CO?IU VERSIOS OF PHOSPHORYLASE b TO PHOSPHORYLASE a IS MT SCLE EXTRACTS*
|
|
|
- Grant Lamb
- 5 years ago
- Views:
Transcription
1 CO?IU VERSIOS OF PHOSPHORYLASE b TO PHOSPHORYLASE a IS MT SCLE EXTRACTS* BY EDMOND H. FISCHER AND EDWIN G. KREBS (From the Department of Biochemistry, University of Washington, Seattle, Washington) (Received for publication, January 17, 1955) In 1943 rabbit skeletal muscle phosphorylase was isolated in the crystalline form by Green and Cori (1). The crystalline enzyme possessed 60 to 70 per cent of its maximal activity in the absence of AMP,l and in this characteristic differed from previously observed muscle phosphorylase that required AMP for activity. The type of phosphorylase represented by the new crystalline enzyme was designated as phosphorylase a, and phosphorylase with the absolute AMP requirement was designated as phosphorylase b (2). An enzyme (PR enzyme), which catalyzed a conversion of phosphorylase a to phosphorylase b in vitro, was discovered in muscle extract, and its action was studied extensively (2-4). Evidence was obtained that during muscle contraction phosphorylase a is changed t.o phosphorylase b and that during the recovery phase phosphorylase a is restored (5). Sutherland reported experiments showing phosphorylase a formation in the isolated muscle diaphragm (6). In the preceding paper (7) it was found that phosphorylase as extracted from resting muscle is predominantly in the b form, i.e. requiring AMP. It has been determined that this enzyme can be converted readily t o phosphorylase a in the cell-free extracts. The requirements for the reaction of phosphorylase b -+ a include a divalent metal ion and, under certain conditions, ATP. Methods The preparation of muscle extracts (Methods 1 to 5) and the determination of activity measurements and phosphorylase units are described in the preceding paper (7). Reaction System for Conversion of Phosphorylase b to Phosphorylase a- 1.0 ml. samples of muscle extract containing phosphorylase b are brought t,o 1.2 ml. final volume with water or various additions. After incubation * Supported by the Initiative 171 Research Fund of the State of Washington and by a research grant (G-2399) from the Xational Institutes of Health, Public Health Service. 1 The abbreviations or contractions used in the paper include AMP, adenosine- B/-phosphate; ADP and ATP, adenosine di- and triphosphate; EDTA, ethylenediaminetetraacetate. 121
2 122 CONVERSION OF PHOSPHORYLASE b TO CZ for specified intervals at 25, samples are removed and diluted in 0.03 M cysteine-0.04 M glycerophosphate buffer, ph 6.0, and kept for 8 minutes at 30 prior to the activity test. The final dilution of the extract in the activity test system is 1:72. Adsorption of Nucleotides with Norit- ml. of crude muscle extract were shaken with 1 gm. of Xorit A (Pfanstiehl) for 5 minutes at 0 and the mixture was filtered through Whatman lyo. 42 paper. Wushing of Filter Paper-18 cm. Whatman Ko. 1 filters were washed with the following solutions added in succession: 50 ml. of 0.01 N HCl (per paper), 50 ml. of 0.01 N NaOH, 50 ml. of H20. The papers were allowed to dry at room temperature before use. Filter Paper Extract-Twenty 18 cm. Whatman No. 1 filters were extracted with successive portions of 0.01 N HCl (total HCl = 1200 ml.). The extract was concentrated and dried in vacua mg. of residue were dissolved in 10 ml. of HsO and neutralized before use. Ash of Filter Paper Extract-Half of the above extract before neutralization was dried in a platinum crucible (23.2 mg.) and ashed for 3 hours at 570. The ash (17.6 mg.) was dissolved in 0.5 ml. of 1 N HCl and dried in vucuo. The residue (21.8 mg.) was dissolved in H20, adjusted to ph 7.0, and brought to 5.0 ml. Results Metal Requirement for Conversion of Phosphorylase b to Phosphorylase a- Fresh crude muscle extracts containing phosphorylase b were found to require only the addition of certain divalent cations and a short period of incubation to bring about complete conversion of the enzyme to phosphorylase a. Table I presents the data from a typical experiment in which the specificity of various metals for the reaction was determined. It can be seen that Ca++, Sr++, Ba*, Zn*, and Mn++ were all effective2 in that the phosphorylase activity, as tested in the absence of added AMP, rose markedly. Metal ions, when added only to the test system, did not affect the relative concentrations of phosphorylases a and b. Total phosphorylase activity (measured with added AMP) also appears to be increased significantly in the transformation of phosphorylase b -+ a; this finding will be discussed in a later section. Nucleotide Requirements for Conversion of Phosphorylase b to a-in crude muscle extracts aged for 24 hours at 3 or kept in the frozen state for several days, phosphorylase b could still be converted to phosphorylase a, but it was found that under these conditions addition of ATP3 was required. 2 The slight effects of Fe++ and Co++ could not be repeated in other experiments. 3 In most of the experiments reported in this paper, Pabst disodium adenosine triphosphate was used. In later experiments crystalline ATP from the same source was used and was found to give identical results.
3 E. H. FISCHER AND E. G. KREBS 123 Dialysis of muscle extracts or treatment with n orit also made the addition of ATP necessary for the conversion, as shown in Table II. Phosphorylase b is no longer converted to phosphorylase a by incubation with Mn++ alone, but the combination of Mn++ and ATP is effective. TABLE Injkence of Metal Ions on Conversion of Phosphorylase b to Phosphorylase a 1.0 ml. portions of muscle extract (Method 1) were brought to 1.2 ml. with water or additions and incubated for 20 minutes at X 10-a M ATP was present except in the first control. Activity measurements in the presence and absence of AMP were performed at a final 1:72 dilution of the extract. Units per ml. are calculated for undiluted extract in each case. Original activity of the extract before incubation, + AMP, 1300 units per ml.; - AMP, 20 units per ml. Addition* Control (- ATP).. (f )... Kf..... Mgft... Ca++... Sr++... Ba Mn++... Fe co Ni Cu++$.... Zn++$.... Hg++$ AMP I units per ml. I unifr per ml * All salts were acetates or chlorides present at a final concentration of 1 X 10m3 M during the incubation. t This quantity divided by 0.65 gives the percentage of phosphorylase a (1). $0.5 X 10-a M during the incubation. I Activity - AMP I -AMP + AMP+ pn cent With the treated extracts (see above) ADP could be substituted for ATP with essentially identical results. AMP was also studied to determine whether it could serve to activate the conversion system of phosphorylase b -+ a; inconsistent behavior of this nucleotide was noted, depending upon the treatment of the extract. In frozen extracts no conversion occurred on incubation with 1 X 1O-3 M AMP and Mn++, although ATP (or ADP) at the same concentration was completely effective. With a lo-fold increase in the AMP concentration partial conversion took place. In Norit-treated extracts AMP was completely ineffective, which made it
4 124 CONVERSION OF PHOSPHORYLASE b TO U appear unlikely that it could act as such in the reaction. Indeed, it was found that AMP could serve in Sorit-t,reated extracts, if phosphocreatine was also added (Table III). As shown, phosphocreatine by itself was TABLE Conversion System in Dialyzed or Norit-Treated Extracts EDTA extract (No. I) from muscle (Method 4) was dialyzed for 3 hours at 5 against distilled Hz0 and then incubated for 20 minutes at 25 with the additions indicated. Extract (No. II) obtained by Method 1 was treated with Norit and then incubated as above. Incubations with untreated control at 5. Extract II Ratio, phosphorylase activity (- AMP/+ AMP) after incubation with No additions 3 X lo-3 M Mn++ 3 X 10-s Y Mn X 10-a Y ATP -- per cent per cenf per cent I. Before dialysis After II. Untreated Norit-treated TABLE Ineffectiveness of AMP without Phosphocreatine Muscle extract was obtained by Method 1 and frozen; it was thawed after 3 days and treated with Norit. Incubations carried out at X 1OF M Mn++ and 1 X 10-a M Ca++ present in each incubation mixture. Phosphorylase activities carried out at 1:80 dilution of the incubation mixture. Addition* III Ratio, phosphorylase activity (- AMP/+ AMP) after incubation for 0 min. I 25 min. I 120 min. per cent ger cent $3.3 cent None Phosphocreatine AMP 24.4t Phosphocreatine + AMP. 31.4t * 1 X 10-S M during incubation for each component. t Norit-treated extracts contain almost no adenylic deaminase (8), and there is partial activation of phosphorylase 6 due to AMP carried over into the activity test system. inactive. Assuming that a trace of ATP was still present in this extract, AMP could give rise to ATP in the presence of phosphocreatine through the myokinase and ATP-creatine transphosphorylase reactions. Although it is difficult to determine the specificity of nucleotides in a crude system in which various transformations and interconversions may
5 E. H. FISCHER AND E. G. KREBS 125 occur, the following nucleotides have also been tested: inosine mono- and triphosphate, uridine mono- and triphosphate, cytidine mono- and triphosphate,4 AMP (3 ), guanosine-3 -phosphate, and cytidine3 -phosphate. Of these, only uridine triphosphate showed a slight effect in activating the conversion reaction of phosphorylase b --+ a; the possibility of traces of ATP in the uridine triphosphate has not been excluded. Extent of Conversion in Di$erent Muscle ExtractsWhen fresh extract is incubated at 25 with added metal ion, the increase in the - AMP/+ AMP phosphorylase activity ratio takes place very rapidly, sometimes reaching TABLE Extent of Conversion of Phosphorylase b to a in Rabbits under various Conditions The mean values are obtained by dividing the sum of the activity units per ml. of crude extract by the number of rabbits. Reaction system for conversion as described under Methods., the activities are determined after 30 minutes incubation at 25. Extract 5 ;Methodl ; Method 1. Strychnine anesthesia; Method Special anesthesia; Methods 3, No. of rabbits IV Mean activities (units per ml.) after incubation with No additions 10-s x Mn++ 10-x M ATP t AMP - AMP t AMI AMP+AMP ~ -AMP I 10-a IA Mn M ATP i-mf AMP a maximum within less than 5 minutes. With continued incubation after conversion is complete, the activities remain relatively stable for several hours; then a definite decline in the ratio occurs. After overnight incubation a phosphorylase b picture usually results. The extent of conversion of phosphorylase b --, a after approximately 30 minutes of incubation was studied with fifteen different muscle extracts with no additions and with added Mn++ plus ATP (Table IV). In most of the experiments incubation was also carried out with ATP alone (omitting Mntf-). The extracts used are separated into four groups (Table IV) according to the temperature at which the animals were kept or to the Pabst Laboratories, Milwaukee. We are indebted to Dr. F. M. Huennekens for providing the samples.
6 126 CONVERSION OF PHOSPHORYLASE b TO U procedure used in killing the rabbits and preparing the extracts. Although they all contained phosphorylase predominantly in the b form originally, conversion to phosphorylase a occurred in each case, even with extracts obtained from strychninized rabbits. It will be noted that, after conversion, the phosphorylase activity measured without AMP exceeds the maximum of 60 to 70 per cent found for crystalline phosphorylase a (1). It is unlikely that this can be accounted for on the basis of a carryover of AMP from the crude extract into the activity test reaction mixture. In an experiment in which the - AMP/+ AMP activity ratio was 100 per cent after conversion of phosphorylase b to a, the reaction mixture was treated with Norit and filtered. The filtrate still showed a ratio of 92 per cent, although absorption at 260 m,u indicated that no detectable amount of nucleotides remained. 5 X lo+ M AMP would be easily detected in the absorption method, and this would be equivalent to less than 1 X lo-- M AMP in the activity test. Total phosphorylase activity is also increased significantly during the conversion of phosphorylase b + a (Table IV), as was confirmed by statistical analysis. Although no definite explanation can be given at this time for this phenomenon, several factors might be involved.6 Activity measurements are carried out at ph 6.0, i.e. below the ph optimum for phosphorylase a or b.s The two enzymes may have different ph dependence curves, and it is possible that the metal ion or the nucleotide added for the conversion affects these curves differently. In crude extracts, deamination of AMP by adenylic deaminase proceeds at such a rate at ph 6.0 that the nucleotide added in the activity test is partially destroyed before the end of the reaction. This might result in values too low for phosphorylase b, whereas phosphorylase a activity would not be affected. This is consistent with the fact that, addition of fluoride, which inhibits the deaminase, increases the total activity of phosphorylase before conversion but not after. Likewise, when a Norit-treated extract is used for the conversion (from which most of the deaminase has been removed), a smaller increase in total activity is observed. Finally, several preparations of crystalline phosphorylase a showed an increased activity when tested after a short incubation in the presence of divalent metal ions (Ca++, Mn++). A similar activation of the enzyme by these ions could occur in the crude extracts and thus contribute to the observed result. 6 Cori and Cori have found that under certain conditions there occurred a 23 per cent decrease in total phosphorylase activity at ph 6.8 when phosphorylase a was converted to b by PR enzyme action (9). 6 The ph optimum for phosphorylase is around 6.7 to 6.8, but, in crude extracts, activation is measured at ph 6.0 to minimize mutase action. Activity at ph 6.0 divided by 0.7 gives activity at ph 6.8 (10).
7 E. H. FISCHER AND E. G. KREBS 127 Properties of Conversion System-Although no definite proof has been obtained that the reaction of phosphorylase b -+ a is enzyme-catalyzed, it has been found that the presence of a protein fraction of the extract is necessary. If the ph of dialyzed muscle extract is lowered to ph 5.8, and the precipitate which forms is removed, the phosphorylase b in the supernatant solution is no longer converted to phosphorylase a by incubation with Mn++ plus ATP. Addition of the fraction removed to the supernatant solution rest ores the reaction. EDTA was found to block completely the reaction of phosphorylase b --+ a, which was to be expected, since the system requires divalent metal ions. When calcium or manganous salts were added in molar excess over EDTA, the conversion system could be reactivated readily. Once phosphorylase b in the extracts had been converted to phosphorylase a, addition of EDTA had no effect on the phosphorylase activity itself. Addition of I? (0.04 M), arsenate (0.015 M), and cysteine (0.06 M) did not affect the degree of phosphorylase b to phosphorylase a conversion after 30 minutes of incubation of extract with Mn* or Mn++ and ATP. In these experiments the extent of conversion at 30 minutes was essentially complete in all cases; hence slight effects on rate would not have been noticed. Evidence of Phosphorylase a Formation during Conversion-It was important to establish beyond doubt that phosphorylase a was actually being formed and that the results observed were not due simply to AMP production and a carryover of this nucleotide into the activity test system. If this occurred, then naturally phosphorylase b could give the a picture. This possibility could be eliminated completely by several types of evidence. If the total non-protein absorption of muscle extracts at 260 rnp is assumed to be due to AMP, the concentration of this nucleotide would be about 1.1 X 1O-3 M in the extract. After dilution for determination of phosphorylase activity the concentration would be approximately 1.5 X 1O-5 M AMP, i.e. a concentration capable of producing less than half maximal activation of phosphorylase b (11). Even in experiments in which adenine nucleotides were added to the conversion reaction mixture to concentrations of 1 X 1O-3 M, the possible carryover of AMP would be too low to give half maximal activation. The nucleotides present in the conversion reaction mixture after incuba- 7 It is of interest that the fraction which is precipitated by this procedure contains the PR enzyme that catalyzes the reaction of phosphorylase a + b (2). Whether this means that the PR enzyme itself participates in the phosphorylase b ---f a process cannot be stated at this time, although it has been determined that purified PR enzyme alone does not catalyze the formation of phosphorylase a from phosphorylase b when incubated with Mn++ plus ATP.
8 128 CONVERSION OF PHOSPHORYLASE b TO U tion, as carried out for phosphorylase a formation, do not include amounts of AMP detectable by the method of Kalckar (12). Even when AMP itself was added to the incubation mixtures in high concentrations (to 6 X 1O-2 M), it had all disappeared within 15 minutes, owing to the action of adenylic deaminase.?iiorit treatment of phosphorylase a after the conversion reaction removed all detectable traces of AMP, but did not alter the high ratio of - AMP/+ AMP activity. Final proof of phosphorylase a formation resulted from its isolation in the crystalline form after conversion: To a crude muscle extract, prepared by Method 1 of the preceding paper (7), sufficient 0.5 M EDTA solution,* ph 7.0, was added to make the final concentration M EDTA. One 450 ml. portion (Sample I) of this extract was carried through the regular steps in the preparation of phosphorylase a (1, lo), with the exception that the filtration step through paper was omitted. Dialysis prior to the removal of PR enzyme was carried out against distilled water.g Capon dialysis of the (NHb)zS04 precipitate, no protein separated from solution within 24 hours. Ultracentrifugal analysis on a similar fraction from a different preparation showed the presence of a single component with s20 = 8.83 in 0.03 M cysteine-0.04 M glycerophosphate buffer, ph 6.8. A second 450 ml. portion (Sample II) of the extract containing EDTA was treated with manganous acetate (final concentration of Mn++ in extract = 3 X 1O-3 M) and ATP (final concentration = 5 X 1O-4 M) and allowed t,o stand for 2 hours at 0 before proceeding with the preparation. The next steps were carried out as described above. With this portion a heavy precipitate of phosphorylase a developed, when the (~HJ)~SO~ precipitate was dialyzed. The enzyme was recrystallized twice in the usual manner. The microscopic appearance of the crystals resembled that shown for phosphorylase a (1). Table V shows the recoveries in the different steps of the preparation. A considerable loss of phosphorylase occurred in the isoelectric precipitation of PR enzyme, but this was compensated for by dissolving the 41 per cent saturated (NH4)&04 fraction in only a small volume of water. The twice recrystallized enzyme was contaminated with traces of PR enzyme, which made ultracentrifugal analysis difficult; however, the major component found (60 per cent of the total) had an s20 of which is in * The EDTA was added so that the control portion of the extract (Sample I) could be carried through the preparation without risk of conversion to phosphorylase a by chance contamination with metals. Dialysis was carried out against distilled water rather than tap water (1) for the same reasons. 9 In 1945 Cori noted an apparent increase in the concentration of phosphorylase a following dialysis of frog and rabbit muscle extracts (5).
9 E. H. FISCHER AND E. G. KREBS 129 close agreement with the known value for phosphorylase a (s20,,, = 13.2) (4). A second component with an s20 of 8.47 comprised 38.2 per rent of the total; this was presumably phosphorylase b (~~0,~ = 8.2) (4). TABLE Phosphorylase Purijkation with and without Conversion to Phosphorylase a Original extract obtained by Method I and EDTA added to final concentration of M. Extract divided into two portions: Sample I, no conversion procedure; Sample II, converted to phosphorylase a as described in the text. step Original extract (with EDTA) After incubation with Mn++ + ATP After dialysis. removal of isoelectric ppt. After dialysis of (NH,)zS04 ppt.. Volume Sample I 450 2,360 / ,990 ~ 200 V Total units x 10-s ~ i 36, TABLE VI - Volume 450 ml , , Filter Paper Effects on Muscle Extract Sample II tmp - AMP Total units 2, ,020, ,000 33, Phosphorylase activities were determined on portions of muscle extract obtained by Method 1 and treated as indicated. Original activity of muscle extract before any treatment, + AMP, 1800 units per ml.; - AMP, 20 units per ml. ~ Treatment Filtration through unwashed paper.... washed paper.... Incubation with filter paper extract.... I ash... units +AMp per nzl. Activity - AMP units per ml. / per cent / I I 63 Effect of Filtration of Muscle Extracts-In the preceding paper (7) it was noted that rabbit, muscle extracts containing only phosphorylase b could serve, nevertheless, for the isolation of crystalline phosphorylase a, when the method of Green and Cori (1) was followed. It was found that the a form of the enzyme appeared following filtration of the extract through
10 130 CONVERSION OF PHOSPHORYLASE b TO U Whatman Xo. 1 paper. If the filtration step was omitted, partial conversion of the original phosphorylase b was found to occur during dialysis9 against cold tap water, but not during dialysis against distilled water. As presented in Table VI the effect of filtration can be accounted for by extraction of metals present in the paper. Washing of the paper abolishes the effect. When filtration is omitted and filter paper extract or ash is added, phosphorylase a is formed after a short incubation period. Incubation with no additions does not change the original activity. KritskiI and Kuvaeva (13), using a procedure based largely on that of Green and Cori (1) but in which filtration through paper and dialysis were avoided, claimed to have obtained a crystalline material showing a phosphorylase b type of activity. Cori found that muscle extracts from rabbits killed with strychnine would not serve for the preparation of crystalline phosphorylase a. In the present study it has been found that the conversion of phosphorylase Z, -+ a does occur in this type of extract when divalent metal ions are added immediately (see Table IV); however, the ph of these extracts (ph 6.0 to 6.1) is lower than those obtained with barbiturate anesthesia and drops rapidly on standing to a point (ph 5.8) at which no conversion occurs with metal alone. When filtration or dialysis serves as the sole source of metal ions, it is possible that they may be introduced too late to effect a conversion. DISCUSSION In this study, evidenoe has been presented showing that the conversion of phosphorylase b to a, as it occurs in cell-free muscle extracts, requires a nucleotide containing high energy phosphate, in addition to a divalent metal ion.1 Whether this implies that during conversion there is a direct phosphorylation of the enzyme or the formation of an active intermediate cannot be stated at this time. It is also possible that the fun&ion of ATP is concerned with the synthesis of a prosthetic group. No definite information is available as to whether phosphorylase b present in crude muscle extract (7) is identical with the phosphorylase b produced from crystalline phosphorylase a with purified PR enzyme. Keller and Cori (4) have found that in this process a halving of the molecular weight lo Rosenfeld and Petrova (14) have reported a conversion of phosphorylase b to a in muscle extracts that were autolyzed for 48 hours at room temperature, stored for several weeks in the refrigerator, and finally alkalinized. It is very difficult to interpret what may have happened under their conditions. In the present study, no spontaneous conversion has been noticed during storage of the extracts. However, on a few occasions fresh crude extracts showed a slight increase in phosphorylase a when made alkaline.
11 E. H. FISCHER AND E. G. KREBS 131 of phosphorylase a occurs. Recent experiments that will be reported in detail in a subsequent publication show, however, that phosphorylase b, obtained by the action of PR enzyme on crystalline phosphorylase a, is also readily converted back to phosphorylase a in the conversion system described in this paper. It is of interest to recall that the dimerization of another muscle protein, that of G-actin to F-actin (15), also requires a divalent metal ion and ATP. In this system the monomer units are thought to be linked together through the nucleotide prosthetic group and the metal. With phosphorylase the possibility of two monomer (phosphorylase b) units being linked together through a metal ATP complex appears to be unlikely, since very little adenine is found in crystalline phosphorylase a (16). Tracer techniques are being used in order to determine whether the metal or any portion of ATP is incorporated during the conversion of phosphorylase b to a in a purified system. Sutherland and Cori (6, 17) have presented evidence that epinephrine exerts its glycogenolytic effect by causing an increased concentration of active phosphorylase in tissues. This increase is due presumably to the conversion of phosphorylase b to CL. Since the requirements for this reaction have now been established, a study of the influence of epinephrine on the mobilization of the various components involved may give a clue to the mode of action of this hormone. The authors wish to thank Mr. Roger Wade for carrying out the ultracentrifugal analyses and Mrs. Jeanne Dills and Mr. Charles Beaudreau for their technical assistance. SUMMARY The conversion of phosphorylase b to phosphorylase a has been accomplished in cell-free muscle extracts. Requirements for the reaction include a protein fraction of the extract, divalent metal ions, and ATP. Crystalline phosphorylase a can be obtained from phosphorylase b after conversion of phosphorylase b -+ a in vitro. BIBLIOGRAPHY 1. Green, A. A., and Cori, G. T., J. Biol. Chem., 161, 21 (1943). 2. Cori, G. T., and Green, A. A., J. Biol. Chem., 161, 31 (1943). 3. Cori, G. T., and Cori, C. F., J. Biol. Chem., 168,321 (1945). 4. Keller, P. J., and Cori, G. T., Biochim. et biophys. acta, 12, 235 (1953). 5. Cori, G. T., J. Biol. Chem., 168,333 (1945). 6. Sutherland, E. W., Ann. New York Acad. SC., 64, 693 (1951). 7. Krebs, E. G., and Fischer, E. H., J. BioZ. Chem., 216, 113 (1955). 8. Cori, G. T., Colowick, S. P., and Cori, C. F., J. BioZ. Chem., 123, 381 (1938).
12 132 CONVERSION OF PHOSPHORYLASE b TO a 9. Cori, C. F., and Cori, G. T., J. Biol. Chem., 168,341 (1945). 10. Illingworth, B., and Cori, G. T., Biochem. Preparations, 3, 1 (1953). 11. Cori, C. F., Cori, G. T., and Green, A. A., J. Biol. Chem., 161,39 (1943). 12. Kalckar, H. M., J. Biol. Chem., 167, 445 (1947). 13. KritskiK, G. A., and Kuvaeva, E. B., Doklady Akad. Nauk S. S. S. R., 64, 549 (1949). 14. Rosenfeld, E. L., and Petrova, A. N., Doklady Akad. Nauk S. S. X. R., 74, 545 (1950). 15. Tsao, T. C., Biochim. et biophys. acta, 11, 227 (1953). 16. Velick, S. F., and Wicks, L. F., J. Biol. Chem., 190, 741 (1951). 17. Sutherland, E. W., and Cori, C. F., J. Biol. Chem., 188, 531 (1951).
Activation of Cardiac Phosphorylase b Kinase*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 241, No. 24, Issue of December 25, PP. 5893-5898, 1966 Printed in U.S.A. Activation of Cardiac Phosphorylase b Kinase* GEORGE I. DRUMMOND AND LOVERNE DUNCAN (Received
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 241, No. 24, Issue of December 25, PP. 5893-5898, 1966 Printed in U.S.A. Activation of Cardiac Phosphorylase b Kinase* GEORGE I. DRUMMOND AND LOVERNE DUNCAN (Received
PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS*
 PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS* BY WALTER H. SEEGERS (Prom the Department of Pathology, State University of Zowa, Iowa City) (Received for publication,
PURIFICATION OF PROTHROMBIN AND THROMBIN : CHEMICAL PROPERTIES OF PURIFIED PREPARATIONS* BY WALTER H. SEEGERS (Prom the Department of Pathology, State University of Zowa, Iowa City) (Received for publication,
CRYSTALLINE PEPSIN BY JOHN H. NORTHROP. (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, iv. J.
 CRYSTALLINE PEPSIN III. PREPARATION OF ACTIVE CRYSTALLINE PEPSIN FROM INACTIVE DENATURED PEPSIN BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton,
CRYSTALLINE PEPSIN III. PREPARATION OF ACTIVE CRYSTALLINE PEPSIN FROM INACTIVE DENATURED PEPSIN BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton,
THE EFFECT OF VARIOUS ACIDS ON THE DIGESTION OF PROTEINS BY PEPSIN.
 Published Online: 20 July, 1919 Supp Info: http://doi.org/10.1085/jgp.1.6.607 Downloaded from jgp.rupress.org on August 20, 2018 THE EFFECT OF VARIOUS ACIDS ON THE DIGESTION OF PROTEINS BY PEPSIN. BY J.
Published Online: 20 July, 1919 Supp Info: http://doi.org/10.1085/jgp.1.6.607 Downloaded from jgp.rupress.org on August 20, 2018 THE EFFECT OF VARIOUS ACIDS ON THE DIGESTION OF PROTEINS BY PEPSIN. BY J.
Polysaccharide phosphorylase
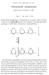 CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES.
 XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES. II. THE PREPARATION OF GLUCOSE UREIDE. BY ALEXANDER HYND. From the Department of Physiology, University of St Andrews. (Received
XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES. II. THE PREPARATION OF GLUCOSE UREIDE. BY ALEXANDER HYND. From the Department of Physiology, University of St Andrews. (Received
Enzymatic Assay of PHOSPHODIESTERASE, 3':5'-CYCLIC NUCLEOTIDE Crude Complex
 PRINCIPLE: 3':5'-cAMP + H 2 O PDE-3':5'-CN > AMP AMP + ATP Myokinase > 2 ADP 2 ADP + 2 PEP Pyruvate Kinase > 2 ATP + 2 Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD Abbreviations
PRINCIPLE: 3':5'-cAMP + H 2 O PDE-3':5'-CN > AMP AMP + ATP Myokinase > 2 ADP 2 ADP + 2 PEP Pyruvate Kinase > 2 ATP + 2 Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD Abbreviations
Enzymatic Assay of PHOSPHORYLASE KINASE (EC )
 PRINCIPLE: Enzymatic Assay of PHOSPHORYLASE KINASE 2 Phosphorylase b + 4 ATP Phosphorylase Kinase > Phosphorylase a + 4 ADP Glycogen n + P i Phosphorylase a > Glycogen n-1 + a-d-glucose 1-Phosphate a-d-glucose
PRINCIPLE: Enzymatic Assay of PHOSPHORYLASE KINASE 2 Phosphorylase b + 4 ATP Phosphorylase Kinase > Phosphorylase a + 4 ADP Glycogen n + P i Phosphorylase a > Glycogen n-1 + a-d-glucose 1-Phosphate a-d-glucose
(From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New Jersey)
 CRYSTALLIZATION OF SALT-FREE CHYMOTRYPSINOGEN AND CHYMOTRYPSIN FROM SOLUTION IN DILUTE ETHYL ALCOHOL BY M. KUNITZ (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New
CRYSTALLIZATION OF SALT-FREE CHYMOTRYPSINOGEN AND CHYMOTRYPSIN FROM SOLUTION IN DILUTE ETHYL ALCOHOL BY M. KUNITZ (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, New
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN
 THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds
 Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds By Edward J. Leonard, M.D., and Stephen Hajdu, M.D. A plasma protein system of mammalian origin which increases the contractile
Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds By Edward J. Leonard, M.D., and Stephen Hajdu, M.D. A plasma protein system of mammalian origin which increases the contractile
A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE*
 A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE* BY SEYMOUR KAUFMAN (From the Laboratory of Cellular Pharmacology, National Institute of Mental Health, United States Department
A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE* BY SEYMOUR KAUFMAN (From the Laboratory of Cellular Pharmacology, National Institute of Mental Health, United States Department
INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT
 Brit. J. Phawmacol. (1951), 6, 289. INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT BY From the Pharmacological Laboratory, University of St. Andrews, Medical School, Dundee (Received February 2, 1951)
Brit. J. Phawmacol. (1951), 6, 289. INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT BY From the Pharmacological Laboratory, University of St. Andrews, Medical School, Dundee (Received February 2, 1951)
On pages 131, 132, 136, and 137, the word conformational should be set off by quotation marks, rather than by parentheses.
 536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the
536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the
ION ANTAGONISMS AFFECTING GLYCOLYSIS BY BACTERIAL SUSPENSIONS*
 ION ANTAGONISMS AFFECTING GLYCOLYSIS BY BACTERIAL SUSPENSIONS* BY HIROSHI TSUYUKIt AND ROBERT A. MAcLEOD (From the Department of Biochemistry, Queen s University, Kingston, Ontario, Canada) (Received for
ION ANTAGONISMS AFFECTING GLYCOLYSIS BY BACTERIAL SUSPENSIONS* BY HIROSHI TSUYUKIt AND ROBERT A. MAcLEOD (From the Department of Biochemistry, Queen s University, Kingston, Ontario, Canada) (Received for
THE RING STRUCTURE OF THYMIDINE
 THE RING STRUCTURE OF THYMIDINE BY P. A. LEVENE AND R. STUART TIPSON (From the Laboratories of The Rockefeller Institute for Medical Research, New York) (Received for publication, March 13, 1935) The 2-desoxy-ribose
THE RING STRUCTURE OF THYMIDINE BY P. A. LEVENE AND R. STUART TIPSON (From the Laboratories of The Rockefeller Institute for Medical Research, New York) (Received for publication, March 13, 1935) The 2-desoxy-ribose
Further study has tended to confirm this interpretation.
 PROPERTIES OF AN ANTICOAGULANT FOUND IN THE BLOOD OF A HEMOPHILIAC By F. L. MUNRO (From the Charlotte Drake Cardeza Foundation, Department of Medicine, College and Hospital, Philadelphia) Jefferson Medical
PROPERTIES OF AN ANTICOAGULANT FOUND IN THE BLOOD OF A HEMOPHILIAC By F. L. MUNRO (From the Charlotte Drake Cardeza Foundation, Department of Medicine, College and Hospital, Philadelphia) Jefferson Medical
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP
 CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
CRYSTALLINE PEPSIN V. ISOLATION OF CRYSTALLINE PEPSIN FROM BOVINE GASTRIC JUICE BY JOHN H. NORTHROP (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J.) (Accepted
METABOLISM OF d-mannohepttjlose. EXCRETION OF THE SUGAR AFTER EATING AVOCADO
 METABOLISM OF d-mannohepttjlose. EXCRETION OF THE SUGAR AFTER EATING AVOCADO BY N. R. BLATHERWICK, HARDY W. LARSON, AND SUSAN D. SAWYER (From the Biochemical Laboratory of the Metropolitan Life Insurance
METABOLISM OF d-mannohepttjlose. EXCRETION OF THE SUGAR AFTER EATING AVOCADO BY N. R. BLATHERWICK, HARDY W. LARSON, AND SUSAN D. SAWYER (From the Biochemical Laboratory of the Metropolitan Life Insurance
THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED TRYPSIN
 Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
THE EFFECT OF DENATURATION ON THE VISCOSITY OF PROTEIN SYSTEMS BY M. L. ANSON A~D A. E. MIRSKY. (Accepted for publication, December 2, 1931)
 THE EFFECT OF DENATURATION ON THE VISCOSITY OF PROTEIN SYSTEMS BY M. L. ANSON A~D A. E. MIRSKY (From tke Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. Y., and the ttospital
THE EFFECT OF DENATURATION ON THE VISCOSITY OF PROTEIN SYSTEMS BY M. L. ANSON A~D A. E. MIRSKY (From tke Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. Y., and the ttospital
RICINOLEATE UPON BACTERIA
 A COMPARATIVE STUDY OF THE ACTION OF SODIUM RICINOLEATE UPON BACTERIA From the Division of Laboratories and Research, New York State Department of Health, Albany Received for publication, May 14, 1928
A COMPARATIVE STUDY OF THE ACTION OF SODIUM RICINOLEATE UPON BACTERIA From the Division of Laboratories and Research, New York State Department of Health, Albany Received for publication, May 14, 1928
THE isolation and availability of crystalline
 Unidentified Factors in Poultry Nutrition. PROPERTIES AND PRELIMINARY FRACTIONATION OF A GROWTH FACTOR IN CONDENSED FISH SOLUBLES H. MENGE, C. A. DENTON, J. R. SIZEMORE, R. J. LILLIE AND H. R. BIRD Bureau
Unidentified Factors in Poultry Nutrition. PROPERTIES AND PRELIMINARY FRACTIONATION OF A GROWTH FACTOR IN CONDENSED FISH SOLUBLES H. MENGE, C. A. DENTON, J. R. SIZEMORE, R. J. LILLIE AND H. R. BIRD Bureau
OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS
 CROSS-HYBRIDIZATION OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS Gabor HOLLGSI*, Sudhir SRIVASTAVA** and Joan WIKMAN-COFFELT University of California, San Francisco Cardiovascular
CROSS-HYBRIDIZATION OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS Gabor HOLLGSI*, Sudhir SRIVASTAVA** and Joan WIKMAN-COFFELT University of California, San Francisco Cardiovascular
THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES
 THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM (From the Departments oj Physiology and Pharmacology and Biochemistry, Duke
THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM (From the Departments oj Physiology and Pharmacology and Biochemistry, Duke
FACTORS INVOLVED IN THE USE OF ORGANIC SOLVENTS AS PRECIPITATING AND DRYING AGENTS OF IMMUNE SERA BY MALCOLM H. MERRILL ni~ MOYER S.
 Published Online: 20 November, 1932 Supp Info: http://doi.org/10.1085/jgp.16.2.243 Downloaded from jgp.rupress.org on November 3, 2018 FACTORS INVOLVED IN THE USE OF ORGANIC SOLVENTS AS PRECIPITATING AND
Published Online: 20 November, 1932 Supp Info: http://doi.org/10.1085/jgp.16.2.243 Downloaded from jgp.rupress.org on November 3, 2018 FACTORS INVOLVED IN THE USE OF ORGANIC SOLVENTS AS PRECIPITATING AND
Enzymatic Assay of CHOLINE KINASE (EC )
 Enzymatic Assay of CHOLINE KINASE PRINCIPLE: Choline + ATP CK > o-phosphocholine + ADP ADP + PEP PK > ATP + Pyruvate Pyruvate + ß-NADH LDH > Lactate + ß-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate
Enzymatic Assay of CHOLINE KINASE PRINCIPLE: Choline + ATP CK > o-phosphocholine + ADP ADP + PEP PK > ATP + Pyruvate Pyruvate + ß-NADH LDH > Lactate + ß-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate
Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength
 Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength By Ada L. Jacobson, Ph.D. ABSTRACT The effect of ouabain (0" 8 to (H M) on the hydrolysis of ATP by beef cardiac myosin B was
Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength By Ada L. Jacobson, Ph.D. ABSTRACT The effect of ouabain (0" 8 to (H M) on the hydrolysis of ATP by beef cardiac myosin B was
Purity Tests for Modified Starches
 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Purity Tests for Modified Starches This monograph was also published in: Compendium
Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Purity Tests for Modified Starches This monograph was also published in: Compendium
THE ABSORPTION OF VOLATILE FATTY ACIDS FROM THE RUMEN
 VOL. 24, Nos. 1 & 2 SEPTEMBER 1947 THE ABSORPTION OF VOLATILE FATTY ACIDS FROM THE RUMEN BY F. V. GRAY From the Division of Biochemistry and General Nutrition of the Council for Scientific and Industrial
VOL. 24, Nos. 1 & 2 SEPTEMBER 1947 THE ABSORPTION OF VOLATILE FATTY ACIDS FROM THE RUMEN BY F. V. GRAY From the Division of Biochemistry and General Nutrition of the Council for Scientific and Industrial
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19
 TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
STUDIES ON THIAMINE ANALOCUES
 STUDIES ON THIAMINE ANALOCUES III. EFFECTS ON ENZYME SYSTEMS* BY STEPHEN EICH AND LEOPOLD R. CERECEDO (From the Department of Biochemistry, Fordham University, New York, New York) (Received for publication,
STUDIES ON THIAMINE ANALOCUES III. EFFECTS ON ENZYME SYSTEMS* BY STEPHEN EICH AND LEOPOLD R. CERECEDO (From the Department of Biochemistry, Fordham University, New York, New York) (Received for publication,
Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL)
 Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL) Patty Lucas AAPFCO Laboratory Services Committee Meeting Friday, August 7, 2015 Fertilizer Sample
Challenges with Chelated &/or Complexed Minerals (Chelated and Soluble Methods of Analysis Used in FL) Patty Lucas AAPFCO Laboratory Services Committee Meeting Friday, August 7, 2015 Fertilizer Sample
SYNTHESIS OF N-SUBSTITUTED GLUCOSAMINES AND THEIR EFFECT ON HEXOKINASE*
 SYNTHESIS OF N-SUBSTITUTED GLUCOSAMINES AND THEIR EFFECT ON HEXOKINASE* BY FRANK MALEY AND HENRY A. LARDY (From the Department of Biochemistry and the Institute for Enzyme Research, University of Wisconsin,
SYNTHESIS OF N-SUBSTITUTED GLUCOSAMINES AND THEIR EFFECT ON HEXOKINASE* BY FRANK MALEY AND HENRY A. LARDY (From the Department of Biochemistry and the Institute for Enzyme Research, University of Wisconsin,
CORESTA RECOMMENDED METHOD N 39
 CORESTA RECOMMENDED METHOD N 39 DETERMINATION OF THE PURITY OF NICOTINE AND NICOTINE SALTS BY GRAVIMETRIC ANALYSIS - TUNGSTOSILICIC ACID METHOD (November 1994) 0. INTRODUCTION Several methods for checking
CORESTA RECOMMENDED METHOD N 39 DETERMINATION OF THE PURITY OF NICOTINE AND NICOTINE SALTS BY GRAVIMETRIC ANALYSIS - TUNGSTOSILICIC ACID METHOD (November 1994) 0. INTRODUCTION Several methods for checking
The incorporation of labeled amino acids into lens protein. Abraham Speclor and Jin H. Kinoshita
 The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
A CHICK GROWTH FACTOR IN COW MANURE VII. ITS STABILITY AND SOLUBILITY BY H. R. BIRD, MAX RUBIN, AND A. C. GROSCHKE
 A CHICK GROWTH FACTOR IN COW MANURE VII. ITS STABILITY AND SOLUBILITY BY H. R. BIRD, MAX RUBIN, AND A. C. GROSCHKE (From the Bureau of Animal Industry, United States Department of Agriculture, Beltsville,
A CHICK GROWTH FACTOR IN COW MANURE VII. ITS STABILITY AND SOLUBILITY BY H. R. BIRD, MAX RUBIN, AND A. C. GROSCHKE (From the Bureau of Animal Industry, United States Department of Agriculture, Beltsville,
Enzyme Action: Testing Catalase Activity
 Enzyme Action: Testing Catalase Activity LabQuest 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Enzyme Action: Testing Catalase Activity LabQuest 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Zeolite Clinoptilolite Powder
 Catalog 2018 Zeolite Clinoptilolite Powder Formula: (Ca,K 2,Na 2,Mg) 4 Al 8 Si 40 O 96.24H 2 O Clinoptilolite content 90-92% TBA - tribomechanical pulverized
Catalog 2018 Zeolite Clinoptilolite Powder Formula: (Ca,K 2,Na 2,Mg) 4 Al 8 Si 40 O 96.24H 2 O Clinoptilolite content 90-92% TBA - tribomechanical pulverized
BRIEFING. Pharmacopeial Discussion Group Sign Off Document Attributes EP JP USP Definition Identification B Identification C + + +
 BRIEFING Edetate Calcium Disodium, USP 29 page 779. The Japanese Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Edetate Calcium Disodium
BRIEFING Edetate Calcium Disodium, USP 29 page 779. The Japanese Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Edetate Calcium Disodium
STUDIES OF THE MECHANISM OF ACTION OF COBAMIDE COENZYMES
 STUDIES OF THE MECHANISM OF ACTION OF COBAMIDE COENZYMES R. H. Abeles and H. A. Lee, Jr. University of Michigan Medical School, Ann Arbor, Mich. Aerobacter aerogenes converts propanediol to propionaldehyde,
STUDIES OF THE MECHANISM OF ACTION OF COBAMIDE COENZYMES R. H. Abeles and H. A. Lee, Jr. University of Michigan Medical School, Ann Arbor, Mich. Aerobacter aerogenes converts propanediol to propionaldehyde,
NEW ONE-STAGE PROCEDURES FOR THE QUANTITATIVE DETERMINATION OF PROTHROMBIN AND LABILE FACTOR*
 NEW ONE-STAGE PROCEDURES FOR THE QUANTITATIVE DETERMINATION OF PROTHROMBIN AND LABILE FACTOR* MARIO STEFANINI, M.D.f From the Department ofbiochemistry, Marquette University School of Medicine, Milwaukee,
NEW ONE-STAGE PROCEDURES FOR THE QUANTITATIVE DETERMINATION OF PROTHROMBIN AND LABILE FACTOR* MARIO STEFANINI, M.D.f From the Department ofbiochemistry, Marquette University School of Medicine, Milwaukee,
Change to read: BRIEFING
 BRIEFING Dibasic Calcium Phosphate Dihydrate, USP 29 page 359. The Japanese Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Dibasic
BRIEFING Dibasic Calcium Phosphate Dihydrate, USP 29 page 359. The Japanese Pharmacopoeia is the coordinating pharmacopeia for the international harmonization of the compendial standards for the Dibasic
Enzymatic Assay of GUANYLATE KINASE (EC )
 PRINCIPLE: GMP + ATP Guanylate Kinase > GDP + ADP ADP + PEP Pyruvate Kinase > ATP + Pyruvate GDP + PEP Pyruvate Kinase > GTP + Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD
PRINCIPLE: GMP + ATP Guanylate Kinase > GDP + ADP ADP + PEP Pyruvate Kinase > ATP + Pyruvate GDP + PEP Pyruvate Kinase > GTP + Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD
THE OCCURRENCE OF SOME PREVIOUSLY UNREPORTED FATTY ACIDS IN PEANUT OIL
 THE OCCURRENCE OF SOME PREVIOUSLY UNREPORTED FATTY ACIDS IN PEANUT OIL BY HELEN L. WIKOFF, JOSEPH M. KAPLAN, AND ALVIN L. BERMAN (From the Department of Physiological Chemistry, The Ohio State University,
THE OCCURRENCE OF SOME PREVIOUSLY UNREPORTED FATTY ACIDS IN PEANUT OIL BY HELEN L. WIKOFF, JOSEPH M. KAPLAN, AND ALVIN L. BERMAN (From the Department of Physiological Chemistry, The Ohio State University,
TEMPORARY INHIBITION OF TRYPSIN*
 TEMPORARY INHIBITION OF TRYPSIN* BY M. LASKOWSKI AND FENG CHI WU (From the Department oj Biochemistry, Marquette University School of Medicine, Milwaukee, Wisconsin) (Received for publication, April 30,
TEMPORARY INHIBITION OF TRYPSIN* BY M. LASKOWSKI AND FENG CHI WU (From the Department oj Biochemistry, Marquette University School of Medicine, Milwaukee, Wisconsin) (Received for publication, April 30,
EFFECT OF HIGH SALT CONCENTRATIONS ON COLOR PRODUCTION OF THE BIURET REACTION FOR PROTEIN ANALYSIS
 EFFECT OF HIGH SALT CONCENTRATIONS ON COLOR PRODUCTION OF THE BIURET REACTION FOR PROTEIN ANALYSIS HAROLD L. ROSENTHAL, PH.D., AND TOYOKO KAWAKAMI, M.T. (ASC1>) Division of Biochemistry, Department of
EFFECT OF HIGH SALT CONCENTRATIONS ON COLOR PRODUCTION OF THE BIURET REACTION FOR PROTEIN ANALYSIS HAROLD L. ROSENTHAL, PH.D., AND TOYOKO KAWAKAMI, M.T. (ASC1>) Division of Biochemistry, Department of
points raised, and the following is an account of what I have done under touched, but my work has fallen under two main heads:
 NOTES ON CREATININE. BY P. C. COLLS, late Assistant Demonstrator in Physiology, King's College, London. (From the Physiological Laboratory, King's College, London.) ABOUT two years ago, a lengthy correspondence
NOTES ON CREATININE. BY P. C. COLLS, late Assistant Demonstrator in Physiology, King's College, London. (From the Physiological Laboratory, King's College, London.) ABOUT two years ago, a lengthy correspondence
Adenosine triphosphate (ATP)
 Adenosine triphosphate (ATP) 1 High energy bonds ATP adenosine triphosphate N NH 2 N -O O P O O P O- O- O O P O- O CH 2 H O H N N adenine phosphoanhydride bonds (~) H OH ribose H OH Phosphoanhydride bonds
Adenosine triphosphate (ATP) 1 High energy bonds ATP adenosine triphosphate N NH 2 N -O O P O O P O- O- O O P O- O CH 2 H O H N N adenine phosphoanhydride bonds (~) H OH ribose H OH Phosphoanhydride bonds
INTERNATIONAL ŒNOLOGICAL CODEX. BENTONITES Bentonita N SIN: 558 (Oeno 11/2003 modified Oeno )
 BENTONITES Bentonita N SIN: 558 (Oeno 11/2003 modified Oeno 441-2011) 1. OBJECT, ORIGIN AND FIELD OF APPLICATION Bentonites are hydrous aluminium silicates belonging to the montmorillonite group. The brute
BENTONITES Bentonita N SIN: 558 (Oeno 11/2003 modified Oeno 441-2011) 1. OBJECT, ORIGIN AND FIELD OF APPLICATION Bentonites are hydrous aluminium silicates belonging to the montmorillonite group. The brute
ON THE DETERMINATION OF UROBILIN IN URINE.
 ON THE DETERMINATION OF UROBILIN IN URINE. PRELIMINARY REPORT. RY S. MARCUSSEN AND SVEND HANSEN. (From the Rigshospitalet, University of Copenhagen, Copenhagen,.) (Received for publication, September 20,
ON THE DETERMINATION OF UROBILIN IN URINE. PRELIMINARY REPORT. RY S. MARCUSSEN AND SVEND HANSEN. (From the Rigshospitalet, University of Copenhagen, Copenhagen,.) (Received for publication, September 20,
Naoki YAMANAKA, Toshio IMANARI,* Zenzo TAMURA,*
 J. Biochem., 73, 993-998 (1973) Uncoupling of Oxidative Phosphorylation of Rat Liver Mitochondria by Chinoform Naoki YAMANAKA, Toshio IMANARI,* Zenzo TAMURA,* and Kunio YAGI Institute of Biochemistry,
J. Biochem., 73, 993-998 (1973) Uncoupling of Oxidative Phosphorylation of Rat Liver Mitochondria by Chinoform Naoki YAMANAKA, Toshio IMANARI,* Zenzo TAMURA,* and Kunio YAGI Institute of Biochemistry,
LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade
 AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
AD0017P-4 (en) 1 LANCE Eu-W1024 ITC Chelate & Europium Standard AD0013 Development grade INTRODUCTION Fluorescent isothiocyanato-activated (ITC-activated) Eu-W1024 chelate is optimized for labelling proteins
colorimetrically by the methylene blue method according to Fogo and manometrically. In the presence of excess sulfur the amount of oxygen taken up
 GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
STUDIES ON CHOLINESTERASE*
 STUDIES ON CHOLINESTERASE* III. PURIFICATION OF THE ENZYME FROM ELECTRIC TISSUE BY FRACTIONAL AMMONIUM SULFATE PRECIPITATION BY MORTIMER A. ROTHENBERG AND DAVID NACHMANSOHN (From the Departments of Neurology
STUDIES ON CHOLINESTERASE* III. PURIFICATION OF THE ENZYME FROM ELECTRIC TISSUE BY FRACTIONAL AMMONIUM SULFATE PRECIPITATION BY MORTIMER A. ROTHENBERG AND DAVID NACHMANSOHN (From the Departments of Neurology
Kit for assay of thioredoxin
 FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
THE EFFECT OF ANTICOAGULANTS ON DETERMINA- TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER
 THE EFFECT OF ANTICOAGULANTS ON DETERMINA TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER (From the Department of Laboratories, Henry Ford Hospital, Detroit) (Received for publication,
THE EFFECT OF ANTICOAGULANTS ON DETERMINA TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER (From the Department of Laboratories, Henry Ford Hospital, Detroit) (Received for publication,
DELFIA Tb-N1 DTA Chelate & Terbium Standard
 AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
AD0029P-1 (en) 1 DELFIA Tb-N1 DTA Chelate & AD0012 Terbium Standard For Research Use Only INTRODUCTION DELFIA Tb-N1 DTA Chelate is optimized for the terbium labeling of proteins and peptides for use in
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE
 METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
SPECTROPHOTOMETRY OF FAIRLEY'S NEW BLOOD PIGMENT, METHEMALBUMIN
 SPECTROPHOTOMETRY OF FAIRLEY'S NEW BLOOD PIGMENT, METHEMALBUMIN Charles L. Fox Jr. J Clin Invest. 1941;20(5):603-606. https://doi.org/10.1172/jci101253. Research Article Find the latest version: http://jci.me/101253-pdf
SPECTROPHOTOMETRY OF FAIRLEY'S NEW BLOOD PIGMENT, METHEMALBUMIN Charles L. Fox Jr. J Clin Invest. 1941;20(5):603-606. https://doi.org/10.1172/jci101253. Research Article Find the latest version: http://jci.me/101253-pdf
belonging to the pseudoglobulins, forming a heat-stable, dialysable vasoconstrictor (Received 2 April 1942)
 284 J. Physiol. (I942) IOI, 284-288 6I2.462.1:6I2.I46 PREPARATION AND SOME PROPERTIES OF HYPERTENSIN (ANGIOTONIN) BY P. EDMAN, U. S. VON EULER, E. JORPES AND 0. T. SJOSTRAND From the Physiology Department
284 J. Physiol. (I942) IOI, 284-288 6I2.462.1:6I2.I46 PREPARATION AND SOME PROPERTIES OF HYPERTENSIN (ANGIOTONIN) BY P. EDMAN, U. S. VON EULER, E. JORPES AND 0. T. SJOSTRAND From the Physiology Department
DELFIA Eu-DTPA ITC Chelate & Europium Standard
 AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
AD0026P-3 (en) 1 DELFIA Eu-DTPA ITC Chelate & AD0021 Europium Standard For Research Use Only INTRODUCTION DELFIA Eu-DTPA ITC Chelate is optimized for the europium labelling of proteins and peptides for
Nitrate and Nitrite Key Words: 1. Introduction 1.1. Nature, Mechanism of Action, and Biological Effects (Fig. 1)
 7 Nitrate and Nitrite Key Words: Nitrate; nitrite; methemoglobin; blood pressure; asphyxia; spinach; spongy cadmium column; zinc metal; sodium nitrate; sodium nitrite; ammonia buffer solution; Jones reductor.
7 Nitrate and Nitrite Key Words: Nitrate; nitrite; methemoglobin; blood pressure; asphyxia; spinach; spongy cadmium column; zinc metal; sodium nitrate; sodium nitrite; ammonia buffer solution; Jones reductor.
THE MILK-CLOTTING ACTION OF PAPAIN*
 THE MILK-CLOTTING ACTION OF PAPAIN* BY A. K. BALLS.4ND SAM R. HOOVER (From the Food Research Division, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington) (Received for
THE MILK-CLOTTING ACTION OF PAPAIN* BY A. K. BALLS.4ND SAM R. HOOVER (From the Food Research Division, Bureau of Chemistry and Soils, United States Department of Agriculture, Washington) (Received for
Colorimetric determination of free
 Colorimetric determination of free fatty acids in biological fluids KOICHI ITAYA and MICHIO UI Department of Biological Chemistry, Faculty of Pharmaceutical Sciences, Hokkaido University School of Medicine,
Colorimetric determination of free fatty acids in biological fluids KOICHI ITAYA and MICHIO UI Department of Biological Chemistry, Faculty of Pharmaceutical Sciences, Hokkaido University School of Medicine,
PRODUCT: RNAzol BD for Blood May 2014 Catalog No: RB 192 Storage: Store at room temperature
 PRODUCT: RNAzol BD for Blood May 2014 Catalog No: RB 192 Storage: Store at room temperature PRODUCT DESCRIPTION. RNAzol BD is a reagent for isolation of total RNA from whole blood, plasma or serum of human
PRODUCT: RNAzol BD for Blood May 2014 Catalog No: RB 192 Storage: Store at room temperature PRODUCT DESCRIPTION. RNAzol BD is a reagent for isolation of total RNA from whole blood, plasma or serum of human
Enzymatic Assay of CREATININASE (EC ) From Pseudomonas species
 PRINCIPLE: Creatinine + H 2 O Creatininase > Creatine Creatine + ATP CPK > Creatine-P + ADP ADP + PEP PK > ATP + Pyruvate Pyruvate + ß-NADH LDH > L-Lactate + ß-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate
PRINCIPLE: Creatinine + H 2 O Creatininase > Creatine Creatine + ATP CPK > Creatine-P + ADP ADP + PEP PK > ATP + Pyruvate Pyruvate + ß-NADH LDH > L-Lactate + ß-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate
Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard. Product Number: AD0013
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1024 ITC Chelate & Europium Standard Product Number: AD0013 INTRODUCTION: Fluorescent isothiocyanato-activated
CARBONIC ANHYDRASE IN THE VITREOUS BODY*
 Brit. J. Ophthal. (1956) 40, 487 CARBONIC ANHYDRASE IN THE VITREOUS BODY* BY J. GLOSTER From the Ophthalmological Unit, Medical Research Council, Institute of Ophthalmology, University oflondon Director
Brit. J. Ophthal. (1956) 40, 487 CARBONIC ANHYDRASE IN THE VITREOUS BODY* BY J. GLOSTER From the Ophthalmological Unit, Medical Research Council, Institute of Ophthalmology, University oflondon Director
Highlights Pentose Phosphate Pathway
 Highlights Pentose Phosphate Pathway 1. The pentose phosphate pathway (PPP) is an interchange of metabolic pathways. 2. It is important to cells as a) an important source of NADPH, b) an important source
Highlights Pentose Phosphate Pathway 1. The pentose phosphate pathway (PPP) is an interchange of metabolic pathways. 2. It is important to cells as a) an important source of NADPH, b) an important source
Enzymatic Assay of GLUCONATE KINASE (EC ) ß-NADPH = ß-Nicotinamide Adenine Dinucleotide Phosphate,
 Enzymatic Assay of GLUCONATE KINASE PRINCIPLE: D-Gluconate + ATP Gluconate Kinase > 6-Phospho-D-Gluconate + ADP 6-Phospho-D-Gluconate + ß-NADP G-PGDH > D-Ribulose-5'-P + ß-NADPH + CO 2 Mg2+ Abbreviations
Enzymatic Assay of GLUCONATE KINASE PRINCIPLE: D-Gluconate + ATP Gluconate Kinase > 6-Phospho-D-Gluconate + ADP 6-Phospho-D-Gluconate + ß-NADP G-PGDH > D-Ribulose-5'-P + ß-NADPH + CO 2 Mg2+ Abbreviations
Chapter 13, 21. The Physiology of Training: Physiological Effects of Strength Training pp Training for Anaerobic Power p.
 Chapter 13, 21 The Physiology of Training: Physiological Effects of Strength Training pp. 267-270 270 Training for Anaerobic Power p. 430-431 431 Types of Contractions Dynamic, Isotonic, or concentric
Chapter 13, 21 The Physiology of Training: Physiological Effects of Strength Training pp. 267-270 270 Training for Anaerobic Power p. 430-431 431 Types of Contractions Dynamic, Isotonic, or concentric
University of Groningen. Magnesium and zinc hydride complexes Intemann, Julia
 University of Groningen Magnesium and zinc hydride complexes Intemann, Julia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Magnesium and zinc hydride complexes Intemann, Julia IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
Recombinant Trypsin, Animal Origin Free
 Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Collin County Community College BIOL Muscle Physiology. Muscle Length-Tension Relationship
 Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Collin County Community College BIOL 2401 Muscle Physiology 1 Muscle Length-Tension Relationship The Length-Tension Relationship Another way that muscle cells can alter their force capability, is determined
Acta Medica Okayama. Masana Ogata FEBRUARY Volume 16, Issue Article 2. Okayama University,
 Acta Medica Okayama Volume 16, Issue 1 1962 Article 2 FEBRUARY 1962 Studies on the protein synthesis in poisoning. III. Labeling of ph-5 enzyme with C14-glycine and the inhibition by para chloromercuribenzoate
Acta Medica Okayama Volume 16, Issue 1 1962 Article 2 FEBRUARY 1962 Studies on the protein synthesis in poisoning. III. Labeling of ph-5 enzyme with C14-glycine and the inhibition by para chloromercuribenzoate
THE TOXICITY OF THE DOUBLE CHLORIDES OF MERCURY AND SODIUM
 325 THE TOXICITY OF THE DOUBLE CHLORIDES OF MERCURY AND SODIUM I. EXPERIMENTS WITH THE MINNOW PHOXINUS PHOXINUS (L.) BY J. R. ERICHSEN JONES Department of Zoology, University College of Wales, Aberystwyth
325 THE TOXICITY OF THE DOUBLE CHLORIDES OF MERCURY AND SODIUM I. EXPERIMENTS WITH THE MINNOW PHOXINUS PHOXINUS (L.) BY J. R. ERICHSEN JONES Department of Zoology, University College of Wales, Aberystwyth
TECHNICAL BULLETIN. Sialic Acid Quantitation Kit. Catalog Number SIALICQ Storage Temperature 2 8 C
 Sialic Acid Quantitation Kit Catalog Number SIALICQ Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description The Sialic Acid Quantitation Kit provides a rapid and accurate determination of total
Sialic Acid Quantitation Kit Catalog Number SIALICQ Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description The Sialic Acid Quantitation Kit provides a rapid and accurate determination of total
The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 24, No. 1, October 1965 Printed in U.S.A. The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle* NAOMI AzUMAt AND SHIZUO WATANABE~ From the
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 24, No. 1, October 1965 Printed in U.S.A. The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle* NAOMI AzUMAt AND SHIZUO WATANABE~ From the
EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH
 Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
FURTHER STUDIES UPON THE PURIFICATION AND PROPERTIES OF MALT AMYLASE
 FURTHER STUDIES UPON THE PURIFICATION AND PROPERTIES OF MALT AMYLASE BY H. C. SHERMAN, M. L. CALDWELL, AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for
FURTHER STUDIES UPON THE PURIFICATION AND PROPERTIES OF MALT AMYLASE BY H. C. SHERMAN, M. L. CALDWELL, AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for
STUDIES ON HEMOGLOBIN. III An Ultra-Micro-method for the Determination of Hemoglobin as a Peroxidase.
 BY STUDIES ON HEMOGLOBIN. III An Ultra-Micro-method for the Determination of Hemoglobin as a Peroxidase. HSIEN WU (From the L' b)oratory of Physiological CCemistry. Peking Union Medical College, Peking)
BY STUDIES ON HEMOGLOBIN. III An Ultra-Micro-method for the Determination of Hemoglobin as a Peroxidase. HSIEN WU (From the L' b)oratory of Physiological CCemistry. Peking Union Medical College, Peking)
Bioenergetics. Chapter 3. Objectives. Objectives. Introduction. Photosynthesis. Energy Forms
 Objectives Chapter 3 Bioenergetics Discuss the function of cell membrane, nucleus, & mitochondria Define: endergonic, exergonic, coupled reactions & bioenergetics Describe how enzymes work Discuss nutrients
Objectives Chapter 3 Bioenergetics Discuss the function of cell membrane, nucleus, & mitochondria Define: endergonic, exergonic, coupled reactions & bioenergetics Describe how enzymes work Discuss nutrients
Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard. Product Number: AD0014
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
FUNDAMENTALS OF BIOCHEMISTRY, CELL BIOLOGY AND BIOPHYSICS Vol. I - Biochemistry of Vitamins, Hormones and Other Messenger Molecules - Chris Whiteley
 BIOCHEMISTRY OF VITAMINS, HORMONES AND OTHER MESSENGER MOLECULES Chris Whiteley Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa Keywords: phosphorylation, phosphorylase,
BIOCHEMISTRY OF VITAMINS, HORMONES AND OTHER MESSENGER MOLECULES Chris Whiteley Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa Keywords: phosphorylation, phosphorylase,
STUDIES OF THE HEMAGGLUTININ OF HAEMOPHILUS PERTUSSIS HIDEO FUKUMI, HISASHI SHIMAZAKI, SADAO KOBAYASHI AND TATSUJI UCHIDA
 STUDIES OF THE HEMAGGLUTININ OF HAEMOPHILUS PERTUSSIS HIDEO FUKUMI, HISASHI SHIMAZAKI, SADAO KOBAYASHI AND TATSUJI UCHIDA The National Institute of Health, Tokyo, Japan (Received: August 3rd, 1953) INTRODUCTION
STUDIES OF THE HEMAGGLUTININ OF HAEMOPHILUS PERTUSSIS HIDEO FUKUMI, HISASHI SHIMAZAKI, SADAO KOBAYASHI AND TATSUJI UCHIDA The National Institute of Health, Tokyo, Japan (Received: August 3rd, 1953) INTRODUCTION
A MICRO TIME METHOD FOR DETERMINATION OF REDUCING SUGARS, AND ITS APPLICATION TO ANALYSIS OF BLOOD AND URINE.
 A MICRO TIME METHOD FOR DETERMINATION OF REDUCING SUGARS, AND ITS APPLICATION TO ANALYSIS OF BLOOD AND URINE. BY JAMES A. HAWKINS. (From Ike Hospital of The Rockefeller Institute for Medical Research,
A MICRO TIME METHOD FOR DETERMINATION OF REDUCING SUGARS, AND ITS APPLICATION TO ANALYSIS OF BLOOD AND URINE. BY JAMES A. HAWKINS. (From Ike Hospital of The Rockefeller Institute for Medical Research,
JORIND 9(2) December, ISSN
 SEQUENTIAL EXTRACTION OF Cu, Cd, Pb AND Zn FROM SOIL AROUND INDUSTRIAL WASTE DUMP SITES IN KADUNA ENVIRON USING SIMPLE AND SEQUENTIAL PROCEDURES. H.A Zakari, D.D. Adams, M Shimbayev, P. Nyam Department
SEQUENTIAL EXTRACTION OF Cu, Cd, Pb AND Zn FROM SOIL AROUND INDUSTRIAL WASTE DUMP SITES IN KADUNA ENVIRON USING SIMPLE AND SEQUENTIAL PROCEDURES. H.A Zakari, D.D. Adams, M Shimbayev, P. Nyam Department
ACETONE DERIVATIVES OF d-ribose. II.
 ACETONE DERIVATIVES OF d-ribose. II. BY P. A. LEVENE AND ERIC T. STILLER* (From the Laboratories of The Rockefeller Institute for Medical Research, New York) (Received for publication, June 14, 1934) The
ACETONE DERIVATIVES OF d-ribose. II. BY P. A. LEVENE AND ERIC T. STILLER* (From the Laboratories of The Rockefeller Institute for Medical Research, New York) (Received for publication, June 14, 1934) The
BCH 4054 Chapter 19 Lecture Notes
 BCH 4054 Chapter 19 Lecture Notes 1 Chapter 19 Glycolysis 2 aka = also known as verview of Glycolysis aka The Embden-Meyerhoff Pathway First pathway discovered Common to almost all living cells ccurs in
BCH 4054 Chapter 19 Lecture Notes 1 Chapter 19 Glycolysis 2 aka = also known as verview of Glycolysis aka The Embden-Meyerhoff Pathway First pathway discovered Common to almost all living cells ccurs in
SOLUBLE ACCESSORY FACTOR NECESSARY FOR THE NUTRITION OF THE RAT.
 LXXVIII. A SECOND THERMOLABILE WATER- SOLUBLE ACCESSORY FACTOR NECESSARY FOR THE NUTRITION OF THE RAT. By VERA READER. From the Department of Biochemistry, University Museum, Oxford. (Received April 30th,
LXXVIII. A SECOND THERMOLABILE WATER- SOLUBLE ACCESSORY FACTOR NECESSARY FOR THE NUTRITION OF THE RAT. By VERA READER. From the Department of Biochemistry, University Museum, Oxford. (Received April 30th,
COMPLEX SALTS OF AMINO ACIDS AND PEPTIDES
 COMPLEX SALTS OF AMINO ACIDS AND PEPTIDES II. DETERMINATION OF Z-PROLINE WITH THE AID OF RHODAN- ILIC ACID. THE STRUCTURE OF GELATIN BY MAX BERGMANN (From the Laboratories of The Rockefeller Institute
COMPLEX SALTS OF AMINO ACIDS AND PEPTIDES II. DETERMINATION OF Z-PROLINE WITH THE AID OF RHODAN- ILIC ACID. THE STRUCTURE OF GELATIN BY MAX BERGMANN (From the Laboratories of The Rockefeller Institute
THE CHEMISTRY OF THE LIPIDS OF TUBERCLE BACILLI
 THE CHEMISTRY OF THE LIPIDS OF TUBERCLE BACILLI LXXII. FATTY ACIDS OCCURRING IN THE WAX PREPARED FROM TUBERCULIN RESIDUES. CONCERNING MYCOCEROSIC ACID* BY LEONARD G. GINGER? AND R. J. ANDERSON (From the
THE CHEMISTRY OF THE LIPIDS OF TUBERCLE BACILLI LXXII. FATTY ACIDS OCCURRING IN THE WAX PREPARED FROM TUBERCULIN RESIDUES. CONCERNING MYCOCEROSIC ACID* BY LEONARD G. GINGER? AND R. J. ANDERSON (From the
ON THE FATTY ACIDS ESSENTIAL IN NUTRITION. III*
 ON THE FATTY ACIDS ESSENTIAL IN NUTRITION. III* BY GEORGE 0. BURR, MILDRED M. BURR, AND ELMER S. MILLER (From the Department of Botany, University of Minnesota, Minneapolis) (Received for publication,
ON THE FATTY ACIDS ESSENTIAL IN NUTRITION. III* BY GEORGE 0. BURR, MILDRED M. BURR, AND ELMER S. MILLER (From the Department of Botany, University of Minnesota, Minneapolis) (Received for publication,
UV Tracer TM Maleimide NHS ester
 UV Tracer TM Maleimide HS ester Product o.: 1020 Product ame: UV-Tracer TM Maleimide-HS ester Chemical Structure: Chemical Composition: C 41 H 67 5 18 Molecular Weight: 1014.08 Appearance: Storage: Yellow
UV Tracer TM Maleimide HS ester Product o.: 1020 Product ame: UV-Tracer TM Maleimide-HS ester Chemical Structure: Chemical Composition: C 41 H 67 5 18 Molecular Weight: 1014.08 Appearance: Storage: Yellow
HISTAMINE AND PROTEOLYTIC ENZYMES. (Received for publication, March 31, 1943)
 HISTAMINE AND PROTEOLYTIC ENZYMES LIBERATION OF HISTAMINE BY PAPAIN BY M. ROCHA E SILVA AND SYLVIA 0. ANDRADE (From the Department of Biochemistry and Pharmacodynamics, Instituto Biologico, &io Paulo,
HISTAMINE AND PROTEOLYTIC ENZYMES LIBERATION OF HISTAMINE BY PAPAIN BY M. ROCHA E SILVA AND SYLVIA 0. ANDRADE (From the Department of Biochemistry and Pharmacodynamics, Instituto Biologico, &io Paulo,
Sansom & Manston, 1963) and rats (Payne & Sansom, 1963). It appeared
 J. Physiol. (1964), 170, pp. 613-620 613 Printed in Great Britain THE RELATIVE TOXICITY IN RATS OF DISODIUM ETHYLENE DIAMINE TETRA-ACETATE, SODIUM OXALATE AND SODIUM CITRATE BY J. M. PAYNE AND B. F. SANSOM
J. Physiol. (1964), 170, pp. 613-620 613 Printed in Great Britain THE RELATIVE TOXICITY IN RATS OF DISODIUM ETHYLENE DIAMINE TETRA-ACETATE, SODIUM OXALATE AND SODIUM CITRATE BY J. M. PAYNE AND B. F. SANSOM
FUNCTION OF PYRIDOXAL PHOSPHATE: RESOLUTION AND PURIFICATION OF THE TRYPTOPHANASE ENZYME OF ESCHERICHIA COLI
 FUNCTION OF PYRIDOXAL PHOSPHATE: RESOLUTION AND PURIFICATION OF THE TRYPTOPHANASE ENZYME OF ESCHERICHIA COLI BY W. A. WOOD,* I. c. GUNSALUS, AND W. W. UMBREIT (From the Laboratory of Bacteriology, College
FUNCTION OF PYRIDOXAL PHOSPHATE: RESOLUTION AND PURIFICATION OF THE TRYPTOPHANASE ENZYME OF ESCHERICHIA COLI BY W. A. WOOD,* I. c. GUNSALUS, AND W. W. UMBREIT (From the Laboratory of Bacteriology, College
The Structure and Function of Biomolecules
 The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
