Single-Dose Oritavancin in the Treatment of Acute Bacterial Skin Infections
|
|
|
- Harvey Quinn
- 6 years ago
- Views:
Transcription
1 The new england journal of medicine original article Single-Dose Oritavancin in the Treatment of Acute Bacterial Skin Infections G. Ralph Corey, M.D., Heidi Kabler, M.D., Purvi Mehra, M.D., Sandeep Gupta, M.D., J. Scott Overcash, M.D., Ashwin Porwal, M.D., Philip Giordano, M.D., Christopher Lucasti, M.D., Antonio Perez, M.D., Samantha Good, Ph.D., Hai Jiang, Ph.D., Greg Moeck, Ph.D., and William O Riordan, M.D., for the SOLO I Investigators* ABSTRACT From Duke University Medical Center, Durham, NC (G.R.C.); Sunrise Hospital and Medical Center, Las Vegas (H.K.); Sharp Chula Vista Medical Center, Chula Vista (P.M., W.O.), and Sharp Grossmont Hospital, San Diego (J.S.O.) both in California; MV Hospital and Research Center, Lucknow (S. Gupta), and Inamdar Multispecialty Hospital, Pune (A. Porwal) both in India; Orlando Health, Orlando, FL (P.G.); and South Jersey Infectious Disease, Somers Point (C.L.), and the Medicines Company, Parsippany (A. Perez, S. Good, H.J., G.M.) both in New Jersey. Address reprint requests to Dr. Corey at Duke University Medical Center, 310 Trent Dr., Box 90519, Durham, NC 27708, or at g.corey@duke.edu. *A complete list of the investigators in the SOLO I trial is provided in the Supplementary Appendix, available at NEJM.org. N Engl J Med 2014;370: DOI: /NEJMoa Copyright 2014 Massachusetts Medical Society. Background Oritavancin is a lipoglycopeptide with bactericidal activity against gram-positive bacteria. Its concentration-dependent activity and prolonged half-life allow for singledose treatment. Methods We conducted a randomized, double-blind trial in which adults with acute bacterial skin and skin-structure infections received either a single intravenous dose of 1200 mg of oritavancin or a regimen of intravenous vancomycin twice daily for 7 to 10 days. Three efficacy end points were tested for noninferiority. The primary composite end point was defined as cessation of spreading or reduction in lesion size, absence of fever, and no need for administration of a rescue antibiotic 48 to 72 hours after administration of oritavancin. Secondary end points were clinical cure 7 to 14 days after the end of treatment, as determined by a study investigator, and a reduction in lesion size of 20% or more 48 to 72 hours after administration of oritavancin. Results The modified intention-to-treat population comprised 475 patients who received oritavancin and 479 patients who received vancomycin. All three efficacy end points met the prespecified noninferiority margin of 10 percentage points for oritavancin versus vancomycin: primary end point, 82.3% versus 78.9% (95% confidence interval [CI] for the difference, 1.6 to 8.4 percentage points); investigator-assessed clinical cure, 79.6% versus 80.0% (95% CI for the difference, 5.5 to 4.7 percentage points); and proportion of patients with a reduction in lesion area of 20% or more, 86.9% versus 82.9% (95% CI for the difference, 0.5 to 8.6 percentage points). Efficacy outcomes measured according to type of pathogen, including methicillinresistant Staphylococcus aureus, were similar in the two treatment groups. The overall frequency of adverse events was also similar, although nausea was more common among those treated with oritavancin. Conclusions A single dose of oritavancin was noninferior to twice-daily vancomycin administered for 7 to 10 days for the treatment of acute bacterial skin and skin-structure infections caused by gram-positive pathogens. (Funded by the Medicines Company; SOLO I ClinicalTrials.gov number, NCT ) 2180
2 The economic burden of acute bacterial skin and skin-structure infections remains substantial 1 and is driven by the high costs of hospitalization 2,3 and by treatment with agents that require dosing once or twice daily for a duration of 7 to 10 days or more. 2,4-12 Treatment of these infections often requires agents that are active against methicillin-resistant Staphylococcus aureus (MRSA), which continues to be an important causative pathogen in many countries. 13,14 Even treatment in an outpatient setting cannot overcome the disadvantage of multiple administrations, incomplete adherence to medication regimens, 15 and the complexity of monitoring therapeutic drug levels. 16 Oritavancin is a lipoglycopeptide antibiotic with three mechanisms of action that result in concentration-dependent bactericidal activity 20 against clinically relevant gram-positive pathogens Oritavancin has a prolonged terminal half-life 24 and is excreted unchanged in both urine and feces. No dose adjustment is required on the basis of age or renal function or for patients with moderate hepatic impairment. 24,25 In two previous phase 3 trials of oritavancin for the treatment of acute bacterial skin and skin-structure infections, in which oritavancin was administered once daily for 3 to 7 days, the data accrued failed to provide substantial evidence of efficacy overall and in the subgroup of patients infected with MRSA. Since the pharmacokinetic pharmacodynamic profile of oritavancin allows for single-dose treatment, 26,27 the phase 3 study presented here (SOLO I) was designed to evaluate the efficacy and safety of a single dose of oritavancin as compared with a regimen of twicedaily vancomycin for 7 to 10 days in adults with acute bacterial skin and skin-structure infections. Methods Study Design SOLO I was an international, randomized, doubleblind study designed to compare the efficacy and safety of a single intravenous dose of oritavancin with intravenous dosing of vancomycin for 7 to 10 days in adults with acute bacterial skin and skin-structure infections (wound infection, cellulitis, or major cutaneous abscess). The study design was consistent with current guidelines for eligibility criteria, end points, assessment methods, and noninferiority margins. The protocol was approved by the institutional review board or ethics committee at each participating site, and all patients provided written informed consent. (The protocol is available with the full text of this article at NEJM.org.) The study was conducted from January 2011 through November Participants underwent randomization in a 1:1 ratio to receive either a single intravenous dose of 1200 mg of oritavancin followed by intravenously administered placebo or an intravenous dose of vancomycin (1 g, or 15 mg per kilogram of body weight) every 12 hours for 7 to 10 days. Randomization was stratified according to geographic region, study site, and presence or absence of diabetes mellitus. Enrollment of patients with major cutaneous abscesses was capped at 30%. Clinical evaluations were performed at the following time points: 48 to 72 hours after the initiation of the study treatment (early clinical evaluation), day 7 to day 10 (end of therapy) or, in the case of early discontinuation, the day the patient stopped receiving the study drug or was switched to a nonstudy drug for primary acute bacterial skin and skin-structure infection; 10 days after the initiation of the study drug; and 7 to 14 days after the end-of-therapy visit (post-therapy evaluation). A follow-up period of 60 days was specified for analysis of safety in order to evaluate the potential effect of the prolonged half-life of oritavancin. Safety data were reviewed by an external independent data and safety monitoring committee, once after 120 patients had been treated and again after 250 patients had been treated. (Definitions of the analysis populations are provided in Fig. 1.) The Medicines Company designed and conducted the study and prepared the statistical analysis plan. Analyses were performed and data interpreted by the Medicines Company in conjunction with the authors. An author who is an employee of the sponsor prepared the first draft of the manuscript. All the authors reviewed and edited the manuscript and made the decision to submit the manuscript for publication. All the authors vouch for the completeness and accuracy of the data and analyses and for the fidelity of study conduct to the protocol. Eligibility Criteria Eligible patients were at least 18 years of age and had received a diagnosis of acute bacterial skin and skin-structure infection that was thought or proven to be caused by a gram-positive pathogen 2181
3 The new england journal of medicine 968 Patients underwent randomization 968 Were included in intention-to-treat population 483 Were assigned to receive oritavancin 485 Were assigned to receive vancomycin 14 Did not receive study drug 8 Were in oritavancin group 6 Were in vancomycin group 2 In the oritavancin group received vancomycin 954 Were included in safety population 473 Received oritavancin 481 Received vancomycin 954 Were included in modified intentionto-treat population Microbiologic evaluation Clinical evaluation 475 Were assigned to and received oritavancin 479 Were assigned to and received vancomycin 475 Were assigned to and received oritavancin 479 Were assigned to and received vancomycin 231 Did not have grampositive pathogen at baseline 237 Did not have grampositive pathogen at baseline 16 Had missing data for primary efficacy end point 23 Had missing data for primary efficacy end point 43 Did not meet criteria for clinical evaluation 244 Were included in the microbiologic intention-to-treat population 41 Did not meet criteria for clinical evaluation 242 Were included in the microbiologic intention-to-treat population 81 Did not meet criteria for clinical evaluation 7 Did not meet inclusion or met exclusion criteria 37 Had treatment duration <7 days 54 Did not undergo investigator assessment at PTE 2 Did not receive treatment as assigned 82 Did not meet criteria for clinical evaluation 3 Did not meet inclusion or met exclusion criteria 58 Had treatment duration <7 days 61 Did not undergo investigator assessment at PTE 201 Were included in the microbiologic evaluation 201 Were included in the microbiologic evaluation 394 Were included in the clinical evaluation 397 Were included in the clinical evaluation 2182
4 Figure 1 (facing page). Study-Group Assignments and Analysis Populations. The intention-to-treat population included all patients who underwent randomization. The modified intention-to-treat population was the primary population for all the efficacy analyses and included all patients who underwent randomization and received either oritavancin or vancomycin. The safety population was the primary population for all safety analyses and consisted of all patients who received the assigned study drug. Treatment classification was based on the actual treatment received. The population that underwent clinical evaluation consisted of all patients in the modified intention-to-treat population who met the criteria for study inclusion, received the full-course of study treatment (for a minimum of 7 days), and underwent an assessment for clinical cure at the post-therapy evaluation (PTE) by the site investigator. Analysis of data for this population was used to confirm the efficacy analyses. The population that underwent microbiologic evaluation consisted of all patients in the modified intention-to-treat population in whom a gram-positive pathogen known to cause acute bacterial skin and skinstructure infections was detected at baseline and who could be evaluated clinically. This population was used for secondary efficacy analyses. Two patients who were randomly assigned to receive oritavancin inadvertently received vancomycin. Patients who did not meet the criteria for clinical evaluation may be included in more than one category. and that required at least 7 days of intravenous therapy. The diagnosis of acute bacterial skin and skin-structure infection required the presence of wound infection (either traumatic or surgical in origin), cellulitis, erysipelas, or a major cutaneous abscess, with each lesion surrounded by erythema, edema, or an induration of at least 75 cm 2. Signs and symptoms of systemic inflammation also had to be present. (Further details regarding the eligibility criteria are available in the Methods section in the Supplementary Appendix, available at NEJM.org.) Efficacy Assessments The primary efficacy end point and the end point approved by the Food and Drug Administration was a composite outcome at the time of the early clinical evaluation that comprised the cessation of spreading or a reduction in the size of the baseline lesion, the absence of fever, and the absence of a need for rescue antibiotic medication. The key secondary end point was clinical cure as assessed by a study investigator at the post-therapy evaluation, as required by the European Medicines Agency. Another important secondary efficacy end point was a decrease in lesion area of 20% or more from baseline to the early clinical evaluation. Outcomes were analyzed for the modified intention-to-treat population, all patients who could be evaluated clinically, patients in the intention-to-treat population who could be evaluated microbiologically, and all patients who could be evaluated both clinically and microbiologically (Fig. 1). Further explanation of the efficacy end points, including the definition of treatment failure, can be found in Section 2.3 in the Supplementary Appendix. Safety Assessments Safety was assessed by monitoring vital signs, performing electrocardiography (ECG), measuring serum chemical and hematologic values, and recording adverse events and serious adverse events in the safety population. Adverse events that developed during treatment were defined as events with an onset or worsening severity at the time of or after the administration of the first dose of the study drug through the safety followup visit on day 60. Statistical Analysis We calculated that a sample of 960 patients (480 per treatment group) would provide at least 90% power to test the noninferiority of oritavancin as compared with vancomycin with respect to the primary efficacy end point; a noninferiority margin of 10 percentage points was used at a onesided alpha level of 0.025, with an assumed rate of 75% for the primary efficacy outcome in both treatment groups. This sample size would also provide at least 90% power to test noninferiority with respect to clinical cure as assessed by investigators at the post-therapy evaluation, with the use of a noninferiority margin of 10 percentage points at a one-sided alpha level of and an assumed rate of 65% for clinical cure in both the oritavancin and vancomycin groups. For the primary efficacy assessment performed at the early clinical evaluation, the investigator s assessment of clinical cure at the post-therapy evaluation, and the determination of whether there was a decrease in lesion area by 20% or more from baseline to the early clinical evaluation, a two-sided 95% confidence interval for the difference in rates between the two treatment groups was derived with the use of a two-group, large-sample normal approximation test of pro- 2183
5 The new england journal of medicine portions. If the lower bound of the two-sided 95% confidence interval for the between-group difference (oritavancin vs. vancomycin) was above 10 percentage points, noninferiority of oritavancin was claimed at a one-sided alpha level of A hierarchical ordering of statistical testing was used, with the primary efficacy end point at the early clinical evaluation tested first, followed by testing of investigator-assessed clinical cure at the post-therapy evaluation; a decrease in the lesion area of 20% or more from baseline to the early clinical evaluation was tested last. The noninferiority margin of 10 percentage points for the primary efficacy end point and for the end point of a decrease in lesion area of 20% or more from baseline was based on studies showing that a treatment effect of 18% for sulfonamides as compared with ultraviolet light can be estimated for the main component of the primary efficacy end point cessation of lesion spread. 28,31,32 This noninferiority margin is justified on the basis of the belief that the control effect of vancomycin is at least as large as that for sulfonamides as compared with ultraviolet light and on the assumption that the control effect on the end point of a decrease in lesion area of 20% or more from baseline in the present study would be similar to the effect on the cessation of lesion spread in the earlier studies. Justification of the noninferiority margin of 10 percentage points for the clinical cure rate was based on analyses conducted by Spellberg et al. 33 The analysis of the primary and secondary efficacy end points was performed in the modified intention-to-treat population. Patients with missing assessments were considered to have had treatment failure with respect to the primary and secondary efficacy end points. For additional end points, 95% confidence intervals were provided for descriptive purposes only. For safety Table 1. Demographic and Baseline Clinical Characteristics of the Study Participants (Modified Intention-to-Treat Population).* Characteristic Age yr Oritavancin (N = 475) Vancomycin (N = 479) Mean 46.2± ±14.50 Median Range Age 65 yr no. (%) 47 (9.9) 38 (7.9) Male sex no. (%) 301 (63.4) 301 (62.8) Race no. (%) White 274 (57.7) 275 (57.4) Black 43 (9.1) 40 (8.4) Asian 153 (32.2) 154 (32.2) Other 5 (1.1) 10 (2.1) Body weight kg BMI Mean 81.9± ±26.52 Median Range Mean 28.7± ±8.67 Median Range Value no. (%) < (34.1) 175 (36.5) 25 to < (33.3) 138 (28.8) (32.6) 166 (34.7) 2184
6 Table 1. (Continued.) Characteristic Infection type Oritavancin (N = 475) Vancomycin (N = 479) Wound no. (%) 92 (19.4) 105 (21.9) Wound with MRSA infection confirmed no./total no. (%) 23/104 (22.1) 20/100 (20.0) Cellulitis no. (%) 243 (51.2) 233 (48.6) Cellulitis with MRSA infection confirmed no./total no. (%) 20/104 (19.2) 23/100 (23.0) Abscess no. (%) 140 (29.5) 141 (29.4) Abscess with MRSA infection confirmed no./total no. (%) 61/104 (58.7) 57/100 (57.0) Diabetes mellitus no. (%) 93 (19.6) 95 (19.8) Temperature 38.0 C no./total no. (%) 68/474 (14.3) 79/478 (16.5) White-cell count >12,000 per mm 3 no./total no. (%) 104/434 (24.0) 85/430 (19.8) Lesion area cm 2 Median Range Lesion area 75 cm 2 no./total no. (%) 473/475 (99.6) 478/478 (100.0) Receipt of permitted medications no. (%) Aztreonam 52 (10.9) 47 (9.8) Metronidazole 15 (3.2) 17 (3.5) Positive infection-site culture no./total no. (%) 290/475 (61.1) 290/479 (60.5) Any gram-positive pathogen 279/290 (96.2) 277/290 (95.5) S. aureus 218/279 (78.1) 210/277 (75.8) Positive blood culture no. (%) 18 (3.8) 9 (1.9) S. aureus 9 0 Positive infection-site and blood cultures for MRSA * Plus minus values are means ±SD. There were no significant between-group differences except for age (P = 0.04, calculated with the use of the Kruskal Wallis test) and a positive blood culture for Staphylococcus aureus (P = 0.002, calculated with the use of Fisher s exact test). Percentages may not add up to 100 because of rounding. MRSA denotes methicillin-resistant S. aureus. Race was self-reported. BMI denotes body-mass index (the weight in kilograms divided by the square of the height in meters). assessments, descriptive analyses were performed in the safety population for all safety variables according to treatment group. Methods of microbiologic assessment are outlined in the Methods section of the Supplementary Appendix. Results Study Population Figure 1 shows the numbers of patients who were screened, randomly assigned to a treatment group, and included in the primary analysis. In the modified intention-to-treat population, the oritavancin and vancomycin groups had similar demographic and clinical characteristics (Table 1). The mean age of the patients was 45 years, and 8.9% were at least 65 years of age. The patients were predominantly white and male. Infection types were balanced in the oritavancin and vancomycin groups, with approximately 50% of patients having cellulitis, 30% having abscess, and 20% having wound infection. The median size of the infection area at baseline was cm 2 in the oritavancin group and cm 2 in the vancomycin group. A pathogen was isolated from approximately 60% of patients in both treatment 2185
7 The new england journal of medicine Subgroup Modified intention-to-treat population Primary efficacy outcome at ECE Investigator-assessed clinical cure at PTE Lesion size reduction 20% at ECE CE population Primary efficacy outcome at ECE Investigator-assessed clinical cure at PTE Lesion size reduction 20% at ECE Patients infected with MRSA in intention-to-treat population with microbiologic evaluation Primary efficacy outcome at ECE Investigator-assessed clinical cure at PTE Lesion size reduction 20% at ECE Patients infected with MSSA in intention-to-treat population with microbiologic evaluation Primary efficacy outcome at ECE Investigator-assessed clinical cure at PTE Lesion size reduction 20% at ECE Oritavancin Vancomycin no. of events/total no. (%) 391/475 (82.3) 378/475 (79.6) 413/475 (86.9) 344/394 (87.3) 357/394 (90.6) 362/394 (91.9) 84/104 (80.8) 86/104 (82.7) 94/104 (90.4) 96/116 (82.8) 89/116 (76.7) 98/116 (84.5) 378/479 (78.9) 383/479 (80.0) 397/479 (82.9) 342/397 (86.1) 352/397 (88.7) 370/397 (93.2) 80/100 (80.0) 83/100 (83.0) 84/100 (84.0) 92/110 (83.6) 88/110 (80.0) 94/110 (85.5) Percentage-Point Difference (95% CI) 3.4 ( 1.6 to 8.4) 0.4 ( 5.5 to 4.7) 4.1 ( 0.5 to 8.6) 1.2 ( 3.6 to 5.9) 1.9 ( 2.3 to 6.2) 1.3 ( 5.0 to 2.3) 0.8 ( 10.1 to 11.7) 0.3 ( 10.7 to 10.0) 6.4 ( 2.8 to 15.5) 0.9 ( 10.6 to 8.9) 3.3 ( 14.0 to 7.4) 1.0 ( 10.3 to 8.3) Vancomycin Better Oritavancin Better Figure 2. Primary and Secondary Efficacy End Points According to Analysis Population and MRSA Subgroup. CE denotes clinical evaluation, ECE early clinical evaluation, MRSA methicillin-resistant Staphylococcus aureus, MSSA methicillin-susceptible S. aureus, and PTE post-therapy evaluation. groups at baseline; 96% of these patients had a gram-positive pathogen known to cause acute bacterial skin and skin-structure infections. S. aureus was the most common pathogen, and MRSA was recovered in 204 patients. Clinical Outcomes The efficacy of a single 1200-mg intravenous dose of oritavancin was similar to that of vancomycin administered twice daily for 7 to 10 days, at both the early clinical evaluation and the post-therapy evaluation. The primary composite end point at the early clinical evaluation (82.3% with oritavancin and 78.9% with vancomycin), the end point of clinical cure at post-therapy evaluation as assessed by a study investigator (79.6% and 80.0%, respectively), and the end point of a reduction in lesion size of 20% or more at early clinical evaluation (86.9% and 82.9%, respectively) met the prespecified noninferiority margin, since the lower limit of the 95% confidence interval for the between-group difference (oritavancin vs. vancomycin) was above 10 percentage points (Fig. 2). The efficacy rates for oritavancin and vancomycin with respect to the primary end point (early clinical evaluation) were similar when analyzed according to body-mass index (BMI, the weight in kilograms divided by the square of the height in meters), presence or absence of diabetes, age, presence or absence of MRSA infection, sex, race, or lesion type (Fig. S1 in the Supplementary Appendix). On the basis of the results of pharmacokinetic analyses, demographic characteristics, including age, race, and sex, had no significant effect on the pharmacokinetic activity of oritavancin. Approximately 34% of patients had a BMI of more than 30, and there were no significant differences between these patients and those with a BMI of 30 or lower with regard to the primary efficacy end point in the oritavancin and vancomycin treatment groups at the early clinical evaluation or with regard to a reduction in lesion size of more than 20% or to investigator-assessed clinical cure at the posttherapy evaluation. The number of patients in whom there was treatment failure and the reasons for failure at both the early clinical evaluation and the posttherapy evaluation were balanced between the two treatment groups (Tables S2 and S3 in the Supplementary Appendix). Overall, 11.6% of patients in the oritavancin group (55 of 475) and 12.7% of patients in the vancomycin group (61 of 479) were classified as having treatment failure 2186
8 Table 2. Primary Efficacy Outcome at Early Clinical Evaluation According to Pathogen Detected at Baseline (Intentionto-Treat Population Who Could Be Evaluated Microbiologically).* Pathogen Oritavancin (N = 244) no./total no. (%) Vancomycin (N = 242) Difference (95% CI) percentage points Detection of at least one pathogen 201/244 (82.4) 196/242 (81.0) 1.4 ( 5.5 to 8.3) Staphylococcus aureus 180/220 (81.8) 172/210 (81.9) 0.1 ( 7.4 to 7.2) MRSA 84/104 (80.8) 80/100 (80.0) 0.8 ( 10.1 to 11.7) MSSA 96/116 (82.8) 92/110 (83.6) 0.9 ( 10.6 to 8.9) Streptococcus species 25/31 (80.6) 31/38 (81.6) 0.9 ( 19.5 to 17.6) S. anginosus group 12/13 (92.3) 14/16 (87.5) S. agalactiae 6/7 (85.7) 8/8 (100.0) S. pyogenes 5/8 (62.5) 5/10 (50.0) S. dysgalactiae 2/3 (66.7) 3/3 (100.0) Enterococcus faecalis 6/7 (85.7) 4/5 (80.0) * The pathogens listed are gram-positive pathogens known to cause acute bacterial skin and skin-structure infections, whether isolated from an infection site-culture or a blood culture. The pathogens listed include only those detected in both treatment groups. Patients with multiple pathogens were counted once in the rows for each pathogen. MSSA denotes methicillin-susceptible S. aureus. Differences and 95% confidence intervals are shown only for speciated pathogens identified from 10 or more patients in each treatment group. This group includes S. anginosus, S. intermedius, and S. constellatus. because of missing data for the end point of investigator-assessed clinical cure. For the majority of these patients (98.3%), treatment was considered to be a failure because the patients did not undergo the post-therapy evaluation. Results in the modified intention-to-treat population were consistent with those in the population of patients who could be evaluated clinically (Fig. 1, and Table S4 in the Supplementary Appendix). Results of sensitivity analyses in which different methods were used to handle missing data are presented in Table S5 in the Supplementary Appendix. In the subpopulation of patients with MRSA infection, similar efficacy was observed in the two treatment groups for the primary and secondary end points (Fig. 2). These efficacy results for patients infected with MRSA were consistent with those in the population of patients who could be evaluated microbiologically (Table S6 in the Supplementary Appendix). Results for the primary end point according to the infecting pathogen at baseline are presented in Table 2. The efficacy of oritavancin against these isolates was similar to that of vancomycin. The mean (±SD) total daily vancomycin dose in the safety population was 2.3±0.94 g, and the mean duration of vancomycin therapy was 8.1±2.43 days. The mean vancomycin level in patients with a measurable trough level (before administration of the fourth dose) was 15.4 µg per milliliter, and the median level 11.1 µg per milliliter. Safety and Side-Effect Profile The incidence of adverse events that developed during treatment, regardless of the relationship of the event to the study drug, was similar in the oritavancin and vancomycin groups (Table 3), and most of the events were mild. The most frequently reported adverse events in the oritavancin group were nausea (11.0%, vs. 8.9% in the vancomycin group), headache (7.2% vs. 7.9%), vomiting (4.9% vs. 3.7%), and diarrhea (4.9% vs. 3.5%) (Table 3). The proportion of patients with an adverse event that led to discontinuation of the study drug was lower in the oritavancin group. The incidence of abnormalities on tests of liver function was 2.3% in the oritavancin group and 1.0% in the vancomycin group. No symptomatic adverse events related to liver function were reported, there were no reports of serious elevations in levels of metabolites related to liver func- 2187
9 The new england journal of medicine Table 3. Patients with Adverse Events (Safety Population).* Adverse Event At least 1 adverse event that developed during treatment Oritavancin (N = 473) no. of patients (%) Vancomycin (N = 481) 284 (60.0) 307 (63.8) Related to study drug 108 (22.8) 151 (31.4) Leading to discontinuation of study drug 18 (3.8) 28 (5.8) Serious adverse event 35 (7.4) 35 (7.3) Related to study drug 3 (0.6) 3 (0.6) Leading to discontinuation of study drug 11 (2.3) 13 (2.7) Death 1 (0.2) 2 (0.4) Most frequently reported adverse events Nausea 52 (11.0) 43 (8.9) Headache 34 (7.2) 38 (7.9) Pruritus 16 (3.4) 44 (9.1) Infusion-site reaction 19 (4.0) 34 (7.1) Infusion-site extravasation 18 (3.8) 23 (4.8) Vomiting 23 (4.9) 18 (3.7) Constipation 19 (4.0) 21 (4.4) Diarrhea 23 (4.9) 17 (3.5) Cellulitis 20 (4.2) 17 (3.5) Pyrexia 15 (3.2) 20 (4.2) Dizziness 15 (3.2) 15 (3.1) Insomnia 14 (3.0) 13 (2.7) Chills 10 (2.1) 12 (2.5) Urticaria 7 (1.5) 15 (3.1) Pruritus, generalized 11 (2.3) 9 (1.9) Subcutaneous abscess 9 (1.9) 11 (2.3) Abscess on limb 13 (2.7) 5 (1.0) Infusion-site phlebitis 8 (1.7) 10 (2.1) Alanine aminotransferase elevation 11 (2.3) 5 (1.0) Fatigue 10 (2.1) 6 (1.2) * A study investigator determined whether there was a causal relationship between an adverse event and the study drug. All serious adverse events are listed, not only those that developed during treatment. Listed are the adverse events that occurred in more than 2% of the patients in either study group during treatment. tion, and none of the patients discontinued the study drug as a result of these adverse events. There was no case in which a patient s hepatic profile met the criteria of Hy s law (a serum alanine or aspartate aminotransferase level that is more than three times the upper limit of the normal range and a serum total bilirubin level that is more than two times the upper limit of the normal range in the absence of initial findings of cholestasis, with no other explanation for the combination of elevated aminotransferase and total bilirubin levels). 34,35 In the oritavancin group, the only adverse event that developed during treatment and led to discontinuation in more than one patient was cellulitis (in two patients). In the vancomycin group, adverse events that developed during treatment and led to discontinuation of the study drug were hypersensitivity (in five patients), cellulitis (in three patients), and sepsis, bacterial skin infection, drug hypersensitivity, pruritus, and rash (in two patients for each condition). The frequency of serious adverse events was similar in the two groups (7.4% with oritavancin and 7.3% with vancomycin), as was the distribution of serious adverse events (Table 3, and Table S13 in the Supplementary Appendix). Three patients died during the study: in the oritavancin group, one patient died from sepsis and septic shock, and in the vancomycin group, one patient died from sepsis and one from advanced dementia with parkinsonism. The incidence of laboratory abnormalities was balanced between the treatment groups. No significant between-group difference in vital signs or ECG findings was identified. Discussion Currently available therapeutic options for the treatment of acute bacterial skin and skin-structure infections require repeat administrations that may result in extended hospitalization and can result in substantial costs to the health care system. A single-dose treatment for acute bacterial skin and skin-structure infections that results in an early and sustained clinical response could have the potential to reduce the complications associated with multiple intravenous administrations in patients with these infections, improve adherence to treatment, improve quality of life, and reduce the utilization of health care resources. 16 In this phase 3 study (SOLO I) involving adults with acute bacterial skin and skin-structure infections, treatment with a single dose of oritavancin met the primary and secondary efficacy end points and had an adverse-event pro- 2188
10 file that was similar to that of the active comparator, vancomycin, which was administered twice daily for 7 to 10 days. Oritavancin was noninferior to vancomycin on the basis of both the early clinical evaluation of efficacy (reduction in lesion size, assessed 48 to 72 hours after the initiation of treatment) and the post-therapy evaluation of efficacy (clinical cure, assessed by the site investigator 7 to 14 days after the end of treatment). These early and late assessments of efficacy were concordant (Table S11 in the Supplementary Appendix). In addition, oritavancin showed efficacy against infection with S. aureus, and MRSA in particular, irrespective of the end point and the analysis population. Treatment-failure rates were balanced between the two groups, and the reasons for failure were similar in the groups. The main reason for failure at the post-therapy evaluation was missing data, and this problem was largely due to a missed follow-up visit. The results of sensitivity analyses (performed in the modified intentionto-treat population and in the population of patients who could be evaluated clinically) in which missing data were either excluded or imputed as indicating successful treatment were consistent with the results of the primary analyses, for both the early clinical evaluation and the posttherapy evaluation. The frequency, distribution, and severity of adverse events that emerged during treatment were similar in the oritavancin and vancomycin groups. Discontinuation of the study treatment because of such events were uncommon. In addition, no clinically significant between-group differences in clinical laboratory values were observed. The prolonged half-life of oritavancin 24 was not associated with any untoward safety issues during the study, which included a followup assessment on day 60. The severity of the baseline infection was underscored both by the need for at least 7 days of intravenous therapy, as determined by the site investigator, and the median lesion area, including surrounding erythema, edema, and induration of approximately 235 cm 2. The fact that approximately 20% of the patients in the study had diabetes mellitus also attests to the complicated nature of the baseline infections. The oritavancin regimen, in which 1200 mg is administered in a single dose, may ensure adherence to treatment, reduce or eliminate hospital stays, and reduce the utilization of health care resources. 16 The results presented here, which show the efficacy of a single dose of oritavancin without obvious consequences for safety, as compared with 7 to 10 days of treatment with intravenous vancomycin, should provide an impetus to determine the costs of outpatient care for patients with acute bacterial skin and skinstructure infections. There are several limitations of the SOLO I study. Whereas the extended half-life of oritavancin provides the physician with a single infusion option for treatment, it also raises concern about extended illness, should a serious reaction to this antibiotic occur. The evidence to date suggests that oritavancin has a safety profile that is similar to the profile for vancomycin, and the 60-day follow-up assessment in our study, involving nearly 500 patients treated with oritavancin, did not identify any such prolonged adverse events; however, experience with this treatment is limited. Similarly, the inability to step down to a beta-lactam antibiotic once the possibility of MRSA infection has been ruled out has the potential to result in increased microbial resistance. Further evaluation of this possibility will be important. Whether patients treated with a single infusion can be discharged and followed on an outpatient basis remains to be determined. Several questions must be answered; for example, is there a risk that outpatient follow-up will delay the diagnosis of serious, deep infections such as necrotizing fasciitis and bacteremia? Serious infections such as bacteremia, which were once considered manageable only in a hospital setting, have since shown the potential to be effectively managed on an outpatient basis once the patient s condition has stabilized. 36 Also, studies are needed to determine whether treatment with oritavancin is effective for other infections, such as bacteremia, osteomyelitis, and prosthetic-joint infections. In conclusion, treatment with a single dose of oritavancin was noninferior to 7 to 10 days of vancomycin in adults with acute bacterial skin and skin-structure infections caused by grampositive pathogens, including MRSA. No significant differences in safety between the two treatment regimens were observed. Supported by the Medicines Company. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. 2189
11 References 1. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, JAMA 2012;308: Jenkins TC, Sabel AL, Sarcone EE, Price CS, Mehler PS, Burman WJ. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis 2010;51: Lee BY, Singh A, David MZ, et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect 2013;19: Garau J, Ostermann H, Medina J, Avila M, McBride K, Blasi F. Current management of patients hospitalized with complicated skin and soft tissue infections across Europe ( ): assessment of clinical practice patterns and real-life effectiveness of antibiotics from the REACH study. Clin Microbiol Infect 2013;19(9): E377-E Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41: [Errata, Clin Infect Dis 2005;41:1830, 2006;42:1219.] 6. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52(3):e18- e Teflaro (ceftaroline fosamil): prescribing information. New York: Forest Laboratories, October 2012 ( pi/teflaro_pi.pdf). 8. Tygacil (tigecycline): prescribing information. New York: Pfizer, November 2012 ( 9. Vancomycin: prescribing information. New York: Pfizer, December 2010 ( labeling.pfizer.com/showlabeling.aspx?id =661). 10. Vibativ (telavancin): prescribing information. San Francisco: Theravance, January 2012 ( VIBATIV_PI_Final.pdf). 11. Zyvox (linezolid): prescribing information. New York: Pfizer, March 2012 ( 12. Cubicin (daptomycin): prescribing information. Lexington, MA: Cubist Pharmaceuticals, January 2013 ( 13. Active Bacterial Core Surveillance (ABCs) report: Emerging Infections Program Network methicillin-resistant Staphylococcus aureus. Atlanta: Centers for Disease Control and Prevention, 2011 ( survreports/mrsa11.pdf). 14. Antimicrobial resistance surveillance in Europe 2011: annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: European Centre for Disease Prevention and Control, 2012 ( en/publications/publications/antimicrobial -resistance-surveillance-europe-2011.pdf). 15. Ball AT, Xu Y, Sanchez RJ, Shelbaya A, Deminski MC, Nau DP. Nonadherence to oral linezolid after hospitalization: a retrospective claims analysis of the incidence and consequence of claim reversals. Clin Ther 2010;32: Tice A. Oritavancin: a new opportunity for outpatient therapy of serious infections. Clin Infect Dis 2012;54:Suppl 3: S239-S Belley A, McKay GA, Arhin FF, et al. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing. Antimicrob Agents Chemother 2010;54: Belley A, Neesham-Grenon E, McKay G, et al. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother 2009;53: Zhanel GG, Schweizer F, Karlowsky JA. Oritavancin: mechanism of action. Clin Infect Dis 2012;54:Suppl 3:S214-S McKay GA, Beaulieu S, Arhin FF, et al. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother 2009;63: Arhin FF, Draghi DC, Pillar CM, Parr TR Jr, Moeck G, Sahm DF. Comparative in vitro activity profile of oritavancin against recent gram-positive clinical isolates. Antimicrob Agents Chemother 2009;53: Mendes RE, Farrell DJ, Sader HS, Jones RN. Oritavancin microbiologic features and activity results from the surveillance program in the United States. Clin Infect Dis 2012;54:Suppl 3:S203-S Ambrose PG, Drusano GL, Craig WA. In vivo activity of oritavancin in animal infection models and rationale for a new dosing regimen in humans. Clin Infect Dis 2012;54:Suppl 3:S220-S Rubino CM, Van Wart SA, Bhavnani SM, Ambrose PG, McCollam JS, Forrest A. Oritavancin population pharmacokinetics in healthy subjects and patients with complicated skin and skin structure infections or bacteremia. Antimicrob Agents Chemother 2009;53: Kumar A, Mann HJ, Keshtgarpour M, et al. In vitro characterization of oritavancin clearance from human blood by low-flux, high-flux, and continuous renal replacement therapy dialyzers. Int J Artif Organs 2011;34: Belley A, Arhin FF, Sarmiento I, Deng H, Rose W, Moeck G. Pharmacodynamics of a simulated single 1,200-milligram dose of oritavancin in an in vitro pharmacokinetic/pharmacodynamic model of methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother 2013;57: Dunbar LM, Milata J, McClure T, Wasilewski MM. Comparison of the efficacy and safety of oritavancin front-loaded dosing regimens to daily dosing: an analysis of the SIMPLIFI trial. Antimicrob Agents Chemother 2011;55: Guidance for Industry acute bacterial skin and skin structure infections: developing drugs for treatment. Silver Spring, MD: Food and Drug Administration, 2010 ( Drugs/GuidanceComplianceRegulatory Information/Guidances/ucm pdf). 29. Addendum to the note for guidance on evaluation of medicinal products indicated for treatment of bacterial infections (CHMP/EWP/558/95 REV 2) to address indication-specific clinical data (draft). London: European Medicines Agency Committee for Medicinal Products for Human Use, 2012 ( en_gb/document_library/scientific_ guideline/2012/07/wc pdf). 30. Talbot GH, Powers JH, Fleming TR, Siuciak JA, Bradley J, Boucher H. Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis 2012;55: Snodgrass WR, Anderson T. Prontosil in erysipelas. BMJ 1937;2: Idem. Sulfanilamide in the treatment of erysipelas. BMJ 1937;2: Spellberg B, Talbot GH, Boucher HW, et al. Antimicrobial agents for complicated skin and skin-structure infections: justification of noninferiority margins in the absence of placebo-controlled trials. Clin Infect Dis 2009;49: Watkins PB, Desai M, Berkowitz SD, et al. Evaluation of drug-induced serious hepatotoxicity (edish): application of this data organization approach to phase III clinical trials of rivaroxaban after total hip or knee replacement surgery. Drug Saf 2011;34: Guidance for Industry druginduced liver injury: premarketing clinical evaluation. Silver Spring, MD: Food and Drug Administration, 2009 ( Guidances/UCM pdf). 36. Rehm S, Campion M, Katz DE, Russo R, Boucher HW. Community-based outpatient parenteral antimicrobial therapy (CoPAT) for Staphylococcus aureus bacteraemia with or without infective endocarditis: analysis of the randomized trial comparing daptomycin with standard therapy. J Antimicrob Chemother 2009;63: Copyright 2014 Massachusetts Medical Society. 2190
Open Forum Infectious Diseases MAJOR ARTICLE
 Open Forum Infectious Diseases MAJOR ARTICLE Efficacy and Safety of Oritavancin Relative to Vancomycin for Patients with Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in the Outpatient Setting:
Open Forum Infectious Diseases MAJOR ARTICLE Efficacy and Safety of Oritavancin Relative to Vancomycin for Patients with Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in the Outpatient Setting:
ESCMID Online Lecture Library. by author
 Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry
 doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
ABSTRACT. n engl j med 370;23 nejm.org june 5,
 The new england journal of medicine established in 1812 june 5, 2014 vol. 370 no. 23 Once-Weekly versus Daily Conventional Therapy for Skin Infection Helen W. Boucher, M.D., Mark Wilcox, M.D., George H.
The new england journal of medicine established in 1812 june 5, 2014 vol. 370 no. 23 Once-Weekly versus Daily Conventional Therapy for Skin Infection Helen W. Boucher, M.D., Mark Wilcox, M.D., George H.
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
Options for Complicated Skin and Skin Structure Infections. Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ.
 Options for Complicated Skin and Skin Structure Infections Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ. Disclosures I have served as a consultant to, researcher/investigator for,
Options for Complicated Skin and Skin Structure Infections Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ. Disclosures I have served as a consultant to, researcher/investigator for,
Consideration of some other specific indications: Bacteremia
 European Medicines Agency Workshop on Antibacterials, London 7-8 February 2011 Consideration of some other specific indications: Bacteremia Harald Seifert Institut für Medizinische Mikrobiologie, Immunologie
European Medicines Agency Workshop on Antibacterials, London 7-8 February 2011 Consideration of some other specific indications: Bacteremia Harald Seifert Institut für Medizinische Mikrobiologie, Immunologie
Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more)
 Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more) Christian Eckmann Antibiotic Stewardship Expert ECDC Chief of Staff Department of General, Visceral and Thoracic Surgery Klinikum
Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more) Christian Eckmann Antibiotic Stewardship Expert ECDC Chief of Staff Department of General, Visceral and Thoracic Surgery Klinikum
Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger
 Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger Cubicin (Daptomycin): A new generation of antibiotics First-in-class cyclic natural lipopeptide Activity against major gram positive pathogens
Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger Cubicin (Daptomycin): A new generation of antibiotics First-in-class cyclic natural lipopeptide Activity against major gram positive pathogens
Paul McGovern, MD Vice President, Clinical Affairs Paratek Pharmaceuticals, Inc. Presented at ECCMID, 22 April 2018, Madrid, Spain.
 A phase-3 randomized, double-blind, multicentre study to compare the safety and efficacy of oral omadacycline to oral linezolid for treating adult subjects with ABSSSI (OASIS-2 study) Paul McGovern, MD
A phase-3 randomized, double-blind, multicentre study to compare the safety and efficacy of oral omadacycline to oral linezolid for treating adult subjects with ABSSSI (OASIS-2 study) Paul McGovern, MD
Treatment of acute bacterial skin and skin structure infection with single-dose dalbavancin in persons who inject drugs
 ORIGINAL RESEARCH A continuous publication, open access, peer-reviewed journal Treatment of acute bacterial skin and skin structure infection with single-dose dalbavancin in persons who inject drugs ACCESS
ORIGINAL RESEARCH A continuous publication, open access, peer-reviewed journal Treatment of acute bacterial skin and skin structure infection with single-dose dalbavancin in persons who inject drugs ACCESS
Michael L. Corrado* INC Research, 580 Union Square Drive, New Hope, PA 18938, USA
 J Antimicrob Chemother 2010; 65 Suppl 4: iv67 71 doi:10.1093/jac/dkq256 Integrated safety summary of CANVAS 1 and 2 trials: Phase III, randomized, double-blind studies evaluating ceftaroline fosamil for
J Antimicrob Chemother 2010; 65 Suppl 4: iv67 71 doi:10.1093/jac/dkq256 Integrated safety summary of CANVAS 1 and 2 trials: Phase III, randomized, double-blind studies evaluating ceftaroline fosamil for
Department of Pharmacy Services, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA; 2
 Pathogens 2015, 4, 599-605; doi:10.3390/pathogens4030599 Article OPEN ACCESS pathogens ISSN 2076-0817 www.mdpi.com/journal/pathogens Assessing the Surrogate Susceptibility of Oxacillin and Cefoxitin for
Pathogens 2015, 4, 599-605; doi:10.3390/pathogens4030599 Article OPEN ACCESS pathogens ISSN 2076-0817 www.mdpi.com/journal/pathogens Assessing the Surrogate Susceptibility of Oxacillin and Cefoxitin for
CLINICAL USE OF GLYCOPEPTIDES. Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel
 CLINICAL USE OF GLYCOPEPTIDES Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel Glycopeptides Natural Vancomycin introduced in 1958 Teicoplanin introduced in Europe
CLINICAL USE OF GLYCOPEPTIDES Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel Glycopeptides Natural Vancomycin introduced in 1958 Teicoplanin introduced in Europe
Cellceutix Corporation Beverly, MA USA Abstract 2969; Presentation 0195; Hall J, 4:00pm. April 27, 2015
 ECCMID 2015 Copenhagen, Denmark 25 28 April 2015 Cellceutix Corporation Beverly, MA USA www.cellceutix.com A Randomized, Double-Blind Study Comparing Single-Dose and Short-Course Brilacidin to Daptomycin
ECCMID 2015 Copenhagen, Denmark 25 28 April 2015 Cellceutix Corporation Beverly, MA USA www.cellceutix.com A Randomized, Double-Blind Study Comparing Single-Dose and Short-Course Brilacidin to Daptomycin
Safety of Dalbavancin in the Treatment of Skin and Skin Structure Infections: A Pooled Analysis of Randomized, Comparative Studies
 Drug Saf (2016) 39:147 157 DOI 10.1007/s40264-015-0374-9 ORIGINAL RESEARCH ARTICLE Safety of Dalbavancin in the Treatment of Skin and Skin Structure Infections: A Pooled Analysis of Randomized, Comparative
Drug Saf (2016) 39:147 157 DOI 10.1007/s40264-015-0374-9 ORIGINAL RESEARCH ARTICLE Safety of Dalbavancin in the Treatment of Skin and Skin Structure Infections: A Pooled Analysis of Randomized, Comparative
ABSSSI Case Study days (clinical evaluation)
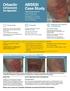 ABSSSI Case Study Cellulitis patient background 44-year-old single father Chronic renal insufficiency Hyperlipidemia, on rosuvastatin Has atrial fibrillation, on apixaban Fell and injured right leg At
ABSSSI Case Study Cellulitis patient background 44-year-old single father Chronic renal insufficiency Hyperlipidemia, on rosuvastatin Has atrial fibrillation, on apixaban Fell and injured right leg At
The Challenge of Managing Staphylococcus aureus Bacteremia
 The Challenge of Managing Staphylococcus aureus Bacteremia M A R G A R E T G R A Y B S P F C S H P C L I N I C A L P R A C T I C E M A N A G E R N O R T H / I D P H A R M A C I S T A L B E R T A H E A
The Challenge of Managing Staphylococcus aureus Bacteremia M A R G A R E T G R A Y B S P F C S H P C L I N I C A L P R A C T I C E M A N A G E R N O R T H / I D P H A R M A C I S T A L B E R T A H E A
ANTIMICROBIALS AVAILABLE FOR
 ORIGINAL CONTRIBUTION Tedizolid Phosphate vs Linezolid for Treatment of Acute Bacterial Skin and Skin Structure Infections The ESTABLISH-1 Randomized Trial Philippe Prokocimer, MD Carisa De Anda, PharmD
ORIGINAL CONTRIBUTION Tedizolid Phosphate vs Linezolid for Treatment of Acute Bacterial Skin and Skin Structure Infections The ESTABLISH-1 Randomized Trial Philippe Prokocimer, MD Carisa De Anda, PharmD
D DAVID PUBLISHING. Evaluation of the Effectiveness of a Vancomycin Nomogram at Predicting Trough Levels within a Therapeutic Range. 1.
 Journal of Pharmacy and Pharmacology 2 (2014) 713-721 doi: 10.17265/2328-2150/2014.12.004 D DAVID PUBLISHING Evaluation of the Effectiveness of a Vancomycin Nomogram at Predicting Trough Levels within
Journal of Pharmacy and Pharmacology 2 (2014) 713-721 doi: 10.17265/2328-2150/2014.12.004 D DAVID PUBLISHING Evaluation of the Effectiveness of a Vancomycin Nomogram at Predicting Trough Levels within
Pharmacologyonline 1: (2010) ewsletter Singh and Kochbar. Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of
 Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of Cefoperazone Sulbactam Singh M*, Kochhar P* Medical & Research Division, Pfizer India. Summary Antimicrobial resistance is associated with
Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of Cefoperazone Sulbactam Singh M*, Kochhar P* Medical & Research Division, Pfizer India. Summary Antimicrobial resistance is associated with
D DAVID PUBLISHING. 1. Introduction. Kathryn Koliha 1, Julie Falk 1, Rachana Patel 1 and Karen Kier 2
 Journal of Pharmacy and Pharmacology 5 (2017) 607-615 doi: 10.17265/2328-2150/2017.09.001 D DAVID PUBLISHING Comparative Evaluation of Pharmacist-Managed Vancomycin Dosing in a Community Hospital Following
Journal of Pharmacy and Pharmacology 5 (2017) 607-615 doi: 10.17265/2328-2150/2017.09.001 D DAVID PUBLISHING Comparative Evaluation of Pharmacist-Managed Vancomycin Dosing in a Community Hospital Following
Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects
 Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects Karolyn S. Horn, Mark H. Gotfried, Judith N. Steenbergen,
Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects Karolyn S. Horn, Mark H. Gotfried, Judith N. Steenbergen,
ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication
 ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication ICD-10-CM Diagnosis Codes Diagnosis INDICATION ORBACTIV (oritavancin) for injection is indicated for the treatment of adult patients
ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication ICD-10-CM Diagnosis Codes Diagnosis INDICATION ORBACTIV (oritavancin) for injection is indicated for the treatment of adult patients
Use of Ceftaroline Fosamil in Osteomyelitis: CAPTURE Study Experience
 Johnson et al. BMC Infectious Diseases (2019) 19:183 https://doi.org/10.1186/s12879-019-3791-z RESEARCH ARTICLE Open Access Use of Ceftaroline Fosamil in Osteomyelitis: CAPTURE Study Experience Leonard
Johnson et al. BMC Infectious Diseases (2019) 19:183 https://doi.org/10.1186/s12879-019-3791-z RESEARCH ARTICLE Open Access Use of Ceftaroline Fosamil in Osteomyelitis: CAPTURE Study Experience Leonard
Comparison of the Microbiology and Antibiotic Treatment Among Diabetic and Nondiabetic Patients Hospitalized for Cellulitis or Cutaneous Abscess
 ORIGINAL RESEARCH Comparison of the Microbiology and Antibiotic Treatment Among Diabetic and Nondiabetic atients Hospitalized for Cellulitis or Cutaneous Abscess Timothy C. Jenkins, MD 1,2,3,4 *, Bryan
ORIGINAL RESEARCH Comparison of the Microbiology and Antibiotic Treatment Among Diabetic and Nondiabetic atients Hospitalized for Cellulitis or Cutaneous Abscess Timothy C. Jenkins, MD 1,2,3,4 *, Bryan
Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH)
 Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH) Clinical Practice Guideline* for the Diagnosis and Management of Acute Bacterial
Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH) Clinical Practice Guideline* for the Diagnosis and Management of Acute Bacterial
Vancomycin Drug Class 1
 Drug Class 1 Antibiotic glycopeptide Spectrum 1 Cross Sensitivities / Allergies 1 Refer to product monograph for complete spectrum Gram positive pathogens (e.g., S. aureus, Enterococcus, S. viridans, methicillinresistant
Drug Class 1 Antibiotic glycopeptide Spectrum 1 Cross Sensitivities / Allergies 1 Refer to product monograph for complete spectrum Gram positive pathogens (e.g., S. aureus, Enterococcus, S. viridans, methicillinresistant
Development of Drugs for Bacteremia
 Development of Drugs for Bacteremia Charles Knirsch, MD, MPH VP, Clinical Research Pfizer Inc 1 Bacteremia Guidance Issues EMA guidance suggests that bacteremia is not a primary diagnosis but represents
Development of Drugs for Bacteremia Charles Knirsch, MD, MPH VP, Clinical Research Pfizer Inc 1 Bacteremia Guidance Issues EMA guidance suggests that bacteremia is not a primary diagnosis but represents
Bariatric Surgery versus Intensive Medical Therapy for Diabetes 3-Year Outcomes
 The new england journal of medicine original article Bariatric Surgery versus Intensive Medical for Diabetes 3-Year Outcomes Philip R. Schauer, M.D., Deepak L. Bhatt, M.D., M.P.H., John P. Kirwan, Ph.D.,
The new england journal of medicine original article Bariatric Surgery versus Intensive Medical for Diabetes 3-Year Outcomes Philip R. Schauer, M.D., Deepak L. Bhatt, M.D., M.P.H., John P. Kirwan, Ph.D.,
Resistance to new anti-grampositive. Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France
 Resistance to new anti-grampositive agents Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France Recently available antimicrobials against MDR Gram-positive infections Cyclic lipopeptide: daptomycin
Resistance to new anti-grampositive agents Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France Recently available antimicrobials against MDR Gram-positive infections Cyclic lipopeptide: daptomycin
Clinical Guidelines for Use of Antibiotics. VANCOMYCIN (Adult)
 VANCOMYCIN (Adult) Please always prescribe VANCOMYCIN in the Variable Dose Antibiotic section of the EPMA SUPPLEMENTARY drug chart (and add a placeholder on the electronic drug chart). 1 Background Vancomycin
VANCOMYCIN (Adult) Please always prescribe VANCOMYCIN in the Variable Dose Antibiotic section of the EPMA SUPPLEMENTARY drug chart (and add a placeholder on the electronic drug chart). 1 Background Vancomycin
Abstract. Introduction
 ORIGINAL ARTICLE Comparison of vancomycin and linezolid in patients with peripheral vascular disease and/or diabetes in an observational European study of complicated skin and soft-tissue infections due
ORIGINAL ARTICLE Comparison of vancomycin and linezolid in patients with peripheral vascular disease and/or diabetes in an observational European study of complicated skin and soft-tissue infections due
Abhijit Chakraborty 1 *, Sandip Roy 1, Juergen Loeffler 2 and Ricardo L. Chaves 2. CH-4056 Basel, Switzerland. Introduction
 Journal of Antimicrobial Chemotherapy (29) 64, 151 158 doi:1.193/jac/dkp155 Advance Access publication 22 April 29 Comparison of the pharmacokinetics, safety and tolerability of in healthy adult volunteers
Journal of Antimicrobial Chemotherapy (29) 64, 151 158 doi:1.193/jac/dkp155 Advance Access publication 22 April 29 Comparison of the pharmacokinetics, safety and tolerability of in healthy adult volunteers
Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia
 Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia Brandon Dionne, PharmD, BCPS, AAHIVP Assistant Clinical Professor Northeastern University Seth Housman, PharmD, MPA Clinical Assistant
Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia Brandon Dionne, PharmD, BCPS, AAHIVP Assistant Clinical Professor Northeastern University Seth Housman, PharmD, MPA Clinical Assistant
ORIGINAL ARTICLE DOI: (e) ISSN Online: (p) ISSN Print: Anand Kumar Singh 1, Poonam Verma 2. Sciences, Dehradun
 ORIGINAL ARTICLE CLINICAL ASSESSMENT OF NEPHROTOXICITY ASSOCIATED WITH VANCOMYCIN TROUGH CONCENTRATIONS DURING TREATMENT OF DEEP-SEATED INFECTIONS: A RETROSPECTIVE ANALYSIS Anand Kumar Singh 1, Poonam
ORIGINAL ARTICLE CLINICAL ASSESSMENT OF NEPHROTOXICITY ASSOCIATED WITH VANCOMYCIN TROUGH CONCENTRATIONS DURING TREATMENT OF DEEP-SEATED INFECTIONS: A RETROSPECTIVE ANALYSIS Anand Kumar Singh 1, Poonam
Evaluation of Vancomycin Continuous Infusion in Trauma Patients
 OBJECTIVES Evaluation of Vancomycin Continuous Infusion in Trauma Patients Brittany D. Bissell, Pharm.D. PGY-2 Critical Care Pharmacy Resident Jackson Memorial Hospital Miami, Florida Evaluate the potential
OBJECTIVES Evaluation of Vancomycin Continuous Infusion in Trauma Patients Brittany D. Bissell, Pharm.D. PGY-2 Critical Care Pharmacy Resident Jackson Memorial Hospital Miami, Florida Evaluate the potential
Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens to tedizolid, a novel oxazolidinone
 Zurenko et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:46 RESEARCH Open Access Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens
Zurenko et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:46 RESEARCH Open Access Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens
profiles Decision: Dalvance
 Patient candidate profiles Decision: Dalvance 2 A series of patient candidate profiles The following patient candidate profiles are hypothetical examples of treatment decisions that could be seen in the
Patient candidate profiles Decision: Dalvance 2 A series of patient candidate profiles The following patient candidate profiles are hypothetical examples of treatment decisions that could be seen in the
ESTABLISH 2 Top Line Data Release
 The Future of Anti-Infectives ESTABLISH 2 Top Line Data Release March 25, 2013 1 Forward Looking Statements Statements contained in this data release regarding matters that are not historical facts are
The Future of Anti-Infectives ESTABLISH 2 Top Line Data Release March 25, 2013 1 Forward Looking Statements Statements contained in this data release regarding matters that are not historical facts are
Cubicin A Guide to Dosing
 Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections
Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections
DRUG UTILIZATION EVALUATION
 DRUG UTILIZATION EVALUATION Prescribing Trends with Daptomycin (Cubicin) For the Treatment of Gram-Positive Infections Noreen H. Chan Tompkins, PharmD, and Stephen J. Harnicar, PharmD INTRODUCTION Daptomycin
DRUG UTILIZATION EVALUATION Prescribing Trends with Daptomycin (Cubicin) For the Treatment of Gram-Positive Infections Noreen H. Chan Tompkins, PharmD, and Stephen J. Harnicar, PharmD INTRODUCTION Daptomycin
Synopsis (C1034T02) CNTO 95 Module 5.3 Clinical Study Report C1034T02
 Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
Module 5.3 Protocol: EudraCT No.: 2004-002130-18 Title of the study: A Phase 1/2, Multi-Center, Blinded, Randomized, Controlled Study of the Safety and Efficacy of the Human Monoclonal Antibody to Human
PFIZER INC. Study Initiation Date and Completion Dates: Information not available (Date of Statistical Report: 16 May 2004)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Pharmacokinetics and Tolerability of Daptomycin at Doses up to 12 Milligrams per Kilogram of Body Weight Once Daily in Healthy Volunteers
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 2006, p. 3245 3249 Vol. 50, No. 10 0066-4804/06/$08.00 0 doi:10.1128/aac.00247-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Pharmacokinetics
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 2006, p. 3245 3249 Vol. 50, No. 10 0066-4804/06/$08.00 0 doi:10.1128/aac.00247-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Pharmacokinetics
Serious MRSA infection: anything new?
 Serious MRSA infection: anything new? Professor Mark H. Wilcox Leeds Teaching Hospitals & University of Leeds, UK Public Health England & NHS Improvement Potential Conflicts of Interest I have received:
Serious MRSA infection: anything new? Professor Mark H. Wilcox Leeds Teaching Hospitals & University of Leeds, UK Public Health England & NHS Improvement Potential Conflicts of Interest I have received:
IV Vancomycin dosing and monitoring Antibiotic Guidelines. Contents. Intro
 IV Vancomycin dosing and Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): as above Authors Division: DCSS & Tertiary Medicine Unique
IV Vancomycin dosing and Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): as above Authors Division: DCSS & Tertiary Medicine Unique
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study
 Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study Daniel J. Cloutier 1, Loren G. Miller 2, Allison S. Komirenko 1,
Plazomicin Versus Meropenem for the Treatment of Complicated Urinary Tract Infection and Acute Pyelonephritis: Results of the EPIC Study Daniel J. Cloutier 1, Loren G. Miller 2, Allison S. Komirenko 1,
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Michael S. Niederman, M.D. Clinical Director Pulmonary and Critical Care Medicine New York Presbyterian Hospital Weill Cornell Medical Center
 CA-MRSA Pneumonia Michael S. Niederman, M.D. Clinical Director Pulmonary and Critical Care Medicine New York Presbyterian Hospital Weill Cornell Medical Center Professor of Clinical Medicine Weill Cornell
CA-MRSA Pneumonia Michael S. Niederman, M.D. Clinical Director Pulmonary and Critical Care Medicine New York Presbyterian Hospital Weill Cornell Medical Center Professor of Clinical Medicine Weill Cornell
VANLID Capsules (Vancomycin hydrochloride)
 Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Evaluation Of The Use Of Daptomycin In Diabetic Foot Infections With Osteomyelitis
 ISPUB.COM The Internet Journal of Infectious Diseases Volume 10 Number 1 Evaluation Of The Use Of Daptomycin In Diabetic Foot Infections With Osteomyelitis R Stienecker, R Wolcott, M Crompton, E Hershberger,
ISPUB.COM The Internet Journal of Infectious Diseases Volume 10 Number 1 Evaluation Of The Use Of Daptomycin In Diabetic Foot Infections With Osteomyelitis R Stienecker, R Wolcott, M Crompton, E Hershberger,
PFIZER INC. Study Center(s): A total of 6 centers took part in the study, including 2 in France and 4 in the United States.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis
 Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Group B streptococcal infection;. Bacteremia without a focus occurs in 80-85%,. July has been recognised as Group B Strep Awareness Month,.
 Group B streptococcal infection;. Bacteremia without a focus occurs in 80-85%,. July has been recognised as Group B Strep Awareness Month,. 12-10-2017 Group B streptococci are uniformly sensitive to penicillin
Group B streptococcal infection;. Bacteremia without a focus occurs in 80-85%,. July has been recognised as Group B Strep Awareness Month,. 12-10-2017 Group B streptococci are uniformly sensitive to penicillin
Summary ID#7029. Clinical Study Summary: Study F1D-MC-HGKQ
 CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
Enhanced EARS-Net Surveillance REPORT FOR 2012 DATA
 Enhanced EARS-Net Surveillance REPORT FOR DATA 1 In this report Main results for Proposed changes to the enhanced programme Abbreviations Used Here BSI Bloodstream Infections CVC Central Venous Catheter
Enhanced EARS-Net Surveillance REPORT FOR DATA 1 In this report Main results for Proposed changes to the enhanced programme Abbreviations Used Here BSI Bloodstream Infections CVC Central Venous Catheter
Vancomycin: Class: Antibiotic.
 Vancomycin: Class: Antibiotic. Indications: Treatment of patients with infections caused by staphylococcal species and streptococcal Species. Available dosage form in the hospital: 1G VIAL, 500MG VIAL.
Vancomycin: Class: Antibiotic. Indications: Treatment of patients with infections caused by staphylococcal species and streptococcal Species. Available dosage form in the hospital: 1G VIAL, 500MG VIAL.
Hospital-wide Impact of Mandatory Infectious Disease Consultation on Staphylococcus aureus Septicemia
 Hospital-wide Impact of Mandatory Infectious Disease Consultation on Staphylococcus aureus Septicemia Amanda Guth 1 Amy Slenker MD 1,2 1 Department of Infectious Diseases, Lehigh Valley Health Network
Hospital-wide Impact of Mandatory Infectious Disease Consultation on Staphylococcus aureus Septicemia Amanda Guth 1 Amy Slenker MD 1,2 1 Department of Infectious Diseases, Lehigh Valley Health Network
Tedizolid Phosphate (Sivextro) A Second-Generation Oxazolidinone to Treat Acute Bacterial Skin and Skin Structure Infections
 DrUG FOrECAST Tedizolid Phosphate (Sivextro) A Second-Generation Oxazolidinone to Treat Acute Bacterial Skin and Skin Structure Infections Elaine Wong, PharmD, BCPS; and Saba rab, PharmD, BCPS Dr. Wong
DrUG FOrECAST Tedizolid Phosphate (Sivextro) A Second-Generation Oxazolidinone to Treat Acute Bacterial Skin and Skin Structure Infections Elaine Wong, PharmD, BCPS; and Saba rab, PharmD, BCPS Dr. Wong
Pharmacokinetics of daptomycin in a patient with severe renal failure not under dialysis
 AAC Accepts, published online ahead of print on 1 April 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.00230-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 Pharmacokinetics
AAC Accepts, published online ahead of print on 1 April 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.00230-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 Pharmacokinetics
Enhanced EARS-Net Surveillance 2017 First Half
 1 Enhanced EARS-Net Surveillance 2017 First Half In this report Main results for 2017, first half Breakdown of factors by organism and resistance subtype Device-association Data quality assessment Key
1 Enhanced EARS-Net Surveillance 2017 First Half In this report Main results for 2017, first half Breakdown of factors by organism and resistance subtype Device-association Data quality assessment Key
Flexible Outpatient. Opportunity. Decision: Dalvance
 Flexible Outpatient Opportunity Decision: Dalvance DALVANCE: an opportunity for use at various SITES OF CARE HOME HOME Outpatient Infusion Site ED One 30-minute infusion a week for two weeks provides a
Flexible Outpatient Opportunity Decision: Dalvance DALVANCE: an opportunity for use at various SITES OF CARE HOME HOME Outpatient Infusion Site ED One 30-minute infusion a week for two weeks provides a
Evidence for the use of new and old agents for MRSA infection. Dr Charis Marwick Ninewells Hospital & Medical School Dundee
 Evidence for the use of new and old agents for MRSA infection Dr Charis Marwick Ninewells Hospital & Medical School Dundee 32 year old man Admitted unwell for few days Cough, fever, short of breath, pleuritic
Evidence for the use of new and old agents for MRSA infection Dr Charis Marwick Ninewells Hospital & Medical School Dundee 32 year old man Admitted unwell for few days Cough, fever, short of breath, pleuritic
Daptomycin in Clinical Practice. Paolo Grossi
 Clinica delle Malattie Infettive e Tropicali Università degli Studi dell Insubria Ospedale di Circolo e Fondazione Macchi, Varese Second Opinion Infettivologica Centro Nazionale Trapianti, ISS, Roma Daptomycin
Clinica delle Malattie Infettive e Tropicali Università degli Studi dell Insubria Ospedale di Circolo e Fondazione Macchi, Varese Second Opinion Infettivologica Centro Nazionale Trapianti, ISS, Roma Daptomycin
Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1
 Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1 Fast onset of pain relief with 7% reduction in visual analog scale (VAS) scores
Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1 Fast onset of pain relief with 7% reduction in visual analog scale (VAS) scores
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
MRSA Micro Scan Pos Combo 6J DADE BEHRING VCM
 PKPD MRSA 1 1 2 1 1 2 17 1 26 17 3 16 vancomycinvcm methicillin-resistant Staphylococcus aureusmrsa 31 pharmacokineticpkparameter retrospective VCM 21 10 PK parameter Mann- Whitney U-test Cmax 37.1 µ gml29.942
PKPD MRSA 1 1 2 1 1 2 17 1 26 17 3 16 vancomycinvcm methicillin-resistant Staphylococcus aureusmrsa 31 pharmacokineticpkparameter retrospective VCM 21 10 PK parameter Mann- Whitney U-test Cmax 37.1 µ gml29.942
Setting The setting was secondary care. The economic study was carried out in the USA.
 Cost-effectiveness of IV-to-oral switch therapy: azithromycin vs cefuroxime with or without erythromycin for the treatment of community-acquired pneumonia Paladino J A, Gudgel L D, Forrest A, Niederman
Cost-effectiveness of IV-to-oral switch therapy: azithromycin vs cefuroxime with or without erythromycin for the treatment of community-acquired pneumonia Paladino J A, Gudgel L D, Forrest A, Niederman
Scottish Medicines Consortium
 Scottish Medicines Consortium anidulafungin 100mg powder and solvent for concentrate for solution for infusion (Ecalta ) No. (465/08) Pfizer Ltd 09 May 2008 The Scottish Medicines Consortium (SMC) has
Scottish Medicines Consortium anidulafungin 100mg powder and solvent for concentrate for solution for infusion (Ecalta ) No. (465/08) Pfizer Ltd 09 May 2008 The Scottish Medicines Consortium (SMC) has
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
SYNOPSIS Final Clinical Study Report for Study AI444031
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Name of Active Ingredient: () Individual Study Table Referring to the Dossier (For National Authority Use Only) SYNOPSIS for Study
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Name of Active Ingredient: () Individual Study Table Referring to the Dossier (For National Authority Use Only) SYNOPSIS for Study
Continuous Infusion of Antibiotics In The ICU: What Is Proven? Professor of Medicine Vice-Chairman, Department of Medicine SUNY at Stony Brook
 Continuous Infusion of Antibiotics In The ICU: What Is Proven? Michael S. Niederman, M.D. Chairman, Department of Medicine Winthrop-University Hospital Mineola, NY Professor of Medicine Vice-Chairman,
Continuous Infusion of Antibiotics In The ICU: What Is Proven? Michael S. Niederman, M.D. Chairman, Department of Medicine Winthrop-University Hospital Mineola, NY Professor of Medicine Vice-Chairman,
MorphiDex (MS:DM) Double-Blind, Multiple-Dose Studies In Chronic Pain Patients
 Vol. 19 No. 1(Suppl.) January 2000 Journal of Pain and Symptom Management S37 Proceedings Supplement NMDA-Receptor Antagonists: Evolving Role in Analgesia MorphiDex (MS:DM) Double-Blind, Multiple-Dose
Vol. 19 No. 1(Suppl.) January 2000 Journal of Pain and Symptom Management S37 Proceedings Supplement NMDA-Receptor Antagonists: Evolving Role in Analgesia MorphiDex (MS:DM) Double-Blind, Multiple-Dose
400 mg IV (over 5 to 60 minutes) every 12 hours. > 30 to mg IV (over 5 to 60 minutes ) every 12 hours. 15 to 30
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
Infections Amenable to OPAT. (Nabin Shrestha + Ajay Mathur)
 3 Infections Amenable to OPAT (Nabin Shrestha + Ajay Mathur) Decisions regarding outpatient treatment of infections vary with the institution, the prescribing physician, the individual patient s condition
3 Infections Amenable to OPAT (Nabin Shrestha + Ajay Mathur) Decisions regarding outpatient treatment of infections vary with the institution, the prescribing physician, the individual patient s condition
Methicillin-Resistant Staphylococcus aureus (MRSA) S urveillance Report 2008 Background Methods
 Methicillin-Resistant Staphylococcus aureus (MRSA) Surveillance Report 2008 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Department of Human Services
Methicillin-Resistant Staphylococcus aureus (MRSA) Surveillance Report 2008 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Department of Human Services
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Vfend /Voriconazole
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Vfend /Voriconazole
GSK Medicine Study Number: Title: Rationale Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and nonapproved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and nonapproved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Risk Factors for Failure of OPAT (Outpatient Parenteral Antimicrobial Therapy) in Veterans with Staphylococcus aureus Bacteremia
 Journal of Pharmacy and Pharmacology 4 (2016) 241-247 doi: 10.17265/2328-2150/2016.06.001 D DAVID PUBLISHING Risk Factors for Failure of OPAT (Outpatient Parenteral Antimicrobial Therapy) in Veterans with
Journal of Pharmacy and Pharmacology 4 (2016) 241-247 doi: 10.17265/2328-2150/2016.06.001 D DAVID PUBLISHING Risk Factors for Failure of OPAT (Outpatient Parenteral Antimicrobial Therapy) in Veterans with
Infections In Cirrhotic patients. Dr Abid Suddle Institute of Liver Studies King s College Hospital
 Infections In Cirrhotic patients Dr Abid Suddle Institute of Liver Studies King s College Hospital Infection in cirrhotic patients Leading cause morbidity/mortality Common: 30-40% of hospitalised cirrhotic
Infections In Cirrhotic patients Dr Abid Suddle Institute of Liver Studies King s College Hospital Infection in cirrhotic patients Leading cause morbidity/mortality Common: 30-40% of hospitalised cirrhotic
! Macrolide antibacterial. Fidaxomicin (Dificid ) package labeling. Optimer Pharmaceuticals, Inc. May 2011.
 Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING
 EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING Commentary provided by: Julie Hall, MHS, MT (ASCP) Assistant Dean, College of Health Professions Assistant Professor, Medical Laboratory Science Grand Valley
EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING Commentary provided by: Julie Hall, MHS, MT (ASCP) Assistant Dean, College of Health Professions Assistant Professor, Medical Laboratory Science Grand Valley
Severe β-lactam allergy. Alternative (use for mild-moderate β-lactam allergy) therapy
 Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Recommended Empirical Antibiotic Regimens for MICU Patients Notes: The antibiotic regimens shown are general guidelines and should not replace clinical judgment. Always assess for antibiotic allergies.
Optimizing Antibiotic Therapy in the ICU For Pneumonia Current and Future Approaches
 Optimizing Antibiotic Therapy in the ICU For Pneumonia Current and Future Approaches Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ. Disclosures I have served as a consultant to, researcher/investigator
Optimizing Antibiotic Therapy in the ICU For Pneumonia Current and Future Approaches Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ. Disclosures I have served as a consultant to, researcher/investigator
ISPUB.COM. C Licitra, A Crespo, D Licitra, M Wallis-Crespo INTRODUCTION
 ISPUB.COM The Internet Journal of Infectious Diseases Volume 9 Number 2 Daptomycin for the Treatment of Osteomyelitis and Prosthetic Joint Infection: Retrospective Analysis of C Licitra, A Crespo, D Licitra,
ISPUB.COM The Internet Journal of Infectious Diseases Volume 9 Number 2 Daptomycin for the Treatment of Osteomyelitis and Prosthetic Joint Infection: Retrospective Analysis of C Licitra, A Crespo, D Licitra,
Endocardite infectieuse
 Endocardite infectieuse 1. Raccourcir le traitement: jusqu où? 2. Proposer un traitement ambulatoire: à partir de quand? Endocardite infectieuse A B 90 P = 0.014 20 P = 0.0005 % infective endocarditis
Endocardite infectieuse 1. Raccourcir le traitement: jusqu où? 2. Proposer un traitement ambulatoire: à partir de quand? Endocardite infectieuse A B 90 P = 0.014 20 P = 0.0005 % infective endocarditis
Sponsor / Company: Sanofi Drug substance(s): SSR103371
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Guidance for Industry Complicated Urinary Tract Infections: Developing Drugs for Treatment
 Guidance for Industry Complicated Urinary Tract Infections: Developing Drugs for Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Guidance for Industry Complicated Urinary Tract Infections: Developing Drugs for Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia
 High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia Review Andrew F. Shorr, MD, MPH, FCCP Department of Medicine Pulmonary and Critical Care Medicine, Washington Hospital Center, Washington,
High-Dose Levofloxacin for the Treatment of Community-Acquired Pneumonia Review Andrew F. Shorr, MD, MPH, FCCP Department of Medicine Pulmonary and Critical Care Medicine, Washington Hospital Center, Washington,
New Insights into Peptidoglycan Biosynthesis. Louis B. Rice Warren Alpert Medical School of Brown University Providence, RI
 New Insights into Peptidoglycan Biosynthesis Louis B. Rice Warren Alpert Medical School of Brown University Providence, RI Outline of Presentation Review role of penicillin-binding proteins in cell wall
New Insights into Peptidoglycan Biosynthesis Louis B. Rice Warren Alpert Medical School of Brown University Providence, RI Outline of Presentation Review role of penicillin-binding proteins in cell wall
Product: Talimogene Laherparepvec Clinical Study Report: Date: 31 October 2018 Page 1
 Date: 31 October 2018 Page 1 2. SYNOPSIS Name of Sponsor: Amgen Inc., Thousand Oaks, CA, USA Name of Finished Product: Imlygic Name of Active Ingredient: Talimogene laherparepvec Title of Study: A Phase
Date: 31 October 2018 Page 1 2. SYNOPSIS Name of Sponsor: Amgen Inc., Thousand Oaks, CA, USA Name of Finished Product: Imlygic Name of Active Ingredient: Talimogene laherparepvec Title of Study: A Phase
Sponsor Novartis. Generic Drug Name Pasireotide. Therapeutic Area of Trial Cushing s disease. Protocol Number CSOM230B2208E1
 Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
Community-acquired pneumonia (CAP)
 Community-acquired pneumonia (CAP) Keith F Barker, MD GlaxoSmithKline 1 CAP: Key issues Definitions and patient population Use of prior antibiotics Microbiologically Evaluable population Efficacy endpoints
Community-acquired pneumonia (CAP) Keith F Barker, MD GlaxoSmithKline 1 CAP: Key issues Definitions and patient population Use of prior antibiotics Microbiologically Evaluable population Efficacy endpoints
ICU Volume 11 - Issue 3 - Autumn Series
 ICU Volume 11 - Issue 3 - Autumn 2011 - Series Impact of Pharmacokinetics of Antibiotics in ICU Clinical Practice Introduction The efficacy of a drug is mainly dependent on its ability to achieve an effective
ICU Volume 11 - Issue 3 - Autumn 2011 - Series Impact of Pharmacokinetics of Antibiotics in ICU Clinical Practice Introduction The efficacy of a drug is mainly dependent on its ability to achieve an effective
Cefotaxime Rationale for the EUCAST clinical breakpoints, version th September 2010
 Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
