Peripherally Acting μ-opioid Receptor Antagonists for the Treatment of Opioid-Related Side Effects: Mechanism of Action and Clinical Implications
|
|
|
- Poppy Crawford
- 5 years ago
- Views:
Transcription
1 Review Article Peripherally Acting μ-opioid Receptor Antagonists for the Treatment of Opioid-Related Side Effects: Mechanism of Action and Clinical Implications Journal of Pharmacy Practice 2018, Vol. 31(6) ª The Author(s) 2017 Article reuse guidelines: sagepub.com/journals-permissions DOI: / journals.sagepub.com/home/jpp John M. Streicher, PhD 1, and Edward J. Bilsky, PhD 2 Abstract Opioid receptors are distributed throughout the central and peripheral nervous systems and on many nonneuronal cells. Therefore, opioid administration induces effects beyond analgesia. In the enteric nervous system (ENS), stimulation of m-opioid receptors triggers several inhibitory responses that can culminate in opioid-induced bowel dysfunction (OBD) and its most common side effect, opioid-induced constipation (OIC). OIC negatively affects patients quality of life (QOL), ability to work, and pain management. Although laxatives are a common first-line OIC therapy, most have limited efficacy and do not directly antagonize opioid effects on the ENS. Peripherally acting m-opioid receptor antagonists (PAMORAs) with limited ability to cross the blood-brain barrier have been developed. The PAMORAs approved by the U S Food and Drug Administration for OIC are subcutaneous and oral methylnaltrexone, oral naloxegol, and oral naldemedine. Although questions of cost-effectiveness and relative efficacy versus laxatives remain, PAMORAs can mitigate OIC and improve patient QOL. PAMORAS may also have applications beyond OIC, including reducing the increased cardiac risk or potential tumorigenic effects of opioids. This review discusses the burden of OIC and OBD, reviews the mechanism of action of new OIC therapies, and highlights other potential opioid-related side effects mediated by peripheral opioid receptors in the context of new OIC therapies. Keywords methylnaltrexone, naloxegol, naldemedine, opioid-induced constipation, PAMORA Introduction Opioid agonists are commonly used for the treatment of moderate to severe chronic cancer and noncancer pain, owing to their analgesic actions, 1 mostly at m-opioid receptors. Opioid receptor locations in the periphery include lung, spleen, kidney, heart, skeletal muscle, cartilage, synovium, periosteum, tendon, bone, liver, thymus, enteric neurons of the gastrointestinal (GI) system, pancreas, adrenal gland, and dorsal root ganglion (Table 1). 2-5 The diverse central nervous system (CNS) and peripheral nervous system (PNS) locations of the m-opioid receptors in particular contribute to the respective side effect profiles of opioid drugs. Due to the distribution of m-opioid receptors in the enteric nervous system (ENS), a common and significant consequence of opioid therapy is opioid-induced bowel dysfunction (OBD), which is most often characterized by opioid-induced constipation (OIC), 6-9 along with decreased appetite, increased reflux, nausea, and vomiting. 6,10 Studies in rats have shown that concentrations of systemically administered morphine are considerably higher in the GI tract than in the CNS, and GI concentrations correlate directly with inhibition of GI transit. 11 In addition, evidence from studies comparing the effects of central and systemic administration of morphine in rats suggests that CNS opioid receptors may have some influence on GI transit as well. 12 The interaction of opioids with m-opioid receptors in the ENS of the GI tract induces multiple effects that lead to impaired GI motility, including slowing GI transit, promoting nonpropulsive motility and segmentation, and promoting contraction of the sphincters. 13,14 The increase in GI transit time, along with the opioid-induced stimulation of mucosal sensory receptors, results in enhanced fluid reabsorption, which further promotes constipation. 13,14 A consensus definition for OIC is lacking. 15 Although not specific to OIC, the American College of Gastroenterology definition of constipation includes infrequent bowel movements and challenges with passing stool (eg, straining and/or 1 Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ, USA 2 Pacific Northwest University of Health Sciences, Yakima, WA, USA Corresponding Author: Edward J. Bilsky, Pacific Northwest University of Health Sciences, Yakima, WA 98901, USA. ebilsky@pnwu.edu
2 Streicher and Bilsky 659 Table 1. Locations and Inferred Functions of Opioid Receptor Subtypes. Anatomical Location Receptor mrna Detected Receptor Protein Subtype(s) Detected Inferred Function References Central Regions a Caudate nucleus μ, δ, κ m: Pain perception, sensorimotor integration 2 Cerebellum μ, d, k d: Motor and cognitive functioning 2 Cerebral cortex m, δ, k k: Feeding, pain perception, neuroendocrine function 2 Nucleus accumbens μ, δ, κ m, d, k: Stress-related activation of endogenous opioid systems 2 Putamen m, δ, κ 2 Spinal cord m, d, k 2 Substantia nigra m, d, k 2 Peripheral Regions Adrenal gland m, d, k Paracrine or autocrine role in adrenal function 2 Bone m Endogenous opioid function in skeletal ontology 3 Cartilage m m 3 Dorsal root ganglion m, d, k Analgesia 2 Enteric neurons m, d, k m, d: Inhibition of adenylate cyclase and nerve terminal Ca 2þ channels, 4 activation of K þ channels, inhibition of submucosal secretomotor neurons k: Inhibition of Ca 2þ channels, inhibition of neurotransmitter release m, d, k: Inhibition of acetylcholine release from enteric interneurons and purine/nitric oxide release from inhibitory motorneurons Heart d, k m, d, k Myocardial performance; regulation of sympathetic and 2,5 parasympathetic control of the heart Kidney d, k 2 Liver k 2 Lung d, k 2 Pancreas m, d, k Modulation of visceral pain 2 Periosteum m Endogenous opioid function in skeletal ontology 3 Skeletal Muscle d, k 2 Spleen k Possible involvement in immune function 2 Synovium m, d, k m, d, k m, d: Modulation of nociception, endogenous analgesia 3 k: Possible role in inflammation Tendon d 3 Thymus d, k Possible involvement in immune function 2 Abbreviations: CNS, central nervous system; d, d-opioid receptor; k, k-opioid receptor; m, m-opioid receptor. a Bolded subtype indicates the brain regions in which the greatest relative expression for that subtype was apparent. incomplete evacuations). The Rome III criteria define functional constipation by the presence of multiple constipation symptoms, including less than 3 bowel movements per week and/or straining, lumpy or hard stools, a feeling of incomplete evacuation and/or anorectal blockage, or manual maneuvers during at least one-quarter of bowel movements. 13,16 There are several tools that can be used to assess the severity of symptoms of OIC and evaluate response to treatment, including the following: the Patient Assessment of Constipation, a 12-item questionnaire that evaluates 3 key areas of OIC (stool characteristics, rectal symptoms, and abdominal symptoms) 17 ;the Bowel Function Index (BFI), a 3-item questionnaire that evaluates the ease of bowel movements, feelings of incomplete evacuation, and patient-rated constipation 18 ; and the bowel function diary, which evaluates multiple constipation-related domains, including frequency and difficulty of bowel movements and medication use. 13,19 Due to the negative impact of OIC on pain relief, patient quality of life (QOL), and health-related outcomes, effective treatments of OIC are needed. Although evidence supporting the management of OIC with laxatives (stool softeners, osmotic agents, and stimulant laxatives) is limited, 10 several guidelines endorse laxatives as a first-line treatment of OIC Additional prospective studies of laxatives versus alternative treatments, including new targeted therapies, may result in the revision of these guidelines for more effective management of OIC. The objectives of this article are to discuss the burden of illness of OIC and OBD, to provide an overview of the mechanism of action of new OIC therapies from basic science and clinical perspectives, and to highlight other potential opioid-related side effects mediated by peripheral opioid receptors and the potential effect of new OIC therapies on them. Burden of Illness OIC is highly prevalent among patients receiving opioids for cancer pain and chronic noncancer pain. Furthermore, unlike other opioid-related side effects, patients typically do not
3 660 Journal of Pharmacy Practice 31(6) develop a tolerance to OIC over time with continued opioid use. 13 In a survey of more than 2000 patients receiving opioids for chronic noncancer pain, the prevalence of OIC was 57% overall and ranged from 17% to 67% depending on the particular opioid used (codeine, hydrocodone, propoxyphene, morphine, oxycodone, or tramadol). 9 In an analysis of 8 trials of patients with chronic noncancer pain receiving opioid treatment for up to 8 weeks, OIC was reported by 41% of patients. 7 That analysis determined that the number needed to harm was 3.4 (95% confidence interval, ), meaning that for every 3 patients treated with opioids, 1 more would have constipation versus those treated with placebo. 7 A study of patients receiving opioids for cancer pain found that 62% had Knowles Eccersley Scott Symptom constipation scores from 9 to 39 points, indicating a problematic level of constipation. 6 In an online survey of patients with OIC, at least 80% of participants would like to improve the following aspects of constipation: the ability to have a pain-free bowel movement or to produce a soft (not loose or watery) stool, to worry less about performing a bowel movement, to experience no rectal straining due to constipation, to feel less bloated or to have less stomach pain, and to be more comfortable using opioid medication without fear of constipation. 23 The symptoms common to OBD and OIC that have been reported at a prevalence of 20% in patients with chronic pain are summarized in Table 2. 6,9,24,25 OBD and OIC negatively affect patient QOL and activities of daily living. Almost all (93%) patients taking opioids for cancer pain who had constipation reported that their OBD symptoms affected their QOL, and 38% rated the effect as moderate to severe. 6 The difference in QOL between patients with and without constipation was significant, as measured by both the condition-specific Patient Assessment of Constipation Quality of Life (PAC-QOL) and the generic Short Form-12 questionnaires. 6 In the Patient Reports of Opioidrelated Bothersome Effects (PROBE) I study (a multinational Internet study to assess OBD), more than half of the patients who reported constipation, passing gas, and bloating noted that these symptoms had a moderate to great or great effect on their QOL. 24 Abdominal pain, bloating, passing gas, reflux, and heartburn were also reported to have a moderate to great or great effect on patient activities of daily living. 24 In a multicountry longitudinal study by Coyne and colleagues, patients with OIC had a lower QOL compared with others with chronic pain alone or other chronic conditions, 38% reported impairment in daily activities, and, of those who were employed, 9% missed work and 32% reported impairment while working due to OIC. 25 OIC may also interfere with pain management. Multiple studies have documented that patients may lower the dose of, switch, or stop taking their opioid medication to ameliorate their GI symptoms, which may in turn compromise pain control. 6,9,23-25 However, constipation often occurs with opioid doses lower than those required for analgesia, so lowering the dose is generally not effective for managing OIC. 13 Although dose reduction to alleviate OIC is not typically feasible, switching opioids may reduce the symptoms of OIC in some patients. 13 Other nonpharmacologic methods of managing constipation, including increasing physical activity and fiber intake, have not been proven to be beneficial in patients with OIC but could offer some relief of symptoms. 13 Shortcomings of OIC Treatment With Laxatives Although laxatives are often used as first-line therapy to treat OIC, they do not address the underlying pathophysiologic mechanism, because they do not oppose opioid actions at enteric opioid receptors. 8 Laxatives have not been well studied in randomized, controlled trials, 26 but published data from patient surveys suggest that most laxatives are not very effective for OIC. 6,8,9,24,25 In a survey by Bell et al of patients with chronic noncancer pain or cancer-related pain who were taking laxatives as well as opioids, 81% reported persistent constipation. 24 Abramowitz and colleagues showed that in patients receiving strong opioids for cancer pain, even though 84.7% were taking laxatives, 85.7% were considered by physicians to be constipated. 6 In a study by Rosti et al of patients receiving analgesics for chronic pain, the majority of which were strong opioids, 63.5% had OBD symptoms despite the fact that 89.5% of those who reported OBD symptoms were taking laxatives. 27 In a multicountry longitudinal study of patients with chronic noncancer pain taking opioids and experiencing OIC, the majority of whom were taking laxatives, Coyne and colleagues found that more than half reported dissatisfaction with their constipation treatment, and most who reported any benefit of their OIC treatment reported it provided little benefit. 25 Patient-reported achievement of the desired outcome at least half of the time with laxatives was 46% in 1 study, 8 and patient dissatisfaction with laxatives was reported by *25% of patients in another. 9 Because OIC is associated with a high burden of disease, cross-comparisons of the effectiveness of laxatives versus the newer, peripherally acting m-opioid receptor antagonists (PAMORAs) in prospective placebo-controlled trials are needed. Targeted Treatment of Opioid-Induced Constipation PAMORAs were developed to address the need to block the GI actions of opioids in the periphery while not affecting their CNS actions, thereby directly targeting the mechanism of OIC. The first PAMORAs approved by the US Food and Drug Administration (FDA) were subcutaneous methylnaltrexone, which was initially approved for OIC in adult patients with advanced illness and later in adult patients with chronic noncancer pain, and alvimopan, which is indicated for accelerating the time to upper and lower GI recovery following surgeries that include partial bowel resection with primary anastomosis Naloxegol was then approved in September 2014 for the treatment of OIC in adult patients with chronic noncancer pain. 31 Naldemedine was approved in March 2017 for the treatment of OIC in adult patients with chronic noncancer pain. 32
4 Streicher and Bilsky 661 Table 2. Common Symptoms of OBD and OIC Reported at 20% Frequency Among Patients Using Opioids for the Treatment of Chronic Pain. Study type Cook et al 9 Bell et al PROBE I Study 24 Abramowitz et al 6 DYONISOS Study Coyne et al 25 US populationbased survey Internet-based survey (US, France, Germany, Italy, Spain, United Kingdom) Cross-sectional, physicianconducted study in France Longitudinal study (US, Canada, Germany, United Kingdom) Patient population Noncancer pain Cancer and noncancer pain using laxatives Cancer pain Noncancer pain N OBD symptoms, % Abdominal pain or 30 a 31 b,20 c 26.2, d 23.6 e 66.9 f, 64.1 g, 51.7 h, 51.7 i discomfort Bloating BM characteristics BM too small/hard ND 50 ND 75.1 j, 63.3 k Evacuation ND 45 l,20 m ND 68.4 l, 59.8 n Rectal symptoms ND ND ND 42.8 o, 26.6 p Straining ND q 82.6 r Decrease or loss of ND appetite Flatulence Heartburn ND ND Nausea ND 22.1 Regurgitation or reflux ND 36.7 Other Abdominal rumblings ND 23 ND ND Fatigue ND 50 ND ND Headache or ND ND ND 39.1 migraine Insomnia ND 40 ND ND Abbreviations: BM, bowel movement; DYONISOS, Dysfonctions Intestinales Induites par les Opioids Forts; ND, not determined; OBD, opioid-induced bowel dysfunction; OIC, opioid-induced constipation; PROBE, Patient Reports of Opioid-Related Bothersome Effects; US, United States. a Includes stomach pain or discomfort. b Lower abdominal discomfort. c Upper abdominal pain/discomfort. d Pelvic discomfort. e Abdominal pain during defecation. f Painful BM. g Abdominal discomfort. h Abdominal pain. I Stomach cramps. j BM too hard. k BM too small. l Incomplete BM. m False alarm BM. n Feeling like had to pass BM but could not. o Burning during or after BM. p Bleeding/tearing during or after BM. q During defecation. r Straining or squeezing to pass BMs. Mechanism of Action of PAMORAs PAMORAs block opioids from binding to peripheral m-opioid receptors, with the goal of restoring ENS function. Although PAMORAs may compete with opioids for m-opioid receptor binding throughout the body, the desired therapeutic mechanism of action involves selective, competitive binding of the drug to enteric m-opioid receptors (Figure 1). 33 The initial strategy in the drug development process was to investigate the use of the m-opioid receptor antagonist naloxone for the treatment of OIC. 34,35 In a dose-ranging study, oral naloxone was given to patients with advanced cancer and OIC who were receiving morphine for pain control. 34 Naloxone at doses that were 20% of the morphine dose provided a clinically significant laxative effect in 9 of 12 patients, but 2 patients experienced generalized withdrawal reactions, 1 of whom also had a return of pain. 34 In a subsequent pilot study, administration of lowdose oral naloxone (2 or 4 mg) 3 times per day to patients with
5 662 Journal of Pharmacy Practice 31(6) Figure 1. Mechanism of action of PAMORAs in the GI tract. 33 Normal GI activity is shown in the left column, the action of opioids in the GI tract is shown in the middle column, and the action of naloxegol in the GI tract is shown in the right column. The first row shows opioids crossing the blood-brain barrier from the blood stream, while naloxegol is restricted to the periphery and thus does not cross the blood-brain barrier or alter centrally mediated analgesia. The second row shows the interaction of opioids with m-opioid receptors in the enteric nervous system, leading to nonpropulsive motility. Naloxegol has a higher affinity for these receptors and, by displacing opioids, can restore normal motility. The third row shows the impact of opioids on secretion of electrolytes and water into the intestinal lumen, leading to dry, hardened stools. Naloxegol counteracts the opioid-induced reduction in intestinal secretion, resulting in softer stools. The fourth row shows the impact of opioids on the sphincter and restoration of sphincter function through the action of naloxegol. GI indicates gastrointestinal; PAMORA, peripherally acting m-opioid receptor antagonists. (Adapted with permission from Poulsen JL et al. 33 ) OIC increased the frequency of bowel movements from baseline in all patients; however, 3 of 6 patients experienced reversal of analgesia. 35 The mixed results in those trials led investigators to pursue this approach with modifications to keep agents from crossing the blood-brain barrier (BBB), which led to the development of PAMORAs. With PAMORAs, entry to the CNS is restricted as the result of various molecular properties or modifications that alter their ability to cross the BBB. Figure 2 shows the chemical structures of approved and investigational agents and the modifications that restrict them to the periphery ,36,37 Methylnaltrexone is a quaternary amine with a positive charge on the nitrogen that increases its polarity and water solubility, reducing its ability to cross the BBB. 30 However, the quaternary amine structure has a significant effect on its m-opioid receptor binding affinity, 38 such that higher doses are required, increasing the risk of possible side effects. Naloxegol is a pegylated form of naloxone 31 and is covalently conjugated with polyethylene glycol. This increases its molecular weight, making the amount of naloxegol that crosses the BBB negligible, while maintaining its m-opioid receptor affinity. 31 The pegylation of naloxegol also results in it becoming a substrate for the P-glycoprotein (PGP) efflux transporter, 31 similar to therapeutic doses of the antidiarrheal opioid agent loperamide, which lacks CNS effects because it is subject to efflux by PGP. 39 PGP effectively pumps loperamide and naloxegol out of the CNS so that pharmacologically active concentrations are not reached. 31,39 Naldemedine is a derivative of naltrexone with an additional side chain that results in increased polar surface area and molecular weight; this side chain reduces the
6 Streicher and Bilsky 663 Figure 2. Structures of approved and investigational PAMORAs ,36,37 NAP indicates naltrexamine derivative; PAMORA, peripherally acting m-opioid receptor antagonists. ability of naldemedine to cross the BBB. 32 Similar to naloxegol, naldemedine is a substrate of the PGP efflux transporter. 32 In theory, PAMORAs may be associated with therapeutic and/or withdrawal-like laxation, diarrhea, or other peripheral effects. No clinically significant opioid withdrawal has been observed in studies of PAMORAs approved for OIC In addition to their therapeutic effect for reducing OIC, it is possible that blockade by PAMORAs may decrease the incidence of opioid-mediated adverse events (AEs) in other tissues, including cardiac tissue. Efficacy and Safety of FDA-Approved PAMORAs for OIC Three of the 4 available PAMORAs are approved by the FDA for OIC, and their efficacy, safety, and dosing schedules are summarized here. Methylnaltrexone Subcutaneous methylnaltrexone (Relistor ; Salix Pharmaceuticals, Inc., Raleigh, North Carolina) is indicated for the treatment of OIC in patients receiving palliative care for advanced illness with insufficient response to laxatives (Table 3), and was approved for the treatment of OIC in patients with chronic noncancer pain in September In addition, oral methylnaltrexone was approved for the treatment of OIC in patients with chronic noncancer pain in July Subcutaneous methylnaltrexone is administered as an every-other-day injection (0.15 mg/kg for patients <38 or >114 kg; 8 mg for patients 38 to <62 kg; 12 mg for patients 62 to 114 kg) in patients with advanced illness receiving palliative care and as a once-daily injection (12 mg) in patients with chronic noncancer pain. Oral methylnaltrexone is administered as a once-daily tablet (450 mg) in the morning. 30 In patients with moderate and severe renal impairment (creatinine clearance [CLcr] <60 ml/min) and those with moderate or severe hepatic impairment, the following reduced dosing is recommended: in patients with advanced illness, an every-other-day subcutaneous injection (0.075 mg/kg for patients <38 or >114 kg; 4 mg for patients 38 to <62 kg; 6 mg for patients 62 to 114 kg); and in patients with chronic noncancer pain, a subcutaneous injection of 6 mg once daily or a 150mgoraltabletoncedaily. 30 The efficacy and safety of subcutaneous methylnaltrexone was investigated in 2 randomized, double-blind, placebocontrolled, phase 3 clinical trials in patients with advanced illness receiving palliative care and OIC, and 1 randomized, double-blind, placebo-controlled, phase 3 trial in patients with noncancer pain and OIC. 41,44,45 In 1 trial, 154 patients with advanced illness receiving palliative care were given a single dose of methylnaltrexone 0.15 or 0.30 mg/kg or placebo. 41 The primary end point was laxation within 4 hours of drug administration. 41 Patients could then enter a 28-day open-label phase to receive methylnaltrexone as needed (starting dose, 0.15 mg/kg) as often as every 24 hours, followed by the option to continue in a 3-month extension. 41 The primary end point was achieved by 62%, 58%, and 14% of patients who received a single dose of methylnaltrexone 0.15 mg/kg, methylnaltrexone 0.30 mg/kg, and placebo, respectively (P <.0001 for both doses vs placebo). The median time to rescue-free laxation (spontaneous bowel movement [SBM] without interventions such as laxatives) was 1.10, 0.8, and >24 hours in these respective groups (P <.0001 for both doses vs placebo). 41 Almost all patients (97%) continued to the open-label phase, and 54% of
7 664 Journal of Pharmacy Practice 31(6) Table 3. Indications, Dosing, and Formulations for FDA-Approved PAMORAs for OIC. PAMORA FDA-Approved Indication(s) Formulation Recommended Dosage Methylnaltrexone (Relistor ) 30 Indicated for the treatment of OIC in adults with chronic noncancer pain Indicated for the treatment of OIC in adults with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient Naloxegol (Movantik ) 31 Naldemedine (Symproic ) 32 Indicated for the treatment of OIC in adults with chronic noncancer pain Indicated for the treatment of OIC in adults with chronic noncancer pain Oral tablets and SC injections For OIC in adult patients with chronic noncancer pain: Tablets: 450 mg once daily Injection: 12 mg SC once daily For OIC in adult patients with advanced illness, the following weight-based dosing is used every other day, as needed: <38 kg: 0.15 mg/kg SC 38 to <62 kg: 8 mg SC 62 to 114 kg: 12 mg SC >114 kg: 0.15 mg/kg SC For patients with moderate to severe renal or hepatic impairment, the following weight-based dosing is used every other day, as needed: <38 kg: mg/kg SC 38 to <62 kg: 4 mg SC 62 to 114 kg: 6 mg SC >114 kg: mg/kg SC Oral tablets 25 mg once daily; if not tolerated, reduce to 12.5 mg once daily Renal impairment (CLcr <60 ml/min): 12.5 mg once daily; increase to 25 mg once daily if tolerated and monitor for adverse reactions Oral tablets 0.2 mg once daily No dose adjustment is needed in patients with mild or moderate hepatic impairment Abbreviations: CLcr, creatinine clearance; FDA, Food and Drug Administration; OIC, opioid-induced constipation; PAMORA, peripherally acting mu-opioid receptor agonist; SC, subcutaneous. those who had previously received placebo achieved laxation within 4 hours of administration of the first open-label dose of methylnaltrexone. 41 The most common AEs that occurred more frequently with methylnaltrexone than placebo were abdominal pain and flatulence. 41 No patient had a significant change in baseline pain score or experienced opioid withdrawal. 41 In this population of patients with advanced illness, the response to methylnaltrexone may have been lower due to the contribution of other nonopioid-related mechanisms to constipation, including reduced fiber intake or immobility. 41 Furthermore, the rate of AEs in the palliative care population was fairly high, and determining the relationship of these AEs to the administered study agent or to the advanced illness was challenging. 41 Nevertheless, this randomized, placebocontrolled study showed that methylnaltrexone was effective for rapidly inducing laxation in this palliative care population and was well tolerated. 41 In the other trial, 134 patients with advanced illness receiving palliative care were given subcutaneous methylnaltrexone (0.15 mg/kg) or placebo every other day for 2 weeks, after which they could opt to continue in a 3-month, open-label extension trial to receive drug as needed up to every 24 hours. 44 The coprimary end points were rescue-free laxation within 4 hours after the first dose and within 4 hours after 2 or more of the first 4 doses. 44 Rescue-free laxation within 4 hours after the first dose was achieved by 48% and 15% of patients who received methylnaltrexone and placebo, respectively, and rescue-free laxation within 4 hours after 2 or more of the first 4doseswasachievedby52% and 8% of patients in these respective groups (P <.001 vs placebo for both comparisons). 44 The median time to rescue-free laxation was 6.3 hours with methylnaltrexone and >48 hours with placebo (P <.001 vs placebo). 44 The most common AEs that occurred more often with methylnaltrexone than placebo were abdominal pain and flatulence. 44 Minimal changes from baseline pain score occurred over time, and no patient experienced withdrawal. 44 The population included in this study was patients with advanced illness who were constipated at baseline despite laxative treatment (median, 2 classes of laxatives). 44 These patients could have responded to a change in their baseline laxative treatment and, thus, did not represent a population that had failed optimal laxative therapy. Furthermore, approximately half of the patients included in this study did not respond to the first dose of methylnaltrexone. 44 As in the former study, this population could have had other contributing factors to their constipation, which would have reduced the response to methylnaltrexone. 44 This randomized, placebo-controlled study showed that methylnaltrexone was associated with rapid laxation in these patients, with no effects on analgesia or evidence of opioid withdrawal. 44 In a trial of 460 patients with noncancer pain and OIC, subcutaneous methylnaltrexone 12 mg or placebo was given
8 Streicher and Bilsky 665 once daily or every other day for 4 weeks. 45 The coprimary end points were rescue-free laxation within 4 hours after the first dose and active injections per patient resulting in rescue-free laxation within 4 hours. 45 Rescue-free laxation within 4 hours after the first dose was achieved by 33%, 35%, and 10% of patients who received methylnaltrexone every day, methylnaltrexone every other day, and placebo, respectively, and the percentage of active injections resulting in rescue-free laxation within 4 hours was 29%, 30%, and9% of patients in these respective groups (P <.001 vs placebo for all comparisons). 45 The median time to laxation was significantly shorter with both schedules of methylnaltrexone versus placebo (P <.001). 45 The most common AEs that occurred more often with methylnaltrexone than placebo were abdominal pain, diarrhea, nausea, and hyperhidrosis. 45 No clinically significant change from baseline in pain intensity or clinically meaningful evidence of withdrawal was found. 45 Evaluation of the benefits of subcutaneous methylnaltrexone on QOL with the PAC-QOL at baseline and end of study showed a mean improvement of 33% with daily methylnaltrexone, 27% with every-other-day methylnaltrexone, and 18% with placebo (P <.001, daily vs placebo; P ¼ 0.014, every other day vs placebo). 45 The doubleblind treatment period in this study was only 4 weeks, which is relatively limited considering the long-term management needed for OIC in patients with chronic, noncancer pain. 45 In addition, no upward titration of opioid analgesics was anticipated in this trial; in a clinical setting, patients could require an increase in their opioid dose to manage worsening pain. 45 Results of this randomized, placebo-controlled study indicate that methylnaltrexone was effective and well tolerated for addressing OIC in patients with chronic noncancer pain. 45 The efficacy and safety of oral methylnaltrexone were demonstrated in a randomized, double-blind, placebocontrolled study of 401 patients with chronic noncancer pain and OIC. Patients received oral methylnaltrexone 450 mg (n ¼ 200) or placebo (n ¼ 201) once daily for 4 weeks, after which treatment was given on an as-needed basis (no more than once daily) for an additional 8 weeks. Treatment response was defined as 3 SBMs per week, with an increase of at least 1 SBM per week over baseline for 3 of the first 4 weeks. The response rate was 52% with oral methylnaltrexone and 38% with placebo. Abdominal pain and diarrhea were the most common AEs with oral methylnaltrexone. 19 In this study and the above-described studies in patients with advanced illness or chronic noncancer pain and OIC, 41,44,45 methylnaltrexone was not compared directly with other first-line treatments such as laxatives. Naloxegol Naloxegol (Movantik ; AstraZeneca Pharmaceuticals LP, Wilmington, Delaware) is an orally administered PAMORA that was approved in September 2014 for the treatment of OIC in patients with chronic noncancer pain (Table 3). Naloxegol is administered orally as a once-daily tablet at a recommended dose of 25 mg. 31 In patients with moderate, severe, or end-stage renal disease (CLcr <60 ml/min), a dose of 12.5 mg once daily is recommended as the starting dose; the dose can be increased to 25 mg once daily if well tolerated. 31 The efficacy and safety of naloxegol were evaluated in 2 identically designed, pivotal, randomized, double-blind, placebo-controlled, phase 3 clinical trials in outpatients with noncancer pain and OIC. 40 Patients were given naloxegol 25 mg, naloxegol 12.5 mg, or placebo once daily for 12 weeks. 40 The primary end point was response rate, with response defined as 3 SBMs per week and an increase of 1 SBM over baseline for 9 of the 12 weeks of treatment and 3 of the last 4 weeks of treatment. 40 In the first study (N ¼ 652), the response rates were 44.4%, 40.8%, and 29.4% with naloxegol 25 mg, naloxegol 12.5 mg, and placebo, respectively (P <.001 for naloxegol 25 mg vs placebo; P ¼.02 for naloxegol 12.5 mg vs placebo). 40 In the second study (N ¼ 700), corresponding response rates were 39.7%, 34.9%, and 29.3% (P ¼.02 for naloxegol 25 mg vs placebo; P ¼.20 for naloxegol 12.5 mg vs placebo). 40 In the first study, the median time to the first SBM was 6 hours with naloxegol 25 mg and 36 hours with placebo; in the second study, values were 12 and 37 hours, respectively (P <.001 for both comparisons). 40 The AEs that occurred more commonly with naloxegol than placebo were abdominal pain, diarrhea, nausea, and flatulence. 40 No clinically significant changes from baseline in pain score were reported, and symptoms of potential withdrawal syndrome were infrequent (1 patient in each treatment group in the first study [0.5% incidence per group]; 1 patient in the naloxegol 12.5-mg group who ran out of opioid medication in the second study [0.4% incidence]; and 4 patients in the naloxegol 25-mg group in the second study [1.7% incidence]). 40 The long-term safety and tolerability of naloxegol 25 mg were evaluated in a 52-week, randomized, open-label, parallel-group, phase 3 clinical trial (N ¼ 844) in outpatients with noncancer pain and OIC, and results indicated that treatment was generally safe and well tolerated. 46 The pivotal phase 3 studies also examined the benefits of naloxegol treatment on a number of symptoms commonly described by patients. 40 Compared with placebo, the first study reported that 25 mg naloxegol produced significant improvements in severity of straining per week (5-point scale; P <.01), stool consistency per week (Bristol stool scale; P <.05), percentage of days per week with a complete SBM, and number of SBMs per week (both end points, P <.001); 12.5 mg naloxegol significantly improved the number of SBMs per week (P <.05). 40 In the second study, both naloxegol doses produced significant improvements in all 4 measures versus placebo: severity of straining per week, stool consistency per week, percentage of days per week with a complete SBM (all end points: 12.5 mg, P <.01; 25 mg, P <.001), and number of SBMs per week (12.5 mg, P <.05;25mg,P <.001). 40 Although the improvements from baseline in SBMs were comparable for both studies, response rates with naloxegol compared with placebo were numerically lower in the second study than in the first, and a significant difference in the response rate between naloxegol 12.5 mg, and placebo was only observed in the first study. 40 The reasons for the differences in response
9 666 Journal of Pharmacy Practice 31(6) between these 2 identically designed studies were not clear. 40 Furthermore, similar to the studies of methylnaltrexone, naloxegol was not directly compared with first-line treatments, including laxatives, in these studies. Nevertheless, this study showed that naloxegol was associated with a strong treatment response, with no reduction in opioid-induced analgesia. 40 Naldemedine Naldemedine (Symproic ; Shionogi Inc., Florham Park, New Jersey) is an orally administered PAMORA that was approved in March 2017 for the treatment of OIC in patients with chronic noncancer pain (Table 3). 32 Naldemedine is administered orally as a once-daily tablet at a recommended dose of 0.2 mg, with or without food. 32 No dose adjustment is required for patients with mild to moderate hepatic impairment, while the use of naldemedine is not recommended in patients with severe hepatic impairment. 32 The efficacy and safety of naldemedine were evaluated in 2 identically designed, pivotal, double-blind, randomized, placebo-controlled, phase 3 studies in adult outpatients with noncancer pain and OIC. 43 Patients were randomized to treatment with oral naldemedine 0.2 mg or placebo once daily for 12 weeks. 43 Similar to the 12-week studies of naloxegol, the primary end point was response rate, with response defined as 3 SBMs per week and an increase of 1 SBM over baseline for 9 of the 12 weeks of treatment and 3 of the last 4 weeks of treatment. 43 In the first study (N ¼ 547), the response rate was 47.6% with naldemedine 0.2 mg and 34.6% with placebo (P ¼.002); in the second study (N ¼ 553), the response rates were 33.6% and 18.9%, respectively (P <.0001). 43 The most common AEs reported with naldemedine in these 2 studies were abdominal pain and diarrhea. 43 The incidence of symptoms of potential opioid withdrawal were uncommon across these 2 studies (1% each in the naldemedine 0.2 mg and placebo groups). 32 Long-term tolerability was comparable in a double-blind, placebo-controlled, 52-week, phase 3 study (N ¼ 1240) in patients with chronic noncancer pain and OIC. 32 Similar to naloxegol, the impact of naldemedine on symptoms of OIC was evaluated in the pivotal phase 3 studies. In the first study, the mean change from baseline in the number of SBMs per week at the last 2 weeks of the 12-week treatment period was 3.4 with naldemedine 0.2 mg and 2.1 with placebo; in the second study, the mean change from baseline in the number of SBMs per week at the last 2 weeks of the 12-week treatment period was 3.6 and 2.2, respectively (P <.0001 for naldemedine vs placebo for both studies). 43 The mean change from baseline in the number of complete SBMs per week at the last 2 weeks of the 12-week treatment period was 2.6 with naldemedine 0.2 mg and 1.6 with placebo in the first study and 2.8 and 1.6, respectively, in the second study (P <.0001 for naldemedine vs placebo for both studies). 43 The mean change from baseline to the last 2 weeks of treatment in the number of SBMs without straining per week was 1.5 with naldemedine and 0.7 with placebo in the first study (P ¼.0003 for naldemedine vs placebo) and 1.9 and 1.1, respectively, in the second study (P ¼.0011 for naldemedine vs placebo). 43 Some of the response rates quoted in the above studies of PAMORAs (*40%) approximate the patient survey results with laxatives discussed previously. Thus, studies need to be performed that directly compare the performance of laxatives versus PAMORAs, to determine whether the use of PAMORAs is justified or whether PAMORAs should replace laxatives as first-line treatments. An additional consideration is cost: laxatives in general are relatively inexpensive, while the cost of PAMORAs is more variable. The cost of methylnaltrexone is similar whether dosed orally (recommended dose, 450 mg [3 tablets] per day [wholesale acquisition cost *US$53 for mg daily dose]) or subcutaneously (recommended dose, 8 mg every other day for patients 38-<62 kg [*US$107 for 1 dose] and 12 mg every other day for patients kg [*US$112 for 1 dose]), while the cost of naloxegol (recommended dose, mg [1 tablet] per day) is *US$11 for to 25-mg daily dose (cost information for naldemedine was not yet available at the time this article was written). 47 These factors require study, and the relatively modest performance of PAMORAs as discussed above suggests that new and more efficacious PAMORAs need to be developed. PAMORAs in Development Oral axelopran (formerly TD-1211), which has been shown in preclinical models to reverse loperamide inhibition in the GI tract and not induce withdrawal in morphine-dependent animals or impair analgesia, 48 is currently in phase 2 clinical development. Other agents in earlier phases of investigation are 6b-naltrexol and 6b-naltrexamine. 6b-naltrexol, the main metabolite of naltrexone, inhibits receptor activation but does not suppress basal signaling, making it a neutral opioid receptor antagonist. When administered intravenously, 6b-naltrexol (AIKO Biotechnology, Portland, Maine) antagonized morphine-mediated GI slowing of orocecal transit time in healthy volunteers 49 and antagonized hydrocodone-induced inhibition of GI transit in mice. 50 Another neutral opioid receptor antagonist, a 6b-Nheterocyclic substituted naltrexamine derivative, is a substrate of PGP and increased intestinal motility in mice following subcutaneous administration. 37 Both molecules appear to be neutral antagonists under a variety of testing conditions (unpublished data), which may enable the use of higher doses to further increase the reversal of opioid constipating effects without precipitating significant withdrawal in an opioiddependent gut. This hypothesis must be verified clinically. General considerations or strategies for future drug discovery involve optimizing the receptor selectivity and affinity of candidate compounds as well as the route of administration. Given the findings that m-opioid receptors demonstrate functional selectivity/biased agonism, 51 functionally selective antagonism with PAMORAs or functionally selective agonists that are biased against b-arrestin-2 52 may be considered as a potential means to target OIC.
10 Streicher and Bilsky 667 Other Potential Uses of PAMORAs The potential therapeutic activity of PAMORAs in the GI tract extends beyond the treatment of OIC. Alvimopan is a substrate for the PGP efflux transport protein, 29 which limits its entry into the CNS. It also has a higher affinity for the m-opioid receptor than methylnaltrexone. 53 Alvimopan is an FDA-approved oral PAMORA indicated to accelerate the time to upper and lower GI recovery following surgeries that include partial bowel resection with primary anastomosis. 29 Its use is restricted to the hospital setting, and it is intended only for short-term use (12 mg given 30 minutes to 5 hours before surgery and 12 mg twice daily starting the day after surgery and continued until discharge, for a maximum of 7 days). 29 In a 12-month study of alvimopan for OIC in patients with chronic noncancer pain, a higher incidence of myocardial infarction (MI) was reported with alvimopan than with placebo, which led to the inclusion of a black box warning related to the increased risk of MI with long-term exposure in the prescribing information for alvimopan. 29 This potential cardiovascular risk has not been reported with other FDA-approved PAMORAs. 30,31 It is not approved by the FDA for OIC. The primary end point in all of the alvimopan registration studies was time to achieve resolution of postoperative ileus, which was a composite of upper and lowergirecovery. 29 Additional nonanalgesic effects of opioids may occur via opioid receptors in other peripheral tissues. Opioid receptor subtypes have been detected in myocardial cells and individual nerve fibers of the heart, 5 and therefore binding of opioid agonists to cardiovascular opioid receptors may cause significant effects. In a comparison of patients using opioids for noncancer pain with those not using opioids, users had a 1.3-fold higher risk of MI versus nonusers, and any cumulative use of 11 to 50 opioid prescriptions conferred a 1.4-fold increased risk of MI versus nonusers. 54 Data from another study indicated that patients using opioids chronically for noncancer pain had a 2.7-fold higher rate of MI and a 2.4- fold higher rate of a combination of MI or coronary revascularization (whichever occurred first) than an untreated, general population of matched individuals. 55 A1.8-foldelevated risk of cardiovascular events, including MI, stroke, heart failure, revascularization, and out-of-hospital cardiac death, was identified in patients taking opioids versus nonsteroidal anti-inflammatory drugs for osteoarthritis or rheumatoid arthritis. 56 A study of opioid-dependent men found a dose-response relationship between lifetime opioid exposure and central cardiovascular age and arterial stiffness, with an acceleration in vascular age of >10% versus controls. 57 Stimulation of m-opioid receptors may depress sinoatrial and atrioventricular node function, leading to bradycardia and reduced heart rate variability. 58 Methadone prolongs the QT interval and may induce torsade de pointes in individuals with cardiovascular risk factors. 58 While these retrospective studies have limitations, the collective data should still inform evolving hypotheses. Future preclinical and clinical trials should explore the potential cardioprotective effects of PAMORAs, 59 which are certainly of interest, given the widespread use of chronic opioid agonists. Activity of opioid receptors may also have implications in patients with cancer, as recent data suggest that PAMORAs could decrease the potential effects of opioid drugs on cancer cell proliferation and metastasis. In human lung cancer cells in vitro, blockade of peripheral m-opioid receptors dose dependently inhibited cell proliferation and migration stimulated by epidermal growth factor. 60 In addition, stimulation of human lung cancer cells by morphine, fentanyl, or a synthetic opioid peptide induced proliferation, migration, and epithelial mesenchymal transition. 60 These findings suggest a role for the m-opioid receptor in these processes. Retrospective evaluations of opioid use and survival in patients with advanced cancer found that a higher opioid requirement was associated with significantly shorter overall survival in patients with stage IIIB or IV non small-cell lung cancer 61 and significantly shorter progression-free and overall survival in patients with stage IV prostate cancer. 62 These associations were independent of other prognostic factors. 61,62 A recent retrospective post hoc analysis of pooled data from 2 placebocontrolled clinical trials of subcutaneous methylnaltrexone found that treatment with methylnaltrexone was associated with significantly longer median overall survival compared to placebo in patients with advanced cancer and OIC. 63 Although data from prospective studies are not yet available, further attention to the relationship of opioid exposure with survival and the potential of PAMORAs to slow disease progression is warranted in the future. Summary and Conclusions Optimal use of opioids for analgesia is often compromised by OIC, which is mediated primarily by m-opioid receptors in the GI tract. OIC exacts a considerable burden on patients in terms of impaired QOL and activities of daily living. Early attempts to treat OIC with naloxone led to the development of PAMORAs. Three currently approved PAMORAs have shown efficacy in treating OIC in patients with chronic pain, and new PAMORAs currently in development are likely to provide more treatment options in this patient population. Consensus guidelines from the American Academy of Pain Medicine recommend the use of prescription therapy for OIC, including PAMORAs, for patients with a BFI score of 30 (out of a possible score of 100) and an inadequate response to first-line treatments (eg, dietary changes, overthe-counter laxatives). 15 Future research should address whether PAMORAs are more efficacious than laxatives and other first-line treatments, and whether PAMORAs have actions in addition to preventing exogenous opioids from binding to m-opioid receptors, such as blocking endogenous tone or acting as inverse agonists. Future research should also address whether PAMORAs can reduce the increased cardiac risk or potential tumorigenic effects of opioids.
11 668 Journal of Pharmacy Practice 31(6) Declaration of Conflicting Interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.B. is a cofounder of Biousian Biosystems Inc. and Aiko Biotechnology. E.B. holds founding shares of Biousian Biosystems Inc. and Aiko Biotechnology. E.B. holds several issued patents around opioid agonists and antagonists. J.M.S. is a cofounder and equity partner of Teleport Pharmaceuticals, LLC. J.M.S. has received contract funding from Stealth Peptides and Depomed, Inc. as a fee for service. E.B. has conducted contracted work through the University of New England for several biotechnology and pharmaceutical companies. Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sponsored by AstraZeneca Pharmaceuticals LP. Editorial support was provided by Stephanie Leinbach, PhD, Diane DeHaven-Hudkins, PhD, and Erica S. Wehner, RPh, Complete Healthcare Communications, LLC (Chadds Ford, PA) and was funded by AstraZeneca Pharmaceuticals LP. References 1. Cheung CW, Qiu Q, Choi SW, et al. Chronic opioid therapy for chronic non-cancer pain: a review and comparison of treatment guidelines. Pain Physician. 2014;17(5): Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012;124(3): Spetea M. Opioid receptors and their ligands in the musculoskeletal system and relevance for pain control. Curr Pharm Des. 2013;19(42): Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl. 2014;2(1): Sobanski P, Krajnik M, Shaqura M, et al. The presence of mu-, delta-, and kappa-opioid receptors in human heart tissue. Heart Vessels. 2014;29(6): Abramowitz L, Beziaud N, Labreze L, et al. Prevalence and impact of constipation and bowel dysfunction induced by strong opioids: a cross-sectional survey of 520 patients with cancer pain: DYONISOS study. J Med Econ. 2013;16(12): Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic noncancer pain: systematic review of efficacy and safety. Pain. 2004; 112(3): Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182(5A suppl):11s-18s. 9. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27(12): Ketwaroo GA, Cheng V, Lembo A. Opioid-induced bowel dysfunction. Curr Gastroenterol Rep. 2013;15(9): Manara L, Bianchi G, Ferretti P, et al. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J Pharmacol Exp Ther. 1986;237(3): Manara L, Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol. 1985;25: Dorn S, Lembo A, Cremonini F. Opioid-induced bowel dysfunction: epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol Suppl. 2014;2(1): Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106(5): Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16(12): Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26;quiz S Frank L, Kleinman L, Farup C, et al. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34(9): Rentz AM, Yu R, Muller-Lissner S, et al. Validation of the Bowel Function Index to detect clinically meaningful changes in opioidinduced constipation. J Med Econ. 2009;12(4): Camilleri M, Rothman M, Ho KF, et al. Validation of a bowel function diary for assessing opioid-induced constipation. Am J Gastroenterol. 2011;106(3): Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014;26(10): Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2): Nelson AD, Camilleri M. Opioid-induced constipation: advances and clinical guidance. Ther Adv Chronic Dis. 2016;7(2): Epstein RS, Cimen A, Benenson H, et al. Patient preferences for change in symptoms associated with opioid-induced constipation. Adv Ther. 2014;31(12): Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 2009; 10(1): Coyne KS, LoCasale RJ, Datto CJ, et al. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res. 2014;6: Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev. 2011;19(1):CD Rosti G, Gatti A, Costantini A, et al. Opioid-related bowel dysfunction: prevalence and identification of predictive factors in a large sample of Italian patients on chronic treatment. Eur Rev Med Pharmacol Sci. 2010;14(12): Pergolizzi JV Jr, Raffa RB, Pappagallo M, et al. Peripherally acting mu-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:
Opioid-Induced Constipation
 Objectives Opioid-Induced Constipation Brianna Jansma, PharmD Alex Smith, PharmD Megan Robinson, PharmD Summarize epidemiology of opioid-induced constipation (OIC) Understand opiates effects on the gastrointestinal
Objectives Opioid-Induced Constipation Brianna Jansma, PharmD Alex Smith, PharmD Megan Robinson, PharmD Summarize epidemiology of opioid-induced constipation (OIC) Understand opiates effects on the gastrointestinal
OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES
 OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES Introduction Introduction Mean faecal weight 128 g / cap / day Mean range 51-796 g Absolute range 15-1505 g Main factors affecting mass are caloric intake,
OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES Introduction Introduction Mean faecal weight 128 g / cap / day Mean range 51-796 g Absolute range 15-1505 g Main factors affecting mass are caloric intake,
CENTENE PHARMACY AND THERAPEUTICS DRUG REVIEW 3Q17 July August
 BRAND NAME Symproic GENERIC NAME Naldemedine MANUFACTURER Shionogi Inc. DATE OF APPROVAL March 23, 2017 PRODUCT LAUNCH DATE Anticipated to launch mid-summer 2017 REVIEW TYPE Review type 1 (RT1): New Drug
BRAND NAME Symproic GENERIC NAME Naldemedine MANUFACTURER Shionogi Inc. DATE OF APPROVAL March 23, 2017 PRODUCT LAUNCH DATE Anticipated to launch mid-summer 2017 REVIEW TYPE Review type 1 (RT1): New Drug
Opioid-related bowel dysfunction: prevalence and identification of predictive factors in a large sample of Italian patients on chronic treatment
 European Review for Medical and Pharmacological Sciences Opioid-related bowel dysfunction: prevalence and identification of predictive factors in a large sample of Italian patients on chronic treatment
European Review for Medical and Pharmacological Sciences Opioid-related bowel dysfunction: prevalence and identification of predictive factors in a large sample of Italian patients on chronic treatment
Movantik (naloxegol), Relistor (methylnaltrexone bromide), Symproic (naldemedine)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: June 22, 2017 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: June 22, 2017 Opioid Antagonist
SYMPROIC (naldemedine tosylate) oral capsule
 SYMPROIC (naldemedine tosylate) oral capsule Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
SYMPROIC (naldemedine tosylate) oral capsule Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
ALVIMOPAN 0.0 OVERVIEW
 ALVIMOPAN 0.0 OVERVIEW A. Alvimopan is a peripherally restricted mu-opioid receptor antagonist. B. DOSING INFORMATION : For the treatment of opioid bowel dysfunction, oral alvimopan doses between 0.5 milligrams
ALVIMOPAN 0.0 OVERVIEW A. Alvimopan is a peripherally restricted mu-opioid receptor antagonist. B. DOSING INFORMATION : For the treatment of opioid bowel dysfunction, oral alvimopan doses between 0.5 milligrams
Movantik (naloxegol), Relistor (methylnaltrexone bromide), Symproic (naldemedine)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 7 Last Review Date: November 30, 2018 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 7 Last Review Date: November 30, 2018 Opioid Antagonist
Horizon Scanning Technology Summary. Methylnaltrexone for opioid induced constipation in advanced illness and palliative care
 Horizon Scanning Technology Summary National Horizon Scanning Centre Methylnaltrexone for opioid induced constipation in advanced illness and palliative care April 2007 This technology summary is based
Horizon Scanning Technology Summary National Horizon Scanning Centre Methylnaltrexone for opioid induced constipation in advanced illness and palliative care April 2007 This technology summary is based
daily; available as 10- mg g PO
 Overview of the PRN: The Pain and Palliative Care PRN of ACCP is an organization of pharmacy practitioners, clinical scientists, pharmacy educators, and others. Its mission is to advance pain and palliative
Overview of the PRN: The Pain and Palliative Care PRN of ACCP is an organization of pharmacy practitioners, clinical scientists, pharmacy educators, and others. Its mission is to advance pain and palliative
Movantik (naloxegol), Relistor (methylnaltrexone bromide)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: December 2, 2016 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: December 2, 2016 Opioid Antagonist
Improving the Diagnosis and Management of Opioid-induced Constipation to Optimize Outcomes of Patients with Chronic Pain
 Improving the Diagnosis and Management of Opioid-induced Constipation to Optimize Outcomes of Patients with Chronic Pain Sponsored by Integrity Continuing Education, Inc. Supported by an educational grant
Improving the Diagnosis and Management of Opioid-induced Constipation to Optimize Outcomes of Patients with Chronic Pain Sponsored by Integrity Continuing Education, Inc. Supported by an educational grant
OIC, opioid-induced constipation.
 Disclosures Charles E. Argoff, MD Speakers Bureau for Allergan, Inc., AstraZeneca plc, Depomed, Inc., Iroko Pharmaceuticals LLC, Janssen Pharmaceuticals, Inc., Millenium Laboratories, and Xenoport Inc.
Disclosures Charles E. Argoff, MD Speakers Bureau for Allergan, Inc., AstraZeneca plc, Depomed, Inc., Iroko Pharmaceuticals LLC, Janssen Pharmaceuticals, Inc., Millenium Laboratories, and Xenoport Inc.
754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011
 754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011 Brief Report Characterization of Abdominal Pain During Methylnaltrexone Treatment of Opioid-Induced Constipation in Advanced Illness:
754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011 Brief Report Characterization of Abdominal Pain During Methylnaltrexone Treatment of Opioid-Induced Constipation in Advanced Illness:
Opioid Use in Palliative Care
 Opioid Use in Palliative Care Relief of pain is one of the core components of palliative care 1,2 Up to 69% of patients with advanced cancer experience pain 3 ~65% of patients dying from nonmalignant disease
Opioid Use in Palliative Care Relief of pain is one of the core components of palliative care 1,2 Up to 69% of patients with advanced cancer experience pain 3 ~65% of patients dying from nonmalignant disease
Summary of the risk management plan (RMP) for Moventig (naloxegol)
 EMA/611606/2014 Summary of the risk management plan (RMP) for Moventig (naloxegol) This is a summary of the risk management plan (RMP) for Moventig, which details the measures to be taken in order to ensure
EMA/611606/2014 Summary of the risk management plan (RMP) for Moventig (naloxegol) This is a summary of the risk management plan (RMP) for Moventig, which details the measures to be taken in order to ensure
The Road to Opioid-Induced Constipation: Pathophysiology and Impact Kenneth C. Jackson, II, PharmD, CPE
 Disclaimer This slide deck in its original and unaltered format is for educational purposes and is current as of March 2015. The content and views presented in this educational activity are those of the
Disclaimer This slide deck in its original and unaltered format is for educational purposes and is current as of March 2015. The content and views presented in this educational activity are those of the
Pharmacy Benefit Determination Policy
 Policy Subject: Opioid Induced Constipation Policy Number: SHS PBD11 Category: GI Agents Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual HMO/POS
Policy Subject: Opioid Induced Constipation Policy Number: SHS PBD11 Category: GI Agents Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual HMO/POS
Horizon Scanning Technology Briefing. Alvimopan (Entrareg ) for opioid-induced bowel disfunction. National Horizon Scanning Centre.
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Alvimopan (Entrareg ) for opioid-induced bowel disfunction August 2006 This technology briefing is based on information available at
Horizon Scanning Technology Briefing National Horizon Scanning Centre Alvimopan (Entrareg ) for opioid-induced bowel disfunction August 2006 This technology briefing is based on information available at
Impact of a Pharmacist Implemented Protocol on Overall Use of Alvimopan (Entereg ) and Length of Stay in Laparoscopic Colorectal Surgeries
 Journal of Pharmacy and Pharmacology 4 (2016) 521-525 doi: 10.17265/2328-2150/2016.10.001 D DAVID PUBLISHING Impact of a Pharmacist Implemented Protocol on Overall Use of Alvimopan (Entereg ) and Length
Journal of Pharmacy and Pharmacology 4 (2016) 521-525 doi: 10.17265/2328-2150/2016.10.001 D DAVID PUBLISHING Impact of a Pharmacist Implemented Protocol on Overall Use of Alvimopan (Entereg ) and Length
Oral Methylnaltrexone for the. Constipation in Patients with Chronic Non-cancer Pain
 Oral Methylnaltrexone for the Treatment of Opioid-induced Constipation in Patients with Chronic Non-cancer Pain Richard L. Rauck, 1 John F. Peppin, 2 Robert J.Israel, 3 Jennifer Carpenito, 3 Jeffrey Cohn,
Oral Methylnaltrexone for the Treatment of Opioid-induced Constipation in Patients with Chronic Non-cancer Pain Richard L. Rauck, 1 John F. Peppin, 2 Robert J.Israel, 3 Jennifer Carpenito, 3 Jeffrey Cohn,
MANAGEMENT OF CHRONIC CONSTIPATION BEYOND LAXATIVES
 Enrique Rey Professor of Medicine Head. Department of Digestive Diseases Hospital Clínico San Carlos Complutense University Madrid, Spain MANAGEMENT OF CHRONIC CONSTIPATION BEYOND LAXATIVES CONSTIPATION:
Enrique Rey Professor of Medicine Head. Department of Digestive Diseases Hospital Clínico San Carlos Complutense University Madrid, Spain MANAGEMENT OF CHRONIC CONSTIPATION BEYOND LAXATIVES CONSTIPATION:
FOOT OFF THE BRAKES. Kerri Novak MD MSc FRCPC. Chronic Constipation: Taking the Foot off the Brakes Dr. Kerri Novak
 CHRONIC CONSTIPATION: TAKING THE FOOT OFF THE BRAKES Kerri Novak MD MSc FRCPC www.seacourses.com 1 OUTLINE Epidemiology i Quality of life Approach Therapies www.seacourses.com 2 DEFINING CHRONIC CONSTIPATION
CHRONIC CONSTIPATION: TAKING THE FOOT OFF THE BRAKES Kerri Novak MD MSc FRCPC www.seacourses.com 1 OUTLINE Epidemiology i Quality of life Approach Therapies www.seacourses.com 2 DEFINING CHRONIC CONSTIPATION
NDA (APPROVED 2008) snda /S-010 (COMPLETE RESPONSE LETTER July 27, 2012)
 Salix Pharmaceuticals, Inc. Briefing Document Relistor (Methylnaltrexone Bromide) Subcutaneous Injection & Oral Tablet RELISTOR (METHYLNALTREXONE BROMIDE) FOR THE TREATMENT OF OPIOID INDUCED CONSTIPATION
Salix Pharmaceuticals, Inc. Briefing Document Relistor (Methylnaltrexone Bromide) Subcutaneous Injection & Oral Tablet RELISTOR (METHYLNALTREXONE BROMIDE) FOR THE TREATMENT OF OPIOID INDUCED CONSTIPATION
THE OPIUM POPPY OPIOID PHARMACOLOGY 2/18/16. PCTH 300/305 Andrew Horne, PhD MEDC 309. Papaver somniferum. Poppy Seeds Opiates
 OPIOID PHARMACOLOGY PCTH 300/305 Andrew Horne, PhD andrew.horne@ubc.ca MEDC 309 THE OPIUM POPPY Papaver somniferum Sleep-bringing poppy Poppy Seeds Opiates Opium Poppy Straw 1 OPIATES VS. OPIOIDS Opiates:
OPIOID PHARMACOLOGY PCTH 300/305 Andrew Horne, PhD andrew.horne@ubc.ca MEDC 309 THE OPIUM POPPY Papaver somniferum Sleep-bringing poppy Poppy Seeds Opiates Opium Poppy Straw 1 OPIATES VS. OPIOIDS Opiates:
Applying Quality to Postsurgical Opioid-Induced Constipation
 Applying Quality to Postsurgical Opioid-Induced Constipation Applying Quality to Postsurgical Opioid-Induced Constipation Ernest J. Dole, PharmD, PhC, FASHP, BCPS Clinical Pharmacist University of New
Applying Quality to Postsurgical Opioid-Induced Constipation Applying Quality to Postsurgical Opioid-Induced Constipation Ernest J. Dole, PharmD, PhC, FASHP, BCPS Clinical Pharmacist University of New
IBS: overview and assessment of pain outcomes and implications for inclusion criteria
 IBS: overview and assessment of pain outcomes and implications for inclusion criteria William D. Chey, MD Professor of Medicine University of Michigan What is the Irritable Bowel Syndrome Symptom based
IBS: overview and assessment of pain outcomes and implications for inclusion criteria William D. Chey, MD Professor of Medicine University of Michigan What is the Irritable Bowel Syndrome Symptom based
Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345
 Naloxegol for treating opioid-induced constipation Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Naloxegol for treating opioid-induced constipation Technology appraisal guidance Published: 22 July 2015 nice.org.uk/guidance/ta345 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
The Journal of International Medical Research 2011; 39: [first published online as 39(1) 9]
![The Journal of International Medical Research 2011; 39: [first published online as 39(1) 9] The Journal of International Medical Research 2011; 39: [first published online as 39(1) 9]](/thumbs/74/71295117.jpg) The Journal of International Medical Research 2011; 39: 41 50 [first published online as 39(1) 9] The Bowel Function Index for Evaluating Constipation in Pain Patients: Definition of a Reference Range
The Journal of International Medical Research 2011; 39: 41 50 [first published online as 39(1) 9] The Bowel Function Index for Evaluating Constipation in Pain Patients: Definition of a Reference Range
Opioids and the Gastroenterologist: A Painful Issue
 Opioids and the Gastroenterologist: A Painful Issue Wendell K. Clarkston, MD Professor of Medicine GI Program Director UMKC School of Medicine Disclosures: No financial relationships to disclose 1 Outline
Opioids and the Gastroenterologist: A Painful Issue Wendell K. Clarkston, MD Professor of Medicine GI Program Director UMKC School of Medicine Disclosures: No financial relationships to disclose 1 Outline
David Leff, DO. April 13, Disclosure. I have the following financial relationships to disclosure:
 David Leff, DO AOMA 94 th Annual Convention April 13, 2016 Disclosure I have the following financial relationships to disclosure: Speaker s Bureau: Allergan Labs, Takeda Pharmaceutical, Valeant Pharmaceutical
David Leff, DO AOMA 94 th Annual Convention April 13, 2016 Disclosure I have the following financial relationships to disclosure: Speaker s Bureau: Allergan Labs, Takeda Pharmaceutical, Valeant Pharmaceutical
Naloxegol an investigational drug for the. Constipation
 Naloxegol an investigational drug for the treatment of Opioid-Induced Constipation October 2012 Cautionary Statement Regarding Forward-Looking Statements t t In order, among other things, to utilise the
Naloxegol an investigational drug for the treatment of Opioid-Induced Constipation October 2012 Cautionary Statement Regarding Forward-Looking Statements t t In order, among other things, to utilise the
UBS Global Healthcare Conference May 19, 2014
 UBS Global Healthcare Conference May 19, 2014 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
UBS Global Healthcare Conference May 19, 2014 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
TABLE 1. Undesirable Effects of Opioids Depression of ventilation Sedation Dysphoria Hypotension, bradycardia Increased skeletal muscle tone Suppressi
 SPECIAL ARTICLE DEVELOPMENT OF PERIPHERAL OPIOID ANTAGONISTS Development of Peripheral Opioid Antagonists: New Insights Into Opioid Effects JONATHAN MOSS, MD, PHD, AND CARL E. ROSOW, MD, PHD The recent
SPECIAL ARTICLE DEVELOPMENT OF PERIPHERAL OPIOID ANTAGONISTS Development of Peripheral Opioid Antagonists: New Insights Into Opioid Effects JONATHAN MOSS, MD, PHD, AND CARL E. ROSOW, MD, PHD The recent
Slide #43. Functional Disorders - An Update 11/8/ MA ACP Annual Scientific Meeting. Functional Disorders: An Update
 Functional Disorders: An Update Anthony Lembo, M.D. Associate Professor of Medicine Beth Israel Deaconess Medical Center Harvard Medical School Boston, MA Disclosure of Financial Relationships Anthony
Functional Disorders: An Update Anthony Lembo, M.D. Associate Professor of Medicine Beth Israel Deaconess Medical Center Harvard Medical School Boston, MA Disclosure of Financial Relationships Anthony
Postoperative Ileus. UCSF Postgraduate Course in General Surgery Maui, HI March 20, 2011
 Postoperative Ileus UCSF Postgraduate Course in General Surgery Maui, HI March 20, 2011 Hobart W. Harris, MD, MPH Introduction Pathophysiology Clinical Research Management Summary Postoperative Ileus:
Postoperative Ileus UCSF Postgraduate Course in General Surgery Maui, HI March 20, 2011 Hobart W. Harris, MD, MPH Introduction Pathophysiology Clinical Research Management Summary Postoperative Ileus:
Drugs in Development for Opioid-Induced Constipation
 7 Drugs in Development for Opioid-Induced Constipation Kelly S. Sprawls, Egilius L.H. Spierings and Dustin Tran MedVadis Research Corporation USA 1. Introduction Opioid-induced bowel dysfunction (OIBD)
7 Drugs in Development for Opioid-Induced Constipation Kelly S. Sprawls, Egilius L.H. Spierings and Dustin Tran MedVadis Research Corporation USA 1. Introduction Opioid-induced bowel dysfunction (OIBD)
Emerging Treatments for IBS-C and Clinical Trial Endpoints
 Emerging Treatments for IBS-C and Clinical Trial Endpoints Lin Chang, M.D. Oppenheimer Family Center for Neurobiology of Stress David Geffen School of Medicine at UCLA Learning Objectives Describe current
Emerging Treatments for IBS-C and Clinical Trial Endpoints Lin Chang, M.D. Oppenheimer Family Center for Neurobiology of Stress David Geffen School of Medicine at UCLA Learning Objectives Describe current
PAIN & ANALGESIA. often accompanied by clinical depression. fibromyalgia, chronic fatigue, etc. COX 1, COX 2, and COX 3 (a variant of COX 1)
 Pain - subjective experience associated with detection of tissue damage ( nociception ) acute - serves as a warning chronic - nociception gone bad often accompanied by clinical depression fibromyalgia,
Pain - subjective experience associated with detection of tissue damage ( nociception ) acute - serves as a warning chronic - nociception gone bad often accompanied by clinical depression fibromyalgia,
Multimodal Approach for Managing Postoperative Ileus: Role of Health- System Pharmacists (ACPE program H01P)
 1. In the normal gastrointestinal tract, what percent of nutrient absorption occurs in the jejunum? a. 20%. b. 40%. c. 70%. d. 90%. 2. According to Dr. Erstad, the four components of gastrointestinal control
1. In the normal gastrointestinal tract, what percent of nutrient absorption occurs in the jejunum? a. 20%. b. 40%. c. 70%. d. 90%. 2. According to Dr. Erstad, the four components of gastrointestinal control
REFERENCE CODE GDHC004POA PUBLICAT ION DATE SEPTEMBER 2013
 REFERENCE CODE GDHC004POA PUBLICAT ION DATE SEPTEMBER 2013 OPIOID-INDUCED CONSTIPATION - Executive Summary The table below presents the key metrics for opioid-induced constipation (OIC) in the six major
REFERENCE CODE GDHC004POA PUBLICAT ION DATE SEPTEMBER 2013 OPIOID-INDUCED CONSTIPATION - Executive Summary The table below presents the key metrics for opioid-induced constipation (OIC) in the six major
Analgesic Drugs PHL-358-PHARMACOLOGY AND THERAPEUTICS-I. Mr.D.Raju,M.pharm, Lecturer
 Analgesic Drugs PHL-358-PHARMACOLOGY AND THERAPEUTICS-I Mr.D.Raju,M.pharm, Lecturer Mechanisms of Pain and Nociception Nociception is the mechanism whereby noxious peripheral stimuli are transmitted to
Analgesic Drugs PHL-358-PHARMACOLOGY AND THERAPEUTICS-I Mr.D.Raju,M.pharm, Lecturer Mechanisms of Pain and Nociception Nociception is the mechanism whereby noxious peripheral stimuli are transmitted to
ACTIVITY DESCRIPTION FACULTY. Opioid Use in Palliative Care. Opioid Analgesic Use in Chronic, Non-cancer Pain
 ACTIVITY DESCRIPTION Target Audience This continuing pharmacy education activity is planned to meet the needs of pharmacists in a variety of practice settings, including large and small healthcare systems,
ACTIVITY DESCRIPTION Target Audience This continuing pharmacy education activity is planned to meet the needs of pharmacists in a variety of practice settings, including large and small healthcare systems,
OCTOBER 7-10 PHILADELPHIA, PENNSYLVANIA
 OMED 17 OCTOBER 7-10 PHILADELPHIA, PENNSYLVANIA 29.5 Category 1-A CME credits anticipated ACOFP / AOA s 122 nd Annual Osteopathic Medical Conference & Exposition Joint Session with ACOFP and Cleveland
OMED 17 OCTOBER 7-10 PHILADELPHIA, PENNSYLVANIA 29.5 Category 1-A CME credits anticipated ACOFP / AOA s 122 nd Annual Osteopathic Medical Conference & Exposition Joint Session with ACOFP and Cleveland
Naloxegol for Opioid-Induced Constipation in Patients with Noncancer Pain
 The new england journal of medicine original article Naloxegol for Opioid-Induced Constipation in Patients with Noncancer Pain William D. Chey, M.D., Lynn Webster, M.D., Mark Sostek, M.D., Jaakko Lappalainen,
The new england journal of medicine original article Naloxegol for Opioid-Induced Constipation in Patients with Noncancer Pain William D. Chey, M.D., Lynn Webster, M.D., Mark Sostek, M.D., Jaakko Lappalainen,
William Chey, MD University of Michigan Ann Arbor, MI
 Lin Chang, MD David Geffen School of Medicine at UCLA Los Angeles, CA William Chey, MD University of Michigan Ann Arbor, MI Mark Pimentel, MD Cedars-Sinai Medical Center Los Angeles, CA Accredited by Jointly
Lin Chang, MD David Geffen School of Medicine at UCLA Los Angeles, CA William Chey, MD University of Michigan Ann Arbor, MI Mark Pimentel, MD Cedars-Sinai Medical Center Los Angeles, CA Accredited by Jointly
Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary
 Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,2 Lotronex (alosetron) a Indication For women with severe diarrheapredominant irritable
Xifaxan, Lotronex and Viberzi Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,2 Lotronex (alosetron) a Indication For women with severe diarrheapredominant irritable
Antidiarrheals Antidiarrheal
 Antidiarrheals Major factors in diarrhea Increased motility of the GI tract. Decreased absorption of fluid. Antidiarrheal drugs include: Antimotility agents. Adsorbents. Drugs that modify fluid and electrolyte
Antidiarrheals Major factors in diarrhea Increased motility of the GI tract. Decreased absorption of fluid. Antidiarrheal drugs include: Antimotility agents. Adsorbents. Drugs that modify fluid and electrolyte
SUPPLEMENTARY INFORMATION Associated with
 Table1: Rome III and Rome IV diagnostic criteria for IBS, functional constipation and functional dyspepsia. Rome III diagnostic criteria 1,2 Rome IV diagnostic criteria 3,4 Diagnostic criteria for IBS
Table1: Rome III and Rome IV diagnostic criteria for IBS, functional constipation and functional dyspepsia. Rome III diagnostic criteria 1,2 Rome IV diagnostic criteria 3,4 Diagnostic criteria for IBS
Efficacy and Safety of Lubiprostone. Laura Wozniak February 23, 2010 K30 Monthly Journal Club
 Efficacy and Safety of Lubiprostone Laura Wozniak February 23, 2010 K30 Monthly Journal Club Objectives Brief overview of constipation Review of article Discussion Constipation in Children 3-5% of all
Efficacy and Safety of Lubiprostone Laura Wozniak February 23, 2010 K30 Monthly Journal Club Objectives Brief overview of constipation Review of article Discussion Constipation in Children 3-5% of all
OST. Pharmacology & Therapeutics. Leo O. Lanoie, MD, MPH, FCFP, CCSAM, ABAM, MRO
 OST Pharmacology & Therapeutics Leo O. Lanoie, MD, MPH, FCFP, CCSAM, ABAM, MRO Disclaimer In the past two years I have received no payment for services from any agency other than government or academic.
OST Pharmacology & Therapeutics Leo O. Lanoie, MD, MPH, FCFP, CCSAM, ABAM, MRO Disclaimer In the past two years I have received no payment for services from any agency other than government or academic.
Clinically proven to quickly relieve symptoms of common gastrointestinal disorders. TERRAGASTRO - Good health starts in the gut
 Clinically proven to quickly relieve symptoms of common gastrointestinal disorders GASTROINTESTINAL DISEASE Referred to as gastrointestinal diseases, they are common disorders which affect the esophagus,
Clinically proven to quickly relieve symptoms of common gastrointestinal disorders GASTROINTESTINAL DISEASE Referred to as gastrointestinal diseases, they are common disorders which affect the esophagus,
Opioid-Induced Constipation: Update on Prevention and Management EDUCATIONALPROGRAM
 EDUCATIONALPROGRAM Recognizing i the Growing Burden of OIC Bill H. McCarberg, MD Founder, Chronic Pain Management Program Kaiser Permanente San Diego Adjunct Assistant Clinical Professor University of
EDUCATIONALPROGRAM Recognizing i the Growing Burden of OIC Bill H. McCarberg, MD Founder, Chronic Pain Management Program Kaiser Permanente San Diego Adjunct Assistant Clinical Professor University of
Physiological processes in the GI tract:
 Gastrointestinal physiology for medical students General principal of gastrointestinal function Motility, nervous control and blood circulation Physiological processes in the GI tract: Motility Secretion
Gastrointestinal physiology for medical students General principal of gastrointestinal function Motility, nervous control and blood circulation Physiological processes in the GI tract: Motility Secretion
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
 June 5, 2018 Synergy Pharmaceuticals Highlights New Data at Digestive Disease Week (DDW) 2018 Linking Uroguanylin Deficiency to Chronic Idiopathic Constipation (CIC) and Irritable Bowel Syndrome with Constipation
June 5, 2018 Synergy Pharmaceuticals Highlights New Data at Digestive Disease Week (DDW) 2018 Linking Uroguanylin Deficiency to Chronic Idiopathic Constipation (CIC) and Irritable Bowel Syndrome with Constipation
7/13/16. Pharmaceuticals that alter GI motility. Financial disclosures. Common pharmaceuticals that alter GI Motility (an incomplete list) None
 Pharmaceuticals that alter GI motility Megan Taylor, ND AANP 2016 Motility Bootcamp Financial disclosures None Common pharmaceuticals that alter GI Motility (an incomplete list) Analgesics Opioids NSAIDs
Pharmaceuticals that alter GI motility Megan Taylor, ND AANP 2016 Motility Bootcamp Financial disclosures None Common pharmaceuticals that alter GI Motility (an incomplete list) Analgesics Opioids NSAIDs
Daniel Canafax, PharmD VP, Clinical Research Theravance, Inc.
 Demonstrates Improvement in Bowel Movement Frequency and Bristol Stool Scores in a Phase 2b Study of Patients with Opioid-Induced Constipation (OIC) Ross Vickery, PhD, 1 Yu-Ping Li, PhD, 1 Ullrich Schwertschlag,
Demonstrates Improvement in Bowel Movement Frequency and Bristol Stool Scores in a Phase 2b Study of Patients with Opioid-Induced Constipation (OIC) Ross Vickery, PhD, 1 Yu-Ping Li, PhD, 1 Ullrich Schwertschlag,
IRONWOOD AND FOREST ANNOUNCE POSITIVE LINACLOTIDE RESULTS FROM PHASE 3 TRIAL IN PATIENTS WITH IRRITABLE BOWEL SYNDROME WITH CONSTIPATION
 FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
Effects of baseline abdominal pain and bloating on response to lubiprostone in patients with irritable bowel syndrome with constipation
 Alimentary Pharmacology and Therapeutics Effects of baseline abdominal pain and bloating on response to lubiprostone in patients with irritable bowel syndrome with constipation L. Chang*, W. D. Chey, D.
Alimentary Pharmacology and Therapeutics Effects of baseline abdominal pain and bloating on response to lubiprostone in patients with irritable bowel syndrome with constipation L. Chang*, W. D. Chey, D.
National Horizon Scanning Centre. Methylnaltrexone (MOA-728) for postoperative ileus. April 2008
 (MOA-728) for postoperative ileus April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
(MOA-728) for postoperative ileus April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
Why does my stomach hurt? Exploring irritable bowel syndrome
 Why does my stomach hurt? Exploring irritable bowel syndrome By Flavio M. Habal, MD, PhD, FRCPC Case In this article: 1. What is IBS? A 45-year-old female is referred to your office with recurrent 2. How
Why does my stomach hurt? Exploring irritable bowel syndrome By Flavio M. Habal, MD, PhD, FRCPC Case In this article: 1. What is IBS? A 45-year-old female is referred to your office with recurrent 2. How
... SELECTED ABSTRACTS...
 ... SELECTED ABSTRACTS... The following abstracts, from medical journals containing literature on irritable bowel syndrome, were selected for their relevance to this supplement. A Technical Review for
... SELECTED ABSTRACTS... The following abstracts, from medical journals containing literature on irritable bowel syndrome, were selected for their relevance to this supplement. A Technical Review for
Primary Management of Irritable Bowel Syndrome
 Primary Management of Irritable Bowel Syndrome Jasmine Zia, MD Acting Instructor, Division of Gastroenterology Current Concepts in Drug Therapy CME Course April 23, 2015 Irritable Bowel Syndrome (IBS)
Primary Management of Irritable Bowel Syndrome Jasmine Zia, MD Acting Instructor, Division of Gastroenterology Current Concepts in Drug Therapy CME Course April 23, 2015 Irritable Bowel Syndrome (IBS)
PALLIATIVE TREATMENT BY DR. KHRONGKAMOL SIHABAN MEDICAL ONCOLOGIST
 PALLIATIVE TREATMENT BY DR. KHRONGKAMOL SIHABAN MEDICAL ONCOLOGIST TREATMENT IN ONCOLOGY Main treatment : surgery Neoadjuvant treatment : RT, CMT Adjuvant treatment : Tx micrometastatic disease -CMT,Targeted
PALLIATIVE TREATMENT BY DR. KHRONGKAMOL SIHABAN MEDICAL ONCOLOGIST TREATMENT IN ONCOLOGY Main treatment : surgery Neoadjuvant treatment : RT, CMT Adjuvant treatment : Tx micrometastatic disease -CMT,Targeted
Elderly Man With Chronic Constipation
 Elderly Man With Chronic Constipation Linda Nguyen, MD Director, Neurogastroenterology and Motility Clinical Assistant Professor Stanford University Overview Normal bowel function Defining Constipation:
Elderly Man With Chronic Constipation Linda Nguyen, MD Director, Neurogastroenterology and Motility Clinical Assistant Professor Stanford University Overview Normal bowel function Defining Constipation:
NALTREXONE DAVID CRABTREE, MD, MPH UNIVERSITY OF UTAH HEALTH, 2018
 NALTREXONE DAVID CRABTREE, MD, MPH TREATMENT OF OPIOID USE DISORDER (OUD) Majority of people who develop OUD are not receiving treatment Only a small fraction of patients are offered treatment with medications
NALTREXONE DAVID CRABTREE, MD, MPH TREATMENT OF OPIOID USE DISORDER (OUD) Majority of people who develop OUD are not receiving treatment Only a small fraction of patients are offered treatment with medications
Methadone Maintenance
 Methadone Maintenance A Practical Guide to Pharmacotherapy Methadone/Buprenorphine 101 Workshop, April 1, 2017 Ron Joe, MD, DABAM Objectives I. Pharmacology Of Methadone II. Practical Application of Pharmacology
Methadone Maintenance A Practical Guide to Pharmacotherapy Methadone/Buprenorphine 101 Workshop, April 1, 2017 Ron Joe, MD, DABAM Objectives I. Pharmacology Of Methadone II. Practical Application of Pharmacology
Validated questionnaire on diagnosis and symptom severity for functional constipation in the Chinese population
 Aliment Pharmacol Ther 2005; 22: 483 488. doi: 10.1111/j.1365-2036.2005.02621.x Validated questionnaire on diagnosis and symptom severity for functional constipation in the Chinese population A. O. O.
Aliment Pharmacol Ther 2005; 22: 483 488. doi: 10.1111/j.1365-2036.2005.02621.x Validated questionnaire on diagnosis and symptom severity for functional constipation in the Chinese population A. O. O.
Drug Evaluation. Use of lubiprostone in constipating disorders and its potential for opioid-induced bowel dysfunction
 Use of lubiprostone in constipating disorders and its potential for opioid-induced bowel dysfunction Lubiprostone is a novel medication, approved by the US FDA for the treatment of chronic idiopathic constipation
Use of lubiprostone in constipating disorders and its potential for opioid-induced bowel dysfunction Lubiprostone is a novel medication, approved by the US FDA for the treatment of chronic idiopathic constipation
Methylnaltrexone for the management of unwanted peripheral opioid effects
 DRUG EVALUATION Methylnaltrexone for the management of unwanted peripheral opioid effects Peter Holzer Medical University of Graz, Research Unit of Translational Neurogastroenterology, Institute of Experimental
DRUG EVALUATION Methylnaltrexone for the management of unwanted peripheral opioid effects Peter Holzer Medical University of Graz, Research Unit of Translational Neurogastroenterology, Institute of Experimental
TRANSPARENCY COMMITTEE OPINION. 10 December 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 RELISTOR 12 mg/0.6 ml solution for injection 1 vial (CIP: 387 365-1) 2 vials + 2 sterile syringes
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 RELISTOR 12 mg/0.6 ml solution for injection 1 vial (CIP: 387 365-1) 2 vials + 2 sterile syringes
Opioid induced constipation in cancer patients: pathophysiology, diagnosis and treatment
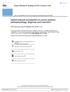 Expert Review of Quality of Life in Cancer Care ISSN: (Print) 2380-9000 (Online) Journal homepage: http://www.tandfonline.com/loi/terq20 Opioid induced constipation in cancer patients: pathophysiology,
Expert Review of Quality of Life in Cancer Care ISSN: (Print) 2380-9000 (Online) Journal homepage: http://www.tandfonline.com/loi/terq20 Opioid induced constipation in cancer patients: pathophysiology,
Constipation. What is constipation? What is the criteria for having constipation? What are the different types of constipation?
 What is constipation? is defined as having a bowel movement less than 3 times per week. It is usually associated with hard stools or difficulty passing stools. You may have pain while passing stools or
What is constipation? is defined as having a bowel movement less than 3 times per week. It is usually associated with hard stools or difficulty passing stools. You may have pain while passing stools or
The ideal drug therapy for postoperative
 Emerging pharmacologic options for treating postoperative ileus The ideal drug therapy for postoperative ileus (POI), a common surgical complication, would have several characteristics. It would selectively
Emerging pharmacologic options for treating postoperative ileus The ideal drug therapy for postoperative ileus (POI), a common surgical complication, would have several characteristics. It would selectively
Naltrexone for the treatment of Crohn s disease
 Naltrexone for the treatment of Crohn s disease DR. NILESH CHANDE COORDINATING EDITOR, IBD REVIEW GROUP; UNIVERSITY OF WESTERN ONTARIO, LONDON, ON CANADA Opioid receptors 3 major types kappa κ delta δ
Naltrexone for the treatment of Crohn s disease DR. NILESH CHANDE COORDINATING EDITOR, IBD REVIEW GROUP; UNIVERSITY OF WESTERN ONTARIO, LONDON, ON CANADA Opioid receptors 3 major types kappa κ delta δ
6/6/2018. Nalbuphine: Analgesic with a Niche. Mellar P Davis MD FCCP FAAHPM. Summary of Advantages. Summary of Advantages
 Nalbuphine: Analgesic with a Niche Mellar P Davis MD FCCP FAAHPM 1 Summary of Advantages Safe in renal failure- fecal excretion Analgesia equal to morphine with fewer side effects Reduced constipation
Nalbuphine: Analgesic with a Niche Mellar P Davis MD FCCP FAAHPM 1 Summary of Advantages Safe in renal failure- fecal excretion Analgesia equal to morphine with fewer side effects Reduced constipation
Constipation and bowel obstruction
 Constipation and bowel obstruction Constipation Infrequent or difficult defecation with reduced number of bowel movements, which may or may not be abnormally hard with increased difficulty or discomfort
Constipation and bowel obstruction Constipation Infrequent or difficult defecation with reduced number of bowel movements, which may or may not be abnormally hard with increased difficulty or discomfort
Overview of Essentials of Pain Management. Updated 11/2016
 0 Overview of Essentials of Pain Management Updated 11/2016 1 Overview of Essentials of Pain Management 1. Assess pain intensity on a 0 10 scale in which 0 = no pain at all and 10 = the worst pain imaginable.
0 Overview of Essentials of Pain Management Updated 11/2016 1 Overview of Essentials of Pain Management 1. Assess pain intensity on a 0 10 scale in which 0 = no pain at all and 10 = the worst pain imaginable.
Irritable Bowel Syndrome Now. George M. Logan, MD Friday, May 5, :35 4:05 PM
 Irritable Bowel Syndrome Now George M. Logan, MD Friday, May 5, 2017 3:35 4:05 PM Dr. Logan indicated no potential conflict of interest to this presentation. He does not intend to discuss any unapproved/investigative
Irritable Bowel Syndrome Now George M. Logan, MD Friday, May 5, 2017 3:35 4:05 PM Dr. Logan indicated no potential conflict of interest to this presentation. He does not intend to discuss any unapproved/investigative
Irritable Bowel Syndrome. Mustafa Giaffer March 2017
 Irritable Bowel Syndrome Mustafa Giaffer March 2017 Introduction First described in 1771. 50% of patients present
Irritable Bowel Syndrome Mustafa Giaffer March 2017 Introduction First described in 1771. 50% of patients present
Lehman Brothers Eighth Annual Global Healthcare Conference March 30, 2005 Bruce A. Peacock President and Chief Executive Officer
 Lehman Brothers Eighth Annual Global Healthcare Conference March 30, 2005 Bruce A. Peacock President and Chief Executive Officer 2005 Adolor Corporation. All rights reserved. Safe Harbor Statement This
Lehman Brothers Eighth Annual Global Healthcare Conference March 30, 2005 Bruce A. Peacock President and Chief Executive Officer 2005 Adolor Corporation. All rights reserved. Safe Harbor Statement This
WR Fentanyl Symposium. Opioids, Overdose, and Fentanyls
 Opioids, Overdose, and Fentanyls Outline: What are opioids? Why are we experiencing and opioid crisis? Potency, purity, and product How do opioids cause overdose and overdose deaths? What is naloxone and
Opioids, Overdose, and Fentanyls Outline: What are opioids? Why are we experiencing and opioid crisis? Potency, purity, and product How do opioids cause overdose and overdose deaths? What is naloxone and
Amitiza. Amitiza (lubiprostone) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.22 Subject: Amitiza Page: 1 of 5 Last Review Date: March 16, 2018 Amitiza Description Amitiza (lubiprostone)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.22 Subject: Amitiza Page: 1 of 5 Last Review Date: March 16, 2018 Amitiza Description Amitiza (lubiprostone)
Substitution Therapy for Opioid Use Disorder The Role of Suboxone
 Substitution Therapy for Opioid Use Disorder The Role of Suboxone Methadone/Buprenorphine 101 Workshop, December 10, 2016 Leslie Lappalainen, MD, CCFP, dip ABAM Prepared by Mandy Manak, MD, ABAM, CCSAM
Substitution Therapy for Opioid Use Disorder The Role of Suboxone Methadone/Buprenorphine 101 Workshop, December 10, 2016 Leslie Lappalainen, MD, CCFP, dip ABAM Prepared by Mandy Manak, MD, ABAM, CCSAM
TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain
 TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain Second Low-Dose SoluMatrix NSAID from Iroko Now Available by Prescription PHILADELPHIA, June 29, 2015 Iroko Pharmaceuticals, LLC,
TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain Second Low-Dose SoluMatrix NSAID from Iroko Now Available by Prescription PHILADELPHIA, June 29, 2015 Iroko Pharmaceuticals, LLC,
OIC. Drug List. Personalized Approach to Opioid induced Constipation. Learning Objectives. Presenter Disclosure Information. Management Options
 1:30 2:45 pm Personalized Approach to OIC: Improving Communication and Managing Treatment SPEAKER Jeffrey A. Gudin, MD Presenter Disclosure Information The following relationships exist related to this
1:30 2:45 pm Personalized Approach to OIC: Improving Communication and Managing Treatment SPEAKER Jeffrey A. Gudin, MD Presenter Disclosure Information The following relationships exist related to this
Pain Management Strategies Webinar/Teleconference
 Pain Management Strategies Webinar/Teleconference Barry K. Baines, MD April 16, 2009 Objectives Describe the principles of pain management. Identify considerations in the use of opioids. Describe the benefits
Pain Management Strategies Webinar/Teleconference Barry K. Baines, MD April 16, 2009 Objectives Describe the principles of pain management. Identify considerations in the use of opioids. Describe the benefits
IBS Irritable Bowel syndrome Therapeutics II PHCL 430
 Salman Bin AbdulAziz University College Of Pharmacy IBS Irritable Bowel syndrome Therapeutics II PHCL 430 Email:- ahmedadel.pharmd@gmail.com Ahmed A AlAmer PharmD R.S is 32-year-old woman experiences intermittent
Salman Bin AbdulAziz University College Of Pharmacy IBS Irritable Bowel syndrome Therapeutics II PHCL 430 Email:- ahmedadel.pharmd@gmail.com Ahmed A AlAmer PharmD R.S is 32-year-old woman experiences intermittent
IBS - Definition. Chronic functional disorder of GI generally characterized by:
 IBS - Definition Chronic functional disorder of GI generally characterized by: 3500 3000 No. of Publications 2500 2000 1500 1000 Irritable Bowel syndrome Irritable Bowel Syndrome 500 0 1968-1977 1978-1987
IBS - Definition Chronic functional disorder of GI generally characterized by: 3500 3000 No. of Publications 2500 2000 1500 1000 Irritable Bowel syndrome Irritable Bowel Syndrome 500 0 1968-1977 1978-1987
Acute Pain NETP: SEPTEMBER 2013 COHORT
 Acute Pain NETP: SEPTEMBER 2013 COHORT Pain & Suffering an unpleasant sensory & emotional experience associated with actual or potential tissue damage, or described in terms of such damage International
Acute Pain NETP: SEPTEMBER 2013 COHORT Pain & Suffering an unpleasant sensory & emotional experience associated with actual or potential tissue damage, or described in terms of such damage International
Pharmacogenetics of Codeine. Lily Mulugeta, Pharm.D Office of Clinical Pharmacology Pediatric Group FDA
 Pharmacogenetics of Codeine Lily Mulugeta, Pharm.D Office of Clinical Pharmacology Pediatric Group FDA 1 Codeine Overview Naturally occurring opium alkaloid Demethylated to morphine for analgesic effect
Pharmacogenetics of Codeine Lily Mulugeta, Pharm.D Office of Clinical Pharmacology Pediatric Group FDA 1 Codeine Overview Naturally occurring opium alkaloid Demethylated to morphine for analgesic effect
Scandinavian Journal of Pain
 Scandinavian Journal of Pain 11 (2016) 111 122 Contents lists available at ScienceDirect Scandinavian Journal of Pain journal homepage: www.scandinavianjournalpain.com Topical review Definition, diagnosis
Scandinavian Journal of Pain 11 (2016) 111 122 Contents lists available at ScienceDirect Scandinavian Journal of Pain journal homepage: www.scandinavianjournalpain.com Topical review Definition, diagnosis
What do we want for pain medications?
 New Trends in Pain Pharmacotherapy Dr. Chi Wai Cheung MBBS(HK), FHKCA, FHKAM(Anaesthesiology), Dip Pain Mgt(HKCA) Clinical Assistant Professor Department of Anaesthesiology The University of Hong Kong
New Trends in Pain Pharmacotherapy Dr. Chi Wai Cheung MBBS(HK), FHKCA, FHKAM(Anaesthesiology), Dip Pain Mgt(HKCA) Clinical Assistant Professor Department of Anaesthesiology The University of Hong Kong
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Immodium / loprarmide
 Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Current evidence in acute pain management. Jeremy Cashman
 Current evidence in acute pain management Jeremy Cashman Optimal analgesia Best possible pain relief Lowest incidence of side effects Optimal analgesia Best possible pain relief Lowest incidence of side
Current evidence in acute pain management Jeremy Cashman Optimal analgesia Best possible pain relief Lowest incidence of side effects Optimal analgesia Best possible pain relief Lowest incidence of side
Karam Darwish. Dr. Munir. Munir Gharaibeh
 7 Karam Darwish Dr. Munir Munir Gharaibeh Opioid Analgesics Pain is an important symptom as it is usually the symptom that brings the patient to the hospital, and an Analgesic is a drug used to relieve
7 Karam Darwish Dr. Munir Munir Gharaibeh Opioid Analgesics Pain is an important symptom as it is usually the symptom that brings the patient to the hospital, and an Analgesic is a drug used to relieve
HOPE. Considerations. Considerations ISING. Safe Opioid Prescribing Guidelines for ACUTE Non-Malignant Pain
 Due to the high level of prescription drug use and abuse in Lake County, these guidelines have been developed to standardize prescribing habits and limit risk of unintended harm when prescribing opioid
Due to the high level of prescription drug use and abuse in Lake County, these guidelines have been developed to standardize prescribing habits and limit risk of unintended harm when prescribing opioid
LESSON ASSIGNMENT. Emetics, Antiemetics, and Antidiarrheals. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 3 Emetics, Antiemetics, and Antidiarrheals. LESSON ASSIGNMENT Paragraphs 3-1 through 3-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 3-1. Given one
LESSON ASSIGNMENT LESSON 3 Emetics, Antiemetics, and Antidiarrheals. LESSON ASSIGNMENT Paragraphs 3-1 through 3-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 3-1. Given one
Knock Out Opioid Abuse in New Jersey:
 Knock Out Opioid Abuse in New Jersey: A Resource for Safer Prescribing GUIDELINE FOR PRESCRIBING OPIOIDS FOR CHRONIC PAIN IMPROVING PRACTICE THROUGH RECOMMENDATIONS CDC s Guideline for Prescribing Opioids
Knock Out Opioid Abuse in New Jersey: A Resource for Safer Prescribing GUIDELINE FOR PRESCRIBING OPIOIDS FOR CHRONIC PAIN IMPROVING PRACTICE THROUGH RECOMMENDATIONS CDC s Guideline for Prescribing Opioids
