Mutation in VMAT2 Causing a Pediatric Neurotransmitter Disease
|
|
|
- Marilyn Walton
- 5 years ago
- Views:
Transcription
1 Mutation in VMAT2 Causing a Pediatric Neurotransmitter Disease by Jennifer Rilstone A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Institute of Medical Science University of Toronto Copyright by Jennifer Rilstone 2015
2 Mutation in VMAT2 Causing a Pediatric Neurotransmitter Disease Abstract Jennifer Rilstone Doctor of Philosophy Institute of Medical Science University of Toronto 2015 This thesis describes a new disease encompassing infantile-onset movement disorder (including severe parkinsonism and nonambulation), mood disturbance, autonomic instability, and developmental delay that was identified in eight cousins of a consanguineous Bedouin family in Saudi Arabia. The autosomal recessive disease was hypothesized to be a disorder of monoamine neurotransmission, and evidence supporting its causation by a mutation in SLC18A2 (which encodes the vesicular monoamine transporter 2 [VMAT2]) was acquired by single nucleotide polymorphism (SNP) genotyping, homozygosity analysis, and exome sequencing. VMAT2 translocates dopamine and serotonin into synaptic vesicles and is essential for motor control, stable mood, and autonomic function. The loss of VMAT2 function was further confirmed through biochemical assay of serotonin uptake. Consistent with a defect in vesicular monoamine transport, treatment of the patients with levodopa was associated with worsening, whereas treatment with direct dopamine agonists was followed by immediate ambulation, near-complete correction of the movement disorder, and resumption of development. Understanding the underlying mechanism of this disorder extends the spectrum of known pediatric neurotransmitter diseases, serves as the first demonstration of mutation in VMAT2 causing a human phenotype, and thereby provides new insight into the role of VMAT2 in monoamine homeostasis. This thesis additionally demonstrates the utility of implementing genomic diagnosis in the clinic, with ii
3 respect to providing simple and effective treatments in a timely manner to improve outcomes for patients with rare inborn errors of metabolism. iii
4 Acknowledgments I would first like to thank my supervisor, Dr. Berge Minassian, for his consistent support throughout this degree, and more importantly infinite patience. He taught me to think foremost about the patients and their families, and that perspective motivated me when the work itself was a struggle. I would also like to thank the members of my advisory committee, Dr. Lucy Osborne and Dr. Stephen Scherer, for timely strategic guidance and their support particularly with the decision to submit to NEJM and the decision to write. I would additionally like to thank the members of the Minassian laboratory. Thank you to Peter Wang and Xiaochu Zhao for their incredible technical expertise. I d also like to thank the summer students who worked with me over the years for giving me the opportunity to teach and for demonstrating enthusiasm for this project: Tarek Abdelhalim, John Bilbily, Ari Damla, and Alex Bilbily. Support and encouragement through completion of this thesis were appreciated, with particular thanks due to all those who routinely asked me whether I was done yet. Finally, I d like to thank my parents, David and Karen Rilstone, for being unendingly supportive through a nonlinear path to a place in which I m settled and happy. I d also like to thank Katie Mercer, mostly for existing, also for being curious and driven and inspiring me through example to pursue new opportunities with confidence. This work was supported by a Vanier Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC). iv
5 Contributions The intellectual content of this thesis, including the planning and direction of experiments, are attributable to the author. In Chapter 2, clinical characterization of the disease was performed in collaboration with Dr. Reem Alkhater. As described throughout the text of the chapter, Dr. Alkhater identified this family in clinical practice, performed neurological examinations and coordinated clinical investigations (including the collection of urine and cerebrospinal fluid), collected written informed consent for participation in the study, and collected blood samples from members of the extended family for genetic analyses. Dr. Alkhater maintained contact with the family and their physicians, providing treatment guidance and obtaining follow-up information. Ethical approval for the study, including blood collection, was sought by the author. Genetic analyses of candidate genes for pediatric neurotransmitter disorder were performed by the author. Interpretations of CSF and urine analyses and the underlying pathophysiology of the disorder were determined through discussions among Dr. Alkhater, Dr. Berge Minassian, and the author. In Chapter 3, all genetic investigations were conceived and coordinated by the author. As described in the methods section of the chapter, single nucleotide polymorphism analysis was contracted through the University of Helsinki, and whole exome sequencing was performed by The Center for Advanced Genomics at the Hospital for Sick Children. All mutation screening, linkage and homozygosity analyses, and analyses of exome data were performed by the author, as well as database searches and bioinformatic analyses of the described variant. In Chapter 4, all functional analyses were conceived and performed by the author. Acknowledgement is due to Dr. Robert Edwards and members of his laboratory for providing v
6 guidance in performing the serotonin uptake assays, some of which were completed by the author in a visit to his laboratory. In Chapter 5, all future experiments have been conceived and planned by the author. Cloning steps and the construction of cell lines described in this chapter were performed by the author, with gratitude to Xiaochu Zhao for troubleshooting and completing the final step of the mouse construct, and to the aforementioned summer students for assistance with cell culture maintenance and PCR. vi
7 Table of Contents Acknowledgments... iv Contributions... v Table of Contents...vii List of Tables... xi List of Figures... xiii List of Abbreviations... xviii Chapter 1 Literature Review Movement Disorder and Monoamine Deficiency Pathophysiology The Biogenic Amines Parkinson s Disease Pediatric Neurotransmitter Diseases GTP Cyclohydrase I (GTPCH1) Deficiency Sepiapterin Reductase (SR) Deficiency Pyruvoyl Tetrahydropterin Synthase (PTPS) Deficiency Dihydropteridine Reductase (DHPR) Deficiency Pterin-4α-Carbinolamine (PCD) Deficiency Tyrosine Hydroxylase (TH) Deficiency Aromatic Amino Acid Decarboxylase (AADC) Deficiency Dopamine Transporter Deficiency Syndrome (DTDS) Dopamine β-hydroxylase Deficiency Secondary neurotransmitter disorders and related diseases Phenotypic Spectrum of Pediatric Neurotransmitter Disorders Diagnosis of Monoamine Deficiencies Vesicular Monoamine Transporters VMAT Isolation VMAT Structure VMAT Biochemistry Role of VMAT2 in Biogenic Amine Physiology vii
8 2.4.1 Vmat2 Knockout Mice Vmat2 Heterozygous Mice Constitutive Overexpression of Vmat2 in Mice Selective Deletion of Vmat2 in Serotonergic Neurons Selective Expression of Vmat2 in Noradrenergic Neurons Role of VMAT2 in Disease Processes VMAT2 and Parkinson s Disease VMAT2, Neuropsychiatric Phenotypes, and Drugs of Abuse Genetic Variation in VMAT Thesis Overview Chapter 2 Clinical Characterization of a New Pediatric Neurotransmitter Disease Introduction Methods Patients General Investigations Clinical Measurement of Neurotransmitter Metabolites AADC Enzyme Test Candidate Gene Sequencing Results Patient History and Neurological Examination Investigations Family Structure Genetic Screening of Candidate Genes Drug Response Summary of Clinical Features and Differential Diagnosis Discussion Chapter 3 Identification of Disease-Causing Genetic Variant Introduction Methods Patient Samples Genotyping of Single Nucleotide Polymorphisms viii
9 2.3 Homozygosity Analysis Linkage Analysis Mutation Screening Whole-Exome Sequencing TaqMan SNP Genotyping Assay Results Homozygosity Analysis Linkage Analysis Characterization of Genes in the Locus Whole-Exome Sequencing of the Proband Identification of VMAT2 Variant as a Causative Candidate Controls Discussion Chapter 4 Functional Characterization of the Disease-Causing Variant Introduction Methods Construct Design and Site-Directed Mutagenesis Cell Culture and Transfection Serotonin Uptake Assay Western Blotting Sucrose Gradient Centrifugation Results Steady-State Protein Levels of Transfected Protein Unaffected by P387L Variant in Heterologous System Highly Reduced Transport Activity of VMAT2 p.p387l in Heterologous System Measurable Residual Transport Activity of VMAT2 p.p387l Protein Subcellular Localization of Protein in Heterologous System Discussion Chapter 5 General Discussion and Future Directions Introduction ix
10 2 Identification of the VMAT2 p.p387l Mutation: Impact on Knowledge of Monoamine Physiology Future Directions and Experiments Subcellular Localization of VMAT2 p.p387l Additional Biochemical Characterization of VMAT2 p.p387l Development of a Mouse Model Expression Vmat2 p.p390l VMAT2 Mutation in PND: Impact on Understanding of PND Pathogenesis Future Directions and Experiments F-DOPA and 11 C-DTBZ PET scans Screening of Patient Cohorts to Identify Novel SLC18A2 Variants VMAT2 Mutation: Impact on Approaches to PND Diagnosis Future Directions and Experiments Development of Methodology to Measure Biogenic Amines Directly in CSF Development of VMAT2 Platelet Activity Assay Use of Next-Generation Sequencing for Genetic Diagnosis of Rare Monogenic Diseases Knowledge Translation Challenges and Strategies for PND Diagnosis Concluding Statement References x
11 List of Tables Table 1 Causative genes and modes of inheritance for monoamine neurotransmitter diseases 11 Table 2 Metabolites of biogenic amine neurotransmitters and pterin profiles in the cerebrospinal fluid of patients with pediatric neurotransmitter diseases Table 3 Primer sequences for the amplification of exons of candidate genes associated with known pediatric neurotransmitter diseases Table 4 Serum metabolic screen revealed no abnormalities Table 5 Cerebrospinal fluid and urine neurotransmitters and their metabolites measured in a younger affected sibling of the proband reveal decreased monoamines and elevated metabolites in urine, but not in cerebrospinal fluid. Values outside reference ranges are presented in bold.. 52 Table 6 Pediatric neurotransmitter disease associated genes screened in the proband revealing no putative mutations Table 7 Age at initation of dopamine agonist affects disease course Table 8 Clinical features common to all affected individuals in the pedigree Table 9 Clinical features of the disease organized by category Table 10 Comparison of clinical features of the present disease with those of closely related monoamine deficiency syndromes Table 11 Comparison of metabolite profiles in cerebrospinal fluid for pediatric neurotransmitter diseases, including VMAT2 deficiency Table 12 Primer sequences for amplification of exons of candidate genes located at 10q Table 13 Primer sequences for amplification of SLC18A2 exons Table 14 Genes of known neuronal function or localization present in the disease-associated bomozygous region xi
12 Table 15 Sequence variants identified among eight candidate genes in the disease-associated locus by direct sequencing of exons Table 16 Primers to amplify SLC18A2 cdna sequence for subcloning into pcdna Table 17 Oligonucleotides to introduce cytosine to thymine substitution at position 1160 of SLC18A2 using site-directed mutagenesis Table 18 Primers for the integration of the HA epitope at Arg94 of VMAT2 using site-direted mutagenesis Table 19 Primer sequences to amplify a probe for Slc18a2 exon 13 in mouse Table 20 Primer sequences to amplify the upstream and downstream arms of the gene-targeting construct 130 Table 21 Oligonucleotide sequences for site-directed mutagenesis of gene-targeting construct to introduce the p.p390l variant in the expressed protein Table 22 Sequencing primer sequences for gene-targeting construct Table 23 Primer sequences for generating labelled probes to identify the neomycin selection cassette by Southern blot xii
13 List of Figures Figure 1 Schematic diagram of the direct and proposed indirect pathways of the basal ganglia; (a) direct/striatonigral pathway, (b) indirect/striatopallidal pathways. The overall effect on motor thalamus of pathways in (b) are equivalent, and opposite in sign to (a)... 3 Figure 2 Monoaminergic neurotransmission exists as a balance of several critical processes: biosynthesis, packaging, release, degradation, and reuptake. VMAT2, vesicular monoamine transporter Figure 3 Common and parallel pathways for the synthesis and degradation of biogenic amines. AADC, aromatic amino acid decarboxylase; BH 4, tetrahydrobiopterin cofactor; COMT, catechol-o-methyltransferase;; DAT, plasma membrane dopamine transporter;; DβH, dopamine β- hydroxylase; 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid; 5-HTP, 5- hydroxytryptophan; MAO, monoamine oxidase; MHPG, 3-methoxy-4-hydroxyphenylglycol; 3-OMD, 3-O-methyldopa; qbh 2, quinonoid dihydrobiopterin; PNMT, phenylethanolamine N-methyltransferase; TH, tyrosine hydroxylase; TPH, tryptophan hydroxylase; VMA, vanillylmandelic acid; VMAT2, vesicular monoamine transporter Figure 4 Synthesis and regeneration of tetrahydrobiopterin the conversion of BH 4 to PCBD is coupled to the generation of dopamine or serotonin. AR, aldose reductase; BH 4, tetrahydrobiopterin; DHPR, dihydropteridine reductase; GTPCH, GTP cyclohydrolase 1; H 2 NP 3, dihydroneopterin triphosphate; PCBD, tetrahydrobiopterin-α-carbinolamine; PCD, pterin-4αcarbinolamine dehydratase; 6-PTP, 6-pyruvoyltetrahydropterin; qbh 2, quinonoid dihydrobiopterin; PTPS, 6-pyruvoyltetrahydropterin synthase; SP, sepiapterin; SR, sepiapterin reductase. 14 Figure 5 T2-weighted magnetic resonance images of proband at age 14 revealed no abnormalities Figure 6 Family structure of kindred demonstrates autosomal recessive mode of inheritance and consanguineous pedigree structure. Proband is labelled V:6. Cerebrospinal fluid and urine neurotransmitter analyses were performed on individual V:10. Black, affected; white, unaffected; square, male; circle, female; diamond, spontaneous abortion. Numbers in squares or xiii
14 circles indicate number of male or female offspring, respectively. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission Figure 7 Single nucleotide polymorphism alleles for all genotyped family members in the homozygous region uniquely shared by affected family members. Black, affected; white, unaffected; square, male; circle, female. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission Figure 8 Multipoint LOD scores estimated across chromosome Figure 9 Multipoint LOD scores estimated across chromosomes 3 and Figure 10 Genes present within the homozygous region shared by all affected individuals in the family. C10orf82, chromosome 10 open reading frame 82; CACUL 1, CDK2-assocaited cullin domain 1; CASC2, cancer susceptibility candidate 2 (non-protein coding); CCDC172, coiled-coil domain containing 172; EIF3A, eukaryotic translation factor 3 subunit A; EMX2, empty spiracles homeobox 2; ENO4, enolase family member 4; FAM204A, family with sequence similarity 204 member A; GFRA1, GDNF family receptor alpha 1; GRK5, G protein coupled kinase 5; HSPA12A, heat shock 70 kda protein 12A; KCNK18, potassium channel subfamily K member 18; KIAA1598, Shootin1; MIR, microrna; PDZD8, PDZ domain containing 8; PNLIP, pancreatic lipase; PNLIPRP, pancreatic lipase related protein; PRDX3, peroxiredoxin 3; PRLHR, prolactin-releasing hormone receptor; RAB11FIP1, RAB11 family interacting protein 2 (class I); SFXN4, sideroflexin 4; SLC18A2, solute carrier family 18 member 2; SNORA19, small nucleolar RNA H/ACA box 19; VAX1, ventral anterior homeobox 1. Image from UCSC Genome Browser [ 84 Figure 11 Electropherogram depicting sequence variation in unaffected and affected family members. Top panel, unaffected sibling with wild-type genomic sequence; middle panel, asymptomatic parent (IV:3) possesses the SLC18A2 c.1160c T variant in heterozygous form;; lower panel, proband (V:6) is homozygous for SLC18A2 c.1160c T. From New England xiv
15 Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission Figure 12 Predicted structure of VMAT2 comprises 12 transmembrane domains, a large lumenal loop including four proposed glycosylation sites, and both N-terminal and C-terminal cytoplasmic regions. Proline residue 387 is located immediately adjacent to the insertion of transmembrane domain X. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission Figure 13 Multiple sequence alignment of VMAT2 in the region of the p.p387l variant. TM9 and TM10 represent sequences associated with transmembrane domains 9 and 10, respectively. Residues that differ from human VMAT2 sequence are indicated in gray. Amino acid position 387 is indicated by an asterisk. Homo, homo sapiens (human); Patient, proband; Pan, Pan troglodytes (chimpanzee); Macaca, Macaca mulatta (Rhesus macaque); Mus, Mus musculus (mouse); Rattus, Rattus norvegicus (rat); Canis, Canis familiaris (dog); Bos, Bos taurus (cow); Monodelphis, Monodelphis domestica (opossum); Gallus, Gallus gallus (chicken); Tetraodon, Tetraodon nigroviridis (pufferfish); Danio, Danio rario (zebrafish); Drosophila, Drosophila melanogaster (fruit fly); C. elegans, Caenorhabditis elegans (nematode); hvmat1, human VMAT1 isoform. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission Figure 14 Visualization of ATP by absorbance at 260 nm to illustrate sucrose gradient linearity 99 Figure 15 Consistent fractionation demonstrated by absorbance at 280 nm of parallel sucrose gradient fractionations Figure 16 Markers of cellular compartments in sucrose gradient fractions Figure 17 Parallel transient transfections of wild-type and p.p387l VMAT2 in Cos7 cells, and vector-transfected control. GAPDH, protein loading control xv
16 Figure 18 Comparison of banding pattern observed for wild-type and p.p387l VMAT2 expressed transiently in Cos7 cells. unt, untransfected; WT, wild-type Figure 19 Vesicular uptake of tritiated serotonin by the wild-type and p.p387l human VMAT2 transiently expressed in a heterologous Cos7 cell system. Inset: Expression of VMAT2 in lysates used for uptake assay. 5-HT, serotonin; wt, wild-type. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission Figure 20 Vesicular uptake of tritiated serotonin by wild-type and p.p387l human VMAT2 transiently expressed in a heterologous Cos7 cell system after 10 minutes with and without the addition of the specific VMAT inhibitor reserpine (10 µm). From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission Figure 21 Sucrose gradient centrifugation of transiently transfected Cos7 lysates Figure 22 Expression of wild-type and p.p387l VMAT2 in stable clones of the Cos7 cell line; band at 37 kda represents GAPDH loading control Figure 23 Expression of wild-type and p.p387l VMAT2 in stable clones of the MN9D cell line 120 Figure 24 Schematic of incorporation of mouse gene targeting construct into mouse genome. Upper panel, wild type mouse genomic sequence of Slc18a2 exons and flanking intronic sequences. Middle panel, mouse genomic sequence after incorporation of the gene-targeting construct by homologous recombination with the long arms of the construct in embryonic stem cells. Slc18a2 c.1169c T mutation, coding for the P390L amino acid substitution, is present in exon 13. Lower panel, mouse genomic sequence after expression of Cre recombinase and excision of the selection cassette. PGKneobpA, neomycin selection cassette; loxp, recombination sites targeted by Cre recombinase xvi
17 Figure 25 Gene targeting construct for the introduction of the c.1169c T mutation into Slc18a2. AmpR, ampicillin resistance gene; F1_origin, origin of replication; loxp, recombination site for Cre recombinase; NeoR/KanR, neomycin and kanamycin resistance gene (of the PGKneobpA selection cassette); NheI, HindIII, restriction sites for insertion of downstream arm of genomic sequence for recombination; NotI, SacII, restriction sites for insertion of upstream arm of genomic sequence for recombination. Gray boxes, mouse intronic sequence of Slc18a2; black boxes, mouse exonic sequence of Slc18a xvii
18 List of Abbreviations AADC aromatic amino acid decarboxylase AR aldose reductase ATP adenosine triphosphate BH 4 tetrahydrobiopterin CHO Chinese hamster ovary CIHR Canadian Institutes of Health Research COMT catechol-o-methyltransferase CP cerebral palsy CSF cerebrospinal fluid DA dopamine DAT plasma membrane dopamine transporter DβH dopamine β-hydroxylase DDC encodes AADC DHPR dihydropteridine reductase DMEM Dulbecco s modified Eagle medium xviii
19 DOPAC 3,4-dihydroxyphenylacetic acid DRD dopa-responsive dystonia DTBZ dihydrotetrabenazine DTDS dopamine transporter deficiency syndrome E epinephrine EEG electroencephalogram ELISA enzyme-linked immunosorbant assay ER endoplasmic reticulum ES embryonic stem GAPDH glyceraldehyde 3-phosphate dehydrogenase GPi globus pallidus internus GCH1 encodes GTPCH1 GTP guanosine-5'-triphosphate GTPCH1 GTP cyclohydrolase I 5-HIAA 5-hydroxyindoleacetic acid HIS histamine xix
20 H 2 NP 3 dihydroneopterin triphosphate HPLC-EC high-performance liquid chromatography with electrochemical detection 5-HT serotonin 5-HTP 5-hydroxytryptophan HVA homovanillic acid kda kilodalton LC locus coerulus LC-MS/MS liquid chromatography tandem mass spectrometry LDCV large dense-core vesicles LOD logarithm of odds MAO monoamine oxidase MFS major facilitator superfamily MHPG 3-methoxy-4-hydroxyphenylglycol MPP + 1-methyl-4-phenylpyridinium; active metabolite of MPTP MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine MRI magnetic resonance imaging xx
21 NBIA neurodegeneration with brain iron accumulation NE norepinephrine NHLBI National Heart, Lung, and Blood Institute 3-OMD 3-O-methyldopa PBS phosphate-buffered saline PCBD tetrahydrobiopterin-α-carbinolamine PCBD encodes PCD PCD pterin-4α-carbinolamine dehydratase PD Parkinson s disease PET positron emission tomography PKU phenylketonuria PLP pyridoxal phosphate PND pediatric neurotransmitter disorder PNMT phenylethanolamine N-methyltransferase PNPO pyridoxamine 5 -phosphate oxidase 6-PTP 6-pyruvoyltetrahydropterin xxi
22 PTPS 6-pyruvoyl tetrahydropterin synthase PTS encodes PTPS PVDF polyvinylidene fluoride qbh 2 quinonoid dihydrobiopterin QDPR encodes DHPR SCA spinocerebellar ataxia SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis SERT serotonin plasma membrane transporter SLC6A3 encodes DAT SLC18A2 encodes VMAT2 SNP single nucleotide polymorphism SNpc substantia nigra pars compacta SNR substantia nigra pars reticulata SP sepiapterin SPR encodes SR SR sepiapterin reductase xxii
23 SSV small synaptic vesicles TBS-T 50 mm Tris-HCl, ph 7.5, 150 mm NaCl, 0.05% Tween 20 TfR transferrin receptor TH tyrosine hydroxylase TMD transmembrane domain TPH tryptophan hydroxylase VAChT vesicular acetylcholine transporter VMA vanillylmandelic acid VMAT vesicular monoamine transporter VTA ventral tegmental area xxiii
24 Chapter 1 Literature Review 1 Movement Disorder and Monoamine Deficiency Movement disorders are a large group of diseases in which patients suffer impairment of the planning, control or execution of movement (Klein, 2005). The clinical spectrum of movement disorders includes, but is not limited to, ataxia, blepharospasm, dysphonia, dystonic disorders, gait disorders, Huntington s disease, myoclonus, Parkinson s disease, spasticity, tardive dyskinesia, tics and Tourette syndrome, and tremor (Klein, 2005). They are clinically, pathologically, and genetically heterogeneous. Genes for many are already known (Klein, 2005). Despite this, the pathogenic mechanisms of the vast majority of these movement disorders are still being elucidated. For example, the prototypical movement disorder, Parkinson s disease, is characterized by neurodegeneration of the dopamine-secreting neurons of the substantia nigra pars compacta (SNpc), and a deficit of dopamine in the brain (see Section 1.3). This results in bradykinesia (slowing of physical movement), rigidity, postural instability, and rest tremor. The molecular mechanisms leading to this pathology are still unclear despite the discovery of 11 distinct genes causing monogenic forms of the disease, as well as a number of associations with additional genes and loci associated with sporadic forms of the disease (Singleton et al., 2013). 1.1 Pathophysiology The underlying pathophysiology of Parkinson s disease and related movement disorders involves misregulation of a series of structures comprising the basal ganglia. 1
25 The basal ganglia function in the fine modulation of motor activity. Input from the cortex is received by the striatum (input nucleus), which in turn regulates the output nuclei the substantia nigra pars reticula (SNR) and globus pallidus internus (GPi). The output nuclei tonically inhibit the motor thalamus, and therefore motor activity. By current models, this occurs through the structures of the basal ganglia via two possible pathways the direct (striatonigral) or indirect (striatopallidal) pathway (Figure 1) (Miller, 2008). Inhibitory striatal neurons can be identified as members of either of these two pathways by the presence of peptide markers (substance P or enkephalin, respectively). 2
26 Figure 1 Schematic diagram of the direct and proposed indirect pathways of the basal ganglia; (a) direct/striatonigral pathway, (b) indirect/striatopallidal pathways. The overall effect on motor thalamus of pathways in (b) are equivalent, and opposite in sign to (a). 3
27 The role of dopamine in the basal ganglia is in regulation of the striatum. Dopaminergic neurons originate in the SNpc, and project their axons to innervate and regulate the striatum via D1- and D2-class receptors. At physiological concentrations, dopamine is sequestered in vesicles of dopaminergic neurons and undergoes regulated exocytotic release at the synapse. However, it has been demonstrated that at higher, pathological concentrations, unsequestered cytosolic dopamine can cause toxicity with the formation of reactive oxygen species generated by dopamine auto-oxidation or monoamine oxidase (MAO) metabolism (Cubells et al., 1994, Graham, 1978, Hastings et al., 1996). This natural dopamine toxicity is a suggested mechanism underlying the progressive nigrostriatal neurodegeneration in Parkinson s disease (see Section 1.3). 1.2 The Biogenic Amines The biogenic amines (also referred to as monoamines) are a class of neurotransmitter that are synthesized enzymatically from amino acids. Serotonin is derived from tryptophan, and is the basis of the serotonergic system. The catecholamines (dopamine, norepinephrine, and epinephrine) are derived from tyrosine. Dopamine is the basis of the dopaminergic system, and norepinephrine and epinephrine underlie the adrenergic system. Histamine is derived from histidine, and is present in neurons of the hypothalamus. The biogenic amines are modulatory in nature (acting on a timescale of seconds to minutes), have mixed effects, and are subject to significant crosstalk. The dopaminergic system comprises the nigrostriatal pathway that projects from the SNpc to the striatum (caudate and putamen), and the mesolimbic and mesocortical projections from the ventral tegmental area (VTA) to the nucleus accumbens and cerebral 4
28 cortex, respectively. There are also dopaminergic projections comprising the tuberoinfundibular pathway. The serotonergic system comprises widespread projections from the dorsal raphe nuclei to many regions of the brain, including the neocortex, amygdala, hippocampus, thalamus, hypothalamus, striatum, cerebellum, brain stem, and spinal cord. The noradrenergic system also involves widespread projections from the locus coeruleus to the neocortex, amygdala, hippocampus, thalamus, hypothalamus, tectum, cerebellar cortex, visceral cranial nuclei, and spinal cord. Both norepinephrine and epinephrine also function peripherally in sympathetic ganglion cells. Histamine is present in the hypothalamus, where it plays an integral role in neurometabolic functions (e.g., wakefulness, appetite regulation, response to pain, and immunological response). Monoaminergic neurotransmission exists as a balance of several critical processes: biosynthesis, packaging, release, degradation, and reuptake. First, transmitters are synthesized from their amino acid precursors in the cytosol. Next, they are accumulated into synaptic vesicles through the action of transporters that are driven by the proton gradient. Further biochemical transformation occurs within the synaptic vesicle in the case of norepinephrine. Neurotransmission occurs by way of regulated exocytotic release from the synaptic vesicles into the synaptic cleft in response to physiologic stimuli. At the synapse, the transmitter interacts with target receptors on the postsynaptic cell to mediate signal transduction. Transmitter at the synapse is then cleared by both degradation and reuptake processes. Specific monoamine metabolizing enzymes are 5
29 present to inactivate the transmitter. Reuptake into the presynaptic cell and glia occurs through plasma membrane monoamine transporters that are driven by Na + and Cl gradients. This model of monoamine neurotransmission and homeostasis is illustrated in Figure 2. The biochemical pathways for the synthesis and degradation of the biogenic amines are presented in Figure 3. 6
30 Figure 2 Monoaminergic neurotransmission exists as a balance of several critical processes: biosynthesis, packaging, release, degradation, and reuptake. VMAT2, vesicular monoamine transporter 2. 7
31 Figure 3 Common and parallel pathways for the synthesis and degradation of biogenic amines. AADC, aromatic amino acid decarboxylase; BH 4, tetrahydrobiopterin cofactor; COMT, catechol-o-methyltransferase; DAT, plasma membrane dopamine transporter;; DβH, dopamine β-hydroxylase; 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid; 5-HTP, 5-hydroxytryptophan; MAO, monoamine oxidase; MHPG, 3-methoxy-4-hydroxyphenylglycol; 3-OMD, 3-O-methyldopa; qbh 2, quinonoid dihydrobiopterin; PNMT, phenylethanolamine N-methyltransferase; TH, tyrosine hydroxylase; TPH, tryptophan hydroxylase; VMA, vanillylmandelic acid; VMAT2, vesicular monoamine transporter 2. 8
32 1.3 Parkinson s Disease Parkinson s disease (PD) is a common, adult-onset neurodegenerative disease characterized by the selective and progressive loss of neuronal subtypes in particular, the nigrostriatal dopaminergic pathway. PD is primarily characterized by the deterioration of motor function, evidenced as bradykinesia, rigidity, postural instability, and rest tremor. In addition, there has been increased recognition of the nonmotor manifestations of PD. These include anosmia, depression, anxiety, sweating, dyspnea, orthostatic hypotension, constipation, pain, genitourinary problems, sexual dysfunction, and sleep disorders (Witjas et al., 2002). These nonmotor symptoms may be grouped into domains: cognitive and psychiatric, autonomic, and sensory/pain. Many of the nonmotor manifestations of PD precede the motor symptoms of the disease, and certainly emerge upon disease progression. Furthermore, the nonmotor symptoms of PD are reported to have a greater impact on patient quality of life (Barone et al., 2009). Evidence of the degeneration of other monoaminergic neuronal types (serotonergic neurons in the raphe nuclei and noradrenergic neurons in the locus coeruleus), as well as cholinergic neurons in the basal forebrain and midbrain tegmentum, establish a broader monoaminergic deficiency phenotype in PD (Hirsch et al., 2003). The etiology and pathogenesis of PD have been the subject of considerable study and reflect significant complexity. PD is primarily sporadic, although multiple monogenic forms of the disease exist (Singleton et al., 2013). Familial and sporadic forms of the disease have nearly identical motor phenotypes. Furthermore, toxins such as 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP) can selectively destroy dopaminergic neurons and acutely cause parkinsonism providing evidence that environmental exposures may 9
33 play a role in the pathogenesis of PD. Notably, the strongest risk factor for PD is advancing age, possibly implicating the aging process in PD pathogenesis. Current thinking regarding PD pathogenesis favors a mixture of pathogenic mechanisms. Multiple avenues of investigation have identified common pathways that may underlie the disease. These include oxidative stress, protein aggregation, defects in the ubiquitin proteasome pathway, autophagy, and alterations in mitochondrial function. The pathologic hallmark of PD is Lewy bodies inclusion bodies comprising α-synuclein and other protein deposits and there has been some evidence of cell-to-cell transfer of these deposits that may be associated with the spread of the disease to other neuronal types (Steiner et al., 2011). 1.4 Pediatric Neurotransmitter Diseases The pediatric neurotransmitter diseases, by contrast, are a group of early onset rare diseases attributable to a disturbance in neurotransmitter metabolism with causative defects in enzymes that are involved in the biosynthesis, degradation, or membrane transport of transmitter. Known disorders specifically of the biogenic amine neuromediators (dopamine, norepinephrine, epinephrine, and serotonin) comprise defects in nine enzymes and one transporter each presenting in early childhood with symptoms and signs referable to the affected neurotransmitter. Deficiency in dopamine results in movement disorder, including parkinsonism and dystonia; deficient norepinephrine and epinephrine cause sympathetic autonomic dysfunction; and serotonin deficiency leads to sleep disturbance and psychiatric disease. The monoamine neurotransmitter disorders of known genetic etiology are summarized in Table 1 Causative genes and modes of inheritance for monoamine neurotransmitter diseases. 10
34 Table 1 neurotransmitter diseases Causative genes and modes of inheritance for monoamine Disorder Causative Gene Mode of Inheritance Autosomal dominant GTP GCH1 Autosomal dominant cyclohydrolase I deficiency (Dopa-responsive dystonia or Segawa disease) Autosomal recessive GTP GCH1 Autosomal recessive cyclohydrolase I deficiency Sepiapterin reductase SPR Autosomal recessive deficiency 6-Pyruvoyltetrahydropterin PTS Autosomal recessive synthase deficiency Dihydropteridine reductase QDPR Autosomal recessive deficiency Pterin-4α-carbinolamine PCD Autosomal recessive dehydratase deficiency Tyrosine hydroxylase TH Autosomal recessive deficiency Aromatic L-amino acid DDC Autosomal recessive decarboxylase deficiency Pyridoxal phosphatedependent PNPO Autosomal recessive epilepsy (PLP- DE) Dopamine transporter deficiency syndrome SLC6A3 Autosomal recessive The multiple deficits of PNDs overlap substantially with the combination of dopaminergic motor dysfunction and the domains of nonmotor symptoms in PD. Similarly, evidence of degeneration of other monoaminergic neuronal types (serotonergic neurons in the raphe nuclei and noradrenergic neurons in the locus coeruleus), as well as cholinergic neurons in the basal forebrain and midbrain tegmentum have established the involvement of multiple monoaminergic systems in PD (Hirsch et al., 2003). However, whereas PND are disorders of infancy and childhood, PD is a disease of aging. It thus 11
35 reflects distinct underlying pathogenic mechanisms. Similarly, the manifestations of PNDs are expected to differ from those of PD because the monoaminergic dysfunction occurs during critical periods of neurological development GTP Cyclohydrase I (GTPCH1) Deficiency The longest recognized monoamine neurotransmitter disorder is autosomal dominant GTP cyclohydrolase I (GTPCH1) deficiency that was first described in the 1970s (Segawa et al., 1976), and is more commonly referred to as dopa-responsive dystonia (DRD) or Segawa disease. DRD results from a defect in tetrahydrobiopterin (BH 4 ) synthesis which is a necessary cofactor for the rate-limiting step in dopamine synthesis (tyrosine hydroxylase conversion of tyrosine to L-DOPA). These patients exhibit dystonic spasms with diurnal fluctuation that begin in the first decade of life. The disease is associated with short stature. Adult-onset cases have also been described, in which the primary features are writer s cramp, torticollis, or generalized rigid hypertonus with tremor, but not postural dystonia (Segawa, 2011). The disorder is extremely responsive to treatment with levodopa in combination with a decarboxylase inhibitor (carbidopa). The disease is caused by heterozygous mutations in GCH1 at 14q22.1 q22.2 (Ichinose et al., 1994). More than 100 mutations have been discovered. The autosomal dominant mutations are dominant negative, as a result of an inability to properly form the native homodecameric form of the protein. Less than 10% of identified mutations are found in homozygous or compound heterozygous form, causing an autosomal recessive form of the disease (Thony and Blau, 2006). Several other disorders of tetrahydrobiopterin synthesis or regeneration present as pediatric movement disorder, including defects in 6-pyruvoyl tetrahydropterin synthase 12
36 (PTPS; PTS), sepiapterin reductase (SR; SPR), dihydropteridine reductase (DHPR; QDPR), and pterin-4α-carbinolamine dehydratase (PCD; PCBD) (Longo, 2009). The pathway for tetrahydrobiopterin synthesis and regeneration is presented in Figure 4. Tetrahydrobiopterin is an essential cofactor for tyrosine hydroxylase and tryptophan hydroxylase, as well as phenylalanine hydroxylase, nitric oxide synthases, and glycerylether monooxygenase. Despite its necessity for phenylalanine hydroxylase activity, fewer than 2% of patients with phenylketonuria have mutations in PTS, QDPR, GCH1, or PCBD (Longo, 2009). Laboratory diagnosis of tetrahydrobiopterin deficiency is based on newborn screening for phenylketonuria, urine or dried blood profiling of pterins, and the measurement of DHPR enzyme activity in blood. 13
37 Figure 4 Synthesis and regeneration of tetrahydrobiopterin the conversion of BH 4 to PCBD is coupled to the generation of dopamine or serotonin. AR, aldose reductase; BH 4, tetrahydrobiopterin; DHPR, dihydropteridine reductase; GTPCH, GTP cyclohydrolase 1; H 2 NP 3, dihydroneopterin triphosphate; PCBD, tetrahydrobiopterin-α-carbinolamine; PCD, pterin-4α-carbinolamine dehydratase; 6-PTP, 6-pyruvoyltetrahydropterin; qbh 2, quinonoid dihydrobiopterin; PTPS, 6-pyruvoyltetrahydropterin synthase; SP, sepiapterin; SR, sepiapterin reductase. 14
38 1.4.2 Sepiapterin Reductase (SR) Deficiency SR deficiency, like GTPCH1 deficiency, is not associated with hyperphenylalaninemia but phenylalanine loading tests are often positive. The disease is caused by mutations in the SPR gene at 2p13, and at least 19 mutations have been identified (Thony and Blau, 2006). Symptoms arise in the first decade of life, including psychomotor retardation, dystonia, oculogyric crises, choreoathetosis, hypotonia, spasticity, tremor, ataxia, parkinsonism, seizures, temperature instability, hypersalivation, microcephaly, and irritability, as well as psychiatric symptoms. These symptoms exhibit diurnal variation, as with GTPCH1 deficiency. Alternatively, a mild form of the disease with mild motor symptoms has been reported, associated with a splicing defect leading to a missense mutation with residual protein function (Arrabal et al., 2011). Metabolite profiles in CSF reveal a disturbance of dopamine, norepinephrine, and serotonin synthesis. SR activity can be measured clinically in skin fibroblasts Pyruvoyl Tetrahydropterin Synthase (PTPS) Deficiency PTPS deficiency is the most common disorder of BH 4 metabolism (Leuzzi et al., 2010), caused by mutations in the PTS gene at 11q More than 50 mutations have been described (Thony and Blau, 2006). It exists as both severe and peripheral forms. The severe form manifests in the first few months of life with delayed developmental milestones and movement disorder, as well as hyperphenylalaninemia and risk of premature birth and low birth weight. The peripheral form involves hyperphenylalaninemia but minor or no disturbance in biogenic amine neurotransmitters, as measured by their metabolites in CSF and a normal neurological course. Genotype phenotype correlation is evident in that mutations with high residual enzyme activity 15
39 cause the peripheral form of the disease, whereas mutations causing gross disturbances to protein function (such as frameshift mutations leading to protein truncation or altered protein zinc binding or oligomerization) lead to the severe form of the disease (Brasil et al., 2011) Dihydropteridine Reductase (DHPR) Deficiency DHPR deficiency is the most severe of the disorders of pterin metabolism, caused by mutations in the QDPR gene at 4p DHPR deficiency reflects a defect in the regeneration of tetrahydrobiopterin. It has neonatal onset, or onset in early infancy. Patients exhibit feeding difficulties, bulbar dysfunction, hypersalivation, and microcephaly. They also exhibit delayed motor and cognitive milestones, truncal and limb hypertonia, dyskinesia, tremor, dystonia, choreoathetosis, and seizures. These patients are at risk of sudden death. There are often white matter abnormalities and basal ganglia calcifications visible by MRI (Longhi et al., 1985, Woody et al., 1989). The severity of this particular defect in biopterin metabolism is thought to be related to the concomitant accumulation of q-dihydrobiopterin and its inhibitory effects on AADC, tyrosine hydroxylase, tryptophan hydroxylase, and phenylalanine hydroxylase, as well as depletion of folate in the brain related to the absence of DHPR and the accumulation of q-dihydrobiopterin. A mild form of the disease affects only serotonin metabolism, and is caused by two specific protein mutations (G151S and F212C) (Blau et al., 1992) Pterin-4α-Carbinolamine (PCD) Deficiency PCD deficiency is also a defect in the regeneration of tetrahydrobiopterin resulting from mutations in PCBD at 10q22. It is associated with a mild hyperphenylalaninemia that 16
40 normalizes after a few months of life. There is usually no neurotransmitter phenotype, but transient neonatal hypotonia has been reported in some patients (Thony et al., 1998) Tyrosine Hydroxylase (TH) Deficiency A deficiency of tyrosine hydroxylase is a progressive and lethal encephalopathy with poor prognosis, involving dystonia and other extrapyramidal movement disorder symptoms. It is a rare autosomal recessive disorder caused by mutations in TH at 11p15.5, and reported in less than 40 patients worldwide thus far. Importantly, mutations reflecting a complete loss of function (such as premature truncations) have not been observed in homozygous form, suggesting that a complete lack of tyrosine hydroxylase is incompatible with life (Willemsen et al., 2010). There are two overlapping subtypes: type A, a progressive extrapyramidal movement disorder (hypokinetic rigid syndrome with dystonia) with onset in infancy or childhood; and type B, a complex encephalopathy with onset in the neonatal period or early infancy (Willemsen et al., 2010). Type A comprises approximately 70% of patients who present in infancy with a parkinsonian phenotype of hypokinesia, bradykinesia, dystonia, and rigidity. There can be diurnal variation in the dystonia. An onset in the first year is associated with some mild cognitive impairment. Some patients develop onset of symptoms later (within the first 5 years), with gait instability and difficulty walking. Type B disease has an onset within the first 3 months of life with hypotonia and severe parkinsonism, in combination with mental retardation, dystonia, oculogyric crises, myoclonus, tremor, dyskinesia, and ptosis. Autonomic features (sweating, temperature instability, hyperpyrexia, and drooling) are also observed. Seizures have also been reported in these patients. Notably, 17
41 MRI investigations are usually normal, though some white matter abnormalities and increased extracerebral CSF spaces have been observed in more severe cases Aromatic Amino Acid Decarboxylase (AADC) Deficiency AADC deficiency disorder, for which there have been less than 100 patients diagnosed worldwide (Brun et al., 2010), results from mutations in the aromatic amino acid decarboxylase gene (DDC; also known as dopa decarboxylase) at 7p These patients present with hypotonia in the first six months of life, and further develop extrapyramidal movement disorder symptoms including dystonia, chorea, blepharospasm, bulbar dysfunction, and myoclonus. They further exhibit developmental delay, irritability, sleep disturbances, autonomic manifestations, temperature instability, irritability, and nasal congestion. Most patients exhibit cognitive impairment. AADC activity can be assayed clinically in plasma. MRI is generally normal, although some nonspecific changes have been reported (Brun et al., 2010). Patients generally do not respond to treatment. Briefly, pyridoxal phosphate is an essential cofactor for AADC. Deficiency in the synthesis of this cofactor resulting from mutations in the gene encoding pyridoxamine 5 - phosphate oxidase (PNPO at 17q21.32) leads to prenatal seizures an a severe, potentially fatal, anticonvulsant-resistant neonatal encephalopathy (Mills et al., 2005). CSF metabolite profiles mimic those of AADC deficiency Dopamine Transporter Deficiency Syndrome (DTDS) Whereas the pediatric disorders described thus far reflect deficiencies in the synthesis of one or more biogenic amine neurotransmitters, dopamine transporter (DAT) deficiency 18
42 syndrome (DTDS) is a defect in monoamine transport. Children with homozygous or compound heterozygous mutations in SLC6A3 (coding for DAT) at 5p15.3 present with a complex motor disorder in early infancy that comprises hyperkinetic symptoms, hypokinesia, or a mixed phenotype that involves progressive dystonia, axial hypotonia, and parkinsonism (Kurian et al., 2011b, Kurian et al., 2009). Eye movement disorder (ocular flutter, saccade initiation failure, eyelid myoclonus, and oculogyric crises) is also observed. Patients also exhibit sleeping difficulties, orthopedic complications, and cardiorespiratory insufficiency. The mean life expectancy for patients with DTDS is 13.6 years. Defects in presynaptic dopamine reuptake reduce presynaptic dopamine stores and an accumulation of synaptic dopamine that is susceptible to catabolism. This is reflected by increased HVA concentrations in the CSF. Increased synaptic dopamine may also lead to downregulation of postsynaptic receptor expression (Blackstone, 2009) Dopamine β-hydroxylase Deficiency Dopamine β-hydroxylase deficiency is characterized as a primary autonomic failure stemming from the inability to convert dopamine to norepinephrine or epinephrine (Senard and Rouet, 2006) Secondary neurotransmitter disorders and related diseases Dopamine and serotonin depletion can occur secondary to certain other neurological disorders, including perinatal asphyxia, disorders of folate metabolism, phenylketonuria, Lesch-Nyhan disease, mitochondrial disorders, epilepsy and infantile spasms, 19
43 opsoclonus-myoclonus, pontocerebellar hypoplasia, leukodystrophies, Rett s syndrome, and certain neuropsychiatric disorders (Kurian et al., 2011a). Additionally, there are several other rare disorders that present in the first decade of life and involve a combination of dystonia and parkinsonism complicated by pyramidal tract involvement (Pearl, 2013, Schneider and Bhatia, 2010). These include Wilson s disease;; parkinsonism associated with mutations in parkin (PARK2), PINK1 (PARK6), and DJ1 (PARK7); x-linked dystonia-parkinsonism/lubag (DYT3); rapid-onset dystoniaparkinsonism (DYT12); DYT16 dystonia; neurodegeneration with brain iron accumulation (NBIA, including PANK2 and PLA2G6/PARK14); neuroferritinopathy; Kufor-Rakeb disease; SENDA syndrome; and autosomal recessive spastic paraplegia with thin corpus collosum (SPG11) Phenotypic Spectrum of Pediatric Neurotransmitter Disorders A phenotypic continuum has been proposed for GTPCH1 deficiency and extended to all pediatric monoamine biosynthetic deficiencies (see Table 3 in Pons, 2009). This spectrum illustrates the variability in the presentation of pediatric neurotransmitters disorders that reflects a range of severities stemming from a common underlying pathophysiology. The severity of presentation depends upon the effect of the mutation on enzyme function and the importance of the affected enzyme to biogenic amine availability, on the extent of monoamine deficiency, on the relative involvement of each of the biogenic amines, and on the age of onset and subsequent effect on neurological development. Additional variability in presentation is observed between patients with common defects. 20
44 Diagnosis of Monoamine Deficiencies In addition to clinical signs, several tests may be useful to the diagnosis of pediatric neurotransmitter disorder. Because tetrahydrobiopterin is also a cofactor for phenylalanine hydroxylase, some monoamine deficiencies include peripheral hyperphenylalanemia that may be identified in serum or in neonatal screening. These include autosomal recessive GTP cyclohydrolase deficiency, pterin-carbinolamine dehydratase deficiency, dihydropteridine reductase deficiency, and pyruvoyltetrahydropterin synthase deficiency. In the absence of peripheral hyperphenylalanemia, an oral phenylalanine loading test, in which the ratio of phenylalanine to tyrosine is measured in serum after loading, may be useful to identify defects in tetrahydrobiopterin metabolism (Bandmann et al., 2003, Opladen et al., 2010). Genetic testing of associated genes is reasonably simple, but may not reveal noncoding mutations with functional relevance, and the functional relevance of novel mutations may be unclear. Enzyme activity tests are available for GTPCH activity in fibroblasts, SR activity in fibroblasts, DHPR activity in blood spots, and AADC activity in plasma. These tests are not considered routine, but may be useful in cases of negative genetic screening. Urine metabolite (HVA, 3-OMD, 5-HIAA, VMA; see Figure 3) and pterin profiles (biopterin and neopterin; see Figure 4) may also aid diagnosis. However, as a measurement of metabolites and pterins in the periphery, urine results are not conclusive. An abnormal pterin profile in the urine may help distinguish diseases with peripheral 21
45 hyperphenylalanemia from phenylketonuria (PKU; phenylalanine hydroxylase deficiency). The most conclusive available diagnostic is CSF neurotransmitter metabolite and pterin profiles (see Table 2). Metabolite levels are indirect measures of each neurotransmitter, as well as an indication of turnover. There are, however, several difficulties with this analysis that prohibit routine testing. CSF collection requires lumbar puncture, with inherent risks. CSF neurotransmitter analysis must be performed in specialized laboratories, of which there are few. CSF is labile, and special collection procedures are necessary, including immediate centrifugation, snap freezing, and preservatives. Metabolites exhibit diurnal variation, and there is a rostrocaudal gradient of metabolite and pterin concentrations that requires precise labeling of collected fractions and careful interpretation of results. In addition, there is variation in concentrations with age, so reference ranges must be defined in age-matched controls. 22
46 Table 2 Metabolites of biogenic amine neurotransmitters and pterin profiles in the cerebrospinal fluid of patients with pediatric neurotransmitter diseases Affected Enzyme HVA 5-HIAA HVA/5-HIAA MHPG 3-OMD Pterin profile Disorders of decrease decrease normal decrease normal abnormal BH4 synthesis (recessive) GTP decrease normal normal normal normal abnormal cyclohydrolase (dominant) Tyrosine decrease normal normal decrease normal normal hydroxylase AADC decrease decrease normal decrease increase normal PNPO decrease decrease normal decrease increase normal Dopamine β- increase normal normal decrease normal normal hydroxylase Dopamine transporter (Kurian et al., 2009, Kurian et al., 2011b) increase normal increase NR NR normal AADC = L-aromatic amino acid decarboxylase; BH 4 = tetrahydrobiopterin; GTP = guanosine-5'-triphosphate;; NR = not reported;; PNPO = pyridoxamine 5 -phosphate oxidase; Note: Data in table derived from Hyland 2008, except where otherwise noted. 2 Vesicular Monoamine Transporters The vesicular monoamine transporters are responsible for the efficient uptake of cytosolic monoamines into storage vesicles. A 10,000-fold concentration of monoamines (up to 0.5 M) can be achieved by active transport. This transport against the concentration gradient is driven by the transmembrane ph and electrochemical gradient generated by the vesicular H + -ATPase in the synaptic membrane. Two closely related vesicular monoamine transporters, VMAT1 and VMAT2, have been characterized. The VMAT1 protein is primarily expressed in neuroendocrine cells, including chromaffin and enterochromaffin cells, and localized to large dense core 23
47 vesicles. The VMAT2 protein is primarily expressed in monoaminergic neurons of the central nervous system, as well as the sympathetic nervous system, mast cells, platelets, β cells of the pancreas, and histaminergic cells of the gut. Both VMAT1 and VMAT2 are expressed in chromaffin cells of the adrenal medulla (Erickson et al., 1996). 2.1 VMAT Isolation The VMATs were initially isolated and characterized from chromaffin granules. The SLC18A2 gene encoding VMAT2 was initially identified from a PC12 cdna library for its ability to confer resistance to the neurotoxin MPP + in MPP + -sensitive Chinese hamster ovary (CHO) cells (Liu et al., 1992). MPTP (of which MPP + is the active metabolite) is a neurotoxin that, in humans and primates, as well as other mammals, produces a very specific phenotype parkinsonism characterized by oxidative damage and progressive neurodegeneration in the substantia nigra region of the brain. It is thought that MPTP represents a greatly accelerated model of natural dopamine toxicity. 2.2 VMAT Structure Both VMAT1 and VMAT2 are acidic glycoproteins with an apparent endogenous molecular weight of 70 kda, and they share 63% overall amino acid identity (the related vesicular acetylcholine transporter, VAChT, has 34% amino acid identity). The predicted secondary structure of the VMATs comprises 12 transmembrane domains (TMDs) (Erickson and Eiden, 1993). This includes a large lumenal loop near the N-terminus with four putative N-linked glycosylation sites. Both the C- and N-termini of the protein are predicted to be cytoplasmic. These regions are the most variable with respect to species conservation. The lumenal loop and C- and N-termini are both subject to the greatest 24
48 sequence divergence between VMAT1 and VMAT2. The VMATs are part of the broader DHS12 superfamily of multidrug transporters of the major facilitator superfamily (MFS) of secondary transporters that include drug-resistance proteins (toxin-extruding antiporters), sugar uniporter proteins, H+ symporters, antiporters of organic phosphate esters, and bacterial permeases (Marger and Saier, 1993). 2.3 VMAT Biochemistry The biochemistry of the VMATs has been extensively reviewed (Schuldiner et al., 1995, Wimalasena, 2011, Eiden and Weihe, 2011, Henry et al., 1994). The inward transport of each cytosolic monoamine is coupled to the efflux of two protons. The first proton is proposed to alter transporter conformation to generate a high-affinity amine-binding site on the cytosolic side. The second proton is proposed to generate a second conformational change to move the amine from the cytosol to the vesicle lumen (Parsons, 2000). The VMATs have several natural substrates: serotonin (5-HT), dopamine (DA), norepinephrine (NE), and epinephrine (E). VMAT2 additionally transports histamine (HIS). VMAT2 has a consistently higher affinity for all monoamine substrates than VMAT1, though the rank order of affinity is the same for both isoforms: serotonin, dopamine, epinephrine (Peter et al., 1994). Other substrates, including MPP + and methamphetamine show a similar pattern. The greatest difference in affinity, however, is for histamine: K m of 3 µm for VMAT2 and 436 µm for VMAT1. Common structural features among native and non-native substrates and inhibitors of VMAT include a positive charge and aromatic ring. Hydroxyl, methoxy, and amino substituents in the ring improve affinity, whereas negative charge in the molecule reduces affinity 25
49 (Schuldiner et al., 1995). Specificity for a wide variety of substrates demonstrates significant plasticity in the binding site. In synaptic vesicles, uptake is dependent upon the magnitude of the ph and electrochemical gradients maintained by the vesicular H + -ATPase, as well as the cytoplasmic concentration of transmitters, the transporter density on the vesicle membrane, and the composition of the extravesicular media (Pothos et al., 2000, Sulzer and Pothos, 2000). The stoichiometry of transport (net transport out of the vesicle of two H + but only one positive charge) leads to a greater dependency on ph than on membrane potential. There are two commonly utilized VMAT inhibitors, reserpine and tetrabenazine. Biochemical study has revealed that these inhibitors function by two different mechanisms. The binding of reserpine is modulated by the proton gradient, as is substrate binding (Darchen et al., 1989), as well as substrate concentration. It is thus suggested that reserpine interacts with the substrate-binding site of VMAT. However, tetrabenazine binding is not affected by the proton gradient or substrate concentration (except at concentrations greater than 100 K m ) and therefore likely binds a unique site of the VMAT protein. Because tetrabenazine inhibits the binding of reserpine, it is further suggested that it binds a distinct conformation of the VMAT protein than that bound by reserpine and monoamine substrates. Notably, VMAT1 is less sensitive to tetrabenazine than VMAT2 (Peter et al., 1994). 26
50 2.4 Role of VMAT2 in Biogenic Amine Physiology Although classically attributed to postsynaptic mechanisms such as receptor number and sensitivity, evidence that receptors are not saturated under physiological conditions provides a role for the dynamic regulation of quantal size to affect synaptic transmission. A quantum is defined as a single vesicle filled with transmitter (Katz, 1971). As reviewed by Edwards (Edwards, 2007), VMAT2 is the key determinant of vesicle filling for serotonin and catecholamines, and therefore a mediator of quantal size for catecholaminergic and serotonergic neurotransmission. The activity of VMAT2 may therefore be a rate-limiting step in monoamine production and release, and its regulation is thus relevant to the pathophysiology of perturbations in monoamine neurotransmission. Indeed, the overexpression of VMAT2 increases quantal size (Pothos et al., 2000). VMAT2 function depends on the cytosolic concentrations of monoamine, the transport mechanism itself, and nonspecific leakage of monoamine across the vesicle membrane. Low cytosolic concentrations of monoamine (possibly reflective of cytosolic toxicity) are compensated by the relatively high affinity of VMAT2 (low K m ). Furthermore, the rapid depletion of vesicular monoamine stores in chromaffin granules with the use of reserpine suggests substantial nonspecific leakage (Kozminski et al., 1998), which further emphasizes and explains the importance of VMAT2 to maintaining quantal size. In addition to its role in synaptic release, VMAT2 has also been found to localize to a subpopulation of vesicles that undergo regulated exocytotic release in dendrites, conferring somatodendritic release of retrograde signals (Li et al., 2005). 27
51 The physiological consequences of VMAT2 deletion and hypomorphism have been explored through a number of mouse models Vmat2 Knockout Mice Complete deletion of Slc18a2 (Vmat2 -/- ) in mice resulted in elimination of exocytotic release and dramatic reduction of monoamine stores in the brain. Vmat2 -/- mice survive only a few days after birth (Fon et al., 1997, Wang et al., 1997). They have extremely low monoamine levels relative to wild-type animals (DA, 1.5%; NE, 6%; 5-HT, 1%), and consequently a severe impairment in monoaminergic signaling that is most likely the underlying cause of postnatal lethality. The pups feed poorly and move little prior to dying within the first postnatal week. However, they are born in normal Mendelian ratios (~1/4) and there are no gross differences in brain morphology. There was also no difference in TH immunohistochemistry between wild-type and Vmat2 -/- mice, indicating that vesicular monoamine transport (and subsequent exocytotic release) are not required for normal development of the mesostriatal projections. In vitro cell cultures derived from mesostriatal cells of Vmat2 -/- mice exhibited effectively no release of dopamine after K + -induced depolarization, demonstrating the requirement of vesicular transport for exocytotic release. The biogenesis and cycling of empty synaptic vesicles were unaffected by Vmat2 deletion (Croft et al., 2005). The motor and feeding phenotype can be partially rescued with amphetamine (Fon et al., 1997), demonstrating that reverse efflux of monoamines through plasma membrane transporters can mimic regulated exocytotic release with temporary success. This effect is most likely related to dopamine. The profound deficit in monoamine levels indicates 28
52 an increased ratio of degradation to synthesis, underscoring the importance of vesicular storage to this balance. Inhibition of monoamine degradation by MAO also rescues behavior and improves survival (Fon et al., 1997). Detailed histology of the early postnatal cerebral cortex shows an increase in developmental programmed cell death in these mice, which is partially rescued by MaoA inhibition (Vmat2-MaoA double knockout mice) with increased serotonin levels (Stankovski et al., 2007) Vmat2 Heterozygous Mice Mice that are heterozygous for Vmat2 (Vmat2 +/- ) exhibit a 50% reduction in Vmat2 expression, and normal viability and physiology compared with wild-type littermates. They also exhibit significant reductions in monoamines (DA, 42%; NE, 23%; 5-HT, 34%) (Fon et al., 1997), demonstrating the importance of vesicular storage capacity to monoamine content. In vitro cell cultures derived from mesostriatal cells of Vmat2 +/- mice exhibited approximately half (46.8%) the dopamine released from cells of wild-type mice after K + -induced depolarization, demonstrating the role of vesicular transport in determining quantal size (Fon et al., 1997). Notably, Vmat2 +/- mice are sensitized to direct and indirect DA agonists such as cocaine and amphetamine (Wang et al., 1997), and are significantly more vulnerable to MPTP and methamphetamine toxicity (Takahashi et al., 1997, Gainetdinov et al., 1998, Fumagalli et al., 1999). Phenotypically, the heterozygotes exhibit moderately elevated heart rate and blood pressure relative to wild-type littermates (Takahashi et al., 1997). Behaviorally, they exhibit no difference in locomotor activity, passive avoidance habit, or stress response 29
53 (Takahashi et al., 1997). However, they do express a depressive phenotype comprising locomotor retardation, anhedonia, immobility in forced swim and tail suspension test, and increased sensitivity to learned helplessness (Fukui et al., 2007). The forced swim and tail suspension phenotypes were ameliorated by antidepressants with serotonergic, dopaminergic, and noradrenergic mechanisms (fluoxetine, bupropion, and imipramine, respectively), suggesting involvement of multiple monoamine systems in the depressive phenotype (Fukui et al., 2007). Additionally, the heterozygous mice were reported to prolonged QT syndrome and an increased rate of sudden death at 2 4 months of age (Fukui et al., 2007, Itokawa et al., 1999) Constitutive Overexpression of Vmat2 in Mice Evidence of the dose dependence of the Vmat2 phenotype was recently complemented by a study investigating the effects of constitutive Vmat2 overexpression. BAC transgenic C57BL/6 mice were generated that possess three additional copies of the complete Slc18a2 gene and flanking regions (Lohr et al., 2014). These mice exhibit 3.5-fold higher Slc18a2 mrna levels, and 3-fold higher Vmat2 protein levels, compared with wild type littermates. The mice exhibited increases both in vesicular uptake and in vesicular capacity, corresponding to increased striatal vesicle volume and increased vesicle size. Resultingly, the mice exhibited increased striatal dopamine content, synaptic dopamine release, and extracellular dopamine. The locomotor and behavioral phenotypes of the mice also corresponded to these increases in dopamine neurotransmission the mice showed increased locomotor activity 30
54 and reduced anxiety-like and depressive-like behaviors (measured by marble-burying assay and forced-swim test, respectively) although these measures were likely also confounded by serotonergic and noradrenergic effects. Finally, these mice with increased Vmat2 expression had demonstrably reduced susceptibility to MPTP neurotoxicity, as measured by loss of striatal dopamine terminal markers (DAT and TH). Evidence of the dose dependence of the Vmat2 phenotype demonstrates its role as an important modulator of monoaminergic function, in contrast to vesicular glutamate transport in which compensatory decreases in synaptic vesicle release result in no net increase in neurotransmission (Daniels et al., 2004). Enhancement of Vmat2 function may therefore be an attractive therapeutic target for disorders resulting from reduced monoaminergic function, including depression and Parkinson s disease Selective Deletion of Vmat2 in Serotonergic Neurons Two models of the selective deletion of Vmat2 in serotonergic neurons have been developed to address the developmental role of serotonin. The Slc18a2 transcript is expressed transiently in several regions of the brain during early postnatal development in mice, in spatial and temporal co-expression with the serotonin plasma membrane transporter (SERT) (Lebrand et al., 1998). In Vmat2 sert-cr e mice, the Slc18a2 gene is selectively deleted in all SERT-expressing cells, causing a profound depletion of central serotonin stores (~95%), a partial depletion of serotonin in the blood, and normal serotonin levels in the gut (Narboux-Neme et al., 2011). In Vmat2 pet1-cre mice, the deletion of Slc18a2 is specifically targeted to raphe neurons, producing a partial (~75%) reduction in brain serotonin levels (Narboux-Neme et al., 2013). In both models, an overall somatic growth defect was observed, consistent with the phenotype of serotonin 31
55 depletion observed in Tph2 -/- mice. Additionally, severe serotonin depletion was shown to cause a mild delay in the development of the upper layers of the cerebral cortex, but no impairment in barrel cortex development. Interestingly, the barrel cortex development defects observed in Vmat2 -/- mice are not reproduced in these mouse models (Stankovski et al., 2007), and therefore likely do not derive from serotonin depletion. Generally, the delay in cortical growth was much more mild than the overall growth defect in these mice. Behavioral testing of Vmat2 sert-cre mice revealed decreased locomotor activity, decreased anxiety, and decreased immobility in the tail suspension test; interestingly, the latter result differs from observations in Vmat2 +/- mice in which all monoamines are affected. These changes were reversed by treatment with a monoamine oxidase inhibitor. Behavioral correlates of Vmat2 depletion specifically in raphe neurons have not yet been reported Selective Expression of Vmat2 in Noradrenergic Neurons A Vmat2 transgene was selectively expressed in noradrenergic neurons of Vmat2 -/- mice (Vmat2 -/-NE+ ) to investigate the role of noradrenergic defects in early postnatal lethality of Vmat2 deletion (Ohara et al., 2013). These mice had a slightly extended lifespan compared with Vmat2 -/- mice (2 3 weeks), but a body weight of approximately half that of wild-type littermates. Behavioral testing revealed pronounced akinesia and almost complete elimination of the normal open field exploratory pattern. The data suggest that noradrenergic function is critical to early neonatal survival, but survival beyond this point is dependent on other monoaminergic systems. 32
56 2.5 Role of VMAT2 in Disease Processes The relationship between VMAT2, affective disorders, and Parkinson s disease stems from the early discovery that chronic administration of reserpine initially introduced for the treatment of hypertension causes central nervous system effects including aspects of depression and Parkinsonism. This observation lead to the monoamine hypothesis of affective disorders (Freis, 1954), and the recognition of the role of dopamine depletion in Parkinson s disease VMAT2 and Parkinson s Disease Dopamine toxicity is a controversial topic suffering a lack of direct evidence in vivo. Importantly, toxic dopamine is that displaced from vesicles and subject to oxidation; greater than 90% of intracellular dopamine is normally sequestered in the reducing environment of vesicles (Eisenhofer et al., 2004). Dopamine is highly reactive, and can yield reactive species including hydroxyl radicals, superoxide, hydrogen peroxide, and dopamine quinones (Graham, 1978, Hastings et al., 1996). Reaction of quinones with proteins can create cysteinyl adducts; L-DOPA and DOPAC cysteinyl adducts are also formed. Superoxide further reacts with nitric oxide to produce reactive nitrogen species. All of these species may contribute to the oxidative stress underlying the multifactorial pathogenesis of Parkinson s disease, and the unique vulnerability of nigrostriatal dopamine neurons (Jenner, 2007). Therefore, VMAT2 function and regulation may be critical to neuroprotection through its role in maintaining dopamine homeostasis. Several lines of indirect evidence suggest a role for VMAT2 in Parkinson s disease pathogenesis. These include the parkinsonian side effects of the specific VMAT 33
57 inhibitor, reserpine; its role in the sequestration of MPP + conferring protection against MPTP-induced parkinsonism; evidence of a direct interaction between VMAT2 and α-synuclein and an increase in cytosolic dopamine and reactive species with α-synuclein overexpression (Lotharius and Brundin, 2002, Mosharov et al., 2006, Guo et al., 2008); and the mechanism of methamphetamine toxicity disrupting vesicular dopamine sequestration (Cubells et al., 1994, Sulzer and Rayport, 1990). Furthermore, a hypomorphic Vmat2 mouse model appears to recapitulate relevant molecular and pathological hallmarks of PD Vmat2 Hypomorphic Mice In addition to the previously described models of Vmat2 deletion, hypomorphic mice were studied to assess the effects of significant Vmat2 deficiency in animals that are able to overcome the barrier of early postnatal lethality exhibited by Vmat2 -/- mice. These mice were created accidentally during the process of generating complete knockout mice, in which the knockout cassette was stably inserted into intron 3 (Mooslehner et al., 2001). Termed the KA1 strain, these mice also unintentionally possessed a spontaneous deletion encompassing the -synuclein locus (not recognized in the initial reports). KA1 mice exhibit 95% reduction in Vmat2 protein levels, and corresponding reductions in tissue monoamines of 92% (dopamine), 87% (norepinephrine), and 82% (serotonin). Interestingly, in vitro assay of electrically stimulated dopamine release measured only a 70% reduction compared with age-matched wild-type animals (Patel et al., 2003). Compared with a 95% reduction in protein levels, this result demonstrates the presence of compensatory mechanisms for the reduction in Vmat2 likely a redistribution of Vmat2 from reserve pools to actively cycling vesicles. 34
58 In KA1 mice, no differences were observed in D1/D2 receptor sensitization, but there was supersensitization of D2/D3 autoreceptors (Colebrooke et al., 2007). In addition, downregulation of TH phosphorylation was observed at residues involved in feedback inhibition. No differences in DAT expression or activity were observed (Colebrooke et al., 2006). Importantly, these mice were found to downregulate substance P expression, and upregulate enkephalin in the striatum, demonstrating differences in underlying architecture of the striatum (Mooslehner et al., 2001). Behaviorally, the KA1 mice exhibit decreased locomotor activity and impairment in motor coordination (measured by beam walking and rotarod) that worsens with age, but normal reactivity in the novelty place preference task (Mooslehner et al., 2001). The motor deficits were responsive to L-DOPA administration. KA1 animals were also predictably more sensitive to MPTP toxicity and amphetamine. No evidence of neurodegeneration was evident in Vmat2-deficient KA1 mice at any age, but this was later attributed to the lack of -synuclein in these mice. A strain of Vmat2- deficient mice was therefore created in which wild-type -synuclein was reintroduced through selective breeding. These Vmat2-deficient mice exhibited an 85% reduction in striatal dopamine with associated reductions in DOPAC and HVA (Caudle et al., 2007). Importantly, age-related declines in striatal dopamine were observed in these mice, and were associated with several hallmarks of PD (Caudle et al., 2007). These mice exhibited molecular markers of oxidative stress and damage, including cysteinyl-dopa and DOPAC adducts at 2 and 12 months of age, and protein carbonyls and 3-nitrotyrosine at 12 months of age. Progressive cell death and loss of TH-positive neurons was observed 35
59 within the SNpc in older animals. More dramatic progressive cell loss was observed in the locus coerulus (LC), preceding that in the SNpc, consistent with observations in human patients with PD (Braak et al., 2003). Additional progressive motor deficits were also observed at 28 months of age, including a shorted forepaw stride length that may mimic shuffling gait. These motor deficits were responsive to L-DOPA. Although age-dependent alterations in the nigrostriatal system were observed in both Vmat2-deficient mouse strains, progressive cell loss was only observed in the Vmat2- deficient mice with intact -synuclein, implicating the latter in the severity of the phenotype and the mechanism of cell death. Nonmotor deficits analogous to the nonmotor symptoms of PD were also assessed in these Vmat2-deficient animals. These mice were observed to have progressive deficits in olfactory discrimination, altered sleep latency, delayed gastric emptying, and anxiety-like and depressive phenotypes (Taylor et al., 2009). As in PD, these nonmotor deficits preceded the severe motor deficits, and the pattern of L-DOPA responsiveness was similar (with the exception of sleep latency, which was L-DOPA responsive in Vmat2- deficient mice and is not responsive in human patients with PD) (Taylor et al., 2009, Taylor et al., 2011) VMAT2, Neuropsychiatric Phenotypes, and Drugs of Abuse That the monoaminergic systems play a role in neuropsychiatric phenotypes is well established, although the precise mechanisms are poorly understood. The particular role of VMAT2 in this respect, however, has not been clearly investigated. As discussed above, mouse models of VMAT2 deficiency (Vmat2 +/- and hypomorphic Vmat2 mice) 36
60 exhibit phenotypes related to depression and anxiety. In addition, SLC18A2 was identified in multiple genome-wide association studies of schizophrenia, as well as alcohol dependency and post-traumatic stress disorder (see Section 2.5.3). Nonetheless, the critical role of VMAT2 in determining quantal size makes it an attractive target for further study into the perturbations of monoaminergic function that underlie these phenotypes. More evidence exists for a role of VMAT2 in the mechanism of action of drugs of abuse. Amphetamines have multiple effects within the synapse that combine to produce non-exocytic release of dopamine. Efflux occurs through reverse transport of dopamine through DAT. The amphetamine- and methamphetamine-induced efflux of dopamine out of cells, however, first requires the desequestration of dopamine from vesicles. Amphetamine prompts this by one or both of two proposed mechanisms: VMAT2 inhibition or eliciting DA reverse transport as a VMAT2 substrate, and by collapsing the synaptic vesicle ph gradient through competition for intravesicular protons (Fleckenstein et al., 2007, Sulzer et al., 2005). In the case of methamphetamine, desequestration was shown to be tetrabenazine-sensitive (Volz et al., 2006). Some evidence has been provided that drug treatments may cause differential redistribution of VMAT2 within striatal synaptic terminals. Administration of methamphetamine was shown to redistribute VMAT2 in striatal synaptosome preparations out of an enriched vesicular fraction in conjunction with a decrease in vesicular DA content (Riddle et al., 2002, Sandoval et al., 2003, Eyerman and Yamamoto, 2005). Cocaine and methylphenidate primarily act as inhibitors of dopamine reuptake. However, both have been shown to redistribute VMAT2 from a membrane- 37
61 associated vesicle fraction to a vesicle-enriched, non membrane-associated fraction (Sandoval et al., 2002, Riddle et al., 2002). Importantly, differential sensitivity to the locomotor effects of amphetamine, cocaine, and ethanol (Takahashi et al., 1997, Wang et al., 1997), and methamphetamine toxicity (Fumagalli et al., 1999), as well as altered ethanol-associated behaviors (Hall et al., 2003, Savelieva et al., 2006), have been demonstrated in Vmat2 +/- mice. Much work remains be done to truly understand the role of VMAT2 in the mechanism of action of drugs of abuse Genetic Variation in VMAT2 Although no direct disease-associated VMAT2 mutations have been identified prior to that reported in this thesis, some associations have been discovered between single nucleotide polymorphism (SNP) alleles in SLC18A2 introns or promoter and Parkinson s disease (Brighina et al., 2013), schizophrenia (Talkowski et al., 2008, Chu and Liu, 2010, Gutierrez et al., 2007, Simons and van Winkel, 2013), post-traumatic stress disorder (Solovieff et al., 2014), and liability to or protection against alcohol dependency (Lin et al., 2005, Fehr et al., 2013). Gain-of-function promoter haplotypes may also be associated with a protective effect against Parkinson s disease in women (Glatt et al., 2006). As expected for traits that are highly polygenic, the magnitudes of effect in each case are not conclusive, and functional validation of mechanistic involvement of VMAT2 would be required to draw further conclusions. 38
62 3 Thesis Overview A new pediatric neurotransmitter disorder was discovered in eight cousins of a consanguineous Bedouin family in Saudi Arabia, and a causative mutation in VMAT2 (SLC18A2) was discovered in the present work. Understanding the underlying mechanism of this disorder extends the spectrum of known pediatric neurotransmitter diseases, serves as the first demonstration of mutation in VMAT2 causing a human phenotype, and thereby provides new insight into the role of VMAT2 in monoamine homeostasis. This thesis additionally demonstrates the utility of implementing genomic diagnosis in the clinic, with respect to providing simple and effective treatments in a timely manner to improve outcomes for patients with rare inborn errors of metabolism. My thesis objectives were the following: (1) To characterize this new pediatric neurotransmitter disorder. The disease was hypothesized to involve a defect in monoamine neurotransmitter pathophysiology. To provide evidence of the underlying pathophysiology of the disease, careful clinical investigations, including genetic analyses of candidate genes, were undertaken to exclude known diagnoses and produce a comprehensive clinical description of the disorder and its progression. In particular, neurotransmitter metabolite profiles were assessed in both CSF and urine. This data informs the differential diagnosis of this particular disease and related disorders; however, genetic and treatment evidence were further required to confirm this hypothesis. 39
63 (2) To identify the genetic mutation causing this disease and characterize the effect of the mutation I hypothesized that a homozygous genetic mutation was causing this autosomal recessive disorder in affected individuals of a consanguineous pedigree. To investigate this hypothesis, whole-genome SNP analysis was performed on 5 patients and 3 unaffected siblings, in combination with linkage and homozygosity analyses, to identify the region containing the disease-causing mutation. Sanger sequencing of candidate genes within the locus identified a nonsynonymous variant in the SLC18A2 gene encoding the vesicular monoamine transporter 2 (VMAT2) protein. The possibility of other variants in the region and in other candidate genes in the genome was excluded by whole-exome sequencing of the proband. (3) To infer treatment targets on the basis of the biochemical and physiological role of the identified disease-causing mutation Patients exhibited an initial positive response to treatment with levodopa/carbidopa followed by a rapid decline and worsening of the phenotype. This poor response to dopamine replacement is consistent with the presumed normal dopamine biosynthetic capacity in these patients combined with aberrant dopamine storage and release. It was then hypothesized on the basis of the known defect in VMAT2 function that treatment with a dopamine agonist would stimulate dopamine receptors while bypassing the requirement for vesicular storage and release. Subsequent treatment with a dopamine agonist, pramipexole, 40
64 resulted in a remarkable response in all patients, with the most substantial recovery occurring in younger patients. Much of the work contained in this thesis and the major conclusions were published in: RILSTONE, J. J., ALKHATER, R. A. & MINASSIAN, B. A Brain dopamine-serotonin vesicular transport disease and its treatment. N Engl J Med, 368,
65 Chapter 2 Clinical Characterization of a New Pediatric Neurotransmitter Disease 1 Introduction The pediatric neurotransmitter diseases (PNDs) mimic the phenotype of other neurological disorders, and are therefore often misdiagnosed or remain without diagnosis for years. Common misdiagnoses include cerebral palsy, hypoxic ischemic encephalopathy, paroxysmal disorders, inherited metabolic diseases, and genetic dystonic or parkinsonian syndromes. Further hindering accurate and timely diagnosis, several of the pediatric neurotransmitter diseases are exceedingly rare. The most similar syndromes to that presented in this thesis (TH deficiency and AADC deficiency) reflect worldwide diagnoses of just 50 and 100 patients, respectively. Increasing awareness in the clinical community of this group of disorders and the clinical spectrum of monoamine deficiency will aid the recognition of these disorders. In this chapter, the clinical investigation and diagnosis of a new autosomal recessive pediatric neurotransmitter disease is described. The diagnosis of monoamine neurotransmitter disorders is based on clinical history, neurological examination, biochemical investigations (including specific cerebrospinal fluid investigations), enzyme analysis, and genetic analysis. Conclusive diagnosis of biogenic amine deficiency in the central nervous system is often demonstrated by clinical measurements of their primary metabolites in cerebrospinal fluid. In the present case, several lines of evidence coincided most importantly, the genetic results described in Chapter 3 to conclusively diagnose this new disease. The relevant investigations are presented in this chapter to characterize this new syndrome, demonstrate the involvement 42
66 of biogenic amines in its etiology, and present a successful treatment strategy. Additionally, difficulties in diagnostic strategy are discussed, including the pitfalls of cerebrospinal fluid metabolite measurements as a conclusive diagnostic method. 2 Methods 2.1 Patients The patients in this study were identified in the Khobar province of Saudi Arabia by Dr. Reem Alkhater, who undertook neurological examinations and relevant investigations. The study was approved by the Hospital for Sick Children s research ethics board. Written informed consent was provided by the parents, with the patients providing their assent for participation in the study. 2.2 General Investigations Investigations presented here were performed as part of regular clinical care for these patients, and included neurological examination, bloodwork and metabolic screens, video-eeg, magnetic resonance imaging (MRI), and MR spectroscopy. 2.3 Clinical Measurement of Neurotransmitter Metabolites Lumbar puncture was performed in a younger affected sibling of the proband at the age of two to allow measurement of monoamine neurotransmitter metabolites, intermediates, and precursors. Samples were drawn sequentially into numbered tubes to ensure collection of the appropriate fraction. The tube for analysis contained 1.0 ml of CSF collected directly from the tap needle after previously dispensing 1.0 ml of CSF. This tube contained appropriate antioxidants for preservation of the analytes. The tube was 43
67 immediately frozen at 80 C and shipped on dry ice for analysis. The sample contained no visible blood contamination. Metabolites were measured at the Service de Génétique Médicale of the Centre Hospitalier Universitaire de Sherbrooke Fleurimont by liquid chromatography tandem mass spectrometry (LC-MS/MS) according to established methods. A urine sample was also collected from the same patient. This sample was analyzed for relevant neurotransmitter metabolites at the Mayo Clinic in Rochester, MN. 2.4 AADC Enzyme Test The activity of aromatic L-amino acid decarboxylase (AADC) was assayed commercially by plasma enzymology at Medical Neurogenetics. Plasma was separated immediately upon collection of a blood sample in an EDTA tube and stored at 80 C before shipping on dry ice for analysis. 2.5 Candidate Gene Sequencing A total of 5 ml of whole blood was collected in EDTA tubes. Genomic DNA was isolated at the Hospital for Sick Children s DNA lab. Commercial sequencing of a panel of genes associated with spinocerebellar ataxia (SCA) and those associated with mitochondrial DNA disorders was performed. Primers were designed to amplify the exons of each gene and approximately 50 bp of flanking intronic sequence (Table 3). The reaction was performed using Picomaxx DNA polymerase (Stratagene) according to the manufacturer s specifications. Cycling conditions incorporated an initial denaturation at 95 C for 5 minutes, followed by 30 cycles of 95 C for 30 s, 55 C for 30 s, and 72 C for 30 s, and a final extension step at 44
68 72 C for 10 minutes. Amplification products were visualized by gel electrophoresis, and purified from bands of the appropriate size using a Qiagen Gel Purification Kit according to manufacturer s specifications. Purified PCR products were sequenced using both forward and reverse primers in separate reactions. Sequences were compared to reference genome sequence using the BLAT tool in the UCSC Genome Browser (Meyer et al., 2013). Putative variants were verified by manual inspection of electropherograms. Table 3 Primer sequences for the amplification of exons of candidate genes associated with known pediatric neurotransmitter diseases Gene Exon Sequence Size PTS 1 AGC GGA GAC GCA CTT CCT A 389 bp GAC ACT CCA GCC CCC ATC 2 TTG GTG AGC TAA AGT AAT AAA TTG 300 bp G TCC GTA AGT TTT CCC ATT CTT 3 AGC TTT TGG GGA CAG ATC TAA 250 bp AAG CAA TAC TGA CTG GAA CAG TTT 4 GGA TGA AGG CAA ATG TGC AA 298 bp CCA GTT CTA TTC ACA AAG TCA TGG 5 GAC AGC TGG GCC TGA CTT TA 380 bp GAA ATT CTA GTT TCG AAA GAT TTC AT 6 TTT GAT TGT TGT GTG ATT TCT GA 392 bp AAT AGG CAC TCC AGA GCA CAA SPR 1 CAG ATC CCA AGG GAA CCA G 600 bp GCT GGA ACA ACT AGG GCT TTA 2 TAG GTT CCC CAG CTG TGT CT 575 bp ATC CTG TGG GTT GTT TCC TG 3 AAG ATG ACG TGT TTC CTC TGG 400 bp CCC TAT GGC AGG GTG TGG QDPR 1 GCC TGG CCG AAG TTA CAG T 428 bp GTG CAA GCA ACA CGA GTC AG 2 TCC CAA AGT GCT GGC ATT AC 298 bp GCC AAA GGA AGA ACA TAC AGC 3 CAA AGC ATT AAT TGC CAG GT 300 bp AGT GCA AAC CCA ATC CTT GT 4 GCC CTG TGC TGT TTG TGT TA 371 bp 45
69 TCC TCA TCC CAT GAA AGT GC 5 CGT CTG ACC TGA AGG AGG AG 363 bp CAG AAC CAG AGG TGA GAG CA 6 TAG CAC CCT CAG TGC CAG A 300 bp GGG AAC ACA GAC TTG TCC TCT 7 TTA AAC AGT CGC TGC TGT GC 377 bp GGA GAG CAA ATG CAT ATT ATG TGA GTCPH1 1 GCG TAC CTT CCT CAG GTG AC 561 bp AGT GAG GCA ACT CCG GAA AC 2 CAG AAA AGG ACT TTG CTA CTT TGA 476 bp GCC TTC TGC TAC TTT GGT TTT G 3 AAC AGT TCC AGA TGT TTT CAA GG 379 bp GTA GGG GAC GAG AAG GAA GG 4 GTC CTT TTT GTT TTA TGA GGA AGG C 288 bp GGT GAT GCA CTC TTA TAA TCT CAG C 5 TGG TGT GTC TTG GCT CTT AAA 300 bp CCT GGT GCT ACA AAA TAT GAG AA 6 CCG CAG TTA CTT TTG CAT GA 396 bp CAC ATC TGT AAC AAT TGA AAA TGG A SLC6A3 1 TGG CTG AAG ACC AAG AGG G 425 bp CTC GTT TCC GTA CGT GCC 2 ATG GAT GGT TGA CTG GGG TA 335 bp TAG CAA AGC AGG GCT GGA T 3 TGG GCT CAG GGT AAT GTC TC 490 bp CCA GAG CAC TAA AGG GAT GG 4 AGT TCC AGG TGG GTT GAC AG 304 bp AGC ACA AAA CCC AAC TGA GG 5 CTC CCA ATC AGA GGA CAA GC 354 bp CCT GGT GTC TGC AAC TCT GA 6 CAC TTC CTG AGG CTG CAT CT 361 bp TCT CAT CCA GGG ACA CCC TA 7 AAA AGT AGC CCC TCC GAA GA 383 bp TGA GGC AGC AAC TCT CAC TG 8 CGC ACC AGC CCT AGT CTC TA 424 bp TCC AGT CAC CAC TCA CTC CA 9 AAA CCC CCT ACC GTG GAT AC 561 bp TGA CTC TGG GAC CAA GCT CT 10 TAC ACG TGG CCC TAA GAA GC 442 bp AGC ACC TCA CTG ATC CCA TC 11 CTT GGG AGT CAG CGA GGA 314 bp AGT CTT GAG GCC CCT GAC TC 12 GAG ACG CTC TGC CAT GAA GT 311 bp CTT TCT GGT GGC CTC ACA CT 13 CTG GCA GTG GGT ACT GGT CT 200 bp 46
70 CTG GGG GCT AAG AAC ACT GA 14 CAG GAG GCT GCA AAC TGT CT 2.2 kb AGG CCG GAA GGA GAG ATG TH 1 GCC TCC CTC CTT CCT CAC 379 bp GGT TTG CAT GGA CCC TGA 2 AAA TGG GTT TTT ATT TAT GGA CCT T 400 bp GGG ACT TGG CAG ACA CCT G 3 CTC AAA AAC GTG CTC TCA TCC 500 bp AGC TGA GGC CTG AGA CTC C 4 AAG AAC GGG ATC TGT GTG CT 479 bp CAC GGA TGT GTA GCA AAA CG 5, 6 CCC CCG GAA GTC TTG TAG G 500 bp GGT CCT CCC CTT TGT CCT T 7 CAC CCT CCT GTC CAT CCT C 293 bp CTC TCC TGT TGT GCC AAG GT 8 ACT GGG GTG GGG CAT TAG 396 bp CAC TGG AAC ACG CGG AAG 9 GGG GAT GGT CAG CCA AGC 297 bp CGC GTA GGA GGG AGA AGG 10 ACT CCC CTG AGC CGT GAG 268 bp AGC AGG CAG CAC ACT TCA C 11 AGG GAA GTG TCC CAG AGA CC 235 bp AGA GGG TGA GGC CTG GAT T 12 TGT CTC TGG GCT GAT GCT G 250 bp AGA GCC TGA GTC CTG GAG GT 13 TGG AGT CAG TGA TGC CAT TG 242 bp CTC AAG GCC AGA AGG AAG G 14 TCT GAG CCA CTG TGA AGG TG 296 bp GTT GGG AAG GGC CCT CAG DDC 1 GGG GAG GCA GAC ACT CTG T 228 bp GGA GGA GAA TTC AGC ACA GC 2 TCC TAC AGA CAT GGA GGG AAA 494 bp TGC CAT AGG GAT TCC TTG AA 3 ACA TTT GGG GAA CTG CAC TC 340 bp CAG GTC CCT TGT GCA TAG GT 4 TCT GGG CTT TAG TGG AAG GTT 300 bp GTG CCT CTT TCC CCA CCT 5 GGA CAC AAA ACA ATA TGT CTT CCA 300 bp TGG TTT GGT TTG AAT TTG ACA 6 CCT TGT GTT TGC AAT GTT GG 300 bp CCC GAG TAG CTG GGA TTA CA 7 CAT TGG GAT CTC GGC TCA T 300 bp GGC AAA CCA TCA CAA TAT GAA 8 CAA GAA GGT GCT CAG ACA GG 299 bp TTG GCT GAA ACA AAC CTC AA 47
71 3 Results 9 CAC TGT GGA TTA GTT GTG CCA TA 296 bp GCA GCA AGC AGT GAG CTA AG 10 CCC AGG TAC TTG GAG CAG AG 390 bp TAC AAG GGC AAA TCC AGG AA 11 GCC TTT GGG CAG TTT TAT TTC 249 bp CGT GCT GAT CAT GAG AGT GG 12 GTT GGC CAC CAG GGA ATC 300 bp GCA GTG AGC TGA GAT TGT GC 13 TGC CAA GAG CGT CTA AAT GA 392 bp CGT GGA AAC AAG GCT GTG TA 14 GTA GGG TTG CCA AGC ACT GT 395 bp CCT GTA GCT GGG TCT GGA CT 15-1 ACC GTG GAA AGA GAG GGA GA 475 bp GTT GCG TGA ACA TTG ATT GC 15-2 GAG GGT TGT GAT TTT GTC TGC 400 bp TGC CGT TTA AAA ACA TCC AA 3.1 Patient History and Neurological Examination The index case was a 16-year-old female with global developmental delay and abnormal movements. She had initially been brought to medical attention at 4 months of age with hypotonia, loss of acquired head control, and paroxysmal stereotyped episodes of persistent eye deviation, and crying lasting hours. Video-EEG monitoring had excluded seizures, and a symptom diagnosis of oculogyric crisis had been made. Development had been normal initially, but had slowed after presentation. She had developed the ability to sit at 30 months, crawled at 4 years, and walked at age 13. Upon presentation at the age of 16, she was experiencing fatigue, excessive diaphoresis, profuse nasal and oropharyngeal secretions, noisy breathing, hypernasal speech, poor distal perfusion, cold extremities, disrupted sleep, hypotonia, ataxia, dysarthria, and incoordination. There was no diurnal variation and no improvement with vitamin B6 or folinic acid. 48
72 Neurological examination of the proband at age 16 revealed ptosis, hypomimia, facial dyskinesia, and limited upward gaze. She had axial hypotonia and appendicular hypertonia specifically involving the extensor muscles of the upper and lower extremities, and her power was 4+/5 in all muscle groups. Deep tendon reflexes were 2+/4 and symmetric. Plantar reflexes were flexor, and there was no clonus. Coordination testing revealed a fine tremor, and dysdiadochokinesia in the upper and lower extremities. Her gait was parkinsonian with typical shuffling, her posture was stooped, and her postural reflexes were diminished. She walked with bilateral alternating dystonia of hands and feet with intermittent toe walking and foot inversion, and was unable to tandem walk. 3.2 Investigations The patient s basic bloodwork and metabolic screens were normal (Table 4). Repeat overnight video-eeg revealed neither seizures nor interictal abnormalities, and MRI and MR spectroscopy were normal (Figure 5). Table 4 Amino acids Vitamin B12 Biotinidase Copper Ceruloplasmin Lead Very long chain fatty acids Carnitine Ammonia Lactate Pyruvate Serum metabolic screen revealed no abnormalities 49
73 Figure 5 T2-weighted magnetic resonance images of proband at age 14 revealed no abnormalities. 50
74 Lumbar puncture was performed in a younger affected sibling at the age of 2 to measure monoamine neurotransmitter metabolites, intermediates, and precursors all of which were normal (Table 5). The urine neurotransmitter profile, however, revealed increased monoamine metabolites [5-HIAA = 17.6 µg/dl (reference range: 0 6 µg/dl); HVA = 14.1 µg/mg Cr ( µg/mg Cr)] and a decrease in measurable urine monoamines [norepinephrine = 1.1 µg/dl (4 29 µg/dl); dopamine = 19 µg/dl ( µg/dl)] (Table 5). Plasma was tested commercially for AADC enzyme activity; this result was also normal. 51
75 Table 5 Cerebrospinal fluid and urine neurotransmitters and their metabolites measured in a younger affected sibling of the proband reveal decreased monoamines and elevated metabolites in urine, but not in cerebrospinal fluid. Values outside reference ranges are presented in bold. Cerebrospinal Fluid 5-HIAA HVA HVA/5-HIAA 3-OMD 5-OHTrp 5-HT Normal Range nm nm <150 nm <25 nm no reference Patient 169 nm 314 nm nm 12 nm <5 nm Urine 5-HIAA Norepinephrine Epinephrine VMA HVA Dopamine Normal Range 0 6 µg/dl 4 29 µg/dl µg/dl µg/mg Cr µg/mg Cr µg/dl Patient 17.6 µg/dl 1.1 µg/dl 0.5 µg/dl 5.5 µg/mg Cr 14.1 µg/mg Cr 19 µg/dl HIAA, hydroxyindoleacetic acid; HVA, homovanillic acid; OMD, O-methyldopa; OHTrp, hydroxytryptophan; HT, hydroxytyramine; Cr, creatinine; VMA, vanillylmandelic acid. 52
76 3.3 Family Structure The proband is a member of a consanguineous Saudi family that includes 6 children sharing an identical clinical picture of complex movement disorder (Figure 6). The proband (V:6) was the eldest of 6 siblings, three of whom also presented with the disease (V:9, V:10, and V:11). Two siblings were unaffected (V:7 and V:8). The parents had also experienced two spontaneous abortions. An additional affected individual (V:3) is a second cousin of the proband. A sixth affected individual (VI:2) was a first cousin once removed of the affected children. The parents of the affected individuals exhibited no movement disorder. The ratio of male to female affected individuals is approximately 1:1, and there is no evidence of maternal inheritance. The mode of inheritance is therefore autosomal recessive. 53
77 Figure 6 Family structure of kindred demonstrates autosomal recessive mode of inheritance and consanguineous pedigree structure. Proband is labelled V:6. Cerebrospinal fluid and urine neurotransmitter analyses were performed on individual V:10. Black, affected; white, unaffected; square, male; circle, female; diamond, spontaneous abortion. Numbers in squares or circles indicate number of male or female offspring, respectively. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission. 54
78 Two additional affected cousins were identified. These two affected individuals were full siblings of each other (VI:3 and VI:4) and first cousins of one of the affected individuals (VI:2). At the time of analysis, the eldest was a 31-month-old boy. In addition to the phenotype of hypotonia, these siblings exhibited bilateral anopthalmia. There is no clear theoretical relationship between the neutrotransmitter phenotype and this developmental phenotype. There is also no documented anopthalmia in other pediatric neurotransmitter diseases.(kurian et al., 2011a, Pons, 2009) Given the consanguineous background of these affected individuals, they may simultaneously express an unrelated autosomal recessive trait caused by an independent genetic mutation. A karyotype should be performed for these siblings to identify any chromosomal abnormality, and these patients may further opt to undergo whole-exome sequencing to identify additional homozygous nonsynonymous variants. 3.4 Genetic Screening of Candidate Genes The clear autosomal recessive mode of inheritance in this consanguineous family belies a single causative genetic mutation. Initial screening of some candidate genes was performed in the clinical laboratory setting. Clinical genetic screening of candidate genes revealed no mutations associated with SCA or mitochondrial disorders. After the suggestion was made of biogenic amine involvement, the causative genes for all known dopamine deficiencies were screened. Genes known to be associated with pediatric neurotransmitter diseases were screened by sequencing of exons and flanking intronic sequences (Table 6). The causative gene for pterin-4α-carbinolamine dehydratase deficiency (PCD) was not sequenced because PCD does not present with 55
79 movement disorder features, and the causative gene for PLP was not sequenced because seizures were not a component of the patients histories. No coding mutations or putative splice variants were identified in any of the sequenced candidate genes. Whole-genome investigations were undertaken to identify the causative mutation, and a mutation was discovered in the SLC18A2 gene encoding vesicular monoamine transporter 2 (VMAT2). These genetic studies are described in Chapter 3. Table 6 Pediatric neurotransmitter disease associated genes screened in the proband revealing no putative mutations Gene Number of Protein Name Associated Disorder Symbol Coding Exons DDC 9 Aromatic L-amino acid AADC deficiency decarboxylase SLC6A3 14 Dopamine transporter (DAT) Dopamine transporter deficiency syndrome (DTDS) QDPR 7 Dihydropyridine Receptor (DHPR) DHPR deficiency GCH1 6 GTP cyclohydrolase I GTPCH1 deficiency Segawa disease Dopamine-responsive dystonia (DRD) PTS 7 6-Pyruvoyltetrahydropterin synthase PTPS deficiency (PTPS) SPR 3 Sepiapterin reductase (SR) SR deficiency TH 13 Tyrosine hydroxylase (TH) TH deficiency 3.5 Drug Response On the basis of the parkinsonism and the diminished urine dopamine, the proband and three of her younger affected siblings were initially treated with L-DOPA/carbidopa (approximately 1 mg/kg/day), which resulted within 1 week in major deterioration, with 56
80 the appearance of intense chorea and worsening of the dystonia. Discontinuation of the medication led to rapid return to baseline in all four children. On the basis of the identification of the underlying VMAT2 mutation (described in Chapter 3), the decision was made to attempt treatment with a direct dopamine receptor agonist (pramipexole; 0.02 mg/kg/day). This treatment resulted within 1 week in dramatic and sustained disappearance of parkinsonism and dystonic attacks, and improvement of other symptoms. The younger affected siblings were then treated. They exhibited even more remarkable recoveries; the younger the particular child at the time of initiation of treatment, the greater the number of symptoms that were corrected and the greater the extent to which symptoms improved. The effect of treatment at each age is presented in Table 7. 57
81 Table 7 Feature Cognition and ability to learn Age at initation of dopamine agonist affects disease course Age at treatment 18 years 11 years 7 years 3 years Mildly improved Mildly Moderately Greatly improved improved improved. Able to make stories from pictures. Occulogyric crises No further events; on a dose higher than her siblings No further events No further events No further events Dystonia Gait dystonia persists Gait dystonia persists Gait dystonia persists Gait dystonia improved Parkinsonism Improved Improved Improved Improved Fine motor skills Improved coordination, able to feed self, drink from cup, and hold a pen. Improved handwriting Improved coordination, learning to hold a pen. Unable to write or read Learning to hold a pen and drink from a cup independently. Unable to write or read Able to write, learning to read Language and speech Dysarthric No language development Mama and Papa Normal language development and mild dysarthria Gait Improved posture and fatigue (had started walking at age 13) Started walking within days of treatment Started walking within days of treatment Started walking within days of treatment 58
82 3.6 Summary of Clinical Features and Differential Diagnosis The salient features of the disorder are summarized in Table 8. The syndrome is onset in early infancy (approximately 4 months of age) with the appearance of hypotonia and the loss of motor milestones. As the affected children age, they develop features of movement disorder, cerebellar symptoms, and autonomic symptoms. As initially observed in the proband (the eldest patient), the movement disorder phenotype exhibited in early childhood (dystonia, oculogyric crises, facial dyskinesia, and chorea) evolves by the age of 11 into parkinsonism characterized by bradykinesia, rigidity, stooped posture, and shuffling gait. Table 8 Clinical features common to all affected individuals in the pedigree Features Onset at age 4 months Hypotonia Hypomimia Paucity of movements Oculogyric crises Attacks of dystonia Dysarthria Ataxia and incoordination Excessive diaphoresis Profuse nasal and oropharyngeal secretions Poor distal profusion and cold extremities Disrupted sleep Mild cognitive impairment No diurnal variation Evolution of the movement disorder by age 11 years into a picture closely resembling Parkinson s disease, with dyskinesias The composite clinical picture in this family includes features that can be attributed to deficiencies of particular biogenic amines. A categorization of the clinical symptoms is 59
83 presented in Table 9. The movement disorder symptoms, as well as ptosis and hypersalivation, are considered signs of dopamine deficiency. The manifestations of serotonin deficiency include temperature instability, sleep disruption, sweating, and possibly dystonia. Many of the autonomic disturbances experienced by these patients may be attributable to the deficiency in norepinephrine. Although this model is useful for understanding, it should be recognized that significant interaction among the biogenic amine pathways clouds these distinctions. Additionally, some features are difficult to directly attribute to the actions of the biogenic amines, such as mild cognitive impairment. Table 9 Clinical features of the disease organized by category Onset 4 7 months hypotonia and developmental regression Movement Disorder oculogyric crises, dystonia, facial dyskinesia, chorea Parkinsonism bradykinesia, rigidity, shuffling gait, stooped posture Cerebellar symptoms ataxia, dysarthria, tremor Autonomic symptoms ptosis, fatigue, diaphoresis, excessive nasal and oropharyngeal secretions, noisy breathing, hypernasal speech, disrupted sleep, poor distal perfusion Medication responsiveness L-DOPA/carbidopa: temporary improvement, rapid decline and worsening of initial phenotype Dopamine agonist (pramipexole): Responsive The multisystemic constellation of symptoms of this disorder fall within the spectrum of pediatric monoamine deficiency. A comparison with the most closely related pediatric monoamine deficiencies is presented in Table 10; as illustrated, a normal CSF neurotransmitter metabolite profile is the major distinguishing feature of VMAT2 60
84 deficiency among these syndromes. As with other PNDs, the motor manifestations dominate the clinical picture of VMAT2 deficiency, and the autonomic features of the syndrome may be overlooked. As such, the underlying monoamine pathophysiology may not be recognized by many physicians, and the most common misdiagnosis would be cerebral palsy. Furthermore, physicians who suspect biogenic amine involvement may opt for confirmatory CSF neurotransmitter analysis, which would produce a negative result, further impeding proper diagnosis. The sole conclusive diagnostic for VMAT2 deficiency at the present time is genetic analysis. These caveats underscore the importance of including genomic investigations in the diagnostic protocol for pediatric movement disorder. In addition to being the sole diagnostic for VMAT2 deficiency, the rate of misdiagnosis (or lack of diagnosis) is high for the majority of pediatric neurotransmitter diseases, and more broadly, all inborn errors of metabolism presenting with motor features. 61
85 Table 10 Comparison of clinical features of the present disease with those of closely related monoamine deficiency syndromes Our Family AADC Deficiency Tyrosine Hydroxylase Deficiency DTDS Age at onset Infancy Infancy Infancy Infancy Muscular hypotonia Dystonic spasms Occulogyric crises Parkinsonism + + infantile onset + Delayed motor milestones Autonomic symptoms Depression + + CSF neurotransmitters a Normal b (Decreased HVA, 5-HIAA, and Abnormal increased 3-OMD) Treatment dopamine agonist dopamine agonist Abnormal (Decreased HVA) L-DOPA Abnormal (Increased HVA/5-HIAA) dopamine agonist (mild success in some patients) AADC = aromatic amino acid decarboxylase; CSF = cerebrospinal fluid; DTDS = dopamine transporter deficiency syndrome; 5-HIAA = 5-hydroxyindoleacetic acid; HVA = homovanillic acid; 3-OMD = 3-Omethyldopa; a b Complete CSF neurotransmitter metabolite profiles are presented in Table 11. Based on a single case. 62
86 4 Discussion Until the identification of dopamine transporter deficiency syndrome (DTDS) in 2009 (Kurian et al., 2009), the spectrum of PNDs included multiple defects in the biosynthesis of one or more of the biogenic amines. The gold standard diagnostic test in patients with suspected diseases of monoamine metabolism is the measurement of monoamine metabolites in the CSF. Because each specific defect results in a particular metabolite profile, this single test specifies the disease (Hyland, 2008, Kurian et al., 2011a, Pons, 2009). The particular metabolite profiles of each disease are presented in Table 11. DTDS was the first PND discovered to result from a defect in biogenic amine transport (specifically, dopamine uptake at the plasma membrane). This syndrome depicted a normal serotonergic profile (normal 5-HIAA), but elevated HVA concentrations. This is also reflected as an increased HVA/5-HIAA ratio, as recently observed in 11 unrelated children with DTDS (range, ; normal range, ) (Kurian et al., 2011b). The elevation of the primary metabolite of dopamine indicates the production of dopamine in relevant quantities, as well as an increased rate of dopamine turnover. The latter is expected to result from increased exposure of dopamine to degradative processes in the extraneuronal space. The measurement of CSF neurotransmitters in 1 patient in the present study revealed metabolite concentrations within the normal ranges (Table 5), including a normal HVA concentration and HVA/5-HIAA ratio (314 nm and 1.9, respectively). Acknowledging the limitation in sample size, this result is similarly distinct to that observed for patients with biosynthetic monoamine deficiencies. 63
87 Defects in monoamine transport processes within the central nervous system would be expected to produce an abnormal distribution of the affected transmitters with respect to the relative quantities present within the vesicles, the cytosol, and the extraneuronal space. Vesicular monoamine transport is driven by the ph gradient across the vesicular membrane; namely, the acidification of the vesicles. In the acidic, reducing environment of the vesicles, the monoamines are protected from oxidative processes. Additionally, they are sequestered from degradative enzymes (MAO and COMT). Aberrant redistribution of the biogenic amines would therefore be expected to increase the rate of turnover, and subsequently increase the ratio of metabolite to transmitter. Importantly, Vmat2 -/- mice exhibit normal levels of DOPAC, HVA, and 5-HIAA despite dramatically reduced levels of brain monoamines (Wang et al., 1997, Fon et al., 1997), consistent with observations in this patient. The biogenic amines are synthesized in the cytosol (with the exception of norepinephrine, which is synthesized intravesicularly from dopamine, which is in turn synthesized in the cytosol). In the case of VMAT2 deficiency, it is possible that the increased cytosolic concentrations of dopamine and serotonin may provide some feedback inhibition on their biosynthesis. This presumed difference in distribution relative to DTDS in which the neuronal reuptake of monoamines is defective may underlie the difference in measured HVA concentrations between DTDS and this case of VMAT2 deficiency (increase in HVA versus normal HVA, respectively). It will be necessary to perform metabolite measurements in the CSF of a larger cohort of patients to conclusively characterize the particular metabolite profile of VMAT2 deficiency. This opportunity may arise as unrelated patients with suspected 64
88 neurotransmitter deficiency phenotypes are screened genetically for VMAT2 mutation. Many of these patients may have previously undergone CSF metabolite profiling, but remain undiagnosed given normal results. CSF neurotransmitter metabolite analysis has been instrumental in the discoveries of the monoamine disorders (Hyland, 2008, Kurian et al., 2011a, Pons, 2009), but not may have captured VMAT2 deficiency because of its normal CSF profile. Table 11 Comparison of metabolite profiles in cerebrospinal fluid for pediatric neurotransmitter diseases, including VMAT2 deficiency Affected HVA 5-HIAA MHPG 3-OMD Enzyme Disorders of decrease decrease decrease normal BH4 synthesis (recessive) GTP decrease normal normal normal cyclohydrolase (dominant) Tyrosine decrease normal decrease normal hydroxylase AADC decrease decrease decrease increase PNPO decrease decrease decrease increase Dopamine β- increase normal decrease normal hydroxylase Dopamine increase normal NR NR transporter (Kurian et al., 2009, Kurian et al., 2011b) VMAT2 (single case) normal normal NR normal AADC = L-aromatic amino acid decarboxylase; BH4 = tetrahydrobiopterin; GTP = ; NR = not reported; PNPO = ; VMAT2 = vesicular monoamine transporter 2 Note: Data in table derived from Hyland 2008, except for the present case and where otherwise noted. 65
89 The specificity of HVA abnormalities in diagnosing primary neurotransmitter disorders has also recently been called into question by the demonstration of decreased HVA in 15.4% of cases and increased HVA in 4.6% of cases in a cohort of 1388 pediatric patients with neurological disorder, whereas genetic dopaminergic deficiency was only discovered in 21 (1.5%) patients by genetic screening (Molero-Luis et al., 2013). The magnitude of the HVA decrease was greater in this study for patients with genetic dopaminergic deficiency compared with patients with other neurological disorders, but the ranges overlapped significantly. The analysis of monoamines or their metabolites in urine is not reliable in the diagnosis of monoamine neurotransmitter diseases (Hyland, 2008, Kurian et al., 2011a, Pons, 2009), except in one AADC deficiency in which increased 3-O-methyldopa (3-OMD) with decreased vanillylmandelic acid (VMA) in the proper clinical context is highly suggestive and generally confirmed by mutation analysis (Brun et al., 2010, Lee et al., 2012, Pons et al., 2004, Swoboda et al., 1999). In the present condition, urine shows abnormalities because VMAT2 also functions outside the central nervous system, including in the peripheral nervous system, adrenal medulla, and platelets (Eiden and Weihe, 2011). The reason abnormalities are detected in urine and not CSF may pertain to differences in monoamine and metabolite stabilities, processing, and ranges of normal values between brain and periphery. In any case, it appears that the pair of metabolically and clinically similar diseases, the AADC and VMAT2 deficiencies, could be screened for by urine testing, and then confirmed by corresponding gene sequencing, obviating lumbar puncturing. 66
90 The initial selection of treatment of the affected children on the basis of clinical phenotype alone (parkinsonism) led to severe, immediate worsening of the movement disorder. Subsequent identification of the underlying pathophysiology allowed the rational selection of an appropriate treatment. It has been suggested that the immediate worsening of the movement disorder observed in these patients upon treatment with L-DOPA may reflect a severe dyskinesia resulting from the overactivation of sensitized receptors. In this hypothesis, the supplementation with exogenous L-DOPA at a high dose would have been sufficient to overcome the vesicle-loading deficiency, resulting in excessive release of dopamine at the synapse. L- DOPA responsiveness has been observed in a pair of AADC-deficient siblings harbouring a G387A mutation (Chang et al., 2004). The mutation affected the binding site of the AADC protein, causing an approximately 60-fold decrease in affinity for the L-DOPA substrate. However, saturation of the enzyme by treatment with exogenous L- DOPA allowed sufficient enzyme activity to improve motor functioning in these patients. Given the partial functioning of VMAT2-P387L observed in vitro (see Chapter 4, Section 3.3), it is plausible that the supplementation of cytosolic dopamine would allow the transport mechanism to come to equilibrium with a sufficient quantal size to allow neurotransmission. The plausibility of this suggestion is supported by the L-DOPA responsiveness of the motor phenotype in VMAT2-LO mice (Caudle et al., 2007, Taylor et al., 2011). Another possibility that may underlie neurotransmission in these patients under conditions of an extreme excess of cytosolic dopamine include reverse efflux through the plasma membrane transporter. 67
91 Willemsen et al. suggested that, as in patients with TH deficiency (Willemsen et al., 2010), careful selection of minimal L-DOPA doses may rescue the dopamine-deficient motor phenotype and avoid extreme dyskinesia. This would have the additional benefit of restoring norepinephrine neurotransmission and ameliorating the autonomic features of the syndrome. As a treatment strategy, smaller doses of L-DOPA may indeed be effective. However, treatment with L-DOPA for an extended period of time in a setting of disrupted vesicular storage confers a potential risk of oxidative damage leading to neurodegeneration, and hypomorphic Vmat2 mice do exhibit age-related neurodegeneration (Caudle et al., 2007, Taylor et al., 2011). An additional clinical feature of note is the very high rate of major depression observed in the patients parents. Heterozygous mice possessing a single Vmat2 allele exhibit no overt motor phenotype, but do express a depressive behavioral phenotype (Fukui et al., 2007). Major depression is also observed in parents of patients with AADC deficiency and thought to be caused by clinically significant reductions in serotonin in these individuals with hemizygous defects in the serotonin pathway (Brun et al., 2010, Lee et al., 2012, Pons et al., 2004, Swoboda et al., 1999). To what extent mutations in the AADC and VMAT2 genes contribute to common depression and its heritability remains to be investigated. 68
92 Chapter 3 Identification of Disease-Causing Genetic Variant 1 Introduction In Chapter 2, the clinical features of a new autosomal recessive pediatric neurotransmitter disease are described, and the difficulties are discussed of determining a precise diagnosis by clinical characterization alone, comprising clinical history, neurological examination, biochemical investigations (including specific urine and cerebrospinal fluid investigations), enzyme analysis, and genetic sequencing of candidate genes. In this chapter, the discovery of the disease-causing variant is presented initially by genome-wide single nucleotide polymorphism (SNP) genotyping, homozygosity analysis, linkage analysis, and mutation screening. Additionally, the advent of whole-exome sequencing allowed the exclusion of the possibility mutations in other genes in the locus by whole-exome sequencing of the proband, as well as demonstrating the possibilities of genomic diagnosis of rare diseases in a clinically relevant timeframe. This is particularly relevant in the present case, in which a group of related diseases has an overlapping phenotypic spectrum that is difficult to discern by clinical investigations alone, but the determination of the underlying genetic etiology precisely determines the successful course of treatment. 69
93 2 Methods 2.1 Patient Samples The study was approved by the Hospital for Sick Children s research ethics board and parents provided informed consent. A total of 5 ml of whole blood was collected in EDTA tubes. Blood samples were stored at 4 C for up to 1 week. Genomic DNA was isolated from each sample using a QIAGEN FlexiGene DNA Kit according to manufacturer s specifications. Briefly, blood samples were diluted in buffer and centrifuged to obtain a cell pellet. The cell pellet was resuspended and incubated with a protease mixture at 65 C for 10 minutes to release genomic DNA into solution. This genomic DNA was then precipitated by the addition of isopropanol to a final concentration of 50% and centrifugation. The resulting genomic DNA pellet was then washed with 70% isopropanol and re-pelleted. After air drying, the genomic DNA was resuspended in water or Buffer FG Genotyping of Single Nucleotide Polymorphisms The genotyping of SNPs was performed by the Finnish Genome Center at the University of Helsinki. A 300K Illumina SNP microarray was used to genotype >300,000 SNPs in the genomic DNA of eight family members (V:2,3,6,7,8,9;VI:2; see Figure 6). 2.3 Homozygosity Analysis Regions of homozygosity across the genome were identified using PLINK v0.99s (Purcell et al., 2007). Raw data from the SNP genotyping array was parsed into the input file format for PLINK using custom PERL scripts. One set of input files was created for 70
94 data from each chromosome (i.e., data was divided among 22 sets of input files), and analysis was performed by chromosome. The parameters specified for homozygosity analysis were a minimum size of 100 kb for each homozygous region. Additionally, a SNP density of at least 1 SNP per 50 kb was mandated. Regions of homozygosity were identified separately in all eight genotyped individuals, and overlap among homozygous regions was then identified between family members. These shared regions of homozygosity were then further classified by the disease affection status of the individuals. 2.4 Linkage Analysis The complexity of this consanguineous family structure prohibited successful power simulation. To perform linkage analysis, it was necessary to alter the analyzed family structure to remove consanguineous connections. A modified family structure lacking consanguineous marriages was therefore used in this analysis. A subset of 2500 SNPs was selected for parametric linkage analysis. To ensure a maximally informative SNP subset, the selected SNPs had a minimal allele frequency of at least 0.4 in the available HapMap dataset (International HapMap Consortium 2003). An average spacing of approximately 1.0 Mb was selected to provide sufficient resolution across the genome. This subset was determined manually, and PERL scripts were designed to parse the data appropriately. 71
95 Parametric linkage analysis was performed using Merlin software (Abecasis et al., 2002). A fully penetrant autosomal recessive mode of inheritance was assumed. Marker allele frequencies were derived from marker allele frequencies in the HapMap dataset, and disease allele frequency was estimated at 1%. Analysis was performed independently for each chromosome. 2.5 Mutation Screening Sanger sequencing of candidate gene exons was performed to identify sequence variants within the homozygous region. Eight genes in the region were selected as candidates for mutation screening on the basis of neuronal function or localization: GRK5, EMX2, KCNK18, PRLHR, SLC18A2, GFRA1, VAX1, and NANOS1. Primers were designed to amplify the exons of each gene and approximately 50 bp of flanking intronic sequence (Table 12 and Table 13). The reaction was performed using Taq DNA polymerase (Stratagene) according to manufacturer s specifications. Cycling conditions incorporated an initial denaturation at 95 C for 5 minutes, followed by 30 cycles of 95 C for 30 s, 55 C for 30 s, and 72 C for 30 s, and a final extension step at 72 C for 10 minutes. Amplification products were visualized by gel electrophoresis, and purified from bands of the appropriate size using a Qiagen Gel Purification Kit according to manufacturer s specifications. Purified PCR products were sequenced using both forward and reverse primers in separate reactions. Sequences were compared to reference genome sequence using the BLAT tool in the UCSC Genome Browser. Putative variants were verified by manual inspection of electropherograms. 72
96 Table 12 Primer sequences for amplification of exons of candidate genes located at 10q26 Gene Exon Primer sequences Size GRFA1 1 TCA CTG GAT GGA GCT GAA CTT 300 bp CTT CCT TCC ACA TCC ACC AC 2 CGA AGG CTG GTC AAG CAT C 497 bp AAG TGA GTG GAG GAT GAG ATC AG 3 GGG GAG AAC AAG GAC AAC AA 398 bp CCA GAA ACA CTG TGC CAT TC VAX1 1 GCG GGG ACA TTC ATT CTT 534 bp GCC AAC AAC TTT CTC CCA AG 2 GCC CTC CAC ACA GTG TCT TT 463 bp CCC AAG GGT AGT TCT GTC CA 3/4 TCT GAA GCA AGC GAA AAA CA 496 bp TCG AGA GCG AAA CAC TCA AG NANOS1 1 AGT GGG CCC GAT AAA AGG 456 bp GTC GTC GTC CTC GTC GTA GT 2 CGC ACA CCA TCA AGT ACT GC 393 bp AGC GCC TCT AAG TTG CCA TA EMX2 1-1 ACC CCA AAC AAA CGA GTC C 490 bp ATG AGC CAG GGG TAG AAG GT 1-2 GGT AGG GGC GTC TAC TCC A 375 bp CCG CCT AGT TTC CCA ACA G 2 GTG AGC CCT TGG GAG GAC 474 bp CAG GCG TGG AAC CAG CTA C 3 GGA GGC TGG ACC TTA GGA CT 422 bp GTG AAC GTG TAT GCG GTT TG KCNK18 1 CAC ACG CAC CAT CCA CTT AG 486 bp CTT AAA GTG CCC AGG CAT GA 2 CAC CGA GCT TTG GTG TTG AT 392 bp GGA AGG GAG GGA AGA AGA GA 3-1 GTA TTT TCA AAA ACA ATG TGT AAA 500 bp ATG GTT GCA GTG TGT TCT GTT TCT C 3-2 GCA GAT GAA GCT GTC CCT CA 598 bp TGT CAC CTG AGA GAT AAT GAA ACC PRLHR 1-1 GTT GTT CTG TGG CCG GTT AT 418 bp AAG TTC GTC ACG TTG TGC AG 1-2 GGC TGA TCG TGC TGC TCT A 427 bp AGC TCC ACG TGA TAG GTG TG 1-3 CGC TAC GTC GTG CTG GTG 495 bp GAG CCA GTG GCA GAG CAG 1-4 GGT GAT CGT GGT GGT GTT C 489 bp CCC AAG CAA AGA GCA AGA CT 73
97 GRK5 1 GGA ATA ATG CGG TAG GCA AG 337 bp CTC TCC GAA GTG TCC TGC TC 2 TGG GAG GAA GAG TGT GTG TG 400 bp TGT GTC CAC TGA TTG GCA TT 3 GAG TTT GCC AGT CAC CTT CC 398 bp CCT CAG ACA ATT TTG CAT TCC 4 CAG GAG AAG GGG GTT GGT C 300 bp CAC TTA ACA GCT CCC CAT GT 5 GCT GCC AGA TGT ACC AGC A 350 bp TGG TAT AAC TTG GCT GAA GCT G 6 GCA TCT GCA TGG GTT GAG A 300 bp CGG GAG GTA ACT GAT TTT TAT ATG 7 CTC GAA GGT CCA GTC TCC AG 391 bp CAA GGC CAC TGA CTC TCT CC 8 AGG CAT CAC TGG GTC CTG 391 bp ATT AGA GGA CCA CGC CCT TC 9 ACT GAA AGG GAG GAG CAG GT 498 bp AGG GCT GAT TCC CAG AGC 10 AGG AGG TGG GAA GGA AAC AC 299 bp CAG GTA CCC AGC ACT GAG C 11 GAG TGG GCA TTT TCC TGT GT 399 bp AGT GCA TGA AGA GGC GAG AG 12 GGG CAC AGA GGA GAG TCA TC 484 bp CTC AGA CCC CTG TCC CTT C 13 AGC CCT GAA GCA AGA CCT TT 369 bp CAC ACT CTG CAC CAG CTC AC 14 CAT AGC AGT TCT GGG GGT GT 353 bp CTG CCG CCA AAC TCA TAT TC 15 CCA GTG GCT TTG CTG CTG 299 bp CAA GGG TCT CTA CTG TGG GTC T 16 CTG AGG GGA GAC TGC AAA AG 377 bp GTT CTA CGT CGA CGG GAT G 74
98 Table 13 Primer sequences for amplification of SLC18A2 exons SLC18A2 Primer sequences Size 0 GAC TGA CGG AGC CCA CTG 266 bp ACT GTG CCA CCT CCC AAA CT 1 GCG CTC GAG GCA GGT GAG 438 bp CCT GGA GGG GAT GTC TTT TT 2 CTC AGC TTC CCA AAG TCC TG 300 bp CCA CCA TGG ATT TTC CAG AC 3 TTT CAG AAA AGT CCA CCA AAC A 279 bp CGC CGT CCT TAG GTT GTA TT 4 AAG CAC AGG GTG GCT AAC AT 298 bp CAA CCA CCA CCA ATA CCT GA 5 AAG GCA CCC ACT TTC CTC TT 365 bp GGA CCT CTG TGT CAG TGC AA 6-8 CCC AAA GCC TTA TTG GAA CA 595 bp GCA GCA AAA CGA AAA TCA GC 9 AGG AGG CAG AAG CCA CTA CA 182 bp GGG GAC AAA TGC TCT TGA AT 10/11 GGG GCT TCG TTT TAT CTG CT 497 bp AAA TGT TTC TTG GTG ATT CTT GC 12 CCA CCC TTC TTC CTC CTG TT 324 bp CCT GCA GCC TCC TTC TAA GAT 13/14 CTG GCA GGG TGG TGA GTT TA 599 bp TTC TCA CCA TTT ATG TTT GAA GG 14 TTG CAT CTT TCA GTC TAC AAG ACA 269 bp CCA TGA GGA AGC TAT CAG GAA 15 CGA TTG CTC CAA ATG ACT GG 398 bp CAA TCG ACC ATA ACC ATG GAA 2.6 Whole-Exome Sequencing Whole-exome sequencing was performed to exclude the possibility of additional mutations present in genes within the homozygous disease-associated region at 10q26. Exon sequences were first enriched using an Agilent SureSelect V4 50-Mb capture kit that uses a biotinylated library of RNA baits and streptavidin-coated magnetic beads to 75
99 specifically capture exon sequences. The exon-enriched samples were sequenced using the Illumina Hiseq 2000 next-generation sequencing system. These protocols were performed by The Centre for Advanced Genomics (TCAG). The resulting sequence data was filtered for coding variants. 2.7 TaqMan SNP Genotyping Assay Genotyping was performed to confirm the absence of the identified mutation in controls. Differentially fluorescently labelled TaqMan probes were synthesized to represent each of the SLC18A2 c.1160c and c.1160t alleles. Within an amplification reaction, fluorescence is released from probes that hybridize to the template DNA through the 5' nuclease activity of DNA polymerase. The ratio of the detected fluorescent labels thereby determines the genotype of the individual at that locus. The samples were processed in 96-well format. This assay was performed by TCAG. 3 Results 3.1 Homozygosity Analysis Homozygosity mapping of the microarray results identified a single homozygous 3.0-Mb interval in 10q that was uniquely shared by the affected family members (chr10:117,937, ,972,346), and not homozygous in the unaffected family members who were genotyped (Figure 7). Because of the consanguinity of the family structure, a large number of homozygous regions were expected in individual genomes. Indeed, an average of 30 homozygous regions of >500 kb were discovered in each genome. These regions of homozygosity in individual genomes ranged from 780 kb to 68 Mb. Several of these regions were 76
100 inherited in their entirety by multiple siblings, and overlapped with homozygous regions inherited by cousins. A limitation of this homozygosity analysis was related to the density of SNPs. Whereas the region at 10q was the only homozygous region shared by the affected individuals, the parameters for the analysis specified a minimum size of 100 kb, and it is possible that smaller homozygous regions would not have been identified. The average size of a gene in the human genome is 27 kb (Lander et al., 2001), smaller than the minimum identifiable region in this analysis. More dense SNP coverage would allow higher confidence assessment of smaller homozygous regions. 77
101 Figure 7 Single nucleotide polymorphism alleles for all genotyped family members in the homozygous region uniquely shared by affected family members. Black, affected; white, unaffected; square, male; circle, female. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission. 78
102 3.2 Linkage Analysis Parametric linkage analysis produced a significant logarithm of odds (LOD) score of 4.1 within the region of 10q25 26 (Figure 8). One additional significant LOD score of 3.1 on chromosome 3 did not correspond to a region of homozygosity shared by all affected individuals. A small positive LOD score was also obtained on chromosome 16, also not representing a region of homozygosity shared by all affected individuals (Figure 9). Methodological limitations, however, were several. These included the difficulty in calculating a logarithm of odds (LOD) score for each putative locus in a highly consanguineous family. For the purposes of calculation, the consanguineous connections in the pedigree had to be removed, thereby not accounting for all of the available inheritance information and reducing the power of the analysis. 79
103 Figure 8 Multipoint LOD scores estimated across chromosome 10 80
104 Figure 9 Multipoint LOD scores estimated across chromosomes 3 and 16 81
105 3.3 Characterization of Genes in the Locus The homozygous region at 10q contained 26 known protein-coding genes (Figure 10), of which eight genes had known neuronal localization or function: EMX2, GFRA1, GRK5, KCNK18, NANOS1, PRLHR, SLC18A2, and VAX1. The known function of these eight genes is summarized in Table 14. The exons and exon intron boundaries of each of these genes were sequenced in one affected patient, and all identified sequence variants are listed in Table 15. Additionally, KIAA1598 was not initially identified as a candidate (and therefore not initially sequenced), but has since been annotated to have neuronal localization and is included in Table 14. Sequencing revealed two nonsynonymous variants among the eight sequenced genes: c.847a G in PRLHR and c.1160c T in SLC18A2. The variant in PRLHR was previously identified in the dbsnp database with a global minor allele frequency of 0.17; this variant is therefore unlikely to be causative of this disease. The c.1160c T variant in SLC18A2, however, appears to be novel and predicts a substitution of proline with leucine at position 387 (p.p387l) in the VMAT2 protein. 82
106 Table 14 Genes of known neuronal function or localization present in the disease-associated bomozygous region Gene Functional Information (Meyer et al., 2013) EMX2 Homeobox-containing transcription factor homologous to empty spiracles gene in Drosophila melanogaster. Expressed in the dorsal telencephalon during development and is proposed to pattern the neocortex into defined functional areas. GFRA1 A receptor for glial cell line derived neurotrophic factor (GDNF) and neurturin (NTN). Candidate gene for Hirschsprung disease. GRK5 G protein coupled receptor kinase 5. Accumulates in Lewy bodies, a histological hallmark of Parkinson s disease (Arawaka et al., 2006). KCNK18 Outward-rectifying potassium channel associated with migraine with aura. KIAA1598 Involved in neuronal polarization NANOS1 Zinc-finger RNA-binding protein expressed in the developing nervous system and adult brain. PRLHR Prolactin-releasing hormone receptor expressed in the brain and pituitary gland. Transcription of this gene was shown to be regulated by dopamine D2 receptor agonist bromocriptine (Ozawa et al., 2002). SLC18A2 Vesicular monoamine transporter 2 responsible for packing monoamines into synaptic vesicles. VAX1 Homeodomain-containing transcription factor that may play a role in development of the anterior ventral forebrain and visual system. 83
107 Figure 10 Genes present within the homozygous region shared by all affected individuals in the family. C10orf82, chromosome 10 open reading frame 82; CACUL 1, CDK2-assocaited cullin domain 1; CASC2, cancer susceptibility candidate 2 (non-protein coding); CCDC172, coiled-coil domain containing 172; EIF3A, eukaryotic translation factor 3 subunit A; EMX2, empty spiracles homeobox 2; ENO4, enolase family member 4; FAM204A, family with sequence similarity 204 member A; GFRA1, GDNF family receptor alpha 1; GRK5, G protein coupled kinase 5; HSPA12A, heat shock 70 kda protein 12A; KCNK18, potassium channel subfamily K member 18; KIAA1598, Shootin1; MIR, microrna; PDZD8, PDZ domain containing 8; PNLIP, pancreatic lipase; PNLIPRP, pancreatic lipase related protein; PRDX3, peroxiredoxin 3; PRLHR, prolactin-releasing hormone receptor; RAB11FIP1, RAB11 family interacting protein 2 (class I); SFXN4, sideroflexin 4; SLC18A2, solute carrier family 18 member 2; SNORA19, small nucleolar RNA H/ACA box 19; VAX1, ventral anterior homeobox 1. Image from UCSC Genome Browser [ chr10 (q25.3-q26.11) 10p14 10p13 p q q21.3 q22.1 q q q25.1 q q26.3 Scale chr10: GFRA1 GFRA1 GFRA1 JA CCDC172 PNLIPRP3 PNLIP PNLIPRP1 PNLIPRP1 PNLIPRP1 PNLIPRP1 PNLIPRP2 PNLIPRP2 PNLIPRP2 1 Mb hg19 118,500, ,000, ,500, ,000, ,500, ,000,000 UCSC Genes (RefSeq, GenBank, CCDS, Rfam, trnas & Comparative Genomics) C10orf82 C10orf82 HSPA12A HSPA12A ENO4 DQ ENO4 KIAA1598 KIAA1598 KIAA1598 KIAA1598 KIAA1598 AK KIAA1598 KIAA1598 VAX1 VAX1 BC MIR3663 KCNK18 SLC18A2 SLC18A2 PDZD8 EMX2 EMX2OS EMX2OS EMX2 RAB11FIP2 RAB11FIP2 CASC2 CASC2 CASC2 CASC2 CASC2 FAM204A FAM204A PRLHR PRLHR Mir_584 CACUL1 CACUL1 NANOS1 PRDX3 EIF3A EIF3A EIF3A PRDX3 SNORA19 SNORA19 FAM45B FAM45B FAM45B FAM45B FAM45B BC SFXN4 SFXN4 SFXN4 GRK5 GRK5 GRK5 84
108 Table 15 Sequence variants identified among eight candidate genes in the disease-associated locus by direct sequencing of exons Gene Segment Sequence Variation Present in dbsnp EMX2 Exon 1 none Exon 2 none Exon 3 none GRK5 Exon 1 none Exon 2 none Exon 3 c.149-6g A rs KCNK18 Exon 1 none Exon 2 none Exon 3 none PRLHR Exon 1 c.847a G (I283V) rs SLC18A2 Exon 1 none Exon 2 none Exon 3 none Exon 4 none Exon 5 none Exon 6 c c T rs Exon 7 c c T rs Exon 8 c c A rs Exon 9 none Exon 10 none Exon 11 none Exon 12 c t C rs Exon 13 c.1160c T (P387L) Exon 14 c a G rs Exon 15 none Exon 16 c t C rs VAX1 Exon 1 none Exon 2 none Exon 3 none Exon 4 none GRFA1 Exon 1 none Exon 2 none Exon 3 none 85
109 3.4 Whole-Exome Sequencing of the Proband We also performed whole-exome sequencing in the proband, and the data were filtered to reveal 9,821 coding variants in the genome. Of these, 3,900 coding variants were present in the homozygous state. This large number reflects the extent of homozygosity in the genome of this individual resulting from her consanguineous background. A total of 70 homozygous, non-coding variants were not previously represented in the NCBI dbsnp database. As a member of an isolated, Bedouin population, there may be poor representation of this patient s particular ethnic background among the ethnically diverse samples genotyped to date. This presents a current limitation of the ability to exclude variants on the basis of their presence in the general population. In the absence of a clear functional candidate, it would be warranted to perform whole-exome sequencing on additional affected and unaffected family members to narrow the list of shared homozygous sequence variants, and identify those that are present homozygously more broadly within the pedigree. However, this analysis independently identified the homozygous variant in SLC18A2 (VMAT2-P387L) and the aforementioned c.847a G variant in PRLHR, and revealed no other novel non-synonymous variant in the linked region of shared homozygosity at 10q Identification of VMAT2 Variant as a Causative Candidate The identified novel variant in SLC18A2 (c.1160c T) was present in exon 13 (Table 15) and predicts a substitution of proline with leucine at position 387 (p.p387l) in the VMAT2 protein. To confirm that the variant segregated with the disease, the region was 86
110 sequenced in all available affected and unaffected family members, including siblings and parents. The mutation was present in homozygous form in all affected family members and was not present, or was present in heterozygous form, in unaffected siblings (Figure 11). All genotyped parents carried the variant in heterozygous form. The SLC18A2 c.1160c T variant has not previously been identified in the literature, and is not listed in dbsnp or the 1000 Genomes Database. 87
111 Figure 11 Electropherogram depicting sequence variation in unaffected and affected family members. Top panel, unaffected sibling with wild-type genomic sequence; middle panel, asymptomatic parent (IV:3) possesses the SLC18A2 c.1160c T variant in heterozygous form;; lower panel, proband (V:6) is homozygous for SLC18A2 c.1160c T. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission. 88
112 3.6 Controls To confirm that the SLC18A2 c.1160c T variant is not present in unaffected members of the extended kindred, TaqMan genotyping was performed on DNA samples isolated from direct relatives of the patients. The SLC18A2 c.1160c T variant is not present in homozygous form in 78 unaffected members of the extended family, 26 of whom do carry it in the heterozygous state. In addition, as one of the most studied candidate genes for involvement in Parkinson s disease, SLC18A2 was previously screened in 704 healthy individuals of diverse ethnic backgrounds and 452 Parkinson s disease patients (Burman et al., 2004, Glatt et al., 2001, Iwasa et al., 2001), none of whom had the c.1160c T change. The 1000 Genomes Database (Abecasis et al., 2010) identifies 44 missense variants in SLC18A2 that are not associated with an overt phenotype, but none within the same codon. 4 Discussion Collectively, the results presented in this chapter identify and confirm SLC18A2 c.1160c T as the disease-causing mutation in this family. Consistent with the observed autosomal recessive mode of inheritance, the mutation is present in homozygous form in all affected individuals, and those who possess the mutation in heterozygous form do not exhibit the movement disorder phenotype. All affected individuals are direct descendants of individuals I:1 and I:2 (see Figure 6). The expression of the movement disorder phenotype begins in the fifth generation of this consanguineous kindred, consistent with a founder mutation likely arising in one of these two individuals. 89
113 No disease-associated mutations have been identified in SLC18A2 (VMAT2) to date. There is no evidence of the SLC18A2 c.1160c T variant in genomic databases of healthy individuals. Additionally, the 1000 Genomes Database identifies no variants predicted to cause a premature stop codon in VMAT2. Of 44 identified VMAT2 missense variants in this database, 12 are predicted to be deleterious ( probably damaging ) by PolyPhen (a further 10 are possibly damaging ). By the SIFT algorithm, 20 were considered deleterious. In the Exome Variant Server of the NHLBI GO Exome Sequencing Project, a single frameshift deletion allele was identified, but this deletion involved the coding sequence for the final amino acid of the protein and may therefore be benign. Importantly, none of the 25 missense variants identified in this database (a subset of the 44 missense variants catalogued in the 1000 Genomes Database) were present in homozygous form. In addition, the screening of SLC18A2 in 704 healthy individuals of diverse ethnic backgrounds and 452 Parkinson s disease patients identified only 5 missense variants (only two of which are not missense variants represented in the 1000 Genomes Database) (Burman et al., 2004, Glatt et al., 2001, Iwasa et al., 2001). Four of these missense variants were functionally screened, and determined to have only modest effects on protein function (Burman et al., 2004). It has been suggested that VMAT2 exhibits a low rate of variation relative to genes of related function, such as the dopamine and serotonin plasma membrane transporters (DAT and SERT, respectively) (Glatt et al., 2001). This implies functional significance of the protein, and therefore suggests that a dramatically deleterious mutation in VMAT2 would have correspondingly dramatic phenotypic effects. Indeed, the 1000 Genomes 90
114 Database reports 151 missense mutations in DAT and 317 missense mutations in SERT, both transmembrane proteins of comparable length to VMAT2 and related function in the central nervous system. Importantly, 726 missense mutations are reported in the 1000 Genomes Database for the non-neuronal VMAT isoform, VMAT1. In comparison with the respective 44 missense mutations currently identified in VMAT2 in the same sample set, these figures therefore illustrate that a functionally deleterious mutation in VMAT2 would be predicted to have significant phenotypic effects particularly if identified in homozygous form, as observed here for the VMAT2 p.p387l variant. Proline residues located adjacent to transmembrane domains have major structural implications, and are overrepresented among disease-causing substitutions in transmembrane domains (Partridge et al., 2004). The P387L substitution is immediately adjacent to a transmembrane segment (Figure 12), and the constraints imposed by the proline residue on the flexibility of the peptide backbone are likely important for insertion of the transmembrane domain into the membrane to form the proper tertiary configuration. Sequence alignment shows that the P387 residue is highly conserved through evolution, and is therefore of likely structural or functional significance. It is also conserved in the VMAT1 paralog, as well as in C. elegans CAT-1 the single vesicular monoamine transporter in nematode (Figure 13) (Duerr et al., 1999). Interestingly, the residue is not conserved in the vesicular acetylcholine transporter (VAChT), which maintains 39% identity to VMAT2, implying that P387 may have a specific implication in monoamine transport. 91
115 Furthermore, the P387L substitution is predicted to be probably damaging by the PolyPhen-2 algorithm (Adzhubei et al., 2010) with a score of This algorithm considers eight sequence-based and three structure-based predictive features, and was developed using large datasets of known damaging (Mendelian disease-causing or protein destabilizing) and nondamaging alleles. The evidence presented in this discussion the segregation of the variant consistent with an autosomal recessive inheritance pattern, the absence of the variant in healthy individuals of ethnically diverse populations, evolutionary conservation of the residue, its potential structural relevance, and the low rate of genetic variation in SLC18A2 together predicts the likely functional relevance of the p.p387l substitution in VMAT2 (SLC18A2 c.1160c T). 92
116 Figure 12 Predicted structure of VMAT2 comprises 12 transmembrane domains, a large lumenal loop including four proposed glycosylation sites, and both N-terminal and C-terminal cytoplasmic regions. Proline residue 387 is located immediately adjacent to the insertion of transmembrane domain X. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission. 93
117 Figure 13 Multiple sequence alignment of VMAT2 in the region of the p.p387l variant. TM9 and TM10 represent sequences associated with transmembrane domains 9 and 10, respectively. Residues that differ from human VMAT2 sequence are indicated in gray. Amino acid position 387 is indicated by an asterisk. Homo, homo sapiens (human); Patient, proband; Pan, Pan troglodytes (chimpanzee); Macaca, Macaca mulatta (Rhesus macaque); Mus, Mus musculus (mouse); Rattus, Rattus norvegicus (rat); Canis, Canis familiaris (dog); Bos, Bos taurus (cow); Monodelphis, Monodelphis domestica (opossum); Gallus, Gallus gallus (chicken); Tetraodon, Tetraodon nigroviridis (pufferfish); Danio, Danio rario (zebrafish); Drosophila, Drosophila melanogaster (fruit fly); C. elegans, Caenorhabditis elegans (nematode); hvmat1, human VMAT1 isoform. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission. 94
118 Chapter 4 Functional Characterization of the Disease-Causing Variant 1 Introduction The identification of the putative disease-causing mutation is described in Chapter 3. As presented in that chapter, the SLC18A2 c.1160c T variant segregates in a manner consistent with the observed autosomal recessive inheritance of the disease. The corresponding VMAT2 p.p387l substitution is predicted to have a structural role within the protein, and the proline residue at this position is conserved in all vesicular monoamine transporters isoforms in all species. The phenotype expressed by the patients harboring SLC18A2 c.1160c T is similar to that expressed by patients with biosynthetic deficiencies of the biogenic amine neurotransmitters, predicting a loss of VMAT2 function that would interrupt vesicular storage in the brain and expose biogenic amines to degradation. The patient CSF neurotransmitter metabolite profile presented in Chapter 2 is consistent with this hypothesis, and the hypothesis is tested in the present chapter. 2 Methods 2.1 Construct Design and Site-Directed Mutagenesis Human VMAT2 cdna sequence was acquired from the Mammalian Gene Collection and subcloned into pcdna3.1a (Invitrogen). The primers used for amplification were designed to incorporate restriction sites flanking the open reading frame (Table 16). The P387L (c.1160c T) mutation was introduced by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis kit (Stratagene). The oligonucleotide sequences 95
119 for site-directed mutagenesis are presented in Table 17. The final sequence of the vector was confirmed by sequencing using standard M13 primers that are complementary to vector sequences. Table 16 Primers to amplify SLC18A2 cdna sequence for subcloning into pcdna3.1 Primer Sequence VMAT2-KpnI-F CAT GGT ACC AGT AGT ATG GCC CTG AGC GAG CTG G VMAT2-PmeI-R CAT GTT TAA ACA CTA CTT CAG TCA CTT TCA GAT TCT TCA TC Table 17 Oligonucleotides to introduce cytosine to thymine substitution at position 1160 of SLC18A2 using site-directed mutagenesis Primer Sequence VMAT2-P387L AAA CAT TTA TGG ACT CAT AGC TCT GAA CTT Quikchange-F TGG AGT TGG TTT TGC VMAT2-P387L GCA AAA CCA ACT CCA AAG TTC AGA GCT ATG Quikchange-R AGT CCA TAA ATG TTT 2.2 Cell Culture and Transfection The wild type and mutant constructs were expressed in Cos7 cells, as there is no endogenous VMAT1 or VMAT2 expressed in these cells. Cos7 cells were maintained in Dulbecco s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) at 37 C and 5% CO 2. Cells were passaged by trypsinization, except immediately prior to transfection for the serotonin uptake assay; cells prior to the uptake assay were removed from the surface of the plate by scraping. In both cases, cells were diluted 1:10 for seeding onto fresh plates. 96
120 Transient transfection of Cos7 cells was performed at 80% confluency using Fugene HD transfection reagent (Roche) according to manufacturer specifications. After 48 hours, cells were washed once in 1 phosphate-buffered saline (PBS), scraped, and pelleted for further experiments. Cells from three 10-cm plates that had been transfected in parallel were combined into a single pellet. 2.3 Serotonin Uptake Assay Vesicular serotonin uptake was assayed in a heterologous cell system. VMAT2 transport activity was measured by incubating membrane preparations with tritiated serotonin, followed by rapid washing and filtration to retain vesicles with trapped substrate. Transiently transfected Cos7 cell pellets were immediately resuspended in 320 mm sucrose-hepes buffer (ph 7.4) and sonicated by 20 1 s pulses. Lysates were centrifuged at 4,000g for 5 min, and supernatants were stored at 80 C until use. Reaction buffer contained 150 mm choline gluconate, 10 mm HEPES-Tris (ph 7.4), 2 mm Mg-ATP, and 90 nm 3 H-serotonin (New England Biolabs). Where indicated, reaction buffer also contained 10 µm reserpine. To each tube was added 10 µl of microsomal lysate, followed by incubation at 30 C for the specified time. Reactions were stopped by rapid filtration, and retained serotonin was measured by scintillation counting. Retained serotonin was also measured from samples incubated on ice and filtered immediately upon addition of the lysate, as well as from reaction buffer alone. 2.4 Western Blotting Western blots were performed by equal loading of protein lysates onto 10% SDS-PAGE gels. Proteins were transferred to PVDF membrane in a Bio-Rad semidry transfer cell at 97
121 1 ma/cm 2 for 45 min. Transfer buffer was 200 mm glycine, 25 mm Tris-HCl, ph 7.5, and 20% methanol. The membrane was blocked for 1 h in 50 mm Tris-HCl, ph 7.5, 150 mm NaCl, and 0.05% Tween 20 (TBS-T) with 5% skim milk. Blots were incubated in blocking solution with primary antibody for 1 h at 4 C, washed three times with TBS-T, and then incubated with secondary antibody in blocking solution for 1 h at 4 C, followed by three TBS-T washing steps and detection by chemiluminescence according to manufacturer s recommendation. The specificity of a commercial antibody against a 20 amino acid C-terminal peptide of VMAT2 (Abcam) was verified by western blot, showing no non-specific signal in untransfected or vector-transfected cultures (see inset to Figure 19). Secondary antibody was horseradish peroxidase conjugated anti-rabbit antibody. 2.5 Sucrose Gradient Centrifugation To compare the subcellular localization of the wild-type and p.p387l VMAT2 proteins in Cos7 cells used for serotonin uptake assays, cell lysates were fractionated by sucrose gradient centrifugation, and the presence of the protein in each fraction was visualized by western blot. Transiently transfected Cos7 cell pellets were immediately resuspended in 320 mm sucrose-hepes buffer (ph 7.4) and sonicated by 20 1 s pulses. Lysates were centrifuged at 4000 g for 5 min, and protein concentrations were measured by Bradford assay to ensure equal protein loading on gradients tested in parallel. Supernatants were immediately applied to a linear sucrose gradient of 20% 55% sucrose (approximately M) in HEPES (ph 7.4) and centrifuged at g for 16 h at 4 C. 98
122 Fractionation was performed using a Brandel Density Gradient Fractionation system. Aliquots of equal volume were separated by SDS-PAGE on 4% 12% Bis-Tris gels (Invitrogen). Blots were prepared as described in Section 2.4. Linear sucrose gradients were generated by tilted tube rotation on a BioComp Gradient Master. The linearity of select gradients was confirmed by the addition of ATP in the heavy phase (50% sucrose) prior to gradient generation, and measurement of absorbance at 260 nm during fractionation (Figure 14). Figure 14 Visualization of ATP by absorbance at 260 nm to illustrate sucrose gradient linearity The consistency of gradient fractionation between samples was demonstrated by the measurement of absorbance at 280 nm during the fractionation process. An initial peak in the first fraction, likely representing free cytosolic contents, is followed by a trimodal peak present approximately between fractions 7 and 12. All fractionations exhibited the same pattern of absorbance, and parallel fractionations produced absorbances of equal intensity (Figure 15). This demonstrates the reproducibility of the 99
123 gradient centrifugation process. Some minor shift in the absorbance peaks relative to particular numbered fractions resulted from variation in the fractionation process. Figure 15 Consistent fractionation demonstrated by absorbance at 280 nm of parallel sucrose gradient fractionations Protein markers of cellular compartments were visualized by western blot. GAPDH was used as a cytosolic marker, and was present only fractions 2 and 3, verifying a lack of 100
124 cytosolic contamination in later fractions. Transferrin receptor (TfR) was used as a marker for recycling endosomes, and was reproducibly visualized at highest concentration in fractions 5 9, with faint signal detectable in later fractions. Calnexin was used as a marker for endoplasmic reticulum, and was present primarily in fractions 9 13, but with signal detectable in all fractions. Overlapping signal demonstrates incomplete separation of the microsomal components of the cell, but the relative order of components is as expected. Figure 16 Markers of cellular compartments in sucrose gradient fractions 3 Results 3.1 Steady-State Protein Levels of Transfected Protein Unaffected by P387L Variant in Heterologous System To determine the effect of the p.p387l mutation on VMAT2 transport activity, we transiently expressed wild-type and mutant human VMAT2 in Cos7 cells. Immunoblotting of membrane preparations confirmed equivalent protein levels of VMAT2 in parallel transfections, suggesting no major defect in protein processing (Figure 17). 101
125 Figure 17 Parallel transient transfections of wild-type and p.p387l VMAT2 in Cos7 cells, and vector-transfected control. GAPDH, protein loading control. 102
126 The wild-type and p.p387l VMAT2 constructs exhibit the same intensity and banding pattern on western blot, indicative of multiple glycosylation forms. This banding pattern is better illustrated in Figure 18. In transiently transfected Cos7 cells, bands of approximately 57 and 45 kda are most prominent, with a lower molecular weight band of lesser intensity at approximately 35 kda. These bands are consistent with those observed in previous studies by Tong et al., who examined the expression of VMAT2 in autopsied human brain tissue, and reported four protein bands of approximately 75, 55, 45, and 35 kda (Tong et al., 2011). A pair of higher molecular weight bands are also visible, but have a molecular weight of approximately 90 kda in this heterologous cell system. The bands less distinct than is observed in brain tissue, and multiple bands of intermediate size are also visible and appear as a smear this was interpreted as an artifact of overexpression in the heterologous system. Because glycosylation does not substantially affect VMAT transport activity (Yelin et al., 1998), no further investigation of the identity of the protein bands was performed. 103
127 Figure 18 Comparison of banding pattern observed for wild-type and p.p387l VMAT2 expressed transiently in Cos7 cells. unt, untransfected; WT, wild-type. 3.2 Highly Reduced Transport Activity of VMAT2 p.p387l in Heterologous System The p.p387l variant of VMAT2 was assayed for its ability to transport tritiated serotonin in a crude vesicle lysate isolated from Cos7 cells transfected in parallel with either the wild-type or p.p387l VMAT2 construct, or vector alone. After incubation with tritiated serotonin, samples were rapidly filtered to retain microsomes, and the trapped substrate retained on the filter was measured by scintillation counting. 104
128 The p.p387l variant of VMAT2 showed dramatically decreased serotonin uptake activity relative to wild-type VMAT2 (Figure 19). The sensitivity of the assay did not permit quantification of the uptake kinetics of p.p387l VMAT2, or quantification of the relative decrease in activity compared with the wild-type protein. In parallel time courses, the activity of the p.p387l mutant of VMAT2 was indistinguishable from background, as measured in lysates isolated from vector-transfected Cos7 cultures. 105
129 Figure 19 Vesicular uptake of tritiated serotonin by the wild-type and p.p387l human VMAT2 transiently expressed in a heterologous Cos7 cell system. Inset: Expression of VMAT2 in lysates used for uptake assay. 5-HT, serotonin; wt, wild-type. From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission. 106
130 3.3 Measurable Residual Transport Activity of VMAT2 p.p387l Protein Because a complete lack of VMAT2 function would be expected to be lethal on the basis of mouse models (Wang et al., 1997), the activity of the p.p387l mutant of VMAT2 was more closely investigated. The specific VMAT inhibitor reserpine was used to better distinguish VMAT2 transport activity from the nonspecific uptake or retention of substrate on the filters observed in lysates from vector-transfected cultures. Microsomal uptake of tritiated serotonin was assayed at the 10-minute timepoint the time at which the reaction begins to saturate (Figure 19) with and without the addition of 10µM reserpine. The p.p387l mutant VMAT2 exhibited weakly measurable uptake that was approximately three times the background level measured with the addition of reserpine (Figure 20). Therefore, the VMAT2 p.p387l mutation reflects a severe, but not complete, loss of protein function. 107
131 Figure 20 Vesicular uptake of tritiated serotonin by wild-type and p.p387l human VMAT2 transiently expressed in a heterologous Cos7 cell system after 10 minutes with and without the addition of the specific VMAT inhibitor reserpine (10 µm). From New England Journal of Medicine, Rilstone JJ, Alkhater RA, Minassian BA, Brain Dopamine Serotonin Vesicular Transport Disease and Its Treatment, 368, Copyright 2013 Massachusetts Medical Society. Reprinted with permission. 108
132 3.4 Subcellular Localization of Protein in Heterologous System Sucrose gradient centrifugation was performed to compare the subcellular localization of wild-type VMAT2 protein with that of VMAT2-P387L in the transiently transfected Cos7 lysates that were used for the serotonin uptake assay. Preliminary data reveals signal in the majority of gradient fractions for both proteins, with the highest concentrations of protein in fractions 7 12 (Figure 21). The broad distribution of VMAT2 protein across gradient fractions is indicative of either broad localization in multiple microsomal components of the cell, or it is reflective of ER contamination in multiple fractions. In either case, the lack of confinement of VMAT2 to its expected compartment recycling endosomes is likely a consequence of overexpression in the transient transfection setting. Given the expected elution of fractions containing the highest proportion of ER in fractions 9 13 relative to the earlier elution of recycling endosomes in fractions 4 9 (Figure 16), extensive misfolding of the mutant protein relative to wild-type VMAT2 would be expected to produce a rightward shift in the distribution of VMAT2 across fractions. No such pattern is observed. This result is therefore inconclusive, but does suggest no significant difference in localization between the wild-type and mutant VMAT2 proteins, and likely a lack of specificity of protein localization as a result of protein overexpression. This preliminary result therefore suggests that the difference in serotonin uptake between wild-type VMAT2 and VMAT2-p.P387L is not reflective of a drastic difference in the subcellular distribution of the proteins, but rather a true difference in transport function. Verification of this observation should be performed 109
133 using an alternative technique such as immunofluorescence. In addition, data regarding the subcellular localization of VMAT2-P387L in the physiological setting should be pursued using cell lines with stable expression of the constructs to avoid artifacts of overexpression. Figure 21 Sucrose gradient centrifugation of transiently transfected Cos7 lysates 110
134 4 Discussion Direct characterization of the mutant VMAT2 protein in a heterologous cell system in this study revealed a severe detriment of vesicular transport function. This could be the result of poor incorporation of the transporter into vesicle membranes or lost transport activity; data regarding the relative subcellular distribution of wild-type and p.p387l VMAT2 was inconclusive, but suggestively not the major factor underlying loss of function. The p.p387l substitution is immediately adjacent to transmembrane domain 10 (see Figure 12). Prolines can have significant structural and functional significance in transmembrane domains because the constraints imposed by their ring structure induce kinks in α-helices. Prolines are also preferentially located at the central and terminal regions of transmembrane segments (Cordes et al., 2002). Though prolines constrain the angle of the peptide backbone, they also induce flexibility in the region through the disruption of stabilizing hydrogen bonds in upstream amino acids, and have therefore been implicated as hinge points with possible functional relevance (Cordes et al., 2002). Prolines are prevalent in the transmembrane domains of ion channels and transport proteins, but not the transmembrane segments of proteins without transport function, inspiring a hypothesis that cis-trans isomerization may underlie the conformational change inherent in the transport process. Additionally, the disruption of hydrogen bonds in the peptide backbone that is imposed by proline exposes a carbonyl group, providing a site for cationic ligand binding or proton translocation (Brandl and Deber, 1986, Williams 111
135 and Deber, 1991). Finally, Partridge et al. reported an overrepresentation of proline substitutions among disease-causing mutations located in transmembrane domains, indicating a high phenotypic propensity for amino acid substitutions of this nature (Partridge et al., 2004). With respect to VMAT2 structure specifically, a hydrogen bond between D400 in transmembrane domain 10 and Y342 in transmembrane domain 8 was predicted in a homology model of the rat protein, rvmat2, and the D400 residue was verified experimentally to be critical to transport activity (Yaffe et al., 2013). The relative configuration of transmembrane domain 10 and transmembrane domain 8 is therefore functionally critical, underscoring the likely importance of proline constraints on the peptide backbone near the insertion of transmembrane domain 10. This, however, does not exclude the possibility of a role for the P387 residue in conformational change, ligand binding of the cationic monoamine substrates, or proton translocation. Direct biochemical characterization in a heterologous system confirms the functional significance of the P387L substitution. This functional biochemical evidence in combination with genetic linkage data, bioinformatic analysis, and the observed treatment effect confirms the causative role of the VMAT2 p.p387l mutation in this autosomal recessive pediatric neurotransmitter disorder. 112
136 1 Introduction Chapter 5 General Discussion and Future Directions This thesis presents the identification of a new pediatric neurotransmitter disease and its causative mutation in the gene encoding the brain vesicular monoamine transporter (VMAT2; SLC18A2), as well as demonstrating the utility of next-generation sequencing for the clinical diagnosis of extremely rare diseases. This discovery extends the spectrum of pediatric neurotransmitter disease pathophysiology to include defects in the vesicular transport and storage of biogenic amines. This is the first demonstration of a clinical phenotype directly associated with VMAT2 deficiency, providing new insight on the physiological consequences of perturbed monoamine homeostasis in humans. The research directions and experiments proposed in this chapter are designed to address open questions stemming from the results presented here pertaining to the role of VMAT2 in monoamine physiology (Section 2), the pathophysiology of VMAT2 deficiency (Section 3), and the diagnosis and treatment of pediatric neurotransmitter diseases (Section 4). In Section 2, a series of experiments are proposed to further probe the dose dependence of VMAT2 function and its role as a modulator of monoamine homeostasis, leveraging the P387L variant to demonstrate the effect of reduced VMAT2 function without the confounding effects of reduced protein levels or off-target effects of drug inhibitors. 113
137 Subcellular localization experiments in relevant cell culture models are proposed to explore any mechanisms of regulation of VMAT2 in the context of reduced function. These results will also inform data derived from a proposed mouse model incorporating the corresponding P390L mutation. In Section 3, some investigations are proposed to further explore the pathophysiology of VMAT2 deficiency by obtaining more data in a clinical context. Positron emission tomography (PET) is proposed to visualize aberrant VMAT2 and dopamine distribution in vivo. Genetic screening of patient cohorts with neurological disorders is also proposed to identify additional patients with VMAT2 mutations and better characterize the range of phenotypes associated with VMAT2 deficiency. In Section 4, challenges in the diagnosis of VMAT2 deficiency are described and some methodologies are described that may address the current limitations of clinical diagnosis. In addition, the promise and challenges of next-generation sequencing for rare disease diagnosis are outlined. In sum, this section outlines three different directions for continued research stemming from the results of this thesis and discusses the relevance of these results to clinical practice. 2 Identification of the VMAT2 p.p387l Mutation: Impact on Knowledge of Monoamine Physiology As the first demonstration of a phenotype associated with VMAT2 deficiency in human patients, the results presented in this thesis confirm that significant loss of VMAT2 114
138 function is associated with a phenotype that is within the spectrum of those caused by biosynthetic monoamine deficiencies. The VMAT2 p.p387l variant, which is associated with viability despite corresponding to a near complete but not complete loss of VMAT2 function as measured by functional assay in a heterologous system, provides an interesting opportunity to further probe the role of VMAT2 in monoamine physiology and homeostasis. In mice, the complete knockout of Vmat2 results in absent exocytotic monoamine neurotransmission, and animals that feed poorly and die within days after birth (Wang et al., 1997, Fon et al., 1997). By contrast, mice that express just 5% of native Vmat2 levels live to adulthood and only develop comparatively minor age-related motor deficits over time (Mooslehner et al., 2001). The phenotypic spectrum of VMAT2 deficiency is therefore broad, and very large decreases in protein function may be required to result in severe, early onset motor symptoms. However, there may be differences in the phenotypes caused by lower protein levels and aberrant protein function, possibly stemming from differences in VMAT2 trafficking and its relative distribution between readily releasable and recycling or reserve pools of synaptic vesicles. Future research should clarify the mechanism of loss of function by confirming whether protein localization to synaptic vesicles is affected by the mutation in vivo. Appropriate cell culture models can be used to investigate VMAT2 sorting. These experiments are discussed in Section Initial confirmation of reduced uptake activity of a single substrate was considered sufficient in the present work to confirm the functional effect of the VMAT2 p.p387l variant. More precise characterization of the p.p387l mutation may be of interest for 115
139 drawing broader insights on VMAT2 structure function relationships. Proposed experiments are described in Section Because VMAT2 density on synaptic vesicles determines quantal size for monoamine neurotransmission (Edwards, 2007, Pothos et al., 2000), and its function is therefore subject to dose dependence, unique insights may be gleaned into monoamine physiology by investigating the effect of the corresponding Vmat2 p.p390l mutation in a mouse model that were not derived from studying one of the existing mouse models. The proposed mouse model is described in Section Future Directions and Experiments Subcellular Localization of VMAT2 p.p387l Subcellular localization of VMAT2-P387L can be assessed in relevant cell culture models using immunofluorescence colocalization and sucrose gradient centrifugation using relevant cell compartment markers (e.g., synaptophysin for synaptic vesicles). To avoid the artifacts of overexpression introduced by transient transfection (as in Chapter 4, Section 3.4), cell lines should be constructed to stably express either wild-type or mutant VMAT2 protein. The following section describes the construction of these cell lines. Stable expression of VMAT2 (wild-type and P387L) was confirmed in both Cos7 cells and the MN9D mesencephalic cell line. MN9D cells were originally derived as a fusion of embryonic ventral mesencephalic and neuroblastoma cells, and are extensively used as a model of dopamine neurons because they express tyrosine hydroxylase and synthesize and release 116
140 dopamine. These cells can be differentiated with exposure to butyric acid, and develop some morphological characteristics of neurons such as visible processes. Because these cells possess synaptic vesicles, they have already been used in studies of VMAT2 function, in which it was shown that overexpression of VMAT2 confers additional protection against MPP + (Chen et al., 2005). Interestingly, co-expression of synaptophysin enhanced this effect possibly through increased vesicular capacity. The co-expression of synaptophysin may therefore also be helpful in these proposed localization experiments Generation of Stable Cell Lines Expressing VMAT2 p.p387l Cell lines were generated to stably express human VMAT2. Independent cell lines were generated to express wild-type VMAT2 and VMAT2-P387L. Additionally, cell lines with genomic incorporation of the vector backbone alone were generated for use as experimental controls. Stable expression of human VMAT2 was generated in the Cos7 cell line. Cos7 cells were maintained as described in Chapter 4 (Section 2.2). The pcdna3.1 vectors containing wild-type VMAT2 and VMAT2-P387L cdna sequence, in addition to empty pcdna3.1 vector, were used in independent transfections. Prior to transfection, the vector was linearized to optimize genomic incorporation of the construct. Transfection was performed with Fugene HD transfection reagent (Roche), according to the manufacturer s specifications. After 48 hours to allow expression of the neomycin cassette on the vector, the cells were trypsinized and a series of dilutions from 1:10 to 1:1000 were plated 117
141 independently in DMEM containing 500 µg/ml of G418. The selection medium was replaced each day with fresh medium until the appearance of visible colonies. Colonies were transferred to 96-well plates containing trypsin and subsequently diluted into DMEM + 10% FBS µg/ml G418. Individual wells were eventually passaged into larger diameter wells and screened for VMAT2 expression by western blot (Figure 22). Positive clones were expanded and frozen at 80 C Figure 22 Expression of wild-type and p.p387l VMAT2 in stable clones of the Cos7 cell line; band at 37 kda represents GAPDH loading control. MN9D cells were maintained in custom DMEM media at ph 7.2 containing 10% Fetal Clone 3 (Hyclone) and 50 U/mL penicillin/streptomycin. They were cultured in flasks at 118
142 37 C and 5% CO 2, and fed every other day by aspiration of the media and replacement with fresh media. Cells were passaged by trypsinization and seeding into new flasks at plating densities of 200,000 per T75 flask and grown to confluence. The number of passages was minimized to avoid morphological changes. Because MN9D cells endogenously express mouse Vmat2, the human VMAT2 protein was tagged internally with an HA epitope to distinguish the isoforms. Initial attempts to incorporate N-terminal and C-terminal FLAG and myc tags led to no detectable protein by western blot. The HA tag was therefore incorporated into the large lumenal loop immediately after Arg94, as described by Thiriot and Ruoho (Thiriot and Ruoho, 2001). The tag was incorporated by site-directed mutagenesis using complementary oligonucleotide sequences that incorporated the HA epitope (Table 18). The transfection and selection of clones was performed as described for generation of the Cos7 stable cell lines. Confirmation of human VMAT2 expression was performed by western blot using an antibody against the HA epitope (Figure 23). Table 18 Primers for the integration of the HA epitope at Arg94 of VMAT2 using site-direted mutagenesis Primer VMAT2-Arg94-HAepitope-upR VMAT2-Arg94-HAepitope-downF Sequence AGC GTA ATC TGG AAC ATC GTA TGG GTA TCT GGT AGC ATT CCC GGT G TAC CCA TAC GAT GTT CCA GAT TAC GCT GAC CTG ACA CTT CAT CAG 119
143 Figure 23 Expression of wild-type and p.p387l VMAT2 in stable clones of the MN9D cell line Additional Biochemical Characterization of VMAT2 p.p387l The precise functional consequences of proline to leucine substitution at residue 387 in VMAT2 can be further probed by comparing the relative uptake of additional substrates (i.e., dopamine, norepinephrine, histamine) either directly or through competitive inhibition of serotonin uptake, and the relative binding of inhibitors (i.e., reserpine, tetrabenazine). Furthermore, the use of valinomycin (a K + ionophore) and bafilomycin (an inhibitor of the vesicular ATPase) can be used to probe the coupling of VMAT2 p.p387l to the electrochemical and proton gradients. 120
Neurotransmitter Disorders.
 Neurotransmitter Disorders Simon.heales@gosh.nhs.uk Chemical Neurotransmission Neurotransmitters Substances that upon release from nerve terminals, act on receptor sites at postsynaptic membranes to produce
Neurotransmitter Disorders Simon.heales@gosh.nhs.uk Chemical Neurotransmission Neurotransmitters Substances that upon release from nerve terminals, act on receptor sites at postsynaptic membranes to produce
Analysis of Neurotransmitters. Simon Heales
 Analysis of Neurotransmitters Simon Heales HealeS@gosh.nhs.uk Tyrosine O 2 L-Dopa Dopamine HVA Tryptophan 5-HTP PLP Serotonin 5-HIAA Phenylalanine Tyrosine BH4 qbh2 BH2 CSF Sample Requirements Tube 1 Tube
Analysis of Neurotransmitters Simon Heales HealeS@gosh.nhs.uk Tyrosine O 2 L-Dopa Dopamine HVA Tryptophan 5-HTP PLP Serotonin 5-HIAA Phenylalanine Tyrosine BH4 qbh2 BH2 CSF Sample Requirements Tube 1 Tube
Genetics of monoamine neurotransmitter disorders
 Review Article Genetics of monoamine neurotransmitter disorders Wai-Kwan Siu Kowloon West Cluster Laboratory Genetic Service, Department of Pathology, Princess Margaret Hospital, Hong Kong SAR, China Correspondence
Review Article Genetics of monoamine neurotransmitter disorders Wai-Kwan Siu Kowloon West Cluster Laboratory Genetic Service, Department of Pathology, Princess Margaret Hospital, Hong Kong SAR, China Correspondence
Clinical aspects of pterin disorders
 Clinical aspects of pterin disorders Thomas Opladen, MD University Children s Hospital Department of Inborn Errors of Metabolism Heidelberg Germany Introductory words Brain function depends on the capacity
Clinical aspects of pterin disorders Thomas Opladen, MD University Children s Hospital Department of Inborn Errors of Metabolism Heidelberg Germany Introductory words Brain function depends on the capacity
Neurotransmitter Systems III Neurochemistry. Reading: BCP Chapter 6
 Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Neurotransmitter Systems III Neurochemistry Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the most important
Neurotransmitters acting on G-protein coupled receptors
 Neurotransmitters acting on G-protein coupled receptors Part 1: Dopamine and Norepinephrine BIOGENIC AMINES Monoamines Diamine Overview of Neurotransmitters and Their Receptors Criteria for defining a
Neurotransmitters acting on G-protein coupled receptors Part 1: Dopamine and Norepinephrine BIOGENIC AMINES Monoamines Diamine Overview of Neurotransmitters and Their Receptors Criteria for defining a
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817.
 Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Making Every Little Bit Count: Parkinson s Disease. SHP Neurobiology of Development and Disease
 Making Every Little Bit Count: Parkinson s Disease SHP Neurobiology of Development and Disease Parkinson s Disease Initially described symptomatically by Dr. James Parkinson in 1817 in An Essay on the
Making Every Little Bit Count: Parkinson s Disease SHP Neurobiology of Development and Disease Parkinson s Disease Initially described symptomatically by Dr. James Parkinson in 1817 in An Essay on the
Basal ganglia Sujata Sofat, class of 2009
 Basal ganglia Sujata Sofat, class of 2009 Basal ganglia Objectives Describe the function of the Basal Ganglia in movement Define the BG components and their locations Describe the motor loop of the BG
Basal ganglia Sujata Sofat, class of 2009 Basal ganglia Objectives Describe the function of the Basal Ganglia in movement Define the BG components and their locations Describe the motor loop of the BG
Making Things Happen 2: Motor Disorders
 Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Phenylketonuria (PKU) the Biochemical Basis. Biol 405 Molecular Medicine
 Phenylketonuria (PKU) the Biochemical Basis Biol 405 Molecular Medicine PKU a history In 1934 Følling identified a clinical condition - imbecillitas phenylpyruvica. Mental retardation associated with this
Phenylketonuria (PKU) the Biochemical Basis Biol 405 Molecular Medicine PKU a history In 1934 Følling identified a clinical condition - imbecillitas phenylpyruvica. Mental retardation associated with this
Neurodegenerative Disease. April 12, Cunningham. Department of Neurosciences
 Neurodegenerative Disease April 12, 2017 Cunningham Department of Neurosciences NEURODEGENERATIVE DISEASE Any of a group of hereditary and sporadic conditions characterized by progressive dysfunction,
Neurodegenerative Disease April 12, 2017 Cunningham Department of Neurosciences NEURODEGENERATIVE DISEASE Any of a group of hereditary and sporadic conditions characterized by progressive dysfunction,
Neurotransmitter Systems I Identification and Distribution. Reading: BCP Chapter 6
 Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Neurotransmitter Systems I Identification and Distribution Reading: BCP Chapter 6 Neurotransmitter Systems Normal function of the human brain requires an orderly set of chemical reactions. Some of the
Objectives. 1. Outline the criteria that need to be met before a molecule can be classified as neurotransmitter
 Neurotransmitters Objectives 1. Outline the criteria that need to be met before a molecule can be classified as neurotransmitter 2. Identify the major neurotransmitter types 3. Mechanism of action of important
Neurotransmitters Objectives 1. Outline the criteria that need to be met before a molecule can be classified as neurotransmitter 2. Identify the major neurotransmitter types 3. Mechanism of action of important
VL VA BASAL GANGLIA. FUNCTIONAl COMPONENTS. Function Component Deficits Start/initiation Basal Ganglia Spontan movements
 BASAL GANGLIA Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo I) Overview How do Basal Ganglia affect movement Basal ganglia enhance cortical motor activity and facilitate movement.
BASAL GANGLIA Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo I) Overview How do Basal Ganglia affect movement Basal ganglia enhance cortical motor activity and facilitate movement.
Exam 2 PSYC Fall (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
 Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
Movement Disorders: A Brief Overview
 Movement Disorders: A Brief Overview Albert Hung, MD, PhD Massachusetts General Hospital Harvard Medical School August 17, 2006 Cardinal Features of Parkinsonism Tremor Rigidity Bradykinesia Postural imbalance
Movement Disorders: A Brief Overview Albert Hung, MD, PhD Massachusetts General Hospital Harvard Medical School August 17, 2006 Cardinal Features of Parkinsonism Tremor Rigidity Bradykinesia Postural imbalance
This is a free sample of content from Parkinson's Disease. Click here for more information or to buy the book.
 A AADC. See Aromatic amino acid decarboxylase AAV. See Adeno-associated virus Acetylcholine (ACh), functional imaging, 174 175 ACh. See Acetylcholine Adaptive immune system central nervous system, 381
A AADC. See Aromatic amino acid decarboxylase AAV. See Adeno-associated virus Acetylcholine (ACh), functional imaging, 174 175 ACh. See Acetylcholine Adaptive immune system central nervous system, 381
Biogenic amines and pterins in cerebrospinal fluid: some pitfalls with interpretation
 CLINICAL FOCUS Special Focus Biogenic amines and pterins in cerebrospinal fluid: some pitfalls with interpretation Birgit Assmann University Children s Hospital, Department of General Pediatrics, Moorenstrasse
CLINICAL FOCUS Special Focus Biogenic amines and pterins in cerebrospinal fluid: some pitfalls with interpretation Birgit Assmann University Children s Hospital, Department of General Pediatrics, Moorenstrasse
Damage on one side.. (Notes) Just remember: Unilateral damage to basal ganglia causes contralateral symptoms.
 Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Classes of Neurotransmitters. Neurotransmitters
 1 Drugs Outline 2 Neurotransmitters Agonists and Antagonists Cocaine & other dopamine agonists Alcohol & its effects / Marijuana & its effects Synthetic & Designer Drugs: Ecstasy 1 Classes of Neurotransmitters
1 Drugs Outline 2 Neurotransmitters Agonists and Antagonists Cocaine & other dopamine agonists Alcohol & its effects / Marijuana & its effects Synthetic & Designer Drugs: Ecstasy 1 Classes of Neurotransmitters
Chemical Control of Behavior and Brain 1 of 9
 Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Chemical Control of Behavior and Brain 1 of 9 I) INTRO A) Nervous system discussed so far 1) Specific 2) Fast B) Other systems extended in space and time 1) Nonspecific 2) Slow C) Three components that
Basal Ganglia. Steven McLoon Department of Neuroscience University of Minnesota
 Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Inherited disorders of amine biosynthesis
 REVIEW Special Focus Inherited disorders of amine biosynthesis Kathryn J Swoboda University of Utah School of Medicine, 50 North Medical Drive, Room 3R210, Salt Lake City, UT 84132, USA Tel.: +1 801 585
REVIEW Special Focus Inherited disorders of amine biosynthesis Kathryn J Swoboda University of Utah School of Medicine, 50 North Medical Drive, Room 3R210, Salt Lake City, UT 84132, USA Tel.: +1 801 585
Movement disorders in neurometabolic disorders handout IN ADDITION to talk (extension of text slides, illustrative videos in talk)
 Movement disorders in neurometabolic disorders handout IN ADDITION to talk (extension of text slides, illustrative videos in talk) Birgit Assmann University Children s Hospital Heidelberg, Germany Dr.med
Movement disorders in neurometabolic disorders handout IN ADDITION to talk (extension of text slides, illustrative videos in talk) Birgit Assmann University Children s Hospital Heidelberg, Germany Dr.med
Autonomic Nervous System. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
Visualization and simulated animations of pathology and symptoms of Parkinson s disease
 Visualization and simulated animations of pathology and symptoms of Parkinson s disease Prof. Yifan HAN Email: bctycan@ust.hk 1. Introduction 2. Biochemistry of Parkinson s disease 3. Course Design 4.
Visualization and simulated animations of pathology and symptoms of Parkinson s disease Prof. Yifan HAN Email: bctycan@ust.hk 1. Introduction 2. Biochemistry of Parkinson s disease 3. Course Design 4.
Background and proposal for a pilot EQA scheme for pterins
 Background and proposal for a pilot EQA scheme for pterins enad Blau Division of Inborn Metabolic Diseases University Children's Hospital Heidelberg Germany Tetrahydrobiopterin (BH 4 ) Brimstone butterfly
Background and proposal for a pilot EQA scheme for pterins enad Blau Division of Inborn Metabolic Diseases University Children's Hospital Heidelberg Germany Tetrahydrobiopterin (BH 4 ) Brimstone butterfly
variant led to a premature stop codon p.k316* which resulted in nonsense-mediated mrna decay. Although the exact function of the C19L1 is still
 157 Neurological disorders primarily affect and impair the functioning of the brain and/or neurological system. Structural, electrical or metabolic abnormalities in the brain or neurological system can
157 Neurological disorders primarily affect and impair the functioning of the brain and/or neurological system. Structural, electrical or metabolic abnormalities in the brain or neurological system can
The motor regulator. 1) Basal ganglia/nucleus
 The motor regulator 1) Basal ganglia/nucleus Neural structures involved in the control of movement Basal Ganglia - Components of the basal ganglia - Function of the basal ganglia - Connection and circuits
The motor regulator 1) Basal ganglia/nucleus Neural structures involved in the control of movement Basal Ganglia - Components of the basal ganglia - Function of the basal ganglia - Connection and circuits
Pathogenesis of Degenerative Diseases and Dementias. D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria)
 Pathogenesis of Degenerative Diseases and Dementias D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria) Dementias Defined: as the development of memory impairment and other cognitive deficits
Pathogenesis of Degenerative Diseases and Dementias D r. Ali Eltayb ( U. of Omdurman. I ). M. Path (U. of Alexandria) Dementias Defined: as the development of memory impairment and other cognitive deficits
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
Genetic mates SNPedia write-ups Final Next-gen sequencing Mike Snyder QA: new technology and the future
 Genetic mates SNPedia write-ups Final Next-gen sequencing Mike Snyder QA: new technology and the future Genetic Mates Jonathan Mortensen/Francisco Gimenez Will need class to upload data Tuesday night Analyze
Genetic mates SNPedia write-ups Final Next-gen sequencing Mike Snyder QA: new technology and the future Genetic Mates Jonathan Mortensen/Francisco Gimenez Will need class to upload data Tuesday night Analyze
Treatment of Parkinson s Disease and of Spasticity. Satpal Singh Pharmacology and Toxicology 3223 JSMBS
 Treatment of Parkinson s Disease and of Spasticity Satpal Singh Pharmacology and Toxicology 3223 JSMBS singhs@buffalo.edu 716-829-2453 1 Disclosures NO SIGNIFICANT FINANCIAL, GENERAL, OR OBLIGATION INTERESTS
Treatment of Parkinson s Disease and of Spasticity Satpal Singh Pharmacology and Toxicology 3223 JSMBS singhs@buffalo.edu 716-829-2453 1 Disclosures NO SIGNIFICANT FINANCIAL, GENERAL, OR OBLIGATION INTERESTS
III./3.1. Movement disorders with akinetic rigid symptoms
 III./3.1. Movement disorders with akinetic rigid symptoms III./3.1.1. Parkinson s disease Parkinson s disease (PD) is the second most common neurodegenerative disorder worldwide after Alzheimer s disease.
III./3.1. Movement disorders with akinetic rigid symptoms III./3.1.1. Parkinson s disease Parkinson s disease (PD) is the second most common neurodegenerative disorder worldwide after Alzheimer s disease.
- Neurotransmitters Of The Brain -
 - Neurotransmitters Of The Brain - INTRODUCTION Synapsis: a specialized connection between two neurons that permits the transmission of signals in a one-way fashion (presynaptic postsynaptic). Types of
- Neurotransmitters Of The Brain - INTRODUCTION Synapsis: a specialized connection between two neurons that permits the transmission of signals in a one-way fashion (presynaptic postsynaptic). Types of
Neurotransmitter Functioning In Major Depressive Disorder
 Neurotransmitter Functioning In Major Depressive Disorder Otsuka Pharmaceutical Development & Commercialization, Inc. 2017 Otsuka Pharmaceutical Development & Commercialization, Inc., Rockville, MD January
Neurotransmitter Functioning In Major Depressive Disorder Otsuka Pharmaceutical Development & Commercialization, Inc. 2017 Otsuka Pharmaceutical Development & Commercialization, Inc., Rockville, MD January
Sepiapterin Reductase Deficiency: Clinical Presentation and Evaluation of Long-Term Therapy
 Sepiapterin Reductase Deficiency: Clinical Presentation and Evaluation of Long-Term Therapy Bernard Echenne, MD*, Agathe Roubertie, MD, PhD*, Birgit Assmann, MD, Thomas Lutz, MD, Johann M. Penzien, MD,
Sepiapterin Reductase Deficiency: Clinical Presentation and Evaluation of Long-Term Therapy Bernard Echenne, MD*, Agathe Roubertie, MD, PhD*, Birgit Assmann, MD, Thomas Lutz, MD, Johann M. Penzien, MD,
Neurophysiology and Neurochemistry in PsychoGeriatrics
 Tel Aviv University Sackler Faculty of Medicine CME in Psychiatry Neurophysiology and Neurochemistry in PsychoGeriatrics Nicola Maggio, MD, PhD Sackler Faculty of Medicine Tel Aviv University Department
Tel Aviv University Sackler Faculty of Medicine CME in Psychiatry Neurophysiology and Neurochemistry in PsychoGeriatrics Nicola Maggio, MD, PhD Sackler Faculty of Medicine Tel Aviv University Department
Sepiapterin reductase dewciency an autosomal recessive DOPA-responsive dystonia
 Molecular Genetics and Metabolism 89 (2006) 116 120 www.elsevier.com/locate/ymgme Sepiapterin reductase dewciency an autosomal recessive DOPA-responsive dystonia Nico G. Abeling a,, Marinus Duran a, Henk
Molecular Genetics and Metabolism 89 (2006) 116 120 www.elsevier.com/locate/ymgme Sepiapterin reductase dewciency an autosomal recessive DOPA-responsive dystonia Nico G. Abeling a,, Marinus Duran a, Henk
Cheyenne 11/28 Neurological Disorders II. Transmissible Spongiform Encephalopathy
 Cheyenne 11/28 Neurological Disorders II Transmissible Spongiform Encephalopathy -E.g Bovine4 Spongiform Encephalopathy (BSE= mad cow disease), Creutzfeldt-Jakob disease, scrapie (animal only) -Sporadic:
Cheyenne 11/28 Neurological Disorders II Transmissible Spongiform Encephalopathy -E.g Bovine4 Spongiform Encephalopathy (BSE= mad cow disease), Creutzfeldt-Jakob disease, scrapie (animal only) -Sporadic:
I. OVERVIEW DIRECT. Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of
 THE CHOLINERGIC NEURON 1 I. OVERVIEW DIRECT Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of ACTING neuron involved in their mechanism of action.
THE CHOLINERGIC NEURON 1 I. OVERVIEW DIRECT Drugs affecting the autonomic nervous system (ANS) are divided into two groups according to the type of ACTING neuron involved in their mechanism of action.
Neuron types and Neurotransmitters
 Neuron types and Neurotransmitters Faisal I. Mohammed. PhD, MD University of Jordan 1 Transmission of Receptor Information to the Brain the larger the nerve fiber diameter the faster the rate of transmission
Neuron types and Neurotransmitters Faisal I. Mohammed. PhD, MD University of Jordan 1 Transmission of Receptor Information to the Brain the larger the nerve fiber diameter the faster the rate of transmission
Brain Neurotransmitters
 Brain Neurotransmitters * Chemical substances released by electrical impulses into the synaptic cleft from synaptic vesicles of presynaptic membrane * Diffuses to the postsynaptic membrane * Binds to and
Brain Neurotransmitters * Chemical substances released by electrical impulses into the synaptic cleft from synaptic vesicles of presynaptic membrane * Diffuses to the postsynaptic membrane * Binds to and
Movement Disorders Will Garrett, M.D Assistant Professor of Neurology
 Movement Disorders Will Garrett, M.D Assistant Professor of Neurology I. The Basal Ganglia The basal ganglia are composed of several structures including the caudate and putamen (collectively called the
Movement Disorders Will Garrett, M.D Assistant Professor of Neurology I. The Basal Ganglia The basal ganglia are composed of several structures including the caudate and putamen (collectively called the
Study Guide Unit 2 Psych 2022, Fall 2003
 Study Guide Unit 2 Psych 2022, Fall 2003 Subcortical Anatomy 1. Be able to locate the following structures and be able to indicate whether they are located in the forebrain, diencephalon, midbrain, pons,
Study Guide Unit 2 Psych 2022, Fall 2003 Subcortical Anatomy 1. Be able to locate the following structures and be able to indicate whether they are located in the forebrain, diencephalon, midbrain, pons,
Elise Cook. BForensics (Hons) Forensic Biology and Toxicology. BSc Biomedical Science and Molecular Biology
 Acute and chronic toxicity of methamphetamine exposure in cultured neuronal cells Elise Cook BForensics (Hons) Forensic Biology and Toxicology BSc Biomedical Science and Molecular Biology This thesis is
Acute and chronic toxicity of methamphetamine exposure in cultured neuronal cells Elise Cook BForensics (Hons) Forensic Biology and Toxicology BSc Biomedical Science and Molecular Biology This thesis is
Strick Lecture 4 March 29, 2006 Page 1
 Strick Lecture 4 March 29, 2006 Page 1 Basal Ganglia OUTLINE- I. Structures included in the basal ganglia II. III. IV. Skeleton diagram of Basal Ganglia Loops with cortex Similarity with Cerebellar Loops
Strick Lecture 4 March 29, 2006 Page 1 Basal Ganglia OUTLINE- I. Structures included in the basal ganglia II. III. IV. Skeleton diagram of Basal Ganglia Loops with cortex Similarity with Cerebellar Loops
Basal Ganglia General Info
 Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Margo J Nell Dept Pharmacology
 Margo J Nell Dept Pharmacology 1 The extra pyramidal system Separation of cortico-spinal system (pyramidal system, (PS)) from the basal ganglia (extra pyramidal motor system (EPS)) because they produce
Margo J Nell Dept Pharmacology 1 The extra pyramidal system Separation of cortico-spinal system (pyramidal system, (PS)) from the basal ganglia (extra pyramidal motor system (EPS)) because they produce
GBME graduate course. Chapter 43. The Basal Ganglia
 GBME graduate course Chapter 43. The Basal Ganglia Basal ganglia in history Parkinson s disease Huntington s disease Parkinson s disease 1817 Parkinson's disease (PD) is a degenerative disorder of the
GBME graduate course Chapter 43. The Basal Ganglia Basal ganglia in history Parkinson s disease Huntington s disease Parkinson s disease 1817 Parkinson's disease (PD) is a degenerative disorder of the
Frontiers in Personalized Medicine. PW-GW-AS DNA sequencing Reverse human genetics
 Frontiers in Personalized Medicine PW-GW-AS DNA sequencing Reverse human genetics Published Genome-Wide Associations through 06/2011, 1,449 published GWA at p 5x10-8 for 237 traits NHGRI GWA Catalog www.genome.gov/gwastudies
Frontiers in Personalized Medicine PW-GW-AS DNA sequencing Reverse human genetics Published Genome-Wide Associations through 06/2011, 1,449 published GWA at p 5x10-8 for 237 traits NHGRI GWA Catalog www.genome.gov/gwastudies
Parkinson s Disease in the Elderly A Physicians perspective. Dr John Coyle
 Parkinson s Disease in the Elderly A Physicians perspective Dr John Coyle Overview Introduction Epidemiology and aetiology Pathogenesis Diagnosis and clinical features Treatment Psychological issues/ non
Parkinson s Disease in the Elderly A Physicians perspective Dr John Coyle Overview Introduction Epidemiology and aetiology Pathogenesis Diagnosis and clinical features Treatment Psychological issues/ non
Adrenergic agonists Sympathomimetic drugs. ANS Pharmacology Lecture 4 Dr. Hiwa K. Saaed College of Pharmacy/University of Sulaimani
 Adrenergic agonists Sympathomimetic drugs ANS Pharmacology Lecture 4 Dr. Hiwa K. Saaed College of Pharmacy/University of Sulaimani 2017-2018 Adrenergic agonists The adrenergic drugs affect receptors that
Adrenergic agonists Sympathomimetic drugs ANS Pharmacology Lecture 4 Dr. Hiwa K. Saaed College of Pharmacy/University of Sulaimani 2017-2018 Adrenergic agonists The adrenergic drugs affect receptors that
Parkinson's Disease. Robert L. Copeland, Ph.D. Howard University College of Medicine Department of Pharmacology
 Parkinson's Disease Robert L. Copeland, Ph.D. Howard University College of Medicine Department of Pharmacology 18 February 2002 Parkinson Disease Neurological disease affecting over four million patients
Parkinson's Disease Robert L. Copeland, Ph.D. Howard University College of Medicine Department of Pharmacology 18 February 2002 Parkinson Disease Neurological disease affecting over four million patients
The Nervous System Mark Stanford, Ph.D.
 The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
The Nervous System Functional Neuroanatomy and How Neurons Communicate Mark Stanford, Ph.D. Santa Clara Valley Health & Hospital System Addiction Medicine and Therapy Services The Nervous System In response
Chapter 3. Biological Processes
 Biological Processes Psychology, Fifth Edition, James S. Nairne What s It For? Biological Solutions Communicating internally Initiating and coordinating behavior Regulating growth and other internal functions
Biological Processes Psychology, Fifth Edition, James S. Nairne What s It For? Biological Solutions Communicating internally Initiating and coordinating behavior Regulating growth and other internal functions
Movement Disorders. Psychology 372 Physiological Psychology. Background. Myasthenia Gravis. Many Types
 Background Movement Disorders Psychology 372 Physiological Psychology Steven E. Meier, Ph.D. Listen to the audio lecture while viewing these slides Early Studies Found some patients with progressive weakness
Background Movement Disorders Psychology 372 Physiological Psychology Steven E. Meier, Ph.D. Listen to the audio lecture while viewing these slides Early Studies Found some patients with progressive weakness
Neurotransmitters acting on G-protein coupled receptors
 Neurotransmitters acting on G-protein coupled receptors Part 2: Serotonin and Histamine BIOGENIC AMINES Monoamines Diamine Indolamines: Serotonin Basic Neurochemistry. FIGURE 15-1: Chemical structure of
Neurotransmitters acting on G-protein coupled receptors Part 2: Serotonin and Histamine BIOGENIC AMINES Monoamines Diamine Indolamines: Serotonin Basic Neurochemistry. FIGURE 15-1: Chemical structure of
Connections of basal ganglia
 Connections of basal ganglia Introduction The basal ganglia, or basal nuclei, are areas of subcortical grey matter that play a prominent role in modulating movement, as well as cognitive and emotional
Connections of basal ganglia Introduction The basal ganglia, or basal nuclei, are areas of subcortical grey matter that play a prominent role in modulating movement, as well as cognitive and emotional
Movement disorders in childhood: assessment and diagnosis. Lucinda Carr
 Movement disorders in childhood: assessment and diagnosis Lucinda Carr Movement disorders in childhood: Assessment Classification Causes Diagnosis Presentation of movement disorders in childhood: Concerns
Movement disorders in childhood: assessment and diagnosis Lucinda Carr Movement disorders in childhood: Assessment Classification Causes Diagnosis Presentation of movement disorders in childhood: Concerns
Introduction to Autonomic
 Part 2 Autonomic Pharmacology 3 Introduction to Autonomic Pharmacology FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM The autonomic nervous system (Figure 3 1) is composed of the sympathetic and parasympathetic
Part 2 Autonomic Pharmacology 3 Introduction to Autonomic Pharmacology FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM The autonomic nervous system (Figure 3 1) is composed of the sympathetic and parasympathetic
Chapter 8. Parkinsonism. M.G.Rajanandh, Dept. of Pharmacy Practice, SRM College of Pharmacy, SRM University.
 Chapter 8 Parkinsonism M.G.Rajanandh, Dept. of Pharmacy Practice, SRM College of Pharmacy, SRM University. Definition of Parkinson s Disease Parkinson's disease is a progressive, neurodegenerative disease
Chapter 8 Parkinsonism M.G.Rajanandh, Dept. of Pharmacy Practice, SRM College of Pharmacy, SRM University. Definition of Parkinson s Disease Parkinson's disease is a progressive, neurodegenerative disease
The Nervous System. Chapter 4. Neuron 3/9/ Components of the Nervous System
 Chapter 4 The Nervous System 1. Components of the Nervous System a. Nerve cells (neurons) Analyze and transmit information Over 100 billion neurons in system Four defined regions Cell body Dendrites Axon
Chapter 4 The Nervous System 1. Components of the Nervous System a. Nerve cells (neurons) Analyze and transmit information Over 100 billion neurons in system Four defined regions Cell body Dendrites Axon
MOVEMENT OUTLINE. The Control of Movement: Muscles! Motor Reflexes Brain Mechanisms of Movement Mirror Neurons Disorders of Movement
 MOVEMENT 2 Dr. Steinmetz 3 OUTLINE The Control of Movement: Muscles! Motor Reflexes Brain Mechanisms of Movement Mirror Neurons Disorders of Movement Parkinson s Disease Huntington s Disease 1 4 TYPES
MOVEMENT 2 Dr. Steinmetz 3 OUTLINE The Control of Movement: Muscles! Motor Reflexes Brain Mechanisms of Movement Mirror Neurons Disorders of Movement Parkinson s Disease Huntington s Disease 1 4 TYPES
First described by James Parkinson in his classic 1817 monograph, "An Essay on the Shaking Palsy"
 Parkinson's Disease First described by James Parkinson in his classic 1817 monograph, "An Essay on the Shaking Palsy" Parkinson s disease (PD) is a neurological disorder characterized by a progressive
Parkinson's Disease First described by James Parkinson in his classic 1817 monograph, "An Essay on the Shaking Palsy" Parkinson s disease (PD) is a neurological disorder characterized by a progressive
The utility of CSF for the diagnosis of primary and secondary monoamine neurotransmitter deficiencies
 In this issue: Recent Advances in Pediatric Laboratory Medicine The utility of CSF for the diagnosis of primary and secondary monoamine neurotransmitter deficiencies A.B. Burlina 1, A. Celato 1, G. Polo
In this issue: Recent Advances in Pediatric Laboratory Medicine The utility of CSF for the diagnosis of primary and secondary monoamine neurotransmitter deficiencies A.B. Burlina 1, A. Celato 1, G. Polo
Chapter 17. Nervous System Nervous systems receive sensory input, interpret it, and send out appropriate commands. !
 Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 17 Sensory receptor Sensory input Integration Nervous System Motor output Brain and spinal cord Effector cells Peripheral nervous system (PNS) Central nervous system (CNS) 28.1 Nervous systems
Chapter 4. Psychopharmacology. Copyright Allyn & Bacon 2004
 Chapter 4 Psychopharmacology This multimedia product and its contents are protected under copyright law. The following are prohibited by law: any public performance or display, including transmission of
Chapter 4 Psychopharmacology This multimedia product and its contents are protected under copyright law. The following are prohibited by law: any public performance or display, including transmission of
COGNITIVE SCIENCE 107A. Motor Systems: Basal Ganglia. Jaime A. Pineda, Ph.D.
 COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
Disorders of Tetrahydrobiopterin and Related Biogenic Amines
 Disorders of Tetrahydrobiopterin and Related Biogenic Amines C H A P T E R 78 Nenad Blau _ Beat ThoÈ ny Richard G. H. Cotton _ Keith Hyland 1. Tetrahydrobiopterin BH 4 ) de ciencies are disorders affecting
Disorders of Tetrahydrobiopterin and Related Biogenic Amines C H A P T E R 78 Nenad Blau _ Beat ThoÈ ny Richard G. H. Cotton _ Keith Hyland 1. Tetrahydrobiopterin BH 4 ) de ciencies are disorders affecting
The Neurobiology of Mood Disorders
 The Neurobiology of Mood Disorders J. John Mann, MD Professor of Psychiatry and Radiology Columbia University Chief, Department of Neuroscience, New York State Psychiatric Institute Mood Disorders are
The Neurobiology of Mood Disorders J. John Mann, MD Professor of Psychiatry and Radiology Columbia University Chief, Department of Neuroscience, New York State Psychiatric Institute Mood Disorders are
Advanced Neurotransmitters & Neuroglia
 Advanced Neurotransmitters & Neuroglia Otsuka Pharmaceutical Development & Commercialization, Inc. 2017 Otsuka Pharmaceutical Development & Commercialization, Inc., Rockville, MD Lundbeck, LLC. February
Advanced Neurotransmitters & Neuroglia Otsuka Pharmaceutical Development & Commercialization, Inc. 2017 Otsuka Pharmaceutical Development & Commercialization, Inc., Rockville, MD Lundbeck, LLC. February
 PSY 302 Lecture 6: The Neurotransmitters (continued) September 12, 2017 Notes by: Desiree Acetylcholine (ACh) CoA + Acetate Acetyl-CoA (mitochondria) (food, vinegar) + Choline ChAT CoA + ACh (lipids, foods)
PSY 302 Lecture 6: The Neurotransmitters (continued) September 12, 2017 Notes by: Desiree Acetylcholine (ACh) CoA + Acetate Acetyl-CoA (mitochondria) (food, vinegar) + Choline ChAT CoA + ACh (lipids, foods)
PHRM20001: Pharmacology - How Drugs Work!
 PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
Parkinson s disease Therapeutic strategies. Surat Tanprawate, MD Division of Neurology University of Chiang Mai
 Parkinson s disease Therapeutic strategies Surat Tanprawate, MD Division of Neurology University of Chiang Mai 1 Scope Modality of treatment Pathophysiology of PD and dopamine metabolism Drugs Are there
Parkinson s disease Therapeutic strategies Surat Tanprawate, MD Division of Neurology University of Chiang Mai 1 Scope Modality of treatment Pathophysiology of PD and dopamine metabolism Drugs Are there
Abstracts and affiliations
 Dopamine Discovery Day August 30, 2012 Rikshospitalet Store auditorium, Oslo, Norway Organized by Linda H. Bergersen & Vidar Gundersen Institute of Basic Medical Sciences & Centre for Molecular Biology
Dopamine Discovery Day August 30, 2012 Rikshospitalet Store auditorium, Oslo, Norway Organized by Linda H. Bergersen & Vidar Gundersen Institute of Basic Medical Sciences & Centre for Molecular Biology
Synaptic transmission
 Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Outline Synaptic transmission Sompol Tapechum M.D., Ph.D. Department of Physiology Faculty of Medicine Siriraj Hospital, Bangkok, Thailand. sisth@mahidol.ac.th 2 Structure of synapse Modes of synaptic
Karam Darwish. Saleh Fayez AL-jbour. Diala
 1 Karam Darwish Saleh Fayez AL-jbour Diala Neurotransmitters Note: anything written with the italic font and present between brackets is from the slides. A neurotransmitter is a chemical substance that
1 Karam Darwish Saleh Fayez AL-jbour Diala Neurotransmitters Note: anything written with the italic font and present between brackets is from the slides. A neurotransmitter is a chemical substance that
Antidepressants and Sedatives. David G. Standaert, M.D., Ph.D. Massachusetts General Hospital Harvard Medical School
 Antidepressants and Sedatives David G. Standaert, M.D., Ph.D. Massachusetts General Hospital Harvard Medical School Depression A frequent problem, affecting up to 5% of the population Common presentations
Antidepressants and Sedatives David G. Standaert, M.D., Ph.D. Massachusetts General Hospital Harvard Medical School Depression A frequent problem, affecting up to 5% of the population Common presentations
Dania Ahmad. Tamer Barakat + Dania Ahmad. Faisal I. Mohammed
 16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
16 Dania Ahmad Tamer Barakat + Dania Ahmad Faisal I. Mohammed Revision: What are the basic types of neurons? sensory (afferent), motor (efferent) and interneuron (equaled association neurons). We classified
Teach-SHEET Basal Ganglia
 Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Lecture XIII. Brain Diseases I - Parkinsonism! Brain Diseases I!
 Lecture XIII. Brain Diseases I - Parkinsonism! Bio 3411! Wednesday!! Lecture XIII. Brain Diseases - I.! 1! Brain Diseases I! NEUROSCIENCE 5 th ed! Page!!Figure!!Feature! 408 18.9 A!!Substantia Nigra in
Lecture XIII. Brain Diseases I - Parkinsonism! Bio 3411! Wednesday!! Lecture XIII. Brain Diseases - I.! 1! Brain Diseases I! NEUROSCIENCE 5 th ed! Page!!Figure!!Feature! 408 18.9 A!!Substantia Nigra in
COGNITIVE IMPAIRMENT IN PARKINSON S DISEASE
 1 GENERAL INTRODUCTION GENERAL INTRODUCTION PARKINSON S DISEASE Parkinson s disease (PD) is a neurodegenerative movement disorder, named after James Parkinson who described some of its characteristic
1 GENERAL INTRODUCTION GENERAL INTRODUCTION PARKINSON S DISEASE Parkinson s disease (PD) is a neurodegenerative movement disorder, named after James Parkinson who described some of its characteristic
Organization of the nervous system. [See Fig. 48.1]
![Organization of the nervous system. [See Fig. 48.1] Organization of the nervous system. [See Fig. 48.1]](/thumbs/90/103926552.jpg) Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Nervous System [Note: This is the text version of this lecture file. To make the lecture notes downloadable over a slow connection (e.g. modem) the figures have been replaced with figure numbers as found
Schizophrenic twin. Normal twin
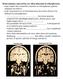 Brain anatomy and activity are often abnormal in schizophrenics - many studies have found the ventricles in schizophrenic patients enlarged (see below). - at the structural level, several brain areas have
Brain anatomy and activity are often abnormal in schizophrenics - many studies have found the ventricles in schizophrenic patients enlarged (see below). - at the structural level, several brain areas have
PETER PAZMANY CATHOLIC UNIVERSITY Consortium members SEMMELWEIS UNIVERSITY, DIALOG CAMPUS PUBLISHER
 PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
Neural Communication. Central Nervous System Peripheral Nervous System. Communication in the Nervous System. 4 Common Components of a Neuron
 Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Neural Communication Overview of CNS / PNS Electrical Signaling Chemical Signaling Central Nervous System Peripheral Nervous System Somatic = sensory & motor Autonomic = arousal state Parasympathetic =
Newborn Screening Study for AADC Deficiency Published. in Molecular Genetics and Metabolism
 Newborn Screening Study for AADC Deficiency Published in Molecular Genetics and Metabolism Study highlights frequency of AADC deficiency Cambridge, MA, June 2, 2016, 7:30 am EST - Agilis Biotherapeutics
Newborn Screening Study for AADC Deficiency Published in Molecular Genetics and Metabolism Study highlights frequency of AADC deficiency Cambridge, MA, June 2, 2016, 7:30 am EST - Agilis Biotherapeutics
ADHD Medications & How They Work. Gail C. Rodin, Ph.D. January 21, 2008
 ADHD Medications & How They Work Gail C. Rodin, Ph.D. January 21, 2008 Agenda How the (ADHD) Brain Works (or doesn t) Neurons and neurotransmitters NE & DA: the major players in ADHD Channel vs. state
ADHD Medications & How They Work Gail C. Rodin, Ph.D. January 21, 2008 Agenda How the (ADHD) Brain Works (or doesn t) Neurons and neurotransmitters NE & DA: the major players in ADHD Channel vs. state
Autonomic nervous system
 Autonomic nervous system Key notes Autonomic: an independent system that runs on its own The ANS is a visceral and involuntary sensory and motor system The visceral motor fibers in the autonomic nerves
Autonomic nervous system Key notes Autonomic: an independent system that runs on its own The ANS is a visceral and involuntary sensory and motor system The visceral motor fibers in the autonomic nerves
Acetylcholine (ACh) Action potential. Agonists. Drugs that enhance the actions of neurotransmitters.
 Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Acetylcholine (ACh) The neurotransmitter responsible for motor control at the junction between nerves and muscles; also involved in mental processes such as learning, memory, sleeping, and dreaming. (See
Drug Therapy of Parkinsonism. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Drug Therapy of Parkinsonism Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Parkinsonism is a progressive neurological disorder of muscle movement, usually
Drug Therapy of Parkinsonism Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Parkinsonism is a progressive neurological disorder of muscle movement, usually
Integrated Cardiopulmonary Pharmacology Third Edition
 Integrated Cardiopulmonary Pharmacology Third Edition Chapter 3 Pharmacology of the Autonomic Nervous System Multimedia Directory Slide 19 Slide 37 Slide 38 Slide 39 Slide 40 Slide 41 Slide 42 Slide 43
Integrated Cardiopulmonary Pharmacology Third Edition Chapter 3 Pharmacology of the Autonomic Nervous System Multimedia Directory Slide 19 Slide 37 Slide 38 Slide 39 Slide 40 Slide 41 Slide 42 Slide 43
CN V! touch! pain! Touch! P/T!
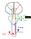 CN V! touch! pain! Touch! P/T! Visual Pathways! L! R! B! A! C! D! LT! E! F! RT! G! hypothalamospinal! and! ALS! Vestibular Pathways! 1. Posture/Balance!!falling! 2. Head Position! 3. Eye-Head Movements
CN V! touch! pain! Touch! P/T! Visual Pathways! L! R! B! A! C! D! LT! E! F! RT! G! hypothalamospinal! and! ALS! Vestibular Pathways! 1. Posture/Balance!!falling! 2. Head Position! 3. Eye-Head Movements
Chapter 2: Cellular Mechanisms and Cognition
 Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Chapter 2: Cellular Mechanisms and Cognition MULTIPLE CHOICE 1. Two principles about neurons were defined by Ramón y Cajal. The principle of connectional specificity states that, whereas the principle
Section: Chapter 5: Multiple Choice. 1. The structure of synapses is best viewed with a(n):
 Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
Section: Chapter 5: Multiple Choice 1. The structure of synapses is best viewed with a(n): p.155 electron microscope. light microscope. confocal microscope. nissle-stained microscopic procedure. 2. Electron
DISORDERS OF THE MOTOR SYSTEM. Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine
 DISORDERS OF THE MOTOR SYSTEM Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine THE MOTOR SYSTEM To understand disorders of the motor system, we need to review how a
DISORDERS OF THE MOTOR SYSTEM Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine THE MOTOR SYSTEM To understand disorders of the motor system, we need to review how a
1. Neuromodula1on* 2. The*role*of*neuromodulators*in*mood,*stress,*depression*and* anxiety*
 SerotoninergicSystem Contribu1onto DepressionandAnxiety Transla1onalNeuromodelingand Computa1onalNeuroeconomicsSeminar FallSemester2013 CarolineJahn Neuromodula1on Mood&neuromodulators Cogni1vemodel Biologicalmodel
SerotoninergicSystem Contribu1onto DepressionandAnxiety Transla1onalNeuromodelingand Computa1onalNeuroeconomicsSeminar FallSemester2013 CarolineJahn Neuromodula1on Mood&neuromodulators Cogni1vemodel Biologicalmodel
