGNORE ref to phosphorylation of glucose as this is taken into account in estimate.
 1 (a) (i) Mark the first answer on each prompt line. If the answer is crect and an additional answer is given that is increct contradicts the crect answer then = 0 marks 1 (a) (ii) W ; Z ; X ; W ; 4 IGNORE
1 (a) (i) Mark the first answer on each prompt line. If the answer is crect and an additional answer is given that is increct contradicts the crect answer then = 0 marks 1 (a) (ii) W ; Z ; X ; W ; 4 IGNORE
UNIVERSITY OF BOLTON SCHOOL OF SPORT AND BIOMEDICAL SCIENCES SPORT PATHWAYS WITH FOUNDATION YEAR SEMESTER TWO EXAMINATIONS 2015/2016
 LH8 UNIVERSITY OF BOLTON SCHOOL OF SPORT AND BIOMEDICAL SCIENCES SPORT PATHWAYS WITH FOUNDATION YEAR SEMESTER TWO EXAMINATIONS 2015/2016 INTRODUCTION TO HUMAN PHYSIOLOGY MODULE NO: SRB3008 Date: Monday
LH8 UNIVERSITY OF BOLTON SCHOOL OF SPORT AND BIOMEDICAL SCIENCES SPORT PATHWAYS WITH FOUNDATION YEAR SEMESTER TWO EXAMINATIONS 2015/2016 INTRODUCTION TO HUMAN PHYSIOLOGY MODULE NO: SRB3008 Date: Monday
Metabolism. Chapter 5. Catabolism Drives Anabolism 8/29/11. Complete Catabolism of Glucose
 8/29/11 Metabolism Chapter 5 All of the reactions in the body that require energy transfer. Can be divided into: Cell Respiration and Metabolism Anabolism: requires the input of energy to synthesize large
8/29/11 Metabolism Chapter 5 All of the reactions in the body that require energy transfer. Can be divided into: Cell Respiration and Metabolism Anabolism: requires the input of energy to synthesize large
4. Which step shows a split of one molecule into two smaller molecules? a. 2. d. 5
 1. Which of the following statements about NAD + is false? a. NAD + is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD + has more chemical energy than NADH. c. NAD + is reduced
1. Which of the following statements about NAD + is false? a. NAD + is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD + has more chemical energy than NADH. c. NAD + is reduced
Anaerobic Pathways. Glycolysis
 Anaerobic Pathways Glycolysis Glucose + 2 ATP 4 ATP + 2 Pyruvate No oxygen required Fairly low energy yield Lactate byproduct Resting levels low Tolerances 40 mmole/kg in humans, 200 mmole/kg in sea turtles
Anaerobic Pathways Glycolysis Glucose + 2 ATP 4 ATP + 2 Pyruvate No oxygen required Fairly low energy yield Lactate byproduct Resting levels low Tolerances 40 mmole/kg in humans, 200 mmole/kg in sea turtles
NCERT SOLUTIONS OF Life Processes
 1 NCERT SOLUTIONS OF Life Processes Question 1: Why is diffusion insufficient to meet the oxygen requirements of multicellular organisms like humans? Answer: The body structure of multicellular organism
1 NCERT SOLUTIONS OF Life Processes Question 1: Why is diffusion insufficient to meet the oxygen requirements of multicellular organisms like humans? Answer: The body structure of multicellular organism
1 CH:14 RESPIRATION IN PLANTS https://biologyaipmt.com/
 1 CH:14 RESPIRATION IN PLANTS https://biologyaipmt.com/ CHAPTER 14 RESPIRATION IN PLANTS All the energy required for 'life' processes is obtained by oxidation of some macromolecules that we call 'food'.
1 CH:14 RESPIRATION IN PLANTS https://biologyaipmt.com/ CHAPTER 14 RESPIRATION IN PLANTS All the energy required for 'life' processes is obtained by oxidation of some macromolecules that we call 'food'.
Oxygen and CO 2 transport. Biochemistry II
 Oxygen and CO 2 transport 2 Acid- base balance Biochemistry II Lecture 9 2008 (J.S.) 1 Transport of O 2 and CO 2 O 2 INSPIRED AIR CO 2 EXPIRED AIR HCO 3 + HHb + Lungs HbO 2 + H + + HCO 3 HbO 2 + H 2 O
Oxygen and CO 2 transport 2 Acid- base balance Biochemistry II Lecture 9 2008 (J.S.) 1 Transport of O 2 and CO 2 O 2 INSPIRED AIR CO 2 EXPIRED AIR HCO 3 + HHb + Lungs HbO 2 + H + + HCO 3 HbO 2 + H 2 O
Physiological Chemistry II Exam IV Dr. Melissa Kelley April 13, 2004
 Name Write your name on the back of the exam Physiological Chemistry II Exam IV Dr. Melissa Kelley April 13, 2004 This examination consists of forty-four questions, each having 2 points. The remaining
Name Write your name on the back of the exam Physiological Chemistry II Exam IV Dr. Melissa Kelley April 13, 2004 This examination consists of forty-four questions, each having 2 points. The remaining
UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017
 LH14 UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017 INTRODUCTION TO SPORT AND EXERCISE PHYSIOLOGY MODULE NO: SPS4002 Date: Thursday
LH14 UNIVERSITY OF BOLTON SPORT AND BIOLOGICAL SCIENCES SPORT AND EXERCISE SCIENCE PATHWAY SEMESTER TWO EXAMINATIONS 2016/2017 INTRODUCTION TO SPORT AND EXERCISE PHYSIOLOGY MODULE NO: SPS4002 Date: Thursday
University of Palestine. Final Exam 2016/2017 Total Grade:
 Part 1 : Multiple Choice Questions (MCQs) 1)Which of the following statements about Michaelis-Menten kinetics is correct? a)k m, the Michaelis constant, is defined as the concentration of substrate required
Part 1 : Multiple Choice Questions (MCQs) 1)Which of the following statements about Michaelis-Menten kinetics is correct? a)k m, the Michaelis constant, is defined as the concentration of substrate required
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Which of the following statements concerning anabolic reactions is FALSE? A. They are generally endergonic. B. They usually require ATP. C. They are part of metabolism. D.
MULTIPLE CHOICE QUESTIONS 1. Which of the following statements concerning anabolic reactions is FALSE? A. They are generally endergonic. B. They usually require ATP. C. They are part of metabolism. D.
Swimbladder gas gland cells cultured on permeable supports regain their characteristic polarity
 The Journal of Experimental Biology 204, 4023 4029 (2001) Printed in Great Britain The Company of Biologists Limited 2001 JEB3672 4023 Swimbladder gas gland cells cultured on permeable supports regain
The Journal of Experimental Biology 204, 4023 4029 (2001) Printed in Great Britain The Company of Biologists Limited 2001 JEB3672 4023 Swimbladder gas gland cells cultured on permeable supports regain
WJEC. Respiration. Questions
 WJEC Respiration Questions 6. Answer one of the following questions. Any diagrams included in your answer must be fully annotated. 13 Examiner only Arholwr yn unig Either, (a)
WJEC Respiration Questions 6. Answer one of the following questions. Any diagrams included in your answer must be fully annotated. 13 Examiner only Arholwr yn unig Either, (a)
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy
 Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
Energy Transformation: Cellular Respiration Outline 1. Sources of cellular ATP 2. Turning chemical energy of covalent bonds between C-C into energy for cellular work (ATP) 3. Importance of electrons and
2/25/2015. Anaerobic Pathways. Glycolysis. Alternate Endpoints. Gluconeogenesis fate of end products
 Anaerobic Pathways Glycolysis Glucose + 2 ATP 4 ATP + 2 Pyruvate No oxygen required Fairly low energy yield Lactate byproduct Resting levels low Tolerances 40 mmole/kg in humans, 200 mmole/kg in sea turtles
Anaerobic Pathways Glycolysis Glucose + 2 ATP 4 ATP + 2 Pyruvate No oxygen required Fairly low energy yield Lactate byproduct Resting levels low Tolerances 40 mmole/kg in humans, 200 mmole/kg in sea turtles
METABOLISM Biosynthetic Pathways
 METABOLISM Biosynthetic Pathways Metabolism Metabolism involves : Catabolic reactions that break down large, complex molecules to provide energy and smaller molecules. Anabolic reactions that use ATP energy
METABOLISM Biosynthetic Pathways Metabolism Metabolism involves : Catabolic reactions that break down large, complex molecules to provide energy and smaller molecules. Anabolic reactions that use ATP energy
CONTEXT POINT 2: Plants and animals transport dissolved nutrients and gases in a fluid medium.
 CONTEXT POINT 2: Plants and animals transport dissolved nutrients and gases in a fluid medium. Identify the form(s) in which each of the following is carried in mammalian blood: Carbon dioxide 70% as hydrogen
CONTEXT POINT 2: Plants and animals transport dissolved nutrients and gases in a fluid medium. Identify the form(s) in which each of the following is carried in mammalian blood: Carbon dioxide 70% as hydrogen
Introduction to Carbohydrate metabolism
 Introduction to Carbohydrate metabolism Some metabolic pathways of carbohydrates 1- Glycolysis 2- Krebs cycle 3- Glycogenesis 4- Glycogenolysis 5- Glyconeogenesis - Pentose Phosphate Pathway (PPP) - Curi
Introduction to Carbohydrate metabolism Some metabolic pathways of carbohydrates 1- Glycolysis 2- Krebs cycle 3- Glycogenesis 4- Glycogenolysis 5- Glyconeogenesis - Pentose Phosphate Pathway (PPP) - Curi
A cell has enough ATP to last for about three seconds.
 Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Energy Transformation: Cellular Respiration Outline 1. Energy and carbon sources in living cells 2. Sources of cellular ATP 3. Turning chemical energy of covalent bonds between C-C into energy for cellular
Acid - base equilibrium
 Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Acid base equilibrium ph concept ph = log [H + ] ph [H+] 1 100 mmol/l D = 90 mmol/l 2 10 mmol/l D = 9 mmol/l 3 1 mmol/l 2 ph = log [H + ] 3 ph ph = log [H + ] ph of capillary blood norm: 7,35 7,45 Sorensen
Page 2 of 51 WJEC/CBAC 2016 pdfcrowd.com
 1. Page 2 of 51 WJEC/CBAC 2016 Page 3 of 51 WJEC/CBAC 2016 2. Page 4 of 51 WJEC/CBAC 2016 Page 5 of 51 WJEC/CBAC 2016 3. Page 6 of 51 WJEC/CBAC 2016 Page 7 of 51 WJEC/CBAC 2016 4. Page 8 of 51 WJEC/CBAC
1. Page 2 of 51 WJEC/CBAC 2016 Page 3 of 51 WJEC/CBAC 2016 2. Page 4 of 51 WJEC/CBAC 2016 Page 5 of 51 WJEC/CBAC 2016 3. Page 6 of 51 WJEC/CBAC 2016 Page 7 of 51 WJEC/CBAC 2016 4. Page 8 of 51 WJEC/CBAC
Cellular Respiration
 Cellular Respiration Chemical Equation 6 O 2 + C 6 H 12 O 6 6 H 2 O + 6 CO 2 + Page 107 Adenosine Triphosphate Adenosine Diphosphate Background Aerobic= requires oxygen Anaerobic= does not require oxygen
Cellular Respiration Chemical Equation 6 O 2 + C 6 H 12 O 6 6 H 2 O + 6 CO 2 + Page 107 Adenosine Triphosphate Adenosine Diphosphate Background Aerobic= requires oxygen Anaerobic= does not require oxygen
Chap 3 Metabolism and Growth
 Chap 3 Metabolism and Growth I. Metabolism Definitions: Metabolism includes two parts: anabolism and catabolism Catabolism: Anabolism: Aerobic metabolism: catabolism anabolis m catabolis anabolis m Anaerobic
Chap 3 Metabolism and Growth I. Metabolism Definitions: Metabolism includes two parts: anabolism and catabolism Catabolism: Anabolism: Aerobic metabolism: catabolism anabolis m catabolis anabolis m Anaerobic
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Collin County Community College BIOL Muscle Physiology. Muscle Length-Tension Relationship
 Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Chapter 44. Regulating the Internal Environment. AP Biology
 Chapter 44. Regulating the Internal Environment Homeostasis Living in the world organisms had a choice: regulate their internal environment maintain relatively constant internal conditions conform to the
Chapter 44. Regulating the Internal Environment Homeostasis Living in the world organisms had a choice: regulate their internal environment maintain relatively constant internal conditions conform to the
Medical Biochemistry and Molecular Biology department
 Medical Biochemistry and Molecular Biology department Cardiac Fuels [Sources of energy for the Cardiac muscle] Intended learning outcomes of the lecture: By the end of this lecture you would be able to:-
Medical Biochemistry and Molecular Biology department Cardiac Fuels [Sources of energy for the Cardiac muscle] Intended learning outcomes of the lecture: By the end of this lecture you would be able to:-
Cell Respiration. Anaerobic & Aerobic Respiration
 Cell Respiration Anaerobic & Aerobic Respiration Understandings/Objectives 2.8.U1: Cell respiration is the controlled release of energy from organic compounds to produce ATP. Define cell respiration State
Cell Respiration Anaerobic & Aerobic Respiration Understandings/Objectives 2.8.U1: Cell respiration is the controlled release of energy from organic compounds to produce ATP. Define cell respiration State
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Metabolism. Metabolism. Energy. Metabolism. Energy. Energy 5/22/2016
 5//016 Metabolism Metabolism All the biochemical reactions occurring in the body Generating, storing and expending energy ATP Supports body activities Assists in constructing new tissue Metabolism Two
5//016 Metabolism Metabolism All the biochemical reactions occurring in the body Generating, storing and expending energy ATP Supports body activities Assists in constructing new tissue Metabolism Two
Fluid and Electrolytes P A R T 4
 Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Fluid and Electrolytes P A R T 4 Mechanisms that control acid-base homeostasis Acids and bases continually enter and leave body Hydrogen ions also result from metabolic activity Acids Hydrogen ion donors
Carbohydrate Metabolism
 Chapter 34 Carbohydrate Metabolism Carbohydrate metabolism is important for both plants and animals. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison,
Chapter 34 Carbohydrate Metabolism Carbohydrate metabolism is important for both plants and animals. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison,
Haemoglobin Revision. May minutes. 85 marks. Page 1 of 32
 Haemoglobin Revision May 103 97 minutes 85 marks Page 1 of 3 Q1. (a) The graph shows a dissociation curve for human haemoglobin at ph 7.4. The position of the curve is different at ph 7.. (i) (ii) Sketch
Haemoglobin Revision May 103 97 minutes 85 marks Page 1 of 3 Q1. (a) The graph shows a dissociation curve for human haemoglobin at ph 7.4. The position of the curve is different at ph 7.. (i) (ii) Sketch
7 Pathways That Harvest Chemical Energy
 7 Pathways That Harvest Chemical Energy Pathways That Harvest Chemical Energy How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of Glucose Metabolism? How Is Energy Harvested
7 Pathways That Harvest Chemical Energy Pathways That Harvest Chemical Energy How Does Glucose Oxidation Release Chemical Energy? What Are the Aerobic Pathways of Glucose Metabolism? How Is Energy Harvested
Chapter 7: How Cells Harvest Energy AP
 Chapter 7: How Cells Harvest Energy AP Essential Knowledge 1.B.1 distributed among organisms today. (7.1) 1.D.2 Organisms share many conserved core processes and features that evolved and are widely Scientific
Chapter 7: How Cells Harvest Energy AP Essential Knowledge 1.B.1 distributed among organisms today. (7.1) 1.D.2 Organisms share many conserved core processes and features that evolved and are widely Scientific
Cellular Respiration. 3. In the figure, which step of the citric acid cycle requires both NAD+ and ADP as reactants? a. Step 1. c. Step 3 b.
 Cellular Respiration 1. Enzymes are organic catalysts. How do they increase the rate of chemical reactions? a. By decreasing the free-energy change of the reaction b. By increasing the free-energy change
Cellular Respiration 1. Enzymes are organic catalysts. How do they increase the rate of chemical reactions? a. By decreasing the free-energy change of the reaction b. By increasing the free-energy change
How Did Energy-Releasing Pathways Evolve? (cont d.)
 How Did Energy-Releasing Pathways Evolve? (cont d.) 7.1 How Do Cells Access the Chemical Energy in Sugars? In order to use the energy stored in sugars, cells must first transfer it to ATP The energy transfer
How Did Energy-Releasing Pathways Evolve? (cont d.) 7.1 How Do Cells Access the Chemical Energy in Sugars? In order to use the energy stored in sugars, cells must first transfer it to ATP The energy transfer
Acid Base Balance. Professor Dr. Raid M. H. Al-Salih. Clinical Chemistry Professor Dr. Raid M. H. Al-Salih
 Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Acid Base Balance 1 HYDROGEN ION CONCENTRATION and CONCEPT OF ph Blood hydrogen ion concentration (abbreviated [H + ]) is maintained within tight limits in health, with the normal concentration being between
Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz )
 Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz ) Biochemistry Submitted by Marie Havlová on 8. February 2012-0:00 Syllabus of Biochemistry Mechanisms of enzyme catalysis.
Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz ) Biochemistry Submitted by Marie Havlová on 8. February 2012-0:00 Syllabus of Biochemistry Mechanisms of enzyme catalysis.
Cellular Respiration Checkup Quiz. 1. Of the following products, which is produced by both anaerobic respiration and aerobic respiration in humans?
 1. Of the following products, which is produced by both anaerobic respiration and aerobic respiration in humans? I. Pyruvate II. III. ATP Lactate A. I only B. I and II only C. I, II and III D. II and III
1. Of the following products, which is produced by both anaerobic respiration and aerobic respiration in humans? I. Pyruvate II. III. ATP Lactate A. I only B. I and II only C. I, II and III D. II and III
7 Cellular Respiration and Fermentation
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 7 Cellular Respiration and Fermentation Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
III. 6. Test. Respiració cel lular
 III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
Energy sources in skeletal muscle
 Energy sources in skeletal muscle Pathway Rate Extent ATP/glucose 1. Direct phosphorylation Extremely fast Very limited - 2. Glycolisis Very fast limited 2-3 3. Oxidative phosphorylation Slow Unlimited
Energy sources in skeletal muscle Pathway Rate Extent ATP/glucose 1. Direct phosphorylation Extremely fast Very limited - 2. Glycolisis Very fast limited 2-3 3. Oxidative phosphorylation Slow Unlimited
SHORT COMMUNICATION USE OF FILTRATION METHODS IN EVALUATION OF THE CONDITION OF FISH RED BLOOD CELLS
 J. exp. Biol. 138, 523-527 (1988) 523 Primed in Great Britain The Company of Biologists Limited 1988 SHORT COMMUNICATION USE OF FILTRATION METHODS IN EVALUATION OF THE CONDITION OF FISH RED BLOOD CELLS
J. exp. Biol. 138, 523-527 (1988) 523 Primed in Great Britain The Company of Biologists Limited 1988 SHORT COMMUNICATION USE OF FILTRATION METHODS IN EVALUATION OF THE CONDITION OF FISH RED BLOOD CELLS
Ch 07. Microbial Metabolism
 Ch 07 Microbial Metabolism SLOs Differentiate between metabolism, catabolism, and anabolism. Fully describe the structure and function of enzymes. Differentiate between constitutive and regulated enzymes.
Ch 07 Microbial Metabolism SLOs Differentiate between metabolism, catabolism, and anabolism. Fully describe the structure and function of enzymes. Differentiate between constitutive and regulated enzymes.
Integration of Metabolism 1. made by: Noor M. ALnairat. Sheet No. 18
 Integration of Metabolism 1 made by: Noor M. ALnairat Sheet No. 18 Data :24/11/2016 SLIDE 2: Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP,
Integration of Metabolism 1 made by: Noor M. ALnairat Sheet No. 18 Data :24/11/2016 SLIDE 2: Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP,
Bell Work. b. is wrong because combining two glucose molecules requires energy, it does not release energy
 Bell Work How is energy made available to the cell to move large starch molecules across the cell membrane through the process of endocytosis? a. removing a phosphate from ATP b. combining two glucose
Bell Work How is energy made available to the cell to move large starch molecules across the cell membrane through the process of endocytosis? a. removing a phosphate from ATP b. combining two glucose
Locomotion and Swimming
 Locomotion and Swimming Different categories Rest Swimming at constant velocity Swim to accelerate See review paper Langerhans and Reznick 2009 Types of Fishes and Habitats Shape and habitat are related
Locomotion and Swimming Different categories Rest Swimming at constant velocity Swim to accelerate See review paper Langerhans and Reznick 2009 Types of Fishes and Habitats Shape and habitat are related
Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration
 Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration Read this: Digestive enzymes are protein-based biological catalysts that play important roles in our lives. They help
Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration Read this: Digestive enzymes are protein-based biological catalysts that play important roles in our lives. They help
Microbial Metabolism. PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 5 Microbial Metabolism Big Picture: Metabolism Metabolism is the buildup and breakdown of nutrients
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 5 Microbial Metabolism Big Picture: Metabolism Metabolism is the buildup and breakdown of nutrients
D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor
![D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor D fini n tion: p = = -log [H+] ph=7 me m an s 10-7 Mol M H+ + (100 nmol m /l); ) p ; H=8 me m an s 10-8 Mol M H+ + (10 (10 n nmol m /l) Nor](/thumbs/75/72756397.jpg) Definition: ph regulation ph = -log [H + ] ph=7 means 10-7 Mol H + (100 nmol/l); ph=8 means 10 Normal plasma value: 7.35-7.45; 7.45; (H Acidosis: ph7.45 Intracellular ph = 7.1-7.3
Definition: ph regulation ph = -log [H + ] ph=7 means 10-7 Mol H + (100 nmol/l); ph=8 means 10 Normal plasma value: 7.35-7.45; 7.45; (H Acidosis: ph7.45 Intracellular ph = 7.1-7.3
AP Biology. Homeostasis. Chapter 44. Regulating the Internal Environment. Homeostasis
 Chapter 44. Regulating the Internal Environment omeostasis Living in the world organisms had a choice: regulate their internal environment maintain relatively constant internal conditions conform to the
Chapter 44. Regulating the Internal Environment omeostasis Living in the world organisms had a choice: regulate their internal environment maintain relatively constant internal conditions conform to the
Neaam Al-Bahadili. Rana J. Rahhal. Mamoun Ahram
 5 Neaam Al-Bahadili Rana J. Rahhal Mamoun Ahram In this sheet we will continue taking about Titration curve and Buffers in human body. Let s begin Titration curve of phosphate buffer: 1. There are 3 buffering
5 Neaam Al-Bahadili Rana J. Rahhal Mamoun Ahram In this sheet we will continue taking about Titration curve and Buffers in human body. Let s begin Titration curve of phosphate buffer: 1. There are 3 buffering
We can see the organelles that participate in photosynthesis with a microscope! Microscope Micro = small Scope = to look at
 We can see the organelles that participate in photosynthesis with a microscope! Microscope Micro = small Scope = to look at How do you use a microscope? 1. Always start on low power! 2. Use the coarse
We can see the organelles that participate in photosynthesis with a microscope! Microscope Micro = small Scope = to look at How do you use a microscope? 1. Always start on low power! 2. Use the coarse
Oxidation of Long Chain Fatty Acids
 Oxidation of Long Chain Fatty Acids Dr NC Bird Oxidation of long chain fatty acids is the primary source of energy supply in man and animals. Hibernating animals utilise fat stores to maintain body heat,
Oxidation of Long Chain Fatty Acids Dr NC Bird Oxidation of long chain fatty acids is the primary source of energy supply in man and animals. Hibernating animals utilise fat stores to maintain body heat,
BIO16 Mapua Institute of Technology
 BIO16 Mapua Institute of Technology The Marathon If somebody challenged you to a run a race, how should you prepare to win? 1. Practice 2. Eat the right foods 3. Drink the right liquids Energy All living
BIO16 Mapua Institute of Technology The Marathon If somebody challenged you to a run a race, how should you prepare to win? 1. Practice 2. Eat the right foods 3. Drink the right liquids Energy All living
How Cells Release Chemical Energy. Chapter 8
 How Cells Release Chemical Energy Chapter 8 Impacts, Issues: When Mitochondria Spin Their Wheels More than forty disorders related to defective mitochondria are known (such as Friedreich s ataxia); many
How Cells Release Chemical Energy Chapter 8 Impacts, Issues: When Mitochondria Spin Their Wheels More than forty disorders related to defective mitochondria are known (such as Friedreich s ataxia); many
Respiration. Respiration. How Cells Harvest Energy. Chapter 7
 How Cells Harvest Energy Chapter 7 Respiration Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs:
How Cells Harvest Energy Chapter 7 Respiration Organisms can be classified based on how they obtain energy: autotrophs: are able to produce their own organic molecules through photosynthesis heterotrophs:
Name Class Date. 1. Cellular respiration is the process by which the of "food"
 Name Class Date Cell Respiration Introduction Cellular respiration is the process by which the chemical energy of "food" molecules is released and partially captured in the form of ATP. Carbohydrates,
Name Class Date Cell Respiration Introduction Cellular respiration is the process by which the chemical energy of "food" molecules is released and partially captured in the form of ATP. Carbohydrates,
ATP. Chapter 7, parts of 48 Cellular Respiration: Gas Exchange, Other Metabolites & Control of Respiration. Cellular Respiration
 Chapter 7, parts of 48 Cellular Respiration: Gas Exchange, Other Metabolites & Control of Respiration Cellular Respiration ATP Gas Exchange O 2 & CO 2 exchange provides O 2 for aerobic cellular respiration
Chapter 7, parts of 48 Cellular Respiration: Gas Exchange, Other Metabolites & Control of Respiration Cellular Respiration ATP Gas Exchange O 2 & CO 2 exchange provides O 2 for aerobic cellular respiration
Cellular Respiration and Fermentation
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 9 Cellular Respiration and Fermentation
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 9 Cellular Respiration and Fermentation
Revision Question Bank
 Revision Question Bank Life Processes 1. Name the passage in sequence through which urine passes from kidney to the outside in human. How is urine prevented from flowing back into the ureter? The passage
Revision Question Bank Life Processes 1. Name the passage in sequence through which urine passes from kidney to the outside in human. How is urine prevented from flowing back into the ureter? The passage
Chapter 6 : How Cells Harvest Energy (B) Dr. Chris Doumen 10/28/14 CITRIC ACID CYCLE. Acetyl CoA CoA CoA CO 2 NAD + FADH 2 NADH FAD + 3 H + ADP + ATP
 Chapter 6 : How Cells Harvest Energy (B) Dr. Chris Doumen Acetyl CoA CoA CoA Oxaloacetate Citrate CITRIC ACID CYCLE CO FADH 3 NAD + FAD 3 NADH ADP + P + 3 1 Pyruvate oxida.on and Citric Acid Cycle Thus
Chapter 6 : How Cells Harvest Energy (B) Dr. Chris Doumen Acetyl CoA CoA CoA Oxaloacetate Citrate CITRIC ACID CYCLE CO FADH 3 NAD + FAD 3 NADH ADP + P + 3 1 Pyruvate oxida.on and Citric Acid Cycle Thus
Use the following diagram to answer the next question. 1. In the diagram above, pressure filtration occurs in a. W b. X c. Y d. Z
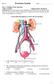 Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Dalkeith High School Higher Human Biology Homework 3
 Dalkeith High School Higher Human Biology Homework 3 1. During which of the following chemical conversions is A T P produced? A B C Amino acids protein Glucose pyruvic acid Haemoglobin oxyhaemoglobin energy
Dalkeith High School Higher Human Biology Homework 3 1. During which of the following chemical conversions is A T P produced? A B C Amino acids protein Glucose pyruvic acid Haemoglobin oxyhaemoglobin energy
CELLULAR RESPIRATION. Glycolysis
 CELLULAR RESPIRATION Glycolysis Sources of Energy Carbohydrates glucose most usable source of energy cells turn to other fuels only if glucose supplies have been depleted stored in glycogen (animal) &
CELLULAR RESPIRATION Glycolysis Sources of Energy Carbohydrates glucose most usable source of energy cells turn to other fuels only if glucose supplies have been depleted stored in glycogen (animal) &
