Safety. What You Need. What to do... Neo/SCI Student s Guide Name... Teacher/Section... Date... Step 1. Step 2
|
|
|
- Dora Gallagher
- 5 years ago
- Views:
Transcription
1 Diffusion & Osmosis Activity 1a Diffusion Lab 1 Background Diffusion occurs whenever concentrations of substances are not even throughout an area. This unequal distribution of particles is called a concentration gradient. Molecules tend to move from areas where they are highly concentrated to areas where there are less molecules present. This process does not require any energy input to move the molecules from one area to another, and so is said to be passive. Cells are surrounded by the plasma membrane, which is made up of a variety of different organic molecules, with the most common component being phospholipids. This lab exercise illustrates the role of the cell membrane in regulating the passive movement of substances between the environment and the cytoplasm. Outside of Cell Carbohydrate chains Objectives Construct models of cells using dialysis tubing to simulate the selectively permeable nature of the plasma membrane. Study the effects of diffusion through a selectively permeable membrane. Predict the characteristics of substances that allow them to pass through a selectively permeable membrane. Inside of Cell (Cytoplasm) Lipid bilayer Protein channel Plasma Membrane Phospholipid molecules are amphipathic, meaning that one end of the molecule is polar (phosphate head), while the other is nonpolar (lipid tail). The phospholipid molecules in a cell membrane line up in two adjacent layers, with phosphate groups on the outside and the nonpolar lipid tails in the center. Since water is a polar molecule, having a slight negative charge at one end and a slight positive charge at the other end, it is attracted to the polar phosphate groups. The lipid tails, which do not mix well with water much like salad oil in water are hidden on the inside of the membrane and do not have to interact with the polar water molecules. This arrangement allows the cell membrane to mix well with the fluids, which are mostly water, both outside and inside the cell. 1
2 The cell membrane has several components besides phospholipids, including proteins that act as channels through the membrane, cholesterol molecules, enzymes, and carbohydrates. The cell membrane is said to be selectively permeable, that is, it lets some substances into and out of the cell, while not allowing other substances to pass at all. Molecules that are large, such as proteins and starch, and charged particles, such as ions, usually are not able to get through the cell membrane unless the appropriate protein gate is present. Molecules that cannot pass from one side of the membrane to the other can become concentrated on one side of the membrane. Solutes of different sizes and/or charges can be separated from one another in this way. This process is called dialysis. Safety Be sure to always wear safety goggles, gloves and a lab apron to protect your eyes and clothing when working with any chemicals. Iodine potassium iodide (IKI) is a corrosive substance and can be a caustic irritant if allowed to come in contact with the skin. Keep the iodine potassium iodide bottle tightly capped and only open it when you are ready to use it. In case of spills or skin contact, inform your teacher immediately and flush areas with running water for 15 minutes. Dispose of any waste materials and clean up your work area as directed by your teacher. What You Need Be sure to always wash your hands before leaving the laboratory. Per student Apron Gloves Goggles Per group 1 Cup, clear 1 Funnel, plastic 15 cm Soaked dialysis tubing 15 ml 15% Glucose/1% starch solution in medicine cup 1 Pipet, plastic 2 Glucose test strips 1 ml Starch indicator solution (IKI) 2 10-cm pieces of string Water, distilled Step 1 Fill the plastic cup with distilled water to within 1-2 cm of the top. Step 2 What to do... Dip a glucose test strip into the water in the cup for 1-2 seconds. Run the test strip along the edge of the cup to remove any excess liquid. Wait approximately 2-3 minutes and observe any color change on the strip. A positive (+) glucose test is indicated by a greenish color on the test strip. No color change will occur if the test result is negative (-). Record the results of the test in Data Table 1. 2
3 Step 3 Caution: IKI solution is a poison. Avoid any skin contact. Be sure to wear proper safety equipment. Using the plastic pipet, carefully add drops of the starch indicator solution (IKI) to the water in the cup. Observe what happens to the indicator solution as it mixes with the water. Record the color of the water in Data Table 1. Step 4 Your teacher will provide you with a soaking dialysis tube segment. Gently rub the tubing between your fingers to open it. Step 5 Tie one end of the tubing tightly with one piece of string. Make sure this end is tied tightly enough to prevent any leaks from the end of the bag. Fill the tubing with water and test it for leaks at a sink. Empty the tubing. Step 9 Completely submerge the model cell into the cup of water and starch indicator solution. Allow osmosis and diffusion to occur for 30 minutes. Step 10 After 30 minutes, test the water in the cup for sugar content, as in Step 2. Note any color changes in the dialysis tubing and in the cup. Record these observations in Data Table 1. Note: A positive (+) result for the presence of starch is indicated by a blue black color of the final solution. If the starch test result is negative (-), the final solution will be an orange brown color. Step 11 Be sure to wash your hands and clean up and dispose of any waste materials as directed by your teacher. Step 6 Insert the tip of the plastic funnel into the open end of the dialysis bag, and pour the 15 ml of 15% glucose/1% starch solution from the medicine cup into the tubing. Step 7 Squeeze all the air bubbles out of the tubing and tie the open end shut with another piece of string. Note the color of the starch-glucose solution in the dialysis tubing and record your observations in Data Table 1. Step 8 Briefly rinse the outside of the bag under running water. Squeeze the bag gently to be sure that there are no leaks. If you find a bag leaking at an end, retie it securely. 3
4 Analysis Data Table 1: Diffusion 3. What types of substances pass easily through living cell membranes? Characteristic Initial Color Water in Cup Solution in Tubing Final Color Initial Glucose (+/-) + Final Glucose (+/-) + Initial Starch (+/-) + Final Starch (+/-) + Initial Solutes Final Solutes Change in Volume Observed 1. What is simple diffusion? Questions 2. What are two characteristics of substances that do not allow them to pass through the selectively permeable membrane of living cells? 4. Did the glucose molecules pass through the dialysis tubing? How do you know? 5. Did the starch molecules pass through the dialysis tubing? What evidence do you have to support this conclusion? 6. Why did we use dialysis tubing as a model for a cell membrane? 7. Why were the results for final starch and final glucose filled in already in Data Table #1? (Hint: Would all of the solutes diffuse out of the dialysis bag? Why or why not?) 4
5 Activity 1b Osmosis However, the concentration gradient across the membrane still exists. Although the solute molecules are nonpermeating, that is, they are unable to diffuse across the membrane, the plasma membrane is permeable to water molecules. This results in water molecules diffusing down their own concentration gradient, from an area where water is more concentrated (fewer solutes) to an area where water is less concentrated (more solutes). The movement of the solvent (in this case, water) down its concentration gradient through a selectively permeable membrane is called osmosis. This lab exercise models the effect of hypertonic and hypotonic solutions on the cell due to the selectively permeable nature of the cell membrane. Objectives Construct models of cells using dialysis tubing to simulate the selectively permeable nature of the plasma membrane. Observe the effects of osmosis on a model cell. Selectively permeable membrane Sugar molecule Water molecule Predict the effect of solute concentration on osmosis. Background Osmosis is a term used to describe diffusion under a particular set of circumstances. Consider what occurs when a concentration gradient exists across a plasma membrane, but the solutes are unable to cross from one side to the other due to size or electrochemical interactions. In this situation, diffusion of solutes cannot occur. Notice that water molecules over time, diffuse across the semipermeable membrane toward the more concentrated sugar solution on the right. When describing the solute concentration of solutions relative to each other, three terms are commonly used: hypertonic, hypotonic, and isotonic. A 20% glucose solution is hypertonic to a 10% glucose solution, because the 20% glucose solution has more solute particles dissolved in the solvent. Two solutions that are isotonic to each other have the same number of solute particles dissolved in them. When determining if a solution is hypertonic, hypotonic, or isotonic to another, the identity of the solute is not important. Tonicity is measured by the total number of solute particles present in the solution, not by how many of each type of solute are present. 5
6 What You Need What to do... Per student Apron Gloves Goggles Per group 6 Cups, clear 6 15-cm dialysis tubing segments cm string segments 1 Medicine cup, plastic 10 ml Distilled water 10 ml 0.2 M Sucrose solution 10 ml 0.4 M Sucrose solution 10 ml 0.6 M Sucrose solution 10 ml 0.8 M Sucrose solution 10 ml 1.0 M Sucrose solution 1 Marking pencil Labeling tape Calculator Paper toweling Shared Materials Balance(s) Distilled water to cover the tubing bags in cups Safety Be sure to always wear safety goggles, gloves and a lab apron to protect your eyes and clothing when working with any chemicals. Dispose of any waste materials and clean up your work area as directed by your teacher. Be sure to always wash your hands before leaving the laboratory. Step 1 Number the plastic cups 1-6 with the marking pencil and labeling tape. Step 2 Your teacher will provide you with 6 soaking dialysis tube segments. Gently rub each piece of tubing between your fingers to open it. Step 3 Tie one end of each piece of tubing tightly with a piece of string. Make sure the knot tight enough to prevent any leaks from the end of the bag. Fill the tubing with water and test it for leaks at a sink. Empty the tubing. Repeat this step for each of the 6 dialysis tubing segments. Step 4 Using the medicine cup provided, measure out 10 ml of the appropriate solution into the dialysis bags as follows: Bag to be placed in cup # Solution 1 Distilled water M Sucrose M Sucrose M Sucrose M Sucrose M Sucrose Rinse the medicine cup between solutions. Be sure to gently squeeze excess air from each bag. Tie off the other end of each dialysis bag with string. 6
7 Step 5 Briefly rinse the outside of each bag under running water. Squeeze each bag gently to be sure that there are no leaks. If you find a bag leaking at an end, retie it securely. Step 6 Dry the outside of the bag with a paper towel, and obtain the mass of each bag. Record the initial mass of each bag in Data Table 2. Step 7 Place the correct model cell into each numbered cup and fill each cup with enough distilled water to completely cover each model cell. Allow osmosis to occur for 30 minutes. Step 11 For each solution, divide the result you got in Step 9 by the initial mass of the bags and multiply by 100. This is the percent change in mass for each bag. Record your results in Data Table 2. Step 12 Calculate the class average percent change in mass of the bags in each solution, and record your results in Data Table 3. Step 13 Construct a graph using Figure 1, illustrating the percent change in mass for both your group s data and the combined class averages. Step 8 After waiting 30 minutes, remove each bag from its cup. Gently blot excess moisture from the surface of each bag with a paper towel and obtain the mass of each bag. Record the final mass of each bag in Data Table 2. Step 9 Be sure to wash your hands and clean up and dispose of any waste materials as directed by your teacher. Step 10 For each of the solutions, subtract the initial mass of the dialysis bag from the final mass of the dialysis bag to obtain the change in mass and record this information in Data Table 2. Be sure to record a positive result if the bags gained mass, and a negative result if the bags lost mass. 7
8 Analysis Data Table 2: Changes in Dialysis Bag Mass due to Osmosis Beaker with: Initial Mass of Bag (g) Final Mass of Bag (g) Change Mass of Bag (g) % Change Mass of Bag (g) Distilled Water 0.2 M Sucrose 0.4 M Sucrose 0.6 M Sucrose 0.8 M Sucrose 1.0 M Sucrose Data Table 3: Calculation of Average % Change in Dialysis Bag Mass % Change in Dialysis Bag Mass Group # Distilled Water 0.2 M Sucrose 0.4 M Sucrose 0.6 M Sucrose 0.8 M Sucrose 1.0 M Sucrose Sum: Average % Change in Mass (Sum/# of Groups) 8
9 The independent variable in a scientific experiment is the condition that the experiment deliberately varies. What condition in this experiment did you allow to vary to see if that had any effect on the experiment s results? In constructing the graph below, use the following instructions: Plot the dependent variable on the Y-axis; plot the independent variable on the X-axis. Independent variable:... The dependent variable in a scientific experiment is what the experimenter designed the experiment to measure. What variable did you measure at the completion of this experiment? Dependent variable:... % Change in Dialysis Bag Mass FIGURE 1: Percent Change in Dialysis Bag Mass in Various Sucrose Solutions After 30 Minutes Distilled Water 0.2 M Sucrose 0.4 M Sucrose 0.6 M Sucrose 0.8 M Sucrose 1.0 M Sucrose Solution 9
10 Questions 1. What is osmosis, and how does it differ from simple diffusion? 2. Did osmosis occur in each treatment? What observation led you to this conclusion? 3. You observed a change in mass of the bags over the course of the experiment. Why was it important to convert the absolute change in mass of the bags to percent change in mass of the bags? 4. Did you see any clear relationship between the molarity of the solutions in the bags and the percent change in the mass of the bag? If so, how would you describe that relationship? 5. If you had placed all 6 bags into a beaker containing 1.2 M sucrose, would your results have differed from what you observed? Why? Describe possible results for each of the bags placed in this solution. 10
11 Activity 1c Water Potential This lab exercise will allow the calculation of water potential in potato tuber tissue placed in solutions of various molarities. Objectives Observe the effects of water potential on cells placed in hypotonic and hypertonic solutions. Calculate the percent change in mass of potato cores over the course of the experiment. Predict the approximate solute concentration inside potato tuber cells. Background Water potential is a concept that describes the tendency of water to move from one area to another, particularly into or out of cells. This term is frequently used to describe the events that occur when plants are exposed to solutions that are not isotonic to their cells. Water potential is represented by ψ, the Greek letter psi. An area with a high concentration of water molecules has high water potential. An area with a low concentration of water molecules has low water potential. In other words, water will tend to move into an area where the water potential is low, from an area where water potential is high. This movement is attributable to osmosis; therefore, water potential is a measure of the degree to which osmosis occurs. Water potential is influenced by the amount of pressure on the container surrounding the solution. This component of water potential is called the pressure potential and is represented by ψ p. Water potential is also influenced by the amount of solute in the solution. This component of water potential is called the solute potential and is represented by ψ s. The equation that describes the magnitude of water potential is as follows: ψ = ψ p + ψ s (water potential) (pressure potential) (solute potential) The unit of measure used to express water potential is the SI units for pressure, either bars or megapascals. Zero bars water potential (ψ = 0) is defined to be that of distilled water at 1 atmosphere pressure. Any solute added to distilled water will cause the water potential of that solution to become negative, because the concentration of water molecules in any solution is less than that of distilled water. This would result in the movement of water into the solution, if possible. Physical pressure on the walls of the container (like the cell wall in plant cells) has the opposite effect on water potential. Physical pressure on the container walls causes the water potential for that solution to become positive. The solution in the container would tend to move from it toward a lower water potential, if possible. It is the combined effect of pressure potential and solute potential that determine the overall water potential of a cell. 11
12 Potato tuber cells can be used to demonstrate the effect of solute concentration on water potential. Remember that each plant cell is surrounded by a cell membrane and a nonliving cellulose cell wall. If we place potato tuber cells into a container of distilled water osmosis will occur. Water molecules will move down the solute potential through the selectively permeable membrane into the potato cells. This will continue until the resistance provided by the cell wall stops any further net movement of water molecules. At this point, the pressure potential (due to the rigid cell wall) and the solute potential are equal. What is your prediction about the water potential changes in potato tuber cells placed in a hypertonic solution? Safety Be sure to always wear safety goggles, gloves and a lab apron to protect your eyes and clothing when working with any chemicals. Use caution when handling sharp lab instruments. Dispose of any waste materials and clean up your work area as directed by your teacher. Be sure to always wash your hands before leaving the laboratory. Per student Apron Gloves Goggles Per group 1 #2 Cork borer (inner diameter of approximately 5mm) 1 Potato 1 Knife or scalpel 1 Centimeter ruler 6 Clear plastic cups 6 Pieces of plastic wrap or aluminum foil 1 Marking pencil Labeling tape 100 ml Distilled water 100 ml 0.2 M Sucrose solution 100 ml 0.4 M Sucrose solution 100 ml 0.6 M Sucrose solution 100 ml 0.8 M Sucrose solution 100 ml 1.0 M Sucrose solution Paper toweling Shared materials Balance What You Need Step 1 Label 6 clear plastic cups with the different solutions used in this lab. Pour 100 ml of the appropriate solution into the cups. Step 2 Push the cork borer completely through the potato. Remove the potato core from the borer. Carefully cut off each end of the core where the potato skin is. Step 3 Lay the core next to a metric ruler. Measure and cut a 3 cm long potato core section. Step 4 What to do... Punch a total of 4 potato cores, each 3 cm in length. Obtain the mass of the 4 cores together to the nearest 0.1 g. If you have to wait to use the balance, be sure to wrap the cores in a piece of plastic wrap or aluminum foil until the balance is available. 12
13 Step 5 Place these 4 cores into one of the labeled plastic cups containing a solution. Record the initial mass of the 4 cores for this solution in Data Table 4. Step 6 Repeat Steps 2-5 with each of the remaining cups. Cover all of the cups with plastic wrap or aluminum foil to keep evaporation to a minimum. Step 7 Place the cups in a location where they won t be disturbed overnight. Step 8 Remove the cores from one of the cups and carefully place them on a paper towel. Dab the cores with the paper towel to dry them. Obtain the mass of the 4 cores together. Record the final mass of the cores in Data Table 4. Step 9 Repeat Step 8 for each of the cups. Step 12 Calculate the class average percent change in mass of the cores in each solution in Data Table 5. Step 13 You will construct a graph of the percent change in mass for both your group s data and the combined class averages in Figure 2. Plot the molarity of the solutions in the beakers on the X-axis, and the % change in mass of the potato cores on the Y-axis. Step 14 Using a ruler, draw a best fit line corresponding to the points on your graph. The point at which your line crosses zero on the Y-axis is an approximation of the molar concentration of solutes inside the potato tuber cells. This point identifies the molarity of a sucrose solution that has the same water potential as that of the potato tuber cells. Step 15 Be sure to wash your hands and clean up and dispose of any waste materials as directed by your teacher. Step 10 For each of the solutions, subtract the initial mass of the cores from the final mass of the cores to obtain the change in mass. Be sure to record a positive result if the cores gained mass, and a negative result if the cores lost mass. Record these calculations in Data Table 4. Step 11 For each solution, calculate the percent change in mass for the cores as you did for the dialysis bags in the previous exercise. Record your results. 13
14 Analysis Data Table 4: Changes in Potato Core Mass Cup containing Initial Mass of Cores (g) Final Mass of Cores (g) Change Mass of Cores (g) % Change Mass of Cores (g) Distilled Water M Sucrose M Sucrose M Sucrose M Sucrose M Sucrose 2.55 Data Table 5: Calculation of Average % Change in Potato Core Mass % Change in Potato Core Mass Group # Distilled Water 0.2 M Sucrose 0.4 M Sucrose 0.6 M Sucrose 0.8 M Sucrose 1.0 M Sucrose Sum: Average % Change in Mass (Sum/# of Groups) 14
15 % Change in Potato Core Mass FIGURE 2: Percent Change in Potato Core Mass as a result of Sucrose Concentration Distilled Water 0.2 M Sucrose 0.4 M Sucrose Solution 0.6 M Sucrose 0.8 M Sucrose 1.0 M Sucrose Instructions for Calculating Water Potential of Potato Tuber Cells Both components of water potential must be considered in order to calculate water potential for the potato cells. First, calculate solute potential of the sucrose solution that has the same water potential as the potato tuber cells using this formula: Note: 1. The ionization constant for sucrose is 1.0, since sucrose is not an ionizing substance. 2. The molar concentration for the sucrose solution with the same water potential as the potato tuber cells was the molarity at the point on the graph where your best fit line crossed zero. 3. The pressure constant (R) = liter bars/mole ºK. 4. Temperature in ºK = ºC of the solution. Be sure to cancel all units of measure except that for pressure as you go through this calculation. Don t forget to include the correct unit in your statement of the value of ψ s. ψ s = -(1.0) ( moles/liter) ( liter bars/mole ºK) ( ºK) ψ s = Since the beaker containing the sucrose solution was not sealed, it is an open system. The pressure potential in an open system is zero; therefore, the pressure potential (ψ p of the sucrose solution is zero. This means that the water potential of the sucrose solution is identical to its solute potential. Further, the water potential of the potato tuber cells was equal to that of this sucrose solution. Potato tuber cell water potential =. ψ s = -icrt where: I = the ionization constant C = Molar concentration R = Pressure constant T = Temperature in ºK 15
16 Questions Notes 1. Why was it necessary to keep the potato tuber cores in a covered container until the balance was available? If you had left them out in the open, how might that have affected your results? 2. Some plants grow in salt marshes, where the concen tration of solutes in the soil water is high. What is one adaptation you might expect to find in these salt-tolerant plants with respect to the solute concentration within their cells? 3. Water is usually pulled through a plant, from roots to leaves. What happens at the leaves to cause this pull? (Hint: Think about the effect of a hot, windy day on a puddle of water.) How would you describe the water potential at the leaf versus the water potential at the root? Why? 16
17 When this happens in animal cells, it is called crenation, and can lead to the shrinkage and death of the cell. In plants, this process is called plasmolysis, and can also lead to the death of the plant cell. Activity 1d Onion Cell Plasmolysis This lab exercise demonstrates the effect of hypertonic and hypotonic solutions on plant cells. Both turgid and plasmolyzed cells will be easily observed. Vacuole Cell Wall Objectives Prepare a wet mount slide of onion epidermal cells. Observe the effects of hypertonic and hypotonic solutions on a plant cell. Predict the conditions under which plasmolysis and turgidity would occur in living plants. Background In a hypertonic environment, water leaves the cell through osmosis and the cell shrinks away from the cell wall. This condition is called plasmolysis. The opposite process, also involving osmosis, can occur if the intracellular fluid (i.e. cytosol) becomes more concentrated than the extracellular fluid. In this case, the concentration of water is greater outside the cell, and once again, water moves across the membrane down its concentration gradient. The extracellular fluid is now hypotonic to the intracellular fluid. The movement of water into the cell can lead to the rupture of the cell membrane in animal cells, a process called lysis. In plant cells, this pressure within the cell pushes the plant cell membrane tightly against the cell wall, leading to a firm plant cell. This pressure is called turgor pressure, and the plant cell is said to be turgid. When a solution becomes more concentrated on one side of a selectively permeable membrane than on the other, osmosis will occur. In living cells, when the extracellular fluid solutes become more concentrated than the solutes in the cytosol, water will leave the cell by osmosis. In this case, the solution surrounding the cell is hypertonic to the cytosol, meaning that it has a greater concentration of solutes than does the cytosol. 17
18 What You Need What to do... Per student Apron Gloves Goggles Per group 1 Microscope slide 1 Coverslip 1 Forceps 1 Pipet Onion leaves 15% NaCl solution in beaker Distilled water in beaker Paper toweling Shared materials Compound light microscope Safety Be sure to always wear safety goggles, gloves and a lab apron to protect your eyes and clothing when working with any chemicals. Step 1 Using the forceps, remove a small piece of onion epidermis ( onion skin ) from an onion leaf. Make a wet mount slide of the epidermis and view at 100X magnification. Sketch a few of the onion epidermis cells in the analysis section. Step 2 Without removing the slide from the microscope stage, place 2-4 drops of the NaCl solution at the left edge of the coverslip. Step 3 While viewing the onion epidermis through the microscope, hold a piece of paper toweling at the right edge of the coverslip, touching the fluid on the slide. This will wick the fluid from the left side of the slide to the right. Note any changes that occur in the cells during this process. Step 4 Now repeat Steps 2-3 using distilled water instead of NaCl solution. Note any changes that occur in the cells during this process. Dispose of any waste materials and clean up your work area as directed by your teacher. Be sure to always wash your hands before leaving the laboratory. 18
19 Analysis 3. Sketch of onion epidermal cells after flooding with distilled water (100X): 1. Sketch of onion epidermal cells, prior to addition of NaCl solution (100X): 4. Describe the changes that took place in the onion epidermal cells after the NaCl solution surrounded them. 2. Sketch of onion epidermal cells after adding NaCl solution (100X): 5. Describe the changes that took place in the onion epidermal cells after they were flooded with distilled water. 19
20 Questions Learn and Read More About It 1. Circle the hypertonic solution in each of the pairs of solutions below: Onion epidermal cells Onion epidermal cells 15% NaCl solution Distilled water 2. Why does a plant lose a wilted appearance after it has been watered well? 3. Why would spraying a brine solution or applying road salt to a slick road kill early spring plants growing near the roadside? 4. Animal cells can lyse if placed in a hypotonic solution. Why don t plant cells lyse when placed in a hypotonic solution? Benjamin, Clinton; Garman, Gregory; and Funston, James. Human Biology. McGraw-Hill Allan J. Tobin and Richard E. Morel. Asking About Cells. HBJ School Div Andres Llamas Ruiz and Luis Rizo. The Life of a Cell (Cycles of Life). Sterling Publications Neat Websites Information of biology related topics in easy to understand format for students html html branes/transport.html Provides a thorough overview of cell structure and function Provides background information and suggested activities to learn more about cells html Provides a wide selection on various types of cells and related information 20
LAB 04 Diffusion and Osmosis
 LAB 04 Diffusion and Osmosis Objectives: Describe the physical mechanisms of diffusion and osmosis. Understand the relationship between surface area and rate of diffusion. Describe how molar concentration
LAB 04 Diffusion and Osmosis Objectives: Describe the physical mechanisms of diffusion and osmosis. Understand the relationship between surface area and rate of diffusion. Describe how molar concentration
Diffusion and Osmosis
 Diffusion and Osmosis Introduction: In this exercise you will measure diffusion of small molecules through dialysis tubing, an example of a semi permeable membrane. The movement of a solute through a semi
Diffusion and Osmosis Introduction: In this exercise you will measure diffusion of small molecules through dialysis tubing, an example of a semi permeable membrane. The movement of a solute through a semi
Name Date. In this lab investigation you will investigate the movement of water through a selectively permeable membrane.
 This lab will be hand-written in your data book AP Osmosis Labs Part A (was done in previous a previous class: Dialysis tube + Starch + Glucose) Part B: Osmosis Unknowns In this lab investigation you will
This lab will be hand-written in your data book AP Osmosis Labs Part A (was done in previous a previous class: Dialysis tube + Starch + Glucose) Part B: Osmosis Unknowns In this lab investigation you will
Name: Bio A.P. Lab Diffusion & Osmosis
 Name: Bio A.P. Lab Diffusion & Osmosis BACKGROUND: Many aspects of the life of a cell depend on the fact that atoms and molecules are constantly in motion (kinetic energy). This kinetic energy results
Name: Bio A.P. Lab Diffusion & Osmosis BACKGROUND: Many aspects of the life of a cell depend on the fact that atoms and molecules are constantly in motion (kinetic energy). This kinetic energy results
Osmosis and Diffusion: How biological membranes are important This page is a lab preparation guide for instructors.
 Osmosis and Diffusion: How biological membranes are important This page is a lab preparation guide for instructors. **All solutions and dialysis bags can easily be prepared prior to lab start to maximize
Osmosis and Diffusion: How biological membranes are important This page is a lab preparation guide for instructors. **All solutions and dialysis bags can easily be prepared prior to lab start to maximize
DIFFUSON AND OSMOSIS INTRODUCTION diffusion concentration gradient. net osmosis water potential active transport
 DIFFUSON AND OSMOSIS NAME DATE INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. Raw materials such as oxygen and sugars needed
DIFFUSON AND OSMOSIS NAME DATE INTRODUCTION The life of a cell is dependent on efficiently moving material into and out of the cell across the cell membrane. Raw materials such as oxygen and sugars needed
Chapter MEMBRANE TRANSPORT
 Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
Chapter 3 I MEMBRANE TRANSPORT The cell membrane, or plasma membrane, is the outermost layer of the cell. It completely surrounds the protoplasm or living portion of the cell, separating the cell s interior
AP Biology Lab 1c Water Potential
 Page 1 of 9 AP Biology Lab 1c Water Potential In this part of the exercise you will use potato cores placed in different molar concentrations of sucrose in order to determine the water potential of potato
Page 1 of 9 AP Biology Lab 1c Water Potential In this part of the exercise you will use potato cores placed in different molar concentrations of sucrose in order to determine the water potential of potato
Big. Cellular Processes: Idea. Energy and Communication DIFFUSION AND OSMOSIS. What causes my plants to wilt if I forget to water them?
 Big Cellular Processes: Idea 2 Energy and Communication INVESTIGATION 4 DIFFUSION AND OSMOSIS What causes my plants to wilt if I forget to water them? BACKGROUND Cells must move materials through membranes
Big Cellular Processes: Idea 2 Energy and Communication INVESTIGATION 4 DIFFUSION AND OSMOSIS What causes my plants to wilt if I forget to water them? BACKGROUND Cells must move materials through membranes
To understand osmosis, we must focus on the behavior of the solvent, not the solute.
 GCC CHM 130LL Osmosis and Dialysis Purpose: The purpose of this experiment is to observe the closely related phenomena of osmosis and diffusion as it relates to dialysis. It is hoped that you will be able
GCC CHM 130LL Osmosis and Dialysis Purpose: The purpose of this experiment is to observe the closely related phenomena of osmosis and diffusion as it relates to dialysis. It is hoped that you will be able
INVESTIGATION : Determining Osmolarity of Plant Tissue
 INVESTIGATION : Determining Osmolarity of Plant Tissue AP Biology This lab investigation has two main components. In the first component, you will learn about the osmolarity of plant tissues and the property
INVESTIGATION : Determining Osmolarity of Plant Tissue AP Biology This lab investigation has two main components. In the first component, you will learn about the osmolarity of plant tissues and the property
Investigation 4: Diffusion and Osmosis Notes From the teacher
 Day 1: Investigation 4: Diffusion and Osmosis Notes From the teacher Before class: Read Learning Objectives through Procedure 1 and complete Day 1 Pre Lab. Pre-Lab: 1. What is diffusion? 2. What is kinetic
Day 1: Investigation 4: Diffusion and Osmosis Notes From the teacher Before class: Read Learning Objectives through Procedure 1 and complete Day 1 Pre Lab. Pre-Lab: 1. What is diffusion? 2. What is kinetic
Cell Diffusion and Osmosis Lab: Directions
 Cell Diffusion and Osmosis Lab: Directions Adapted from AP bio lab 4 http://media.collegeboard.com/digitalservices/pdf/ap/bio-manual/bio_lab4-diffusionandosmosis.pdf Please return Background: Most cells
Cell Diffusion and Osmosis Lab: Directions Adapted from AP bio lab 4 http://media.collegeboard.com/digitalservices/pdf/ap/bio-manual/bio_lab4-diffusionandosmosis.pdf Please return Background: Most cells
Principles & Practice of Diffusion & Osmosis. Storage: Store entire experiment at room temperature. EXPERIMENT OBJECTIVE
 The Biotechnology Education Company Storage: Store entire experiment at room temperature. 281 EDVO-Kit # Principles & Practice of Diffusion & Osmosis EXPERIMENT OBJECTIVE The objective of this experiment
The Biotechnology Education Company Storage: Store entire experiment at room temperature. 281 EDVO-Kit # Principles & Practice of Diffusion & Osmosis EXPERIMENT OBJECTIVE The objective of this experiment
DIFFUSION AND OSMOSIS
 Lab 5 DIFFUSION AND OSMOSIS OBJECTIVES Describe the process of diffusion at the molecular level; State the physical factors that determine the direction and rate of diffusion; Discuss why diffusion rates,
Lab 5 DIFFUSION AND OSMOSIS OBJECTIVES Describe the process of diffusion at the molecular level; State the physical factors that determine the direction and rate of diffusion; Discuss why diffusion rates,
BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION. READING: Please read pages & in your text prior to lab.
 BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION READING: Please read pages 27-31 & 83-86 in your text prior to lab. INTRODUCTION: All living things depend on water. A water molecule is made up of an oxygen atom
BIOLOGY 1101 LAB 1: OSMOSIS & DIFFUSION READING: Please read pages 27-31 & 83-86 in your text prior to lab. INTRODUCTION: All living things depend on water. A water molecule is made up of an oxygen atom
Osmosis. Computer OBJECTIVES
 Osmosis Computer 22 In order to survive, all organisms need to move molecules in and out of their cells. Molecules such as gases (e.g., O 2, CO 2 ), water, food, and wastes pass across the cell membrane.
Osmosis Computer 22 In order to survive, all organisms need to move molecules in and out of their cells. Molecules such as gases (e.g., O 2, CO 2 ), water, food, and wastes pass across the cell membrane.
Lab #6: Cellular Transport Mechanisms Lab
 Lab #6: Cellular Transport Mechanisms Lab OVERVIEW One of the major functions of the plasma membrane is to regulate the movement of substances into and out of the cell. This process is essential in maintaining
Lab #6: Cellular Transport Mechanisms Lab OVERVIEW One of the major functions of the plasma membrane is to regulate the movement of substances into and out of the cell. This process is essential in maintaining
Cellular Transport Worksheet
 Cellular Transport Worksheet Name Section A: Cell Membrane Structure 1. Label the cell membrane diagram. You ll need to draw lines to some of the structures. **Draw cholesterol molecules in the membrane.**
Cellular Transport Worksheet Name Section A: Cell Membrane Structure 1. Label the cell membrane diagram. You ll need to draw lines to some of the structures. **Draw cholesterol molecules in the membrane.**
Osmosis. Evaluation copy
 Osmosis Computer 1B In order to survive, all organisms need to move molecules in and out of their cells. Molecules such as gases (e.g., O 2, CO 2 ), water, food, and wastes pass across the cell membrane.
Osmosis Computer 1B In order to survive, all organisms need to move molecules in and out of their cells. Molecules such as gases (e.g., O 2, CO 2 ), water, food, and wastes pass across the cell membrane.
EXERCISE Transport Mechanisms in the Body
 EXERCISE Transport Mechanisms in the Body 2 OBJECTIVES After completing these activities, you should be able to: Understand the differences between passive and active processes of transport Define diffusion,
EXERCISE Transport Mechanisms in the Body 2 OBJECTIVES After completing these activities, you should be able to: Understand the differences between passive and active processes of transport Define diffusion,
LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE
 LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE NAME: PERIOD: DATE: Building Background Knowledge: 1) SELECTIVELY PERMEABLE MEMBRANE: Every cell is surrounded by a selectively permeable membrane
LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE NAME: PERIOD: DATE: Building Background Knowledge: 1) SELECTIVELY PERMEABLE MEMBRANE: Every cell is surrounded by a selectively permeable membrane
LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE
 LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE NAME: PERIOD: DATE: Building Background Knowledge: 1) SELECTIVELY PERMEABLE MEMBRANE: Every cell is surrounded by a selectively permeable membrane
LAB: DIFFUSION ACROSS A SELECTIVELY PERMEABLE MEMBRANE NAME: PERIOD: DATE: Building Background Knowledge: 1) SELECTIVELY PERMEABLE MEMBRANE: Every cell is surrounded by a selectively permeable membrane
Diffusion and Osmosis
 Diffusion and Osmosis OBJECTIVES: 1. To explore how different molecules move by diffusion and osmosis through semi-permeable membranes. 2. To understand how concentration affects the movement of substances
Diffusion and Osmosis OBJECTIVES: 1. To explore how different molecules move by diffusion and osmosis through semi-permeable membranes. 2. To understand how concentration affects the movement of substances
Diffusion across a Selectively Permeable Membrane
 Diffusion across a Selectively Permeable Membrane Each cell is surrounded by a selectively permeable cell membrane Cell Membrane which regulates what gets into and out of the cell. A selectively permeable
Diffusion across a Selectively Permeable Membrane Each cell is surrounded by a selectively permeable cell membrane Cell Membrane which regulates what gets into and out of the cell. A selectively permeable
Cell Diffusion & Permeability: See-Through Eggs Student Advanced Version
 Cell Diffusion & Permeability: See-Through Eggs Student Advanced Version In this lab, students will learn about the permeability of the cell membrane. By studying the ability of a shell-less egg to absorb
Cell Diffusion & Permeability: See-Through Eggs Student Advanced Version In this lab, students will learn about the permeability of the cell membrane. By studying the ability of a shell-less egg to absorb
Diffusion and Osmosis
 Diffusion and Osmosis During your first year of residency at Mountainside Hospital, you are treating a group of patients that exhibit signs of dehydration. You have to be sure to take note of all the solutes
Diffusion and Osmosis During your first year of residency at Mountainside Hospital, you are treating a group of patients that exhibit signs of dehydration. You have to be sure to take note of all the solutes
Cell Diffusion & Permeability: See-Through Eggs Teacher Version
 Cell Diffusion & Permeability: See-Through Eggs Teacher Version In this lab, students will learn about the permeability of the cell membrane. By studying the ability of a shell-less egg to absorb various
Cell Diffusion & Permeability: See-Through Eggs Teacher Version In this lab, students will learn about the permeability of the cell membrane. By studying the ability of a shell-less egg to absorb various
Biology Movement across the Cell Membrane
 Biology 160 - Movement across the Cell Membrane Prelab Information Movement is one of the characteristics of life. The ability to control the movement of material across the cell membrane is an incredibly
Biology 160 - Movement across the Cell Membrane Prelab Information Movement is one of the characteristics of life. The ability to control the movement of material across the cell membrane is an incredibly
1. All cells have a that acts as a between the outside and inside of the cell.
 https://www.youtube.com/watch?v=cnbzdcibegy 1. All cells have a that acts as a between the outside and inside of the cell. 1 2. Cell membranes are primarily made of which are large molecules. 3. It is
https://www.youtube.com/watch?v=cnbzdcibegy 1. All cells have a that acts as a between the outside and inside of the cell. 1 2. Cell membranes are primarily made of which are large molecules. 3. It is
Experimental Design and Investigating Diffusion and Osmosis
 Bio 101 Name: Experimental Design and Investigating Diffusion and Osmosis OBJECTIVES: To practice applying hypothesis testing. To further your understanding of experimental design. To gain a better understanding
Bio 101 Name: Experimental Design and Investigating Diffusion and Osmosis OBJECTIVES: To practice applying hypothesis testing. To further your understanding of experimental design. To gain a better understanding
Lab 4: Osmosis and Diffusion
 Page 4.1 Lab 4: Osmosis and Diffusion Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Page 4.1 Lab 4: Osmosis and Diffusion Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Measuring Osmotic Potential
 Measuring Osmotic Potential INTRODUCTION All cells require essential materials to ensure their survival. Chemical, physical, and biological processes are used to move these materials inside of cells. Similar
Measuring Osmotic Potential INTRODUCTION All cells require essential materials to ensure their survival. Chemical, physical, and biological processes are used to move these materials inside of cells. Similar
Investigating Osmosis By Amy Dewees,Jenkintown.High School and Dr. Ingrid Waldron, Department of Biology, University of Pennsylvania, 20091
 Investigating Osmosis By Amy Dewees,Jenkintown.High School and Dr. Ingrid Waldron, Department of Biology, University of Pennsylvania, 20091 What is diffusion? What does it mean to say that a membrane is
Investigating Osmosis By Amy Dewees,Jenkintown.High School and Dr. Ingrid Waldron, Department of Biology, University of Pennsylvania, 20091 What is diffusion? What does it mean to say that a membrane is
Biology Movement Across the Cell Membrane
 Biology 160 - Movement Across the Cell Membrane Prelab Information Movement is one of the characteristics of life. The ability to control the movement of material across the cell membrane is an incredibly
Biology 160 - Movement Across the Cell Membrane Prelab Information Movement is one of the characteristics of life. The ability to control the movement of material across the cell membrane is an incredibly
Cell Membranes: Diffusion and Osmosis
 STO-112 Cell Membranes: Diffusion and Osmosis Part 1: Diffusion Diffusion is a process by which molecules move into or out of cells. To diffuse into or out of a cell, molecules must pass through the cell
STO-112 Cell Membranes: Diffusion and Osmosis Part 1: Diffusion Diffusion is a process by which molecules move into or out of cells. To diffuse into or out of a cell, molecules must pass through the cell
Name: Date Block Selective Permeability
 LAB Name: Date Block Selective Permeability OBJECTIVES: Observe the selective permeability of an artificial membrane. Observe diffusion of substances across an artificial membrane. Devise a model for the
LAB Name: Date Block Selective Permeability OBJECTIVES: Observe the selective permeability of an artificial membrane. Observe diffusion of substances across an artificial membrane. Devise a model for the
AP Lab Four: Water Potential and Osmosis
 AP Biology AP Lab Four: Water Potential and Osmosis Name Atoms and molecules are constantly in motion, bumping off of membranes, barriers, each other, without end. The results of this among other phenomena
AP Biology AP Lab Four: Water Potential and Osmosis Name Atoms and molecules are constantly in motion, bumping off of membranes, barriers, each other, without end. The results of this among other phenomena
Research Experiences for Teachers (RET) 2012 LESSON PLAN TEMPLATE
 LESSON PLAN TEMPLATE MODULE TOPIC: Inquiry based learning- Osmosis and Diffusion The acquisition of biochemical and life sustaining compounds is a major theme in life science. This lesson provides students
LESSON PLAN TEMPLATE MODULE TOPIC: Inquiry based learning- Osmosis and Diffusion The acquisition of biochemical and life sustaining compounds is a major theme in life science. This lesson provides students
LAB Potato Cores Honors Biology, Newton North High
 Name Date Block LAB Potato Cores Honors Biology, Newton North High BACKGROUND: Osmosis is a type of passive transport. No input of energy is needed in order for water to pass through a selectively permeable
Name Date Block LAB Potato Cores Honors Biology, Newton North High BACKGROUND: Osmosis is a type of passive transport. No input of energy is needed in order for water to pass through a selectively permeable
Name: There are two things that will determine which particles will pass through and which will not:
 18 Diffusion and Osmosis in Living Systems Name: Problem: How do substances move into and out of cells? Introduction: In order for cells to carry on their life processes, they must take in materials and
18 Diffusion and Osmosis in Living Systems Name: Problem: How do substances move into and out of cells? Introduction: In order for cells to carry on their life processes, they must take in materials and
Distilled Water Balance Ruler Plastic wrap
 The following lab taken from: http://www.utsouthwestern.edu/edumedia/edufiles/education_training/programs/stars/osmosis-demo-lab.pdf Background Osmosis is the process whereby water moves across a cell
The following lab taken from: http://www.utsouthwestern.edu/edumedia/edufiles/education_training/programs/stars/osmosis-demo-lab.pdf Background Osmosis is the process whereby water moves across a cell
STATION 4: TONICITY due to OSMOSIS / Turgor Pressure in Plants
 STATION 4: TONICITY due to OSMOSIS / Turgor Pressure in Plants Tonicity is the concentration of solutions that determines the direction water will move across a semi-permeable membrane. A solution is a
STATION 4: TONICITY due to OSMOSIS / Turgor Pressure in Plants Tonicity is the concentration of solutions that determines the direction water will move across a semi-permeable membrane. A solution is a
Introduction diffusion osmosis. imbibe Diffusion The Cell Membrane and Osmosis selectively permeable membrane Osmosis 1. Isotonic 2.
 Topic 6. Diffusion Introduction: This exercise explores the physical phenomenon of diffusion and osmosis. Osmosis is simply the diffusion of water through a selectively permeable membrane. We will also
Topic 6. Diffusion Introduction: This exercise explores the physical phenomenon of diffusion and osmosis. Osmosis is simply the diffusion of water through a selectively permeable membrane. We will also
Table of Contents Title Page Number Due Date Stamp
 1 Table of Contents Title Page Number Due Date Stamp Calendar 3 Warm - Ups 4 Carbon Based Molecules 5 02/20/2018 Notes Cell Membrane Notes 8 02/20/2018 Membrane Structure and Cell Signaling Worksheet Diffusion
1 Table of Contents Title Page Number Due Date Stamp Calendar 3 Warm - Ups 4 Carbon Based Molecules 5 02/20/2018 Notes Cell Membrane Notes 8 02/20/2018 Membrane Structure and Cell Signaling Worksheet Diffusion
BIO 322/122L Laboratory Plant Water Relations
 BIO 322/122L Laboratory Plant Water Relations I. Water Potential. The cytoplasm of the plant cell, with its enclosed vacuole, is contained within a membrane that is more permeable to water than to most
BIO 322/122L Laboratory Plant Water Relations I. Water Potential. The cytoplasm of the plant cell, with its enclosed vacuole, is contained within a membrane that is more permeable to water than to most
LAB 4: OSMOSIS AND DIFFUSION
 Page 4.1 LAB 4: OSMOSIS AND DIFFUSION Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Page 4.1 LAB 4: OSMOSIS AND DIFFUSION Cells need to obtain water and other particles from the fluids that surround them. Water and other particles also move out of cells. Osmosis (for water) and diffusion
Movement of substances across the cell membrane
 Ch 4 Movement of substances across the cell membrane Think about (Ch 4, p.2) 1. The structure of the cell membrane can be explained by the fluid mosaic model. It describes that the cell membrane is mainly
Ch 4 Movement of substances across the cell membrane Think about (Ch 4, p.2) 1. The structure of the cell membrane can be explained by the fluid mosaic model. It describes that the cell membrane is mainly
Quotes from Next Generation Science Standards, available at
 Teacher Preparation Notes for Diffusion across a Selectively Permeable Membrane Drs. Jennifer Doherty and Ingrid Waldron, Department of Biology, University of Pennsylvania, 2015 1 Students investigate
Teacher Preparation Notes for Diffusion across a Selectively Permeable Membrane Drs. Jennifer Doherty and Ingrid Waldron, Department of Biology, University of Pennsylvania, 2015 1 Students investigate
INTERNATIONAL TURKISH HOPE SCHOOL ACADEMIC YEAR CHITTAGONG SENIOR SECTION BIOLOGY HANDOUT OSMOSIS, DIFFUSION AND ACTIVE TRANSPORT CLASS 9
 INTERNATIONAL TURKISH HOPE SCHOOL 2014 2015 ACADEMIC YEAR CHITTAGONG SENIOR SECTION BIOLOGY HANDOUT OSMOSIS, DIFFUSION AND ACTIVE TRANSPORT CLASS 9 Name :... Date:... d) Movement of substances into and
INTERNATIONAL TURKISH HOPE SCHOOL 2014 2015 ACADEMIC YEAR CHITTAGONG SENIOR SECTION BIOLOGY HANDOUT OSMOSIS, DIFFUSION AND ACTIVE TRANSPORT CLASS 9 Name :... Date:... d) Movement of substances into and
Passive Transport Lab: Diffusion and Osmosis
 Name Date Period Passive Transport Lab: Diffusion and Osmosis OBJECTIVE: Apply your understanding of the processes of diffusion and osmosis to explain observational data. PART A: Starch and Iodine MATERIALS
Name Date Period Passive Transport Lab: Diffusion and Osmosis OBJECTIVE: Apply your understanding of the processes of diffusion and osmosis to explain observational data. PART A: Starch and Iodine MATERIALS
Passive Cellular Transport. Unit 2 Lesson 4
 Unit 2 Lesson 4 Students will be able to: Define passive transport Enumerate the three types of passive transport Described each type of passive transport: osmosis, diffusion, and facilitated diffusion
Unit 2 Lesson 4 Students will be able to: Define passive transport Enumerate the three types of passive transport Described each type of passive transport: osmosis, diffusion, and facilitated diffusion
Bio10 Lab 2: Cells. Using your text and the cell models and posters in the lab, sketch an animal cell and a plant cell on the group results sheet.
 Bio10 Lab 2: Cells Cells are the smallest living things and all living things are composed of cells. They are able to perform all necessary metabolic functions as well as specialized tasks such as moving,
Bio10 Lab 2: Cells Cells are the smallest living things and all living things are composed of cells. They are able to perform all necessary metabolic functions as well as specialized tasks such as moving,
Passive Transport. Does not expend cellular energy for the movement to take place. Ex-rolling down a hill
 Passive Transport Fluid Mosaic Model Passive Transport Does not expend cellular energy for the movement to take place Ex-rolling down a hill Parts of a Solution Solute: what gets dissolved Solvent: What
Passive Transport Fluid Mosaic Model Passive Transport Does not expend cellular energy for the movement to take place Ex-rolling down a hill Parts of a Solution Solute: what gets dissolved Solvent: What
1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4
 Topic 3: Movement of substances across cell membrane 1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4 Directions: Questions 2 and 3 refer to
Topic 3: Movement of substances across cell membrane 1. How many fatty acid molecules combine with a glycerol to form a phospholipid molecule? A. 1 B. 2 C. 3 D. 4 Directions: Questions 2 and 3 refer to
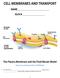
CELL BOUNDARIES. Cells create boundaries through: Cell Membranes made of the phospholipid bilayer Cell Walls made of cellulose in plants
 CELL BOUNDARIES CELL BOUNDARIES Cells create boundaries through: Cell Membranes made of the phospholipid bilayer Cell Walls made of cellulose in plants TYPES OF MEMBRANES Some substances = too large or
CELL BOUNDARIES CELL BOUNDARIES Cells create boundaries through: Cell Membranes made of the phospholipid bilayer Cell Walls made of cellulose in plants TYPES OF MEMBRANES Some substances = too large or
CELL TRANSPORT and THE PLASMA MEMBRANE. SB1d. Explain the impact of water on life processes (i.e., osmosis, diffusion).
 CELL TRANSPORT and THE PLASMA MEMBRANE SB1d. Explain the impact of water on life processes (i.e., osmosis, diffusion). What if What would happen if an organism could not get energy or get rid of wastes?
CELL TRANSPORT and THE PLASMA MEMBRANE SB1d. Explain the impact of water on life processes (i.e., osmosis, diffusion). What if What would happen if an organism could not get energy or get rid of wastes?
Chapter 3: Exchanging Materials with the Environment. Cellular Transport Transport across the Membrane
 Chapter 3: Exchanging Materials with the Environment Cellular Transport Transport across the Membrane Transport? Cells need things water, oxygen, balance of ions, nutrients (amino acids, sugars..building
Chapter 3: Exchanging Materials with the Environment Cellular Transport Transport across the Membrane Transport? Cells need things water, oxygen, balance of ions, nutrients (amino acids, sugars..building
Plasma Membrane Function
 Plasma Membrane Function Cells have to maintain homeostasis, they do this by controlling what moves across their membranes Structure Double Layer of phospholipids Head (polar) hydrophiliclikes water -
Plasma Membrane Function Cells have to maintain homeostasis, they do this by controlling what moves across their membranes Structure Double Layer of phospholipids Head (polar) hydrophiliclikes water -
Identification of Organic Compounds Lab
 Identification of Organic Compounds Lab Introduction All organic compounds contain the element carbon (C). Organic compounds usually also contain oxygen (O) or hydrogen (H) or both. They may also contain
Identification of Organic Compounds Lab Introduction All organic compounds contain the element carbon (C). Organic compounds usually also contain oxygen (O) or hydrogen (H) or both. They may also contain
Diffusion & Osmosis - Exercise 4
 Diffusion & Osmosis - Exercise 4 Objectives -Define: Solvent, Solute, and Solution -Define: Diffusion, Selectively permeable membrane, Osmosis, and Dialysis -Understand rule of thumb: Concentration will
Diffusion & Osmosis - Exercise 4 Objectives -Define: Solvent, Solute, and Solution -Define: Diffusion, Selectively permeable membrane, Osmosis, and Dialysis -Understand rule of thumb: Concentration will
[S] [S] Hypertonic [H O] [H 2 O] g. Osmosis is the diffusion of water through membranes! 15. Osmosis. Concentrated sugar solution
![[S] [S] Hypertonic [H O] [H 2 O] g. Osmosis is the diffusion of water through membranes! 15. Osmosis. Concentrated sugar solution [S] [S] Hypertonic [H O] [H 2 O] g. Osmosis is the diffusion of water through membranes! 15. Osmosis. Concentrated sugar solution](/thumbs/88/115710711.jpg) Concentrated sugar solution Sugar molecules (Water molecules not shown) 100ml 100ml Hypertonic [S] g [H2 Hypotonic [H O] 2 O] [H 2 O] g Semipermeable Dilute sugar solution (100ml) Time 125ml Osmosis 75ml
Concentrated sugar solution Sugar molecules (Water molecules not shown) 100ml 100ml Hypertonic [S] g [H2 Hypotonic [H O] 2 O] [H 2 O] g Semipermeable Dilute sugar solution (100ml) Time 125ml Osmosis 75ml
Science Biology Unit 04 Exemplar Lesson 02: Homeostasis and Membrane Transport
 Science Unit 04 Exemplar Lesson 02: Homeostasis and Membrane Transport Science Unit: 04 Lesson: 02 Suggested Duration: 7 days This lesson is one approach to teaching the State Standards associated with
Science Unit 04 Exemplar Lesson 02: Homeostasis and Membrane Transport Science Unit: 04 Lesson: 02 Suggested Duration: 7 days This lesson is one approach to teaching the State Standards associated with
8.8b Osmosis Project. Grade 8 Activity Plan
 8.8b Osmosis Project Grade 8 Activity Plan Reviews and Updates 2 8.8b Osmosis Project Objectives: 1. To demonstrate osmosis and the permeability of the cell membrane. 2. Use plant cells to demonstrate
8.8b Osmosis Project Grade 8 Activity Plan Reviews and Updates 2 8.8b Osmosis Project Objectives: 1. To demonstrate osmosis and the permeability of the cell membrane. 2. Use plant cells to demonstrate
Cells & Transport. Chapter 7.1, 7.2, & 7.4
 Cells & Transport Chapter 7.1, 7.2, & 7.4 Do Now How big is a cell? How many cells are we made of? How many cells is the smallest living organism made of? Objectives Describe how cells were discovered
Cells & Transport Chapter 7.1, 7.2, & 7.4 Do Now How big is a cell? How many cells are we made of? How many cells is the smallest living organism made of? Objectives Describe how cells were discovered
LAB #3 - DIFFUSION AND OSMOSIS
 DIFFUSION EXPERIMENT - pg. 4-6 LAB #3 - DIFFUSION AND OSMOSIS Definition of DIFFUSION - The natural tendency of particles to move from areas of high concentration to areas of lower concentration START
DIFFUSION EXPERIMENT - pg. 4-6 LAB #3 - DIFFUSION AND OSMOSIS Definition of DIFFUSION - The natural tendency of particles to move from areas of high concentration to areas of lower concentration START
Constant Motion of Molecules. Kinetic Theory of Matter Molecules move randomly and bump into each other and other barriers
 CELL TRANSPORT Constant Motion of Molecules Kinetic Theory of Matter Molecules move randomly and bump into each other and other barriers Solution homogenous liquid throughout which two or more substances
CELL TRANSPORT Constant Motion of Molecules Kinetic Theory of Matter Molecules move randomly and bump into each other and other barriers Solution homogenous liquid throughout which two or more substances
Chapter 5 Homeostasis and Cell Transport
 Chapter 5 Homeostasis and Cell Transport Palabra Palooza! Role #1: The Definer says: The word can be explained as Role #2: The Re-stater says: Then I understand (word) to mean Words: Passive transport
Chapter 5 Homeostasis and Cell Transport Palabra Palooza! Role #1: The Definer says: The word can be explained as Role #2: The Re-stater says: Then I understand (word) to mean Words: Passive transport
Diffusion, Osmosis and Active Transport
 Diffusion, Osmosis and Active Transport Part A: Diffusion A living cell interacts constantly with the environmental medium that surrounds it. The plasma membrane surrounding a cell is a living, selectively
Diffusion, Osmosis and Active Transport Part A: Diffusion A living cell interacts constantly with the environmental medium that surrounds it. The plasma membrane surrounding a cell is a living, selectively
FIGURE A. The phosphate end of the molecule is polar (charged) and hydrophilic (attracted to water).
 PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
The Plasma Membrane. 5.1 The Nature of the Plasma Membrane. Phospholipid Bilayer. The Plasma Membrane
 5.1 The Nature of the Plasma Membrane The Plasma Membrane Four principal components in animals Phospholipid bilayer Molecules of cholesterol interspersed within the bilayer. Membrane proteins embedded
5.1 The Nature of the Plasma Membrane The Plasma Membrane Four principal components in animals Phospholipid bilayer Molecules of cholesterol interspersed within the bilayer. Membrane proteins embedded
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes
 BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes
 BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
Chapter 3.4 & 3.5 Cell Transport (Osmosis and Diffusion) = only some molecules can get in or out of the cell
 Chapter 3.4 & 3.5 Cell Transport (Osmosis and Diffusion) I. Cell Membrane (cells need an inside and outside) a. separate cell from its environment b. cell membrane is the boundary c. cell membrane controls
Chapter 3.4 & 3.5 Cell Transport (Osmosis and Diffusion) I. Cell Membrane (cells need an inside and outside) a. separate cell from its environment b. cell membrane is the boundary c. cell membrane controls
BIO 12 UNIT 04: The Cell Membrane BCLN Rev. July, 2015
 Project 1: Osmosis Lab Name: Potential Credits: /50 Project Goals: to gain and demonstrate a better understanding of osmosis Instructions Please read through the Unit 4 Lessons, paying particular attention
Project 1: Osmosis Lab Name: Potential Credits: /50 Project Goals: to gain and demonstrate a better understanding of osmosis Instructions Please read through the Unit 4 Lessons, paying particular attention
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes
 BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
BIOLOGY 12 - Cell Membrane and Cell Wall Function: Chapter Notes The cell membrane is the gateway into the cell, and must allow needed things such as nutrients into the cell without letting them escape.
What kind of things must pass into and out of cells?? Be careful not to go too fast.
 1. A membrane s molecular organization results in selective permeability What kind of things must pass into and out of cells?? Be careful not to go too fast. Permeability of a molecule through a membrane
1. A membrane s molecular organization results in selective permeability What kind of things must pass into and out of cells?? Be careful not to go too fast. Permeability of a molecule through a membrane
What kind of things must pass into and out of cells?? Be careful not to go too fast.
 1. A membrane s molecular organization results in selective permeability What kind of things must pass into and out of cells?? Be careful not to go too fast. Permeability of a molecule through a membrane
1. A membrane s molecular organization results in selective permeability What kind of things must pass into and out of cells?? Be careful not to go too fast. Permeability of a molecule through a membrane
Plasma Membrane Structure and Function
 Plasma Membrane Structure and Function The plasma membrane separates the internal environment of the cell from its surroundings. The plasma membrane is a phospholipid bilayer with embedded proteins. The
Plasma Membrane Structure and Function The plasma membrane separates the internal environment of the cell from its surroundings. The plasma membrane is a phospholipid bilayer with embedded proteins. The
250-mL beakers. iodine solution metric ruler. 10-mL graduated cylinders pipettes. (Read the Procedure first to answer the Questions)
 Detecting Diffusion Introduction A cell membrane is a selectively permeable barrier. Some particles can pass through the cell membrane while other particles are held back. Solutes that can move across
Detecting Diffusion Introduction A cell membrane is a selectively permeable barrier. Some particles can pass through the cell membrane while other particles are held back. Solutes that can move across
The Role of the Cell Membrane in Transport
 The Role of the Cell Membrane in Transport diffusion: the spontaneous movement of particles from an area of higher concentration to an area of lower concentration Many people, young and old, enjoy a nice
The Role of the Cell Membrane in Transport diffusion: the spontaneous movement of particles from an area of higher concentration to an area of lower concentration Many people, young and old, enjoy a nice
Phospholipids. Extracellular fluid. Polar hydrophilic heads. Nonpolar hydrophobic tails. Polar hydrophilic heads. Intracellular fluid (cytosol)
 Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Module 2C Membranes and Cell Transport All cells are surrounded by a plasma membrane. Eukaryotic cells also contain internal membranes and membrane- bound organelles. In this module, we will examine the
Passive Transport: Practice Problems PAP BIOLOGY
 Passive Transport: Practice Problems PAP BIOLOGY #1 Draw a diagram where the cell has low concentration of salt molecules and the environment it is in has a high concentration of salt molecules in a water
Passive Transport: Practice Problems PAP BIOLOGY #1 Draw a diagram where the cell has low concentration of salt molecules and the environment it is in has a high concentration of salt molecules in a water
UNIT 4 CELL BOUNDARIES AND TRANSPORT. Unit 4 test: October 16, 2018
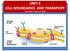 UNIT 4 CELL BOUNDARIES AND TRANSPORT Unit 4 test: October 16, 2018 Cell Wall CELL BOUNDARIES support protect & the cell cell membrane Lies outside of the Is made of & carbohydrates proteins Plant cell
UNIT 4 CELL BOUNDARIES AND TRANSPORT Unit 4 test: October 16, 2018 Cell Wall CELL BOUNDARIES support protect & the cell cell membrane Lies outside of the Is made of & carbohydrates proteins Plant cell
Observing Osmosis Lab
 Observing Osmosis Lab Background Information: Molecules are in constant motion, and tend to move from areas of higher concentrations to areas of lower concentrations. Diffusion is defined as the movement
Observing Osmosis Lab Background Information: Molecules are in constant motion, and tend to move from areas of higher concentrations to areas of lower concentrations. Diffusion is defined as the movement
Cell Membranes. Q: What components of the cell membrane are in a mosaic pattern?
 Cell Membranes The cell / plasma membrane is. Selective in that it allows things in and some things out of the cell. Recall that phospholipids have hydrophobic and hydrophilic. The term to describe this
Cell Membranes The cell / plasma membrane is. Selective in that it allows things in and some things out of the cell. Recall that phospholipids have hydrophobic and hydrophilic. The term to describe this
Maintained by plasma membrane controlling what enters & leaves the cell
 CELL TRANSPORT AND HOMEOSTASIS Homeostasis Balanced internal condition of cells Also called equilibrium Maintained by plasma membrane controlling what enters & leaves the cell Functions of Plasma Membrane
CELL TRANSPORT AND HOMEOSTASIS Homeostasis Balanced internal condition of cells Also called equilibrium Maintained by plasma membrane controlling what enters & leaves the cell Functions of Plasma Membrane
GCSE Biology Coursework Osmosis : - The Potato Experiment
 GCSE Biology Coursework Osmosis : - The Potato Experiment Background Information Osmosis can be defined as the movement of water across a semi-permeable membrane from a region of high water concentration
GCSE Biology Coursework Osmosis : - The Potato Experiment Background Information Osmosis can be defined as the movement of water across a semi-permeable membrane from a region of high water concentration
Concept 7.1: Cellular membranes are fluid mosaics of lipids and proteins
 Concept 7.1: Cellular membranes are fluid mosaics of lipids and proteins Lipids: Non-polar substances such as fat that contain C, H, O. Phospholipids: Lipid with phosphate group, very abundant in plasma
Concept 7.1: Cellular membranes are fluid mosaics of lipids and proteins Lipids: Non-polar substances such as fat that contain C, H, O. Phospholipids: Lipid with phosphate group, very abundant in plasma
BELLRINGER DAY In which types of cell is a cell membrane located? 2. What is the function of the cell membrane?
 BELLRINGER DAY 01 1. In which types of cell is a cell membrane located? 2. What is the function of the cell membrane? THE CELL MEMBRANE S T R U C T U R E A N D F U N C T I O N CELL MEMBRANE FUNCTIONS Cell
BELLRINGER DAY 01 1. In which types of cell is a cell membrane located? 2. What is the function of the cell membrane? THE CELL MEMBRANE S T R U C T U R E A N D F U N C T I O N CELL MEMBRANE FUNCTIONS Cell
Cell Transport. Movement of molecules
 Cell Transport Movement of molecules TEKS Students will investigate and explain cellular processes, including homeostasis and transport of molecules Homeostasis The maintaining of a stable body system
Cell Transport Movement of molecules TEKS Students will investigate and explain cellular processes, including homeostasis and transport of molecules Homeostasis The maintaining of a stable body system
Unit 1 Matter & Energy for Life
 Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different
Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different
The Phospholipids Between Us (Part 2) Transport through Cell Membranes
 The Phospholipids Between Us (Part 2) Transport through Cell Membranes Lesson Plan developed by Kai Orton, PhD and Apurva Naik, PhD (Northwestern University) and based on the PhET Interactive Simulation:
The Phospholipids Between Us (Part 2) Transport through Cell Membranes Lesson Plan developed by Kai Orton, PhD and Apurva Naik, PhD (Northwestern University) and based on the PhET Interactive Simulation:
Diffusion and Osmosis Lab AP LAB 4
 Diffusion and Osmosis Lab AP LAB 4 Part 1: Surface Area and Cell Size Which do you think has a greater influence on the rate of diffusion in a cell surface area or volume? You will calculate surface are-to-volume
Diffusion and Osmosis Lab AP LAB 4 Part 1: Surface Area and Cell Size Which do you think has a greater influence on the rate of diffusion in a cell surface area or volume? You will calculate surface are-to-volume
Ch. 7 Diffusion, Osmosis, and Movement across a Membrane
 Ch. 7 Diffusion, Osmosis, and Movement across a Membrane Diffusion Spontaneous movement of particles from an area of high concentration to an area of low concentration Does not require energy (exergonic)
Ch. 7 Diffusion, Osmosis, and Movement across a Membrane Diffusion Spontaneous movement of particles from an area of high concentration to an area of low concentration Does not require energy (exergonic)
The Cell Membrane. Also known as the Plasma Membrane
 Student Objectives Know the different parts of the cell membrane Understand the role of the cell membrane in cellular transport Understand diffusion and osmosis Determine what will happen to plant and
Student Objectives Know the different parts of the cell membrane Understand the role of the cell membrane in cellular transport Understand diffusion and osmosis Determine what will happen to plant and
Lab 4. Diffusion and Osmosis in Selectively Permeable Membranes
 Lab 4. Diffusion and Osmosis in Selectively Permeable Membranes Prelab Assignment Before coming to lab, read carefully the introduction and the procedures for each part of the experiment, and then answer
Lab 4. Diffusion and Osmosis in Selectively Permeable Membranes Prelab Assignment Before coming to lab, read carefully the introduction and the procedures for each part of the experiment, and then answer
Chapter 7-3 Cell Boundaries
 Chapter 7-3 Cell Boundaries The Plasma Membrane: Cell Membrane Regulates what enters and leaves the cell. Provides protection and support. Highly selective barrier!!!! What the plasma membrane is made
Chapter 7-3 Cell Boundaries The Plasma Membrane: Cell Membrane Regulates what enters and leaves the cell. Provides protection and support. Highly selective barrier!!!! What the plasma membrane is made
