Tedizolid Phosphate (Sivextro) A Second-Generation Oxazolidinone to Treat Acute Bacterial Skin and Skin Structure Infections
|
|
|
- Dina Willis
- 5 years ago
- Views:
Transcription
1 DrUG FOrECAST Tedizolid Phosphate (Sivextro) A Second-Generation Oxazolidinone to Treat Acute Bacterial Skin and Skin Structure Infections Elaine Wong, PharmD, BCPS; and Saba rab, PharmD, BCPS Dr. Wong is Assistant Professor of Pharmacy Practice at Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Long Island University, Brooklyn, New York. Dr. Rab is Clinical Pharmacist and Academic Supervisor of Pharmacy Students at Maimonides Medical Center, Brooklyn. Drug Forecast is a regular column coordinated by Alan Caspi, PhD, PharmD, MBA, President of Caspi & Associates in New York, New York. Disclosure: The authors report that they have no commercial or financial relationships in regard to this article. INTRODUCTION Between 2001 and 2003, acute bacterial skin and skin structure infections (ABSSSIs) accounted for more than 11 million ambulatory care visits, with a visit rate of per 10,000 persons. 1 As a broader term, ABSSSI encompasses the following (Table 1): Cellulitis/erysipelas 2. Wound infection 3. Major cutaneous abscess This infection is characteristically identified by a measurable lesion (based on edema, redness, and induration) of at least 75 cm 2 with systemic signs of infection and/or lymphadenopathy. 2 4 For major abscesses and wound infections, the area of erythema or induration must also extend 5 cm or more from the peripheral margin of the lesion. 3,4 A common contributing pathogen in this setting is Staphylococcus aureus, particularly methicillin-resistant S. aureus (MRSA). 1,4 Several pharmacological options, including oxazolidinones, are available for use (Table 2). 5,6 In 2000, linezolid (Zyvox, Pfizer) became the first oxazolidinone approved by the Food and Drug Administration (FDA). Its mechanism of action entails inhibition of bacterial protein synthesis by binding 23S ribosomal RNA within the 50S subunit. Indications for use include the management of skin and skin structure infections (complicated and uncomplicated), communityacquired pneumonia, nosocomial pneumonia, and vancomycin-resistant Enterococcus faecium infections. Linezolid is bacteriostatic against staphylococci and enterococci, and bactericidal against streptococci strains. Conveniently dosed at 600 mg twice daily, it can be administered intravenously or orally in a 1:1 ratio. Notable warnings and precautions are serotonin syndrome, myelosuppression, and postmarketing reports of hypoglycemia with concomitant use of antihyperglycemic agents. 6 Since its release, however, there have been reports of MRSA strains resistant to linezolid due to the acquisition of a natural resistance gene known as cfr (chloramphenicol-florfenicol resistance). As a result, Cfr methyltransferase is expressed and alters the 23S rrna component of the ribosomal subunit in bacteria. 7,8 Introducing a unique challenge for the management of multidrugresistant gram-positive organisms, new drug developments have been explored. In June 2014, the FDA approved tedizolid phosphate (Sivextro, Cubist Pharmaceuticals) as a second-generation oxazolidinone with potentially four- to 16-fold potency against MRSA when compared with linezolid Favorable results from clinical trials have led to its indication for the treatment of ABSSSIs in adult patients with susceptible bacteria. 11 This article reviews the information available to date on this product in relation to safety and efficacy. CHEMICAL STRUCTURE The full chemical name of tedizolid is (5R)-3-{3-fluoro-4-[6-(2-methyl-2H- tetrazol-5-yl)pyridine-3-yl]phenyl}- 5-(hydroxymethyl)-1,3-oxazolidin-2-one. 9 Figure 1 illustrates the chemical structures Table 1 Characteristics of ABSSSIs Cellulitis/ erysipelas Wound infection Cutaneous abscess Tedizolid phosphate Linezolid Figure 1 Chemical Structures 6,11 of tedizolid phosphate and linezolid. CLINICAL PHARMACOLOGY Tedizolid phosphate is a prodrug that is converted by plasma phosphatases to microbiologically active tedizolid in vivo. It interacts with the bacterial 23S ribosome initiation complex and binds to the 50S subunit to prevent the formation of the 70S complex. As a result, tedizolid inhibits bacterial translation and protein synthesis. 9,10 MICROBIOLOGICAL ACTIVITY Tedizolid has activity against clinically relevant gram-positive pathogens such as MRSA, methicillin-susceptible S. aureus (MSSA), Streptococcus pyogenes, Streptococcus agalactiae, enterococci, and coagulase-negative staphylococci (Table 3). In addition, this drug has demonstrated activity against linezolid-resistant staphylococci (Table 4) Specific to S. aureus strains in vitro, tedizolid had a minimum inhibitory concentration (MIC) range of to 0.5 mcg/ml. Diffuse skin infection characterized by spreading of edema, redness, and heat 2,5 May accompany lymphangitis and regional lymph node inflammation 5 Erysipelas may be differentiated with raised skin lesions and clear demarcation line of affected and unaffected areas 5 Purulent drainage with edema, redness, and/or induration of the surrounding wound 2 Involves the dermis and deeper skin tissues in the presence of pus collections 2,5 Vol. 39 No. 8 August 2014 P&T 555
2 Table 2 Examples of Antibacterial Drugs Used Against MRSA 5 Class Drug Beta-lactam Ceftaroline Folate antagonist Trimethoprim-sulfamethoxazole Glycolipopeptide Telavancin Glycopeptide Vancomycin Lincosamide Clindamycin Lipopeptide Daptomycin Oxazolidinone Linezolid Streptogramin Quinupristin-dalfopristin Tetracycline Minocycline Table 3 Spectrum of Activity for Tedizolid 11 Aerobic and facultative Staphylococcus aureus (MRSA and MSSA) gram-positive bacteria Streptococcus pyogenes Streptococcus agalactiae Streptococcus anginosus group Enterococcus faecalis Aerobic and facultative anaerobic gram-positive bacteria Further evaluation showed that 95% of MRSA isolates and 72% of MSSA isolates had a MIC of 0.25 mcg/ml or less. 12 For penicillin-resistant streptococcus pneumoniae, in vitro susceptibility results showed a MIC of to 0.25 mcg/ml and MIC 50 (the MIC at which 50% of the isolates were inhibited) of 0.25 mcg/ml. When considering MIC 90 (the MIC at which 90% of the isolates were inhibited), tedizolid was four times more potent than linezolid, with values of 0.25 mcg/ml and 1 mcg/ml, respectively. 14 PHARMACODYNAMICS AND PHARMACOKINETICS Using a neutropenic murine S. aureus pneumonia model, the mean free drug area under the curve (fauc) over 24 hours divided by the MIC, or fauc/mic ratio, associated with a bacteriostatic endpoint was comparable between tedizolid and linezolid: 20 versus 19, respectively. The average fauc/mic ratio for a bactericidal or 1-log unit kill endpoint was about two-fold higher when compared with bacteriostatic endpoints, with no difference between treatment groups (tedizolid, 34.6, and linezolid, 46.1, respectively; P = 0.334). 15 Moreover, tedizolid has been shown to be bacteriostatic Staphylococcus epidemidis Staphylococcus haemolyticus Staphylococcus lugdenesis Enterococcus faecium at 24 hours and bactericidal by day 3 of treatment in S. aureus isolates. 16 A prospective, open-label, multiple-dose study evaluated the pharmaco kinetics of tedizolid phosphate 200 mg by mouth (PO) once daily for three days. A total of 20 healthy adult patients completed the study, with an average age of 28 ± 9 years and weight of 82.4 ± 12.5 kg. There was no difference in drug concentrations obtained immediately prior to the third dose and at 24 hours (P = 0.947), indicating steady-state concentrations. Plasma pharmacokinetic data obtained from bronchoalveolar samples on day 3 revealed a maximum drug concentration (C max ) of 2.4 ± 0.4 mcg/ml, time to C max (T max ) of two hours (range: 0.5 to 4 hours), terminal half-life (t ½ ) of 9.2 ± 2 hours, and protein binding of ± 1.58%. This study also described higher concentrations in the epithelial lining fluid and alveolar macrophages compared to free plasma concentrations, suggesting extensive penetration into both extracellular and intracellular pulmonary compartments. 17 Sahre et al. evaluated a single 600-mg oral dose of tedizolid phosphate in 12 healthy volunteers. Pharmacokinetic analysis of drug in plasma reported a C max of 5.4 ± 1.5 mg/l, T max of 2.4 ± 1.1 hours, t ½ of 8.1 hours (range: 5.9 to 12.8 hours), and protein binding of 87.3 ± 1.3%. It was also found that tedizolid freely distributes into adipose and muscle tissue following the single dose, with similar levels detected in free plasma concentrations. 18 Active tedizolid had a mean total clearance (CL) of 8.28 L/hr and distribution clearance (CLd) of 2.95 L/hr. A central compartment volume (V1) of 71.4 L and peripheral compartment volume (V2) of 27.9 L were reported. 10 In summary, according to the prescribing information, peak plasma concentrations are attained within approximately three hours after oral tedizolid therapy and one hour following a tedizolid infusion. The absolute bioavailability is approx imately 91% for both formulations, with a reported 70% to 90% plasma protein binding capacity. The volume of distribution at steady state for a single intravenous (IV) dose is on average 67 to 80 L. Tedizolid seems to be an unlikely substrate of the CYP450 enzymes. However, a majority of the compound is hepatically eliminated. 11 Table 5 compares the pharmacokinetic parameters of tedizolid phosphate and linezolid. PIVOTAL CLINICAL TRIALS Phase 2 Trial Prokocimer et al. conducted a randomized, double-blind, dose-ranging study involving tedizolid phosphate at 12 U.S. sites. Eligible patients had to be 18 to 75 years old with complicated skin and skin structure infection (csssi) and a suspected or confirmed gram-positive pathogen. Types of infections included were deep extensive cellulitis, abscesses, and surgical or post-traumatic wounds. 10 Table 4 Comparison of Tedizolid vs. Linezolid-Resistant Staphylococci 13 Coagulase-negative staphylococci Staphylococcus aureus MIC Range (mg/l) Resistant Strains Tedizolid 0.06 to 16 Linezolid 8 to > Tedizolid 0.5 Linezolid 8 to P&T August 2014 Vol. 39 No. 8
3 The main difference between csssi and ABSSSI is a distinguishing factor of lesion size present with the latter. Patients were randomized in a 1:1:1 ratio to receive 200, 300, or 400 mg of oral tedizolid phosphate daily for five to seven days. The primary objective was clinical response rates in each group at the test-of-cure visit in the clinically evaluable (CE) and clinical modified-intent-to-treat (cmitt) populations. A total of 188 patients were included in the modified intent-totreat (MITT) and cmitt analysis (200-mg group, n = 63; 300-mg group, n = 63; 400-mg group, n=62). The most common pathogen isolated was S. aureus (90.3%), with a majority identified as MRSA strains (80.6%). Patients were treated for an average of 6.4 days. The clinical response rates were similar in all dosing arms: MITT, 87.8%; CE, 95.7%; microbiologically evaluable, 96.2%. Data trends were also similar irrespective of lesion type or size and infection severity. 10 Table 5 Mean (Standard Deviation) Pharmacokinetic Parameters Tedizolid Phosphate 11 (200 mg PO once daily) C max (mg/l) 2.2 ± ± 5.78 T max (hours) 3.5 (1.0 to 6.0) 1.03 ± 0.62 T ½ (hours) ± 2.06 Clearance (L/hr) 8.4 ± ± 1.74 AUC (mcg*hr/ml) 25.6 ± ± 42.1 Median (range) Phase 3 Trials ESTABLISH-1 The efficacy and safety of tedizolid phosphate versus linezolid in ABSSSI were evaluated in a multicenter, randomized, double-blind, double-dummy, noninferiority, phase 3 trial. Patients had to be 18 years of age or older with cellulitis/erysipelas, major cutaneous abscess, or wound infection. Along with erythema and a total lesion size of at least 75 cm 2, patients also had to have at least one local, regional, or systemic sign of infection and a documented or suspected gram-positive bacterium. Patients were excluded if they had an uncomplicated ABSSSI, a vascular catheter site associated ABSSSI, thrombophlebitis, or surgery other than clean surgery; if they received any other topical or systemic antibiotics with similar spectrum of activity within 96 hours of the first dose of study drug; or if they previously failed therapy at the same infection site. Eligible patients were randomized on a 1:1 basis to receive either tedizolid phosphate 200 mg PO once daily for six days or linezolid 600 mg PO twice daily for 10 days. The primary endpoint was an early clinical response at the 48- to 72-hour assessment in the intent-to-treat (ITT) analysis group. A treatment responder was defined as a patient who was afebrile; had no growth in lesion area, width, or length from baseline; did not receive other antimicrobial therapy identified by the exclusion criteria; and did not die of any cause. 12 A total of 667 patients were randomized to tedizolid phosphate (n = 332) or linezolid (n = 335). The median age was 43 years (range: 18 to 100 years). A majority of patients had cellulitis/erysipelas (41.1%) versus major cutaneous abscess (29.7%) or wound infections (29.25%). S. aureus was the most common pathogen detected, with MRSA isolated in both tedizolid phosphate (42.1%) and linezolid (43.1%) groups. Treatment response rates were similar at the 48- to 72-hour assessment in those taking tedizolid phosphate (79.5%; 95% confidence interval [CI], 74.8 to 83.7%) and linezolid (79.4%; 95% CI, 74.7 to 83.6%). Non inferiority was achieved with an absolute treatment difference of 0.1% (95% CI, 6.1 to 6.2%). Sustained clinical success rates at the end of treatment using the ITT data were comparable between the tedizolid phosphate (69.3%; 95% CI, 64 to 74.2%) and linezolid (79.1%; 95% CI, 66.8 to 76.7%) groups (abso lute treatment difference, 2.6%; 95% CI, 9.6 to 4.2%). Similar trends were noted in clinical responses assessed by investigators. 12 ESTABLISH-2 A subsequent randomized, doubleblind, parallel-group, noninferiority phase 3 trial evaluated the use of IV tedizolid phosphate or linezolid with an option to switch to oral therapy. Patients had to be 12 years of age or older with a minimum lesion area of 75 cm 2, a documented or suspected gram-positive infection, and at least one regional or systemic sign of infection. The margin of abscess or wound, if present, had to extend 5 cm or more from the abscess and wound, induration, erythema, or edema. Exclusion criteria were analogous to the ESTABLISH-1 trial. Eligible patients received randomly, in a 1:1 ratio, IV tedizolid phosphate 200 mg once daily for Linezolid 6 (600 mg PO twice daily) six days or IV linezolid 600 mg twice daily for 10 days. Patients who received at least two doses of therapy were eligible to switch to oral therapy if they met at least two of the following criteria: no increase in lesion size compared with baseline, temperature of 37.7 C or less, or absence of worsening signs and symptoms of the affected area. Additional therapy to broaden coverage for gram-negative or anaerobic bacteria was permitted for wound infections at the discretion of the investigator. The primary outcome was early clinical response 48 to 72 hours following the initiation of treatment. Treatment responders were defined as patients who had a 20% or more reduction in the primary lesion from baseline, did not receive any other systemic antibiotic therapy with overlapping grampositive activity, and did not die from any cause within 72 hours of receiving the first dose of study drug. 19 A total of 666 patients received either tedizolid phosphate (n = 332) or linezolid (n = 334). There was no difference in baseline characteristics between the groups. The median age of patients receiving tedizolid phosphate was 46 years (range: 17 to 86). Cellulitis or erysipelas accounted for a majority of the ABSSSIs (tedizolid phosphate, n = 166, 50%; linezolid, n = 168, 50%). Other reported infections included major cutaneous abscess (tedizolid phosphate, n = 68, 20%; linezolid, n = 68, 20%) and infected wounds (tedizolid phosphate, n = 98, 30%; and linezolid, n = 98, 29%). Approximately 59.5% of the study population had at least one gram-positive organism identified at baseline (tedizolid phosphate, n = 197, 59%; linezolid, n = 202, 60%). At 81.5%, S. aureus was the most common organism identified (tedizolid phosphate, n = 158, 80%; linezolid, n = 167, 83%), with 27.5% being MRSA isolates and 54% MSSA isolates. Other gram-positive bacteria identified were beta-hemolytic streptococci, Vol. 39 No. 8 August 2014 P&T 557
4 Streptococcus agninosus group, and Enterococcus faecalis. The mean time to switch to oral therapy was similar between groups (tedizolid phosphate, 1.7 ± 1.18 days; linezolid, 1.8 ± 1.35 days, P = 0.99). There were also no statistically significant differences in the mean durations of IV treatment, which for the U.S. were 2.2 ± 2.17 days and 2.0 ± 2.03 days with tedizolid phosphate and linezolid, respectively. However, there was a disparity between patients managed outside the U.S., suggesting different clinical practices overseas. Only 18% of patients received IV study drug for the entire treatment duration. Early clinical response at 48 to 72 hours was achieved in 85% (n = 283) of patients receiving tedizolid phosphate and 83% (n = 276) of patients receiving linezolid therapy. Thus, noninferiority was attained with an absolute difference of 2.6% (95% CI, 3.0 to 8.2). 19 SAFETY PROFILE Adverse Events Tedizolid phosphate was well tolerated following the oral administration of a oncedaily 200-mg dose for three days. No serious adverse events (AEs) were reported; the most commonly reported AEs were mild bradycardia (n = 2), headache (n = 1), and nausea (n = 1). 17 In the ESTABLISH-1 trial, the most common AEs associated with tedizolid phosphate were gastrointestinal in nature (nausea, vomiting, and diarrhea), although they occurred at a lesser incidence than with linezolid (15.7% for tedizolid phosphate vs. 24.8% for linezolid). Headaches were also reported (6.3% for tedizolid phosphate vs. 5.1% for linezolid). The rates of serious AEs were low in both arms. However, alanine aminotransferase elevations greater than or equal to twice the baseline or upper limit of normal were noted in 24 patients (4.1% for tedizolid phosphate vs. 3.5% for linezolid). Patients continued therapy regardless of this abnormality and there were no implications of hepatic dysfunction or toxicity. Other laboratory data revealed that patients who received tedizolid phosphate (2.3%) had a lower incidence of abnormal platelet counts compared with linezolid (4.9%). Platelet counts normalized without medical intervention. 12 Gastrointestinal effects (nausea, vomiting, and diarrhea) and headaches were also seen with tedizolid phosphate in the ESTABLISH-2 trial; however, there were no statistically significant differences 558 P&T August 2014 Vol. 39 No. 8 when compared with linezolid. Nine percent of patients in the tedizolid phosphate group and 13% of patients in the linezolid group had a platelet count below the lower limit of normal (less than 150 x 109/L; P = 0.071). A smaller percentage of patients had an absolute neutrophil count below the lower limit of normal (less than 1.6 x 109/L) with tedizolid phosphate (nine of 305 patients, 3%) compared with linezolid (21 of 299 patients, 7%; P = 0.024). 19 Warnings and Precautions Due to a lack of efficacy and safety data in the setting of neutropenia, clinicians should consider alternative therapies when managing a patient with neutropenia and ABSSSIs. 11 Special Populations Tedizolid phosphate is classified as pregnancy category C. Animal data have shown the potential to induce toxic effects on fetal development. In rats, tedizolid was excreted in breast milk. 11 DOSAGE AND ADMINISTRATION Based on the findings of the doseranging study conducted by Prokocimer et al., the recommended dose for tedizolid phosphate in adult patients (18 years of age or older) is 200 mg once daily either orally or intravenously for six days. Dosage adjustments are not necessary for patients with hepatic impairment, renal impairment, or undergoing hemodialysis. 10,11 The IV solution is prepared from a sterile, lyophilized powder, single-use 200-mg vial. It must be reconstituted with 0.9% sodium chloride injection, USP, and administered as an infusion over one hour; it is not to be given as an IV push or bolus. 11 DRUG DRUG AND DRUG FOOD INTERACTIONS Tedizolid (200 mg daily) has a 50% inhibitory concentration (IC 50 ) of 8.7 µm and 5.7 µm for monoamine oxidase (MAO)-A and MAO-B, respectively. Linezolid (600 mg twice daily), on the other hand, seemed to have a higher overall IC 50 for these enzymes (MAO-A = 46 µm, MAO- B = 2.1 µm). These data show that tedizolid is a seemingly weak, reversible inhibitor of MAO in vitro. 20 A tyramine sensitivity study was conducted in 30 healthy patients to determine the concentration of tyramine needed to increase systolic blood pressure by 30 mmhg or greater (TYR 30 ). As a result, a geometric mean tyramine sensitivity ratio (placebo TYR 30 divided by tedizolid phosphate TYR 30 ) of 1.33 was identified (90% CI, 1.05 to 1.69). Of note, a ratio of two or more was deemed to be clinically relevant. Although tedizolid phosphate was generally well tolerated, at least 25 of 30 patients experienced palpitations, a known AE in tyramine-based studies. This study also concluded that 550 mg or more of tyramine is needed to observe a 30 mmhg increase in systolic blood pressure with tedizolid phosphate. It is therefore unlikely to see clinically relevant pressor effects in patients taking a tyramine-rich meal while receiving tedizolid phosphate therapy. 20 Tedizolid phosphate also does not seem to have meaningful effects on blood pressure when coadministered with pseudoephedrine. 20 However, as the data are still scarce, further studies are needed to determine tedizolid phosphate s potential for other adrenergic and serotonergic interactions observed with linezolid. COST AND AVAILABILITY Tedizolid phosphate is available as 200-mg tablets in a unit dose blister pack of six tablets or a bottle of 30 tablets. It is also available as a 200-mg single-use vial for injection. The average wholesale prices for six days of oral versus IV therapy are $2,212 and $1,692, respectively. 21 P&T COMMITTEE CONSIDERATIONS Tedizolid phosphate has demonstrated good activity against clinically relevant gram-positive organisms such as MRSA. Potential advantages with the available data include enhanced potency, longer half-life, weak and reversible inhibition of MAO enzymes, and an improved safety profile when compared to linezolid. Although several agents with a similar spectrum of activity are currently available (Table 2), tedizolid phosphate may have a role in treating infections involving antimicrobial resistance. Additional efficacy and safety data are needed before P&T committees can delineate its specific role in clinical practice and make a formulary decision. CONCLUSION Tedizolid phosphate is a novel secondgeneration oxazolidinone with activity against clinically relevant gram-positive organisms. It has recently been approved continued on page 579
5 continued from page 558 for the management of ABSSSIs in adults. Additional data are needed on the safety of this therapy when used in the setting of neutropenia and on coadministration with serotonergic agents. As studies continue, it will also be interesting to see the effectiveness of tedizolid phosphate in other indications such as nosocomial pneumonias (NCT ). 22 REFERENCES 1. McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureusassociated skin and soft tissue infections in ambulatory care. Emerg Infect Dis 2006;12(11): Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment. Available at: fda.gov/downloads/drugs/.../guidances/ ucm pdf. Accessed February 4, Itani KM, Shorr AF. FDA guidance for ABSSSI trials: implications for conducting and interpreting clinical trials. Clin Infect Dis 2014;58(S1):S Corey GR, Stryjewski ME. New rules for clinical trials of patients with acute bacterial skin and skin-structure infections: do not let the perfect be the enemy of the good. Clin Infect Dis 2011;52(S7):S469 S Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41: Zyvox (linezolid) prescribing information. New York, New York; Pharmacia & Upjohn Co.; Available at: Accessed February 4, Toh S-M, Xiong L, Arias CA, et al. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol Microbiol 2007;64(6): Morales G, Picazo JJ, Baos E, et al. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolidresistant Staphylococcus aureus. Clin Infect Dis 2010;50(6): Kanafani Z, Corey GR. Tedizolid (TR-701): a new oxazolidinone with enhanced potency. Expert Opin Investig Drugs 2012;21(4): Prokocimer P, Bien P, Surber J, et al. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother 2011;55(2): Sivextro (tedizolid phosphate) prescribing information. Lexington, Massachusetts; Cubist Pharmaceuticals U.S.; Available at: Accessed July 9, Prokocimer P, De Anda C, Fang E, et al. Tedizolid phosphate vs. linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA. 2013;306(6) Rodriguez-Avial I, Culebras E, Betriu C, et al. In vitro activity of tedizolid (TR-700) against linezolid-resistant staphylococci. J Antimicrob Chemother 2012;67(1): Choi S, Im W, Bartizal K. Activity of tedizolid phosphate (TR-701) in murine models of infection with penicillin-resistant and penicillin-sensitive Streptococcus pneumoniae. Antimicrob Agents Chemother 2012;56(9): Lepak AJ, Marchillo K, Pichereau S, et al. Comparative pharmacodynamics of the new oxazolidinone tedizolid phosphate and linezolid in a neutropenic murine Staphylococcus aureus pneumonia model. Antimicrob Agents Chemother 2012;56(11): Keel RA, Tessier PR, Crandon JL, Nicolau DP. Comparative efficacies of human simulated exposures of tedizolid and linezolid against Staphylococcus aureus in the murine thigh infection model. Antimicrob Agents Chemother 2012;56(8): Housman ST, Pope JS, Russomanno J, et al. Pulmonary disposition of tedizolid following administration of once-daily oral 200-milligram tedizolid phosphate in healthy adult volunteers. Antimicrob Agents Chemother 2012;56(5): Sahre M, Sabarinath S, Grant M, et al. Skin and soft tissue concentrations of tedizolid (formerly torezolid), a novel oxazolidinone, following a single oral dose in healthy volunteers. Int J Antimicrob Agents. 2012;40(1): Moran GJ, Fang E, Corey GR, et al. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomized, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis doi: /S (14) [Epub ahead of print]. 20. Flanagan S, Bartizal K, Minassian SL, et al. In vitro, in vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. Antimicrob Agents Chemother 2013;57(7): Red book Online. Ann Arbor, Michigan: Truven Health Analytics. Accessed July 14, ClinicalTrials.gov. TR-701 FA vs linezolid for the treatment of nosocomial pneumonia [July 13, 2014]. Available at: NCT ?term=tr-701&rank=7. Accessed July 14, n Vol. 39 No. 8 August 2014 P&T 579
ESCMID Online Lecture Library. by author
 Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
Options for Complicated Skin and Skin Structure Infections. Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ.
 Options for Complicated Skin and Skin Structure Infections Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ. Disclosures I have served as a consultant to, researcher/investigator for,
Options for Complicated Skin and Skin Structure Infections Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ. Disclosures I have served as a consultant to, researcher/investigator for,
Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens to tedizolid, a novel oxazolidinone
 Zurenko et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:46 RESEARCH Open Access Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens
Zurenko et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:46 RESEARCH Open Access Use of linezolid susceptibility test results as a surrogate for the susceptibility of Gram-positive pathogens
SIVEXTRO (tedizolid phosphate) for injection, for intravenous use SIVEXTRO (tedizolid phosphate) tablet, for oral use Initial U.S.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SIVEXTRO safely and effectively. See full prescribing information for SIVEXTRO. SIVEXTRO (tedizolid
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SIVEXTRO safely and effectively. See full prescribing information for SIVEXTRO. SIVEXTRO (tedizolid
Tedizolid Phosphate: a Next-Generation Oxazolidinone
 Infect Dis Ther (2015) 4:1 14 DOI 10.1007/s40121-015-0060-3 REVIEW Tedizolid Phosphate: a Next-Generation Oxazolidinone Jeffrey M. Rybak Karrine Roberts To view enhanced content go to www.infectiousdiseases-open.com
Infect Dis Ther (2015) 4:1 14 DOI 10.1007/s40121-015-0060-3 REVIEW Tedizolid Phosphate: a Next-Generation Oxazolidinone Jeffrey M. Rybak Karrine Roberts To view enhanced content go to www.infectiousdiseases-open.com
Resistance to new anti-grampositive. Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France
 Resistance to new anti-grampositive agents Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France Recently available antimicrobials against MDR Gram-positive infections Cyclic lipopeptide: daptomycin
Resistance to new anti-grampositive agents Roland Leclercq, Microbiology, CHU Cote de Nacre, Caen, France Recently available antimicrobials against MDR Gram-positive infections Cyclic lipopeptide: daptomycin
OBJECTIVES DESIGN SETTING PARTICIPANTS INTERVENTION MEASUREMENTS AND MAIN RESULTS
 Pharmacokinetics of Tedizolid Following Oral Administration: Single and Multiple Dose, Effect of Food, and Comparison of Two Solid Forms of the Prodrug Shawn D. Flanagan, 1, * Paul A. Bien, 1 Kelly A.
Pharmacokinetics of Tedizolid Following Oral Administration: Single and Multiple Dose, Effect of Food, and Comparison of Two Solid Forms of the Prodrug Shawn D. Flanagan, 1, * Paul A. Bien, 1 Kelly A.
ALTARGO TM Retapamulin. QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w)
 ALTARGO TM Retapamulin QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) PHARMACEUTICAL FORM Ointment. CLINICAL PARTICULARS Indications ALTARGO (retapamulin) 1% ointment is indicated
ALTARGO TM Retapamulin QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) PHARMACEUTICAL FORM Ointment. CLINICAL PARTICULARS Indications ALTARGO (retapamulin) 1% ointment is indicated
Paul McGovern, MD Vice President, Clinical Affairs Paratek Pharmaceuticals, Inc. Presented at ECCMID, 22 April 2018, Madrid, Spain.
 A phase-3 randomized, double-blind, multicentre study to compare the safety and efficacy of oral omadacycline to oral linezolid for treating adult subjects with ABSSSI (OASIS-2 study) Paul McGovern, MD
A phase-3 randomized, double-blind, multicentre study to compare the safety and efficacy of oral omadacycline to oral linezolid for treating adult subjects with ABSSSI (OASIS-2 study) Paul McGovern, MD
ANTIMICROBIALS AVAILABLE FOR
 ORIGINAL CONTRIBUTION Tedizolid Phosphate vs Linezolid for Treatment of Acute Bacterial Skin and Skin Structure Infections The ESTABLISH-1 Randomized Trial Philippe Prokocimer, MD Carisa De Anda, PharmD
ORIGINAL CONTRIBUTION Tedizolid Phosphate vs Linezolid for Treatment of Acute Bacterial Skin and Skin Structure Infections The ESTABLISH-1 Randomized Trial Philippe Prokocimer, MD Carisa De Anda, PharmD
Resistance to linezolid in enterococci and staphylococci referred to the national reference laboratory
 Resistance to linezolid in enterococci and staphylococci referred to the national reference laboratory Dr Danièle Meunier, AMRHAI BSAC - Antimicrobial Susceptibility Testing User Days Oxazolidinone Linezolid
Resistance to linezolid in enterococci and staphylococci referred to the national reference laboratory Dr Danièle Meunier, AMRHAI BSAC - Antimicrobial Susceptibility Testing User Days Oxazolidinone Linezolid
Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia
 Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia Brandon Dionne, PharmD, BCPS, AAHIVP Assistant Clinical Professor Northeastern University Seth Housman, PharmD, MPA Clinical Assistant
Superhero or Superzero? Vancomycin vs. Linezolid for MRSA Pneumonia Brandon Dionne, PharmD, BCPS, AAHIVP Assistant Clinical Professor Northeastern University Seth Housman, PharmD, MPA Clinical Assistant
Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects
 Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects Karolyn S. Horn, Mark H. Gotfried, Judith N. Steenbergen,
Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects Karolyn S. Horn, Mark H. Gotfried, Judith N. Steenbergen,
Linezolid - Tigecycline
 Linezolid - Tigecycline Paul M. Tulkens, MD, PhD Cellular and Molecular Pharmacology Louvain Drug Research Institute Catholic University of Louvain, Brussels, Belgium With the support of Wallonie-Bruxelles-International
Linezolid - Tigecycline Paul M. Tulkens, MD, PhD Cellular and Molecular Pharmacology Louvain Drug Research Institute Catholic University of Louvain, Brussels, Belgium With the support of Wallonie-Bruxelles-International
Cubicin A Guide to Dosing
 Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections
Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections
Open Forum Infectious Diseases MAJOR ARTICLE
 Open Forum Infectious Diseases MAJOR ARTICLE Efficacy and Safety of Oritavancin Relative to Vancomycin for Patients with Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in the Outpatient Setting:
Open Forum Infectious Diseases MAJOR ARTICLE Efficacy and Safety of Oritavancin Relative to Vancomycin for Patients with Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in the Outpatient Setting:
ESTABLISH 2 Top Line Data Release
 The Future of Anti-Infectives ESTABLISH 2 Top Line Data Release March 25, 2013 1 Forward Looking Statements Statements contained in this data release regarding matters that are not historical facts are
The Future of Anti-Infectives ESTABLISH 2 Top Line Data Release March 25, 2013 1 Forward Looking Statements Statements contained in this data release regarding matters that are not historical facts are
! Macrolide antibacterial. Fidaxomicin (Dificid ) package labeling. Optimer Pharmaceuticals, Inc. May 2011.
 Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
Disclosure! I have no conflicts of interest related to this presentation Nina Naeger Murphy, Pharm.D., BCPS Clinical Pharmacy Specialist Infectious Diseases MetroHealth Medical Center Learning Objectives!
Clinical Pharmacokinetics and Pharmacodynamics of Telavancin Compared with the Other Glycopeptides
 Clin Pharmacokinet (2018) 57:797 816 https://doi.org/10.1007/s40262-017-0623-4 REVIEW ARTICLE Clinical Pharmacokinetics and Pharmacodynamics of Telavancin Compared with the Other Glycopeptides Valentin
Clin Pharmacokinet (2018) 57:797 816 https://doi.org/10.1007/s40262-017-0623-4 REVIEW ARTICLE Clinical Pharmacokinetics and Pharmacodynamics of Telavancin Compared with the Other Glycopeptides Valentin
Disclosure. Patient Case. Objectives. Patient Case. Patient Case 7/25/2015. An update on the treatment of skin and soft tissue infections
 Disclosure 49th Annual Meeting An update on the treatment of skin and soft tissue infections I do not have a vested interest in or affiliation with any corporate organization offering financial support
Disclosure 49th Annual Meeting An update on the treatment of skin and soft tissue infections I do not have a vested interest in or affiliation with any corporate organization offering financial support
WARNING: FETAL RISK See full prescribing information for complete boxed warning.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VIBATIV safely and effectively. See full prescribing information for VIBATIV. VIBATIV (telavancin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VIBATIV safely and effectively. See full prescribing information for VIBATIV. VIBATIV (telavancin)
Daptomycin in Clinical Practice. Paolo Grossi
 Clinica delle Malattie Infettive e Tropicali Università degli Studi dell Insubria Ospedale di Circolo e Fondazione Macchi, Varese Second Opinion Infettivologica Centro Nazionale Trapianti, ISS, Roma Daptomycin
Clinica delle Malattie Infettive e Tropicali Università degli Studi dell Insubria Ospedale di Circolo e Fondazione Macchi, Varese Second Opinion Infettivologica Centro Nazionale Trapianti, ISS, Roma Daptomycin
ALTARGO. Retapamulin. Retapamulin 10 mg per gram (1% w/w)
 ALTARGO Retapamulin 1. QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) 2. PHARMACEUTICAL FORM Ointment 3. CLINICAL PARTICULARS 3.1 Indications Retapamulin is indicated for
ALTARGO Retapamulin 1. QUALITATIVE AND QUANTITATIVE COMPOSITION Retapamulin 10 mg per gram (1% w/w) 2. PHARMACEUTICAL FORM Ointment 3. CLINICAL PARTICULARS 3.1 Indications Retapamulin is indicated for
Received 30 March 2005; returned 16 June 2005; revised 8 September 2005; accepted 12 September 2005
 Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
AXITAB-CV TAB. COMPOSITION :
 AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger
 Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger Cubicin (Daptomycin): A new generation of antibiotics First-in-class cyclic natural lipopeptide Activity against major gram positive pathogens
Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger Cubicin (Daptomycin): A new generation of antibiotics First-in-class cyclic natural lipopeptide Activity against major gram positive pathogens
life-threatening infections
 Vancomycin Vancomycin has become increasingly important in the treatment of life-threatening infections. MRSA infections. Methicillin-resistant Staphylococcus epidermidis (MRSE) infections Enterococcal
Vancomycin Vancomycin has become increasingly important in the treatment of life-threatening infections. MRSA infections. Methicillin-resistant Staphylococcus epidermidis (MRSE) infections Enterococcal
Pharmacologyonline 1: (2010) ewsletter Singh and Kochbar. Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of
 Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of Cefoperazone Sulbactam Singh M*, Kochhar P* Medical & Research Division, Pfizer India. Summary Antimicrobial resistance is associated with
Optimizing Pharmacokinetic/Pharmacodynamics Principles & Role of Cefoperazone Sulbactam Singh M*, Kochhar P* Medical & Research Division, Pfizer India. Summary Antimicrobial resistance is associated with
Mupirocin Rationale for the EUCAST clinical breakpoints, version th July 2010
 Mupirocin Rationale for the EUCAST clinical breakpoints, version 1.0 6 th July 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Mupirocin Rationale for the EUCAST clinical breakpoints, version 1.0 6 th July 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
profiles Decision: Dalvance
 Patient candidate profiles Decision: Dalvance 2 A series of patient candidate profiles The following patient candidate profiles are hypothetical examples of treatment decisions that could be seen in the
Patient candidate profiles Decision: Dalvance 2 A series of patient candidate profiles The following patient candidate profiles are hypothetical examples of treatment decisions that could be seen in the
Cellceutix Corporation Beverly, MA USA Abstract 2969; Presentation 0195; Hall J, 4:00pm. April 27, 2015
 ECCMID 2015 Copenhagen, Denmark 25 28 April 2015 Cellceutix Corporation Beverly, MA USA www.cellceutix.com A Randomized, Double-Blind Study Comparing Single-Dose and Short-Course Brilacidin to Daptomycin
ECCMID 2015 Copenhagen, Denmark 25 28 April 2015 Cellceutix Corporation Beverly, MA USA www.cellceutix.com A Randomized, Double-Blind Study Comparing Single-Dose and Short-Course Brilacidin to Daptomycin
ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication
 ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication ICD-10-CM Diagnosis Codes Diagnosis INDICATION ORBACTIV (oritavancin) for injection is indicated for the treatment of adult patients
ICD-10 diagnostic codes consistent with ORBACTIV (oritavancin) indication ICD-10-CM Diagnosis Codes Diagnosis INDICATION ORBACTIV (oritavancin) for injection is indicated for the treatment of adult patients
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
Continuous Infusion of Antibiotics In The ICU: What Is Proven? Professor of Medicine Vice-Chairman, Department of Medicine SUNY at Stony Brook
 Continuous Infusion of Antibiotics In The ICU: What Is Proven? Michael S. Niederman, M.D. Chairman, Department of Medicine Winthrop-University Hospital Mineola, NY Professor of Medicine Vice-Chairman,
Continuous Infusion of Antibiotics In The ICU: What Is Proven? Michael S. Niederman, M.D. Chairman, Department of Medicine Winthrop-University Hospital Mineola, NY Professor of Medicine Vice-Chairman,
Macrolides & Ketolides. Objectives. Protein Synthesis
 Macrolides & Ketolides Elizabeth D. Hermsen, Pharm.D. Infectious Diseases Research Fellow University of Minnesota College of Pharmacy bjectives Participant should be able to explain macrolide/ketolide
Macrolides & Ketolides Elizabeth D. Hermsen, Pharm.D. Infectious Diseases Research Fellow University of Minnesota College of Pharmacy bjectives Participant should be able to explain macrolide/ketolide
Other β-lactam. A. Carbapenems:
 A. Carbapenems: Other β-lactam Carbapenems are synthetic β-lactam antibiotics Differ in structure from the penicillins in that the sulfur atom of the thiazolidine ring. Imipenem, meropenem, doripenem,
A. Carbapenems: Other β-lactam Carbapenems are synthetic β-lactam antibiotics Differ in structure from the penicillins in that the sulfur atom of the thiazolidine ring. Imipenem, meropenem, doripenem,
Initial U.S. Approval: 2010
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
Evidence for the use of new and old agents for MRSA infection. Dr Charis Marwick Ninewells Hospital & Medical School Dundee
 Evidence for the use of new and old agents for MRSA infection Dr Charis Marwick Ninewells Hospital & Medical School Dundee 32 year old man Admitted unwell for few days Cough, fever, short of breath, pleuritic
Evidence for the use of new and old agents for MRSA infection Dr Charis Marwick Ninewells Hospital & Medical School Dundee 32 year old man Admitted unwell for few days Cough, fever, short of breath, pleuritic
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Henry A. McKinnell, Jr., Ph.D. Chairman of the Board and Chief Executive
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Henry A. McKinnell, Jr., Ph.D. Chairman of the Board and Chief Executive
SCMID Online Lecture Library. by author. Optimizing antimicrobial therapy in the elderly. Dose Finding - The Past
 Optimizing antimicrobial therapy in the elderly Johan W. Mouton MD PhD FIDSA Professor pharmacokinetics and pharmacodynamics Dosing should be such that the level of antmicrobial activity is associated
Optimizing antimicrobial therapy in the elderly Johan W. Mouton MD PhD FIDSA Professor pharmacokinetics and pharmacodynamics Dosing should be such that the level of antmicrobial activity is associated
Updated Clostridium difficile Treatment Guidelines
 Updated Clostridium difficile Treatment Guidelines Arielle Arnold, PharmD, BCPS Clinical Pharmacist Saint Alphonsus Regional Medical Center September 29 th, 2018 Disclosures Nothing to disclose Learning
Updated Clostridium difficile Treatment Guidelines Arielle Arnold, PharmD, BCPS Clinical Pharmacist Saint Alphonsus Regional Medical Center September 29 th, 2018 Disclosures Nothing to disclose Learning
Steven D. Brown* and Maria M. Traczewski. The Clinical Microbiology Institute, 9725 SW Commerce Circle, Wilsonville, Oregon 97070
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2010, p. 2063 2069 Vol. 54, No. 5 0066-4804/10/$12.00 doi:10.1128/aac.01569-09 Copyright 2010, American Society for Microbiology. All Rights Reserved. Comparative
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2010, p. 2063 2069 Vol. 54, No. 5 0066-4804/10/$12.00 doi:10.1128/aac.01569-09 Copyright 2010, American Society for Microbiology. All Rights Reserved. Comparative
ICU Volume 11 - Issue 3 - Autumn Series
 ICU Volume 11 - Issue 3 - Autumn 2011 - Series Impact of Pharmacokinetics of Antibiotics in ICU Clinical Practice Introduction The efficacy of a drug is mainly dependent on its ability to achieve an effective
ICU Volume 11 - Issue 3 - Autumn 2011 - Series Impact of Pharmacokinetics of Antibiotics in ICU Clinical Practice Introduction The efficacy of a drug is mainly dependent on its ability to achieve an effective
ABSSSI Case Study days (clinical evaluation)
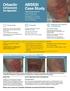 ABSSSI Case Study Cellulitis patient background 44-year-old single father Chronic renal insufficiency Hyperlipidemia, on rosuvastatin Has atrial fibrillation, on apixaban Fell and injured right leg At
ABSSSI Case Study Cellulitis patient background 44-year-old single father Chronic renal insufficiency Hyperlipidemia, on rosuvastatin Has atrial fibrillation, on apixaban Fell and injured right leg At
Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry
 doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH)
 Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH) Clinical Practice Guideline* for the Diagnosis and Management of Acute Bacterial
Rochester Patient Safety C. difficile Prevention Collaborative: Long Term Care Antimicrobial Stewardship (funded by NYSDOH) Clinical Practice Guideline* for the Diagnosis and Management of Acute Bacterial
Mesporin TM. Ceftriaxone sodium. Rapid onset, sustained action, for a broad spectrum of infections
 Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Beta-lactamase production should have no effect on Azithromycin activity.
 AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Action Azithromycin
AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Action Azithromycin
ZINEX. Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg
 ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
Received 19 January 2010/Returned for modification 9 June 2010/Accepted 2 November 2010
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 2011, p. 583 592 Vol. 55, No. 2 0066-4804/11/$12.00 doi:10.1128/aac.00076-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Phase 2,
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 2011, p. 583 592 Vol. 55, No. 2 0066-4804/11/$12.00 doi:10.1128/aac.00076-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Phase 2,
Flexible Outpatient. Opportunity. Decision: Dalvance
 Flexible Outpatient Opportunity Decision: Dalvance DALVANCE: an opportunity for use at various SITES OF CARE HOME HOME Outpatient Infusion Site ED One 30-minute infusion a week for two weeks provides a
Flexible Outpatient Opportunity Decision: Dalvance DALVANCE: an opportunity for use at various SITES OF CARE HOME HOME Outpatient Infusion Site ED One 30-minute infusion a week for two weeks provides a
Randomized Comparison of Linezolid (PNU ) versus Oxacillin-Dicloxacillin for Treatment of Complicated Skin and Soft Tissue Infections
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 2000, p. 3408 3413 Vol. 44, No. 12 0066-4804/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Randomized Comparison of (PNU-100766)
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Dec. 2000, p. 3408 3413 Vol. 44, No. 12 0066-4804/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Randomized Comparison of (PNU-100766)
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. ZYVOX Zyvox 600 mg Film coated tablets (Tablets) Zyvox 20 mg/ml Granules for oral suspension (Granules) Zyvox 2 mg/ml Solution for infusion (Injection) 2. QUALITATIVE AND QUANTITATIVE
NEW ZEALAND DATA SHEET 1. ZYVOX Zyvox 600 mg Film coated tablets (Tablets) Zyvox 20 mg/ml Granules for oral suspension (Granules) Zyvox 2 mg/ml Solution for infusion (Injection) 2. QUALITATIVE AND QUANTITATIVE
VIBATIV - telavancin hydrochlorideâ injection, powder, lyophilized, for solutionâ Theravance, Inc
 VIBATIV - telavancin hydrochloride injection, powder, lyophilized, for solution Theravance, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed
VIBATIV - telavancin hydrochloride injection, powder, lyophilized, for solution Theravance, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed
Optimizing Antibiotic Therapy in the ICU For Pneumonia Current and Future Approaches
 Optimizing Antibiotic Therapy in the ICU For Pneumonia Current and Future Approaches Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ. Disclosures I have served as a consultant to, researcher/investigator
Optimizing Antibiotic Therapy in the ICU For Pneumonia Current and Future Approaches Andrew F. Shorr, MD, MPH Washington Hospital Center Georgetown Univ. Disclosures I have served as a consultant to, researcher/investigator
CLINICAL USE OF GLYCOPEPTIDES. Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel
 CLINICAL USE OF GLYCOPEPTIDES Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel Glycopeptides Natural Vancomycin introduced in 1958 Teicoplanin introduced in Europe
CLINICAL USE OF GLYCOPEPTIDES Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel Glycopeptides Natural Vancomycin introduced in 1958 Teicoplanin introduced in Europe
Pharmacodynamic indices in targeting therapy of critical infections
 Pharmacodynamic indices in targeting therapy of critical infections P.M. Tulkens Cellular and Molecular Pharmacology, Catholic University of Louvain, Brussels, Belgium & International Society of Anti-infective
Pharmacodynamic indices in targeting therapy of critical infections P.M. Tulkens Cellular and Molecular Pharmacology, Catholic University of Louvain, Brussels, Belgium & International Society of Anti-infective
Vancomycin Pharmacokinetics. Myrna Y. Munar, Pharm.D., BCPS Associate Professor of Pharmacy
 Vancomycin Pharmacokinetics Myrna Y. Munar, Pharm.D., BCPS Associate Professor of Pharmacy Goals Review the PK properties of vancomycin Compare and contrast methods of dosage regimen design for vancomycin
Vancomycin Pharmacokinetics Myrna Y. Munar, Pharm.D., BCPS Associate Professor of Pharmacy Goals Review the PK properties of vancomycin Compare and contrast methods of dosage regimen design for vancomycin
Cefotaxime Rationale for the EUCAST clinical breakpoints, version th September 2010
 Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Cefotaxime Rationale for the EUCAST clinical breakpoints, version 1.0 26 th September 2010 Foreword EUCAST The European Committee on Antimicrobial Susceptibility Testing (EUCAST) is organised by the European
Zyvox linezolid injection, linezolid tablets, linezolid for oral suspension
 linezolid injection linezolid tablets linezolid for oral suspension DESCRIPTION ZYVOX I.V. Injection, ZYVOX Tablets, and ZYVOX for Oral Suspension contain linezolid, which is a synthetic antibacterial
linezolid injection linezolid tablets linezolid for oral suspension DESCRIPTION ZYVOX I.V. Injection, ZYVOX Tablets, and ZYVOX for Oral Suspension contain linezolid, which is a synthetic antibacterial
Protein Synthesis Inhibitors. Ass Prof. Dr. Naza M. Ali 15 Nov 2018 Lec 8
 Protein Synthesis Inhibitors Ass Prof. Dr. Naza M. Ali 15 Nov 2018 Lec 8 These drugs selectively inhibit bacterial protein synthesis. The selectivity is due to the differences between bacterial and human
Protein Synthesis Inhibitors Ass Prof. Dr. Naza M. Ali 15 Nov 2018 Lec 8 These drugs selectively inhibit bacterial protein synthesis. The selectivity is due to the differences between bacterial and human
PRODUCT MONOGRAPH LINEZOLID INJECTION. 2 mg/ml. Sterile Solution. Antibacterial Agent. 165 Galaxy Blvd, Suite 100 December 4, 2017 Toronto, ON M9W 0C8
 PRODUCT MONOGRAPH Pr LINEZOLID INJECTION 2 mg/ml Sterile Solution Antibacterial Agent Fresenius Kabi Canada Ltd. Date of Revision: 165 Galaxy Blvd, Suite 100 December 4, 2017 Toronto, ON M9W 0C8 Control
PRODUCT MONOGRAPH Pr LINEZOLID INJECTION 2 mg/ml Sterile Solution Antibacterial Agent Fresenius Kabi Canada Ltd. Date of Revision: 165 Galaxy Blvd, Suite 100 December 4, 2017 Toronto, ON M9W 0C8 Control
400 mg IV (over 5 to 60 minutes) every 12 hours. > 30 to mg IV (over 5 to 60 minutes ) every 12 hours. 15 to 30
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO. TEFLARO (ceftaroline
Tedizolid: a novel treatment for Gram + infections and its potential role in clinical practice
 Tedizolid: a novel treatment for Gram + infections and its potential role in clinical practice Paul M. Tulkens, MD, PhD Cellular and Molecular Pharmacology & Centre for Clinical Pharmacy Louvain Drug Research
Tedizolid: a novel treatment for Gram + infections and its potential role in clinical practice Paul M. Tulkens, MD, PhD Cellular and Molecular Pharmacology & Centre for Clinical Pharmacy Louvain Drug Research
LINEZOLID APOTEX Tablets. (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]- acetamide
![LINEZOLID APOTEX Tablets. (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]- acetamide LINEZOLID APOTEX Tablets. (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]- acetamide](/thumbs/83/88174044.jpg) LINEZOLID APOTEX Tablets NAME OF THE MEDICINE Active Ingredient: Chemical Name: Linezolid (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]- acetamide Structural Formula: Molecular
LINEZOLID APOTEX Tablets NAME OF THE MEDICINE Active Ingredient: Chemical Name: Linezolid (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]- acetamide Structural Formula: Molecular
EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING
 EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING Commentary provided by: Julie Hall, MHS, MT (ASCP) Assistant Dean, College of Health Professions Assistant Professor, Medical Laboratory Science Grand Valley
EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING Commentary provided by: Julie Hall, MHS, MT (ASCP) Assistant Dean, College of Health Professions Assistant Professor, Medical Laboratory Science Grand Valley
NEW ZEALAND DATA SHEET
 1 PRODUCT NAME Linezolid Kabi solution for intravenous infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Linezolid 2 mg/ml For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution
1 PRODUCT NAME Linezolid Kabi solution for intravenous infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Linezolid 2 mg/ml For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution
(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5- oxazolidinyl]methyl]-acetamide
![(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5- oxazolidinyl]methyl]-acetamide (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5- oxazolidinyl]methyl]-acetamide](/thumbs/96/128476325.jpg) PRODUCT INFORMATION Zyvox 2 mg/ml injection, 400 mg and 600 mg film coated tablets and 20 mg/ml granules for oral suspension NAME OF THE DRUG Non-proprietary name: Chemical name: CAS Number: Linezolid
PRODUCT INFORMATION Zyvox 2 mg/ml injection, 400 mg and 600 mg film coated tablets and 20 mg/ml granules for oral suspension NAME OF THE DRUG Non-proprietary name: Chemical name: CAS Number: Linezolid
PRODUCT MONOGRAPH ZYVOXAM. Linezolid Tablets 600 mg. Linezolid Injection 2 mg/ml. Linezolid Powder for Oral Suspension 100 mg/5ml when reconstituted
 PRODUCT MONOGRAPH Pr ZYVOXAM Linezolid Tablets 600 mg Linezolid Injection 2 mg/ml Linezolid Powder for Oral Suspension 100 mg/5ml when reconstituted Antibacterial Agent Pfizer Canada Inc 17,300 Trans-Canada
PRODUCT MONOGRAPH Pr ZYVOXAM Linezolid Tablets 600 mg Linezolid Injection 2 mg/ml Linezolid Powder for Oral Suspension 100 mg/5ml when reconstituted Antibacterial Agent Pfizer Canada Inc 17,300 Trans-Canada
Vancomycin Rationale for the EUCAST clinical breakpoints, version June 2010
 Vancomycin Rationale for the EUCAST clinical breakpoints, version 2.1 17 June 2010 Introduction The glycopeptides are a class of agents composed of amino acid residues and attached sugars. Glycopeptides
Vancomycin Rationale for the EUCAST clinical breakpoints, version 2.1 17 June 2010 Introduction The glycopeptides are a class of agents composed of amino acid residues and attached sugars. Glycopeptides
PACKAGE INSERT USP ANTIBIOTIC
 Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
PRODUCT INFORMATION TARGOCID
 NAME OF THE MEDICINE Non-proprietary Name teicoplanin PRODUCT INFORMATION TARGOCID DESCRIPTION Teicoplanin is a glycopeptide-antibiotic produced by Actinoplanes teichomyceticus. It is presented as a sterile,
NAME OF THE MEDICINE Non-proprietary Name teicoplanin PRODUCT INFORMATION TARGOCID DESCRIPTION Teicoplanin is a glycopeptide-antibiotic produced by Actinoplanes teichomyceticus. It is presented as a sterile,
FULL PRESCRIBING INFORMATION: CONTENTS*
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OTIPRIO safely and effectively. See full prescribing information for OTIPRIO. OTIPRIO (ciprofloxacin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OTIPRIO safely and effectively. See full prescribing information for OTIPRIO. OTIPRIO (ciprofloxacin
Chemical name: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl]-acetamide.
![Chemical name: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl]-acetamide. Chemical name: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl]-acetamide.](/thumbs/81/84077786.jpg) PRODUCT INFORMATION ZYVOX linezolid NAME OF THE MEDICINE Non-proprietary name: Linezolid. Chemical name: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl]-acetamide. The empirical
PRODUCT INFORMATION ZYVOX linezolid NAME OF THE MEDICINE Non-proprietary name: Linezolid. Chemical name: (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl]-acetamide. The empirical
Anti-Microbial Drugs
 Name: Date: Monday March 7 th 2011 Class: I "Pharmacology Anti-Microbial Drugs Lecture 5 د. حيدر الشكرجي Macrolides: Anti-Microbial Drugs Erythromycin was the 1 st of macrolides to find clinical application,
Name: Date: Monday March 7 th 2011 Class: I "Pharmacology Anti-Microbial Drugs Lecture 5 د. حيدر الشكرجي Macrolides: Anti-Microbial Drugs Erythromycin was the 1 st of macrolides to find clinical application,
Antibiotics to treat multi-drug-resistant bacterial infections
 Antibiotics to treat multi-drug-resistant bacterial infections July 2013 JUNE 2013 Copyright 2013 Tetraphase Pharmaceuticals, Inc. 1 Forward Looking Statements and Other Important Cautions Any statements
Antibiotics to treat multi-drug-resistant bacterial infections July 2013 JUNE 2013 Copyright 2013 Tetraphase Pharmaceuticals, Inc. 1 Forward Looking Statements and Other Important Cautions Any statements
Disclosures. Efficacy of the drug. Optimizing Dosing Based on PKPD- An overview. Dose Finding - The Past
 Disclosures Optimizing Dosing Based on PKPD- An overview Johan W. Mouton MD PhD FIDSA FAAM Professor pharmacokinetics and pharmacodynamics Research grants advisory boards speaker This Patient Needs Antibiotics.
Disclosures Optimizing Dosing Based on PKPD- An overview Johan W. Mouton MD PhD FIDSA FAAM Professor pharmacokinetics and pharmacodynamics Research grants advisory boards speaker This Patient Needs Antibiotics.
Development of C sporins. Beta-lactam antibiotics - Cephalosporins. Second generation C sporins. Targets - PBP s
 Beta-lactam antibiotics - Cephalosporins Development of C sporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability properties
Beta-lactam antibiotics - Cephalosporins Development of C sporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability properties
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
Single- and Multiple-Dose Study to Determine the Safety, Tolerability, Pharmacokinetics,
 AAC Accepted Manuscript Posted Online 6 February 2017 Antimicrob. Agents Chemother. doi:10.1128/aac.02181-16 Copyright 2017 American Society for Microbiology. All Rights Reserved. Single- and Multiple-Dose
AAC Accepted Manuscript Posted Online 6 February 2017 Antimicrob. Agents Chemother. doi:10.1128/aac.02181-16 Copyright 2017 American Society for Microbiology. All Rights Reserved. Single- and Multiple-Dose
Shirin Abadi, B.Sc.(Pharm.), ACPR, Pharm.D. Clinical Pharmacy Specialist & Pharmacy Education Coordinator, BC Cancer Agency Clinical Associate
 Shirin Abadi, B.Sc.(Pharm.), ACPR, Pharm.D. Clinical Pharmacy Specialist & Pharmacy Education Coordinator, BC Cancer Agency Clinical Associate Professor of Pharmacy & Associate Member of Medicine, UBC
Shirin Abadi, B.Sc.(Pharm.), ACPR, Pharm.D. Clinical Pharmacy Specialist & Pharmacy Education Coordinator, BC Cancer Agency Clinical Associate Professor of Pharmacy & Associate Member of Medicine, UBC
Aciphin Ceftriaxone Sodium
 Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
Xerava (eravacycline) NEW PRODUCT SLIDESHOW
 Xerava (eravacycline) NEW PRODUCT SLIDESHOW Introduction Brand name: Xerava Generic name: Eravacycline Pharmacological class: Tetracycline antibiotic Strength and Formulation: 50mg; per vial; lyophilized
Xerava (eravacycline) NEW PRODUCT SLIDESHOW Introduction Brand name: Xerava Generic name: Eravacycline Pharmacological class: Tetracycline antibiotic Strength and Formulation: 50mg; per vial; lyophilized
Infectious Disease in the Critically Ill Patient
 Infectious Disease in the Critically Ill Patient Heather L. Evans, MD MS FACS Director of Surgical Infectious Disease Harborview Medical Center Asst. Professor UW Department of Surgery New Antibiotics:
Infectious Disease in the Critically Ill Patient Heather L. Evans, MD MS FACS Director of Surgical Infectious Disease Harborview Medical Center Asst. Professor UW Department of Surgery New Antibiotics:
VANLID Capsules (Vancomycin hydrochloride)
 Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
PHARMACOKINETIC & PHARMACODYNAMIC OF ANTIBIOTICS
 PHARMACOKINETIC & PHARMACODYNAMIC OF ANTIBIOTICS SITI HIR HURAIZAH MD TAHIR Bpharm (UKM), MSc (Clinical Microbiology) (UoN) CLINICAL PHARMACIST HOSPITAL MELAKA WHY STUDY PHARMACOKINETICS (PK) AND PHARMACODYNAMICS
PHARMACOKINETIC & PHARMACODYNAMIC OF ANTIBIOTICS SITI HIR HURAIZAH MD TAHIR Bpharm (UKM), MSc (Clinical Microbiology) (UoN) CLINICAL PHARMACIST HOSPITAL MELAKA WHY STUDY PHARMACOKINETICS (PK) AND PHARMACODYNAMICS
ZIN EN ONZIN VAN ANTIBIOTICASPIEGELS BIJ NEONATEN
 ZIN EN ONZIN VAN ANTIBIOTICASPIEGELS BIJ NEONATEN Anne Smits Fellow neonatologie UZ Leuven Use of antibiotics in neonates 50 European hospitals 23 non-european hospitals Countries n = 14 n = 9 Pediatric
ZIN EN ONZIN VAN ANTIBIOTICASPIEGELS BIJ NEONATEN Anne Smits Fellow neonatologie UZ Leuven Use of antibiotics in neonates 50 European hospitals 23 non-european hospitals Countries n = 14 n = 9 Pediatric
TOUGH ON IMPETIGO GENTLE ON THE1 PATIENT. Demonstrated efficacy and safety1. New treatment option now available!
 New treatment option now available! TOUGH ON IMPETIGO GENTLE ON THE1 PATIENT Demonstrated efficacy and safety1 Home Efficacy Safety Convenience (ozenoxacin) is indicated for the topical treatment of impetigo
New treatment option now available! TOUGH ON IMPETIGO GENTLE ON THE1 PATIENT Demonstrated efficacy and safety1 Home Efficacy Safety Convenience (ozenoxacin) is indicated for the topical treatment of impetigo
Analysis of the Phase 3 ESTABLISH Trials: Tedizolid Versus Linezolid in Acute Bacterial Skin
 AAC Accepts, published online ahead of print on 24 November 2014 Antimicrob. Agents Chemother. doi:10.1128/aac.03688-14 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 2 Analysis
AAC Accepts, published online ahead of print on 24 November 2014 Antimicrob. Agents Chemother. doi:10.1128/aac.03688-14 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 2 Analysis
(telavancin) Healthcare Professional s Guide. Version 2, 4 November 2014
 VIBATIV (telavancin) Healthcare Professional s Guide Version 2, 4 November 2014 1 Table of Contents Introduction... 3 About Vibativ / Therapeutic indications... 3 Antimicrobial spectrum of activity for
VIBATIV (telavancin) Healthcare Professional s Guide Version 2, 4 November 2014 1 Table of Contents Introduction... 3 About Vibativ / Therapeutic indications... 3 Antimicrobial spectrum of activity for
Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more)
 Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more) Christian Eckmann Antibiotic Stewardship Expert ECDC Chief of Staff Department of General, Visceral and Thoracic Surgery Klinikum
Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more) Christian Eckmann Antibiotic Stewardship Expert ECDC Chief of Staff Department of General, Visceral and Thoracic Surgery Klinikum
PEDIATRIC PHARMACOTHERAPY
 PEDIATRIC PHARMACOTHERAPY Volume 23 Number 6 June 2017 C Ceftaroline: An Alternative Broad Spectrum Antibiotic for Pediatric Infections Marcia L. Buck, PharmD, FCCP, FPPAG, BCPPS eftaroline was approved
PEDIATRIC PHARMACOTHERAPY Volume 23 Number 6 June 2017 C Ceftaroline: An Alternative Broad Spectrum Antibiotic for Pediatric Infections Marcia L. Buck, PharmD, FCCP, FPPAG, BCPPS eftaroline was approved
The Challenge of Managing Staphylococcus aureus Bacteremia
 The Challenge of Managing Staphylococcus aureus Bacteremia M A R G A R E T G R A Y B S P F C S H P C L I N I C A L P R A C T I C E M A N A G E R N O R T H / I D P H A R M A C I S T A L B E R T A H E A
The Challenge of Managing Staphylococcus aureus Bacteremia M A R G A R E T G R A Y B S P F C S H P C L I N I C A L P R A C T I C E M A N A G E R N O R T H / I D P H A R M A C I S T A L B E R T A H E A
Protein Synthesis Inhibitors
 Frank Lowy Protein Synthesis Inhibitors This lecture discusses a diverse group of antibiotics that are grouped together because they all have a common mechanism of action they are protein synthesis inhibitors.
Frank Lowy Protein Synthesis Inhibitors This lecture discusses a diverse group of antibiotics that are grouped together because they all have a common mechanism of action they are protein synthesis inhibitors.
NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP)
 NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP) NEOSPORIN SOLUTION DESCRIPTION NEOSPORIN Ophthalmic Solution (neomycin and polymyxin B
NEOSPORIN Ophthalmic Solution Sterile (neomycin and polymyxin B sulfates and gramicidin ophthalmic solution, USP) NEOSPORIN SOLUTION DESCRIPTION NEOSPORIN Ophthalmic Solution (neomycin and polymyxin B
Linezolid-related adverse effects in clinical practice in children
 Original article Arch Argent Pediatr 2017;115(5):470-475 / 470 Linezolid-related adverse effects in clinical practice in children Nuri Bayram, M.D., Associate professor a, Mine Düzgöl, M.D. a, Ahu Kara,
Original article Arch Argent Pediatr 2017;115(5):470-475 / 470 Linezolid-related adverse effects in clinical practice in children Nuri Bayram, M.D., Associate professor a, Mine Düzgöl, M.D. a, Ahu Kara,
