Pax-2 controls multiple steps of urogenital development
|
|
|
- Annabel Ramsey
- 6 years ago
- Views:
Transcription
1 Development 121, (1995) Printed in Great Britain The Company of Biologists Limited 1995 DEV Pax-2 controls multiple steps of urogenital development Miguel Torres 1, Emilia Gómez-Pardo 1, Gregory R. Dressler 2 and Peter Gruss 1, * 1 Abteilung Molekulare Zellbiologie, Max-Planck-Institut für Biophysikalische Chemie, Göttingen 37077, Germany 2 Howard Hughes Medical Institute and Department of Pathology, University of Michigan, Ann Arbor, MI 48109, USA *Author for correspondence SUMMARY Urogenital system development in mammals requires the coordinated differentiation of two distinct tissues, the ductal epithelium and the nephrogenic mesenchyme, both derived from the intermediate mesoderm of the early embryo. The former give rise to the genital tracts, ureters and kidney collecting duct system, whereas mesenchymal components undergo epithelial transformation to form nephrons in both the mesonephric (embryonic) and metanephric (definitive) kidney. Pax-2 is a transcriptional regulator of the paired-box family and is widely expressed during the development of both ductal and mesenchymal components of the urogenital system. We report here that Pax-2 homozygous mutant newborn mice lack kidneys, ureters and genital tracts. We attribute these defects to dysgenesis of both ductal and mesenchymal components of the developing urogenital system. The Wolffian and Müllerian ducts, precursors of male and female genital tracts, respectively, develop only partially and degenerate during embryogenesis. The ureters, inducers of the metanephros are absent and therefore kidney development does not take place. Mesenchyme of the nephrogenic cord fails to undergo epithelial transformation and is not able to form tubules in the mesonephros. In addition, we show that the expression of specific markers for each of these components is de-regulated in Pax-2 mutants. These data show that Pax-2 is required for multiple steps during the differentiation of intermediate mesoderm. In addition, Pax-2 mouse mutants provide an animal model for human hereditary kidney diseases. Key words: Pax-2, urogenital development, gene targeting, mouse INTRODUCTION Pax genes encode a family of transcriptional regulators specifically expressed during the development of a wide range of structures and organs (Gruss and Walther, 1992). As indicated by both human and mouse mutations, at least five Pax genes have critical morphogenetic functions during the development of complex tissues (Chalepakis et al., 1992; Strachan and Read, 1994). These functions include the development of paraxial mesoderm derivatives (Pax-1) (Balling et al., 1988), neural crest cells and pre-myoblast migration (Pax-3) (Epstein et al., 1991; Bober et al., 1994), B-cell and midbrain development (Pax-5) (Urbanek et al., 1994), and eye and nose development (Pax-6) (Hill et al., 1991; Hadler et al., 1995). A remarkable characteristic is the semidominant character of loss-of-function mutations for three of the members of the family, Pax-1, Pax- 3 and Pax-6. This feature suggests that the dosage of Pax protein is essential to ensure wild-type function. The urogenital system is derived predominantly from the intermediate mesoderm of the early embryo. This mesoderm lies between paraxial and lateral mesoderms and undergoes extensive epithelial transformation to generate the ducts and tubules that compose urogenital tracts and kidneys (see Saxén, 1987, for review). The first event in the differentiation of intermediate mesoderm is the formation of the Wolffian duct, which progresses rostrocaudally from the cervical level towards the cloaca. The duct runs parallel to a tract of intermediate mesoderm, the nephrogenic cord. Subsequently, three embryonic kidneys, pronephros, mesonephros and metanephros, develop in spatial and temporal sequence. First and most anterior, the pronephros forms by an epithelial transformation of the nephrogenic cord mesenchyme and results in a linear array of tubules emptying into the Wolffian duct. While the pronephros seems to represent a true excretory organ in fish and amphibians, it remains a rudimentary and transitory structure in the mouse. The mesonephros appears after and just posterior to pronephros and develops in a very similar manner. However, mesonephric tubules may be functional during the embryonic life of mammals. Metanephric development starts when the ureter buds out from the posterior Wolffian duct and contacts the metanephric mesenchyme in the caudal part of the nephrogenic cord. Signals from the ureter induce metanephric mesenchyme to condense and proliferate at the ureter tips and reciprocal signals from the mesenchyme induce the ureter to grow and branch, thus forming the kidney collecting system (Grobstein, 1953, 1955). The induced mesenchyme at the tips of the branches undergoes transformation to generate glomerular, proximal tubular and distal tubular epithelium. Mutations in genes expressed during urogenital development disrupt different aspects of metanephros development.
2 4058 M. Torres and others The transcription factor WT-1 is expressed in uninduced mesenchyme and is required for the induction response (Kreidberg et al., 1993). The tyrosine kinase receptor c-ret (Schuchardt et al., 1994) is essential for ureter growth and branching. The secreted factor Wnt-4 is required for metanephric tubule formation (Stark et al., 1994). However, besides a reduction in the number of mesonephric tubules reported in WT-1 mutants, other epithelial derivatives of intermediate mesoderm develop normally in these mutants. In the male, mesonephric tubules and the Wolffian duct lose their excretory function and are transformed into the genital tract. Genital tracts originate in females from the Müllerian ducts, which are also derived from the intermediate mesoderm and develop parallel to the Wolffian duct much later in development. Soon after ducts appear, sexual dimorphism is evident and the Wolffian duct and mesonephros degenerate in the female while the Müllerian duct degenerates in the male (Jost and Magre, 1984). Pax-2 is expressed in both ductal and mesenchymal components deriving from the intermediate mesoderm during the development of pronephros, mesonephros and metanephros (Dressler et al., 1990). In vitro experiments have suggested a role for Pax-2 in the conversion of mesenchyme to epithelium during metanephros development (Rothenpieler and Dressler, 1993). Recently, kidney hypoplasia has been reported associated with heterozygosity for a human PAX-2 point mutation (Sanyanusin et al., 1995) and a mouse chromosome 19 deletion (Krd) spanning about 400 genes, which includes the Pax-2 gene (Keller et al., 1994). These data suggest a role for Pax-2 in kidney organogenesis and adds Pax-2 to the class of haploinsufficient Pax genes. We report here the generation and analysis of a mouse Pax- 2 null mutation (Pax-2 ). Our results indicate that Pax-2 is essential for multiple steps during intermediate mesoderm development including the differentiation of both ductal and mesenchymal components of mesonephros and metanephros and their derivatives. All mutations reported to date affecting urogenital development exclusively disrupt metanephros development with only one of the two components involved, ureter or mesenchyme. These data show that Pax-2 is a primary regulator of urogenital development. We suggest that Pax-2 may control a regulatory hierarchy of genes involved in the differentiation of intermediate mesoderm. MATERIALS AND METHODS Targeting Pax-2 locus A positive-negative selection construct was designed. R1 ES cells were electroporated and selected as described (Wurst and Joyner, 1993) 234 independent resistant clones were screened by genomic southern blot using probe a (Fig. 1) and BamHI digestion. One clone (no. 132) gave the expected recombinant band. Clone 132 was used to generate chimeras by morula aggregation (Nagy and Rossant, 1993) and germline transmission of the Pax-2 mutation. F 1 and F 2 generations were genotyped by genomic southern blot using either probe a and BamHI digestion or probe b and HindIII digestion. Embryo dissections Fetuses were collected in PBS and yolk sacs or tails were removed for DNA analysis by Southern blot. Urogenital systems were dissected out and cleaned from main blood vessels. Histology E12.5 embryos were dissected out in PBS and embryos were fixed in Fig. 1. Generation of Pax-2 mutant mice. (A) The targeting construct and expected recombination event with sizes of restriction enzyme digests characteristic for wild-type and mutant alleles. Homologous recombination would remove about 4 kb between the NotI site in exon one, 20 nucleotides before the ATG, and the PvuII site inside the paired box-encoding region in exon two. B, BamHI; N, NotI; H, HindIII; P, PvuII; Bg, BglII; WT, wild type; Mut, mutant. (B) Comparative Southern Blot analysis shows the wild-type bands in the parental ES cell line and both wild-type and expected mutant bands in clone 132. (C) Results obtained from an F2 litter genotyped with probe b and HindIII digestion. Mendelian segregation of the wild-type and mutant allele is observed at birth.
3 Pax-2 required for urogenital development 4059 Fig. 2. Analysis of the urogenital system defects in Pax-2 mutant mice at E17.5 (A-D) Whole mounts of dissected complete urogenital systems at E17.5 in male (A,C) and female (B,D) homozygous and heterozygous mutants and wild-type fetus. Note the reduced size of kidneys in heterozygous female shown in B. (E,F) Detail of the distal region of male urogenital tracts. (G,H) Detail of gonads and proximal part of genital tracts in female and male, mutant and wild-type fetus. Note the absence of seminal vesicles and vas deferens in F and the normal appearance of gonads in both sexes (G,H). Note that endodermderived components: bladder, urethra and prostatic glands (not shown) are unaffected. Mutant gonads are surrounded by a remnant of the urogenital ridge (*) which is blunt at both ends. ad, adrenal glands; ur, ureter; u, urethra; b, bladder; t, testis; e, epididymis; ut, uterus; o, ovary; od, oviduct; mt, mesonephric tubules developing into ductuli efferentes; v, vas deferens; sv, seminal vesicle.
4 4060 M. Torres and others Fig. 3. Analysis of the expression of c-ret during the development of the Wolffian duct in Pax-2 mutant embryos. (A,B) Vibratome cross sections of E9.5 embryos after wholemount in situ hybridization with the c-ret probe; (C,D) similar but correspond to E10.5. (E) Dissected posterior regions of whole-mount E11 wild-type and mutant embryos hybridized with a c-ret probe. wd white arrowhead, Wolffian duct; ub, ureter bud; black arrow, nephrogenic cord. 4% paraformaldehyde (PFA) and, after dehydration, embedded in paraffin and sectioned at 8 to 10 µm. Sections were stained with haematoxylin and Eosin. E16.5 embryos were treated similarly except they were fixed in Bouin s fixative and stained by Mallory s tetrachromic procedure. Antibody staining E13.5 embryos were fixed overnight in PFA, immersed in sucrose 30% for 24 hours, embedded in tissue tek and cryostat sectioned at 10 µm. Sections were incubated with a Pax-2 polyclonal antibody as described (Dressler and Douglas, 1992) using a Cy3-conjugated secondary antibody. Whole-mount in situ hybridizations were performed as described (Wilkinson, 1992) using probes previously described for Pax-2 (Dressler et al., 1990) and Six-2 (Oliver et al., 1995), and a 700 bp EcoRI fragment from the pmcret10 plasmid of c-ret (Pachnis et al., 1993). After in situ hybridization embryos were embedded in gelatin and vibratome sectioned at 30 µm. RESULTS Pax-2 mutants lack kidneys, ureters and genital tracts In order to study Pax-2 function, we have generated a null allele by homologous recombination in embryonic stem (ES) cells (Fig. 1). Pax-2 heterozygous (Pax-2 +/ ) and homozygous (Pax-2 / ) mutant animals are born at the expected Mendelian proportions. Homozygous mutant animals invariably lack both ureters and kidneys while heterozygous mutants frequently show a reduction in kidney size, which ranges from 1/10 of the normal size to wild-type size (Fig. 2). In addition, homozygous mutants of both sexes completely lack the entire genital tracts; females lack oviducts, uterus and vagina and males lack ductuli efferentes, epididymis, vas deferens and seminal vesicles. Gonads appear, in mutants from both sexes, surrounded by a blunt-ended remnant of the genital ridge. Kidney defects seem to be the only trait affected in the urogenital system of heterozygous mutants, genital tracts appear normal in these embryos. All the structures missing in the Fig. 4. Histological analysis at E12.5. (A,C) Cross sections through genital ridges of E12.5 control embryos at, respectively, mesonephric and infra-mesonephric levels (B,D) Similar sections from mutant embryos. Black arrowhead shows the place of the degenerating (B) or missing (D) Wolffian duct in mutants, and white arrowhead the place of the missing mesonephric tubules in mutants. wd, Wolffian duct; mt, mesonephric tubules. mutants are intermediate mesoderm-derived. In contrast, the endoderm-derived structures: urethra, bladder and prostatic glands appear normal. Development of genital tracts in Pax-2 mutants In males, the epididymis, vas deferens and seminal vesicles are derived from the Wolffian duct and in females, the oviducts, uteri and upper part of the vagina are derived from the Müllerian duct. To study the primary cause of the defects observed, we
5 Pax-2 required for urogenital development 4061 Fig. 5. Histological analysis of wolffian and Müllerian ducts development at E17.0. Cross sections of female wildtype (A), female mutant (B), male wildtype (C) and male mutant embryos (D). Note in B the degeneration of the Wolffian (*) and Müllerian (arrow) ducts in females and the normal appearance of the ovary. (D) Wolffian duct (*) presents a similarly degenerated aspect in males. E to H show details of the Wolffian ducts in wild-type and mutant females (E,F, respectively) and wild-type and mutant males (G,H, respectively). Degenerated ducts are found inside the remnant of the urogenital ridge shown in Fig. 2. wd, Wolffian duct; md, Müllerian duct; k, kidney; vd, vas deferens.
6 4062 M. Torres and others Fig. 6. (A,B) Cross sections of females at sacral level of respectively wild-type and mutant E17.0 embryos; (C,D) similar sections at a pelvic level. Observe that mutants lack uterine horns, vagina and ureters. Arrows mark the places of the missing structures. r, rectum; ur, ureter; ut, uterine horn; b, bladder; v, vagina. Fig. 7. Pax-2 expression in the developing Wolffian and Müllerian ducts. (A,B) Phase contrast and fluorescent images, respectively, of cross sections through the Wolffian and Müllerian ducts at E13.5 showing antibody detection of Pax-2 protein expression in wild-type embryos. wd, Wolffian duct; md, Müllerian duct. followed the development of these ducts using histological analysis. In addition, we used the c-ret gene as a marker, a tyrosine kinase receptor expressed in the Wolffian duct and ureters (Pachnis et al., 1993). At 9.5 days of embryonic development (E9.5), when most rostral parts of Wolffian duct are being generated, there are no differences between mutant and wild-type embryos in morphology and expression of c-ret (Fig. 3). However, while the Wolffian duct has reached the cloaca and strongly expresses c-ret at E10.5 in Pax-2 +/+ embryos, in Pax-2 / littermates the Wolffian ducts do not reach the cloaca and have lost c-ret expression (Fig. 3). A similar situation is Fig. 8. Analysis of Pax-2 and Six-2 expression during mesonephric development. (A) A vibratome cross section at E9.5 after wholemount in situ hybridization with Pax-2 probe. The white arrowhead marks the Wolffian duct and the black arrow, the nephrogenic cord. (B,C) Cross sections of wild-type and mutant embryos, respectively, at E9.5 after whole-mount in situ hybridization with a Six-2 probe. observed in E11.5 embryos where the Wolffian ducts do not extend beyond somite 25 in the mutants (Fig. 3). In E12.5 embryos, the parts of the Wolffian duct initially formed are degenerating in discontinuous spherical epithelial bodies (Fig. 4). Müllerian ducts develop in parallel to Wolffian ducts around E13.0. At E13.5 Müllerian ducts are found in wild-type embryos along the entire length of the urogenital
7 Pax-2 required for urogenital development 4063 Fig. 9. Metanephric development in Pax-2 mutants. (A,B) Cross sections at a metanephric level of E12.5 wild-type and mutant embryos, respectively. wd, Wolffian duct; ur, ureter; mm, metanephric mesenchyme. Arrowhead marks the place of the missing Wolffian duct and asterisk marks metanephric mesenchymes in the mutants. (C,D) Kidney cross sections at the level where the ureter stems from the calyx of, respectively, wild-type and heterozygous E17.0 embryos. c, calix; ur, ureter. ridge parallel to the Wolffian ducts and reaching the cloaca. At the same stage, Müllerian ducts are only present at the upper most levels of the genital ridge in Pax-2 / embryos (data not shown). By E16.5 the part of the Müllerian duct initially formed has degenerated and the spherical bodies remaining from the degeneration of the Wolffian duct have lost the epithelial morphology in both sexes (Fig. 5). The degenerated remains from both ducts are contained within the remnant of the urogenital ridges flanking the gonads (Fig. 5). Sections at a lower level at this stage show the absence of uterine horns and vagina in mutant females (Fig. 6). We have analysed the expression of Pax-2 protein in the developing urogenital ridge at E13.5 when both Wolffian and Müllerian ducts are present. At this stage, Pax-2 is expressed in both developing Wolffian and Müllerian ducts, suggesting an autonomous function of Pax-2 in the development of both ducts (Fig. 7). Development of mesonephros in Pax-2 mutant embryos The mesonephros is a transient embryonic kidney consisting of a linear array of mesonephric tubules connecting to the Wolffian duct (Saxén, 1987). Formation of the mesonephric tubules requires epithelial transformation of the nephrogenic cord mesenchyme flanking the Wolffian duct and is concomitant with Pax-2 expression (Dressler et al., 1990 and Fig. 8). Pax-2 / embryos do not develop mesonephric tubules (Fig. 4), Fig. 10. Expression analysis of Six-2 and c-ret during metanephros development in Pax-2 mutant embryos. Vibratome cross sections at the metanephric level of E11.5 embryos hybridized with c-ret (A,B) or Six-2 probes (C,D). (A,C) Wild-type embryos; (B,D) mutant embryos. ur, ureter; w, Wolffian duct at the level where the ureter buds out. therefore, it is likely that Pax-2 is needed for the epithelial transition in the nephrogenic cord. We have analyzed tubule formation using the homeobox gene Six-2 as a marker (Oliver et al., 1995). While initially weakly expressed in the nephrogenic cord, Six-2 is upregulated precluding tubule formation at about E10 (Fig. 8). In contrast, Pax-2 / embryos keep a low expression level of Six- 2 at E10, not showing the up-regulation typical of this stage. Mesonephric tubules regress in the female but persist in the male where they form the ductuli efferentes, also missing in the mutants (Fig. 2). Metanephros development in Pax-2 mutants Development of the definitive kidney starts at about E11 when the ureter buds out from the posterior part of the Wolffian duct and contacts the metanephric mesenchyme, a morphologically distinct group of posterior nephrogenic cord cells (Grobstein, 1953, 1955). Ultimately, the mesenchyme will undergo epithelial transformation to generate the definitive nephrons (Eckblom et al., 1981). Pax-2 is expressed in both the ureter and induced mesenchyme (Dressler et al., 1990). In Pax-2 / embryos, metanephric development does not take place since ureter buds are absent (Fig. 9), and the ureter marker c-ret is not expressed (Fig. 10). However, there is a morphologically distinct metanephric mesenchyme (Fig. 9). Paralleling mesonephric development, Six-2 is expressed weakly in uninduced mesenchyme and strongly up-regulated after invasion of the mesenchyme by the ureter bud at E11.5. In contrast, mutant embryos still express Six-2 at similar levels to the uninduced mesenchyme (Fig. 10). The function of Pax-2 in metanephric development appears
8 4064 M. Torres and others so crucial that even one wild-type copy of the gene is not enough to provide normal kidney development. Pax-2 +/ animals frequently develop hypoplastic kidneys mainly due to reduced calyces and upper part of the ureters, suggesting defects in ureter branching and/or cell proliferation, but also to a thinner cortical region where a reduced number of developing nephrons is found, suggesting a decreased rate of epithelial transformation and/or cell proliferation (Fig. 9). DISCUSSION Pax-2 and the development of epithelial components of the intermediate mesoderm The defects found in urogenital system development in Pax- 2 mutant mice point to a critical function during intermediate mesoderm differentiation. This mesoderm undergoes a series of sequential inductions that transform it into different epithelial components of the kidney and the urogenital tracts. The mesenchymal cells of the intermediate mesoderm are unique in this respect, as most other epithelial tissues are formed by branching morphogenesis of pre-existing epithelium. Pax-2 is expressed during formation and differentiation of all epithelial structures derived from intermediate mesoderm, consistent with the developmental defects in Pax-2 mutants. In Pax-2 mutants, we observe defects not only in the formation of the Wolffian duct and ureter but also in tubule formation during mesonephric development. The Wolffian duct appears normal at E9-10 but fails to extend caudally towards the cloaca. Rather, by E11, the duct begins to degenerate and appears as discontinuous epithelium with enlarged lumens. Although duct formation appears normal in the region of the mesonephros, Pax-2 mutant mice fail to form mesonephric tubules. The mutant Wolffian duct may not be competent to induce tubule formation despite its normal morphology. However, it is likely that the Pax-2 mutant mesonephric mesenchyme is unable to undergo epithelial transformation, as mesonephric tubule formation can occur independent of the Wolffian duct (Etheridge, 1968; Croisille et al., 1976). Therefore, the defects in mesonephric tubules observed in Pax-2 / embryos most probably result from an autonomous function of Pax-2 in tubule formation. Likewise, the defects observed in the Wolffian duct formation and posterior degeneration are most likely due to an autonomous function of Pax-2 in the duct cells. However, we cannot exclude the possibility that the defects in the Wolffian duct may result at least partially from an impaired interaction with the mutant mesenchyme. Because the ureter fails to form, it is not possible to study tubule formation during metanephric development in Pax-2 mutant embryos. However, in vitro experiments using Pax-2 antisense oligonucleotides (Rothenpieler and Dressler, 1993) have suggested that Pax-2 is also necessary for epithelial transformation in metanephric tubule formation. In our studies and those by Keller et al. (1994), heterozygous mice carrying a normal Pax-2 allele show reduced kidney size and an attenuated nephrogenic zone. Thus, a Pax-2 function during proliferation and epithelial differentiation of the metanephric mesenchyme is clearly indicated. The function of Pax-2 is thus essential for the development of all epithelial components derived from the intermediate mesoderm, irrespective of their time of appearance or mechanism of induction. Supporting this view, the development of the Müllerian duct is also impaired. The Müllerian duct and the Wolffian duct both originate from the intermediate mesoderm; however, each duct consists of unique cell types since they react in opposite ways to sex hormones (Jost and Magre, 1984). Epithelial components are not the only derivatives from the intermediate mesoderm. Mesenchymal cells from the mesonephros migrate during gonadal development and populate testis where they contribute to interstitial cell populations (Buehr et al., 1993). Quite remarkably, in Pax-2 mutants, testis appear completely normal in structure and cell type composition (data not shown), supporting the view that Pax-2 function is specifically needed for development of the epithelial components of the intermediate mesoderm. Among the genes required for urogenital development are c- ret, WT-1 and Wnt-4. WT-1 is expressed in the uninduced metanephric mesenchyme and enables these cells to respond to induction by the ureteric bud. In the absence of WT-1 function in the mesenchyme, there is no ureteric bud outgrowth from the adjacent Wolffian duct. Thus, the outgrowth of the duct may depend on positional cues originating from the metanephric mesenchyme. These cues may be received by the receptor-type kinase c-ret, which is expressed in the Wolffian duct and subsequently in the tips of the growing ureter bud. Finally, in the absence of the secreted factor Wnt-4, tubule formation does not take place in metanephros, despite normal ureter growth and branching (Stark et al., 1994). Because Pax-2 is a transcription factor, it may be required to regulate the expression of genes essential for the differentiation of the epithelial components of the urogenital system. We have observed alterations in the expression pattern of c-ret and Six-2 during the development of the affected structures. However, it is likely that these alterations are the result of impaired development of the structures implicated, rather than implying direct control by Pax-2 of the expression of these genes. In support of this view, expression of the c-ret gene was normal in the Wolffian duct and altered only after the duct started degeneration. Pax-2 and kidney disease There is suggestive evidence that links human kidney diseases with altered Pax-2 expression. During normal kidney development, Pax-2 is repressed after tubule formation (Dressler et al., 1990). Overexpression of Pax-2 is incompatible with normal kidney development in transgenic mice and results in kidney abnormalities similar to human nephrotic syndromes (Dressler et al., 1993). Persistent Pax-2 expression is found in Wilm s tumor (Dressler and Douglas, 1992; Eccles et al., 1992) and in adult renal carcinoma where it is required for continued proliferation (Gnarra and Dressler, 1995). Partial loss of function by reducing the Pax-2 gene dosage through mutation of one of the alleles, either in humans or mice, results in hypoplastic kidneys (Keller et al., 1995; Sanyanusin et al., 1995; this report). Appropriate timing and dosage of Pax-2 expression is therefore essential to accomplish proper development of the kidney. There are at least four haplo-insufficient Pax genes, Pax-1, Pax-2, Pax-3 and Pax-6. Single gene haploinsufficiency is rather rare; for the majority of genes one copy is enough to ensure wild-type function. The unusual high incidence of haplo-insufficient phenotypes among Pax genes suggests that, despite the heterogeneity in their developmental functions, an underlying common molecular mechanism for
9 Pax-2 required for urogenital development 4065 Pax protein function may exist that depends quantitatively on the level of protein present in the cell. Pax-2 is also expressed during central nervous system (CNS) development in eye, ear and midbrain (Nornes et al., 1990; Püschel et al., 1993). We have observed defects in the development of these three structures in Pax-2 mutant mice (unpublished data). Eye defects have also been reported in Krd mice and human PAX-2 mutants. Therefore, Pax-2 mutant mice provide an excellent animal model for the study of both CNS and urogenital system human hereditary diseases. We thank S. Mashur M. Breuer and R. Scholz for excellent technical assistance, G. Oliver, M. Kessel, F. Pituello, K. Chowdhury and E. Stuart for critical comments on the manuscript, B. Sosa-Pineda for advice on the cryostat sectioning, F. Constantini for the c-ret probe, G. Oliver for the Six-2 probe and R. Altschäffel for excellent photographic work. M. T. received support from EMBO and European Community Human Genome Analysis program. REFERENCES Balling, R., Deutsch, U. and Gruss, P. (1988). undulated, a mutation affecting the development of the mouse skeleton has a point mutation in the paired box of Pax-1. Cell 55, Bober, E., Franz, T., Arnold, H. H., Gruss, P. and Tremblay, P. (1994). Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development 120, Buehr, M., Gu, S. and McLaren, A. (1993). Mesonephric contribution to testis differentiation in the fetal mouse. Development 117, Chalepakis, G., Tremblay, P., and Gruss, P. (1992). Pax genes, mutants and molecular function. J. Cell Sci. Supplement 16, Croisille, Y., Gumpel-Pinot, M. and Martin, C. (1976). Embryologie expérimentale. La differenciation des tubes sécréteurs du rein chez les oiseaux: effects des inducteurs hétérogènes. C. R. Acad. Sci. Paris Ser. 282, Dressler, G. R., Deutsch, U., Chowdhury, K., Nornes, H. O. and Gruss, P. (1990). Pax-2, anew paired-box-containing gene and its expression in the developing excretory system. Development 109, Dressler, G. R. and Douglas, E. C. (1992). Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc. Nat. Acad. Sci. USA 89, Dressler, R., Wilkinson, J. E., Rothenpieler, U. W., Patterson, L. T., Williams-Simons, L. and Westphal, H. (1993). Deregulation of Pax-2 expression in transgenic mice generates kidney abnormalities. Nature 362, Eccles, M. R., Wallis, L. J., Fidler, A. E., Spur, N. K., Goodfellow, P. J. and Reeve, A. E. (1992). Expression of the Pax-2 gene in human fetal kidney and Wilm s tumor. Cell Growth Diff. 3, Eckblom, P., Miettinen, A., Virtanen, I., Wahlström, A. D. and Saxén, L. (1981). In Vitro segregation of the metanephric nephron. Dev. Biol. 84, Epstein, D. J., Vekemans, M. and Gros P. (1991). Splotch (Sp 2h ), a mutation affecting the development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell 67, Etheridge, A. L. (1968). Determination of the mesonephric kidney. J. Exp. Zool. 169, (1968) Gnarra, J. R. and Dressler, G. R. (1995). Expression of Pax-2 in renal cell carcinoma and growth inhibition by antisense oligonucleotides. Cancer Res. (In Press. Grobstein, C. (1953). Inductive epithelio-mesenchymal interaction in cultured organ rudiments of the mouse Science 118, Grobstein, C. (1955). Tissue interactions in the morphogenesis of the mouse metanephros. J. Exp. Zool. 130, Gruss, P. and Walther, C. (1992). Pax in development. Cell 69, Hadler, G., Callaerts, P. and Gehring, W. J. (1995). Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267, Hill, R. E., Favor, J., Hogan, B. L. M., Ton, C. C. T., Saunders, G. F., Hanson, I. M., Prosser, J., Jordan, T., Hastie, N. D. and Van Heyningen, V. (1991). Mouse Small eye results from mutations in a paired-like homeobox-containing gene. Nature 354, Jost, A. and Magre, S. (1984). Testicular development phases and dual hormonal control of sexual organogenesis. In Sexual Differentiation: Basic and clinical Aspects. (Ed. Serio et al.) pp New York: Raven Press. Keller, S. A., Jones, J. M., Boyle, A. Barrow, L. L., Killen, P. D., Green, D. G., Kapousta, N. V., Hitchcock, P. F., Swank, R. T. and Meisler, M. H. (1994). Kidney and retinal defects (Krd), a transgene-induced mutation with a deletion of mouse chromosome 19 that includes the Pax-2 locus. Genomics 23, Kreidberg, J. A., Sariola, H., Loring, J. M., Maeda, M., Pelletier, J., Housman, D. and Jaenisch, R. (1993). WT-1 is required for early kidney development. Cell 74, Nagy, A. and Rossant, J. (1993). Production of completely ES cell-derived fetuses. In Gene Targeting, A Practical Approach. (Ed. A. Joyner) pp Oxford: IRL Press. Nornes, H. O., Dressler, G. R., Knapik, E. W., Deutsch, U. and Gruss, P. (1990). Spatially and temporally restricted expression of Pax-2 during murine neurogenesis. Development 109, Oliver, G., Wehr, R., Jenkins, N. A., Copeland, N. G., Cheyette, B. N. R., Hartenstein, V., Zipursky, S. L. and Gruss, P. (1995). Homeobox genes and connective tissue patterning. Development 121, Pachnis, V., Mankoo, B. and Constantini, F. (1993). Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119, Püschel, A. W., Westerfield, M. and Dressler, G. R. (1992). Comparative analysis of Pax-2 protein distributions during neurulation in mice and Zebrafish. Mech. Dev. 38, Rothenpieler, U. W. and Dressler, G. R. (1993). Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development 119, Sanyanusin, P., Schimenti, L. A., McNoe, L. A., Ward, T. A., Pierpont, M. E. M., Sullivan, M. J., Dobyns, W. B. and Eccles, M. R. (1995). Mutation of the Pax-2 gene in a family with optic nerve colobomas, renal anomalies and vesiculoureteral reflux. Nature Genet. 9, Saxén, L. (1987). Organogenesis of the Kidney. Developmental and Cell Biology Series 19 (ed. P. W. Barlow, P. B. Green and C. C. White) Cambridge: Cambridge University Press. Schuchardt, A., D Agati, V., Larsson-Blomberg, L., Constantini, F. and Pachnis, V. (1994). Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, Stark, K., Vainio, S., Vassileva, G. and McMahon, A. P. (1994). Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, Strachan, T. and Read A. P. (1994). Pax genes. Curr. Op. Dev. Biol. 4, Urbanek, P.,Wang, Z., Fetka, I., Wagner, E. F. and Busslinger, M. (1994). Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax-5/BSAP. Cell 79, Wilkinson, D. G. (1992). Whole mount in situ hybridization of vertebrate embryos. In In situ hybridization, A Practical Approach. (ed. D. Wilkinson) pp Oxford: IRL Press. Wurst, W. and Joyner, A. L. (1993). Production of targeted embryonic stem cell clones. In Gene Targeting, A Practical Approach. (ed. A. Joyner) pp Oxford: IRL Press. (Accepted 12 September 1995)
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
Formation of Urine: Formation of Urine
 The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
Development of the Urinary System. 3 Distinct Embryonic Kidney Structures
 Development of the Urinary System Excretory portion of urinary system derived from intermediate mesoderm Week 4: 1 st nephrons/renal corpuscles form Nephrotomes form and develop hollow lumens to form nephric
Development of the Urinary System Excretory portion of urinary system derived from intermediate mesoderm Week 4: 1 st nephrons/renal corpuscles form Nephrotomes form and develop hollow lumens to form nephric
Development of the Urinary System
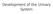 Development of the Urinary System Lecture Objectives Understand the development of the kidney and related organs of the urinary system. Define the pronephrons, mesonephrons and metanephrons. Understand
Development of the Urinary System Lecture Objectives Understand the development of the kidney and related organs of the urinary system. Define the pronephrons, mesonephrons and metanephrons. Understand
Urinary system development. Male ( ) and Female ( ) Reproductive Systems Development
 Urinary system development Male ( ) and Female ( ) Reproductive Systems Development Urogenital system develops from mesodermal uro-genital ridge (intermediate mesoderm) development of male and female genital
Urinary system development Male ( ) and Female ( ) Reproductive Systems Development Urogenital system develops from mesodermal uro-genital ridge (intermediate mesoderm) development of male and female genital
Urinary System. J. H. Lue. intermediate mesoderm cloaca coelomic epithelium
 Urinary System J. H. Lue Primordium: intermediate mesoderm cloaca coelomic epithelium 1 3w(18d) 3 w 4w (24d) 4w (26d) 2 Intermediate mesoderm 3 Intermediate mesoderm intermediate mesoderm urogenital ridge
Urinary System J. H. Lue Primordium: intermediate mesoderm cloaca coelomic epithelium 1 3w(18d) 3 w 4w (24d) 4w (26d) 2 Intermediate mesoderm 3 Intermediate mesoderm intermediate mesoderm urogenital ridge
11. SEXUAL DIFFERENTIATION. Germinal cells, gonocytes. Indifferent stage INDIFFERENT STAGE
 11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
Development of the Genital System
 Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Animal Science 434 Reproductive Physiology"
 Animal Science 434 Reproductive Physiology" Embryogenesis of the Pituitary and Sexual Development: Part A Development of the Pituitary Gland" Infundibulum" Brain" Rathke s Pouch" Stomodeum" Germ Cell Migration"
Animal Science 434 Reproductive Physiology" Embryogenesis of the Pituitary and Sexual Development: Part A Development of the Pituitary Gland" Infundibulum" Brain" Rathke s Pouch" Stomodeum" Germ Cell Migration"
Renal agenesis and hypodysplasia in ret-k mutant mice result from defects in ureteric bud development
 Development 122, 1919-1929 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3412 1919 Renal agenesis and hypodysplasia in ret-k mutant mice result from defects in ureteric bud
Development 122, 1919-1929 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3412 1919 Renal agenesis and hypodysplasia in ret-k mutant mice result from defects in ureteric bud
under its influence, male development occurs; in its absence, female development is established.
 Sex differentiation is a complex process that involves many genes, including some that are autosomal. The key to sexual dimorphism is the Y chromosome, which contains the testis determining gene called
Sex differentiation is a complex process that involves many genes, including some that are autosomal. The key to sexual dimorphism is the Y chromosome, which contains the testis determining gene called
Development of the urogenital system
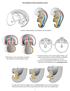 Development of the urogenital system Location of the pronephros, mesonephros and metanephros Differentiation of the intermedierm mesoderm into nephrotome and mesonephric tubules Connection between aorta
Development of the urogenital system Location of the pronephros, mesonephros and metanephros Differentiation of the intermedierm mesoderm into nephrotome and mesonephric tubules Connection between aorta
Development of the urinary system
 Development of the urinary system WSO School of Biomedical Sciences, University of Hong Kong. 3 sets of kidneys developing in succession (temporally and spatially) : Pronephros ] Mesonephros ]- Intermediate
Development of the urinary system WSO School of Biomedical Sciences, University of Hong Kong. 3 sets of kidneys developing in succession (temporally and spatially) : Pronephros ] Mesonephros ]- Intermediate
W.S. O University of Hong Kong
 W.S. O University of Hong Kong Development of the Genital System 1. Sexual differentiation 2. Differentiation of the gonads a. Germ cells extragonadal in origin b. Genital ridge intermediate mesoderm consisting
W.S. O University of Hong Kong Development of the Genital System 1. Sexual differentiation 2. Differentiation of the gonads a. Germ cells extragonadal in origin b. Genital ridge intermediate mesoderm consisting
Comparative Anatomy Urogenital System. Note Set 11 Chapter 15
 Comparative Anatomy Urogenital System Note Set 11 Chapter 15 Urogenital System Ducts of excretory and reproductive systems are intimately associated Figure 14.1: Embryonic and evolutionary development
Comparative Anatomy Urogenital System Note Set 11 Chapter 15 Urogenital System Ducts of excretory and reproductive systems are intimately associated Figure 14.1: Embryonic and evolutionary development
Animal Science 434 Reproductive Physiology
 Animal Science 434 Reproductive Physiology Development of the Pituitary Gland Lec 5: Embryogenesis of the Pituitary and Sexual Development Stomodeum Brain Infundibulum Rathke s Pouch Germ Cell Migration
Animal Science 434 Reproductive Physiology Development of the Pituitary Gland Lec 5: Embryogenesis of the Pituitary and Sexual Development Stomodeum Brain Infundibulum Rathke s Pouch Germ Cell Migration
Six1 is required for the early organogenesis of mammalian kidney
 Development 130, 3085-3094 2003 The Company of Biologists Ltd doi:10.1242/dev.00536 3085 Six1 is required for the early organogenesis of mammalian kidney Pin-Xian Xu 1, *, Weiming Zheng 1, Li Huang 1,
Development 130, 3085-3094 2003 The Company of Biologists Ltd doi:10.1242/dev.00536 3085 Six1 is required for the early organogenesis of mammalian kidney Pin-Xian Xu 1, *, Weiming Zheng 1, Li Huang 1,
Urogenital System. PUMC Dept. of Anat. Hist. & Embry. 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College
 Urogenital System 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College intermediate mesoderm urogenital ridge mesonephric ridge genital ridge I. Urinary System 1. kidney
Urogenital System 钱晓菁 XIAO-JING QIAN Dept. of Anatomy, Histology & Embryology Peking Union Medical College intermediate mesoderm urogenital ridge mesonephric ridge genital ridge I. Urinary System 1. kidney
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
CHARA CTERS* nearly sterile. As in other similar combinations the sex glands are reduced ZOOLOGY: E. WITSCHI
 ZOOLOGY: E. WITSCHI VOL. 23, 1937 35 STIMULATIVE AND INHIBITIVE INDUCTION IN THE DEVELOPMENT OF PRIMARY AND SECONDARY SEX CHARA CTERS* By EMIL WITSCHI DEPARTMENT OF ZOOLOGY, STATE UNIVERSITY OF IOWA Read
ZOOLOGY: E. WITSCHI VOL. 23, 1937 35 STIMULATIVE AND INHIBITIVE INDUCTION IN THE DEVELOPMENT OF PRIMARY AND SECONDARY SEX CHARA CTERS* By EMIL WITSCHI DEPARTMENT OF ZOOLOGY, STATE UNIVERSITY OF IOWA Read
Development of the Urinary System
 Development of the Urinary System ## every things is written in this sheet so u don t have to go back to the slide. ## I write everything and I hope everything will be clear,, if some note doesn t clear
Development of the Urinary System ## every things is written in this sheet so u don t have to go back to the slide. ## I write everything and I hope everything will be clear,, if some note doesn t clear
Sex Determination and Development of Reproductive Organs
 Sex Determination and Development of Reproductive Organs Sex determination The SRY + gene is necessary and probably sufficient for testis development The earliest sexual difference appears in the gonad
Sex Determination and Development of Reproductive Organs Sex determination The SRY + gene is necessary and probably sufficient for testis development The earliest sexual difference appears in the gonad
Growth pattern of the sex ducts in foetal mouse hermaphrodites
 /. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
/. Embryol. exp. Morph. 73, 59-68, 1983 59 Printed in Great Britain The Company of Biologists Limited 1983 Growth pattern of the sex ducts in foetal mouse hermaphrodites By C. YDING ANDERSEN 1, A. G. BYSKOV
Embryology /organogenesis/ Week 4 Development and teratology of reproductive system.
 Embryology /organogenesis/ Week 4 Development and teratology of reproductive system. Male or female sex is determined by spermatozoon Y in the moment of fertilization SRY gene, on the short arm of the
Embryology /organogenesis/ Week 4 Development and teratology of reproductive system. Male or female sex is determined by spermatozoon Y in the moment of fertilization SRY gene, on the short arm of the
Obstetrics Content Outline Obstetrics - Fetal Abnormalities
 Obstetrics Content Outline Obstetrics - Fetal Abnormalities Effective February 2007 10 16% renal agenesis complete absence of the kidneys occurs when ureteric buds fail to develop Or degenerate before
Obstetrics Content Outline Obstetrics - Fetal Abnormalities Effective February 2007 10 16% renal agenesis complete absence of the kidneys occurs when ureteric buds fail to develop Or degenerate before
DISSECTION 8: URINARY AND REPRODUCTIVE SYSTEMS
 8546d_c01_1-42 6/25/02 4:32 PM Page 38 mac48 Mac 48: 420_kec: 38 Cat Dissection DISSECTION 8: URINARY AND REPRODUCTIVE SYSTEMS Typically, the urinary and reproductive systems are studied together, because
8546d_c01_1-42 6/25/02 4:32 PM Page 38 mac48 Mac 48: 420_kec: 38 Cat Dissection DISSECTION 8: URINARY AND REPRODUCTIVE SYSTEMS Typically, the urinary and reproductive systems are studied together, because
Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development
 Research article 2809 Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development Akio Kobayashi 1,2, Kin-Ming Kwan 2, Thomas
Research article 2809 Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development Akio Kobayashi 1,2, Kin-Ming Kwan 2, Thomas
The functional anatomy of the urinary system. Human Anatomy Department Dr. Anastasia Bendelic
 The functional anatomy of the urinary system Human Anatomy Department Dr. Anastasia Bendelic Plan Development of the kidneys and their abnormalities Development of the urinary ways and their abnormalities
The functional anatomy of the urinary system Human Anatomy Department Dr. Anastasia Bendelic Plan Development of the kidneys and their abnormalities Development of the urinary ways and their abnormalities
FORMS OF EMBRYONIC PRIMORDIA
 FORMS OF EMBRYONIC PRIMORDIA BY PROF. ANTHONY OBIOMA NWAOPARA UNIVERSITY OF MEDICAL SCIENCES ONDO CITY, ONDO STATE LEARNING OBJECTIVES To recognise the different forms of embryonic primordia. To recognise
FORMS OF EMBRYONIC PRIMORDIA BY PROF. ANTHONY OBIOMA NWAOPARA UNIVERSITY OF MEDICAL SCIENCES ONDO CITY, ONDO STATE LEARNING OBJECTIVES To recognise the different forms of embryonic primordia. To recognise
Fig General Structural Features of a kidney
 Chapter 11 Development of the Mesodermal Organs in Vertebrates 11.1. Subdivisions of the Mesoderm There are 4 subdivisions of the mesoderm: 1. The Notochord develops into the centrum of the vertebral column.
Chapter 11 Development of the Mesodermal Organs in Vertebrates 11.1. Subdivisions of the Mesoderm There are 4 subdivisions of the mesoderm: 1. The Notochord develops into the centrum of the vertebral column.
1. Both asexual and sexual reproduction occur in the animal kingdom
 1. Both asexual and sexual reproduction occur in the animal kingdom Asexual reproduction involves the formation of individuals whose genes all come from one parent. There is no fusion of sperm and egg.
1. Both asexual and sexual reproduction occur in the animal kingdom Asexual reproduction involves the formation of individuals whose genes all come from one parent. There is no fusion of sperm and egg.
Lesson 31. Lesson Outline:
 Lesson 31 Lesson Outline: The Urogenital System Urinary System o Embryonic Origins o Phylogenetic Trends Function Urinary Bladder Implications for the Evolution of the Vertebrates o Freshwater or Seawater
Lesson 31 Lesson Outline: The Urogenital System Urinary System o Embryonic Origins o Phylogenetic Trends Function Urinary Bladder Implications for the Evolution of the Vertebrates o Freshwater or Seawater
The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
 Development 126, 5771-5783 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9673 5771 The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
Development 126, 5771-5783 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9673 5771 The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
Male Reproduction Organs. 1. Testes 2. Epididymis 3. Vas deferens 4. Urethra 5. Penis 6. Prostate 7. Seminal vesicles 8. Bulbourethral glands
 Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
Outline Terminology Human Reproduction Biol 105 Lecture Packet 21 Chapter 17 I. Male Reproduction A. Reproductive organs B. Sperm development II. Female Reproduction A. Reproductive organs B. Egg development
a) They are the most common cause of pediatric kidney failure. b) They are always symptomatic. c) They can be asymmetric.
 Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats
 Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Chapter 7 DEVELOPMENT AND SEX DETERMINATION
 Chapter 7 DEVELOPMENT AND SEX DETERMINATION Chapter Summary The male and female reproductive systems produce the sperm and eggs, and promote their meeting and fusion, which results in a fertilized egg.
Chapter 7 DEVELOPMENT AND SEX DETERMINATION Chapter Summary The male and female reproductive systems produce the sperm and eggs, and promote their meeting and fusion, which results in a fertilized egg.
The role of GDNF in patterning the excretory system
 Developmental Biology 283 (2005) 70 84 www.elsevier.com/locate/ydbio The role of GDNF in patterning the excretory system Reena Shakya a, Eek-hoon Jho a,1, Pille Kotka a,2, Zaiqi Wu a, Nikolai Kholodilov
Developmental Biology 283 (2005) 70 84 www.elsevier.com/locate/ydbio The role of GDNF in patterning the excretory system Reena Shakya a, Eek-hoon Jho a,1, Pille Kotka a,2, Zaiqi Wu a, Nikolai Kholodilov
Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney
 The EMBO Journal (2006) 25, 5214 5228 & 2006 European Molecular Biology Organization All Rights Reserved 0261-4189/06 www.embojournal.org Six2 is required for suppression of nephrogenesis and progenitor
The EMBO Journal (2006) 25, 5214 5228 & 2006 European Molecular Biology Organization All Rights Reserved 0261-4189/06 www.embojournal.org Six2 is required for suppression of nephrogenesis and progenitor
Urinary System. ectoderm. notochord. mesonephric tubules. Nephrogenic Cord (left)
 Urinary System NOTE: Urine proion requires an increased capillary surface area (glomeruli), epithelial s to collect plasma filtrate and extract desirable constituents, and a system to convey urine away
Urinary System NOTE: Urine proion requires an increased capillary surface area (glomeruli), epithelial s to collect plasma filtrate and extract desirable constituents, and a system to convey urine away
Transgenic Mice and Genetargeting
 Transgenic Mice and Genetargeting mice In Biomedical Science Techniques of transgenic and gene-targeting mice are indispensable for analyses of in vivo functions of particular genes and roles of their
Transgenic Mice and Genetargeting mice In Biomedical Science Techniques of transgenic and gene-targeting mice are indispensable for analyses of in vivo functions of particular genes and roles of their
Chapter 16: Steroid Hormones (Lecture 17)
 Chapter 16: Steroid Hormones (Lecture 17) A) 21 or fewer carbon atoms B) Precursor: 27 carbon cholesterol C) major classes of steroid hormones 1) progestagens a) progesterone- prepares lining of uterus
Chapter 16: Steroid Hormones (Lecture 17) A) 21 or fewer carbon atoms B) Precursor: 27 carbon cholesterol C) major classes of steroid hormones 1) progestagens a) progesterone- prepares lining of uterus
SISTEMA REPRODUCTOR (LA IDEA FIJA) Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
SISTEMA REPRODUCTOR (LA IDEA FIJA) How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development,
- production of two types of gametes -- fused at fertilization to form zygote
 Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
Male reproductive system I. Sexual reproduction -- overview - production of two types of gametes -- fused at fertilization to form zygote - promotes genetic variety among members of a species -- each offspring
PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception
 PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception REVIEW OF PELVIS I Pelvic brim, inlet Pelvic outlet True pelvis-- --viscera Tilt forward Mid-sagital views-- --how the
PELVIS II: FUNCTION TABOOS (THE VISCERA) Defecation Urination Ejaculation Conception REVIEW OF PELVIS I Pelvic brim, inlet Pelvic outlet True pelvis-- --viscera Tilt forward Mid-sagital views-- --how the
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Outline. Male Reproductive System Testes and Sperm Hormonal Regulation
 Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Outline Male Reproductive System Testes and Sperm Hormonal Regulation Female Reproductive System Genital Tract Hormonal Levels Uterine Cycle Fertilization and Pregnancy Control of Reproduction Infertility
Mohammad Sha ban. Basheq Jehad. Hamzah Nakhleh
 11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
11 Mohammad Sha ban Basheq Jehad Hamzah Nakhleh Physiology of the reproductive system In physiology, we are concerned with the mechanisms in which the system functions, and how the system responds to different
Chapter 14 The Reproductive System
 Biology 12 Name: Reproductive System Per: Date: Chapter 14 The Reproductive System Complete using BC Biology 12, page 436-467 14. 1 Male Reproductive System pages 440-443 1. Distinguish between gametes
Biology 12 Name: Reproductive System Per: Date: Chapter 14 The Reproductive System Complete using BC Biology 12, page 436-467 14. 1 Male Reproductive System pages 440-443 1. Distinguish between gametes
DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT. Reading Assignment: The Developing Human, Clinically Oriented Embryology pp
 Developmental Anatomy Steven M. Hill, Ph.D. 9/16/13 DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT Reading Assignment: The Developing Human, Clinically Oriented Embryology pp. 264-273. Objectives:
Developmental Anatomy Steven M. Hill, Ph.D. 9/16/13 DEVELOPMENT OF THE ADRENAL GLAND; TESTES AND MESONEPHRIC DUCT Reading Assignment: The Developing Human, Clinically Oriented Embryology pp. 264-273. Objectives:
Biology of Reproduction-Biol 326
 Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro
 Kidney International, Vol. 67 (25), pp. 42 431 GENETIC DISORDERS DEVELOPMENT Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro JASON E. CAIN,THIBAULD NION,DOMINIQUE
Kidney International, Vol. 67 (25), pp. 42 431 GENETIC DISORDERS DEVELOPMENT Exogenous BMP-4 amplifies asymmetric ureteric branching in the developing mouse kidney in vitro JASON E. CAIN,THIBAULD NION,DOMINIQUE
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
Basic Body Structure
 Basic Body Structure The Cell All life consists of microscopic living structures called cells. They perform various functions throughout the body. All cells are similar in structure, but not identical.
Basic Body Structure The Cell All life consists of microscopic living structures called cells. They perform various functions throughout the body. All cells are similar in structure, but not identical.
Differential regulation of two sets of mesonephric tubules by WT-1
 Development 124, 1293-1299 (1997) Printed in reat ritain The Company of iologists Limited 1997 DEV3617 1293 Differential regulation of two sets of mesonephric tubules by WT-1 Kirsi Sainio 1,2, *, Paavo
Development 124, 1293-1299 (1997) Printed in reat ritain The Company of iologists Limited 1997 DEV3617 1293 Differential regulation of two sets of mesonephric tubules by WT-1 Kirsi Sainio 1,2, *, Paavo
Kidney development proceeds through a complex series
 Pax2 and Pax8 Regulate Branching Morphogenesis and Nephron Differentiation in the Developing Kidney Melina Narlis, David Grote, Yaned Gaitan, Sami K. Boualia, and Maxime Bouchard McGill Cancer Centre and
Pax2 and Pax8 Regulate Branching Morphogenesis and Nephron Differentiation in the Developing Kidney Melina Narlis, David Grote, Yaned Gaitan, Sami K. Boualia, and Maxime Bouchard McGill Cancer Centre and
Chapter 18 Development. Sexual Differentiation
 Chapter 18 Development Sexual Differentiation There Are Many Levels of Sex Determination Chromosomal Sex Gonadal Sex Internal Sex Organs External Sex Organs Brain Sex Gender Identity Gender Preference
Chapter 18 Development Sexual Differentiation There Are Many Levels of Sex Determination Chromosomal Sex Gonadal Sex Internal Sex Organs External Sex Organs Brain Sex Gender Identity Gender Preference
Sexual Development. 6 Stages of Development
 6 Sexual Development 6 Stages of Development Development passes through distinct stages, the first of which is fertilization, when one sperm enters one ovum. To enter an ovum, a sperm must undergo the
6 Sexual Development 6 Stages of Development Development passes through distinct stages, the first of which is fertilization, when one sperm enters one ovum. To enter an ovum, a sperm must undergo the
T analysis of purely genetical problems. Students of other biological sciences
 THE MORPHOLOGICAL MANIFESTATIONS OF A DOMINANT MUTATION IN MICE AFFECTING TAIL AND UROGENITAL SYSTEM S. GLUECKSOHN-SCHOENHEIMER' Columbia University Received March 24, 1943 HE study of hereditary variations
THE MORPHOLOGICAL MANIFESTATIONS OF A DOMINANT MUTATION IN MICE AFFECTING TAIL AND UROGENITAL SYSTEM S. GLUECKSOHN-SCHOENHEIMER' Columbia University Received March 24, 1943 HE study of hereditary variations
Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant
 Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant Reporting Period July 1, 2011 June 30, 2012 Formula Grant Overview The Children's Hospital of Pittsburgh received $958,038 in
Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant Reporting Period July 1, 2011 June 30, 2012 Formula Grant Overview The Children's Hospital of Pittsburgh received $958,038 in
Urinary System Chapter 16
 Urinary System Chapter 16 1 Urology- the branch of medicine that treats male and female urinary systems as well as the male reproductive system. Nephrology- the scientific study of the anatomy, physiology,
Urinary System Chapter 16 1 Urology- the branch of medicine that treats male and female urinary systems as well as the male reproductive system. Nephrology- the scientific study of the anatomy, physiology,
Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells
 AND DISEASE RESEARCH ARTICLE 2995 Development 133, 2995-3004 (2006) doi:10.1242/dev.02442 Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation
AND DISEASE RESEARCH ARTICLE 2995 Development 133, 2995-3004 (2006) doi:10.1242/dev.02442 Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation
Pelvis MCQs. Block 1. B. Reproductive organs. C. The liver. D. Urinary bladder. 1. The pelvic diaphragm includes the following muscles: E.
 Pelvis MCQs Block 1 1. The pelvic diaphragm includes the following muscles: A. The obturator internus B. The levator ani C. The coccygeus D. The external urethral sphincter E. The internal urethral sphincter
Pelvis MCQs Block 1 1. The pelvic diaphragm includes the following muscles: A. The obturator internus B. The levator ani C. The coccygeus D. The external urethral sphincter E. The internal urethral sphincter
Sexual differentiation:
 Abnormal Development of Female Genitalia Dr. Maryam Fetal development of gonads, external genitalia, Mullerian ducts and Wolffian ducts can be disrupted at a variety of points, leading to a wide range
Abnormal Development of Female Genitalia Dr. Maryam Fetal development of gonads, external genitalia, Mullerian ducts and Wolffian ducts can be disrupted at a variety of points, leading to a wide range
Urogenital system - Development. Aleš Hampl
 Urogenital system - Development Aleš Hampl cloaca Urogenital system Overall picture Urogenital system Reminder Urogenital system Intermediate mesoderm Urogenital system Early forms of kidneys - Pronephros
Urogenital system - Development Aleš Hampl cloaca Urogenital system Overall picture Urogenital system Reminder Urogenital system Intermediate mesoderm Urogenital system Early forms of kidneys - Pronephros
LECTURE 4. Anatomy of the urinary and genital organs.
 LECTURE 4 Anatomy of the urinary and genital organs. SKELETOTOPY OF THE KIDNEYS Left kidney upper pole of the kidney - ThXI lower pole of the kidney LIII Right kidney is located on the half vertebra
LECTURE 4 Anatomy of the urinary and genital organs. SKELETOTOPY OF THE KIDNEYS Left kidney upper pole of the kidney - ThXI lower pole of the kidney LIII Right kidney is located on the half vertebra
PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION
 PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION Prof. Dr med. Jolanta Słowikowska-Hilczer Department of Andrology and Reproductive Endocrinology Medical University of Łódź, Poland Sexual determination
PHYSIOLOGY AND PATHOLOGY OF SEXUAL DIFFERENTIATION Prof. Dr med. Jolanta Słowikowska-Hilczer Department of Andrology and Reproductive Endocrinology Medical University of Łódź, Poland Sexual determination
Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret
 Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret article Ekatherina Batourina, Christopher Choi, Neal Paragas, Natalie Bello, Terry Hensle, Frank D. Costantini,
Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret article Ekatherina Batourina, Christopher Choi, Neal Paragas, Natalie Bello, Terry Hensle, Frank D. Costantini,
18 Urinary system. 19 Male reproductive system. Female reproductive system. Blok 11: Genital and Urinary Tract Diseases
 Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase
 Human Sexuality Exam II Review Material Gametogenesis: Oogenesis 1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase 2. Know the relative
Human Sexuality Exam II Review Material Gametogenesis: Oogenesis 1. Be able to characterize the menstrual cycle from the perspective of the ovary a. Follicular phase b. Luteal phase 2. Know the relative
Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature
 REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
REPRODUCTION Testes (male gonads) -Produce sperm -Produce sex hormones -Found in a sac called the scrotum -Suspended outside of the body cavity for temperature reduction -Testes wall made of fibrous connective
Male Reproductive System
 Male Reproductive System The male reproductive system consists of a number of sex organs that are part of the reproductive process. The following sections describe the function of each part of the male
Male Reproductive System The male reproductive system consists of a number of sex organs that are part of the reproductive process. The following sections describe the function of each part of the male
Repression of Interstitial Identity in Nephron Progenitor Cells by Pax2 Establishes the Nephron- Interstitium Boundary during Kidney Development
 Article Repression of Interstitial Identity in Nephron Progenitor Cells by Pax2 Establishes the Nephron- Interstitium Boundary during Kidney Development Graphical Abstract Authors Natalie Naiman, Kaoru
Article Repression of Interstitial Identity in Nephron Progenitor Cells by Pax2 Establishes the Nephron- Interstitium Boundary during Kidney Development Graphical Abstract Authors Natalie Naiman, Kaoru
UMR 7221CNRS/MNHN Evolution des régulations endocriniennes Muséum National d Histoire Naturelle Paris - France
 Role of Foxl2 and Dlx5/6 on uterine development and function: implications for BPES UMR 7221CNRS/MNHN Evolution des régulations endocriniennes Muséum National d Histoire Naturelle Paris - France TAKE HOME
Role of Foxl2 and Dlx5/6 on uterine development and function: implications for BPES UMR 7221CNRS/MNHN Evolution des régulations endocriniennes Muséum National d Histoire Naturelle Paris - France TAKE HOME
Bio Section IV Late Development. Development of the Tetrapod Limb. Pattern Formation. Skeletal pattern formation. Gilbert 9e Chapter 13
 Bio 127 - Section IV Late Development Development of the Tetrapod Limb Gilbert 9e Chapter 13 Pattern Formation Limbs show an amazing aspect of development Similarities: Architectural symmetry in all four
Bio 127 - Section IV Late Development Development of the Tetrapod Limb Gilbert 9e Chapter 13 Pattern Formation Limbs show an amazing aspect of development Similarities: Architectural symmetry in all four
Animal Reproduction. Hypothalamic-pituitary-gonadal axis. # lectures for cumulative test # 01 book 01
 Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 05 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Animal Reproduction JP Advis DVM, Ph.D. Bartlett Hall, Animal Sciences, Cook, (732) 932-9240, advis@aesop.rutgers.edu 05 Course website: rci.rutgers.edu/~advis Material to be covered: About lecture Meetings
Effect of the T-mutation on histogenesis of the mouse embryo under the testis capsule
 /. Embryol. exp. Morph. Vol. 5, pp. -3, 79 Printed in Great Britain Company of Biologists Limited 79 Effect of the T-mutation on histogenesis of the mouse embryo under the testis capsule By H. FUJIMOTO
/. Embryol. exp. Morph. Vol. 5, pp. -3, 79 Printed in Great Britain Company of Biologists Limited 79 Effect of the T-mutation on histogenesis of the mouse embryo under the testis capsule By H. FUJIMOTO
4.05 Remember the structures of the reproductive system
 4.05 Remember the structures of the reproductive system 4.05 Remember the structures of the reproductive system Essential question What are the structures of the reproductive system? 2 Structures of the
4.05 Remember the structures of the reproductive system 4.05 Remember the structures of the reproductive system Essential question What are the structures of the reproductive system? 2 Structures of the
COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival
 2330 Development 139, 2330-2339 (2012) doi:10.1242/dev.076299 2012. Published by The Company of Biologists Ltd COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival
2330 Development 139, 2330-2339 (2012) doi:10.1242/dev.076299 2012. Published by The Company of Biologists Ltd COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival
Early nephron formation in the developing mouse kidney
 J. Anat. (2001) 199, pp. 385 392, with 7 figures Printed in the United Kingdom 385 Early nephron formation in the developing mouse kidney JONATHAN B. L. BARD 1, ADELE GORDON, LINDA SHARP AND WILLIAM I.
J. Anat. (2001) 199, pp. 385 392, with 7 figures Printed in the United Kingdom 385 Early nephron formation in the developing mouse kidney JONATHAN B. L. BARD 1, ADELE GORDON, LINDA SHARP AND WILLIAM I.
MRC-Holland MLPA. Description version 12; 13 January 2017
 SALSA MLPA probemix P219-B3 PAX6 Lot B3-0915: Compared to version B2 (lot B2-1111) two reference probes have been replaced and one additional reference probe has been added. In addition, one flanking probe
SALSA MLPA probemix P219-B3 PAX6 Lot B3-0915: Compared to version B2 (lot B2-1111) two reference probes have been replaced and one additional reference probe has been added. In addition, one flanking probe
Developmental Changes of Müllerian and Wolffian Ducts in Domestic Cat Fetuses
 Exp. Anim. 58(1), 41 45, 2009 Note Developmental Changes of Müllerian and Wolffian Ducts in Domestic Cat Fetuses Tomo INOMATA 1), Hiroyoshi NINOMIYA 1), Katsuyasu SAKITA 1), Naomi KASHIWAZAKI 2), Junya
Exp. Anim. 58(1), 41 45, 2009 Note Developmental Changes of Müllerian and Wolffian Ducts in Domestic Cat Fetuses Tomo INOMATA 1), Hiroyoshi NINOMIYA 1), Katsuyasu SAKITA 1), Naomi KASHIWAZAKI 2), Junya
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Multistep nature of cancer development. Cancer genes
 Multistep nature of cancer development Phenotypic progression loss of control over cell growth/death (neoplasm) invasiveness (carcinoma) distal spread (metastatic tumor) Genetic progression multiple genetic
Multistep nature of cancer development Phenotypic progression loss of control over cell growth/death (neoplasm) invasiveness (carcinoma) distal spread (metastatic tumor) Genetic progression multiple genetic
Study Guide Answer Key Reproductive System
 Biology 12 Human Biology Textbook: BC Biology 12 Study Guide Answer Key Reproductive System 1. Distinguish between a gamete and a gonad using specific examples from the male and female systems. Gonads
Biology 12 Human Biology Textbook: BC Biology 12 Study Guide Answer Key Reproductive System 1. Distinguish between a gamete and a gonad using specific examples from the male and female systems. Gonads
The Reproductive System
 Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
Essentials of Human Anatomy & Physiology Elaine N. Marieb Seventh Edition Chapter 16 The Reproductive System Slides 16.1 16.20 Lecture Slides in PowerPoint by Jerry L. Cook The Reproductive System Gonads
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Generation of a conditional allele of the Kindlin-2 gene. (A) A restriction map of the relevant genomic region of Kindlin-2 (top), the targeting construct
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Generation of a conditional allele of the Kindlin-2 gene. (A) A restriction map of the relevant genomic region of Kindlin-2 (top), the targeting construct
Midgut. Over its entire length the midgut is supplied by the superior mesenteric artery
 Gi Embryology 3 Midgut the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct or yolk stalk Over its entire length the
Gi Embryology 3 Midgut the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct or yolk stalk Over its entire length the
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Normal and Abnormal Development of the Genital Tract. Dr.Raghad Abdul-Halim
 Normal and Abnormal Development of the Genital Tract Dr.Raghad Abdul-Halim objectives: Revision of embryology. Clinical presentation, investigations and clinical significance of most common developmental
Normal and Abnormal Development of the Genital Tract Dr.Raghad Abdul-Halim objectives: Revision of embryology. Clinical presentation, investigations and clinical significance of most common developmental
The Reproductive System
 16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
16 PART A The Reproductive System PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reproductive
(Stratagene, La Jolla, CA) (Supplemental Fig. 1A). A 5.4-kb EcoRI fragment
 SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
SUPPLEMENTAL INFORMATION Supplemental Methods Generation of RyR2-S2808D Mice Murine genomic RyR2 clones were isolated from a 129/SvEvTacfBR λ-phage library (Stratagene, La Jolla, CA) (Supplemental Fig.
Case Report Unilateral hypoplastic kidney and ureter associated with diverse mesonephric remnant hyperplasia
 Am J Clin Exp Urol 2015;3(2):107-111 www.ajceu.us /ISSN:2330-1910/AJCEU0009935 Case Report Unilateral hypoplastic kidney and ureter associated with diverse mesonephric remnant hyperplasia Guang-Qian Xiao
Am J Clin Exp Urol 2015;3(2):107-111 www.ajceu.us /ISSN:2330-1910/AJCEU0009935 Case Report Unilateral hypoplastic kidney and ureter associated with diverse mesonephric remnant hyperplasia Guang-Qian Xiao
10. Development of genital system. Gonads. Genital ducts. External genitalia.
 10. Development of genital system. Gonads. Genital ducts. External genitalia. Gonads, genital ducts and the external genital organs initially pass through an indifferent period of development, which is
10. Development of genital system. Gonads. Genital ducts. External genitalia. Gonads, genital ducts and the external genital organs initially pass through an indifferent period of development, which is
A road to kidney tubules via the Wnt pathway
 Pediatr Nephrol (2000) 15:151 156 IPNA 2000 DEVELOPMENTAL BIOLOGY REVIEW Seppo J. Vainio Marika S. Uusitalo A road to kidney tubules via the Wnt pathway Received: 21 June 1999 / Revised: 31 March 2000
Pediatr Nephrol (2000) 15:151 156 IPNA 2000 DEVELOPMENTAL BIOLOGY REVIEW Seppo J. Vainio Marika S. Uusitalo A road to kidney tubules via the Wnt pathway Received: 21 June 1999 / Revised: 31 March 2000
Human Anatomy Unit 3 REPRODUCTIVE SYSTEM
 Human Anatomy Unit 3 REPRODUCTIVE SYSTEM In Anatomy Today Male Reproductive System Gonads = testes primary organ responsible for sperm production development/maintenan ce of secondary sex characteristics
Human Anatomy Unit 3 REPRODUCTIVE SYSTEM In Anatomy Today Male Reproductive System Gonads = testes primary organ responsible for sperm production development/maintenan ce of secondary sex characteristics
Primary sex organs (gonads): testes and ovaries. Accessory reproductive organs: ducts, glands, and external genitalia
 Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Male Reproductive System Primary sex organs (gonads): testes and ovaries Produce sex cells (gametes) Secrete steroid sex hormones Androgens (males) Estrogens and progesterone (females) Accessory reproductive
Development of the Liver and Pancreas
 Development of the Liver and Pancreas Professor Alfred Cuschieri Department of Anatomy University of Malta Three glandular buds arise from the distal end of the foregut during the fourth week Day 22 -The
Development of the Liver and Pancreas Professor Alfred Cuschieri Department of Anatomy University of Malta Three glandular buds arise from the distal end of the foregut during the fourth week Day 22 -The
Urinary System. Chapter 17 7/19/11. Introduction
 7/19/11 Chapter 17 Urinary System Introduction A. The urinary system consists of two kidneys that filter the blood, two ureters, a urinary bladder, and a urethra to convey waste substances to the outside.
7/19/11 Chapter 17 Urinary System Introduction A. The urinary system consists of two kidneys that filter the blood, two ureters, a urinary bladder, and a urethra to convey waste substances to the outside.
