Post-Exposure Prophylaxis (PEP) Guidelines for children and adolescents potentially exposed to blood-borne viruses
|
|
|
- Cornelius Holland
- 5 years ago
- Views:
Transcription
1 Post-Exposure Prophylaxis (PEP) Guidelines for children and adolescents potentially exposed to blood-borne viruses Authors: C Foster, G Tudor-Williams, A Bamford Date reviewed: June 2017 Next review date: June 2019 Scope of Guideline: Following exposure to blood-borne viruses, it should be remembered that the risk of transmission is highest for Hepatitis B, then Hepatitis C and then HIV. As this document has been prepared for the CHIVA website its focus is on HIV. However, it is important to consider risk of pregnancy and sexually transmitted infections following high-risk sexual exposure, and any safeguarding concerns. New in Guideline 2015 update: 1. Switch from Kaletra -based PEP to raltegravir-based regimens for older children who are able to swallow tablets and inclusion of chewable raltegravir as an option in younger children in centres where chewable raltegravir is available. The switch is in line with the change in adult guidelines prompted by MHRA warnings against the use of antiemetics in conjunction with ritonavir-containing regimens, due to the increased risk of cardiac events (prolonged QT interval) in adults. Raltegravir based ART was recommended by the Public Health England Expert Advisory Group on AIDS as it has been extensively tested in HIV infected adults with no significant safety issues and is better tolerated than Kaletra so switching is likely to improve adherence and hence the efficacy of PEP. 2. Reduction in the window period for repeat HIV testing from 12 weeks to 8 weeks post completion of PEP using 4 th generation antigen/antibody assays following the BASHH/EAGA statement on HIV window period in October 2014 ( Fig 1. Immediate Action Algorithm 1
2 Background The risk of community acquired HIV in children is extremely low. However, children and adolescents are potentially at risk of contracting HIV from a variety of exposures, including needlestick injury, sexual abuse and consensual sexual activity in adolescence. 1 There have been no reported school-related transmissions. The HIV status of the source is often unknown and difficult to establish. Body fluids presenting a risk of HIV transmission include blood, breast milk, semen or any body fluid if visibly bloodstained. 2 The risks of HIV being transmitted from a variety of exposures are shown in Table 1. HIV-infected fluids cannot penetrate intact skin. Sexual abuse represents a particular risk because of possible multiple exposures, mucosal trauma and the cervical ectopy and vaginal epithelial thinness found in children. 3 Up to 40% of 15 year olds in the UK are sexually active. Following the widespread use of HAART (Highly Active Antiretroviral Therapy) children with perinatally acquired HIV-1 infection are surviving into adolescence and entering sexual relationships with their HIV negative peers who may present for PEPSE (Post Exposure Prophylaxis following Sexual Exposure). Please refer to BASHH Guidelines 4 however it should be noted that following consensual unprotected vaginal sex PEPSE is no longer recommended where the HIV positive partner is known to be on effective antiretroviral therapy with an undetectable plasma HIV viral load. 4. Please note: at time of writing of this 2
3 guideline the BASHH PEPSE guidance is under review, the forthcoming updated recommendation will include raltegravir as discussed above. Table 1. Estimated risks of HIV transmission according to type of exposure from a known HIV positive individual with detectable HIV viral load 4,5 Type of HIV exposure Occupational needlestick injury that punctures skin Risk of transmission 0.3% or 1 in 333 Unprotected receptive anal sex 1.11% or 1 in 90 Unprotected receptive vaginal intercourse 0.1% or 1 in 1000 If the HIV status of the source is not known, the risk can be calculated from the following formula: *Risk of HIV transmission = Risk that source is HIV positive x Risk of exposure The risks of transmission of Hepatitis B (HBV) and Hepatitis C (HCV) from a community acquired needle stick injury are significantly higher than for HIV. Table 2. UK seroprevalence data for blood-borne infections in people who use intravenous drugs (from 2014 report) 6 : London Outside London HIV Prevalence 3.9% 0.9% HBV Prevalence 32% 17% HCV Prevalence 59% 49% IVDU HIV seropositivity rate in London in 2013 was 3.9% (several fold higher than rates in rest of UK). 6 Given a 0.3% risk of transmission if the source was HIV positive, the risk from a community acquired needle stick injury can therefore be assessed as about 1:10,000 in London to less than 1:50,000 elsewhere. Note that quoted risks are based on injuries from needles contaminated with fresh blood: old blood in a syringe and needle found in the park is likely to carry a lower risk of transmission. In studies where a small amount of blood is retained in a syringe, viable HIV cannot be detected after 24 hours 7. The risk of HBV seroconversion following a needle-stick from known high risk HBV infected source (HBe Ag +ve) is 37-62% 8 and around 5% following needle-stick from a known low risk HBV infected source (HBe Ag ve). The average HCV seroconversion rate following needle- 3
4 stick from known HCV positive source is 1.8% 8. Data for risk of transmission of HBV or HCV from single sexual exposure are not robust. HCV is inefficiently transmitted. Risks from high risk HBV infected source may be as high as 50% for seroconversion (lower for clinically symptomatic HBV infection). Mechanism of action of HIV PEP The presumed mechanism for HIV PEP is that shortly after an exposure to HIV a window period exists during which antiretroviral therapy may help to diminish or end viral replication. In a small case controlled study, AZT reduced the transmission rate of HIV by 79%. 9 In addition, combination antiretroviral therapy has been shown to markedly reduce vertical transmission of HIV from mother-to-child 10 Previously Department of Health recommendations for PEP in adults were Truvada (a combination of tenofovir + emtricitabine) and Kaletra (lopinavir/ritonavir), a reflection of the rapid genital tract penetration of tenofovir and efficacy of Truvada /Kaletra against most current viral isolates in the UK. 11 There are no DOH recommendations for PEP in children. However, recently adult national PEP guidelines have moved to the combination of raltegravir with Truvada prompted by MHRA warnings against the use of antiemetics in conjunction with ritonavir containing antiretroviral regimens due to the increased risk of cardiac events (prolonged QT interval) in adults. Raltegravir is an integrase inhibitor licensed for use as first line therapy for treatment naïve adults and in treatment experienced children. Raltegravir is dosed at full adult dose of 400mg (single pink film coated tablet) twice daily for children from 25kg. Alternative chewable formulations of raltegravir are available for children over 11kg (see table 4 for dosing). It should be noted that the film coated tablet and the chewable preparations are not bioequivalent. Given the safety of HBV vaccination, the risk-benefit ratio favours vaccinating all exposed children following needle stick injuries or sexual assault, unless they have a documented prior history of successful HBV immunisation. Baseline testing and 3-month serological follow-up testing for HCV and HBV are recommended. It is recommended to request HCV IgG, and HBsAg, HBsAb and HBcAb. Procedure for Children and Adolescents presenting with possible exposure to HIV 1. Risk assessment Careful history and examination to assess the risk of exposure to HIV. Establish whether exposure occurred within the last 72 hours. Detailed plan in Immediate Action Algorithm (Fig 1). 2. Investigations 4
5 Source In rare situations the source may be known and if the individual gives consent HIV, HBV and HCV serology may be tested. If the source is already known to be HIV positive, obtain details of present and past antiretroviral medications, known previous resistance mutations and consider further resistance testing (if viral load detectable), although the latter should not delay commencement of PEP. Most virologists now do NOT recommend testing of source materials such as needles found in public places, since the test results are of low sensitivity and should not be used to guide management. Child/Adolescent Obtain baseline HCV, HBV and HIV antibody status. If antiretroviral therapy is to be started also request FBC, U&E and LFTs. Ascertainment that the child / adolescent is not already HIV infected is important, as treatment with PEP in that circumstance would be inappropriate (although awaiting this result should not delay PEP as it can be started and subsequently stopped or switched if necessary). The baseline HIV test result on the child/adolescent should be available at the first follow up visit (within hours of PEP initiation). Baseline Point of Care testing (POCT) is not recommended in this situation. 3. Management HIV PEP HIV PEP is most effective if started within 1 hour of exposure, but may be beneficial up to 72 hours after. The child s family should be counselled about likely side effects (Table 4) and given contact phone numbers in case of concerns during or after the treatment period. An appointment to see a paediatrician/hiv physician ideally within hours of starting HIV PEP should be made. Initially 5 days of PEP should be prescribed. A full 4 weeks should NOT be prescribed at the first appointment. A further prescription for a total of 4 weeks should be given at consultant review if PEP is to be continued. PEP regimens may sometimes need modification if the index case is known to or likely to harbour drug resistant virus. Seek expert help but do not delay starting PEP. Regimens The regimens below are based on age bandings; however accurate weight and height measurements should be used to calculate individual drug doses as per Table 4 or the CHIVA antiretroviral dosing table ( Preferred regimens reflect changes in adult PEP guidance, however the start of PEP should not be delayed whilst obtaining paediatric formulations of newer agents and hence alternative regimens are provided. In centres where chewable raltegravir is not readily available, if a child is unable to tolerate Kaletra, zidovudine and lamivudine, referral to a tertiary centre for consideration of an alternative regimen is recommended. Table 3. Suggested PEP regimens (see dose table below) 5
6 Age PEP - preferred PEP - alternative Notes (years) 10 + Raltegravir + Truvada (emtricitabine 200mg/tenofovir disoproxil fumarate 300mg) NB if under 35kg, in centres where TDF paediatric 1. Raltegravir + lamivudine 150mg/zidovudine 300mg combined tablet 2. Kaletra + As per adult guideline with an alternative for tenofovir in those with renal insufficiency. formulations are immediately available, we would recommend age and weight appropriate dosing of raltegravir + TDF + lamivudine lamivudine 150mg/zidovudine 300mg combined tablet 6-9 Raltegravir + lamivudine + zidovudine 1.Kaletra + lamivudine + zidovudine 2.Raltegravir or Kaletra + paediatric tenofovir + lamivudine Adult dose raltegravir for children above 25kg. Note that the chewable formulation of raltegravir is not bioequivalent to the tablets (see table 4) 2- <6 Kaletra + lamivudine + zidovudine 1. Raltegravir+ lamivudine + zidovudine 2. Raltegravir or Alternative option for centres with access to chewable raltegravir Kaletra + paediatric tenofovir + lamivudine <2 Kaletra + lamivudine + zidovudine Liquid formulations Notes: 1. Young people from 10 years of age and over 35 kg who are able to swallow tablets should receive PEP as for adults:- raltegravir 400mg (1 tablet) bd + Truvada 1 tablet od 2. Young people 10 years of age or older with renal insufficiency should not receive tenofovir and should therefore be given:- raltegravir 400mg (1 tablet) bd + a fixed dose combination of lamivudine 150mg/zidovudine 300mg 1 tablet bd 3. Tenofovir should be avoided in the context of renal impairment at any age if at all possible (seek expert advice) 4. Although raltegravir is currently licensed in children younger than 6 years, experience of use in children in this age group is very limited and chewable formulation is rarely immediately available even in specialist centres. For these reasons Kaletra remains first line recommendation in children under 6 years of age with chewable raltegravir as an alternative. 6
7 Table 4 HIV PEP Drugs, Doses and Side effects Dosing is correct as per date of guideline publication but for updated dosing please see CHIVA ART dosing table Drug Formulation Dose Side Effects * Raltegravir (RAL) Tablet: 400mg Tablet: Rash, nausea, hepatitis NOTE: formulations are not bioequivalent; use chewable tabs for children 11-25kg and children >25kg who cannot swallow tablets Chewable tablet: 25mg, 100mg (can be chewed or swallowed) From 25kg: 400mg BD Chewable tablet: 11-14kg 75 mg BD 14-20kg 100mg BD 20-28kg 150mg BD 28-40kg 200mg BD >40kg 300mg BD Zidovudine (AZT, ZDV) Capsule: 100mg, 250mg Liquid: 10mg/ml Capsule or liquid: 180mg/m 2 /dose BD to a maximum dose of 250mg BD (max. 300mg BD when Granulocytopenia and/or anaemia, nausea, headache, myopathy, hepatitis, neuropathy. used in combination products) Lamivudine (3TC) Tablet: 100mg, 150mg Liquid: 10mg/ml Tablet or liquid: 4mg/kg/dose BD to a maximum dose of 150mg Peripheral neuropathy, nausea, diarrhoea, headache. BD Truvada (TDF+FTC) Do not use if known renal impairment Combined tablet: TDF 300mg/FTC 200mg Combined tablet: >35kg 1 tablet OD Headache, diarrhoea, nausea, vomiting, renal tubular dysfunction, bone demineralization 7
8 Tenofovir (TDF) Tablet TDF (TD): Tablet: Do not use if known Note: 300mg 300mg (245mg) >35kg 300mg OD renal impairment tenofovir disoproxil Paed tab TDF (TD): Paed tab: fumarate (TDF) = 150mg (123mg) 200mg 17-22kg 150mg OD 245mg tenofovir (163mg) 250mg (204mg) 23-28mg 200mg OD disoproxil (TD) Powder TDF (TD): 28-34kg 250mg OD All doses expressed 40mg (33mg) per 1g Powder: as TDF scoop 2-12 yrs 10-12kg 2 scoops 12-14kg 2.5 scoops 14-17kg 3 scoops 17-19kg 3.5 scoops 19-22kg 4 scoops 22-24kg 4.5 scoops 24-27kg 5 scoops 27-29kg 5.5 scoops 29-32kg 6 scoops 32-34kg 6.5 scoops 34-35kg 7 scoops 35kg 7.5 scoops Lamivudine Combined tablet: Combined tablet: As for ZDV and 3TC 150mg/zidovudine 3TC 150mg/ZDV 300mg >30kg 1 tablet BD 300mg (Combivir or generic equiv) Kaletra (LPV/RTV) Liquid: Liquid: Diarrhoea, abdominal 2 adult tabs = 4 paed tabs = 5ml of liquid LPV 80mg/RTV 20mg per ml 300mg/m 2 /dose BD Dose in mls = (300 SA)/80 pain, nausea, vomiting, headache. **All doses based on LPV** Paed tablet: LPV 100mg/RTV 25mg (yellow) Paed tablet: 15-25kg 2 tabs BD 25-35kg 3 tabs BD >35kg 4 tabs BD Adult tablet: LPV Adult tablet: 200mg/RTV 50mg >35kg 2 tabs BD (orange) 8
9 *This list of side effects is not exhaustive refer to product datasheet for detailed information on side effects, interactions with other medicines and other cautions for use. Drug interactions that may reduce the effectiveness of raltegravir: Rifampicin within the preceeding 2 weeks Aluminium/ magnesium containing antacids Avoid co-administration of ritonavir with steroids including nasal/inhaled preparations of fluticasone and budesonide due to the interaction with ritonavir producing extremely high steroid levels impacting on bone metabolism. Further information on drug interactions with antiretrovirals can be obtained at or discuss with a pharmacist. Antiemetics: Gastrointestinal side effects are more likely to occur with regimens that contain Kaletra when compared to raltegravir. For those with nausea and vomiting on Kaletra based PEP a switch to paediatric raltegravir should be considered. Alternatively the addition of an anti-emetic to a Kaletra based regimen requires a risk benefit discussion with the family (including discussion regarding the unknown risk of prolonged QT in the paediatric population inferred from adult data) and specialist advice from a tertiary centre and/or HIV pharmacist is recommended. HBV For a significant exposure to an unknown source an accelerated course of HBV immunisation (Day 0, 1 month and 2 months) should be offered. The HPA recommends the use of intramuscular hepatitis B immunoglobulin only if the source is known to be HBV infected, although would agree to its use with an unknown source if compelling circumstances existed. HCV There is no recognised PEP for HCV. Families may be counselled that, in the event of HCV seroconversion, therapy is increasingly successful. Tetanus The need for tetanus injection/booster should be assessed per usual practice. 4. Emergency contraception and screening for sexually transmitted infections In cases of sexual assault refer to BASHH guidelines on management of adult and adolescent complainants of sexual assault Following sexual exposure it is important to consider emergency contraception in girls of reproductive age who may require a double dose of levonorgestrol if receiving Kaletra based PEP and the need for screening/prophylaxis for other sexually transmitted infections. See BASHH Guidelines. 4 9
10 NB: Children under 18 presenting with non-consensual sexual activity should be referred to the Child Protection Co-ordinator. For those cases where sexual trauma has occurred in a child with a risk of HIV transmission, those carrying out testing and PEP care need to be sensitive to reducing possibility of creating extra trauma or exacerbating distress. e.g. blood tests/investigations should be in a paediatric setting if younger child. 5. Follow-up Prior to discharge from A&E families embarking on HIV PEP should have the following: An outpatient appointment, preferably within the next 72 hours to see a named clinician with experience in prescribing antiretroviral drugs Contact telephone numbers in case of concerns about any aspect of the HIV PEP including out-of-hours number. 5 days of antiretroviral therapy A letter for their GP, with patients/parents consent. Clear guidance should be provided for family/child as well as involved services about what details will be communicated between services (those dealing with original abuse/rape or other incident and those managing the PEP). Outpatients Visits Within 72hrs: Review in clinic, assess adherence and toxicity, decide whether PEP should continue for the full four-week course. Document and give baseline HIV, HBV, HCV Ab results. Arrange psychological support as necessary. Newly diagnosed Hepatitis B infection: If the exposed patient is HBsAg positive there is a risk of flare of hepatitis after Truvada is stopped and specialist advice should be sought prior to the cessation of PEP Day 14: Review in clinic, assess adherence and toxicity, check FBC, U&E, LFTs. Day 28: Review in clinic, assess adherence and toxicity, check FBC, U&E, LFTs (if abnormalities on previous blood tests or clinically indicated). A minimum of 4 weeks AFTER PEP completion (8 weeks from high risk exposure): Follow-up HIV testing should be undertaken with a fourth generation combined HIV antibody/ antigen assay. Antibody screening for Hepatitis B and C is also recommended. Optimally this should be performed 4-8 weeks after completing the 3 doses of HBV vaccine, so that infection can be excluded (HBsAg and HBcAb) and to ascertain that the vaccine response was satisfactory (HBsAb >10mIU/ml). If ongoing risk of exposure to HBV then a 4 th dose of HBV vaccine should be given at 12 months. If further HBV vaccination required arrange appropriate follow up (either clinic or GP based). 10
11 Acknowledgments With thanks to members of the Family Clinic team at Imperial College Healthcare NHS Trust particularly Dr Hermione Lyall (Consultant in Paediatric Infectious Diseases), Neil Tickner and Penny Fletcher (Lead Pharmacists Paediatrics), Rosy Weston (Lead HIV Pharmacist), David Muir (Consultant Virologist), and Senthuran Thillainathan (Medical Student). Thanks also to Nicola Husain (Highly Specialised Paediatric Pharmacist) at Evelina London Children s Hospital. References 1. Merchant RC and Keshavarz R. Human immunodeficiency virus post-exposure prophylaxis for adolescents and children. Pediatrics. 2001; 108(2): Jeffries DJ. Occupational exposure and treatment options. J HIV Comb Ther. 1997; 2(3): Gutman L.T., Herman-Giddens M.E. et al. Pediatric acquired immunodeficiency syndrome. Barriers to recognising the role of child sexual abuse. Am J Dis Child. 1993; 147: Clinical Effectiveness Group (British Association of Sexual Health and HIV). UK Guideline For The Use Of Post-Exposure Prophylaxis For HIV Following Sexual Exposure (2011). Available at 5. Bell D M. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med. 1997;102(Suppl. 5B): HPA: Data Tables of the Unlinked Anonymous HIV and Viral Hepatitis monitoring among people who inject drugs: 2014 report. 4_hivUAM.pdf. 7. Abdala N, Stephens PC, Griffith BP, Heimer R. Survival of HIV-1 in syringes. J Acquir Immune Defic Syndr Hum Retrovirol 1999;20: Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Post-exposure Prophylaxis. MMWR 2001; 50: (RR-11): La Fon SW, Mooney BD et al. A double-blind, placebo-controlled study of the safety and efficacy of Retrovir (Zidovudine) as a chemoprophylactic agent in health care workers. 30th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC, 1990; Abstr Townsend C et al. Earlier initiation of ART and further decline in mother-to-child transmission rates, AIDS 2014;28:
12 11. HIV post-exposure prophylaxis: guidance from the UK Chief Medical Officers' Expert Advisory Group on AIDS DOH, September e/dh_
MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE
 Sandyford Protocols MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE www.hiv-druginteractions.org If you require information on occupational exposure to blood borne viruses, including HIV, please refer to the
Sandyford Protocols MANAGEMENT OF SEXUAL EXPOSURE TO HIV: PEPSE www.hiv-druginteractions.org If you require information on occupational exposure to blood borne viruses, including HIV, please refer to the
CCC Guidance for Pediatric HIV PEP Outside of the Perinatal Period
 CCC Guidance for Pediatric HIV PEP Outside of the Perinatal Period There are currently no published guidelines for post-exposure prophylaxis from the CDC/PHS specific to the pediatric population. This
CCC Guidance for Pediatric HIV PEP Outside of the Perinatal Period There are currently no published guidelines for post-exposure prophylaxis from the CDC/PHS specific to the pediatric population. This
HIV Occupational Transmission and Exposure. Marsh Gelbart 2010
 HIV Occupational Transmission and Exposure Marsh Gelbart 2010 Rationale for Post Exposure Prophylaxis (PEP) It was estimated that the risk for HIV transmission after percutaneous exposures involving larger
HIV Occupational Transmission and Exposure Marsh Gelbart 2010 Rationale for Post Exposure Prophylaxis (PEP) It was estimated that the risk for HIV transmission after percutaneous exposures involving larger
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
What does the HIV Pharmacy Team need to know about PEP
 What does the HIV Pharmacy Team need to know about PEP Rosy Weston Senior Lead Pharmacist Sexual Health and HIV St. Mary s Hospital Imperial College Healthcare NHS Trust London November 2008 Objectives
What does the HIV Pharmacy Team need to know about PEP Rosy Weston Senior Lead Pharmacist Sexual Health and HIV St. Mary s Hospital Imperial College Healthcare NHS Trust London November 2008 Objectives
HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland. An update for registered practitioners September 2017
 HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland An update for registered practitioners September 2017 Key Messages HIV is a major public health challenge for Scotland with an annual average of 359
HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland An update for registered practitioners September 2017 Key Messages HIV is a major public health challenge for Scotland with an annual average of 359
FAMILY HIV CENTER NJ CARES SANE
 HIV, Hepatitis B & C Postexposure Prophylaxis for Sexual Assault Victims Update for Case Managers Susan Burrows-Clark, RN, MSN, CNS, C. 10/17/11 Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug
HIV, Hepatitis B & C Postexposure Prophylaxis for Sexual Assault Victims Update for Case Managers Susan Burrows-Clark, RN, MSN, CNS, C. 10/17/11 Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug
DEPARTMENT OF GENITO-URINARY MEDICINE HEPATITIS B
 DEPARTMENT OF GENITO-URINARY MEDICINE MAIN TRANSMISSION ROUTES HEPATITIS B Sexual transmission Occurs in unvaccinated/non immune men who have sex with men and correlates with multiple partners, unprotected
DEPARTMENT OF GENITO-URINARY MEDICINE MAIN TRANSMISSION ROUTES HEPATITIS B Sexual transmission Occurs in unvaccinated/non immune men who have sex with men and correlates with multiple partners, unprotected
Post-Sexual Exposure Prophylaxis (npep)
 Projeto Praça Onze Universidade Federal do Rio de Janeiro Post-Sexual Exposure Prophylaxis (npep) Mauro Schechter Principal Investigator, Projeto Praça Onze Professor of Infectious Diseases Universidade
Projeto Praça Onze Universidade Federal do Rio de Janeiro Post-Sexual Exposure Prophylaxis (npep) Mauro Schechter Principal Investigator, Projeto Praça Onze Professor of Infectious Diseases Universidade
Post Exposure Prophylaxis for HIV following Sexual Exposure (PEPSE) Assessment Proforma PART A: FOR COMPLETION AT INITIAL PRESENTATION
 Addressograph or Name D.O.B Address Clinic no DOB Post Exposure Prophylaxis for HIV following Sexual Exposure (PEPSE) Assessment Proforma PART A: FOR COMPLETION AT INITIAL PRESENTATION The use of PEP following
Addressograph or Name D.O.B Address Clinic no DOB Post Exposure Prophylaxis for HIV following Sexual Exposure (PEPSE) Assessment Proforma PART A: FOR COMPLETION AT INITIAL PRESENTATION The use of PEP following
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses
 Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
Guidance for management of exposure events where there is a risk of transmission of blood borne viruses (HIV, Hepatitis B and Hepatitis C) in the community SUMMARY Where a child is thought to have had
PrEP for HIV Prevention. Adult Clinical Guideline from the New York State Department of Health AIDS Institute
 PrEP for HIV Prevention Adult Clinical Guideline from the New York State Department of Health AIDS Institute www.hivguidelines.org Purpose of the PrEP Guideline Raise awareness of PrEP among healthcare
PrEP for HIV Prevention Adult Clinical Guideline from the New York State Department of Health AIDS Institute www.hivguidelines.org Purpose of the PrEP Guideline Raise awareness of PrEP among healthcare
Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP)
 Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) For Healthcare Providers About Emtricitabine/Tenofovir Disoproxil
Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) For Healthcare Providers About Emtricitabine/Tenofovir Disoproxil
PEDIATRIC EMERGENCY DEPARTMENT CLINICAL GUIDELINE: NON- OCCUPATIONAL EXPOSURE TO BLOOD-BORNE PATHOGENS (HIV, Hepatitis B, AND Hepatitis C)
 General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
determine need but regimen does not change based on risk factor ED medicine attending meets
 HIV PEP Guidelines after Sexual Assault Harborview Medical Center January 2015 Initial risk assessment and discussion with patient about risk and PEP is by clinician who does exam (SANE, Ob-Gyn resident,
HIV PEP Guidelines after Sexual Assault Harborview Medical Center January 2015 Initial risk assessment and discussion with patient about risk and PEP is by clinician who does exam (SANE, Ob-Gyn resident,
Disclosure. Learning Objectives. Epidemiology. Transmission. Risk of Transmission PRE-EXPOSURE PROPHYLAXIS (PREP) FOR HIV PREVENTION 50,000.
 Disclosure PRE-EXPOSURE PROPHYLAXIS (PREP) FOR HIV PREVENTION I have no financial interest in and/or affiliation with any external organizations in relation to this CE program. DaleMarie Vaughan, PharmD
Disclosure PRE-EXPOSURE PROPHYLAXIS (PREP) FOR HIV PREVENTION I have no financial interest in and/or affiliation with any external organizations in relation to this CE program. DaleMarie Vaughan, PharmD
Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers
 Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers About emtricitabine/tenofovir disoproxil fumarate for HIV-1 PrEP
Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers About emtricitabine/tenofovir disoproxil fumarate for HIV-1 PrEP
PROTOCOL FOR THE MANAGAMENT OF SEXUAL EXPOSUE TO HIV, HBV AND HCV: POST EXPOSURE PROPHYLAXIS FOR HIV (PEPSE) AND HEPATITIS B
 PROTOCOL FOR THE MANAGAMENT OF SEXUAL EXPOSUE TO HIV, HBV AND HCV: POST EXPOSURE PROPHYLAXIS FOR HIV (PEPSE) AND HEPATITIS B Printed copies must not be considered the definitive version DOCUMENT CONTROL
PROTOCOL FOR THE MANAGAMENT OF SEXUAL EXPOSUE TO HIV, HBV AND HCV: POST EXPOSURE PROPHYLAXIS FOR HIV (PEPSE) AND HEPATITIS B Printed copies must not be considered the definitive version DOCUMENT CONTROL
Blood-Borne Pathogens and Post-Exposure Prophylaxis
 Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Management of sharps injuries in the healthcare setting
 Link to this article online for CPD/CME credits Management of sharps injuries in the healthcare setting Anna Riddell, 1 Ioana Kennedy, 2 C Y William Tong 1 3 1 Department of Infection, Barts Health NHS
Link to this article online for CPD/CME credits Management of sharps injuries in the healthcare setting Anna Riddell, 1 Ioana Kennedy, 2 C Y William Tong 1 3 1 Department of Infection, Barts Health NHS
Medications to Reduce the Risk of HIV Infection
 Medications to Reduce the Risk of HIV Infection INFORMATION ABOUT EARLY DRUG TREATMENT AFTER CONTACT WITH BLOOD OR BODY FLUIDS AUGUST 2018 THERE ARE MEDICATIONS (medicines) that you should take soon after
Medications to Reduce the Risk of HIV Infection INFORMATION ABOUT EARLY DRUG TREATMENT AFTER CONTACT WITH BLOOD OR BODY FLUIDS AUGUST 2018 THERE ARE MEDICATIONS (medicines) that you should take soon after
HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital
 HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital HIV continues to be a major global public health issue, Approximately
HIV prophylaxis for health care providers. Dr. A. K. M. Humayon Kabir Assistant Professor Department of Medicine Dhaka Medical College Hospital HIV continues to be a major global public health issue, Approximately
PAEDIATRIC HIV INFECTION. Dr Ashendri Pillay Paediatric Infectious Diseases Specialist
 PAEDIATRIC HIV INFECTION Dr Ashendri Pillay Paediatric Infectious Diseases Specialist Paediatric HIV Infection Epidemiology Immuno-pathogenesis Antiretroviral therapy Transmission Diagnostics Clinical
PAEDIATRIC HIV INFECTION Dr Ashendri Pillay Paediatric Infectious Diseases Specialist Paediatric HIV Infection Epidemiology Immuno-pathogenesis Antiretroviral therapy Transmission Diagnostics Clinical
Guidelines for Implementing Pre-Exposure Prophylaxis For The Prevention of HIV in Youth Peter Havens, MD MS Draft:
 Guidelines for Implementing Pre-Exposure Prophylaxis For The Prevention of HIV in Youth Peter Havens, MD MS Draft: 10-2-2015 Clinical studies demonstrate that when a person without HIV infection takes
Guidelines for Implementing Pre-Exposure Prophylaxis For The Prevention of HIV in Youth Peter Havens, MD MS Draft: 10-2-2015 Clinical studies demonstrate that when a person without HIV infection takes
Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
 PROPHYLAXISFOLLOWINGHIV, HEPB ANDC EXPOSURES: WHAT SNEW Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
PROPHYLAXISFOLLOWINGHIV, HEPB ANDC EXPOSURES: WHAT SNEW Antonio E. Urbina, M.D. Associate Medical Director Center for Comprehensive Care, West 17 th Street Clinic St. Luke s Roosevelt Hospital DISCLOSURES
Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication. For Healthcare Providers
 Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication For Healthcare Providers About TRUVADA for a PrEP Indication INDICATION AND PRESCRIBING CONSIDERATIONS TRUVADA,
Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication For Healthcare Providers About TRUVADA for a PrEP Indication INDICATION AND PRESCRIBING CONSIDERATIONS TRUVADA,
POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV
 POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV DISCLOSURE Relevant relationships with commercial entities none Potential for conflicts of interest within this presentation none
POST-EXPOSURE PROPHYLAXIS, PRE-EXPOSURE PROPHYLAXIS, & TREATMENT OF HIV DISCLOSURE Relevant relationships with commercial entities none Potential for conflicts of interest within this presentation none
National Institute for Health and Clinical Excellence. Tenofovir disoproxil fumarate for the treatment of hepatitis B
 National Institute for Health and Clinical Excellence Tenofovir disoproxil fumarate for the treatment of hepatitis B Comment 1: The draft remit Comments received on the draft scope Appropriateness As liver
National Institute for Health and Clinical Excellence Tenofovir disoproxil fumarate for the treatment of hepatitis B Comment 1: The draft remit Comments received on the draft scope Appropriateness As liver
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Centre for Clinical Practice SCOPE Clinical guideline title: Hepatitis B (chronic): diagnosis and management of chronic hepatitis B in children, young
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Centre for Clinical Practice SCOPE Clinical guideline title: Hepatitis B (chronic): diagnosis and management of chronic hepatitis B in children, young
ANTIRETROVIRAL (ART) DRUG INFORMATION FOR HEALTH CARE PROFESSIONAL
 ZIDOVUDINE (AZT, Retrovir ) Benefits of zidovudine: Zidovudine (ZDV) is an antiretroviral drug that slows the growth of the HIV virus. It has shown in a large randomized, multicentre study to decrease
ZIDOVUDINE (AZT, Retrovir ) Benefits of zidovudine: Zidovudine (ZDV) is an antiretroviral drug that slows the growth of the HIV virus. It has shown in a large randomized, multicentre study to decrease
Practical Scenarios Calculating doses for newborns. Karen Buckberry
 Practical Scenarios Calculating doses for newborns Karen Buckberry Real Life Midwife phones you HIV+ mother in labour Needs you to tell Dr what meds to prescribe for baby What info do you need? Info needed
Practical Scenarios Calculating doses for newborns Karen Buckberry Real Life Midwife phones you HIV+ mother in labour Needs you to tell Dr what meds to prescribe for baby What info do you need? Info needed
TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication. Training Guide for Healthcare Providers
 TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication Training Guide for Healthcare Providers About TRUVADA for a PrEP indication to reduce the risk of sexually acquired HIV-1 infection in high-risk
TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication Training Guide for Healthcare Providers About TRUVADA for a PrEP indication to reduce the risk of sexually acquired HIV-1 infection in high-risk
South African Guidelines for the Safe Use of. Dr. Oscar Radebe
 South African Guidelines for the Safe Use of PrEP in MSM Dr. Oscar Radebe Background Globally MSM(men who have sex with men) have been disproportionately at high risk of HIV transmission. Biological &
South African Guidelines for the Safe Use of PrEP in MSM Dr. Oscar Radebe Background Globally MSM(men who have sex with men) have been disproportionately at high risk of HIV transmission. Biological &
WHAT S NEW IN THE 2015 PERINATAL HIV GUIDELINES?
 WHAT S NEW IN THE 2015 PERINATAL HIV GUIDELINES? Today s Webinar will be starting soon For the audio portion of this meeting: Dial 1-855-702-5382 Enter participant code 596-825-4701# Guidelines for online
WHAT S NEW IN THE 2015 PERINATAL HIV GUIDELINES? Today s Webinar will be starting soon For the audio portion of this meeting: Dial 1-855-702-5382 Enter participant code 596-825-4701# Guidelines for online
Victorian guidelines for post-exposure prophylaxis following non-occupational exposure to HIV
 Victorian guidelines for post-exposure prophylaxis following non-occupational exposure to HIV Victorian NPEP Guidelines 2016 Victorian NPEP Service Alfred Hospital Written by Dr Anna Pierce Endorsed by
Victorian guidelines for post-exposure prophylaxis following non-occupational exposure to HIV Victorian NPEP Guidelines 2016 Victorian NPEP Service Alfred Hospital Written by Dr Anna Pierce Endorsed by
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
Case year old single gay man First contact with clinic in Nov Unprotected sex 60 hours previously Received PEP Remained HIV-negative
 PrEP Case Studies Case 1 62 year old single gay man First contact with clinic in Nov 2011 Unprotected sex 60 hours previously Received PEP Remained HIV-negative Repeat exposures requiring PEP in 2013,
PrEP Case Studies Case 1 62 year old single gay man First contact with clinic in Nov 2011 Unprotected sex 60 hours previously Received PEP Remained HIV-negative Repeat exposures requiring PEP in 2013,
National guidelines for post-exposure prophylaxis after non-occupational exposure to HIV
 National guidelines for post-exposure prophylaxis after non-occupational exposure to HIV These guidelines outline the management of individuals who have been exposed (or suspect they have been exposed)
National guidelines for post-exposure prophylaxis after non-occupational exposure to HIV These guidelines outline the management of individuals who have been exposed (or suspect they have been exposed)
TO DECREASE THE CHANCE OF GETTING
 POST ASSAULT MEDICATIONS Terri Stewart RN Naomi Sugar MD TO DECREASE THE CHANCE OF GETTING Pregnant STDs Chlamydia Gonorrhea Trichomonas Hep B HIV EMERGENCY CONTRACEPTION Plan B Levonorgestrel PLAN B Plan
POST ASSAULT MEDICATIONS Terri Stewart RN Naomi Sugar MD TO DECREASE THE CHANCE OF GETTING Pregnant STDs Chlamydia Gonorrhea Trichomonas Hep B HIV EMERGENCY CONTRACEPTION Plan B Levonorgestrel PLAN B Plan
HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland
 HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland An update for registered practitioners November 2017 Rationale for resource This resource is designed to support registered practitioners involved in
HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland An update for registered practitioners November 2017 Rationale for resource This resource is designed to support registered practitioners involved in
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents
 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents 1 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in
Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in treatment of HIV positive adults and adolescents 1 Clinical Commissioning Policy: Use of cobicistat (Tybost ) as a booster in
patients with blood borne viruses Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled Document Lead:
 CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
CONTROLLED DOCUMENT Procedure for the management of patients with blood borne viruses CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 4 Controlled Document Sponsor: Controlled
HIV PrEP in Ireland. Information booklet for people who are accessing PrEP themselves or are considering accessing PrEP
 HIV PrEP in Ireland Information booklet for people who are accessing PrEP themselves or are considering accessing PrEP The HSE Sexual Health and Crisis Pregnancy Programme (SHCPP) and the HIV PrEP working
HIV PrEP in Ireland Information booklet for people who are accessing PrEP themselves or are considering accessing PrEP The HSE Sexual Health and Crisis Pregnancy Programme (SHCPP) and the HIV PrEP working
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Centre for Clinical Practice SCOPE Clinical guideline title: Hepatitis B (chronic): diagnosis and management of chronic hepatitis B in children, young
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Centre for Clinical Practice SCOPE Clinical guideline title: Hepatitis B (chronic): diagnosis and management of chronic hepatitis B in children, young
Study finds PEP not 100% effective in preventing HIV infection
 From TreatmentUpdate 152 Study finds PEP not 100% effective in preventing HIV infection Some doctors and nurses who care for PHAs may sustain needle-stick injuries. This raises the possibility that they
From TreatmentUpdate 152 Study finds PEP not 100% effective in preventing HIV infection Some doctors and nurses who care for PHAs may sustain needle-stick injuries. This raises the possibility that they
AMERICAN ACADEMY OF PEDIATRICS. Guidance for the Clinician in Rendering Pediatric Care. Peter L. Havens, MD, and the Committee on Pediatric AIDS
 AMERICAN ACADEMY OF PEDIATRICS CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Peter L. Havens, MD, and the Committee on Pediatric AIDS Postexposure Prophylaxis in Children and Adolescents
AMERICAN ACADEMY OF PEDIATRICS CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Peter L. Havens, MD, and the Committee on Pediatric AIDS Postexposure Prophylaxis in Children and Adolescents
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Paediatric antiretroviral therapy.
 Paediatric antiretroviral therapy. Guidelines exist for the choice of first line agents, but do not cover second line agents, other than recommending regimes of adequate potency. We would follow PENTA
Paediatric antiretroviral therapy. Guidelines exist for the choice of first line agents, but do not cover second line agents, other than recommending regimes of adequate potency. We would follow PENTA
THE SOUTH AFRICAN ANTIRETROVIRAL TREATMENT GUIDELINES 2010
 THE SOUTH AFRICAN ANTIRETROVIRAL TREATMENT GUIDELINES 2010 The South African Antiretroviral Treatment Guidelines 2010 Goals of the programme Achieve best health outcomes in the most cost-efficient manner
THE SOUTH AFRICAN ANTIRETROVIRAL TREATMENT GUIDELINES 2010 The South African Antiretroviral Treatment Guidelines 2010 Goals of the programme Achieve best health outcomes in the most cost-efficient manner
Guidance for cost-sensitive HIV therapy prescribing in NHS Scotland 2017
 Guidance for cost-sensitive HIV therapy prescribing in NHS Scotland 2017 Scottish Health Protection Network HIV Clinical Leads FINAL Approved for circulation 17 May 2017 Version No. 6.2 (04/05/17) Date
Guidance for cost-sensitive HIV therapy prescribing in NHS Scotland 2017 Scottish Health Protection Network HIV Clinical Leads FINAL Approved for circulation 17 May 2017 Version No. 6.2 (04/05/17) Date
CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE
 CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE Chronic Hepatitis B virus (HBV) is an important public health problem globally and a leading cause of liver
CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE Chronic Hepatitis B virus (HBV) is an important public health problem globally and a leading cause of liver
For further information, please contact BC Women s Sexual Assault Service at Thank-you.
 The following guideline has been developed for use within BC Women s Hospital and Health Centre. There are support systems at BC Women s Hospital and Health Centre that may not exist in other clinical
The following guideline has been developed for use within BC Women s Hospital and Health Centre. There are support systems at BC Women s Hospital and Health Centre that may not exist in other clinical
An International Antiviral Society-USA
 Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
What is the most important information I should know about tenofovir? What should I discuss with my healthcare provider before taking tenofovir?
 1 of 6 6/10/2016 4:33 PM Generic Name: tenofovir (ten OF oh vir) Brand Name: Viread What is tenofovir? Tenofovir is an antiviral medicine that prevents human immunodeficiency virus (HIV) or hepatitis B
1 of 6 6/10/2016 4:33 PM Generic Name: tenofovir (ten OF oh vir) Brand Name: Viread What is tenofovir? Tenofovir is an antiviral medicine that prevents human immunodeficiency virus (HIV) or hepatitis B
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
One of your office personnel
 Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Guideline on the use of Hepatitis A&B Immunisations for Immunocompromised and Immunocompetent Hepatology Patients
 Guideline on the use of Hepatitis A&B Immunisations for Immunocompromised and Immunocompetent Hepatology Patients WOMEN AND CHILDREN S DIRECTORATE ACUTE SERVICES DIVISION GUIDELINES TITLE Guideline on
Guideline on the use of Hepatitis A&B Immunisations for Immunocompromised and Immunocompetent Hepatology Patients WOMEN AND CHILDREN S DIRECTORATE ACUTE SERVICES DIVISION GUIDELINES TITLE Guideline on
1. E-learning: NHIVNA HIV modules on the NHIVNA website
 Vers Jan 18 E LEARNING sessions to complete for STIF NHIVNA Core Competency 1. E-learning: NHIVNA HIV modules on the NHIVNA website http://www.nhivna.org/nhivna-hiv-nursing-modules.aspx The NHIVNA HIV
Vers Jan 18 E LEARNING sessions to complete for STIF NHIVNA Core Competency 1. E-learning: NHIVNA HIV modules on the NHIVNA website http://www.nhivna.org/nhivna-hiv-nursing-modules.aspx The NHIVNA HIV
2nd line failure, provincial evaluation process for 3rd line therapy, 3rd line treatment options James Nuttall
 2nd line failure, provincial evaluation process for 3rd line therapy, 3rd line treatment options James Nuttall Paediatric Infectious Diseases Unit, Red Cross War Memorial Children s Hospital & University
2nd line failure, provincial evaluation process for 3rd line therapy, 3rd line treatment options James Nuttall Paediatric Infectious Diseases Unit, Red Cross War Memorial Children s Hospital & University
POLICY, PROCEDURE & GUIDELINES FOR POST EXPOSURE PROPHYLAXIS IN CUHG.
 POLICY, PROCEDURE & GUIDELINES FOR POST EXPOSURE PROPHYLAXIS IN CUHG. Reference Number: PPG CUH CUH Revision No: 1 Review Cycle: 2 Years Author (Lead): Dr Colm Kerr, Infectious Owner: Dr A Jackson, Infectious
POLICY, PROCEDURE & GUIDELINES FOR POST EXPOSURE PROPHYLAXIS IN CUHG. Reference Number: PPG CUH CUH Revision No: 1 Review Cycle: 2 Years Author (Lead): Dr Colm Kerr, Infectious Owner: Dr A Jackson, Infectious
EQuIP 5 - This version June 2011 Revision Due June 2014
 Preamble Rockingham Peel Group Non-Occupational Post-Exposure Prophylaxis (NPEP) To Prevent HIV in Western Australia Protocol The purpose of this protocol is to clarify the appropriate use and methods
Preamble Rockingham Peel Group Non-Occupational Post-Exposure Prophylaxis (NPEP) To Prevent HIV in Western Australia Protocol The purpose of this protocol is to clarify the appropriate use and methods
AWMSG Secretariat Assessment Report Advice no Darunavir (Prezista
 AWMSG Secretariat Assessment Report Advice no. 0311 Darunavir (Prezista ) for the treatment of HIV-1 infection in treatment-experienced children and adolescents This assessment report is based on evidence
AWMSG Secretariat Assessment Report Advice no. 0311 Darunavir (Prezista ) for the treatment of HIV-1 infection in treatment-experienced children and adolescents This assessment report is based on evidence
Objectives. HIV in the Trenches HIV Update for the Primary Care Provider, An Overview The HIV Continuum of Care.
 1:30 2:30pm HIV Update SPEAKER Gordon Dickinson, MD Presenter Disclosure Information The following relationships exist related to this presentation: Gordon Dickinson, MD, has no financial relationships
1:30 2:30pm HIV Update SPEAKER Gordon Dickinson, MD Presenter Disclosure Information The following relationships exist related to this presentation: Gordon Dickinson, MD, has no financial relationships
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
James Nuttall Paediatric Infectious Diseases Unit Red Cross Children s Hospital
 James Nuttall Paediatric Infectious Diseases Unit Red Cross Children s Hospital Paediatric HIV Infection Symposium, August 21, 2010, Red Cross Children s Hospital Overview of talk The 2010 paediatric guidelines
James Nuttall Paediatric Infectious Diseases Unit Red Cross Children s Hospital Paediatric HIV Infection Symposium, August 21, 2010, Red Cross Children s Hospital Overview of talk The 2010 paediatric guidelines
Greater Glasgow and Clyde. Blood Borne Viruses: Some important basic facts
 Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts A programme developed by Greater Glasgow and Clyde Health
Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts Greater Glasgow and Clyde Blood Borne Viruses: Some important basic facts A programme developed by Greater Glasgow and Clyde Health
Wales Neonatal Network Guideline CARE OF THE BABY WHO HAS BEEN BORN TO AN HIV POSITIVE MOTHER
 CARE OF THE BABY WHO HAS BEEN BORN TO AN HIV POSITIVE MOTHER A plan of care is written for all babies to be born to a woman with HIV infection. These are placed in the maternal notes within the Baby Pack
CARE OF THE BABY WHO HAS BEEN BORN TO AN HIV POSITIVE MOTHER A plan of care is written for all babies to be born to a woman with HIV infection. These are placed in the maternal notes within the Baby Pack
I M ENDING HIV PATIENT INFORMATION. endinghiv.org.au/prep
 I M ENDING HIV PrEP PATIENT INFORMATION endinghiv.org.au/prep THIS BOOKLET PROVIDES YOU WITH INFORMATION ABOUT Pre-Exposure Prophylaxis (PrEP) for HIV. CONTENTS 06 Who will benefit from PrEP? 04 What is
I M ENDING HIV PrEP PATIENT INFORMATION endinghiv.org.au/prep THIS BOOKLET PROVIDES YOU WITH INFORMATION ABOUT Pre-Exposure Prophylaxis (PrEP) for HIV. CONTENTS 06 Who will benefit from PrEP? 04 What is
Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis).
 LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
LTHT Infection Control Policies Needlestick Policy and Actions to be taken after Exposure to Blood and Body Fluids (including HIV Post- Exposure Prophylaxis). This policy covers the immediate actions to
HIV and PEP. LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED
 HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
43. Guidelines on Needle stick Injury
 43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
Post-Exposure Prophylaxis Review for International Visitors
 Post-Exposure Prophylaxis Review for International Visitors Case Presentation 27 yo nurse presents to Urgent Care for a needlestick 2 days ago from a diabetic lancet. Source patient (SP): 35 yo male known
Post-Exposure Prophylaxis Review for International Visitors Case Presentation 27 yo nurse presents to Urgent Care for a needlestick 2 days ago from a diabetic lancet. Source patient (SP): 35 yo male known
HIV Management Update 2015
 9/30/15 HIV Management Update 2015 Larry Pineda, PharmD, PhC, BCPS Visiting Assistant Professor Pharmacy Practice and Administrative Science ljpineda@salud.unm.edu Pharmacist Learning Objectives Describe
9/30/15 HIV Management Update 2015 Larry Pineda, PharmD, PhC, BCPS Visiting Assistant Professor Pharmacy Practice and Administrative Science ljpineda@salud.unm.edu Pharmacist Learning Objectives Describe
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
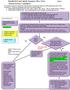 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
EAST LONDON INTEGRATED CARE
 CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE Chronic Hepatitis B virus (HBV) is an important public health problem globally and a leading cause of liver
CITY & HACKNEY ELIC EAST LONDON INTEGRATED CARE MANAGEMENT OF CHRONIC HEPATITIS B IN PRIMARY CARE Chronic Hepatitis B virus (HBV) is an important public health problem globally and a leading cause of liver
Mycobacterial Infections and HIV
 HIV Pediatrics 2017 Paris, France Mycobacterial Infections and HIV Complex cases from Imperial College Healthcare NHS Trust, St. Mary s Hospital, London, UK Presented by Gareth Tudor-Williams, with thanks
HIV Pediatrics 2017 Paris, France Mycobacterial Infections and HIV Complex cases from Imperial College Healthcare NHS Trust, St. Mary s Hospital, London, UK Presented by Gareth Tudor-Williams, with thanks
ESCA: Cinacalcet (Mimpara )
 ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
Post exposure prophylaxis following exposure to HIV. Paul Benn Mortimer Market Centre, Camden PCT HIVPA study day Tuesday 18 th November 2008 SOAS
 Post exposure prophylaxis following exposure to HIV Paul Benn Mortimer Market Centre, Camden PCT HIVPA study day Tuesday 18 th November 2008 SOAS Overview Hypothesis PEP? Awareness & uptake PEP/PEPSE Ensuring
Post exposure prophylaxis following exposure to HIV Paul Benn Mortimer Market Centre, Camden PCT HIVPA study day Tuesday 18 th November 2008 SOAS Overview Hypothesis PEP? Awareness & uptake PEP/PEPSE Ensuring
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS
 NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
NEEDLESTICK INJURIES, BLOOD OR BODY FLUID EXPOSURE INFORMATION AND TEST FORMS Hepatitis B, Hepatitis C and HIV may be contracted through exposure to any body fluid, particularly blood. IMMEDIATE ACTION
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens
 EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
Exposure. Blood. Department of Health & Human Services
 Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Pre-Exposure Prophylaxis (PrEP) Stefanie La Manna, PhD, MPH, APRN, FNP-C, AGACNP-BC October 12, 2018
 Pre-Exposure Prophylaxis (PrEP) Stefanie La Manna, PhD, MPH, APRN, FNP-C, AGACNP-BC October 12, 2018 Disclosures I have no financial disclosures to report Objectives Identify the need for HIV prevention
Pre-Exposure Prophylaxis (PrEP) Stefanie La Manna, PhD, MPH, APRN, FNP-C, AGACNP-BC October 12, 2018 Disclosures I have no financial disclosures to report Objectives Identify the need for HIV prevention
Management of Acute HCV Infection
 Management of Acute HCV Infection This section provides guidance on the diagnosis and medical management of acute HCV infection, which is defined as presenting within 6 months of the exposure. During this
Management of Acute HCV Infection This section provides guidance on the diagnosis and medical management of acute HCV infection, which is defined as presenting within 6 months of the exposure. During this
NEW CANADIAN. GUIDELINES: Public health and community perspectives. Camille Arkell, Moderator Dr. Darrell Tan, Gilles Charette, Dr.
 NEW CANADIAN HIV PrEP/nPEP GUIDELINES: Public health and community perspectives PRESENTED BY Camille Arkell, Moderator Dr. Darrell Tan, Gilles Charette, Dr. Joss Reimer May 16, 2018 Webinar Agenda (1 hour)
NEW CANADIAN HIV PrEP/nPEP GUIDELINES: Public health and community perspectives PRESENTED BY Camille Arkell, Moderator Dr. Darrell Tan, Gilles Charette, Dr. Joss Reimer May 16, 2018 Webinar Agenda (1 hour)
Considerations for Antiretroviral Use in Patients with Hepatitis B Virus & Human Immunodeficiency Syndrome Coinfection
 Considerations for Antiretroviral Use in Patients with Hepatitis B Virus & Human Immunodeficiency Syndrome Coinfection Mahnaz Arian, MD Assistant Professor in infectious Disease Mashhad university of Medical
Considerations for Antiretroviral Use in Patients with Hepatitis B Virus & Human Immunodeficiency Syndrome Coinfection Mahnaz Arian, MD Assistant Professor in infectious Disease Mashhad university of Medical
Bloodbourne Pathogens (BBP) Occupational Post-Exposure Chemophrophylaxis
 Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Prevention of and Immunisation against Hepatitis B and C
 Prevention of and Immunisation against Hepatitis B and C January 2014 Quality Education for a Healthier Scotland 1 Learning Outcomes Participants will be able to:- Demonstrate an increased knowledge and
Prevention of and Immunisation against Hepatitis B and C January 2014 Quality Education for a Healthier Scotland 1 Learning Outcomes Participants will be able to:- Demonstrate an increased knowledge and
Antiretroviral Dosing in Renal Impairment
 Protease Inhibitors (PIs) Atazanavir Reyataz hard capsules 300 mg once daily taken with ritonavir 100 mg once daily No dosage adjustment is needed for atazanavir in renal impairment Atazanavir use in haemodialysis
Protease Inhibitors (PIs) Atazanavir Reyataz hard capsules 300 mg once daily taken with ritonavir 100 mg once daily No dosage adjustment is needed for atazanavir in renal impairment Atazanavir use in haemodialysis
PrEP in Scotland. PrEP. in Scotland. PrEP. PrEP. PrEP. PrEP is a combination pill that prevents HIV.
 PrEP in Scotland PrEP PrEP PrEP PrEP is a combination pill that prevents HIV. 1 Contents Introduction 3 What is PrEP 3 Who should take PrEP 4 Getting PrEP in Scotland 5 Side effects and interactions with
PrEP in Scotland PrEP PrEP PrEP PrEP is a combination pill that prevents HIV. 1 Contents Introduction 3 What is PrEP 3 Who should take PrEP 4 Getting PrEP in Scotland 5 Side effects and interactions with
Sunny Smiles Clinical Guidelines
 Sunny Smiles Clinical Guidelines Accidental Inoculation Injury - Guidance for healthcare professionals on dealing with needle-stick injuries to members Date Written: April 2010 Contents 1 Local Contacts...3
Sunny Smiles Clinical Guidelines Accidental Inoculation Injury - Guidance for healthcare professionals on dealing with needle-stick injuries to members Date Written: April 2010 Contents 1 Local Contacts...3
Primary Care Services for blood borne viral hepatitis prevention, treatment and care
 Primary Care Services for blood borne viral hepatitis prevention, treatment and care Author: Josie Smith, Marion Lyons Page 1 of 13 October 2006 Status : Final Contents: Page: Executive Summary 3 Introduction
Primary Care Services for blood borne viral hepatitis prevention, treatment and care Author: Josie Smith, Marion Lyons Page 1 of 13 October 2006 Status : Final Contents: Page: Executive Summary 3 Introduction
NHS public health functions agreement Service specification No.1 Neonatal hepatitis B immunisation programme
 NHS public health functions agreement 2017-18 Service specification No.1 Neonatal hepatitis B immunisation programme NHS public health functions agreement 2017-18 Service specification No.1Neonatal hepatitis
NHS public health functions agreement 2017-18 Service specification No.1 Neonatal hepatitis B immunisation programme NHS public health functions agreement 2017-18 Service specification No.1Neonatal hepatitis
PEP, PREP, HPTN052 and MLN2238
 PEP, PREP, HPTN052 and MLN2238 Understanding the alphabet soup of HIV prevention and cure strategies Christina G Rivera, PharmD, BCPS Pharmacy Grand Rounds August 15, 2017 2017 MFMER slide-1 Presentation
PEP, PREP, HPTN052 and MLN2238 Understanding the alphabet soup of HIV prevention and cure strategies Christina G Rivera, PharmD, BCPS Pharmacy Grand Rounds August 15, 2017 2017 MFMER slide-1 Presentation
PEP and PREP. Dr David Hawkins Chelsea and Westminster Hospital
 PEP and PREP Dr David Hawkins Chelsea and Westminster Hospital Opportunities for biomedical interventions YEARS HOURS 72 HOURS YEARS Prior to exposure Exposure (pre-coital/ coital) Exposure (pre-coital/
PEP and PREP Dr David Hawkins Chelsea and Westminster Hospital Opportunities for biomedical interventions YEARS HOURS 72 HOURS YEARS Prior to exposure Exposure (pre-coital/ coital) Exposure (pre-coital/
Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents
 Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents Visit the AIDSinfo website to access the most up-to-date guideline. Register for e-mail notification of guideline
Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents Visit the AIDSinfo website to access the most up-to-date guideline. Register for e-mail notification of guideline
SFAF CLINICAL PROTOCOLS
 SFAF CLINICAL PROTOCOLS Page 1 of Supersedes Date: December 31, 2016 Original Date: August 20, 2014 Version: 03 Policy Section: Patient Care Non-Occupational Post Exposure Prophylaxis Program Back ground:
SFAF CLINICAL PROTOCOLS Page 1 of Supersedes Date: December 31, 2016 Original Date: August 20, 2014 Version: 03 Policy Section: Patient Care Non-Occupational Post Exposure Prophylaxis Program Back ground:
Duration of treatment All DMARDs are long term treatments. Clinical benefit may take up to 6 months. 1
 Leflunomide Traffic light classification- Amber 1 Information sheet for Primary Care Prescribers Part of the Shared Care Protocol: Management of Rheumatological Conditions with Disease-Modifying Anti Rheumatic
Leflunomide Traffic light classification- Amber 1 Information sheet for Primary Care Prescribers Part of the Shared Care Protocol: Management of Rheumatological Conditions with Disease-Modifying Anti Rheumatic
HIV Update Objectives. Epidemiology. Epidemiology, Transmission and Natural History. Transmission Risk by Exposure. Transmission 9/29/2014
 Objectives HIV Update 2014 Jay Sizemore, MD, MPH Medical Director Chattanooga CARES Assistant Professor UTCOM Chattanooga 2October 2014 Review HIV epidemiology and screening/testing guidelines Discuss
Objectives HIV Update 2014 Jay Sizemore, MD, MPH Medical Director Chattanooga CARES Assistant Professor UTCOM Chattanooga 2October 2014 Review HIV epidemiology and screening/testing guidelines Discuss
