Introduction to Biochemistry Midterm exam )ومن أحياها(
|
|
|
- Emory Leonard
- 5 years ago
- Views:
Transcription
1 Introduction to Biochemistry Midterm exam )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2. Which of the following has a sulfur in its side chain? * a. Met b. ala c. ile d. val 3. Which of the following plays a significant role in blood buffering a. phosphate * b. Bicarbonate c. ammonia d. protein 4. Collagen is an example of which of the following? * c. rod-like protein 5. Which of the following is a polar amino acid but NOT charged? *a. Ser b. leu c. met d. arg 6. Which amino acid is neither D nor L in configuration? a. proline b. alanine *c. Glycine d. tyrosine 1
2 7. The ratio of a weak acid and its conjugate base at the point of maximum buffering capacity is: * a.1/1 b. 10/1 c. 1/10 d. 100/1 8. Which of the following is an amino acid NOT found in proteins a. glycine * b. ornithine c. lysine d. proline 9.Which amino acid has a benzene-like ring? a. histidine b. glycine c. serine d. arginine *e. Tyrosine 10.How many different tripeptides can be assembled using one molecule each of the amino acids glycine, glutamic acid, and lysine? a. 1 *b. 6 c. 3 d What is Aspartame? * a. is a dipeptide derivative used as an artificial sweetener b. contain phenylalanine and arginine c. is a tripeptide used as an artificial sweetener 12. Which of the following forces are involved in maintaining the primary structure of a protein? *a. covalent bonds b. ionic bond c. hydrogen d. hydrophobic bond 2
3 13. Fibrous proteins are typically used in this function: a. transport *b. Structural roles. c. protection 14. Why does myoglobin have a histidine that prevents both O 2 and CO from binding perpendicularly to the heme plane? *c. This lessens the difference in myoglobin's affinity for CO versus O These are all differences between hemoglobin (Hb) and myoglobin (Mb). The choices are: 1. Hb transports oxygen while Mb stores it. 2. Mb has quaternary structure but Hb does not. 3. Mb displays simple kinetics of binding while Hb displays cooperativity. The answers are: a. only 1 b. only 2 c. both 1 and 2 *d. Both 1 and The Bohr effect for oxygen binding states that: The choices are 1. Mb binds oxygen more tightly than Hb 2. Hb will bind oxygen very tightly when the CO 2 concentration is high 3. As the ph goes down, Hb binds oxygen less tightly The answers are a. number 1 b. number 2 and 3 *c. Number 3. d. none of them 17. Binding of bisphosphoglyceric acid (BPG) has the following effects on hemoglobin. The choices are 3
4 1. Leads to tighter binding of oxygen 2. Explains why maternal (adult) Hb binds oxygen more tightly than fetal Hb 3. Causes oxygen to dissociate from Hb The answers are a. number 1 b. number 2 *c. Number 3. d. all of them 18. The affinity of fetal hemoglobin for oxygen a. is lower than that of maternal hemoglobin b. is the same than that of maternal hemoglobin * d. Is higher than that of maternal hemoglobin 19. The purity of an enzyme at various stages of purification is best measured by: a. total activity for the enzyme b. percent recovery * c. Specific activity of the enzyme. d. volume 20. Which would be best to separate a protein that binds strongly to its substrate? a. anion, cation exchange *b. Affinity chromatography c. gel fractionation d. anion exchange 21. Which would be best to separate positively charged proteins? a. anion exchange b. gel fractionation *c. Cation exchange d. affinity chromatography 22. In sickle-cell anemia hemoglobin (Hb S) β-chains: a. glutamic acid replaces valine b. valine replaces arginine 4
5 *c. valine replaces glutamic acid d. glutamic acid replaces histidine 23.The typical order for the major steps of enzyme isolation would be (from first to last): a. salt fractionation, column chromatography. Cell lysis, electrophoresis b. cell lysis, electrophoresis, salt fractionation, column chromatography *c. Cell lysis, salt fractionation, column chromatography, electrophoresis d. electrophoresis, cell lysis, column chromatography, salt lysis 24. Enkephalins are natural pain killers are an example of: a. tripeptide b. dipeptide c. hexapeptide *d. pentapeptide 25. Which of the following happens as a protein is purified? a. the percent recovery increases and the fold purification increases b. the percent recovery increases and the fold purification increases c. the percent recovery decreases and the fold purification decreases *d. the percent recovery decreases and the fold purification increases 26. Which of the following amino acids is not a branched chain amino acid? *a. lysine b. valine c. Isoleucine d. Leucine 27. Which of the following amino acids is an essential amino acid in children and not in adults? *a. Histidine b. valine c. isoleucine d. lysine 5
6 28. Which of the following has the lowest electronegativity? * a. hydrogen b. carbon c. oxygen d. nitrogen 29. Which of the following in NOT a hydrophobic molecule? a. hexane b. fatty acid c. cholestrol *d. phosphate esters 30. Which of the following is NOT a strong acid? a. HNO 3 b. HI *c. H3PO 4 d. HClO Which of the following neutralizes stomach acid? a. NaOH b. KOH *c. Mg(OH) 2 d. MgCl Sour milk, sour cream, yogurt, and cottage cheese have which acid *a. lactic acid b. malic acid c. citric acid d. acetic acid 33. Which of the following is NOT a body electrolyte? a. Na + b. K+ c. Cld. HCO 3 - *e. Ca 2 + 6
7 34. Which of the following buffer systems is NOT present in kidneys? a. phosphate b. ammonia c. bicarbonate *d. protein 35. Thyroxine is a hormone present in the thyroid gland, it is a modified structure of which of the following amino acids? a. lysine b. proline c. arginine *d. tyrosine 36. Milk is rich in which of the following amino acids? a. alanine b. glycine c. arginine *b. tryptophan 37. Glutathione is a tripeptide scavenger for oxidizing agents (antioxidant) is tripeptide composed of which amino acids? a. glutamic acid, glycine, and serine b. asparagine, glycine and serine * c. glutamic acid, glycine and cysteine d. glutamic acid, valine and cysteine 38. The difference between the structure of oxytocin and vasopressin is: *a. oxytocin has isoleucine at position 3 and leucine at position 8 while vasopressin has phenylalanine at position 3 & arginine at position 8. b. oxytocin has isoleucine at position 8 and leucine at position 3 while vasopressin has phenylalanine at position 8 & arginine at position 3. c. vasopressin has isoleucine at position 3 and leucine at position 8 while oxytocin has phenylalanine at position 3 & arginine at position 8. 7
8 39. Collagen has a high concentration of which amino acids? * a. glycine and proline b. glycine and lysine c. lysine and proline d. serine and glycine 40. Glycosylated hemoglobin concentration in blood is about: a. 7% * b. 5 % c. 6% d. 8% 41. Diethylaminomethyl (DEAE) is what kind of media? a. weakly acidic b. strong basic c. strong acidic *d. weakly basic 42. Trypsin act on a polypeptide chains at which site? * a. Carboxyl side of lysine and arginine residues b. amino side of lysine and arginine residues c. carboxyl side of proline and arginine residues 43. How many stereoisomers are possible for a linear aldohexose? a. 8 b. 4 c. 6 * d What are epimers? a. stereoisomers that differ from each other in their configuration at two carbon atoms *b. stereoisomers that differ from each other in their configuration at a single carbon atom c. identical structures d. completely different structures 8
9 45. When sugars form ring structures, the additional chiral carbon is called: *a. Anomeric b. penultimate c. epimer d.isomer 46. Which of the following is the most common biopolymer? a. glycogen *b. Cellulose c. starch d. chitin 47. Which of the following monosaccharides is a ketose? a. glucose * b. fructose c. mannose d. galactose 48. Cellulose is not a source of glucose to humans because? a. humans do not have the enzymes needed to hydrolyze the a- glycosidic linkages between the monomer units b. humans don t benefit from cellulose *c. humans do not have the enzymes needed to hydrolyze the b- glycosidic linkages between the monomer units 49. Which of the following sugars have the lowest sweetness? a. maltose b. glucose *c. Lactose d. sucrose 50. HY + XOH HOH + XY In the above equation, what is X? *a. A cation b. base c. acid d. an anion 9
Short polymer. Dehydration removes a water molecule, forming a new bond. Longer polymer (a) Dehydration reaction in the synthesis of a polymer
 HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
HO 1 2 3 H HO H Short polymer Dehydration removes a water molecule, forming a new bond Unlinked monomer H 2 O HO 1 2 3 4 H Longer polymer (a) Dehydration reaction in the synthesis of a polymer HO 1 2 3
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
The Structure and Function of Macromolecules
 The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
The Structure and Function of Macromolecules Macromolecules are polymers Polymer long molecule consisting of many similar building blocks. Monomer the small building block molecules. Carbohydrates, proteins
Four Classes of Biological Macromolecules. Biological Macromolecules. Lipids
 Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
Biological Macromolecules Much larger than other par4cles found in cells Made up of smaller subunits Found in all cells Great diversity of func4ons Four Classes of Biological Macromolecules Lipids Polysaccharides
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Macromolecules Structure and Function
 Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
Macromolecules Structure and Function Within cells, small organic molecules (monomers) are joined together to form larger molecules (polymers). Macromolecules are large molecules composed of thousands
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A
 Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Biological systems interact, and these systems and their interactions possess complex properties. STOP at enduring understanding 4A Homework Watch the Bozeman video called, Biological Molecules Objective:
Dr. Nafith Abu Tarboush
 11 Dr. Nafith Abu Tarboush July 3 rd 2013 Marah Dannoun Continuation of amino acids applications in life other than tryptophan and tyrosine: 1. Glutamate (glutamic acid): it s modified to give monosodium
11 Dr. Nafith Abu Tarboush July 3 rd 2013 Marah Dannoun Continuation of amino acids applications in life other than tryptophan and tyrosine: 1. Glutamate (glutamic acid): it s modified to give monosodium
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination # 5: Section Five May 7, Name: (print)
 Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall 2013 Instructor: Professor Gopal Examination # 5: Section Five May 7, 2013 Name: (print) Directions: Make sure your examination contains TEN total pages (including this
Moorpark College Chemistry 11 Fall Instructor: Professor Gopal. Examination #5: Section Five December 7, Name: (print) Section:
 Moorpark College Chemistry 11 Fall 2011 Instructor: Professor Gopal Examination #5: Section Five December 7, 2011 Name: (print) Section: alkene < alkyne < amine < alcohol < ketone < aldehyde < amide
Moorpark College Chemistry 11 Fall 2011 Instructor: Professor Gopal Examination #5: Section Five December 7, 2011 Name: (print) Section: alkene < alkyne < amine < alcohol < ketone < aldehyde < amide
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
2. Which of the following is NOT true about carbohydrates
 Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
Chemistry 11 Fall 2011 Examination #5 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response questions
Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
Concept 5.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 50% of the dry mass of most cells Protein functions include structural support, storage,
االمتحان النهائي لعام 1122
 االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
AP Bio. Protiens Chapter 5 1
 Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
Concept.4: Proteins have many structures, resulting in a wide range of functions Proteins account for more than 0% of the dry mass of most cells Protein functions include structural support, storage, transport,
(30 pts.) 16. (24 pts.) 17. (20 pts.) 18. (16 pts.) 19. (5 pts.) 20. (5 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Moorpark College Chemistry 11 Spring 2009 Instructor: Professor Torres Examination # 5: Section Five April 30, 2009 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total
Chapter 5: Structure and Function of Macromolecules AP Biology 2011
 Chapter 5: Structure and Function of Macromolecules AP Biology 2011 1 Macromolecules Fig. 5.1 Carbohydrates Lipids Proteins Nucleic Acids Polymer - large molecule consisting of many similar building blocks
Chapter 5: Structure and Function of Macromolecules AP Biology 2011 1 Macromolecules Fig. 5.1 Carbohydrates Lipids Proteins Nucleic Acids Polymer - large molecule consisting of many similar building blocks
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
9/16/15. Properties of Water. Benefits of Water. More properties of water
 Properties of Water Solid/Liquid Density Water is densest at 4⁰C Ice floats Allows life under the ice Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form Benefits
Properties of Water Solid/Liquid Density Water is densest at 4⁰C Ice floats Allows life under the ice Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds break and re-form Benefits
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site. Still having trouble understanding the material? Check
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site. Still having trouble understanding the material? Check
Name. The following exam contains 44 questions, valued at 2.6 points/question. 2. Which of the following is not a principal use of proteins?
 Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Raghad Abu Jebbeh. Amani Nofal. Mamoon Ahram
 ... 14 Raghad Abu Jebbeh Amani Nofal Mamoon Ahram This sheet includes part of lec.13 + lec.14. Amino acid peptide protein Terminology: 1- Residue: a subunit that is a part of a large molecule. 2- Dipeptide:
... 14 Raghad Abu Jebbeh Amani Nofal Mamoon Ahram This sheet includes part of lec.13 + lec.14. Amino acid peptide protein Terminology: 1- Residue: a subunit that is a part of a large molecule. 2- Dipeptide:
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Methionine (Met or M)
 Fig. 5-17 Nonpolar Fig. 5-17a Nonpolar Glycine (Gly or G) Alanine (Ala or A) Valine (Val or V) Leucine (Leu or L) Isoleucine (Ile or I) Methionine (Met or M) Phenylalanine (Phe or F) Polar Trypotphan (Trp
Fig. 5-17 Nonpolar Fig. 5-17a Nonpolar Glycine (Gly or G) Alanine (Ala or A) Valine (Val or V) Leucine (Leu or L) Isoleucine (Ile or I) Methionine (Met or M) Phenylalanine (Phe or F) Polar Trypotphan (Trp
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Chapter 21 Lecture Outline
 Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Human Biochemistry Option B
 Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Q1: Circle the best correct answer: (15 marks)
 Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
Lipids: diverse group of hydrophobic molecules
 Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Lipids: diverse group of hydrophobic molecules Lipids only macromolecules that do not form polymers li3le or no affinity for water hydrophobic consist mostly of hydrocarbons nonpolar covalent bonds fats
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2009 Examination #5 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Polypeptides. Dr. Mamoun Ahram Summer, 2017
 Polypeptides Dr. Mamoun Ahram Summer, 2017 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.72-78) and 4 Definitions and concepts A residue: each amino acid in a (poly)peptide
Polypeptides Dr. Mamoun Ahram Summer, 2017 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.72-78) and 4 Definitions and concepts A residue: each amino acid in a (poly)peptide
Biology 12 - Biochemistry Practice Exam
 Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
1. Denaturation changes which of the following protein structure(s)?
 Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
Chem 11 Fall 2008 Examination #5 ASWER KEY MULTIPLE CICE (20 pts. total; 2 pts. each) 1. Denaturation changes which of the following protein structure(s)? a. primary b. secondary c. tertiary d. both b
1. (38 pts.) 2. (25 pts.) 3. (15 pts.) 4. (12 pts.) 5. (10 pts.) Bonus (12 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2010 Instructor: Professor Torres Examination #5: Section Five May 4, 2010 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2010 Instructor: Professor Torres Examination #5: Section Five May 4, 2010 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total pages
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
Biological Sciences 4087 Exam I 9/20/11
 Name: Biological Sciences 4087 Exam I 9/20/11 Total: 100 points Be sure to include units where appropriate. Show all calculations. There are 5 pages and 11 questions. 1.(20pts)A. If ph = 4.6, [H + ] =
Name: Biological Sciences 4087 Exam I 9/20/11 Total: 100 points Be sure to include units where appropriate. Show all calculations. There are 5 pages and 11 questions. 1.(20pts)A. If ph = 4.6, [H + ] =
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Chapter 20 and GHW#10 Questions. Proteins
 Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
The. Crash Course. Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O)
 The Biochemistry Crash Course Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O) This exercise is designed to familiarize you with
The Biochemistry Crash Course Basically, almost all living things are made up of these 4 Elements: - Carbon (C) - Nitrogen (N) - Hydrogen (H) - Oxygen (O) This exercise is designed to familiarize you with
Chem Exam 2 (A) Name
 Chem 4511 Exam 2 (A) Name No credit will be given for answers (or work) that are on the backsides of the pages. You may use the backsides as scratch paper, but put all of your answers on the front sides.
Chem 4511 Exam 2 (A) Name No credit will be given for answers (or work) that are on the backsides of the pages. You may use the backsides as scratch paper, but put all of your answers on the front sides.
CS612 - Algorithms in Bioinformatics
 Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Spring 2016 Protein Structure February 7, 2016 Introduction to Protein Structure A protein is a linear chain of organic molecular building blocks called amino acids. Introduction to Protein Structure Amine
Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond
 Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Lecture 4. Grouping Amino Acid 7/1/10. Proteins. Amino Acids. Where Are Proteins Located. Nonpolar Amino Acids
 Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Biomolecules. Presented by Amelia McCutcheon
 Biomolecules Presented by Amelia McCutcheon Fats Carbohydrates Proteins Vitamins Fats Also known as lipids Fats are solids (high mel=ng point) ils are liquids (low mel=ng point) Mainly consist of Carbon
Biomolecules Presented by Amelia McCutcheon Fats Carbohydrates Proteins Vitamins Fats Also known as lipids Fats are solids (high mel=ng point) ils are liquids (low mel=ng point) Mainly consist of Carbon
Slide 1. Slide 2. Slide 3. So far... All living things are primarily made up of four classes of Macromolecules
 Slide 1 So far... 1. Biology is the study of life - All life is based on the cell - The Earth, organisms, cells are all aqueous 2. Water s uniqueness stems from its internal polarity - Solvent, Co/Adhesion,
Slide 1 So far... 1. Biology is the study of life - All life is based on the cell - The Earth, organisms, cells are all aqueous 2. Water s uniqueness stems from its internal polarity - Solvent, Co/Adhesion,
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
BIOLOGICALLY IMPORTANT MOLECULES
 BIOLOGICALLY IMPORTANT MOLECULES ( use with printout from zerobio website) Note: images from internet and used for educational purposes only CARBOHYDRATES: MONOSACCHARIDES H GLUCOSE FRUCTOSE GALACTOSE
BIOLOGICALLY IMPORTANT MOLECULES ( use with printout from zerobio website) Note: images from internet and used for educational purposes only CARBOHYDRATES: MONOSACCHARIDES H GLUCOSE FRUCTOSE GALACTOSE
Introduction to proteins and protein structure
 Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Introduction to proteins and protein structure The questions and answers below constitute an introduction to the fundamental principles of protein structure. They are all available at [link]. What are
Cells. Variation and Function of Cells
 Cells Variation and Function of Cells Plasma Membrane= the skin of a cell, it protects and nourishes the cell while communicating with other cells at the same time. Lipid means fat and they are hydrophobic
Cells Variation and Function of Cells Plasma Membrane= the skin of a cell, it protects and nourishes the cell while communicating with other cells at the same time. Lipid means fat and they are hydrophobic
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
The Structure and Function of Large Biological Molecules
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 5 The Structure and Function of
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 5 The Structure and Function of
Macromolecules. Note: If you have not taken Chemistry 11 (or if you ve forgotten some of it), read the Chemistry Review Notes on your own.
 Macromolecules Note: If you have not taken Chemistry 11 (or if you ve forgotten some of it), read the Chemistry Review Notes on your own. Macromolecules are giant molecules made up of thousands or hundreds
Macromolecules Note: If you have not taken Chemistry 11 (or if you ve forgotten some of it), read the Chemistry Review Notes on your own. Macromolecules are giant molecules made up of thousands or hundreds
PROTEINS. Amino acids are the building blocks of proteins. Acid L-form * * Lecture 6 Macromolecules #2 O = N -C -C-O.
 Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
Proteins: Linear polymers of amino acids workhorses of the cell tools, machines & scaffolds Lecture 6 Macromolecules #2 PRTEINS 1 Enzymes catalysts that mediate reactions, increase reaction rate Structural
THE UNIVERSITY OF MANITOBA. DATE: Oct. 22, 2002 Midterm EXAMINATION. PAPER NO.: PAGE NO.: 1of 6 DEPARTMENT & COURSE NO.: 2.277/60.
 PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
Tala Saleh. Tamer Barakat. Diala Abu-Hassan
 13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
Introduction to Protein Structure Collection
 Introduction to Protein Structure Collection Teaching Points This collection is designed to introduce students to the concepts of protein structure and biochemistry. Different activities guide students
Introduction to Protein Structure Collection Teaching Points This collection is designed to introduce students to the concepts of protein structure and biochemistry. Different activities guide students
Chemistry 107 Exam 3 Study Guide
 Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Amino acids & Protein Structure Chemwiki: Chapter , with most emphasis on 16.3, 16.4 and 16.6
 Amino acids & Protein Structure Chemwiki: Chapter 16. 16.1, 16.3-16.9 with most emphasis on 16.3, 16.4 and 16.6 1 1. Most jobs (except information storage) in cells are performed by proteins. 2. Proteins
Amino acids & Protein Structure Chemwiki: Chapter 16. 16.1, 16.3-16.9 with most emphasis on 16.3, 16.4 and 16.6 1 1. Most jobs (except information storage) in cells are performed by proteins. 2. Proteins
CHY2026: General Biochemistry. Unit 4:Amino Acid Chemistry
 CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
Chapter 5 Structure and Function Of Large Biomolecules
 Formation of Macromolecules Monomers Polymers Macromolecules Smaller larger Chapter 5 Structure and Function Of Large Biomolecules monomer: single unit dimer: two monomers polymer: three or more monomers
Formation of Macromolecules Monomers Polymers Macromolecules Smaller larger Chapter 5 Structure and Function Of Large Biomolecules monomer: single unit dimer: two monomers polymer: three or more monomers
So where were we? But what does the order mean? OK, so what's a protein? 4/1/11
 So where were we? We know that DNA is responsible for heredity Chromosomes are long pieces of DNA DNA turned out to be the transforming principle We know that DNA is shaped like a long double helix, with
So where were we? We know that DNA is responsible for heredity Chromosomes are long pieces of DNA DNA turned out to be the transforming principle We know that DNA is shaped like a long double helix, with
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL
 Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL For Questions 1-10 choose ONE INCORRECT answer. 1. Which ONE of the following statements concerning the
Multiple-Choice Questions Answer ALL 20 multiple-choice questions on the Scantron Card in PENCIL For Questions 1-10 choose ONE INCORRECT answer. 1. Which ONE of the following statements concerning the
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
Fatty acids and phospholipids
 PYS 4xx Intro 2 1 PYS 4xx Intro 2 - Molecular building blocks We now describe in more detail the nomenclature and composition of several classes of compounds of relevance to the cell, including: membrane
PYS 4xx Intro 2 1 PYS 4xx Intro 2 - Molecular building blocks We now describe in more detail the nomenclature and composition of several classes of compounds of relevance to the cell, including: membrane
1.4. Lipids - Advanced
 1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
1.4. Lipids - Advanced www.ck12.org In humans, triglycerides are a mechanism for storing unused calories, and their high concentration in blood correlates with the consumption of excess starches and other
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
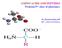 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Unit 3: Chemistry of Life Mr. Nagel Meade High School
 Unit 3: Chemistry of Life Mr. Nagel Meade High School IB Syllabus Statements 3.2.1 Distinguish between organic and inorganic compounds. 3.2.2 Identify amino acids, glucose, ribose and fatty acids from
Unit 3: Chemistry of Life Mr. Nagel Meade High School IB Syllabus Statements 3.2.1 Distinguish between organic and inorganic compounds. 3.2.2 Identify amino acids, glucose, ribose and fatty acids from
Amino Acids. Review I: Protein Structure. Amino Acids: Structures. Amino Acids (contd.) Rajan Munshi
 Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
Review I: Protein Structure Rajan Munshi BBSI @ Pitt 2005 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2005 Amino Acids Building blocks of proteins 20 amino acids
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Classification of amino acids: -
 Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Page 1 of 8 P roteinogenic amino acids, also known as standard, normal or primary amino acids are 20 amino acids that are incorporated in proteins and that are coded in the standard genetic code (subunit
Biological Molecules
 The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
The Chemical Building Blocks of Life. Chapter 3
 The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
