Effects of Maintenance Immunosuppression With Sirolimus After Liver Transplant for Hepatocellular Carcinoma
|
|
|
- Grace Hawkins
- 5 years ago
- Views:
Transcription
1 ORIGINAL ARTICLE YANIK ET AL. Effects of Maintenance Immunosuppression With Sirolimus After Liver Transplant for Hepatocellular Carcinoma Elizabeth L. Yanik, 1 Srinath Chinnakotla, 2 Sally K. Gustafson, 5 Jon J. Snyder, 3,5 Ajay K. Israni, 3-5 Dorry L. Segev, 5,6 and Eric A. Engels 1 1 Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD; Department of 2 Surgery and 3 Epidemiology and Community Health, 4 Hennepin County Medical Center, School of Medicine, University of Minnesota, Minneapolis, MN; 5 Scientific Registry of Transplant Recipients, Minneapolis Medical Research Foundation, Minneapolis, MN; and 6 School of Medicine, Johns Hopkins University, Baltimore, MD For recipients of liver transplantations (LTs) for hepatocellular carcinoma (HCC), HCC recurrence after transplantation remains a major concern. Sirolimus (SRL), an immunosuppressant with anticarcinogenic properties, may reduce HCC recurrence and improve survival. In our study, the US Scientific Registry of Transplant Recipients was linked to pharmacy claims. For liver recipients transplanted for HCC, Cox regression was used to estimate associations of early SRL use with recurrence, cancer-specific mortality, and all-cause mortality, adjusting for recipient ethnicity, calendar year of transplant, total tumor volume, alpha-fetoprotein, transplant center size, use of interleukin 2 induction therapy, and allocated and calculated Model for End-Stage Liver Disease score. We performed stratified analyses among recipients who met Milan criteria, among those without renal failure, among those with deceased liver donors, by age at transplantation, and by tumor size. Among the 3936 included HCC LTs, 234 (6%) were SRL users. In total, there were 242 recurrences and 879 deaths, including 261 cancer-related deaths. All-cause mortality was similar in SRL users and nonusers (adjusted hazard ratio [ahr], 1.01; 95% CI, ). HCC recurrence and cancer-specific mortality rates appeared lower in SRL users, but associations were not statistically significant (recurrence ahr, 0.86; 95% CI, ; cancer-specific mortality ahr, 0.80; 95% CI, ). Among recipients >55 years old, associations were suggestive of better outcomes for SRL users (all-cause mortality ahr, 0.62; 95% CI, ; recurrence ahr, 0.52; 95% CI, ; cancer-specific mortality ahr, 0.34; 95% CI, ), whereas among recipients 55 years old, SRL users had worse outcomes (all-cause mortality ahr, 1.76; 95% CI, ; recurrence ahr, 1.49; 95% CI, ; cancer-specific mortality ahr, 1.54; 95% CI, ). In conclusion, among HCC liver recipients overall, SRL did not appear beneficial in reducing all-cause mortality. However, there were suggestions of reductions in recurrence and cancer-specific mortality, and effects appeared to be modified by age at transplantation. Liver Transplantation AASLD. Received October 5, 2015; accepted January 7, SEE EDITORIAL ON PAGE 582 Abbreviations: AFP, alpha-fetoprotein; ahr, adjusted hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; IL, interleukin; INR, international normalized ratio; IQR, interquartile range; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; MMRF, Minneapolis Medical Research Foundation; mtor, mammalian target of rapamycin; SRL, sirolimus; SRTR, Scientific Registry of Transplant Recipients. Address reprint requests to Elizabeth L. Yanik, Ph.D., Division of Cancer Epidemiology and Genetics, National Cancer Institute, 9609 Medical Center Drive, Room 6E-216, Rockville, MD Telephone: ; FAX: ; elizabeth. yanik@nih.gov A growing fraction of all liver transplantations (LTs) are performed for the indication of hepatocellular carcinoma (HCC). (1) Among these transplant recipients, approximately 10% experience a recurrence of the primary HCC following transplantation, making recurrence a leading cause of morbidity and mortality. (2-5) Mammalian target of rapamycin (mtor) is a protein that plays a key role in a signaling pathway that controls cellular growth and proliferation. (6) Because this pathway is often hyperactivated in malignant cells, ORIGINAL ARTICLE 627
2 YANIK ET AL. LIVER TRANSPLANTATION, May 2016 including HCC, (6-9) use of medications that inhibit mtor might hinder HCC recurrence. Sirolimus (SRL) is an mtor inhibitor with immunosuppressant properties and is used to prevent graft rejection among solid organ recipients, but use of this medication in LT remains controversial. In 2002, a randomized trial showed higher rates of graft loss, hepatic artery thrombosis, and death among liver recipients randomized to SRL. (10) However, these trial results have not since been replicated, and retrospective cohort studies have found lower or similar rates of adverse outcomes among liver recipients treated with SRL. (10,11) Additionally, the benefits of mtor inhibitors may outweigh the risks among transplant recipients with high cancer risk, such as those who receive a LT as treatment for HCC. (11) Along these lines, observational studies of liver recipients with HCC have reported lower cancer recurrence rates and better survival among SRL users. (12-14) However, most of these studies came from single institutions, which have a restricted range of practice patterns and experience systematic changes in immunosuppression protocols over time. In particular, SRL use was typically restricted to the most recent calendar years in these studies and is therefore potentially correlated with improvements in HCC staging and surgical practice over time. (13,15,16) Using the Scientific Registry of Transplant Recipients (SRTR; the US national transplant registry), 1 prior study demonstrated better survival associated with SRL use among liver recipients with HCC. (12) However, the study relied upon immunosuppression Elizabeth L. Yanik and Eric A. Engels were supported by the Intramural Research Program of the National Cancer Institute. The Scientific Registry of Transplant Recipients (SRTR) was managed by Minneapolis Medical Research Foundation (MMRF) in Minneapolis, MN (contract HHSH C). Research support for the linkage between the Scientific Registry of Transplant Recipients and IMS Health pharmacy claims was provided by Pfizer. The data reported here have been supplied by the MMRF as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. Copyright VC 2016 by the American Association for the Study of Liver Diseases. View this article online at wileyonlinelibrary.com. DOI /lt Potential conflict of interest: Nothing to report. data provided by the transplant centers at the time of initial hospital discharge, which may be unreliable. Additionally, this study did not consider associations with HCC recurrence or cancer-specific death. To address the limitations of these prior studies, we conducted a large observational study linking the SRTR to pharmacy claims data to estimate the effect of SRL-based immunosuppression on HCC recurrence, cancer-specific mortality, and overall mortality. Patients and Methods We identified liver recipients through the SRTR, a database of all US solid organ transplant recipients, which includes information on organ recipients demographic characteristics, indications for transplant, donor organ characteristics, and transplant outcomes including death. (17) We included liver recipients in the SRTR who had received wait-list exception points for HCC and for whom the SRTR had information on tumor size. The SRTR was linked with national pharmacy claims obtained from IMS Health (18) to capture immunosuppressant medication use after hospital discharge. The analysis was restricted to HCC liver recipients transplanted during with IMS claims for immunosuppressant medications in the first 3 months after transplantation. Recipients with SRL use reported in both their SRTR discharge regimen and IMS Health claims in the first 3 months after transplantation were considered SRL users. Recipients with no SRL reported in their SRTR discharge regimen and no SRL claims in the first 3 months were considered SRL nonusers. Recipients with discrepant SRL information (SRL reported only in SRTR or only in IMS) were excluded from analyses. We conducted an intention-to-treat analysis, in which recipients treated with SRL in the first 3 months after transplantation were considered SRL exposed for the duration of subsequent follow-up regardless of subsequent claims, whereas recipients not using SRL in the first 3 months were considered SRL unexposed. Although SRL use may have changed over time after transplantation, these changes might have been triggered by clinical events related to an incipient recurrence. We chose not to account for these changes in order to avoid introducing bias into the analyses. However, we did find that among the SRL users in our study with pharmacy claims beyond 3 months after transplant, the majority (74%) continued to have SRL claims beyond 3 months. 628 ORIGINAL ARTICLE
3 LIVER TRANSPLANTATION, Vol. 22, No. 5, 2016 YANIK ET AL. The outcomes of interest were death from any cause, cancer-related death, and HCC recurrence, as ascertained through SRTR. Cancer-related death was defined as any death classified as caused by a cancer, excluding posttransplant lymphoproliferative disorder. At-risk time for the outcomes began at 3 months following transplantation and ended at the first of HCC recurrence (in analyses in which that was the outcome of interest), death, or last date of recipient follow-up information. Recipients who experienced HCC recurrence in the first 3 months after transplantation were excluded from all analyses because these recurrences could have influenced early SRL use. We also assessed associations of SRL with noncancer cause-specific deaths as secondary outcomes of interest, specifically deaths from graft failure, infection, and cardiovascular disease. Rates of all-cause mortality, cancer-related mortality, and recurrence were calculated among the SRL exposed and unexposed. For each group, we constructed Kaplan-Meier curves to estimate overall survival proportions and cumulative incidence curves accounting for the competing risk of death to evaluate the proportion of recipients with an HCC recurrence across time. (19) Associations with SRL were estimated using Cox regression adjusting for variables selected based on correlations with SRL use in our data and a priori knowledge of potential predictors of mortality and recurrence: ethnicity, calendar year of transplant, total tumor volume, alpha-fetoprotein (AFP) level, allocated Model for End-Stage Liver Disease (MELD) score based on exception points, calculated MELD score based on laboratory values, use of interleukin (IL) 2 induction therapy, and transplant center volume. For characteristics that could change over time, such as total tumor volume and AFP level, we used the most proximal values collected before transplant. The time scale for Cox regression was time since LT. We evaluated potential changes in the effect of SRL over time by testing interaction terms of SRL use with the log of time since transplantation in the multivariate model. SRL users and nonusers had some characteristics that appeared to differ between the 2 groups but that included categories too infrequent to include in a parsimonious multivariate model. We therefore conducted sensitivity analyses after excluding these infrequent categories, in order to evaluate the effects on SRL associations. Specifically, we estimated SRL associations among the following: (1) recipients who met Milan criteria, (20) (2) recipients without a history of renal failure, and (3) recipients with a deceased liver donor. We decided a priori to evaluate potential effect modification by recipient age and total tumor volume at transplant, and we present stratified analyses using cutoffs near the median values for these characteristics (55 or >55 years for recipient age, and <7 cm 3 or 7cm 3 for total tumor volume). In a sensitivity analysis, we adjusted for the variables included in the initial multivariate regression model by using them to calculate propensity scores. We then ran the proportional hazards models assessing the associations of SRL with posttransplant outcomes stratifying on deciles of the propensity score. All statistical analyses were done using SAS, version 9.3 (SAS Institute, Cary, NC). Ethical approval or exemption from review was given by institutional review boards at the National Cancer Institute and Health Resources and Services Administration. Results Between 2002 and 2012, there were 13,991 US LTs performed in recipients who had received HCC exception points while on the waiting list. Of these, 4480 liver recipients had claims data on immunosuppressant medications in the first 3 months after transplantation. Among these recipients, 75 were excluded because they had a HCC recurrence, died, or had their last follow-up information before 3 months after transplantation. Additionally, 241 recipients had discrepant SRL information in SRTR and IMS Health, including 38 recipients for whom SRL use was only reported in SRTR and 203 recipients for whom SRL use was only reported in IMS Health claims. Tumor size information was not available for 228 recipients. After these exclusions, 3936 HCC liver recipients remained in our study population with a median follow-up of 2.8 years starting 3 months after transplantation (maximum follow-up of 11 years). Among these recipients, 234 (6%) received SRL in their initial immunosuppressant regimen (Table 1). For the majority of these recipients (62%), SRL claims were identified within a week following transplantation. Recipients using SRL were similar to those not using SRL with regard to sex, race, and age (Table 1). Before transplantation, SRL users and nonusers had similar total tumor volumes, AFP levels, and allocation MELD scores. Liver recipients on SRL underwent transplantation in more recent calendar years than nonusers (median 2009 versus 2008), and they were ORIGINAL ARTICLE 629
4 YANIK ET AL. LIVER TRANSPLANTATION, May 2016 TABLE 1. Characteristics of Liver Recipients With HCC and Outcomes Following Transplantation Stratified by SRL Use Characteristic SRL Exposed Recipients (n 5 234) SRL Unexposed Recipients (n ) P Value Sex 0.97 Female 54 (23) 858 (23) Male 180 (77) 2844 (77) Race 0.73 White 190 (81) 3026 (82) Black 25 (11) 344 (9) Other 19 (8) 332 (9) Ethnicity 0.02 Hispanic 40 (17) 446 (12) Non-Hispanic 194 (83) 3256 (88) Age at transplantation in years 57 (53-62) 57 (53-62) 0.96 Prior renal failure 0.16 Yes 1 (0)** 59 (2) No 233 (100) 3643 (98) Type of donor 0.17 Living 1 (0)** 57 (2) Deceased 233 (100) 3645 (98) Calendar year at transplantation 2009 ( ) 2008 ( ) <0.001 Transplant center volume* <0.001 < (30)** 1078 (29) (9)** 1737 (47) (62)** 887 (24) Total tumor volume 7.7 ( ) 7.2 ( ) 0.13 Met Milan criteria <0.001 Yes 209 (89) 3526 (95) No 25 (11) 176 (5) AFP, ng/ml 13 (5-55) 11 (5-40) 0.33 AFP < 500 ng/ml 0.54 Yes 210 (94) 3219 (95) No 13 (6) 166 (5) Allocation MELD score 25 (22-25) 24 (22-28) 0.12 Calculated MELD score 13 (10-16) 12 (9-16) 0.04 Serum creatinine in mg/dl 0.9 ( ) 0.9 ( ) 0.95 Bilirubin in mg/dl 1.8 ( ) 1.6 ( ) 0.08 INR 1.4 ( ) 1.3 ( ) Anti-IL2 induction therapy Yes 168 (72) 1821 (49) No 66 (28) 1881 (51) <0.001 Polyclonal antibody induction therapy Yes 16 (7) 294 (8) No 218 (93) 3408 (92) 0.54 Days from transplant to first SRL claim 6 (5-15) Outcomes following transplantation Deaths, n Rate per 100 person-years, (95% CI) 6.43 ( ) 6.03 ( ) Cancer-specific deaths, n Rate per 100 person-years, (95% CI) 1.78 ( ) 1.96 ( ) Recurrences, n Rate per 100 person-years (95% CI) 1.54 ( ) 1.71 ( ) NOTE: Chi-square tests were used to calculate P values for categorical variables. Wilcoxon rank sum tests were used to calculate P values for continuous variables. Data are given as median (IQR) or n (%) unless otherwise noted. *Transplant center volume was measured as the number of liver transplants done for recipients with a HCC diagnosis between 2002 and recipients (8.3%) did not have information on AFP. Values were based on most recent measurement/score before LT. **For some categories with greater than 0 recipients, 0% is given due to rounding. Percentages may also add up to greater than 100% due to rounding. more frequently transplanted at large centers. Also, SRL users were more frequently Hispanic, had higher calculated MELD scores, more frequently had tumors that did not meet Milan criteria (11% versus 5%), and more frequently received anti-il2 induction therapy at transplantation (Table 1). 630 ORIGINAL ARTICLE
5 LIVER TRANSPLANTATION, Vol. 22, No. 5, 2016 YANIK ET AL. Among recipients who lived at least 3 months after LT, there were 879 subsequent deaths, including 261 deaths attributable to cancer. SRL users did not differ FIG. 1. Overall survival and cumulative incidence of HCC recurrence among SRL users and nonusers. The upper curves depict the overall survival probability across time after 3 months after transplant for SRL users and nonusers. The lower curves depict the cumulative incidence of HCC recurrence after accounting for the competing risk of death. from nonusers in terms of all-cause mortality (adjusted hazard ratio [ahr], 1.01; 95% confidence interval [CI], ; Fig. 1). Although cancer-specific mortality appeared lower in SRL users, the association was not significant (ahr, 0.80; 95% CI, ; see Tables 1 and 2 for supporting details). The estimates of association with all-cause mortality and cancer-specific mortality did not vary over time since transplantation (P values for interaction and 0.14, respectively). When other causes of death were assessed, SRL was associated with a nonsignificant increase in death from graft failure (Table 3). There was a total of 242 HCC recurrences observed across follow-up, of which 11 occurred in SRL users (Table 1). The HCC recurrence rate in SRL users appeared lower, but again the reduction was not statistically significant (ahr, 0.86; 95% CI, ); the magnitude of the association with SRL did not change over time since transplantation (P value for interaction ; Fig. 1). In a sensitivity analysis using propensity scores to adjust for potential differences in SRL users and nonusers, the associations of SRL with posttransplant outcomes were similar to the main analysis (all-cause mortality ahr, 1.02; 95% CI, ; cancerspecific mortality ahr, 0.85; 95% CI, ; HCC recurrence ahr, 0.76; 95% CI, ). Similarly, there were no clear differences in all-cause mortality after limiting to recipients who met Milan criteria (ahr, 0.98; 95% CI, ), recipients TABLE 2. Associations of SRL Use With All-Cause Mortality, Cancer-Specific Mortality, and HCC Recurrence for All Recipients and Within Subgroups n All-Cause Mortality Cancer-Specific Mortality HCC Recurrence Unadjusted HR (95% CI) ahr (95% CI)* Unadjusted HR (95% CI) ahr (95% CI)* Unadjusted HR (95% CI) ahr (95% CI)* Overall ( ) Recipients who met Milan criteria ( ) Recipients without renal failure ( ) Recipients with deceased donors ( ) Recipients with total tumor volume <7 cm 3 ( ) Recipients with total tumor volume 7 cm 3 ( ) Recipients 55 years of age at transplant ( ) Recipients >55 years of age at transplant ( ) 1.01 ( ) 0.98 ( ) 1.01 ( ) 1.00 ( ) 0.83 ( ) 1.09 ( ) 1.76 ( ) 0.62 ( ) 0.88 ( ) 0.77 ( ) 0.90 ( ) 0.89 ( ) 1.18 ( ) 0.74 ( ) 1.63 ( ) 0.43 ( ) 0.80 ( ) 0.77 ( ) 0.80 ( ) 0.81 ( ) 1.11 ( ) 0.66 ( ) 1.54 ( ) 0.34 ( ) 0.83 ( ) 0.77 ( ) 0.84 ( ) 0.76 ( ) 0.84 ( ) 0.79 ( ) 1.39 ( ) 0.48 ( ) 0.86 ( ) 0.91 ( ) 0.87 ( ) 0.78 ( ) 0.96 ( ) 0.78 ( ) 1.49 ( ) 0.52 ( ) *Models are adjusted for ethnicity, calendar year of transplant, total tumor volume, AFP, allocated MELD score, calculated MELD score, transplant center size, and use of IL2 induction therapy. ORIGINAL ARTICLE 631
6 YANIK ET AL. LIVER TRANSPLANTATION, May 2016 TABLE 3. Associations of SRL With Noncancer Causes of Death Cause of Death without a history of renal failure (ahr, 1.01; 95% CI, ), and recipients with a deceased liver donor (ahr, 1.00; 95% CI, ; Table 2). Nonetheless, cancer-specific mortality and recurrence rates were nonsignificantly lower among SRL users in these subgroups, mirroring the primary analyses. Total tumor volume before transplantation did not appear to modify the effect of SRL (P value for interaction >0.20 for all outcomes; Table 2). Results differed by recipient age at transplantation, particularly for SRL associations with all-cause mortality (P value for interaction ). For recipients 55 years of age or younger at transplantation, SRL was associated with higher all-cause mortality (ahr, 1.76; 95% CI, ). Among these younger recipients, SRL users also had higher recurrence and cancer-specific mortality rates, though these associations were not statistically significant (Table 2). For recipients older than 55 years of age, SRL had a borderline association with lower all-cause mortality (ahr, 0.62; 95% CI, ). In this older age group, SRL was also associated with nonsignificant reductions in cancer-specific mortality (ahr, 0.34; 95% CI, ) and recurrence (ahr, 0.52; 95% CI, ). Discussion SRL Users Compared to Nonusers Unadjusted HR (95% CI) ahr (95% CI)* Graft failure 1.78 ( ) 1.80 ( ) Infection 0.39 ( ) 0.41 ( ) Cardiovascular disease 1.00 ( ) 1.18 ( ) *Models are adjusted for ethnicity, calendar year of transplant, total tumor volume, AFP, allocated MELD score, calculated MELD score, transplant center size, and use of IL2 induction therapy. In this large US study in which we used 2 independent sources of data on the use of immunosuppressant medications, we did not observe clear evidence for a benefit of SRL use for recipients of LTs for HCC. All-cause mortality rates were the same for SRL users and nonusers. Although recurrence rates and cancer-specific mortality rates tended to be lower among SRL users, these differences did not achieve statistical significance. Among younger recipients, SRL users had higher mortality and recurrence rates, whereas there was a suggestion that SRL may reduce recurrence and mortality among older recipients. However, no statistically significant reductions were seen in any subgroup of recipients. Although mtor inhibitors have anticarcinogenic properties, use of SRL may lack meaningful benefit in this population for a number of reasons. Although mutations leading to hyperactivation of the mtor pathway have been characterized in HCC, (8) hyperactivation is not uniformly present in all HCC tumors (9,21) and was likely absent in some of the HCC cases in our study. Inhibiting mtor would have no effect on tumors that are not dependent on the mtor pathway, thus diluting the overall effect observed among liver recipients with HCC. It is also possible that SRL doses were too low to be effective for cancer prevention because doses of mtor inhibitors used to prevent graft rejection are typically lower than doses used in cancer treatment. In addition, HCC tumors are known to have high levels of within-tumor heterogeneity, (22) which may allow the tumor to evolve resistance in response to mtor inhibition. Providing further evidence of the limits of HCC control through mtor inhibition, a recently completed randomized trial of SRL-based immunosuppression in liver recipients with HCC did not find evidence of improved recurrence-free survival. (23) Additionally, a randomized trial of patients with advanced HCC treated outside the transplant setting found no benefit associated with the use of everolimus (an mtor inhibitor that is molecularly similar to SRL). (24) Although the prior observational study using SRTR data reported a survival benefit associated with SRL use in HCC liver recipients, (12) we were unable to reproduce this result even when we attempted to replicate their study design and methods. Specifically, when we limited our study population to fit the inclusion criteria of the prior observational study and only used SRTR data on immunosuppressant medications to define SRL use, we estimated that 5-year survival was 75% among both SRL users and nonusers, in contrast to the previously reported results showing 83% survival among SRL users and 69% survival among SRL nonusers. (12) The lack of a reduction in all-cause mortality may also be partly due to adverse effects of SRL use, which might negate any improvements in survival due to reduced HCC recurrence. Such adverse events might include hepatic artery thrombosis, as observed in the prior randomized trial of liver recipients. (10) Although we could not directly assess these events in our study, we did 632 ORIGINAL ARTICLE
7 LIVER TRANSPLANTATION, Vol. 22, No. 5, 2016 YANIK ET AL. observe a nonsignificant increase in deaths from graft failure in SRL users, some of which could potentially be attributable to hepatic artery thrombosis. Higher rates of hyperlipidemia and hyperglycemia have also been observed in liver recipients treated with mtor inhibitors, (25) and among kidney recipients, nephrotoxicity has been observed when SRL is used in conjunction with calcineurin inhibitors. (26) Notably, another study that used SRTR data to assess outcomes related to SRL use in liver recipients but that was not restricted to HCC patients found that SRL was associated with significantly higher mortality among recipients with hepatitis C infection. (27) It is more difficult to interpret the associations that we observed for cancer-specific mortality and HCC recurrence, for which there was suggestive but inconclusive evidence of a benefit. Although the effect was marginal and nonsignificant for the overall group of liver recipients, mtor inhibitors may still be useful in particular subgroups. In our study, among people older than 55 years of age at transplantation, SRL users had lower recurrence, cancer-specific mortality, and all-cause mortality rates, though these associations were still not statistically significant. Importantly, among the younger recipients, all-cause mortality was higher with SRL use, indicating that SRL use may actually be detrimental in this subgroup. This difference may reflect varying molecular characteristics of tumors in different age groups, or differences in recipients susceptibility to adverse effects of the various immunosuppressant medication options. Associations in stratified analyses could also be due to chance and should be interpreted with caution. In contrast to our study, the recent trial in liver recipients with HCC found that SRL use appeared most beneficial among younger recipients, specifically those less than 60 years of age. (23) We speculate that, although beyond the scope of our study, molecular subtyping of tumors might best identify a subset of recipients who would benefit from the use of SRL or everolimus, given that mtor inhibitors would be expected to be most effective against tumors with mtor pathway hyperactivation. Another group of recipients who may be more likely to benefit from mtor inhibitors are those who have already experienced a recurrence after HCC LT. Once a recurrence occurs, it is far more likely that the recipient will die from cancer than from other causes, and switching to an mtor inhibitor based immunosuppressant regimen may be beneficial. Our large study of HCC liver recipients has a number of strengths. Unlike most prior observational studies of SRL among HCC liver recipients, (14) our study drew from numerous transplant centers across the United States, so we were able to evaluate SRL users and nonusers transplanted during the same calendar periods. We capitalized on the availability of pharmacy claims data in addition to the information from the SRTR in order to confidently categorize recipients as SRL users or nonusers. The identification of a number of discrepancies between SRTR information and pharmacy claims confirmed that a single source of information may not always be reliable. We evaluated multiple outcomes, including recurrence and cancer-specific mortality, in order to more clearly assess whether SRL had an effect on the course of HCC. We were also able to evaluate these outcomes over an extended follow-up period after transplantation, which was longer than in typical randomized trials. We acknowledge that there were also several limitations. Although measures such as AFP and total tumor volume did not differ notably between SRL users and nonusers, we lacked information on other measures of HCC severity, such as HCC tumor grade. Clinicians may have disproportionately prescribed SRL to recipients with more severe HCC, thus leading to a greater risk of recurrence and mortality among SRL users. We also did not have information on ablative treatment before transplantation, which could affect outcomes after transplantation. Although our study was one of the largest to date evaluating SRL use among HCC liver recipients, we still had a small number of SRL users. Given our sample size and length of follow-up, we only had 80% power to detect a 42% decrease in all-cause mortality and a 75% decrease in HCC recurrence among SRL users. Thus, our ability to detect more modest effects, which might still be clinically meaningful, was limited. The available sample also limited our ability to assess the effects of SRL in some subgroups of recipients. For example, SRL may be more useful in recipients who are transplanted when their tumors do not meet Milan criteria at transplantation, but in our study, there were only 25 SRL users who met this description. In conclusion, our study did not indicate a clear benefit of SRL use among liver recipients with HCC in terms of overall survival, although there was a suggestive reduction in cancer-specific mortality and HCC recurrence. Of interest, effects appeared to vary by age at transplant, and SRL use in our study was associated with significantly worse overall survival in younger recipients. Currently, randomized clinical trials evaluating SRL and everolimus use among liver recipients with HCC are ongoing (clinicaltrials.gov: NCT , NCT ). (28) Completion of these trials will ORIGINAL ARTICLE 633
8 YANIK ET AL. LIVER TRANSPLANTATION, May 2016 provide further evidence to inform the use of mtor inhibitors in this population. Even if these trials fail to demonstrate benefit for recipients with HCC overall, mtor inhibitors could still play a role in treating certain subsets of recipients, such as recipients with molecularly characterized tumors indicating mtor pathway hyperactivation or those who have already experienced a recurrence after transplantation. Acknowledgements: The authors acknowledge the support and assistance by individuals at the SRTR. REFERENCES 1) Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. United States Organ Transplantation: OPTN & SRTR Annual Data Report Liver. Rockville, MD: Division of Transplantation, Healthcare Systems Bureau,Health Resources and Services Administration, US Department of Health and Human Services; ) Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther 2010;31: ) Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant 2011;11: ) Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, et al. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol 1999; 17: ) Castroagudın JF, Molina-Perez E, Ferreiro-Iglesias R, Abdulkader I, Otero-Anton E, Tome S, Varo-Perez E. Late recurrence of hepatocellular carcinoma after liver transplantation: is an active surveillance for recurrence needed? Transplant Proc 2012;44: ) Faivre S, Kroemer G, Raymond E. Current development of mtor inhibitors as anticancer agents. Nat Rev Drug Discov 2006;5: ) Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004;4: ) Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol 2011;7: ) Zhou L, Huang Y, Li J, Wang Z. The mtor pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol 2010;27: ) Massoud O, Wiesner RH. The use of sirolimus should be restricted in liver transplantation. J Hepatol 2012;56: ) McKenna GJ, Trotter JF. Sirolimus--it doesn t deserve its bad Rap(a). J Hepatol 2012;56: ) Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010;51: ) Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl 2009;15: ) Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2013;37: ) Zhou J, Wang Z, Wu ZQ, Qiu SJ, Yu Y, Huang XW, et al. Sirolimus-based immunosuppression therapy in liver transplantation for patients with hepatocellular carcinoma exceeding the Milan criteria. Transplant Proc 2008;40: ) Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, Kam I. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl 2008;14: ) Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 2013;27: ) IMS Health. Accessed February 10, ) Lin G, So Y, Johnston G. Analyzing survival data with competing risks using SAS Software. SAS Global Forum 2012 Conference, Cary, NC: SAS Institute Inc. 20) Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334: ) Sieghart W, Fuereder T, Schmid K, Cejka D, Werzowa J, Wrba F, et al. Mammalian target of rapamycin pathway activity in hepatocellular carcinomas of patients undergoing liver transplantation. Transplantation 2007;83: ) Friemel J, Rechsteiner M, Frick L, B ohm F, Struckmann K, Egger M, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res 2015;21: ) Geissler EK, Schnitzbauer AA, Z ulke C, Lamby PE, Proneth A, Duvoux C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, openlabel phase 3 trial. Transplantation 2016;100: ) Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312: ) Klintmalm GB, Nashan B. The role of mtor inhibitors in liver transplantation: reviewing the evidence. J Transplant 2014;2014: ) Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004;351: ) Watt KD, Dierkhising R, Heimbach JK, Charlton MR. Impact of sirolimus and tacrolimus on mortality and graft loss in liver transplant recipients with or without hepatitis C virus: an analysis of the Scientific Registry of Transplant Recipients Database. Liver Transpl 2012;18: ) Schnitzbauer AA, Zuelke C, Graeb C, Rochon J, Bilbao I, Burra P, et al. A prospective randomized, open-labeled, trial comparing sirolimus-containing versus mtor-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer 2010;10: ORIGINAL ARTICLE
Historically, hepatocellular carcinoma (HCC)
 Delayed Hepatocellular Carcinoma Model for End-Stage Liver Disease Exception Score Improves Disparity in Access to Liver Transplant in the United States Julie K. Heimbach, 1 Ryutaro Hirose, 2 Peter G.
Delayed Hepatocellular Carcinoma Model for End-Stage Liver Disease Exception Score Improves Disparity in Access to Liver Transplant in the United States Julie K. Heimbach, 1 Ryutaro Hirose, 2 Peter G.
Geographic Differences in Event Rates by Model for End-Stage Liver Disease Score
 American Journal of Transplantation 2006; 6: 2470 2475 Blackwell Munksgaard C 2006 The Authors Journal compilation C 2006 The American Society of Transplantation and the American Society of Transplant
American Journal of Transplantation 2006; 6: 2470 2475 Blackwell Munksgaard C 2006 The Authors Journal compilation C 2006 The American Society of Transplantation and the American Society of Transplant
ORIGINAL ARTICLE. Eric F. Martin, 1 Jonathan Huang, 3 Qun Xiang, 2 John P. Klein, 2 Jasmohan Bajaj, 4 and Kia Saeian 1
 LIVER TRANSPLANTATION 18:914 929, 2012 ORIGINAL ARTICLE Recipient Survival and Graft Survival are Not Diminished by Simultaneous Liver-Kidney Transplantation: An Analysis of the United Network for Organ
LIVER TRANSPLANTATION 18:914 929, 2012 ORIGINAL ARTICLE Recipient Survival and Graft Survival are Not Diminished by Simultaneous Liver-Kidney Transplantation: An Analysis of the United Network for Organ
Optimizing Patient Selection, Organ Allocation, and Outcomes in Liver Transplant (LT) Candidates with Hepatocellular Carcinoma (HCC)
 XXVI SETH Congress- 30 November 2017 Optimizing Patient Selection, Organ Allocation, and Outcomes in Liver Transplant (LT) Candidates with Hepatocellular Carcinoma (HCC) Neil Mehta, MD University of California,
XXVI SETH Congress- 30 November 2017 Optimizing Patient Selection, Organ Allocation, and Outcomes in Liver Transplant (LT) Candidates with Hepatocellular Carcinoma (HCC) Neil Mehta, MD University of California,
Chapter 6: Transplantation
 Chapter 6: Transplantation Introduction During calendar year 2012, 17,305 kidney transplants, including kidney-alone and kidney plus at least one additional organ, were performed in the United States.
Chapter 6: Transplantation Introduction During calendar year 2012, 17,305 kidney transplants, including kidney-alone and kidney plus at least one additional organ, were performed in the United States.
Research Article A Decade of Experience Using mtor Inhibitors in Liver Transplantation
 Transplantation Volume 2011, Article ID 913094, 7 pages doi:10.1155/2011/913094 Research Article A Decade of Experience Using mtor Inhibitors in Liver Transplantation Jeffrey Campsen, 1, 2 Michael A. Zimmerman,
Transplantation Volume 2011, Article ID 913094, 7 pages doi:10.1155/2011/913094 Research Article A Decade of Experience Using mtor Inhibitors in Liver Transplantation Jeffrey Campsen, 1, 2 Michael A. Zimmerman,
Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients
 Pediatr Transplantation 2013: 17: 436 440 2013 John Wiley & Sons A/S. Pediatric Transplantation DOI: 10.1111/petr.12095 Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients
Pediatr Transplantation 2013: 17: 436 440 2013 John Wiley & Sons A/S. Pediatric Transplantation DOI: 10.1111/petr.12095 Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients
Liver Sharing and Organ Procurement Organization Performance
 LIVER TRANSPLANTATION 21:293 299, 2015 ORIGINAL ARTICLE Liver Sharing and Organ Procurement Organization Performance Sommer E. Gentry, 1,2 Eric K. H. Chow, 1 Allan Massie, 1,3 Xun Luo, 1 David Zaun, 4
LIVER TRANSPLANTATION 21:293 299, 2015 ORIGINAL ARTICLE Liver Sharing and Organ Procurement Organization Performance Sommer E. Gentry, 1,2 Eric K. H. Chow, 1 Allan Massie, 1,3 Xun Luo, 1 David Zaun, 4
Factors predicting survival after post-transplant hepatocellular carcinoma recurrence
 J Hepatobiliary Pancreat Sci (2013) 20:342 347 DOI 10.1007/s00534-012-0528-4 ORIGINAL ARTICLE Factors predicting survival after post-transplant hepatocellular carcinoma recurrence Christian Toso Sonia
J Hepatobiliary Pancreat Sci (2013) 20:342 347 DOI 10.1007/s00534-012-0528-4 ORIGINAL ARTICLE Factors predicting survival after post-transplant hepatocellular carcinoma recurrence Christian Toso Sonia
Liver Transplantation for Alcoholic Liver Disease in the United States: 1988 to 1995
 Liver Transplantation for Alcoholic Liver Disease in the United States: 1988 to 1995 Steven H. Belle, Kimberly C. Beringer, and Katherine M. Detre T he Scientific Liver Transplant Registry (LTR) was established
Liver Transplantation for Alcoholic Liver Disease in the United States: 1988 to 1995 Steven H. Belle, Kimberly C. Beringer, and Katherine M. Detre T he Scientific Liver Transplant Registry (LTR) was established
21/02/2014. Disclosures. HCC: predicting recurrence. Outline. Liver transplant: Beyond Milan?
 Disclosures HCC: predicting recurrence Peter Ghali, MD, FRCPC, MSc (epid) None relevant to this talk other than off-label use of sirolimus Toronto, February 2014 Outline Recurrence after what? Locoregional
Disclosures HCC: predicting recurrence Peter Ghali, MD, FRCPC, MSc (epid) None relevant to this talk other than off-label use of sirolimus Toronto, February 2014 Outline Recurrence after what? Locoregional
Screening for HCCwho,
 Screening for HCCwho, how and how often? Catherine Stedman Associate Professor of Medicine, University of Otago, Christchurch Gastroenterology Department, Christchurch Hospital HCC Global Epidemiology
Screening for HCCwho, how and how often? Catherine Stedman Associate Professor of Medicine, University of Otago, Christchurch Gastroenterology Department, Christchurch Hospital HCC Global Epidemiology
The pediatric end-stage liver disease (PELD) score
 Selection of Pediatric Candidates Under the PELD System Sue V. McDiarmid, 1 Robert M. Merion, 2 Dawn M. Dykstra, 2 and Ann M. Harper 3 Key Points 1. The PELD score accurately predicts the 3 month probability
Selection of Pediatric Candidates Under the PELD System Sue V. McDiarmid, 1 Robert M. Merion, 2 Dawn M. Dykstra, 2 and Ann M. Harper 3 Key Points 1. The PELD score accurately predicts the 3 month probability
Review Article The Role of mtor Inhibitors in Liver Transplantation: Reviewing the Evidence
 Hindawi Publishing Corporation Journal of Transplantation Volume 2014, Article ID 845438, 45 pages http://dx.doi.org/10.1155/2014/845438 Review Article The Role of mtor Inhibitors in Liver Transplantation:
Hindawi Publishing Corporation Journal of Transplantation Volume 2014, Article ID 845438, 45 pages http://dx.doi.org/10.1155/2014/845438 Review Article The Role of mtor Inhibitors in Liver Transplantation:
Worldwide Causes of HCC
 Approach to HCV Treatment in Patients with HCC JORGE L. HERRERA, MD, MACG UNIVERSITY OF SOUTH ALABAMA COLLEGE OF MEDICINE Worldwide Causes of HCC 60% 50% 40% 54% 30% 20% 10% 31% 15% 0% Hepatitis B Hepatitis
Approach to HCV Treatment in Patients with HCC JORGE L. HERRERA, MD, MACG UNIVERSITY OF SOUTH ALABAMA COLLEGE OF MEDICINE Worldwide Causes of HCC 60% 50% 40% 54% 30% 20% 10% 31% 15% 0% Hepatitis B Hepatitis
Living Donor Liver Transplantation for Hepatocellular Carcinoma: It Is All about Donors?
 Original Article Living Donor Liver Transplantation for Hepatocellular Carcinoma: It Is All about Donors? R. F. Saidi 1 *, Y. Li 2, S. A. Shah 2, N. Jabbour 2 1 Division of Organ Transplantation, Department
Original Article Living Donor Liver Transplantation for Hepatocellular Carcinoma: It Is All about Donors? R. F. Saidi 1 *, Y. Li 2, S. A. Shah 2, N. Jabbour 2 1 Division of Organ Transplantation, Department
2017 BANFF-SCT Joint Scientific Meeting. Personalized Medicine in Liver Transplantation
 2017 BANFF-SCT Joint Scientific Meeting Personalized Medicine in Liver Transplantation Miquel Navasa Liver Transplant Unit. Hospital Clínic. Barcelona. Barcelona, March 2017 Disclosures Consultant for
2017 BANFF-SCT Joint Scientific Meeting Personalized Medicine in Liver Transplantation Miquel Navasa Liver Transplant Unit. Hospital Clínic. Barcelona. Barcelona, March 2017 Disclosures Consultant for
Long-term prognosis of BK virus-associated nephropathy in kidney transplant recipients
 Original Article Kidney Res Clin Pract 37:167-173, 2018(2) pissn: 2211-9132 eissn: 2211-9140 https://doi.org/10.23876/j.krcp.2018.37.2.167 KIDNEY RESEARCH AND CLINICAL PRACTICE Long-term prognosis of BK
Original Article Kidney Res Clin Pract 37:167-173, 2018(2) pissn: 2211-9132 eissn: 2211-9140 https://doi.org/10.23876/j.krcp.2018.37.2.167 KIDNEY RESEARCH AND CLINICAL PRACTICE Long-term prognosis of BK
Long-term Clinical Outcomes and Risk of Hepatocellular Carcinoma in Chronic Hepatitis B Patients with HBsAg Seroclearance
 Long-term Clinical Outcomes and Risk of Hepatocellular Carcinoma in Chronic Hepatitis B Patients with HBsAg Seroclearance Gi-Ae Kim, Han Chu Lee *, Danbi Lee, Ju Hyun Shim, Kang Mo Kim, Young-Suk Lim,
Long-term Clinical Outcomes and Risk of Hepatocellular Carcinoma in Chronic Hepatitis B Patients with HBsAg Seroclearance Gi-Ae Kim, Han Chu Lee *, Danbi Lee, Ju Hyun Shim, Kang Mo Kim, Young-Suk Lim,
Liver transplantation for hepatocellular carcinoma: outcomes and treatment options for recurrence
 INVITED REVIEW Annals of Gastroenterology (2015) 28, 1-8 Liver transplantation for hepatocellular carcinoma: outcomes and treatment options for recurrence Robert S. Rahimi, James F. Trotter Baylor University
INVITED REVIEW Annals of Gastroenterology (2015) 28, 1-8 Liver transplantation for hepatocellular carcinoma: outcomes and treatment options for recurrence Robert S. Rahimi, James F. Trotter Baylor University
Research Article New Onset Diabetes Mellitus in Living Donor versus Deceased Donor Liver Transplant Recipients: Analysis of the UNOS/OPTN Database
 Transplantation Volume 2013, Article ID 269096, 7 pages http://dx.doi.org/10.1155/2013/269096 Research Article New Onset Diabetes Mellitus in Living Donor versus Deceased Donor Liver Transplant Recipients:
Transplantation Volume 2013, Article ID 269096, 7 pages http://dx.doi.org/10.1155/2013/269096 Research Article New Onset Diabetes Mellitus in Living Donor versus Deceased Donor Liver Transplant Recipients:
ORIGINAL ARTICLE. Hung-Tien Kuo, 1,2 Erik Lum, 1 Paul Martin, 3 and Suphamai Bunnapradist ORIGINAL ARTICLE
 ORIGINAL ARTICLE Effect of Diabetes and Acute Rejection on Liver Transplant Outcomes: An Analysis of the Organ rocurement and Transplantation Network/United Network for Organ Sharing Database Hung-Tien
ORIGINAL ARTICLE Effect of Diabetes and Acute Rejection on Liver Transplant Outcomes: An Analysis of the Organ rocurement and Transplantation Network/United Network for Organ Sharing Database Hung-Tien
Liver Transplantation: The End of the Road in Chronic Hepatitis C Infection
 University of Massachusetts Medical School escholarship@umms UMass Center for Clinical and Translational Science Research Retreat 2012 UMass Center for Clinical and Translational Science Research Retreat
University of Massachusetts Medical School escholarship@umms UMass Center for Clinical and Translational Science Research Retreat 2012 UMass Center for Clinical and Translational Science Research Retreat
PATIENTS AND METHODS. Data Source
 LIVER TRANSPLANTATION 20:1045 1056, 2014 ORIGINAL ARTICLE Waiting Time Predicts Survival After Liver Transplantation for Hepatocellular Carcinoma: A Cohort Study Using the United Network for Organ Sharing
LIVER TRANSPLANTATION 20:1045 1056, 2014 ORIGINAL ARTICLE Waiting Time Predicts Survival After Liver Transplantation for Hepatocellular Carcinoma: A Cohort Study Using the United Network for Organ Sharing
Organ allocation for liver transplantation: Is MELD the answer? North American experience
 Organ allocation for liver transplantation: Is MELD the answer? North American experience Douglas M. Heuman, MD Virginia Commonwealth University Richmond, VA, USA March 1998: US Department of Health and
Organ allocation for liver transplantation: Is MELD the answer? North American experience Douglas M. Heuman, MD Virginia Commonwealth University Richmond, VA, USA March 1998: US Department of Health and
BK virus infection in renal transplant recipients: single centre experience. Dr Wong Lok Yan Ivy
 BK virus infection in renal transplant recipients: single centre experience Dr Wong Lok Yan Ivy Background BK virus nephropathy (BKVN) has emerged as an important cause of renal graft dysfunction in recent
BK virus infection in renal transplant recipients: single centre experience Dr Wong Lok Yan Ivy Background BK virus nephropathy (BKVN) has emerged as an important cause of renal graft dysfunction in recent
Worldwide Causes of HCC
 Approach to HCV Treatment in Patients with HCC Mark W. Russo, MD, MPH, FACG Carolinas HealthCare System Charlotte Worldwide Causes of HCC 60% 50% 40% 30% 20% 10% 0% 54% 31% 15% Hepatitis B Hepatitis C
Approach to HCV Treatment in Patients with HCC Mark W. Russo, MD, MPH, FACG Carolinas HealthCare System Charlotte Worldwide Causes of HCC 60% 50% 40% 30% 20% 10% 0% 54% 31% 15% Hepatitis B Hepatitis C
Salvage Liver Transplantation for Recurrent Hepatocellular Carcinoma after Liver Resection: Retrospective Study of the Milan and Hangzhou Criteria
 Salvage Liver Transplantation for Recurrent Hepatocellular Carcinoma after Liver Resection: Retrospective Study of the Milan and Hangzhou Criteria Zhenhua Hu 1,2,3, Jie Zhou 1,2,3, Zhiwei Li 1,2,3, Jie
Salvage Liver Transplantation for Recurrent Hepatocellular Carcinoma after Liver Resection: Retrospective Study of the Milan and Hangzhou Criteria Zhenhua Hu 1,2,3, Jie Zhou 1,2,3, Zhiwei Li 1,2,3, Jie
Liver grafts for transplantation from donors with diabetes: an analysis of the Scientific Registry of Transplant Recipients database
 Title Liver grafts for transplantation from donors with diabetes: an analysis of the Scientific Registry of Transplant Recipients database Author(s) Zheng, J; Xiang, J; Zhou, J; Li, Z; Hu, Z; Lo, CM; Wang,
Title Liver grafts for transplantation from donors with diabetes: an analysis of the Scientific Registry of Transplant Recipients database Author(s) Zheng, J; Xiang, J; Zhou, J; Li, Z; Hu, Z; Lo, CM; Wang,
NIH Public Access Author Manuscript J Surg Res. Author manuscript; available in PMC 2011 May 18.
 NIH Public Access Author Manuscript Published in final edited form as: J Surg Res. 2011 April ; 166(2): 189 193. doi:10.1016/j.jss.2010.04.036. Hepatocellular Carcinoma Survival in Uninsured and Underinsured
NIH Public Access Author Manuscript Published in final edited form as: J Surg Res. 2011 April ; 166(2): 189 193. doi:10.1016/j.jss.2010.04.036. Hepatocellular Carcinoma Survival in Uninsured and Underinsured
AASLD Washington DC, USA Dr. Alexander Kim Chief Vascular and Interventional Radiology, Medstar Georgetown University Hospital
 AASLD 2017 - Washington DC, USA Dr. Alexander Kim Chief Vascular and Interventional Radiology, Medstar Georgetown University Hospital THE CHANGING LANDSCAPE IN THE TREATMENT OF HCC DISCLAIMER Please note:
AASLD 2017 - Washington DC, USA Dr. Alexander Kim Chief Vascular and Interventional Radiology, Medstar Georgetown University Hospital THE CHANGING LANDSCAPE IN THE TREATMENT OF HCC DISCLAIMER Please note:
Access and Outcomes Among Minority Transplant Patients, , with a Focus on Determinants of Kidney Graft Survival
 American Journal of Transplantation 2010; 10 (Part 2): 1090 1107 Wiley Periodicals Inc. Special Feature No claim to original US government works Journal compilation C 2010 The American Society of Transplantation
American Journal of Transplantation 2010; 10 (Part 2): 1090 1107 Wiley Periodicals Inc. Special Feature No claim to original US government works Journal compilation C 2010 The American Society of Transplantation
Potential Catalysts in Therapeutics
 LIVER TRANSPLANTATION 20:S22 S31, 2014 SUPPLEMENT Potential Catalysts in Therapeutics Bruce A. Luxon Division of Gastroenterology-Hepatology, University of Iowa, Iowa City, IA Received July 23, 2014; accepted
LIVER TRANSPLANTATION 20:S22 S31, 2014 SUPPLEMENT Potential Catalysts in Therapeutics Bruce A. Luxon Division of Gastroenterology-Hepatology, University of Iowa, Iowa City, IA Received July 23, 2014; accepted
OPTN/SRTR 2015 Annual Data Report: Deceased Organ Donation
 OPTN/SRTR 2015 Annual Data Report: Deceased Organ Donation A. K. Israni 1,2,3, D. Zaun 1, C. Bolch 1, J.D. Rosendale 4,5, C. Schaffhausen 3, J. J. Snyder 1,2, and B. L. Kasiske 1,3 1 Scientific Registry
OPTN/SRTR 2015 Annual Data Report: Deceased Organ Donation A. K. Israni 1,2,3, D. Zaun 1, C. Bolch 1, J.D. Rosendale 4,5, C. Schaffhausen 3, J. J. Snyder 1,2, and B. L. Kasiske 1,3 1 Scientific Registry
Early Changes in Kidney Distribution under the New Allocation System
 Early Changes in Kidney Distribution under the New Allocation System Allan B. Massie,* Xun Luo,* Bonnie E. Lonze,* Niraj M. Desai,* Adam W. Bingaman, Matthew Cooper, and Dorry L. Segev* *Department of
Early Changes in Kidney Distribution under the New Allocation System Allan B. Massie,* Xun Luo,* Bonnie E. Lonze,* Niraj M. Desai,* Adam W. Bingaman, Matthew Cooper, and Dorry L. Segev* *Department of
Key Words: Everolimus; Living donor liver transplantation; Hepatocellular carcinoma; Hepatic artery thrombosis
 Ann Hepatobiliary Pancreat Surg 2017;21:205-211 https://doi.org/10.14701/ahbps.2017.21.4.205 Original Article Assessing the role of everolimus in reducing hepatocellular carcinoma recurrence after living
Ann Hepatobiliary Pancreat Surg 2017;21:205-211 https://doi.org/10.14701/ahbps.2017.21.4.205 Original Article Assessing the role of everolimus in reducing hepatocellular carcinoma recurrence after living
9/10/2018. Liver Transplant for Hepatocellular Carcinoma (HCC): What is New? DISCLOSURES
 UCSF Transplant 2018: Pioneering Advances in Transplantation DISCLOSURES Liver Transplant for Hepatocellular Carcinoma (HCC): What is New? I have no relevant commercial interests or relationships to report
UCSF Transplant 2018: Pioneering Advances in Transplantation DISCLOSURES Liver Transplant for Hepatocellular Carcinoma (HCC): What is New? I have no relevant commercial interests or relationships to report
What Is the Real Gain After Liver Transplantation?
 LIVER TRANSPLANTATION 15:S1-S5, 9 AASLD/ILTS SYLLABUS What Is the Real Gain After Liver Transplantation? James Neuberger Organ Donation and Transplantation, NHS Blood and Transplant, Bristol, United Kingdom;
LIVER TRANSPLANTATION 15:S1-S5, 9 AASLD/ILTS SYLLABUS What Is the Real Gain After Liver Transplantation? James Neuberger Organ Donation and Transplantation, NHS Blood and Transplant, Bristol, United Kingdom;
Progress in Pediatric Kidney Transplantation
 Send Orders for Reprints to reprints@benthamscience.net The Open Urology & Nephrology Journal, 214, 7, (Suppl 2: M2) 115-122 115 Progress in Pediatric Kidney Transplantation Jodi M. Smith *,1 and Vikas
Send Orders for Reprints to reprints@benthamscience.net The Open Urology & Nephrology Journal, 214, 7, (Suppl 2: M2) 115-122 115 Progress in Pediatric Kidney Transplantation Jodi M. Smith *,1 and Vikas
Literature Review Transplantation
 Literature Review 2010- Transplantation Alexander Wiseman, M.D. Associate Professor, Division of Renal Diseases and Hypertension Medical Director, Kidney and Pancreas Transplant Programs University of
Literature Review 2010- Transplantation Alexander Wiseman, M.D. Associate Professor, Division of Renal Diseases and Hypertension Medical Director, Kidney and Pancreas Transplant Programs University of
Clinical Study Use of Adjuvant Sorafenib in Liver Transplant Recipients with High-Risk Hepatocellular Carcinoma
 Transplantation, Article ID 913634, 5 pages http://dx.doi.org/10.1155/2014/913634 Clinical Study Use of Adjuvant Sorafenib in Liver Transplant Recipients with High-Risk Hepatocellular Carcinoma Kirti Shetty,
Transplantation, Article ID 913634, 5 pages http://dx.doi.org/10.1155/2014/913634 Clinical Study Use of Adjuvant Sorafenib in Liver Transplant Recipients with High-Risk Hepatocellular Carcinoma Kirti Shetty,
Natural History and Treatment Trends in Hepatocellular Carcinoma Subtypes: Insights From a National Cancer Registry
 2015;112:872 876 Natural History and Treatment Trends in Hepatocellular Carcinoma Subtypes: Insights From a National Cancer Registry PETER L. JERNIGAN, MD, KOFFI WIMA, MS, DENNIS J. HANSEMAN, PhD, RICHARD
2015;112:872 876 Natural History and Treatment Trends in Hepatocellular Carcinoma Subtypes: Insights From a National Cancer Registry PETER L. JERNIGAN, MD, KOFFI WIMA, MS, DENNIS J. HANSEMAN, PhD, RICHARD
Developing a Kidney Waiting List Calculator
 Developing a Kidney Waiting List Calculator Jon J. Snyder, PhD* Nicholas Salkowski, PhD, Jiannong Liu, PhD, Kenneth Lamb, PhD, Bryn Thompson, MPH, Ajay Israni, MD, MS, and Bertram Kasiske, MD, FACP *Presenter
Developing a Kidney Waiting List Calculator Jon J. Snyder, PhD* Nicholas Salkowski, PhD, Jiannong Liu, PhD, Kenneth Lamb, PhD, Bryn Thompson, MPH, Ajay Israni, MD, MS, and Bertram Kasiske, MD, FACP *Presenter
kidney OPTN/SRTR 2012 Annual Data Report:
 kidney wait list 18 deceased donation 22 live donation 24 transplant 26 donor-recipient matching 28 outcomes 3 pediatric transplant 33 Medicare data 4 transplant center maps 43 A. J. Matas1,2, J. M. Smith1,3,
kidney wait list 18 deceased donation 22 live donation 24 transplant 26 donor-recipient matching 28 outcomes 3 pediatric transplant 33 Medicare data 4 transplant center maps 43 A. J. Matas1,2, J. M. Smith1,3,
SELECTED ABSTRACTS. All (n) % 3-year GS 88% 82% 86% 85% 88% 80% % 3-year DC-GS 95% 87% 94% 89% 96% 80%
 SELECTED ABSTRACTS The following are summaries of selected posters presented at the American Transplant Congress on May 5 9, 2007, in San Humar A, Gillingham KJ, Payne WD, et al. Review of >1000 kidney
SELECTED ABSTRACTS The following are summaries of selected posters presented at the American Transplant Congress on May 5 9, 2007, in San Humar A, Gillingham KJ, Payne WD, et al. Review of >1000 kidney
Following the introduction of adult-to-adult living
 LIVER FAILURE/CIRRHOSIS/PORTAL HYPERTENSION Liver Transplant Recipient Survival Benefit with Living Donation in the Model for Endstage Liver Disease Allocation Era Carl L. Berg, 1 Robert M. Merion, 2 Tempie
LIVER FAILURE/CIRRHOSIS/PORTAL HYPERTENSION Liver Transplant Recipient Survival Benefit with Living Donation in the Model for Endstage Liver Disease Allocation Era Carl L. Berg, 1 Robert M. Merion, 2 Tempie
During the past 2 decades, an increase in the ageadjusted
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:104 110 Racial Differences in Survival of Hepatocellular Carcinoma in the United States: A Population-Based Study JESSICA A. DAVILA* and HASHEM B. EL SERAG*,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2006;4:104 110 Racial Differences in Survival of Hepatocellular Carcinoma in the United States: A Population-Based Study JESSICA A. DAVILA* and HASHEM B. EL SERAG*,
ORIGINAL ARTICLE. Sarah K. Alver, 1 Douglas J. Lorenz, 1 Michael R. Marvin, 2 and Guy N. Brock 1 SEE EDITORIAL ON PAGE 1321 ORIGINAL ARTICLE 1343
 ORIGINAL ARTICLE Projected Outcomes of 6-Month Delay in Exception Points Versus an Equivalent Model for End-Stage Liver Disease Score for Hepatocellular Carcinoma Liver Transplant Candidates Sarah K. Alver,
ORIGINAL ARTICLE Projected Outcomes of 6-Month Delay in Exception Points Versus an Equivalent Model for End-Stage Liver Disease Score for Hepatocellular Carcinoma Liver Transplant Candidates Sarah K. Alver,
Literature Review: Transplantation July 2010-June 2011
 Literature Review: Transplantation July 2010-June 2011 James Cooper, MD Assistant Professor, Kidney and Pancreas Transplant Program, Renal Division, UC Denver Kidney Transplant Top 10 List: July Kidney
Literature Review: Transplantation July 2010-June 2011 James Cooper, MD Assistant Professor, Kidney and Pancreas Transplant Program, Renal Division, UC Denver Kidney Transplant Top 10 List: July Kidney
Karnofsky Performance Score and Its Use in Risk Adjustment of Transplant Outcomes in the United States
 Karnofsky Performance Score and Its Use in Risk Adjustment of Transplant Outcomes in the United States Jon J Snyder, PhD* Nicholas J Salkowski, PhD Kenneth E Lamb, PhD David A Zaun, MS Taqee A Khaled,
Karnofsky Performance Score and Its Use in Risk Adjustment of Transplant Outcomes in the United States Jon J Snyder, PhD* Nicholas J Salkowski, PhD Kenneth E Lamb, PhD David A Zaun, MS Taqee A Khaled,
Autoimmune Hepatitis: Defining the need for Liver Transplantation
 Autoimmune Hepatitis: Defining the need for Liver Transplantation Michael A Heneghan, MD, MMedSc, FRCPI. Institute of Liver Studies, King s College Hospital, London Outline Autoimmune Hepatitis Background
Autoimmune Hepatitis: Defining the need for Liver Transplantation Michael A Heneghan, MD, MMedSc, FRCPI. Institute of Liver Studies, King s College Hospital, London Outline Autoimmune Hepatitis Background
Heart Transplantation ACC Middle East Conference Dubai UAE October 21, 2017
 Heart Transplantation ACC Middle East Conference Dubai UAE October 21, 2017 Randall C Starling MD MPH FACC FAHA FESC FHFSA Professor of Medicine Kaufman Center for Heart Failure Department of Cardiovascular
Heart Transplantation ACC Middle East Conference Dubai UAE October 21, 2017 Randall C Starling MD MPH FACC FAHA FESC FHFSA Professor of Medicine Kaufman Center for Heart Failure Department of Cardiovascular
Offer Acceptance Practices and Geographic Variability in Allocation Model for End-Stage Liver Disease at Transplant
 ORIGINAL ARTICLE Offer Acceptance Practices and Geographic Variability in Allocation Model for End-Stage Liver Disease at Transplant Andrew Wey, 1 Joshua Pyke, 1 David P. Schladt, 1 Sommer E. Gentry, 2,3
ORIGINAL ARTICLE Offer Acceptance Practices and Geographic Variability in Allocation Model for End-Stage Liver Disease at Transplant Andrew Wey, 1 Joshua Pyke, 1 David P. Schladt, 1 Sommer E. Gentry, 2,3
Ammonia level at admission predicts in-hospital mortality for patients with alcoholic hepatitis
 Gastroenterology Report, 5(3), 2017, 232 236 doi: 10.1093/gastro/gow010 Advance Access Publication Date: 1 May 2016 Original article ORIGINAL ARTICLE Ammonia level at admission predicts in-hospital mortality
Gastroenterology Report, 5(3), 2017, 232 236 doi: 10.1093/gastro/gow010 Advance Access Publication Date: 1 May 2016 Original article ORIGINAL ARTICLE Ammonia level at admission predicts in-hospital mortality
Total Tumor Volume and Alpha Fetoprotein for selection of transplant candidates. with hepatocellular carcinoma: a prospective validation
 Total Tumor Volume and Alpha Fetoprotein for selection of transplant candidates with hepatocellular carcinoma: a prospective validation Christian Toso 1, Glenda Meeberg 2, Roberto Hernandez-Alejandro 3,
Total Tumor Volume and Alpha Fetoprotein for selection of transplant candidates with hepatocellular carcinoma: a prospective validation Christian Toso 1, Glenda Meeberg 2, Roberto Hernandez-Alejandro 3,
Hepatocellular Carcinoma Surveillance
 Amit G. Singal, MD, MS Hepatocellular Carcinoma Surveillance Postgraduate Course: Challenges in Management of Common Liver Diseases 308 1 Patient Case 69 year-old otherwise healthy male with compensated
Amit G. Singal, MD, MS Hepatocellular Carcinoma Surveillance Postgraduate Course: Challenges in Management of Common Liver Diseases 308 1 Patient Case 69 year-old otherwise healthy male with compensated
Pancreas After Islet Transplantation: A First Report of the International Pancreas Transplant Registry
 American Journal of Transplantation 2016; 16: 688 693 Wiley Periodicals Inc. Brief Communication Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons doi:
American Journal of Transplantation 2016; 16: 688 693 Wiley Periodicals Inc. Brief Communication Copyright 2015 The American Society of Transplantation and the American Society of Transplant Surgeons doi:
ORIGINAL ARTICLE. Received April 30, 2007; accepted June
 LIVER TRANSPLANTATION 13:1405-1413, 2007 ORIGINAL ARTICLE Human Leukocyte Antigen and Adult Living- Donor Liver Transplantation Outcomes: An Analysis of the Organ Procurement and Transplantation Network
LIVER TRANSPLANTATION 13:1405-1413, 2007 ORIGINAL ARTICLE Human Leukocyte Antigen and Adult Living- Donor Liver Transplantation Outcomes: An Analysis of the Organ Procurement and Transplantation Network
Hepatocellular Carcinoma: Transplantation, Resection or Ablation?
 Hepatocellular Carcinoma: Transplantation, Resection or Ablation? Roberto Gedaly MD Chief, Abdominal Transplantation Transplant Service Line University of Kentucky Nothing to disclose Disclosure Objective
Hepatocellular Carcinoma: Transplantation, Resection or Ablation? Roberto Gedaly MD Chief, Abdominal Transplantation Transplant Service Line University of Kentucky Nothing to disclose Disclosure Objective
Liver Transplantation Evaluation: Objectives
 Liver Transplantation Evaluation: Essential Work-Up Curtis K. Argo, MD, MS VGS/ACG Regional Postgraduate Course Williamsburg, VA September 13, 2015 Objectives Discuss determining readiness for transplantation
Liver Transplantation Evaluation: Essential Work-Up Curtis K. Argo, MD, MS VGS/ACG Regional Postgraduate Course Williamsburg, VA September 13, 2015 Objectives Discuss determining readiness for transplantation
Waitlist Priority for Hepatocellular Carcinoma Beyond Milan Criteria: A Potentially Appropriate Decision Without a Structured Approach
 American Journal of Transplantation 2014; 14: 79 87 Wiley Periodicals Inc. C Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons doi: 10.1111/ajt.12530
American Journal of Transplantation 2014; 14: 79 87 Wiley Periodicals Inc. C Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons doi: 10.1111/ajt.12530
Optimization of immunosuppressive medication upon liver transplantation against HCC recurrence
 Review Article Optimization of immunosuppressive medication upon liver transplantation against HCC recurrence Shirin Elizabeth Khorsandi, Nigel Heaton Institute of Liver Studies, King s Healthcare Partners
Review Article Optimization of immunosuppressive medication upon liver transplantation against HCC recurrence Shirin Elizabeth Khorsandi, Nigel Heaton Institute of Liver Studies, King s Healthcare Partners
Diabetes, Hypertension and Hyperlipidemia: Prevalence Over Time and Impact on Long-Term Survival After Liver Transplantation
 American Journal of Transplantation 2012; 12: 2181 2187 Wiley Periodicals Inc. C Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons doi: 10.1111/j.1600-6143.2012.04077.x
American Journal of Transplantation 2012; 12: 2181 2187 Wiley Periodicals Inc. C Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons doi: 10.1111/j.1600-6143.2012.04077.x
2 Biostatistics and 3 Surgery, University of Michigan, Ann
 LIVER TRANSPLANTATION 22:71 79, 2016 ORIGINAL ARTICLE Propensity Score-Based Survival Benefit of Simultaneous Liver-Kidney Transplant Over Liver Transplant Alone for Recipients With Pretransplant Renal
LIVER TRANSPLANTATION 22:71 79, 2016 ORIGINAL ARTICLE Propensity Score-Based Survival Benefit of Simultaneous Liver-Kidney Transplant Over Liver Transplant Alone for Recipients With Pretransplant Renal
LIVER TRANSPLANTATION FOR OVERLAP SYNDROMES OF AUTOIMMUNE LIVER DISEASES
 LIVER TRANSPLANTATION FOR OVERLAP SYNDROMES OF AUTOIMMUNE LIVER DISEASES No conflict of interest Objectives Introduction Methods Results Conclusions Objectives Introduction Methods Results Conclusions
LIVER TRANSPLANTATION FOR OVERLAP SYNDROMES OF AUTOIMMUNE LIVER DISEASES No conflict of interest Objectives Introduction Methods Results Conclusions Objectives Introduction Methods Results Conclusions
Increasing Trends in Transplantation of HCV-positive Livers into Uninfected Recipients
 Accepted Manuscript Increasing Trends in Transplantation of HCV-positive Livers into Uninfected Recipients George Cholankeril, MD, Andrew A. Li, MD, Brittany B. Dennis, PhD, Alice E. Toll, MS, Donghee
Accepted Manuscript Increasing Trends in Transplantation of HCV-positive Livers into Uninfected Recipients George Cholankeril, MD, Andrew A. Li, MD, Brittany B. Dennis, PhD, Alice E. Toll, MS, Donghee
Reconsidering Liver Transplantation for HCC in a Era of Organ shortage
 Reconsidering Liver Transplantation for HCC in a Era of Organ shortage Professor Didier Samuel Centre Hépatobiliaire Inserm-Paris Sud Research Unit 1193 Departement Hospitalo Universitaire Hepatinov Hôpital
Reconsidering Liver Transplantation for HCC in a Era of Organ shortage Professor Didier Samuel Centre Hépatobiliaire Inserm-Paris Sud Research Unit 1193 Departement Hospitalo Universitaire Hepatinov Hôpital
User Guide. A. Program Summary B. Waiting List Information C. Transplant Information
 User Guide This report contains a wide range of useful information about the kidney transplant program at (FLMR). The report has three main sections: A. Program Summary B. Waiting List Information The
User Guide This report contains a wide range of useful information about the kidney transplant program at (FLMR). The report has three main sections: A. Program Summary B. Waiting List Information The
10-Year Mortality of Older Acute Myocardial Infarction Patients Treated in U.S. Community Practice
 10-Year Mortality of Older Acute Myocardial Infarction Patients Treated in U.S. Community Practice Ajar Kochar, MD on behalf of: Anita Y. Chen, Puza P. Sharma, Neha J. Pagidipati, Gregg C. Fonarow, Patricia
10-Year Mortality of Older Acute Myocardial Infarction Patients Treated in U.S. Community Practice Ajar Kochar, MD on behalf of: Anita Y. Chen, Puza P. Sharma, Neha J. Pagidipati, Gregg C. Fonarow, Patricia
Prelisting Prescription Narcotic Use: Survival Implications in Liver Transplantation
 Prelisting Prescription Narcotic Use: Survival Implications in Liver Transplantation American Transplant Congress June 13, 2016 H Randall, MD, 1 KL Lentine, MD, PhD, 1 DL Segev, MD, PhD, 2 D Axelrod, MD,
Prelisting Prescription Narcotic Use: Survival Implications in Liver Transplantation American Transplant Congress June 13, 2016 H Randall, MD, 1 KL Lentine, MD, PhD, 1 DL Segev, MD, PhD, 2 D Axelrod, MD,
Reduced graft function (with or without dialysis) vs immediate graft function a comparison of long-term renal allograft survival
 Nephrol Dial Transplant (2006) 21: 2270 2274 doi:10.1093/ndt/gfl103 Advance Access publication 22 May 2006 Original Article Reduced graft function (with or without dialysis) vs immediate graft function
Nephrol Dial Transplant (2006) 21: 2270 2274 doi:10.1093/ndt/gfl103 Advance Access publication 22 May 2006 Original Article Reduced graft function (with or without dialysis) vs immediate graft function
Locoregional treatment Session Oral Abstract Presentation Saulo Brito Silva
 Locoregional treatment Session Oral Abstract Presentation Saulo Brito Silva Background Post-operative radiotherapy (PORT) improves disease free and overall suvivallin selected patients with breast cancer
Locoregional treatment Session Oral Abstract Presentation Saulo Brito Silva Background Post-operative radiotherapy (PORT) improves disease free and overall suvivallin selected patients with breast cancer
Influence of kidney offer acceptance behavior on metrics of allocation efficiency
 Accepted: 11 July 2017 DOI: 10.1111/ctr.13057 ORIGINAL ARTICLE Influence of kidney offer acceptance behavior on metrics of allocation efficiency Andrew Wey 1 Nicholas Salkowski 1 Bertram L. Kasiske 1,2
Accepted: 11 July 2017 DOI: 10.1111/ctr.13057 ORIGINAL ARTICLE Influence of kidney offer acceptance behavior on metrics of allocation efficiency Andrew Wey 1 Nicholas Salkowski 1 Bertram L. Kasiske 1,2
Pretransplant Risk Factors for New-Onset Diabetes Mellitus After Transplant in Pediatric Liver Transplant Recipients
 LIVER TRANSPLANTATION 16:1249-1256, 2010 ORIGINAL ARTICLE Pretransplant Risk Factors for New-Onset Diabetes Mellitus After Transplant in Pediatric Liver Transplant Recipients Hung-Tien Kuo, 1,2 Christine
LIVER TRANSPLANTATION 16:1249-1256, 2010 ORIGINAL ARTICLE Pretransplant Risk Factors for New-Onset Diabetes Mellitus After Transplant in Pediatric Liver Transplant Recipients Hung-Tien Kuo, 1,2 Christine
ORIGINAL ARTICLES LIVER, PANCREAS, AND BILIARY TRACT
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2011;9:989 994 ORIGINAL ARTICLES LIVER, PANCREAS, AND BILIARY TRACT Level of -Fetoprotein Predicts Mortality Among Patients With Hepatitis C Related Hepatocellular
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2011;9:989 994 ORIGINAL ARTICLES LIVER, PANCREAS, AND BILIARY TRACT Level of -Fetoprotein Predicts Mortality Among Patients With Hepatitis C Related Hepatocellular
Understanding Pharmaceutical Care Needs of Living Kidney Donors through Linked Transplant Registry and Pharmacy Claims Data
 Understanding Pharmaceutical Care Needs of Living Kidney Donors through Linked Transplant Registry and Pharmacy Claims Data The SRTR Living Donor Collective Lentine KL, 1 Gustafson SK, 2 Schnitzler MA,
Understanding Pharmaceutical Care Needs of Living Kidney Donors through Linked Transplant Registry and Pharmacy Claims Data The SRTR Living Donor Collective Lentine KL, 1 Gustafson SK, 2 Schnitzler MA,
Does Viral Cure Prevent HCC Development
 Does Viral Cure Prevent HCC Development Prof. Henry LY Chan Head, Division of Gastroenterology and Hepatology Director, Institute of Digestive Disease Director, Center for Liver Health Assistant Dean,
Does Viral Cure Prevent HCC Development Prof. Henry LY Chan Head, Division of Gastroenterology and Hepatology Director, Institute of Digestive Disease Director, Center for Liver Health Assistant Dean,
Program- specific transplant rate ratios: Association with allocation priority at listing and posttransplant outcomes
 Received: 20 August 2017 Revised: 25 January 2018 Accepted: 28 January 2018 DOI: 10.1111/ajt.14684 ORIGINAL ARTICLE Program- specific transplant rate ratios: Association with allocation priority at listing
Received: 20 August 2017 Revised: 25 January 2018 Accepted: 28 January 2018 DOI: 10.1111/ajt.14684 ORIGINAL ARTICLE Program- specific transplant rate ratios: Association with allocation priority at listing
Hepatocellular Carcinoma: Can We Slow the Rising Incidence?
 Hepatocellular Carcinoma: Can We Slow the Rising Incidence? K.Rajender Reddy M.D. Professor of Medicine Director of Hepatology Medical Director of Liver Transplantation University of Pennsylvania Outline
Hepatocellular Carcinoma: Can We Slow the Rising Incidence? K.Rajender Reddy M.D. Professor of Medicine Director of Hepatology Medical Director of Liver Transplantation University of Pennsylvania Outline
NIH Public Access Author Manuscript World J Urol. Author manuscript; available in PMC 2012 February 1.
 NIH Public Access Author Manuscript Published in final edited form as: World J Urol. 2011 February ; 29(1): 11 14. doi:10.1007/s00345-010-0625-4. Significance of preoperative PSA velocity in men with low
NIH Public Access Author Manuscript Published in final edited form as: World J Urol. 2011 February ; 29(1): 11 14. doi:10.1007/s00345-010-0625-4. Significance of preoperative PSA velocity in men with low
Donor Hypernatremia Influences Outcomes Following Pediatric Liver Transplantation
 8 Original Article Donor Hypernatremia Influences Outcomes Following Pediatric Liver Transplantation Neema Kaseje 1 Samuel Lüthold 2 Gilles Mentha 3 Christian Toso 3 Dominique Belli 2 Valérie McLin 2 Barbara
8 Original Article Donor Hypernatremia Influences Outcomes Following Pediatric Liver Transplantation Neema Kaseje 1 Samuel Lüthold 2 Gilles Mentha 3 Christian Toso 3 Dominique Belli 2 Valérie McLin 2 Barbara
Does Kidney Donor Risk Index implementation lead to the transplantation of more and higher-quality donor kidneys?
 Nephrol Dial Transplant (2017) 32: 1934 1938 doi: 10.1093/ndt/gfx257 Advance Access publication 21 August 2017 Does Kidney Donor Risk Index implementation lead to the transplantation of more and higher-quality
Nephrol Dial Transplant (2017) 32: 1934 1938 doi: 10.1093/ndt/gfx257 Advance Access publication 21 August 2017 Does Kidney Donor Risk Index implementation lead to the transplantation of more and higher-quality
Protocol Development: The Guiding Light of Any Clinical Study
 Protocol Development: The Guiding Light of Any Clinical Study Susan G. Fisher, Ph.D. Chair, Department of Clinical Sciences 1 Introduction Importance/ relevance/ gaps in knowledge Specific purpose of the
Protocol Development: The Guiding Light of Any Clinical Study Susan G. Fisher, Ph.D. Chair, Department of Clinical Sciences 1 Introduction Importance/ relevance/ gaps in knowledge Specific purpose of the
In the United States, the Model for End-Stage Liver. Re-weighting the Model for End-Stage Liver Disease Score Components
 GASTROENTEROLOGY 2008;135:1575 1581 Re-weighting the Model for End-Stage Liver Disease Score Components PRATIMA SHARMA,* DOUGLAS E. SCHAUBEL,, CAMELIA S. SIMA,, ROBERT M. MERION,, and ANNA S. F. LOK* *Division
GASTROENTEROLOGY 2008;135:1575 1581 Re-weighting the Model for End-Stage Liver Disease Score Components PRATIMA SHARMA,* DOUGLAS E. SCHAUBEL,, CAMELIA S. SIMA,, ROBERT M. MERION,, and ANNA S. F. LOK* *Division
Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Is There a Place for Resection?
 ORIGINAL ARTICLE Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Is There a Place for Resection? Elena Fernandez-Sevilla, 1 Marc-Antoine Allard, 1-3 Jasmijn Selten, 1 Nicolas Golse,
ORIGINAL ARTICLE Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Is There a Place for Resection? Elena Fernandez-Sevilla, 1 Marc-Antoine Allard, 1-3 Jasmijn Selten, 1 Nicolas Golse,
K For patients who have never been tested for HCV, it is. K It is suggested that HCV-infected patients not previously
 http://www.kidney-international.org & 2008 DIGO Guideline 4: Management of HCV-infected patients before and after kidney transplantation idney International (2008) 73 (Suppl 109), S53 S68; doi:10.1038/ki.2008.87
http://www.kidney-international.org & 2008 DIGO Guideline 4: Management of HCV-infected patients before and after kidney transplantation idney International (2008) 73 (Suppl 109), S53 S68; doi:10.1038/ki.2008.87
Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany
 Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany PHC 2018 - www.aphc.info Disclosures Advisory boards:
Hepatitis C: Difficult-to-treat Patients 11th Paris Hepatology Conference 16th January 2018 Stefan Zeuzem, MD University Hospital, Frankfurt, Germany PHC 2018 - www.aphc.info Disclosures Advisory boards:
The New Kidney Allocation Policy: Implications for Your Patients and Your Practice
 The New Kidney Allocation Policy: Implications for Your Patients and Your Practice Clinical Practice Today CME Co-provided by Learning Objectives Upon completion, participants should be able to: Explain
The New Kidney Allocation Policy: Implications for Your Patients and Your Practice Clinical Practice Today CME Co-provided by Learning Objectives Upon completion, participants should be able to: Explain
3 Workshop on HCV THERAPY ADVANCES New Antivirals in Clinical Practice
 3 Workshop on HCV THERAPY ADVANCES New Antivirals in Clinical Practice Rome, 13 December 2013 Management and monitoring of HCC in the future era of DAA s Prof. Massimo Colombo Chairman Department of Liver,
3 Workshop on HCV THERAPY ADVANCES New Antivirals in Clinical Practice Rome, 13 December 2013 Management and monitoring of HCC in the future era of DAA s Prof. Massimo Colombo Chairman Department of Liver,
Disparities in Liver Transplant Allocation: An Update on MELD Allocation System
 Disparities in Liver Transplant Allocation: An Update on MELD Allocation System Naudia L. Jonassaint, MD MHS Assistant Professor of Medicine and Surgery University of Pittsburgh School of Medicine Historical
Disparities in Liver Transplant Allocation: An Update on MELD Allocation System Naudia L. Jonassaint, MD MHS Assistant Professor of Medicine and Surgery University of Pittsburgh School of Medicine Historical
Survival of Liver Transplant Recipients With Hemochromatosis in the United States
 GASTROENTEROLOGY 2007;133:489 495 Survival of Liver Transplant Recipients With Hemochromatosis in the United States LEI YU*, and GEORGE N. IOANNOU*, *Division of Gastroenterology, Department of Medicine
GASTROENTEROLOGY 2007;133:489 495 Survival of Liver Transplant Recipients With Hemochromatosis in the United States LEI YU*, and GEORGE N. IOANNOU*, *Division of Gastroenterology, Department of Medicine
Hepatocellular Carcinoma Recurrence After Liver Transplantation: an Analysis of Risk Factors and Incidence from Oregon Health & Science University
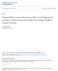 Portland State University PDXScholar University Honors Theses University Honors College 2016 Hepatocellular Carcinoma Recurrence After Liver Transplantation: an Analysis of Risk Factors and Incidence from
Portland State University PDXScholar University Honors Theses University Honors College 2016 Hepatocellular Carcinoma Recurrence After Liver Transplantation: an Analysis of Risk Factors and Incidence from
Increased hepatocellular carcinoma recurrence in women compared to men with high alpha fetoprotein at liver transplant
 ORIGINAL ARTICLE July-August, Vol. 15 No. 4, 2016: 545-549 545 The Official Journal of the Mexican Association of Hepatology, the Latin-American Association for Study of the Liver and the Canadian Association
ORIGINAL ARTICLE July-August, Vol. 15 No. 4, 2016: 545-549 545 The Official Journal of the Mexican Association of Hepatology, the Latin-American Association for Study of the Liver and the Canadian Association
Despite recent advances in the care of patients with
 Liver Transplantation for Hepatocellular Carcinoma: Lessons from the First Year Under the Model of End- Stage Liver Disease (MELD) Organ Allocation Policy Francis Y. Yao, 1,2 Nathan M. Bass, 1 Nancy L.
Liver Transplantation for Hepatocellular Carcinoma: Lessons from the First Year Under the Model of End- Stage Liver Disease (MELD) Organ Allocation Policy Francis Y. Yao, 1,2 Nathan M. Bass, 1 Nancy L.
INTRODUCTION. Journal of Surgical Oncology 2014;109:
 2014;109:533 541 Transplant Versus Resection for the Management of Hepatocellular Carcinoma Meeting Milan Criteria in the MELD Exception Era at a Single Institution in a UNOS Region with Short Wait Times
2014;109:533 541 Transplant Versus Resection for the Management of Hepatocellular Carcinoma Meeting Milan Criteria in the MELD Exception Era at a Single Institution in a UNOS Region with Short Wait Times
Supervivencia a 5 años de Pacientes Coinfectados por VHC-VIH Trasplantados Hepáticos: un Estudio de Casos y Controles
 I Congreso de GESIDA Madrid, 21-24 de Octubre del 2009. Supervivencia a 5 años de Pacientes Coinfectados por VHC-VIH Trasplantados Hepáticos: un Estudio de Casos y Controles José M. Miró, 1 Miguel Montejo,
I Congreso de GESIDA Madrid, 21-24 de Octubre del 2009. Supervivencia a 5 años de Pacientes Coinfectados por VHC-VIH Trasplantados Hepáticos: un Estudio de Casos y Controles José M. Miró, 1 Miguel Montejo,
Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008
 Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Comparing Living Donor and Deceased Donor Liver Transplantation: A Matched National Analysis From 2007 to 2012
 LIVER TRANSPLANTATION 20:1347 1355, 2014 ORIGINAL ARTICLE Comparing Living Donor and Deceased Donor Liver Transplantation: A Matched National Analysis From 2007 to 2012 Richard S. Hoehn, 1 Gregory C. Wilson,
LIVER TRANSPLANTATION 20:1347 1355, 2014 ORIGINAL ARTICLE Comparing Living Donor and Deceased Donor Liver Transplantation: A Matched National Analysis From 2007 to 2012 Richard S. Hoehn, 1 Gregory C. Wilson,
RECURRENT HEPATITIS C CIRRHOSIS AFTER LIVER TRANSPLANTATION: A NATURAL HISTORY STUDY
 RECURRENT HEPATITIS C CIRRHOSIS AFTER LIVER TRANSPLANTATION: A NATURAL HISTORY STUDY By VIRGINIA C. CLARK A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF
RECURRENT HEPATITIS C CIRRHOSIS AFTER LIVER TRANSPLANTATION: A NATURAL HISTORY STUDY By VIRGINIA C. CLARK A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF
Underutilization of Living Donor Liver Transplantation in the United States: Bias against MELD 20 and Higher
 Original Article Underutilization of Living Donor Liver Transplantation in the United States: Bias against MELD 20 and Higher Ryan B. Perumpail 1, Eric R. Yoo 2, George Cholankeril 3, Lupe Hogan 1, Melodie
Original Article Underutilization of Living Donor Liver Transplantation in the United States: Bias against MELD 20 and Higher Ryan B. Perumpail 1, Eric R. Yoo 2, George Cholankeril 3, Lupe Hogan 1, Melodie
