Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus pallidal circuits
|
|
|
- Gwendolyn Jordan
- 6 years ago
- Views:
Transcription
1 Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus pallidal circuits Leonid L. Rubchinsky*, Nancy Kopell, and Karen A. Sigvardt* *Center for Neuroscience and Department of Neurology, University of California, Davis, CA 95616; and Center for BioDynamics and Department of Mathematics, Boston University, Boston, MA Contributed by Nancy Kopell, September 29, 2003 The motor symptoms of Parkinson s disease (PD) implicate the basal ganglia (BG) in some aspect of motor control, although the role the BG play in regulation of motor behavior is not completely understood. The modeling study presented here takes advantage of available cellular, systems, and clinical data on BG and PD to begin to build a biophysically based network model of pallidosubthalamic circuits of BG, to integrate this information and better understand the physiology of the normal BG and PD pathophysiology. The model reflects the experimentally supported hypothesis that the BG are involved in facilitation of the desired motor program and inhibition of competing motor programs that interfere with the desired movement. Our model network consists of subthalamic and pallidal (both external and internal segments) neural assemblies, with inputs from cortex and striatum. Functional subsets within each of the BG nuclei correspond to the desired motor program and the unwanted motor programs. A single compartment conductance-based model represents each subset. This network can discriminate between competing signals for motor program initiation, thus facilitating a single motor program. This ability depends on metabotropic -aminobutyric acid B projections from the external pallidum to subthalamic nucleus and rebound properties of subthalamic cells, as well as on the structure of projections between pallidum and subthalamus. The loss of this ability leads to hypokinesia, known PD motor deficits characterized by a slowness or inability to switch between motor programs. The basal ganglia (BG) play a significant role in motor control. Nevertheless, the physiological mechanisms responsible for this control and the pathophysiology responsible for Parkinson s disease (PD) motor symptoms remain unclear. The structures that make up the BG include the striatum, internal and external segments of the globus pallidus (GPi and GPe, respectively), substantia nigra pars compacta and pars reticulata (SNc and SNr), and subthalamic nucleus (STN). The standard models of BG function, such as that shown in Fig. 1, are based on the hypothesis that the BG consist of two processing streams: the direct and indirect pathways (1, 2). This model was originally formulated (1, 3) to provide a simple framework for understanding the hypo- and hyperkinetic extrapyramidal movement disorders (e.g., PD and Huntington s disease, respectively). However, the model fails to adequately explain control of motor programs, PD motor symptomatology, and the outcome of surgical interventions in BG (reviewed in refs. 4 6). The conceptual framework of the box-and-arrow standard model is that of a steady state model based only on anatomical connections and, thus, cannot adequately address either the dynamics associated with different ionic currents present in BG neurons or the spatiotemporal organization of activity within BG nuclei. The need for biophysically based modeling of BG motor control is widely acknowledged (see, e.g., refs. 7 and 8). Development of computational models of the BG to date has primarily focused on reinforcement learning in striatum or learning of sequences (e.g., refs. 9 11) and on the development Fig. 1. Standard model of primate BG. Filled arrows correspond to inhibitory connections, open arrows correspond to excitatory connections, and hatched arrows represent the dopaminergic innervation of the striatum. Cortical activation of the direct pathway (striatum GPi thalamus) facilitates movement by releasing the motor thalamus from inhibition from GPi and allowing excitation of corresponding cortical motor areas. Activation of the indirect pathway (striatum GPe STN GPi thalamus) has the opposite effects. of simplified models (12 14), none of which incorporate dynamics of ionic channels. A recent beginning of biophysically based modeling (15) examined the dynamics of model networks of only GPe and STN neurons and found that the biophysical properties of the neurons in this simple model network can support a wide spectrum of spatiotemporal oscillatory activity patterns. This study is an important step toward understanding BG function, but it was not designed to model the control of motor behavior. In studies of the relationships between behavior and neural activity in the BG, recordings have revealed many different patterns of activity, differently (and somewhat inconsistently) timed in relation to movement onset (see ref. 16 for review). Based on a series of experimental observations (17 19), Mink (4) suggested that the function of the BG is to control competing motor programs, inhibiting those that interfere with the voluntary movement being executed, and focusing disinhibition on this desired movement. This hypothesis was supported by evidence that movement-related activity in GPi occurs late compared with those of movement-related activity in motor regions of the cortex (16), and that 70% of GPi neurons with movement-related activity have an increased firing rate with movement while 30% have a decreased firing rate, consistent with the idea that GPi Abbreviations: BG, basal ganglia; GABA, -aminobutyric acid; GPe, globus pallidus pars externa; GPi, globus pallidus pars interna; PD, Parkinson s disease; STN, subthalamic nucleus. To whom correspondence should be addressed at: Center for Neuroscience, University of California, 1544 Newton Court, Davis, CA lrubchinsky@ucdavis.edu by The National Academy of Sciences of the USA NEUROSCIENCE cgi doi pnas PNAS November 25, 2003 vol. 100 no
2 Fig. 2. Diagram of motor control model BG network (filled arrowheads correspond to inhibitory connections, and open arrowheads correspond to excitatory connections). The depicted pattern of corticostriatal input corresponds to the execution of motor program 1 ( wanted ) and the inhibition of motor program 2 ( unwanted ). The reversed, complementary structure of corticostriatal input (i.e., input to GPe1, STN2, and GPi2) will reverse wanted and unwanted motor programs: motor program 2 will be promoted, and motor program 1 will be inhibited. output inhibits more of its thalamic targets than it disinhibits (20 22). Modeling of the BG s role in motor control requires a study of how BG networks respond to cortical input to influence thalamocortical circuits responsible for motor control. This article presents the development and analysis of a minimal biophysically based model of BG motor control circuitry. Our model is constructed within an experimentally motivated theory in which the BG are hypothesized to facilitate the motor programs to be executed and to inhibit motor programs that may interfere with the ongoing movement (4, 23 25). In this context, PD results in an inability to correctly inhibit unwanted motor programs and support wanted motor programs, leading to akinesia, bradykinesia, and rigidity. The modeling was developed to elucidate possible mechanisms for both normal motor control and the pathophysiology associated with PD. Methods: Construction of the Model Network Architecture for Motor Control BG Circuits. To begin, we presume that groups of STN, GPe, and GPi neurons are transiently formed by input from cortex and striatum to participate in execution of a particular motor program. Other groups of pallidal and subthalamic neurons, at that moment, act to suppress motor programs that may interfere with the desired motion. This pattern of activation during movement is supported by experimental data recorded from non-human primates (e.g., 20). Subsequently, a new movement would require formation of new subsets of neurons within each nucleus. In our model circuit (Fig. 2), we have two subsets of GPe, STN, and GPi, each of which represents a transiently formed group of neurons. Input from the cortex and striatum determine which subset in their target structures are to be activated or suppressed. Obviously, the simple circuit considered here (Fig. 2) is limited to the control of only two motor programs, the goal being to represent the transient execution of only one motor program at a time ( wanted ) and the suppression of the other ( unwanted ). The connectivity of the circuit is based on the known anatomy of BG (reviewed in refs. 26 and 27). What we have added to this proposed circuit is the way in which pallidal and STN subsets are connected to one another. As will be discussed below, the cross-connections were chosen to provide a mechanism for discriminating between corticostriatal signals for the desired movement and inappropriate corticostriatal inputs. In our minimal model, striatal and cortical inputs are represented as external signals to our model system with assigned timing targeted to specific subsets of the circuit. These input signals are not derived from biophysical modeling of corticostriatal networks. Instead, we use simple forms of spatial and temporal organization of input signals to GP STN networks (in this study spatial is limited to only two subsets within each nucleus). This strategy simplifies the analysis of the model. Further support of this strategy is grounded in the fact that the GPi and STN are the effective sites for surgical procedures to alleviate the motor symptoms of PD. The striatum has been studied extensively (reviewed in refs ) and will provide the necessary experimental constraints on the timing and structure of corticostriatal input to pallidum and STN for more detailed modeling. Modeling Pallidal STN Subsets. The cellular physiology of STN and GPe has been studied extensively (STN in refs , and GPe in refs ). Those studies gave rise to the formulation of conductance-based models of STN and GPe cells (15). We use these models for STN and pallidal subsets in our network. Experimental data available on GPi cell physiology is limited. Therefore, our GPi model has the same form as the GPe model, but its parameters are adjusted in such a way that the network behavior produces tonic GPi activity with a spiking rate similar to that observed in vivo. All these models include standard sodium, potassium, and leak currents and incorporate low threshold T-type Ca 2 current, high-threshold Ca 2 current, and Ca 2 -activated voltage-independent afterhyperpolarization K current. The equation for membrane potential is C dv dt I L I K I Na I T I Ca I AHP I syn I app, where leak current is I L g L (V V L ), fast potassium and sodium currents are I K g K n 4 (V V K ) and I Na g Na m 3 (V)h(V V Na ), calcium currents are I T g T a 3 (V)b 2 (r)(v V Ca ) and I Ca g Ca s 2 (V)(V V Ca ), the afterhyperpolarization current is I AHP g AHP ([Ca] [Ca] k 1 )(V V K ), where [Ca] is concentration of intracellular Ca 2 ions, and the equation of the calcium balance is d[ca] dt ( I Ca I T k Ca [Ca]). n, h and r are slow gating variables described by first-order kinetic equations, and m, a and s are instantaneously activated gating variables and depend on voltage (see ref. 15 for details). The model parameters are from ref. 15, with the following differences: h 2.0, 0 1 n 0.005, n 0.31, h 0.1, and n The parameters for GPi model are the same as for GPe model with the exception of g T 0.1, g Ca 0.03, g AHP 10.0, and n 0.05, which makes the firing rate of GPi cells higher, as has been observed experimentally (see below). Modeling Synaptic Connections: Ionotropic and Metabotropic Synapses. All connections present in Fig. 2 represent ionotropic synapses [excitatory glutamatergic and inhibitory GABAergic (GABA, -aminobutyric acid)]. These synapses are modeled by first-order kinetic equations describing the fraction of activated receptors as in ref. 15: ds dt H V presyn g 1 s s, where sigmoidal function H 1 (1 exp[ (V H g ) H g ]). The synaptic current from a single connection is given by cgi doi pnas Rubchinsky et al.
3 I syn g syn V V syn s. The values of parameters for ionotropic synapses are taken from ref. 15. In our model, we include GPe STN inhibitory metabotropic GABA B synapses (37, 38). The addition of these slow-acting synapses can inhibit STN for a sufficiently long time to induce a strong rebound after release from inhibition. The model of GABA B synapses consists of two kinetic equations, which include an equation describing the concentration of activated G protein (39): dr dt k 1T 1 R k 2 R dg dt k 3R k 4 G, where R is the fraction of activated receptors and T T max H. The synaptic current is given by G 4 I syn g B G 4 k V V syn. The values of parameters for GABA B synapses follow those considered in ref. 40 and are T max 0.5, g B 6.0, k 1 0.5, k , k , k , k 100, and V syn Modeling of Corticostriatal Input to BG Network. The input signal from cortex to STN and from striatum to GPe and GPi is modeled as a train of spikes in the form of f (t )exp( t ) (for simplicity, when the new spike occurs f is set to zero). The current injected into BG model subsets is I app g f, with g g Cx, g Str e, and g Str i for current injected to STN, GPe, and GPi, respectively. The parameters of the input currents are g Cx 80.0, g Str e 50.0, g Str i 50.0, 1 ms, and the spiking rate is 100 spikes per second. Experimental Constraints on Model Circuit Dynamics. Synaptic strength in the model network cannot be directly estimated from available experimental data. Therefore, to find values appropriate for the present modeling study, synaptic strength was adjusted in a series of simulations in such a way that the patterns of activity of the modeled neurons are physiologically reasonable. A recent paper (40) provides the results of simultaneous recordings from rodent STN and GP in several in vivo conditions. Firing patterns of BG structures in normal and Parkinsonian primates are reviewed in refs. 4 and 25. The values for synaptic strengths are g stn3gpe 4.0, g gpe3stn 1.0, g stn3gpi 15.0, and g gpe3gpi 0.5. The model network behavior is robust for these parameter values, although the chosen values are not a unique combination of parameters that yield the appropriate spiking rates in the model network. Results: Behavior of BG Model Circuit in Movement Control In the absence of any inputs, all subsets of the model network discharge tonically (Fig. 3). Note that GPi has a relatively high firing rate in the baseline regime, when no motor program is being executed (4) (in vivo GPi projects to thalamus and inhibits thalamocortical motor circuits to prevent movement execution). Model Circuit Under Corticostriatal Input: Execution of Single Movements and Switching Between Movements. A command to execute a single movement is modeled as a train of spikes arriving simultaneously in GPe2, STN1, and GPi1 (input 1), leading to the following restructuring of the network s behavior: STN1 firing frequency increases strongly because of the cortical input. In turn, STN firing leads to an increased frequency in GPe1, Fig. 3. Dynamics of isolated model circuit. Traces of membrane potential for GPe, STN, and GPi subsets are presented (in the absence of input, the dynamics of the network is symmetric: the activity in subsets 1 and 2 is the same). In all of the figures, horizontal axes are time in milliseconds, and vertical axes are membrane potential V in millivolts. which inhibits GPi1 and STN2 (so STN2 does not excite GPi1). Striatal input to GPe2 releases STN1 and GPi2 from inhibition, and striatal input to GPi1 helps to suppress GPi1 firing. As a result, the following is observed in the output nucleus of BG network (Fig. 4): GPi1 ceases to fire spikes, and the GPi2 firing rate increases. After input signals vanish, firing rates return to the normal values after a period of transient dynamics (there is a short period of silence in GPi2, probably due to a rebound in GPe2, which inhibits GPi2; at the same time there is a period of increased firing in GPi1). So, during the movement, GPi1 is silent (releasing the corresponding parts of motor thalamus from inhibition, thus facilitating the desired movement) and GPi2 is overactive, thus inhibiting thalamocortical circuits responsible for unwanted motor programs. Because our goal was to develop a model relevant both to normal behavior and behavioral deficits in PD, we chose to simulate motor switching (e.g., simple opposing movements such as reaching and withdrawing or pronation supination of the simple neurological examination), which is critically impaired in PD (41, 42). We simulated the dynamics of the model circuit by switching corticostriatal input from one set of network subsets to the complementary one. So, input 2 will drive GPe1, STN2, Fig. 4. Execution of a single movement. The traces of membrane potential represent the output of GPi subsets of our model circuit in Fig. 2 in response to corticostriatal signals lasting from t 1 2 sec. In all of the figures, horizontal bars indicate the duration of input to the network (solid, input 1; dashed, input 2). NEUROSCIENCE Rubchinsky et al. PNAS November 25, 2003 vol. 100 no
4 Fig. 5. Switching between different movements. (A) Corticostriatal input 1 is active from t 1 2 sec, and input 2 (reversed, complementary signal) is active from t 2 3 sec. (B and C) Overlapping corticostriatal inputs. Input 1 is active from t sec, and input 2 is active from t 2 3 sec. Dynamics of GPi and STN are shown. and GPi2. In this case (Fig. 5A), GPi1 ceases to fire for the time when input 1 is present and fires with a higher-than-baseline rate when input 2 is active, whereas GPi2 has the opposite behavior. As a result, motor program 1 is facilitated and motor program 2 is inhibited during activation of input 1, and when the input is switched to facilitate motor program 2 and to suppress the previously executed motor program 1, the pattern of GPi activity follows it. One can see that, even though the switching of the input signal to a complementary one is instantaneous, the switching in GPi output is not. GPi1 spiking appears 100 ms after input 1 vanishes and input 2 becomes active. So the temporal precision of motor program control by our model subthalamopallidal network is limited. In a series of simulations, we examined the effect of varying the duration of input 2 (followed by a reversal back to input 1) on the behavior of the network. If is 200 ms and smaller, then GPi2 continues to produce high-frequency spiking during that time and input 2 is not detected. For larger, the short alternative signal is reflected in the GPi output and, so, the corresponding motor program is facilitated, whereas the competing motor program is inhibited. The value of the limit for temporal precision depends on the characteristics of rebound properties in STN (on the properties of GABA B synapses and I T current). Model Circuit Under Conflicting Corticostriatal Inputs. We now consider the dynamics of subthalamopallidal networks under conflicting input signals, i.e., when two inputs, which correspond to conflicting motor programs 1 and 2, coexist for a certain amount of time. For numerical simulations of conflicting corticostriatal inputs, we consider input signals overlapped in time: input 2 starts some period before input 1 stops (the characteristics of input currents are the same for both inputs). A typical example is presented in the Fig. 5B. Here, input 1 ends 500 ms after input 2 is introduced. The spiking in GPi2 disappears, so new movement (movement 2) can start when input 2 starts, whereas the ongoing movement 1 should be suppressed because of intense GPi1 spiking. Thus, the output pattern changes to terminate ongoing movement and promote the new movement, even though the input signal for the old movement is still active. This is because high-frequency cortical input to STN2 at t 2 sec depolarizes STN2, which leads to a strong rebound burst of spikes in STN2 (Fig. 5C). This rebound spiking in STN2 excites GPi1 and excites GPe2 (despite inhibitory input to GPe2, which is still present). This, in turn, helps to inhibit GPi2 and inhibit STN1 (which, otherwise, would excite GPi2), even though STN1 continues to receive cortical excitation. This is how spatial symmetry is broken in the network in response to temporal asymmetry: the network is symmetric (subsets 1 and 2 are identical in each nucleus) and the structure of inputs 1 and 2 is symmetric (have identical characteristics and target complementary subsets), but the timing of inputs is different, and this is reflected in the differences in the dynamics of the GPi subsets. Slow GABA B Synapses Influence Model Circuit Behavior. The detection of the new signal in two overlapping signals relies on the slow metabotropic GABA B synaptic projection from GPe to STN. This synapse leads to hyperpolarization of STN as long as GPe produces intense spiking. When GPe stops intense spiking, STN is released from inhibition, resulting in a strong rebound burst due to the T-type transient calcium current in STN. Numerical simulations with a network with weak GABA B synapses or without GABA B synapses at all confirm this role of the slow GABA B synapse (Fig. 6). The network without these GABA B synapses is subject to the same overlapping corticostriatal inputs considered above. The dynamics of GPi are not as extreme as in the example in Fig. 5 (silence vs. high-frequency spiking) because the total amount of pallidal inhibition of STN has been changed, but the general pattern of GPi dynamics is easy to see. When there is only one input present at any moment of time, GPi activity facilitates motor program 1 and inhibits motor program 2. However, when the input signals overlap in time, both subsets of the BG output nuclei, GPi1 and GPi2, produce high-frequency spiking (Fig. 6 A and B). Therefore, both thalamocortical circuits, corresponding to two different motor programs, are inhibited and no movement is performed. This can be interpreted as an akinetic condition. In this case, there is no rebound burst, which was provided by GABA B synapses and transient calcium T current, in STN (Fig. 6C). As a consequence, spatial symmetry cannot be broken in response to the break in temporal symmetry, and the network loses the ability to discriminate between inputs. Nonreciprocal Structure of GPe STN Connections Is Crucial for Model Circuit Function. The network architecture considered in this work involves nonreciprocal coupling between GPe and STN subsets. This hypothesized organization of the network helps to discriminate between competing corticostriatal inputs to the pallidosubthalamic network. We studied whether reciprocal links between GPe and STN (in addition to the nonreciprocal links in the network in Fig. 2) affect this ability. The addition of the reciprocal links with weak synaptic strengths (5% of nonreciprocal links strengths) does not affect the performance of the network in response to the conflicting inputs, but the ability to discriminate between conflicting motor programs is gradually lost with the increase of the synaptic strengths of reciprocal links cgi doi pnas Rubchinsky et al.
5 Fig. 6. Dynamics of BG model network with weak GABA B synapses (g B 1) (A and C) and without GABA B synapses (g B 0) (B). The configuration of the inputs is the same as in Fig. 5 B and C. In the network without GABA B synapses, the strength of synaptic connections was modified to compensate for the absence of GABA B inhibition of STN by GPe. As a result, the network behavior is similar to the one observed with weak GABA B synapses (Fig. 7): both GPi segments are activated, which leads to the inhibition of thalamus and prevention of the execution of both old and new motor programs, an akinesia-like condition. A plausible explanation for this effect is that reciprocal links provide a negative feedback to STN, when STN is being excited by cortex. Therefore, the STN firing rate becomes low, there is no rebound burst of activity at the time of initiation of the corticostriatal signal 2 (Fig. 7), and the ability to discriminate between corticostriatal inputs is lost. Note that even though in the present situation reciprocal connections do not affect the tonic behavior of model subsets, for other parameter values they can contribute to the occurrence of oscillatory activity in the baseline regime [oscillations in STN GPe model networks with various types of coupling have been considered Fig. 7. Dynamics of BG model with additional reciprocal links between GPe and STN (the synaptic strength of these links is half the strength of nonreciprocal links). GPi and STN membrane potential traces are shown. (15); the relation of these oscillations to PD tremor remains unclear]. Discussion Facilitation of Desired Motor Program and Inhibition of Competing Motor Programs. According to the present understanding of BG physiology, releasing thalamocortical circuits from pallidal inhibition allows movement execution. In our model network, there are two different subsets in GPi; activation of both subsets corresponds to the absence of movement, whereas deactivation of one or the other corresponds to the execution of one of two different motor programs. In the baseline dynamics of the model circuit (Fig. 3), both subsets of GPi discharge tonically at a high rate so that all thalamocortical circuits are under pallidal inhibition and no movement is possible. Incoming corticostriatal signals act to suppress the activity of one of the GPi subsets (depending on the spatial pattern of this signal, which subsets it targets). As simulations show, the network is able to perform such discrimination and facilitate only one motor program because of the action of slow GABA B synapses, rebound properties of STN neurons, and nonreciprocal architecture of connections between STN and GPe subsets. Network models of action selection in BG have been suggested (12, 43). In these networks, the most salient input is selected by using lateral inhibition, and there is a hard-wired correspondence between specific neurons and specific motor programs. In particular, the study discussed in ref. 43 supposes that malfunction of lateral inhibition in striatum (acting by a winner-take-all mechanism) is directly responsible for failure of proper control of motor programs in PD. In contrast, the present model is not designed to detect the most salient action among many in striato-pallido-subthalamic pathways. Rather, it deals with the suppression of motor programs that interfere with the motor program being performed. A group of neurons within the pallidum or STN is dynamically formed in response to corticostriatal activity. The model subset representing this group can consist of different sets of real neurons from one moment to the next. Hypokinetic Behavior of BG Model Network. PD motor symptoms include hypokinetic behavior: akinesia and bradykinesia. Akinesia-like behavior is exhibited in the model network when both subsets of GPi become active and significantly inhibit thalamocortical circuits, thus preventing facilitation of any motor program. This situation was observed during numerical simulation of the disrupted model network under the influence of competing corticostriatal inputs. If the GABA B GPe to STN projection is weak or absent, then both GPi subsets are active, a signature NEUROSCIENCE Rubchinsky et al. PNAS November 25, 2003 vol. 100 no
6 of hypokinetic behavior. The prediction about the effect of GABA B synapses on network function made in this modeling study calls for confirmation in in vivo or in vitro experiments. A related prediction from the model is that the Parkinsonian symptoms should be accompanied by a deficit in the function of metabotropic GABAergic projections from GPe to STN. Determining whether this happens in the Parkinsonian BG requires studies of changes in BG with Parkinsonian dopaminergic degeneration. The introduction into the model network of reciprocal connections between BG nuclei subsets, in addition to nonreciprocal connections, also leads to akinetic dynamics. In PD, there is a loss of specificity of neuronal responses to passive movements in GPi (22, 44) and increased correlations between activity in distant neural territories (45). The action of reciprocal connections (which would be inhibited under normal conditions) may be a mechanism that contributes to the loss of specificity. This third prediction from the modeling could be tested by examining the changing structure of connectivity that might accompany the expansion of receptive fields. Further predictions about the role of the observed loss of specificity in hypokinetic behavior may result from modeling studies of larger versions of the network presented here. Studies of PD patients performance on specific motor tasks show that although the component movements of a motor sequence are performed more slowly than healthy subjects, the slowing is disproportionately greater if that sequence involves switching between muscle groups (41, 42). The observed change in the behavior of the network that accompanies deficits in switching between different motor programs is reminiscent of this slowness in motor switching. The function of the GABA B receptors and rebound properties of STN cells in vivo is unknown; it is possible that these phenomena in the model provide a mechanism to avoid hypokinetic behavior. BG Model Networks for Control of Many Motor Programs. Our minimal model describes the control of only two different motor programs. We believe that this network is scalable to the large number of pallidal and STN subsets needed to investigate the mechanisms responsible for focused activation and rapid transition between the individual motor programs that accompany the fluid movements during ongoing behavior. In the larger network, the spatial pattern of connections could be implemented. The network would include off-center inhibitory projections from GPe to STN, allowing for inhibition of motor programs interfering with ongoing motion. However, full understanding of BG motor program control will also require more detailed modeling of the spatial and temporal patterns of input into the pallidal STN networks from the cortex and striatum, which are necessary for the transient formation of the appropriate cell groups within the subthalamopallidal networks. We thank Drs. J. W. Mink and C. J. Wilson for comments on a previous version of the manuscript. This work was supported by National Institutes of Health Grants NS39121 and MH Albin, R. L., Young, A. B. & Penney, J. B. (1989) Trends Neurosci. 12, Alexander, G. E., Crutcher, M. D. & DeLong, M. R. (1990) Prog. Brain Res. 85, DeLong, M. R. (1900) Trends Neurosci. 13, Mink, J. W. (1996) Prog. Neurobiol. 50, Wichmann, T. & DeLong, M. R. (1996) Curr. Opin. Neurobiol. 6, Levy, R., Hazrati, L. N., Herrero, M. T., Vila, M., Hassani, O. K., Mouroux, M., Ruberg, M., Asensi, H., Agid, Y., Feger, J., et al. (1997) Neuroscience 76, Bar-Gad, I. & Bergman, H. (2001) Curr. Opin. Neurobiol. 11, Bergman, H. & Deuschl, G. (2002) Movement Disorders 17, Suppl., S28 S Houk, J., Adams, J. & Barto, A. (1995) in Models of Information Processing in the Basal Ganglia, eds. Houk, J. C., Davis, J. L. & Beiser, D. G. (MIT Press, Cambridge, MA), pp Schultz, W., Dayan, P. & Montague, P. R. (1997) Science 275, Berns, G. S. & Sejnowski, T. J. (1998) J. Cognit. Neurosci. 10, Gurney, K., Prescott, T. J. & Redgrave, P. (2001) Biol. Cybern. 84, Gillies, A. J. & Willshaw, D. J. (1998) Proc. R. Soc. London Ser. B 265, Humphries, M. D. & Gurney, K. N. (2001) Neural Netw. 14, Terman, D., Rubin, J. E., Yew, A. C. & Wilson, C. J. (2002) J. Neurosci. 22, Kimura, M. & Matsumoto, N. (1997) Adv. Neurol. 74, Georgopoulos, A. P., DeLong, M. R. & Crutcher, M. D. (1983) J. Neurosci. 3, Anderson, M. E. & Horak, F. B. (1985) J. Neurophysiol. 54, Mink, J. W. & Thach, W. T. (1991) J. Neurophysiol. 65, Turner, R. S. & Anderson, M. E. (1997) J. Neurophysiol. 77, Wenger, K. K., Musch, K. L. & Mink, J. W. (1999) J. Neurophysiol. 82, Boraud, T., Bezard, E., Bioulac, B. & Gross, C. E. (2000) J. Neurophysiol. 83, Mink, J. W. & Thach, W. T. (1993) Curr. Opin. Neurobiol. 3, Hikosaka, O., Takikawa, Y. & Kawagoe, R. (2000) Physiol. Rev. 80, Boraud, T., Bezard, E., Bioulac, B. & Gross, C. E. (2002) Prog. Neurobiol. 66, Wilson, C. J. (1998) in Synaptic Organization of the Brain, ed. Shepherd, G. M. (Oxford Univ. Press, New York), pp Bolam, J. P., Hanley, J. J., Booth, P. A. & Bevan, M. D. (2000) J. Anat. 196, Gerfen, C. R. (1992) Annu. Rev. Neurosci. 15, Flaherty, A. W. & Graybiel, A. M. (1994) in Movement Disorders 3, eds. Marsden, C. D. & Fahn, S. (Buterworth-Heinemann, Oxford), pp Miller, R. & Wickens, J. R., eds. (2000) Brain Dynamics and Striatal Complex (Harwood Academic, Amsterdam). 31. Bevan, M. D. & Wilson, C. J. (1999) J. Neurosci. 19, Bevan, M. D., Wilson, C. J., Bolam, J. P. & Magill, P. J. (2000) J. Neurophysiol. 83, Beurrier, C., Bioulac, B. & Hammond, C. (2000) J. Neurophysiol. 83, Kita, H. & Kitai, S. T. (1991) Brain Res. 564, Nambu, A. & Llinas, R. (1994) J. Neurophysiol. 72, Cooper, A. J. & Stanford, I. M. (2000) J. Physiol. 527, Charara, A., Heilman, T. C., Levey, I. A. & Smith, Y. (2000) Neuroscience 95, Smith, Y., Charara, A., Paquet, M., Kieval, J. Z., Pare, J. F., Hanson, J. E., Hubert, G. W., Kuwajima, M. & Levey A. I. (2002) J. Chem. Neuroanat. 22, Destexhe, A., Mainen, Z. F. & Sejnowski, T. J. (1998) in Methods in Neuronal Modeling: From Ions to Channels, eds. Koch, C. & Segev, I. (MIT Press, Cambridge, MA), pp Magill, P. J., Bolam, J. P. & Bevan, M. D. (2001) Neuroscience 106, Benecke, R., Rothwell, J., Dick, J., Day, B. & Marsden, C. (1987) Brain 110, Weiss, P., Stelmach, G. E. & Hefter, H. (1997) Brain 120, Wickens, J. (1993) A Theory of the Striatum (Pergamon, Oxford). 44. Filion, M., Tremblay, L. & Bedard, P. J. (1988) Brain Res. 444, Bergman, H., Feingold, A., Nini, A., Raz, A., Slovin, H., Abeles, M. & Vaadia, E. (1998) Trends Neurosci. 21, cgi doi pnas Rubchinsky et al.
Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease
 Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease Abstract Parkinson's disease (PD) is a neurodegenerative disease affecting the dopaminergic neurons of the
Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease Abstract Parkinson's disease (PD) is a neurodegenerative disease affecting the dopaminergic neurons of the
COGNITIVE SCIENCE 107A. Motor Systems: Basal Ganglia. Jaime A. Pineda, Ph.D.
 COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
Basal Ganglia. Introduction. Basal Ganglia at a Glance. Role of the BG
 Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
On the Origin of Tremor in Parkinson s Disease
 On the Origin of Tremor in Parkinson s Disease Andrey Dovzhenok 1, Leonid L Rubchinsky 1,2 1 Department of Mathematical Sciences and Center for Mathematical Biosciences, Indiana University Purdue University
On the Origin of Tremor in Parkinson s Disease Andrey Dovzhenok 1, Leonid L Rubchinsky 1,2 1 Department of Mathematical Sciences and Center for Mathematical Biosciences, Indiana University Purdue University
Anatomy of the basal ganglia. Dana Cohen Gonda Brain Research Center, room 410
 Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Teach-SHEET Basal Ganglia
 Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
GBME graduate course. Chapter 43. The Basal Ganglia
 GBME graduate course Chapter 43. The Basal Ganglia Basal ganglia in history Parkinson s disease Huntington s disease Parkinson s disease 1817 Parkinson's disease (PD) is a degenerative disorder of the
GBME graduate course Chapter 43. The Basal Ganglia Basal ganglia in history Parkinson s disease Huntington s disease Parkinson s disease 1817 Parkinson's disease (PD) is a degenerative disorder of the
NS219: Basal Ganglia Anatomy
 NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
This is a repository copy of Basal Ganglia. White Rose Research Online URL for this paper:
 This is a repository copy of. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/114159/ Version: Accepted Version Book Section: Prescott, A.J. orcid.org/0000-0003-4927-5390,
This is a repository copy of. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/114159/ Version: Accepted Version Book Section: Prescott, A.J. orcid.org/0000-0003-4927-5390,
Modeling the interplay of short-term memory and the basal ganglia in sequence processing
 Neurocomputing 26}27 (1999) 687}692 Modeling the interplay of short-term memory and the basal ganglia in sequence processing Tomoki Fukai* Department of Electronics, Tokai University, Hiratsuka, Kanagawa
Neurocomputing 26}27 (1999) 687}692 Modeling the interplay of short-term memory and the basal ganglia in sequence processing Tomoki Fukai* Department of Electronics, Tokai University, Hiratsuka, Kanagawa
Making Things Happen 2: Motor Disorders
 Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Electrophysiology of Subthalamic Nucleus in Normal and Parkinson s Disease
 Continuing Medical Education 206 Electrophysiology of Subthalamic Nucleus in Normal and Parkinson s Disease Chun-Hwei Tai and Chung-Chin Kuo Abstract- Subthalamic nucleus (STN) has been known to play an
Continuing Medical Education 206 Electrophysiology of Subthalamic Nucleus in Normal and Parkinson s Disease Chun-Hwei Tai and Chung-Chin Kuo Abstract- Subthalamic nucleus (STN) has been known to play an
Basal ganglia Sujata Sofat, class of 2009
 Basal ganglia Sujata Sofat, class of 2009 Basal ganglia Objectives Describe the function of the Basal Ganglia in movement Define the BG components and their locations Describe the motor loop of the BG
Basal ganglia Sujata Sofat, class of 2009 Basal ganglia Objectives Describe the function of the Basal Ganglia in movement Define the BG components and their locations Describe the motor loop of the BG
Basal ganglia macrocircuits
 Tepper, Abercrombie & Bolam (Eds.) Progress in Brain Research, Vol. 160 ISSN 0079-6123 Copyright r 2007 Elsevier B.V. All rights reserved CHAPTER 1 Basal ganglia macrocircuits J.M. Tepper 1,, E.D. Abercrombie
Tepper, Abercrombie & Bolam (Eds.) Progress in Brain Research, Vol. 160 ISSN 0079-6123 Copyright r 2007 Elsevier B.V. All rights reserved CHAPTER 1 Basal ganglia macrocircuits J.M. Tepper 1,, E.D. Abercrombie
Network Effects of Deep Brain Stimulation for Parkinson s Disease A Computational. Modeling Study. Karthik Kumaravelu
 Network Effects of Deep Brain Stimulation for Parkinson s Disease A Computational Modeling Study by Karthik Kumaravelu Department of Biomedical Engineering Duke University Date: Approved: Warren M. Grill,
Network Effects of Deep Brain Stimulation for Parkinson s Disease A Computational Modeling Study by Karthik Kumaravelu Department of Biomedical Engineering Duke University Date: Approved: Warren M. Grill,
Connections of basal ganglia
 Connections of basal ganglia Introduction The basal ganglia, or basal nuclei, are areas of subcortical grey matter that play a prominent role in modulating movement, as well as cognitive and emotional
Connections of basal ganglia Introduction The basal ganglia, or basal nuclei, are areas of subcortical grey matter that play a prominent role in modulating movement, as well as cognitive and emotional
Basal Ganglia George R. Leichnetz, Ph.D.
 Basal Ganglia George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the brain structures which constitute the basal ganglia, and their interconnections 2. To understand the consequences (clinical manifestations)
Basal Ganglia George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the brain structures which constitute the basal ganglia, and their interconnections 2. To understand the consequences (clinical manifestations)
Learning Working Memory Tasks by Reward Prediction in the Basal Ganglia
 Learning Working Memory Tasks by Reward Prediction in the Basal Ganglia Bryan Loughry Department of Computer Science University of Colorado Boulder 345 UCB Boulder, CO, 80309 loughry@colorado.edu Michael
Learning Working Memory Tasks by Reward Prediction in the Basal Ganglia Bryan Loughry Department of Computer Science University of Colorado Boulder 345 UCB Boulder, CO, 80309 loughry@colorado.edu Michael
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
SYNCHRONIZATION OF PALLIDAL ACTIVITY IN THE MPTP PRIMATE MODEL OF PARKINSONISM IS NOT LIMITED TO OSCILLATORY ACTIVITY
 SYNCHRONIZATION OF PALLIDAL ACTIVITY IN THE MPTP PRIMATE MODEL OF PARKINSONISM IS NOT LIMITED TO OSCILLATORY ACTIVITY Gali Heimer, Izhar Bar-Gad, Joshua A. Goldberg and Hagai Bergman * 1. INTRODUCTION
SYNCHRONIZATION OF PALLIDAL ACTIVITY IN THE MPTP PRIMATE MODEL OF PARKINSONISM IS NOT LIMITED TO OSCILLATORY ACTIVITY Gali Heimer, Izhar Bar-Gad, Joshua A. Goldberg and Hagai Bergman * 1. INTRODUCTION
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
The control of spiking by synaptic input in striatal and pallidal neurons
 The control of spiking by synaptic input in striatal and pallidal neurons Dieter Jaeger Department of Biology, Emory University, Atlanta, GA 30322 Key words: Abstract: rat, slice, whole cell, dynamic current
The control of spiking by synaptic input in striatal and pallidal neurons Dieter Jaeger Department of Biology, Emory University, Atlanta, GA 30322 Key words: Abstract: rat, slice, whole cell, dynamic current
Dynamic Behaviour of a Spiking Model of Action Selection in the Basal Ganglia
 Dynamic Behaviour of a Spiking Model of Action Selection in the Basal Ganglia Terrence C. Stewart (tcstewar@uwaterloo.ca) Xuan Choo (fchoo@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca) Centre
Dynamic Behaviour of a Spiking Model of Action Selection in the Basal Ganglia Terrence C. Stewart (tcstewar@uwaterloo.ca) Xuan Choo (fchoo@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca) Centre
Damage on one side.. (Notes) Just remember: Unilateral damage to basal ganglia causes contralateral symptoms.
 Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making
 Neural Networks 19 (2006) 1120 1136 www.elsevier.com/locate/neunet 2006 Special Issue Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making Michael J. Frank,1 Department
Neural Networks 19 (2006) 1120 1136 www.elsevier.com/locate/neunet 2006 Special Issue Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making Michael J. Frank,1 Department
VL VA BASAL GANGLIA. FUNCTIONAl COMPONENTS. Function Component Deficits Start/initiation Basal Ganglia Spontan movements
 BASAL GANGLIA Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo I) Overview How do Basal Ganglia affect movement Basal ganglia enhance cortical motor activity and facilitate movement.
BASAL GANGLIA Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo I) Overview How do Basal Ganglia affect movement Basal ganglia enhance cortical motor activity and facilitate movement.
Hold Your Horses: A Dynamic Computational Role for the Subthalamic Nucleus in Decision Making
 Frank 1 Hold Your Horses: A Dynamic Computational Role for the Subthalamic Nucleus in Decision Making Michael J. Frank Dept of Psychology and Program in Neuroscience University of Arizona 1503 E University
Frank 1 Hold Your Horses: A Dynamic Computational Role for the Subthalamic Nucleus in Decision Making Michael J. Frank Dept of Psychology and Program in Neuroscience University of Arizona 1503 E University
Basal Ganglia. Steven McLoon Department of Neuroscience University of Minnesota
 Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Visualization and simulated animations of pathology and symptoms of Parkinson s disease
 Visualization and simulated animations of pathology and symptoms of Parkinson s disease Prof. Yifan HAN Email: bctycan@ust.hk 1. Introduction 2. Biochemistry of Parkinson s disease 3. Course Design 4.
Visualization and simulated animations of pathology and symptoms of Parkinson s disease Prof. Yifan HAN Email: bctycan@ust.hk 1. Introduction 2. Biochemistry of Parkinson s disease 3. Course Design 4.
Bifurcation analysis points towards the source of beta neuronal oscillations in Parkinson s disease
 2011 50th IEEE Conference on Decision and Control and European Control Conference (CDC-ECC) Orlando, FL, USA, December 12-15, 2011 Bifurcation analysis points towards the source of beta neuronal oscillations
2011 50th IEEE Conference on Decision and Control and European Control Conference (CDC-ECC) Orlando, FL, USA, December 12-15, 2011 Bifurcation analysis points towards the source of beta neuronal oscillations
The motor regulator. 1) Basal ganglia/nucleus
 The motor regulator 1) Basal ganglia/nucleus Neural structures involved in the control of movement Basal Ganglia - Components of the basal ganglia - Function of the basal ganglia - Connection and circuits
The motor regulator 1) Basal ganglia/nucleus Neural structures involved in the control of movement Basal Ganglia - Components of the basal ganglia - Function of the basal ganglia - Connection and circuits
Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning
 1 Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Sensory-motor loop The essence
1 Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Sensory-motor loop The essence
Basal Ganglia General Info
 Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Strick Lecture 4 March 29, 2006 Page 1
 Strick Lecture 4 March 29, 2006 Page 1 Basal Ganglia OUTLINE- I. Structures included in the basal ganglia II. III. IV. Skeleton diagram of Basal Ganglia Loops with cortex Similarity with Cerebellar Loops
Strick Lecture 4 March 29, 2006 Page 1 Basal Ganglia OUTLINE- I. Structures included in the basal ganglia II. III. IV. Skeleton diagram of Basal Ganglia Loops with cortex Similarity with Cerebellar Loops
The role of intra-thalamic and thalamocortical circuits in action selection
 The role of intra-thalamic and thalamocortical circuits in action selection M D Humphries and K N Gurney Department of Psychology, University of Sheffield, Sheffield, S10 2TP,UK E-mail: m.d.humphries@shef.ac.uk,k.gurney@shef.ac.uk
The role of intra-thalamic and thalamocortical circuits in action selection M D Humphries and K N Gurney Department of Psychology, University of Sheffield, Sheffield, S10 2TP,UK E-mail: m.d.humphries@shef.ac.uk,k.gurney@shef.ac.uk
THE ROBOT BASAL GANGLIA: Action selection by an embedded model of the basal ganglia
 To appear in Nicholson, L. F. B. & Faull, R. L. M. (eds.) Basal Ganglia VII, Plenum Press, 2002 THE ROBOT BASAL GANGLIA: Action selection by an embedded model of the basal ganglia Tony J. Prescott, Kevin
To appear in Nicholson, L. F. B. & Faull, R. L. M. (eds.) Basal Ganglia VII, Plenum Press, 2002 THE ROBOT BASAL GANGLIA: Action selection by an embedded model of the basal ganglia Tony J. Prescott, Kevin
Gangli della Base: un network multifunzionale
 Gangli della Base: un network multifunzionale Prof. Giovanni Abbruzzese Centro per la Malattia di Parkinson e i Disordini del Movimento DiNOGMI, Università di Genova IRCCS AOU San Martino IST Basal Ganglia
Gangli della Base: un network multifunzionale Prof. Giovanni Abbruzzese Centro per la Malattia di Parkinson e i Disordini del Movimento DiNOGMI, Università di Genova IRCCS AOU San Martino IST Basal Ganglia
Deep Brain Stimulation: Surgical Process
 Deep Brain Stimulation: Surgical Process Kia Shahlaie, MD, PhD Assistant Professor Bronte Endowed Chair in Epilepsy Research Director of Functional Neurosurgery Minimally Invasive Neurosurgery Department
Deep Brain Stimulation: Surgical Process Kia Shahlaie, MD, PhD Assistant Professor Bronte Endowed Chair in Epilepsy Research Director of Functional Neurosurgery Minimally Invasive Neurosurgery Department
High Frequency Stimulation of the Subthalamic Nucleus Eliminates Pathological Thalamic Rhythmicity in a Computational Model
 Journal of Computational Neuroscience 16, 211 235, 2004 c 2004 Kluwer Academic Publishers. Manufactured in The Netherlands. High Frequency Stimulation of the Subthalamic Nucleus Eliminates Pathological
Journal of Computational Neuroscience 16, 211 235, 2004 c 2004 Kluwer Academic Publishers. Manufactured in The Netherlands. High Frequency Stimulation of the Subthalamic Nucleus Eliminates Pathological
Network effects of subthalamic deep brain stimulation drive a unique mixture of responses in basal ganglia output
 Section: CECN Special Issue Proposed Associate Editor: Tomoki Fukai Network effects of subthalamic deep brain stimulation drive a unique mixture of responses in basal ganglia output Abbreviated title:
Section: CECN Special Issue Proposed Associate Editor: Tomoki Fukai Network effects of subthalamic deep brain stimulation drive a unique mixture of responses in basal ganglia output Abbreviated title:
MODELING FUNCTIONS OF STRIATAL DOPAMINE MODULATION IN LEARNING AND PLANNING
 MODELING FUNCTIONS OF STRIATAL DOPAMINE MODULATION IN LEARNING AND PLANNING Suri R. E. 1,*, Bargas J. 2, and Arbib M.A. 1 1 USC Brain Project, Los Angeles CA 989-252 2 Departamento de Biofisica, Instituto
MODELING FUNCTIONS OF STRIATAL DOPAMINE MODULATION IN LEARNING AND PLANNING Suri R. E. 1,*, Bargas J. 2, and Arbib M.A. 1 1 USC Brain Project, Los Angeles CA 989-252 2 Departamento de Biofisica, Instituto
Short-Term Plasticity Shapes the Response to Simulated Normal and Parkinsonian Input Patterns in the Globus Pallidus
 The Journal of Neuroscience, June 15, 2002, 22(12):5164 5172 Short-Term Plasticity Shapes the Response to Simulated Normal and Parkinsonian Input Patterns in the Globus Pallidus Jesse E. Hanson and Dieter
The Journal of Neuroscience, June 15, 2002, 22(12):5164 5172 Short-Term Plasticity Shapes the Response to Simulated Normal and Parkinsonian Input Patterns in the Globus Pallidus Jesse E. Hanson and Dieter
This is a repository copy of Frequency and function in the basal ganglia: the origins of beta and gamma band activity.
 This is a repository copy of Frequency and function in the basal ganglia: the origins of beta and gamma band activity. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/113391/
This is a repository copy of Frequency and function in the basal ganglia: the origins of beta and gamma band activity. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/113391/
Lecture XIII. Brain Diseases I - Parkinsonism! Brain Diseases I!
 Lecture XIII. Brain Diseases I - Parkinsonism! Bio 3411! Wednesday!! Lecture XIII. Brain Diseases - I.! 1! Brain Diseases I! NEUROSCIENCE 5 th ed! Page!!Figure!!Feature! 408 18.9 A!!Substantia Nigra in
Lecture XIII. Brain Diseases I - Parkinsonism! Bio 3411! Wednesday!! Lecture XIII. Brain Diseases - I.! 1! Brain Diseases I! NEUROSCIENCE 5 th ed! Page!!Figure!!Feature! 408 18.9 A!!Substantia Nigra in
Functional reorganization in thalamocortical networks: Transition between spindling and delta sleep rhythms
 Proc. Natl. Acad. Sci. USA Vol. 93, pp. 15417 15422, December 1996 Neurobiology Functional reorganization in thalamocortical networks: Transition between spindling and delta sleep rhythms D. TERMAN*, A.BOSE*,
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 15417 15422, December 1996 Neurobiology Functional reorganization in thalamocortical networks: Transition between spindling and delta sleep rhythms D. TERMAN*, A.BOSE*,
The Basal Ganglia and Motor Control
 NEURAL PLASTICITY VOLUME 10, NO. 1-2, 2003 The Basal Ganglia and Motor Control Henk J. Groenewegen Department ofanatomy, Research Institute Neurosciences Vrije Universiteit, Medical Center, Amsterdam,
NEURAL PLASTICITY VOLUME 10, NO. 1-2, 2003 The Basal Ganglia and Motor Control Henk J. Groenewegen Department ofanatomy, Research Institute Neurosciences Vrije Universiteit, Medical Center, Amsterdam,
Basal Ganglia. Today s lecture is about Basal Ganglia and it covers:
 Basal Ganglia Motor system is complex interaction between Lower motor neurons (spinal cord and brainstem circuits) and Upper motor neurons (pyramidal and extrapyramidal tracts) plus two main regulators
Basal Ganglia Motor system is complex interaction between Lower motor neurons (spinal cord and brainstem circuits) and Upper motor neurons (pyramidal and extrapyramidal tracts) plus two main regulators
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Temporal patterning of neural synchrony in the basal ganglia in Parkinson s disease
 Temporal patterning of neural synchrony in the basal ganglia in Parkinson s disease Shivakeshavan Ratnadurai-Giridharan 1, S. Elizabeth Zauber 2, Robert M. Worth 1,3, Thomas Witt 3, Sungwoo Ahn 1,5, Leonid
Temporal patterning of neural synchrony in the basal ganglia in Parkinson s disease Shivakeshavan Ratnadurai-Giridharan 1, S. Elizabeth Zauber 2, Robert M. Worth 1,3, Thomas Witt 3, Sungwoo Ahn 1,5, Leonid
Symbolic Reasoning in Spiking Neurons: A Model of the Cortex/Basal Ganglia/Thalamus Loop
 Symbolic Reasoning in Spiking Neurons: A Model of the Cortex/Basal Ganglia/Thalamus Loop Terrence C. Stewart (tcstewar@uwaterloo.ca) Xuan Choo (fchoo@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca)
Symbolic Reasoning in Spiking Neurons: A Model of the Cortex/Basal Ganglia/Thalamus Loop Terrence C. Stewart (tcstewar@uwaterloo.ca) Xuan Choo (fchoo@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca)
Reciprocal inhibition controls the oscillatory state in thalamic networks
 Neurocomputing 44 46 (2002) 653 659 www.elsevier.com/locate/neucom Reciprocal inhibition controls the oscillatory state in thalamic networks Vikaas S. Sohal, John R. Huguenard Department of Neurology and
Neurocomputing 44 46 (2002) 653 659 www.elsevier.com/locate/neucom Reciprocal inhibition controls the oscillatory state in thalamic networks Vikaas S. Sohal, John R. Huguenard Department of Neurology and
Functional Correlations between Neighboring Neurons in the Primate Globus Pallidus Are Weak or Nonexistent
 4012 The Journal of Neuroscience, May 15, 2003 23(10):4012 4016 Brief Communication Functional Correlations between Neighboring Neurons in the Primate Globus Pallidus Are Weak or Nonexistent Izhar Bar-Gad,
4012 The Journal of Neuroscience, May 15, 2003 23(10):4012 4016 Brief Communication Functional Correlations between Neighboring Neurons in the Primate Globus Pallidus Are Weak or Nonexistent Izhar Bar-Gad,
Basics of Computational Neuroscience: Neurons and Synapses to Networks
 Basics of Computational Neuroscience: Neurons and Synapses to Networks Bruce Graham Mathematics School of Natural Sciences University of Stirling Scotland, U.K. Useful Book Authors: David Sterratt, Bruce
Basics of Computational Neuroscience: Neurons and Synapses to Networks Bruce Graham Mathematics School of Natural Sciences University of Stirling Scotland, U.K. Useful Book Authors: David Sterratt, Bruce
Intro. Comp. NeuroSci. Ch. 9 October 4, The threshold and channel memory
 9.7.4 The threshold and channel memory The action potential has a threshold. In figure the area around threshold is expanded (rectangle). A current injection that does not reach the threshold does not
9.7.4 The threshold and channel memory The action potential has a threshold. In figure the area around threshold is expanded (rectangle). A current injection that does not reach the threshold does not
Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects
 Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects Jonathan E. Rubin, University of Pittsburgh Cameron C. McIntyre, Cleveland Clinic Robert S Turner,
Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects Jonathan E. Rubin, University of Pittsburgh Cameron C. McIntyre, Cleveland Clinic Robert S Turner,
Research Article A Biologically Inspired Computational Model of Basal Ganglia in Action Selection
 Computational Intelligence and Neuroscience Volume 25, Article ID 8747, 24 pages http://dx.doi.org/.55/25/8747 Research Article A Biologically Inspired Computational Model of Basal Ganglia in Action Selection
Computational Intelligence and Neuroscience Volume 25, Article ID 8747, 24 pages http://dx.doi.org/.55/25/8747 Research Article A Biologically Inspired Computational Model of Basal Ganglia in Action Selection
Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons
 1916 The Journal of Neuroscience, March 1, 2003 23(5):1916 1923 Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons Takao Hashimoto, 1,2 Christopher M. Elder, 1 Michael
1916 The Journal of Neuroscience, March 1, 2003 23(5):1916 1923 Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons Takao Hashimoto, 1,2 Christopher M. Elder, 1 Michael
Kinematic Modeling in Parkinson s Disease
 Kinematic Modeling in Parkinson s Disease Alexander Hui Department of Bioengineering University of California, San Diego La Jolla, CA 92093 alexhui@ucsd.edu Abstract Parkinson s disease is a slowly progressing
Kinematic Modeling in Parkinson s Disease Alexander Hui Department of Bioengineering University of California, San Diego La Jolla, CA 92093 alexhui@ucsd.edu Abstract Parkinson s disease is a slowly progressing
An Interactive Channel Model of the Basal Ganglia: Bifurcation Analysis Under Healthy and Parkinsonian Conditions
 Journal of Mathematical Neuroscience (2013) 3:14 DOI 10.1186/2190-8567-3-14 RESEARCH OpenAccess An Interactive Channel Model of the Basal Ganglia: Bifurcation Analysis Under Healthy and Parkinsonian Conditions
Journal of Mathematical Neuroscience (2013) 3:14 DOI 10.1186/2190-8567-3-14 RESEARCH OpenAccess An Interactive Channel Model of the Basal Ganglia: Bifurcation Analysis Under Healthy and Parkinsonian Conditions
神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心.
 神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心 http://www.ym.edu.tw/~cflu OUTLINE Components and Pathways of the Basal Nuclei Functions and Related Disorders of the Basal Nuclei
神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心 http://www.ym.edu.tw/~cflu OUTLINE Components and Pathways of the Basal Nuclei Functions and Related Disorders of the Basal Nuclei
Expectation of reward modulates cognitive signals in the basal ganglia
 articles Expectation of reward modulates cognitive signals in the basal ganglia Reiko Kawagoe, Yoriko Takikawa and Okihide Hikosaka Department of Physiology, School of Medicine, Juntendo University, 2-1-1
articles Expectation of reward modulates cognitive signals in the basal ganglia Reiko Kawagoe, Yoriko Takikawa and Okihide Hikosaka Department of Physiology, School of Medicine, Juntendo University, 2-1-1
Dysfunctional and compensatory synaptic plasticity in Parkinson s disease
 European Journal of Neuroscience European Journal of Neuroscience, Vol. 39, pp. 688 702, 2014 doi:10.1111/ejn.12434 DISORDERS OF THE NERVOUS SYSTEM Dysfunctional and compensatory synaptic plasticity in
European Journal of Neuroscience European Journal of Neuroscience, Vol. 39, pp. 688 702, 2014 doi:10.1111/ejn.12434 DISORDERS OF THE NERVOUS SYSTEM Dysfunctional and compensatory synaptic plasticity in
Multi-objective Evolutionary Algorithms to Investigate Neurocomputational Issues: The Case Study of Basal Ganglia Models
 Multi-objective Evolutionary Algorithms to Investigate Neurocomputational Issues: The Case Study of Basal Ganglia Models Jean Liénard, Agnès Guillot, and Benoît Girard Institut des Systèmes Intelligents
Multi-objective Evolutionary Algorithms to Investigate Neurocomputational Issues: The Case Study of Basal Ganglia Models Jean Liénard, Agnès Guillot, and Benoît Girard Institut des Systèmes Intelligents
The Wonders of the Basal Ganglia
 Basal Ganglia The Wonders of the Basal Ganglia by Mackenzie Breton and Laura Strong /// https://kin450- neurophysiology.wikispaces.com/basal+ganglia Introduction The basal ganglia are a group of nuclei
Basal Ganglia The Wonders of the Basal Ganglia by Mackenzie Breton and Laura Strong /// https://kin450- neurophysiology.wikispaces.com/basal+ganglia Introduction The basal ganglia are a group of nuclei
Effects of nicotine on neuronal firing patterns in human subthalamic nucleus. Kim Scott Mentor: Henry Lester SURF seminar, January 15, 2009
 Effects of nicotine on neuronal firing patterns in human subthalamic nucleus Kim Scott Mentor: Henry Lester SURF seminar, January 15, 2009 Smoking tobacco protects against Parkinson s Disease (PD). Identical
Effects of nicotine on neuronal firing patterns in human subthalamic nucleus Kim Scott Mentor: Henry Lester SURF seminar, January 15, 2009 Smoking tobacco protects against Parkinson s Disease (PD). Identical
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817.
 Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Whether deep brain stimulation can dramatically. Deep brain stimulation: How does it work?
 JERROLD L. VITEK, MD, PhD Director, Neuromodulation Research Center, Department of Neurosciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH Deep brain stimulation: How does it work? ABSTRACT
JERROLD L. VITEK, MD, PhD Director, Neuromodulation Research Center, Department of Neurosciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH Deep brain stimulation: How does it work? ABSTRACT
Exam 2 PSYC Fall (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
 Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
Exam 2 PSYC 2022 Fall 1998 (2 points) What 2 nuclei are collectively called the striatum? (2 points) Match a brain structure that is located closest to the following portions of the ventricular system
1/2/2019. Basal Ganglia & Cerebellum a quick overview. Outcomes you want to accomplish. MHD-Neuroanatomy Neuroscience Block. Basal ganglia review
 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the
This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the
This manuscript version is distributed under the CC-BY-NC-ND (Creative Commons) license.
 This manuscript version is distributed under the CC-BY-NC-ND (Creative Commons) license. It has appeared in final form as: Van Albada SJ, Gray RT, Drysdale PM, Robinson PA. Mean-field modeling of the basal
This manuscript version is distributed under the CC-BY-NC-ND (Creative Commons) license. It has appeared in final form as: Van Albada SJ, Gray RT, Drysdale PM, Robinson PA. Mean-field modeling of the basal
Basal ganglia output to the thalamus: still a paradox
 Basal ganglia output to the thalamus: still a paradox The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher
Basal ganglia output to the thalamus: still a paradox The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher
Modeling synaptic facilitation and depression in thalamocortical relay cells
 College of William and Mary W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 5-2011 Modeling synaptic facilitation and depression in thalamocortical relay cells Olivia
College of William and Mary W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 5-2011 Modeling synaptic facilitation and depression in thalamocortical relay cells Olivia
STRUCTURE AND CIRCUITS OF THE BASAL GANGLIA
 STRUCTURE AND CIRCUITS OF THE BASAL GANGLIA Rastislav Druga Department of Anatomy, Second Faculty of Medicine 2017 Basal ganglia Nucleus caudatus, putamen, globus pallidus (medialis et lateralis), ncl.
STRUCTURE AND CIRCUITS OF THE BASAL GANGLIA Rastislav Druga Department of Anatomy, Second Faculty of Medicine 2017 Basal ganglia Nucleus caudatus, putamen, globus pallidus (medialis et lateralis), ncl.
Different inhibitory effects by dopaminergic modulation and global suppression of activity
 Different inhibitory effects by dopaminergic modulation and global suppression of activity Takuji Hayashi Department of Applied Physics Tokyo University of Science Osamu Araki Department of Applied Physics
Different inhibitory effects by dopaminergic modulation and global suppression of activity Takuji Hayashi Department of Applied Physics Tokyo University of Science Osamu Araki Department of Applied Physics
A Role for Dopamine in Temporal Decision Making and Reward Maximization in Parkinsonism
 12294 The Journal of Neuroscience, November 19, 2008 28(47):12294 12304 Behavioral/Systems/Cognitive A Role for Dopamine in Temporal Decision Making and Reward Maximization in Parkinsonism Ahmed A. Moustafa,
12294 The Journal of Neuroscience, November 19, 2008 28(47):12294 12304 Behavioral/Systems/Cognitive A Role for Dopamine in Temporal Decision Making and Reward Maximization in Parkinsonism Ahmed A. Moustafa,
Signal enhancement in the output stage of the basal ganglia by synaptic short-term plasticity in the direct, indirect, and hyperdirect pathways
 COMPUTATIONAL NEUROSCIENCE ORIGINAL RESEARCH ARTICLE published: 19 June 2013 doi: 10.3389/fncom.2013.00076 Signal enhancement in the output stage of the basal ganglia by synaptic short-term plasticity
COMPUTATIONAL NEUROSCIENCE ORIGINAL RESEARCH ARTICLE published: 19 June 2013 doi: 10.3389/fncom.2013.00076 Signal enhancement in the output stage of the basal ganglia by synaptic short-term plasticity
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Investigation of Physiological Mechanism For Linking Field Synapses
 Investigation of Physiological Mechanism For Linking Field Synapses Richard B. Wells 1, Nick Garrett 2, Tom Richner 3 Microelectronics Research and Communications Institute (MRCI) BEL 316 University of
Investigation of Physiological Mechanism For Linking Field Synapses Richard B. Wells 1, Nick Garrett 2, Tom Richner 3 Microelectronics Research and Communications Institute (MRCI) BEL 316 University of
Synfire chains with conductance-based neurons: internal timing and coordination with timed input
 Neurocomputing 5 (5) 9 5 www.elsevier.com/locate/neucom Synfire chains with conductance-based neurons: internal timing and coordination with timed input Friedrich T. Sommer a,, Thomas Wennekers b a Redwood
Neurocomputing 5 (5) 9 5 www.elsevier.com/locate/neucom Synfire chains with conductance-based neurons: internal timing and coordination with timed input Friedrich T. Sommer a,, Thomas Wennekers b a Redwood
Parkinson s Disease and Cortico-Basal Ganglia Circuits
 Continuing Medical Education 213 Parkinson s Disease and Cortico-Basal Ganglia Circuits Ming-Kai Pan, Chun-Hwei Tai, Chung-Chin Kuo Abstract- Cortico-basal ganglia circuit model has been studied extensively
Continuing Medical Education 213 Parkinson s Disease and Cortico-Basal Ganglia Circuits Ming-Kai Pan, Chun-Hwei Tai, Chung-Chin Kuo Abstract- Cortico-basal ganglia circuit model has been studied extensively
Embryological origin of thalamus
 diencephalon Embryological origin of thalamus The diencephalon gives rise to the: Thalamus Epithalamus (pineal gland, habenula, paraventricular n.) Hypothalamus Subthalamus (Subthalamic nuclei) The Thalamus:
diencephalon Embryological origin of thalamus The diencephalon gives rise to the: Thalamus Epithalamus (pineal gland, habenula, paraventricular n.) Hypothalamus Subthalamus (Subthalamic nuclei) The Thalamus:
Modèles mathématiques et corrélats anatomiques du mouvement (2/2)
 17 mars 2016 Modèles mathématiques et corrélats anatomiques du mouvement (2/2) Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris,
17 mars 2016 Modèles mathématiques et corrélats anatomiques du mouvement (2/2) Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris,
Synaptic Transmission: Ionic and Metabotropic
 Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
Synaptic Transmission: Ionic and Metabotropic D. Purves et al. Neuroscience (Sinauer Assoc.) Chapters 5, 6, 7. C. Koch. Biophysics of Computation (Oxford) Chapter 4. J.G. Nicholls et al. From Neuron to
arxiv: v1 [math.fa] 18 Jun 2009
![arxiv: v1 [math.fa] 18 Jun 2009 arxiv: v1 [math.fa] 18 Jun 2009](/thumbs/93/111682225.jpg) Averaging Transformations of Synaptic Potentials on Networks H. R. Noori,a arxiv:0906.3404v1 [math.fa] 18 Jun 2009 Abstract a Interdisciplinary Center for Scientific Computing, University of Heidelberg,
Averaging Transformations of Synaptic Potentials on Networks H. R. Noori,a arxiv:0906.3404v1 [math.fa] 18 Jun 2009 Abstract a Interdisciplinary Center for Scientific Computing, University of Heidelberg,
Down regulation of the HCN2 channel in rat models of Parkinson s disease
 Down regulation of the HCN2 channel in rat models of Parkinson s disease Abstract The basal ganglia is a key control centre involved with the regulation of movement. It is therefore a primary interest
Down regulation of the HCN2 channel in rat models of Parkinson s disease Abstract The basal ganglia is a key control centre involved with the regulation of movement. It is therefore a primary interest
Modelling Decision Making under Uncertainty Machine Learning and Neural Population Techniques. Elaine Duffin
 Modelling Decision Making under Uncertainty Machine Learning and Neural Population Techniques Elaine Duffin Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University
Modelling Decision Making under Uncertainty Machine Learning and Neural Population Techniques Elaine Duffin Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University
Synaptic Interactions
 1 126 Part 111: Articles Synaptic Interactions Alain Destexhe, Zachary F. Mainen, and Terrence J. Sejnowski 7 Modeling synaptic interactions in network models poses a particular challenge. Not only should
1 126 Part 111: Articles Synaptic Interactions Alain Destexhe, Zachary F. Mainen, and Terrence J. Sejnowski 7 Modeling synaptic interactions in network models poses a particular challenge. Not only should
MODULE 6: CEREBELLUM AND BASAL GANGLIA
 MODULE 6: CEREBELLUM AND BASAL GANGLIA This module will summarize the important neuroanatomical and key clinical concepts from Chapters 15 and 16 of the textbook for the course. The first part of this
MODULE 6: CEREBELLUM AND BASAL GANGLIA This module will summarize the important neuroanatomical and key clinical concepts from Chapters 15 and 16 of the textbook for the course. The first part of this
Simplified Model of Machado-Joseph Disease in Comparison to Parkinson s Disease and Normal Models
 Simplified Model of Machado-Joseph Disease in Comparison to Parkinson s Disease and Normal Models 1 2 3 4 5 6 7 8 9 10 Anjulie Agrusa M.S. Student of Bioengineering UC San Diego San Diego CA Julia Hardy
Simplified Model of Machado-Joseph Disease in Comparison to Parkinson s Disease and Normal Models 1 2 3 4 5 6 7 8 9 10 Anjulie Agrusa M.S. Student of Bioengineering UC San Diego San Diego CA Julia Hardy
The basal ganglia (BG) are a group of nuclei located subcortically
 The Basal Ganglia and Disorders of Movement: Pathophysiological Mechanisms José A. Obeso, 1 María C. Rodríguez-Oroz, 1 Manuel Rodríguez, 4 Javier Arbizu, 2 and José M. Giménez-Amaya 3 Departments of 1
The Basal Ganglia and Disorders of Movement: Pathophysiological Mechanisms José A. Obeso, 1 María C. Rodríguez-Oroz, 1 Manuel Rodríguez, 4 Javier Arbizu, 2 and José M. Giménez-Amaya 3 Departments of 1
Mechanisms of choice in the primate brain: a quick look at positive feedback
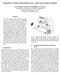 Mechanisms of choice in the primate brain: a quick look at positive feedback JM Chambers, K Gurney, M Humphries, A Prescott Department of Psychology, University of Sheffield Western Bank, Sheffield, S10
Mechanisms of choice in the primate brain: a quick look at positive feedback JM Chambers, K Gurney, M Humphries, A Prescott Department of Psychology, University of Sheffield Western Bank, Sheffield, S10
G eneralised dystonia is characterised by sustained or
 1509 PAPER Childhood onset generalised dystonia can be modelled by increased gain in the indirect basal ganglia pathway T D Sanger...... Correspondence to: Dr Terence D Sanger, Department of Neurology
1509 PAPER Childhood onset generalised dystonia can be modelled by increased gain in the indirect basal ganglia pathway T D Sanger...... Correspondence to: Dr Terence D Sanger, Department of Neurology
Multi-site Stimulation of Subthalamic Nucleus Diminishes Thalamocortical Relay Errors in a Biophysical Network Model
 Multi-site Stimulation of Subthalamic Nucleus Diminishes Thalamocortical Relay Errors in a Biophysical Network Model Yixin Guo a,, Jonathan E. Rubin b a Department of Mathematics, Drexel University, Philadelphia,
Multi-site Stimulation of Subthalamic Nucleus Diminishes Thalamocortical Relay Errors in a Biophysical Network Model Yixin Guo a,, Jonathan E. Rubin b a Department of Mathematics, Drexel University, Philadelphia,
Towards a Better Treatment of Parkinson's disease
 Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 3-2014 Towards a Better Treatment
Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 3-2014 Towards a Better Treatment
Membrane Channel Interactions Underlying Rat Subthalamic Projection Neuron Rhythmic and Bursting Activity
 J Neurophysiol 95: 2352 2365, 2006. First published September 7, 2005; doi:10.1152/jn.00525.2005. Membrane Channel Interactions Underlying Rat Subthalamic Projection Neuron Rhythmic and Bursting Activity
J Neurophysiol 95: 2352 2365, 2006. First published September 7, 2005; doi:10.1152/jn.00525.2005. Membrane Channel Interactions Underlying Rat Subthalamic Projection Neuron Rhythmic and Bursting Activity
Neurons! John A. White Dept. of Bioengineering
 Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Neurons! John A. White Dept. of Bioengineering john.white@utah.edu What makes neurons different from cardiomyocytes? Morphological polarity Transport systems Shape and function of action potentials Neuronal
Basal Ganglia Anatomy, Physiology, and Function. NS201c
 Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The Classical Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The Classical Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate
The basal ganglia optimize decision making over general perceptual hypotheses
 The basal ganglia optimize decision making over general perceptual hypotheses 1 Nathan F. Lepora 1 Kevin N. Gurney 1 1 Department of Psychology, University of Sheffield, Sheffield S10 2TP, U.K. Keywords:
The basal ganglia optimize decision making over general perceptual hypotheses 1 Nathan F. Lepora 1 Kevin N. Gurney 1 1 Department of Psychology, University of Sheffield, Sheffield S10 2TP, U.K. Keywords:
The Basal Ganglia in Parkinson s Disease: Current Concepts and Unexplained Observations
 The Basal Ganglia in Parkinson s Disease: Current Concepts and Unexplained Observations Jose A. Obeso, PhD, MD, 1,2 Concepcio Marin, PhD, MD, 2,3 C. Rodriguez-Oroz, PhD, MD, 1,2 Javier Blesa, 1,2 B. Benitez-Temiño,
The Basal Ganglia in Parkinson s Disease: Current Concepts and Unexplained Observations Jose A. Obeso, PhD, MD, 1,2 Concepcio Marin, PhD, MD, 2,3 C. Rodriguez-Oroz, PhD, MD, 1,2 Javier Blesa, 1,2 B. Benitez-Temiño,
Relationships between the Prefrontal Cortex and the Basal Ganglia in the Rat: Physiology of the Cortico-Nigral Circuits
 The Journal of Neuroscience, June 1, 1999, 19(11):4674 4681 Relationships between the Prefrontal Cortex and the Basal Ganglia in the Rat: Physiology of the Cortico-Nigral Circuits Nicolas Maurice, 1 Jean-Michel
The Journal of Neuroscience, June 1, 1999, 19(11):4674 4681 Relationships between the Prefrontal Cortex and the Basal Ganglia in the Rat: Physiology of the Cortico-Nigral Circuits Nicolas Maurice, 1 Jean-Michel
