FH9-A 2016 Kit Instructions CAP 2016
|
|
|
- Cameron Atkins
- 5 years ago
- Views:
Transcription
1 FH9-A 2016 Kit Instructions CAP 2016 Comprehensive Hematology Survey Automated Differential FH9 FH9P Table of Contents Kit Contents Kit Contents... 1 Important: Before You Begin... 1 Detailed Testing Instructions... 2 Reporting Your Results... 6 Biohazard Warning... 8 For Assistance... 9 Master Lists... 9 FH9 FH9P FH9-01 FH9-05 FH9-01 FH9-05 BCP-01 BCP-10 Important: The Kit Contents lists all possible orderable programs for this mailing. Your laboratory will only receive the programs it ordered. Important: Before You Begin New for this Mailing 1. Beginning with this mailing, the MCH, MCHC, and MPV challenges will be graded and primary and secondary mode data will be combined for each instrument peer group. 2. The handling instructions have been updated. Please review them carefully prior to testing. Storage and Stability Instructions 1. Store whole blood specimens at 2-10 C until testing can be performed. 2. Testing must be performed within 48 hours after opening. 3. For best results, follow mixing instructions precisely. 4. Material exposed to room temperature for long periods of time may produce questionable values. 5. The supernatant in these specimens is expected to be a dark color. This does not indicate damage during shipment. Critical Reporting Information 1. A unit of measure (UOM) must be filled in for HGB, HCT, MCHC, and RDW challenges for proper evaluation. 2. Please verify which Control you are using and fill in the appropriate bubble when reporting nucleated red blood cells (nrbc). Refer to the alert on the result form. 3. Sysmex XE-2100D/L users: Use code 1986 for regular calibration. Use code 1357 for blood center calibration. See Instrument Master List for details. 4. Sysmex XN-series users: Review the footnote on the master list and enter the appropriate code on the result form. Important: See the Biohazard Warning at the end of these instructions. Customer Contact Center option 1 (domestic) or option 1 (international) 1
2 5. For RDW: a. If your instrument is able to report RDW-CV (%) or RDW-SD (fl), use the reference ranges below: The RDW-CV reference range in adults is typically %. The RDW-SD reference range in adults is typically fl. b. Sysmex users: If your instrument is able to report RDW-CV (%) and RDW-SD (fl), select the appropriate UOM based on patient reporting. For all other Sysmex instruments, fill the bubble for fl (RDW-SD [fl]). c. RDW-CV (%) and RDW-SD (fl) are graded for all instruments. Reporting Code Changes The following manufacturers have either deleted or updated codes for this mailing: Sysmex Detailed Testing Instructions 1. Flags are likely to accompany the differential results when specimens are tested in the patient mode. Although these flags may trigger manual differential review on a patient specimen, the user should ignore the differential flagging that is generated; do not perform a manual differential review. Report the automated instrument differential. 2. Elevated and/or flagged MCHC results do not indicate the presence of cold agglutinins. Do not incubate specimens. 3. These specimens are not suitable for methods for which platelet-rich plasma is used. Handling Instructions 1. Allow vial to stand at room temperature for 15 minutes before mixing. 2. Hold the vial vertically and roll each vial between the palms of the hands for 15 to 20 seconds. 3. Continue to mix by holding the vial by the ends between the thumb and finger, rapidly inverting the vial 20 times end-over-end using a very quick turning motion of the wrist. 4. Sample from the vial using the same technique as with a patient specimen. 5. Perform analysis of all specimens using the aspiration mode that the majority of your patient specimens are processed in: either closed vial or open vial mode. Instrument instructions: A. XE-2100/XE-2100L, XE-5000, XE-HST, XE-Alpha, XT-4000i, and XT-2000i/XT-1800i: Sysmex XE-2100, XE-5000, and XT-Series analyzers Note: This CAP Survey must be analyzed in the QC program to obtain white blood cell (WBC) differential data. Read and follow the specimen handling instructions before analyzing the specimens. Closed and Manual Open Mode a. Click on the Controller icon on the Main Menu. The X-Series Controller Menu will be displayed. b. Double-click the Setting icon in the X-Series Controller Menu. c. Click on the Sampler Stop Conditions tab. d. The current sampler stop conditions will be displayed on the Sampler Stop Conditions screen. 2 Comprehensive Hematology 2016
3 e. If a check mark is present in the box next to Unregistered QC Sample, then click on the box to remove the check mark. f. Click Apply. g. Click Menu to return to Main Menu. h. Closed Mode Manual Open Mode Place specimens in a rack and start the auto mode sampler. For XE-HST users, analyze the specimen using the Single Mode. Run specimens in Manual Open Mode as usual, reading the QC code 128 bar code label with the handheld bar code reader. Note: XE-Alpha and XE-HST users: When the CAP Manual specimen analysis is completed, go to Manual, enter a specimen ID number such as 1, and press ENTER. Then the XE will exit the QC LOCAL mode and return the Manual status to Ready. i. When specimen analysis has been completed, go into Explorer and print the results to the graphic printer (GP). Record the results on the form provided by CAP. j. When all data have been recorded, follow steps a through d to access the Sampler Stop Conditions screen. Click on the box next to Unregistered QC Sample to place the check mark back in the box. k. Click OK. l. Click Menu to return to Main Menu. B. Sysmex XN Series (XN-1000, XN-1000 R, XN-1000 BR (with SA-01 sampler), XN-2000, XN-3000, and XN-9000) XN-1000 XN-1000 R or XN-1000 BR (with SA-01 sampler) XN-2000 or XN-3000 XN-9000 Note: This CAP Survey must be analyzed using the QC Mode and the QC bar code label (code 128 label) that is on the vial to obtain the WBC differential data. Read the following specimen handling directions before analyzing the specimens. a. Click on the Analyzer Setting icon on the Main Menu. b. Select Sampler Analysis Stop. Customer Contact Center option 1 (domestic) or option 1 (international) 3
4 c. If a check mark is present in the box next to Unregistered QC Sample, then click on the box to remove the check mark. d. Click Apply. e. Click OK to return to Main Menu. f. XN-1000 XN-1000 R or XN-1000 BR (with SA-01 sampler) Place specimens in a rack and load onto sampler unit. The sampler unit will autostart. Place CAP samples in a rack and load onto right sampler pool unit. Select the Sampler Mode Icon on the IPU and select Start to start the sampler. XN-2000 or XN-3000 Power down one of the XN modules by opening the front cover and toggling the on/off switch to the off position. This will ensure the Survey specimen will be run on only the XN analyzer that is still operational in the sampler mode. Place specimens in a rack and load onto sampler unit. The sampler unit will autostart. XN-9000 To run QC on ONE XN analyzer, access the Off Line mode: Press the mode switch button on the conveyor. Place the rack on the right rack pool next to the labeled rack area. The sampler unit will automatically start. Remove the rack when analysis is completed. Cancel the Off Line mode by pressing the mode switch button on the conveyor. Note: A Control is not entered error will display due to the fact that this particular control is not set up in a QC file. Touch Accept to clear the error. This will not affect your results. g. When specimen analysis has been completed, go into Explorer and print the validated results to the GP. Record the results on the form provided by CAP. Note: If the QC result is not validated in Explorer, click on the specimens and click on the Validate icon. h. When all data have been recorded, follow steps a and b to access Sampler Analysis Stop. Click on the box next to Unregistered QC Sample to place the check mark back in the box. i. Click Apply. j. Click OK to return to Main Menu. k. XN-2000 or XN-3000 only: Power on the XN module previously turned off by opening the front cover and toggling the on/off switch to the on position. C. Sysmex XS-Series Note: This Survey must be analyzed using the QC Mode and the QC bar code label (code 128 label) that is on the vial. Closed Mode (for XS-1000i With Autoloader) Manual Open Mode (for XS-1000i without Autoloader (see CBC+DIFF on page 5)) Closed Mode (for XS-1000i With Autoloader) 4 Comprehensive Hematology 2016
5 a. Click on the Controller icon on the Main Menu. The XS-Series Controller Menu will be displayed. b. Click on the Setting icon in the XS-Series Controller Menu. c. Click on the Sampler Stop Conditions tab. d. The current sampler stop conditions will be displayed on the Sampler Stop Conditions screen. e. If a check mark is present in the box next to Unregistered QC Sample, then click on the box to remove the check mark. f. Click Apply. g. Click Menu to return to Main Menu. h. Place specimen vials into a sampler rack in positions 6, 7, 8, 9, and 10, and place the rack into sampler rack position 1 or 2. Rack notch must be facing right side of instrument. If rack is already in sampler, place tubes into rack and note rack number and tube position number. i. Close Sampler Cover. Click the Sampler icon. j. Click on the rack and tube position number in Sampler Sample No. dialog box to initiate where the instrument will begin processing. k. Click OK then press the Start button. Note: A control entry error will display in the Error List dialog box due to the fact that this particular control product is not set up in a QC file. Click on Accept and Close. This will not affect your results. l. A dialog box will display when specimen measurement has completed. Click OK. m. When specimen analysis has been completed, click on Explorer, deselect Last 20, highlight the specimens, and print the results to the GP. Record the results on the form. n. When all data have been recorded, follow steps a through d to access the Sampler Stop Conditions screen. Click on the box next to Unregistered QC Sample to place the check mark back in the box. o. Click OK. p. Click Menu to return to Main Menu. Manual Open Mode With Autoloader and Manual Open Mode Without Autoloader (CBC+DIFF): a. With Ready LED green, click the Manual icon or the [F2] function key on the keyboard. b. Enter the CAP bar coded number into the Sample No. field, using: Handheld bar code reader to scan the label. Note: Confirm Caps Lock on the keyboard is OFF. or Keyboard to manually type the number, always type the prefix QCbefore the bar coded number (example: QC ). Note: QC must always be uppercase and followed with a hyphen. c. Click OK, or press Enter on the keyboard. Note: A control entry error will display in the Error List dialog box due to the fact that this particular control product is not set up in a QC file. Click on Accept and Close. This will not affect your results. d. Manual Open Mode With Autoloader only: Open the Sampler Cover. e. Select the appropriate specimen tube adapter and place it in the specimen position area of the XS-1000i with the red mark facing up. Turn the adapter clockwise until there is a click (about 45 ) to secure it. f. Place the well mixed (10 times by inversion) CAP vial into the XS specimen adapter. Customer Contact Center option 1 (domestic) or option 1 (international) 5
6 g. Press the white Start button on the right near the specimen position inside the sampler cover. h. A dialog box will display when specimen measurement has completed. i. When specimen analysis has been completed, click on Explorer, deselect Last 20, highlight the specimens, and print the results to the GP. Record the results on the form. 6. White cell count (manual): These specimens contain simulated leukocytes. For the WBC, count all unlysed cells and/or particles other than platelets. 7. Hematocrit: Laboratories reporting HCT by the microhematocrit centrifugation method should spin specimens for exactly 5 minutes, regardless of the observed HCT. The packing behavior of specimens may be different than fresh human blood. The 5-minute centrifugation process is intended to minimize interlaboratory difference. This instruction does not pertain to Critspin/Statspin or Hematastat II/C-70 users. 8. Flow Through Differential: Do not attempt a manual differential on these specimens. 9. Nucleated Red Blood Cells: Laboratories have the option of reporting nrbcs as an absolute and/or percentage. To ensure that your laboratory is properly evaluated, you must select the type of control used in your laboratory (echeck, echeck XE, or XN-Check) by filling in the bubbles provided on the result form. 10. RDW: See Critical Reporting Information section for details. Immature Platelet Fraction (IPF) The IPF will not be formally evaluated; however, the data will be available in the participant summary (PS). Reporting Your Results General Reporting Instructions Failure to specify an instrument or selection of an inappropriate instrument may result in comparison with the wrong peer group and unacceptable performance. 1. All laboratories subject to Clinical Laboratory Improvement Amendments (CLIA) regulations: If your laboratory is discontinuing testing on any CMS-regulated analyte, you must check your CMS Analyte Reporting Selections to ensure no changes are needed. You can maintain your laboratory s current reporting preferences by accessing the application via e-lab Solutions Suite. 2. Each mailing, verify the accuracy of your reporting codes (eg, manufacturer, method, instrument, reagent) by reviewing the online result form or the Method Summary Page attached to the front of your result form. 3. For any testing that you do not routinely perform in your laboratory, leave all reporting areas for that test blank unless otherwise noted. 4. Exception Codes: If you must report an analytical problem for a test or individual analyte, leave the result area for that section blank and fill one of the following bubbles on the result form within that section. The exception code bubble that you fill in will apply only to the result area(s) left blank. Documentation on the use of these codes is the responsibility of the laboratory and should be kept internally. 11 Unable to analyze Use code 11 to indicate why specimens were not analyzed (eg, instrument not functioning, reagents not available). 22 Result is outside the method/instrument reportable range Use code 22 if you obtain a high or low result outside the reportable range of your method or instrument. Do not use this code if there is the appropriate option to fill a bubble for a greater than or less than result. 6 Comprehensive Hematology 2016
7 Per CLIA, as published by the United States Federal Register Proficiency Testing (PT) specimens must be tested with the laboratory s regular workload, using routine methods, and testing the PT specimens the same number of times it routinely tests patient specimens. If referral for testing is routinely performed for patient specimens, the practice cannot be followed for PT specimens. Referral is considered to be movement of the specimen from a laboratory with a CLIA identification number to another laboratory that has a different CLIA identification number. Laboratories must ensure that personnel do not share results or refer PT specimens for any reflex or testing outside their CLIA identification number. Disclaimer Survey specimens, their progeny, unmodified derivatives, or modifications thereof may not be transferred or incorporated into a program intended for sale. Survey specimens, their progeny, unmodified derivatives, or modifications thereof, reagents, and disposable equipment used in PT, when disposed of, should be autoclaved or incinerated and disposed of as hazardous waste. Disposal must follow local regulations, if more stringent than regulations enforced by the CDC or the FDA. 33 Specimen unsatisfactory To use code 33, you must contact the CAP. If you fill an exception code bubble and enter data on the result form, the data will be graded. 5. Corrections can be made at any time prior to the due date printed on the result form. Review all entries for accuracy prior to online approval or before sending by fax or mail. For results that are approved online, corrections must also be done online. Faxed or mailed corrections will not be accepted. 6. Multiple selections are not permitted and may result in an incorrect comparison of your results or an incorrect evaluation. 7. Quantitative results for this Survey can now be automatically transmitted using e-lab Solutions Connect. To learn more, visit cap.org and search for e-lab Solutions Connect. Blood Cell Identification 1. This section contains photographs and online images. The online images are intended to provide educational value. For consistency, report all blood cell identification challenges from the photographs and not the online images. 2. The first 5 photographs will be formally evaluated if there is at least 80% referee or participant consensus. The last 5 photographs are educational; they will not be formally evaluated, but referee and participant performance will be provided in the PS. 3. The review of photographs must be performed by a single individual who may seek the assistance of a supervisor, lead tech, or pathologist if that is the laboratory s standard procedure for patient slide review. 4. Only 1 selection is permitted for each photograph. 5. The Hematology Blood Cell Identification Master List includes all identifications that will be required. Consult the current Hematology Glossary before selecting a code from the master list. 6. Locate your identification on the Hematology Blood Cell Identification Master List and enter the code on the result form. Please select answers from this master list, as it is periodically revised. Please double-check to ensure that the correct code has been selected. 7. For further description of the Hematology Blood Cell Identification Master List choices, please refer to the Blood Cell Identification section of the current CAP Hematology and Clinical Microscopy Glossary, which can be accessed at cap.org. a. Under the Get Involved tab, click on Councils and Committees. b. Click on Hematology/Clinical Microscopy Resource Committee. c. Click on Hematology Topic Center. d. Click on 2016 Hematology and Clinical Microscopy Glossary. 8. After submission and receipt of PT results, a group review of the images provides an excellent continuing education opportunity. Submitting Results 1. Results must be received at the CAP no later than midnight, Central Time by the due date on the result form. Results cannot be accepted if received after the due date. 2. Your laboratory must establish a laboratory Web account, referred to as Opting In, to submit results online. Information about opting in and a unique PIN was mailed to all laboratory directors. If your laboratory director does not have this information, please contact the CAP for a replacement letter. Customer Contact Center option 1 (domestic) or option 1 (international) 7
8 3. Laboratory staff who will enter results online must first establish a personal Web account. Once a personal Web account is established, laboratory staff can request access to their laboratory s information. Biohazard Warning All Survey specimens should be treated as if potentially infectious and should be handled as if they are capable of transmitting disease. Survey specimens are prepared from blood or other source material obtained from human donors or animals. When working with Survey specimens, precautions should be taken to protect yourself and others from accidental exposure to infectious agents such as HIV, HBV, and HCV. HIV can be transmitted through accidental parenteral inoculation, mucous membranes, or non-intact skin contact with HIV infected blood or body fluids. HBV and HCV can be transmitted through accidental parenteral inoculation, mucous membranes, non-intact skin contact, aerosolization or ingestion. Precautions described in CDC and FDA recommendations and OSHA blood borne pathogen rules should be followed at all times when handling Survey specimens and reagents. Such precautions include the following: Gloves should be put on before opening the container and should be kept on throughout the period specimens are handled. Replace gloves if contaminated, or if their ability to function as a barrier is compromised. At high altitudes, specimens should be opened in a hood or biologic safety cabinet. There should be no eating, drinking, or smoking in the laboratory. Hands should be washed after removing gloves and before leaving the testing area. Survey specimens and reagents should be kept in separate refrigerators from those containing blood or blood components for transfusion. Survey specimens, reagents, and disposable equipment used in testing should be autoclaved or incinerated and disposed of as hazardous waste. Disposal must follow local regulations, if more stringent than regulations enforced by the CDC or the FDA. Warning: This Survey may contain a chemicals known to the State of California to cause cancer. If there has been an accident in which you have been exposed to the Survey s materials, please call the CAP Hot Line at (domestic) or (international) at any time. You can access Safety Data Sheets (SDS/MSDS) by logging on to cap.org, clicking on the Laboratory Improvement tab, then Catalog and Ordering Information. 8 Comprehensive Hematology 2016
9 For Assistance For replacement materials, please contact the CAP within 10 calendar days of the ship date for information. Provide your CAP number and contact information with all correspondence. Participants in countries serviced by a designated CAP distributor should contact their distributor s customer service department. Telephone: option 1 (Monday - Friday, 7:00 am 5:30 pm US Central Time) International Participants: option 1 Website: Address: contactcenter@cap.org cap.org CAP Surveys Program 325 Waukegan Road Northfield, IL USA Instrument Master List Deleted codes None New/Updated codes None 1410 Microhematocrit (all PCV) Waived 1986 Sysmex XE-2100, XE-2100D, XE-2100L* 1828 Sysmex XE-2100C, XE-2100DC 1357 Sysmex XE-2100D/L (Blood Center)** 2015 Sysmex XE Sysmex XN-series [EXCEPT XN-series (RL App)] 1198 Sysmex XN-series (RL App)*** 1922 Sysmex XS-series (EXCEPT XS-1000iC) 1829 Sysmex XS-1000iC 1441 Sysmex XT-1800i, XT-2000i 1443 Sysmex XT-4000i 0010 Other, specify on result form * Sysmex XE-2100D/L - Regular Calibration: Use instrument code 1986 ** Sysmex XE-2100D/L - Blood Center Calibration: Use instrument code 1357 *** Sysmex XN-series - For reference laboratories using CELLSHEATH C: Use instrument code 1198 Inclusion on this master list does not imply US FDA approval. Customer Contact Center option 1 (domestic) or option 1 (international) 9
10 Hematology Blood Cell Identification Master List Important: Consult the current Hematology and Clinical Microscopy Glossary, which can be accessed at cap.org, for brief descriptions or definitions. Note: Please read the history that accompanies the photographs or whole slide images before making your selection. If microorganisms, inclusions, or other intracellular findings are seen, choose an identification that indicates their presence. 108 Immature or abnormal cell, would refer for identification (Code 108 should be used only if you would routinely send the cell in question to an outside laboratory with another CLIA number.) Erythrocytic Cells 140 Acanthocyte (spur cell) 245 Bite cell 299 Blister cell/prekeratocyte 287 Echinocyte (burr cell, crenated cell) 135 Erythrocyte, normal 279 Erythrocyte with overlying platelet 288 Fragmented red cell (schistocyte, helmet cell, keratocyte, triangular cell) 298 Hypochromasia 229 Macrocyte oval or round (excluding polychromatophilic red cells) 249 Microcyte (with increased central pallor) 253 Nucleated red cell, normal or abnormal morphology 146 Ovalocyte (elliptocyte) 134 Polychromatophilic (non-nucleated) red cell 178 Red cell agglutinates 188 Rouleaux 147 Sickle cell (drepanocyte) 148 Spherocyte 149 Stomatocyte 150 Target cell (codocyte) 151 Teardrop cell (dacrocyte) Erythrocytic Cell Inclusions 152 Basophilic stippling (coarse) 153 Hemoglobin C crystal 155 Howell-Jolly body (Wright stain) 157 Pappenheimer bodies (iron stain) 225 Pappenheimer bodies, presumptive (Wright stain) Myeloid: Granulocytic and Monocytic Cells 208 Basophil, any stage 209 Eosinophil, any stage 117 Mast cell 236 Monocyte 237 Monocyte, immature (promonocyte, monoblast) 284 Neutrophil, segmented or band 259 Neutrophil, toxic (to include toxic granulation and/or Döhle bodies, and/or toxic vacuolization) 122 Neutrophil with hypersegmented nucleus 240 Neutrophil with Pelger-Huët nucleus (acquired or congenital) 161 Neutrophil, polyploid 239 Neutrophil with dysplastic nucleus and/or hypogranular cytoplasm 191 Neutrophil necrobiosis (degenerated neutrophil) 121 Neutrophil, giant band or giant metamyelocyte 112 Neutrophil, metamyelocyte 111 Neutrophil, myelocyte 241 Neutrophil, promyelocyte 238 Neutrophil, promyelocyte, abnormal with/without Auer rod(s) 246 Myeloblast with Auer rod Lymphocytic and Plasmacytic Cells 265 Lymphocyte 307 Lymphocyte, large granular 266 Lymphocyte, reactive (to include plasmacytoid and immunoblastic forms) 162 Malignant lymphoid cell (other than blast) 223 Plasma cell (to include morphologically mature, abnormal, and with inclusion, eg, Dutcher body, Russell body, etc) Megakaryocytic Cells and Platelets 279 Erythrocyte with overlying platelet 212 Megakaryocytic cell (normal, abnormal, or nuclear fragment) 263 Platelet, giant 285 Platelet, hypogranular 171 Platelet, normal 264 Platelet satellitism Microorganisms 282 Babesia sp. 268 Bacteria (cocci or rod), extracellular 269 Bacteria (spirochete), extracellular 270 Fungi, extracellular 196 Leukocyte with Anaplasma/Ehrlichia 271 Leukocyte with phagocytized bacteria 231 Leukocyte with phagocytized fungi 283 Microfilaria 156 Plasmodium sp. (malaria) 233 Protozoan (non-malarial) 311 Parasite(s) seen, referred for definitive identification (Code 311 should be used only if you would routinely send cell in question to an outside laboratory with another CLIA number) Miscellaneous 174 Blast cell 200 Cryoglobulin 118 Leukocyte with Alder (Alder-Reilly) anomaly inclusions 119 Leukocyte with Chediak-Higashi anomaly inclusion(s) 184 Metastatic tumor cell or tumor cell clump 204 Mitotic figure 213 Squamous epithelial cell/endothelial cell Artifacts 192 Basket cell/smudge cell 220 Stain precipitate 10 Comprehensive Hematology 2016
11 This page intentionally left blank. Customer Contact Center option 1 (domestic) or option 1 (international) 11
12 This page intentionally left blank. 12 Comprehensive Hematology 2016
Contents. Section Editor David Blomberg, MD
 Contents A Closer Look At Discussions...viii Heme CAPsules Video...ix Foreword...x Preface to First Edition...xii Preface to Second Edition...xiii Contributors...xiv Current and Past HCMRC Members...xv
Contents A Closer Look At Discussions...viii Heme CAPsules Video...ix Foreword...x Preface to First Edition...xii Preface to Second Edition...xiii Contributors...xiv Current and Past HCMRC Members...xv
G-B 2016 Kit Instructions CAP 2016
 G-B 2016 Kit Instructions CAP 2016 Syphilis Serology Survey Table of Contents Kit Contents Kit Contents... 1 Important: Before You Begin... 1 Detailed Testing Instructions... 1 Reporting Your Results...
G-B 2016 Kit Instructions CAP 2016 Syphilis Serology Survey Table of Contents Kit Contents Kit Contents... 1 Important: Before You Begin... 1 Detailed Testing Instructions... 1 Reporting Your Results...
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TEST PROGRAM Glass Slide - November 2016
 NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TEST PROGRAM Glass Slide - November 2016 Results from this proficiency test event are available at: http://www.wadsworth.org/regulatory/clep/pt/summaries SLIDE
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TEST PROGRAM Glass Slide - November 2016 Results from this proficiency test event are available at: http://www.wadsworth.org/regulatory/clep/pt/summaries SLIDE
Blood Cell Identification Graded
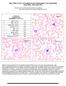
 BCP-21 Blood Cell Identification Graded Case History The patient is a 37-year-old female with a history of multiple sickle cell crises. She now presents with avascular necrosis of the left hip. Laboratory
BCP-21 Blood Cell Identification Graded Case History The patient is a 37-year-old female with a history of multiple sickle cell crises. She now presents with avascular necrosis of the left hip. Laboratory
Differential Blood Smear H3
 Verein für Association pour le Associazione per il medizinische Qualitätskontrolle contrôle de qualité médical controllo di qualità medico Report Differential Blood Smear H3 MQ 2015-4 MQ, Institut für
Verein für Association pour le Associazione per il medizinische Qualitätskontrolle contrôle de qualité médical controllo di qualità medico Report Differential Blood Smear H3 MQ 2015-4 MQ, Institut für
q,a;e Fc ) ORIGINAL EVALUATION. FH9-B 2017 Hematology Auto Differentials, FH9
 etiee INSTITUTION: ATTENTION: CAP NUMBER: KIT INFORMATION: COPIED TO: LKF Laboratorium fur Klinische Forschung GmbH Schwentinental GE 24223 Volker El-Samalouti PhD 7234136-01 Kit# 1 Kit ID: Kit Mailed:
etiee INSTITUTION: ATTENTION: CAP NUMBER: KIT INFORMATION: COPIED TO: LKF Laboratorium fur Klinische Forschung GmbH Schwentinental GE 24223 Volker El-Samalouti PhD 7234136-01 Kit# 1 Kit ID: Kit Mailed:
Blood Cell Identification Graded
 Blood Cell Identification Graded Case History The patient is a 20-year-old female with sickle cell disease who presents with bilateral leg pain for 3 days. She is scheduled to have bilateral hip and leg
Blood Cell Identification Graded Case History The patient is a 20-year-old female with sickle cell disease who presents with bilateral leg pain for 3 days. She is scheduled to have bilateral hip and leg
Differential Blood Smear H3
 Verein für Association pour le Associazione per il medizinische Qualitätskontrolle contrôle de qualité médical controllo di qualità medico Report Differential Blood Smear H3 MQ 2018-1 MQ, Institut für
Verein für Association pour le Associazione per il medizinische Qualitätskontrolle contrôle de qualité médical controllo di qualità medico Report Differential Blood Smear H3 MQ 2018-1 MQ, Institut für
Full Blood Count analysis Is a 3 part-diff good enough? Dr Marion Münster, Sysmex South Africa
 Full Blood Count analysis Is a 3 part-diff good enough? Dr Marion Münster, Sysmex South Africa The Role of the FBC in clinical decision making History Examination Investigations Decision 70% FBC Laboratory
Full Blood Count analysis Is a 3 part-diff good enough? Dr Marion Münster, Sysmex South Africa The Role of the FBC in clinical decision making History Examination Investigations Decision 70% FBC Laboratory
Differential Blood Smear H3
 Verein für Association pour le Associazione per il medizinische Qualitätskontrolle contrôle de qualité médical controllo di qualità medico Report Differential Blood Smear H3 MQ 2014-2 MQ, Institut für
Verein für Association pour le Associazione per il medizinische Qualitätskontrolle contrôle de qualité médical controllo di qualità medico Report Differential Blood Smear H3 MQ 2014-2 MQ, Institut für
HEMATOLOGIC MORPHOLOGY- AECOM HEMATOLOGY COURSE
 Log Out Help current login :lcytryn@montefiore.org HEMATOLOGIC MORPHOLOGY- AECOM HEMATOLOGY COURSE Lawrence Cytryn, M.D. - Course Director 1998 Edward Burns, M.D. Images used by permission within AECOM
Log Out Help current login :lcytryn@montefiore.org HEMATOLOGIC MORPHOLOGY- AECOM HEMATOLOGY COURSE Lawrence Cytryn, M.D. - Course Director 1998 Edward Burns, M.D. Images used by permission within AECOM
Interpreting the CBC. Robert Miller PA Assistant Professor of Clinical Pediatrics and Family Medicine USC Keck School of Medicine Retired
 Interpreting the CBC Robert Miller PA Assistant Professor of Clinical Pediatrics and Family Medicine USC Keck School of Medicine Retired The CBC 3 Cell Lines RBCs WBCs Platelets Assess general health Make
Interpreting the CBC Robert Miller PA Assistant Professor of Clinical Pediatrics and Family Medicine USC Keck School of Medicine Retired The CBC 3 Cell Lines RBCs WBCs Platelets Assess general health Make
Participants Identification No. % Evaluation. Mitotic figure Educational Erythrocyte precursor, abnormal 1 0.
 Cell Identification Mitotic figure 212 99.5 Educational Erythrocyte precursor, abnormal BMD-02 The arrowed cell is a mitotic figure. It was correctly identified by 99.5% of the participants. A cell containing
Cell Identification Mitotic figure 212 99.5 Educational Erythrocyte precursor, abnormal BMD-02 The arrowed cell is a mitotic figure. It was correctly identified by 99.5% of the participants. A cell containing
Hematopathology Lab. Third year medical students
 Hematopathology Lab Third year medical students Objectives Identify the lesion Know the specific name of the lesion Know associated disease Know relevant pathologic background Spherocytes: appear small,
Hematopathology Lab Third year medical students Objectives Identify the lesion Know the specific name of the lesion Know associated disease Know relevant pathologic background Spherocytes: appear small,
Lymphoma Tumor Board Quiz! Laboratory Hematology: Basic Cell Morphology
 Lymphoma Tumor Board Quiz! Laboratory Hematology: Basic Cell Morphology CABOT RINGS Cabot rings in a patient with hemolytic anemia. Cabot ring (red arrow) and Howell-Jolly body (blue arrow). Observed in
Lymphoma Tumor Board Quiz! Laboratory Hematology: Basic Cell Morphology CABOT RINGS Cabot rings in a patient with hemolytic anemia. Cabot ring (red arrow) and Howell-Jolly body (blue arrow). Observed in
EDUCATIONAL COMMENTARY MORPHOLOGIC CHANGES IN PERIPHERAL BLOOD CELLS
 EDUCATIONAL COMMENTARY MORPHOLOGIC CHANGES IN PERIPHERAL BLOOD CELLS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
EDUCATIONAL COMMENTARY MORPHOLOGIC CHANGES IN PERIPHERAL BLOOD CELLS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
ORIGINAL EVALUATION. FH9-C 2017 Hematology Auto Differentials, FH9
 ft Cit e * 4> ee e e e *111» * * PAT H 0 LO GI STS INSTITUTION: ATTENTION: CAP NUMBER: KIT INFORMATION: COPIED TO: LKF Laboratorium fur Klinische Forschung GmbH Schwentinental GE 24223 Volker El-Samalouti
ft Cit e * 4> ee e e e *111» * * PAT H 0 LO GI STS INSTITUTION: ATTENTION: CAP NUMBER: KIT INFORMATION: COPIED TO: LKF Laboratorium fur Klinische Forschung GmbH Schwentinental GE 24223 Volker El-Samalouti
Proper Slide Preparation
 Hematology Essentials: A Foundation for WBC Review Using Case Studies Christine Hinz, MS, MLS(ASCP) CM Proper Slide Preparation smooth, homogenous film 1/2 to 3/4 the slide length straight feather edge
Hematology Essentials: A Foundation for WBC Review Using Case Studies Christine Hinz, MS, MLS(ASCP) CM Proper Slide Preparation smooth, homogenous film 1/2 to 3/4 the slide length straight feather edge
Indication of peripheral blood smear exmination:
 Indication of peripheral blood smear exmination: 1. For carried out differential WBC count. 2. For differential diagnosis of anemia. 3. For detection of parasites. 4. For diagnosis of leucemoid reaction.
Indication of peripheral blood smear exmination: 1. For carried out differential WBC count. 2. For differential diagnosis of anemia. 3. For detection of parasites. 4. For diagnosis of leucemoid reaction.
Hematology Unit Lab 1 Review Material
 Hematology Unit Lab 1 Review Material - 2018 Objectives Laboratory instructors: 1. Assist students during lab session Students: 1. Review the introductory material 2. Study the case histories provided
Hematology Unit Lab 1 Review Material - 2018 Objectives Laboratory instructors: 1. Assist students during lab session Students: 1. Review the introductory material 2. Study the case histories provided
EDUCATIONAL COMMENTARY DISTINGUISHING MORPHOLOGIC LOOK-ALIKES
 EDUCATIONAL COMMENTARY DISTINGUISHING MORPHOLOGIC LOOK-ALIKES Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
EDUCATIONAL COMMENTARY DISTINGUISHING MORPHOLOGIC LOOK-ALIKES Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
LeadCare BLOOD LEAD ANALYZER. Quick Reference Guide
 LeadCare II BLOOD LEAD ANALYZER Quick Reference Guide Precautions Precautions Caution The LeadCare II Blood Lead Analyzer is a CLIA-waived device. Facilities that perform tests with the LeadCare II System
LeadCare II BLOOD LEAD ANALYZER Quick Reference Guide Precautions Precautions Caution The LeadCare II Blood Lead Analyzer is a CLIA-waived device. Facilities that perform tests with the LeadCare II System
VETERINARY HEMATOLOGY ATLAS OF COMMON DOMESTIC AND NON-DOMESTIC SPECIES COPYRIGHTED MATERIAL SECOND EDITION
 VETERINARY HEMATOLOGY ATLAS OF COMMON DOMESTIC AND NON-DOMESTIC SPECIES SECOND EDITION COPYRIGHTED MATERIAL CHAPTER ONE HEMATOPOIESIS GENERAL FEATURES All blood cells have a finite life span, but in normal
VETERINARY HEMATOLOGY ATLAS OF COMMON DOMESTIC AND NON-DOMESTIC SPECIES SECOND EDITION COPYRIGHTED MATERIAL CHAPTER ONE HEMATOPOIESIS GENERAL FEATURES All blood cells have a finite life span, but in normal
HISTOLOGY VIRTUAL LABORATORY BLOOD AND LYMPHATICS SYSTEM
 HISTOLOGY VIRTUAL LABORATORY BLOOD AND LYMPHATICS SYSTEM Login: http://histopath.westernu.edu Histology Atlas AND Virtual Histology links. I. HEMATOLOGY - PERIPHERAL BLOOD Purpose: To be able to identify
HISTOLOGY VIRTUAL LABORATORY BLOOD AND LYMPHATICS SYSTEM Login: http://histopath.westernu.edu Histology Atlas AND Virtual Histology links. I. HEMATOLOGY - PERIPHERAL BLOOD Purpose: To be able to identify
Guide to the 1-3 Minute Blood Film Microscopic Review: Why and How?
 Guide to the 1-3 Minute Blood Film Microscopic Review: Why and How? Dennis B. DeNicola, DVM, PhD, DACVP Chief Veterinary Educator IDEXX Laboratories, Inc. Westbrook, ME USA Adjunct Professor of Veterinary
Guide to the 1-3 Minute Blood Film Microscopic Review: Why and How? Dennis B. DeNicola, DVM, PhD, DACVP Chief Veterinary Educator IDEXX Laboratories, Inc. Westbrook, ME USA Adjunct Professor of Veterinary
Collect and label sample according to standard protocols. Gently invert tube 8-10 times immediately after draw. DO NOT SHAKE. Do not centrifuge.
 Complete Blood Count CPT Code: CBC with Differential: 85025 CBC without Differential: 85027 Order Code: CBC with Differential: C915 Includes: White blood cell, Red blood cell, Hematocrit, Hemoglobin, MCV,
Complete Blood Count CPT Code: CBC with Differential: 85025 CBC without Differential: 85027 Order Code: CBC with Differential: C915 Includes: White blood cell, Red blood cell, Hematocrit, Hemoglobin, MCV,
CELL-DYN Strength in Technology, Proven Reliability. Optical WBC Technology. Patented M.A.P.S.S. Differential. Multiple Technologies
 CELL-DYN 3700 Strength in Technology, Proven Reliability Optical WBC Technology Patented M.A.P.S.S. Differential Multiple Technologies CELL-DYN 3700 Multiple Technologies One Superior Result Multiple Technologies
CELL-DYN 3700 Strength in Technology, Proven Reliability Optical WBC Technology Patented M.A.P.S.S. Differential Multiple Technologies CELL-DYN 3700 Multiple Technologies One Superior Result Multiple Technologies
EDUCATIONAL COMMENTARY BLOOD CELL IDENTIFICATION
 EDUCATIONAL COMMENTARY BLOOD CELL IDENTIFICATION Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click
EDUCATIONAL COMMENTARY BLOOD CELL IDENTIFICATION Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click
Step-by-Step Instructions For OraQuick HCV Rapid Antibody Test
 Step-by-Step Instructions For OraQuick HCV Rapid Antibody Test Complexity: WAIVED for fingerstick whole blood and venipuncture whole blood. A Certificate of CLIA Waiver is required to perform the test
Step-by-Step Instructions For OraQuick HCV Rapid Antibody Test Complexity: WAIVED for fingerstick whole blood and venipuncture whole blood. A Certificate of CLIA Waiver is required to perform the test
A Look Into the Determination of Cell Morphology in Hematology in the 21 st Century. Ramon Simon-Lopez, MD Global Scientific Director Beckman Coulter
 A Look Into the Determination of Cell Morphology in Hematology in the 21 st Century Ramon Simon-Lopez, MD Global Scientific Director Beckman Coulter Is cell morphology important? AML M7 CLL CD5 CD19 NHL
A Look Into the Determination of Cell Morphology in Hematology in the 21 st Century Ramon Simon-Lopez, MD Global Scientific Director Beckman Coulter Is cell morphology important? AML M7 CLL CD5 CD19 NHL
Continuing Education Questions
 FOCUS: INTERPRETING THE COMPLETE BLOOD COUNT Continuing Education Questions SUMMER 2017 1. A methodical approach to CBC interpretation that is aimed at medical laboratory professionals differs from one
FOCUS: INTERPRETING THE COMPLETE BLOOD COUNT Continuing Education Questions SUMMER 2017 1. A methodical approach to CBC interpretation that is aimed at medical laboratory professionals differs from one
Blood Cell Identification Graded
 Blood Cell Identification Graded Case History The patient was a five-day-old girl with an elevated unconjugated bilirubin and a weakly positive direct antiglobulin test (DAT). Her CBC showed: WBC = 11.0
Blood Cell Identification Graded Case History The patient was a five-day-old girl with an elevated unconjugated bilirubin and a weakly positive direct antiglobulin test (DAT). Her CBC showed: WBC = 11.0
SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
 MEDICAL ELECTRONIC SYSTEMS 5757 W. Century Blvd. Suite 805 Los Angeles, CA. 90045 Remember, it ALL Started with a Sperm! www.mes global.com SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
MEDICAL ELECTRONIC SYSTEMS 5757 W. Century Blvd. Suite 805 Los Angeles, CA. 90045 Remember, it ALL Started with a Sperm! www.mes global.com SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
Performance evaluation of Celltac G: a new automated hematology analyzer
 Original Article Performance evaluation of Celltac G: a new automated hematology analyzer Masaaki Sugiyama 1, Tsukasa Kobayashi 1, Yuki Jisyage 2, Shigeko Yamamoto 2, Yutaka Nagai 2,3 and Hiroshi Kondo
Original Article Performance evaluation of Celltac G: a new automated hematology analyzer Masaaki Sugiyama 1, Tsukasa Kobayashi 1, Yuki Jisyage 2, Shigeko Yamamoto 2, Yutaka Nagai 2,3 and Hiroshi Kondo
EDUCATIONAL COMMENTARY DIFFERENTIATING IMMATURE PERIPHERAL BLOOD CELLS
 Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on the left side of the
Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on the left side of the
3. Blood Cell Histograms:
 LECTURE MODULE 6c: ELECTRONIC CELL COUNTING PART III 3. Blood Cell Histograms: a. The Coulter cell counters today provides size distributions of the cellular content: 1) volume given in µm 3 or fl vs relative
LECTURE MODULE 6c: ELECTRONIC CELL COUNTING PART III 3. Blood Cell Histograms: a. The Coulter cell counters today provides size distributions of the cellular content: 1) volume given in µm 3 or fl vs relative
The LaboratoryMatters
 Laboratory Medicine Newsletter for clinicians, pathologists & clinical laboratory technologists. A Initiative. Complete Blood Count This issue highlights: CBC, while ubiquitous, is an excellent diagnostic
Laboratory Medicine Newsletter for clinicians, pathologists & clinical laboratory technologists. A Initiative. Complete Blood Count This issue highlights: CBC, while ubiquitous, is an excellent diagnostic
2014 Continuing Compliance Master Series Best Practices in Alternative Assessment of Performance
 2014 Continuing Compliance Master Series Best Practices in Alternative Assessment of Performance Brad S. Karon, MD, PhD, FCAP November 19, 2014 www.cap.org Today s Presenter Brad S. Karon, MD, PhD, FCAP
2014 Continuing Compliance Master Series Best Practices in Alternative Assessment of Performance Brad S. Karon, MD, PhD, FCAP November 19, 2014 www.cap.org Today s Presenter Brad S. Karon, MD, PhD, FCAP
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
2018 Hematology and Clinical Microscopy Glossary
 2018 Hematology and Clinical Microscopy Glossary Table of Contents 2018 Hematology, Clinical Microscopy, and Body Fluids Glossary Blood Cell Identification Introduction... 1 Granulocytes and Monocytes...
2018 Hematology and Clinical Microscopy Glossary Table of Contents 2018 Hematology, Clinical Microscopy, and Body Fluids Glossary Blood Cell Identification Introduction... 1 Granulocytes and Monocytes...
Standard Operating Procedure
 Subject Differential: Counting and Morphology Index Number Lab-5074 Section Laboratory Subsection Hematology Automated Laboratory Category Departmental Contact Moldenhauer, Emily C Last Revised 11/28/2017
Subject Differential: Counting and Morphology Index Number Lab-5074 Section Laboratory Subsection Hematology Automated Laboratory Category Departmental Contact Moldenhauer, Emily C Last Revised 11/28/2017
QUICK REFERENCE INSTRUCTIONS
 QUICK REFERENCE INSTRUCTIONS For use with the Sofia Analyzer only. CLIA Complexity: WAIVED Nasal Swab and Nasopharyngeal Swab specimens ONLY. Study the Package Insert and User Manual thoroughly before
QUICK REFERENCE INSTRUCTIONS For use with the Sofia Analyzer only. CLIA Complexity: WAIVED Nasal Swab and Nasopharyngeal Swab specimens ONLY. Study the Package Insert and User Manual thoroughly before
EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES
 EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
EDUCATIONAL COMMENTARY MORPHOLOGIC ABNORMALITIES IN LEUKOCYTES Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE
Lavender Top Management SUCCESS BEYOND FINANCES
 Lavender Top Management Alfonso Ziccardi Ass.Lab.Operation Manager for AP/CP/Safety Officer New York Hospital Medical Center of Queens SUCCESS BEYOND FINANCES 1 . VISION Mission/ Vision To successfully
Lavender Top Management Alfonso Ziccardi Ass.Lab.Operation Manager for AP/CP/Safety Officer New York Hospital Medical Center of Queens SUCCESS BEYOND FINANCES 1 . VISION Mission/ Vision To successfully
Drop of Blood Unravels Mysteries. Prof. Salma Afrose Department of Hematology Dhaka Medical College
 Drop of Blood Unravels Mysteries Prof. Salma Afrose Department of Hematology Dhaka Medical College Peripheral Blood Film (PBF) PBF is a laboratory workup that involves cytology of Peripheral blood cell
Drop of Blood Unravels Mysteries Prof. Salma Afrose Department of Hematology Dhaka Medical College Peripheral Blood Film (PBF) PBF is a laboratory workup that involves cytology of Peripheral blood cell
Hematology 101. Cindy Rogers, MT(ASCP) Diagnostics System Specialist
 Hematology 101 Cindy Rogers, MT(ASCP) Diagnostics System Specialist More Acronyms...» CBC» RBC» HGB» HCT» WBC» MPV» PLT» RDW» DIFF» H&H» Complete Blood Count» Red Blood Cell» Hemoglobin» Hematocrit» White
Hematology 101 Cindy Rogers, MT(ASCP) Diagnostics System Specialist More Acronyms...» CBC» RBC» HGB» HCT» WBC» MPV» PLT» RDW» DIFF» H&H» Complete Blood Count» Red Blood Cell» Hemoglobin» Hematocrit» White
PROFICIENCY TESTING POLICY
 Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Hematology 101. Blanche P Alter, MD, MPH, FAAP Clinical Genetics Branch Division of Cancer Epidemiology and Genetics Bethesda, MD
 Hematology 101 Blanche P Alter, MD, MPH, FAAP Clinical Genetics Branch Division of Cancer Epidemiology and Genetics Bethesda, MD Hematocrits Plasma White cells Red cells Normal, Hemorrhage, IDA, Leukemia,
Hematology 101 Blanche P Alter, MD, MPH, FAAP Clinical Genetics Branch Division of Cancer Epidemiology and Genetics Bethesda, MD Hematocrits Plasma White cells Red cells Normal, Hemorrhage, IDA, Leukemia,
Incorporating Differentials Into Every Complete Blood Count. Paige Flowers, LVT Dogwood Veterinary Internal Medicine
 Incorporating Differentials Into Every Complete Blood Count Paige Flowers, LVT Dogwood Veterinary Internal Medicine Complete Blood Count Diagnostic performed to evaluate the quantity and morphology of
Incorporating Differentials Into Every Complete Blood Count Paige Flowers, LVT Dogwood Veterinary Internal Medicine Complete Blood Count Diagnostic performed to evaluate the quantity and morphology of
2008 CAP TODAY Q & A
 2008 CAP TODAY Q & A Q. How often should we document hematology competencies to ensure consistency of morphologic observations for manual differentials and fluids? Should we do this every six months or
2008 CAP TODAY Q & A Q. How often should we document hematology competencies to ensure consistency of morphologic observations for manual differentials and fluids? Should we do this every six months or
i-stat CHEM8 + Cartridge Quick Reference Guide
 i-stat CHEM8 + Cartridge Quick Reference Guide For use with a Certificate of Waiver The CHEM8+ cartridge has tests for sodium, potassium, chloride, total carbon dioxide, ionized calcium, glucose, urea
i-stat CHEM8 + Cartridge Quick Reference Guide For use with a Certificate of Waiver The CHEM8+ cartridge has tests for sodium, potassium, chloride, total carbon dioxide, ionized calcium, glucose, urea
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
NEPHROCHECK Liquid Control Kit Package Insert
 NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
XN series. Case interpretation. Gebruikersdag Vlaanderen- 6 oktober 2016
 XN series Case interpretation Gebruikersdag Vlaanderen- 6 oktober 2016 Fluorescence flow cytometry RET channel PLT-F channel WDF channel WPC channel WNR channel Case 1 Case 1: Initial measurement Patient
XN series Case interpretation Gebruikersdag Vlaanderen- 6 oktober 2016 Fluorescence flow cytometry RET channel PLT-F channel WDF channel WPC channel WNR channel Case 1 Case 1: Initial measurement Patient
Participants Identification No. % Evaluation. Mitotic figure Educational Erythrocyte precursor, abnormal/
 Cell Identification BMD-09 Participants Identification No. % Evaluation Mitotic figure 233 96.7 Educational Erythrocyte precursor, abnormal/ 4 1.7 Educational dysplastic nuclear features Erythrocyte precursor
Cell Identification BMD-09 Participants Identification No. % Evaluation Mitotic figure 233 96.7 Educational Erythrocyte precursor, abnormal/ 4 1.7 Educational dysplastic nuclear features Erythrocyte precursor
LESSON ASSIGNMENT. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 4 Morphology of Blood Cells. TEXT ASSIGNMENT Paragraphs 4-1 through 4-13. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Select the statement that
LESSON ASSIGNMENT LESSON 4 Morphology of Blood Cells. TEXT ASSIGNMENT Paragraphs 4-1 through 4-13. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Select the statement that
CELL-DYN 3700 Strength in Technology, Proven Reliability
 CELL-DYN 3700 Strength in Technology, Proven Reliability Optical WBC Technology Patented MAPSS Differential Multiple Technologies CELL-DYN 3700 Multiple Technologies One Superior Result Multiple Technologies
CELL-DYN 3700 Strength in Technology, Proven Reliability Optical WBC Technology Patented MAPSS Differential Multiple Technologies CELL-DYN 3700 Multiple Technologies One Superior Result Multiple Technologies
Lavender Top Management SUCCESS BEYOND FINANCES. Mission/ Vision
 Lavender Top Management Alfonso Ziccardi Lab.Operation Manager for AP/CP/Safety Officer New York Hospital Medical Center of Queens SUCCESS BEYOND FINANCES Mission/ Vision. VISION To successfully develop
Lavender Top Management Alfonso Ziccardi Lab.Operation Manager for AP/CP/Safety Officer New York Hospital Medical Center of Queens SUCCESS BEYOND FINANCES Mission/ Vision. VISION To successfully develop
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only
 QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
Biology 218 Human Anatomy. Adapted form Martini Human Anatomy 7th ed. Chapter 20 The Cardiovascular System: Blood
 Adapted form Martini Human Anatomy 7th ed. Chapter 20 The Cardiovascular System: Blood Introduction The cardiovascular system functions as a system to transport numerous substances throughout the body
Adapted form Martini Human Anatomy 7th ed. Chapter 20 The Cardiovascular System: Blood Introduction The cardiovascular system functions as a system to transport numerous substances throughout the body
Quality control in the hematology laboratory for the sake of the patient. Dr Marion Münster Manager Medical & Scientific Affairs Sysmex South Africa
 Quality control in the hematology laboratory for the sake of the patient Dr Marion Münster Manager Medical & Scientific Affairs Sysmex South Africa The FBC. Most frequently requested lab test Usually delegated
Quality control in the hematology laboratory for the sake of the patient Dr Marion Münster Manager Medical & Scientific Affairs Sysmex South Africa The FBC. Most frequently requested lab test Usually delegated
Detail PRINCIPLE: Body fluids other than blood and urine will be analyzed according to their site of origin, and the providers specific orders.
 Subject Body Fluid Analysis - Affiliate Index Number Lab-8760 Section Laboratory Subsection Regional/Affiliates Category Departmental Contact Munson, Karen Last Revised 6/28/2017 References Required document
Subject Body Fluid Analysis - Affiliate Index Number Lab-8760 Section Laboratory Subsection Regional/Affiliates Category Departmental Contact Munson, Karen Last Revised 6/28/2017 References Required document
Hematology Essentials: A Foundation for Accurate Smear Reviews. Christine Hinz, MS, MLS(ASCP) CM
 Hematology Essentials: A Foundation for Accurate Smear Reviews Christine Hinz, MS, MLS(ASCP) CM Differential Training Program Current Challenges System Wide Approach Standardization Training Area How does
Hematology Essentials: A Foundation for Accurate Smear Reviews Christine Hinz, MS, MLS(ASCP) CM Differential Training Program Current Challenges System Wide Approach Standardization Training Area How does
Sputum DNA Collection, Preservation and Isolation Kit 50 Individual Devices
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Sputum DNA Collection, Preservation and Isolation Kit 50 Individual
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Sputum DNA Collection, Preservation and Isolation Kit 50 Individual
Safety Data Sheet Product Name: Heparin / PF4 Serum Panel - For In Vitro Diagnostic Use Only
 Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
Section 1 Identification Product Name Catalog Numbers Heparin / PF4 Assayed Antibody Serum Panel 4036027, 2 Member QC Panel 4036028, Multi-Member Qualification Panel (6 member) Company Identification AKERS
Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual
 Page 1 / 14 Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual ZV-3051-0200-15 200 Page 2 / 14 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST
Page 1 / 14 Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual ZV-3051-0200-15 200 Page 2 / 14 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST
Lifeblood Lab Activity
 History of Blood: It is the universal symbol of horror, of death, yet it is the one thing that keeps you living. It is the blood that is coursing through your veins. But, what do you really know about
History of Blood: It is the universal symbol of horror, of death, yet it is the one thing that keeps you living. It is the blood that is coursing through your veins. But, what do you really know about
Rapid-VIDITEST. Influenza A+B
 Rapid-VIDITEST Influenza A+B (One step Influenza A+B blister Test for the detection of Influenza type A and type B from nasal swabs, nasal wash or nasal aspirate specimens). Instruction manual Producer:
Rapid-VIDITEST Influenza A+B (One step Influenza A+B blister Test for the detection of Influenza type A and type B from nasal swabs, nasal wash or nasal aspirate specimens). Instruction manual Producer:
EHE1-16 Participants Identification No. % Evaluation
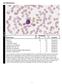 Cell Identification EHE1-16 Participants Identification No. % Evaluation Blast cell 154 90.1 Educational Lymphocyte 4 2.3 Educational Lymphocyte, reactive 1 0.6 Educational Lymphoma cell (malignant) 1
Cell Identification EHE1-16 Participants Identification No. % Evaluation Blast cell 154 90.1 Educational Lymphocyte 4 2.3 Educational Lymphocyte, reactive 1 0.6 Educational Lymphoma cell (malignant) 1
Requirements: Glass slides Leishman stain Microscopes Disposable needles Vials containing anticoagulants Methylated-spirit Staining rack
 Aim: To make a blood smear and to count the different types of leucocytes present in a stained blood smear and express their relative counts in percentage Principle - The blood contains various types of
Aim: To make a blood smear and to count the different types of leucocytes present in a stained blood smear and express their relative counts in percentage Principle - The blood contains various types of
Blood Glucose Monitoring System. User Guide
 Blood Glucose Monitoring System User Guide Table of Contents Introduction...2 Important Safety Instructions...2 About ipet PRO Blood Glucose Monitoring System...3 About ipet PRO Meter...4 About the ipet
Blood Glucose Monitoring System User Guide Table of Contents Introduction...2 Important Safety Instructions...2 About ipet PRO Blood Glucose Monitoring System...3 About ipet PRO Meter...4 About the ipet
Blood Cell Identification Graded
 Blood Cell Identification Graded Case History A 51-year-old female presented with dyspnea on exertion. Laboratory results were as follows: WBC=5.5 X 10 9 /L; Hgb=4.2 g/dl; Hct=13.9%; MCV=78.7fL; RDW=30;
Blood Cell Identification Graded Case History A 51-year-old female presented with dyspnea on exertion. Laboratory results were as follows: WBC=5.5 X 10 9 /L; Hgb=4.2 g/dl; Hct=13.9%; MCV=78.7fL; RDW=30;
DR SUDHIR MEHTA MD,MNAMS,FICP. Senior Professor & Head Medical Unit SMS Medical College & Hospital Jaipur
 DR SUDHIR MEHTA MD,MNAMS,FICP Senior Professor & Head Medical Unit SMS Medical College & Hospital Jaipur s.smehta@hotmail.com CBC..What is the Utility of performing this basic Hematology Test? 10/31/2010
DR SUDHIR MEHTA MD,MNAMS,FICP Senior Professor & Head Medical Unit SMS Medical College & Hospital Jaipur s.smehta@hotmail.com CBC..What is the Utility of performing this basic Hematology Test? 10/31/2010
By Dr. Mohamed Saad Daoud
 By Dr. Mohamed Saad Daoud Part I Introduction Types of White Blood Cells Genesis of the White Blood Cells Life Span of the White Blood Cells Dr. Mohamed Saad Daoud 2 Leucocytes Introduction: Infectious
By Dr. Mohamed Saad Daoud Part I Introduction Types of White Blood Cells Genesis of the White Blood Cells Life Span of the White Blood Cells Dr. Mohamed Saad Daoud 2 Leucocytes Introduction: Infectious
ADx Bone Marrow Report. Patient Information Referring Physician Specimen Information
 ADx Bone Marrow Report Patient Information Referring Physician Specimen Information Patient Name: Specimen: Bone Marrow Site: Left iliac Physician: Accession #: ID#: Reported: 08/19/2014 - CHRONIC MYELOGENOUS
ADx Bone Marrow Report Patient Information Referring Physician Specimen Information Patient Name: Specimen: Bone Marrow Site: Left iliac Physician: Accession #: ID#: Reported: 08/19/2014 - CHRONIC MYELOGENOUS
Cotinine (Mouse/Rat) ELISA Kit
 Cotinine (Mouse/Rat) ELISA Kit Catalog Number KA2264 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Cotinine (Mouse/Rat) ELISA Kit Catalog Number KA2264 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.04 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+/133+ Progenitor Cells, cryopreserved Cryopreserved
Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.04 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+/133+ Progenitor Cells, cryopreserved Cryopreserved
EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric)
 EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE Uses: The EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric)
EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE Uses: The EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric)
r). SUPPLEMENTARY/SECOND OPPORTUNITY EXAMINATION PAPER nnmlbih UNIVERSITY Sophia Blaauw INSTRUCTIONS FACULTY OF HEALTH AND APPLIED SCIENCES
 r). nnmlbih UNIVERSITY OF SCIEFICE nnd TECHNOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF HEALTH SCIENCES QUALIFICATION: BACHELOR OF MEDICAL LABORATORY SCIENCES QUALIFICATION CODE: 08BMLS
r). nnmlbih UNIVERSITY OF SCIEFICE nnd TECHNOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF HEALTH SCIENCES QUALIFICATION: BACHELOR OF MEDICAL LABORATORY SCIENCES QUALIFICATION CODE: 08BMLS
Blood Cell Identification Graded
 Blood Cell Identification Graded Case History An 11-year-old girl presented to the emergency room with wheezing and was diagnosed as having asthma. Her CBC showed WBC = 12.5 x 10 9 /L; Hgb = 12.4g/dL;
Blood Cell Identification Graded Case History An 11-year-old girl presented to the emergency room with wheezing and was diagnosed as having asthma. Her CBC showed WBC = 12.5 x 10 9 /L; Hgb = 12.4g/dL;
Table of Contents. Contour Diabetes App User Guide
 Table of Contents Introduction... 3 About the CONTOUR Diabetes App... 3 System and Device Requirements... 3 Intended Use... 3 Getting Started... 3 Downloading CONTOUR... 3 Apple... 3 Android... 4 Quick
Table of Contents Introduction... 3 About the CONTOUR Diabetes App... 3 System and Device Requirements... 3 Intended Use... 3 Getting Started... 3 Downloading CONTOUR... 3 Apple... 3 Android... 4 Quick
ab HIV-1 Protease Inhibitor Screening Kit (Fluorometric)
 Version 1 Last updated 2 March 2017 ab211106 HIV-1 Protease Inhibitor Screening Kit (Fluorometric) For the rapid, sensitive and accurate screening of potential HIV-1 Protease inhibitors. This product is
Version 1 Last updated 2 March 2017 ab211106 HIV-1 Protease Inhibitor Screening Kit (Fluorometric) For the rapid, sensitive and accurate screening of potential HIV-1 Protease inhibitors. This product is
Monitoring the Health of Transplanted Organs DONOR GENOTYPING MANUAL
 Monitoring the Health of Transplanted Organs DONOR GENOTYPING MANUAL 10101 Innovation Drive. Suite 600. Wauwatosa, WI 53226 1-888-214-3151 MKT-008 v3.0 MKT-008 v3.0 TABLE OF CONTENTS I Intended Use...
Monitoring the Health of Transplanted Organs DONOR GENOTYPING MANUAL 10101 Innovation Drive. Suite 600. Wauwatosa, WI 53226 1-888-214-3151 MKT-008 v3.0 MKT-008 v3.0 TABLE OF CONTENTS I Intended Use...
ANTIBODY SCREENING by Uni-Gold Recombigen HIV
 ANTI-HIV SPECIMEN 1 REQUIREMENTS ANTIBODY SCREENING by Uni-Gold Recombigen HIV PRINCIPLE: The Uni-Gold Recombigen HIV was designed as a rapid immunoassay and is intended to detect antibodies to HIV- 1
ANTI-HIV SPECIMEN 1 REQUIREMENTS ANTIBODY SCREENING by Uni-Gold Recombigen HIV PRINCIPLE: The Uni-Gold Recombigen HIV was designed as a rapid immunoassay and is intended to detect antibodies to HIV- 1
HumaCount 5D. Outperforming 5-part Hematology System. CoreLab DX. Hematology
 HumaCount 5D Outperforming 5-part Hematology System Direct Capillary Process by OptimalCount Technology Distinct 5-part Differentiation Definite Immature Cell Count (LIC, ALY) CoreLab DX Hematology 5-part
HumaCount 5D Outperforming 5-part Hematology System Direct Capillary Process by OptimalCount Technology Distinct 5-part Differentiation Definite Immature Cell Count (LIC, ALY) CoreLab DX Hematology 5-part
Chapter 21 Outline. General Composition and Functions of Blood Blood Plasma Formed Elements in the Blood Hemopoiesis: Production of Formed Elements
 Chapter 21 Outline General Composition and Functions of Blood Blood Plasma Formed Elements in the Blood Hemopoiesis: Production of Formed Elements Introduction Blood serves many functions. Some examples
Chapter 21 Outline General Composition and Functions of Blood Blood Plasma Formed Elements in the Blood Hemopoiesis: Production of Formed Elements Introduction Blood serves many functions. Some examples
QUICK REFERENCE INSTRUCTIONS. THYROCHEK TSH Cassette
 QUICK REFERENCE INSTRUCTIONS THYROCHEK TSH Cassette A certificate of CLIA waiver is required to perform the testing in a waived setting. If the laboratory does not have a Certificate of Waiver, the Application
QUICK REFERENCE INSTRUCTIONS THYROCHEK TSH Cassette A certificate of CLIA waiver is required to perform the testing in a waived setting. If the laboratory does not have a Certificate of Waiver, the Application
QUICK REFERENCE INSTRUCTIONS For use with Sofia only.
 QUICK REFERENCE INSTRUCTIONS For use with Sofia only. CLIA Complexity: Waived Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package
QUICK REFERENCE INSTRUCTIONS For use with Sofia only. CLIA Complexity: Waived Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package
PROCEDURE. TITLE: Bedside Glucose Monitoring PC Laboratory. Issuing Department: Clinical Director Signature: Departments Involved:
 PROCEDURE TITLE: Bedside Glucose Monitoring Issuing Department: Clinical Director Signature: Departments Involved: Laboratory Nursing Effective Date: 10/97 Review Dates: 09/01, 07/02, 05/13 Revision Dates:
PROCEDURE TITLE: Bedside Glucose Monitoring Issuing Department: Clinical Director Signature: Departments Involved: Laboratory Nursing Effective Date: 10/97 Review Dates: 09/01, 07/02, 05/13 Revision Dates:
Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister
 Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister One
Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister One
Formation of Blood Cells
 Hematopoiesis Lecture Objectives Name organs responsible for hematopoiesis in the fetus. List the developmental stages of hematopoiesis both prenatally and postnatally. Outline the major steps of post
Hematopoiesis Lecture Objectives Name organs responsible for hematopoiesis in the fetus. List the developmental stages of hematopoiesis both prenatally and postnatally. Outline the major steps of post
For research or further manufacturing use only. Not for injection or diagnostic procedures.
 PRIME-XV T cell Expansion XSFM PRIME-XV T Cell Expansion XSFM is a xeno-free, serum-free medium optimized for the activation and expansion of human T lymphocytes. This medium contains gentamicin and requires
PRIME-XV T cell Expansion XSFM PRIME-XV T Cell Expansion XSFM is a xeno-free, serum-free medium optimized for the activation and expansion of human T lymphocytes. This medium contains gentamicin and requires
Product Training & Certification
 B R A N A N M E D I C A L C O R P O R A T I O N Product Training & Certification Oratect III Oral Fluid Drug Screen Device Catalog # HM11 & HM12 For Forensic Use Only Branan Medical Corporation 140 Technology
B R A N A N M E D I C A L C O R P O R A T I O N Product Training & Certification Oratect III Oral Fluid Drug Screen Device Catalog # HM11 & HM12 For Forensic Use Only Branan Medical Corporation 140 Technology
Rotavirus Test Kit. Instructions For Use. Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA
 Rotavirus Test Kit Instructions For Use Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA * Please read the instructions carefully before use INTENDED USE Velotest Rotavirus Test is
Rotavirus Test Kit Instructions For Use Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA * Please read the instructions carefully before use INTENDED USE Velotest Rotavirus Test is
namib la UnIVERSITY OF SCIEnCE AnD TECHnOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF HEALTH SCIENCES
 namib la UnIVERSITY OF SCIEnCE AnD TECHnOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF HEALTH SCIENCES QUALIFICATION: BACHELOR OF BIOMEDICAL SCIENCES QUALIFICATION CODE: SOBBMS LEVEL: 6 COURSE
namib la UnIVERSITY OF SCIEnCE AnD TECHnOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF HEALTH SCIENCES QUALIFICATION: BACHELOR OF BIOMEDICAL SCIENCES QUALIFICATION CODE: SOBBMS LEVEL: 6 COURSE
BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens]
![BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens] BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens]](/thumbs/73/68341667.jpg) BLOODBORNEPATHOGENS CAP Safety Meetings Revision: 10-2011 2011 Copyright - PEC/Premier Safety Management, Inc. All Rights Reserved Revision: [10-2011] 1 THEBLOODBORNEPATHOGENSSTANDARD The Bloodborne Pathogens
BLOODBORNEPATHOGENS CAP Safety Meetings Revision: 10-2011 2011 Copyright - PEC/Premier Safety Management, Inc. All Rights Reserved Revision: [10-2011] 1 THEBLOODBORNEPATHOGENSSTANDARD The Bloodborne Pathogens
