For Health Professionals Who Care For Cancer Patients EDITOR S CHOICE
|
|
|
- Mariah Byrd
- 5 years ago
- Views:
Transcription
1 Inside This Issue: For Health Professionals Who Care For Cancer s March 2019 Vol. 22 No. 3 Editor s Choice New Programs: Rituximab Protocols in CLL/SLL Updated (LYCLLBENDR, LYCLLCHLR, LYCLLCVPR, LYCLLFLUDR, LYFCR); Revised Programs: Expanded Eligibility for Ibrutinib in the First-Line Treatment of CLL/SLL Drug Update Medical Assistance Programs Updated Communities Oncology Network OSCAR Billing Deadline Benefit Drug List New: LYCLLBENDR, LYCLLCHLR, LYCLLCVPR, LYCLLFLUDR, Pediatric Protocol; Revised: LYFCR, LYASPMEDEX, LYSMILE, LYBEND, LYBENDR, LYCLLBEND, LYCLLFBR; Deleted: GIEFUPRT Cancer Drug Manual New: AGS-16C3F; Revised: Cetuximab, Interferon, Lenalidomide List of New and Revised Protocols, Provincial Pre-Printed Orders and s New: LYCHOP, LYCLLBENDR, LYCLLCHLR, LYCLLCVPR, LYCLLFLUDR, LYCVP; Revised: GIAJCAPOX, GIAJFFOX, GIAVTZCAP, GICAPIRI, GICAPOX, GICART, GICOXB, GIEFFOXRT, GIENDO2, GIFFOXB, GIFIRINOX, GIFOLFIRI, GIFOLFOX, GIGAVCC, GIGAVCOX, GIGAVFFOXT, GIPE, GOBEP, GOCXCATB, GOEP, UGOOVBEVG, GOOVPLDC, GOTDEMACO, GOTDLR, GUBEP, GUEP, GUSCPERT, UGUTAXGEM, UGUTIP, GUVEIP, GUVIP2, HNLACETRT, HNNAVFUFA, UHNOTLEN, LKATOATRA, LKATOP, ULKMDSA, ULKMDSL, LUAVPEM, LUSCPERT, LYABVD, LYASPMEDEX, LYBEND, LYBENDR, LYCHLRR, LYCHOPR, LYCLLBEND, LYCLLFBR, LYCODOXMR, LYCVPR, LYFCR, ULYFIBRU, LYFLUDR, ULYMIBRU, LYRITUX, LYRMTN, LYSMILE, UMYCARDEX, UMYCARLD, UMYDARBD, UMYDARLD, UMYLDR, UMYDREL, UMYLENMTN, MYMPBOR, UMYPOMDEX, SAAVGS, USANADENO, SCIMMUNE Website Resources and Contact Information EDITOR S CHOICE NEW PROGRAMS Effective 01 March 2019, the BC Cancer Provincial Systemic Therapy Program has approved the following treatment programs: Lymphoma: All Rituximab-Containing Chemotherapy Protocols for CLL/SLL Updated (LYCLLBENDR, LYCLLCHLR, LYCLLCVPR, LYCLLFLUDR, LYFCR) Previously, all BC Cancer rituximab-containing protocols used the rituximab dose of 375 mg/m 2 IV (or 1400 mg SC) given every cycle, which is the standard approved dosing for non-hodgkin Lymphoma. However, evidence suggests that increasing rituximab dosing to 500 mg/m 2 IV (or 1600 mg SC) for cycle 2 and beyond is preferred in CLL/SLL patients. 1-3 To align with this best evidence-based practice, separate rituximab-containing chemotherapy protocols have been created for CLL/SLL to reflect this modified dosing rituximab 375 mg/m 2 IV in cycle 1, followed by 500 mg/m 2 IV (or 1600 mg SC) in cycle 2 and beyond. 1-3 References: 1. O Brien SM, Kantarjian H, Thomas DA, et al: Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 2001;19: Hallek M, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 1
2 EDITOR S CHOICE open-label, phase 3 trial. Lancet 2010;376: Robak T, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol 2010;28: REVISED PROGRAMS Effective 01 March 2019, the BC Cancer Provincial Systemic Therapy Program has revised the following treatment program: Lymphoma: Expanded Eligibility for Ibrutinib in the First-Line Treatment of Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) (ULYFIBRU) Ibrutinib had previously been restricted at BC Cancer for the first-line treatment of CLL/SLL patients with chromosome 17p deletion. 1 In addition to this highrisk cohort, the Lymphoma Tumour Group has now expanded the eligibility of ibrutinib to include previously untreated CLL/SLL patients who are ineligible for fludarabine therapy (FCR). FCR ineligibility is defined as patients over 65 years of age and/or a strong clinical reason that the patient is ineligible for FCR. In a phase III randomized controlled trial, ibrutinib monotherapy demonstrated improved 2-year progression-free survival (PFS) compared to bendamustine plus rituximab (87% vs. 74%, HR % CI ). 2 At a median follow-up of 38 months, the median PFS was not yet reached for ibrutinib. There was no difference in median overall survival (OS) between treatment groups. Additionally, this trial included a third treatment arm involving ibrutinib plus rituximab, which demonstrated no improved PFS or OS compared to ibrutinib alone. While ibrutinib exhibited higher rates of grades 3 and 4 nonhematological toxicities (74% versus 63%), particularly atrial fibrillation, hypertension, and bleeding, bendamustine exhibited higher rates of grades 3 and 4 hematological toxicities (61% versus 41%). 2 References: 1. Burger JA, Tedeschi A, Barr PM et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015;373: Woyach JA, Rupper AS, Heerema NA et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 2018;379: DRUG UPDATE MEDICAL PATIENT ASSISTANCE PROGRAMS UPDATED The listing of oncology medical patient assistance programs offered by pharmaceutical companies has been and can be found at: *Located on the BC Cancer Systemic Therapy website under Health Professionals > Systemic Therapy > Reimbursement & Forms BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 2
3 COMMUNITIES ONCOLOGY NETWORK OSCAR BILLING DEADLINE APRIL 4, 2019 The fiscal year will end on Sunday, March 31, To meet the deadlines for external reporting to the Ministry of Health, all claims for drug reimbursement for the fiscal year must be invoiced by 11:59 pm on Thursday April 4, 2019 via OSCAR (Online System for Cancer drugs Adjudication and Reimbursement). Any claims invoiced after this date will not be eligible for reimbursement. For more information, please contact NEW PROGRAMS BENEFIT DRUG LIST Effective 01 March 2019, the following treatment programs have been added to the BC Cancer Benefit Drug List: Protocol Code Benefit Status Treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma with Bendamustine and Rituximab LYCLLBENDR Class I Treatment of Chronic Lymphocytic Leukemia with Chlorambucil and Rituximab LYCLLCHLR Class I Treatment of Relapsed Chronic Lymphocytic Leukemia using Cyclophosphamide, Vincristine, Prednisone and Rituximab LYCLLCVPR Class I Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and Rituximab LYCLLFLUDR Class I Treatment of Recurrent/Refractory Rhabdomysarcoma in Pediatric s with Cyclophosphamide, Vinorelbine and Temsirolimus on the COG ARST0921 Protocol N/A [Pediatric Protocol] Class I REVISED PROGRAMS Effective 01 March 2019, subcutaneous rituximab injectable 1600 mg (RITUXAN SC) has been added to the BC Cancer Benefit Drug List for the following protocol: Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine, Cyclophosphamide and Rituximab Protocol Code LYFCR BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 3
4 BENEFIT DRUG LIST Effective 01 March 2019, pegaspargase has been added to the BC Cancer Benefit Drug List for the following protocols: Treatment of Refractory or Relapsing Extranodal Natural Killer or T-Cell Lymphoma using Pegaspargase, Methotrexate and Dexamethasone Treatment of Natural Killer or T-Cell Lymphoma using Dexamethasone, Methotrexate, Ifosfamide, Pegaspargase and Etoposide Protocol Code LYASPMEDEX LYSMILE Effective 01 March 2019, bendamustine-based protocols have been changed to Class I status on the BC Cancer Benefit Drug List: Treatment of Non-Hodgkin Lymphoma with Bendamustine Treatment of Non-Hodgkin Lymphoma with Bendamustine and Rituximab Treatment of Relapsed Chronic Lymphocytic Leukemia with Bendamustine Treatment of Previously Untreated Chronic Lymphocytic Leukemia with Bendamustine and Rituximab Protocol Code LYBEND LYBENDR LYCLLBEND LYCLLFBR DELETED PROGRAMS Effective 01 March 2019, the following treatment program has been deleted from the BC Cancer Benefit Drug List: Combined Modality Therapy for Locally Advanced Esophageal Cancer using Fluorouracil and Cisplatin Protocol Code GIEFUPRT BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 4
5 NEW MONOGRAPHS AND PATIENT HANDOUTS CANCER DRUG MANUAL The following drug is NOT a BC Cancer Benefit Drug and requires application to the BC Cancer Compassionate Access Program (CAP). Its corresponding Interim Monograph is made available for reference only. All BC Cancer drug monographs and patient handouts can be accessed on the Cancer Drug Manual Drug Index. AGS-16C3F Interim Monograph has been developed. AGS-16C3F is an antibody-drug conjugate comprised of a fully human monoclonal antibody conjugated to a microtubule disrupting agent. Outside of clinical trials, AGS-16C3F is only available through the Health Canada Special Access Programme (SAP). The usual dosing is 1.8 mg/kg IV given in a three-weekly schedule. AGS-16C3F has now been added to the Chemotherapy Preparation and Stability Chart and the Hazardous Drug List. Highlights from this document: Visual and ocular disorders are reported in approximately 85% of patients. The most frequently reported events include dry eyes, blurry vision and keratitis. However, diplopia, reduced acuity, photophobia, eye pain/irritation/swelling, and other ocular toxicities have also been reported. Infusion reactions are reported in up to 24% of patients. Reactions are mainly grades 1 to 2 in severity and may include pruritus, flushing, upper body rash or fever. Following a reaction, suitable premedication should be used prior to each subsequent infusion. REVISED MONOGRAPHS AND PATIENT HANDOUTS Highlights of key changes and/or updates to the Monographs, s and Chemotherapy Preparation and Stability Chart (CPSC) are listed below: Cetuximab CPSC: Update manufacturer from BMS to Eli Lilly Interferon Monograph and CPSC: Supply and Storage, CPSC deleted multidose pens and 18 MU vial of lyophilized powder as they are no longer available in Canada Lenalidomide Monograph and : Side Effects added progressive multifocal encephalopathy (PML) Dosing references and added BC Cancer protocols added PML to bullets in Emergency section BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 5
6 LIST OF REVISED PROTOCOLS, PRE-PRINTED ORDERS AND PATIENT HANDOUTS BC Cancer Protocol Summaries, Provincial Pre-Printed Orders (PPPOs) and s are revised periodically. New, revised or deleted protocols, PPPOs and patient handouts for this month are listed below. Protocol codes for treatment requiring BC Cancer Compassionate Access Program approval are prefixed with the letter U. NEW PROTOCOLS, PPPOS AND PATIENT HANDOUTS (AFFECTED DOCUMENTS ARE CHECKED) LYCHOP Treatment of Lymphoma with Doxorubicin, Cyclophosphamide, Vincristine and Prednisone LYCLLBENDR LYCLLCHLR LYCLLCVPR LYCLLFLUDR LYCVP Treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma with Bendamustine and Rituximab Treatment of Chronic Lymphocytic Leukemia with Chlorambucil and Rituximab Treatment of Relapsed Chronic Lymphocytic Leukemia using Cyclophosphamide, Vincristine, Prednisone and Rituximab Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and Rituximab Treatment of Advanced Indolent Lymphoma using Cyclophosphamide, Vincristine, Prednisone GIAJCAPOX Eligibility, Tests, Treatment, Contact Information, Side Effects management Adjuvant combination chemotherapy for stage III and stage IIB colon cancer using oxaliplatin and capecitabine GIAJFFOX GIAVTZCAP Eligibility, Tests, Treatment, Contact Information, Side Effects management, Eligibility, Contact Physician Adjuvant Combination Chemotherapy for Stage III and Stage IIB Colon Cancer using Oxaliplatin, Fluorouracil, and Leucovorin Palliative Therapy of Metastatic Neuroendocrine Cancer using Temozolomide and Capecitabine BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 6
7 GICAPIRI and Logo, Eligibility, Tests, Treatment, Contact Information Metastatic Colorectal Cancer using Irinotecan and Capecitabine in s Unsuitable for GIFOLFIRI GICAPOX and Logo, Eligibility, Tests, Treatment, Contact Information, Side Effects management Metastatic Colorectal Cancer using Oxaliplatin, and Capecitabine GICART Minor typo corrected GICOXB Treatment Combined Modality Therapy for Carcinoma of the Anal Canal using Mitomycin, Capecitabine and Radiation Therapy Metastatic Colorectal Cancer using Oxaliplatin, Bevacizumab and Capecitabine GIEFFOXRT and Logo, Eligibility, Treatment, Contact Information Combined Modality Therapy for Locally Advanced Esophageal Cancer using Oxaliplatin, Fluorouracil, Leucovorin, and Radiation Therapy GIENDO2 Eligibility GIFFOXB Treatment Palliative Therapy for Pancreatic Endocrine Tumours using Streptozocin and Doxorubicin Metastatic Colorectal Cancer using Oxaliplatin, Fluorouracil, Leucovorin, and Bevacizumab GIFIRINOX GIFOLFIRI GIFOLFOX GIGAVCC Eligibility, Tests, Treatment, Contact Information Eligibility, Tests, Treatment, Contact Information, Side Effects management Eligibility, Treatment, Contact Information and Logo, Title, Eligibility, Treatment Advanced Pancreatic Adenocarcinoma using Irinotecan, Oxaliplatin, Fluorouracil and Leucovorin Metastatic Colorectal Cancer using Irinotecan, Fluorouracil and Leucovorin Metastatic Colorectal Cancer using Oxaliplatin, Fluorouracil, and Leucovorin Metastatic or Locally Advanced Gastric, Gastroesophageal Junction Adenocarcinoma, or Esophageal Squamous Cell Carcinoma using Cisplatin and Capecitabine BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 7
8 GIGAVCOX Eligibility Palliative Treatment of Metastatic or Locally Advanced Gastric, Gastroesophageal Junction, or Esophageal Adenocarcinoma using Capecitabine, and Oxaliplatin GIGAVFFOXT Minor typo corrected Palliative Treatment of Metastatic or Locally Advanced Gastric, Gastroesophageal Junction, or Esophageal Adenocarcinoma using Oxaliplatin, Fluorouracil, Leucovorin, and Trastuzumab GIPE GOBEP GOCXCATB GOEP, Tests, Carboplatin Dosing, Test, Test, Test Palliative Therapy of Neuroendocrine Tumours using Cisplatin and Etoposide Therapy of Non-Dysgerminomatous Ovarian Germ Cell Cancer using Bleomycin, Etoposide, and Cisplatin Treatment of Metastatic or Recurrent Cancer of the Cervix with Bevacizumab, Carboplatin and Paclitaxel Therapy of Dysgerminomatous Ovarian Germ Cell Cancer using Cisplatin and Etoposide UGOOVBEVG Tests Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Gemcitabine GOOVPLDC, Test, Hypersensitivity information added Treatment of Epithelial Ovarian Cancer Relapsing After Primary Treatment using Doxorubicin Pegylated Liposomal (CAELYX) and Carboplatin GOTDEMACO Test GOTDLR Test GUBEP Test Therapy for High Risk Gestational Trophoblastic Neoplasia using Etoposide, Methotrexate, Leucovorin (Folinic Acid), Dactinomycin, Cyclophosphamide and Vincristine Therapy for Low Risk Gestational Trophoblastic Cancer using Dactinomycin and Methotrexate Curative Therapy for Germ Cell Cancer using Bleomycin, Etoposide, Cisplatin GUEP GUSCPERT, Test Cisplatin infusion time Etoposide-Cisplatin Protocol for Germ Cell Cancers Therapy of Genitourinary Small Cell Tumors with a Platinum and Etoposide with Radiation BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 8
9 UGUTAXGEM, Test Palliative Therapy for Germ Cell Cancers using Paclitaxel and Gemcitabine UGUTIP GUVEIP GUVIP2 HNLACETRT HNNAVFUFA UHNOTLEN LKATOATRA LKATOP, Test, Test, Test Magnesium supplement Weekly treatment Dispensed quantity, Tests, Premedication, Support Medications, Tests, Premedication, Support Medications Advanced Therapy for Relapsed Testicular Germ Cell Cancer using Paclitaxel, Ifosfamide and Cisplatin Consolidation and Salvage Treatment for Germ Cell Cancer using Vinblastine, Cisplatin, Ifosfamide and Mesna Consolidation and Salvage Therapy for Nonseminoma using Etoposide, Cisplatin, Ifosfamide and Mesna Combined Cetuximab and Radiation Treatment for Locally Advanced Squamous Cell Carcinoma of the Head and Neck Treatment of Recurrent or Metastatic Nasopharyngeal Cancer with Fluorouracil and Leucovorin Therapy for Locally Recurrent or Metastatic, RAI- Refractory Differentiated Thyroid Cancer using Lenvatinib First-Line Induction and Consolidation Therapy of Acute Promyelocytic Leukemia using Arsenic Trioxide and Tretinoin (All-Trans Retinoic Acid) First-Line Induction and Consolidation Therapy of Acute Promyelocytic Leukemia using Arsenic Trioxide, Tretinoin (All-Trans Retinoic Acid) and Daunorubicin ULKMDSA Tests Therapy of Myelodysplastic Syndrome using Azacitidine ULKMDSL LUAVPEM LUSCPERT Dose Modification Treatment duration, Cisplatin infusion time Therapy of Myelodysplastic Syndrome using Lenalidomide Second-Line Chemotherapy of Advanced Non- Small Cell Lung Cancer with Pemetrexed Therapy of Limited Stage Small Cell Lung Cancer using Cisplatin and Etoposide with Radiation BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 9
10 LYABVD Tests and Treatment cycles Treatment of Hodgkin Lymphoma with Doxorubicin, Bleomycin, Vinblastine, and Dacarbazine LYASPMEDEX LYBEND LYBENDR Title, Tests, Supportive Medications, and Treatment drugs Eligibility, Protocol Code and Institutional name Eligibility, Protocol Code and Institutional name Treatment of Refractory or Relapsing Extranodal Natural Killer or T-Cell Lymphoma using Pegaspargase, Methotrexate and Dexamethasone Treatment of Non-Hodgkin Lymphoma with Bendamustine Treatment of Non-Hodgkin Lymphoma with Bendamustine and Rituximab LYCHLRR Eligibility Treatment of Indolent B-Cell Lymphoma and Chronic Lymphocytic Leukemia with Chlorambucil and Rituximab LYCHOPR LYCLLBEND LYCLLFBR LYCODOXMR Treatment cycles Eligibility and Protocol Code Eligibility, Protocol Code, Bendamustine Dose and Contact Physician, Tests and Cyclophosphami de infusion time Treatment of Lymphoma with Doxorubicin, Cyclophosphamide, Vincristine, Prednisone and Rituximab Treatment of Relapsed Chronic Lymphocytic Leukemia with Bendamustine Treatment of Previously Untreated Chronic Lymphocytic Leukemia with Bendamustine and Rituximab Treatment of Burkitt Lymphoma and Leukemia with Cyclophosphamide, Vincristine, Doxorubicin, Methotrexate, Leucovorin (CODOX-M) and Rituximab LYCVPR Eligibility Treatment of Advanced Indolent Lymphoma using Cyclophosphamide, Vincristine, Prednisone and Rituximab LYFCR Rituximab dosing, Eligibility, Reference and note on renal function Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine, Cyclophosphamide and Rituximab BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 10
11 ULYFIBRU Eligibility and Title Treatment of Previously Untreated Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma using Ibrutinib LYFLUDR Eligibility ULYMIBRU Eligibility LYRITUX Minor typo corrected Treatment Relapsed Indolent Lymphoma with Fludarabine and Rituximab Treatment of Relapsed/Refractory Mantle-Cell Lymphoma using Ibrutinib Treatment of Lymphoma with Single Agent Rituximab LYRMTN Eligibility Maintenance Rituximab for Indolent Lymphoma LYSMILE UMYCARDEX UMYCARLD UMYDARBD Title, Tests, Supportive Medications, and Treatment drugs Pre-treatment metrics Pre-treatment metrics Eligibility, Tests and Dexamethasone dose, minor typo corrected Treatment of Natural Killer or T-Cell Lymphoma using Dexamethasone, Methotrexate, Ifosfamide, Pegaspargase and Etoposide Therapy of Multiple Myeloma using Carfilzomib and Dexamethasone with or without Cyclophosphamide Therapy of Multiple Myeloma using Carfilzomib, Lenalidomide with Dexamethasone Treatment of Relapsed and Refractory Multiple Myeloma with Daratumumab in Combination with Bortezomib and Dexamethasone with or without Cyclophosphamide UMYDARLD Eligibility, Tests, Treatment section and Dexamethasone dose, minor typo corrected Treatment of Relapsed and Refractory Multiple Myeloma with Daratumumab in Combination with Lenalidomide and Dexamethasone UMYLDF UMYLDREL UMYLENMTN Abbreviations corrected Abbreviations corrected Abbreviations corrected Treatment of Previously Untreated Multiple Myeloma and Not Eligible for Stem Cell Transplant using Lenalidomide with Low-dose Dexamethasone Therapy of Relapsed Multiple Myeloma using Lenalidomide with Dexamethasone Maintenance Therapy of Multiple Myeloma using Lenalidomide BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 11
12 MYMPBOR Dexamethasone option added Treatment of Multiple Myeloma using Melphalan, Prednisone and Weekly Bortezomib with the Option of Substituting Cyclophosphamide for Melphalan UMYPOMDEX Abbreviations corrected Therapy of Multiple Myeloma using Pomalidomide with Dexamethasone SAAVGS Minor typo corrected Second Line Treatment of Advanced C-Kit Positive Gastrointestinal Stromal Cell Tumours after Imatinib using Sunitinib USANADENO, Test Denosumab for Neoadjuvant Use in s with Non-Metastatic Operable Giant Cell Tumour of the Bone SCIMMUNE Alert card added Management of Immune-Mediated Adverse Reactions to Checkpoint Inhibitors Immunotherapy BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 12
13 WEBSITE RESOURCES AND CONTACT INFORMATION CONTACT INFORMATION PHONE FAX Systemic Therapy Update Editor x bulletin@bccancer.bc.ca Provincial Systemic Therapy Program x mlin@bccancer.bc.ca To update contact information of any CON sites, please contact: bulletin@bccancer.bc.ca Oncology Drug Information druginfo@bccancer.bc.ca Nurse Educators x nursinged@bccancer.bc.ca Library/Cancer Information Toll Free x 8003 requests@bccancer.bc.ca Pharmacy Professional Practice x mlin@bccancer.bc.ca Provincial Professional Practice Nursing BCCancerPPNAdmin@ehcnet.phsa.ca OSCAR oscar@bccancer.bc.ca Compassionate Access Program (CAP) cap_bcca@bccancer.bc.ca Pharmacy Oncology Certification x rxchemocert@bccancer.bc.ca BC Cancer-Abbotsford BC Cancer-Prince George (Centre for the North) BC Cancer-Surrey BC Cancer-Kelowna BC Cancer-Vancouver BC Cancer-Victoria Toll Free Toll Free Toll Free Toll Free Toll Free Toll Free EDITORIAL REVIEW BOARD Sally Waignein, PharmD (Editor) Mario de Lemos, PharmD, MSc(Oncol) Caroline Lohrisch, MD Ava Hatcher, RN, MN, CON(c) Naren Bollipalli, BSc(Pharm) BC Cancer Provincial Systemic Therapy Program Update March 2019 Vol. 22 No. 3 Page 13
For Health Professionals Who Care For Cancer Patients EDITOR S CHOICE
 Inside This Issue: For Health Professionals Who Care For Cancer Patients April 2019 Vol. 22 No. 4 Editor s Choice New Programs: Ipilimumab-Nivolumab Combination for Metastatic Melanoma (USMAVIPNI) Drug
Inside This Issue: For Health Professionals Who Care For Cancer Patients April 2019 Vol. 22 No. 4 Editor s Choice New Programs: Ipilimumab-Nivolumab Combination for Metastatic Melanoma (USMAVIPNI) Drug
For Health Professionals Who Care For Cancer Patients
 March 2018 Volume 21, No. 3 For Health Professionals Who Care For Cancer s Inside This Issue: Editor s Choice New Programs: Nivolumab for Squamous Cell Cancer of the Head and Neck, Ibrutinib for Mantle-Cell
March 2018 Volume 21, No. 3 For Health Professionals Who Care For Cancer s Inside This Issue: Editor s Choice New Programs: Nivolumab for Squamous Cell Cancer of the Head and Neck, Ibrutinib for Mantle-Cell
For Health Professionals Who Care For Cancer Patients
 April 2018 Volume 21, No. 4 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Palbociclib for Metastatic Breast Cancer, Adjuvant Capecitabine for Breast
April 2018 Volume 21, No. 4 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Palbociclib for Metastatic Breast Cancer, Adjuvant Capecitabine for Breast
For Health Professionals Who Care For Cancer Patients
 February 2018 Volume 21, No. 2 For Health Professionals Who Care For Cancer s Inside This Issue: Editor s Choice New Programs: Everolimus for Neuroendocrine Tumours, Pembrolizumab for Non-Small Cell Lung
February 2018 Volume 21, No. 2 For Health Professionals Who Care For Cancer s Inside This Issue: Editor s Choice New Programs: Everolimus for Neuroendocrine Tumours, Pembrolizumab for Non-Small Cell Lung
For Health Professionals Who Care For Cancer Patients EDITOR S CHOICE
 Inside This Issue: For Health Professionals Who Care For Cancer s September 2018 Vol. 21 No. 9 Editor s Choice New Programs: Carboplatin with CAELYX for First-Line Treatment of Epithelial Ovarian Cancer
Inside This Issue: For Health Professionals Who Care For Cancer s September 2018 Vol. 21 No. 9 Editor s Choice New Programs: Carboplatin with CAELYX for First-Line Treatment of Epithelial Ovarian Cancer
For Health Professionals Who Care For Cancer Patients
 May 2014 Volume 17, Number 5 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Breast Trastuzumab Emtansine for Metastatic Breast Cancer (UBRAVKAD);
May 2014 Volume 17, Number 5 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Breast Trastuzumab Emtansine for Metastatic Breast Cancer (UBRAVKAD);
For Health Professionals Who Care For Cancer Patients EDITOR S CHOICE
 Inside This Issue: For Health Professionals Who Care For Cancer Patients February 2019 Vol. 22 No. 2 Editor s Choice New Programs: Daratumumab in Combination with Bortezomib (UMYDARBD) or Lenalidomide
Inside This Issue: For Health Professionals Who Care For Cancer Patients February 2019 Vol. 22 No. 2 Editor s Choice New Programs: Daratumumab in Combination with Bortezomib (UMYDARBD) or Lenalidomide
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
For Health Professionals Who Care For Cancer Patients
 July 2017 Volume 20, No. 7 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice Drug Update: Shortage of Vinorelbine and Parenteral Sodium Bicarbonate New Programs:
July 2017 Volume 20, No. 7 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice Drug Update: Shortage of Vinorelbine and Parenteral Sodium Bicarbonate New Programs:
For Health Professionals Who Care For Cancer Patients
 March 2017 Volume 20, Number 3 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Nivolumab for Advanced Renal Cell Carcinoma (UGUAVNIV), Nivolumab for
March 2017 Volume 20, Number 3 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Nivolumab for Advanced Renal Cell Carcinoma (UGUAVNIV), Nivolumab for
For Health Professionals Who Care For Cancer Patients
 August 2016 Volume 19, Number 8 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: EMA-CO for High-Risk Gestational Trophoblastic Neoplasia (GOTDEMACO);
August 2016 Volume 19, Number 8 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: EMA-CO for High-Risk Gestational Trophoblastic Neoplasia (GOTDEMACO);
For Health Professionals Who Care For Cancer Patients
 February 2014 Volume 17, Number 2 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice Highlights of Changes in Protocols, PPOs, and Patient Handouts: Reclassification
February 2014 Volume 17, Number 2 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice Highlights of Changes in Protocols, PPOs, and Patient Handouts: Reclassification
I NSIDE THIS ISSUE. Volume 3, Number 6 for health professionals who care for cancer patients June 2000
 Volume 3, Number 6 for health professionals who care for cancer patients June 2000 I NSIDE THIS ISSUE Alert! GUEP Pre-printed Order Misinterpretation, LUCAV, LUALTE, LUALTL Pre-printed Order Error Protocol
Volume 3, Number 6 for health professionals who care for cancer patients June 2000 I NSIDE THIS ISSUE Alert! GUEP Pre-printed Order Misinterpretation, LUCAV, LUALTE, LUALTL Pre-printed Order Error Protocol
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
National Horizon Scanning Centre. Temsirolimus (Torisel) for mantle cell lymphoma - relapsed and/or refractory. January 2008
 Temsirolimus (Torisel) for mantle cell lymphoma - relapsed and/or refractory January 2008 This technology summary is based on information available at the time of research and a limited literature search.
Temsirolimus (Torisel) for mantle cell lymphoma - relapsed and/or refractory January 2008 This technology summary is based on information available at the time of research and a limited literature search.
ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
I NSIDE THIS ISSUE. CEF expanded eligibility Effective March 5, 2001, the adjuvant program for
 Volume 4, Number 3 for health professionals who care for cancer patients March 2001 Available on website http://bccancer.com/providerhome.cfm I NSIDE THIS ISSUE Benefit Drug List - CEF expanded eligibility,
Volume 4, Number 3 for health professionals who care for cancer patients March 2001 Available on website http://bccancer.com/providerhome.cfm I NSIDE THIS ISSUE Benefit Drug List - CEF expanded eligibility,
I NSIDE THIS ISSUE. Volume 2, Number 9 for health professionals who care for cancer patients Nov 1999
 Volume 2, Number 9 for health professionals who care for cancer patients Nov 1999 I NSIDE THIS ISSUE Protocol Update ANTIEM-A, ANTIEM-B, UBRAVTR, CNCCV, UCNIME, CNVCD2, GIFUA, GIFUC, GOOVMHI, GOSMCC2,
Volume 2, Number 9 for health professionals who care for cancer patients Nov 1999 I NSIDE THIS ISSUE Protocol Update ANTIEM-A, ANTIEM-B, UBRAVTR, CNCCV, UCNIME, CNVCD2, GIFUA, GIFUC, GOOVMHI, GOSMCC2,
National Cancer Drugs Fund List - Approved
 National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
National Cancer Drugs Fund List - Approved DRUG Abiraterone Aflibercet Albumin Bound Paclitaxel Axitinib CDF INDICATION (EXCLUDING APPROVED CRITERIA ) Metastatic Prostate Cancer Metastatic Colorectal Cancer
New Evidence reports on presentations given at EHA/ICML Bendamustine in the Treatment of Lymphoproliferative Disorders
 New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal drainage, after hepatic resection, 159 160 Ablation, radiofrequency, for hepatocellular carcinoma, 160 161 Adenocarcinoma, pancreatic.
Index Note: Page numbers of article titles are in boldface type. A Abdominal drainage, after hepatic resection, 159 160 Ablation, radiofrequency, for hepatocellular carcinoma, 160 161 Adenocarcinoma, pancreatic.
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Standard Regimens for Haematology
 Regimens for Haematology ChlVPP Chlorambucil 6mg/m 2 PO D1 to 14 Vinblastine 6mg/m 2 (max 10mg) IV on D1 & 8 Procarbazine 100mg/m 2 PO on D1 to 14 Prednisolone 40mg PO D1 to 14 ABVD Doxorubicin 25mg/m
Regimens for Haematology ChlVPP Chlorambucil 6mg/m 2 PO D1 to 14 Vinblastine 6mg/m 2 (max 10mg) IV on D1 & 8 Procarbazine 100mg/m 2 PO on D1 to 14 Prednisolone 40mg PO D1 to 14 ABVD Doxorubicin 25mg/m
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
FEATURE TOPIC IMPORTANT CHANGES IN PACLITAXEL PRICING
 Volume 6, Number 6 for health professionals who care for cancer patients June 2003 Available on website www.bccancer.bc.ca FEATURE TOPIC IMPORTANT CHANGES IN PACLITAXEL PRICING A recent Federal Court ruling
Volume 6, Number 6 for health professionals who care for cancer patients June 2003 Available on website www.bccancer.bc.ca FEATURE TOPIC IMPORTANT CHANGES IN PACLITAXEL PRICING A recent Federal Court ruling
Chronic Lymphocytic Leukemia Update. Learning Objectives
 Chronic Lymphocytic Leukemia Update Ashley Morris Engemann, PharmD, BCOP, CPP Clinical Associate Adult Stem Cell Transplant Program Duke University Medical Center August 8, 2015 Learning Objectives Recommend
Chronic Lymphocytic Leukemia Update Ashley Morris Engemann, PharmD, BCOP, CPP Clinical Associate Adult Stem Cell Transplant Program Duke University Medical Center August 8, 2015 Learning Objectives Recommend
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
SPECIAL AUTHORIZATION REQUEST FOR COVERAGE OF HIGH COST CANCER DRUGS
 SPECIAL AUTHORIZATION REQUEST FOR COVERAGE OF HIGH COST CANCER DRUGS (Filgrastim, Capecitabine, Imatinib, Dasatinib, Erolotinib, Sunitinib, Pazopanib, Fludarabine, Sorafenib, Crizotinib, Tretinoin, Nilotinib,
SPECIAL AUTHORIZATION REQUEST FOR COVERAGE OF HIGH COST CANCER DRUGS (Filgrastim, Capecitabine, Imatinib, Dasatinib, Erolotinib, Sunitinib, Pazopanib, Fludarabine, Sorafenib, Crizotinib, Tretinoin, Nilotinib,
Gazyva (obinutuzumab)
 STRENGTH DOSAGE FORM ROUTE GPID 1000mg/40mL Vial Intravenous 35532 MANUFACTURER Genentech, Inc. INDICATION(S) Gazyva (obinutuzumab) is a CD20- directed cytolytic antibody and is indicated, in combination
STRENGTH DOSAGE FORM ROUTE GPID 1000mg/40mL Vial Intravenous 35532 MANUFACTURER Genentech, Inc. INDICATION(S) Gazyva (obinutuzumab) is a CD20- directed cytolytic antibody and is indicated, in combination
For Health Professionals Who Care For Cancer Patients Available online at:
 September 2011 Volume 14, Number 9 For Health Professionals Who Care For Cancer Patients Available online at: www.bccancer.bc.ca/hpi/chemotherapyprotocols/stupdate Inside This Issue: Editor s Choice 2011-2012
September 2011 Volume 14, Number 9 For Health Professionals Who Care For Cancer Patients Available online at: www.bccancer.bc.ca/hpi/chemotherapyprotocols/stupdate Inside This Issue: Editor s Choice 2011-2012
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center
 Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
Active Cancer Studies by Approval Date For additional information on any one of these studies contact the Lancaster General Cancer Center 717-544-3113 PROTOCOL NO STUDY TITLE PRINCIPAL INVESTIGATOR ECOG
PROTOCOL UPDATE PATIENT HANDOUTS
 Volume 2, Number 2 for health professionals who care for cancer patients February 1999 I NSIDE THIS ISSUE Benefit drug list: Paclitaxel and carboplatin (GOOVCATX), tamoxifen Clinical trial update: Letrozole
Volume 2, Number 2 for health professionals who care for cancer patients February 1999 I NSIDE THIS ISSUE Benefit drug list: Paclitaxel and carboplatin (GOOVCATX), tamoxifen Clinical trial update: Letrozole
Azacitidine Vidaza Non-transplant myelodysplastic syndrome Funded Funded Funded Funded Funded Funded Not Funded
 Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
Provincial Fundin Summary The interim Joint Oncoloy Dru Review (ijodr) was the precursor oncoloy dru review process prior to pcodr, which provided evidence-based recommendation for cancer treatments from
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Docetaxel. Class: Antineoplastic agent, Antimicrotubular, Taxane derivative.
 Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
trial update clinical
 trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY NFORMATON n format provided by Melero et al. (AUGUST 2015) Supplementary nformation S3 Combinations including two or more immunotherapy agents based on PD-1/PD-L1 blockade. (Source: https://clinicaltrials.gov/
SUPPLEMENTARY NFORMATON n format provided by Melero et al. (AUGUST 2015) Supplementary nformation S3 Combinations including two or more immunotherapy agents based on PD-1/PD-L1 blockade. (Source: https://clinicaltrials.gov/
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Product Visual Guide
 Product Visual Guide Teamwork A team with an unflinching faith in one another is one of our core strength. Excellence Achieving excellence is not the end result, we begin with excelling in any endeavor.
Product Visual Guide Teamwork A team with an unflinching faith in one another is one of our core strength. Excellence Achieving excellence is not the end result, we begin with excelling in any endeavor.
CCC Chemotherapy Protocols V9.0
 CCC Chemotherapy Protocols V9.0 General observations 3 Breast cancer 5 Gastrointestinal cancer Oesophagus 17 Gastric 19 Pancreas 22 Cholangiocarcinoma 24 Hepatocellular carcinoma 25 Neuroendocrine tumours
CCC Chemotherapy Protocols V9.0 General observations 3 Breast cancer 5 Gastrointestinal cancer Oesophagus 17 Gastric 19 Pancreas 22 Cholangiocarcinoma 24 Hepatocellular carcinoma 25 Neuroendocrine tumours
Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens. Low Grade Lymphoma
 Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for low grade lymphoma and Hairy Cell leukemia used in the palliative
Palliative Low Grade Lymphoma & Hairy Cell Leukemia Regimens The following table lists the evidence-informed regimens (both IV and non-iv) for low grade lymphoma and Hairy Cell leukemia used in the palliative
Volume 7, Number 7 for health professionals who care for cancer patients July 2004 Website access at
 Volume 7, Number 7 for health professionals who care for cancer patients July 2004 Website access at http://www.bccancer.bc.ca/stupdate/ I NSIDE THIS ISSUE Highlights of Protocol Changes New Adjuvant Therapy
Volume 7, Number 7 for health professionals who care for cancer patients July 2004 Website access at http://www.bccancer.bc.ca/stupdate/ I NSIDE THIS ISSUE Highlights of Protocol Changes New Adjuvant Therapy
Velcade (bortezomib)
 Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 03/09/2004 Current Effective Date: 03/01/2018 POLICY A. INDICATIONS The indications below
Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 03/09/2004 Current Effective Date: 03/01/2018 POLICY A. INDICATIONS The indications below
Notification to Implement Issued by pcodr: December 14, 2012
 PROVINCIAL FUNDING SUMMARY Bendamustine hydrochloride (Treanda) for indolent Non-Hodgkin Lymphoma and Mantle Cell Lymphoma (first-line and relapsed/refractory) perc Recommendation: Recommends For further
PROVINCIAL FUNDING SUMMARY Bendamustine hydrochloride (Treanda) for indolent Non-Hodgkin Lymphoma and Mantle Cell Lymphoma (first-line and relapsed/refractory) perc Recommendation: Recommends For further
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
World Health Organization: Essential Medicines and Devices for Cancer:
 World Health Organization: Essential Medicines and Devices for Cancer: Principles, Methodologies, and Outcomes Lawrence N. Shulman, MD Abramson Cancer Center, University of Pennsylvania Partners In Health
World Health Organization: Essential Medicines and Devices for Cancer: Principles, Methodologies, and Outcomes Lawrence N. Shulman, MD Abramson Cancer Center, University of Pennsylvania Partners In Health
Corporate Medical Policy
 Corporate Medical Policy Monoclonal Antibodies for Non-Hodgkin Lymphoma and Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: monoclonal_antibodies_for_non_hodgkin_lymphoma_acute_myeloid_leukemia
Corporate Medical Policy Monoclonal Antibodies for Non-Hodgkin Lymphoma and Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: monoclonal_antibodies_for_non_hodgkin_lymphoma_acute_myeloid_leukemia
NCCN Non Hodgkin s Lymphomas Guidelines V Update Meeting 06/14/12 and 06/15/12
 NCCN Non Hodgkin s Lymphomas Guidelines V.1.213 Update Meeting 6/14/12 and 6/15/12 Guidelines Page and Request Chronic Lymphocytic Leukemia/ Small Lymphocytic Lymphoma (CLL/SLL) Panel Discussion References
NCCN Non Hodgkin s Lymphomas Guidelines V.1.213 Update Meeting 6/14/12 and 6/15/12 Guidelines Page and Request Chronic Lymphocytic Leukemia/ Small Lymphocytic Lymphoma (CLL/SLL) Panel Discussion References
Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona
 Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona Solid Tumor: USON 15180 / A Multicenter, Open-Label Phase 1b/2 Trial of Lenvatinib (E7080) Plus Pembrolizumab
Open and Pending Trials Listing For Arizona Oncology Associates P.C. HOPE Division/ Tucson, Arizona Solid Tumor: USON 15180 / A Multicenter, Open-Label Phase 1b/2 Trial of Lenvatinib (E7080) Plus Pembrolizumab
Initial Recommendation for Ibrutinib (Imbruvica) for Mantle Cell Lymphoma perc Meeting: June 16, pcodr PAN-CANADIAN ONCOLOGY DRUG REVIEW 4
 although ibrutinib was associated with significant improvements in PFS and QoL, adverse events such as fatigue and bleeding were still observed in patients who received ibrutinib. perc concluded that overall,
although ibrutinib was associated with significant improvements in PFS and QoL, adverse events such as fatigue and bleeding were still observed in patients who received ibrutinib. perc concluded that overall,
London Cancer New Drugs Group. February London Cancer New Drugs Group (LCNDG) Work Plan for the London Cancer Drugs Fund list.
 February 2013 London Cancer New s Group (LCNDG) Work Plan for the London Cancer s Fund London Cancer s Fund List This Cancer s Fund (CDF) list of medicines and s is in two parts. 1. The standard list of
February 2013 London Cancer New s Group (LCNDG) Work Plan for the London Cancer s Fund London Cancer s Fund List This Cancer s Fund (CDF) list of medicines and s is in two parts. 1. The standard list of
Mantle cell lymphoma An update on management
 Mantle cell lymphoma An update on management Dr Kim Linton Consultant Medical Oncologist The Christie NHS Foundation Trust 6 th October 2016 This educational meeting is organised and sponsored by Janssen-Cilag
Mantle cell lymphoma An update on management Dr Kim Linton Consultant Medical Oncologist The Christie NHS Foundation Trust 6 th October 2016 This educational meeting is organised and sponsored by Janssen-Cilag
Adult Intravenous Systemic Anticancer Therapy (SACT) Section A. SUMMARY of SCHEME QIPP Reference
 CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
1 The Cancer Programs Regulation (AR 242/98) is amended by this Regulation.
 Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Clinical Policy: Bendamustine (Bendeka, Treanda) Reference Number: CP.PHAR.307 Effective Date: Last Review Date: 11.18
 Clinical Policy: (Bendeka, Treanda) Reference Number: CP.PHAR.307 Effective Date: 02.01.17 Last Review Date: 11.18 Coding Implications Revision Log Line of Business: Medicaid, HIM-Medical Benefit See Important
Clinical Policy: (Bendeka, Treanda) Reference Number: CP.PHAR.307 Effective Date: 02.01.17 Last Review Date: 11.18 Coding Implications Revision Log Line of Business: Medicaid, HIM-Medical Benefit See Important
Susan O Reilly, MB, FRCPC Provincial Systemic Program Leader
 Volume 1, Number 5 for health professionals who care for cancer patients November 1998 I NSIDE THIS ISSUE Benefit drug list: Gemcitabine Cancer Drug Manual: Paclitaxel Drug update: Fluorouracil bolus administration,
Volume 1, Number 5 for health professionals who care for cancer patients November 1998 I NSIDE THIS ISSUE Benefit drug list: Gemcitabine Cancer Drug Manual: Paclitaxel Drug update: Fluorouracil bolus administration,
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT
 NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including accrual, and
NCIC CLINICAL TRIALS GROUP DATA SAFETY MONITORING COMMITTEE Friday, 1 May 2009 SUMMARY REPORT The NCIC CTG DSMC reviewed the following trials with respect to safety, trial conduct, including accrual, and
FAX request form and IN TOUCH phone list are provided if additional information is needed. EDITOR S CHOICE
 Volume 8, Number 8 for health professionals who care for cancer patients August 2005 Website access at http://www.bccancer.bc.ca/stupdate/ I NSIDE THIS ISSUE Editor s Choice: Adjuvant Oxaliplatin for Colon
Volume 8, Number 8 for health professionals who care for cancer patients August 2005 Website access at http://www.bccancer.bc.ca/stupdate/ I NSIDE THIS ISSUE Editor s Choice: Adjuvant Oxaliplatin for Colon
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Medical Therapies in Ovarian Cancer The Arabic Perspectives. Mezghani Bassem -Tunisia
 Tunisian Health System: Social Welfare with a Public insurance for all citizens including Indigent persons. (± Additional private insurance) Choice: Public Hospital/Private Clinics (Indigents Public H)
Tunisian Health System: Social Welfare with a Public insurance for all citizens including Indigent persons. (± Additional private insurance) Choice: Public Hospital/Private Clinics (Indigents Public H)
I NSIDE THIS ISSUE. Susan O Reilly, MB, FRCPC Provincial Systemic Program Leader
 Volume 6, Number 7 for health professionals who care for cancer patients July 2003 Available on website www.bccancer.bc.ca I NSIDE THIS ISSUE Highlights of Protocol Changes Benefit Drug List Busulfan,
Volume 6, Number 7 for health professionals who care for cancer patients July 2003 Available on website www.bccancer.bc.ca I NSIDE THIS ISSUE Highlights of Protocol Changes Benefit Drug List Busulfan,
Summary of Research and Writing Activities in Oncology
 Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
CURRENTLY ENROLLING ONCOLOGY TREATMENT STUDIES (as of 4/27/2017)
 CURRENTLY ENROLLING ONCOLOGY TREATMENT STUDIES (as of 4/27/2017) Leukemia AALL0932 closed after Induction Treatment of Patients with Newly Diagnosed Standard Risk B-Lymphoblastic Leukemia (B-ALL) or Localized
CURRENTLY ENROLLING ONCOLOGY TREATMENT STUDIES (as of 4/27/2017) Leukemia AALL0932 closed after Induction Treatment of Patients with Newly Diagnosed Standard Risk B-Lymphoblastic Leukemia (B-ALL) or Localized
Erbitux. Erbitux (cetuximab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.84 Subject: Erbitux Page: 1 of 6 Last Review Date: December 2, 2016 Erbitux Description Erbitux (cetuximab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.84 Subject: Erbitux Page: 1 of 6 Last Review Date: December 2, 2016 Erbitux Description Erbitux (cetuximab)
Background. Approved by FDA and EMEA for CLL and allows for treatment without chemotherapy in all lines of therapy
 Updated Efficacy and Safety From the Phase 3 RESONATE-2 Study: Ibrutinib As First-Line Treatment Option in Patients 65 Years and Older With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Abstract
Updated Efficacy and Safety From the Phase 3 RESONATE-2 Study: Ibrutinib As First-Line Treatment Option in Patients 65 Years and Older With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Abstract
CURRENTLY ENROLLING ONCOLOGY TREATMENT STUDIES (as of )
 Leukemia AALL0932 closed after Induction CURRENTLY ENROLLING ONCOLOGY TREATMENT STUDIES (as of 10.10.2017) Treatment of Patients with Newly Diagnosed Standard Risk B-Lymphoblastic Leukemia (B-ALL) or Localized
Leukemia AALL0932 closed after Induction CURRENTLY ENROLLING ONCOLOGY TREATMENT STUDIES (as of 10.10.2017) Treatment of Patients with Newly Diagnosed Standard Risk B-Lymphoblastic Leukemia (B-ALL) or Localized
BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab
 BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab Protocol Code Tumour Group Contact Physicians LYCLLFLUDR Lymphoma Dr.
BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab Protocol Code Tumour Group Contact Physicians LYCLLFLUDR Lymphoma Dr.
TRANSPARENCY COMMITTEE OPINION. 27 January 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 January 2010 TORISEL 25 mg/ml, concentrate for solution and diluent for solution for infusion Box containing 1
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 27 January 2010 TORISEL 25 mg/ml, concentrate for solution and diluent for solution for infusion Box containing 1
For Health Professionals Who Care For Cancer Patients
 November 2012 Volume 15, Number 11 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Bendamustine for Relapsed Indolent Non-Hodgkin Lymphoma and Mantle
November 2012 Volume 15, Number 11 For Health Professionals Who Care For Cancer Patients Inside This Issue: Editor s Choice New Programs: Bendamustine for Relapsed Indolent Non-Hodgkin Lymphoma and Mantle
BC Cancer Benefit Drug List April 2018
 Page 1 of 55 DEFNTONS Class estricted Funding () eimbursed for active cancer or approved treatment or approved indication only. eimbursed for approved indications only. Completion of the BC Cancer Compassionate
Page 1 of 55 DEFNTONS Class estricted Funding () eimbursed for active cancer or approved treatment or approved indication only. eimbursed for approved indications only. Completion of the BC Cancer Compassionate
Medication Policy Manual. Topic: Arzerra, ofatumumab Date of Origin: January 15, 2010
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru196 Topic: Arzerra, ofatumumab Date of Origin: January 15, 2010 Committee Approval Date: January
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru196 Topic: Arzerra, ofatumumab Date of Origin: January 15, 2010 Committee Approval Date: January
An introduction to the Essential Medicines concept: balancing innovation with public health priorities
 An introduction to the Essential Medicines concept: balancing innovation with public health priorities WHO HQ, Geneva 11 October 2017 Nicola Magrini Secretary, WHO Expert Committee on the Selection and
An introduction to the Essential Medicines concept: balancing innovation with public health priorities WHO HQ, Geneva 11 October 2017 Nicola Magrini Secretary, WHO Expert Committee on the Selection and
Working Formulary January 2013 Oncology Chemotherapy Regimens
 Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
University of Groningen. Health economics of targeted cancer therapies Mihajlovic, Jovan
 University of Groningen Health economics of targeted therapies Mihajlovic, Jovan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Health economics of targeted therapies Mihajlovic, Jovan IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
CLINICAL TRIALS ACC. Jul 2016
 CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
CLINICAL TRIALS ACC Jul 2016 Glioblastoma BRAIN A071102 A Phase II/III Randomized Trial of Veliparib or Placebo in Combination With Temozolomide in Newly Diagnosed Glioblastoma With MGMT Promoter Hypermethylation
H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS
 H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS Each of you should have 37 new cards: 7 orange cards for antimetabolites 11 white cards for miscellaneous drugs (2 DNA synthesis inhibitors, 1 enzyme,
H&HD ANTINEOPLASTIC DRUG CARD ASSEMBLY INSTRUCTIONS Each of you should have 37 new cards: 7 orange cards for antimetabolites 11 white cards for miscellaneous drugs (2 DNA synthesis inhibitors, 1 enzyme,
RITUXAN (rituximab and hyaluronidase human)
 Drug Prior Authorization Guideline RITUXIMAB products J9310 RITUXAN (rituximab and hyaluronidase human) PA9847 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria
Drug Prior Authorization Guideline RITUXIMAB products J9310 RITUXAN (rituximab and hyaluronidase human) PA9847 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria
CancerPACT Cancer Patients Alliance for Clinical Trials
 TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Salinas Valley Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
TM CancerPACT Cancer Patients Alliance for Clinical Trials Listing of Ongoing Cancer Clinical Trials in the Salinas Valley Winter 2008 I. Solid Tumors 1. Breast p.1 2. Central Nervous System p.2 3. Gastrointestinal
Inpatient Antineoplastic Medication Administration And Associated Drug Costs: Institution of a Hospital Policy Limiting Inpatient Administration
 Inpatient Antineoplastic Medication Administration And Associated Drug Costs: Institution of a Hospital Policy Limiting Inpatient Administration Alexandra E. Foster, PharmD; and David J. Reeves, PharmD,
Inpatient Antineoplastic Medication Administration And Associated Drug Costs: Institution of a Hospital Policy Limiting Inpatient Administration Alexandra E. Foster, PharmD; and David J. Reeves, PharmD,
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Rituxan (rituximab) Policy Number: MP-031-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Issue Date: 11/01/2017
CLINICAL MEDICAL POLICY Policy Name: Rituxan (rituximab) Policy Number: MP-031-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Issue Date: 11/01/2017
Disease Site Sub-Disease Site Intent Regimen Code Regimen Details Status (as of October 17, 2017) Breast Not Applicable Adjuvant / Curative
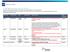 ST-QBP Regimen Request Status for 2017/18 Below are status of regimen requests submitted for funding considerations in FY 17/18 Q1 & Q2. Requests with status will be updated on the ST-QBP website and will
ST-QBP Regimen Request Status for 2017/18 Below are status of regimen requests submitted for funding considerations in FY 17/18 Q1 & Q2. Requests with status will be updated on the ST-QBP website and will
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Herceptin) Reference Number: ERX.SPA.42 Effective Date: 07.01.16 Last Review Date: 05/17 Line of Business: Commercial [Prescription Drug Plan] Revision Log See Important Reminder at the
Clinical Policy: (Herceptin) Reference Number: ERX.SPA.42 Effective Date: 07.01.16 Last Review Date: 05/17 Line of Business: Commercial [Prescription Drug Plan] Revision Log See Important Reminder at the
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Venclexta) Reference Number: CP.PHAR.129 Effective Date: 07.17.18 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Venclexta) Reference Number: CP.PHAR.129 Effective Date: 07.17.18 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this
MEDICAL PRIOR AUTHORIZATION
 MEDICAL PRIOR AUTHORIZATION TAXOTERE (docetaxel) DOCEFREZ(docetaxel) docetaxel (generic) POLICY I. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
MEDICAL PRIOR AUTHORIZATION TAXOTERE (docetaxel) DOCEFREZ(docetaxel) docetaxel (generic) POLICY I. INDICATIONS The indications below including FDA-approved indications and compendial uses are considered
Highlights from the 2017 Annual Meeting of the American Society of Hematology
 Latest NEWS IN BLOOD CANCER RESEARCH Highlights from the 2017 Annual Meeting of the American Society of Hematology CancerCare Connect Booklet Series www.cancercare.org The CancerCare Connect Booklet Series
Latest NEWS IN BLOOD CANCER RESEARCH Highlights from the 2017 Annual Meeting of the American Society of Hematology CancerCare Connect Booklet Series www.cancercare.org The CancerCare Connect Booklet Series
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
CENTENE PHARMACY AND THERAPEUTICS DRUG REVIEW 3Q17 April May
 BRAND NAME Keytruda GENERIC NAME Pembrolizumab MANUFACTURER Merck & Co., Inc. DATE OF APPROVAL Non-small cell lung cancer (NSCLC) indication: May 10, 2017 Urothelial carcinoma indication: May 18, 2017
BRAND NAME Keytruda GENERIC NAME Pembrolizumab MANUFACTURER Merck & Co., Inc. DATE OF APPROVAL Non-small cell lung cancer (NSCLC) indication: May 10, 2017 Urothelial carcinoma indication: May 18, 2017
Velcade (bortezomib)
 Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 03/09/2004 Current Effective Date: 05/01/2017 POLICY A. INDICATIONS The indications below
Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 03/09/2004 Current Effective Date: 05/01/2017 POLICY A. INDICATIONS The indications below
Antiemetic protocol for rare emetogenic chemotherapy - see protocol SCNAUSEA
 BCCA Protocol Summary for Treatment of Previously Untreated Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma with obinutuzumab and Chlorambucil Protocol Code Tumour Group Contact Physician
BCCA Protocol Summary for Treatment of Previously Untreated Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma with obinutuzumab and Chlorambucil Protocol Code Tumour Group Contact Physician
National Horizon Scanning Centre. Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer. December 2007
 Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
Aflibercept (VEGF Trap) for advanced chemo-refractory epithelial ovarian cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
Myeloma care and proteasome inhibitors. Brendan M. Weiss, MD Abramson Cancer Center University of Pennsylvania
 Myeloma care and proteasome inhibitors Brendan M. Weiss, MD Abramson Cancer Center University of Pennsylvania Why care about CV toxicities in MM? Median age 72 years About 2/3 have CV disease at baseline
Myeloma care and proteasome inhibitors Brendan M. Weiss, MD Abramson Cancer Center University of Pennsylvania Why care about CV toxicities in MM? Median age 72 years About 2/3 have CV disease at baseline
National Horizon Scanning Centre. Rituximab (MabThera) for chronic lymphocytic leukaemia. September 2007
 Rituximab (MabThera) for chronic lymphocytic leukaemia This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Rituximab (MabThera) for chronic lymphocytic leukaemia This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
