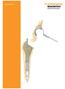
Section of Modular Hip Prostheses cemented. TMC-3 Modular Hip Prosthesis, cemented. TMC-3 Modular Hüftprothese, zementiert
|
|
|
- Edward Smith
- 6 years ago
- Views:
Transcription
1 Section of Modular Hip Prostheses cemented TMC-3 Modular Hip Prosthesis, cemented TMC-3 Modular Hüftprothese, zementiert Prothèse de hanche modulaire TMC-3, cimentée Indication : The TMC-3 Modular hip prosthesis is a solution for total hip replacement namely with the concept of a hybrid system combined with distal centralizer. The form and the length of prostheses are ideal-together with distal centralizer in order to ensure self-centring. This prosthesis is design of conical wedge form with rounded edges in the proximal section corresponds with the anatomical and biomechanical requirements. These are, retention and utilisation of the morphology of the intertrochanteric region with resultant rotatory stability, self centering and a favourable zone for cementing. The main part of the cement mantle is subject to compressive forces. Tensile forces are restricted to the lateral shoulder where the cement merely has the function of a filling material. The implant does not have any sharp edges, as a result of which stress concentrations and resultant cement cracks are avoided. This prosthesis consist of an appropriate number of stem sizes which enable one to obtain the filling of the medullary canal. Furthermore, an optimum thickness of the cement layer can also be achieved. Tapered distal design helps reduce strains in the cement as compared to conventional stem. Also cutted edge at the end of the stem avoid the pain in the distal part of femur and permit wide range of motion.this stem is mostly indicated for all cases of primary implants and all cases in which there is a contraindication for an uncemented stem. Contraindications is revision cases with extensive proximal osseous defect. The prosthesis feature has a 35º degree stem/neck angle for an anatomic reconstruction. The neck has a taper 2/4 allowing standard metal heads to be used. Material : The TMC-3 modular hip prosthesis is manufactured from casting Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This alloy is characterized by demonstrating excellent biocompatibility together with a high resistance of corrosion and superiormechanical strenght. Co.Cr-alloy Stem K mm Stem Length L mm Cone mm Neck Angle /4 2/4 2/4 2/4 2/4 35º 35º 35º 35º 35º A-
2 Section of Modular Hip Prostheses cemented TMC-4 Modular Hip Prosthesis with collar, cemented TMC-4 Modular Hüftprothese mit Kragen, zementiert Prothèse de hanche modulaire avec collerette TMC-4, cimentée Indication : The TMC-4 modular hip prosthesis with collar is a solution for total hip replacement namely with the concept of a hybrit system combined with distal centralizer. This prosthesis is designed to provide an anatomic precise fit.the presence of a broad medial collar together with a precise calcar preparation, allows excellent collar/calcar contact for load distribution and cement pressurization. A collarless version is also available (TMC-3) for clinical application where a collared prosthesis is inappropriate. Tapered distal design helps reduce strain in the cement as compared to conventional stem and cutted edge at the end of the stem avoid the pain at the distal part of the femur and permit wide range of motion. The form and the length of prosthesis are ideal - together with distal centralizer. This prosthesis is used for pirimary total hip arthroplasty when a collared design is preferred. Modular femoral heads available in diameters 28 mm with three different neck sizes S-M-L. Various stem size with interchangeable heads options enabling the surgeon to select the best combination for the long-term satisfactory result. The prosthesis feature has a 35º degree stem/neck angle for an anatomic reconstruction. The neck has a taper 2/4 allowing standard metal heads to be used. Material : The TMC-4 modular hip prosthesis is manufactured from casting Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This alloy is characterized by demonstrating excellent biocompatibility together with a high resistance of corrosion and superiormechanical strenght. Co.Cr-alloy Stem K mm Stem Length L mm Cone mm Neck Angle /4 2/4 2/4 2/4 2/4 35º 35º 35º 35º 35º A-2
3 Cement Plug for TMC-3 / TMC-4 Indication : Reduced the potential for distal migration of bone cement within the medullary canal during prosthetic implantation, thus enhancing the chance for lateral displacement of bone cement and trabecular interstitial penetration. Firmly anchored at the desired depth within the medullary canal via the wedging action of 6-7 rows of circumferential fins. Material : UHMWPE Ultra Heavy Molecular Weight Polyethylyene meets ASTM F.6 and ISO 5834/2 standards. Selected for its, high degree of purity, good biotolerance, good mechanical performance and friction properties (ø) Distal Centralizer for TMC-3 / TMC-4 Indication : The distal centralizer with a three point - star configuration, helps improve cortical diaphyseal contact and stem alignment. Material : PMMA Polymethylmethacrylate (ø) Combining Stem 6 mm stem 8 mm stem 0 mm stem 2 mm stem 4 mm stem A-3
4 TMC-3 / TMC-4 Modular Hip Prosthesis Complete Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces T-Handle Medullary Awl Reamer Plug for Introducing Rod Plug for Introducing Rod Plug for Introducing Rod Plug for Introducing Rod Introducing Rod for Medullary Plug Cement Pusher Ruler Test Head ø 28 mm 2/4 Test Head ø 28 mm 2/4 Test Head ø 28 mm 2/4 Repositioning Lever Repositioning Lever Synthetic Top Repositioning Top S M L ø Rasp Bar Modular Rasp Handle Rasps Rasps Rasps Rasps Rasps Handle for Setting Device Impactor Impactor & Extractor Femoral Head Extractor Curved Chisel Straight Chisel Gauge for Femoral Head A-4
5 Prosthesis Rasp Modular Rasp Handle (ø) Medullary Awl Reamer Test Heads Rasp Bar S M L Handle for Setting Device T-Handle Gauge For Femoral Head Straight Chisel Curved Chisel A-5
6 Impactor & Extractor Impactor Ruler Cement Pusher Repositioning Lever Repositioning Lever Synthetic Top Repositioning Top Plug for Introducing Rod Femoral Head Extractor (ø) Introducing Rod for Medullary Plug A-6
7 Section of Modular Hip Prostheses uncemented TAU- Anatomical Modular Stem with Porous Coating, uncemented TAU- Anatomische Modular Schaft mit Porous, unzementiert Tige anatomic modulaire en Porous TAU-, non-cimentée Indication : The TAU- Anatomical modular hip prosthesis system is designed to improve initial fixation stability and stress distribution at fixation interfaces, enchanging long-term fixation durability. The cervico-diapyseal angle of 35º is closed to resulting force of mechanical axis of the femur and limits varus stresses. Optimal fit and fill of metaphysis in all three planes ensures perfect rotational stability. Diaphyseal part with anatomic anterior curvature ensures proper centring of the stem in the medullary canal. 8 sizes right & left femoral stem components are proportional to physiological shape of the proximal femur to improve initial fixation stability and stress distribution at fixation interfaces. Three dimensional porous coating uses the osteo-conductive qualities of this efficient biomaterial, which produce the mineral fraction of natural bone. This similarity allows; - To mislead the organism defenses - To avoid fibrous encapsulation-the natural response to the introduction of any foreign substance. - To create a remarkable link, of chemichal nature, which appears shortly after operation and is very strong. Anatomical design of neck cone 2/4 taper 5º 42. Material : It is manufactured from casting Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This alloy is characterized by demonstrating excellent biocompatibility together with a high resistance of corrosion and superiormechanical strenght. Stem Stem Length Co.Cr-alloy K mm L mm Cone Side /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 Right Right Right Right Right Right Right Right /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 Left Left Left Left Left Left Left Left A-7
8 Section of Modular Hip Prostheses uncemented TAU- Anatomical Modular Stem with Ti. Plasma Spray & H.A Coating, uncemented TAU- Anatomische Modular Schaft mit Ti. Plasma Spray und H.A, unzementiert Tige anatomic modulaire en Ti. Plasma Spray & H.A TAU-, non-cimentée Indication : The TAU- Anatomical modular hip prosthesis system is designed to improve initial fixation stability and stress distribution at fixation interfaces, enchanging long-term fixation durability. The cervico-diapyseal angle of 35º is closed to resulting force of mechanical axis of the femur and limits varus stresses. Optimal fit and fill of metaphysis in all three planes ensures perfect rotational stability. Diaphyseal part with anatomic anterior curvature ensures proper centring of the stem in the medullary canal. 8 sizes right & left femoral stem components are proportional to physiological shape of the proximal femur to improve initial fixation stability and stress distribution at fixation interfaces. Three dimensional Titanium plasma spray coating with H.A (hydroxapatite) uses the osteo-conductive qualities of this efficient biomaterial, which produce the mineral fraction of natural bone. This similarity allows; - To mislead the organism defenses - To avoid fibrous encapsulation-the natural response to the introduction of any foreign substance. - To create a remarkable link, of chemichal nature, which appears shortly after operation and is very strong. Anatomical design of neck cone 2/4 taper 5º 42. Material : It is manufactured from casting Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This alloy is characterized by demonstrating excellent biocompatibility together with a high resistance of corrosion and superiormechanical strenght. Stem Stem Length Co.Cr-alloy mm L mm Cone Side /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 Right Right Right Right Right Right Right Right /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 Left Left Left Left Left Left Left Left A-8
9 Section of Modular Hip Prostheses cemented TAU-2 Anatomical Modular Stem, cemented TAU-2 Anatomische Modular Schaft, zementiert Tige anatomic modulaire TAU-2, cimentée Indication : The TAU-2 Anatomical modular cemented hip prosthesis system is designed to improve initial fixation stability and stress distribution at fixation interfaces, enchanging long-term fixation durability. The cervico-diapyseal angle of 35º is closed to resulting force of mechanical axis of the femur and limits varus stresses. Optimal fit and fill of metaphysis in all three planes ensures perfect rotational stability. Diaphyseal part with anatomic anterior curvature ensures proper centring of the stem in the medullary canal. 8 sizes right & left femoral stem components are proportional to the physiological shape of the proximal femur to improve initial fixation stability and stress distribution at fixation interfaces. Three dimensional interlock grit blast on the proximal area of prosthesis improve mechanical properties of cement and stress distribution at the fixation interfaces. Material : It is manufactured from casting Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This alloy is characterized by demonstrating excellent biocompatibility together with a high resistance of corrosion and superiormechanical strenght. Co.Cr-alloy Stem K mm Stem Length L mm Cone Side /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 Right Right Right Right Right Right Right Right /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 Left Left Left Left Left Left Left Left A-9
10 TAU Anatomical Modular Stem Compete Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces Conical Test Head ø28 mm 2/ Conical Test Head ø28 mm 2/ Conical Test Head ø28 mm 2/ Bone Hook Blunt Hohmann Extractor Hohmann Extractor Curved Chisel Straight Chisel Guj Curette Curette Spoon Curette Femoral Head Extractor Femoral Head Impactor Impactor Gauge for Femoral Head S M L No. No Rasp Bar Modular Rasp Handle Anatomical Stem Rasp (Right) Anatomical Stem Rasp (Right) Anatomical Stem Rasp (Right) Anatomical Stem Rasp (Right) Anatomical Stem Rasp (Right) Anatomical Stem Rasp (Left) Anatomical Stem Rasp (Left) Anatomical Stem Rasp (Left) Anatomical Stem Rasp (Left) Anatomical Stem Rasp (Left) Medullary Awl Reamer A-0
11 Anatomical Stem Rasp (Left) (ø) Anatomical Stem Rasp (Right) Rasp Bar (ø) Modular Rasp Handle Bone Hook Blunt Gauge For Femoral Head Straight Chisel Curved Chisel A-
12 Guj Curette Femoral Head Impactor Spoon Curette Curette Hohmann Retractor Hohmann Retractor Femoral Head Extractor Impactor Medullary Awl Reamer Test Heads S M L A-2
13 Section of Modular Hip Prostheses uncemented TPD Modular Straight Stem, uncemented TPD Modular Geradschaft, unzementiert Tige droite modulaire TPD, non-cimentée Indication : It is identical in shape to the TAU- anatomical modular stem but has no anatomical anterior curvature on stem. It is totally straight. This uncemented prosthesis is designed to improve initial fixation stability and stress distribution at fixation interfaces, enchanging long-term fixation stability. Three dimensional porous coating uses the osteo-conductive qualities of this efficient biometerial, which produce the mineral fraction of natural bone. This prosthesis is available with porous coating and hydroxapatite (C.H.A) coating together. Its C.H.A coating uses the osteoconductive qualities of this efficient biomaterial, which reproduces the mineral fraction of nature bone. This similarity allows, - to mislead the organism defenses, - to avoid fibrous encapsulation-the natural response to the introduction of any foreign substance - to create a remarkable link, of chemical nature, which appears shortly after the operation, and is very strong. It ensures secondary, biological, active stability. This prosthesis can be used for all classical indications of total hip arthroplasty, such primary or secondary hip osteoarthritis, rheumatic coxitis, avascular necroses and broken femoral neck. The prosthesis feature has a 35º stem/neck angle for an anatomic reconstruction. The neck has a taper 2/4 allowing standard metal heads to be used. Material : It is manufactured from casting Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This alloy is characterized by demonstrating excellent biocompatibility together with a high resistance of corrosion and superiormechanical strenght. Porous Coated Ti.Pl.S. + HA Stem K mm Stem Length L mm Cone /4 2/4 2/4 2/4 2/4 A-3
14 TPD Modular Straight Stem Compete Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces Conical Test Head ø28 mm 2/4 Conical Test Head ø28 mm 2/4 Conical Test Head ø28 mm 2/4 Bone Hook Sharp Hohmann Extractor Hohmann Extractor Curved Chisel Straight Chisel Guj Curette Curette Spoon Curette Femoral Head Impactor Impactor T-Handle S M L No. No Rasp Bar Modular Rasp Handle Stem Rasp Stem Rasp Stem Rasp Stem Rasp Stem Rasp Medullary Awl Reamer Gauge for Femoral Head A-4
15 Stem Rasp Modular Rasp Handle (ø) Rasp Bar Impactor Bone Hook Sharp Gauge For Femoral Head Straight Chisel Curved Chisel A-5
16 Guj Curette Femoral Head Impactor Spoon Curette Curette Hohmann Retractor Hohmann Retractor Medullary Awl Reamer Test Heads T-Handle S M L A-6
17 Section of Modular Hip Prostheses uncemented Modular Straight Stem Titanium, uncemented Modular Geradschaft Titanium, unzementiert Tige droite modulaire, non-cimentée Indication : It is a non-cemented, total hip prosthesis. It can be used for all the classical indications of total hip arthoplasty such as primary or secondary hip osteoarthritis, rheumatic coxitis, avascular necroses and broken femoral neck. Neither age, osteoporosis nor modifications to non-cemented implants of this type. In revision surgery with limited amount of available bone repeated cementings are illogical and disappointing. With the addition of cancellous bone grafts, this prosthesis is a very efficient alternative. This prosthesis is available with hydroxapatite (C.H.A) coating. Its C.H.A coating uses the osteo-conductive qualities of this efficient biomaterial, which reproduces the mineral fraction of nature bone. This similarity allows, - to mislead the organism defenses, - to avoid fibrous encapsulation-the natural response to the introduction of any foreign substance and to create a remarkable link, of chemical nature, which appears shortly after the operation, and is very strong. It ensures secondary, biological, active stability. The fixation of the stem in the proximal area should quarantee that transmission of the force is retained within this area. the interposition of the cancellous structure reduces the danger of the bond between bone and prosthesis becoming stiff. On introduction, the axial ribs exercise a cutting action and promote stability. In order to avoid tension peaks, the surface of the distal part of the stem are well rounded. The shape of distal part of the stem is kept relatively slender, thusdirect contact with the cortex is where possible avoided. This means greatly reducted incidence of pain for the patient, plus the trabecular structures can form between the cortex and implant, which eventually result in osseointegration. Material : Forged titanium alloy according to ASTM F.36 - ISO 5832/3 Selected for its; modulus of elasticity closes to that of living bone, high fatique strength and perfect biocompatibility. Titanium Titanium (H.A.) Stem s K mm Stem Length L mm Cone mm /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 A-7
18 Modular Straight Stem Uncemented Complete Instrumentation Set Ref.Number Description Modular Prosthesis Rasp Modular Prosthesis Rasp Modular Prosthesis Rasp Modular Prosthesis Rasp Modular Prosthesis Rasp Modular Prosthesis Rasp Medullary Awl Reamer Modular Rasp Handle Rasp Bar Test Head ø 28 mm 2/4 Test Head ø 28 mm 2/4 Test Head ø 28 mm 2/4 Gauge For Femoral Head S M L Pieces Ref.Number Description Repositioning Lever Repositioning Lever Synthetic Top Repositioning Top Impactor Femoral Head Extractor Spoon Curette Curette Guj Curette Straight Chisel Curved Chisel Hohmann Retractor Hohmann Retractor No. No.2 Pieces A-8
19 Prosthesis Rasp Medullary Awl Reamer (ø) Modular Rasp Handle Rasp Bar Impactor Spoon Curette Curette Straight Chisel Curved Chisel A-9
20 Guj Curette Impactor Hohmann Retractor Hohmann Retractor Repositioning Lever Repositioning Lever Synthetic Top Repositioning Top Gauge For Femoral Head Femoral Head Extractor Test Heads S M L A-20
21 Section of Modular Hip Prostheses uncemented DHP Hip Prosthesis, uncemented DHP Hüftprothese, unzementiert Prothèse de DHP, non-cimentée Indication : The DHP hip prosthesis primary is used for difficult bone conditions at the proximal end of the femur and where it has the most important indication. In particular these are; - Cylindrical configuration of the proximal medullary cavity where implanting conventionally shaped prosthesis stem can lead to splitting of the bone. These conditions are mainly found in congenitally dislocated hips and in coxa vara congenita, in addition with very fine bone structures, often combined with coxa valga. - Increased anterversion of the neck of the femur, with congenitally dislocated hips, where, even after resection of the neck of the femur. The cross-section of the proximal medullary cavity forces stem with a flat or oval profile into anterversion. With circular cross-section of the shaft of the DHP prosthesis, the anterversion angle can be as desired. - Deformities and osseous cicatrization of the proximal end of the femur after osteotomies, fractures and growth disorders or with congenital deformities. The DHP hip prostheses with diameters 3 mm and 4 mm may not be used with a head for hemi prostheses. Material : The DHP hip prosthesis is manufactured from titanium Ti6Al4v alloy according to ASTM F.36 - ISO 5832/3 which has been specifically designed for manufacture of orthopaedic implants. This alloy is characterized by demonstrating excellent biocompatibility together with a high resistance of corrosion and superiormechanical strenght. The roughened surface finish also plays a vital role in the osseointegration. Titanium Stem s Stem Length L mm Cone mm /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 A-2
22 Section of Modular Hip Prostheses uncemented Revision Stem, uncemented Revisionsschaft, unzementiert Tige de reprise, non-cimentée Indication : Revision of a loose prostheses (primary of revision care) in which proximal anchoring in the metaphysis is no longer possible owing to pronounced damage to the bony implant bed, and in which anchoring of a new implant must occur exlusively in the diaphysis. -Revision in periprosthetic fractures -Primary care in multi segmental fractures of the proximal femur, with simultaneous severe coxarthrosis. The revision prosthesis has been develop for cementless application. The conical design permits stable anchorage of the prosthesis within the femoral medullary canal. The revision stem is available in four lengths with different diameters, enabling the surgeon to select the most appropriate implants for each individual procedure upon the severity of the bone loss and width of the medullary canal. The prosthesis feature eight longitudinal ribs or equal heigh,which guarantees rotational stability of the implant after impaction. The wedging principle is facilitated by the conical and straight stem geometry. The wedging effect is magnified should any subsidence occur, ensuring prosthetic stability. The 45º cervicodiapyseal angle has been selected as it permits smaller torque forces on the femur, which significant advantages, especially during standing from a chair and / or climbing stairs. The cone angle is a standard 2/4 taper, allowing standard metal heads to be used. Material : The revision stem is manufactured from titanium Ti6Al4V according to ASTM F.36 - ISO 5832/3 which has been specifically designed for the manufacture of orthopaedic implants. This alloy is characterized by demonstrating excellent biocompatibility, together with a high resistance of corrosion and superiormechanical strength. The roughened surface finish also plays a vital role in the osseointegration. Titanium Stem D mm Cone mm 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 Stem Length L mm ØD L A-22
23 Section of Modular Hip Prostheses cemented TMK- Modular Revision Stem, cemented TMK- Modular Revisionsschaft, zementiert Tige de reprise modulaire TMK-, cimentée Indication : Used for hip arthroplasty in cases of hip fractures when collapse of the neck stucture has occured and revision arthroplasty where there is significant loss of bone stock in the region of the calcar. Specially for large deficits in the calcar region and severe proximal bone loss, the calcar replacement femoral stem allows customization via its proximal segments. The calcar series offers 38- and 58 mm resection levels to compensate for bone deficiencies in the proximal/medial portion of the femur. In adittion to the three resection levels, the stems are offered in three different diameters mm. Diameter 8 mm component has standard 40 mm stem length, other stem diameters 0-2 mm offered with 50 mm stem lenght. These components are specifically designed to conform and match with the patient s anatomy. The keel, located below the medial collar provides the additional rotational stability that has been reduced due to medial bone loss. Three dimensional Interlock grit blast all over on the stem surface provides increased fixation at the prosthesis/cement interface compared with that of traditional smooth surface implants. The angle of the neck is 35º for all size, while neck length increases in line with the prosthesis diameter. Also prosthesis has a cone 2/4 taper 5º 42. Material : It is manufactured from casting Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This alloy is characterized by demonstrating excellent biocompatibility together with a high resistance of corrosion and superiormechanical strenght. Co.Cr-alloy mm Stem Stem Length L mm Cone mm Resection Level A mm Small Medium Large Small Medium Large Small Medium Large /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/ A-23
24 TMK- Modular Revision Stem Complete Instrumentation Set Ref.Number Description Prothesis Body Rasp Prothesis Body Rasp Prothesis Body Rasp T-handle Medullary Awl Reamer Medullary Awl Reamer Medullary Awl Reamer Test Head Ø 28 mm 2/4 Test Head Ø 28 mm 2/4 Test Head Ø 28 mm 2/4 Rasp Bar Curette Spoon Curette ø 8 ø 0 ø 2 ø 8 ø 0 ø 2 S M L Pieces Ref.Number Description Bone Hook Blunt Straight Chisel Curved Chisel Guj Curette Impactor Hohmann Retractor Hohmann Retractor Impactor Pim Slotted Hammer Canal Chisel Modular Rasp Handle Femoral Head Extractor Gauge for Femoral Head No. No.2 Pieces A-24
25 Prosthesis Body Rasp Gauge For Femoral Head (ø) Medullary Awl Reamer Modular Rasp Handle Rasp Bar (ø) Curette Spoon Curette Bone Hook Blunt Straight Chisel Curved Chisel A-25
26 Guj Curette Impactor Hohmann Retractor Hohmann Retractor Impactor Pim Slotted Hammer Canal Chisel T-Handle Femoral Head Extractor Test Heads S M L A-26
27 Section of Modular Hip Prostheses uncemented T-2 Revision Hip Prosthesis, uncemented T-2 Revision Hüftprothese, unzementiert Tige de reprise T-2, non-cimentée Indication : In revision arthroplasty especially in cases of ostelysis or large cement mantels the extended femoral defects can cause intraoperatively shaft fractures when removing the stem. The defects and fractures must be bridged by a long stem. The implant has to be addapted exactly to the diameter of the cortical tube and has to be placed distally beyond the pathologic area where stress pattern become normal. The modular hip revision implant system Ti6Al4V Eli-alloy consist of the single modular elements, stems, proximal body (with or without tendon holes) and safety screw. All elements are able to be fixed rotationally free. The stems are available in three different length of mm straight with eight different diameters. These components do really quarantee to solve all intraoperative situations as soon as the surgeon sees the defect during revision. A torque limiter fixes the compression force between the single modules always at the same value of torque. The type 2 revision hip surgery has been proved very successfuly for femoral deficiency. Modular concept of the system allows to manage each intraoperative findings and especially changing situations. Each step of the operation is reversible and intraoperative changing situation can be easlly managed proceeding operation. The modular aspects of the design allow appropriate intraoperative adjustment of the length of prosthesis and angle of anterversion of the neck. Material : The T-2 revision hip prosthesis is manufactured from titanium Ti6Al4V according to ASTM F.36 - ISO 5832/3 which has been specifically designed for the manufacture of orthopaedic implants. This alloy is characterized by demonstrating excellent biocompatibility, together with a high resistance of corrosion and superiormechanical strength. The roughened surface finish also plays a vital role in the osseointegration. A-27
28 T-2 Revision Hip Prosthesis Body with Tendon Holes T-2 Revision Hip Prosthesis Body Standard Titanium Length L mm Titanium Length L mm X Short Short Medium Long X Short Short Medium Long T-2 Revision Hip Prosthesis Stem uncemented T-2 Revision Hip Prosthesis Fixation Bolt Titanium Stem D mm Stem Length L mm Cone mm /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 Titanium X Short Short Medium Long Length L mm A-28
29 T-2 Revision Hip Prosthesis Complete Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces Modular Head Impactor T-Handle Hammer Impactor Bar Shaft Impactor Body Impactor Reamer Reamer Reamer Reamer Reamer Reamer Cortical Screwdriver /82 T-2 Revision Stem Gauge for Femoral Head Long Modular Test Head ø 28mm 2/4 Modular Test Head ø 28mm 2/4 Modular Test Head ø 28mm 2/4 Modular Head ø 28mm 2/4 Modular Head ø 28mm 2/4 Modular Head ø 28mm 2/4 T2 Revision Hip Prosthesis Test Body T2 Revision Hip Prosthesis Test Body T2 Revision Hip Prosthesis Test Body T2 Revision Hip Prosthesis Body T2 Revision Hip Prosthesis Body T2 Revision Hip Prosthesis Body T-2 Fixation Bolt 29mm T-2 Fixation Bolt 35mm T-2 Fixation Bolt 45mm S M L S M L XS S M XS S M XS S M A-29
30 T-2 Revision Hip Prosthesis Body Standard Titanium K mm Length L mm X Short Short Medium T-2 Revision Hip Prosthesis Test Body X Short Short Medium Reamer T-2 Revision Hip Prosthesis Fixation Bolt (ø) Titanium K mm Length L mm X Short Short Medium Long T-2 Revision Hip Prosthesis Stem uncemented Titanium Stem K mm Stem Length L mm Cone mm /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 A-30
31 Screwdriver (Long) Hammer (500gr) Shaft Impactor Gauge For Femoral Head Body Impactor Modular Head Impactor T-Handle Impactor Bar Modular Head ø28 mm 2/ Modular Test Head S M L S M L A-3
32 Section of Modular Hip Prostheses cemented Modular Tumor Resection Hip Prosthesis, cemented Modulare Tumor Resektion Hüftprothese, zementiert Tige modulaire de tumeur résection, cimentée Indication : The cemented modular tumor resection prosthesis system was designed for cases of large resection of the proximal femur as primary tumors, metastases, revisions with bone resorption and severe epiphysial trauma. In order to optain the highest number solution with a low number of components, a modular structure made up of stem, buttom cover, spacer and single proximal femoral body, has been conceived, where every component is assembled by a fixation screw connection. The stem feature eight longitudinal rips or equal high, which guarantees rotational stability of the implant after impaction. The roughened surface finish also plays a vital role in the osseointegration. Suitable for both right and left femur. Material : The modular tumor resection hip prosthesis system is manufactured from titanium Ti6Al4V according to ASTM F.36 - ISO 5832/3 which has been specifically designed for the manufacture of orthopaedic implants. This alloy is characterized by demonstrating excellent biocompatibility, together with a high resistance of corrosion and superiormechanical strength. The roughened surface finish also plays a vital role in the osseointegration. ExtansionPieces Titanium Length (mm) A-32
33 Proximal Femur Body Fixation Bolt Titanium Length ( L mm) Modular Femoral Stem Buttom Cover Titanium (ø) Length (mm) Titanium (mm) A-33
34 Modular Tumor Resection Implants & Instrumentation Set Ref.Number Description Pieces Ref.Number Description Pieces T-handle for Reamer Femoral Stem Reamer Femoral Stem Reamer Femoral Stem Reamer Femoral Stem Reamer Impactor Screwdriver Cup Inserting Device Positioning Metal Cup Curette Femoral Head Extractor Shaft Wrench for Straight Chisel Curved Chisel Guj Curette Spoon Curette Hohmann Retractor Hohmann Retractor Bone Hook Blunt Impactor Bar Impactor Shaft Fixation Bolt No. No Porximal Femoral Body Femoral Stem Femoral Stem Femoral Stem Femoral Stem Femoral Stem Femoral Stem Femoral Stem Femoral Stem Extension Pieces Extension Pieces Extension Pieces Extension Pieces Extension Pieces Bottom Cover Bottom Cover Bottom Cover Bottom Cover Modular Head ø 28-2/4 Modular Head ø 28-2/4 Modular Head ø 28-2/4 Modular Head 2/4 Titanium Modular Head 2/4 Titanium Modular Head 2/4 Titanium Modular Head 2/4 Titanium Modular Head 2/4 Titanium Modular Head 2/4 Titanium Modular Head 2/4 Titanium Gauge for Femoral Head x245 x285 2x245 2x285 4x245 4x285 6x245 6x S M L A-34
35 Femoral Stem Reamer Gauge For Femoral Head (ø) Positioning Metal Cup Cup Inserting Device Shaft Impactor Bone Hook Blunt Wrench for Impactor (ø) A-35
36 Spoon Curette Curette Guj Curette Hexagonal Head Screw Driver Femoral Head Extractor T-Handle Straight Chisel Curved Chisel Hohmann Retractor Hohmann Retractor Shaft Impactor Impactor Bar A-36
37 Section of Modular Hip Prostheses cemented Leinbach Hip Prosthesis, cemented Leinbach Hüftprothese, zementiert Prothèse de Leinbach, cimentée Indication : The Leinbach hip prosthesis can be used as a salvage procedure when a collapse of bone neck structure has occured. It is a procedure in compensating for bone loss. A slightly thicker and longer stem affords stability. The proshesis feature has a 35º degree stem/neck angle for an anatomic reconstruction. The neck has a taper 2/4 to accept a variety of head components. Three different length of stem available. Material : The Leinbach hip prosthesis is manufactured from certified Stainless steel according to ASTM F.38 - ISO 5832/. Co.Cr-alloy according to ASTM F.75 - ISO 5832/4 Stainless Steel Co.Cr-alloy Cone Stem Length L mm s /4 2/4 2/ Short Medium Long A-37
38 Leinbach Hip Prosthesis Complete Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces Bone Hook Blunt Hohmann Extractor Hohmann Extractor Curved Chisel Straight Chisel Guj Curette Curette Spoon Curette Impactor T-Handle Rasp Bar Femoral Head Extractor Shaft Wrench for No. No Monoblock Prosthesis Rasp Monoblock Prosthesis Rasp Cup Inserting Device Femoral Head Impactor Positioning Metal Top Positioning Metal Top Gauge for Femoral Head Short Long A-38
39 Stem Rasp Gauge For Femoral Head (ø) Short Long Positioning Metal Cup Cup Inserting Device Rasp Bar (ø) Impactor Shaft Wrench for Bone Hook Blunt (ø) A-39
40 Spoon Curette Curette Guj Curette Femoral Head Impactor Femoral Head Extractor T-Handle Straight Chisel Curved Chisel Hohmann Retractor Hohmann Retractor A-40
41 Section of Modular Hip Prostheses cemented Modular Straight Stem, cemented Modular Geradschaft, zementiert Tige droite modulaire, cimentée Indication : The modular straight stem hip prosthesis is designed to be used as a primary and revision total hip. The 4 stem sizes allow accurate matching of prosthesis to the medullary canal. The prosthesis offers dual fixation by combinig the locking effect of a stem to bone contact, plus minimal bone cement support. May be used in conjuction with modular head cone 2/4 and polyethylene acetabular components for total articular replacement. Material : Certified Stainless Steel according to ASTM F.38 ISO 5832/. Available in titanium according to ASTM F.36 ISO 5832/3 and Co.Cr-alloy according to ASTM F.75 - ISO 5832/4 Stainless Steel Titanium Co.Cr-alloy Stem K mm Cone mm Stem Length L mm /4 2/4 2/4 2/ A-4
42 Section of Modular Hip Prostheses cemented Modular Long Straight Stem, cemented Modular Lang Geradschaft, zementiert Tige droite modulaire long, cimentée Indication : The modular long straight stem hip prosthesis allows for an increase in cross-sectional stem dimensions to more proportionately fill the intra-medullary canal and ensure implant stability. The stem can be used in either primary or revision cases where bone dimensions are too large for the standard primary hip. May be used in conjuction with modular head and polyethylene acetabular components for total articular replacement. Material : Certified Stainless Steel according to ASTM F.38 ISO 5832/. Available in titanium according to ASTM F.36 ISO 5832/3 and Co.Cr-alloy according to ASTM F.75 - ISO 5832/4 Stainless Steel Titanium Co.Cr-alloy Stem K mm Cone mm Stem Length L mm /4 2/4 2/4 2/ /4 2/4 2/4 2/ /4 2/4 2/4 2/ /4 2/4 2/4 2/ A-42
43 Modular Straight Stem Complete Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces Bone Hook Blunt Bone Hook Sharp Hohmann Extractor Hohmann Extractor Curved Chisel Straight Chisel Guj Curette Curette Spoon Curette Impactor T-Handle Rasp Bar Femoral Head Extractor Shaft Wrench for No. No Monoblock Prosthesis Rasp Monoblock Prosthesis Rasp Monoblock Prosthesis Rasp Monoblock Prosthesis Rasp Cup Inserting Device Femoral Head Impactor Positioning Metal Top Positioning Metal Top Positioning Metal Top Gauge for Femoral Head A-43
44 Stem Rasp Positioning Metal Cup (ø) Cup Inserting Device Rasp Bar (ø) Impactor Shaft Wrench for Bone Hook Sharp Bone Hook Blunt (ø) A-44
45 Spoon Curette Curette Guj Curette Femoral Head Impactor Femoral Head Extractor T-Handle Straight Chisel Curved Chisel Hohmann Retractor Hohmann Retractor Gauge for Femoral Head A-45
46 Section of Modular Hip Prostheses cemented CDH Modular Straight Stem, cemented CDH Modular Geradschaft, zementiert Tige droite modulaire CDH, cimentée Indication : It is cemented total hip prosthesis. CDH prosthesis is adapted to the narrow condition in the proximal medullary canal. Fixation is achieved by self-locking of the largest possible stem into the medullary cavity and using bone cement. The modular CDH staight stem hip prosthesis is designed for using detachable head for total hip replacement as well. This prosthesis combines the self-locking feature with the versalitiy of detachable heads with ø22 mm - 2/4 cone. 3 size of heads available (S-M-L). Detachable heads mean different sizes may be fitted without removing entire unit and enabling the surgeon to select the most appropriate implants for each individual procedure.the CDH straight stem with modular head has been used in combination with a ultra-high molecular weight (U.H.M.W.P.E.) polyethylene acetabular cup provides for an exceptionally stable total hip replacement. Cups to be used : Polyethylene acetabular cup diam. 22 mm. See page A-64 Heads to be used : Metal stainless steel or Co.Cr-alloy heads diam. 22mm. See page A-62 Material : The modular CDH straight stem is manufactured from titanium Ti6AL4V Alloy according to ASTM F.36 - ISO 5832/3 Titanium Stem K mm Stem Length L mm Cone mm /4 2/4 2/4 2/4 2/4 A-46
47 Section of Monoblock Hip Prostheses cemented CDH Straight Stem, cemented CDH Geradschaft, zementiert Tige droite CDH, cimentée Indication : It is cemented total hip prosthesis. CDH prosthesis is adapted to the narrow condition in the proximal medullary canal. Fixation is achieved by self-locking of the largest possible stem into the medullary cavity and using bone cement. Proximal stem configuration and 22 mm head diameter are designed for special conditions of dysplastic hip.the CDH straight stem with 22 mm head has been used in combination with a ultra-high molecular weight (U.H.M.W.P.E.) 22 mm polyethylene acetabular cup provides for an exceptionally stable total hip replacement. Material : Certified Stainless Steel according to ASTM F.38 ISO 5832/ Stainless Steel Stem K mm Head D mm Stem Length L mm P.E acetabular cups to be used U.H.M.W Inside Diam mm Outside Diam mm A-47
48 Section of Acetabular Implants uncemented Press fit Cup, uncemented Pressfitpfannen, unzementiert Cotyle press-fit, non-cimentée Indication : In principal for all forms of arthrosis, advance wear and tear of tne hip joint because of degeneratif, posttraumatic or rheumatoid arthritis fracture of avascular necrosis of the head of femur. Sequelae of earlier operations such as osteosynthesis, joint reconstraction, arthrodesis, hemiarthroplastic or total hip replacement. Press-fit cup, is an acetabular implant for uncemented fixation. The standard cup made of Ti6Al4V alloy with three dimensional interlock grit blast present an hemispherical shape with polar deflection to enhance the perifery distribution of the stresses effecting the cup. The titanium cup has three holes on superolateral side. This sensibly reduce the extrubtion of UHMWPE owing to a wide contact surface between metal and P.E and consequent low specific pressure. The screws have a spheric head and have always a good contact with their seatings, even if the introduction angle may be different. This prevent the fratting corrosion and assure the screw stability. In spite of the different possible introduction angles of the screw. There is no risk of damages to vessels or nerves if the acetabular metal cup have been conceived for a press-fit implant. Therefore if the acetabular socket has a regular confirmation, the use of screws is not necessary and cup must implanted with the holes in inferomedial. The unused screw holes can be sealed with a screw plug. In this way there is no contact discontinuity between metal and P.E and the extrusion of UHMWPE in the screws seals is avoid. Chosen as the basic material was Ti6Al4V which has been tried and tested over many years and is outstandingly biocompatible. For the bone side contact surface Titanium Plasma Spray coating enhances initial fixation and long term bone apposition. The porosity of plasma spray is ideal for osseointegration. Optional, available with Titanium plasma spray + HA.C Hydroxapatite coating. Material : Certified Titanium alloy according to ASTM F.36 - ISO 5832/3. It is selected for its, modulus of elasticity closest to that living bone, high fatique strength and perfect biocompatibility. Titanium Grit Blast Ti. Plasma Spray Ti.P.S. + HA Diam D mm A-
49 Section of Acetabular Implants uncemented P.E Insert for Press fit Cup, uncemented P.E Einsartz für Pressfitpfannen, unzementiert Noyau P.E pour Cotyle press-fit, non-cimentée Indication : The UHMWPE insert is snappe into the metal acetabular cup. However, the liner can be removed via the two slots in the rim and be reused again. The press fit acetabular cup can reduce the possibility of wear debris from the inner surface of the metal cup. It is done by machining and polishing the inner surface after the coating process, thus preventing a roug metal surface from coming into contact with polyethylene. The good precision of manufacture assures moreover the direct contact between the UHMWPE insertand inner surface of the metal cup reducing micromotions and fretting The polyethylene insert is produced with a 0º rim to avoid dislocation. Material : UHMWPE Ultra Heavy Molecular Weight Polyethylene meets ASTM F.6 and ISO 5834/2 standards. Selected for its; - High degree fo purity - Good biotolerance - Good mechanical performance - Friction properties P.E Inside Diam mm Outside Diam D mm Starex Titanium Fixation Screw Titanium Diam D mm Lenght mm Starex Titanium Fixationschrauben Vis starex de fixation en Titane Indication : If bone quality is such that the titanium cup has to be secured with screws, special bone screws with spheric head and cancellous thread (ø 6.5 mm) are used for fixation. For fixation, flexible drill and universal joint screw driver are avaliable. The screw have always a good contact with their seatings, even if the introduction angle may be different. This prevent the fretting corrosion and assure the screw stability. Material : Certified Titanium alloy according to ASTM F.36 and ISO 5832/3. A-49
50 Press Fit Cup Instrumentation Set : A-50
51 Press Fit Cup Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces Guj Curette Curette Spoon Curette Tap Gauge for Femoral Head Gyroscopic Drill Guide Depth Gauge Handle for Test Cup Test Cup Test Cup Test Cup Test Cup Test Cup Test Cup Test Cup Test Cup Test Cup Self Holding Forceps Starex Screw Holding Forceps Extraction Sleeve Press Fit Cup Handle Impactor Attachment Impactor Attachment Impactor Attachment Impactor Attachment Impactor Attachment Impactor Attachment Impactor Attachment Impactor Attachment Impactor Attachment Hexagonal Long Screwdriver Universal Joint Screwdriver ø Shaft T-Handle (Fiber) Drill ø 3.5 x 25 x 40 Drill ø 3.5 x 30 x 50 Replacement Handle Insert Control Guide Insert Control Guide Insert Control Guide Insert Control Guide Insert Control Guide Insert Control Guide Insert Control Guide Insert Control Guide Insert Control Guide Flexible Shaft Universal Joint Tap Handle Bone Hook Blunt Hohmann Retractor Hohmann Retractor Hohmann Retractor Wrench Hexagonal Wrench Screw-in Attachment No.5 No. No.2 2 A-5
52 Tap Gyroscopic Drill Guide Gauge for Femoral Head Depth Gauge Self Holding Forceps Extraction Sleeve Starex Screw Holding Forceps Press Fit Cup Handle Flexible Shaft Replacement Handle Universal Joint Tap Handle Bone Hook Blunt A-52
53 Spoon Curette Curette Guj Curette Hexagonal Long Screwdriver Hexagonal Wrench Universal Joint Screwdriver Wrench T-Handle Screw-in Attachment Hohmann Retractor Hohmann Retractor Hohmann Retractor A-53
54 Handle for Test Cup Drill Test Cup Impactor Attachment Insert Control Guide (ø) 3.5x25x40 3.5x30x40 (ø) (ø) (ø) Shaft (ø) A-54
55 Section of Acetabular Implants uncemented Expansion Cup, uncemented Spreizchale, unzementiert Cupule à expansion, non-cimentée Indication : The expansion cup is characterised by extreme easy implantation. When inserted, six point pedals that expand equatorially assure an immediate primary stability. The hemisferical shape with polar deflection quarantees that the stress is distributed on lateral portions of the cup. The outer surface of the cup is microstructured by sandblasting with corundic stream to enhance the biological fixation. Internally is sandblasted too. Titanium D Material : Certified Titanium alloy according to ASTM F.36 - ISO 5832/3. It is selected for its, modulus of elasticity closest to that living bone, high fatique strength and perfect biocompatibility. P.E Insert for Expansion Cup P.E Einsatz für Spreizchale Noyau P.E pour la cupule à expansion Indication : The insert is installeted into titanium expansion cup. The rotationel stability is insured by this special profile both on external surface of the liner and internal surface of the cup. In addition, the polyethylene does not have any expansive function, in order to avoid the formation of debris due to excessive between cup and polyethylene insert. Material : UHMWPE Ultra Heavy Molecular Weight Polyethylyene meets ASTM F.6 and ISO 5834/2 standards. Selected for its, high degree of purity, good biotolerance, good mechanical performance and friction properties. P.E D A-55
56 Expansion Cup Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces Compession Pliers Positioning Guide for Lateral Posit. Guide for Postero-Lateral Acetabular Remer Shaft Setting Device Setting Device Handle Expansion Cone Expansion Cone Expansion Cone Expansion Cone Expansion Cone Expansion Cone Expansion Cone Expansion Cone T-Handle Ratchet Wrench Wrench Screw-in Attachment ø46- Screw-in Attachment ø50-62 Extraction Sleeve A-56
57 Compression Pilers Positioning Guide for Postero-Lateral Positioning Guide Shaft Handle Wrench T-Handle Extraction Sleeve A-57
58 Ratchet Wrench Screw-in Attachment Expansion Cone (ø) (ø) Setting Device (ø) (ø) A-58
59 Section of Acetabular Components uncemented Modular Bipolar Cup, uncemented Modulare Bipolarkopf, unzementiert Tête modulaire bipolaire, non-cimentée Indication : Placement of cup together with a femoral head on stem gives an articulated partial prosthesis. The modular outher shell is manufactured from certified stainless steel and inner side, the insert is manufactured from Ultra High Molecular Weight Polyetylene UHMWPE. Material : UHMWPE meets ASTM F.6 and ISO 5834/2 standards. Selected for its, high degree of purity, good biotolerance, good mechanical performance and friction properties. Metal part made from certified stainless steel meets ASTM F.38 - ISO 5832/ standards. Titanium Inside Diam D mm Outside Diam D2 mm Bipolar Cup Clamp Required for replace or remove the inner C ring of the bipolar cup during operation. A-59
60 Modular Bipolar Cup Instrumentation Set : Ref.Number Description Pieces Ref.Number Description Pieces Bipolar Modular Test head Bipolar Modular Test head Bipolar Modular Test head Bipolar Modular Test head Bipolar Modular Test head Bipolar Modular Test head Bipolar Modular Test head Bipolar Modular Test head Bipolar Modular Test head Inner Taper Trial Inner Taper Trial Inner Taper Trial Cup Impactor Curved Attachment for Cup Impactor Insert Remover Gauge for Femoral Head Bipolar Clamp S M L A-60
61 Bipolar Modular Test Head Inner Taper Trial Head Cone 2/ (ø) Attachment for Cup Impactor S M L Cup Impactor Curved Insert Remover Gauge For Femoral Head Bipolar C Ring Clamp A-6
62 Section of Modular Heads for Total Hip Prostheses Modular Head for Total Hip Prosthesis Modulare Kopf, für Hüft-totalprothese Tête modulaire pour prothèse total de hanche Indication : Placement of the head on the stem gives an articulated partial prosthesis. The head is set on the taper of stem giving required movement whithin the iner semi-sphere of the acetabulum. The T psan modular heads avaliable with cone 2/4 and mm outside diameter with 3 sizes (Short - Medium - Long) Material : Certified Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This raw metarial has excellent biomechanical properties. Combined with a modern manufacturing process and outstanding quality control the result is a carbide free, high-polished surface femoral head. This ensure maximum function and reliability for both surgeon and patient. Advantages; Biologically compatible, meets stringent CE quality control standards, fractures resistance. The femoral heads are also avaliable from certified Stainless Steel according to ASTM F.38 - ISO 5832/ Stainless Steel Co.Cr-alloy Head Diam mm Cone mm /4 2/4 2/4 S M L /4 2/4 2/4 S M L Stainless Steel Co.Cr-alloy Head Diam mm Cone mm 2/4 2/4 2/4 S M L A-62
63 Section of Modular Heads for Total Hip Prostheses Unipolar Head for Partial Prothesis Unipolarkopf, für Partialprothese Tête unipolar pour prothèse partial Indication : Placement of the head on the stem gives an articulated partial prosthesis. The head is set on the taper of stem giving required movement whithin the iner semi-sphere of the acetabulum. The T psan unipolar head has are avaliable with 20 outside diameters and with cone 2/4. Material : Certified Co.Cr-alloy according to ASTM F.75 - ISO 5832/4. This raw metarial has excellent biomechanical properties. Combined with a modern manufacturing process and outstanding quality control the result is a carbide free, high-polished surface femoral head. This ensure maximum function and reliability for both surgeon and patient. Advantages; Biologically compatible, meets stringent CE quality control standards, fractures resistance. The femoral heads are also avaliable from certified Stainless Steel according to ASTM F.38 - ISO 5832/ Stainless Steel Co.Cr-alloy Head Diam mm Cone mm /4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 2/4 A-63
64 Section of Acetabular Implants cemented Accetabular Cup, cemented Pfannen, zementiert Cotyle, cimentée Indication : The cups are placed in degerated anatomical acetabulum and requires cement for fixation. Provided with radiopaque wire for postoperatory visualisation. The standard 50 mm acetabular cup meets the anatomical requirements of major patients, while the 44 mm cup is used if the ilium is narrow in cases of high dislocation with migration of the acetabulum. Two larger sizes of acetabular cups, 54, 58 mm have been added to tha range to meet special requirements of large boned patients. The U.H.M.W. polyethylene cup has wellproved tissue tolerance, elasticity and a very low coefficient of friction. Particular attention is paid to the precision machining and surface finish of the acetabular component, so that, when combined with femoral component, and exceptionally stable total hip replacement is achieved with minimal risk of dislocation and minimum wear rate on the acetabular component. Material : UHMWPE Ultra Heavy Molecular Weight Polyethylyene meets ASTM F.6 and ISO 5834/2 standards. Selected for its, high degree of purity, good biotolerance, good mechanical performance and friction properties. U.H.M.W Inside Diam D mm Outside Diam D mm A-64
65 Section of Acetabular Components uncemented Acetabular Ring Pfannendach - Schale Anneau de soutien Indication : Acetabular ring is indicated in total hip replacement in cases of osteoporosis, rheumatoid arthritis, acetabular roof cysts, simultaneous bone grafting, after arthrodeses and in socket revision. An acetabular reinforcement ring is also recommended in combination with polyethylene cups with low wall thickness. In revision cases with severe bone loss and protrusion, the usage of a acetabular reinforcement cage is recommended. After the preparation of the acetabulum, an acetabular ring with diameter of 4 mm smaller than the last reamer is normaly used. The implant is wedge into the acetabulum with the metal impactor.the ring is positioned correctly if its lateral rim lies on the border of acetabulum. It is imperative that the area of the screw holes and lower border of the ring should have sufficient bone contact. The acetabular ring is fixed with 4 or 6 cancellous screws. These are positioned in the os ilim in the direction of the sacral-iliae woint and are inclined approximately 20 medially and posteriorly in relation to the long axis of the body. The polyethylene cup has an inclination of approximately 40 which corresponds in most cases with the position of the ring. Material : Certified Stainless Steel according to ASTM F.38 and ISO 5832/. On request can be produced from pure Titanium according to ISO 5832/3. Pure titanium is known to be one of the most corrosion-resistance, most biocompatible metal implant materials available. Its elasticity makes it ideal for acetabular cups. Pure titanium with a defined rough surface enhances the growth of bone directly onto the implant surface. (Avaliable on special request ) Stainless Steel Titanium Diam D mm A-65
66 Section of Acetabular Components uncemented Acetabular Reinforcement Cage Pfannenstützschale Armature de soutien Indication : The reinforcement cage is indicated for cup revision with considerable osseous defects or protrusion, if it is no longer possible to achieve adequate primary stability with any other implant. The intact, resistance bone is made usable again; forces acting on the bone are spread over a large area. The reinforcement cage permits undisturbed incorporation of bone grafts. The reinforcement cage is implanted without cement; the cement serves only to fix the cup in the cage. The sievelike cage tapers to flange cranially and to a nose caudally. The dorsal edge is turned out in order to ensure better rotational stability. The holes in the flange serve for subsequent fixing in the pelvic wall. The distal nose is hammered through from the inside of the cup into the ischium. The reinforcement cage can be adapted to the anatomy of pelvis by bending the flange. The inclination of the cage does not determine that of the cup. The cup can be cemented into correct position. Right Left Material : Certified Stainless Steel according to ASTM F.38 and ISO 5832/. On request can be produced from pure Titanium according to ISO 5832/3. Pure titanium is known to be one of the most corrosion-resistance, most biocompatible metal implant materials available. Its elasticity makes it ideal for acetabular cups. Pure titanium with a defined rough surface enhances the growth of bone directly onto the implant surface. (Avaliable on special request ) Stainless Steel Titanium Diam D mm Style Right Right Right Right Right Right Right Right Left Left Left Left Left Left Left Left A-66
67 Section of Monoblock Hip Prostheses cemented ØD Standard Moore Hip Prosthesis Hüftprothese nach Moore Prothesè de Moore Indication : The Standard Moore hip prosthesis is designed to use for degenerated femoral head replacement. The designed of this applience approximates the anatomical shape of femur. The fenestrated stem permits packing of bone grafts and bony ingrowth for improved fixation. The availability of head sizes in fractional and millimeter increments offers, the surgeon a wide selection for a more accurate fit to the acetabulum. The stem shape as well as the fin along, the outer side helps to prevent rotation of the prosthesis. The loop at the top of stem is provided for extracting the stem. The prosthesis feature has a 35º degree stem/neck angle for an anatomic reconstruction. L Material : Certified Stainless Steel according to ASTM F.38 ISO 5832/. Available in titanium according to ASTM F.36 ISO 5832/3 and Co.Cr-alloy according to ASTM F.75 ISO 5832/4 Stainless Steel Titanium Co.Cr-alloy Head s Diameter D mm Stem Length L mm A mm B mm C mm mm 39 mm 40 mm 4 mm 42 mm 43 mm 44 mm 45 mm 46 mm 47 mm mm 49 mm 50 mm 5 mm 52 mm 53 mm 54 mm 55 mm 56 mm 57 mm 58 mm , ,5 45,5 38 A-67
68 Section of Monoblock Hip Prostheses cemented ØD Moore Hip Prosthesis, Solid Stem Hüftprothese nach Moore, vollschaft Prothèse de Moore, tige pleine Indication : The solid stem Moore prostheses are exact duplicates of the regular stems respectively, eccept that fenestration in the stem have been filled in for the surgeon who wishes to use with bone cement. It is totally designed for cemented apllication. The availability of head sizes in fractional and millimeter increments offers, the surgeon a wide selection for a more accurate fit to the acetabulum. The stem shape as well as the fin along, the outer side helps to prevent rotation of the prosthesis. The loop at the top of stem is provided for extracting the stem. The prosthesis feature has a 35º degree stem/neck angle for an anatomic reconstruction. L Material : Certified Stainless Steel according to ASTM F.38 ISO 5832/. Available in titanium according to ASTM F.36 ISO 5832/3 and Co.Cr-alloy according to ASTM F.75 ISO 5832/4 Stainless Steel Titanium Co.Cr-alloy Head s Diameter D mm Stem Length L mm A mm B mm C mm mm 39 mm 40 mm 4 mm 42 mm 43 mm 44 mm 45 mm 46 mm 47 mm mm 49 mm 50 mm 5 mm 52 mm 53 mm 54 mm 55 mm 56 mm 57 mm 58 mm , A-68
69 Moore Hip Prosthesis Complete Instrumentation Set : Ref.Number Description Moore Rasp Moore Rasp Moore Mortising Chisel Femoral Head Impactor Femoral Head Extractor Universal Head Extractor Rasp Bar Hammer Gauge for Femoral Head Long Short 500gr Pieces A-69
70 Moore Rasps Long Short Moore Mortising Chisel Modular Head Impactor Femoral Head Extractor Universal Driver Extractor Rasp Bar Hammer (500gr) Gauge for Femoral Head A-70
71 Section of Monoblock Hip Prostheses cemented ØD Moore Hip Prosthesis, Narrow Stem Hüftprothese nach Moore, schmalschaft Prothèse de Moore, tige étroite Indication : The narrow stem Moore hip prosthesis is designed to use when the medullary canal is to small to accept the standard Moore hip prosthesis. This prosthesis has the same features as the standard stem but is narrower then the regular stem in the medial/lateral direction. The designed of this applience approximates the anatomical shape of femur. The fenestrated stem permits packing of bone grafts and bony ingrowth for improved fixation. The availability of head sizes in fractional and millimeter increments offers, the surgeon a wide selection for a more accurate fit to the acetabulum. The stem shape as well as the fin along, the outer side helps to prevent rotation of the prosthesis. The loop at the top of stem is provided for extracting the stem. The prosthesis feature has a 35º degree stem/neck angle for an anatomic reconstruction. Material : Certified Stainless Steel according to ASTM F.38 ISO 5832/ Available in titanium according to ASTM F.36 ISO 5832/3 and Co.Cr-alloy according to ASTM F.75 ISO 5832/4 L Stainless Steel Titanium Co.Cr-alloy Head s Diameter D mm Stem Length L mm A mm B mm C mm mm 39 mm 40 mm 4 mm 42 mm 43 mm 44 mm 45 mm 46 mm 47 mm mm 49 mm 50 mm 5 mm 52 mm 53 mm 54 mm 55 mm 56 mm 57 mm 58 mm A-7
72 Narrow Stem Moore Hip Prosthesis Complete Instrumentation Set : Ref.Number Description Narrow Stem Moore Rasp Narrow Stem Moore Rasp Narrow Moore Mortising Chisel Femoral Head Impactor Femoral Head Extractor Universal Head Extractor Rasp Bar Hammer Gauge for Femoral Head Long Short 500gr Pieces A-72
73 Narrow Stem Moore Rasps Long Short Narrow Moore Mortising Chisel Modular Head Impactor Femoral Head Extractor Universal Driver Extractor Rasp Bar Hammer (500gr) Gauge for Femoral Head A-73
74 Section of Monoblock Hip Prostheses cemented ØD Thompson Hip Prosthesis Hüftkopfprothese nach Thompson Prothèse de Thompson Indication : Used for replacement of the degenerated femoral head. The wedge of the stem provides great resistance to rotation and its broad collar gives excellent weight distribution on the calcar.the availability of head sizes in fractional and millimeter increments offers the surgeon a wide selection for a more accurate fit to the acetabulum. The Curved Trapezoidal Stem resists rotation. The prosthesis features has a 35º degree stem/neck angle for an anatomic reconstruction. Material : Certified Stainless Steel according to ASTM F.38 ISO 5832/ Available in titanium according to ASTM F.36 ISO 5832/3 and Co.Cr-alloy according to ASTM F.75 ISO 5832/4 L Stainless Steel Titanium Co.Cr-alloy Head s Diameter D mm Stem Length L mm A mm B mm mm 39 mm 40 mm 4 mm 42 mm 43 mm 44 mm 45 mm 46 mm 47 mm mm 49 mm 50 mm 5 mm 52 mm 53 mm 54 mm 55 mm 56 mm 57 mm 58 mm 04 28,5 46 A-74
75 Thompson Hip Prosthesis Complete Instrumentation Set : Ref.Number Description Rasp Bar Thompson Rasp Impactor Femoral Head Extractor Curette Gauge for Femoral Head Pieces A-75
76 Thompson Rasp Rasp Bar Impactor Femoral Head Extractor Gauge For Femoral Head Curette A-76
77 Section of Monoblock Hip Prostheses cemented ØD Müller Type Hip Prosthesis Hüftkopfprothese nach Müller Prothèse de Müller C Indication : The standard stem configuration will match %90 of all indications. There are 3 different neck lengths which allow implantation without removal of the greater trochanter. This curved stem is designed to provide the greatest metal strength laterally where strength is needed most. Also the structured surface offers maximum cement/stem interface. The tapered shaft helps permit easy removal, if removal becomes necessary.müller type hip prostheses with a 32 mm/28 mm diameter head in combination with the ultra-high moleculer weight (U.H.M.W.P.E.) polyethylene cup provides for an exceptionally stable total hip replacement. The eliptical neck allows for greater clearance and flexion, and the collar is recessed at the base to provide greater surface area contact for the cement. The prosthesis feature has a 35º degree stem/neck angle for an anatomic reconstruction. L Material : Certified Stainless Steel according to ASTM F.38 - ISO 5832/ Stainless Steel Neck Length C mm Head s Diameter D mm Stem Length L mm A mm B mm mm 34 mm 40 mm 28 mm 34 mm 40 mm 32 mm 32 mm 32 mm 28 mm 28 mm 28 mm A-77
78 Hohmann Retractor Instruments for Hip Surgery Distap tip of retractor placed under shaft of bone to retract soft tissue and elevate bone. Hohmann Retractor Hohmann Retractor Hohmann Retractor Hohmann Retractor Hohmann Retractor A-78
Section of Modular Hip Prostheses cemented. TMC-3 Modular Hip Prosthesis, cemented. TMC-3 Modular Hüftprothese, zementiert
 Section of Modular Hip Prostheses cemented TMC-3 Modular Hip Prosthesis, cemented TMC-3 Modular Hüftprothese, zementiert Prothèse de hanche modulaire TMC-3, cimentée Indication : The TMC-3 Modular hip
Section of Modular Hip Prostheses cemented TMC-3 Modular Hip Prosthesis, cemented TMC-3 Modular Hüftprothese, zementiert Prothèse de hanche modulaire TMC-3, cimentée Indication : The TMC-3 Modular hip
Hip Implants. Hip Implants. 6 Your partner for implants and trauma products. Euromed Implants GmbH General Catalogue
 6 Modular Straight Cemented Stem The modular straight cemented stem hip prosthesis is designed to be used as a primary and revision total hip. The 4 stem sizes allow accurate matching of prosthesis to
6 Modular Straight Cemented Stem The modular straight cemented stem hip prosthesis is designed to be used as a primary and revision total hip. The 4 stem sizes allow accurate matching of prosthesis to
Ref. Number Ti.P.S. + HA. Ref. Number Ti. Plasma Spray
 Section of Acetabular Implants uncemented Press fit Cup, uncemented Pressfitpfannen, unzementiert Cotyle press-fit, non-cimentée Indication : In principal for all forms of arthrosis, advance wear and tear
Section of Acetabular Implants uncemented Press fit Cup, uncemented Pressfitpfannen, unzementiert Cotyle press-fit, non-cimentée Indication : In principal for all forms of arthrosis, advance wear and tear
Surgical Technique. Hip System
 Surgical Technique Hip System INDICATIONS FOR USE The TaperSet Hip System is designed for total or partial hip arthroplasty and is intended to be used with compatible components of the Consensus Hip System.
Surgical Technique Hip System INDICATIONS FOR USE The TaperSet Hip System is designed for total or partial hip arthroplasty and is intended to be used with compatible components of the Consensus Hip System.
THE NATURAL FIT. Surgical Technique. Hip Knee Spine Navigation
 THE NATURAL FIT Surgical Technique Hip Knee Spine Navigation MiniMAX Surgical Technique Hip Knee Spine Navigation INTRODUCTION The MiniMAX TM is a cementless anatomic stem available in 9 right sizes and
THE NATURAL FIT Surgical Technique Hip Knee Spine Navigation MiniMAX Surgical Technique Hip Knee Spine Navigation INTRODUCTION The MiniMAX TM is a cementless anatomic stem available in 9 right sizes and
Encina Taper Stem. Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA
 Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Table of Contents Introduction 3 Features 4 Surgical Technique 5 Preoperative
Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Table of Contents Introduction 3 Features 4 Surgical Technique 5 Preoperative
CAUTION: Ceramic liners are not approved for use in the United States.
 Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS
 SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
*smith&nephew SL-PLUS Cementless Femoral Hip System. Product Information
 Product Information *smith&nephew SL-PLUS Cementless Femoral Hip System First Came the Philosophy to develop a universal hip system that could be used in almost every indication, immaterial to the patient
Product Information *smith&nephew SL-PLUS Cementless Femoral Hip System First Came the Philosophy to develop a universal hip system that could be used in almost every indication, immaterial to the patient
HELIOS h i p s y s t e m
 HELIOS h i p s y s t e m Design The Helios stem is a highly polished, High Nitrogen Stainless Steel (ISO5832-9) dual tapered cemented stem. The design of the stem is based on the clinically lly successful
HELIOS h i p s y s t e m Design The Helios stem is a highly polished, High Nitrogen Stainless Steel (ISO5832-9) dual tapered cemented stem. The design of the stem is based on the clinically lly successful
28 Surgical Technique
 Surgical Technique 10 12 14 16 18 20 22 24 28 26 Technique described by James L. Guyton, MD Campbell Clinic Memphis, Tennessee James W. Harkess, MD Campbell Clinic Memphis, Tennessee David G. LaVelle,
Surgical Technique 10 12 14 16 18 20 22 24 28 26 Technique described by James L. Guyton, MD Campbell Clinic Memphis, Tennessee James W. Harkess, MD Campbell Clinic Memphis, Tennessee David G. LaVelle,
Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA
 Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Encina HA Stem Table of Contents Introduction 3 Encina HA Stem Features 4 Surgical
Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Encina HA Stem Table of Contents Introduction 3 Encina HA Stem Features 4 Surgical
Cement Polished Tapered Stems of 12/14 Taper. 96 mm 98 mm 104 mm 110 mm 116 mm 122 mm 128 mm. Ceramic Femoral Head. Outer Diameter
 Design Philosophy Cementless Stems of 12/14 Taper Stem Length Neck shaft Angle STEM-N3 STEM-N4 STEM-N5 STEM-N6 STEM-N7 STEM-N8 STEM-N9 123 mm 125 mm 130 mm 135 mm 140 mm 145 mm 150 mm 132 Cement Polished
Design Philosophy Cementless Stems of 12/14 Taper Stem Length Neck shaft Angle STEM-N3 STEM-N4 STEM-N5 STEM-N6 STEM-N7 STEM-N8 STEM-N9 123 mm 125 mm 130 mm 135 mm 140 mm 145 mm 150 mm 132 Cement Polished
Optimum implant geometry
 Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique VPLWK QHSKHZ 1$126 1HFN 3UHVHUYLQJ +LS 6WHP 1716-e_NANOS_OPT.indd :27
 Surgical Technique NANOS Neck Preserving Hip Stem Table of Contents Introduction... 3 Development/Concept... 4 Indications/Contraindications... 5 Preoperative Planning... 5 Surgical Technique... 6 Prosthesis
Surgical Technique NANOS Neck Preserving Hip Stem Table of Contents Introduction... 3 Development/Concept... 4 Indications/Contraindications... 5 Preoperative Planning... 5 Surgical Technique... 6 Prosthesis
Section of total knee replacement. Total Knee Replacement System. Knieendoprothesen System. Système de prothèse totale de genou
 Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Aesculap Trilliance Triple Tapered Polished Hip Stem
 Aesculap Trilliance Triple Tapered Polished Hip Stem Aesculap Orthopaedics Trilliance Triple Tapered Polished Hip Stem CONTENTS 2 Contents Page Trilliance Philosophy 4 Trilliance Design 6 Trilliance Implants
Aesculap Trilliance Triple Tapered Polished Hip Stem Aesculap Orthopaedics Trilliance Triple Tapered Polished Hip Stem CONTENTS 2 Contents Page Trilliance Philosophy 4 Trilliance Design 6 Trilliance Implants
CSS Combined Spine System
 CSS Combined Spine System H- CSS Combined Spine System H- CSS Combined Spinal System : 800000 H-3 CSS Combined Spinal System : 800000 Ref.Number Description Pieces Ref.Number Description Pieces 00000 Open
CSS Combined Spine System H- CSS Combined Spine System H- CSS Combined Spinal System : 800000 H-3 CSS Combined Spinal System : 800000 Ref.Number Description Pieces Ref.Number Description Pieces 00000 Open
Optimum implant geometry
 Design Rationale Optimum implant geometry Extending the proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Design Rationale Optimum implant geometry Extending the proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Cementless Tapered Femoral Stem Surgical technique
 Cementless Tapered Femoral Stem Surgical technique Contents Operative summary 4 Pre-operative planning 5 Femoral neck osteotomy 5 Femoral canal preparation 5 Intra-medullary (IM) reamer 6 Sequential rasping
Cementless Tapered Femoral Stem Surgical technique Contents Operative summary 4 Pre-operative planning 5 Femoral neck osteotomy 5 Femoral canal preparation 5 Intra-medullary (IM) reamer 6 Sequential rasping
PLR. Proximal Loading Revision Hip System
 PLR Proximal Loading Revision Hip System The PLR splined revision stem is designed to recreate the natural stresses in the revised femur, where proximal bone may be compromised. PLR Hip System Design Considerations
PLR Proximal Loading Revision Hip System The PLR splined revision stem is designed to recreate the natural stresses in the revised femur, where proximal bone may be compromised. PLR Hip System Design Considerations
Cementless Tapered Femoral Stem Surgical technique
 Cementless Tapered Femoral Stem Surgical technique Contents Operative summary 4 Pre-operative planning 5 Femoral neck osteotomy 5 Femoral canal preparation 5 Intra-medullary (IM) reamer 6 Sequential rasping
Cementless Tapered Femoral Stem Surgical technique Contents Operative summary 4 Pre-operative planning 5 Femoral neck osteotomy 5 Femoral canal preparation 5 Intra-medullary (IM) reamer 6 Sequential rasping
ADDRESSING CLINICAL ISSUES OF CEMENTLESS HIP ARTHROPLASTY
 E C H E L O N P R I M A R Y H I P S Y S T E M P R O D U C T R A T I O N A L E ADDRESSING CLINICAL ISSUES OF CEMENTLESS HIP ARTHROPLASTY Echelon Primary Total Hip System HIGH OFFSET STANDARD OFFSET Cementless
E C H E L O N P R I M A R Y H I P S Y S T E M P R O D U C T R A T I O N A L E ADDRESSING CLINICAL ISSUES OF CEMENTLESS HIP ARTHROPLASTY Echelon Primary Total Hip System HIGH OFFSET STANDARD OFFSET Cementless
Optimum implant geometry
 Design Rationale Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Design Rationale Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Cementless femoral stem type SF
 Cementless femoral stem type SF Cementless Femoral Hip Joint Components ARTHROPLASTY Implant Description Surgical Technique Instrumentation Set Catalogue Preface The cementless stem of a total hip joint
Cementless femoral stem type SF Cementless Femoral Hip Joint Components ARTHROPLASTY Implant Description Surgical Technique Instrumentation Set Catalogue Preface The cementless stem of a total hip joint
UNDERSTANDING TRADITION, MASTERING INNOVATION. Surgical Technique
 UNDERSTANDING TRADITION, MASTERING INNOVATION Surgical Technique Joint Spine Sports Med MasterLoc Surgical Technique Joint Spine Sports Med INTRODUCTION This document describes the Surgical Technique for
UNDERSTANDING TRADITION, MASTERING INNOVATION Surgical Technique Joint Spine Sports Med MasterLoc Surgical Technique Joint Spine Sports Med INTRODUCTION This document describes the Surgical Technique for
Operating Instruction. Müller Hip Stem
 Müller Hip Stem Ortho Select GmbH Eltastrasse 2 D 78573 Wurmlingen Germany Tel: +49-(0)7461-96632-30 Fax: +49-(0)7461-96632-35 info@ortho-select.de www.ortho-select.de 12009 Introduction and product description
Müller Hip Stem Ortho Select GmbH Eltastrasse 2 D 78573 Wurmlingen Germany Tel: +49-(0)7461-96632-30 Fax: +49-(0)7461-96632-35 info@ortho-select.de www.ortho-select.de 12009 Introduction and product description
Bone Preservation Stem
 TRI-LOCK Bone Preservation Stem Featuring GRIPTION Coating Surgical Technique Implant Geometry Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
TRI-LOCK Bone Preservation Stem Featuring GRIPTION Coating Surgical Technique Implant Geometry Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
Progeny Hip Stem. Surgical Protocol and Product Specifications
 Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Revision. Hip Stem. Surgical Protocol
 U2 TM Revision Hip Stem Surgical Protocol U2 Revision Hip Stem Table of Contents Introduction... 1 Preoperative Planning... 2 Femoral Preparation... 3 Trial Reduction... 5 Implant Insertion... 6 Ordering
U2 TM Revision Hip Stem Surgical Protocol U2 Revision Hip Stem Table of Contents Introduction... 1 Preoperative Planning... 2 Femoral Preparation... 3 Trial Reduction... 5 Implant Insertion... 6 Ordering
ACOR cementless anatomical femoral stem with a modular neck
 ACOR cementless anatomical femoral stem with a modular neck ACOR stem: important components of intra-medullary part Stem-neck modular connection via an elliptical Morse taper. Threaded bottom of the oblong
ACOR cementless anatomical femoral stem with a modular neck ACOR stem: important components of intra-medullary part Stem-neck modular connection via an elliptical Morse taper. Threaded bottom of the oblong
operative technique Kent Hip
 operative technique Kent Hip The Kent Hip Operative Technique The Kent Hip was developed by Mr Cliff Stossel, FRCS in Maidstone, Kent, UK and first implanted in 1986. It was designed to deal with problems
operative technique Kent Hip The Kent Hip Operative Technique The Kent Hip was developed by Mr Cliff Stossel, FRCS in Maidstone, Kent, UK and first implanted in 1986. It was designed to deal with problems
asterloc Surgical Technique HIP SYSTEM UNDERSTANDING TRADITION, MASTERING INNOVATION Hip Knee Spine Navigation
 asterloc HIP SYSTEM UNDERSTANDING TRADITION, MASTERING INNOVATION Surgical Technique Hip Knee Spine Navigation Masterloc Surgical Technique Hip Knee Spine Navigation INTRODUCTION This document describes
asterloc HIP SYSTEM UNDERSTANDING TRADITION, MASTERING INNOVATION Surgical Technique Hip Knee Spine Navigation Masterloc Surgical Technique Hip Knee Spine Navigation INTRODUCTION This document describes
Surgical Technique. Unisyn Hip System
 Surgical Technique Unisyn Hip System INDICATIONS AND USAGE Significantly impaired joints resulting from rheumatoid, osteo, and post-traumatic arthritis. Revision of failed femoral head replacement, hip
Surgical Technique Unisyn Hip System INDICATIONS AND USAGE Significantly impaired joints resulting from rheumatoid, osteo, and post-traumatic arthritis. Revision of failed femoral head replacement, hip
Optimizing function Maximizing survivorship Accelerating recovery
 Surgical Technique Optimizing Function Maximizing Survivorship Accelerating Recovery The company believes in an approach to patient treatment that places equal importance on: Optimizing function Maximizing
Surgical Technique Optimizing Function Maximizing Survivorship Accelerating Recovery The company believes in an approach to patient treatment that places equal importance on: Optimizing function Maximizing
Answer. Hip System Design Features:
 Table of Contents Alliance Revision Balance Stanmore Bio-Moore II Taperloc Mallory-Head P.O. Box 587, Warsaw, IN 46581-0587 574.267.6639 2003 Biomet, Inc. All Rights Reserved web site: www.biomet.com email:
Table of Contents Alliance Revision Balance Stanmore Bio-Moore II Taperloc Mallory-Head P.O. Box 587, Warsaw, IN 46581-0587 574.267.6639 2003 Biomet, Inc. All Rights Reserved web site: www.biomet.com email:
HIP SYSTEM SURGICAL TECHNIQUE
 HIP SYSTEM SURGICAL TECHNIQUE Introduction...2 Preoperative Planning...3 Preoperative Planning...3 Templating and Radiographs...4 Determination of Leg Length Discrepancy...5 Determining Acetabular Cup
HIP SYSTEM SURGICAL TECHNIQUE Introduction...2 Preoperative Planning...3 Preoperative Planning...3 Templating and Radiographs...4 Determination of Leg Length Discrepancy...5 Determining Acetabular Cup
9800 Metric Blvd. Austin, Texas
 rev. A Encore Orthopedics, Inc. 1998 www.encoremed.com 9800 Metric Blvd. Austin, Texas 78758 512-832-9500 1 contents How to use the Foundation Hip 1 2 Plan your approach Select your hardware Preparing
rev. A Encore Orthopedics, Inc. 1998 www.encoremed.com 9800 Metric Blvd. Austin, Texas 78758 512-832-9500 1 contents How to use the Foundation Hip 1 2 Plan your approach Select your hardware Preparing
Surgical Technique. Cup System
 Surgical Technique Cup System INDICATIONS AND USAGE Indications for the use of the CS2 ACETABULAR CUP SYSTEM must be carefully considered with respect to the patient s entire evaluation and alternative
Surgical Technique Cup System INDICATIONS AND USAGE Indications for the use of the CS2 ACETABULAR CUP SYSTEM must be carefully considered with respect to the patient s entire evaluation and alternative
Absolut TM Cemented Stem. Surgical Technique
 Absolut TM Cemented Stem Surgical Technique Contents ABSOLUT Cemented Stem 2 Absolut Confidence 2 Absolut Reproducibility 2 Absolut Choice 2 Pre-Operative Planning 3 Suggested Templating Method 3 Surgical
Absolut TM Cemented Stem Surgical Technique Contents ABSOLUT Cemented Stem 2 Absolut Confidence 2 Absolut Reproducibility 2 Absolut Choice 2 Pre-Operative Planning 3 Suggested Templating Method 3 Surgical
Surgical technique MkII instrumentation
 MiniHip Surgical technique MkII instrumentation Contents Operative summary 4 Overview 5 Pre-operative templating 6 Operative technique 7 1. Intra-operative templating 7 2. Neck starter awl 7 3. Curved
MiniHip Surgical technique MkII instrumentation Contents Operative summary 4 Overview 5 Pre-operative templating 6 Operative technique 7 1. Intra-operative templating 7 2. Neck starter awl 7 3. Curved
Design Rationale. ECHELON Primary Hip System
 Design Rationale ECHELON Primary Hip System ECHELON Primary Total Hip System Addressing clinical issues of cementless hip arthroplasty Cementless total hip arthroplasty has provided a proven method of
Design Rationale ECHELON Primary Hip System ECHELON Primary Total Hip System Addressing clinical issues of cementless hip arthroplasty Cementless total hip arthroplasty has provided a proven method of
VerSys LD/Fx Cemented and Press-Fit Hip Prostheses. Surgical Technique IMAGE TO COME. Versatile solutions for total and partial hip replacement
 VerSys LD/Fx Cemented and Press-Fit Hip Prostheses Surgical Technique IMAGE TO COME Versatile solutions for total and partial hip replacement VerSys LD/Fx Cemented and Press-Fit Hip Prostheses VerSys
VerSys LD/Fx Cemented and Press-Fit Hip Prostheses Surgical Technique IMAGE TO COME Versatile solutions for total and partial hip replacement VerSys LD/Fx Cemented and Press-Fit Hip Prostheses VerSys
LOCKING TEP LOCKING TITANIUM ELASTIC PIN INTRAMEDULLARY NAIL
 LOCKING TEP LOCKING TITANIUM ELASTIC PIN INTRAMEDULLARY NAIL ... Index -3 3-8 8 9 9 0 7 Introduction Features Indicatiıons Surgical Technique Femoral Surgical Technique Tibial Surgical Technique Ulna Radius
LOCKING TEP LOCKING TITANIUM ELASTIC PIN INTRAMEDULLARY NAIL ... Index -3 3-8 8 9 9 0 7 Introduction Features Indicatiıons Surgical Technique Femoral Surgical Technique Tibial Surgical Technique Ulna Radius
CC TRIO VERSAFITCUP. Surgical Technique. each to their own. Hip Knee Spine Navigation
 VERSAFITCUP CC TRIO each to their own Surgical Technique Hip Knee Spine Navigation Versafitcup CC TRIO Surgical Technique Hip Knee Spine Navigation EACH TO THEIR OWN The Versafitcup CC Trio is a range
VERSAFITCUP CC TRIO each to their own Surgical Technique Hip Knee Spine Navigation Versafitcup CC TRIO Surgical Technique Hip Knee Spine Navigation EACH TO THEIR OWN The Versafitcup CC Trio is a range
SURGICAL TECHNIQUE. Alpine Cementless Hip Stem
 SURGICAL TECHNIQUE Alpine Cementless Hip Stem The following technique is a general guide for the instrumentation of the Alpine Cementless Hip Stem. It is expected that the surgeon is already familiar with
SURGICAL TECHNIQUE Alpine Cementless Hip Stem The following technique is a general guide for the instrumentation of the Alpine Cementless Hip Stem. It is expected that the surgeon is already familiar with
AUTOBLOQUANTE AUTOBLOQUANTE. Product Rationale Surgical Technique
 AUTOBLOQUANTE AUTOBLOQUANTE Product Rationale Surgical Technique AUTOBLOQUANTE The Product of Long-Term Clinical Experience The AUTOBLOQUANTE femoral component is a direct descendant of the original straight
AUTOBLOQUANTE AUTOBLOQUANTE Product Rationale Surgical Technique AUTOBLOQUANTE The Product of Long-Term Clinical Experience The AUTOBLOQUANTE femoral component is a direct descendant of the original straight
WAGNER CONE PROSTHESIS. Surgical Technique
 WAGNER CONE TM PROSTHESIS Surgical Technique 1 WAGNER CONE PROSTHESIS SURGICAL TECHNIQUE CONTENTS INDICATIONS... 3 CONTRAINDICATIONS... 3 BIOMECHANICAL CONCEPT... 4 THE IMPLANT... 5 INSTRUMENTS... 6 PREOPERATIVE
WAGNER CONE TM PROSTHESIS Surgical Technique 1 WAGNER CONE PROSTHESIS SURGICAL TECHNIQUE CONTENTS INDICATIONS... 3 CONTRAINDICATIONS... 3 BIOMECHANICAL CONCEPT... 4 THE IMPLANT... 5 INSTRUMENTS... 6 PREOPERATIVE
Lubinus Classic Plus Hip Prosthesis System
 Lubinus Classic Plus Hip Prosthesis System Presented by: Waldemar Link GmbH & Co. KG Barkhausenweg 10 22339 Hamburg, Germany P.O. Box 63 05 52 22315 Hamburg, Germany Tel.: +49 40 53995-0 Fax: +49 40 5386929
Lubinus Classic Plus Hip Prosthesis System Presented by: Waldemar Link GmbH & Co. KG Barkhausenweg 10 22339 Hamburg, Germany P.O. Box 63 05 52 22315 Hamburg, Germany Tel.: +49 40 53995-0 Fax: +49 40 5386929
DHS Plate 135º. DHS Platte 135º
 Section of hip fixation plate compression system DHS Plate 3º DHS Platte 3º Plaque de DHS 3º Indication : The compression hip plate is primarly indicated for intertrochanteric fractures. However, it can
Section of hip fixation plate compression system DHS Plate 3º DHS Platte 3º Plaque de DHS 3º Indication : The compression hip plate is primarly indicated for intertrochanteric fractures. However, it can
Surgical Technique r5.indd 1 12/8/10 10:36 AM
 Surgical Technique The science of simplicity With more than 700,000 implantations and two and a half decades of clinical success, the Corail Total Hip System now has the most extensive experience with
Surgical Technique The science of simplicity With more than 700,000 implantations and two and a half decades of clinical success, the Corail Total Hip System now has the most extensive experience with
Zimmer NexGen MIS Tibial Component. Cemented Surgical Technique IMAGE TO COME
 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique IMAGE TO COME Zimmer NexGen MIS Tibial Component Cemented Surgical Technique 1 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique
Zimmer NexGen MIS Tibial Component Cemented Surgical Technique IMAGE TO COME Zimmer NexGen MIS Tibial Component Cemented Surgical Technique 1 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique
Surgical procedure. METS Modular Proximal Femur
 Surgical procedure METS Modular Proximal Femur Surgical Procedure Contents 1.0 2.0 3.0 4.0 5.0 Device information 2 3 1.1 Product overview 1.2 Indications 1.3 Absolute contra-indications 1.4 Relative
Surgical procedure METS Modular Proximal Femur Surgical Procedure Contents 1.0 2.0 3.0 4.0 5.0 Device information 2 3 1.1 Product overview 1.2 Indications 1.3 Absolute contra-indications 1.4 Relative
CONSENSUS ORTHOPEDICS INC.
 CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
PROCOTYL E Acetabular Cup System. Modular to Fit Patient s Anatomy. Versatile for Revisions.
 PROCOTYL E Acetabular Cup System Modular to Fit Patient s Anatomy. Versatile for Revisions. TM PROCOTYL E Shell is a revision modular shell manufactured from titanium alloy. It is ovoid-shaped, with a
PROCOTYL E Acetabular Cup System Modular to Fit Patient s Anatomy. Versatile for Revisions. TM PROCOTYL E Shell is a revision modular shell manufactured from titanium alloy. It is ovoid-shaped, with a
Hype Femoral stems. Surgical technique
 Hype Femoral stems Surgical technique Femoral implant Instrumentation Offset 6 mm Geometry Femoral broaches L 45 The neck length increases with each stem size. 130 (standard and offset stems) 120 (coxa
Hype Femoral stems Surgical technique Femoral implant Instrumentation Offset 6 mm Geometry Femoral broaches L 45 The neck length increases with each stem size. 130 (standard and offset stems) 120 (coxa
Metha Short Hip Stem System
 Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Cemented femoral stem - type CSC
 Cemented femoral stem - type CSC Cemented Femoral Hip Joint Components ARTHROPLASTY Implant Description Preface The cemented femoral stem type CSC with centralizer was designed using the latest knowledge
Cemented femoral stem - type CSC Cemented Femoral Hip Joint Components ARTHROPLASTY Implant Description Preface The cemented femoral stem type CSC with centralizer was designed using the latest knowledge
*smith&nephew SBG Anatomical Hip Stem Prosthesis. Product Information
 Product Information *smith&nephew SBG Anatomical Hip Stem Prosthesis For a Perfect Fit Because Anatomy Matters On account of its anatomical shape the cementless SBG stem, which has been used successfully
Product Information *smith&nephew SBG Anatomical Hip Stem Prosthesis For a Perfect Fit Because Anatomy Matters On account of its anatomical shape the cementless SBG stem, which has been used successfully
U2 PSA. Revision Knee. Surgical Protocol
 U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
Furlong H-A.C. THR System
 Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
Arcos Modular Femoral Revision System
 Arcos Modular Femoral Revision System Arcos System Simplify the Complex The Arcos Modular Femoral Revision System meets the demands of complex hip revision surgery by offering surgeons and OR staff the
Arcos Modular Femoral Revision System Arcos System Simplify the Complex The Arcos Modular Femoral Revision System meets the demands of complex hip revision surgery by offering surgeons and OR staff the
Bone Bangalore
 Dr Suresh Annamalai MBBS, MRCS(Edn), FRCS( Tr & Orth)(Edn), FEBOT(European Board), Young Hip and Knee Fellowship(Harrogate, UK) Consultant Arthroplasty and Arthroscopic Surgeon Manipal Hospital, Whitefield,
Dr Suresh Annamalai MBBS, MRCS(Edn), FRCS( Tr & Orth)(Edn), FEBOT(European Board), Young Hip and Knee Fellowship(Harrogate, UK) Consultant Arthroplasty and Arthroscopic Surgeon Manipal Hospital, Whitefield,
AML Hip System. Design Rationale/ Surgical Technique
 AML Hip System Design Rationale/ Surgical Technique Design Rationale Evolution In 1977, DePuy Synthes Companies introduced the original cementless total hip. The AML Hip launched in order to solve one
AML Hip System Design Rationale/ Surgical Technique Design Rationale Evolution In 1977, DePuy Synthes Companies introduced the original cementless total hip. The AML Hip launched in order to solve one
*smith&nephew SL-PLUS
 Surgical Technique *smith&nephew SL-PLUS Cementless Femoral Hip System SL-PLUS Standard and Lateral Stem Table of Contents Notes from the Author s Clinic... 3 Indications... 4 Contraindications... 5 Preoperative
Surgical Technique *smith&nephew SL-PLUS Cementless Femoral Hip System SL-PLUS Standard and Lateral Stem Table of Contents Notes from the Author s Clinic... 3 Indications... 4 Contraindications... 5 Preoperative
Restoration Modular. Revision Hip System Surgical Protocol
 Orthopaedics Restoration Modular Revision Hip System Surgical Protocol Restoration Modular Cone Body/Conical Distal Stem Femoral Components Using the Restoration Modular Instrument System Restoration Modular
Orthopaedics Restoration Modular Revision Hip System Surgical Protocol Restoration Modular Cone Body/Conical Distal Stem Femoral Components Using the Restoration Modular Instrument System Restoration Modular
Preoperative Planning. The primary objectives of preoperative planning are to:
 Preoperative Planning The primary objectives of preoperative planning are to: - Determine preoperative leg length discrepancy. - Assess acetabular component size and placement. - Determine femoral component
Preoperative Planning The primary objectives of preoperative planning are to: - Determine preoperative leg length discrepancy. - Assess acetabular component size and placement. - Determine femoral component
Featuring. Technology. Product Rationale
 Featuring Technology Product Rationale 2 Optimum implant geometry Extending proven TRI-LOCK heritage The original TRI-LOCK Stem was introduced in 1981. This implant was the first proximally coated tapered-wedge
Featuring Technology Product Rationale 2 Optimum implant geometry Extending proven TRI-LOCK heritage The original TRI-LOCK Stem was introduced in 1981. This implant was the first proximally coated tapered-wedge
pact SYSTEM Surgical Technique HEMISPHERICAL CEMENTLESS CUP SYSTEM MULTI-HOLE & RIM-HOLE Hip Knee Spine Navigation
 pact SYSTEM HEMISPHERICAL CEMENTLESS CUP SYSTEM MULTI-HOLE & RIM-HOLE Surgical Technique Hip Knee Spine Navigation Mpact Surgical Technique Hip Knee Spine Navigation PREFACE The Mpact Multi-hole and the
pact SYSTEM HEMISPHERICAL CEMENTLESS CUP SYSTEM MULTI-HOLE & RIM-HOLE Surgical Technique Hip Knee Spine Navigation Mpact Surgical Technique Hip Knee Spine Navigation PREFACE The Mpact Multi-hole and the
Fixed and Variable Geometry Total Shoulder Arthroplasty
 Fixed and Variable Geometry Total Shoulder Arthroplasty RECOVERY FUNCTION SURVIVORSHIP DePuy believes in an approach to total shoulder replacement that places equal importance on recovery, function and
Fixed and Variable Geometry Total Shoulder Arthroplasty RECOVERY FUNCTION SURVIVORSHIP DePuy believes in an approach to total shoulder replacement that places equal importance on recovery, function and
Surgical Technique. *smith&nephew POLARSTEM Cementless Stem System
 Surgical Technique *smith&nephew POLARSTEM Cementless Stem System POLARSTEM Cementless Stem System Contents Introduction... 3 Indications... 4 Contraindications... 4 Case Studies... 5 Preoperative Planning...
Surgical Technique *smith&nephew POLARSTEM Cementless Stem System POLARSTEM Cementless Stem System Contents Introduction... 3 Indications... 4 Contraindications... 4 Case Studies... 5 Preoperative Planning...
Anterior Approach Surgical Technique. Paragon Stem System. enabling people to enjoy life
 Anterior Approach Surgical Technique Paragon Stem System enabling people to enjoy life Contents Pre-Operative Planning... 2 Suggested Templating Method... 2 Surgical Technique... 3 Surgical Approach...
Anterior Approach Surgical Technique Paragon Stem System enabling people to enjoy life Contents Pre-Operative Planning... 2 Suggested Templating Method... 2 Surgical Technique... 3 Surgical Approach...
Integral 180 Surgical Technique
 Integral 180 Surgical Technique The Integral 180 and 225 are part of the Alliance Family Total Hip System. The Integral 225 femoral component is marketed for use with bone cement in the United States.
Integral 180 Surgical Technique The Integral 180 and 225 are part of the Alliance Family Total Hip System. The Integral 225 femoral component is marketed for use with bone cement in the United States.
EXTENDED TROCHANTERIC OSTEOTOMY SURGICAL TECHNIQUE FPO EXTENSIVELY COATED FIXATION
 EXTENDED TROCHANTERIC OSTEOTOMY SURGICAL TECHNIQUE FPO EXTENSIVELY COATED FIXATION SINCE 1983 PREOPERATIVE PLANNING EXPLANTATION OPTIONS the cement from inside the cement canal until the bone/ cement bond
EXTENDED TROCHANTERIC OSTEOTOMY SURGICAL TECHNIQUE FPO EXTENSIVELY COATED FIXATION SINCE 1983 PREOPERATIVE PLANNING EXPLANTATION OPTIONS the cement from inside the cement canal until the bone/ cement bond
Alloclassic Zweymüller Stem
 Alloclassic Zweymüller Stem Surgical Technique A proven concept Disclaimer This document is intended exclusively for physicians and is not intended for laypersons. Information on the products and procedures
Alloclassic Zweymüller Stem Surgical Technique A proven concept Disclaimer This document is intended exclusively for physicians and is not intended for laypersons. Information on the products and procedures
Revision Modular Stem type RMD
 Revision Modular Stem type RMD Cementless Femoral Hip Joint Components Revision Systems Implant Description Surgical Technique Instrumentation Set Catalogue Preface Revision modular stem (RMD) is a solution
Revision Modular Stem type RMD Cementless Femoral Hip Joint Components Revision Systems Implant Description Surgical Technique Instrumentation Set Catalogue Preface Revision modular stem (RMD) is a solution
Integra. Titan Modular Shoulder System, 2.5
 Titan Modular Shoulder System, 2.5 Limit uncertainty with a shoulder implant system that redefines modularity, addresses multiple indications, and allows for reproducible results. Titan Modular Shoulder
Titan Modular Shoulder System, 2.5 Limit uncertainty with a shoulder implant system that redefines modularity, addresses multiple indications, and allows for reproducible results. Titan Modular Shoulder
Clinical Evaluation Surgical Technique
 Clinical Evaluation Surgical Technique Table of Contents EMPERION Specifications 3 EMPERION Surgical Technique 9 EMPERION Catalog 18 Nota Bene: This technique description herein is made available to the
Clinical Evaluation Surgical Technique Table of Contents EMPERION Specifications 3 EMPERION Surgical Technique 9 EMPERION Catalog 18 Nota Bene: This technique description herein is made available to the
Screw Set. Ref. Number : F-1
 Screw Set : 8008000 F- Screw Set : 8008000 Ref.Number Description Pieces Ref.Number Description Pieces 0800003 080000 0800005 0800006 080000 0800008 0800009 080000 08000 08000 080003 08000 080005 080006
Screw Set : 8008000 F- Screw Set : 8008000 Ref.Number Description Pieces Ref.Number Description Pieces 0800003 080000 0800005 0800006 080000 0800008 0800009 080000 08000 08000 080003 08000 080005 080006
Lubinus Classic Plus Hip Prosthesis System
 Lubinus Classic Plus Hip Prosthesis System Presented by: WALDEMAR LINK GmbH & Co. KG Barkhausenweg 10 22339 Hamburg, Germany P. O. Box 63 05 52 22315 Hamburg, Germany Tel.: +49 40 53995-0 Fax: +49 40 5386929
Lubinus Classic Plus Hip Prosthesis System Presented by: WALDEMAR LINK GmbH & Co. KG Barkhausenweg 10 22339 Hamburg, Germany P. O. Box 63 05 52 22315 Hamburg, Germany Tel.: +49 40 53995-0 Fax: +49 40 5386929
Efficacy Innovation ABG II. Brochure. Surgical Protocol. Cemented Stem
 Efficacy Innovation ABG II Cemented Stem Brochure Surgical Protocol 2 The ABG Hip System The ABG hip implant range has been progressively extended and enhanced over the past years, harnessing extensive
Efficacy Innovation ABG II Cemented Stem Brochure Surgical Protocol 2 The ABG Hip System The ABG hip implant range has been progressively extended and enhanced over the past years, harnessing extensive
Bone Conserving Hip Replacement Surgical technique
 MiniHip Bone Conserving Hip Replacement Surgical technique MiniHip Contents Operative summary 4 Overview 5 Pre-operative templating 6 Operative technique 7 Neck resection 7 Femoral canal preparation 7
MiniHip Bone Conserving Hip Replacement Surgical technique MiniHip Contents Operative summary 4 Overview 5 Pre-operative templating 6 Operative technique 7 Neck resection 7 Femoral canal preparation 7
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM
 CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
comprehensive Design Rationale shoulder system Knees Hips Extremities Cement and Accessories PMI TEchnology
 comprehensive shoulder system Design Rationale Knees Hips Extremities Cement and Accessories PMI TEchnology . INTRODUCTION The Comprehensive Shoulder System is an evolutionary design based on the successful
comprehensive shoulder system Design Rationale Knees Hips Extremities Cement and Accessories PMI TEchnology . INTRODUCTION The Comprehensive Shoulder System is an evolutionary design based on the successful
FIRST STEM SPECIFICALLY DESIGNED FOR AMIS. Surgical Technique
 FIRST STEM SPECIFICALLY DESIGNED FOR AMIS Surgical Technique Joint Spine Sports Med AMIStem Surgical Technique Joint Spine Sports Med INTRODUCTION This document describes the Surgical Technique for the
FIRST STEM SPECIFICALLY DESIGNED FOR AMIS Surgical Technique Joint Spine Sports Med AMIStem Surgical Technique Joint Spine Sports Med INTRODUCTION This document describes the Surgical Technique for the
ACETABULAR CUP SURGICAL TECHNIQUE
 ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
AVANTEON. Operative Technique & Catalogue Information AVANTEON
 AVANTEON Operative Technique & Catalogue Information AVANTEON H I P S Y S T E M Pre-operative Planning The overall aim of pre-operative planning is to establish anatomical data from the patient to guide
AVANTEON Operative Technique & Catalogue Information AVANTEON H I P S Y S T E M Pre-operative Planning The overall aim of pre-operative planning is to establish anatomical data from the patient to guide
Zimmer Segmental System
 Zimmer Segmental System Simple solutions for solving complex salvage cases A Step Forward The Zimmer Segmental System is designed to address patients with severe bone loss associated with disease, trauma
Zimmer Segmental System Simple solutions for solving complex salvage cases A Step Forward The Zimmer Segmental System is designed to address patients with severe bone loss associated with disease, trauma
MetaFix. Cementless Total Hip Replacement Surgical technique
 MetaFix Cementless Total Hip Replacement Surgical technique Contents Operative summary 4 Acetabular preparation 5 Pre-operative templating 5 Operative technique 6 Femoral neck osteotomy 6 Femoral canal
MetaFix Cementless Total Hip Replacement Surgical technique Contents Operative summary 4 Acetabular preparation 5 Pre-operative templating 5 Operative technique 6 Femoral neck osteotomy 6 Femoral canal
MIS Cemented Tibial Component
 MIS Cemented Tibial Component NexGen Complete Knee Solution Surgical Technique Table of Contents Surgical Exposure... 2 Finish the Tibia... 2 Position Based on Anatomic Landmarks... 3 Lateral Posterior
MIS Cemented Tibial Component NexGen Complete Knee Solution Surgical Technique Table of Contents Surgical Exposure... 2 Finish the Tibia... 2 Position Based on Anatomic Landmarks... 3 Lateral Posterior
A further enhanced classic. Wagner SL Revision Hip Stem
 A further enhanced classic Wagner SL Revision Hip Stem The original Wagner SL Revision Stem offers a time-proven solution in the treatment of revision hips. While its underlying anchorage philosophy and
A further enhanced classic Wagner SL Revision Hip Stem The original Wagner SL Revision Stem offers a time-proven solution in the treatment of revision hips. While its underlying anchorage philosophy and
Approach Patients with Confidence
 Surgical Technique Approach Patients with Confidence The ACTIS Total Hip System is the first DePuy Synthes stem specifically designed to be utilized with tissue sparing approaches, such as the anterior
Surgical Technique Approach Patients with Confidence The ACTIS Total Hip System is the first DePuy Synthes stem specifically designed to be utilized with tissue sparing approaches, such as the anterior
SURGICAL TECHNIQUE. Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM
 SURGICAL TECHNIQUE Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM Important: This essential product information does not include all of the information necessary for selection and use of a device.
SURGICAL TECHNIQUE Protrusio Cage A COMPREHENSIVE ACETABULAR REVISION SYSTEM Important: This essential product information does not include all of the information necessary for selection and use of a device.
Surgical Technique. *smith&nephew POLARSTEM Cementless and Cemented Stem System
 Surgical Technique *smith&nephew POLARSTEM Cementless and Cemented Stem System 2 POLARSTEM Contents Introduction... 4 Indications... 5 Contraindications... 5 Case Studies... 6 Preoperative Planning...
Surgical Technique *smith&nephew POLARSTEM Cementless and Cemented Stem System 2 POLARSTEM Contents Introduction... 4 Indications... 5 Contraindications... 5 Case Studies... 6 Preoperative Planning...
EVOLVING OUR HERITAGE, MEETING YOUR NEEDS. Surgical Technique
 EVOLVING OUR HERITAGE, MEETING YOUR NEEDS Surgical Technique Joint Spine Sports Med Mpact DM Surgical Technique Joint Spine Sports Med INTRODUCTION The Mpact DM is part of the Mpact Acetabular System and
EVOLVING OUR HERITAGE, MEETING YOUR NEEDS Surgical Technique Joint Spine Sports Med Mpact DM Surgical Technique Joint Spine Sports Med INTRODUCTION The Mpact DM is part of the Mpact Acetabular System and
FIRST STEM SPECIFICALLY DESIGNED FOR AMIS. Surgical Technique
 FIRST STEM SPECIFICALLY DESIGNED FOR AMIS Surgical Technique Joint Spine Sports Med AMIStem Surgical Technique Joint Spine Sports Med INTRODUCTION This document describes the Surgical Technique for the
FIRST STEM SPECIFICALLY DESIGNED FOR AMIS Surgical Technique Joint Spine Sports Med AMIStem Surgical Technique Joint Spine Sports Med INTRODUCTION This document describes the Surgical Technique for the
Bi-Polar 22.2mm & 28mm System - Operative technique
 Disclaimer Biomet UK Ltd, as the manufacturer of this device, does not practice medicine and does not recommend any particular surgical technique for use on a specific patient. The surgeon who performs
Disclaimer Biomet UK Ltd, as the manufacturer of this device, does not practice medicine and does not recommend any particular surgical technique for use on a specific patient. The surgeon who performs
DESIGN RATIONALE AND SURGICAL TECHNIQUE
 DESIGN RATIONALE AND SURGICAL TECHNIQUE ANCHOR PEG GLENOID DESIGN RATIONALE In total shoulder arthroplasty, most cases of clinical and radiographic loosening involve failure of the fixation of the glenoid
DESIGN RATIONALE AND SURGICAL TECHNIQUE ANCHOR PEG GLENOID DESIGN RATIONALE In total shoulder arthroplasty, most cases of clinical and radiographic loosening involve failure of the fixation of the glenoid
The proven, simple solution. CPT 12/14 Hip System
 The proven, simple solution CPT 12/14 Hip System Primary CPT Hip The proven, simple solution 5mm The collarless, polished, doubletaper design concept used in the CPT 12/14 Hip System has proven itself
The proven, simple solution CPT 12/14 Hip System Primary CPT Hip The proven, simple solution 5mm The collarless, polished, doubletaper design concept used in the CPT 12/14 Hip System has proven itself
