Activation of Cardiac Phosphorylase b Kinase*
|
|
|
- Denis Cannon
- 6 years ago
- Views:
Transcription
1 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 241, No. 24, Issue of December 25, PP , 1966 Printed in U.S.A. Activation of Cardiac Phosphorylase b Kinase* GEORGE I. DRUMMOND AND LOVERNE DUNCAN (Received for publication, July 8, 1966) From the Department of Pharmacology, School of Medicine, University of British Columbia, Vancouver 8, Canada SUMMARY Nonactivated, partially purified bovine heart phosphorylase b kinase was activated by incubation with adenosine triphosphate and Mg+f ion prior to assay. Adenosine 3,5 -cyclic pgosphate increased the rate of activation, but there was no absolute requirement for the cyclic nucleotide. Activation was particularly prominent when the subsequent assay for kinase activity was carried out at ph 7.0, where the enzyme has low activity. Significant activation was also evident when the assay was performed at ph 8.2, where the nonactivated enzyme is partially active. The response to adenosine 3,5 -cyclic phosphate was linear from 1 x lo-* to 2 x 10W7 M. Other ribonucleoside 3,5 -cyclic phosphates were without effect except at much higher concentrations. Supramaximal doses of epinephrine (2 pg), when injected into perfused rat hearts, caused a modest but significant activation of phosphorylase b kinase as compared with controls. A S-fold increase in enzyme activity occurred as determined by assaying 30,000 x g supernatant fractions at ph 6.8. Activation was also apparent, but proportionately less, when extracts were assayed at ph 8.2. Activation was characterized by a 3-fold increase in ph 6.8 to 8.2 activity ratios. The activation occurred rapidly, was maximal at 3 set after epinephrine injection, and rose well ahead of the contractile response, which reached a maximum in 10 sec. Phosphorylase b kinase, the enzyme which catalyzes the conversion of phosphorylase b to phosphorylase a, can be extracted from skeletal muscle (1, 2) and cardiac muscle (3, 4) in a form which has very low activity when assayed at physiological ph (ph 6.8 or 7). At ph values above this, activity rises sharply to an optimum at ph 8.5. Krebs et al. (5) have referred to the enzyme with this ph profile as nonactivated phosphorylase b kinase. Nonactivated kinase from both skeletal muscle (1, 2) and cardiac muscle (3, 4, 6) is markedly activated by incubation with Ca+f ion. Activation is especially marked when the enzyme is assayed at ph values of 6.8 and below and is less apparent when assays are conducted at ph 8.2. The ratio of activity at ph 6.8 to that at ph 8.2 has been used as a useful index of the degree of activation. The nonactivated enzyme is * This work was supported by grants from the Life Insurance Medical Research Fund, Philadelphia, and the British Columbia Heart Foundation. also activated by incubation with adenosine triphosphate and adenosine 3,5 -cyclic phosphate (cyclic adenosine monophosphate). Activation of the skeletal muscle enzyme by cyclic AMP has been extensively studied by Krebs et al. (5), and the reaction constitutes a sensitive method for the assay of the cyclic nucleotide in biological materials (7). The action of cyclic AMP on the cardiac enzyme has not been studied in detail although evidence is available (3, 4) that it may be similar to that of the skeletal muscle enzyme. It is now established that epinephrine stimulates the formation of cyclic AMP in beating hearts (3, 8, 9) and causes the conversion of phosphorylase b to the a form. It has been assumed that increased phosphorylase a levels result from the activation of phosphorylase b kinase by the cyclic AMP formed by epinephrine stimulation. If this assumption is correct, it follows that epinephrine should activate phosphorylase b kinase when administered to beating hearts. Recently, Hammermeister, Yunis, and Krebs (3) have reported that epinephrine failed to activate the enzyme significantly in perfused rabbit hearts. This report describes the activation of purified cardiac phosphorylase b kinase by ATP and cyclic AMP. Evidence is provided that epinephrine causes a significant activation of the enzyme in perfused rat hearts. EXPERIMENTAL PROCEDURE MaterialsRabbit muscle phosphorylase b, adenylic deaminase, glycogen, and glucose l-phosphate were prepared or purified by methods used previously (4). Ribonucleoside 3, 5 -cyclic phosphates, other than cyclic AMP (which was obtained from Calbiochem), were prepared by synthetic procedures (10, 11). Phosphorylase b kinase was purified from bovine hearts (6). The 100,000 X g precipitate fraction was used in the present study and was stored in 500/, glycerol at -18. When necessary, it was suitably diluted in 15 mu neutral cysteine immediately before use. Measurement of Kinase Activation-Activation of purified kinase was followed by measuring the increase in enzyme units which occurred when the nonactivated enzyme was incubated together with ATP, Mg++ ion, and cyclic AMP. The standard activation mixture contained M Tris M sodium /3- glycerophosphate at ph 7.0, 0.05 ml; 50 mu magnesium acetate containing 50 mu theophylline, 0.05 ml; 5 MM ATP, 0.05 ml; 0.25 mu cyclic AMP (when present), 0.05 ml, in a final volume 1 The purified enzyme contained traces of adenosine 3,5 -cyclic phosphate die&erase activity. Theophylline was therefore added to avoid danger of hydrolysis of the cyclic nucleotide. 5893
2 58 34 Cardiac Phosphorylase b Kinase Vol. 241, No NO ATP NO 5,SAMP 1o-4 1o-3 1o-2 FIG. 1. Activation of cardiac phosphorvlase b kinase bv ATP and cyclic AMP. Standard activation conditions were used except that ATP concentration was varied. Cyclic AMP concentration was 5 X lo+ M. Following the activation, the diluted mixtures were assayed for kinase at ph 7.0. Curve A, ATP plus cyclic AMP; Curve B, no cyclic AMP. ATP of 0.25 ml. Tubes were assembled in an ice bath, and then, after a l-mm equilibration at 30, 0.05 ml of phosphorylase b kinase solution (500 ph 8.2 units) was added, and the tubes were incubated at 30 for 10 min. The reaction was terminated by the addition of 5 ml of ice-cold neutral 15 mu cysteine. The tubes were placed in ice and a 0.2-ml aliquot was used immediately for kinase assay at ph 6.0, 7.0, or 8.2. In order to avoid delay between the activation reaction and assay for kinase activity, tubes containing all components of the kinase assay were assembled during the activation reaction and were kept in ice. Phosphorylase b Kinase Assay-The assay used was described previously (6) and is essentially that elaborated by Krebs et al. (5) for the skeletal muscle enzyme. Four times recrystallized phosphorylase b (10,000 Cori units) was used as substrate. Assays were conducted at 30 for 5 min at ph 6.0, 6.8, 7.0, or 8.2 as required. A final assay ph of 8.2 was achieved by using ph 8.5 buffer. Following the kinase incubation, a O.l-ml aliquot was transferred to a tube containing 1.9 ml of dilution buffer consisting of 40 mu sodium P-glycerophosphate and 30 mm cysteine, ph 6.8, to which had been added adenylic deaminase (200 pg of protein) (6). After 2 min at room temperature, a 0.2-ml aliquot of this diluted incubation mix was assayed for phosphorylase a (5). In studies involving purified kinase, a control containing phosphorylase b and all other components of the assay except phosphorylase b kinase was carried for each assay at each ph. When crude extracts of perfused rat hearts were assayed, a modified control was also used. This will be described later. The unit of phosphorylase b kinase was defined previously ( 3). Perfusion and Epinephrine Stimulation of Rat Heart-Female (M) Wistar rats weighing 190 to 220 g were stunned and decapitated; the heart was rapidly removed, rinsed, and attached via the aorta to a glass cannula of a Langendorf perfusion system and perfused with oxygenated Tyrode s solution at 37 at a pressure of 50 cm of water. Contractile activity was measured by a Statham force displacement transducer attached to the apex of the ventricle and adjusted to provide an initial diastolic tension of 5 g to each heart. Recordings were made on a Grass polygraph. Hearts were perfused for a stablizing period of 10 min before administering epinephrine. During this interval an epinephrine solution containing 10 pg per ml was prepared by diluting a stock solution (1 mg per ml in 0.1 y0 sodium metabisulfite) with Tyrode s solution. The freshly prepared dilution (0.2 ml containing 2.0 pg) was injected through thin polyethylene tubing into the cannula immediately above the heart. For controls, 0.2 ml of Tyrode s solution was administered. All injections were delivered evenly over a IO-see interval. At the desired time (measured from the instant of completion of injection), the heart was frozen by crushing between two aluminum blocks precooled in liquid NZ. Control hearts were frozen 10 set after completion of Tyrode s injection. The frozen wafers were immersed in liquid NB; ventricular tissue was chiseled from the remainder of the heart and stored in liquid Nz until used (usually 1 to 3 days). Preparation of Extracts-Each frozen ventricle was weighed (400 to 600 mg) and then homogenized in a Potter-Elvehjem homogenizer for 3 min at 4 with 10 volumes of a medium consisting of 50 mm Tris-HCl, 50 rnm NaF, and 2 mm EDTA at ph 7.0. In initial experiments a port,ion (3.2 ml) of the resulting homogenate was centrifuged at 30,000 x g for 15 min. The supernatant fluid was removed and kept in ice. The precipitate was suspended in 1.0 ml of homogenizing medium. In later experiments, the entire homogenate was centrifuged at 30,000 x g for 15 min and the precipitate was discarded. All preparations were assayed for kinase activity at ph 6.8 and 8.2 within 5 min after centrifugation. RESULTS Activation of Cardiac Phosphorylase b Kinase by ATP and Cyclic AMP-Previous studies (4, 6) have shown that nonactivated cardiac phosphorylase b kinase possesses relatively low activity at ph 7.0 and below. Above ph 7.0, activity rises sharply to an optimum at ph 8.5. It has been previously reported that crude extracts of rabbit heart (3) and acid precipitate fractions from bovine heart (4) were activated by ATP and cyclic AMP. When the purified nonactivated enzyme was incubated with ATP and Mg++ ion prior to dilution and assay at ph 7.0, significant activation occurred (Fig. 1, Curve B). Maximum activation occurred at 1 m&i ATP, but a wide range of concentrations were effective. When cyclic AMP (5 x lop5 $1) was present in addition to ATP and Mg++ ion, activation was considerably increased (Curve 21). The cyclic nucleotide in the absence of ATP caused only slight activation; ATP was inactive in the absence of Mg++ ion. Activation was most evident when the assay for kinase was conducted at ph 7.0 (Fig. 2), although significant activation was also apparent when assays were performed at ph 8.2. Usually a total activation of 6- to g-fold was achieved when both ATP and the cyclic nucleotide were present in the activation reaction as manifested by kinase assay at ph 7.0. E$ect of Time on Activation of Cardiac,Phosphorylase b Kinase-
3 Issue of December 25, 1966 G. I. Drummond and L. Duncan 5895 In the experiments just described, the activation reaction was terminated after 10 mm. When ATP and cyclic AMP were present together, activation occurred rapidly, reaching completion essentially in 10 min, with very little further change occurring up to 60 min (Fig. 3, Curve A). When ATP and Mg++ ion were present alone, activation occurred at a slower rate (Curve B) but reached essentially the same maximum as when cyclic AMP was present. Thus, as with the skeletal muscle enzyme, there seems to be no absolute requirement for cyclic AMP; activation simply occurs at an enhanced rate when it is present. Effect of ph on Activation of Phosphorylase b Kinase-Routinely, the activation reaction was carried out at p1-i 7.0. An experiment was performed in which the ph of the activation reaction was varied. Following dilution of the activation mixtures, kinase assays were performed at ph 7.0. Fig. 4 shows that the rate of activation in the presence of ATP and Mg++ ion increased with increasing ph to an optimum in the area of ph 8.5 (Curve B). When cyclic AMP was present also (Curve A), the rate of activation again rose with increasing ph, but the increase caused by the cyclic nucleotide was less marked at higher ph. The greatest effect of cyclic AMP over that produced by ATP was seen at ph 7.0. Effect of Cyclic AMP Concentration and Speci$city of Activation -The cardiac enzyme, like the skeletal muscle enzyme, was found to be sensitive to very minute amounts of the cyclic nucleotide. In the presence of 1 mm ATP, the response to cyclic AMP was linear over the range 1 X 10-S to 2 X 10m7 M. Concentrations as high as 10e3 M had no additional effect. Halfmaximal activation occurred at approximately 5 X 10-g M. The reaction appeared to be highly specific for cyclic AMP. When added to the activation reaction in the presence of 1 mm 0 I I I I I I ACTIVATION TIME MINUTES FIG. 3. Effect of time on activation.of cardiac phosphorylase b kinase. The concentration of cyclic AMP, where present, was 5 X lo+ M; ATP, 1 mm. At the time periods noted, the activation mixtures were diluted and assayed for phosphorylase kinase immediately at ph 7.0. Curve A, ATP and Mg++ plus cyclic AMP; Curve B, no cyclic AMP; and Curve C, no ATP, no cyclic AMP Z G 2.0 v) k Z PH FIG. 2. Effect of ph on activated cardiac phosphorylase 6 kinase. Standard activation conditions were used except that cyclic AMP concentration was 5 X 10-e M, and ATP concentration was 5 X 1O-3 M. Diluted activation mixtures were assayed at ph 6.0,7.0, and 8.2. Open bars, no ATP, no cyclic AMP; hatched bars, ATP, no cyclic AMP; and solid bars, ATP plus cyclic AMP.. C I I I I ACTIVATION FIG. 4. Effect of ph on phosphorylase b kinase activation. Standard activation conditions were used except that the ph of the activation mixtures was varied. Curve A, ATP and Mg++ plus cyclic AMP; Curve B, no cyclic AMP; Curve C, no ATP, no cyclic AMP. Following dilution of the activation reaction mixtures, kinase assays were conducted at ph 7.0. ATP, the ribonucleoside 3,5 cyclic phosphates of uridine, cytidine, guanosine, deoxyadenosine, deoxycytidine, thymidine, and deoxyguanosine were all inactive at 10-S M. When present at 1 X lo-4 M, 3,5 -cyclic UMP, 3,5 -cyclic CMP, 3,5 - ph
4 Cardiac Phosphorylase b Kinase Vol. 241, No. 24 cyclic GMP, and 3, 5 -cyclic deoxy-amp produced activation equivalent to 100, 80, 75, and 40%, respectively, of that produced by 1 X lop7 M cyclic ilmp. This activation could possibly be due to trace amounts of cyclic AMP resulting from contamination of the parent 5 -nucleotides with minute amounts of 5 -AMP. When present with ATP and Mg++ ion, 5 -AMP, 5 -CMP, 5 -GMP, 5 -UMP, 5 -IMP, 5 -deoxy-amp, 2,3 -CMP, and 5 -deoxy-gmp produced no activation at 1 X 10e4 M. Phosphorylase b Kinase in Perfused Rat Hearts Treated with Epinephrine-It can be concluded that cyclic AMP facilitates the activation in vitro of cardiac phosphorylase b kinase in a manner similar to that of the skeletal muscle enzyme. Recently Robison et al. (8) and Cheung and Williamson (9) have shown that in rat hearts treated with epinephrine, cyclic AMP rose within seconds; peak levels were obtained before the contractile response reached a maximum. It was of interest, therefore, to determine whether phosphorylase b kinase was activated by epinephrine in beating rat hearts. To this end, extracts were prepared from control and epinephrine-treated perfused rat hearts and assayed for kinase activity at ph 6.8 and 8.2. A dose of 2 pg of epinephrine was chosen for initial experiments. This dose is supramaximal with respect to contractile activity and is known to activate phosphorylase almost fully in perfused rat hearts (12). Hammermeister, Yunis, and Krebs (3) have reported that a considerable amount of rabbit heart kinase was not readily extractable and appeared in the precipitate after sedimentation of a homogenate at 10,000 x g. Moreover, the sedimentable enzyme appeared largely active as manifest by high ph 6.8 to ph 8.2 ratios of activity (0.53 and 0.57 as compared with 0.11 and 0.14 for 10,000 X g supernatant fractions). In the kinase assay used by the above authors, control tubes contained phosphorylase b but no heart extract (see Reference 2). In earlier experiments (4) we have shown that erroneous values were obtained when partially purified heart kinase preparations were assayed because of the conversion of ATP to AMP by contaminating enzymes. These errors could be minimized by treating the diluted kinase reaction mixtures with adenylic deaminase. The possibility existed that values obtained in the assay of uncentri- fuged homogenates and particulate preparations with the use of phosphorylase alone in the control could be considerably in error. In the present experiments, therefore, two controls were prepared for each experimental tube at each ph. The first (Control A) contained phosphorylase (equivalent to that in the experimental) and all other components of the kinase assay except heart extract (2). In the second (Control B), heart extract and all components of the kinase assay except phosphorylase were present during the incubation interval. After dilution (which effectively terminates the kinase reaction) an amount of phosphorylase equivalent to that in the experimental tube was added (0.1 ml of a 1:6 dilution of the stock enzyme solution). Control tubes were then carried through the phosphorylase a assay as usual. Apparent kinase activity in each heart fraction was calculated applying each control as a correction. The results are shown in Table I. Apparent kinase activity differed markedly depending on which control was used as a correction. Kinase values calculated with Control B were much lower than those calculated with Control A, especially at ph 6.8. This resulted because of the much higher levels of inorganic phosphate present in the Control B tubes. Specifically, with Control B, kinase activity of the uncentrifuged homogenate at ph 6.8 was only 23% of that calculated with Control A, and in the 30,000 x g precipitate fraction it was only 9%. The increased inorganic phosphate present in Control B tubes was not due to phosphorylase present in the heart extract. Thus, when a third set of controls was prepared in which heart extract was present during the incubation and phosphorylase subsequently omitted, values for inorganic phosphate were insignificantly above the phosphorylase reagent control. The data indicated that during the kinase incubation heart extract caused the formation of some factor or factors which when carried over into the phosphorylase assay resulted in phosphorylase b activity. It was shown chromatographically that ATP was largely degraded (to the extent of 60%) by the uncentrifuged homogenate and the 30,000 x g precipitate fraction during the kinase incubation interval. The products of this degradation were ADP and AMP. The discrepancy was not likely to be due to AMP since in these assays TABLE I Apparent phosphorylase b kinase in extracts of control and epinephrine-treated perfused rat hearts Seven rat hearts were used as a control and seven were injected with epinephrine (2 pg). The epinephrine-treated hearts were frosen at 9 set when contractile response was at a maximum. Uncentrifuged homogenates and 30,000 X g supernatant fractions (see Experimental Procedure ) were diluted 1:4, and the 30,000 X g precipitate fractions were diluted 1:3 with 15 mm neutral cysteine and 0.2-ml aliquots used in the kinase assay. Both controls were carried for each experimental tube at each ph. All assays as well as all controls were performed in duplicate. Values are expressed as units of phosphorylase b kinase per ml of undiluted fraction. Fraction Control hearts Uncentrifuged homogenate... 30,000 X g supernatant. 30,000 X g precipitate fraction. Epinephrine-treated hearts Uncentrifuged homogenate.. 30,000 X g supernatant.. 30,000 X g precipitate fraction. ph 6.8 units/ml 69.1 f * f f f f 11.4 Control method A (phosphorylase - - ph 8.2 units/ml f f f f j, ~ 15.2 alone) Ratio of activities, ph 6.8:pH 8.2 Control method B (phosphorylase added after kinase reaction) ph 6.8 units/ml f f f f f f f f zlz f f f 1.5 ph 8.2 wits/ml f f f f f f 5.6 Ratio of activities, ph 6.8:pH f f f f f f 0.049
5 Issue of December 25, 1966 G. I. Drummond and L. Duncan 5897 adenylic deaminase was present in the dilution buffer, and it was shown chromatographically that the AMP produced was converted entirely to IMP. The latter nucleotide has no effect on phosphorylase b activity at the concentrations that would be present here. In other experiments, control tubes containing heart homogenate were placed in a boiling water bath for 2 min after the kinase incubation interval and prior to dilution and addition of phosphorylase. These tubes gave final inorganic phosphate values identical with the unboiled controls. The contaminating material thus appeared to be heat-stable. Other experiments indicated that it might be ADP. It was estimated from chromatograms that sufficient ADP was formed during the kinase incubation to yield a final carryover of 5 X lop5 M into the phosphorylase a assay. In the presence of 2.5 X 10-4 M Mg++ ion (concentration of carryover), 5 X lo+ M ADP added to the phosphorylase a assay caused a rise in phosphorylase b activity sufficient to account for the inorganic phosphate levels of Control B. It is clear that controls employing phosphorylase alone do not constitute an adequate correction when assaying crude heart extracts for kinase activity, especially at ph 6.8, where the enzyme has low activity. Much of the apparent activity is an artifact, due to the formation of substances which increase phosphorylase 6 activity. A much more reliable control is that of Method B, in which this action of crude heart extracts is compensated for. It should be emphasized that the discrepancy between the two methods is considerably less for the 30,000 x g supernatant fraction in which most of the particulate material has been removed. Our data indicate that kinase of heart muscle is fully soluble by the homogenizing procedure used here and that no insoluble form of the enzyme exists. The small amount of activity remaining in the 30,000 X g precipitate fraction (as determined with Control B) was easily removed by washing the precipitate once with buffer. Assay of 30,000 x g supernatant fractions with Control B would, therefore, seem to give reliable values of total kinase in heart muscle when assayed at either ph 6.8 or 8.2. It can be seen in Table I that, when Control A was used, epinephrine appeared to produce a small but unimpressive increase in apparent kinase activity, as evidenced by increased ph 6.8 to 8.2 ratios, especially in the 30,000 X g supernatant fraction. However, a quite different picture emerged when the activities were calculated with Control B. Phosphorylase b kinase activities in the 30,000 X g supernatant fraction rose from 7.36 units per ml in control hearts to 28.8 units per ml in epinephrinetreated hearts. Increase in activity was also evident at ph 8.2, but the proportion of this increase was less so that the ph 6.8 to 8.2 ratios rose from in the control to in the epinephrine-treated hearts. Although this degree of activation is certainly not the maximal which might be predicited from ph 6.8 to 8.2 ratios, it is highly significant statistically. Differences between 6.8 values and between ph 6.8 to 8.2 ratios for control and epinephrine-treated hearts were subjected to statistical analysis. As determined by the Student t test, P values in each case were less than A larger dose of epinephrine (5 pg) produced no greater activation of the enzyme. Rate of Activation of Phosphorylase b Kinase by Epinephrine- In the previous experiment, hearts were frozen at 9 set after injection of epinephrine, when the contractile response reached a maximum. In order to further clarify the nature of the activation, additional hearts were injected with this dose of epinephrine and frozen at 3, 9, 16, 38, and 65 set after completion of the KINASE pti 6.8 KINASE ph 8.2 PH 6.8/8.2 RATIO ,oo.J.o TIME IN SECONDS i -.lo -.06 Fxo. 5. Time course of phosphorylase b kinase activation by 2 fig of epinephrine in perfused rat hearts. Perfused rat hearts were treated with epinephrine and frozen at 3, 9, 16, 38, and 65 set after completion of the injection. Control hearts were frozen 10 set after injection of Tyrode s solution (0.2 ml). The number of hearts used for each experiment were: control, 14; 3-set, 12; 9-set, 10; 16-see, 12; 3%set, 6; and 65-set, 5. Each ventricle was homogenized, and the resulting 30,900 X g supernatant fluid was immediately diluted 1:4 with 15 mm ice-cold neutral cvsteine. and 0.2-ml aliquots were used for the assays at ph 6.8 and 8.2 with Control B. All assavs, including all controls at each nh. were uerformed in duplicate. The ver&az bars represent the standard error of the mean. Phosphorylase b kinase activity is expressed as units per ml of undiluted extract. injection. During this interval the contractile response rose to a maximum and then receded. A 30,000 x g supernatant fraction from each heart was prepared and assayed for phosphorylase b kinase at ph 6.8 and 8.2 with Control B described above. The data in Fig. 5 show that a 5-fold increase in phosphorylase kinase activity occurred in the epinephrine-treated hearts over the control as determined by assays at ph 6.8. Activation was also apparent in the assays at ph 8.2 but of lesser t.otal magnitude as compared with the control. The result was a a-fold increase in the ph 6.8 to 8.2 ratios. Moreover, these changes occurred with striking rapidity and were maximal at 3 see when the first hearts were frozen. The contractile response did not reach a maximum until 10 set after the injection and then fell rapidly. Kinase activity remained maximal until after 10 set and then decreased somewhat more slowly than the contractile response. It should be noted that this supramaximal dose of epinephrine causes a
6 5898 Cardiac Phosphorylase b Kinase Vol. 241, No. 24 latent depression of perfused rat hearts which results in an initial rapid decline in contractile activity. At no time was phosphorylase kinase fully activated as could be predicted from ph 6.8 to 8.2 ratios, i.e. these ratios never approached 1. The maximum ph 6.8 to 8.2 ratios obtained were The changes, however, are highly significant statistically. All differences between the control (ph 6.8 values, ph 8.2 values, and ph 6.8 to 8.2 ratios) and epinephrine-treated hearts at the 3-, 9-, and 16.set intervals were subjected to statistical analysis by the Student t test. In every case the P values obtained were less than DISCUSSIOS The data presented establish that nonactivated cardiac phosphorylase b kinase is activated by preincubation with ATP and Mg++ ion. The rate of the activation reaction was increased by cyclic AMP, but there was no absolute requirement for the cyclic nucleotide. Activation was especially apparent when the assays were conducted at ph 7.0, where the nonactivated enzyme has low activity, but was also evident when assays were performed at ph 8.2. The activation process is, therefore, characterized by an increase in ph 7.0 to 8.2 activity ratios. The response to cyclic AMP was linear over the range, 1 x 10-g to 2 x 10-7 M. The reaction was highly specific for cyclic AMP; other cyclic nucleotides produced no activation above that of ATP at concentrations below 1 X lop4 M. In all respects, the activation of cardiac phosphorylase b kinase is qualitatively similar to the activation of the enzyme from skeletal muscle (5). There are obvious difficulties in assaying phosphorylase b kinase in crude heart extracts especially at ph 6.8. The difliculties arise from contaminating enzymes in these crude extracts which produce a substance or substances which when carried over into the phosphorylase assay stimulate phosphorylase b activity. Because of this, crude heart extracts cannot be reliably assayed with phosphorylase alone in the control. In the present study, a control was used in which heart extract was present during the kinase incubation and phosphorylase was added after dilution to stop the kinase reaction. The data obtained with this control clearly give a more reliable measure of phosphorylase kinase activity in crude heart extracts, especially in assays conducted at ph 6.8. With the use of this procedure, ph 6.8 to 8.2 activity ratios of about 0.06 were obtained in control perfused rat hearts. One can conclude that epinephrine causes a modest (5-fold at ph 6.8) but highly significant activation of phosphorylase b kinase in perfused rat hearts. Activation relative to the control was greater at ph 6.8 than at ph 8.2 so that ph 6.8 to 8.2 activity ratios increased 3-fold. The activation was maximal at 3 set after injection of epinephrine when the first hearts were frozen. The contractile response reached a maximum after 9 sec. Evidence exists that phosphorylase a levels rise later than the contractile response and lag well behind the rise in cyclic AMP level in hearts injected with supramaximal doses of epinephrine (8, 9). It is generally felt that phosphorylase activation is dissociated from the contractile response. The rapid activation of phosphorylase b kinase would suggest that this reaction follows closely the rise in cyclic AMP levels (8) and clearly precedes the contractile response. Although the dose of epinephrine chosen was deliberately supramaximal (with respect to contractile response), the data lend support to the idea that epinephrine-induced phosphorylase activation in the myocardium may result from activation of phosphorylase b kinase by cyclic AMP. Additional studies with physiological doses of epinephrine are needed to define more clearly the relationship between phosphorylase b kinase activation, increased cyclic AMP levels, and the inotropic response. Such a study is the subject of the succeeding report (13). REFERENCES 1. KREBS, E. G., GRAVES, i: J., AND FISCHER, E. H., J. Biol. Chem., 234, 2867 (1959). 2. MEYER, W. L., FISCHER, E. H., AND KREBS, E. G., Biochemistry, 3, 1033 (1964). 3. HA&ERMEI&ER, k. E., YUNIS, A. A., AND KREBS, E. G., J. Biol. Chem DRUMMOND, 6. I.; D&CAN, L., AND FRIESEN, A. J. D., J. Biol. Chem., 240, 2778 (1965). 5. KREBS, E. G., LOVE, D. S., BRATVOLD, G. E., TRAYSER, K. A., MEYER, W. L., AND FISCHER, E. H., Biochemistry, 3, 1022 (1964). 6. DRUMMOND, G. I., AND DUNCAN, L., J. Biol. Chem., 241, 3097 (1966). 7. Po~NE~, J. B., HAMMERMEISTER, K. E., BRATVOLD, G. E., AND KREBS. E. G.. Biochemistru ROBISON, G. k., B&CHER, R. I.; MORGAN, H. E., AND SUTHERLAND, E. W., Molecular Pharmacol., 1,168 (1965). 9. CREUNG, W. Y., AND WILLIAMSON, J. R., Nature, 207, 979 (1965). 10. SMITH, M., DRUMMOND, G. I., AND KHORANA, H. G., J. Am. Chem. Sot., 83, 698 (1961). 11. DRUMMOND, G. I., GILGAN, M. W., REINER, E. J., AND SMITE, M., J. Am. Chem. Sot., (1964). 12. DRUMMOND, G. I., VALADARES, J. R. E., AND DUNCAN, L., Proc. Sot. Exptl. Biol. Med., 117, 307 (1964). 13. DRUMMOND, G. I., DUNCAN, L., AND HERTZMAN, E., J. Biol. Chem., 241, 5899 (1966).
CO?IU VERSIOS OF PHOSPHORYLASE b TO PHOSPHORYLASE a IS MT SCLE EXTRACTS*
 CO?IU VERSIOS OF PHOSPHORYLASE b TO PHOSPHORYLASE a IS MT SCLE EXTRACTS* BY EDMOND H. FISCHER AND EDWIN G. KREBS (From the Department of Biochemistry, University of Washington, Seattle, Washington) (Received
CO?IU VERSIOS OF PHOSPHORYLASE b TO PHOSPHORYLASE a IS MT SCLE EXTRACTS* BY EDMOND H. FISCHER AND EDWIN G. KREBS (From the Department of Biochemistry, University of Washington, Seattle, Washington) (Received
Enzymatic Assay of PHOSPHORYLASE KINASE (EC )
 PRINCIPLE: Enzymatic Assay of PHOSPHORYLASE KINASE 2 Phosphorylase b + 4 ATP Phosphorylase Kinase > Phosphorylase a + 4 ADP Glycogen n + P i Phosphorylase a > Glycogen n-1 + a-d-glucose 1-Phosphate a-d-glucose
PRINCIPLE: Enzymatic Assay of PHOSPHORYLASE KINASE 2 Phosphorylase b + 4 ATP Phosphorylase Kinase > Phosphorylase a + 4 ADP Glycogen n + P i Phosphorylase a > Glycogen n-1 + a-d-glucose 1-Phosphate a-d-glucose
Enzymatic Assay of PHOSPHODIESTERASE, 3':5'-CYCLIC NUCLEOTIDE Crude Complex
 PRINCIPLE: 3':5'-cAMP + H 2 O PDE-3':5'-CN > AMP AMP + ATP Myokinase > 2 ADP 2 ADP + 2 PEP Pyruvate Kinase > 2 ATP + 2 Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD Abbreviations
PRINCIPLE: 3':5'-cAMP + H 2 O PDE-3':5'-CN > AMP AMP + ATP Myokinase > 2 ADP 2 ADP + 2 PEP Pyruvate Kinase > 2 ATP + 2 Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD Abbreviations
Synopsis. Received March 2, adrenaline. Mosinger and Kujalova (1964) reported that adrenaline-induced lipolysis
 Studies on Reduction of Lipolysis in Adipose Tissue on Freezing and Thawing YASUSHI SAITO1, NoBUO MATSUOKA1, AKIRA KUMAGAI1, HIROMICHI OKUDA2, AND SETSURO FUJII3 Chiba University, Chiba 280, Japan, 2Department
Studies on Reduction of Lipolysis in Adipose Tissue on Freezing and Thawing YASUSHI SAITO1, NoBUO MATSUOKA1, AKIRA KUMAGAI1, HIROMICHI OKUDA2, AND SETSURO FUJII3 Chiba University, Chiba 280, Japan, 2Department
B. 15 mm Ouabain Solution (Ouabain) (Prepare 10 ml in Reagent A using Ouabain Octahydrate, Sigma Prod. No. O3125.)
 SIGMA QUALITY CONTROL TEST PROCEDURE Sigma Prod. No. A7510 PRINCIPLE: ATP + H 2 O ATPase > ADP + P i Abbreviations used: ATPase = Adenosine 5'-Triphosphatase ATP = Adenosine 5'-Triphosphate ADP = Adenosine
SIGMA QUALITY CONTROL TEST PROCEDURE Sigma Prod. No. A7510 PRINCIPLE: ATP + H 2 O ATPase > ADP + P i Abbreviations used: ATPase = Adenosine 5'-Triphosphatase ATP = Adenosine 5'-Triphosphate ADP = Adenosine
PhosFree TM Phosphate Assay Biochem Kit
 PhosFree TM Phosphate Assay Biochem Kit (Cat. # BK050) ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 To order by e-mail: cservice@cytoskeleton.com Technical
PhosFree TM Phosphate Assay Biochem Kit (Cat. # BK050) ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 To order by e-mail: cservice@cytoskeleton.com Technical
Enzymatic Assay of RIBONUCLEIC ACID POLYMERASE 1 (EC )
 PRINCIPLE: Enzymatic Assay of RIBONUCLEIC ACID POLYMERASE 1 DNA + NTP RNA Polymerase > DNA + RNA + PP i PP i + UDPG UDPG Pyrophosphorylase > UTP + Glucose 1-Phosphate Glucose 1-Phosphate Phosphoglucomutase
PRINCIPLE: Enzymatic Assay of RIBONUCLEIC ACID POLYMERASE 1 DNA + NTP RNA Polymerase > DNA + RNA + PP i PP i + UDPG UDPG Pyrophosphorylase > UTP + Glucose 1-Phosphate Glucose 1-Phosphate Phosphoglucomutase
A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT
 A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT BY M. L. CALDWELL AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for publication,
A STUDY OF THE CONCENTRATION AND PROPERTIES OF TWO AMYLASES OF BARLEY MALT BY M. L. CALDWELL AND S. E. DOEBBELING (From the Department of Chemistry, Columbia University, New York) (Received for publication,
A Protein Kinase Inhibitor in Brown Adipose Tissue of Developing Rats
 Biochem. J. (1974) 138, 195-199 Printed in Great Britain 195 A Protein Kinase Inhibitor in Brown Adipose Tissue of Developing Rats By JOSEF P. SKALA, GEORGE I. DRUMMOND and PETER HAHN Departments ofpaediatrics,
Biochem. J. (1974) 138, 195-199 Printed in Great Britain 195 A Protein Kinase Inhibitor in Brown Adipose Tissue of Developing Rats By JOSEF P. SKALA, GEORGE I. DRUMMOND and PETER HAHN Departments ofpaediatrics,
Delta-9-tetrahydrocannabinol and human spermatozoa
 J. Biosci., Vol. 1, Number 3, September 1979, pp. 289 293. Printed in India. Delta-9-tetrahydrocannabinol and human spermatozoa INDIRA CHAKRAVARTY*, GIRISH SHAH**, ANIL R. SHETH** and JAGAT J. GHOSH *
J. Biosci., Vol. 1, Number 3, September 1979, pp. 289 293. Printed in India. Delta-9-tetrahydrocannabinol and human spermatozoa INDIRA CHAKRAVARTY*, GIRISH SHAH**, ANIL R. SHETH** and JAGAT J. GHOSH *
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN
 THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
DIHYDROSTREPTOMYCIN, VITAMIN K2-COUPLED
 JOURNAL OF BACTERIOLOGY Vol. 88, No. 4, p. 1019-1023 October, 1964 Copyright 1964 American Society for Microbiology Printed in U.S.A. DIHYDROSTREPTOMYCIN, VITAMIN K2-COUPLED TETRAZOLIUM REDUCTION, AND
JOURNAL OF BACTERIOLOGY Vol. 88, No. 4, p. 1019-1023 October, 1964 Copyright 1964 American Society for Microbiology Printed in U.S.A. DIHYDROSTREPTOMYCIN, VITAMIN K2-COUPLED TETRAZOLIUM REDUCTION, AND
Enzymatic Assay of POLYGALACTURONASE (EC )
 PRINCIPLE: Polygalacturonic Acid + H 2 O PG > Reducing Sugars Abbreviations: PG = Polygalacturonase CONDITIONS: T = 30 C, ph 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric REAGENTS: A. 50 mm Sodium
PRINCIPLE: Polygalacturonic Acid + H 2 O PG > Reducing Sugars Abbreviations: PG = Polygalacturonase CONDITIONS: T = 30 C, ph 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric REAGENTS: A. 50 mm Sodium
THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES
 THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM (From the Departments oj Physiology and Pharmacology and Biochemistry, Duke
THE EFFECT OF TITANIUM ON THE OXIDATION OF SULFHYDRYL GROUPS BY VARIOUS TISSUES BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM (From the Departments oj Physiology and Pharmacology and Biochemistry, Duke
PFK Activity Assay Kit (Colorimetric)
 PFK Activity Assay Kit (Colorimetric) Catalog Number KA3761 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
PFK Activity Assay Kit (Colorimetric) Catalog Number KA3761 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
FUNDAMENTALS OF BIOCHEMISTRY, CELL BIOLOGY AND BIOPHYSICS Vol. I - Biochemistry of Vitamins, Hormones and Other Messenger Molecules - Chris Whiteley
 BIOCHEMISTRY OF VITAMINS, HORMONES AND OTHER MESSENGER MOLECULES Chris Whiteley Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa Keywords: phosphorylation, phosphorylase,
BIOCHEMISTRY OF VITAMINS, HORMONES AND OTHER MESSENGER MOLECULES Chris Whiteley Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa Keywords: phosphorylation, phosphorylase,
Experiment 1. Isolation of Glycogen from rat Liver
 Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19
 TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
TRANSAMINASES IN SMOOTH BRUCELLA ABORTUS, STRAIN 19 BY ROBERT A. ALTENBERN AND RILEY D. HOUSEWRIGHT (From the Chemical Corps Biological Laboratories, Camp Detrick, Frederick, Maryland) (Received for publication,
Experiment 3: Activity Determination
 Experiment 3: Activity Determination Introduction: Specific activity is a method for measuring enzymatic activity and the enzyme purity in a mixture. In order to determine the specific activity of an enzyme,
Experiment 3: Activity Determination Introduction: Specific activity is a method for measuring enzymatic activity and the enzyme purity in a mixture. In order to determine the specific activity of an enzyme,
Human PKA (Protein Kinase A) Activity Assay Kit
 Human PKA (Protein Kinase A) Activity Assay Kit CATALOG NO: IRAAKT2532 LOT NO: SAMPLE INTENDED USE The PKA (Protein Kinase A) Activity kit is designed to quantitatively measure PKA activity in a variety
Human PKA (Protein Kinase A) Activity Assay Kit CATALOG NO: IRAAKT2532 LOT NO: SAMPLE INTENDED USE The PKA (Protein Kinase A) Activity kit is designed to quantitatively measure PKA activity in a variety
Enzymatic Assay of GLUCONATE KINASE (EC ) ß-NADPH = ß-Nicotinamide Adenine Dinucleotide Phosphate,
 Enzymatic Assay of GLUCONATE KINASE PRINCIPLE: D-Gluconate + ATP Gluconate Kinase > 6-Phospho-D-Gluconate + ADP 6-Phospho-D-Gluconate + ß-NADP G-PGDH > D-Ribulose-5'-P + ß-NADPH + CO 2 Mg2+ Abbreviations
Enzymatic Assay of GLUCONATE KINASE PRINCIPLE: D-Gluconate + ATP Gluconate Kinase > 6-Phospho-D-Gluconate + ADP 6-Phospho-D-Gluconate + ß-NADP G-PGDH > D-Ribulose-5'-P + ß-NADPH + CO 2 Mg2+ Abbreviations
Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds
 Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds By Edward J. Leonard, M.D., and Stephen Hajdu, M.D. A plasma protein system of mammalian origin which increases the contractile
Protection and Reactivation of Cardioglobulin-A by High Energy Phosphate Compounds By Edward J. Leonard, M.D., and Stephen Hajdu, M.D. A plasma protein system of mammalian origin which increases the contractile
On pages 131, 132, 136, and 137, the word conformational should be set off by quotation marks, rather than by parentheses.
 536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the
536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the
Enzymatic Assay of GUANYLATE KINASE (EC )
 PRINCIPLE: GMP + ATP Guanylate Kinase > GDP + ADP ADP + PEP Pyruvate Kinase > ATP + Pyruvate GDP + PEP Pyruvate Kinase > GTP + Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD
PRINCIPLE: GMP + ATP Guanylate Kinase > GDP + ADP ADP + PEP Pyruvate Kinase > ATP + Pyruvate GDP + PEP Pyruvate Kinase > GTP + Pyruvate 2 Pyruvate + 2 ß-NADH Lactic Dehydrogenase > 2 Lactate + 2 ß-NAD
CYTIDINE. Enzymatic synthesis of cytidine diphosphate diglyceride
 Enzymatic synthesis of cytidine diphosphate diglyceride JAMES R. CARTER* and EUGENE P. KENNEDY Department of Biological Chemistry, Harvard Medical School, Boston, Massachusetts ABSTRACT Evidence is presented
Enzymatic synthesis of cytidine diphosphate diglyceride JAMES R. CARTER* and EUGENE P. KENNEDY Department of Biological Chemistry, Harvard Medical School, Boston, Massachusetts ABSTRACT Evidence is presented
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
GLYCOGEN BEFORE THE LAB YOU HAVE TO READ ABOUT:
 GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS
 PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM* (From the Departments of Physiology and Biochemistry, Duke University School of Medicine, Durham)
PYRROLE AS A CATALYST FOR CERTAIN BIOLOGICAL OXIDATIONS BY FREDERICK BERNHEIM AND MARY L. C. BERNHEIM* (From the Departments of Physiology and Biochemistry, Duke University School of Medicine, Durham)
A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE*
 A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE* BY SEYMOUR KAUFMAN (From the Laboratory of Cellular Pharmacology, National Institute of Mental Health, United States Department
A NEW COFACTOR REQUIRED FOR THE ENZYMATIC CONVERSION OF PHENYLALANINE TO TYROSINE* BY SEYMOUR KAUFMAN (From the Laboratory of Cellular Pharmacology, National Institute of Mental Health, United States Department
MRP2 TR ATPase Assay Protocol CAT. NO. SBAT03
 MRP2 TR ATPase CAT. NO. SBAT03 Page 1 of 18 Determination of the interaction of drugs with the human MRP2 (ABCC2) transporter using the ATPase Assay For the following membrane products: SB-MRP2-Sf9-ATPase
MRP2 TR ATPase CAT. NO. SBAT03 Page 1 of 18 Determination of the interaction of drugs with the human MRP2 (ABCC2) transporter using the ATPase Assay For the following membrane products: SB-MRP2-Sf9-ATPase
Kinin forming and destroying activities in human bile and mucous membranes of the biliary tract
 Br. J. Pharmac. (1969), 37, 172-177. Kinin forming and destroying activities in human bile and mucous membranes of the biliary tract H. M. NIELSEN Surgical Department L, Aarhus Municipal Hospital, University
Br. J. Pharmac. (1969), 37, 172-177. Kinin forming and destroying activities in human bile and mucous membranes of the biliary tract H. M. NIELSEN Surgical Department L, Aarhus Municipal Hospital, University
ASSAY OF USING BETA-GLUCAZYME TABLETS
 ASSAY OF endo-β-glucanases USING BETA-GLUCAZYME TABLETS T-BGZ 12/12 Note: Changed assay format for malt β-glucanase Megazyme International Ireland 2012 SUBSTRATE: The substrate employed is Azurine-crosslinked
ASSAY OF endo-β-glucanases USING BETA-GLUCAZYME TABLETS T-BGZ 12/12 Note: Changed assay format for malt β-glucanase Megazyme International Ireland 2012 SUBSTRATE: The substrate employed is Azurine-crosslinked
MEK1 Assay Kit 1 Catalog # Lot # 16875
 MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
AMPK Assay. Require: Sigma (1L, $18.30) A4206 Aluminum foil
 AMPK Assay Require: Acetone Sigma (1L, $18.30) A4206 Aluminum foil Ammonium sulfate Fisher BP212R-1 AMP Sigma A1752 ATP Sigma A6144 (alt. use A7699) Beta-mercaptoethanol Sigma M6250 (alt. use M7154) Bio-Rad
AMPK Assay Require: Acetone Sigma (1L, $18.30) A4206 Aluminum foil Ammonium sulfate Fisher BP212R-1 AMP Sigma A1752 ATP Sigma A6144 (alt. use A7699) Beta-mercaptoethanol Sigma M6250 (alt. use M7154) Bio-Rad
colorimetrically by the methylene blue method according to Fogo and manometrically. In the presence of excess sulfur the amount of oxygen taken up
 GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength
 Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength By Ada L. Jacobson, Ph.D. ABSTRACT The effect of ouabain (0" 8 to (H M) on the hydrolysis of ATP by beef cardiac myosin B was
Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength By Ada L. Jacobson, Ph.D. ABSTRACT The effect of ouabain (0" 8 to (H M) on the hydrolysis of ATP by beef cardiac myosin B was
Enzymatic Assay of CHOLINE KINASE (EC )
 Enzymatic Assay of CHOLINE KINASE PRINCIPLE: Choline + ATP CK > o-phosphocholine + ADP ADP + PEP PK > ATP + Pyruvate Pyruvate + ß-NADH LDH > Lactate + ß-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate
Enzymatic Assay of CHOLINE KINASE PRINCIPLE: Choline + ATP CK > o-phosphocholine + ADP ADP + PEP PK > ATP + Pyruvate Pyruvate + ß-NADH LDH > Lactate + ß-NAD Abbreviations used: ATP = Adenosine 5'-Triphosphate
AZO-WHEAT ARABINOXYLAN
 www.megazyme.com ASSAY OF endo-1,4-b-xylanase using AZO-WHEAT ARABINOXYLAN S-AWAXP S-AWAXL 05/17 Megazyme 2017 PRINCIPLE: This assay procedure is specific for endo-1,4-β-d-xylanase activity. On incubation
www.megazyme.com ASSAY OF endo-1,4-b-xylanase using AZO-WHEAT ARABINOXYLAN S-AWAXP S-AWAXL 05/17 Megazyme 2017 PRINCIPLE: This assay procedure is specific for endo-1,4-β-d-xylanase activity. On incubation
INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT
 Brit. J. Phawmacol. (1951), 6, 289. INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT BY From the Pharmacological Laboratory, University of St. Andrews, Medical School, Dundee (Received February 2, 1951)
Brit. J. Phawmacol. (1951), 6, 289. INSULIN AND THE SUPRARENAL GLAND OF THE RABBIT BY From the Pharmacological Laboratory, University of St. Andrews, Medical School, Dundee (Received February 2, 1951)
GAA Activity Assay Kit (Colorimetric)
 GAA Activity Assay Kit (Colorimetric) Catalog Number KA3959 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 General
GAA Activity Assay Kit (Colorimetric) Catalog Number KA3959 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 General
AZO-XYLAN (BIRCHWOOD)
 ASSAY OF endo-1,4-ß-xylanase using AZO-XYLAN (BIRCHWOOD) S-AXBP S-AXBL 10/07 Megazyme International Ireland 2007 PRINCIPLE: This assay procedure is specific for endo-1,4-ß-d-xylanase activity. On incubation
ASSAY OF endo-1,4-ß-xylanase using AZO-XYLAN (BIRCHWOOD) S-AXBP S-AXBL 10/07 Megazyme International Ireland 2007 PRINCIPLE: This assay procedure is specific for endo-1,4-ß-d-xylanase activity. On incubation
XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES.
 XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES. II. THE PREPARATION OF GLUCOSE UREIDE. BY ALEXANDER HYND. From the Department of Physiology, University of St Andrews. (Received
XXVI. STUDIES ON THE INTERACTION. OF AMINO-COMPOUNDS AND CARBOHYDRATES. II. THE PREPARATION OF GLUCOSE UREIDE. BY ALEXANDER HYND. From the Department of Physiology, University of St Andrews. (Received
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH
 Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
Enzymatic Assay of NAD-PYROPHOSPHORYLASE (EC )
 Enzymatic Assay of NAD-PYROPHOSPHORYLASE PRINCIPLE: ß-NMN + ATP NAD-Pyrophosphorylase > ß-NAD + PP ß-NAD + Ethanol ADH > ß-NADH + Acetaldehyde Abbreviations used: ATP = Adenosine 5'-Triphosphate ADH =
Enzymatic Assay of NAD-PYROPHOSPHORYLASE PRINCIPLE: ß-NMN + ATP NAD-Pyrophosphorylase > ß-NAD + PP ß-NAD + Ethanol ADH > ß-NADH + Acetaldehyde Abbreviations used: ATP = Adenosine 5'-Triphosphate ADH =
A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* Previous studies (1, 2) have shown that after the ingestion of caffeine
 A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* BY HERBERT H. CORNISH AND A. A. CHRISTMAN (From the Department of Biological Chemistry, Medical School, University of Michigan,
A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* BY HERBERT H. CORNISH AND A. A. CHRISTMAN (From the Department of Biological Chemistry, Medical School, University of Michigan,
ASSAY OF using AZO-FRUCTAN S-AZFR5 11/17
 www.megazyme.com ASSAY OF endo-fructanase using AZO-FRUCTAN S-AZFR5 11/17 Megazyme 2017 PRINCIPLE: The substrate is the high molecular weight fraction of chicory fructan (DP ~ 20-60) dyed with an azo-dye
www.megazyme.com ASSAY OF endo-fructanase using AZO-FRUCTAN S-AZFR5 11/17 Megazyme 2017 PRINCIPLE: The substrate is the high molecular weight fraction of chicory fructan (DP ~ 20-60) dyed with an azo-dye
ALTERATIONS OF ASPARTATE- AND ALANINE- TRANSAMINASE IN MICE WITH HEREDITARY MUSCULAR DYSTROPHY
 The Japanese Journal of Physiology 17, pp. 57-64, 1967 ALTERATIONS OF ASPARTATE- AND ALANINE- TRANSAMINASE IN MICE WITH HEREDITARY MUSCULAR DYSTROPHY Shigekatsu TSUJI AND Hiroshi MATSUSHITA Department
The Japanese Journal of Physiology 17, pp. 57-64, 1967 ALTERATIONS OF ASPARTATE- AND ALANINE- TRANSAMINASE IN MICE WITH HEREDITARY MUSCULAR DYSTROPHY Shigekatsu TSUJI AND Hiroshi MATSUSHITA Department
Polysaccharide phosphorylase
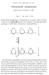 CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
 Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
J. Biosci., Vol. 7, Number 2, March 1985, pp Printed in India.
 J. Biosci., Vol. 7, Number 2, March 1985, pp. 123 133. Printed in India. Irreversibility of the interaction of human growth hormone with its receptor and analysis of irreversible reactions in radioreceptor
J. Biosci., Vol. 7, Number 2, March 1985, pp. 123 133. Printed in India. Irreversibility of the interaction of human growth hormone with its receptor and analysis of irreversible reactions in radioreceptor
Positive Inotropic Effects of Dibutyryl Cyclic Adenosine 3',5'-Monophosphate
 Positive Inotropic Effects of Dibutyryl Cyclic Adenosine 3',5'-Monophosphate By C. Lynn Skelton, M.D., Gerald S. Levey, M.D., and Stephen E. Epstein, M.D. ABSTRACT The positive inotropic effects of catecholamines
Positive Inotropic Effects of Dibutyryl Cyclic Adenosine 3',5'-Monophosphate By C. Lynn Skelton, M.D., Gerald S. Levey, M.D., and Stephen E. Epstein, M.D. ABSTRACT The positive inotropic effects of catecholamines
Biochemical Techniques 06 Salt Fractionation of Proteins. Biochemistry
 . 1 Description of Module Subject Name Paper Name 12 Module Name/Title 2 1. Objectives Understanding the concept of protein fractionation Understanding protein fractionation with salt 2. Concept Map 3.
. 1 Description of Module Subject Name Paper Name 12 Module Name/Title 2 1. Objectives Understanding the concept of protein fractionation Understanding protein fractionation with salt 2. Concept Map 3.
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE
 METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
METABOLISM OF CARBOHYDRATES BY PSEUDOMONAS SACCHAROPHILA1 II. NATURE OF THE KINASE REACTON INVOLVING FRUCTOSE NORBERTO J. PALLERONI, REBECCA CONTOPOULOU, AND MICHAEL DOUDOROFF Department of Bacteriology,
possibilities occurs. It has been found that the organism acquires addition of vitamin B1 to cells of P. pentosaceum which had
 ADAPTATION OF THE PROPIONIC-ACID BACTERIA TO VITAMIN B1 SYNTHESIS INCLUDING A METHOD OF ASSAY M. SILVERMAN AND C. H. WERKMAN Bacteriology Section, Industrial Science Research Institute, Iowa State College,
ADAPTATION OF THE PROPIONIC-ACID BACTERIA TO VITAMIN B1 SYNTHESIS INCLUDING A METHOD OF ASSAY M. SILVERMAN AND C. H. WERKMAN Bacteriology Section, Industrial Science Research Institute, Iowa State College,
ab ATP Synthase Enzyme Activity Microplate Assay Kit
 ab109714 ATP Synthase Enzyme Activity Microplate Assay Kit Instructions for Use For the quantitative measurement of ATP Synthase activity in samples from Human, Rat and Cow This product is for research
ab109714 ATP Synthase Enzyme Activity Microplate Assay Kit Instructions for Use For the quantitative measurement of ATP Synthase activity in samples from Human, Rat and Cow This product is for research
Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection. EPL-BAS Method No.
 Page 1 of 10 Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection EPL-BAS Method No. 205G881B Method Summary: Residues of 6-CPA are
Page 1 of 10 Determination of 6-Chloropicolinic Acid (6-CPA) in Crops by Liquid Chromatography with Tandem Mass Spectrometry Detection EPL-BAS Method No. 205G881B Method Summary: Residues of 6-CPA are
Enzymatic Assay of ß-GLUCOSIDASE (EC )
 PRINCIPLE: ß-D-Glucoside + H 2 O ß-Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric 1 REAGENTS: A. 100 mm Sodium Acetate Buffer, ph 5.0
PRINCIPLE: ß-D-Glucoside + H 2 O ß-Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD: Colorimetric 1 REAGENTS: A. 100 mm Sodium Acetate Buffer, ph 5.0
2. Langendorff Heart
 2. Langendorff Heart 2.1. Principle Langendorff heart is one type of isolated perfused heart which is widely used for biochemical, physiological, morphological and pharmacological researches. It provides
2. Langendorff Heart 2.1. Principle Langendorff heart is one type of isolated perfused heart which is widely used for biochemical, physiological, morphological and pharmacological researches. It provides
ab ORAC Assay Kit
 Version 1 Last updated 10 April 2018 ab233473 ORAC Assay Kit For the measurement of ORAC activity in cell lysate, plasma, serum, tissue homogenates and food extracts. This product is for research use only
Version 1 Last updated 10 April 2018 ab233473 ORAC Assay Kit For the measurement of ORAC activity in cell lysate, plasma, serum, tissue homogenates and food extracts. This product is for research use only
ASSAY OF using CELLAZYME C TABLETS T-CCZ 01/17
 www.megazyme.com ASSAY OF endo-cellulase using CELLAZYME C TABLETS T-CCZ 01/17 Megazyme 2017 SUBSTRATE: The substrate employed is azurine-crosslinked HE-cellulose (AZCL-Cellulose). This substrate is prepared
www.megazyme.com ASSAY OF endo-cellulase using CELLAZYME C TABLETS T-CCZ 01/17 Megazyme 2017 SUBSTRATE: The substrate employed is azurine-crosslinked HE-cellulose (AZCL-Cellulose). This substrate is prepared
Β-FRUCTOFURANOSIDASE ENZYME
 KINETICS ANALYSIS OF Β-FRUCTOFURANOSIDASE ENZYME 2-The effects of enzyme concentration on the rate of an enzyme catalyzed reaction. Systematic names and numbers β-fructofuranosidase (EC 3.2.1.26) Reactions
KINETICS ANALYSIS OF Β-FRUCTOFURANOSIDASE ENZYME 2-The effects of enzyme concentration on the rate of an enzyme catalyzed reaction. Systematic names and numbers β-fructofuranosidase (EC 3.2.1.26) Reactions
High-density Lipoprotein Cholesterol (HDL-C) Assay Kit
 (FOR RESEARCH USE ONLY. DO NOT USE IT IN CLINICAL DIAGNOSIS!) High-density Lipoprotein Cholesterol (HDL-C) Assay Kit (Double reagents) Catalog No: E-BC-K221 Method: Colorimetric method Specification: 96T
(FOR RESEARCH USE ONLY. DO NOT USE IT IN CLINICAL DIAGNOSIS!) High-density Lipoprotein Cholesterol (HDL-C) Assay Kit (Double reagents) Catalog No: E-BC-K221 Method: Colorimetric method Specification: 96T
TECHNICAL BULLETIN. Sialic Acid Quantitation Kit. Catalog Number SIALICQ Storage Temperature 2 8 C
 Sialic Acid Quantitation Kit Catalog Number SIALICQ Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description The Sialic Acid Quantitation Kit provides a rapid and accurate determination of total
Sialic Acid Quantitation Kit Catalog Number SIALICQ Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description The Sialic Acid Quantitation Kit provides a rapid and accurate determination of total
EFFECTS OF FREEZING ON PARTICULATE ENZYMES OF RAT LIVER*
 EFFECTS OF FREEZING ON PARTICULATE ENZYMES OF RAT LIVER* BY VIVIAN S. PORTER, NANCY P. DEMING, RITA C. WRIGHT, AND E. M. SCOTT (From the Arctic Health Research Center, United States Public Health Service,
EFFECTS OF FREEZING ON PARTICULATE ENZYMES OF RAT LIVER* BY VIVIAN S. PORTER, NANCY P. DEMING, RITA C. WRIGHT, AND E. M. SCOTT (From the Arctic Health Research Center, United States Public Health Service,
THE EFFECT OF ANTICOAGULANTS ON DETERMINA- TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER
 THE EFFECT OF ANTICOAGULANTS ON DETERMINA TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER (From the Department of Laboratories, Henry Ford Hospital, Detroit) (Received for publication,
THE EFFECT OF ANTICOAGULANTS ON DETERMINA TIONS OF INORGANIC PHOSPHATE AND PROTEIN IN PLASMA BY OLIVER HENRY GAEBLER (From the Department of Laboratories, Henry Ford Hospital, Detroit) (Received for publication,
STUDIES ON CHOLINESTERASE*
 STUDIES ON CHOLINESTERASE* III. PURIFICATION OF THE ENZYME FROM ELECTRIC TISSUE BY FRACTIONAL AMMONIUM SULFATE PRECIPITATION BY MORTIMER A. ROTHENBERG AND DAVID NACHMANSOHN (From the Departments of Neurology
STUDIES ON CHOLINESTERASE* III. PURIFICATION OF THE ENZYME FROM ELECTRIC TISSUE BY FRACTIONAL AMMONIUM SULFATE PRECIPITATION BY MORTIMER A. ROTHENBERG AND DAVID NACHMANSOHN (From the Departments of Neurology
METHEMOGLOBIN BLOCKADE OF CORONARY ARTERIAL SOLUBLE GUANYLATE CYCLASE ACTIVATION BY NITROSO COMPOUNDS AND ITS REVERSAL WITH DITHIOTHREITOL
 METHEMOGLOBIN BLOCKADE OF CORONARY ARTERIAL SOLUBLE GUANYLATE CYCLASE ACTIVATION BY NITROSO COMPOUNDS AND ITS REVERSAL WITH DITHIOTHREITOL Eliot H. OHLSTEIN, Barbara K. BARRY, Darlene Y. GRUETTER and Louis
METHEMOGLOBIN BLOCKADE OF CORONARY ARTERIAL SOLUBLE GUANYLATE CYCLASE ACTIVATION BY NITROSO COMPOUNDS AND ITS REVERSAL WITH DITHIOTHREITOL Eliot H. OHLSTEIN, Barbara K. BARRY, Darlene Y. GRUETTER and Louis
For the quantitative measurement of ATP Synthase Specific activity in samples from Human, Rat and Cow
 ab109716 ATP Synthase Specific Activity Microplate Assay Kit Instructions for Use For the quantitative measurement of ATP Synthase Specific activity in samples from Human, Rat and Cow This product is for
ab109716 ATP Synthase Specific Activity Microplate Assay Kit Instructions for Use For the quantitative measurement of ATP Synthase Specific activity in samples from Human, Rat and Cow This product is for
Basic Building Blocks of Cells Course 1 / Lecture 119
 Basic Building Blocks of Cells Course 1 / Lecture 119 vladimira.kvasnicova@lf3.cuni.cz Department of biochemistry the 4 th floor office 411 Biogenic elements = elements essential for structure and function
Basic Building Blocks of Cells Course 1 / Lecture 119 vladimira.kvasnicova@lf3.cuni.cz Department of biochemistry the 4 th floor office 411 Biogenic elements = elements essential for structure and function
liberated in the body is probably less than 1 part in a million. The
 547.435-292: 577.153 KINETICS OF CHOLINE ESTERASE. By A. J. CLARK, J. RAVENT6S, E. STEDMAN, and ELLEN STEDMAN. From the Departments of Pharmacology and Medical Chemistry, University of Edinburgh. (Received
547.435-292: 577.153 KINETICS OF CHOLINE ESTERASE. By A. J. CLARK, J. RAVENT6S, E. STEDMAN, and ELLEN STEDMAN. From the Departments of Pharmacology and Medical Chemistry, University of Edinburgh. (Received
ASSAY OF SPHINGOMYELINASE ACTIVITY
 ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
endo-1,4-beta-xylanase
 www.megazyme.com ASSAY OF endo-1,4-beta-xylanase using XYLAZYME TABLETS T-XYZ 03/14 Megazyme International Ireland 2014 SUBSTRATE: The substrate employed is azurine-crosslinked arabinoxylan (AZCL- Arabinoxylan),
www.megazyme.com ASSAY OF endo-1,4-beta-xylanase using XYLAZYME TABLETS T-XYZ 03/14 Megazyme International Ireland 2014 SUBSTRATE: The substrate employed is azurine-crosslinked arabinoxylan (AZCL- Arabinoxylan),
KE-SIALIQ Sialic Acid Quantitation Kit. SialiQuant Sialic Acid Quantitation Kit
 SialiQuant Sialic Acid Quantitation Kit Part Number KE-SIALIQ Certification of Analysis Lot Number 706.1A Kit Storage Kits should be stored at 4 C. Kit Contents Kit contains all the reagents to quickly
SialiQuant Sialic Acid Quantitation Kit Part Number KE-SIALIQ Certification of Analysis Lot Number 706.1A Kit Storage Kits should be stored at 4 C. Kit Contents Kit contains all the reagents to quickly
Enzymatic Assay of FRUCTOSE-6-PHOSPHATE KINASE, PYROPHOSPHATE DEPENDENT (EC ) from Mung Bean
 PRINCIPLE: PP i + F-6-P PP i -PFK > F-1,6-DP + P i F-2,6-DP 1 F-1,6-DP Aldolase > GAP + DHAP GAP TPI > DHAP 2DHAP + 2 ß-NADH GDH > 2 Glycerol-3-Phosphate + 2 ß-NAD Abbreviations used: PP i = Pyrophosphate
PRINCIPLE: PP i + F-6-P PP i -PFK > F-1,6-DP + P i F-2,6-DP 1 F-1,6-DP Aldolase > GAP + DHAP GAP TPI > DHAP 2DHAP + 2 ß-NADH GDH > 2 Glycerol-3-Phosphate + 2 ß-NAD Abbreviations used: PP i = Pyrophosphate
B. 1% (w/v) Salicin Substrate Solution (Salicin) (Prepare 50 ml in Reagent A using Salicin, Sigma Prod. No. S-0625.)
 SIGMA QUALITY CONTROL TEST PROCEDURE (Q]\PDWLFÃ$VVD\ÃRIÃ */8&26,'$6( PRINCIPLE: 'Glucoside + H 2 O Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD:
SIGMA QUALITY CONTROL TEST PROCEDURE (Q]\PDWLFÃ$VVD\ÃRIÃ */8&26,'$6( PRINCIPLE: 'Glucoside + H 2 O Glucosidase > D-Glucose + an Alcohol CONDITIONS: T = 37 C, ph = 5.0, A 540nm, Light path = 1 cm METHOD:
MIXED XYLANASE, β-glucanase ENZYME PREPARATION, produced by a strain of HUMICOLA INSOLENS
 MIXED XYLANASE, β-glucanase ENZYME PREPARATION, produced by a strain of HUMICOLA INSOLENS New specifications prepared at the 61st JECFA (2003) and published in FNP 52 Add 11 (2003). An ADI not specified
MIXED XYLANASE, β-glucanase ENZYME PREPARATION, produced by a strain of HUMICOLA INSOLENS New specifications prepared at the 61st JECFA (2003) and published in FNP 52 Add 11 (2003). An ADI not specified
Prom the Department of Pharmacology, McGill University, Montreal, Canada
 365 J. Physiol. (I95I) II3, 365-37I EFFECTS OF NORADRENALINE ON CORONARY FLOW AND HEART CONTRACTION, AS RECORDED CONCURRENTLY IN THE ISOLATED RABBIT HEART BY F. C. LU* AND K. I. MELVILLE Prom the Department
365 J. Physiol. (I95I) II3, 365-37I EFFECTS OF NORADRENALINE ON CORONARY FLOW AND HEART CONTRACTION, AS RECORDED CONCURRENTLY IN THE ISOLATED RABBIT HEART BY F. C. LU* AND K. I. MELVILLE Prom the Department
PROTAZYME AK TABLETS
 www.megazyme.com ASSAY OF endo-protease using PROTAZYME AK TABLETS T-PRAK 05/16 Megazyme International Ireland 2016 SUBSTRATE: The substrate employed is Azurine-crosslinked casein (AZCL-casein). This substrate
www.megazyme.com ASSAY OF endo-protease using PROTAZYME AK TABLETS T-PRAK 05/16 Megazyme International Ireland 2016 SUBSTRATE: The substrate employed is Azurine-crosslinked casein (AZCL-casein). This substrate
Superoxide Dismutase Kit
 Superoxide Dismutase Kit Catalog Number: 7500-100-K Reagent kit for the analysis of Superoxide Dismutase in cell extracts. Sufficient reagents for 100 experimental tests, 50 negative controls, and 50 positive
Superoxide Dismutase Kit Catalog Number: 7500-100-K Reagent kit for the analysis of Superoxide Dismutase in cell extracts. Sufficient reagents for 100 experimental tests, 50 negative controls, and 50 positive
ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric)
 Version 10 Last updated 19 December 2017 ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric) For the measurement of triglycerides in various samples. This product is for research
Version 10 Last updated 19 December 2017 ab65336 Triglyceride Quantification Assay Kit (Colorimetric/ Fluorometric) For the measurement of triglycerides in various samples. This product is for research
The incorporation of labeled amino acids into lens protein. Abraham Speclor and Jin H. Kinoshita
 The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
Human Alpha 1 microglobulin ELISA Kit
 Human Alpha 1 microglobulin ELISA Kit Catalogue No.: EH4144 Size: 48T/96T Reactivity: Human Range:0.625-40ng/ml Sensitivity:
Human Alpha 1 microglobulin ELISA Kit Catalogue No.: EH4144 Size: 48T/96T Reactivity: Human Range:0.625-40ng/ml Sensitivity:
Nitrate and Nitrite Key Words: 1. Introduction 1.1. Nature, Mechanism of Action, and Biological Effects (Fig. 1)
 7 Nitrate and Nitrite Key Words: Nitrate; nitrite; methemoglobin; blood pressure; asphyxia; spinach; spongy cadmium column; zinc metal; sodium nitrate; sodium nitrite; ammonia buffer solution; Jones reductor.
7 Nitrate and Nitrite Key Words: Nitrate; nitrite; methemoglobin; blood pressure; asphyxia; spinach; spongy cadmium column; zinc metal; sodium nitrate; sodium nitrite; ammonia buffer solution; Jones reductor.
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Glucose 6 Phosphate Assay Kit (Colorimetric)
 ab83426 Glucose 6 Phosphate Assay Kit (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of Glucose 6 Phosphate levels in various samples This product is for research
ab83426 Glucose 6 Phosphate Assay Kit (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of Glucose 6 Phosphate levels in various samples This product is for research
RICINOLEATE UPON BACTERIA
 A COMPARATIVE STUDY OF THE ACTION OF SODIUM RICINOLEATE UPON BACTERIA From the Division of Laboratories and Research, New York State Department of Health, Albany Received for publication, May 14, 1928
A COMPARATIVE STUDY OF THE ACTION OF SODIUM RICINOLEATE UPON BACTERIA From the Division of Laboratories and Research, New York State Department of Health, Albany Received for publication, May 14, 1928
Note: During 30 minute incubation; proceed thru appropriate sections below (e.g. sections II, III and V).
 LEGEND MAX β Amyloid x 40 LEGEND MAX β Amyloid x 40 ELISA Kit Components and Protocol Kit Components Capture Antibody Coated Plate 1 stripwell plate 1 40 Standard (2) 20μg vial 5X Wash Buffer 125mL Standard
LEGEND MAX β Amyloid x 40 LEGEND MAX β Amyloid x 40 ELISA Kit Components and Protocol Kit Components Capture Antibody Coated Plate 1 stripwell plate 1 40 Standard (2) 20μg vial 5X Wash Buffer 125mL Standard
CHANGES IN PHOSPHATE AND CARBOHYDRATE METABOLISM IN SHOCK
 CHANGES IN PHOSPHATE AND CARBOHYDRATE METABOLISM IN SHOCK BY E. S. GORANSON,* J. E. HAMILTON, AND R. E. HAIST (From the Department of Physiology, University of Toronto, Toronto, Canada) (Received for publication,
CHANGES IN PHOSPHATE AND CARBOHYDRATE METABOLISM IN SHOCK BY E. S. GORANSON,* J. E. HAMILTON, AND R. E. HAIST (From the Department of Physiology, University of Toronto, Toronto, Canada) (Received for publication,
Aperto Cell Lysis and Protein Solubilization Users Manual
 Aperto Cell Lysis and Protein Solubilization Users Manual Revision 2 THIS MANUAL APPLIES TO THE FOLLOWING PRODUCTS: 3A8600 Aperto, 5X Cell Lysis Buffer. 20mL 3A8610 Aperto, 5X Cell Lysis Buffer. 100mL
Aperto Cell Lysis and Protein Solubilization Users Manual Revision 2 THIS MANUAL APPLIES TO THE FOLLOWING PRODUCTS: 3A8600 Aperto, 5X Cell Lysis Buffer. 20mL 3A8610 Aperto, 5X Cell Lysis Buffer. 100mL
Kit for assay of thioredoxin
 FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
FkTRX-02-V2 Kit for assay of thioredoxin The thioredoxin system is the major protein disulfide reductase in cells and comprises thioredoxin, thioredoxin reductase and NADPH (1). Thioredoxin systems are
THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED TRYPSIN
 Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
Published Online: 20 January, 1934 Supp Info: http://doi.org/10.1085/jgp.17.3.393 Downloaded from jgp.rupress.org on November 8, 2018 THE EQUILIBRIUM BETWEEN ACTIVE NATIVE TRYPSIN AND INACTIVE DENATURED
decarboxylation. Further work with the enzyme systems involved has shown
 THE BACTERIAL OXIDATION OF AROMATIC COMPOUNDS IV. STITDIES ON THE MECHANISM OF ENZYMATC DEGRADATION OF PROTOCATECHuiC ACID' R. Y. STANIER Department of Bacteriology, University of California, Berkeley,
THE BACTERIAL OXIDATION OF AROMATIC COMPOUNDS IV. STITDIES ON THE MECHANISM OF ENZYMATC DEGRADATION OF PROTOCATECHuiC ACID' R. Y. STANIER Department of Bacteriology, University of California, Berkeley,
Alkaline Phosphatase Assay Kit (Fluorometric)
 Alkaline Phosphatase Assay Kit (Fluorometric) Catalog Number KA0820 500 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
Alkaline Phosphatase Assay Kit (Fluorometric) Catalog Number KA0820 500 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
CHEMICAL STUDIES ON BACTERIAL AGGLUTINATION II. THE IDENTITY OF PRECIPITIN AND AGGLUTININ* BY MICHAEL HEIDELBERGER, PH.D., AND ELVIN A.
 CHEMICAL STUDIES ON BACTERIAL AGGLUTINATION II. THE IDENTITY OF PRECIPITIN AND AGGLUTININ* BY MICHAEL HEIDELBERGER, PH.D., AND ELVIN A. KABAT (From the Laboratories of the Departments of Medicine and Biological
CHEMICAL STUDIES ON BACTERIAL AGGLUTINATION II. THE IDENTITY OF PRECIPITIN AND AGGLUTININ* BY MICHAEL HEIDELBERGER, PH.D., AND ELVIN A. KABAT (From the Laboratories of the Departments of Medicine and Biological
CELLULASE from PENICILLIUM FUNICULOSUM
 CELLULASE from PENICILLIUM FUNICULOSUM Prepared at the 55th JECFA (2000) and published in FNP 52 Add 8 (2000), superseding tentative specifications prepared at the 31st JECFA (1987) and published in FNP
CELLULASE from PENICILLIUM FUNICULOSUM Prepared at the 55th JECFA (2000) and published in FNP 52 Add 8 (2000), superseding tentative specifications prepared at the 31st JECFA (1987) and published in FNP
