Altered sleep and EEG power in the P301S Tau transgenic mouse model
|
|
|
- Leslie Alexander
- 6 years ago
- Views:
Transcription
1 Washington University School of Medicine Digital Open Access Publications 2017 Altered sleep and EEG power in the P301S Tau transgenic mouse model Jerrah K. Holth Washington University School of Medicine in St. Louis Thomas E. Mahan Washington University School of Medicine in St. Louis Grace O. Robinson Washington University School of Medicine in St. Louis Andreia Rocha Washington University School of Medicine in St. Louis David M. Holtzman Washington University School of Medicine in St. Louis Follow this and additional works at: Recommended Citation Holth, Jerrah K.; Mahan, Thomas E.; Robinson, Grace O.; Rocha, Andreia; and Holtzman, David M.,,"Altered sleep and EEG power in the P301S Tau transgenic mouse model." Annals of Clinical and Translational Neurology.4, (2017). This Open Access Publication is brought to you for free and open access by Digital It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital For more information, please contact
2 RESEARCH ARTICLE Altered sleep and EEG power in the P301S Tau transgenic mouse model Jerrah K. Holth, Thomas E. Mahan, Grace O. Robinson, Andreia Rocha & David M. Holtzman Department of Neurology, Hope Center for Neurological Disorders, and the Knight Alzheimer s Disease Research Center, Washington University School of Medicine, St. Louis, Missouri Correspondence David M. Holtzman, Department of Neurology, Washington University, 660 S. Euclid Ave, Box 8111, St. Louis, MO Tel: ; Fax: ; holtzman@neuro.wustl.edu Funding Information This work was funded by grants from the National Institute of Health-NINDS F32NS (J. K. H.) and P01NS (D. M. H.) as well as the Tau Consortium (D. M. H.), Cure Alzheimer s Fund (D. M. H.), and the JPB Foundation (D. M. H.). Received: 29 October 2016; Revised: 21 December 2016; Accepted: 27 December 2016 Annals of Clinical and Translational Neurology 2017; 4(3): doi: /acn3.390 Abstract Objective: Sleep disturbances are prevalent in human tauopathies yet despite the importance of sleep, little is known about its relationship with tau pathology. Here, we investigate this interaction by analyzing sleep and tau pathology throughout tauopathy disease progression in P301S human tau transgenic mice. Methods: P301S and wild-type mice were analyzed by electroencephalography (EEG)/electromyography at 3, 6, 9, and 11 months of age for sleep/wake time, EEG power, and homeostatic response. Cortical volume and tau pathology was also assessed by anti-phospho-tau AT8 staining. Results: P301S tau mice had significantly decreased rapid eye movement (REM) sleep at 9 months of age and decreased REM and non-rem (NREM) sleep as well as increased wakefulness at 11 months. Sleep loss was characterized by fewer wake, REM, and NREM bouts, increased wake bout duration, and decreased sleep bout duration. Decreased REM and NREM sleep was associated with increased brainstem tau pathology in the sublaterodorsal area and parafacial zone, respectively. P301S mice also showed increased EEG power at 6 and 9 months of age and decreased power at 11 months. Decreased EEG power was associated with decreased cortical volume. Despite sleep disturbances, P301S mice maintained homeostatic response to sleep deprivation. Interpretation: Our results indicate that tau pathology is associated with sleep disturbances that worsen with age and these changes may be related to tau pathology in brainstem sleep regulating regions as well as neurodegeneration. Tau-induced sleep changes could affect disease progression and be a marker for therapeutic efficacy in this and other tauopathy models. Introduction Abnormal phosphorylation and aggregation of the microtubule-binding protein tau is a hallmark of a class of neurodegenerative diseases known as tauopathies. Tauopathies include diseases such as Alzheimer s disease (AD), which is characterized by aggregation of tau and amyloid-b (Ab), some forms of frontotemporal dementia (FTD) such as Pick s disease, and pure tauopathies such as progressive supranuclear palsy (PSP) and corticobasal degeneration. Sleep disturbances are prevalent in tauopathies with up to 76% of FTD and 67% of AD patients exhibiting clinical sleep disturbances of some kind and as many as 20% of PSP patients displaying rapid eye movement (REM) sleep specific disorders. 1,2 Although varied in presentation and pathology, tauopathies are associated with an overall decrease in sleep. 3 7 Sleep disturbance is one of the leading causes of institutionalization in AD, however, the direct effect of tau pathology on sleep remains poorly understood. 8 Tau abnormalities are among the earliest observable neurodegenerative pathologies associated with AD and in both AD and PSP tau pathology is prevalent in sleep centers of the brain For example, AD-type tau pathology is shown to begin accumulating in the brainstem early in life, with 70% of year olds studied having abnormal tau pathology in the locus coeruleus (LC). 9,10 Early abnormal tau pathology progresses to other parts of the brainstem and hypothalamus, including many sleep regulating regions, prior to any cortical detection of tau or Ab aggregation. 9,11 In AD, decreases in sleep efficiency 180 ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
3 J. K. Holth et al. Altered Sleep and EEG Power in P301S Tau Mice precede the onset of dementia, while reduced sleep and increased sleep fragmentation are associated with an increased risk of developing AD Tau pathology in sleep regions of the brain may play a role in these preclinical AD sleep disturbances. Furthermore, tau pathology develops in sleep-regulating brain regions throughout the clinical course of AD and PSP, suggesting that tau pathology could also play a role in the severity of sleep disturbances in clinical tauopathy disease progression. 6,12,13,17,18 Sleep disturbances have been identified in mice expressing both tau and amyloid pathology as well as mice expressing tau pathology alone in the forebrain Furthermore, tau knockout mice have decreased sleep suggesting that loss of endogenous tau function may also contribute to sleep disturbances. 23 However, the direct effect of tau pathology throughout the brain on sleep has not been studied. To determine if tau pathology alone is sufficient to alter sleep we characterized sleep over time in a mouse model of FTD with Parkinsonism linked to chromosome 17 containing a human tau transgene with the P301S mutation. We further investigate tau pathology in brainstem sleep regions of P301S Tau transgenic (tg) mice over time to determine if pathology in these areas may contribute to changes in sleep. Methods Animals Male P301S mice and wild-type (WT) littermates were studied at 3 4, 6 7, 9 10, and months of age. P301S mice (PS19) contain a human tau transgene with a P301S mutation on the B6C3 background and were obtained from Jackson Labs (Bar Harbor, ME USA). 24 Mice were housed in a 12 h light/dark cycle with food ad libitum. All animal procedures and studies were approved by the Animal Studies Committee at Washington University School of Medicine. and habituated for 3 days after which EEG/EMG was recorded for 2 days. For sleep deprivation studies, a third day of recording was conducted in which mice were manually kept awake by gentle touching with a paintbrush for 6 h following lights on and further recorded for five more hours to test homeostatic response. During recording, mice were disturbed only during the 1 h prior to lights off, this time was omitted from all analysis. EEG and EMG signals were acquired by a P511K A.C. Preamplifier (Grass-Telefactor Instruments, Warwick, RI USA), digitized with a Digi- Data 1440A Data Acquisition System (Molecular Devices, Sunnyvale, CA USA), and recorded digitally using pclamp 10.2 (Molecular Devices). EEG/EMG analysis and statistics EEG/EMG recordings were scored manually for wake, non-rapid eye movement (NREM) sleep, and REM sleep in 10 second epochs using SleepSign (Kissei Comtec Co., Ltd, Matsumoto Japan) and all statistics performed in Graphpad Prism 5 (GraphPad Software Inc., La Jolla, CA USA) unless otherwise stated. Sleep time was averaged over 2 days (23 h/day) and significance at each age determined by Student s t-test. Sleep/wake bout number and duration analysis as well as power analysis was completed on day 2 of recording only. Significance in bout number and duration at each time point was determined by Student s t-test. FFT significance was determined by two-way analysis of variance (ANOVA) and followed with Bonferroni post hoc when a significant interaction was observed. In power analysis, two P301S outliers were detected using Grubb s test (GraphPad QuickCalcs) between all P301S animals at that time point. Animals excluded from analysis include one 6 month animal fornrempoweronlyandone11monthanimalforrem power only. For sleep deprivation studies, sleep time and power for 5 h following sleep deprivation was normalized to the previous day and analyzed as stated above. All data are expressed as mean standard error of the mean (SEM). EEG sleep/wake monitoring Electroencephalography (EEG)/electromyography (EMG) sleep/wake monitoring was performed as previously described. 22,25 EEG was recorded by stainless steel bone screws placed over the right frontal bone (Bregma: +1.0 mm, 1.0 mm lateral to midline) and right parietal bone (Bregma: 3.0 mm, 2.5 mm lateral to midline) and EMG recorded from two electrode wires placed on the right and left neck musculature. All signals were grounded to a bone screw electrode placed over the cerebellum midline. Following electrode implantation, mice were housed in 12 h light/dark conditions for 10 days. Mice were transferred to EEG/EMG cages, attached to recording cables, Immunohistochemistry Following the recording session mice were anesthetized with pentobarbatol (200 mg/kg, i.p.) and perfused with cold Dulbecco s phosphate-buffered saline (PBS) with heparin (3 U/mL). The left brain hemisphere was fixed in 4% paraformaldehyde and cryoprotected in 30% sucrose in PBS. Brain hemispheres were sliced coronally in 30 lm sections from the most rostral region of the cortex caudally through the brain stem using a freezing slice microtome. Sections were stored at 20 C in cryoprotectant (0.2 mol/ L PBS, 30% sucrose, 30% ethylene glycol) until use. Immunohistochemistry was performed in five batches that each contained a control sample for normalization. ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association. 181
4 Altered Sleep and EEG Power in P301S Tau Mice J. K. Holth et al. Every sixth hemisphere section was selected from the rostral cortex through the brainstem and placed in netwells (6 slices/well). Slices were washed three times for 5 min in Tris-buffered saline (TBS), incubated in 0.3% hydrogen peroxide in TBS for 10 min, and washed three times. Samples were then blocked for 1 h in 3% milk in TBS with 0.25% triton (vol/vol) (TBS-X) and incubated overnight at 4 C with biotinylated AT8 antibody (1:500, Thermo-Fisher, Rockford, IL USA) in 1% milk in TBS-X. AT8 recognizes tau phosphorylated at Ser 202 and Thr Following antibody incubation, slices were washed, bound to avidin using Vectastain ABC elite (Vector Laboratories, Burlingame, CA USA), washed, and developed in DAB (Sigma, St. Louis, MO USA). Slices were mounted on gelatin coated slides and rehydrated in increasing concentrations of ethanol and xylene. All samples were imaged using a Nanozoomer slide scanner (Hamamatsu Photonics, Hamamatsu Japan). Cortex volume and tau pathology analysis AT8-stained slices were used to determine cortex volume and tau pathology. Cortex volume was determined using NDP viewer (Hamamatsu Photonics). Every sixth slice was analyzed from the crossing of the corpus callosum (Bregma: +1.1 mm) to the dorsal end of the hippocampus (Bregma: 3.9 mm) and volume was determined stereologically. Significance was determined by one-way ANOVA with Tukey s post hoc. To analyze tau pathology, slices were converted to gray scale and analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA). For each region a threshold was determined to accurately depict positive staining and the percent area covered determined by ImageJ. One slice/animal was used to determine pathology in the sublaterodorsal area (SLD) (approximately Bregma: 5.3 mm) and parafacial zone (PZ) (approximately Bregma: 5.6 mm) and three slices/ animal separated by 180 lm used to determine entorhinal cortex and piriform cortex area pathology (approximately Bregma: 1.75, 1.93, 2.11). All % area covered values were normalized to appropriate staining control to eliminate batch variation. Significant differences between age points were determined by one-way ANOVA with Tukey s post hoc and significant correlation identified by Pearson r. Results P301S Tau tg mice have decreased sleep, increased wakefulness, and disrupted sleep architecture that worsens with age P301S Tau tg mice display a progressive development of tau phosphorylation and aggregation with onset of tau pathology around 5 months of age and brain atrophy beginning around 8 months. 24 To determine if tau pathology throughout the brain is sufficient to alter sleep, P301S and WT mice at 3 months (prepathology), 6 months (early pathology), 9 months (late pathology, early atrophy), and 11 months (late pathology, late atrophy) of age were analyzed by EEG/EMG recording and the amount of time spent in wake, REM sleep, and NREM sleep quantified. No significant changes in sleep or wake time were observed between genotypes at 3 or 6 months of age (Fig. 1). At 9 months of age, REM sleep was significantly decreased by 28% in P301S compared to WT mice but no changes were observed in wake or NREM sleep (Fig. 1). Loss of REM sleep worsened at 11 months of age with P301S REM sleep decreased 61% compared to WT mice (Fig. 1B). In addition to changes in REM sleep, 11 month P301S mice showed a significant decrease in NREM sleep and a significant increase in wake (Fig. 1A and C). Despite loss of sleep time, P301S mice maintained circadian sleep rhythms with increased sleep during light periods compared to dark and increased wake during dark periods compared to light, suggesting that these changes in sleep time are not due to lack of circadian regulation (data not shown). The observed changes in sleep time were associated with altered wake, REM, and NREM bout number as well as bout duration. P301S mice had significantly fewer wake bouts and significantly longer wake bout duration at both 9 and 11 months of age (Fig. 2A and D). These results demonstrate that P301S mice do not transition out of wake into sleep states as often as WT mice, suggesting a breakdown in sleep-generating/wake-inhibiting pathways in P301S mice. Furthermore, decreased REM sleep in P301S mice (Fig. 1B) was associated with fewer REM bouts at 9 and 11 months of age and a shorter REM bout duration at 11 months (Fig. 2B and E). NREM bouts were also affected with significantly fewer entries into NREM sleep at 9 and 11 months of age, which was countered by increased NREM bout duration at 9 months but not at 11 months (Fig. 2C and F). Taken together, these changes demonstrate that sleep architecture is significantly disrupted in P301S mice beginning by 9 months of age and sleep disturbances worsen with disease progression. Decreased REM and NREM sleep in P301S Tau tg mice is associated with tau pathology in brainstem sleep regulating regions In tauopathies, sleep regulating regions of the brain accumulate tau pathology and in AD specifically, tau pathology develops in many regions prior to cortical tau or amyloid aggregation. 9,11 13 To test if tau pathology in sleep centers of the brain may contribute to sleep disturbances in P301S 182 ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
5 J. K. Holth et al. Altered Sleep and EEG Power in P301S Tau Mice Tau tg mice we analyzed tau pathology, as identified by AT8 staining, in 3-, 6-, 9-, and 11-month-old P301S mice. Many brain regions contribute to the maintenance of sleep/wake architecture. 27 Due to the observed decrease in REM and NREM sleep as well as decreased sleep bouts in P301S mice, we analyzed tau pathology in two brain regions known to play a substantial role in generating REM and NREM sleep. The SLD is a REM-generating region located in the pons/ brainstem (Fig. 3A) and loss of glutamatergic neurons in this region results in decreased REM sleep. 28,29 P301S mice displayed a significant increase in tau pathology in the SLD at 9 and 11 months of age, the ages at which REM sleep was decreased in these mice (Fig. 3B, one-way ANOVA, P < ). Furthermore, SLD AT8 staining was negatively correlated with total REM sleep time (Fig. 3C, Pearson r, P < ). The GABAergic PZ, located in the brainstem (Fig. 3D), is a slow wave sleep- or NREM sleep-promoting region. 30 P301S mice showed a significant increase in PZ tau pathology at 11 months of age, the same age at which NREM sleep time was significantly decreased (Fig. 3E, oneway ANOVA, P < ). PZ AT8 pathology was also negatively correlated with total NREM sleep time (Fig. 3F, Pearson r, P < ). These results suggest that brainstem tau pathology may play a role in the sleep loss observed in P301S mice. Due to the global neuronal expression of the P301S Tau transgene and progressive pathology development in P301S mice, correlation between sleep time and tau pathology was not limited to these two brainstem regions. However, AT8 pathology in the entorhinal cortex, a forebrain region heavily affected with tau pathology, as well as the piriform cortex did not correlate with REM or NREM sleep times in P301S mice (data not shown, Pearson r, P > 0.05). The entorhinal cortex is well studied in AD and is an early location of tau pathology progression in P301S mice as well as human forebrain AD tau pathology. 9,24 The lack of association between sleep time and entorhinal or piriform cortex tau pathology further strengthens the possible role of brainstem pathology in P301S sleep disturbances. P301S Tau tg mice exhibit changes in EEG delta and theta power Figure 1. P301S Tau tg mice have decreased sleep that worsens with age. Analysis of the average minutes/hour spent in wake (A), REM sleep (B), and NREM sleep (C) for P301S and WT mice at 3 (n = 6), 6 (n = 9), 9 (n = 11 P301S, 10 WT), and 11 months of age (n = 14). P301S mice had a significant loss of REM sleep at 9 months of age that worsened at 11 months (B). Eleven-monthold P301S mice also spent significantly less time in NREM sleep and increased time in wake (A and C). **P < 0.01, ***P < 0.001, ****P < Student s t-test at each age. All values represent mean SEM. REM, rapid eye movement; NREM, non-rem; WT, wild-type. EEG power from 1 to 20 Hz was determined for wake, REM, and NREM vigilance states in P301S and WT mice. No differences in EEG power were observed at 3 months of age between P301S and WT mice (Fig. 4A C). At 6 months of age, P301S NREM EEG power was significantly increased in the delta frequencies (1 4 Hz) compared to WT but wake and REM sleep were not significantly altered (Fig. 4D F, two-way ANOVA, NREM interaction: P = 0.019, genotype: P < ). P301S NREM EEG delta power remained significantly increased ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association. 183
6 Altered Sleep and EEG Power in P301S Tau Mice J. K. Holth et al. Figure 2. Wake, REM, and NREM bouts are altered in P301S mice. The number of wake (A), REM (B), and NREM (C) bouts was significantly decreased in P301S mice compared to WT mice at 9 (n = 11 P301S, 10 WT) and 11 months of age (n = 14) but not at 3 (n = 6) and 6 months (n = 9). (D) Wake bout duration was significantly increased at 9 and 11 months of age. (E) P301S REM bout duration was unchanged at 3, 6, and 9 months of age but significantly decreased at 11 months. (F) P301S NREM bout duration was significantly increased at 9 months of age but unchanged at 3, 6, and 11 months. *P < 0.05, **P < 0.01, ****P < Student s t-test at each age. All values represent mean SEM. REM, rapid eye movement; NREM, non-rem; WT, wild-type. at 9 months of age and REM sleep EEG power was also significantly increased in the theta frequencies (4 8 Hz) (Fig. 4H and I, two-way ANOVA, REM interaction: P = , NREM interaction: P = , REM and NREM genotype: P < ). Wake EEG power was not affected at 9 months of age (Fig. 4G). However, at 11 months of age, P301S mice showed a marked and significant decrease in delta EEG power during wake and NREM sleep, and theta EEG power during REM sleep compared to WT mice (Fig. 4J L, two-way ANOVA, Wake interaction: P = , REM interaction: P = 0.001, NREM interaction: P = , all genotype: P < ). EEG power in WT mice did not change with age (Fig. 5A C) and is therefore not responsible for the observed changes in P301S EEG power. However, in P301S Tau tg mice, 11-month-old animals had significantly decreased EEG power compared to all other age points in the delta and theta frequencies of wake and NREM sleep and in theta frequencies only for REM sleep (Fig. 5D F, two-way ANOVA, all interaction and age: P < ). These measures were not significantly different when comparing 3, 6, and 9 month P301S mice. Cortex volume is associated with EEG power in P301S Tau tg mice P301S Tau tg mice have neurodegeneration and develop brain atrophy beginning around 8 months of age that results in loss of cortex volume by 12 months. 24 To test if the observed decrease in P301S EEG power at 11 months of age is associated with atrophy we analyzed hemispheric cortical volume at 3, 6, 9, and 11 months of age. In this study, 11-month-old P301S mice had significantly decreased cortical hemispheric volume compared to 3- and 6-month-old P301S mice, the same age at which decreased EEG power was observed in these mice (Fig. 6A, one-way ANOVA, P < ). Furthermore, cortical hemispheric volume was significantly correlated with NREM EEG delta power in P301S mice of all ages combined, demonstrating a possible interaction between cortical volume and EEG power (Fig. 6B, Pearson r, P = ,). NREM delta power was analyzed due to the profound and early changes observed at this frequency range in P301S mice (Fig. 4). When analyzed at independent age points, P301S cortical hemispheric volume was significantly correlated with NREM delta power at 11 month of age, but this correlation was not significant at 9 months (Fig. 6C and D, Pearson r, 11 months: P = , 9 months: P = ). The observed correlation at 11 months of age when EEG power is significantly decreased but not at 9 months when EEG power is elevated suggests that neurodegeneration-induced brain atrophy may play a role in the loss of EEG power observed at 11 months of age but may not explain the increased EEG power observed at earlier time points. 184 ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
7 J. K. Holth et al. Altered Sleep and EEG Power in P301S Tau Mice Figure 3. Decreased REM and NREM sleep in P301S Tau tg mice is associated with tau pathology development in sleep regulating regions of the brain. (A) Representative image of the SLD, a REM sleep-generating region of the brain. (B) AT8 staining in the SLD was significantly increased at 9(n = 11) and 11 (n = 12) months of age compared to 3 (n = 8) and 6 (n = 11) months. (C) Time in REM sleep was negatively correlated with AT8 SLD staining (n = 38). (D) Representative image of the PZ, a slow wave/nrem sleep-promoting region. (E) AT8 staining in the PZ was significantly increased at 11 month of age and (F) NREM sleep time negatively correlated with AT8 PZ staining. *P < 0.05, **P < 0.01, ***P < One-way ANOVA with Tukey s post hoc, all values represent mean SEM (B and E). Pearson r (C and F). All AT8 staining values were normalized to a batch control. REM, rapid eye movement; NREM, non-rem; SLD, sublaterodorsal area; PZ, parafacial zone; ANOVA, analysis of variance. Eleven-month-old P301S Tau tg mice maintain homeostatic response to sleep deprivation Sleep is homeostatically regulated in that prolonged wakefulness leads to a compensatory increase in sleep as well as increased NREM sleep slow wave activity. 27 Since P301S mice have decreased sleep time and decreased EEG power at 11 months of age, we further characterized homeostatic sleep response in these mice as another way to assess their sleep/wake cycle. Sleep and wake were analyzed in P301S and WT mice following 6 h of manual sleep deprivation. When sleep and wake time was normalized to the previous baseline day of recording, 11-monthold P301S mice had less of a decrease in wake than WT mice, but changes in REM and NREM sleep were not significantly different (Fig. 7A C). Eleven-month P301S mice maintained an elevated level of sleep similar to controls following sleep deprivation. Furthermore, NREM EEG power normalized to the previous day was increased in a similar manner in the delta frequencies of both 11- month-old P301S and WT mice (Fig. 7D). These results demonstrate that even at advanced stages of disease when sleep time and EEG power is significantly decreased, P301S mice maintain homeostatic sleep responses. Discussion Sleep is an important biological function that is commonly altered in neurodegenerative disease. 18,31 Despite the importance of sleep and the prevalence of sleep disturbances observed across tauopathies, little is known about the effects of disease associated tau aggregation throughout the brain on sleep. Here, we demonstrate that a mouse model expressing human P301S Tau throughout the brain and tau pathology in both forebrain and brainstem regions, exhibits changes in sleep with disease progression. We have shown that P301S mice have a progressive decrease in sleep and increase in wakefulness, and that loss of sleep is associated with tau pathology in brainstem sleep regulating regions. Particularly interesting is the early decrease in REM sleep. Furthermore, P301S mice have changes in EEG power over time that are associated with decreased cortical volume. Despite changes in sleep and EEG power, P301S mice maintain homeostatic response to sleep deprivation. ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association. 185
8 Altered Sleep and EEG Power in P301S Tau Mice J. K. Holth et al. Figure 4. EEG power is altered in P301S Tau tg mice. Analysis of EEG power during wake, REM sleep, and NREM sleep at 3 (n = 6 P301S and WT), 6 (n = 8 P301S [D and E], n = 7 P301S [F], n = 8 WT), 9 (n = 11 P301S, n = 10 WT), and 11 months of age (n = 12 P301S [J and L], n = 11 P301S [K], n = 12 WT) in P301S and WT mice. EEG power was not significantly altered at 3 months of age in wake (A), REM (B) or NREM sleep (C). EEG power was unchanged at 6 months of age during wake (D) and REM sleep (E), but power in P301S mice was significantly increased during NREM sleep in the delta frequencies (1 4 Hz) (C). Nine-month-old P301S mice exhibited significantly increased EEG power in the theta frequencies (4 8 Hz) during REM sleep (H) and delta frequencies in NREM sleep (I) but power was not significantly affected during wake (G). At 11 months of age, P301S delta EEG power was significantly decreased in wake (J) and NREM sleep (L) and theta EEG power significantly decreased during REM sleep (K). *P < 0.05, **P < 0.01, ***P < Two-way ANOVA with Bonferroni post hoc. All values represent mean SEM. EEG, electroencephalography; REM, rapid eye movement; NREM, non-rem; WT, wild-type; ANOVA, analysis of variance. Changes in sleep time and association with brainstem sleep regions FTD, PSP, and AD patients all have decreased sleep and we have demonstrated that this phenotype is recapitulated in the P301S tauopathy mouse model. 3 7 The progression of sleep disturbances over time has not been reported in tauopathy mouse models and we show here that sleep deficits worsen with age and disease progression. Sleep analysis of a different FTD model expressing tau specifically in the forebrain (PLB2 Tau ) has also reported loss of NREM sleep and increases in wake, but unlike P301S mice shown here, changes in REM sleep were not observed in PLB2 Tau mice. 20 Sleep changes in PLB2 Tau mice support our findings that tau pathology is sufficient to induce sleep changes and further suggests that the REM sleep disruption we observe in P301S mice may be due to brainstem pathology present in P301S but not PLB2 Tau models. 20 This is supported by our observation that AT8-positive tau pathology in the SLD is negatively correlated with REM sleep time. Tau knock-out mice also only show changes in wake and NREM sleep, suggesting a role for tau pathology, and not tau loss of function, in REM sleep disruption. 23 In addition to REM sleep, areas 186 ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
9 J. K. Holth et al. Altered Sleep and EEG Power in P301S Tau Mice Figure 5. EEG power does not change with age in WT mice but is significantly decreased in 11-month-old P301S mice. WT mice at 3, 6, 9, and 11 months of age showed no changes in wake (A), REM (B), or NREM (C) EEG power with age. However, P301S mice had significantly decreased wake (D), REM (E), and NREM (F) EEG power at 11 months of age compared to 3, 6, and 9 month P301S mice. *P < 0.05, **P < 0.01, ***P < Two-way ANOVA. All significance compared to 11 month P301S mice by Bonferroni post hoc (D-F). All values represent mean SEM. EEG, electroencephalography; REM, rapid eye movement; NREM, non-rem; ANOVA, analysis of variance; WT, wild-type. in the brainstem also regulate NREM sleep and wake. Despite the presence of NREM decreases in PLB2 Tau forebrain expressing mice, our results highlight that the effect of tau pathology on brainstem regions such as the PZ, which negatively correlated with NREM sleep time in P301S mice, should not be overlooked. 20 The brainstem and other sleep-regulating regions of the brain are affected by tau pathology in taupathies. 9 12,32 In AD, abnormal tau pathology can be seen in brainstem sleep/wake regions such the LC, dorsal raphe, and parabrachial nucleus as well as hypothalamic sleep regions prior to the onset of cortical tau or amyloid Other sleep regions, such as the pedunculopontine tegmental nucleus (PPT), develop tau pathology in clinical stages of disease. 17 In AD, neuronal loss and tau neurofibrillary tangles occur in the PPT, but not amyloid pathology. 13,17 In PSP, REM sleep is highly affected and loss of REM sleep is suggested to result from tau pathology-induced degeneration of the PPT, demonstrating the importance of brainstem regions in sleep and their vulnerability to tau pathology. 6,13 Early tau abnormalities in the brainstem are not recapitulated in mouse models and despite pathological analysis of some ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association. 187
10 Altered Sleep and EEG Power in P301S Tau Mice J. K. Holth et al. Figure 6. Cortical hemispheric volume decreases with age and correlates with NREM delta power in P301S Tau tg mice. (A) Analysis of cortical hemispheric volume in 3 (n = 8), 6 (n = 11), 9 (n = 11), and 11 (n = 12) month-old P301S mice showed a significant decrease at 11 months. Cortical hemispheric volume was significantly correlated with NREM delta power when all P301S mice ages were combined (B, n = 36) and 11 months of age only (D, n = 12), but not at 9 months of age (C, n = 11), suggesting that cortical volume loss may play a role in the decreased EEG power observed at 11 months of age. **P < One-way ANOVA with Tukey s post hoc, all values represent mean SEM (A). Pearson r (B D). NREM, non-rapid eye movement; EEG, electroencephalography; ANOVA, analysis of variance. Figure 7. P301S Tau tg mice maintain homeostatic response to sleep deprivation. Eleven-month-old P301S and WT mice (n = 6) were sleep deprived for 6 h and EEG analyzed for 5 h following sleep deprivation. All data were normalized to the time matched previous day of recording. Time spent in wake (A), REM sleep (B), and NREM sleep (C) following sleep deprivation. (D) NREM EEG power was similarly increased in the delta frequencies of both P301S and WT mice. *P < Student s t-test (A C). Two-way ANOVA (D). All values represent mean SEM. EEG, electroencephalography; REM, rapid eye movement; NREM, non- REM; ANOVA, analysis of variance; WT, wild-type. brainstem regions in human clinical AD, many specific sleep regions, including the SLD and PZ analyzed here, have yet to be studied. However, the association of pathology onset in sleep-generating brainstem regions with the onset of sleep loss as well as significant correlation between these factors is strengthened by the proximity of these brain regions to others known to be affected by AD tau pathology and is suggestive of a role for brainstem tau pathology in P301S sleep disturbances. Here, we analyzed the SLD and PZ based on their ability to modulate REM and NREM sleep and our results demonstrate, for the first time, the likely importance of brainstem tau pathology in tau-induced sleep deficits. Eliminating glutamatergic signaling in the SLD significantly decreases REM sleep by ~40%, similar to what was observed here in P301S mice. 28 However, SLD Vglut knockdown also increases sleep bout frequency, which we did not observe in the P301S Tau tg model. 28 These similarities and differences demonstrate that tau pathology may affect many regions of the brain, a combination of which is likely responsible for sleep/wake changes observed in P301S mice. In P301S mice we also observed fewer sleep bouts as well as fewer and longer wake bouts. The decreased transition to sleep from wake suggests breakdown of sleep-promoting/wake-inhibiting pathways. The PZ promotes slow wave sleep by inhibiting the wake-promoting parabrachial nucleus and its basal forebrain cortical circuit. 30 Thus, loss of PZ function is a possible mechanism for tau pathology-induced decrease in sleep bouts and this is supported by the negative correlation between NREM sleep time and PZ tau pathology. It is likely that other sleep regions of the brain, including brainstem and thalamic regions, are also affected by tau pathology and contribute to the observed sleep disturbances. More work is needed to determine if tau pathology in the SLD, PZ, or other sleep regions alone is sufficient to induce changes in sleep and if tau pathologyinduced sleep changes can affect disease progression. EEG power and cortical volume Here, we demonstrated that P301S mice have changes in EEG power, with elevated EEG power compared to 188 ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
11 J. K. Holth et al. Altered Sleep and EEG Power in P301S Tau Mice WT at 6 and 9 months of age and significantly decreased power at 11 months. These changes are not due to changes in WT EEG power but to a significant decrease in P301S EEG power at 11 months of age that corresponds with a significant decrease in cortical volume. Changes in EEG power over time have not previously been reported in tauopathy mice. Increased EEG power during disease progression could represent a compensatory response to pathological tau aggregation. Although more work is needed to understand the mechanism of increased power in P301S mice, the observed correlation between cortex volume and EEG power demonstrates that neurodegenerative cortical atrophy may be responsible for the severe decrease in EEG power observed in 11-monthold P301S mice. Furthermore, we have shown that even at severe stages of disease when sleep time and EEG power are decreased, P301S mice maintain a homeostatic response to sleep deprivation. We surprisingly observed similar increases in REM and NREM sleep time as well as increased NREM slow wave delta power in aged P301S and WT mice. The mechanisms and exact sleep factors resulting in homeostatic regulation of sleep are not fully elucidated. Adenosine build up and signaling in the basal forebrain and other regions is known to play a role, but it is likely not the only factor. 27 Despite this, alterations in sleep and EEG power are well documented. 27 The maintenance of homeostatic response of NREM EEG power in P301S aged mice may indicate that despite regional brain atrophy and decreased EEG power, the existing neurons and neural networks maintain the ability to respond and increase activity to homeostatic sleepdrive cues. Conclusions and Implications We have demonstrated for the first time that tau pathology throughout the brain, including both the forebrain and brainstem, can progressively cause sleep disruption and changes in EEG power over time. We have further shown that the brainstem may play an important role in the development of sleep disturbances. Previously, Ab pathology has been shown to not only induce sleep disruption but pathology is also accelerated by sleep deprivation and decreased by sleep improvement. 22,25,33 Therefore, tau-induced decreases in REM and NREM sleep may directly affect amyloid AD pathology, interact with Ab-induced sleep problems, and could play a role in worsening disease. In the brain interstitial fluid, levels of tau, like Ab, are increased with elevated neuronal activity, suggesting that increased wakefulness may elevate tau secretion and disease spreading in tauopathies. 25,34,35 Whether changes in sleep affect tau pathology directly, as has been seen with Ab, remains to be studied. 25,33 However, even if changes in sleep do not directly affect tau pathology, decreasing sleep could play a role in accelerating other AD disease mechanisms such as amyloid pathology and secondarily affect tau. Furthermore, quantitative assessment of sleep and EEG power in the P301S Tau tg model could be a useful method to functionally assess the effects of anti-tau and other neuroprotective therapies, in addition to evaluation of cognitive and motor outcomes. P301S mice and the sleep disturbances observed could therefore serve as a valuable way to assess for disease progression and as a functional tool in assessing disease outcome. Acknowledgments The authors thank Clifford Saper (Harvard University) for guidance and expertise regarding sleep regulation and neuroanatomy. This work was funded by grants from the National Institute of Health-NINDS F32NS (J. K. H.) and P01NS (D. M. H.) as well as the Tau Consortium (D. M. H.), Cure Alzheimer s Fund (D. M. H.), and the JPB Foundation (D. M. H.). Author Contributions J. K. H. and D. M. H. conceived, designed, and analyzed the studies as well as wrote and edited the manuscript. All authors contributed to designing and performing experiments. Conflict of Interest D. M. H. reports grants from NIH, Tau Consortium, The JPB Foundation, Cure Alzheimer s Fund, Eli Lilly, Denali, AbbVie, and C2N Diagnostics during the conduct of the study. D. M. H. is on the scientific advisory board of C2N Diagnostics, Denali, and Neurophage and consults for Genentech, AbbVie, and Eli Lilly. D. M. H. is a co-inventor on a patent licensed by Washington University to C2N Diagnostics for anti-tau antibodies. C2N Diagnostics has licensed these antibodies to AbbVie. D. M. H. is a cofounder of C2N Diagnostics and has equity in the company. References 1. Guarnieri B, Adorni F, Musicco M, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical crosssectional study on 431 patients. Dement Geriatr Cogn Disord 2012;33: Arnulf I, Merino-Andreu M, Bloch F, et al. REM sleep behavior disorder and REM sleep without atonia in patients with progressive supranuclear palsy. Sleep 2005;28: ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association. 189
12 Altered Sleep and EEG Power in P301S Tau Mice J. K. Holth et al. 3. Anderson KN, Hatfield C, Kipps C, et al. Disrupted sleep and circadian patterns in frontotemporal dementia. Eur J Neurol 2009;16: Bonakis A, Economou NT, Paparrigopoulos T, et al. Sleep in frontotemporal dementia is equally or possibly more disrupted, and at an earlier stage, when compared to sleep in Alzheimer s disease. J Alzheimers Dis 2014;38: Bonanni E, Maestri M, Tognoni G, et al. Daytime sleepiness in mild and moderate Alzheimer s disease and its relationship with cognitive impairment. J Sleep Res 2005;14: Montplaisir J, Petit D, Decary A, et al. Sleep and quantitative EEG in patients with progressive supranuclear palsy. Neurology 1997;49: Vitiello MV, Prinz PN, Williams DE, et al. Sleep disturbances in patients with mild-stage Alzheimer s disease. J Gerontol 1990;45:M131 M Bianchetti A, Scuratti A, Zanetti O, et al. Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia 1995;6: Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011;70: Braak H, Del Tredici K. The pathological process underlying Alzheimer s disease in individuals under thirty. Acta Neuropathol 2011;121: Stratmann K, Heinsen H, Korf HW, et al. Precortical phase of Alzheimer s disease (AD)-related tau cytoskeletal pathology. Brain Pathol 2016;26: Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 1999;246(Suppl 2):II6 II Jellinger K. The pedunculopontine nucleus in Parkinson s disease, progressive supranuclear palsy and Alzheimer s disease. J Neurol Neurosurg Psychiatry 1988;51: Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol 2013;70: Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry 2013;22: Lim AS, Kowgier M, Yu L, et al. Sleep fragmentation and the risk of incident Alzheimer s disease and cognitive decline in older persons. Sleep 2013;36: Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer s disease. Ann Neurol 2001;49: Stern AL, Naidoo N. Wake-active neurons across aging and neurodegeneration: a potential role for sleep disturbances in promoting disease. Springerplus 2015;4: Jyoti A, Plano A, Riedel G, Platt B. Progressive age-related changes in sleep and EEG profiles in the PLB1 Triple mouse model of Alzheimer s disease. Neurobiol Aging 2015;36: Koss DJ, Robinson L, Drever BD, et al. Mutant Tau knock-in mice display frontotemporal dementia relevant behaviour and histopathology. Neurobiol Dis 2016;91: Platt B, Drever B, Koss D, et al. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PLoS One 2011;6:e Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of b-amyloid in mice with Alzheimer s disease pathology. Sci Transl Med 2012;4:150ra Cantero JL, Hita-Ya~nez E, Moreno-Lopez B, et al. Tau protein role in sleep-wake cycle. J Alzheimers Dis 2010;21: Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007;53: Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009;326: Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 1995;189: Saper CB, Fuller PM, Pedersen NP, et al. Sleep state switching. Neuron 2010;68: Krenzer M, Anaclet C, Vetrivelan R, et al. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PLoS One 2011;6:e Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature 2006;441: Anaclet C, Ferrari L, Arrigoni E, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci 2014;17: Petit D, Gagnon JF, Fantini ML, et al. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res 2004;56: Pan XD, Chen XC. Clinic, neuropathology and molecular genetics of frontotemporal dementia: a mini-review. Transl Neurodegener 2013;2: Roh JH, Jiang H, Finn MB, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer s disease. J Exp Med 2014;211: Yamada K, Holth JK, Liao F, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med 2014;211: Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 2005;48: ª 2017 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Sleep and Circadian Rhythms in Neurodegenerative Disorders
 Sleep and Circadian Rhythms in Neurodegenerative Disorders Erik S. Musiek, MD, PhD Department of Neurology Washington University in St. Louis U13 Bench to Bedside Sleep Conference 2015 Disclosures Funding:
Sleep and Circadian Rhythms in Neurodegenerative Disorders Erik S. Musiek, MD, PhD Department of Neurology Washington University in St. Louis U13 Bench to Bedside Sleep Conference 2015 Disclosures Funding:
Neuropathology of Neurodegenerative Disorders Prof. Jillian Kril
 Neurodegenerative disorders to be discussed Alzheimer s disease Lewy body diseases Frontotemporal dementia and other tauopathies Huntington s disease Motor Neuron Disease 2 Neuropathology of neurodegeneration
Neurodegenerative disorders to be discussed Alzheimer s disease Lewy body diseases Frontotemporal dementia and other tauopathies Huntington s disease Motor Neuron Disease 2 Neuropathology of neurodegeneration
EEG Sleep Circadian rhythms Learning Objectives: 121, 122
 EEG Sleep Circadian rhythms Learning Objectives: 121, 122 Zoltán Lelkes Electroencenphalography Hans Berger pen time amplifier electrodes 1 The waves of the EEG gamma > 30 Hz beta: 13-30 Hz Mental activity:
EEG Sleep Circadian rhythms Learning Objectives: 121, 122 Zoltán Lelkes Electroencenphalography Hans Berger pen time amplifier electrodes 1 The waves of the EEG gamma > 30 Hz beta: 13-30 Hz Mental activity:
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR
 Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR In Physiology Today What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR In Physiology Today What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may
The Marmoset Monkey as Model for Neurological Disorders
 The Marmoset Monkey as Model for Neurological Disorders Jan Langermans and Ingrid Philippens From Laboratory to Clinic Disease models neuroscience: Parkinson, Sleep, Stress, Alzheimer, MS MS Models: rhmog
The Marmoset Monkey as Model for Neurological Disorders Jan Langermans and Ingrid Philippens From Laboratory to Clinic Disease models neuroscience: Parkinson, Sleep, Stress, Alzheimer, MS MS Models: rhmog
NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07)
 NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07) 1. Revisitation of Bremer s 1936 Isolated Brain Studies Transected the brain: a. Cut between the medulla and the spinal cord ( encephale isole ) Note: recall
NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07) 1. Revisitation of Bremer s 1936 Isolated Brain Studies Transected the brain: a. Cut between the medulla and the spinal cord ( encephale isole ) Note: recall
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR
 Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may have as many as 200,000
Physiology Unit 2 CONSCIOUSNESS, THE BRAIN AND BEHAVIOR What the Brain Does The nervous system determines states of consciousness and produces complex behaviors Any given neuron may have as many as 200,000
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature06310 SUPPLEMENTARY INFORMATION www.nature.com/nature 1 www.nature.com/nature 2 www.nature.com/nature 3 Supplementary Figure S1 Spontaneous duration of wake, SWS and REM sleep (expressed
doi: 10.1038/nature06310 SUPPLEMENTARY INFORMATION www.nature.com/nature 1 www.nature.com/nature 2 www.nature.com/nature 3 Supplementary Figure S1 Spontaneous duration of wake, SWS and REM sleep (expressed
9.01 Introduction to Neuroscience Fall 2007
 MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
The Spectrum of Age-Associated Astroglial Tauopathies. Dennis W. Dickson MD Department of Neuroscience Mayo Clinic, Jacksonville, FL
 The Spectrum of Age-Associated Astroglial Tauopathies Dennis W. Dickson MD Mayo Clinic, Jacksonville, FL Thorn-shaped astrocytes TSA were first reported by Ikeda (1995), as tau-positive astrocytes in various
The Spectrum of Age-Associated Astroglial Tauopathies Dennis W. Dickson MD Mayo Clinic, Jacksonville, FL Thorn-shaped astrocytes TSA were first reported by Ikeda (1995), as tau-positive astrocytes in various
! slow, progressive, permanent loss of neurologic function.
 UBC ! slow, progressive, permanent loss of neurologic function.! cause unknown.! sporadic, familial or inherited.! degeneration of specific brain region! clinical syndrome.! pathology: abnormal accumulation
UBC ! slow, progressive, permanent loss of neurologic function.! cause unknown.! sporadic, familial or inherited.! degeneration of specific brain region! clinical syndrome.! pathology: abnormal accumulation
Outline of Lecture 2/13/2018
 Allan I. Pack, M.B.Ch.B., Ph.D., FRCP John Miclot Professor of Medicine Director, Center for sleep and Circadian Neurobiology University of Pennsylvania Perelman School of Medicine Philadelphia, Pennsylvania
Allan I. Pack, M.B.Ch.B., Ph.D., FRCP John Miclot Professor of Medicine Director, Center for sleep and Circadian Neurobiology University of Pennsylvania Perelman School of Medicine Philadelphia, Pennsylvania
Nature Neuroscience: doi: /nn Supplementary Figure 1. Large-scale calcium imaging in vivo.
 Supplementary Figure 1 Large-scale calcium imaging in vivo. (a) Schematic illustration of the in vivo camera imaging set-up for large-scale calcium imaging. (b) High-magnification two-photon image from
Supplementary Figure 1 Large-scale calcium imaging in vivo. (a) Schematic illustration of the in vivo camera imaging set-up for large-scale calcium imaging. (b) High-magnification two-photon image from
BIO333 Comparative Physiology and Pharmacology of Sleep. Genetics of Sleep December 3, Raphaelle Winsky-Sommerer, PhD, PD
 BIO333 Comparative Physiology and Pharmacology of Sleep Genetics of Sleep December 3, 2011 Raphaelle Winsky-Sommerer, PhD, PD r.winsky-sommerer@surrey.ac.uk Genetics of Sleep Quantitative traits are determined
BIO333 Comparative Physiology and Pharmacology of Sleep Genetics of Sleep December 3, 2011 Raphaelle Winsky-Sommerer, PhD, PD r.winsky-sommerer@surrey.ac.uk Genetics of Sleep Quantitative traits are determined
FDG-PET e parkinsonismi
 Parkinsonismi FDG-PET e parkinsonismi Valentina Berti Dipartimento di Scienze Biomediche, Sperimentali e Cliniche Sez. Medicina Nucleare Università degli Studi di Firenze History 140 PubMed: FDG AND parkinsonism
Parkinsonismi FDG-PET e parkinsonismi Valentina Berti Dipartimento di Scienze Biomediche, Sperimentali e Cliniche Sez. Medicina Nucleare Università degli Studi di Firenze History 140 PubMed: FDG AND parkinsonism
Protocol for Rat Sleep EEG
 Protocol for Rat Sleep EEG Subjects Male Spraue Dawley rats weihin 250-300 rams at the time of surery are used. Food and water are available ad libitum throuhout the experiment. Rats are roup housed prior
Protocol for Rat Sleep EEG Subjects Male Spraue Dawley rats weihin 250-300 rams at the time of surery are used. Food and water are available ad libitum throuhout the experiment. Rats are roup housed prior
SLEEP DISORDERS IN HUNTINGTON S DISEASE. Gary L. Dunbar, Ph.D.
 SLEEP DISORDERS IN HUNTINGTON S DISEASE Gary L. Dunbar, Ph.D. Executive Director, Field Neurosciences Institute Co-Director, Program in Neuroscience Central Michigan University Pre-Talk Test 1. Which type
SLEEP DISORDERS IN HUNTINGTON S DISEASE Gary L. Dunbar, Ph.D. Executive Director, Field Neurosciences Institute Co-Director, Program in Neuroscience Central Michigan University Pre-Talk Test 1. Which type
Introduction to Physiological Psychology
 Introduction to Physiological Psychology Psych 260 Kim Sweeney ksweeney@cogsci.ucsd.edu cogsci.ucsd.edu/~ksweeney/psy260.html What could possibly go wrong? n Causes of Narcolepsy Uncertain, but appears
Introduction to Physiological Psychology Psych 260 Kim Sweeney ksweeney@cogsci.ucsd.edu cogsci.ucsd.edu/~ksweeney/psy260.html What could possibly go wrong? n Causes of Narcolepsy Uncertain, but appears
Sleep, Dreaming and Circadian Rhythms
 Sleep, Dreaming and Circadian Rhythms People typically sleep about 8 hours per day, and spend 16 hours awake. Most people sleep over 175,000 hours in their lifetime. The vast amount of time spent sleeping
Sleep, Dreaming and Circadian Rhythms People typically sleep about 8 hours per day, and spend 16 hours awake. Most people sleep over 175,000 hours in their lifetime. The vast amount of time spent sleeping
DEMENTIA 101: WHAT IS HAPPENING IN THE BRAIN? Philip L. Rambo, PhD
 DEMENTIA 101: WHAT IS HAPPENING IN THE BRAIN? Philip L. Rambo, PhD OBJECTIVES Terminology/Dementia Basics Most Common Types Defining features Neuro-anatomical/pathological underpinnings Neuro-cognitive
DEMENTIA 101: WHAT IS HAPPENING IN THE BRAIN? Philip L. Rambo, PhD OBJECTIVES Terminology/Dementia Basics Most Common Types Defining features Neuro-anatomical/pathological underpinnings Neuro-cognitive
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1180962/dc1 Supporting Online Material for Amyloid-β Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle Jae-Eun Kang, Miranda M. Lim, Randall J. Bateman, James
www.sciencemag.org/cgi/content/full/1180962/dc1 Supporting Online Material for Amyloid-β Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle Jae-Eun Kang, Miranda M. Lim, Randall J. Bateman, James
TGF-ß1 pathway as a new pharmacological target for neuroprotection in AD. Filippo Caraci
 Department of Clinical and Molecular Biomedicine Section of Pharmacology and Biochemistry Department of Educational Sciences University of Catania TGF-ß1 pathway as a new pharmacological target for neuroprotection
Department of Clinical and Molecular Biomedicine Section of Pharmacology and Biochemistry Department of Educational Sciences University of Catania TGF-ß1 pathway as a new pharmacological target for neuroprotection
Cephalization. Nervous Systems Chapter 49 11/10/2013. Nervous systems consist of circuits of neurons and supporting cells
 Nervous Systems Chapter 49 Cephalization Nervous systems consist of circuits of neurons and supporting cells Nervous system organization usually correlates with lifestyle Organization of the vertebrate
Nervous Systems Chapter 49 Cephalization Nervous systems consist of circuits of neurons and supporting cells Nervous system organization usually correlates with lifestyle Organization of the vertebrate
Carlson (7e) PowerPoint Lecture Outline Chapter 9: Sleep and Biological Rhythms
 Carlson (7e) PowerPoint Lecture Outline Chapter 9: Sleep and Biological Rhythms This multimedia product and its contents are protected under copyright law. The following are prohibited by law: any public
Carlson (7e) PowerPoint Lecture Outline Chapter 9: Sleep and Biological Rhythms This multimedia product and its contents are protected under copyright law. The following are prohibited by law: any public
Role of TDP-43 in Non-Alzheimer s and Alzheimer s Neurodegenerative Diseases
 Role of TDP-43 in Non-Alzheimer s and Alzheimer s Neurodegenerative Diseases Keith A. Josephs, MD, MST, MSc Professor of Neurology 13th Annual Mild Cognitive Impairment (MCI) Symposium: Alzheimer and Non-Alzheimer
Role of TDP-43 in Non-Alzheimer s and Alzheimer s Neurodegenerative Diseases Keith A. Josephs, MD, MST, MSc Professor of Neurology 13th Annual Mild Cognitive Impairment (MCI) Symposium: Alzheimer and Non-Alzheimer
Potential role of extracellular tau in development and spread of tauopathy: Effects of anti-tau antibodies
 Potential role of extracellular tau in development and spread of tauopathy: Effects of anti-tau antibodies David M. Holtzman, MD Professor and Chair, Dept. of Neurology Washington University School of
Potential role of extracellular tau in development and spread of tauopathy: Effects of anti-tau antibodies David M. Holtzman, MD Professor and Chair, Dept. of Neurology Washington University School of
Clinicopathologic and genetic aspects of hippocampal sclerosis. Dennis W. Dickson, MD Mayo Clinic, Jacksonville, Florida USA
 Clinicopathologic and genetic aspects of hippocampal sclerosis Dennis W. Dickson, MD Mayo Clinic, Jacksonville, Florida USA The hippocampus in health & disease A major structure of the medial temporal
Clinicopathologic and genetic aspects of hippocampal sclerosis Dennis W. Dickson, MD Mayo Clinic, Jacksonville, Florida USA The hippocampus in health & disease A major structure of the medial temporal
Biological Rhythms, Sleep, and Dreaming. Elaine M. Hull
 Biological Rhythms, Sleep, and Dreaming Elaine M. Hull Rhythms of Waking and Sleeping Animals generate 24 hour cycles of wakefulness and sleep. Some animals generate endogenous circannual rhythms (yearly
Biological Rhythms, Sleep, and Dreaming Elaine M. Hull Rhythms of Waking and Sleeping Animals generate 24 hour cycles of wakefulness and sleep. Some animals generate endogenous circannual rhythms (yearly
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Deletion of the Mammalian Circadian Clock Gene BMAL1/Mop3 Alters Baseline Sleep Architecture and the Response to Sleep Deprivation
 CIRCADIAN RHYTHMS Deletion of the Mammalian Circadian Clock Gene BMAL1/Mop3 Alters Baseline Sleep Architecture and the Response to Sleep Deprivation Aaron Laposky, PhD 1 ; Amy Easton, PhD 1 ; Christine
CIRCADIAN RHYTHMS Deletion of the Mammalian Circadian Clock Gene BMAL1/Mop3 Alters Baseline Sleep Architecture and the Response to Sleep Deprivation Aaron Laposky, PhD 1 ; Amy Easton, PhD 1 ; Christine
Dementia. Stephen S. Flitman, MD Medical Director 21st Century Neurology
 Dementia Stephen S. Flitman, MD Medical Director 21st Century Neurology www.neurozone.org Dementia is a syndrome Progressive memory loss, plus Progressive loss of one or more cognitive functions: Language
Dementia Stephen S. Flitman, MD Medical Director 21st Century Neurology www.neurozone.org Dementia is a syndrome Progressive memory loss, plus Progressive loss of one or more cognitive functions: Language
Special Lecture 6 Effects of Sleep on Seizures ; A Path to sleep neurology (Somno-neurology)
 Special Lecture 6 Effects of Sleep on Seizures ; A Path to sleep neurology (Somno-neurology) Jun Kohyama, MD Tokyo Bay Urayasi/Ichikawa Medical Center Taiwan Pediatric Epilepsy Congress 2010 Dec. 26, 2010,
Special Lecture 6 Effects of Sleep on Seizures ; A Path to sleep neurology (Somno-neurology) Jun Kohyama, MD Tokyo Bay Urayasi/Ichikawa Medical Center Taiwan Pediatric Epilepsy Congress 2010 Dec. 26, 2010,
Final Scientific Progress Report
 CUREPSP Final Scientific Progress Report Tau in Peripheral Tissues of PSP and CBD. Brittany Dugger, PhD; University of California San Francisco Specific Aim: Using immunohistochemical methods on autopsy
CUREPSP Final Scientific Progress Report Tau in Peripheral Tissues of PSP and CBD. Brittany Dugger, PhD; University of California San Francisco Specific Aim: Using immunohistochemical methods on autopsy
Lecture 8. Arousal & Sleep. Cogs17 * UCSD
 Lecture 8 Arousal & Sleep Cogs17 * UCSD Arousal in the Brain Stimulated by sensory input Initiated, maintained endogenously Basal Forebrain Delivers ACh throughout cortex Arousal in the Brain Lateral Hypothalamus
Lecture 8 Arousal & Sleep Cogs17 * UCSD Arousal in the Brain Stimulated by sensory input Initiated, maintained endogenously Basal Forebrain Delivers ACh throughout cortex Arousal in the Brain Lateral Hypothalamus
Announcing a new era in Alzheimer s
 EMBARGOED UNTIL 16:00 Monte Carlo Time 30 October 2012 Announcing a new era in Alzheimer s The time for Tau is now as Phase 3 clinical trials get under way with a second-generation Tau Aggregation Inhibitor
EMBARGOED UNTIL 16:00 Monte Carlo Time 30 October 2012 Announcing a new era in Alzheimer s The time for Tau is now as Phase 3 clinical trials get under way with a second-generation Tau Aggregation Inhibitor
Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo
 Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo Shannon L. Macauley, 1 Molly Stanley, 1 Emily E. Caesar, 1 Steven A. Yamada, 1 Marcus E. Raichle, 1,2 Ronaldo
Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo Shannon L. Macauley, 1 Molly Stanley, 1 Emily E. Caesar, 1 Steven A. Yamada, 1 Marcus E. Raichle, 1,2 Ronaldo
Sleep. No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness,
 Sleep Neil B. Kavey, MD Columbia Presbyterian Medical Center No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness, It is an active physiologic
Sleep Neil B. Kavey, MD Columbia Presbyterian Medical Center No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness, It is an active physiologic
Sleep. No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness,
 Sleep Neil B. Kavey, MD Columbia Presbyterian Medical Center No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness, It is an active physiologic
Sleep Neil B. Kavey, MD Columbia Presbyterian Medical Center No longer think of sleep as an isolated block of time at the end of the day. Sleep is not just the absence of wakefulness, It is an active physiologic
Neuro degenerative PET image from FDG, amyloid to Tau
 Neuro degenerative PET image from FDG, amyloid to Tau Kun Ju Lin ( ) MD, Ph.D Department of Nuclear Medicine and Molecular Imaging Center, Chang Gung Memorial Hospital ( ) Department of Medical Imaging
Neuro degenerative PET image from FDG, amyloid to Tau Kun Ju Lin ( ) MD, Ph.D Department of Nuclear Medicine and Molecular Imaging Center, Chang Gung Memorial Hospital ( ) Department of Medical Imaging
CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX:
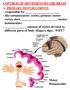 CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to different parts of body
CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to different parts of body
UPDATE ON RESEARCH IN PARKINSON S DISEASE
 UPDATE ON RESEARCH IN PARKINSON S DISEASE Charles H. Adler, M.D., Ph.D. Professor of Neurology Mayo Clinic College of Medicine Co-Principal Investigator Arizona Parkinson s Disease Consortium Arizona Study
UPDATE ON RESEARCH IN PARKINSON S DISEASE Charles H. Adler, M.D., Ph.D. Professor of Neurology Mayo Clinic College of Medicine Co-Principal Investigator Arizona Parkinson s Disease Consortium Arizona Study
A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to
 CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to different parts of body
CONTROL OF MOVEMENT BY THE BRAIN A. PRIMARY MOTOR CORTEX: - responsible for - like somatosensory cortex, primary motor cortex show (motor homunculus) - amount of cortex devoted to different parts of body
EEG Electrode Placement
 EEG Electrode Placement Classifying EEG brain waves Frequency: the number of oscillations/waves per second, measured in Hertz (Hz) reflects the firing rate of neurons alpha, beta, theta, delta Amplitude:
EEG Electrode Placement Classifying EEG brain waves Frequency: the number of oscillations/waves per second, measured in Hertz (Hz) reflects the firing rate of neurons alpha, beta, theta, delta Amplitude:
Dementia and Healthy Ageing : is the pathology any different?
 Dementia and Healthy Ageing : is the pathology any different? Professor David Mann, Professor of Neuropathology, University of Manchester, Hope Hospital, Salford DEMENTIA Loss of connectivity within association
Dementia and Healthy Ageing : is the pathology any different? Professor David Mann, Professor of Neuropathology, University of Manchester, Hope Hospital, Salford DEMENTIA Loss of connectivity within association
Neuroscience Optional Lecture. The limbic system the emotional brain. Emotion, behaviour, motivation, long-term memory, olfaction
 Neuroscience Optional Lecture The limbic system the emotional brain Emotion, behaviour, motivation, long-term memory, olfaction Emotion Conscious experience intense mental activity and a certain degree
Neuroscience Optional Lecture The limbic system the emotional brain Emotion, behaviour, motivation, long-term memory, olfaction Emotion Conscious experience intense mental activity and a certain degree
Stem Cells and the Study of Neurodegeneration. Tracy Young-Pearse, PhD September 12, 2014!
 Stem Cells and the Study of Neurodegeneration Tracy Young-Pearse, PhD September 12, 2014! Techniques for studying mechanisms of neurological disease Animal models Human subjects Postmortem analyses, imaging
Stem Cells and the Study of Neurodegeneration Tracy Young-Pearse, PhD September 12, 2014! Techniques for studying mechanisms of neurological disease Animal models Human subjects Postmortem analyses, imaging
Neurodegenerative Disease. April 12, Cunningham. Department of Neurosciences
 Neurodegenerative Disease April 12, 2017 Cunningham Department of Neurosciences NEURODEGENERATIVE DISEASE Any of a group of hereditary and sporadic conditions characterized by progressive dysfunction,
Neurodegenerative Disease April 12, 2017 Cunningham Department of Neurosciences NEURODEGENERATIVE DISEASE Any of a group of hereditary and sporadic conditions characterized by progressive dysfunction,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Splenic atrophy and leucopenia caused by T3 SCI.
 Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
Supplementary Figure 1 Splenic atrophy and leucopenia caused by T3 SCI. (a) Gross anatomy of representative spleens from control and T3 SCI mice at 28 days post-injury. (b and c) Hematoxylin and eosin
from sleep to attention lecture 4 April 9, 2012 control of sleep/wake state production II
 from sleep to attention lecture 4 April 9, 2012 control of sleep/wake state production II "From the moment of my birth, the angels of anxiety, worry, and death stood at my side, followed me out when I
from sleep to attention lecture 4 April 9, 2012 control of sleep/wake state production II "From the moment of my birth, the angels of anxiety, worry, and death stood at my side, followed me out when I
The Role of Adenosine in Sleep-Wake Regulation. Adam Painter. Copyright 2014 Adam Painter and Dr. Koni Stone
 The Role of Adenosine in Sleep-Wake Regulation Adam Painter Copyright 2014 Adam Painter and Dr. Koni Stone The Role of Adenosine in Sleep-Wake Regulation Sleep is one of the few experiences in life that
The Role of Adenosine in Sleep-Wake Regulation Adam Painter Copyright 2014 Adam Painter and Dr. Koni Stone The Role of Adenosine in Sleep-Wake Regulation Sleep is one of the few experiences in life that
CASE 49. What type of memory is available for conscious retrieval? Which part of the brain stores semantic (factual) memories?
 CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
Chronic Traumatic Encephalopathy Provider and Parent Essentials
 Chronic Traumatic Encephalopathy Provider and Parent Essentials Concussion Global Cast July 30, 2014 John Lockhart, MD Seattle Children s Hospital Chronic Traumatic Encephaly (CTE) Working Definition Chronic
Chronic Traumatic Encephalopathy Provider and Parent Essentials Concussion Global Cast July 30, 2014 John Lockhart, MD Seattle Children s Hospital Chronic Traumatic Encephaly (CTE) Working Definition Chronic
Page 1 L 58. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems /2013 RETICULAR FORMATION
 Page 1 L 58 Douglas L. Oliver, Ph.D. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems 1 2012/2013 RETICULAR FORMATION Lecture Lecture: Douglas Oliver
Page 1 L 58 Douglas L. Oliver, Ph.D. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems 1 2012/2013 RETICULAR FORMATION Lecture Lecture: Douglas Oliver
COGNITIVE IMPAIRMENT IN PARKINSON S DISEASE
 1 GENERAL INTRODUCTION GENERAL INTRODUCTION PARKINSON S DISEASE Parkinson s disease (PD) is a neurodegenerative movement disorder, named after James Parkinson who described some of its characteristic
1 GENERAL INTRODUCTION GENERAL INTRODUCTION PARKINSON S DISEASE Parkinson s disease (PD) is a neurodegenerative movement disorder, named after James Parkinson who described some of its characteristic
EEG and some applications (seizures and sleep)
 EEG and some applications (seizures and sleep) EEG: stands for electroencephalography and is a graphed representation of the electrical activity of the brain. EEG is the recording of electrical activity
EEG and some applications (seizures and sleep) EEG: stands for electroencephalography and is a graphed representation of the electrical activity of the brain. EEG is the recording of electrical activity
III./3.1. Movement disorders with akinetic rigid symptoms
 III./3.1. Movement disorders with akinetic rigid symptoms III./3.1.1. Parkinson s disease Parkinson s disease (PD) is the second most common neurodegenerative disorder worldwide after Alzheimer s disease.
III./3.1. Movement disorders with akinetic rigid symptoms III./3.1.1. Parkinson s disease Parkinson s disease (PD) is the second most common neurodegenerative disorder worldwide after Alzheimer s disease.
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE
 TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Brown EN, Lydic R, Schiff ND, et al. General anesthesia, sleep,
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Brown EN, Lydic R, Schiff ND, et al. General anesthesia, sleep,
Basic Mechanism for Generation of Brain Rhythms
 203 Continuing Medical Education Basic Mechanism for Generation of Brain Rhythms Wei-Hung Chen Abstract- Study of the basic mechanism of brain rhythms adds to our understanding of the underlying processes
203 Continuing Medical Education Basic Mechanism for Generation of Brain Rhythms Wei-Hung Chen Abstract- Study of the basic mechanism of brain rhythms adds to our understanding of the underlying processes
Overview of neurological changes in Alzheimer s disease. Eric Karran
 Overview of neurological changes in Alzheimer s disease Eric Karran Alzheimer s disease Alois Alzheimer 1864-1915 Auguste D. 1850-1906 Case presented November 26 th 1906 Guildford Talk.ppt 20 th March,
Overview of neurological changes in Alzheimer s disease Eric Karran Alzheimer s disease Alois Alzheimer 1864-1915 Auguste D. 1850-1906 Case presented November 26 th 1906 Guildford Talk.ppt 20 th March,
Neural Communication. Central Nervous System Peripheral Nervous System. Communication in the Nervous System. 4 Common Components of a Neuron
 Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Imaging of Alzheimer s Disease: State of the Art
 July 2015 Imaging of Alzheimer s Disease: State of the Art Neir Eshel, Harvard Medical School Year IV Outline Our patient Definition of dementia Alzheimer s disease Epidemiology Diagnosis Stages of progression
July 2015 Imaging of Alzheimer s Disease: State of the Art Neir Eshel, Harvard Medical School Year IV Outline Our patient Definition of dementia Alzheimer s disease Epidemiology Diagnosis Stages of progression
ON-LINE REPOSITORY MATERIAL DIFFERENCES IN SLEEP-INDUCED HYPOXIA BETWEEN A/J AND DBA/2J MOUSE STRAINS
 37 ON-LINE REPOSITORY MATERIAL DIFFERENCES IN SLEEP-INDUCED HYPOXIA BETWEEN A/J AND DBA/2J MOUSE STRAINS Arnon E. Rubin, Vsevolod Y. Polotsky, Alex Balbir, Jerry A. Krishnan, Alan R. Schwartz, Philip L.
37 ON-LINE REPOSITORY MATERIAL DIFFERENCES IN SLEEP-INDUCED HYPOXIA BETWEEN A/J AND DBA/2J MOUSE STRAINS Arnon E. Rubin, Vsevolod Y. Polotsky, Alex Balbir, Jerry A. Krishnan, Alan R. Schwartz, Philip L.
Sleep and Consciousness
 * Sleep and Dreaming The Neural Basis of Consciousness Sleep and Consciousness 1 Sleep and Dreaming readily observable, consists of different levels of consciousness, and can be studied scientifically
* Sleep and Dreaming The Neural Basis of Consciousness Sleep and Consciousness 1 Sleep and Dreaming readily observable, consists of different levels of consciousness, and can be studied scientifically
Synaptic changes in dementia: links to cognition and behaviour
 Synaptic changes in dementia: links to cognition and behaviour Paul T Francis, PhD Professor of Neurochemistry Director, Brains for Dementia Research Agenda Discuss synaptic changes in various dementias
Synaptic changes in dementia: links to cognition and behaviour Paul T Francis, PhD Professor of Neurochemistry Director, Brains for Dementia Research Agenda Discuss synaptic changes in various dementias
TAKEDA NEUROSCIENCE BRINGING INNOVATIVE MEDICINES TO PATIENTS FOR WHOM THERE ARE NO TREATMENTS AVAILABLE
 TAKEDA NEUROSCIENCE BRINGING INNOVATIVE MEDICINES TO PATIENTS FOR WHOM THERE ARE NO TREATMENTS AVAILABLE EMILIANGELO RATTI, PHD Head, Neuroscience Therapeutic Area WE HAVE TAKEN ON THE CHALLENGE TO ALLEVIATE
TAKEDA NEUROSCIENCE BRINGING INNOVATIVE MEDICINES TO PATIENTS FOR WHOM THERE ARE NO TREATMENTS AVAILABLE EMILIANGELO RATTI, PHD Head, Neuroscience Therapeutic Area WE HAVE TAKEN ON THE CHALLENGE TO ALLEVIATE
Chemical Control of Behavior and Brain 1 of 9
 Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Physiology of Sleep. Dr Nervana
 Physiology of Sleep Dr Nervana Objectives: 1. Explain the difference between sleep and coma. 2. Define NREM (non-rapid eye movement, SWS) and REM (rapid eye movement) sleep. 3. Describe how NREM and REM
Physiology of Sleep Dr Nervana Objectives: 1. Explain the difference between sleep and coma. 2. Define NREM (non-rapid eye movement, SWS) and REM (rapid eye movement) sleep. 3. Describe how NREM and REM
Brain Circuitry Controlling Sleep and Wakefulness Richard L. Horner, PhD; John H. Peever, PhD
 Review Article Brain Circuitry Controlling Sleep and Wakefulness Richard L. Horner, PhD; John H. Peever, PhD ABSTRACT Purpose of Review: This article outlines the fundamental brain mechanisms that control
Review Article Brain Circuitry Controlling Sleep and Wakefulness Richard L. Horner, PhD; John H. Peever, PhD ABSTRACT Purpose of Review: This article outlines the fundamental brain mechanisms that control
TAKEDA NEUROSCIENCE BRINGING INNOVATIVE MEDICINES TO PATIENTS FOR WHOM THERE ARE NO TREATMENTS AVAILABLE
 TAKEDA NEUROSCIENCE BRINGING INNOVATIVE MEDICINES TO PATIENTS FOR WHOM THERE ARE NO TREATMENTS AVAILABLE EMILIANGELO RATTI, PHD Head, Neuroscience Therapeutic Area WE HAVE TAKEN ON THE CHALLENGE TO ALLEVIATE
TAKEDA NEUROSCIENCE BRINGING INNOVATIVE MEDICINES TO PATIENTS FOR WHOM THERE ARE NO TREATMENTS AVAILABLE EMILIANGELO RATTI, PHD Head, Neuroscience Therapeutic Area WE HAVE TAKEN ON THE CHALLENGE TO ALLEVIATE
Type 2 Diabetes and Brain Disease in Older Adults. Erin L. Abner, PhD, MPH Asst. Professor University Of Kentucky
 Type 2 Diabetes and Brain Disease in Older Adults Erin L. Abner, PhD, MPH Asst. Professor University Of Kentucky Disclosures to Participants Requirements for Successful Completion: For successful completion,
Type 2 Diabetes and Brain Disease in Older Adults Erin L. Abner, PhD, MPH Asst. Professor University Of Kentucky Disclosures to Participants Requirements for Successful Completion: For successful completion,
International Institute for Integrative Sleep Medicine
 International Institute for Integrative Sleep Medicine Director Masashi Yanagisawa Sound sleep for everyone in the world ~ Solving the mystery of sleep ~ 76 We spend nearly one-third of our lives asleep.
International Institute for Integrative Sleep Medicine Director Masashi Yanagisawa Sound sleep for everyone in the world ~ Solving the mystery of sleep ~ 76 We spend nearly one-third of our lives asleep.
Index SLEEP MEDICINE CLINICS. Note: Page numbers of article titles are in boldface type.
 299 SLEEP MEDICINE CLINICS Sleep Med Clin 1 (2006) 299 303 Note: Page numbers of article titles are in boldface type. A Acid reflux, sleep disturbances in older adults related to, 238 Aging, alterations
299 SLEEP MEDICINE CLINICS Sleep Med Clin 1 (2006) 299 303 Note: Page numbers of article titles are in boldface type. A Acid reflux, sleep disturbances in older adults related to, 238 Aging, alterations
Nervous System. Oct 15 10:00 AM
 Nervous System Oct 15 10:00 AM 1 Nerve net = series of interconnected nerve cells Nerve = axons of many nerve cells Oct 15 10:10 AM 2 bilateral organisms exhibit cephalization (evolutionary trend towards
Nervous System Oct 15 10:00 AM 1 Nerve net = series of interconnected nerve cells Nerve = axons of many nerve cells Oct 15 10:10 AM 2 bilateral organisms exhibit cephalization (evolutionary trend towards
Exclusion criteria and outlier detection
 1 Exclusion criteria and outlier detection 1 2 Supplementary Fig. 1 31 subjects complied with the inclusion criteria as tested during the familiarization session. The upper part of the figure (ovals) indicates
1 Exclusion criteria and outlier detection 1 2 Supplementary Fig. 1 31 subjects complied with the inclusion criteria as tested during the familiarization session. The upper part of the figure (ovals) indicates
Intracranial Studies Of Human Epilepsy In A Surgical Setting
 Intracranial Studies Of Human Epilepsy In A Surgical Setting Department of Neurology David Geffen School of Medicine at UCLA Presentation Goals Epilepsy and seizures Basics of the electroencephalogram
Intracranial Studies Of Human Epilepsy In A Surgical Setting Department of Neurology David Geffen School of Medicine at UCLA Presentation Goals Epilepsy and seizures Basics of the electroencephalogram
Lewy Bodies in the Amygdala
 ORIGINAL CONTRIBUTION Lewy Bodies in the Amygdala Increase of -Synuclein Aggregates in Neurodegenerative Diseases With Tau-Based Inclusions Anca Popescu, MD; Carol F. Lippa, MD; Virginia M.-Y. Lee, PhD;
ORIGINAL CONTRIBUTION Lewy Bodies in the Amygdala Increase of -Synuclein Aggregates in Neurodegenerative Diseases With Tau-Based Inclusions Anca Popescu, MD; Carol F. Lippa, MD; Virginia M.-Y. Lee, PhD;
NEOCORTEX. Laminar pattern 6 layers billion neurons 95 % surface of the hemisphere
 THE CEREBRAL CORTEX NEOCORTEX Laminar pattern 6 layers 10 20 billion neurons 95 % surface of the hemisphere Six Layers of Cortex LGN input Parvo Magno B-Slide 4 NEOCORTEX, types of neurons Pyramidal neurons
THE CEREBRAL CORTEX NEOCORTEX Laminar pattern 6 layers 10 20 billion neurons 95 % surface of the hemisphere Six Layers of Cortex LGN input Parvo Magno B-Slide 4 NEOCORTEX, types of neurons Pyramidal neurons
Normal sleep mechanisms & why do we sleep?
 4 rd Congress of the European Academy of Neurology Lisbon, Portugal, June 16-19, 2018 Teaching Course 18 Basics of sleep medicine - Level 1 Normal sleep mechanisms & why do we sleep? Rolf Fronczek Leiden,
4 rd Congress of the European Academy of Neurology Lisbon, Portugal, June 16-19, 2018 Teaching Course 18 Basics of sleep medicine - Level 1 Normal sleep mechanisms & why do we sleep? Rolf Fronczek Leiden,
Simulated brain biopsy for diagnosing neurodegeneration using autopsy-confirmed cases
 Acta Neuropathol (2011) 122:737 745 DOI 10.1007/s00401-011-0880-5 ORIGINAL PAPER Simulated brain biopsy for diagnosing neurodegeneration using autopsy-confirmed cases Sriram Venneti John L. Robinson Subhojit
Acta Neuropathol (2011) 122:737 745 DOI 10.1007/s00401-011-0880-5 ORIGINAL PAPER Simulated brain biopsy for diagnosing neurodegeneration using autopsy-confirmed cases Sriram Venneti John L. Robinson Subhojit
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Supplementary Methods
 1 Supplementary Methods Social Preference Test Subjects Seventy-four Long-Evans, male rats served as subjects (S-rats) in the foodpreference test, with 40 assigned to the CXT-Same (CXT-S) Condition and
1 Supplementary Methods Social Preference Test Subjects Seventy-four Long-Evans, male rats served as subjects (S-rats) in the foodpreference test, with 40 assigned to the CXT-Same (CXT-S) Condition and
C. G. Diniz Behn, N. Kopell, E. N. Brown, T. Mochizuki and T. E. Scammell
 C. G. Diniz Behn, N. Kopell, E. N. Brown, T. Mochizuki and T. E. Scammell J Neurophysiol 99:3090-3103, 2008. First published Apr 16, 2008; doi:10.1152/jn.01243.2007 You might find this additional information
C. G. Diniz Behn, N. Kopell, E. N. Brown, T. Mochizuki and T. E. Scammell J Neurophysiol 99:3090-3103, 2008. First published Apr 16, 2008; doi:10.1152/jn.01243.2007 You might find this additional information
The Neural Control of Behavioral State
 The Neural Control of Behavioral State Learning objectives: Introduction - Behavioral State; why do we sleep? A Clinical Case - The Sleeping Beauty of Oak Park. EEG - A Neural Signature of Behavioral State.
The Neural Control of Behavioral State Learning objectives: Introduction - Behavioral State; why do we sleep? A Clinical Case - The Sleeping Beauty of Oak Park. EEG - A Neural Signature of Behavioral State.
Sleep, Circadian Rhythms, and Aging: Translational Perspective
 Bedside to Bench Conference Sleep, Circadian Rhythms, and Aging: New Avenues for Improving Brain Health, Physical Health and Functioning October 5, 2015 Sleep, Circadian Rhythms, and Aging: Translational
Bedside to Bench Conference Sleep, Circadian Rhythms, and Aging: New Avenues for Improving Brain Health, Physical Health and Functioning October 5, 2015 Sleep, Circadian Rhythms, and Aging: Translational
Author Manuscript Faculty of Biology and Medicine Publication
 Serveur Académique Lausannois SERVAL serval.unil.ch Author Manuscript Faculty of Biology and Medicine Publication This paper has been peer-reviewed but does not include the final publisher proof-corrections
Serveur Académique Lausannois SERVAL serval.unil.ch Author Manuscript Faculty of Biology and Medicine Publication This paper has been peer-reviewed but does not include the final publisher proof-corrections
Poor Sleep Before Dementia: A Risk Factor for Cognitive Decline and Clinical Conversion?
 15th ANNUAL MCI SYMPOSIUM Session 3: Sleep Disorders and MCI Poor Sleep Before Dementia: A Risk Factor for Cognitive Decline and Clinical Conversion? Bryce Mander, PhD Sleep and Neuroimaging Laboratory
15th ANNUAL MCI SYMPOSIUM Session 3: Sleep Disorders and MCI Poor Sleep Before Dementia: A Risk Factor for Cognitive Decline and Clinical Conversion? Bryce Mander, PhD Sleep and Neuroimaging Laboratory
Pathogenesis of Degenerative Diseases and Dementias. D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria)
 Pathogenesis of Degenerative Diseases and Dementias D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria) Dementias Defined: as the development of memory impairment and other cognitive deficits
Pathogenesis of Degenerative Diseases and Dementias D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria) Dementias Defined: as the development of memory impairment and other cognitive deficits
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH- TITLE: PRINCIPAL INVESTIGATOR: CONTRACTING ORGANIZATION: University of REPORT DATE: TYPE OF REPORT: PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick,
AD Award Number: W81XWH- TITLE: PRINCIPAL INVESTIGATOR: CONTRACTING ORGANIZATION: University of REPORT DATE: TYPE OF REPORT: PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick,
Why sleep should not be ignored in Alzheimer s Disease
 15 th ANNUAL MCI SYMPOSIUM Public Education Forum: New concepts in Alzheimer s Cause, Progression and Therapy Why sleep should not be ignored in Alzheimer s Disease Bryce Mander, PhD Sleep and Neuroimaging
15 th ANNUAL MCI SYMPOSIUM Public Education Forum: New concepts in Alzheimer s Cause, Progression and Therapy Why sleep should not be ignored in Alzheimer s Disease Bryce Mander, PhD Sleep and Neuroimaging
The Parkinson s You Can t See
 The Parkinson s You Can t See We principally see the motor phenomena of Parkinson's disease, but is there an early stage without visible features? Might this provide a window for disease-modifying therapy?
The Parkinson s You Can t See We principally see the motor phenomena of Parkinson's disease, but is there an early stage without visible features? Might this provide a window for disease-modifying therapy?
Neurophysiology & EEG
 Neurophysiology & EEG PG4 Core Curriculum Ian A. Cook, M.D. Associate Director, Laboratory of Brain, Behavior, & Pharmacology UCLA Department of Psychiatry & Biobehavioral Sciences Semel Institute for
Neurophysiology & EEG PG4 Core Curriculum Ian A. Cook, M.D. Associate Director, Laboratory of Brain, Behavior, & Pharmacology UCLA Department of Psychiatry & Biobehavioral Sciences Semel Institute for
Development of the Nervous System. Leah Militello, class of 2018
 Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
A new approach to Common Sporadic Alzheimer s, Post-Traumatic Alzheimer s, and CTE:
 A new approach to Common Sporadic Alzheimer s, Post-Traumatic Alzheimer s, and CTE: Roles of Aβ, Tau, ApoE, and Regulatory Signaling in Elucidating Pathogenesis and Experimental Therapeutics Sam Gandy,
A new approach to Common Sporadic Alzheimer s, Post-Traumatic Alzheimer s, and CTE: Roles of Aβ, Tau, ApoE, and Regulatory Signaling in Elucidating Pathogenesis and Experimental Therapeutics Sam Gandy,
Supplementary Materials for. c-abl Activation Plays a Role in α-synucleinopathy Induced Neurodegeneration
 Supplementary Materials for c-abl Activation Plays a Role in α-synucleinopathy Induced Neurodegeneration Saurav Brahmachari, Preston Ge, Su Hyun Lee, Donghoon Kim, Senthilkumar S. Karuppagounder, Manoj
Supplementary Materials for c-abl Activation Plays a Role in α-synucleinopathy Induced Neurodegeneration Saurav Brahmachari, Preston Ge, Su Hyun Lee, Donghoon Kim, Senthilkumar S. Karuppagounder, Manoj
UNIT 5 REVIEW GUIDE - NERVOUS SYSTEM 1) State the 3 functions of the nervous system. 1) 2) 3)
 UNIT 5 REVIEW GUIDE - NERVOUS SYSTEM State the 3 functions of the nervous system. Briefly describe the general function(s) of each of the following neuron types: a) SENSORY NEURONS: b) INTERNEURONS: c)
UNIT 5 REVIEW GUIDE - NERVOUS SYSTEM State the 3 functions of the nervous system. Briefly describe the general function(s) of each of the following neuron types: a) SENSORY NEURONS: b) INTERNEURONS: c)
Interventions on Lifestyle and Social Support Factors: Sleep Quality and Disorders
 Interventions on Lifestyle and Social Support Factors: Sleep Quality and Disorders Preventing Dementia and Cognitive Impairment: A Workshop Susan M. McCurry, PhD Northwest Research Group on Aging Department
Interventions on Lifestyle and Social Support Factors: Sleep Quality and Disorders Preventing Dementia and Cognitive Impairment: A Workshop Susan M. McCurry, PhD Northwest Research Group on Aging Department
Cheyenne 11/28 Neurological Disorders II. Transmissible Spongiform Encephalopathy
 Cheyenne 11/28 Neurological Disorders II Transmissible Spongiform Encephalopathy -E.g Bovine4 Spongiform Encephalopathy (BSE= mad cow disease), Creutzfeldt-Jakob disease, scrapie (animal only) -Sporadic:
Cheyenne 11/28 Neurological Disorders II Transmissible Spongiform Encephalopathy -E.g Bovine4 Spongiform Encephalopathy (BSE= mad cow disease), Creutzfeldt-Jakob disease, scrapie (animal only) -Sporadic:
Hippocampal mechanisms of memory and cognition. Matthew Wilson Departments of Brain and Cognitive Sciences and Biology MIT
 Hippocampal mechanisms of memory and cognition Matthew Wilson Departments of Brain and Cognitive Sciences and Biology MIT 1 Courtesy of Elsevier, Inc., http://www.sciencedirect.com. Used with permission.
Hippocampal mechanisms of memory and cognition Matthew Wilson Departments of Brain and Cognitive Sciences and Biology MIT 1 Courtesy of Elsevier, Inc., http://www.sciencedirect.com. Used with permission.
