Enzyme Cut-outs Activity
|
|
|
- Marjory Knight
- 6 years ago
- Views:
Transcription
1 Name Date Group Block Enzyme Cut-outs Activity Objective: Enzymes are proteins that help chemical reactions occur at a faster rate by lowering the energy needed for the reactions. First, the enzymes react with a substrate to form an enzymesubstrate complex (like a lock and key). Once this complex is formed, the substrate becomes a product or products and leaves the enzyme. The enzyme can then repeat the reaction with more substrate. The enzyme is shaped so it will react with only one specific substrate. On the next page are shapes of enzymes, substrates, and products. Your job will be to cut them out, manipulate them, glue them, and explain the reaction that occurs. Complete Parts A, B, C, D. Materials directions and class notes construction paper glue cut-out sheet scissors pen or pencil Part A Vocabulary define the following terms (hint: you can use your notes!) enzyme catalyst chemical reaction activation energy substrate active site denatured THEN fold your card strip into four sections. NEXT open your card strip and draw a line lengthwise along the middle THEN: color the diagrams on the white cardstock according to the following: Enzyme PINK Triangular Substrate - PURPLE Square Substrate 1 - YELLOW Product 1 - BLUE Square Substrate 2 - BLUE Product 2 - RED Rectangular Product - GREEN Part B: HYDROLYSIS 1. In the first block of your strip, label the TOP with the word Hydrolysis Then - Using the enzyme cut-out card stock paper, cut out all of the triangular shaped enzymes, substrates, and products. 2. ACROSS THE TOP: Organize the cut outs on the remaining blocks of your strip so the pieces demonstrate this equation: enzyme + substrate enzyme-substrate complex enzyme + product 1 + product 2 3. Glue the cut outs in the appropriate places on the construction paper and then label each block with the above terms.
2 Part C: DEHYDRATION SYNTHESIS 1. In the first block of your strip, label the BOTTOM with the words Dehydration Synthesis Then - Using the enzyme cut-out card stock paper, cut out all of the square/rectangular shaped enzymes, substrates, and products. 2. ACROSS THE BOTTOM: Organize the cut outs on the remaining blocks of your strip so the pieces demonstrate this equation: Enzyme + substrate 1 + substrate 2 enzyme-substrate complex enzyme + product Part D: Graphing Each enzyme works best at a certain temperature and ph. Below or above an enzyme s optimal temperature or optimal ph, the reaction is slower. 1. Using the data table, graph the data to determine the optimum temperature for the enzyme catalase which speeds up the following reaction: H2O 2 H 2 O + O 2. ** Don t forget to include a title! 2. Describe the line that you graphed; what happens as the temperature increases? 3. What is the optimum temperature for which the enzyme activity is the greatest for this reaction? Temp ( o C) Reaction Rate (mol/min)
3 3. As with the previous sheet, glue and label the cutouts as the following compounds: Enzyme = Sucrase Substrates = glucose and fructose Product = sucrose 4. With the above terms and equation, explain what happened (write your explanation on the same side of construction paper). Use as many vocabulary words from Part A as you can. 4. Label the cutouts that you glued as the following compounds: Enzyme = lactase Substrate = lactose Products = glucose and galactose 5. With the above terms and equation, explain what happened (write your explanation on the same side of construction paper). Use as many vocabulary words from Part A as you can.
4 Part D Each enzyme works best at a certain temperature and ph. Below or above an enzyme s optimal temperature or optimal ph, the reaction is slower. 1. Using the table and grid below, graph the data to determine the optimum temperature for the enzyme catalase which speeds up the following reaction: H2O 2 H 2 O + O 2. Don t forget to include the title. 2. Describe the line that you just drew; what happens as temperature increases? 3. What is the optimum temperature for which the enzyme activity is the greatest for this reaction? Temp ( o C) Reaction Rate (mol/min)
5
Name Date Block. Lactase Lab. (Adapted from Lactase Investigation Philadelphia Public Schools and Enzymes Help Us Digest Food from Bryn Mawr)
 Name Date Block Lactase Lab (Adapted from Lactase Investigation Philadelphia Public Schools and Enzymes Help Us Digest Food from Bryn Mawr) BACKGROUND INFORMATION: The food we eat contains many different
Name Date Block Lactase Lab (Adapted from Lactase Investigation Philadelphia Public Schools and Enzymes Help Us Digest Food from Bryn Mawr) BACKGROUND INFORMATION: The food we eat contains many different
Enzymes Help Us Digest Food 1
 Enzymes Help Us Digest Food 1 Introduction to Sugars and Enzymes The food you eat contains many different types of molecules, including two types of sugar molecules: monosaccharides and disaccharides.
Enzymes Help Us Digest Food 1 Introduction to Sugars and Enzymes The food you eat contains many different types of molecules, including two types of sugar molecules: monosaccharides and disaccharides.
Human Biochemistry. Enzymes
 Human Biochemistry Enzymes Characteristics of Enzymes Enzymes are proteins which catalyze biological chemical reactions In enzymatic reactions, the molecules at the beginning of the process are called
Human Biochemistry Enzymes Characteristics of Enzymes Enzymes are proteins which catalyze biological chemical reactions In enzymatic reactions, the molecules at the beginning of the process are called
Biology Unit 3 Review. Objective 1. Describe the important functions of organic molecules Carbohydrates Lipids Proteins Nucleic acids
 Biology Unit 3 Review Name Objective 1. Describe the important functions of organic molecules Carbohydrates Lipids Proteins Nucleic acids 1. What is the difference between organic and inorganic molecules?
Biology Unit 3 Review Name Objective 1. Describe the important functions of organic molecules Carbohydrates Lipids Proteins Nucleic acids 1. What is the difference between organic and inorganic molecules?
Notes 2-4. Chemical Reactions and Enzymes
 Notes 2-4 Chemical Reactions and Enzymes Chemical Reaction: A process that changes one set of chemicals into another set of chemicals Reactants: Elements entered into the reaction Products: Elements or
Notes 2-4 Chemical Reactions and Enzymes Chemical Reaction: A process that changes one set of chemicals into another set of chemicals Reactants: Elements entered into the reaction Products: Elements or
Do Now #1. Name: Enzymes & ph. 1. Enzymes, hormones and cell receptors are examples of which type of macromolecule?
 Name: Do Now #1 Enzymes & ph 1. Enzymes, hormones and cell receptors are examples of which type of macromolecule? 2. What do you think enzymes do for the body? Chemical reactions with enzymes are used
Name: Do Now #1 Enzymes & ph 1. Enzymes, hormones and cell receptors are examples of which type of macromolecule? 2. What do you think enzymes do for the body? Chemical reactions with enzymes are used
Molecules. Background
 Background A molecule is a group of two or more atoms. Compounds are also two or more atoms. Compounds are made from different types of atoms. can be made of different types of atoms or can also be made
Background A molecule is a group of two or more atoms. Compounds are also two or more atoms. Compounds are made from different types of atoms. can be made of different types of atoms or can also be made
2.5 Dehydration Synthesis and Hydrolysis Activity Name Date Block
 2.5 Dehydration Synthesis and Hydrolysis Activity Name Date Block Instructions: Complete the printout of this activity sheet with your lab partner. Show your work to your instructor when completed. Switch
2.5 Dehydration Synthesis and Hydrolysis Activity Name Date Block Instructions: Complete the printout of this activity sheet with your lab partner. Show your work to your instructor when completed. Switch
09 Enzymes. December 04, Chapter 9 Enzymes. Mr. C Biology 1
 Chapter 9 Enzymes Mr. C Biology 1 Chapter 9 Enzymes Metabolism is the sum of all chemical reactions in the body. Your metabolism is controlled by enzymes. Enzymes are proteins made in the ribosome from
Chapter 9 Enzymes Mr. C Biology 1 Chapter 9 Enzymes Metabolism is the sum of all chemical reactions in the body. Your metabolism is controlled by enzymes. Enzymes are proteins made in the ribosome from
Coat Color in Labrador Retrievers
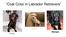 Coat Color in Labrador Retrievers Sunny Cocoa Midnight Metabolic Networks Introduction On your note sheet, write a definition for what you think metabolism means. HINT: my mom used to tell me when I was
Coat Color in Labrador Retrievers Sunny Cocoa Midnight Metabolic Networks Introduction On your note sheet, write a definition for what you think metabolism means. HINT: my mom used to tell me when I was
Enzymes Topic 3.6 & 7.6 SPEED UP CHEMICAL REACTIONS!!!!!!!
 Enzymes Topic 3.6 & 7.6 SPEED UP CHEMICAL REACTIONS!!!!!!! Key Words Enzyme Substrate Product Active Site Catalyst Activation Energy Denature Enzyme-Substrate Complex Lock & Key model Induced fit model
Enzymes Topic 3.6 & 7.6 SPEED UP CHEMICAL REACTIONS!!!!!!! Key Words Enzyme Substrate Product Active Site Catalyst Activation Energy Denature Enzyme-Substrate Complex Lock & Key model Induced fit model
9. At about 0 C., most enzymes are (1.) inactive (2.) active (3.) destroyed (4.) replicated
 Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Pharmex Pharmaceuticals
 MEMO to students Pharmex Pharmaceuticals To: All Research Team Members From: Principal Investigator Date: 11/22/11 Re: Testing the efficacy of our new enzyme product I commend all research team members
MEMO to students Pharmex Pharmaceuticals To: All Research Team Members From: Principal Investigator Date: 11/22/11 Re: Testing the efficacy of our new enzyme product I commend all research team members
Unit II Written Response Set-Up
 Unit II Written Response Set-Up On the next blank page in your notebook, put the title Unit II Written Responses If your title page is the front of a page, skip the back of the page and the front of the
Unit II Written Response Set-Up On the next blank page in your notebook, put the title Unit II Written Responses If your title page is the front of a page, skip the back of the page and the front of the
G.T. College G10 Term One Biology Form Test 2
 G.T. College 2018 19 G10 Term One Biology Form Test 2 Total marks: 40 marks Time allowed: 35 minutes Date: Name: Class: ( ) Section A: Multiple choice questions (10 marks@ 1 mark each) 1. Given a solution
G.T. College 2018 19 G10 Term One Biology Form Test 2 Total marks: 40 marks Time allowed: 35 minutes Date: Name: Class: ( ) Section A: Multiple choice questions (10 marks@ 1 mark each) 1. Given a solution
Lab Activity 30. Digestive Enzymes. Portland Community College BI 233
 Lab Activity 30 Digestive Enzymes Portland Community College BI 233 Cellular Reactions All molecular bonds have energy barriers that prevent spontaneous breakdown Enzymes lowering these activation energy
Lab Activity 30 Digestive Enzymes Portland Community College BI 233 Cellular Reactions All molecular bonds have energy barriers that prevent spontaneous breakdown Enzymes lowering these activation energy
Enzyme Action. Intermediate 2 Biology Unit 1: Living Cells
 Enzyme Action Intermediate 2 Biology Unit 1: Living Cells Learning Objectives Describe 2 ways in which chemical reactions can be speeded up. Name the products of the breakdown of hydrogen peroxide. State
Enzyme Action Intermediate 2 Biology Unit 1: Living Cells Learning Objectives Describe 2 ways in which chemical reactions can be speeded up. Name the products of the breakdown of hydrogen peroxide. State
Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating enzymatic activity.
 Name: Enzymes in Action Objectives: You will use the model pieces in the kit to: Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating
Name: Enzymes in Action Objectives: You will use the model pieces in the kit to: Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating
Terminology-Amino Acids
 Enzymes 1 2 Terminology-Amino Acids Primary Structure: is a polypeptide (large number of aminoacid residues bonded together in a chain) chain of amino acids linked with peptide bonds. Secondary Structure-
Enzymes 1 2 Terminology-Amino Acids Primary Structure: is a polypeptide (large number of aminoacid residues bonded together in a chain) chain of amino acids linked with peptide bonds. Secondary Structure-
Lab: Enzymes and the factors that affect their function
 Name Date Hour Lab: Enzymes and the factors that affect their function INTRODUCTION: What would happen to your cells if they made a poisonous chemical? You might think that they would die. If fact, your
Name Date Hour Lab: Enzymes and the factors that affect their function INTRODUCTION: What would happen to your cells if they made a poisonous chemical? You might think that they would die. If fact, your
BIO3X. General Certificate of Education Advanced Subsidiary Examination June AS Externally Marked Practical Assignment.
 Centre Number Surname Other Names Candidate Signature Candidate Number For Examiner s Use Total EMPA mark Examiner s Initials General Certificate of Education Advanced Subsidiary Examination June 2010
Centre Number Surname Other Names Candidate Signature Candidate Number For Examiner s Use Total EMPA mark Examiner s Initials General Certificate of Education Advanced Subsidiary Examination June 2010
Chapter 6. Flow of energy through life. Chemical reactions of life. Examples. Examples. Chemical reactions & energy 9/7/2012. Enzymes & Metabolism
 Flow of energy through life Chapter 6 Life is built on chemical reactions Enzymes & Metabolism Chemical reactions of life Examples Metabolism Forming bonds between molecules Dehydration synthesis Anabolic
Flow of energy through life Chapter 6 Life is built on chemical reactions Enzymes & Metabolism Chemical reactions of life Examples Metabolism Forming bonds between molecules Dehydration synthesis Anabolic
EXERCISE 5. Enzymes H amylase + starch + amylase-starch complex maltose+ amylase.
 EXERCISE 5 Enzymes LEARNING OBJECTIVES Demonstrate enzyme activity by the hydrolysis of starch by amylase. Determine the effect of different temperatures on the rate of starch hydrolysis. Determine the
EXERCISE 5 Enzymes LEARNING OBJECTIVES Demonstrate enzyme activity by the hydrolysis of starch by amylase. Determine the effect of different temperatures on the rate of starch hydrolysis. Determine the
Classwork #10 - Enzymes Key Vocabulary protein enzyme catalyst reactant substrate active site product
 Biology 2017-2018 Noble efforts change lives. Name: Excellence. Tenacity. Community. Reflection. Classwork #10 - Enzymes Key Vocabulary protein enzyme catalyst reactant substrate active site product Pre-Reading
Biology 2017-2018 Noble efforts change lives. Name: Excellence. Tenacity. Community. Reflection. Classwork #10 - Enzymes Key Vocabulary protein enzyme catalyst reactant substrate active site product Pre-Reading
Investigation: Enzymes
 Investigation: Enzymes INTRODUCTION: What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemicals. They
Investigation: Enzymes INTRODUCTION: What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemicals. They
2-2 Properties of Water
 2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
DNA and Protein Synthesis Practice
 Biology 12 DNA and Protein Synthesis Practice Name: 1. DNA is often called the "code of life". Actually it contains the code for a) the sequence of amino acids in a protein b) the sequence of base pairs
Biology 12 DNA and Protein Synthesis Practice Name: 1. DNA is often called the "code of life". Actually it contains the code for a) the sequence of amino acids in a protein b) the sequence of base pairs
Enzymes. Ms. Paxson. From food webs to the life of a cell. Enzymes. Metabolism. Flow of energy through life. Examples. Examples
 From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions sun transforming energy from one form to another solar energy ATP & organic molecules
From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions sun transforming energy from one form to another solar energy ATP & organic molecules
Amylase: a sample enzyme
 Amylase: a sample enzyme Objectives: After completion of this laboratory exercise you will be able to: 1. Explain the importance of enzymes in biology. 2. Explain the basic properties of an enzyme as a
Amylase: a sample enzyme Objectives: After completion of this laboratory exercise you will be able to: 1. Explain the importance of enzymes in biology. 2. Explain the basic properties of an enzyme as a
Work in groups of 3 to 4 students (enough materials for 5 groups total)
 Chemical and Physical Processes of Digestion Exercise 39A / 39 (begins page 597 in 9 th &10 th eds, page 595 in 11 th edition, page 599 in 12 th edition) Lab 7 Objectives Read lab Exercise 39A / 39 Do
Chemical and Physical Processes of Digestion Exercise 39A / 39 (begins page 597 in 9 th &10 th eds, page 595 in 11 th edition, page 599 in 12 th edition) Lab 7 Objectives Read lab Exercise 39A / 39 Do
the Bone Teacher Pages Classroom Activities Grade Level 4-6
 Grade Level 4-6 Building Big Bones Students will work in pairs to investigate how the light, spongy layer around the bone s marrow makes the bones lighter for easier movement, by comparing cardboard tubes
Grade Level 4-6 Building Big Bones Students will work in pairs to investigate how the light, spongy layer around the bone s marrow makes the bones lighter for easier movement, by comparing cardboard tubes
Student Handout. green B 1. foam pieces. ) into a single unit to model the substrate in this reaction.
 Student Handout Introduction Enzymes are specialized proteins that catalyze or speed up chemical reactions within cells. The substance upon which an enzyme acts is called a substrate. Substrates are small
Student Handout Introduction Enzymes are specialized proteins that catalyze or speed up chemical reactions within cells. The substance upon which an enzyme acts is called a substrate. Substrates are small
Enzymes. Essential Question: Why are the actions of an enzymes important to us?
 Enzymes 18.11- Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors such as ph and temperature, and their effect on enzyme activity. Enzymes
Enzymes 18.11- Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors such as ph and temperature, and their effect on enzyme activity. Enzymes
AP Biology Protein Structure and Enzymes
 AP Biology Protein Structure and Enzymes Connection to the Nitrogen-cycle Amino acids (protein) Nucleic acids (RNA and DNA) ATP 78% 1. Assimilation of nitrate by photosynthetic eukaryotes 2. Nitrogen fixation
AP Biology Protein Structure and Enzymes Connection to the Nitrogen-cycle Amino acids (protein) Nucleic acids (RNA and DNA) ATP 78% 1. Assimilation of nitrate by photosynthetic eukaryotes 2. Nitrogen fixation
Chapter 6. Metabolism & Enzymes. AP Biology
 Chapter 6. Metabolism & Enzymes Flow of energy through life Life is built on chemical reactions Chemical reactions of life Metabolism forming bonds between molecules dehydration synthesis anabolic reactions
Chapter 6. Metabolism & Enzymes Flow of energy through life Life is built on chemical reactions Chemical reactions of life Metabolism forming bonds between molecules dehydration synthesis anabolic reactions
Examples. Chapter 8. Metabolism & Enzymes. Flow of energy through life. Examples. Chemical reactions of life. Chemical reactions & energy
 WH Examples dehydration synthesis Chapter 8 Metabolism & Enzymes + H 2 O hydrolysis + H 2 O Flow of energy through life Life is built on chemical reactions Examples dehydration synthesis hydrolysis 2005-2006
WH Examples dehydration synthesis Chapter 8 Metabolism & Enzymes + H 2 O hydrolysis + H 2 O Flow of energy through life Life is built on chemical reactions Examples dehydration synthesis hydrolysis 2005-2006
AP Biology. Metabolism & Enzymes
 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic molecules
Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic molecules
CHEMISTRY OF LIFE 05 FEBRUARY 2014
 CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
CHEMISTRY OF LIFE 05 FEBRUARY 2014 In this lesson we will: Lesson Description Discuss inorganic compounds and their importance Discuss organic compounds and their biological importance. Summary Inorganic
REVISION: CHEMISTRY OF LIFE 19 MARCH 2014
 REVISION: CHEMISTRY OF LIFE 19 MARCH 2014 Lesson Description In this lesson we revise: The Chemistry of Life Food tests Summary Inorganic Nutrients Water Solvent Medium in which chemical reactions occur
REVISION: CHEMISTRY OF LIFE 19 MARCH 2014 Lesson Description In this lesson we revise: The Chemistry of Life Food tests Summary Inorganic Nutrients Water Solvent Medium in which chemical reactions occur
GRU3L1 Metabolism & Enzymes. AP Biology
 GRU3L1 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions u transforming energy from one form to organic molecules
GRU3L1 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions u transforming energy from one form to organic molecules
1) DNA unzips - hydrogen bonds between base pairs are broken by special enzymes.
 Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
BIOMOLECULES. Ms. Bosse Fall 2015
 BIOMOLECULES Ms. Bosse Fall 2015 Biology Biology is the study of the living world. Bio = life Major Molecules of Life Macromolecules giant molecules found in living cells; made from thousands of smaller
BIOMOLECULES Ms. Bosse Fall 2015 Biology Biology is the study of the living world. Bio = life Major Molecules of Life Macromolecules giant molecules found in living cells; made from thousands of smaller
Enzymes: Helper Protein molecules
 Enzymes: Helper Protein molecules 2009-2010 Flow of energy through life Life is built on chemical reactions Chemical reactions of life Processes of life building molecules synthesis + breaking down molecules
Enzymes: Helper Protein molecules 2009-2010 Flow of energy through life Life is built on chemical reactions Chemical reactions of life Processes of life building molecules synthesis + breaking down molecules
Enzymes. Ch 3: Macromolecules
 Enzymes Ch 3: Macromolecules Living things use different chemical reactions to get the energy needed for life Chemical Reactions Reactants = substance that is changed Products = new substance that forms
Enzymes Ch 3: Macromolecules Living things use different chemical reactions to get the energy needed for life Chemical Reactions Reactants = substance that is changed Products = new substance that forms
Extra AQA Questions on 1.1 Biological Molecules (the answers are at the end of the document)
 Extra AQA Questions on. Biological Molecules (the answers are at the end of the document) Q. Read the following passage. During the course of a day, we come into contact with many poisonous substances.
Extra AQA Questions on. Biological Molecules (the answers are at the end of the document) Q. Read the following passage. During the course of a day, we come into contact with many poisonous substances.
Biology Unit 3 Review. Objective 1. Describe the important functions of organic molecules Carbohydrates Lipids Proteins Nucleic acids
 Biology Unit 3 Review Name Objective 1. Describe the important functions of organic molecules Carbohydrates Lipids Proteins Nucleic acids 1. What is the difference between organic and inorganic molecules?
Biology Unit 3 Review Name Objective 1. Describe the important functions of organic molecules Carbohydrates Lipids Proteins Nucleic acids 1. What is the difference between organic and inorganic molecules?
6 The chemistry of living organisms
 Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
4. Summarize the biological importance of the information displayed in the graph below
 GOT LACTASE: DATA ANALYSIS, POST LAB? s: 1. Describe the relationship between enzyme, substrate and product. It may be helpful to do this by creating an illustration of the general equation. 2. What class
GOT LACTASE: DATA ANALYSIS, POST LAB? s: 1. Describe the relationship between enzyme, substrate and product. It may be helpful to do this by creating an illustration of the general equation. 2. What class
The Chemistry of Life
 The Chemistry of Life Biomolecules Warm-up List the percentages of each: Total Fats Saturated Fats 25% Carbohydrates 10% Protein 7% 20% What Biomolecule would cholesterol be classified as? Lipids (fats)
The Chemistry of Life Biomolecules Warm-up List the percentages of each: Total Fats Saturated Fats 25% Carbohydrates 10% Protein 7% 20% What Biomolecule would cholesterol be classified as? Lipids (fats)
Biochemistry. Definition-
 Biochemistry Notes Biochemistry Definition- the scientific study of the chemical composition of living matter AND of the chemical processes that go on in living organisms. Biochemistry Facts 1. The human
Biochemistry Notes Biochemistry Definition- the scientific study of the chemical composition of living matter AND of the chemical processes that go on in living organisms. Biochemistry Facts 1. The human
Chapter 5 Metabolism: Energy and Enzymes
 Biology 12 Name: Cell Biology Per: Date: Chapter 5 Metabolism: Energy and Enzymes Complete using BC Biology 12, pages 154-175 Diagnostic Questions (mark using the answer key on page 533) 1. B 2. B 3. C
Biology 12 Name: Cell Biology Per: Date: Chapter 5 Metabolism: Energy and Enzymes Complete using BC Biology 12, pages 154-175 Diagnostic Questions (mark using the answer key on page 533) 1. B 2. B 3. C
a. What is the stimulus? Consuming a large pumpkin spice muffin and caramel macchiato.
 : Homeostasis and Macromolecules Unit Study Guide Homeostasis 1. Define homeostasis and give an example. Homeostasis is the ability of the body to maintain relatively constant internal physical and chemical
: Homeostasis and Macromolecules Unit Study Guide Homeostasis 1. Define homeostasis and give an example. Homeostasis is the ability of the body to maintain relatively constant internal physical and chemical
MILK HOW SWEET IS IT?
 MILK HOW SWEET IS IT? The Making of the Fittest: Natural INTRODUCTION Selection and Adaptation In the film, The Making of the Fittest: The Co-Evolution of Genes and Culture, Dr. Dallas Swallow determines
MILK HOW SWEET IS IT? The Making of the Fittest: Natural INTRODUCTION Selection and Adaptation In the film, The Making of the Fittest: The Co-Evolution of Genes and Culture, Dr. Dallas Swallow determines
Dalkeith High School Higher Human Biology Homework 3
 Dalkeith High School Higher Human Biology Homework 3 1. During which of the following chemical conversions is A T P produced? A B C Amino acids protein Glucose pyruvic acid Haemoglobin oxyhaemoglobin energy
Dalkeith High School Higher Human Biology Homework 3 1. During which of the following chemical conversions is A T P produced? A B C Amino acids protein Glucose pyruvic acid Haemoglobin oxyhaemoglobin energy
Neatness 0 1 Accuracy Completeness Lab Class Procedure Total Lab Score
 New Paltz High School Science Department Name:.... Due Date:... Lab Title:.Big Ol Biomolecules..Lab #... Lab Partners: Your Lab Score will be based on the following: Neatness: All labs must be well-written
New Paltz High School Science Department Name:.... Due Date:... Lab Title:.Big Ol Biomolecules..Lab #... Lab Partners: Your Lab Score will be based on the following: Neatness: All labs must be well-written
Metabolism & Enzymes. From food webs to the life of a cell. Flow of energy through life. Life is built on chemical reactions
 Metabolism & Enzymes 2007-2008 From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic
Metabolism & Enzymes 2007-2008 From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic
Chapter 2: Biochemistry
 Chapter 2: Biochemistry Biochemistry Biochemistry is the study of chemical makeup and reactions of living matter All chemicals in the body are either organic & inorganic Organic compounds contain carbon
Chapter 2: Biochemistry Biochemistry Biochemistry is the study of chemical makeup and reactions of living matter All chemicals in the body are either organic & inorganic Organic compounds contain carbon
Diffusion and Osmosis Lab AP LAB 4
 Diffusion and Osmosis Lab AP LAB 4 Part 1: Surface Area and Cell Size Which do you think has a greater influence on the rate of diffusion in a cell surface area or volume? You will calculate surface are-to-volume
Diffusion and Osmosis Lab AP LAB 4 Part 1: Surface Area and Cell Size Which do you think has a greater influence on the rate of diffusion in a cell surface area or volume? You will calculate surface are-to-volume
AP Biology Lab Reports
 AP Biology Lab Reports Your Lab Notebook Some labs will be worksheets that you write down data, do calculations and answer questions. Inquiry-based labs, where you design experiments yourself, will be
AP Biology Lab Reports Your Lab Notebook Some labs will be worksheets that you write down data, do calculations and answer questions. Inquiry-based labs, where you design experiments yourself, will be
Review for Test #1: Biochemistry
 Review for Test #1: Biochemistry 1. Know and understand the definitions and meanings of the following terms. Be able to write complete definitions for the terms in BOLD: Biology triglyceride metabolism
Review for Test #1: Biochemistry 1. Know and understand the definitions and meanings of the following terms. Be able to write complete definitions for the terms in BOLD: Biology triglyceride metabolism
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *0063574818* BIOLOGY 5090/02 Paper 2 Theory May/June 2007 1 hour 45 minutes Candidates answer Section
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *0063574818* BIOLOGY 5090/02 Paper 2 Theory May/June 2007 1 hour 45 minutes Candidates answer Section
3. Which of the following cannot increase metabolic rate? A. Reading B. Sleeping C. Talking D. Jogging
 Topic 4: Enzymes and metabolism 1. An is only required in a small amount because A. its shape is specific. B. it speeds up both forward and backward reactions. C. it is made of proteins. D. it is not consumed
Topic 4: Enzymes and metabolism 1. An is only required in a small amount because A. its shape is specific. B. it speeds up both forward and backward reactions. C. it is made of proteins. D. it is not consumed
D. glycerol and fatty acids 4. Which is an example of an inorganic compound?
 Name: ate: 1. Glucose and maltose are classified as organic compounds because they are both 3. Which process is most directly responsible for the production of O 2 in these sugar solutions?. carbon-containing
Name: ate: 1. Glucose and maltose are classified as organic compounds because they are both 3. Which process is most directly responsible for the production of O 2 in these sugar solutions?. carbon-containing
Name: Regents Exam Preparation: Vocabulary Winter Break
 Name: Regents Exam Preparation: Vocabulary Winter Break 2016-2017 I. Scientific Method Design an experiment and answer the following questions below: Kyle wanted to know if watching the Giants play football
Name: Regents Exam Preparation: Vocabulary Winter Break 2016-2017 I. Scientific Method Design an experiment and answer the following questions below: Kyle wanted to know if watching the Giants play football
Carbon. p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms
 Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
 We ve seen that organisms have to work hard to maintain optimal conditions for their cells. But how exactly does that work?? What happens if something changes? http://galeri12.uludagsozluk.com/511/ekg_730911.jpg
We ve seen that organisms have to work hard to maintain optimal conditions for their cells. But how exactly does that work?? What happens if something changes? http://galeri12.uludagsozluk.com/511/ekg_730911.jpg
June 3, 2015 By Dr. Jenny Williams & Dr. Nora Swenson
 Math Strategies for Grades 3-5 IDEAS 2015 June 3, 2015 By Dr. Jenny Williams & Dr. Nora Swenson Sense of Number (Exemplars) Basic Counting Understanding Size Number Relationships Patterns Operations Place
Math Strategies for Grades 3-5 IDEAS 2015 June 3, 2015 By Dr. Jenny Williams & Dr. Nora Swenson Sense of Number (Exemplars) Basic Counting Understanding Size Number Relationships Patterns Operations Place
The ideas which form the background to this case study are listed in the following table.
 Enzymes Prerequisites The ideas which form the background to this case study are listed in the following table. Topic Book page Amino acid 343 Protein 344 Peptide bond 344 Rate of reaction and concentration
Enzymes Prerequisites The ideas which form the background to this case study are listed in the following table. Topic Book page Amino acid 343 Protein 344 Peptide bond 344 Rate of reaction and concentration
Biochemistry Macromolecules and Enzymes. Unit 02
 Biochemistry Macromolecules and Enzymes Unit 02 Organic Compounds Compounds that contain CARBON are called organic. What is Carbon? Carbon has 4 electrons in outer shell. Carbon can form covalent bonds
Biochemistry Macromolecules and Enzymes Unit 02 Organic Compounds Compounds that contain CARBON are called organic. What is Carbon? Carbon has 4 electrons in outer shell. Carbon can form covalent bonds
Review of Energetics Intro
 Review of Energetics Intro Learning Check The First Law of Thermodynamics states that energy can be Created Destroyed Converted All of the above Learning Check The second law of thermodynamics essentially
Review of Energetics Intro Learning Check The First Law of Thermodynamics states that energy can be Created Destroyed Converted All of the above Learning Check The second law of thermodynamics essentially
Chapter 8.4, 8.5. Enzymes. AP Biology
 Chapter 8.4, 8.5 Enzymes Activation energy Breaking down large molecules requires an initial input of energy activation energy large biomolecules are stable must absorb energy to break bonds cellulose
Chapter 8.4, 8.5 Enzymes Activation energy Breaking down large molecules requires an initial input of energy activation energy large biomolecules are stable must absorb energy to break bonds cellulose
1. Cut the copier paper into strips 8.5 inches long and half an inch wide, to make at least 40 strips.
 1 Unit 2: Lesson 2 Case Studies: Influenza and HIV Activity 1: Influenza Antigenic Drift Materials 4 highlighter pens colored red, green, blue and yellow Tape or glue Scissors Paper clips 2 sheets copier
1 Unit 2: Lesson 2 Case Studies: Influenza and HIV Activity 1: Influenza Antigenic Drift Materials 4 highlighter pens colored red, green, blue and yellow Tape or glue Scissors Paper clips 2 sheets copier
½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = X X= 325 g
 BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
Biochemical Concepts. Section 4.6 The Chemistry of Water. Pre-View 4.6. A Covalent Polar Molecule
 Biochemical Concepts Section 4.6 The Chemistry of Water Pre-View 4.6 Polar molecule a molecule that has a partial positive charge on one end and a partial negative charge on the other end Hydrogen bond
Biochemical Concepts Section 4.6 The Chemistry of Water Pre-View 4.6 Polar molecule a molecule that has a partial positive charge on one end and a partial negative charge on the other end Hydrogen bond
Bridging task for 2016 entry. AS/A Level Biology. Why do I need to complete a bridging task?
 Bridging task for 2016 entry AS/A Level Biology Why do I need to complete a bridging task? The task serves two purposes. Firstly, it allows you to carry out a little bit of preparation before starting
Bridging task for 2016 entry AS/A Level Biology Why do I need to complete a bridging task? The task serves two purposes. Firstly, it allows you to carry out a little bit of preparation before starting
Macromolecules Biomolecules Concept Map. The Big 4. Chapter 6. Color the molecule of carbon.
 Biomolecules Concept Map Using the terms provided below, complete the concept map showing the characteristics of organic compounds Carbohydrates DNA Enzymes Fats Lipids Monosaccharides Nucleic Acids Nucleotides
Biomolecules Concept Map Using the terms provided below, complete the concept map showing the characteristics of organic compounds Carbohydrates DNA Enzymes Fats Lipids Monosaccharides Nucleic Acids Nucleotides
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4672819309* BIOLOGY 0610/61 Paper 6 Alternative to Practical October/November 2017 1 hour Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4672819309* BIOLOGY 0610/61 Paper 6 Alternative to Practical October/November 2017 1 hour Candidates
increase rate of reaction without being consumed reduce activation energy don t change free energy ( G) released or required
 Enzymes Enzymes Biological catalysts proteins (& RNA) facilitate chemical reactions increase rate of reaction without being consumed reduce activation energy don t change free energy ( G) released or required
Enzymes Enzymes Biological catalysts proteins (& RNA) facilitate chemical reactions increase rate of reaction without being consumed reduce activation energy don t change free energy ( G) released or required
The Chromosomes of a Frimpanzee: An Imaginary Animal
 The Chromosomes of a Frimpanzee: An Imaginary Animal Introduction By now, you have heard the terms chromosome, mitosis, and meiosis. You probably also know that chromosomes contain genetic information
The Chromosomes of a Frimpanzee: An Imaginary Animal Introduction By now, you have heard the terms chromosome, mitosis, and meiosis. You probably also know that chromosomes contain genetic information
Unit 7 Part I: Introductions to Biochemistry
 Unit 7 Part I: Introductions to Biochemistry Chemical Reactions, Enzymes and ATP 19 March 2014 Averett 1 Reaction Graphs Every chemical reaction involves bond breaking and bond forming. In order for bonds
Unit 7 Part I: Introductions to Biochemistry Chemical Reactions, Enzymes and ATP 19 March 2014 Averett 1 Reaction Graphs Every chemical reaction involves bond breaking and bond forming. In order for bonds
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *4300711309* BIOLOGY 5090/62 Paper 6 Alternative to Practical October/November 2017 1 hour Candidates answer on the Question Paper. No Additional
Cambridge International Examinations Cambridge Ordinary Level *4300711309* BIOLOGY 5090/62 Paper 6 Alternative to Practical October/November 2017 1 hour Candidates answer on the Question Paper. No Additional
BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) CARBOHYDRATES
 AP BIOLOGY BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) NAME DATE PERIOD CARBOHYDRATES GENERAL CHARACTERISTICS: Polymers of simple sugars Classified according to number of simple sugars Sugars 3
AP BIOLOGY BIOCHEMISTRY UNIT 2 Part 4 ACTIVITY #4 (Chapter 5) NAME DATE PERIOD CARBOHYDRATES GENERAL CHARACTERISTICS: Polymers of simple sugars Classified according to number of simple sugars Sugars 3
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Cambridge International Examinations Cambridge International Advanced Level
 Cambridge International Eaminations Cambridge International Advanced Level *8932538116* BIOLOGY 9700/53 Paper 5 Planning, Analysis and Evaluation October/November 2014 1 hour 15 minutes Candidates answer
Cambridge International Eaminations Cambridge International Advanced Level *8932538116* BIOLOGY 9700/53 Paper 5 Planning, Analysis and Evaluation October/November 2014 1 hour 15 minutes Candidates answer
Paper Reference. Advanced Subsidiary Unit Test 1. Monday 6 June 2005 Morning Time: 1 hour
 Centre No. Candidate No. Paper Reference(s) 6101/01 Edexcel GCE Biology Biology (Human) Advanced Subsidiary Unit Test 1 Monday 6 June 2005 Morning Time: 1 hour Materials required for examination Ruler
Centre No. Candidate No. Paper Reference(s) 6101/01 Edexcel GCE Biology Biology (Human) Advanced Subsidiary Unit Test 1 Monday 6 June 2005 Morning Time: 1 hour Materials required for examination Ruler
Carbon. Has four valence electrons Can bond with many elements. Can bond to other carbon atoms. Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
 Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Teacher Preparation Notes for Enzymes Help Us Digest Food 1
 Teacher Preparation Notes for Enzymes Help Us Digest Food 1 Students learn about enzyme function, enzyme specificity and the molecular basis of lactose intolerance through experiments with the enzyme lactase
Teacher Preparation Notes for Enzymes Help Us Digest Food 1 Students learn about enzyme function, enzyme specificity and the molecular basis of lactose intolerance through experiments with the enzyme lactase
BACKGROUND INFORMATION:
 BIOLOGY 12 ENZYMES NAME: BACKGROUND INFORMATION: Energy: is defined as the ability to do or bring about change. A living organism must constantly perform work in order to maintain its organization, to
BIOLOGY 12 ENZYMES NAME: BACKGROUND INFORMATION: Energy: is defined as the ability to do or bring about change. A living organism must constantly perform work in order to maintain its organization, to
Essential Biology 3.2 Carbohydrates, Lipids, Proteins. 1. Define organic molecule.
 1. Define organic molecule. An organic molecule is a molecule that contains carbon and is found in living things. There are many organic molecules in living things. The same (or very similar) molecules
1. Define organic molecule. An organic molecule is a molecule that contains carbon and is found in living things. There are many organic molecules in living things. The same (or very similar) molecules
Ch 5 Metabolism and enzymes
 Ch 5 Metabolism and enzymes Think about (Ch 5, p.2) 1. Enzymes are proteins that act as biological catalysts to speed up metabolic reactions. 2. Enzymes catalyse the breakdown of cellulose fibres of the
Ch 5 Metabolism and enzymes Think about (Ch 5, p.2) 1. Enzymes are proteins that act as biological catalysts to speed up metabolic reactions. 2. Enzymes catalyse the breakdown of cellulose fibres of the
17 Cell Differentiation and Gene Expression In m ost h u m a n cells, the nucleus contains a full set of 23 pairs of chromosomes,
 17 Cell Differentiation and Gene Expression In m ost h u m a n cells, the nucleus contains a full set of 23 pairs of chromosomes, which carry 20,000 25,000 genes. These genes are identical from cell to
17 Cell Differentiation and Gene Expression In m ost h u m a n cells, the nucleus contains a full set of 23 pairs of chromosomes, which carry 20,000 25,000 genes. These genes are identical from cell to
Identify and describe the circulation system that is missing from the organizer above.
 Lesson 15.1 NOTES: The Circulatory System (Unlock) Essential Question: -What are the structures and functions of the circulatory system? Learning Target(s): -I can identify structures and explain functions
Lesson 15.1 NOTES: The Circulatory System (Unlock) Essential Question: -What are the structures and functions of the circulatory system? Learning Target(s): -I can identify structures and explain functions
Diffusion across a Selectively Permeable Membrane
 Diffusion across a Selectively Permeable Membrane Each cell is surrounded by a selectively permeable cell membrane Cell Membrane which regulates what gets into and out of the cell. A selectively permeable
Diffusion across a Selectively Permeable Membrane Each cell is surrounded by a selectively permeable cell membrane Cell Membrane which regulates what gets into and out of the cell. A selectively permeable
Bell Work 11/27/17. Do Not Touch the Lab Supplies! What is Energy?
 UNIT 3 - ENERGY Bell Work 11/27/17 Do Not Touch the Lab Supplies! What is Energy? Calorimetry Lab 1. Energy is a property of objects that can be transferred from one object to another or converted from
UNIT 3 - ENERGY Bell Work 11/27/17 Do Not Touch the Lab Supplies! What is Energy? Calorimetry Lab 1. Energy is a property of objects that can be transferred from one object to another or converted from
Catalase Lab - A Bio ENZYME ACTIVITY Investigation Created by Gen Nelson, modified by Dr G
 Catalase Lab - A Bio ENZYME ACTIVITY Investigation Created by Gen Nelson, modified by Dr G INTRODUCTION Hydrogen peroxide (H 2O 2) is a poisonous byproduct of metabolism that can damage cells if it is
Catalase Lab - A Bio ENZYME ACTIVITY Investigation Created by Gen Nelson, modified by Dr G INTRODUCTION Hydrogen peroxide (H 2O 2) is a poisonous byproduct of metabolism that can damage cells if it is
The graph should contain 5 major parts: the title, the independent variable, the dependent variable, the scales for each variable, and a legend.
 BLIZZARD BAG #2 Please complete the activity below. You will need to print out this activity sheet (pages 2-5 only) OR Write out the answers on a separate piece of paper and graph on a piece of graphing
BLIZZARD BAG #2 Please complete the activity below. You will need to print out this activity sheet (pages 2-5 only) OR Write out the answers on a separate piece of paper and graph on a piece of graphing
Biology Unit 2 Elements & Macromolecules in Organisms Date/Hour
 Biology Unit 2 Name Elements & Macromolecules in rganisms Date/our Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body
Biology Unit 2 Name Elements & Macromolecules in rganisms Date/our Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body
Part 1: Energy and Metabolism
 Part 1: Energy and Metabolism Life is highly organized rganisms need free energy to survive, grow, and reproduce In each system, the arrow is pointing in the direction of spontaneous change. Why? 1 2 More
Part 1: Energy and Metabolism Life is highly organized rganisms need free energy to survive, grow, and reproduce In each system, the arrow is pointing in the direction of spontaneous change. Why? 1 2 More
2.3: Carbon-Based Molecules Notes
 2.3: Carbon-Based Molecules Notes Carbon-based molecules are the of life. Bonding Properties of Carbon Carbon forms bonds with up to other atoms, including other carbon atoms. QUESTION: What types of elements
2.3: Carbon-Based Molecules Notes Carbon-based molecules are the of life. Bonding Properties of Carbon Carbon forms bonds with up to other atoms, including other carbon atoms. QUESTION: What types of elements
Digestion and Human Health
 Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
