Kidney development proceeds through a complex series
|
|
|
- Annis Merritt
- 6 years ago
- Views:
Transcription
1 Pax2 and Pax8 Regulate Branching Morphogenesis and Nephron Differentiation in the Developing Kidney Melina Narlis, David Grote, Yaned Gaitan, Sami K. Boualia, and Maxime Bouchard McGill Cancer Centre and Biochemistry Department, McGill University, Montreal, Quebec, Canada Pax genes are important regulators of kidney development. In the mouse, homozygous Pax2 inactivation results in renal agenesis, a phenotype that has largely precluded the analysis of Pax gene function during metanephric kidney development. To address this later function, kidney development was analyzed in embryos that were compound heterozygous for Pax2 and for Pax8, a closely related member of the Pax gene family. Both genes are coexpressed in differentiating nephrons and collecting ducts. At the morphological level, Pax2 / Pax8 / metanephric kidneys are severely hypodysplastic and characterized by a reduction in ureter tips and nephron number in comparison with wild-type or Pax2 / kidneys. In developing nephrons, the molecular analysis of Pax2 / Pax8 / kidneys reveals a strong reduction in the expression levels of Lim1, a key regulator of nephron differentiation, accompanied by an increase in apoptosis. At a more mature stage, the reduction of Pax2/8 gene dosage severely affects distal tubule formation, revealing a role for Pax genes in the differentiation of specific nephron segments. At the ureter tips, the expression of Wnt11, a target of glial cell derived neurotrophic factor Ret signaling, is significantly reduced, whereas the expression levels of Ret and GDNF remain normal. Together, these results demonstrate a crucial role for Pax2 and Pax8 in nephron differentiation and branching morphogenesis of the metanephros. J Am Soc Nephrol 18: , doi: /ASN Kidney development proceeds through a complex series of mesenchymal epithelial interactions whereby the epithelial ureteric bud initially evaginates from the nephric duct and invades the metanephric mesenchyme at the level of the hindlimb bud. The ureteric bud subsequently undergoes multiple branching cycles to form the collecting duct system, which is responsible for urine flow to the bladder. In turn, the ureter tips induce the surrounding mesenchyme to condense, epithelialize, and differentiate into mature nephrons through a succession of morphogenetic steps that are referred to as renal vesicle, comma-shaped body, and S-shaped body. Several signaling and transcriptional regulators are necessary for these early steps of kidney development. Among them, the glial cell derived neurotrophic factor (GDNF)/Ret-GFR 1 pathway is crucial for ureter budding and branching (1 6), whereas Fgf8 and Wnt4 are necessary for nephron differentiation past the renal vesicle stage (7 9). At the transcriptional level, Lim1 acts downstream of Fgf8 in nephron differentiation and is part of an intricate network of transcriptional regulation in renal epithelia, together with Pax, Gata, and homeodomain family members (10 12). However, the cellular mechanisms by which this transcriptional network operates during kidney morphogenesis and differentiation are still largely unknown. Received July 14, Accepted January 5, Published online ahead of print. Publication date available at M.N. and D.G. contributed equally to this work. Address correspondence to: Dr. Maxime Bouchard, McGill Cancer Centre and Biochemistry Department, McGill University, 3655 Promenade Sir-William-Osler, Montreal, Quebec H3G 1Y6, Canada. Phone: ; Fax: ; maxime.bouchard@mcgill.ca The Pax2 and Pax8 transcription factors are central regulators of kidney development. In the transient pro- and mesonephros of the mouse, they both are expressed in the mesenchymal primordium as well as in the epithelial components. In the metanephros, Pax2 is expressed in the collecting duct, nephron progenitors, and all epithelial components of the developing nephron. In contrast, Pax8 expression starts at the renal vesicle stage and is maintained, together with Pax2, until the end of nephron differentiation. In terms of function, Pax2/8 genes are necessary and sufficient to induce the nephric lineage. Embryos that are mutant for both genes indeed fail to generate the nephric (Wolffian) duct and therefore are defective in the formation of all three embryonic kidneys (pro-, meso-, and metanephros) (13). Both genes act in a cooperative and dosagedependent manner as revealed by the correlation between the number of Pax2/8 alleles and the severity of renal defects. In contrast to double-knockout embryos, Pax2 / embryos initially form the pro/mesonephros but fail to induce the metanephric kidney, largely because of the role of Pax2 in nephric duct maintenance (10,14) and GDNF regulation in the metanephric mesenchyme (15). A milder reduction in Pax gene dosage in Pax2 / mice allows metanephros formation but results in hypoplastic kidneys (60 to 75% of the normal size), a phenotype also found in Pax2 / patients with renal coloboma (16). Finally, Pax8 inactivation alone does not result in any kidney malformation (17). The relatively mild kidney phenotype of Pax2 / mice and the complete absence of metanephros in Pax2 / mice have largely obscured the role of Pax genes in branching morphogenesis and nephron differentiation. Organ culture experiments and Pax2 heterozygote kidney analysis could nonetheless reveal the role of Pax2 in cell survival and Copyright 2007 by the American Society of Nephrology ISSN: /
2 1122 Journal of the American Society of Nephrology J Am Soc Nephrol 18: , 2007 mesenchymal-to-epithelial transition leading to nephron formation (18 21). To understand better the role of Pax genes in early metanephros development, we performed a detailed analysis of Pax2 / Pax8 / mice, which consistently form kidneys that are severely reduced in size (25 to 50% of the normal size) (13) and therefore represent an intermediate between Pax2 / and Pax2 / phenotypes. We found that Pax2 / Pax8 / kidneys show a significant reduction in nephron number, which correlates with a downregulation of the key developmental regulator Lim1 (11) and an increase in the apoptotic index of nephron progenitor cells. In mature nephrons, the reduction of Pax2/8 gene dosage results in a differentiation defect of the distal convoluted tubules. In addition, Pax2 / Pax8 / kidneys display branching defects that are associated with a downregulation of Wnt11 gene expression, a target of Ret signaling. From these data, we conclude that Pax genes are important regulators of both nephron differentiation and branching morphogenesis in the developing kidney. Materials and Methods Mice Pax2 lacz and Pax8 neo mice were maintained in a C3H/He background and genotyped as described previously (13,18). Immunohistochemistry and Terminal Deoxynucleotidyl Transferase Mediated Digoxigenin-Deoxyuridine Nick-End Labeling Assays Metanephric kidneys were dissected in cold PBS and fixed for 2 h in 4% paraformaldehyde at 4 C. Samples were processed for cryosection and subsequent immunohistochemistry as described previously (18). The following primary detection reagents were used: Rat anti E-cadherin antibody (1:400; Zymed Laboratories, San Francisco, CA), rabbit anti-laminin antibody (1:2000; Sigma, St. Louis, MO), rabbit anti phospho-h3 antibody (1:200; Upstate Biotechnology, Lake Placid, NY), and biotinylated Lotus tetragonolobus lectin (1:500; Vector Laboratories, Burlingame, CA). Secondary detection was done with anti-rat or anti-rabbit secondary antibodies that were labeled with Alexa488, Alexa568 (1:200; Molecular Probes, Eugene, OR), or FITC-conjugated streptavidin (Zymed Laboratories). Sections were occasionally counterstained with hematoxylin or DAPI at 50 g/ml in SlowFade Light mounting medium (Molecular Probes). Terminal deoxynucleotidyl transferase mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay was performed using the In Situ Cell Death Detection Kit according to manufacturer s instruction (Roche, Basel, Switzerland). Apoptotic indexes were obtained from 12- m-thick frozen sections of embryonic day 16.5 (E16.5) wild-type, Pax2 /,orpax2 / Pax8 / kidneys that were immunostained against E-cadherin and counterstained with DAPI. Peripheral mesenchyme was defined by dense DAPI staining, absence of epithelial morphology, and peripheral location relative to the ureter tips. Cellular morphology, low E-cadherin staining, and location in proximity to the ureter trunk identified early differentiating nephrons. High E-cadherin levels and cell morphology defined collecting duct cells. A minimum of 20 independent fields ( 10 sections) from four kidneys of each genotype was used for statistical analysis (Student t test). In Situ Hybridization and Histology Metanephric kidneys were prepared as for immunohistochemical staining (see previous section). In situ hybridization with digoxigenindutp RNA probes was performed as described previously (22), using the following probes: Pax2 and Pax8 (13), Wnt4 (23), Wt1 (24), Fgf8 (25), Ret (26), GDNF (27), and Wnt11 (28). Additional probes for Lim1 (NM_008498), Nkcc2 (NM_011389), Ncc (NM_019415), and Ncx1 (NM_011406) were generated from E18.5 kidney cdna with the following primer pairs and cloned in pgem-t-easy: Lim1 (Lhx1) 5 -TGGCGTCTTC- CTTTTCGTCTG-3 and 5 -CTTTGGCGACACTGCTGTTACTC-3 ; Nkcc2 (Slc12a1) 5 -TCATTCGCCTCTCCTGGATTG-3 and 5 -CCTTGCACA- GAGCCTGGAATAC-3 ; Ncc (Slc12a3) 5 -TGGCTGACCTGCATTCAT- TCC-3 and 5 -GCTGGGAGCATTCTGTGAAGTTC-3 ; and Ncx1 (Slc8a1) 5 -ACGACGGTGAGAATCTGGAACG-3 and 5 -CAAACACAGTGGT- GCTCAAGTCG-3. Glomerular and ureter tip counts were performed by in situ hybridization with Wt1 and Ret probes, respectively. Wt1 and Ret stainings were obtained from adjacent 10- m-thick cryosections while quantification was performed every 50 m (minimizing double counts) on four entire kidneys of each genotype (15 to 25 sections per kidney). Samples were scored for high Wt1 expression (maturing nephrons) or Retpositive ureter tips and subjected to statistical analysis (Student t test). Hematoxylin and eosin stainings were performed on 6- m-thick paraffin sections using standard procedures. Results Growth Impairment in Pax2 / Pax8 / Kidneys Pax2 and Pax8 were previously shown to cooperate genetically as compound heterozygous mutations result in abnormal kidney development (13). To understand better the role of Pax2 and Pax8 in metanephros formation, we initially performed histologic analysis of Pax2 / Pax8 / compared with Pax2 / and wild-type kidneys at various developmental stages. By day 13.5 post coitum (E13.5), Pax2 / Pax8 / kidneys were smaller than controls, with very few detectable nephron progenitors (Figure 1, A through C). At this stage, both wild-type and Pax2 / kidneys already showed several comma- and S-shaped bodies (Figure 1, A and B). By E16.5, control kidneys had formed mature glomeruli, and proximal and distal tubules were readily visible (Figure 1, D and E). In contrast, Pax2 / Pax8 / kidneys harbored only a few maturing nephrons and tubules at this stage, and certain areas were completely devoid of differentiating nephrons (Figure 1F). This marked dysplasia and delay in nephron differentiation was also observed at E18.5, when the number of differentiated nephron structures was irregular and still significantly reduced (Figure 1, G through I, data not shown). Immunohistochemical analysis with the epithelial markers E-cadherin and laminin revealed that collecting ducts and nephrons epithelialized normally in Pax2 / Pax8 / kidneys at E16.5 (Figure 1, J through L). The dysplastic phenotype and nephrogenesis delay that was specifically found in Pax2 / Pax8 / but not in Pax2 / kidneys prompted us to quantify both branching morphogenesis and nephron differentiation in these samples. For this, we counted the number of Ret( ) ureter tips and Wt1( ) glomeruli (including progenitors), throughout E16.5 wild-type, Pax2 /, and Pax2 / Pax8 / kidneys. Pax2 / Pax8 / kidneys displayed a 51 and 33% reduction in ureter tip number when compared with wild-type (P 0.007) and Pax2 / (P 0.092), respectively. In addition, the number of glomeruli was reduced by 63% (P 0.002) compared with wildtype and 42% compared with Pax2 / (P 0.007; Figure 2). Importantly, the striking reduction in Pax2 / Pax8 / glomeruli
3 J Am Soc Nephrol 18: , 2007 Pax2/8 in Metanephros Development 1123 Figure 1. Metanephros development in Pax2;Pax8 mutant embryos. Hematoxylin and eosin stainings (A through I) and immunohistochemical analysis (J through L) of wild-type (A, D, G, and J), Pax2 / (B, E, H, and K), and Pax2 / Pax8 / (C, F, I, and L) kidneys at embryonic day 13.5 (E13.5; A through C), E16.5 (D through F and J through L), and E18.5 (G through I). (A through C) At E13.5, wildtype and Pax2 / kidneys have initiated nephron differentiation, whereas Pax2 / Pax8 / kidneys are smaller and exhibit little signs of nephron differentiation. (D through E) By E16.5, both wild-type and Pax2 / metanephros harbor several differentiated nephrons. (F) At the same stage, Pax2 / Pax8 / kidneys have initiated nephrogenesis, but few nephrons have matured past the S-shaped body stage. (G through I) At E18.5, Pax2 / Pax8 / kidneys develop differentiated nephrons, but these are reduced in number and disorganized when compared with control wild-type and Pax2 / kidneys. (J through L) Immunohistochemical staining against E-cadherin (green) and laminin (red) reveal a normal epithelialization of the collecting duct and early differentiating nephrons at E16.5 in wildtype Pax2 / and Pax2 / Pax8 / kidneys. cb, comma-shaped body; cd, collecting duct; g, glomerulus; sb, S-shaped body; pct, proximal convoluted tubule. number was associated with dysplasia that was characterized by an unequal distribution of nephrons throughout the kidney (Figure 1F). This phenotype, which is not observed in Pax2 / kidneys, is indicative of a nephrogenesis defect independent of the ureter branching delay that is also observed in Pax2 / Pax8 / kidneys. These data point to a role for Pax2 and Pax8 in both branching morphogenesis and nephron formation. Pax2 and Pax8 Are Coexpressed and Regulated Independently within the Collecting Duct and Differentiating Nephrons The involvement of Pax8 in branching morphogenesis was unexpected because, until recently, Pax8 expression had been Figure 2. Ureter tips and glomerular counts. Counts of ureter tips and glomeruli were obtained throughout E16.5 kidneys (every 50 m) using in situ hybridization with Ret and Wt1 crna probes, respectively. Glomerular progenitor counts, normally reaching a mean value of 839 in control kidneys (wildtype or Pax8 / ), are reduced by 63% to 306 in Pax2 / Pax8 / kidneys (P 0.002). This reduction represents a 42% decrease in comparison with Pax2 / kidneys (with 525 glomeruli progenitors; P 0.007). The ureter tip count of Pax2 / Pax8 / kidneys (277 tips) is a 51% reduction in reference to wild-type (566 tips; P 0.007) and by 31% compared with Pax2 / (415 tips; P 0.092). SD is indicated above each column; n 4 entire kidneys for each genotype. reported only in the renal vesicle and subsequent steps of nephron differentiation (29,30). Although the expression in nephron progenitors could, in principle, influence collecting duct elongation and branching, the possibility remained that the defect is intrinsic to the collecting duct. To clarify the issue, we used in situ hybridization to reexamine Pax8 expression in the developing metanephros. As expected, we found high expression of Pax8 in renal vesicles, comma- and S-shaped bodies, and more differentiated structures (Figure 3, A and C). It is interesting, however, that Pax8 expression was also found at low levels in the collecting duct of E13.5 and E16.5 kidneys (Figure 3, A through D), in accordance with a recent report (31). This expression of Pax8 was thus similar to Pax2 expression in the metanephros, with the exception of the condensing mesenchyme (before renal vesicle stage), which is positive for Pax2 but negative for Pax8 (Figure 3, C and E). To verify whether the expression of Pax2 and Pax8 in the metanephros was subjected to auto- or cross-regulation, we then compared their expression levels in wild-type and Pax2 / Pax8 / and found no significant difference in staining intensity (Figure 3). These results are in agreement with the normal expression of Pax2 in Pax8 / kidneys (data not shown) and Pax8 in Pax2 / mesonephros (12,13). Together, these data suggest that Pax2 and Pax8 are coexpressed independently in the collecting duct and regulate branching morphogenesis within these cells rather than indirectly from differentiating nephrons.
4 1124 Journal of the American Society of Nephrology J Am Soc Nephrol 18: , 2007 Figure 3. Expression of Pax8 and Pax2 in the metanephros. In situ hybridizations on wild-type and Pax2 / Pax8 / kidneys with Pax8 (A through D) and Pax2 (E and F) crna probes at E13.5 (A and B) and E16.5 (C through F). The expression of Pax8 is found in the differentiating nephrons but also in the collecting duct of wild-type embryos at E13.5 (A) and E16.5 (C). Despite a reduction in the number of differentiating nephrons, this expression is essentially unchanged in Pax2 / Pax8 / kidneys (B and D). In comparison, Pax2 is expressed in the collecting duct and differentiating nephrons as well as in the condensing mesenchyme of wild-type kidneys (E). This expression is unaltered in Pax2 / Pax8 / samples (F), arguing against an auto- or cross-regulation of Pax genes in this system. cm, condensing mesenchyme; csb, comma-shaped body; lh, loop of Henle; rv, renal vesicle. Reduction of Pax2 and Pax8 Gene Dosage Is Associated with a Downregulation of Wnt11, a Target of Ret Signaling Because the role of Pax2 and Pax8 in branching morphogenesis may be related to the GDNF-Ret signaling pathway, central to this process, we looked at Ret expression and signaling at E13.5 and E16.5. In the metanephros, Ret expression is restricted to the newly branched ureter tips (Figure 4, A and C). This restricted expression pattern was essentially unchanged in Pax2 / Pax8 / kidneys (Figure 4, B and D). Unexpectedly, however, the expression levels of Wnt11 were markedly decreased in E12.5 Pax2 / Pax8 / kidneys when compared with wild-type and Pax2 / controls (Figure 4, E and F). This downregulation was even more pronounced at E16.5, when a significant number of ureter tips showed complete loss of Wnt11 expression (Figure 4, G and H). Because this result suggests an effect of Pax genes on GDNF-Ret signaling, we tested the expression of mesenchymal Ret ligand GDNF in double-heterozy- Figure 4. Regulation of branching morphogenesis in Pax2; Pax8 kidneys. In situ hybridization for Ret (A through D), Wnt11 (E through H), and GDNF (I and J) at the indicated stages. At E13.5, the expression of Ret is restricted to ureter tips of wild-type kidneys (A). This expression pattern is maintained in Pax2 / Pax8 / kidneys (B). At E16.5, the expression of Ret remains normal in Pax2 / Pax8 / kidneys (C and D). At E12.5, the Ret signaling target Wnt11 is downregulated in Pax2 / Pax8 / (F), when compared with wildtype expression pattern at the ureter tips (E) or with Pax2 / (insert in E). At E16.5, Wnt11 downregulation is even more pronounced (H) in comparison with wild-type (G) or Pax2 / (insert in G). GDNF expression levels are similar between wild-type (I) and Pax2 / Pax8 / (J) kidneys. The different thickness of the GDNF signal reflects an increase in metanephric mesenchyme resulting from reduced branching morphogenesis in Pax2 / Pax8 / kidneys. mm, metanephric mesenchyme; ut, ureter tip.
5 J Am Soc Nephrol 18: , 2007 Pax2/8 in Metanephros Development 1125 gous animals. For this, in situ hybridization with a GDNF crna probe was performed on E16.5 kidneys but revealed no significant alteration in expression levels in Pax2 / Pax8 / samples compared with controls (Figure 4, I and J). The GDNF expression domain was, however, more prominent in Pax2 / Pax8 / kidneys, reflecting a larger metanephric mesenchyme region. Similar expression results were obtained for the Ret co-receptor GFR 1 (data not shown). We therefore conclude that the GDNF-Ret target gene Wnt11 requires Pax gene for normal expression, which is in accordance with the branching morphogenetic defect observed in Pax2 / Pax8 / kidneys. Pax Genes Regulate Nephron Differentiation To investigate directly the reduction of nephron number that was observed in Pax2 / Pax8 / kidneys, we looked at the expression of the key nephron differentiation regulators Wnt4 (9), Lim1 (11), and Fgf8 (7,8). In the developing nephron, Wnt4 is predominantly expressed at the renal vesicle stage, in the medullary part of the collecting duct, and in the surrounding mesenchyme (Figure 5A). This expression was maintained in Figure 5. Regulation of nephron differentiation in Pax2;Pax8 kidneys. In situ hybridization for the nephron differentiation regulators Wnt4 (A and B), Fgf8 (C and D), and Lim1 (E and F) at E16.5. (A) Wnt4 is normally expressed in the renal vesicles, medullary collecting duct, and surrounding stromal cells. (B) This expression pattern is unaffected in Pax2 / Pax8 / kidneys. (C and D) The pattern of Fgf8 expression is similar in wild-type and Pax2 / Pax8 / kidneys. (E and F) In contrast, Lim1 expression is strongly reduced in Pax2 / Pax8 / nephron progenitors in reference to wild-type or Pax2 / kidneys (insert in E). Figure 6. Apoptosis and proliferation analysis. Cell survival (A, B, and E) was assayed by terminal deoxynucleotidyl transferase mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining (red), and proliferation index (C and D) was determined by phospho-histone H3 immunodetection (red). In all assays, epithelia were immunolabeled with E-cadherin (green), and nuclei were revealed by DAPI stain (blue). (A and B) The basal level of apoptotic signals (white arrows) that were observed in wild-type kidneys was significantly increased in Pax2 / Pax8 / kidneys. (C and D) Proliferation index was unchanged between wild-type and Pax2 / Pax8 / samples. (E) Quantification of TUNELpositive signals in the peripheral mesenchyme, collecting duct, and differentiating nephrons of wild-type, Pax2 /, and Pax2 / Pax8 / show a strong increase in cell death in all three domains of Pax2 / Pax8 / kidneys. This effect is especially pronounced in differentiating nephrons, where apoptosis more than doubled compared with control Pax2 / samples. *Statistically significant values (P 0.05) in reference to controls. SD is indicated above each column. Pax2 / Pax8 / kidneys (Figure 5B). Similarly, Fgf8 expression was not affected in Pax2 / Pax8 / kidneys (Figure 5, C and D), indicating that the initial formation of renal vesicles is not significantly perturbed by a reduction in Pax2/8 gene dosage. In contrast, the expression of Lim1, normally strong in nephron progenitors and weak in the collecting duct, was severely
6 1126 Journal of the American Society of Nephrology J Am Soc Nephrol 18: , 2007 downregulated in Pax2 / Pax8 / kidneys when compared with wild-type or Pax2 / controls (Figure 5, E and F). These kidneys indeed showed large and irregular regions of low or absent Lim1 expression. Such distribution is consistent with the dysplastic distribution of differentiated nephrons that was observed in these kidneys (Figure 1, D through I). The genetic regulation of Lim1 by the Pax2/8 genes was confirmed in Pax2 / Pax8 / mesonephros, in which Lim1 expression was virtually lost despite the presence of differentiating mesonephric tissue (Y.G., unpublished data). Together, these results identify an important role for Pax genes in nephron formation through the regulation of Lim1, a key component of nephron differentiation and patterning (11). Regulation of Cell Survival by Pax2/8 To elucidate further the nephron differentiation defects of compound heterozygote kidneys, we examined the apoptosis and proliferation indexes of Pax2 / Pax8 / kidneys using TUNEL and phospho-histone H3 assays, respectively. These experiments revealed a strong increase of apoptotic signals in Pax2 / Pax8 / kidneys compared with controls (Figure 6, A and B), whereas the proliferation index remained unchanged (Figure 6, C and D). To characterize better the effect of Pax genes on cell survival, we quantified the number of TUNELpositive signals in different compartments of wild-type, Pax2 /, and Pax2 / Pax8 / kidneys. As previously reported, Pax2 / kidneys showed an increase in apoptotic signals in the peripheral mesenchyme, collecting duct, and differentiating nephrons (Figure 6E) (18 20). However, this effect was markedly higher in Pax2 / Pax8 / kidneys, with the strongest effect observed in differentiating nephrons. This finding is in agreement with the high expression levels of both Pax2 and Pax8 that are normally present in this structure (Figure 3). Interestingly, the increase in TUNEL-positive signals that was observed in the peripheral mesenchyme of Pax2 / Pax8 / kidneys revealed a non cell-autonomous effect of Pax genes on cell survival as Pax8 is not expressed in these cells (Figure 3) (29,30). Together, these results demonstrate a high sensitivity of nephron progenitors to Pax gene dependent cell survival and suggest a cellular explanation for the decrease in nephron number and overall kidney size that are observed in Pax2/8 compound heterozygote embryos. Pax Genes Regulate Distal Convoluted Tubule Differentiation The results presented so far involved primarily the early stages of nephron morphogenesis. To determine the developmental outcome of reduced Pax gene dosage on nephron differentiation, we looked at late differentiation markers for the main segments of the nephron (glomerulus, proximal convoluted tubule, loop of Henle, and distal convoluted tubule). As expected, Wt1 expression marking the podocytes of the glomerulus was not affected in the developing nephrons, in accordance with the absence of Pax gene expression in these cells (Figure 7, A and B). Similarly, the lectin Lotus tetragonolobus and the Nkcc2 gene, which mark the proximal convoluted tubule and the loop of Henle, respectively, were still expressed in Figure 7. Late nephron marker analysis in Pax2;Pax8 kidneys. In situ hybridization (A, B, and E through J) and lectin staining (C and D) of the glomerular marker Wt1 (A and B), proximal tubule marker Lotus tetragonolobus (C and D), loop of Henle marker Nkcc2 (E and F), and distal tubule markers Ncc (G and H) and Ncx1 (I and J). A and B are E16.5 and E through H are E18.5 kidneys. (A and B) The expression of Wt1 is maintained at normal levels in Pax2 / Pax8 / kidneys, despite a general reduction in glomerular counts. (C and D) The number and patterning of proximal tubules are affected in Pax2 / Pax8 / kidneys, but the tubules are still detectable. Note the large dysplastic region in the left kidney side. (E and F) Nkcc2 shows a normal expression pattern in a reduced number of loops of Henle. (G and H) The expression of Ncc reveals a specific defect in the formation of distal convoluted tubules in Pax2 / Pax8 / kidneys compared with Pax2 / and wild-type. (I and J) The distal convoluted tubule defect is also observed with Ncx1, an independent marker of this structure. alh, ascending loop of Henle; dct, distal convoluted tubule.
7 J Am Soc Nephrol 18: , 2007 Pax2/8 in Metanephros Development 1127 Pax2 / Pax8 / kidneys, despite the overall reduction in nephron number (Figure 7, C through F). Strikingly, however, the expression of the distal convoluted tubule marker Ncc was severely affected in Pax2 / Pax8 / kidneys, with very few and small regions of expression compared with controls (Figure 7, G and H). This result was confirmed by in situ staining with Ncx1, another marker primarily expressed in distal convoluted tubules (Figure 7, I and J). Hence, these results reveal a specific role for Pax2 and Pax8 in the differentiation of the distal part of the nephron. Discussion Pax genes have been implicated in several aspects of kidney development ranging from nephric lineage specification to mesenchymal-to-epithelial transition and control of cell survival (18 21). The crucial role that is played by Pax genes during mesonephros development and metanephros induction has hindered the study of their function during later kidney development. The hypoplastic phenotype of Pax2 / kidneys has provided some important information, notably about the implication of Pax genes in cell survival. However, mainly because of the persistence of Pax8 expression, Pax2 / mice only allow the study of some aspects of Pax gene function in the metanephros. To specifically address the role of Pax genes during metanephros development, we took advantage of the strong phenotype that is observed in Pax2 / Pax8 / metanephric kidneys (13). These kidneys were hypodysplastic and characterized by a strong reduction in branching morphogenesis associated with a failure to activate Wnt11. In addition, they showed a significant reduction in the potential of Pax2 / Pax8 / progenitor cells to differentiate into mature nephrons, a defect that is accompanied by a severe downregulation of Lim1 gene expression and by an increase in cell death of the progenitor population. It is interesting that the distal segment of the developing nephrons was especially affected by a reduction in Pax gene dosage. Together, these results identify a key role for Pax genes in branching morphogenesis and nephron differentiation. Recent analyses using a conditional inactivation in the mesenchymal components of the metanephros showed that Lim1 is strictly required for nephron development past the renal vesicle stage (11). The observation that nephron differentiation occurred irregularly in Pax2 / Pax8 / kidneys correlates well with the absence or low expression of Lim1 that is observed in most Pax2 / Pax8 / nephron progenitor cells and thus indicates that Lim1 is sensitive to Pax gene dosage. Consistent with this is the loss of Lim1 expression from the mesonephros of Pax / Pax8 / embryos in which the mesonephric tissue is present but Pax gene dosage is minimal (Y.G., unpublished results) (12). The genetic regulation of Lim1 by Pax genes in the mesonephros, however, is not observed in Pax2;Pax8 compound heterozygote embryos, which excludes an effect on the phenotype described here. Accordingly, Pax2 / Pax8 / embryos show a normal elongation and budding of the ureter (data not shown). Our finding that Lim1 is genetically regulated by Pax genes adds to a recent model of early nephron differentiation implicating Fgf8, Wnt4, and Lim1 (7 9,11). According to this model, the autocrine activity of Fgf8 in renal vesicles is necessary for Lim1 and Wnt4 expression, both of which are required for nephrogenesis (9,11). Our results raise the question of whether the activity of Pax genes on Lim1 is mediated through this pathway. The persistent expression of Wnt4 in Pax2 / Pax8 / kidneys, however, does not support such a model. In addition, the expression of Fgf8 was found to be normal in compound heterozygote kidneys, suggesting that Pax genes do not act upstream of the Fgf8 pathway. A complete elimination of Pax genes from nephron progenitors would be necessary to definitively conclude on this issue. The other possibility suggested by the model is that Fgf8 would exert its regulatory effect on Lim1 through Pax gene activity. However, Pax2 is still expressed at normal levels, and Pax8 is only slightly downregulated in Fgf8-deficient renal vesicles (7) (U. Grieshammer and G. Martin, personal communication). From these data, we therefore conclude that Fgf8 and Pax genes act in parallel to activate Lim1 in renal vesicles, thereby allowing nephron differentiation to proceed. The increase in apoptotic cell death that was observed in Pax2 / Pax8 / kidneys is in accordance with previous studies implicating Pax genes in cell survival (18 20). Because Pax8 expression is turned on at the renal vesicle stage, it was somewhat expected to find the highest increase in cell death in these cells. It is interesting, however, to correlate this increased apoptosis in nephron progenitor cells with the loss of distal convoluted tubules later during development. It is indeed tempting to speculate that some parts of the differentiating nephron are more sensitive to Pax-dependent cell survival. Alternatively, Pax proteins may regulate key factors such as Brn1, which is involved in the differentiation of specific nephron segments (32,33). Another interesting aspect of the cell survival analysis is the significant increase of apoptosis in the peripheral mesenchyme that does not express Pax8. This finding suggests that the survival signal that is regulated by Pax proteins in the ureter tip or renal vesicle has a non cell-autonomous component. Such non cell-autonomous survival signal was also proposed downstream of Pax2 and Pax8 in the intermediate mesoderm (13) and may be mediated by members of the FGF or TGF superfamilies (7,34). The specific effect of Pax genes on Ret signaling is still unclear. One interpretation of our results is that Pax genes are necessary for the expression of a critical component of the Ret signaling pathway. Alternatively, Pax genes could act in parallel to the Ret signal to mediate part of its transcriptional response, notably on Wnt11. The current data do not allow us to discriminate between these possibilities, which would require a detailed analysis of Ret signaling. Nonetheless, the downregulation of Wnt11 that was observed in Pax2 / Pax8 / kidneys is significant per se as this gene was shown to be necessary for normal branching morphogenesis in the kidney (28). Although some aspects of the role of Pax2/8 in metanephros development remain to be clarified, our results unambiguously identified a cooperative role for Pax2 and Pax8 in metanephric branching morphogenesis and nephron differentiation.
8 1128 Journal of the American Society of Nephrology J Am Soc Nephrol 18: , 2007 Acknowledgments M.B. is supported by the Canadian Institutes for Health Research and the Terry Fox Foundation and holds a Canada Research Chair in Kidney Disease. We thank Meinrad Busslinger for the Pax8 neo mice and Uta Grieshammer and Gail Martin for sharing unpublished data (Department of Anatomy, University of California at San Francisco, San Francisco, CA; May 2005). Thanks also to David Cotnoir-White and Denise Cook for the cloning of Ncc, Ncx1, and Nkcc2 in situ probes. Disclosures None. References 1. Schuchardt AD, Agati V, Larsson-Blomberg L, Costantini F, Pachnis V: Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367: , Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A: Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76 79, Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H: Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382: 73 76, Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M: Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382: 70 73, Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A: GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 21: 53 62, Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Milbrandt J: GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 21: , Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR: FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132: , Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni- Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M: Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132: , Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: , Bouchard M: Transcriptional control of kidney development. Differentiation 72: , Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR: Distinct and sequential tissuespecific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132: , Grote D, Souabni A, Busslinger M, Bouchard M: Pax2/8- regulated Gata3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133: 53 61, Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M: Nephric lineage specification by Pax2 and Pax8. Genes Dev 16: , Torres M, Gomez-Pardo E, Dressler GR, Gruss P: Pax-2 controls multiple steps of urogenital development. Development 121: , Brophy PD, Ostrom L, Lang KM, Dressler GR: Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128: , Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR: Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 9: , 1995 [published erratum appears in Nat Genet 13: 129, 1996] 17. Mansouri A, Chowdhury K, Gruss P: Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19: 87 90, Bouchard M, Pfeffer P, Busslinger M: Functional equivalence of the transcription factors Pax2 and Pax5 in mouse development. Development 127: , Ostrom L, Tang MJ, Gruss P, Dressler GR: Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev Biol 219: , Porteous S, Torban E, Cho NP, Cunliffe H, Chua L, McNoe L, Ward T, Souza C, Gus P, Giugliani R, Sato T, Yun K, Favor J, Sicotte M, Goodyer P, Eccles M: Primary renal hypoplasia in humans and mice with PAX2 mutations: Evidence of increased apoptosis in fetal kidneys of Pax2(1Neu) /- mutant mice. Hum Mol Genet 9: 1 11, Rothenpieler UW, Dressler GR: Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development 119: , Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish- Horowicz D: Expression of a Delta homologue in prospective neurons in the chick. Nature 375: , Gavin BJ, McMahon JA, McMahon AP: Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes Dev 4: , Buckler AJ, Pelletier J, Haber DA, Glaser T, Housman DE: Isolation, characterization, and expression of the murine Wilms tumor gene (WT1) during kidney development. Mol Cell Biol 11: , Crossley PH, Minowada G, MacArthur CA, Martin GR: Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell 84: , Pachnis V, Mankoo B, Costantini F: Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119: , Srinivas S, Wu Z, Chen CM, D Agati V, Costantini F: Dominant effects of RET receptor misexpression and ligand-independent RET signaling on ureteric bud development. Development 126: , Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP: Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130: , Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL,
9 J Am Soc Nephrol 18: , 2007 Pax2/8 in Metanephros Development 1129 Gruss P: Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 110: , Poleev A, Fickenscher H, Mundlos S, Winterpacht A, Zabel B, Fidler A, Gruss P, Plachov D: PAX8, a human paired box gene: Isolation and expression in developing thyroid, kidney and Wilms tumors. Development 116: , Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP: Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: , Nakai S, Sugitani Y, Sato H, Ito S, Miura Y, Ogawa M, Nishi M, Jishage K, Minowa O, Noda T: Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development 130: , Bouchard M, Grote D, Craven SE, Sun Q, Steinlein P, Busslinger M: Identification of Pax2-regulated genes by expression profiling of the mid-hindbrain organizer region. Development 132: , Dudley AT, Godin RE, Robertson EJ: Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13: , 1999
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
Formation of Urine: Formation of Urine
 The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney
 The EMBO Journal (2006) 25, 5214 5228 & 2006 European Molecular Biology Organization All Rights Reserved 0261-4189/06 www.embojournal.org Six2 is required for suppression of nephrogenesis and progenitor
The EMBO Journal (2006) 25, 5214 5228 & 2006 European Molecular Biology Organization All Rights Reserved 0261-4189/06 www.embojournal.org Six2 is required for suppression of nephrogenesis and progenitor
Development of the Urinary System. 3 Distinct Embryonic Kidney Structures
 Development of the Urinary System Excretory portion of urinary system derived from intermediate mesoderm Week 4: 1 st nephrons/renal corpuscles form Nephrotomes form and develop hollow lumens to form nephric
Development of the Urinary System Excretory portion of urinary system derived from intermediate mesoderm Week 4: 1 st nephrons/renal corpuscles form Nephrotomes form and develop hollow lumens to form nephric
Renal agenesis and hypodysplasia in ret-k mutant mice result from defects in ureteric bud development
 Development 122, 1919-1929 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3412 1919 Renal agenesis and hypodysplasia in ret-k mutant mice result from defects in ureteric bud
Development 122, 1919-1929 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3412 1919 Renal agenesis and hypodysplasia in ret-k mutant mice result from defects in ureteric bud
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats
 Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
a) They are the most common cause of pediatric kidney failure. b) They are always symptomatic. c) They can be asymmetric.
 Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Six1 is required for the early organogenesis of mammalian kidney
 Development 130, 3085-3094 2003 The Company of Biologists Ltd doi:10.1242/dev.00536 3085 Six1 is required for the early organogenesis of mammalian kidney Pin-Xian Xu 1, *, Weiming Zheng 1, Li Huang 1,
Development 130, 3085-3094 2003 The Company of Biologists Ltd doi:10.1242/dev.00536 3085 Six1 is required for the early organogenesis of mammalian kidney Pin-Xian Xu 1, *, Weiming Zheng 1, Li Huang 1,
Development of the human fetal kidney begins at approximately
 Suppression of Ureteric Bud Apoptosis Rescues Nephron Endowment and Adult Renal Function in Pax2 Mutant Mice Alison Dziarmaga,* Michael Eccles, and Paul Goodyer* Departments of *Human Genetics and Pediatrics,
Suppression of Ureteric Bud Apoptosis Rescues Nephron Endowment and Adult Renal Function in Pax2 Mutant Mice Alison Dziarmaga,* Michael Eccles, and Paul Goodyer* Departments of *Human Genetics and Pediatrics,
Pax-2 controls multiple steps of urogenital development
 Development 121, 4057-4065 (1995) Printed in Great Britain The Company of Biologists Limited 1995 DEV2038 4057 Pax-2 controls multiple steps of urogenital development Miguel Torres 1, Emilia Gómez-Pardo
Development 121, 4057-4065 (1995) Printed in Great Britain The Company of Biologists Limited 1995 DEV2038 4057 Pax-2 controls multiple steps of urogenital development Miguel Torres 1, Emilia Gómez-Pardo
Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development
 Research article 2809 Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development Akio Kobayashi 1,2, Kin-Ming Kwan 2, Thomas
Research article 2809 Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development Akio Kobayashi 1,2, Kin-Ming Kwan 2, Thomas
Mouse kidney development
 Alan J. Davidson, Center for Regenerative Medicine, Harvard Medical School and Harvard Stem Cell Institute, Massachusetts General Hospital, Boston, MA 02114, USA Table of Contents 1. Overview of kidney
Alan J. Davidson, Center for Regenerative Medicine, Harvard Medical School and Harvard Stem Cell Institute, Massachusetts General Hospital, Boston, MA 02114, USA Table of Contents 1. Overview of kidney
Repression of Interstitial Identity in Nephron Progenitor Cells by Pax2 Establishes the Nephron- Interstitium Boundary during Kidney Development
 Article Repression of Interstitial Identity in Nephron Progenitor Cells by Pax2 Establishes the Nephron- Interstitium Boundary during Kidney Development Graphical Abstract Authors Natalie Naiman, Kaoru
Article Repression of Interstitial Identity in Nephron Progenitor Cells by Pax2 Establishes the Nephron- Interstitium Boundary during Kidney Development Graphical Abstract Authors Natalie Naiman, Kaoru
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons
 Research article 3847 FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons Uta Grieshammer 1, *, Cristina Cebrián 2, *, Roger
Research article 3847 FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons Uta Grieshammer 1, *, Cristina Cebrián 2, *, Roger
How Does the Ureteric Bud Branch?
 How Does the Ureteric Bud Branch? Sanjay K. Nigam* and Mita M. Shah Departments of *Pediatrics, Medicine, and Cellular and Molecular Medicine, University of California, San Diego, San Diego, California
How Does the Ureteric Bud Branch? Sanjay K. Nigam* and Mita M. Shah Departments of *Pediatrics, Medicine, and Cellular and Molecular Medicine, University of California, San Diego, San Diego, California
Early nephron formation in the developing mouse kidney
 J. Anat. (2001) 199, pp. 385 392, with 7 figures Printed in the United Kingdom 385 Early nephron formation in the developing mouse kidney JONATHAN B. L. BARD 1, ADELE GORDON, LINDA SHARP AND WILLIAM I.
J. Anat. (2001) 199, pp. 385 392, with 7 figures Printed in the United Kingdom 385 Early nephron formation in the developing mouse kidney JONATHAN B. L. BARD 1, ADELE GORDON, LINDA SHARP AND WILLIAM I.
Transcriptional Control of Epithelial Differentiation during Kidney Development
 J Am Soc Nephrol 14: S9 S15, 2003 Transcriptional Control of Epithelial Differentiation during Kidney Development DAVID RIBES,* EVELYNE FISCHER, AMÉLIE CALMONT,* and JEROME ROSSERT* *INSERM U489 and The
J Am Soc Nephrol 14: S9 S15, 2003 Transcriptional Control of Epithelial Differentiation during Kidney Development DAVID RIBES,* EVELYNE FISCHER, AMÉLIE CALMONT,* and JEROME ROSSERT* *INSERM U489 and The
COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival
 2330 Development 139, 2330-2339 (2012) doi:10.1242/dev.076299 2012. Published by The Company of Biologists Ltd COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival
2330 Development 139, 2330-2339 (2012) doi:10.1242/dev.076299 2012. Published by The Company of Biologists Ltd COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
REVIEW ARTICLE. Kidney Development Branches Out DEVELOPMENTAL GENETICS 24: (1999) 1999 WILEY-LISS, INC.
 DEVELOPMENTAL GENETICS 24:189 193 (1999) REVIEW ARTICLE Kidney Development Branches Out GREGORY R. DRESSLER* Department of Pathology, University of Michigan, Ann Arbor, Michigan ABSTRACT For more than
DEVELOPMENTAL GENETICS 24:189 193 (1999) REVIEW ARTICLE Kidney Development Branches Out GREGORY R. DRESSLER* Department of Pathology, University of Michigan, Ann Arbor, Michigan ABSTRACT For more than
Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells
 AND DISEASE RESEARCH ARTICLE 2995 Development 133, 2995-3004 (2006) doi:10.1242/dev.02442 Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation
AND DISEASE RESEARCH ARTICLE 2995 Development 133, 2995-3004 (2006) doi:10.1242/dev.02442 Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation
Kidney Development in the Absence of Gdnf and Spry1 Requires Fgf10
 Kidney Development in the Absence of Gdnf and Spry1 Requires Fgf10 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Kidney Development in the Absence of Gdnf and Spry1 Requires Fgf10 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
The role of GDNF in patterning the excretory system
 Developmental Biology 283 (2005) 70 84 www.elsevier.com/locate/ydbio The role of GDNF in patterning the excretory system Reena Shakya a, Eek-hoon Jho a,1, Pille Kotka a,2, Zaiqi Wu a, Nikolai Kholodilov
Developmental Biology 283 (2005) 70 84 www.elsevier.com/locate/ydbio The role of GDNF in patterning the excretory system Reena Shakya a, Eek-hoon Jho a,1, Pille Kotka a,2, Zaiqi Wu a, Nikolai Kholodilov
SLIT2-Mediated ROBO2 Signaling Restricts Kidney Induction to a Single Site
 Developmental Cell, Vol. 6, 709 717, May, 2004, Copyright 2004 by Cell Press SLIT2-Mediated ROBO2 Signaling Restricts Kidney Induction to a Single Site Short Article Uta Grieshammer, 1,3 Le Ma, 2,3 Andrew
Developmental Cell, Vol. 6, 709 717, May, 2004, Copyright 2004 by Cell Press SLIT2-Mediated ROBO2 Signaling Restricts Kidney Induction to a Single Site Short Article Uta Grieshammer, 1,3 Le Ma, 2,3 Andrew
The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
 Development 126, 5771-5783 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9673 5771 The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
Development 126, 5771-5783 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9673 5771 The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Downregulation of Spry-1, an inhibitor of GDNF/Ret, causes angiotensin II-induced ureteric bud branching
 http://www.kidney-international.org & 2008 International Society of Nephrology original article Downregulation of Spry-1, an inhibitor of GDNF/Ret, causes angiotensin II-induced ureteric bud branching
http://www.kidney-international.org & 2008 International Society of Nephrology original article Downregulation of Spry-1, an inhibitor of GDNF/Ret, causes angiotensin II-induced ureteric bud branching
Active Hedgehog Signaling Regulates Renal Capsule Morphogenesis
 Active Hedgehog Signaling Regulates Renal Capsule Morphogenesis by Hovhannes Martirosyan A thesis submitted in conformity with the requirements for the degree of Master of Science Laboratory Medicine and
Active Hedgehog Signaling Regulates Renal Capsule Morphogenesis by Hovhannes Martirosyan A thesis submitted in conformity with the requirements for the degree of Master of Science Laboratory Medicine and
Urinary System. J. H. Lue. intermediate mesoderm cloaca coelomic epithelium
 Urinary System J. H. Lue Primordium: intermediate mesoderm cloaca coelomic epithelium 1 3w(18d) 3 w 4w (24d) 4w (26d) 2 Intermediate mesoderm 3 Intermediate mesoderm intermediate mesoderm urogenital ridge
Urinary System J. H. Lue Primordium: intermediate mesoderm cloaca coelomic epithelium 1 3w(18d) 3 w 4w (24d) 4w (26d) 2 Intermediate mesoderm 3 Intermediate mesoderm intermediate mesoderm urogenital ridge
Development of the urinary system
 Development of the urinary system WSO School of Biomedical Sciences, University of Hong Kong. 3 sets of kidneys developing in succession (temporally and spatially) : Pronephros ] Mesonephros ]- Intermediate
Development of the urinary system WSO School of Biomedical Sciences, University of Hong Kong. 3 sets of kidneys developing in succession (temporally and spatially) : Pronephros ] Mesonephros ]- Intermediate
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Structural and cellular changes in fetal renal papilla during development
 www.jpnim.com Open Access eissn: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2017;6(1):e060136 doi: 10.7363/060136 Received: 2016 May 19; revised: 2016 Jun 06; accepted: 2016 Jul
www.jpnim.com Open Access eissn: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2017;6(1):e060136 doi: 10.7363/060136 Received: 2016 May 19; revised: 2016 Jun 06; accepted: 2016 Jul
The Ebf1 knockout mouse and glomerular maturation
 Repository of the Max Delbrück Center for Molecular Medicine (MDC) Berlin (Germany) http://edoc.mdc-berlin.de/14009/ The Ebf1 knockout mouse and glomerular maturation Schmidt-Ott, K.M. Published in final
Repository of the Max Delbrück Center for Molecular Medicine (MDC) Berlin (Germany) http://edoc.mdc-berlin.de/14009/ The Ebf1 knockout mouse and glomerular maturation Schmidt-Ott, K.M. Published in final
The functional anatomy of the urinary system. Human Anatomy Department Dr. Anastasia Bendelic
 The functional anatomy of the urinary system Human Anatomy Department Dr. Anastasia Bendelic Plan Development of the kidneys and their abnormalities Development of the urinary ways and their abnormalities
The functional anatomy of the urinary system Human Anatomy Department Dr. Anastasia Bendelic Plan Development of the kidneys and their abnormalities Development of the urinary ways and their abnormalities
Inhibitory Effects of Torin2 on Proximal Tubular Development of the Xenopus laevis Pronephric Kidney
 John Carroll University Carroll Collected Senior Honors Projects Theses, Essays, and Senior Honors Projects Spring 2013 Inhibitory Effects of Torin2 on Proximal Tubular Development of the Xenopus laevis
John Carroll University Carroll Collected Senior Honors Projects Theses, Essays, and Senior Honors Projects Spring 2013 Inhibitory Effects of Torin2 on Proximal Tubular Development of the Xenopus laevis
Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro
 Kidney International, Vol. 67 (25), pp. 42 431 GENETIC DISORDERS DEVELOPMENT Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro JASON E. CAIN,THIBAULD NION,DOMINIQUE
Kidney International, Vol. 67 (25), pp. 42 431 GENETIC DISORDERS DEVELOPMENT Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro JASON E. CAIN,THIBAULD NION,DOMINIQUE
Urinary system development. Male ( ) and Female ( ) Reproductive Systems Development
 Urinary system development Male ( ) and Female ( ) Reproductive Systems Development Urogenital system develops from mesodermal uro-genital ridge (intermediate mesoderm) development of male and female genital
Urinary system development Male ( ) and Female ( ) Reproductive Systems Development Urogenital system develops from mesodermal uro-genital ridge (intermediate mesoderm) development of male and female genital
Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant
 Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant Reporting Period July 1, 2011 June 30, 2012 Formula Grant Overview The Children's Hospital of Pittsburgh received $958,038 in
Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant Reporting Period July 1, 2011 June 30, 2012 Formula Grant Overview The Children's Hospital of Pittsburgh received $958,038 in
Lesson 31. Lesson Outline:
 Lesson 31 Lesson Outline: The Urogenital System Urinary System o Embryonic Origins o Phylogenetic Trends Function Urinary Bladder Implications for the Evolution of the Vertebrates o Freshwater or Seawater
Lesson 31 Lesson Outline: The Urogenital System Urinary System o Embryonic Origins o Phylogenetic Trends Function Urinary Bladder Implications for the Evolution of the Vertebrates o Freshwater or Seawater
CHAPTER 6 SUMMARIZING DISCUSSION
 CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
Not all renal stem cell niches are the same: anatomy of an evolution
 eissn: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2016;5(2):e050225 doi: 10.7363/050225 Received: 2015 Sept 11; revised: 2015 Dec 21; accepted: 2016 Mar 01; published online: 2016
eissn: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2016;5(2):e050225 doi: 10.7363/050225 Received: 2015 Sept 11; revised: 2015 Dec 21; accepted: 2016 Mar 01; published online: 2016
Implication of Wt1 in the Pathogenesis of Nephrogenic Failure in a Mouse Model of Retinoic Acid-Induced Caudal Regression Syndrome
 American Journal of Pathology, Vol. 166, No. 5, May 2005 Copyright American Society for Investigative Pathology Cardiovascular, Pulmonary and Renal Pathology Implication of Wt1 in the Pathogenesis of Nephrogenic
American Journal of Pathology, Vol. 166, No. 5, May 2005 Copyright American Society for Investigative Pathology Cardiovascular, Pulmonary and Renal Pathology Implication of Wt1 in the Pathogenesis of Nephrogenic
Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret
 Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret article Ekatherina Batourina, Christopher Choi, Neal Paragas, Natalie Bello, Terry Hensle, Frank D. Costantini,
Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret article Ekatherina Batourina, Christopher Choi, Neal Paragas, Natalie Bello, Terry Hensle, Frank D. Costantini,
It has been hypothesized that low nephron numbers in the
 Nephron Number, Renal Function, and Arterial Pressure in Aged GDNF Heterozygous Mice Luise A. Cullen-McEwen, Michelle M. Kett, John Dowling, Warwick P. Anderson, John F. Bertram Abstract The loss of one
Nephron Number, Renal Function, and Arterial Pressure in Aged GDNF Heterozygous Mice Luise A. Cullen-McEwen, Michelle M. Kett, John Dowling, Warwick P. Anderson, John F. Bertram Abstract The loss of one
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen. Yusuke Murasawa, Pi-chao Wang
 Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen Yusuke Murasawa, Pi-chao Wang Abstract Although tissue engineering of artificial organs such as skin or cartilage
Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen Yusuke Murasawa, Pi-chao Wang Abstract Although tissue engineering of artificial organs such as skin or cartilage
Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy
 Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Chapter 25: Urinary System
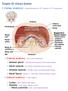 Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Fstl1 Antagonizes BMP Signaling and Regulates Ureter Development
 Fstl1 Antagonizes BMP Signaling and Regulates Ureter Development Jingyue Xu 1., Xin Qi 1., Jianfeng Gong 1, Mingyan Yu 1, Fangxiong Zhang 3, Haibo Sha 1, Xiang Gao 1,2 * 1 MOE Key Laboratory of Model Animal
Fstl1 Antagonizes BMP Signaling and Regulates Ureter Development Jingyue Xu 1., Xin Qi 1., Jianfeng Gong 1, Mingyan Yu 1, Fangxiong Zhang 3, Haibo Sha 1, Xiang Gao 1,2 * 1 MOE Key Laboratory of Model Animal
Development of the Genital System
 Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Histological differentiation of human fetal kidney
 Original Research Article Histological differentiation of human fetal kidney Mamatha Hosapatnaa 1, Hemalatha Bangera 2, Anne D Souza 3, Aswin Das 4, Supriya 4, Antony Sylvan D Souza 5, Vrinda Hari Ankolekar
Original Research Article Histological differentiation of human fetal kidney Mamatha Hosapatnaa 1, Hemalatha Bangera 2, Anne D Souza 3, Aswin Das 4, Supriya 4, Antony Sylvan D Souza 5, Vrinda Hari Ankolekar
Kidneycentric. Follow this and additional works at:
 Washington University School of Medicine Digital Commons@Becker All Kidneycentric 2014 Renal Dysplasia Halana V. Whitehead Washington University School of Medicine in St. Louis Follow this and additional
Washington University School of Medicine Digital Commons@Becker All Kidneycentric 2014 Renal Dysplasia Halana V. Whitehead Washington University School of Medicine in St. Louis Follow this and additional
Development of the Urinary System
 Development of the Urinary System Lecture Objectives Understand the development of the kidney and related organs of the urinary system. Define the pronephrons, mesonephrons and metanephrons. Understand
Development of the Urinary System Lecture Objectives Understand the development of the kidney and related organs of the urinary system. Define the pronephrons, mesonephrons and metanephrons. Understand
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Betaglycan Is Required for the Establishment of Nephron Endowment in the Mouse
 Betaglycan Is Required for the Establishment of Nephron Endowment in the Mouse Kenneth A. Walker 1,2 *, Sunder Sims-Lucas 1,3, Georgina Caruana 1, Luise Cullen-McEwen 1, Jinhua Li 1, Mai A. Sarraj 2, John
Betaglycan Is Required for the Establishment of Nephron Endowment in the Mouse Kenneth A. Walker 1,2 *, Sunder Sims-Lucas 1,3, Georgina Caruana 1, Luise Cullen-McEwen 1, Jinhua Li 1, Mai A. Sarraj 2, John
A Common RET Variant Is Associated with Reduced Newborn Kidney Size and Function
 A Common RET Variant Is Associated with Reduced Newborn Kidney Size and Function Zhao Zhang,* Jackie Quinlan,* Wendy Hoy, Michael D. Hughson, Mathieu Lemire, Thomas Hudson, Pierre-Alain Hueber,* Alice
A Common RET Variant Is Associated with Reduced Newborn Kidney Size and Function Zhao Zhang,* Jackie Quinlan,* Wendy Hoy, Michael D. Hughson, Mathieu Lemire, Thomas Hudson, Pierre-Alain Hueber,* Alice
A road to kidney tubules via the Wnt pathway
 Pediatr Nephrol (2000) 15:151 156 IPNA 2000 DEVELOPMENTAL BIOLOGY REVIEW Seppo J. Vainio Marika S. Uusitalo A road to kidney tubules via the Wnt pathway Received: 21 June 1999 / Revised: 31 March 2000
Pediatr Nephrol (2000) 15:151 156 IPNA 2000 DEVELOPMENTAL BIOLOGY REVIEW Seppo J. Vainio Marika S. Uusitalo A road to kidney tubules via the Wnt pathway Received: 21 June 1999 / Revised: 31 March 2000
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Journal Club. 03/04/2012 Lama Nazzal
 Journal Club 03/04/2012 Lama Nazzal NOTCH and the kidneys Is an evolutionarily conserved cell cell communication mechanism. Is a regulator of cell specification, differentiation, and tissue patterning.
Journal Club 03/04/2012 Lama Nazzal NOTCH and the kidneys Is an evolutionarily conserved cell cell communication mechanism. Is a regulator of cell specification, differentiation, and tissue patterning.
Heart Development. Origins of congenital heart defects Properties of cardiac progenitor cells. Robert G. Kelly
 ESC CBCS Summer School on Cardiovascular Sciences Heart Development 19th June 2013 Origins of congenital heart defects Properties of cardiac progenitor cells Robert G. Kelly Animal models of heart development
ESC CBCS Summer School on Cardiovascular Sciences Heart Development 19th June 2013 Origins of congenital heart defects Properties of cardiac progenitor cells Robert G. Kelly Animal models of heart development
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.
 Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Supplementary Figure 1: Hsp60 / IEC mice are embryonically lethal (A) Light microscopic pictures show mouse embryos at developmental stage E12.5 and E13.5 prepared from uteri of dams and subsequently genotyped.
Branching morphogenesis of the lung: new molecular insights into an old problem
 86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang 1 and Andrew P. McMahon 2 1 Cardiovascular Research
86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang 1 and Andrew P. McMahon 2 1 Cardiovascular Research
LDL Receptor Related Protein 6 Modulates Ret Proto-Oncogene Signaling in Renal Development and Cystic Dysplasia
 LDL Receptor Related Protein 6 Modulates Ret Proto-Oncogene Signaling in Renal Development and Cystic Dysplasia Yongping Wang,* Arjun Stokes,* Zhijian Duan,* Jordan Hui, Ying Xu, YiPing Chen, Hong-Wu Chen,*
LDL Receptor Related Protein 6 Modulates Ret Proto-Oncogene Signaling in Renal Development and Cystic Dysplasia Yongping Wang,* Arjun Stokes,* Zhijian Duan,* Jordan Hui, Ying Xu, YiPing Chen, Hong-Wu Chen,*
The Good and Bad of β-catenin in Kidney Development and Renal Dysplasia
 REVIEW published: 22 December 2015 doi: 10.3389/fcell.2015.00081 The Good and Bad of β-catenin in Kidney Development and Renal Dysplasia FelixJ.Boivin,SanjaySarin,J.ColinEvansandDarrenBridgewater* Department
REVIEW published: 22 December 2015 doi: 10.3389/fcell.2015.00081 The Good and Bad of β-catenin in Kidney Development and Renal Dysplasia FelixJ.Boivin,SanjaySarin,J.ColinEvansandDarrenBridgewater* Department
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and
 Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and Edn1-knockout (Edn1-KO) (c, d) hearts. The boxed areas
Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and Edn1-knockout (Edn1-KO) (c, d) hearts. The boxed areas
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
P215 Spring 2018: Renal Physiology Chapter 18: pp , Chapter 19: pp ,
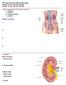 P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
P215 Spring 2018: Renal Physiology Chapter 18: pp. 504-520, 525-527 Chapter 19: pp. 532-548, 553-560 I. Main Components of the Renal System 1. kidneys 2. ureters 3. urinary bladder 4. urethra 4 Major Functions
PAX2 and PAX8 Expression in Primary and Metastatic Renal Tumors. A Comprehensive Comparison
 PAX2 and PAX8 Expression in Primary and Metastatic Renal Tumors A Comprehensive Comparison Ayhan Ozcan, MD; Gustavo de la Roza, MD; Jae Y. Ro, MD, PhD; Steven S. Shen, MD, PhD; Luan D. Truong, MD N Context.
PAX2 and PAX8 Expression in Primary and Metastatic Renal Tumors A Comprehensive Comparison Ayhan Ozcan, MD; Gustavo de la Roza, MD; Jae Y. Ro, MD, PhD; Steven S. Shen, MD, PhD; Luan D. Truong, MD N Context.
BCL11B Regulates Epithelial Proliferation and Asymmetric Development of the Mouse Mandibular Incisor
 BCL11B Regulates Epithelial Proliferation and Asymmetric Development of the Mouse Mandibular Incisor Kateryna Kyrylkova 1, Sergiy Kyryachenko 1, Brian Biehs 2 *, Ophir Klein 2, Chrissa Kioussi 1 *, Mark
BCL11B Regulates Epithelial Proliferation and Asymmetric Development of the Mouse Mandibular Incisor Kateryna Kyrylkova 1, Sergiy Kyryachenko 1, Brian Biehs 2 *, Ophir Klein 2, Chrissa Kioussi 1 *, Mark
Basic Urinary Tract Anatomy and Histology
 Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Heart Development. Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy
 ESC CBCS Summer School on Cardiovascular Sciences 15th June 2011 Heart Development Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy Animal models of heart development Tinman/Nkx2.5
ESC CBCS Summer School on Cardiovascular Sciences 15th June 2011 Heart Development Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy Animal models of heart development Tinman/Nkx2.5
Transgenic Mice and Genetargeting
 Transgenic Mice and Genetargeting mice In Biomedical Science Techniques of transgenic and gene-targeting mice are indispensable for analyses of in vivo functions of particular genes and roles of their
Transgenic Mice and Genetargeting mice In Biomedical Science Techniques of transgenic and gene-targeting mice are indispensable for analyses of in vivo functions of particular genes and roles of their
JINNAH SINDH MEDICAL UNIVERSITY
 MODULE TITLE INTRODUCTION TARGET STUDENTS DURATION MODULE OUTCOMES Spiral -1 Renal and Excretory-1 Module The Renal and Excretory-1 Module is designed to help the learners understand how the urinary system
MODULE TITLE INTRODUCTION TARGET STUDENTS DURATION MODULE OUTCOMES Spiral -1 Renal and Excretory-1 Module The Renal and Excretory-1 Module is designed to help the learners understand how the urinary system
Urinary Anatomy. Lab 40. Kidneys. Nephrons. Renal Corpuscle
 Urinary Anatomy Lab 40. Urinary Anatomy and Kidney Dissection Kidneys: filters blood, produces urine Ureters: convey urine to bladder Bladder: holding tank Urethra: carries urine to the outside for elimination
Urinary Anatomy Lab 40. Urinary Anatomy and Kidney Dissection Kidneys: filters blood, produces urine Ureters: convey urine to bladder Bladder: holding tank Urethra: carries urine to the outside for elimination
URINARY SYSTEM ANATOMY
 URINARY SYSTEM ANATOMY Adapted from Human Anatomy & Physiology Marieb and Hoehn (9 th ed.) OVERVIEW Metabolism of nutrients by the body produces wastes that must be removed from the body. Although excretory
URINARY SYSTEM ANATOMY Adapted from Human Anatomy & Physiology Marieb and Hoehn (9 th ed.) OVERVIEW Metabolism of nutrients by the body produces wastes that must be removed from the body. Although excretory
CHAPTER 25 URINARY. Urinary system. Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1. functions
 CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
Pkd1 Inactivation Induced in Adulthood Produces Focal Cystic Disease
 JASN Express. Published on September 5, 2008 as doi: 10.1681/ASN.2007101139 Pkd1 Inactivation Induced in Adulthood Produces Focal Cystic Disease Ayumi Takakura, Leah Contrino, Alexander W. Beck, and Jing
JASN Express. Published on September 5, 2008 as doi: 10.1681/ASN.2007101139 Pkd1 Inactivation Induced in Adulthood Produces Focal Cystic Disease Ayumi Takakura, Leah Contrino, Alexander W. Beck, and Jing
Obstetrics Content Outline Obstetrics - Fetal Abnormalities
 Obstetrics Content Outline Obstetrics - Fetal Abnormalities Effective February 2007 10 16% renal agenesis complete absence of the kidneys occurs when ureteric buds fail to develop Or degenerate before
Obstetrics Content Outline Obstetrics - Fetal Abnormalities Effective February 2007 10 16% renal agenesis complete absence of the kidneys occurs when ureteric buds fail to develop Or degenerate before
Quantification of early stage lesions for loss of p53 should be shown in the main figures.
 Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Anatomy Unit 3 URINARY SYSTEM
 Human Anatomy Unit 3 URINARY SYSTEM In Anatomy Today Components Kidneys Ureters Urinary bladder Urethra Functions Storage of urine Bladder stores up to 1 L of urine Excretion of urine Transport of urine
Human Anatomy Unit 3 URINARY SYSTEM In Anatomy Today Components Kidneys Ureters Urinary bladder Urethra Functions Storage of urine Bladder stores up to 1 L of urine Excretion of urine Transport of urine
The Beauty of the Skin
 The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
Urinary System. Dr. Ahmed Maher Dr. Ahmed Manhal
 Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Copyright 2003 Pearson Education, Inc. publishing as Benjamin Cummings. Dr. Nabil Khouri
 Dr. Nabil Khouri Objectives: General objectives: - to identify the kidney s structures, function and location - to analyze the relationship between microscopic structure and function Specific objectives:
Dr. Nabil Khouri Objectives: General objectives: - to identify the kidney s structures, function and location - to analyze the relationship between microscopic structure and function Specific objectives:
H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M
 SCPA 602- Anatomical Basis For Pathological Study H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M S O M P H O N G N A R K P I N I T, M. D. D E P A R T M E N T O F P A T H O B I O L O G Y F A C U
SCPA 602- Anatomical Basis For Pathological Study H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M S O M P H O N G N A R K P I N I T, M. D. D E P A R T M E N T O F P A T H O B I O L O G Y F A C U
Chapter 11 Development and Diseases
 Chapter 11 Development and Diseases Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Axon Growth/Target innervation
Chapter 11 Development and Diseases Fertilization Development of the Nervous System Cleavage (Blastula, Gastrula) Neuronal Induction- Neuroblast Formation Cell Migration Axon Growth/Target innervation
The PAX2 Transcription Factor Is Expressed in Cystic and Hyperproliferative Dysplastic Epithelia in Human Kidney Malformations
 The PAX2 Transcription Factor Is Expressed in Cystic and Hyperproliferative Dysplastic Epithelia in Human Kidney Malformations Paul J.D. Winyard,* R. Anthony Risdon, Virginia R. Sams, Gregory R. Dressler,
The PAX2 Transcription Factor Is Expressed in Cystic and Hyperproliferative Dysplastic Epithelia in Human Kidney Malformations Paul J.D. Winyard,* R. Anthony Risdon, Virginia R. Sams, Gregory R. Dressler,
Urinary System and Fluid Balance. Urine Production
 Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
