X-irradiation reduces lesion scarring at the contusion site of adult rat spinal cord
|
|
|
- Lee Blankenship
- 6 years ago
- Views:
Transcription
1 Histol Histopathol (2005) 20: Histology and Histopathology Cellular and Molecular Biology X-irradiation reduces lesion scarring at the contusion site of adult rat spinal cord S.X. Zhang 1, J.W. Geddes 2, J.L. Owens 1,3 and E.G. Holmberg 1,3 1 Spinal Cord Society Research Center, Fort Collins, Colorado, USA, 2 Spinal Cord and Brain Injury Research Center, University of Kentucky College of Medicine, Lexington, Kentucky, USA and 3 University of Alaska Anchorage, Anchorage, Alaska, USA Summary. Spinal cord injury (SCI) results in cell death and tissue destruction, and ultimately cavitation followed by the formation of lesion scars at the injury site. The lesion scars include an astrocytic component (glial scar) and a fibroblastic component (connective tissue scar). The purpose of the present study is to determine if X-irradiation could minimize the formation of lesion scars and reduce the levels of chondroitin sulfate proteoglycans (CSPGs) in the contusion SCI model of the adult rat. Two weeks after SCI, a connective tissue scar formed at the injury site consisting primarily of fibroblasts and exhibits strong CSPG immunoreactivity. The fibroblasts might originate from the connective tissue of pia mater or arachnoid mater. At the same time, reactive astrocytes in the spared tissue accumulate surrounding the lesion cavity to form a thick glial scar with significant enhancement of glial fibrillary acidic protein (GFAP) and CSPG immunoreactivity. After X-irradiation (40 Gy) of the injury site 2 days postinjury, that results in an attenuated dose to the lesion, the connective tissue scar was not observed, and accordingly, almost no CSPG immunoreactivity was detected at this area. Meanwhile, the glial scar and its CSPG immunoreactivity were prominently reduced. X- irradiation did not show significant improvement in locomotor recovery, but resulted in a slight delay of body weight recovery following injury. This preparative treatment could be used to reduce secondary scarring in the lesion resulting in an enriched site for further treatment such as growth related transplantation. Key words: X-irradiation, Spinal cord injury (SCI), Lesion scar, Chondroitin sulfate proteoglycans (CSPGs), Glial fibrillary acidic protein (GFAP) Offprint requests to: Shu-xin Zhang, M.D., Ph.D., SCS Research Center, 2401 Research Blvd., Suite 104, Fort Collins, CO, USA. szhang@scs.cbeyond.com Introduction Traumatic spinal cord injury (SCI) involves both initial mechanical injury and subsequent secondary events which include ischemia, hemorrhage, edema, and inflammation, resulting in extensive neuronal and glial degeneration and final cavitation followed by lesion scarring (Tator, 1991; Bunge et al., 1994; Liu et al., 1997; von Euler et al., 1997; Fitch et al., 1999; Verdu et al., 2003). The lesion scars that represent a combined physical and molecular barrier to axonal regeneration (Davies et al., 2004), include an astrocytic component (glial scar) and a fibroblastic component (connective tissue scar). Within two weeks following SCI in rats, reactive astrocytes accumulate at the margin of the spared tissue and contribute to the formation of glial scar (Reier et al., 1983, 1989). Although astrocytes have neuritepromoting and supportive roles, they can also be inhibitory, depending on the age and differentiation stage of the astrocytes (Smith et al., 1990; Fawcett and Asher, 1999). The reactive astrocytes actively inhibit the growth of regenerating axons by forming a physical barrier or by expressing growth inhibitory substances (Schnell and Schwab, 1990; Bartholdi et al., 1997; Silver and Miller, 2004). Implants to central nervous system (CNS) are often surrounded by similar astrocytic phenotypes (Jakeman and Reier, 1991; Schnell et al., 1999). As first proposed by Ramon y Cajal [see(ramon y Cajal, 1928)], the glial scar is thought to exert a powerful inhibitory effect on neurite regeneration following SCI, representing a major obstacle for the axonal elongation, therefore this structure has become an important target for regeneration research in spinal cord and brain injury (Schwab and Bartholdi, 1996; Fawcett and Asher, 1999; Silver and Miller, 2004). After spinal cord or brain injury, a dense connective tissue scaring develops at the injury site (Krikorian et al., 1981; Fernandez and Pallini, 1985; Maxwell et al., 1990; Hermanns et al., 2001) in rats, mice and humans (Bunge et al., 1994; Fujiki et al., 1995; Bunge et al., 1997; Croul
2 520 X-ray reduces lesion scarring after SCI and Flanders, 1997; Masanneck et al., 2003). The scar tissue is composed primarily of fibroblasts and collagen fibers, as well as Schwann cells (Schwab and Bartholdi, 1996; Bruce et al., 2000). The fibrous scar tissue contains an extensive supply of blood vessels and is rich in basic fibroblast growth factor and transforming growth factor-ß1 (Koshinaga et al., 1993; Logan and Berry, 1993; Follesa et al., 1994), which may influence neovascularization and the formation of connective tissue scar (Folkman and Klagsbrun, 1987; Logan et al., 1994). The fibroblasts, which invade the injury site to form a dense connective tissue scar, may originate from the meninges or the epidural connective tissue (Krikorian et al., 1981; Fernandez and Pallini, 1985; Maxwell et al., 1990). The fibroblasts in lesion center have been found expressing NG2 (Tang et al., 2003). Neoformation of connective tissue occurring at the level of SCI is considered a major factor in the failure of regeneration in the mammalian spinal cord (Fernandez and Pallini, 1985). Chondroitin sulfate proteoglycans (CSPGs) are potent inhibitory molecules in the injured CNS and are present in areas of reactive gliosis following adult CNS injury (McKeon et al., 1991; Bovolenta et al., 1993; Levine, 1994; Zuo et al., 1998; Fawcett and Asher, 1999; Lemons et al., 1999; McKeon et al., 1999). They are thought to be secreted by reactive astrocytes, fibroblasts, as well as other cell types (Yamada et al., 1994; Lemons et al., 1999). Recent studies demonstrate that while axonal regeneration of implanted sensory neurons in adult rat spinal cord is robust within normal white matter and white matter undergoing fulminant Wallerian degeneration despite intimate contact with myelin, it is inhibited in the glial scar containing high concentration of CSPGs (Davies et al., 1997, 1999). These data suggest that CSPGs may be key inhibitory molecules to axonal elongation. Recent attempts to reduce the lesion scarring and promote the functional recovery have involved the administration of X-irradiation to the injury site of experimental SCI. It was reported that X-irradiation following unilateral transection of the adult rat olfactory bulb dramatically reduces the number of reactive astrocytes, prevents tissue degeneration adjacent to the lesion, and preserves normal tissue morphology (Kalderon et al., 1990, 2001; Kalderon and Fuks, 1996). Following a crush-freeze lesion of dorsal root axons, X- irradiation prevents glial scar formation and supports regrowth of dorsal root axons into the spinal cord (Sims and Gilmore, 1994). Regeneration of CNS neurons is enhanced in glial cell deficient environments, produced by X-irradiation (Savio and Schwab, 1990; Sims and Gilmore, 1994; Schwegler et al., 1995; Kalderon and Fuks, 1996; Wilson et al., 2000). Together, these results suggest that X-irradiation may minimize glial scar formation and convert a normally non-permissive environment into one conductive for axonal regrowth and functional recovery (Gimenez y Ribotta et al., 1998; Vanek et al., 1998; Ridet et al., 2000; Li et al., 2003). However, the ability of X-irradiation to reduce the lesion scar formation and the levels of CSPGs in a contusion injury model (in contrast to transection or freeze/crush) has not been well documented previously. Therefore, the goal of the present study is to determine if a single dose of 40 Gy of X-irradiation could reduce the formation of lesion scars and the expression of inhibitory CSPGs, and improve the locomotor recovery following contusion SCI in adult rat. Materials and methods Spinal cord injury Following anesthesia with sodium pentobarbital (50 mg/kg, ip), female Long-Evans rats weighing g received a dorsal laminectomy to expose the spinal cord at thoracic (T) level T10 with the dura intact. The vertebral column was stabilized through clamping the spinous process at vertebra T7 and T10. The 10 g impact rod of the NYU (New York University) impactor device was dropped from a distance of 25 mm, inflicting a moderate contusion injury that resulted in hindlimb locomotor deficits as previously described (Constantini and Young, 1994). After surgery, buprenorphine analgesia (0.02 mg/kg, sc) was administered twice a day for 2 days. Polyflex (Ampicillin) antibiotic (50 mg/kg, sc) was injected once prophylactically, with treatment continuing if blood was detected in urine. Saline solution (0.9% NaCl, 10 ml, sc) was administered daily for one week to prevent dehydration. Urinary bladders were manually expressed twice a day until adequate spontaneous voiding returned. Rats were weighed daily during the first two weeks postinjury and once a week afterward. X-irradiation Radiation was delivered with a Phillips MG-100 X- ray generator to the injury site of the rat spinal cord the second day post-injury. The rats were anesthetized and covered with a protective lead shield with a slit of 25x20 mm (lenght x width) in correspondence with the spinal cord. A single X-ray dose of 40 Gy was administered under the following conditions: 100 kv, 15 ma, a 50 mm focus distance, and a 0.5 mm Cu filter. In preliminary studies, we examined various postinjury time points (1, 2, 3, 7, and 18 days) and found that the maximal reduction in glial scarring was obtained with the 2 and 3 days time points. The 2d time point was chosen for subsequent studies. A recent report revealed that 20 or 40 Gy of X-irradiation may increase its capacity of support neurite regeneration in vitro due to the result of alterations in the glial cell populations in the post-irradiated tissues (Pinjuh and Bedi, 2003). In preliminary studies we also examined various doses of X-irradiation (20, 40, 60, and 80 Gy) and found that a dose of 40 Gy is the highest dose that could be used without causing prominent radiation burns to the skin.
3 X-ray reduces lesion scarring after SCI 521 Actually, after penetrating the tissue over the spinal cord the dose of 40 Gy delivered at skin level has been attenuated to 22 Gy at the center of the spinal cord (Ridet et al., 2000). Eighteen rats (n=12 for 2 wks time point; n=6 for 4 wks time point) received X-irradiation after spinal cord injury. Controls included uninjured rats (n=4) and rats that received spinal cord contusion, but no X-irradiation (total n=18; n=12 for 2 wks time point; n=6 for 4 wks time point). Tissue processing Experimental and control rats were euthanized with an overdose of sodium pentobarbital (90 mg/kg, i.p.) 2 or 4 weeks after contusion injury. The rats were transcardially perfused with saline followed by phosphate-buffered 4% paraformaldehyde fixative. A 30 mm long spinal cord containing the lesion site was cut into ten 3 mm blocks and post-fixed in the same fixative overnight. Following dehydration with graded alcohol, blocks were cleared in xylene and embedded in paraffin. Eight-µm cross sections of each block were cut with a microtome and mounted on glass slides for hematoxylineosin (H.E.) staining or immunostaining. Immunohistochemistry Monoclonal anti-chondroitin sulfate proteoglycans (CS-56, 1:500, Sigma) and a monoclonal antibody against 49 kd glial fibrillary acidic protein (GFAP, 1: 2000; Boehringer, Germany) of astrocytes were used. The sections were deparaffinized and rehydrated, then were boiled in the Antigen Retrieval Citra solution (BioGenex, San Ramon, CA) for 15 min. To quench endogenous peroxidase, the sections were covered with 3% H 2 O 2 in 50 mm Tris-buffered saline containing 0.1% Triton X-100 (TTBS) ph 7.5, for 30 min at room temperature (RT). The non-specific antigen was blocked by incubating in 2% normal goat serum in TTBS for 60 min at RT. The sections were incubated in primary antibodies diluted in TTBS with 1% normal goat serum in a humid chamber overnight at RT. The sections were then washed and incubated in 1:200 biotinylated goat anti-mouse IgM (for CS-56) or IgG (for GFAP) (Jackson) for 60 min and then in 1:500 streptavidin peroxidase (Jackson) for 60 min in the same buffer. The reaction product was visualized by incubating in 3,3 - diaminobenzidine solution (DAB, 0.5 mg in 1 ml TTBS) with 0.01% H 2 O 2 for 3-5 min. Some sections were counter-stained with hematoxylin and eosin. The sections were rinsed, dehydrated, cleared, and coverslipped. Negative controls were treated similarly, except the primary antibody was omitted. GFAP immunoreactivity was quantitatively analyzed with NIH image program. The density of GFAP immunoreactivity in the white matter of control spinal cord tissue and in the spared tissue of injured spinal cord was measured. Three histological sections for each case were examined and the results were compared between normal control group and experimental groups with different conditions. Light and electron microscopy Paraffin sections from each group were stained with hematoxylin and eosin for general histology evaluation and cell counting. The ventral white matter of the normal control cords and the ventral spared tissue at the lesion center of the spinal cord were selected as the cell counting areas. Cells were counted in four fields of 0.1 mm 2 per section, and three sections from each case were examined. In order to ultrastructurally identify the cell type of the connective tissue scar, two extra rats at the second post-injury week were transcardially perfused for electron microscopic study with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, ph 7.4. One-mm thick blocks from the injury site were treated for electron microscopic evaluation as described previously (Spurr, 1969; Guth et al., 1994). The ultrathin sections stained with uranyl acetate and lead citrate were examined under a Hitachi H-7000 transmission electron microscope. Behavioral analysis Hindlimb locomotor recovery was observed by video tape-recording and evaluated using the BBB locomotor rating scale (Basso et al., 1995, 1996). Open field locomotor scores were collected 1 day, 3 days, 1 week, 2 weeks, 3 weeks, and 4 weeks after injury. Statistical analysis All quantified data were treated and expressed as mean ± SEM. Comparison of the X-irradiated to nonirradiated injured animals was performed with a onefactor ANOVA with post hoc multiple comparisons using the Tukey test. Cell counts were compared using the Student t test. Statistical evaluation was performed using the Statview program. In all cases, statistical significance was established with a p value of < Results Two weeks following SCI, a dense connective tissue scar, a cavity, and a glial scar were observed at the lesion epicenter. The spared tissue contained numerous degenerating or demyelinated axons. Connective tissue scarring The connective tissue scar was localized in the dorsal part of the spinal cord. Similar to a dense scar tissue found in the skin wound, the connective tissue scar consists primarily of reactive fibroblasts and contains a rich supply of blood vessels (Fig. 1A,B). It was negative for GFAP immunoreactivity, indicating that the scar tissue is different in structure from the CNS
4 522 X-ray reduces lesion scarring after SCI
5 X-ray reduces lesion scarring after SCI 523 Fig. 2. The presumptive cellular origin of connective tissue scar. This is a section from a rat spinal cord one week after contusion labeled with GFAP immunostaining (brown) and H.E. counter staining. Reactive fibroblasts in the proliferating connective tissue (CTPA) of the pia mater and arachnoid invade the spinal cord parenchyma to form a connective tissue scar (CT Scar). The negative GFAP immunoreactivity along the border (arrowheads, B) of spinal cord parenchyma indicates the lack of the glial limitans (arrows). The dura mater in A was pointed by arrowheads. Scale bars: A, 100 µm; B, 50 µm. Fig. 3. Schwann cells in the connective tissue scar. A. Toluidine blue-stained semithin plastic section at the injury site from a rat spinal cord two weeks post-injury. Schwann cells (arrowheads) formed fascicles, which were surrounded by a layer of fibroblasts (arrows). The scar tissue was rich in blood vessels (*). Scale bar: 50 µm. B. Representative electron micrograph of a Schwann cell. The Schwann cell myelinated an axon (Ax); the cell was covered by a layer of basal lamina (arrows) and surrounded by collagen fibers (*). Nu: nucleus of Schwann cell; Fb: cytoplasm of the fibroblast. Scale bar: 1 µm. Fig. 1. Effect of X-irradiation on minimizing the lesion scar formation. Paraffin sections from lesion site two weeks after SCI were stained with hematoxylin and eosin. The connective tissue scar (CT Scar) was located on the dorsal part of injured cord, containing densely packed fibroblasts and blood vessels (A, B). The lesion cavity (Cav) was filled with macrophages (Mφ, C) and walled off by the glial scar (arrows, A). The thick glial scar in the spared tissue (ST) had a darker eosin staining and high cell density including many reactive astrocytes (arrowheads, C). Four weeks after SCI, the glial scar and connective tissue scar were similar to that observed at the two-week time point after injury, but the cell density was increased in some areas (D). In X-irradiated animals, the connective tissue scar was not observed (E), except for few fibroblasts (Fb) scattered among the macrophages (Mφ, F); the glial scar was also prominently reduced (G). Ax: degenerating axon. Scale bars: A, E, 200 µm; B-D, F, G, 10 µm. H. The total cell counts per unit area (0.1 µm 2 ) in the ventral spared white matter demonstrated the significantly decreased cell density in X-irradiated injured rats, as compared to nonirradiated injured animals two weeks and four weeks following injury (** p< 0.01).
6 524 X-ray reduces lesion scarring after SCI (Fig. 2). The scar tissue was blended with the proliferating connective tissue of the pia mater and arachnoid, and there was no glia limitans between them (Fig. 2), suggesting that they might share the same phenotype. Schwann cells were also found in the scar tissue two weeks after SCI. Schwann cells formed fascicles, which were surrounded by a layer of fibroblasts (Fig. 3A). Under the electron microscope, the Schwann cells were myelinating, or ensheathing, the regenerating or demyelinated axons (Fig. 3B). In the normal rat spinal cord, the CSPG Fig. 4. CSPGs after SCI and X-irradiation. Paraffin sections from lesion site were stained with monoclonal antibody CS-56. Two weeks following SCI, CSPGs are strongly expressed not only in the connective tissue scar (A, B), but also in the glial scar (arrows, C). After X-irradiation, the connective tissue scar was not apparent, resulting in the significant decrease in CSPG immunoreactivity in the area where the connective tissue scar used to occur (D, E). The CSPG immunoreactivity is also decreased in the glial scar with X-irradiation (arrows, F). ST: spared tissue; Cav: cavity. Scale bars: A, D, 200 µm; B, C, E, 10µm; F, 50 µm.
7 X-ray reduces lesion scarring after SCI 525 immunoreactivity is pronounced at the glia limitans, but is weak in the white and gray matter (Lemons et al., 1999). The connective tissue scar expressed strong CSPG immunoreactivity (Fig. 4A), which is located adjacent to and surrounding the fibroblasts and blood vessels (Fig. 4B). In addition, the proliferating Fig. 5. GFAP immunoreactivity after SCI and X-irradiation. Paraffin sections were stained with a monoclonal antibody against GFAP. In normal spinal cord, GFAP immunoreactivity appeared weak in both gray (GM) and white matter (WM, A, B). Two weeks after injury, the intensity of GFAP immunoreactivity in the spared tissue (ST) was greatly increased (C), but there was no GFAP immunoreactivity in the connective tissue scar (CT Scar). The dark-staining zone along the wall of the cavity represented the glial scar in the spared tissue (ST, D). Following X-irradiation, the density of GFAP immunoreactivity in the spared tissue (ST) was reduced, and the dark-staining zone along the wall of the cavity was not observed (E, F). Scale bars: A, C, E, 200 µm; B, D, F, 10 µm.
8 526 X-ray reduces lesion scarring after SCI connective tissue of the pia mater and arachnoid, as well as the thickened dura matter also showed strong positive of CSPG immunoreactivity (Fig. 4A). Glial scarring Two weeks after SCI, reactive astrocytes accumulated along the edge of the spared tissue and formed a thick glial scar, walling off the lesion cavity filled with macrophages. Its superficial cells appeared as epithelium-like cells in morphology. The scar tissue had a high cell density (Fig. 1A, C, H) and exhibited strong GFAP immunoreactivity, which sharply demarcated the spared tissue from the cavity (Fig. 5C,D). The quantitative analysis of GFAP immunoreactivity showed that there was a 4.8 fold increasing two weeks after SCI (Fig. 6). In addition to the strong GFAP immunoreactivity, the glial scar also showed strong CSPG immunoreactivity along the border between the cavity and spared tissue, but the CSPG immunoreactivity was found to be quite weak in the rest area of the spared tissue (Fig. 4A,C). Four weeks after SCI the connective tissue scar and glial scar in both morphology and immunostaining were similar to that observed at two weeks post-injury. However, the total cell density was higher in some areas (Fig. 1D). At the same time, more Schwann cells were observed in the connective tissue scar (data not shown). Effects of X-irradiation on the formation of lesion scars Fig. 6. Quantitative analysis of GFAP immunoreactivity density. Two weeks after SCI, the density of GFAP immunoreactivity in the spared tissue prominently increased (about 4.8 fold; SCI-2w) compared with the normal control spinal cord (control), but significantly reduced (** p<0.01) by X-irradiation (SCI+X-ray/2w). Points represent mean ± SEM. By two weeks post-injury, in X-irradiated animals the dense connective tissue scar at the injury site was not observed, and no blood vessels were present, except for few fibroblasts scattered among the macrophages, which were still numerous and filled the cavity (Fig. 1E, F). Accordingly, the strong CSPG immunoreactivity in this area was ameliorated (Fig. 4D-F). Schwann cells were not observed in the area where the connective tissue scar once occurred, but they could be seen in the spared tissue of X-irradiated spinal cords four weeks after contusion injury. The total cell density in the spared tissue including glial scar was significantly increased at two and four weeks post-injury as compared with uninjured control animals. In X-irradiated animals, the magnitude of the increase in total cell density was reduced by 28% (p< 0.01) two weeks, and by 36% (p<0.01) four weeks after Fig. 7. Effects of X-irradiation on the body weight recovery after SCI. Animals were weighed daily at the first two weeks post-injury and weekly afterward. The body weight obtained immediately before SCI was considered as 100%. X-irradiation resulted in one-week delay (three weeks after SCI) in the recovery of pre-injury body weight compared with non-irradiated injured rats that took two weeks to regain their body weight after SCI. Points represent mean ± SEM. Fig. 8. Effects of X-irradiation on the recovery of hindlimb locomotor after SCI. The BBB scores of hindlimb locomotor were collected 1, 3 days, 1, 2, 3 and 4 weeks following contusion SCI. The analysis did not show significant difference of the locomotor recovery between the X- irradiated and non-irradiated groups observed in the present study (p> 0.05). Points represent mean ± SEM.
9 X-ray reduces lesion scarring after SCI 527 SCI (Fig. 1C, G, H). This more than likely includes the reduction of reactive astrocytes in substantial numbers, which did not accumulate to form a thick wall along the cavity edge of the spared tissue (Fig. 1G). The density of GFAP immunoreactivity in the spared tissue was significantly reduced (Fig. 6) and there was no prominent dark-staining zone along the edge (Fig. 5E, F). Body weight recovery The rat body weight obtained immediately before SCI was considered as 100%. Following contusive SCI the body weight started to decline, and lost by approximately 10% at 5-6 days after injury. By the end of the second post-injury week, rats had regained their body weight, exceeding their pre-injury weight after three weeks. In X-irradiated rats, there was a greater loss of body weight, reaching a maximum of 15% loss at 9-10 days post-injury. Recovery was also delayed with their pre-injury body weight not regained until the end of the third post-injury week. However, observed weight differences were not significant four weeks post-injury (p>0.05, Fig. 7). Locomotor recovery All rats with spinal cord contusion injury had hindlimb paralysis (BBB scores 0) the first day following injury, but started to show recovery from the third day post-injury. By the end of fourth week, the BBB scores averaged 9.4±0.6 in animals not receiving X-irradiation. In rats that received spinal cord injury plus X-irradiation, there was a similar recovery of hindlimb locomotion (10.0±0.8). The comparison between these two groups did not show significant difference (p>0.05, Fig. 8) Discussion The results of the present study demonstrate that X- irradiation on a spinal cord contusion site 2 days postinjury effectively minimizes the formation of a connective tissue scar and its expression of CSPGs. In addition, exposure reduces the cell density in the lesion site, more than likely including reactive astrocytes, to result in a partially attenuated astrocytic scar and CSPG secretion. A recent study (Zeman et al., 2001) reported that localized X-irradiation (20 Gy) of contusion site (25 mm height setting) enhanced recovery of locomotor function (final BBB score 7.1 ~ 8.2 from 2.3 in control) and increased the tissue sparing (to almost 50% from 20% in control). In contrast to the report, X-irradiation in the present study did not significantly benefit the locomotor recovery following injury, but caused a slight delay in body weight recovery. The reason for the discrepancy is not clear. Following contusion injury, the locomotor recovery depends on spared spinal cord tissue; greater tissue sparing is highly correlated with final locomotor performance using the BBB rating scale (Basso et al., 1995, 1996). Accordingly, injured rats with spared cord tissue around 20% and 50% should have a higher BBB score (at least 11 and 19 respectively); rats receiving 25 mm drop contusion should have about 10% spared tissue and BBB score of 10 (Basso et al., 1995, 1996; Young, 2002). In the cited report, however, rats with 25 mm drop contusion had more than 20% spared tissue and BBB score of 2.3 in the control rats (unirradiated) six weeks after injury, in comparison with X-irradiated rats with spared tissue more than 40% and BBB score of 8.2 (Zeman et al., 2001). It seems quite different from the results by Beattie s group (Basso et al., 1996). After contusion injury to the rat spinal cord, the connective tissue scar and glial scar formed within the first two weeks. In pilot studies, X-irradiation at early time points (2-3 days post-injury) was much more effective than later time points (7 day and 18 day postinjury) in minimizing glial scar formation (data not shown). In addition, a number of mitotic cells, which possibly include astrocytes, were observed at the injury site two days after spinal cord contusion injury, consistent with the effects of X-irradiation at this time point. X-irradiation reduced the cell density in the spared tissue, more than likely due to a decrease in astrocytes within the glial scar. It has been reported that, the twoday time point after rat spinal cord compression injury was considered as the optimal time for X-irradiation (Ridet et al., 2000). A response to SCI is the appearance of collagenous connective tissue at the injury site. This has been described as connective tissue scarring, fibrocytic scar, or connective tissue mass, and has been reported to occur in injured spinal cords in humans, rats, and mice (Bunge et al., 1994, 1997; Fujiki et al., 1995; Croul and Flanders, 1997). The connective tissue scarring occurred in all SCI cases of rat contused using NYU impactor with a 25 mm height setting in our studies. The scar tissue consists primarily of reactive fibroblasts and collagen fibers with a rich supply of blood vessels. The fibroblasts are thought to originate from outside of the CNS after breakdown of the spinal cord glia limitans (Fernandez and Pallini, 1985; Maxwell et al., 1990). Our results are in agreement with those findings. We observed that following a contusion injury, the connective tissue of the pia mater or arachnoid was activated, and numerous reactive fibroblasts invaded the damaged spinal cord tissue through the breakdown of the dorsal glia limitans. No boundary between the invading connective tissue and the arachnoid tissue could be seen, suggesting that the glia limitans was no longer present in this area. Because the dura mater was intact in all cases we observed, it is improbable that the fibroblasts were derived from the epidural tissue (Krikorian et al., 1981). The present study confirmed that fibroblasts are one of the cell types that produce CSPGs in the injured spinal cord. We also observed that fibroblasts in the thickened dura mater over the injury site expressed strong CSPG
10 528 X-ray reduces lesion scarring after SCI immunoreactivity. The dense connective tissue of the scar and its CSPGs may function as both physical and molecular barriers to the regeneration of injured axons. Our results revealed that the connective tissue scar was susceptible to X-irradiation. Fibroblasts, which separated Schwann cells to aid in forming small fascicles, may be beneficial to the endogenous structure recovery after SCI, but also contribute to dense scar tissue. X- irradiation delayed, but did not block, the migration of Schwann cells, which migrate into the injury site and myelinate or ensheath the injured axons following SCI (Bresnahan, 1978; Beattie et al., 1997; Bunge et al., 1997; Franklin and Barnett, 1997). In normal spinal cords, astrocytes play an important role in the maintenance of tissue structure, provide neurons with metabolic support, and help regulate the extracellular ionic milieu (Hatten et al., 1991). Following SCI, astrocytes produce interleukin-3 and other cytokines that stimulate microglia to divide (Gimenez y Ribotta et al., 1995; Sugita et al., 1999). The microglia may have both beneficial and detrimental effects on recovery from injury (Streit et al., 1999). The glial scar might play two different roles in the injured spinal cord, preventing further tissue damage and providing neurotrophic support and acting as a physical barrier for axonal regeneration (Davies et al., 1999). The CSPGs produced by the glial scar are likely the key reason, that scarring results in the failure of axonal regeneration (Davies et al., 1997, 1999). Astrocytic hypertrophy may also restore the blood-brain barrier after injury (Bush et al., 1999). It might be necessary for astrocytes in CNS to restore some degree of structural and physiological integrity at the lesion site (Reier et al., 1989; Gimenez y Ribotta et al., 2002). Thus, a moderate dose of X-irradiation (40 Gy) was chosen to reduce astrocytic number sufficiently to attenuate glial scar formation, but to maintain sufficient numbers of astrocytes for structural integrity and neurotrophic support. Treatment with chondroitinase ABC to degrade CSPGs reduced growth inhibition associated with many CSPGs, and profoundly enhanced axonal regeneration (Zuo et al., 1998; Chung et al., 2000; Asher et al., 2001; Bradbury et al., 2002; Morgenstern et al., 2002). Enzymatic inactivation of inhibitory CSPGs may provide a means to convert the injured spinal cord to a microenvironment more permissive to axonal regeneration (Zuo et al., 1998; Asher et al., 2002). The lesion scars also represent a major barrier to axonal growth from transplanted neurons in the injured spinal cord (Davies et al., 1999). While insufficient to promote locomotor recovery in itself, our findings suggest that X-irradiation may help establish or maintain a microenvironment for axonal regeneration or elongation. Thus, combination of X-irradiation with transplantation or strategies to promote axonal regeneration may provide improved functional recovery after SCI. Acknowledgements. This study was supported by Kentucky Spinal Cord and Head Injury Research Trust grant GA-9601-K (J.W.G.), the Paralysis Project of America grant (S.X.Z.), and Spinal Cord Society. We thank Dr. Mansoor M. Ahmed and Dr. Ali S. Meigooni (University of Kentucky, Lexington, KY) for assistance in X-irradiation. We also acknowledge Dr. Diane Snow, Dr. George Smith, and Dr. Alexander Rabchevsky (University of Kentucky, Lexington, KY) for their valuable opinions. References Asher R.A., Morgenstern D.A., Moon L.D. and Fawcett J.W. (2001). Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog. Brain Res. 132, Asher R.A., Morgenstern D.A., Shearer M.C., Adcock K.H., Pesheva P. and Fawcett J.W. (2002). Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J. Neurosci. 22, Bartholdi D., Rubin B.P. and Schwab M.E. (1997). VEGF mrna induction correlates with changes in the vascular architecture upon spinal cord damage in the rat. Eur. J. Neurosci. 9, Basso D.M., Beattie M.S. and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, Basso D.M., Beattie M.S. and Bresnahan J.C. (1996). Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, Beattie M.S., Bresnahan J.C., Komon J., Tovar C.A., Van Meter M., Anderson D.K., Faden A.I., Hsu C.Y., Noble L.J., Salzman S.and Young W. (1997). Endogenous repair after spinal cord contusion injuries in the rat. Exp. Neurol. 148, Bovolenta P., Wandosell F. and Nieto-Sampedro M. (1993). Characterization of a neurite outgrowth inhibitor expressed after CNS injury. Eur. J. Neurosci. 5, Bradbury E.J., Moon L.D., Popat R.J., King V.R., Bennett G.S., Patel P.N., Fawcett J.W. and Mcmahon S.B. (2002). Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, Bresnahan J.C. (1978). An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J. Neurol. Sci. 37, Bruce J.H., Norenberg M.D., Kraydieh S., Puckett W., Marcillo A. and Dietrich D. (2000). Schwannosis: role of gliosis and proteoglycan in human spinal cord injury. J. Neurotrauma 17, Bunge M.B., Holets V.R., Bates M.L., Clarke T.S. and Watson B.D. (1994). Characterization of photochemically induced spinal cord injury in the rat by light and electron microscopy. Exp. Neurol. 127, Bunge R.B., Puckett W.R. and Hiester E.D. (1997). Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv. Neurol. 72, Bush T.G., Puvanachandra N., Horner C.H., Polito A., Ostenfeld T., Svendsen C.N., Mucke L., Johnson M.H. and Sofroniew M.V. (1999). Leukocyte infiltration, neuronal degeneration, and neurite
11 X-ray reduces lesion scarring after SCI 529 outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23, Chung K.Y., Taylor J.S., Shum D.K. and Chan S.O. (2000). Axon routing at the optic chiasm after enzymatic removal of chondroitin sulfate in mouse embryos. Development 127, Constantini S. and Young W. (1994). The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J. Neurosurg. 80, Croul S.E. and Flanders A.E. (1997). Neuropathology of human spinal cord injury. Adv. Neurol. 72, Davies J.E., Tang X., Denning J.W., Archibald S.J..and Davies S.J. (2004). Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur. J. Neurosci. 19, Davies S.J., Fitch M.T., Memberg S.P., Hall A.K., Raisman G. and Silver J. (1997). Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390, Davies S.J., Goucher D.R., Doller C. and Silver J. (1999). Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 19, Fawcett J.W. and Asher R.A. (1999). The glial scar and central nervous system repair. Brain Res. Bull. 49, Fernandez E. and Pallini R. (1985). Connective tissue scarring in experimental spinal cord lesions: significance of dural continuity and role of epidural tissues. Acta Neurochir. 76, Fitch M.T., Doller C., Combs C.K., Landreth G.E. and Silver J. (1999). Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 19, Folkman J. and Klagsbrun M. (1987). Angiogenic factors. Science 235, Follesa P., Wrathall J.R. and Mocchetti I. (1994). Increased basic fibroblast growth factor mrna following contusive spinal cord injury. Brain Res. Mol. Brain Res. 22, 1-8. Franklin R.J. and Barnett S.C. (1997). Do olfactory glia have advantages over Schwann cells for CNS repair? J. Neurosci. Res. 50, Fujiki M., Zhang Z., Guth L. and Steward O. (1995). Absence of progressive necrosis and cavitation after spinal cord crush injury in mice. Soc. Neurosci. Abstr. 21, Gimenez Y Ribotta M., Gaviria M., Menet V. and Privat A. (2002). Strategies for regeneration and repair in spinal cord traumatic injury. Prog. Brain Res. 137, Gimenez Y Ribotta M., Orsal D., Feraboli-Lohnherr D. and Privat A. (1998). Recovery of locomotion following transplantation of monoaminergic neurons in the spinal cord of paraplegic rats. Ann. NY Acad. Sci. 860, Gimenez Y Ribotta M., Rajaofetra N., Morin-Richaud C., Alonso G., Bochelen D., Sandillon F., Legrand A., Mersel M. and Privat A. (1995). Oxysterol (7 beta-hydroxycholesteryl-3-oleate) promotes serotonergic reinnervation in the lesioned rat spinal cord by reducing glial reaction. J. Neurosci. Res. 41, Guth L., Zhang Z., Diprospero N.A., Joubin K. and Fitch M.T. (1994). Spinal cord injury in the rat: treatment with bacterial lipopolysaccharide and indomethacin enhances cellular repair and locomotor function. Exp. Neurol. 126, Hatten M.E., Liem R.K., Shelanski M.L., Mason C.A. (1991). Astroglia in CNS injury. Glia 4, Hermanns S., Klapka N. and Muller H.W. (2001). The collagenous lesion scar--an obstacle for axonal regeneration in brain and spinal cord injury. Restor. Neurol. Neurosci. 19, Jakeman L.B. and Reier P.J. (1991). Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J. Comp. Neurol. 307, Kalderon N. and Fuks Z. (1996). Structural recovery in lesioned adult mammalian spinal cord by x- irradiation of the lesion site. Proc. Natl. Acad. Sci. USA 93, Kalderon N., Alfieri A.A. and Fuks Z. (1990). Beneficial effects of x- irradiation on recovery of lesioned mammalian central nervous tissue. Proc. Natl. Acad. Sci. USA 87, Kalderon N., Xu S., J.A. K. and Fuks Z. (2001). Fractionated radiation facilitates repair and functional motor recovery after spinal cord transection in rat. Brain Res. 904, Koshinaga M., Sanon H.R. and Whittemore S.R. (1993). Altered acidic and basic fibroblast growth factor expression following spinal cord injury. Exp. Neurol. 120, Krikorian J.G., Guth L and Donati E.J. (1981). Origin of the connective tissue scar in the transected rat spinal cord. Exp. Neurol. 72, Lemons M.L., Howland D.R. and Anderson D.K. (1999). Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp. Neurol. 160, Levine J.M. (1994). Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J. Neurosci. 14, Li G., Wang J.Z., Li X.G., Zhang Q.L., Jia D.Z. and Gong S.F. (2003). Study of X-irradiation to enhance the functional and structural recovery of the injured spinal cord of rat. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 25, (in Chinesse). Liu X.Z., Xu X.M., Hu R., Du C., Zhang S.X., Mcdonald J.W., Dong H.X., Wu Y.J., Fan G.S., Jacquin M.F., Hsu C.Y. and Choi D.W. (1997). Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 17, Logan A. and Berry M. (1993). Transforming growth factor-beta 1 and basic fibroblast growth factor in the injured CNS. Trends Pharmacol. Sci. 14, Logan A., Berry M., Gonzalez A.M., Frautschy S.A., Sporn M.B. and Baird A. (1994). Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur. J. Neurosci. 6, Masanneck C., Muller D., Heyden A., Hermanns S., Kury P. and Muller H. (2003). Suppression of fibrous scar formation after spinal cord injury (SCI) effects inhibitory proteoglycan deposition. Soc. Neurosci. Abstr. Maxwell W.L., Follows R., Ashhurst D.E. and Berry M. (1990). The response of the cerebral hemisphere of the rat to injury. I. The mature rat. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 328, Mckeon R.J., Jurynec M.J. and Buck C.R. (1999). The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J. Neurosci. 19, Mckeon R.J., Schreiber R.C., Rudge J.S. and Silver J. (1991). Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 11, Morgenstern D.A., Asher R.A. and Fawcett J.W. (2002). Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 137,
12 530 X-ray reduces lesion scarring after SCI Pinjuh D. and Bedi K.S. (2003). X-irradiation of adult spinal cord increases its capacity to support neurite regeneration in vitro. Int. J. Dev. Neurosci. 21, Ramon Y Cajal S. (1928) Cajal's degeneration and regeneration of the nervous system. In: History of Neuroscience. Defilipe J.A.J. (ed). Oxford University Press. New York. p 769. Reier P.J., Eng L.F. and Jakeman L. (1989) Reactive astrocyte and axonal outgrowth in the injured CNS: is gliosis really an impediment to regeneration? In: Neural regeneration and transplantation. Seil F.J. (ed). Alan R. Liss, Inc. pp Reier P.J., Perlow M.J. and Guth L. (1983). Development of embryonic spinal cord transplants in the rat. Brain Res. 312, Ridet J.L., Pencalet P., Belcram M., Giraudeau B., Chastang C., Philippon J., Mallet J., Privat A. and Schwartz L. (2000). Effects of spinal cord X-irradiation on the recovery of paraplegic rats. Exp. Neurol. 161, Savio T. and Schwab M.E. (1990). Lesioned corticospinal tract axons regenerate in myelin-free rat spinal cord. Proc. Natl. Acad. Sci. USA 87, Schnell L., Fearn S., Schwab M.E., Perry V.H. and Anthony D.C. (1999). Cytokine-induced acute inflammation in the brain and spinal cord. J. Neuropathol. Exp. Neurol. 58, Schnell L. and Schwab M.E. (1990). Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 343, Schwab M.E. and Bartholdi D. (1996). Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 76, Schwegler G., Schwab M.E. and Kapfhammer J.P. (1995). Increased collateral sprouting of primary afferents in the myelin-free spinal cord. J. Neurosci. 15, Silver J. and Miller J.H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, Sims T.J. and Gilmore S.A. (1994). Regrowth of dorsal root axons into a radiation-induced glial-deficient environment in the spinal cord. Brain Res. 634, Smith G.M., Rutishauser U., Silver J. and Miller R.H. (1990). Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev. Biol. 138, Spurr A.R. (1969). A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, Streit W.J., Walter S.A., Pennell N.A. (1999). Reactive microgliosis. Prog. Neurobiol. 57, Sugita Y., Zhao B., Shankar P., Dunbar C.E., Doren S., Young H.A. and Schwartz J.P. (1999). CNS interleukin-3 (IL-3) expression and neurological syndrome in antisense-il-3 transgenic mice. J. Neuropathol. Exp. Neurol. 58, Tang X., Davies J.E. and Davies S.J. (2003). Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-c during acute to chronic maturation of spinal cord scar tissue. J. Neurosci. Res. 71, Tator C.H. (1991). Review of experimental spinal cord injury with emphasis on the local and systemic circulatory effects. Neurochirurgie 37, Vanek P., Thallmair M., Schwab M.E. and Kapfhammer J.P. (1998). Increased lesion-induced sprouting of corticospinal fibres in the myelin-free rat spinal cord. Eur. J. Neurosci. 10, Verdu E., Garcia-Alias G., Fores J., Vela J.M., Cuadras J., Lopez-Vales R. and Navarro X. (2003). Morphological characterization of photochemical graded spinal cord injury in the rat. J. Neurotrauma 20, von Euler M., Sundstrom E. and Seiger A. (1997). Morphological characterization of the evolving rat spinal cord injury after photochemically induced ischemia. Acta Neuropathol. (Berl) 94, Wilson N., Esfandiary E. and Bedi K.S. (2000). Cryosections of preirradiated adult rat spinal cord tissue support axonal regeneration in vitro. Int. J. Dev. Neurosci. 18, Yamada H., Watanabe K., Shimonaka M. and Yamaguchi Y. (1994). Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J. Biol. Chem. 269, Young W. (2002). Spinal cord contusion models. Prog. Brain Res. 137, Zeman R.J., Feng Y., Peng H., Visintainer P.F., Moorthy C.R., Couldwell W.T. and Etlinger J.D. (2001). X-irradiation of the contusion site improves locomotor and histological outcomes in spinal cord-injured rats. Exp. Neurol. 172, Zuo J., Ferguson T.A., Hernandez Y.J., Stetler-Stevenson W.G. and Muir D. (1998). Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite- inhibiting chondroitin sulfate proteoglycan. J. Neurosci. 18, Accepted December 29, 2004
X-ray therapy promotes structural regeneration after spinal cord injury in a rat model
 Liu et al. Journal of Orthopaedic Surgery and Research (2016) 11:6 DOI 10.1186/s13018-015-0327-0 RESEARCH ARTICLE X-ray therapy promotes structural regeneration after spinal cord injury in a rat model
Liu et al. Journal of Orthopaedic Surgery and Research (2016) 11:6 DOI 10.1186/s13018-015-0327-0 RESEARCH ARTICLE X-ray therapy promotes structural regeneration after spinal cord injury in a rat model
regenerative medicine in the brain and the spinal cord spinal cord injuries
 regenerative medicine in the brain and the spinal cord spinal cord injuries primary and secondary events during SCI traumatic spinal cord injury (SCI) traumatic spinal cord injury (SCI) main goal is to
regenerative medicine in the brain and the spinal cord spinal cord injuries primary and secondary events during SCI traumatic spinal cord injury (SCI) traumatic spinal cord injury (SCI) main goal is to
Progress Report for NJCSCR (Yu-Wen Chang)
 Progress Report for NJCSCR (Yu-Wen Chang) Overall Plan Summary: Traumatic injury to the spinal cord initiates a cascade of degenerative processes, known as secondary injury, which include various inflammatory
Progress Report for NJCSCR (Yu-Wen Chang) Overall Plan Summary: Traumatic injury to the spinal cord initiates a cascade of degenerative processes, known as secondary injury, which include various inflammatory
Symptoms of spinal cord injury:
 Symptoms of spinal cord injury: involuntary muscle spasms loss of voluntary movement sensation, balance control of breathing autonomic functions (blood pressure) bladder, sexual, bowel control All due
Symptoms of spinal cord injury: involuntary muscle spasms loss of voluntary movement sensation, balance control of breathing autonomic functions (blood pressure) bladder, sexual, bowel control All due
Helen R Barbour 2, Christine D Plant 1, Alan R Harvey 3 and Giles W Plant 1,2*
 Barbour et al. BMC Neuroscience 2013, 14:106 RESEARCH ARTICLE Open Access Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model
Barbour et al. BMC Neuroscience 2013, 14:106 RESEARCH ARTICLE Open Access Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model
Role of endogenous Schwann cells in tissue repair after spinal cord injury*
 NEURAL REGENERATION RESEARCH Volume 8, Issue 2, January 2013 www.nrronline.org doi:10.3969/j.issn.1673-5374.2013.02.011 [http://www.nrronline.org; http://www.sjzsyj.org] Zhang SX, Huang FF, Gates M, Holmberg
NEURAL REGENERATION RESEARCH Volume 8, Issue 2, January 2013 www.nrronline.org doi:10.3969/j.issn.1673-5374.2013.02.011 [http://www.nrronline.org; http://www.sjzsyj.org] Zhang SX, Huang FF, Gates M, Holmberg
Strategies for nerve regeneration after CNS injury
 Strategies for nerve regeneration after CNS injury Xiaodan JIANG 1 *, Shengbin Kou 1#, Zhiqiang Fa 1#, Yehai Li 1#, Zhenzhou Chen 1, Shanshan Song 2, Hongbo GUO 1, Yiquan KE 1, Ruxiang XU1, Zhicheng XIAO
Strategies for nerve regeneration after CNS injury Xiaodan JIANG 1 *, Shengbin Kou 1#, Zhiqiang Fa 1#, Yehai Li 1#, Zhenzhou Chen 1, Shanshan Song 2, Hongbo GUO 1, Yiquan KE 1, Ruxiang XU1, Zhicheng XIAO
Human Anatomy and Physiology I Laboratory
 Human Anatomy and Physiology I Laboratory Histology of Nervous Tissue and The Spinal Cord This lab involves two laboratory exercises: 1) Histology of Nervous Tissue, and 2) Spinal Cord, Spinal Nerves,
Human Anatomy and Physiology I Laboratory Histology of Nervous Tissue and The Spinal Cord This lab involves two laboratory exercises: 1) Histology of Nervous Tissue, and 2) Spinal Cord, Spinal Nerves,
Olfactory ensheathing glia
 Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Role of the Chondroitin Sulfate Proteoglycan, Neurocan, in Inhibition of Sensory Neurite Regeneration
 University of Kentucky UKnowledge Lewis Honors College Capstone Collection Lewis Honors College 2016 Role of the Chondroitin Sulfate Proteoglycan, Neurocan, in Inhibition of Sensory Neurite Regeneration
University of Kentucky UKnowledge Lewis Honors College Capstone Collection Lewis Honors College 2016 Role of the Chondroitin Sulfate Proteoglycan, Neurocan, in Inhibition of Sensory Neurite Regeneration
BioMed Central. Jeannette E Davies*, Christoph Pröschel, Ningzhe Zhang, Mark Noble, Margot Mayer-Pröschel and Stephen JA Davies* Research article
 BioMed Central Open Access Research article Transplanted astrocytes derived from BMP- or CNTF-treated glialrestricted precursors have opposite effects on recovery and allodynia after spinal cord injury
BioMed Central Open Access Research article Transplanted astrocytes derived from BMP- or CNTF-treated glialrestricted precursors have opposite effects on recovery and allodynia after spinal cord injury
The Nervous System PART C. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7 PART C Protection of the Central Nervous System Scalp and skin Skull and vertebral
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7 PART C Protection of the Central Nervous System Scalp and skin Skull and vertebral
Nerve tissue & the Nervous System
 Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Adult Nervous System
 Adult Nervous System What is the capacity of the PNS and CNS for repair? WHY? Why discuss this now? Potential for repair depends on cellular properties of nerve and glial cells. http://neuroscience.uth.tmc.edu/s1/chapter09.html
Adult Nervous System What is the capacity of the PNS and CNS for repair? WHY? Why discuss this now? Potential for repair depends on cellular properties of nerve and glial cells. http://neuroscience.uth.tmc.edu/s1/chapter09.html
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AD Award Number: W81XWH-11-1-77 TITLE: Translational Pharmacologic Efficacy Studies of Glial Growth Factor 2 (GGF2) in Spinal Cord Injury Models and in the Veterinary Clinical Setting PRINCIPAL INVESTIGATOR:
AD Award Number: W81XWH-11-1-77 TITLE: Translational Pharmacologic Efficacy Studies of Glial Growth Factor 2 (GGF2) in Spinal Cord Injury Models and in the Veterinary Clinical Setting PRINCIPAL INVESTIGATOR:
Chapter 2 Microenvironment Within the Injured Spinal Cord Focusing on IL-6
 Chapter 2 Microenvironment Within the Injured Spinal Cord Focusing on IL-6 Masaya Nakamura Abstract In recent years, a variety of studies have been conducted towards the goal of achieving regeneration
Chapter 2 Microenvironment Within the Injured Spinal Cord Focusing on IL-6 Masaya Nakamura Abstract In recent years, a variety of studies have been conducted towards the goal of achieving regeneration
SUPPLEMENTARY MATERIAL. Sample preparation for light microscopy
 SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
Supplementary Information
 Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
RECEIVED. W. M. Keck Center for Collaborative Neuroscience 604 Allison Road, D-251 Piscataway, NJ (732)
 Principal Investigator: Dongming Sun, W. M. Keck Center for Collaborative Neuroscience 604 Allison Road, D-251 Piscataway, NJ 08854 (732) 445-1832 RECEIVED NJ COMMISSION ON SPINAL CORD RESEARCH The overall
Principal Investigator: Dongming Sun, W. M. Keck Center for Collaborative Neuroscience 604 Allison Road, D-251 Piscataway, NJ 08854 (732) 445-1832 RECEIVED NJ COMMISSION ON SPINAL CORD RESEARCH The overall
Human Histology The Nervous System. Dr. Rawaa Salim Hameed
 Human Histology The Nervous System Dr. Rawaa Salim Hameed The organization of the nervous system Anatomically, the nervous system is divided into:- Neurohistology Structurally, nerve tissue consists of
Human Histology The Nervous System Dr. Rawaa Salim Hameed The organization of the nervous system Anatomically, the nervous system is divided into:- Neurohistology Structurally, nerve tissue consists of
SUPPLEMENTARY FIG. S2. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of dnscs.
 Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Nervous system. Dr. Rawaa Salim Hameed
 Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
B-cell. Astrocyte SCI SCI. T-cell
 RF #2015 P-01 PI: Azizul Haque, PhD Grant Title: Targeting Enolase in Spinal Cord Injury 12-month Technical Progress Report Progress Report (First Six Months): Enolase is one of the most abundantly expressed
RF #2015 P-01 PI: Azizul Haque, PhD Grant Title: Targeting Enolase in Spinal Cord Injury 12-month Technical Progress Report Progress Report (First Six Months): Enolase is one of the most abundantly expressed
Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury
 Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury James Guest MD, PhD, FACS Clinical Professor of Neurological Surgery, Neurosurgery and the Miami Project to
Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury James Guest MD, PhD, FACS Clinical Professor of Neurological Surgery, Neurosurgery and the Miami Project to
Growth-modulating molecules are associated with invading Schwann cells and not astrocytes in human traumatic spinal cord injury
 doi:10.1093/brain/awl374 Brain (2007), 130,940^953 Growth-modulating molecules are associated with invading Schwann cells and not astrocytes in human traumatic spinal cord injury Armin Buss, 1 Katrin Pech,
doi:10.1093/brain/awl374 Brain (2007), 130,940^953 Growth-modulating molecules are associated with invading Schwann cells and not astrocytes in human traumatic spinal cord injury Armin Buss, 1 Katrin Pech,
Cells of the nervous system
 Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
Treatments for Acute Spinal Cord Injury. Jim Geddes Spinal Cord and Brain Injury Research Center University of Kentucky
 Treatments for Acute Spinal Cord Injury Jim Geddes Spinal Cord and Brain Injury Research Center University of Kentucky FDA Approved Drugs for Acute SCI Progress to date 5017 140 NIH funding ~$142 million/yr
Treatments for Acute Spinal Cord Injury Jim Geddes Spinal Cord and Brain Injury Research Center University of Kentucky FDA Approved Drugs for Acute SCI Progress to date 5017 140 NIH funding ~$142 million/yr
Nervous system. 1. Neurons :
 Nervous system nervous system is composed of billions of cells, the most essential being the nerve cells or neurons. There are estimated to be as many as 100 billion neurons in our nervous system. Two
Nervous system nervous system is composed of billions of cells, the most essential being the nerve cells or neurons. There are estimated to be as many as 100 billion neurons in our nervous system. Two
SCIRF Award #2016 I-03 PI: Azizul Haque, PhD Grant Title: Neuron-specific Enolase and SCI
 SCIRF Award #2016 I-03 PI: Azizul Haque, PhD Grant Title: Neuron-specific Enolase and SCI 10-month Technical Progress Report Enolase is a multifunctional glycolytic enzyme involved in growth control, hypoxia,
SCIRF Award #2016 I-03 PI: Azizul Haque, PhD Grant Title: Neuron-specific Enolase and SCI 10-month Technical Progress Report Enolase is a multifunctional glycolytic enzyme involved in growth control, hypoxia,
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
effect on the upregulation of these cell surface markers. The mean peak fluorescence intensity
 SUPPLEMENTARY FIGURE 1 Supplementary Figure 1 ASIC1 disruption or blockade does not effect in vitro and in vivo antigen-presenting cell activation. (a) Flow cytometric analysis of cell surface molecules
SUPPLEMENTARY FIGURE 1 Supplementary Figure 1 ASIC1 disruption or blockade does not effect in vitro and in vivo antigen-presenting cell activation. (a) Flow cytometric analysis of cell surface molecules
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System. Assoc.Prof. E.Elif Güzel, M.D.
 Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
The 7 th lecture. Anatomy and Physiology For the. 1 st Class. By Dr. Ala a Hassan Mirza
 The 7 th lecture In Anatomy and Physiology For the 1 st Class By Dr. Ala a Hassan Mirza Nervous System (part I) The Nerve Tissue and the Nervous System The Tissues of the Body There are 4 types of tissues
The 7 th lecture In Anatomy and Physiology For the 1 st Class By Dr. Ala a Hassan Mirza Nervous System (part I) The Nerve Tissue and the Nervous System The Tissues of the Body There are 4 types of tissues
8.2. Types of Neurons
 Chapter 8 Nervous Tissue The neuron is the functional and the structural unit of the nervous system. It displays two highly developed physiological traits: 1. Irritability - the capacity to generate a
Chapter 8 Nervous Tissue The neuron is the functional and the structural unit of the nervous system. It displays two highly developed physiological traits: 1. Irritability - the capacity to generate a
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
Major Structures of the Nervous System. Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors
 Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Spinal cord injury (SCI) throughout history
 Overview of Cellular Therapies in Spinal Cord Injuries Jesse OWENS Biomedical Program, University of Alaska, Anchorage, Alaska Spinal cord injury (SCI) throughout history has been considered untreatable.
Overview of Cellular Therapies in Spinal Cord Injuries Jesse OWENS Biomedical Program, University of Alaska, Anchorage, Alaska Spinal cord injury (SCI) throughout history has been considered untreatable.
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy and Physiology- Problem Drill 04: Tissues of the Body
 Human Anatomy and Physiology- Problem Drill 04: Tissues of the Body Question No. 1 of 10 A biopsy sample is obtained from a lesion on the right cheek of a male patient. A technician in the histology lab
Human Anatomy and Physiology- Problem Drill 04: Tissues of the Body Question No. 1 of 10 A biopsy sample is obtained from a lesion on the right cheek of a male patient. A technician in the histology lab
The failure of axon regeneration after CNS injury is due to an inadequate or inappropriate
 CHAPTER 2 The Glial Response to Injury and Its Role in the Inhibition of CNS Repair James W. Fawcett The failure of axon regeneration after CNS injury is due to an inadequate or inappropriate regenerative
CHAPTER 2 The Glial Response to Injury and Its Role in the Inhibition of CNS Repair James W. Fawcett The failure of axon regeneration after CNS injury is due to an inadequate or inappropriate regenerative
Nerve Cells and Behavior
 Nerve Cells and Behavior 27 th September, 2016 Touqeer Ahmed Ph.D. Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Nervous System and Behavior Nervous system
Nerve Cells and Behavior 27 th September, 2016 Touqeer Ahmed Ph.D. Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Nervous System and Behavior Nervous system
The Pathology of Human Spinal Cord Injury: Defining the Problems. MICHAEL D. NORENBERG, JON SMITH, and ALEX MARCILLO
 JOURNAL OF NEUROTRAUMA Volume 21, Number 4, 2004 Mary Ann Liebert, Inc. Pp. 429 440 The Pathology of Human Spinal Cord Injury: Defining the Problems MICHAEL D. NORENBERG, JON SMITH, and ALEX MARCILLO ABSTRACT
JOURNAL OF NEUROTRAUMA Volume 21, Number 4, 2004 Mary Ann Liebert, Inc. Pp. 429 440 The Pathology of Human Spinal Cord Injury: Defining the Problems MICHAEL D. NORENBERG, JON SMITH, and ALEX MARCILLO ABSTRACT
The CNS Part II pg
 The CNS Part II pg. 455-474 Protection of the Brain Objectives Describe how the meninges, cerebrospinal fluid, and the blood brain barrier protect the CNS. Explain how Cerebrospinal fluid is formed, and
The CNS Part II pg. 455-474 Protection of the Brain Objectives Describe how the meninges, cerebrospinal fluid, and the blood brain barrier protect the CNS. Explain how Cerebrospinal fluid is formed, and
REGENERATION BEYOND THE GLIAL SCAR
 REGENERATION BEYOND THE GLIAL SCAR Jerry Silver and Jared H. Miller After injury to the adult central nervous system (CNS), injured axons cannot regenerate past the lesion. In this review, we present evidence
REGENERATION BEYOND THE GLIAL SCAR Jerry Silver and Jared H. Miller After injury to the adult central nervous system (CNS), injured axons cannot regenerate past the lesion. In this review, we present evidence
Role of the lesion scar in the response to damage and repair of the central nervous system
 Cell Tissue Res (2012) 349:169 180 DOI 10.1007/s00441-012-1336-5 REVIEW Role of the lesion scar in the response to damage and repair of the central nervous system Hitoshi Kawano & Junko Kimura-Kuroda &
Cell Tissue Res (2012) 349:169 180 DOI 10.1007/s00441-012-1336-5 REVIEW Role of the lesion scar in the response to damage and repair of the central nervous system Hitoshi Kawano & Junko Kimura-Kuroda &
Rapamycin suppresses astrocytic and microglial activation and reduces development of neuropathic pain after spinal cord injury in mice.
 Rapamycin suppresses astrocytic and microglial activation and reduces development of neuropathic pain after spinal cord injury in mice. Satoshi Tateda, MD, Haruo Kanno, MD, PhD, Hiroshi Ozawa, MD, PhD,
Rapamycin suppresses astrocytic and microglial activation and reduces development of neuropathic pain after spinal cord injury in mice. Satoshi Tateda, MD, Haruo Kanno, MD, PhD, Hiroshi Ozawa, MD, PhD,
Semmelweis University of Budapest. Department of Anatomy, Histology and Embryology. Béla Ajtai, M.D. COMPARATIVE MORPHOLOGY OF REACTIVE GLIOSIS
 Semmelweis University of Budapest Department of Anatomy, Histology and Embryology Béla Ajtai, M.D. COMPARATIVE MORPHOLOGY OF REACTIVE GLIOSIS Theses for PhD degree (short form) Tutor: Dr Mihály Kálmán,
Semmelweis University of Budapest Department of Anatomy, Histology and Embryology Béla Ajtai, M.D. COMPARATIVE MORPHOLOGY OF REACTIVE GLIOSIS Theses for PhD degree (short form) Tutor: Dr Mihály Kálmán,
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM
 ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM Introduction The nervous system is the major controlling, regulatory, and communicating system in the body. It is the center of all mental
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM Introduction The nervous system is the major controlling, regulatory, and communicating system in the body. It is the center of all mental
Internship Research Paper. Locomotion and Motor Neuron Plasticity after Incomplete Spinal Cord Injury. Completed at St.
 Internship Research Paper Locomotion and Motor Neuron Plasticity after Incomplete Spinal Cord Injury Completed at St. Joseph s Hospital Barrow Neurological Institute Dr. Thomas Hamm By Anivarya Kumar Introduction
Internship Research Paper Locomotion and Motor Neuron Plasticity after Incomplete Spinal Cord Injury Completed at St. Joseph s Hospital Barrow Neurological Institute Dr. Thomas Hamm By Anivarya Kumar Introduction
Cells of the Nervous System
 Cells of the Nervous System Layout of the Nervous System Central Nervous System (CNS) Brain (in the skull) Spinal Cord (in the spine) Interprets sensory input, initiates movement, and mediates complex
Cells of the Nervous System Layout of the Nervous System Central Nervous System (CNS) Brain (in the skull) Spinal Cord (in the spine) Interprets sensory input, initiates movement, and mediates complex
Strategies for Neuronal Regeneration after Spinal Cord Injury
 Magnetic Shield Corporation. (1997). Electromagnetic Shielding. Riley, K. (1995). Tracing EMFs in Building wiring and grounding. Tucson, AZ: Magnetic Sciences International. Posted in Uncategorized Tags:
Magnetic Shield Corporation. (1997). Electromagnetic Shielding. Riley, K. (1995). Tracing EMFs in Building wiring and grounding. Tucson, AZ: Magnetic Sciences International. Posted in Uncategorized Tags:
The Nervous System. Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes =
 The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
NURSE-UP INTRODUCTION TO THE NERVOUS SYSTEM
 NURSE-UP INTRODUCTION TO THE NERVOUS SYSTEM FUNCTIONS OF THE NERVOUS SYSTEM Body s primary communication and control system. Integrates and regulates body function Collects information specialized nervous
NURSE-UP INTRODUCTION TO THE NERVOUS SYSTEM FUNCTIONS OF THE NERVOUS SYSTEM Body s primary communication and control system. Integrates and regulates body function Collects information specialized nervous
Bridging a Complete Transection Lesion of Adult Rat Spinal Cord
 (C) Freund Publishing House Ltd., 1994 Bridging a Complete Transection Lesion of Adult Rat Spinal Cord with Growth Factor-Treated Nitrocellulose Implants John D. Houle and Mei Kheng Ziegler Department
(C) Freund Publishing House Ltd., 1994 Bridging a Complete Transection Lesion of Adult Rat Spinal Cord with Growth Factor-Treated Nitrocellulose Implants John D. Houle and Mei Kheng Ziegler Department
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury
 Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury Bastian OW, Koenderman L, Alblas J, Leenen LPH, Blokhuis TJ. Neutrophils contribute to
Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System
 Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System Question No. 1 of 10 The human body contains different types of tissue. The tissue is formed into organs and organ systems.
Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System Question No. 1 of 10 The human body contains different types of tissue. The tissue is formed into organs and organ systems.
Axon Guidance. Matthew Blewitt
 Axon Guidance Matthew Blewitt Overview Axonal development PNS regeneration CNS regeneration Future directions Brain Repair Axonal Development Neurite growth cone www.anatomy.unimelb.edu.au http://kalil.anatomy.wisc.edu/articles/kalil_dent_curropneurobiol_2005.pdf
Axon Guidance Matthew Blewitt Overview Axonal development PNS regeneration CNS regeneration Future directions Brain Repair Axonal Development Neurite growth cone www.anatomy.unimelb.edu.au http://kalil.anatomy.wisc.edu/articles/kalil_dent_curropneurobiol_2005.pdf
CNS third year med students Summary of midterm material H Awad
 CNS third year med students 2018 Summary of midterm material H Awad Dear All This presentation summaries the main important topics covered in the midterm material ( lectures 1-6) There will be two questions
CNS third year med students 2018 Summary of midterm material H Awad Dear All This presentation summaries the main important topics covered in the midterm material ( lectures 1-6) There will be two questions
Yara Saddam. Amr Alkhatib. Ihsan
 1 Yara Saddam Amr Alkhatib Ihsan NOTE: Yellow highlighting=correction/addition to the previous version of the sheet. Histology (micro anatomy) :- the study of tissues and how they are arranged into organs.
1 Yara Saddam Amr Alkhatib Ihsan NOTE: Yellow highlighting=correction/addition to the previous version of the sheet. Histology (micro anatomy) :- the study of tissues and how they are arranged into organs.
Neurogenesis in Adult Central Nervous System: Death of a Dogma
 Aristotle University of Thessaloniki, Greece, Nov. 2007 Neurogenesis in Adult Central Nervous System: Death of a Dogma Anton B. Tonchev Division of Cell Biology, Varna University of Medicine, Bulgaria
Aristotle University of Thessaloniki, Greece, Nov. 2007 Neurogenesis in Adult Central Nervous System: Death of a Dogma Anton B. Tonchev Division of Cell Biology, Varna University of Medicine, Bulgaria
April 29, Neurophysiology. Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University,
 April 29, 2016 Neurophysiology Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University, Cells in the brain Neurons glia 1. Astrocytes 2. Microglia
April 29, 2016 Neurophysiology Chul-Kyu Park, Ph.D. Assistant Professor Department of Physiology, Graduate School of Medicine, Gachon University, Cells in the brain Neurons glia 1. Astrocytes 2. Microglia
Cellular components of CNS
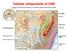 Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Neural stem cells and the neurobiology of ageing. Chen Siyun 1, Dawe G.S. 2
 ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung
 IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung Se-Ran Yang, Samantha Valvo, Hongwei Yao, Aruna Kode, Saravanan Rajendrasozhan, Indika Edirisinghe, Samuel
IKKα Causes Chromatin Modification on Pro-Inflammatory Genes by Cigarette Smoke in Mouse Lung Se-Ran Yang, Samantha Valvo, Hongwei Yao, Aruna Kode, Saravanan Rajendrasozhan, Indika Edirisinghe, Samuel
The Nervous System An overview
 Nervous System The Nervous System An overview Includes Nerve tissue Sense organs Functions to Sense environment Process information it receives Respond to information 1 Copyright 2009 Pearson Education,
Nervous System The Nervous System An overview Includes Nerve tissue Sense organs Functions to Sense environment Process information it receives Respond to information 1 Copyright 2009 Pearson Education,
CHONDROITINASE ABC TREATMENT AND THE PHENOTYPE OF NEURAL
 CHONDROITINASE ABC TREATMENT AND THE PHENOTYPE OF NEURAL PROGENITOR CELLS ISOLATED FROM INJURED RAT SPINAL CORD L.Slovinska 1, I.Novotna 1, D.Cizkova 1 1 Institute of Neurobiology, Center of Excellence,
CHONDROITINASE ABC TREATMENT AND THE PHENOTYPE OF NEURAL PROGENITOR CELLS ISOLATED FROM INJURED RAT SPINAL CORD L.Slovinska 1, I.Novotna 1, D.Cizkova 1 1 Institute of Neurobiology, Center of Excellence,
HISTOLOGY د.عبد الجبار فالح الربيعي
 Nervous System The human Nervous system is the most complex system in the human body, is formed by a network of more than 100 million nerve cells (neurons) assisted by many more glial cells. Anatomically
Nervous System The human Nervous system is the most complex system in the human body, is formed by a network of more than 100 million nerve cells (neurons) assisted by many more glial cells. Anatomically
NERVOUS TISSUE. 1. Functional units of the nervous system; receive, process, store and transmit information to other neurons, muscle cells or glands.
 NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
25/12/50. Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury
 2 Karimi-Abdolrezaee et al. J Neuroscience 26(13):3377-89; (2006) Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury
2 Karimi-Abdolrezaee et al. J Neuroscience 26(13):3377-89; (2006) Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury
Chapter 12 The Nervous System INTRODUCTION TO THE NERVOUS SYSTEM. Central Nervous System (CNS): STRUCTURE BRAIN SPINAL CORD NERVES
 Chapter 12 The Nervous System PowerPoint by John McGill Supplemental Notes by Beth Wyatt INTRODUCTION TO THE NERVOUS SYSTEM STRUCTURE BRAIN SPINAL CORD NERVES Central Nervous System (CNS): Brain Spinal
Chapter 12 The Nervous System PowerPoint by John McGill Supplemental Notes by Beth Wyatt INTRODUCTION TO THE NERVOUS SYSTEM STRUCTURE BRAIN SPINAL CORD NERVES Central Nervous System (CNS): Brain Spinal
Characteristic features of CNS pathology. By: Shifaa AlQa qa
 Characteristic features of CNS pathology By: Shifaa AlQa qa Normal brain: - The neocortex (gray matter): six layers: outer plexiform, outer granular, outer pyramidal, inner granular, inner pyramidal, polymorphous
Characteristic features of CNS pathology By: Shifaa AlQa qa Normal brain: - The neocortex (gray matter): six layers: outer plexiform, outer granular, outer pyramidal, inner granular, inner pyramidal, polymorphous
The Nervous System. PowerPoint Lecture Slides C H A P T E R 7. Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slides Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College C H A P T E R 7 The Nervous System NERVOUS SYSTEM OVERVIEW Essential Question: What are the primary functions
PowerPoint Lecture Slides Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College C H A P T E R 7 The Nervous System NERVOUS SYSTEM OVERVIEW Essential Question: What are the primary functions
Terminology. Terminology. Terminology. Terminology. Terminology. Bromodeoxyuridine
 Kateřina Náměstková, Zuzana Šimonová, Eva Syková Behavioural Brain Research Bromodeoxyuridine : Doublecortin : DCX Glial Fibrillary Acidic Protein : GFAP Trace eye blink conditioning 1 Volume 163 : pp.
Kateřina Náměstková, Zuzana Šimonová, Eva Syková Behavioural Brain Research Bromodeoxyuridine : Doublecortin : DCX Glial Fibrillary Acidic Protein : GFAP Trace eye blink conditioning 1 Volume 163 : pp.
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
POST-INJURY INTERVALS 1
 POST-INJURY INTERVALS 1 Introduction 1 Contusion dating 2 Skin 2 Brain 5 Hypoxic/ischemic injury and increased intracranial pressure 18 Brain incidentals (non-injurious) 21 Sexual violence 27 INTRODUCTION
POST-INJURY INTERVALS 1 Introduction 1 Contusion dating 2 Skin 2 Brain 5 Hypoxic/ischemic injury and increased intracranial pressure 18 Brain incidentals (non-injurious) 21 Sexual violence 27 INTRODUCTION
Pathogenesis of Cervical Myelopathy in Chronic Cervical Cord Compression Model of Rat
 Pathogenesis of Cervical Myelopathy in Chronic Cervical Cord Compression Model of Rat Shigeru Kobayashi,MD,PhD 1, Masafumi Kubota, PT 1, Hisao Iwamoto, MD,PhD 2, Riya Kosaka,MD,PhD 3, Katsuhiko Hayakawa,MD,PhD
Pathogenesis of Cervical Myelopathy in Chronic Cervical Cord Compression Model of Rat Shigeru Kobayashi,MD,PhD 1, Masafumi Kubota, PT 1, Hisao Iwamoto, MD,PhD 2, Riya Kosaka,MD,PhD 3, Katsuhiko Hayakawa,MD,PhD
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Chapter 7 Nerve tissue 1 Liu Jiamei
 Chapter 7 Nerve tissue 1 Liu Jiamei General description: nerve tissue nerve cells (neurons): show numerous long processes receive the stimulation make contact with each other, conduct the nerve impulse
Chapter 7 Nerve tissue 1 Liu Jiamei General description: nerve tissue nerve cells (neurons): show numerous long processes receive the stimulation make contact with each other, conduct the nerve impulse
(3) Chemical synapse ---structure
 (3) Chemical synapse ---structure LM: in silver preparation dark brown color button-liked on the surface of cell body and dendrites called synaptic button LM: synaptic button (3) Chemical synapse ---structure
(3) Chemical synapse ---structure LM: in silver preparation dark brown color button-liked on the surface of cell body and dendrites called synaptic button LM: synaptic button (3) Chemical synapse ---structure
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
NACC Vascular Consortium. NACC Vascular Consortium. NACC Vascular Consortium
 NACC Vascular Consortium NACC Vascular Consortium Participating centers: Oregon Health and Science University ADC Rush University ADC Mount Sinai School of Medicine ADC Boston University ADC In consultation
NACC Vascular Consortium NACC Vascular Consortium Participating centers: Oregon Health and Science University ADC Rush University ADC Mount Sinai School of Medicine ADC Boston University ADC In consultation
Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided
 Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided into: CNS (central nervous system) = brain + spinal cord
Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided into: CNS (central nervous system) = brain + spinal cord
Glial Cells: The Other Cells of the Nervous System
 Glial Cells: The Other Cells of the Nervous System 2. Astrocytes - Star Performers in the Neural Tissue Medha S Rajadhyaksha and Daya Manghani Medha S Rajadhyaksha is a reader in life sciences at Sophia
Glial Cells: The Other Cells of the Nervous System 2. Astrocytes - Star Performers in the Neural Tissue Medha S Rajadhyaksha and Daya Manghani Medha S Rajadhyaksha is a reader in life sciences at Sophia
Unit I Problem 9 Histology: Basic Tissues of The Body
 Unit I Problem 9 Histology: Basic Tissues of The Body - What is the difference between cytology and histology? Cytology: it is the study of the structure and functions of cells and their contents. Histology:
Unit I Problem 9 Histology: Basic Tissues of The Body - What is the difference between cytology and histology? Cytology: it is the study of the structure and functions of cells and their contents. Histology:
Nervous Tissue Mediates Perception and Response *
 OpenStax-CNX module: m46057 1 Nervous Tissue Mediates Perception and Response * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end
OpenStax-CNX module: m46057 1 Nervous Tissue Mediates Perception and Response * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end
ApoE and S-100 expression and its significance in the brain tissue of rats with focal contusion
 ApoE and S-100 expression and its significance in the brain tissue of rats with focal contusion Z.L. Wang 1 *, R.F. Chai 2 *, W.S. Yang 3, Y. Liu 1, H. Qin 1, H. Wu 1, X.F. Zhu 1, Y.X. Wang 1 and G. Dangmurenjiafu
ApoE and S-100 expression and its significance in the brain tissue of rats with focal contusion Z.L. Wang 1 *, R.F. Chai 2 *, W.S. Yang 3, Y. Liu 1, H. Qin 1, H. Wu 1, X.F. Zhu 1, Y.X. Wang 1 and G. Dangmurenjiafu
Histology of the CNS
 Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Development of Spinal Cord & Vertebral Column. Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla
 Development of Spinal Cord & Vertebral Column Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla OBJECTIVES At the end of the lecture, students should be able to: q Describe the development of the spinal cord
Development of Spinal Cord & Vertebral Column Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla OBJECTIVES At the end of the lecture, students should be able to: q Describe the development of the spinal cord
removed replaced inflammation scar tissue
 HOMEOSTASIS Normal maintenance and renewal of differentiated cells in many tissues This does NOT involve leukocytes. Leukocytes and inflammation occurs in response to damage NEED FOR REPAIR When tissue
HOMEOSTASIS Normal maintenance and renewal of differentiated cells in many tissues This does NOT involve leukocytes. Leukocytes and inflammation occurs in response to damage NEED FOR REPAIR When tissue
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
12 Anatomy and Physiology of Peripheral Nerves
 12 Anatomy and Physiology of Peripheral Nerves Introduction Anatomy Classification of Peripheral Nerves Sensory Nerves Motor Nerves Pathologies of Nerves Focal Injuries Regeneration of Injured Nerves Signs
12 Anatomy and Physiology of Peripheral Nerves Introduction Anatomy Classification of Peripheral Nerves Sensory Nerves Motor Nerves Pathologies of Nerves Focal Injuries Regeneration of Injured Nerves Signs
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer
 Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
2015 Diagnostic Slide Session
 2015 Diagnostic Slide Session Case 3 R.S. Tashjian, MD A.M. Langer-Gould, MD S. Natarajan, MD B.K. Kleinschmidt-DeMasters, MD H.V. Vinters, MD Disclosures No financial disclosures or conflicts of interest
2015 Diagnostic Slide Session Case 3 R.S. Tashjian, MD A.M. Langer-Gould, MD S. Natarajan, MD B.K. Kleinschmidt-DeMasters, MD H.V. Vinters, MD Disclosures No financial disclosures or conflicts of interest
Chapter 12b. Overview
 Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Rewiring of hindlimb corticospinal neurons after spinal cord injury
 Rewiring of hindlimb corticospinal neurons after spinal cord injury Arko Ghosh, Florent Haiss, Esther Sydekum, Regula Schneider, Miriam Gullo, Matthias T. Wyss, Thomas Mueggler, Christof Baltes, Markus
Rewiring of hindlimb corticospinal neurons after spinal cord injury Arko Ghosh, Florent Haiss, Esther Sydekum, Regula Schneider, Miriam Gullo, Matthias T. Wyss, Thomas Mueggler, Christof Baltes, Markus
Effect of rolipram-loaded polymeric micelle nanoparticle on camp level in hypoxia condition and in rat SCI model
 Effect of rolipram-loaded polymeric micelle nanoparticle on camp level in hypoxia condition and in rat SCI model 1 Christian Macks, 1 So Jung Gwak, PhD, 2 Michael Lynn, MD, 1 JeoungSoo Lee, PhD. 1 Bioengineering,
Effect of rolipram-loaded polymeric micelle nanoparticle on camp level in hypoxia condition and in rat SCI model 1 Christian Macks, 1 So Jung Gwak, PhD, 2 Michael Lynn, MD, 1 JeoungSoo Lee, PhD. 1 Bioengineering,
Introduction. Acute sodium overload produces renal tubulointerstitial inflammation in normal rats
 Acute sodium overload produces renal tubulointerstitial inflammation in normal rats MI Roson, et al. Kidney International (2006) Introduction Present by Kanya Bunnan and Wiraporn paebua Tubular sodium
Acute sodium overload produces renal tubulointerstitial inflammation in normal rats MI Roson, et al. Kidney International (2006) Introduction Present by Kanya Bunnan and Wiraporn paebua Tubular sodium
Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall
 Histol Histopath (1 990) 5: 161-1 67 Histology and Histopathology Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall Reinhold Kittelberger,
Histol Histopath (1 990) 5: 161-1 67 Histology and Histopathology Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall Reinhold Kittelberger,
Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma
 Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma Y.-J. Hu 1, X.-Y. Luo 2, Y. Yang 3, C.-Y. Chen 1, Z.-Y. Zhang 4 and X. Guo 1 1 Department
Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma Y.-J. Hu 1, X.-Y. Luo 2, Y. Yang 3, C.-Y. Chen 1, Z.-Y. Zhang 4 and X. Guo 1 1 Department
The cells of the nervous system
 The cells of the nervous system LESSON N.9 - PSYCHOBIOLOGY because of the location and volume as compared to our body, the brain has always been a matter of conjecture about its fundamental role in the
The cells of the nervous system LESSON N.9 - PSYCHOBIOLOGY because of the location and volume as compared to our body, the brain has always been a matter of conjecture about its fundamental role in the
