Integra Forefoot Mid & HindFoot Products Catalogue. Products for sale in Europe, Middle-East and Africa only Catalogue des ventes
|
|
|
- Shana Harrison
- 6 years ago
- Views:
Transcription
1 Integra Forefoot Mid & HindFoot Products Catalogue Products for sale in Europe, Middle-East and Africa only Catalogue des ventes
2 Table of contents Table of Contents Warranty Information 5 Integra Forefoot Solutions 7 Forefoot Set 8 Bold Compression Screw dia. 2.5 mm / 3.0 mm / 3.7 mm 14 QWIX Fixation Screw dia. 3.0 mm 14 SPIN Screw dia. 2.0 mm / 2.7 mm 15 Uni-Clip Compressive Staple 16 Solustaple Standard Staple 16 Osteoguides 17 K-FIX - K-wire End Protector 17 Weil Clamp 17 B-Bop Lock plate 18 IPP-ON Interphalangeal Arthrodesis Implant 19 METIS Metatarso-phalangeal prosthesis K2 Hemi Toe Implant 22 Movement Great Toe system 23 HALLU -FIX MTP Arthrodesis System 24 HALLU Plates 24 HALLU SNAP-OFF Screws 25 HALLU -FIX Instruments 25 HALLU -LOCK Arthrodesis System 26 HALLU -REAM set 28 DPR System Minimal Invasive Foot Surgery 29 Post-Operative Shoes 2
3 Ordering and Warranty Table Information of contents Integra Mid & HindFoot Solutions 33 Subtalar Arthrodesis 34 QWIX Fixation screw 34 Large QWIX Fixation and Positioning Screws 35 I.CO.S. Ideal Compression Screw 36 Flatfoot implants 39 KALIX II Flat Foot Implant 39 MBA Titanium Subtalar Implant Dorsal Lisfranc plate & Medial Lisfranc plate 41 Advansys Lisfranc Plate 41 UNI-CP Compression Plate 43 Ankle Solutions 44 Total Ankle Replacement Hintegra prosthesis 44 Ankle Fusion 54 PANTA Nail 54 Advansys Plate 59 Tibiaxys Plating System 61 Minimally Invasive Achilles Tendon Ruptures 64 ACHILLON System 64 Surgical Instruments 65 Hintermann Distractors 65 Osteoguides 66 K-FIX Protector 66 Weil Clamp 66 General Optional Instruments 67 Post-Operatives Shoes 68 Product s 71 3
4 Table of contents 4
5 Ordering and Warranty Information Pricing: Prices stated in specific written quotations are firm for thirty days from the date given, and are otherwise subject to change without prior notice. Pricing terms stated on written agreements are governed by such agreements. Minimum Order Requirements: Minimum order requirement is 2 (a minimum order requirement charge will be added to any order under 2). Ordering Procedure: A written purchase order on the customer s form may be requested for purchases of Integra LifeSciences products. Acceptance of Orders: Orders are accepted upon approval by Integra Customer Service. Return Policy: Authorization from Customer Service must be obtained prior to returning product. Sterile product must be returned in unopened, undamaged cartons, packed to prevent damage. Non-sterile product must be returned in unused saleable condition in original package. Custom or special order products will not be accepted for credit. Credit will be issued for goods returned prior to ninety days from ship date with a % restocking charge. This assumes that the product returned is not damaged and can be verified to have not been used or opened. Integra Limited Warranty Integra products INTEGRA LIFESCIENCES CORPORATION and its wholly owned subsidiaries ( IN- TEGRA ) warrant to INTEGRA authorized distributors and the original purchaser only that each new INTEGRA Surfix product is free from manufacturing defects in material and workmanship when used for its intended surgical purpose. For purposes of products sold by INTEGRA through an authorized distributor of IN- TEGRA, original purchaser shall include the purchaser of INTEGRA products to whom the distributor first sells the product. If any covered defect occurs, the purchaser or distributor should communicate directly with INTEGRA. If purchaser or distributor seeks to invoke the terms of this warranty, the product must be returned to INTEGRA. The defective product should be returned promptly, properly packaged and postage prepaid. Loss or damage in return shipment to INTEGRA shall be at sender s risk. INTEGRA s sole responsibility under this warranty shall be repair or replacement, at INTEGRA s sole discretion at INTEGRA s expense, subject to the terms of this warranty and applicable agreements. INTEGRA provides for no commercial or additional warranty whatsoever, except as may be otherwise expressly agreed in a separate commercial agreement or undertaking duly signed by an INTEGRA authorised representative. IN NO EVENT SHALL INTEGRA BE LIABLE FOR ANY INCIDENTAL, INDIRECT, CONSEQUENTIAL OR PUNITIVE DAMAGES IN CONNECTION WITH THE AC- QUISITION OR USE OF ANY INTEGRA PRODUCT. Further, this warranty shall not apply to, and INTEGRA shall not be responsible for, any loss arising in connection with the purchase or use of any INTEGRA product that has been repaired by anyone other than an authorized INTEGRA service representative or altered in any way so as, in INTEGRA s judgment, to affect its stability or reliability, or which has been subject to misuse, negligence or accident, or which has been used otherwise than in accordance with the instructions furnished by INTEGRA. Customer Service Contact Information: International +33 (0) (0) (Fax) csemea@integralife.com Benelux +32 (0) (0) (Fax) custsvcbenelux@integralife.com France +33 (0) (0) (Fax) cs-ortho@integralife.com Switzerland +41 (0) (0) (Fax) custsvcsuisse@integralife.com United Kingdom +44 (0) (0) (Fax) custsvcs.uk@integralife.com THIS INTEGRA LIMITED WARRANTY IS EXCLUSIVE AND IN LIEU OF ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, AND OF ALL OTHER OBLIGATIONS OR LI- ABILITIES ON INTEGRA S PART OR THE PART OF ITS DISTRIBUTORS, AND INTE- GRA NEITHER ASSUMES NOR AUTHORIZES ANY REPRESENTATIVE OR OTHER PERSON TO ASSUME FOR IT ANY OTHER LIABILITY IN CONNECTION WITH INTEGRA S PRODUCTS. INTEGRA DISCLAIMS ALL OTHER WARRANTIES, EXPRESS OR IMPLIED INCLUD- ING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR OF FITNESS FOR A PARTICULAR PURPOSE OR APPLICATION OR WARRANTY OF QUALITY AS WELL AS ANY EXPRESS OR IMPLIED WARRANTY TO PATIENTS. No warranty or guarantee may be created by any act or statement nor may this Standard Warranty be modified in any way, except as a result of a writing signed by an officer of INTEGRA. These limitations on the creation or modification of this warranty may not be waived or modified orally or by any conduct. IN NO EVENT SHALL INTEGRA AUTHORIZED DISTRIBUTORS BE LIABLE TO- WARDS THE ORIGINAL PURCHASER FOR ANY INCIDENTAL, INDIRECT, CONSE- QUENTIAL OR PUNITIVE DAMAGES IN CONNECTION WITH THE ACQUISITION OR USE OF ANY INTEGRA PRODUCT. Further, this warranty shall not apply to, and INTEGRA authorized distributors shall not be responsible towards the original purchaser for, any loss, arising in connection with the purchase or use of any INTEGRA product that has been repaired by anyone other than an authorized INTEGRA service representative or altered in any way so as to affect its stability or reliability, or which has been subject to misuse, negligence or accident, or which has been used otherwise than in accordance with the instructions furnished by INTEGRA. THIS INTEGRA DISTRIBUTOR LIMITED WARRANTY IS EX- CLUSIVE AND IN LIEU OF ALL OTHER WARRANTIES TOWARDS THE ORIGINAL PURCHASER, EXPRESS OR IMPLIED, AND OF ALL OTHER OBLIGATIONS OR LI- ABILITIES TOWARDS THE ORIGINAL PURCHASER ON INTEGRA AUTHORIZED DISTRIBUTOR S PART. INTEGRA AUTHORISED DISTRIBUTORS DISCLAIM ALL OTHER WARRANTIES TOWARDS THE ORIGINAL PURCHASER, EXPRESS OR IMPLIED INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR OF FITNESS FOR A PARTICU- LAR PURPOSE OR APPLICATION OR WARRANTY OF QUALITY AS WELL AS ANY EXPRESS OR IMPLIED WARRANTY TO PATIENTS. All the references numbers mentioned on this document are CE marked according to European council directive 93/42/EEC on medical devices and its relatives, unless specifically identified as NOT CE MARKED. 5
6
7 Integra Forefoot Solutions Products for sale in Europe, Middle-East and Africa only Catalogue des ventes
8 Forefoot Solutions Forefoot Set A new modular and scalable forefoot instruments set: Bold Compression screw. QWIX 3.0 Fixation screw. Spin Spin break off screws. Solustaple Standard staple. Uni-clip Compression Staple. 0 1 Compression Screws Bold Fixation Screws QWIX Standard Staple Compression Staple Break Off screws 1 Solustaple Uni-Clip Spin Screw instrumentation module (details page 8) Staple instrumentation module (details page 9) Generic module (details page ) Implant rack module (details page ) Frame Frame - 2 floors 2299 Frame - 3 floors 2299 Forefoot II- lid- generic 8
9 Forefoot Solutions Staple instrumentation - Screw instrumentation Non sterile Implants - Staple instrumentation - Screw instrumentation Non sterile Implants - Generic module - Screw instrumentation 9
10 Forefoot Solutions Forefoot Set Screw Module 1195 Scarf clamp 2 Screwdriver - Hexa AO Screwdriver - Hexa AO Attachment - Cannulated 3 Screwdriver - T7 Star - Cannulated 4 Screwdriver - AO - T7 Star - Cannulated 9971 Depth Gauge AO Straight Handle 2211 Longitudinal Cutting Guide (Optional) 2212 Transversal Cutting Guide (Optional) 2291 Ruler Sterilization Screws Container including: Tray 2299 Lid Mat Drill Corresponding Product 2 in 1 Diameter Cannulated Length AO attachment (S) Bold 3.0 / Bold mm 12 mm (S) Bold 3.0 / Bold mm 22 mm (S) Bold 3.0 / Bold mm 32 mm (S) Bold 3.0 / Bold mm 12 mm (S) Bold 3.0 / Bold mm 22 mm (S) Bold 3.0 / Bold mm 32 mm (S) Bold 3.0 / Bold mm 12 mm (S) Bold 3.0 / Bold mm 22 mm (S) Bold 3.0 / Bold mm 32 mm (S) Bold 3.0 / Bold mm 12 mm (S) Bold 3.0 / Bold mm 22 mm (S) Bold 3.0 / Bold mm 32 mm S Bold mm 12 mm S Bold mm 22 mm S Bold mm 12 mm S Bold mm 22 mm S Bold mm 12 mm S Bold mm 22 mm S Bold mm 12 mm S Bold mm 22 mm K-wire Corresponding Product Diameter Length (S) Bold 3.0 / Bold mm mm (S) Bold 3.0 / Bold mm 0 mm S Bold mm mm All drills and K-wires are delivered sterile or non sterile except Bold 2.5 K-wire which is only delivered in sterile. (S) means available in sterile and non sterile
11 Forefoot Solutions 2 Forefoot Set Staple Module Uni-Clip Associated Instrument 2291 Uni-Clip Drilling guide 2292 Uni-Clip impactor Uni-Clip spreading forceps Solustaple Associated Instrument 2292 Solustaple impactor & Solustaple drilling guide 9971 Depth gauge - dia 2.0 X L. - mm Sterilization Container Staples container includes: Tray 2299 lid Drill Corresponding Product Diameter Cannulated Length AO attachment (S) Uni-Clip 2.2 mm 34 mm (S) Uni-Clip 2.2 mm 34 mm (S) Uni-Clip 2.2 mm 34 mm (S) Uni-Clip 2.2 mm 34 mm K-wire Corresponding Product Diameter Length (S) Uni-Clip 1.0 mm mm (S) Uni-Clip 1.0 mm 0 mm All drills and K-wires are delivered sterile or non sterile except Bold 2.5 K-wire which is only delivered in sterile. 1 0 (S) means available in sterile and non sterile 11
12 Forefoot Solutions Forefoot Set Implant Module Sterilization Container Forefoot II - tray - non sterile implants Forefoot II - rack - non sterile implants
13 Forefoot Solutions 2 Forefoot Set Generic Module Sterilization Container Forefoot II - tray - generic Forefoot II - mat - generic
14 Forefoot Solutions Bold Compression Screw Dia. 2.5 mm / 3.0 mm / 3.7 mm (for associated instrumentation, see p. 8) For fixation of bone fractures or for bone reconstruction. Totally intra-osseous implants. Large range of sizes: > > to mm for Bold 2.5 screws by 2 mm increment. > > to 34 mm for Bold 3.0 screws by 2 mm increment. > > 14 to 34 mm for Bold 3.7 screws by 2 mm increment. K-Wire guided drilling and insertion. Self tapping. Bold dia 2.5 mm True & controlled compression. Length Dual threads. 00(S) L. mm Material: Titanium alloy TA6V. 0012(S) L. 12 mm 0014(S) 0016(S) 0018(S) 00(S) 0022(S) 0024(S) 0026(S) 0028(S) 00(S) L. 14 mm L. 16 mm L. 18 mm L. mm L. 22 mm L. 24 mm L. 26 mm L. 28 mm L. mm Bold dia 3.0 mm 11(S) 1112(S) 1114(S) 1116(S) 1118(S) 11(S) 1122(S) 1124(S) 1126(S) 1128(S) 11(S) 1132(S) 1134(S) Length L. mm L. 12 mm L. 14 mm L. 16 mm L. 18 mm L. mm L. 22 mm L. 24 mm L. 26 mm L. 28 mm L. mm L. 32 mm L. 34 mm Bold dia 3.7 mm Length 1314(S) L. 14 mm 1316(S) L. 16 mm 1318(S) L. 18 mm 13(S) L. mm 1322(S) L. 22 mm 1324(S) L. 24 mm 1326(S) L. 26 mm 1328(S) L. 28 mm 13(S) L. mm 1332(S) L. 32 mm 1334(S) L. 34 mm QWIX Fixation Screw, Dia. 3.0 mm (for associated instrumentation, see p. 8) For fixation of bone fractures or for bone reconstruction. Self-drilling and self-tapping. Totally intra-osseous fixation. K-Wire guided drilling and insertion. Material: Titanium alloy TA6V. QWIX Dia. 3.0 mm Length (S) L. 12 mm (S) L. 14 mm (S) L. 16 mm (S) L. 18 mm 1113(S) L. mm (S) L. 22 mm (S) L. 24 mm (S) L. 26 mm (S) L. 28 mm 1113(S) L. mm (S) L. 32 mm (S) L. 34 mm (S) means available in sterile and non sterile 14
15 Forefoot Solutions 2 Spin Break Off Screw, Dia. 2.0 mm (for associated instrumentation, see p. 8) For bone fixation. Break-off barrel. Self-drilling and self-tapping. Material: Titanium alloy TA6V. 1111(S) Spin Dia. 2.0 x L. 11 mm 1112(S) Spin Dia. 2.0 x L. 12 mm 1113(S) Spin Dia. 2.0 x L. 13 mm 1114(S) Spin Dia. 2.0 x L. 14 mm Spin Break Off Screw, Dia. 2.7 mm (for associated instrumentation, see p. 8) Spin 2.7 screw is recommended for soft or osteoporotic bones fixation. Break-off barrel. Self-drilling and self-tapping. Material: Titanium alloy TA6V. Length (S) Spin Dia. 2.7 x L. 11 mm (S) Spin Dia. 2.7 x L. 12 mm (S) Spin Dia. 2.7 x L. 13 mm (S) Spin Dia. 2.7 x L. 14 mm 1 0 Color coded (S) means available in sterile and non sterile 15
16 Forefoot Solutions Uni-Clip Notched Staples (for associated instrumentation, see p. 9) For fixation of bone fractures or for bone reconstruction. Range sizes: > > Interaxis: 11, 12, 13, 15 and mm. > > Leg lengths: 12, 13, 14, 15, 16, 17 and mm. Material: Stainless steel 316L (S) (S) (S) (S) (S) Uni-Clip interaxis 11.0 x L. 13 mm Uni-Clip interaxis 11.0 x L. 14 mm Uni-Clip interaxis 11.0 x L. 15 mm Uni-Clip interaxis 11.0 x L. 16 mm Uni-Clip interaxis 11.0 x L. 17 mm (S) (S) (S) (S) (S) Uni-Clip interaxis 12.0 x L. 13 mm Uni-Clip interaxis 12.0 x L. 14 mm Uni-Clip interaxis 12.0 x L. 15 mm Uni-Clip interaxis 12.0 x L. 16 mm Uni-Clip interaxis 12.0 x L. 17 mm (S) (S) (S) (S) (S) Uni-Clip interaxis 13.0 x L. 13 mm Uni-Clip interaxis 13.0 x L. 14 mm Uni-Clip interaxis 13.0 x L. 15 mm Uni-Clip interaxis 13.0 x L. 16 mm Uni-Clip interaxis 13.0 x L. 17 mm (S) Uni-Clip interaxis 15.0 x L. 12 mm (S) Uni-Clip interaxis.0 x L. mm Solustaple Standard Staple (for associated instrumentation, see p. 9) Static staple for fixation of mono cortical phalangeal osteotomies. Two shapes of staples (26 and ) are available. Self-perforating. Material: stainless steel 316L. 12(S) 14(S) 1123(S) 1125(S) Solustaple interaxis 8 mm Solustaple interaxis mm Solustaple 26 interaxis 8 mm Solustaple 26 interaxis mm (S) means available in sterile and non sterile 16
17 Forefoot Solutions 2 Osteoguides Suggested use: > > Scarf osteotomy. > > Chevron osteotomy. > > Basal osteotomy. > > Arthrodesis. > > Wedge osteotomies (Akin ). Details: > > Hole for K-Wire: Diameter 1.0 to 1.6 mm. > > Knurled handle for optimal grip. > > Blade thickness maxi 0.8 mm. > > Positioning tip Longitudinal 2212 Transversal Transversal osteotomy guide Longitudinal osteotomy guide K-FIX K-Wire End Protector The K-Fix pin protector is a simple system to protect patients or hospital staff from K-Wires sharp ends. Delivered sterile Length K-FIX for K-WIRE - Dia. 0.8 mm to 2.0 mm (5 units per pack) Weil Clamp Length 1196 Weil Clamp 17
18 Forefoot Solutions B-BOP LOCK Plate B-BOP -LOCK plate is indicated for fixation of osteotomy of the basis of the first metatarsal. Examples include: Moderate to severe Hallux valgus. Hallux varus. Implants 3 0S Basal plantar plate B-Bop Lock right 3 0S Basal plantar plate B-Bop Lock left 295 1S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 12 mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 14 mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 16 mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 18 mm 295 1S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 22 mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 24 mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 26 mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 28 mm 295 1S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 32 mm S Screw and Lock screw titanium B-Bop Lock Dia 3.0 x L 34 mm 195 0S Lock screw B-Bop Lock Instrumentation Drilling guide Surfix Alpha Dia 2.0 mm Screwdriver Torx T Screwdriver AO blade Torx T Drill AO Dia 2.0 mm Drill Dia 2.0 mm Bending forceps Forceps K-wire Dia 1.0 mm x L mm K-wire Dia 1.6 mm x L 1 mm Depth gauge B-Bop Lock Wedge Wedge Wedge Container Container including: Basis Silicone mat 9960 Lid 18
19 Forefoot Solutions 2 IPP-ON Interphalangeal Arthrodesis Implant IPP-ON interphalangeal implant is an implant which respects anatomical flexion to achieve interphalangeal arthrodesis of the lesser toes in case of: Fixed or semi-fixed hammer toe. Revision in case of arthrodesis or arthroplasty failure. Shortening in case of length excess of the second toe. Material: Stainless steel: 316 LVM. 2 sizes S IPP-ON Interphalangeal Implant Size 1 02S IPP-ON Interphalangeal Implant Size IPP-ON Instruments 2311 Distal Hand Drill Size Proximal Hand Drill Size Distal Hand Drill Size Proximal Hand Drill Size 2 23 Clamp 23 Implant Holder Sterilization Container 0 IPP-ON sterilization container including: 1 Base 9960 Lid 2782 Mat 1199 Silicone wedge
20 Forefoot Solutions METIS Metatarso-Phalangeal Prosthesis The three-component METIS prosthesis replaces the articular surfaces of the great toe joint. Metatarsal component: > > 7 sizes (0 to 6). > > Material: Cobalt-Chromium alloy (CoCr). > > Coating: Porous Titanium & Hydroxyapatite (Ti and HAP). PE inlay: > > Ultra High Molecular Weight Polyethylene (UHMWPE). > > 5 sizes (1 to 5), 3 thicknesses (1 to 3). Phalangeal implant: > > 5 sizes (1 to 5). > > Material: Titanium alloy TA6V. > > Coating: Porous Titanium (Ti) Metatarsal component size 0 21 Metatarsal component size 1 22 Metatarsal component size 2 23 Metatarsal component size 3 24 Metatarsal component size 4 25 Metatarsal component size 5 26 Metatarsal component size 6 21 Phalangeal component size 1 22 Phalangeal component size 2 23 Phalangeal component size 3 24 Phalangeal component size 4 25 Phalangeal component size METIS Polyethylene Inlay - Size 1 - Thickness METIS Polyethylene Inlay - Size 1 - Thickness METIS Polyethylene Inlay - Size 1 - Thickness METIS Polyethylene Inlay - Size 2 - Thickness METIS Polyethylene Inlay - Size 2 - Thickness METIS Polyethylene Inlay - Size 2 - Thickness METIS Polyethylene Inlay - Size 3 - Thickness METIS Polyethylene Inlay - Size 3 - Thickness METIS Polyethylene Inlay - Size 3 - Thickness METIS Polyethylene Inlay - Size 4 - Thickness METIS Polyethylene Inlay - Size 4 - Thickness METIS Polyethylene Inlay - Size 4 - Thickness METIS Polyethylene Inlay - Size 5 - Thickness METIS Polyethylene Inlay - Size 5 - Thickness METIS Polyethylene Inlay - Size 5 - Thickness 3
21 Forefoot Solutions 2 METIS Instruments METIS Instruments 1397 Metatarsal Osteotome 1391 Implant holder Locking screw 1392 Phalangeal reamer starter size Phalangeal reamer size 1 to Metatarsal sizer size Metatarsal sizer size 1 to Metatarsal cutting guide size Metatarsal cutting guide size 1 to Metatarsal impactor Visualization guide Visualization guide wire Screwdriver hexa 3.5 mm Screwdriver hexa 2.5 mm K-Wire Dia. 1.6 x L. 1 mm Drill Dia. 2.5 x L. 1 mm 1399 METIS sterilization container including: Metatarsal base Phalangeal base Base Lid 1199 Silicone wedge METIS Trial Implants 13 6 Metatarsal trial implant size 0 to Trial inlay size 1 thickness 1 to Trial inlay size 2 thickness 1 to Trial inlay size 3 thickness 1 to Trial inlay size 4 thickness 1 to Trial inlay size 5 thickness 1 to Phalangeal trial implant size 1 to
22 Forefoot Solutions K2 Hemi Toe Implant The K2 Hemi Toe Implant System is an anatomically designed, metallic, one-piece surface replacement system for the base of the hallux. 4 sizes are available. K2 Implants Phalangeal, Size 1 Phalangeal, Size 2 Phalangeal, Size 3 Phalangeal, Size K2 Instruments Size 1 Trial Size 2 Trail Size 3 Trial Size 4 Trial Size 1 Shaper Size 2 Shaper Size 3 Shaper Size 4 Shaper 30 Cutting Guide 30 Sizer/Drill Guide Broach Handle Impactor 00 Sterilization tray 30 Reamer mm mm drill bit 22
23 Forefoot Solutions 2 Movement Great Toe System Hemi Proximal Phalanx Implant. Hemi Metatarsal Implant. Total Great Toe Implant. MGT-8-PPH Hemi Proximal Phalanx, Size MGT-8-PPH Hemi Proximal Phalanx, Size MGT-8-PPH Hemi Proximal Phalanx, Size MGT-8-PPH Hemi Proximal Phalanx, Size MGT-8-MT Hemi Metatarsal, Size MGT-8-MT Hemi Metatarsal, Size MGT-8-MT Hemi Metatarsal, Size MGT-8-MT Hemi Metatarsal, Size MGT-8-PPT Phalangeal Total, Size MGT-8-PPT Phalangeal Total, Size MGT-8-PPT Phalangeal Total, Sixe MGT-8-PPT Phalangeal Total, Size INS-8-00 MIS-8-00 PIS-8-00 GDW-8-00 Instrument Set Metatarsal Implant Sizer Proximal Phalanx Implant Sizer Guide Pin, 2.0 mm X 125 mm MSR-8-/ Metatarsal Surface Reamer, Size / MSR-8-/ Metatarsal Surface Reamer, Size / PSR-8-/ Proximal Phalanx Surface Reamer, Size / PSR-8-/ Proximal Phalanx Surface Reamer, Size / DRL-8-00 DCG-8-00 Cannulated Drill, 4.5 mm Metatarsal Dorsal Cutting Guide TRL-8-MT Metatarsal Trial, Size TRL-8-MT Metatarsal Trial, Size TRL-8-MT Metatarsal Trial, Size TRL-8-MT Metatarsal Trial, Size TRL-8-PPH Proximal Phalanx Hemi Trial, Size TRL-8-PPH Proximal Phalanx Hemi Trial, Size TRL-8-PPH Proximal Phalanx Hemi Trial, Size TRL-8-PPH Proximal Phalanx Hemi Trial, Size TRL-8-PPT Phalangeal Total Trial, Size TRL-8-PPT Phalangeal Total Trial, Size TRL-8-PPT Phalangeal Total Trial, Size TRL-8-PPT Phalangeal Total Trial, Size IMP-8-00MT IMP-8-00PP ALG-8-00 Metatarsal Impactor Proximal Phalanx Impactor Sizer Alignment Guide
24 Forefoot Solutions Hallu -fix MTP Arthrodesis System The Hallu -fix solution is a system dedicated to first MTP arthrodesis. Pre-bent plate. Snap-Off screw fixation. Color coded. 3 sizes (3 left / 3 right). Material: Titanium alloy TA6V HALLU -C Plate HALLU -C Plates 1173(S) HALLU -C Plate Right-Size 1: L. mm - 4 holes (S) HALLU -C Plate Right-Size 2: L. 45 mm - 5 holes 1173(S) HALLU -C Plate Right-Size 3: L. mm - 6 holes (S) (S) 1174(S) HALLU -C Plate Left-Size 1: L. mm - 4 holes HALLU -C Plate Left-Size 2: L. 45 mm - 5 holes HALLU -C Plate Left-Size 3: L. mm - 6 holes HALLU -S Plate HALLU -S Plates 1171(S) (S) 1171(S) HALLU -S Plate Right-Size 1: L. 45 mm - 3 holes HALLU -S Plate Right-Size 2: L. mm - 4 holes HALLU -S Plate Right-Size 3: L. 55 mm - 5 holes 1172(S) (S) 1172(S) HALLU -S Plate Left-Size 1: L. 45 mm - 3 holes HALLU -S Plate Left-Size 2: L. mm - 4 holes HALLU -S Plate Left-Size 3: L. 55 mm - 5 holes 24
25 Forefoot Solutions 2 HALLU Snap-Off Screws 2 2 diameters: 2.7 mm & 3.0 mm (for porous bone). Self-tapping. Color code for length identification. Material: Titanium alloy TA6V. HALLU Snap-Off screws Dia. 3.0 mm 1171(S) Length mm HALLU Snap-Off screws Dia. 2.7 mm 11(S) Length mm (S) 12 mm 1112(S) 12 mm (S) 14 mm 1114(S) 14 mm (S) 16 mm 1116(S) 16 mm (S) 18 mm 1118(S) 18 mm 11(S) mm (S) 22 mm 1124(S) 24 mm (S) 26 mm 1128(S) 28 mm 1 11(S) mm 1132(S) 32 mm (S) 34 mm 1 Hallu -fix Instruments 1 1 HALLU -Ream Metatarsal HALLU -Ream Phalangeal HALLU Associated instruments Diameter Diameter Dia. 14 mm Dia. 14 mm 1297 Quick coupling Dia. 16 mm Dia. 18 mm 1297 Dia. mm Dia. 22 mm Dia. 16 mm Dia. 18 mm 1297 Dia. mm Dia. 22 mm Snap-Off Cannulated screwdriver - Dia. 2.7 mm and 3.0 mm Plate Bender Left Plate Bender Right Drilling Guide Dia. 1.9 mm Screwdriver tip Depth gauge Drill Dia. 1.9 x L. 0 mm K-Wire Dia. 1.6 x L. 1 mm 1 K-Wire Dia. 1.0 x L. 0 mm Sterilization container 1299 Hallu -fix Sterilization Container including: Hallu -fix Basis HALLU -S Plate Insert HALLU -C Plate Insert 9960 Lid Hallu -fix screws rack 1199 Silicone wedge (Optional) (S) means available in sterile and non sterile 25
26 Forefoot Solutions HALLU -LOCK MTP Arthrodesis System The HALLU -LOCK solution is a locked system dedicated to first MTP arthrodesis: Pre-bent plate. Color coded. 3 sizes (3 left / 3 right). Material: Titanium alloy TA 6V. Monobloc fixation with Surfix locking system. Surfix locking screw 2 diameters: 2.7 mm & 3.0 mm (for porous bone). Standard and orientable screws. Color code for length identification. Material: Titanium alloy TA 6V. Sterily Packaged Surfix standard screw Dia. 2.7 mm + lock-screw Length 2852S mm S 12 mm S 14 mm S 16 mm S 18 mm 2852S mm Surfix standard screw Dia. 3.0 mm + lock-screw Length 2851S mm S 12 mm S 14 mm S 16 mm S 18 mm 2851S mm Hallu -Lock C plate 23S 2345S 23S 24S 2445S 24S Right-Size 1: L. mm 4 holes Right-Size 2: L.45 mm 5 holes Right-Size 3: L. mm 6 holes Left-Size 1: L. mm 4 holes Left-Size 2: L.45 mm 5 holes Left-Size 3: L. mm 6 holes S 22 mm S 22 mm S S 1850S 24 mm 26 mm lock-screw S S 1850S 24 mm 26 mm lock-screw Hallu -Lock S plate Number of holes excludes the 4 extremity holes 21S Right-Size 1: L.45 mm 3 holes Surfix Alpha variable angle screw Dia. 2.7 mm + lock-screw Length 2952S mm S 12 mm Surfix Alpha variable angle screw Dia. 3.0 mm + lock-screw Length 2951S mm S 12 mm 2155S 21S 22S 2255S 22S Right-Size 2: L. mm 3 holes Right-Size 3: L.55 mm 3 holes Left-Size 1: L.45 mm 3 holes Left-Size 2: L. mm 3 holes Left-Size 3: L.55 mm 3 holes S S S 2952S S S S 1950S 14 mm 16 mm 18 mm mm 22 mm 24 mm 26 mm lock-screw S S S 2951S S S S 1950S 14 mm 16 mm 18 mm mm 22 mm 24 mm 26 mm lock-screw QWIX transarticular compressive screw Dia. 3.0 mm Length S 28 mm 1113S mm S 32 mm S 34 mm 26
27 Forefoot Solutions 2 HALLU -LOCK Instruments HALLU -REAM System Metatarsal Reamer Dia. 14 mm Metatarsal Reamer Dia. 16 mm Metatarsal Reamer Dia. 18 mm 1297 Metatarsal Reamer Dia. mm Metatarsal Reamer Dia. 22 mm Phalangeal Reamer Dia. 14 mm Phalangeal Reamer Dia. 16 mm Phalangeal Reamer Dia. 18 mm 1297 Phalangeal Reamer Dia. mm Phalangeal Reamer Dia. 22 mm HALLU -Lock associated instruments 1297 Quick coupling 99 Quick-Coupling AO 29 Left bender 29 Right bender 2127 SURFIX Alpha drilling guide Dia. 2.0 mm SURFIX drilling guide Dia. 2.0 mm Screwdriver torx T AO Screwdriver tip torx T7 29 Depth Gauge Handle with AO Attachment Dia. 3.0 mm AO Screwdriver tip dia 3.0 mm 29 Trial plate holder 2991 Trial plate-s Right - Size Trial plate-s Right - Size Trial plate-s Right - Size Trial plate-s Left - Size Trial plate-s Left - Size Trial plate-s Left - Size Trial plate-c Right - Size Trial plate-c Right - Size Trial plate-c Right - Size Trial plate-c Left - Size Trial plate-c Left - Size Trial plate-c Left - Size Drill Dia. 2.0 x L. 0 mm 29 AO Drill AO Dia. 2.0 x L. 0 mm 1527S 2 in 1 cannulated drill AO dia 2.2/3.0 x L. 12 mm sterile 11 K-Wire Dia. 1.0 x L. mm 1 K-wire Dia. 1.0 x L. 0 mm K-Wire Dia. 1.6 x L. 1 mm HALLU -LOCK sterilization container 2990 HALLU -Lock sterilization container 2991 Base 2993 Trial implants tray 2994 K-Wire tray 9960 Lid 1199 Silicone wedge
28 Forefoot Solutions HALLU -Ream Set Metatarsal Reamer Dia. 14 mm Metatarsal Reamer Dia. 16 mm Metatarsal Reamer Dia. 18 mm 1297 Metatarsal Reamer Dia. mm Metatarsal Reamer Dia. 22 mm Phalangeal Reamer Dia. 14 mm Phalangeal Reamer Dia. 16 mm Phalangeal Reamer Dia. 18 mm 1297 Phalangeal Reamer Dia. mm Phalangeal Reamer Dia. 22 mm Instruments 1297 Quick coupling K-Wire Dia. 1.6 x L. 1 mm Sterilization container HALLU -Ream sterilization container including: HALLU -Ream Basis 9960 Lid
29 Forefoot Solutions 2 DPR System Minimal Invasive Foot Surgery DPR System minimal invasive foot surgery was born from our collaboration with minimal invasive foot surgeons. Single use: Sterile packaging. Safety: Protection by silicone ring. Identification: Color coded. Material: Stainless Steel 316L DPR System Minimal Invasive Foot Surgery burrs 22S Burr straight Dia. 2.0 x L. 15 mm 27S Burr conical Dia. 3.1/1.0 x L. 12 mm 25S Burr straight Dia. 1.9 x L. mm 28S Burr conical Dia. 4.1/1.0 x L. 12 mm 29S Burr cylindrical Dia. 4.1 x L. 15 mm 27S Burr cylindrical Dia. 3.1 x L. 15 mm DPR System Minimal Invasive Foot Surgery curettes Curette Medium - Down Curette Medium - Up Curette Large - Down Curette Large - Up 27 Sleeve protection Cap 2781 Sleeve protection Dia. 3.1 mm 2789 Sleeve protection Dia. 4.1 mm Sterilization container 2780 DPR System Minimal Invasive Foot Surgery sterilization container 2781 Base 9960 Lid 2782 Mat 1199 Silicone wedge Please contact us if you need a MIS motor offer. 29
30 Forefoot Solutions Post-Operative Shoes Flat Post Operative Shoe 9901 Small 9902 Medium 9903 Large 9904 X-Large Wedged Hell Post Operative Shoe 99 Size 4 to Size 7 to Size 9 to
31
32
33 Integra Mid & HindFoot Solutions Products for sale in Europe, Middle-East and Africa only Catalogue des ventes
34 Subtalar Arthrodesis QWIX Fixation Screw, dia. 4.3 mm For the fixation of bone fractures or for bone reconstruction. Self-drilling and self-tapping. Intra-osseous fixation. Variable lag length for extremity fixation. Material: Titanium alloy TA6V QWIX dia. 4.3 mm s s s s 1114s s s s s 1114s s 1114s s 1114s Length L. 22 mm L. 24 mm L. 26 mm L. 28 mm L. mm L. 32 mm L. 34 mm L. 36 mm L. 38 mm L. mm L. 45 mm L. mm L. 55 mm L. mm QWIX dia. 4.3 mm Instruments 1194 Screwdriver tip Short Drill dia. 2.6/5.0 x L. 0 mm Quick coupling handle Long cannulated drill dia. 2.6 x L. 1 mm K-Wire dia. 1.6 x L. 1 mm Sterilization container QWIX 4.3 sterilization container including: Cylinder Base Mat 99 Lid 34
35 Subtalar Arthrodesis 2 Large QWIX Fixation and Positioning Screws dia. 5.5 mm / 7.5 mm Fixation and positioning screws dia. 5.5 and 7.5 mm. Self-drilling and self-tapping. Intra-osseous fixation. Material: Titanium alloy TA6V. Sterile packaged Large QWIX Fixation screws dia. 7.5 mm Large QWIX Positioning screws dia. 7.5 mm 0 Length Length s s L. mm L. 45 mm 1217s s L. mm L. 45 mm s s 1117s s L. mm L. 55 mm L. mm L. 65 mm Large QWIX Fixation screws dia. 5.5 mm Length 1217s s 1217s s L. mm L. 55 mm L. mm L. 65 mm Large QWIX Positioning screws dia. 5.5 mm Length s s L. mm L. 75 mm 1115s s L. mm L. 35 mm 1217s s L. mm L. 75 mm 1215s s L. mm L. 35 mm s s L. mm L. 85 mm 1115s s L. mm L. 45 mm 1217s s L. mm L. 85 mm 1215s s L. mm L. 45 mm s s L. mm L. 95 mm 1115s s L. mm L. 55 mm 1217s s L. mm L. 95 mm 1215s s L. mm L. 55 mm s 1115s L. 0 mm L. 5 mm 1115s s L. mm L. 65 mm 1s 1215s L. 0 mm L. 5 mm 1215s s L. mm L. 65 mm s s L. 1 mm L. 115 mm 1115s s L. mm L. 75 mm 1218s s L. 1 mm L. 115 mm 1215s s L. mm L. 75 mm s L. 1 mm 1115s L. mm 1218s L. 1 mm 1215s L. mm 0 Large QWIX Instruments Length 1191 Measurer 0 and 2 mm Internal protection sleeve dia. 1.8 mm Drilling guide dia. 4.5 mm External protection sleeve dia. 7.3 mm Cannulated drill dia. 1.8/4.2 x L. 1 mm Cannulated 2 in 1 drill dia. 4.2/5.8 x L. 113 mm Hexa screwdriver Tip 3 mm Internal protection sleeve dia. 2.7 mm Drilling guide dia. 5.8 mm External protection sleeve dia. 9.3 mm Cannulated drill dia. 2.7/5.5 x L. 2 mm Cannulated 2 in 1 drill dia. 5.5/7.0 x L. 128 mm Hexa screwdriver Tip 4 mm T handle quick coupling K-Wire dia. 1.6 x L. 0 mm 5261 K-Wire dia. 2.5 x L. 2 mm Sterilization container 1219 LARGE QWIX sterilization container including: Base 9960 Lid 1191 Cylinder 1199 Silicone wedge 35
36 Subtalar Arthrodesis I.CO.S. Ideal Compression Screw Cannulated Titanium 4.0 mm and 6.5 mm diameter compression screw are dedicated to fixation of osteotomies or for fractures stabilization. K-Wire guidance for drilling, tapping and screw setting (dia. 1.6 or 2.5 mm). Adjustable and controlled static compression. Dynamic compression by additional head screwing. Intra osseous implant. Large range of sizes : > > I.CO.S dia. 4.0 mm:»» L. 26 to mm by 2 mm»» L. to mm by 5 mm > > I.CO.S dia. 6.5 mm:»» L. to mm by 5 mm Material: Titanium alloy TA6V. Cortico-spongious thread. Long non threaded shaft (lag screws) allowing compression I.CO.S. dia. 4.0 mm Length 5426 L. 26 mm 5428 L. 28 mm 54 L. mm 5432 L. 32 mm 5434 L. 34 mm 5436 L. 36 mm 5438 L. 38 mm 54 L. mm 5445 L. 45 mm 54 L. mm 5455 L. 55 mm 54 L. mm I.CO.S. dia. 6.5 mm Length 56 L. mm 5645 L. 45 mm 56 L. mm 5655 L. 55 mm 56 L. mm 5665 L. 65 mm 56 L. mm 5675 L. 75 mm 56 L. mm 5685 L. 85 mm 56 L. mm 36
37 Subtalar Arthrodesis 2 I.CO.S. dia. 4.0 mm Instruments 1191 Protection Sleeve dia. 6 mm 1196 Drilling Guide dia. 1.8 mm 1195 Measurer Long cannulated drill dia. 2.6 x L. 1 mm Short cannulated Drill dia. 5.0 x L. 1 mm 1297 Quick-coupling Tap Internal screwdriver dia. 4.0 x L. 0 mm Screwdriver tip dia. 4.0 x L. 0 mm External screwdriver dia. 6.4 x L. 1 mm I.CO.S. 4.0 sterilization Container I.CO.S. 4.0 sterilization container including: Base Screw rack Metallic lid for screws (Optional) 9960 Lid 1199 Silicone wedge I.CO.S. dia. 6.5 mm Instruments Drilling Guide dia. 6.5 x L. 0 mm Internal protection sleeve dia. 6.5 mm External protection sleeve dia. 6.5 mm Long cannulated drill dia. 4.6 x L. 1 mm Short cannulated drill dia. 6.5 x L. 118 mm Internal screwdriver dia. 6.5 x L. 1 mm Screwdriver tip dia. 6.5 x L. 1 mm External screwdriver dia..1 x L. 1 mm Tap 1195 Measurer 1297 Quick coupling K-Wire dia. 2.5 x L. 0 mm I.CO.S. 6.5 sterilization Container I.CO.S. 6.5 sterilization Container including: Base Screws rack Metallic lid for screws (Optional) Lid 1199 Silicone wedge 37
38 Subtalar Arthrodesis I.CO.S. Removal Set This removal set contains instrumentation required to remove I.CO.S. screws dia. 4.0 and 6.5 mm Removal set Instruments (for all diameters) Instruments for I.CO.S. dia. 4.0 mm K-Wire dia. 1.6 x L. 1 mm Internal screwdriver dia. 4.0 x L. 0 mm 1195 Trephine dia. 4.0 x L. mm Internal Extractor dia. 4.0 x L. mm (single use) Instruments for I.CO.S. dia. 6.5 mm K-Wire dia. 2.5 x L. 0 mm Internal screwdriver dia. 6.5 x L. 1 mm 1195 Trephine dia. 6.5 x L. mm Internal Extractor dia. 6.5 x L. mm (single use) Sterilization container I.CO.S. Removal Sterilization container including Lid Base K-Wire cylinder 0 38
39 Flatfoot implants 2 KALIX II Flat Foot Implant Expansion endorthesis for flat foot correction in child or adult. 2 2 Progressive expansion. Material: > > Titanium alloy TA6V > > Polyethylene (UHMWPE) Color coded. Sterily packaged. Ext. diameter 9 dia. 9 mm 14 dia. mm 1411 dia. 11 mm 1412 dia. 12 mm 1413 dia. 13 mm 1414 dia. 14 mm 1415 dia. 15 mm 1417 dia. 17 mm KALIX II Flat Foot Implant Instruments Viladot Lever 1198 Impactor Trial implant holder KALIX II Trial implant dia. 9.0 mm 1198 KALIX II Trial implant dia..0 mm KALIX II Trial implant dia. 11 mm KALIX II Trial implant dia. 12 mm KALIX II Trial implant dia. 13 mm KALIX II Trial implant dia. 14 mm KALIX II Trial implant dia. 15 mm KALIX II Trial implant dia. 17 mm KALIX II sterilization container 1198 KALIX II sterilization container including Lid Base
40 Flatfoot Implants MBA Titanium Subtalar Implant The Subtalar MBA System is indicated for use in treatment of the hyperpronated foot and stabilization of the subtalar joint. It is designed to block the posterior and inferior displacement of the talus, thus allowing normal subtalar joint motion while blocking excessive pronation and the resulting sequela. Severely pronated foot. Walking intemperance. Calcaneal stance position greater than 5. Manually correctable deformities. Mid-tarsal breech (arch pain). Forefoot varus greater than Removal set Instruments (for all diameters) Instruments tray Guide pin insert holder Trial 6 mm Trial 8 mm Trial 9 mm Trial mm Trial 12 mm Yellow probe mm Sizer mm Sizer mm Sizer 05- mm Sizer mm Sizer 05- Insertion Device Guide pin 2 mm Sterilization tray Implants MBA 6 mm implant MBA 8 mm implant MBA 9 mm implant MBA mm implant MBA 12 mm implant
41 Dorsal Lisfranc plate & Medial Lisfranc plate 2 ADVANSYS Dorsal Lisfranc Plate 2 2 The Dorsal Lisfranc Plates are intended for fractures, fusions, osteotomies and replantations of small bones at the tarso-metatarsal joints (Lisfranc joints). 3 sizes of implants: 3 right & 3 left plates (small, medium and large). 3.5 mm diameter screws available in lengths from 8 mm to 34 mm in 2 mm increments. 3.5 mm lock-screws. Material: > > Plate: Stainless Steel 316LVM. > > Screw: Titanium TA6V. ADVANSYS Dorsal Lisfranc Plates 1821s ADVANSYS DLP small-right 1822s ADVANSYS DLP medium-right 1823s ADVANSYS DLP large-right 1831s ADVANSYS DLP small-left 1832s ADVANSYS DLP medium-left 1833s ADVANSYS DLP large-left Surfix Locking screw Sterile Titanium dia.3.5 mm + lock-screw 2853s L. mm s L. 12 mm s L. 14 mm s L. 16 mm s L. 18 mm 2853s L. mm s L. 22 mm s L. 24 mm s s L. 26 mm L. 28 mm s s L. mm L. 32 mm s 1850s L. 34 mm Lock-screw dia.3.5 mm 1 Optional instrument 1 MA3511 Surfix screw extractor 3.5 mm 1 1 ADVANSYS Medial Lisfranc Plate 1 The Medial Lisfranc Plates are intended to be used for bone fixation such as: Lisfranc arthrodesis. Mono or bi-cortical osteotomies or fractures near the 1 st metatarso-cuneiform joint. 2 sizes of implants: 2 right & 2 left plates (small and large sizes). 3.5 mm diameter screws available in lengths from 8 mm to 34 mm in 2 mm increments. 3.5 mm lock-screws. Material: > > Plate: Stainless Steel 316LVM. > > Screw: Titanium TA6V. Sterily packaged. ADVANSYS Medial Lisfranc Plate 1841s ADVANSYS MLP small-right 1842s ADVANSYS MLP large-right 1851s ADVANSYS MLP small-left 1852s ADVANSYS MLP large-left Surfix Locking screw Sterile Titanium dia. 3.5 mm + lock-screw 2853s L. mm s L. 12 mm s L. 14 mm s L. 16 mm s L. 18 mm 2853s L. mm s L. 22 mm s L. 24 mm s L. 26 mm s L. 28 mm s L. mm s L. 32 mm s 1850s L. 34 mm Lock-screw dia.3.5 mm Optional instrument MA3511 Surfix screw extractor 3.5 mm 41
42 Dorsal Lisfranc plate & Medial Lisfranc plate ADVANSYS Medial Lisfranc Plate Instruments 1 K-Wire dia. 1.0 x L. 0 mm Depth gauge hole dia. 3.5 mm AO Screwdriver tip hexa 2.0 x L. 76 mm AO drill dia. 2.7 x L. 125 mm Drilling guide dia. 2.7 mm Bending forceps L. 171 hole - dia 3.5 mm Screwdriver hexa 2.0 x L. 1 mm 1641 MLP trial implant right-small 1642 MLP trial implant right-large 1651 MLP trial implant left-small 1652 MLP trial implant left-large ADVANSYS Midfoot sterilization container 1692 ADVANSYS Midfoot sterilization container includes: Base 9960 Lid 1696 Cylinder 1697 DLP/MLP Trial implant module 1199 Silicone wedge DLP trial plates 1621 Dorsal trial plate, right, small 1622 Dorsal trial plate, right, medium 1623 Dorsal trial plate, right, large 1631 Dorsal trial plate, left, small 1632 Dorsal trial plate, left, medium 1633 Dorsal trial plate, left, large Optional instrument MA3511 Surfix screw extractor 3.5 mm 42
43 Dorsal Lisfranc plate & Medial Lisfranc plate 2 UNI-CP Compression plate 2 The UNI-CP compression plate is indicated for fixation of bone fractures or for bone reconstruction: 4 plate configurations: > > 2 hole design (17,, 25, and mm interaxis) > > 4 hole design (, 25, and mm interaxis) > > 4 hole T-shape design ( mm interaxis) > > 4 hole U-shape design (17x, 19x22 5, 21x25 mm interaxis) Range of screw lengths: from 12 mm to mm in 2 mm increments. Surfix Locking Technology. Material > > Plate: Stainless Steel 316LVM. > > Screw: Titanium TA6V. UNI-CP Compression Plate 34S UNI-CP 4 holes - mm 3425S UNI-CP 4 holes - 25 mm 34S UNI-CP 4 holes - mm 32S UNI-CP 2 holes - mm 3225S UNI-CP 2 holes - 25 mm 32S UNI-CP 2 holes - mm 3217S UNI-CP 2 holes -17 mm 30S UNI-CP T shaped plate 3021S 4 holes - 17x mm U shaped plate 3023S 4 holes - 19x22.5 mm U shaped plate 3025S 4 holes - 21x25 mm U shaped plate Surfix Sterile Titanium screw dia. 3.5 mm + lock-screw s L. 12 mm s L. 14 mm s L. 16 mm s L. 18 mm 2853s L. mm s L. 22 mm s L. 24 mm s L. 26 mm s L. 28 mm 2853s L. mm s L. 32 mm s L. 34 mm s L. 36 mm s L. 38 mm s L. mm 1850s Surfix lock screw dia. 3.5 mm 1 UNI-CP Compression plate Instruments UNI-CP plate 4 holes UNI-CP plate 2 holes UNI-CP T-shape plate UNI-CP U-shape plate Depth gauge hole dia. 3.5 mm AO Screwdriver tip hexa 2.0 x L. 76 mm AO drill dia. 2.7 x L. 125 mm Drilling guide dia. 2.7 mm Bending forceps L. 171 hole - dia 3.5 mm Screwdriver hexa 2.0 x L. 1 mm 3301 Compression forceps 3304 Trial implant for T and U plates 3305 Trial implant for 2 and 4 holes plates 3303 Holder 1151 K-Wire dia. 1.0 x L. 0 mm Optional instrument MA3511 Surfix screw extractor 3.5 mm UNI-CP sterilization container 3399 UNI-CP sterilization container including: 9960 Lid 3399 Base 1696 Cylinder 1199 Silicone wedge Optional instrument MA3511 Surfix screw extractor 3.5 mm 43
44 Total Ankle Replacement HINTEGRA Total Ankle Prosthesis Primary Surgery The HINTEGRA Total Ankle Prosthesis is a three components design anatomical prosthesis. Materials: > > Talar & tibial components: Cobalt Chromium alloy (CoCr), with double coating:»» Porous Titanium & Hydroxyapatite (Ti and HAP), for optimal bone ingrowth (osteointegration). > > PE inlay: UHMWPE. > > Fixation screws (optional): Stainless steel MNW HINTEGRA Total Ankle Prosthesis Tibial Component Primary Surgery Minimum contact stress to the bone. Materials: > > Cobalt Chromium alloy (CoCr) with double coating: Porous Titanium & Hydroxyapatite (Ti and HAP), for optimal bone ingrowth (osteointegration). 7 sizes right & 7 sizes left. HINTEGRA - Tibial Component - Right HINTEGRA tibial component-right - size 0 11 HINTEGRA tibial component-right - size 1 12 HINTEGRA tibial component-right - size 2 13 HINTEGRA tibial component-right - size 3 14 HINTEGRA tibial component-right - size 4 15 HINTEGRA tibial component-right - size 5 16 HINTEGRA tibial component-right - size 6 HINTEGRA - Tibial Component - Left 20 HINTEGRA tibial component-left - size 0 21 HINTEGRA tibial component-left - size 1 22 HINTEGRA tibial component-left - size 2 23 HINTEGRA tibial component-left - size 3 24 HINTEGRA tibial component-left - size 4 25 HINTEGRA tibial component-left - size 5 26 HINTEGRA tibial component-left - size 6 44
45 Total Ankle Replacement 2 HINTEGRA Total Ankle Prosthesis PE Inlay Primary Surgery Ultra High Molecular Weight Polyethylene (UHMWPE). X-rays markers (Titanium TA6V rods). Same size as talar component: 6 sizes with 4 thicknesses (5, 6, 7, 9 mm). HINTEGRA - PE inlay - size HINTEGRA PE inlay - size 0 - thickness 5 mm 0006 HINTEGRA PE inlay - size 0 - thickness 6 mm 0007 HINTEGRA PE inlay - size 0 - thickness 7 mm 0009 HINTEGRA PE inlay - size 0 - thickness 9 mm HINTEGRA - PE inlay - size 1 05 HINTEGRA PE inlay - size 1 - thickness 5 mm 06 HINTEGRA PE inlay - size 1 - thickness 6 mm 07 HINTEGRA PE inlay - size 1 - thickness 7 mm 09 HINTEGRA PE inlay - size 1 - thickness 9 mm HINTEGRA - PE inlay - size 2 05 HINTEGRA PE inlay - size 2 - thickness 5 mm 06 HINTEGRA PE inlay - size 2 - thickness 6 mm HINTEGRA - PE inlay - size 4 05 HINTEGRA PE inlay - size 4 - thickness 5 mm 06 HINTEGRA PE inlay - size 4 - thickness 6 mm 07 HINTEGRA PE inlay - size 4 - thickness 7 mm 09 HINTEGRA PE inlay - size 4 - thickness 9 mm HINTEGRA - PE inlay - size 5 05 HINTEGRA PE inlay - size 5 - thickness 5 mm 06 HINTEGRA PE inlay - size 5 - thickness 6 mm 07 HINTEGRA PE inlay - size 5 - thickness 7 mm 09 HINTEGRA PE inlay - size 5 - thickness 9 mm HINTEGRA - PE inlay - size 6 05 HINTEGRA PE inlay - size 6 - thickness 5 mm 06 HINTEGRA PE inlay - size 6 - thickness 6 mm HINTEGRA PE inlay - size 2 - thickness 7 mm 09 HINTEGRA PE inlay - size 2 - thickness 9 mm 07 HINTEGRA PE inlay - size 6 - thickness 7 mm 09 HINTEGRA PE inlay - size 6 - thickness 9 mm 1 HINTEGRA - PE inlay - size 3 05 HINTEGRA PE inlay - size 3 - thickness 5 mm 1 06 HINTEGRA PE inlay - size 3 - thickness 6 mm 07 HINTEGRA PE inlay - size 3 - thickness 7 mm 1 09 HINTEGRA PE inlay - size 3 - thickness 9 mm 1 HINTEGRA Total Ankle Prosthesis Talar component with Pegs Primary Surgery Double coating: Porous Titanium & Hydroxyapatite (Ti and HAP), for optimal bone ingrowth (osteointegration). Cobalt Chromium alloy (CoCr). Anatomical conical shape. 7 sizes right and 7 sizes left components. HINTEGRA - Talar Component with Pegs - Right 11 HINTEGRA talar component with pegs-right - size HINTEGRA talar component with pegs-right - size HINTEGRA talar component with pegs-right - size HINTEGRA talar component with pegs-right - size HINTEGRA talar component with pegs-right - size HINTEGRA talar component with pegs-right - size HINTEGRA talar component with pegs-right - size 6 HINTEGRA - Talar Component with Pegs - Left 21 HINTEGRA talar component with pegs-left - size HINTEGRA talar component with pegs-left - size HINTEGRA talar component with pegs-left - size HINTEGRA talar component with pegs-left - size HINTEGRA talar component with pegs-left - size HINTEGRA talar component with pegs-left - size HINTEGRA talar component with pegs-left - size HINTEGRA Total Ankle Prosthesis (Optional) Tibial cortical screw Primary Surgery Stainless steel MNW. Optionally to be put only with tibial components. 3328S HINTEGRA cortical screw - Dia. 3.5 mm x L. 28 mm - tibia - size 0 33S HINTEGRA cortical screw - Dia. 3.5 mm x L. mm - size S HINTEGRA cortical screw - Dia. 3.5 mm x L. 34 mm - size S HINTEGRA cortical screw - Dia. 3.5 mm x L. 38 mm - size 3 33S HINTEGRA cortical screw - Dia. 3.5 mm x L. mm - size S HINTEGRA cortical screw - Dia. 3.5 mm x L. 42 mm - size 5 & 6 45
46 Total Ankle Replacement HINTEGRA Total Ankle Prosthesis Associated Instruments for Primary Surgery Generic base (upper tray) Trial tray 1 0 Talar base 46
47 Total Ankle Replacement 2 HINTEGRA Total Ankle Prosthesis Associated Instruments for Primary Surgery Instruments for LOWER TRAY Talar Base Drilling guide talus - Size 0 91 Drilling guide talus - Size 1 92 Drilling guide talus - Size 2 93 Drilling guide talus - Size 3 94 Drilling guide talus - Size 4 95 Drilling guide talus - Size 5 96 Drilling guide talus - Size 6 93 Tal. cut. guide-right - Size Tal. cut. guide-right - Size Tal. cut. guide-right - Size Tal. cut. guide-right - Size Tal. cut. guide-right - Size Tal. cut. guide-right - Size Tal. cut. guide-right - Size 6 93 Tal. cut. guide-left - Size Tal. cut. guide-left - Size Tal. cut. guide-left - Size Tal. cut. guide-left - Size Tal. cut. guide-left - Size Tal. cut. guide-left - Size Tal. cut. guide-left - Size 6 91 Screwdriver tibial screws 98 Spacer h 12 mm 1199 Talar impactor Cutting guide tibial revision Reamer talar dia. 6 mm 99 Peg drill talus - dia. 4.5 mm 9697 Holder trial talar implant 9699 Impactor trial talar implant Trial Tray HINTEGRA Tibial Trial-Right Tibial Trial-Right - Size Tibial Trial-Right - Size Tibial Trial-Right - Size Tibial Trial-Right - Size Tibial Trial-Right - Size Tibial Trial-Right - Size Tibial Trial-Right - Size 6 HINTEGRA Tibial Trial-Left Tibial Trial-Left - Size Tibial Trial-Left - Size Tibial Trial-Left - Size Tibial Trial-Left - Size Tibial Trial-Left - Size Tibial Trial-Left - Size Tibial Trial-Left - Size 6 HINTEGRA Talar Trial-Right 96 Talar Trial-Right - Size Talar Trial-Right - Size Talar Trial-Right - Size Talar Trial-Right - Size Talar Trial-Right - Size Talar Trial-Right - Size Talar Trial-Right - Size 6 HINTEGRA Talar Trial-Left 96 Talar Trial-Left - Size Talar Trial-Left - Size Talar Trial-Left - Size Talar Trial-Left - Size Talar Trial-Left - Size Talar Trial-Left - Size Talar Trial-Left - Size 6 HINTEGRA Trial inlay Trial inlay - thickness 5mm Trial inlay - thickness 6mm Trial inlay - thickness 7mm Trial inlay - thickness 9 mm Instruments for UPPER TRAY 9625 Tibial v positioning 96 V translation block Distractor Hintermann big model Tibial - Talar Holder Tibial impactor 94 Pins L. mm 95 Pins L. mm 96 Pins L. 45 mm 97 Depth gauge tibial Drilling guide dia. 2.7mm 9615 Alignment rod tibial 96 Connector v - tibial rod 9622 Saw blade Aesculap attachment 9623 Saw blade AO Synthes attachment 9624 Saw blade Stryker 6 attachment 9626 Saw blade Stryker attachment 9627 Saw blade Conmed Linvatec attachment 9635 Tib. cut. guide large 9636 Tib. cut. guide medium / standard 9637 Tib. cut. guide small 9645 Screwdriver Hex dia. 3.5 mm 9652 Imp / Extract pins 9656 Cutting block-talar cut / Medium - Standard 9657 Cutting block-talar cut - Small 9661 Holder PE inlays 9753 Cutting block proximal - tibial 9773 Cutting block distal - tibial 9934 Curved chisel mm rmm 9935 Straight chisel mm 2755 Parallel pliers 7-1/ Drill dia. 2.5 mm x L. 1 mm K-Wire dia. 1.6 x L. 1 mm K-Wire dia. 2.5 x L. 0 mm Sterilization container 90 HINTEGRA Container (Upper + Lower) including: 92 Stainless steel base upper, including: 94 Stainless steel base lower, including: 99 Generic base 99 Talar base 99 Pins insert 99 Thermo insert trial implants 9942 Lid pins insert 99 Lid trial implants 9963 Lid 1199 Silicone wedge 47
48 Total Ankle Replacement HINTEGRA Total Ankle Prosthesis Flat Cut Revision Surgery Double coating: Porous Titanium & Hydroxyapatite (Ti and HAP), for optimal bone ingrowth (osteointegration) HINTEGRA Total Ankle Prosthesis Flat Cut Tibial Component Revision Surgery Double coating: Porous Titanium & Hydroxyapatite (Ti and HAP), for optimal bone ingrowth (osteointegration). Two thicknesses: 8 and 12 mm. HINTEGRA - revision tibial component - right - 8 mm 1222 HINTEGRA revision tibial component-right-8 mm - size HINTEGRA revision tibial component-right-8 mm - size HINTEGRA revision tibial component-right-8 mm - size HINTEGRA revision tibial component-right -8 mm - size 5 HINTEGRA - revision tibial component - left - 8 mm 2222 HINTEGRA revision tibial component-left-8 mm - size HINTEGRA revision tibial component-left-8 mm - size HINTEGRA revision tibial component-left-8 mm - size HINTEGRA revision tibial component-left-8 mm - size 5 HINTEGRA - revision tibial component - right - 12 mm 1232 HINTEGRA revision tibial component-right-12 mm - size HINTEGRA revision tibial component-right-12 mm - size HINTEGRA revision tibial component-right-12 mm - size HINTEGRA revision tibial component-right-12 mm - size 5 HINTEGRA - revision tibial component - left - 12 mm 2232 HINTEGRA revision tibial component-left-12 mm - size HINTEGRA revision tibial component-left-12 mm - size HINTEGRA revision tibial component-left-12 mm - size HINTEGRA revision tibial component-left-12 mm - size HINTEGRA Total Ankle Prosthesis Flat Cut Talar Component Revision Surgery / Flat Talus For flat talus and revision surgeries. Double coating: Porous Titanium & Hydroxyapatite (Ti and HAP), for optimal bone ingrowth (osteointegration). HINTEGRA - flat cut talar component - right 1121 HINTEGRA revision talar component-right - size HINTEGRA revision talar component-right - size HINTEGRA revision talar component-right - size HINTEGRA revision talar component-right - size HINTEGRA revision talar component-right - size 5 HINTEGRA - flat cut talar component - left 2121 HINTEGRA revision talar component-left - size HINTEGRA revision talar component-left - size HINTEGRA revision talar component-left - size HINTEGRA revision talar component-left - size HINTEGRA revision talar component-left - size 5 HINTEGRA Total Ankle Prosthesis Cortical screw Stainless steel: MNW. Sterile. 3312S HINTEGRA cortical screw - dia. 3.5 mm x l. 12 mm - talus 3316S HINTEGRA cortical screw - dia. 3.5 mm x l. 16 mm - talus 33S HINTEGRA cortical screw - dia. 3.5 mm x l. mm - talus 3334S HINTEGRA cortical screw - dia. 3.5 mm x l. 34 mm - tibia - size S HINTEGRA cortical screw - dia. 3.5 mm x l. 38 mm - tibia - size 3 33S HINTEGRA cortical screw - dia. 3.5 mm x l. mm - tibia - size S HINTEGRA cortical screw - dia. 3.5 mm x l. 42 mm - tibia - size 5 & 6 48
49 Total Ankle Replacement 2 HINTEGRA Total Ankle Prosthesis PE Inlay Revision Surgery (See page 19 for product references.) Size of the PE inlay has to be the same as the talar component HINTEGRA Total Ankle Prosthesis Flat Cut Instruments for Revision Surgery 1 1 Instruments 1197 FC Talar Trial for Drilling-Right - Size FC Talar Trial for Drilling-Right - Size FC Talar Trial for Drilling-Right - Size FC Talar Trial for Drilling-Right - Size FC Talar Trial for Drilling-Right - Size Drill dia. 4.5 mm x L. 22 mm FC Talar trial for drilling Impactor Sterilization container HINTEGRA FC revision container includes: Container pocket HINTEGRA FC container base Lid for HINTEGRA FC container base FC Talar Trial for Drilling-Left - Size FC Talar Trial for Drilling-Left - Size FC Talar Trial for Drilling-Left - Size FC Talar Trial for Drilling-Left - Size FC Talar Trial for Drilling-Left - Size FC Spacer 4 mm for revision Tibial Component 8 mm FC Spacer 8 mm for revision Tibial Component 12 mm FC spacer 1197 FC Talar Cutting Guide-Right - Size FC Talar Cutting Guide-Right - Size FC Talar Cutting Guide-Right - Size FC Talar Cutting Guide-Right - Size FC Talar Cutting Guide-Right - Size FC Talar Cutting Guide-Left - Size FC Talar Cutting Guide-Left - Size FC Talar Cutting Guide-Left - Size FC Talar Cutting Guide-Left - Size FC Talar Cutting Guide-Left - Size Tibial depth gauge for tibial component 8 mm 9712 Tibial depth gauge for tibial component 12 mm 49
50 Total Ankle Replacement HINTEGRA Sensitive Total Ankle Prosthesis Primary Surgery The Hintegra Sensitive Total Ankle Prosthesis is also a three component design specially made for allergic patients. Materials: > > Talar & Tibial components:»» Cobalt Chromium alloy (CoCr) with Titanium Nitride (TiN) coating. > > PE inlay:»» Polyethylene (UHMWPE) HINTEGRA Sensitive Total Ankle Prosthesis Tibial Component Primary Surgery Anatomical shape for minimal contact stress to the bone. Cobalt Chromium alloy (CoCr) with Titanium Nitride (TiN) coating. 6 right and 6 left components. HINTEGRA sensitive - tibial component - right HINTEGRA sensitive - tibia-right - size HINTEGRA sensitive - tibia-right - size HINTEGRA sensitive - tibia-right - size HINTEGRA sensitive - tibia-right - size HINTEGRA sensitive - tibia-right - size HINTEGRA sensitive - tibia-right - size 6 HINTEGRA sensitive - tibial component - left HINTEGRA sensitive - tibia-left - size HINTEGRA sensitive - tibia-left - size HINTEGRA sensitive - tibia-left - size HINTEGRA sensitive - tibia-left - size HINTEGRA sensitive - tibia-left - size HINTEGRA sensitive - tibia-left - size 6
51 Total Ankle Replacement 2 HINTEGRA Sensitive Total Ankle Prosthesis Talar component with Pegs Primary Surgery Cobalt Chromium alloy (CoCr) with Titanium Nitride (TiN) coating. Anatomical conical shape. 5 sizes and 5 left components HINTEGRA - talar component with pegs - right HINTEGRA sensitive - talus -right - size HINTEGRA sensitive - talus -right - size HINTEGRA sensitive - talus -right - size HINTEGRA sensitive - talus -right - size HINTEGRA sensitive - talus -right - size 6 HINTEGRA - talar component with pegs - left HINTEGRA sensitive - talus-left - size HINTEGRA sensitive - talus-left - size HINTEGRA sensitive - talus-left - size HINTEGRA sensitive - talus-left - size HINTEGRA sensitive - talus-left - size HINTEGRA Sensitive Total Ankle Prosthesis PE Inlay (See page 45 for product references.) HINTEGRA PE inlays are sensitive compatible since metallic rods are made of titanium Alloy (TA6V). Size of the PE inlay has to be the same as the talar component HINTEGRA Sensitive Total Ankle Prosthesis (Optional) Tibial cortical screw Titanium alloy (TA6V). Optionally to be put only with tibial components. 35S Cortical Screw Ti - Dia. 3.5 mm x L. mm - Tibia - Size S Cortical Screw Ti - Dia. 3.5 mm x L. 34 mm - Tibia - Size S Cortical Screw Ti - Dia. 3.5 mm x L. 38 mm - Tibia - Size 3 35S Cortical Screw Ti - Dia. 3.5 mm x L. mm - Tibia - Size S Cortical Screw Ti - Dia. 3.5 mm x L. 42 mm - Tibia - Size 5 & 6 51
52 Total Ankle Replacement HINTEGRA Sensitive Total Ankle Prosthesis Flat Cut Revision Surgery The HINTEGRA Sensitive Total Ankle Prosthesis is also a three component design specially made for allergic patients. Materials: > > Talar & Tibial components:»» Cobalt Chromium alloy (CoCr) with Titanium Nitride (TiN) coating. > > PE inlay:»» Polyethylene (UHMWPE) HINTEGRA Sensitive Total Ankle Prosthesis Flat Cut Tibial Component Revision Surgery Anatomical shape for minimal contact stress to the bone. Cobalt Chromium alloy (CoCr) with Titanium Nitride (TiN) coating. 4 right and 4 left components HINTEGRA - Revision Tibial Component - Right - 8 mm HINTEGRA sensitive FC - tibia-right 8 mm - size HINTEGRA sensitive FC - tibia-right 8 mm - size HINTEGRA sensitive FC - tibia-right 8 mm - size HINTEGRA sensitive FC - tibia-right 8 mm - size 5 HINTEGRA - Revision Tibial Component - Left - 8 mm HINTEGRA sensitive FC - tibia-left 8 mm - size HINTEGRA sensitive FC - tibia-left 8 mm - size HINTEGRA sensitive FC - tibia-left 8 mm - size HINTEGRA sensitive FC - tibia-left 8 mm - size 5 HINTEGRA - Revision Tibial Component - Right - 12 mm HINTEGRA sensitive FC - tibia-right 12 mm - size HINTEGRA sensitive FC - tibia-right 12 mm - size HINTEGRA sensitive FC - tibia-right 12 mm - size HINTEGRA sensitive FC - tibia-right 12 mm - size 5 HINTEGRA - Revision Tibial Component - Left - 12 mm HINTEGRA sensitive FC - tibia-left 12 mm - size HINTEGRA sensitive FC - tibia-left 12 mm - size HINTEGRA sensitive FC - tibia-left 12 mm - size HINTEGRA sensitive FC - tibia-left 12 mm - size 5 52
53 Total Ankle Replacement 2 HINTEGRA Sensitive Total Ankle Prosthesis Flat Cut Talar component with Pegs Revision Surgery / Flat talus Cobalt Chromium alloy (CoCr) with Titanium Nitride (TiN) coating. 4 right and 4 left components HINTEGRA - Talar Component with Pegs - Right HINTEGRA sensitive FC - talus-right - size HINTEGRA sensitive FC - talus-right - size HINTEGRA sensitive FC - talus-right - size HINTEGRA sensitive FC - talus-right - size 5 HINTEGRA - Talar Component with Pegs - Left HINTEGRA sensitive FC - talus-left - size HINTEGRA sensitive FC - talus-left - size HINTEGRA sensitive FC - talus-left - size HINTEGRA sensitive FC - talus-left - size HINTEGRA Sensitive Total Ankle Prosthesis PE Inlay (See page 45 for product references.) HINTEGRA PE inlays are sensitive compatible since metallic rods are made of titanium Alloy (TA6V). Size of the PE inlay has to be the same as the talar component HINTEGRA Sensitive Total Ankle Prosthesis Flat Cut Cortical screw Titanium alloy (TA6V). 3512S Cortical Screw Ti - dia.3.5 x L. 12 mm - Talus 3516S Cortical Screw Ti - dia.3.5 x L. 16 mm - Talus 35S Cortical Screw Ti - dia.3.5 x L. mm - Talus 3534S Cortical Screw Ti - dia. 3.5 x L. 34 mm - Tibia - Size S Cortical Screw Ti - dia. 3.5 x L. 38 mm - Tibia - Size 3 35S Cortical Screw Ti - dia. 3.5 x L. mm - Tibia - Size S Cortical Screw Ti - dia. 3.5 x L. 42 mm - Tibia - Size 5 53
54 Ankle Fusion PANTA / PANTA XL Arthrodesis Nail The PANTA Nail is intended for use in tibiotalocalcaneal arthrodesis and treatment of trauma of the hindfoot and distal tibia. Cannulated Titanium Nail. Radio-transparent instrumentation system to apply compression. Multi-axial fixation: > > Oblique antero-posterior calcaneal screws. > > Medio-lateral tibial screws. 2 kind of screws: > > Oblique antero-posterior calcaneal screws.»» fully threaded»» partially threaded 5 mm diameter Titanium screw. Screw lengths: to 1 mm. Material of the nail, the screws and the end cap: Titanium alloy TA6V PANTA Nails (sterile) Dia. - length 00 Dia. mm - L. 1 mm 00 Dia. mm - L. 1 mm 01 Dia. 11 mm - L. 1 mm 01 Dia. 11 mm - L. 1 mm 02 Dia. 12 mm - L. 1 mm 02 Dia. 12 mm - L. 1 mm 03 Dia. 13 mm - L. 1 mm 03 Dia. 13 mm - L. 1 mm PANTA XL Nails (sterile) To be used only with Partially threaded Screws Dia. - length 5111 Dia. 11mm - L 2 mm 5141 Dia. 11mm - L 2 mm 5211 Dia. 12 mm - L 2 mm 5241 Dia. 12 mm - L 2 mm 5311 Dia. 13mm - L 2 mm 5341 Dia. 13mm - L 2 mm End cap (sterile) 0001 End cap 5005 Locking end cap 54
55 Ankle Fusion 2 PANTA Arthrodesis Nail Fully threaded screws dia. 5 mm (not for use with PANTA XL Arthrodesis Nail) 2 2 Length L. mm 22 L mm 25 L. 25 mm 27 L mm L. mm 35 L. 35 mm L. mm 45 L. 45 mm L. mm 55 L. 55 mm L. mm 65 L. 65 mm L. mm 75 L. 75 mm L. mm 85 L. 85 mm L. mm 95 L. 95 mm 10 L. 0 mm 15 L. 5 mm 11 L. 1 mm PANTA / PANTA XL Arthrodesis Nail Partially threaded lag screws dia. 5 mm 1 Length 51 L. mm 5122 L mm 5125 L. 25 mm 5127 L mm 51 L. mm 5135 L. 35 mm 51 L. mm 5145 L. 45 mm 51 L. mm 5155 L. 55 mm 51 L. mm 5165 L. 65 mm 51 L. mm 5175 L. 75 mm 51 L. mm 5185 L. 85 mm 51 L. mm 5195 L. 95 mm 5 L. 0 mm 5115 L. 5 mm 5111 L. 1 mm 0 55
56 Ankle Fusion PANTA Arthrodesis Nail Instruments 5103 Drill dia. 4.3 mm for Calcaneum Screws 5104 Drill dia. 4.3 mm for Tibial Screws 5105 Drill dia. 5.0 mm AO Attachment 5106 Long drill for calcaneus screws dia. 4.3 mm 5107 Cannulated Drill dia. 7.0 mm 5109 Cannulated Drill dia. 9.0 mm 5111 Reamer dia. 11 mm 5112 Reamer dia. 12 mm 5113 Reamer dia. 13 mm 5114 Reamer dia..5 mm 5115 Reamer dia mm 5116 Reamer dia mm 5117 Reamer dia mm 5121 T Handle AO Attachment 5128 Internal Protecting Sleeve dia. 3.2 mm 5129 Central Internal Protecting Sleeve dia. 9.0 mm 51 External Protecting Sleeve 5132 Pin dia. 3.2 mm x L. 0 mm 51 Trocard Awl 5191 Support Device 5191 Nail Fixation Axis Toothed Wheel Threaded Axis 5191 Compression Device Teflon Ring Compression Wheel 5191 Depth Gauge Compression Rod Short Drill Guide dia. 4.3 mm L. mm 5191 Long Drill Guide dia. 4.3 mm L. 1 mm Drill Guide dia. 5.0 mm Short Drill Guide dia. 7.0 mm L. mm Drill Guide dia. 7.0 mm L. 1 mm 5192 Hexagonal screwdriver tip dia. 3.5 mm Hexagonal screwdriver dia. 3.5 mm 5315 Tap dia. 6.5 mm PANTA Optional Instruments 51 Reamer dia. mm 51 Quick Coupling AO 5102 Short drill for calcaneus screws used with medium drill guide dia. 4.3 mm 5108 Long drill for calcaneus screws used with medium drill guide dia. 4.3 mm 5134(S) Guide wire dia. 3.2 mm, L. 0 mm (sterile upon request) Medium drill guide dia. 4.3 mm Medium soft tissue protector dia. 7 mm PANTA XL Associated Instruments (optional) 53 Conical reamer dia..5 mm 5321 Conical reamer dia mm 5322 Conical reamer dia mm 5323 Conical reamer dia mm Reamer holder PANTA Sterilization Container 5199 PANTA sterilization container including: 5199 PANTA base PANTA Insert 5199 Lid 1199 Silicone wedge (S) means available in sterile and non sterile 56
57 Ankle Fusion 2 PANTA Arthrodesis Nail - Associated Instruments PANTA XL conical reamers (optional) 1 0 PANTA XL reamer holder (optional) 57
58 Ankle Fusion PANTA Removal Set 5199 Sliding hammer 5192 Handle extractor 9645 Screwdriver Hex dia.3.5 mm External protection sleeve dia.6.5 mm Sterilization container 5199 PANTA Removal sterilization container 1199 Silicone wedge Basis 9960 Lid
59 Ankle Fusion 2 Advansys TTC Fusion Plate The TTC Plates are intended for arthrodesis of the ankle joint and calcanea, osteotomies, fusions and replantations of small bones in the ankle and distal tibia. 4 sizes of implants > > 4 right & 4 left plates (in 6,7,8,9 holes configurations). Screw dia.4.5 mm for fixation of the tibia and talus Length 14 mm to 65 mm. Screw dia.6.5 mm for fixation of the calcaneus Length mm to 65 mm. Lock-screws for 4.5 mm dia. screws. Lock-screws for 6.5 mm dia. screws. Material: > > Plate: Stainless Steel 316LVM. > > Screw: Titanium TA6V Advansys TTC Fusion Plate 11S ADVANSYS TTCP Right-6 holes 12S ADVANSYS TTCP Right-8 holes 13S ADVANSYS TTCP Right-7 holes 14S ADVANSYS TTCP Right-9 holes 1811S ADVANSYS TTCP Left-6 holes 1812S ADVANSYS TTCP Left-8 holes 1813S ADVANSYS TTCP Left-7 holes 1814S ADVANSYS TTCP Left-9 holes Surfix Locking screw Sterile Titanium dia. 4.5 mm + lock-screw Length s L. 14 mm s L. 16 mm s L. 18 mm 2854s L. mm s L. 22 mm s L. 24 mm s L. 26 mm s L. 28 mm 2854s L. mm s L. 35 mm 2854s L. mm s L. 45 mm 2854s L. mm s L. 55 mm 2854s L. mm s L. 65 mm 1850s SURFIX Lock Screw dia. 4.5 mm Surfix Locking screw Sterile Titanium dia. 6.5 mm + lock-screw Length 2856s L. mm s L. 25 mm 2856s L. mm s L. 35 mm 2856s L. mm s L. 45 mm 2856s L. mm s L. 55 mm 2856s L. mm s L. 65 mm 1850s SURFIX lock Screw dia. 6.5 mm Optional instrument MA3511 Surfix screw extractor 3.5 mm 0 59
60 Ankle Fusion ADVANSYS TTC Fusion Plate Instruments Screwdriver hexa 2.5 x L. 191 mm Screwdriver hexa dia.3.5 x L. 2 mm AO Screwdriver tip hexa dia.2.5 x L. 76 mm AO Screwdriver tip hexa dia.3.5 x L. 76 mm Drilling guide dia.3.0 mm Drilling guide dia.3.5 mm AO Drill dia.3.0 x L. 1 mm Drill dia.3.5 x L. 0 mm 2 Compression forceps L. 2 mm Bending forceps L hole dia.6.5 mm Depth gauge hole dia.4.5 mm Depth gauge hole dia.6.5 mm K-Wire dia.1.6 x L. 1 mm K-Wire dia.2.5 x L. 0 mm 1 TTCP trial implant 6 holes 2 TTCP trial implant 8 holes 3 TTCP trial implant 7 holes 4 TTCP trial implant 9 holes Sterilization container 1691 ADVANSYS Hindfoot sterilization container including: Base 9960 Lid 1694 Cylinder 1695 TTCP trial implant module 1199 Silicone wedge Optional instrument MA3511 Surfix screw extractor 3.5 mm
61 Ankle Fusion 2 TIBIAXYS Ankle Arthrodesis Plating System TIBIAXYS Distal Tibia and Fibula Osteotomy Plating Systems The Tibiaxys Plates are intended for fixation of arthrodesis osteotomies and fractures of ankle joint, distal tibia and fibula. Each plate is anatomically contoured and available in 1 size. 2 systems of 2 anterior plates for ankle fusion (green left and blue right systems, including 1 antero-medial and 1 antero-lateral plates). 3 different plates for distal tibia osteotomies (medial plate, left lateral / right medial anterior plate, left medial/ right lateral anterior plate). 2 different plates for fibula osteotomies (4 and 6 holes). dia. 3.5 mm Surfix or Surfix -Alpha screws and lock-screws. dia. 4.0 mm cortical screws (only for 1 hole in each plate for the ankle fusion). Material: > > Plates: Titanium alloy TA6V. > > Surfix and Surfix -Alpha screws and lock-screws: Titanium alloy TA6V. > > Cortical screws: Titanium alloy TA6V ARTHRODESIS Ankle Arthrodesis Plates OSTEOTOMY Distal Tibia Osteotomy Plates s TIBIAXYS Arthrodesis Plate / Left antero-medial 1 1 Left antero-lateral plate S Left: green system Antero-Medial / Antero-Lateral plates 11s s 11s 11s TIBIAXYS Arthrodesis Plate / Left antero-lateral TIBIAXYS Arthrodesis Plate / right antero-medial TIBIAXYS Arthrodesis Plate / right antero-lateral s s TIBIAXYS Osteotomy Plate / Lateral-right / Medial-left 1 Left antero-medial plate s 11s TIBIAXYS Osteotomy Plate / Medial-right / Lateral-left s TIBIAXYS Osteotomy Plate / Medial 1 1 Right antero-medial plate 11s Right: blue system Medial plate 1514s 1516s TIBIAXYS Fibula Osteotomy Plate 4 holes TIBIAXYS Fibula Osteotomy Plate 6 holes 0 Medial plate s Optional instrument MA3511 Surfix screw extractor 3.5 mm Right antero-lateral plate 11S Fibula Osteotomy Plates 6 holes 1516s 4 holes 1514s 61
62 Ankle Fusion TIBIAXYS Ankle Arthrodesis Plating System TIBIAXYS Distal Tibia and Fibula Osteotomy Plating Systems Surfix -Alpha variable angle locking screws Sterile Titanium dia. 3.5 mm + lock-screw Length Surfix Locking screws Sterile Titanium dia. 3.5 mm + lock-screw Length Sterile Titanium cortical screw dia. 4.0 mm Length s s L. mm L. 12 mm 2853s s L. mm L. 12 mm 12s 1242s L. mm L. 42 mm s s L. 14 mm L. 16 mm s s L. 14 mm L. 16 mm 1246s 12s L. 46 mm L. mm s 2953s L. 18 mm L. mm s 2853s L. 18 mm L. mm 1255s 12s L. 55 mm L. mm s s L. 22 mm L. 24 mm s s L. 22 mm L. 24 mm 1265s 12s L. 65 mm L. mm s s L. 26 mm L. 28 mm s s L. 26 mm L. 28 mm 1275s 12s L. 75 mm L. mm s s L. mm L. 32 mm 2853s s L. mm L. 32 mm 1285s 12s L. 85 mm L. mm s s L. 34 mm L. 36 mm s S L. 34 mm L. 36 mm s L. 0 mm s 2953s L. 38 mm L. mm S 2853S L. 38 mm L. mm s s L. 44 mm L. 48 mm S S L. 44 mm L. 48 mm Optional instrument 2953s 1950s L. mm Lock-screw dia. 3.5 mm 2853S 1850s L. mm Lock-screw dia. 3.5 mm MA3511 Surfix screw extractor 3.5 mm 62
63 Ankle Fusion 2 Tibiaxys Instruments K-Wire dia.1.6 x L. 1 mm K-Wire dia.2.5 x L. 0 mm 1593 Wedge thickness 3 mm 1596 Wedge thickness 6 mm 1599 Wedge thickness 9 mm 1591 Cortical screw drilling guide dia.3.0 mm 15 Depth gauge for dia.4.0 mm screws Compression forceps guide 1597 Compression forceps screw dia.4.0 x L. mm Compression forceps screw dia.4.0 x L. 55 mm 1597 Compression forceps screw dia.4.0 x L. mm 2135 Surfix Alpha drilling guide dia.2.7 mm Screwdriver hexalobular T Depth gauge for dia. 3.5 mm screws AO Screwdriver tip hexa 2.0 x L. 76 mm AO Screwdriver tip hexa dia.2.5 x L. 76 mm AO drill dia.2.7 x L. 125 mm AO Drill dia.3.0 x L. 1 mm Drilling guide dia.2.7 mm Screwdriver hexa dia. 2.0 x L. 1 mm Screwdriver hexa dia. 2.5 x L. 191 mm 2199 Compression forceps L. 2 mm 15 Trial plate Left Antero-Medial 15 Trial plate Left Antero-Lateral 1591 Trial plate Right Antero-Medial (optional) 1591 Trial plate Right Antero-Lateral (optional) Sterilization container Tibiaxys sterilization container including 1599 Base 9960 Lid 1199 Silicone wedge Trial container 15 Left medial anterior trial plate 15 Left lateral anterior trial plate 1591 Right medial anterior trial plate 15 Right lateral anterior trial plate Container 1571 Base 9942 Lid Optional instrument MA3511 Surfix screw extractor 3.5 mm 63
64 Minimally Invasive Achilles Tendon Ruptures ACHILLON Minimal Invasive Achilles Tendon Suture System Single Use The ACHILLON System is a method to treat acute Achilles tendon ruptures. Needle driver. Suture size: USP (3.5 metric). Materials: > > Polycarbonate. > > Stainless steel 316LVM. 11 ACHILLON + 2 Needles and 1 Needle Driver
65 Surgical Instruments 2 Hintermann Distractors Range of 4 spreading forceps. 2 2 For each of the 4 models, two pins (2.5 mm for the large model, and 1.6 or 2.5 mm diameter for the small one) are slid into the appropriate holes of the spreading forceps, and are then introduced at appropriate localisation into each of the two bone fragments to be outspread. The spreader is then distracted, and locked when the distance between the two fragments is reached. The flexible positioning of these spreading forceps enables them not to interfere with the main procedure. These spreaders are available in two different sizes (15 cm long, or 21 cm long), and with closed or outspread arms, making them fitting many possible indication. Hintermann Distractor open handle Hintermann Small Distractor 15 cm Hintermann Distractor Small open handle Hintermann Distractor Small closed handle Hintermann Large Distractor 21 cm Hintermann Distractor Big model Hintermann Distractor Large closed handle Hintermann Distractor closed handle 0 65
66 Surgical Instruments Osteoguides Suggested use: > > Scarf osteotomy. > > Chevron osteotomy. > > Basal osteotomy. > > Arthrodesis. > > Wedge osteotomies (Akin ). Details: > > Hole for K-Wire: Diameter 1.0 to 1.6 mm. > > Knurled handle for optimal grip. > > Blade thickness maxi 0.8 mm. > > Positioning tip Longitudinal 2212 Transversal Transversal osteotomy guide Longitudinal osteotomy guide K-FIX K-Wire End Protector The K-Fix pin protector is a simple system to protect patients or hospital staff from K-Wires sharp ends. Delivered sterile. 11 Length K-FIX for K-WIRE - Dia. 0.8 mm to 2.0 mm (5 units per pack) Weil Clamp Length 1196 Weil Clamp 66
67 Surgical Instruments 2 General Optional Instruments Scalpel Handle N 3 92 Scalpel Handle N Beyer Bone Rongeur Forceps 94 Backhaus Towel Forceps 2 95 Beckmann Mini Retractor 96 Museux Forceps (14 cm) 0 97 Lange- Hohmann Retractor 98 Liston Bone Cutting Forceps 1 99 Ombredanne Square Points 9911 Wolkmann Retractor Wolkmann Curette 9913 Lambotte Raspatory 12 mm MA3511 Surfix screw extractor 3.5 mm Guillaume Gouge 8 mm 9915 Mac Even Hammer Redon Needle 9917 Finochieto Needle Holder 13 cm Pauwels Osteotomes 15 mm 9919 Farabeuf Retractor 12 cm 1 99 Dissection Forceps (GR) 14 cm 9921 Dissection Forceps Curved 14 cm Metzenbaum Scissors 9923 Straight Scissors Mayo Scissors 9925 Leriche Forceps Verbrugge Bone Holding Forceps 9927 Poirier Gouge (14 mm) Metatarsal Head Extractor MA3511 Surfix screw 3.5 mm extractor Hintermann Distractor / Large Closed Jaws Hintermann Distractor / Small Open Jaws Hintermann Distractor / Small Closed Jaws 1196 Weil clamp 9928 Inge Retractor 9929 Banaleck Forceps Right 99 Banaleck Forceps Left 1199 Silicone wedge Instrument Tray 67
68 Surgical Instruments Post-Operative Shoes Flat Post Operative Shoe 9901 Small 9902 Medium 9903 Large 9904 X-Large Wedged Hell Post Operative Shoe 99 Size 4 to Size 7 to Size 9 to
Integra Extremity Reconstruction Lower Extremity Product Catalogue
 Integra Extremity Reconstruction Lower Extremity Product Catalogue Table of contents 2 Table of contents Integra Forefoot Solutions 5 Forefoot Set 6 BOLD Compression Screw dia. 2.5 mm / 3.0 mm / 3.7 mm
Integra Extremity Reconstruction Lower Extremity Product Catalogue Table of contents 2 Table of contents Integra Forefoot Solutions 5 Forefoot Set 6 BOLD Compression Screw dia. 2.5 mm / 3.0 mm / 3.7 mm
Integra. Solustaple Standard staple SURGICAL TECHNIQUE. Uni-Clip Compression staple. Products for sale in Europe, Middle-East and Africa only.
 eng Integra Solustaple Standard staple SURGICAL TECHNIQUE Uni-Clip Compression staple Table of Contents Solustaple Standard staple...04 Implants Details...04 Indications...04 Instruments Details...04 Surgical
eng Integra Solustaple Standard staple SURGICAL TECHNIQUE Uni-Clip Compression staple Table of Contents Solustaple Standard staple...04 Implants Details...04 Indications...04 Instruments Details...04 Surgical
Integra. surgical technique. Advansys Midfoot Plating System. eng. D.L.P. Dorsal Lisfranc Plate. M.L.P. Medial Lisfranc Plate
 eng Integra Advansys Midfoot Plating System surgical technique D.L.P. Dorsal Lisfranc Plate M.L.P. Medial Lisfranc Plate Table of Contents Advansys Medial Lisfranc Plate (DLP)... 4 Indications... 4 Contraindications...
eng Integra Advansys Midfoot Plating System surgical technique D.L.P. Dorsal Lisfranc Plate M.L.P. Medial Lisfranc Plate Table of Contents Advansys Medial Lisfranc Plate (DLP)... 4 Indications... 4 Contraindications...
Cover Back Cover EXTREMITY Germany (Fax)
 Cover EXTREMITY Table of Contents Ordering and Policy Information... ii - iv Soft Tissue Products 3-12 Integra Bilayer Matrix Wound Dressing... 3 Integra Dermal Regeneration Template... 4 Integra Flowable
Cover EXTREMITY Table of Contents Ordering and Policy Information... ii - iv Soft Tissue Products 3-12 Integra Bilayer Matrix Wound Dressing... 3 Integra Dermal Regeneration Template... 4 Integra Flowable
Product Catalog. newdeal HALLU -FIX. New ideas for foot surgery
 Reconstructive Surgery Product Catalog newdeal New ideas for foot surgery HALLU -FIX Table of Contents Ordering and Policy Information... ii - iv Soft Tissue Products 3-9 Integra Bilayer Matri Wound Dressing...
Reconstructive Surgery Product Catalog newdeal New ideas for foot surgery HALLU -FIX Table of Contents Ordering and Policy Information... ii - iv Soft Tissue Products 3-9 Integra Bilayer Matri Wound Dressing...
Integra BraIn MaPPIng
 Integra BraIn MaPPIng Product catalogue Innovations. For Life. brain mapping Ordering and Warranty Information Pricing: Prices stated in specific written quotations are firm for thirty days from the date
Integra BraIn MaPPIng Product catalogue Innovations. For Life. brain mapping Ordering and Warranty Information Pricing: Prices stated in specific written quotations are firm for thirty days from the date
Integra. ADVANSYS Plating System SURGICAL TECHNIQUE
 Integra ADVANSYS Plating System SURGICAL TECHNIQUE Table of Contents Dorsal Lisfranc Plate Indications...2 Contraindications...2 Description...2 Surgical Technique...3 Surgical Site Preparation...3 Step
Integra ADVANSYS Plating System SURGICAL TECHNIQUE Table of Contents Dorsal Lisfranc Plate Indications...2 Contraindications...2 Description...2 Surgical Technique...3 Surgical Site Preparation...3 Step
Astrid Modular Forefoot System. Surgical Technique
 Astrid Modular Forefoot System Surgical Technique Table of Contents Astrid a System for Surgery of the Forefoot... Scarf Osteotomies...6 Stabilization by Means of Two Parallel Double-Threaded Screws Introduced
Astrid Modular Forefoot System Surgical Technique Table of Contents Astrid a System for Surgery of the Forefoot... Scarf Osteotomies...6 Stabilization by Means of Two Parallel Double-Threaded Screws Introduced
Merete BLP. Surgical Technique. - Bunion Locking Plate - Low Profile Locking Bone Plate System
 Merete BLP - Bunion Locking Plate - Low Profile Locking Bone Plate System Surgical Technique Merete Medical, Inc. 49 Purchase Street Rye, N.Y. 10580 Phone: 914 967-1532 www.merete-medical.com - Surgical
Merete BLP - Bunion Locking Plate - Low Profile Locking Bone Plate System Surgical Technique Merete Medical, Inc. 49 Purchase Street Rye, N.Y. 10580 Phone: 914 967-1532 www.merete-medical.com - Surgical
ADVANSYS Midfoot plating system
 T.T.C. Tibio-Talo-Calcaneus plate TTC ENGLISH ADVANSYS Midfoot plating system Surgical technique Or t h o pa e d i c s LOWER EXTREMITY T.T.C. (DORSAL LISFRANC PLATE) Introduction Indications The Newdeal
T.T.C. Tibio-Talo-Calcaneus plate TTC ENGLISH ADVANSYS Midfoot plating system Surgical technique Or t h o pa e d i c s LOWER EXTREMITY T.T.C. (DORSAL LISFRANC PLATE) Introduction Indications The Newdeal
eng Integra surgical technique Large Qwix Products for sale in Europe, Middle-East and Africa only.
 eng Integra Large Qwix surgical technique Positionning Positioning and Fixation fixation Screw screw Table of Contents Concept...04 Indications...04 Contraindications...04 Implant details...05 Description...06
eng Integra Large Qwix surgical technique Positionning Positioning and Fixation fixation Screw screw Table of Contents Concept...04 Indications...04 Contraindications...04 Implant details...05 Description...06
ORTHOLOC CROSSCHECK TECHNOLOGY OVERVIEW FOR HOSPITAL VALUE ANALYSIS COMMITTEE
 ORTHOLOC CROSSCHECK TECHNOLOGY OVERVIEW FOR HOSPITAL VALUE ANALYSIS COMMITTEE Indications for Use The ORTHOLOC 3Di Foot Reconstruction System is intended for use in stabilization of fresh fractures, revision
ORTHOLOC CROSSCHECK TECHNOLOGY OVERVIEW FOR HOSPITAL VALUE ANALYSIS COMMITTEE Indications for Use The ORTHOLOC 3Di Foot Reconstruction System is intended for use in stabilization of fresh fractures, revision
Foot Plating System Product rationale
 Product rationale CONTENTS Introduction 2 Foot plating system 2.7mm Universal Locking Plate 2.7mm 3 Metatarsophalangeal Plate 4 Opening Wedge Locking Plate 5 Opening Wedge Osteotomy Plate 5 Foot plating
Product rationale CONTENTS Introduction 2 Foot plating system 2.7mm Universal Locking Plate 2.7mm 3 Metatarsophalangeal Plate 4 Opening Wedge Locking Plate 5 Opening Wedge Osteotomy Plate 5 Foot plating
ToeMobile. Surgical Technique. Great Toe Endoprosthesis
 Great Toe Endoprosthesis Surgical Technique Merete Medical, Inc. 99 Purchase Street Rye, N.Y. 10580 Phone: 914 967-1532 Fax: 914-967-1542 E-Mail: service@merete-medical.com www.merete-medical.com - Description
Great Toe Endoprosthesis Surgical Technique Merete Medical, Inc. 99 Purchase Street Rye, N.Y. 10580 Phone: 914 967-1532 Fax: 914-967-1542 E-Mail: service@merete-medical.com www.merete-medical.com - Description
large qwix Positioning and fixation screws Surgical Technique lower large qwix English PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY
 large qwix Positioning and fixation screws Dia. 7.5 mm Dia. 5.5 mm large qwix English Surgical Technique orthopedics lower extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY LARGE qwix
large qwix Positioning and fixation screws Dia. 7.5 mm Dia. 5.5 mm large qwix English Surgical Technique orthopedics lower extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY LARGE qwix
Surgical technique. Angular Stable X-Plate and 2-Hole Plate. For osteotomies, arthrodeses and fractures of the foot.
 Surgical technique Angular Stable X-Plate and 2-Hole Plate. For osteotomies, arthrodeses and fractures of the foot. Table of Contents Indications 4 Implants 5 X-plate: Crescentic osteotomy 6 X-plate:
Surgical technique Angular Stable X-Plate and 2-Hole Plate. For osteotomies, arthrodeses and fractures of the foot. Table of Contents Indications 4 Implants 5 X-plate: Crescentic osteotomy 6 X-plate:
LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System.
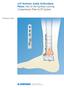 LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction
LCP Anterior Ankle Arthrodesis Plates. Part of the Synthes Locking Compression Plate (LCP) System. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction
Merete PlantarMAX Lapidus Plate Surgical Technique. Description of Plate
 Merete PlantarMAX Lapidus Plate Surgical Technique Description of Plate Merete Medical has designed the PlantarMax; a special Plantar/Medial Locking Lapidus plate which places the plate in the most biomechanically
Merete PlantarMAX Lapidus Plate Surgical Technique Description of Plate Merete Medical has designed the PlantarMax; a special Plantar/Medial Locking Lapidus plate which places the plate in the most biomechanically
CHARLOTTE Lisfranc. Recontruction System SURGICAL TECHNIQUE
 CHARLOTTE Lisfranc Recontruction System SURGICAL TECHNIQUE Contents Preface 3 Chapter 1 4 Chapter 2 4 4 4 4 4 Chapter 3 5 5 5 6 6 6 7 7 8 Chapter 4 8 8 9 10 Appendix 10 Introduction Indications and Contraindications
CHARLOTTE Lisfranc Recontruction System SURGICAL TECHNIQUE Contents Preface 3 Chapter 1 4 Chapter 2 4 4 4 4 4 Chapter 3 5 5 5 6 6 6 7 7 8 Chapter 4 8 8 9 10 Appendix 10 Introduction Indications and Contraindications
NeoFit. Metatarsophal angeal Arthrodesis Pl ate
 NeoFit Metatarsophal angeal Arthrodesis Pl ate 2 NEOFIT - TABLE OF CONTENTS PRODUCT DESCRIPTION 3 INDICATIONS 6 Download «PRINT» version (white background) SURGICAL TECHNIQUE 7 1 - Incision 8 2 - Preparation
NeoFit Metatarsophal angeal Arthrodesis Pl ate 2 NEOFIT - TABLE OF CONTENTS PRODUCT DESCRIPTION 3 INDICATIONS 6 Download «PRINT» version (white background) SURGICAL TECHNIQUE 7 1 - Incision 8 2 - Preparation
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
The Flower Medial Column Fusion Plate
 The Flower Medial Column Fusion Plate PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION
The Flower Medial Column Fusion Plate PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION
MICA. Minimally Invasive Foot Surgery CHEVRON OSTEOTOMY SURGIC AL TECHNIQUE
 MICA Minimally Invasive Foot Surgery CHEVRON OSTEOTOMY SURGIC AL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 5 Indications and Warnings Chapter 3 6 Patient Positioning and Set Up Chapter 4 7
MICA Minimally Invasive Foot Surgery CHEVRON OSTEOTOMY SURGIC AL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 5 Indications and Warnings Chapter 3 6 Patient Positioning and Set Up Chapter 4 7
The Vilex FUZETM. Dual Thread Screw & Intramedullary Nail in One Implant. The Ultimate TTC Arthrodesis Internal Fixator
 The Vilex FUZETM Dual Thread Screw & Intramedullary Nail in One Implant The Ultimate TTC Arthrodesis Internal Fixator Introduction The Vilex FUZE TM TTC Arthrodesis Compression Nail combines the attributes
The Vilex FUZETM Dual Thread Screw & Intramedullary Nail in One Implant The Ultimate TTC Arthrodesis Internal Fixator Introduction The Vilex FUZE TM TTC Arthrodesis Compression Nail combines the attributes
Integra. Solutions for Hand and Plastic Surgeons. Products for sale in Europe, Middle-East and Africa only
 Integra Solutions for Hand and Plastic Surgeons Products for sale in Europe, Middle-East and Africa only nerve injury > > Wrapping technique > > Entubulation technique wrist Arthritis & deformity > > Limited
Integra Solutions for Hand and Plastic Surgeons Products for sale in Europe, Middle-East and Africa only nerve injury > > Wrapping technique > > Entubulation technique wrist Arthritis & deformity > > Limited
Surgical Technique Guide
 Guide CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The Align Anterior Ankle Fusion Plate is intended to facilitate arthrodesis of the
Guide CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The Align Anterior Ankle Fusion Plate is intended to facilitate arthrodesis of the
Foot & Ankle. EasyClip. Osteosynthesis Compression Staples. Foot & Ankle
 Foot & Ankle EasyClip Osteosynthesis Compression Staples Foot & Ankle Operative Technique EasyClip Osteosynthesis Compression Staples 2 This publication sets forth detailed recommended procedures for using
Foot & Ankle EasyClip Osteosynthesis Compression Staples Foot & Ankle Operative Technique EasyClip Osteosynthesis Compression Staples 2 This publication sets forth detailed recommended procedures for using
TIBIAXYS ANKLE FUSION
 TIBIAXYS ANKLE FUSION SURGICAL TECHNIQUE TIBIAXYS Ankle Fusion Plate features Anatomically contoured plates The plates are designed to approximate the patient s bony and soft tissue anatomy The plate designs
TIBIAXYS ANKLE FUSION SURGICAL TECHNIQUE TIBIAXYS Ankle Fusion Plate features Anatomically contoured plates The plates are designed to approximate the patient s bony and soft tissue anatomy The plate designs
CHARLOTTE. 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE
 CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGICAL TECHNIQUE Surgical Technique as described by: Robert Anderson, MD Bruce
CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGICAL TECHNIQUE Surgical Technique as described by: Robert Anderson, MD Bruce
DuaFit. Proximal Interphal angeal Impl ant
 DuaFit Proximal Interphal angeal Impl ant DUAFIT - TABLE OF CONTENTS PRODUCT DESCRIPTION 3 INDICATIONS 6 SURGICAL TECHNIQUE #1 7 Without guide wire (DuaFit 0 10 17 ) SURGICAL TECHNIQUE #2 14 With guide
DuaFit Proximal Interphal angeal Impl ant DUAFIT - TABLE OF CONTENTS PRODUCT DESCRIPTION 3 INDICATIONS 6 SURGICAL TECHNIQUE #1 7 Without guide wire (DuaFit 0 10 17 ) SURGICAL TECHNIQUE #2 14 With guide
Contents - Merete Foot Family
 Merete Foot Family Contents - Merete Foot Family MetaFix I MetaFix BLP MetaCun DuoMetaCun MetaFix OpenWedge Page Page Page 6 Page 7 Page 8 MetaFix Ludloff MetaFix MTP Page 0 Page ToeMobile DuoThread Scarf
Merete Foot Family Contents - Merete Foot Family MetaFix I MetaFix BLP MetaCun DuoMetaCun MetaFix OpenWedge Page Page Page 6 Page 7 Page 8 MetaFix Ludloff MetaFix MTP Page 0 Page ToeMobile DuoThread Scarf
SHOULDER EXTRACTION INSTRUMENTS
 SHOULDER EXTRACTION INSTRUMENTS PRODUCT OVERVIEW Introducing the orthopaedic industry s first dedicated Shoulder Extraction Instrument set. DePuy Synthes Joint Reconstruction has created this set of instruments
SHOULDER EXTRACTION INSTRUMENTS PRODUCT OVERVIEW Introducing the orthopaedic industry s first dedicated Shoulder Extraction Instrument set. DePuy Synthes Joint Reconstruction has created this set of instruments
Variable Angle LCP Forefoot/Midfoot System 2.4/2.7. Procedure specific plates for osteotomies, arthrodeses and fractures of the foot.
 Variable Angle LCP Forefoot/Midfoot System 2.4/2.7. Procedure specific plates for osteotomies, arthrodeses and fractures of the foot. Compression technology Variable angle locking technology Anatomic and
Variable Angle LCP Forefoot/Midfoot System 2.4/2.7. Procedure specific plates for osteotomies, arthrodeses and fractures of the foot. Compression technology Variable angle locking technology Anatomic and
Flower Medium Headless & Cannulated Screws
 Flower Medium Headless & Cannulated Screws PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION
Flower Medium Headless & Cannulated Screws PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation.
 LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
SURGICAL TECHNIQUE CHI. Cannulated Hemi Implants 1 ST MPJ ARTHROPLASTY
 CHI Cannulated Hemi Implants 1 ST MPJ ARTHROPLASTY SURGICAL TECHNIQUE CHI Cannulated Hemi Implants Design Rationale Pain of the great toe joint often leads to altered lifestyles. The goal of MPJ implant
CHI Cannulated Hemi Implants 1 ST MPJ ARTHROPLASTY SURGICAL TECHNIQUE CHI Cannulated Hemi Implants Design Rationale Pain of the great toe joint often leads to altered lifestyles. The goal of MPJ implant
Integra. Uni CP Compression Plating System PRODUCTS FOR SALE IN EUROPE, MIDDLE EAST AND AFRICA ONLY
 TM Integra Uni CP Compression Plating System S U R G I C A L T E C H N I Q U E Uni CP Compression Plating System Table of contents Indications...03 Contraindications...03...03 Surgical Technique... 04
TM Integra Uni CP Compression Plating System S U R G I C A L T E C H N I Q U E Uni CP Compression Plating System Table of contents Indications...03 Contraindications...03...03 Surgical Technique... 04
Pre-Operative Planning. Positioning of the Patient
 Surgical Technique Pre-Operative Planning Decide upon the size and angle of the barrel plate to be used from measuring the x-rays. To maximise the sliding action when using shorter lag screws, the Short
Surgical Technique Pre-Operative Planning Decide upon the size and angle of the barrel plate to be used from measuring the x-rays. To maximise the sliding action when using shorter lag screws, the Short
Use of the 20 Memory Staple in Osteotomies of Fusions of the Forefoot
 168 Forefoot Reconstruction Use of the 20 Memory Staple in Osteotomies of Fusions of the Forefoot Definition, History, Generalities This staple first provides a permanent compression both in the prongs
168 Forefoot Reconstruction Use of the 20 Memory Staple in Osteotomies of Fusions of the Forefoot Definition, History, Generalities This staple first provides a permanent compression both in the prongs
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
AxSOS Locking Plate System
 AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment 1 2 Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5mm Compression Plates 5 Reconstruction and 1/3 Tubular Locking
AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment 1 2 Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5mm Compression Plates 5 Reconstruction and 1/3 Tubular Locking
Distal Radius Plate Instrument and Implant Set. Discontinued December 2017 DSUS/TRM/0916/1063(1)
 Distal Radius Plate Instrument and Implant Set Surgical Technique Discontinued December 2017 DSUS/TRM/0916/1063(1) The Distal Radius Plates Indications For fixation of fractures and osteotomies, including
Distal Radius Plate Instrument and Implant Set Surgical Technique Discontinued December 2017 DSUS/TRM/0916/1063(1) The Distal Radius Plates Indications For fixation of fractures and osteotomies, including
Forefoot/Midfoot Plating System
 Surgical Technique Forefoot/Midfoot Plating System Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that
Surgical Technique Forefoot/Midfoot Plating System Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that
MPJ Implant System. Patents Pending Worldwide
 MPJ Implant System Patents Pending Worldwide OVERVIEW ACTIVE STABILIZATION The DynaFORCE Active Stabilization System is the first to offer a hybrid implant system designed to address the most common rearfoot,
MPJ Implant System Patents Pending Worldwide OVERVIEW ACTIVE STABILIZATION The DynaFORCE Active Stabilization System is the first to offer a hybrid implant system designed to address the most common rearfoot,
DYNAMIC, TRANSVERSE COMPRESSION. Low Profile, Anatomic Design, Type II Anodized. Mechanical Compression Designed to Stimulate the Fusion Process
 CoLink_XP_ST_080218.pdf 1 8/2/18 8:36 AM SURGICAL TECHNIQUE CoLink XP Plates DYNAMIC, TRANSVERSE COMPRESSION C M Y CM MY CY CMY K MTP Std. Plate MTP Revision Plate Lapidus Standard +1mm and +2mm Y Plate
CoLink_XP_ST_080218.pdf 1 8/2/18 8:36 AM SURGICAL TECHNIQUE CoLink XP Plates DYNAMIC, TRANSVERSE COMPRESSION C M Y CM MY CY CMY K MTP Std. Plate MTP Revision Plate Lapidus Standard +1mm and +2mm Y Plate
Clinical. Solutions. Synthes Solutions. Foot and Ankle.
 Clinical Solutions Foot and Ankle. Foot and Ankle. Fractures of the tibial shaft Fractures of the distal fibula Fractures of the distal tibia Fractures and osteotomies of the calcaneus Arthrodesis Fractures,
Clinical Solutions Foot and Ankle. Foot and Ankle. Fractures of the tibial shaft Fractures of the distal fibula Fractures of the distal tibia Fractures and osteotomies of the calcaneus Arthrodesis Fractures,
Forefoot/Midfoot Plating System. Surgical Technique
 Forefoot/Midfoot Plating System Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that
Forefoot/Midfoot Plating System Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that
Technique Guide. 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot.
 Technique Guide 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot. Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
Technique Guide 6.5 mm Midfoot Fusion Bolt. For intramedullary fixation of the medial column of the foot. Table of Contents Introduction 6.5 mm Midfoot Fusion Bolt 2 AO Principles 4 Indications 5 Surgical
Anchorage. Foot & Ankle. Plating System. Operative Technique
 Foot & Ankle Anchorage Plating System Operative Technique Foot & Ankle Anchorage Plate System MTP Plates Lapidus Plates Lisfranc Plates Basal Osteotomy Plates Utility Plates Anchorage Cross Plate System
Foot & Ankle Anchorage Plating System Operative Technique Foot & Ankle Anchorage Plate System MTP Plates Lapidus Plates Lisfranc Plates Basal Osteotomy Plates Utility Plates Anchorage Cross Plate System
LCP Distal Tibia Plate
 Surgical Technique LCP Locking Compression Plate Original Instruments and Implants of the Association for the Study of Internal Fixation AO/ASIF Table of contents Indications 3 Implants/Instruments 5 Surgical
Surgical Technique LCP Locking Compression Plate Original Instruments and Implants of the Association for the Study of Internal Fixation AO/ASIF Table of contents Indications 3 Implants/Instruments 5 Surgical
MetaFix Ludloff Plate
 Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
MEDICAL ADVANCED TECHNOLOGY FOOT SURGERY: COMPRESSION AND VARISATION STAPLE SYSTEM FOOT SURGERY STAPLE FIXATION
 MEDICAL ADVANCED TECHNOLOGY FOOT SURGERY: COMPRESSION AND VARISATION STAPLE SYSTEM FOOT SURGERY STAPLE FIXATION FOOT SURGERY STAPLE FIXATION Design rationale & Main features Progressive controlled compression
MEDICAL ADVANCED TECHNOLOGY FOOT SURGERY: COMPRESSION AND VARISATION STAPLE SYSTEM FOOT SURGERY STAPLE FIXATION FOOT SURGERY STAPLE FIXATION Design rationale & Main features Progressive controlled compression
Integra. DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE
 Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
3. Insert Tocar Sleeves Insert the NCB tissue protection sleeve assembly 1.6 to 10mm through a skin incision (Fig. 38).
 NCB Proximal Humerus Plating System Surgical Technique 19 2. Temporary Plate Fixation The plate can be temporary fixed to the bone with 1.6mm K-wire through the proximal cannulated fixation screw of the
NCB Proximal Humerus Plating System Surgical Technique 19 2. Temporary Plate Fixation The plate can be temporary fixed to the bone with 1.6mm K-wire through the proximal cannulated fixation screw of the
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
hintegra Total Ankle Prosthesis Surgical Technique For Hintegra total-ankle prosthesis extremity lower extremity Orthopaedics HINTEGRA English
 hintegra Total Ankle Prosthesis EGRA English Surgical Technique For Hintegra total-ankle prosthesis Orthopaedics lower extremity Orthopaedics lower extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and
hintegra Total Ankle Prosthesis EGRA English Surgical Technique For Hintegra total-ankle prosthesis Orthopaedics lower extremity Orthopaedics lower extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and
SpeedTip CCS 2.2, 3.0
 PRODUCT INFORMATION SpeedTip CCS 2.2, 3.0 Cannulated Compression Screws APTUS 2 SpeedTip CCS 2.2, 3.0 Cannulated Compression Screws SpeedTip CCS * 2.2, 3.0 Cannulated Compression Screws A new generation
PRODUCT INFORMATION SpeedTip CCS 2.2, 3.0 Cannulated Compression Screws APTUS 2 SpeedTip CCS 2.2, 3.0 Cannulated Compression Screws SpeedTip CCS * 2.2, 3.0 Cannulated Compression Screws A new generation
3.5 mm LCP Olecranon Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Flower Opening Wedge Plate
 Flower Opening Wedge Plate PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE
Flower Opening Wedge Plate PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE
Asnis III. Cannulated Screw System. Operative Technique. 4.0mm 5.0mm 6.5/8.0mm. Shoulder. Elbow. Hand & Wrist. Hip. Pelvis. Femur.
 Shoulder Elbow Asnis III Cannulated Screw System Hand & Wrist Operative Technique 4.0mm 5.0mm 6.5/8.0mm Hip Pelvis Femur Tibia & Fibula Foot & Ankle Asnis III Cannulated Screws Content This publication
Shoulder Elbow Asnis III Cannulated Screw System Hand & Wrist Operative Technique 4.0mm 5.0mm 6.5/8.0mm Hip Pelvis Femur Tibia & Fibula Foot & Ankle Asnis III Cannulated Screws Content This publication
Surgical Technique. Customer Service:
 Patent and Patent Pending CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The Axis Charcot Fixation System in diameters of 5.5, 6.5 and 7.5mm
Patent and Patent Pending CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The Axis Charcot Fixation System in diameters of 5.5, 6.5 and 7.5mm
IPP-ON. Interphalangeal implant. Surgical Technique. l o w e r e x tre mit y. Or t h o pa e d i c s. IPP-ON English
 IPP-ON Interphalangeal implant IPP-ON English Surgical Technique Or t h o pa e d i c s l o w e r e x tre mit y IPP-ON Rationale Indications The proximal interphalangeal fusion is a challenging surgery.
IPP-ON Interphalangeal implant IPP-ON English Surgical Technique Or t h o pa e d i c s l o w e r e x tre mit y IPP-ON Rationale Indications The proximal interphalangeal fusion is a challenging surgery.
Foot & Ankle. Smart Toe II. Intramedullary Implant. Operative Technique. Foot & Ankle
 Foot & Ankle Smart Toe II Intramedullary Implant Operative Technique Foot & Ankle Smart Toe This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments.
Foot & Ankle Smart Toe II Intramedullary Implant Operative Technique Foot & Ankle Smart Toe This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments.
PEDUS-L. Locking Plantar Lapidus Plate
 PEDUS-L Locking Plantar Lapidus Plate Page 1 PEDUS-L - Locking Plantar Lapidus Plate Table of Contents Implants 3 System 4 Operation manual 5 Approach 5 Identification of the TMT 1 joint with a cannula
PEDUS-L Locking Plantar Lapidus Plate Page 1 PEDUS-L - Locking Plantar Lapidus Plate Table of Contents Implants 3 System 4 Operation manual 5 Approach 5 Identification of the TMT 1 joint with a cannula
Locking Forefoot/Midfoot Plating System
 Locking Forefoot/Midfoot Plating System Locking Forefoot/Midfoot Plating System Acumed is an industry leader in innovative solutions for extremities and trauma. We are dedicated to pioneering solutions
Locking Forefoot/Midfoot Plating System Locking Forefoot/Midfoot Plating System Acumed is an industry leader in innovative solutions for extremities and trauma. We are dedicated to pioneering solutions
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Surgical Technique 4.5/8.5MM BEAMING SYSTEM. Customer Service:
 Patent and Patent Pending CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The 4.5/8.5 screw system is intended for fixation arthrodesis of
Patent and Patent Pending CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The 4.5/8.5 screw system is intended for fixation arthrodesis of
PL ATING SYSTEM. Pre-contoured implants Versatile fixation system Hexalobe screw recess design Locked fixation
 I N N O VA T I O N ME A NS M OT I O N FOOTMOTION PL ATING SYSTEM Pre-contoured implants Versatile fixation system Hexalobe screw recess design Locked fixation FOOTMOTION PLATING SYSTEM Indications: The
I N N O VA T I O N ME A NS M OT I O N FOOTMOTION PL ATING SYSTEM Pre-contoured implants Versatile fixation system Hexalobe screw recess design Locked fixation FOOTMOTION PLATING SYSTEM Indications: The
PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique
 PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique Surgical Technique Contour Femur Plate The technique description herein is made available to the healthcare professional to illustrate the
PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique Surgical Technique Contour Femur Plate The technique description herein is made available to the healthcare professional to illustrate the
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal
DARCO MFS. Locked plating system for reconstructive forefoot surgery.
 DARCO MFS Locked plating system for reconstructive forefoot surgery. DARCO MFS Locked plating system for reconstructive forefoot surgery System Basics The DARCO MFS plating system for the forefoot has
DARCO MFS Locked plating system for reconstructive forefoot surgery. DARCO MFS Locked plating system for reconstructive forefoot surgery System Basics The DARCO MFS plating system for the forefoot has
Low Bend Distal Tibia Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
Endolog Implant for Correction of Hallux Valgus
 Endolog Implant for Correction of Hallux Valgus Surgical Technique Distributed by: Simple and precise Mini-invasive For mild, moderate and severe HV Surgical Indications Endolog implant is proposed for
Endolog Implant for Correction of Hallux Valgus Surgical Technique Distributed by: Simple and precise Mini-invasive For mild, moderate and severe HV Surgical Indications Endolog implant is proposed for
FLT105 12/02.
 FLT105 12/02 www.biometmerck.co.uk Disclaimer Biomet Merck Ltd, as the manufacturer of this device, does not practice medicine and does not recommend this or any other surgical technique for use on a
FLT105 12/02 www.biometmerck.co.uk Disclaimer Biomet Merck Ltd, as the manufacturer of this device, does not practice medicine and does not recommend this or any other surgical technique for use on a
The Titanium Tibial Nail System
 The Titanium Tibial Nail System Solid Tibial Nails (UTN) and Cannulated Tibial Nails (CTN) Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved
The Titanium Tibial Nail System Solid Tibial Nails (UTN) and Cannulated Tibial Nails (CTN) Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved
DRACO FOOT & ANKLE SYSTEM PRODUCT INFORMATION
 DRACO FOOT & ANKLE SYSTEM PRODUCT INFORMATION 2 DRACO FOOT & ANKLE SYSTEM www.hnmtotal.com DRACO Foot & Ankle System CONTENTS MetaFuse L Plate 3 MetaFuse BLP Plate 4 MetaFuse Midfoot/Lisfranc Plate 5 MetaFuse
DRACO FOOT & ANKLE SYSTEM PRODUCT INFORMATION 2 DRACO FOOT & ANKLE SYSTEM www.hnmtotal.com DRACO Foot & Ankle System CONTENTS MetaFuse L Plate 3 MetaFuse BLP Plate 4 MetaFuse Midfoot/Lisfranc Plate 5 MetaFuse
Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems
 Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems Designed to allow customized system configurations Variable Angle (VA) and Locking Compression Plate (LCP ) options
Compact Distal Radius System. Consolidated solution for Distal Radius Plating Systems Designed to allow customized system configurations Variable Angle (VA) and Locking Compression Plate (LCP ) options
Lesser MPJ Hemi Implant
 Lesser MPJ Hemi Implant Surgical Technique Contents Product The BioPro Lesser MPJ Hemi Implant is a simple, durable, metallic hemiarthroplasty resurfacing prosthesis for the treatment of arthritis, Freiberg
Lesser MPJ Hemi Implant Surgical Technique Contents Product The BioPro Lesser MPJ Hemi Implant is a simple, durable, metallic hemiarthroplasty resurfacing prosthesis for the treatment of arthritis, Freiberg
Contents. Chapter 1 4 Chapter Chapter Chapter Chapter 5 15
 Contents Chapter 1 4 Chapter 2 5 5 Chapter 3 6 6 7 Chapter 4 8 8 8 8 9 10 10 11 12 13 14 14 Chapter 5 15 Introduction Intended Use Indications Device Description Implant Options and Sizing Instrumentation
Contents Chapter 1 4 Chapter 2 5 5 Chapter 3 6 6 7 Chapter 4 8 8 8 8 9 10 10 11 12 13 14 14 Chapter 5 15 Introduction Intended Use Indications Device Description Implant Options and Sizing Instrumentation
Technique Guide. 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
3.5 mm LCP Tibial Plateau Sets. Configuration options.
 3.5 mm LCP Tibial Plateau Sets. Configuration options. Multiple trays Multiple levels Multiple options 3.5 mm LCP Tibial Plateau Sets The tibial plateau trays allow the hospital to build a custom - ized
3.5 mm LCP Tibial Plateau Sets. Configuration options. Multiple trays Multiple levels Multiple options 3.5 mm LCP Tibial Plateau Sets The tibial plateau trays allow the hospital to build a custom - ized
DARCO. Bow 2 Plate SURGIC AL TECHNIQUE
 DARCO Bow 2 Plate SURGIC AL TECHNIQUE Contents 2 Preface 3 Chapter 1 4 Chapter 2 5 6 7 8 9 Appendix 10 10 11 Intended Use Indications/Contraindications Design Rationale Preoperative Planning Surgical Technique
DARCO Bow 2 Plate SURGIC AL TECHNIQUE Contents 2 Preface 3 Chapter 1 4 Chapter 2 5 6 7 8 9 Appendix 10 10 11 Intended Use Indications/Contraindications Design Rationale Preoperative Planning Surgical Technique
Asnis III Cannulated Screw System
 Trauma Asnis III Cannulated Screw System Operative Technique 4.0mm, 5.0mm, 6.5mm and 8.0mm diameter implants Contents Introduction 4 Features & Benefits 5 Contraindications 7 Additional Information 7 Operative
Trauma Asnis III Cannulated Screw System Operative Technique 4.0mm, 5.0mm, 6.5mm and 8.0mm diameter implants Contents Introduction 4 Features & Benefits 5 Contraindications 7 Additional Information 7 Operative
The Flower Forefoot PROCEDURE GUIDE.
 The Flower Forefoot PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE MTP
The Flower Forefoot PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE MTP
Long Volar Plates for Diaphyseal-Metaphyseal Radius Fractures LCP. Dia-Meta Volar Distal Radius Plates. Surgical Technique
 Long Volar Plates for Diaphyseal-Metaphyseal Radius Fractures LCP Dia-Meta Volar Distal Radius Plates Surgical Technique Table of Contents Introduction LCP Dia-Meta Volar Distal Radius Plates 2 AO Principles
Long Volar Plates for Diaphyseal-Metaphyseal Radius Fractures LCP Dia-Meta Volar Distal Radius Plates Surgical Technique Table of Contents Introduction LCP Dia-Meta Volar Distal Radius Plates 2 AO Principles
3.5 mm LCP Hook Plate
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Hook Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Hook Plate 2 AO Principles 4 Indications 5 Clinical
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Hook Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Hook Plate 2 AO Principles 4 Indications 5 Clinical
Technique Guide. TomoFix Osteotomy System. A comprehensive plating system for stable fixation of osteotomies around the knee.
 Technique Guide TomoFix Osteotomy System. A comprehensive plating system for stable fixation of osteotomies around the knee. Table of Contents Introduction TomoFix Osteotomy System 2 AO Principles 4 Indications
Technique Guide TomoFix Osteotomy System. A comprehensive plating system for stable fixation of osteotomies around the knee. Table of Contents Introduction TomoFix Osteotomy System 2 AO Principles 4 Indications
Technique Guide. Locking Attachment Plate. For treatment of periprosthetic fractures.
 Technique Guide Locking Attachment Plate. For treatment of periprosthetic fractures. Table of Contents Introduction Locking Attachment Plate 2 Indications 4 Surgical Technique Patient Positioning 5 Preparation
Technique Guide Locking Attachment Plate. For treatment of periprosthetic fractures. Table of Contents Introduction Locking Attachment Plate 2 Indications 4 Surgical Technique Patient Positioning 5 Preparation
Telya. Varisation staple. Surgical Technique. Irish Distributer: O'Mara Medical Tel
 Surgical Technique Indications The varisation is used to stabilize an osteotomy of the great toe proximal phalanx (Akin osteotomy), in order to correct : Hallux valgus interphalangeus, Phalangeal pronation,
Surgical Technique Indications The varisation is used to stabilize an osteotomy of the great toe proximal phalanx (Akin osteotomy), in order to correct : Hallux valgus interphalangeus, Phalangeal pronation,
Surgical Technique. Calcaneal Locking Plate
 Surgical Technique Calcaneal Locking Plate PERI-LOC Locked Plating System Calcaneal Locking Plate Surgical TechniqueCatalog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Calcaneal Locking Plate PERI-LOC Locked Plating System Calcaneal Locking Plate Surgical TechniqueCatalog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
SURGICAL TECHNIQUE. Compression screw FOREFOOT SOLUTIONS
 SURGICAL TECHNIQUE Compression screw FOREFOOT SOLUTIONS SCARF OSTEOTOMY The Scarf osteotomy consists of a horizontal cut and two transversal cuts of the first metatarsal, allowing for a broad range of
SURGICAL TECHNIQUE Compression screw FOREFOOT SOLUTIONS SCARF OSTEOTOMY The Scarf osteotomy consists of a horizontal cut and two transversal cuts of the first metatarsal, allowing for a broad range of
TABLE OF CONTENTS
 TABLE OF CONTENTS 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18-24 Hallux Interphalangeal (IP) Joint Arthrodesis Akin Osteotomy First Metatarsophalangeal (MTP) Joint Arthrodesis Chevron Osteotomy/ Bunionectomy
TABLE OF CONTENTS 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18-24 Hallux Interphalangeal (IP) Joint Arthrodesis Akin Osteotomy First Metatarsophalangeal (MTP) Joint Arthrodesis Chevron Osteotomy/ Bunionectomy
AxSOS. Locking Plate System. Operative Technique. Small Fragment Basic Fragment
 AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment Stryker Plating Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5 Compression Plates 5 Reconstruction and 1/3
AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment Stryker Plating Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5 Compression Plates 5 Reconstruction and 1/3
Hand Fracture System. Surgical Technique
 Hand Fracture System Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve
Hand Fracture System Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve
Surgical Technique. Lower Extremity Plates and Straight Plates
 Surgical Technique Lower Extremity Plates and Straight Plates 2 Table of contents Overview...4 Indications... 4 Contraindications... 4 Screw Options... 5 Straight Plate Options... 6 Proximal Tibia Plate
Surgical Technique Lower Extremity Plates and Straight Plates 2 Table of contents Overview...4 Indications... 4 Contraindications... 4 Screw Options... 5 Straight Plate Options... 6 Proximal Tibia Plate
HALLU-FIX ENGLISH. HALLU -FIX M.T.P. Arthrodesis System. Surgical Technique
 HALLU-FIX ENGLISH HALLU -FIX M.T.P. Arthrodesis System Surgical Technique Or t h o pa e d i c s LOWER EXTREMITY HALLU-FIX Table of content Introduction...03 Indications...03 Contre-indications...03 Surgical
HALLU-FIX ENGLISH HALLU -FIX M.T.P. Arthrodesis System Surgical Technique Or t h o pa e d i c s LOWER EXTREMITY HALLU-FIX Table of content Introduction...03 Indications...03 Contre-indications...03 Surgical
2.7 mm/3.5 mm LCP Distal Fibula Plate System. Part of the Synthes locking compression plate (LCP) system.
 2.7 mm/3.5 mm LCP Distal Fibula Plate System. Part of the locking compression plate (LCP) system. Anatomically contoured Multiple screw options for fixedangle support Coaxial holes minimize screw head
2.7 mm/3.5 mm LCP Distal Fibula Plate System. Part of the locking compression plate (LCP) system. Anatomically contoured Multiple screw options for fixedangle support Coaxial holes minimize screw head
COMPARING KIRSCHNER WIRE FIXATION TO A NEW DEVICE USED FOR PROXIMAL INTERPHALANGEAL FUSION
 C H A P T E R 3 0 COMPARING KIRSCHNER WIRE FIXATION TO A NEW DEVICE USED FOR PROXIMAL INTERPHALANGEAL FUSION Scott R. Roman, DPM INTRODUCTION The most common fixation for proximal interphalangeal fusion
C H A P T E R 3 0 COMPARING KIRSCHNER WIRE FIXATION TO A NEW DEVICE USED FOR PROXIMAL INTERPHALANGEAL FUSION Scott R. Roman, DPM INTRODUCTION The most common fixation for proximal interphalangeal fusion
LCP Medial Proximal Tibial Plate 4.5/5.0. Part of the Synthes LCP periarticular plating system.
 LCP Medial Proximal Tibial Plate 4.5/5.0. Part of the Synthes LCP periarticular plating system. Technique Guide This publication is not intended for distribution in the USA. Instruments and implants approved
LCP Medial Proximal Tibial Plate 4.5/5.0. Part of the Synthes LCP periarticular plating system. Technique Guide This publication is not intended for distribution in the USA. Instruments and implants approved
