Tala Saleh. Tamer Barakat. Diala Abu-Hassan
|
|
|
- Jeffry Dawson
- 5 years ago
- Views:
Transcription
1 13 Tala Saleh Tamer Barakat Diala Abu-Hassan
2 Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome is a set of symptoms (chills, headaches and dizziness) that some people have after eating Chinese food where MSG is used a lot. Questions on amino acids: 1) What is specific about proline? Proline is an imino acid unique in that it contains a secondary nitrogen in its backbone resulted from the bonding of the amino group to the R group, thus it is a secondary amine rather than a primary amine like in other amino acids. 2) Which amino acids are found charged at physiological conditions? 5 amino acids have a side chain which can be charged at physiological conditions. 2 are negatively charged: aspartic acid and glutamic acid (acidic side chains), and 3 are positively charged: lysine, arginine and histidine (basic side chains). 3) Name amino acids that share the same functional group in their side chain. Glutamine and Asparagine Share the amide group. Serine, Threonine and Tyrosine Share the hydroxyl group.
3 Amino acid and protein molecular weight The average molecular weight of an amino acid residue is about 110. The molecular weights of most proteins are between 5,500 and 220,000. We refer to the mass of a polypeptide in the unit of Daltons (Da) or kilo Daltons (kda). A 10,000 MW protein has a mass of 10,000 Da / 10 kda. Amino acids stereoisomers / optical isomers Because the alpha carbon has 4 different groups, amino acids can exist as stereoisomers in proteins (except glycine since its alpha carbon is attached to 2 H atoms). The molecules are mirror image to each other and cannot be superimposed, they rotate polarized light in opposite directions. (Right D- dexter, Left L-laevus) Both isomers (D-, L-) are found in nature however, all amino acids in proteins have L- configuration. D- Amino acids are found in bacteria cell walls and in some antibiotics. Remember: we have D-sugars in our bodies. Ionization of amino acids The main structure of amino acid contains a carboxyl group and an amino group, these 2 groups can be ionized.
4 Note that ionization is the process by which an atom or a molecule acquires a negative or a positive charge. Therefore: Protonation of the carboxyl group neutral COO - COOH Protonation of the amino group ionized NH 2 NH3 + De-protonated carboxyl group ionized COO - De-protonated amino group neutral NH 2 Zwitterion: is a molecule that is charged but the total net charge is zero. At physiological ph, amino acids (without acidic/basic groups) are electrically neutral. Isoelectric point (pi): is the specific ph when the molecule has a total net charge of zero (Zwitterion). Any molecule with equal opposite charges becomes a zwitterion at the isoelectric point. At a very low ph "abundant amount of H" : Carboxyl group is protonated -COOH uncharged Amino group is protonated -NH3 + charged Net charge of the amino acid= +1 ph << pka Protonated groups are abundant At a very High ph "low amount of H" : Carboxyl group is de-protonated COO - charged Amino group is de-protonated NH2 uncharged Net charge of the amino acid= -1 ph >> pka Deprotonated groups are abundant
5 Note the following curve and the numbered structures: At very low ph all the amino acids will be 1. As the ph increases, the carboxyl group will start losing its proton. Decreases while 1 2 increases. At physiological ph, all amino acids will be in their zwitterionic form 2. At high ph the amino group will start losing its proton. 2 Decreases while 3 starts appearing. At very high ph all the amino acids will be Point 1 Point 2 Point 1: It is the pka of the carboxyl group, when half of the amino acids carboxyl group is de-protonated and charged while the other half is protonated and uncharged. Point 2: It is the pka of the amino group, when half of the amino acids amino group is protonated and charged while the other half is de-protonated and uncharged.
6 Amino acids as buffers Amino acids contain a COOH group which acts as a weak acid, and a NH2 group which acts as a weak base, hence amino acids acting as buffers. The curve is not shown as a one large step because of the different pka values of these 2 groups. -COOH undergoes titration before NH2 because COOH is a stronger acid compared to NH2. Amino acids have a minimum of 2 buffering capacities: 1) For the carboxyl group at low ph. 2) For the amino group at high ph. 3) For the R group if it's ionizable. Check this titration curve of alanine (its R group is not ionizable). In this example, OH is added to the amino acid, therefore deprotonation will be taking place: This midpoint "where ph=pka" is the pka of the amino group 9 This midpoint "where ph=pka" is the pka of the carboxyl group 2
7 1) As the ph increases (H atoms decrease), the carboxyl group loses a proton thus resisting the change in ph acting as a buffer which is the 1 st plateau in the graph. 2) When we reach the end of the 1 st buffering capacity, a slight addition of OH causes a sharp increase in the ph, which stops after reaching the 2 nd buffering capacity of the amine group. 3) The amino group of the zwitterion loses a proton thus resisting the change in ph acting as a buffer again which is the 2 nd plateau in the graph. Any further addition of OH will increase the ph sharply with no more buffer zones. The Henderson-Hasselbalch equation can be used to calculate and predict the ratio of base to acid. Example: which form of amino acid is dominant at a ph of 8 "using the graph before"? From the graph we can tell that both the zwitterion "acid" and the anion "conjugate base" exist at the ph of 8. The deprotonation of NH3 is involved so its pka, which equals 9, is used. Thus, from the equation the base to acid ratio will be -1 The Acid is more abundant than the base (10 folds) Zwitterion is more abundant at the ph of 8. Note that in both cases we didn t care about the carboxyl group because its 100% in its deprotonated (charged form) Isoelectric Point (pi) The ph where the net charge of molecules such as an amino acid or protein is zero is known as the Isoelectric Point.
8 The isoelectric point for polar and non-polar amino acids with 2 pka values can be calculated using this equation: From the alanine graph above we can see that the pi value is the average between both pka1 and pka2, which almost equals using the equation above: pi = = We can also deduce the pi from the graph, it's in equal distances away from both the pka values. Amino Acids with more than 2 pka values Some amino acids "9 in total" have ionizable R group, therefore there are 3 different pka values (The R groups' pka will be referred to as pk R ). Such as: tyrosine, cysteine, arginine, lysine, histidine, serine, threonine, and aspartic and glutamic acids. The pi will be calculated in a different method by taking the average of the pka values of the amino acids forms that have the opposite charges (So that the net charge is 0). Considering pka for NH2 =9 and the pka for COOH =2 in all amino acids.
9 Example 1: Glutamic Acid Glutamic acid has a carboxyl group, which is ionizable, in its side chain As the ph increases the following happens: 1) The backbone carboxyl group will be deprotonated. At a ph of 2, half of the amino acid will be of the fully protonated form and the other half will be a zwitterion. 2) Then the carboxyl group of the R group will be deprotonated. A ph of 4 will be its midpoint. So, the pk R = 4. 3) At a high ph, the amino group will be deprotonated. Giving the fully deprotonated form of glutamic acid. 4) To calculate the isoelectric point, we must take pka values of 2 amino acids where their net charges =0. So, we calculate the total charge of each form and pick 2 forms that have equal and opposite charges (form 1 and 3 ) (pkr and pk1). pi = = 3 In other words, we take the average of the pka values around the zwitterion form. This is not the same method mentioned by the doctor.
10 Example 2: Histidine Histidine has an amine group in its ring, so it has a basic ionizable R group. Thus, as you increase the ph the following happens: 1) The carboxyl group loses a proton. (pka1 = 2) 2) The amine group in the R group loses a proton. (pka2 = 6) 3) The original amine group loses a proton. (pka3 = 9) Note how the amino acid in the R group is a stronger acid compared to the original amino acid. 4) Calculating the pi by taking pka2 and pka3 (pka values around the zwitterion): pi = = 7. 5 So for now what we are reqiured to know is: 1) Understanding any titration curves given. 2) Determing which for is predominat at a certain ph. 3) Calculating the isoelectric point of different amino acids. 4) Names of amino acids, their special features and their 3 letter abbrevietion. 5) Identifying different aminno acids structures. 6) The uncommon amino acids, their precursor and their functions. 7) The R groups wether they are acidic, basic or near neutral.
11 Questions: 1) Draw the titration curve of histidine. Using the pka values: pka1= 1.82, pkr= 6 and pka2= 9.17 Keeping in mind that the buffering capacity= pka ± 1 2) What is the ratio of conjugate base to acid of glutamate at ph= 4.5. ph = pka + log base acid 4. 5 = log base acid Base to acid ratio = 1.78 Note that we used the pka value that is in range of the ph. Since ph is 4.5, the pka must be between 3.5 and 5,5 3) What is the total charge of lysine at ph= 7 In amino acids the pi is defined as the ph at which the amino acid has no net charge. Therefore, when ph> pi, an amino acid has a negative net charge and when ph< pi, an amino acid has a positive net charge. In this question, ph= 7 and pi= 9.74 Total charge of lysine is +1
12 Peptides Important definitions: Residue: a subunit that is part of a larger molecule (such as glucose in glycogen, amino acid in a polypeptide). We use the prefix (Di,Tri,Tetra ) to designate the number of aminoacids linked together (Dipeptide, Tripeptide, Tetrapeptide ) Oligopeptide: a short sequence of amino acids connected to each other (short chain of amino acids). Polypeptide: a longer peptide with no particular structure. Protein: a polypeptide chain with an organized 3D structure and a specific function (usually more than 100 residues). The average molecular weight of an amino acid= 110 Daltons Example 1: a protein has a MW = 5500, how many amino acid residues does it contain? Number of amino acids = proteins MW amino acid MW Number of amino acids = = 50 amino acids Example 2: a protein composed of 100 amino acid, what is the MW of this protein? Proteins MW = amino acid MW number of amino acids Proteins MW = = 11 kda
13 Peptide Bond The linkage between two amino acids (between the alpha carboxyl group and the amine group of another amino acid) as a result of a condensation reaction where a H2O molecule is eliminated, is known as the Peptide bond. Also known chemically as an amide bond. Features of the peptide bond It is a zigzag structure It has a double bond resonance making the peptide bond: rigid, planar and charged. Resonance of this double bond is found because the double bond between the C and O moves to the peptide linkage of C and N. Hydrogen Bonds are formed between amino acids. (Oxygen of the carbonyl group is the H bond acceptor and the H atom of the amino group is the H bond donor). Except proline, due to its cyclic structure the Nitrogen has only 1 H atom which is used in forming the peptide bond. So, proline can't be a hydrogen bond donor, however it can be a hydrogen bond acceptor.
14 Question: the protein has to be flexible in order to take different forms, but the peptide bond is rigid (due to the resonance hybrid, it cannot be rotated), how does the protein make its structure possible? The peptide bond itself is rigid and can t be rotated, but the bonds within the amino acid itself (phi and psi) are flexible and can be rotated, therefore the protein can be folded into its 3D structure. Note that the phi is the bond between the alpha carbon and the nitrogen while the psi is the bond between the alpha carbon and the carbonyl carbon, both are flexible unlike the peptide bond. The Peptide Chain The backbone of a peptide chain consists of: 1) α-amide nitrogen 2) α-carbon 3) α-carbonyl carbon atom The R groups in the peptide chain are branched, orientated toward the outside and in a trans configuration. (Which provides less steric hindrance between the functional groups attached to the alpha carbon, the steric hindrance would be greater if it was in a cis configuration). Except in proline, where both cis and trans conformations have equivalent energies (repulsion will occur anyway). Thus, proline is found in the cis configuration more frequently than other amino acids.
15 The peptide chain starts with the N- terminus and ends with the C- terminus. The amino acids are added to the C- terminus (Cterminus can be modified while the N- terminus can't be modified). The polarity of the peptide backbone is due to the N- terminus (positive end) and the C- terminus (negative end). Examples of functional and exceptional peptides Carnosine Glutathione Enkephalins Oxytocin and vasopressin(adh) Gramicidin S and Tyrocidine A Aspartame 1) Carnosine (β-alanyl-l-histidine) It is a dipeptide constructed of two amino acids (β-alanine and L-histidine). The amine group is bonded to the β carbon of alanine.
16 The difference between β-alanine and α-alanine is that the amine group is linked with the α -carbon in α-alanine. Functions: Protection of the cells from ROS (radical oxygen species) and peroxides. Contraction of muscles. It is highly concentrated in muscle and brain tissues. 2) Glutathione (γ-glutamyl-l-cysteinylglycine) It is a tripeptide, made up of 3 amino acids (γ-glutamate-l-cysteine-glycine) Note that it is a γ-glutamyl amino acid because γ -carboxyl group of the glutamic acid is involved in the peptide bond with the amine group of cysteine, which is bonded to the amine group of glycine. Function: Glutathione has a major role as it functions as an anti-oxidant. It removes oxidizing agents like reactive oxygen species.
17 How does Glutathione work as an anti-oxidant? Two molecules of the reduced glutathione molecules form the oxidized form of glutathione by forming a disulfide bond between the SH groups of the two cysteine residues (two reduced molecules, linked by a disulfide bond = 1 oxidized molecule). So, what happened is that the 2 glutathione molecules donated their extra electrons in their thiol group to the reactive oxygen species. Thus, protecting other structures from them. Note that there are some enzymes that regenerate glutathione from its oxidized form making it functional again. 3) Enkephalins They are penta-peptides which functions as a pain reliever (analgesic). Two types of Enkephalin are found in the brain, they only differ in their C- terminal amino acids: Met-enkephalin: Tyr-Gly-Gly-Phe-Met Leu-enkephalin: Tyr-Gly-Gly-Phe-Leu The aromatic side chains of tyrosine and phenylalanine play a role in their activities.
18 Enkephalin and morphine There are similarities between the 3D structures of opiates (which are drugs derived from opium) such as morphine and enkephalin. Morphine is an addictive pain reliever that is wildly used in hospitals. Due to the similar 3D shape of it to that of the enkephalin, it binds to the same receptors that enkephalin binds to. 4) Oxytocin and Vasopressin They are hormones with a cyclic structure due to the (S-S link) between Cys amino acids of the same peptide. Both have a modification of an amide group at the C-terminus. Both are nona-peptides (made up of 9 amino acids). Name of the Hormone Oxytocin Vasopressin (ADH) The different amino acids 8Leucine, 3Isoleucine 8Arginine, 3Phenylalanine Functions 1) Regulates the contraction of the uterine muscles when delivering the baby (labor contraction) 2) It is present in males and have a pleasant feeling 1) Regulates the contraction of smooth muscle 2) Increases water retention 3) Increases blood pressure 4) Renal function 5) Maintain salt concentration
19 Question: what is the primary structure of the figure below? Arginine Phenylalanine Try to spot the special groups, here we can see arginine and phenylalanine Is it oxytocin or vasopressin? 5) Gramicidin S & Tyrocidine A They are cyclic deca-peptides (made up of 10 amino acids). Produced by: Bacterium Bacillus brevis and acts as an antibiotic. Features: Both contain D-and L-amino acids. Both contain the amino acid ornithine (Orn). Ornithine doesn t occur in proteins. The 20 amino acids that we studied are the only ones that occur in protein synthesis.
20 6) Aspartame (L-Aspartyl-L-phenylalanine / methyl ester) It is a dipeptide (L-aspartic acid and L-phenylalanine). The carboxyl end of phenylphthaline is modified by a methyl group. It is 200 times sweeter than sugar (used as a sweetener.) If the D-amino acid is substituted for either one of the amino acid or for both of them, the resulting derivative is bitter rather than sweet. Phenylketonuria (PKU) PKU is a hereditary inborn error of metabolism, caused by defection of the enzyme phenylalanine hydroxylase. It causes accumulation of phenyl-pyruvate, which causes mental retardation. Sources of phenylalanine such as aspartame and milk must be limited, and as a substitution of aspartame, alatame is used which contains alanine rather than phenylalanine. Normally in the body, phenylalanine has to be converted to tyrosine, but when phenylalanine hydroxylase enzyme is defected, phenylalanine gets converted to phenyl-pyruvate instead, and it gets accumulated. Its accumulation is dangerous because it can get to the CNS and damage the brain causing retardation. Good Luck
Polypeptides. Dr. Mamoun Ahram Summer, 2017
 Polypeptides Dr. Mamoun Ahram Summer, 2017 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.72-78) and 4 Definitions and concepts A residue: each amino acid in a (poly)peptide
Polypeptides Dr. Mamoun Ahram Summer, 2017 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.72-78) and 4 Definitions and concepts A residue: each amino acid in a (poly)peptide
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
Raghad Abu Jebbeh. Amani Nofal. Mamoon Ahram
 ... 14 Raghad Abu Jebbeh Amani Nofal Mamoon Ahram This sheet includes part of lec.13 + lec.14. Amino acid peptide protein Terminology: 1- Residue: a subunit that is a part of a large molecule. 2- Dipeptide:
... 14 Raghad Abu Jebbeh Amani Nofal Mamoon Ahram This sheet includes part of lec.13 + lec.14. Amino acid peptide protein Terminology: 1- Residue: a subunit that is a part of a large molecule. 2- Dipeptide:
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
PEPTIDES and PROTEINS
 PEPTIDES and PROTEINS - Learned basic chemistry of amino acids structure and charges - Chemical nature/charges of amino acids is CRUCIAL to the structure and function of proteins - Amino acids can assemble
PEPTIDES and PROTEINS - Learned basic chemistry of amino acids structure and charges - Chemical nature/charges of amino acids is CRUCIAL to the structure and function of proteins - Amino acids can assemble
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Dr. Nafith Abu Tarboush
 11 Dr. Nafith Abu Tarboush July 3 rd 2013 Marah Dannoun Continuation of amino acids applications in life other than tryptophan and tyrosine: 1. Glutamate (glutamic acid): it s modified to give monosodium
11 Dr. Nafith Abu Tarboush July 3 rd 2013 Marah Dannoun Continuation of amino acids applications in life other than tryptophan and tyrosine: 1. Glutamate (glutamic acid): it s modified to give monosodium
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture -02 Amino Acids II
 Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Amino Acids. Lecture 4: Margaret A. Daugherty. Fall Swiss-prot database: How many proteins? From where?
 Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
2018/9/24 AMINO ACIDS. Important. 436 Notes Original slides. 438 notes Extra information
 2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
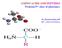 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Maha AbuAjamieh. Tamara Wahbeh. Mamoon Ahram
 12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
12 Maha AbuAjamieh Tamara Wahbeh Mamoon Ahram - - Go to this sheet s last page for definitions of the words with an asterisk above them (*) - You should memorise the 3-letter abbreviations, of all the
Content Lecture 1 Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 Lecture 4
 Good Morning Class! Content Lecture 1 - Basic knowledge for Biochemistry - Cell: Structure and Function - Water Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 - Protein Structure and Function
Good Morning Class! Content Lecture 1 - Basic knowledge for Biochemistry - Cell: Structure and Function - Water Lecture 2 - Amino acid Peptide and Polypeptide Lecture 3 - Protein Structure and Function
استاذ الكيمياءالحيوية
 قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Biochemistry 210 Chapter 22
 Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Organic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins
 rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
Chapter 21 Lecture Outline
 Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
BIO 311C Spring Lecture 15 Friday 26 Feb. 1
 BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
Structure of -amino acids. Stereoisomers of -amino acids. All amino acids in proteins are L-amino acids, except for glycine, which is achiral.
 amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Introduction. Basic Structural Principles PDB
 BCHS 6229 Protein Structure and Function Lecture 1 (October 11, 2011) Introduction Basic Structural Principles PDB 1 Overview Main Goals: Carry out a rapid review of the essentials of protein structure
BCHS 6229 Protein Structure and Function Lecture 1 (October 11, 2011) Introduction Basic Structural Principles PDB 1 Overview Main Goals: Carry out a rapid review of the essentials of protein structure
Sheet #1 Dr. Mamoun Ahram 01/07/2014. Amino Acids
 Amino Acids Dr s office in physiology department in floor #1 Office hours: every day after 1 *How to study biochemistry for Dr. Mamoun s material: Slides will be sent before the lecture, try to read it
Amino Acids Dr s office in physiology department in floor #1 Office hours: every day after 1 *How to study biochemistry for Dr. Mamoun s material: Slides will be sent before the lecture, try to read it
CHAPTER 3 Amino Acids, Peptides, Proteins
 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Introduction to proteins and protein structure
 Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Amino Acids. Review I: Protein Structure. Amino Acids: Structures. Amino Acids (contd.) Rajan Munshi
 Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
A Chemical Look at Proteins: Workhorses of the Cell
 A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
Peptides and Proteins:
 Peptides and Proteins: Interesting Peptide in Biological Systems: 1. Glutathione a. Tripeptide of glutamate, cysteine, glycine b. Regulates oxidation/reduction reactions in cells c. Destroys destructive
Peptides and Proteins: Interesting Peptide in Biological Systems: 1. Glutathione a. Tripeptide of glutamate, cysteine, glycine b. Regulates oxidation/reduction reactions in cells c. Destroys destructive
Lecture 4. Grouping Amino Acid 7/1/10. Proteins. Amino Acids. Where Are Proteins Located. Nonpolar Amino Acids
 Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Human Biochemistry Option B
 Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Practice Problems 3. a. What is the name of the bond formed between two amino acids? Are these bonds free to rotate?
 Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
Life Sciences 1a Practice Problems 3 1. Draw the oligopeptide for Ala-Phe-Gly-Thr-Asp. You do not need to indicate the stereochemistry of the sidechains. Denote with arrows the bonds formed between the
number Done by Corrected by Doctor Dr.Diala
 number 32 Done by Mousa Salah Corrected by Bahaa Najjar Doctor Dr.Diala 1 P a g e In the last lecture we talked about the common processes between all amino acids which are: transamination, deamination,
number 32 Done by Mousa Salah Corrected by Bahaa Najjar Doctor Dr.Diala 1 P a g e In the last lecture we talked about the common processes between all amino acids which are: transamination, deamination,
Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds.
 Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
2. Which of the following is NOT true about carbohydrates
 Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Ali Yaghi مها أبو عجمية. Mamon Ahram
 13 Ali Yaghi مها أبو عجمية Mamon Ahram 0 P a g e This sheet is the last sheet we are supposed to study for the midterm exam. Let s begin. Biological significance of amino acids: Amino acids are used for
13 Ali Yaghi مها أبو عجمية Mamon Ahram 0 P a g e This sheet is the last sheet we are supposed to study for the midterm exam. Let s begin. Biological significance of amino acids: Amino acids are used for
Section 1 Proteins and Proteomics
 Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
MBB 694:407, 115:511. Please use BLOCK CAPITAL letters like this --- A, B, C, D, E. Not lowercase!
 MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Amino Acids االحماض األمينية
 Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
Activities for the α-helix / β-sheet Construction Kit
 Activities for the α-helix / β-sheet Construction Kit The primary sequence of a protein, composed of amino acids, determines the organization of the sequence into the secondary structure. There are two
Activities for the α-helix / β-sheet Construction Kit The primary sequence of a protein, composed of amino acids, determines the organization of the sequence into the secondary structure. There are two
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
PROTEINS. Amino acids are the building blocks of proteins. Acid L-form * * Lecture 6 Macromolecules #2 O = N -C -C-O.
 Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Classification of amino acids: -
 Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
AMINO ACIDS. Qualitative Tests
 AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
Proteins: Structure and Function 2/8/2017 1
 Proteins: Structure and Function 2/8/2017 1 outline Protein functions hemistry of amino acids Protein Structure; Primary structure Secondary structure Tertiary structure Quaternary structure 2/8/2017 2
Proteins: Structure and Function 2/8/2017 1 outline Protein functions hemistry of amino acids Protein Structure; Primary structure Secondary structure Tertiary structure Quaternary structure 2/8/2017 2
Chapter 2 Biosynthesis of Enzymes
 Chapter 2 Biosynthesis of Enzymes 2.1 Basic Enzyme Chemistry 2.1.1 Amino Acids An amino acid is a molecule that has the following formula: The central carbon atom covalently bonded by amino, carboxyl,
Chapter 2 Biosynthesis of Enzymes 2.1 Basic Enzyme Chemistry 2.1.1 Amino Acids An amino acid is a molecule that has the following formula: The central carbon atom covalently bonded by amino, carboxyl,
Chapter 20 and GHW#10 Questions. Proteins
 Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
(30 pts.) 16. (24 pts.) 17. (20 pts.) 18. (16 pts.) 19. (5 pts.) 20. (5 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Date: EXERCISE 4. Figure 1. Amino acid structure.
 Student s name: Date: Points: Assistant s signature: Index numer: /6 EXERISE 4 AMIN AIDS AND PRTEINS. Amino acids are structural units (monomers) of proteins. There are 20 different amino acids coded for
Student s name: Date: Points: Assistant s signature: Index numer: /6 EXERISE 4 AMIN AIDS AND PRTEINS. Amino acids are structural units (monomers) of proteins. There are 20 different amino acids coded for
PROTEIN. By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM
 PROTEIN By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM OBJECTIVES OF THE LECTURE By the end of this lecture, student can: Define
PROTEIN By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM OBJECTIVES OF THE LECTURE By the end of this lecture, student can: Define
CHY2026: General Biochemistry. Unit 4:Amino Acid Chemistry
 CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
Chemistry 20 Chapter 14 Proteins
 Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Understand how protein is formed by amino acids
 Identify between fibrous and globular proteins Understand how protein is formed by amino acids Describe the structure of proteins using specific examples Functions of proteins Fibrous proteins Globular
Identify between fibrous and globular proteins Understand how protein is formed by amino acids Describe the structure of proteins using specific examples Functions of proteins Fibrous proteins Globular
Distribution of the amino acids in Nature Frequency in proteins (%)
 Distribution of the amino acids in ature Amino Acid Frequency in proteins (%) Leucine Alanine Glycine erine Valine Glutamic acid Threonine Arginine Lysine Aspartic acid Isoleucine Proline Asparagine Glutamine
Distribution of the amino acids in ature Amino Acid Frequency in proteins (%) Leucine Alanine Glycine erine Valine Glutamic acid Threonine Arginine Lysine Aspartic acid Isoleucine Proline Asparagine Glutamine
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination #5: Section Five December 7, Name: (print) Section:
 Moorpark College Chemistry 11 Fall 2011 Instructor: Professor Gopal Examination #5: Section Five December 7, 2011 Name: (print) Section: alkene < alkyne < amine < alcohol < ketone < aldehyde < amide
Moorpark College Chemistry 11 Fall 2011 Instructor: Professor Gopal Examination #5: Section Five December 7, 2011 Name: (print) Section: alkene < alkyne < amine < alcohol < ketone < aldehyde < amide
INTRODUCTION TO BIOCHEMISTRY/POLYMERS. 3. With respect to amino acids, polypeptides, and proteins, know:
 INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
INTRDUCTIN T BICEMISTRY/PLYMERS A STUDENT SULD BE ABLE T: 1. With respect to lipids, know: The characteristic common to members of the class (solubility in nonpolar solvents) The functional groups most
Name. The following exam contains 44 questions, valued at 2.6 points/question. 2. Which of the following is not a principal use of proteins?
 Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
